Abstract
Among natural products, essential oils from aromatic plants have been reported to possess potent anticancer properties. In this work, we aimed to perform the cytotoxic concentration range screening and antiproliferative activity screening of chemically characterized Thymus vulgaris L. essential oil. In vivo bioassay was conducted using the brine shrimp lethality test (BSLT). In vitro evaluation of antiproliferative activity was carried out on three human tumor cell lines: breast adenocarcinoma MCF-7, lung carcinoma H460 and acute lymphoblastic leukemia MOLT-4 using MTT assay. Essential oil components thymol (36.7%), p-cymene (30.0%), γ-terpinene (9.0%) and carvacrol (3.6%) were identified by gas chromatography/mass spectrometry. Analyzed essential oil should be considered as toxic/highly toxic with LC50 60.38 µg/mL in BSLT and moderate/weakly cytotoxic with IC50 range 52.65–228.78 µg/mL in vitro, according to evaluated cytotoxic criteria. Essential oil induced a dose-dependent inhibition of cell proliferation in all tested tumor cell lines and showed different sensitivity. Dose dependent toxicity observed in bioassay as well as the in vitro assay confirmed that brine shrimp lethality test is an adequate method for preliminary toxicity testing of Thymus vulgaris L. essential oil in tumor cell lines.
Subject terms: Cancer, Plant sciences
Introduction
According to official data from the World Health Organization, cancer is the second leading cause of death in the world population and it is estimated that 9.6 million people died from this disease in 20181. According to the latest issue of the International Agency for Research on Cancer (IARC), September 2018, lung cancer dominates, as the most common form of cancer and is also the most common cause of death among men. It is followed by prostate cancer and colorectal cancers by incidence and liver and stomach cancers by mortality2. When it comes to women, breast cancer is the most commonly diagnosed cancer and is the leading cause of cancer mortality, followed by colorectal and lung cancer by incidence. Cervical cancer ranks fourth in incidence and mortality2. Acute lymphoblastic leukemia is the most common hematological cancer in children and adolescents, representing 20% of all cancers diagnosed in persons aged < 20 years in the United States3.
More than two thirds of drugs currently used as anticancer agents have plant origin. Key hallmarks of anticancer therapy approach include inducing cancers cell death and reducing their sustained proliferative signaling and growth4.
Certain essential oils (EOs) have been labeled as promising anticancer agents and are currently being investigated for their cytotoxic and antiproliferative activities in cancer cell lines or experimental animals5. Different mechanisms have been reported for cytotoxic effects of EOs or their constituents. These include induction of cell death by apoptosis and/or necrosis, antimutagenic, antiproliferative, antioxidant properties, cell cycle arrest, and loss of key organelle functions6. According to the research findings, essential oils possess anticancer potential against mouth, breast, lung, prostate, liver, colon, and brain cancer and even in leukemia7. Studies indicate that the specific components of essential oils increase the cytotoxic activity of chemotherapeutic drugs (docetaxel, paclitaxel, 5-fluorouracil) on different cell lines and thus open the possibility of reducing their dose while providing the same effect8.
Oxygenated monoterpenes and monoterpene hydrocarbons represent the main chemical components of Thymus vulgaris L. essential oil. The natural terpenoid thymol and its phenol isomer carvacrol are the most abundant compounds of this oil. Non-clinical data reported that thyme essential oil exhibits strong antibacterial, antifungal, spasmolytic, antioxidant, anti-inflammatory activities9. On the basis of a summary review of available data, it is evident that thymol may be a promising plant-derived cancer chemotherapeutic agent10. T. vulgaris L. extracts are reported to possess anticancer potential, according to the several researches. These include significant free radical scavenging activity and proapoptotic effects in the human BC T47D cell line11, inhibition of proliferation of colorectal HCT116 cancer cell line12, and tumor inhibitory effects on human leukemia THP-1 cells13. The capability of the T. vulgaris L. essential oil to significantly inhibit the growth of human oral cavity squamous cell carcinoma has been reported14. Evaluation of cytotoxic activity of thyme essential oil demonstrated antiproliferative and proapoptotic effects on the two independent human breast adenocarcinoma cell lines15. However, a further investigation is needed in order to estimate the anticancer potential of thyme essential oil.
It has been proven that the brine shrimp lethality test (BSLT) has a good correlation with cytotoxic activity in some human solid tumors16. Application of BSLT in cytotoxic assays has been described for several essential oils17. Although the BSLT is able to identify strong anticancer activity of tested compound, its main limitation is its sensitivity to distinguish between strong to moderate and weak anti-cancer potentials18. Therefore, the BSLT represents a screening tool for potential cytotoxins, but a more sensitive distinction of anticancer activity requires testing on human cancer cells.
This paper focuses on the analysis of the antiproliferative activity of the chemically characterized T. vulgaris L. essential oil against breast adenocarcinoma (MCF-7) cell line, lung carcinoma (H460) cell line and acute lymphoblastic leukemia (MOLT-4) cell line. Moreover, cytotoxic concentration range screening and cytotoxicity validation in cancer cell lines and brine shrimp lethality of thyme essential oil were tested and evaluated.
Results
Chemical composition analysis
The essential oil of T. vulgaris L. isolated by hydro-distillation was of yellowish color, clear with an aromatic odor of thymol, in total yield of 1.5% (v/w) on dry weight basis. Qualitative and quantitative analytical results were obtained using gas chromatography/mass spectrometry (GC–MS) (see Supplementary Fig. S1). Table 1 shows the compounds identified in the essential oil of T. vulgaris L. in order of elution on ZB-5 MS capillary column. The percentage content of the individual components, retention indices and chemical class distribution are summarized.
Table 1.
Chemical composition of Thymus vulgaris L. essential oil.
| Component no | Componentsa | RT (minutes) | RIb | RIc | Percentage (%) |
|---|---|---|---|---|---|
| 1 | Methyl isobutyrate | 2.77 | 780 | 780 | 0.40 |
| 2 | α-Thujene | 5.25 | 930 | 930 | 1.00 |
| 3 | α-Pinene | 5.45 | 932 | 934 | 1.50 |
| 4 | Camphene | 5.86 | 946 | 956 | 1.00 |
| 5 | β-Pinene | 6.53 | 974 | 978 | 0.70 |
| 6 | 1-Octen-3-ol | 6.53 | 979 | 981 | 0.70 |
| 7 | 3-Octanone | 6. 67 | 983 | 986 | 0.10 |
| 8 | Myrcene | 6.78 | 987 | 990 | 1.50 |
| 9 | 3-Octanol | 6.99 | 988 | 991 | 0.10 |
| 10 | α-Terpinene | 7.60 | 1014 | 1020 | 1.90 |
| 11 | p-Cymene | 7.85 | 1024 | 1029 | 30.00 |
| 12 | Limonene | 7.97 | 1024 | 1033 | 0.70 |
| 13 | 1,8-Cineole | 8.07 | 1026 | 1037 | 1.40 |
| 14 | γ-Terpinene | 8.83 | 1059 | 1062 | 9.00 |
| 15 | Linalool | 10.12 | 1096 | 1100 | 2.40 |
| 16 | Camphor | 11.73 | 1146 | 1152 | 0.70 |
| 17 | Borneol | 12.56 | 1165 | 1167 | 1.70 |
| 18 | Terpinen-4-ol | 12.83 | 1147 | 1183 | 0.90 |
| 19 | α-Terpineol | 13.32 | 1186 | 1196 | 0.40 |
| 20 | Methyl thymyl ether | 14.43 | 1235 | 1231 | 0.30 |
| 21 | Methyl carvacryl ether | 14.74 | 1244 | 1241 | 0.40 |
| 22 | Bornyl acetate | 16.32 | 1287 | 1286 | 0.30 |
| 23 | Thymol | 16.54 | 1290 | 1292 | 36.70 |
| 24 | Carvacrol | 16.78 | 1299 | 1298 | 3.60 |
| 25 | β-Caryophyllene | 20.71 | 1417 | 1420 | 0.40 |
| Total identified | 100 | ||||
| Monoterpene hydrocarbons | 47.60 | ||||
| Oxygenated monoterpenes | 49.20 | ||||
| Other monoterpenes | 1.00 | ||||
| Aliphatic compounds | 1.30 | ||||
| Sesquiterpene hydrocarbons | 0.40 | ||||
| Oxygenated sesquiterpene | 0.50 |
aCompounds listed in order of eluation from a ZB-5 MS column.
bLiterature retention indices.
cIndices relative to C8-C20 n-alkanes on the ZB-5 MS column.
Out of a total of 32 identified components, 25 components presented in Table 1 represent 97.8% of the total content of the thyme essential oil. This oil was characterized by high percentage of monoterpene hydrocarbons (47.6%) and oxygenated monoterpenes (49.2%), represented in almost equal quantities, and made up ~ 97% of the oil composition. In contrast, aliphatic compounds (1.30%), sesquiterpene hydrocarbons (0.4%) and oxygenated sesquiterpene (0.5%) fractions were lower. As shown in Table 1, p-cymene (30.0%) and γ-terpinene (9.0%) were the major components within the monoterpene hydrocarbon fraction. The most significant components within the oxygeneted monoterpene fraction were thymol (36.7%) and carvacrol (3.6%).
Brine shrimp lethality test
The LC50 values obtained from brine shrimp lethality bioassay for T. vulgaris L. essential oil and thymol as positive control were 60.38 μg/mL and 16.67 μg/mL, respectively. The valorized toxicity of essential oil by comparison to Meyer’s and Clarkson’s toxicity index is summarized in Table 2.
Table 2.
The results of cytotoxic activity of Thymus vulgaris L. essential oil and positive control thymol.
| Compound | Concentration (µg/mL) | LC50 (µg/mL) | 95% confidence interval | Toxicity class (Meyer/Clarkson) | ||
|---|---|---|---|---|---|---|
| Thymus vulgaris L. essential oil | [3.9–1000] | [60.38] | [27.12–134.26] | Toxic/highly toxic | ||
| Thymol | [3.9–1000] | [16.67] | [5.20–56.29] | Toxic/highly toxic | ||
Antiproliferative evaluation
Results of the antiproliferative evaluation of tested essential oil and of the doxorubicin, as referent drug are presented by (Fig. 1a,b, 2a,b). Essential oil of the T. vulgaris L. at 72 h after treatment had inhibitory potential on proliferation of tested cancer cells. The results of the antiproliferative activity suggested that T. vulgaris L. essential oil induced a dose-dependent inhibition of cell proliferation in all tested tumor cell lines, although each culture showed different sensitivity, in accordance to determined IC50.
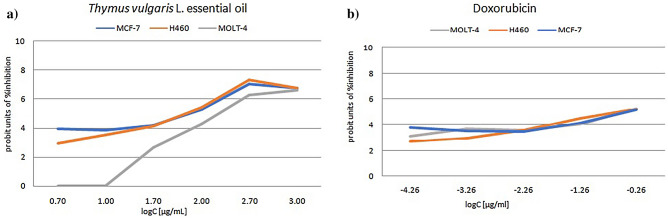
Figure 1.
Probit units of growth inhibition of cancer cell lines. (a) Probit units of growth inhibition of MCF-7, H460 and MOLT-4 cells in vitro, assessed by MTT assay 72 h after the addition of Thymus vulgaris L. essential oil. (b) Probit units of growth inhibition of MOLT-4, H460 and MCF-7 cells in vitro, assessed by MTT assay 72 h after the addition of doxorubicin.
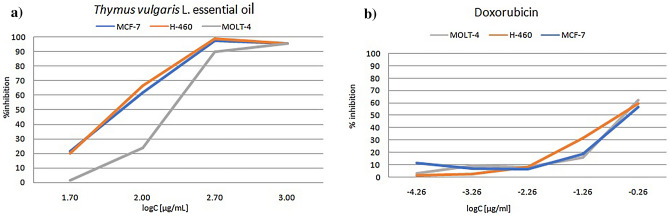
Figure 2.
Growth inhibition of cancer cell lines. (a) Growth inhibition of MCF-7, H460 and MOLT-4 cells in vitro, assessed by MTT assay 72 h after the addition of Thymus vulgaris L. essential oil. (b) Growth inhibition of MOLT-4, H460 and MCF-7 cells in vitro, assessed by MTT assay 72 h after the addition of doxorubicin.
Table 3 presents the IC50 values determined from the dose–response curves of the thyme essential oil and the used anticancer drug on MOLT-4, MCF-7 and H460 cells lines. The strongest antiproliferative activity of T. vulgaris L. essential oil was observed against MCF-7 cell line (IC50 52.65 μg/mL) followed by H460 cell line (IC50 68.59 μg/mL) and MOLT-4 cells (IC50 228.78 μg/mL).
Table 3.
In vitro screening cytotoxic activity of Thymus vulgaris L. essential oil and positive control doxorubicin.
| IC50 (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Compound | Cell lines | |||||
| MOLT-4 | 95% confidence interval | MCF-7 | 95% confidence interval | H460 | 95% confidence interval | |
| Thymus vulgaris L. essential oil | [228.78] | [118.23–442.66] | [52.65] | [11.35–244.13] | [68.59] | [22.49–209.09] |
| Doxorubicin | [0.399] | [0.295–0.504] | [0.459] | [0.455–0.463] | [0.381] | [0.377–0.386] |
IC50: the concentration that causes 50% growth inhibition, as assessed by MTT assay, 72 h after the addition of the test substances. The results are shown as mean values of quadruplicates.
Discussion
Dried Thymi herba contains up to 2.5% essential oil9, which corresponds to the total yield of essential oil obtained in our study (1.5%) (v/w). According to the literature, different chemotypes of T. vulgaris L. have been reported, providing evidence of intraspecific chemical variability of this plant species. There are at least 6 chemotypes of T. vulgaris L.19, with different compositions of the essential oil. The main components are thymol, carvacrol, p-cymene, γ-terpinene, linalool, β- myrcene and terpinen-4-ol9. The above results show that chemical composition of the T. vulgaris L. essential oil, native to Bosnia and Herzegovina, was dominated by thymol (36.7%), p-cymene (30.0%), γ-terpinene (9.0%), carvacrol (3.6%). These results are in agreement with previous published data on thyme essential oil from different geographical regions in Europe20, reporting that monoterpene alcohol and monoterpene hydrocarbon fractions were the most abundant constituents in the oil of T. vulgaris L. The sum of phenolic compounds (thymol and carvacrol) in the studied oils varied from 19.4 to 84.4%, and the sum of their precursors (p-cymene and γ-terpinene) ranged from 5.7 to 38.5%21. The effect of different geographical zone on percentage composition was also confirmed by the research of Satyal et al. who worked on thyme collected from different regions22. The result of their study showed that the T. vulgaris L. collected from France showed higher percentage of linalool (76.2%) and linalyl acetate (14.3%) where the thyme obtained from Serbia showed the higher presence of geraniol (59.8%) and geranyl acetate (16.7%). The chemical composition analysis obtained suggests that T. vulgaris L. essential oil isolated in this study can be classified as ‘thymol’ chemotype, with thymol as the predominant compound, which complies with the definition in the European Pharmacopoeia23. Sum of the contents of thymol and carvacrol also meets the requirements of European Pharmacopoeia (minimum 40.0%)23.
The variations during the vegetative cycle in yield and percentage of chemical composition content is also reported20. Hudaib et el. confirmed that young plant, collected in June/July just before the end of the vegetative cycle, provided the best oil yield with also the highest % content of the monoterpene fractions (thymol and carvacrol)20. In this study, the appropriate harvest time of thyme material is chosen in order to obtain an essential oil with of appropriate quality and quantity.
The significant correlation between the BSLT and in vitro growth inhibition of human solid tumor cell lines demonstrated by the National Cancer Institute (NCI, USA) is significant because it shows the value of this bioassay as a pre-screening tool for antitumor drug research24. Hence, we assumed that this method could be used in evaluating essential oil toxicity for predicting cytotoxic concentration range and cytotoxicity validation in cancer cell lines. The toxicity of tested essential oil by comparison to Meyer’s and Clarkson’s toxicity index showed that analyzed essential oil should be considered as toxic/highly toxic with LC50 60.38 μg/mL in BSLT. T. vulgaris L. essential oil showed 100% toxicity in brine shrimp model assay provided by Bogavac et al.25. Although the essential oil is obtained from the same plant species (T. vulgaris L.) the difference in LC50 values and % toxicity of the thyme essential oil exists, compared to our study. By comparing the GC–MS analysis of the tested oils, a difference in proportions of monoterpene alcohol and monoterpene hydrocarbon fractions is observed. In contrast to our results, the contribution of monoterpene alcohols (thymol and carvacrol), in EO obtained from Bogavac et al.25, is doubled, whereas the contribution of monoterpene hydrocarbons (p-cymene and γ-terpinene) is significantly decreased. The toxicity effect in BSLT of above mentioned EOs can be assumed, regarding the high percentage of thymol and carvacrol. Differences in the LC50 values of essential oils from different species of the genus Thymus in BSLT has also been evaluated. Thymus serpyllum L. essential oil showed LC50 value of 37.99 µg/mL17, compared to higher LC50 values (60.38 µg/mL) of T. vulgaris L. essential oil in our study. Toxicity of different extracts of T. vulgaris L. is also reported26. Al-Balushi et al. concluded that non polar organic extracts (chloroform and petroleum ether extracts) are toxic against the brine shrimp nauplii, whereas polar fractions are not showing toxic activity (hydroalcoholic extract)26. All in all, the thyme essential oil showed activity in the brine shrimp assay with LC50 values as relevant preliminary toxicity parameter for further toxicity studies.
In order to gain relevant data on cytotoxicity concentration range of thyme essential oil, it was necessary that in vitro testing was involved. Until now, no study had been conducted testing antiproliferative activity of thyme essential oil on lung carcinoma cell line H460 and acute lymphoblastic leukemia cell line MOLT-4. Our research contributes mainly to defining the IC50 values of tested essential oil for listed cell lines.
Our data demonstrated that T. vulgaris L. essential oil inhibited the viability of MCF-7, H460 and MOLT-4 cell lines in a concentration-dependent manner. At the concentrations ranging from 5 to 1000 μg/mL an antiproliferative effect was observed for each cell line MCF-7, H460 and MOLT-4 with IC50 values 52.65 µg/mL, 68.59 µg/mL, 228.78 µg/mL, respectively. Based on the results of the antiproliferative effect of thyme essential oil on the observed cell lines presented in (Fig. 1a,b), it is obvious that solid tumor cell lines MCF-7 and H460 showed very similar sensitivity to the essential oil. The hematological tumor cell line MOLT-4 is less sensitive to essential oil than solid tumor cell lines MCF-7 and H460, which correlates with the obtained IC50 values. Our research with T. vulgaris L. essential oil, native to Bosnia and Herzegovina with its unique chemical composition, contributes to previously published statements on good correlation between BSLT bioassay and cytotoxic activity in human solid tumors16. Although, there is a difference in sensitivity of solid and hematological tumor cell line to thyme essential oil, it can be noticed that plateau effect of percentage of inhibition of cell proliferation is observed in concentrations of 500–1000 µg/mL. For all tested cell lines, a high level of inhibition of cell proliferation in the indicated concentrations was approximately 96%. T. vulgaris L. essential oil showed moderate cytotoxic activity against MCF-7 and H460 cell lines, compared to weak cytotoxic activity against MOLT-4 cell line, according to a criteria based on NCI and Geran protocols27. Beside our research results, T. vulgaris L. essential oil has been observed to significantly inhibit growth of human oral cavity squamous cell carcinoma with IC50 value of 369.55 µg/mL14. Cytotoxicity of thyme essential oil towards head and neck squamous cell lines (HNSCC) was evaluated by standard 2,3-bis [2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt (XTT) assay, after 72 h incubation. In vitro study performed by Kubatka et al. suggested the proapoptotic potential of T. vulgaris L. essential oil, by showing the activation of a mitochondria-induced apoptosis pathway in two independent human breast adenocarcinoma cell lines (MCF-7 and MDA-MB-231)15. All these studies, were conducted with T. vulgaris L. essential oil of similar chemical composition to the one used in this study.
Given the limited data on an in vitro antiproliferative activity of thyme essential oil, the obtained IC50 values for the tested tumor cell lines represent a significant contribution to the database on a cytotoxic activity of essential oils. In some cases, this activity was attributed to specific components of the oil. The chemical complexity of the essential oils as a phyto-complex contributes to its biological effects, as each constituent takes part in the overall outcome and may modulate the effects of the others. Studies on individual ingredients might report results that do not recapitulate the effect of treatment with the phyto-complex as a whole.
Our GC–MS analysis showed that the most abundant metabolites in thyme essential oil are thymol and p-cymene. Based on previously reported in vivo and in vitro results, both molecules could be possible candidates for lead molecules of cytotoxic activity14,15. However, the cytotoxic effect of other components present at lower percentages in the essential oil must be considered. From the early reports, thymol has been reported to exert anti-carcinogenic activity through a different mechanism of action on the cancer cells lines. These include oxidative stress and cancer cell death28, apoptotic cancer cell death29 and antiproliferative effects on cancer cells30. Contrary, antioxidant activity31, protective effects32, antiapoptotic effects31, anti-inflammatory/immunomodulatory effects33 and antigenotoxic effects34 may be the key mechanisms of thymol’s anti-carcinogenic activity in normal cells. The interesting observation reported by Oliviero et al.35 was that the same treatments with thyme extract that had no effect on viability of normal human epithelial cells, were able to induce necrotic cell death in H460 lung cancer cell line. The growth of A17 triple negative breast carcinoma cells transplanted into Friend leukemia virus B (FVB) syngeneic mice was remarkably inhibited by the ruthenium (II) p-cymen complex throught inhibition of the tumor infiltration of regulatory T cells36. The induction of p53 protein expression in MCF-7 cells and reduction of their ability to invade have also been reported for ruthenium (II) p-cymene complexes37. Ferraz et al. demonstrated that p-cymene was cytotoxic to mouse melanoma B16-F10 cell lines, showing IC50 = 20.06 µg/mL38. Carvacrol induced apoptosis via mitochondrial membrane permeabilization in the metastatic breast cancer cell line (MDA-MB-231)39. These data, together with the results obtained by our group, demonstrate that thymol, p-cymene and T. vulgaris L. essential oil as a phyto-complex have a potential therapeutic significance and should not be overlooked as possible therapeutics in treating cancer. The benefit of thyme essential oil as a chemotherapeutic of plant origin is reflected in the different mechanism of action on normal cells compared to cancer cells40. During the use of thyme oil expectorant, spasmolytic, antioxidant, antimicrobial, invigorating, eupeptic, appetizing and choleretic properties have been manifested9. When administered in the recommended posology, Thymus vulgaris L. essential oil is recognized as safe9. The Food and Drug Administration (FDA) has stated for thyme oil (for which thymol is a component) that it is Generally Recognized as Safe (GRAS) essential oil41.
Conclusions
Dose dependent toxicity was observed in brine shrimp lethality assay as well as the in vitro assay conducted in cancer cell lines supporting the presence of bioactive compounds in T. vulgaris L. essential oil. The results of our study show that the essential oil of T. vulgaris L. has antiproliferative activity against several malignant cell lines (MOLT-4, MCF-7 and H460). This study has, for the first time, demonstrated cytotoxic activity of thyme essential oil to a lung carcinoma and an acute lymphoblastic leukemia cell line. We suggest that the antiproliferative activity of phytochemicals present in T. vulgaris L. essential oil is cancer cell line dependent. Moreover, we assumed that results obtained in this study point to the activity of the more abundant compounds thymol and p-cymene in analyzed thyme oil. The previously stated positive correlation between BSLT and in vitro testing on cancer cell lines are in accordance with results obtained in this study. This confirms, that BSLT is an adequate method for preliminary toxicity testing in tumor cell lines.
Methods
Chemical and materials
Plant material
Aerial parts of wild growing plant, Thymus vulgaris L., in the first year of growth, were collected in June 2018 in Bosnia and Herzegovina (location: Višići, near the city Čapljina, Latitude N 43° 04′ 02″; Longitude E 17° 42′ 44″). The permission for collecting Thymus vulgaris L. was obtained by the Federal Ministry of Enviroment and Tourism, Bosnia and Herzegovina. The identification of the experimental plant material,Voucher specimen No. 115/18, was carried out by the plant taxonomist Dr. Samir Đug, and voucher specimen deposited in the official herbarium of the Department of Biology and Ecology, Faculty of Sciences, University of Sarajevo. After plant material (1 kg) had been collected, the samples were dried at room temperature. Plant material was stored under nitrogen.
Isolation of the essential oil
A total of 30 g air-dried herb (leaf, flower and stem) of T. vulgaris L. was mixed with 400 mL of distilled water. The mixture was subjected to hydro distillation using a Clevenger-type apparatus (Klaus Hofmann GmbH, Germany) according to the European Pharmacopoeia 8.0. at flow rate of 2.5 ml/min for 2 h23. The essential oil was collected, determined volumetrically, dried under anhydrous sodium sulphate and stored in a refrigerator at 4 °C in a vial prior to analysis. Distillations were performed at least three times and the mean content of essential oil was calculated.
Gas chromatography-mass spectrometry (GC–MS) analysis of the essential oil
The chemical components of the analyzed essential oil were separated by the gas chromatography and identified by the mass spectrometry using Thermo Scientific GC–MS system (DSQ II GC/MS with Trace GC Ultra, Palo Alto, USA). Samples were analyzed on the capillary column ZB-5 MS 30 m × 0.25 mm, film thickness 0.2 μm, (Phenomenex, Torrance, USA). GC–MS settings were as follows: the initial oven temperature was held at 60 °C for 1 min and ramped at 4 °C/min rising to 250 °C; helium was used as carrier gas at a flow rate of 1 mL/min; the sample (0.1 μL) was injected manually at the split/splitless injector temperature of 260 °C, with a split ratio 1:50 and transfer line temperature was set to 270 °C for GC–MS analyses. Mass spectra were obtained at 70 eV (EI), the scan range was 45–350 m/z and Xcalibur version 2.0.7. was used for result processing and quantification. The components of the essential oil were identified by obtained GC–MS spectra and retention indices (RI) relative to C8-C20 n-alkanes. AMDIS computer program version 2.62 was used for GC–MS data processing using NIST library version 2.0. Spectra and obtained retention indices were compared with data already available in the literature42.
Brine shrimp lethality test (BSLT)
The brine shrimp lethality test was performed according to the protocol of Asaduzzaman et al.43. The method estimates in vivo lethality in a simple zoological organism, using nauplii of the brine shrimp Artemia salina.
Artificial sea water (ASW) was prepared by mixing 27 g commercially available salt mixture (Dohse Aquaristik GmbH & CO. KG, Germany) with 900 mL of distilled water, per instructions given. Artemia salina eggs (Dohse Aquaristik GmbH & CO. KG, Germany) were added in the small commercial tank for nauplii hatching and incubated in ASW under a halogen lamp, providing direct light and warmth. Twenty-four hours were allowed to hatch the shrimp and to be matured as nauplii. The hatched shrimps are attracted to the light and nauplii free from brine shrimp eggs were collected from the illuminated compartment of the tank. Ten nauplii were counted macroscopically in the stem of the graduated Pasteur pipette against a lighted background and transferred to test tubes. By dissolving the essential oil sample in a convenient solvent (DMSO), a stock solution is made (2000 µg/mL), which was used for serial dilutions to prepare the concentrations 1000; 500; 250; 125; 62.5; 31.25; 15,625; 7.81; 3.9 µg/mL. Each test tube contained essential oil sample dilution in DMSO (2.5 mL) and commercial salt mixture with 10 brine shrimp nauplii (2.5 mL). DMSO and thymol were used as negative and positive controls, respectively. The test was conducted with three replicates for each treatment and ten nauplii per replicate. Each test tube was maintained under illumination during 24 h. Survivors were counted macroscopically, by two independent counters.
Antiproliferative experiments
The antiproliferative effect of the T. vulgaris L. essential oil was determined using MTT assay44. In vitro investigation of antiproliferative activity of the T. vulgaris L. essential oil was conducted in Laboratory for Systemic Biomedicine, Division of Molecular Medicine, “Ruđer Bošković” Institute in Zagreb, Croatia. The aim of this study was to evaluate the antiproliferative activity of thyme essential oil by determining the its activity in different concentrations on selected cell lines. For this purpose, three well-characterized cell lines (hematological and solid) were selected according to tumor type, proliferation rate, genotype, growth characteristics and general sensitivity and drug resistance (cell lines used by the National Cancer Institute (NCI) cell line screen). The original substance, as well as stock solution (250 mg/mL/DMSO) were kept in the dark at 4 °C prior to MTT assay. An antitumor drug, doxorubicin, was set up as a positive control (reference compound according to NCI protocol), in parallel with the tested essential oil44.
Three human tumor cell lines were used in this study: breast adenocarcinoma (MCF7 ATCC HTB-22), lung carcinoma—large cell lung cancer (NCI-H460 ATCC HTB-177) and acute lymphoblastic leukemia (MOLT-4 ATCC CRL-1582). Cell lines were obtained from American Type Culture Collection (ATCC). All cell lines were routinely cultured as monolayers (MCF-7 and H460) or suspension (MOLT-4) and maintained in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), l-glutamine (2 mM), penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37 °C in a humidified atmosphere with 5% CO244.
The effect of the thyme essential oil on the selected cell lines was assessed using the tetrazolium colorimetric MTT assay according to a slightly modified protocol used at the National Cancer Institute45. The cells were inoculated at a density of 1.2 × 104 cells/mL (H460), 3 × 104 cells/mL (MCF-7) or 1 × 105 cells/mL (MOLT-4) onto the 96-well microtiter plates on day 0, depending on the doubling times of the specific cell line. Subsequently, six different concentrations (5, 10, 50, 100, 500, and 1000 μg/mL) of the analyzed essential oil were added to the wells, and the plates were incubated for further 72 h. As a control, the reference compound doxorubicin was tested in parallel in five ten-fold dilutions (from 10–10 to 10-6 M). Working dilutions were prepared on the day of testing. The dimethyl-sulfoxide (DMSO) solvent was also tested for possible inhibitory effect by adjusting its concentration to be the same as in working concentration (concentration of DMSO never exceeded 0.1%). After 72 h of incubation, the cell growth rate was assessed by performing an MTT assay. Next, the essential oil treated medium was discarded and MTT was added to each well at a concentration 20 µg/40 µL and incubated for 4 h. After incubation time, the precipitates were dissolved in 160 μL of DMSO. The absorbance (A) was measured at 570 nm using a microplate reader. The experiment was done in quadruplicate.
A formazan absorbance is in direct correlation with tumor cell viability. The percentage growth (PG) of cell lines was calculated according to the following formulas:
where A tzero is the average absorbance before exposure of cells to the test supstances, A test is the average absorbance after the incubation period (72 h), A cont is the average absorbance after 72 h with no exposure of cells to the test supstances.
Data analysis
All the experimental results in brine shrimp lethality test (BSLT) were as mean ± SD of three parallel measurements. The percent of mortality (% M) of the brine shrimp nauplii was calculated for each concentration. The median lethal concentration (LC50) of the test samples and 95% confidence intervals were determined from the twenty-four hours count using the probit analysis method described by Finney46. The LC50was derived by linear regression method from plotting probit units against correspondent log of concentration. Probit is a unit of measurement of statistical probability based on deviations from the mean of a normal distribution. The obtained LC50 values are compared to Meyer’s and Clarkson’s toxicity criteria47,48.
The screening of cytotoxic effects in vitro of the thyme essential oil against tested cell lines (MOLT-4, MCF-7, H460) were estimated in terms of growth inhibition percentage and expressed as IC50. 95% confidence intervals were determined. Percent growth inhibition of cells exposed to treatments was calculated as follows:% Inhibition = (100 − PG)/200 × 100. Percent of growth inhibition was transformed by probit analysis method described by Finney46. The IC50was derived by linear regression method from plotting probit units against correspondent log concentration. The criteria used to categorize the cytotoxicity of thyme essential oil based on U.S. National Cancer Institute (NCI) and Geran’s protocol was as follows: IC50 ≤ 20 µg/mL = highly cytotoxic, IC50 ranged between 21 and 200 µg/mL = moderately cytotoxic, IC50 ranged between 201 and 500 µg/mL = weakly cytotoxic and IC50 > 501 µg/mL = not cytotoxicity27. All the experimental results were as mean ± SD of quadruplicates.
Ethics declaration
None.
Supplementary Information
Acknowledgements
The authors would like to thank plant taxonomist Dr. Samir Đug from the Department of Biology and Ecology, Faculty of Sciences, University of Sarajevo for his help with identification of the experimental plant material.
Author contributions
Conceptualization, H.N. and K.D.; methodology, H.N., K.D., I.G. and E.K.; formal analysis, E.O., I.G. and E.K.; investigation, H.N., K.D., I.G. and E.K.; resources, F.B.; data curation, H.N., K.D.; writing—original draft preparation, I.G. and E.K.; writing—review and editing, H.N., B.M. and K.D.; visualization, S.M. and B.M.; supervision, F.B. and S.M. All authors have read and approved the final manuscript.
Data availability
All data generated/analysed during this study are included in this manuscript (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92679-x.
References
- 1.World Health Organization (WHO): Fact sheets, Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer (2018). Accessed 8 June 2020.
- 2.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics. Cancer J. Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 4.Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R. Overview of major classes of plant-derived anticancer drugs. Int. J. Biomed. Sci. 2009;5:1–11. [PMC free article] [PubMed] [Google Scholar]
- 5.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Int. J. Devot. Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 6.Blowman K, Magalhães M, Lemos MF, Cabral C, Pires IM. Anticancer properties of essential oils and other natural products. Evid. Based Complement. Altern. Med. 2008;2018:1–12. doi: 10.1155/2018/3149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautam N, Mantha AK, Mittal S. Essential oils and their constituents as anticancer agents: A mechanistic view. Biomed. Res. Int. 2014;2014:1–23. doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carnesecchi S, et al. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J. Pharmacol. Exp. Ther. 2001;298:197–200. [PubMed] [Google Scholar]
- 9.European Medicines Agency (EMA). Assessment report on Thymus vulgaris L. Thymus zygis Loefl.ex.L. aetheroleum. https://www.fitoterapia.net/archivos/201910/draft-assessment-report-thymus-vulgaris-l-thymus-zygis-l-aetheroleum-revision-1_en.pdf (2010). Accessed 20 May 2020.
- 10.Islam MT, et al. Anticancer activity of thymol: A literature-based review and docking study with Emphasis on its anticancer mechanisms. IUBMB Life. 2019;71:9–19. doi: 10.1002/iub.1935. [DOI] [PubMed] [Google Scholar]
- 11.Heidari Z, Salehzadeh A, Shandiz SA, Tajdoost S. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech. 2018;8:177. doi: 10.1007/s13205-018-1199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Menhali A, et al. Thymus vulgaris (thyme) inhibits proliferation, adhesion, migration, and invasion of human colorectal cancer cells. J. Med. Food. 2015;18:54–59. doi: 10.1089/jmf.2013.3121. [DOI] [PubMed] [Google Scholar]
- 13.Ayesh BM, Abed AA, Doaa MF. In vitro inhibition of human leukemia THP-1 cells by Origanumsyriacum L. and Thymus vulgaris L. extracts. BMC. Res. Notes. 2014;7:612. doi: 10.1186/1756-0500-7-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sertel S, Eichhorn T, Plinkert PK, Efferth T. Cytotoxicity of Thymus vulgaris essential oil towards human oral cavity squamous cell carcinoma. Anticancer Res. 2011;31:81–87. [PubMed] [Google Scholar]
- 15.Kubatka P, et al. Anticancer activities of Thymus vulgaris L. in experimental breast carcinoma in vivo and in vitro. Int. J. Mol. Sci. 2019;7:1749. doi: 10.3390/ijms20071749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mclaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug. Inf. J. 1998;32:513–524. doi: 10.1177/009286159803200223. [DOI] [Google Scholar]
- 17.Bajracharya GB, Tuladhar SM. Brine-shrimp bioassay for assessment of anticancer property of essential oils from spices. Nepal J. Sci. Technol. 2011;12:163–170. doi: 10.3126/njst.v12i0.6495. [DOI] [Google Scholar]
- 18.Colegate SM, Molyneux R. Bioactive Natural Products: Detection, Isolation, and Structural Determination. CRC Press; 2007. pp. 18–20. [Google Scholar]
- 19.Thompson JD, Chalchat JC, Michet A, Linhart YB, Ehlers B. Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J. Chem. Ecol. 2003;29:859–880. doi: 10.1023/A:1022927615442. [DOI] [PubMed] [Google Scholar]
- 20.Hudaib M, Speroni E, Di Pietra AM, Cavrini V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. Anal. 2002;29:691–700. doi: 10.1016/S0731-7085(02)00119-X. [DOI] [PubMed] [Google Scholar]
- 21.Raal A, Arak E, Orav A. Comparative chemical composition of the essential oil of Thymus vulgaris L. from different geographical sources. Herba Pol. 2005;1:10–17. [Google Scholar]
- 22.Satyal P, Murray BL, McFeeters RL, Setzer WN. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods. 2016;5:70. doi: 10.3390/foods5040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Council of Europe European (COE). European Pharmacopeia 8th ed 1403–1405 (Strasbourg, 2013)
- 24.Anderson JE, Goetz CM, McLaughlin JL, Suffness M. A blind comparison of simple bench-top bioassays and human tumour cell cytotoxicities as antitumor prescreens. Phytochem. Anal. 1991;2:107–111. doi: 10.1002/pca.2800020303. [DOI] [Google Scholar]
- 25.Bogavac M, et al. Alternative treatment of vaginal infections—In vitro antimicrobial and toxic effects of Coriandrumsativum L. and Thymus vulgaris L. essential oils. J. Appl. Microbiol. 2015;119:697–710. doi: 10.1111/jam.12883. [DOI] [PubMed] [Google Scholar]
- 26.Al-Balushi AH, et al. Antibacterial and cytotoxic activities of Thymus vulgaris leaves grown in Oman. Int. J. Pharm. Sci. Res. 2013;4:4253–4257. [Google Scholar]
- 27.Geran RI, Greenberg NH, Macdonald MM, Schumacher AM. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972;3:59–61. [Google Scholar]
- 28.Satooka H, Kubo I. Effects of thymol on B16–F10 melanoma cells. J. Agric. Food Chem. 2012;60:2746–2752. doi: 10.1021/jf204525b. [DOI] [PubMed] [Google Scholar]
- 29.Deb DD, Parimala G, Devi SS, Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem. Biol. Interact. 2011;193:97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Mastelic J, et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008;56:3989–3996. doi: 10.1021/jf073272v. [DOI] [PubMed] [Google Scholar]
- 31.Mapelli M, Calo R, Marabini L. Thymol and Thymus vulgaris extract protects human keratinocyte cell line (HaCaT) from UVA and UVB damage. OxidAntioxid. Med. Sci. 2016;5:39–48. [Google Scholar]
- 32.Hsu SS, et al. Effect of thymol on Ca2+ homeostasis and viability in human glioblastoma cells. Eur. J. Pharmacol. 2011;670:85–91. doi: 10.1016/j.ejphar.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Gholijani N, Gharagozloo M, Kalantar F, Ramezani A, Amirghofran Z. Modulation of cytokine production and transcription factors activities in human jurkat T cells by thymol and carvacrol. Adv. Pharm. Bull. 2015;5:653–660. doi: 10.15171/apb.2015.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slamenova D, Horvathova E, Sramkova M, Marsalkova L. DNA-protective effects of two components of essential plant oils carvacrol and thymol on mammalian cells cultured in vitro. Neoplasma. 2007;54:108–112. [PubMed] [Google Scholar]
- 35.Oliviero M, et al. Evaluations of thyme extract effects in human normal bronchial and tracheal epithelial cell lines and in human lung cancer cell line. Chem. Biol. Interact. 2016;256:125–133. doi: 10.1016/j.cbi.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Montani M, et al. The water soluble ruthenium (II) organometallic compound [Ru (p-cymene)(bis (3, 5 dimethylpyrazol-1-yl) methane) Cl] Cl suppresses triple negative breast cancer growth by inhibiting tumor infiltration of regulatory T cells. Pharmacol. Res. 2016;107:282–290. doi: 10.1016/j.phrs.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Rahman FU, et al. Dimetallic Ru (II) arene complexes appended on bis-salicylaldimine induce cancer cell death and suppress invasion via p53-dependent signaling. Eur. J. Med. Chem. 2018;157:1480–1490. doi: 10.1016/j.ejmech.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 38.Ferraz RP, et al. Cytotoxic effect of leaf essential oil of LippiagracilisSchauer (Verbenaceae) Phytomedicine. 2013;20:615–621. doi: 10.1016/j.phymed.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Arunasree KM. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine. 2010;17:581–588. doi: 10.1016/j.phymed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Islam MT. Anticancer activity of thymol: A literature-based review and docking study with emphasis on its anticancer mechanisms. IUBMB. 2019;71:9–19. doi: 10.1002/iub.1935. [DOI] [PubMed] [Google Scholar]
- 41.Food and Drug Administration (FDA). Substances generally recognized as safe. https://www.govinfo.gov/content/pkg/CFR-2019-title21-vol3/xml/CFR-2019-title21-vol3-sec182-20.xml (2019). Accessed 21 June 2020.
- 42.Adams, R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry (Allured Publishing Corporation, 2007).
- 43.Asaduzzaman M, Rana MS, Hasan SM, Hossain MM, Das N. Cytotoxic (brine shrimp lethality bioassay) and antioxidant investigation of BarringtoniaAcutangula (L.) Int. J. Pharm. Sci. Res. 2015;6:1179–1185. [Google Scholar]
- 44.Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Drug Des. Discov. 2011;716:157–168. doi: 10.1007/978-1-61779-012-6_9. [DOI] [PubMed] [Google Scholar]
- 45.Developmental Therapeutics Program (DTP). https://dtp.cancer.gov. Accessed 10 June 2020.
- 46.Finney DJ. A Statistical Treatment of the Sigmoid Response Curve 2nd ed. 2. New York-London: Cambridge University Press; 1952. p. 318. [Google Scholar]
- 47.Meyer BN, et al. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 48.Clarkson C, et al. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated/analysed during this study are included in this manuscript (and its Supplementary Information files).