Abstract
Background
Little is known about clinical outcomes other than transition to psychosis in people at Clinical High-Risk for psychosis (CHR-P). Our aim was to comprehensively meta-analytically evaluate for the first time a wide range of clinical and functional outcomes beyond transition to psychosis in CHR-P individuals.
Methods
PubMed and Web of Science were searched until November 2020 in this PRISMA compliant meta-analysis (PROSPERO:CRD42020206271). Individual longitudinal studies conducted in individuals at CHR-P providing data on at least one of our outcomes of interest were included. We carried out random-effects pairwise meta-analyses, meta-regressions, and assessed publication bias and study quality. Analyses were two-tailed with α=0.05.
Findings
75 prospective studies were included (n=5,288, age=20.0 years, females=44.5%). Attenuated positive symptoms improved at 12 (Hedges’ g=0.753, 95%CI=0.495-1.012) and 24 (Hedges’ g=0.836, 95%CI=0.463-1.209), but not ≥36 months (Hedges’ g=0.315. 95%CI=-0.176–0.806). Negative symptoms improved at 12 (Hedges’ g=0.496, 95%CI=0.315–0.678), but not 24 (Hedges’ g=0.499, 95%CI=-0.137–1.134) or ≥36 months (Hedges’ g=0.033, 95%CI=-0.439–0.505). Depressive symptoms improved at 12 (Hedges’ g=0.611, 95%CI=0.441–0.782) and 24 (Hedges’ g=0.583, 95%CI=0.364–0.803), but not ≥36 months (Hedges’ g=0.512 95%CI=-0.337–1.361). Functioning improved at 12 (Hedges’ g=0.711, 95%CI=0.488–0.934), 24 (Hedges’ g=0.930, 95%CI=0.553–1.306) and ≥36 months (Hedges’ g=0.392, 95%CI=0.117–0.667). Remission from CHR-P status occurred in 33.4% (95%CI=22.6–44.1%) at 12 months, 41.4% (95%CI=32.3–50.5%) at 24 months and 42.4% (95%CI=23.4–61.3%) at ≥36 months. Heterogeneity across the included studies was significant and ranged from I2=53.6% to I2=96.9%. The quality of the included studies (mean±SD) was 4.6±1.1 (range=2-8).
Interpretation
CHR-P individuals improve on symptomatic and functional outcomes over time, but these improvements are not maintained in the longer term, and less than half fully remit. Prolonged duration of care may be needed for this patient population to optimize outcomes.
Funding
None.
Research in context.
Evidence before this study
To date, studies of the clinical outcomes of individuals at clinical high-risk for psychosis (CHR-P) have mainly focused on transition to psychosis, while the magnitude and consistency of other outcomes have not been comprehensively evaluated. To overcome this limitation, we searched with a wide combinations of search terms PubMed and Web of Science databases until November 2020, looking for individual studies that evaluated longitudinally attenuated positive symptoms, negative symptoms, depressive symptoms, level of functioning and overall remission. Meta-analytic random-effects models, meta-regression analyses, publication bias assessment and quality assessment using a modified version of the Newcastle-Ottawa Scale (NOS) for cohort studies were carried out.
Added value of this study
This first meta-analysis comprehensively evaluating the magnitude and consistency of clinical outcomes other than transition to psychosis in CHR-P individuals identified 75 studies and showed that most clinical outcomes improve within the first two years, but such improvement is not maintained in the longer term. Functional improvements are maintained, yet at a smaller magnitude after three years. Less than half of the subjects fully remit from CHR-P status.
Implications of all the available evidence
The present work advances clinical knowledge about the longitudinal outcomes in CHR-P individuals, which will inform the clinical management of this group.
Alt-text: Unlabelled box
1. Introduction
At present, indicated prevention in individuals at clinical high-risk for psychosis (CHR-P) [1] is one of the most promising primary preventive approaches in psychiatry [2,3]. The CHR-P paradigm is grounded in three concatenated components: detection, prognosis and intervention [4]. CHR-P individuals are adolescents and young adults, and typically accumulate risk factors for psychotic disorders [[5], [6], [7]], present with subtle clinical symptoms [8] and suffer from functional impairment [9]. Because of these problems (as well as comorbid psychiatric conditions), they often seek help at mental health clinics [10,11], where specialised psychometric instruments are used to formulate a group-level prognosis [12].
Understanding the exact prognosis and longitudinal trajectories of this patient group is pivotal for informing their clinical care [13]. To date, transition to psychosis has been the primary outcome of interest in this field [[14], [15], [16]]. However, the CHR-P group is clinically heterogeneous [17] and display several other outcomes beyond transition to psychosis. These outcomes include varying levels of attenuated positive [18] and negative [19] symptoms, depressive symptoms [20], impairments in functioning [21] and quality of life [22], and overall remission [23]. Many recent studies have examined these outcomes (in addition to transition to psychosis), but the findings have been mixed. Some meta-analyses have addressed the magnitude and consistency of these other outcomes in CHR-P individuals. Still, these meta-analyses have been either limited to randomised controlled trials (e.g. positive [24] and negative [25] symptoms, depression [26]), provided only baseline results (e.g. functioning [9]), or are more than five years old (e.g. remission [23]). To the best of our knowledge, there is no recent comprehensive meta-analysis addressing the magnitude and consistency of clinical outcomes other than transition to psychosis in CHR-P cohorts.
The current meta-analysis seeks to fill this knowledge gap by evaluating comprehensively for the first time longitudinal outcomes beyond transition to psychosis in CHR-P individuals at different follow-up periods while testing for potential outcome moderators.
2. Methods
The protocol for this systematic review and meta-analysis was registered on PROSPERO (CRD42020206271). This study was conducted following the "Preferred Reporting Items for Systematic reviews and Meta-Analyses" (PRISMA [27]) checklist.
2.1. Search strategy and selection criteria
A multi-step literature search was performed by three independent researchers (GSdP, VA, JP) in PubMed and Web of Science database (Clarivate Analytics), incorporating the Web of Science Core Collection, BIOSIS Citation Index, KCI-Korean Journal Database, MEDLINE, Russian Science Citation Index, and SciELO Citation Index, as well as Cochrane Central Register of Reviews and Ovid/PsycINFO databases, from inception until November 1st, 2020. Different combinations of search terms related to CHR-P individuals, their outcomes and progression were applied (eMethods 1). The references for previously published articles were reviewed using MEDLINE, and additional relevant titles were included. Same authors screened the articles as abstracts. After excluding those that did not meet our inclusion criteria, the full texts of the remaining articles were assessed for eligibility and decisions were made regarding their final inclusion in the review.
2.2. Condition and individuals being studied
Studies included were: a) individual longitudinal studies; b) conducted in individuals that fulfilled criteria for CHR-P according to psychometric instruments, which are established in the literature (eMethods 2); c) providing data on at least one of our outcomes of interest (see below); d) published in English. Studies excluded were: a) clinical cases, conference proceedings, study protocols, grey literature or reviews; b) studies with a cross-sectional design; c) studies conducted in samples not fulfilling CHR-P criteria− with or without formal assessment with CHR-P instruments−, such as those at genetic risk for psychosis (e.g., twins, first or second-degree relatives) or schizotypal personality disorder without fulfilling CHR-P instruments’ functional decline criteria; d) data from samples including either non-transitioning or transitioning CHR-P individuals only; e) studies in a language other than English; f) overlapping samples for a given outcome. No additional exclusion criterion was applied for CHR-P individuals transitioning to psychosis, as long as they did not report transitioning CHR-P samples only. For clinical trials, only data from the placebo/needs-based intervention arm was included, while data from the experimental intervention arms were excluded. When two or more studies from the same cohort were found, we contacted corresponding authors to clarify whether there was an overlap in the respective samples. The largest and most recently published sample was retained for each of the outcomes. Disagreements in selection criteria were resolved through discussion and consensus.
2.3. Outcome Measures and Data Extraction
At least two independent researchers (JV-S, AC, JP, LS, FC, SK, JDS) extracted data from all the included studies into an excel file. When the agreement in the data extraction of the different variables was <95% between researchers, a third independent researcher (GSdP, FP, VA) cross-checked the data to ensure accuracy of the extraction. After that step, data extraction disagreements were resolved through discussion and consensus between both extractors and with the study leads. From each study, a predetermined set of variables was included (eMethods 3): first author and year of publication, country, design, CHR-P sample size, CHR-P subgroups [i.e. Attenuated Psychosis Symptoms (APS); Brief Intermittent Psychotic Symptoms (BIPS)/ Brief Limited Intermittent Psychotic Symptoms (BLIPS); Genetic Risk and Deterioration syndrome (GRD); Basic symptoms (BS)], age, sex, CHR-P assessment tool (eMethods 2), follow-up period and outcome. Outcomes measured were: (a) change in severity of attenuated positive symptoms; (b) change in severity of negative symptoms; (c) change in severity of depressive symptoms; (d) change in level of functioning; (e) remission. All outcomes were operationalised as indicated in eTable 1. For outcomes (a)-(d), raw data, including mean value and standard deviation (SD), were extracted at baseline and then at 12/24/≥36 months of follow-up. For outcome (e), we extracted the raw counts of CHR-P individuals in remission (i.e., not fulfilling CHR-P criteria anymore) at 12/24/≥36 months follow-up.
2.4. Risk of bias (quality) assessment
The quality of the included studies was evaluated using a modified version of the Newcastle-Ottawa Scale (NOS) for cohort studies, which has been frequently employed in systematic reviews and meta-analysis in the field [9,15], [28,29] (see eTable 2).
2.5. Data analysis
Outcomes were meta-analysed using Meta and Metaprop packages of Stata statistical software version 16 (StataCorp) [30] and Comprehensive Meta-Analysis software, version 3 (Biostat, Inc) [31], whenever at least two studies per outcome and time point were available. The primary effect size for outcomes (a)-(d) was Hedges’ g33, indicating the magnitude and direction of change from baseline to each follow-up time point (12/24/≥36 months) for each outcome. Positive values of Hedges’ g indexed improvements in the outcome of interest from baseline to follow-up. Hedges’ g=0.2 was interpreted as a small effect size, Hedges’ g=0.5 as a medium effect size and Hedges’ g=0.8 as a large effect size [32,33]. The primary effect size for outcome (e) was the meta-analytical proportion of the categorical event remission at each time point (12/24/≥36 months follow-up). Because the studies were expected to be heterogeneous, meta-analytic random-effects models were used. Heterogeneity among study point estimates was assessed with the Q statistic. The I2 index evaluated the magnitude of heterogeneity with I2>50% and p<0.10 [34] indicating significant heterogeneity. For outcomes (a)-(d), publication bias was evaluated by visually inspecting funnel plots and performing Egger's test [35]. When small effect bias was detected, "trim and fill" sensitivity analyses were employed [36]. Publication bias is not typically assessed for proportions –outcome (e)–, as there are generally no "negative" or "undesirable" results or study characteristics that may have biased publications [37].
Meta-regression analyses were performed when ≥7 studies per outcome were available. We investigated the influence of the following factors: continent (Europe vs North America vs Asia vs other), psychometric instrument (Comprehensive Assessment of At-Risk Mental States [38] -CAARMS- vs Structured Interview for Psychosis-risk Syndromes -SIPS [39,40]- vs other), quality of the included studies (NOS total score), mean age, sex (% female), year of publication, follow-up duration, duration of untreated psychotic symptoms, proportion of APS, proportion of BIPS/BLIPS, proportion of GRD, proportion of BS, functional level, frequency of baseline ICD or DSM-defined comorbidity and exposure to baseline interventions (antipsychotics, antidepressants, other psychotropics, psychotherapy) (eMethods 3). Analyses were two-tailed with α=0.05. In meta-regression analyses, we used α=0.01 to correct for multiple testing.
Role of the funding: There was no funding source for this study. All authors had access to the study data and the corresponding author and lead author had final responsibility for the decision to submit for publication.
3. Results
3.1. Database
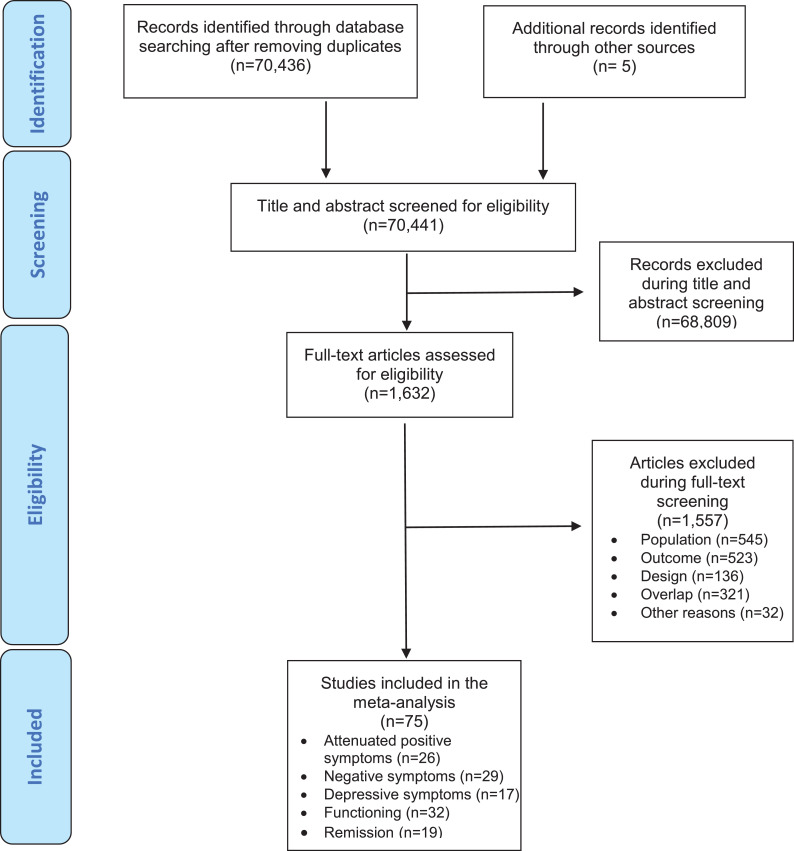
The literature search yielded 70,441 citations after removing duplicates; 1,632 were assessed for eligibility at full text. After excluding 1,557 studies, a final set of 75 studies were included in at least one of the meta-analyses per timepoint (in descending order of frequency): 32 studies evaluated functioning, 29 negative psychotic symptoms, 26 attenuated positive symptoms, 19 remission and 17 studies depressive symptoms (Figure 1; eTable 3). Sixty-one (81.3%) studies were longitudinal cohorts, 12 (16.0%) were randomised clinical trials and two (2.7%) were non-randomised clinical trials. Twenty-seven (36.0%) studies were conducted in Europe, 20 (26.7%) in North America, 14 (18.7%) in Asia, four (5.3%) in Australia, and 10 (13.3%) in more than one country. The mean duration of the follow-up in the included studies was 25.3 months (median=18 months; IQR=12-34 months; range 6.7-192 months). The overall database comprised 5,288 non-overlapping CHR-P individuals (mean age=20.0 years, 44.5% females) (eTable 3). 22.6% were on antipsychotics, 25.2% on antidepressants and 9.3% on anxiolytics at baseline.
Figure 1.
Study selection and inclusion.
3.2. Psychopathological outcomes
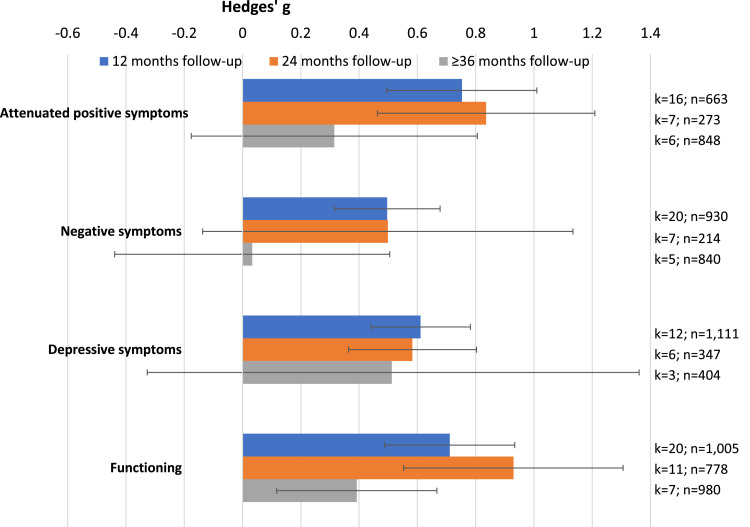
Attenuated positive symptoms in CHR-P individuals had improved at 12 (k=16, n=663, Hedges’ g=0.753, 95%CI=0.495–1.012) and 24 months follow-up (k=7, n=273, Hedges’ g=0.836, 95%CI=0.463-1.209), but not at ≥36 months (k=6, n=848 Hedges’ g=0.315 95%CI=-0.176–0.806). Negative symptoms had improved in CHR-P individuals at 12 (k=20, n=930, Hedges’ g=0.496, 95%CI=0.315–0.678), but not at 24 (k=7, n=214, Hedges’ g=0.499, 95%CI=-0.137–1.134) or ≥36 months follow-up (k=5, n=840, Hedges’ g=0.033, 95%CI=-0.439–0.505). Depressive symptoms had improved at 12 (k=12, n=1,111, Hedges’ g=0.611, 95%CI=0.441–0.782) and 24 months follow-up (k=6, n=347, Hedges’ g=0.583, 95%CI=0.364–0.803), but not at ≥36 months (k=3, n=404, Hedges’ g=0.512 95%CI=-0.337–1.361) (Table 1, Figure 2).
Table 1.
Meta-analytic outcomes other psychosis in CHR-P individuals: psychopathology, functioning and remission
| Outcome, follow-up period |
No. of Studiesa |
Sample size |
Hedges’ g |
z Score |
p-values |
Test for Heterogeneity |
Funnel plot asymmetry |
Egger´s test p |
Trim and fill bias | ||||||||||||
| Mean | 95% CI | Q | I2 | p-values | |||||||||||||||||
| Change in attenuated positive symptoms from baseline to follow-up | |||||||||||||||||||||
| 12 months follow-up | 16 | 663 | 0.753 | 0.495 | 1.012 | 5.709 | <0.001 | 121.798 | 87.685 | <0.001 | Yes | 0.002* | No change | ||||||||
| 24 months follow-up | 7 | 273 | 0.836 | 0.463 | 1.209 | 4.089 | <0.001 | 38.643 | 84.473 | <0.001 | No | 0.828 | D.n.a | ||||||||
| ≥36 months follow-up | 6 | 848 | 0.315 | -0.176 | 0.806 | 1.256 | 0.209 | 159.749 | 96.870 | <0.001 | No | 0.671 | D.n.a | ||||||||
| Change in negative symptoms from baseline to follow-up | |||||||||||||||||||||
| 12 months follow-up | 20 | 930 | 0.496 | 0.315 | 0.678 | 5.360 | <0.001 | 118.240 | 83.931 | <0.001 | Yes | 0.085 | D.n.a | ||||||||
| 24 months follow-up | 7 | 214 | 0.499 | -0.137 | 1.134 | 1.537 | 0.124 | 96.214 | 93.764 | <0.001 | No | 0.187 | D.n.a | ||||||||
| ≥36 months follow-up | 5 | 840 | 0.033 | -0.439 | 0.505 | 0.138 | 0.890 | 124.240 | 96.780 | <0.001 | No | 0.835 | D.n.a | ||||||||
| Changes in depressive symptoms from baseline to follow-up | |||||||||||||||||||||
| 12 months follow-up | 12 | 1,111 | 0.611 | 0.441 | 0.782 | 7.018 | <0.001 | 79.473 | 53.588 | <0.001 | No | 0.213 | D.n.a | ||||||||
| 24 months follow-up | 6 | 347 | 0.583 | 0.364 | 0.803 | 5.201 | <0.001 | 14.535 | 65.601 | 0.013 | No | 0.491 | D.n.a | ||||||||
| ≥36 months follow-up | 3 | 404 | 0.512 | -0.337 | 1.361 | 1.182 | 0.237 | 65.376 | 96.941 | <0.001 | Yes | 0.497 | D.n.a | ||||||||
| Changes in functioning from baseline to follow-up | |||||||||||||||||||||
| 12 months follow-up | 20 | 1005 | 0.711 | 0.488 | 0.934 | 6.239 | <0.001 | 171.658 | 88.931 | <0.001 | Yes | 0.059 | D.n.a | ||||||||
| 24 months follow-up | 11 | 778 | 0.930 | 0.553 | 1.306 | 4.838 | <0.001 | 166.286 | 93.986 | <0.001 | No | 0.257 | D.n.a | ||||||||
| ≥36 months follow-up | 7 | 980 | 0.392 | 0.117 | 0.667 | 2.793 | 0.005 | 81.221 | 92.613 | <0.001 | Yes | 0.134 | D.n.a | ||||||||
| Outcome, follow-up period |
No. of Studiesa |
Sample size |
Proportion |
z Score |
P |
Test for Heterogeneity |
Funnel plot asymmetry |
Egger´s test p |
Trim and fill bias | ||||||||||||
| % | 95% CI | Q | I2 | P | |||||||||||||||||
| Remission | |||||||||||||||||||||
| 12 months follow-up | 9 | 572 | 0.334 | 0.226 | 0.441 | D.n.a | D.n.a | 54.091 | 85.210 | <0.001 | D.n.a | D.n.a | D.n.a | ||||||||
| 24 months follow-up | 6 | 1,317 | 0.414 | 0.323 | 0.505 | D.n.a | D.n.a | 34.039 | 85.311 | <0.001 | D.n.a | D.n.a | D.n.a | ||||||||
| ≥36 months follow-up | 3 | 199 | 0.424 | 0.234 | 0.613 | D.n.a | D.n.a | 15.378 | 86.994 | <0.001 | D.n.a | D.n.a | D.n.a | ||||||||
D.n.a: does not apply
Overlapping samples can contribute with different outcomes data at different follow-up periods.
Figure 2.
Meta-analytic outcomes other than psychosis in CHR-P individuals: change in psychopathology and functioning. Error bars indicate 95% CI. Blue colour indicates 12 months follow-up; orange colour indicates 24 months follow-up; grey colour indicates ≥36 months follow-up.
3.3. Functioning
Functioning in CHR-P individuals was improved at 12 (k=20, n=1,005, Hedges’ g=0.711, 95%CI=0.488–0.934), 24 (k=11, n=778, Hedges’ g=0.930, 95%CI=0.553–1.306) and ≥36 months follow-up (k=7, n=980, Hedges’ g=0.392, 95%CI=0.117–0.667) (Table 1, Figure 1).
3.4. Remission from CHR-P status
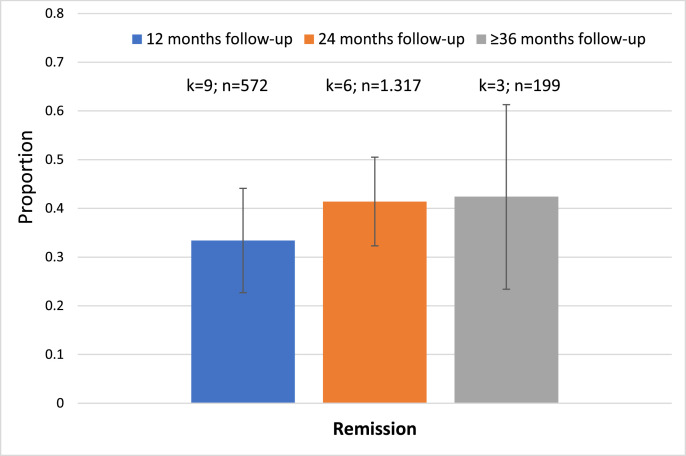
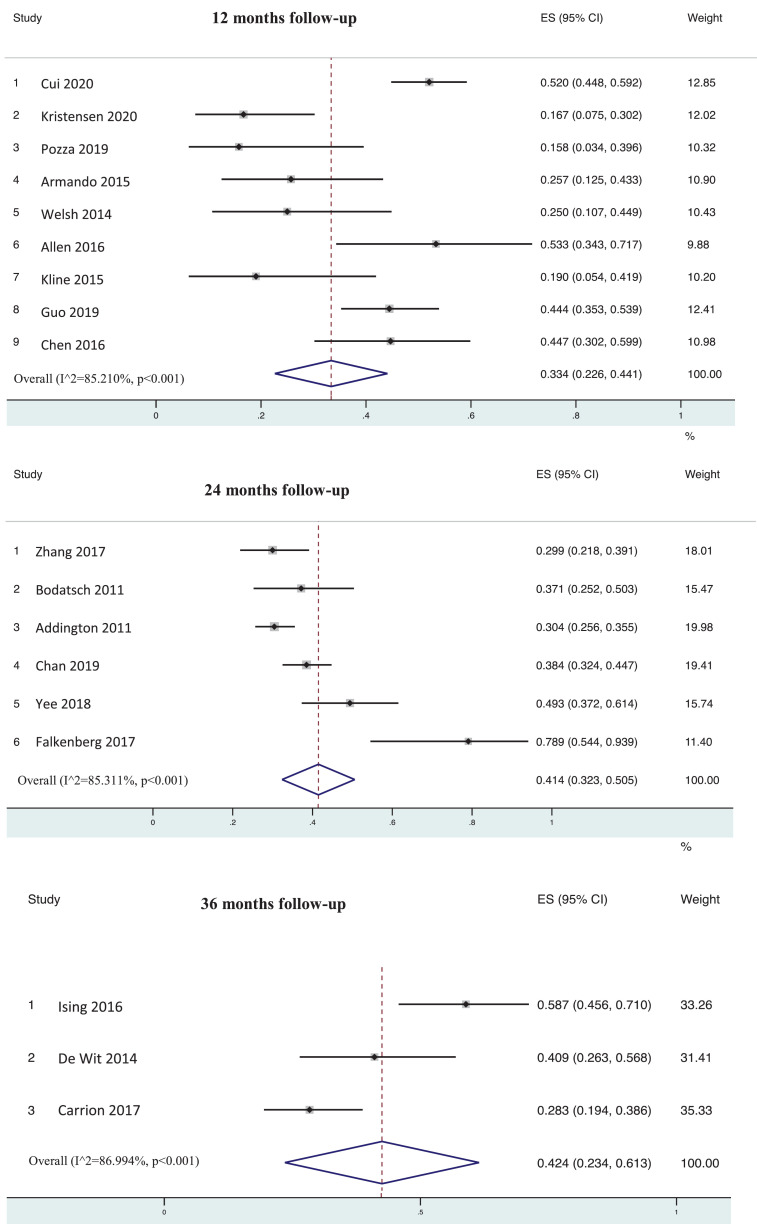
Remission was observed in 33.4% of subjects (95%CI=22.6–44.1%) after 12 (k=9, n=572), 41.4% (95%CI=32.3–50.5%) after 24 (k=6, n=1,317) and 42.4% (95%CI=23.4–61.3%) after ≥36 months follow-up (k=3, n=199) (Table 1, Figure 3, Figure 4).
Figure 3.
Meta-analytic outcomes other than psychosis in CHR-P individuals: remission at follow-up. Error bars indicate 95% CI. Blue colour indicates 12 months follow-up; orange colour indicates 24 months follow-up; colour indicates ≥36 months follow-up.
Figure 4.
Forest plot remission at follow-up in CHR-P individuals. Error bars indicate 95% CI. Lines indicate individual study results; diamonds indicate overall meta-analytical results. Follow up was considered at 12, 24 and 36 months.
3.5. Heterogeneity and Publication bias
Heterogeneity across the included studies was statistically significant for all the outcomes (p<0.05), ranging from I2=53.6% (depressive symptoms at 12 months follow-up) to I2=96.9% (depressive symptoms at ≥36 months follow-up). Egger's test was significant for attenuated positive symptoms at 12 months follow-up (p=0.002). However, results remained the same when the trim and fill method was applied (Table 1).
3.6. Quality Assessment and Meta-regression analyses
The quality of the included studies (mean±SD) was 4.6±1.1 (range=2-8). A higher baseline exposure to antipsychotics (β=0.032, p<0.001) was associated with greater improvement in attenuated positive symptoms at the last available follow-up for each sample. A lower functional level (β=-0.080, p<0.001) was associated with greater improvement in negative symptoms at the last available follow-up for each sample. The meta-regression analyses did not reveal any other significant association (all p>0.05) (eTable 4).
4. Discussion
To our knowledge, this is the first meta-analysis of CHR-P individuals to comprehensively evaluate longitudinal outcomes other than transition to psychosis, including attenuated positive symptoms, negative symptoms, depressive symptoms, functioning and remission. The main finding is that while attenuated positive symptoms, negative symptoms, depressive symptoms and functioning improved during the first two years of follow-up, these improvements were not maintained at/beyond three years. Furthermore, less than half of the subjects achieved remission.
This meta-analysis is based on a large dataset encompassing 75 studies and 5,288 CHR-P individuals. The mean age of 20 years and minimally higher frequency of males (55.5%) in the CHR-P individuals of the meta-analysed cohorts is consistent with the typical sociodemographic profile of this group [11]. The large number of studies identified indicates that, as well as transition to psychosis, other outcomes are increasingly being evaluated in prospective studies of CHR-P subjects.
The first key finding is that psychopathology and functioning consistently improved within the first 1-2 years in individuals at CHR-P. These improvements appear to be most marked for attenuated positive symptoms (medium to large Hedges’ g=0.8 at 1-2 years), followed by depressive symptoms (medium to large Hedges’ g=0.6 at 1-2 years) and then negative symptoms (medium Hedges’ g=0.5 at 1 year only). This is similar to the pattern of symptom improvement in first episode psychosis, where positive symptoms improve more than depressive or negative symptoms [41,42]. This pattern is paralleled by similar functional improvements, with medium to large effects (Hedges’ g=0.7-0.9) in the first two years. Beyond the defining attenuated positive symptoms, CHR-P individuals frequently present with high levels of baseline negative symptoms− particularly social isolation19−, which are often the first presenting symptoms [43]. Similarly, functional impairments in CHR-P individuals can be frequent and severe, arguably similar to functional impairments in other psychiatric disorders [9]. These findings provide some indirect support for the clinical staging model of psychotic disorders, which postulates more marked improvements during the earlier stages [2].
Interpretation of these findings is complex. A first hypothesis is that the observed improvements could be secondary to the effects of specific preventive interventions implemented in CHR-P services (70.7% of the studies involved CHR-P clinical services). However, at present, there is no robust evidence to favour any specific preventive intervention over the others for improving the severity of attenuated positive symptoms [24], negative symptoms [25], depressive symptoms [26] or functioning [44,45] in CHR-P individuals [4,16,46]. Furthermore, not all the studies included in the current meta-analysis implemented recommended preventive interventions. An alternative hypothesis may also be that these improvements represent the natural history of the condition, independent of clinical input. However, as noted below, the improvement trajectory is typically discontinued in the long-term. A third hypothesis may be that these symptomatic and functional improvements could also be related to the care that is often provided by CHR-P clinical services, which can include clinical monitoring, crisis management, support, case management, psychosocial assistance, psychoeducation and medications [[47], [48], [49]]. However, as antipsychotic medications typically used in CHR-P clinics do not reach the minimum effective dosage for treating psychotic symptoms [50], their impact on outcomes is questionable.
Furthermore, the possibility of a negative effect of systematically truncated care for CHR-P individuals is supported by our second core finding that the observed improvements were not maintained in the long-term (i.e. at ≥3 years). Improvements in attenuated positive and depressive symptoms were not maintained after 3-year follow-up, and in negative symptoms not even beyond the first year. Similarly, the magnitude of functional improvement was reduced at 3 years (from large to small effect size, Hedges’ g=0.4). It is thus possible that some of the initial improvements are diminished following discharge from clinical CHR-P services. A similar effect has previously been observed for transition to psychosis, the risk of which persists after the cessation of clinical care from CHR-P services [50]. Notably, the baseline severity of attenuated positive, negative/depressive symptoms and functioning are the strongest meta-analytic predictors of outcome in CHR-P samples (attenuated positive symptoms SMD=0.35, global functioning SMD=-0.29, negative symptoms SMD=0.39) [7]. For example, depression has been associated with a reduced likelihood of remission [51] (although not with an increased risk of transition to psychosis [51,52]). Not surprisingly, the lack of sustained symptomatic and functional improvement at 3 years aligns with our additional analysis showing that only 42.4% of CHR-P individuals were in remission at this timepoint. Nevertheless, this estimate is higher than in our previous meta-analysis (1-3 year follow-up: remission of 35.4% [23]). Overall, these findings caution against the argument of “false positives” frequently leveraged to criticise the CHR-P paradigm, particularly in those with help-seeking behaviour, indicating that this group is at risk of displaying several persisting poor mental health outcomes beyond transition to psychosis [53], which are not currently well addressed. In particular, the suboptimal proportion of those remitting from CHR-P status [54] calls for urgent clinical research on this outcome. The development of a clear and widely established definition of favourable outcomes, including remission or recovery, is essential. Furthermore, research on the likely even larger subgroup of people with poor functional outcome may be even more important than whether or not individuals retained or lost their CHR-P status. Establishing clinical outcomes across multiple domains has the advantages of maximising the numerator of preventivables targets against the denominator of efforts and costs [75].
There was high heterogeneity across the observed outcomes. This finding is partially due to the fact that, in contrast to transition to psychosis, there are no standardised criteria to define non-psychotic outcomes in CHR-P groups. Future global collaborative initiatives could take the opportunity to operationalise good outcomes [55,56], such as clinically significant symptomatic and/or functional improvements, remission (or recovery [57], which is hardly ever reported in the current CHR-P literature) and promotion of good mental health [58]. Our meta-regression analyses showed a positive association between baseline antipsychotic exposure and the magnitude of improvement of attenuated positive symptoms. This is in line with evidence that the therapeutic effects of antipsychotic medications are much greater on positive symptoms than on negative symptoms [57,59]. However, two recent meta-analyses of randomised controlled trials conducted in CHR-P individuals found that antipsychotics were not superior to other interventions for improving APS [24,60]. It is also likely that antipsychotics are initially prescribed to those CHR-P individuals who have higher levels of APS [11,61] and are perceived as being at higher risk of developing psychosis, and therefore have more chances to display relative improvements over follow-up time. Although in the past antipsychotics have been compared to placebo in CHR-P individuals [47], they are currently not recommended by clinical guidelines for CHR-P individuals due to the lack of preventive evidence and low benefit to risk ratio [4].
The main clinical implication of this study is to provide evidence for extending clinical CHR-P care in the long-term, beyond 2 years. This conclusion is supported by evidence that other negative outcomes keep increasing in the long-term (after 2 years), including the risk of psychosis onset, informal and compulsory hospital admissions and cumulative exposure to psychotropic medications [50]. Extending the duration of care might be able to address many of these risks and poor outcomes. Although there is currently no evidence that any specific intervention is more effective than others, our findings suggest that prolonged intervention could be recommended. Such extended care would ideally take place in a stepped care/individualized fashion, providing longer care to those who are in greater need and also varying the content and intensity of specialized or of need-based intervention elements to target individualized goals, as has been suggested for first episode/early psychosis services [62]. These efforts should be refined by precision medicine approaches, which could personalise the efficacy of long-term provision of care, accounting for the individual variability in clinical outcomes [63]. While a plethora of prediction models have been developed and validated on the transition to psychosis and psychosis onset in CHR-P [[64], [65], [66], [67], [68], [69], [70], [71], [72]], only a few have evaluated non-psychotic outcomes [73,74]. Future prognostic and interventional research should address this gap.
This study has several limitations. First, the evidence for some of the evaluated outcomes at some follow-up periods was limited, for instance, for depressive symptoms and remission after ≥36 months follow-up (only three cohorts provided this data). However, the database was large and sufficiently powered to test our a priori defined outcomes at most time points. Second, other outcomes that could have been relevant were not assessed, including quality of life, categorically defined functional outcomes or other psychiatric disorders. Third, there was high heterogeneity across studies and samples, which we tried to address in the meta-regression analyses. Fourth, we included a wide range of CHR-P individuals, some of which transitioned to psychosis, who are expected to present worse outcomes throughout the follow-up. Future studies should evaluate these outcomes in both individuals who transition and who do not transition to psychosis. Finally, limited data precluded certain meta-regression analyses. For example, we could not conduct meta-regression analyses for the duration of untreated APS , proportion of individuals with BS, exposure to antidepressants, exposure to other psychotropics, exposure to psychotherapy, or type of comorbidity.
Funding
There was no funding source for this study.
Data sharing statement
The studies included in this review were publicly available. Data are available upon reasonable request to the lead or corresponding author.
Contributors
GSdP had full access to the data and take responsibility for its accuracy and reporting. GSdP, FP, VA, JV-S, AC, JP, LS, FC, SK and JDS did the literature search, data selection, and data extraction. All the authors critically revised the manuscript for important intellectual content. Senior academic PF-P provided expert supervision during all stages of elaboration of the study.
Declaration of Competing Interest
Dr Salazar de Pablo has received honoraria from Janssen Cilag and grants from Alicia Koplowitz Foundation. Dr Vaquerizo-serrano has received grants from Alicia Koplowitz Foundation. Dr Moreno has been a consultant to or has received honoraria from Janssen Cilaq, Angelini, Servier, Nuvelution, Otsuka, Lundbeck, Pfizer and Esteve outside the submitted work. Prof Gonzalez-Pinto has received grants from the Spanish Ministry of Science and the European Framework and has been a consultant to or has received honoraria from Janssen-Cilag, Angelini, and Roche, support for attending meetings from Janssen, partecipations n a data advisory board for Jenssen, Takeda and Angelini and is president of the Spanish society of Biological Psichiatry and of the Spanish Foundation of Psichiatry and mental health. Dr Díaz-Caneja has received honoraria from AbbVie, Sanofi, Exeltis and Lundbeck. Dr Solmi has been a consultant to or has received honoraria from Angelini and Lundbeck. Prof Arango has received grants or contracts from Bristol-Myers Squibb, Narsad, Sumitomo Dainipon Pharma and Stanley Foundation. He has been a consultant to or has received honoraria or grants from Acadia, Angelini, AstraZeneca, Bristol-Myers Squibb, Gedeon Richter, Janssen Cilag, Lundbeck, Otsuka, Roche, Sage, Servier, Shire, Schering Plough, Stanley Foundation, Takeda and Alicia Koplowitz Foundation. Prof Correll has been receiving grants from Janssen, royalties form UpToDate, consulting fees from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva, payment or honoraria for lectures from Angelini, Gedeon Richter, Janssen/J&J, Lundbeck, Mitsubishi Tanabe Pharma, Mylan, Sumitomo Dainippon. Otsuka, Recordati, Sunovion (no speakers bureau), payment for expert testimony from Janssen and Otsuka, participation on a data safety monitoring Board for Lundbeck, Rovi, Supernus, and Teva, leadership or fiduciary role in other boards for ASCP, receipt of equipment from Takeda and is a shareholder of LB Pharma.
Prof Fusar-Poli has received research fees from Lundbeck and honoraria from Lundbeck, Angelini and Menarini outside the current study. All the other authors report no conflict of interests.
Acknowledgments
Prof McGuire and Prof Fusar-Poli are supported by the PSYSCAN project through the European commission. Dr Salazar de Pablo and Dr Vaquerizo Serrano are supported by the Alicia Koplowitz Foundation. Dr Moreno, Prof Arango, and Dr Díaz-Caneja are supported by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III, European Regional Development Fund ‘A way of making Europe’, Centro de Investigación Biomédica en Red Salud Mental, Madrid Regional Government; and Fundación Mutua Madrileña.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100909.
Appendix. Supplementary materials
References
- 1.Fusar-Poli P. The Clinical High-Risk State for Psychosis (CHR-P), Version II. Schizophr Bull. 2017;43(1):44–47. doi: 10.1093/schbul/sbw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16(3):251–265. doi: 10.1002/wps.20446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arango C, Díaz-Caneja CM, McGorry PD. Preventive strategies for mental health. Lancet Psychiatry. 2018;5(7):591–604. doi: 10.1016/S2215-0366(18)30057-9. [DOI] [PubMed] [Google Scholar]
- 4.Fusar-Poli P, Salazar de Pablo G, Correll CU. Prevention of Psychosis: Advances in Detection, Prognosis, and Intervention. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2019.4779. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Tantardini M, De Simone S. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. European Psychiatry. 2017;40:65–75. doi: 10.1016/j.eurpsy.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Radua J, Ramella-Cravaro V, Ioannidis JPA. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry. 2018;17(1):49–66. doi: 10.1002/wps.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver D, Reilly TJ, Baccaredda Boy O. What Causes the Onset of Psychosis in Individuals at Clinical High Risk? A Meta-analysis of Risk and Protective Factors. Schizophr Bull. 2019 doi: 10.1093/schbul/sbz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Raballo A, Parnas J. What Is an Attenuated Psychotic Symptom? On the Importance of the Context. Schizophrenia Bulletin. 2017;43(4):687–692. doi: 10.1093/schbul/sbw182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusar-Poli P, Rocchetti M, Sardella A. Disorder, not just state of risk: meta-analysis of functioning and quality of life in people at high risk of psychosis. British Journal of Psychiatry. 2015;207(3):198–206. doi: 10.1192/bjp.bp.114.157115. [DOI] [PubMed] [Google Scholar]
- 10.Falkenberg I, Valmaggia L, Byrnes M. Why are help-seeking subjects at ultra-high risk for psychosis help-seeking? Psychiatry Research. 2015;228(3):808–815. doi: 10.1016/j.psychres.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Salazar de Pablo G, Estradé A, Cutroni M, Andlauer O, Fusar-Poli P. Establishing a clinical service to prevent psychosis: What, how and when? Systematic review. Transl Psychiatry. 2021;11(1):43. doi: 10.1038/s41398-020-01165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Cappucciati M, Rutigliano G. At risk or not at risk? A meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry. 2015;14(3):322–332. doi: 10.1002/wps.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusar-Poli P, Hijazi Z, Stahl D, Steyerberg EW. The Science of Prognosis in Psychiatry: A Review. JAMA Psychiatry. 2018;75(12):1289–1297. doi: 10.1001/jamapsychiatry.2018.2530. [DOI] [PubMed] [Google Scholar]
- 14.Kempton MJ, Bonoldi I, Valmaggia L, McGuire P, Fusar-Poli P. Speed of Psychosis Progression in People at Ultra-High Clinical Risk: A Complementary Meta-analysis. JAMA psychiatry. 2015;72(6):622–623. doi: 10.1001/jamapsychiatry.2015.0094. [DOI] [PubMed] [Google Scholar]
- 15.Salazar de Pablo G, Catalan A, Fusar-Poli P. Clinical Validity of DSM-5 Attenuated Psychosis Syndrome: Advances in Diagnosis, Prognosis, and Treatment. JAMA Psychiatry. 2019 doi: 10.1001/jamapsychiatry.2019.3561. [DOI] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Davies C, Solmi M. Preventive Treatments for Psychosis: Umbrella Review (Just the Evidence) Front Psychiatry. 2019;10:764. doi: 10.3389/fpsyt.2019.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fusar-Poli P, Cappucciati M, Borgwardt S. Heterogeneity of Psychosis Risk Within Individuals at Clinical High Risk A Meta-analytical Stratification. Jama Psychiatry. 2016;73(2):113–120. doi: 10.1001/jamapsychiatry.2015.2324. [DOI] [PubMed] [Google Scholar]
- 18.Calkins ME, Woods SW, Bearden CE. Concordance and factor structure of subthreshold positive symptoms in youth at clinical high risk for psychosis. Schizophr Res. 2020 doi: 10.1016/j.schres.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr Res. 2004;68(1):37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 20.DeVylder JE, Yang LH, Harkavy-Friedman JM, Azimov N, Walder DJ, Corcoran CM. Assessing depression in youth at clinical high risk for psychosis: a comparison of three measures. Psychiatry Res. 2014;215(2):323–328. doi: 10.1016/j.psychres.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99(1-3):119–124. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechdolf A, Pukrop R, Köhn D. Subjective quality of life in subjects at risk for a first episode of psychosis: a comparison with first episode schizophrenia patients and healthy controls. Schizophr Res. 2005;79(1):137–143. doi: 10.1016/j.schres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Simon AE, Borgwardt S, Riecher-Rössler A, Velthorst E, de Haan L, Fusar-Poli P. Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res. 2013;209(3):266–272. doi: 10.1016/j.psychres.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Davies C, Radua J, Cipriani A. Efficacy and Acceptability of Interventions for Attenuated Positive Psychotic Symptoms in Individuals at Clinical High Risk of Psychosis: A Network Meta-Analysis. Front Psychiatry. 2018;9:187. doi: 10.3389/fpsyt.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devoe DJ, Peterson A, Addington J. Negative Symptom Interventions in Youth at Risk of Psychosis: A Systematic Review and Network Meta-analysis. Schizophr Bull. 2018;44(4):807–823. doi: 10.1093/schbul/sbx139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. doi: 10.1136/bmj.f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusar-Poli P, Tantardini M, De Simone S. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry. 2017;40:65–75. doi: 10.1016/j.eurpsy.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Catalan A, Salazar de Pablo G, Vaquerizo Serrano J. Annual Research Review: Prevention of psychosis in adolescents - systematic review and meta-analysis of advances in detection, prognosis and intervention. J Child Psychol Psychiatry. 2020 doi: 10.1111/jcpp.13322. [DOI] [PubMed] [Google Scholar]
- 30.Nyaga V, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borenstein M, Hedges L, Higgins J, Rothstein H. Biostat; Englewood, NJ: 2013. Comprehensive Meta-Analysis Version 3. [Google Scholar]
- 32.Hedges LV. Effect sizes in cluster-randomized designs. Journal of Educational and Behavioral Statistics. 2007;32:341–370. [Google Scholar]
- 33.Cohen J. Routledge Academic.; New York, NY: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 34.Sackett D. Oxford; (UK): 1994. The Cochrane Collaboration Handbook. [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins J, Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0; [Google Scholar]
- 37.Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32(2):419–436. doi: 10.1016/j.ridd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Yung AR, Yuen HP, McGorry PD. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 39.Fusar-Poli P, Cappucciati M, Rutigliano G. Towards a Standard Psychometric Diagnostic Interview for Subjects at Ultra High Risk of Psychosis: CAARMS versus SIPS. Psychiatry J. 2016;2016 doi: 10.1155/2016/7146341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGlashan T WB, Woods S. Oxford University; Oxford: 2010. The psychosis-risk syndrome: handbook for diagnosis and follow-up. [Google Scholar]
- 41.Fusar-Poli P, Papanastasiou E, Stahl D. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr Bull. 2015;41(4):892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Németh G, Laszlovszky I, Czobor P. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103–1113. doi: 10.1016/S0140-6736(17)30060-0. [DOI] [PubMed] [Google Scholar]
- 43.Metzak PD, Devoe DJ, Iwaschuk A, Braun A, Addington J. Brain changes associated with negative symptoms in clinical high risk for psychosis: A systematic review. Neurosci Biobehav Rev. 2020;118:367–383. doi: 10.1016/j.neubiorev.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 44.Devoe DJ, Farris MS, Townes P, Addington J. Interventions and social functioning in youth at risk of psychosis: A systematic review and meta-analysis. Early Interv Psychiatry. 2019;13(2):169–180. doi: 10.1111/eip.12689. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt SJ, Schultze-Lutter F, Schimmelmann BG. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):388–404. doi: 10.1016/j.eurpsy.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Bosnjak Kuharic D, Kekin I, Hew J, Rojnic Kuzman M, Puljak L. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11) doi: 10.1002/14651858.CD012236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGorry PD, Yung AR, Phillips LJ. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 48.Amminger GP, Schäfer MR, Papageorgiou K. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 49.Yung AR, McGorry PD, Francey SM. PACE: a specialised service for young people at risk of psychotic disorders. Medical Journal of Australia. 2007;187(7):S43–SS6. doi: 10.5694/j.1326-5377.2007.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 50.Fusar-Poli P, De Micheli A, Signorini L, Baldwin H, de Pablo GS, McGuire P. Real-world long-term outcomes in individuals at clinical risk for psychosis: The case for extending duration of care. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kline ER, Seidman LJ, Cornblatt BA. Depression and clinical high-risk states: Baseline presentation of depressed vs. non-depressed participants in the NAPLS-2 cohort. Schizophr Res. 2018;192:357–363. doi: 10.1016/j.schres.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fusar-Poli P, Nelson B, Valmaggia L, Yung A, McGuire P. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40(1):120–131. doi: 10.1093/schbul/sbs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimbrón J, Ruiz de Azúa S, Khandaker GM. Clinical and sociodemographic comparison of people at high-risk for psychosis and with first-episode psychosis. Acta Psychiatr Scand. 2013;127(3):210–216. doi: 10.1111/acps.12000. [DOI] [PubMed] [Google Scholar]
- 54.Beck K, Andreou C, Studerus E. Clinical and functional long-term outcome of patients at clinical high risk (CHR) for psychosis without transition to psychosis: A systematic review. Schizophr Res. 2019;210:39–47. doi: 10.1016/j.schres.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 55.Petros N, Cullen AE, Vieira S. Examining service-user perspectives for the development of a good outcome checklist for individuals at clinical high risk for psychosis. Early Interv Psychiatry. 2020 doi: 10.1111/eip.12991. [DOI] [PubMed] [Google Scholar]
- 56.Petros N, Mechelli A, Fusar-Poli P, Vieira S, Rowland E, McGuire P. Towards a framework for good outcome in people at clinical high risk for psychosis: A Delphi consensus study. Schizophrenia Research. 2019;208:209–216. doi: 10.1016/j.schres.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 57.Jääskeläinen E, Juola P, Hirvonen N. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–1306. doi: 10.1093/schbul/sbs130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salazar de Pablo G, De Micheli A, Nieman DH. Universal and selective interventions to promote good mental health in young people: Systematic review and meta-analysis. Eur Neuropsychopharmacol. 2020;41:28–39. doi: 10.1016/j.euroneuro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Woodward TS, Jung K, Smith GN. Symptom changes in five dimensions of the Positive and Negative Syndrome Scale in refractory psychosis. Eur Arch Psychiatry Clin Neurosci. 2014;264(8):673–682. [Google Scholar]
- 60.Devoe DJ, Farris MS, Townes P, Addington J. Attenuated psychotic symptom interventions in youth at risk of psychosis: A systematic review and meta-analysis. Early Intervention in Psychiatry. 2019;13(1):3–17. doi: 10.1111/eip.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salazar de Pablo G, Guinart D, Cornblatt BA. DSM-5 Attenuated Psychosis Syndrome in Adolescents Hospitalized With Non-psychotic Psychiatric Disorders. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.568982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Correll CU, Galling B, Pawar A. Comparison of Early Intervention Services vs Treatment as Usual for Early-Phase Psychosis: A Systematic Review, Meta-analysis, and Meta-regression. JAMA Psychiatry. 2018;75(6):555–565. doi: 10.1001/jamapsychiatry.2018.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salazar de Pablo G, Studerus E, Vaquerizo-Serrano J. Implementing Precision Psychiatry: A Systematic Review of Individualized Prediction Models for Clinical Practice. Schizophr Bull. 2020 doi: 10.1093/schbul/sbaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bedi G, Carrillo F, Cecchi GA. Automated analysis of free speech predicts psychosis onset in high-risk youths. Npj Schizophrenia. 2015;1 doi: 10.1038/npjschz.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrion RE, Cornblatt BA, Burton CZ. Personalized Prediction of Psychosis: External Validation of the NAPLS-2 Psychosis Risk Calculator With the EDIPPP Project. American Journal of Psychiatry. 2016;173(10):989–996. doi: 10.1176/appi.ajp.2016.15121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciarleglio AJ, Brucato G, Masucci MD. A predictive model for conversion to psychosis in clinical high-risk patients. Psychol Med. 2019;49(7):1128–1137. doi: 10.1017/S003329171800171X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malda A, Boonstra N, Barf H. Individualized Prediction of Transition to Psychosis in 1,676 Individuals at Clinical High Risk: Development and Validation of a Multivariable Prediction Model Based on Individual Patient Data Meta-Analysis. Frontiers in Psychiatry. 2019;10 doi: 10.3389/fpsyt.2019.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perkins DO, Jeffries CD, Cornblatt BA. Severity of thought disorder predicts psychosis in persons at clinical high-risk. Schizophr Res. 2015;169(1-3):169–177. doi: 10.1016/j.schres.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramyead A, Studerus E, Kometer M. Prediction of psychosis using neural oscillations and machine learning in neuroleptic-naïve at-risk patients. World J Biol Psychiatry. 2016;17(4):285–295. doi: 10.3109/15622975.2015.1083614. [DOI] [PubMed] [Google Scholar]
- 70.Corcoran CM, Carrillo F, Fernandez-Slezak D. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17(1):67–75. doi: 10.1002/wps.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koutsouleris N, Borgwardt S, Meisenzahl EM, Bottlender R, Moller HJ. Riecher-Rossler A. Disease Prediction in the At-Risk Mental State for Psychosis Using Neuroanatomical Biomarkers: Results From the FePsy Study. Schizophrenia Bulletin. 2012;38(6):1234–1246. doi: 10.1093/schbul/sbr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zarogianni E, Storkey AJ, Johnstone EC, Owens DGC, Lawrie SM. Improved individualized prediction of schizophrenia in subjects at familial high risk, based on neuroanatomical data, schizotypal and neurocognitive features. Schizophrenia Research. 2017;181:6–12. doi: 10.1016/j.schres.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 73.de Wit S, Ziermans TB, Nieuwenhuis M. Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: Applying machine learning techniques to brain imaging data. Human Brain Mapping. 2017;38(2):704–714. doi: 10.1002/hbm.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koutsouleris N, Kambeitz-Ilankovic L, Ruhrmann S. Prediction Models of Functional Outcomes for Individuals in the Clinical High-Risk State for Psychosis or With Recent-Onset Depression A Multimodal, Multisite Machine Learning Analysis. Jama Psychiatry. 2018;75(11):1156–1172. doi: 10.1001/jamapsychiatry.2018.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fusar-Poli P, Correll CU, Arango C, Berk M, Patel V, Ioannidis JPA. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry. 2021;20(2):200–221. doi: 10.1002/wps.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.