Abstract
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent and increasing liver disease, which encompasses a variety of liver diseases of different severity. NAFLD can lead to liver cirrhosis with all its complications as well as hepatocellular carcinoma (HCC). Steatosis of the liver is not only related to obesity and other metabolic risk factors, but can also be caused by several drugs, including certain cytotoxic chemotherapeutic agents. In patients undergoing liver surgery, hepatic steatosis is associated with an increased risk of post-operative morbidity and mortality. This review paper summarizes implications of hepatic steatosis on the management of patients with cancer. Specifically, we discuss the epidemiological trends, pathophysiological mechanisms, and management of NAFLD, and its role as a leading cause of liver cancer. We elaborate on factors promoting immunosuppression in patients with NAFLD-related HCC and how this may affect the efficacy of immunotherapy. We also summarize the mechanisms and clinical course of chemotherapy-induced acute steatohepatitis (CASH) and its implications on cancer treatment, especially in patients undergoing liver resection.
Key words: non-alcoholic fatty liver disease, hepatocellular carcinoma, cancer, hepatic steatosis, chemotherapy-induced steatohepatitis
Highlights
-
•
Non-alcoholic fatty liver disease can lead to cirrhosis with all its complications, including hepatocellular carcinoma.
-
•
Chemotherapy-associated acute steatohepatitis is a side-effect of chemotherapeutic agents and may limit treatment options.
-
•
In this review we summarize current clinical concepts of NAFLD and CASH that help clinicians in their clinical practice.
Introduction
Obesity—a major health problem globally1—is not only associated with the development of cardiovascular complications,2 but also increases the risk for liver diseases, including non-alcoholic fatty liver disease (NAFLD) and liver cancer.3,4 Obesity is also associated with at least 12 other tumor types (i.e. esophageal, gastric, colorectal, gallbladder, pancreatic, breast, corpus uteri, ovarian, renal cell, thyroid, multiple myeloma, and meningioma).5 NAFLD encompasses a variety of liver diseases of different severity, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), and eventually results in liver cirrhosis and/or hepatocellular carcinoma (HCC) in a significant proportion of affected individuals.6 Underlying liver cirrhosis impacts on the prognosis and management of patients with HCC, as more advanced liver function impairment limits therapeutic options and worsens outcome.7
Hepatic steatosis is not only related to obesity and other metabolic risk factors, but can also occur as a feature of drug-induced liver injury.8,9 Several drugs are known to promote hepatic fat accumulation, including certain cytotoxic chemotherapeutic agents.10 This can have implications for the treatment of cancer patients, especially for those undergoing liver surgery for metastatic disease.11
In this review, we discuss the epidemiology, pathophysiology, and management of NAFLD, and its role as a leading cause of liver cancer. We summarize emerging evidence indicating that NAFLD may be associated with reduced efficacy of immunotherapy in HCC. Moreover, we elaborate on the mechanisms and clinical course of chemotherapy-induced steatohepatitis and its implications for the management of cancer patients.
NAFLD: epidemiology, pathophysiology, diagnosis, and treatment
NAFLD has by some been referred to as the most rapidly emerging liver disease of the 21st century, with prevalence rates ranging between 23% and 32% in most parts of the world.3,12,13 Based on histology, NAFLD can range from simple steatosis without evidence of hepatocellular injury—referred to as non-alcoholic fatty liver (NAFL)—to steatosis with hepatic inflammation and hepatocyte injury, reflected by ballooning—referred to as non-alcoholic steatohepatitis (NASH).14 Risk factors for the development of NAFLD mainly include components of metabolic syndrome, such as diabetes mellitus, obesity, and hyperlipidemia.14 In consideration of the large contribution of metabolic risk factors to the evolvement of NAFLD and its disease severity, an international expert panel has recently proposed the new term ‘Metabolic Dysfunction-Associated Fatty Liver Disease’ (MAFLD) for patients with hepatic steatosis and type 2 diabetes or presence of at least two metabolic risk abnormalities.15,16 However, it remains unknown how quickly this newly proposed definition will be adapted into daily clinical practice. Genetic risk factors have also been associated with an increased risk for developing NAFL, NASH, and its associated complications such as advanced fibrosis, cirrhosis, and HCC, with a genetic variation in the patatin-like phospholipase domain-containing protein 3 (PNPLA3) being the most widely studied.17, 18, 19
In a patient with suspected NAFLD, thorough review of the medical history and exclusion of other obvious causes of chronic liver disease (i.e. the most common being viral hepatitis, alcoholic liver disease, autoimmune-associated liver disease, cholestatic liver disease, drug-induced liver disease) is mandatory.
Apart from distinguishing whether a patient has NAFL or NASH, evaluation of the fibrosis stage is crucial, as fibrosis per se rather than the presence of NASH on liver biopsy seems to be the leading driver of major hepatic outcomes (i.e. liver-related mortality or hepatic decompensation such as ascites, hepatic encephalopathy, or variceal bleeding).20,21 Even though several non-invasive methods to evaluate fibrosis have proven to effectively rule-in/-out advanced fibrosis or cirrhosis in patients with NAFLD, liver biopsy remains the gold standard.14,22 Nevertheless, non-invasive methods such as vibration controlled transient elastography (VCTE; FibroScan, Echosense, Paris, France)23,24 and magnetic resonance elastography (MRE)25,26 or laboratory based scores such as FIB-4 or the NAFLD Fibrosis Score (NFS) are increasingly accepted and implemented in clinical routine.14,22,27
Given that the clinical benefit may be limited due to lacking therapeutic options outside of clinical trials, the indication of liver biopsy needs to be evaluated by a hepatology specialist, and pros and cons should be discussed with the patient. We usually perform non-invasive tests (VCTE or MRE, FIB-4 or NFS; see Table 1) first. If these are suggestive of advanced chronic liver disease (i.e. fibrosis stage ≥3), we recommend confirmatory liver biopsy, especially when evaluating eligibility for clinical trials.
Table 1.
Bullet points on risk factors, diagnostic tools, histological readouts, and treatment options for patients with non-alcoholic fatty liver disease (NAFLD)
| Prevalence? | NAFLD prevalence is estimated to range between 23% and 32% in most regions of the world. NAFLD summarizes two distinct disease courses:
|
| Risk factors? | Lifestyle factors, obesity, diabetes mellitus, hyperlipidemia, arterial hypertension (= metabolic syndrome) and genetic risk factors (polymorphisms in PNPLA3, TM6SF2, HSD17B13) |
| Liver histology? | Should be evaluated for degree of steatosis (grade 0-3), inflammation (grade 0-3), ballooning (grade 0-2), and stage of fibrosis (stage 0-4)
|
| Non-invasive tools? |
|
| Staging/grading? | Stage and grade according to NAS and fibrosis stage, i.e. NASH patient with advanced fibrosis would be staged/graded: NAS 6, F3 |
| Treatment options? |
|
FIB-4, fibrosis 4; HCC, hepatocellular carcinoma; HSD17B13, 17B-hydroxysteroid dehydrogenase type 13; MRE, magnetic resonance elastography; NAFLD, non-alcoholic fatty liver disease; NAS, non-alcoholic fatty liver disease activity score; PNPLA3, patatin-like phospholipase domain-containing protein 3; TM6SF2, transmembrane 6 superfamily 2 human gene; VCTE, vibration controlled transient elastography.
The liver specimen is graded according to the NAFLD activity score (NAS),28,29 which comprises the sum of the grade of steatosis (0-3), hepatocyte ballooning (0-2), and inflammation (0-3). Ranging from 0 to 8 points, a NAS score of ≥5 is highly suggestive of NASH.29 Liver fibrosis should be staged on a five-point scale: no fibrosis (stage 0), pericellular fibrosis (stage 1), pericellular and portal fibrosis (stage 2), bridging fibrosis (stage 3), or cirrhosis (stage 4),28 with ‘advanced fibrosis’ implicating stages 3 and 4.
Once diagnosis of NAFLD is made, treatment options aiming to reduce histopathological features as well as fibrosis should be discussed with the patient. Lifestyle interventions including weight loss and hypocaloric diet are the basis of all therapeutic interventions. Current guidelines recommend losing at least 3%-5% of body weight, as this was associated with improvement of steatosis. However, a reduction of around 7%-10% is usually needed to really impact histopathological features of NASH and fibrosis.14 In overweight/obese patients with advanced chronic liver disease, 16 weeks of diet and moderate exercise even reduced portal pressure.30 If this translates into a reduced number of hepatic events (i.e. liver-related mortality and/or hepatic decompensation) needs further evaluation. Besides weight loss, treatment of components of the metabolic syndrome, including hypertension, diabetes, and hyperlipidemia, represents another mainstay in the management of patients with NAFLD.14,22 Finally, several pharmacological treatments have been studied in patients with NASH, but only pioglitazone and vitamin E are recommended for selected patients by current practice guidelines.14,22
In patients with advanced fibrosis or cirrhosis, the presence of clinically significant portal hypertension (CSPH) needs to be evaluated. Hepatic venous pressure gradient (HVPG) measurement via hepatic vein catheterization represents the gold standard, and an HVPG of 10 mmHg or higher denotes CSPH. Indirect markers of CSPH include gastroesophageal varices in endoscopy as well as thrombocytopenia plus splenomegaly. Management includes evaluation and treatment of complications of CSPH (i.e. gastroesophageal varices, ascites, and hepatic encephalopathy).31, 32, 33
HCC in NAFLD: incidence, screening, and treatment
Patients with advanced liver disease due to NAFLD show two main liver-related complications in the course of their disease—both leading to increased morbidity and mortality: (i) hepatic decompensation, including development of gastroesophageal varices and associated variceal bleeding, ascites, and hepatic encephalopathy, all of which almost exclusively occur in patients with advanced chronic liver disease (i.e. cirrhosis), and (ii) HCC, which may also occur in NAFLD patients without cirrhosis.32,34
Cancer-related mortality is among the top three causes of death in NAFLD patients.14,35 Overall cancer incidence is 783 per 100 000 person years in patients with NAFLD compared with 593 without NAFLD.36 However, this increased cancer incidence in NAFLD patients seems to be primarily driven by HCC development rather than extrahepatic cancers, as Simon and colleagues have shown that the contribution of extrahepatic cancers to the cancer incidence in NAFLD patients was modest at best.37 Liver cancer is the sixth most commonly diagnosed cancer worldwide and the fourth in leading causes for cancer-related death.38 HCC (75%-85%) and intrahepatic cholangiocarcinoma (10%-15%) include the majority of cases.38 Incidence and mortality rates are two to three times higher among men.38
Incidence rates of HCC in NAFLD-associated cirrhosis range between 1% and 3% per year,39, 40, 41 and on average, an incidence rate of >1.5% per year can be expected.42
HCC can also occur in non-cirrhotic NAFLD, although numbers are lower.43 HCC in non-cirrhotic livers is more frequent in those with metabolic syndrome and NAFLD compared with other etiologies.43,44 In a USA cohort of non-cirrhotic NAFLD patients, the HCC incidence rate was 0.21/1000 person years (= 0.02% annual risk).45 On the other hand, in patients with NAFLD-related HCC, up to 42%-54% developed in a non-cirrhotic liver, compared with only 2.8% in subjects with hepatitis C virus-associated HCC,46, 47, 48, 49 although a referral bias cannot be excluded.6
Apart from progression to advanced fibrosis/cirrhosis with the accompanying risk for HCC, diabetes mellitus—often associated with NAFLD—puts NAFLD patients at significant risk for developing HCC.6,43,50 Notably, antidiabetic drugs could have an impact on HCC risk. While metformin use is associated with a reduced risk of developing HCC, insulin may increase liver cancer risk.51
Genetic risk factors, mainly PNPLA3, transmembrane 6 superfamily 2 human gene (TM6SF2), and 17B-hydroxysteroid dehydrogenase type 13 (HSD17B13), have been associated with an increased HCC risk not only in NAFLD patients,52, 53, 54, 55 but also in the general population.56
Carcinogenesis in NAFLD is a complex, multifactorial process involving genetic and lifestyle factors (i.e. obesity, high fat diet) as well as small intestinal bacterial overgrowth. These factors induce cell death, cause genetic and epigenetic alterations, and activate pathways related to inflammation, cell proliferation, and hepatic energy metabolism. This results in the development of NASH and hepatic fibrosis, and eventually promotes hepatocarcinogenesis.57, 58, 59 Recent evidence suggests that obesity can promote HCC independently of NASH via STAT-3 signaling.60,61
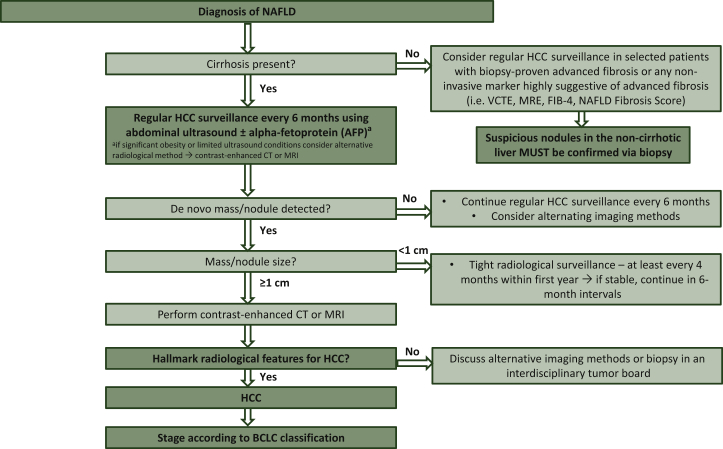
Surveillance for HCC in NAFLD patients is recommended for individuals with liver cirrhosis and may be considered in non-cirrhotic patients with advanced fibrosis (fibrosis grade F3) based on an individual risk assessment (Figure 1).34,42 Screening should be carried out in 6-month intervals by ultrasound.34 While European guidelines do not recommend additional assessment of serum alpha-fetoprotein (AFP) during screening due to reasons of cost-effectiveness,34 its use is optional according to American guidelines.62 Other potential biomarkers, such as des-gamma-carboxy prothrombin (DCP), the AFP isoform AFP-L3, or glypican-3, have not been recommended for routine clinical use by current guidelines.34,62 Similarly, scores to detect early HCC (e.g. GALAD model63) need prospective validation before adoption in clinical routine.62
Figure 1.
Surveillance algorithm for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease.
BCLC, Barcelona Clinic Liver Cancer; CT, computed tomography scan; FIB-4, Fibrosis-4; HCC, hepatocellular carcinoma; MRE, magnetic resonance elastography; MRI, magnetic resonance imaging; NAFLD, non-alcoholic fatty liver disease; VCTE, vibration controlled transient elastography.
Notably, ultrasound depends on the operator and patient’s body composition, which can impair diagnostic accuracy, especially in overweight and obesity, a common clinical problem in NAFLD patients.64,65 Therefore, in cases where ultrasound is unreliable, practice guidelines recommend alternative imaging methods such as computed tomography (CT) scan or magnetic resonance imaging (MRI).42
If a nodule is detected on ultrasound, further steps depend on the size of the lesion. A nodule <1 cm in diameter should be followed at 4-month intervals in the first year, and if there is no increase in size or number, surveillance can be returned to the usual 6-month interval. For tumors <1 cm with typical HCC characteristics on CT or MRI, the optimal management has not been clarified yet. Thus, current guidelines recommend discussion within a local multidisciplinary tumor board. Tumors ≥1 cm need to be evaluated by multiphasic contrast-enhanced CT or MRI. In patients with liver cirrhosis, HCC can be diagnosed by imaging only if certain hallmarks are met.34 However, especially in small tumors, intrahepatic cholangiocarcinoma and HCC may show similar enhancement patterns,66 which could lead to a false diagnosis by imaging only. The lack of tissue samples also complicates the identification of predictive biomarkers to guide treatment decisions in HCC. Thus, at least in clinical studies, tumor biopsies should become mandatory.67 In non-cirrhotic livers, diagnosis must always be confirmed by histology.34
Staging and treatment of HCC depend on tumor burden, liver function, and performance status of the patient. The Barcelona Clinic Liver Cancer (BCLC) Staging System has been endorsed by current practice guidelines and recommends ablation, resection, and liver transplantation with curative intent for early stages, while palliative treatments (i.e. transarterial chemoembolization, systemic therapy) are indicated for intermediate-advanced stage HCC.34,68 Generally, surgical resection is recommended as treatment of choice in non-cirrhotic livers, which explains why NASH-associated HCC represents an emerging indication for resection.34 However, as up to 50% of NASH-HCCs occur in patients without cirrhosis, HCC is often diagnosed incidentally outside of screening programs and thus, at more advanced cancer stages with limited curative treatment options.47,69 Notably, NAFLD impairs functional recovery after liver resection, and the risk of major post-operative complications is higher in NAFLD patients—even if severe fibrosis is absent—compared with those with normal underlying liver.70, 71, 72 Hence, concomitant NAFLD not only affects the management of patients with primary liver cancer but also that of patients undergoing resection of liver metastasis from other cancer types (i.e. colorectal cancer).73
HCC in NAFLD and immunotherapy
Immunotherapy with immune checkpoint blockers has been recently added to the treatment armamentarium of HCC.74,75 While monotherapy with programmed cell death protein 1 (PD-1)-targeted antibodies failed in phase III trials in both first-line and second-line,76,77 the combination of atezolizumab plus bevacizumab improved both primary endpoints overall and progression-free survival over sorafenib.78 Even though this combination represents the new reference standard in front-line HCC treatment, some patients should still receive tyrosine kinase inhibitors in first-line due to safety reasons (i.e. patients with a history of organ transplantation or severe autoimmune disease).75
There is also emerging preclinical and clinical evidence that immunotherapy may be less effective in patients with underlying NAFLD/NASH.75 Subgroup analyses from both first-line phase III trials of advanced stage HCC testing nivolumab monotherapy or combined atezolizumab/bevacizumab demonstrated that immunotherapy was more efficacious versus sorafenib in patients with underlying viral etiologies compared with non-viral diseases (including NAFLD).76,77 Similar data were reported in the phase III trial testing pembrolizumab monotherapy versus placebo in sorafenib-pretreated patients with HCC.76 A meta-analysis including these three phase III studies with a total of 1656 subjects confirmed that immunotherapy was superior versus control arm in patients with hepatitis-B- and hepatitis-C-related HCC, but not in patients with non-viral underlying etiologies {hazard ratio (HR) [95% confidence interval (CI)] for pooled hepatitis B virus/hepatitis C virus and non-viral: 0.64 (0.48-0.94) and 0.92 (0.77-1.11); P of interaction = 0.03}.79 Notably, this meta-analysis was not based on individual patient data. In two retrospective cohorts of patients with advanced stage HCC treated with PD-(L)1-targeted immunotherapy (n = 130 and n = 118, respectively), those with NAFLD/NASH-related HCC had a significantly shorter survival than patients with any other etiology.79
Mechanistically, NASH impacts the hepatic immune environment (Figure 2).80 For instance, NASH promotes a pro-tumorigenic milieu driven by exhausted, unconventionally activated CD8+PD-1+ T cells. In mouse models of NASH, anti-PD-1 treatment increased hepatic and tumoral CD8+PD-1+ T-cell accumulation, but failed to induce regression of liver tumors. Instead, mice experienced enhanced liver damage and hepatocarcinogenesis. Depletion of CD8+ T cells decreased anti-PD-1-induced tissue damage and HCC incidence.79 Moreover, NAFLD induces loss of hepatic CD4+ T lymphocytes, which hampers tumor immunosurveillance and fosters HCC development.81 In preclinical models with diet-induced steatohepatitis and intrahepatic injection of melanoma or colon cancer cells, immunotherapy with an RNA-based vaccine or an antibody against OX40 failed to inhibit intrahepatic tumor growth. Tumors of mice with steatohepatitis showed fewer CD4+ T cells and effector memory cells compared with tumors of mice on regular diet. Prevention of intratumoral T-cell loss recovered efficacy of immunotherapy.82
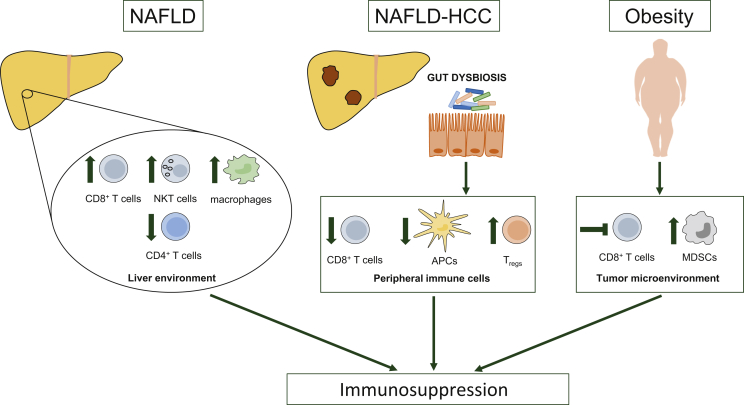
Figure 2.
Factors promoting immunosuppression in patients with non-alcoholic fatty liver disease.
NAFLD impacts the liver immune microenvironment. While the number of CD4+ T cells with antitumor functions is reduced, CD8+ T cells, NKT cells, and macrophages with tumor-promoting properties expand in NAFLD. Gut dysbiosis in NAFLD-related hepatocellular carcinoma promotes peripheral immunosuppression, characterized by reduced numbers of CD8+ T cells and antigen-presenting cells and expansion of regulatory T cells. Obesity is a risk factor for NAFLD and thus frequently present in patients with NAFLD. Obesity impairs the function of CD8+ T cells and enhances the immunosuppressive potency of tumor-infiltrating MDSCs.
APCs, antigen-presenting cells; HCC, hepatocellular carcinoma; MDSCs, myeloid-derived suppressor cells; NAFLD, non-alcoholic fatty liver disease; NKT cells, natural killer T cells; Tregs, regulatory T cells.
Gut microbiota has been implicated in modulating response to immunotherapy.83 Recent evidence suggests that altered gut microbiome in NASH-related HCC may hamper immunotherapy efficacy by modulating peripheral immune responses. Accordingly, gut dysbiosis in patients with NASH-HCC resulted in peripheral immunosuppression (reduced CD8+ T cells and antigen-presenting cells, increased regulatory T cells)—at least partly via increased short-chain fatty acid production84 (Figure 2). Early findings in melanoma patients suggest that approaches to modulate the gut microbiome (i.e. fecal microbiota transplant) may help to overcome immunotherapy resistance and render tumors more susceptible to immune checkpoint blockers (ICBs).85,86
Obesity—typically associated with NAFLD—can hamper antitumor immunity (Figure 2). In murine models, high fat diet (HFD) increased the accumulation of myeloid-derived suppressor cells (MDSCs) via leptin and enhanced the immunosuppressive activity of tumor-infiltrating MDSC; MDSCs enhanced cancer progression by preventing T-cell activation.87 Moreover, HFD-induced obesity impaired the function of CD8+ T cells via induction of metabolic changes in the tumor microenvironment, resulting in enhanced cancer growth.88 In another preclinical study, obesity promoted PD-1-mediated T-cell dysfunction—partly via leptin signaling—and tumor growth. However, obesity was associated with increased responsiveness of tumors to anti-PD-(L)1 treatment,89 suggesting that obesity-mediated immunosuppression can be reversed by ICBs. This is in line with several clinical reports showing better response rates and survival for obese patients with advanced cancers treated with immunotherapy.90, 91, 92
Together, these data support the notion that NAFLD is associated with reduced immunotherapy efficacy, not only in HCC but also in hepatic metastases from other tumor entities. Potential deleterious effects of PD-1-targeted therapy on NAFLD progression could also affect immunotherapy-treated patients with extrahepatic cancer types who suffer from concomitant NAFLD. Besides NASH-associated changes of the hepatic immune milieu, gut dysbiosis-related immunosuppression may hamper immunotherapy efficacy in NASH-HCC. If modulation of the gut microbiota can render tumors more susceptible to ICBs needs to be addressed in future studies. Obesity-induced immunosuppression may be reversed by ICBs.
Chemotherapy-associated steatohepatitis: mechanisms and clinical implications
Chemotherapy-induced acute steatohepatitis (CASH) describes inflammation with hepatocyte injury and steatosis of the liver in patients receiving systemic chemotherapy, possibly leading to liver-related complications such as sinusoidal obstruction syndrome (SOS) or nodular regenerative hyperplasia (NRH).10,11,93,94 Most data on chemotherapy-associated steatohepatitis and liver injury comes from patients with colorectal liver metastases where irinotecan- and oxaliplatin-based treatments have been associated with liver injury.11,93,95 In this setting, liver injury increases post-operative morbidity and liver-surgery-specific complications.11 Mainly oxaliplatin treatment was linked to severe sinusoidal dilatation, which resulted in an increased rate of major morbidity.11 However, various other systemic chemotherapeutics can induce CASH-like liver injury, the most common being methotrexate, 5-fluorouracil, irinotecan, tamoxifen, and l-asparaginase.10 CASH is usually reversible once treatment is stopped. However, liver injury per se can persist for a long time even after cessation of chemotherapy. For instance, while SOS and NRH regressed after 9 months, steatosis and steatohepatitis persisted.94 Mechanisms behind CASH are not entirely clear but seem to be based on mitochondrial dysfunction. Mitochondrial and peroxisomal beta-oxidation lead to lipid peroxidation via reactive oxygen species, which induces stellate cell activation, fibrosis, cell death, and ultimately CASH.10
Apart from the chosen chemotherapy regimen, obvious risk factors for CASH—which overlap with risk factors for NAFLD—include components of the metabolic syndrome, above-average alcohol intake, and previous chronic liver disease of any etiology.10 Additionally, genetic polymorphisms that play a key role in hepatic fat metabolism (i.e. PNPLA3) seem to influence the risk for developing CASH.96
CASH can be particularly problematic in patients who underwent downstaging with chemotherapy before resection, as steatohepatitis increases the risk of post-operative morbidity and mortality.97,98 Thus, a risk-benefit assessment regarding tumor progression during the chemotherapy-free period versus the risk for post-operative complications should be done before deciding on the proper timing of surgery.10 Generally speaking, the longer the interval between chemotherapy and hepatic resection, the lower the risk of liver-related post-operative complications.10,99
In patients scheduled for a chemotherapy regimen with increased risk of CASH, preexisting liver diseases, potential risk factors for CASH, and pre-treatment liver function should be evaluated. The latter includes blood tests and imaging (i.e. ultrasound), and non-invasive fibrosis assessment (i.e. FIB-4, VCTE) if underlying liver disease is suspected. During chemotherapy, liver function should be monitored on a regular basis. In case of suspected liver injury, other potential causes (i.e. hepatotoxic co-medication, viral hepatitis, autoimmunological liver diseases, alcohol abuse, biliary obstruction, tumor progression) should be excluded. Liver biopsy may be indicated based on the results of non-invasive tests and severity of liver damage. In case of liver surgery after chemotherapy, the condition of the liver should also be evaluated preoperatively (Table 2).10
Table 2.
Chemotherapy-associated acute steatohepatitis (CASH)—bullet points
| Chemotherapeutics associated with CASH? |
|
| Risk factors for developing CASH? |
|
| Diagnostic work-up? |
|
| Monitoring during chemotherapy? |
|
PNPLA3, Patatin-like phospholipase domain-containing protein 3.
Conclusion
Hepatic steatosis induced by cytotoxic chemotherapy (CASH) is usually reversible after cessation of therapy, even though it may persist in some cases.94 In contrast, NAFLD is a highly prevalent and further increasing liver disease that can progress to liver cirrhosis and HCC. Due to a lack of effective drug treatments, management of NAFLD mainly focuses on lifestyle interventions.14 NASH-related HCC often occurs in non-cirrhotic patients with well-preserved liver function, which would be optimal conditions for surgical resection. However, due to the lack of robust screening recommendations in non-cirrhotic NASH patients, tumors are often diagnosed at an advanced stage, where only systemic therapies can be applied.47 The recent approval of the combination of atezolizumab plus bevacizumab represents a milestone in the systemic management of patients with advanced stage HCC. Emerging data suggest that changes in the local immune microenvironment and gut dysbiosis may hamper the efficacy of immunotherapy in NASH-HCC.79,82,84 These data are preliminary and need validation in prospective studies. Hence, based on the current evidence, immunotherapy should not be withheld from patients with NASH-HCC.
Pharmacological therapy for NASH is researched extensively. While we are still waiting for a striking breakthrough, we can only speculate about potential benefits of drugs to reverse hepatic steatosis, inflammation, and fibrosis. In NAFLD patients, they may prevent disease progression to cirrhosis and HCC. They may reprogram the immune microenvironment in patients with NASH-HCC, which could have implications on treatment efficacy and outcome. Depending on the mode of action (e.g. anti-inflammatory), some of these drugs could even be tested as a prophylactic treatment in cancer patients with a high risk for CASH.
Acknowledgments
Funding
None declared.
Disclosure
MT received speaker fees from Bristol-Myers Squibb (BMS), Falk Foundation, Gilead, Intercept, and Merck Sharp & Dohme (MSD); advisory board fees from Albireo, Boehringer Ingelheim, BiomX, Falk Pharma GmbH, Genfitt, Gilead, Intercept, Janssen, MSD, Novartis, Phenex, Regulus and Shire; travel grants from AbbVie, Falk, Gilead, and Intercept; and research grants from Albireo, CymaBay, Falk, Gilead, Intercept, MSD, and Takeda. He is also co-inventor of patents on the medical use of norUDCA filed by the Medical University of Graz. MP is an investigator for Bayer, BMS, Lilly, and Roche; he received speaker honoraria from Bayer, BMS, Eisai, Lilly, and MSD; he is a consultant for Bayer, BMS, Ipsen, Eisai, Lilly, MSD, and Roche; he received travel support from Bayer and BMS. All other authors have declared no conflicts of interest.
References
- 1.Swinburn B.A., Sacks G., Hall K.D. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.Van Gaal L.F., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z., Anstee Q.M., Marietti M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskaran K., Douglas I., Forbes H. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B., Scoccianti C., Loomis D. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anstee Q.M., Reeves H.L., Kotsiliti E. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 7.Pinter M., Trauner M., Peck-Radosavljevic M. Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open. 2016;1:e000042. doi: 10.1136/esmoopen-2016-000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dash A., Figler R.A., Sanyal A.J. Drug-induced steatohepatitis. Expert Opin Drug Metab Toxicol. 2017;13:193–204. doi: 10.1080/17425255.2017.1246534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel V., Sanyal A.J. Drug-induced steatohepatitis. Clin Liver Dis. 2013;17:533–546. doi: 10.1016/j.cld.2013.07.012. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meunier L., Larrey D. Chemotherapy-associated steatohepatitis. Ann Hepatol. 2020;19:597–601. doi: 10.1016/j.aohep.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J., van Mierlo K.M.C., Gómez-Ramírez J. Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases. Br J Surg. 2017;104:990–1002. doi: 10.1002/bjs.10572. [DOI] [PubMed] [Google Scholar]
- 12.Younossi Z.M., Marchesini G., Pinto-Cortez H. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation. 2019;103:22–27. doi: 10.1097/TP.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 13.Blachier M., Leleu H., Peck-Radosavljevic M. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 15.Eslam M., Sanyal A.J., George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 16.Eslam M., Newsome P.N., Sarin S.K. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Romeo S., Kozlitina J., Xing C. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paternostro R., Staufer K., Traussnigg S. Combined effects of PNPLA3, TM6SF2 and HSD17B13 variants on severity of biopsy-proven non-alcoholic fatty liver disease. Hepatol Int. 2021 doi: 10.1007/s12072-021-10200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagstrom H., Nasr P., Ekstedt M. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Dulai P.S., Singh S., Patel J. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui M.S., Vuppalanchi R., Van Natta M.L. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–163.e2. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staufer K., Halilbasic E., Spindelboeck W. Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United European Gastroenterol J. 2019;7:1113–1123. doi: 10.1177/2050640619865133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajmera V.H., Liu A., Singh S. Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease. Hepatology. 2020;71:849–860. doi: 10.1002/hep.30974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dulai P.S., Sirlin C.B., Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaswala D.H., Lai M., Afdhal N.H. Fibrosis assessment in nonalcoholic fatty liver disease (NAFLD) in 2016. Dig Dis Sci. 2016;61:1356–1364. doi: 10.1007/s10620-016-4079-4. [DOI] [PubMed] [Google Scholar]
- 28.Kleiner D.E., Brunt E.M., Van Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Brunt E.M., Kleiner D.E., Wilson L.A. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berzigotti A., Albillos A., Villanueva C. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 31.Reiberger T., Puspok A., Schoder M. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III) Wien Klin Wochenschr. 2017;129:135–158. doi: 10.1007/s00508-017-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 33.De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 34.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Younossi Z., Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778–1785. doi: 10.1053/j.gastro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Kim G.A., Lee H.C., Choe J. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Simon T.G., Roelstraete B., Sharma R. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: a population-based cohort study. Hepatology. 2021 doi: 10.1002/hep.31845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 39.Marrero J.A., Kulik L.M., Sirlin C.B. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 40.Ascha M.S., Hanouneh I.A., Lopez R. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 41.Yatsuji S., Hashimoto E., Tobari M. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 42.Loomba R., Lim J.K., Patton H. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158:1822–1830. doi: 10.1053/j.gastro.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Younes R., Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol. 2018;68:326–334. doi: 10.1016/j.jhep.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Paradis V., Zalinski S., Chelbi E. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 45.Kanwal F., Kramer J.R., Mapakshi S. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–1837.e2. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanyal A., Poklepovic A., Moyneur E. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 47.Piscaglia F., Svegliati-Baroni G., Barchetti A. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 48.Ertle J., Dechêne A., Sowa J.P. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 49.Yasui K., Hashimoto E., Komorizono Y. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–433. doi: 10.1016/j.cgh.2011.01.023. quiz e50. [DOI] [PubMed] [Google Scholar]
- 50.Alexander M., Loomis A.K., van der Lei J. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y.Y., Zhu G.Q., Liu T. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep. 2016;6:33743. doi: 10.1038/srep33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedrich K., Wannhoff A., Kattner S. PNPLA3 in end-stage liver disease: alcohol consumption, hepatocellular carcinoma development, and transplantation-free survival. J Gastroenterol Hepatol. 2014;29:1477–1484. doi: 10.1111/jgh.12540. [DOI] [PubMed] [Google Scholar]
- 53.Trepo E., Romeo S., Zucman-Rossi J. PNPLA3 gene in liver diseases. J Hepatol. 2016;65:399–412. doi: 10.1016/j.jhep.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Trépo E., Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol. 2020;72:1196–1209. doi: 10.1016/j.jhep.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Bianco C., Jamialahmadi O., Pelusi S. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2020;74:775–782. doi: 10.1016/j.jhep.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gellert-Kristensen H., Richardson T.G., Davey Smith G. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatology. 2020;72:845–856. doi: 10.1002/hep.31238. [DOI] [PubMed] [Google Scholar]
- 57.Kanda T., Goto T., Hirotsu Y. Molecular mechanisms: connections between nonalcoholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int J Mol Sci. 2020;21:1525. doi: 10.3390/ijms21041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kutlu O., Kaleli H.N., Ozer E. Molecular pathogenesis of nonalcoholic steatohepatitis- (nash-) related hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2018;2018:8543763. doi: 10.1155/2018/8543763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piccinin E., Villani G., Moschetta A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: the role of PGC1 coactivators. Nat Rev Gastroenterol Hepatol. 2019;16:160–174. doi: 10.1038/s41575-018-0089-3. [DOI] [PubMed] [Google Scholar]
- 60.Grohmann M., Wiede F., Dodd G.T. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell. 2018;175:1289–1306.e20. doi: 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhanasekaran R., Felsher D.W. A tale of two complications of obesity: NASH and hepatocellular carcinoma. Hepatology. 2019;70:1056–1058. doi: 10.1002/hep.30649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heimbach J.K., Kulik L.M., Finn R.S. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 63.Johnson P.J., Pirrie S.J., Cox T.F. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–153. doi: 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]
- 64.Del Poggio P., Olmi S., Ciccarese F. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1927–1933.e2. doi: 10.1016/j.cgh.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 65.Simmons O., Fetzer D.T., Yokoo T. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:169–177. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang B., Wu L., Lu X.Y. Small intrahepatic cholangiocarcinoma and hepatocellular carcinoma in cirrhotic livers may share similar enhancement patterns at multiphase dynamic MR imaging. Radiology. 2016;281:150–157. doi: 10.1148/radiol.2016151205. [DOI] [PubMed] [Google Scholar]
- 67.Pinter M., Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;48:598–609. doi: 10.1111/apt.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogel A., Cervantes A., Chau I. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 69.Younossi Z.M., Koenig A.B., Abdelatif D. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 70.Hoppe S., von Loeffelholz C., Lock J.F. Nonalcoholic steatohepatitis and liver steatosis modify partial hepatectomy recovery. J Invest Surg. 2015;28:24–31. doi: 10.3109/08941939.2014.971206. [DOI] [PubMed] [Google Scholar]
- 71.Cauchy F., Zalinski S., Dokmak S. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100:113–121. doi: 10.1002/bjs.8963. [DOI] [PubMed] [Google Scholar]
- 72.de Meijer V.E., Kalish B.T., Puder M. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331–1339. doi: 10.1002/bjs.7194. [DOI] [PubMed] [Google Scholar]
- 73.Gomez D., Malik H.Z., Bonney G.K. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 74.Pinter M., Jain R.K., Duda D.G. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol. 2021;7:113–123. doi: 10.1001/jamaoncol.2020.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinter M., Scheiner B., Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70:204–214. doi: 10.1136/gutjnl-2020-321702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finn R.S., Ryoo B.Y., Merle P. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 77.Yau T., Park J.W., Finn R.S. CheckMate 459: a randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (PTS) with advanced hepatocellular carcinoma. Ann Oncol. 2019;30(suppl 5):v874–v875. [Google Scholar]
- 78.Finn R.S., Qin S., Ikeda M. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 79.Pfister D., Núñez N.G., Pinyol R. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma C., Zhang Q., Greten T.F. Nonalcoholic fatty liver disease promotes hepatocellular carcinoma through direct and indirect effects on hepatocytes. FEBS J. 2018;285:752–762. doi: 10.1111/febs.14209. [DOI] [PubMed] [Google Scholar]
- 81.Ma C., Kesarwala A.H., Eggert T. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heinrich B., Brown Z.J., Diggs L.P. Steatohepatitis impairs T-cell-directed immunotherapies against liver tumors in mice. Gastroenterology. 2021;160:331–345.e6. doi: 10.1053/j.gastro.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gopalakrishnan V., Spencer C.N., Nezi L. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Behary J., Amorim N., Jiang X.T. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. doi: 10.1038/s41467-020-20422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baruch E.N., Youngster I., Ben-Betzalel G. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 86.Davar D., Dzutsev A.K., McCulloch J.A. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clements V.K., Long T., Long R. Frontline science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol. 2018;103:395–407. doi: 10.1002/JLB.4HI0517-210R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ringel A.E., Drijvers J.M., Baker G.J. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183:1848–1866.e26. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z., Aguilar E.G., Luna J.I. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cortellini A., Ricciuti B., Tiseo M. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer. 2020;8:e001403. doi: 10.1136/jitc-2020-001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kichenadasse G., Miners J.O., Mangoni A.A. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McQuade J.L., Daniel C.R., Hess K.R. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robinson S.M., Wilson C.H., Burt A.D. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–4299. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vigano L., De Rosa G., Toso C. Reversibility of chemotherapy-related liver injury. J Hepatol. 2017;67:84–91. doi: 10.1016/j.jhep.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 95.Zorzi D., Laurent A., Pawlik T.M. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 96.Casper M., Zimmermann S., Weber S.N. Risk of chemotherapy-associated liver injury (CALI) in PNPLA3 p.148M allele carriers: preliminary results of a transient elastography-based study. Dig Liver Dis. 2020;52:102–106. doi: 10.1016/j.dld.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Veteläinen R., van Vliet A., Gouma D.J. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20–30. doi: 10.1097/01.sla.0000225113.88433.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vauthey J.N., Pawlik T.M., Ribero D. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 99.Aloia T., Sebagh M., Plasse M. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]