ABSTRACT
Infections caused by extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli are a significant cause of morbidity and health care costs. Globally, the prevailing clonal type is ST131 in association with the blaCTX-M-15 β-lactamase gene. However, other ESBLs, such as blaCTX-M-14 and blaCTX-M-27, can also be prevalent in some regions. We identified ST38 ESBL-producing E. coli from different regions in the United States which carry blaCTX-M-27 embedded on two distinct plasmid types, suggesting the potential emergence of new ESBL lineages.
KEYWORDS: Escherichia coli, β-lactamases, ST38, IncF plasmid, CTX-M-27, ESBL, extended-spectrum β-lactamases, ceftriaxone
INTRODUCTION
Infections caused by multidrug-resistant organisms present a major threat to health care systems worldwide (1). Disease caused by extended-spectrum-β-lactamase (ESBL)-producing organisms (such as Escherichia coli) is on the rise in the United States, with estimated costs to the health care system of $1.2 billion in 2017 (2). ESBLs confer resistance to most β-lactam antibiotics, including penicillins, oxyimino-cephalosporins (e.g., ceftriaxone), and monobactams, and can cause difficult-to-treat nosocomial- and community-acquired infections (3, 4). Globally, sequence type (ST) 131 is the predominant ESBL-producing E. coli isolated from urinary tract infections (UTIs) and bloodstream infections (BSIs), although other STs have been associated with ESBL carriage (i.e., ST38, ST648, ST405, ST10, and ST1193) (5–7). In the United States and elsewhere, a subclone of ST131 (C2/H30Rx) that carries the blaCTX-M-15 β-lactamase gene is often reported (8). Another ST131 clade (C1-M27) was first observed in 2006 in Japan and subsequently emerged as a major lineage; C1-M27 is also reported in Europe (5, 6). C1-M27 carries the blaCTX-M-27 gene on IncF[F1:A2:B20]-type plasmids (9, 10). Little is known about the epidemiology and genetic context of the blaCTX-M-27 gene among clonal types other than ST131.
In 2017, we performed whole-genome sequencing (WGS) of 89 ceftriaxone-resistant E. coli urine and sterile site isolates collected by our laboratory (“URMC ESBL”; Table 1), which serves several counties in western New York. We identified ST38 as a frequent lineage second only to ST131 (ST131, 41/89 [46.1%] isolates; ST38, 14/89 [15.7%] isolates) (11). ST38 is a phylogroup D lineage that encompasses a variety of O:H serotypes and has been described as a hybrid uropathogenic/enteroaggregative strain (12). ST38 is far less characterized than ST131, and clear CTX-M-associated ST38 lineages have not been defined, although blaCTX-M-14 and blaCTX-M-15 have been found in ST38 (6, 7, 13–15). In contrast, in our 2017 study, ST38 was strongly associated with blaCTX-M-27, which was found in 10/14 (71.4%) of ST38 isolates compared to 11/41 (26.8%) of ST131 isolates. In ST38, blaCTX-M-27 was typically associated with IncF[F2:A-:B10] replicon plasmids, whereas in ST131, it was found on IncF[F1:A2:B20] plasmids (11, 16).
TABLE 1.
Summary of ST38 isolates included in this study
| Parent collection | Description (reference no.) | Yr(s) isolated | Location | No. of ST38 isolates included in current study |
|---|---|---|---|---|
| URMC ESBL | 89 ceftriaxone-resistant surveillance isolates (11) | 2017 | NY | 14 |
| CDC EIP | 97 isolates from 5 EIP surveillance sitesa | 2017 | NY, CO, NM | 12 |
| URMC 2018–2019 | Additional ceftriaxone-resistant ST38 isolates found in clinical archive | 2018–2019 | NY | 11 |
| Total | 35b |
Resistant to ceftazidime, cefotaxime, or ceftriaxone, and nonresistant to all carbapenems tested and confirmed to be ESBL positive.
Two ST38 URMC isolates were submitted to the CDC EIP program (35 unique isolates in total).
In the current study, we sought to determine whether the occurrence of blaCTX-M-27 in ST38 was regional or more widespread. To address this question, 12 ST38 isolates collected in 2017 from Colorado, New Mexico, and New York by the Centers for Disease Control and Prevention (CDC) Emerging Infections Program (EIP) (17) were sequenced alongside their purified plasmids. These isolates were ceftazidime, cefotaxime, or ceftriaxone resistant, susceptible to all carbapenems tested, and confirmed to be ESBL positive (“CDC EIP” in Table 1). We compared the CDC EIP isolates to our previous ST38 E. coli isolated in 2017 (“URMC ESBL” in Table 1) and to 11 more recent (2018 to 2019) ST38 isolates (“URMC 2018–2019” in Table 1) from our institution in New York.
Library preparation and Nanopore- and Illumina-based sequencing for University of Rochester Medical Center (URMC) isolates (11) and EIP isolates (18) were performed as described previously. Only URMC and EIP isolates from 2017 were sequenced on the MinION platform (Oxford Nanopore Technologies, Cambridge, MA). Plasmid DNA was purified using the QIAfilter plasmid midi kit (Qiagen, Germantown, MD) from 100-ml Luria-Bertani (LB) cultures incubated for ∼18 h at 37°C shaking. Read processing, assembly, single-nucleotide polymorphism (SNP) calling (GenBank accession no. NZ_CP026723.1), and phylogenetic analysis were done as described (18). The average coverage of the reference strain was 87.3% (74.7% to 95.1%). Illumina-Nanopore hybrid read assemblies were generated with Unicycler (19). Sequence contigs were screened for antibiotic resistance genes (ARGs) and putative virulence factors using ABRicate (including ResFinder and VFDB databases) (https://github.com/tseemann/abricate). Plasmids were aligned with Mauve (20), and sequence identity was depicted with Easyfig (21). This study was approved with a waiver of consent by the University of Rochester institutional review board (IRB) office.
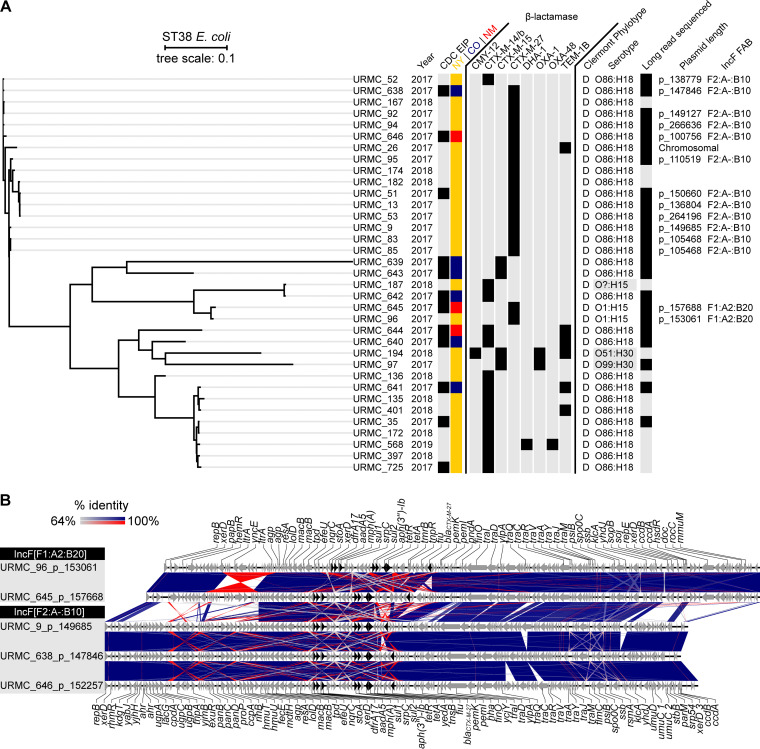
The CDC’s EIP ESBL surveillance found that 12 of 97 (12.4%) isolates typed as ST38 (6 from Colorado, 3 from New Mexico, and 3 from New York). Two of these 3 New York E. coli isolates were isolated from our hospital laboratory and were described previously as URMC_35 and URMC_51 (11). The third, URMC_725, was isolated from another local hospital system. An SNP-based phylogenetic tree was constructed using the 12 EIP ST38 isolates and the 23 other E. coli ST38 isolated and sequenced in our laboratory between 2017 and 2019 (35 isolates in total) (Table 1; Fig. 1A). Isolates appeared to group by β-lactamase type rather than state of origin. The most frequent β-lactamase gene detected was blaCTX-M-27 (total, 17/35 [48.6%]; New York, 14/26 [53.8%]; Colorado, 1/6 [16.6%]; New Mexico, 2/3 [66.7%]), followed by blaCTX-M-14 (total, 14/35 [40.0%]; New York, 10/26 [38.5%]; Colorado, 3/6 [50.0%]; New Mexico, 1/3 [33.3%]). Less prevalent β-lactamase genes detected included blaTEM-1B (6/35 [17.1%]), blaCTX-M-15 (4/35 [11.4%]), blaCMY-12 (1/35 [2.9%]), blaDHA-1 (1/35 [2.9%]), and blaOXA-48 (1/35 [2.9%]). All isolates with blaTEM-1B also carried one other β-lactamase gene, either blaCTX-M-27 (1/6 [16.7%]), blaCTX-M-15 (1/6 [16.7%]), or blaCTX-M-14 (4/6 [66.7%]). The blaOXA-48 carbapenemase gene was detected in 1 ST38 E. coli (URMC_568) isolated from New York, which also carried blaDHA-1 and blaCTX-M-14.
FIG 1.
Whole-genome sequence SNP-based phylogenomic tree of ST38 E. coli isolates obtained in New York, New Mexico, and Colorado with alignments and sequence identity of related plasmids harboring the blaCTX-M-27 gene. (A) Phylogenomic tree of 35 unique ST38 isolates showing year isolated, inclusion in the CDC EIP program, collection site, detection of β-lactamase genes (including only those with >95% sequence coverage), predicted phylotype and serotype, and plasmid name (with size in bp) and replicon type (fast atom bombardment [FAB] nomenclature). (B) Gene schematic and linear alignment of complete plasmids encoding the blaCTX-M-27 gene from ST38 (with genes shown as arrows). Antibiotic resistance genes are show in black. All blaCTX-M-27 plasmids identified in CDC EIP isolates are depicted (URMC_645_p_157668, URMC_638_p_147846, and URMC_646_p_152257) alongside selected previously described plasmids of the same FAB replicon type (URMC_96_p_153061 and URMC_9_p_149685) (11).
The median SNP distance between the 17 isolates carrying the blaCTX-M-27 gene was 199 SNPs (or 101 SNPs, excluding URMC_96 and URMC_645) compared to a median distance of 2,727 SNPs between all isolates carrying blaCTX-M-14. Isolates URMC_96 and URMC_645 were more distant (median of 4,153 SNPs) from the other blaCTX-M-27-carrying E. coli. Long-read sequencing established that these also carried the blaCTX-M-27 gene on plasmids (URMC_96_p_153061 and URMC_645_p_157668) of a different plasmid multilocus sequence typing (pMLST) type (IncF[F1:A2:B20]) compared to blaCTX-M-27-carrying plasmids (IncF[F2:A-:B10]) found in the other ST38 isolates (Fig. 1B). Despite this, these different plasmid types shared some synteny (generally, ∼92% identity over 42% of the conserved tra gene backbone versus ∼100% identity over ∼78% of the conserved ARG region). In addition to blaCTX-M-27, which was flanked by insertion elements in an arrangement known in ST131 (i.e., IS26-ΔISEcp1-blaCTX-M-27-ΔIS903D-IS26) (9), other ARGs found on these plasmids putatively included those for resistance to tetracyclines [tet(A)], sulfonamides (sul1, sul2), trimethoprim (dfrA17), aminoglycosides [aadA5, aph(6)-Id, aph(3″)-Ib, ant(3″)-Ia], and macrolides [mph(A)]. A class 1 integron harbored the dfrA17, aadA5, mph(A), and sul1 genes (22).
This work suggests the emergence of blaCTX-M-27 in ST38 on a newly described and conserved plasmid backbone (IncF[F2:A-:B10]) across three states from different regions of the United States. Also identified were two ST38 isolates with the gene on a plasmid type (IncF[F1:A2:B20]) already known in ST131 (10). This finding suggests two pathways for horizontal transfer of the β-lactamase among ST38. The association between blaCTX-M-27 and ST38 may potentially result in the emergence of new ESBL-producing clones and lead to an increase in antibiotic-resistant UTIs and BSIs.
Data availability.
The sequence information presented in this study has been deposited under NCBI BioProject accession nos. PRJNA692174 and PRJNA510429.
ACKNOWLEDGMENTS
We thank the clinical microbiology staff at UR Medicine Central Laboratory for specimen collection and antimicrobial susceptibility testing. We thank Nadezhda Duffy for her work coordinating strain sharing between URMC and the CDC MUGSI program. We thank Christopher Czaja and Erin C. Phipps for agreeing to share isolates from Colorado and New Mexico, respectively. We also thank Alison Laufer-Halpin and Maria Karlsson for project oversight.
Funding from the Department of Pathology and Laboratory Medicine, University of Rochester Medical Center supported this study.
N.D.P. conceived the study and collected clinical isolates. J.W. isolated plasmid DNA and performed sequencing. S.T. performed and managed bioinformatics analyses and pipelines. S.T., A.C., R.M., and N.D.P. analyzed sequence data. R.M., A.C., and N.D.P. analyzed overall data/results and wrote the first draft of the manuscript. N.D.P. provided funding and resources. R.A.S. did bioinformatics analysis. J.B.D. did sequencing work. D.C. performed AST and database management; J.D.L. organized CDC ESBL surveillance. All authors participated in editing and reviewing the manuscript and approved the final manuscript. All authors contributed to the article and approved the submitted version.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 3.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. 2018. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev 31:e00079-17. 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson DL, Bonomo RA. 2005. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev 18:657–686. 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan ER, Jones AM, Hawkey PM. 2017. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 72:2145–2155. 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 6.Day MJ, Hopkins KL, Wareham DW, Toleman MA, Elviss N, Randall L, Teale C, Cleary P, Wiuff C, Doumith M, Ellington MJ, Woodford N, Livermore DM. 2019. Extended-spectrum beta-lactamase-producing Escherichia coli in human-derived and food chain-derived samples from England, Wales, and Scotland: an epidemiological surveillance and typing study. Lancet Infect Dis 19:1325–1335. 10.1016/S1473-3099(19)30273-7. [DOI] [PubMed] [Google Scholar]
- 7.Yasir M, Farman M, Shah MW, Jiman-Fatani AA, Othman NA, Almasaudi SB, Alawi M, Shakil S, Al-Abdullah N, Ismaeel NA, Azhar EI. 2020. Genomic and antimicrobial resistance genes diversity in multidrug-resistant CTX-M-positive isolates of Escherichia coli at a health care facility in Jeddah. J Infect Public Health 13:94–100. 10.1016/j.jiph.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas-Chanoine M-H, Bertrand X, Madec J-Y. 2014. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev 27:543–574. 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumura Y, Johnson JR, Yamamoto M, Nagao M, Tanaka M, Takakura S, Ichiyama S, Group K, Matsumura Y, Yamamoto M. 2015. CTX-M-27-and CTX-M-14-producing, ciprofloxacin-resistant Escherichia coli of the H 30 subclonal group within ST131 drive a Japanese regional ESBL epidemic. J Antimicrob Chemother 70:1639–1649. 10.1093/jac/dkv017. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura Y, Pitout JD, Gomi R, Matsuda T, Noguchi T, Yamamoto M, Peirano G, DeVinney R, Bradford PA, Motyl MR. 2016. Global Escherichia coli sequence type 131 clade with blaCTX-M-27 gene. Emerg Infect Dis 22:1900–1907. 10.3201/eid2211.160519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mostafa HH, Cameron A, Taffner SM, Wang J, Malek A, Dumyati G, Hardy DJ, Pecora ND. 2020. Genomic surveillance of ceftriaxone-resistant Escherichia coli in western New York suggests the extended-spectrum β-lactamase blaCTX-M-27 is emerging on distinct plasmids in ST38. Front Microbiol 11:1747. 10.3389/fmicb.2020.01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattaway MA, Jenkins C, Ciesielczuk H, Day M, DoNascimento V, Day M, Rodríguez I, van Essen-Zandbergen A, Schink AK, Wu G, Threlfall J, Woodward MJ, Coldham N, Kadlec K, Schwarz S, Dierikx C, Guerra B, Helmuth R, Mevius D, Woodford N, Wain J. 2014. Evidence of evolving extraintestinal enteroaggregative Escherichia coli ST38 clone. Emerg Infect Dis 20:1935–1937. 10.3201/eid2011.131845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guenther S, Semmler T, Stubbe A, Stubbe M, Wieler LH, Schaufler K. 2017. Chromosomally encoded ESBL genes in Escherichia coli of ST38 from Mongolian wild birds. J Antimicrob Chemother 72:1310–1313. 10.1093/jac/dkx006. [DOI] [PubMed] [Google Scholar]
- 14.Shaik S, Ranjan A, Tiwari SK, Hussain A, Nandanwar N, Kumar N, Jadhav S, Semmler T, Baddam R, Islam MA, Alam M, Wieler LH, Watanabe H, Ahmed N. 2017. Comparative genomic analysis of globally dominant ST131 clone with other epidemiologically successful extraintestinal pathogenic Escherichia coli (ExPEC) lineages. mBio 8:e01596-17. 10.1128/mBio.01596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peirano G, van der Bij AK, Gregson DB, Pitout JD. 2012. Molecular epidemiology over an 11-year period (2000 to 2010) of extended-spectrum beta-lactamase-producing Escherichia coli causing bacteremia in a centralized Canadian region. J Clin Microbiol 50:294–299. 10.1128/JCM.06025-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh H, Bunk B, Doijad S, Schmiedel J, Falgenhauer L, Spröer C, Imirzalioglu C, Overmann J, Chakraborty T. 2017. Complete genome sequence of blaCTX-M-27-encoding Escherichia coli strain H105 of sequence type 131 lineage C1/H30R. Genome Announc 5:e00736-17. 10.1128/genomeA.00736-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magill SS, Dumyati G, Ray SM, Fridkin SK. 2015. Evaluating epidemiology and improving surveillance of infections associated with health care, United States. Emerg Infect Dis 21:1537. 10.3201/eid2109.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanton RA, McAllister G, Daniels JB, Breaker E, Vlachos N, Gable P, Moulton-Meissner H, Halpin AL. 2020. Development and application of a core genome multilocus sequence typing scheme for the health care-associated pathogen Pseudomonas aeruginosa. J Clin Microbiol 58:e00214-20. 10.1128/JCM.00214-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darling AC, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillings M, Boucher Y, Labbate M, Holmes A, Krishnan S, Holley M, Stokes HW. 2008. The evolution of class 1 integrons and the rise of antibiotic resistance. J Bacteriol 190:5095–5100. 10.1128/JB.00152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence information presented in this study has been deposited under NCBI BioProject accession nos. PRJNA692174 and PRJNA510429.