Abstract
Purpose of Review
Time-restricted eating (TRE) is a form of intermittent fasting that involves confining the eating window to 4–10 h and fasting for the remaining hours of the day. The purpose of this review is to summarize the current literature pertaining to the effects of TRE on body weight and cardiovascular disease risk factors.
Recent Findings
Human trial findings show that TRE reduces body weight by 1–4% after 1–16 weeks in individuals with obesity, relative to controls with no meal timing restrictions. This weight loss results from unintentional reductions in energy intake (~350–500 kcal/day) that occurs when participants confine their eating windows to 4–10 h/day. TRE is also effective in lowering fat mass, blood pressure, triglyceride levels, and markers of oxidative stress, versus controls. This fasting regimen is safe and produces few adverse events.
Summary
These findings suggest that TRE is a safe diet therapy that produces mild reductions in body weight and also lowers several key indicators of cardiovascular disease in participants with obesity.
Keywords: Intermittent fasting, Time-restricted eating, Cardiovascular disease, Body weight, Cholesterol, Blood pressure
Introduction
Intermittent fasting has greatly increased in popularity over the past decade owing to its ability to produce clinically significant weight loss and confer protection against cardiovascular disease [1–3]. The most popular form of intermittent fasting is time-restricted eating (TRE). Indeed, TRE is currently one of the most researched diets on the internet for weight loss [4]. TRE typically involves confining the eating window to 4–10 h and fasting for the remaining hours of the day (14–20-h fast). During the eating window, individuals are not required to count calories or monitor food intake in any way. During the fasting window, individuals are encouraged to drink plenty of water. Energy-free beverages, such as black coffee and black tea, are also permitted during the fasting window.
TRE is a unique weight loss regimen in that it does not require calorie counting. Participants are merely asked to consume all of their energy needs for the day within a specified window of time and fast for the remaining hours of the day. Accumulating evidence suggests that TRE produces a ~350–500 kcal/day energy deficit by simply limiting the eating window to 4–10 h/day [5•, 6•, 7••]. From a clinical standpoint, these findings are highly significant. One of the main reasons for subject attrition with traditional dieting, i.e., daily calorie restriction (CR), is frustration with having to vigilantly monitor energy intake on a regular basis [8, 9]. TRE regimens are able to side-step this requirement by allowing participants to simply watch the clock instead of monitoring calories, while still producing significant weight loss. This feature of TRE has the ability to greatly improve long-term adherence to the diet, and in turn, produce lasting weight control in adults with obesity.
Despite the growing popularity of TRE, only a handful of human trials [5•, 6•, 7••, 10–19] have examined the effect of this fasting regimen on cardiovascular endpoints. The purpose of this review is to summarize the current literature pertaining to the effects of TRE on body weight, body composition, and cardiovascular disease risk factors, including blood pressure, plasma lipids, and markers of inflammation and oxidative stress. The safety of the diet will also be reviewed.
Methods
A Medline search was conducted using the following key words: “time restricted eating,” “time restricted feeding,” “intermittent fasting,” “fasting,” “meal timing,” “meal frequency,” “intermittent energy restriction,” “clinical trial,” “human.” Inclusion criteria for research articles were as follows: (1) adult male and female subjects, (2) randomized controlled trials and non-randomized trials, and (3) endpoints that included body weight changes and relevant metabolic health markers. The following exclusion criteria were applied: (1) cohort and observational studies, (2) fasting performed as a religious practice (Ramadan or Seventh Day Adventist), (3) studies of other forms of intermittent fasting, i.e., alternate day fasting or the 5:2 diet, (4) trial durations of less than 1 week. Our search retrieved 13 trials of TRE [5•, 6•, 7••, 10–19] which are displayed in Table 1.
Table 1.
Time-restricted eating: effect on cardiovascular disease risk factors
| Reference | Subjects | Diet length | Design and intervention groups | Body weight | Fat mas | Lean mass | Visceral fat mass | Blood pressure | Plasma lipids | Inflammation | Oxidative stress | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDL | HDL | TG | |||||||||||
| 4-h time-restricted eating | |||||||||||||
| Carlson 2007 [10] | n = 15, MF | 8 weeks | RCT: Cross-over | 1. ∅ | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Normal wt | Isocaloric/eucaloric | 2. ∅ | |||||||||||
| No diabetes | 1. 4-h TRE (4–8pm) | ||||||||||||
| 2. Control (no meal timing restrictions) | |||||||||||||
| Tinsley 2016 [11] | n = 18, MF | 8 weeks | RCT: Parallel-arm | 1. ∅ | 1. ∅ | 1. ∅ | -- | -- | -- | -- | -- | -- | -- |
| Normal wt | 1. 4-h TRE (4–8pm) + Resistance training | 2. ∅ | 2. ∅ | 2. ∅ | |||||||||
| No diabetes | |||||||||||||
| 2. Control (no meal timing restrictions) + Resistance training | |||||||||||||
| Cienfuegos 2020 [5•] | n = 58, MF | 8 weeks | RCT: Parallel-arm | 1. ↓3%† | 1. ↓ † | 1. ↓ † | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ TNF-α, IL-6 | 1. ↓8-iso† |
| Obese | 1. 4-h TRE (3–7pm) | 2. ∅ | 2.0 | 2.0 | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ TNF-α, IL-6 | 2. ∅ | ||
| No diabetes | 2. Control (no meal timing restrictions) | ||||||||||||
| 6-h time-restricted eating | |||||||||||||
| Sutton 2018 [12] | n = 8,M | 5 weeks | RCT: Cross-over | 1. ∅ | -- | -- | -- | 1. ↓ SBP† | 1. ∅ | 1. ∅ | 1. ↑† | 1. ∅ CRP IL-6 | 1. ↓ 8-iso† |
| Obese | Isocaloric/eucaloric | 2. ∅ | ↓ DBP† | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ CRP, IL-6 | 2. ∅ | |||||
| Prediabetes | 1. 6-h TRE (~8am–2pm) | 2. ∅ | |||||||||||
| 2. Control (12-h eating window) | |||||||||||||
| Cienfuegos 2020 [5•] | n = 58, MF | 8 weeks | RCT: Parallel-arm | 1. ↓ 3%† | 1. ↓ † | 1. ↓ † | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ TNF-α, IL-6 | 1. ↓8-iso† |
| Obese | 1. 6-h TRE (1–7pm) | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ TNF-α, IL-6 | 2. ∅ | ||
| No diabetes | 2. Control (no meal timing restrictions) | ||||||||||||
| 8-h time-restricted eating | |||||||||||||
| Anton 2019 [13] | n = 10, MF | 4 weeks | Single-arm | 1. ↓ 2%* | -- | -- | -- | 1. ∅ | -- | -- | -- | -- | -- |
| Obese | 1. 8-h TRE (self-select) | ||||||||||||
| No diabetes | |||||||||||||
| Moro 2016 [14] | n = 34, M | 8 weeks | RCT: Parallel-arm | 1. ∅ | 1. ↓ † | 1. ∅ | -- | -- | 1. ∅ | 1. ∅ | 1. ↓† | 1. ∅ TNF-α, IL-6 | -- |
| Normal wt | Isocaloric/eucaloric | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. TNF-α, IL-6 | |||||
| No diabetes | 1. 8-h TRE (12–8pm) + Resistance training | ||||||||||||
| 2. Control (8am–8pm) + Resistance training | |||||||||||||
| Tinsley 2019 [15] | n = 40, F | 8 weeks | RCT: Parallel-arm | 1. ↑ 2%† | 1. ↓ † | 1. ↑ † | -- | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | -- | -- |
| Normal wt | 1. 8-h TRE (12–8pm) + Resistance training | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | |||||
| No diabetes | |||||||||||||
| 2. Control (no meal timing restrictions) + Resistance training | |||||||||||||
| Gabel 2018 [6•] | n = 46, MF | 12 weeks | CT: Parallel-arm | 1. ↓ 3%† | 1. ∅ | 1. ∅ | 1. ∅ | 1. ↓ SBP† | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ Hcy | -- |
| Obese | 1. 8-h TRE (10am–6pm) | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | ∅ DBP | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ Hcy | |||
| No diabetes | 2. Control (no meal timing restrictions) | 2. ∅ | |||||||||||
| Chow 2020 [16] | n = 20, MF | 12 weeks | RCT: Parallel-arm | 1. ↓ 4%† | 1. ∅ | 1. ↓ † | 1. ↓ † | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | -- | -- |
| Obese | 1. 8-h TRE (self-select) | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | ||||
| No diabetes | 2. Control (no meal timing restrictions) | ||||||||||||
| Lowe 2020 [17] | n = 116, MF | 12 weeks | RCT: Parallel-arm | 1. ∅ | 1. ∅ | 1. LM: ↓ † | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | 1. ∅ | -- | -- |
| Obese | 1. 8-h TRE (12–8pm) | 2. ∅ | 2. ∅ | 2. LM: ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | 2. ∅ | ||||
| No diabetes | 2. Control (no meal timing restrictions) | ||||||||||||
| 9-h time-restricted eating (continued) | |||||||||||||
| Hutchison 2020 [18] | n = 15, M | 1 week | RT: Cross-over | 1. ↓ 1%* | -- | -- | -- | 1. ∅ | -- | -- | 1. ↓* | -- | -- |
| Obese | 1. 9-h TRE (8am–5pm) | 2. ↓ 1%* | 2. ∅ | 2. ↓* | |||||||||
| Prediabetes | 2. 9-h TRE (12–9pm) | ||||||||||||
| 10-h time-restricted eating | |||||||||||||
| Wilkinson 2020 [19] | n = 19, M | 12 weeks | Single-arm | 1. ↓ 3%* | 1. ↓* | -- | 1. ↓* | 1. ↓ SBP* | 1. ↓* | 1.∅ | 1.∅ | 1. ∅ CRP | -- |
| Overweight | 1. 10-h TRE (self-select) | ↓ DBP* | |||||||||||
| Prediabetes | |||||||||||||
| Gill & Panda 2015 [7••] | n = 8,M | 16 weeks | Single-arm | 1. ↓3%* | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Overweight | 1. 10-h TRE (self-select) | ||||||||||||
| No diabetes | |||||||||||||
Non-significant change
P < 0.05, significantly different from baseline (within group effect)
P < 0.05, significantly different from the control or comparison group (between group effect). When control group present, only significant changes versus control reported
Abbreviations: 8-iso 8-isoprostane, A1c hemoglobin A1c, CRP C-reactive protein, CT controlled trial, DBP diastolic blood pressure, F female, Hcy homocysteine, HDL high-density lipoprotein cholesterol, LDL low-density lipoprotein cholesterol, LM lean mass, M male, RT randomized trial, RCT randomized controlled trial, SBP systolic blood pressure, TG triglycerides, TRE time-restricted eating (prescribed eating window shown in parentheses)
Body Weight
Mild to moderate weight loss (5–10%) is associated with improvements in several cardiovascular disease parameters, including blood pressure, triglycerides, and LDL cholesterol levels [20–22]. Whether TRE can produce clinically significant weight loss (>5% from baseline [23, 24]) compared to a non-TRE group remains unclear. All of the 13 human trials included in this review evaluated the effects of TRE on body weight (Table 1). Most of these studies permitted ad libitum intake during the eating window. However, some studies [10, 12, 14] required subjects to follow isocaloric and eucaloric diets during TRE, to ensure that weight remained stable during the intervention period. Since this section focuses on weight loss with TRE, only the studies that allowed for ad libitum intake will be discussed here [5•, 6•, 7••, 11, 13, 15–19].
A few recent trials have evaluated the effects of shorter eating windows (4-h TRE and 6-h TRE) on body weight [5•, 11]. In a recent study by Cienfuegos et al. [5•], similar reductions in body weight were noted by 4-h TRE (3–7 pm) and 6-h TRE (1–7 pm) after 8 weeks in participants with obesity (3% weight loss for both diets), when compared to a group with no meal timing restrictions. In contrast, Tinsley et al. [11] observed no weight changes when normal weight men and women followed a 4-h TRE regimen (4–8 pm, 4 days/week) combined with resistance training, relative to controls with no meal timing restrictions.
The effect of a slightly longer eating window (8-h TRE) on body weight has also been investigated. As demonstrated in the trials by Anton et al. [13], Gabel et al. [6•], and Chow et al. [16], 8-h TRE produces 2–4% weight loss after 4–12 weeks of intervention in participants with obesity, relative to controls with no meal timing restrictions. However, when 8-h TRE (12–8 pm) was combined with resistance training in the trial by Tinsley et al. [15], body weight slightly increased (+2%) after 8 weeks of treatment, versus a non-TRE group. In the study by Lowe et al. [17], 12 weeks of 8-h TRE (12–8 pm) produced no weight loss, versus controls. The trial by Lowe et al. [17] has some methodological limitations however. For instance, the control group was told to consume all of their food for the day as 3 meals/day. It is well known that individuals do not naturally conform to a 3 meal/day eating pattern, as demonstrated in the eloquent meal timing study by Gill & Panda [7••]. Indeed, subjects tend to consume food every 1–2 h over a ~15-h period with no distinct breakfast-lunch-dinner pattern [7••]. Since the controls in the study by Lowe et al. [17] were required to drastically change their eating pattern to conform to the 3 meal/day paradigm, they lost similar amounts of weight over time (~1%) as the 8-h TRE group (~1%), yielding no statistically significant differences between groups. It can be assumed that if the authors implemented a true “usual diet” control group, the controls would have remained weight stable, and the body weight reductions experienced by the 8-h TRE group would have been significantly different versus controls.
Changes in body weight during 9-h TRE and 10-h TRE have also been tested. Hutchison et al. [18] compared the effects of early 9-h TRE (8 am–5 pm) versus late 9-h TRE (12–9 pm) in subjects with obesity and prediabetes. After 1 week of diet, participants lost 1% of body weight, and this effect did not vary according to the timing of the eating window (early versus late) [18]. Two trials have evaluated whether 10-h TRE can facilitate weight loss [7••, 19]. Following 12 weeks of 10-h TRE (with a self-selected eating window), participants with overweight and prediabetes lost 3% of body weight, relative to baseline, in the single-arm study by Wilkinson et al. [19]. Similarly, after 16 weeks of unsupervised 10-h TRE (with a self-selected eating window), men and women with overweight observed 3% weight loss, relative to baseline, in the single-arm trial by Gill & Panda [7••].
Taken together, TRE (4–10 h) produces 1–4% weight loss in subjects with overweight and obesity over short trial durations (1–16 weeks), relative to a non-TRE condition. Unfortunately, these reductions in body weight do not meet the threshold for clinically significant weight loss (>5%) [23, 24]. It will be of interest to see if longer durations of TRE can produce body weight reductions beyond 4%. The weight loss noted in these TRE trials was the result of unintentional reductions in energy intake. More specifically, confining the period of eating to 4–10 h/day was shown to reduce energy intake by ~350–500 kcal/day, without calorie counting [5•, 6•, 7••, 11, 19]. Though the data are limited, it does not appear as though shorter eating windows (4 h and 6 h) produce greater degrees of weight loss compared to longer eating windows (8 h and 10 h). It also remains uncertain whether the timing of the eating window (early versus late) impacts weight loss. At present, only one study [18] has examined this, and no difference in weight loss was noted when early TRE was compared to late TRE. Interestingly, when TRE is combined with resistance exercise, the weight loss effects of the diet appear to be negated [11, 15]. Since exercise has been shown to occasionally augment energy consumption and appetite [25, 26], this could explain why no change in body weight was noted in these exercise studies. Whether similar effects would occur when TRE is combined with endurance exercise has yet to be elucidated.
Body Composition
Changes in fat mass, lean mass, and visceral fat mass were assessed in the majority of these studies [5•, 6•, 7••, 11, 14–19]. When an individual loses weight with traditional dieting, i.e., daily CR, approximately 75% of the weight lost is fat mass, and 25% is lean mass [27–29]. Based on the trials reviewed here, it would appear as though TRE generally produces a similar ratio of fat to lean mass loss, as CR, relative to controls. Interestingly, when TRE is combined with resistance training, no additional lean mass retention is observed [11, 14, 15]. This is surprising as resistance exercise usually helps preserve muscle mass during periods of dietary restriction [30–32]. As for visceral fat mass, no changes were observed with any TRE regimen [5•, 6•, 16, 17, 19], versus controls with no meal timing restrictions. It is likely, however, that greater degrees of weight loss (>5%) would be needed to see consistent reductions in this body composition parameter [33–35].
The only trial that observed drastically different body composition changes by TRE was the study by Lowe et al. [17]. After 12 weeks of 8-h TRE (12–8pm) men and women with obesity lost ~1% of body weight compared to baseline [17]. Remarkably, approximately 65% of the weight loss was lean mass [17]. This degree of lean mass loss far exceeds the normal range generally reported during dietary weight loss interventions [27–29]. The authors of this trial [17] speculate that these reductions in lean mass occurred because subjects reduced protein intake, but oddly, protein intake was never measured at any point in the trial. This is a major limitation of the study. It is more likely that these unexpected changes in lean mass occurred due to measurement error via dual-energy X-ray absorptiometry (DXA). Any state of abnormal hydration can lead to an error in lean mass quantification by DXA [36–38]. Lowe et al. [17] attempted to control for hydration status by having the participants fast for 12 h prior to measuring body composition. However, it is unclear if the fast also prohibited water intake. Thus, more data on the hydration status of these participants pre-and post-intervention are needed to fully comprehend why these dramatic reductions in lean mass occurred. The findings by Lowe et al. [17] are also highly questionable considering that no other 8-h TRE trial observed these extreme reductions in lean mass [6•, 14–16]. Moreover, all previous studies [6•, 14–16] used food records to assess dietary intake and saw no change in protein intake at any point during the trial. Strangely, Lowe et al. [17] failed to acknowledge any of the body composition or dietary intake data from other 8-h TRE studies that contradicted their findings.
Blood Pressure
Several studies have examined how TRE impacts blood pressure (Table 1). Reductions in systolic blood pressure (4–9%) and diastolic blood pressure (7–9%) have been reported during TRE [6•, 12, 19], relative to a non-TRE group, but not always [5•, 15–18]. Improvements were observed with shorter eating windows (i.e., 6-h TRE [12]) and also with longer eating windows (i.e., 8-h TRE [6•] and 10-h TRE [19]). Decreases in blood pressure were more commonly seen in studies where participants lost at least 3% of baseline body weight [6•, 19]. However, in the early 6-h TRE study by Sutton et al. [12], blood pressure was reduced despite no change in body weight. It would also appear as though TRE only exerts beneficial effects on blood pressure in those with perturbed baseline values. For instance, the only studies that noted improvements in blood pressure included subjects with borderline hypertension (i.e., systolic blood pressure: >120 mm Hg and diastolic blood pressure: >80 mm Hg) [6•, 12, 19]. In view of this, it will be of interest for future research to examine how the effects of TRE vary according to hypertensive status.
Plasma Lipids
Elevated levels of circulating LDL cholesterol and triglycerides, in conjunction with low levels of HDL cholesterol, greatly increase the risk of myocardial infarction and atherosclerotic cardiovascular disease [39–42]. The effects of TRE on plasma lipid concentrations were evaluated in the majority of the trials reviewed here (Table 1). In the trial by Wilkinson et al. [19], LDL cholesterol concentrations decreased after 12 weeks of 10-h TRE, relative to baseline, in subjects with metabolic syndrome. In contrast, LDL cholesterol concentrations remained unchanged, relative to controls, in all the other trials that examined this parameter [5•, 6•, 12, 14–17]. HDL cholesterol levels also remained unaffected by TRE, relative to controls [5•, 6•, 12, 16, 17]. Interestingly, when TRE was combined with exercise [14, 15], no increases in HDL cholesterol levels were observed, versus a non-TRE control group. This is somewhat surprising as moderate intensity resistance training generally increases HDL cholesterol levels [43, 44].
As for triglycerides, reductions in this lipid parameter were demonstrated in the trials by Moro et al. [14] and Hutchison et al. [18]. In both of these studies, triglyceride levels were reduced by approximately 10% from baseline. The majority of other trials, in contrast, showed no change in triglycerides levels, versus controls. This lack of effect was demonstrated with both shorter (4–6 h TRE) [5•] and longer eating windows (8–10 h TRE) [6•, 15–17, 19]. It should be noted, however, that participants in most of these studies had triglyceride levels within the normal range at baseline. Since the subjects already had healthy concentrations of triglycerides at the onset of the intervention, this could explain why no additional improvements were observed. In the trial by Sutton et al. [12], triglyceride levels increased after 5 weeks of early 6-h TRE (~8am–2pm), relative to a control intervention (12-h eating window). The authors speculate that this elevation resulted from an extended acute fast prior to the blood draw. More specifically, subjects in this trial [12] fasted for 18 h before blood collection, while subjects in the other TRE studies fasted for shorter durations (8–10 h) before testing [5•, 6•, 14–19]. Acute fasting has been shown to stimulate lipolysis, and in turn, produce sharp elevations in triglycerides and fatty acids [45–48]. Therefore, it is possible that the acute fast produced this spike in triglyceride concentrations, rather than the chronic TRE intervention itself.
Inflammatory Markers
Changes in inflammatory markers were measured in several trials of TRE [5•, 6•, 12, 14, 19].
High circulating levels of C-reactive protein (CRP) and homocysteine are strong independent predictors of adverse cardiovascular events, including myocardial infarction and ischemic stroke [49–52]. In the studies by Sutton et al. [12] and Wilkinson et al. [19], CRP concentrations did not change after 5–12 weeks of TRE. As for homocysteine, no changes were observed after 12 weeks of 8-h TRE in the trial by Gabel et al. [6•] versus controls with no meal timing restrictions. Proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-alpha), may increase the risk of atherosclerosis by promoting vascular inflammation [53–55]. As demonstrated in the studies by Cienfuegos et al. [5•], Sutton et al. [12], and Moro et al. [14], 5–8 weeks of TRE had no effect on circulating levels of IL-6 or TNF-alpha, versus a non-TRE group. Altogether, it would appear as though TRE does not affect circulating markers of inflammation (i.e., CRP, homocysteine, IL-6, and TNF-alpha) in human subjects.
Oxidative Stress
Oxidative stress can be defined as an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to detoxify these reactive products. When ROS production is left unchecked, and circulating levels of these markers accumulate, this can lead to the initiation and progression of atherosclerotic disease [56–58]. Data from short-term trials suggest that TRE may produce consistent reductions in markers of oxidative stress [5•, 12]. For instance, 8-isoprostane (a marker of oxidative stress to lipids) was reduced by 14% in the early 6-h TRE study by Sutton et al. [12], versus controls (12-h eating window). Similarly, 8-isoprostane levels were markedly reduced by 37% by 4-h TRE and 6-h TRE in the study by Cienfuegos et al. [5•], versus controls with no meal timing restrictions. These promising preliminary findings show that TRE may offer some benefit in helping to regulate the production of ROS in humans.
Safety Considerations
Accumulating evidence suggests that TRE is a safe diet therapy that results in little or no adverse events. Occurrences of diarrhea, constipation, nausea, headaches, irritability, and fatigue did not change from baseline to post-treatment in several short-term studies of TRE [5•, 6•, 19]. However, a few minor cases of headaches, increased thirst, and diarrhea were reported in the early 6-h TRE trial by Sutton et al. [12]. Complete blood count (CBC), which is used as an indicator of general health, was not changed during 12 weeks of 8-h TRE in the study by Gabel et al. [6•]. Disordered eating symptoms also remained unaltered during 8-h TRE, suggesting that this fasting regimen may not increase the risk of eating disorders [6•]. It should be noted, however, that subjects with a history of eating disorders were excluded from this study [6•]. Whether TRE augments disordered eating behaviors in those with a history of this condition remains unknown. In addition, sleep is not negatively impacted by this form of intermittent fasting. Recent findings show that sleep quality and duration remained unchanged after 12–16 weeks of 8-h or 10-h TRE in several recent trials [6•, 7••, 19]. Thus, preliminary findings indicate that TRE is generally safe in human subjects.
Limitations
It is obvious that the data regarding the efficacy of TRE in modulating human health is quite limited at present. Only about a dozen studies have examined the effects of this diet on body weight and other metabolic disease risk parameters in human subjects. While these findings offer important preliminary evidence, they are limited in several ways. First, all of these studies were quite short, with the longest trial running for only 16 weeks. Second, the sample size in each study was quite small, which could severely limit the ability to detect significant differences in secondary outcome measures, such as blood pressure, plasma lipids, and inflammatory markers. Third, no trial to date has compared the weight loss efficacy of TRE to a traditional dieting approach, such as daily CR. A head-to-head comparison between TRE and CR will be needed to further clarify whether this fasting regimen is indeed beneficial for weight loss. Fourth, some of these trials were cross-over studies. Cross-over studies are not an appropriate design when a change in health status such as weight loss is expected. It is possible that subjects did not return to their baseline weight prior to the beginning of the new intervention period, which may have affected the findings. Fifth, no trial to date has examined whether TRE can be used to maintain weight loss. Future parallel-arm trials which run for longer durations (>6 months), with larger sample sizes, which directly compare TRE to other weight loss approaches, will be needed before solid conclusions can be reached.
Conclusion
In summary, human evidence suggests that TRE produces mild weight loss (1–4% from baseline) in individuals with overweight and obesity (Fig. 1). Interestingly, shorter eating windows (4–6-h TRE) do not produce greater weight loss, compared to longer eating windows (8–10-h TRE). Reductions in body weight by TRE generally result from decreases in fat mass, rather than lean mass. TRE is unique in that it produces a daily energy deficit of 350–500 kcal, without calorie counting. This finding could have important clinical implications for the long-term feasibility of the diet. As for cardiovascular benefits, TRE produces somewhat consistent reductions in blood pressure and markers of oxidative stress (8-isoprostane). The effects of this fasting regimen on plasma lipids are less clear, with some studies showing reductions in LDL cholesterol and triglycerides, and others showing no effect. Based on available findings to date, TRE does not seem to modulate HDL cholesterol levels or inflammatory markers, such as CRP, homocysteine, IL-6, or TNF-alpha. Very few adverse events are reported during TRE, suggesting that the diet is generally safe in human subjects. Taken together, preliminary evidence from human trials shows that TRE is a safe and effective diet therapy to lower body weight and several key indicators of cardiovascular disease in participants with obesity.
Fig. 1.
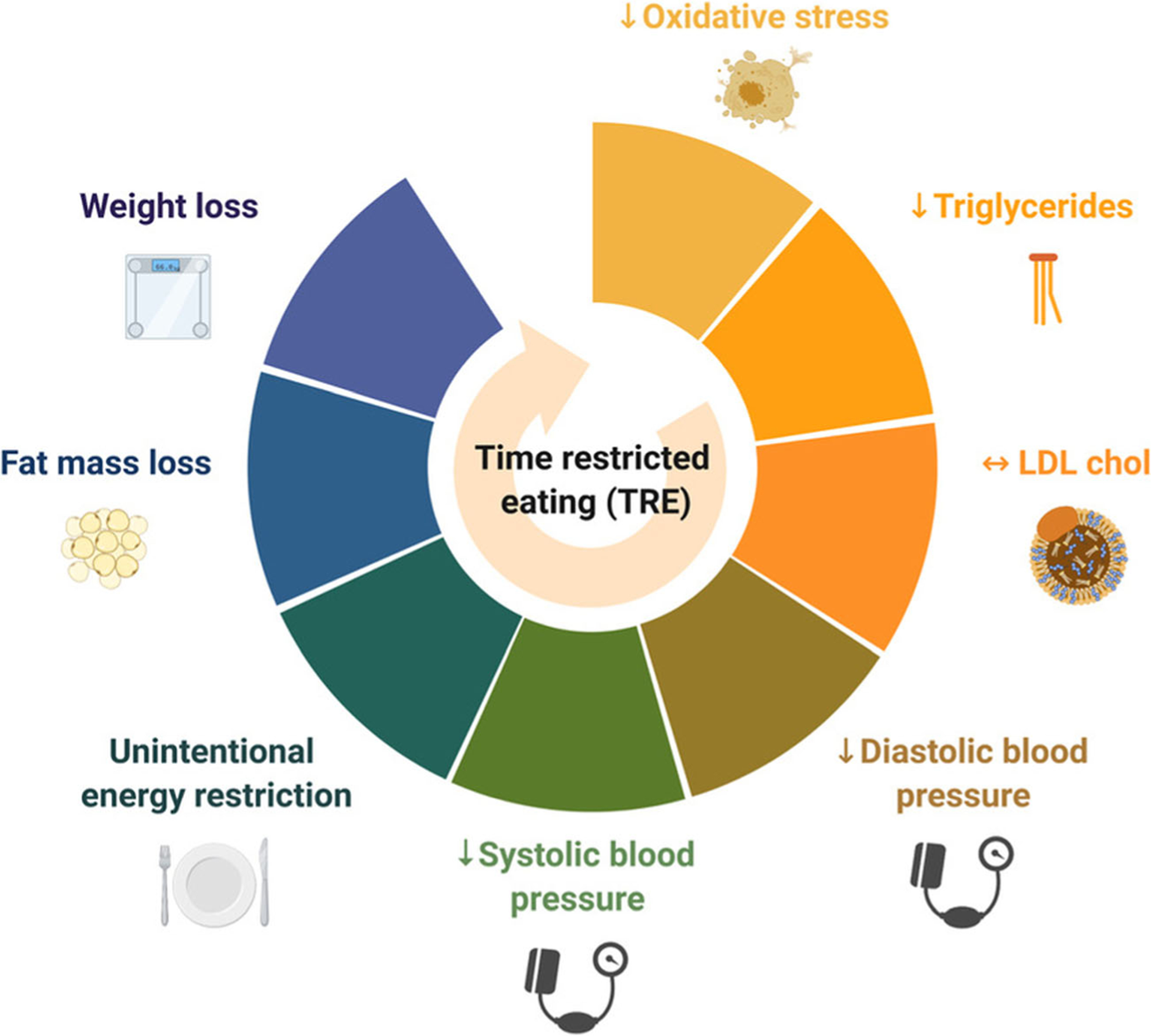
Effects of time-restricted eating on cardiovascular risk factors. Time-restricted eating reduces body weight, fat mass, energy intake, blood pressure, triglycerides, and makers of oxidative stress in individuals with obesity
Funding
This review was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. R01DK119783).
Footnotes
This article is part of the Topical Collection on Nutrition
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson MP, de Cabo R. Effects of intermittent fasting on health, aging, and disease. Reply. N Engl J Med. 2020;382(18):1773–4. [DOI] [PubMed] [Google Scholar]
- 4.Google Trends Data. Top 5 Diet Trends. Google. 2020. Available from: https://trends.google.com/trends/yis/2019/US/?utm_source=socialinfluencer&utm_medium=social&utm_campaign=yis2020. Accessed 12 Oct 2020 [Google Scholar]
- 5.•.Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4-and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to show that 4-h TRE and 6-h TRE are safe and effective interventions for weight loss and metabolic disease risk reduction in human subjects.
- 6.•.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345–53 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to show that 8-h TRE is a safe and effective diet therapy for weight loss in subjects with obesity.
- 7.••.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–98 [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study show that the daily eating pattern in healthy adults is highly variable from day to day. Most people do not adhere to a breakfast-lunch-dinner meal pattern and more than half of adults eat for 15 h or longer every day.
- 8.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85(4):1023–30. [DOI] [PubMed] [Google Scholar]
- 10.Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–7. [DOI] [PubMed] [Google Scholar]
- 12.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–21 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, et al. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, et al. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring). 2020;28(5):860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180:1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27(5):724–32. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92–104 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beavers DP, Beavers KM, Lyles MF, Nicklas BJ. Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2013;68(6):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JD, Buscemi J, Milsom V, Malcolm R, O’Neil PM. Effects on cardiovascular risk factors of weight losses limited to 5–10. Transl Behav Med. 2016;6(3):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dow CA, Thomson CA, Flatt SW, Sherwood NE, Pakiz B, Rock CL. Predictors of improvement in cardiometabolic risk factors with weight loss in women. J Am Heart Assoc. 2013;2(6):e000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring). 2015;23(12):2319–20. [DOI] [PubMed] [Google Scholar]
- 24.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16(Suppl 1):67–76. [DOI] [PubMed] [Google Scholar]
- 26.Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab. 2010;57(Suppl 2):36–42. [DOI] [PubMed] [Google Scholar]
- 27.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15(4): 310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2015;23(3):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, et al. A 2-Year Randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell WW, Haub MD, Wolfe RR, Ferrando AA, Sullivan DH, Apolzan JW, et al. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity (Silver Spring). 2009;17(7):1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101(5):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sardeli AV, Komatsu TR, Mori MA, Gaspari AF, Chacon-Mikahil MPT. Resistance training prevents muscle loss induced by caloric restriction in obese elderly individuals: a systematic review and meta-analysis. Nutrients. 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall KD, Hallgreen CE. Increasing weight loss attenuates the preferential loss of visceral compared with subcutaneous fat: a predicted result of an allometric model. Int J Obes (Lond). 2008;32(4):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallgreen CE, Hall KD. Allometric relationship between changes of visceral fat and total fat mass. Int J Obes (Lond). 2008;32(5): 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan DH, Yockey SR. Weight Loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barreira TV, Tseh W. The effects of acute water ingestion on body composition analyses via Dual-Energy X-Ray Absorptiometry. Clin Nutr. 2020. [DOI] [PubMed] [Google Scholar]
- 37.De Feo P, Horber FF, Haymond MW. Meal stimulation of albumin synthesis: a significant contributor to whole body protein synthesis in humans. Am J Physiol. 1992;263(4 Pt 1):E794–9. [DOI] [PubMed] [Google Scholar]
- 38.Horber FF, Thomi F, Casez JP, Fonteille J, Jaeger P. Impact of hydration status on body composition as measured by dual energy X-ray absorptiometry in normal volunteers and patients on haemodialysis. Br J Radiol. 1992;65(778):895–900. [DOI] [PubMed] [Google Scholar]
- 39.Chiavaroli L, Nishi SK, Khan TA, Braunstein CR, Glenn AJ, Mejia SB, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog Cardiovasc Dis. 2018;61(1):43–53. [DOI] [PubMed] [Google Scholar]
- 40.Kosmas CE, Martinez I, Sourlas A, Bouza KV, Campos FN, Torres V, et al. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context. 2018;7:212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liou L, Kaptoge S. Association of small, dense LDL-cholesterol concentration and lipoprotein particle characteristics with coronary heart disease: a systematic review and meta-analysis. PLoS One 2020;15(11):e0241993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson JC, Tsiligiannis S, Panay N. Cardiovascular risk in perimenopausal women. Curr Vasc Pharmacol. 2019;17(6):591–4. [DOI] [PubMed] [Google Scholar]
- 43.Lira FS, Yamashita AS, Uchida MC, Zanchi NE, Gualano B, Martins E Jr, et al. Low and moderate, rather than high intensity strength exercise induces benefit regarding plasma lipid profile. Diabetol Metab Syndr. 2010;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44(2):211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antoni R, Johnston KL, Collins AL, Robertson MD. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br J Nutr. 2016;115(6):951–9. [DOI] [PubMed] [Google Scholar]
- 46.Browning JD, Baxter J, Satapati S, Burgess SC. The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J Lipid Res. 2012;53(3): 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol(1985). 2005;99(6): 2128–36. [DOI] [PubMed] [Google Scholar]
- 48.Salgin B, Marcovecchio ML, Humphreys SM, Hill N, Chassin LJ, Lunn DJ, et al. Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am J Physiol Endocrinol Metab. 2009;296(3):E454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–97. [DOI] [PubMed] [Google Scholar]
- 50.Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008;83(11): 1203–12. [DOI] [PubMed] [Google Scholar]
- 51.Karger AB, Steffen BT, Nomura SO, Guan W, Garg PK, Szklo M, et al. Association between homocysteine and vascular calcification incidence, prevalence, and progression in the MESA cohort. J Am Heart Assoc. 2020;9(3):e013934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237–42. [DOI] [PubMed] [Google Scholar]
- 53.Fatkhullina AR, Peshkova IO, Koltsova EK. The role of cytokines in the development of atherosclerosis. Biochemistry (Mosc). 2016;81(11):1358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22(3):147–51. [DOI] [PubMed] [Google Scholar]
- 55.Ramji DP, Davies TS. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26(6):673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13: 757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25(1):29–38. [DOI] [PubMed] [Google Scholar]
- 58.Vichova T, Motovska Z. Oxidative stress: predictive marker for coronary artery disease. Exp Clin Cardiol. 2013;18(2):e88–91. [PMC free article] [PubMed] [Google Scholar]


