Abstract
There is growing interest in the alterations in body composition (BC) that accompany rheumatoid arthritis (RA). The purpose of this review is to (i) investigate how BC is currently measured in RA patients, (ii) describe alterations in body composition in RA patients and (iii) evaluate the effect on nutrition, physical training, and treatments; that is, corticosteroids and biologic Disease Modifying Anti-Rheumatic Disease (bDMARDs), on BC in RA patients.
The primary-source literature for this review was acquired using PubMed, Scopus and Cochrane database searches for articles published up to March 2021. The Medical Subject Headings (MeSH) terms used were ‘Arthritis, Rheumatoid’, ‘body composition’, ‘sarcopenia’, ‘obesity’, ‘cachexia’, ‘Absorptiometry, Photon’ and ‘Electric Impedance’. The titles and abstracts of all articles were reviewed for relevant subjects.
Whole-BC measurements were usually performed using dual energy x-ray absorptiometry (DXA) to quantify lean- and fat-mass parameters. In RA patients, lean mass is lower and adiposity is higher than in healthy controls, both in men and women. The prevalence of abnormal BC conditions such as overfat, sarcopenia and sarcopenic obesity is significantly higher in RA patients than in healthy controls; these alterations in BC are observed even at an early stage of the disease. Data on the effect treatments on BC in RA patients are scarce. In the few studies published, (a) creatine supplementation and progressive resistance training induce a slight and temporary increase in lean mass, (b) exposure to corticosteroids induces a gain in fat mass and (c) tumour necrosis factor alpha (TNFα) inhibitors might be associated with a gain in fat mass, while tocilizumab might be associated with a gain in lean mass.
The available data clearly demonstrate that alterations in BC occur in RA patients, but data on the effect of treatments, especially bDMARDs, are inconsistent and further studies are needed in this area.
Keywords: body composition, body fat and lean mass, dual energy x-ray absorptiometry, rheumatoid arthritis
Background
Rheumatoid arthritis (RA) is a systemic, immune-mediated disease, characterised by synovitis and/or inflammation of periarticular structures and systemic inflammation. 1 RA is the most prevalent inflammatory rheumatic disease, with estimated prevalence rates in European adults ranging from 3.1 to 8.5 cases per 10,000. 2 Patients with RA exhibit alterations in quality of life and a high prevalence of fatigue and depression.3,4 The disease also constitutes a major global health burden, as measured in disability-adjusted life years (DALYs). 5
Twenty years ago, RA often led to joint destruction and considerable disability. Since then, scientific progress has prompted major advances in the treatment of the disease. Disease Modifying Anti-Rheumatic Drugs (DMARDs), such as conventional synthetic DMARDs (csDMARDs, e.g. methotrexate), biologic DMARDs [bDMARDs, e.g. tumour necrosis factor alpha (TNFα) inhibitors] and targeting synthetic DMARDs [tsDMARDs, e.g. janus kinase (JAK) inhibitors], can control disease activity and thus prevent joint destruction.6–9 Moreover, in the last 20 years, RA has become milder, with patients exhibiting fewer severe extra-articular manifestations and lower mortality rates.10,11 However, patients with RA still exhibit excess mortality, especially from cardiovascular disease. 12 As such, for RA patients, better evaluation of comorbidities and patients’ cardio-metabolic profiles is essential.13,14
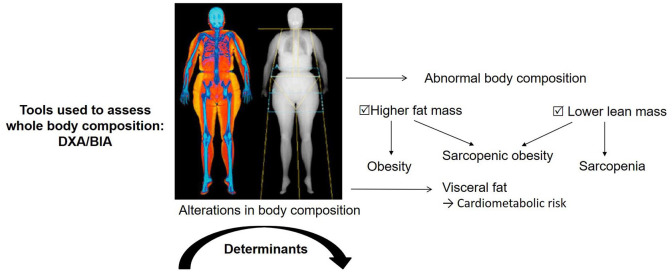
In addition to joint damage and comorbidities, alterations in body composition have been observed in patients with RA.15,16 Systemic inflammation is the main factor underlying these alterations, but several other factors, such as malnutrition, physical disability, comorbidities, corticosteroids and bDMARDs, may also induce alterations in body composition in RA patients.17,18 Evidence suggests that the proportions and distribution of fat and lean mass throughout the body have important implications for health. Low lean mass and excess body fat are predictors of poor health outcomes in the general population. 19 Low lean mass at its most extreme (a condition known as cachexia) may lead to weakness, disability and metabolic abnormalities.20–22 Gains in fat mass may predispose to obesity, diabetes, hypertension and risk of cardiovascular disease.12,23–25 A better understanding of the alterations in body composition that occur in RA patients (including those receiving bDMARDs) is important because of the many potential implications in terms of outcome, such as disability and cardio-metabolic risk.
The purpose of this review is three-fold: (i) to investigate how body composition is currently measured in RA patients, and which parameters are associated with abnormal body composition conditions in those patients, (ii) to investigate alterations in body composition in RA patients compared with healthy controls and (iii) to investigate the effect of nutrition, physical training and treatments (corticosteroids and bDMARDs) on body composition in RA patients.
Methods
Data sources
For this manuscript, the authors searched the PubMed, Scopus and Cochrane databases for articles published up to March 2021. We also searched for conference abstracts from selected Rheumatology meetings: European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR); and from Nutrition meetings: International Congress of Nutrition, European Nutrition Conference and American Society of Nutrition, from 2018 to 2020. We manually reviewed them for inclusion.
Search terms
The Medical Subject Headings (MeSH) terms used were ‘Arthritis, Rheumatoid’, ‘body composition’, ‘sarcopenia’, ‘obesity’, ‘cachexia’, ‘Absorptiometry, Photon’ and ‘Electric Impedance’, in the following combination: [‘Arthritis, Rheumatoid’ (Mesh)] AND [‘Body Composition’ (Mesh) OR ‘Electric Impedance’ (Mesh) OR ‘Obesity’ (Mesh) OR ‘Cachexia’ (Mesh) OR ‘Sarcopenia’ (Mesh)].
Inclusion/exclusion criteria
Searches were restricted to adults ⩾18 years old and human studies in the English language. All types of original studies, including clinical trials, cohort studies, case-control studies, conference abstracts and cross-sectional studies, as well as meta-analyses and reviews were included and analysed. As the definition of sarcopenia has changed with time, we included studies in which sarcopenia is fully described and publications in which patients are reported as having sarcopenia defined as low muscle mass. Protocols, case reports and studies which did not assess whole-body composition were excluded. The terms ‘bone assessment’ and ‘osteoporosis’ were excluded from our search. Indeed, bone fragility in patients with RA is beyond the scope of this paper and a detailed discussion of this topic can be found in excellent reviews elsewhere.26–28
The results of the bibliographic search are shown in Supplemental Figure 1.
Measurement of body composition in RA patients
Definition of body composition
The term ‘body composition’ does not refer to an anatomic view of the body, with a description of each tissue and its localization. Instead, it refers to a description of the body from a chemical point of view. For example, the three-compartment model includes two major components (fat mass and fat-free mass) and a minor component (mineral content). 29 Body composition is not stable. It is dynamic and variable, with changes occurring in response to external or internal stimuli. 30 Body composition is a ‘living memory’ of what we have eaten or experienced. Experiences that affect body composition include trauma, infections and chronic systemic inflammation. 31 The assessment of body composition provides insights into both the nutritional status and functional capacity of the human body. 32 Several techniques are used to measure it. In this review, we focus on those techniques that are used to assess whole-body composition, such as dual energy x-ray absorptiometry (DXA) and bioelectrical impedance analysis (BIA). Body parts assessment of body composition using computed tomography (CT), (high resolution) peripheral quantitative CT, bedside ultrasound (US), or magnetic resonance imaging (MRI) is beyond the scope of this review.
Currently available techniques (Supplemental Table 1)
Dual energy x-ray absorptiometry
Dual energy x-ray absorptiometry (DXA) was primarily used to assess bone mineral density (BMD) and to diagnose osteoporosis. 33 DXA measures energy absorption at two X-ray photon energy levels. By measuring the intensities of the absorbed energy in this way, it is possible to differentiate between bone, fat mass and fat-free mass (FFM), which is also known as ‘soft tissue lean mass’ (i.e. non-bone and non-fat soft tissue), as since these tissues have different X-ray attenuation profiles. 34 Nowadays, DXA is recognized as the gold standard for assessing body composition. 35 It provides a means of quantifying fat and lean masses, both in a single body region (e.g. arms and legs) and at the whole-body level. With the latest generation of densitometers, body composition can be assessed with a single whole-body scan, with low exposure to radiation [1 µSv, less than a standard X-ray (100 µSv)] and fast acquisition times. 34 Other benefits of this technology include simplicity, moderate cost, availability and accuracy. DXA is also used to assess BMD and visceral adipose tissue (VAT). VAT is associated with metabolic syndrome and cardiovascular risk.36–39 However, there are limitations to using DXA to assess body composition: trunk muscles, for instance, are difficult to evaluate using DXA. Therefore, if DXA fat mass and lean mass measurements are derived from arms and legs, they might over- or under-estimate the extent of low muscle mass and obesity. 40 In addition, oedema or dehydration can bias DXA muscle and fat measurements. 41
Bioelectrical impedance analysis
BIA is used to assess body composition by passing a low intensity (500–800 mA), high frequency (50 kHz) current through the body and then measuring several electrical parameters (resistance, reactance, impedance and phase angle). The measurements obtained provide indirect estimates of whole-body composition. BIA assesses total body water and can predict the amount of FFM. Its use is based on several assumptions, namely that (1) the human body is divided into five cylinders, that is, the trunk and the upper and lower extremities, with uniform electric conductivity; (2) FFM contains virtually all of the water and conduction electrolytes in the body; (3) FFM hydration is constant and (4) conductive length equates to stature. 42 BIA can be used to assess body composition in elderly and obese patients, as well as in patients with cancer or infections.42–44 The benefits of BIA include ease of use, affordability, portability of the device, absence of radiation exposure and non-invasiveness of the technique. It appears to correlate well with DXA and CT.38,45 The limitations of BIA as a means of assessing body composition include the fact that (1) it is an indirect method, (2) it cannot be used to assess BMD and VAT and (3) every specific population assessment requires its own equation. 45
Parameters measured by DXA
Of all the techniques used to assess body composition, DXA seems to be the best compromise between accuracy and accessibility. As DXA is the technique used in most of the studies included in this review, it is important to explain the main parameters of this technique.
In 2019, the International Society for Clinical Densitometry (ISCD) published an updated official position on whole-body composition assessment with DXA. 46 According to the ISCD, where adults are concerned, total body (with head) values of body mass index (BMI), total body mass (TBM), total lean mass (TLM), total fat mass (FM) and percent body fat (PBF) should appear on all reports (Supplemental Table 2). Moreover, optional DXA measurements of adiposity and lean mass include VAT (cm²), fat mass index (FMI: FM/height²), appendicular lean mass (ALM, kg), appendicular lean mass index (ALMI: ALM/height²) and skeletal muscle index (SMI: TLM/height²). Data are also lacking on changes in VAT. Indeed, using DXA to measure VAT is a recent practice, but not all DXA machines are designed to do so. Fat-free mass and fat-free mass index (FFMI: FFM/height²) are sometimes reported. The clinical utility of these latter measurements (FFM and FFMI) is currently uncertain. As such, only the recommended DXA parameters and optional DXA parameters, if available, are reported from the studies included in this review.
Categories of abnormal body composition conditions
Overfat
The term ‘obesity’ is commonly used to refer to a BMI ⩾ 30 kg/m². However, in RA studies, the term ‘overfat’ is used to refer to excess adiposity (PBF). For instance, in the criteria proposed by Cesari et al., 47 people aged 60–79 years-old were defined as ‘overfat’ if their PBF was >31% in white men, >29% in African American men, >29% in Asian men, >43% in white women, >41% in African American women and >41% in Asian women.
Sarcopenia
Sarcopenia is a condition that is commonly associated with ageing. It is characterised by the loss of muscle mass and muscle strength, leading to impaired muscle function. However, in RA studies on body composition, ‘sarcopenia’ is generally used to refer to low lean mass (ALM, ALMI or SMI) alone using various cut-offs (Supplemental Table 3).19,35,48–55 For instance, in the criteria proposed by Baumgartner et al., 48 the mean ALMIs of young male and female reference groups, minus two standard deviations (SDs), were defined as the gender-specific cut-offs for sarcopenia, that is, 7.26 kg/m² for men and 5.5 kg/m² for women.
A quantitative assessment is not enough to establish a diagnosis of sarcopenia. A comprehensive assessment of muscle, muscle strength and muscle function are also required. Sarcopenia is characterised by a loss of lean mass leading to impaired muscle function. 56 It could also lead to physical disability, falls, fractures and even cardiovascular diseases.57,58 While many definitions of sarcopenia have been proposed, the most commonly accepted definition is the one published in 2010 by the European Working Group on Sarcopenia in Older People (EWGSOP). 35 In 2019, the EWGSOP published an update to the definition (Supplemental Figure 2). Sarcopenia is now considered a muscle disease (muscle failure), with low muscle strength superseding low muscle mass as a principal determinant. If there is a suspicion of sarcopenia, muscle strength must be assessed. 19 Sarcopenia is probable if low muscle strength is established (i.e. grip strength <26 kg for men and <16 kg for women OR chair stand >15s for five rises). The diagnosis is confirmed if low muscle mass (i.e. ALM/height² <7.0 kg/m² for men and <5.5 kg/m² for women, OR ALM <20 kg for men and <15 kg for women:) or quality (i.e. gait speed ⩽0.8 m/s, Short Physical Performance Battery ⩽8 point score, Timed-up-and-go test ⩾20s or non-completion or ⩾6 min for completion of 400 m walk test) is also established. When low muscle strength, low muscle mass/quality and low physical performance are all established, sarcopenia is considered severe. 19
Sarcopenic obesity
In RA studies on whole-body composition, the term ‘sarcopenic obesity’ is used to refer to a loss of lean mass concurrent with excess adiposity (PBF). For instance, according to Giles et al., 16 sarcopenic obesity was defined as fulfilling the criteria for both sarcopenia (as proposed by Janssen et al. 49 ) and overfat (as proposed by Cesari et al. 47 ).
Rheumatoid cachexia
The term ‘classic cachexia’ is used to refer to the condition that is characterised by severe loss of weight, fat and muscle mass, accompanied by an increase in protein catabolism due to underlying disease(s). 59
Despite numerous publications on ‘rheumatoid cachexia’ (RC), there is no consensus on the clinical criteria for its diagnosis. RC is a dramatic change in body composition characterised by the loss of muscle mass with or without loss of fat mass, resulting in no or limited changes in BMI. 60
The most used criteria for establishing RC are those proposed by Engvall et al., 61 that is FFMI below the 10th percentile and FMI above the 25th percentile. Data from a Swiss population sample of healthy adults (2986 men and 2649 women) were used to establish a body composition index that determines RC.
Sarcopenia and rheumatoid cachexia: two different conditions!
As the loss of muscle mass is commonly evaluated in both RC and sarcopenia, these syndromes are often confused with one another. However, the diagnosis of sarcopenia is established based on an assessment of muscle alone, while the diagnosis of RC is based on an assessment of both muscle and fat.
Alterations in body composition in rheumatoid arthritis
Body composition in RA patients was assessed (mainly by DXA) in several studies. We drew a distinction between studies that included patients with early RA and those that included patients with established RA. Moreover, data on the prevalence of abnormal body composition conditions (sarcopenia, overfat, sarcopenic obesity and RC) are also reported, if available. Results are summarized in Table 1.
Table 1.
Body composition in patients with early and established RA versus controls.
| References | Patients (n) | Controls | Mean age in years | Disease duration | Conclusion |
|---|---|---|---|---|---|
| Book et al. 15 | 132 (95F/37M) | 132 (95F/37M) matched for current smoking when possible. | 58.4 ± 15.8 (F) | 7.5 ± 2.8 months (F) | Lower ALM, higher BMI, higher FM and higher trunk fat mass in Female RA patients |
| 64.5 ± 9.7 (M) | 7.3 ± 2.7 months (M) | Lower TLM and lower ALM in Male RA patients | |||
| Book et al. 62 | 63 patients (45F/18M) 63 controls matched for sex and age |
Early treatment with DMARDs | 58.3 ± 15.7 in F patients 66.2 ± 11.5 in M patients |
7.2 ± 2.9 in F patients | In both men and women TLM decreased significantly less in patients compared with controls, p = 0.002 for women and p = 0.002 for men |
| 7.6 ± 3.0 in M patients | FM increased significantly less in female patients compared with female controls (p = 0.002) | ||||
| Dao et al. 63 | 105 F | 105 matched for age | 56.3 ± 8.7 | 21.6± 2.8 months | Higher BMI, FM and PBF in RA patients Higher prevalence of sarcopenia, overfat and sarcopenic obesity in Female RA patients. |
| Turk et al. 64 | 317 (219F/98M) | 1268 (875F/393M) from the Rotterdam Study II 3 and matched for ethnicity, gender and age | 61.0 ± 7.0 | 7 (3–22) months | Lower ALMI (both in F and M), higher BMI and FMI (F) |
| Sarcopenia 4–5 times more common in RA patients than in controls | |||||
| Müller et al. 65 | 91 (66F/25M) | 328 matched for sex and age | 52 ± 2 | 215 ± 24 days | Higher PBF and lower in RA patients |
| Giles et al. 16 | 189 (117F/72M) | 189 matched for sex, race and age | 59.9 ± 8.4 | 9 (5–17) years | Higher BMI, higher FM, higher FMI, higher trunk fat mass, higher PBF and lower TLM/FM in Female RA patients |
| Higher prevalence of sarcopenia, overfat and sarcopenic obesity in Female RA patients. | |||||
| Lower ALM in Male RA patients | |||||
| Reina et al. 66 | 89F | 100 matched for age | 62 ± 8 | 13.7 ± 9.5 years | ALM, ALMI and TLM lower in RA patients |
| Higher prevalence of sarcopenia in RA patients than in controls | |||||
| Elkan et al. 67 | 60 (50F/10M) | Age- and sex-matched European reference population | F: 65.5 (60.0–75.0) M: 60.5 (55.0–67.0) |
F: 13.0 (9.0–23.0) years M: 15.5 (6.0–24.0) years |
Higher BMI and lower FFM/FFMI in F RA patients. Higher FM and FMI in RA patients compared with reference population |
| Lemmey et al. 68 | 82 (53F/29M) | 85 matched for sex and age | 60.9 ± 11.7 | 23.8 ± 19.0 months | Higher PBF and FM and lower % ALM in RA patients |
| Delgado-Frías et al. 69 | 100F | 98 matched for age | 55.6 ± 9.3 | NR | No difference between the two groups |
| Ngeuleu et al. 70 | 123 (107F/16M) | No controls | 52.3 ± 13.2 | 9.8 ± 8 years | 40% of patients diagnosed with sarcopenia |
| Vliestra et al. 71 | 82 (60F/15M) | 75 patients with osteoarthritis (45F/30M) | 61.1 (13.3) | NR | 17.1% of RA patients diagnosed with sarcopenia versus 29.3% of patients with osteoarthritis (non-significant) |
| Higher prevalence of sarcopenic obesity in patients with osteoarthritis | |||||
| Torii et al. 72 | 388F | No controls | 65 (54.3–72.0) | 9 (4.0–21.0) years | 37.1% of patients classified as having sarcopenia (14.7%: severe sarcopenia, 22.4%: sarcopenia) |
| Lin et al. 73 | 457 (378F/79M) | 1860 non matched | 49.5 ± 13.1 | 54 (24–118) months | Lower ALMI in RA patients |
| Tada et al. 74 | 100 (78F/22M) | No controls | 68.0 (59.0, 76.0) | 5.5 (1.2, 11.3) years | 28% of patients diagnosed with sarcopenia (F: 26.9%; M: 31.8%). |
| Mochizuki et al. 75 | 240 (189F/51) | No controls | 75.0 ± 6.2 | 13.8 ± 11.1 | 29.6% of patients diagnosed with sarcopenia. |
| Feklistov et al. 76 | 40F | 40F controls | 63 ± 7 | NR | Sarcopenia occurred in 10 (25%) RA patients and in 5 (12.5%) people without RA (p > 0,05) |
| Kondrashov et al. 77 | 110M | 30 controls matched for age and BMI | 59 (53; 65) | NR | Sarcopenia detected in 66 (60%) of RA patients, whereas in the control group it was absent. |
| Casabella et al. 78 | 55F | 55 controls | 58 ± 12 | NR | Nineteen RA patients (35%) presented sarcopenia |
| Park et al. 79 | 294 | No controls | NR | NR | 8.2% of 294 RA patients had sarcopenia at the time of enrolment |
| El Maghraoui et al. 80 | 178 (147F/31M) | No controls | 54.1 ± 11.5 | 8.9 ± 7.4 years | 53.9% of patients diagnosed with RC (F: 53.7% and M: 54.8%) |
| Engvall et al. 61 | 60 (50F/10 M) | No controls | F: 66.0 (63.0–69.0) M: 60.0 (51.0–70.0) |
F: 13 (9–23) years M: 16.0 16 (6–24) years |
38% of patients categorized as having RC |
| Hugo et al. 81 | 57 (41F/16M) | No controls | 57.6 ± 10.2 | 3.8 ± 3.0 years | 18% of patients categorized as having RC |
| Elkan et al. 82 | 80 (61F/19M) | No controls | F: 60.8 (57.3–64.4) M: 63.4 (59.8– 66.9) |
F: 6.0 (2.0–15.0) M: 5.0 (3.0–9.0) |
18% of F patients and 21% of M patients categorized as having RC |
| Santo et al. 83 | 90 (78F/12M) | No controls | 56.5 ± 7.3 | 8.5 (3.0–18.0) years | 13.3% of patients categorized as having RC |
Results are expressed as mean ± standard deviation or median (25th–75th percentile).
ALM, appendicular lean mass; ALMI, appendicular lean mass index; BC, body composition; BMI, body mass index; F, female; FFM, fat free mass; FFMI, fat free mass index; FM, total fat mass; FMI, fat mass index; M, male; NR, not reported; PBF, body fat percentage; RA, rheumatoid arthritis; RC, rheumatoid cachexia; TLM, total lean mass.
Early rheumatoid arthritis
We found four case-control studies (n = 105–317 cases) in which alterations in body composition were assessed in patients with early RA.15,62–65
In 2009, Book et al. 15 compared with 132 early RA patients (women: 72%; mean age: ~60 years old; disease duration: ⩽1 year) with controls matched one-to-one for age and sex. All of the patients who had been treated with corticosteroids or csDMARDs prior to inclusion had been treated for fewer than 30 days. Fewer women (31%) than men (38%) had been treated with corticosteroids, with a mean daily dose of 6.5 and 8.0 mg, respectively. Body composition was assessed by DXA. Appendicular lean mass was lower in RA patients compared with controls in both women and men (women: 16.8 versus 17.6 kg, p = 0.007; men: 23.0 versus 25.8 kg, p < 0.001). TLM was significantly lower in RA men (but not RA women) (54.4 versus 58.3 kg, p = 0.012), whereas weight, BMI and FM were significantly higher in RA women (but not RA men) (68.1 versus 64.0 kg, p = 0.016; 25.1 versus 23.7 kg/m², p = 0.012; and 26.1 versus 23.0 kg, p = 0.014, respectively).
In a longitudinal follow-up of their previous study, Book et al. 62 compared 63 RA patients (women: 71%; mean age of women: 58.3 years; mean age of men: 60.7 years; mean disease duration: 7 months) with 63 healthy controls matched for age and sex. At 2 years, the changes observed in the RA group were generally less pronounced than those observed in the control group. In both men and women, loss of TLM was significantly less in RA patients compared with controls (women: −0.29 versus −1.48 kg, p = 0.002; men: 0.09 versus −2.94 kg, p = 0.002) and gain in FM was significantly less in female patients compared with female controls (0.44 versus 2.83 kg, p = 0.002). 62 However, due to the small sample size and the modest follow-up time, no definitive conclusions can be drawn and this study was conducted in the pre-bDMARDs era.
The occurrence of alterations in body composition in early RA was confirmed in three studies.63–65 In 105 Vietnamese women with RA (disease duration ⩽3 years) compared with healthy age-matched controls, mean weight and BMI values were similar, but higher FM (19.1 versus 16.9 kg, p = 0.007) and lower ALM (12.9 versus 14.1 kg, p = 0.02) were observed. 63 Moreover, the prevalence of abnormal body composition conditions was significantly higher in RA patients compared with controls (all p < 0.001): (i) sarcopenia (18.1 versus 9.5%, using the criteria proposed by Hull et al. 84 ), (ii) overfat (41.9 versus 31.4%, using the criteria proposed by Gallagher et al. 85 ) and (iii) sarcopenic obesity (12.4 versus 3.8%, using the criteria for both sarcopenia and overfat). 63 In another recent study, the cohort was composed of 317 early arthritis patients (symptom duration ⩽2 years; 84% RA according to 2010 ACR/EULAR criteria 86 ) with no prior treatment with DMARDs. 64 Mean age was 61 ± 7 years and mean BMI was 28.0 kg/m². The cohort was compared with 1268 non-arthritis controls from the Rotterdam study, matched one-to-four for age, gender and ethnicity (Caucasian, African or Asian). 87 Compared with controls, BMI and FMI were higher (both p < 0.01) in arthritic women (but not in men), ALMI was lower in both sexes (p < 0.01) and sarcopenia was 4–5 times more common in early arthritis patients than in controls (women: 5.5 versus 1.3%; men: 8.2 versus 1.5%; both p < 0.01; using the criteria proposed by Baumgartner et al. 48 ). Similar results (higher PBF and lower ALM in RA patients) were found in an Estonian study. 65 In that study, the authors compared the DXA-acquired body composition data of 91 early RA patients (72% women) with those of 328 controls (54%) women. 41.8% of the early RA patients and 19.8% of the controls were classified as having sarcopenia and 68.1% of the early RA patients and 47.3% of the controls were overfat.
Compared with healthy controls, patients with early RA exhibit alterations in body composition, with higher fat mass and lower lean mass leading to abnormal body composition conditions.
Established rheumatoid arthritis
We found five case-control studies (n = 60–189 cases) in which alterations in body composition were assessed in patients with established RA.16,66–69
In 2008, Giles et al. 16 reported alterations in body composition in a cohort of 189 RA patients (women: 62%; mean disease duration: 9 years; no previous exposure to bDMARDs: 55%). The RA patients were compared with non-RA controls matched for age, gender, weight and ethnicity. Weight, BMI and FM were significantly higher in women with RA (all p < 0.05), but not in men, compared with controls. No significant differences were found between men with RA and controls in any body composition parameter except for ALM, which was lower (24.7 versus 26.1 kg, p = 0.039). A significant association was found between RA status and greater prevalence of abnormal body composition conditions in women, but not in men; (i) sarcopenia (21.4 versus 7.7%, p = 0.004, using the criteria proposed by Janssen et al. 49 ), (ii) overfat (57.3 versus 35.0%, p = 0.001, using the criteria proposed by Cesari et al. 47 ) and (iii) sarcopenic obesity (11.1 versus 2.6%, p = 0.008).
These results were confirmed in most studies,66–68 but not all. 69 Reina et al. 66 reported alterations in body composition in a case-control study of 89 women with RA (mean disease duration: 13.7 years). The control group was composed of 100 patients with non-inflammatory rheumatic disorders. TLM and ALM were significantly lower (p < 0.001) in RA patients compared with controls and no difference in FM was observed. RA patients fulfilled the criteria for sarcopenia in 44% of cases versus 19% of controls (p < 0.001, using the criteria proposed by Janssen et al. 49 ). In a study of 60 RA patients (women: 83%; mean disease duration: 13 years; receiving DMARDs such as methotrexate, sulfasalazine, leflunomide and TNFα inhibitors: 68%). Elkan et al. 67 reported higher FM (p < 0.05) both in women and men, and lower TLM (p < 0.001) in women (but not in men) compared with the reference population. In a case-control study, Lemmey et al. 68 compared 82 RA patients treated exclusively with csDMARDs (mainly methotrexate) in a treat-to-target (T2T) protocol with 85 matched controls. Although the RA patients responded well to the treatment (mean DAS28=2.8, with 49% in remission), they had lower ALM (−9.9%, p < 0.001) and higher FM (+26.5%, p < 0.001) than controls. 68
Compared with controls, patients with established RA also have higher fat mass and lower lean mass leading to abnormal body composition conditions. Moreover, the benefits of the T2T strategy in reducing inflammation (i.e. disease activity) failed to improve body composition parameters.
Sarcopenia in patients with RA
We found ten cross-sectional studies (n = 40–457 RA patients) that specifically sought to evaluate the prevalence of sarcopenia in established RA.70–79
Although sarcopenia seems to be more frequent in RA patients compared with controls,15,16,63,64,66 it is difficult to provide conclusive evidence since the studies used different definitions for sarcopenia and involved different populations. In a cross-sectional study of 123 Moroccan RA patients (women: 87%; mean disease duration: 9.8 years; patients receiving csDMARDs: 90.2%; patients receiving bDMARDs: 8.1%), 70 the prevalence of sarcopenia was reported to be 39.8% (using the criteria proposed by Baumgartner et al. 48 ), but muscle strength and physical performance were not evaluated. In another cross-sectional study involving 82 patients with RA (women: 73.2%), Vlietstra et al. 71 found that the prevalence of sarcopenia (using the criteria proposed by Studenski et al. 55 ) and sarcopenic obesity (using the criteria proposed by Gallagher et al. 85 ) were 17.1% and 15.9% respectively. On the other hand, in a cross-sectional study of 388 consecutive women with RA, conducted by Torii et al., 72 sarcopenia was defined as the presence of low muscle mass and either low muscle strength or low physical performance, as per the consensus report of the Asian Working Group for Sarcopenia (AWGS) 54 and 37.1% of the patients were classified as having sarcopenia. 72 In another cross-sectional study, Lin et al. 73 evaluated body composition by BIA in 457 Chinese RA patients (women: 82.7%; mean age: 49.5 years) and found that low ALMI, with sarcopenia in 45.1% of the patients, using the AWGS criteria, 54 was the main characteristic of Chinese RA patients. In two other studies using BIA and the same criteria for sarcopenia,74,75 the prevalence of sarcopenia among Japanese patients with RA was found to be 28% (n = 100; mean age: 66.1 years; women: 78%) and 29.6% (n = 240; mean age: 75.0 years; women: 78.8%), respectively.
The prevalence of sarcopenia in RA patients ranged from 17.1% to 60%, depending on the population that was evaluated and the assessment technique and diagnostic criteria that were used.
Rheumatoid cachexia
We found four cross-sectional studies80–83 (n = 57–178 patients) that specifically sought to evaluate the prevalence of RC using DXA and the criteria proposed by Engvall et al. 61
As already mentioned, RC is another alteration in body composition observed in RA. Engvall et al. defined RC as FFMI < 10th percentile and FMI > 25th percentile. 61 The prevalence of RC ranged from 18% to 53.9%, depending on the cohort. El Maghraoui et al. 80 found the highest prevalence (53.9%), while Santo et al. 83 found the lowest prevalence (13.3%). Since this alteration in body composition is characterised by a loss of lean mass, RC is also associated with physical disability and mortality. 68
The effect of treatments on body composition in patients with RA
Since body composition depends on several factors that can be potentially modified, this raises the question as to whether acting on these factors modifies body composition in RA. In this section, we investigate the effect of non-pharmaceutical treatments (i.e. nutrition and physical activity) and pharmaceutical treatments (i.e. corticosteroids and bDMARDs) on body composition in patients with RA. The results are summarized in Table 2.
Table 2.
Effect of nutrition, physical training and DMARDs on body composition in RA.
| References | Type of study | Patients | Treatment | Mean age (years) | Disease duration | Conclusion |
|---|---|---|---|---|---|---|
| Wilkinson et al. 88 | 24-week, double-blind, randomized, placebo-controlled trial | 35 (15 in treatment group and 20 in placebo group) | Creatine supplementation: 20 grams (4 × 5 g/day) for the initial 5 days (loading dose) followed by 3 g/day for the remainder of the 12-week period (maintenance dose) | 63.1 ± 10.0 years in creatine group | 112.5 ± 82.8 months in creatine group | Significant increase in ALM in the creatine group (0.52 ± 0.13 kg), with no change in the placebo group (0.05 ± 0.13 kg); between-group p = 0.004). 12 weeks after cessation of supplementation, no significant difference in ALM or TLM between the two groups |
| 57.26 ± 10.4 years in placebo group | 141.46 ± 160.1 months in placebo group | |||||
| Marcora et al. 89 | Randomized controlled trial | 20 patients in supplementation group 20 in placebo group |
b-hydroxy-b-methylbutyrate, glutamine and arginine for 12 weeks | 54 ± 10 | NR | At 12 weeks, no significant difference between the two groups |
| Aryaeian et al. 90 | Double-blind, randomized, placebo-controlled trial | 31 patients in supplementation group 31 in placebo group |
1500 mg barberry extract for 12 weeks | 48.61 ± 11.69 in supplementation group 47.1 ± 10.75 in placebo group |
NR | At 12 weeks, significant decrease in PBF in the supplementation group versus placebo group |
| Siqueira et al. 91 | Randomized, blinded, prospective, 16-week controlled trial | 100 female patients (33 in land-based exercise group, 33 in water-based exercise group, and 34 in control group) | Water-based aerobic group | 55 ± 6 in water-based exercise group 54 ± 5.1 in land-based exercise group 53.2 ± 7 in control group |
9.2 ± 3.1 years in water-based exercise group 7.7 ± 2.9 years in land-based exercise group 8.5 ± 4 years in control group |
No significant differences in total body composition (including body weight, lean or fat mass) in any of the groups during the period of intervention. |
| Marcora et al. 92 | Randomized controlled trial | 10 patients in high-intensity progressive resistance training 10 patients in control group |
High-intensity progressive resistance training | 53 ± 13 in intervention group 54 ± 10 in control group |
8.9 ± 5.7 years in intervention group 7.3 ± 5.3 years in control group |
At 12 weeks, significant increase in TLM and significant decrease in PBF in the high-intensity progressive resistance training group compared with controls. |
|
Lemmey et al.
93
Lemmey et al. 94 |
Randomized controlled trial Follow up of the previous trial |
13 patients in high-intensity progressive resistance training 15 patients in control group |
High-intensity progressive resistance training | 55.6 ± 8.3 | 74 ± 76 months | Significant increase in TLM (p = 0.006) and ALM (p = 0.004) in the high-intensity progressive resistance training group compared with controls. No difference between the two groups after 3 years of follow up |
| Cooney et al. 95 | Prospective study | 10 patients (8F/2M) | 8 weeks of exercise | 64 ± 6 | 11 ± 12 years | Significant decrease in PBF |
| Konijn et al. 96 | Ancillary study of COBRA-light (non-inferiority) trial | 54 patients in COBRA-light group 54 patients in COBRA group |
COBRA-light group (prednisolone 30 mg/day, tapered off to 7.5 mg/day in 8 weeks) COBRA group (prednisolone 60 mg/day, tapered off to 7.5 mg/day in 6 weeks) |
51 ± 12 in COBRA-light group | 20 (9–40) weeks in COBRA-light group | No difference between the two groups. Significant increase in total body and fat masses in all patients. However, no change in trunk/peripheral fat ratio |
| 54 ± 13 in COBRA group | 16 (9–33) weeks in COBRA group | |||||
| Engvall et al. 17 | Controlled cross-sectional study | 50 patients treated with prednisolone 50 patients never treated with prednisolone |
Prednisolone | 63 (56–68) in prednisolone group 64 (55–68) in control group |
7.5 (4–12) years in prednisolone group 8 (5–18) years in control group |
Significant increase in FM and PBF in prednisolone group compared with control group No difference in lean mass between the two group |
| Resmini et al. 97 | Case-control study | 26 patients in RA 78 non matched controls |
Low doses of prednisone (5 mg every day or 10 mg every 2 days) | 62.0 ± 10.1 in RA group | NR | Significant decrease in TLM in RA group |
| Engvall et al. 98 | Ancillary study of Swefot (open, multicentre, randomised study) | 22 patients in MTX-SLZ-HCQ group 18 patients in MTX-IFX group |
To compare MTX-SLZ-HCQ versus MTX-IFX | 59.5 (58.0–67.0) in MTX-SLZ-HCQ group 56.0 (42.0–73.0) in MTX-IFX |
5.4 ± 2.9 months in MTX-SLZ-HCQ group 4.9 ± 3.4 months in MTX-IFX |
Increase in fat mass (+3.77 kg, 1.63–5.90; p = 0.040) with infliximab at 24 months. Changes in lean mass did not differ significantly between the treatment groups |
| Marcora et al. 99 | Randomized phase II controlled trial | 12 patients in ETN group 12 patients in MTX group |
ETN | 54 ± 11 in ETN group 50 ± 15 in MTX group |
<6 months | At 24 weeks, only arm lean mass was significantly higher with etanercept compared with MTX |
| Serelis et al. 100 | Prospective study | 19 F patients | TNFα inhibitors | 54 ± 18 | 9 ± 7 years | At 1 year, no change in body composition |
| Toussirot et al. 18 | Single-centre, prospective, open-label study over 2 years | Eight patients with RA | TNFα inhibitors (IFX, ADA, ETN) | 60.5 ± 3.4 in RA group | 8.4 ± 2.5 years in RA group | In RA group, significant increase in BMI (p = 0.05), and trend towards significance for weight (p = 0.07) and visceral fat (p = 0.059). |
| Metsios et al. 101 | Prospective study | 20 patients in treatment group 12 controls matched for age, sex and BMI |
TNFα inhibitors | 61.1± 6.8 | 17.3± 11.4 years | At 12 weeks, no significant change in body composition |
| Tournadre et al. 102 | 1 year open follow-up study | 21 patients with RA 21 controls matched for age, sex, BMI and criteria for metabolic syndrome |
Tocilizumab | 57.8 ± 10.5 | 8.5 (1.7–21.5) years | At 1 year, significant gain in weight and BMI. In contrast, TLM, ALM and FFMI increased at 1 year. No change in VAT |
| Toussirot et al. 103 | Multicentre open-label study | 107 patients (78F/29M) | Tocilizumab | 56.6 ± 13.5 | 9.9 ± 8.1 years | No change in FM and PBF during the study |
| Vial et al. 104 | 1-year open monocentric follow-up study | 52 RA patients in TNFα inhibitors 23 RA patients in non TNFα inhibitors 21 RA patients in csDMARDs |
TNFα inhibitor Non TNFα inhibitors csDMARDs |
TNFα inhibitors: 56.9 ± 11.2 Non TNFα inhibitors: 56.9 ± 11.2 csDMARDs: 57.9 ± 10.8 |
TNFα inhibitors: 4.2 (1.6–12.0) Non TNFα inhibitors: 5.4 (2.3–14.8) csDMARDs: 0.3 (0.1–3.8) |
TLM, FFMI and ALMI increased significantly in TNFα inhibitors group No change in body composition was observed in non TNFα inhibitors group No change in body composition was observed in csDMARDs group |
| Chikugo et al. 105 | 3-month prospective study | 8 female patients with RA | Tofacitinib versus bDMARDs | Tofacitinib: 55.3 ± 19.5 bDMARDs: 57.0 ±14.7 |
8.5± 6.6 years in tofacitinib group 5.8 ± 10.8 years in bDMARDs group |
At 3 months, body weight of patients in tofacitinib group tended to increase (p = 0.06). Significant increase in PBF (p < 0.05), but no change in TLM. No change in body composition in bDMARDs group |
Results are expressed as mean ± standard deviation or median (25th–75th percentile).
ADA, adalimumab; ALM, appendicular lean mass; ALMI, appendicular lean mass index; bDMARDs, biological disease-modifying anti-rheumatic drugs; BIA, bioelectrical impedance; BMI, body mass index; COBRA, combinatietherapie bij reumatoide artritis; csDMARDs, conventional synthetic disease modifying anti-rheumatic drugs; DXA, dual X-ray absorptiometry; ETN, etanercept; F, female; FFM, fat free mass; FFMI, fat free mass index; FM, total fat mass; FMI, fat mass index; HCQ, hydroxychloroquine; IFX, infliximab; M, male; MTX, methotrexate; NR, not reported; PBF, body fat percentage; RA, rheumatoid arthritis; SLZ, sulfasalazine; TLM, total lean mass; TNFα, tumour necrosis factor alpha; VAT, visceral adipose tissue.
Nutritional intervention
We found two small double-blind randomised placebo-controlled trials (n = 35–40)88,89 in which DXA was used to evaluate changes in body composition in RA patients 12 weeks after an anabolic nutritional intervention.
Wilkinson et al. 88 investigated whether anabolic nutritional supplementation could increase ALM. The participants were randomized to receive a drink containing either supplementary creatine (n = 15) or placebo (n = 20) for 12 weeks. A slight increase in ALM (+0.52 ± 0.13 kg) was observed in the creatine supplementation group, while no significant changes were observed in the placebo group (between-group, p = 0.004). Twelve weeks after cessation of the creatinine supplementation regimen, no significant differences in ALM were observed between the two groups. Other body composition parameters, such as FM, were not affected by creatine supplementation. Moreover, anabolic nutritional supplementation had no effect on muscle strength and physical performance, either immediately or 12 weeks after cessation of supplementation. In another study, Marcora et al. 89 compared the efficacy on body composition (with ALM as the primary outcome) of a mixture of β-hydroxy-β-methylbutyrate, glutamine and arginine (HMB/GLN/ARG) and a mixture of other non-essential amino acids used as placebo. At 12 weeks, the authors found no significant differences in ALM, or in any of the measured body composition parameters, between the intervention group and the placebo group.
The findings reported in these studies suggest that oral creatine supplementation induces a slight and temporary increase in ALM, with no changes in any of the other body composition parameters. However, due to the limitations of these studies (small number of patients and short period of time), no definitive conclusions can be drawn.
We found one small double-blind randomised placebo-controlled trial (n = 70) 90 in which BIA was used to evaluate changes in BFP in RA patients 3 months after barberry extract (6 capsules of 500 mg per day). The results showed a slight decrease in BFP (−1.03 versus 0.37, p = 0.05) in the intervention group compared with the placebo group. No lean mass measurements were performed.
Physical training
We found three small randomised controlled trials (n = 20–100)91–94 in which DXA was used to evaluate changes in body composition in RA patients 12–24 weeks after a physical training intervention.
Siqueira et al. randomized 100 women with RA to three groups: one with land-based exercises, one with water-based exercises and one without exercise (control group). After 16 weeks, no significant changes in body composition or in muscle strength were observed between the groups. 91 In a pilot trial, 10 patients participating in a supervised progressive resistance training (PRT) program were compared with 10 patients with no training program. At 12 weeks, a significant gain in TLM (primary outcome) (+1.2 kg, p = 0.005) and a significant loss of PBF (−1.1%, p = 0.047) were observed. 92 Moreover, it was the increase in ALM that accounted for the significant increase in lean mass; changes in body composition were found to be associated with improvements in various physical function parameters. 92 In a randomised controlled trial involving 28 patients, Lemmey et al. confirmed the efficacy of high-intensity PRT in restoring muscle mass and function in patients with RA. 93 At 24 weeks, the authors observed a significant gain in TLM (+1.5 kg, p < 0.01) and ALM (+1.2 kg, p < 0.01) in the high-intensity PRT group, but no changes in the control group (home-based range of movement exercise). 93 In a 3-year follow-up study, the authors reported that long-term resumption of normal activity resulted in the loss of PRT-induced improvements in lean mass and strength-related function. 94 In a small prospective non-controlled study assessing 8 weeks of exercise (aerobic exercise followed by PRT 3 times per week) in 10 RA patients (eight women; age 64 ± 6 years; disease duration 11 ± 12 years), a significant decrease in PBF assessed by BIA was found (−9%). 95 No lean mass measurements were performed.
The findings reported in these physical training intervention studies demonstrate that PRT improves lean mass and physical function.
Corticosteroids
Chronic exposure to corticosteroids is known to induce cushingoid appearance and weight gain. 106 Despite this, corticosteroids are recommended at the beginning of treatment for RA, but only as a short-term treatment. They should be tapered off as quickly as clinically feasible. 107
We found three studies (one prospective study and two cross-sectional studies)17,96,97 in which DXA was used to evaluated changes in body composition (particularly fat mass and redistribution) in RA patients following corticosteroid treatment.
In a prospective study, Konijn et al. investigated the effects of two different high-dose, step-down prednisolone regimens on body composition in early RA patients after 26 weeks of treatment. This study was part of the larger multicentre COBRA-light trial (Combinatietherapie Bij Reumatoide Artritis), which assessed the non-inferiority of COBRA-light therapy (prednisolone 30 mg/day, tapered off to 7.5 mg/day in 8 weeks, and methotrexate (MTX) escalated to 25 mg/week in 8 weeks) versus COBRA therapy (prednisolone 60 mg/day, tapered off to 7.5 mg/day in 6 weeks, MTX 7.5 mg/week and salazopyrine 2 g/day). Overall, 108 patients were evaluated (n = 54 in each group). All patients exhibited a significant increase in total body and fat mass (TBM + 1.6 kg, p < 0.001 and FM + 1.3, p < 0.001 respectively). Surprisingly, no changes in fat redistribution (trunk/peripheral fat ratio) were observed. 96 No significant changes in lean mass (total and appendicular) were observed in either group. In a cross-sectional study, body composition was assessed by DXA in 100 RA patients (50 women) with a median [interquartile (IQR)] disease duration of 8 (4–15) years. Fifty had been treated with prednisolone (5–7.5 mg) for at least 2 years and 50 gender- and age-matched patients had not. A significant increase in FM and PBF was observed in the prednisolone group compared with the control group, but no significant differences in fat redistribution and TLM were observed between the two groups. 17 In another study, body composition was evaluated after endogenous (Cushing’s syndrome) and exogenous (RA women, n = 26) exposure to glucocorticoids (5 mg of prednisone every day or 10 mg every 2 days). 97 However, due to the study design, no definitive conclusions could be drawn.
The findings reported in these studies demonstrate that exposure to corticosteroids increases total body and fat mass but, surprisingly, induces no changes in fat redistribution and lean mass.
Biologic disease modifying anti-rheumatic drugs
Individually-tailored treatment strategies featuring early and aggressive DMARDs use and frequent monitoring of treatment response to achieve low disease activity (preferably clinical remission) form the cornerstone of pharmacological treatment in RA. 107 Conventional synthetic DMARDs are recommended as part of the first-line treatment strategy. 107
To date, no studies have been published that have specifically sought to investigate the effect of csDMARDs on body composition in RA patients. Biologic DMARDs are considered if the response to csDMARDs is insufficient or if the latter are contraindicated. 107 Most of the data on body composition were from studies on TNFα inhibitors.
We found two randomised controlled trials98,99 and four small prospective studies18,100,101 in which the effect of TNFα inhibitors on body composition in RA patients was assessed, mainly using DXA.
Engvall et al. investigated 40 early-RA patients in whom treatment with methotrexate (MTX) at a dose of up to 20 mg/week for 3 months had failed. The patients were randomised to an ‘addition of sulfasalazine and hydroxychloroquine’ group (n = 22) or an ‘addition of TNFα inhibitors (infliximab)’ group (n = 18). At 24 months, a significant increase in FM was observed in the infliximab treatment group (+3.8 versus +0.4 kg, p = 0.04), while changes in lean mass (both ALM and TLM) did not differ significantly between the two groups. 98
Marcora et al. conducted a study to assess the effect of etanercept on body composition. Twenty-four patients with early RA (disease duration <6 months) were randomized to two groups. In one group, the patients were treated with etanercept (n = 12) and in the other with MTX (n = 12). At 24 weeks, no significant changes in body composition were found. 99 Similar findings (i.e. no significant changes in body composition) were reported with TNFα inhibitors in three small prospective studies (n = 8–20) with various follow-up intervals (12 weeks to 2 years) and without control groups.18,100,101 On the other hand, in a 1-year open follow-up study involving 83 RA patients (75% women; mean age 58.5 years; median disease duration 3.7 years), Tournadre et al. 104 found significant changes in lean mass parameters in patients treated with TNFα inhibitors. In that study, the body composition parameters of 47 bDMARDs-naïve patients (mainly etanercept, n = 35) with active RA were compared with those of patients treated with csDMARDs alone (n = 18) or with non-TNFα inhibitor bDMARDs (n = 18 including 2 with tocilizumab, 10 with rituximab and 6 with abatacept). No significant difference in BMI changes over time was observed in any of the treatment group. After 1 year of TNFα inhibitors, lean mass as assessed by TLM (p = 0.015), FFMI (p = 0.013) and ALMI (p = 0.010) increased significantly and was associated with an improvement in muscle function and strength. No change in body composition was observed at 12 months in patients treated with non-TNFα inhibitor bDMARDs or csDMARDs. 104 However, due to the lack of randomisation and the small sample size of the controls, no definitive conclusions can be drawn. Moreover, 45% of patients were on steroids (median dose 6.5 mg/day) with a heterogeneous distribution between the groups (72.2% in the non-TNF inhibitor bDMARDs group versus 38.3% in the TNF inhibitor groups versus 33.3% in the csDMARDs group).
In two open, prospective 1-year follow-up studies, treatment with the interleukin-6 (IL-6) inhibitor, tocilizumab, was reported to be associated with a gain in lean mass, but with no change in fat mass.102,103
Tournadre et al. 102 investigated the effect of tocilizumab on body composition in 21 patients with RA. 2 At baseline, the patients were compared with 21 controls matched for age, sex, BMI and metabolic syndrome criteria. After 1 year of treatment with tocilizumab, the authors observed a significant gain in weight and BMI (+1.9 kg, p < 0.01 and +1.2 kg/m², p < 0.01 respectively). Significant gains in ALM and TLM were observed at 12 months (+1.0 kg, p < 0.001 and +1.1 kg, p < 0.05 respectively). In contrast, no changes in FM were observed. 104 These findings in respect of lean mass were recently confirmed in a French multicentric study (the ADIPRAT study) conducted by Toussirot et al. 103 In that study, the authors assessed 107 RA patients who had begun treatment with tocilizumab. BC was evaluated by DXA at baseline and at 6 and 12 months. Significant increases in TLM were observed at 6 and 12 months (+1.0 kg, p = 0.01 and +1.3 kg, p = 0.02 respectively). Fat parameters did not change over time.
IL-6 is a myokine (i.e. a cytokine produced and released by myocytes under the action of contractile activity). 108 During exercise, its secretion induces an increase in the production of hepatic glucose, which serves as an energy source for contracting muscles. IL-6 also mediates the crosstalk between insulin-sensitive tissues. 108 However, chronic exposure to IL-6 has been reported to be associated with muscle atrophy. 109 Inhibition of IL-6 attenuates muscular atrophy and dystrophy in several preclinical models.110,111
There are no data available on the effect of other bDMARDs (such as abatacept or rituximab) on body composition in RA patients. Data on JAK inhibitors are too scarce to draw any definitive conclusions on their effect on body composition. 105
Patients with RA exhibit a significant loss of lean mass and a significant gain in fat mass (Figure 1). The use of TNF inhibitors might be associated with a further gain in fat mass with no major change in lean mass, while the use of the IL-6 inhibitor, tocilizumab, might be associated with a gain in lean mass with no further change in fat mass. However, there are limitations to the studies that sought to investigate the effect of tocilizumab, most notably the small number of patients, no randomization and no assessment of nutritional status and physical performance.
Figure 1.
Body composition alterations in rheumatoid arthritis.
Limitations
Alterations in body composition in RA patients is a growing field of interest. However, there are several limitations to the studies we reviewed. Firstly, only four studies focused on patients with early RA. As a result, few conclusions can be drawn. In addition, in three of the four studies, the number of patients was small (less than 150). Moreover, the prevalence of sarcopenia in RA patients is difficult to estimate since different criteria were used to define sarcopenia. Further studies are needed with the new EWGSOP criteria, which assess muscle function, mass and strength. 19 Interventional studies are also lacking, especially on the effect of nutrition, exercise, corticosteroids and DMARDs on body composition in patients with RA. The studies assessing bDMARDs also lack statistical power, as few patients were included (less than 100 patients), limiting the conclusions that could be drawn.
Conclusion
In this review we identified several clinical studies on the assessment of and alterations in whole-body composition in patients with RA. However, the studies had several methodological limitations due to inconsistent definitions of altered body composition conditions. The findings reported in the studies demonstrate that (i) RA is characterized by alterations in body composition, and specifically a loss of lean mass and a gain in adiposity compared with healthy controls, regardless of sex, (ii) the prevalence of abnormal body composition conditions, such as overfat, sarcopenia, sarcopenic obesity and RC, is significantly higher in RA patients and (iii) these alterations in body composition are observed even at an early stage of the disease (i.e. before initiation of DMARDs).
Data on the effect of nutritional intervention, physical training and treatments (i.e. corticosteroids and bDMARDs) on body composition in RA patients are scarce. The findings reported in the studies demonstrate that (i) creatine supplementation and PRT programs induce a slight and temporary gain in lean mass, (ii) exposure to corticosteroids induces a gain in fat mass and (iii) the use of TNF inhibitors might be associated with a gain in fat mass, while the use of tocilizumab might be associated with a gain in lean mass.
Longitudinal studies are needed to evaluate whether alterations in body composition are more pronounced in RA patients compared with controls. There are still many gaps in our understanding of the alterations in body composition that occur in RA patients, including their initiation and progression, their determinants and outcomes and their treatment.
Supplemental Material
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-4-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-5-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing. As this work is a review, there was no need to seek the approval of an Ethical Review Board.
All authors declare that the submitted work has not been published before (neither in English nor in any other language) and that the work is not under consideration for publication elsewhere.
Footnotes
Authors’ note: The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Author Contributions: Conceptualization: J.G.L. and J.P.; methodology: J.P.; writing: original draft preparation: J.G.L.; writing: review and editing: J.P., R.M.F, AT and B.C.; visualization, J.P., R.M.F, A.T. and B.C.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jean-Guillaume Letarouilly  https://orcid.org/0000-0003-2793-6303
https://orcid.org/0000-0003-2793-6303
Julien Paccou  https://orcid.org/0000-0001-6599-8623
https://orcid.org/0000-0001-6599-8623
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jean-Guillaume Letarouilly, University of Lille F-59000 Lille, CHU Lille F-59000 Lille, France; University of Littoral Côte d’Opale F-62200 Boulogne-sur-Mer, France; Marrow Adiposity and Bone Lab - MABLab ULR4490Lille, France; Department of Rheumatology, University of Lille, CHU Lille, F-59000 Lille, France.
René-Marc Flipo, Department of Rheumatology, University of Lille, CHU Lille, F-59000 Lille, France.
Bernard Cortet, University of Lille F-59000 Lille, CHU Lille F-59000 Lille, France; University of Littoral Côte d’Opale F-62200 Boulogne-sur-Mer, France; Marrow Adiposity and Bone Lab - MABLab ULR4490Lille, France; Department of Rheumatology, University of Lille, CHU Lille, F-59000 Lille, France.
Anne Tournadre, University of Clermont Auvergne, CHU Clermont-Ferrand, UNH-UMR 1019, INRA Department of Rheumatology, F-63003 Clermont-Ferrand, France.
Julien Paccou, MABlab ULR 4490, Department of Rheumatology, CHU Lille, 2, Avenue Oscar Lambret - 59037 Lille Cedex; Department of Rheumatology, University of Lille, CHU Lille, F-59000 Lille, France.
References
- 1. Oude Voshaar MAH, Das Gupta Z, Bijlsma JWJ, et al. International consortium for health outcome measurement set of outcomes that matter to people living with inflammatory arthritis: consensus from an international working group. Arthritis Care Res (Hoboken) 2019; 71: 1556–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 2006; 36: 182–1888. [DOI] [PubMed] [Google Scholar]
- 3. Choy EH, Dures E. Fatigue in rheumatoid arthritis. Rheumatology 2019; 58: v1–v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang L, Cai P, Zhu W. Depression has an impact on disease activity and health-related quality of life in rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis 2020; 23: 285–293. [DOI] [PubMed] [Google Scholar]
- 5. Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis 2019; 78: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 6. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primer 2018; 4: 18001. [DOI] [PubMed] [Google Scholar]
- 7. Kerschbaumer A, Sepriano A, Smolen JS, et al. Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2020; 79: 744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebina K, Hirano T, Maeda Y, et al. Drug retention of 7 biologics and tofacitinib in biologics-naïve and biologics-switched patients with rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther 2020; 22: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sullivan E, Kershaw J, Blackburn S, et al. Biologic disease-modifying antirheumatic drug prescription patterns for rheumatoid arthritis among United States physicians. Rheumatol Ther 2020; 7: 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lacaille D, Avina-Zubieta JA, Sayre EC, et al. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population-closing the mortality gap. Ann Rheum Dis 2017; 76: 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartels CM, Bell CL, Shinki K, et al. Changing trends in serious extra-articular manifestations of rheumatoid arthritis among United State veterans over 20 years. Rheumatol Oxf Engl 2010; 49: 1670–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshida T, Hashimoto M, Kawahara R, et al. Non-obese visceral adiposity is associated with the risk of atherosclerosis in Japanese patients with rheumatoid arthritis: a cross-sectional study. Rheumatol Int 2018; 38: 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chodara AM, Wattiaux A, Bartels CM. Managing cardiovascular disease risk in rheumatoid arthritis: clinical updates and three strategic approaches. Curr Rheumatol Rep 2017; 19: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017; 76: 17–28. [DOI] [PubMed] [Google Scholar]
- 15. Book C, Karlsson MK, Akesson K, et al. Early rheumatoid arthritis and body composition. Rheumatol Oxf Engl 2009; 48: 1128–1132. [DOI] [PubMed] [Google Scholar]
- 16. Giles JT, Ling SM, Ferrucci L, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 2008; 59: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engvall I-L, Brismar K, Hafström I, et al. Treatment with low-dose prednisolone is associated with altered body composition but no difference in bone mineral density in rheumatoid arthritis patients: a controlled cross-sectional study. Scand J Rheumatol 2011; 40: 161–168. [DOI] [PubMed] [Google Scholar]
- 18. Toussirot É, Mourot L, Dehecq B, et al. TNFα blockade for inflammatory rheumatic diseases is associated with a significant gain in android fat mass and has varying effects on adipokines: a 2-year prospective study. Eur J Nutr 2014; 53: 951–961. [DOI] [PubMed] [Google Scholar]
- 19. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui C, Mackey RH, Shaaban CE, et al. Associations of body composition with incident dementia in older adults: cardiovascular health study-cognition study. Alzheimers Dement 2020; 16: 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tournadre A, Vial G, Capel F, et al. Sarcopenia. Joint Bone Spine 2019; 86: 309–134. [DOI] [PubMed] [Google Scholar]
- 22. Radkowski MJ, Sławiński P, Targowski T. Osteosarcopenia in rheumatoid arthritis treated with glucocorticosteroids - essence, significance, consequences. Reumatologia 2020; 58: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanley A, Schuna J, Yang S, et al. Distinct phenotypic characteristics of normal-weight adults at risk of developing cardiovascular and metabolic diseases. Am J Clin Nutr 2020; 112: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shetty S, Kapoor N, Thomas N, et al. DXA measured visceral adipose tissue, total fat, anthropometric indices and its association with cardiometabolic risk factors in mother-daughter Pairs from India. J Clin Densitom 2020; 24: 146–155. [DOI] [PubMed] [Google Scholar]
- 25. Chen Y, He D, Yang T, et al. Relationship between body composition indicators and risk of type 2 diabetes mellitus in Chinese adults. BMC Public Health 2020; 20: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adami G, Saag KG. Osteoporosis pathophysiology, epidemiology, and screening in rheumatoid arthritis. Curr Rheumatol Rep 2019; 21: 34. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Zhao R, Gu Z, et al. Effects of glucocorticoids on osteoporosis in rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos Int 2020; 31: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 28. Raterman HG, Bultink IE, Lems WF. Osteoporosis in patients with rheumatoid arthritis: an update in epidemiology, pathogenesis, and fracture prevention. Expert Opin Pharmacother 2020; 21: 1725–1737. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Wang Z-M, Heymsfield SB. History of the study of human body composition: a brief review. Am J Hum Biol 1999; 11: 157–165. [DOI] [PubMed] [Google Scholar]
- 30. Mazzoccoli G. Body composition: where and when. Eur J Radiol 2016; 85: 1456–1460. [DOI] [PubMed] [Google Scholar]
- 31. Ward LC. Human body composition: yesterday, today, and tomorrow. Eur J Clin Nutr 2018; 72: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 32. Kuriyan R. Body composition techniques. Indian J Med Res 2018; 148: 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rothman MS, Miller PD, Lewiecki EM, et al. Bone density testing: science, the media, and patient care. Curr Osteoporos Rep 2014; 12: 227–229. [DOI] [PubMed] [Google Scholar]
- 34. Bazzocchi A, Diano D, Ponti F, et al. A 360-degree overview of body composition in healthy people: relationships among anthropometry, ultrasonography, and dual-energy x-ray absorptiometry. Nutrition 2014; 30: 696–701. [DOI] [PubMed] [Google Scholar]
- 35. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piché M-E, Tchernof A, Després J-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020; 126: 1477–1500. [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi M, Ogawa S, Tayama J, et al. Intra-abdominal fat accumulation is an important predictor of metabolic syndrome in young adults. Medicine (Baltimore) 2020; 99: e22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tewari N, Awad S, Macdonald IA, et al. A comparison of three methods to assess body composition. Nutrition 2018; 47: 1–5. [DOI] [PubMed] [Google Scholar]
- 39. Xu L, Cheng X, Wang J, et al. Comparisons of body-composition prediction accuracy: a study of 2 bioelectric impedance consumer devices in healthy Chinese persons using DXA and MRI as criteria methods. J Clin Densitom 2011; 14: 458–464. [DOI] [PubMed] [Google Scholar]
- 40. Micklesfield LK, Goedecke JH, Punyanitya M, et al. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obes Silver Spring 2012; 20: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morrell GR, Ikizler TA, Chen X, et al. Psoas muscle cross-sectional area as a measure of whole-body lean muscle mass in maintenance hemodialysis patients. J Ren Nutr 2016; 26: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ceniccola GD, Castro MG, Piovacari SMF, et al. Current technologies in body composition assessment: advantages and disadvantages. Nutrition 2018; 62: 25–31. [DOI] [PubMed] [Google Scholar]
- 43. Böhm A, Heitmann BL. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur J Clin Nutr 2013; 67(Suppl. 1): S79–S85. [DOI] [PubMed] [Google Scholar]
- 44. Buffa R, Mereu E, Succa V, et al. Specific BIVA recognizes variation of body mass and body composition: two related but different facets of nutritional status. Nutrition 2017; 35: 1–5. [DOI] [PubMed] [Google Scholar]
- 45. Bosaeus I, Wilcox G, Rothenberg E, et al. Skeletal muscle mass in hospitalized elderly patients: comparison of measurements by single-frequency BIA and DXA. Clin Nutr 2014; 33: 426–431. [DOI] [PubMed] [Google Scholar]
- 46. Shuhart CR, Yeap SS, Anderson PA, et al. Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, periprosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom 2019; 22: 453–471. [DOI] [PubMed] [Google Scholar]
- 47. Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity, and inflammation—results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors study. Am J Clin Nutr 2005; 82: 428–434. [DOI] [PubMed] [Google Scholar]
- 48. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 49. Janssen I, Baumgartner RN, Ross R, et al. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004; 159: 413–421. [DOI] [PubMed] [Google Scholar]
- 50. Delmonico MJ, Harris TB, Lee J-S, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007; 55: 769–774. [DOI] [PubMed] [Google Scholar]
- 51. Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010; 29: 154–159. [DOI] [PubMed] [Google Scholar]
- 52. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011; 12: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen L-K, Liu L-K, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 55. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014; 11: 177–180. [PMC free article] [PubMed] [Google Scholar]
- 57. Beaudart C, Zaaria M, Pasleau F, et al. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One 2017; 12: e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chin SO, Rhee SY, Chon S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One 2013; 8: e60119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rohm M, Zeigerer A, Machado J, et al. Energy metabolism in cachexia. EMBO Rep 2019; 20: e47258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Santo RCE, Fernandes KZ, Lora PS, et al. Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2018; 9: 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Engvall I-L, Elkan A-C, Tengstrand B, et al. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand J Rheumatol 2008; 37: 321–328. [DOI] [PubMed] [Google Scholar]
- 62. Book C, Karlsson MK, Nilsson J-Å, et al. Changes in body composition after 2 years with rheumatoid arthritis. Scand J Rheumatol 2011; 40: 95–100. [DOI] [PubMed] [Google Scholar]
- 63. Dao H-H, Do Q-T, Sakamoto J. Abnormal body composition phenotypes in Vietnamese women with early rheumatoid arthritis. Rheumatol Oxf Engl 2011; 50: 1250–1258. [DOI] [PubMed] [Google Scholar]
- 64. Turk SA, van Schaardenburg D, Boers M, et al. An unfavorable body composition is common in early arthritis patients: a case control study. PLoS One 2018; 13: e0193377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Müller R, Kull M, Põlluste K, et al. Factors associated with low lean mass in early rheumatoid arthritis: a cross- sectional study. Med Kaunas Lith 2019; 55: 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reina D, Gómez-Vaquero C, Díaz-Torné C, et al.; Rheumatology Service. Hospital Moisès Broggi. Assessment of nutritional status by dual X-Ray absorptiometry in women with rheumatoid arthritis: a case-control study. Medicine (Baltimore) 2019; 98: e14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elkan A-C, Engvall I-L, Tengstrand B, et al. Malnutrition in women with rheumatoid arthritis is not revealed by clinical anthropometrical measurements or nutritional evaluation tools. Eur J Clin Nutr 2008; 62: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 68. Lemmey AB, Wilkinson TJ, Clayton RJ, et al. Tight control of disease activity fails to improve body composition or physical function in rheumatoid arthritis patients. Rheumatology 2016; 55: 1736–1745. [DOI] [PubMed] [Google Scholar]
- 69. Delgado-Frías E, González-Gay MA, Muñiz-Montes JR, et al. Relationship of abdominal adiposity and body composition with endothelial dysfunction in patients with rheumatoid arthritis. Clin Exp Rheumatol 2015; 33: 516–523. [PubMed] [Google Scholar]
- 70. Ngeuleu A, Allali F, Medrare L, et al. Sarcopenia in rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int 2017; 37: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 71. Vlietstra L, Meredith-Jones K, Stebbings S, et al. Sarcopenic obesity is more prevalent in osteoarthritis than rheumatoid arthritis: are different processes involved? Rheumatol Oxf Engl 2017; 56: 1816–1818. [DOI] [PubMed] [Google Scholar]
- 72. Torii M, Hashimoto M, Hanai A, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol. Epub ahead of print 11 September 2018. DOI: 10.1080/14397595.2018.1510565. [DOI] [PubMed] [Google Scholar]
- 73. Lin J-Z, Liang J-J, Ma J-D, et al. Myopenia is associated with joint damage in rheumatoid arthritis: a cross-sectional study. J Cachexia Sarcopenia Muscle 2019; 10: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tada M, Yamada Y, Mandai K, et al. Matrix metalloprotease 3 is associated with sarcopenia in rheumatoid arthritis - results from the CHIKARA study. Int J Rheum Dis 2018; 21: 1962–1969. [DOI] [PubMed] [Google Scholar]
- 75. Mochizuki T, Yano K, Ikari K, et al. Sarcopenia-associated factors in Japanese patients with rheumatoid arthritis: a cross-sectional study. Geriatr Gerontol Int 2019; 19: 907–912. [DOI] [PubMed] [Google Scholar]
- 76. Feklistov A, Demin N, Toroptsova N. AB0330 osteoporosis, sarcopenia and osteosarcopenia in women with rheumatoid arthritis. Ann Rheum Dis 2018; 77(Suppl. 2): 1340. [Google Scholar]
- 77. Kondrashov A, Shostak N, Muradyants A. Fri0052 body composition in men with rheumatoid arthritis. Ann Rheum Dis 2019; 78: 687–688. [Google Scholar]
- 78. Casabella A, Ruaro B, Seriolo C, et al. Sat0549 sarcopenia in rheumatoid arthritis female patients: relationship between whole body and trabecular bone score. Ann Rheum Dis 2019; 78(Suppl. 2): 1367. [Google Scholar]
- 79. Park D-J, Kang J-H, Xu H, et al. Thu0152 sarcopenia is associated with persistent disease activity during follow-up of rheumatoid arthritis. Ann Rheum Dis 2019; 78(Suppl. 2): 349. [Google Scholar]
- 80. El Maghraoui A, Sadni S, Rezqi A, et al. Does rheumatoid cachexia predispose patients with rheumatoid arthritis to osteoporosis and vertebral fractures? J Rheumatol 2015; 42: 1556–1562. [DOI] [PubMed] [Google Scholar]
- 81. Hugo M, Mehsen-Cetre N, Pierreisnard A, et al. Energy expenditure and nutritional complications of metabolic syndrome and rheumatoid cachexia in rheumatoid arthritis: an observational study using calorimetry and actimetry. Rheumatology (Oxford) 2016; 55: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 82. Elkan A-C, Håkansson N, Frostegård J, et al. Rheumatoid cachexia is associated with dyslipidemia and low levels of atheroprotective natural antibodies against phosphorylcholine but not with dietary fat in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 2009; 11: R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Santo RC, Silva JM, Lora PS, et al. Cachexia in patients with rheumatoid arthritis: a cohort study. Clin Rheumatol 2020; 39: 3603–3613. [DOI] [PubMed] [Google Scholar]
- 84. Hull HR, Thornton J, Wang J, et al. Fat-free mass index: changes and race/ethnic differences in adulthood. Int J Obes 2005. 2011; 35: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000; 72: 694–701. [DOI] [PubMed] [Google Scholar]
- 86. Aletaha D, Neogi T, Silman AJ, et al. 2010. rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 87. Hofman A, Brusselle GGO, Darwish Murad S, et al. The Rotterdam study: 2016 objectives and design update. Eur J Epidemiol 2015; 30: 661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wilkinson TJ, Lemmey AB, Jones JG, et al. Can creatine supplementation improve body composition and objective physical function in rheumatoid arthritis patients? A randomized controlled trial. Arthritis Care Res 2016; 68: 729–737. [DOI] [PubMed] [Google Scholar]
- 89. Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr 2005; 24: 442–454. [DOI] [PubMed] [Google Scholar]
- 90. Aryaeian N, Sedehi SK, Khorshidi M, et al. Effects of hydroalcoholic extract of Berberis Integerrima on the anthropometric indices and metabolic profile in active rheumatoid arthritis patients. Complement Ther Med 2020; 50: 102331. [DOI] [PubMed] [Google Scholar]
- 91. Siqueira US, Orsini Valente LG, de Mello MT, et al. Effectiveness of aquatic exercises in women with rheumatoid arthritis: a randomized, controlled, 16-week intervention-the HydRA trial. Am J Phys Med Rehabil 2017; 96: 167–175. [DOI] [PubMed] [Google Scholar]
- 92. Marcora SM, Lemmey AB, Maddison PJ. Can progressive resistance training reverse cachexia in patients with rheumatoid arthritis? Results of a pilot study. J Rheumatol 2005; 32: 1031–1039. [PubMed] [Google Scholar]
- 93. Lemmey AB, Marcora SM, Chester K, et al. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 2009; 61: 1726–1734. [DOI] [PubMed] [Google Scholar]
- 94. Lemmey AB, Williams SL, Marcora SM, et al. Are the benefits of a high-intensity progressive resistance training program sustained in rheumatoid arthritis patients? A 3-year followup study. Arthritis Care Res 2012; 64: 71–75. [DOI] [PubMed] [Google Scholar]
- 95. Cooney JK, Ahmad YA, Moore JP, et al. The impact of cardiorespiratory fitness on classical cardiovascular disease risk factors in rheumatoid arthritis: a cross-sectional and longitudinal study. Rheumatol Int 2019; 39: 1759–1766. [DOI] [PubMed] [Google Scholar]
- 96. Konijn NPC, van Tuyl LHD, Boers M, et al. The short-term effects of two high-dose, step-down prednisolone regimens on body composition in early rheumatoid arthritis. Rheumatol Oxf Engl 2016; 55: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 97. Resmini E, Farkas C, Murillo B, et al. Body composition after endogenous (Cushing’s syndrome) and exogenous (rheumatoid arthritis) exposure to glucocorticoids. Horm Metab Res 2010; 42: 613–618. [DOI] [PubMed] [Google Scholar]
- 98. Engvall I-L, Tengstrand B, Brismar K, et al. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomised study over 21 months. Arthritis Res Ther 2010; 12: R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marcora SM, Chester KR, Mittal G, et al. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr 2006; 84: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 100. Serelis J, Kontogianni MD, Katsiougiannis S, et al. Effect of anti-TNF treatment on body composition and serum adiponectin levels of women with rheumatoid arthritis. Clin Rheumatol 2008; 27: 795–797. [DOI] [PubMed] [Google Scholar]
- 101. Metsios GS, Stavropoulos-Kalinoglou A, Douglas KMJ, et al. Blockade of tumour necrosis factor-alpha in rheumatoid arthritis: effects on components of rheumatoid cachexia. Rheumatol Oxf Engl 2007; 46: 1824–1827. [DOI] [PubMed] [Google Scholar]
- 102. Tournadre A, Pereira B, Dutheil F, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle 2017; 8: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Toussirot E, Marotte H, Mulleman D, et al. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: a 12-month multicentre study. Arthritis Res Ther 2020; 22: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vial G, Lambert C, Pereira B, et al. The effect of TNF and non-TNF-targeted biologics on body composition in rheumatoid arthritis. J Clin Med 2021; 10: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chikugo M, Sebe M, Tsutsumi R, et al. Effect of Janus kinase inhibition by tofacitinib on body composition and glucose metabolism. J Med Invest 2018; 65: 166–170. [DOI] [PubMed] [Google Scholar]
- 106. Burt MG, Gibney J, Ho KKY. Characterization of the metabolic phenotypes of Cushing’s syndrome and growth hormone deficiency: a study of body composition and energy metabolism. Clin Endocrinol (Oxf) 2006; 64: 436–443. [DOI] [PubMed] [Google Scholar]
- 107. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 108. Manole E, Ceafalan LC, Popescu BO, et al. Myokines as possible therapeutic targets in cancer cachexia. J Immunol Res 2018; 2018: 8260742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Moresi V, Adamo S, Berghella L. The JAK/STAT pathway in skeletal muscle pathophysiology. Front Physiol 2019; 10: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yakabe M, Ogawa S, Ota H, et al. Inhibition of interleukin-6 decreases atrogene expression and ameliorates tail suspension-induced skeletal muscle atrophy. PLoS One 2018; 13: e0191318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wada E, Tanihata J, Iwamura A, et al. Treatment with the anti-IL-6 receptor antibody attenuates muscular dystrophy via promoting skeletal muscle regeneration in dystrophin-/utrophin-deficient mice. Skelet Muscle 2017; 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-4-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-5-tab-10.1177_1759720X211015006 for Body composition in patients with rheumatoid arthritis: a narrative literature review by Jean-Guillaume Letarouilly, René-Marc Flipo, Bernard Cortet, Anne Tournadre and Julien Paccou in Therapeutic Advances in Musculoskeletal Disease