Abstract
Objective.
To develop an evidence-based guideline for the pharmacologic and nonpharmacologic treatment of psoriatic arthritis (PsA), as a collaboration between the American College of Rheumatology (ACR) and the National Psoriasis Foundation (NPF).
Methods.
We identified critical outcomes in PsA and clinically relevant PICO (population/intervention/comparator/outcomes) questions. A Literature Review Team performed a systematic literature review to summarize evidence supporting the benefits and harms of available pharmacologic and nonpharmacologic therapies for PsA. GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology was used to rate the quality of the evidence. A voting panel, including rheumatologists, dermatologists, other health professionals, and patients, achieved consensus on the direction and the strength of the recommendations.
Results.
The guideline covers the management of active PsA in patients who are treatment-naive and those who con tinue to have active PsA despite treatment, and addresses the use of oral small molecules, tumor necrosis factor inhibitors, interleukin-12/23 inhibitors (IL-12/23i), IL-17 inhibitors, CTLA4-Ig (abatacept), and a JAK inhibitor (tofaciti nib). We also developed recommendations for psoriatic spondylitis, predominant enthesitis, and treatment in the presence of concomitant inflammatory bowel disease, diabetes, or serious infections. We formulated recommendations for a treat-to-target strategy, vaccinations, and nonpharmacologic therapies. Six percent of the recommendations were strong and 94% conditional, indicating the importance of active discussion between the health care provider and the patient to choose the optimal treatment.
Conclusion.
The 2018 ACR/NPF PsA guideline serves as a tool for health care providers and patients in the selection of appropriate therapy in common clinical scenarios. Best treatment decisions consider each individual patient situation. The guideline is not meant to be proscriptive and should not be used to limit treatment options for patients with PsA.
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disease associated with psoriasis, manifesting most commonly with peripheral arthritis, dactylitis, enthesitis, and spondylitis. Nail lesions, including pitting and onycholysis, occur in ~80–90% of patients with PsA. The incidence of PsA is ~6 per 100,000 per year, and the prevalence is ~1–2 per 1,000 in the general population (1). The annual incidence of PsA in patients with psoriasis is 2.7% (2), and the reported prevalence of PsA among patients with psoriasis has varied between 6% and 41% (1). In the majority of patients the skin symptoms develop first, followed by the arthritis; however, in some patients the skin and joint symptoms present at the same time, and in 10–15% the arthritis presents first (2).
PsA affects men and women equally. The distribution of the peripheral arthritis varies from asymmetric oligoarthritis (involving ≤4 joints) to symmetric polyarthritis (involving ≥5 joints). Distal interphalangeal joints are commonly affected and, in some patients, are the only affected joints. Axial disease, when present, usually occurs together with peripheral arthritis. Some patients present with rapidly progressive and destructive PsA–arthritis mutilans. PsA is associated with an adverse impact on health-related quality of life (3–5) and high health care costs and utilization (6,7). Greater disease activity is associated with progressive joint damage and higher mortality (8–11). Early identification of PsA and early initiation of therapy are important for improving long-term outcomes (12).
Both nonpharmacologic and pharmacologic treatment can ameliorate PsA symptoms and can occasionally result in disease remission (Figure 1). Clinicians and patients can now choose from a wide variety of pharmacologic therapies, including symptomatic treatments such as nonsteroidal antiinflammatory drugs (NSAIDs) and intraarticular injections, as well as immunomodulatory therapies.
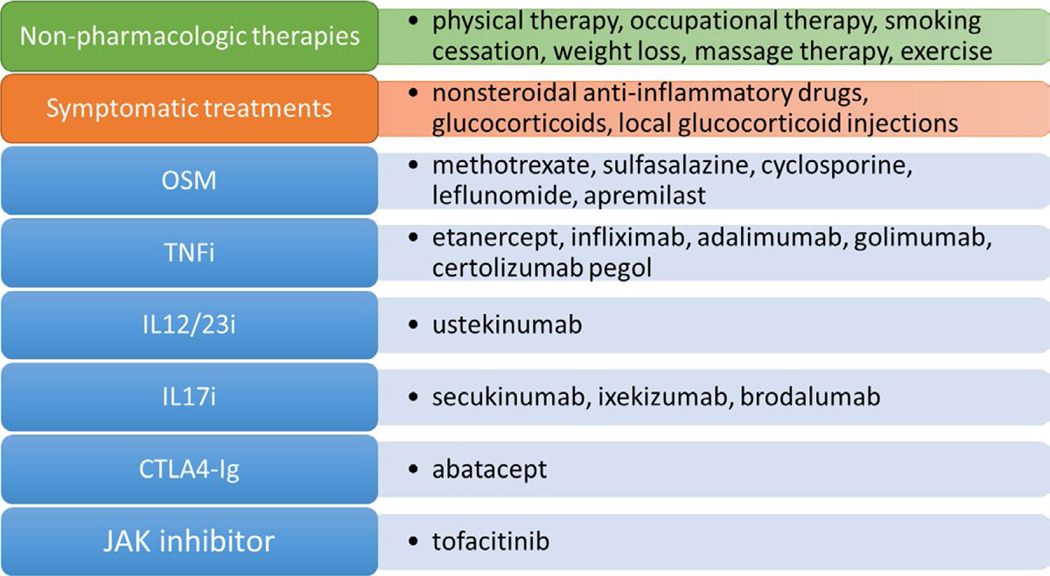
Figure 1.
Pharmacologic, nonpharmacologic, and symptomatic therapies for psoriatic arthritis. Pharmacologic therapies are displayed in the blue boxes and include oral small molecules (OSMs), tumor necrosis factor inhibitor (TNFi) biologics, interleukin-17 inhibitor (IL-17i) biologics, an IL-12/23i biologic, CTLA4-immunoglobulin, and a JAK inhibitor. While there are numerous nonpharmacologic therapies available, 6 of these are addressed in this guideline. Symptomatic therapies include nonsteroidal antiinflammatory drugs, systemic glucocorticoids, and local glucocorticoid injections. Systemic glucocorticoids or local injections are not addressed in this guideline.
The presentation of PsA is heterogeneous, and health care providers frequently face challenges when considering the various treatment options. Our objective was to develop evidence-based treatment recommendations for the management of active PsA in adults, using pharmacologic and nonpharmacologic therapies. These PsA treatment recommendations can help guide both clinicians and patients to arrive at optimal management decisions.
METHODS
Methodology overview.
This guideline followed the American College of Rheumatology (ACR) guideline development process (http://www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines). This process includes using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology (13–15) (www.gradeworkinggroup.org) to rate the quality of the available evidence and to develop the recommendations. ACR policy guided disclosures and the management of conflicts of interest. The full methods are presented in detail in Supplementary Appendix 1, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract.
This work involved 4 teams selected by the ACR Quality of Care Committee after reviewing individual and group volunteer applications in response to an open request for proposals announcement: 1) a Core Leadership Team, which supervised and coordinated the project and drafted the clinical questions and manuscript; 2) a Literature Review Team, which completed the literature search and abstraction; 3) an Expert Panel, composed of patients, patient advocates, rheumatologists, dermatologists, 1 dermatologist-rheumatologist, and 1 rheumatology nurse practitioner, which developed the clinical questions (PICO [population/intervention/comparator/outcomes] questions) and decided on the scope of the guideline project; and 4) a Voting Panel, which included rheumatologists, 1 dermatologist, 1 dermatologist-rheumatologist, 1 rheumatology physician assistant, and 2 patients (1 of whom was also a physical therapist), who provided input from the patient perspective and voted on the recommendations. Additionally, a Patient Panel consisting of 9 adults with PsA reviewed the evidence and provided input on their values and preferences, which was reviewed before discussion of each section of PsA management (e.g., treatment-naive, treated, comorbidities), and was incorporated into discussions and formulation of recommendations. Supplementary Appendix 2 (http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract) presents rosters of the team and panel members. In accordance with ACR policy, the principal investigator and the leader of the literature review team were free of conflicts, and within each team, >50% of the members were free of conflicts.
Framework for the PsA guideline development and scope of the guideline.
Because there are numerous topics within PsA that could be addressed, at the beginning of the process the guideline panels made several decisions regarding the focus of this guideline and how to define aspects of the disease (e.g., active disease). At an initial scoping meeting, the Voting Panel and Expert Panel agreed that the project would include the management of patients with active PsA, defined as symptoms at an unacceptably bothersome level as reported by the patient and judged by the examining health care provider to be due to PsA based on the presence of at least 1 of the following: actively inflamed joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and/or extraarticular manifestations such as uveitis or inflammatory bowel disease (IBD). The health care provider may, in deciding if symptoms are due to active PsA, consider information beyond the core information from the history and physical examination, such as inflammation markers (C-reactive protein [CRP] or erythrocyte sedimentation rate [ESR]) and imaging results. At the scoping meeting, the panels decided that the guideline would address both pharmacologic and nonpharmacologic therapies for the treatment of PsA. We examined evidence regarding vaccinations, treatment in the presence of common comorbidities, and implementing a treat-to-target strategy.
In addressing pharmacologic therapies, we focused on immunomodulatory agents for long-term management rather than addressing acute symptom management (i.e., through intraarticular injections and the use of systemic glucocorticoids). Tofacitinib and ixekizumab were submitted for review and potential approval by the US Food and Drug Administration (FDA) at the time of formulation of this guideline (16,17) and for this reason, these drugs were addressed in the guideline. Both drugs have been approved for PsA since then (18,19). Tofacitinib is not included within the oral small molecules (OSM) category since its benefit/risk profile differs from that of the rest of the OSMs, especially with regard to risks (20–22), and consistent with its being considered separately in other treatment guidelines (23,24). Additionally, the panel addressed alternatives in patient subpopulations (e.g., patients with predominant enthesitis, axial disease, dactylitis, comorbidities), and greater versus lesser disease severity.
There are currently no widely agreed-upon definitions of disease severity in PsA or psoriasis. Thus, health care providers and patients should judge PsA and psoriasis severity on a case-by-case basis. For the purpose of these recommendations, severity includes not only the level of disease activity at a given time point, but also the presence or absence of poor prognostic factors and long-term damage. Examples of severe PsA disease include the presence of 1 or more of the following: a poor prognostic factor (erosive disease, dactylitis, elevated levels of inflammation markers such as ESR and CRP attributable to PsA), long-term damage that interferes with function (e.g., joint deformities), highly active disease that causes a major impairment in quality of life (i.e., active psoriatic inflammatory disease at many sites [including dactylitis, enthesitis] or function-limiting inflammatory disease at few sites), and rapidly progressive disease (Figure 2). In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) (25) score of ≥12 and a body surface area score of ≥10. However, because it is cumbersome, physicians seldom use the PASI in clinical practice. Examples of definitions of severe PsA and severe psoriasis are shown in Figure 2. Finally, because the National Psoriasis Foundation (NPF) and American Academy of Dermatology are concurrently developing psoriasis treatment guidelines, the treatment of skin psoriasis separately from the inflammatory arthritis was not included in the current ACR/NPF PsA guideline.
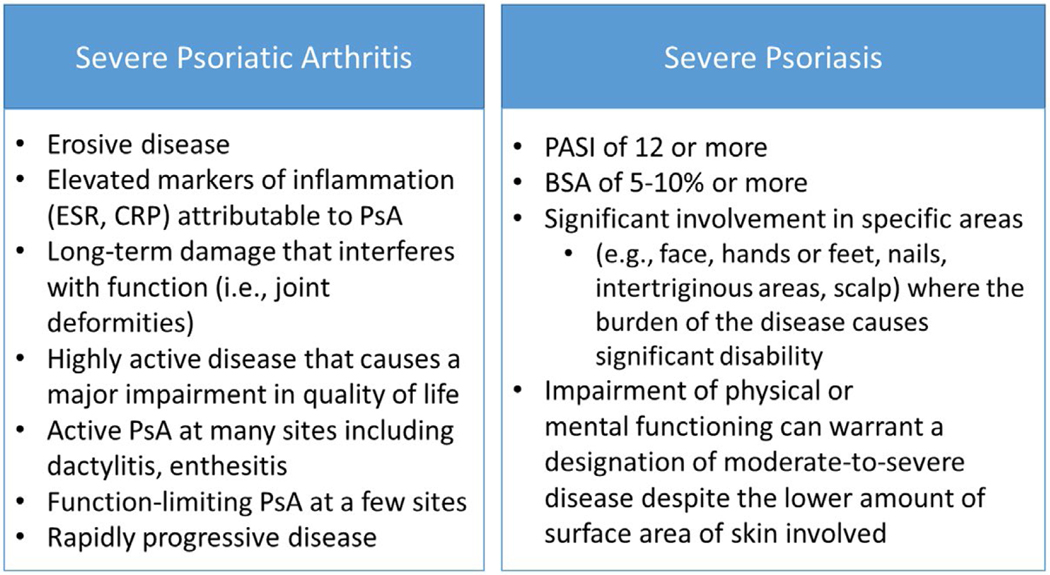
Figure 2.
Examples of “severe” psoriatic arthritis (PsA) and psoriasis. The guideline development group defined severe PsA and psoriasis as the presence of 1 or more of the items listed. This is not a formal definition. There have been many definitions of severe psoriasis used over time—the items here are adapted from the 2007 National Psoriasis Foundation expert consensus statement for moderate-to-severe psoriasis (68). In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score of ≥12 and a body surface area (BSA) score of ≥10 (25). ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
Systematic synthesis of the literature.
Systematic searches of the published English-language literature included Ovid Medline, PubMed, Embase, and the Cochrane Library (including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and Health Technology Assessments) from the beginning of each database through November 15, 2016 (Supplementary Appendix 3, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract); we conducted updated searches on May 2, 2017 and again on March 8, 2018. DistillerSR software (https://distillercer.com/products/distillersr-systematic-reviewsoftware/) (Supplementary Appendix 4; http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract) was used to facilitate duplicate screening of literature search results. Reviewers entered extracted data into RevMan software (http://tech.cochrane.org/revman), and evaluated the risk of bias in primary studies using the Cochrane risk of bias tool (http://handbook.cochrane.org/). We exported RevMan files into GRADEpro software to formulate a GRADE summary of findings table (Supplementary Appendix 5; http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract) for each PICO question (26). Additionally, a network meta-analysis was performed when sufficient studies were available. GRADE criteria provided the framework for judging the overall quality of evidence (13).
The panels chose the critical outcomes for all comparisons at the initial scoping; these included the American College of Rheumatology 20% response criteria (ACR20) (the primary outcome for most PsA clinical trials), the Health Assessment Questionnaire disability index (a measure of physical function), the PASI 75% response criteria (PASI75) (a measure of skin psoriasis improvement), and serious infections. Both the ACR20 and the PASI75 are accepted outcome measures specified by regulatory agencies, including the US FDA, for the approval of treatments for PsA (27). Serious infections are among the issues of greatest concern for patients and physicians when selecting among therapies. Other specific harms (e.g., liver toxicity with methotrexate [MTX]) were included as critical outcomes for individual comparisons. We included other outcomes, such as total infections (regardless of severity), when appropriate.
Moving from evidence to recommendations.
GRADE methodology specifies that panels make recommendations based on the balance of benefits and harms, the quality of the evidence (i.e., confidence in effect estimates), and patients’ values and preferences. Deciding on the balance between desirable and undesirable outcomes requires estimating the relative value patients place on those outcomes. When the literature provided very limited guidance, the experience of the Voting Panel members (including physicians, a rheumatology physician assistant, and the 2 patients present) in managing the relevant cases and issues became an important source of evidence. Values and preferences, crucial to all recommendations, derived from input from the members of the Patient Panel were particularly salient in such situations. GRADE methodology allows the panels the possibility of not coming to a decision, and a summary of the discussion is noted in such cases. However, during the development of this guideline, the Voting Panel came to a conclusion in each case scenario, and such a situation did not arise.
Consensus building.
The Voting Panel voted on the direction and strength of the recommendation related to each PICO question. Recommendations required a 70% level of agreement, as used previously in other similar processes (28) and in the previous ACR guidelines (23,29,30); if 70% agreement was not achieved during an initial vote, the panel members held additional discussions before revoting. For all conditional recommendations, a written explanation is provided, describing the reasons for the decision and conditions under which the alternative choice may be preferable.
Moving from recommendations to practice.
These recommendations are designed to help health care providers work with patients in selecting therapies. The presence or absence of concomitantly occurring conditions, such as IBD, uveitis, diabetes, and serious infections, and the knowledge of previous therapies, influence decisions regarding optimal management. In the context of PsA, the physical examination, which is also required for selecting therapy, includes assessment of the peripheral joints (including for dactylitis), the entheses, the spine, the skin, and the nails. Health care providers and patients must take into consideration all active disease domains, comorbidities, and the patient’s functional status in choosing the optimal therapy for an individual at a given point in time.
RESULTS/RECOMMENDATIONS
How to interpret the recommendations
A strong recommendation means that the panel was confident that the desirable effects of following the recommendation outweigh the undesirable effects (or vice versa), so the course of action would apply to all or almost all patients, and only a small proportion of clinicians/patients not wanting to follow the recommendation. We use the phrase “should use” or “should be used” for strong recommendations.
A conditional recommendation means that the panel believed the desirable effects of following the recommendation probably outweigh the undesirable effects, so the course of action would apply to the majority of the patients, but a small proportion of clinicians/patients may not want to follow the recommendation. Because of this, conditional recommendations are preference sensitive and always warrant a shared decision-making approach. We use the phrase “is recommended over” or “is/would be recommended” for conditional recommendations. We specify conditions under which the less preferred drug may be used by using the phrase “may be used” or “may consider” or “Y (less preferred drug) may be used instead of X (preferred drug)” or “may consider Y instead of X (preferred drug)” for conditional recommendations.
Conditional recommendations were usually based on low- to very-low-quality evidence (in rare instances, moderate-quality evidence). Strong recommendations were typically based on moderate- or high-quality evidence.
For each recommendation, Supplementary Appendix 5 (on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract) provides details regarding the PICO questions and the GRADE evidence tables.
In each case, the Voting Panel’s recommendation was based on a judgment of the most likely net benefit, i.e.,1) more benefit with the medication conditionally recommended with no difference in harms between the medications being compared (e.g., choosing a TNFi over OSMs in treatment-naive patients) or 2) less harm with the medication conditionally recommended and no difference in benefit (e.g., choosing abatacept over a TNFi in patients at risk of or with a history of previous infections, or preferring a different OSM over MTX in patients with PsA and diabetes due to an increased risk of liver toxicity in this subpopulation).
This is an evidence-based guideline, in that we explicitly use the best evidence available and present that in a transparent manner for the clinician reader/user (31,32). In some instances, this includes a randomized trial directly comparing the interventions under consideration. In other cases, in the absence of any published evidence, the best evidence comes from the collective experience of the Voting Panel and patient panel members, which in the GRADE system is rated as “very-low-quality” evidence.
Recommendations for pharmacologic interventions
Active PsA in treatment-naive patients (Table 1 and Figure 3).
Table 1.
Recommendations for the initial treatment of patients with active psoriatic arthritis who are OSM- and other treatment–naive (PICOs 9–15)*
| Level of evidence (evidence [refs.] reviewed)† | |
|---|---|
| In OSM- and other treatment–naive patients with active PsA, | |
| 1. Treat with a TNFi biologic over an OSM (MTX, SSZ, LEF, CSA, or APR) (PICO 10a–e) | Low (53–66) |
| Conditional recommendation based on low- quality evidence; may consider an OSM if the patient does not have severe PsA,‡ does not have severe psoriasis,§ prefers oral therapy, has concern over starting a biologic as the first therapy, or has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 2. Treat with a TNFi biologic over an IL-17i biologic (PICO 14) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider an IL-17 i biologic if the patient has severe psoriasis or has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 3. Treat with a TNFi biologic over an IL-12/23i biologic (PICO 13) | Very low |
| Conditional recommendation based on very- low- quality evidence; may consider an IL-12 /23i biologic if the patient has severe psoriasis, prefers less frequent drug administration, or has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 4. Treat with an OSM over an IL-17i biologic (PICO 12) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider an IL- 17 i biologic if the patient has severe psoriasis and/or severe PsA. | |
| 5. Treat with an OSM over an IL-12/23i biologic (PICO 11) | Very low |
| Conditional recommendation based on very- low- quality evidence; may consider an IL-12 /23i biologic if the patient has concomitant IBD and/or severe psoriasis and/or severe PsA or prefers less frequent drug administration. | |
| 6. Treat with MTX over NSAIDs (PICO 9) | Very low (67) |
| Conditional recommendation based on very- low- quality evidence; may consider NSAIDs before starting MTX in patients with less active disease, after careful consideration of cardiovascular risks and renal risks of NSAIDs. | |
| 7. Treat with an IL-17i biologic over an IL-12/23i biologic (PICO 15) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider an IL-12 /23i biologic if the patient has concomitant IBD or prefers less frequent drug administration. |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease (IBD). Oral small molecules (OSMs) are defined as methotrexate (MTX), sulfasalazine (SSZ), leflunomide (LEF), cyclosporine (CSA), or apremilast (APR) and do not include tofacitinib, which was handled separately since its efficacy/safety profile is much different from that of other OSMs listed above. OSM- and other treatment–naive is defined as naive to treatment with OSMs, tumor necrosis factor inhibitors (TNFi,) interleukin-17 inhibitors (IL-17i), and IL-12/23i; patients may have received nonsteroidal antiinflammatory drugs (NSAIDs), glucocorticoids, and/or other pharmacologic and nonpharmacologic interventions.
When there were no published studies, we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
Because there are currently no widely agreed-upon definitions of disease severity, PsA severity should be established by the health care provider and patient on a case-by-case basis. For the purposes of these recommendations, severity is considered a broader concept than disease activity in that it encompasses the level of disease activity at a given time point, as well as the presence of poor prognostic factors and long-term damage. Examples of severe PsA disease include the presence of ≥1 of the following: a poor prognostic factor (erosive disease, elevated levels of inflammation markers such as C-reactive protein or erythrocyte sedimentation rate attributable to PsA), long-term damage that interferes with function (e.g., joint deformities, vision loss), highly active disease that causes major impairment in quality of life (i.e., active psoriatic inflammatory disease at many sites [including dactylitis, enthesitis] or function-limiting inflammatory disease at few sites), and rapidly progressive disease.
Because there are currently no widely agreed-upon definitions of disease severity, psoriasis severity should be established by the health care provider and patient on a case-by-case basis. In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score (25) of ≥12 and a body surface area score of ≥10. In clinical practice, however, the PASI tool is not standardly utilized given its cumbersome nature. In 2007, the National Psoriasis Foundation published an expert consensus statement, which defined moderate-to-severe disease as a body surface area of ≥5% (68). In cases in which the involvement is in critical areas, such as the face, hands or feet, nails, intertriginous areas, scalp, or where the burden of the disease causes significant disability or impairment of physical or mental functioning, the disease can be severe despite the lower amount of surface area of skin involved. The need to factor in the unique circumstances of the individual patient is of critical importance, but this threshold provides some guidance in the care of patients.
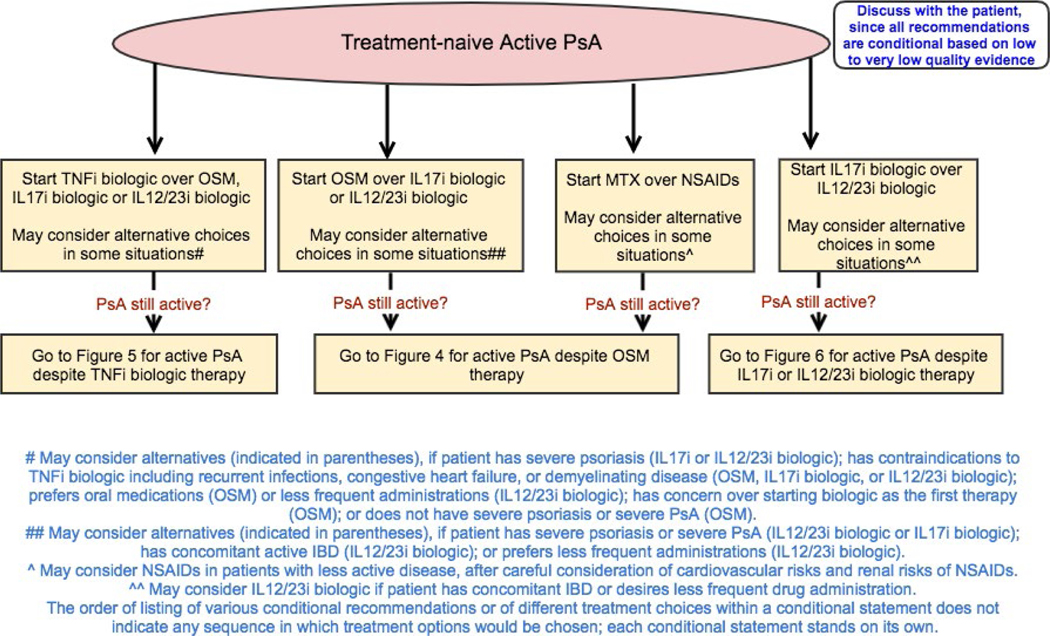
Figure 3.
Recommendations for the treatment of patients with active psoriatic arthritis (PsA) who are treatment-naive (no exposure to oral small molecules [OSMs] or other treatments). All recommendations are conditional based on low- to very-low-quality evidence. A conditional recommendation means that the panel believed the desirable effects of following the recommendation probably outweigh the undesirable effects, so the course of action would apply to the majority of the patients, but some may not want to follow the recommendation. Because of this, conditional recommendations are preference sensitive and always warrant a shared decision-making approach. Due to the complexity of management of active PsA, not all clinical situations and choices could be depicted in this flow chart, and therefore we show only the key recommendations. For a complete list of recommendations, please refer to the Results section of the text. For the level of evidence supporting each recommendation, see Table 1 and the related section in the Results. This figure is derived from recommendations based on PICO (population/intervention/comparator/outcomes) questions that are based on the common clinical situations. Active PsA was defined as symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining health care provider to be due to PsA based on the presence of at least 1 of the following: actively inflamed joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and/or extraarticular manifestations such as uveitis or inflammatory bowel disease (IBD). TNFi = tumor necrosis factor inhibitor; IL-17i = interleukin-17 inhibitor; MTX = methotrexate; NSAIDs = nonsteroidal antiinflammatory drugs.
All recommendations for treatment-naive patients with active PsA are conditional based on low- to very-low-quality evidence.
In treatment-naive patients with active PsA, a TNFi biologic agent is recommended over an OSM as a first-line option (Table 1). OSMs may be used instead of a TNFi biologic in patients without severe PsA and without severe psoriasis (as defined in Methods and Figure 2; final determination of severity to be made by the patient and the health care provider), those who prefer an oral drug instead of parenteral therapy, or those with contraindications to TNFi treatment, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease.
For treatment-naive patients with active PsA, the use of a TNFi biologic or OSM is recommended over an interleukin-17 inhibitor (IL-17i) or IL-12/23i biologic. An IL-17i or IL-12/23i biologic may be used instead of TNFi biologics in patients with severe psoriasis or contraindications to TNFi biologics, and may be used instead of OSMs in patients with severe psoriasis or severe PsA. MTX is recommended over NSAIDs in treatment-naive patients with active PsA. NSAIDs may be used instead of MTX after consideration of possible contraindications and side effect profile in patients without evidence of severe PsA or severe psoriasis and in those at risk for liver toxicity (Table 1 and Figure 3). An IL-17i biologic is recommended over an IL-12/23i biologic. IL-12/23i biologics may be used in patients who have concomitant IBD or who desire less frequent drug administration.
Active PsA despite treatment with an OSM (Table 2 and Figure 4).
Table 2.
Recommendations for treatment of patients with active psoriatic arthritis despite treatment with an OSM (PICOs 16–25; 67–69; 76–78)*
| Level of evidence (evidence [refs.] reviewed)† | |
|---|---|
| In adult patients with active PsA despite treatment with an OSM, | |
| 1. Switch to a TNFi biologic over a different OSM (PICO 23) | Moderate (62–66, 69–86) |
| Conditional recommendation based on moderate- quality evidence; may consider switching to a different OSM if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, if the patient prefers an oral versus parenteral therapy, or in patients without evidence of severe PsA‡ or severe psoriasis.§ | |
| 2. Switch to a TNFi biologic over an IL-17i biologic (PICO 17) | Moderate (62–66, 72–78, 87–97) |
| Conditional recommendation based on moderate- quality evidence; may consider an IL- 17i if the patient has severe psoriasis and/or has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, and/or a family history of demyelinating disease such as multiple sclerosis. | |
| 3. Switch to a TNFi biologic over an IL-12/23i biologic (PICO 16) | Moderate (62–66, 72–78, 97–102) |
| Conditional recommendation based on moderate- quality evidence; may consider an IL- 12/23i if the patient has severe psoriasis and/or contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, or prefers less frequent drug administration. | |
| 4. Switch to a TNFi biologic over abatacept (PICO 67) | Low (62–66, 72–78, 103, 104) |
| Conditional recommendation based on low- quality evidence; may consider abatacept if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 5. Switch to a TNFi biologic over tofacitinib (PICO 76) | Low (62–66, 72–78, 105) |
| Conditional recommendation based on low- quality evidence; may consider tofacitinib if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, or prefers oral medication. | |
| 6. Switch to an IL-17i over a different OSM (PICO 25) | Low (79–87, 89–95) |
| Conditional recommendation based on low-quality evidence; may consider switch- ing to a different OSM if the patient prefers an oral versus parenteral therapy or in patients without evidence of severe PsA or severe psoriasis. | |
| 7. Switch to an IL-17i biologic over an IL-12/23i biologic (PICO 18) | Moderate (87, 89–95, 98–100, 106, 107) |
| Conditional recommendation based on moderate-quality evidence; may consider an IL-12 /23i biologic if the patient has concomitant IBD or prefers less frequent drug administration. | |
| 8. Switch to an IL-17i biologic over abatacept (PICO 69) | Low (89–95, 103, 104) |
| Conditional recommendation based on low-quality evidence; may consider abata- cept in patients with recurrent or serious infections. | |
| 9. Switch to an IL-17i biologic over tofacitinib (PICO 78) | Low (89–95, 105) |
| Conditional recommendation based on low- quality evidence; may consider tofacitinib if the patient prefers an oral therapy or has a history of recurrent Candida infections. | |
| 10. Switch to an IL-12/23i biologic over a different OSM (PICO 24) | Low (79–86, 98–100) |
| Conditional recommendation based on low-quality evidence; may consider switch- ing to a different OSM if the patient prefers an oral versus parenteral therapy or in patients without evidence of severe PsA or severe psoriasis. | |
| 11. Switch to an IL-12/23i biologic over abatacept (PICO 68) | Low (98–100, 103, 104) |
| Conditional recommendation based on low-quality evidence; may consider abata- cept in patients with recurrent or serious infections. | |
| 12. Switch to an IL-12/23i biologic over tofacitinib (PICO 77) | Low (98–100, 105) |
| Conditional recommendation based on low-quality evidence; may consider tofaci- tinib if the patient prefers an oral therapy. | |
| 13. Add apremilast to current OSM therapy over switching to apremilast (PICO 22b) | Low (83, 84, 108) |
| Conditional recommendation based on low- quality evidence; may consider switching to apremilast if the patient has intolerable side effects with the current OSM. | |
| 14. Switch to another OSM (except apremilast) over adding another OSM (except apremilast) to current treatment (PICO 22a) | Low (83, 84, 108) |
| Conditional recommendation based on low-quality evidence; may consider adding another OSM (except apremilast) to current treatment if the patient has demonstrated partial response to the current OSM. | |
| 15. Switch to a TNFi biologic monotherapy over MTX and a TNFi biologic combi nation therapy (PICO 19) | Low (109–111) |
| Conditional recommendation based on low- quality evidence; may consider MTX and TNFi biologic combination therapy if the patient has severe skin manifestations, has had a partial response to current MTX therapy, has concomitant uveitis (since uveitis may respond to MTX therapy), and if the current TNFi biologic is infliximab or adalimumab. | |
| 16. Switch to an IL-17i biologic monotherapy over MTX and an IL-17i biologic combination therapy (PICO 21) | Very low |
| Conditional recommendation based on very- low-quality evidence; may consider MTX and an IL-17 i biologic combination therapy if the patient has severe skin manifestations, has had a partial response to current MTX therapy, or has concomitant uveitis (since uveitis may respond to MTX therapy). | |
| 17. Switch to an IL-12/23i biologic monotherapy over MTX and an IL-12/23i bio logic combination therapy (PICO 20) | Very low |
| Conditional recommendation based on very- low- quality evidence; may consider MTX and an IL-12 /23i biologic combination therapy if the patient has severe skin manifestations, has had a partial response to current MTX therapy, or has concomitant uveitis (since uveitis may respond to MTX therapy). |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease (IBD). Oral small molecules (OSMs) are defined as methotrexate (MTX), sulfasalazine, leflunomide, cyclosporine, or apremilast and do not include tofacitinib, which was handled separately since its efficacy/safety profile is much different from that of other OSMs listed above. TNFi = tumor necrosis factor inhibitor; IL-17i = interleukin-17 inhibitor.
When there were no published studies, we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
Because there are currently no widely agreed-upon definitions of disease severity, PsA severity should be established by the health care provider and patient on a case-by-case basis. For the purposes of these recommendations, severity is considered a broader concept than disease activity in that it encompasses the level of disease activity at a given time point, as well as the presence of poor prognostic factors and long-term damage. Examples of severe PsA disease include the presence of ≥1 of the following: a poor prognostic factor (erosive disease, elevated levels of inflammation markers such as C-reactive protein or erythrocyte sedimentation rate attributable to PsA), long-term damage that interferes with function (e.g., joint deformities, vision loss), highly active disease that causes major impairment in quality of life (i.e., active psoriatic inflammatory disease at many sites [including dactylitis, enthesitis] or function-limiting inflammatory disease at few sites), and rapidly progressive disease.
Because there are currently no widely agreed-upon definitions of disease severity, psoriasis severity should be established by the health care provider and patient on a case-by-case basis. In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score (25) of ≥12 and a body surface area score of ≥10. In clinical practice, however, the PASI tool is not standardly utilized given its cumbersome nature. In 2007, the National Psoriasis Foundation published an expert consensus statement, which defined moderate-to-severe disease as a body surface area of ≥5% (68). In cases in which the involvement is in critical areas, such as the face, hands or feet, nails, intertriginous areas, scalp, or where the burden of the disease causes significant disability or impairment of physical or mental functioning, the disease can be severe despite the lower amount of surface area of skin involved. The need to factor in the unique circum stances of the individual patient is of critical importance, but this threshold provides some guidance in the care of patients.
Figure 4.
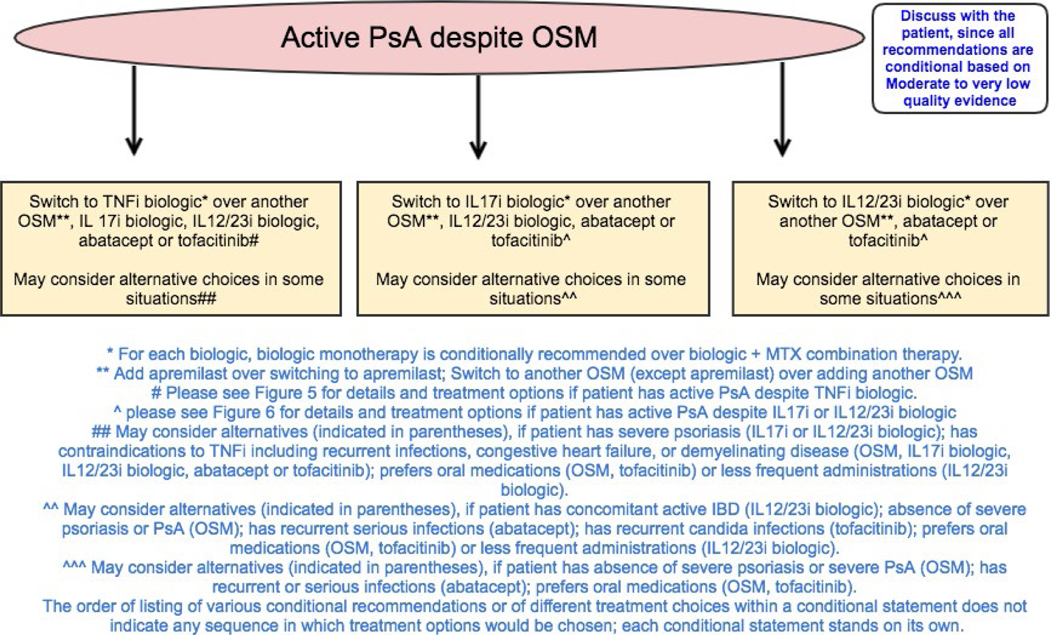
Recommendations for the treatment of patients with active psoriatic arthritis (PsA) despite treatment with oral small molecules (OSMs). All recommendations are conditional based on low- to very-low-quality evidence. A conditional recommendation means that the panel believed the desirable effects of following the recommendation probably outweigh the undesirable effects, so the course of action would apply to the majority of the patients, but some may not want to follow the recommendation. Because of this, conditional recommendations are preference sensitive and always warrant a shared decision-making approach. Due to the complexity of management of active PsA, not all clinical situations and choices could be depicted in this flow chart, and therefore we show only the key recommendations. For a complete list of recommendations, please refer to the Results section of the text. For the level of evidence supporting each recommendation, see Table 2 and the related section in the Results. TNFi = tumor necrosis factor inhibitor; IL-17i = interleukin-17 inhibitor; MTX = methotrexate.
All recommendations for patients with active PsA despite treatment with an OSM are conditional based on mostly low- to very-low-quality evidence and, in a few instances, moderate-quality evidence.
In patients with active PsA despite OSM therapy, switching to a TNFi, an IL-17i, or an IL-12/23i biologic is recommended over switching to a different OSM (Table 2 and Figure 4). A different OSM may be used rather than a TNFi, IL-17i, or IL-12/23i in patients who prefer an oral medication or those without evidence of severe PsA or severe psoriasis; a different OSM may be used rather than a TNFi in the presence of contraindications to TNFi biologics. A TNFi biologic is recommended over an IL-17i biologic, an IL-12/23i biologic, abatacept, or tofacitinib. An IL-17i biologic is recommended over an IL-12/23i biologic, abatacept, or tofacitinib. An IL-12/23i is recommended over abatacept or tofacitinib. In patients with contraindications to TNFi agents, an IL-12/23i, an IL-17i, abatacept, or tofacitinib may be used instead of a TNFi. In patients with severe psoriasis, an IL-12/23i or an IL-17i may be used instead of a TNFi. Tofacitinib may be used instead of a TNFi in patients preferring oral medication who do not have severe psoriasis.
Switching to another OSM is recommended over adding another OSM to the current treatment (except in the case of apremilast). Adding another OSM (except apremilast) to current treatment may be considered if the patient has exhibited partial response to the current OSM. Adding apremilast to the current OSM therapy is recommended over switching to apremilast monotherapy since most evidence for benefits of apremilast pertains to apremilast combination therapy. Switching to apremilast monotherapy may be considered instead of apremilast combination therapy if the patient has intolerable side effects with the current OSM.
Biologic monotherapy is recommended over biologic combination therapy with MTX (the most commonly used OSM in combination therapy). When switching to biologic monotherapy, stopping the OSM or tapering of the OSM are both reasonable options and depend on patient and health care provider preferences. A biologic agent in combination with MTX may be used instead of biologic monotherapy if the patient has severe psoriasis, has had a partial response to current MTX therapy, or has concomitant uveitis (since uveitis may respond to MTX therapy), or in patients receiving treatment with a monoclonal antibody TNFi biologic, especially infliximab and adalimumab, to potentially delay or prevent the formation of antidrug antibodies.
Active PsA despite treatment with a TNFi biologic agent as monotherapy or in combination therapy (Table 3 and Figure 5).
Table 3.
Recommendations for treatment of patients with active psoriatic arthritis despite treatment with a TNFi biologic, as monotherapy or in combination with MTX (PICOs 26–35; 70–75)*
| Level of evidence (evidence [refs.] reviewed)† | |
|---|---|
| In adult patients with active PsA despite treatment with a TNFi biologic monotherapy, | |
| 1. Switch to a different TNFi biologic over switching to an IL-17i biologic (PICO 28) | Low (72, 73, 90–93, 95) |
| Conditional recommendation based on low-quality evidence; may consider an IL- 17 i if the patient had a primary TNFi biologic efficacy failure or a TNFi biologic–associated serious adverse event or severe psoriasis.‡ | |
| 2. Switch to a different TNFi biologic over switching to an IL-12/23i biologic (PICO 27) | Low (72, 73, 99, 100) |
| Conditional recommendation based on low-quality evidence; may consider an IL- 12 /23i if the patient had a primary TNFi biologic efficacy failure or a TNFi biologic–associated serious adverse effect or prefers less frequent drug administration. | |
| 3. Switch to a different TNFi biologic over switching to abatacept (PICO 70) | Low (72, 73, 103, 104) |
| Conditional recommendation based on low- quality evidence; may consider abatacept if the patient had a primary TNFi biologic efficacy failure or TNFi biologic–associated serious adverse effect. | |
| 4. Switch to a different TNFi biologic over switching to tofacitinib (PICO 73) | Low (62–66, 72–78, 105) |
| Conditional recommendation based on low-quality evidence; may consider tofacitinib if the patient prefers an oral therapy or had a primary TNFi biologic efficacy failure or a TNFi biologic–associated serious adverse effect. | |
| 5. Switch to a different TNFi biologic (with or without MTX) over adding MTX to the same TNFi biologic monotherapy (PICO 26 and 26A) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider adding MTX when patients have demonstrated partial response to the current TNFi biologic therapy, especially if the TNFi biologic is a monoclonal antibody. | |
| 6. Switch to an IL-17i biologic over switching to an IL-12/23i biologic (PICO 29) | Low (90–93, 95, 99, 100) |
| Conditional recommendation based on low-quality evidence; may consider an IL- 12 /23i if the patient has IBD or if the patient prefers less frequent drug administration. | |
| 7. Switch to an IL-17i biologic over abatacept (PICO 72) | Low (90–93, 95, 103, 104, 112) |
| Conditional recommendation based on low-quality evidence; may consider abatacept if the patient prefers IV dosing or in patients with recurrent or serious infections. | |
| 8. Switch to an IL-17i biologic over tofacitinib (PICO 75) | Low (90–93, 105) |
| Conditional recommendation based on low-quality evidence; may consider tofacitinib if the patient prefers an oral therapy or in patients with concomitant IBD or a history of recurrent Candida infections. | |
| 9. Switch to an IL-12/23i biologic over abatacept (PICO 71) | Low (99, 100, 103, 104) |
| Conditional recommendation based on of low-quality evidence; may consider abatacept if the patient prefers IV dosing or in patients with recurrent or serious infections. | |
| 10. Switch to an IL-12/23i biologic over tofacitinib (PICO 74) | Low (98–100, 105) |
| Conditional recommendation based on low-quality evidence; may consider tofacitinib if the patient prefers an oral therapy. | |
| 11. Switch to a different TNFi biologic monotherapy over switching to a different TNFi biologic and MTX combination therapy (PICO 30) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to a TNFi biologic and MTX combination therapy if the current TNFi biologic is infliximab. | |
| 12. Switch to an IL-17i biologic monotherapy over switching to an IL-17i biologic and MTX combination therapy (PICO 32) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to an IL-17i biologic and MTX combination therapy in patients with concomitant uveitis, as uveitis may respond to MTX therapy. | |
| 13. Switch to an IL-12/23i biologic monotherapy over switching to an IL-12/23i biologic and MTX combination therapy (PICO 31) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to an IL-12 /23i biologic and MTX combination therapy if the patient has severe psoriasis. | |
| In adult patients with active PsA despite treatment with a TNFi biologic and MTX combination therapy, | |
| 14. Switch to a different TNFi biologic + MTX over switching to a different TNFi biologic monotherapy (PICO 33) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to a different TNFi biologic monotherapy if the patient has demonstrated MTX-associated adverse events, prefers to receive fewer medications, or perceives MTX as a burden. | |
| 15. Switch to an IL-17i biologic monotherapy over an IL-17i biologic and MTX combina tion therapy (PICO 35) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to an IL-17 i biologic and MTX combination therapy if the patient had had a partial response to the existing regimen or in patients with concomitant uveitis, as uveitis may respond to MTX therapy. Continuing MTX during the transition to an IL-17 i biologic was discussed as potentially beneficial to allow the new therapy time to work. | |
| 16. Switch to IL-12/23i biologic monotherapy over IL-12/23i biologic and MTX combina tion therapy (PICO 34) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to an IL-12 /23i biologic and MTX combination therapy if the patient had had a partial response to the existing regimen or in patients with concomitant uveitis, as uveitis may respond to MTX therapy. Continuing MTX during the transition to an IL-12 /23i biologic was discussed as potentially beneficial to allow the new therapy time to work. |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease (IBD). TNFi = tumor necrosis factor inhibitor; MTX = methotrexate; IL-17i = interleukin-17 inhibitor; IV = intravenous.
When there were no published studies, we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
Because there are currently no widely agreed-upon definitions of disease severity, psoriasis severity should be established by the health care provider and patient on a case-by-case basis. In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score (25) of ≥12 and a body surface area score of ≥10. In clinical practice, however, the PASI tool is not standardly utilized given its cumbersome nature. In 2007, the National Psoriasis Foundation published an expert consensus statement, which defined moderate-to-severe disease as a body surface area of ≥5% (68). In cases in which the involvement is in critical areas, such as the face, hands or feet, nails, intertriginous areas, scalp, or where the burden of the disease causes significant disability or impairment of physical or mental functioning, the disease can be severe despite the lower amount of surface area of skin involved. The need to factor in the unique circumstances of the individual patient is of critical importance, but this threshold provides some guidance in the care of patients.
Figure 5.
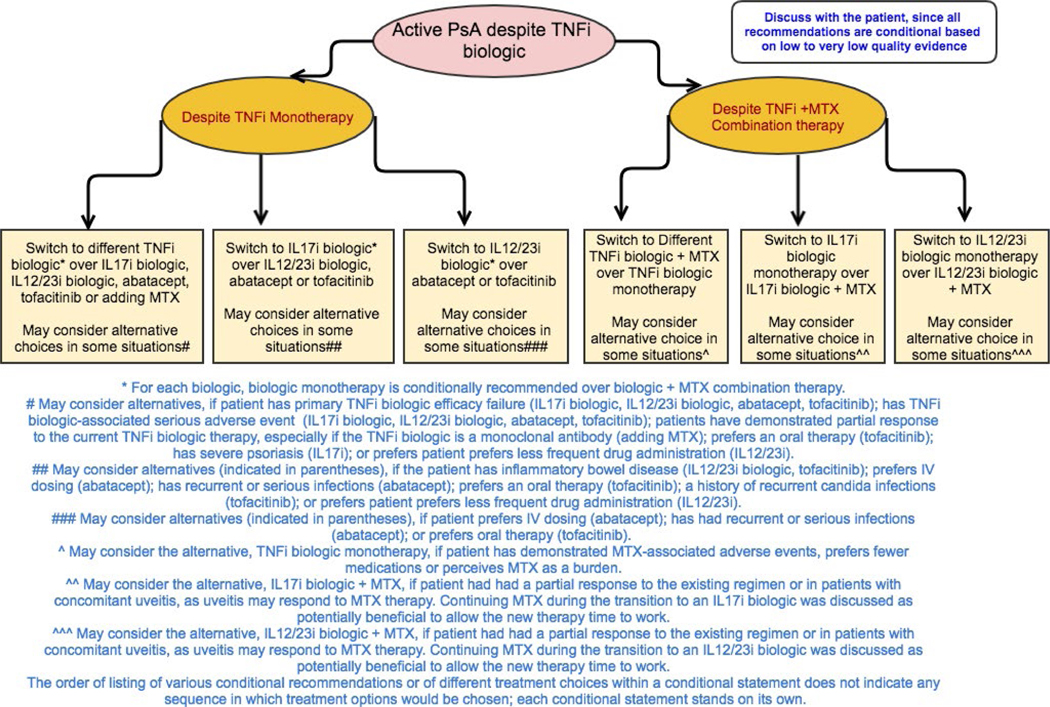
Recommendations for the treatment of patients with active psoriatic arthritis (PsA) despite treatment with a tumor necrosis factor inhibitor (TNFi) as monotherapy or as combination therapy with methotrexate (MTX). All recommendations are conditional based on low- to very-low-quality evidence. A conditional recommendation means that the panel believed the desirable effects of following the recommendation probably outweigh the undesirable effects, so the course of action would apply to the majority of the patients, but some may not want to follow the recommendation. Because of this, conditional recommendations are preference sensitive and always warrant a shared decision-making approach. Due to the complexity of management of active PsA, not all clinical situations and choices could be depicted in this flow chart, and therefore we show only the key recommendations. For a complete list of recommendations, please refer to the Results section of the text. For the level of evidence supporting each recommendation, see Table 3 and the related section in the Results. IL-17i = interleukin-17 inhibitor; IV = intravenous.
All recommendations for patients with active PsA despite TNFi biologic treatment are conditional based on low- to very-low-quality evidence.
In patients with active PsA despite treatment with TNFi biologic monotherapy, switching to a different TNFi biologic monotherapy is recommended over switching to IL-12/23i biologic, an IL-17i biologic, abatacept, or tofacitinib monotherapy or adding MTX to the current TNFi biologic (Table 3 and Figure 5). An IL-12/23i biologic, IL-17i biologic, abatacept, or tofacitinib may be used instead of a different TNFi biologic monotherapy in the case of a primary TNFi biologic failure or a serious adverse event due to the TNFi biologic. An IL-17i or IL-12/23i biologic may be used instead of a different TNFi biologic, particularly in the presence of severe psoriasis. Abatacept may be used instead of a TNFi biologic in patients with recurrent or serious infections in the absence of severe psoriasis, based on indirect evidence of fewer hospitalized infections with abatacept compared to TNFi biologics in a population with rheumatoid arthritis (33). Tofacitinib may be used instead of a TNFi biologic if oral therapy is preferred by the patient.
In patients with active PsA despite treatment with TNFi biologic monotherapy, an IL-17i biologic is recommended over an IL-12/23i biologic, abatacept, or tofacitinib, and an IL-12/23i biologic is recommended over abatacept or tofacitinib. An IL-12/23i biologic may be considered instead of an IL-17i biologic if the patient has IBD or desires less frequent drug administration. Abatacept may be considered instead of an IL-17i or IL-12/23i biologic in patients with recurrent or serious infections. Tofacitinib may be considered instead of an IL-17i biologic in patients who prefer oral therapy or have a history of recurrent or severe Candida infections. Tofacitinib may be considered instead of an IL-12/23i biologic in patients who prefer oral therapy. For each biologic (TNFi, IL-12/23i, or IL-17i), monotherapy is recommended over combination with MTX. Combination therapy with biologic and MTX may be used instead of biologic monotherapy in the presence of severe psoriasis, partial response to current MTX therapy, concomitant uveitis (since uveitis may respond to MTX therapy), and if the current TNFi biologic is infliximab or adalimumab (for immunogenicity prevention).
Under circumstances in which combination therapy with a TNFi biologic and MTX is used and active PsA persists, switching to a different TNFi with MTX is recommended over monotherapy with a different TNFi. Continuing MTX treatment during TNFi transition was seen as beneficial because TNFi biologics may have more sustained efficacy when used in combination with MTX, but evidence is limited (34). Monotherapy with a different TNFi biologic may be used if the patient has had MTX-associated adverse events, prefers to receive fewer medications, or perceives MTX treatment as a burden. IL-12/23i or IL-17i biologic monotherapy is recommended over either of these agents in combination with MTX. Combination therapy with an IL-17i or IL-12/23 biologic and MTX may be used instead of switching to biologic monotherapy if the patient had a partial response to the existing regimen and/or has concomitant uveitis that might respond to MTX therapy.
Active PsA despite treatment with an IL-17i biologic agent as monotherapy (Table 4 and Figure 6).
Table 4.
Recommendations for treatment of patients with active psoriatic arthritis despite treatment with an IL-17i or an IL-12/23i biologic monotherapy (PICOs 36–43)*
| Level of evidence† | |
|---|---|
| In adult patients with active PsA despite treatment with an IL-17i biologic monotherapy, | |
| 1. Switch to a TNFi biologic over switching to an IL-12/23i biologic (PICO 39) | Very low |
| Conditional recommendation based on very-low- quality-evidence; may consider switching to IL- 12/23i if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, or prefers less frequent drug administration. | |
| 2. Switch to a TNFi biologic over switching to a different IL-17i biologic (PICO 42) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to a differ ent IL-17 i if the patient had had a secondary efficacy failure to current IL-17 i, or severe psoriasis, or contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 3. Switch to a TNFi biologic over adding MTX to an IL-17i biologic (PICO 41) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider adding MTX to an IL- 17i if the patient had had a partial response to the existing regimen or if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 4. Switch to an IL-12/23i biologic over switching to a different IL-17i biologic (PICO 43) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to a different IL-17 i if the patient had had a secondary efficacy failure to current IL-17 i or severe psoriasis,‡ or if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 5. Switch to an IL-12/23i biologic over adding MTX to an IL-17i biologic (PICO 40) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider adding MTX to an IL- 17i if the patient had had a partial response to the existing regimen. | |
| In adult patients with active PsA despite treatment with an IL-12/23i biologic monotherapy, | |
| 6. Switch to a TNFi biologic over switching to an IL-17i biologic (PICO 38) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider an IL-17 i if the patient has severe psoriasis or contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 7. Switch to a TNFi biologic over adding MTX to an IL-12/23i biologic (PICO 36) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider adding MTX in patients in whom the severe psoriasis is not responding to the current therapy, or if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 8. Switch to an IL-17i biologic over adding MTX to an IL-12/23i biologic (PICO 37). | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider adding MTX in pa- tients with only partial response to the current therapy or in those who potentially have not had enough time to adequately respond. |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease. IL-17i = interleukin-17 inhibitor; TNFi = tumor necrosis factor inhibitor; MTX = methotrexate.
When there were no published studies—as was the case with all of the recommendations presented in this table—we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
Because there are currently no widely agreed-upon definitions of disease severity, psoriasis severity should be established by the health care provider and patient on a case-by-case basis. In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score (25) of ≥12 and a body surface area score of ≥10. In clinical practice, however, the PASI tool is not standardly utilized given its cumbersome nature. In 2007, the National Psoriasis Foundation published an expert consensus statement, which defined moderate-to-severe disease as a body surface area of ≥5% (68). In cases in which the involvement is in critical areas, such as the face, hands or feet, nails, intertriginous areas, scalp, or where the burden of the disease causes significant disability or impairment of physical or mental functioning, the disease can be severe despite the lower amount of surface area of skin involved. The need to factor in the unique circumstances of the individual patient is of critical importance, but this threshold provides some guidance in the care of patients.
Figure 6.
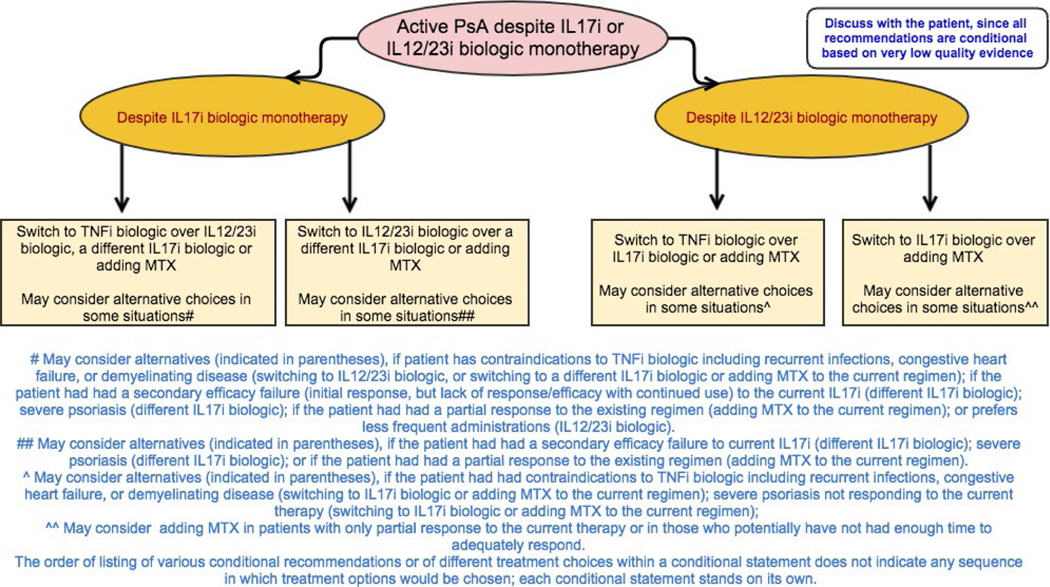
Recommendations for the treatment of patients with active psoriatic arthritis (PsA) despite treatment with interleukin-17 inhibitor (IL-17i) or IL-12/23i biologic monotherapy. All recommendations are conditional based on low- to very-low-quality of evidence. A conditional recommendation means that the panel believed the desirable effects of following the recommendation probably outweigh the undesirable effects, so the course of action would apply to the majority of the patients, but some may not want to follow the recommendation. Because of this, conditional recommendations are preference sensitive and always warrant a shared decision-making approach. Due to the complexity of management of active PsA, not all clinical situations and choices could be depicted in this flow chart, and therefore we show only the key recommendations. For a complete list of recommendations, please refer to the Results section of the text. For the level of evidence supporting each recommendation, see Table 4 and the related section in the Results. TNFi = tumor necrosis factor inhibitor; MTX = methotrexate.
All recommendations for patients with active PsA despite IL-17i biologic treatment are conditional based on very-low-quality evidence.
In patients with active PsA despite treatment with an IL-17i biologic, switching to a TNFi biologic is recommended over switching to an IL-12/23i biologic, adding MTX to the current IL-17i biologic, or switching to a different IL-17i biologic (Table 4 and Figure 6). Switching to an IL-12/23i biologic is recommended over adding MTX to the current IL-17i biologic or switching to a different IL-17i biologic. Treatment may be switched to an IL-12/23i biologic instead of a TNFi biologic if the patient has severe psoriasis or a contraindication to TNFi biologic treatment. Another IL-17i biologic may be used instead of switching to a TNFi or IL-12/23i biologic if the patient had a secondary efficacy failure with the current IL-17i biologic, severe psoriasis, or a contraindication to TNFi treatment. MTX may be added to the current IL-17i regimen instead of switching to a TNFi or IL-12/23i biologic in patients who have had a partial response to the current IL-17i biologic.
Active PsA despite treatment with an IL-12/23i biologic agent as monotherapy (Table 4 and Figure 6).
All recommendations for patients with active PsA despite IL-12/23i biologic treatment are conditional based on very-low-quality evidence.
In patients with active PsA despite treatment with an IL-12/23i biologic, switching to a TNFi biologic is recommended over adding MTX to the current regimen or switching to an IL-17i biologic (Table 4 and Figure 6). Switching to an IL-17i biologic is recommended over adding MTX to the current therapy. Treatment may be switched to an IL-17i biologic instead of a TNFi biologic if the patient has severe psoriasis or a contraindication to TNFi biologic treatment. MTX may be added to the current IL-12/23i biologic therapy instead of switching to a TNFi or an IL-17i biologic in patients with a partial response to the current therapy; MTX may also be added to the current IL-12/23i biologic therapy instead of switching to a TNFi biologic in the presence of contraindications to TNFi biologics.
Treat-to-target (Table 5).
Table 5.
Recommendations for treatment of patients with active psoriatic arthritis including treat-to-target, active axial disease, enthesitis, or active inflammatory bowel disease (PICOs 44–55; 58–62)*
| Level of evidence (evidence [refs.] reviewed)† | |
|---|---|
| In adult patients with active PsA, | |
| 1. Use a treat-to-target strategy over not following a treat-to-target strategy (PICO 44) | Low (113) |
| Conditional recommendation based on low- quality evidence; may consider not following a treat-to- target strategy in patients in whom higher frequency and/or severity of adverse events, higher cost of therapy, or higher patient burden of medications with tighter control are a concern. | |
| In patients with active PsA with psoriatic spondylitis/axial disease despite treatment with NSAIDs,‡ | |
| 2. Switch to a TNFi biologic over switching to an IL-17i biologic (PICO 46) | Very low |
| Conditional recommendation based on very- low- quality evidence; may consider switching to an IL- 17i biologic if the patient has contraindications to TNFi biologics, congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, or if the patient has severe psoriasis.§ | |
| 3. Switch to a TNFi biologic over switching to an IL-12/23i biologic (PICO 45) | Very low |
| Conditional recommendation based on very-low- quality evidence; switching to an IL-12 /23i biologic is not considered since recent trials in axial SpA were stopped. | |
| 4. Switch to an IL-17i biologic over switching to an IL-12/23i (PICO 47) | Very low |
| Conditional recommendation based on very-low- quality evidence; switching to an IL-12 /23i biologic is not considered since recent trials in axial SpA were stopped. | |
| In adult patients with active PsA and predominant enthesitis who are both OSM- and biologic treatment–naive,¶ | |
| 5. Start oral NSAIDs over an OSM (specifically apremilast) (PICO 48) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider starting an OSM (specifically apremilast) if the patient has active joint disease and/or skin disease or contraindications to the use of NSAIDs, including cardiovascular disease, peptic ulcer disease, or renal disease or impairment. | |
| 6. Start a TNFi biologic over an OSM (specifically apremilast) (PICO 48A) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider starting an OSM (specifically apremilast) if the patient prefers an oral treatment as the first therapy or the patient has contraindications to TNFi biologics, including recurrent infections, congestive heart failure, or demyelinating disease. | |
| 7. Start tofacitinib over an OSM (specifically apremilast) (PICO 55) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider starting an OSM (specifically apremilast) if the patient has recurrent infections. | |
| In adult patients with active PsA and predominant enthesitis despite treatment with OSM, | |
| 8. Switch to a TNFi biologic over an IL-17i biologic (PICO 53) | Low (72, 73, 76, 89, 90, 92) |
| Conditional recommendation based on low-quality evidence; may consider switching to an IL- 117i if the patient has severe psoriasis or contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 9. Switch to a TNFi biologic over an IL-12/23i biologic (PICO 52) | Low (72, 73, 76, 98, 100) |
| Conditional recommendation based on low-quality evidence; may consider switching to an IL- 12/23i if the patient has severe psoriasis or contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, or if the patient prefers less frequent drug administration. | |
| 10. Switch to a TNFi biologic over switching to another OSM (PICO 49) | Low (72, 73, 76, 83–85) |
| Conditional recommendation based on low-quality evidence; may consider switching to another OSM# if the patient prefers an oral medication over an injection, or if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| 11. Switch to an IL-17i biologic over an IL-12/23i biologic (PICO 54) | Low (89, 90, 92, 93, 98–100) |
| Conditional recommendation based on low- quality evidence; may consider switching to an IL- 12/23i if the patient has concomitant IBD or if the patient prefers less frequent drug administration. | |
| 12. Switch to an IL-17i biologic over switching to another OSM (PICO 51) | Low (83–86, 89, 90, 92, 93) |
| Conditional recommendation based on low-quality evidence; may consider switching to another OSM if the patient prefers an oral medication. | |
| 13. Switch to an IL-12/23i biologic over switching to another OSM (PICO 50) | Low (83–86, 98, 100) |
| Conditional recommendation based on low-quality evidence; may consider switching to an- other OSM# if the patient prefers an oral medication over an injection, or if there are contraindications to an IL- 12/23i, such as severe recurrent infections. | |
| In adult patients with active PsA and concomitant active IBD who are both OSM- and biologic treatment–naive, | |
| 14. Start a monoclonal antibody TNFi biologic over an OSM (PICO 62) | Very low (114) |
| Conditional recommendation based on very-low- quality evidence; may consider starting an OSM if the patient prefers an oral medication, or if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease. | |
| In adult patients with active PsA and concomitant active IBD despite treatment with an OSM, | |
| 15. Switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic (i.e., etanercept) (PICO 58) | Moderate (115–117) |
| Strong recommendation supported by moderate- quality evidence, showing TNFi monoclonal antibody biologics are effective in IBD but indirect evidence shows a TNFi biologic soluble receptor biologic is not effective for the treatment of IBD. | |
| 16. Switch to a TNFi monoclonal antibody biologic over an IL-17i biologic (PICO 59) | Moderate (50) |
| Strong recommendation supported by moderate- quality evidence showing monoclonal antibody TNFi biologics are effective for IBD while an IL- 17i biologic is not effective for IBD. | |
| 17. Switch to a TNFi biologic monoclonal antibody biologic over an IL-12/23i biologic (PICO 61) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider switching to an IL- 12/23i biologic if the patient has contraindications to TNFi biologics, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease, or prefers less frequent drug administration. | |
| 18. Switch to an IL-12/23i biologic over switching to an IL-17i biologic (PICO 60) | Moderate (50) |
| Strong recommendation supported by moderate- quality evidence showing IL- 12/23i biologic is effective for IBD while an IL- 17i biologic is not effective for IBD. |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease (IBD).
When there were no published studies, we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
Axial disease is generally treated according to the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for spondyloarthritis (SpA).
Because there are currently no widely agreed-upon definitions of disease severity, psoriasis severity should be established by the health care provider and patient on a case-by-case basis. In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score (25) of ≥12 and a body surface area score of ≥10. In clinical practice, however, the PASI tool is not standardly utilized given its cumbersome nature. In 2007, the National Psoriasis Foundation published an expert consensus statement, which defined moderate-to-severe disease as a body surface area of ≥5% (68). In cases in which the involvement is in critical areas, such as the face, hands or feet, nails, intertriginous areas, scalp, or where the burden of the disease causes significant disability or impairment of physical or mental functioning, the disease can be severe despite the lower amount of surface area of skin involved. The need to factor in the unique circumstances of the individual patient is of critical importance, but this threshold provides some guidance in the care of patients.
Oral small molecules (OSMs) are defined as methotrexate (MTX), sulfasalazine, leflunomide, cyclosporine, or apremilast and do not include tofacitinib, which was handled separately since its efficacy/safety profile is much different from that of other OSMs listed above. OSM- and biologic treatment–naive is defined as naive to treatment with OSMs, tumor necrosis factor inhibitors (TNFi,), interleukin-17 inhibitors (IL-17i), and IL-12/23i; patients may have received nonsteroidal antiinflammatory drugs (NSAIDs), glucocorticoids, and/or other pharmacologic and nonpharmacologic interventions.
It should be noted that for the enthesitis questions (PICO 49, 50, and 51), the existing evidence was mainly drawn from the apremilast studies, as no randomized controlled trial report described enthesitis outcomes for the other OSMs.
This recommendation for patients with active PsA is conditional based on low-quality evidence.
In patients with active PsA, using a treat-to-target strategy is recommended over not following a-treat-to-target strategy. One may consider not using a treat-to-target strategy in patients in whom there are concerns related to increased adverse events, costs of therapy, and patient burden of medications associated with tighter control.
Active PsA with psoriatic spondylitis/axial disease despite treatment with NSAIDs (Table 5).
All recommendations for patients with active PsA with psoriatic spondylitis/axial disease despite NSAID treatment are conditional based on very-low-quality evidence.
The ACR/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for patients with axial spondyloarthritis (35) should be followed for patients with axial PsA. OSMs are not effective for axial disease (35). In patients with active axial PsA despite NSAID treatment, a TNFi biologic is recommended over an IL-17i or IL-12/23i biologic, and an IL-17i biologic is recommended over an IL-12/23i biologic. An IL-17i biologic may be used instead of a TNFi biologic if the patient has severe psoriasis or a contraindication to TNFi biologic treatment (Table 5). We recommend not using an IL-12/23i biologic since 3 randomized trials of an IL-12/23i biologic (ustekinumab) in patients with axial spondyloarthritis (a related condition) were stopped because the key primary and secondary end points were not achieved (36–38); the safety profile was reportedly consistent with that observed in past ustekinumab studies.
Active PsA with predominant enthesitis in treatment-naive patients and despite treatment with an OSM (Table 5).
All recommendations for patients with active PsA with predominant enthesitis are conditional based on low- to very-low-quality evidence. (This section names apremilast among all OSMs specifically for recommendations, since of the OSMs, only apremilast has shown efficacy for enthesitis.)
In treatment-naive PsA patients with predominant enthesitis, a TNFi biologic is recommended over an OSM as a first-line option. Apremilast may be used instead of a TNFi biologic if the patient prefers an oral therapy or has contraindications to TNFi. Oral NSAIDs are recommended over starting an OSM unless the patient has cardiovascular disease, peptic ulcer disease, renal disease (or impairment), or severe psoriasis or PsA, in which case apremilast may be given instead of NSAIDs. Tofacitinib is recommended over apremilast for treatment-naive patients with predominant enthesitis. Apremilast may be used instead of tofacitinib in patients with recurrent infections.
In patients with active PsA with predominant enthesitis despite treatment with an OSM (used for other manifestations of PsA), a TNFi biologic, an IL-17i biologic, or an IL-12/23i biologic is recommended over switching to another OSM. Apremilast may be used in patients who prefer oral therapy or who have recurrent infections or contraindications to TNFi biologics. A TNFi biologic is recommended over an IL-17i or IL-12/23i biologic. An IL-17i or IL-12/23i biologic may be used instead of a TNFi biologic in patients with severe psoriasis or contraindications to TNFi. An IL-17i biologic is recommended over an IL-12/23i biologic. An IL-12/23i biologic may be used instead of a TNFi biologic in patients who prefer less frequent drug administration, and instead of an IL-17i biologic in patients with concomitant IBD or who prefer less frequent drug administration.
Active PsA with concomitant active IBD (Table 5).
All recommendations for patients with active PsA with concomitant active IBD are strong based on moderate-quality evidence, except for 2 conditional recommendations based on very-low-quality evidence.
Active PsA in OSM- and biologic treatment–naive patients with concomitant active IBD. In patients with active PsA with concomitant active IBD who have not received OSM or biologic treatment, a monoclonal antibody TNFi biologic (excludes etanercept, which is a fusion molecule/soluble receptor biologic) is recommended over an OSM (Table 5). An OSM may be used in patients without severe PsA who prefer oral therapy or have contraindications to TNFi biologics.
Active PsA despite treatment with an OSM in patients with concomitant active IBD. In patients with active PsA with concomitant active IBD despite treatment with an OSM, a monoclonal antibody TNFi biologic or an IL-12/23i biologic should be used over an IL-17i biologic, and a monoclonal antibody TNFi biologic should be used over a TNFi soluble receptor biologic (etanercept) (all strong recommendations [Table 5]). A monoclonal antibody TNFi biologic is recommended over an IL-12/23i biologic (conditional recommendation). An IL-12/23i biologic may be used instead of a monoclonal antibody TNFi biologic in patients with contraindications to TNFi biologics or who prefer less frequent drug administration.
Active PsA with comorbidities (Table 6).
Table 6.
Recommendations for treatment of patients with active psoriatic arthritis and comorbidities, including concomitant diabetes and recurrent serious infections (PICOs 63–66)*
| Level of evidence (evidence [refs.] reviewed)† | |
|---|---|
| In adult patients with active PsA and diabetes who are both OSM- and biologic treatment–naive,‡ | |
| 1. Start an OSM other than MTX over a TNFi biologic (PICO 63a) | Very low (118, 119) |
| Conditional recommendation based on very-low- quality evidence; may consider starting a TNFi, if the patient has severe PsA§ or severe/active skin disease,¶ when diabetes is well controlled. | |
| In adult patients with active PsA and frequent serious infections who are both OSM- and biologic treatment–naive, | |
| 2. Start an OSM over a TNFi biologic (PICO 64) | Moderate (33, 120) |
| Strong recommendation supported by moderate- quality evidence, including a black box warning against the use of a TNFi biologic with regard to increased risk of serious infection. | |
| 3. Start an IL-12/23i biologic over a TNFi biologic (PICO 65) | Very low (33) |
| Conditional recommendation based on very-low- quality evidence; may consider starting a TNFi if the patient has severe PsA. | |
| 4. Start an IL-17i biologic over a TNFi biologic (PICO 66) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider starting a TNFi biologic in patients with concomitant IBD. |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease (IBD).
When there were no published studies, we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
Oral small molecules (OSMs) are defined as methotrexate (MTX), sulfasalazine, leflunomide, cyclosporine, or apremilast and do not include tofacitinib, which was handled separately since its efficacy/safety profile is much different from that of other OSMs listed above. OSM- and other treatment–naive is defined as naive to treatment with OSMs, tumor necrosis factor inhibitors (TNFi), interleukin-17 inhibitors (IL-17i), and IL-12/23i; patients may have received nonsteroidal antiinflammatory drugs, glucocorticoids, and/or other pharmacologic and nonpharmacologic interventions.
Because there are currently no widely agreed-upon definitions of disease severity, PsA severity should be established by the health care provider and patient on a case-by-case basis. For the purposes of these recommendations, severity is considered a broader concept than disease activity in that it encompasses the level of disease activity at a given time point, as well as the presence of poor prognostic factors and long-term damage. Examples of severe PsA disease include the presence of ≥1 of the following: a poor prognostic factor (erosive disease, elevated levels of inflammation markers such as C-reactive protein or erythrocyte sedimentation rate attributable to PsA), long-term damage that interferes with function (e.g., joint deformities, vision loss), highly active disease that causes major impairment in quality of life (i.e., active psoriatic inflammatory disease at many sites [including dactylitis, enthesitis] or function-limiting inflammatory disease at few sites), and rapidly progressive disease.
Because there are currently no widely agreed-upon definitions of disease severity, psoriasis severity should be established by the health care provider and patient on a case-by-case basis. In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score (25) of ≥12 and a body surface area score of ≥10. In clinical practice, however, the PASI tool is not standardly utilized given its cumbersome nature. In 2007, the National Psoriasis Foundation published an expert consensus statement, which defined moderate-to-severe disease as a body surface area of ≥5% (68). In cases in which the involvement is in critical areas, such as the face, hands or feet, nails, intertriginous areas, scalp, or where the burden of the disease causes significant disability or impairment of physical or mental functioning, the disease can be severe despite the lower amount of surface area of skin involved. The need to factor in the unique circumstances of the individual patient is of critical importance, but this threshold provides some guidance in the care of patients.
All recommendations for patients with active PsA with comorbidities are conditional based on low- to very-low-quality evidence, except those for patients with serious infections, which are strong based on moderate-quality evidence.
Active PsA in OSM- and biologic treatment–naive patients with concomitant diabetes. In patients with active PsA with concomitant active diabetes who have not received OSM or biologic treatment, an OSM other than MTX is recommended over a TNFi biologic, due to the concern about the higher prevalence of fatty liver disease and liver toxicity with MTX use in this patient population (39,40) (Table 6). A TNFi biologic may be used instead of an OSM in the presence of severe PsA or severe psoriasis or when diabetes is well controlled (i.e., with a potentially lower risk of infections).
Active PsA in OSM- and biologic treatment–naive patients with frequent serious infections.
In patients with active PsA who have frequent serious infections and have not received OSM or biologic treatment, an OSM should be used over a TNFi biologic as a first-line treatment since there is a black box warning against the use of a TNFi biologic in patients with frequent serious infections (strong recommendation). An IL-12/23i or IL-17i biologic is recommended over a TNFi biologic (conditional recommendation [Table 6]). A TNFi biologic may be used instead of an IL-12/23i biologic in patients with severe PsA and instead of an IL-17i biologic in patients with concomitant IBD.
Active PsA in patients requiring killed or live attenuated vaccinations when starting biologic treatment (Table 7).
Table 7.
Recommendations for vaccination in patients with active psoriatic arthritis (PICOs 56–57*)
| Level of evidence (evidence [refs.] reviewed)† | |
|---|---|
| In adult patients with active PsA needing vaccinations,‡ | |
| 1. Start the biologic and administer killed vaccines over delaying the start of biologic to administer killed vaccines (PICO 56) | Very low (121–126) |
| Conditional recommendation based on very-low- quality evidence; may consider delaying the start of biologic to administer killed vaccines due to patient preference based on patient belief about vaccine efficacy. | |
| 2. Delay the start of biologic to administer live attenuated vaccines over starting the biologic and administering live attenuated vaccines (PICO 57) | Very low (127) |
| Conditional recommendation based on very-low- quality evidence; may consider starting the biologic and administering live attenuated vaccines in patients with very active severe joint§ or skin¶ disease who prefer no delay in biologic initiation. |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease.
When there were no published studies, we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
Vaccines as indicated by patient age, sex, and immunization history per recommendations from the Centers for Disease Control and Prevention and available at: https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf.
Because there are currently no widely agreed-upon definitions of disease severity, PsA severity should be established by the health care provider and patient on a case-by-case basis. For the purposes of these recommendations, severity is considered a broader concept than disease activity in that it encompasses the level of disease activity at a given time point, as well as the presence of poor prognostic factors and long-term damage. Examples of severe PsA disease include the presence of ≥1 of the following: a poor prognostic factor (erosive disease, elevated levels of inflammation markers such as C-reactive protein or erythrocyte sedimentation rate attributable to PsA), long-term damage that interferes with function (e.g., joint deformities, vision loss), highly active disease that causes major impairment in quality of life (i.e., active psoriatic inflammatory disease at many sites [including dactylitis, enthesitis] or function-limiting inflammatory disease at few sites), and rapidly progressive disease.
Because there are currently no widely agreed-upon definitions of disease severity, psoriasis severity should be established by the health care provider and patient on a case-by-case basis. In clinical trials, severe psoriasis has been defined as a Psoriasis Area and Severity Index (PASI) score (25) of ≥12 and a body surface area score of ≥10. In clinical practice, however, the PASI tool is not standardly utilized given its cumbersome nature. In 2007, the National Psoriasis Foundation published an expert consensus statement, which defined moderate-to-severe disease as a body surface area of ≥5% (68). In cases in which the involvement is in critical areas, such as the face, hands or feet, nails, intertriginous areas, scalp, or where the burden of the disease causes significant disability or impairment of physical or mental functioning, the disease can be severe despite the lower amount of surface area of skin involved. The need to factor in the unique circumstances of the individual patient is of critical importance, but this threshold provides some guidance in the care of patients.
All recommendations for vaccinations in patients with active PsA are conditional based on very-low-quality evidence.
It is recommended that the biologic treatment be started and the killed vaccines administered (as indicated based on patient age, sex, and immunization history per recommendations of the Centers for Disease Control and Prevention [41]) in patients with active PsA over delaying the biologic to give the killed vaccines. Delaying the start of the biologic is recommended over not delaying to administer a live attenuated vaccination in patients with active PsA (Table 7). If PsA manifestations are severe and delaying the start of the biologic is not desirable, starting the biologic and administering the live attenuated vaccines at the same time might be considered.
Recommendations for nonpharmacologic interventions in patients with active PsA regardless of pharmacologic treatment status (Table 8)
Table 8.
Recommendations for treatment of patients with active psoriatic arthritis with nonpharmacologic interventions (PICOs 1–8)*
| Level of evidence (evidence [refs.] reviewed)† | |
|---|---|
| In adult patients with active PsA, | |
| 1. Recommend exercise over no exercise (PICO 1) | Low (128) |
| Conditional recommendation based on low-quality evidence; may consider no exercise in pa- tients with existing muscle/tendon injury or multiple inflamed symptomatic joints with worsening pain with exercise. | |
| 2. Recommend low-impact exercise (e.g., tai chi, yoga, swimming) over high-impact exer cise (e.g., running) (PICO 2) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider high-impact exercise due to patient preference. | |
| 3. Recommend physical therapy over no physical therapy (PICO 3) | Very low |
| Conditional recommendation based on very-low- quality evidence; may consider no physical therapy due to patient preference, out-of- pocket cost, distance to physical therapy site, or lack of transportation. | |
| 4. Recommend occupational therapy over no occupational therapy (PICO 4) | Low (129, 130) |
| Conditional recommendation based on low-quality evidence; may consider no occupational therapy due to patient preference, out-of- pocket cost, distance to occupational therapy site, or lack of transportation. | |
| 5. Recommend weight loss over no weight loss for patients who are overweight/obese (PICO 5) | Low (131–133) |
| Conditional recommendation based on low-quality evidence; may consider no weight loss due to additional patient burden involved with weight-loss program. | |
| 6. Recommend massage therapy over no massage therapy (PICO 7) | Very low (134) |
| Conditional recommendation based on very-low- quality evidence; may consider no massage therapy due to associated costs. | |
| 7. Recommend acupuncture over no acupuncture (PICO 8) | Very low (135) |
| Conditional recommendation based on very-low- quality evidence; may consider no acupuncture due to associated costs. | |
| 8. Recommend smoking cessation over no smoking cessation (PICO 6) | Moderate (136, 137) |
| Strong recommendation supported by moderate- quality evidence, rated down for indirectness. |
Active psoriatic arthritis (PsA) is defined as disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease.
When there were no published studies, we relied on the clinical experience of the panelists, which was designated very-low-quality evidence.
All recommendations for nonpharmacologic interventions for patients with active PsA are conditional based on low- to very-low-quality evidence, except that for smoking cessation, which is a strong recommendation.
It is recommended that patients with active PsA use some form or combination of exercise, physical therapy, occupational therapy, massage therapy, and acupuncture over not using these modalities as tolerated. Low-impact exercise (e.g., tai chi, yoga, swimming) is recommended over high-impact exercise (e.g., running). High-impact exercises may be performed instead of low-impact exercises by patients who prefer the former and have no contraindications to high-impact exercises (Table 8). Clinicians should encourage patients to stop smoking, offering cessation aids, due to a demonstrated effectiveness of smoking cessation in randomized trials in other conditions and in the general population (42–44) (strong recommendation). In PsA patients who are overweight or obese, weight loss is recommended in order to potentially increase pharmacologic response.
All strong recommendations in this guideline are also listed separately in Supplementary Appendix 6, at http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract.
DISCUSSION
We present herein the first ACR/NPF guideline for the treatment of psoriatic arthritis. The goal of this guideline is to assist health care providers in managing active PsA in their patients, including optimizing therapy. PsA is a heterogeneous and multifaceted inflammatory disease, and its different clinical features (e.g., peripheral arthritis, psoriasis, nail disease, enthesitis, dactylitis, axial disease) sometimes respond differently to therapy. Despite an expansion in the number of new therapies for PsA, there remains limited comparative efficacy/effectiveness evidence to inform treatment decisions. Thus, most of our recommendations are based on low-quality evidence and are conditional. The conditional recommendations convey that, although the suggested course of action will be best for many patients, there will be some patients in whom, considering their comorbidities and/or their values and preferences, the alternative represents the best choice. The guideline will be updated as new evidence from comparative studies becomes available.
A Patient Panel meeting was held prior to the Voting Panel meeting to gain insight into patients’ values and preferences for the pharmacologic/nonpharmacologic intervention comparisons being addressed. We recognize that patient preferences are an important part of our treatment recommendations. Findings from the Patient Panel meeting were discussed throughout the Voting Panel meeting to ensure that patient input was incorporated into the final PsA guideline. Examples of patient feedback included strong value on therapies that are effective (e.g., prevent further damage, and improve quality of life, social participation, and function) and safe (especially having low adverse event profiles). In particular, patients discussed the negative impact of adverse events (e.g., fatigue, nausea, and malaise) on quality of life and social participation, and thus the risk for these adverse events weighed heavily in patients’ decision-making. The concept of treat-to-target was challenging for patients. Although they saw value in improved outcomes, they also thought this strategy could increase costs to the patient (e.g., copayments, time traveling to more frequent appointments, etc.) and potentially increase adverse events. Therefore, a detailed conversation with the patient is needed to make decisions regarding treat-to-target. To help ensure that the recommendations were patient-centered, 2 patients were members of the Voting Panel.
While using a treat-to-target approach over not using a treat-to-target approach was discussed by the Voting Panel, we did not address specific targets to be recommended or used. There have been 2 international meetings to discuss potential targets: the use of either minimal disease activity (MDA) or disease activity in psoriatic arthritis (DAPSA) (45,46). The treatment target for PsA would likely be MDA or DAPSA, although a different target may be chosen through patient–provider discussion.
The ACR/NPF PsA guideline conditionally recommends a TNFi biologic over an OSM agent in patients with active PsA. The available low-quality evidence is inconclusive regarding the efficacy of OSMs in management of PsA, whereas there is moderate-quality evidence of the benefits of TNFi biologics, in particular regarding their impact on the prevention of disease progression and joint damage. In making their recommendation, the panel recognized the cost implications, but put considerations of quality of evidence for benefit over other considerations. This guideline provides recommendations for early and aggressive therapy in patients with newly diagnosed PsA.
The recommendation is, however, conditional, and the panel recognized several potential exceptions to it. Circumstances in which a patient may choose an OSM over a TNFi biologic may include mild-to-moderate disease, a preference of oral over parenteral therapy, or concerns regarding adverse effects of a biologic. A TNFi biologic would not be a good choice in patients with contraindications, including congestive heart failure, previous serious infections, recurrent infections, or demyelinating disease.
During the development of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis recommendations (47) and the European League Against Rheumatism (EULAR) recommendations (48) for the treatment of PsA, panel members also challenged the decision to put OSMs first in those recommendations. For the EULAR recommendations, the final decision was made based on the lower cost of these medications, a consideration our panel placed lower than the quality of evidence for benefit.
In patients with concomitant IBD, the Voting Panel made strong recommendations favoring a monoclonal antibody TNFi or an IL-12/23i biologic over an IL-17i biologic or a TNFi receptor biologic (etanercept). This was based on moderate-quality evidence showing that TNFi biologics and ustekinumab (an IL-12/23i biologic) are effective for the management of IBD, whereas etanercept (a TNFi receptor biologic) and secukinumab (an IL-17i biologic) are not (49,50).
When the evidence was low or very-low quality, the panel could not be confident in the judgment of net benefit—thus the conditional recommendation. Often, low- or very-low-quality evidence came from indirect evidence, for instance from rheumatoid arthritis (33) or, in the absence of studies, from clinical experience (Supplementary Appendix 5, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract). When data on comparative benefits and comparative harms were similar between two medications, the panel explicitly preferred and recommended the medication for which longer-term harms were more well-known, and in which the physician experience in patients with PsA was longer, supplementing with harms data/experience from related rheumatic conditions, where these medications are commonly used. In each case, judgments of net benefit involved explicit consideration of values and preferences, including input from Patient Panel members of the Voting Panel as well as the full Patient Panel that met prior to the Voting Panel meeting.
We recognize that these recommendations do not account for the full complexity of PsA or the full range of possible therapies (e.g., glucocorticoids were not addressed). The high degree of heterogeneity in the presentation and course of PsA coupled with the involvement of multiple domains in a single patient cannot be captured in a single algorithm. In addition, reporting of disease measures and differences in inclusion/exclusion criteria in PsA clinical trials makes it difficult to compare therapies across trials. The impact of alternative therapies on important outcomes such as joint damage still remains to be elucidated. Vaccination recommendations with tofacitinib were not included, as it was not yet approved for PsA when the PICO questions were drafted and only a limited number of PICO questions could be feasibly included for voting. Additional topics, including vaccination in the setting of tofacitinib, will be addressed in a subsequent guideline update.
The ACR has decided to use GRADE methodology in the development of guidelines for the management of rheumatic diseases. The GRADE methodology specifies that panels make recommendations based on a consideration of the balance of relative benefits and harms of the treatment options under consideration, the quality of the evidence (i.e., confidence in the evidence based on the lowest quality of the critical outcomes—high, moderate, low, or very low), and patients’ values and preferences. The rating of the quality of evidence for each clinical situation (PICO question) helped to inform the strength of the recommendation (strong or conditional) (51).
The use of GRADE (not used in other PsA treatment recommendations) allowed an explicit consideration of the overall evidence, including the balance of benefits and harms of treatments, the incorporation of patient values and preferences, and cost considerations to judge the tradeoff. This approach led to transparency in decision making by the Voting Panel for each clinical scenario and the formulation of these recommendations. Consistent with GRADE guidance, the Voting Panel usually offered a strong recommendation in the presence of moderate- or high-quality rating of the evidence, and a conditional recommendation in the presence of very-low or low-quality evidence (although recommendations can also be conditional in the setting of moderate-quality evidence, and in certain circumstances strong in the face of low-quality evidence) (15). The other merits of the ACR/NPF process undertaken included a comprehensive literature search, the consideration of each comparison in light of the available evidence, the diverse composition of the Voting Panel, the inclusion of all of the available therapies (e.g., IL-17i biologics, an IL-12/23i biologic, abatacept, and tofacitinib) in the decision-making process (including those approved for psoriasis or rheumatoid arthritis but not yet for PsA, ensuring that the guideline would not be out of date by the time it was published), and the inclusion of population subsets, such as those with predominant enthesitis and/or IBD.
Limitations of the guideline include the limited comparative evidence to inform selection of therapies (i.e., primary comparative benefit/efficacy and harms evidence) and the inability to include all possible clinical scenarios due to the necessity of keeping the task feasible. Because the American Academy of Dermatology and the NPF are currently developing a guideline addressing therapy for psoriasis, our guideline did not address treatment of isolated psoriasis. Another limitation is that we searched only English-language literature. The major limitation of the work arises from the limitations in the evidence.
In this guideline, we often used indirect comparisons among trials/therapies, frequently relying on network meta-analysis. Stratified analyses among subgroups (e.g., treatment-naive, inadequate response to a TNFi biologic agent) were rarely reported separately in primary trials, limiting our ability to perform network meta-analyses in these important subgroups. For most clinical scenarios (PICO questions) there were few or no head-to-head comparison studies identified in the literature review. Thus, the quality of evidence was most often low or very low, and only occasionally moderate (Supplementary Appendix 5; http://onlinelibrary.wiley.com/doi/10.1002/art.40726/abstract). This led to nearly all recommendations being conditional, with a few strong recommendations in cases in which there was sufficient evidence (including that from outside of PsA) to make the Voting Panel confident in selecting one option over the comparator. A flow chart or ranking of treatments requires strong recommendation; when recommendations are conditional/weak it means that the right course of action differs between patients. When the right course of action differs between patients, it is inappropriate to make the flow chart and establish treatment ranking or a hierarchy of treatment options (14).
The 2018 ACR/NPF guideline for the treatment of PsA will assist patients and their health care providers in making challenging disease management decisions. More comparative data are needed to inform treatment selection. Several ongoing trials, including a trial to compare a TNFi biologic combination therapy with a TNFi biologic monotherapy and MTX monotherapy (52), will inform treatment decisions. We anticipate future updates to the guideline when new evidence is available.
Supplementary Material
Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to the recommendations within this guideline to be voluntary, with the ultimate determination regarding their application to be made by the health care provider in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed and endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice. ACR recommendations are not intended to dictate payment or insurance decisions. These recommendations cannot adequately convey all uncertainties and nuances of patient care.
The American College of Rheumatology is an independent, professional, medical and scientific society that does not guarantee, warrant, or endorse any commercial product or service.
ACKNOWLEDGMENTS
We thank Ms Emily Boyd with the NPF for her involvement throughout the guideline development process. We thank Dr. Maureen Dubreuil for leading the Patient Panel meeting, as well the patients who participated in this guideline project: Eddie Applegate, Steve Bishkoff, Christy Gephart, Lisa Medlin, Gail C. Richardson, Richard F. Seiden, Delores Teddleton, and Hilary Wilson. We thank the ACR staff, including Ms Regina Parker for assistance in organizing the face-to-face meeting and coordinating the administrative aspects of the project and Ms Robin Lane for assistance in manuscript preparation. We thank Ms Janet Joyce for help in developing the literature search strategy and performing the literature search and updates, and Ms Tamara Rader for peer-reviewing the literature search strategy. We thank Dr. Jonathan Treadwell for his statistical contributions to the network meta-analyses that were done as part of the literature review.
Supported by the American College of Rheumatology and the National Psoriasis Foundation.
Footnotes
This article is published simultaneously in Arthritis Care & Research and the Journal of Psoriasis and Psoriatic Arthritis.
Dr. Singh has received consulting fees from Savient, Takeda, Regeneron, Merz, Iroko, Bioiberica, Crealta/Horizon, Allergan Pharmaceuticals, WebMD, UBM LLC, Medscape, and Fidia Pharmaceuticals (less than $10,000 each) and has received research support from Takeda and Savient Pharmaceuticals. Dr. Ogdie has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol-Myers Squibb, Lilly, Novartis, Pfizer, and Takeda (less than $10,000 each). Dr. Gladman has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Pfizer, Novartis, and UCB (less than $10,000 each). Dr. Deodhar has received consulting fees, speaking fees, and/or honoraria from Eli Lilly and Novartis (more than $10,000 each) and from AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, and UCB (less than $10,000 each) and has received research support from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Dr. Husni has received consulting fees, speaking fees, and/or honoraria from AbbVie, Janssen, Sanofi Genzyme/Regeneron, UCB, Novartis, and Lilly (less than $10,000 each) and is a coinventor on a patent for a psoriatic arthritis questionnaire (Psoriatic Arthritis Screening Evaluation), for which she receives royalties. Dr. Mease has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Celgene, Janssen, Lilly, Novartis, Pfizer, and UCB (more than $10,000 each) and from Genentech, Merck, and Sun (less than $10,000 each) and has served as a paid consultant to investment analysis companies Gerson Lehman and Guidepoint. Dr. Merola has received consulting fees, speaking fees, and/or honoraria from AbbVie, Eli Lilly, Novartis, Pfizer, UCB, Celgene, Sanofi, and Regeneron (more than $10,000 each) and from Merck, Biogen Idec, and Janssen (less than $10,000 each) and has served as a paid consultant for investment analysis companies Cowen Group and GLG. Dr. Ritchlin has received consulting fees from AbbVie, Amgen, Janssen, Novartis, Pfizer, UCB, and Celgene (less than $10,000 each) and has received research support from AbbVie, UCB, and Amgen. Mr. Smith has received consulting fees from the American Academy of Physician Assistants/Medical Logix for educational product development related to psoriatic arthritis CME courses (less than $10,000). Dr. Van Voorhees has received consulting fees, speaking fees, and/or honoraria from Dermira, Novartis, Derm Tech, WebMD, Celgene, AbbVie, Allergan, Valeant, and Merck (less than $10,000 each) and owns stock or stock options in Merck. Dr. Coates has received consulting fees from AbbVie (more than $10,000) and from Amgen, Bristol-Myers Squibb, Celgene, Pfizer, UCB, MSD, Novartis, Lilly, Janssen, Sun Pharma, Prothena, and Galapogos (less than $10,000 each). Dr. Gottlieb has received consulting fees, speaking fees, and/or honoraria from Janssen, Lilly, AbbVie, and UCB (more than $10,000 each) and from Sun, Celgene, Bristol-Myers Squibb, Beiersdorf, Incyte, Reddy, Valeant, Dermira, Allergan, and Novartis (less than $10,000 each) and has received research support from Janssen and Incyte. Dr. Nowell owns stock or stock options in AbbVie, Lilly, and Johnson & Johnson. Dr. Orbai has received consulting fees, speaking fees, and/or honoraria from Eli Lilly, Janssen, Novartis, Pfizer, and UCB (less than $10,000 each) and has received research support from Celgene, Eli Lilly, Horizon, Janssen, Novartis, and Pfizer. Dr. Reddy has received consulting fees, speaking fees, and/or honoraria from Novartis, AbbVie, and Pfizer (less than $10,000 each). Dr. Scher has received consulting fees from Janssen, UCB, AbbVie, and Novartis (less than $10,000 each). Dr. E. Siegel has received consulting fees, speaking fees, and/or honoraria from AbbVie, Lilly, and Novartis (more than $10,000 each) and from Amgen, Janssen, and Celgene (less than $10,000 each). Dr. Walsh has received consulting fees from Novartis, AbbVie, and Pfizer (less than $10,000 each).
REFERENCES
- 1.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:2095–6. [DOI] [PubMed] [Google Scholar]
- 3.Husted JA, Gladman DD, Farewell VT, Cook RJ. Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum 2001;45:151–8. [DOI] [PubMed] [Google Scholar]
- 4.Adams R, Walsh C, Veale D, Bresnihan B, FitzGerald O, Barry M. Understanding the relationship between the EQ-5D, SF-6D, HAQ and disease activity in inflammatory arthritis. Pharmacoeconomics 2010;28:477–87. [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Strand V. Spondyloarthritis is associated with poor function and physical health-related quality of life. J Rheumatol 2009;36:1012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javitz HS, Ward MM, Farber E, Nail L, Vallow SG. The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol 2002;46:850–60. [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Strand V. Health care utilization in patients with spondyloarthropathies. Rheumatology (Oxford) 2009;48:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bond SJ, Farewell VT, Schentag CT, Gladman DD. Predictors for radiological damage in psoriatic arthritis: results from a single centre. Ann Rheum Dis 2007;66:370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cresswell L, Chandran V, Farewell VT, Gladman DD. Inflammation in an individual joint predicts damage to that joint in psoriatic arthritis. Ann Rheum Dis 2011;70:305–8. [DOI] [PubMed] [Google Scholar]
- 10.Gladman DD, Farewell VT, Wong K, Husted J. Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum 1998;41:1103–10. [DOI] [PubMed] [Google Scholar]
- 11.Gladman DD. Mortality in psoriatic arthritis. Clin Exp Rheumatol 2008;26 Suppl 51:S62–5. [PubMed] [Google Scholar]
- 12.Gladman DD. Early psoriatic arthritis. Rheum Dis Clin North Am 2012;38:373–86. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–25. [DOI] [PubMed] [Google Scholar]
- 15.Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation—determinants of a recommendation’s direction and strength. J Clin Epidemiol 2013;66:726–35. [DOI] [PubMed] [Google Scholar]
- 16.Pfizer. Press release: Pfizer Announces U.S. FDA Filing acceptance of supplemental new drug application for Xeljanz (tofacitinib citrate) for the treatment of adult patients with active psoriatic arthritis. 2017. URL: https://press.pfizer.com/press-release/pfizer-announces-us-fda-filing-acceptance-supplemental-new-drug-application-xeljanz-to.
- 17.Reuters. Brief: Eli Lilly files supplemental biologics license application with FDA for Taltz. 2017. URL: http://www.reuters.com/article/brief-eli-lilly-files-supplemental-biolo-idUSFWN1JC0KM.
- 18.National Psoriasis Foundation. FDA approves Xeljanz for psoriatic arthritis. 2017. URL: https://www.psoriasis.org/advance/fda-approves-xeljanz-psoriatic-arthritis.
- 19.National Psoriasis Foundation. FDA approves Taltz for psoriatic arthritis. 2017. URL: https://www.psoriasis.org/advance/fda-approves-taltz-psoriatic-arthritis.
- 20.Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strand V, Ahadieh S, French J, Geier J, Krishnaswami S, Menon S, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo CM, Tung TH, Wang SH, Chi CC. Efficacy and safety of tofacitinib for moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol 2018;32:355–62. [DOI] [PubMed] [Google Scholar]
- 23.Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 24.Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 25.Feldman SR. A quantitative definition of severe psoriasis for use in clinical trials. J Dermatolog Treat 2004;15:27–9. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 27.European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of psoriatic arthritis. 2006. URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003413.pdf.
- 28.Jaeschke R, Guyatt GH, Dellinger P, Schunemann H, Levy MM, Kunz R, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744. [DOI] [PubMed] [Google Scholar]
- 29.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. [DOI] [PubMed] [Google Scholar]
- 30.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying anti rheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann A, Akl E, Vandvik P, Agoritsas T, Alonso-Coello P, Rind D, et al. How to use a patient management recommendation: clinical practice guidelines and decision analyses. Users’ guides to the medical literature: a manual for evidence-based clinical practice. New York (NY): McGraw-Hill; 2014. [Google Scholar]
- 32.Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, et al. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol 2016;72:45–55. [DOI] [PubMed] [Google Scholar]
- 33.Yun H, Xie F, Delzell E, Levitan EB, Chen L, Lewis JD, et al. Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in Medicare. Arthritis Rheumatol 2016;68:56–66. [DOI] [PubMed] [Google Scholar]
- 34.Favalli EG, Selmi C, Becciolini A, Biggioggero M, Ariani A, Santilli D, et al. Eight-year retention rate of first-line tumor necrosis factor inhibitors in spondyloarthritis: a multicenter retrospective analysis. Arthritis Care Res (Hoboken) 2017;69:867–74. [DOI] [PubMed] [Google Scholar]
- 35.Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016;68:282–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Research Janssen & Development LLC, sponsor. An efficacy and safety study of ustekinumab in participants with active nonradiographic axial spondyloarthritis. ClinicalTrials.gov identifier: NCT02407223; 2017. [Google Scholar]
- 37.Research Janssen & Development LLC, sponsor. A study to evaluate the efficacy and safety of ustekinumab in the treatment of anti-TNFα naive participants with active radiographic axial spondyloarthritis . ClinicalTrials.gov identifier: NCT02437162; 2018. [Google Scholar]
- 38.Research Janssen & Development LLC, sponsor. A study to evaluate the efficacy and safety of ustekinumab in the treatment of anti-TNFα refractory participants with active radiographic axial spondyloarthritis. ClinicalTrials.gov identifier: NCT02438787; 2018. [Google Scholar]
- 39.Maybury CM, Jabbar-Lopez ZK, Wong T, Dhillon AP, Barker JN, Smith CH. Methotrexate and liver fibrosis in people with psoriasis: a systematic review of observational studies. Br J Dermatol 2014;171:17–29. [DOI] [PubMed] [Google Scholar]
- 40.Miele L, Vallone S, Cefalo C, La Torre G, Di Stasi C, Vecchio FM, et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol 2009;51:778–86. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Recommended immunization schedules for adults aged 19 years or older. URL: https://www.cdc.gov/vaccines/schedules/hcp/adult.html. [Google Scholar]
- 42.Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev 2004:CD003041. [DOI] [PubMed] [Google Scholar]
- 43.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 44.Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 46.Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–8. [DOI] [PubMed] [Google Scholar]
- 47.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta-Felquer ML, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 48.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 49.Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ 2017;357:j2505. [DOI] [PubMed] [Google Scholar]
- 50.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 52.Mease PJ, Gladman DD, Samad AS, Coates LC, Liu LX, Aras GA, et al. Design and rationale of the Study of Etanercept and Methotrexate in Combination or as Monotherapy in Subjects with Psoriatic Arthritis (SEAM-PsA). RMD Open 2018;4:e000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baranauskaite A, Raffayova H, Kungurov NV, Kubanova A, Venalis A, Helmle L, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis 2012;71:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heiberg MS, Kaufmann C, Rodevand E, Mikkelsen K, Koldingsnes W, Mowinckel P, et al. The comparative effectiveness of anti-TNF therapy and methotrexate in patients with psoriatic arthritis: 6 month results from a longitudinal, observational, multicentre study. Ann Rheum Dis 2007;66:1038–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eder L, Thavaneswaran A, Chandran V, Gladman DD. Tumour necrosis factor α blockers are more effective than methotrexate in the inhibition of radiographic joint damage progression among patients with psoriatic arthritis. Ann Rheum Dis 2014;73:1007–11. [DOI] [PubMed] [Google Scholar]
- 56.Barker J, Hoffmann M, Wozel G, Ortonne JP, Zheng H, van Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol 2011;165:1109–17. [DOI] [PubMed] [Google Scholar]
- 57.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008;158:558–66. [DOI] [PubMed] [Google Scholar]
- 58.Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012;51:1368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta AK, Grober JS, Hamilton TA, Ellis CN, Siegel MT, Voorhees JJ, et al. Sulfasalazine therapy for psoriatic arthritis: a double blind, placebo controlled trial. J Rheumatol 1995;22:894–8. [PubMed] [Google Scholar]
- 60.Combe B, Goupille P, Kuntz JL, Tebib J, Liote F, Bregeon C. Sulphasalazine in psoriatic arthritis: a randomized, multicentre, placebo-controlled study. Br J Rheumatol 1996;35:664–8. [DOI] [PubMed] [Google Scholar]
- 61.Farr M, Kitas GD, Waterhouse L, Jubb R, Felix-Davies D, Bacon PA. Sulphasalazine in psoriatic arthritis: a double-blind placebo-controlled study. Br J Rheumatol 1990;29:46–9. [DOI] [PubMed] [Google Scholar]
- 62.Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. [DOI] [PubMed] [Google Scholar]
- 63.Gladman DD, Mease PJ, Cifaldi MA, Perdok RJ, Sasso E, Medich J. Adalimumab improves joint-related and skin-related functional impairment in patients with psoriatic arthritis: patient-reported outcomes of the Adalimumab Effectiveness in Psoriatic Arthritis Trial. Ann Rheum Dis 2007;66:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. [DOI] [PubMed] [Google Scholar]
- 65.Mease PJ, Woolley JM, Singh A, Tsuji W, Dunn M, Chiou CF. Patient-reported outcomes in a randomized trial of etanercept in psoriatic arthritis. J Rheumatol 2010;37:1221–7. [DOI] [PubMed] [Google Scholar]
- 66.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385–90. [DOI] [PubMed] [Google Scholar]
- 67.Abu-Shakra M, Gladman DD, Thorne JC, Long J, Gough J, Farewell VT. Longterm methotrexate therapy in psoriatic arthritis: clinical and radiological outcome. J Rheumatol 1995;22:241–5. [PubMed] [Google Scholar]
- 68.Pariser DM, Bagel J, Gelfand JM, Korman NJ, Ritchlin CT, Strober BE, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol 2007;143:239–42. [DOI] [PubMed] [Google Scholar]
- 69.Karanikolas GN, Koukli EM, Katsalira A, Arida A, Petrou D, Komninou E, et al. Adalimumab or cyclosporine as monotherapy and in combination in severe psoriatic arthritis: results from a prospective 12-month nonrandomized unblinded clinical trial. J Rheumatol 2011;38:2466–74. [DOI] [PubMed] [Google Scholar]
- 70.Bachelez H, van de Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386:552–61. [DOI] [PubMed] [Google Scholar]
- 71.Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol 2017;31:507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gladman D, Fleischmann R, Coteur G, Woltering F, Mease PJ. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken) 2014;66:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA, et al. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 2007;34:1040–50. [PubMed] [Google Scholar]
- 75.Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four–week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 77.Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227–36. [DOI] [PubMed] [Google Scholar]
- 78.Torii H, Nakagawa H, Japanese Infliximab Study Investigators. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis: a randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 2010;59:40–9. [DOI] [PubMed] [Google Scholar]
- 79.Nash P, Thaci D, Behrens F, Falk F, Kaltwasser JP. Leflunomide improves psoriasis in patients with psoriatic arthritis: an in-depth analysis of data from the TOPAS study. Dermatology 2006;212:238–49. [DOI] [PubMed] [Google Scholar]
- 80.Kaltwasser JP, Nash P, Gladman D, Rosen CF, Behrens F, Jones P, et al. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum 2004;50:1939–50. [DOI] [PubMed] [Google Scholar]
- 81.Strand V, Schett G, Hu C, Stevens RM. Patient-reported health-related quality of life with apremilast for psoriatic arthritis: a phase II, randomized, controlled study. J Rheumatol 2013;40:1158–65. [DOI] [PubMed] [Google Scholar]
- 82.Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2012;64:3156–67. [DOI] [PubMed] [Google Scholar]
- 83.Edwards CJ, Blanco FJ, Crowley J, Birbara CA, Jaworski J, Aelion J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 2016;75:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cutolo M, Myerson GE, Fleischmann RM, Liote F, Diaz-Gonzalez F, Van den Bosch F, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 Trial. J Rheumatol 2016;43:1724–34. [DOI] [PubMed] [Google Scholar]
- 85.Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol 2015;42:479–88. [DOI] [PubMed] [Google Scholar]
- 86.Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014;73:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. J Drugs Dermatol 2015;14:821–33. [PubMed] [Google Scholar]
- 88.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- 89.Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17 A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 91.McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 2014;73:349–56. [DOI] [PubMed] [Google Scholar]
- 92.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 93.Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014;370:2295–306. [DOI] [PubMed] [Google Scholar]
- 94.Nakagawa H, Niiro H, Ootaki K, Japanese Brodalumab Study Group. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci 2016;81:44–52. [DOI] [PubMed] [Google Scholar]
- 95.Papp K, Menter A, Strober B, Kricorian G, Thompson EH, Milmont CE, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2015;72:436–9. [DOI] [PubMed] [Google Scholar]
- 96.Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541–51. [DOI] [PubMed] [Google Scholar]
- 97.Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CE, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2017;176:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. [DOI] [PubMed] [Google Scholar]
- 99.Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373:633–40. [DOI] [PubMed] [Google Scholar]
- 100.Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010;362:118–28. [DOI] [PubMed] [Google Scholar]
- 102.Gupta AK, Daigle D, Lyons DC. Network meta-analysis of treatments for chronic plaque psoriasis in Canada. J Cutan Med Surg 2014;18:371–8. [DOI] [PubMed] [Google Scholar]
- 103.Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum 2011;63:939–48. [DOI] [PubMed] [Google Scholar]
- 104.Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017;76:1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menter MA, Papp KA, Cather J, Leonardi C, Pariser DM, Krueger JG, et al. Efficacy of tofacitinib for the treatment of moderate-to-severe chronic plaque psoriasis in patient subgroups from two randomised Phase 3 trials. J Drugs Dermatol 2016;15:568–80. [PubMed] [Google Scholar]
- 106.Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015;373:1318–28. [DOI] [PubMed] [Google Scholar]
- 107.Thaci D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015;73:400–9. [DOI] [PubMed] [Google Scholar]
- 108.Fraser AD, van Kuijk AW, Westhovens R, Karim Z, Wakefield R, Gerards AH, et al. A randomised, double blind, placebo controlled, multicentre trial of combination therapy with methotrexate plus ciclosporin in patients with active psoriatic arthritis. Ann Rheum Dis 2005;64:859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Combe B, Behrens F, McHugh N, Brock F, Kerkmann U, Kola B, et al. Comparison of etanercept monotherapy and combination therapy with methotrexate in psoriatic arthritis: results from 2 clinical trials. J Rheumatol 2016;43:1063–7. [DOI] [PubMed] [Google Scholar]
- 110.Zachariae C, Mork NJ, Reunala T, Lorentzen H, Falk E, Karvonen SL, et al. The combination of etanercept and methotrexate increases the effectiveness of treatment in active psoriasis despite inadequate effect of methotrexate therapy. Acta Derm Venereol 2008;88:495–501. [DOI] [PubMed] [Google Scholar]
- 111.Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Lexberg AS, Rodevand E, et al. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis 2014;73:132–7. [DOI] [PubMed] [Google Scholar]
- 112.Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017;389:2317–27. [DOI] [PubMed] [Google Scholar]
- 113.Coates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O’Dwyer JL, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Narula N, Marshall JK, Colombel JF, Leontiadis GI, Williams JG, Muqtadir Z, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol 2016;111:477–91. [DOI] [PubMed] [Google Scholar]
- 115.Stidham RW, Lee TC, Higgins PD, Deshpande AR, Sussman DA, Singal AG, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Ther 2014;39:1349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stidham RW, Lee TC, Higgins PD, Deshpande AR, Sussman DA, Singal AG, et al. Systematic review with network meta-analysis: the efficacy of anti-tumour necrosis factor-α agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther 2014;39:660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, et al. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2001;121:1088–94. [DOI] [PubMed] [Google Scholar]
- 118.Rosenberg P, Urwitz H, Johannesson A, Ros AM, Lindholm J, Kinnman N, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol 2007;46:1111–8. [DOI] [PubMed] [Google Scholar]
- 119.Malatjalian DA, Ross JB, Williams CN, Colwell SJ, Eastwood BJ. Methotrexate hepatotoxicity in psoriatics: report of 104 patients from Nova Scotia, with analysis of risks from obesity, diabetes and alcohol consumption during long term follow-up. Can J Gastroenterol 1996;10:369–75. [DOI] [PubMed] [Google Scholar]
- 120.US Food and Drug Administration. FDA drug safety communication: drug labels for the tumor necrosis factor-α (TNFα) blockers now include warnings about infection with Legionella and Listeria bacteria. 2011. URL: https://www.fda.gov/Drugs/DrugSafety/ucm270849.htm. [Google Scholar]
- 121.Kivitz AJ, Schechtman J, Texter M, Fichtner A, de Longueville M, Chartash EK. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase IV trial. J Rheumatol 2014;41:648–57. [DOI] [PubMed] [Google Scholar]
- 122.Franca IL, Ribeiro AC, Aikawa NE, Saad CG, Moraes JC, Goldstein-Schainberg C, et al. TNF blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology (Oxford) 2012;51:2091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elkayam O, Bashkin A, Mandelboim M, Litinsky I, Comaheshter D, Levartovsky D, et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum 2010;39:442–7. [DOI] [PubMed] [Google Scholar]
- 124.Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol 2007;34:272–9. [PubMed] [Google Scholar]
- 125.Ribeiro AC, Laurindo IM, Guedes LK, Saad CG, Moraes JC, Silva CA, et al. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:476–80. [DOI] [PubMed] [Google Scholar]
- 126.Migita K, Akeda Y, Akazawa M, Tohma S, Hirano F, Ideguchi H, et al. Effect of abatacept on the immunogenicity of 23-valent pneumococcal polysaccharide vaccination (PPSV23) in rheumatoid arthritis patients. Arthritis Res Ther 2015;17:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012;308:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baillet A, Zeboulon N, Gossec L, Combescure C, Bodin LA, Juvin R, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2010;62:984–92. [DOI] [PubMed] [Google Scholar]
- 129.Knittle K, Maes S, de Gucht V. Psychological interventions for rheumatoid arthritis: examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2010;62:1460–72. [DOI] [PubMed] [Google Scholar]
- 130.Siegel S, Tencza M, Apodaca B, Poole J. Effectiveness of occupational therapy interventions for adults with rheumatoid arthritis: a systematic review. Am J Occup Ther 2017;71:1–11. [DOI] [PubMed] [Google Scholar]
- 131.Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis 2014;73:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gisondi P, Del Giglio M, Di Francesco V, Zamboni M, Girolomoni G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr 2008;88:1242–7. [DOI] [PubMed] [Google Scholar]
- 133.Al-Mutairi N, Nour T. The effect of weight reduction on treatment outcomes in obese patients with psoriasis on biologic therapy: a randomized controlled prospective trial. Expert Opin Biol Ther 2014;14:749–56. [DOI] [PubMed] [Google Scholar]
- 134.Nelson L, Churilla J. Massage therapy for pain and function in patients with arthritis: a systematic review of randomized controlled trials. Am J Phys Med Rehabil 2017;96:665–72. [DOI] [PubMed] [Google Scholar]
- 135.Manyanga T, Froese M, Zarychanski R, Abou-Setta A, Friesen C, Tennenhouse M, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med 2014;14:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005;142:233–9. [DOI] [PubMed] [Google Scholar]
- 137.Mons U, Muezzinler A, Gellert C, Schottker B, Abnet CC, Bobak M, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015;350:h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.