Abstract
Background
The mechanisms underlying disease pathogenesis in chronic spontaneous urticaria (CSU) and improvement with omalizumab are incompletely understood.
Objective:
To examine if the rate of clinical remission is concordant with baseline basophil features or the rate of change of IgE-dependent functions of basophils and/or plasmacytoid dendritic cells (pDC) during omalizumab therapy.
Methods
Adults (n=18) with refractory CSU were treated with omalizumab 300 mg monthly for 90 days. Subjects recorded daily Urticaria Activity Scores (UAS), and clinical assessments with blood sampling occurred at baseline and on days 1, 3, 6, 10, 20, 30, 60, and 90 following omalizumab. At baseline, subjects were categorized by basophil functional phenotypes, determined by in vitro histamine release (HR) responses to anti-IgE antibody, as CSU-responder (CSU-R) or CSU-non-responder (CSU-NR), as well as basopenic (B) or non-basopenic (NB).
Results
CSU-R/NB subjects demonstrated the most rapid and complete symptom improvement. By day 6, CSU-R/NB and CSU-NR/NB had increased anti-IgE mediated basophil HR relative to baseline, and these shifts did not correlate with symptom improvement. In contrast, CSU-NR/B basophil HR did not change during therapy. The kinetics of the decrease in surface IgE/FcεRI was similar in all 3 phenotypic groups and independent of the timing of the clinical response. Likewise, pDC surface IgE/FcεRI decline and TLR-9 induced IFN-α responses did not reflect clinical change.
Conclusions
Changes in basophil IgE based HR, surface IgE or FcεRI, bear no relationship to the kinetics in the change in clinical symptoms. Baseline basophil count and basophil functional phenotype, as determined by HR, may be predictive of responsiveness to omalizumab.
Keywords: Chronic Spontaneous Urticaria, Omalizumab, Basophil, FcεRI, IgE, Plasmacytoid Dendritic Cell, Histamine Release, Basophil Activation Test
Capsule Summary:
Baseline basophil count and basophil functional phenotype, as determined in response to anti-IgE, may be predictive of responsiveness to omalizumab.
Introduction
Chronic spontaneous urticaria (CSU) is a skin disease characterized by recurrent hives and/or angioedema for at least 6 weeks with an estimated prevalence of 1% 1. Symptoms of pruritus and wheals result from skin mast cell (SMC) degranulation of histamine and other mediators, leading to vasodilation and infiltration by basophils, lymphocytes, monocytes, neutrophils and eosinophils 2. IgG autoantibodies targeting either IgE or FcεRI are reported in approximately 30–40% of cases with an unclear role in pathogenesis 1. Over the decades, a role for basophils in CSU pathophysiology has emerged 3. Basopenia is a feature of active CSU4, is related to disease severity, 5–7 and may reflect the recruitment of basophils to skin lesions8. In addition, blood basophils have altered IgE receptor-mediated histamine degranulation 9, 10 that has recently been classified into two basophil functional phenotypes 11. These phenotypes are defined based on the magnitude of histamine release observed after optimal activation of their IgE receptor: CSU-responders (CSU-R) release ≥ 10% of cellular histamine content whereas CSU-non-responders (CSU-NR) demonstrate < 10% release 11. A third group, termed CSU basopenics (CSU-B) are unable to be classified due to extreme basopenia 6, 11, 12. These basophil functional phenotypes are stable during disease, however, in remission, enhanced degranulation mediated through FcεRI is observed along with normalized basophil counts 7, 13. A longitudinal study of basophil functional subsets and disease outcomes revealed that CSU-B and CSU-NR experience more severe disease but of shorter duration as compared to CSU-R6.
First-line therapy of CSU is second-generation H1 antihistamines up-dosed to fourfold approved dosing 14, however, this regimen is insufficient to control symptoms in half of patients 15. Omalizumab, a monoclonal IgG anti-human IgE antibody, is approved for antihistamine-refractory patients at 150 mg or 300 mg subcutaneously every 4 weeks, an approach different from allergic asthma where dosing is based upon the patient’s baseline serum IgE level and weight. By binding to free IgE, omalizumab prevents its attachment to FcεRI on FcεRI bearing mast cells and basophils, leading to down-regulation of FcεRI levels on these cells16, 17. Overall phase III clinical trials in CSU demonstrated nearly 40% of subjects treated with 300 mg of omalizumab achieved well-controlled urticaria (weekly urticaria activity score (UAS-7) ≤ 6) at 2 weeks18. However, an understanding of the IgE dependent pathways of CSU disease that underlie the rapid response to a fixed dose of omalizumab is lacking19. To date, biomarkers associated with a poor response to omalizumab include low baseline serum IgE20, 21, low basophil FcεRI expression 22 and positive BHRA and/or ASST results 23, 24, but an underlying basis for these findings is lacking.
In allergic disease, omalizumab induced reductions of basophil FcεRI expression and allergen responses occur more rapidly than for mast cells, and both cellular shifts contribute to the clinical effects 16, 25–27. Similar changes have been noted in omalizumab treated CSU patients28, however, the timing of cellular shifts relative to a rapid clinical response is unknown. Dendritic cells also express FcεRI which can modulate cellular function and can be rapidly altered by omalizumab therapy29. For the purposes of understanding the underlying mechanisms behind the therapeutic efficacy of omalizumab in CSU, a study of dendritic cell and basophil behavior during treatment is warranted. The primary objective of this study was to examine whether the rate of clinical remission with omalizumab therapy in CSU subjects would be concordant with baseline basophil features or the rate of change of IgE-dependent functions of basophils. Additionally, for the first time, we will explore the potential role of dendritic cell functional changes during omalizumab treatment.
Methods
Study Design
This phase IV, single site, open-label clinical trial approved by the Johns Hopkins Institutional Review Board was conducted between October 2017 and February 2020. A total of 11 visits occurred over 15 weeks, and consisted of a screening period (day −21 to −14), a run-in period (day −14 to day 0) and a treatment period of omalizumab 300 mg every 4 weeks for a total of 3 doses (day 0 to day 90) (Figure 1). Frequent visits on days 1, 3, 6, 10, 20 and 30 after omalizumab initiation aimed to capture the rate of clinical changes as they related to IgE bearing cell measures. Subjects recorded twice daily urticaria activity score (UAS) scores using electronic and paper diaries and weekly UAS-7 scores were calculated. The UAS is a validated measure of CSU disease activity calculated from the average of twice daily recorded itch (0 – 3; 0= no itch, 1= mild itch, 2= moderate itch, 3= severe itch) and hives (0 – 3, 0= no hives, 1= 1–6 hives, 2= 7–12 hives, 3= > 12 hives in the past 12 hours) scores with a daily maximum of 6. The UAS-7 is the sum of 7 daily average UAS scores with a maximum value of 42 30. Subjects with a UAS-7 ≥ 16 during screening or run-in and a clinic visit UAS ≥ 2 were eligible for enrollment. Blood was collected at each visit. Non-lesional skin punch biopsies were obtained at day 0, at the time of 50% symptom reduction as determined by the UAS-7 between days 6 to 30, and again at day 90. For subjects that did not achieve 50% symptom reduction by day 30, a non-lesional skin biopsy was obtained at day 30. The biopsy results will not be part of this report.
Figure 1:
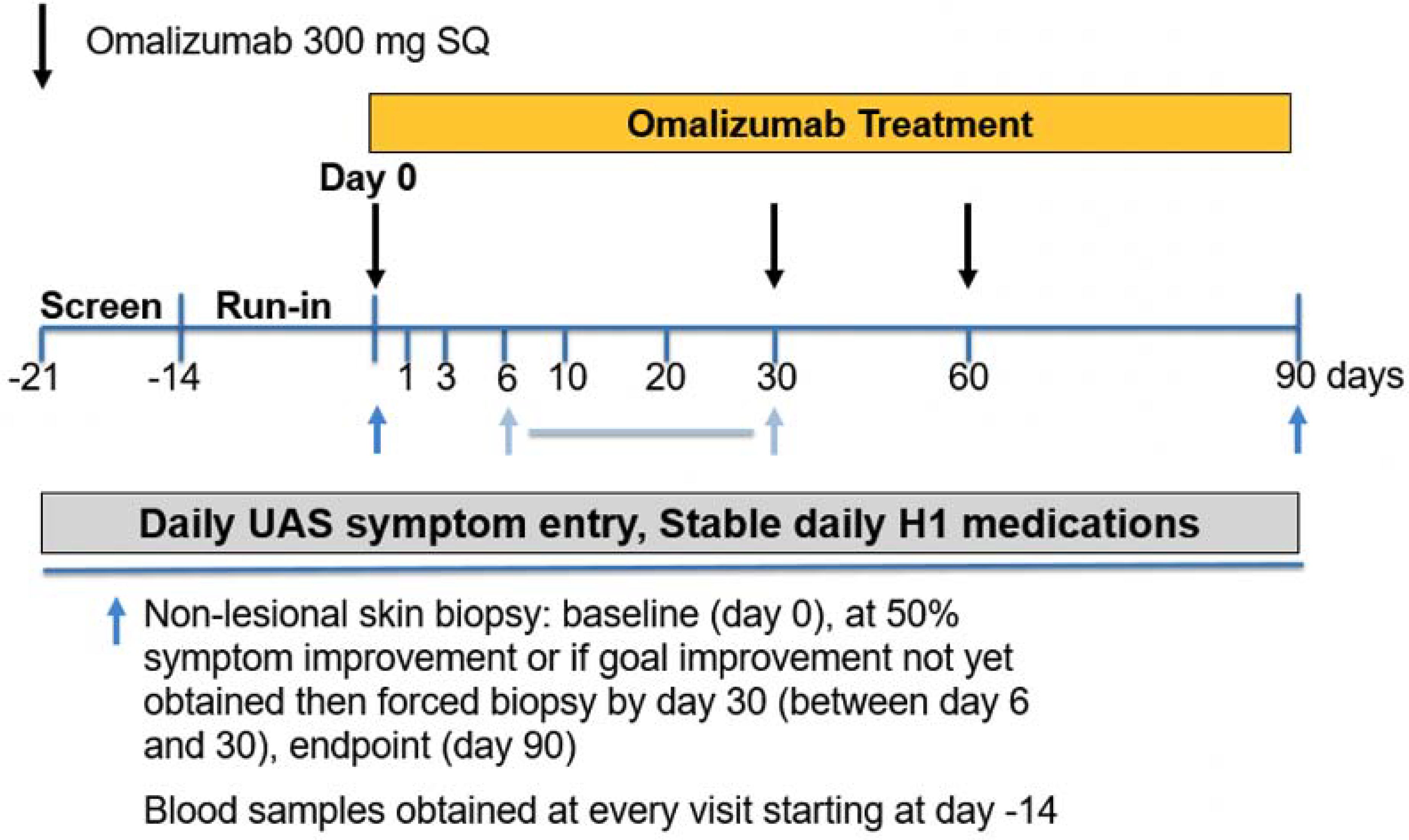
Study design. Depicted days indicate visits with laboratory studies. Black arrows indicate days of omalizumab dosing. Blue arrows indicate timing of skin biopsies. The second skin biopsy occurred between day 6 and 30 at the time of 50% symptom reduction relative to baseline UAS-7. If 50% symptom reduction was not achieved by day 30 then biopsy performed at day 30.
Subjects
Subjects ≥ 18 years of age with at least a 3-month history of CSU were recruited from allergy and dermatology clinics. Subjects were required to have active symptoms despite the use of 6 weeks of a daily 2nd generation H1-antihistamine up to 4-fold of the approved dose and maintained a stable dose of antihistamines throughout the study. Exclusion criteria included active atopic dermatitis, inducible urticaria, use of doxepin within 14 days of screening, or use of omalizumab within 6 months of screening. Subjects were not permitted to use systemic steroids or immuno-suppressants during the study or within 4 weeks prior to enrollment. Use of H2-antihistamines and/or leukotriene receptor antagonists was not permitted within 7 days of screening or during the trial unless these medications were previously indicated for treatment of GERD, asthma or allergic rhinitis.
Basophil Histamine Release (BHR) Assay
Single Percoll density gradient sedimentation was used to enrich blood basophils of patients from venous samples 11. Polyclonal goat antihuman IgE antibody (0.001 – 1.0 μg/mL; DACI Lab, Baltimore, MD) was used to stimulate basophils for HR in calcium-containing buffers for 45 minutes at 37° C. Basophils were also stimulated using fMLP (10−6 M) as a non-IgE receptor pathway stimulus for basophil degranulation. Supernatants were collected and analyzed via automated fluorometry11. Results are presented as the percentages of total histamine content of cell lysates of leukocyte aliquots after spontaneous HR was subtracted. Based on previous studies, maximum in vitro induced HR to an optimal concentration of anti-IgE antibody, was used to classify basophil functional phenotypes with CSU-R releasing ≥ 10% of cellular histamine content, and CSU-NR releasing <10% of cellular histamine content11. Patients with blood basophil counts less than 8000 cells per ml blood were classified as basopenic (CSU-B) based on Ward hierarchical clustering (see below, baseline basophil phenotype, Figure E1 and Table 1).
Table 1:
Study population baseline characteristics.
| Subject | Age | Gender | Duration | IgE | Dosing | BslUAS7 | MidPtDay | BasoPheno | BasoCount |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | 5 | 191 | 0.019 | 24.5 | 20 | NR | B |
| 2 | 34 | F | <1 | 347 | 0.013 | 16.5 | 10 | R | NB |
| 3 | 50 | F | 4 | 133 | 0.024 | 23.5 | 20 | NR | B |
| 4 | 32 | F | <1 | 21.4 | 0.238 | 32 | 6 | R | NB |
| 5 | 62 | F | 4 | 78.6 | 0.027 | 22 | 10 | R | NB |
| 6 | 39 | F | <1 | 5.3 | 0.821 | 32 | 10 | NR | B |
| 7 | 31 | M | 2 | 610 | 0.004 | 20.5 | 6 | R | NB |
| 8 | 33 | F | 16 | 1558 | 0.002 | 25.5 | 20 | R | B |
| 9 | 39 | M | 1 | < 2 | 2.054 | 42 | 30* | NR | B |
| 10 | 52 | M | 50 | 153 | 0.020 | 30.5 | 30* | Unclassified | B |
| 11 | 35 | M | <1 | < 2 | 1.586 | 35.5 | 20 | NR | B |
| 12 | 54 | M | 1 | 13.5 | 0.289 | 36 | 30* | NR | B |
| 13 | 67 | F | 10 | 70.8 | 0.059 | 29 | 6 | NR | NB |
| 14 | 36 | F | 20 | 146 | 0.025 | 21.5 | 6 | R | NB |
| 15 | 71 | F | 2 | 149 | 0.037 | 41.5 | 30 | NR | NB |
| 16 | 29 | M | 8 | 4.2 | 0.854 | 32 | 30* | NR | B |
| 17 | 36 | F | 1 | 197 | 0.016 | 34 | 6 | NR | NB |
| 18 | 22 | F | 6 | 56.3 | 0.084 | 23.5 | 30 | R | NB |
| Average | 44 | 67% F | 7.4 | 207 | 0.340 | 29 | 17.7 | ||
| R/NB | 36 | 83% F | 5.6 | 210 | 0.065 | 23^ | 11.3 | ||
| NR/NB | 58 | 100% F | 4.3 | 139 | 0.037 | 35 | 14.0 | ||
| NR/B | 44 | 43% F | 2.9 | 50 | 0.807 | 32 | 22.9 |
Indicates subjects that did not reach 50% UAS-7 reduction by day 30.
ANOVA p=0.0473
Duration = years of disease
Dosing = calculated dose of omalizumab in mg, per Kg body weight per IU IgE
BslUAS-7 = baseline UAS-7 score
MidPtDay = day at which symptom drop was greater than 50%
BasoPheno = anti-IgE functional category for the subject (NR = non-responder, R = responder)
BasoCount = Basophil count (B = basopenic, NB = non-basopenic)
Basophil Activation Test (BAT)
At the time of blood sampling for BHR, samples were also collected into heparinized tubes (BD vacutainer) for BAT studies. Heparinized blood was incubated with either buffer, doses of anti-IgE antibody or FMLP identical to those used in the BHR assay for 30 minutes at 37° C. Samples were then washed and labeled with CD123 APC (ThermoFisher) and either CCR3 PE (ThermoFisher) or CD63 PE (Beckman Coulter). Cells were subsequently lysed and fixed (Beckman Coulter) and then analyzed on a BD FACS Calibur flow cytometer. Basophils were gated using CCR3+, CD123+ cell subset and results expressed as CD63 % positive relative to buffer.
Basophil surface IgE, FcεRI Quantification
A separate aliquot of blood was used for receptor analysis. After Percoll isolation, cells were fixed in 2% paraformaldehyde for 20 minutes at room temperature, and the cell suspension was stored at 4°C. Cells were blocked with 0.2 mg/ml non-specific human IgG and labeled first with either nonspecific goat IgG, nonspecific mouse IgG1 isotype control, goat anti-IgE antibody, 22E7, or 15A5 mIgG1 antibodies (gift from Hoffman-LaRoche) at concentrations previously determine to be optimal for flow cytometry (25 minute incubation). A second 25 minute incubation with anti-mouseIgG1-alexa647, or anti-goat-alexa647 with anti-CD123-FITC and CCR3 (PE-labeled) (1/30 dilution from eBioscience stock solution) was followed by washing and analysis. When receptor expression was high enough, it was possible to alternatively gate the samples to derive the plasmacytoid dendritic cell IgE surface expression and determine the kinetics of change in these two parameters.
Dendritic Cell Surface IgE, FcεRI and Function
Additional blood samples (~50 ml drawn in EDTA, 10mM final) were obtained at baseline and at the time of second skin biopsy. These specimens were subjected to 2-step Percoll density centrifugation, which produces both basophil-depleted cell (BDC) and basophil-enriched cell suspensions 31. The BDC suspensions were washed 4 times to remove platelets and then immediately depleted of monocytes using CD14+ immunobeads with two passes over LS columns (both from Miltenyi-Biotec, Gaithersburg, MD). The column flow-thru cells, collected during monocyte depletion, were then subjected to BDCA4+ (CD304) selection (Miltenyi-Biotec). Cells retrieved from these LS columns were enriched for pDC and underwent testing for TLR9-dependent IFN-α production using stimulation with CpG-2216 oligonucleotide (TriLink Biotechnologies, San Diego, CA), as described 32. Supernatants were harvested after 20 hours and assayed for IFN-α protein by ELISA (R&D Systems, Piscataway, NJ). Surface IgE and FcεRIα were detected on pDC, as previously described29. In brief, 1×107 washed BDCs were fixed in 1 ml buffered 4% paraformaldehyde and stored at −80°C, which allowed for pre and post treatment preparations to be concurrently analyzed by flow cytometry. Staining for pDC was done by using anti-BDCA-2/allophycocyanin (Miltenyi Biotec, Auburn, Calif.). Co-expression of cell surface IgE and FcεRIα was individually assessed using goat anti-human IgE/fluorescein isothiocyanate (KPL, Gaithersburg, MD), and anti-FcεRIα/phycoerythrin (clone AER-37; ThermoFisher, Grand Island, NY), respectively. Analyses were performed using a Becton-Dickinson (BD) Accuri C6 Plus flow cytometer, with normalized mean fluorescence intensity (nMFI) determined after subtracting isotype control values.
Statistical Analysis
Generally, ANOVA was the statistical test applied to the results. A primary design categorization was based on the basophil phenotype. As noted below, the 3 basophil phenotypes were not necessarily independent. Therefore, the statistical analysis required independent comparisons of non-releasers/releasers and baso-normal/basopenic as two dichotomous categories. Tukey-Kramer analyses were utilized for comparing post-hoc group characteristics. Chi-square was used for comparison of categorical values. To determine the T1/2 metric for changes in daily UAS scores, the daily score values were first smoothed using a smoothing window of ±1 day. Once the data was normalized to the average of day −14 to 0, the crossing point for a value of 0.5 was extrapolated for the T1/2 of the UAS metric. Without the smoothing step, a similar procedure derived the T1/2 results for basophil surface IgE and FcεRI.
Results
Subjects
Of 27 subjects recruited, 8 failed to meet screening criteria, 1 was lost to follow-up and 18 subjects completed treatment with omalizumab (Figure E2) with demographics shown in Table 1. Two-thirds of subjects were females with an average age of 44 years with 7 years of CSU disease. The average baseline UAS-7 was 29 despite the use of antihistamines. All subjects received omalizumab 300 mg every 4 weeks, yielding a broad range in omalizumab dose (mg/kg/IgE) given the wide range of IgE levels (<2 to 1558 kU/L).
Baseline Basophil Phenotype and Symptom Response
Subjects were next categorized by two baseline basophil characteristics, circulating numbers of basophils and IgE-mediated histamine release profile (Table I). These then were examined for their clinical symptom response to omalizumab. As noted, Ward hierarchial clustering was used to set the threshold for basopenia (< 8000 basophils/mL). There were two clear clusters (non-basopenic (n=9) average basophil count 28600 basophils/mL, basopenic (n=9) average basophil count 2670 basophils/mL) separated by a 10-fold difference in basophil counts (Figure E1). Basopenics were more likely to be males (p =0.038, Chi-square), and received a higher average monthly omalizumab dose (p = 0.039, ANOVA) whereas no difference was found in age, baseline UAS, disease duration or total IgE. Daily UAS scores from Day −7 to 50 after omalizumab declined slower in basopenic subjects leading to a later day for a skin biopsy at the time of 50% symptom decline (23 days basopenic subjects vs. 12 days non-basopenic subjects, p = 0.043 ANOVA) (Figure 2A).
Figure 2.
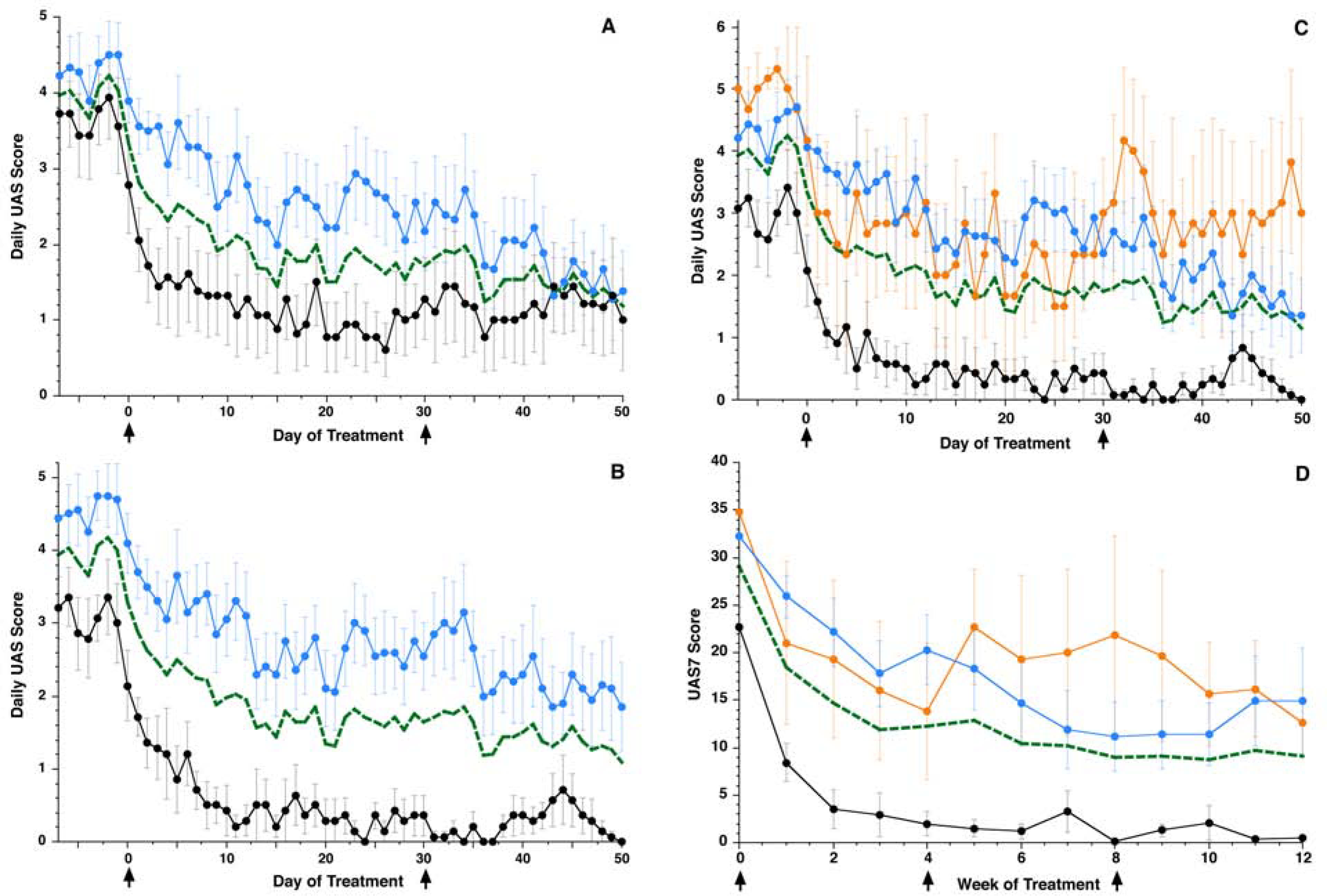
Clinical response during treatment represented by daily UAS scores for subjects. Black arrows indicate days of omalizumab dosing. (A) Single parameter classification based on basopenic status: Blue line (n=9) – basopenics average, Black line (n=9) – non-basopenics average, Green dashed line (n=18) – Average. (B) Single parameter classification based on basophil histamine response: Black line (n=7) – responders average, Blue line (n=10) – non-responders average, Green line (n=17) – Average. (C) Daily UAS scores during treatment with omalizumab according to the dual parameter basophil categorization of basopenia and responder status. (D) Weekly UAS7 scores during treatment. Black arrows indicate days of omalizumab dosing. For both panels 2C and 2D, Black line (n=6) – CSU-R/NB average, Orange line (n=3) – CSU-NR/NB average, Blue line (n=7) – CSU-NR/B average, Green line (n=16) – Average.
The second baseline basophil characteristic examined was the functional phenotype (CSU-R vs. CSU-NR) 11. For the responder/non-responder dichotomy, there were 10 basophil CSU-NR, 7 CSU-R and one subject that could not be classified due to extreme basopenia (subject 10, Table 1). With this classification, CSU-NR subjects had a significantly higher baseline UAS-7 (33 vs. 23, p < 0.01, ANOVA) but no other differences were found (i.e. age, IgE, omalizumab dosing, gender, or duration of disease). UAS symptom reduction was faster and more robust for CSU-R as compared to CSU-NR (Figure 2B) with an earlier average day of 50% UAS-7 reduction (day 12. 5 vs day 20 CSU-NR). However, it became apparent that there was a strong association between the non-responder status and the presence of basopenia (Table E1, chi-square p= 0.004 shown in companion paper as Table 2C). Therefore, we next used two-parameter categories and examined 3 sub-groups defined as a CSU-NR, non-basopenic (n=3, CSU-NR/NB) vs. CSU-NR with basopenia (n=7, CSU-NR/B) vs. CSU-R non-basopenic (n=6, labeled CSU-R/NB) for the remainder of the analyses. Two subjects were excluded from this classification, one due to extreme basopenia (subject #10) and another as the sole basopenic CSU-responder (Subject #8).
CSU-R/NB had the lowest mean baseline UAS-7 score of 23 of all 3 groups, indicative of the mildest symptoms; this was significantly different from the other two groups (p=0.016, Table 1). Of the three groups, NR/B received the highest dose of omalizumab, 0.807 mg/kg/IgE (ANOVA comparison of 3 groups, p = 0.0473, Tukey-Kramer HSD post-hoc analysis). Daily UAS scores from −7 to 50 days are shown in Figure 2C whereas weekly UAS-7 for the entire study are shown in Figure 2D. Classifying subjects according to both basophil characteristics, it is apparent that the distinguishing phenotype is the responder status; both CSU-NR/B and CSU-NR/NB were similar in their response to treatment (Figure 2C). Of interest, the CSU-R/NB group had the fastest and most complete symptom decline in response to omalizumab with an average UAS-7 reduction of 61% from baseline at week 1 and a 92% reduction at week 4 (Figure 2D). In contrast, CSU-NR/NB (43% week 1, progressing to 63% at week 4) and CSU-NR/B (17% week 1, progressing to 38% at week 4) experienced slower and less complete symptom decline on treatment with omalizumab. By the end of week 12, the overall reduction from baseline UAS-7 symptoms was 98% for CSU-R/NB, 64% for CSU-NR/NB, and 51% for CSU-NR/B. The sole patient with CSU-R and basopenia status followed the clinical response profile of CSU-R/NB subjects (data not shown).
Basophil Histamine Release and Basophil Activation Test Measures
Using the dual parameter classifications, we next examined the shifts in basophil anti-IgE triggered histamine response during omalizumab treatment relative to symptom changes. By day 6 of omalizumab therapy, both CSU-R/NB (Figure 3A) and CSU-NR/NB (Figure 3B) demonstrated an elevation in their anti-IgE mediated BHR as compared to baseline. The greater increase occurred in subjects with the CSU-NR/NB profile, but did not correspond to the greatest symptom improvement. This elevation persisted throughout the remainder of the study, through day 90. The similar timing for a rise in basophil histamine release in the corresponding CSU-R/NB and CSU-NR/NB groups and lack of concordance with the timing of clinical changes or changes in surface IgE or FcεRI suggests that this change is the result other variables in the basophil response. The notable lack of change in the CSU-NR/B anti-IgE antibody triggered response during omalizumab therapy is presented and discussed in a companion manuscript (Figure 3C and companion paper). However, no difference in HR was identified between the three groups when stimulated by fMLP, a non-IgE mediated pathway during treatment (Figure E3A). Similar shifts in anti-IgE induced profiles were noted by day 6 using BAT measures along with no change in BAT FMLP induced response (Figure E4 and E3B).
Figure 3:
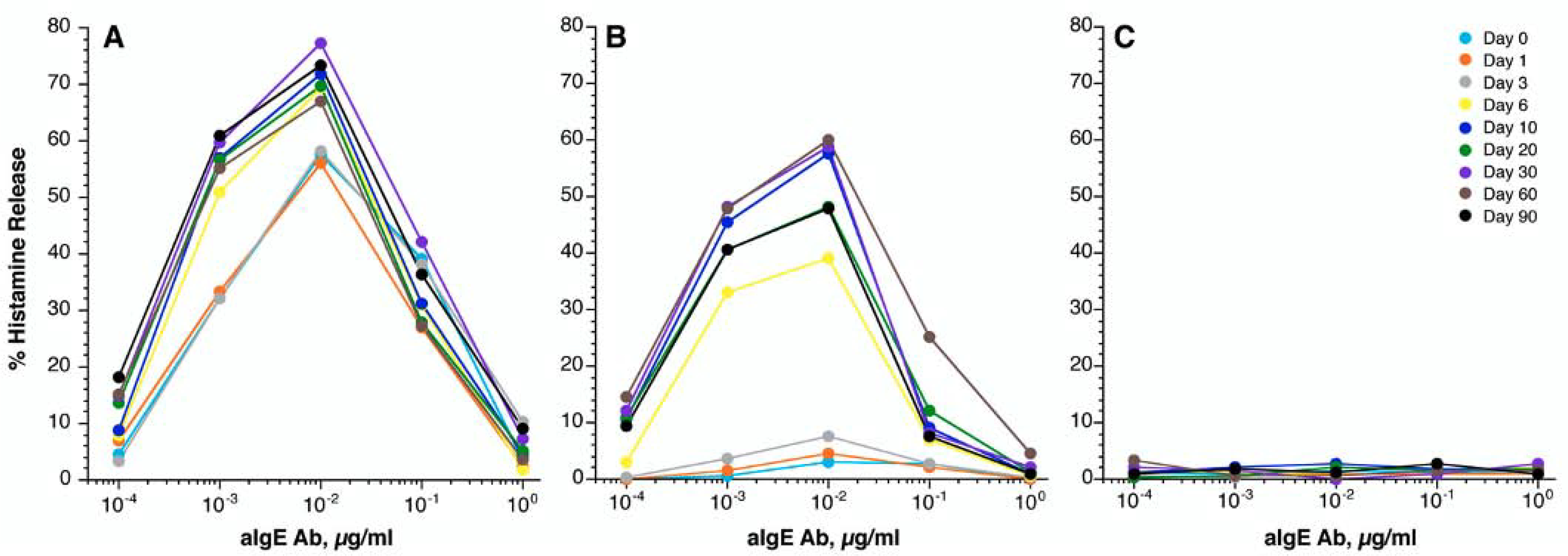
In vitro basophil histamine release in response to anti-IgE antibody stimulation at the indicated day of study. (A) Responder/Non-basopenics (CSU-R/NB), (n=6); (B) Non-responder/Non-basopenics (CSU-NR/NB), (n=3); (C) Non-responder/basopenics (CSU-NR/B) (n=7). The colored lines represent day of study.
Basophil Surface IgE and FcεRI data
Basophil surface IgE and FcεRI levels were reduced in all subjects following treatment with omalizumab. The kinetics of the decrease in surface IgE/FcεRI was similar in all 3 phenotypic groups (CSU-R/NB, -NR/NB, -NR/B) and did not follow the different timelines for the clinical response (Figure 4A and 4B). Although the serum IgE levels for the 3 groups did not show a statistically significant difference, differences were apparent in the starting surface IgE levels; the CSU-NR/B group had lower surface IgE at baseline (ANOVA, p<0.0001, Tukey-Kramer HSD). In the figure 4A and B insets, the percentage of baseline surface IgE and receptor levels are shown. While there was a similar percentage reduction of surface IgE in all groups, the percentage of baseline receptor in the CSU-NR/B group displayed a rising curve after an initial fall on therapy. This pattern of change in FcεRI in low receptor expressing basophils at baseline has been noted by Deza et al22. However, a strong correlation was noted between serum IgE and the baseline cell surface IgE level for the entire study group (see companion report). It is also worth noting that the CSU-NR/B group experienced minimal change in in vitro anti-IgE antibody responses either by HR or BAT and showed the greatest expression of auto-antibodies (presented in a companion report).
Figure 4.
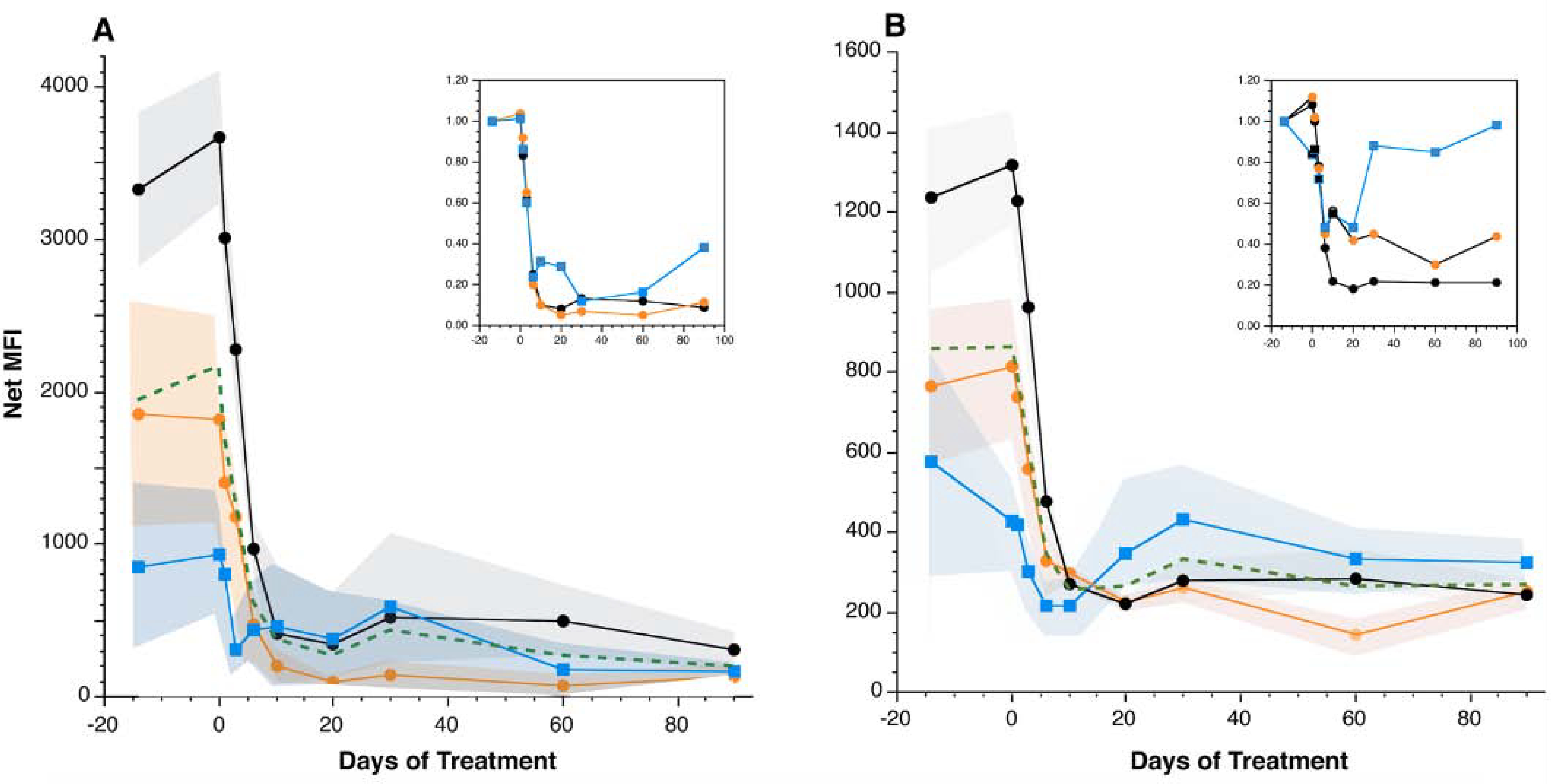
Omalizumab-induced reductions in basophil surface IgE (A) and FcεRI (B) were similar amongst basophil subsets. Inset figures represent change relative to baseline for the 3 categories (2-parameter categories). For both panels, Black line – CSU-R/NB average, Orange line – CSU-NR/NB average, Blue line – CSU-NR/B average, Green line – Average. Colored regions surrounding each line represent the SEM bands for the averages.
Our interest in the relationship between the loss of basophil surface IgE or FcεRI led to analyzing the rate of change in UAS daily symptom scores expressed as T1/2 of daily UAS (see statistical methods, i.e, the day at which symptoms were reduced by 50%,) vs. the T1/2 for reduction in either basophil surface IgE or surface FcεRI. Figures 5A&B show these relationships and the results suggest that there is no correlation between changes in symptoms and the rate of change in basophil IgE or FcεRI. Indeed, anecdotally, we could find individuals whose symptoms were reduced to 25% of their baseline with no changes yet occurring in the cell surface IgE or FcεRI. The online repository (Figure E5A and E5B) presents these results a different way, showing the kinetic curves for groups assembled on the basis of the rate of clinical change and the conclusion is similar.
Figure 5:
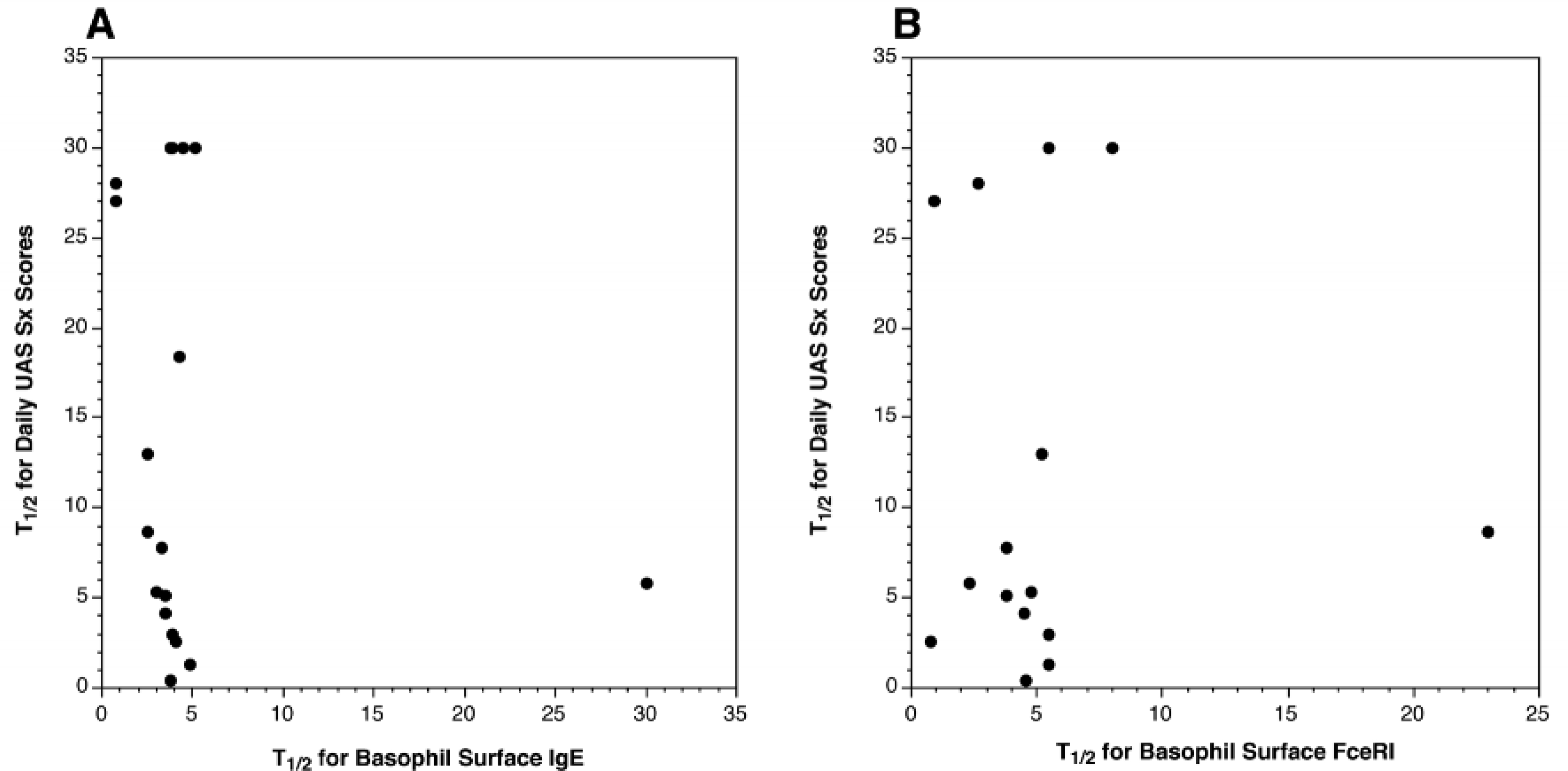
Relationship between rates of reduction (T1/2) in basophil surface IgE (A)or FcεRI (B) versus the rate of reduction (T1/2) in clinical symptoms. Each dot represents one subject.
Basophil Counts
From the perspective of the starting levels of circulating basophils, treatment with omalizumab did not, on average, increase basophil numbers in blood (Figure E6). In addition, there were no consistent changes in the subgroups (CSU-R/NB, -NR/NB, -NR/B). There were, however, significant increases among individual subjects that were consistent across multiple measurements during treatment. The most notable changes in some individual statistics occurred in the basopenic subjects but the increases still did not rise above the thresholds set for being classified as basopenic.
Dendritic Cells
Like basophils, other IgE-bearing cells are known to down-regulate FcεRIα following omalizumab administration, including pDCs from CSU subjects33. In this recent study, there were no apparent changes in pDC frequency before and after treatment; we also observed a similar stability in the frequency of pDCs (determined by flow cytometry, Figure 6E) and isolated numbers of pDCs before and after therapy (data not shown). To extend these observations, we examined pDC for functional responses before and after omalizumab treatment by focusing on their capacity to secrete IFN-α in response to TLR9-dependent activation. When classified according to the dual parameter basophil phenotypes, we observed that pDCs of the CSU-R/NB subjects had greater baseline TLR9 induced IFN responses as compared to the other 2 groups, and this pattern was maintained at the time of second biopsy on omalizumab (Figure 6A). When simply comparing IFN-α levels between the CSU-R and the CSU-NR using a dichotomous basophil R/NR classification, we observed significant IFN-α differences between these 2 groups both pre- and post-therapy, but no significant changes within groups (Figure 6B). We further confirmed a decline in FcεRI and surface IgE and at the indicated timepoints for functional assays (Figure 6C, 6D). Similar to basophils, in a subset of subjects, we found surface IgE on pDC decreased rapidly in those samples where there was confidence in the FcεRI expression with re-gating. In these cells, the loss of surface IgE was very rapid, a T1/2 of 0.9±0.3 days (Figure E7A). Total FcεRI decreased with a slightly slower time course (1.8 days) and unoccupied receptors increased (Figure E7B and E7C).
Figure 6:
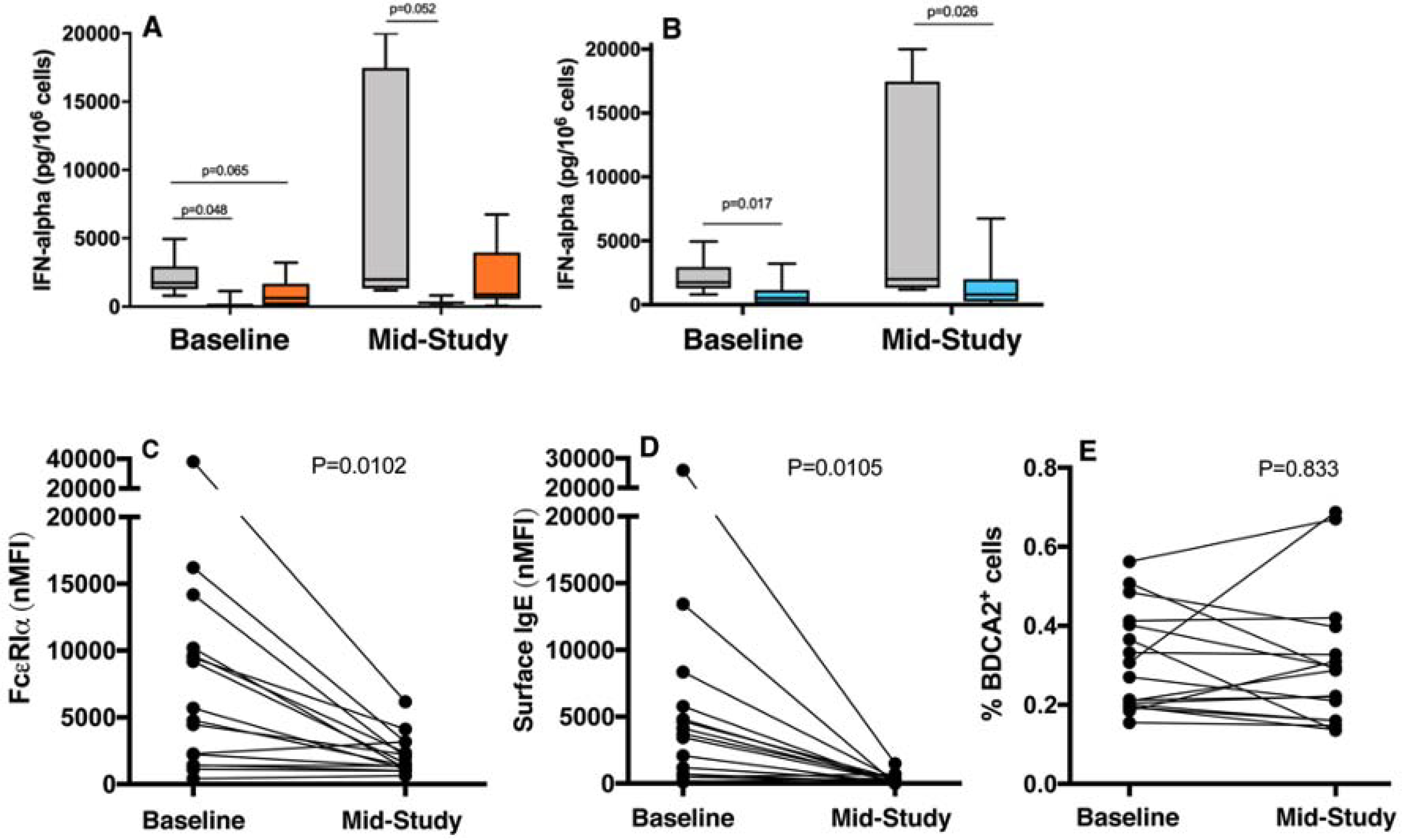
TLR9-mediated secretion of IFN-α, in vitro, from isolated pDCs at baseline and at mid-study measurement. Grouped by dual parameter basophil classifications (A):CSU-R/NB (n=6, grey bar), CSU-NR/NB (n=3, blue bar), NR/B (n=6, orange bar). (B): CSU-R (n=6, grey bar), CSU-NR (n=9, blue bar). (C) FcεRI (D) surface IgE, and (E) percent BDCA2+ pDC by flow at baseline and mid-study timepoints.
Discussion
At present, an understanding of disease pathways in CSU is limited to a role for skin mast cell activation via unknown triggering mechanisms. Among the proposed triggers for SMCs are IgG auto-antibodies to IgE and FcεRI in a subset of patients. A role for basophils in CSU disease has emerged given the detection of these cells at CSU skin lesions, linkage of basopenia to disease severity, and altered basophil IgE pathway phenotypes1, 3. These altered basophil features, basopenia and altered IgE pathways improve in disease remission7, 13. The rapid efficacy of omalizumab in mitigating CSU symptoms has focused attention on the contributions of basophils to CSU disease as well as other IgE bearing cell types such as pDCs. Past studies of omalizumab in allergic subjects have noted a faster reduction in IgE receptor levels and allergen activation of basophils as compared to mast cells, highlighting the possible contributions of basophils in clinical symptom relief in CSU 25, 26.
In this study, we confirmed the two expected basophil characteristics in CSU: basopenia and distinct anti-IgE functional phenotypes. A primary goal of the study was to determine if these well-defined basophil phenotypes would predict the clinical response to omalizumab. Furthermore, we also compared the onset of clinical symptom relief during omalizumab treatment relative to changes in basophil features to identify factors relevant to disease expression. When comparing the clinical responses to omalizumab, basopenic subjects had slower responses (Figure 2A) as did those with a CSU-NR profile (Figure 2B). Given the large overlap in these 2 basophil characteristics (basopenia with CSU-NR functional status), we examined the clinical response in subjects classified according to both parameters. We found that the functional phenotype was the dominant parameter and that the CSU subjects with a responder phenotype had the most rapid and complete clinical response to omalizumab (Figure 2A–D). The underlying disease mechanisms for the basophil CSU-R phenotype are not fully understood but appear to reveal a CSU subset that is dependent on IgE pathways for disease expression. This is the first description of this baseline basophil characteristic as a positive predictor for an omalizumab response. We have previously established the stability of this phenotype over time in active CSU patients13, and the association of this phenotype with milder but more prolonged CSU disease course, profiles that were as observed in this study.6
Among the CSU-NR subjects, we found a large overlap with basopenia and both of these features are associated with more severe CSU disease6. Prior studies have also suggested that low serum IgE 20, 21and basophil surface FcεRI22 levels are negative predictors for omalizumab response. As the basopenic group in this study also had the lowest serum IgE and basophil surface FcεRI expression, our study supports these prior observations. Due to the small group numbers, the statistical analysis suggested no difference in the serum IgE levels among the 3 groups (Table I) but the trend was evident and there was a significant reduction in surface IgE at baseline on CSU-NR/B (Figure 4A). At baseline, a correlation between serum IgE and surface IgE was indeed present when analyzing all study participants (see companion paper).
A second goal of the study was to determine if changes in the basophil compartment, notably with respect to cell surface IgE or FcεRI density, or functional phenotypes, would explain the rapidity of the response of CSU patients to omalizumab. This latter goal was predicated on characteristics of omalizumab to rapidly reduce FcεRI on human basophils (while causing a much slower decrease on mast cells) and the published observations that symptom reduction occurs rapidly in CSU patients being treated18. Our data suggests that the answer is that the two outcomes (rate of surface IgE reductions and symptom decreases) are not related and consistent with the recent observations of Aghdam33. The rate that cell surface IgE or FcεRI decreases during treatment is approximately the same for all subjects regardless of how one groups their basophil phenotypes (Figure 4A&B) or their rate of symptom change (Figure 5A&B, E5A–B). Notably, the rate of receptor decrease is consistent with prior studies in asthma or peanut allergy26, 34.
With regard to functional basophil changes, increased IgE receptor reactivity was noted in both the basophil CSU-R/NB and CSU-NR/NB groups, with the latter having the greater increase. The timing of change did not fit the timing of clinical response and may be explained by underlying changes in IgE pathway regulators such as SYK (see companion manuscript). In these non-basopenic subjects, these changes are consistent with the behavior we have found in allergics and asthmatics. Our findings agree with the Aghdam study which noted greater increase in basophil IgE reactivity in CSU subjects with modest to limited clinical response, whereas more modest basophil change was seen in those with the greatest clinical benefits33. Using the BAT assay, we were able to mirror basophils functional phenotypes based on histamine release profiles and reflect timing of omalizumab shifts. In contrast to other groups12, 33, 35, we did not include IL-3 in BAT assay buffers as this may impact the functional readouts of basophils.
Additionally, we explored the effect of omalizumab on alternative IgE bearing cell types such as pDCs. Prior literature demonstrates that innate immune responses are impaired in pDCs first exposed to IgE cross-linking stimuli, with these pDCs exhibiting a reduced capacity to secrete IFN-α upon subsequent stimulation with TLR9 agonists 32. pDC from CSU subjects are likewise reported to have impaired capacity to make IFN-α36. Although clinical trials demonstrate that omalizumab administration protects allergic asthmatics from viral-induced exacerbations37, the impact of omalizumab in CSU subjects in regards to this IFN-α production defect has not been previously reported in relation to symptom relief of urticaria. In our study, following treatment with omalizumab, enhanced IFN-α secretion was observed, although this did not reach statistical significance. Furthermore, this innate immune response was predicted by basophil phenotypes both at baseline and following treatment with omalizumab, with the pDCs from CSU-R/NB secreting greater levels of IFN-α compared to those of CSU-NR/NB and CSU-NR/B. CSU-NR subjects have reductions in TLR9 induced IFN-α response, which may be related to the known down-regulation of this pathway with IgE receptor cross-linking. An explanation for impaired TLR-9 response linked to IgE pathway profiles between two independent IgE bearing cell types is not obvious. The potential for IgG auto-antibodies targeting and limiting pDC functions such as for basophils (see related manuscript) has not been previously reported and requires further study. The clinical implication of blunted pDC interferon response in CSU subjects has also not been explored. Like the recent Aghdam study 33, pDC IgE/FcεRI receptors declined and no differences in isolated pDC numbers were noted pre and post omalizumab.
Previous studies of CSU have noted an increase in basophil counts during remission and that treatment with omalizumab may also cause increases7, 13, 38. The underlying basis for basal basophil circulating numbers is not well understood. It is known that an acute response to systemic glucocorticoids include a marked reduction in circulating basophils but the mechanism underlying that response is also unknown39. As a leukocyte that marginates into tissues in response to stimulation, it stands to reason that processes that activate the cell may lead to its margination. Previous larger phase III clinical studies have observed that circulating basophil numbers increase during treatment of CSU patients with omalizumab after 12 and 24 weeks of therapy38. In this study, the increases were inconsistent and in no group were the increases statistically significant, however, like Aghdam et al we observed an increase in the early timeframe post initiation of therapy33. When examined as a baseline characteristic, basopenic subjects had higher symptom scores and a more gradual symptom improvement as compared to non-basopenic subjects. This is consistent with previous studies noting basopenia as a marker of disease severity6. There were changes in some individuals in the basopenic group but the increases were never sufficient to change the basopenic status of the patient.
The potential clinical implications of our study should be mentioned. At present, basophil phenotyping via histamine release or BAT is only available in research centers. With the potential for more widespread use of the BAT assay, basophil functional phenotyping may be completed with greater ease, enabling the expansion of personalized medicine. Although we report CSU-R had the most rapid and complete clinical response to omalizumab, we would like to emphasize that all subjects experienced some degree of benefit, although without a placebo arm, it is unclear whether these responses were related to treatment. This data should therefore be utilized to inform providers and patients of potential timing and degree of response to omalizumab. Future studies are necessary to confirm if basopenia is a negative predictor for response to omalizumab.
In summary, these results demonstrate that changes in basophil surface IgE or FcεRI bear no relationship to the kinetics in the change in clinical symptoms. Whether the results were analyzed in the context of the 3 basophil phenotypes or the general relationships among the kinetics of decreases in symptoms, surface IgE or FcεRI, the results do not suggest a linkage between the receptor changes and clinical behavior. However, the study did identify a potential predictor of highly successful and rapid response to treatment, and that is the basophil functional responder phenotype. Two measurements, a basophil count and response of a subject’s basophils to an in vitro stimulation with crosslinking anti-IgE antibody would predict the rapidity of the response and its efficacy. It is unclear why this phenotype is predictive because it doesn’t appear to be related to what is happening to surface IgE or FcεRI.
Supplementary Material
Figure E1: Basophil counts (alcian blue-based) distribution for all subjects (n=18) at baseline.
Figure E2: Consort diagram of subject enrollment.
Figure E3. In vitro response kinetics for stimulation with FMLP during treatment with omalizumab. (A) Histamine release in response to 1 μM fMLP for the 3 groups (2-parameter categorization). (B) BAT CD63 response to 1 μM FMLP for the 3 groups. For both panels, gray line – CSU-R/NB average, Orange line – CSU-NR/NB average, Blue line – CSU-NR/B average.
Figure E4: In vitro basophil CD63 expression response to anti-IgE stimulation at the indicated day of study. (A) Responder/Non-basopenics (CSU-R/NB), (n=6). (B) Non-responder/Non-basopenics (CSU-NR/NB) (N=3). (C) Non-responder/basopenics (CSU-NR/B) (n=7). The colored lines represent each visit day.
Figure E6: Kinetics of basophil counts during treatment. Average counts grouped by 2-parameter categories. Black line (n=6)– CSU-R/NB average, Orange line (n=3) – CSU-NR/NB average, Blue line (n=7) – CSU-NR/B average, Green dashed line (n=16) – Average.
Figure E5: Kinetics of the decrease in symptom scores vs. the kinetics of the decrease in basophil surface IgE. Three groups defined by the relationships; IgE T1/2 << UAS T1/2, IgE T1/2 ≈ UAS T1/2, IgE T1/2 >> UAS T1/2. (A) The symptom change relative to baseline in these 3 groups and (B) kinetics of the basophil surface IgE changes relative to baseline in the same 3 groups as A. Arrows indicate 50% of measure.
Figure E7: (A) Kinetics of pDC surface IgE, (B) total FceRI and (C) unoccupied FceRI during treatment for 5 subjects.
Table E1: Association between responder status (R, NR) and basopenic status (NB,B). Chi-squared analysis, p < 0.004.
Clinical Implications or Key Messages.
Baseline basophil count and basophil functional phenotype, as determined in response to anti-IgE, may be predictive of responsiveness to omalizumab.
Acknowledgements:
We wish to acknowledge the excellent technical assistance of Valerie Alexander and the support of drug by Novartis for this study.
Funding:
AI116658, AI115703 (JTS, AB) AI116658-S1(ETO)
Abbreviations:
- CSU
Chronic Spontaneous Urticaria
- SMC
Skin mast cell
- FcεRI
High-affinity IgE receptor
- UAS
Urticaria Activity Score
- UAS-7
Urticaria Activity Score 7 day
- BHR
Basophil histamine release
- HR
Histamine release
- BAT
Basophil activation test
- CSU-R
Chronic spontaneous urticaria responder
- CSU-NR
Chronic spontaneous urticaria non-responder
- CSU-B
Chronic spontaneous urticaria basopenic
- CSU-R/NB
Chronic spontaneous urticaria responder/non-basopenic
- CSU-NR/NB
Chronic spontaneous urticaria non-responder/non-basopenic
- CSU-NR/B
Chronic spontaneous urticaria non-responder/basopenic
- pDC
Plasmacytoid dendritic cell
- nMFI
Normalized mean fluorescent intensity
- BDC
Basophil depleted cells
Footnotes
Conflicts of Interest:
The authors, other than Sarbjit S. Saini MD, declare that they have no relevant conflicts of interest. S. S. Saini has consulted for Allakos, Celltrion, Eli Lilly, Gbio, Genentech, Medimmune, Novartis, Regeneron, Sanofi & Pfizer and has received funding for research from the NIH, Novartis, Eli Lilly and Regeneron.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saini SS. Chronic spontaneous urticaria: etiology and pathogenesis. Immunol Allergy Clin North Am 2014; 34:33–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol 2002; 109:694–700. [DOI] [PubMed] [Google Scholar]
- 3.Saini SS. Basophil responsiveness in chronic urticaria. Curr Allergy Asthma Rep 2009; 9:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rorsman H Basopenia in urticaria. Acta Allergol 1961; 16:185–215. [DOI] [PubMed] [Google Scholar]
- 5.Grattan CE, Dawn G, Gibbs S, Francis DM. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy 2003; 33:337–41. [DOI] [PubMed] [Google Scholar]
- 6.Huang AH, Chichester KL, Saini SS. Association of basophil parameters with disease severity and duration in chronic spontaneous urticaria (CSU). J Allergy Clin Immunol Pract 2020; 8:793–5 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver ET, Sterba PM, Saini SS. Interval shifts in basophil measures correlate with disease activity in chronic spontaneous urticaria. Allergy 2015; 70:601–3. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy 2011; 66:1107–13. [DOI] [PubMed] [Google Scholar]
- 9.Greaves MW, Plummer VM, McLaughlan P, Stanworth DR. Serum and cell bound IgE in chronic urticaria. Clin Allergy 1974; 4:265–71. [DOI] [PubMed] [Google Scholar]
- 10.Kern F, Lichtenstein LM. Defective histamine release in chronic urticaria. J Clin Invest 1976; 57:1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vonakis BM, Vasagar K, Gibbons SP Jr., Gober L, Sterba PM, Chang H, et al. Basophil FcepsilonRI histamine release parallels expression of Src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. J Allergy Clin Immunol 2007; 119:441–8. [DOI] [PubMed] [Google Scholar]
- 12.Rauber MM, Pickert J, Holiangu L, Mobs C, Pfutzner W. Functional and phenotypic analysis of basophils allows determining distinct subtypes in patients with chronic urticaria. Allergy 2017; 72:1904–11. [DOI] [PubMed] [Google Scholar]
- 13.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol 2008; 128:1956–63. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol 2014; 133:1270–7. [DOI] [PubMed] [Google Scholar]
- 15.van den Elzen MT, van Os-Medendorp H, van den Brink I, van den Hurk K, Kouznetsova OI, Lokin A, et al. Effectiveness and safety of antihistamines up to fourfold or higher in treatment of chronic spontaneous urticaria. Clin Transl Allergy 2017; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol 2004; 114:527–30. [DOI] [PubMed] [Google Scholar]
- 17.MacGlashan DW Jr., Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of FceRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997; 158:1438–45. [PubMed] [Google Scholar]
- 18.Kaplan A, Ferrer M, Bernstein JA, Antonova E, Trzaskoma B, Raimundo K, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol 2016; 137:474–81. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan AP, Gimenez-Arnau AM, Saini SS. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy 2017; 72:519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straesser MD, Oliver E, Palacios T, Kyin T, Patrie J, Borish L, et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract 2018; 6:1386–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2017. [DOI] [PubMed] [Google Scholar]
- 22.Deza G, Bertolin-Colilla M, Pujol RM, Curto-Barredo L, Soto D, Garcia M, et al. Basophil FcepsilonRI Expression in Chronic Spontaneous Urticaria: A Potential Immunological Predictor of Response to Omalizumab Therapy. Acta Derm Venereol 2017; 97:698–704. [DOI] [PubMed] [Google Scholar]
- 23.Gericke J, Metz M, Ohanyan T, Weller K, Altrichter S, Skov PS, et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol 2017; 139:1059–61 e1. [DOI] [PubMed] [Google Scholar]
- 24.Palacios T, Stillman L, Borish L, Lawrence M. Lack of basophil CD203c-upregulating activity as an immunological marker to predict response to treatment with omalizumab in patients with symptomatic chronic urticaria. J Allergy Clin Immunol Pract 2016; 4:529–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol 2010; 125:889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paterniti MO, Breslin LM, Courneya JP, Sterba PM, Hamilton RG, MacGlashan DW Jr., et al. Differences in effects of omalizumab on late-phase responses to allergen challenge in the skin and nose at the time of basophil hyporesponsiveness. J Invest Dermatol 2014; 134:1743–4. [DOI] [PubMed] [Google Scholar]
- 28.Metz M, Staubach P, Bauer A, Brehler R, Gericke J, Kangas M, et al. Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcepsilonRI-positive cells in the skin. Theranostics 2017; 7:1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroeder JT, Bieneman AP, Chichester KL, Hamilton RG, Xiao H, Saini SS, et al. Decreases in human dendritic cell-dependent T(H)2-like responses after acute in vivo IgE neutralization. J Allergy Clin Immunol 2010; 125:896–901. [DOI] [PubMed] [Google Scholar]
- 30.Mathias SD, Crosby RD, Zazzali JL, Maurer M, Saini SS. Evaluating the minimally important difference of the urticaria activity score and other measures of disease activity in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 2012; 108:20–4. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder JT, Bieneman AP. Isolation of Human Basophils. Curr Protoc Immunol 2016; 112:7 24 1–7 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, et al. TLR9- and Fc{epsilon}RI-Mediated Responses Oppose One Another in Plasmacytoid Dendritic Cells by Down-Regulating Receptor Expression. J Immunol 2005; 175:5724–31. [DOI] [PubMed] [Google Scholar]
- 33.Alizadeh Aghdam M, Knol EF, van den Elzen M, den Hartog Jager C, van Os-Medendorp H, Knulst AC, et al. Response of FcepsilonRI-bearing leucocytes to omalizumab in chronic spontaneous urticaria. Clin Exp Allergy 2020; 50:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacGlashan DW Jr., Saini SS. Syk expression and IgE-mediated histamine release in basophils as biomarkers for predicting the clinical efficacy of omalizumab. J Allergy Clin Immunol 2017; 139:1680–2 e10. [DOI] [PubMed] [Google Scholar]
- 35.Rauber MM, Pickert J, Holiangu L, Mobs C, Pfutzner W. Omalizumab response correlates with reduced IFN-gamma-, IL-10- and IL-31-secreting cells in chronic spontaneous urticaria. J Eur Acad Dermatol Venereol 2020. [DOI] [PubMed] [Google Scholar]
- 36.Futata E, Azor M, Dos Santos J, Maruta C, Sotto M, Guedes F, et al. Impaired IFN-alpha secretion by plasmacytoid dendritic cells induced by TLR9 activation in chronic idiopathic urticaria. Br J Dermatol 2011; 164:1271–9. [DOI] [PubMed] [Google Scholar]
- 37.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr., Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 2015; 136:1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saini SS, Omachi TA, Trzaskoma B, Hulter HN, Rosen K, Sterba PM, et al. Effect of Omalizumab on Blood Basophil Counts in Patients with Chronic Idiopathic/Spontaneous Urticaria. J Invest Dermatol 2017; 137:958–61. [DOI] [PubMed] [Google Scholar]
- 39.Dunsky EH, Zweiman B, Fischler E, Levy DA. Early effects of corticosteroids on basophils, leukocyte histamine and tissue histamine. J Allergy Clin Immunol 1979; 63(6):426–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1: Basophil counts (alcian blue-based) distribution for all subjects (n=18) at baseline.
Figure E2: Consort diagram of subject enrollment.
Figure E3. In vitro response kinetics for stimulation with FMLP during treatment with omalizumab. (A) Histamine release in response to 1 μM fMLP for the 3 groups (2-parameter categorization). (B) BAT CD63 response to 1 μM FMLP for the 3 groups. For both panels, gray line – CSU-R/NB average, Orange line – CSU-NR/NB average, Blue line – CSU-NR/B average.
Figure E4: In vitro basophil CD63 expression response to anti-IgE stimulation at the indicated day of study. (A) Responder/Non-basopenics (CSU-R/NB), (n=6). (B) Non-responder/Non-basopenics (CSU-NR/NB) (N=3). (C) Non-responder/basopenics (CSU-NR/B) (n=7). The colored lines represent each visit day.
Figure E6: Kinetics of basophil counts during treatment. Average counts grouped by 2-parameter categories. Black line (n=6)– CSU-R/NB average, Orange line (n=3) – CSU-NR/NB average, Blue line (n=7) – CSU-NR/B average, Green dashed line (n=16) – Average.
Figure E5: Kinetics of the decrease in symptom scores vs. the kinetics of the decrease in basophil surface IgE. Three groups defined by the relationships; IgE T1/2 << UAS T1/2, IgE T1/2 ≈ UAS T1/2, IgE T1/2 >> UAS T1/2. (A) The symptom change relative to baseline in these 3 groups and (B) kinetics of the basophil surface IgE changes relative to baseline in the same 3 groups as A. Arrows indicate 50% of measure.
Figure E7: (A) Kinetics of pDC surface IgE, (B) total FceRI and (C) unoccupied FceRI during treatment for 5 subjects.
Table E1: Association between responder status (R, NR) and basopenic status (NB,B). Chi-squared analysis, p < 0.004.


