Abstract
Mucoadhesive polymers represent a major part of site-specific and localized retention strategies in oral drug delivery. The present research was designed to synthesize and characterize a novel mucoadhesive carbohydrate polymer (thiolated gum ghatti; TGG), which was employed to formulate mucoadhesive tablets of domperidone using an industrially viable compression coating technique. Thiolation of gum ghatti was achieved by the ester formation (esterification) between the hydroxyl group and the carboxyl group of gum ghatti and thioglycolic acid. TGG was characterized by various physicochemical techniques such as FTIR, XRD, SEM, and DSC. In rheological studies, the observed viscosities of pure gum mucin were 45.45 and 71.75 mPa·s and those of the thiolated gum were 78.7 and 112.58 mPa·s, respectively, in water and simulated gastric fluid. A significant increase in viscosity for thiolated gum may be attributed to increased macromolecular interactions responsible for enhanced mucoadhesive potential of thiolated gum. In silico studies corroborate the role of mucin gum interaction and energetic stabilization for enhanced mucoadhesion properties of thiolated gum. Ex vivo mucoadhesion strength of gum ghatti- and TGG-coated tablets was found to be ranging between 45.77 ± 1.49 and 88.16 ± 1.75 and 115.32 ± 2.36 and 184.65 ± 2.07 mN, respectively. In an acute oral toxicity study, TGG did not show any toxicity on the vital organs of the Wistar rat and proved to be a safe polymer. TGG may be regarded as a promising polymer for developing different mucoadhesive drug delivery systems.
1. Introduction
Mucoadhesion is the phenomenon involving interactions between the polymer and mucosal surface or mucin. Mucoadhesive interactions lead to the development of a strong mucoadhesive bond due to electrostatic, mechanical/physical cross-linking, chemical bonding, wetting, or adsorption interactions.1,2 Mucoadhesive drug delivery systems could be designed for active targeting of different biological locations such as nasal,3 buccal,4,5 gastrointestinal,6 rectal,7 vaginal, and so forth.8,9 Mucoadhesive polymers could be used alone or in combination for providing sufficient mucoadhesive property to the drug delivery system.2,10 Polymer composites and chemical modification of polymers could enhance the mucoadhesive capacity of the polymers.11
Gum ghatti is a high-molecular weight, anionic polysaccharide obtained from Anogeissus latifolia, family Combretaceae. The primary structure of gum ghatti is composed of d-glucuronic acid, d-xylose, d-mannose, d-galactose, and l-arabinose. Gum ghatti is widely used in paper production, pharmaceutical, and food industries due to its thickening and emulsification properties. It is employed as a sustained release, matrix-forming, film-forming, and mucoadhesive polymer for developing pharmaceutical formulations.12,13
Thiomers or thiolated polymers are important for mucoadhesive polymers, exhibiting the capability to form inter- and intrachain disulfide bonds within the polymeric network and strongly improve cohesive properties. Thiol/disulfide chemical reactions with cysteine-rich mucin lead to the formation of strong covalent bonds in thiomers.14,15 Thiomers when compared with the unmodified polymers show a strong adhesive strength which is sufficient to localize dosage forms at a given specific site for a prolonged period. Apart from the improvement in mucoadhesive properties, thiolated polymers have also been reported to exhibit permeation enhancing, enzyme inhibition, controlled release, and thermal stability effects.16 The thiolation procedure has been successfully implemented for enhancing the mucoadhesive potential of various gums viz. karaya gum,17 moringa gum,18 xanthan gum,19 gellan gum,20 tamarind gum,21 and psyllium husk.22 Through the literature search, it was found that the thiolation of gum ghatti and its mucoadhesive potential has not been documented.
The present research was intended to perform the synthesis of thiolated gum ghatti followed by characterization of the modified gum by various techniques such as Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), X-ray diffraction analysis (XRD), and scanning electron microscopy (SEM). Rheological studies were performed to observe the behavior of the polymer and sensitivity at given temperatures. The molecular transitions within the polymer were characterized using molecular mechanics analysis. The polymer mucin interaction study indicates the mucoadhesion property of modified gum in comparison to the native gum. An acute oral toxicity study was performed in rats for establishing a safety profiling of thiolated gum ghatti. Domperidone core tablets were press-coated with pure gum ghatti and thiolated gum ghatti. The formulated compression-coated tablets were evaluated for various parameters, ex vivo mucoadhesion and in vitro drug release studies.
2. Results and Discussion
2.1. Synthesis and Determination of Thiol Content of Thiolated Gum Ghatti
2.1.1. Determination of Thiol Content
Thiolation processes can strengthen the mucoadhesive properties of natural gums. In the present research, thiolation of gum ghatti was performed, followed by characterization of the synthesized thiomers. Thiolated gum ghatti was found to contain 4.5 mM of thiol groups in 2 mg/mL of polymeric solution, as determined by Ellman’s method.
2.2. Characterization of Gum Ghatti and Thiolated Gum Ghatti
2.2.1. Fourier Transform Infrared Spectroscopy
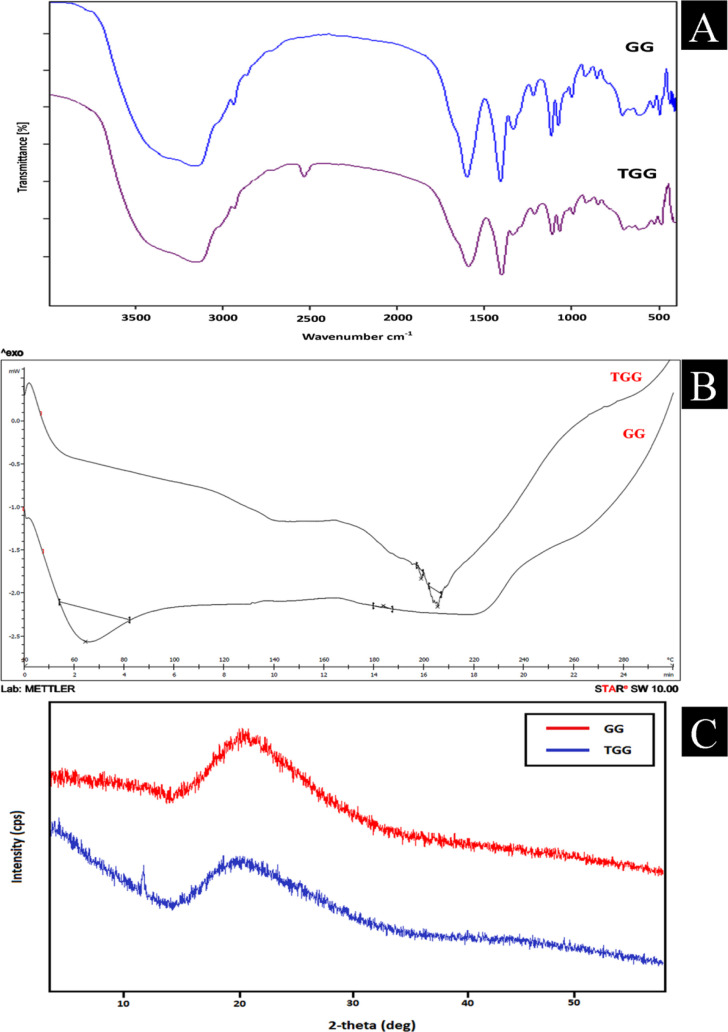
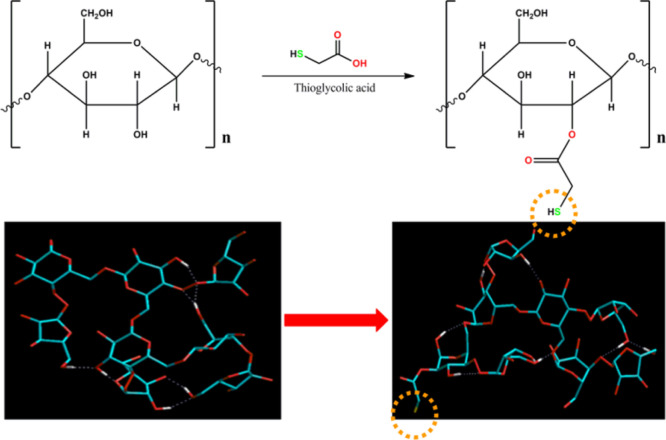
The FTIR spectra of gum ghatti and thiolated gum ghatti are depicted in Figure 1A. The FTIR spectra of gum ghatti showed a prominent peak at 3441.74 cm–1 with a stretching vibration of −OH at 3441.74 cm–1, −OH at 3161.30 cm–1, −CH at 2932.40 cm–1, C=O alkene at 1669.32 cm–1, C–H alkane at 1450.02 cm–1, and 1035.51 cm–1 attributed to C–O primary alcohol.23 All the characteristic peaks of gum ghatti were found in thiolated gum ghatti. However, the presence of an additional −SH stretch at 2571.48 cm–1 confirmed the thiolation of gum ghatti. Thiolation of the gum ghatti was authorized by the formation of ester bonds between carboxyl group of thioglycolic acid and the hydroxyl group of gum ghatti.17,24,25 The FTIR spectra (Figure S1) of the physical mixture of domperidone with different components added in the tablets (core tablet and compressed tablet) did not show any shift in characteristic bands or appearance of new bands, indicating the compatibility of the drug with all the tablet components.26,27
Figure 1.
(A) FTIR spectra of pure gum ghatti and thiolated gum ghatti; (B) DSC thermogram of pure gum ghatti and thiolated gum ghatti; and (C) XRD diffractogram of pure and thiolated gum ghatti.
2.2.2. Differential Scanning Calorimetry
Figure 1B shows the DSC thermogram of the gum ghatti and thiolated gum ghatti. The thermogram of the pure gum ghatti showed an endothermic peak at 64.42 °C (onset 53.97 °C, end set 81.97 °C, enthalpy −39.36 mJ/g) and 183.73 °C (onset 182.93 °C, end set 185.38 °C, enthalpy 0.16 mJ/g). DSC thermogram of the thiolated gum ghatti depicted endothermic peaks at 199.73 °C (onset 198.39 °C, end set 200.55 °C, enthalpy −0.24 mJ/g) and 206.39 °C (onset 204.43 °C, end set 207.62 °C, enthalpy −1.41 mJ/g). The increase in endothermic transition temperature and heat of fusion in the thiolated gum ghatti indicated thiol modification of the gum ghatti.19
2.2.3. X-ray Diffraction Analysis
The X-ray diffractogram (Figure 1C) of the gum ghatti depicted the amorphous nature of the gum with no sharp peaks. However, the XRD diffractogram of the thiolated gum ghatti exhibited an additional sharp peak at 12.01 (2θ), indicating a relative increase in the crystalline behavior of the thiolated gum compared to the pure gum ghatti.20,26
2.2.4. Scanning Electron Microscopy
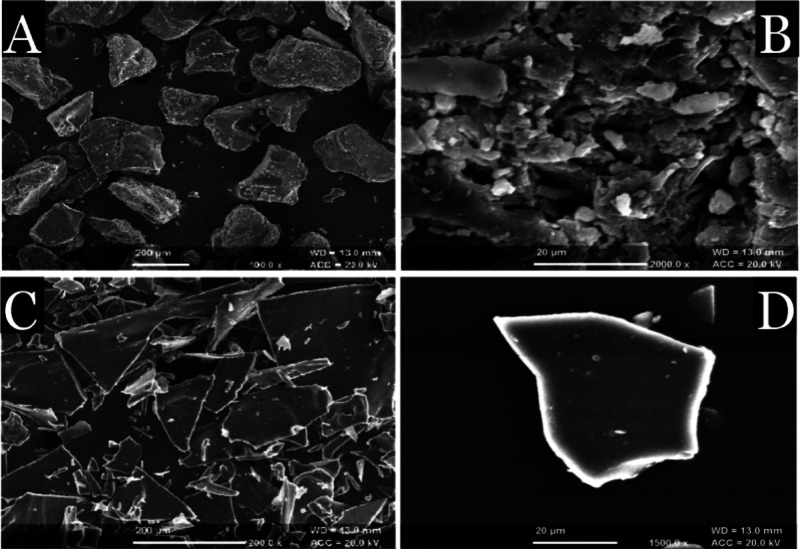
SEM examined the surface morphology of gum ghatti and thiolated gum ghatti (Figure 2). SEM of the pure gum ghatti indicated the presence of polyhedral flakes with a rough surface morphology. However, thiolated gum ghatti showed sharp lucent crystalline flakes with a relatively smooth surface. The relative smooth surface of the thiolated gum ghatti may be helpful in providing a larger surface area for interactions with the mucosal layer, and hence, it may be responsible for enhanced mucoadhesive interactions. The results were in line with the findings reported by Ahuja and his co-workers.18
Figure 2.
SEM images of pure gum ghatti (A,B) and thiolated gum ghatti (C,D) at different magnifications.
2.2.5. Rheological Measurements
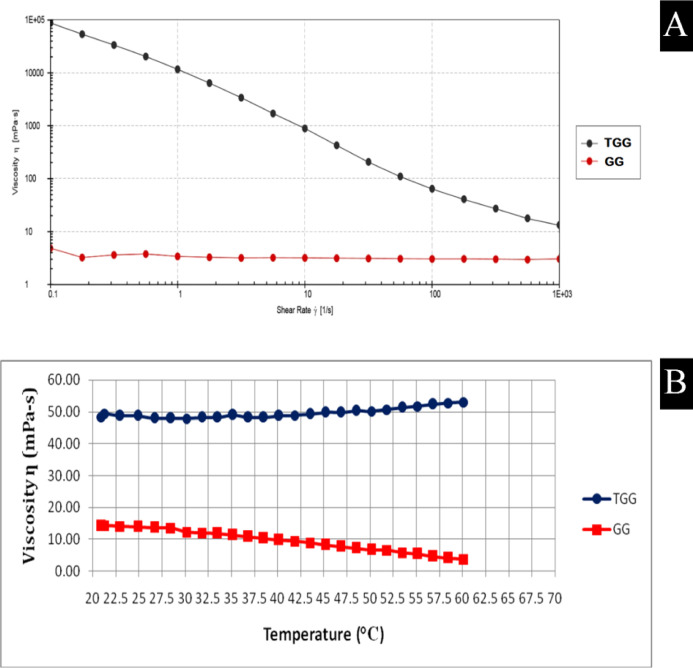
The gum ghatti and thiolated gum ghatti were evaluated for rheological measurements and studied for shear rate sweep and temperature sweep analysis, as shown in Figure 3A,B. The pure gum ghatti was observed to show almost a Newtonian behavior. A sample of thiolated gum ghatti was found to exhibit shear thinning behavior across the given experimental conditions due to the orientation of the microstructures in the direction of given deformation.27
Figure 3.
Rheological measurements: (A) Shear rate sweep analysis (n = 3) and (B) temperature sweep analysis (n = 3).
The pure gum ghatti was observed to be sensitive to given temperatures, as a continuous drop in viscosity could be observed from Figure 3B. However, the viscosity of thiolated gum ghatti was found to be unaffected with the increase in temperature. It may be deduced that the viscosity and the mucoadhesive property of thiolated gum were not affected by temperature rise when compared with the pure gum ghatti. The DSC results indicating increased endothermic transition temperature and heat of fusion in thiolated gum ghatti also corroborate with the temperature sweep analysis results.
2.2.6. Polymer Mucin Interaction Study
The significant enhancement in mucoadhesive interactions was reported between mucin and native gum/thiolated gum in a simulated gastric fluid. It prominently indicated that the native gum/thiolated gum was pH-dependent and showed molecular interaction and viscosity enhancement in SGF. The viscosity of mucin, pure gum (gum ghatti), thiolated gum (gum ghatti), mucin and gum (mucin + pure gum), and mucin and thiolated gum (mucin + thiolated gum) in water was found to be 7.65, 21.62, 34.20, 45.45, and 78.70 mPa·s and in SGF 11.85, 34.90, 55.11, 71.75, and 112.58 mPa·s, respectively. The mixture of mucin and pure gum in water exhibited 29.27 and 45.45 mPa·s values of ηexp and ηobs, respectively, with a ηenhance of 16.18 mPa·s. Similarly, the mixture of mucin and the thiolated gum showed ηexp and ηobs to be 41.85 and 78.70 mPa·s with a ηenhance of 24.85 mPa·s, respectively. In SGF, the mixture of mucin and thiolated gum showed ηexp and ηobs to be 66.96 and 112.58 mPa·s with a ηenhance of 45.62 mPa·s, respectively. The bioadhesion force of the mucin and gum (mucin + pure gum) and mucin and thiolated gum (mucin + thiolated gum) in water was found to be 64.07 and 98.41 mPa and in SGF 99.00 and 180.65 mPa, respectively (Tables 1 and 2). The bioadhesion force, however, seems to be depending on the initial viscosity and environmental pH. The bioadhesion force of the pure and the thiolated gum ghatti with mucin was high at lower pH (SGF), in contrast to that in water.28 These observations were corroborated by in silico mucoadhesion profiling where it was found that the increase in the viscosity component could be attributed to the molecular interactions between the macromolecules. The TGG–MUC (thiolated gum ghatti–mucin) complex showed much higher energy of stabilization (total energy) in contrast to the GG–MUC (gum ghatti–mucin) complex (proteosaccharide). The energetic and geometrical stabilization was mainly attributed to electrostatic interactions. Interestingly, while GG–MUC was supported by OH–HO hydrogen bonding, TGG–MUC interaction included −OH–HN– and −SH–HN–H– bonding and hence showed a better mucoadhesion profile (Figure 4).
Table 1. Apparent Viscosity of Samples in Water and SGF at Shear Rate 3.96 s–1 and Total Minimized Energy.
| S.no. | sample | viscosity (mPa s) (in water) | viscosity (mPa s) (in SGF) | total minimized energy (MM+) |
|---|---|---|---|---|
| 1. | mucin (5%) | 7.65 | 11.85 | –166.81 |
| 2. | pure gum (1%) | 21.62 | 34.90 | –39.48 |
| 3. | thiolated gum (1%) | 34.20 | 55.11 | –13.73 |
| 4. | mucin + pure gum | 45.45 | 71.75 | –258.41 (ΔE = −52.12) |
| 5. | mucin + thiolated gum | 78.70 | 112.58 | –267.84 (ΔE = −87.29) |
Table 2. Different Parameters of Viscosities, Expected Viscosity (ηexp), Observed Viscosity (ηobs), Enhanced Viscosity (ηenhance), Relative Viscosity (ηrel), and Force of Bioadhesion was Calculated in Water and in SGF.
| water |
SGF |
|||
|---|---|---|---|---|
| parameter | mucin + pure gum | mucin + thiolated gum | mucin + pure gum | mucin + thiolated gum |
| ηexp(mPa·s) | 29.27 | 41.85 | 46.75 | 66.96 |
| ηobs(mPa·s) | 45.45 | 78.70 | 71.75 | 112.58 |
| ηenhance (mPa·s) | 16.18 | 24.85 | 25 | 45.62 |
| ηrel(mPa·s) | 1.55 | 1.88 | 1.53 | 1.68 |
| F (mPa) | 64.07 | 98.41 | 99 | 180.65 |
Figure 4.
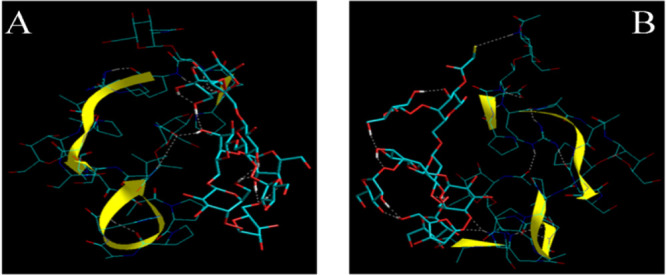
In silico mucoadhesion visualization of (A) gum ghatti and (B) thiolated gum ghatti with glycosylated mucin (stick rendering with yellow ribbon). The gum and its derivative are represented by tube rendering. The H-bonds are represented by white broken lines (- - -).
Interactions of thiolated gum with mucin resulted in the formation of a strong mucoadhesive bond via disulfide exchange. Formation of inter- and/or intramolecular disulfide bonds could be held responsible for the enhanced mucoadhesive and drug release retardant property of thiolated gum. Similar findings of solubility, dissolution, prolonged residence time, and increased mucoadhesive property were reported by Nowak et al. and Jalil et al.(29,30)
Esterification between the hydroxyl groups in gum ghatti and the sulfur groups in TGA was achieved by the covalent bond attachment of thioglycolic acid to gum ghatti. The mean yield of thiolated gum ghatti was found to be 92% after optimizing the critical process parameters.
2.2.7. In Vivo Toxicity Study
Histopathological images of the stomach and intestinal tissues of rats after oral administration of the gum ghatti and thiolated gum ghatti are shown in Figure 5. In the histopathological images of the stomach, the gastric gland surface epithelium was found to be normal with no ulcerative spots. No deformations were observed in lacteals and goblet cells of the duodenum. Further, the villi and microvilli (hair-like projections) were in normal condition. It was affirmed that both the stomach and intestinal tissues did not show any evidence of toxicity after a single oral dose (300 and 2000 mg/kg body weight) administration of the pure and thiolated gum ghatti in the Wistar rats.
Figure 5.
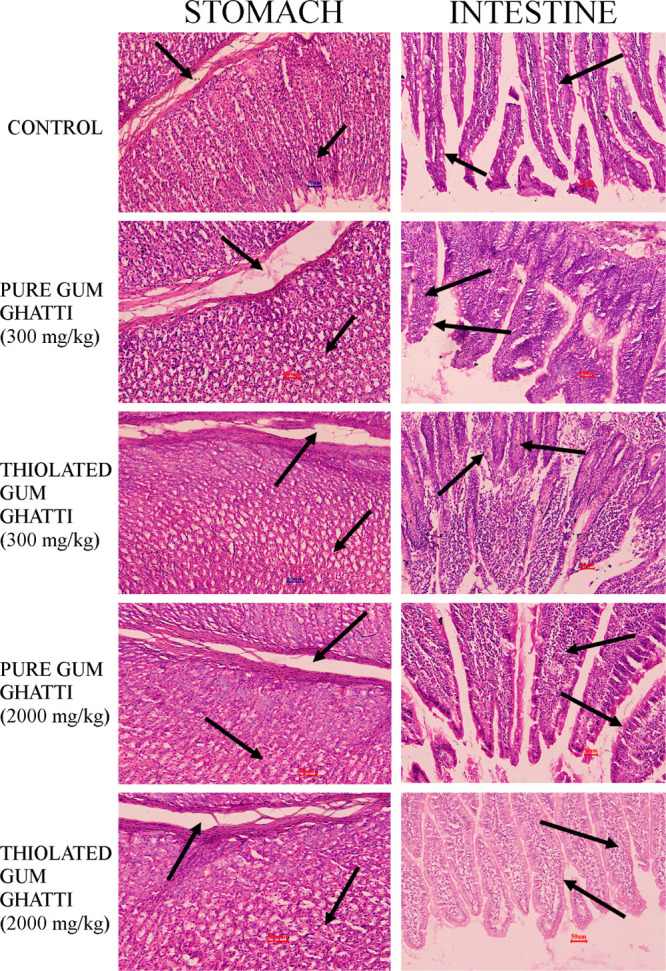
Histopathological examination of the stomach and intestinal tissues of the Wistar rats. Row 1: control; row 2: pure gum ghatti (300 mg/kg); row 3: thiolated gum ghatti (300 mg/kg); row 4: pure gum ghatti (2000 mg/kg); row 5: thiolated gum ghatti (2000 mg/kg).
2.3. Evaluation of Core Tablets
The prepared core tablet of domperidone was evaluated by various evaluation parameters. The inner core tablets of domperidone were found to be 80 ± 5 mg in weight. The thickness of the core tablets was found to be 1.82 ± 0.10 mm, and the hardness and friability of the core tablets were 3.7 ± 0.50 kg/cm2 and 0.79 ± 0.15%, respectively. The drug content in the core tablet was found to be in the range between 96.14 ± 0.56 and 98.95 ± 0.88%. The absorbance spectrum of domperidone is depicted in Supporting Information,Figure S2. The evaluation indicated the significant quality attributes in core tablets. All assessments were performed in triplicate (n = 3).
2.4. Evaluation of Compression-Coated Tablets
2.4.1. Ex Vivo Determination of Mucoadhesive Strength
The coated tablets were compressed and prepared using gums (pure and thiolated) in varied proportions to coat previously prepared inner core tablets of domperidone. The detachment force (Fmax) of F1GG to F4GG was found to be between 45.77 ± 1.49 and 88.16 ± 1.75 mN. The Fmax for F1TGG to F4TGG was found to be ranging between 115.32 ± 2.36 and 184.65 ± 2.07 mN. The results depicted a notable increase in the mucoadhesive property of thiolated gum compared to pure gum. Additionally, mucoadhesive strength was directly proportional to the concentration of thiolated gum which was used as a coating material for developing mucoadhesive tablets of domperidone.
2.4.2. In Vitro Drug Release
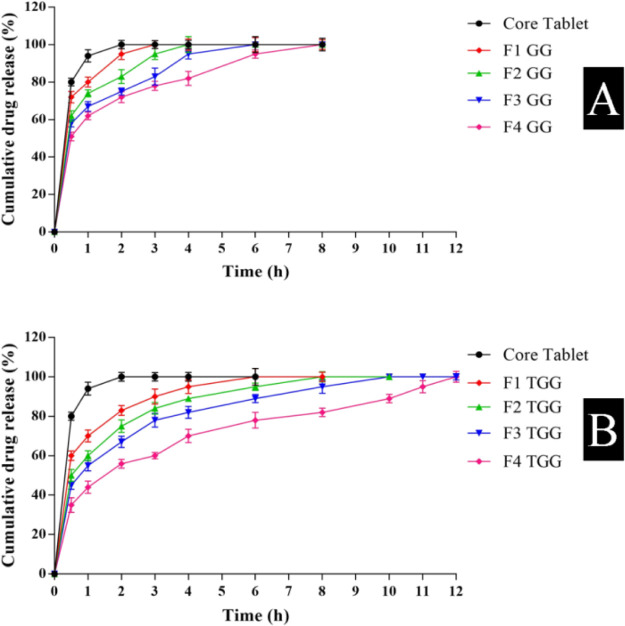
For comparing drug release rates, the in vitro dissolution study was performed on core tablets and core tablets coated with gum ghatti and thiolated gum ghatti in different concentrations, as shown in Figure 6. Core tablets released 80.19 and 94.63% domperidone in 30 and 60 min, respectively. Core tablets compression-coated with gum ghatti (F4GG) depicted 51.85 and 95.33% drug release in 0.5 and 6 h, respectively. However, the core tablets coated with thiolated gum ghatti (F4TGG) exhibited 35.28 and 78.95% drug release after 0.5 and 6 h, respectively. The significantly improved drug release retardant properties of thiolated gum ghatti compared to pure gum ghatti could be attributed to increased polymer cross-linking after thiolation due to the formation of inter-/intrachain di-sulfide bonds. This may increase the drug diffusional path length within the polymer matrix, resulting in a better controlled/sustained drug release property of the modified gum. Compression-coated batches F1GG, F2GG, F1TGG, and F2TGG exhibited first order to be the best fitted model. However, batches F3GG, F4GG, F3TGG, and F4TGG displayed Korsmeyer–Peppas to be the best obeyed model. It could be deduced that at lower polymer concentrations, the best fit model was first order, and at higher polymer concentrations, Korsmeyer–Peppas was the best fit model for explaining the mechanism of drug release from the formulation, as shown in Table 3.31,32
Figure 6.
In vitro drug release from core tablets and different batches of compression-coated tablets of (A) gum ghatti (n = 3) and (B) thiolated gum ghatti (n = 3).
Table 3. In vitro Drug Release Data of the Formulated Batches.
| zero
order |
first
order |
Higuchi
model |
Hixson
Crowell model |
Korsmeyer–Peppas model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batches | r2 | k0 | r2 | k1 | r2 | kH | r2 | kHC | r2 | Kkp | n |
| core tablet | 0.293 | 0.111 | 0.993 | –0.020 | 0.566 | 3.615 | 0.957 | –0.047 | 0.695 | 1.828 | 0.070 |
| F1GG | 0.394 | 0.128 | 0.962 | –0.010 | 0.678 | 3.915 | 0.888 | –0.022 | 0.862 | 1.688 | 0.126 |
| F2GG | 0.505 | 0.143 | 0.948 | –0.006 | 0.779 | 4.155 | 0.889 | –0.014 | 0.935 | 1.540 | 0.182 |
| F3GG | 0.604 | 0.152 | 0.850 | –0.003 | 0.851 | 4.211 | 0.884 | –0.009 | 0.975 | 1.449 | 0.211 |
| F4GG | 0.671 | 0.153 | 0.940 | –0.003 | 0.894 | 4.138 | 0.890 | –0.006 | 0.994 | 1.359 | 0.238 |
| F1TGG | 0.474 | 0.078 | 0.960 | –0.004 | 0.719 | 2.891 | 0.884 | –0.010 | 0.922 | 1.569 | 0.159 |
| F2TGG | 0.595 | 0.090 | 0.969 | –0.003 | 0.824 | 3.158 | 0.896 | –0.007 | 0.957 | 1.398 | 0.220 |
| F3TGG | 0.688 | 0.096 | 0.968 | –0.002 | 0.890 | 3.268 | 0.903 | –0.005 | 0.986 | 1.292 | 0.254 |
| F4TGG | 0.816 | 0.101 | 0.958 | –0.001 | 0.960 | 3.275 | 0.911 | –0.003 | 0.994 | 1.076 | 0.318 |
The value of release exponent (n) was found to be less than 0.5, indicating diffusion to be the lead mechanism responsible for release of drug through the polymer matrix. Imbibition of media may cause polymeric chains to relax and swell, leading to the formation of a swollen gelatinous transition state of the polymer acting as a barrier for diffusional transport of the drug from within the polymer matrix. Subsequent dissolution of polymeric chain and development of pore/channel also contributed toward the release of the drug.33,34
3. Conclusions
Thiolation of the gum ghatti was achieved by the ester formation (esterification) between the carboxyl group and hydroxyl group of thioglycolic acid and gum ghatti. Different techniques (FTIR, DSC, XRD, and SEM analysis) were employed for characterization analysis of thiolated gum ghatti, and rheological studies were executed using a rheometer for studying viscosity parameters and their role in mucoadhesion. The compression-coated method, a solvent-free technique, was employed for coating the core tablets of domperidone for developing mucoadhesive sustained release tablets. Thiolated biopolymers exhibited a significant bioadhesive and drug release retardant property for developing mucoadhesive drug delivery systems and targeting different biological locations viz. gastrointestinal, vaginal, ocular, rectal, pulmonary, and buccal for effective drug delivery. Basic properties of the thiolated biopolymers could be altered for being used as a potential candidate for developing 3D printed drug delivery systems. Considering the toxicological and regulatory issues related to the modified biopolymers, successful commercial exploitation of the same could be positively explored.
4. Materials and Methods
4.1. Materials
Gum ghatti was gifted by Hydrocolloid Plantations (New Delhi, India). Domperidone was kindly gifted by Kwality Pharmaceuticals, Amritsar, Punjab, India. Thioglycolic acid, potassium dihydrogen orthophosphate, and sodium chloride were acquired from LobaChemie Pvt. Ltd. (Mumbai, India). 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC), 5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB (Ellman’s Reagent), and dialysis membrane (width–31.13 mm and diameter–21.5 mm) were received from Hi-Media Laboratories Pvt. Ltd. (Mumbai, India).
4.2. Synthesis of Thiolated Gum Ghatti
Pure gum ghatti (2 g) was first dissolved in 50 mL of deionized water, followed by the addition of EDAC (50 mM) and thioglycolic acid (4 g). The aforementioned reaction mixture was kept undisturbed for 3 h at room temperature. Further, the reaction mixture was added in a dialysis membrane and dialyzed against 5 mM hydrochloric acid (HCl) at 10 ± 1 °C for 1 h, against 5 mM HCl containing sodium chloride (1%) for 2 h at room temperature, and against 1 mM HCl containing sodium chloride (1%) for 2 h at room temperature. Afterward, the reaction mixture was collected and lyophilized (Allied frost, Delhi, India) at −30 ± 1 °C under 10.01 mbar pressure, and the mixture was kept at +4 °C.35 Chemical reaction depicting the synthesis of thiolated polymer is shown in Figure 7.
Figure 7.
Chemical synthesis of the thiolated biopolymer.
4.3. Determination of Thiol Group Content
The degree of substitution in thiolated biopolymers was determined by spectrophotometric methods using Ellman’s reagent. Ellman’s reagent is a water soluble compound used to detect free sulfhydryl groups in solution. A yellow-colored product is produced when this compound reacts with sulfhydryl groups, and the rate of reaction depends on factors such as pH, pKa of sulfhydryl, and electrostatic effects.36,37 In brief, a polymeric solution of 2 mg/mL was prepared in purified water. Further, 250 μL of the sample was added to 250 μL of 0.5 M phosphate buffer saline having pH 8, followed by the addition 500 μL of Ellman’s reagent. The resulted reaction was preceded at room temperature for 2 h. The absorbance of the aforementioned solution was determined at 412 nm using a UV spectrophotometer. The thiol content was calculated using the standard curve, which was plotted between 0.25 and 2 mM of thioglycolic acid in water (Figure S3).38,39
4.4. Characterization of Gum Ghatti and Thiolated Gum Ghatti
4.4.1. Fourier Transform Infrared Spectroscopy
Powder samples of the pure gum ghatti and thiolated gum ghatti were subjected to FTIR analysis using an FTIR spectrophotometer (Alpha, Bruker, Japan). Sample pellets were prepared with KBr, and FTIR spectra were recorded in the frequency range of 4000–400 cm–1. The possible interactions between the drug and different components of the tablet formulation were also evaluated by FTIR analysis.
4.4.2. Differential Scanning Calorimetry
DSC thermograms of pure gum ghatti and thiolated gum ghatti were recorded using a differential scanning calorimeter (MettlerToledo Star System, 305, Switzerland). A required amount of the sample was crimped in a standard aluminum pan and heated over a temperature range of 40 to 300 °C at a heating rate 10 °C per minute in a nitrogen atmosphere.
4.4.3. X-ray Diffraction Analysis
XRD patterns of the powdered pure gum ghatti and thiolated gum ghatti (dialyzed using a dialysis membrane with double distilled water and ethanol to remove the residual salts) were traced/recorded using an X-ray diffractometer (Miniflex 2, Rigaku, Japan) with Ni-filtered Cu (Kα) radiations, with a voltage rate of 45 kV and a current of 40 mA. The samples (gum ghatti and thiolated gum ghatti) were analyzed over the 2θ range of 0 to 80° with a scan step size of 0.0170° (2θ), scan step time of 25 s, and scan speed 0.05 min–1.
4.4.4. Scanning Electron Microscopy
The external morphology (shape and surface) of gum ghatti and thiolated gum ghatti was determined by a scanning electron microscope (Joel, fine coat ion sputter, JFC-1100). A double-sided adhesive tape was used to adhere the gold palladium alloy (150–200 A°)-coated samples onto the stubs of microscope.
4.4.5. Rheological Measurements
Rheological behavior of gum ghatti and thiolated gum ghatti was analyzed using a rheometer (MCR 92, Anton Paar, Austria). For temperature sweep analysis, the samples were analyzed in the temperature range of 20 to 60 °C with a 2 °C/min constant shear rate of 10 s–1. The samples were carried out under shear rate sweep analysis ranging from 0.1 to 1000 s–1 to evaluate the flow behavior, with a data acquisition duration varying from 30 s on a logarithmic scale at a constant temperature of 25 °C.40
4.4.6. Polymer Mucin Interaction Study
For the polymer–mucin interaction study, pure gum (gum ghatti) (1% w/v), thiolated gum (gum ghatti) (1% w/v), and mucin (5% w/v) solutions were prepared in SGF (simulated gastric fluid) medium without enzymes (0.2% w/v sodium chloride in 0.7% v/v HCl).28 The experiments (viscometry) were performed on pure gum, thiolated gum, mucin, pure gum mucin mixture, and thiolated gum mucin mixture solutions. All the mixture solutions were allowed to stand for at least 1 h at 37.0 ± 0.1 °C (prior to analysis). The rheological measurements were performed using a Brookfield viscometer (Model DV-III, Brookfield, USA). Each sample (mixture solutions) was added to the viscometer and equilibrated for 2 min. The measurement was made with the shear rate up to about 25 s–1, which was given as per following equation
where τ is the shear stress and Υ is the shear rate. Apparent viscosity was measured at a shear rate of 3.96 s–1.
The effect of mucin and polymer on viscosity enhancement was studied by various parameters of viscosity such as expected viscosity (ηexp), observed viscosity (ηobs), enhanced viscosity (ηenhance), and the relative viscosity (ηrel), which were calculated as per following equations
where ηp and ηm are the viscosity of polymer and mucin, respectively. The polymer–mucin interaction was studied by the force of mucoadhesion by using the formula
where F is the force of mucoadhesion, ηb is viscosity components of bioadhesion, and σ is shear rate (s–1).38 For in silico evaluation, gum ghatti and thiolated gum ghatti were made to interact with glycosylated mucin (Avogadro 1.2 platform) using molecular mechanics simulations (MM + force field; Polak–Ribere conjugate gradient; ChemLite3.0., FL, USA).41
4.4.7. In Vivo Toxicity Study
For the in vivo toxicity study, Wistar rats (150–200 g body weight) were obtained from LalaLajpat Rai University of Veterinary & Animal Sciences, India, and were kept under standard housing conditions following balanced diet and water ad libitum. The study protocol was approved by the animal ethics committee of the institute (reg.no. 1181/PO/ReBi/S/08/CPCSEA; vide Protocol no. IAEC/CCP/20/01/PR-004). The single dose in vivo acute oral toxicity study on pure gum ghatti and thiolated gum ghatti was performed as per Organization for Economic Co-operation and Development (OECD) 423 guidelines. The animals were divided into five different groups: group-I (control group; n = 3), group-II (pure gum ghatti; dose–300 mg/kg; n = 3), group-III (thiolated gum ghatti; dose–300 mg/kg; n = 3), group-IV (pure gum ghatti; dose–2000 mg/kg; n = 3), and group-V (thiolated gum ghatti; dose–2000 mg/kg; n = 3). The sample was administered orally by feeding needles made of stainless steel. On the 14th day of the experimental procedure, the animals were sacrificed by cervical dislocation for histological examination of the stomach and intestine.42,43
4.5. Preparation of the Core Tablet of Domperidone
The core tablet (80 mg) of domperidone was formulated using domperidone (10 mg) as an active ingredient and Avicel 112 (63 mg), PVP K30 (5 mg), talc (1 mg), and magnesium stearate (1 mg) as tablet excipients. All ingredients were sieved (60#) and blended using double cone blender for 15–20 min. Tablets with 80 mg weight were prepared using a multiple station tablet punching machine equipped with 6 mm concave round die-punch tooling (A.K. Industries, Nakodar, India).44,45
4.6. Compression Coating of Core Tablets
An appropriate blend of coating polymers (pure gum ghatti and thiolated gum ghatti) was press-coated over the formulated core tablet as per the composition given in Table 4. Avicel-112 was added in sufficient quantity for making the total tablet weight equal to 600 mg. The die cavity was first half filled with the polymer (coating material), then the core tablet was placed in the die, and the remaining coating material was added over the core tablet. Compression coating was performed using a multipunch tableting machine having 8.5 mm concave punches at an applied force of 5000 kg.45,46
Table 4. Compression Coating Composition for Mucoadhesive Tablet Batches of Pure Gum Ghatti and Thiolated Gum Ghatti.
| batches | gum ghatti (mg) | thiolated gum ghatti (mg) | Avicel 112 (mg) | PVP K30 (mg) | talc (mg) |
|---|---|---|---|---|---|
| F1GG | 350 | 145 | 20 | 5 | |
| F2GG | 400 | 95 | 20 | 5 | |
| F3GG | 450 | 45 | 20 | 5 | |
| F4GG | 500 | 20 | 5 | ||
| F1TGG | 350 | 145 | 20 | 5 | |
| F2TGG | 400 | 95 | 20 | 5 | |
| F3TGG | 450 | 45 | 20 | 5 | |
| F4TGG | 500 | 20 | 5 |
4.7. Evaluation of Core and Compression-Coated Tablets
To ensure the uniformity and mechanical integrity of prepared tablets of the pure gum ghatti and thiolated gum ghatti, the following parameters such as thickness, weight variation, friability, drug content, in vitro release study, and ex vivo mucoadhesion strength were measured.
4.7.1. Hardness and Friability
Hardness and friability of 20 tablets were measured using the Monsanto hardness tester (Model VMT- 1, VinSyst Technologies, Mumbai, India) and the Roche friabilator (Campbell Electronics, Mumbai, India), respectively. Pre-weighed tablets were placed in the friabilator. The friability test machine (Roche friabilator) was rotated for 4 min at 25 rpm (100 revolutions). Afterward, the tablets were again weighed, and the values were calculated using the formula given below.
4.7.2. Thickness
The thickness of formulated tablets was deliberately considered using Digital Vernier Caliper (Mitutoyo Absolute Digimatic Caliper, Japan). From each formulated batch, five tablets were taken, and the average value was calculated.
4.7.3. Drug Content
The formulated tablets were weighed individually and crushed in a mortar and pestle. The powder equivalent to average weight of tablets was initially weighed and then transferred to volumetric flask containing buffer solution (0.1 N HCl). The dispersion was stirred for at least 2–3 h followed by filtration using a Whatman filter paper. The drug content was observed at absorbance 287 nm after dilution using UV–vis double beam spectrophotometer (AU 2701, Systronics, Mumbai, India).
4.7.4. Ex Vivo Determination of Mucoadhesive Strength
Mucoadhesion testing of compression-coated tablets of domperidone was performed using two different polymers (gum ghatti and thiolated gum ghatti) and was executed employing a texture analyzer (TA.XT plus, Stable MicroSystems, UK). The tablet was attached to a cylindrical probe with the help of a double side adhesive tape. The pig stomach (tissue) was equilibrated at 37.0 ± 0.5 °C for 15 min before placing onto the holder stage. The probe attached with tablet was dispersed into the medium for framed time proceeding to the test. Afterward, the hydrated disc was shifted to the downward direction to get in contact with the rinsed tissue at a defined force and sustained until the time specified. The probe was uplifted at a predetermined test speed and maximum detaching force (Fmax) required to separate the tablet equipped with probe from tissue, which can be determined from software (texture exponent 32). The precursor settings of the instrument were tested with different parameters such as test speed (0.5 mm/s), contact time (60 s), contact force (1.0 N), and distance (15 mm). The probe without the attached sample (tablet) was also assessed to examine the animal tissue uniformity.47,48
4.7.5. In Vitro Drug Release
An in vitro dissolution study of compressed domperidone tablets was executed using USP-II Paddle type dissolution apparatus (DS 8000, Lab India, India) with a rotating speed (50 rpm at 37 ± 0.5 °C) using dissolution medium 0.1 N HCl (pH 1.2). At fixed time intervals, the samples (5 mL) were taken out and filtered through a membrane filter (0.45 μm). Further, it was diluted and analyzed using a UV double beam spectrophotometer (AU 2701, Systronics, Mumbai, India) at 287 nm. Drug release cumulative percentage was deliberated using an equation derived from calibration curve. Pharmacokinetic models such as zero order, first order, Higuchi, Kosmeyer–Peppas, and Hixon–Crowell were fitted with release data of prepared tablets to perceive kinetic drug release modeling.49
Acknowledgments
The authors kindly acknowledge support and institutional facilities extended by Chitkara College of Pharmacy, Chitkara University, Punjab, India.
Glossary
Abbreviations
- Cu
copper
- DSC
differential scanning calorimetry
- DTNB
5,5′-dithiobis-(2-nitrobenzoic acid)
- EDAC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- FTIR
Fourier-transform infrared spectroscopy
- GG
gum ghatti
- HCl
hydrochloric acid
- KBr
potassium bromide
- MUC
mucin
- Ni
nickel
- OECD
organisation for economic co-operation and development
- PBS
phosphate buffer saline
- PVP K30
polyvinylpyrrolidone
- SEM
scanning electron microscope
- SGF
simulated gastric fluid
- TGA
thioglycolic acid
- TGG
thiolated gum ghatti
- UV–vis
ultraviolet–visible
- XRD
X-ray powder diffraction
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01328.
FTIR spectra of pure drug and tablet components: drug (domperidone), Avicel-112, PVP K30, talc, magnesium stearate, drug with Avicel-112, drug with PVP K30, drug with talc, and drug with magnesium stearate; UV absorption maxima of domperidone; and standard plot of thioglycolic acid (PDF)
Author Contributions
V.P.: Experimentation, manuscript writing, analysis, and validation. A.S.: Manuscript writing and editing, methodology, and resources. P.K.: conceptualization, supervision, revisions, and data curation. I.S.: conceptualization, supervision, administration, and designing experimentation. K.H.: administration, analysis, manuscript editing, and funding acquisition.
The authors received no external funding.
The authors declare no competing financial interest.
Supplementary Material
References
- Roy S.; Pal K.; Anis A.; Pramanik K.; Prabhakar B. Polymers in mucoadhesive drug-delivery systems: a brief note. Des. Monomers Polym. 2009, 12, 483–495. 10.1163/138577209x12478283327236. [DOI] [Google Scholar]
- Singh I.; Rana V. Enhancement of mucoadhesive property of polymers for drug delivery applications. Rev. Adhes. Adhes. 2013, 1, 271–290. 10.7569/raa.2013.097307. [DOI] [Google Scholar]
- Badhe R. V.; Nipate S. S.. Bioadhesives in Drug Delivery; Scrivener Publishing LLC: Hoboken, New Jersey, US, 2020; Chapter 10, pp 259–305, ISBN 9781119640196. [Google Scholar]
- Sharma M.; Rathore A.; Sharma S.; Sadhu V.; Reddy K. R.; Kulkarni R. V.. Nanomaterials in Diagnostic Tools and Devices; Elsevier Inc, 2020; Chapter 8, pp 213–240. [Google Scholar]
- Puri V.; Sharma A.; Maman P.; Rathore N.; Singh I. Overview of mucoadhesive biopolymers for buccal drug delivery systems. Int. J. Appl. Pharm. 2019, 11, 18–29. 10.22159/ijap.2019v11i6.35438. [DOI] [Google Scholar]
- Mendes A. C.; Sevilla Moreno J.; Hanif M.; Douglas T. E. L.; Chen M.; Chronakis I. S. Morphological, mechanical and mucoadhesive properties of electrospun chitosan/phospholipid hybrid nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. 10.3390/ijms19082266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Tam M.; Samaei S.; Lerouge S.; Barralet J.; Stevenson M. M.; Cerruti M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017, 48, 247–257. 10.1016/j.actbio.2016.10.026. [DOI] [PubMed] [Google Scholar]
- de Araújo Pereira R. R.; Bruschi M. L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. 10.3109/03639045.2011.623355. [DOI] [PubMed] [Google Scholar]
- Deshkar S. S.; Shirolkar S. V.; Patil A. T.. Bioadhesives in Drug Delivery; Scrivener Publishing LLC: Hoboken, New Jersey, US, 2020; Chapter 11, pp 307–369, ISBN 9781119640196. [Google Scholar]
- Asati S.; Jain S.; Choubey A. Bioadhesive or mucoadhesive drug delivery system: a potential alternative to conventional therapy. J. Drug Deliv. Therapeut. 2019, 9, 858–867. 10.22270/jddt.v9i4-A.3708. [DOI] [Google Scholar]
- Brannigan R. P.; Khutoryanskiy V. V. Progress and current trends in the synthesis of novel polymers with enhanced mucoadhesive properties. Macromol. Biosci. 2019, 19, 1900194. 10.1002/mabi.201900194. [DOI] [PubMed] [Google Scholar]
- Deshmukh A. S.; Setty C. M.; Badiger A. M.; Muralikrishna K. S. Gum ghatti: A promising polysaccharide for pharmaceutical applications. Carbohydr. Polym. 2012, 87, 980–986. 10.1016/j.carbpol.2011.08.099. [DOI] [Google Scholar]
- Zhang P.; Zhao Y.; Zhang X.; Zhu L.; Fang Z.; Shi Q. Thermodynamic properties and state diagram of gum ghatti-based edible films: Effects of glycerol and nisin. Polymers 2020, 12, 449. 10.3390/polym12020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner V. M.; Walker G. F.; Bernkop-Schnürch A. Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur. J. Pharm. Biopharm. 2003, 56, 207–214. 10.1016/s0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Netsomboon K.; Jalil A.; Laffleur F.; Hupfauf A.; Gust R.; Bernkop-Schnürch A. Thiolatedchitosans: Are Cys-Cys ligands key to the next generation?. Carbohydr. Polym. 2020, 242, 116395. 10.1016/j.carbpol.2020.116395. [DOI] [PubMed] [Google Scholar]
- Puri V.; Sharma A.; Kumar P.; Singh I. Thiolation of biopolymers for developing drug delivery systems with enhanced mechanical and mucoadhesive properties: A review. Polymers 2020, 12, 1803. 10.3390/polym12081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahulkar S. S.; Munot N. M.; Surwase S. S. Synthesis, characterization of thiolatedkaraya gum and evaluation of effect of pH on its mucoadhesive and sustained release properties. Carbohydr. Polym. 2015, 130, 183–190. 10.1016/j.carbpol.2015.04.064. [DOI] [PubMed] [Google Scholar]
- Grewal P.; Mundlia J.; Ahuja M. Thiol modified Moringa gum–A potential bioadhesive polymer. Carbohydr. Polym. 2019, 209, 400–408. 10.1016/j.carbpol.2018.12.100. [DOI] [PubMed] [Google Scholar]
- Bhatia M.; Ahuja M.; Mehta H. Thiol derivatization of Xanthan gum and its evaluation as a mucoadhesive polymer. Carbohydr. Polym. 2015, 131, 119–124. 10.1016/j.carbpol.2015.05.049. [DOI] [PubMed] [Google Scholar]
- Yadav S.; Ahuja M.; Kumar A.; Kaur H. Gellan–thioglycolic acid conjugate: Synthesis, characterization and evaluation as mucoadhesive polymer. Carbohydr. Polym. 2014, 99, 601–607. 10.1016/j.carbpol.2013.08.068. [DOI] [PubMed] [Google Scholar]
- Kaur H.; Yadav S.; Ahuja M.; Dilbaghi N. Synthesis, characterization and evaluation of thiolated tamarind seed polysaccharide as a mucoadhesive polymer. Carbohydr. Polym. 2012, 90, 1543–1549. 10.1016/j.carbpol.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Bhatia M.; Ahuja M. Thiol modification of psyllium husk mucilage and evaluation of its mucoadhesive applications. Sci. World J. 2013, 2013, 284182. 10.1155/2013/284182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani P.; Sen G.; Mishra S.; Jha U. Microwave assisted synthesis of polyacrylamide grafted gum ghatti and its application as flocculant. Carbohydr. Polym. 2012, 89, 275–281. 10.1016/j.carbpol.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Stulzer H. K.; Rodrigues P. O.; Cardoso T. M.; Matos J. S. R.; Silva M. A. S. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J. Therm. Anal. Calorim. 2008, 91, 323–328. 10.1007/s10973-006-7935-1. [DOI] [Google Scholar]
- Rus L.; Tomuţă I.; Iuga C. A.; Maier C.; Kacso I.; Borodi G.; Bratu I.; Bojita M. Compatibility studies of indapamide/pharmaceutical excipients used in tablet preformulation. Farmacia 2012, 60, 92–101. [Google Scholar]
- Naveen N. R.; Gopinath C.; Kurakula M. Okra-Thioglycolic Acid Conjugate—Synthesis, Characterization, and Evaluation as a Mucoadhesive Polymer. Processes 2020, 8, 316. 10.3390/pr8030316. [DOI] [Google Scholar]
- Vieira J. M.; Mantovani R. A.; Raposo M. F. J.; Coimbra M. A.; Vicente A. A.; Cunha R. L. Effect of extraction temperature on rheological behavior and antioxidant capacity of flaxseed gum. Carbohydr. Polym. 2019, 213, 217–227. 10.1016/j.carbpol.2019.02.078. [DOI] [PubMed] [Google Scholar]
- Thirawong N.; Kennedy R. A.; Sriamornsak P. Viscometric study of pectin–mucin interaction and its mucoadhesive bond strength. Carbohydr. Polym. 2008, 71, 170–179. 10.1016/j.carbpol.2007.05.026. [DOI] [Google Scholar]
- Nowak J.; Laffleur F.; Bernkop-Schnürch A. Preactivated hyaluronic acid: a potential mucoadhesive polymer for vaginal delivery. Int. J. Pharm. 2015, 478, 383–389. 10.1016/j.ijpharm.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Jalil A.; Asim M. H.; Le N.-M. N.; Laffleur F.; Matuszczak B.; Tribus M.; Bernkop–Schnürch A. S-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films. Int. J. Biol. Macromol. 2019, 130, 148–157. 10.1016/j.ijbiomac.2019.02.092. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A.; Kast C. E.; Richter M. F. Improvement in the mucoadhesive properties of alginate by the covalent attachment of cysteine. J. Controlled Release 2001, 71, 277–285. 10.1016/s0168-3659(01)00227-9. [DOI] [PubMed] [Google Scholar]
- Laffleur F.; Michalek M. Modified xanthan gum for buccal delivery—a promising approach in treating sialorrhea. Int. J. Biol. Macromol. 2017, 102, 1250–1256. 10.1016/j.ijbiomac.2017.04.123. [DOI] [PubMed] [Google Scholar]
- Barmpalexis P.; Kachrimanis K.; Malamataris S. Statistical moments in modelling of swelling, erosion and drug release of hydrophilic matrix-tablets. Int. J. Pharm. 2018, 540, 1–10. 10.1016/j.ijpharm.2018.01.052. [DOI] [PubMed] [Google Scholar]
- Hattori Y.; Takaku T.; Otsuka M. Mechanochemical effect on swelling and drug release of natural polymer matrix tablets by X-ray computed tomography. Int. J. Pharm. 2018, 539, 31–38. 10.1016/j.ijpharm.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Medeiros Borsagli F. G. L.; Carvalho I. C.; Mansur H. S. Amino acid-grafted and N-acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications. Int. J. Biol. Macromol. 2018, 114, 270–282. 10.1016/j.ijbiomac.2018.03.133. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A.; Hornof M.; Zoidl T. Thiolated polymers—thiomers: synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. 10.1016/s0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- Hornof M.; Kast C. E.; Bernkop-Schnurch A. In vitro evaluation of the viscoelastic properties of chitosan–thioglycolic acid conjugates. Eur. J. Pharm. Biopharm. 2003, 55, 185–190. 10.1016/s0939-6411(02)00162-5. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A.; Kast C. E.; Guggi D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems. J. Controlled Release 2003, 93, 95–103. 10.1016/j.jconrel.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Anitha A.; Deepa N.; Chennazhi K. P.; Nair S. V.; Tamura H.; Jayakumar R. Development of mucoadhesive thiolated chitosan nanoparticles for biomedical applications. Carbohydr. Polym. 2011, 83, 66–73. 10.1016/j.carbpol.2010.07.028. [DOI] [Google Scholar]
- Luo Q.; Han Q.; Wang Y.; Zhang H.; Fei Z.; Wang Y. The thiolated chitosan: Synthesis, gelling and antibacterial capability. Int. J. Biol. Macromol. 2019, 139, 521–530. 10.1016/j.ijbiomac.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Amaral M.; Martins A. S.; Catarino J.; Faísca P.; Kumar P.; Pinto J. F.; Pinto R.; Correia I.; Ascensão L.; Afonso R. A.; Gaspar M. M.; Charmier A. J.; Figueiredo I. V.; Reis C. P. How Can Biomolecules Improve Mucoadhesion of Oral Insulin? A Comprehensive Insight using Ex-Vivo, In Silico, and In Vivo Models. Biomolecules 2020, 10, 675. 10.3390/biom10050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronpot R. R.; Davis J.; Moser G.; Giri D. K.; Hayashi S.-m. Evaluation of 90-day oral rat toxicity studies on the food additive, gum ghatti. Food Chem. Toxicol. 2013, 51, 215–224. 10.1016/j.fct.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Publisher Organization for Economic Cooperation and Development (OECD) Guidelines for the Testing of Chemicals, OECD 423. Acute Oral Toxicity-Acute Toxic Class Method. Organization for Economic Cooperation and Development: Paris, 2001. https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed on 05 03 2021).
- Raza A.; Shen N.; Li J.; Chen Y.; Wang J.-Y. Formulation of zein based compression coated floating tablets for enhanced gastric retention and tunable drug release. Eur. J. Pharm. Sci. 2019, 132, 163–173. 10.1016/j.ejps.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Giri P.; Singh I. Development and evaluation of mucoadhesive tablets of cinnarizine using carboxymethylated guar gum by compression coating technique. Biointerface Res. Appl. Chem. 2020, 10, 6365–6376. 10.33263/briac105.63656376. [DOI] [Google Scholar]
- Malik D.; Singh I. Formulation and evaluation of press coated tablets of esomeprazole for colonic delivery. Asian J. Pharm. 2012, 6, 252–258. 10.4103/0973-8398.107560. [DOI] [Google Scholar]
- Thirawong N.; Nunthanid J.; Puttipipatkhachorn S.; Sriamornsak P. Mucoadhesive properties of various pectins on gastrointestinal mucosa: an in vitro evaluation using texture analyzer. Eur. J. Pharm. Biopharm. 2007, 67, 132–140. 10.1016/j.ejpb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sogias I. A.; Williams A. C.; Khutoryanskiy V. V. Chitosan-based mucoadhesive tablets for oral delivery of ibuprofen. Int. J. Pharm. 2012, 436, 602–610. 10.1016/j.ijpharm.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Costa P.; Sousa Lobo J. M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.