Abstract
Background
Pheochromocytoma and paraganglioma (PPGL) have currently only limited treatment options available for patients in the metastatic phase (mPPGL) in either post-surgery or inoperable settings. However, these rare tumors overexpress somatostatin receptors and can thus be treated with peptide receptor radionuclide therapy (PRRT). We present data about our 10-year experience treating 46 consecutive mPPGL patients with 90Y-DOTATOC or 177Lu-DOTATATE.
Patients and methods
All patients (20 men and 26 women, median age 52 years) showed positive scintigraphic imaging at 111In-octreotide or 68Ga-DOTATOC positron emission tomography/computed tomography (PET/CT). 90Y-DOTATOC was administered in 12 patients, with cumulative dosages ranging from 7.4 to 11 GBq, while 34 patients received 18.5 or 27.5GBq of 177Lu-DOTATATE. We used Southwest Oncology Group Response Evaluation Criteria in Solid Tumors criteria to evaluate treatment efficacy and Common Terminology Criteria for Adverse Events criteria to assess toxicity. The prognostic role of primary tumor site, hormone secretion, succinate dehydrogenase (SDHx) mutation, and metastatic involvement was also evaluated.
Results
Both 90Y-DOTATOC and 177Lu-DOTATATE PRRT were well tolerated by patients without significant renal or bone marrow toxicity. The median follow-up was 73 months (range 5-146 months). The overall disease control rate (DCR) was 80% [95% confidence interval (CI) 68.9% to 91.9%] with a mean five cycles of therapy. However, 177Lu-DOTATATE patients showed a longer median overall survival (mOS) than those receiving 90Y-Dotatoc and a better DCR when higher dosages were administered, even if a direct comparison was not carried out. Syndromic patients had a poorer mOS. SDHx mutations did not interfere with treatment efficacy.
Conclusions
PRRT is safe and effective for the treatment of patients with progressive mPPGL, especially at higher dosages. The longer mOS of 177Lu-DOTATATE-treated patients in our protocols indicates the former radiopharmaceutical as the better candidate for further clinical application.
Key words: phaeochromocytoma, paraganglioma, peptide receptor radionuclide therapy, 177Lu-DOTATATE, 90Y-DOTATOC
Highlights
-
•
PPGLs are rare tumors with limited therapeutic options.
-
•
PRRT could be an optimal candidate to treat these rare tumors.
-
•
mPPGL patients treated with 90Y- or 177LU-PRRT obtained high median progression-free survival and mOS in a long follow-up.
-
•
The safety profile is consistent with previous data on PRRT.
Introduction
Phaeochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors (NETs) arising from the cells of the neural crest, with an incidence of 0.6 cases per 100 000 persons per year. These tumors can occur anywhere in the parasympathetic and sympathetic autonomic nervous system from the base of the skull to the pelvis.1 In particular, sympathetic PPGL cells produce and release catecholamines that may cause cardiovascular and gastrointestinal problems.2,3
Surgery is the curative approach for local disease. However, 15%-20% of patients with PPGL have metastases, a rare condition with only 100-200 new cases diagnosed annually in the USA.4 Patient prognosis is fairly heterogeneous and it is difficult to predict the clinical behavior of each case given that only 50%-60% of patients with metastatic PPGLs (mPPGLs) are still alive 5 years after their initial diagnosis.5
In 30% of cases, PPGLs are associated with mutations of SDHB, SDHC, SDHD, SDHAF2, SDHA, TMEM, MAX, and VH genes. There are >20 different genes with both germline or sporadic driver mutations and an increasing number of potential disease-modifying genes. Mutations in the succinate dehydrogenase (SDHx) gene predispose up to 70% of patients to aggressive phenotypes, resulting in distant metastasis, tumor multiplicity and disease recurrence, thus highlighting the need for effective therapies against SDHx-mutated PPGLs.6 Other treatment options including thermal ablation, chemotherapy, targeted therapy and external beam radiation have not shown a clear efficacy in validated prospective clinical trials.7
In a retrospective experience, temozolomide, an alkylating agent with a safety profile and activity similar to those of dacarbazine, showed a 50% partial response rate in SDHB carriers (n = 10), suggesting greater efficacy in this patient subgroup.8 A theranostic approach based on metaiodobenzylguanidine, a norepinephrine precursor, has also been evaluated. Metaiodobenzylguanidine labeled with Iodine-123 for diagnostic purposes and with Iodine-131 for therapy obtained appreciable results in the diagnostic field, but the therapeutic application was burdened by difficult handling and significant side-effects.9
The overexpression of somatostatin receptor in gastroenteropancreatic neuroendocrine tumors (GEP-NETs) forms the rationale for the theranostic application of radiolabeled somatostatin analogs in these tumors. A diagnostic approach with 68Ga Dota-peptide PET/CT10 and the therapeutic use of 90Y-DOTATOC and 177Lu-DOTATATE11, 12, 13 in GEP-NETs has paved the way for their use in other NET histologies, including paragangliomas.14, 15, 16
In this scenario, peptide receptor radionuclide therapy (PRRT) experiences in PPGLs are prevalently retrospective, performed in small cohorts of patients and administered with highly variable activities and low numbers of cycles. Recently, however, several prospective studies have used more homogeneous dosages and numbers of cycles, obtaining encouraging results and good disease control (85%).17
The two most frequently used radiopharmaceuticals differ in terms of the physical characteristics of the radionuclides and the sensitivity for somatostatin receptor 2 (SSTr2) (widely expressed in NETs), which is nine-fold higher for octreotate than for octreotide. In particular, 90Y (T/2 2.67 days and energy 2.3 MeV) has a tissue penetration of 5-7 mm, which makes it more suitable for larger lesions. Conversely, 177Lu (T/2 6.7 days and energy 0.5 MeV) is generally preferred in small lesions and in the presence of risk factors for renal toxicity.
In the present study, we focused on mPPGL patients enrolled in prospective homogenous therapy protocols characterized by low dosages with a higher mean number of cycles. In these prospective trials, we evaluated the activity and safety of the two most commonly used radiopharmaceuticals, 90Y-DOTATOC and 177Lu-DOTATATE, and herein present the results as well as the impact of important prognostic factors in a large case series of mPPGL patients with long-term follow-up.
Patients and methods
Study design and participants
Forty-seven patients with SSTr2-positive progressive locally advanced or metastatic PPGLs were consecutively treated with PRRT from March 2008 to March 2018. Patients were treated with 90Y-DOTATOC or 177Lu-DOTATATE in our institute [IRST Istituto Romagnolo per lo Studio dei Tumori (IRCCS)] in Meldola according to the phase II protocol available in the enrolment period.
All patients signed an informed consent before study procedures. The protocols (Eudract no. 2007-005517-20, Eudract no. 2011-002891-18, Eudract no. 2015-004727-31 and Eudract no. 2012-003155-11) were approved by the local ethics committees of the centers taking part and the Italian Medicines Agency (AIFA). All the studies were carried out in compliance with the ethical standards laid down in the 1964 Declaration of Helsinki and the principles of Good Clinical Practice guidelines (including written informed consent).
Procedures
Patients with SSTr2-positive mPPGL (confirmed by 111In-octreotide or 68Ga-dotapeptide PET/CT) without active therapy in the previous 4 weeks were enrolled and treated with 4/5 cycles of PRRT performed 8-10 weeks apart. 90Y-DOTATOC was administered at dosages of 1.1 or 1.85 GBq/cycle, while 177Lu-DOTATATE was administered at dosages of 3.7 or 5.5 GBq/cycle. The lower dosages were used for patients with risk factors for bone marrow or renal toxicity, generally represented by hypertension, diabetes, renal dysfunction, previous chemotherapy and anemia, according to our previous experience of PRRT in GEP-NETs.11,18,19
Block randomization was used for Eudract no. 2015-004727-31 study. For the other studies, the different treatment allocation was based on the presence of one of the risk factors at investigators' judgment.
Radiopharmaceutical labeling and administration modality, renal protection protocol, inclusion and exclusion criteria, disease progression criteria (RECIST) and the follow-up program have been described elsewhere.11 Efficacy was assessed using RECIST criteria version 1.1.
Briefly, follow-up generally consisted of blood tests, instrumental exams and clinical evaluation every 3 months for the first 2 years and then every 6 months thereafter until disease progression. For liver and bone metastases, we divided our case series into metastatic and non-metastatic patients and evaluated renal and bone marrow toxicity according to Common Terminology Criteria for Adverse Events guidelines. We offered genetic counseling and molecular analyses for PPGL susceptibility genes, including RET, VHL, MAX, SDHA, SDHB, SDHC, SDHD, SDHAF2, and TMEM127. We performed a DNA Sanger sequencing for all the exons of these genes and searched for large deletions or rearrangements by multiplex ligation-dependent probe amplification. Patients were classified as mutation-negative if they had no pathogenic DNA variant in these genes.
Statistical analysis
The results for this case series of consecutively enrolled patients with mPPGL were evaluated considering the general objectives proposed in the different studies: disease control rate (DCR), progression-free survival (PFS), overall survival (OS) and safety. DCR was defined in all patients as the percentage of complete response (CR) plus partial response (PR) plus stable disease response over a period of at least 12 months from the start of therapy. DCR was calculated with an exact 95% two-sided confidence interval (95% CI) using standard methods based on binomial distribution.
The association between categorical variables was assessed using the chi-square test or the Fisher's exact test, as appropriate. PFS was calculated from the first day of each therapy to the date of progression disease or death, whichever occurred first, or last tumor evaluation. OS was calculated from the start of therapy until death or the last follow-up. Survival curves were estimated with the Kaplan–Meier method and were compared using the log-rank test; 95% CIs for median time were calculated with non-parametric methods. The difference in activity was analyzed with exploratory intent.
All P values were two-sided, and a P < 0.05 was considered statistically significant. Statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC).
Results
We prospectively enrolled 47 patients with mPPGLs. One patient underwent a single PRRT cycle and was only considered for the safety analysis, as per the protocol. Of the remaining 46 patients, 20 were men and 26 women, with a median age of 52 years. Twelve were treated with 90Y-DOTATOC, median cumulative dosage of 9.2 GBq, and 34 patients underwent treatment with 177Lu-DOTATATE, median cumulative dosage of 24.42 GBq. A mean of five cycles was administered in both cases.
The overall response to treatment evaluated on 31 June 2020 after a median follow-up of 73 months (range 5-146 months) (Table 1) showed 4 PR, 33 stable disease and 9 progressive disease (PD), with a DCR of 80.4% (95% CI 68.9% to 91.9%). Median PFS (mPFS) was not reached (nr), while median OS (mOS) was 142.6 months (95% CI 103.1-146.2 months) (Tables 1 and 2; Figure 1).
Table 2.
Overall response and response related to radiopharmaceuticals
| Response | Overall |
90Y-DOTATOC |
177Lu-DOTATATE |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| PR | 4 (8.7) | 1 (8.3) | 3 (8.9) |
| SD | 33 (71.7) | 8 (66.7) | 25 (73.5) |
| DCR (PR + SD) | 37 (80.4) | 9 (75.0) | 28 (82.4) |
| PD | 9 (19.6) | 3 (25.0) | 6 (17.6) |
DCR, disease control rate; PD, progressive disease; PR, partial response; SD, stable disease.
Table 1.
Main clinical and response parameters
| No. patients | No. events | Median PFS, months (95% CI) | P | No. events | Median OS, months (95% CI) | P | |
|---|---|---|---|---|---|---|---|
| Overall | 46 | 19 | nr | — | 9 | 142.6 (103.1-146.2) | — |
| Sex | |||||||
| Male | 20 | 8 | nr | — | 4 | 143.5 (103.1-146.2) | — |
| Female | 26 | 11 | 74.5 (27.5-nr) | 0.803 | 5 | 142.6 (85.9-142.6) | 0.442 |
| Therapy | |||||||
| 90Y-DOTATOC | 12 | 5 | 74.5 (8.4-nr) | — | 2 | 92.1 (57.1-92.1) | — |
| 177Lu-DOTATATE | 34 | 14 | nr | 0.678 | 7 | 143.5 (103.1-146.2) | 0.192 |
| Origin | |||||||
| Sympathetic | 16 | 11 | 27.5 (14.5-51.5) | — | 7 | 142.6 (76.1-146.2) | — |
| Parasympathetic | 30 | 8 | nr | 0.0005a | 2 | nr | 0.104 |
| Symptomatic | |||||||
| No | 33 | 11 | nr | 4 | 142.6 (142.6-146.2) | ||
| Yes | 13 | 8 | 27.5 (8.4-nr) | 0.027a | 5 | 103.1 (25.5-143.5) | 0.099 |
| Mutation | |||||||
| No (wild-type) | 16 | 7 | 51.5 (27.5-nr) | 1 | Nr | — | |
| Yes (SDHD/SDHB) | 20 | 4 | nr | 0.030a | 0 | Nr | 0.248 |
| Liver lesions | |||||||
| 0 | 38 | 15 | nr | — | 7 | 142.6 (92.1-146.2) | — |
| ≥1 | 8 | 4 | nr | 0.359 | 2 | 143.5 (76.1-143.5) | 0.753 |
| Bone lesions | |||||||
| 0 | 27 | 9 | nr | 3 | 142.6 (103.1-142.6) | ||
| ≥1 | 19 | 10 | 48.5 (22.5-nr) | 0.062 | 6 | 143.5 (57.1-146.2) | 0.586 |
CI, confidence interval; nr, not reached; OS, overall survival; PFS, progression-free survival
Overall median follow-up: 73 months (range 5-146 months).
Median follow-up 177Lu: 73 months (range 6-146 months); 90Y: 76 months (range 5-91 months).
See mPFS figure in Supplementary Files, available at https://doi.org/10.1016/j.esmoop.2021.100171.
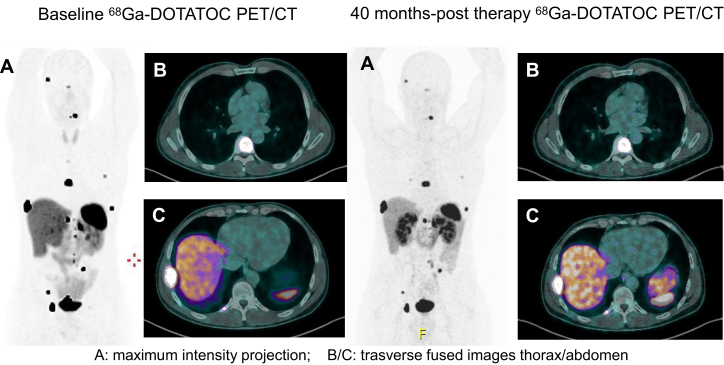
Figure 1.
A durable PRRT response in a PPGL patient with high metastatic tumor volume.
A 47-year-old man with diffuse metastatic paraganglioma (lymph node, left adrenal gland and bone metastases) with primary tumor already surgically treated in 2012. The patient carried out a pre-therapy 68Gallium-DOTATOC (68Ga) positron emission tomography/computed tomography (PET/CT) on March 2016 (left A-C). Then the patient received 177Lu-DOTATATE treatment (cumulative dose: 24.8 GBq). After >40 months progression-free survival, the patient still maintains the response obtained (right A-C).
PPGL, pheochromocytoma and paraganglioma; PRRT, peptide receptor radionuclide therapy.
At a median follow-up of 76 months (range 5-91 months) in the 12 patients treated with 90Y-DOTATOC, we observed 1 PR, 8 stable disease and 3 PD, with a 75.0% (95% CI 50.5% to 99.5%) DCR. mPFS was 74.5 months (95% CI 8.4-nr months), while mOS was 92 months (95% CI 57.1-92.1 months) (Figure 2). The three 90Y-DOTATOC patients with risk factors treated with a median dosage of 6.4 GBq showed a 33.3% DCR (95% CI 0% to 86.6%), while the nine 90Y-DOTATOC patients in which we administered a median dosage of 9.2 GBq showed a DCR of 88.9% (95% CI 68.4% to 100%). mPFS in the same two groups was 8.4 months (95% CI 5.5-51.5 months) versus nr, and mOS was 74.6 months (95% CI 57.1-92.0 months) versus nr, respectively.
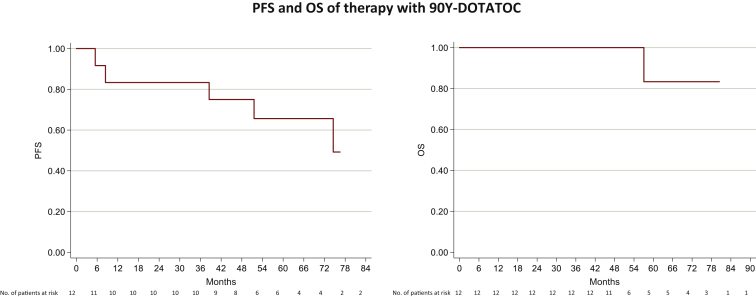
Figure 2.
mPPGL patients treated with 90Y showed an mPFS of 74.5 months (95% CI 8.4-nr months) (right) and a mOS of 92 months (95% CI 57.1-92.1 months) (left).
CI, confidence interval; mOS, median overall survival; mPFS, median progression-free survival; mPPGL, metastatic pheochromocytoma and paraganglioma; nr, not reached.
At a median follow-up of 73 months (range 6-146 months), the 34 patients treated with 177Lu-DOTATATE showed 3 PR, 25 stable disease and 6 PD, with a DCR of 82.4% (95% CI 69.6% to 95.2%) (Table 1). mPFS was not reached while mOS was 143.5 months (95% CI 143.5-146.2 months) (Figure 3). The 9 177Lu-Dotatate patients with risk factors treated with a median dosage of 15.9 GBq showed a 55% DCR (95% CI 23.1% to 88.1%), while a 92% DCR (95% CI 81.4% to 100%) was observed in the 25 patients without risk factors treated with a median dosage of 25.9 GBq. mPFS in the same two groups was 27.5 months (95% CI 7.0-nr months) versus nr, respectively, and mOS was 143.5 months (95% CI 25.5-146.2 months) versus 142.6 (95% CI 103.1-142.6 months), respectively.
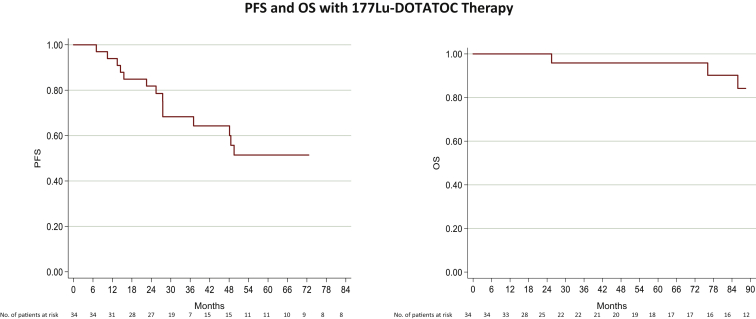
Figure 3.
mPPGL patients treated with 177Lu the mPFS was nr (right).
The mOS of this population was 143.5 months (95% CI 143.5-146.2 months) (left).
CI, confidence interval; mOS, median overall survival; mPFS, median progression-free survival; mPPGL, metastatic pheochromocytoma and paraganglioma; nr, not reached.
A DCR of 68.8% (95% CI 46.1% to 91.5%) was observed in the 16 patients with PPGLs of sympathetic origin, while that of the 30 patients with parasympathetic tumors was 86.7% (95% CI 74.6% to 98.8%). mPFS in the same two groups was significantly different, 27.5 months (95% CI 14.5% to 51.5%) versus nr, respectively (P = 0.0005), but the difference was no longer evident in the mOS 142.6 months (95% CI 76.1-146.2 months) versus nr.
All of the 13 patients with functioning tumors had stable disease from a syndromic point of view (all were undergoing specific therapy) and did not have complications from PRRT administration. DCR in this group was 77% (95% CI 54.0% to 99.8%), while mPFS was 27.5 months (95% CI 8.4-nr months) and mOS was 103.1 months (95% CI 25.5-143.5 months). In the 33 patients with non-functioning tumors, DCR was 82% (95% CI 68.6% to 95.0%), mPFS was nr and mOS was 142.6 months (95% CI 142.6-146.2 months). In the two groups, we had a statistically significant difference in mPFS (P = 0.027) while the mOS was not significant (P = 0.099).
Mutational data was available in 36 (78%) of the 46 patients. Sixteen had succinate dehydrogenase wild-type tumors and 20 showed SDHD/SDHB mutations. The former had a 93.8% DCR (95% CI 82.0% to 100%) and the latter, a 95% DCR (95% CI 85.4% to 100%). mPFS of wild-type patients was 51.5 months (95% CI 27.5-nr months), while that of the mutated group was not reached (P = 0.030). In both groups, mOS were not reached. No significant differences in DCR, mPFS or mOS were seen in relation to the presence or not of liver metastases and the tumor radiopharmaceutical concentration. The 27 patients without bone metastases had a DCR of 89% (95% CI 77.1% to 100.0%) compared with 69% (95% CI 47.5% to 89.3%) in the 19 patients with bone involvement. mPFS was nr and 48.5 months (95% CI 22.5-nr months), respectively (P = 0.062), the non-significant difference persisting for mOS [nr versus 143.5 months (95% CI 57.1-146.2 months), respectively].
DCR percentage according to site of origin, presence of mutation, different dosage of two radiopharmaceuticals and presence or not of liver or bone lesions has been listed in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100171.
Toxicity
No cases of grade (G) 3/4 bone marrow toxicity were observed in any of the treated patients. G1 and G2 bone marrow toxicity was observed in 19 and 4 patients, respectively. All patients recovered during the interval between cycles, without the need for interference in the treatment schedule. Two patients had G1 renal toxicity and one patient G2 toxicity. The latter patient started treatment (2008) with 1.53 creatinine at baseline and registered a 1.88 creatinine level at the third cycle, at which point therapy was interrupted (cumulative dosage 11.1 GBq 177Lu-DOTATATE). She still has stable disease after >10 years, but a worsening of renal function led to the need for weekly hemodialysis from 2019. No significant differences in toxicity were reported in patients treated with 90Y-DOTATOC or 177Lu-DOTATATE (Table 3).
Table 3.
Radiopharmaceutical-related toxicity
|
90Y-DOTATOC |
177Lu-DOTATATE |
|
|---|---|---|
| n (%) | n (%) | |
| HB | ||
| G1 | 8 (23.5) | 2 (16.7) |
| G2 | 1 (2.9) | 0 |
| Plt | ||
| G1 | 5 (14.7) | 0 |
| G2 | 1 (2.9) | 0 |
| Neutro | ||
| G1 | 2 (5.9) | 2 (16.7) |
| G2 | 2 (5.9) | 0 |
| Creat | ||
| G1 | 2 (5.9) | 0 |
| G2 | 1 (2.9) | 0 |
Creat, creatinine; HB, hemoglobin; Neutro, neutrophils; Plt, platelets.
Discussion
Over the past 10 years, PRRT has confirmed its role in the therapeutic landscape of G1/G2 GEP-NETs. The results from the NETTER-1 trial led to the registration of 177Lu-DOTATATE in this setting and set a milestone in PRRT in neuroendocrine neoplasia. In the present study, we focused our efforts on exploring the potential usefulness of PRRT in mPPGL, a rare and orphan setting generally studied in sporadic and mainly retrospective experiences15, 16, 17, 18 using 90Y-DOTATOC and 177Lu-DOTATATE. We also analyzed the role of prognostic and predictive biomarkers not previously adequately investigated. Finally, we collected data on patients treated with 90Y-DOTATOC or 177Lu-DOTATATE PRRT according to the protocol being used at that time, and with reduced cumulative dosages in individuals with risk factors for bone marrow and/or kidney toxicity.
In our paper, we observed an overall DCR of 80% in our group of 46 consecutively enrolled patients with mPPGL, which is in line with results obtained in GEP-NETs, while mPFS and mOS showed even better results after a median follow-up of 73 months. Better patient outcomes with respect to those previously reported in mainly retrospective works can be attributed to treatment standardization in terms of cumulative dosage and number of performed cycles. Our data correlate strongly with those of centers using a similar study design16,17 and were confirmed by the better disease control obtained with the higher activities administered to patients without risk factors. In fact, we obtained a 92% DCR using the full dosage of 177Lu-Dotatate and a DCR of 55% when the reduced dosages were used. mPFS was nr versus 27.5 months, respectively.
The relatively small number of enrolled patients and the complex study design did not justify carrying out a multiparametric analysis. However, we observed a slight, albeit not significant, benefit from full-dosage 177Lu-DOTATATE that was also seen in patients treated with 90Y-DOTATOC.
The better mOS obtained with 177Lu-DOTATATE compared with 90Y-DOTATOC (143 versus 92 months, respectively) would seem to favor the use of the former radiopharmaceutical in subsequent studies and in therapeutic associations. Its efficacy may be attributable to higher tyr3-octreotate affinity for SSTr2 receptors, but also to the longer residence time of 177Lu-DOTATATE in the tumor.
With regard to prognostic factors, we observed a different therapeutic activity in sympathetic PGLs with respect to parasympathetic tumors. This finding was confirmed by the poorer mPFS of patients with symptomatic tumors (P = 0.027) and is logically correlated with the syndromic nature of the disease, which can be affected by the clinical consequences of hormonal hyperproduction. In our series of mPPGLs, mutated tumors were just as numerous as un-mutated tumors, confirming their greater aggressiveness. The appreciable and similar response of the two groups to treatment can be ascribable to the high efficacy of PRRT.
We did not register significant differences with regard to the presence or not of hepatic lesions. In case of skeletal involvement, an interesting difference was observed in terms of DCR and mPFS (P = 0.062), which was 48 months in the metastatic group and not reached in the non-metastatic group, respectively, confirming results from previous reports.14 It is worthy of note that the difference normalized when looking at mOS, which was similar in patients with and without bone metastases and linked to the long survival of these patients. No differences were reported in radiopharmaceutical concentrations in tumor, which were generally high. FDG PET/CT-positivity tested as a possible prognostic factor was suspended after observing its positivity in 19/20 cases. This finding was not considered as an indicator of higher tumor aggressiveness and was hypothesized as being related to succinate or norepinephrine hypersecretion.20
Unlike other experiences,16 we observed a very low frequency of toxicity in both 177Lu-DOTATATE and 90Y-DOTATOC PRRT patients. This may have been the result of using a reduced cumulative dosage for patients with reduced kidney function or bone marrow reserve and from administering therapy in five cycles rather than the normal four. The only patient at risk of renal toxicity was treated with a reduced dosage but required treatment interruption after the third cycle of PRRT. The patient is still clinically stable, but her kidney failure progressed slowly, leading to the need for dialysis over the past year. These data emphasize the possibility of using low dosage PRRT in patients with renal or bone marrow risk factors who would otherwise normally be excluded from PRRT.
Efforts to further improve treatment response, especially in patients with unfavorable prognostic factors, could now focus on the area of therapeutic associations with sensitizing or alkylating agents such as capecitabine21 or temozolomide,8 respectively. The association of PRRT with PARP inhibitors or bortezomib-type proteasome inhibitors, both involved in DNA repair, can also be hypothesized in neuroendocrine neoplasms because of the slow proliferation of tumor cells, providing sufficient time for the repair of radiation damage.
The current study presents some limitations. Despite close collaboration with other experienced centers, the rarity of the histological type influenced patient enrolment, which extended over a long period and through different experimental phase II protocols. Of note, all the studies, stratified by tumor type, were designed with a similar therapeutic strategy, resulting in a homogeneous treatment modality. Notwithstanding these limits, we consider our data both accurate and important because of the substantial sample size and the long follow-up. Furthermore, a direct comparison between 177-Lu- and 90Y-PRRT was not planned and this could be of great value.
Of note, our study presents prospective data of long-term efficacy and safety of PRRT in mPPGL patients focusing also on the clinical and biological prognostic and risk factors useful to tailor the therapeutic strategy in patients with a rare disease.
Conclusions
In a long-term follow-up of patients with mPPGL, PRRT demonstrated a tolerability and efficacy comparable to those obtained in GEP-NETs. Our results highlight the need for an appropriate cumulative administered dosage to achieve a very prolonged result and underline the importance of specific symptoms and presence of bone lesions as predictive factors. Given the substantial number of patients evaluated over time, the superior mOS of 177Lu-DOTATATE with respect to 90Y-DOTATOC indicates the former radiopharmaceutical as the better candidate for new prospective studies of single or associated therapies.
Acknowledgements
The authors thank all patients and their families and all staff for participating in this trial. The authors thank Gráinne Tierney for editorial assistance.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary data
References
- 1.Lam A.K. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28:213–227. doi: 10.1007/s12022-017-9484-5. [DOI] [PubMed] [Google Scholar]
- 2.Lenders J.W., Duh Q.Y., Eisenhofer G. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–1942. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 3.Thosani S., Ayala-Ramirez M., Roman-Gonzalez A. An overlooked, unmanaged symptom of patients with pheochromocytoma and sympathetic paraganglioma. Eur J Endocrinol. 2015;173:377–387. doi: 10.1530/EJE-15-0456. [DOI] [PubMed] [Google Scholar]
- 4.Ayala-Ramirez M., Feng L., Johnson M.M. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–725. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 5.Lenders J.W., Eisenhofer G., Mannelli M., Pacak K. Phaechromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 6.Fishbein L., Leshchiner I., Walter V. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31:181–193. doi: 10.1016/j.ccell.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plouin P.F., Fitzgerald P., Rich T. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm Metab Res. 2012;44:390–399. doi: 10.1055/s-0031-1299707. [DOI] [PubMed] [Google Scholar]
- 8.Hadoux J., Favier J., Scoazec J.Y. SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer. 2014;135:2711–2720. doi: 10.1002/ijc.28913. [DOI] [PubMed] [Google Scholar]
- 9.Thorpe M.P., Kane A., Zhu J., Morse M.A., Wong T., Borges-Neto S. Long-term outcomes of 125 patients with metastatic pheochromocytoma or paraganglioma treated with 131I-mIBG. J Clin Endocrinol Metab. 2019;105:dgz074. doi: 10.1210/clinem/dgz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taïeb D., Hicks R.J., Hindié E. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46:2112–2137. doi: 10.1007/s00259-019-04398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sansovini M., Severi S., Ianniello A. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-DOTATATE. Eur J Nucl Med Mol Imaging. 2017;44:490–499. doi: 10.1007/s00259-016-3533-z. [DOI] [PubMed] [Google Scholar]
- 12.Baum R.P., Kluge A.W., Kulkarni H. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-octreotide ((177)Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: a phase-II study. Theranostics. 2016;6:501–510. doi: 10.7150/thno.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strosberg J., El Haddad G., Wolin E. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolasinska-Ćwikła A., Pęczkowska M., Ćwikła J.B. Clinical efficacy of PRRT in patients with advanced, nonresectable, paraganglioma-pheochromocytoma, related to SDHx gene mutation. J Clin Med. 2019;8:E952. doi: 10.3390/jcm8070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinato D.J., Black J.R., Ramaswami R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol. 2016;33:47. doi: 10.1007/s12032-016-0737-9. [DOI] [PubMed] [Google Scholar]
- 16.Satapathy S., Mittal B.R., Bhansali A. Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: a systematic review and meta-analysis. Clin Endocrinol. 2019;91:718–727. doi: 10.1111/cen.14106. [DOI] [PubMed] [Google Scholar]
- 17.Zandee W.T., Feelders R.A., Smit Duijzentkunst D.A. Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur J Endocrinol. 2019;181:45–53. doi: 10.1530/EJE-18-0901. [DOI] [PubMed] [Google Scholar]
- 18.Zovato S., Kumanova A., Dematte S. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL) Horm Metab Res. 2012;44:411–414. doi: 10.1055/s-0032-1311637. [DOI] [PubMed] [Google Scholar]
- 19.Bodei L., Cremonesi M., Ferrari M. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–1856. doi: 10.1007/s00259-008-0778-1. [DOI] [PubMed] [Google Scholar]
- 20.Abdul Sater Z., Jha A., Hamimi A. Pheochromocytoma and paraganglioma patients with poor survival often show brown adipose tissue activation. J Clin Endocrinol Metab. 2020;105:1176–1185. doi: 10.1210/clinem/dgz314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav M.P., Ballal S., Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res. 2019;9:13. doi: 10.1186/s13550-019-0484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.