Abstract
Background
The Ad26.COV2.S vaccine is a recombinant, replication-incompetent human adenovirus type 26 vector encoding full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein in a prefusion-stabilized conformation.
Methods
In an international, randomized, double-blind, placebo-controlled, phase 3 trial, we randomly assigned adult participants in a 1:1 ratio to receive a single dose of Ad26.COV2.S (5×1010 viral particles) or placebo. The primary end points were vaccine efficacy against moderate to severe–critical coronavirus disease 2019 (Covid-19) with an onset at least 14 days and at least 28 days after administration among participants in the per-protocol population who had tested negative for SARS-CoV-2. Safety was also assessed.
Results
The per-protocol population included 19,630 SARS-CoV-2–negative participants who received Ad26.COV2.S and 19,691 who received placebo. Ad26.COV2.S protected against moderate to severe–critical Covid-19 with onset at least 14 days after administration (116 cases in the vaccine group vs. 348 in the placebo group; efficacy, 66.9%; adjusted 95% confidence interval [CI], 59.0 to 73.4) and at least 28 days after administration (66 vs. 193 cases; efficacy, 66.1%; adjusted 95% CI, 55.0 to 74.8). Vaccine efficacy was higher against severe–critical Covid-19 (76.7% [adjusted 95% CI, 54.6 to 89.1] for onset at ≥14 days and 85.4% [adjusted 95% CI, 54.2 to 96.9] for onset at ≥28 days). Despite 86 of 91 cases (94.5%) in South Africa with sequenced virus having the 20H/501Y.V2 variant, vaccine efficacy was 52.0% and 64.0% against moderate to severe–critical Covid-19 with onset at least 14 days and at least 28 days after administration, respectively, and efficacy against severe–critical Covid-19 was 73.1% and 81.7%, respectively. Reactogenicity was higher with Ad26.COV2.S than with placebo but was generally mild to moderate and transient. The incidence of serious adverse events was balanced between the two groups. Three deaths occurred in the vaccine group (none were Covid-19–related), and 16 in the placebo group (5 were Covid-19–related).
Conclusions
A single dose of Ad26.COV2.S protected against symptomatic Covid-19 and asymptomatic SARS-CoV-2 infection and was effective against severe–critical disease, including hospitalization and death. Safety appeared to be similar to that in other phase 3 trials of Covid-19 vaccines. (Funded by Janssen Research and Development and others; ENSEMBLE ClinicalTrials.gov number, NCT04505722.)
Since emerging in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused high morbidity and mortality, with new variants rapidly spreading.1-4 Vaccines to prevent coronavirus disease 2019 (Covid-19) have been developed with unprecedented speed.5,6
The Ad26.COV2.S vaccine comprises a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector7 encoding a full-length, membrane-bound SARS-CoV-2 spike protein in a prefusion-stabilized conformation.8,9 Other Ad26-based vaccines, including an approved Ebola vaccine, are safe and have induced durable immune responses.8,10-13 Ad26.COV2.S induced durable protection at low doses in preclinical SARS-CoV-2 challenge studies,8,14 and initial clinical data showed that a single dose at 5×1010 viral particles was safe and induced excellent humoral and cellular immune responses.9 Ad26.COV2.S can be stored for up to 2 years in a standard freezer and up to 3 months at refrigerator temperatures, which simplifies transport, storage, and use in a pandemic.
We are conducting an ongoing phase 3 trial (ENSEMBLE) to evaluate the safety and efficacy of a single dose of Ad26.COV2.S at 5×1010 viral particles for the prevention of Covid-19 and SARS-CoV-2 infection in adults. Here, we report the results of the primary analyses.
Methods
Trial Design and Oversight
We are conducting this ongoing, 2-year, multicenter, randomized, double-blind, placebo-controlled, phase 3, pivotal trial in Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, and the United States. All the participants provided written informed consent. The trial adheres to the principles of the Declaration of Helsinki and to the Good Clinical Practice guidelines of the International Council for Harmonisation. The protocol (available with the full text of this article at NEJM.org) and amendments were approved by institutional review boards according to local regulations. An unblinded independent data and safety monitoring board continuously monitors safety, including monitoring for vaccine-associated enhanced respiratory disease.
The trial is a collaboration between the sponsor, Janssen Research and Development, which is an affiliate of Janssen Vaccines and Prevention and part of the Janssen pharmaceutical companies of Johnson & Johnson, and the Operation Warp Speed Covid-19 Rapid Response Team (which includes the Biomedical Advanced Research and Development Authority, the National Institutes of Health, the Covid-19 Prevention Trials Network, and the Department of Defense). The trial was designed and conducted, and the data analysis and data interpretation were performed, by the sponsor and collaborators. Trial-site investigators collected and contributed to the interpretation of the data. All the data were available to the authors, who vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. Medical writers who were funded by the sponsor assisted in drafting the manuscript.
Trial Participants
Stages 1a and 2a of the trial were conducted in parallel and included 2000 adults 18 to 59 years of age and 60 years of age or older, respectively, who were in good or stable health and did not have coexisting conditions that have been associated with an increased risk of severe Covid-19. After a 3-day safety review by the data and safety monitoring board, stages 1b and 2b were initiated. Those stages additionally included adults of the same respective age ranges who had stable and well-controlled coexisting conditions. The eligibility criteria are provided in the Supplementary Methods section in the Supplementary Appendix, available at NEJM.org. Participants were not excluded on the basis of SARS-CoV-2 infection or serostatus.
Procedures
Details of the trial procedures are provided in the Supplementary Methods section. Participants were randomly assigned in a 1:1 ratio, with the use of randomly permuted blocks, to receive either Ad26.COV2.S or saline placebo. Randomization was conducted with an interactive Web-response system and stratified according to trial site, age group, and the presence or absence of coexisting conditions that have been associated with an increased risk of severe Covid-19.
Vaccine or placebo was administered on day 1. Ad26.COV2.S was supplied in single-use vials at a concentration of 1×1011 viral particles per milliliter and was administered at a dose of 5×1010 viral particles as a single intramuscular injection (0.5 ml) by a health care worker who was unaware of the group assignment.
Participants reported Covid-19 symptoms electronically using the Symptoms of Infection with Coronavirus-19 questionnaire (methods described in Fig. S1 in the Supplementary Appendix). Participants and trial staff obtained nasal swabs, which were tested with the use of a Food and Drug Administration (FDA) Emergency Use Authorization reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay for SARS-CoV-2 at a local laboratory and subsequently confirmed centrally (m-2000 SARS-CoV-2 real-time RT-PCR, Abbott). Seropositivity for SARS-CoV-2 was evaluated by means of a SARS-CoV-2 nucleocapsid (N) immunoassay (Elecsys, Roche) at trial entry and on days 29 and 71. Assays were performed according to the manufacturers’ protocols.
Primary and key secondary efficacy evaluations were based on centrally confirmed cases of Covid-19. Owing to the high incidence of Covid-19 and the time taken for central confirmation, not all cases had been centrally confirmed at the time of the primary analysis. A supplementary analysis of RT-PCR–positive cases from all sources, whether centrally confirmed or not, was therefore performed for subgroups, hospitalizations, and deaths.
Safety Assessments
Serious adverse events and adverse events leading to withdrawal from the trial are being recorded throughout the trial. In a safety subpopulation comprising approximately 6000 participants (see below), data on solicited local and systemic adverse events were recorded in an electronic diary for 7 days after administration and unsolicited adverse events for 28 days after administration.
Efficacy Assessments
The two primary end points were the efficacy of the Ad26.COV2.S vaccine against the first occurrence of centrally confirmed moderate to severe–critical Covid-19 with an onset at least 14 days after administration and at least 28 days after administration in the per-protocol population (see below). All the potential cases of severe–critical Covid-19 and cases of moderate Covid-19 with at least three signs or symptoms were classified as being severe–critical by an independent Clinical Severity Adjudication Committee whose members were unaware of the group assignments. This committee adjudicated cases on the basis of clinical judgment (e.g., a single low oxygen-saturation measurement was not classified as indicating severe Covid-19 unless other clinical findings were consistent with a severe classification). The case definitions for Covid-19 and the protocol-defined secondary and exploratory end points are described in the Supplementary Appendix.
Statistical Analysis
The full analysis set included all the participants who underwent randomization and received a dose of trial vaccine or placebo. The per-protocol population comprised participants who received a dose of trial vaccine or placebo, were seronegative or had an unknown serostatus at the time that the vaccine or placebo was administered, and had no protocol deviations that were likely to affect vaccine efficacy. Participants who were RT-PCR–positive between days 1 and 14 or between days 1 and 28 were excluded from the analysis of cases with an onset at least 14 days after administration and at least 28 days after administration, respectively. The per-protocol population was the main population for the efficacy analyses. Safety analyses were conducted in the full analysis set, including the safety subpopulation.
The null hypothesis was that the efficacy of Ad26.COV2.S would be no higher than 30% for each primary end point, as evaluated with a truncated sequential probability ratio test15,16 at a one-sided significance level of 0.025. The sample size was reduced from 60,000 to approximately 40,000 on the basis of the high incidence of Covid-19 during the trial. The primary analysis was triggered on a positive recommendation from the data and safety monitoring board, after the FDA-specified median 8-week follow-up was reached and prespecified data requirements were met.
If the null hypothesis was rejected for both primary end points, secondary objectives were evaluated against a null hypothesis that used a lower limit of vaccine efficacy of more than 0% with prespecified multiplicity adjustments for familywise type I error control (Fig. S2). Exact Poisson regression17 was used for the analysis of vaccine efficacy and the associated confidence interval calculations, with accounting for follow-up time. The cumulative incidence over time was estimated with the use of Kaplan–Meier methods to evaluate the onset of vaccine efficacy and vaccine efficacy over time. Participants had their data censored at the end of their follow-up.
The frequency of serious adverse events was tabulated in the full analysis set. The frequency and severity of solicited and unsolicited adverse events were tabulated in the safety subpopulation.
Results
Participants
The trial began enrollment on September 21, 2020, and the data-cutoff date for the present analysis was January 22, 2021. A total of 44,325 participants underwent randomization, of whom 43,783 received vaccine or placebo; the per-protocol population included 39,321 SARS-CoV-2–negative participants, of whom 19,630 received Ad26.COV2.S and 19,691 received placebo (Fig. S3). The demographic characteristics and coexisting conditions of the participants at baseline were balanced across the two groups (Table 1 and S4). A total of 9.6% of the participants were SARS-CoV-2–seropositive at baseline. The median follow-up was 58 days (range, 1 to 124), and 55% of participants had at least 8 weeks of follow-up; later and slower recruitment of participants 60 years of age or older with coexisting conditions resulted in a shorter duration of follow-up in this subgroup (Table S5).
Table 1. Characteristics of the Trial Participants at Baseline (Full Analysis Set).*.
| Characteristic | Ad26.COV2.S (N=21,895) |
Placebo (N=21,888) |
Total (N=43,783) |
|---|---|---|---|
| Age | |||
| Median (range) — yr | 52 (18−100) | 52 (18−94) | 52 (18−100) |
| Distribution — no. (%) | |||
| 18−59 yr | 14,564 (66.5) | 14,547 (66.5) | 29,111 (66.5) |
| ≥60 yr | 7,331 (33.5) | 7,341 (33.5) | 14,672 (33.5) |
| Sex — no. (%) | |||
| Female | 9,820 (44.9) | 9,902 (45.2) | 19,722 (45.0) |
| Male | 12,071 (55.1) | 11,982 (54.7) | 24,053 (54.9) |
| Nonbinary | 2 (<0.1) | 4 (<0.1) | 6 (<0.1) |
| Unknown | 2 (<0.1) | 0 | 2 (<0.1) |
| Race or ethnic group — no. (%)† | |||
| American Indian or Alaskan Native | 92 (0.4) | 95 (0.4) | 187 (0.4) |
| Indigenous South American | 1,991 (9.1) | 1,965 (9.0) | 3,956 (9.0) |
| Asian | 743 (3.4) | 687 (3.1) | 1,430 (3.3) |
| Black | 4,251 (19.4) | 4,264 (19.5) | 8,515 (19.4) |
| Native Hawaiian or other Pacific Islander | 58 (0.3) | 48 (0.2) | 106 (0.2) |
| White | 12,858 (58.7) | 12,838 (58.7) | 25,696 (58.7) |
| Multiracial | 1,204 (5.5) | 1,245 (5.7) | 2,449 (5.6) |
| Not reported, unknown, or missing | 698 (3.2) | 746 (3.4) | 1,444 (3.3) |
| Hispanic ethnic group — no. (%)† | |||
| Hispanic | 9,874 (45.1) | 9,963 (45.5) | 19,837 (45.3) |
| Non-Hispanic | 11,472 (52.4) | 11,362 (51.9) | 22,834 (52.2) |
| Not reported, unknown, or missing | 549 (2.5) | 563 (2.6) | 1,112 (2.5) |
| Country or region — no. (%) | |||
| Latin America | 8,954 (40.9) | 8,951 (40.9) | 17,905 (40.9) |
| Argentina | 1,498 (6.8) | 1,498 (6.8) | 2,996 (6.8) |
| Brazil | 3,644 (16.6) | 3,634 (16.6) | 7,278 (16.6) |
| Chile | 563 (2.6) | 570 (2.6) | 1,133 (2.6) |
| Colombia | 2,125 (9.7) | 2,123 (9.7) | 4,248 (9.7) |
| Mexico | 238 (1.1) | 241 (1.1) | 479 (1.1) |
| Peru | 886 (4.0) | 885 (4.0) | 1,771 (4.0) |
| South Africa | 3,286 (15.0) | 3,290 (15.0) | 6,576 (15.0) |
| United States | 9,655 (44.1) | 9,647 (44.1) | 19,302 (44.1) |
| SARS-CoV-2 serostatus — no. (%) | |||
| Positive | 2,151 (9.8) | 2,066 (9.4) | 4,217 (9.6) |
| Negative | 19,104 (87.3) | 19,191 (87.7) | 38,295 (87.5) |
| Missing | 640 (2.9) | 631 (2.9) | 1,271 (2.9) |
| Body-mass index‡ | |||
| Median | 27.0 | 27.0 | 27.0 |
| ≥30 — no./total no. (%) | 6264/21,871 (28.6) | 6217/21,853 (28.4) | 12,481/43,724 (28.5) |
| ≥1 Coexisting condition — no. (%) | 8,936 (40.8) | 8,922 (40.8) | 17,858 (40.8) |
The full analysis set included all the participants who underwent randomization and received a dose of Ad26.COV2.S vaccine or placebo. Percentages may not total 100 because of rounding. SARS-CoV-2 denotes severe acute respiratory coronavirus 2.
Race and ethnic group were reported by the participants. American Indian or Alaskan Native was reported only by participants residing in the United States.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. A BMI of 30 or higher indicates obesity.
Safety
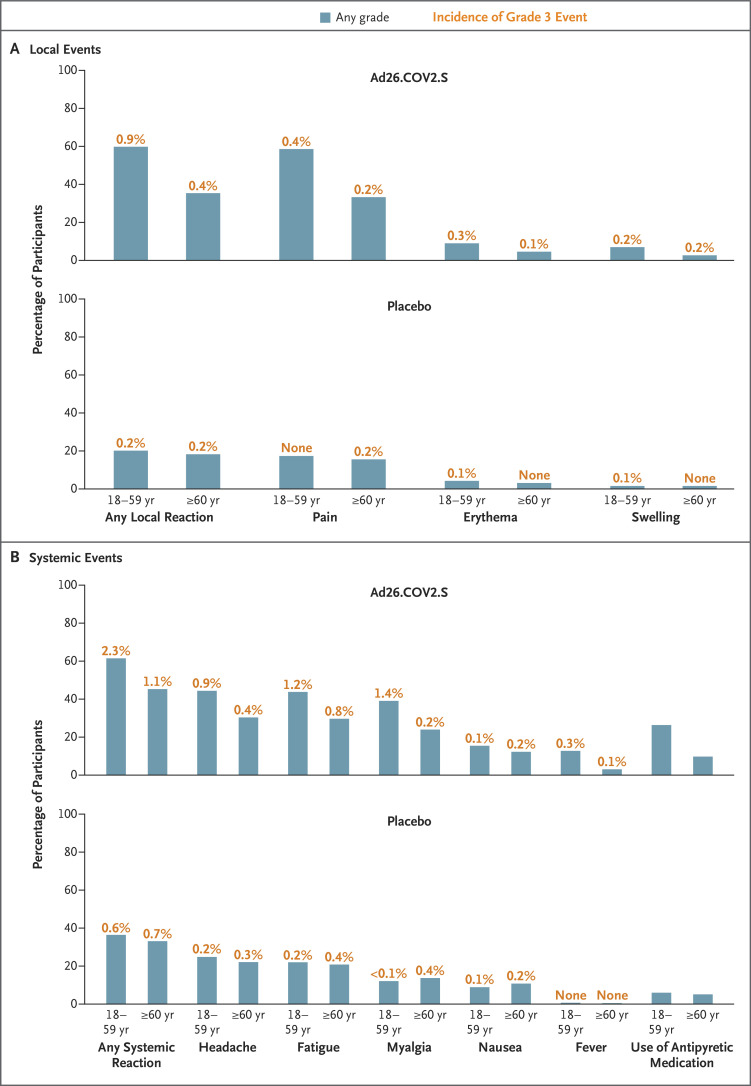
The safety subpopulation included 3356 participants in the vaccine group and 3380 in the placebo group. During the 7-day period after the administration of vaccine or placebo, more solicited adverse events were reported by Ad26.COV2.S recipients than by placebo recipients and by participants 18 to 59 years of age than by those 60 years of age or older (Figure 1). In the vaccine group, injection-site pain was the most common local reaction (in 48.6% of the participants); the most common systemic reactions were headache (in 38.9%), fatigue (in 38.2%), myalgia (in 33.2%), and nausea (in 14.2%).
Figure 1. Solicited Local and Systemic Adverse Events Reported within 7 days after the Administration of Vaccine or Placebo (Safety Subpopulation).
Most solicited local and systemic adverse events occurred within 1 to 2 days after the administration of vaccine or placebo and had a median duration of 1 to 2 days. No grade 4 local or systemic adverse events were reported. There were no local or systemic reactogenicity differences between participants who were seronegative at baseline and those who were seropositive (data not shown). Pain was categorized as grade 1 (mild; does not interfere with activity), grade 2 (moderate; requires modification of activity or involves discomfort with movement), grade 3 (severe; inability to perform usual activities), or grade 4 (potentially life-threatening; hospitalization or inability to perform basic self-care). Erythema and swelling were categorized as grade 1 (mild; 25 to 50 mm), grade 2 (moderate; 51 to 100 mm), grade 3 (severe; >100 mm), or grade 4 (potentially life-threatening; necrosis or leading to hospitalization). Systemic events were categorized as grade 1 (mild; minimal symptoms), grade 2 (moderate; notable symptoms not resulting in loss of work or school time), grade 3 (severe; incapacitating symptoms resulting in loss of work or school time), or grade 4 (life-threatening; hospitalization or inability to perform basic self-care). Fever was defined as grade 1 (mild; ≥38.0 to 38.4°C), grade 2 (moderate; ≥38.5 to 38.9°C), grade 3 (severe; ≥39.0 to 40.0°C), or grade 4 (potentially life-threatening; >40°C).
The adverse events of at least grade 3 that were considered by the investigators to be possibly related to Ad26.COV2.S or placebo are listed in Table S6. Serious adverse events, excluding those related to Covid-19, were reported by 83 of 21,895 vaccine recipients (0.4%) and by 96 of 21,888 placebo recipients (0.4%). Seven serious adverse events were considered by the investigators to be related to vaccination in the Ad26.COV2.S group (Table S7).
A numeric imbalance was observed for venous thromboembolic events (11 in the vaccine group vs. 3 in the placebo group). Most of these participants had underlying medical conditions and predisposing factors that might have contributed to these events (Table S8). Imbalances were also observed with regard to seizure (which occurred in 4 participants in the vaccine group vs. 1 in the placebo group) and tinnitus (in 6 vs. 0). A causal relationship between these events and Ad26.COV2.S cannot be determined. These events will be monitored in the post-marketing setting.
Three deaths were reported in the vaccine group and 16 in the placebo group, all of which were considered by the investigators to be unrelated to the trial intervention (Table S7). No deaths related to Covid-19 were reported in the vaccine group, whereas 5 deaths related to Covid-19 were reported in the placebo group. Transverse sinus thrombosis with cerebral hemorrhage and a case of the Guillain–Barré syndrome were each seen in 1 vaccine recipient.
Efficacy
In the per-protocol at-risk population, 468 centrally confirmed cases of symptomatic Covid-19 with an onset at least 14 days after administration were observed, of which 464 were moderate to severe–critical (116 cases in the vaccine group vs. 348 in the placebo group), which indicated vaccine efficacy of 66.9% (adjusted 95% confidence interval [CI], 59.0 to 73.4) (Table 2). In terms of the primary end point of disease onset at least 28 days after administration, 66 cases of moderate to severe–critical Covid-19 in the vaccine group and 193 cases in the placebo group were observed, which indicated vaccine efficacy of 66.1% (adjusted 95% CI, 55.0 to 74.8) (Table 2).
Table 2. Vaccine Efficacy against Covid-19 with Onset at Least 14 Days and at Least 28 Days after the Administration of Vaccine or Placebo (Per-Protocol at-Risk Population).*.
| Variable | ≥14 Days after Administration† | ≥28 Days after Administration‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ad26.COV2.S (N=19,514) |
Placebo (N=19,544) |
Vaccine Efficacy (95% CI) |
Ad26.COV2.S (N=19,306) |
Placebo (N=19,178) |
Vaccine Efficacy (95% CI) |
|||||
| no. of cases | person-yr | no. of cases | person-yr | % | no. of cases | person-yr | no of cases | person-yr | % | |
| Moderate to severe–critical Covid-19 | 116 | 3116.6 | 348 | 3096.1 | 66.9 (59.0–73.4) | 66 | 3102.0 | 193 | 3070.7 | 66.1 (55.0–74.8) |
| 18−59 yr | 95 | 2106.8 | 260 | 2095.0 | 63.7 (53.9–71.6) | 52 | 2097.6 | 152 | 2077.0 | 66.1 (53.3–75.8) |
| ≥60 yr | 21 | 1009.8 | 88 | 1001.2 | 76.3 (61.6–86.0) | 14 | 1004.4 | 41 | 993.6 | 66.2 (36.7–83.0) |
| Symptomatic Covid-19 of any severity | 117 | 3116.5 | 351 | 3095.9 | 66.9 (59.1–73.4) | 66 | 3102.0 | 195 | 3070.5 | 66.5 (55.5–75.1) |
| Mild | 1 | 3116.5 | 3 | 3095.9 | NC§ | 0 | 3102.0 | 2 | 3070.5 | NC§ |
| Moderate | 102 | 3116.6 | 288 | 3096.1 | 64.8 (55.8–72.2) | 61 | 3102.0 | 159 | 3070.7 | 62.0 (48.7–72.2) |
| Severe–critical | 14 | 3125.1 | 60 | 3122.0 | 76.7 (54.6–89.1) | 5 | 3106.2 | 34 | 3082.6 | 85.4 (54.2–96.9) |
| Severity-adjusted symptomatic Covid-19¶ | 117 | 3116.5 | 351 | 3095.9 | 68.1 (60.3–74.3) | 66 | 3102.0 | 195 | 3070.5 | 69.0 (56.7–77.6) |
| 18−59 yr | 95 | 2106.8 | 260 | 2095.0 | 65.8 (56.2–73.1) | 52 | 2097.6 | 152 | 2077.0 | 69.3 (57.4–77.7) |
| ≥60 yr | 22 | 1009.6 | 91 | 1001.0 | 74.5 (57.9–84.3) | 14 | 1004.4 | 43 | 993.5 | 67.9 (38.2–82.8) |
| Moderate to severe–critical Covid-19, including noncentrally confirmed cases | 173 | 3113.9 | 509 | 3089.1 | 66.3 (59.9–71.8) | 113 | 3100.3 | 324 | 3065.9 | 65.5 (57.2–72.4) |
| Covid-19, according to FDA harmonized definition‖ | 114 | 3116.6 | 345 | 3,096.3 | 67.2 (59.3–73.7) | 65 | 3102.0 | 193 | 3070.6 | 66.7 (55.6–75.2) |
| Moderate to severe–critical Covid-19, according to Cox proportional-hazards model** | 116 | 3116.6 | 348 | 3,096.1 | 66.9 (59.1–73.2) | 66 | 3102.0 | 193 | 3070.7 | 66.2 (55.3–74.4) |
All cases of coronavirus disease 2019 (Covid-19) were centrally confirmed unless stated otherwise and occurred in participants who had been seronegative at baseline and negative on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) testing before 14 or 28 days after the administration of vaccine or placebo, for the respective end points, and were therefore at risk for Covid-19. The follow-up time for each participant was defined as the time from the administration of vaccine or placebo to the onset of Covid-19 or the last available trial measurement (January 22, 2021). Adjusted 95% confidence intervals are shown for moderate and severe–critical Covid-19, severe–critical Covid-19, severity-adjusted Covid-19, and moderate to severe–critical Covid-19, including non–centrally confirmed cases; unadjusted 95% confidence intervals are shown for other end points. The adjusted confidence interval was calculated with implementation of type I error control for multiple testing. Adjusted confidence intervals are presented for the end points that were prespecified for inferential evaluation at the primary analysis and on reaching the associated minimal required number of cases for that end point. Mild cases of Covid-19 were defined as a positive result on RT-PCR testing and the presence of at least one of the following symptoms: fever (body temperature, ≥38.0°C), sore throat, malaise, headache, myalgia, gastrointestinal symptoms, cough, chest congestion, runny nose, wheezing, skin rash, eye irritation or discharge, chills, loss of taste or smell, red or bruised looking feet or toes, or shaking chills or rigors. Moderate cases were defined as a positive RT-PCR test and either the presence of at least two of the following symptoms: fever (≥38.0°C), heart rate of at least 90 beats per minute, shaking chills or rigors, sore throat, cough, malaise, headache, myalgia, gastrointestinal symptoms, loss of taste or smell, or red or bruised-looking feet or toes; or the presence at least one of the following symptoms: respiratory rate of at least 20 breaths per minute, abnormal oxygen saturation (but >93% while the patient was breathing ambient air at sea level), clinical or radiologic evidence of pneumonia, radiologic evidence of deep-vein thrombosis, or shortness of breath or difficulty breathing. Severe–critical cases were defined as a positive RT-PCR test and the presence of at least one of the following features: clinical signs at rest that were indicative of severe systemic illness (respiratory rate of ≥30 breaths per minute, heart rate of ≥125 beats per minute, oxygen saturation of ≤93% while the patient was breathing ambient air at sea level, or partial pressure of oxygen divided by the fraction of inspired oxygen, <300 mm Hg); respiratory failure (defined as the use of high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation); shock; clinically meaningful acute renal, hepatic, or neurologic dysfunction; intensive care unit admission; or death.
The at-risk population for this analysis excluded participants who were RT-PCR–positive between days 1 and 14 after the administration of vaccine or placebo.
The at-risk population for this analysis excluded participants who were RT-PCR–positive between days 1 and 28 after the administration of vaccine or placebo.
The vaccine efficacy was not calculated (NC) if fewer than 6 cases were observed for an end point.
Shown is the weighted version of the estimates of vaccine efficacy against mild, moderate, and severe–critical Covid-19.18
The Food and Drug Administration (FDA) harmonized definition of Covid-19 was defined as a positive RT-PCR test and the presence of Covid-19 symptoms consistent with the FDA harmonized definition at the time that the protocol was written: fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, or diarrhea.
A supportive analysis with the use of a Cox proportional-hazards regression model of the time to moderate to severe–critical Covid-19 was used to estimate vaccine efficacy.
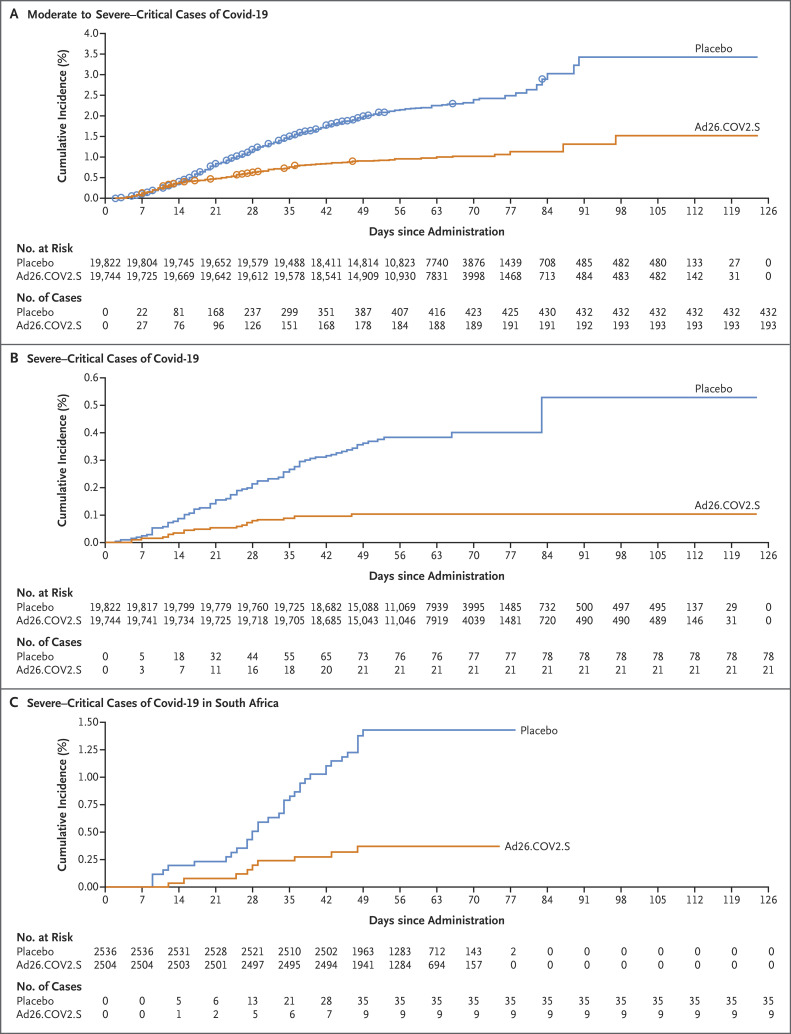
The cumulative incidence of the first occurrence of moderate to severe–critical Covid-19 diverged between the two trial groups at approximately 14 days after the administration of vaccine or placebo, which indicates an early onset of protection with the vaccine (Figure 2A). Fewer cases in the vaccine group were observed after day 14 while cases continued to accrue in the placebo group, which led to increasing vaccine efficacy over time (Fig. S4A). Efficacy against disease with an onset at least 28 days after administration was similar across age groups, but efficacy against disease with an onset 14 days after administration was higher among older participants than among younger participants (Table 2). This discrepancy probably resulted from differences in follow-up duration or from smaller sample sizes in subgroups. The number of primary end-point cases was similar to the number of cases of symptomatic Covid-19 as defined according to the FDA harmonized definition (Table 2); thus, the primary end-point analyses captured most of the cases of symptomatic Covid-19. Estimates of vaccine efficacy in the analyses of the two primary end points and the secondary end points of centrally confirmed cases differed by less than 2 percentage points from the estimates in analyses of positive cases from all sources, and the confidence intervals were similar (Table 2 and Table 3). Vaccine-efficacy estimates in the full analysis set were generally lower than those in the per-protocol population because the estimates included cases that occurred at or after 1 day after administration, when immunity was building (Table S9).
Figure 2. Cumulative Incidence of Covid-19 with Onset at Least 1 Day after Vaccination and Vaccine Efficacy over Time.
Panel A shows the cumulative incidence of moderate to severe–critical cases of coronavirus disease 2019 (Covid-19); circles indicate severe–critical cases. Panel B shows the cumulative incidence of severe–critical cases. Cases included in the analyses in Panels A and B were centrally confirmed cases in the full analysis set among participants who were seronegative at baseline. Panel C shows the cumulative incidence of severe–critical cases in South Africa among participants who were seronegative at baseline; these cases were those that were positive on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) testing from all sources, whether centrally confirmed or not.
Table 3. Vaccine Efficacy against Covid-19 with Onset at Least 14 Days and at Least 28 Days after Administration of Vaccine or Placebo, According to Country (Per-Protocol at-Risk Population).*.
| Variable | ≥14 Days after Administration† | ≥28 Days after Administration‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ad26.COV2.S | Placebo | Vaccine Efficacy (95% CI) |
Ad26.COV2.S | Placebo | Vaccine Efficacy (95% CI) |
|||||
| no. | person-yr | no. | person-yr | % | no. | person-yr | no. | person-yr | % | |
| Worldwide | ||||||||||
| No. of participants | 19,514 | 19,544 | 19,306 | 19,178 | ||||||
| Moderate to severe–critical Covid-19 | 173 | 3113.9 | 509 | 3089.1 | 66.3 (59.9 to 71.8) | 113 | 3100.3 | 324 | 3065.9 | 65.5 (57.2 to 72.4) |
| Severe–critical Covid-19 | 19 | 3124.7 | 80 | 3121.0 | 76.3 (57.9 to 87.5) | 8 | 3106.0 | 48 | 3082.0 | 83.5 (54.2 to 96.9) |
| United States | ||||||||||
| No. of participants | 9,119 | 9,086 | 8,958 | 8,835 | ||||||
| Moderate to severe–critical Covid-19 | 51 | 1414.0 | 196 | 1391.3 | 74.4 (65.0 to 81.6) | 32 | 1403.4 | 112 | 1375.6 | 72.0 (58.2 to 81.7) |
| Severe–critical Covid-19 | 4 | 1417.2 | 18 | 1404.8 | 78.0 (33.1 to 94.6) | 1 | 1405.2 | 7 | 1382.2 | 85.9 (−9.4 to 99.7) |
| Brazil | ||||||||||
| No. of participants | 3,370 | 3,355 | 3,354 | 3,312 | ||||||
| Moderate to severe–critical Covid-19 | 39 | 555.7 | 114 | 548.8 | 66.2 (51.0 to 77.1) | 24 | 554.8 | 74 | 546.1 | 68.1 (48.8 to 80.7) |
| Severe–critical Covid-19 | 2 | 558.9 | 11 | 556.8 | 81.9 (17.0 to 98.1) | 1 | 556.2 | 8 | 549.8 | 87.6 (7.8 to 99.7) |
| South Africa | ||||||||||
| No. of participants | 2,473 | 2,496 | 2,449 | 2,463 | ||||||
| Moderate to severe–critical Covid-19 | 43 | 377.6 | 90 | 379.2 | 52.0 (30.3 to 67.4) | 23 | 376.1 | 64 | 376.9 | 64.0 (41.2 to 78.7) |
| Severe–critical Covid-19 | 8 | 380.2 | 30 | 382.9 | 73.1 (40.0 to 89.4) | 4 | 377.0 | 22 | 379.0 | 81.7 (46.2 to 95.4) |
All cases of Covid-19 occurred in participants who had been seronegative at baseline and RT-PCR–negative before 14 or 28 days after the administration of vaccine or placebo, for the respective end points, and were therefore at risk for Covid-19; these participants were positive on RT-PCR testing from all sources. Adjusted 95% confidence intervals are shown for Covid-19 cases worldwide; unadjusted 95% confidence intervals are shown for country-specific end points. The adjusted confidence interval implements type I error control for multiple testing.
The at-risk population for this analysis excluded participants who were RT-PCR–positive between days 1 and 14 after the administration of vaccine or placebo.
The at-risk population for this analysis excluded participants who were RT-PCR–positive between days 1 and 28 after the administration of vaccine or placebo.
With regard to severe–critical Covid-19, vaccine efficacy was 76.7% (adjusted 95% CI, 54.6 to 89.1) against disease with onset at least 14 days after administration and 85.4% (adjusted 95% CI, 54.2 to 96.9) against disease with onset at least 28 days after administration (Table 2). The cumulative-incidence curves began to separate approximately 7 days after administration; vaccine efficacy increased with longer follow-up and was 92.4% after day 42 (post hoc calculation) (Figures 2B and S4B).
The analysis of vaccine efficacy against asymptomatic infection included all the participants with a newly positive N-immunoassay result at day 71 (i.e., those who had been seronegative or had no result available at day 29 and who were seropositive at day 71). Only 2650 participants had an N-immunoassay result available at day 71, and therefore only a preliminary analysis could be performed. A total of 18 asymptomatic infections were identified in the vaccine group and 50 in the placebo group (vaccine efficacy, 65.5%; 95% CI, 39.9 to 81.1).
Vaccine efficacy against Covid-19 involving medical intervention ranged from 75.0 to 100.0% (Table S10). Two cases of Covid-19 with onset at least 14 days after administration in the Ad26.COV2.S group and 29 such cases in the placebo group led to hospitalization (vaccine efficacy, 93.1%; 95% CI, 72.7 to 99.2) (Fig. S5). No hospitalizations for cases with an onset at least 28 days after administration occurred in the vaccine group, as compared with 16 hospitalizations in the placebo group (vaccine efficacy, 100%; 95% CI, 74.3 to 100.0).
Participants with moderate Covid-19 who had received Ad26.COV2.S most frequently reported 4 to 6 symptoms, as compared with 7 to 9 symptoms in participants who had received placebo (Fig. S6). The total mean symptom-severity score as reported on the Symptoms of Infection with Coronavirus-19 questionnaire was 24% (95% CI, −1 to 46) lower among vaccine recipients than among placebo recipients at day 1 after symptom onset, 47% (95% CI, 23 to 66) lower at day 7 after symptom onset, and 53% (95% CI, 0 to 81) lower at day 14 after symptom onset among participants with an onset of moderate illness at least 28 days after administration (Fig. S1).
The estimates of vaccine efficacy against severe–critical disease were consistently high across countries that had sufficient cases for analysis (Table 3). On the basis of interim sequencing data from 512 unique RT-PCR–positive samples obtained from 714 participants (71.7%) with SARS-CoV-2 infection, the reference sequence (Wuhan-Hu-1 including the D614G mutation) was detected predominantly in the United States (190 of 197 sequences [96.4%]) and the 20H/501Y.V2 variant (also called B.1.351) was detected predominantly in South Africa (86 of 91 sequences [94.5%]), whereas in Brazil, the reference sequence was detected in 38 of 124 sequences (30.6%) and the reference sequence with the E484K mutation (P.2 lineage) was detected in 86 of 124 sequences (69.4%). Despite the high prevalence of the 20H/501Y.V2 variant in South Africa and in Covid-19 cases in the trial, vaccine efficacy was maintained (52.0% against moderate to severe–critical disease and 73.1% against severe–critical disease with onset ≥14 days after administration; 64.0% against moderate to severe–critical disease and 81.7% against severe–critical disease with onset at ≥28 days after administration) (Figure 2C and Table 3). In South Africa, no hospitalizations of participants with an onset of Covid-19 at least 28 days after administration occurred in the vaccine group, as compared with 6 hospitalizations in the placebo group. All five Covid-19–related deaths in the trial occurred in the placebo group in South Africa.
No meaningful differences in vaccine efficacy were observed among subgroups defined according to sex, race, or ethnic group (Fig. S7 and Table S11). A lower point estimate of vaccine efficacy was observed among participants 60 years of age or older with coexisting conditions in the analysis of cases with onset at least 28 days after administration (15 cases of moderate to severe–critical Covid-19 among vaccine recipients vs. 26 cases among placebo recipients) but not in the analysis of cases with onset at least 14 days after administration (22 vs. 63 cases) (Fig. S7). Estimates of efficacy over time that were based on Kaplan–Meier analysis were similar among participants 60 years of age or older with coexisting conditions and those without coexisting conditions (Figs. S4C and S8). Two participants 60 years of age or older with coexisting conditions in the vaccine group were hospitalized, as compared with 11 such participants in the placebo group (vaccine efficacy, 81.6%; 95% CI, 15.8 to 98.0).
Discussion
This international, phase 3 ENSEMBLE trial showed the efficacy of a single dose of the Ad26.COV2.S vaccine in preventing Covid-19. Efficacy against moderate to severe–critical Covid-19 was 67% against disease with onset at least 14 days after administration and 66% against disease with onset 28 days after administration. Because the number of primary end-point cases was similar to the number of cases according to the FDA harmonized definition, this estimate essentially captures most of the cases of symptomatic Covid-19. Higher efficacy against severe–critical Covid-19 was observed, with vaccine efficacy of 77% against disease with onset at least 14 days after administration and 85% against disease with onset at least 28 days after administration.
The onset of efficacy was evident as of 14 days after administration for moderate to severe–critical disease and as of 7 days after administration for severe–critical disease. Efficacy continued to increase through approximately 8 weeks after administration, especially for severe–critical Covid-19. No evidence of waning efficacy was noted among the approximately 3000 participants who were followed for 11 weeks or among 1000 participants who were followed for 15 weeks, a finding that is consistent with the persistence of humoral immunity that was observed in a phase 1–2a trial.9
Efficacy against severe–critical Covid-19 was consistently high overall and in individual countries that had sufficient cases for analysis, which is particularly important because severe disease has the greatest effect on individual persons and health care systems.19 Efficacy against Covid-19 involving hospitalization was 93% with regard to onset at least 14 days after administration (2 cases in the vaccine group and 29 in the placebo group) and 100% with regard to onset at least 28 days after administration (no hospitalizations in the vaccine group and 16 in the placebo group). Although hospitalization can be influenced by local practice and resource availability, all the hospitalizations that were reported were justified by clear clinical findings and were consistent across countries. Moreover, identical management practices would have applied to the Ad26.COV2.S group and the placebo group in each country. Five deaths that were related to Covid-19 occurred in the placebo group, but there were no such deaths in the vaccine group. The reduction in the incidence of death and the high efficacy against hospitalization are expected to substantially reduce the effect of this disease on individual persons and dramatically decrease the burden on health care systems.
Vaccine recipients with breakthrough Covid-19 reported fewer and less severe symptoms than did placebo recipients with Covid-19, which suggests that illness is milder after vaccination. The data are consistent with studies reporting higher efficacy of the influenza vaccine against more severe influenza20-22 and the attenuation of influenza among vaccinees.23-25 A preliminary analysis indicated that Ad26.COV2.S provided at least 66% protection against serologically confirmed asymptomatic infection with SARS-CoV-2. The effect on the incidence of symptomatic and asymptomatic SARS-CoV-2 infection by the vaccine suggests that it might be useful in reducing community-wide transmission.
New SARS-CoV-2 virus lineages have emerged, with mutations in the N-terminal and receptor-binding domains of the spike protein that are known targets for neutralizing antibodies; in particular, the E484K mutation is associated with reduced neutralization sensitivity.26-31 Of main concern are variants that were first identified in Brazil, South Africa, and the United Kingdom.2-4 In our trial, 95% of the Covid-19 cases in South Africa in which SARS-CoV-2 was sequenced were caused by the 20H/501Y.V2 variant, whereas a variant from the P.2 lineage carrying the E484K mutation was identified in 69% of the cases in Brazil with a sequenced sample. However, despite the high prevalence of SARS-CoV-2 variants of concern, vaccine efficacy remained high. This finding shows that a Covid-19 vaccine that was based on the original Wuhan-Hu-1 strain can elicit cross-protective efficacy against new variants in South Africa and Brazil. Nonneutralizing antibodies against SARS-CoV-2 variants are probably preserved because they are not limited to the N-terminal or receptor-binding domains, where most mutations occur. Antibodies with Fc-mediated functions are induced by Ad26.COV2.S against SARS-CoV-2 in humans,32 and these Fc functional antibodies show no decrease in potency against new variants (personal communication: G. Alter and D. Barouch). In addition, CD8+ T-cell responses to the SARS-CoV-2 spike protein were seen in a phase 1–2a trial.9 T-cell epitopes were shown to be conserved between SARS-CoV-2 variants according to immunoinformatics analyses.33-35 These factors might contribute to the high efficacy against severe–critical disease, hospitalization, and death in South Africa, where the relatively neutralization-resistant 20H/501Y.V2 variant predominates.26,36
Efficacy against symptomatic infection was similar among younger and older participants and among participants with coexisting conditions and those without coexisting conditions. A subgroup analysis involving participants 60 years of age or older showed that vaccine efficacy against symptomatic disease with onset at least 14 days after administration was similar in subgroups defined according to the presence or absence of coexisting conditions. With regard to onset at least 28 days after administration, vaccine efficacy appeared lower among participants with coexisting conditions than among those without coexisting conditions. This finding can be attributed to imprecision owing to fewer cases and shorter follow-up in this subgroup. Furthermore, Kaplan–Meier curves indicated that the cumulative incidence of cases among vaccine recipients 60 years of age or older with coexisting conditions was similar to that in the overall trial population, which suggests a similar vaccine efficacy. Vaccine efficacy against hospitalization among vaccine recipients 60 years of age or older with coexisting conditions was 82%, a finding consistent with this result.
This trial confirmed the findings from a phase 1–2a trial9 showing that Ad26.COV2.S had an acceptable safety and reactogenicity profile. Reactogenicity to Ad26.COV2.S was transient, was lower in older participants than in younger participants, and resolved quickly. Severe reactogenicity (grade ≥3) was uncommon, and serious adverse events were rare. Data from the current trial are supported by long-term and robust safety data on the Ad26 platform.10-12
A key strength of this trial is that it showed vaccine efficacy in an ethnically and geographically diverse population, including participants in regions with emerging SARS-CoV-2 variants, as well as in participants with coexisting conditions that have been associated with an increased risk of severe Covid-19. A limitation of the trial is the relatively short follow-up, which was necessitated, as in other Covid-19 vaccine trials, by the urgent need for vaccine. The data do not suggest a waning of protection. Long-term unblinded follow-up is planned to compare results in initial Ad26.COV2.S recipients with those in placebo recipients who are expected to receive Ad26.COV2.S after a protocol amendment has been approved.
This trial was conducted during a time of an extraordinarily high incidence of SARS-CoV-2 infection. Lower vaccine efficacy has been associated with a higher incidence of disease.37-39 This situation, combined with the emergence of viral variants, precludes the comparison of vaccine trials. In this trial, we robustly field-tested a simple regimen under high attack-rate conditions on three continents and consistently found early and increasing protection from severe disease.
In this trial, we found that a single dose of Ad26.COV2.S protected against symptomatic Covid-19 and was particularly efficacious against severe–critical disease (including hospitalization and death), including in countries where variants that are considered to be relatively resistant to antibody neutralization predominate. Safety appeared to be similar to that seen in previous phase 3 trials of Covid-19 vaccines. The single-dose schedule and favorable storage conditions of this vaccine provide major advantages in its deployment and effect worldwide.
Acknowledgments
We thank all the participants in this trial, the staff members at the trial locations, the members of the data and safety monitoring board, all the investigators at the clinical sites, the COV3001 study team (Richard Gorman, Carmen A. Paez, Edith Swann, James Kublin, Simbarashe G. Takuva, Alex Greninger, Pavitra Roychoudhury, Robert W. Coombs, Keith R. Jerome, Flora Castellino, Xiaomi Tong, Corrina Pavetto, Teletha Gipson, Tina Tong, Marina Lee, James Zhou, Michael Fay, Kelly McQuarrie, Chimeremma Nnadi, Obiageli Sogbetun, Nina Ahmad, Ian De Proost, Cyrus Hoseyni, Paul Coplan, Najat Khan, Peter Ronco, Dawn Furey, Jodi Meck, Johan Vingerhoets, Boerries Brandenburg, Jerome Custers, Jenny Hendriks, Jarek Juraszek, Marit de Groot, Griet Van Roey, Dirk Heerwegh, and Ilse Van Dromme), and Mary L. Greenacre (An Sgriobhadair) and Jill E. Kolesar and Kurt Kunz (Cello Health Communications–MedErgy) for writing and editorial assistance, funded by Janssen Global Services, with an earlier version of the manuscript.
Research Summary
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on April 21, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Janssen Research and Development, an affiliate of Janssen Vaccines and Prevention and part of the Janssen pharmaceutical companies of Johnson & Johnson, and in whole or in part by federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Other Transaction Agreement HHSO100201700018C, and from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI68614), the HVTN Statistics and Data Management Center (UM1 AI68635), the HVTN Laboratory Center (UM1 AI68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI68636), the Infectious Diseases Clinical Research Consortium Leadership Group (UM1 AI148684), and Vaccine and Therapeutic Evaluation Units (UM1 AI148576, UM1 AI148373, UM1 AI148685, and UM1 AI148452).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. (https://coronavirus.jhu.edu/map.html).
- 2.Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. December 22, 2020. (https://www.medrxiv.org/content/10.1101/2020.12.21.20248640v1). preprint.
- 3.Voloch CM, da Silva Francisco R Jr, de Almeida LGP, et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J Virol 2021. March 1 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 4.Public Health England. Investigation of novel SARS-CoV-2 variant: variant of concern 202012/01: technical briefing 3. 2021. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950823/Variant_of_Concern_VOC_202012_01_Technical_Briefing_3_-_England.pdf).
- 5.Krammer F. SARS-CoV-2 vaccines in development. Nature 2020;586:516-527. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. April 9, 2021. (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
- 7.Abbink P, Lemckert AAC, Ewald BA, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 2007;81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020;5:91-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018;392:232-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Custers J, Kim D, Leyssen M, et al. Vaccines based on replication incompetent Ad26 viral vectors: standardized template with key considerations for a risk/benefit assessment. Vaccine 2020. October 3 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams K, Bastian AR, Feldman RA, et al. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥60 years. J Infect Dis 2020;222:979-988. [DOI] [PubMed] [Google Scholar]
- 13.Salisch NC, Stephenson KE, Williams K, et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an Ad26-vectored anti-Zika virus vaccine. Ann Intern Med 2021. February 16 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 14.Mercado NB, Zahn R, Wegmann F, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020;586:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dragalin V, Fedorov V, Cheuvart B. Statistical approaches to establishing vaccine safety. Stat Med 2002;21:877-893. [DOI] [PubMed] [Google Scholar]
- 16.Dragalin V, Fedorov V. Multistage designs for vaccine safety studies. J Biopharm Stat 2006;16:539-553. [DOI] [PubMed] [Google Scholar]
- 17.Nauta J. Statistics in clinical vaccine trials. New York: Springer-Verlag, 2011:95-97. [Google Scholar]
- 18.Mehrotra DV, Janes HE, Fleming TR, et al. Clinical endpoints for evaluating efficacy in COVID-19 vaccine trials. Ann Intern Med 2021;174:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2021;21(2):e26-e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain VK, Rivera L, Zaman K, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013;369:2481-2491. [DOI] [PubMed] [Google Scholar]
- 21.Claeys C, Zaman K, Dbaibo G, et al. Prevention of vaccine-matched and mismatched influenza in children aged 6-35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health 2018;2:338-349. [DOI] [PubMed] [Google Scholar]
- 22.Beran J, Reynales H, Poder A, et al. Prevention of influenza during mismatched seasons in older adults with an MF59-adjuvanted quadrivalent influenza vaccine: a randomised, controlled, multicentre, phase 3 efficacy study. Lancet Infect Dis 2021. February 9 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 23.Arriola C, Garg S, Anderson EJ, et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis 2017;65:1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castilla J, Godoy P, Domínguez A, et al. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013;57:167-175. [DOI] [PubMed] [Google Scholar]
- 25.Deiss RG, Arnold JC, Chen W-J, et al. Vaccine-associated reduction in symptom severity among patients with influenza A/H3N2 disease. Vaccine 2015;33:7160-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021. March 2 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 27.Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife 2020;9:e61312-e61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021. February 23 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees-Spear C, Muir L, Griffith SA, et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Rep 2021;34:108890-108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu K, Werner AP, Moliva JI, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. January 25, 2021. (https://www.biorxiv.org/content/10.1101/2021.01.25.427948v1). preprint.
- 31.Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med 2021. February 8 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 32.Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 2021. March 11 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prakash S, Srivastava R, Coulon P-G, et al. Genome-wide asymptomatic B-cell, CD4+ and CD8+ T-cell epitopes, that are highly conserved between human and animal coronaviruses, identified from SARS-CoV-2 as immune targets for pre-emptive pan-coronavirus vaccines. September 28, 2020. (https://www.biorxiv.org/content/10.1101/2020.09.27.316018v1.full). preprint. [DOI] [PMC free article] [PubMed]
- 34.Lee E, Sandgren K, Duette G, et al. Identification of SARS-CoV-2 nucleocapsid and spike T-cell epitopes for assessing T-cell immunity. J Virol 2021;95(6):e02002-20-e02002-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CH, Pinho MP, Buckley PR, et al. Potential CD8+ T cell cross-reactivity against SARS-CoV-2 conferred by other coronavirus strains. Front Immunol 2020;11:579480-579480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GISAID. Map of tracked variant occurrence. 2021. (https://www.gisaid.org/hcov19-variants).
- 37.Langwig KE, Gomes MGM, Clark MD, et al. Limited available evidence supports theoretical predictions of reduced vaccine efficacy at higher exposure dose. Sci Rep 2019;9:3203-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes MGM, Gordon SB, Lalloo DG. Clinical trials: the mathematics of falling vaccine efficacy with rising disease incidence. Vaccine 2016;34:3007-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margheri A, Rebelo C, Gomes MGM. Heterogeneity in disease risk induces falling vaccine protection with rising disease incidence. Dyn Syst 2017;32:148-163. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.