Abstract
A surge in research focused on understanding the physical principles governing the formation, properties, and function of membraneless compartments has occurred over the past decade. Compartments such as the nucleolus, stress granules, and nuclear speckles have been designated as biomolecular condensates to describe their shared property of spatially concentrating biomolecules. Although this research has historically been carried out in animal and fungal systems, recent work has begun to explore whether these same principles are relevant in plants. Effectively understanding and studying biomolecular condensates require interdisciplinary expertise that spans cell biology, biochemistry, and condensed matter physics and biophysics. As such, some involved concepts may be unfamiliar to any given individual. This review focuses on introducing concepts essential to the study of biomolecular condensates and phase separation for biologists seeking to carry out research in this area and further examines aspects of biomolecular condensates that are relevant to plant systems.
Keywords: protein phase separation, biomolecular condensates, membraneless organelles
1. INTRODUCTION
Compartmentalization of subcellular space allows cells to carry out processes in ways that would otherwise not be possible within a homogeneous intracellular environment (59, 74, 121). Scientists have spent decades furthering our understanding of the unique environments and biochemical processes that are characteristic of various subcellular compartments. Initially focused on membrane-bound compartments, researchers have recently turned their attention to understanding membraneless compartments. These compartments are not new to the scientific community, with literature on the nucleolus dating back to the 1800s (96). However, modern advances in our capacity to observe subcellular organization have dramatically increased the number of known membraneless compartments (82). Furthermore, numerous recent breakthroughs in our understanding of how membraneless compartments form and the properties they adopt have resulted in a renewed interest in membraneless compartments within the scientific community (23).
Membraneless compartments represent a diverse collection of cellular bodies (Figure 1). While some are constitutively present and found in virtually all cell types, others show cell or tissue specificity or may form only in response to specific environmental stimuli (102, 132, 143). Generic membraneless organelles have been given many names that range from general descriptors, such as cellular aggregates, puncta, cellular bodies, and protein assemblies, to names of specific compartments, such as the nucleolus, Cajal bodies, processing bodies, and stress granules (4, 13, 47, 62, 103). These compartments can be nuclear or cytoplasmic and have been implicated in multiple cellular processes, including mRNA processing and export (41), small nuclear ribonucleoprotein biogenesis (snRNP) biogenesis (88), ribosomal RNA (rRNA) biosynthesis and ribosome biogenesis (58), DNA damage response (112), microRNA (miRNA) processing (70), mRNA decay and mRNA translation suppression (72), and stress response (19). Recently, membraneless compartments were grouped together and termed biomolecular condensates (or just condensates) to describe their capacity to concentrate biological molecules (4). The term biomolecular condensate, by its original definition, simply describes the phenomenon whereby biomolecules are spatially concentrated in nonstoichiometric assemblies within a cell, and in this review, that is how we use the term (4). Importantly, usage of the term biomolecular condensate in this way does not require attribution to the underlying mechanism by which a condensate forms, the condensate morphology, or the condensate physical properties. This is advantageous because individuals studying biomolecular condensates can refer to the wide range of various membraneless compartments by using this single term. To avoid confusion, throughout this review we refer to reconstituted membraneless compartments in vitro as assemblies and reserve the term condensate to refer to in vivo membraneless compartments.
Figure 1.
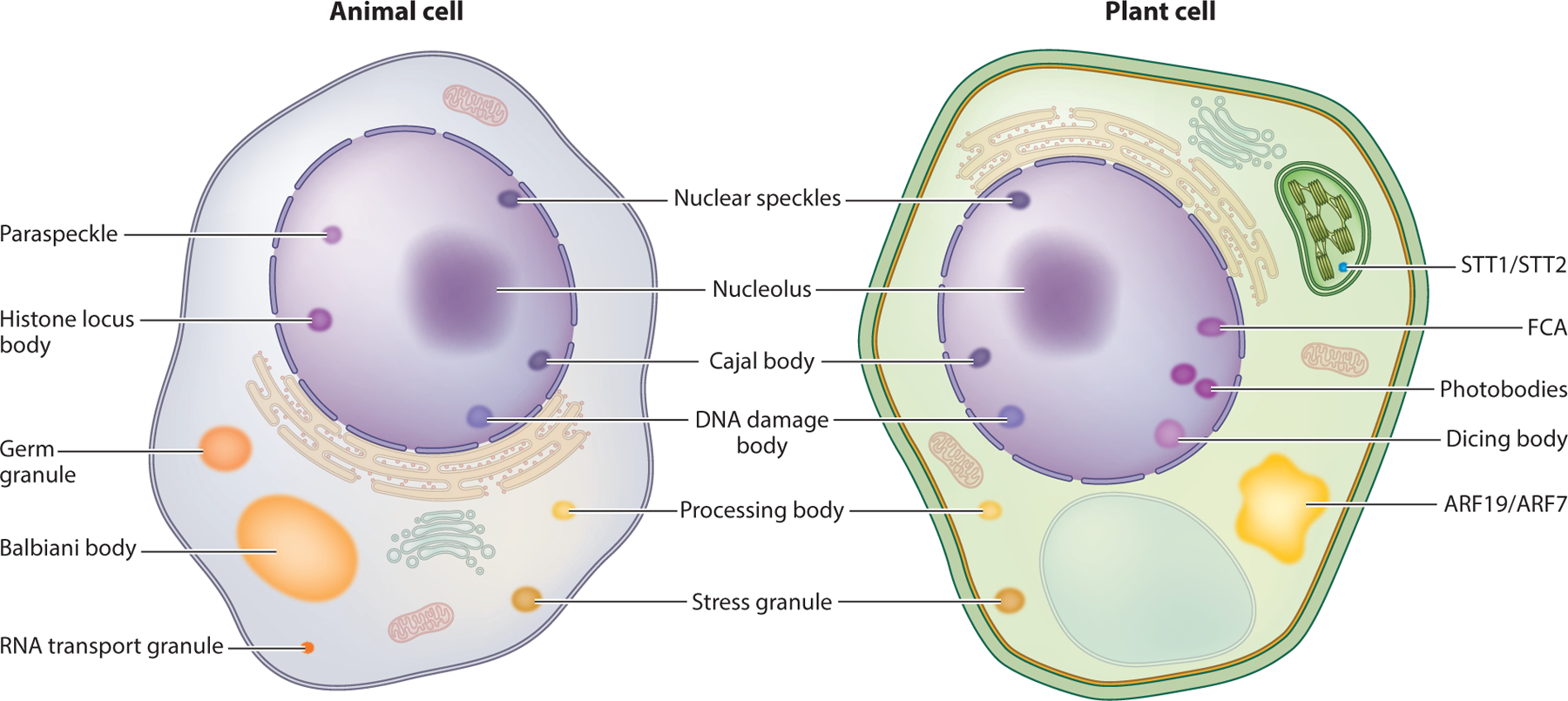
Plant and animal cells contain many biomolecular condensates. A simplified animal cell (left) and plant cell (right) with various biomolecular condensates are depicted. Notably, some condensates appear to be conserved between plants and animals (terms between the two cells), whereas others appear to be specific to either animals (terms to the left of the animal cell) or plants (terms to the right of the plant cell). Abbreviations: ARF, AUXIN RESPONSE FACTOR; FCA, FLOWERING CONTROL LOCUS A.
Biomolecular condensate:
a non-membrane-bound and nonstoichiometric compartment made of one or more biological molecules concentrated relative to their surroundings
Although using the term condensate does not require attribution to how a condensate forms, increasing evidence suggests that many condensates form through a process termed liquid-liquid phase separation (LLPS) (23). LLPS describes the phenomenon whereby a solution spontaneously demixes into two (or more) distinct phases that stably coexist with one another (54). Our understanding of the importance of phase separation and more generally condensate formation from a biological perspective has increased dramatically over the previous decade, specifically in animal and fungal systems (17). In animal systems, such insight has shed light on previously elusive aspects of fundamental cellular processes as well as underlying biological mechanisms that drive disease (1). Similarly, in the plant community, phase separation is emerging as a potential mechanism to clarify previously difficult-to-explain observations (91, 102). Numerous examples of apparent condensates exist in plants; more recently, literature examining condensates and phase separation in plant systems has been published (35, 48, 61, 70, 91, 102, 107, 129) (Figure 1). However, few in-depth reviews on phase separation and condensate formation focus on plants (32). To address this need, this review provides an introductory set of information that is critical for understanding LLPS and biomolecular condensates, with the objective of creating a resource for biologists interested in studying this phenomenon. We start the review by introducing LLPS and biomolecular condensates, with a focus on general concepts that are critical for those seeking to study LLPS in any biological system. We then summarize current approaches that are used to study condensates in general as well as approaches that are specific to plant systems. Finally, we end the review by summarizing the current state of the phase separation field in plant biology.
Liquid-liquid phase separation (LLPS):
a specific type of phase separation in which a homogeneous solution reorganizes into two (or more) compositionally distinct solutions
Phase separation:
the process whereby a system reorganizes into two (or more) spatially distinct regions with distinct and uniform physical properties and/or compositions
2. LIQUID-LIQUID PHASE SEPARATION AND BIOMOLECULAR CONDENSATES
Although observations of what we now refer to as biomolecular condensates stretch back many decades, breakthroughs in our understanding of the underlying physical principles governing condensate formation did not occur until recently. In a pioneering 2009 paper, Brangwynne et al. (16) demonstrated that germline P granules in Caenorhabditis elegans exhibit liquid-like behaviors, suggesting that these P granules could form through phase separation. The realization that P granule assembly is well described by the physics of phase separation provided a cogent physical explanation for the cellular positioning of P granules in the absence of directed cytoplasmic flows. This possibility of making well-defined predictions based on robust, general models is what motivates the description of biomolecular condensates through the physics of phase separation. Rather than simply providing a new set of vocabulary to describe existing phenomena, phase transition physics offers an existing and well-established physical framework to interpret, understand, and predict extant biological events.
Phase transition:
the process whereby a collection of molecules change from one phase of matter (solid, liquid, gas or plasma) to another
Since the original work on P granules, significant effort to elucidate and assess the physics underlying intracellular phase transitions has been expended. In this context, the application of both solution thermodynamics and polymer physics has contributed substantially to our understanding of how condensates form in cells (9, 17, 23, 54). This section focuses on a high-level review of the concepts critical for those seeking to study biological phase separation as applied to biomolecular condensates.
2.1. Liquid-Liquid Phase Separation
LLPS, in its simplest form, is the process whereby a solute (such as a protein, a polymer, or any other molecule) that is initially homogeneously distributed throughout a solution demixes into two distinct, stable phases: a solute-rich phase (the dense phase) and a solute-poor phase (the dilute phase). To greatly oversimplify, phase separation occurs under conditions in which it is more energetically favorable for solute-solute interactions to occur than solute-solvent interactions (reviewed in 4, 9, 11, 17, 46). In the context of condensates, the solute in question is often a protein or nucleic acid, and the solvent is typically the cytoplasm or nucleoplasm. In reality, condensates typically consist of many different proteins, RNAs, and other cellular components.
Dense phase:
in a (pseudo-)two-component system, the dense phase is the solution found inside a dense droplet
Dilute phase:
in a (pseudo-)two-component system, the dilute phase is the solution found around a dense droplet
It is useful to introduce the physical intuition associated with phase separation by using a hypothetical simplified two-component system that contains only solvent and polymer. This system type maps approximately to an in vitro system containing only a buffer solution and protein. For such a system, under certain solution conditions (i.e., temperature, pressure, concentration), the protein is homogeneously mixed throughout the system. The set of conditions over which the protein is homogeneously mixed define the one-phase regime for the system, which are the conditions under which the protein is completely soluble. Starting at a low total protein concentration within the one-phase regime, if we hold all other parameters fixed and systematically increase the protein concentration, at some protein concentration we may see spontaneous protein-dense droplet formation (i.e., demixing). At this point, the system has undergone LLPS, and we have entered the two-phase regime. In the two-phase regime, the system contains a dilute phase that has a low concentration of the protein and a dense phase (the protein-dense droplets) that has a higher concentration of the protein. The concentration of a molecule (in this example a protein) at which the system enters the two-phase regime is known as the saturation concentration (often abbreviated as Csat). Once the total concentration (Ctotal) exceeds Csat, additional protein added to the system will partition into the dense phase. In a simplified system that has undergone LLPS, the protein concentrations in the dilute phase and the dense phase do not change as additional protein is added to the system. Instead, additional protein will result in an increased volume of the dense phase and a corresponding decrease in the volume of the dilute phase (reviewed in 49). Notably, while in this example we varied protein concentration, other parameters, including temperature, pH, and salt concentration, influence whether the system will be in the one-phase or the two-phase regime (reviewed in 115). Similarly, Csat inherently depends on all of these possible parameters.
Demixing:
the process of separating into two (or more) distinct phases (i.e., the physical behavior observed during liquid-liquid phase separation)
Saturation concentration (Csat):
the concentration on the low-concentration arm of the binodal that represents the solubility limit of the system under fixed conditions
2.2. Phase Diagrams Describe Conditions Under Which a Molecule Phase Separates
Phase diagrams are a convenient way to depict the phase space that maps a set of solution conditions to the physical state of the system. The term system here refers to the contents within whatever container we are describing. For in vitro experiments, this system may be a test tube or microcentrifuge tube, and in vivo the system would be the intracellular contents contained within the cell. Phase diagrams are generated by examining the solution homogeneity with respect to a molecule of interest (in this context, typically a protein or nucleic acid) as a function of different solution conditions. In effect, a phase diagram is a two-dimensional map that allows the reader to know the type of solution (one phase or two phase) that will be arrived at should they start their experiment under some condition set that corresponds to a given location on that map.
Phase diagram:
a graphical tool that describes the phase behavior of a system as a function of one or more control parameters
The most common phase diagram type in the context of biological phase transitions is that of the (pseudo-)two-component system—one in which there is a given molecule of interest (i.e., a specific protein or nucleic acid) and the concentration dependence of assembly is assessed as a function of some control parameter, such as temperature, pH, salt, or concentration of molecular crowder. The two components in this type of system are solvent and solute—i.e., buffer and protein. It is worthwhile to emphasize that we designate this type of phase diagram as a pseudo-two-component system (as opposed to a two-component system) because, for a typical in vitro experiment, the solvent is not a single chemical species, but rather an aqueous solution that includes various soluble osmolytes (salts, buffer, reducing agents). Nevertheless, it is convenient and often reasonable to approximate buffer as a homogeneous solvent. More problematic is a pseudo-two-component system in which the control parameter is effectively determining the concentration of a third component—salt, crowder, even pH reports on the proton concentration. For these multicomponent systems in camouflage, the striking simplicity of a pseudo-one-component system can mask complex underlying behavior, as has been described and demonstrated (22, 109).
(Pseudo-)two-component system:
a convenient representation where a multicomponent system is defined in terms of just two components (e.g., protein and solvent)
Multicomponent:
a term used commonly to describe systems with more than two components being explicitly assessed
For a (pseudo-)two-component system, a phase diagram is constructed by systematically altering the control parameter while also varying the solute concentration, effectively performing a two-dimensional titration around phase space. As these experiments are performed, conditions under which the solution is monodisperse (irrespective of Ctotal) represent the one-phase regime of the phase diagram. In contrast, conditions in which two distinct phases coexist are the two-phase regime. Typically, two coexisting phases manifest as a dense assembly surrounded by a dilute phase (Figure 2a). However, at sufficiently high Ctotal, an inversion of these two phases occurs such that a dilute droplet appears in a majority dense phase (Figure 2a). On the basis of the observations made during these experiments, a typical phase diagram can be constructed by plotting molecule concentration on the x axis and some other parameter on the y axis and by designating measured points as one phase or two phase. The boundary that separates these two regions is termed the binodal or the coexistence curve.
Figure 2.

Liquid-liquid phase separation (LLPS) of a simplified in vitro pseudo-two-component system. (a) LLPS is dependent on protein concentration, c (x axis), and other factors such as salt concentration; pH; or, as depicted here, temperature (y axis). At a fixed temperature (denoted by the horizontal line in the graph), as protein concentration increases from a relatively low concentration (c1) to a higher concentration (any concentration greater than c2 in this example), the protein will go from being homogeneously distributed throughout the system (the white one-phase regime) to existing in a two-phase regime (blue). In the two-phase regime, a protein-poor dilute phase and a protein-rich dense phase coexist with one another (❶ to ❷). In this example, c2 corresponds to the saturation concentration at the specified temperature associated with the horizontal line. In the two-phase regime, as overall protein concentration increases past c2, additional protein will be recruited into the dense phase of the system, resulting in an increase in the volume of the dense phase and a corresponding decrease in the volume of the dilute phase (❷ to ❸), Importantly, the protein concentration in the dense phase (c3) and dilute phase (c2) remains constant. As the total protein concentration increases further and while still in the two-phase regime, it will become more energetically favorable for the system to undergo an inversion transition, whereby dilute droplets exist in a majority dense phase (❸ to ❹). Finally, as protein concentration increases further, the system will exit the two-phase regime, exceeding the dense phase concentration (c3) such that the system will once again be homogeneously distributed in a one-phase regime. Once reentrance into the one-phase regime has occurred, the total concentration can continue to increase and exceed the concentration observed inside the dense-phase droplet (➎). (b) Phase diagrams built by changing different parameters can result in tie-lines having different slopes. Tie-lines are generated by connecting the solution conditions of the dense phase to the dilute phase. (i) For a tie-line generated in a system in which salt concentration is altered across the phase diagram, the concentration of salt in the dense phase may be different from that in the dilute phase, resulting in a tie-line that is not horizontal. (ii) In contrast, when temperature is altered, the temperature within the dilute and dense phases is expected to be equal, allowing for generation of a horizontal tie-line.
Binodal:
also known as the coexistence curve; delineates between the dense and dilute phases on a phase diagram
For a phase diagram generated in the temperature/concentration plane, if the protein concentration in the dense phase and the dilute phase can be measured, then a horizontal line between these two concentrations can be drawn (Figure 2b). This line is known as a tie-line and connects the dilute phase concentration to the dense phase concentration on a phase diagram (Figure 2b). Any experiment that starts at solution conditions that lie on the tie-line will undergo LLPS to form a dense phase and a dilute phase with concentrations that match those where the tie-line intersects the binodal on the dense and dilute arms, respectively. A horizontal tie-line in the temperature-versus-concentration phase diagram can be intuitively explained in that the dense and dilute phases must be at the same temperature. However, in a salt-versus-concentration phase diagram, there should be no expectation for the same salt concentration in the two phases such that we might expect an angled tie-line. This is similarly true for any other pseudo-two-component phase diagram in which the control parameter reports on a third species (e.g., pH, crowder, osmolyte). For more in-depth discussion on phase diagrams and the experimental phase diagram construction, see References 49, 97, 101, and 134.
Tie-line:
a line that connects the low-concentration arm and high-concentration arm of a phase diagram to describe two coexisting concentrations
2.3. Multivalency Promotes Phase Separation
Nearly any biomolecule can theoretically phase separate in vitro if the concentration is sufficiently high and solution conditions are appropriately tuned. Whether or not these concentrations and solution conditions are physiologically relevant for a given biomolecule is a separate and more nuanced issue. This discussion leads to an obvious question: What properties must a molecule possess to promote phase separation? Ultimately, the propensity of a molecule to undergo phase separation relies on multivalency: the ability of a molecule to simultaneously engage in multiple intermolecular interactions (reviewed in 4, 9, 23). In general, a molecule with greater multivalency has an increased capacity to phase separate, although the impact of multivalency depends on the relative strength of the binding sites that encode multivalency.
Multivalency:
a characteristic in which species (e.g., protein, RNA, polymer, molecular complex, small molecule) can simultaneously interact with multiple other species
Multivalency was first examined explicitly in a stylized manner by exploring a protein class referred to as linear multivalent proteins. In this protein class, defined folded binding domains are connected by flexible linkers such that the valency of a given molecule conveniently maps to the number of a given binding domains present. The Rosen group (67) generated two flavors of linear multivalent proteins: those with multiple repeats of the Src-homology 3 (SH3) domain and those with multiple repeats of a proline-rich motif (PRM). SH3 and PRM domains bind one another but do not bind themselves. Csat was highly dependent on the number of SH3 and PRM modules present for mixtures of poly-SH3 and poly-PRM molecules. Subsequent work demonstrated that the change in Csat followed the behavior expected from theory, demonstrating that remarkably simple models quantitatively reproduce the emergent polypeptide behavior (46).
More recently, multivalency has been cast in terms of the stickers and spacers model for associative polymers, as first developed by Semenov & Rubinstein (120) and Rubinstein & Dobrynin (113) for synthetic polymers and popularized in the context of biomolecular polymers by Choi, Pappu, and colleagues (24, 134; reviewed in 23). In this model, stickers represent molecule regions that facilitate intermolecular interactions, whereas spacers connect the stickers but do not significantly contribute to the attractive intermolecular interactions that contribute to higher-order assembly. The stickers and spacers model is appealing in that it does not prescribe an underlying physical chemistry or a length scale to the stickers and spacers. Consequently, it can be applied to a wide range of systems, offering a general framework through which the impact of changes in valency can be interpreted.
Stickers and spacers model:
a model in which multivalent molecules are delineated as containing stickers (regions that drive interaction) and spacers (regions that do not)
Four general mechanisms can provide protein multivalency: by multiple interaction interfaces on a single folded domain, by a series of modular interaction domains, by branched multivalent interactions, or by interactions facilitated by intrinsically disordered regions (IDRs) (reviewed in 101) (Figure 3). However, multivalency is not limited to proteins, and other biological polymers such as DNA or RNA can also encode multivalency through which other nucleic acids or proteins can interact (4). Indeed, multivalent interactions facilitated by nucleic acids frequently participate in biological phase separation, and phase separation may be an important mechanism for regulating not only localization of proteins but also mRNA (reviewed in 64).
Figure 3.
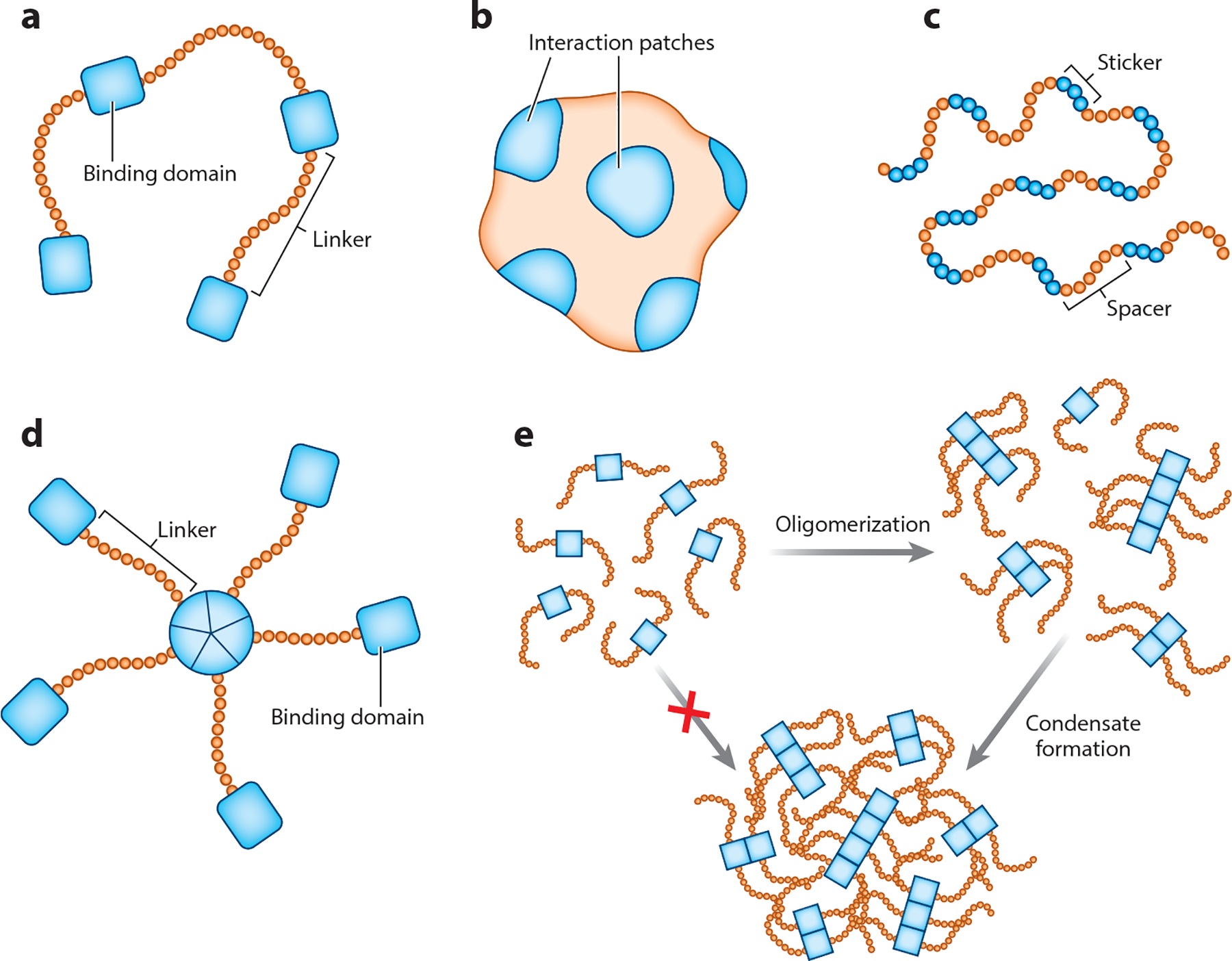
Multivalency can come in many different forms. Individual protein molecules can encode multivalency through various ways. (a) Linear multivalent molecules contain regions that facilitate inter- and intramolecular interactions (depicted as rectangular binding domains) connected by linkers that do not participate in multivalent interactions (linker regions). (b) Folded proteins can encode multivalency through many distinct interaction patches that facilitate multivalent interactions. (c) Intrinsically disordered proteins (or intrinsically disordered regions within a protein) can contain regions that facilitate multivalent interactions (stickers) separated by regions that do not participate in multivalent interactions (spacers). (d) Multiple individual proteins that are not multivalent on their own can come together through a central oligomerization domain, resulting in formation of a branched multivalent protein complex that can encode multivalency. (e) Oligomerization domains can contribute to phase separation both by contributing to multivalency and by bringing proteins within proximity of each other, increasing the likelihood of additional multivalent interactions such that oligomerization is a prerequisite for phase separation.
Intrinsically disordered region (IDR):
a protein region that in isolation cannot fold into a fixed tertiary structure and exists as a collection of interconverting conformations
2.4. Intrinsically Disordered Regions and Phase Separation
Recently, substantial attention has focused on IDRs as protein regions that encode multivalency to promote biological phase separation and condensate formation (reviewed in 78, 128). IDRs are protein regions that, instead of adopting a fixed three-dimensional native state, exist in an ensemble of conformations that are in flux (reviewed in 89). The IDR of many proteins alone is often both necessary and sufficient for LLPS in vitro, and proteins containing IDRs have been found in many condensates to date (49). However, proteins that encode multivalency through means other than IDRs also phase separate (6, 67, 124). More generally, just because a protein is intrinsically disordered or contains an IDR does not mean that it will phase separate (77). IDRs can have a wide range of properties, and different IDRs can promote or suppress phase separation, depending on the IDR sequence (reviewed in 49, 77). In fact, IDRs have successfully been used as solubility tags to prevent protein aggregation during protein purification, and some IDRs are more effective as solubility tags than traditionally used tags such as maltose-binding protein (117). The frequency at which IDR-containing proteins are found in condensates is likely due to the multivalent interactions that IDRs can facilitate, and it is this multivalency and not the intrinsic disorder that is important for phase separation (reviewed in 49).
2.5. The Relationship Between Liquid-Liquid Phase Separation and Biomolecular Condensates
By its original definition, the term biomolecular condensate essentially acts as a catchall for anything that appears to be a concentrated assembly of biomolecules within a cell (4). An assembly does not need to form via LLPS to be considered a biomolecular condensate. The relationship between LLPS and in vivo condensate formation is complex in that it is difficult to unambiguously demonstrate that a condensate forms via LLPS in vivo due to the complex and dynamic nature of the intracellular environment (80). Nonetheless, growing evidence supports the hypothesis that many condensates may form via LLPS in vivo. Many proteins that are integral to the formation of a specific condensate undergo unambiguous LLPS in vitro, and the characteristics of the in vitro phase-separated assemblies often resemble those of the in vivo condensates (38, 51, 73, 90, 105, 108, 141). Furthermore, proteins with mutations that alter in vitro LLPS often show altered in vivo condensate formation and disrupted cellular function (91, 94, 108). Finally, in vivo condensates display many properties that would be expected if they were to form via LLPS (reviewed in 4). All of these considerations support the notion that in vivo condensates can form via LLPS; however, it is at least possible that condensates may also form through some other mechanism that has yet to be determined, and this unknown mechanism may simply be well represented by and behave similarly to LLPS.
It may be tempting to dismiss the notion of phase separation in cells as a new collection of language to describe a previously reported phenomenon. This is neither an unreasonable nor an inaccurate concern in some cases. However, we suggest that, rather than being simply a new collection of language, phase separation is a general physical framework that provides predictive power. It is not a new collection of language, but an old collection of physics. By understanding how well-established physical principles map onto biological systems, extant experimental data can be interpreted and novel predictions made. Whether or not those predictions are accurate provides insight into the extent to which an observed phenomenon follows expected rules, although convolved in those predictions are both our understanding of the system as a whole and our ability to wield the relevant physics. Descriptive language does not offer such a general model for the underling physics that governs weak multivalent interactions, instead requiring bespoke molecular mechanisms for each observed phenomenon. Phase separation provides a general class of mechanisms that may or may not be accurate but unambiguously offers predictions that can be tested.
2.6. The Scaffold and Client Model for Condensates
Any given biomolecular condensate can harbor hundreds or even thousands of distinct proteins and nucleic acids. The multicomponent nature of condensates makes understanding their behavior and the specific interactions that contribute to their biophysical properties incredibly challenging (53, 61, 116). Not all components within a condensate may be required for condensate formation, and many components often do not phase separate at physiologically relevant concentrations on their own (25, 45). In contrast, some condensate components are essential for condensate formation. The difference between components that preferentially localize to a condensate and those that are necessary for condensate formation is well described by the scaffold and client model, in which components that preferentially localize to condensates are referred to as client molecules and the components that are required for condensate formation are termed scaffold molecules (reviewed in 5, 101). Client molecules tend to harbor domains that allow for their interaction with scaffold molecules (5, 25). In contrast, scaffold molecules contribute the multivalency that is necessary for condensate formation, and these molecules can sometimes be identified by their sequence features (101, 134). Strictly speaking, while a client molecule may not be necessary for condensate formation, it may still impact condensate behavior. For example, if a client molecule is interacting with a scaffold molecule such that it is sterically occupying a site at which another scaffold molecule would otherwise interact, the client molecule is effectively lowering the valency of the scaffold molecule that it is interacting with, which would impact the condensate properties (114). Therefore, whereas client molecules may be dispensable for condensate formation, they can impact condensate behavior.
Scaffold and client model:
a model in which molecular components are scaffolds (drivers of biomolecular condensates) or clients (which passively partition into condensates)
2.7. Condensates Formed by Liquid-Liquid Phase Separation May Not Retain Liquid-Like Properties
It might be reasonable to expect that a condensate formed via LLPS will possess liquid-like characteristics; however, condensates adopt a wide range of physical properties and are capable of undergoing progressive hardening and even liquid-to-solid transitions (reviewed in 2). The process by which condensates transition from a viscous liquid to a viscous-elastic solid is broadly known as condensate maturation; many condensates that begin as liquid-like droplets mature to have less dynamic, solid-like properties (14, 94, 99, 118, 139). These solid-like condensates are frequently referred to as gels (with a liquid-to-solid transition sometimes termed gelation), although given the broad and field-specific definitions for a gel, we refrain from referring to these solid-like assemblies as gels in the interest of clarity. It is currently thought that some condensates exhibiting stable, solid-like properties initially form through LLPS such that, while initially surface tension is minimized to form spherical assemblies for which molecular rearrangement is possible, over time these condensates mature to eventually display nondynamic properties (reviewed in 140). Early work driven by an interest in the relationship between liquid-to-solid transitions and protein aggregation implied that the acquisition of slowed condensate dynamics was a pathological hallmark, notably associated with neurodegenerative diseases. However, more recently there is growing evidence that maturation of some condensates into solid-like states is important for their biological function (reviewed in 140).
3. STUDYING BIOMOLECULAR CONDENSATES AND LIQUID-LIQUID PHASE SEPARATION
Over the previous decade, our understanding of biomolecular condensates and the underlying mechanism governing their formation has progressed substantially, and the approaches and tools used to study condensates have similarly advanced. In this section, we review some common tools used to study condensate formation and LLPS. We also discuss some drawbacks and strengths of the various approaches. Finally, we also consider some approaches that are specific to plant systems.
3.1. Computational Approaches
At this time, our ability to computationally predict whether or not a given biomolecule will phase separate under physiologically relevant conditions is limited. However, this does not mean that there are not useful computational tools that can be used to inform experimental design for studying a biomolecule that appears to be undergoing phase separation. Given the importance of multivalency for phase separation, the computational identification of protein regions that may contribute to multivalent interactions is a powerful means to aid in determining which protein regions are important for condensate formation. Nonetheless, it is important to remember that these tools have limitations, and they should be used as a means to inform experimental design, and not as absolute truth. In this section, we briefly overview some common, accessible computational tools that can be used in the study of proteins suspected of contributing to condensate formation.
3.2. Tools for General Protein Information
Multiple possible resources can be used to obtain information on protein attributes such as length, amino acid sequence, posttranslational modifications, and additional protein annotations. A challenge is knowing which resource to explore. As a first port of call for knowledge-based protein annotations, UniProt (https://www.uniprot.org) is a powerful and centralized resource that integrates distinct protein annotations into a single interactive web resource. UniProt holds information—including predicted protein sequence features and experimental data—for more than 120 million proteins across hundreds of proteomes (127). In addition to integrating many distinct proteome-wide annotation data sets together, UniProt contains hundreds of thousands of manually curated protein features that are extracted from the literature (127).
Features accessible through UniProt may be of interest in the context of exploring phase behavior. Such features may include protein-protein interaction information, whether or not a protein binds DNA or RNA, protein localization, amino acid modifications, secondary structure information, protein domains or repeats, protein compositional biases, and (for some proteins) predicted disordered regions. Furthermore, it is easy to determine whether any feature for a given protein was manually curated or automatically generated, and where possible, the specific publication for any protein feature is referenced, allowing the user to obtain more information. In summary, UniProt is a key pillar of modern bioinformatics and provides convenient and constantly evolving access to proteome-wide analysis.
3.3. Tools for Predicting Intrinsically Disordered Regions and Their Behavior
Whereas IDRs are not necessary for phase separation, determining the predicted disordered regions for a protein of interest is generally useful when designing protein variants that may alter the capacity of a given protein to phase separate. Numerous readily available tools such as IUPred2A (34), ESpritz (133), and PrDOS (55) exist for assessing predicted protein disorder. Each predictor uses a different approach to predict protein disorder, so it is useful to be able to identify consensus regions where the various predictors agree. This can be done using consensus protein disorder databases such as D2P2 and MobiDB, which are databases of disordered protein predictions (87, 100). Once a protein region is predicted to be disordered, additional information on the behavior of the region can be obtained using CIDER, which provides information regarding the biophysical behavior of a disordered protein region (42, 50).
3.4. Predicting Protein Regions That Contribute to Phase Separation
There is no simple computational method that can predict whether or not a protein will phase separate in vivo. Whereas our understanding of the physical chemistry that drives phase separation is undeniably incomplete, our inability to predict whether a region can phase separate in cells reflects a more general challenge in that this is a conceptually ill-posed question (reviewed in 77). The poly-SH3/poly-PRM system introduced previously is a convenient system with which to illustrate this limitation. Solutions of poly-SH3 or poly-PRM alone are monodisperse—even at high protein concentrations—because these proteins do not strongly interact with themselves. In contrast, when a ternary solution (solvent + poly-SH3 + poly-PRM) is generated, robust phase separation is observed, making it clear that poly-SH3 can undergo phase separation in the presence of an appropriate partner. Could we have predicted this from sequence/structural information alone? To take poly-SH3 and confidently assess whether it can phase separate is akin to asking whether, in the possible universe of multivalent macromolecules, there exists any multivalent molecule that can interact with SH3 domains. If we extend this logic to an arbitrary protein or nucleic acid, it is perhaps inconceivable that there exists any biomacromolecule that would not phase separate when mixed with the appropriate complementary partner, where this hypothetical partner could be any conceivable multivalent molecule. Consequently, in the absence of information on every possible molecular interaction that a biomacromolecule of interest could engage in, knowing whether or not that biomacromolecule can phase separate is a fundamentally unanswerable question.
A more limited but tractable question is to ask whether a given protein or protein region will undergo phase separation with itself, in a process referred to a homotypic phase separation (77). This again is conceptually ill defined inasmuch as the solution conditions will also dictate whether or not phase separation can occur, but by prescribing a set of standard in vitro solution conditions, we can at least pose an answerable question. While the importance of homotypic interactions in cells may be limited on a molecular level, on the level of physical chemistry this question allows us to assess how certain modes of intermolecular interaction can contribute to phase separation. The physical principles uncovered through the exploration of homotypic phase separation have been instrumental in advancing our understanding of the molecular grammar of biological phase transitions, notably in the context of disordered protein regions (28, 30, 78, 93, 119, 134). With this in mind, a number of first-generation phase separation predictors have emerged. These predictors involve, either directly or indirectly, assessing homotypic chemical interactions to construct sequence-specific metrics that report on the likelihood of homotypic assembly (reviewed in 131).
A common theme that has risen from studying the phase separation of many different proteins is that cation-pi and pi-pi interactions facilitated by aromatic amino acids within IDRs appear to be major contributors to phase separation (49, 86, 130). The PScore predictor is a tool that predicts phase separation behavior based on interactions mediated by sp2-hybridized carbon atoms (http://pound.med.utoronto.ca/~JFKlab/Software/psp.htm) (130). PScore is trained on structural data from the Protein Data Bank such that, while sp2-hybridized carbon atoms are found in canonical pi systems (such as aromatic rings and arginine guanidinium side chains), they are also found in other residues such as histidine, glutamine, and asparagine. Interactions mediated by aromatic residue side chains take place in a variety of contexts. However, these interactions are not the only predictor of phase separation for any given protein—indeed, many distinct sequence features within an IDR contribute to whether or not it will promote or suppress phase separation (reviewed in 49).
An additional observation from identifying proteins that undergo biological phase separation is that many contain low-complexity prion-like domains (PLDs) (reviewed in 39). Low-complexity domains (LCDs) are regions within proteins that contain little amino acid diversity (reviewed in 79). LCDs are typically, although not always, disordered, and in general LCD is taken to be synonymous with disordered LCD (20). PLDs are a subset of LCDs that are intrinsically disordered and enriched in polar amino acids (notably glutamine, asparagine, glycine, and serine). PLDs were first identified in the context of prion-forming proteins in Saccharomyces cerevisiae, although it is important and somewhat confusing to note that a PLD refers to the amino acid composition, as opposed to an ability to act as a prion. At a practical level, PLDs are effectively defined by their identification by using the sequence analysis tool PLAAC (http://plaac.wi.mit.edu). Many PLD-containing proteins undergo phase separation in vitro (63). Despite the association of PLDs with phase separation, PLD presence is not a guaranteed indicator of phase separation propensity (reviewed in 39). While PLDs are one flavor of LCD, others exist. These include basic-acidic domains, also known as polyampholytic tracts, which undergo phase separation as a function of composition, length, and patterning, all of which can be computed directly from sequence (10, 43, 68). Elastin-like polypeptides are another LCD class that undergo biological phase transitions, with recent work providing general principles from which phase behavior can be inferred from sequence (106, 115).
3.5. Considerations for Studying Phase Separation In Vitro
Commonly, in vitro approaches are used to study aspects of condensate formation and dynamics. While a powerful route for the elucidation of physical chemistry, important factors need to be considered when the phase separation behavior of a purified molecule in vitro is examined.
Most importantly, just because a biomolecule phase separates in vitro does not mean that phase separation is relevant to the biological question being investigated or that the biomolecule in question phase separates in vivo. Phase separation reflects an intricate competition between entropy, solvent-solute interactions, and solute-solute interactions. Although in vitro conditions provide a well-defined and controllable environment suitable for the elucidation of quantitative physical chemistry, the repertoire of solvent and solutes available in buffer is dramatically different from that in vivo. Consequently, the ability of a protein to undergo bona fide phase separation in vitro is solely (a) definitive proof of the ability to undergo phase separation in vitro and (b) a modest suggestion about its behavior elsewhere. The corollary is also true: The inability of a biomolecule to undergo phase separation in vitro says nothing of its ability to form or be recruited to condensates in vivo. As such, effective experimental design is critical if in vitro insights are to be used to uncover biologically relevant insights.
From the perspective of obtaining recombinant protein, posttranslational modifications of IDRs appear to be a mechanism used by organisms to modulate the propensity of a given protein to phase separate (reviewed in 92). Therefore, if the protein is expressed heterologously in a system that does not recapitulate the native posttranslational modifications, then the purified protein may display altered phase behavior relative to the native protein. Furthermore, given that many proteins that phase separate also bind nucleic acids (notably RNA), purification protocols designed to obtain pure protein should include explicit steps to ensure that all RNA is removed, as can ultimately be assessed by UV absorbance (A260/A280). These are two of many practical considerations that must be taken when designing in vitro experiments for studying biological phase separation, and we encourage further reading for those designing in vitro experiments (reviewed in 3).
Although in vitro approaches have been integral to our understanding of biological phase separation, phase-separated assemblies that form in simplified in vitro systems do not perfectly resemble condensates that form in vivo. In vitro experiments typically analyze a single protein for its capacity to phase separate. Therefore, the in vitro reconstituted condensate is unlikely to include all of the different biomolecules that interact with and are within the condensate in vivo. In vivo condensates form through a combination of homotypic interactions (intermolecular interactions between two identical types of molecules) and heterotypic interactions (intermolecular interactions between two different types of molecules). Therefore, in vitro experiments often fail to recapitulate behaviors that condensates adopt in vivo due to the absence of essential heterotypic interactions. Indeed, for multicomponent systems (systems in which many components phase separate together), Csat for a given component is not fixed but instead depends on the concentration of all coexisting components, a conclusion predicted by theory and simulation and shown directly in cells (22, 56, 109).
In vitro studies, despite their limitations, can provide critical insight without the confounding influence of an arbitrary number of unknown variables. As such, in general a combined approach in which in vitro and in vivo experiments are integrated (ideally with theory and simulation) offers the most robust route toward a high-resolution mechanistic understanding of biologically relevant phenomena. Importantly, as with other areas in biology, the approaches, assumptions, and limitations tolerated must be tailored to the questions being investigated.
3.6. Expectations for Biomolecules Undergoing Liquid-Liquid Phase Separation In Vitro
Whereas rigorously demonstrating that a condensate forms through LLPS in vivo is challenging, demonstrating phase separation in vitro is a much more tractable pursuit. For a pseudo-two-component system (biomolecules + solvent), Csat represents a value that, under fixed solution conditions, should be independent of the total protein concentration (see Section 2.2). This fixed Csat is an example of the fact that—at equilibrium—the binodal (the line dividing the one-phase and two-phase regimes in a phase diagram) should follow a well-defined path described by analytical theory. More generally, in vitro phase separation is demonstrable if experimental results can be explained and interpreted using physical principles that emerge from the expansive literature on phase transition theory.
On a practical note, Csat can be determined by keeping all aspects of a system constant except for protein concentration, which is systematically altered. Experimentally, the system is typically incubated for a fixed amount of time, after which it is examined for evidence of phase separation, commonly by light microscopy (135). In addition to light microscopy, turbidity assays can also be used to determine whether phase separation of a molecule has occurred (135). Turbidity assays take advantage of the increase in light scattering upon phase separation of a molecule and are amenable to higher-throughput analyses than microscopy. However, turbidity assays measure only changes in light scattering. Thus, information such as morphology of the resulting assemblies is not obtained in these assays, and visual confirmation via microscopy is essential. Alternatively, solutions of different Ctotal of protein can be generated and allowed to equilibrate, subject to gentle centrifugation to pellet the dense phase, and the supernatant measured spectroscopically to obtain the protein concentration in the dilute phase. When in the two-phase regime, the dilute phase concentration must equal Csat such that, when Ctotal > Csat, the supernatant concentration should be independent of Ctotal and equal to Csat. All three of these methods can be used to build the low-concentration arm of a phase diagram if measurements are made as a function of a control parameter such as temperature, salt, or pH.
Obtaining the high-concentration arm of the binodal is more challenging. One commonly applied approach is to generate sufficient total amounts of protein such that coexisting dense and dilute phases can be seen by eye and the dense phase can be directly extracted, diluted, and measured spectroscopically (15, 18, 78, 83). Another is to use fluorescence-based approaches such as fluorescence correlation spectroscopy (FCS), which, through careful calibration that accounts for the impact of dye quenching, can be used to infer protein concentrations (78, 137). Measuring the dense phase concentrations by using fluorescence-based approaches can be challenging for a variety of reasons such that, while direct measurement though large-volume samples can be tedious, it potentially avoids a range of challenging pitfalls (81, 97).
3.7. Using In Vitro Systems to Infer Liquid-Liquid Phase Separation as a Mechanism for Condensate Formation
A common application of assessing phase separation in vitro is to identify specific protein regions that contribute to phase separation. While this process can also be done in vivo, expression of a truncated or otherwise altered protein in vivo may alter other components of the system (other proteins or nucleic acids) or parameters (such as changes to proteome composition or intracellular state). These alterations could change phase separation; therefore, in vitro analysis of protein variants allows for a controlled systematic investigation of protein regions that are contributing to phase separation. If a protein variant is identified that can no longer undergo in vitro LLPS, it can be leveraged to further investigate whether those same types of interactions contribute to the formation of an associated condensate of interest in vivo. If the LLPS-deficient variant no longer forms condensates in vivo, then the notion that the same interactions that drive LLPS in vitro underlie condensate formation in vivo is supported. This result is consistent with a model in which LLPS underlies condensate formation, although this result is neither necessary nor sufficient.
Although a lack of condensate formation upon in vivo expression of a LLPS-deficient protein variant is consistent with a model in which LLPS reflects the underlying mechanism for condensate formation, it is difficult to interpret the biological importance of these results. For example, if the LLPS-deficient protein variant is unable to rescue a phenotype that is associated with loss of function for that protein, one may be tempted to suggest that LLPS is an important underlying mechanism for whatever processes are associated with the protein being investigated. However, it is possible that the inability of the LLPS-deficient protein variant to function is unrelated to the diminished capacity to phase separate and is instead simply due to loss of protein function in general. Specifically, if protein function is linked to protein-protein interactions, then disentangling a loss-of-function phenotype driven by an inability to bind a partner from loss of function through an inability to self-assemble is extremely challenging. This can be even more challenging when attempting to alter the phase behavior of IDR-containing proteins, given that IDRs frequently require large-scale mutational changes to alter phase separation behavior (reviewed in 49). In sum, it is important to not overinterpret results from experiments in which protein variants with altered in vitro phase separation propensity are expressed in vivo, and careful measures need to be taken to ensure that other aspects of protein function are not compromised in LLPS-deficient protein variants.
3.8. Analyzing Material Properties of In Vitro Phase-Separated Assemblies
Condensates adopt a wide array of material properties. These states include dynamic liquid-like condensates that readily fuse with one another and nondynamic solid-like condensates that do not appear to exchange molecules with their surroundings. These properties are important to investigate because they can provide information with regard to how a given condensate functions (reviewed in 136). Numerous approaches can be used to assess the material properties of in vitro phase-separated assemblies (reviewed in 82).
One of the most straightforward approaches is to utilize time-lapse differential interference contrast (DIC) imaging to capture fusion of in vitro assemblies. By analyzing the rate at which fusion occurs, information with regard to the viscosity and the surface tension of the assembly can be acquired (31). An alternative method that can lend insight into the viscosity of an assembly is particle tracking microrheology (reviewed in 138). This method tracks the movement of extremely small fluorescent beads inside of an assembly and uses the movement of the beads to determine the bulk viscosity of the assembly, assuming that the beads are sufficiently large relative to the mesh size of the assembly (38).
Finally, one of the most common approaches to glean insight into the material properties of an in vitro assembly is fluorescence recovery after photobleaching (FRAP) (126). Careful considerations—such as whether an entire assembly or only part of an assembly is photobleached and the assumptions used for different models meant to generate information with regard to diffusion of fluorophore-labeled molecules within an assembly—must be made when interpreting results obtained using this method (126). It is important to note that after LLPS occurs the material properties of the assemblies that form can change over time from liquid like to solid like, and the rate at which the material properties of the assembly change can depend on the experimental conditions used and the specific biomolecule(s) being investigated. Therefore, during all experiments that seek to assess the material properties of an assembly in vitro, it is extremely important to keep the amount of time after phase separation occurs constant.
3.9. Challenges in Studying Biomolecular Condensates In Vivo
Studying any given condensate in vivo comes with numerous complexities that do not need to be considered when utilizing in vitro approaches. However, to understand the biological function of a condensate, it is imperative that we examine its behavior in vivo.
One of the biggest challenges in studying a condensate in vivo is visualizing the condensate. A common approach is to use fluorescently tagged proteins that localize to a condensate of interest. However, addition of a fluorescent protein to a protein can alter the phase separation properties, and the extent of this impact varies substantially from protein to protein. A robust protocol to mitigate this effect is lacking, although fluorophore impact can be minimized by considering the relative position (N or C termini) to fuse the fluorophore as well as the properties of the fluorophore itself. Some fluorophores form low-affinity oligomers, and others function as obligate dimers or tetramers (122). Even the commonly used enhanced green fluorescent protein weakly dimerizes (84). This property artificially increases the valency of the protein that the fluorophore is fused to, and this increase in turn will affect phase separation of the protein. Therefore, use of monomeric fluorophores is crucial when studying phase-separating proteins. Additionally, if the fluorophore is fused too closely to the protein region facilitating the multivalent interactions integral to condensate formation, the fluorophore may alter interaction valency. A best practice approach would include assessing the Csat of both N- and C-terminally tagged versions of the fusion protein compared to the native protein in vitro to determine fluorophore impact. Additional visualization methods that do not require altering the native protein, such as immunolabeling, also assure that condensation behavior is not an artifact of the fluorescent protein tag.
3.10. Considerations in Using Transgenic Lines to Study Condensates
Condensate formation by phase separation is intimately tied to protein concentration. Thus, it is imperative that any transgenic lines generated to visualize a protein of interest accumulate tagged proteins to levels comparable to the native protein. Ideally, this goal could be accomplished by CRISPR-based alteration of the genomic DNA sequence such that the fusion protein is expressed in its native context; however, this approach is not equally feasible across all organisms. Notably, in plants, CRISPR-based approaches are not currently the primary approach used for visualizing fluorophore-tagged proteins due to the low efficiency of precise gene editing (reviewed in 76). In the model plant Arabidopsis thaliana, the most common method for generating lines expressing protein-fluorophore fusions is integration of a transgene into the genome. However, this approach is subject to many pitfalls that can result in altered expression of the transgene in comparison to the native copy (reviewed in 98). Quantifying protein levels and comparing fusion protein levels to the native protein levels can also be complicated by the use of whole seedling extracts that may mask tissue-level variation in the transgenic plant. This tissue-level variation may result in protein accumulation to levels higher than physiologically relevant levels, which may lead to aberrant condensate formation. Examination of multiple transgenic lines can be helpful in determining whether the insertion site affects expression in this manner.
3.11. Transient Expression Systems for Studying Condensates in Plants
Although generation of stable plant lines is time consuming, plants are amenable to transient expression systems that can be advantageous in studying various aspects of protein behavior. These systems allow for protein expression and visualization in a matter of hours to days, which is substantially faster than the months to years required for generating stable transgenic lines in various plant systems. Whereas these transient expression systems more closely resemble the native environment than in vitro systems, they come with numerous drawbacks that need to be considered when one is interpreting results related to protein phase separation.
A common transient expression system for visualizing proteins of interest is tissue infiltration of Nicotiana benthamiana leaves, with Agrobacterium tumefaciens carrying a vector with the protein of interest fused to a fluorescent protein (66, 85). However, in the context of studying phase separation, this system has many potential pitfalls, particularly as highly active constitutive promoters are often used. If the protein of interest is from a species other than N. benthamiana and relies heavily on heterotypic interactions for condensate formation, this assay would rely on the compatibility and presence of the homologous N. benthamiana protein. Furthermore, the process of infiltrating the tobacco leaves with A. tumefaciens can be destructive, resulting in a defense response on top of the immune response that is induced from A. tumefaciens (104), altering redox status and plant hormone levels (110), among multiple other factors. Finally, dramatic cell-to-cell variation in protein expression levels for an infiltrated N. benthamiana leaf makes comparison of condensate behavior between cells difficult (8). These factors may lead to artifactual condensate formation in N. benthamiana, particularly if protein accumulation is much higher than in its native context.
Transient protein expression in protoplasts is also used to study protein behavior (144). Whereas this system has multiple benefits over tobacco infiltration, there are also considerations for data interpretation with regard to protein condensation. If one assumes that the protoplasts are made from the same species from which the expressed protein originates, this system reduces the likelihood that heterotypic interaction proteins are absent. However, protoplasts derived from different tissues display distinct promoter activity and protein sorting, suggesting that they retain cell identity information (37). Thus, the cell type used for protoplast generation will impact condensate formation and behavior. In addition, protoplast isolation can result in alterations in cytosolic pH and other stress responses (7), which also impact condensate formation. Finally, protoplasts typically express proteins at levels far higher than physiologically relevant levels, which could result in condensate formation by proteins in protoplasts that would not otherwise form condensates in planta. Therefore, while both tissue infiltration of N. benthamiana and transient expression in protoplasts can be useful tools for studying a protein suspected of forming condensates, caution should be exercised when interpreting results in these systems.
3.12. In Vivo Approaches for Studying Biomolecular Condensates
Although studying condensates in vivo is challenging, some questions simply cannot be answered using in vitro approaches. Critically, understanding the biological importance and function of a condensate can be accomplished only by using in vivo approaches. Isolation of condensates to determine their composition of proteins and nucleic acids offers a unique set of challenges caused by the lack of a membrane and the propensity for condensates to disassemble due to their inherent sensitivity and concentration dependence. Despite these challenges, experimental determination of condensate composition by using various approaches, including fluorescence-activated particle sorting, centrifugation, tandem affinity purification, and proximity labeling, can be successful (53, 60, 61, 145).
Like determination of condensate composition, dynamic cell type–dependent and condition-dependent condensate formation can be determined only in vivo. Many condensates form only in certain cell types or in response to specific environmental triggers (102, 108, 132, 143). Understanding the cell types and the conditions under which a condensate is present can lend substantial insight into its biological function. Additionally, the subcellular localization of the condensate can be determined only through in vivo observations. In plants, condensates have been found in the nucleus (35), the cytoplasm (102), and chloroplasts (91); identifying their subcellular localization has been integral to determining biological function.
Lastly, some approaches can be used for either in vitro or in vivo studies. Specifically, many microscopy approaches can be used both in vitro and in vivo. Microscopy-based approaches that are amenable to studying condensates in vivo include DIC time-lapse imaging to monitor condensate fusion (40), FCS to quantify protein diffusion (40), FRAP (126), and numbers and brightness (N&B) analysis (27) to assess the distribution of oligomers within a condensates (102). It is important to note that any approach utilizing a fluorophore-protein fusion (including FRAP, FCS, and N&B) is subject to the limitation that only information on the labeled protein be obtained. Distinct proteins and nucleic acids within an individual condensate may display substantially different mobilities from one another (12). Therefore, when interpreting results from experiments using methods that visualize only individual components of a condensate, it is important to remember that the information obtained will be representative only of the behavior of the component visualized and not the behavior of the overall condensate.
4. THE CURRENT STATE OF UNDERSTANDING PROTEIN PHASE SEPARATION IN PLANT BIOLOGY
4.1. Possible Roles for Biomolecular Condensates
As discussed, phase separation is dependent on many environmental parameters, including pH, temperature, and ion concentration. Therefore, condensate formation can be dynamic and responsive to external stimuli (reviewed in 143). Responsive to the cellular environment, condensate formation can rapidly and reversibly alter the spatial distribution of various biomolecules within the cell, providing an attractive mechanism to regulate various cellular processes.
In addition to having a dynamic and reversible nature, condensates adopt properties that make them uniquely suited to regulate specific cellular processes. For example, condensates can sequester specific biomolecules to prevent a biological process from occurring, by limiting either scaffold or client availability, as seen in stress granules that sequester mRNAs stalled during translation in response to various stressors (19).
In addition to biomolecule sequestration, phase separation can be another mechanism to buffer the concentration of a biomolecule. In simplified in vitro systems, protein concentrations in the dense and dilute phases remain constant as additional protein is added to the system (see Figure 2). This system provides tremendous buffering capacity to maintain a near-constant concentration of a specific protein within a cell. Because cells are more complex than simplified in vitro systems, this buffering capacity is likely to be complicated within a cellular context. An alternative benefit of condensate formation is the spatial concentration of components involved in a specific biochemical reaction to regulate reaction kinetics (95). Finally, because changes in temperature, pH, and other parameters can alter phase separation occurrence, phase separation itself could be used as an environmental sensor (39).
4.2. Current Examples of Condensates or Phase Separation in Plant Systems
At the time of this writing, to our knowledge there are five published examples in which condensate formation or phase separation has been explicitly examined in plants (35, 57, 91, 102, 146) (Figure 4). These studies implicate condensate formation in plant hormone signaling (102), intrachloroplast cargo sorting (91), polyadenylation (35), plant immune response (146), and temperature sensing (57). We briefly summarize aspects of these studies in the following sections.
Figure 4.

Examples of phase separation in plant biology. Plants use phase separation in the regulation of numerous fundamental plant growth and developmental processes. Nuclei are in purple; proteins of interest in blue. Nuclei appear blue when the protein of interest is diffusely in the nucleus. (a) FLOWERING CONTROL LOCUS A (FCA) forms subnuclear condensates and has been associated with regulation of flowering time in Arabidopsis (35). FLX-LIKE 2 (FLL2) (AT1G67170) regulates FCA condensate formation, and a recent study identified an FLL2 mutant that resulted in reduced FCA condensate formation and that had a delayed flowering phenotype, implicating FCA condensate formation in the regulation of flowering time (35). (b) NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) is involved in regulation of effector-triggered immunity and forms oligomers in the cytoplasm through intramolecular disulfide bonds when there is no oxidative stress (125). However, upon pathogen attack, intracellular redox changes result in an oligomer-to-monomer transition for NPR1, resulting in dispersion of cytoplasmic oligomers (125). (c) STT1 (At5G40160) and STT2 (At5G66055) mediate translocation of proteins in the chloroplast twin-arginine translocation (cpTat) pathway from the stroma to the thylakoid membranes via a phase separation–dependent mechanism (91). STT1 and STT2 form condensates within the chloroplast, and disruption of STT1 and STT2 phase separation results in disruption of cpTat substrate translocation across the stroma (91). (d) AUXIN RESPONSE FACTOR (ARF)7 and ARF19 are localized to the nucleus near the root tip but are localized to cytoplasmic condensates in the upper root, where cell growth has ceased (102). The localization of ARF7/ARF19 to cytoplasmic condensates attenuates auxin response in the upper root and is important for regulation of auxin responsiveness in the root (102). (e) PhyB photobodies are subnuclear condensates that form in response to red light (52). PhyB photobodies have been implicated in the regulation of the circadian clock, photomorphogenesis, and thermomorphogenesis (52). Abbreviations: C, chloroplast; N, nucleus.
Careful regulation of plant hormone signaling is vital to plant growth and development. Recent work highlighted a role for the formation of nondynamic, cytoplasmic condensates in regulating tissue responsiveness to the phytohormone auxin (102). Auxin is a plant hormone involved in essentially all aspects of plant growth and development. Two transcription factors involved in auxin signaling, AUXIN RESPONSE FACTOR (ARF)7 and ARF19, are diffuse and nuclear in the root tip, where active growth occurs, yet form cytoplasmic condensates in the upper root, where growth has largely ceased (102). Cells in which ARF7 and ARF19 form condensates (i.e., the upper root) are largely unresponsive to exogenously applied auxin, whereas cells in which ARF7 and ARF19 are nuclear and diffuse (i.e., the root tip) are highly auxin responsive (102).
Notably, expressing an ARF19 variant with severely compromised condensate formation reveals that ARF19 nucleocytoplasmic partitioning regulates auxin responsiveness. Rather than forming condensates in the upper root, this ARF19 variant remains diffuse and localizes to nuclei. Plants expressing this constitutively nuclear ARF19 variant display auxin responsiveness in cell types not responsive to auxin in wild type (i.e., in the upper root, where wild-type ARF19 localizes to condensates) (102), suggesting a model whereby ARF19 condensate formation is integral to regulating auxin responsiveness in the root.
Protein phase separation is implicated in intrachloroplast cargo sorting (91). Two ankyrin-repeat proteins, STT1 (At5G40160) and STT2 (At5G66055), were identified through a screen to find interactors of the chloroplast twin-arginine translocation (cpTat) substrate OXYGEN EVOLVING COMPLEX SUBUNIT 23 KDA (OE23) (91). Incubating STT1 or STT2 with OE23 results in LLPS in vitro (91). Furthermore, fluorescently tagged STT1 and STT2 expressed behind their respective native promoters form chloroplastic condensates (91). Some STT1 and STT2 variants are compromised in their capacity to undergo LLPS in vitro. When these variants are expressed in planta, they are less able to facilitate translocation of cpTat substrates, a result consistent with a model whereby phase separation of STT1 and STT2 is important in the sorting of cargo in the cpTat pathway. Finally, HIGH CHLOROPHYLL FLUORESCENCE 106 (Hcf106) is an additional binding partner that interacts with STT1 and STT2. Addition of Hcf106 to STT1, STT2, and OE23 in vitro suppresses LLPS and in vivo alters cpTat pathway cargo translocation (91). These data support a model in which regulated phase separation of STT1 and STT2 is important to intrachloroplast cargo sorting.
The RNA-binding protein FLOWERING CONTROL LOCUS A (FCA), which regulates flowering in Arabidopsis (65), forms subnuclear condensates with liquid-like properties (35). FCA undergoes LLPS in vitro; the PLD is necessary and sufficient for FCA phase separation (35). FLX-LIKE 2 (FLL2), a protein involved in FCA-mediated repression of FLOWERING LOCUS C, colocalizes with FCA in nuclear condensates and undergoes LLPS in vitro (35). A semidominant fll2 mutant contains fewer and smaller FCA condensates, whereas FLL2 overexpression results in increased FCA condensate size (35), suggesting that FLL2 affects FCA phase separation properties. Additional FCA-interacting proteins have roles in polyadenylation and RNA 3′-end processing and colocalize with FCA in nuclear condensates (35), suggesting that FCA-containing nuclear condensates may phase separate to form subcompartments enriched for proteins involved in RNA 3′-end processing (29).
Protein phase separation plays an unexpected role in plant immunity. In response to the plant hormone salicylic acid (SA), NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1) forms cytoplasmic condensates termed SA-induced NPR1 condensates (SINCs) (146). The NPR1 protein is integral to systemically acquiring resistance in response to pathogen infection. When in the SINC, NPR1 facilitates SA-responsive ubiquitination of target proteins to promote cell survival. Recruitment of NPR1 to SINCs is determined by three redox-sensitive IDRs (146). Thus, SINCs serve as a site for recruitment and ubiquitylation of key members of the stress response machinery, targeting them for degradation, attenuating inappropriate apoptosis, and promoting plant survival during infection and other stresses.
Temperature response and regulation are integral to plant survival. Phase separation is an attractive mechanism by which plants can sense fluctuating temperatures because it is an inherently temperature-responsive process. EARLY FLOWERING3 (ELF3) undergoes phase separation to form nuclear condensates in a reversible and temperature-dependent manner (57). ELF3 functions as a transcriptional repressor; elevated temperature drives ELF3 into nuclear condensates, relieving repression of target genes (57). Occupancy of ELF3 at target genes decreases as temperature increases (57). The ELF3 PLD reversibly phase separates in a temperature-dependent manner in vitro (57) and is both necessary and sufficient to provide this thermosensor behavior. ELF3 temperature-sensitive regulation of gene targets mediated by protein phase separation is an elegant mechanism to tie gene expression to temperature sensing.
In addition to the plant biology examples above, in which protein phase separation was directly interrogated, many additional protein condensates have been described in plants, even if the authors did not describe their observations by using this terminology. For example, plants house many condensates shared with other eukaryotic organisms; such condensates include the nucleolus (13, 58), nuclear speckles (107, 123), Cajal bodies (26, 69), processing bodies (62, 75), stress granules (19), and DNA damage foci (112). Although LLPS has been implicated in the formation of some of these condensates in other systems, whether LLPS is an underlying mechanism for the formation of any of these condensates in plants remains unknown.
Additionally, many published reports of protein localization are consistent with condensates unique to plants, such as dicing bodies (70) and photobodies (129). Dicing bodies are dynamic condensates that are involved in miRNA processing, although we understand very little about their biological importance (70). In contrast to dicing bodies, photobodies have been heavily studied. Out of the various proteins involved in light signal transmission in Arabidopsis, two out of three CRYPTOCHROMES localize to photobodies, and all five PHYTOCHROMES (PHYs) localize to photobodies (reviewed in 111). Notably, mutants in PLASTIC TRANSCRIPTIONALLY ACTIVE 12 (21) or in PHOTOPERIODIC CONTROL OF HYPOCOTYL 1 (52) are deficient in forming PhyB photobodies. These mutants are defective in aspects of red light response, indirectly suggesting that PhyB photobody formation is important in light responsiveness (21, 33, 52). PhyB photobodies may also play roles in thermomorphogenesis and photomorphogenesis, as well as in regulation of the circadian clock (44, 52). Investigating whether plants use LLPS as an underlying mechanism in the formation of these condensates and those shared with other organisms will provide insight into the evolutionary conservation of mechanisms underlying the formation of these protein bodies.
5. CONCLUDING REMARKS
Research to understand aspects of phase separation and biomolecular condensate formation has matured rapidly over the previous decade; however, exploration of phase separation in the context of plant biology has only recently gained traction. The field of biological phase separation has advanced considerably since its inception. Consequently, plant biologists have the opportunity to adapt methods optimized in other systems to plants as well as to avoid pitfalls that early research in the field may have encountered. Despite these advantages, plants offer unique challenges to studying biomolecular condensates and phase separation, and it will be important for those involved to consider potential pitfalls of various plant-specific methods that have not been encountered in other systems.
To date, only a few studies have directly examined plant biology in the context of phase separation and condensate formation. However, phase separation has already been implicated in several fundamental plant growth and developmental processes, including regulation of phytohormone responsiveness (102), flowering time (35), plant immune response (146), temperature sensing (57), and transport of substrates within the chloroplast (91). Additionally, observations from studies that were not focused on biological phase separation have identified apparent condensates in plants associated with a range of different processes. Such processes include thermomorphogenesis, photomorphogenesis, and the circadian clock (52); the stress response (61); the DNA damage response (48); miRNA biogenesis (36); and phytohormone signaling (71, 142).
Whereas many condensates appear to be shared between plants and animals, plant-specific condensates, including dicing bodies and photobodies, have been identified. Additional plant-specific condensates are likely to be discovered in the coming years. Plants have remarkably distinct lifestyles relative to animals, raising the possibility that plants may have evolved use of protein condensation in unprecedented ways. One already apparent example is the dynamic regulation of photobodies in a light-dependent manner. Plants also face unique environmental challenges relative to animals, such as plants’ inability to regulate internal temperature or fluctuations in water availability. Both of these perturbations are well-established physical determinants of phase behavior; thus, it seems almost inconceivable that plants have not adapted novel mechanisms to regulate intracellular phase separation in response to these environmental changes. This is a topic that will be of interest not only in the context of plant biology, but also for the phase separation field as a whole and for our broader understanding of adaptation and robustness in biology. The exploration of phase separation in plants is essentially wide open, and we anticipate a flurry of new and exciting discoveries in the coming years.
SUMMARY POINTS.
Biomolecular condensates are ubiquitous across biology and represent an emerging mechanism for cellular organization and regulation.
There is strong evidence that the interactions that drive phase separation in vitro underlie the formation of biomolecular condensates in vivo.
The physics of phase separation offers a physical framework through which in vitro and in vivo results can be interpreted and predictions made.
Until recently, study of phase separation was carried out largely in nonplant systems, and thus phase separation in plant biology is a new and exciting area of research.
FUTURE ISSUES.
To what extent is LLPS implicated as the underlying mechanism in the formation of condensates that are shared between organisms from different kingdoms?
How has the inability to regulate temperature impacted how plants regulate intracellular phase transitions?
Do plants use phase separation as a mechanism for sensing environmental changes?
What unique condensates have plants evolved?
How conserved are various condensates within the plant kingdom between different plant species?
How do plants regulate phase separation, given that they can experience sudden changes in water availability (which impacts intracellular volume and pressure)?
ACKNOWLEDGMENTS
This research was funded by the William H. Danforth Plant Science Fellowship (to R.J.E.), the National Science Foundation (IOS-1453650 to L.C.S.), the National Institutes of Health (R35GM136338 to L.C.S.), and start-up funds from Washington University in St. Louis (to A.S.H.). We would like to thank Debbie Maizels of Zoobotanica Scientific Illustration for artwork.
DISCLOSURE STATEMENT
A.S.H. is a scientific consultant with Dewpoint Therapeutics. R.J.E. and L.C.S. are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Alberti S, Dormann D. 2019. Liquid-liquid phase separation in disease. Annu. Rev. Genet 53:171–94 [DOI] [PubMed] [Google Scholar]
- 2.Alberti S, Gladfelter A, Mittag T. 2019. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176:419–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti S, Saha S, Woodruff JB, Franzmann TM, Wang J, Hyman AA. 2018. A user’s guide for phase separation assays with purified proteins. J. Mol. Biol 430:4806–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banani SF, Lee HO, Hyman AA, Rosen MK. 2017. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol 18:285–98 [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensively reviews biomolecular condensates.
- 5.Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, et al. 2016. Compositional control of phase-separated cellular bodies. Cell 166:651–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banjade S, Rosen MK. 2014. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife 3:e04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes AC, Elowsky CG, Roston RL. 2019. An Arabidopsis protoplast isolation method reduces cytosolic acidification and activation of the chloroplast stress sensor SENSITIVE TO FREEZING 2. Plant Signal. Behav 14:1629270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bashandy H, Jalkanen S, Teeri TH. 2015. Within leaf variation is the largest source of variation in agroinfiltration of Nicotiana benthamiana. Plant Methods 11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry J, Brangwynne CP, Haataja M. 2018. Physical principles of intracellular organization via active and passive phase transitions. Rep. Prog. Phys 81:046601. [DOI] [PubMed] [Google Scholar]
- 10.Bishof I, Dammer EB, Duong DM, Kundinger SR, Gearing M, et al. 2018. RNA-binding proteins with basic-acidic dipeptide (BAD) domains self-assemble and aggregate in Alzheimer’s disease. J. Biol. Chem 293:11047–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, et al. 2018. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28:420–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeynaems S, Holehouse AS, Weinhardt V, Kovacs D, Van Lindt J, et al. 2019. Spontaneous driving forces give rise to protein-RNA condensates with coexisting phases and complex material properties. PNAS 116:7889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. 2007. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol 8:574–85 [DOI] [PubMed] [Google Scholar]
- 14.Boke E, Ruer M, Wuhr M, Coughlin M, Lemaitre R, et al. 2016. Amyloid-like self-assembly of a cellular compartment. Cell 166:637–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brady JP, Farber PJ, Sekhar A, Lin YH, Huang R, et al. 2017. Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell–specific protein on phase separation. PNAS 114:E8194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, et al. 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324:1729–32 [DOI] [PubMed] [Google Scholar]; The original paper to propose that liquid-liquid phase separation can drive cellular organization.
- 17.Brangwynne CP, Tompa P, Pappu RV. 2015. Polymer physics of intracellular phase transitions. Nat. Phys 11:899–904 [Google Scholar]; Reviews the process of biological phase separation from the perspective of polymer physics.
- 18.Burke KA, Janke AM, Rhine CL, Fawzi NL. 2015. Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol. Cell 60:231–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X, Jin X, Liu B. 2020. The involvement of stress granules in aging and aging-associated diseases. Aging Cell 19:e13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cascarina SM, Elder MR, Ross ED. 2020. Atypical structural tendencies among low-complexity domains in the Protein Data Bank proteome. PLOS Comput. Biol 16:e1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M, Galvao RM, Li M, Burger B, Bugea J, et al. 2010. Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell 141:1230–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J-M, Dar F, Pappu RV. 2019. LASSI: a lattice model for simulating phase transitions of multivalent proteins. PLOS Comput. Biol 15:e1007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi J-M, Holehouse AS, Pappu RV. 2020. Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys 49:107–33 [DOI] [PMC free article] [PubMed] [Google Scholar]; Key review outlines the thermodynamic principles that underlie phase separation in multivalent biomolecules.
- 24.Choi J-M, Hyman AA, Pappu RV. 2020. Generalized models for bond percolation transitions of associative polymers. Phys. Rev. E 102:042403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, et al. 2009. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell 33:717–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collier S, Pendle A, Boudonck K, van Rij T, Dolan L, Shaw P. 2006. A distant coilin homologue is required for the formation of Cajal bodies in Arabidopsis. Mol. Biol. Cell 17:2942–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutrale F, Rodriguez D, Hortiguela V, Chiu CL, Otterstrom J, et al. 2019. Using enhanced number and brightness to measure protein oligomerization dynamics in live cells. Nat. Protoc 14:616–38 [DOI] [PubMed] [Google Scholar]
- 28.Dignon GL, Best RB, Mittal J. 2020. Biomolecular phase separation: from molecular driving forces to macroscopic properties. Annu. Rev. Phys. Chem 71:53–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan Y, Du A, Gu J, Duan G, Wang C, et al. 2019. PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins. Cell Res. 29:233–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzuricky M, Rogers BA, Shahid A, Cremer PS, Chilkoti A. 2020. De novo engineering of intracellular condensates using artificial disordered proteins. Nat. Chem 12:814–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC, Eckmann CR, et al. 2015. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. PNAS 112:7189–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emenecker RJ, Holehouse AS, Strader LC. 2020. Emerging roles for phase separation in plants. Dev. Cell 55:69–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enderle B, Sheerin DJ, Paik I, Kathare PK, Schwenk P, et al. 2017. PCH1 and PCHL promote photomorphogenesis in plants by controlling phytochrome B dark reversion. Nat. Commun 8:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdos G, Dosztanyi Z. 2020. Analyzing protein disorder with IUPred2A. Curr. Protoc. Bioinform 70:e99. [DOI] [PubMed] [Google Scholar]
- 35.Fang X, Wang L, Ishikawa R, Li Y, Fiedler M, et al. 2019. Arabidopsis FLL2 promotes liquid-liquid phase separation of polyadenylation complexes. Nature 569:265–69 [DOI] [PMC free article] [PubMed] [Google Scholar]; Discusses the role of phase separation in the formation of subnuclear condensates involved in polyadenylation.
- 36.Fang Y, Spector DL. 2007. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol 17:818–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faraco M, Di Sansebastiano GP, Spelt K, Koes RE, Quattrocchio FM. 2011. One protoplast is not the other! Plant Physiol. 156:474–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, et al. 2016. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165:1686–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franzmann TM, Alberti S. 2019. Prion-like low-complexity sequences: key regulators of protein solubility and phase behavior. J. Biol. Chem 294:7128–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujioka Y, Alam JM, Noshiro D, Mouri K, Ando T, et al. 2020. Phase separation organizes the site of autophagosome formation. Nature 578:301–5 [DOI] [PubMed] [Google Scholar]
- 41.Galganski L, Urbanek MO, Krzyzosiak WJ. 2017. Nuclear speckles: molecular organization, biological function and role in disease. Nucleic Acids Res. 45:10350–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginell GM, Holehouse AS. 2020. Analyzing the sequences of intrinsically disordered regions with CIDER and localCIDER. Methods Mol. Biol 2141:103–26 [DOI] [PubMed] [Google Scholar]
- 43.Greig JA, Nguyen TA, Lee M, Holehouse AS, Posey AE, et al. 2020. Arginine-enriched mixed-charge domains provide cohesion for nuclear speckle condensation. Mol. Cell 77:1237–50.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahm J, Kim K, Qiu Y, Chen M. 2020. Increasing ambient temperature progressively disassembles Arabidopsis phytochrome B from individual photobodies with distinct thermostabilities. Nat. Commun 11:1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanazawa M, Yonetani M, Sugimoto A. 2011. PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans. J. Cell Biol 192:929–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harmon TS, Holehouse AS, Rosen MK, Pappu RV. 2017. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife 6:e30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hebert MD. 2013. Signals controlling Cajal body assembly and function. Int. J. Biochem. Cell Biol 45:1314–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirakawa T, Matsunaga S. 2019. Characterization of DNA repair foci in root cells of Arabidopsis in response to DNA damage. Front. Plant Sci 10:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holehouse AS. 2019. IDPs and IDRs in biomolecular condensates. In Intrinsically Disordered Proteins, ed. Salvi N, pp. 209–55. Cambridge, MA: Elsevier [Google Scholar]
- 50.Holehouse AS, Das RK, Ahad JN, Richardson MO, Pappu RV. 2017. CIDER: resources to analyze sequence-ensemble relationships of intrinsically disordered proteins. Biophys. J 112:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hondele M, Sachdev R, Heinrich S, Wang J, Vallotton P, et al. 2019. DEAD-box ATPases are global regulators of phase-separated organelles. Nature 573:144–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H, McLoughlin KE, Sorkin ML, Burgie ES, Bindbeutel RK, et al. 2019. PCH1 regulates light, temperature, and circadian signaling as a structural component of phytochrome B-photobodies in Arabidopsis. PNAS 116:8603–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hubstenberger A, Courel M, Benard M, Souquere S, Ernoult-Lange M, et al. 2017. P-body purification reveals the condensation of repressed mRNA regulons. Mol. Cell 68:144–57.e5 [DOI] [PubMed] [Google Scholar]
- 54.Hyman AA, Weber CA, Jülicher F. 2014. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol 30:39–58 [DOI] [PubMed] [Google Scholar]; Reviews various physical concepts that are foundational to understanding condensate formation.
- 55.Ishida T, Kinoshita K. 2007. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res 35:W460–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs WM, Frenkel D. 2013. Predicting phase behavior in multicomponent mixtures. J. Chem. Phys 139:024108. [DOI] [PubMed] [Google Scholar]
- 57.Jung JH, Barbosa AD, Hutin S, Kumita JR, Gao M, et al. 2020. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 585:256–60 [DOI] [PubMed] [Google Scholar]; Arpion-like domain–containing protein forms condensates in response to temperature fluctuation in plants.
- 58.Kalinina NO, Makarova S, Makhotenko A, Love AJ, Taliansky M. 2018. The multiple functions of the nucleolus in plant development, disease and stress responses. Front. Plant Sci 9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kao YT, Gonzalez KL, Bartel B. 2018. Peroxisome function, biogenesis, and dynamics in plants. Plant Physiol. 176:162–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R. 2017. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol. Cell 68:808–20.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kosmacz M, Gorka M, Schmidt S, Luzarowski M, Moreno JC, et al. 2019. Protein and metabolite composition of Arabidopsis stress granules. New Phytol. 222:1420–33 [DOI] [PubMed] [Google Scholar]
- 62.Kulkarni M, Ozgur S, Stoecklin G. 2010. On track with P-bodies. Biochem. Soc. Trans 38:242–51 [DOI] [PubMed] [Google Scholar]
- 63.Lancaster AK, Nutter-Upham A, Lindquist S, King OD. 2014. PLAAC: a web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 30:2501–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langdon EM, Gladfelter AS. 2018. A new lens for RNA localization: liquid-liquid phase separation. Annu. Rev. Microbiol 72:255–71 [DOI] [PubMed] [Google Scholar]
- 65.Lee HJ, Jung JH, Cortes Llorca L, Kim SG, Lee S, et al. 2014. FCA mediates thermal adaptation of stem growth by attenuating auxin action in Arabidopsis. Nat. Commun 5:5473. [DOI] [PubMed] [Google Scholar]
- 66.Leuzinger K, Dent M, Hurtado J, Stahnke J, Lai H, et al. 2013. Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. J. Vis. Exp 23:50521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li P, Banjade S, Cheng HC, Kim S, Chen B, et al. 2012. Phase transitions in the assembly of multivalent signalling proteins. Nature 483:336–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin YH, Chan HS. 2017. Phase separation and single-chain compactness of charged disordered proteins are strongly correlated. Biophys. J 112:2043–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu JL, Wu Z, Nizami Z, Deryusheva S, Rajendra TK, et al. 2009. Coilin is essential for Cajal body organization in Drosophila melanogaster. Mol. Biol. Cell 20:1661–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Q, Shi L, Fang Y. 2012. Dicing bodies. Plant Physiol. 158:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH. 2003. AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev. 17:410–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo Y, Na Z, Slavoff SA. 2018. P-bodies: composition, properties, and functions. Biochemistry 57:2424–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, et al. 2017. TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95:808–16.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mackenzie S, McIntosh L. 1999. Higher plant mitochondria. Plant Cell 11:571–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maldonado-Bonilla LD. 2014. Composition and function of P bodies in Arabidopsis thaliana. Front. Plant Sci 5:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mao Y, Botella JR, Liu Y, Zhu J-K. 2019. Gene editing in plants: progress and challenges. Natl. Sci. Rev 6:421–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin EW, Holehouse AS. 2020. Intrinsically disordered protein regions and phase separation: sequence determinants of assembly or lack thereof. Emerg. Top. Life Sci 4:307–29 [DOI] [PubMed] [Google Scholar]
- 78.Martin EW, Holehouse AS, Peran I, Farag M, Incicco JJ, et al. 2020. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367:694–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin EW, Mittag T. 2018. Relationship of sequence and phase separation in protein low-complexity regions. Biochemistry 57:2478–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McSwiggen DT, Mir M, Darzacq X, Tjian R. 2019. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev. 33:1619–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milkovic NM, Mittag T. 2020. Determination of protein phase diagrams by centrifugation. Methods Mol. Biol 2141:685–702 [DOI] [PubMed] [Google Scholar]
- 82.Mitrea DM, Chandra B, Ferrolino MC, Gibbs EB, Tolbert M, et al. 2018. Methods for physical characterization of phase-separated bodies and membrane-less organelles. J. Mol. Biol 430:4773–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murthy AC, Dignon GL, Kan Y, Zerze GH, Parekh SH, et al. 2019. Molecular interactions underlying liquid-liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol 26:637–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakagawa C, Nishimura S, Senda-Murata K, Sugimoto K. 2012. A rapid and simple method of evaluating the dimeric tendency of fluorescent proteins in living cells using a truncated protein of importin alpha as fusion tag. Biosci. Biotechnol. Biochem 76:388–90 [DOI] [PubMed] [Google Scholar]
- 85.Norkunas K, Harding R, Dale J, Dugdale B. 2018. Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana. Plant Methods 14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, et al. 2015. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57:936–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oates ME, Romero P, Ishida T, Ghalwash M, Mizianty MJ, et al. 2013. D2P2: database of disordered protein predictions. Nucleic Acids Res. 41:D508–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohtani M 2017. Plant snRNP biogenesis: a perspective from the nucleolus and Cajal bodies. Front. Plant Sci 8:2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oldfield CJ, Dunker AK. 2014. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu. Rev. Biochem 83:553–84 [DOI] [PubMed] [Google Scholar]
- 90.Oshidari R, Huang R, Medghalchi M, Tse EYW, Ashgriz N, et al. 2020. DNA repair by Rad52 liquid droplets. Nat. Commun 11:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouyang M, Li X, Zhang J, Feng P, Pu H, et al. 2020. Liquid-liquid phase transition drives intrachloroplast cargo sorting. Cell 180:1144–59.e20 [DOI] [PubMed] [Google Scholar]; Phase separation is involved in intrachloroplast cargo sorting
- 92.Owen I, Shewmaker F. 2019. The role of post-translational modifications in the phase transitions of intrinsically disordered proteins. Int. J. Mol. Sci 20:5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pak CW, Kosno M, Holehouse AS, Padrick SB, Mittal A, et al. 2016. Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol. Cell 63:72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, et al. 2015. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162:1066–77 [DOI] [PubMed] [Google Scholar]
- 95.Pavlovic M, Plucinski A, Zhang J, Antonietti M, Zeininger L, Schmidt B. 2020. Cascade kinetics in an enzyme-loaded aqueous two-phase system. Langmuir 36:1401–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pederson T 2011. The nucleolus. Cold Spring Harb. Perspect. Biol 3:a000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peran I, Martin EW, Mittag T. 2020. Walking along a protein phase diagram to determine coexistence points by static light scattering. Methods Mol. Biol 2141:715–30 [DOI] [PubMed] [Google Scholar]
- 98.Pérez-González A, Elena C. 2016. Hindrances to the efficient and stable expression of transgenes in plant synthetic biology approaches. In Systems Biology Application in Synthetic Biology, ed. Singh S, pp. 79–89. New Delhi: Springer [Google Scholar]
- 99.Peskett TR, Rau F, O’Driscoll J, Patani R, Lowe AR, Saibil HR. 2018. A liquid to solid phase transition underlying pathological huntingtin exon1 aggregation. Mol. Cell 70:588–601.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piovesan D, Tabaro F, Paladin L, Necci M, Micetic I, et al. 2018. MobiDB 3.0: more annotations for intrinsic disorder, conformational diversity and interactions in proteins. Nucleic Acids Res. 46:D471–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Posey AE, Holehouse AS, Pappu RV. 2018. Phase separation of intrinsically disordered proteins. Methods Enzymol. 611:1–30 [DOI] [PubMed] [Google Scholar]
- 102.Powers SK, Holehouse AS, Korasick DA, Schreiber KH, Clark NM, et al. 2019. Nucleo-cytoplasmic partitioning of ARF proteins controls auxin responses in Arabidopsis thaliana. Mol. Cell 76:177–90.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]; Biomolecular condensates regulate tissue responsiveness to the plant hormone auxin.
- 103.Protter DSW, Parker R. 2016. Principles and properties of stress granules. Trends Cell Biol. 26:668–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pruss GJ, Nester EW, Vance V. 2008. Infiltration with Agrobacterium tumefaciens induces host defense and development-dependent responses in the infiltrated zone. Mol. Plant Microbe Interact 21:1528–38 [DOI] [PubMed] [Google Scholar]
- 105.Qu W, Wang Z, Zhang H. 2020. Phase separation of the C. elegans Polycomb protein SOP-2 is modulated by RNA and sumoylation. Protein Cell 11:202–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quiroz FG, Chilkoti A. 2015. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater 14:1164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reddy AS, Day IS, Gohring J, Barta A. 2012. Localization and dynamics of nuclear speckles in plants. Plant Physiol. 158:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Riback JA, Katanski CD, Kear-Scott JL, Pilipenko EV, Rojek AE, et al. 2017. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168:1028–40.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riback JA, Zhu L, Ferrolino MC, Tolbert M, Mitrea DM, et al. 2020. Composition-dependent thermodynamics of intracellular phase separation. Nature 581:209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rico A, Bennett MH, Forcat S, Huang WE, Preston GM. 2010. Agroinfiltration reduces ABA levels and suppresses Pseudomonas syringae–elicited salicylic acid production in Nicotiana tabacum. PLOS ONE 5:e8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ronald J, Davis SJ. 2019. Focusing on the nuclear and subnuclear dynamics of light and circadian signalling. Plant Cell Environ. 42:2871–84 [DOI] [PubMed] [Google Scholar]
- 112.Rothkamm K, Barnard S, Moquet J, Ellender M, Rana Z, Burdak-Rothkamm S. 2015. DNA damage foci: meaning and significance. Environ. Mol. Mutagen 56:491–504 [DOI] [PubMed] [Google Scholar]
- 113.Rubinstein M, Dobrynin AV. 1997. Solutions of associative polymers. Trends Polym. Sci 5:181–86 [Google Scholar]
- 114.Ruff KM, Dar F, Pappu RV. 2020. Ligand effects on phase separation of multivalent macromolecules. bioRxiv. 10.1101/2020.08.15.252346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruff KM, Roberts S, Chilkoti A, Pappu RV. 2018. Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. J. Mol. Biol 430:4619–35 [DOI] [PubMed] [Google Scholar]
- 116.Saitoh N, Spahr CS, Patterson SD, Bubulya P, Neuwald AF, Spector DL. 2004. Proteomic analysis of interchromatin granule clusters. Mol. Biol. Cell 15:3876–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santner AA, Croy CH, Vasanwala FH, Uversky VN, Van YY, Dunker AK. 2012. Sweeping away protein aggregation with entropic bristles: Intrinsically disordered protein fusions enhance soluble expression. Biochemistry 51:7250–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmidt HB, Görlich D. 2015. Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore–like permselectivity. eLife 4:e04251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schuster BS, Dignon GL, Tang WS, Kelley FM, Ranganath AK, et al. 2020. Identifying sequence perturbations to an intrinsically disordered protein that determine its phase-separation behavior. PNAS 117:11421–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Semenov AN, Rubinstein M. 1998. Thermoreversible gelation in solutions of associative polymers. 1. Statics. Macromolecules 31:1373–85 [Google Scholar]
- 121.Shimada T, Takagi J, Ichino T, Shirakawa M, Hara-Nishimura I. 2018. Plant vacuoles. Annu. Rev. Plant Biol 69:123–45 [DOI] [PubMed] [Google Scholar]
- 122.Snapp EL. 2009. Fluorescent proteins: a cell biologist’s user guide. Trends Cell Biol. 19:649–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spector DL, Lamond AI. 2011. Nuclear speckles. Cold Spring Harb. Perspect. Biol 3:a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su X, Ditlev JA, Hui E, Xing W, Banjade S, et al. 2016. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352:595–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, et al. 2008. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 321:952–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taylor NO, Wei MT, Stone HA, Brangwynne CP. 2019. Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophys. J 117:1285–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Consort UniProt. 2019. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47:D506–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uversky VN. 2019. Intrinsically disordered proteins and their “mysterious” (meta)physics. Front. Phys 7; 10.3389/fphy.2019.00010 [DOI] [Google Scholar]
- 129.Van Buskirk EK, Decker PV, Chen M. 2012. Photobodies in light signaling. Plant Physiol. 158:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, et al. 2018. Pi-pi contacts are an overlooked protein feature relevant to phase separation. eLife 7:e31486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vernon RM, Forman-Kay JD. 2019. First-generation predictors of biological protein phase separation. Curr. Opin. Struct. Biol 58:88–96 [DOI] [PubMed] [Google Scholar]
- 132.Voronina E, Seydoux G, Sassone-Corsi P, Nagamori I. 2011. RNA granules in germ cells. Cold Spring Harb. Perspect. Biol 3:a002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walsh I, Martin AJ, Di Domenico T, Tosatto SC. 2012. ESpritz: accurate and fast prediction of protein disorder. Bioinformatics 28:503–9 [DOI] [PubMed] [Google Scholar]
- 134.Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, et al. 2018. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174:688–99.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Z, Zhang G, Zhang H. 2018. Protocol for analyzing protein liquid–liquid phase separation. Biophys. Rep 5:1–9 [Google Scholar]
- 136.Weber SC. 2017. Sequence-encoded material properties dictate the structure and function of nuclear bodies. Curr. Opin. Cell Biol 46:62–71 [DOI] [PubMed] [Google Scholar]
- 137.Wei M-T, Elbaum-Garfinkle S, Holehouse AS, Chen CC-H, Feric M, et al. 2017. Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat Chem. 9:1118–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weihs D, Mason TG, Teitell MA. 2006. Bio-microrheology: a frontier in microrheology. Biophys. J 91:4296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Woodruff JB, Ferreira Gomes B, Widlund PO, Mahamid J, Honigmann A, Hyman AA. 2017. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169:1066–77.e10 [DOI] [PubMed] [Google Scholar]
- 140.Woodruff JB, Hyman AA, Boke E. 2018. Organization and function of non-dynamic biomolecular condensates. Trends Biochem. Sci 43:81–94 [DOI] [PubMed] [Google Scholar]
- 141.Xue S, Gong R, He F, Li Y, Wang Y, et al. 2019. Low-complexity domain of U1–70K modulates phase separation and aggregation through distinctive basic-acidic motifs. Sci. Adv 5:eaax5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang Z, Tian L, Latoszek-Green M, Brown D, Wu K. 2005. Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Mol. Biol 58:585–96 [DOI] [PubMed] [Google Scholar]
- 143.Yoo H, Triandafillou C, Drummond DA. 2019. Cellular sensing by phase separation: using the process, not just the products. J. Biol. Chem 294:7151–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoo SD, Cho YH, Sheen J. 2007. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc 2:1565–72 [DOI] [PubMed] [Google Scholar]
- 145.Youn JY, Dunham WH, Hong SJ, Knight JDR, Bashkurov M, et al. 2018. High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell 69:517–32.e11 [DOI] [PubMed] [Google Scholar]
- 146.Zavaliev R, Mohan R, Chen T, Dong X. 2020. Formation of NPR1 condensates promotes cell survival during the plant immune response. Cell 182:1093–108.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]; The formation of biomolecular condensates induced by salicylic acid are involved in the regulation of effector-triggered immunity.


