Abstract
Traditional drug development and discovery has not kept pace with threats from emerging and re-emerging diseases such as Ebola virus, MERS-CoV and more recently, SARS-CoV-2. Among other reasons, the exorbitant costs, high attrition rate and extensive periods of time from research to market approval are the primary contributing factors to the lag in recent traditional drug developmental activities. Due to these reasons, drug developers are starting to consider drug repurposing (or repositioning) as a viable alternative to the more traditional drug development process. Drug repurposing aims to find alternative uses of an approved or investigational drug outside of its original indication. The key advantages of this approach are that there is less developmental risk, and it is less time-consuming since the safety and pharmacological profile of the repurposed drug is already established. To that end, various approaches to drug repurposing are employed. Computational approaches make use of machine learning and algorithms to model disease and drug interaction, while experimental approaches involve a more traditional wet-lab experiments. This review would discuss in detail various ongoing drug repurposing strategies and approaches to combat the current COVID-19 pandemic, along with the advantages and the potential challenges.
Keywords: COVID-19, Drug Repurposing, Emerging Viruses, Antivirals, Clinical Trials
1. Introduction
Despite technological and scientific advances in the understanding of human disease, drug discovery and the translation of research findings into new therapies has not kept pace with the investments and resources undertaken by global pharmaceutical companies. From target identification to market authorization, analyses across all therapeutic areas for drug development takes about 12 years on average and costs over millions of dollars (Van Norman, 2016). It is also not an exception for antiviral agents used for treating emerging and re-emerging viral infectious diseases. From 2015 to 2020, the Food and Drug Administration (FDA) had only approved 14 new antiviral drugs, of which two-thirds are for the treatment of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) (https://www.fda.gov/media/144982/download) (Fig. 1 ).
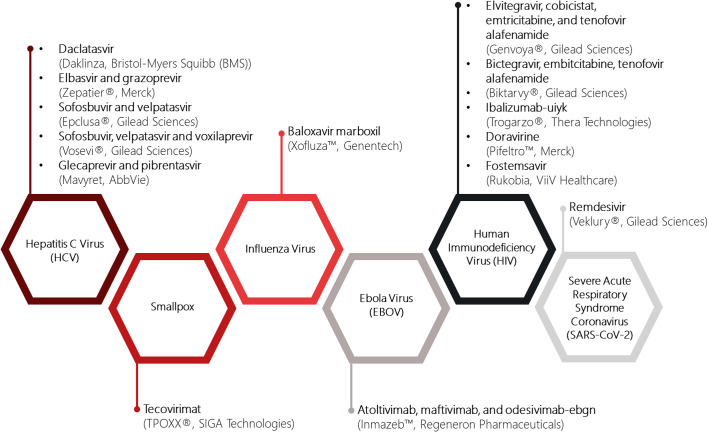
Fig. 1.
List of FDA-approved antiviral drugs from 2015 to 2020. From 2015 to 2020, 15 novel antiviral drugs have been approved for treatment against hepatitis C virus (HCV), smallpox, influenza, ebola virus (EBOV), human immunodeficiency virus (HIV) and recently, for severe acute respiratory virus coronavirus 2 (SARS-CoV-2). The majority of new drugs approved are for HCV and HIV. The active pharmaceutical ingredient, brand name and biopharmaceutical company that manufactures these drugs are indicated accordingly.
In contrast, the pandemic potential of (re)emerging viruses has been on the rise over the past decade, as evidenced by outbreaks of Ebola virus (EBOV), Middle East respiratory virus (MERS-CoV) and Zika virus (ZIKV), for which most still lack specific treatments. In 2020, World Health Organization (WHO) efforts to be “the global guardian of health” is again thwarted by the advent of coronavirus disease 2019 (COVID-19). With over 157 million confirmed cases and 3.2 million deaths worldwide and counting, the outbreak was declared a pandemic by WHO in March 2020 (https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic). Although significant advances have been made to understand and treat COVID-19, the current pandemic has, to a large extent, overwhelmed pharmaceutical companies to expedite the development of vaccines and drugs against this novel disease. The slow pace in novel drug development, coupled with high attrition rates and increasing regulatory hurdles, has prompted drug developers to consider an alternate drug development strategy: drug repurposing.
Drug repurposing, otherwise known as drug repositioning, is a strategy that seeks to identify new indications and targets for approved or investigational drugs that are beyond the scope of its original medical indication (Ashburn & Thor, 2004). One successful repurposing example is recently seen in the FDA approval of remdesivir (Veklury®; Gilead Sciences) for the treatment of COVID-19 (https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness), paving the way for many approved and investigational drugs with repurposing potential under clinical trials for COVID-19, such as dexamethasone (Group et al., 2020) and tocilizumab (Toniati et al., 2020). From the research perspective, repurposing of ‘old’ drugs to treat new diseases is an attractive proposition, especially in the case of an emerging infectious viral disease, as it is more advantageous to de novo drug discovery. First, the development risk is significantly lower, because repurposing candidates would have already been through several stages of clinical development and have well-established safety and pharmacological profiles, thereby translating to lower costs and faster development times. For certain drug candidates, it may be possible to bypass preclinical trials and enter directly into phase II clinical trials if safety assessment and formulation development is already completed. Second, academic and small laboratories can play a major role the drug-discovery process, owing to the availability of drug screening libraries produced by biopharmaceutical companies. This will accelerate the time needed for a biopharma to scour through the pharmacopeia in search of suitable repurposing candidates. Once a suitable repurposing candidate is identified, the formulation and manufacturing chains in the pharmaceutical companies will be ready for large-scale production for emergency use, thereby avoiding the launching cost required for a de novo drug. Third, combination strategies may be deployed with repurposed drugs to delay or reduce monotherapy resistance. Together, the above advantages have the potential to reduce the risks and accelerate return on investment, keeping the cost of bringing a repurposed drug to market to ~10% of the cost needed to market a new drug (Nosengo, 2016). Moreover, drug repurposing allows the use of molecules with novel antiviral properties as molecular tools to unravel the mechanism and pathogenesis of the (re)emerging virus, helping to uncover new therapeutic strategies from previously unexplored cellular pathways, even though not all identified targets may not eventually make it into clinical therapy.
However, beyond the clear advantages presented by drug repurposing, this popular strategy of drug discovery is not without pitfalls. In most cases, the target patient population of a drug repurposing candidate will differ significantly from the one for which the drug has been approved for, lengthening the repurposing workflow required before the drug can undergo clinical trials. To further complicate matters, pharmaceutical companies are often prevented or disincentivised from taking over the further development and costs of clinical trials for some of the identified drugs, as they are protected by intellectual property and/or programs. Even in cases where the agent is generically available, the formulation, possibly even the route of administration, must be changed to generate a new product – both factors necessitate Phase I trials. For combination repurposing, regulators will require Phase I trials in which the repurposed agent will be tested in untried combinations to ensure that there are no unacceptable toxicities. Furthermore, target identification is often indirect and identified drugs may act on multiple targets, making them unusable for repurposing. Herein, we provide an overview of the common approaches and strategies used in drug repurposing. We will discuss how drug repurposing have helped to address the urgent need for antiviral drugs against COVID-19 using representative repurposing examples. Finally, we will discuss major challenges facing drug repurposing and provide recommendations that could maximise the potential of drug repurposing during this unprecedented time.
2. Drug repurposing approaches
As with all drug repurposing projects, drug repurposing for COVID-19 undergoes these three steps before it can be considered for progression through the development pipeline: candidate drug identification; mechanistic evaluation of the drug effect in preclinical models; and evaluation of candidate drugs' efficacy in phase II clinical trials (Pushpakom et al., 2019). Among these three steps, the first step – the identification of a drug for COVID-19 with a high repurposing potential – is the most crucial, as this would determine the repurposing success of the candidate drug. As such, a systematic approach is commonly used to identify the right drug with high confidence.
The systematic approach to drug repurposing can be broadly divided into computational and experimental approaches, although it should be noted that these approaches may be used synergistically to achieve the best repurposing outcome (Fig. 2 ).
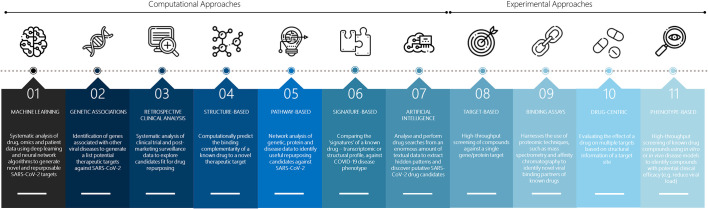
Fig. 2.
Approaches to drug repurposing. Drug repurposing approaches can be broadly divided into computational and experimental approaches. Here, computational approaches may be used individually or in combination to analyse COVID-19 disease pathogenesis to obtain useful information for screening putative COVID-19 drug candidates in the experimental approach. The vector images have been designed using resources from Flaticon.com.
2.1. Computational approaches
2.1.1. Machine learning (ML)-based
Computational approaches, in general, largely utilizes machine learning to capture data dependencies from exponential volumes of biological data. The systematic analysis of omics (e.g. genomics, transcriptomics, proteomics, metabolomics) data, chemical structure, molecular docking studies and past SARS-CoV and MERS-CoV clinical data will then help derive novel drug repurposing hypotheses against SARS-CoV-2. In machine learning (ML)-based drug repurposing, researchers apply a variety of ML-based techniques, such as deep learning and neural network, to discover repurposable candidates.
In this pandemic, several deep-learning algorithms has been developed to identify novel drug-protein interactions, as in the use of the molecule transformer-drug target interaction (MT-DTI), a deep learning model designed for predicting drug-protein binding affinity. Using this model, Beck and colleagues showed that atazanavir is a promising inhibitor against SARS-CoV-2 3C-like proteinase with a dissociation constant of 94.94 nM (Beck, Shin, Choi, Park, & Kang, 2020). In another report, an integrative technique combining network-based and deep-learning approaches, termed CoV-KGE, has identified a total of 41 repurposable drugs, of which some has been validated through transcriptomics and proteomics data derived from existing clinical trials (Zeng et al., 2020). Besides existing approved drugs, novel compounds such as anti-HCV drug IDX-184, have also been identified for SARS-CoV-2 using virtual screening and supervised machine learning methods (Kadioglu, Saeed, Greten, & Efferth, 2021).
To develop a graph neural network for COVID-19 drug treatment, nodes are used as distinct biomedical entities (e.g. drug, protein and disease), while the edges represents the relationship between the entities (e.g. disease-protein interaction) (Gysi et al., 2020). Using this technique, Hsieh and co-workers has reported a simplified drug repurposing workflow that incorporates SARS-CoV-2-drug interactions and deep graph neural networks to prioritize promising repurposing candidates. From there, a total of 22 drugs and various drug-combinations have been identified for COVID-19 treatment (Hsieh et al., 2020).
There is a limitation to ML-based drug repurposing. To generate a reliable prediction of repurposable candidates, researchers must include multiple sources of data during ML-based repurposing as computational drug-repurposing are, in general, sensitive to the type and quantity of datasets used. Since COVID-19 emerged only in late 2019 and is constantly evolving, there is currently no consensus on what type of patient information should be included to predict and screen drug candidates, resulting in each group reporting a subset of potential repurposing candidates. Hence, it is vital for researchers to develop an efficient ML-based repurposing algorithm that can evolve and respond to future disease outbreaks over time using standardized patient information.
2.1.2. Genetic associations
Over the past decade, the insurgence of genome-wide association studies (GWAS) has led to a greater understanding into the pathogenesis of many infectious disease and the genetic variants that are associated with it. With these biological data becoming increasingly available, it is now possible to identify novel targets for clinically approved drugs to treat disease phenotypes studied by GWAS, leading to drug repurposing. Progress has been made to understand the genetic factors which are involved in the development of COVID-19 using GWAS. A GWAS study, conducted by the Severe Covid-19 GWAS Group involving 1980 severely ill COVID-19 patients, identified 3p21.31 gene cluster as a genetic susceptibility locus in COVID-19 patients with respiratory failure (Severe Covid et al., 2020). The 3p21.31 gene cluster consists of the following genes: SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6 and XCR1, which may be novel drug targets for COVID-19.
While results from the GWAS study has presented several repurposing opportunities, the primary problem with GWAS (not only in respect to COVID-19) lies in the huge data body it generates that associations will always be found, and therefore each must be investigated by orthogonal methods to verify it. In addition, GWAS is also highly dependent on having sufficient genomes to analyse to overcome the multiple testing problem in GWAS, so this requires collaborative effort among clinicians and researchers who are residing in COVID-19 hot spots to make GWAS possible since travelling is limited during the pandemic. Another important concern with the validity of GWAS is the population stratification issue, as GWAS signals detected have mostly been attributed to cryptic population stratification (McClellan & King, 2010). For this reason, researchers should try to overcome the stratification issue by adopting family based GWAS or perform correction for population substructure (Tam et al., 2019). So, while GWAS is a powerful method in identifying genetic factors with COVID-19, researchers should exercise rational use of GWAS and conduct further functional studies to determine the direction of effect (agonist or antagonist) for the gene variant before the prediction of repurposing targets (Sanseau et al., 2012).
2.1.3. Retrospective clinical analyses
Dubbed the famous “blue pill”, sildenafil (Viagra®; Pfizer) is the most successful example of a repurposed candidate drug from retrospective clinical analyses. Sildenafil, a phosphodiesterase 5 (PDE5) inhibitor, was originally developed and indicated for angina. Unexpectedly, the drug dilates the blood vessels in the penis and cause erections during a phase I clinical trial conducted in the 1990s (Ashburn & Thor, 2004). While the drug has failed for its primary indication, sildenafil was later rescued and repurposed for erectile dysfunction. Today, drug repurposing through retrospective clinical analyses has led to numerous repurposing success stories, such as the use of propranolol for osteoporosis and melanoma (De Giorgi et al., 2011; Wiens, Etminan, Gill, & Takkouche, 2006), and the use of raloxifene and metformin for breast cancer (Jiralerspong et al., 2009; Lippman et al., 2006).
In the COVID-19 pandemic, retrospective clinical analyses have also been employed to elucidate SARS-CoV-2 pathogenesis and disease manifestations through network medicine, clinical and multi-omics observations. Using this approach, several promising repurposing candidates have since been identified, such as melatonin, carvedilol (Zhou et al., 2020) and umifenovir (Lian et al., 2020). In another study focusing on the retrospective comparison of COVID-19 drugs, it was reported that hydroxychloroquine exhibits the potential for the disease management of COVID-19, while oseltamivir may be used in combination with hydroxychloroquine (J. Tan et al., 2021). There are merits to using retrospective clinical analyses – it represents an opportunity to convert previously failed trials into a promising one and provide an impetus to thrust a borderline successful trial further into the next stage of clinical trials, if the trial was previously dropped due to a lack of competitive advantage against its competitors.
In addition, retrospective clinical data is available from several sources, such as the WHO database for adverse drug reactions (VigiBase), FDA Adverse Event Reporting System (FAERS), clinical trial data and post-market surveillance data. The wealth of data from retrospective clinical data, when analysed, may help indicate drug repurposing leads. However, there are major challenges in accessing and analysing retrospective clinical data, largely due to patient confidentiality, legal hurdles, and limited research capability on retrospective clinical datasets.
2.1.4. Structure-based
Perhaps one of the most popular drug repurposing approach in the COVID-19 pandemic, structure-based screening studies for inhibitors against SARS-CoV-2 has been highly reported from various research groups around the world, due to high computational power and the availability of 3D structures of drug and receptor targets (Abo-Zeid, Ismail, McLean, & Hamdy, 2020; Hall Jr. & Ji, 2020; Jo, Kim, Kim, Kim, & Shin, 2020; Nguyen, Thai, Truong, & Li, 2020; Talluri, 2020). Structure-based screening is a computational approach used in the early stages of a drug repurposing campaign to scrutinise a compound library for bioactive molecules against a certain drug target based on binding site complementarity (Q. Li & Shah, 2017). This virtual screening proceeds in two steps. First, molecular docking is performed on a collection of drug candidates to bind the 3D structure of a molecular target obtained through NMR, X-ray methods, or computational modelling (Q. Li & Shah, 2017). Next, a scoring function is applied to predict the likelihood of each ligand that binds the molecular target with high affinity, generating valuable repurposing hits.
In general, structure-based approaches can be categorized into either antiviral target-based or host target-based approaches. Antiviral target-based approach involves using the 3D structure of the viral proteins to screen for potential inhibitors while host target-based approach involves using the 3D structure of the host proteins instead. To further clarify the methods used, both antiviral target-based approach and host target-based approach can be sub-divided into either drug-centric approach or target-centric approach. For drug-centric approach, the drug candidate is known, and molecular docking is performed to screen for potential docking site (or molecular target) of the drug candidate. For target-centric approach, the target protein is known, and molecular docking is performed to screen for potential inhibitory ligands of the said target.
In the case of SARS-CoV-2, this target-centric approach is highly advantageous since knowledge on its predecessors (SARS-CoV and MERS-CoV) and cellular targets are well known, therefore potentially obviating the need to elucidate the mode of action of the hits. As such, multiple drugs can be interrogated against a particular target (for instance Angiotensin-Converting Enzyme 2, ACE2), as in conventional docking. As an example of antiviral target-based, target-centric approach, Elfiky utilised conventional molecular docking methods involving SARS-CoV-2 RNA-dependent RNA polymerase (RdRP) to screen for potential inhibitors of SARS-CoV-2, screening various currently approved anti-polymerase drugs and found ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir as potent inhibitors of RdRP, with binding energies between −7.0 to −7.8 kcal/mol (Elfiky, 2020). Conversely, drug-centric approach may also be used to identify novel interactions that can provide new insights into COVID-19. As an example of antiviral target-based, drug-centric approach, Martin and Cheng (2020) first identified toremifene as a potential drug candidate for SARS-CoV-2, followed by molecular docking to identify the molecular targets of toremifene. They successfully identified two potential targets, namely the spike glycoprotein and NSP14 (Martin & Cheng, 2020).
On the other hand, host target-based, target-centric approach has also been utilised to screen for potential inhibitors of SARS-CoV-2. For example, ACE2 has previously been documented to interact with SARS-CoV-2 spike glycoprotein and facilitate viral entry (Basit, Ali, & Rehman, 2020). Using this information, Khelfaoui et al. performed molecular docking using DrugBank database to identify compounds which show high binding affinity with ACE2, as well as to study the binding affinity of the selected compounds with the SARS-CoV-2 spike glycoprotein/ACE2 complex (Spike/ACE2 complex) (Khelfaoui, Harkati, & Saleh, 2020). From this study, they identified a few compounds, namely ramipril, delapril and lisinopril which show better binding affinity to ACE2 and the Spike/ACE2 complex as compared to chloroquine and hydroxychloroquine.
Aside from that, there are a number of online resources currently available which are dedicated to the collection, arrangement and management of modelling results, such as the GHDDI COVID-19 information sharing portal and COVIDScholar. Interestingly, a web server is created for the masses to try their hand on molecular docking using a database consisting of various viral and human proteins involved in viral infection (Shi et al., 2020).
That being said, structure-based screening can be also challenging since targeting a single protein or gene is often overly simplifying a disease progression. Oftentimes, multiple factors are involved in a disease progression and as such, inhibiting a single target may not be sufficient. Aside from that, since the screening is done in a cell-free system, there is also a possibility that the hits may not be viable for application in a living system, owing to its cytotoxicity, bioavailability or half-life. Therefore, the hits from a structure-based screen are typically further validated in cell-based assays. As a specific example, the molecular docking study involving toremifene conducted by Martin and Cheng would need to be further validated in vitro to confirm the efficacy of toremifene in inhibiting the action of SARS-CoV-2 spike glycoprotein and NSP14 during live disease progression (Martin & Cheng, 2020). Furthermore, to fully ascertain that the target of toremifene is indeed the spike glycoprotein and NSP14, co-crystal structure of toremifene binding to spike glycoprotein or NSP14 should be resolved.
2.1.5. Pathway-based
Pathway mapping has proved to be a major cornerstone in drug repurposing during this pandemic (Mousavi, Rahmanian, & Sami, 2020; Zhou et al., 2020). This approach is a crucial complement to GWAS data, as potential gene targets identified by GWAS may not be fully amenable as drug targets. In these circumstances, pathway analysis of the genes upstream or downstream of a GWAS-associated target may be used to identify promising candidate drugs for repurposing opportunities (Greene & Voight, 2016).
In pathway mapping, information such as gene expression data, protein interactions and metabolic pathways are used to construct drug and disease networks to define the lead targets for drug repurposing based on shared molecular mechanisms (Park, 2019). Using network proximity analyses between drug targets and HCoV-host interactions in the interactome, Zhou and colleagues identified 16 potential repurposable drugs, including sirolimus, irbesartan and melatonin (Zhou, Hou, Shen, Huang, et al., 2020).
That being said, pathway mapping is not without its limitations. One major limitation is the dependency of pathway mapping methods on gene annotations currently available in databases (Khatri, Sirota, & Butte, 2012). Gene annotations in databases (such as GO database) are still far from completion, with thousands of genes not yet annotated, and thousands more requiring update on their annotations as more experimental evidence surface (GO database statistics can be viewed at http://geneontology.org/stats.html). A significant portion of the annotations require manual curation and therefore, complete annotation of human genes would not be available within the next few years. Due to the incomplete nature of human gene annotations, pathway mapping would possibly fail to completely map the gene interactions between the target gene and its downstream and upstream effectors, missing out crucial genes as potential drug targets.
2.1.6. Signature-based
Signature based approach compares the unique characteristics or ‘signatures’ of a drug with that of another drug, disease or clinical phenotype (Hieronymus et al., 2006; Keiser et al., 2009). Drug signatures may be acquired from omics data, chemical structures and adverse event profiles (Pushpakom et al., 2019). Here, drug-disease comparison, drug-drug comparison and chemical structure-biological activity comparison may be adopted to derive useful information on repurposing candidates from signature matching (Dudley, Deshpande, & Butte, 2011; Iorio, Rittman, Ge, Menden, & Saez-Rodriguez, 2013). In drug-disease comparison, a candidate drug's unique transcriptomic signature is first derived by comparing the gene expression profile of a cell or tissue before and after drug treatment. This transcriptomic signature is then used to compare against a disease-associated expression profile, which is derived from comparison between healthy and diseased conditions. Through this process, differentially expressed genes (DEGs) would help to determine if the candidate drug may have a potential effect on the disease (Dudley et al., 2011; Sirota et al., 2011).
On the other hand, drug-drug comparisons aim to identify similar modes of action between unrelated drugs (drugs of a different pharmacological class or dissimilar chemical structures), which may help uncover alternative drug targets and off-target effects of a particular drug which can be investigated for clinical use (Keiser et al., 2009). For these two approaches, the connectivity map (CMap) is a useful resource, as it comprises of more than 1.5 million gene expression profiles of ~5000 small molecule compounds in multiple cell lines (https://www.broadinstitute.org/connectivity-map-cmap). Finally, in chemical structure-biological activity comparison, the chemical structure of two drugs may be compared to investigate potential shared biological activity between structural similarities.
The use of drug-disease comparison approach for SARS-CoV-2 infection was adopted by Mousavi and colleagues (Mousavi et al., 2020). In this study, DEGs detected in A549 cells and primary human lung epithelial cell line NHBE were identified and applied in CMap to detect similar acting drug candidates. To this end, lansoprazole, folic acid and many other clinically approved drugs were identified as potent drugs against SARS-CoV-2, exemplifying the usefulness of signature matching as a tool in drug repurposing (Mousavi et al., 2020).
However, signature-based drug repurposing has its limitations as well. Due to the nature of the ‘signatures’ being differentially expressed genes (DEG's), it raises the question of what threshold to adopt when judging whether a gene is expressed significantly different as compared to its untreated counterpart. While a threshold of ≥1.5 log fold change is often adopted (Chan, Wang, Turner, Baldwin, & Gu, 2019), a generalised threshold does not take into account the fundamentally different gene expression levels of vastly different subjects. In addition, there is also the question of which and how many genes to choose when determining the signatures of a certain drug or diseases. For example, a study by Johnstone and colleagues regarding CNS injury and a study by Cheng and colleagues regarding glioblastoma both utilised genetic signatures to draw insight regarding the disease and treatment progression (Cheng et al., 2015; Johnstone et al., 2012). However, while the former study monitored 21 genes as the signatures, the latter study monitored 1000 genes. This vast difference illustrates the lack of standardization with regards to signature-based studies and possibly one of the reasons why signature-based studies are often met with limited real-world clinical application.
2.1.7. Artificial intelligence-based
In this big data era, artificial intelligence (AI)-based drug repurposing has been thrust into the global spotlight during the pandemic. This was made possible by the formidable computing power of AI models and algorithms in analysing and processing massive amounts of infectious disease and public health surveillance data (Wong, Zhou, & Zhang, 2019). Since then, this technology has becoming increasingly popular among researchers to leverage on millions of patient and clinical trial records to reveal data insights, with some AI algorithms used include deep learning architecture and graph representation learning (reviewed in (Zhou, Wang, Tang, Nussinov, & Cheng, 2020).
Global research efforts are now underway to apply AI-based drug repurposing for COVID-19, with the development of web servers and resources as in the case of CLAIRE Innovation Network (https://covid19.claire-ai.org/). While AI-based drug repurposing is at its infancy, several reports have shown encouraging results. For instance, using BenevolentAI's knowledge graph, Richardson and co-workers identified baricitinib as a potential therapeutic for SARS-CoV-2 from a list of approved drugs (Richardson et al., 2020). In another report, an AI platform established by Ke and colleagues has predicted over 80 potential repurposing dug candidates, of which eight drugs had been validated to show in vitro antiviral properties against feline infectious peritonitis (FIP) virus in Fcwf-4 cells (Ke et al., 2020). AI may also be used in combination with other computational approaches to hasten the screening of drug libraries. For example, AI and molecular docking was carried out in two separate studies to identify potential drugs against COVID-19 (Ton, Gentile, Hsing, Ban, & Cherkasov, 2020; Zhang, Wu, Zhang, Deng, & Peng, 2020).
The results presented here, although encouraging, has presented unique challenges, stemming from the shortage of training data at the start of the COVID-19 outbreak, making it difficult to generate accurate, predictive AI algorithms at ironically the time where disease forecasting and drug screening is the most crucial (Santosh, 2020). Further, current AI-driven tools are mostly limited to one data type (Santosh, 2020). Given the highly complex nature of COVID-19, such AI-based prediction may misrepresent the overall effectiveness of potential repurposing candidates if assessments are made based on a single data input. Hence, this exemplifies the need to develop better AI tools in drug repurposing that could make use of multitudinal and multimodal data so that researchers can support each drug repurposing decision with high confidence. In this context, the Multimodal Restricted Boltzmann Machine approach (MM-RBM) has been reported to have the capability to connect multiple modalities for drug repurposing applications (Hooshmand et al., 2020).
2.2. Experimental approaches
2.2.1. Target-based
In a target-based screening, the efficacy of the drugs is evaluated based on how well the drug is able to inhibit a single target crucial for the disease progression, typically a protein or a gene (Sams-Dodd, 2005). In this case, the mechanism of action is already known. Target-based drug screening typically consists of a few steps. The first step is target identification where pathways or cellular processes crucial for disease progression is first identified, followed by target validation to verify the importance of the target to the disease. The next step is assay development, where the target-disease interaction is captured in a model, typically utilising cell-free binding assay, to enable screening of the drugs. Subsequently, hit identification is carried out where various drugs are tested and evaluated based on their efficacy in inhibiting the said target. The last step is to optimize the hits by chemically modifying the hits to improve their efficacy, safety, and stability.
Many drug repurposing projects adopt target-based screening due to the relatively simpler requirements. One example is the drug repurposing project by Xia et al. targeting spike glycoprotein of SARS-CoV-2 which induces cell-cell fusion and mediates viral entry into the host cell (Xia et al., 2020). The study identified SARS-CoV-2 spike glycoprotein to be a viable drug target, and then expressed the spike protein by transfecting HuH-7 cells with a plasmid containing the SARS-CoV-2 spike glycoprotein gene. The transfected cells were then used for cell fusion assay where the spike protein would initiate formation of syncytia. Using this cell fusion assay, the effectiveness of peptide EK1 along with its lipopeptide derivatives in reducing the formation of syncytia were examined and lipopeptide EK1C4 was found to be a potent cell fusion inhibitor. Further validation study was then carried out by utilising plaque reduction assay involving Vero-E6 cells infected with live SARS-CoV-2 virus, followed by murine model study. The lipopeptide EK1C4 was then identified as a potential SARS-CoV-2 therapeutic agent. This study illustrates the basic principles of target-based drug screening, as well as how the logistical demands can be met. The initial cell fusion assay can be carried out in a Biosafety Level (BSL)-2 facility since it only involved the spike glycoprotein and not the infectious SARS-CoV-2 virus. Subsequently, only when a promising candidate was discovered, a more stringent validation was then carried out in a BSL-3 facility involving infectious SARS-CoV-2 virus.
2.2.2. Binding assays
To identify the binding partners for clinically approved drugs, mass-spectrometry based proteomic techniques have been successfully utilized in studying earlier pandemics and epidemics such as SARS-CoV, MERS-CoV and ZIKV (Raj et al., 2015; Scaturro, Kastner, & Pichlmair, 2019). In the case of COVID-19, Gordon and co-workers performed affinity purification mass spectrometry and identified 332 high-confidence protein-protein interactions between SARS-CoV-2 and proposed 29 FDA-approved compounds which may be used to treat COVID-19 (Gordon et al., 2020). While binding assays has shown its potential as a key COVID-19 research tool, development of rapid and sensitive mass spectrometry techniques are not fully mature yet to realize their full potential in the COVID-19 pandemic.
2.2.3. Drug-centric
In this strategy, a drug with known activity against a particular disease is chosen and its mechanism of action is scrutinized for potential activity against other diseases (Parisi et al., 2020). One classical example is thalidomide, a drug prescribed to cure morning sickness in pregnant women but recalled within five years due to the associated birth defects. While unfortunate, it has been discovered much later on that the robust anti-angiogenic properties of thalidomide are useful for treating multiple myeloma (Ashburn & Thor, 2004). In this case, while the drug is intended to be a form of sedative, its off-target effects has proven to be beneficial for cancer treatment. Another example would be valproic acid, originally developed to help alleviate the symptoms of bipolar disorder and seizures. It was discovered later on that valproic acid has an off-target effect on histone deacetylase 2, a protein crucial for cancer development. Based on this finding, further experiments were conducted and owing to its success, valproic acid has now been prescribed to treat cancerous growth such as familial adenomatous polyposis (Huang & Guo, 2006). This example further illustrates how the mechanism of action of a known drug can be used to predict its curative properties on other diseases as well.
Perhaps the most famous antiviral against SARS-CoV-2 today would be remdesivir, which is a product of drug-centric drug repurposing. Developed by US-based Gilead Sciences under the trade name of Veklury®, remdesivir is a broad-spectrum antiviral in the form of adenosine analogue. Remdesivir exerts its effect by binding to the viral RdRP and incorporating itself into the nascent RNA chain, resulting in premature termination of the RNA chain and thus inhibiting viral RNA replication (Wang et al., 2020). Originally, remdesivir was first developed as an antiviral against HCV but was found to be ineffective and therefore repurposed for the treatment of EBOV (Tchesnokov, Feng, Porter, & Gotte, 2019). In 2017, repurposing study done by Sheahan et al. proved that remdesivir is also effective against human coronaviruses, namely the original SARS-CoV and MERS-CoV (Sheahan et al., 2017). Based on the mechanism of action by targeting viral RdRP, and the proven effectiveness against human coronaviruses, Wang et al. conducted efficacy studies on remdesivir against SARS-CoV-2 and it was found that remdesivir was also effective in inhibiting SARS-CoV-2 infection (Wang et al., 2020). Following this finding, a series of clinical trials were conducted and the final report, published on November 5, 2020 by Beigel et al., found that remdesivir treatment successfully shortened the recovery time of adults infected with SARS-CoV-2 as compared with the placebo treatment (Beigel et al., 2020). This example illustrates how drug-centric drug screening has been successful in discovering a therapeutic agent against SARS-CoV-2 infection.
2.2.4. Phenotype-based
In a phenotype-based screening, the efficacy of a drug is evaluated against the phenotype – or observable characteristics – in a living system. Established animal- or cell-based models of diseases are utilised to mimic a naturally acquired infection, followed by introduction of various drugs in order to observe the effects brought about by the drugs. Should the disease progression be halted upon the addition of a particular drug, the said drug is considered to be a ‘hit’ and further downstream studies can be carried out, typically to elucidate the mechanism of action of the drug. In this approach, the screening is based on the fact that the disease progression is hindered by the drug, but the mechanism of action is less of a concern at the screening stage.
Phenotype-based screening has enjoyed a resurgence of interest over recent years for several reasons. Firstly, this approach allows for a wide range of drugs to be tested without having a limiting factor in the form of its mechanism of action. As a result, a considerable portion of phenotype-based drug screening results in first-in-class drugs, paving the way for subsequent screening of the drugs belonging in the same class (Moffat, Vincent, Lee, Eder, & Prunotto, 2017). Secondly, this approach does not require prior identification of the drug target or a hypothesis about its role in disease, thus preventing screening bias (Moffat et al., 2017). Lastly, repurposing opportunities for drugs identified through phenotypic screening can be readily pursued (Pushpakom et al., 2019). As such, phenotypic screening of compound libraries has been widely reported during the COVID-19 pandemic.
Using phenotypic screening of 4 different compound libraries, Mok and colleagues identified 121 compounds which exhibited anti-SARS-CoV-2 activity, of which calcitriol was reported to show a potent inhibition against SARS-CoV-2 in Vero E6 cells (Mok et al., 2020). On the other hand, a recent work by Riva and colleagues described a large-scale phenotype-based screening for SARS-CoV-2 antiviral. In this drug repurposing work, a library of 12,000 clinical-stage or FDA-approved small molecules were screened against SARS-CoV-2-infected Vero E6 cells (Riva et al., 2020). This study successfully identified approximately 100 compounds which successfully inhibited SARS-CoV-2 replication by at least 40%, and 21 compounds among them has shown dose-dependent inhibition. From these 21 compounds, 13 among them have been shown to be effective within acceptable therapeutic dose, and therefore show promise towards the development of SARS-CoV-2 antiviral.
That being said, phenotype-based screening is often quoted as time-consuming since the mechanism of action of the ‘hits’ is unknown and therefore, must be investigated later. The investigation would prove to be challenging, especially for diseases which involve multiple pathways in its progression. In addition, developing a robust drug target deconvolution study may prove to be challenging since target identification is not always possible in all cases (Kotz, 2012). While FDA has established stringent experimental and trial guidelines before market approval, complete elucidation of the drug's mechanism of action (MOA) is not one of them owing to its difficulty. In fact, an estimated 7% to 18% of FDA-approved drugs do not actually have a definitively identified molecular target (Moffat et al., 2017). Considering this, in cases where the drug's MOA cannot be elucidated, results are often judged based on the drug's performance in relevant in vivo models. This on itself poses a new hurdle since modelling a particular disease accurately in vivo can be especially challenging, let alone recapitulating the drug's molecular actions. Fortunately, for SARS-CoV-2, a clinically relevant mouse model has been established and will be discussed briefly later.
When possible, pinpointing the molecular target of the drug should be done as accurately as possible. A defined molecular target would prove to be hugely beneficial for further drug optimization since with a known target, molecular modifications could be performed to increase the drug's efficacy and specificity towards the identified target (Isgut et al., 2018). This optimization would enhance the safety and efficacy profile of the drug candidate, hence improving the likelihood of the drug succeeding in getting market approval.
Specific to the case of SARS-CoV-2, an additional challenge is the handling of SARS-CoV-2 viral particles which must be conducted in a BSL-3 facility with BSL-3 practices and precautions (CDC recommendations, available at https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html). Considering the limited access to BSL-3 facilities, target-based screening might be a more viable option since the drug target of SARS-CoV-2 protein can be individually expressed and isolated without needing the use of a live SARS-CoV-2 virus.
2.3. Animal models for SARS-CoV-2 drug repurposing
While not exactly an approach to drug repurposing, animal models has been proven to be invaluable in the quest of finding potential therapeutic agents for SARS-CoV-2. Among many, ferrets, Syrian hamster, mice and non-human primates are routinely used as clinically relevant animal models to capture the disease progression in vivo (Takayama, 2020). In this section, we will focus primarily on transgenic mice and non-human primate (NHP) model.
Bao and co-workers reported the use of hACE2 transgenic mice as a clinically relevant mouse model of SARS-CoV-2 infection (Bao et al., 2020). hACE2 transgenic mice was developed from ICR mice which express human ACE2 receptors, therefore enabling accurate recapitulation of SARS-CoV-2 spike glycoprotein interaction with human ACE2 receptor in vivo. The study reported that unlike the mock-infected hACE2 mice or the wild-type mice, SARS-CoV-2-infected hACE2 mice displayed weight loss and slightly bristled fur. Moreover, viral load was successfully detected by RT-qPCR in the lungs of the hACE2 mice. Following this, infectious SARS-CoV-2 was successfully isolated from the lungs of the infected hACE2 mice (and not from mock-infected or wild-type infected mice), suggesting that ACE2 receptor is crucial for SARS-CoV-2 viral entry into the host cell. In addition, this study also fulfils Koch's Postulate, establishing that SARS-CoV-2 is indeed the causative agent of COVID-19.
Following the success of this model, Zheng and colleagues utilised K18-hACE2 transgenic mice, which expresses human ACE2 receptor under the cytokeratin 18 (K18) promoter to establish a mouse model with more severe symptoms (Zheng et al., 2021). Originally developed in 2006 as a mouse model for the original SARS-CoV (McCray Jr. et al., 2007), this mouse model displays more severe symptoms upon inoculation with SARS-CoV-2, including severe disease in the lungs and on some cases, the brain. The mice also display severe thrombosis and vasculitis after the onset of severe pneumonia. This mouse model proves to be invaluable in studying the pathological aspects of severe SARS-CoV-2 infection, as well as to test various therapeutic agents.
Another clinically relevant animal model involves the use of NHPs. A rhesus macaque model established by Chandrashekar and colleagues has been proved to be suitable to study acute infection of SARS-CoV-2 (Chandrashekar et al., 2020). Upon infection with SARS-CoV-2, the macaques displayed high viral load in the upper and lower respiratory tract, viral pneumonia as well as significant immune response. Using this model, they proved that upon primary infection and recovery, reinfection of the macaques with SARS-CoV-2 resulted in significantly lower viral titre in the bronchoalveolar lavage and nasal mucosa, thus proving that primary infection of SARS-CoV-2 brings about protective immunity towards subsequent infection in NHPs. Aside from that, the rhesus macaque model was also used to assess the efficacy of remdesivir against SARS-CoV-2 (Williamson et al., 2020). From the study, it was discovered that different from the vehicle-treated macaques, remdesivir treatment results in the absence of respiratory disease upon infection of SARS-CoV-2. Moreover, the viral titre in the bronchoalveolar lavages were lower as compared to the vehicle-treated macaques, along with lower lung viral load and less lung tissue damage.
In addition, this rhesus model can also be used to test the efficacy of various vaccine candidates. Van Doremalen and co-workers tested the efficacy of the ChAdOx1 nCOV-19 vaccine in the rhesus macaque model, and discovered that upon vaccination, SARS-CoV-2 challenge resulted in lower viral load as compared to the unvaccinated control, as well as the absence of pneumonia in the macaques (van Doremalen et al., 2020). Similarly, Yu and colleagues used rhesus macaque model to test the efficacy of a DNA vaccine coding for SARS-CoV-2 spike protein (Yu et al., 2020). In the study, it was reported that the vaccination was successful in stimulating humoral and cellular immunity, along with production of neutralizing antibodies in the macaques. Upon challenge with SARS-CoV-2, the vaccinated macaques displayed lower viral loads, and that the vaccine-elicited neutralizing antibody titre was found to be correlated to the protection against SARS-CoV-2 infection.
That being said, despite the overwhelming importance of animal models, they have their limitations as well. Firstly, owing to significant species and genetic differences, animal models (especially small animals such as rodents) may not accurately capture the disease progression that is observed in humans (Subbarao et al., 2004). As a specific example, wild-type mice are found to be less susceptible to SARS-CoV-2 infection owing to their lack of ACE2 receptors (Bao et al., 2020) and as a result, humanized ACE2 (hACE2) mice must be developed to serve as an animal model. While hACE2 mice is widely available now, developing such transgenic mice took significant cost, time and effort to complete and therefore, when the next novel pandemic hits, the suitable animal model may not be readily available.
To bridge the significant genetic differences between humans and animal models, researchers may also consider using NHPs as animal models owing to their relatively higher genetic similarity to humans (Lu et al., 2008). However, housing, caring and conducting experiments on NHP may prove to be significantly costly and may not be easily accessible to many research groups. In addition, due to the costs incurred, typically only a small number of NHP are involved, hence limiting the statistical reliability of such experiments. Moreover, since NHP are typically outbred animals, there will be slight but significant genetic differences in between animals of the same species. This genetic difference might result in high variability in experimental outcome, hence complicating data interpretation from the experiment (Lu et al., 2008).
3. Drug repurposing libraries and repurposing scenarios
Drugs are typically identified through screening of small-molecule compound libraries which are available commercially and at public institutions. These drug repurposing compound libraries can be broadly categorized into FDA-approved and antiviral drug libraries (Table 1 ). Depending on previously known antiviral activity of the repurposed candidate drug, three antiviral drug repurposing scenarios can be described below:
Table 1.
Small-molecule compound libraries used in antiviral drug repurposing.
| Library (Vendor) | Description |
|---|---|
| FDA Drug Libraries | |
| FDA-approved Drug Library (Selleck Chemicals) | 2747 FDA-approved drugs |
| FDA-approved Drug Library (MedChemExpress) | >2331 bioactive compounds, including 2278 FDA-approved drugs |
| FDA-approved Drug Library (TargetMol) | 1403 FDA-approved drugs |
| DiscoveryProbe™ FDA-approved drug library (APExBIO) | 1971 FDA-approved drugs |
| FDA-Approved Drugs Screening Library (Cayman Chemical) | ~875 FDA-approved drugs |
| SCREEN-WELL® FDA approved drug library V2 (Enzo Life Sciences) |
>770 FDA-approved drugs |
| The Library of Pharmacologically Active Compounds (LOPAC, Sigma-Aldrich) | 1280 pharmacologically active compounds including FDA-approved drugs |
| Prestwick Chemical Library | 1520 off-patent small molecules, including FDA-approved drugs |
| Spectrum Collection (Microsource) | 2560 compounds, including FDA-approved drugs |
| Antiviral Compound Library | |
| Anti-COVID-19 Compound Library (TargetMol) | 2448 compounds with confirmed or potential anti-SARS-CoV-2 activity; part of them are broad-spectrum antiviral agents |
| Antiviral Compound Library (SelleckChem) | 458 antiviral compounds |
| DiscoveryProbe™ Anti-virus Compound Library (APExBIO) | 264 antiviral compounds |
| Antiviral Compound Library (MedChemExpress) | 530 antiviral compounds targeting SARS-CoV, HBV, HCV, HIV, HSV and influenza virus |
| Antiviral Library (ChemDiv) | 87,000 compounds, antiviral targets include CoV, HIV, influenza, HCV |
3.1. Same target - new virus
This scenario is a viable option for drug repurposing when an existing antiviral targeting a specific cellular or viral function/pathway of one virus is also implicated in the infection of other viruses (Mercorelli, Palu, & Loregian, 2018). In the case of SARS-CoV-2, remdesivir, a broad-spectrum antiviral that was previously developed for HCV, has been a remarkable drug during the Ebola outbreak, and now for the COVID-19 pandemic (Beigel et al., 2020; Consortium et al., 2021; Tchesnokov et al., 2019; Wang et al., 2020). A prodrug of an adenosine triphosphate (ATP) analog, remdesivir works by competing with ATP for incorporation into RNA and inhibits the viral RdRP in HCV, EBOV and SARS-CoV-2 viruses (Gordon et al., 2020; Tchesnokov et al., 2019). Although remdesivir was unsuccessfully tested in subsequent clinical trials for EBOV (Mulangu, et al., 2019), it was consequently repurposed for SARS-CoV-2 after studies have reported its high potency with SARS-CoV-2 (Choy et al., 2020; Gordon, Tchesnokov, et al., 2020). On the other hand, antimalaria drugs chloroquine and hydroxychloroquine have also exhibited antiviral activity against SARS-CoV-2 (Gautret et al., 2020; Liu et al., 2020; Wang et al., 2020). Recently, the interim WHO Solidarity Trial on remdesivir and hydroxychloroquine has reported little to no effect on COVID-19 hospitalized patients in terms of overall mortality, ventilation initiation and length of hospital stays (Consortium et al., 2021).
3.2. Same target – new indications
This scenario for drug repurposing occurs when a known drug has the ability to modulate the pharmacological molecule or pathway essential in a pathogenic process of a viral infection (Mercorelli et al., 2018). An example is tocilizumab, an immunosuppressive drug originally indicated for cytokine release syndrome. Tocilizumab inhibits both soluble and membrane-bound interleukin 6 receptors from activating pro-inflammatory pathways in severe COVID-19 infection.
3.3. New target – new indications
This last scenario of drug repurposing occurs when a known drug with an established bioactivity and mechanism of action is identified with a new molecular target essential for virus replication (Mercorelli et al., 2018). While candidate drugs for SARS-CoV-2 have not been found by this scenario, antimicrobial agents teicoplanin, itraconazole and nitazoxanide were discovered to inhibit virus-infected cells previously (Colson & Raoult, 2016; Strating et al., 2015).
4. Drug repurposing candidates for COVID-19
Various COVID-19 clinical trials are currently ongoing to determine the repurposing potential of clinically approved drugs. There are 5619 registered clinical trials for COVID-19 as of May 8, 2021, which includes drug safety evaluation, vaccine and repurposing studies (https://clinicaltrials.gov/ct2/results?cond=COVID-19). Generally, the drugs which are being evaluated for repurposing falls into one of the three major categories: 1) inhibit one or several stages of the CoV replication cycle, 2) counteract the effects brought about by SARS-CoV-2 infection and 3) indirectly alleviate effects of COVID-19 infection through manipulating the cellular immune and metabolic pathways (Fig. 3 ).
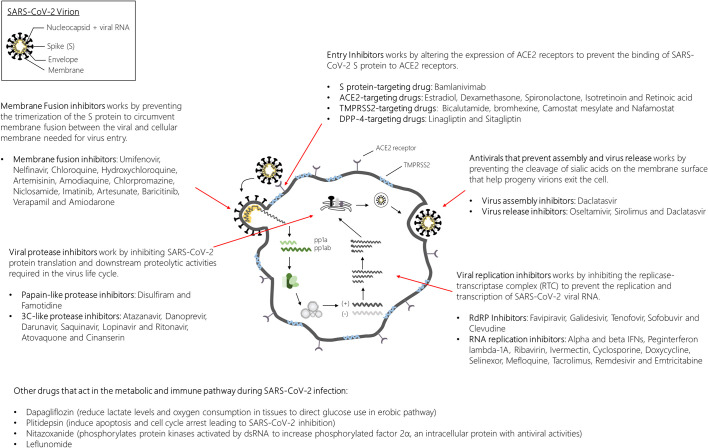
Fig. 3.
Overview of investigational antiviral drugs for SARS-CoV-2 targeting the various stages of the virus life cycle. SARS-CoV-2 infections are typically initiated by the binding of the spike (S) protein to the ACE2 receptor decorated on the surface of the target cell. With the help of the TMPRSS2 protein, the S protein undergoes a series of conformational changes to allow membrane fusion and release of the viral genome into the host cytoplasm. Once the viral genome is released into the host cytoplasm, protein translation quickly ensues to produce the replicase proteins pp1a and pp1ab. Proteolytic processing of pp1a and pp1ab sets the stage for the assembly of the replicase-transcriptase complex for the replication and transcription of the viral genome and subgenomic RNAs. Protein products of the translated RNAs in the endoplasmic reticulum will be transported into the endoplasmic-reticulum Golgi intermediate complex (ERGIC) for virus assembly. The virus is then transported in vesicles for egress by exocytosis. ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease serine 2; pp1a, polyprotein 1a; pp1ab, polyprotein 1ab.
4.1. Drug inhibiting the SARS-CoV-2 replication cycle
4.1.1. Virus attachment and entry into host cells
Inhibiting virus entry into host cells is often the first targetable step in any virus replication cycle. For SARS-CoV-2, its entry into host cells is facilitated by the binding interaction of the CoV spike (S) protein to the target cell's ACE2 receptor and transmembrane protease serine 2 (TMPRSS2), or through endocytosis (Hoffmann et al., 2020). As such, compounds which have been theorised to influence the expression of ACE2 and TMPRSS2 were considered as potential repurposing candidates in the treatment of COVID-19. Examples of compounds in this category includes estradiol, dexamethasone, spironolactone, isotretinoin and retinoic acid (for ACE2) (Cadegiani, 2020; Sarohan, 2020; Seeland et al., 2020; S. Sinha et al., 2020), and bicalutamide, bromhexine, camostat mesylate and nafamostat (for TMPRSS2) (Hoffmann et al., 2020; Pooja, Reddy, Hema, Dodoala, & Koganti, 2020; Olaleye, Kaur, & Onyenaka, 2020; “Repurposing Cancer Drugs for COVID-19,”, 2020). Inhibitors against dipeptidyl-peptidase 4 (DPP4), the cognate receptor for MERS-CoV, is also found to be a druggable target for mild COVID-19 cases. Example of drugs that target DPP-4 under clinical trials are linagliptin (www.clinicaltrials.gov/ct2/show/NCT04371978) and sitagliptin (www.clinicaltrials.gov/ct2/show/NCT04365517).
Besides looking at compounds which act on ACE2 and TMPRSS2, antiviral drugs which target the SARS-CoV-2 S protein were also explored. Bamlanivimab, a recombinant neutralising human IgG1κ monoclonal antibody targeting the receptor-binding domain of the SARS-CoV-2 S protein, has not been approved but is authorised for emergency use by FDA for COVID-19 (Mahase, 2020). Umifenovir (Arbidol®, JSC Pharmstandard), a broad-spectrum antiviral used previously for prophylaxis and treatment of influenza, has been shown to inhibit S protein trimerization (Huang et al., 2020), while nelfinavir (VIRACEPT®, Pfizer), an antiretroviral drug for HIV, was reported to inhibit SARS-CoV-2 membrane fusion in the replication cycle (Musarrat et al., 2020).
Compounds which inhibit SARS-CoV-2 entry into host cells via endocytosis were found to be useful for drug repurposing, such as chloroquine, hydroxychloroquine, artemisinin, amodiaquine, chlorpromazine, niclosamide, imatinib, artesunate, baricitinib, verapamil and amiodarone (Bobrowski et al., 2020; Bronte et al., 2020; Cortegiani, Ingoglia, Ippolito, Giarratano, & Einav, 2020; Emadi, Chua, Talwani, Bentzen, & Baddley, 2020; Li et al., 2020; Lin et al., 2020; Sanchis-Gomar et al., 2020; Sinha & Balayla, 2020; Stip, 2020). The majority of these drugs are hypothesized to act by inhibiting endocytic proteins and increasing the endosomal pH to inhibit fusion between SARS-CoV-2 and the host cell membranes (Owens, 2020).
4.1.2. Viral replication
Upon gaining entry into the host cell, the next phase of the SARS-CoV-2 life cycle is the release of viral RNA genome into the cytoplasm and translation of the replicase genes that forms the replicase-transcriptase complex (RTC) (van Hemert et al., 2008). Therein, the RdRP embedded within the RTC is responsible for the replication of RNA and transcription of sub-genomic RNA. Antivirals which inhibit the RdRP, such as favipiravir, galidesivir, tenofovir, sofobuvir and clevudine have been proposed as COVID-19 repurposing candidates (Elfiky, 2020; Rafi et al., 2020; Sultana et al., 2020). On the other hand, antivirals inhibiting RNA replication, such as remdesivir and emtricitabine, have shown promising clinical trial outcomes (Ayerdi et al., 2020; Daoud, Alabed, & Dahabiyeh, 2021).
Following viral RNA replication comes the translation and proteolytic processing of viral proteins. Here, HIV protease inhibitors such as atazanavir, saquinavir and ritonavir were considered as repurposing candidates against SARS-CoV-2's 3C-like protease alongside danoprevir, a hepatitis C protease inhibitor (Chen et al., 2020; Mahdi et al., 2020). Disulfiram and famotidine were also proposed as repurposing candidates against SARS-CoV-2's papain-like protease (Lin et al., 2018; Samimagham et al., 2020). Further, autoimmune drugs such as interferons have been suggested as a potential treatment for COVID-19 due to their antiviral properties (Consortium et al., 2020). Other drugs have also been evaluated based on their ability to inhibit cell fusion and virus replication, including doxycycline, a tetracycline derivative (Gendrot et al., 2020).
4.1.3. Virus assembly and egress
After the synthesis and processing of the viral structural proteins, progeny viruses are assembled in the endoplasmic reticulum-Golgi intermediate complex and transported in vesicles to be released by exocytosis. Antiviral drugs targeting this stage of the SARS-CoV-2 replication are also proposed as repurposing candidates. Examples of these drugs are oseltamivir, daclatasvir (Eslami et al., 2020; Tan et al., 2020) and sirolimus (www.clinicaltrials.gov/ct2/show/NCT04341675).
5. Host-targeting therapies for SARS-CoV-2
One key aspect of SARS-CoV-2 infection is the severe lung tissue damage due to inflammation and cytokine storm (Nikolich-Zugich et al., 2020). To address this issue, anti-inflammatory compounds can potentially be utilised towards the treatment of SARS-CoV-2 infection. For example, Wu and Yang studied the effects of fedratinib as a potential anti-inflammatory treatment (Wu & Yang, 2020). Originally developed to treat myelofibrosis, fedratinib was approved by FDA to treat myeloproliferative neoplasm by selectively inhibiting JAK-2 (Pardanani et al., 2015), which is later found to be involved in the progression of cytokine storm caused by SARS-CoV-2 infection. Upon treatment with fedratinib, IL-17 expression by murine TH17 is found to be significantly downregulated, hence potentially ameliorating the impact of TH17-mediated cytokine storm and acute inflammation in patients. Moreover, fedratinib was also found not to interfere with IL-21 mediated B-cell response, hence immunoprotective antibodies against SARS-CoV-2 will still be produced during fedratinib treatment.
Another example of host-targeting therapeutics involves targeting the human dihydroorotate dehydrogenase (DHODH), which is an enzyme involved in the fourth and rate-limiting step of pyrimidine-based nucleotide biosynthesis (Reis, Calil, Feliciano, Pinheiro, & Nonato, 2017). Targeting DHODH has shown previous successes, with DHODH-targeting leflunomide being proven to be effective to treat rheumatoid arthritis (Herrmann, Schleyerbach, & Kirschbaum, 2000). Together with brequinar, leflunomide have passed clinical trials and are currently used to treat cancer and autoimmune disease (Lolli et al., 2018). Owing to the attractiveness of DHODH as a host target, Xiong and co-workers utilised DHODH-targeting compounds, S312 and S416, previously developed by Diao and colleagues (Diao et al., 2012) to test its efficacy as SARS-CoV-2 antiviral (Xiong et al., 2020). The study demonstrated that the two compounds successfully impaired viral genome replication, translation and transcription, but the impairment is not extensive enough to disrupt host genome-related processes. As such, the compounds exhibited remarkable antiviral activity and very low toxicity, making the two compounds very potent inhibitors of SARS-CoV-2 in cell-based assay. Moreover, the compounds showed potent antiviral activities against Influenza A, ZIKV and EBOV as well, further proving that the compounds displayed good broad-spectrum antiviral activity.
That being said, host-targeting compounds often suffer from cytotoxicity issues owing to their target being the host cell machinery. In the case of kinase inhibitors such as JAK inhibitors, toxicity is a major issue since kinases are often pivotal in regulating a vast number of cellular processes (Ji & Li, 2020). Moreover, owing to the crucial role kinases play in host cell machinery, kinases are often highly conserved and therefore, kinase-targeting drugs are prone to having off-target effects which may further worsen their cytotoxic effects. As for ACE2 inhibitors, while they may be potent therapeutics for SARS-CoV-2 infection, toxicity remains one of the major hurdles towards their widespread use. Through virtual screening and molecular docking, several potent inhibitors of ACE2 can be identified (Benitez-Cardoza & Vique-Sanchez, 2020). However, it was also reported that a number of the compounds, despite showing good docking scores, displayed acute theoretical toxicity due to carcinogenicity, mutagenicity or hepatoxicity. Moreover, ACE2 inhibitor compounds have been reported to have varying effects depending on the host's gender and genetic make-up (Ferreira & Raizada, 2008), hence developing an effective, broad-range ACE2 inhibitor might prove to be a major challenge.
6. Challenges in drug repurposing
Drug repurposing studies are not without its drawbacks and challenges. The first and maybe the most critical one is the limited repository of drugs to be repurposed, largely owing to the high attrition rate in drug development and approval (Polamreddy & Gattu, 2019). From 2010 to 2018, an average of 41 therapeutics per annum was approved by the FDA, out of hundreds of drug candidate applications received each year (Darrow, Avorn, & Kesselheim, 2020). This high attrition rate and slow approval is largely due to the stringent evaluation process which is crucial to ensure the safety and efficacy of the drug candidates. With only a limited number of novel drugs being approved each year, drug repurposing study can be challenging due to the lack of new drugs to be repurposed. Besides, regulatory and patent considerations and limited efficacy of repurposed drugs are compounding factors to successful drug repurposing, which will be discussed below.
6.1. Regulatory and patent considerations
In a clinical setting, physicians in many countries are permitted to prescribe drugs outside of its indicated use if the drug has exhibited some beneficial effects for their patients. Regulatory agencies often disapprove this “off-label” use for a drug, as the drug efficacies and safety profiles for the off-label indication are not well-established. Intrinsically, biopharmaceuticals who are keen to pursue a drug repurposing campaign would have to make a substantial investment towards the research and clinical trial programs for the repurposed drug. A patent would also have to be filed to ensure the return on investment on the sale of the repurposed drug by preventing others from producing and marketing the drug under the new indication as the patentee.
Under the Discovering New Therapeutic Uses for Existing Molecules initiative of the National Institute of Health (NIH)’s National Center for Advancing Translational Sciences (NCATS), repurposed drugs can be categorised into four types, depending on whether the candidate drug has any remaining patent life and if any approval status was given prior to the time of application (www.ncats.nih.gov/ntu). For the repurposing candidates with remaining patent life but is either never approved for human use or is currently approved for other indications, development can only be proceeded by the holder of the drug patent or when the license is acquired by another company, while therapeutics with no patent life can be become viable when a new use for the drug candidate fits into a patentable criterion (that is, non-obvious). Under these circumstances, companies may be discouraged to take over the development costs of repurposing a drug since the drug's remaining patent life belongs to another company. On the other hand, while providing a repurposed medical use for a known drug is also possible, many potential repurposing uses may already be well known in clinical practice or in scientific literature, thus the patentee must be able to differentiate their patent claims from publicly available information in order to obtain patent protection.
Furthermore, when a marketed drug is patented for a new indication, enforcing the patent may also be a major issue if the drug has been commonly used in practice for the new indication (Pushpakom et al., 2019). Since this may adversely impact the revenue generated from the patent, drug repurposing activities are often discouraged, especially for profit-driven entities. Moreover, in the case of generic drugs which are produced by multiple pharmaceutical companies, enforcing the patent rights might be challenging due to the practice of skinny-labelling. In this practice, companies may evade patent rights infringement as long as the drug label does not encourage usage of the drug for the new indication while still being effective for the said indication. Clinicians privy to this knowledge, therefore, might still prescribe the said drug for the new indication despite the skinny labelling. As a result of the poor revenue prospects, drug repurposing activities may not be a priority in big pharmaceutical companies.
Finally, drug repurposing projects might also be hindered by clinical trials. While big pharmaceutical companies might find it to be less of an issue, smaller biotechnology companies or even non-profit organizations and academia entities might find it hard to afford expensive clinical trials (Pushpakom et al., 2019). Moreover, in the case of rare diseases, traditional clinical trials might not be possible even though drug repurposing projects might be advantageous in finding therapeutic agents for rare diseases. As such, it might be difficult for drug repurposing projects to reach approval by regulatory bodies.
6.2. Limited efficacy of repurposed drugs
For successful repurposing, molecular modifications would often have to be performed in order to increase the efficacy of the said drug towards its repurposed target, as the efficacy of a drug towards its new target is often times not as strong as its efficacy towards its primary target (Oprea & Mestres, 2012). As a result, this steers many drug repurposing projects to end up repurposing the drug towards a target belonging to the same class as the primary target, resulting in a limited range of disease for which the therapeutic agent can be discovered through drug repurposing. Furthermore, a repurposed drug may need to be reformulated to maximise its drug efficacy, and this requires a combination of pharmaceutical and clinical knowledge to ensure the reformulation achieves localized drug delivery. Finding novel formulations and delivery mechanisms in the face of the COVID-19 pandemic is therefore challenging, as the scientific community is confronted with newly produced data every single day.
7. Concluding remarks and future perspectives
The traditional drug discovery process is often associated with exorbitant costs, long timeframes and high failure rates. In the context of today's COVID-19 pandemic, where finding suitable therapeutics is of the highest priority, drug repurposing may provide an essential tool to help us fight this global virus outbreak. Existing FDA-approved drugs present the perfect opportunity for repurposing to treat COVID-19, by leveraging on their known clinical and safety profiles. The challenge is to determine the most suitable dosage and most efficient delivery system to transport the repurposed drug to the target site. Additional considerations to study may also include novel interactions between the repurposed drug and COVID-19, and differences in target populations or dosing schedules which may have safety implications. However, in order to realise the full potential of drug repurposing, better access is required to pre-clinical and clinical compounds, as well as their associated clinical trial data, for research and data analysis. Governmental and industrial support are also needed to break down the barriers of patent and regulatory processes to incentivize drug repurposing. Despite the challenges for drug repurposing, the opportunities of identifying new uses for old drugs in a relatively shorter period of time, while being cost-effective with reduced attrition rates, provide significant incentive for a focus on drug repurposing for COVID-19.
Conflict of interest statement
Y.L. Ng, C.K. Salim and J.J.H. Chu declare no conflict of interest.
Acknowledgments
This study was supported by Ministry of Education (1) Singapore (SG) MOE2017-T2-1-078; (2) Ministry of Education Singapore; MOE2017-T2-2-014; (3) NUHSRO/2020/R05+5/NUHS-COVID14; (4) NUHSRO/2020/006/NUSMED COVID BSL3 COVID Research.
Editor: P. Holzer
References
- Abo-Zeid Y., Ismail N.S.M., McLean G.R., Hamdy N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. European Journal of Pharmaceutical Sciences. 2020;153:105465. doi: 10.1016/j.ejps.2020.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nature Reviews. Drug Discovery. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Ayerdi O., Puerta T., Clavo P., Vera M., Ballesteros J., Fuentes M.E.…Sandoval Study G. Preventive efficacy of tenofovir/emtricitabine against severe acute respiratory syndrome coronavirus 2 among pre-exposure prophylaxis users. Open Forum Infectious Diseases. 2020;7 doi: 10.1093/ofid/ofaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L.…Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Basit A., Ali T., Rehman S.U. Truncated human angiotensin converting enzyme 2; a potential inhibitor of SARS-CoV-2 spike glycoprotein and potent COVID-19 therapeutic agent. Journal of Biomolecular Structure & Dynamics. 2020:1–10. doi: 10.1080/07391102.2020.1768150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Computational and Structural Biotechnology Journal. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C.…Members A.-S.G. Remdesivir for the treatment of Covid-19 - final report. The New England Journal of Medicine. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Cardoza C.G., Vique-Sanchez J.L. Potential inhibitors of the interaction between ACE2 and SARS-CoV-2 (RBD), to develop a drug. Life Sciences. 2020;256:117970. doi: 10.1016/j.lfs.2020.117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowski T., Chen L., Eastman R.T., Itkin Z., Shinn P., Chen C.…Muratov E.N. Discovery of Synergistic and antagonistic drug combinations against SARS-CoV-2 in vitro. bioRxiv. 2020 doi: 10.1101/2020.06.29.178889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Cane S.…Olivieri O. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. The Journal of Clinical Investigation. 2020;130:6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadegiani F.A. Can spironolactone be used to prevent COVID-19-induced acute respiratory distress syndrome in patients with hypertension? American Journal of Physiology. Endocrinology and Metabolism. 2020;318:E587–E588. doi: 10.1152/ajpendo.00136.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Wang X., Turner J.A., Baldwin N.E., Gu J. Breaking the paradigm: Dr Insight empowers signature-free, enhanced drug repurposing. Bioinformatics. 2019;35:2818–2826. doi: 10.1093/bioinformatics/btz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L.…Barouch D.H. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zhang Z., Wang L., Huang Z., Gong F., Li X.…Wu J.J. First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.W., Liang Y.H., Kuo Y.L., Chuu C.P., Lin C.Y., Lee M.H.…Huang C.Y. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death & Disease. 2015;6 doi: 10.1038/cddis.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y.…Yen H.L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Research. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Raoult D. Fighting viruses with antibiotics: an overlooked path. International Journal of Antimicrobial Agents. 2016;48:349–352. doi: 10.1016/j.ijantimicag.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium W.H.O.S.T., Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V.…Swaminathan S. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. The New England Journal of Medicine. 2020;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium W.H.O.S.T., Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V.…Swaminathan S. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. The New England Journal of Medicine. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. Journal of Critical Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud S., Alabed S.J., Dahabiyeh L.A. Identification of potential COVID-19 main protease inhibitors using structure-based pharmacophore approach, molecular docking and repurposing studies. Acta Pharmaceutica. 2021;71:163–174. doi: 10.2478/acph-2021-0016. [DOI] [PubMed] [Google Scholar]
- Darrow J.J., Avorn J., Kesselheim A.S. FDA approval and regulation of pharmaceuticals, 1983-2018. JAMA. 2020;323:164–176. doi: 10.1001/jama.2019.20288. [DOI] [PubMed] [Google Scholar]
- De Giorgi V., Grazzini M., Gandini S., Benemei S., Lotti T., Marchionni N., Geppetti P. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Archives of Internal Medicine. 2011;171:779–781. doi: 10.1001/archinternmed.2011.131. [DOI] [PubMed] [Google Scholar]
- Diao Y., Lu W., Jin H., Zhu J., Han L., Xu M.…Li H. Discovery of diverse human dihydroorotate dehydrogenase inhibitors as immunosuppressive agents by structure-based virtual screening. Journal of Medicinal Chemistry. 2012;55:8341–8349. doi: 10.1021/jm300630p. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R.…Munster V.J. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J.T., Deshpande T., Butte A.J. Exploiting drug-disease relationships for computational drug repositioning. Briefings in Bioinformatics. 2011;12:303–311. doi: 10.1093/bib/bbr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J.T., Sirota M., Shenoy M., Pai R.K., Roedder S., Chiang A.P.…Butte A.J. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Science Translational Medicine. 2011;3 doi: 10.1126/scitranslmed.3002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sciences. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi A., Chua J.V., Talwani R., Bentzen S.M., Baddley J. Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:897. doi: 10.1186/s13063-020-04819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami G., Mousaviasl S., Radmanesh E., Jelvay S., Bitaraf S., Simmons B.…Mobarak S. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. The Journal of Antimicrobial Chemotherapy. 2020;75:3366–3372. doi: 10.1093/jac/dkaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A.J., Raizada M.K. Are we poised to target ACE2 for the next generation of antihypertensives? Journal of Molecular Medicine (Berlin, Germany) 2008;86:685–690. doi: 10.1007/s00109-008-0339-x. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M.…Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gendrot M., Andreani J., Jardot P., Hutter S., Delandre O., Boxberger M.…Pradines B. In vitro antiviral activity of doxycycline against SARS-CoV-2. Molecules. 2020;25 doi: 10.3390/molecules25215064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. The Journal of Biological Chemistry. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M.…Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C.S., Voight B.F. Pathway and network-based strategies to translate genetic discoveries into effective therapies. Human Molecular Genetics. 2016;25:R94–R98. doi: 10.1093/hmg/ddw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L.…Landray M.J. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. The New England Journal of Medicine. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysi D.M., Do Valle I., Zitnik M., Ameli A., Gan X., Varol O.…Barabasi A.L. 2020. Network Medicine Framework for Identifying Drug Repurposing Opportunities for COVID-19. (ArXiv) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D.C., Jr., Ji H.F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Medicine and Infectious Disease. 2020;35:101646. doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert M.J., van den Worm S.H., Knoops K., Mommaas A.M., Gorbalenya A.E., Snijder E.J. SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathogens. 2008;4 doi: 10.1371/journal.ppat.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M.L., Schleyerbach R., Kirschbaum B.J. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology. 2000;47:273–289. doi: 10.1016/s0162-3109(00)00191-0. [DOI] [PubMed] [Google Scholar]
- Hieronymus H., Lamb J., Ross K.N., Peng X.P., Clement C., Rodina A.…Golub T.R. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Hofmann-Winkler H., Smith J.C., Kruger N., Sorensen L.K., Sogaard O.S.…Pohlmann S. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. bioRxiv. 2020 doi: 10.1101/2020.08.05.237651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S.…Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshmand S.A., Zarei Ghobadi M., Hooshmand S.E., Azimzadeh Jamalkandi S., Alavi S.M., Masoudi-Nejad A. A multimodal deep learning-based drug repurposing approach for treatment of COVID-19. Molecular Diversity. 2020 doi: 10.1007/s11030-020-10144-9. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K.L., Wang Y., Chen L., Zhao Z., Savitz S., Jiang X.…Kim Y. 2020. Drug repurposing for COVID-19 using graph neural network with genetic, mechanistic, and epidemiological validation. (Res Sq) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Yu H., Wang T., Yang H., Yao R., Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Journal of Medical Virology. 2020;93:481–490. doi: 10.1002/jmv.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Guo B. Adenomatous polyposis coli determines sensitivity to histone deacetylase inhibitor-induced apoptosis in colon cancer cells. Cancer Research. 2006;66:9245–9251. doi: 10.1158/0008-5472.CAN-06-0887. [DOI] [PubMed] [Google Scholar]
- Iorio F., Rittman T., Ge H., Menden M., Saez-Rodriguez J. Transcriptional data: A new gateway to drug repositioning? Drug Discovery Today. 2013;18:350–357. doi: 10.1016/j.drudis.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgut M., Rao M., Yang C., Subrahmanyam V., Rida P.C.G., Aneja R. Application of combination high-throughput phenotypic screening and target identification methods for the discovery of natural product-based combination drugs. Medicinal Research Reviews. 2018;38:504–524. doi: 10.1002/med.21444. [DOI] [PubMed] [Google Scholar]
- Ji X., Li Z. Medicinal chemistry strategies toward host targeting antiviral agents. Medicinal Research Reviews. 2020;40:1519–1557. doi: 10.1002/med.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiralerspong S., Palla S.L., Giordano S.H., Meric-Bernstam F., Liedtke C., Barnett C.M.…Gonzalez-Angulo A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. Journal of Clinical Oncology. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim S., Kim D.Y., Kim M.S., Shin D.H. Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. Journal of Enzyme Inhibition and Medicinal Chemistry. 2020;35:1539–1544. doi: 10.1080/14756366.2020.1801672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone A.L., Reierson G.W., Smith R.P., Goldberg J.L., Lemmon V.P., Bixby J.L. A chemical genetic approach identifies piperazine antipsychotics as promoters of CNS neurite growth on inhibitory substrates. Molecular and Cellular Neurosciences. 2012;50:125–135. doi: 10.1016/j.mcn.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu O., Saeed M., Greten H.J., Efferth T. Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Computers in Biology and Medicine. 2021;133:104359. doi: 10.1016/j.compbiomed.2021.104359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y.Y., Peng T.T., Yeh T.K., Huang W.Z., Chang S.E., Wu S.H.…Chen C.T. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomedical Journal. 2020;43:355–362. doi: 10.1016/j.bj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser M.J., Setola V., Irwin J.J., Laggner C., Abbas A.I., Hufeisen S.J.…Roth B.L. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P., Sirota M., Butte A.J. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Computational Biology. 2012;8 doi: 10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelfaoui H., Harkati D., Saleh B.A. Molecular docking, molecular dynamics simulations and reactivity, studies on approved drugs library targeting ACE2 and SARS-CoV-2 binding with ACE2. Journal of Biomolecular Structure & Dynamics. 2020:1–17. doi: 10.1080/07391102.2020.1803967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz J. Phenotypic screening, take two. Science-Business eXchange. 2012;5 [Google Scholar]
- Li G., Yuan M., Li H., Deng C., Wang Q., Tang Y.…Song J. Safety and efficacy of artemisinin-piperaquine for treatment of COVID-19: An open-label, non-randomised and controlled trial. International Journal of Antimicrobial Agents. 2020:106216. doi: 10.1016/j.ijantimicag.2020.106216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Shah S. Structure-Based Virtual Screening. Methods in Molecular Biology. 2017;1558:111–124. doi: 10.1007/978-1-4939-6783-4_5. [DOI] [PubMed] [Google Scholar]
- Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clinical Microbiology and Infection. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.H., Moses D.C., Hsieh C.H., Cheng S.C., Chen Y.H., Sun C.Y., Chou C.Y. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Research. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wu F., Xie Z., Song X., Zhu Q., Wei J.…Gong B. Clinical study of artesunate in the treatment of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:417–420. doi: 10.3760/cma.j.cn121430-20200312-00412. [DOI] [PubMed] [Google Scholar]
- Lippman M.E., Cummings S.R., Disch D.P., Mershon J.L., Dowsett S.A., Cauley J.A., Martino S. Effect of raloxifene on the incidence of invasive breast cancer in postmenopausal women with osteoporosis categorized by breast cancer risk. Clinical Cancer Research. 2006;12:5242–5247. doi: 10.1158/1078-0432.CCR-06-0688. [DOI] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H.…Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolli M.L., Sainas S., Pippione A.C., Giorgis M., Boschi D., Dosio F. Use of human dihydroorotate dehydrogenase (hDHODH) inhibitors in autoimmune diseases and new perspectives in cancer therapy. Recent Patents on Anti-Cancer Drug Discovery. 2018;13:86–105. doi: 10.2174/1574892812666171108124218. [DOI] [PubMed] [Google Scholar]
- Lu Y.R., Wang L.N., Jin X., Chen Y.N., Cong C., Yuan Y.…Cheng J.Q. A preliminary study on the feasibility of gene expression profile of rhesus monkey detected with human microarray. Transplantation Proceedings. 2008;40:598–602. doi: 10.1016/j.transproceed.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Mahase E. Covid-19: FDA authorises neutralising antibody bamlanivimab for non-admitted patients. BMJ. 2020;371:m4362. doi: 10.1136/bmj.m4362. [DOI] [PubMed] [Google Scholar]
- Mahdi M., Motyan J.A., Szojka Z.I., Golda M., Miczi M., Tozser J. Analysis of the efficacy of HIV protease inhibitors against SARS-CoV-2’s main protease. Virology Journal. 2020;17:190. doi: 10.1186/s12985-020-01457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W.R., Cheng F. Repurposing of FDA-approved toremifene to treat COVID-19 by blocking the spike glycoprotein and NSP14 of SARS-CoV-2. Journal of Proteome Research. 2020;19:4670–4677. doi: 10.1021/acs.jproteome.0c00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan J., King M.C. Genetic heterogeneity in human disease. Cell. 2010;141:210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L.…Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. Journal of Virology. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercorelli B., Palu G., Loregian A. Drug repurposing for viral infectious diseases: How far are we? Trends in Microbiology. 2018;26:865–876. doi: 10.1016/j.tim.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J.G., Vincent F., Lee J.A., Eder J., Prunotto M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nature Reviews. Drug Discovery. 2017;16:531–543. doi: 10.1038/nrd.2017.111. [DOI] [PubMed] [Google Scholar]
- Mok C.K., Ng Y.L., Ahidjo B.A., Lee R.C.H., Loe M.W.C., Liu J.…Chu J.J.H. Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis. bioRxiv. 2020 doi: 10.1101/2020.06.21.162396. [DOI] [Google Scholar]
- Mousavi S.Z., Rahmanian M., Sami A. A connectivity map-based drug repurposing study and integrative analysis of transcriptomic profiling of SARS-CoV-2 infection. Infection, Genetics and Evolution. 2020;86:104610. doi: 10.1016/j.meegid.2020.104610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D.…Team P.C.S. A Randomized, controlled trial of ebola virus disease therapeutics. The New England Journal of Medicine. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musarrat F., Chouljenko V., Dahal A., Nabi R., Chouljenko T., Jois S.D., Kousoulas K.G. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. Journal of Medical Virology. 2020;92:2087–2095. doi: 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.L., Thai N.Q., Truong D.T., Li M.S. Remdesivir strongly binds to both RNA-dependent RNA polymerase and main protease of SARS-CoV-2: Evidence from molecular simulations. The Journal of Physical Chemistry. B. 2020;124:11337–11348. doi: 10.1021/acs.jpcb.0c07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534:314–316. doi: 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- Olaleye O.A., Kaur M., Onyenaka C.C. Ambroxol hydrochloride inhibits the interaction between severe acute respiratory syndrome coronavirus 2 spike protein’s receptor binding domain and recombinant human ACE2. bioRxiv. 2020 doi: 10.1101/2020.09.13.295691. [DOI] [Google Scholar]
- Oprea T.I., Mestres J. Drug repurposing: far beyond new targets for old drugs. The AAPS Journal. 2012;14:759–763. doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens B. Excitement around hydroxychloroquine for treating COVID-19 causes challenges for rheumatology. Lancet Rheumatol. 2020;2 doi: 10.1016/S2665-9913(20)30089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A., Harrison C., Cortes J.E., Cervantes F., Mesa R.A., Milligan D.…Tefferi A. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: A randomized clinical trial. JAMA Oncology. 2015;1:643–651. doi: 10.1001/jamaoncol.2015.1590. [DOI] [PubMed] [Google Scholar]
- Parisi D., Adasme M.F., Sveshnikova A., Bolz S.N., Moreau Y., Schroeder M. Drug repositioning or target repositioning: A structural perspective of drug-target-indication relationship for available repurposed drugs. Computational and Structural Biotechnology Journal. 2020;18:1043–1055. doi: 10.1016/j.csbj.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. A review of computational drug repurposing. Translational and Clinical Pharmacology. 2019;27:59–63. doi: 10.12793/tcp.2019.27.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polamreddy P., Gattu N. The drug repurposing landscape from 2012 to 2017: evolution, challenges, and possible solutions. Drug Discovery Today. 2019;24:789–795. doi: 10.1016/j.drudis.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Pooja M., Reddy G.J., Hema K., Dodoala S., Koganti B. Unravelling high-affinity binding compounds towards transmembrane protease serine 2 enzyme in treating SARS-CoV-2 infection using molecular modelling and docking studies. European Journal of Pharmacology. 2020;173688 doi: 10.1016/j.ejphar.2020.173688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A.…Pirmohamed M. Drug repurposing: Progress, challenges and recommendations. Nature Reviews. Drug Discovery. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Rafi M.O., Bhattacharje G., Al-Khafaji K., Taskin-Tok T., Alfasane M.A., Das A.K.…Rahman M.S. Combination of QSAR, molecular docking, molecular dynamic simulation and MM-PBSA: Analogues of lopinavir and favipiravir as potential drug candidates against COVID-19. Journal of Biomolecular Structure & Dynamics. 2020:1–20. doi: 10.1080/07391102.2020.1850355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Lamers M.M., Smits S.L., Demmers J.A., Mou H., Bosch B.J., Haagmans B.L. Identification of protein receptors for coronaviruses by mass spectrometry. Methods in Molecular Biology. 2015;1282:165–182. doi: 10.1007/978-1-4939-2438-7_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis R.A.G., Calil F.A., Feliciano P.R., Pinheiro M.P., Nonato M.C. The dihydroorotate dehydrogenases: Past and present. Archives of Biochemistry and Biophysics. 2017;632:175–191. doi: 10.1016/j.abb.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Repurposing Cancer Drugs for COVID-19 Cancer Discovery. 2020;10:OF2. doi: 10.1158/2159-8290.CD-ND2020-016. [DOI] [PubMed] [Google Scholar]
- Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A.…Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L.…Chanda S.K. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimagham H.R., Hassani Azad M., Haddad M., Arabi M., Hooshyar D., KazemiJahromi M. The efficacy of famotidine in improvement of outcomes in hospitalized COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:848. doi: 10.1186/s13063-020-04773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams-Dodd F. Target-based drug discovery: Is something wrong? Drug Discovery Today. 2005;10:139–147. doi: 10.1016/S1359-6446(04)03316-1. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F., Lavie C.J., Morin D.P., Perez-Quilis C., Laukkanen J.A., Perez M.V. Amiodarone in the COVID-19 era: Treatment for symptomatic patients only, or drug to prevent infection? American Journal of Cardiovascular Drugs. 2020;20:413–418. doi: 10.1007/s40256-020-00429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanseau P., Agarwal P., Barnes M.R., Pastinen T., Richards J.B., Cardon L.R., Mooser V. Use of genome-wide association studies for drug repositioning. Nature Biotechnology. 2012;30:317–320. doi: 10.1038/nbt.2151. [DOI] [PubMed] [Google Scholar]
- Santosh K.C. Ai-Driven tools for coronavirus outbreak: Need of active learning and cross-population train/test models on multitudinal/multimodal data. Journal of Medical Systems. 2020;44:93. doi: 10.1007/s10916-020-01562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarohan A.R. COVID-19: Endogenous retinoic acid theory and retinoic acid depletion syndrome. Medical Hypotheses. 2020;144:110250. doi: 10.1016/j.mehy.2020.110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaturro P., Kastner A.L., Pichlmair A. Chasing Intracellular Zika virus using proteomics. Viruses. 2019;11 doi: 10.3390/v11090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeland U., Coluzzi F., Simmaco M., Mura C., Bourne P.E., Heiland M.…Preissner S. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Medicine. 2020;18:369. doi: 10.1186/s12916-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severe Covid G.G., Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A.…Karlsen T.H. Genomewide association study of severe Covid-19 with respiratory failure. The New England Journal of Medicine. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B.…Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Science Translational Medicine. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang X., Mu K., Peng C., Zhu Z., Wang X.…Zhu W. D3Targets-2019-nCoV: a webserver for predicting drug targets and for multi-target and multi-site based virtual screening against COVID-19. Acta Pharmaceutica Sinica B. 2020;10:1239–1248. doi: 10.1016/j.apsb.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N., Balayla G. Hydroxychloroquine and COVID-19. Postgraduate Medical Journal. 2020;96:550–555. doi: 10.1136/postgradmedj-2020-137785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Cheng K., Schaffer A.A., Aldape K., Schiff E., Ruppin E. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Molecular Systems Biology. 2020;16 doi: 10.15252/msb.20209628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota M., Dudley J.T., Kim J., Chiang A.P., Morgan A.A., Sweet-Cordero A.…Butte A.J. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Science Translational Medicine. 2011;3:96ra77. doi: 10.1126/scitranslmed.3001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stip E. Psychiatry and COVID-19: The role of chlorpromazine. Canadian Journal of Psychiatry. 2020;65:739–740. doi: 10.1177/0706743720934997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strating J.R., van der Linden L., Albulescu L., Bigay J., Arita M., Delang L.…van Kuppeveld F.J. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Reports. 2015;10:600–615. doi: 10.1016/j.celrep.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K.…Murphy B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. Journal of Virology. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana J., Crisafulli S., Gabbay F., Lynn E., Shakir S., Trifiro G. Challenges for drug repurposing in the COVID-19 pandemic era. Frontiers in Pharmacology. 2020;11:588654. doi: 10.3389/fphar.2020.588654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K. In vitro and animal models for SARS-CoV-2 research. Trends in Pharmacological Sciences. 2020;41:513–517. doi: 10.1016/j.tips.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talluri S. Molecular docking and virtual screening based prediction of drugs for COVID-19. Combinatorial Chemistry & High Throughput Screening. 2020;24(5):716–728. doi: 10.2174/1386207323666200814132149. [DOI] [PubMed] [Google Scholar]
- Tam V., Patel N., Turcotte M., Bosse Y., Pare G., Meyre D. Benefits and limitations of genome-wide association studies. Nature Reviews. Genetics. 2019;20:467–484. doi: 10.1038/s41576-019-0127-1. [DOI] [PubMed] [Google Scholar]
- Tan J., Yuan Y., Xu C., Song C., Liu D., Ma D., Gao Q. A retrospective comparison of drugs against COVID-19. Virus Research. 2021;294:198262. doi: 10.1016/j.virusres.2020.198262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q., Duan L., Ma Y., Wu F., Huang Q., Mao K.…Jin Y. Is oseltamivir suitable for fighting against COVID-19: In silico assessment, in vitro and retrospective study. Bioorganic Chemistry. 2020;104:104257. doi: 10.1016/j.bioorg.2020.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11 doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton A.T., Gentile F., Hsing M., Ban F., Cherkasov A. Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds. Molecular Informatics. 2020;39 doi: 10.1002/minf.202000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F.…Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmunity Reviews. 2020;19:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman G.A. Drugs, devices, and the FDA: Part 1: An overview of approval processes for drugs. JACC: Basic to Translational Science. 2016;1:170–179. doi: 10.1016/j.jacbts.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M.…Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens M., Etminan M., Gill S.S., Takkouche B. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. Journal of Internal Medicine. 2006;260:350–362. doi: 10.1111/j.1365-2796.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J.…de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585:273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Z.S.Y., Zhou J., Zhang Q. Artificial intelligence for infectious disease big data analytics. Infection, Disease & Health. 2019;24:44–48. doi: 10.1016/j.idh.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of Microbiology, Immunology, and Infection. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S.…Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Research. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R., Zhang L., Li S., Sun Y., Ding M., Wang Y.…Xu K. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein & Cell. 2020;11:723–739. doi: 10.1007/s13238-020-00768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H.…Barouch D.H. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Song X., Ma T., Pan X., Zhou Y., Hou Y.…Cheng F. Repurpose open data to discover therapeutics for COVID-19 using deep learning. Journal of Proteome Research. 2020;19:4624–4636. doi: 10.1021/acs.jproteome.0c00316. [DOI] [PubMed] [Google Scholar]
- Zhang D.H., Wu K.L., Zhang X., Deng S.Q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. Journal of Integrative Medicine. 2020;18:152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Wong L.R., Li K., Verma A.K., Ortiz M.E., Wohlford-Lenane C.…Perlman S. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589:603–607. doi: 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discovery. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Mehra R., Kallianpur A., Culver D.A.…Cheng F. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biology. 2020;18 doi: 10.1371/journal.pbio.3000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang F., Tang J., Nussinov R., Cheng F. Artificial intelligence in COVID-19 drug repurposing. The Lancet Digital Health. 2020;2:e667–e676. doi: 10.1016/S2589-7500(20)30192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]