Abstract
The occurrence of cardiac implantable electronic devices (CIED) infections and related adverse outcomes have an important financial impact on the healthcare system, with hospitalization length of stay (2–3 weeks on average) being the largest cost driver, including the cost of device system extraction and device replacement accounting for more than half of total costs. In the recent literature, the economic profile of the TYRX™ absorbable antibacterial envelope was analysed taking into account both randomized and non-randomized trial data. Economic analysis found that the envelope is associated with cost-effectiveness ratios below USA and European benchmarks in selected patients at increased risk of infection. Therefore, the TYRX™ envelope, by effectively reducing CIED infections, provides value according to the criteria of affordability currently adopted by USA and European healthcare systems.
Keywords: Antibiotics, Cardiac implantable electronic devices, Cost, Cost-effectiveness, Economics, Hospitalization, Infections
Introduction
Infections associated with cardiac implantable electronic devices (CIEDs) are clinically relevant in view of the increasing number of devices implanted owing to population ageing,1,2 with the number of implants worldwide estimated to be ∼1.5 million per year.3 Despite the use of antibiotic prophylaxis at the time of device implantation, device-related infection rates increased in recent years, CIED-associated infections rising to alert levels. Cardiac implantable electronic device-related infections have the specific features of infection on prosthetic materials, and are currently recognized as a major concern for health care systems, with over two million cases annually in the USA, causing substantial morbidity and lengthy hospital admissions.4 Assessments of the incidence, morbidity and mortality are key to improve our knowledge about CIED infections but to have a more complete picture of the adverse impact on the community, the economic consequences also need to be defined. Studies assessing the economic impact of diseases have grown exponentially since the mid-1960s, when the first framework of economic analysis, in the form ‘cost-of-illness’ evaluations, was defined.5
Financial burden of infections associated with cardiac electronic implantable devices
The incidence of infections associated with CIEDs is reported to be ∼1–4%,6–8 negatively impacting both the patient and health care system due to the need for hospitalization with use of intensive care unit stay, expensive diagnostics, prolonged antibiotic therapy, as well as the frequent need for device and lead extraction, and re-implantation. Mortality has been reported as 20–25% at 1 year9,10 and up to 50% at 3 years despite state-of-the-art infection management, consisting11 of device and lead removal, antibiotic treatment, and subsequent device and lead re-implantation when indicated. Approximately 3–15% of patients refuse or are considered not suitable for lead extraction and are managed with suppressive antibiotic therapy as a palliative measure.6,12,13
Cardiac implantable electronic device infections and their adverse outcomes also heavily impact on healthcare systems. The clinical profile is associated to the overall expenditure, as shown in a retrospective cohort analysis of 5401 Medicare patients which captured an average $62 638 for patients requiring CIED extraction and replacement, $50 079 for those extracted but not re-implanted, $77 397 for patients hospitalized for CIED infection not undergoing removal, and $22 856 for patients who had no infection-related hospitalization.10 The main driver of cost was represented by hospitalization (included cost of device system extraction and device replacement) and non-extracted patients with infection-related hospitalization actually had the longest hospital stay and the largest use of resources.10 In 2011, Sohail et al.14 reported on >200 000 Medicare patients admitted for CIED implantation, replacement, or revision during year 2007. A total of 5817 admissions of patients with CIED infection were recorded, with an important burden of hospitalizations and substantial adjusted in-hospital and long-term mortality. The standardized adjusted incremental cost ($14 360–$16 498) and total admission cost ($28 676–$53 349) depended upon device type and was significantly impacted by intensive care stays.14 More recently an analysis of CIED implants in a MarketScan Commercial Claims and Medicare Supplemental database from the USA during the calendar years 2009–2012 identified a cumulative incidence of infection at 1 year post-implant of 1.18% for initial CIED implants and 2.37% for replacements.15 Median time to infection was 35 days for initial implants and 23 days for replacements, with incremental healthcare expenditures at 1 year depending on treatment intensity, ranging from $16 651 (no inpatient admission or device procedure) to $279 744 (inpatient admission, device procedure and concomitant sepsis) for initial implants, and from $26 857 to $362 606 for CIED replacement procedures.15
The main reports published in the literature on the costs associated with CIED infections, with a focus on Europe, are shown in Table 1.16–22 The important financial burden associated with CIED infections highlighted in North America is confirmed by analyses performed in France, Germany, Spain, and the UK. The direct costs of infections are related to the duration of ICU or non ICU care, diagnostic testing, antibiotic treatment, extraction procedure, post-extraction hospital stay, use of temporary pacing or a wearable external defibrillator, CIED reimplantation procedural, and device costs. The retrospective analyses performed in Europe highlight substantial variability in reported costs, according to the device type, need for extraction, patient profile, and setting of care with frequent occurrence of costs >€30 000 (Table 1). There is an urgent need to prevent the occurrence of CIED infections among all higher risk patients.23
Table 1.
CIED infection associated costs
| Authors (year) | Study design | Country | Study period | Population | Infection associated costs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahsan et al. (2014)16 | A retrospective audit of all cardiac device infections in a single tertiary referral institution | UK | 2004–2007 |
2779 CIEDs implants or CIED-related procedures: 30 infections requiring CIEDs removal |
Average cost: £30 958.40 per incident infection | Cost of cardiac device infection: |
|
|||||
| Brough et al. (2018)17 | Retrospective, patient level service line analysis of a single UK extraction centre, during a complete financial year | UK | 2013–2014 | 74 patients required transvenous extraction (47 infected and 27 non-infected; 156 leads)
|
Mean cost of extraction: £9228 (±4099): |
|
When considering additional costs of device reimplantation: |
|
||||
| Clémenty et al. (2018)18 | Retrospective analysis using medico-administrative hospital discharge database | France | 2012–2015 | 78 267 CIED patients (72% de novo implants) | 65 553 (84%) PM implantation |
|
11 845 (15%) Defibrillators |
|
2 years infection de novo mean cost per patient |
|
2 years infection replacement mean cost per patient |
|
| Egea et al. (2018)19 | Cost analysis based on Delphi panel results on health resource utilization associated with the management of chronic pacemaker complications at the hospital level | Spain | 2017 | NR | Mean cost per infection requiring extraction and considering a 100% reimplantation rate: |
|
Cost for inpatient length of stay: |
|
Mean cost of intravenous antibiotic treatment previous and after system: €833.23/patient | |||
| Ludwig et al. (2018)20 | Case-controlled analysis using PSM in patients with ICD/CRT-D using German health insurance claims data | Germany | 2010–2014 |
158/4699 (3.4%) infections Risk of cardiac device infections 12 months post-implant: 3.4% overall, 2.9% for de novo procedures vs. 4.4% for replacement procedures |
Mean 3-year incremental expenditure per patient for patients with cardiac devices infections: |
|
Mean incremental expenditure: €59 419 per patient with a major infection | |||||
| Ahmed et al. (2019)21 | Retrospective analysis of clinical case records of pts undergoing extraction for CIED infection at a single tertiary cardiothoracic centre | UK | 2013–2015 | 84 CIED patients
|
|
|||||||
| Burnhope et al. (2019)22 | Retrospective cohort of HFrEF patients undergoing ICD or CRT procedures | UK | 2014–2017 | 5/157 patients with CIED infection | Average cost of a CIED infection inpatient admission: £41 820 (range £28 377–£56 498) |
CIED, cardiac implantable electronic device; CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; DCP, dual-chamber pacemaker; ICD, implantable cardioverter-defibrillator; HFrEF, heart failure with reduced ejection fraction; PM, pacemaker; PMS, propensity score matching; SCP, single-chamber pacemaker.
Economic perspectives and economic analysis: what are the implications for policy makers?
The background of economic analysis in health care is the scarcity of available resources and the inability to meet all demands, which challenges healthcare providers to allocate resources to maximize the outcome in terms of good health, thus justifying the investment made.24
Due to rising healthcare costs over time, there is a need for healthcare systems to find ways to obtain the highest value for the financial investments made. This may be defined as the maximum health benefit obtained for a given level of healthcare spending. Other factors such as equity and social justice also need to be considered.25 Hence, decision-makers—such as clinicians, hospital formulary committees, local or national health technology assessment (HTA) organizations, governmental health departments or health insurance companies—need to develop explicit and reliable assessments of the value of healthcare products and services to make good decisions.26
Health economics refers to a discipline of economics that provides standardized methods for estimating the costs and benefits of healthcare interventions.
Different types of economic evaluations have been used in the literature. Cost-minimization analysis is used when the clinical effectiveness of two interventions is equivalent.4 More frequently, cost-effectiveness analysis (CEA) is employed, since it compares consequences of alternative intervention/strategies, assessed in natural clinical units such as life-years gained. As a derivation of CEA, cost-utility analysis measures health benefit by considering both length and quality of life, usually represented by the quality-adjusted life-year (QALY) (calculated by multiplying the utility as a measure of preference for a person’s overall quality of life by the duration of time spent in that situation or health state).24,27 The QALYs may be compared for different types of interventions to have a basis for decision making on resources allocation. Another type of analysis is cost-benefit analysis, which is rarely applied in healthcare because of concerns with having all health benefits expressed in monetary terms.
Economic evaluations in healthcare often report an incremental cost-effectiveness ratio (ICER), usually but not always expressed as the cost per QALY gained for a new intervention compared to the pre-existing standard of care.28 In Figure 1, the principles of CEA are briefly summarized. Uniquely, the ICER combines comparative data on costs and outcomes into a single metric which represents value for money. A QALY represents years of survival adjusted for quality of life, using a scale of utility ranging from 0 (equivalent to death) to 1 (perfect health).29 Whether or not a treatment provides sufficient benefits to justify its added costs may be evaluated by comparing the cost per QALY gained with other interventions in a so-called league table, or with a notional cap called the cost-effectiveness threshold. The US Panel on Cost-Effectiveness in Health and Medicine and the National Institute of Health and Care Excellence (NICE) in UK have both endorsed the QALY for their ‘reference case’, a standardized methodological approach to promote comparability in CEAs of different healthcare interventions.30,31
Figure 1.
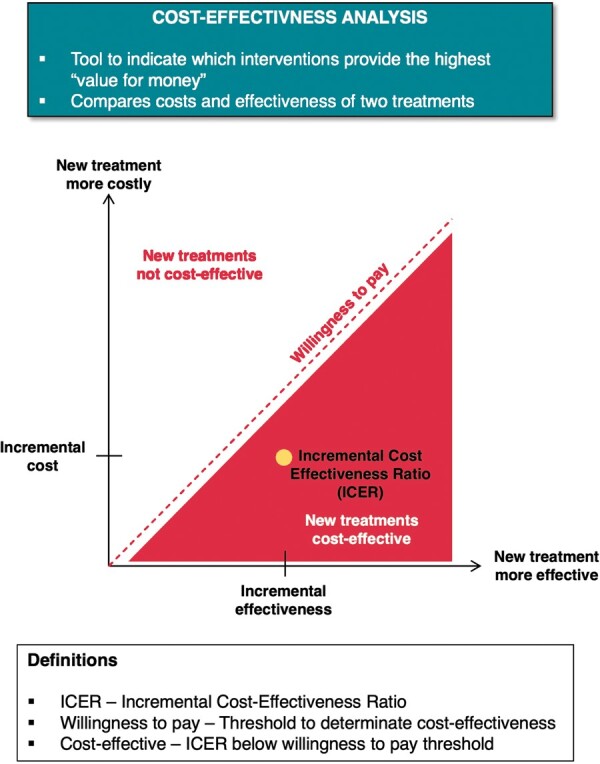
The principles of cost effectiveness analysis in a schematic view.
In Germany, the Institute for Quality and Efficiency in Health Care (IQWiG) has defined methods for health economic evaluation which also compare health outcomes and costs between treatments and calculate ICERs, however, IQWiG restricts its analyses to the estimation of ‘efficiency frontiers’ within individual therapeutic areas.32 The ICER of a new treatment (‘A’) is calculated compared to the next effective intervention (‘B’), and the ICER should not be higher than that of the existing treatment (‘B’) compared to its next effective alternative (‘C’) on the efficiency frontier. While IQWiG does not explicitly exclude QALYs as a measure of health benefit, it criticizes their use based on ethical and methodological grounds. Meanwhile, IQWiG has no need for a universal cost-effectiveness threshold based on QALYs, given its narrower focus on the estimation of efficiency frontiers within individual therapeutic areas.32
Cost-effectiveness ratios can be difficult to estimate from clinical studies, with or without the use of QALYs, and the results of such analyses are subject to uncertainty. In practice, reimbursement decisions are multifactorial and rarely based solely on the comparison of a treatment’s ICER to another treatment’s ICER or a country’s cost-effectiveness threshold value.28 The budget impact for payers and healthcare providers necessarily is critical, and consequently the capability of healthcare systems to create budgetary headroom for new technologies is important, by increasing efficiency and realizing cost-savings.
In the USA, $50 000 per QALY emerged as an early benchmark for a cost-effectiveness threshold, based on the approval in the 1970s by Congress to provide dialysis treatment for patients with end-stage renal disease under the publicly funded Medicare program.33 It can be argued this value is now out-of-date, first because an inflation adjustment is required and second because the cost of renal dialysis is now much higher: an analysis published 2009 estimated an ICER of $129 090 per QALY gained.34
The World Health Organization (WHO) has suggested benchmarks based on the Gross Domestic Product (GDP) per capita in a given country, stating that interventions that avert one disability-adjusted life-year (DALY, similar to a QALY) for less than average per capita income for a given country or region are considered very cost-effective; interventions that cost less than three times average per capita income per DALY averted are still considered cost-effective; and those that exceed this level are considered not cost-effective.35
In 2014, the American College of Cardiology/American Heart Association (ACC/AHA) published a statement on cost/value methodology in Clinical Practice Guidelines, which translated the WHO guideline into present-day US dollar values, as shown in Table 2.29
Table 2.
References for cost effectiveness thresholds
| Level of value | ICER (per QALY gained) | USA | UK | Germany | Italy |
|---|---|---|---|---|---|
| Official threshold | None | £20 000–£30 000 | None |
None Proposed €25 000–40 000 |
|
| High value/highly cost-effective | Less than GDP per capita | <$50 000 | £33 100 | €41 500 | €29 600 |
| Intermediate value/cost-effective | Between 1 and 3 times GDP per capita | $50 000 to <$150 000 | £33 100 to <£99 400 | €41 500 to <€124 500 | €29 600 to <€88 900 |
| Low value/not cost-effective | Greater than 3 × GDP per capita | >$150 000 | >£99 400 | >€124 500 | >€88 900 |
US values based on ACC/AHA statement on cost/value methodology.29
UK values based on National Institute for Health and Care Excellence methods and WHO guidance.
Italy values based on Italian Health Economics Association (AIES) recommendation and WHO guidance.
ACC/AHA, American College of Cardiology/American Heart Association; GDP, Gross Domestic Product; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; WHO, World Health Organization.
An ICER (per QALY gained) of <$50 000 was considered highly cost-effective, between $50 000 and $150 000 was considered as intermediate cost effectiveness, and >$150 000 was considered not cost-effective.
Since its inception in 1999, the United Kingdom’s National Institute for Health and Care Excellence has used an explicit cost-effectiveness threshold of £20 000–£30 000.36 This represented 1.1–1.7 times the per-capita GDP in 1999 (GDP £17 720), though it is only 0.6–0.9 times the corresponding value in 2019 (GDP £33 141). Reviews of past NICE decisions suggest the threshold in practice may be higher than stated by NICE, around £35 000–£40 000 per QALY.37 A multiple of 1–3 times GDP per capita suggests thresholds of £33 100–£99 400.
Official cost-effectiveness thresholds do not exist in Germany, for reasons already explained. In Italy, there is no officially established value, but guidelines from the Italian Health Economics Association (AIES) recommended in 2009 a threshold of €25 000–€40 000 be used.38
Per-capita GDP in 2019 was €41 508 in Germany and €29 661 in Italy, so a multiple of 1–3 times GDP per capita suggests thresholds for cost effectiveness of €41 500–€124 500 for Germany and €29 600–€88 900 for Italy.39
The economic evaluations allow to assess whether strategies and/or treatments with proven clinical efficacy correspond to good value for money and these analyses are a specific and important component of HTA.40 The HTA process refers to the systematic evaluation of characteristics, scientific validation, effect, and impact of health technologies and is a multidisciplinary process to evaluate the social, economic, organizational, and ethical issues of a health intervention or health technology, to inform policy decisions.40 Health technology assessment is particularly important when innovative devices, with new functions are proposed with a need to assess their value and possible implementation.41 In Europe, the basic principle on which consensus Guidelines are constructed, by the European Society of Cardiology is ‘separating science from economics’42 and therefore while evidence is global, decisions for implementation are made locally and have to be considered at the level of national or regional policymakers. In this perspective, the purpose of HTA is to inform policy decisions about investments, coverage, and reimbursement processes.
Economic analyses of TYRX™ antibacterial envelope
The TYRX™ Absorbable Antibacterial Envelope (Medtronic, Inc., Minneapolis, MN, USA) is a sterile, single-use surgical mesh envelope intended to securely hold a pacemaker pulse generator or defibrillator [implantable cardioverter-defibrillator (ICD)] in order to create a stable environment and that employs the antibiotics rifampicin and minocycline to reduce infections following surgical implant of a pacemaker or defibrillator.43,44 In Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT), a randomized, controlled clinical trial, the safety and efficacy of the TYRX™ envelope in reducing the infection rate in patients undergoing CIED replacement or upgrade or initial implantation of a cardiac resynchronization therapy defibrillator (CRT-D) or CIED pocket/lead revision in patients not having pocket assessment in the past 365 days.44 The primary endpoint (infection resulting in system extraction or revision, long-term antibiotic therapy with infection recurrence, or death, within 12 months) was significantly reduced in the envelope group vs. the control group [0.7% and 1.2%; hazard ratio, 0.60; 95% confidence interval (CI), 0.36–0.98; P = 0.04]. The low infection rate in the control group compared to real-world evidence from registries and healthcare administrative/claims datasets has been attributed to certain study exclusion criteria (CIED infection in prior 12 months, haemodialysis or peritoneal dialysis, or treatment with chronic oral immunosuppressive agents), to TYRX™ commercial availability potentially having lead investigators to exclude the highest‐risk candidates, to the study operators and centres generally being highly experienced, and to the Hawthorne effect.
According to these results, the international consensus document on how to prevent, diagnose, and treat CIED infections promoted by EHRA23 reported that the TYRX™ antibiotic envelope is recommended in high-risk situations, as defined in the WRAP-IT study population (patients undergoing pocket or lead revision, generator replacement, system upgrade, or initial CRT-D implantation) and in patients with other high-risk factors such as dialysis or treatment with immunosuppressive agents, and considering also the local CIED infection incidence.
To evaluate the economic implications of the use of the TYRX™ cardiac absorbable antibiotic envelope, a literature review was performed to identify related costs and CEAs. This analysis may be helpful in the assessment and adoption of this innovative technology, and is in accordance with the approach of HTA. A general search was carried out using key words including: ‘envelope’ or ‘TYRX’ in combination with ‘cost’ or ‘economic’. This was supplemented with manual review of references in relevant literature. Fifty-eight articles were screened, leading to the selection of five studies that provided comparative cost or cost-effectiveness data for the antibiotic envelope vs. the current standard of care. A summary of these studies is provided in Table 3.
Table 3.
Studies containing comparative cost analyses with TYRX™ antibiotic envelope
| Authors (year) | Setting | Population | Perspective | Comparison | Time horizon | Results |
|---|---|---|---|---|---|---|
| Boriani et al. (2021)48 | UK, Germany, Italy | High-risk cohorts (WRAP-IT study subgroups and PADIT risk score cohorts) | Healthcare systems (Payers). No indirect costs. Thresholds of €50 000 Germany, €40 000 Italy, £30 000 UK | TYRX™adjunctive to SoC vs. SoC | Lifetime |
Table 4 for full details. Infection rates SoC: high power replacement 2.9%, PADIT score ≥6 points 3.3% Mortality 3 years: 41.4% (infected), 18.0% (not infected) ICER: TYRXTM cost-effective for ICD and CRT-D replacements, PADIT score ≥6 points (all devices), and some other high-risk groups (all devices) |
| Wilkoff et al. (2020)46 | USA |
High-risk patients (WRAP-IT study population). Included subgroup analyses. |
Healthcare system (provider-owned health plan). No indirect costs. Threshold of $150 000. |
TYRX™ adjunctive to SoC vs. SoC | Lifetime |
Infection rate: 1.2% SoC. TYRX™ hazard ratio 0.6 Mortality 1 year: 14.6% (infected) and 5.2% (not infected) Costs: $37 598 (TYRX™) vs. $36 929 (SoC) QALYs: 6.925 (TYRX™) vs. 6.919 (SoC) ICER: $112 603 |
| Kay et al. (2018)47 | UK | High-risk and ‘all-comers’ (lower risk) patients, sourced from observational studies of TYRX™ | National Health Service (Payer). No indirect costs. Threshold of £30 000 | TYRX™ adjunctive to SoC vs. SoC | 12 months and lifetime |
Infection rate: 3.3% (SoC) vs. 0.6% TYRX™ Mortality 1 year: 0.211 (infected), 0.064 (no infection) ICER: IPG £12 711, ICD £4348, CRT-P £11 248, CRT-D £6261. TYRX™ cost-effective at infection rates above 1.65% (CRT-D), 1.95% (CRT-P), 1.87% (IPG), 1.38% (ICD). |
| Burnhope et al. (2019)22 | UK | All ICD and CRT procedures, from a single implanting centre (‘all-comers’) | National Health Service (Payer). No indirect costs. | TYRX™ adjunctive to SoC vs. SoC | 12 months |
Infection rate: 3.14% SoC vs. 1.02% TYRX™ cost savings estimated £184–£624 per patient QALYs and ICERs: not reported |
| Shariff et al. (2015)50 | USA | All ICD and CRT procedures, from a single implanting centre (‘all-comers’) | Hospital costs only. No indirect costs. | TYRX™ adjunctive to SoC vs. SoC | 6 months |
Infection rates: 0% (TYRX™) vs. 1.71% (SoC) Mortality: 15.7% (infected) vs. 4.5% (not infected) Costs: $318 991 (TYRX™) vs. $342 854 (SoC) QALYs and ICERs: not reported Infection rate of 1.59% calculated as break-even |
CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; ICER, incremental cost-effectiveness ratio; PADIT, Prevention of Arrhythmia Device Infection Trial; QALY, quality-adjusted life-year; SoC, standard of care; WRAP-IT, Worldwide Randomized Antibiotic Envelope Infection Prevention Trial.
Cost-effectiveness analyses based on randomized trial data
Two CEAs have been conducted by clinical study investigators involved in the WRAP-IT.45,46 Both analyses were based on a prior model47 and because CEA was pre-specified for the WRAP-IT study, both analyses used prospectively collected data for patients’ quality of life (EQ-5D) and healthcare resource use, in addition to clinical endpoints.43 One study tailored the resource use and cost inputs for the US healthcare system45 while the second analysis presented data for three European countries (UK, Germany, and Italy).48
A decision tree structure with a lifetime horizon was used. The models compare costs and health outcomes for patients receiving the antibacterial envelope adjunctive to ‘standard of care’ infection prophylaxis, vs. patients treated without the envelope. Total costs and QALYs were calculated for each pathway and ICERs were estimated for the whole study cohort and selected sub-groups. The same structure was used to model cost-effectiveness in the USA as in Germany, Italy, and UK. What changed between the analyses was the (i) country-specific unit costs/prices used; (ii) selection of different patient subgroups; (iii) cost-effectiveness thresholds designated for each country; and (iv) resulting conclusions about cost-effectiveness.
A mortality analysis of the WRAP-IT trial compared the risk of death at 12 months and throughout all follow-up in patients with and without CIED major infections. Death occurred in 10 of the 67 patients in the infection group (12-month Kaplan–Meier estimate: 16%), and 345 of the 6836 patients in the no infection group (12-month Kaplan–Meier estimate: 5%).46
The risk-adjusted hazard ratio was 3.41 (95% CI, 1.81–6.41); P < 0.001 at 1 year, and through all follow-up it remained elevated at 2.30 (95% CI, 1.29–4.07); P = 0.004.46
Quality of life was collected using EQ-5D at baseline, infection diagnosis, 1, 3, and 6 months after diagnosis, and at 12 months after the index procedure. Utility scores calculated using the EQ-5D were significantly reduced at time of infection and did not normalize until 6 months later.46
For the USA, mean hospital costs were $55 547 per infection: costs varied from $16 592 for 5 infections treated without extraction, $45 694 for 12 infections treated with extraction and no replacement, and $67 586 for 26 infections treated with extraction and replacement. The ICER of the antibacterial envelope was considered to be cost effective in the overall WRAP-IT population: the ICER was estimated to be $112 603 per QALY gained, based on an overall baseline infection rate of 1.2% and a 40% reduction in major CIED infection with the antibacterial envelope in the trial.46
Subgroup analyses estimated the antibacterial envelope was cost-effective (ICER below $150 K threshold) for patients with a risk of infection ≥1.0%; is highly cost-effective (ICER below $50 K) when the risk of infection is ≥2.0%; and is cost-saving when the risk of infection is ≥4.0%. As examples, use in patients with prior CIED infection was cost-saving; use in immunocompromized patients was highly cost-effective; use in patients with renal dysfunction implanted with High Power devices was cost-effective; and use for initial CRT-D implants in patients without risk factors was not cost-effective.46
For Europe, resource use data (e.g. hospital length of stay) came from the WRAP-IT study, including specific resources like temporary pacing, wearable defibrillators, and leadless devices.48 Significant differences in hospital length of stay were observed between patients at US and non-US sites, so the non-US data were used in the European analysis. Intensive care stays were 4.0 days and general ward stays were 23 days in the European analysis. Costs were estimated for each country based on various sources for each country, including Diagnostic Related Group (DRG) tariffs and national costing datasets.48 As an example, the costs of complete extraction and replacement of an infected CRT-D device were estimated to be at €42 921 (Germany), €45 560 (Italy), and £37 633 (UK). The cost of the envelope was €945 in Germany and Italy, and £800 in UK. An additional analysis was included which builds into the analysis a risk-sharing agreement because the manufacturer provides a replacement device, leads, and envelope free-of-charge in case of occurrence of a CIED infection when the envelope was used.
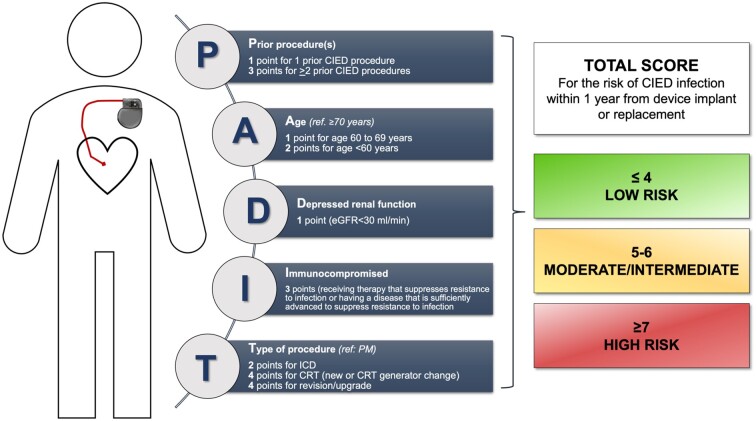
Additional scenarios were performed in the European analysis to estimate the cost-effectiveness of the antibacterial envelope using risks of infection based on the Prevention of Arrhythmia Device Infection Trial (PADIT) risks score (Figure 2).49 Three such subgroups were defined: PADIT score ≥5 points, ≥6 points, or ≥7 points. Furthermore, the infection rate for High Power Replacement procedures (2.9%) used in this analysis was sourced from a subgroup analysis of the Western European sites (n = 313) of the WRAP-IT study, as a significantly higher rate of infection was observed than in the overall study.
Figure 2.
PADIT risk score. From Ref. 49. A correction was published to the original risk score (https://doi.org/10.1016/j.jacc.2020.06.001), the information above is based on the corrected version and the online calculator (https://padit-calculator.ca/). PADIT, Prevention of Arrhythmia Device Infection Trial.
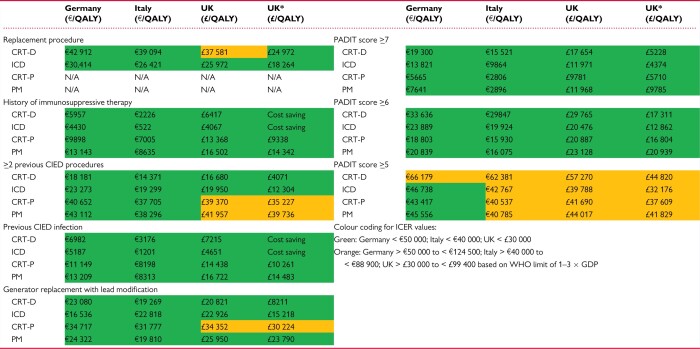
Incremental cost-effectiveness ratios from the European analysis are reproduced in Table 4 (colour-coding added by the authors of this paper to aid interpretation)48 Among the WRAP-IT subgroups, the Italian and German analyses indicate the envelope was cost-effective at thresholds of €40 000 and €50 000 per QALY, respectively, across all subgroups and device types. The results for UK showed the envelope also was cost-effective, with the following caveats: (i) for high power replacement when the risk-sharing program was included but not otherwise; (ii) for patients with ≥ 2 previous CIED procedures it was cost-effective for high power devices but not low power devices; and (iii) for generator replacements with lead modification it was cost-effective for all device types except cardiac resynchronization therapy-pacemaker (CRT-P).
Table 4.
TYRX™ incremental cost effectiveness ratios
| Germany (€/QALY) | Italy (€/QALY) | UK (£/QALY) | UK* (£/QALY) | Germany (€/QALY) | Italy (€/QALY) | UK (£/QALY) | UK* (£/QALY) | ||
|---|---|---|---|---|---|---|---|---|---|
|
CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; GDP, Gross Domestic Product; ICER, incremental cost-effectiveness ratio; ICD, implantable cardioverter-defibrillator; PM, pacemaker; PADIT, Prevention of Arrhythmia Device Infection Trial; QALY, quality-adjusted life-year; WHO, World Health Organization. *results for scenario with risk-sharing programme
From Ref. 48.
Considering the analyses based on the PADIT risk score, for both low and high-power devices, the envelope was more cost-effective in patients with higher PADIT scores (i.e. at higher baseline risk of infection). This is indicated by lower ICER values for patients with higher PADIT scores. Incremental cost-effectiveness ratios for patients with PADIT scores ≥6 were below the cost-effectiveness thresholds used in the respective countries.
Sensitivity analyses are used in CEAs to identify what factors are most important to making the results positive or negative. This type of analysis indicated that the main drivers of the model in all three European countries were baseline rates of major CIED infections, the efficacy of the envelope, and the excess mortality associated with CIED infection.48
Economic analyses based on non-randomized trial data
A CEA of the TYRX™ absorbable antibacterial envelope, from the perspective of the English NHS, was performed based on a combination of two prospective and four retrospective observational studies of TYRX™.47 The analysis modelled infection rates associated with ‘high-risk’ patients (3.3%) and an ‘all-comers’ category (1.9%) based on these observational studies and a relative risk associated with TYRX™ of 0.163 (84% risk reduction). The analyses suggested that over a 12-month time horizon, TYRX™ use in high-risk patients was cost-saving in patients with an ICD or CRT-D, and associated with ICERs of £46 548 and £21 768 per QALY gained in patients with an IPG or CRT-P, respectively. The structure of this model was the basis for the two analyses described above, which incorporated higher quality data inputs after the WRAP-IT study was completed.
A single-centre retrospective analysis in the UK reported data on the costs of CIED-related infections and estimated cost savings with TYRX™.22 Five infections were identified within 12 months amongst 159 (3.14%) ICD/CRT procedures over a period spanning 2014–2017, without the use of TYRX™. An average cost of £41 820 was estimated to be directly attributable to CIED infection, based on patient-level costing data. A secondary analysis estimated the excess total healthcare costs for patients with CIED infection at £62 214. Modelling a potential reduction in the number of infections using TYRX™, by applying an odds ratio of 0.31 (based on a meta-analysis), a cost-saving of £624 per patient was estimated. The main limitations of this analysis were the small sample size and that the efficacy of TYRX™ was modelled based on non-randomized data. A large single-centre retrospective analysis in the USA reported a cost analysis based on CIED infections observed amongst patients treated with or without TYRX™.50 A total of 1476 patients having CIED procedures were followed up: 1111 patients treated without TYRX™ and 365 patients treated with TYRX™. A propensity score matching analysis led to a comparison of 362 of the patients treated with TYRX™ matched to 362 patients with similar risk profiles who did not receive TYRX™. The infection rates in this analysis were 0% with TYRX™ compared to 1.9% without TYRX™. Costs were estimated from hospital length of stay, use of home intravenous antibiotics, and the LifeVest®: the average cost was $54 926 per CIED infection. The cost of treating CIED infections in patients treated without TYRX™ was reported to be similar to the cost of using TYRX™ and having no infections: $340 000 vs. $320 000, respectively.
The CEA results for TYRX™ are similar to those reported for other cardiovascular therapies. In NYHA Class III patients with wide QRS duration, ICERs of £24 875–£28 646 were estimated for CRT-D compared to CRT-P in a comprehensive analysis from the UK National Health Service perspective.51 A recent analysis for Germany reported an ICER of €24 659 for CRT-D compared to CRT-P.52 An analysis from a US Medicare perspective reported an ICER of $43 678 for CRT-D vs. CRT-P, based on the REVERSE trial.53 The CardioMems implantable pulmonary artery pressure monitor was estimated to have an ICER of $71 K in NYHA Class III patients in the USA,54 and ICER between £22 342–£25 464 per QALY gained (€28 709–32 721) from the UK National Health Service perspective.55
Conclusions
The occurrence of CIED infections and related adverse outcomes have an important financial impact on the healthcare system, with hospitalization length of stay (2–3 weeks on average) being the largest cost driver, including the cost of device system extraction and device replacement accounting for more than half of total costs. In a recent analysis, the economic profile of the TYRX™ absorbable antibacterial envelope was analysed taking into account both randomized and non-randomized trial data. Economic analysis found that the envelope is associated with cost-effectiveness ratios below US and European benchmarks in selected patients at increased risk of infection. Therefore, the TYRX™ envelope, by effectively reducing CIED infections, provides value according to the criteria of affordability currently adopted by US and European healthcare systems.
Funding
This article was published as part of a supplement supported by an educational grant from Medtronic.
Conflict of interest: G.B. received small speaker’s fees from Medtronic, Boston, Biotronik, Boehringer, and Bayer, outside of the submitted work. B.B. is an employee of Medtronic International Trading Sàrl. M.B. received small educational and speaker’s fees by Biotronik, Boston Scientific, Medtronic, and Zoll outside the submitted work. K.G.T. performed Consulting/Advisory Board work for Medtronic. B.L.W. receives honoraria as a consultant to Medtronic, Abbott, and Philips.
References
- 1. Zecchin M, Torre M, Carrani E, Sampaolo L, Ciminello E, Ortis B et al. Seventeen-year trend (2001–2017) in pacemaker and implantable cardioverter-defibrillator utilization based on hospital discharge database data: An analysis by age groups. European Journal of Internal Medicine 2021;84:38–45. 10.1016/j.ejim.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 2. Voigt A, Shalaby A, Saba S.. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–9. [DOI] [PubMed] [Google Scholar]
- 3. Mond HG, Proclemer A.. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009 – a World Society of Arrhythmia's project. Pacing Clin Electrophysiol 2011;34:1013–27. [DOI] [PubMed] [Google Scholar]
- 4. Rennert-May E, Conly J, Leal J, Smith S, Manns B.. Economic evaluations and their use in infection prevention and control: a narrative review. Antimicrob Resist Infect Control 2018;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tarricone R. Cost-of-illness analysis. What room in health economics? Health Policy 2006;77:51–63. [DOI] [PubMed] [Google Scholar]
- 6. Diemberger I, Mazzotti A, Biffi M, Massaro G, Martignani C, Ziacchi M. et al. From lead management to implanted patient management: systematic review and meta-analysis of the last 15 years of experience in lead extraction. Expert Rev Med Devices 2013;10:551–73. [DOI] [PubMed] [Google Scholar]
- 7. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT. et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 8. Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S. et al. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the National Cardiovascular Data Registry. Circulation 2014;130:1037–43. [DOI] [PubMed] [Google Scholar]
- 9. Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL.. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014;16:1490–5. [DOI] [PubMed] [Google Scholar]
- 10. Greenspon AJ, Eby EL, Petrilla AA, Sohail MR.. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol 2018;41:495–503. [DOI] [PubMed] [Google Scholar]
- 11. Rizwan Sohail M, Henrikson CA, Jo Braid-Forbes M, Forbes KF, Lerner DJ.. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol 2015;38:231–9. [DOI] [PubMed] [Google Scholar]
- 12. Sandoe JAT, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, British Society for Echocardiography et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother 2015;70:325–59. [DOI] [PubMed] [Google Scholar]
- 13. Diemberger I, Biffi M, Martignani C, Boriani G.. From lead management to implanted patient management: indications to lead extraction in pacemaker and cardioverter–defibrillator systems. Expert Rev Med Devices 2011;8:235–55. [DOI] [PubMed] [Google Scholar]
- 14. Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ.. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011;171:1821–8. [DOI] [PubMed] [Google Scholar]
- 15. Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ.. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US Payer database 1-year post implantation. Circ Arrhythm Electrophysiol 2016;9:e003929. doi: 10.1161/CIRCEP.116.003929. [DOI] [PubMed] [Google Scholar]
- 16. Ahsan SY, Saberwal B, Lambiase PD, Koo CY, Lee S, Gopalamurugan AB. et al. A simple infection-control protocol to reduce serious cardiac device infections. Europace 2014;16:1482–9. [DOI] [PubMed] [Google Scholar]
- 17. Brough CEP, Rao A, Haycox AR, Cowie MR, Wright DJ.. Real-world costs of transvenous lead extraction: the challenge for reimbursement. Europace 2019;21:290–7. [DOI] [PubMed] [Google Scholar]
- 18. Clementy N, Carion PL, Leotoing L, Lamarsalle L, Wilquin-Bequet F, Brown B. et al. Infections and associated costs following cardiovascular implantable electronic device implantations: a nationwide cohort study. Europace 2018;20:1974–80. [DOI] [PubMed] [Google Scholar]
- 19. Egea M, Urra FG, Bellver A, Alvarez M, Waweru C, Quesada A. Economic impact associated with complications of cardiac implantable electronic devices in Spain. In Poster Presentation EHRA Congress 2018, 2018.
- 20. Ludwig S, Theis C, Brown B, Witthohn A, Lux W, Goette A.. Incidence and costs of cardiac device infections: retrospective analysis using German health claims data. J Comp Eff Res 2018;7:483–92. [DOI] [PubMed] [Google Scholar]
- 21. Ahmed FZ, Fullwood C, Zaman M, Qamruddin A, Cunnington C, Mamas MA. et al. Cardiac implantable electronic device (CIED) infections are expensive and associated with prolonged hospitalisation: UK Retrospective Observational Study. PLoS One 2019;14:e0206611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burnhope E, Rodriguez-Guadarrama Y, Waring M, Guilder A, Malhotra B, Razavi R. et al. Economic impact of introducing TYRX amongst patients with heart failure and reduced ejection fraction undergoing implanted cardiac device procedures: a retrospective model based cost analysis. J Med Econ 2019;22:464–70. [DOI] [PubMed] [Google Scholar]
- 23. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG. et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020;22:515–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maniadakis N, Vardas P, Mantovani LG, Fattore G, Boriani G.. Economic evaluation in cardiology. Europace 2011;13Suppl 2:ii3–8. [DOI] [PubMed] [Google Scholar]
- 25. Owens DK, Qaseem A, Chou R, Shekelle P, Physicians CGCotACo. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med 2011;154:174–80. [DOI] [PubMed] [Google Scholar]
- 26. Wilkinson T, Sculpher MJ, Claxton K, Revill P, Briggs A, Cairns JA. et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health 2016;19:921–8. [DOI] [PubMed] [Google Scholar]
- 27. Fattore G, Maniadakis N, Mantovani LG, Boriani G.. Health technology assessment: what is it? Current status and perspectives in the field of electrophysiology. Europace 2011;13Suppl 2:ii49–53. [DOI] [PubMed] [Google Scholar]
- 28. Cameron D, Ubels J, Norström F.. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action 2018;11:1447828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ. et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2304–22. [DOI] [PubMed] [Google Scholar]
- 30. Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC.. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996;276:1172–7. [PubMed] [Google Scholar]
- 31.Excellence NIfHaC. Guide to the Methods of Technology Appraisal 2013, Process and methods [PMG9] 2013. https://www.nice.org.uk/process/pmg9/chapter/foreword. [PubMed]
- 32.Institute for Quality and Efficiency in Health Care (IQWiG). IfQuWiG. General methods, 2017. https://www.iqwig.de/en/. [PubMed]
- 33. Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ.. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ 2004;13:429–36. [DOI] [PubMed] [Google Scholar]
- 34. Lee CP, Chertow GM, Zenios SA.. An empiric estimate of the value of life: updating the renal dialysis cost-effectiveness standard. Value Health 2009;12:80–7. [DOI] [PubMed] [Google Scholar]
- 35. Hutubessy R, Chisholm D, Edejer TT.. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc 2003;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Appleby J, Devlin N, Parkin D.. NICE's cost effectiveness threshold. BMJ 2007;335:358–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Griffiths EA, Hendrich JK, Stoddart SD, Walsh SC.. Acceptance of health technology assessment submissions with incremental cost-effectiveness ratios above the cost-effectiveness threshold. Clinicoecon Outcomes Res 2015;7:463–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fattore G. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. Pharmacoeconomics Ital Res Articles 2009;11:83–93. [Google Scholar]
- 39. Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP. et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ 2016;94:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boriani G, Maniadakis N, Auricchio A, Müller-Riemenschneider F, Fattore G, Leyva F. et al. Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European Heart Rhythm Association. Eur Heart J 2013;34:1869–74. [DOI] [PubMed] [Google Scholar]
- 41. Hatz MH, Schreyögg J, Torbica A, Boriani G, Blankart CR.. Adoption decisions for medical devices in the field of cardiology: results from a European Survey. Health Econ 2017;26Suppl 1:124–44. [DOI] [PubMed] [Google Scholar]
- 42. Priori SG, Klein W, Bassand JP, 2002-2004 ECfPG, 2000-2002 ECfPG, 2002-2004 ESoC. Medical Practice Guidelines. Separating science from economics. Eur Heart J 2003;24:1962–4. [DOI] [PubMed] [Google Scholar]
- 43. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole J, Stromberg K. et al. Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial (WRAP-IT). Am Heart J 2016;180:12–21. [DOI] [PubMed] [Google Scholar]
- 44. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E. et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- 45. Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR. et al. Cost-effectiveness of an antibacterial envelope for cardiac implantable electronic device infection prevention in the US healthcare system from the WRAP-IT Trial. Circ Arrhythm Electrophysiol 2020;13:e008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR. et al. Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT Trial. Circ Arrhythm Electrophysiol 2020;13:e008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kay G, Eby EL, Brown B, Lyon J, Eggington S, Kumar G. et al. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ 2018;21:294–300. [DOI] [PubMed] [Google Scholar]
- 48. Boriani G, Kennergren C, Tarakji KG, Wright DJ, Ahmed FZ, McComb JM. et al. Cost-effectiveness analyses of an absorbable antibacterial envelope for use in patients at increased risk of cardiac implantable electronic device infection in three European countries. Value in Health 2021; DOI: 10.1016/j.jval.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 49. Birnie DH, Wang J, Alings M, Philippon F, Parkash R, Manlucu J. et al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol 2019;74:2845–54. [DOI] [PubMed] [Google Scholar]
- 50. Shariff N, Eby E, Adelstein E, Jain S, Shalaby A, Saba S. et al. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol 2015;26:783–9. [DOI] [PubMed] [Google Scholar]
- 51. Mealing S, Woods B, Hawkins N, Cowie MR, Plummer CJ, Abraham WT. et al. Cost-effectiveness of implantable cardiac devices in patients with systolic heart failure. Heart 2016. 1;102:1742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hadwiger M, Frielitz FS, Eisemann N, Elsner C, Dagres N, Hindricks G. et al. Cardiac resynchronisation therapy in patients with moderate to severe heart failure in Germany: a cost-utility analysis of the additional defibrillator. Appl Health Econ Health Policy 2021;19:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gold MR, Padhiar A, Mealing S, Sidhu MK, Tsintzos SI, Abraham WT.. Economic value and cost-effectiveness of cardiac resynchronization therapy among patients with mild heart failure: projections from the REVERSE long-term follow-up. JACC Heart Fail 2017;5:204–12. [DOI] [PubMed] [Google Scholar]
- 54. Sandhu AT, Goldhaber-Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA.. Cost-effectiveness of implantable pulmonary artery pressure monitoring in chronic heart failure. JACC Heart Fail 2016;4:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cowie MR, Simon M, Klein L, Thokala P.. The cost-effectiveness of real-time pulmonary artery pressure monitoring in heart failure patients: a European perspective. Eur J Heart Fail 2017;19:661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]