Abstract
Systemic buprenorphine and topical antiseptics such as chlorhexidine are frequently used in research animals to aid in pain control and to reduce infection, respectively. These therapeutics are controversial, especially when used in wound healing studies, due to conflicting data suggesting that they delay wound healing. Low-level laser therapy (LLLT) has been used to aid in wound healing without exerting the systemic effects of therapies such as buprenorphine. We conducted 2 studies to investigate the effects of these common treatment modalities on the rate of wound healing in mice. The first study used models of punch biopsy and dermal abrasion to assess whether buprenorphine HCl or 0.12% chlorhexidine delayed wound healing. The second study investigated the effects of sustained-released buprenorphine, 0.05% chlorhexidine, and LLLT on excisional wound healing. The rate of wound healing was assessed by obtaining photographs on days 0, 2, 4, 7, and 9 for the punch biopsy model in study 1, days 0, 1, 2, 4, 6, 8, 11, and 13 for the dermal abrasion model in study 1, and days 0, 3, 6, and 10 for the mice in study 2. Image J software was used to analyze the photographed wounds to determine the wound area. When comparing the wound area on the above days to the original wound area, no significant differences in healing were observed for any of the treatment groups at any time period for either study. Given the results of these studies, we believe that systemic buprenorphine, topical chlorhexidine, and LLLT can be used without impairing or delaying wound healing in mice.
Abbreviations: low-level laser therapy (LLLT)
A recent retrospective analysis using a medical insurance dataset estimated that approximately 8.2 million people experienced wounds ranging from acute to chronic conditions within the particular year analyzed, and estimated that the cost of acute and chronic wound treatments ranged from $28.1 to $96.8 billion dollars.52 The projected rise in the number of people experiencing wounds and the cost of wound care products52 have made wound healing a growing area of interest in both clinical medicine and research. Wound healing is a complex process that involves many overlapping, intricate physiologic processes. Each step can have associated deviations that may lead to enhanced, altered, impaired, or delayed healing. Animal research has been used to develop a better understanding of the basic, physiologic mechanisms of wound healing. Mice are the most commonly used animal in biomedical research, and they are used to model a host of conditions, including wound healing. Despite known anatomic and physiologic differences between murine and human skin,17,53 this species is commonly used due to their small size, ease of handling, and relatively low cost. In addition, the overlapping phases of the wound healing process are similar in mice and humans, making mice a valuable model.65
Pain is inherent to the development of wound models. Pain receptors in the skin are sensitized during the actual wounding process and during the inflammatory response that occurs immediately after wounding.19 Pain can also occur during the cleansing and treatment of wounds.19 Just as managing wound pain is critical in human patients, The Guide for the Care and Use of Laboratory Animals (the Guide)30 and other federal guidelines and regulations governing the care and use of laboratory animals strongly encourages the use of analgesics for animals that experience pain and/or distress.30 Pain, which can also cause stress, may evoke a persistent catabolic state and may ultimately delay wound healing.19,28,31,43 Therefore, adequate pain control is necessary to avoid negatively affecting or altering the wound healing process.
As in human medicine, opioids are commonly used to provide analgesia to research rodents. Buprenorphine, a mixed agonist-antagonist opioid,26,54 is a common analgesic that acts as a very weak partial agonist of the mu opioid receptor and an antagonist of the κ opioid receptor.26 Buprenorphine is frequently used in animals as both a pre- and post-operative analgesic. It works by binding to the opioid receptors in the skin and other tissues. This ligand-receptor binding regulates the physiologic responses of nociception and inflammation,7 which are key factors in the process of healing and regeneration. Buprenorphine is often used instead of full mu-opioid receptor agonist drugs, such as morphine or hydromorphone, because it has fewer systemic side effects.28 Despite their common use as analgesics, reports are mixed in terms of whether opioids, as a class, delay or impair wound healing.11,28,35,40
In addition to controlling pain, minimizing wound contamination and preventing infection is critical to wound healing. The use of antiseptics is often favored over the use of antibiotics as the former presents less chance for developing antibiotic resistance.6 As an antiseptic, chlorhexidine is commonly used to irrigate, cleanse, and treat cutaneous wounds. Chlorhexidine has high antimicrobial activity against gram-positive and gram-negative bacteria and some fungi and viruses.4 Although considered to be relatively safe, reports are conflicting with regard to whether chlorhexidine delays or impairs wound healing.4,9,50,57
Laser techniques have been used medically for many years, and their powerful, but precise capabilities have rendered them a unique surgical and therapeutic modality. In brief, when the electrons of atoms move to higher energy levels, these electrons absorb energy. This excited energy state is unstable and temporary. The natural return of electrons to their more stable ground state releases energy in the form of photons or light. Light Amplification by Stimulated Emission of Radiation (LASERS) are characterized by the photon stimulation of an already excited electron. This stimulation causes the emitted light to be amplified, as demonstrated by the intense, bright light that is emitted from lasers.63 The concept of low-level laser therapy (LLLT) has garnered interest as a therapeutic modality in both human and veterinary medicine. Specifically characterized as laser therapy using a low power output and a low power range, LLLT is distinguished from other forms of laser therapies by certain parameters such as wavelength, pulse rate and duration, total irradiation time, and dose.44 Although the mechanism of action for LLLT is not completely understood,46,64 the absorption of red and near infrared light energy may reduce detrimental, inflammatory substances13,15,24,56 while simultaneously stimulating restorative processes.15,24,46,64 The reduced photothermal impact of LLLT44 is reported to produce beneficial physiologic and biologic effects including analgesia, reduction in inflammation, and acceleration of healing.48 The initial report of LLLT as a therapeutic modality found accelerated wound healing and fur regrowth in mice exposed to LLLT.13,44,46,64 LLLT has since been used as a sole or adjunct therapy for a variety of conditions including tooth root resorption,55 traumatic brain injuries,58 and tendon, muscle, and bone injuries.2,3,25,38
Studies conducted to assess the effects of LLLT on healing often use parameters of normal wound healing to analyze how LLLT influences those parameters in comparison to healthy, undamaged tissue and damaged tissue not receiving laser therapy. Despite the numerous studies designed to investigate the effects of LLLT on wound healing, conflicting reports exist regarding its efficacy.15,17,46,22,23,24,29,34,38,39,55,56,60,64 A recent study in dogs reported accelerated healing and improved cosmetic appearance of a hemilaminectomy surgical site after LLLT,60 while other canine studies reported no significant differences in the healing of surgically induced skin wounds between dogs that did and did not receive LLLT.22,34 Similarly, in an attempt to study the effects of LLLT in pigs, an animal with skin very similar to that of humans, no significant differences were reported in the healing of surgically created skin wounds between swine that did and did not receive LLLT.29 Studies using diabetic rats with excisional cutaneous wounds reported accelerated wound healing,17,46 and beneficial results were reported in a similar study using diabetic mice.56,64 While fewer studies have been conducted on the use of LLLT in rodents without concomitant comorbidities, LLLT has been reported to accelerate wound healing in healthy rodents.15,24 Conversely, some studies found that LLLT does not accelerate or significantly improve wound healing in rodents.24,39
We performed 2 separate studies to investigate the effects of a commonly used opioid, a topical antiseptic solution, and LLLT on excisional wound healing in mice. At the time the initial study (study 1) was conducted, some of our investigators were reluctant to use the recommended analgesic, buprenorphine, due to concern about interference with their study outcomes. Therefore, we conducted study 1 to determine if a single dose of peri-operative buprenorphine would delay healing of a full-thickness excisional wound or a partial-thickness felt wheel dermal abrasion. We also examined the effects of topical chlorhexidine solution on wound healing. The chlorhexidine concentrations used in study 1 were prepared using our standard operating procedure at that time. Study 2 was conducted after study 1, with the design expanded to evaluate a sustained release buprenorphine formulation and LLLT. Study 2 used a full-thickness excisional biopsy to determine the effect of LLLT on excisional wound healing. Commonly used doses of systemic Buprenorphine Sustained Release (SR) and topical chlorhexidine were also included to evaluate their effect on excisional wound healing. The concentration of chlorhexidine in the revised, approved standard operating procedure had been decreased due to literature suggesting that higher concentrations may inhibit healing.4,49,61 For both studies, we hypothesized that the use of buprenorphine and chlorhexidine would have no effect on the rate of wound healing, and that LLLT would accelerate wound healing in a full-thickness excision as compared with a control.
Materials and Methods
Animals.
Both studies used a total of 120 experimentally naïve, female B6(Cg)-Tyrc-2J/J mice (stock no. 000058, Jackson Laboratory, Bar Harbor, ME). These mice were excess animals obtained from an inhouse investigator and were used after a 1-week acclimation period. Study 1 used 30 16-wk-old mice, and study 2 used 90 mice, ranging between 8 to 12 wk of age. All mice were group housed in individually ventilated cages (Techniplast, West Chester, PA) on Alpha-Dri bedding (Shepard Specialty Papers, Watertown, TN) with autoclaved Enviro-dri (Shepard Specialty Papers, Watertown, TN) for nesting. Mice in study 1 were housed 5 per cage, and mice in study 2 were housed 3 per cage. Mice were maintained on a 12:12-h light: dark cycle at 22 ± 0.5 °C and relative humidity of 40% to 60%. Mice were provided ad libitum autoclaved rodent diet (NIH31, Teklad Laboratories, Madison, WI) and deionized water treated by reverse osmosis. All mice tested negative for mouse hepatitis virus, sendai virus, pneumonia virus of mice, mouse parvovirus 1 and 2, epizootic diarrhea of infant mice, mouse norovirus, Mycoplasma pulmonis, Helicobacter spp., and endo- and ectoparasites upon receipt; no pathogens were detected in indirect sentinel mice testing during either study. Sentinel mice (1 cage per double sided rack) were exposed to used bedding from principal cages biweekly during routine cage change. Per our established sentinel program standard, sentinel mice were tested serologically on months 1 and 2 of the quarter, and necropsies were performed quarterly (month 3). Sentinel pairs were replaced every 6 mo. In addition to serology testing and quarterly necropsies, fecal PCR was performed for murine pinworms every 6 mo, and environmental testing for infectious agents was performed on a case-by-case basis. All animal procedures were reviewed and approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee. All animals were housed, cared for, and used in compliance with the Guide for the Care and Use of Laboratory Animals30 in an AAALAC International accredited program.
Wounding Preparation.
On the day prior to wounding, each mouse was individually weighed and was then anesthetized using 3% isoflurane gas with 1 L/min oxygen. After applying eye lubricant, each mouse was placed in ventral recumbency. Clippers were used to remove the majority of fur from the dorsum. Commercially available depilating cream (Nair; Church and Dwight, Ewing, NJ), was then applied to the shaved region using cotton swabs. Nair is a commonly used to remove any remaining fur after shaving and to ensure that a hairless surface was present prior to surgical wounding.66 Using a cotton swab applicator, Nair was applied continuously, in a circular motion, for 15 to 20 s or until the remaining fur started to depilate. A square cotton gauze with saline was used to remove the excess depilatory cream from the mice. Mice were individually identified by ear punch and by tail numbers written using permanent markers, and then were returned to their cages to recover on supplemental heat.
Wounding.
The day after skin preparation, each mouse was anesthetized and eye lubrication was applied as described above.
Study 1.
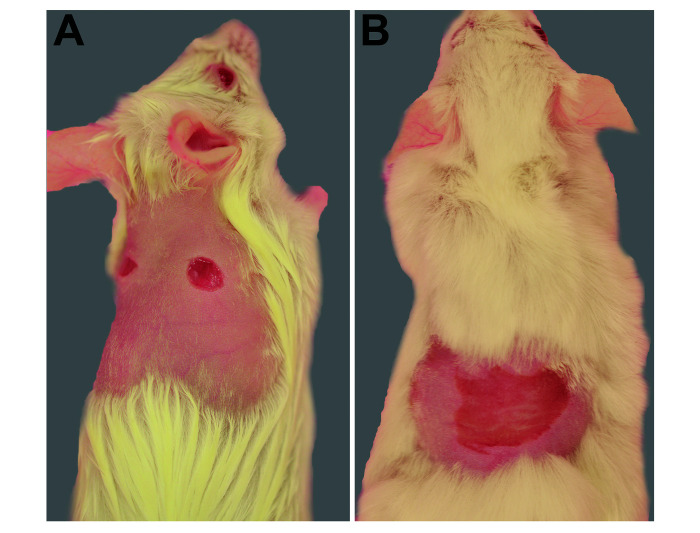
Two wound types were used, a punch biopsy and a dermal abrasion. For the punch biopsy, 10 out of 20 mice received a single preoperative dose of 0.1 mg/kg buprenorphine hydrochloride (0.3 mg/mL) (Pfizer, NY, NY) in a volume of 0.1 mL/10g subcutaneously in the flank or lower back, away from the intended wound site. The remaining 10 animals received an equivalent volume of normal saline subcutaneously. All mice were prepared for surgery using Prevantics swab sticks (Prevantics, PDI, Orangeburg, NY) containing chlorhexidine (3.15%) and isopropyl alcohol (70%) prior to wounding. After confirming a surgical plane of anesthesia as indicated by lack of toe-pinch response on all 4 paws, a sterile 4 mm punch biopsy tool (Miltex, York, PA) was used to create 2 full thickness punch biopsies on the dorsum. Sterile tissue scissors were used to remove the remainder of the biopsy tissues (Figure 1). For the abrasion model, 5 of 10 mice received buprenorphine as described above, and the remaining 5 mice received saline as described above. After surgical preparation and confirmation of a surgical plane of anesthesia as previously described, a 2.0 cm2 area of the dorsum was gently abraded using a felt wheel on a rotary tool (Dremel 3000 corded rotary tool (Racine, WI), creating a shiny, pink, bloodless abraded area (Figure 1 B).
Figure 1.
(A) Full thickness punch biopsies on the dorsum of animals used in study 1. (B) Partial thickness dermal abrasion on the dorsum of animals used in study 1.
Study 2.
Seventy-two of 90 mice received 1 mg/kg (1.0 mg/mL) of Buprenorphine SR-LAB (sustained release formula, ZooPharm, Laramie, WY) subcutaneously in either the flank or lower back to avoid injection near the shaved area intended for wounding. The remaining 18 mice received 0.9% saline subcutaneously (0.05 mL each) in the locations described above. Mice were aseptically prepared for surgery using alternating scrubs of 10% povidone-iodine swab sticks (Medline Industries, Dallas, Tx) and 70% alcohol (Webcol alcohol pads, Cardinal Health, Dublin, OH). After confirming a surgical plane of anesthesia, each mouse was placed in left lateral recumbency, and the shaved skin along the dorsum was tented and folded over on to a firm, flat surface. Due to minor difficulties in obtaining 2 full thickness punch biopsies on the dorsum on animals in study 1, the process was refined for study 2. For study 2, a sterile, 4mm, disposable punch biopsy instrument (Miltex, York, PA) was used to create a single, dorsal, midline, full thickness wound, instead of 2 dorsal wounds. Sterile tissue scissors were used to remove any remaining biopsy tissue.
After the wounding procedures, each mouse in both study 1 and study 2 was photographed and placed back in its home cage with supplemental heat. All animals from both studies were weighed twice weekly, and general health conditions were monitored daily.
Treatments.
Study 1.
For the punch biopsy, a total of 20 mice were divided into 4 treatment groups (5 mice per treatment group). The treatment groups were as follows: (S)-Saline only; (SC)-Saline and Chlorhexidine; (B)-Buprenorphine only (diluted in sterile water to 0.1 mg/mL); and (BC)- Buprenorphine and Chlorhexidine (Figure 2 A). All chlorhexidine applications were provided by the same person throughout the study. For the dermal abrasion, a total of 10 mice were divided into 2 treatment groups (5 mice per treatment group). The treatment groups for the abrasion model were: (S)- Saline only and (B)- Buprenorphine only (Figure 2 A). Prior to wounding, mice were arbitrarily assigned to treatment groups. In order to achieve blind assignments to the treatment groups, a person unaffiliated with the study arbitrarily selected a mouse from the original home cage and assigned the animal to an experimental (treatment) cage.
Figure 2.
(A)Study 1 schematic. (B)Study 2 schematic. (C)Study 2 schematic with division of study 2 animals into 2 batches.
Mice receiving topical chlorhexidine therapy were treated once daily for 5 d. Chlorhexidine solution (2%) (Vedco, St Joseph, Missouri), diluted in sterile reverse osmosis deionized water (0.12%), was applied using a soaked gauze that was held over the wound for 10 to 20 s. Treatments were performed using manual restraint on days that photographs were not obtained. On days that photographs were obtained (days 0, 2, 4, 7, and 9 for the punch biopsy and days 0, 1, 2, 6, 8, 11, and 13 for the abrasion), chlorhexidine was applied while mice were anesthetized.
Mice receiving perioperative Buprenorphine HCl only or perioperative saline only did not receive any other treatments throughout the duration of the study. However, these mice were manually restrained on non-photography days to control for restraint stress. These mice were also anesthetized and photographed on the days previously mentioned.
Study 2.
Mice were divided into 5 treatment groups (18 mice per treatment group). The treatment groups were as follows: (BLC)- Buprenorphine SR + low level laser therapy+ chlorhexidine; (BL)- Buprenorphine SR+ low level laser therapy; (BC)- Buprenorphine SR (1.0 mg/mL) + chlorhexidine; (B)- Buprenorphine SR only; (S)- saline only. All mice were individually weighed twice weekly. Due to the large number of mice used in study 2, 45 mice were equally allocated to the 5 treatment groups and underwent experimentation; the second cohort began the test beginning 2 calendar days after the first cohort finished (Figure 2 B and C). All preparation and procedures were performed in exactly the same manner for both cohorts of mice. However, the second cohort were 10 to 12 wk of age when used rather than the 8 to 10 wk old ages of the first cohort. Mice were assigned to experimental treatment groups as described in study 1. All chlorhexidine and laser treatments were performed by the same person throughout the study. The statistical model adjusted for potential batch effects (see “Statistical Analysis” below).
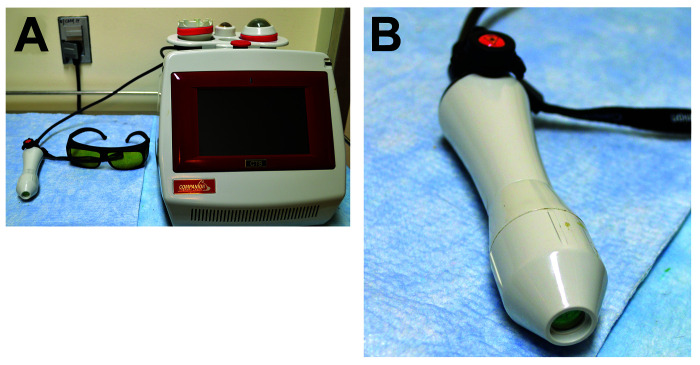
LLLT was administered using a class IV LASER, Companion Therapy System, CTS model (Companion Animal Health, Newark, DE) (Figure 3 A) with a wavelength of 980 nm. A continuous wave mode at an energy of 10 Joules (1 W of power for 10 s) was applied the CTS-provided small dermal treatment attachment (Figure 3 B). Laser treatment was performed 3 d per week (Monday, Wednesday, and Friday), beginning on the first day after wounding. To reduce the number of times mice were placed under general anesthesia, each mouse receiving LLLT was manually restrained using a Mouse DecapiCone (Braintree Scientific, Braintree, MA) with the wound exposed, and black electrical tape covering the narrow end to prevent ocular exposure to laser beams (Figure 4). On days 3, 6, and 10 after wounding, when photos were obtained, mice received laser therapy under isoflurane anesthesia.
Figure 3.
(A) Companion Therapy System (CTS) class IV laser therapy used to administer low-level laser therapy to animals used in study 2. (B). CTS provided small, dermal treatment attachment used to administer low-level laser therapy to animals used in study 2.
Figure 4.

Modified decapicone with black electrical tape used to cover the eyes, and a whole cut out to access wounds. This was used to administer laser therapy to animals without having to anesthetize animals daily.
Mice were treated once daily for 5 d with 1.0 mL of 0.05% dilute chlorhexidine on a cotton gauze square. Mice were manually restrained in a modified DecapiCone as described above, and the chlorhexidine-soaked gauze square was held over the wounded area for 10 to 20 s. Chlorhexidine treatment was performed after laser therapy on days when mice received both treatments. On days 3, 6, and 10 after wounding, photographs were taken under isoflurane anesthesia, immediately after laser therapy, but before chlorhexidine application.
Mice receiving perioperative Buprenorphine SR-LAB only or perioperative saline only were manually restrained in the modified DecapiCone daily, for 10 s each, and received no other treatments throughout the duration of the study. On days 3, 6, and 10 after wounding, photos were obtained while mice were under isoflurane anesthesia.
Documenting and Image Analysis.
Wounds were photographed while mice were anesthetized. All photographs were taken with standard exposures and focal lengths using a digital camera (Nikon D3200) equipped with a fixed focal length lens (zoom lens set at 55 mm) and mounted on a tripod with a set height of 60 centimeters. A micrometer or ruler was included in all photographs and was used as a reference to calibrate the measurements obtained by the software. Image analysis software (Image J/Fiji, National Institutes of Health, Bethesda, MD) was used to analyze all photographs. For all images, measurements and analyses were based on the wound being specifically defined by the edges of the punch biopsy lesion, and not by associated erythema or scabbing.
In study 1, wounds were photographed on experimental days 0, 2, 4, 7, and 9 for the punch biopsies, and on days 0,1, 2, 4, 6, 8, 11, and 13 for the abrasions. In study 2, wounds were photographed on experimental days 0, 3, 6, and 10. Using the Image J software, all photographs were analyzed to calculate the total wound surface area (Figure 5).
Figure 5.
Wounds were photographed on specific days for each study. Image J software was used to determine the total wound surface area.
Tissue preparation and histopathology.
Histopathology was not performed on mice from study 1 because the focus for this study was the visual and clinical measurement of wound healing. For study 2, on days 3, 6, and 10 after wounding, 6 mice from each treatment group were randomly selected and euthanized using 100% carbon dioxide, per AVMA guidelines. By day 10, all mice from study 2 had been submitted for necropsy. A 2.0-centimeter lateral section of skin was taken from the lesion site of each mouse and was stapled to an index card to prevent curling. The skin on the index cards were fixed in 10% neutral buffered formalin for 48 h. After fixation, each section of skin was removed from the index card and trimmed to 1.0-centimeter-long including the lesion. Each section of skin was bisected through the lesion and placed in a labeled cassette and processed. Two slides were made from each cassette. One was stained with H and E and one stained with Masson Trichrome.
Histopathologic evaluation.
Skin samples were histopathologically assessed using a previous publication39 as a reference for scoring specific parameters of wound healing, including edema, leukocytes, macrophages, granulation tissue, fibroblasts/collagen, and epithelialization. However, we did not directly use the specific scoring guideline used in that publication. We were unable to use a semiquantitative scoring guideline due to the presence of unidentified foreign material that was found in a very high percentage of mice across all treatment groups and time points. The foreign material induced an inflammatory response, and the pathologist could not definitively distinguish between microscopic features of normal wound healing and those associated with the foreign material. Instead, the histopathologist provided a microscopic description of the samples.
Statistical analysis.
The average total surface area of the lesions of each treatment group was calculated for each photographed time point. To standardize and assess the rate of healing between the treatment groups, the percentage of the wound area was compared with that of the initial wound area rather than using absolute surface area measurements. The total surface area of the wound was analyzed using a linear mixed model with random intercept for each animal. For study 2, batch effects were adjusted for by including batch and interactions between batch and time (days after wounding) as a fixed effect. Other fixed effect predictors included time, treatment group, and interactions between time and treatment groups. A likelihood ratio test was used to evaluate treatment effects and interactions between treatment and time. Posthoc comparison with Tukey adjustment was used to compare healing between treatment groups at each time point. Statistical significance was defined as P < 0.05.
Results
No incidences of mouse death, illness, or injury occurred during any of the studies. The average wound areas per treatment group and per time point are displayed as a percentage of the initial wound area with corresponding standard error bars (Figures 6, through 8). Wound closure was complete in all mice by the last day of the study.
Figure 6.
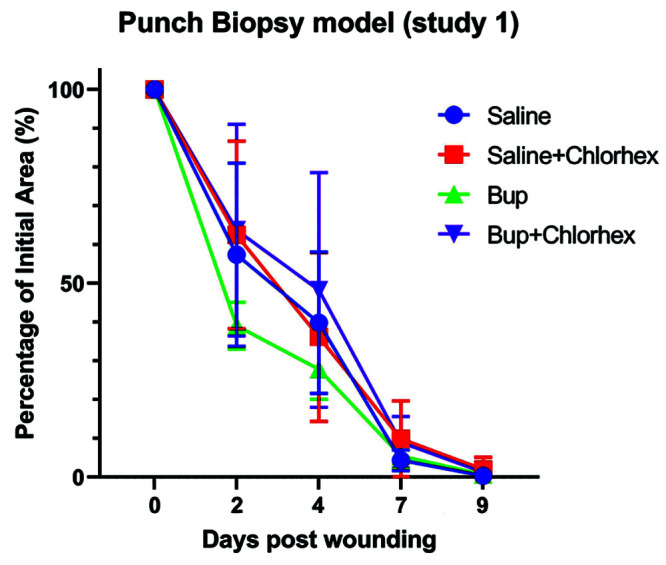
The average wound area of mice used in the punch biopsy model of study 1 on days 2,4,7, and 9 in comparison to the initial wound area (day 0). Shown with corresponding standard error bars.
Figure 7.
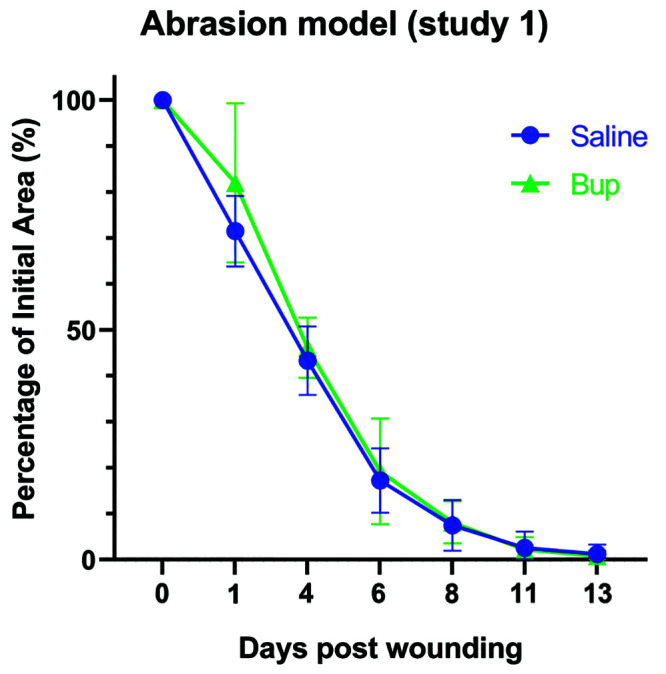
The average wound area of mice used in the dermal abrasion model of study 1 on days 1,4,6, 8, 11, and 13 in comparison to the initial wound area (day 0). Shown with corresponding standard error bars.
Figure 8.
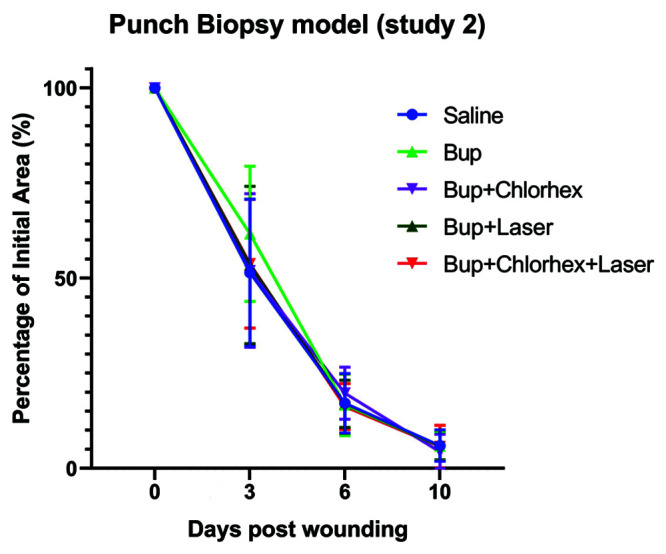
The average wound area of mice used in the punch biopsy model of study 2 on days 3,6, and 10 in comparison to the initial wound area (day 0). Shown with corresponding standard error bars.
Study 1.
On day 2 after the punch biopsy wound (Figure 6), the average wound area of mice in the B group was 39% of the original wound area, whereas mice in the S group had an average wound area of 57% of the original wound area. Wounds of mice in the BC and SC groups were 64% and 62% of the initial wound area, respectively.
By day 4 after wounding, the average wound area of mice in the B- and S-only groups had fallen to 28% and 40% of the initial wound area, respectively. The average wound area of mice in the BC group was 48% of the initial wound area, while the average wound area of mice in the SC group was 36% of the initial wound area.
At 7 days after wounding, the average wound area of mice in the B group was 5% of the original wound area and the average wound area of mice in the S group was 4% of the original wound area. Mice in the BC group had an average wound area that was 9% of the original area, while mice in the SC group had an average wound area of 10% of the original area.
At 9 days after wounding, which was the designated end of the study, all wounds appeared clinically healed as indicated by absence of erythema and scabbing, with negligible scarring. The average wound area of all mice in all treatment groups was 2% or less that the initial wound area. Mice in the B- and S-only groups had an average wound area that was less than 1% of the initial wound area. Mice in the BC and SC treatment groups had average wound areas of 1% and 2% of the initial wound area, respectively.
For punch biopsies, no statistically significant differences were found among any of the treatment groups at any of the measured timepoints. The study compared each treatment group to the saline control group, and also compared treatment groups to each other. All Tukey adjusted P = values were greater than 0.25, and neither overall treatment effects (P = 0.4) nor treatment by time interaction effects (P = 0.3) were significant.
For the abrasion model (Figure 7), on day 1 after wounding, the B-only group had an average wound area of 82% of the original wound area, as compared with an average wound area of 71% of the original wound area for mice in the S-only group. On day 4 after wounding, mice in the B- and S-only treatment groups had similar average wound areas of 46% and 43% of the original wound area, respectively. Six days after wounding, the average wound area of the B-only group was 19% of the original wound area, and the average wound area of the S-only group was 17% of the original wound area. On day 8 after wounding, average wound areas were similar, with mice in the B-group having an area 8% of the original wound area and mice in the saline group having an area 7% of the original surface area. The B-only group had an average total surface area of 2% of the initial wound area on day 11, and the saline only group had a similar average total surface area of 3%. By day 13, the average wound surface area was 0.8% of the initial wound area for the B-only group and 1.2% of the initial wound area for the S-only group. All wounds were clinically considered to have healed by day 13 after wounding.
No statistically significant differences were detected between the treatment groups at any of the measured timepoints for the dermal abrasions performed in study 1. All adjusted P values were greater than 0.8, and neither treatment effects (P = 0.4) nor treatment by time interaction effects (P = 0.5) were significant.
Study 2.
For study 2 (Figure 8), at day 3 after wounding, the average wound area of mice in the B group was 62% of the original wound area and 52% in the BC group. Wounds of mice in the BL and BLC groups were 53% and 54% of the initial wound area, respectively. The average wound area of mice in the saline control group was 51% of the initial wound area.
By day 6 after wounding, the average wound area of mice in the B and BLC groups had fallen to 17% and 16%, respectively. The average wound area of mice in the BC group was 20%, and the averages of mice in the BL and saline groups were both 17%.
At 10 days after wounding, which was designated as the end of the study, all wounds appeared to be clinically healed as indicated by absence of erythema or scabbing and negligible scarring. The average wound area of all mice in all treatment groups was 6% or less of the initial surface area. Mice in the B and BC group had average wound areas of 6% and 4% of the initial wound area, respectively. Similarly, mice in the BL and BLC both had average wound areas of 6%, as did mice in the saline group.
The average percentage of initial wound area for each group is shown on the line graph with standard error bars (Figure 8). No significant treatment effects (P = 0.9) or interactions between treatment and time (P = 0.88) were detected, indicating that the rate of wound healing was not different between any of the 5 treatment groups. No statistically significant differences were detected between treatment groups at any of the timepoints (all adjusted P values were greater than 0.4).
Histopathology.
Samples collected from mice 3 days after wounding were easily and consistently identified by a focal area lacking epithelium (biopsy site) covered by layers of fibrinous material with degenerate neutrophils and cellular debris, consistent with scabbing, of varied thickness (Figure 9 A and B). The histologic appearance of day 3 samples was similar between treatment groups. Skin samples of mice at 6 d after wounding also lacked epithelium, and the biopsy site generally appeared slightly smaller than that seen in day 3 mice (Figure 10 A and B), concordant with clinical observations and image analysis of surface area measurements. Slight reepithelialization of the skin adjacent to the biopsy site was occasionally observed, characterized by thickened epithelium and minimal hyperkeratosis. Generally, samples from day 6 were histologically comparable, with no remarkable differences between treatment groups. By 10 d after wounding, many samples had completely reepithelialized (Figure 11 A and B). The reepithelialized area contained thickened epithelium and minimal hyperkeratosis; most dermal adnexal structures were absent. Day 10 samples were microscopically similar, with no distinct differences noted between treatment groups. Unexpected inflammation associated with foreign bodies (hair and/or other undetermined material) was present in the majority of samples from day 3 and day 6 mice, including the saline control groups. Fewer foreign bodies were noted and associated inflammation was less in day 10 samples. An objective scoring rubric, similar to previously published work,39 and trichrome analysis, were precluded due to the confounding factor of foreign material identified in the majority of skin samples.
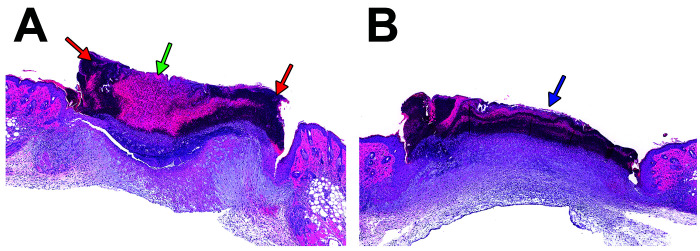
Figure 9.
(A) Biopsy site from saline-treated mouse 3 d post wounding at 3.1× magnification. Note focal fibrinous material (green arrow) admixed with regions of hypercellularity (red arrows) consistent with a scab. (B) Biopsy site from buprenorphine and chlorhexidine-treated mouse 3 d post wounding with similar appearing scab (blue arrow) at 3.1× magnification.
Figure 10.
(A) Biopsy site from saline-treated mouse 6 d post wounding at 3.1× magnification. Note focal fibrinous material (green arrow) admixed with regions of hypercellularity (red arrow) consistent with a scab. (B) Biopsy site from buprenorphine and chlorhexidine-treated mouse 6 d post wounding with less-cellular appearing scab (blue arrow) at 3.1× magnification.
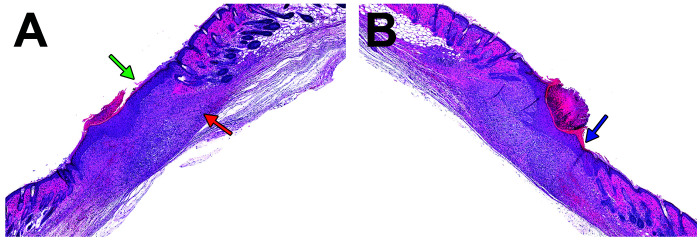
Figure 11.
(A) Biopsy site from saline-treated mouse 10 d post wounding at 3.1× magnification. Note reepithelialization of epidermis (green arrow) with scab to left of arrow and absence of adnexal structures in the subjacent dermis (red arrow). (B) Biopsy site from buprenorphine and laser-treated mouse 10 d post wounding with similar appearing epidermal reepithelialization (blue arrow) with scab to left of arrow at 3.1× magnification.
Discussion
In these studies, we investigated the effects of a commonly used analgesic, a topical antiseptic solution, and a novel LLLT to evaluate their impact on wound healing. Study 1 investigated standard buprenorphine hydrochloride and chlorhexidine in 2 different wound models. The 2 agents were studied individually and in combination. Study 2 investigated suspended release buprenorphine, chlorhexidine, and LLLT in a surgical wound model. We found no significant difference in the rate of wound healing in any of the groups.
The growing awareness and concern regarding wound healing and the economic cost of wound treatment has garnered interest and research in effective treatment and management of wounds. While a host of animal species have been used to model wound healing, most studies have been conducted using either pigs or mice.23 Pig skin shares many anatomic features with human skin, including healing by the process of epithelization,23,29 thereby making them a good preclinical and translational model to study wound healing that is relevant to humans. However, due to their size, pigs are more expensive to house and maintain, require more advanced anesthetic and surgical protocols, and are not feasible for some research facilities.23 In contrast, rodents are small, easy to house, easy to handle, and relatively inexpensive, although they also have loose skin and a subcutaneous panniculus carnosus muscle that allows them to heal relatively quickly.12,65 Unlike humans, who heal mostly by epithelization, the majority of the healing process in mice occurs by contraction.24,62 Despite these differences, the overall healing process is similar in mice and humans. In both species, normal cutaneous wound healing is a complex process with several, converging, phases including inflammation, proliferation, and remodeling.1,45,44,64 The inflammatory phase is characterized by the formation of a platelet plug to achieve hemostasis, and the subsequent influx of inflammatory cells such as neutrophils, macrophages, and lymphocytes to the wound.39,44 During the proliferation stage, macrophages and fibroblasts increase in number,39,44 while the population of neutrophils and lymphocytes begins to decline. Fibroblasts are responsible for creating the extracellular matrix and collagen fibers that are seen during the remodeling phase of wound healing.39,44 However, many factors can affect wound healing including age of the animal, the overall clinical condition, nutritional status, oxygenation of tissue, the presence of infection, and stress.
To mechanistically model these various aspects of wound healing, several murine models have been developed. Some of the most common in mice are ischemia/reperfusion, skin fibrosis/scarring, dermal abrasion, and incisional/excisional wounds. Mouse models of ischemia and reperfusion injury are used to simulate pressure sores and investigate impairments in neovascularization and angiogenesis, which are key components of wound healing.5,12,24,42,62 This model is accomplished using either a skin flap or insertion of a metal disc or magnet beneath the skin.12,24,42,62 Skin fibrosis and hypertrophic scarring are a broad model used to investigate a host of fibroproliferative diseases and dermal changes after burn, radiation, chemical injury, and general cutaneous trauma.42,62 Dermal abrasion models use either a scalpel blade or rotary tool to induce a partial thickness wound in which only the upper most layers of skin are damaged.24 Incisional and excisional wounds involve damage or removal of a full thickness section of skin, usually from the dorsum; excisional wounds may or may not subsequently be splinted to prevent contraction. This model is commonly used to study acute wounds and chronic, nonhealing ulcers such as those seen in diabetic or hypothyroid patients.11,12,23,42,62 Excisional wounds are particularly common as the wound creation is reproducible, and topical medications or compounds can be applied directly to the wounds.
Our studies used excisional skin biopsy and dermal abrasion because these models are currently used at our institute. The analgesic component of the study was designed to address our investigators’ concern that buprenorphine would interfere with the healing process. However, the control of pain in rodent research is an ethical and legal responsibility,8,20 and stress and pain can also impede the wound healing.37
Study 1 used a single dose of buprenorphine hydrochloride. Buprenorphine is a widely used opioid in rodents, but its duration of analgesia varies and is rarely reported to be effective longer than 8 h.21,32,36 Study 2 used a sustained release formulation of buprenorphine. Sustained release formulations have been documented to provide serum concentrations of 1 ng/mL in rodents for as long as 48 to 72 h14,20 and provide analgesia in rats for 72 h.20 Our studies found that neither formulation of buprenorphine affected the wound healing rate.
We incorporated chlorhexidine into our treatment plan because it is a commonly used antiseptic for the treatment of experimental wounds, fight wounds and dermatitis in mice at this institute. Study 1 used a chlorhexidine concentration of 0.12%, which was our standard treatment concentration at the time. A previous report found that tissue healing was inhibited in-vitro by 0.12% chlorhexidine.10,33 Similarly, an in vivo report showed inhibited wound contraction in adult Wistar rats treated with 0.05% chlorhexidine as compared with rats treated with tap water and sterile saline.49 However, another study designed to compare 2 concentrations of chlorhexidine, 0.005% and 0.05%, reported that neither concentration inhibited or enhanced wound healing.50
We hypothesized that LLLT would quicken the healing process. All mice used in LLLT studies were young (16 wk of age or younger) and otherwise healthy with no known diseases or comorbidities. Young animals, in general, heal faster than adult or aged animals.47 As mentioned above, mice heal primarily by wound contracture. In future studies, splinting the wound at the time of surgery would minimize the contributions of contracture in this murine model.16 Similarly, comorbidities, such as diabetes1,46,51,64 and hypothyroidism18,41 inherently delay wound healing. Some evidence suggests that LLLT and other photostimulatory therapies promote healing.1,2,15,46,55 We may have seen effects on the rate of wound healing had we used adult or aged mice or mice with comorbidities. Similarly, we used only one strain of mouse for both studies. This albino strain is commonly used at our institute for blastocyst injections. The lack of pigment allows for easy identification of chimera mice. Due to lack of pigment, these mice are genetically prone to retinal degeneration.27,59 However, we are not aware of evidence that this anomaly has any inherent effect on cutaneous wound healing. A repeat of our experimental design using a different mouse strain may render different results as some mouse strains may be more resistant to healing or more responsive to LLLT than others.15 Moreover, the type of laser and the wavelength used can factor into the efficacy of LLLT. In comparing varying wavelengths ranging from 635 nm to 820 nm, the most effective wound healing was obtained using the 820 nm wavelength.15 With the specific CTS laser used for this study, using a wavelength other than 980 nm may elicit different results.
For study 2, the microscopic changes described were consistent between treatment groups and at each time point. No signs of infection were noted during clinical examination of the mice; however, the presence of foreign material in the samples prevented the objective histopathologic scoring assessment of findings such as inflammation and fibrosis as being due to wound healing as compared with foreign body responses. The hair fragments and other undetermined material may have been secondary to social interaction or self-grooming of the animals, or due to the presence of residual shaved fur during either wounding or administration of treatments. Single housing of animals and the use of an alternate preparation protocol may have prevented this confounding factor and should be considered for future studies of this nature.
Based on our studies, the lack of significant differences in the rate of wound closure between treatment groups at any of the timepoints suggests that for these wound types, buprenorphine, chlorhexidine, and/or LLLT do not alter wound healing. Previously published studies with results contrary to ours vary with regard to strain, age, and health status of the mice used, specifications of the particular laser used, and housing/management of the mice after wounding. All of these should all be taken into consideration when evaluating the results of our study and in planning for future studies. Specifically, future work using this experimental design should consider use of a splint in the punch biopsy, and while certain conclusions can be deduced, LLLT-only and chlorhexidine-only treatment groups should be included in future studies. Future studies should also evaluate these therapeutics in wound healing of aged mice and mice with comorbidities (such as diabetes, hypothyroidism, etc.) and should use varying strains of mice and/or rats. In addition, mice should be singly housed to minimize any inadvertent wound contamination. Furthermore, future studies should be designed to answer questions about the analgesic properties of LLLT in wound healing and consider extrapolation of these treatment modalities to common conditions such as ulcerative dermatitis and fight wounds.
In conclusion, our studies indicate that a single dose of standard systemic buprenorphine hydrochloride or sustained release buprenorphine formulation does not impair or delay excisional wound healing in young, healthy mice. Similarly, acute use of dilute, topical chlorhexidine at a concentration of either 0.05% or 0.12% does not impair or delay excisional wound healing. Our studies have also shown that in young, healthy mice with no co-morbidities, LLLT does not accelerate excisional wound healing in these models.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (#ZIAES102825-11), the Division of the National Toxicology Program (ES103319-05), and the National Institute of Environmental Health Sciences. This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions, or conclusions contained herein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the United States government. The authors thank Angela Dickerson, David Goulding, Ashley Miller, and Page Myers for their help in preparing animals and materials prior to the wounding procedures, and for their help with obtaining photos on designated days. We also thank Daniel Eldridge for his help with data collection in Study 1. We thank the NIEHS necropsy and histopathology core (ES102505-13) for their assistance in processing the Study 2 skin samples, and Natasha Clayton for her assistance in organizing the logistics of the necropsy/sample collection as well as for providing the details regarding tissue preparation. We also thank Eli Ney for her help with ensuring that all images and figures were of appropriate quality for publication standards, and Amy Johnson for her thorough proof reading and assistance in finalizing the manuscript.
References
- 1.Ahmed OM, Mohamed T, Moustafa H, Hamdy H, Ahmed RR, Aboud E. 2018. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacother 101:58–73. 10.1016/j.biopha.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Alessi Pissulin CN, Henrique Fernandes AA, Sanchez Orellana AM, Rossi e Silva RC, Michelin Matheus SM. 2017. Low-level laser therapy (LLLT) accelerates the sternomastoid muscle regeneration process after myonecrosis due to bupivacaine. J Photochem Photobiol B 168:30–39. 10.1016/j.jphotobiol.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Assis L, Yamashita F, Magri AMP, Fernandes KR, Yamauchi L, Renno ACM. 2015. Effect of low-level laser therapy (808 nm) on skeletal muscle after endurance exercise training in rats. Braz J Phys Ther 19:457–465. 10.1590/bjpt-rbf.2014.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti C, Kallenberger A. 1980. Influence of chlorhexidine rinsing on the healing of oral mucosa and osseous lesions. J Clin Periodontol 7:443–456. [DOI] [PubMed] [Google Scholar]
- 5.Batool A, Karimi N, Wu XN, Chen SR, Liu YX. 2019. Testicular germ cell tumor: a comprehensive review. Cell Mol Life Sci 76:1713–1727. 10.1007/s00018-019-03022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. 2017. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg 44:260–268. 10.1016/j.ijsu.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 7.Bigliardi PL, Dancik Y, Neumann C, Bigliardi-Qi M. 2016. Opioids and skin homeostasis, regeneration and ageing— What's the evidence? Exp Dermatol 25:586–591. 10.1111/exd.13021. [DOI] [PubMed] [Google Scholar]
- 8.Blankenship-Paris TL, Dutton JW, Goulding DR, McGee CA, Kissling GE, Myers PH. 2016. Evaluation of buprenorphine hydrochloride Pluronic(®) gel formulation in male C57BL/6NCrl mice. Lab Anim (NY) 45:370–379. 10.1038/laban.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan SS, Foster ME, Leaper DJ. 1986. Antiseptic toxicity in wounds healing by secondary intention. J Hosp Infect 8:263–267. 10.1016/0195-6701(86)90122-2. [DOI] [PubMed] [Google Scholar]
- 10.Brennan SS, Leaper DJ. 1985. The effect of antiseptics on the healing wound: A study using the rabbit ear chamber. Br J Surg 72:780–782. [DOI] [PubMed] [Google Scholar]
- 11.Chang PJ, Chen MY, Huang YS, Lee CH, Huang CC, Lam CF, Tsai YC. 2010. Morphine enhances tissue content of collagen and increases wound tensile strength. J Anesth 24:240–246. 10.1007/s00540-009-0845-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen JS, Longaker MT, Gurtner GC. 2013. Murine models of human wound healing. Methods Mol Biol 1037:265–274. 10.1007/978-1-62703-505-7_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. 2011. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40:516–533. 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark TS, Clark DD, Hoyt RF, Jr. 2014. Pharmacokinetic comparison of sustained-release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53:387–391. [PMC free article] [PubMed] [Google Scholar]
- 15.Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR. 2007. Low-level light stimulates excisional wound healing in mice. Lasers Surg Med 39:706–715. 10.1002/lsm.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn L, Prosser HCG, Tan JTM, Vanags LZ, Ng MKC, Bursill CA. 2013. Murine model of wound healing. J Vis Exp: e50265. doi: 10.3791/50265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eissa M, Salih WHM. 2017. The influence of low-intensity He-Ne laser on the wound healing in diabetic rats. Lasers Med Sci 32:1261–1267. 10.1007/s10103-017-2230-x. [DOI] [PubMed] [Google Scholar]
- 18.Firouzi A, Norozian M, Amini A, Abdollahifar MA, Abbaszadeh HA, Fadaei Fathabadi F. 2018. Combined effect of low-level laser treatment and levothyroxine on wound healing in rats with hypothyroidism. J Lasers Med Sci 9:268–273. 10.15171/jlms.2018.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleck CA. 2007. Managing wound pain: today and in the future. Adv Skin Wound Care 20:138, 141–142, 145. [DOI] [PubMed] [Google Scholar]
- 20.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 21.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13. [PubMed] [Google Scholar]
- 22.Gammel JE, Biskup JJ, Drum MG, Newkirk K, Lux CN. 2018. Effects of low-level laser therapy on the healing of surgically closed incisions and surgically created open wounds in dogs. Vet Surg 47:499–506. 10.1111/vsu.12795. [DOI] [PubMed] [Google Scholar]
- 23.Grada A, Mervis J, Falanga V. 2018. Research techniques made simple: animal models of wound healing. J Invest Dermatol 138:2095–2105.e1. 10.1016/j.jid.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Dai T, Hamblin MR. 2014. Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med Sci 29:257–265. 10.1007/s10103-013-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haslerud S, Lopes-Martins RA, Frigo L, Bjordal JM, Marcos RL, Naterstad IF, Magnussen LH, Joensen J. 2017. Low-level laser therapy and cryotherapy as mono- and adjunctive therapies for achilles tendinopathy in rats. Photomed Laser Surg 35:32–42. 10.1089/pho.2016.4150. [DOI] [PubMed] [Google Scholar]
- 26.Heavner JE, Cooper DM. 1997. Pharmacology of analgesics. Chapter 4. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editor. Anesthesia and analgesia in laboratory animals. Burlington (MA): Elsevier. [Google Scholar]
- 27.Hughes ED, Qu YY, Genik SJ, Lyons RH, Pacheco CD, Lieberman AP, Samuelson LC, Nasonkin IO, Camper SA, Van Keuren ML, Saunders TL. 2007. Genetic variation in C57BL/6 ES cell lines and genetic instability in the Bruce4 C57BL/6 ES cell line. Mamm Genome 18:549–558. 10.1007/s00335-007-9054-0. [DOI] [PubMed] [Google Scholar]
- 28.Huss MK, Felt SA, Pacharinsak C. 2019. Influence of pain and analgesia on orthopedic and wound-healing models in rats and mice. Comp Med 69:535–545. 10.30802/AALAS-CM-19-000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter J, Wilson LLR, Snider G, Dixon J. 1984. Effects of low energy laser on wound healing in a procine model. Lasers Surg Med 3:285–290. 10.1002/lsm.1900030404. [DOI] [PubMed] [Google Scholar]
- 30.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 31.Jirkof P, Durst M, Klopfleisch R, Palme R, Thone-Reineke C, Buttgereit F, Schmidt-Bleek K, Lang A. 2019. Administration of tramadol or buprenorphine via the drinking water for post-operative analgesia in a mouse-osteotomy model. Sci Rep 9:1–16. 10.1038/s41598-019-47186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jirkof P, Tourvieille A, Cinelli P, Arras M. 2015. Buprenorphine for pain relief in mice: repeated injections vs sustained-release depot formulation. Lab Anim 49:177–187. 10.1177/0023677214562849. [DOI] [PubMed] [Google Scholar]
- 33.Karpiński TM, Szkaradkiewicz AK. 2015. Chlorhexidine—pharmaco-biological activity and application. Eur Rev Med Pharmacol Sci 19:1321–1326. [PubMed] [Google Scholar]
- 34.Kurach LM, Stanley BJ, Gazzola KM, Fritz MC, Steficek BA, Hauptman JG, Seymour KJ. 2015. The effect of low-level laser therapy on the healing of open wounds in dogs. Vet Surg 44:988–996. 10.1111/vsu.12407. [DOI] [PubMed] [Google Scholar]
- 35.Lam CF, Chang PJ, Huang YS, Sung YH, Huang CC, Lin MW, Liu YC, Tsai YC. 2008. Prolonged use of high-dose morphine impairs angiogenesis and mobilization of endothelial progenitor cells in mice. Anesth Analg 107:686–692. 10.1213/ane.0b013e31817e6719. [DOI] [PubMed] [Google Scholar]
- 36.Leach MC, Forrester AR, Flecknell PA. 2010. Influence of preferred foodstuffs on the antinociceptive effects of orally administered buprenorphine in laboratory rats. Lab Anim 44:54–58. 10.1258/la.2009.009029. [DOI] [PubMed] [Google Scholar]
- 37.McGuire L, Heffner K, Glaser R, Needleman B, Malarkey W, Dickinson S, Lemeshow S, Cook C, Muscarella P, Melvin WC, Ellison EC, Kiecolt-Glaser JK. 2006. Pain and wound healing in surgical patients. Ann Behav Med 31:165–172. [DOI] [PubMed] [Google Scholar]
- 38.Moreira GS, Machado Alves PH, Esper LA, Sbrana MC, da Silva Dalben G, Neppelenbroek KH, Fraga de Almeida ALP. 2018. Effect of low-level laser on the healing of bone defects filled with autogenous bone or bioactive glass: in vivo study. Int J Oral Maxillofac Implants 33:169–174. 10.11607/jomi.5900. [DOI] [PubMed] [Google Scholar]
- 39.Nussbaum EL, Mazzulli T, Pritzker KPH, Heras FL, Jing F, Lilge L. 2009. Effects of low intensity laser irradiation during healing of skin lesions in the rat. Lasers Surg Med 41:372–381. 10.1002/lsm.20769. [DOI] [PubMed] [Google Scholar]
- 40.Ozkan D, Seker D, Ergil J, Yalcindag A, Han U, Ginis Z, Akinci M, Delibaş N. 2013. The effects of tramadol infiltration on wound healing in rats. Acta Chir Belg 113:434–438. 10.1080/00015458.2013.11680959. [DOI] [PubMed] [Google Scholar]
- 41.Paraguassú GM, Xavier FCA, Cangussu MCT, Ramalho MJP, Cury PR, Dos Santos JN, Pinheiro ALB, Ramalho LMP. 2014. Effect of laser phototherapy (λ660 nm) on type i and III collagen expression during wound healing in hypothyroid rats: An immunohistochemical study in a rodent model. Photomed Laser Surg 32:281–288. 10.1089/pho.2013.3604. [DOI] [PubMed] [Google Scholar]
- 42.Peplow PV, Chung TY, Baxter GD. 2010. Laser photobiomodulation of wound healing: A review of experimental studies in mouse and rat animal models. Photomed Laser Surg 28:291–325. 10.1089/pho.2008.2446. [DOI] [PubMed] [Google Scholar]
- 43.Peterson NC, Nunamaker EA, Turner PV. 2017. To treat or not to treat: the effects of pain on experimental parameters. Comp Med 67:469–482. [PMC free article] [PubMed] [Google Scholar]
- 44.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. 2005. Low level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–340. 10.1097/00042728-200503000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Ramos FS, Maifrino LBM, Alves S, da Costa Aguiar Alves, Perez MM, Feder D, Azzalis LA, Junqueira VBC, Fonseca FLA. 2018. The effects of transcutaneous low-level laser therapy on the skin healing process: an experimental model. Lasers Med Sci 33:967–976. 10.1007/s10103-017-2429-x. [DOI] [PubMed] [Google Scholar]
- 46.Reddy GK, Stehno-Bittel L, Enwemeka CS. 2001. Laser Photostimulatiohn accelerates wound healing in diabetic rats. Wound Repair Regen 9:248–255. 10.1046/j.1524-475x.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- 47.Reed MJ, Karres N, Eyman D, Vernon RB, Edelberg JM. 2006. Age-related differences in repair of dermal wounds and myocardial infarcts attenuate during the later stages of healing.In vivo 20:801–806. [PubMed] [Google Scholar]
- 48.Riegel RJ. 2008. Laser therapy in the companion animal practice: Mechanisms and protocols for class IV laser therapy. Newark (DE): LiteCure. [Google Scholar]
- 49.Salami AA, Imosemi IO, Owoeye OO. 2006. A comparison of the effect of chlorhexidine, tap water and normal saline on healing wounds. Int J Morphol 24:673–676. [Google Scholar]
- 50.Sanchez IR, Swaim SF, Nusbaum KE, Hale AS, Henderson RA, McGuire JA. 1988. Effects of chlorhexidine diacetate and povidone-iodine on wound healing in dogs. Vet Surg 17:291–295. 10.1111/j.1532-950X.1988.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 51.Sato H, Ebisawa K, Takanari K, Yagi S, Toriyama K, Yamawaki-Ogata A, Kamei Y. 2015. Skin-derived precursor cells promote wound healing in diabetic mice. Ann Plast Surg 74:114–120. 10.1097/SAP.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 52.Sen CK. 2019. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle) 8:39–48. 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sousa RG, Batista Kde N. 2016. Laser therapy in wound healing associated with diabetes mellitus - review. An Bras Dermatol 91:489–493. 10.1590/abd1806-4841.20163778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein C, Küchler S. 2012. Non-analgesic effects of opioids: peripheral opioid effects on inflammation and wound healing. Curr Pharm Des 18:6053–6069. 10.2174/138161212803582513. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki SS, Garcez AS, Suzuki H, Ervolino E, Moon W, Ribeiro MS. 2016. Low-level laser therapy stimulates bone metabolism and inhibits root resorption during tooth movement in a rodent model. J Biophotonics 9:1222–1235. 10.1002/jbio.201600016. [DOI] [PubMed] [Google Scholar]
- 56.Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, Damasceno Maia F, do Nascimento NRF, Driusso P, Parizotto NA. 2016. Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B 164:96–102. 10.1016/j.jphotobiol.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas GW, Rael LT, Bar-Or R, Shimonkevitz R, Mains CW, Slone DS, Craun ML, Bar-Or D. 2009. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma 66:82–90, discussion 90–81. 10.1097/TA.0b013e31818b146d. [DOI] [PubMed] [Google Scholar]
- 58.Thunshelle C, Hamblin MR. 2016. Transcranial low-level laser (light) therapy for brain injury. Photomed Laser Surg 34:587–598. 10.1089/pho.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Townsend D, Witkop CJ, Jr, Mattson J. 1981. Tyrosinase subcellular distribution and kinetic parameters in wild type and C-locus mutant C57BL/6J mice. J Exp Zool 216:113–119. 10.1002/jez.1402160112. [DOI] [PubMed] [Google Scholar]
- 60.Wardlaw JL, Gazzola KM, Wagoner A, Brinkman E, Burt J, Butler R, Gunter JM, Senter LH. 2018. Laser therapy for incision healing in 9 dogs. Front Vet Sci 5:1–8. 10.3389/fvets.2018.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watts R, Solomons T, Curtin University . 2017. Evidence summary: Wound management – Chlorhexidine. Wound practice & research 25:49–51. [Google Scholar]
- 62.Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. 2011. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011:1–8. 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodford C. [Internet]. 2020. Lasers. [Cited 06 March 2020]. Available at: https://www.explainthatstuff.com/lasers.html.
- 64.Yu W, Nairn JO, Lanzafame RJ. 1997. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg Med 20:56–63. . [DOI] [PubMed] [Google Scholar]
- 65.Zomer HD, Trentin AG. 2018. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 90:3–12. 10.1016/j.jdermsci.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Zudaire E, Cuttitta F, editors. 2012. The textbook of angiogenesis and lymphangiogenesis: methods and applications. London: Springer. [Google Scholar]