Abstract
Background
Growing evidence has implicated DNA methylation (DNAm) in the regulation of body adiposity; a recent epigenome-wide association study (EWAS) identified a genetic variant determining DNAm at the SREBF1 gene that affected body mass index (BMI).
Objective
In the present study, we tested interactions between DNAm variant rs752579 and methylation metabolism-related B-vitamins (folate, vitamin B2, vitamin B6, and vitamin B12) on longitudinal change in BMI in the Women’s Health Initiative Memory Study (WHIMS).
Design
A total of 5687 white women aged 65–79 from WHIMS with genotyping data on SNP rs752579 were included in the analysis. B-vitamins intakes were estimated by a self-report semi-quantitative food frequency questionnaire. BMI was measured at baseline and 6-year follow-up.
Result
We found significant interactions between the SREBF1 rs752579 genotype and intake of food source B-vitamins on 6-year change in BMI (p interaction <0.01 for all). BMI changes (kg/m2) per DNAm-increasing (C) allele were −0.29, 0.06, and 0.11 within subgroups of increasing tertiles of food source folate intake; and the corresponding BMI changes (kg/m2) were −0.25, −0.01, and 0.15 for vitamin B2 intake; −0.17, −0.16, and 0.21 for vitamin B6 intake; and −0.12, −0.23, and 0.26 for vitamin B12 intake, respectively. Similar gene–diet interaction patterns were observed on the change in body weight.
Conclusions
Our data suggest that habitual intake of food source B-vitamins may modify the effect of DNAm-related variant on long-term adiposity change.
Introduction
More than one-third of US population is currently obese [1]. The rapid rise in obesity over the past 30 years is considered to be due to interactions of changing lifestyle and inherent susceptibility, which is largely determined by genetic or epigenetic variations [2–9].
Growing evidence has implicated DNA methylation (DNAm) in the regulation of body adiposity [10–13]. DNAm is a major epigenetic process by which methyl groups are added to the C-5 position of the cytosine ring of DNA by DNA methyltransferases (DNMTs) [14], and may affect the gene transcription. In a recent epigenome-wide association study, Mendelson et al. [15] identified one CpG site, cg11024682 (intronic to sterol regulatory element binding transcription factor 1 [SREBF1]), was associated with body mass index (BMI) through lipid metabolism pathway [15]. DNAm is profoundly affected by nutrients involved in methyl-metabolism, such as B-vitamins, including folate, riboflavin (B2), pyridoxine (B6), and B12 [16–18]. Interestingly, in a previous study, it was found intakes of B-vitamins significantly interacted with the methylation-associated HIF3A genotype on adiposity change [19]; thus, we hypothesized that similar interactions might exist between B-vitamins and the SREBF1 genotype.
In this study, we analyzed whether the methylation-related genetic variant at SREBF1 interacted with habitual intakes of B-vitamins on long-term change in BMI among participants from the Women’s Health Initiative Memory Study (WHIMS).
Subjects and methods
Study population
The Women’s Health Initiative (WHI) is a long-term national health study that involved 161,808 postmenopausal women aged 50–79 between 1993 and 1998 [20, 21]. The eligibility criteria and recruitment procedures have been reported elsewhere previously [20, 22]. The WHI mainly consists of three clinical trials and one observational study. The Women’s Health Initiative Memory Study (WHIMS) is an ancillary study to WHI Hormone Trials, including a subset of participants from the WHI. Some of the participants from the WHIMS were also enrolled in other one or two WHI trials, namely, calcium and vitamin D intervention, and/or diet modification. Thus, all analyses in the current study adjusted for treatment allocation. We access the data from the National Center for Biotechnology Information (NCBI) database of Genotypes and Phenotypes (dbGap: available at https://www.ncbi.nlm.nih.gov/gap). Phenotype data were extracted from the WHI Clinical Trial and Observational Study (dbGaP study accession number: phs000200.v10.p3). Genotype data were obtained from the WHI Memory Study+GWAS (phs000675.v2.p3). Informed consent was obtained from all participants. The current study includes 5687 white postmenopausal women from WHIMS with available genotype information at baseline. Of them, 5675 (99.8%) provided complete dietary information and nearly half consumed supplemental B-vitamins (supplemental folic acid: 46.5%; vitamin B2: 47%; vitamin B6: 48%; vitamin B12: 47.8%).
Single-nucleotide polymorphism selection and genotyping
The single-nucleotide polymorphism rs752579 within gene SREBF1 was found to be associated with a methylation site at cg11024682 according to an epigenome-wide association study [15]. Genotyping was performed by HumanOmniExpress Exome-8v1_B and imputed to the 1000 genomes. Minimal sample call rate was 97%, and minimum SNP call rate was 98%. Hardy Weinberg p-value less than 1e-4 was excluded.
Assessment of dietary factors and covariates
Dietary intake was assessed by a 145-item validated semi-quantitative food frequency questionnaire (FFQ) that asked about the frequency and portion size of foods or food groups over the past 3 months. Energy and nutrients were calculated by a database derived from University of Minnesota Nutrition Coding Center (MINNESOTA NUTRITION DATA SYSTEM, version 30, Minneapolis, MN). Supplemental source B-vitamins were measured by the pills brought by the participants and calculated by combining intake from supplements, B-complex mixtures, and multivitamins [23]. Due to the mandatory folic acid fortification of grain products in 1996, those whose baseline FFQs were returned post-fortification, folate intake was adjusted to account for the folic acid content change [24, 25]. B-vitamins were adjusted for overall caloric intake. Total energy intake and alternative healthy eating index (AHEI) from the FFQ were included as covariates. AHEI was calculated from each completed FFQ. Details about the measurement characteristics of the WHI FFQ have been published elsewhere [23]. Smoking history was categorized as never smoked, past smoker, and current smoker. Past smoker was defined as those who smoked greater or equal to 100 cigarettes but currently do not smoke. Drinking status was categorized as non-drinker, past drinker, less than one drink per month, less than one drink per week, 1–7 drinks per week, and more than seven drinks per week. Past drinker was defined as those who had consumed greater or equal to 12 alcoholic beverages in the lifetime but do not drink currently. Physical activity was expressed as total energy expended from recreational physical activity per week by incorporating walking, mild, moderate, and strenuous recreational physical activity [26].
Assessment of adiposity and 6-year change in BMI
Height, weight, hip, and waist circumference were measured at the study-designated clinical site by trained personnel according to standardized protocols and calibrated equipment [21]. Height was measured at baseline. Weight, hip, and waist circumference were measured at baseline and annual follow-up after that. BMI was calculated as weight/height2 (kg/m2). A single time measure of adiposity cannot represent the gradual changes, whereas changes in BMI can better reflect the dynamic response of gene–environment interactions [19, 27]. Thus in the current study, we analyzed the 6-year change in BMI as the main outcome.
Statistical analysis
Descriptive statistics were conducted to evaluate the mean and standard deviation for continuous data and frequencies and proportions for categorical data. General linear regression models were used to test the association between rs752579 and adiposity measures, changes of adiposity measures and baseline B-vitamins intake. Multivariable models were adjusted for age (years, continuous), baseline drinking (never, past drinker, <1 drink/month, <1 drink/week, 1–7 drinks/week, 7+ drinks/week), smoking status (never, past, current), total energy intake (kcal) and physical activity (MET-h/week, continuous), AHEI, calcium and vitamin D intervention and diet modification intervention allocation, and other B-vitamins (mutually). Interactions between rs752579 and baseline B-vitamins intake on adiposity measurements were tested by including a multiplicative interaction term in the model. The Bonferroni correction was conducted for multiple comparisons (0.05/8). p-Value was presented as two-sided, and 0.05 were deemed to be significant. All the statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC).
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the WHIMS participants. The mean ± SD age was 68.05 ± 5.89 years. The baseline means (SD) for BMI, body weight, waist, and hip are 28.33 (5.53) kg/m2, 73.74 (15.01) kg, 88.28 (13.18) cm, and 106.96 (11.41) cm respectively. There was no difference in age, body weight, height, waist, hip, physical activity, diet pattern, smoking, drinking, B-vitamins intake, and intervention assignment according to the SREBF1 genotype (data not shown). People with the CC genotype showed lower BMI than other two groups (p = 0.03) and higher total energy intake (p = 0.04) compared to those with TT and CT genotype (data not shown). The (C) allele frequency of SREBF1 rs752579 was 0.39 in WHIMS. All the participants were currently using hormone therapy, 59.24% of them were on calcium and vitamin D trial and 23.23% of them were on diet modification trial.
Table 1.
Baseline characteristics of the study participants (n = 5687)
| Baseline characteristic | Mean (SD) or n (%) |
|---|---|
| Age (years) | 68.05 (5.89) |
| Body weight (kg) | 73.74 (15.01) |
| Baseline BMI | 28.33 (5.53) |
| Waist (cm) | 88.28 (13.18) |
| Hip (cm) | 106.96 (11.41) |
| Energy intake (kcal/day) | 1602 (654.22) |
| Physical activity (MET-h/week) | 11.65 (13.17) |
| Alternative Health Eating Index | 53.52 (10.61) |
| Total folic acid (μg/day) | 454.69 (269.21) |
| Total vitamin B2 (mg/day) | 5.46 (15.92) |
| Total vitamin B6 (mg/day) | 7.49 (31.58) |
| Total vitamin B12 (μg/day) | 19.66 (65.67) |
| Supplemental source vitamins | |
| Supplemental folic acid (μg/day) | 406.61 (169.79) |
| Supplemental vitamin B2 (mg/day) | 7.28 (22.60) |
| Supplemental vitamin B6 (mg/day) | 12.29 (44.70) |
| Supplemental vitamin B12 (μg/day) | 29.00 (93.03) |
| Food source vitamins | |
| Food source folic acid (μg/day) | 265.88 (124.79) |
| Food source vitamin B2 (mg/day) | 2.04 (0.91) |
| Food source vitamin B6 (mg/day) | 1.59 (0.68) |
| Food source vitamin B12 (μg/day) | 5.9 (3.60) |
| Smoking status | |
| Never smoker | 2901 (51.70) |
| Past smoker | 2304 (41.06) |
| Current smoker | 406 (7.24) |
| Drinking | |
| Non-drinker | 626 (11.09) |
| Past drinker | 949 (16.81) |
| <1 drink per month | 743 (13.16) |
| <1 drink per week | 1074 (19.03) |
| 1 to <7 drinks per week | 1463 (25.92) |
| 7+ drinks per week | 790 (13.99) |
| Calcium and Vitamin D Trial (yes) | 3369 (59.24) |
| Diet Modification Trial (yes) | 1321 (23.23) |
| rs752579 | |
| TT | 2109 (37.08) |
| CT | 2737 (48.13) |
| CC | 841 (14.79) |
Data were expressed as mean ± SD, or n (%)
Association of SREBF1 variant with adiposity measures and B-vitamins
We observed a significant association of SREBF1 rs752579 with 6 years change of hip circumference and borderline significant association with waist circumference, but no associations with body weight, BMI, and WHR (Table 2).
Table 2.
Association of rs752579 with measures of adiposity and B-vitamins in WHIMS
| TT | CT | CC | ||
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | p | |
| 6 years change of adiposity measuresa | ||||
| Weight | −0.11 ± 0.25 | −0.39 ± 0.23 | −0.02 ± 0.35 | 0.85 |
| BMI | 0.37 ± 0.09 | 0.19 ± 0.08 | 0.41 ± 0.12 | 0.63 |
| Waist | 1.48 ± 0.41 | 0.86 ± 0.39 | 0.51 ± 0.58 | 0.07 |
| Hip | 0.48 ± 0.37 | −0.07 ± 0.35 | −0.58 ± 0.52 | 0.04 |
| WHR | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.94 |
| Food source B-vitaminsb | ||||
| Folate | 263.08 ± 2.45 | 263.28 ± 2.29 | 262.47 ± 3.39 | 0.91 |
| Vitamin B2 | 2.04 ± 0.01 | 2.03 ± 0.01 | 2.03 ± 0.02 | 0.40 |
| Vitamin B6 | 1.54 ± 0.01 | 1.55 ± 0.01 | 1.57 ± 0.01 | 0.13 |
| Vitamin B12 | 6.07 ± 0.06 | 5.98 ± 0.06 | 5.98 ± 0.08 | 0.16 |
| Supplemental B-vitaminsb | ||||
| Folate | 186.45 ± 6.37 | 188.78 ± 5.96 | 171.79 ± 8.81 | 0.25 |
| Vitamin B2 | 3.58 ± 0.38 | 3.42 ± 0.36 | 3.57 ± 0.53 | 0.89 |
| Vitamin B6 | 5.74 ± 0.75 | 5.92 ± 0.7 | 6.28 ± 1.04 | 0.63 |
| Vitamin B12 | 13.81 ± 1.78 | 13.24 ± 1.67 | 17.47 ± 2.47 | 0.30 |
The general linear regression model was used to test the association of DNA methylation variants with measures of adiposity after adjustment for age, smoking, drinking, Alternative Health Eating Index, B-vitamins, total energy intake, physical activity, calcium and vitamin D intervention, and diet modification intervention. SNP rs752579 was treated as a continuous variable when calculated p-value
The general linear regression model was used to test the association of DNA methylation variants with food sourced B-vitamin and supplemental B-vitamin separately after adjustment for age, smoking, drinking, Alternative Health Eating Index, total energy intake, physical activity, calcium and vitamin D intervention, diet modification intervention, and other B-vitamins (mutually adjusted)
The genetic variant was not associated with the food source or supplemental B-vitamins intake.
Association of B-vitamins intake with 6-year change in BMI and DNAm variant
We found significant associations of baseline total vitamin B12 intake with 6-year BMI change (p for trend 0.04) (Supplementary table 1). Baseline total vitamin B12 was also found to be associated with 6-year body weight change (p = 0.04) and waist circumference (p = 0.04). Across tertiles of vitamin B12 intake, the mean (SD) of BMI changes were 0.06 (0.10) kg/m2, 0.33 (0.10) kg/m2, and 0.37 (0.10) kg/m2, respectively. We did not observe significant associations between baseline intake of folate, vitamin B2, and vitamin B6 with BMI.
Interactions between food source B-vitamins intake and the SREBF1 genotype on 6-year change in BMI
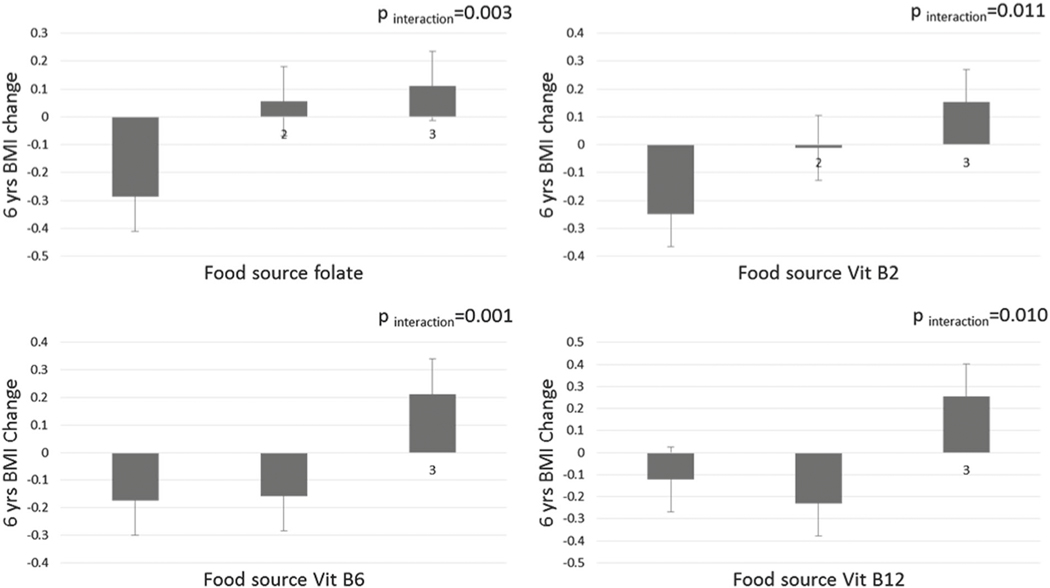
We observed significant interactions between the rs752579 genotype and food source B-vitamins on 6-year change in BMI (p for interaction ≤0.01 for all). BMI changes (standard error [SE]) per DNAm-increasing (C) allele were −0.29 (0.11), 0.06 (0.09), and 0.11 (0.11) kg/m2 within increasing tertiles of food source folate intake; −0.25 (0.1), −0.01 (0.11), and 0.15 (0.1) kg/m2 in tertiles of vitamin B2 intake; −0.17 (0.11), −0.16 (0.1), and 0.21 (0.1) kg/m2 in tertiles of vitamin B6 intake; and −0.12 (0.11), −0.23 (0.1), and 0.26 (0.11) kg/m2 in tertiles of vitamin B12 intake, respectively (Fig. 1). Similar relationships were also found between 6-year body weight change (Table 3), but not for changes of 6-year waist circumference, hip circumference, and WHR. When total or supplement B-vitamins were analyzed, the interactions appeared to be not significant.
Fig. 1.
Differences in 6-year change in BMI allele C of rs7525279 according to baseline intake of food source B-vitamins. Data are beta coefficient ± SE. The general linear model was used to test the genetic association of baseline food source intake of B-vitamins with 6-year change in BMI after adjusted for age, total energy intake, physical activity (MET), baseline BMI, drinking, smoking, alternative health eating index, calcium and vitamin D intervention, diet modification intervention and other B-vitamins (mutually adjusted)
Table 3.
Differences in the 6-year change of adiposity measures per allele of rs752579 according to baseline intake of food source B-vitamins
| Tertile of food source B-vitamins intake | ||||
|---|---|---|---|---|
| T1 | T2 | T3 | p for interaction | |
| BMI change in 6 years | ||||
| Folate | −0.29 ± 0.11 | 0.06 ± 0.09 | 0.11 ± 0.11 | 0.003 |
| Vitamin B2 | −0.25 ± 0.10 | −0.01 ± 0.11 | 0.15 ± 0.10 | 0.011 |
| Vitamin B6 | −0.17 ± 0.11 | −0.16 ± 0.10 | 0.21 ± 0.10 | 0.001 |
| Vitamin B12 | −0.12 ± 0.11 | −0.23 ± 0.10 | 0.26 ± 0.11 | 0.009 |
| Weight change in 6 years | ||||
| Folate | −0.59 ± 0.29 | 0.11 ± 0.26 | 0.31 ± 0.28 | 0.011 |
| Vitamin B2 | −0.89 ± 0.40 | −0.52 ± 0.28 | −0.01 ± 0.29 | 0.024 |
| Vitamin B6 | −0.27 ± 0.29 | −0.46 ± 0.26 | 0.60 ± 0.28 | 0.007 |
| Vitamin B12 | −0.18 ± 0.28 | −0.55 ± 0.26 | 0.63 ± 0.29 | 0.016 |
Data are β ± SE. The general linear model was used to test the genetic association of baseline food source vitamin B intake with 6-year change in adiposity measures after adjustment for age, smoking, drinking, alternative health eating index, total energy intake, physical activity, calcium and vitamin D intervention, and diet modification intervention, and other B-vitamins (mutually adjusted). SNP was treated as a continuous variable when calculating the p-value for interaction
Discussion
In this prospective cohort, we observed significant interactions between DNAm-associated variant at the SREBF1 locus and habitual intakes of food source folate, vitamin B2, vitamin B6, and vitamin B12 on 6-year change in BMI. The DNAm-increasing (C) allele was associated with an increase in BMI among participants with higher intakes of food source B-vitamins (folate, B2, B6, and B12).
The findings from the current analyses are consistent with a previous study in which significant interactions were found between intakes of B-vitamins and DNAm-related genetic variant at the HIF3A locus on the 10-year change in adiposity in the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) [19]. Of note, in the current study, significant interactions were observed only between food source B-vitamins and the SREBF1 genotype. Even though there are no data available to account for the potential mechanisms underlying such observations, we assumed that the discrepancy might be due to several reasons: first, the study populations were considerably different, for example in age—the WHI (on average 68 years) was older than the NHS and HPFS (on average 46 and 55 years, respectively). Aging is among the major factors affecting absorption of B-vitamins. For example, B12 and folate are absorbed better in supplements than from natural foods in older populations. In addition, products encoded by these two genes, HIF3A and SREBF1, have distinct biological functions [28, 29]. It is feasible that these different gene products may interact with B-vitamins through discrete pathways.
Multiple epidemiological studies have found that DNAm was positively associated with BMI [2, 11–13]. In the current study, although we did not measure DNAm directly, the genetic variant acted as a proxy of DNAm according to Mendelian randomization theory [30]. Also, B-vitamins intakes have been related to adiposity in some studies [31–34], although the associations are not entirely consistent. Our result showed that the methylation-increasing (C) allele was associated to opposite-direction BMI change in people with low- vs high habitual B-vitamin intake, indicating modification effects of these methyl-metabolism-related nutrients. Although the mechanism underlying the observed interactions between DNAm variant and food source B-vitamins remained unclear, such interplays are biologically feasible. Vitamin B2, vitamin B12, and folate are the source of coenzymes involved in one-carbon metabolism. In this process, methionine was converted into S-adenosylmethionine (SAM), then DNMTs covalently attach the methyl groups from SAM to carbon-5 position of cytosine bases of DNA [35]. The role of nutrition in one-carbon metabolism and DNAm has been documented in several animal studies [36–39]. In human studies, there are several studies analyzed the relationship between B-vitamins and DNAm at the global level and gene-specific level. Several randomized clinical trials suggested that folic acid supplementation increases DNAm in leukocytes and colorectal mucosa [40–43]. Also, animal studies showed that methyl supplements during the maternal period could increase the methylation level of the offspring [37, 39, 44]. However, there is conflicting evidence showing that exposure to folate could lead to decreased DNAm level, both globally and gene specifically [45, 46]. Vivo studies examining the effect of vitamin B intakes on epigenetic changes in the SREBF1 gene is needed.
The major strengths of this study include the prospective study design, well-validated assessments of nutrient and food intake, the large sample size, and the repeated longitudinal measurements of adiposity. However, several limitations need to be acknowledged. First, we did not measure DNAm directly. However, the genetic marker has been related to methylation levels and can be used as a surrogate measure according to the Mendelian randomization theory [15, 30]. Second, the dietary data were from self-reported food frequency questionnaire (FFQ), measurement errors and recall bias of B-vitamins and other diet factors were inevitable. However, the FFQ data had been well validated [47]. Third, although we have adjusted for several lifestyles and dietary factors in the analysis, residual confounding might still exist due to unmeasured and unknown factors. Fourth, our population was restricted to white women aged 50–79 of European ancestry; whether the result would be generalized to other populations of different age and sex are unknown. Fifth, our analyses were hypothesis-driven and the results were biologically plausible, because B-vitamins play an important role in regulating DNAm and body weight. Even though, we acknowledged that replications were needed to further verify our findings, especially in other racial/ethnic populations. Sixth, some of the interactions would not be considered as statistically significant after the Bonferroni correction. However, the Bonferroni correction is concerned with the stringent null hypothesis that all the multiple tests are independent [48]; notably in our study the dietary factors were not independent. Therefore, a Bonferroni correction might over-adjust multiple comparisons and increase the type II error.
In summary, we found that the DNAm-related SREBF1 genotype interacted with food source B-vitamins, including folate, vitamin B2, vitamin B6, and vitamin B12, are in relation to long-term BMI change. Further studies of other ancestries within different age and sex are warranted to validate our findings.
Supplementary Material
Acknowledgements
We appreciate all the participants in WHIMS for their continued cooperation.
Funding The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States—Israel Binational Science Foundation Grant 2011036. LQ was a recipient of the American Heart Association Scientist Development Award (0730094N).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41366-018-0106-1) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS. Epigenetics and human obesity. Int J Obes (Lond). 2015;39:85–97. [DOI] [PubMed] [Google Scholar]
- 3.Lindgren CM, Heid IM, Randall JC, Lamina C, Steinthorsdottir V, Qi L, et al. Genome-wide association scan meta-analysis identifies three loci influencing adiposity and fat distribution. PLoS Genet. 2009;5:e1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. [DOI] [PubMed] [Google Scholar]
- 5.Qi L, Kraft P, Hunter DJ, Hu FB. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet. 2008;17:3502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal eighteen new loci associated with body mass index. Nat Genet. 2010;42:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Qi Q, Zhang C, Smith SR, Hu FB, Sacks FM, et al. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes. 2012;61:3005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones PA. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–70. [DOI] [PubMed] [Google Scholar]
- 11.Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aïssi D, Wahl S, et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet. 2014;383:1990–8. [DOI] [PubMed] [Google Scholar]
- 12.Martínez JA, Milagro FI, Claycombe KJ, Schalinske KL. Epigenetics in adipose tissue, obesity, weight loss, and diabetes 1, 2. Adv Nutr. 2014;5:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronica L, Joan A, Brennan K, Mi J, Gardner C, Haile RW, et al. Systematic review of studies of DNA methylation in the context of a weight loss intervention. Epigenomics. 2017;9:769–87. [DOI] [PubMed] [Google Scholar]
- 14.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–10. [DOI] [PubMed] [Google Scholar]
- 15.Mendelson MM, Marioni RE, Joehanes R, Liu C, Hedman ÅK, Aslibekyan S, et al. Association of body mass index with DNA methylation and gene expression in blood cells and relations to cardiometabolic disease: a Mendelian randomization approach. PLoS Med. 2017;14:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ly A, Hoyt L, Crowell J, Kim Y-I. Folate and DNA methylation. Antioxid Redox Signal. 2012;17:302–26. [DOI] [PubMed] [Google Scholar]
- 17.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012;23:853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelnutt KP, Kauwell GPA, Gregory JF III, Maneval DR, Quinlivan EP, Theriaque DW, et al. Methylenetetrahydrofolate reductase 677C--T polymorphism affects DNA methylation in response to controlled folate intake in young women. J Nutr Biochem. 2004;15:554–60. [DOI] [PubMed] [Google Scholar]
- 19.Huang T, Zheng Y, Qi Q, Xu M, Ley SH, Li Y et al. DNA methylation variants at HIF3A locus, B-vitamin intake, and long-term weight change: gene-diet interactions in two U.S. Cohorts. Diabetes 2015. 10.2337/db15-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 22.Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–21. [DOI] [PubMed] [Google Scholar]
- 23.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 24.Kessler DA, Shalala DE. Federal Register/Vol. 61, No. 44/Tuesday, March 5, 1996/Rules and Regulations. 1996; 61. [Google Scholar]
- 25.Agnew-Blais JC, Wassertheil-Smoller S, Kang JH, Hogan PE, Coker LH, Snetselaar LG, et al. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the Women’s Health Initiative Memory Study. J Acad Nutr Diet. 2015;115:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer A-M, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi L. Gene-diet interaction and weight loss. Curr Opin Lipidol. 2014;25:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Main AM, Gillberg L, Jacobsen AL, Nilsson E, Gjesing AP, Hansen T, et al. DNA methylation and gene expression of HIF3A: cross-tissue validation and associations with BMI and insulin resistance. Clin Epigenetics. 2016;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi L. Mendelian randomization in nutritional epidemiology. Nutr Rev. 2009;67:439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates CJ, Mansoor MA, Gregory J, Pentiev K, Prentice A. Correlates of plasma homocysteine, cysteine and cysteinyl-glycine in respondents in the British National Diet and Nutrition Survey of young people aged 4–18 years, and a comparison with the survey of people aged 65 years and over. Br J Nutr. 2002;87:71–79. [DOI] [PubMed] [Google Scholar]
- 32.De Laet C, Wautrecht JC, Brasseur D, Dramaix M, Boeynaems JM, Decuyper J, et al. Plasma homocysteine concentration in a Belgian school-age population. Am J Clin Nutr. 1999; 69:968–72. [DOI] [PubMed] [Google Scholar]
- 33.Gunanti IR, Marks GC, Al-Mamun A, Long KZ. Low serum vitamin B-12 and folate concentrations and low thiamin and riboflavin intakes are inversely associated with greater adiposity in Mexican American children. J Nutr. 2014;144:2027–33. [DOI] [PubMed] [Google Scholar]
- 34.Hassapidou M, Fotiadou E, Maglara E, Papadopoulou SK. Energy intake, diet composition, energy expenditure, and body fatness of adolescents in northern Greece. Obesity (Silver Spring). 2006;14:855–62. [DOI] [PubMed] [Google Scholar]
- 35.Folate Selhub J., vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- 36.Vasicek TJ, Zeng L, Guan XJ, Zhang T, Costantini F, Tilghman SM. Two dominant mutations in the mouse fused gene are the result of transposon insertions. Genetics. 1997;147:777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waterland RA, Dolinoy DC, Lin J-R, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;44:401–6. [DOI] [PubMed] [Google Scholar]
- 38.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8:59–65. [DOI] [PubMed] [Google Scholar]
- 39.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pufulete M, Al-Ghnaniem R, Khushal A, Appleby P, Harris N, Gout S, et al. Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut. 2005;54:648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cravo ML, Pinto AG, Chaves P, Cruz JA, Lage P, Nobre Leitao C, et al. Effect of folate supplementation on DNA methylation of rectal mucosa in patients with colonic adenomas: correlation with nutrient intake. Clin Nutr. 1998;17:45–49. [DOI] [PubMed] [Google Scholar]
- 42.Cravo M, Fidalgo P, Pereira AD, Gouveia-Oliveira A, Chaves P, Selhub J, et al. DNA methylation as an intermediate biomarker in colorectal cancer: modulation by folic acid supplementation. Eur J Cancer Prev. 1994;3:473–9. [DOI] [PubMed] [Google Scholar]
- 43.Kim YI, Baik HW, Fawaz K, Knox T, Lee YM, Norton R, et al. Effects of folate supplementation on two provisional molecular markers of colon cancer: a prospective, randomized trial. Am J Gastroenterol. 2001;96:184–95. [DOI] [PubMed] [Google Scholar]
- 44.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–400S. [DOI] [PubMed] [Google Scholar]
- 45.Vineis P, Chuang S-C, Vaissiere T, Cuenin C, Ricceri F, Johansson M, et al. DNA methylation changes associated with cancer risk factors and blood levels of vitamin metabolites in a prospective study. Epigenetics. 2011;6:195–201. [DOI] [PubMed] [Google Scholar]
- 46.Hoyo C, Murtha AP, Schildkraut JM, Jirtle RL, Demark-Wahnefried W, Forman MR, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 48.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.