Abstract
Chronic pain affects one in five of the general population and is the third most important cause of disability-adjusted life-years globally. Unfortunately, treatment remains inadequate due to poor efficacy and tolerability. There has been a failure in translating promising preclinical drug targets into clinic use. This reflects challenges across the whole drug development pathway, from preclinical models to trial design. Nociceptors remain an attractive therapeutic target: their sensitization makes an important contribution to many chronic pain states, they are located outside the blood–brain barrier, and they are relatively specific. The past decade has seen significant advances in the techniques available to study human nociceptors, including: the use of corneal confocal microscopy and biopsy samples to observe nociceptor morphology, the culture of human nociceptors (either from surgical or post-mortem tissue or using human induced pluripotent stem cell derived nociceptors), the application of high throughput technologies such as transcriptomics, the in vitro and in vivo electrophysiological characterization through microneurography, and the correlation with pain percepts provided by quantitative sensory testing. Genome editing in human induced pluripotent stem cell-derived nociceptors enables the interrogation of the causal role of genes in the regulation of nociceptor function. Both human and rodent nociceptors are more heterogeneous at a molecular level than previously appreciated, and while we find that there are broad similarities between human and rodent nociceptors there are also important differences involving ion channel function, expression, and cellular excitability. These technological advances have emphasized the maladaptive plastic changes occurring in human nociceptors following injury that contribute to chronic pain. Studying human nociceptors has revealed new therapeutic targets for the suppression of chronic pain and enhanced repair. Cellular models of human nociceptors have enabled the screening of small molecule and gene therapy approaches on nociceptor function, and in some cases have enabled correlation with clinical outcomes. Undoubtedly, challenges remain. Many of these techniques are difficult to implement at scale, current induced pluripotent stem cell differentiation protocols do not generate the full diversity of nociceptor populations, and we still have a relatively poor understanding of inter-individual variation in nociceptors due to factors such as age, sex, or ethnicity. We hope our ability to directly investigate human nociceptors will not only aid our understanding of the fundamental neurobiology underlying acute and chronic pain but also help bridge the translational gap.
Keywords: nociceptors, transcriptomics, IPSC derived nociceptors, patch-clamp, microneurography
Middleton et al. review advances in the molecular profiling, functional analysis and clinical assessment of human nociceptors. Improved knowledge of human nociceptor subpopulations, inter-species differences and mechanisms underlying hyper-excitability should ultimately translate to improved pain management.
Introduction
Sherrington1 was the first to coin the term nociceptor as the neural apparatus responsible for detecting noxious stimuli. Noxious stimuli are those stimuli that can cause tissue injury including extremes of temperature, mechanical, and chemical insults. Many nociceptors are polymodal (responding to combinations of these stimulus types) although there has been recent debate regarding the extent of polymodality in rodent studies2,3; this may partly depend on whether nociceptors innervate superficial versus deep targets.4 A subgroup of nociceptors referred to as mechanically-insensitive, or silent nociceptors, are unresponsive to all modalities in the naïve state and only respond to stimuli in the presence of inflammation, revealing the capacity of nociceptors to become sensitized in disease states.5
In healthy systems, the detection of noxious stimuli begins with afferent activation wherein sufficient signal results in a complex pain percept with discriminative and affective components. Peripheral and/or central sensitization of this system—as seen in patients with chronic pain—can be debilitating; in such a situation there is often a poor correlation between the stimulus intensity/degree of tissue injury and pain percept. Chronic pain affects up to 20% of the general population and causes significant reductions in quality of life; unfortunately, treatment remains inadequate.6
Nociceptors are an attractive therapeutic target of painful conditions: their sensitization makes an important contribution to many chronic pain states, they are located outside the blood–brain barrier, and they exhibit relative specificity. Even so, several promising preclinical drug targets have failed to translate to clinical use. This reflects challenges spanning the whole drug development process from evolutionary divergence between humans and animal models to the difficulty in assessing pain in analgesic drug trials.
Here, we address the fundamental neurobiology of human nociceptors in both acute and chronic pain states. The molecular profiling, available model systems, and functional assessment of the nociceptive system are discussed drawing on species comparisons, where applicable, and considering therapeutic implications (Fig. 1 and Table 1). Preclinical models show functional age and sex differences in nociceptors7-11 and although there are age and sex dependent differences in pain perception in humans,12 these have yet to be directly linked to differences in nociceptors. We look forward to the addition of these datasets, as they will significantly deepen our understanding of human nociceptor function. Our hope is that these emerging techniques and greater availability of cell/tissue samples available to study human nociceptors will help bridge the translational gap seen in pain research.
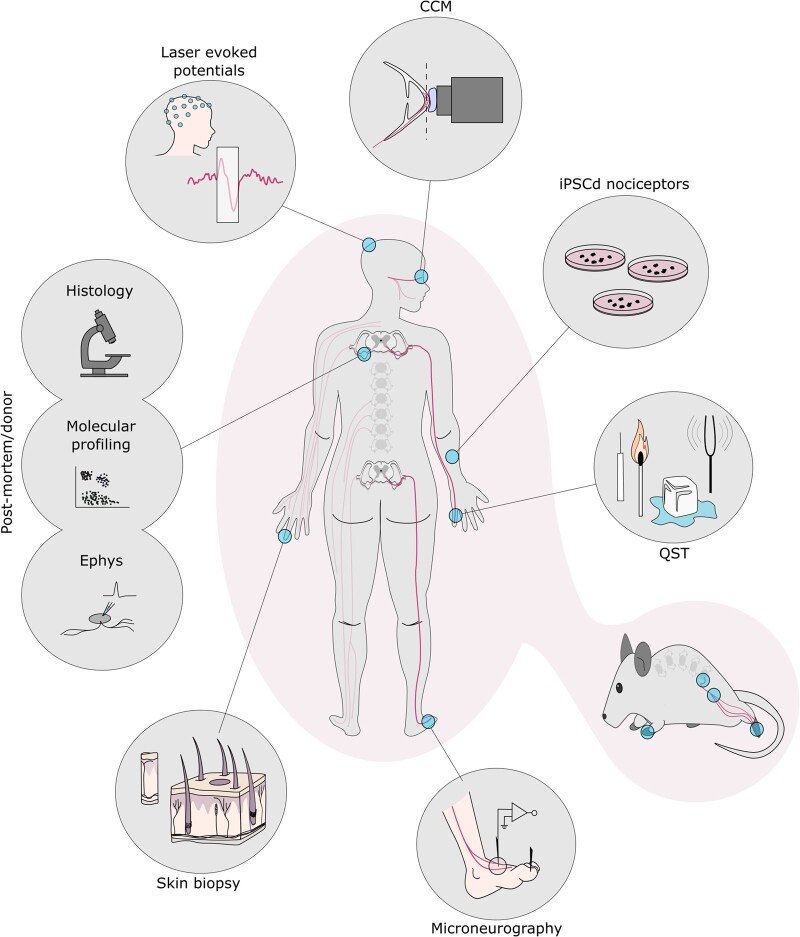
Figure 1.
Highlighted methods to study human nociceptor anatomy and physiology. Clockwise from top: corneal confocal microscopy (CCM): focal plane (dashed line) lands on the subbasal nerve plexus; IPSC-derived nociceptors; quantitative sensory testing (QST); numerous rodent assays mirror those seen in humans with parallels highlighted throughout this review; microneurography schematic of a peroneal nerve recording; skin biopsy schematic with primary afferents shown in black; post-mortem and donor tissue can be used across applications: histology, molecular profiling and electrophysiology (Ephys); laser evoked potentials, recorded through EEG, measures cortical output in response to heat stimuli.
Table 1.
Technical summary for the study of human nociceptors
| Technique | Tissue accessa | Clinical availability | Advantages | Disadvantages |
|---|---|---|---|---|
| CCM | In vivo | Yes | Non-invasive, rapid, longitudinal tracking, simple analysis, diagnostic relevance | Macrostructure, small-fibre specific |
| Skin biopsy/IENFD | Biopsy | Yes | Minimally invasive, time course possible, simple analysis, diagnostic relevance, terminal morphology visible | Terminal focus, antibody penetration in the skin is limited, antibody specificity, limited to cutaneous fibres, time course studies require repeated biopsies |
| Donor IHC/ISH | Donor | No | Multiplexing allows for molecular characterization, in situ localization | Tissue availability, antibody specificity, time between death and tissue collection is variable |
| Deep molecular sequencing | Donor | No | Deep molecular profiling, single cell resolution, data rich | Cell (and nuclei) size provide a technical challenge, cost, computationally complex, variable sequencing depth |
| IPSC-derived nociceptor cultures | Biopsy/blood sample | Nob | Minimally-invasive sample, scalable, functional readouts, personalized interrogation of variants, genome editing allows interrogation of causal genes, culture-based system for therapeutic screening | Many different protocols, the full heterogeneity of human DRG lacking, in vitro model, technical expertise required, long maturation time |
| Human DRG cultures | Donor | No | Functional readouts, access to the full diversity of DRG neurons, allows therapeutic assessment in human tissue | In vitro model, requires tissue dissociation, technical expertise required, low throughput, not scalable |
| Human DRG cultures | Surgery | Yes | Functional readouts, access to the full diversity of DRG neurons, allows therapeutic assessment in human tissue, known pain phenotype of patient | In vitro model, requires tissue dissociation, technical expertise required, low throughput, not scalable, surgeries are rare |
| Microneurography | In vivo | Yes | In vivo functional characterization of cutaneous afferents (sensory and sympathetic), detailed stimulus-response functions possible, can be used in combination with psychophysical tools, pre-post drug treatment recordings possible | Technical expertise required, demanding for experimenter and participant, sampling biases exist, limited to cutaneous fibres, Aδ-fibres difficult to study, informs regional nerve activity only, more invasive than nerve conduction studies |
This list does not include clinical/experimental assays targeting sensory percepts [e.g. quantitative sensory testing (QST) or laser evoked potentials]. Techniques available in clinic are specified. CCM = corneal confocal microscopy; IENFD = intraepidermal nerve fibre density; IHC = immunohistochemistry; ISH = in situ hybridization; scRNA-seq = single cell RNA sequencing.
Fresh donor tissue access is limited.
IPSCd nociceptors are not routinely studied clinically but participants have benefited from this personalized approach (see ‘IPSC-derived nociceptors derived from patients living with chronic pain’ section).
Human nociceptor morphology and anatomical clinical assessment
The soma of nociceptors reside in dorsal root ganglia (DRG) or trigeminal ganglia (TG), with axons extending terminals into innervation targets such as the epithelium. These terminals are widely distributed in the skin, joints, deep tissues, and cornea as free nerve endings.13
Classically, nociceptors can be classified as either C- or A-fibres. C-fibres, comprising the majority of nociceptors, are unmyelinated small diameter neurons with a slower conduction velocity than their A-fibre counterparts. These neurons are commonly subdivided through molecular and electrophysiological criteria, and these distinctions are highlighted throughout this review. Along their axons, C-fibres are closely associated with non-myelinating Schwann cells, forming Remak bundles.14 Recently, a novel class of cutaneous Schwann cells have been identified in mice.15 They are closely associated with C-fibre nerve endings in the skin and participate in the transduction of high threshold mechanical stimuli. The presence of this class of Schwann cell has yet to be confirmed in humans.
By contrast, Aδ-fibres are thinly myelinated with a faster conduction velocity.16 A subset of Aδ-fibres form free nerve endings in the epithelial layer and respond to noxious stimuli. Aβ-nociceptors form a unique group of thickly myelinated high threshold mechanosensors. These neurons have conduction velocities above that seen with Aδ-fibres and are largely insensitive to low-threshold brush stimuli.17 While sometimes overlooked compared to C- and Aδ-fibres, these nociceptors have been described across species, from mouse to human.16,17 Unique molecular markers have yet to be shown, and the proportion of Aδ/Aβ nociceptors remains vague because of sampling bias and varying definitions in conduction velocity. In animal models, Aβ nociceptors comprise anywhere from 18% to 65% of nociceptive A-fibres.16
The morphological and electrophysiological differences between fibre types contribute to the end percept. Generally, A-fibre activation results in a rapid, sharp pain. Correspondingly, nociceptors with C-fibres contribute to enduring, persisting pain sensations.18 However, it is possible that on-going A-fibre nociceptor activity may also result in burning pain. Electrophysiological recordings of monkey Aδ nociceptors have informed on their branching and structure, suggesting it is possible the long unmyelinated peripheral branches of Aδ nociceptors may blur the temporal separation between Aδ and C-fibre nociceptors.19 This has yet to be fully resolved, but Nagi and colleagues17 provide support for A-fibre nociceptor activation, in humans, resulting in fast, sharp pain.
From their innervating target tissues, nociceptors extend central axons to second-order neurons in the trigeminal subnucleus caudalis or the dorsal horn of the spinal cord. In the spinal cord, A-fibres connect with neurons in laminae I, II and V, while C-fibres form synapses in laminae I and II before modulation and/or transmission to the brain.13,16 In broad anatomical strokes, there are many conserved features between humans and experimental mammals, including general fibre types and projection pathways. Nevertheless, notable differences exist. Compared to mouse and rat for example, human DRGs are larger, contain more satellite glia, and have more connective tissue between neurons.20,21 There is also a relatively smaller number of unmyelinated axons per human Remak bundle compared to rat.22
Cutaneous nociceptors and intraepidermal nerve fibre density
Nociceptors innervating the skin are commonly studied clinically and experimentally. Morphologically, afferents project through the dermis into the epidermis as epidermal nerve fibres. Prior to penetrating the basement membrane (bordering the dermal and epidermal layers in the skin), these fibre bundles project horizontally and branch extensively. This dense neural network is referred to as the sub-epidermal nerve plexus.
Clinically, the intraepidermal nerve fibre density (IENFD), a measure of individual small fibre terminals per area, can be used to monitor nociceptor density through minimally-invasive skin biopsy23 and decreased IENFD was developed as a diagnostic measure particularly in small fibre neuropathy.24,25 Decreased IENFD has been demonstrated in many painful conditions and diseases including diabetic polyneuropathy,26,27 carpal tunnel syndrome (nerve entrapment),28 Charcot-Marie-Tooth disease type 1A,29 chronic ischaemic pain,30 complex regional pain syndrome,31 Guillain-Barré syndrome,32 Fabry disease,33 and more (for a review see Sommer and Lauria34) (Fig. 1). Preclinically, cutaneous nerve fibre densities can also be used to examine fibre changes in mice, providing a translatable parallel.35
As well as IENFD quantification, skin biopsies can show morphological changes such as axonal swellings. Axonal swellings are markers of axonal degeneration and contain watery axoplasm, neurofilaments and abnormal mitochondria.36 An increase in axonal swellings may predict nerve fibre loss.37 The molecular properties of nociceptors can be interrogated, including expression analyses of regeneration associated genes such as GAP43.38
Skin biopsy has also revealed small fibre pathology in conditions which were mainly thought to have central sensitization as a cause of chronic pain, such as fibromyalgia,39 and conditions thought to mainly relate to cutaneous pathology, such as the blistering condition dystrophic epidermolysis bullosa.40
Corneal nociceptors and corneal confocal microscopy
Corneal nerves originate from the TG and like the skin, form a sub-basal nerve plexus of axons before projecting terminals into the epithelium. The human cornea has an extremely high nerve density and the development of corneal confocal microscopy (CCM) has allowed clinicians and researchers to examine corneal nociceptors in a non-invasive manner. Here, images of nerve fibres within the sub-basal nerve plexus are studied.
Like skin epidermal afferents, nerve density in the plexus is reduced under pathological conditions: the corneal plexus nerve density, visualized though CCM is reduced in patients with neuropathies such as small fibre neuropathy, diabetic neuropathy, and CMT1A (Fig. 1, CCM),41–45 and has also been reported in fibromyalgia.46 The ease and non-invasiveness of CCM enable repeated longitudinal measures. These strengths are particularly evident when applied to trials which involve disease-modifying therapies.
Nociceptor subpopulations and gene expression profiles in the human dorsal root ganglion
The simplistic distinction of C- and A-fibre nociceptors on morphological and electrophysiological ground fails to capture the true diversity of primary afferents now demonstrated by molecular profiling. Pain neuroscientists often refer to the peptidergic and non-peptidergic populations of nociceptors. These subpopulations are defined by expression of calcitonin gene-related peptide (CGRP) and/or substance P (SP) for the peptidergic population and binding of isolectin B4 (IB4) and/or P2X3 receptor expression for the non-peptidergic population.47 In mice, these populations are mostly distinct entities, particularly when distinguished by protein expression. Both in situ hybridization and single cell RNA sequencing experiments in mice largely support the presence of these two, distinct subpopulations. Single cell RNA sequencing experiments in mice have further divided these populations into distinct subgroups48,49 and delineated their developmental lineages.50 In rats, the segregation of these populations is blurred as there is more overlap between CGRP expression and IB4 binding.51 There is also overlap in the central projection termination of these afferents in the rat that is not seen in mice.52
Efforts to identify the molecular identity of human nociceptors have been hindered by the lack of IB4 binding to human sensory neurons (there are reports of binding in the Rhesus macaque53) and difficulties with antibody specificity on human tissues. However, several recent studies have overcome these technical issues, finding differences between murine and human nociceptors.21,54–56 The most important of these is that CGRP (CALCA gene) and P2X3 (P2RX3 gene) strongly overlap in human DRG (Fig. 2A). There is substantial overlap also in rats,51,52 but not as extensive as seen in humans. In humans, this likely occurs because a greater proportion of human nociceptors express the CALCA gene.56 In mouse nociceptors, the capsaicin receptor, TRPV1, is nearly exclusively localized to the peptidergic class of nociceptors.57 In rats, TRPV1 is expressed in both peptidergic and non-peptidergic nociceptors.51,58–60 However, in human DRG, TRPV1 is found in a substantially larger proportion of neurons suggesting that most human nociceptors express TRPV1.56 Similar results have been observed for TrkA (NTRK1 gene), the high affinity nerve growth factor (NGF) receptor,21 which is exclusively expressed in the peptidergic subset of nociceptors in adult mouse DRG.47 On the other hand, TRPA1 (an ion channel activated by environmental irritants), which is mostly found in the non-peptidergic nociceptor population in mouse DRG,61 is found in a smaller subset of human DRG neurons, and this population overlaps with TRPV1 expression.56
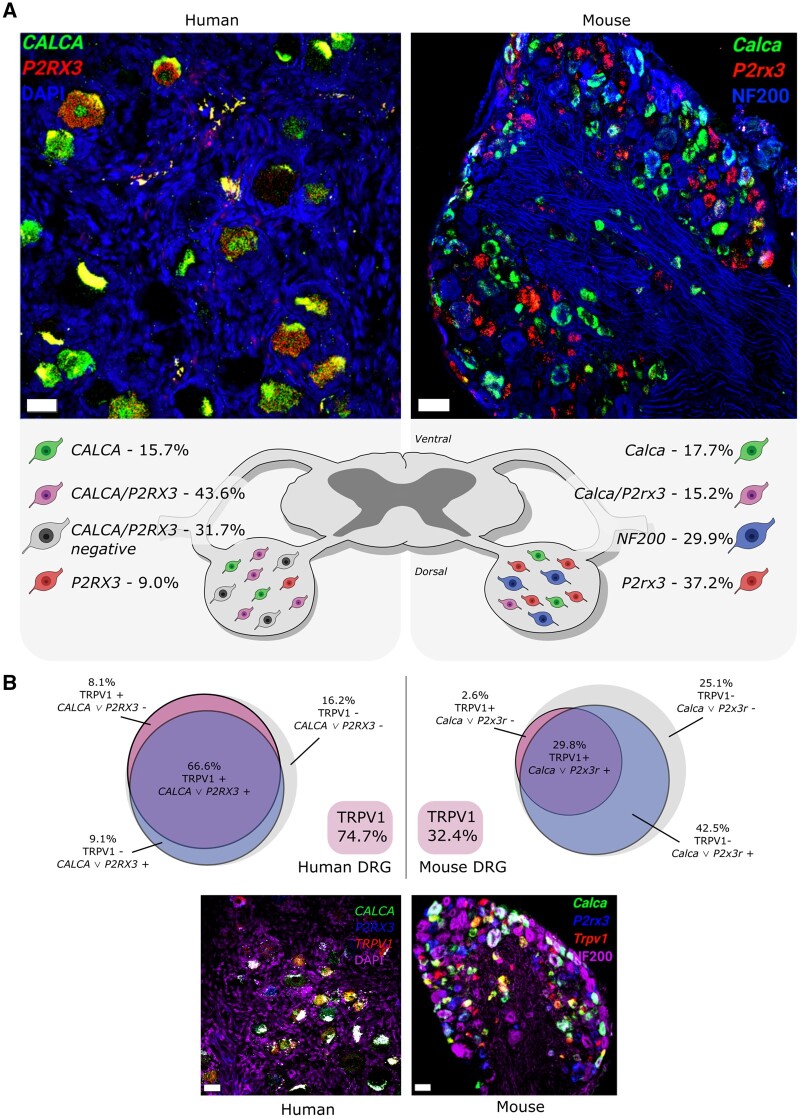
Figure 2.
Classic sensory neuron subpopulations are different between mouse and human DRG. (A) Mouse and human DRG labelled (at ×20) with RNAscope in situ hybridization for CALCA (CGRP; green) and P2RX3 (P2X3R; red) mRNA. Mouse and human DRGs were co-stained for NF200 protein (blue) and DAPI (blue), respectively. Human DRG had a large population of CALCA/P2RX3 co-expressing neurons (43.6%) that was much smaller in mouse (15.2%). This was due to more CALCA being found in human DRG than mouse. These data indicate that the peptidergic (CGRP-positive) and non-peptidergic (P2X3R-positive) neuronal subclasses are not separate entities in human DRG and support that the mouse and human nociceptor phenotypes may be divergent. (B) Mouse and human DRG (at ×20) labelled with RNAscope in situ hybridization for CALCA (CGRP; green), P2RX3 (P2X3R; blue) and TRPV1 (TRPV1; red) mRNA. Mouse and human DRGs were co-stained for NF200 protein (purple) and DAPI (purple), respectively. Trpv1 is expressed in a small percentage of neurons (32.4%) in mouse DRG, most of which are Calca and/or P2rx3 positive (29.8%). However, in human DRG, TRPV1 is expressed in a much larger population (74.7%), the majority of which are positive for CALCA and/or P2RX3. Data shown are summarized from Shiers et al.56 Scale bar = 50 μm.
Larger diameter, Aβ low-threshold mechanoreceptor neurons play an important role in neuropathic pain because they contribute to mechanical allodynia.62 In mice, these neurons can be labelled with antibodies against heavy chain neurofilament (NF200).63 In human DRG, antibodies against this protein label all neurons.21 A potential marker for Aβ low threshold human DRG neurons is KCNS1, which encodes the Kv9.1 β subunit of voltage-gated K+ channels (Fig. 2B). This mRNA is selectively expressed in non-CGRP, non-P2X3 mRNA-positive human DRG neurons whereas it is expressed in some peptidergic and non-peptidergic neurons in mice.56
From these studies, we conclude that most human nociceptors likely have a peptidergic phenotype (Fig. 2A and B). A subset of human DRG neurons show mixed expression of genes that are found in both the peptidergic and non-peptidergic subsets of nociceptors in mice. Rat nociceptor subtypes may be more closely related to human nociceptors but there are differences, such as the lower proportion of TRPV1-expressing nociceptors in rat versus human.51,56,58,59,64 Our understanding of populations of human DRG nociceptors would be greatly improved by single cell RNA sequencing datasets. These have been difficult to generate owing to the large size of human DRG neurons, including their relatively large nuclei. However, similar datasets in non-human primates were recently generated. These show broad conservation of sensory neuron populations between primate and mouse; however, there are differences at the individual gene level. Used in combination with human genetic analysis this data provides insight into the cellular origin of human chronic pain with multiple chronic pain conditions appearing to converge on two populations.65 Similar datasets in rats, which do not currently exist to our knowledge, would enable decisions about appropriate species choice for back-translation studies that will be important for target validation and mechanistic insight. Bulk sequencing datasets have been generated in rats and offer insights into changes in DRG transcriptomes in response to injury.66,67 Overcoming these technical challenges and gaps in knowledge will advance the field dramatically.
Functional impact of differences between human, murine and rat nociceptor subpopulations
Elegant molecular genetic manipulations in mouse have demonstrated that peptidergic neurons are primarily responsible for thermal pain while the non-peptidergic subset of neurons mediate mechanical pain.57,68 More complex behavioural analysis in mice complicates this issue somewhat, revealing that while the TRPV1+, peptidergic population is responsible for reflexive heat behaviours, these neurons also mediate ‘coping’ behaviours in response to a variety of different stimuli.69 These distinct sets of murine sensory neurons also innervate the dorsal horn differently. The peptidergic subset of mouse nociceptors terminate in lamina I and the outer portion of lamina II. These neurons make synaptic contact with projection neurons indicating that they are capable of driving direct activation of a nociceptive relay to the brain.70–72 On the other hand, non-peptidergic mouse nociceptors terminate mostly in inner lamina II and do not make direct synaptic connections with projection neurons. Instead, these neurons terminate on interneurons that form a dense network of synaptic connections that regulate the output of projection neurons.70,72 Synaptic arrangements of human nociceptors in the spinal cord have not been delineated, but immunohistochemical studies suggest that CGRP terminals are homogenous throughout lamina II,73,74 as are TRPV1-positive nerve terminals.64 Such a difference is consistent with expression of CGRP and TRPV1 in a larger population of human nociceptors.56,64 Interestingly, both mechanically sensitive and insensitive human nociceptors respond to the TRPV1 agonist capsaicin,75 consistent with an expansion of TRPV1 expression in subsets of human nociceptors.56
In rats, IB4-saporin has been used to ablate the non-peptidergic population of neurons in adult animals. This approach leads to a different phenotype in rats than would be expected from more recent work in mice. These rats show deficits in both mechanical and thermal pain, both at baseline, and in response to injury.76,77 This is again consistent with species differences between rats and mice in nociceptor populations that have important impacts on behaviour.
The differences described above have important implications for pharmacology. In mice, the mu and delta subtypes of opioid receptors are distinctly expressed in peptidergic and non-peptidergic subsets of nociceptors, respectively. Consistent with this anatomy, intrathecally applied mu agonists selectively inhibit thermal pain while delta agonists inhibit mechanical pain.78 A recent study of human nociceptors taken from organ donors suggests that there is substantial overlap of mu and delta opioid receptor functional expression in the human DRG.54 These mu and delta opioid receptors were both localized to neurons that expressed TRPV1 mRNA, although not all these neurons were capsaicin responsive. Pharmacological differences between mouse and human nociceptors have also been described for nicotinic acetylcholine receptors.79 While some studies have found important differences in receptor expression, signalling mechanisms between species appear to be conserved. For instance, opioid receptor activation is linked to inhibition of voltage-gated Ca2+ channels in mice and humans54 and activation of type II metabotropic glutamate receptors (mGluR) inhibits prostaglandin E2-mediated nociceptor hyperexcitability in both species.80 Interestingly, while type I mGluRs are prominently expressed in murine nociceptors,81 mGluR5 (GRM5 gene) is absent from human DRG.55
Another important consideration is nociceptor synaptic connectivity in the dorsal horn. Mouse genetics is now revealing the logic of how distinct subtypes of sensory neurons wire to specific types of dorsal horn neurons.82 An important next step for this line of work will be understanding the synaptic adhesion or other molecules that determine these synaptic arrangements. A critical piece of this puzzle will be to gain insight into precisely how mouse and human DRG neurons differ in their molecular features so that reasonable hypotheses can be built about wiring diagrams in the human spinal cord. Single cell sequencing technologies, including spatial transcriptomics,83 will play an important role in making these discoveries and species-to-species translations possible.
Genes with enriched expression in the human dorsal root ganglion
A possible route to effective pain therapeutics is drug discovery centred on genes that are specifically expressed in DRG neurons and mostly not found in other tissues. For instance, the voltage-gated Na+ (Nav) channels 1.7, 1.8 and 1.9 are relatively specifically expressed in DRG nociceptors and have been key pain drug targets for decades. Molecules specifically targeting these channels are now in clinical trials. The vast wealth of RNA sequencing data generated across laboratories and fields is uniquely amenable to computational methods that allow for identification of gene expression enrichment in specific tissues. This technique was recently applied to the human DRG to identify ∼140 genes that showed enrichment in human DRG compared to other tissues from the Genotype-Tissue Expression (GTEx) consortium transcriptome resource.55,84 Many of these enriched genes had been identified by previous studies, in particular studies that used subtractive cloning techniques to find DRG-enriched ion channels and G-protein coupled receptors (GPCRs) prior to genome sequencing.85 They also include transcription factors such as PRDM1286,87 and others that are involved in specifying the nociceptor lineage.
The vast majority of these DRG enriched transcripts are conserved in human and mouse, although with varying degrees of enrichment for many genes.55,88 Two examples are the CCKAR and IL31RA genes, which have been implicated in pain and itch, respectively. In humans, CCKAR transcripts are more specifically expressed in DRG as compared to mice where a broader expression pattern is seen.55IL31RA is enriched in both human and mouse DRG but its expression level is far higher in human DRG (Fig. 3). In mouse DRG, the interleukin 31 (IL31) receptor is expressed in a selective set of neurons that also express Nppb and are associated with itch.89 In human DRG, a far greater proportion of nociceptors express the IL31RA mRNA90 suggesting a broader role in pain in humans. IL31 can produce both pain and itch behaviours in mice.91 A final example is the nicotinic alpha 9 receptor encoded by the CHRNA9 gene (Fig. 3). This gene is highly enriched in human DRG but not expressed in mouse DRG at all.55 In human DRG, CHRNA9 is expressed in a subset of nociceptors.56 Antagonist studies for this receptor suggest an efficacy against chemotherapy-induced neuropathy in both mice and rats92 and drug discovery efforts against this receptor are underway for neuropathic pain. It will be interesting to determine the impact of alpha 9 modulation on excitability of human nociceptors given the species expression differences.
Figure 3.
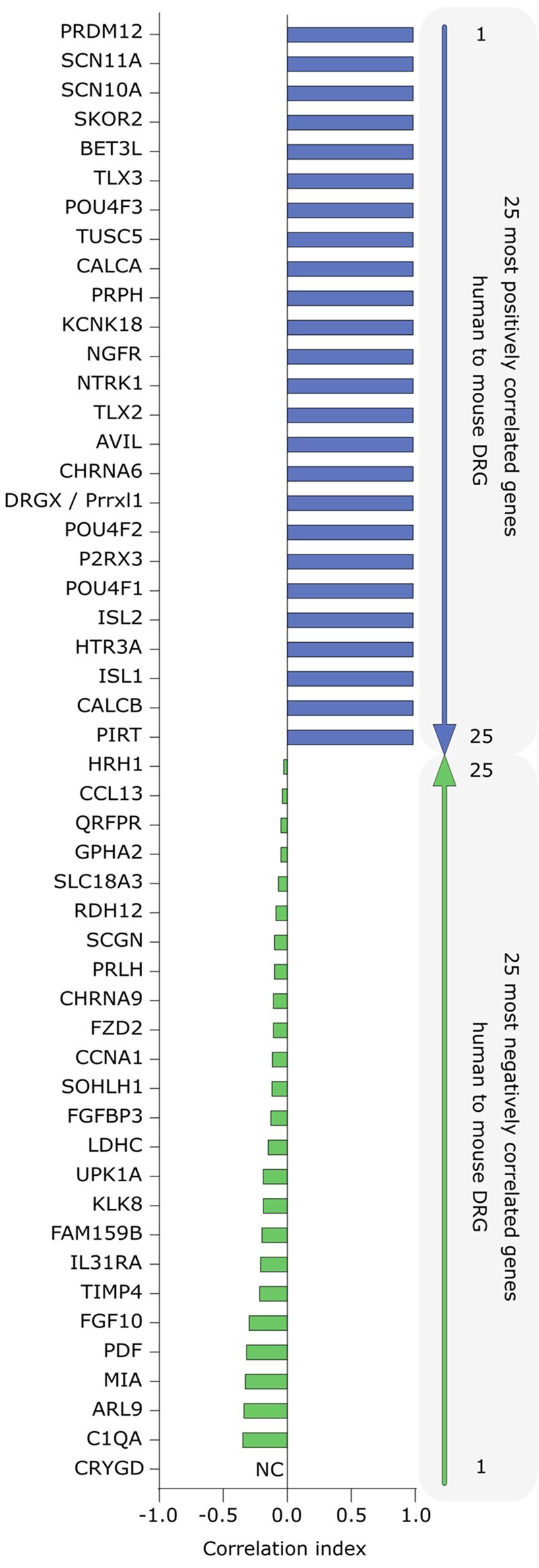
Top 25 positively and negatively correlated DRG-enriched gene expression profiles between pharmacologically relevant human and mouse tissues from RNA sequencing. The figure shows the top 25 most positively and negatively correlated (in blue and green respectively) DRG-enriched gene abundance profiles in 12 tissues across human and mouse gene orthologues. The tissues include the DRG, neural tissue (such as spinal cord and brain subregions), and pharmacologically relevant non-neural tissues (such as skeletal muscle and liver). Only genes with relative abundance >0.1 transcripts per kilobase million (TPM) in human DRGs are shown in either list. The methods are described in detail in Ray et al.55 Among the positively correlated genes, the list is populated with many of the most widely studied genes in the pain and somatosensation fields. Among the anticorrelated genes that are species-specifically enriched in human DRG, most of these genes are relatively unstudied and/or have been validated at the cellular level in independent experiments (e.g. CHRNA9 and IL31RA, discussed in main text). Gene orthologues with different names in human and mice show both names. NC = not calculated since the gene is not expressed in any of the profiled mouse tissues.
Plasticity of human dorsal root ganglion transcriptome in neuropathic pain
Developing therapeutic strategies for neuropathies would be enhanced by a better understanding of molecular changes that occur in the DRG that potentially drive neuropathic pain. To this end, a study was conducted on patients with or without neuropathic pain undergoing vertebrectomy surgery.93 This surgery provides a unique opportunity because patients have their DRGs removed as part of the surgery. Phenotyping before the surgery allows for pairing of DRG transcriptomes with patient-specific pain information matched to the DRG dermatome. This study led to some striking conclusions about underlying changes in gene expression that may drive neuropathic pain.
The first conclusion was that there are likely important sex differences in neuro-immune interactions in neuropathic pain. Rodent studies indicate that monocyte lineage cells, macrophages, and microglia play a critical role in development of neuropathic pain distinctly in males.94,95 Human transcriptomic findings broadly support this conclusion.93 However, in female DRGs associated with neuropathic pain, such striking changes in genes associated with macrophages were not observed. Rather, we find upregulation of a number of GPCRs. One of these, the adenosine type 3 receptor (A3AR), encoded by the ADORA3 gene, also shows some important species differences. The receptor is not expressed in the mouse DRG,55 but in rats it is expressed by nociceptors, where its activation inhibits voltage-gated Ca2+ channels.96 In mice, agonists of the A3AR reduce neuropathic pain through an action on non-neuronal cells that increase production and release of interleukin 10 (IL10) that then acts on pain circuitry.97 Therefore, in female patients with neuropathic pain an inhibitory action on nociceptors coupled with a trophic action on pain-resolving immune cells may have a dramatic analgesic effect.
Another important finding stemming from these studies is discovery of immune-derived factors that are associated with neuropathic pain in patients. Some of these are well-known from the preclinical literature, but others have not been widely studied. A prime example of the latter is oncostatin M (OSM gene).93,98 OSM is an IL6 family cytokine that acts via the OSM receptor and the GP130 signalling receptor.99 Its gene expression is increased in DRGs associated with pain and its likely cellular origin is macrophages.93 Our expression analysis profiling suggests that human nociceptors and satellite glial cells express OSM receptor components (Shiers and Price, unpublished observations). OSM may be an important mediator of neuropathic pain that has not emerged from similar experiments done on rodent DRGs in neuropathic pain models. As the study continues to increase sample sizes from this unique patient cohort, it is expected to have increased power to detect additional potential mediators of neuro-immune interactions driving pain in patients, including potentially sex-specific mediators.
Using human induced pluripotent stem cell-derived nociceptors to understand nociceptor function
As human DRGs are often not readily available, the ability to differentiate human induced pluripotent stem cells (IPSCs) into sensory neurons provides the opportunity to study their function in both naïve and disease states. They can be derived from skin biopsy or a blood sample and are thus less invasive than studying native human DRG neurons (discussed below). They also have the advantage of scalability, investigation of multiple tissue types, and the application of genome engineering. Here, we will briefly outline some of the state-of-art protocols available to generate IPSC-derived sensory neurons and how they have been used to model pain disorders (Table 2). We will also delineate how these in vitro models have informed therapeutic design for chronic pain states (Fig. 4). Electrophysiological properties are discussed in a later section.
Table 2.
Stem cell differentiation protocols for human sensory neurons/nociceptors and their use in modelling painful pathologies
| Original cells | Resulting cellsa | Exogenous factors | Disease modelling | Reference |
|---|---|---|---|---|
| Direct induction | ||||
| Fibroblasts (mouse/human) | Nociceptive sensory neurons (iNoc) | Ascl1, Myt1l, Ngn1, Isl1, Klf7 | Familial dysautonomia | Wainger et al.100 |
| Fibroblasts | Induced peripheral sensory neurons (iSNs) | Brn3a, Ngn1 or Ngn2 | – | Blanchard et al.101 |
| IPSC protocols | ||||
| IPSCs (or ESCs) | IPSC-derived nociceptors | OKSM factors, LDN19318, SB431542, CHIR99021, SU5402, DAPT, Y27632, EGF, FGF, NTFs (Chamber’s protocol) | – | Chambers et al.102 |
| Migraine | Pettingill et al.103 | |||
| IPSCs | IPSC-derived nociceptors | Chamber’s protocol + minor modifications | IEM | Meents et al.104 |
| IPSCs | IPSC-derived nociceptors | Chamber’s protocol + minor modifications | Small fibre neuropathy | Namer et al.105 |
| IPSCs | IPSC-derived nociceptors | Chamber’s protocol + minor modifications | CIP | McDermott et al.106 |
| IPSCs | IPSC-derived peripheral sensory neurons | Chamber’s protocol + minor modifications | Varicella-zoster virus infection | Lee et al.107 |
| IPSCs (from PBMCs) | IPSC-derived peripheral sensory neurons | Chamber’s protocol + minor modifications | IEM | Cao et al.108 |
| IPSCs | IPSC-derived peripheral sensory neurons | Chamber’s protocol + minor modifications | Remyelination therapy | Cai et al.109 |
| IPSCs (from PBMCs) | IPSC-derived peripheral sensory neurons | Chamber’s protocol + minor modifications | IEM | Mis et al.110 |
| IPSCs | IPSC-derived peripheral sensory mechanoreceptors | Chamber’s protocol + minor modifications | – | Guimarães et al.111 |
| IPSCs | IPSC-derived proprioceptive neurons | Chamber’s protocol + minor modifications | Afferent ataxia | Dionisi et al.112 |
| IPSCs | IPSC-derived trigeminal neurons | Chamber’s protocol + minor modifications | HSV-1 infection | Zimmer et al.113 |
| IPSCs | Peripheral mechanoreceptors | OKSM factors, NTFs | – | Schrenk-Siemens et al.114 |
| IPSCs | NC-iSN mechanosensory neurons | OKSM factors, Y27632, SB431542, Brn3a, Ngn2, NTFs | Piezo2 channelopathy | Nickolls et al.115 |
| IPSCs | IPSC-NCd sensory neurons | Noggin, LDN193189, SB431542, Y27632, NTFs | – | Umehara et al.116 |
| ESC/other protocols | ||||
| hEPI-NCSC | Peptidergic nociceptive Sensory neurons | SHH, CHIR99021, LDN193189, DAPT, HGF, NTFs | – | Wilson et al.117 |
| iNPCs | Peripheral sensory neurons (iSNs) | LDN193189, SB431542, CHIR9902, DAPT, SU5402, NTFs | Chemotherapy-induced neuropathy | Vojnits et al.118 |
| ESCs | Peripheral sensory neurons | LDN193189, SB431542, CHIR9902, DAPT, SU5402, Y27632, NTFs | Peripheral nerve injury | Jones et al.119 |
| ESCs | Peripheral sensory neurons | SB431542, CHIR9902, BMP2, Y27632, NTFs | – | Alshawaf et al.120 |
A substantial number of IPSC protocols are largely based on Chambers et al.102 and have since undergone small modifications; for protocol adaptation refer to each original publication. CIP = congenital insensitivity to pain; ESC = embryonic stem cell; hEPI-NCSC = human epidermal neural crest stem cells; iNPC = induced neural progenitor cells; iSNs = induced sensory neurons; NCd = neural crest-derived; NC-iSN = neural crest derived induced sensory neurons; NTF = neurotrophic factors; PBMCs = peripheral blood mononuclear cells.
Note that ‘Resulting cells’ is the nomenclature used by each original publication.
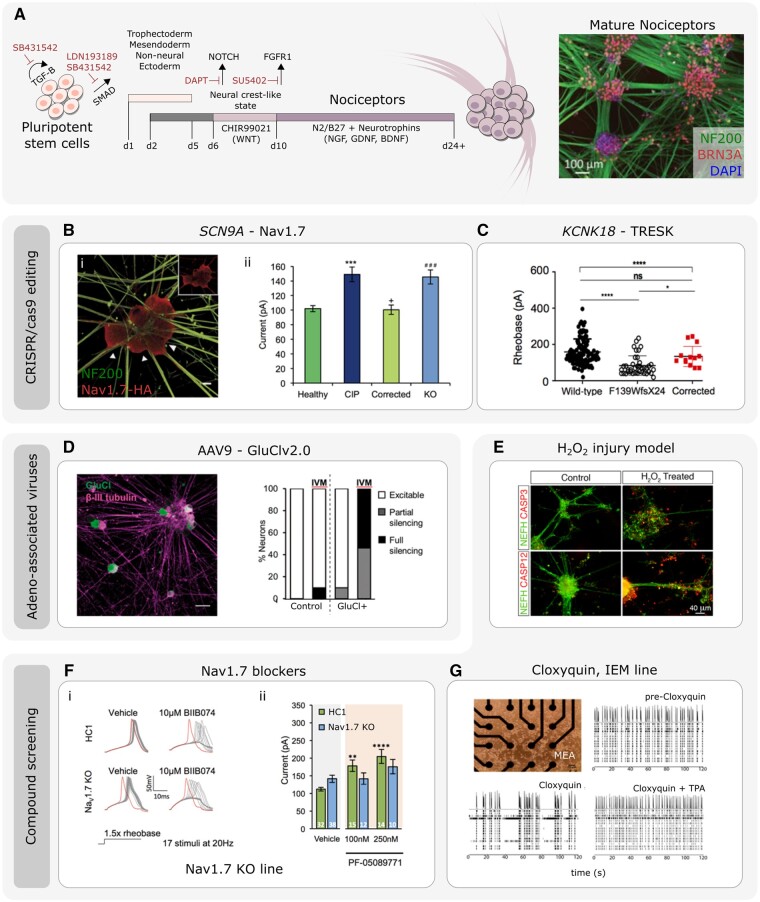
Figure 4.
Using IPSC-derived nociceptors to develop therapeutics for pain disorders. (A) Illustration of the protocol and timeline of events to generate mature IPSC-derived nociceptors (adapted from Chambers et al.102 and Meentz et al.104; immunomicrograph adapted from Clark et al.121). [B(i)] The use of CRISPR/cas9 gene editing to generate IPSC-derived nociceptors edited to constituently express HA-epitope tagged Nav1.7. (ii) Current-clamp analysis of the current require to elicit and action potential of IPSC-derived nociceptors derived from healthy participants and congenital insensitivity to pain (CIP, due to Nav1.7 loss-of-function) participants. CIP participant IPSC-derived nociceptors were hypoexcitable. The CIP participant hypoexcitability was corrected using CRISPR/Cas9 IPSC editing. CRISPR/Cas9 was used to generate Nav1.7-KO IPSCs that were differentiated into nociceptors that mimicked CIP hypoexcitability (adapted from McDermott et al.106). (C) Current-clamp analysis of the rheobase (minimum current required to elicit and action potential) of IPSC-derived nociceptors from wild-type participants, and participants carrying the F139WfsX24 TRESK loss-of-function mutation. IPSC-derived nociceptors from F139WfsX24 are hyperexcitable compared to wild-type nociceptors. This nociceptor hyperexcitability was rescued when using CRISPR/cas9 to correct the TRESK mutation (adapted from Pettingill et al.103). (D) IPSC-derived nociceptors transduced with the chemogenetic silencing tool GluCl. Scale bar = 50 µm. Patch clamp analysis revealed that following application of the GluCl agonist ivermectin (IVM), GluCl+ nociceptors were rendered either fully silent or partially silent to depolarizing current injections (adapted from Weir et al.122). (E) Example of stem cell derived nociceptors that have been used to model a chemical injury model through the addition of H2O2. H2O2 treatment leads increased caspase expression and neurite retraction/disassembly (adapted from Jones et al.119) [F(i)] IPSC-derived nociceptors derived from healthy control (HC1) or CRIPSR/cas9 engineered Nav1.7 KO lines. The Nav1.7 blocker BIIB074 promoted action potential failure in both HC1 and Nav1.7 KO lines. (ii) Current clamp recordings of HC1 and Nav1.7 KO nociceptors in the presence of vehicle or PF-05089771. Only HC1 but not Nav1.7 KO nociceptors demonstrated changes in excitability in response to the PF-05089771 compound (adapted from McDermott et al.106). (G) Multi-electrode arrays (MEAs) used to assess spontaneous activity of IPSC-derived nociceptors from an IEM participant. Activity at electrodes was reduced after addition of the TRESK activator cloxyquin. Spontaneous activity recovery was lost following co-addition of TPA (TRESK inhibitor) and cloxyquin (adapted from Pettingill et al.103).
IPSC differentiation into nociceptors and peripheral sensory neurons
In the past two decades, there have been significant advances in the ability to reprogram rodent and human tissues to generate IPSCs.123–127 The pioneering work by Chambers et al.128 demonstrated that the synergistic action of Noggin and SB431542, inhibitors of the SMAD and TFG-β signalling, respectively, were sufficient to differentiate both human embryonic stem cells and human IPSCs into peripheral neurons at an efficiency of >80%.128 This work and subsequent studies led to differentiation protocols that convert stem cells into sensory neuron populations, in particular nociceptors (Table 2).
While the Chambers et al.102 protocol was the first, other differentiation protocols have since followed (Table 2). Two groups simultaneously published their protocols for reprogramming both rodent and human fibroblasts into induced nociceptors (iNoc) and induced peripheral sensory neurons (iSN) using transcription-mediated lineage conversion.100,101 Both exhibited molecular and physiological properties of mature DRG neurons such as tetrodotoxin (TTX)-sensitive (TTX-s) and resistant (TTX-r) sodium currents and the expression of DRG molecular markers. Wainger et al.100 also demonstrated that human iNoc were able to identify deficits in neurite outgrowth and branching in a patient with familial dysautonomia. However, this protocol has not been as widely adopted as the Chambers’ one, likely because of a lower efficiency when generating nociceptors.
Alternative approaches have included using human embryonic stem cells to derive neural crest progenitors, which can then be further differentiated into DRG sensory neurons (using media supplemented with a cocktail of neurotrophins).120,129 Analyses revealed the presence of neuronal subpopulations after 3 weeks of differentiation, suggesting a heterogeneous culture. Distinct subpopulation responses to algogens, temperature, and osmolarity (mechanosensory) changes supports this notion.120 Recently, it was also proposed that human epidermal neural crest stem cells (hEPI-NCSC) can differentiate into hEPI-NCSC derived nociceptors.117 Functional assays demonstrated that 30% of these sensory neurons responded to capsaicin.
Across differentiation protocols, derived cells are examined for a variety of sensory neuron markers using transcript expression and functional assays. Detailed RNA sequencing has been performed on IPSC-derived nociceptors from the Chambers’ protocol, suggesting a DRG transcript ‘signature’ when compared to published human RNA sequencing data.55,106 The ion channel expression profile in mature human IPSC-derived nociceptors (Chambers’ protocol) closely resembled that of adult human DRGs. For instance, microarray data suggest 84% of human DRG ion channel genes were expressed, such as those encoding for Kv7.2/7.3, which play a role in controlling neuronal resting membrane potential,130 and have been recently recognized for their role in the peripheral pain pathway.131 Additionally, microarray data also showed the expression of HCN1 and HCN3 ion channel subunit genes, as well as the presence of acid-sensing ion channels (ASICs).130 ASICs are highly expressed in nociceptors, with a putative role in inflammatory pain in mice and humans,132 while modulating HCN ion channels (and in turn nociceptor excitability) might represent a potential avenue for treating chronic pain.133
Given the known similarity of mature mouse DRG and TG transcript expression profiles,134 DRG differentiation protocols are likely informative regarding TG and vice versa. For example, the Chambers’ protocol is frequently discussed in the context of DRG, but it has also been used to study migraine-associated channelopathies.103 An IPSC protocol to derive ‘TG’-like sensory neurons has also been developed.113
Single differentiation protocols do not recapitulate the full complexity of sensory neuron subpopulations. As we learn more about human DRG expression profiles through transcript analyses, IPSC differentiation protocols may be tailored further. It is advisable that researchers consider the small number of DRG versus TG-specific differences when selecting a differentiation protocol, as well as verify expression patterns to eliminate possible caveats.
While this review is largely focused on nociceptive sensory neurons, it is important to also note that groups have designed novel protocols to generate a variety of human IPSC-derived mechanoreceptors used to study touch biology.114,115 There is increasing evidence that changes in mechanoreceptors (and their circuitry) post-injury may underlie tactile allodynia.135,136 Therefore, using IPSC-derived nociceptors and, when appropriate, IPSC-derived mechanoreceptors, may facilitate the successful translation of therapeutics targeted towards chronic pain.
IPSC-derived nociceptors derived from patients living with chronic pain
The use of IPSC nociceptors derived from individuals living with chronic pain has been invaluable in the study of human nociception, novel therapeutics, and even new directions in personalized medicine. For instance, IPSC-derived nociceptors have been generated from participants with inherited erythromelalgia (IEM) due to gain of function mutations in Nav1.7.104,108,110 IEM participants experience episodes of severe pain and erythema localized to the extremities and exacerbated by warm temperature. IEM is due to Nav1.7 gain-of-function mutations increasing nociceptor excitability.137 IEM IPSC-derived nociceptors have thus enabled valuable insight into the Nav1.7-dependent mechanisms of IEM in a humanized sensory system. These included aberrant ectopic activity, reduced action potential thresholds, and hyperexcitability to heat stimuli.104,108,110 Further studies have been able to use these IEM patient IPSC-derived nociceptors to investigate the efficacy of drug interventions. For instance, the development of Nav1.7 blockers is an attractive avenue to treat IEM, as such, and thus IEM patient IPSC-derived nociceptors are an excellent platform to test the functionality of Nav1.7-specific blockers. A Nav1.7-specific blocker was administered to IEM IPSC-derived nociceptors and showed efficacy in reducing their hyperexcitability.108 This led to a small, randomized, double-blind, placebo-controlled, cross-over study which demonstrated efficacy of this small molecule in reducing heat evoked pain.108 However, another Nav1.7 blocker (BIIB074) failed to reach the primary end point in a phase 2 trigeminal neuralgia clinical trial.138,139 This might reflect the recent data from IPSC-derived nociceptors that lack Nav1.7, which raised questions over BIIB074’s specificity for Nav1.7 (discussed below).106
IPSC-derived nociceptors from participants with IEM have also enabled identification of peripheral mechanisms that underlie interindividual differences and indeed resilience to chronic pain. An example was the study of two IEM participants, both carrying the same Nav1.7 mutation (S241T) but reporting very different levels of pain. This work identified an additional genetic variant, a Kv7.2 gain-of-function mutation (T730A), which reduced nociceptor excitability and was only present in the participant that experienced the least pain.110
Other groups have generated IPSCs from participants with different chronic pain conditions, including painful small fibre neuropathy. This study was based on an individual subject with small fibre neuropathy where genetic sequencing revealed the presence of two mutations (a common Nav1.9 variant, N1169S, and a rare Nav1.8 variant, R923H) although it was unclear as to the relative contributions of these mutations to the pain experienced.105 Regardless, IPSC-derived nociceptors from this subject exhibited increased spontaneous activity compared to controls, and mimicked the increased incidence of spontaneously active C-fibres revealed by microneurography.105 Lacosamide, an anti-epileptic thought to act by blocking voltage-gated sodium channels, reduced the incidence of spontaneously active IPSC-derived nociceptors and peripheral C-fibres. In this single patient, lacosamide also provided effective pain relief,105 demonstrating that patient IPSC-derived nociceptor characteristics can guide effective treatment design—a step towards personalized medicine.
IPSC-derived nociceptors have been proven useful also to investigate the sensory system in patients with other sensory dysfunctions. For instance, it has been reported that patients with Parkinson’s disease can develop sensory deficits that precede motor symptoms.140 Taken together, this exemplifies how IPSC-derived nociceptors are a powerful tool that can be used to understand pain mechanisms in classical and non-classical disorders of pain.
CRISPR/Cas9 gene editing in human IPSC-derived nociceptors
CRISPR/Cas9 is a fast and accurate method to edit genomic DNA.141 This can be applied to human IPSCs to unequivocally link a gene variant to functional outcomes with clinical relevance in the IPSC-derived-nociceptors. It can also be used for experimental manipulations such as tagging endogenous proteins to determine trafficking, and may in itself provide therapeutic opportunities in the future (Fig. 4A).
The first study to use CRISPR/Cas9 editing in human IPSC-derived nociceptors involved IPSCs generated from participants with congenital insensitivity to pain (CIP) due to loss-of-function mutations in SCN9A (encoding Nav1.7).106 When assessed using patch clamp electrophysiology, IPSC-derived nociceptors from patients with CIP were hypoexcitable to both threshold and suprathreshold electrical stimuli, supporting Nav1.7’s role in nociceptor electrogenesis and excitability.106,142 CRISPR/Cas9 was subsequently used to generate a Nav1.7 knock-out (KO) IPSC line, that when differentiated into nociceptors, recapitulated the patient electrophysiological phenotype [Fig. 4B(ii)]. CRISPR/Cas9 gene editing was then used to correct one SCN9A allele from one CIP patient. This correction recovered the hypoexcitability phenotype of patient IPSC-derived nociceptors [Fig. 4B(ii)].106 The same study also generated novel IPSC tools to interrogate human Nav1.7 in a humanized system. A tagged human Nav1.7 IPSC line was generated by introducing a haemagglutinin (HA) epitope at the C-terminus of human Nav1.7 to allow visualization of the subcellular localization of native Nav1.7 in human IPSC-derived nociceptors [Fig. 4B(i)].
Genome editing was also used in IPSCs generated from participants who suffer from migraine in order to understand the causal impact of gene variants in relation to IPSC-derived nociceptor function. Migraine was due to loss-of-function mutations in the KCNK18 gene, which encodes for the two-pore domain potassium channel TRESK. TRESK mediates a K+ leak current which stabilizes the resting membrane potential and attenuates depolarizing stimuli. Patient-derived IPSC nociceptors that carried the F139WfsX24 TRESK mutation displayed a loss of functional TRESK currents and neuronal hyperexcitability. This phenotype was reversed using CRISPR/Cas9 to correct the F139WfsX24 mutation (Fig. 4C).103
Human IPSC-derived nociceptors as screening tools to test drug and gene therapy efficacy
While CRISPR/Cas9 is a powerful technique for genome editing, many challenges need to be overcome before clinical application, not least the possibility of off target effects. Virus mediated genetic therapies are an alternative that have shown promise clinically.143 Groups have used adeno-associated viruses (AAVs) to deliver transgenes to human IPSC-derived nociceptors to explore pain therapies in a humanized system. As the availability of human donor nociceptors is limited, IPSC-derived nociceptors can be used, at least in part, to close this translational gap. One approach to treat chronic pain includes silencing of primary afferent activity. Expression of an engineered glutamate-gated chloride channel (GluCl), in the presence of its non-toxic agonist ivermectin, resulted in the silencing of mouse nociceptor activity, inhibition of evoked pain in a mouse model of neuropathic pain, and suppression of post-injury ectopic primary afferent activity.122 AAV serotype 9 (AAV9) containing GluCl was used to effectively transduce human IPSC-derived sensory neurons (Fig. 4D). Notably, GluCl was able to functionally suppress nociceptor excitability following application of its agonist ivermectin (Fig. 4D).122 This study demonstrated efficacy of a gene therapeutic in rodent models and a humanized in vitro system. Several groups have developed other AAV-transgene mediated silencing strategies to suppress DRG activity; however, to our knowledge their efficacy has yet to be examined in a humanized system.144,145
The development of new treatments which show promise in preclinical rodent models often do not successfully translate for human use. This can be due to many factors, including both toxicity and lack of efficacy in human neurons. The use of IPSC-derived nociceptors provides opportunity to interrogate these factors in a humanized system. Numerous efforts have been made to use stem cell technology to screen novel drugs and assess neurotoxicity using human IPSC-derived neurons.146,147 Some groups have begun to use human IPSC-derived nociceptors (rather than general CNS neurons) as screening tools for drug efficacy and toxicity; for example the use of IPSC-derived nociceptors identified chemotherapeutic and neurotoxic agents that impaired neurite outgrowth.148,149
Stem cell-derived nociceptors have also been used to model peripheral nerve injury. For instance, when the reactive oxygen species, hydrogen peroxide (H2O2), was added to human embryonic stem cell-derived nociceptors, it resulted in nociceptor cluster disassembly and neurite retraction. This was prevented by co-addition of pan-caspase inhibitors (Fig. 4E).119 Others have used peripheral blood mononuclear cell-derived, or IPSC-derived sensory neurons, to investigate chemotherapy-induced neuropathy, and offer their high throughput methods as drug-discovery and phenotypic screening platforms.118,150
The use of stem cell derived nociceptors has facilitated analysis and investigation into compound toxicity and drug efficacy, but CRISPR/Cas9 targeted KO lines (discussed above) have offered the possibility to test clinically relevant and novel compounds for off target effects in a humanized system. For instance, the Nav1.7 blocker BIIB074 has undergone phase II clinical trials for trigeminal neuralgia.139 However, this compound was found to be non-selective for human Nav1.7 at clinically relevant concentrations, and following high frequency stimuli BIIB074 altered the excitability of Nav1.7 KO IPSC-derived nociceptors [Fig. 4F(i)].106 On the other hand, the selective Nav1.7 blocker PF-05089771, which has shown efficacy in preventing spontaneous activity of IEM IPSC-derived nociceptors, and has undergone phase II clinical trials for IEM and painful diabetic neuropathy,108,151 altered the rheobase only in control but not Nav1.7 KO IPSC-derived nociceptors [Fig. 4F(ii)], suggesting greater human Nav1.7 specificity.106
Others have used IEM IPSC-derived nociceptors to examine efficacy of the TRESK activator cloxyquin in IEM and migraine.103 Using IPSC-derived nociceptors cultured on multi-electrode arrays, the addition of cloxyquin significantly reduced spontaneous activity detected at electrodes (Fig. 4G).103 This was prevented by the co-addition of tetrapentylammonium bromide (TPA), a TRESK inhibitor, confirming cloxyquin’s specificity for TRESK (Fig. 4G).103 Together, this suggested TRESK activation offers therapeutic potential to human pain disorders where ectopic activity and hyperexcitability are key contributors.
Insights into human nociceptor function through direct electrophysiological recordings
In vitro human dorsal root ganglion recordings
It has become clear that the occurrence of ongoing action potentials, spontaneous activity, arising either in the distal endings of primary afferent fibres and/or ectopically from DRG somata, drives multiple chronic pain states.93,152–154 Importantly, ectopic spontaneous activity has been identified in multiple models of inflammation and neuropathic pain in rat155–157 and mouse158–161 that appear to share key physiological processes. Spontaneous activity was recently also shown to occur in human DRG neurons, and found to be specifically associated with spontaneous ongoing neuropathic pain.93,162 The shared physiological processes in rat and human DRG neurons with spontaneous activity include a depolarization of resting membrane potential, a hyperpolarization of action potential threshold, and the occurrence of depolarizing spontaneous fluctuations in membrane potential.162 These changes have also been identified in mouse DRG and TG neurons after axotomy.160,163 Intriguingly, these membrane events have not been the focus of therapeutic discovery up to now, but given that they are subthreshold, they could have a wide therapeutic safety index. Thus, a key area of study is to define the similarities and differences in the neurophysiological properties of human and rodent DRG.21 A necessary focus in this regard will be to understand both the expression and function of the various ion channels critical in the regulation of DRG neuron hyperexcitability and spontaneous activity.20 It should be noted that spontaneous activity has also been observed in vitro from monkey skin-nerve preparations after nerve ligation.164 To our knowledge, studies of in vitro recordings from monkey DRG neurons (with or without the use of experimental pain models) have not been performed.
Recent work on human neurons has shown an important role for voltage-gated sodium (Nav) and calcium (Cav) channels.165 Human DRG were reported to express a higher proportion of the TTX-s channel Nav1.7 (∼50% of total Nav expression) but lower proportion of the TTX-r channel Nav1.8 (∼12% of total Nav expression) than in mouse (∼18% Nav1.7 and ∼45% Nav1.8 of total expression, respectively).166 Similar results were reported when comparing peak TTX-s and TTX-r currents between human and rat DRG.167 Furthermore, there are species differences in channel properties. Human Nav1.8 demonstrates slower inactivation kinetics and produces larger persistent and ramp currents than other species, resulting in longer lasting action potentials.168 In addition, differences between rodents and humans in the expression of Nav1.7 and Nav1.8 in the periphery, as well as comparably longer axons, could also contribute to functional differences. The expression of Nav1.8 in mouse (and monkey) appears to be similarly concentrated in distal portions near the terminal end of peripheral nerves169 and it is possible that the longer sensory axons could accentuate differences. Nav1.7 is expressed in epidermal nerve fibres of the skin of both humans and rats.170,171
Changes in expression of both Nav and Cav channels have been described in numerous preclinical models of chronic pain.137,172 For instance, in the context of a model of painful chemotherapy-induced peripheral neuropathy a comparison of immunohistochemistry images of DRG from vehicle- [Fig. 5A(i–iii)] and paclitaxel-treated rats [Fig. 5A(iv-vi)] show that the expression of Nav1.7, Cav3.2, and TRPV1 is increased with the induction of chemotherapy-induced peripheral neuropathy.173–175 Similar increases in expression of these ion channels have been shown in other rodent models of neuropathic and inflammatory pain.176–179 Human DRG neurons show a similar increase in expression of all three channels when that DRG is associated with neuropathic pain due to nerve root compression. The tissue was donated by a patient at MD Anderson Cancer Center who had surgical resection of metastatic oncological disease that involved ligation of bilateral dorsal roots, but only one of which innervated an area of the body with ongoing neuropathic pain. Immunohistochemistry comparing these DRG, one from the side without pain [Fig. 5A(vii–ix)] and one from a side of the body with neuropathic pain [Fig. 5A(x–xii)], reveals that the overall expression of Nav1.7, Cav3.2, and TRPV1 was markedly increased in the DRG where pain was observed.173–175 Thus, both rats and humans demonstrate similarity in upregulation of these ion channels in chronic pain states.
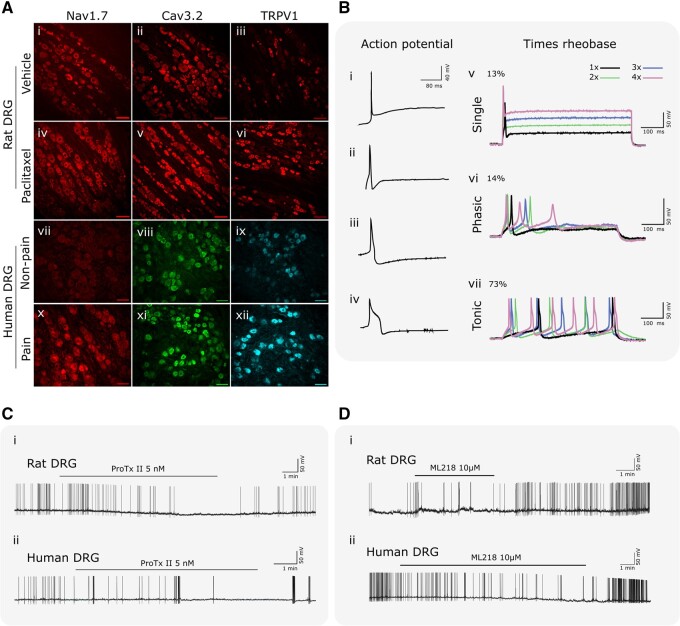
Figure 5.
Properties of human versus rat DRG neurons and their response to selective blockers in vitro. (A) The representative immunohistochemistry illustrates that Nav1.7, Cav3.2 and TRPV1 are expressed in naïve rat DRG (i–iii) and this expression is elevated in rats with neuropathic pain produced using paclitaxel treatment (iv–vi).173–175 All three channels are also expressed at relatively low levels in human DRG where neuropathic pain is absent (vii–ix), but also become markedly elevated when neuropathic pain is present (x–xii).173–175 (B) The representative analogue traces in i–iv show four common types of action potential waveforms observed in whole cell recordings from human DRG neurons. The representative analogue recordings in v–vii show the three typical patterns of response shown by human DRG neurons to intracellular currents using graded steps in intensity from rheobase as colour coded at top right. (C) The representative analogue recordings in show that the Nav1.7 inhibitor ProTxII, given at the time indicated by the solid line over each trace, inhibits spontaneous activity in both rat (i) and human (ii) neurons. Note the downward (hyperpolarizing) shift in the resting membrane potential in the rat DRG neuron recording during ProTxII administration. (D) The representative analogue recordings in show that the Cav3.2 inhibitor ML218, given at the time indicated by the solid line over each trace, inhibits spontaneous activity in both rat (i) and human (ii) neurons. Note the downward (hyperpolarizing) shift in the resting membrane potential in the human DRG neuron recording during ML218 administration.
The membrane neurophysiological properties of DRG neurons from rats and humans with and without neuropathic pain further the theme that DRG neurons in rodents and humans share many characteristics, but also demonstrate pronounced differences. Examination of data from previous published studies93,174,175 reveals that rat and human DRG neurons develop spontaneous activity during neuropathic pain and that each species shows the same three logical alterations in neurophysiological properties that would be expected to drive this spontaneous activity.162 First, both rat and human DRG neurons with spontaneous activity show a similar depolarization of resting membrane potential (rat: −52.01 ± 1.21 mV; human: −50.12 ± 1.91 mV) compared to neurons from the same DRG without spontaneous activity (rat: −59.1 ± 1.6 mV; human: −56.8 ± 1.8 mV) or compared to DRG not associated with neuropathic pain (rat: −58.1 ± 1.3 mV; human −57.9 ± 0.9 mV). Second, the action potential threshold in both rodent and human DRG with spontaneous activity became more hyperpolarized (rat: −24.4 ± 0.9 mV; human: −26.5 ± 1.19 mV) than that in neurons from the same DRG without spontaneous activity (rat: −9.6 ± 1.4 mV; human: −19.6 ± 2.25 mV) or in neurons from DRG not associated with neuropathic pain (rat: −10.2 ± 0.9 mV; human: −18.2 ± 0.9 mV). Third, the cells with spontaneous activity show large, irregular depolarizing spontaneous fluctuations in resting membrane potential that are not present in DRG neurons without spontaneous activity.93,162 Other changes in membrane properties are also shared between rat and human DRG neurons that develop spontaneous activity and that drive spontaneous pain including action potential amplitude, action potential overshoot, rise time, and magnitude of after-hyperpolarization. Even so, DRG neurons with spontaneous activity in rats and humans show subtle differences in neurophysiological properties. By example, action potential fall time in rat DRG neurons with spontaneous activity (12.24 ± 1.32 ms) was markedly shorter than that in humans (20.9 ± 1.41 ms) and the action potential width at 0 mV in rats (5.4 ± 0.71 ms) was markedly shorter than that in humans (7.72 ± 0.61 ms).
Active electrophysiological properties of human DRG neurons also show both similarities as well as some distinct differences with rat DRG neurons. Action potential waveforms in rat DRG neurons have been categorized into nine distinct types primarily based on action potential duration, the presence or absence of a declining phase shoulder, and after-hyperpolarization duration.180 Examples in Fig. 5B(i–iv) show four distinct types of action potential waveforms observed in human DRG to date based on the same criteria. Three of these are similar to that reported for rat DRG neurons, but at least one fairly typical waveform is distinct given the very pronounced declining phase shoulder [Fig. 5B(iv)], prolonged action potential duration, and prolonged after-hyperpolarization. This distinctive waveform was observed in at least one other study on human DRG neurons obtained from organ donors.181 The spike-burst responses of rat and human DRG also show both similarities and distinct differences. Examples of the three patterns of response of human neurons to intracellular current injection are shown in Fig. 5B(v–vii). A tonic firing pattern that grades with increasing current strength is the most common pattern, shown by ∼70% of human DRG neurons. The remaining neurons show an even split between either a single spike regardless of current strength, or a brief phasic burst of action potentials. These patterns are also reported in studies of rat neurons, but the reported proportions of neurons showing different types of responses ranged widely. One study in rats reported proportions similar to that observed in human DRG, though no distinction was made between single spike and phasic response types in that study.162 Two studies in rats reported an apparently equal split between tonic and single spike responses182,183; while a third study also in rats reported a proportion of single spike and tonic responses that were the exact opposite of that observed in humans.184 Combined, these physiological studies suggest that even though the general neurophysiology of DRG neurons in rodents and humans is shared, the underlying molecular biological processes that generate these alterations may be divergent.
A more detailed examination of the neurophysiological characteristics of activation and blockade of Nav1.7 and Cav3.2 illustrates the discrepancy between species. In Fig. 5C(i and ii) both rat and human DRG neurons with spontaneous action potentials showed a significant reduction in spontaneous activity after bath application of the voltage-gated sodium channel 1.7 (Nav1.7) blocker, ProTxII (5 nM). In neurons with a positive response to ProTxII, action potential properties were analysed before, during, and after washout of ProTxII. In rat DRG neurons, ProTxII hyperpolarizes the resting membrane potential (from −38.5 ± 0.6 to −48.8 ± 1.9 mV) as seen in the representative trace, hyperpolarizes action potential threshold (from −20.1 ± 1.1 to −27.9 ± 1.8 mV), induces a shorter action potential width at 0 mV (from 8.4 ± 1.1 to 5.0 ± 0.3 ms), and also causes a significant decrease in after-hyperpolarization amplitude (from 20.1 ± 1.6 to 15.2 ± 1.4 mV). In contrast, no significant changes in action potential characteristics were observed in human neurons. Thus, while ProTxII decreased neuronal hyperexcitability in both rat and human DRG neurons, contribution of Nav1.7 to different aspects of the action potential in hyperexcitable neurons is not consistent across species.
T-type calcium channels, also called low-voltage-activated calcium channels (LVACCs), are found in primary sensory neurons and activated by minor depolarization of the membrane to initiate action potentials and regulate subthreshold excitability in CNS neurons.185–187 In Fig. 5D(i and ii), both rat and human DRG neurons with spontaneous action potentials showed a significant reduction in spontaneous activity after bath application of the voltage-gated calcium channel 3.2 (Cav3.2) blocker, ML218 hydrochloride (10 µM).174 Like above, action potential properties were analysed in detail for neurons with a positive response to ML218 hydrochloride. The results were the opposite of that when using the Nav1.7 inhibitor. ML218 had no effect on any action potential properties in rat neurons. In contrast, human DRG neurons showed a hyperpolarization of resting membrane potential (from −38.7 ± 0.6 to −43.4 ± 0.9 mV) as seen for the representative example, and decreased after-hyperpolarization amplitude (from 17.7 ± 0.4 to 14.2 ± 0.9 mV). An alternative selective T-type calcium channel blocker, ABT-639, showed preclinical promise in numerous rat188 and mouse189 models. However, it failed to show significant analgesia compared to placebo in a phase 2 trial for diabetic peripheral neuropathic pain.190 This failure was supported by in vivo microneurography recordings, with no significant differences in human nociceptor spontaneous activity following the administration of a single dose versus placebo.191 This suggests that further work is required to identify why in vivo efficacy of T-type calcium channel blockers is limited in neuropathic pain and where their promise lies.
The examples shown here are not isolated. Differences between rat and human have also been shown in the currents evoked by GABAA and nicotinic acetylcholine receptor agonists.79,192 This suggests that specific ion channels have evolved to produce different functional outcomes in rats versus humans, and that targeting an ion channel because of its upregulation or modification in a rat model of pain would not necessarily translate effectively in human trials. Future work that may benefit and, in part, overcome this translation hurdle, is combining these in vitro human DRG recordings with single cell RNA sequencing of the same cell (Patch-Seq). Patch-Seq allows single cell analysis of both functional and molecular read-outs, which could revolutionize the way we identify promising clinical targets and define nociceptor populations.193,194
Electrophysiological characterization of IPSC-derived nociceptors
While in vitro human DRG recordings are ideal when investigating compound efficacy, sourcing human nociceptors is not always possible, especially when studying patients who live with chronic pain. The basic electrophysiological properties of IPSC-derived nociceptors and the nociceptor specific ion channels that they express was discussed earlier. While IPSC-derived nociceptors constitute a powerful model, there may be issues with their maturity. Eberhardt et al.195 observed that differentiated IPSC-derived nociceptors showed a discrete presence of Nav1.5 (a TTX-r Nav that is normally expressed developmentally and then downregulated) in addition to Nav1.8 and Nav1.9. Such TTX-resistant currents showed a more hyperpolarized voltage dependence, in contrast to their counterparts from rodent DRGs, probably due to the different expression of Nav1.5. Voltage-clamp recordings also revealed Nav-mediated inward currents, followed by sustained outward currents, indicating the ready excitability of IPSC-sensory neurons.195 Taken together, these data suggest that IPSC-derived nociceptors, at least around 25 days post differentiation, may exhibit an embryonic-like expression pattern of the TTX-r currents, along with specific excitability characteristics. It is therefore important to account for age and maturity of IPSC-derived nociceptors when used in electrophysiological studies. For this reason, many studies have used IPSC-derived nociceptors at 50–70 days post-differentiation, when their maturity and gene expression changes have plateaued.106,122,130
Acknowledging the caveat of maturity, IPSC-derived nociceptors can successfully recapitulate nociceptor action potentials and thus allow the direct investigation of neuronal excitability. IPSC-derived nociceptors can generate single and tonic action potentials in response to current injections.104–106,108,110,122 This has also been shown for direct fibroblast to nociceptor conversion methods and embryonic stem cell-derived nociceptors.100,119 This is similar to responses observed in human DRG neurons. However, the identification of all four distinct types of action potential waveforms seen in human DRG neurons (above) are lacking in IPSC-derived nociceptors. This is likely due to the limitation that protocols do not give rise to the full range of DRG neuronal subpopulations.
Importantly, IPSC-derived nociceptors, in particular those derived from patients living with chronic pain, exhibit increased incidences of spontaneous activity (as discussed above) which has been valuable for understanding disease mechanisms. In addition, they can also be used in screening pharmacology on an individualized basis, and may potentially be extended into medium throughput screens, given their scalability.103,105,110
In vivo human nociceptor afferent recordings
Microneurography is the only technique in which direct (in vivo) recording of nociceptor axonal electrical activity in humans is possible. The use of microneurography in healthy participants and patients living with chronic pain can identify novel insights into the peripheral basis of nociception and chronic pain.
Microneurography can provide exquisite detail of C-fibre nociceptor activity in vivo, and most of our knowledge of axon conduction properties of human nociceptors is based on the study of cutaneous afferents, as they are readily accessible. However, visceral pain is a large clinical problem and there exists a large body of rodent literature demonstrating differences in somatic versus visceral nociceptors. Therefore, the development of new techniques to study visceral afferents innervating resected human bowel tissue should ultimately aid understanding of human visceral afferents.196
While C-fibre nociceptors are studied using microneurography, Aδ nociceptors remain difficult to study.197 Only recently were a class of human nociceptors with Aβ conduction velocities identified.17 Hence, we have a limited understanding of human A-fibre nociceptor physiology and their role in chronic pain states. As such, the role of A-fibres and A-fibre nociceptors are often studied in rodents. While anaesthetized microneurography and teased-fibre recordings in rats and monkeys has been achieved and offers comparison to human work, it is not widely practiced.19,198–202 In the mouse, the ex vivo skin-nerve preparation is often used to obtain a detailed characterization of mouse primary afferents at the single fibre level,203,204 offering primary afferent characterization following genetic or pharmacological manipulation, alongside comparison with human nociceptors.
C-fibre nociceptor subpopulations identified using microneurography
Human cutaneous C-fibre nociceptors are divided into two major classes on functional grounds, C-mechanosensitive and C-mechanoinsensitive afferents, according to their response (or lack of response) to mechanical, electrical, thermal, and chemical stimuli (Table 3).205–212
Table 3.
Characterization of human C-fibre nociceptors using microneurography
| Nociceptor afferents and subpopulations | Response characteristics | Neurophysiological properties | Receptive fields |
|---|---|---|---|
| C-mechanosensitive (‘polymodal’) |
|
|
|
| C-mechano-heat | Mean temperature response ∼40°C; >45°C linear response between stimulus temperature and firing frequency | ||
| C-mechano-heat-cold | Also respond to cold <20°C | ||
| C-high threshold mechanoreceptors | Only respond to mechanical stimuli; no thermal response | ||
| C-mechanoinsensitive |
|
|
500 mm2, discontinuous patches, irregular in shape, heterogeneous physiological response properties across receptive field |
| C-mechanoinsensitive-heat- insensitive (‘silent’) | Unresponsive to mechanical/thermal stimulus; can be sensitized after algogen (mustard oil/capsaicin) application | ||
| C-mechanoinsensitive-histamine-positive (‘pruriceptors’, C-itch afferents) | Very sensitive to histamine, no response to cowhage |
From human in vivo microneurography studies C-fibre nociceptive afferents comprise two classes. This table summarizes key differences between C-mechanosensitive nociceptors and C-mechanoinsensitive afferent responses to mechanical, thermal, and chemical stimuli. C-mechanosensitive nociceptive afferents are also called type 1A fibres and C-mechanoinsensitive type 1B fibres. ADS = activity-dependent slowing; CV = conduction velocity.
Algogens include mustard oil, capsaicin, bradykinin, acetylcholine, serotonin, prostaglandin E2, and endothelin 1.
In response to monopolar skin surface stimulation.
Most C-mechanosensitive afferents are polymodal (Table 3) and it is thought that they are responsible for signalling mechanically induced pain in uninjured skin. While they are activated by mechanical stimuli below the intensity for evoking painful sensations,213,214 a correlation exists between the increasing force of mechanical stimuli into the noxious range and the perception of mechanical pain.215 These findings suggest that coding of mechanically evoked pain by C-mechanosensitive afferents is intensity dependent.197
Most C-mechanosensitive afferents are termed C-mechano-heat afferents (Table 3)5 and are thought to encode heat pain. Similar to mechanical stimuli, activation temperatures are below the noxious range,207,212,216,217 and suprathreshold encoding appears important in the signalling of heat pain.212,217,218 However, the relationship between firing rate and the sensation of pain is complex, with both temporal and spatial summation playing a role.
A smaller proportion of C-mechanosensitive afferents, termed C-mechano-heat-cold afferents (Table 3), are activated by heat and prolonged stimulation to cold (∼20°C) stimuli.219 It is thought they may mediate the burning sensation felt at extreme hot or cold temperatures.197 Their maximum firing frequency to cold stimuli is lower than for mechanical and heat stimuli.217 This suggests a unique mechanism for the encoding of noxious cold temperatures.
C-mechanoinsensitive afferents are activated by intense mechanical stimulation and the majority respond to heat (Table 3).212 A subset of C-mechanoinsensitive afferents are ‘silent’ nociceptors and only respond after chemical sensitization.5,209,220 However, it is important to recognize that thermal assessment of (unsensitized) silent afferents might be limited by sensory ending depth and the necessity to keep skin temperature <50°C. The observation that capsaicin injections activate most ‘silent’ afferents,75 suggests they might indeed be capable of detecting heat stimuli. Whether or not silent afferents are heat sensitive in humans remains an open question. In monkeys silent afferents make up ∼30% of cutaneous C-fibres,201 in rats they make up ∼15–26% of cutaneous C-fibres,221 and in mice they make up ∼10% of cutaneous C-fibres.222 Particularly in mice, this is lower than that observed in humans (∼24%)5 and an important translational point to consider. Recently, a population of murine silent nociceptors were identified and shown to innervate deeper structures, such as muscle, viscera and joints, but not skin.223 Another subpopulation of nociceptive C-mechanoinsensitive afferents are termed pruriceptors, as they signal itch and are sensitive to histamine.224–226 Taken together, C-mechanoinsensitive afferent discharges are related to pain evoked by tonic pressure227 and to chemically induced sensations.75,225,228 It is likely that C-mechanoinsensitive afferents signal ongoing pain after tissue injury, where they become sensitized after the release of inflammatory mediators. While both C-mechanosensitive and C-mechanoinsensitive afferents are activated by a variety of algogens (pain) and pruritogens (itch), stronger and more sustained responses are elicited from C-mechanoinsensitive afferents.75,224–226,228–231
C-mechanosensitive and C-mechanoinsensitive afferents exhibit distinctive changes during low frequency electrical stimulation (Table 3), and thus this can be used to distinguish the fibres in the absence of peripheral natural stimulation of the skin.209,212,232–234 Using repetitive, low frequency electrical stimulation, C-fibres exhibit the phenomenon termed activity dependent slowing, where C-fibre conduction velocity slowing occurs (C-fibre latency increases). C-mechanoinsensitive afferents exhibit greater activity dependent slowing (Table 3), which is maintained under pathological conditions.202,235–238
C-fibre nociceptor subpopulations play different roles in nociceptive physiology in both health and disease. Microneurography provides valuable in vivo information that can link findings from in vitro studies of human nociceptors. For example, human in vivo findings that the majority of C-fibre nociceptors respond to capsaicin75 parallels the recent molecular profiling that revealed a broad (∼75%) TRPV1 distribution within human DRG56 (Fig. 2). As novel molecular and in vitro electrophysiological profiling of nociceptor subpopulations becomes available, other C-fibre nociceptor subpopulations may be identified and characterized using microneurography.
The clinical relevance of microneurography
Microneurography has identified fundamental mechanisms that govern human nociception. It has also facilitated the direct investigation of nociceptor afferent changes in chronic neuropathic pain states. For instance, C-mechanoinsensitive afferents are sensitized and display increased spontaneous activity under pathological conditions, whereas C-mechanosensitive afferents do not display such marked changes.202,235,236,238–240 An increase in spontaneous activity and mechanical sensitization of C-mechanoinsensitive afferents was related to neuropathic pain in patients with painful polyneuropathy.235 A similar pattern was seen in patients with small fibre neuropathy, fibromyalgia, and IEM,238,239 where there is no evidence of tissue injury or inflammation. The presence of aberrant activity seen in vivo mirrors that seen in vitro when studying human DRG neurons or IPSC-derived nociceptors from patients living with chronic pain. The findings that pharmacological suppression of aberrant activity within nociceptor afferents leads to lower reported pain supports the link between nociceptor afferent activity and chronic pain.105,153 In this regard, microneurography can be used to assess aberrant C-fibre activity in clinical studies using compounds that target peripheral hyperexcitability, especially when guided by efficacy data from in vitro human nociceptor models.
The use of microneurography in the study of participants with rare high impact genetic mutations, such as IEM and CIP,106,239 is clinically relevant. These studies have yielded insights for those disorders, and can also be more broadly applied to common causes of neuropathic pain, such as painful diabetic neuropathy.241 This has been an area of limited study and a number of unanswered questions remain. For instance, what are the pathophysiological mechanisms of human afferent sensitization, how does abnormal afferent activity relate to the intensity and presence of chronic neuropathic pain/sensory profile, and what roles do A-fibres play in chronic neuropathic pain?
Conclusions and future directions
We have reviewed the increasingly diverse means to study human nociceptors with a focus on chronic pain pathophysiology. While we acknowledge that other methods such a laser evoked potentials and quantitative sensory testing can be used to engage the nociceptive system, these techniques do not only measure nociception and nociceptor activity; they often rely on stimulus saliency, and the engagement of the entire nociceptive pathway (for reviews, see Valeriani et al.242 and Treede243). It is clear that there are important differences between human, mouse, and rat nociceptors, both in terms of molecular profile and functional properties. There is more that can be learned about the molecular profiles of nociceptors across species and new technologies enable this important characterization. Rodent models have a critical place in pain research. Rodents still provide the unique opportunity to assess the integrative action of the nociceptive system. Similarly, new imaging methodologies in freely behaving rodents and circuit level approaches provide deep mechanistic insight into nociception and pain. These should be seen as important complementary approaches, but we have to better understand how these models relate to clinical translation in quantitative ways. Assays of human nociceptors (both in vitro and in vivo) provide a critical stepping stone in the translational pathway. These studies can confirm and identify novel pathophysiological mechanisms, as well as target engagement and potential biomarkers in experimental medicine studies. Publicly accessible databases are likely to be a potent research resources for understanding with high fidelity gene and protein expression in human DRG and the distinct nociceptor populations. Ultimately, these need to be expanded to relate such features to genotype and demographic variables, functional properties, and clinical phenotype using transcriptome and proteome wide association studies. We are still in the infancy of understanding sex, ethnicity, lifestyle, and age-related changes in human nociceptor function. Finally, we need to develop better techniques to functionally interrogate the ‘hard to reach’ human nociceptors such as joint and visceral afferents, given that dysfunction in such neurons is responsible for such a great deal of human morbidity.
Funding
T.J.P. acknowledges National Institutes of Health (NIH) NS111929 and NS065926, and the Eugene McDermott Professorship from UT Dallas. P.M.D. acknowledges NIH CA200263 and NS111929, the Thompson Family Foundation Initiative, and the H.E.B. Professorship in Cancer Research. D.L.B. is a senior Wellcome clinical scientist (202747/Z/16/Z) and a member of the Wellcome Pain Consortium (102645). D.L.B. and A.T.C. are members of the DOLORisk Consortium funded by the European Commission Horizon 2020 (ID633491) and the International Diabetic Neuropathy Consortium, funded by the Novo Nordisk Foundation (NNF14SA0006). A.C.T. is supported by Academy of Medical Sciences Starter Grant SGL022\1086. D.L.B. also acknowledges funding support of Diabetes UK (grant ref. 19/0005984). D.L.B. and S.J.M. acknowledge funding from the Medical Research Council (grant ref. MR/T020113/1). A.M.B. is a student in the OXION programme supported by the Wellcome (215145/Z/18/Z) and a GTC MSDTC Scholarship.
Competing interests
D.L.B. has acted as a consultant in the last 2 years for Amgen, Bristows, CODA therapeutic, Lilly, Mundipharma, Orion, Regeneron, and Theranexus on behalf of Oxford University Innovation. He has an MRC Industrial Partnership grant with AstraZeneca funded by the BBSRC. T.J.P. has acted as a consultant in the last 2 years for Grunenthal and Synerkine Pharma and holds a research grant from Merck and Hoba Therapeutics. T.J.P. is cofounder of 4E Therapeutics, PARMedics and Doloromics. P.R.R. is cofounder of Doloromics.
Glossary
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglion
- IEM
inherited erythromelalgia
- IPSC
induced pluripotent stem cell
- TG
trigeminal ganglia
- TTX-r/s
tetrodotoxin-resistant/sensitive
References
- 1. Sherrington CS. The integrative action of the nervous system. C. Scribner's sons; 1906. [Google Scholar]
- 2. Chisholm KI, Khovanov N, Lopes DM, La Russa F, McMahon SB.. Large scale in vivo recording of sensory neuron activity with GCaMP6. eNeuro. 2018;5(1):1–14. ENEURO.0417-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emery EC, Luiz AP, Sikandar S, Magnúsdóttir R, Dong X, Wood JN.. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Sci Adv. 2016;2(11):e1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawson SN, Fang X, Djouhri L.. Nociceptor subtypes and their incidence in rat lumbar dorsal root ganglia (DRGs): Focussing on C-polymodal nociceptors, Abeta-nociceptors, moderate pressure receptors and their receptive field depths. Curr Opin Physiol. 2019;11:125–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H.. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15(1):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D.. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. [DOI] [PubMed] [Google Scholar]
- 7. Ferrari LF, Khomula EV, Araldi D, Levine JD.. Marked sexual dimorphism in the role of the ryanodine receptor in a model of pain chronification in the rat. Sci Rep. 2016;6(1):31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gagliese L, Melzack R.. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev. 2000;24(8):843–854. [DOI] [PubMed] [Google Scholar]
- 9. Hendrich J, Alvarez P, Joseph EK, Ferrari LF, Chen X, Levine JD.. In vivo and in vitro comparison of female and male nociceptors. J Pain. 2012;13(12):1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mecklenburg J, Zou Y, Wangzhou A, et al. Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep. 2020;10(1):15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mogil JS. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. [DOI] [PubMed] [Google Scholar]
- 12. Magerl W, Krumova EK, Baron R, Tölle T, Treede R-D, Maier C.. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605. [DOI] [PubMed] [Google Scholar]
- 13. Dubin AE, Patapoutian A.. Nociceptors: The sensors of the pain pathway. J Clin Invest. 2010;120(11):3760–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harty BL, Monk KR.. Unwrapping the unappreciated: Recent progress in Remak Schwann cell biology. Curr Opin Neurobiol. 2017;47:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdo H, Calvo-Enrique L, Lopez JM, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365(6454):695–699. [DOI] [PubMed] [Google Scholar]
- 16. Djouhri L, Lawson SN.. Aβ-fiber nociceptive primary afferent neurons: A review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev. 2004;46(2):131–145. [DOI] [PubMed] [Google Scholar]
- 17. Nagi SS, Marshall AG, Makdani A, et al. An ultrafast system for signaling mechanical pain in human skin. Sci Adv. 2019;5(7):eaaw1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beissner F, Brandau A, Henke C, et al. Quick discrimination of Adelta and C fiber mediated pain based on three verbal descriptors. PLoS One. 2010;5(9):e12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng YB, Ringkamp M, Campbell JN, Meyer RA.. Electrophysiological assessment of the cutaneous arborization of Adelta-fiber nociceptors. J Neurophysiol. 1999;82(3):1164–1177. [DOI] [PubMed] [Google Scholar]
- 20. Haberberger RV, Barry C, Dominguez N, Matusica D.. Human dorsal root ganglia. Front Cell Neurosci. 2019;13:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rostock C, Schrenk-Siemens K, Pohle J, Siemens J.. Human vs. mouse nociceptors - similarities and differences. Neuroscience. 2018;387:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murinson BB, Griffin JW.. C-fiber structure varies with location in peripheral nerve. J Neuropathol Exp Neurol. 2004;63(3):246–254. [DOI] [PubMed] [Google Scholar]
- 23. McCarthy BG, Hsieh S, Stocks A, et al. Cutaneous innervation in sensory neuropathies: Evaluation by skin biopsy. Neurology. 1995;45(10):1848–1855. [DOI] [PubMed] [Google Scholar]
- 24. Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142(12):3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI.. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care. 2004;27(8):1974–1979. [DOI] [PubMed] [Google Scholar]
- 27. Themistocleous AC, Ramirez JD, Shillo PR, et al. The Pain in Neuropathy Study (PiNS): A cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157(5):1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmid AB, Bland JDP, Bhat MA, Bennett DLH.. The relationship of nerve fibre pathology to sensory function in entrapment neuropathy. Brain. 2014;137(12):3186–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartmannsberger B, Doppler K, Stauber J, et al. Intraepidermal nerve fibre density as biomarker in Charcot-Marie-Tooth disease type 1A. Brain Commun. 2020;2(1):fcaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gröne E, Üçeyler N, Abahji T, et al. Reduced intraepidermal nerve fiber density in patients with chronic ischemic pain in peripheral arterial disease. Pain. 2014;155(9):1784–1792. [DOI] [PubMed] [Google Scholar]
- 31. Rasmussen VF, Karlsson P, Drummond PD, et al. Bilaterally reduced intraepidermal nerve fiber density in unilateral CRPS-I. Pain Med. 2018;19(10):2021–2030. [DOI] [PubMed] [Google Scholar]
- 32. Martinez V, Fletcher D, Martin F, et al. Small fibre impairment predicts neuropathic pain in Guillain–Barré syndrome. Pain. 2010;151(1):53–60. [DOI] [PubMed] [Google Scholar]
- 33. Üçeyler N, He L, Schönfeld D, et al. Small fibers in Fabry disease: Baseline and follow-up data under enzyme replacement therapy. J Peripheral Nerv Syst. 2011;16(4):304–314. [DOI] [PubMed] [Google Scholar]
- 34. Sommer C, Lauria G.. Skin biopsy in the management of peripheral neuropathy. Lancet Neurol. 2007;6(7):632–642. [DOI] [PubMed] [Google Scholar]
- 35. Hedstrom KL, Murtie JC, Albers K, Calcutt NA, Corfas G.. Treating small fiber neuropathy by topical application of a small molecule modulator of ligand-induced GFRα/RET receptor signaling. Proc Natl Acad Sci USA. 2014;111(6):2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebenezer GJ, McArthur JC, Thomas D, et al. Denervation of skin in neuropathies: The sequence of axonal and Schwann cell changes in skin biopsies. Brain. 2007;130(Pt 10):2703–2714. [DOI] [PubMed] [Google Scholar]
- 37. Lauria G, Morbin M, Lombardi R, et al. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 2003;61(5):631–636. [DOI] [PubMed] [Google Scholar]
- 38. Bursova S, Dubovy P, Vlckova-Moravcova E, et al. Expression of growth-associated protein 43 in the skin nerve fibers of patients with type 2 diabetes mellitus. J Neurol Sci. 2012;315(1-2):60–63. [DOI] [PubMed] [Google Scholar]
- 39. Evdokimov D, Frank J, Klitsch A, et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol. 2019;86(4):504–516. [DOI] [PubMed] [Google Scholar]
- 40. von Bischhoffshausen S, Ivulic D, Alvarez P, et al. Recessive dystrophic epidermolysis bullosa results in painful small fibre neuropathy. Brain. 2017;140(5):1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brines M, Culver DA, Ferdousi M, et al. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci Rep. 2018;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bucher F, Schneider C, Blau T, et al. Small-fiber neuropathy is associated with corneal nerve and dendritic cell alterations. Cornea. 2015;34(9):1114–1119. [DOI] [PubMed] [Google Scholar]
- 43. Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: A non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–688. [DOI] [PubMed] [Google Scholar]
- 44. Malik RA, Veves A, Tesfaye S, et al. Small fibre neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev. 2011;27(7):678–684. [DOI] [PubMed] [Google Scholar]
- 45. Tavakoli M, Marshall A, Banka S, et al. Corneal confocal microscopy detects small-fiber neuropathy in Charcot-Marie-Tooth disease type 1A patients. Muscle Nerve. 2012;46(5):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramírez M, Martínez-Martínez LA, Hernández-Quintela E, Velazco-Casapía J, Vargas A, Martínez-Lavín M.. Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Sem Arthritis Rheum. 2015;45(2):214–219. [DOI] [PubMed] [Google Scholar]
- 47. Basbaum AI, Bautista DM, Scherrer G, Julius D.. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–153. [DOI] [PubMed] [Google Scholar]
- 49. Zeisel A, Hochgerner H, Lonnerberg P, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174(4):999–1014.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD.. The emergence of transcriptional identity in somatosensory neurons. Nature. 2020;577(7790):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Price TJ, Flores CM.. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8(3):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor-Blake B, Zylka MJ.. Prostatic acid phosphatase is expressed in peptidergic and nonpeptidergic nociceptive neurons of mice and rats. PLoS One. 2010;5(1):e8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pawlowski SA, Gaillard S, Ghorayeb I, Ribeiro-da-Silva A, Schlichter R, Cordero-Erausquin M.. A novel population of cholinergic neurons in the macaque spinal dorsal horn of potential clinical relevance for pain therapy. J Neurosci. 2013;33(9):3727–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moy JK, Hartung JE, Duque MG, et al. Distribution of functional opioid receptors in human dorsal root ganglion neurons. Pain. 2020;161(7):1636–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ray P, Torck A, Quigley L, et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research. Pain. 2018;159(7):1325–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shiers S, Klein RM, Price TJ.. Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain. 2020a;161(10):2410–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cavanaugh DJ, Lee H, Lo L, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106(22):9075–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo A, Simone DA, Stone LS, Fairbanks CA, Wang J, Elde R.. Developmental shift of vanilloid receptor 1 (VR1) terminals into deeper regions of the superficial dorsal horn: Correlation with a shift from TrkA to Ret expression by dorsal root ganglion neurons. Eur J Neurosci. 2001;14(2):293–304. [DOI] [PubMed] [Google Scholar]
- 59. Guo A, Vulchanova L, Wang J, Li X, Elde R.. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11(3):946–958. [DOI] [PubMed] [Google Scholar]
- 60. Zylka MJ, Dong X, Southwell AL, Anderson DJ.. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100(17):10043–10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barabas ME, Kossyreva EA, Stucky CL.. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7(10):e47988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abrahamsen B, Zhao J, Asante CO, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321(5889):702–705. [DOI] [PubMed] [Google Scholar]
- 63. Le Pichon CE, Chesler AT.. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat. 2014;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shiers SI, Sankaranarayanan I, Jeevakumar V, Cervantes A, Reese JC, Price TJ.. Convergence of peptidergic and non-peptidergic protein markers in the human dorsal root ganglion and spinal dorsal horn . J Comp Neurol. 2020;2021:25122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kupari J, Usoskin D, Parisien M, et al. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain.. Nat Commun. 2021;12(1):1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tran PV, Johns ME, McAdams B, Abrahante JE, Simone DA, Banik RK.. Global transcriptome analysis of rat dorsal root ganglia to identify molecular pathways involved in incisional pain. Mol Pain. 2020;16:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yin C, Hu Q, Liu B, et al. Transcriptome profiling of dorsal root ganglia in a rat model of complex regional pain syndrome type-I reveals potential mechanisms involved in pain. J Pain Res. 2019;12:1201–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ.. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78(1):138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang T, Lin SH, Malewicz NM, et al. Identifying the pathways required for coping behaviours associated with sustained pain. Nature. 2019;565(7737):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Braz J, Solorzano C, Wang X, Basbaum AI.. Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82(3):522–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nahin RL, Humphrey E, Hylden JL.. Evidence for calcitonin gene-related peptide contacts on a population of lamina I projection neurons. J Chem Neuroanat. 1991;4(2):123–129. [DOI] [PubMed] [Google Scholar]
- 72. Wang H, Zylka MJ.. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29(42):13202–13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harmann PA, Chung K, Briner RP, Westlund KN, Carlton SM.. Calcitonin gene-related peptide (CGRP) in the human spinal cord: A light and electron microscopic analysis. J Comp Neurol. 1988;269(3):371–380. [DOI] [PubMed] [Google Scholar]
- 74. Jakab G, Salamon I, Petrusz P, Rethelyi M.. Termination patterns of calcitonin gene-related peptide-immunoreactive nerve fibers in the dorsal horn of the human spinal cord. Exp Brain Res. 1990;80(3):609–617. [DOI] [PubMed] [Google Scholar]
- 75. Schmelz M, Schmid R, Handwerker HO, Torebjork HE.. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123(3):560–571. [DOI] [PubMed] [Google Scholar]
- 76. Tarpley JW, Kohler MG, Martin WJ.. The behavioral and neuroanatomical effects of IB4-saporin treatment in rat models of nociceptive and neuropathic pain. Brain Res. 2004;1029(1):65–76. [DOI] [PubMed] [Google Scholar]
- 77. Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN.. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108(1):143–155. [DOI] [PubMed] [Google Scholar]
- 78. Scherrer G, Imamachi N, Cao YQ, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137(6):1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang X, Hartung JE, Friedman RL, Koerber HR, Belfer I, Gold MS.. Nicotine evoked currents in human primary sensory neurons. J Pain. 2019;20(7):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davidson S, Golden JP, Copits BA, et al. Group II mGluRs suppress hyperexcitability in mouse and human nociceptors. Pain. 2016;157(9):2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bhave G, Karim F, Carlton SM, Gereau RWt.. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4(4):417–423. [DOI] [PubMed] [Google Scholar]
- 82. Moehring F, Halder P, Seal RP, Stucky CL.. Uncovering the cells and circuits of touch in normal and pathological settings. Neuron. 2018;100(2):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stark R, Grzelak M, Hadfield J.. RNA sequencing: The teenage years. Nat Rev Genet. 2019;20(11):631–656. [DOI] [PubMed] [Google Scholar]
- 84. Mele M, Ferreira PG, Reverter F, et al. Human genomics. The human transcriptome across tissues and individuals. Science. 2015;348(6235):660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Akopian AN, Sivilotti L, Wood JN.. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379(6562):257–262. [DOI] [PubMed] [Google Scholar]
- 86. Chen YC, Auer-Grumbach M, Matsukawa S, et al. Transcriptional regulator PRDM12 is essential for human pain perception. Nat Genet. 2015;47(7):803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nagy V, Cole T, Van Campenhout C, et al. The evolutionarily conserved transcription factor PRDM12 controls sensory neuron development and pain perception. Cell Cycle. 2015;14(12):1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wangzhou A, McIlvried LA, Paige C, et al. Pharmacological target-focused transcriptomic analysis of native versus cultured human and mouse dorsal root ganglia. Pain. 2020;161(7):1497–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mishra SK, Hoon MA.. The cells and circuitry for itch responses in mice. Science. 2013;340(6135):968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hassler SN, Kume M, Mwirigi J, et al. The cellular basis of protease activated receptor type 2 (PAR2) evoked mechanical and affective pain. JCI Insight. 2020;5(11):e137393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Romero HK, Christensen SB, Di Cesare Mannelli L, et al. Inhibition of alpha9alpha10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc Natl Acad Sci U S A. 2017;114(10):E1825–E1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. North RY, Li Y, Ray P, et al. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain. 2019;142(5):1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mapplebeck JCS, Dalgarno R, Tu Y, et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018;159(9):1752–1763. [DOI] [PubMed] [Google Scholar]
- 95. Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Coppi E, Cherchi F, Fusco I, et al. Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons. Pain. 2019;160(5):1103–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wahlman C, Doyle TM, Little JW, et al. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain. 2018;159(6):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Langeslag M, Constantin CE, Andratsch M, Quarta S, Mair N, Kress M.. Oncostatin M induces heat hypersensitivity by gp130-dependent sensitization of TRPV1 in sensory neurons. Mol Pain. 2011;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Taga T. Gp130, a shared signal transducing receptor component for hematopoietic and neuropoietic cytokines. J Neurochem. 2002;67(1):1–10. [DOI] [PubMed] [Google Scholar]
- 100. Wainger BJ, Buttermore ED, Oliveira JT, et al. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat Neurosci. 2015;18(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Blanchard JW, Eade KT, Szűcs A, et al. Selective conversion of fibroblasts into peripheral sensory neurons. Nat Neurosci. 2015;18(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chambers SM, Qi Y, Mica Y, et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30(7):715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pettingill P, Weir GA, Wei T, et al. A causal role for TRESK loss of function in migraine mechanisms. Brain. 2019;142(12):3852–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Meents JE, Bressan E, Sontag S, et al. The role of Nav1.7 in human nociceptors: Insights from human induced pluripotent stem cell-derived sensory neurons of erythromelalgia patients. Pain. 2019;160(6):1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Namer B, Schmidt D, Eberhardt E, et al. Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors. EBioMedicine. 2019;39:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. McDermott LA, Weir GA, Themistocleous AC, et al. Defining the functional role of NaV1.7 in human nociception. Neuron. 2019;101(5):905–919.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee KS, Zhou W, Scott-McKean JJ, et al. Human sensory neurons derived from induced pluripotent stem cells support varicella-zoster virus infection. PLoS One. 2012;7(12):e53010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cao L, McDonnell A, Nitzsche A, et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med. 2016;8(335):335ra56. [DOI] [PubMed] [Google Scholar]
- 109. Cai S, Han L, Ao Q, Chan YS, Shum DK.. Human induced pluripotent cell-derived sensory neurons for fate commitment of bone marrow-derived Schwann cells: Implications for remyelination therapy. Stem Cells Transl Med. 2017;6(2):369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mis MA, Yang Y, Tanaka BS, et al. Resilience to pain: A peripheral component identified using induced pluripotent stem cells and dynamic clamp. J Neurosci. 2019;39(3):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Guimarães MZP, De VR, Vitória G, et al. Generation of iPSC-derived human peripheral sensory neurons releasing substance P elicited by TRPV1 agonists. Front Mol Neurosci. 2018;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dionisi C, Rai M, Chazalon M, Schiffmann SN, Pandolfo M.. Primary proprioceptive neurons from human induced pluripotent stem cells: A cell model for afferent ataxias. Sci Rep. 2020;10(1):7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zimmer B, Ewaleifoh O, Harschnitz O, et al. Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection. Proc Natl Acad Sci U S A. 2018;115(37):E8775–E8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schrenk-Siemens K, Wende H, Prato V, et al. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat Neurosci. 2015;18(1):10–16. [DOI] [PubMed] [Google Scholar]
- 115. Nickolls AR, Lee MM, Espinoza DF, et al. Transcriptional programming of human mechanosensory neuron subtypes from pluripotent stem cells. Cell Rep. 2020;30(3):932–946 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Umehara Y, Toyama S, Tominaga M, et al. Robust induction of neural crest cells to derive peripheral sensory neurons from human induced pluripotent stem cells. Sci Rep. 2020;10(1):4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wilson R, Ahmmed AA, Poll A, Sakaue M, Laude A, Sieber-Blum M.. Human peptidergic nociceptive sensory neurons generated from human epidermal neural crest stem cells (hEPI-NCSC). PLoS One. 2018;13(6):e0199996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vojnits K, Mahammad S, Collins TJ, Bhatia M.. Chemotherapy-induced neuropathy and drug discovery platform using human sensory neurons converted directly from adult peripheral blood. Stem Cells Transl Med. 2019;8(11):1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jones I, Yelhekar TD, Wiberg R, et al. Development and validation of an in vitro model system to study peripheral sensory neuron development and injury. Sci Rep. 2018;8(1):15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Alshawaf AJ, Viventi S, Qiu W, et al. Phenotypic and functional characterization of peripheral sensory neurons derived from human embryonic stem cells. Sci Rep. 2018;8(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Clark AJ, Kaller MS, Galino J, Willison HJ, Rinaldi S, Bennett DLH.. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain. 2017;140(4):898–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Weir GA, Middleton SJ, Clark AJ, et al. Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source. Brain. 2017;140(10):2570–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 124. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. [DOI] [PubMed] [Google Scholar]
- 125. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 126. Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. [DOI] [PubMed] [Google Scholar]
- 127. Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L.. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Denham M, Hasegawa K, Menheniott T, et al. Multipotent caudal neural progenitors derived from human pluripotent stem cells that give rise to lineages of the central and peripheral nervous system. Stem Cells. 2015;33(6):1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Young GT, Gutteridge A, Fox HDE, et al. Characterizing human stem cell-derived sensory neurons at the single-cell level reveals their ion channel expression and utility in pain research. Mol Ther. 2014;22(8):1530–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Du X, Gao H, Jaffe D, Zhang H, Gamper N.. M-type K(+) channels in peripheral nociceptive pathways. Br J Pharmacol. 2018;175(12):2158–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wemmie JA, Taugher RJ, Kreple CJ.. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14(7):461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dini L, Del Lungo M, Resta F, et al. Selective blockade of HCN1/HCN2 channels as a potential pharmacological strategy against pain. Front Pharmacol. 2018;9:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lopes DM, Denk F, McMahon SB.. The molecular fingerprint of dorsal root and trigeminal ganglion neurons. Front Mol Neurosci. 2017;10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dhandapani R, Arokiaraj CM, Taberner FJ, et al. Control of mechanical pain hypersensitivity in mice through ligand-targeted photoablation of TrkB-positive sensory neurons. Nat Commun. 2018;9(1):1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Xu ZZ, Kim YH, Bang S, et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med. 2015;21(11):1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD.. The role of voltage-gated sodium channels in pain signaling. Physiol Rev. 2019;99(2):1079–1151. [DOI] [PubMed] [Google Scholar]
- 138. Alsaloum M, Higerd GP, Effraim PR, Waxman SG.. Status of peripheral sodium channel blockers for non-addictive pain treatment. Nat Rev Neurol. 2020;16(12):689–705. [DOI] [PubMed] [Google Scholar]
- 139. Zakrzewska JM, Palmer J, Morisset V, Giblin GM, et al. Safety and efficacy of a Nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: A double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol. 2017;16(4):291–300. [DOI] [PubMed] [Google Scholar]
- 140. Schwab AJ, Ebert AD.. Neurite aggregation and calcium dysfunction in iPSC-derived sensory neurons with Parkinson's disease-related LRRK2 G2019S mutation. Stem Cell Rep. 2015;5(6):1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Alexandrou AJ, Brown AR, Chapman ML, et al. Subtype-selective small molecule inhibitors reveal a fundamental role for Nav1.7 in nociceptor electrogenesis, axonal conduction and presynaptic release. PLoS One. 2016;11(4):e0152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Anguela XM, High KA.. Entering the modern era of gene therapy. Annu Rev Med. 2019;70(1):273–288. [DOI] [PubMed] [Google Scholar]
- 144. Iyer SM, Vesuna S, Ramakrishnan C, et al. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep. 2016;6(1):30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Saloman JL, Scheff NN, Snyder LM, Ross SE, Davis BM, Gold MS.. Gi-DREADD expression in peripheral nerves produces ligand-dependent analgesia, as well as ligand-independent functional changes in sensory neurons. J Neurosci. 2016;36(42):10769–10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Elitt MS, Barbar L, Tesar PJ.. Drug screening for human genetic diseases using iPSC models. Hum Mol Genet. 2018;27(R2):R89–R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Farkhondeh A, Li R, Gorshkov K, et al. Induced pluripotent stem cells for neural drug discovery. Drug Discov Today. 2019;24(4):992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hoelting L, Klima S, Karreman C, et al. Stem cell-derived immature human dorsal root ganglia neurons to identify peripheral neurotoxicants. Stem Cells Transl Med. 2016;5(4):476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Krug AK, Balmer NV, Matt F, Schönenberger F, Merhof D, Leist M.. Evaluation of a human neurite growth assay as specific screen for developmental neurotoxicants. Arch Toxicol. 2013;87(12):2215–2231. [DOI] [PubMed] [Google Scholar]
- 150. Stacey P, Wassermann AM, Kammonen L, Impey E, Wilbrey A, Cawkill D.. Plate-based phenotypic screening for pain using human iPSC-derived sensory neurons. SLAS Discov. 2018;23(6):585–596. [DOI] [PubMed] [Google Scholar]
- 151. McDonnell A, Collins S, Ali Z, et al. Efficacy of the Nav1.7 blocker PF-05089771 in a randomised, placebo-controlled, double-blind clinical study in subjects with painful diabetic peripheral neuropathy. Pain. 2018;159(8):1465–1476. [DOI] [PubMed] [Google Scholar]
- 152. Baron R, Hans G, Dickenson AH.. Peripheral input and its importance for central sensitization. Ann Neurol. 2013;74(5):630–636. [DOI] [PubMed] [Google Scholar]
- 153. Haroutounian S, Nikolajsen L, Bendtsen TF, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain. 2014;155(7):1272–1279. [DOI] [PubMed] [Google Scholar]
- 154. Pitcher GM, Henry JL.. Governing role of primary afferent drive in increased excitation of spinal nociceptive neurons in a model of sciatic neuropathy. Exp Neurol. 2008;214(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN.. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26(4):1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ma C, LaMotte RH.. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27(51):14059–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Study RE, Kral MG.. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65(2-3):235–242. [DOI] [PubMed] [Google Scholar]
- 158. Fan N, Sikand P, Donnelly DF, Ma C, Lamotte RH.. Increased Na+ and K+ currents in small mouse dorsal root ganglion neurons after ganglion compression. J Neurophysiol. 2011;106(1):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gabay E, Wolf G, Shavit Y, Yirmiya R, Tal M.. Chronic blockade of interleukin-1 (IL-1) prevents and attenuates neuropathic pain behavior and spontaneous ectopic neuronal activity following nerve injury. Eur J Pain. 2011;15(3):242–248. [DOI] [PubMed] [Google Scholar]
- 160. Liu CN, Devor M, Waxman SG, Kocsis JD.. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J Neurophysiol. 2002;87(4):2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Xie RG, Zheng DW, Xing JL, et al. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS One. 2011;6(4):e18681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Odem MA, Bavencoffe AG, Cassidy RM, et al. Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain. Pain. 2018;159(11):2347–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M.. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110(1-2):290–298. [DOI] [PubMed] [Google Scholar]
- 164. Ali Z, Ringkamp M, Hartke TV, et al. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81(2):455–466. [DOI] [PubMed] [Google Scholar]
- 165. Waxman SG, Zamponi GW.. Regulating excitability of peripheral afferents: Emerging ion channel targets. Nat Neurosci. 2014;17(2):153–163. [DOI] [PubMed] [Google Scholar]
- 166. Chang W, Berta T, Kim YH, Lee S, Lee SY, Ji RR.. Expression and role of voltage-gated sodium channels in human dorsal root ganglion neurons with special focus on Nav1.7, species differences, and regulation by paclitaxel. Neurosci Bull. 2018;34(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhang X, Priest BT, Belfer I, Gold MS.. Voltage-gated Na+ currents in human dorsal root ganglion neurons. eLife. 2017;6:e23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Han C, Estacion M, Huang J, et al. Human Na(v)1.8: Enhanced persistent and ramp currents contribute to distinct firing properties of human DRG neurons. J Neurophysiol. 2015;113(9):3172–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Klein AH, Vyshnevska A, Hartke TV, De Col R, et al. Sodium channel Nav1.8 underlies TTX-resistant axonal action potential conduction in somatosensory C-fibers of distal cutaneous nerves. J Neurosci. 2017;37(20):5204–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Black JA, Frezel N, Dib-Hajj SD, Waxman SG.. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain. 2012;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rice FL, Albrecht PJ, Wymer JP, et al. Sodium channel Nav1.7 in vascular myocytes, endothelium, and innervating axons in human skin. Mol Pain. 2015;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Vicario N, Turnaturi R, Spitale FM, et al. Intercellular communication and ion channels in neuropathic pain chronicization. Inflamm Res. 2020;69(9):841–850. [DOI] [PubMed] [Google Scholar]
- 173. Li Y, Adamek P, Zhang H, et al. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci. 2015;35(39):13487–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Li Y, Tatsui CE, Rhines LD, et al. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain. 2017;158(3):417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Li Y, North RY, Rhines LD, et al. DRG voltage-gated sodium channel 1.7 is upregulated in paclitaxel-induced neuropathy in rats and in humans with neuropathic pain. J Neurosci. 2018;38(5):1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chattopadhyay M, Mata M, Fink DJ.. Continuous delta-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J Neurosci. 2008;28(26):6652–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hong S, Morrow TJ, Paulson PE, Isom LL, Wiley JW.. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J Biol Chem. 2004;279(28):29341–29350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sekiguchi F, Kawabata A.. T-type calcium channels: functional regulation and implication in pain signaling. J Pharmacol Sci. 2013;122(4):244–250. [DOI] [PubMed] [Google Scholar]
- 179. Szallasi A, Blumberg PM.. Vanilloid receptors: New insights enhance potential as a therapeutic target. Pain. 1996;68(2-3):195–208. [DOI] [PubMed] [Google Scholar]
- 180. Petruska JC, Napaporn J, Johnson RD, Cooper BY.. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neuroscience. 2002;115(1):15–30. [DOI] [PubMed] [Google Scholar]
- 181. Davidson S, Copits BA, Zhang J, Page G, Ghetti A, Gereau RW.. Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain. 2014;155(9):1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Amir R, Michaelis M, Devor M.. Burst discharge in primary sensory neurons: Triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J Neurosci. 2002;22(3):1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yu YQ, Chen XF, Yang Y, Yang F, Chen J.. Electrophysiological identification of tonic and phasic neurons in sensory dorsal root ganglion and their distinct implications in inflammatory pain. Physiol Res. 2014;63(6):793–799. [DOI] [PubMed] [Google Scholar]
- 184. Sculptoreanu A, de Groat WC.. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp Neurol. 2007;205(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58(1):329–348. [DOI] [PubMed] [Google Scholar]
- 186. Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–161. [DOI] [PubMed] [Google Scholar]
- 187. Todorovic SM, Jevtovic-Todorovic V.. The role of T-type calcium channels in peripheral and central pain processing. CNS Neurol Disord Drug Targets. 2006;5(6):639–653. [DOI] [PubMed] [Google Scholar]
- 188. Jarvis MF, Scott VE, McGaraughty S, et al. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Biochem Pharmacol. 2014;89(4):536–544. [DOI] [PubMed] [Google Scholar]
- 189. Picard E, Carvalho FA, Agosti F, et al. Inhibition of Cav 3.2 calcium channels: A new target for colonic hypersensitivity associated with low-grade inflammation. Br J Pharmacol. 2019;176(7):950–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ziegler D, Duan WR, An G, Thomas JW, Nothaft W.. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain. 2015;156(10):2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Serra J, Duan WR, Locke C, Solà R, Liu W, Nothaft W.. Effects of a T-type calcium channel blocker, ABT-639, on spontaneous activity in C-nociceptors in patients with painful diabetic neuropathy: A randomized controlled trial. Pain. 2015;156(11):2175–2183. [DOI] [PubMed] [Google Scholar]
- 192. Zhang X-L, Lee K-Y, Priest BT, Belfer I, Gold MS.. Inflammatory mediator-induced modulation of GABAA currents in human sensory neurons. Neuroscience. 2015;310:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bardy C, Van Den Hurk M, Kakaradov B, et al. Predicting the functional states of human iPSC-derived neurons with single-cell RNA-seq and electrophysiology. Mol Psychiatry. 2016;21(11):1573–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Cadwell CR, Palasantza A, Jiang X, et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol. 2016;34(2):199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Eberhardt E, Havlicek S, Schmidt D, et al. Pattern of functional TTX-resistant sodium channels reveals a developmental stage of human iPSC- and ESC-derived nociceptors. Stem Cell Rep. 2015;5(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. McGuire C, Boundouki G, Hockley JRF, et al. Ex vivo study of human visceral nociceptors. Gut. 2018;67(1):86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ackerley R, Watkins RH.. Microneurography as a tool to study the function of individual C-fiber afferents in humans: Responses from nociceptors, thermoreceptors, and mechanoreceptors. J Neurophysiol. 2018;120(6):2834–2846. [DOI] [PubMed] [Google Scholar]
- 198. Campbell JN, Meyer RA.. Sensitization of unmyelinated nociceptive afferents in monkey varies with skin type. J Neurophysiol. 1983;49(1):98–110. [DOI] [PubMed] [Google Scholar]
- 199. LaMotte RH, Torebjork HE, Robinson CJ, Thalhammer JG.. Time-intensity profiles of cutaneous pain in normal and hyperalgesic skin: A comparison with C-fiber nociceptor activities in monkey and human. J Neurophysiol. 1984;51(6):1434–1450. [DOI] [PubMed] [Google Scholar]
- 200. Leem JW, Willis WD, Chung JM.. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69(5):1684–1699. [DOI] [PubMed] [Google Scholar]
- 201. Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN.. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res. 1991;561(2):252–261. [DOI] [PubMed] [Google Scholar]
- 202. Serra J, Bostock H, Solà R, et al. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153(1):42–55. [DOI] [PubMed] [Google Scholar]
- 203. Koltzenburg M, Stucky CL, Lewin GR.. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78(4):1841–1850. [DOI] [PubMed] [Google Scholar]
- 204. Zimmermann K, Hein A, Hager U, et al. Phenotyping sensory nerve endings in vitro in the mouse. Nat Protoc. 2009;4(2):174–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol. 1990;426(1):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Schmelz M, Schmidt R, Ringkamp M, Handwerker HO, Torebjörk HE.. Sensitization of insensitive branches of C nociceptors in human skin. J Physiol. 1994;480(2):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Schmidt R, Schmelz M, Ringkamp M, Handwerker HO, Torebjörk HE.. Innervation territories of mechanically activated C nociceptor units in human skin. J Neurophysiol. 1997;78(5):2641–2648. [DOI] [PubMed] [Google Scholar]
- 208. Schmidt R, Schmelz M, Weidner C, Handwerker HO, Torebjörk HE.. Innervation territories of mechano-insensitive C nociceptors in human skin. J Neurophysiol. 2002;88(4):1859–1866. [DOI] [PubMed] [Google Scholar]
- 209. Serra J, Campero M, Bostock H, Ochoa J.. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol. 2004;91(6):2770–2781. [DOI] [PubMed] [Google Scholar]
- 210. Torebjörk HE. Afferent G units responding to mechanical, thermal and chemical stimuli in human non-glabrous skin. Acta Physiol Scand. 1974;92(3):374–390. [DOI] [PubMed] [Google Scholar]
- 211. Torebjörk HE, Hallin RG.. Identification of afferent C units in intact human skin nerves. Brain Res. 1974;67(3):387–403. [DOI] [PubMed] [Google Scholar]
- 212. Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjörk HE.. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19(22):10184–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Van Hees J, Gybels JM.. Pain related to single afferent C fibers from human skin. Brain Res. 1972;48:397–400. [DOI] [PubMed] [Google Scholar]
- 214. Watkins RH, Wessberg J, Backlund Wasling H, et al. Optimal delineation of single C-tactile and C-nociceptive afferents in humans by latency slowing. J Neurophysiol. 2017;117(4):1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Koltzenburg M, Handwerker H.. Differential ability of human cutaneous nociceptors to signal mechanical pain and to produce vasodilatation. J Neurosci. 1994;14(3 Pt 2):1756–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Gybels J, Handwerker HO, Van Hees J.. A comparison between the discharges of human nociceptive nerve fibres and the subject's ratings of his sensations. J Physiol. 1979;292(1):193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yarnitsky D, Simone DA, Dotson RM, Cline MA, Ochoa JL.. Single C nociceptor responses and psychophysical parameters of evoked pain: Effect of rate of rise of heat stimuli in humans. J Physiol. 1992;450(1):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Hallin RG, Torebjörk HE, Wiesenfeld Z.. Nociceptors and warm receptors innervated by C fibres in human skin. J Neurol Neurosurg Psychiatry. 1982;45(4):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Campero M, Serra J, Ochoa JL.. C-polymodal nociceptors activated by noxious low temperature in human skin. J Physiol. 1996;497(2):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Schmelz M, Schmidt R, Ringkamp M, Forster C, Handwerker HO, TorebjöRk HE.. Limitation of sensitization to injured parts of receptive fields in human skin C-nociceptors. Exper Brain Res. 1996;109(1):141–147. [DOI] [PubMed] [Google Scholar]
- 221. Kress M, Koltzenburg M, Reeh PW, Handwerker HO.. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68(2):581–595. [DOI] [PubMed] [Google Scholar]
- 222. Wetzel C, Hu J, Riethmacher D, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445(7124):206–209. [DOI] [PubMed] [Google Scholar]
- 223. Prato V, Taberner FJ, Hockley JRF, et al. Functional and molecular characterization of mechanoinsensitive “Silent”. Nociceptors. Cell Rep. 2017;21(11):3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Handwerker HO, Forster C, Kirchhoff C.. Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol. 1991;66(1):307–315. [DOI] [PubMed] [Google Scholar]
- 225. Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE.. Specific C-receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO.. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89(5):2441–2448. [DOI] [PubMed] [Google Scholar]
- 227. Schmidt R, Schmelz M, Torebjörk HE, Handwerker HO.. Mechano-insensitive nociceptors encode pain evoked by tonic pressure to human skin. Neuroscience. 2000;98(4):793–800. [DOI] [PubMed] [Google Scholar]
- 228. Namer B, Schick M, Kleggetveit IP, et al. Differential sensitization of silent nociceptors to low pH stimulation by prostaglandin E2 in human volunteers. Eur J Pain. 2015;19(2):159–166. [DOI] [PubMed] [Google Scholar]
- 229. Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M.. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100(4):2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Namer B, Hilliges M, Ørstavik K, et al. Endothelin1 activates and sensitizes human C-nociceptors. Pain. 2008;137(1):41–49. [DOI] [PubMed] [Google Scholar]
- 231. Olausson B. Recordings of human polymodal single C-fiber afferents following mechanical and argon-laser heat stimulation of inflamed skin. Exper Brain Res. 1998;122(1):55–61. [DOI] [PubMed] [Google Scholar]
- 232. Namer B, Barta B, Ørstavik K, et al. Microneurographic assessment of C-fibre function in aged healthy subjects. J Physiol. 2009;587(2):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Obreja O, Ringkamp M, Namer B, et al. Patterns of activity-dependent conduction velocity changes differentiate classes of unmyelinated mechano-insensitive afferents including cold nociceptors, in pig and in human. Pain. 2010;148(1):59–69. [DOI] [PubMed] [Google Scholar]
- 234. Serra J, Campero M, Ochoa J, Bostock H.. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. 1999;515(3):799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Kleggetveit IP, Namer B, Schmidt R, et al. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 2012;153(10):2040–2047. [DOI] [PubMed] [Google Scholar]
- 236. Ørstavik K, Weidner C, Schmidt R, et al. Pathological C‐fibres in patients with a chronic painful condition. Brain. 2003;126(Pt 3):567–578. [DOI] [PubMed] [Google Scholar]
- 237. Schmidt R, Kleggetveit IP, Namer B, et al. Double spikes to single electrical stimulation correlates to spontaneous activity of nociceptors in painful neuropathy patients. Pain. 2012;153(2):391–398. [DOI] [PubMed] [Google Scholar]
- 238. Serra J, Collado A, Solà R, et al. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol. 2014;75(2):196–208. [DOI] [PubMed] [Google Scholar]
- 239. Namer B, Ørstavik K, Schmidt R, et al. Specific changes in conduction velocity recovery cycles of single nociceptors in a patient with erythromelalgia with the I848T gain-of-function mutation of Nav1.7. Pain. 2015;156(9):1637–1646. [DOI] [PubMed] [Google Scholar]
- 240. Ørstavik K, Namer B, Schmidt R, et al. Abnormal function of C-fibers in patients with diabetic neuropathy. J Neurosci. 2006;26(44):11287–11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Blesneac I, Themistocleous AC, Fratter C, et al. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain. 2018;159(3):469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Valeriani M, Pazzaglia C, Cruccu G, Truini A.. Clinical usefulness of laser evoked potentials. Neurophysiol Clin. 2012;42(5):345–353. [DOI] [PubMed] [Google Scholar]
- 243. Treede RD. The role of quantitative sensory testing in the prediction of chronic pain. Pain. 2019;160(1):S66–S69. [DOI] [PubMed] [Google Scholar]