Abstract
Reductions of baseline cerebral blood flow (CBF) of ∼10–20% are a common symptom of Alzheimer’s disease (AD) that appear early in disease progression and correlate with the severity of cognitive impairment. These CBF deficits are replicated in mouse models of AD and recent work shows that increasing baseline CBF can rapidly improve the performance of AD mice on short term memory tasks. Despite the potential role these data suggest for CBF reductions in causing cognitive symptoms and contributing to brain pathology in AD, there remains a poor understanding of the molecular and cellular mechanisms causing them. This review compiles data on CBF reductions and on the correlation of AD-related CBF deficits with disease comorbidities (e.g. cardiovascular and genetic risk factors) and outcomes (e.g. cognitive performance and brain pathology) from studies in both patients and mouse models, and discusses several potential mechanisms proposed to contribute to CBF reductions, based primarily on work in AD mouse models. Future research aimed at improving our understanding of the importance of and interplay between different mechanisms for CBF reduction, as well as at determining the role these mechanisms play in AD patients could guide the development of future therapies that target CBF reductions in AD.
Keywords: Alzheimer’s disease, cerebral blood flow, cognitive impairment, magnetic resonance imaging, in vivo 2-photon imaging
Introduction
The metabolic demand of the brain is narrowly met by the cerebral blood flow (CBF) it receives, with very little local energy reserve, making brain function particularly sensitive to reductions in CBF. It is well acknowledged that disruption of normal blood supply, largely characterized by regional hypoperfusion, is an early and persistent symptom in the development of Alzheimer’s disease (AD) and other neurodegenerative diseases.1–4 In addition to such hypoperfusion, there are other dysfunctions of the cerebrovascular system that have also been linked to AD and other neurodegenerative diseases, such as disintegrated blood brain barrier (BBB) (reviewed in Sweeney et al.5) and impaired autoregulation and neurovascular coupling (reviewed in Iadecola6). These vascular contributions to cognitive impairment and dementia have recently received much attention.7 While an increasing number of studies link vascular dysfunction, including baseline CBF reductions, to AD and cognitive decline,2,8 the mechanisms leading to these CBF reductions remain poorly understood. This review will focus on the causes and consequences of changes to baseline CBF in AD. Specifically, we review the alterations in CBF found in patients and mouse models of AD, the correlations of these CBF deficits with the progression or severity of the brain pathology and cognitive impacts, and the potential molecular and cellular mechanisms that may underlie the CBF reduction found in AD.
Cerebrovascular structure and physiology
Regulation and maintenance of CBF are crucial for proper brain function. The blood flow supply to the brain is fed by two internal carotid arteries that bifurcate from the common carotid arteries and the two vertebral arteries. These arteries feed the Circle of Willis, located at the base of the brain, which then gives rise to the anterior, middle, and posterior cerebral arteries that support a large proportion of the brain, including the cerebral cortex. We note, however, that there is significant variability between species and between individuals in the general pattern of large cerebral vessel arrangement.9,10 In the cortex, the three cerebral arteries give rise to a network of pial surface vessels that then branch into penetrating arterioles that plunge into the brain. These penetrating arterioles feed the capillary network, where most of the oxygen and nutrient exchange occurs.11 The capillary beds have the smallest diameter vessels, such that blood cells (leucocytes and red blood cells (RBCs)) need to deform to flow through capillary segments, and they thus contribute the most to overall vascular resistance.12 The capillaries converge on ascending venules that return blood to the cortical surface, where it is drained out of the brain by surface venules.5 This review will mostly focus on the AD-related CBF disruptions in the cortex, where imaging tools in humans and animal models have enabled the most detailed studies.
Not only does the diameter and connectivity vary across different vessel classes, but also the cellular structure of the vessel wall and milieu of nearby associated cells, termed the neurovascular unit (NVU),6 changes between arterioles, capillaries, and venules.13 Endothelial cells line all blood vessels, are anchored by a continuous basal membrane, and are connected to each other by tight junction proteins, thereby forming the BBB. While small, lipid-soluble molecules (e.g. oxygen) can passively diffuse in and out of the brain through the BBB, the entry and exit of larger molecules is inhibited and transport proteins (e.g. insulin transporter) are required for BBB crossing.5 The cells of the NVU found adjacent to the endothelium vary across vessel classes. Arterioles are surrounded by a tight layer of smooth muscle cells, astrocytic endfeet, and then the brain parenchyma (containing neurons, astrocytes, microglia, and other cells), whereas the capillaries are covered sparsely by pericytes, and then by astrocytic endfeet.5 Capillaries are the blood vessels that are the closest, on average, to neurons and other brain cells. Venules are surrounded by a sparse layer of smooth muscle cells and astrocytic endfeet. Cerebral vessels are surrounded by a perivascular space, where extracellular fluid containing proteins and other solutes are transported.14 In addition to different vessel classes having different cellular compositions, vascular cells show different transcriptional profiles in different vessel classes. This zonation of the cerebral vasculature was recently described in detail.15
These differences in topology and cellular structure along the vasculature reflects varying functional roles of different vessel classes. The cerebral vascular system is very dynamic and actively regulates CBF. Several cell-types of the NVU, including neurons, glial cells (astrocytes and macrophages/microglia), mural cells (smooth muscle cells and pericytes), and endothelial cells, are implicated in brain blood flow regulation.5 Changes in blood pressure are sensed in endothelial cells and drive vessel diameter changes that maintain constant blood flow – a process named autoregulation.16 Endothelial cells can signal adjacent endothelial cells, as well as nearby smooth muscle cells, to coordinate such modulation of vascular tone through gap-junctions and nitric oxide signaling.17,18
CBF also increases locally in response to increases in neuronal activity – a process termed neurovascular coupling.13 Smooth muscle cells surrounding arterioles and pericytes surrounding capillaries are the contractile cells around vessels that are critical for this flow regulation.19–22 Several studies have shown that neuronal activity causes Ca2+ release in astrocytic endfeet that drives vasodilation and/or vasoconstriction in arterioles,23 while other studies suggest astrocytic Ca2+ dynamics can also influence capillary diameter through pericyte contractility.24–27 Thus, it is the interplay of sensing and signaling across multiple cell types in the NVU that coordinates the tight regulation of CBF in the brain. It is widely appreciated that these flow regulation mechanisms are disrupted in AD patients28,29 and in mouse models of AD.6 In this review we will mostly focus on changes in baseline blood flow in AD and how this may impact disease progression.
During healthy aging, brain blood flow reaches its maximum value at the age of 4–6 years, and then decreases to about 60–70% of the maximum value by 50–60 years of age.30 Some structural changes occur in cerebral blood vessels with aging, including increased collagen deposition and calcification in arterioles, leading to increased vessel wall thickness, decreased vessel elasticity, and overall increased resistance.31,32 These vascular changes are correlated with increased levels of reactive oxygen species (ROS) and vascular inflammation.33,34 There is also an age-dependent decrease in capillary density.35,36 These structural changes in vessels are found to be more severe in patients with neurodegenerative disease, including AD,6,37 and likely contribute to the increase in vascular resistance, hypoperfusion, and alterations in cerebrovascular regulation seen in AD37 (Figure 1(a)).
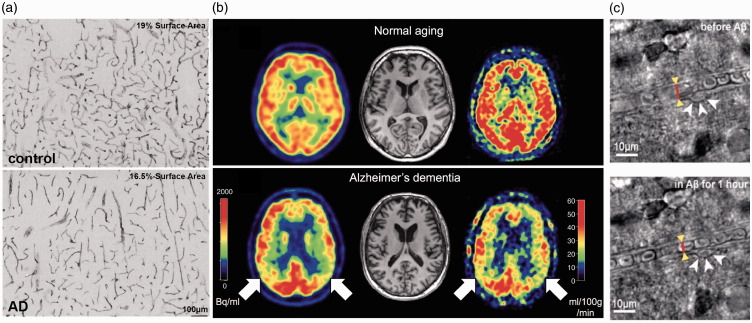
Figure 1.
Vascular alterations and brain blood flow reductions in AD patients. (a) Heparan sulfate proteoglycan mmunohistochemistry in the cortex of a control subject (upper panel) and an AD patient (lower panel), showing reduced vascular density with AD.36 (b) Images of brain perfusion using ASL-MRI from a normal aged subject (top images), and an AD patient (bottom images). Red colors represent higher perfusion, while blue colors represent lower perfusion.167 (c) Bright field images of a capillary segment from a human brain slice before (left) and after (right) application of 72 nM Aβ1-42 onto the slice, which triggered activation of a pericyte (white arrows) and constriction of the capillary (red line and yellow arrowheads).157
Cerebral blood flow reductions in patients with Alzheimer’s disease
Many studies have shown the correlation between reduced CBF, impaired cognitive function, and an increased probability of developing dementia, including AD, later in life. The blood flow reductions correlated with increased risk for dementia exceed the levels of flow reduction associated with normal, healthy aging.38 Here, we focus on brain blood flow reductions in dementia, and in particular in AD, that are not associated with the incidence of a clinically-diagnosed stroke (which in AD patients is associated with more severe cognitive impairments,39 and is reviewed in Pendlebury and Rothwell40).
The largest study of the correlation between CBF reductions and dementia comes from the ongoing Rotterdam Study. Data from 1,730 patients, aged 55 years or older, showed that higher baseline CBF, as measured by transcranial laser Doppler of the middle cerebral artery, was associated with lower risk of being diagnosed with dementia 6.5 years later in life, and also with slower cognitive decline over this time, as compared to patients with lower baseline CBF.41,42 Using single-photon emission computerized tomography to spatially resolve changes in CBF, lower flow within the medial parietal cortex was found to develop before other symptoms of AD, indicating that reduced CBF occurs at early stages of disease progression.43,44 In agreement with this, CBF reductions have been found to precede the onset of detectable memory deficits in patients at predementia stages who later developed AD.8,45,46 Arterial spin-labeled magnetic resonance imaging (ASL-MRI) has provided even greater spatial resolution for imaging CBF changes. With this approach, patients that had mild cognitive impairment (MCI) or AD showed progressively worse disease symptoms with more severe regional CBF reductions in the left hippocampus and right amygdala.47 Interestingly, this study found variable impacts of MCI and AD on regional CBF in the inferior parietal, left lateral, left superior, and left orbitofrontal cortices, and also found an increase in perfusion within the anterior cingulate gyrus. Further studies have largely shown CBF reductions ranging from 2 to 32%, with regional variability, in patients with MCI and/or AD, as compared to healthy controls48–50 (Table 1 and Figure 1(b)). However, some studies report no changes in CBF or even hyperperfusion, for example in the hippocampus, putamen, caudate, lentiform, and thalamus, in patients with AD.51,52 These differing results might be due to subtleties of various CBF measurement techniques, the disease stage at which participants are evaluated, or the inclusion/exclusion criteria for selecting participants – it is known, for example, that cardiovascular risk factors can have complex effects on CBF.
Table 1.
CBF changes in patients with MCI and AD.
| Brain region | Patients | Age (years) |
CBF change (% decrease) |
Imaging modality | Citation | |
|---|---|---|---|---|---|---|
| AD | MCI | |||||
| Cortex | 4759 | 61.3 | 6 | Phase-contrast MRI | 42 | |
| 17 | 71 ± 7.7 | 22 | SPECT | 43 | ||
| 15 | 69.3 | 13 | SPECT | 44 | ||
| 37 | 83.6 ± 3.5 | 22 | 12 | ASL-MRI | 47 | |
| 107 | ∼65 | 11 | ASL-MRI | 45 | ||
| 17 | 72.2 ± 6.8 | 11 | ASL-MRI | 49 | ||
| 20 | 72.9 | 10 | No change | ASL-MRI | 169 | |
| 12 | 70.7 ± 8.7 | 32 | ASL-MRI | 48 | ||
| 22 | 74.5 ± 8.6 | 21 | ASL-MRI | 170 | ||
| 71 | 65 ± 7 | 18 | 11 | ASL-MRI | 50 | |
| 24 | 74.6 ± 6.7 | 11 | 9 | ASL-MRI | 52 | |
| Hippocampus | 4759 | 61.3 | Dementia (AD) 15 | Phase-contrast MRI | 42 | |
| 37 | 83.6 ± 3.5 | 2 | −36 (increase) | ASL-MRI | 47 | |
| 22 | 75.6 ± 9.2 | −4.8 (increase) | ASL-MRI | 51 | ||
| 71 | 6.25 ± 7 | 18 | Similar | ASL-MRI | 50 | |
| Thalamus | 4759 | 61.3 | Dementia (AD) 18 | −21 (increase) | Phase-contrast MRI | 42 |
| 37 | 83.6 ± 3.5 | 17.5 | ASL-MRI | 47 | ||
| 24 | 74.6 ± 6.7 | −12 (increase) | ASL-MRI | 52 | ||
| Parahippocampal gyrus | 4759 | 61.3 | 12.5 | Phase-contrast MRI | 42 | |
| 12 | 70.7 ± 8.7 | 28 | ASL-MRI | 48 | ||
Single-photon emission computed tomography (SPECT); arterial spin labeled MRI (ASL-MRI); Alzheimer’s disease (AD); mild cognitive impairment (MCI).
The deposition of tau has also been found to influence CBF. A recent study including MCI and AD patients that analyzed the association of CBF and cognition with both tau and amyloid deposition showed that while increased tau deposition was linked to lower CBF and more severe cognitive impairment, this association was much stronger in patients with high amyloid burdens as compared to those with lower amounts of amyloid.53
While characterization of structural changes in the vasculature associated with AD can be assessed using post-mortem tissue, there are few approaches that can be used to quantify hypoperfusion using such histological approaches. One approach is measuring the ratio of the concentration of myelin-associated glycoprotein to proteolipid protein 1, which has been found to decrease with chronic hypoperfusion.54 This ratio was found to be reduced in the frontal cortex of tissue from AD patients, as compared to matched controls.55 Scaling this approach could allow the use of large sample sizes from tissue banks to explore, for example, genetic, gender, age, or disease marker progression of regional brain hypoperfusion, as well as to correlate hypoperfusion with other pathological changes. Such data could complement the in vivo imaging data on CBF in AD patients that is available.
Reduced CBF has also been linked to increased levels of amyloid deposition and more severe brain pathology in AD patients.56 Measuring brain amyloid levels using positron emission tomography with the amyloid-labeling Pittsburgh Compound B (PiB) revealed that higher levels of Aβ deposition was correlated to global CBF reductions,57 and that even regional CBF deficits were linked to regional increases in Aβ deposition.58 In addition, a study by Huang et al. has shown a correlation between brain regions with CBF deficits and brain atrophy in AD patients.59 The severity of white matter hyperintensities, areas of white matter damage linked to hypoperfusion that are identified through MRI, is exacerbated in AD patients.60 These studies show that there are correlations between reduced regional CBF and AD pathology.
These reductions in CBF in AD patients are frequently correlated with the development of vascular pathology. Cerebral amyloid angiopathy (CAA) is defined as Aβ deposition around the vessel walls of cortical arteries, arterioles, capillaries, and, rarely, veins.61,62 CAA is present in more than 80% of patients diagnosed with AD,63 and is linked to pathological changes in the vasculature and increased incidence of microhemorrhages and microinfarcts.64 It is thought that CAA contributes to impaired neurovascular regulation and perivascular flow by reducing the elasticity of vessels,6 and by disrupting smooth muscle cell function.65,66 Only a few studies have correlated CBF with CAA burden in AD patients, and they found that the presence of CAA was associated with more severe CBF reductions.67,68 Microvascular pathologies, including pericyte loss, BBB breakdown, and reduced vascular density (Figure 1(a)), have also been found at higher incidence in AD patients, as compared to controls.69,70 The brain microinfarcts and microhemorrhages that are consequences of microvascular occlusions and ruptures are also found in increased number in AD patients as compared to controls.71
The apolipoprotein E (APOE) ε4 allele increases the risk of AD by 3–8-fold and lowers the onset age of AD symptoms by 7–15 years.72,73 The ε4 allele of APOE is also linked to cerebral hypoperfusion. A study by Thambisetty M, et al., showed that APOE ε4 carries have 2–6% reduction in CBF across several brain regions, as compared to non-carries, as well as impaired memory function.73 Similar results were observed in other studies of APOE ε4 carries,57,74 although newer findings have suggested APOE ε4 carries have regional brain hyperperfusion in mid-life.75
Some of the cerebrovascular pathology seen in AD may be exacerbated by the presence of cardiovascular risk factors. Indeed, most cardiovascular risk factors, including hypertension, type 2 diabetes, hypercholesterolemia, as well as metabolic syndrome and obesity (especially when present in mid-life) have been shown to increase the risk for developing AD and severity of symptoms in AD patients.76,77 Cardiovascular risk scores taken during mid and late age in patients can predict cognitive decline later in life.42,55 In a prospective study, the Framingham cardiovascular risk score was predictive of the degree of cognitive decline over a year in a cohort of 254 patients with AD,78 while other studies have correlated the Framingham score with cognitive decline in the middle-age general population.79 Patients with hypertension frequently also have decreased brain perfusion and an increased probability of developing AD later in life.80 A recent meta-analysis studied this correlation in more depth and showed that, in particular, stage one (BP > 140 mm Hg/90 mm Hg (systolic/diastolic)) or two (BP > 160 mm Hg/95 mm Hg) systolic hypertension, but not diastolic hypertension (> 90 mm Hg distolic), was associated with more severe AD.81 These cardiovascular risk factors are all associated with brain blood flow reductions and increased microemboli,82 and it may be, in part, through these flow reductions that these cardiovascular risk factors influence AD progression. In addition, cardiovascular disease, such as coronary heart disease or small vessel disease, is associated with brain hypoperfusion (largest in watershed regions, including the basal ganglia, white matter, and hippocampus83–85) and has been shown to increase the risk of developing MCI and dementia.86 CBF increase, however, is not always associated with improved cognitive function. For example, while moderate exercise is consistently associated with improved cognitive performance, this is not always accompanied by an increase in CBF.87–89 While these studies were not conducted in AD patients, they suggest a complex interplay between CBF and cognitive performance.
In addition to alterations in baseline CBF, the dynamic regional and global regulation of brain blood flow may be impaired in AD patients. While there have been many reports of regional and global differences in blood oxygen level dependent (BOLD) functional MRI measurements in AD patients as compared to controls,90,91 only a few studies have linked performance on a memory-encoding task during functional MRI imaging sessions to the degree of functional hyperemia in the brain regions functionally linked to the task. These studies showed muted CBF increases during task performance in AD patients, as compared to healthy individuals, indicating a deficit in CBF regulation.92–94 More studies will be needed on task- and context-dependent CBF changes between healthy controls and AD patients to shed light on the mechanisms causing these impairments in neurovascular regulation.
In summary, MCI and AD patients tend to:
have reduced cerebral blood flow across a variety of brain regions
show correlation between the severity of hypoperfusion and cognitive impairment
have microvascular pathology and brain microinfarcts
exhibit defects in CBF regulation as well as baseline flow
The presence of cardiovascular risk factors, some years earlier, has been shown to increase the risk and severity of AD and this exacerbation of AD may be related, in part, to the CBF deficits often associated with these risk factors. Broadly speaking, the mechanisms underlying the reductions in CBF seen in AD patients remain poorly understood and remain difficult to study since the origin is likely multifactorial.
Cerebral blood flow reductions in mouse models of AD
Mouse models of AD offer the opportunity to elucidate the mechanistic links between CBF reductions and AD pathology. Hypoperfusion has been repeatedly demonstrated in multiple different mouse models of amyloid precursor protein (APP) overexpression, including PS2APP, TgCRND8, APP/PS1dE9, 5xFAD, Tg2576, and J20 mice (Table 2).95–99 In addition, CBF deficits were found in knock-in mouse models that drive mutant human APP under the endogenous mouse APP promoter.100 Flow deficits in AD mice relative to wild-type animals varied from 13% to 55% across these studies. High field ASL-MRI (7–11.4 T) allows regional CBF to be assessed in mice, and several brain regions in AD mouse models have been shown to exhibit reduced brain blood flow using this approach, including the occipital cortex,101,102 cerebral cortex, hippocampus, and thalamus.97,103,104 However, one study showed increased CBF in the frontoparietal cortex and thalamus,105 while another found no deficits in CBF at eight months of age in APP/PS1 mice,106 but a reduction at 15 months of age compared to age-matched wild-type mice.106,107 The APOE4 allele is the largest risk factor for AD, and targeted replacement knock-in mouse models carrying the human APOE allele are associated with CBF reductions in the cortex, hippocampus, thalamus, and the white matter.108–110 The CBF reductions associated with APOE manipulation were found to correlate with pericyte loss,111 decreased capillary density,112 and BBB breakdown via the CypA-MMP9 pathway,109,113 suggesting the possibility of mechanistic ties between different microvascular dysfunctions. In patients who carry the APOE4 allele, increased pericyte loss and BBB breakdown was also observed, as well as more severe cognitive decline.114,115
Table 2.
CBF changes in AD mouse models.
| Animal model | Mutation | Brain Region | CBF change (% decrease) | Age measured (months) | Age of cognitive deficits | Imaging modalities | Citation |
|---|---|---|---|---|---|---|---|
| J20 | APP Swedish, Indiana | cortex | 20 | 3 | 4 | ASL-MRI | 101 |
| 5xFAD | APP Swedish, Florida, LondonPSEN1 M146L and L286V | cortex | 17 | 5–8 | 4 | 2PEF | 97 |
| TgCRND8 | APP Swedish and Indiana | cortex | 20 | 8 | 4 | ASL-MRI | 171 |
| TgCRND8 | APP Swedish and Indiana | cortex | 25 | 7 | 4 | ASL-MRI | 159 |
| APP/PS1Δ9 | APP SwedishPSEN1 L166P | cortex, HC | 24 | 6–7 | 6–9 | ASL-MRI | 97 |
| APP knock-in | APP Swedish, Dutch, Londoncrossed in PS1 M146 | cortex | 21 | 8 | 8 | ASL-MRI | 100 |
| PS2APP | APP SwedishPSEN PSEN2 N141I | cortex, HC | 28 | 10 | 8–9 | ASL (CASL) MRI | 95 |
| J20 | APP Swedish, Indiana | cortex | 45 | 16 | 4 | Laser doppler | 156 |
| APP/PS1Δ9 | APP SwedishPSEN1 L166P | cortex | 28 | 15 | 6–9 | Contrast enhanced MRI | 106 |
| APP/PS1Δ9 | APP SwedishPSEN1 L166P | cortex | no change | 0 | 6–9 | Contrast enhanced MRI | 107 |
| APP/PS1 | APP SwedishPSEN1 L166P | cortex | 20 | 6 | 6–9 | ASL-MRI | 172 |
| Tg1130H | APP695, V717I, V721A, M722V | cortex | 20 | 2–3 | Laser doppler | 173 | |
| Tg2576 | APP KM670/671NL | cortex | 15 | 2–3 | 6 | Laser doppler | 148 |
| APP/PS1Δ9 | K594N/M595LPSEN1 L166P | cortex, HC | −20 (increase) | 2–8 | 6–9 | ASL-MRI | 105 |
| APOE4-TR | APOE Knock-In | Cortex, HC | 35 | 9 | autoradiography | 109 | |
| APOE4-TR | APOE Knock-In | Cortex | 20 | 3–4 | ASL-MRI2PEF | 108 |
Two-photon excited fluorescence microscopy (2PEF); arterial spin labeled MRI (ASL-MRI); Continuous ASL-MRI (CASAL); hippocampus (HC).
Overall, these data suggest a broad consensus that hypoperfusion occurs in mouse models of AD, but the degree of hypoperfusion and the brain regions most affected are very heterogeneous across the published literature. This variability, including findings of normoperfusion or hyperperfusion, could be a result of differences in age, sex, genetic background, transgenic mouse strain, controls, and imaging modality, much of which is underreported in the existing literature.
Impact of chronic hypoperfusion on AD-like pathology and behavior in mice
Chronic hypoperfusion has been shown, on its own, to cause cognitive deficits in mice, as well as to recreate some of the brain inflammation associated with AD, although the hypoperfusion sufficient to cause these effects is more severe than that found in AD patients or mouse models. We briefly review these findings because they suggest how CBF deficits may exacerbate AD-related pathology. To induce chronic hypoperfusion in mice, common methods include the unilateral common carotid artery occlusion (UCCAO) and the bilateral carotid artery stenosis (BCAS) model, which lead to steady-state reductions of between ∼30% and ∼70%, depending on coil diameter, although often with more severe hypoperfusion just after coil implantation.18,116,117 After 1–4 months, these CBF decreases lead to deficits in short-term memory.116–118 Such chronic cerebral hypoperfusion also induced hyperphosphorylation of the mouse tau protein after 2.5 months.117 When hypoperfusion is induced in mouse models of AD, the pathological phenotypes associated with the AD-related pathology are accelerated and the cognitive impact is worsened.119,120 For example, in 9-month old J20 mice, one month of chronic hypoperfusion led to more severe deficits in spatial short-term memory measured using a Barnes maze, as compared to sham surgery controls.121 Interestingly, this study showed a decrease in the density of diffuse amyloid plaques and a decrease in the concentration of Aß(1-42) in the hypoperfused mice.121 In APP23 mice, hypoperfusion was also found to cause more severe cognitive deficits, but the hypoperfusion was associated with an increase in the density of amyloid plaques.122 In 10–11 months old female Tg2576 AD mice 8 weeks of chronic hypoperfusion led to impaired learning in the Morris water maze.123 In the PS1V97L mouse model of AD chronic hypoperfusion led to increased BBB permeability, caused by a reduction in tight junction proteins, which was attributed to enhanced oxidative stress.124 Similarly, an increase in the concentration of amyloid species was found in APP/PS1 mice with induced hypoxia,125 although the impact of microvascular obstructions on the aggregation state and density of Aß can be complex.126 Several studies have also suggested that cerebral hypoperfusion exacerbates CAA in the Tg-SwDI mouse model.127,128 However, there are differences in the reported impact of hypoperfusion across AD mouse models that might be explained by the underlying disease-driving mutations, promoters driving these transgenes, or the strategies that were used to reduce brain blood flow. In addition, the magnitude of CBF reduction caused by these chronic induced hypoperfusion models is larger than that typically seen in AD patients,129 and these more severe CBF reductions could activate pathogenetic mechanisms that are not involved with the more modest CBF reductions found in AD. Recognizing this, new models that use either larger diameter coils or alternative surgical approaches (e.g. asymmetric common carotid artery surgery) have been developed.129 These models caused CBF reductions of about 30% and this CBF deficit was still associated with memory deficits, as measured using a Y-maze, after 28 days.130
Taken together, these findings indicate that chronic reductions in brain blood flow in wild-type mice cause AD-like memory deficits and brain pathologies, although this has generally required more severe CBF deficits than seen in AD patients. In addition, inducing hypoperfusion in mouse models of AD leads to an acceleration of disease progression, as characterized by amyloid burden, brain pathology, and cognitive deficits.
Potential mechanisms contributing to reduced CBF in AD that have emerged from mouse model studies
While several mechanisms have been proposed as contributing to brain blood flow reductions in AD, the physiological cause remains poorly understood. Disease-related structural changes in vessels as well as variance in the anatomy of large vessels can be important factors in CBF changes linked to neurodegeneration. For example, the hippocampal blood supply is provided by the collateral branches of the posterior cerebral artery and the anterior choroidal artery. Recent studies have shown that variations in the anatomy of the anterior choroidal artery in individuals led to impaired vascular reserve that was associated with increased risk for neurodegeneration and more precipitous cognitive decline with age.131,132 In addition, several studies from human AD patients have shown reduced vascular density and structural vascular alterations that could increase flow resistance, such as increased tortuosity,133 vessel wall thinning,134 string vessel formation.135 In mouse models, the data on structural changes in cerebral vessels is less homogenous. Several studies found decreased density of capillaries in the APP23 transgenic AD model136 and in the hippocampus of 12–15 month old APP/PS1 mice.137 Another study has also shown that capillary density decreases in close proximity to amyloid plaques in the Tg2576 model, suggesting direct Aß-mediated damage to the vasculature.138 Mouse models of APP overexpression also exhibit increased tortuosity in cortical ascending venules, increasing vascular resistance.139 In contrast, mice that overexpress a human mutant tau protein linked to frontotemporal dementia and which leads to the formation of neurofibrillary tangles in neurons were found to have increased capillary length, diameter, and density, as compared to controls.140 Overexpression of non-mutant tau, which does not aggregate into neurofibrillary tangles, did not cause such vascular changes. In this same study, APP overexpression models showed no difference in these measures of vascular structure as compared to controls, indicating different impacts of Aß and tau overexpression on vascular structure (Figure 2(a)).140
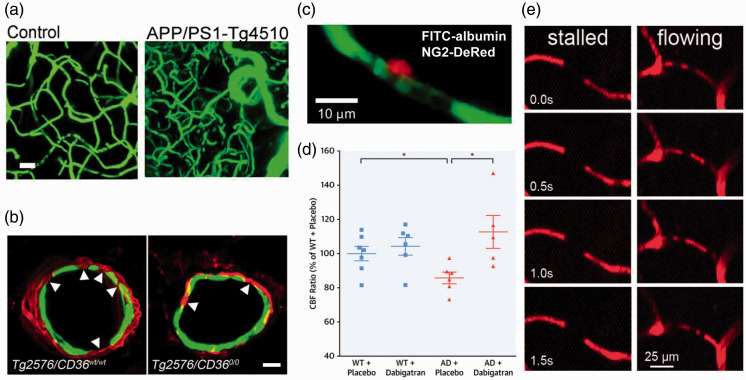
Figure 2.
Mechanisms contributing to CBF reductions in mouse models of Alzheimer’s disease. (a) Images from 15 month old WT (left) and APP/PS1-Tg4510 mice (right; overexpresses mutant APP and mutant tau), showing clearly increased vascular volume in this AD mouse model containing both APP and tau-related mutations (Scale bar, 20 μm).140 (b) Fragmentation of smooth muscle cells (arrows) along a cortical arteriole in Tg2576 mice (left image), which is attenuated by CD36 deletion (right image) (Scale bar, 10 μm; anti-α-actin: green, anti-Aβ: red).168 (c) Example image of a pericyte (red) constricting around a cortical capillary (blood plasma shown in green), with blood cell flow blocked, from an AD mouse.157 (d) Cerebral perfusion was maintained in TgCRND8 mice treated with dabigatran.159 (e) Image sequences showing stalled (left panels) and flowing (right panels) capillaries, where the darker spots in vessels are due to unlabeled blood cells within the fluorescently-labeled blood plasma. Stalled capillaries, where blood cells do not move frame-to-frame, occurred at higher incidence in APP/PS1 mice, as compared to controls.97
Cardiovascular risk factors have also been shown to accelerate disease progression in mouse models of AD, including by exacerbating cerebrovascular dysfunction.141,142 A reduction in vascular density was found in APP/PS1 mice with hypertension induced by angiotensin II infusion, as compare to normotensive APP/PS1 mice.143 Interestingly this study did not show a difference in vascular density at baseline between wild-type and APP/PS1 mice.143 The links between AD and cardiovascular risk factors may be bidirectional; a one-month infusion of Aβ1-40 was found to induce hypertension in Wistar rats and lead to a reduction in carotid artery blood flow.144
Loss of appropriate regulation of vascular diameter, either in the resting state or in response to vasoactive mediators, such as hypercapnia, blood pressure change, or increased neural activity, can also lead to CBF deficits. Superfusion of monomeric species of Aβ1-40 onto the somatosensory cortex of wild-type mice was found to induce vasoconstriction.145,146 In the Tg2576 mouse model of AD, autoregulation and neurovascular coupling were impaired early in disease progression, before the appearance of amyloid-beta deposits.99,147,148 However, other studies have found no differences in neurovascular regulation between Tg2576 and age-matched wild-type mice when the mice were young, with the AD mice showing impairment only later in disease progression when amyloid deposition along vessels was noted.149 In vitro experiments show that Aβ application to endothelial cells causes an increase in reactive oxygen species (ROS),150 and subsequent work has implicated ROS in the impairment of neurovascular regulation. In particular, the scavenger receptor CD36 has been found to bind Aβ and mediate activation of NOX2-containing NAPDH oxidase leading to the production of ROS, which not only causes endothelial dysfunction and impairs neurovascular regulation, but also drives smooth muscle cell degeneration in cortical arterioles later in disease progression (Figure 2(b)).151,152 Interestingly, another NOX protein, NOX1, has been implicated as a dominant source of ROS within the hippocampus in a mouse model of vascular dementia, raising the possibility that different brain regions and/or different disease states involve distinct, but related, molecular players that cause ROS-mediated vascular dysfunction.153,154 In the J20 mouse model of AD, treatment with simvastatin restored neurovascular coupling and this was associated with improvements in spatial memory, as measured by the Morris water maze.155 In stark contrast, Nicolakakis N et al. used the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in J20 mice, which similarly restored neurovascular coupling, but led to no improvements in spatial memory.156 These findings suggest that rescuing neurovascular coupling may not be sufficient to improve cognition in this mouse model. While the magnitude of the change in CBF due to neural activation was restored with these two drugs in J20 mice, the impact on baseline CBF was not assessed.
The use of in vivo microscopy tools has enabled the microvascular contributions to CBF reductions in AD to be explored. Recently, three different cellular mechanisms acting at the microvascular level have been proposed to contribute to baseline CBF reductions in AD. First, a recent study showed, using human brain tissue taken from neuro-oncological biopsies, that Aβ1-42 oligomers induced ROS production that led to release of endothelin-1 and constriction of capillaries via pericyte contraction, likely contributing to increased vascular resistance and thus reduced CBF (Figure 1(c) and 2(c)).157 The authors also analyzed capillary diameters near pericyte locations in APPNL-G-F mice,157 a knock-in mouse model that expresses mutant human APP under the endogenous mouse APP promoter, and found that diameters were smaller in APPNL-G-F mice, as compared to wild-type controls, further implicating Aβ-induced capillary constriction as a potential mechanism of CBF reduction of AD (Figure 2(c)).157 Interestingly a recent study has shown that, in the capillary bed of the retina and cortex, pericytes communicate via tunneling nanotubes. These tunnels were implicated in coordinated pericyte constrictions in response to neural activation, likely contributing to neurovascular coupling.158 These nanotubes could contribute to the orchestration of pericytes mediated capillary constrictions seen in the neuro-oncological biopsies of AD patients and in APPNL-G-F mice.157
Second, AD mouse models show hypercoagulability and increased stability of fibrin clots that could cause vascular obstructions that reduce CBF.159,160 For example, inducing fibrin clot formation in TgCRND8 mouse models of AD by injections of FeCl3 led to earlier and more occlusions at lower doses, as compared to wild-type mice. Another study by the same group demonstrated, in female TgCRND8 mice, that treatment for one year with dabigatran, a reversible thrombin inhibitor, lead to improvements in cognition and reductions in inflammation and amyloid load that were correlated with increases in CBF (Figure 2(d)).159
Third, we have recently found a small but significantly increased number of capillaries with transiently stalled blood flow in the cortex of APP/PS1 mice as compared to wild-type controls.97 A similar increase in stalled capillaries was also recently described in a mouse model of vascular dementia.161 In the APP/PS1 mice, the stalled capillaries were found to be caused by neutrophils that became stuck and plugged flow in individual capillary segments. Administration of antibodies that unstuck the neutrophils lead to a ∼20–30% increase in CBF within minutes. Strikingly, this CBF increase was accompanied by a rapid restoration of short-term memory within hours (Figure 2(e)).162 There is currently no data establishing whether or not such capillary stalls occur in human patients with AD or whether they share the same cellular mechanism. Interestingly, in patients with diabetic retinopathy leucocyte adhesion has been shown to occlude retina capillaries, with low-level vascular inflammation implicated as the cause.163 Finding leukocyte-mediated obstruction of capillaries in human retina lends credence to the possibility that similar capillary obstructions could contribute to the CBF reduction in AD patients, as we have shown in AD mouse models. Because the retina is, in some sense, a “window into the brain,” looking for capillary obstructions in the retina of AD patients may be the first path to establishing whether they occur in AD patients. In fact, the Alzheimer’s Association has launched an initiative to further explore the possibility of following progression of AD pathology, including blood flow deficits, in the eye.164 It is likely that all three of these mechanisms contribute to CBF reductions in AD, and that these mechanisms reinforce each other.
These mouse models do not, of course, capture the full spectrum of pathogenic factors that contribute to AD. For example, these studies rely on mouse models that overexpress the mutant form of APP or tau, and results may be challenging to transfer in the clinic. The mice also do not have contributions from cardiovascular risk factors, although these have been layered on to AD mice in some studies.165 New, second generation mouse models of AD have also been generated to improve the quality of disease modeling by, for example, knocking in human mutant APP at the mouse APP locus to provide more normal APP expression and regulation than in overexpression models.166
Conclusion
CBF deficits are frequently found in AD patients, with some inconsistency across studies (Table 1). Generally, larger CBF deficits appear to be correlated with poorer cognitive performance in AD patients. CBF also decreases in healthy aging, although not as much as in AD patients, and these flow decreases do not seem sufficient to cause cognitive impairment. This suggests that CBF deficits are not the sole driver of cognitive impairment and, more likely, multiple pathogenic factors contribute to the cognitive decline seen in AD. This does not imply, however, that treating CBF deficits in AD patients would not lead to cognitive improvement. More longitudinal data are clearly needed to further investigate CBF changes over disease progression and to determine the correlation with cognitive deficits. The regional differences in blood flow in the brain of AD patients, as well as how CBF deficits differ in AD patients according to sex, race, ethnicity, cardiovascular risk factors, and other relevant factors needs to be further explored. Increased transparency in reporting details of subjects and methods would likely help to resolve remaining inconsistencies.
Mouse models of APP overexpression replicate the CBF deficits found in AD patients, including CBF decreases early in disease progression and ∼20% CBF reductions, on average (Table 2). Recent studies link these CBF deficits to a few potential cellular mechanisms, including tonic constriction of capillaries by activated pericytes, hypercoagulability that leads to vascular obstructions, and increased stalling of capillary segments by circulating white blood cells. Substantial evidence points toward some of these cellular mechanisms being a result of ROS-mediated dysfunction in microvascular cells. More research is needed to determine the role of, and interactions between, different cell types within the NVU that may drive these cellular mechanisms that contribute to CBF deficits in AD, and may cause other microvascular dysfunction, such as BBB failure and neurovascular dysregulation. In AD mouse models, there remains a lack of data on CBF deficits from brain regions other than the cortex, as well as on differences in CBF deficits with sex and across different mouse models of AD. In addition, the contribution of aggregated tau to brain blood flow reductions in AD is severely understudied. Still, given the dramatic structural impacts on the brain vasculature caused by tau aggregation, it is critical to understand its impacts on CBF, as well as the interplay with mutant APP expression.
The correlation of CBF deficits with more severe cognitive outcomes in AD patients as well as the improvement in memory performance in AD mouse models with rescued blood flow both suggest that improving CBF could be a potential symptomatic treatment for AD, especially in early phases of disease progression. Elucidation of the detailed molecular and cellular mechanisms that underlie the CBF deficits in AD could suggest novel therapeutic targets. However, there remains only sparse data analyzing the mechanisms of CBF reduction in AD, and much more research is warranted to fully understand these mechanisms and their links to other aspects of cerebrovascular dysfunction in AD. Recent advances in optical imaging modalities and genetically-targeted molecular labeling and manipulation tools make some of these critical studies a possibility.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health grants AG049952, NS108472 (CBS), and NS097805 (LP), the BrightFocus Foundation (CBS), and the DFG German Research Foundation (OB). We thank Costantino Iadecola, Lianne Trigiani, and Nancy E. Ruiz-Uribe for critical reading of this manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Nozomi Nishimura https://orcid.org/0000-0003-4342-9416
References
- 1.Roher AE, Debbins JP, Malek-Ahmadi M, et al. Cerebral blood flow in Alzheimer’s disease. Vasc Health Risk Manag 2012; 8: 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hays CC, Zlatar ZZ, Wierenga CE.The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell Mol Neurobiol 2016; 36: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang N, Gordon ML, Goldberg TE.Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci Biobehav Rev 2017; 72: 168–175. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney MD, Kisler K, Montagne A, et al. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 2018; 21: 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimers Dement 2019; 15: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C.The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corriveau RA, Bosetti F, Emr M, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol 2016; 36: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leijenaar JF, Maurik IS, Kuijer JPA, et al. Lower cerebral blood flow in subjects with alzheimer's dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimer’s Dement Diagn Assess Dis Monitor 2017; 9: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seghier ML, Price CJ.Interpreting and utilising intersubject variability in brain function. Trends Cogn Sci 2018; 22: 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viviani R.A digital atlas of middle to large brain vessels and their relation to cortical and subcortical structures. Front Neuroanat 2016; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh Y, Suzuki N.Control of brain capillary blood flow. J Cereb Blood Flow Metab 2012; 32: 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould IG, Tsai P, Kleinfeld D, et al. The capillary bed offers the largest hemodynamic resistance to the cortical blood supply. J Cereb Blood Flow Metab 2017; 37: 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iadecola C.Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Benveniste H, Nedergaard M, et al.; Colleagues From the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel Disease. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16: 137–153. [DOI] [PubMed] [Google Scholar]
- 15.Vanlandewijck M, He L, Mae MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018; 554: 475–480. [DOI] [PubMed] [Google Scholar]
- 16.Armstead WM.Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin 2016; 34: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen BR, Kozberg MG, Bouchard MB, et al. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 2014; 3: e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraco G, Hochrainer K, Segarra SG, et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 2019; 574: 686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro Dias M, Mapunda JA, Vladymyrov M, et al. Structure and junctional complexes of endothelial, epithelial and glial brain barriers. Int J Mol Sci 2019; 20: 5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthiaume AA, Hartmann DA, Majesky MW, et al. Pericyte structural remodeling in cerebrovascular health and homeostasis. Front Aging Neurosci 2018; 10: 210–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacVicar BA, Newman EA.Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol 2015; 7: a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen MB, Yang AC, Yousef H, et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep 2020; 30: 4418–4432.e4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 2006; 9: 260–267. [DOI] [PubMed] [Google Scholar]
- 24.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra A, Reynolds JP, Chen Y, et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 2016; 19: 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisler K, Nelson AR, Rege SV, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 2017; 20: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant RI, Hartmann DA, Underly RG, et al. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab 2019; 39: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarantini S, Tran CHT, Gordon GR, et al. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 2017; 94: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolakakis N, Hamel E.Neurovascular function in Alzheimer’s disease patients and experimental models. J Cereb Blood Flow Metab 2011; 31: 1354–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C, Honarmand AR, Schnell S, et al. Age-related changes of normal cerebral and cardiac blood flow in children and adults aged 7 months to 61 years. J Am Heart Assoc 2016; 5: e002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikael LR, Paiva AMG, Gomes MM, et al. Vascular aging and arterial stiffness. Arq Bras Cardiol 2017; 109: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Wang B, Ren C, et al. Age-related impairment of vascular structure and functions. Aging Dis 2017; 8: 590–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guzik TJ, Touyz RM.Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017; 70: 660–667. [DOI] [PubMed] [Google Scholar]
- 34.Park L, Anrather J, Girouard H, et al. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab 2007; 27: 1908–1918. [DOI] [PubMed] [Google Scholar]
- 35.Hase Y, Ding R, Harrison G, et al. White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathol Commun 2019; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buee L, Hof PR, Bouras C, et al. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol 1994; 87: 469–480. [DOI] [PubMed] [Google Scholar]
- 37.Klohs J.An integrated view on vascular dysfunction in Alzheimer’s disease. Neurodegener Dis 2020; 19: 109–127. [DOI] [PubMed] [Google Scholar]
- 38.Tarumi T, Zhang R.Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem 2018; 144: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalaria RN, Akinyemi R, Ihara M.Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta 2016; 1862: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pendlebury ST, Rothwell PM.Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 41.Ruitenberg A, den Heijer T, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 2005; 57: 789–794. [DOI] [PubMed] [Google Scholar]
- 42.Wolters FJ, Zonneveld HI, Hofman A, et al. Heart-Brain Connection Collaborative Research Group. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017; 136: 719–728. [DOI] [PubMed] [Google Scholar]
- 43.Perani D, Di Piero V, Vallar G, et al. Technetium-99m HM-PAO-SPECT study of regional cerebral perfusion in early Alzheimer’s disease. J Nucl Med 1988; 29: 1507–1514. [PubMed] [Google Scholar]
- 44.Johnson KA, Mueller ST, Walshe TM, et al. Cerebral perfusion imaging in Alzheimer’s disease. Use of single photon emission computed tomography and iofetamine hydrochloride I 123. Arch Neurol 1987; 44: 165–168. [DOI] [PubMed] [Google Scholar]
- 45.Binnewijzend MA, Benedictus MR, Kuijer JP, et al. Cerebral perfusion in the predementia stages of Alzheimer’s disease. Eur Radiol 2016; 26: 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016; 7: 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai W, Lopez OL, Carmichael OT, et al. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asllani I, Habeck C, Scarmeas N, et al. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. J Cereb Blood Flow Metab 2008; 28: 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alsop DC, Detre JA, Grossman M.Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol 2000; 47: 93–100. [PubMed] [Google Scholar]
- 50.Binnewijzend MA, Kuijer JP, Benedictus MR, et al. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 2013; 267: 221–230. [DOI] [PubMed] [Google Scholar]
- 51.Alsop DC, Casement M, de Bazelaire C, et al. Hippocampal hyperperfusion in Alzheimer’s disease. Neuroimage 2008; 42: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding B, Ling HW, Zhang Y, et al. Pattern of cerebral hyperperfusion in Alzheimer’s disease and amnestic mild cognitive impairment using voxel-based analysis of 3D arterial spin-labeling imaging: initial experience. Clin Interv Aging 2014; 9: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albrecht D, Isenberg AL, Stradford J, et al. Associations between vascular function and tau PET are associated with global cognition and amyloid. J Neurosci 2020; 40: 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barker R, Wellington D, Esiri MM, et al. Assessing white matter ischemic damage in dementia patients by measurement of myelin proteins. J Cereb Blood Flow Metab 2013; 33: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas T, Miners S, Love S.Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer’s disease and vascular dementia. Brain 2015; 138: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 56.Mattsson N, Tosun D, Insel PS, et al.; Alzheimer’s Disease Neuroimaging Initiative. Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain 2014; 137: 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michels L, Warnock G, Buck A, et al. Arterial spin labeling imaging reveals widespread and abeta-independent reductions in cerebral blood flow in elderly apolipoprotein epsilon-4 carriers. J Cereb Blood Flow Metab 2016; 36: 581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gietl AF, Warnock G, Riese F, et al. Regional cerebral blood flow estimated by early PiB uptake is reduced in mild cognitive impairment and associated with age in an amyloid-dependent manner. Neurobiol Aging 2015; 36: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 59.Huang CW, Hsu SW, Chang YT, et al. Cerebral perfusion insufficiency and relationships with cognitive deficits in Alzheimer’s disease: a multiparametric neuroimaging study. Sci Rep 2018; 8: 1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee S, Viqar F, Zimmerman ME, et al.; Dominantly Inherited Alzheimer Network. White matter hyperintensities are a core feature of Alzheimer’s disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016; 79: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dietrich HH, Xiang C, Han BH, et al. Soluble amyloid-beta, effect on cerebral arteriolar regulation and vascular cells. Mol Neurodegener 2010; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 2008; 34: 131–144. [DOI] [PubMed] [Google Scholar]
- 63.Jellinger KA.Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm (Vienna) 2002; 109: 813–836. [DOI] [PubMed] [Google Scholar]
- 64.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, et al. Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol 2020; 16: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunter JM, Kwan J, Malek-Ahmadi M, et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS One 2012; 7: e36893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christie R, Yamada M, Moskowitz M, et al. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol 2001; 158: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung Y-A, Hyun O J, Kim J-Y, et al. Hypoperfusion and ischemia in cerebral amyloid angiopathy documented by 99mTc-ECD brain perfusion SPECT. J Nucl Med 2009; 50: 1969–1974. [DOI] [PubMed] [Google Scholar]
- 68.Smith EE, Greenberg SM.Beta-amyloid, blood vessels, and brain function. Stroke 2009; 40: 2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sengillo JD, Winkler EA, Walker CT, et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol 2013; 23: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaswal G, Swardfager W, Gao FQ, et al. Reduced substantia innominata volume mediates contributions of microvascular and macrovascular disease to cognitive deficits in Alzheimer’s disease. Neurobiol Aging 2018; 66: 23–31. [DOI] [PubMed] [Google Scholar]
- 71.Vermeer SE Longstreth WT Jr.andKoudstaal PJ.. Silent brain infarcts: a systematic review. Lancet Neurol 2007; 6: 611–619. [DOI] [PubMed] [Google Scholar]
- 72.Rawle MJ, Davis D, Bendayan R, et al. Apolipoprotein-E (apoe) epsilon4 and cognitive decline over the adult life course. Transl Psychiatry 2018; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thambisetty M, Beason-Held L, An Y, et al. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010; 67: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Filippini N, Ebmeier KP, MacIntosh BJ, et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 2011; 54: 602–610. [DOI] [PubMed] [Google Scholar]
- 75.McKiernan EF, Mak E, Dounavi ME, et al. Regional hyperperfusion in cognitively normal. J Neurol Neurosurg Psychiatry 2020; 91: 861–866. [DOI] [PubMed] [Google Scholar]
- 76.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017; 317: 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Heus RAA, de Jong DLK, Sanders ML, et al. Dynamic regulation of cerebral blood flow in patients with Alzheimer disease. Hypertension 2018; 72: 139–150. [DOI] [PubMed] [Google Scholar]
- 78.Viticchi G, Falsetti L, Buratti L, et al. Framingham risk score can predict cognitive decline progression in Alzheimer’s disease. Neurobiol Aging 2015; 36: 2940–2945. [DOI] [PubMed] [Google Scholar]
- 79.Kaffashian S, Dugravot A, Nabi H, et al. Predictive utility of the Framingham general cardiovascular disease risk profile for cognitive function: evidence from the Whitehall II study. Eur Heart J 2011; 32: 2326–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldstein CA.Association between chronic blood pressure changes and development of Alzheimer’s disease. J Alzheimers Dis 2012; 32: 753–763. [DOI] [PubMed] [Google Scholar]
- 81.Lennon MJ, Makkar SR, Crawford JD, et al. Midlife hypertension and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2019; 71: 307–316. [DOI] [PubMed] [Google Scholar]
- 82.Kietaibl C, Engel A, Horvat Menih I, et al. Detection and differentiation of cerebral microemboli in patients undergoing major orthopaedic surgery using transcranial doppler ultrasound. Br J Anaesth 2017; 118: 400–406. [DOI] [PubMed] [Google Scholar]
- 83.Corey-Bloom J, Sabbagh MN, Bondi MW, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology 1997; 48: 154–160. [DOI] [PubMed] [Google Scholar]
- 84.Payabvash S, Souza LC, Wang Y, et al. Regional ischemic vulnerability of the brain to hypoperfusion: the need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke 2011; 42: 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Sullivan M, Summers PE, Jones DK, et al. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 2001; 57: 2307–2310. [DOI] [PubMed] [Google Scholar]
- 86.Tublin JM, Adelstein JM, Del Monte F, et al. Getting to the heart of Alzheimer disease. Circ Res 2019; 124: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brisswalter J, Collardeau M, René A.Effects of acute physical exercise characteristics on cognitive performance. Sports Med 2002; 32: 555–566. [DOI] [PubMed] [Google Scholar]
- 88.McMorris T, Sproule J, Turner A, et al. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta-analytical comparison of effects. Physiol Behav 2011; 102: 421–428. [DOI] [PubMed] [Google Scholar]
- 89.Ogoh S, Tsukamoto H, Hirasawa A, et al. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol Rep 2014; 2: e12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleisher AS, Sherzai A, Taylor C, et al. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage 2009; 47: 1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sperling R.Potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiol Aging 2011; 32 Suppl 1: S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu G, Antuono PG, Jones J, et al. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology 2007; 69: 1650–1656. [DOI] [PubMed] [Google Scholar]
- 93.Alsop DC, Press DZ.Activation and baseline changes in functional MRI studies of Alzheimer disease. Neurology 2007; 69: 1645–1646. [DOI] [PubMed] [Google Scholar]
- 94.Fleisher AS, Podraza KM, Bangen KJ, et al. Cerebral perfusion and oxygenation differences in Alzheimer’s disease risk. Neurobiol Aging 2009; 30: 1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weidensteiner C, Metzger F, Bruns A, et al. Cortical hypoperfusion in the B6.PS2APP mouse model for Alzheimer’s disease: comprehensive phenotyping of vascular and tissular parameters by MRI. Magn Reson Med 2009; 62: 35–45. [DOI] [PubMed] [Google Scholar]
- 96.Dorr A, Sahota B, Chinta LV, et al. Amyloid-beta-dependent compromise of microvascular structure and function in a model of Alzheimer’s disease. Brain 2012; 135: 3039–3050. [DOI] [PubMed] [Google Scholar]
- 97.Cruz Hernandez JC, Bracko O, Kersbergen CJ, et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci 2019; 22: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klohs J, Rudin M, Shimshek DR, et al. Imaging of cerebrovascular pathology in animal models of Alzheimer’s disease. Front Aging Neurosci 2014; 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niwa K, Kazama K, Younkin SG, et al. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis 2002; 9: 61–68. [DOI] [PubMed] [Google Scholar]
- 100.Li H, Guo Q, Inoue T, et al. Vascular and parenchymal amyloid pathology in an Alzheimer disease knock-in mouse model: interplay with cerebral blood flow. Mol Neurodegener 2014; 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hebert F, Grand'maison M, Ho MK, et al. Cortical atrophy and hypoperfusion in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 2013; 34: 1644–1652. [DOI] [PubMed] [Google Scholar]
- 102.Poisnel G, Herard AS, El Tannir El Tayara N, et al. Increased regional cerebral glucose uptake in an APP/PS1 model of Alzheimer’s disease. Neurobiol Aging 2012; 33: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu EX, Tang H, Asai T, et al. Regional cerebral blood volume reduction in transgenic mutant APP (V717F, K670N/M671L) mice. Neurosci Lett 2004; 365: 223–227. [DOI] [PubMed] [Google Scholar]
- 104.Wiesmann M, Capone C, Zerbi V, et al. Hypertension impairs cerebral blood flow in a mouse model for Alzheimer’s disease. Curr Alzheimer Res 2015; 12: 914–922. [DOI] [PubMed] [Google Scholar]
- 105.Guo Y, Li X, Zhang M, et al. Age and brain region associated alterations of cerebral blood flow in early Alzheimer’s disease assessed in AbetaPPSWE/PS1DeltaE9 transgenic mice using arterial spin labeling. Mol Med Rep 2019; 19: 3045–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hooijmans CR, Rutters F, Dederen PJ, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched typical Western diet (TWD). Neurobiol Dis 2007; 28: 16–29. [DOI] [PubMed] [Google Scholar]
- 107.Hooijmans CR, Van der Zee CE, Dederen PJ, et al. DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis 2009; 33: 482–498. [DOI] [PubMed] [Google Scholar]
- 108.Koizumi K, Hattori Y, Ahn SJ, et al. Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun 2018; 9: 3809–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin AL, Parikh I, Yanckello LM, et al. APOE genotype-dependent pharmacogenetic responses to rapamycin for preventing Alzheimer’s disease. Neurobiol Dis 2020; 139: 104834–104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Halliday MR, Rege SV, Ma Q, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 2016; 36: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alata W, Ye Y, St-Amour I, et al. Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 2015; 35: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Main BS, Villapol S, Sloley SS, et al. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol Neurodegener 2018; 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020; 581: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 2018; 24: 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Zuloaga KL, Zhang W, Yeiser LA, et al. Neurobehavioral and imaging correlates of hippocampal atrophy in a mouse model of vascular cognitive impairment. Transl Stroke Res 2015; 6: 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao Y, Gu JH, Dai CL, et al. Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice. Front Aging Neurosci 2014; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kitagawa K, Yagita Y, Sasaki T, et al. Chronic mild reduction of cerebral perfusion pressure induces ischemic tolerance in focal cerebral ischemia. Stroke 2005; 36: 2270–2274. [DOI] [PubMed] [Google Scholar]
- 119.Pimentel-Coelho PM, Michaud JP, Rivest S.Effects of mild chronic cerebral hypoperfusion and early amyloid pathology on spatial learning and the cellular innate immune response in mice. Neurobiol Aging 2013; 34: 679–693. [DOI] [PubMed] [Google Scholar]
- 120.Qiu L, Ng G, Tan EK, et al. Chronic cerebral hypoperfusion enhances tau hyperphosphorylation and reduces autophagy in Alzheimer’s disease mice. Sci Rep 2016; 6: 23964–23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamada M, Ihara M, Okamoto Y, et al. The influence of chronic cerebral hypoperfusion on cognitive function and amyloid beta metabolism in APP overexpressing mice. PLoS One 2011; 6: e16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhai Y, Yamashita T, Nakano Y, et al. Chronic cerebral hypoperfusion accelerates Alzheimer’s disease pathology with cerebrovascular remodeling in a novel mouse model. J Alzheimers Dis 2016; 53: 893–905. [DOI] [PubMed] [Google Scholar]
- 123.Lee JS, Im DS, An YS, et al. Chronic cerebral hypoperfusion in a mouse model of Alzheimer’s disease: an additional contributing factor of cognitive impairment. Neurosci Lett 2011; 489: 84–88. [DOI] [PubMed] [Google Scholar]
- 124.Yang H, Wang W, Jia L, et al. The effect of chronic cerebral hypoperfusion on blood-brain barrier permeability in a transgenic Alzheimer’s disease mouse model (PS1V97L). J Alzheimers Dis 2020; 74: 261–275. [DOI] [PubMed] [Google Scholar]
- 125.Zhang X, Zhou K, Wang R, et al. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem 2007; 282: 10873–10880. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Y, Bander ED, Lee Y, et al. Microvessel occlusions alter amyloid-beta plaque morphology in a mouse model of Alzheimer’s disease. J Cereb Blood Flow Metab 2019; 40: 2115–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okamoto Y, Yamamoto T, Kalaria RN, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol 2012; 123: 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salvadores N, Searcy JL, Holland PR, et al. Chronic cerebral hypoperfusion alters amyloid-beta peptide pools leading to cerebral amyloid angiopathy, microinfarcts and haemorrhages in Tg-SwDI mice. Clin Sci (Lond) 2017; 131: 2109–2123. [DOI] [PubMed] [Google Scholar]
- 129.Washida K, Hattori Y, Ihara M.Animal models of chronic cerebral hypoperfusion: from mouse to primate. Int J Mol Sci 2019; 20: 6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hattori Y, Enmi J, Kitamura A, et al. A novel mouse model of subcortical infarcts with dementia. J Neurosci 2015; 35: 3915–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spallazzi M, Dobisch L, Becke A, et al. Hippocampal vascularization patterns: a high-resolution 7 tesla time-of-flight magnetic resonance angiography study. Neuroimage Clin 2019; 21: 101609–102018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perosa V, Priester A, Ziegler G, et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain 2020; 143: 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buee L, Hof PR, Delacourte A.Brain microvascular changes in Alzheimer’s disease and other dementias. Ann N Y Acad Sci 1997; 826: 7–24. [DOI] [PubMed] [Google Scholar]
- 134.Beskow J, Hassler O, Ottosson JO.Cerebral arterial deformities in relation to senile deterioration. Acta Psychiatr Scand Suppl 1971; 221: 111–119. [DOI] [PubMed] [Google Scholar]
- 135.Brown WR.A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimers Dis 2010; 21: 725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meyer EP, Ulmann-Schuler A, Staufenbiel M, et al. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc Natl Acad Sci U S A 2008; 105: 3587–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Janota CS, Brites D, Lemere CA, et al. Glio-vascular changes during ageing in wild-type and Alzheimer’s disease-like APP/PS1 mice. Brain Res 2015; 1620: 153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kouznetsova E, Klingner M, Sorger D, et al. Developmental and amyloid plaque-related changes in cerebral cortical capillaries in transgenic Tg2576 Alzheimer mice. Int J Dev Neurosci 2006; 24: 187–193. [DOI] [PubMed] [Google Scholar]
- 139.Lai AY, Dorr A, Thomason LA, et al. Venular degeneration leads to vascular dysfunction in a transgenic model of Alzheimer’s disease. Brain 2015; 138: 1046–1058. [DOI] [PubMed] [Google Scholar]
- 140.Bennett RE, Robbins AB, Hu M, et al. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc Natl Acad Sci U S A 2018; 115: E1289–E1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bink DI, Ritz K, Aronica E, et al. Mouse models to study the effect of cardiovascular risk factors on brain structure and cognition. J Cereb Blood Flow Metab 2013; 33: 1666–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Faraco G, Park L, Zhou P, et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab 2016; 36: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cifuentes D, Poittevin M, Dere E, et al. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension 2015; 65: 218–224. [DOI] [PubMed] [Google Scholar]
- 144.Palmer JC, Tayler HM, Dyer L, et al. Zibotentan, an endothelin a receptor antagonist, prevents amyloid-beta-induced hypertension and maintains cerebral perfusion. JAD 2020; 73: 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park L, Zhou P, Koizumi K, et al. Brain and circulating levels of Abeta1-40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke 2013; 44: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Park L, Anrather J, Zhou P, et al. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci 2005; 25: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Niwa K, Kazama K, Younkin L, et al. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol 2002; 283: H315–H323. [DOI] [PubMed] [Google Scholar]
- 148.Niwa K, Younkin L, Ebeling C, et al. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A 2000; 97: 9735–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shin HK, Jones PB, Garcia-Alloza M, et al. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain 2007; 130: 2310–2319. [DOI] [PubMed] [Google Scholar]
- 150.Thomas T, Thomas G, McLendon C, et al. Beta-amyloid-mediated vasoactivity and vascular endothelial damage. Nature 1996; 380: 168–171. [DOI] [PubMed] [Google Scholar]
- 151.Park L, Wang G, Zhou P, et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci U S A 2011; 108: 5063–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park L, Zhou P, Pitstick R, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A 2008; 105: 1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi DH, Lee KH, Kim JH, et al. NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal 2014; 21: 533–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim HA, Miller AA, Drummond GR, et al. Vascular cognitive impairment and Alzheimer’s disease: role of cerebral hypoperfusion and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol 2012; 385: 953–959. [DOI] [PubMed] [Google Scholar]
- 155.Tong XK, Lecrux C, Rosa-Neto P, et al. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J Neurosci 2012; 32: 4705–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nicolakakis N, Aboulkassim T, Ongali B, et al. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 2008; 28: 9287–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nortley R, Korte N, Izquierdo P, et al. Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 2019; 365: eaav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, et al. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature 2020; 585: 91–95. [DOI] [PubMed] [Google Scholar]
- 159.Cortes-Canteli M, Kruyer A, Fernandez-Nueda I, et al. Long-Term dabigatran treatment delays Alzheimer’s disease pathogenesis in the TgCRND8 mouse model. J Am Coll Cardiol 2019; 74: 1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cortes-Canteli M, Paul J, Norris EH, et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron 2010; 66: 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yoon J-H, Shin P, Joo J, et al. Increased capillary stalling is associated with endothelial glycocalyx loss in subcortical vascular dementia. bioRxiv 2020. 2020.2004.2008.031187. DOI: 10.1101/2020.04.08.031187. [DOI] [PMC free article] [PubMed]
- 162.Bracko O, Njiru BN, Swallow M, et al. Increasing cerebral blood flow improves cognition into late stages in Alzheimer’s disease mice. J Cereb Blood Flow Metab 2019: 271678X19873658. DOI: 10.1177/0271678X19873658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mesquida M, Drawnel F, Fauser S.The role of inflammation in diabetic eye disease. Semin Immunopathol 2019; 41: 427–445. [DOI] [PubMed] [Google Scholar]
- 164.Lim JK, Li QX, He Z, et al. The eye as a biomarker for Alzheimer’s disease. Front Neurosci 2016; 10: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bracko O, Vinarcsik LK, C, Hernandez JC, et al. High fat diet worsens Alzheimer’s disease-related behavioral abnormalities and neuropathology in APP/PS1 mice, but not by synergistically decreasing cerebral blood flow. Sci Rep 2020; 10: 9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Foidl BM, Humpel C.Can mouse models mimic sporadic Alzheimer’s disease? Neural Regen Res 2020; 15: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haller S, Zaharchuk G, Thomas DL, et al. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 2016; 281: 337–356. [DOI] [PubMed] [Google Scholar]
- 168.Park L, Zhou J, Zhou P, et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci U S A 2013; 110: 3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 2005; 234: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yoshiura T, Hiwatashi A, Yamashita K, et al. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. AJNR Am J Neuroradiol 2009; 30: 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Decker Y, Muller A, Nemeth E, et al. Analysis of the vasculature by immunohistochemistry in paraffin-embedded brains. Brain Struct Funct 2018; 223: 1001–1015. [DOI] [PubMed] [Google Scholar]
- 172.Lu X, Moeini M, Li B, et al. A pilot study investigating changes in capillary hemodynamics and its modulation by exercise in the APP-PS1 Alzheimer mouse model. Front Neurosci 2019; 13: 1261–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Iadecola C, Zhang F, Niwa K, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 1999; 2: 157–161. [DOI] [PubMed] [Google Scholar]