INTRODUCTION
Since the first reports of smell and taste loss were linked to the COVID‐19 pandemic in March 2020, the occurrence of true gustatory impairment has been a matter of debate. 1 This is particularly so because studies incorporating reliable psychophysical evaluation of taste are scarce. In a meta‐analysis concerning almost 30,000 patients, Saniasiaya et al. found only four reliable psychophysical studies out of 775 studies on taste loss and COVID‐19. 2 Furthermore, these studies may have missed part of the picture as they were only based on a quick screening three‐ or four‐item gustatory identification test, not taking into account taste threshold function. With prevalence ranging from 4% to 90%, there is a crucial need for a more accurate understanding of taste disturbance due to COVID‐19. Moreover, the physiological mechanism underlying potential dysgeusia is still poorly understood. Some authors suggested that COVID‐19 patients likely confuse taste loss with flavor loss due to impaired retronasal olfaction as this has been largely shown in past research on taste perception. 3 , 4 In contrast, other authors support the idea of a genuine peripheral gustatory impairment by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). They highlight that ACE2 receptors have been shown to be highly expressed in epithelial cells of oral mucosa and that SARS‐CoV‐2 is frequently found in saliva. 2 This study's objectives are to explore the prevalence rate, subjective and psychophysical evaluation, and evolution in time of dysgeusia in a group of patients reporting chemosensory loss caused by COVID‐19 infection using an extensive 16‐item psychophysical gustatory test combining threshold and identification tasks.
METHODS
Adult patients with sudden chemosensory loss since September 2020 were prospectively recruited via public call from two Belgian institutions (Saint‐Pierre University and EpiCURA Hospitals). Three clinical presentations were accepted for patient eligibility: isolated sudden loss of smell, isolated sudden loss of taste, or concomitant sudden loss of smell and taste. In addition, each patient had to show evidence of concurrent confirmed SARS‐CoV‐2 diagnosis by reverse transcription polymerase chain reaction (RT‐PCR) analysis. Eligible patients were then asked to come to the clinic for subjective rating and psychophysical testing. At Saint‐Pierre Hospital, all patients were invited to perform a second session after 4 weeks. Patients were also asked to self‐assess their smell, taste, and ability to identify “sweet/salty taste” from 0 (absent) to 10 (normal function).
Gustatory and olfactory functions were evaluated by the “Taste Strips” test and the “Sniffin’ Sticks” identification test (Medisense, Groningen, Netherlands), respectively. Both are validated, widely used, and easy‐to‐perform psychophysical tests. Their methodology and scoring system were described previously: normogeusia (>8), hypogeusia (1–8), and ageusia (0); normosmia (≥12), hyposmia (9–11), and anosmia (<9). 5 , 6 , 7
Categorical variables were compared using chi‐square tests, and continuous variables using Wilcoxon signed‐rank test. Spearman correlation was used to analyze the relationship between subjective and psychophysical scores. Statistical significance was fixed at α = 0.05. All analyses were performed using the Statistical Package for the Social Sciences (SPSS version 25; IBM Corp, Armonk, NY, USA).
RESULTS
A total of 93 patients completed the first evaluation, on average 13 ± 2.7 days after the onset of smell/taste dysfunction. Mean age was 42 ± 12 years with a sex ratio of 66 females to 27 males. Thirty‐nine patients (42%) reported having normal taste at day 1 of chemosensory loss, compared with 48 patients (52%) at the time of evaluation. Mean scores of self‐assessed taste, smell, and sweet/salty functions were 6.6 ± 2.7, 6.0 ± 2.7, and 8.7 ± 1.9, respectively. The median gustatory score was 13/16 (interquartile range [IQR] = 11.5–14.5) with 82 normogeusic (88%), 11 hypogeusic (12%), and no ageusic patients. Regarding odor identification testing, median score was 11/16 (IQR = 9‐13), with 43 normosmic (46%), 28 hyposmic (30%), and 22 anosmic (24%) patients, respectively.
Olfactory scores correlated better with subjective smell (r = 0.535, p < 0.0005) and taste (r = 0.387, p < 0.0005) than subjective sweet/salty function (r = 0.287, p = 0.008). On the other hand, gustatory score did not correlate with olfactory score, subjective taste, and sweet/salty rating. Within olfactory groups, there was a significant difference in subjective smell/taste rating and olfactory score, but not in subjective sweet/salty rating and gustatory score (Figure 1).
FIGURE 1.
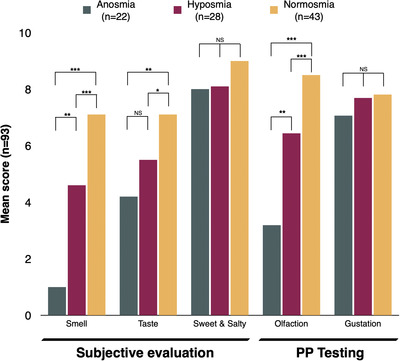
Subjective scores about smell, taste and sweet/salty identification ranged from 0 (absent) to 10 (normal function). Psychophysical scores on olfaction (1–48) and gustation (0–4) were adjusted to a 0–10 scale to allow visual comparisons with subjective scores. NS, not significant; PP, psychophysical. *p < 0.05; **p < 0.01; ***p < 0.005
Thirty‐four out of 37 patients completed the second evaluation 4 weeks after the first testing. We compared their self‐rated taste/smell function at the time of follow‐up with their psychophysical score at the 1st and 2nd visit. Cochran's Q test showed a significant difference among the three proportions (χ2 (2) = 18, p < 0.001; Figure 2). Post hoc comparisons showed the proportion of patients self‐reporting hypogeusia (N = 21) was significantly higher than the proportion of patients diagnosed hypogeusic on testing (N = 4; p = 0.001). After 4 weeks, the median gustatory score remained at 13/16 (IQR = 11.75‐14.25), and the ratio of normogeusia to hypogeusia was almost identical. In contrast, median olfactory scores significantly increased from 11/16 to 13/16 (IQR = 11.88–14.12; p = 0.001, N = 34) with 79% normosmic, 18% hyposmic, and 3% anosmic patients. In addition, the McNemar–Bowker test of symmetry showed a significant difference in the proportion of patients between the 1st and 2nd olfactory tests (χ2 (3) = 9.45, p = 0.024).
FIGURE 2.
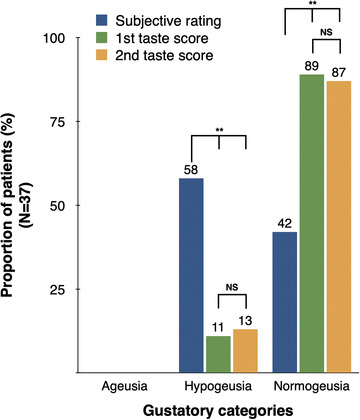
Self‐rating of taste function is compared with psychophysical gustatory scores at the first and second visit (i.e., after 4 weeks). High rate of subjective loss of taste contrasted with a low rate of dysgeusic patients. NS, not significant. **p < 0.01
DISCUSSION
To the best of our knowledge, the present study is the first to investigate gustation with a full 16‐item test in COVID‐19 patients. We found that although half of patients considered themselves to have abnormal taste function right before testing, only 12% of them were found to be dysgeusic following psychophysical evaluation. We also asked patients to rate both “taste” and “sweet/salty” functions in order to differentiate taste and flavor. Interestingly, we observed that olfactory score—rather than gustatory score—correlated better with the patients’ self‐assessed taste rating, and there was a weak correlation with their sweet/salty rating. Of note, there was no correlation between ratings of sweet/salty tastes and gustatory scores.
These results support the idea that most patients with COVID‐19 tend to unconsciously conflate taste loss and olfactory loss, even when taste function is clearly defined to them beforehand as only referring to the perception of sweet, salty, sour, and bitter tastes. This may be explained in part by the fact that eating is intuitively a unique integrated experience and not the addition of three separate chemosensory modalities. 8 The fact that many languages tend to use the same word for flavor and for gustation, such as in English (taste), French (goût), or Chinese (味道), adds on to the biological innate confusion. This study suggests that the reported high prevalence of true dysgeusia might be overestimated. However, due to the nature of patient inclusion in this study, the exact prevalence of initial gustatory loss in the COVID‐19 population cannot be clearly identified.
Supporting the theory that true dysgeusia may occur initially, impairment of trigeminal chemosensation has been reported, suggesting that chemosensory modalities other than olfaction can be affected by this virus. 7 , 10 Nevertheless, it does not resolve the question of why patients keep reporting decreased taste function while having a normal gustatory score. Ultimately, it may be a combination of both scenarios, that is, an initial transient peripheral gustatory loss combined with a biological and semiological conflation of loss of taste with loss of flavor.
The main limitation of this study is the timeframe of 13 days on average between the reported taste loss and gustatory function evaluation. As taste receptor turnover happens every 7 to 10 days, this may explain in part why an initial loss of taste may not be observed after this timepoint, as it was the case in this study. 9 Gustatory testing and/or taste bud biopsies on the day of chemosensory loss may be the final piece of the controversy jigsaw about gustatory loss in COVID‐19.
ETHICS APPROVAL
The study was performed in accordance with the Declaration of Helsinki on Biomedical Studies Involving Human Subjects. The study protocol was approved by the Review Board of CHU Saint‐Pierre, Brussels, Belgium (CHUSP210119).
CONFLICT OF INTERESTS
The authors have no conflict of interests.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Le Bon S‐D, Payen L, Prunier L, et al. Making scents of loss of taste in COVID‐19: Is self‐reported loss of taste due to olfactory dysfunction? A prospective study using psychophysical testing. Int Forum Allergy Rhinol. 2021;11:1504–1507. 10.1002/alr.22815
Jérôme R. Lechien and Sven Saussez contributed equally to this work. Author order was determined in order of increasing seniority.
REFERENCES
- 1. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saniasiaya J, Islam MA, Abdullah B. Prevalence and characteristics of taste disorders in cases of COVID‐19: a meta‐analysis of 29,349 patients. Otolaryngol Neck Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 3. Hintschich CA, Wenzel JJ, Hummel T, et al. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID‐19 patients. Int Forum Allergy Rhinol. 2020;10(9):1105‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitcroft KL, Hummel T. Olfactory dysfunction in COVID‐19. JAMA. 2020;323(24):2512. [DOI] [PubMed] [Google Scholar]
- 5. Landis BN, Welge‐Luessen A, Brämerson A, et al. Taste strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol. 2009;256(2):242‐248. [DOI] [PubMed] [Google Scholar]
- 6. Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated sniffin’ sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276(3):719‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Bon S‐D, Pisarski N, Verbeke J, et al. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID‐19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. 2021;278(1):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spence C. Multisensory flavor perception. Cell. 2015;161(1):24‐35. [DOI] [PubMed] [Google Scholar]
- 9. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID‐19 patients: single‐center experience on 72 cases. Head Neck. 2020;42(6):1252‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parma V, Ohla K, Veldhuizen MG, et al. More than smell—COVID‐19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020;45(7):609‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]


