Abstract
We aimed to estimate the coronavirus disease 2019 (COVID-19) vaccine acceptance rate and identify predictors associated with acceptance. To this end, we searched PubMed, Web of Science, Cochrane Library, and Embase databases until November 4, 2020. Meta-analyses were performed to estimate the rate with 95% confidence intervals (CI). Predictors were identified to be associated with vaccination intention based on the health belief model framework. Thirty-eight articles, with 81,173 individuals, were included. The pooled COVID-19 vaccine acceptance rate was 73.31% (95%CI: 70.52, 76.01). Studies using representative samples reported a rate of 73.16%. The pooled acceptance rate among the general population (81.65%) was higher than that among healthcare workers (65.65%). Gender, educational level, influenza vaccination history, and trust in the government were strong predictors of COVID-19 vaccination willingness. People who received an influenza vaccination in the last year were more likely to accept COVID-19 vaccination (odds ratio: 3.165; 95%CI: 1.842, 5.464). Protecting oneself or others was the main reason for willingness, and concerns about side effects and safety were the main reasons for unwillingness. National- and individual-level interventions can be implemented to improve COVID-19 vaccine acceptance before large-scale vaccine rollout. Greater efforts could be put into addressing negative predictors associated with willingness.
Keywords: COVID-19, Vaccine, Acceptance, Predictors, Systematic review
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is currently the most pressing global issue. COVID-19 has led to 1.75 million deaths worldwide (World Health Organization [WHO], 2021) and exerted an unprecedented impact across every aspect of life globally.
Effective interventions are key to controlling COVID-19 spread, and vaccinations are considered a routine and effective measure for controlling infectious diseases (Hajj et al., 2015). Recently, several studies have reported the effectiveness of novel COVID-19 vaccines. The efficacy of the BNT162b2 mRNA vaccine has been reported to be 95% (Polack et al., 2020), that of the mRNA-1273 vaccine is 94.1% (Baden et al., 2021), that of the Gam-COVID-Vac (Sputnik V) is 91.6% (Logunov et al., 2021), and that of the ChAdOx1 nCoV-19 vaccine is 70.4% (Voysey et al., 2021). Overall, the reported efficacy is far higher than the standard (50% efficacy) developed by the U.S. Food and Drug Administration (Food and Drug Administration, 2020).
In addition to the effectiveness and safety of the COVID-19 vaccine, there are a few important considerations: (1) how can the vaccine be equitably allocated globally? (2) and what is the population's acceptance of the COVID-19 vaccine? Allocation and distribution frameworks for COVID-19 vaccines have been proposed in previous studies (Emanuel et al., 2020; Wang et al., 2020a, Wang et al., 2020b). Six core ethical principles (human well-being, equal respect, global equity, national equity, reciprocity, and legitimacy) have been proposed by the WHO to guide the distribution of vaccines (WHO, 2020b). The “Allocation Mechanism for COVID-19 Vaccines Global Access (COVAX) Facility Vaccines” document suggested that, for each country, proportional allocation - subject to country readiness and the availability of doses - should be used to achieve a vaccination rate of 20% of the total population in phase I, and that weighted allocation (considering vulnerability and COVID-19 threat) should be used when beyond 20% of phase II (WHO, 2020a).
Even the most effective vaccine has a limited impact on the spread of a disease if people refuse to take it. In 2018, the largest measles outbreak occurred in New York City, America in nearly 30 years (Yang, 2020). A total of 148,279 cases were reported by the European Centre for Disease Prevention and Control in European Union countries between 2010 and 2019 (Nicolay et al., 2020). The low uptake of the measles vaccine has contributed to continuous measles transmission (Nicolay et al., 2020; Yang, 2020). Concerns about side effects, mistrust in the government, and religious beliefs, among others, were found to be key factors resulting in vaccine hesitancy (Díaz et al., 2020).
COVID-19 vaccination intentions have been surveyed and reported in previous studies (Dror et al., 2020; Fisher et al., 2020; Lazarus et al., 2020). The proportion of willingness to undergo COVID-19 vaccination was 68.4% based on a meta-analysis (Wang et al., 2020a, Wang et al., 2020b). However, several problems were not examined or explored, and the representativeness of the samples was unclear. The bias in the sample may have an impact on the estimated willingness rate. However, the predictors associated with willingness were not identified in this study. By identifying associated predictors, governments and health authorities can inform the development of evidence-based guidelines and specific vaccine campaigns to effectively address COVID-19 vaccine hesitancy and improve vaccine uptake.
The purpose of this systematic review was to estimate the COVID-19 vaccine acceptance rate and identify predictors associated with COVID-19 vaccine acceptance.
2. Methods
2.1. Search strategy and selection criteria
A systematic search was performed using the PRISMA checklist (http://www.prisma-statement.org/) on four English databases (PubMed, Web of Science, Cochrane Library, and Embase) on November 4, 2020. The PRISMA checklist facilitated transparent and complete reporting of systematic reviews (Page et al., 2021). We used the following search terms: “COVID-19” OR “SARS-CoV-2” OR “novel coronavirus” OR “coronavirus disease 2019” AND “vaccin*” OR “immunization”. All search records were first screened by title and abstract; after exclusion of duplicate records, no relevant records were excluded. Two researchers (QW and LQY) reviewed the full text of all potential eligible studies. The peer-reviewed studies included in this review reported on at least two of the following three topics: the total number of surveyed persons, the number of persons intending to receive vaccination against COVID-19, and the COVID-19 vaccine acceptance rate. We did not limit the types of studies included.
We excluded studies that did not involve COVID-19 vaccine acceptance or did not provide specific survey numbers for pooling. Duplicate studies and data were excluded. Two researchers (QW and LQY) independently performed article selection, and disputes were settled by a third researcher (HJ). Additionally, we manually scanned the references of all articles in which full-text reading was performed to avoid missing any additional articles. The review protocol is available on PROSPERO (ID: CRD42020226875).
2.2. Data abstraction and quality assessment
We extracted the following information from the included articles: title, first author, journal, article type, survey period, surveyed location, sampling method, sample representativeness, survey method, survey population, questions about vaccination, and measurement method (such as 5-point Likert scale). When the original study claimed that a representative sample of a country (countries) or city (cities) was used, we verified this claim by assessing their surveying strategies before reporting it. All predictors of vaccine acceptance reported in the included studies were extracted according to the health belief model (HBM) framework (Lin et al., 2020; Prematunge et al., 2012; Janz and Becker, 1984). The framework has been used to explain the factors associated with immunization behaviors, such as seasonal influenza vaccination (Prematunge et al., 2012) and human papillomavirus vaccination (Batista et al., 2015) to predict vaccination uptake (Prematunge et al., 2012), and can provide good support for complex and effective interventions (Craig et al., 2008). Based on the HBM constructs, the predictors of vaccination willingness included perceived susceptibility and severity of COVID-19, perceived benefits and risks of vaccination acceptance (reasons for vaccination willingness or unwillingness), modified factors (such as socio-demographics and knowledge), and cues to action (internal and external stimuli for promoting vaccination) (Prematunge et al., 2012).
The STROBE statement, which offers guidance for observational research reporting, was used to assess the quality of the included studies (Von et al., 2007). The quality of the included studies was assessed using the 22 items in the STROBE statement (1 point for each item, for a total of 22 points). Two researchers (QW and LQY) performed the data abstraction and quality assessment independently, and the two results were compared to identify differences. If the data extracted for each article differed, the article was re-read. If the quality assessment scores for an article differed, the assistance of a third researcher (LL) was sought to resolve disputes.
2.3. Statistical analysis
All analyses were performed using Stata 14.0 software. We used a double arcsine transformation on the data and reported the pooled acceptance rate and 95% confidence interval (CI) with extracted variables, the total number of surveyed persons, and the number of persons who accepted vaccination against COVID-19. If the number of persons accepting vaccination against COVID-19 was not provided in the study, we calculated it by multiplying the total number by the willingness rate. DerSimonian–Laird random effects were used in the pooling process. Stratified subgroups and meta-regression analyses were conducted to explore the sources of heterogeneity according to the study characteristics. Some studies only reported the inclusion criteria of study population was adults. We used the term “mixed general population” instead of “general population” to account for the inclusion of studies in special populations such as healthcare workers (HCWs).
We analyzed the relationship between acceptance rates and the number of cumulative infections and daily increase in the number of infections in the global context or surveyed country during the survey period. More specific details regarding our calculations are available in the supplemental materials.
We systematically identified predictors associated with vaccination intention. Predictors reported in two or more studies were analyzed. The odds ratio (OR) was calculated and pooled when the data were appropriate and sufficient (e.g., gender). We described the predictors without pooling in the following cases: (1) the divisions of predictors were complex, such as income, and (2) the predictors were insufficient. We further compared our data with previous systematic reviews that reported the influencing factors associated with pandemic influenza vaccination.
3. Results
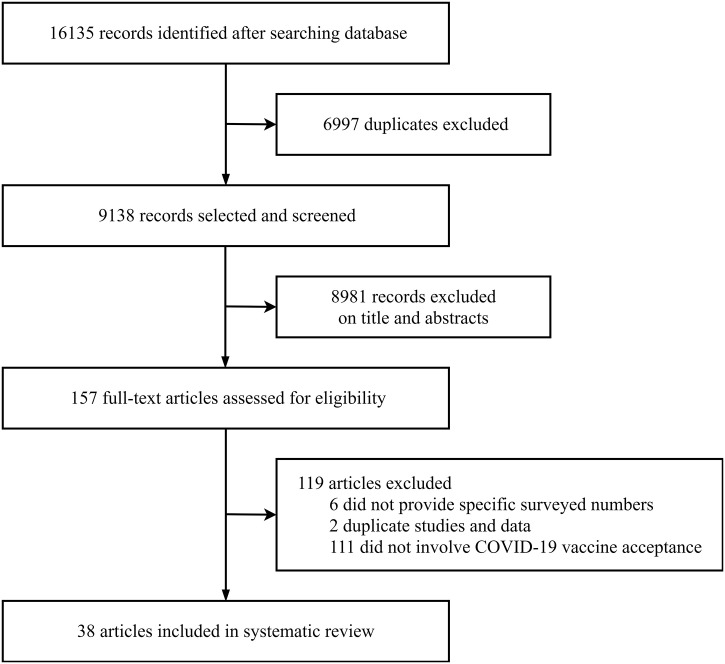
A total of 16,135 records were returned, of which 38 articles were included in the meta-analysis (Fig. 1 ). Most of the included studies (34/38) were performed online and by telephone (Table S1). Of the 38 studies, 28 were original articles, and 10 were brief communications, correspondences, or editorials. These studies reported cross-sectional surveys from 36 countries and regions with 81,173 surveyed individuals. The survey time included 38 studies covering February to September 2020. The quality assessment scores ranged from 10 to 20, with an average of 15.07 ± 2.91 (Table S2).
Fig. 1.
Flowchart of study selection.
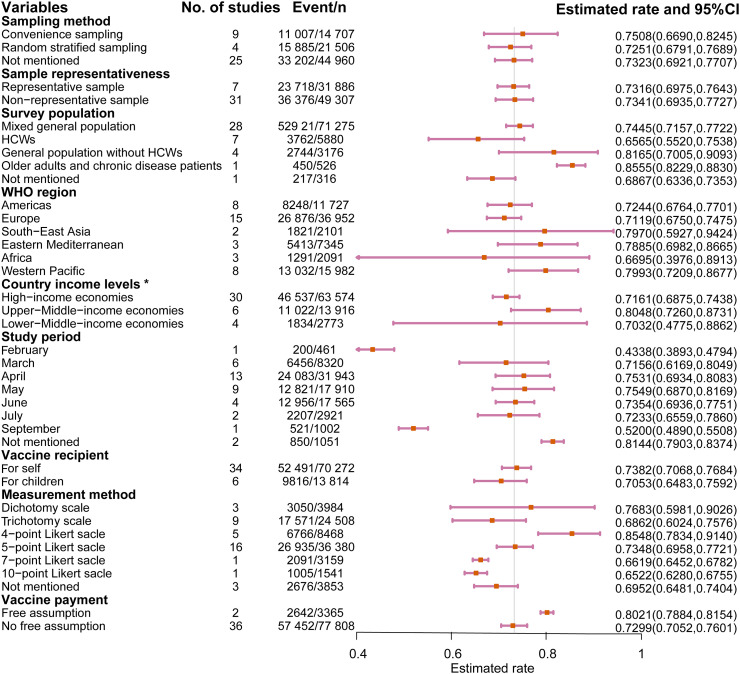
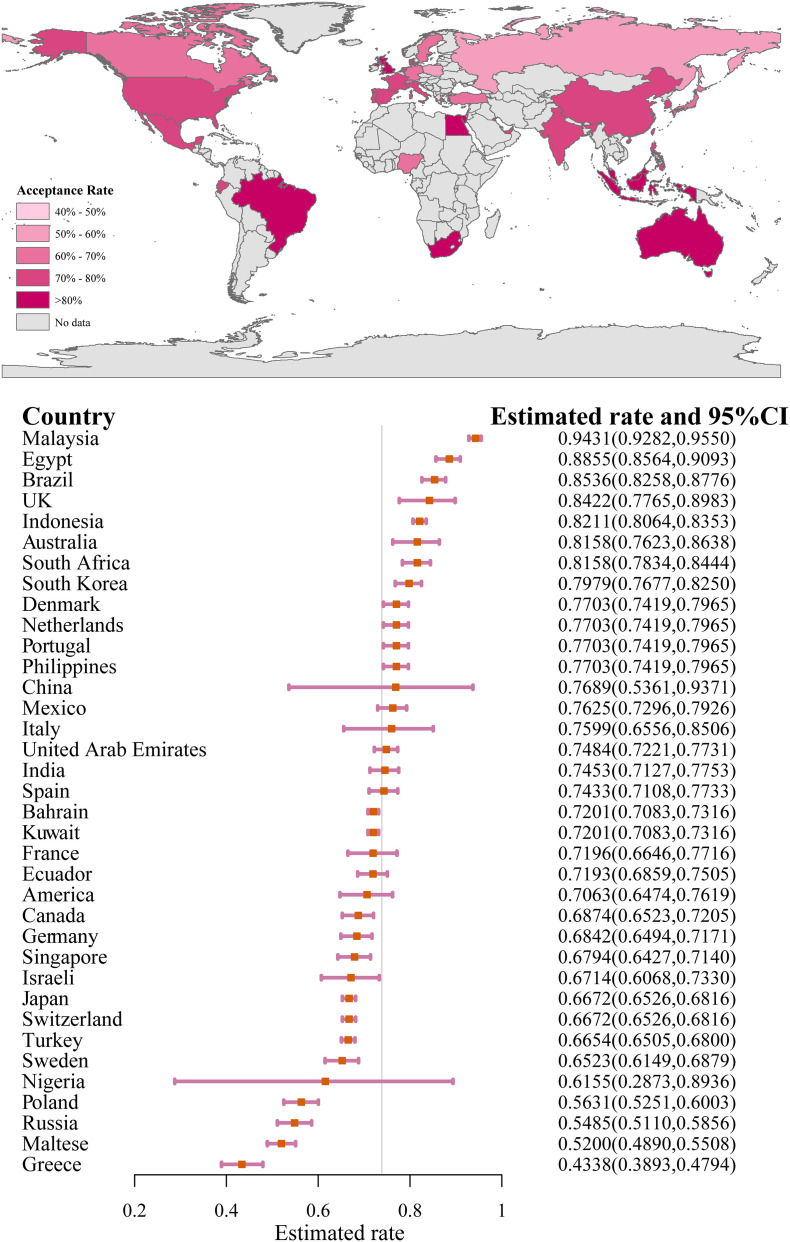
The COVID-19 vaccination acceptance rate was 73.31% (95%CI: 70.52, 76.01), as shown in Fig. S1. Studies using representative samples reported a rate of 73.16% (Fig. 2 ,). The acceptance rates were 74.45% (95%CI: 71.57, 77.22) and 81.65% (95%CI: 70.05, 90.93) among the mixed general population and general population without HCWs, respectively; both of which were higher than those of HCWs (65.65%, 95%CI: 55.20, 75.38). The willingness proportion ranged from 75.49% (May) to 43.38% (February) over the course of 7 months. Among the 38 studies, two used the assumption that the COVID-19 vaccine was free; when using this assumption, the proportion of willingness to vaccinate (80.21%) was significantly higher than when not using this assumption (72.99%). As shown in Fig. 3 , the results from Malaysia reported the highest proportion of willingness (94.31%), and the results from Greece reported the lowest proportion (43.38%).
Fig. 2.
COVID-19 vaccination acceptance rate by subgroup†.
†HCWs: Healthcare workers, the vertical bar represents the overall pooled rate (0.7331), there were no data in August being reported. *Division came from https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
Fig. 3.
Estimated COVID-19 vaccination acceptance rate worldwide†.
†The vertical bar represents the overall pooled rate (0.7331).
3.1. Meta-analysis regression
Table S3 shows that the survey population, income levels of the surveyed country, study period, and measurement method significantly affected the heterogeneity of the meta-analysis results (adjusted R2 = 28.02%). The differences among the surveyed populations explained the largest amount of heterogeneity (adjusted R2 = 14.56%).
3.2. Relationship with number of infections
We found no correlations between the proportion of vaccination acceptance, number of cumulative infections (r = −0.037, p = 0.842), and daily increase in the number of infections (r = −0.077, p = 0.674) in the global context. Similarly, correlations between vaccination acceptance proportion, number of cumulative infections (r = −0.062, p = 0.668), and daily increase in the number of infections (r = −0.092, p = 0.523) in each surveyed country were not significant. There was no significant correlation between COVID-19 vaccine acceptance and cumulative/ daily infections when lagged days were considered (Table S4).
3.3. Analysis of predictors
3.3.1. Perceived susceptibility and severity of COVID-19
The frequencies of the predictors are shown in Table S5. Seven studies reported an association between the perceptions of COVID-19 infection risk and COVID-19 willingness. Four studies found a positive relationship between perceived COVID-19 infection risk and COVID-19 vaccine willingness (Detoc et al., 2020; Faasse and Newby, 2020; Salali and Uysal, 2020; Wong et al., 2020); three studies provided evidence that this relationship was not significant (Fisher et al., 2020; Harapan et al., 2020; Wang et al., 2020a, Wang et al., 2020b). Two studies performed in Australia and Italy reported that concern about COVID-19 outbreak was not significantly associated with vaccination willingness (Faasse and Newby, 2020; Pierantoni et al., 2020).
3.3.2. Perceived benefits and risks of acceptance
Of the 38 studies, six explored the reasons for vaccination willingness or unwillingness. Protecting oneself or others was the most common reason for willingness (benefits of acceptance), and concerns about side effects and safety were the most common reasons for unwillingness (risks of acceptance).
Regarding the reasons for willingness in each study, the most common was “how well the vaccine works” (Reiter et al., 2020), followed by “protect person being vaccinated” (Bell et al., 2020), and “protect child” (Goldman et al., 2020a). Regarding reasons for refusal and hesitancy in each study, the most common was “side effects, safety,” followed by “don't believe in, want, or feel comfortable with vaccines” (Fisher et al., 2020), “suspicion on efficacy, effectiveness or safety” (Bell et al., 2020), “novelty” (Goldman et al., 2020a), “concerned about vaccine efficacy and safety” (Rhodes et al., 2020), “I'm concerned about potential side effects” and “new/rushed vaccine/ not enough evidence” (Neumann-Bohme et al., 2020).
3.3.3. Modified factors
In our review, the modified factors included the following: (1) socio-demographics; (2) knowledge, attitude, beliefs, and prior experience with COVID-19 and vaccination; and (3) trust.
3.3.4. Socio-demographics
Gender (17/38), age (16/38), and educational level (13/38) were the most frequent predictive factors reported in the included studies. Compared to men, women were less likely to accept the vaccine (OR: 0.728, 95%CI: 0.613, 0.865). We estimated the proportion of acceptance to be 83.00% in those aged ≥60 years, and it was higher than that observed in those aged <60 years (72.09%). Compared to people with high school education (equivalent) or below, people with a college degree or higher education were more likely to accept COVID-19 vaccination (OR–:1.613, 95%CI: 1.212, 2.145).
Higher household income has been found to be a positive predictor of COVID-19 vaccination willingness (Bell et al., 2020; Lazarus et al., 2020; Reiter et al., 2020; Ward et al., 2020). Some studies have found no significant relationship between respondent's household income and willingness (Fisher et al., 2020; Harapan et al., 2020; Pierantoni et al., 2020; Rhodes et al., 2020; Wong et al., 2020). In two articles using the “free vaccine assumption,” one showed that higher income was positively associated with COVID-19 vaccination willingness in America (Reiter et al., 2020), whereas the other concluded the association was not significant in Indonesia (Harapan et al., 2020). With regard to race/ethnicity, black people were less likely to accept COVID-19 vaccination than white people (OR:0.425, 95%CI: 0.312, 0.580). There was no significant difference between people living in urban and rural areas (OR–: 1.154; 95%CI: 0.955, 1.395; urban as the reference).
Employment status was not significantly associated with vaccination willingness (Bell et al., 2020; Fisher et al., 2020; Head et al., 2020; Malik et al., 2020). In one study conducted among a representative sample of Australian parents, employed people were more likely to accept vaccination than unemployed people (Rhodes et al., 2020). In contrast, unemployed people were more likely to accept vaccination in one study conducted among Israeli HCWs and the general population (Dror et al., 2020). No association between marital status and vaccination willingness was reported in three studies performed in America and Indonesia (Fisher et al., 2020; Harapan et al., 2020; Reiter et al., 2020). There was contradictory evidence on whether the status of having a child (or children) was an effective predictor. Having a child (or children) was negatively associated with vaccination acceptance in a study conducted in Turkey (Salali and Uysal, 2020), but was not significantly associated in studies conducted in America and China (Dong et al., 2020; Head et al., 2020). Differences in willingness rates were not significant among individuals with different occupations (Harapan et al., 2020; Wong et al., 2020).
3.3.5. Knowledge, attitude, beliefs, and prior experience
There was no significant relationship between people with chronic diseases and willingness to receive the COVID-19 vaccine among the general population in France and Malaysia (Detoc et al., 2020; Wong et al., 2020). Chronic conditions were positively associated with vaccination acceptance among nurses in Hong Kong (Wang et al., 2020a, Wang et al., 2020b). The difference between vaccination rates among people with different self-rated health grades was not significant in the United States and Indonesia (Faasse and Newby, 2020; Fisher et al., 2020). Influenza vaccination in the past season was an effective predictor of COVID-19 vaccination acceptance; in all four studies reporting data, people who received influenza vaccination in the last season were more likely to accept COVID-19 vaccination (OR:3.165, 95%CI: 1.842, 5.464).
3.3.6. Trust
Trust in the government was a positive predictive factor of vaccination willingness in 20 surveyed countries (Lazarus et al., 2020; Faasse and Newby, 2020).
3.3.7. Cues to action
The relationship between family members or friends having COVID-19 and vaccination willingness has not been demonstrated (Lazarus et al., 2020; Reiter et al., 2020; Wong et al., 2020). People with greater exposure to media reports about COVID-19 were more likely to accept COVID-19 vaccination in the UK, Turkey, and Australia (Faasse and Newby, 2020; Salali and Uysal, 2020). Furthermore, compared to those with conservative political leanings (views), those with liberal political leanings showed stronger COVID-19 vaccine willingness among the general population in America (Head et al., 2020; Reiter et al., 2020); however, one study conducted in America that did not report a survey population found no significant association between political leanings and vaccination willingness (Pogue et al., 2020).
3.3.8. Comparisons of reviews
We compared the predictors with factors influencing pandemic influenza vaccination reported by four previous systematic reviews (Table S5) (Brien et al., 2012; Prematunge et al., 2012; Bish et al., 2011; Nguyen et al., 2011). Seasonal influenza vaccination history was a positively effective predictor. In four studies, the evidence of socio-demographics associated with willingness was mixed. Perception of risk was not significantly associated with vaccination willingness, and concerns about safety and side effects were the main barriers to vaccination.
4. Discussion
The estimated COVID-19 vaccine acceptance rate was 73.31%, ranging from 43.38% to 94.31% across countries and regions. This number was similar with data reported by a previous study (Wang et al., 2020a, Wang et al., 2020b). Gender, educational level, influenza vaccination history, and trust in the government were strong predictors of COVID-19 vaccination willingness.
To date, the proportion of the population that must be vaccinated against COVID-19 to begin inducing herd immunity is yet unknown; a modelling study showed that to end this ongoing epidemic, the vaccines need to have an efficacy rate of at least 80% with a coverage of 75% (Bartsch et al., 2020). However, even though we found some countries/regions had high pooled rates, policy makers should be concerned about the acceptance of COVID-19 vaccination for the following reasons: 1) there are significant regional and local differences in the acceptance of COVID-19 vaccination. The pooled rates did not represent the rates within the countries or areas; countries/areas that have low coverage rates will continue to struggle with the fight against the disease; 2) Learning from prior experience with vaccinations, some existing barriers are expected to reduce actual vaccine uptake in certain subpopulations (Fisher et al., 2020; Ward et al., 2020).
HCWs should be given priority in receiving COVID-19 vaccines as they are key to COVID-19 responses and are considered to be at high risk of infection (Emanuel et al., 2020; McClung et al., 2020). However, hesitancy and refusal among HCWs to receive a COVID-19 vaccine remains a major concern. A previous study suggested that recommendations by HCWs were associated with uptake in the general population (Wang et al., 2018). If the COVID-19 vaccine is not mandatory, low uptake among HCWs might contribute to low uptake among the general population. Vaccine hesitancy and refusal among HCWs mainly originate from concerns regarding the safety and effectiveness of COVID-19 vaccines (Dror et al., 2020; Wang et al., 2020a, Wang et al., 2020b). Systematic strategies should be implemented to improve COVID-19 vaccine acceptance and uptake among HCWs. A previous review showed that a few combined interventions, including education and training sessions, easy vaccine accessibility, and rewards after vaccination, could increase influenza vaccination uptake (Rashid et al., 2016). We suggest that health education and better public health messaging could be used to eliminate HCWs' concerns about vaccine safety. Furthermore, HCWs should be informed about the benefits of protecting themselves, their patients, and their family and friends after vaccination. Additionally, the supply of COVID-19 vaccines to HCWs needs to be ensured to improve accessibility.
Vaccine acceptance predictors were comprehensively analyzed in this study. Among socio-demographic factors, gender, and education level were effective predictors. Moreover, our results demonstrated that women were less likely to accept the COVID-19 vaccine than men. A previous systematic review also demonstrated that women were less likely to be vaccinated during the 2009 global influenza pandemic (Bish et al., 2011). The reason for this may be that men engage in riskier behaviors than women (Goldman et al., 2020a). Furthermore, women's refusal and hesitancy to accept the COVID-19 vaccine may make vaccinating children difficult, as women play a key role in child vaccination when the COVID-19 vaccine is accessible to children (COCONEL Group, 2020).
The association between age and willingness to vaccinate is not conclusive (Brien et al., 2012; Bish et al., 2011; Nguyen et al., 2011). Vaccination intention may be related to different age groups. Most studies, including ours, have shown that older people (> 60 years) are more likely to accept vaccination (Bish et al., 2011). This is promising news given that the elderly are at a high risk of being infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Consistent with one review of barriers to vaccination (Guzman-Holst et al., 2020), low education levels might also contribute to low vaccine acceptance.
Influenza vaccination history is a strong predictor of vaccine acceptance. Our study and a previous review have shown that people with histories of seasonal influenza vaccination are more likely to be vaccinated against COVID-19 and pandemic influenza (Prematunge et al., 2012; Brien et al., 2012; Bish et al., 2011; Nguyen et al., 2011). Influenza vaccination history is an interesting indicator of people's attitudes toward vaccines (Schmid et al., 2017). It has been reported that willingness to be vaccinated against influenza was higher in 2020 than in the past (Goldman et al., 2020b). In China, as of November 9, 2020, the national usage of influenza vaccine was approximately 25 million doses, close to its usage in 2019 (Chinese National Health Commission, 2020). Perception of a higher likelihood of SARS-CoV-2 infection (Wang et al., 2020a, Wang et al., 2020b) and considering COVID-19 as a community threat (Sturm et al., 2021) were positively associated with influenza vaccination acceptance. We suggest that the present moment is an ideal opportunity to improve influenza vaccination uptake by using guided scientific public health strategies. Trust in the government also had an impact on the intention to be vaccinated. Previous reviews have demonstrated that trust in health authorities is associated with vaccination willingness (Bish et al., 2011; Prematunge et al., 2012). Disease control relies on credible information and guidance (Lazarus et al., 2020). In our study, the main reasons for unwillingness to undergo vaccination were safety considerations. A transparent, robust, and reasonable immunization process can improve public confidence in the COVID-19 vaccine (Lazarus et al., 2020).
We suggest that national and individual interventions be undertaken to improve COVID-19 vaccine acceptance and future uptake. At the national level, governments should inculcate public confidence in vaccines using scientific vaccine programs. Moreover, governments should be cautious and aware of potential trends in anti-vaccine movements. In particular, the Internet has expanded the audience for the anti-vaccine movement (Johnson et al., 2020), and it is possible that the explosive growth in anti-vaccination views will hinder the development and uptake of vaccines (Johnson et al., 2020).
At the individual level, people with vaccine hesitancy should be identified using easily available and effective predictors, and a series of combined interventions can be implemented to persuade these people to be vaccinated (Rashid et al., 2016). Among the predictors, some (such as media exposure) are more amendable than others (age). In terms of behavioral changes, these predictors require different interventions. Behavioral change theories such as the HBM and social marketing, which have been effectively adapted to improve individual use of medical interventions, should be considered when developing community engagement strategies for vaccine rollout (Lin et al., 2020; Opel et al., 2009).
Our study has several limitations. First, although this study included 36 countries and regions, it may not be representative of the global reaction to the COVID-19 vaccine. Indeed, data from low-income countries are very limited, and there is an urgent need for rapid research to support vaccine rollout worldwide. Second, the representability of the sample was uncertain in most studies, and the accessibility of the Internet (to complete an online survey) may become a barrier to generalizing the findings. Therefore, the selection of sample bias needs to be carefully considered. According to the sampling method and sample representability, we performed a stratified subgroup analysis and concluded that our findings have acceptable validity. We analyzed and compared the pooled willingness rate for representative samples (73.16%) versus non-representative samples (73.41%) and found that the gap was very small, and the results of the different sampling methods were similar. Additionally, sample representativeness and the sampling method did not significantly affect heterogeneity in the meta-regression. Third, attitudes to vaccination are influenced by many complex factors, including insurance and price, which might vary across different times, policies, and health systems. Finally, the effects of some predictors were not explored due to limitations of the data. Meanwhile, some predictors were not examined on a finer scale because the reported data did not provide sufficient details. As reported, the willingness rate in individuals aged <60 years was higher than that in those aged <60 years. However, the associated factors were not sufficiently granular to be explored further. Greater efforts to report data on a finer scale are needed in future research.
5. Conclusions
More than 70% of people reported that they were willing to be vaccinated against COVID-19. However, the acceptability rate still needs more attention. Gender, education level, and influenza vaccination history are positive predictors of COVID-19 vaccine acceptance. National-and-individual-level interventions should be undertaken to improve the COVID-19 acceptance rate and uptake in the future.
Funding
This study was supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant number KYCX20_0153); Wuxi City Technology Development Fund (grant number N20191007); UK Foreign, Commonwealth and Development Office and Wellcome [grant number 215373/A/19/Z]. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Contributors
Wang, Jin, and Lin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Wang, Jin, and Lin.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Wang and Lin.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Wang.
Obtained funding: Wang, Jin, and Lin.
Administrative, technical, or material support: Wang.
Supervision: Jin and Lin.
Ethical compliance
The underlying work is based on systematic reviews of published data and thus does not require ethical review approval.
Data sharing
No additional data available.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2021.106694.
Appendix A. Supplementary data
Supplementary material
References
- Baden L.R., Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch S.M., O’Shea K.J., Ferguson M.C., et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am. J. Prev. Med. 2020;59(4):493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista F.H., Audrey S., Trotter C., Hickman M. An appraisal of theoretical approaches to examining behaviours in relation to human papillomavirus (HPV) vaccination of young women. Prev. Med. 2015;81:122–131. doi: 10.1016/j.ypmed.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S., Clarke R., Mounier-Jack S., Walker J.L., Paterson P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. 2020;38(49):7789–7798. doi: 10.1016/j.vaccine.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish A., Yardley L., Nicoll A., Michie S. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29(38):6472–6484. doi: 10.1016/j.vaccine.2011.06.107. [DOI] [PubMed] [Google Scholar]
- Brien S., Kwong J.C., Buckeridge D.L. The determinants of 2009 pandemic a/H1N1 influenza vaccination: a systematic review. Vaccine. 2012;30(7):1255–1264. doi: 10.1016/j.vaccine.2011.12.089. [DOI] [PubMed] [Google Scholar]
- Chinese National Health Commission . 2020. About 25 million doses of influenza vaccine were administered nationwide as at 9 November.http://health.people.com.cn/n1/2020/1112/c14739-31929122.html [Google Scholar]
- COCONEL Group A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect. Dis. 2020;20(7):769–770. doi: 10.1016/S1473-3099(20)30426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P., Dieppe P., Macintyre S., et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detoc M., Bruel S., Frappe P., Tardy B., Botelho-Nevers E., Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38(45):7002–7006. doi: 10.1016/j.vaccine.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz C.M.E., Ghirotto L., Sisson H., et al. A meta-synthesis study of the key elements involved in childhood vaccine hesitancy. Public Health. 2020;180:38–45. doi: 10.1016/j.puhe.2019.10.027. [DOI] [PubMed] [Google Scholar]
- Dong D., Xu R.H., Wong E.L., et al. Public preference for COVID-19 vaccines in China: a discrete choice experiment. Health Expect. 2020;23(6):1543–1578. doi: 10.1111/hex.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror A.A., Eisenbach N., Taiber S., et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel E.J., Persad G., Kern A. An ethical framework for global vaccine allocation. Science. 2020;369:1309–1312. doi: 10.1126/science.abe2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faasse K., Newby J. Public perceptions of COVID-19 in Australia: perceived risk, knowledge, health-protective behaviors, and vaccine intentions. Front. Psychol. 2020;11:551004. doi: 10.3389/fpsyg.2020.551004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration Coronavirus (COVID-19) Update: FDA Takes Action to Help Facilitate Timely Development of Safe, Effective COVID-19 Vaccines. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-help-facilitate-timely-development-safe-effective-covid
- Goldman R.D., Yan T.D., Seiler M., et al. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine. 2020;38(48):7668–7673. doi: 10.1016/j.vaccine.2020.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R.D., McGregor S., Marneni S.R., et al. Willingness to vaccinate children against influenza after the coronavirus disease 2019 pandemic. J. Pediatr. 2020;S0022-3476(20) doi: 10.1016/j.jpeds.2020.08.005. 30987–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Holst A., DeAntonio R., Prado-Cohrs D., Juliao P. Barriers to vaccination in Latin America: a systematic literature review. Vaccine. 2020;38(3):470–481. doi: 10.1016/j.vaccine.2019.10.088. [DOI] [PubMed] [Google Scholar]
- Hajj H.I., Chams N., Chams S., et al. Vaccines through centuries: major cornerstones of global health. Front. Public Health. 2015;3:269. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan H., Wagner A.L., Yufika A., et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Front. Public Health. 2020;8:381. doi: 10.3389/fpubh.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head K.J., Kasting M.L., Sturm L.A., Hartsock J.A., Zimet G.D. A National Survey Assessing SARS-CoV-2 vaccination intentions: implications for future public health communication efforts. Sci. Commun. 2020;42(5):698–723. doi: 10.1177/1075547020960463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz N.K., Becker M.H. The health belief model: a decade later. Health Educ. Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Johnson N.F., Velásquez N., Restrepo N.J., et al. The online competition between pro- and anti-vaccination views. Nature. 2020;582(7811):230–233. doi: 10.1038/s41586-020-2281-1. [DOI] [PubMed] [Google Scholar]
- Lazarus J.V., Ratzan S.C., Palayew A., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020;1-4 doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Sun R., Yao T., Zhou X., Harbarth S. Factors influencing inappropriate use of antibiotics in outpatient and community settings in China: a mixed-methods systematic review. BMJ Glob. Health. 2020;5(11) doi: 10.1136/bmjgh-2020-003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A.A., McFadden S.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:100495. doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung N., Chamberland M., Kinlaw K., et al. The advisory committee on immunization practices’ ethical principles for allocating initial supplies of COVID-19 vaccine - United States, 2020. Morb. Mortal. Wkly. Rep. 2020;69(47):1782–1786. doi: 10.15585/mmwr.mm6947e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Bohme S., Varghese N.E., Sabat I., et al. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur. J. Health Econ. 2020;21(7):977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Henningsen K.H., Brehaut J.C., Hoe E., Wilson K. Acceptance of a pandemic influenza vaccine: a systematic review of surveys of the general public. Infect. Drug. Resist. 2011;4:197–207. doi: 10.2147/IDR.S23174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay N., Mirinaviciute G., Mollet T., Celentano L.P., Bacci S. Epidemiology of measles during the COVID-19 pandemic, a description of the surveillance data, 29 EU/EEA countries and the United Kingdom, January to may 2020. Euro. Surveill. 2020;25(31):2001390. doi: 10.2807/1560-7917.ES.2020.25.31.2001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel D.J., Diekema D.S., Lee N.R., Marcuse E.K. Social marketing as a strategy to increase immunization rates. Arch. Pediatr. Adolesc. Med. 2009;163(5):432–437. doi: 10.1001/archpediatrics.2009.42. [DOI] [PubMed] [Google Scholar]
- Page M.J., Moher D., Bossuyt P.M., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantoni L., Lenzi J., Lanari M., et al. Nationwide COVID-19 survey of Italian parents reveals useful information on attitudes to school attendance, medical support, vaccines and drug trials. Acta Paediatr. 2020;110(3):942–943. doi: 10.1111/apa.15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue K., Jensen J.L., Stancil C.K., et al. Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines (Basel). 2020;8(4):582. doi: 10.3390/vaccines8040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prematunge C., Corace K., McCarthy A., Nair R.C., Pugsley R., Garber G. Factors influencing pandemic influenza vaccination of healthcare workers--a systematic review. Vaccine. 2012;30(32):4733–4743. doi: 10.1016/j.vaccine.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Rashid H., Yin J.K., Ward K., King C., Seale H., Booy R. Assessing interventions to improve influenza vaccine uptake among health care workers. Health Aff (Millwood). 2016;35(2):284–292. doi: 10.1377/hlthaff.2015.1087. [DOI] [PubMed] [Google Scholar]
- Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38(42):6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes A., Hoq M., Measey M.A., Danchin M. Intention to vaccinate against COVID-19 in Australia. Lancet Infect. Dis. 2020;21(5) doi: 10.1016/S1473-3099(20)30724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salali G.D., Uysal M.S. COVID-19 vaccine hesitancy is associated with beliefs on the origin of the novel coronavirus in the UK and Turkey. Psychol. Med. 2020;1-3 doi: 10.1017/S0033291720004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P., Rauber D., Betsch C., Lidolt G., Denker M.L. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm L., Kasting M.L., Head K.J., Hartsock J.A., Zimet G.D. Influenza vaccination in the time of COVID-19: a national U.S. survey of adults. Vaccine. 2021;39(14):1921–1928. doi: 10.1016/j.vaccine.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von E.E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Yue N., Zheng M., et al. Influenza vaccination coverage of population and the factors influencing influenza vaccination in mainland China: a meta-analysis. Vaccine. 2018;36(48):7262–7269. doi: 10.1016/j.vaccine.2018.10.045. [DOI] [PubMed] [Google Scholar]
- Wang K., Wong E.L.Y., Ho K.F., et al. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. 2020;38(45):7049–7056. doi: 10.1016/j.vaccine.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wu Q., Yang J., et al. Global, regional, and national estimates of target population sizes for covid-19 vaccination: descriptive study. BMJ. 2020;371:m4704. doi: 10.1136/bmj.m4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.K., Alleaume C., Peretti-Watel P. The French public’s attitudes to a future COVID-19 vaccine: the politicization of a public health issue. Soc. Sci. Med. 2020;265:113414. doi: 10.1016/j.socscimed.2020.113414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L.P., Alias H., Wong P.-F., Lee H.Y., AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Human. Vaccin. Immunother. 2020;16(9):2204–2214. doi: 10.1080/21645515.2020.1790279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Allocation Mechanism for COVAX Facility Vaccines.https://www.who.int/docs/default-source/coronaviruse/allocation-of-covax-f-vaccines-explainer-v3-db.pdf?sfvrsn=516b3714_16&download=true [Google Scholar]
- World Health Organization . 2020. WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination.https://apps.who.int/iris/bitstream/handle/10665/334299/WHO-2019-nCoV-SAGE_Framework-Allocation_and_prioritization-2020.1-eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- World Health Organization Coronavirus Disease 2019 (COVID-19) Situation Report. 2021. https://www.who.int/
- Yang W. Transmission dynamics of and insights from the 2018-2019 measles outbreak in New York City: a modeling study. Sci. Adv. 2020;6(22):eaaz4037. doi: 10.1126/sciadv.aaz4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material