Abstract
Background:
Low-calorie sweeteners (LCSs) provide sweetness without sugar or calories and are used to replace added sugars by many children with type 1 diabetes (T1D). However, the role of LCSs in diabetes management and cardiometabolic health is unclear.
Objective:
The Diabetes Research in Kids Study (DRINK-T1D) study aims to investigate effects of LCS restriction on glycemic variability, visceral adiposity, lipid profiles, and systemic inflammation among children 6–12 years old with T1D.
Methods:
Children with T1D, who report habitual consumption of foods and beverages containing LCSs, are recruited from the Washington Nationals Diabetes Care Complex (DCC) at Children’s National Hospital (CNH) in Washington, DC. Following a phone screening and two-week run-in period involving continuation of usual LCS intake, children are randomized to 12 weeks of LCS restriction (replacement of diet beverages with still or sparkling water and avoidance of other sources of LCSs) or continued usual LCS intake (control). The primary outcome is the difference in change in glycemic variability in the LCS restriction group versus the control group. Change in glycemic variability will be assessed as the difference in daily average time-in-range (TIR), measured using continuous glucose monitoring (CGM) during two weeks at the end of the 12-week intervention, compared with during the two-week run-in period prior to randomization. Participants also complete a variety of anthropometric, metabolic, dietary, and behavioral assessments throughout the 14-week study.
Conclusions:
DRINK-T1D is an innovative, randomized controlled trial, evaluating effects of LCS restriction on glycemic variability and cardiometabolic health in children with T1D. Findings of DRINK-T1D will support or challenge the common practice of recommending LCS use in this patient population and will have clinically relevant implications for pediatric T1D management.
Keywords: diet soda, artificial sweeteners, pediatrics, type 1 diabetes, glycemic control
1. Introduction
Type 1 diabetes (T1D) is a lifelong metabolic disorder that affects 1 out of every 500 children in the United States annually [1]. Children diagnosed with T1D before the age of ten are at 11 times higher risk of cardiovascular disease (CVD), six times higher risk of stroke, and most notably, 31 times higher risk of coronary heart disease and myocardial infarction, compared with similar-aged children without T1D [2]. Furthermore, cardiometabolic perturbations, including abnormalities in circulating inflammatory cytokines, are observable in children with T1D despite their young age [3]. As demonstrated in the SEARCH cohort [4], a common strategy for T1D management is replacement of added sugars with low-calorie sweeteners (LCSs), which provide sweetness without calories and at a reduced glycemic load [5]. However, effects of LCSs on glycemic control and cardiometabolic health are controversial [6], and while use of LCSs instead of added sugars reduces beverage calories and sugar content, this replacement may paradoxically worsen glycemic variability and exacerbate cardiometabolic health.
While LCSs do not acutely raise blood glucose when administered alone, LCSs are metabolically active in humans [7, 8]. For example, sucralose reduces estimated insulin sensitivity [9] and dysregulates inflammatory pathways in adipose tissue in humans [10], accelerates intestinal glucose absorption in rodents when consumed with caloric sugars [11], and promotes glucose intolerance [12] and metabolic dysfunction [13] in mice, which may be induced via effects on gut microbiota [12–14]. Alterations of the gut microbiota and elevated fasting glucose levels [15] have also been reported in rodents following prolonged exposure to aspartame. Furthermore, incubation of human mesenchymcal stem cells with sucralose at clinically relevant dosages upregulates expression of glucose transporter genes, and markers of fat accumulation, inflammation, and oxidative stress [16, 17]. This is alarming because inflammation and hyperglycemia drive elevations in cardiometabolic risk in children with T1D [18] and positive associations between LCS consumption, metabolic syndrome [19, 20], CVD [21], and stroke [21, 22] are consistently reported in observational studies in adults without diabetes. These findings are particularly concerning for patients with T1D, who already struggle with glycemic variability and are at heightened CVD risk. However, in the absence of conclusive evidence demonstrating adverse effects of LCS on cardiometabolic risk factors, LCS consumption continues to be encouraged for pediatric T1D management.
1.1. Scientific premise
While the use of LCSs in place of added sugars reduces the calorie and sugar content of foods and beverages, this replacement may paradoxically worsen glycemic variability and cardiometabolic health among already at-risk children with T1D. This is particularly concerning because hyperglycemia and inflammation play a key role in accelerated CVD onset in T1D [18]. Meanwhile, little is known about effects of LCSs in children [5, 23], and the role of LCSs specifically among children with T1D is severely understudied.
1.2. LCS restriction
Because many children with T1D already consume LCSs, conducting a trial where children are assigned to consume LCSs is challenging. Further, a growing body of predominantly preclinical evidence suggests that LCS consumption may counterintuitively exacerbate cardiometabolic health [6], and thus, provision of LCSBs may induce unintended harm among children with T1D, who are already at elevated risk for development of cardiometabolic disease [24]. We are therefore investigating effects of LCS restriction among those who habitually consume LCSBs (the predominant contributors to LCS intake among children [25]). To our knowledge, the Diabetes Research in Kids (DRINK-T1D) study is the first to examine effects of LCS restriction on glycemic variability, visceral fat, lipid profiles, and systemic inflammation in metabolically-vulnerable children with T1D.
1.3. Study aims and hypotheses
DRINK-T1D is intended to serve as an initial step in elucidating the role of LCSs in cardiometabolic health among children with T1D. The study aims are as follows. Aim 1: To examine the effects of replacement of usual LCSB consumption with unsweetened still or seltzer water for 12 weeks on glycemic variability. Aim 2: To examine the effects of replacement of usual LCSB consumption with unsweetened still or seltzer water for 12 weeks on visceral adiposity, lipid profiles, and systemic inflammation. Aim 3: To examine the feasibility and acceptability of the DRINK-T1D intervention, in which children with T1D are asked to replace LCSB consumption with unsweetened alternatives and avoid other sources of LCSs (e.g., LCS-containing foods, condiments, and packets) for 12 weeks. We hypothesize that average daily time in the target glycemic range (TIR) will be increased and HbA1c reduced after 12 weeks of LCS restriction, compared with after 12 weeks of usual LCS consumption. We further hypothesize that visceral adiposity and circulating concentrations of inflammatory biomarkers will be reduced, and lipid profiles improved, in the intervention (LCS restriction), compared with the control group (usual LCS intake). The primary study outcome is the difference in change in glycemic variability in the LCS restriction group versus control, at the end of the 12-week intervention compared with before the intervention. Secondary outcomes include hemoglobin A1c (HbA1C), inflammatory cytokines (C-reactive protein (CRP), tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6)), lipid profiles (triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), free fatty acids (FFA)), and visceral adiposity (VAT), as well as intervention feasibility, acceptability, and adherence to LCS restriction. The study aims, hypotheses, and outcomes are summarized in Table 1 below.
Table 1.
Overview of study aims, hypotheses, and outcomes.
| Aim | Hypothesis | Outcomes of Interest |
|---|---|---|
| Aim 1: To examine the effects of replacement of usual LCSB consumption with unsweetened still or seltzer water for 12 weeks on glycemic variability. | Hypothesis 1: Average daily time in the target glycemic range (TIR) will be increased and HbA1c reduced after 12 weeks of LCS restriction, compared with after 12 weeks of usual LCS consumption. | Glycemic variability HbA1c |
| Aim 2: To examine the effects of replacement of usual LCSB consumption with unsweetened still or seltzer water for 12 weeks on visceral adiposity, lipid profiles, and systemic inflammation. | Hypothesis 2: Visceral adiposity and circulating concentrations of inflammatory biomarkers will be reduced, and lipid profiles improved after 12 weeks of LCS restriction, compared with after 12 weeks of usual LCS consumption. | Visceral adiposity Inflammatory cytokines1 (CRP, IL-6, TNF-α) Lipid profiles2 (TG, LDL, HDL, FFA) |
| Aim 3: To examine the feasibility and acceptability of the DRINK-T1D intervention, in which children with T1D are asked to replace LCSB consumption with unsweetened alternatives and avoid other sources of LCSs (e.g., LCS-containing foods, condiments, and packets) for 12 weeks. | Hypothesis 3: We hypothesize that we will achieve recruitment of ≥70% of eligible patients, ≥80% adherence to LCS restriction, and retention of ≥80% of enrolled subjects. We further hypothesize that LCS restriction will be acceptable and welltolerated. | Percentage of eligible participants recruited Percent adherence based on daily self-report questionnaires Percent adherence based on objective measures of urinary LCS concentrations Percentage of enrolled participants who complete the study |
CRP = C-reactive protein; IL-6 = interleukin-6; TNF- α = tumor necrosis factor alpha
TG = triglycerides; LDL = low-density lipoprotein; HDL = high-density lipoprotein; FFA = free fatty acids
2. Research Design and Methods
The DRINK-T1D study is a two-arm, parallel group, RCT, of 12 weeks duration (Figure 1). After two weeks of usual LCS intake (“run-in period”), children with T1D are randomized to replace their usual LCSB intake with unsweetened still or seltzer water and avoid other sources of LCSs, or to continue their usual LCS consumption, for 12 weeks.
Figure 1. Overview of the study design.
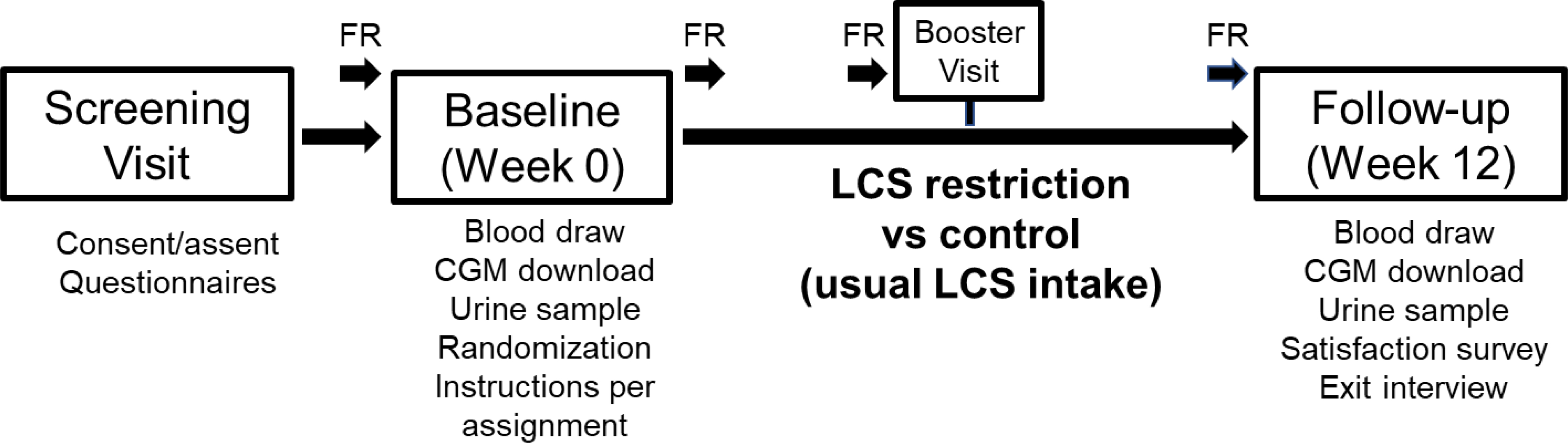
In DRINK-T1D, children with T1D are randomized to replace their usual LCSB intake with unsweetened still or seltzer water and avoid other sources of LCSs, or to continue their usual LCS consumption, for 12 weeks. Glycemic variability is monitored using continuous glucose monitoring (CGM) during a 2-week run-in period, prior to randomization, and for 2-weeks at the end of the 12-week intervention. Participants are also instructed to complete detailed, 7-day photo-assisted food records (FR) at four time points throughout the study.
This study is registered on ClinicalTrials.gov (NCT0438588). The Institutional Review Board (IRB) at Children’s National Hospital (CNH) reviewed and approved the study protocol. All participants provide informed consent (parent) and assent (child) prior to beginning the study procedures.
2.1. Study population
The target sample size is 60 children with T1D, 6–12 years of age, seen in the Washington Nationals Diabetes Care Complex (DCC) at Children’s National Hospital (CNH) in Washington D.C. Children are eligible if they are: (1) between the ages of 6 and 12 years old; (2) have had physician-diagnosed T1D for at least 12 months; (3) have reliable phone and internet access; (4) are seen for their T1D at CNH or CNH-affiliated satellite clinics; (5) use a DEXCOM™ continuous glucose monitor (CGM) and (6) report usual consumption of at least 12 ounces of LCSBs, sweetened with either sucralose (with or without acesulfame-potassium (ace-K)) or aspartame in combination with ace-K, on most days of the week (≥ 5 days/week). Exclusion criteria also include use of medications impacting cardiometabolic outcomes, other than insulin. The 6–12-year-old age range was selected because school-aged children consume LCSBs more frequently than younger children, and also have more parental oversight compared with adolescents, which is likely to facilitate intervention adherence. In addition, most females 6–12 years have not yet reached menarche, minimizing the likelihood of confounding by the menstrual cycle.
Children who report consumption of foods with LCS (e.g. sugar-free ice cream) or condiments with LCS (e.g. sugar-free syrup) are included, but are instructed to avoid consumption of these products for 12 weeks if randomized to LCSB restriction. This is because frequent ingestion of LCS from non-beverage sources would confound assessment of changes in the study outcomes. Children reporting consumption of LCS packets containing sucralose (e.g. Splenda™) or aspartame + ace-K (e.g. Equal™) are included, because packets are typically added to beverages and their consumption among children is generally low (<1%) [26].
2.2. Recruitment and screening
A trained research assistant (hereafter RA) at CNH (HRM) reviews clinic schedules on a weekly basis, and identifies potentially-eligible children, based on review of electronic medical records. A recruitment letter with study information is mailed to the potentially eligible child’s parent/guardian (hereafter parent). Approximately one week later, a RA (HRM or JHK) calls the parent to confirm receipt of the letter and gauge initial study interest. If the parent expresses interest, the RA (HRM or JHK) administers a brief LCS screener questionnaire and study eligibility checklist. While recruitment has taken place primarily virtually, due to the COVID-19 pandemic, the above-described recruitment and screening procedures (e.g., confirmation of letter receipt, determination of eligibility) may also take place in-person in the clinic waiting room.
2.3. Randomization and study intervention
Participants are randomly assigned to either LCS restriction (intervention group) or continuation of their usual LCS intake (control), for 12 weeks. One-to-one block randomization, stratified by the type of LCSBs habitually consumed (sweetened with either sucralose or aspartame + ace-K) is used to assign children to the intervention or control. A stratified randomization approach is used because different LCSs are likely to have different cardiometabolic effects. Parents of children randomized to the intervention (LCS restriction) are given a brochure on avoiding LCSs, which includes a list of specific foods and beverages containing LCSs. The child is also provided with several varieties of still and seltzer water samples to take home, in order to encourage adherence. Children randomized to the control group do not receive information and resources for avoiding LCSs. Rather, children in the control group are instructed to continue their usual LCS intake, consistent with standard clinical guidance provided by dietitians in the DCC, and are given a variety of sample LCSBs.
In both groups, participants and their parent undergo a brief, 20-minute, counseling session with a RA (HRM). During the session, the RA provides guidance on general healthy eating strategies, consistent with standard dietary guidance provided by the dietitians at CNH, as well as randomization-specific instructions to avoid or continue usual LCS intake.
2.4. Data collection schedule
A schedule of study procedures is shown in Table 2. Participants attend four study visits throughout the 14-week study: a virtual, enrollment visit conducted via Zoom™; an in-person, baseline visit at CNH (prior to randomization); a virtual, mid-intervention booster visit via Zoom™; and, an in-person, follow-up visit at CNH (end of intervention week 12). In addition, participants complete a variety of remote assessments at pre-determined timepoints throughout the study, including CGM, daily electronic questionnaires, and photo-assisted food records.
Table 2.
Schedule of the study procedures
| Timepoint | Location | Assessments | Procedures |
|---|---|---|---|
| Enrollment visit | Via Zoom™ | Eligibility determination, Enrollment, Questionnaires | Consent, assent, self-care (SCI), PROMIS questionnaire, COVID-19 questionnaire, LCS screener |
| Run-in period | Remotely | Diet assessment, glycemic variability | Food record, CGM, daily questionnaire |
| Baseline visit | CNH | Glycemic control (HbA1c), lipids, inflammation, visceral adiposity, anthropometrics | Height/weight, spot urine, blood draw, CGM download, DXA (subset) |
| Weeks 1–5 | Remotely | Adherence | Food record, daily questionnaire |
| Week 6 | Via Zoom™ | Diet assessment, adherence, intervention booster | Food record, daily questionnaire, spot urine1 |
| Week 7–10 | Remotely | Adherence | Daily questionnaire |
| Week 11–12 | Remotely | Diet assessment, glycemic variability, adherence | Food record, CGM, daily questionnaire |
| Follow-up visit | CNH | Glycemic control (HbA1c), lipids, inflammation, visceral adiposity, anthropometrics, acceptability, feasibility | Height/weight, spot urine, blood draw, SCI, satisfaction survey, qualitative interview, CGM download (for weeks 11 and 12), DXA (subset) |
Participants are provided with a bag containing materials for collecting and shipping a mid-intervention (week 6) urine sample at home, and returning it by mail to the study team. Postage is prepaid by the study team.
2.4.1. Enrollment visit
During the enrollment visit, informed consent and assent are obtained electronically, after which, the parent is asked to complete several questionnaires via RedCap™. Questionnaires include the Diabetes Self Care Inventory (SCI)[27], Patient Reported Outcomes Measurement Information System – Physical Activity survey (PROMIS-PA)[28], and a survey pertaining to how COVID-19 has impacted the child’s diabetes management. In addition, a detailed LCS intake questionnaire is administered by the RA (HRM or JHK). Following questionnaire completion, the parent is provided with instructions for completing the electronic beverage survey (Table 3), which is sent via email or text message (per participant preference) each day throughout the study. The daily beverage survey is used to assess the child’s beverage intake during the two-week run-in period and monitor adherence throughout the 12-week intervention.
Table 3.
Daily electronic beverage questionnaire
| 1. Did you drink any beverage(s) with artificial or low-calorie sweeteners (e.g., diet or light fruit drinks, diet soda, reduced sugar or “zero“ sports drinks, diet iced tea) today? | YES OR NO | |
| If yes, what beverage(s) with artificial or low-calorie sweeteners did you drink today and how much? | ||
| 2. Did you consume any food with low-calorie or artificial sweetener (e.g., sugar-free cookies, sugar-free desserts, or sugar-free candy, light yogurt, sugar-free oatmeal) today? | YES OR NO | |
| If yes, what sugar-free food(s) or foods with low-calorie or artificial sweeteners did you consume today and how much? | ||
| 3. Did you consume any low-calorie or artificial sweetener packets (e.g., Splenda™, Equal, Sweet N Low™, Truvia™) today? | YES OR NO | |
| If yes, what low-calorie or artificial sweetener packets did you consume today and how many? | ||
At the end of the enrollment visit, participants are instructed to continue their usual LCS intake and CGM use until their baseline visit. The baseline visit is scheduled for at least two weeks later, to allow for 14 days of CGM data collection prior to randomization. Participants are also instructed to complete a photo-assisted, 7-day, food record during the week prior to their baseline visit (see Measures), for which the RA provides detailed verbal instructions. The child and their parent are also provided with child- and parent-oriented food record completion instructions and a fillable electronic food record form.
2.4.2. Baseline Visit
Participants are scheduled for a baseline visit in the Clinical Research Unit (CRU) at CNH. The child’s height, weight, and vital signs are measured by a trained pediatric research nurse using standard procedures, and a spot urine sample is collected for measurement of urinary LCS concentrations. Next, the child’s CGM data for the two weeks prior are downloaded by the RA (HRM) using the DEXCOM Clarity™ software package. A fasted blood draw is performed by the research nurse, using standard venipuncture procedures. A subset of participants (n=30) also undergoes dual X-ray absorptiometry (DXA) for assessment of visceral adiposity, which is performed by a trained radiology technician at CNH. The subset is determined by asking each participant if they are interested in having DXA, until a sample of 15 participants in each randomized treatment group (n=30 total) undergoes DXA at both baseline and 12 weeks. Following the blood draw (and DXA, as applicable), participants are randomly assigned to either LCS restriction (intervention group) or continuation of usual LCS intake, for 12 weeks. Treatment-group specific counseling on avoiding LCSs or continuing usual LCS intake is provided by the RA (HRM), and children randomized to the LCS restriction group are provided with sample carbonated and still water beverages to take home, along with a brochure containing additional guidance for avoiding LCSs. The parent is instructed to continue completion of the daily electronic questionnaires throughout the intervention and is also asked to complete a second photo-assisted food record over the next 7 days, in order to monitor the child’s dietary intake during the first week of the intervention period. Food record instructions provided at the enrollment visit are reinforced and the parent is provided with additional instructions and food log forms, if necessary. The parent is also provided with a folder containing randomization-specific reminders and a bag containing materials for collecting a mid-intervention (week 6) urine sample at home and returning it, by mail, to the study team.
2.4.3. Mid-intervention booster visit
During week 6, the child and their parent attend a virtual, mid-intervention booster visit, which takes place via Zoom™. During the booster visit, a RA (HRM or JHK) reinforces treatment group specific instructions, per the child’s randomization. The parent is reminded to return their child’s mid-intervention spot urine sample by mail (for the purpose of monitoring adherence), if they have not done so already, using pre-paid shipping materials provided by the study team. Participants are also reminded to complete a third (mid-intervention, week 6) photo-assisted 7-day food record, using the instructions and food record forms previously provided.
2.4.4. Follow-up visit
At the beginning of week 12, a RA reminds the participant to complete their final, photo-assisted, 7-day, food record, prior to returning to CNH for their follow-up visit, which is scheduled at the end of that week, and at approximately the same time of day as their baseline visit. The child’s height, weight, and vital signs are again measured, another spot urine sample is provided, and a second fasted blood sample is collected, identical to at baseline. Children who underwent DXA at baseline have a second DXA scan, and their CGM data are again downloaded by the RA using the DEXCOM Clarity™ software.
The parent is then asked to complete a 5-minute satisfaction survey about the acceptability of the study, and children randomized to LCS restriction (intervention group) are purposefully sampled and asked to complete a 20-minute qualitative interview, together with their parent. Qualitative interviews, approximately 20 minutes in duration, are conducted by the PI (ACS) using a semi-structured interview guide. Questions focus on children’s and parents’ experiences with LCS restriction, as well as aspects of the intervention that were most challenging. Interviews are audio-recorded to facilitate accurate transcription for analysis.
2.5. Measures
2.5.1. Questionnaires
At the enrollment visit, the parent also completes several questionnaires including: a brief demographic questionnaire to obtain information on the child’s sex, race/ethnicity, and family income; the Patient Reported Outcomes Measurement Information System – Physical Activity survey (PROMIS-PA) [28], which is a 4-item parent-report of their child’s physical activity; a COVID-19 survey developed by investigators at CNH to examine the impact of the pandemic on children’s diabetes management and overall family functioning; and, a LCS intake survey developed by the research team, which assesses the child’s usual intake of LCS from beverages, foods, condiments, and packets. The parent also completes the SCI [27] a 14-item child and parent report measure of adherence with diabetes self-care behaviors, at both the enrollment and follow-up visits, in order to determine whether effects of LCS restriction differ based on adherence to diabetes self-care.
2.5.2. Glycemic variability
Participants are instructed to continue wearing their personal DEXCOM G6™ CGM throughout the study. CGM data are recorded every 5 min for up to 14 days with DEXCOM G6™, and no calibration is needed. CGM data are used to calculate average daily “time in range (TIR),” over the 14 days prior to baseline assessments, and during the final two weeks of the 12-week intervention. HbA1c is also measured at baseline and 12-week follow-up as an additional measure of glycemic control (see Laboratory analyses in section 2.5.4 below).
2.5.3. Dietary intake
Participants are instructed to complete photo-assisted 7-day food records during four specific weeks throughout the study. The first is the week prior to their baseline visit. The other three are during weeks 1, 6 (mid-intervention), and 12 (follow-up) of the intervention. The parent is instructed to provide a detailed record of all foods and beverages consumed by the child for 7 days, including portion sizes and brand information, and is asked to submit photos of each eating occasion via email or text message. Detailed written instructions for food record completion are provided to both the child and parent and include guidance for estimating portion sizes, and reminders to specify portion sizes and brand names, as well as to provide the corresponding photos.
2.5.4. Laboratory analyses
Urinary sucralose and ace-K are measured using liquid chromatography-mass spectrometry (LC-MS) by the Clinical Mass Spectrometry Laboratory at the National Institutes of Health (NIH). Assays are performed with an Acquity I-Class UPLC (Waters, Milford, MA, USA), coupled with a Q-Exactive MS (Thermo Scientific, Waltham, MA, USA). Blood samples are centrifuged at 4°C and the whole blood, plasma, and serum are sent to LabCorp for assessment of cardiometabolic outcomes, including HbA1c, CRP, IL-6, TNF-alpha, TG, LDL, HDL, and FFA, or stored at −80 degrees Celsius for future analyses. Assays for all cardiometabolic outcomes are conducted using commercially available assay kits and standard LabCorp (a large, nationwide, commercial laboratory) procedures.
2.5.5. Visceral adiposity
Dual X-ray absorptiometry (DXA) is performed by trained radiology technician, using a Hologic Horizons (Hologic, Inc.) DXA machine, and used to determine whether LCS restriction affects VAT. Total fat (g), lean mass (g) and fat mass in specific regions (e.g., legs, trunk) are recorded and visceral adiposity mass (g), volume (cm3) and area (cm2) are calculated using the Apex 5.6.0.4 software program provided by Hologic, Inc.
2.5.6. Adherence
Adherence will be assessed using several approaches. The primary method of assessing adherence will be using electronic questionnaires sent to the parent daily via text message or email (per participant preference) each evening throughout the study. Intervention adherence will also be monitored objectively through collection of spot-urine samples for measurement of LCS concentrations at baseline (in-person at CNH), during week 6 (urine sample collected at home and mailed to research team) and week 12 (in-person at CNH), which will allow for verification of the self-reported adherence data collected using daily electronic questionnaires. If discrepancies between the objectively measured (urinary LCS concentrations) and self-reported (daily questionnaires) data are identified, adherence will be further assessed using 7-day photo-assisted food records, which are completed electronically and emailed to the study team, during the week before and after baseline, as well as during weeks 6 (mid-intervention) and 12 (follow-up) of the intervention. Participants who are having difficulty with adherence are contacted by the RA, who suggests potential strategies for increasing adherence. Strategies for encouraging adherence are also discussed in detail with the child and their parent during the virtual, mid-intervention (week 6), booster visit.
2.6. Data analyses
2.6.1. Sample Size
Our primary outcome is the difference in change in glycemic variability in the LCS restriction group versus in the control group, calculated as difference in average daily TIR in weeks 11 and 12 (end of intervention period) compared with during the two-week run-in prior to randomization. Because no prior study has assessed glycemic variability before and after LCS restriction, we powered our study using changes before and after LCS consumption [12] in seven volunteers consuming saccharin (equivalent to 4–5 diet sodas per day) for 1 week. Based on this effect size (1564 ± 1852 (mg·dL·120 mins)−1 increase in glucose AUC post-LCS versus baseline), 13 participants per group provides 80% power to detect differences in glycemic control before and after LCS restriction versus control. Conservatively accounting for 20% attrition and the observation that only 60% (4 of 7) of participants in the prior study responded to LCS [12], 48 participants are needed to complete the analyses. However, because prior studies conducted in this patient population have generated 70–80% usable CGM data [29], we conservatively plan to enroll a total of 60 participants (n=30 per group).
2.6.2. Cardiometabolic outcomes
Data for all cardiometabolic outcomes, including CGM and laboratory assays are extracted by a RA from the DEXCOM Clarity™ or laboratory reports, respectively, and entered into RedCap™. Given the large volume of CGM assessments, missing data are likely, and the maximum number of days of glycemic data will be used without imputation. However, multilevel generalized linear modeling (required for analyses detailed below) is good at handling missing data and does not require the same number of observations per person. Univariate analyses are used to assess the distribution of all outcomes. All analyses are conducted using SAS 9.4 using an α-level of 0.05 (two-sided). Bivariate associations will be examined using odds ratios, chi-squared, boxplots, mean differences, and ANOVA, as appropriate. We will identify and validate outliers and non-parametric tests will be used for non-normal data. By establishing strengths of associations and highlighting irregular data, descriptive analyses will inform more complex models. To account for repeated measures within individuals, multilevel generalized linear models, with random subject intercepts will be used, to account for similarity of repeated observations taken from an individual. Analyses of all cardiometabolic outcomes will be performed using an intention to treat (ITT) approach. Adherence is computed as proportions, based on daily electronic questionnaires. Proportions will reflect the number of days in which participants consumed or avoided LCS, per their randomization, based on daily electronic questionnaires. Measures of adherence and assessments at enrollment, including age, sex, race/ethnicity, weight status, type and amount of usual LCS intake, and SCI scores, will be included as covariates in all models assessing responses to LCS restriction versus control. We will also conduct sensitivity analyses excluding participants with < 50% reported adherence.
2.6.3. Feasibility and acceptability
Rates of recruitment, enrollment, and completion will be used to examine feasibility. To assess acceptability, attrition is examined, and qualitative interviews are conducted at follow-up to probe for acceptability, feasibility, and situations where adherence was challenging. Qualitative interviews are transcribed, and subsequently coded by two coders. The two coders independently code a subset of the transcripts using the NVivo Pro software package (version 12; QSR International, Inc.; Burlington, MA, USA) and then create a shared codebook. New codes are added as they emerge, in order to develop the final codebook. Transcripts are then reviewed by the PI to ensure that coding of all transcripts is in accordance with the final codebook. Any discrepancies between coders are discussed until consensus is reached or the disagreement is resolved by a third team member, as necessary. The two coders independently identify preliminary emergent themes and subthemes, which are then discussed with a third team member. Themes and subthemes are further organized and refined, and quotations representative of each theme and subtheme are then selected.
2.7. Safety
The risks associated with the study procedures, including LCS restriction, CGM, blood draws, urine sample collection, DXA, dietary data collection, qualitative interviews, and questionnaire completion, are considered to be minimal, and do not exceed the exceed the risks encountered in routine medical procedures or in usual activities of daily life. The PI (ACS), study physician (FRC), and an independent data safety officer monitor adverse events. Adverse events are any untoward medical occurrences in participants undergoing a study-related procedure and believed reasonably to be caused by a study-related procedure. Adverse events are reviewed upon completion of each study participant and are classified as follows, with respect to the likelihood that they are related to the intervention or study procedures: definite, probable, possible, unlikely, or unrelated. Any adverse events identified are then graded based on their severity.
3. Discussion
DRINK-T1D is an ongoing RCT comparing a novel LCS restriction intervention with continuation of usual LCS intake among already metabolically-vulnerable children with T1D. LCSB intake has been previously reported to be associated with higher HbA1c and plasma triglycerides in children with T1D [4], yet no prior intervention study has investigated effects of LCS on cardiometabolic risk factors in this patient population. DRINK-T1D is the first study to examine effects of LCS restriction on cardiometabolic outcomes including glycemic variability, visceral fat, lipid profiles, and systemic inflammation. Given that reliance on LCSs is widespread among children with diabetes, results of DRINK-T1D are likely to have high clinical and public health relevance.
A growing and compelling body of preclinical evidence [17, 30, 31] and human observational [21, 32] and intervention [9, 33] studies demonstrate that LCS consumption may paradoxically increase cardiometabolic risk factors [34]. Meanwhile, the majority of RCTs investigating LCS effects on cardiometabolic health to date have been conducted in adults, and most focus on changes in body weight and adiposity [34]. Few RCTs have specifically evaluated LCS effects in individuals with an existing cardiometabolic disease, and the evidence surrounding cardiometabolic effects of LCS consumption in children is particularly scarce. As a result, scientific organizations including the American Heart Association (AHA) [35] and American Academy of Pediatrics (AAP) [36] have recently cautioned against use of LCSs among children; yet, LCSs, and specifically LCSBs, continue to be advised for pediatric T1D management. It is paramount to rigorously investigate the relationship between LCS consumption and cardiometabolic risk factors in this already at-risk patient population.
4. Strengths and Limitations
A key strength of our study is assessment of intervention adherence using both objective (urinary LCS concentrations) and self-report (questionnaires) approaches. While collection of urine samples at three timepoints does not provide comprehensive information about participants’ LCS intake throughout the study, urine LCS concentrations will allow us to verify the self-reported data collected. Another important strength of our study is collection of photo-assisted 7-day food records. Despite inherent limitations of self-report dietary assessment [37], collection of photographs will improve accuracy and provide detailed information on children’s diets prior to randomization, and at several timepoints throughout the intervention. Another important limitation is that the participants and intervention staff are not blinded to treatment allocation. However, the outcome assessors are blinded and measures of cardiometabolic outcomes (e.g., CGM data, laboratory assays) are objective. Furthermore, participants in both groups receive identical intervention content, except for whether to avoid or consume LCSs. Finally, although the time commitment required for study completion is significant, the use of mobile technologies for data collection reduces participant burden and facilitates maintenance of frequent contact with participants throughout the study, in order encourage adherence and enhance retention.
5. Conclusions
Findings of DRINK T1D are expected to justify or challenge the standard practice of encouraging LCSB use among children with T1D. This and future studies will have significant impacts on clinical diabetes management, and will inform how nutritional guidance is delivered at the time of T1D diagnosis and over the course of clinical care.
Acknowledgements
This work is being supported by the National Institutes of Diabetes and Digestive and Kidney Diseases, R21 DK12234501A1.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests
Trial Registration: ClinicalTrials.gov Identifier NCT04385888.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Menke A, Orchard TJ, Imperatore G, Bullard KM, Mayer-Davis E, Cowie CC, The prevalence of type 1 diabetes in the United States. Epidemiology 2013, 24, 773–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, Eliasson B, Gudbjornsdottir S, Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Snell-Bergeon JK, West NA, Mayer-Davis EJ, Liese AD, Marcovina SM, D’Agostino RB Jr., Hamman RF, Dabelea D, Inflammatory markers are increased in youth with type 1 diabetes: the SEARCH Case-Control study. J Clin Endocrinol Metab 2010, 95, 2868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bortsov AV, Liese AD, Bell RA, Dabelea D, D’Agostino RB Jr., Hamman RF, Klingensmith GJ, Lawrence JM, Maahs DM, McKeown R, Marcovina SM, Thomas J, Williams DE, Mayer-Davis EJ, Sugar-sweetened and diet beverage consumption is associated with cardiovascular risk factor profile in youth with type 1 diabetes. Acta Diabetol 2011, 48, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sylvetsky A, Rother KI, Brown R, Artificial sweetener use among children: epidemiology, recommendations, metabolic outcomes, and future directions. Pediatr Clin North Am 2011, 58, 1467–1480, xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Swithers SE, Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab 2013, 24, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sylvetsky AC, Brown RJ, Blau JE, Walter M, Rother KI, Hormonal responses to non-nutritive sweeteners in water and diet soda. Nutr Metab (Lond) 2016, 13, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S, Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 2013, 36, 2530–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lertrit A, Srimachai S, Saetung S, Chanprasertyothin S, Chailurkit LO, Areevut C, Katekao P, Ongphiphadhanakul B, Sriphrapradang C, Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: a randomized, double-blind, placebo-controlled trial. Nutrition 2018, 55–56, 125–130. [DOI] [PubMed] [Google Scholar]
- [10].Sylvetsky AC, Sen S, Merkel P, Dore F, Stern DB, Henry CJ, Cai H, Walter PJ, Crandall KA, Rother KI, Hubal MJ, Consumption of Diet Soda Sweetened with Sucralose and Acesulfame-Potassium Alters Inflammatory Transcriptome Pathways in Females with Overweight and Obesity. Mol Nutr Food Res 2020, 64, e1901166. [DOI] [PubMed] [Google Scholar]
- [11].Mace OJ, Affleck J, Patel N, Kellett GL, Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 2007, 582, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E, Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [DOI] [PubMed] [Google Scholar]
- [13].Olivier-Van Stichelen S, Rother KI, Hanover JA, Maternal Exposure to Non-nutritive Sweeteners Impacts Progeny’s Metabolism and Microbiome. Front Microbiol 2019, 10, 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bian X, Chi L, Gao B, Tu P, Ru H, Lu K, Gut Microbiome Response to Sucralose and Its Potential Role in Inducing Liver Inflammation in Mice. Front Physiol 2017, 8, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Palmnas MS, Cowan TE, Bomhof MR, Su J, Reimer RA, Vogel HJ, Hittel DS, Shearer J, Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS One 2014, 9, e109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sen S, Rouphael C, Houston S, Sylvetsky AC, Rother KI, 75th Scientific Sessions of the American Diabetes Association, Boston, MA 2016, p. A560.
- [17].Kundu N, Domingues CC, Patel J, Aljishi M, Ahmadi N, Fakhri M, Sylvetsky AC, Sen S, Sucralose promotes accumulation of reactive oxygen species (ROS) and adipogenesis in mesenchymal stromal cells. Stem Cell Res Ther 2020, 11, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heier M, Margeirsdottir HD, Brunborg C, Hanssen KF, Dahl-Jorgensen K, Seljeflot I, Inflammation in childhood type 1 diabetes; influence of glycemic control. Atherosclerosis 2015, 238, 33–37. [DOI] [PubMed] [Google Scholar]
- [19].Lutsey PL, Steffen LM, Stevens J, Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008, 117, 754–761. [DOI] [PubMed] [Google Scholar]
- [20].Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR Jr., Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009, 32, 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gardener H, Rundek T, Markert M, Wright CB, Elkind MS, Sacco RL, Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med 2012, 27, 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pase MP, Himali JJ, Beiser AS, Aparicio HJ, Satizabal CL, Vasan RS, Seshadri S, Jacques PF, Sugar- and Artificially Sweetened Beverages and the Risks of Incident Stroke and Dementia: A Prospective Cohort Study. Stroke 2017, 48, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Archibald AJ, Dolinsky VW, Azad MB, Early-Life Exposure to Non-Nutritive Sweeteners and the Developmental Origins of Childhood Obesity: Global Evidence from Human and Rodent Studies. Nutrients 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee SI, Patel M, Jones CM, Narendran P, Cardiovascular disease and type 1 diabetes: prevalence, prediction and management in an ageing population. Ther Adv Chronic Dis 2015, 6, 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA, Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. Journal of the Academy of Nutrition and Dietetics 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA, Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J Acad Nutr Diet 2017, 117, 441–448.e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lewin AB, LaGreca AM, Geffken GR, Williams LB, Duke DC, Storch EA, Silverstein JH, Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: the Self-Care Inventory (SCI). J Pediatr Psychol 2009, 34, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tucker CA, Bevans KB, Becker BD, Teneralli R, Forrest CB, Development of the PROMIS Pediatric Physical Activity Item Banks. Phys Ther 2020, 100, 1393–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tully CB, Toaff M, Herbert L, DiPietro L, Henderson C, Cogen F, Streisand R, Acceptability and Feasibility of Examining Physical Activity in Young Children with Type 1 Diabetes. J Pediatr Health Care 2018, 32, 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shearer J, Swithers SE, Artificial sweeteners and metabolic dysregulation: Lessons learned from agriculture and the laboratory. Rev Endocr Metab Disord 2016, 17, 179–186. [DOI] [PubMed] [Google Scholar]
- [31].Plows JF, Morton-Jones J, Bridge-Comer PE, Ponnampalam A, Stanley JL, Vickers MH, Reynolds CM, Consumption of the Artificial Sweetener Acesulfame Potassium throughout Pregnancy Induces Glucose Intolerance and Adipose Tissue Dysfunction in Mice. J Nutr 2020. [DOI] [PubMed] [Google Scholar]
- [32].O’Connor L, Imamura F, Lentjes M, Khaw K, Wareham N, Forouhi N, Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia 2015, 58, 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, Gomez-Diaz RA, Almeda-Valdes P, Sucralose decreases insulin sensitivity in healthy subjects: a randomized controlled trial. Am J Clin Nutr 2018, 108, 485–491. [DOI] [PubMed] [Google Scholar]
- [34].Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiander M, MacKay DS, McGavock J, Wicklow B, Zarychanski R, Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Johnson RK, Lichtenstein AH, Anderon AM, Carson JA, Despres JP, Hu FB, Kris-Etherton PM, Otten JJ, Towfighi A, Wylie-Rosett J, Low-calorie Sweetened Beverages and Cardiometabolic Health: A Science Advisory from the American Heart Association Circulation 2018, 138. [DOI] [PubMed] [Google Scholar]
- [36].Baker-Smith CM, de Ferranti SD, Cochran WJ, Committee On Nutrition SOGH, Nutrition, The Use of Nonnutritive Sweeteners in Children. Pediatrics 2019, 144. [DOI] [PubMed] [Google Scholar]
- [37].Murakami K, Livingstone MB, Prevalence and characteristics of misreporting of energy intake in US children and adolescents: National Health and Nutrition Examination Survey (NHANES) 2003–2012. Br J Nutr 2016, 115, 294–304. [DOI] [PubMed] [Google Scholar]


