Abstract
Background
Milk feedings can be given via nasogastric tube either intermittently, typically over 10 to 20 minutes every two or three hours, or continuously, using an infusion pump. Although the theoretical benefits and risks of each method have been proposed, their effects on clinically important outcomes remain uncertain.
Objectives
To examine the evidence regarding the effectiveness of continuous versus intermittent bolus tube feeding of milk in preterm infants less than 1500 grams.
Search methods
We used the standard search strategy of Cochrane Neonatal to run comprehensive searches in the Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 7) in the Cochrane Library; Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions; and CINAHL (Cumulative Index to Nursing and Allied Health Literature) on 17 July 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐RCTs.
Selection criteria
We included RCTs and quasi‐RCTs comparing continuous versus intermittent bolus nasogastric milk feeding in preterm infants less than 1500 grams.
Data collection and analysis
Two review authors independently assessed all trials for relevance and risk of bias. We used the standard methods of Cochrane Neonatal to extract data. We used the GRADE approach to assess the certainty of evidence. Primary outcomes were: age at full enteral feedings; feeding intolerance; days to regain birth weight; rate of gain in weight, length and head circumference; and risk of necrotising enterocolitis (NEC).
Main results
We included nine randomised trials (919 infants) in this updated Cochrane Review. One study is awaiting classification. Seven of the nine included trials reported data from infants with a maximum weight of between 1000 grams and 1400 grams. Two of the nine trials included infants weighing up to 1500 grams.
Type(s) of milk feeds varied, including human milk (either mother's own milk or pasteurised donor human milk), preterm formula, or mixed feeding regimens. In some instances, preterm formula was initially diluted. Earlier studies also used water to initiate feedings.
We judged six trials as unclear or high risk of bias for random sequence generation. We judged four trials as unclear for allocation concealment. We judged all trials as high risk of bias for blinding of care givers, and seven as unclear or high risk of bias for blinding of outcome assessors. We downgraded the certainty of evidence for imprecision, due to low numbers of participants in the trials, and/or wide 95% confidence intervals, and/or for risk of bias.
Continuous compared to intermittent bolus (nasogastric and orogastric tube) milk feeding
Babies receiving continuous feeding may reach full enteral feeding almost one day later than babies receiving intermittent feeding (mean difference (MD) 0.84 days, 95% confidence interval (CI) ‐0.13 to 1.81; 7 studies, 628 infants; low‐certainty evidence).
It is uncertain if there is any difference between continuous feeding and intermittent feeding in terms of number of days of feeding interruptions (MD ‐3.00 days, 95% CI ‐9.50 to 3.50; 1 study, 171 infants; very low‐certainty evidence).
It is uncertain if continuous feeding has any effect on days to regain birth weight (MD ‐0.38 days, 95% CI ‐1.16 to 0.41; 6 studies, 610 infants; low‐certainty evidence). The certainty of evidence is low and the 95% confidence interval is consistent with possible benefit and possible harm.
It is uncertain if continuous feeding has any effect on rate of gain in weight compared with intermittent feeding (standardised mean difference (SMD) 0.09, 95% CI ‐0.27 to 0.46; 5 studies, 433 infants; very low‐certainty evidence).
Continuous feeding may result in little to no difference in rate of gain in length compared with intermittent feeding (MD 0.02 cm/week, 95% CI ‐0.04 to 0.08; 5 studies, 433 infants; low‐certainty evidence).
Continuous feeding may result in little to no difference in rate of gain in head circumference compared with intermittent feeding (MD 0.01 cm/week, 95% CI ‐0.03 to 0.05; 5 studies, 433 infants; low‐certainty evidence).
It is uncertain if continuous feeding has any effect on the risk of NEC compared with intermittent feeding (RR 1.19, 95% CI 0.67 to 2.11; 4 studies, 372 infants; low‐certainty evidence). The certainty of evidence is low and the 95% confidence interval is consistent with possible benefit and possible harm.
Authors' conclusions
Although babies receiving continuous feeding may reach full enteral feeding slightly later than babies receiving intermittent feeding, the evidence is of low certainty. However, the clinical risks and benefits of continuous and intermittent nasogastric tube milk feeding cannot be reliably discerned from current available randomised trials. Further research is needed to determine if either feeding method is more appropriate for the initiation of feeds. A rigorous methodology should be adopted, defining feeding protocols and feeding intolerance consistently for all infants. Infants should be stratified according to birth weight and gestation, and possibly according to illness.
Plain language summary
Continuous nasogastric milk feeding versus intermittent bolus milk feeding for preterm infants less than 1500 grams
Review question
Is continuously feeding through a tube placed into the stomach through the nose or mouth better than feedings given every two to three hours through a tube, in premature, very low birth weight babies?
Background
Preterm infants born weighing less than 1500 grams are not able to coordinate sucking, swallowing, and breathing. Feeding into the stomach (enteral feeding) helps with gastrointestinal tract development and growth. Therefore, in addition to feeding through a tube into a vein (parenterally), preterm infants may be fed milk through a tube placed either up their nose and into the stomach (nasogastric feeding) or through their mouth and into the stomach (orogastric feeding). Usually, a set amount of milk is given over 10 to 20 minutes every two to three hours (intermittent bolus gavage feeding). Some clinicians prefer to feed preterm infants continuously. Each feeding method has potential beneficial effects but may also have harmful effects.
Study characteristics
We included nine studies that involved 919 babies. One further study is awaiting classification. Seven of the nine included trials reported data from infants with a maximum weight of between 1000 grams and 1400 grams. Two of the nine trials included infants weighing up to 1500 grams. The search is up to date as of 17 July 2020.
Key results
Babies receiving continuous feeding may reach full enteral feeding slightly later than babies receiving intermittent feeding. Full enteral feeding is defined as the baby taking a specified volume of human or formula milk feeds by the required route. This promotes the development of the gastrointestinal system, reduces the risk of infection from intravenous catheters used to deliver parenteral nutrition, and may reduce the length of hospital stay.
It is uncertain if there is any difference between continuous feeding and intermittent feeding in terms of number of days to regain birth weight, days of feeding interruptions, and rate of gain in weight.
Continuous feeding may result in little to no difference in rate of gain in length or head circumference compared with intermittent feeding.
It is uncertain if continuous feeding has any effect on the risk of necrotising enterocolitis (a common and serious intestinal disease among premature babies) compared with intermittent feeding.
Certainty of evidence
The certainty of the evidence is low to very low because of the low numbers of babies in the studies and because the studies were conducted in ways that may have introduced errors in their results.
Summary of findings
Summary of findings 1. Continuous compared to intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all preterm infants less than 1500 grams.
| Continuous compared to intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all preterm infants less than 1500 grams | ||||||
| Patient or population: preterm infants less than 1500 grams Setting: neonatal units in maternity hospitals in the USA, Israel, UK, the Netherlands and India Intervention: continuous Comparison: intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants | Risk with Continuous | |||||
| Age at full enteral feedings (days) | The mean age at full enteral feedings in the intermittent group ranged from 8 to 28.8 days | MD 0.84 days more (0.13 fewer to 1.81 more) | ‐ | 628 (7 RCTs) | ⊕⊕⊝⊝ LOWa, b | Continuous feeding may result in a slight increase in age at full enteral feedings compared to intermittent feeding. |
| Feeding intolerance: number of days of feeding interruptions | The mean number of days of feeding interruptions in the intermittent group was 13 | MD 3 days lower (9.5 lower to 3.5 higher) | ‐ | 171 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc, d | It is uncertain if continuous feeding has any effect on number of days of feeding interruptions compared to bolus feeding. |
| Days to regain birth weight | The mean time to regain birth weight in the intermittent group ranged from 7.8 to 25 days | MD 0.38 days fewer (1.16 fewer to 0.41 more) | ‐ | 610 (6 RCTs) | ⊕⊕⊝⊝ LOWa, b | It is uncertain if continuous feeding has any effect on days to regain birth weight compared to intermittent feeding. |
| End of intervention: rate of gain in weight | ‐ | SMD 0.09 SD higher (0.27 lower to 0.46 higher) | ‐ | 433 (5 RCTs) | ⊕⊝⊝⊝ VERY LOWb, e | It is uncertain if continuous feeding has any effect on rate of gain in weight compared to intermittent feeding. (SMD < 0.20 = trivial effect; SMD 0.20 to 0.49 = small effect; SMD 0.50 to 0.79 = moderate effect; SMD > 0.80 = large effect). Heterogeneity was significant (P value for Chi2 was 0.004). |
| End of intervention: rate of gain in length (cm/week) | The mean rate of gain in length in the intermittent group ranged from 0.62 to 1.05 cm/week | MD 0.02 cm/week higher (0.04 lower to 0.08 higher) | ‐ | 433 (5 RCTs) | ⊕⊕⊝⊝ LOWe, f | Continuous feeding may result in little to no difference in rate of gain in length compared to intermittent feeding. |
| End of intervention: rate of gain in head circumference (cm/week) | The mean rate of gain in head circumference in the intermittent group ranged from 0.53 to 0.99 cm/week | MD 0.01 days higher (0.03 lower to 0.05 higher) | ‐ | 433 (5 RCTs) | ⊕⊕⊝⊝ LOWe, f | Continuous feeding may result in little to no difference in rate of gain in head circumference compared to intermittent feeding. |
| Necrotising enterocolitis (NEC) | Study population | RR 1.19 (0.67 to 2.11) | 372 (4 RCTs) | ⊕⊕⊝⊝ LOWa, g | It is uncertain if continuous feeding has any effect on the risk of NEC compared to intermittent feeding. | |
| 96 per 1000 | 106 per 1000 (64 to 202) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio; SMD: standardised mean difference; NEC: necrotising enterocolitis | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for risk of bias: unclear randomisation and allocation concealment; high risk of bias due to lack of blinding of care givers and due to incomplete outcome data bDowngraded one level for imprecision: wide 95% CI spans possible benefit and possible harm cDowngraded one level for risk of bias: unclear randomisation and high risk of bias due to lack of blinding of care givers dDowngraded two levels for imprecision: very few infants and wide 95% CI that is consistent with possible benefit and possible harm eDowngraded two levels for risk of bias: unclear randomisation, and high risk of bias due to lack of blinding, incomplete outcome data and selective reporting fAlthough the 95% CI spans possible benefit and possible harm, we did not downgrade for imprecision because the difference on either side would not be clinically important gDowngraded one level for imprecision: few events and wide 95% CI that is consistent with possible benefit and possible harm
Background
Description of the condition
Tube feeding is necessary for most preterm infants less than 1500 grams because of their inability to coordinate sucking, swallowing, and breathing (Bertoncelli 2012; Schanler 1999), and the danger of aspiration (Bertoncelli 2012; Valman 1972).
Description of the intervention
The conventional tube feeding method is intermittent bolus gavage feeding, where a prescribed volume of milk is given over a short period of time (Aynsley‐Green 1982), usually over 10 to 20 minutes by gravity. The first reported use of the continuous nasogastric tube feeding method for preterm infants was in 1972 (Valman 1972). Some clinicians prefer the continuous nasogastric feeding method for feeding preterm infants less than 1300 grams birth weight. However, intermittent bolus gavage feeding is the method more commonly used in practice (Toce 1987).
How the intervention might work
Theoretical risks and benefits of both continuous nasogastric milk feeding and intermittent bolus milk feeding have been proposed. Continuous nasogastric feedings may improve energy efficiency (by increasing energy absorbed and decreasing energy expenditure) (Grant 1991), reduce feeding intolerance, improve nutrient absorption, and improve growth (Toce 1987). However, continuous infusion of milk into the gastrointestinal tract could alter the cyclical pattern of release of gastrointestinal tract hormones, which might affect metabolic homeostasis, and growth (Aynsley‐Green 1982), and may result in loss of nutrients from feeds into tubing which might affect growth and nutrient accretion (Rogers 2010). Furthermore, a properly functioning lower oesophageal sphincter is an important barrier against the reflux of stomach contents into the oesophagus and aspiration. Apnoea, reflux and aspiration may be compounded in the preterm infant receiving continuous nasogastric feedings (Corvaglia 2014; Newell 1988). Not only do these infants have reduced lower oesophageal sphincter pressure (Newell 1988), but the nasogastric tube remains in situ preventing complete closure of the sphincter.
Milk feedings given by the intermittent bolus gavage method are thought to be more physiologic because they promote the cyclical surges of gastrointestinal tract hormones normally seen in healthy term infants (Aynsley‐Green 1982; Aynsley‐Green 1990). Gastrointestinal hormones such as gastrin, gastric inhibitory peptide, and enteroglucagon are trophic and require the presence of intraluminal nutrients to stimulate secretion. Surges in plasma concentrations of gastrointestinal tract hormones postnatally may be important for gastrointestinal tract development (Aynsley‐Green 1989; Lucas 1986). On the other hand, functional limitations of the preterm infant's gastrointestinal system, such as delayed gastric emptying or intestinal transit, could hinder the preterm infant's ability to handle bolus milk feeds, resulting in feeding intolerance. Additionally, this feeding regimen alternates between periods of feeding and fasting which may challenge the preterm infant's ability to maintain metabolic homeostasis and, therefore, decrease growth (Aynsley‐Green 1982).
The effects of the feeding method on feeding tolerance, weight gain, or days to regain birth weight were examined in two non‐randomised controlled trials (Krishnan 1981; Urrutia 1983). In a retrospective study, Krishnan 1981 found that infants fed milk by continuous nasogastric tube feeding reached enteral intakes of 90 kcal/kg/day almost twice as quickly as those infants fed milk by intermittent bolus gavage feeding (16 +/‐ 6 versus 26 +/‐ 17 days, respectively). In addition, infants in the continuous group achieved steady weight gain sooner than infants in the intermittent group (24 +/‐ 10 versus 32 +/‐ 14 days). Unfortunately, these findings are difficult to interpret due to study design and methodologic limitations. First, the non‐random assignment of infants allows for selection bias. Second, energy intake was not controlled and may have influenced feeding tolerance and weight gain. Third, a convenience sample rather than a predetermined sample size was used, making it difficult to achieve both clinical and statistical significance in a study. Hence, it is difficult to make generalisations regarding these findings to similar populations of infants (Raudonis 1995).
Urrutia 1983 conducted a non‐randomised prospective study of continuous versus intermittent nasogastric tube milk feedings. They found no difference between groups in days to regain birth weight. These findings are also difficult to interpret because infants were allocated to the continuous or intermittent group based on neonatologists' preference rather than random assignment, and a convenience sample was used.
Why it is important to do this review
It is important to determine the clinical risks and benefits of each method of feeding to enable clinicians to make informed decisions regarding the most appropriate feeding method for an individual infant. New studies have been completed since the previous version of this review. Therefore, it is important to incorporate their findings to ensure the review provides up‐to‐date evidence.
Objectives
To examine the evidence regarding the effectiveness of continuous versus intermittent bolus tube feeding of milk in preterm infants less than 1500 grams.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised trials which compared continuous versus intermittent tube milk feeding, delivered via either the nasogastric or orogastric route, as primary feeding strategies in preterm infants less than 1500 grams.
Types of participants
We included infants born with birth weight less than 1500 grams who had no prior history of feeding or feeding intolerance, and no congenital anomalies that might interfere with establishing enteral feeds.
Types of interventions
We included continuous nasogastric feeding versus intermittent feeding with human milk or infant formula for the initiation of feeds and advancement to full enteral feeds. We included trials where infants in the comparator group received intermittent feeding through either nasogastric or orogastric tube feeding.
Types of outcome measures
Primary outcomes
Age at full enteral feedings (days)
Feeding intolerance as measured by number of days of feeding interruptions
Days to regain birth weight
Rate of gain in weight (grams/week)
Rate of gain in length (cm/week)
Rate of gain in head circumference (cm/week)
Necrotising enterocolitis (NEC), including suspected and confirmed (Bell's Stage II or greater)
Secondary outcomes
Days to discharge to referral hospital or home
Episodes of apnoea
Days on total parenteral nutrition
Search methods for identification of studies
Electronic searches
We conducted a comprehensive update search in July 2020, including: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 7) in the Cochrane Library; Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions (1 January 2011 to 17 July 2020); and Cumulative Index to Nursing and Allied Health Literature (CINAHL, via EBSCOhost; 1 January 2011 to 17 July 2020). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), and the United States' National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the ISRCTN registry (www.isrctn.com/) from 2011 onwards, the Australian New Zealand Clinical Trials Registry (ANZCTR), EU Clinical Trials Register (EU‐CTR), and the Clinical Trial Registry – India (CTRI) (the latter three from February 2020 onwards) for any unique trials not found through the Cochrane CENTRAL search.
This is the third update of this review. Our previous search details are listed in Appendix 2.
Searching other resources
We cross‐referenced relevant literature, including identified trials and existing review articles, in order to identify additional relevant trials.
Data collection and analysis
The systematic review followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020, hereafter referred to as the Cochrane Handbook).
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as an RCT or as Not an RCT; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs; and if appropriate, Cochrane Crowd – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence.
For more information about Screen4Me, please go to community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal/crs-videos-and-quick-reference-guides#Screen4Me. Detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; and Thomas 2020.
At least two review authors independently assessed relevance of all the articles that were retrieved from the complete search. Criteria for relevance included trials that utilised experimental or quasi‐experimental designs, compared continuous nasogastric tube milk feeding versus intermittent bolus nasogastric tube milk feeding, and reviewed clinically relevant outcomes as stated in the objectives.
We resolved differences through discussion and consensus of the review authors.
Data extraction and management
Two of the three review authors (SSP, LC, or FS) independently extracted data from studies. We resolved discrepancies through discussion, and if required, by consulting the third review author. We entered data into Review Manager software (Review Manager 2020), and checked for accuracy.
We contacted investigators for additional information or clarification, or both, where necessary.
Assessment of risk of bias in included studies
Two of the three review authors (SSP, LC, or FS) independently assessed the risk of bias of all included trials using the Cochrane risk of bias tool (Higgins 2011), for these domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
any other bias.
We resolved any disagreements through discussion or by consulting a third assessor. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We used the standard methods of Cochrane Neonatal. We performed statistical analyses using Review Manager 5 software (Review Manager 2020). We analysed categorical outcomes such as the incidence of necrotising enterocolitis using risk ratio (RR) and 95% confidence intervals (CIs).
We reported mean differences (MD) and 95% CIs for continuous outcomes such as days of feeding intolerance.
Where different scales were used to measure continuous outcomes, we combined the data using standardised mean difference (SMD), with the following interpretation:
SMD greater than or equal to 0.2 and less than 0.5 = small effect;
SMD greater than or equal to 0.5 and less than 0.8 = moderate effect;
SMD greater than 0.8 = large effect.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials, and an infant was considered only once in the analysis. For trials with three arms (e.g. continuous feeding versus bolus feeding by gravity versus bolus feeding by infusion), where continuous outcomes were reported, we divided the intervention group denominator by two in order to avoid double‐counting in the meta‐analysis.
The participating neonatal unit or section of a neonatal unit or hospital was the unit of analysis in cluster‐randomised trials. In future updates, if we identify eligible cluster‐RCTs, we will analyse them using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial or from a study with a similar population, as described in the Cochrane Handbook (Higgins 2020). If we use ICCs from a similar trial or from a study with a similar population, we will report this and conduct sensitivity analysis to investigate the effect of variation in the ICC.
We planned to only combine results from cluster‐RCTs with individually randomised trials in the same analysis if there was little heterogeneity between the study designs, and the interaction between the effect of the intervention and the choice of randomisation unit was considered to be unlikely. We planned to investigate any possible heterogeneity in the randomisation unit, and perform sensitivity analysis to investigate possible effects of the randomisation unit.
Dealing with missing data
We made every effort to contact study authors to ask for data that were missing from their published reports; for example, where P values only were reported instead of presenting the data in full.
Where studies reported median (interquartile range (IQR)), we used the methods described in Wan 2014 to convert to mean and SD. If there were no substantial differences between the median and mean, we included the mean and standard deviation (SD) in meta‐analysis.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic, according to the following guidance in the Cochrane Handbook (Higgins 2020).
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
If we detected statistical heterogeneity, we explored the possible causes (for example, differences in risk of bias, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
In future updates, if we identify 10 or more trials for meta‐analysis, we will assess possible publication bias by inspection of a funnel plot. If we uncover reporting bias that could, in the opinion of the review authors, introduce serious bias, we will conduct a sensitivity analysis to determine the effect of including and excluding these studies in the analysis.
Data synthesis
Where we identified studies that were similar enough in terms of population, intervention and comparator, we conducted fixed‐effect meta‐analysis using Review Manager 5 (Review Manager 2020). Where we identified substantial statistical heterogeneity, we conducted random‐effects meta‐analysis.
For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse‐variance method.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses for primary outcomes based on birth weight groups (< 1000 grams, 1000 to 1249 grams and 1250 to 1499 grams) where there were sufficient data.
Sensitivity analysis
We performed sensitivity analyses for primary outcomes in the following situations.
We removed studies where more than half of the risk of bias domains were judged as unclear or high risk.
Where the analysis included trials whose interventions were delivered by a mix of nasogastric and orogastric feeding, we removed those trials, leaving only data from infants fed by nasogastric feeding in the analysis.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes.
Age at full enteral feedings (days).
Feeding intolerance as measured by number of days of feeding interruptions.
Days to regain birth weight.
Rate of gain in weight (grams/week).
Rate of gain in length (cm/week).
Rate of gain in head circumference (cm/week).
Necrotising enterocolitis, including suspected and confirmed (Bell's Stage II or greater).
Two review authors (SSP and FS) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create Table 1 to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
Results of the search
Our search identified a total of 7848 search results (see Figure 1). In assessing the studies, we used Cochrane’s Screen4Me workflow to help identify potential reports of randomised trials. The results of the Screen4Me assessment process can be seen in Figure 2. We then assessed the remaining 2596 records left after Screen4Me. The review author team (at least two of SSP, LC and FS) screened these records. From these, we obtained 17 full texts for further screening. We identified two new studies (three reports) to include in the review (Neelam 2018; Rövekamp‐Abels 2015).
1.
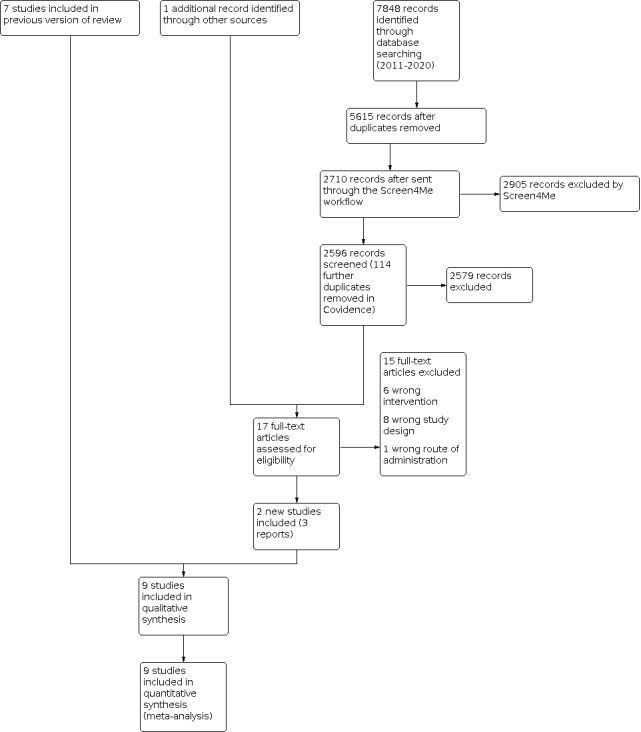
Study flow diagram
2.
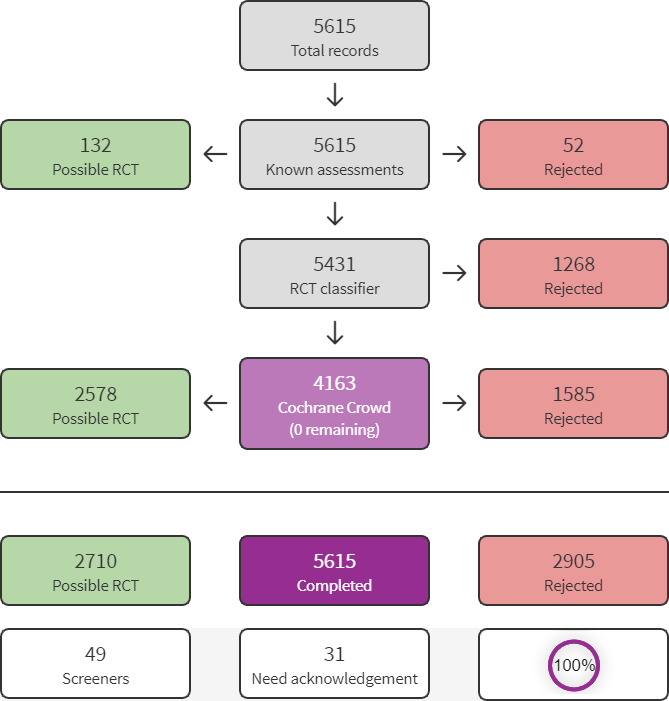
Screen4Me summary diagram
Included studies
We included nine studies (919 randomised infants) in this updated review.
See Characteristics of included studies for full details.
Study design
All but one of the nine included studies are RCTs (Toce 1987). Toce 1987 used alternative assignment rather than randomisation to allocate infants to treatment groups.
Setting
Eight studies took place in high‐resource settings: four in the USA (Akintorin 1997; Schanler 1999; Silvestre 1996; Toce 1987); and one each in Israel (Dollberg 2000); Sweden (Dsilna 2005); the United Kingdom (UK) (Macdonald 1992); and the Netherlands (Rövekamp‐Abels 2015). One study took place in a lower‐middle‐income setting, India (Neelam 2018).
Participants
Three studies included infants up to 1250 grams (Akintorin 1997; Dollberg 2000; Neelam 2018). In one study, the upper weight limit was 1200 grams (Dsilna 2005), and in another the limit was 1400 grams (Macdonald 1992). Two studies included infants weighing up to 1500 grams (Silvestre 1996; Toce 1987). In one study, the upper weight limit was 1750 grams; however, the authors provided data from the subgroup of infants weighing less than 1000 grams which met our inclusion criteria of infants weighing less than 1500 grams (Rövekamp‐Abels 2015). One study did not specify an upper weight limit, but it included only babies between 26 and 30 weeks' gestation (Schanler 1999). Four other studies included infants only within specific gestational age ranges: 24 to 29 weeks (Dsilna 2005); 27 to 34 weeks (Silvestre 1996); and up to 32 weeks (Rövekamp‐Abels 2015; Neelam 2018). All studies excluded infants with major congenital anomalies.
Sample size
Sample sizes ranged from 28 randomised infants (Dollberg 2000), to 250 (Rövekamp‐Abels 2015).
Interventions
In five studies, the continuous feeds were described as being delivered by infusion pump (Akintorin 1997; Dollberg 2000; Dsilna 2005; Neelam 2018; Toce 1987). One study described the continuous feeding method as semi‐continuous feeds every quarter of an hour, volume fed by gravity every 15 minutes over a 24‐hour period (Rövekamp‐Abels 2015). Three studies did not describe the continuous feeding method in detail (Macdonald 1992; Schanler 1999; Silvestre 1996).
In the comparator groups, the infants received nasogastric bolus feeds by gravity. In one study, the comparator group had either an orogastric tube placed for each feeding or indwelling nasogastric tube feedings (Dsilna 2005). In the comparator groups, the feeds were given for 15 to 30 minutes. The feeds were given every three hours (Akintorin 1997; Dsilna 2005; Rövekamp‐Abels 2015; Schanler 1999; Silvestre 1996; Toce 1987), every two hours (Neelam 2018), or every two hours for infants who weighed 501 grams to 750 grams, and every three hours for all other infants (Dollberg 2000). In one study, the frequency of bolus feeding was unclear (Macdonald 1992).
Type(s) of milk feeds varied, including human milk (Dollberg 2000; Neelam 2018; Rövekamp‐Abels 2015; Schanler 1999), either mother's own milk or pasteurised donor human milk (Dsilna 2005), preterm formula (Akintorin 1997; Dollberg 2000; Macdonald 1992; Neelam 2018; Rövekamp‐Abels 2015; Schanler 1999; Silvestre 1996; Toce 1987), and mixed feeding regimens (Dollberg 2000). In some instances, preterm formula was initially diluted (Dollberg 2000; Schanler 1999; Silvestre 1996; Toce 1987). Earlier studies also used water to initiate feedings (Silvestre 1996; Toce 1987).
Feeding for infants was initiated at the following times.
On day of birth (Rövekamp‐Abels 2015).
Within 30 hours of birth (Dsilna 2005).
Day two after birth (Macdonald 1992).
Day two or three after birth (Silvestre 1996).
At less than 96 hours of age (Schanler 1999).
Between day two and five after birth (Dollberg 2000).
Before day 10 after birth (Akintorin 1997).
Two studies did not specify the timing of feeds in the protocol (Neelam 2018; Toce 1987).
Outcomes
All studies except two reported age at full enteral feedings (Macdonald 1992; Toce 1987).
Other outcomes were reported by at least one study:
number of days of feeding interruptions: one study (Schanler 1999);
days to regain birth weight: six studies (Akintorin 1997;Dsilna 2005 Neelam 2018; Rövekamp‐Abels 2015; Schanler 1999; Silvestre 1996);
rate of gain in weight: five studies (Macdonald 1992; Neelam 2018; Schanler 1999; Silvestre 1996; Toce 1987);
rate of gain in length: five studies (Macdonald 1992; Neelam 2018; Schanler 1999; Silvestre 1996; Toce 1987);
rate of gain in head circumference: five studies (Macdonald 1992; Neelam 2018; Schanler 1999; Silvestre 1996; Toce 1987);
necrotising enterocolitis: four studies (Akintorin 1997; Dsilna 2005; Schanler 1999; Toce 1987);
days to discharge: two studies (Schanler 1999; Silvestre 1996);
apnoea: two studies (Schanler 1999; Toce 1987);
days on total parenteral nutrition: two studies (Dsilna 2005; Schanler 1999).
Study dates
The majority of the studies took place in the 1990s (Akintorin 1997; Macdonald 1992; Schanler 1999; Silvestre 1996), and 2000s (Dollberg 2000; Dsilna 2005; Neelam 2018; Rövekamp‐Abels 2015). One study took place in the 1980s (Toce 1987).
Funding sources
One study received funding from a commercial company (Toce 1987); three studies received funding from government or charitable grants (Dsilna 2005; Schanler 1999; Silvestre 1996); and the remaining studies did not report any details about their funding sources.
Declarations of interest
One study stated that the authors had no conflicts of interest to declare (Rövekamp‐Abels 2015), while the remaining studies did not mention authors' declarations of interest at all.
Excluded studies
We excluded 15 studies because they were either not eligible study designs, they did not include eligible interventions, or the route of administration was not eligible. See Characteristics of excluded studies for further information.
Studies awaiting classification
One study is awaiting classification (Corbin 2011). We contacted the trial investigators in November 2020 to ask for information about the inclusion criteria for trial participants, but we have not received any response.
See Characteristics of studies awaiting classification for further information.
Risk of bias in included studies
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Three studies described using robust random sequence generation methods and were judged as low risk of bias (Akintorin 1997; Dollberg 2000; Dsilna 2005). Five studies did not provide sufficient information about their randomisation methods, so we judged them as unclear risk of bias (Macdonald 1992; Neelam 2018; Rövekamp‐Abels 2015; Schanler 1999; Silvestre 1996). One study used alternative allocation and was judged as high risk of bias because the sequence of allocation to treatment was not truly random (Toce 1987).
Allocation concealment
We judged five studies as low risk of bias because they reported using methods such as opaque sealed envelopes to ensure allocation to treatment could not be predicted (Akintorin 1997; Dollberg 2000; Dsilna 2005; Rövekamp‐Abels 2015; Schanler 1999). The other studies did not provide enough information about allocation concealment methods used, so we judged them as unclear risk of bias.
Blinding
Blinding of participants and care givers
We judged all studies as high risk of bias because blinding of participants and care givers was not possible in any of the trials, and knowledge of treatment allocation could have an influence on the outcomes.
Blinding of outcome assessment
Two studies reported using blinded outcome assessors, so we judged them as low risk of bias (Dsilna 2005; Schanler 1999). Two studies reported that outcome assessment was not blinded, so we judged them as high risk of bias (Dollberg 2000; Rövekamp‐Abels 2015). The other studies provided insufficient information about blinding of outcome assessment, so we judged them as unclear risk of bias.
Incomplete outcome data
Five studies had low and non‐differential attrition, so we judged them as low risk of bias (Akintorin 1997; Dsilna 2005; Macdonald 1992; Rövekamp‐Abels 2015; Schanler 1999). We judged the other studies as high risk of bias because they reported high or differential attrition, or both (Dollberg 2000; Neelam 2018; Silvestre 1996), or because they did not explain substantial amounts of missing data (Toce 1987).
Selective reporting
We judged two studies as high risk of bias because they did not report all of the outcomes that they stated would be measured (Neelam 2018; Rövekamp‐Abels 2015). We judged the remaining studies as low risk of bias, because despite the lack of published protocols or prospective trial registrations, they reported in full, all outcomes that would be reasonably expected. Additionally, all of the studies we judged as low risk of reporting bias were published before 2010 and would not be expected to have published protocols or to have been registered prospectively.
Other potential sources of bias
None of the studies had any other aspects that could indicate other sources of bias, so we judged all of them as low risk of bias.
Effects of interventions
See: Table 1
Primary outcomes
Age at full enteral feedings (days)
Babies receiving continuous feeding may reach full enteral feeding slightly later than babies receiving intermittent feeding (MD 0.84 days, 95% CI ‐0.13 to 1.81; 7 studies, 628 infants; I2 = 41%; low‐certainty evidence; Analysis 1.1). For transparency, we have also reported the median (IQR) data from the two studies whose data we converted to mean (SD) (Neelam 2018; Rövekamp‐Abels 2015) (see Analysis 1.2).
1.1. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 1: Age at full enteral feedings (days)
1.2. Analysis.
Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 2: Age at full enteral feedings (days) (median, IQR)
| Age at full enteral feedings (days) (median, IQR) | |||
| Study | Continuous infusion | Intermittent bolus | Measure |
| Neelam 2018 | 16 days (12‐23.2) 35 infants |
by infusion: 16 (14.5‐26), 33 infants by gravity: 15 (11.5‐ 17.5), 29 infants |
Median (IQR) |
| Rövekamp‐Abels 2015 | 8 days (7‐11) 49 infants |
8 days (6‐10) 47 infants |
Median (IQR) |
Sensitivity analysis removing the studies whose comparator groups included infants who received orogastric feeding did not change the effect estimate substantially (MD 0.41 days, 95% CI ‐0.60 to 1.42; 5 studies, 336 infants; I2 = 48%; Analysis 1.3). For this sensitivity analysis, we removed all the data from two studies (Neelam 2018; Schanler 1999), and from another study we removed the comparison between the continuous nasogastric and intermittent orogastric groups (Dsilna 2005).
1.3. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 3: Age at full enteral feedings (days): sensitivity analysis removing trials with mix of nasogastric and orogastric
Sensitivity analysis removing the studies where more than half of the risk of bias domains were unclear or high risk (Neelam 2018; Rövekamp‐Abels 2015; Silvestre 1996), changed the direction of effect but made it less precise, with a wide 95% CI that is consistent with possible benefit and possible harm (MD 1.33 days, 95% CI ‐1.17 to 3.84; 4 studies, 342 infants; I2 = 64%).
The test for subgroup differences did not suggest there may be variation in effect according to birth weight (P = 0.09, I2 = 54.4%; Analysis 1.4).
1.4. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 4: Age at full enteral feedings (days): subgroup analysis
The subgroup analysis has five fewer infants in the intervention arm and 13 fewer in the control arm, compared with the main analysis. This discrepancy is because one study provided data for infants in the less than 1000 grams birth weight category but not for infants in the upper birth weight categories (Dsilna 2005).
Feeding intolerance: number of days of feeding interruptions
It is uncertain if there is any difference between continuous feeding and intermittent feeding in terms of number of days of feeding interruptions (MD ‐3.00, 95% CI ‐9.50 to 3.50; 1 study, 171 infants; very low‐certainty evidence; Analysis 1.5). Only one trial reported number of days of feeding interruptions, so we could not conduct sensitivity or subgroup analysis.
1.5. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 5: Feeding intolerance: number of days of feeding interruptions
Another trial reported the number of infants with feeding interruptions during the study: 44/54 in the continuous group and 36/54 in the intermittent group (Rövekamp‐Abels 2015).
One trial reported 17/35 infants in the continuous group and 36/62 in the intermittent group with any kind of feeding intolerance during the study period (https://revman.cochrane.org/#/074400092812461148/htmlView/6.49.7#STD‐Neelam‐2018).
Days to regain birth weight
It is uncertain if there is any difference between continuous feeding and intermittent feeding in terms of the number of days to regain birth weight (MD ‐0.38 days, 95% CI ‐1.16 to 0.41; 6 studies, 610 infants; I2 = 0%; low‐certainty evidence; Analysis 1.6). The certainty of evidence is low and the 95% CI includes possible benefit and possible harm.
1.6. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 6: Days to regain birth weight
Sensitivity analysis removing the studies whose comparator groups included infants who received orogastric feeding did not change the effect estimate substantially (MD ‐0.42 days, 95% CI ‐1.27 to 0.43; 5 studies, 489 infants; I2 = 0%; Analysis 1.7). For this sensitivity analysis, we removed all the data from one study (Neelam 2018), and from another study we removed the comparison between the continuous nasogastric and intermittent orogastric groups (Dsilna 2005).
1.7. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 7: Days to regain birthweight: sensitivity analysis removing trials with mix of nasogastric and orogastric
Sensitivity analysis removing the studies where more than half of the risk of bias domains were unclear or high risk (Neelam 2018; Rövekamp‐Abels 2015; Silvestre 1996), did not change the effect estimate substantially (MD ‐0.41 days, 95% CI ‐1.49 to 0.67; 3 studies, 319 infants; I2 = 0%).
The test for subgroup differences did not suggest there may be variation in effect according to birth weight (P = 0.99; I2 = 0%; Analysis 1.8). The subgroup analysis has five fewer infants in the intervention arm and 13 fewer in the control arm, compared with the main analysis. This discrepancy is because one study provided data for infants in the less than 1000 grams birth weight category but not for infants in the upper birth weight categories (Dsilna 2005).
1.8. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 8: Days to regain birth weight: subgroup analysis
Rate of gain in weight
It is uncertain if continuous feeding has any effect on rate of gain in weight compared with intermittent feeding (SMD 0.09, 95% CI ‐0.27 to 0.46; 5 studies, 433 infants; I2 = 66%; very low‐certainty evidence; Analysis 1.9). We used the random‐effects model for this analysis due to the high I2. According to the interpretation of SMD we have used, this effect estimate does not suggest an important difference between the groups. However, since the evidence is very low certainty, it is very likely that further studies might change the effect estimate substantially.
1.9. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 9: End of intervention: rate of gain in weight
The statistical heterogeneity may be due to the results in the lower birth weight infants, which favour continuous feeding, while the results in higher birth weight infants and the trials that did not stratify by birth weight have 95% confidence intervals that are consistent with possible benefit and possible harm.
Sensitivity analysis removing one trial that used orogastric feeding (Neelam 2018), did not change the effect estimate substantially (SMD 0.01, 95% CI ‐0.48 to 0.47).
Sensitivity analysis removing the studies where more than half of the risk of bias domains were unclear or high risk (Macdonald 1992; Neelam 2018; Silvestre 1996; Toce 1987), changed the effect estimate substantially such that continuous feeding may lead to less gain in weight compared to intermittent feeding, with the interpretation that SMD equal to or greater than 0.2 and less than 0.5 suggests a moderate effect (SMD ‐0.44, 95% CI ‐0.74 to ‐0.13; 1 study, 171 infants).
The result of the test for subgroup differences suggests there may be a variation in effect between birth weight categories (P = 0.01, I2 = 72.4%). However, since only one trial (93 infants) provided data stratified by birth weight category, the difference between subgroups may be due to chance.
In addition to rate of gain in weight at the end of the intervention, one trial also reported rate of gain in weight at discharge (MD ‐0.18 days, 95% CI ‐1.61 to 1.25; 1 study, 92 infants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 10: At discharge: rate of gain in weight g/kg/day
Rate of gain in length
Continuous feeding may result in little to no difference in rate of gain in length compared with intermittent feeding (MD 0.02 cm/week, 95% CI ‐0.04 to 0.08; 5 studies, 433 infants; I2 = 3%; low‐certainty evidence; Analysis 1.11).
1.11. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 11: End of intervention: rate of gain in length (cm/week)
Sensitivity analysis removing one trial that used a mix of nasogastric and orogastric feeding (Neelam 2018), did not change the effect estimate substantially (MD 0.07 cm/week, 95% CI ‐0.02 to 0.15; 4 studies, 341 infants; I2 = 0%).
Sensitivity analysis removing the studies where more than half of the risk of bias domains were unclear or high risk (Macdonald 1992; Neelam 2018; Silvestre 1996; Toce 1987), did not change the effect estimate substantially (MD 0.10 cm/week, 95% CI ‐0.09 to 0.29; 1 study, 171 infants).
The test for subgroup differences did not suggest there may be variation in effect according to birth weight (P = 0.54, I2 = 0%).
In addition to rate of gain in length at the end of the intervention, one trial also reported rate of gain in length at discharge (MD ‐0.01 cm/week, 95% CI ‐0.07 to 0.05; 1 study, 125 infants; Analysis 1.12).
1.12. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 12: At discharge: rate of gain in length (cm/week)
Rate of gain in head circumference
Continuous feeding may result in little to no difference in rate of gain in head circumference compared with intermittent feeding (MD 0.01 cm/week, 95% CI ‐0.03 to 0.05; 5 studies, 433 infants; I2 = 0%; low‐certainty evidence; Analysis 1.13).
1.13. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 13: End of intervention: rate of gain in head circumference (cm/week)
Sensitivity analysis removing one trial that used a mix of nasogastric and orogastric feeding (Neelam 2018), did not change the effect estimate substantially (MD ‐0.00 cm/week, 95% CI ‐0.05 to 0.05; 4 studies, 341 infants; I2 = 0%).
Sensitivity analysis removing the studies where more than half of the risk of bias domains were unclear or high risk (Macdonald 1992; Neelam 2018; Silvestre 1996; Toce 1987), did not change the effect estimate substantially (MD ‐0.04 cm/week, 95% CI ‐0.12 to 0.04; 1 study, 171 infants).
The test for subgroup differences did not suggest there may be variation in effect according to birth weight (P = 0.72, I2 = 0%).
In addition to rate of gain in head circumference at the end of the intervention, one trial also reported rate of gain in head circumference at discharge (MD 0.10 cm/week, 95% CI 0.04 to 0.16; 1 study, 91 infants; Analysis 1.14).
1.14. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 14: At discharge: rate of gain in head circumference (cm/week)
Necrotising enterocolitis
It is uncertain if continuous feeding has any effect on the risk of necrotising enterocolitis compared with intermittent feeding (RR 1.19, 95% CI 0.67 to 2.11; 4 studies, 372 infants; I2 = 0%; low‐certainty evidence; Analysis 1.15). The evidence is low certainty and the 95% confidence interval is consistent with possible benefit and possible harm.
1.15. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 15: Necrotolising enterocolitis
One trial reported zero cases of confirmed necrotising enterocolitis (Bell's stage II or greater) in both arms (Silvestre 1996; 0/45 and 0/48).
Sensitivity analysis removing the data from infants receiving orogastric feeding (Dsilna 2005), did not change the effect estimate substantially (RR 1.16, 95% CI 0.64 to 2.08).
Sensitivity analysis removing the studies where more than half of the risk of bias domains were unclear or high risk (Toce 1987), did not change the effect estimate substantially (RR 1.20, 95% CI 0.65 to 2.20; 3 studies, 319 infants; I2 = 0%).
None of the studies contributing data to this outcome reported data by birth weight category, so we did not do subgroup analysis.
Secondary outcomes
Days to discharge to referral hospital or home
It is uncertain if continuous feeding has any effect on days to discharge compared to intermittent feeding (MD ‐1.55 days, 95% CI ‐5.13 to 2.02; 2 studies, 264 infants; I2 = 28%; Analysis 1.16).
1.16. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 16: Days to discharge
Apnoea
It is uncertain if continuous feeding has any effect on the number of apnoea episodes compared with intermittent feeding (SMD 0.08, 95% CI ‐0.44 to 0.60; Analysis 1.17). Since this SMD is less than 0.20, and the 95% CI spans little effect to possible harm, we cannot be certain about this result.
1.17. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 17: Apnoea episodes
Another study reported little difference in median (IQR) episodes of apnoea between the two groups (Analysis 1.18) (Rövekamp‐Abels 2015). We have not combined these data with those reported as mean (SD) because we judged the converted means were so different from the data reported as medians that it would be misleading to include them in the analysis (converted means: 4.03 and 4.4 episodes).
1.18. Analysis.
Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 18: Number of apnoea episodes per day (median, IQR)
| Number of apnoea episodes per day (median, IQR) | ||
| Study | Continuous infusion | Intermittent bolus |
| Rövekamp‐Abels 2015 | 3.9 (2.5‐5.7) 54 infants |
3.6 (2.5‐7.1) 54 infants |
Days on total parenteral nutrition
Continuous feeding may result in fewer days on total parenteral nutrition compared to intermittent feeding (MD ‐4.77 days, 95% CI ‐9.52 to ‐0.03; 2 studies, 239 infants; I2 = 30%; Analysis 1.19).
1.19. Analysis.

Comparison 1: Continuous versus intermittent bolus milk feeding, Outcome 19: Days on total parenteral nutrition
Discussion
Summary of main results
This updated review now includes evidence from nine trials (involving 919 infants), compared to seven trials (involving 511 infants) in the previous version of this review (Premji 2011).
We found low‐certainty evidence that continuous feeding may result in infants reaching full enteral feeding slightly later than with intermittent feeding. There may be little to no difference between continuous and intermittent feeding in terms of rate of gain in length and head circumference.
It is uncertain if there is any difference between continuous feeding and intermittent feeding in terms of number of days to regain birth weight and risk of necrotising enterocolitis, because the certainty of evidence is low and the 95% confidence intervals are consistent with possible benefit and possible harm.
The evidence regarding feeding intolerance, measured by number of days of feeding interruptions, and rate of gain in weight is very low certainty. Therefore, we cannot be certain if there is any difference between continuous feeding and intermittent feeding for these two outcomes.
Overall completeness and applicability of evidence
Despite the inclusion of data from an additional 408 infants, compared with the previous version of this review, the evidence remains largely uncertain about the effect of continuous feeding compared with intermittent feeding for preterm infants weighing less than 1500 grams.
Several of the studies in this review utilised the occurrence of gastric residuals as a major criterion for determining feeding intolerance. Three studies reported higher incidence of residuals in infants fed by continuous tube feeding which might have resulted in more feeding interruptions or slower increases in feeds, or both, thereby increasing the time taken to reach full feeds (Akintorin 1997; Dollberg 2000; Schanler 1999). The necessity of checking gastric residuals has been challenged in recent years, as there is a paucity of evidence to support this practice, and wide variation in practice regarding acceptable volumes of residuals, and how they should be managed (Li 2014). One recent study of preterm infants born at 32 weeks' gestation or earlier and weighing 1250 grams or more at birth, found that not checking gastric residuals prior to feeding as compared with checking residuals prior to feeding was associated with more rapid advancement of enteral feeding and higher intake of nutrients at some time points (Rysavy 2020). Therefore, a change in the practice of using gastric residuals to guide feeding advancement in preterm infants might affect outcomes such as feeding tolerance and time to full feeds when comparing continuous tube feeding to intermittent bolus tube feeding.
In practice, when utilising continuous feedings of human milk, concerns have been raised related to a loss of nutrients when human milk sits in tubing for a length of time (typically four hours). This has been studied in vitro by mimicking intermittent and continuous feedings using available feeding systems, and measuring nutrient contents of feedings before and after running feeds through a feeding system set to mimic either continuous or intermittent feedings. One study comparing nutrient loss suggested that continuous feeding of fortified human milk resulted in significant losses of calcium, phosphorus, protein, and fat, especially when using a bovine source human milk fortifier (Rogers 2010). Another in vitro study measured nutrient loss during continuous feedings with or without the use of a priming volume of feeding which would be discarded after the feeding. Researchers found that they could reduce the loss of fat (decreased from 16.7% to 8.2%), protein (decreased from 3.4% to 0), and calories (decreased from 9.2% to 3.3%) by preparing and infusing the exact feeding volume using air to clear the milk from the tubing at the end of the feed, as compared to discarding the priming volume of milk which remained in the tubing at the end of the feeding (Davidson 2020). These studies were performed in vitro and therefore, could not assess the impact of continuous feedings on outcomes such as growth and nutrient retention, and further studies to address this issue would be useful. In the current systematic review, the evidence is uncertain about differences in the rates of growth in weight, length, or head circumference in infants fed by either continuous or intermittent tube feeding. The use of parenteral nutrition to supplement enteral nutrition while establishing feedings might have diminished any potential variation.
Quality of the evidence
The assessment of risk of bias was limited by lack of information reported in the trials, particularly in terms of random sequence generation, allocation concealment, and blinding of outcome assessors. It was not possible for care givers to be blinded to group allocation in any of the studies, which could have had an impact on outcomes. There was high risk of bias in terms of incomplete outcome data in four of the nine trials, and we judged two trials to be at high risk of selective reporting.
As a result of serious concerns about risk of bias, we downgraded the certainty of the evidence. We also downgraded the certainty of evidence because of serious concerns about imprecision due to low numbers of infants in the included trials, wide confidence intervals leading to lack of precision in the effect estimates, or both.
Potential biases in the review process
To reduce the risk of bias in the review, we conducted a comprehensive literature search with no limitations in terms of language of publication or publication status. While we recognise that there may be studies that were not retrieved by our literature searches, we made every attempt to identify relevant unpublished studies and to obtain missing data from published studies.
Two review authors independently carried out study selection, data extraction, risk of bias assessment, and GRADE assessment, with recourse to the third review author to resolve any discrepancies.
Agreements and disagreements with other studies or reviews
We were able to locate only one systematic review examining feeding methods (continuous versus intermittent bolus) in low birth weight infants under 2500 grams (Wang 2020). This systematic review also reported that infants (n = 707) fed by continuous feeding method took longer to achieve full feedings (weighted mean difference 0.98 days, 95% CI 0.26 to 1.71; P = 0.008) when compared to those fed by intermittent bolus feeding method.
Authors' conclusions
Implications for practice.
Although babies receiving continuous feeding may reach full enteral feeding slightly later than babies receiving intermittent feeding, the evidence is of low certainty. However, the clinical risks and benefits of continuous and intermittent nasogastric tube milk feeding cannot be reliably discerned from current available randomised trials.
Implications for research.
Further research is needed to determine if either feeding method is more appropriate for the initiation of feeds, and if either method may be better tolerated by infants who experience feeding intolerance, a question not addressed in the current review. A rigorous methodology should be adopted, defining feeding protocols and feeding intolerance consistently for all infants. Infants should be stratified according to birth weight and gestation, and possibly according to illness.
What's new
| Date | Event | Description |
|---|---|---|
| 17 July 2020 | New citation required but conclusions have not changed |
|
| 17 July 2020 | New search has been performed |
|
History
Protocol first published: Issue 4, 1999 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 4 August 2011 | New citation required but conclusions have not changed | Search was updated in July 2011. No new trials identified. Risk of Bias tables completed. No changes to conclusions. |
| 4 August 2011 | New search has been performed | This review updates the existing review "Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams", published in the Cochrane Database of Systematic Reviews (Premji 2004). |
| 5 February 2008 | New search has been performed | This review updates the existing review "Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams", published in the Cochrane Database of Systematic Reviews, Issue 4, 2004 (Premji 2004). Two new trials were identified as a result of the most recent search completed October 26, 2007. The previous conclusion of no significant difference in somatic growth of infants fed by continuous versus intermittent bolus tube feeds remains unchanged. |
| 15 January 2008 | Amended | Converted to new review format. |
| 5 March 2004 | New search has been performed | Substantive amendment |
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor, Jane Cracknell, Assistant Managing Editor, Roger Soll, Co‐coordinating editor, and William McGuire, Co‐coordinating Editor, who provided editorial and administrative support. Carol Friesen, Information Specialist, designed and conducted the literature searches, and Colleen Ovelman peer reviewed the Ovid MEDLINE search strategy.
Sarah Hodgkinson and William McGuire have peer reviewed and offered feedback on this review.
We thank Hamilton Health Sciences Corporation Foundation for funding the initial review.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Anna Noel‐Storr, Stefanie Rosumeck, Karen Ma, Stella Maria O'Brien, Nikolaos Sideris, Anna Resolver, Lai Ogunsola, Luma Haj Kassem, Thidar Aung, Sarah Moore, Sunu Alice Cherian, Brian Duncan, Charlotte Lake, Ana María Rojas Gómez, Lyle Croyle, Ana Beatriz Pizarro, Cathy Wellan, Chet Chaulagai, Artem Oganesyan, Mohammad Aloulou, Ana‐Marija Ljubenković, Jorge MM Teixeira, Iltimass Gouazar, Selin Bicer, Dulce Estêvão, Ya‐Ying Wang, Mateus Falco, Jehath Syed, Chloe Thomson, Ahlam Jamal Alhemedi, Paula Miguel, and Nirupa Sundaravadanan.
We would like to acknowledge and thank Dr John C Sinclair for his mentorship and guidance with the initial systematic review (Premji 2004).
Appendices
Appendix 1. 2020 Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2020). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist; please see the Search Methodology section at https://neonatal.cochrane.org/resources-authors/author-resources-new-reviews.
CENTRAL via CRS Web:
Date ranges: 01 January 2011 to 17 July 2020 Terms: 1 MESH DESCRIPTOR Enteral Nutrition EXPLODE ALL AND CENTRAL:TARGET 2 (((bolus or continuous* or intermittent* or enteral* or early or nasogastric*) and (feed* or fed or tube‐feed* or tube‐fed or nutrition)) or feeding method* or feeding strateg*) AND CENTRAL:TARGET 3 #2 OR #1 AND CENTRAL:TARGET 4 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 5 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birthweight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 6 #5 OR #4 AND CENTRAL:TARGET 7 #6 AND #3 AND CENTRAL:TARGET 8 2011 TO 2020:YR AND CENTRAL:TARGET 9 #8 AND #7 AND CENTRAL:TARGET
MEDLINE via Ovid:
Date ranges: 01 January 2011 to 17 July 2020 Terms: 1. exp Enteral Nutrition/ 2. (((bolus or continuous* or intermittent* or enteral* or early or nasogastric*) and (feed* or fed or tube‐feed* or tube‐fed or nutrition)) or feeding method* or feeding strateg*).mp. 3. 1 or 2 4. exp infant, newborn/ 5. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birthweight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 6. 4 or 5 7. randomized controlled trial.pt. 8. controlled clinical trial.pt. 9. randomized.ab. 10. placebo.ab. 11. drug therapy.fs. 12. randomly.ab. 13. trial.ab. 14. groups.ab. 15. or/7‐14 16. exp animals/ not humans.sh. 17. 15 not 16 18. 6 and 17 19. randomi?ed.ti,ab. 20. randomly.ti,ab. 21. trial.ti,ab. 22. groups.ti,ab. 23. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 24. placebo*.ti,ab. 25. 19 or 20 or 21 or 22 or 23 or 24 26. 5 and 25 27. limit 26 to yr="2018 ‐Current" 28. 18 or 27 29. 3 and 28 30. limit 29 to yr="2011 ‐Current"
CINAHL via EBSCOhost:
Date ranges: 01 January 2011 to 17 July 2020 Terms: (((bolus or continuous* or intermittent* or enteral* or early or nasogastric*) and (feed* or fed or tube‐feed* or tube‐fed or nutrition)) or feeding method* or feeding strateg*) AND (infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birthweight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Limiters ‐ Published Date: 20110101‐20201231
ISRCTN:
Date ranges: 01 January 2011 to 17 July 2020 Terms: "Continuous nasogastric" AND feeding within Participant age range: Neonate "intermittent bolus" AND feeding within Participant age range: Neonate
Australian New Zealand Clinical Trials Registry (ANZCTR):
Date ranges: 1 February 2020 to 17 July 2020 Terms: "Continuous nasogastric" AND feeding "intermittent bolus" AND feeding
EU Clinical Trials Register (EU‐CTR):
Date ranges: 1 February 2020 to 17 July 2020 Terms: "Continuous nasogastric" AND feeding "intermittent bolus" AND feeding
Clinical Trial Registry – India (CTRI):
Date ranges: 1 February 2020 to 17 July 2020 Terms: "Continuous nasogastric" AND feeding "intermittent bolus" AND feeding
Appendix 2. Previous search methods
Computerised searches were conducted by both review authors up to July 2011. The databases that were searched included the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2011), MEDLINE back to 1966, CINAHL back to 1982 and HealthSTAR back to 1975. The following MeSH headings were used to conduct the searches: continuous, intermittent, enteral nutrition, enteral feeding, feeding, enteral nursing, enteroinsular axis, infant‐premature‐metabolism, feeding methods, gastric residuals, feeding intolerance. The searches were limited with terms such as infant‐newborn and infant, very low birthweight.
We also searched: www.clinicaltrials.gov and www.controlled‐trials.com; terms: (infant OR newborn) AND (continuous OR intermittent) AND (nutrition OR feeding OR nursing OR enteroinsular OR metabolism OR gastric).
All potentially relevant titles and abstracts identified in the searches by either review author were retrieved. The reference list of each article was reviewed independently for additional relevant titles and abstracts and these were also retrieved.
Appendix 3. ‘Risk of bias’ tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Continuous versus intermittent bolus milk feeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Age at full enteral feedings (days) | 7 | 628 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐0.13, 1.81] |
| 1.2 Age at full enteral feedings (days) (median, IQR) | 2 | Other data | No numeric data | |
| 1.3 Age at full enteral feedings (days): sensitivity analysis removing trials with mix of nasogastric and orogastric | 5 | 336 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.60, 1.42] |
| 1.4 Age at full enteral feedings (days): subgroup analysis | 7 | 610 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.13, 1.74] |
| 1.4.1 Birth weight < 1000 grams | 4 | 216 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.69, 1.54] |
| 1.4.2 Birth weight ≥ 1000 grams and < 1249 grams | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐2.54, 2.21] |
| 1.4.3 Birth weight ≥ 1250 grams and < 1500 grams | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [‐0.48, 10.48] |
| 1.4.4 Data not stratified by birth weight category | 3 | 291 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [0.58, 6.01] |
| 1.5 Feeding intolerance: number of days of feeding interruptions | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐9.50, 3.50] |
| 1.6 Days to regain birth weight | 6 | 610 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.16, 0.41] |
| 1.7 Days to regain birthweight: sensitivity analysis removing trials with mix of nasogastric and orogastric | 5 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.27, 0.43] |
| 1.8 Days to regain birth weight: subgroup analysis | 6 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.14, 0.46] |
| 1.8.1 Birth weight < 1000 grams | 3 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐2.11, 1.84] |
| 1.8.2 Birth weight ≥ 1000 grams and < 1249 grams | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.45, 1.66] |
| 1.8.3 Birth weight ≥ 1250 grams and < 1500 grams | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐3.53, 3.53] |
| 1.8.4 Data not stratified by birth weight category | 3 | 369 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.40, 0.60] |
| 1.9 End of intervention: rate of gain in weight | 5 | 433 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.27, 0.46] |
| 1.9.1 Birth weight < 1000 grams | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.97 [0.20, 1.75] |
| 1.9.2 Birth weight ≥ 1000 grams and < 1249 grams | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [0.04, 1.52] |
| 1.9.3 Birth weight ≥ 1250 grams and < 1500 grams | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.69, 0.69] |
| 1.9.4 Data not stratified by birth weight category | 4 | 340 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.47, 0.15] |
| 1.10 At discharge: rate of gain in weight g/kg/day | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.61, 1.25] |
| 1.11 End of intervention: rate of gain in length (cm/week) | 5 | 433 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 1.11.1 Birth weight < 1000 grams | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.08, 0.22] |
| 1.11.2 Birth weight ≥ 1000 grams and < 1249 grams | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.15, 0.15] |
| 1.11.3 Birth weight ≥ 1250 grams and < 1500 grams | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.08, 0.36] |
| 1.11.4 Data not stratified by birth weight category | 4 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 1.12 At discharge: rate of gain in length (cm/week) | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 1.13 End of intervention: rate of gain in head circumference (cm/week) | 5 | 433 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 1.13.1 Birth weight < 1000 grams | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| 1.13.2 Birth weight ≥ 1000 grams and < 1249 grams | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.52, 0.52] |
| 1.13.3 Birthweight ≥ 1250 grams and < 1500 grams | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.10, 0.10] |
| 1.13.4 Data not stratified by birth weight category | 4 | 340 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 1.14 At discharge: rate of gain in head circumference (cm/week) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.04, 0.16] |
| 1.15 Necrotolising enterocolitis | 4 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.67, 2.11] |
| 1.16 Days to discharge | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐1.55 [‐5.13, 2.02] |
| 1.17 Apnoea episodes | 2 | 224 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.44, 0.60] |
| 1.18 Number of apnoea episodes per day (median, IQR) | 1 | Other data | No numeric data | |
| 1.19 Days on total parenteral nutrition | 2 | 239 | Mean Difference (IV, Fixed, 95% CI) | ‐4.77 [‐9.52, ‐0.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akintorin 1997.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Cook County Children’s Hospital, Chicago Country: USA |
|
| Participants | 89 infants randomised. 9 post‐randomisation exclusions.
80 infants analysed. Inclusion: infants 700 to 1250 grams, haemodynamically stable and ready to start enteral feeds. Exclusion: Apgar score < 3 at 5 minutes, to receive breast milk, documented sepsis, NEC or unable to start feeding before day 10 of life. |
|
| Interventions | Feeding did not begin until umbilical arterial catheter removed. Continuous feeding by nasogastric feeding tube and infusion pump. Intermittent feeding given every 3 hours for 15 to 30 minutes by gravity via indwelling nasogastric feeding tube. Feeding protocol for each 50 to 100 grams weight category. Protocol to manage feeding intolerance (feeds held > 12 hours). Energy and protein intake kept identical between groups. Feeds: undiluted preterm formula (20 kcal/ounce). Timing of feeds
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes | Sample size calculation based on 35% decrease in number of days to full feeds in continuous group. Did not exclude SGA infants. Uncertain when feeds changed from continuous to bolus feeding. Numbers unbalanced. Exclusions: 4 Continuous (none due to protocol violation) and 5 Bolus (3 due to protocol violation). Larger proportion of infants whose feeds were held in the continuous group had residuals, whereas in the bolus group, infants had apnoea/bradycardia. Guidelines for residuals may allow larger volumes than some other studies. Study dates: April 1994 to July 1995 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Infants were randomly assigned within each weight group to either CNG or IBG by using sequentially numbered opaque sealed envelopes using a table of random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote: “sequentially numbered opaque sealed envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of care givers is not possible. Outcomes may be influence by care givers’ knowledge of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/43 and 5/46 were not included in the analysis. Attrition is not differential and reasons for excluding from analysis do not seem to be related to the intervention. Group A (continuous): 2 severe congenital syphilis, 1 switched to breastfeeding, 1 required surgery for intestinal malrotation. Group B (intermittent): protocol not followed in 3 participants, 1 switched to breastfeeding, 1 transferred to another hospital before completing the protocol. |
| Selective reporting (reporting bias) | Low risk | No published protocol but outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Dollberg 2000.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Lis Maternity Hospital, Tel Aviv, and Sheba Medical Center, Tel Hashomer Country: Israel |
|
| Participants | 28 infants randomised
5 post‐randomisation exclusions
23 infants analysed Inclusion: AGA, < 48 hours postnatal age, no major congenital malformations, and informed consent |
|
| Interventions | Continuous feeding with nasogastric feeding tube by syringe pump
Intermittent feeding with nasogastric feeding tube by gravity every 2 hours in 501 to 750 grams group; every 3 hours in other infants Feeding protocol for each weight group Protocol to manage feeding intolerance (gastric residual > 20% of the volume fed over the previous 4 hours) Feeds: undiluted human milk, preterm formula (initially diluted), or both Timing of feeds
|
|
| Outcomes |
|
|
| Notes | Pilot study No sample size calculation. 5 post‐randomisation exclusions Regression analysis suggested mode of feeding as the only variable affecting feeding tolerance Awaiting subgroup data Study dates: January to September 1998 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “assigned randomly (using random numbers) to CGI or IGB. The randomization assignment was performed using sealed opaque envelopes that were grouped in an even blocked design, by the stratification variable (birth weight)” |
| Allocation concealment (selection bias) | Low risk | Quote: “sealed opaque envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote “Investigators were not blinded to the study group assignment, but caregivers responsible for the infants' care and for feeding protocol performance were not part of the investigation.” Not possible to blind caregivers. Knowledge of treatment allocation could influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Investigators were not blinded to the study group assignment” Assuming investigators are also outcomes assessors, lack of blinding may affect the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Text states that 4/12 and 1/16 were removed from analysis (3 and 1 deaths per group, and 1 protocol violation in group A) but the denominators for the outcome data are not clear |
| Selective reporting (reporting bias) | Low risk | No published protocol but outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Dsilna 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: 3 independent neonatal units at Karolinska University Hospital, Stockholm Country: Sweden |
|
| Participants | 70 infants randomised
2 post‐randomisation exclusions. 68 infants analysed Inclusion: gestational age 24 to 29 weeks and birth weight < 1200 grams, stable respiratory status (i.e. arterial‐alveolar oxygen tension ratio ≥ 0.18), no major congenital malformations, maternal ability to read in Swedish, and residing within geographical catchment area of 3 independent neonatal units at Karolinska University Hospital. |
|
| Interventions | Feeding initiated before 30 hours postnatal age. Actual not stated Feeds: mother's own milk or pasteurised donor human milk from the local milk bank Continuous feedings with an indwelling nasogastric feeding tube by electric infusion pump and intermittent feedings given every third hour over a period of 15 to 40 minutes using orogastric tube placed for each feeding or indwelling nasogastric tube feedings. Duration of feeding based on volume given and feeding difficulties experienced by infant. Protocol, based on infant's birth weight, followed for increasing feedings. At postmenstrual age of 32 weeks' continuous nasogastric feedings weaned to intermittent feeding. Feeding intolerance managed by (quote:) “clinical routine” which included reducing volume of feeding or temporarily withholding feeding Fortification of human milk was initiated for all infants when total parenteral nutrition was discontinued |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | Infants in the continuous feeding method group compared with infants from the 2 control groups (intermittent orogastric group and intermittent nasogastric group) combined as the 2 control groups did not (quote:) “differ in primary outcome, demographic and birth‐related factors, and duration of feedings” (p. 45).
No significant difference in protein and energy intakes Sample size calculation based on 40% difference in time to achieving full enteral feedings Did not exclude SGA infants Exclusions ‐ 2 post‐randomisation because of diagnosed malformations Switched intermittent orogastric feeding to continuous nasogastric feeding for 14 days (N = 1). Switched intermittent orogastric feeding to intermittent nasogastric feeding for 13 days (N = 1). Switched intermittent nasogastric feeding to intermittent orogastric feeding for 6 days (N = 1). (Not clear if intention‐to‐treat) Mortality ‐ 1 infant in each feeding group (total 3) died in the early intervention phase due to respiratory and circulatory collapse (not accounted for in sample of the study). 2 infants died after postmenstrual ages of 33 and 47 weeks due to septicaemia combined with severe respiratory and circulatory distress and chronic lung disease. Both infants were in the continuous nasogastric feeding group (accounted for in the sample of the study). Study dates: February 1998 to November 2001 Funding sources: supported by grants from the Vårdal Foundation, the Mjölkdroppen Foundation, and the Frimurare Barnhuset Foundation, Stockholm, Sweden Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was achieved by using opaque envelopes in blocks of 12 randomly ordered in sequence by a person not connected to the study” |
| Allocation concealment (selection bias) | Low risk | Quote: “opaque envelopes in blocks of 12 randomly ordered in sequence by a person not connected to the study” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Care givers not blinded as would not be feasible. Knowledge of treatment allocation may influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only radiographic assessors for the outcome of NEC were blinded to participant group assignment. “Misclassification of outcome was unlikely, even though the study was not blinded with regard to the study group” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition is low and not differential. 2/70 were excluded from analysis because of (quote:) “diagnosed malformations” Quote: “Data analysis was performed by intention to treat; that is, all infants were included as randomized” |
| Selective reporting (reporting bias) | Low risk | No published protocol but outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Macdonald 1992.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Royal Maternity Hospital, Glasgow Country: United Kingdom |
|
| Participants | 43 infants randomised. 9 post‐randomisation exclusions. 34 infants analysed Inclusion: infants < 1400 grams Exclusion: infants who received expressed breast milk, major congenital malformations, developed hydrocephalus, and if there was intrauterine viral infections |
|
| Interventions | Milk feeding started on day 2 of life with 1 mL/hr of SMA low birth weight formula (Wyeth). Increased 0.5 to 1.0 mL/hr until tolerating 150 mL/kg/day (supplemented with total parenteral nutrition). Unclear frequency of bolus nasogastric feeding, frequency of increase in feeds, equipment for delivery of continuous feeds. Selected method used until infant attained weight of 1600 grams (Quote:) “Each infant's daily energy input per kilogram, achieved in the form of milk, was calculated for the first four weeks or until exit from the study at 1600 g or whichever occurred first.” Feeds: SMA low birth weight formula (Wyeth). No energy supplements during study period. Continuous nasogastric (n = 13) Bolus nasogastric (n = 15) Continuous transpyloric (n = 15) |
|
| Outcomes |
|
|
| Notes | No sample size calculation Did not exclude SGA infants Feeding intervention not described in detail 5 exclusions in transpyloric group, 1 in continuous group and 3 in bolus group Report pooled standard deviations for days to full feedings (awaiting mean and standard deviation) Extra abdominal radiographs (n = 31) were performed for transpyloric tube placements. Aspiration (1 in continuous group) occurred when babies were over 1600 grams, hence were being fed by bolus nasogastric route. Gastric bleeding (1 in bolus group) occurred before milk feedings were started. Staphylococcus epidermidis ‐ 1 case in each group ‐ unclear if this was during study period or during length of stay in NICU. Sample size for each group obtained from author Study dates: January to December 1987 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The word ‘randomised’ appears in the title and abstract but there is no further information about how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Quote: “On day 2 the feeding route was determined for each baby by opening a sealed envelope” Unclear if anyone could have predicted the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind care givers. Outcomes could be influenced by care givers’ knowledge of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/15, 1/13 and 3/15 did not complete the study due to death in the first week and were not included in the analysis. Attrition is not differential and reasons for withdrawing from the study do not appear to be related to the intervention |
| Selective reporting (reporting bias) | Low risk | No published protocol but outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Neelam 2018.
| Study characteristics | ||
| Methods |
Study design: randomised design Setting: Sir Ganga Ram Hospital, New Delhi Country: India |
|
| Participants | Target sample size was 171 129 enrolled (not stated how many were randomised), 97 analysed (for some outcomes 35, 33 and 29 are the numbers analysed per group) Inclusion criteria: all haemodynamically stable infants < 32 weeks at birth and birth weight ≤ 1250 grams in whom enteral feeding can be started Exclusion criteria: gross congenital anomalies, including those with anomalies of gastrointestinal tract (omphalocele, gastroschisis, anorectal malformation, congenital diaphragmatic hernia, congenital intestinal obstruction) |
|
| Interventions |
Group A (n = 33*): continuous infusion (CI). (Quote:) “The amount of milk to be given to the infant in the next 24 hours will be calculated and will be loaded periodically in a 50 ml syringe. Feeds will be infused hourly by electric infusion pump through orogastric tube. The syringe will be loaded with freshly prepared formula milk every 2 hours and in case of EBM every 6 hourly. Intervention till infant is taken on direct (breast/paladi) feed.” Group B (n = 31*): intermittent bolus by infusion (IBI). (Quote:) “The amount of milk to be given in every 2 hours will be calculated and delivered by a 50 ml syringe. Feeds will be infused over a period of 15 minutes every 2 hours by electric infusion pump. Intervention till infant is taken on direct (breast/paladi) feed.” Group C (n = 28*): Intermittent bolus by gravity (IBG). (Quote:) “The amount of milk to be given in every 2 hours will be calculated and delivered by a 10 ml syringe via orogastric tube. The orogastric tube will be pinched while the syringe is loaded with milk and pinch is released, allowing milk to flow through orogastric tube over a period of a few minutes. Time taken for each feeds will be noted with a stop watch. Intervention till infant is taken on direct (breast/paladi) feed.” Feeds: freshly prepared formula or EBM *is the analysed group (number randomised is not reported) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding sources: not reported Declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:“randomly assigned” – no further information |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible. Knowledge of treatment allocation could have an influence on outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 129 enrolled (not stated how many were randomised), 97 analysed |
| Selective reporting (reporting bias) | High risk | Not all outcomes that are stated as having been measured, are included in the results |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Rövekamp‐Abels 2015.
| Study characteristics | ||
| Methods |
Study design: single centre RCT Setting: Sophia Children’s Hospital, Rotterdam Country: Netherlands |
|
| Participants | 250 infants randomised Inclusion criteria: birth weight (BW) < 1750 grams, gestational age < 32 weeks, and born in or admitted to the neonatal intensive care unit (NICU) of the Sophia Children’s Hospital of the Erasmus Medical Centre in Rotterdam within 24 hours of birth Exclusion criteria: congenital gastrointestinal obstructions (e.g. duodenal atresia, anal atresia) and suspected metabolic, endocrine, or renal disorders |
|
| Interventions |
Group A (n = 125): semi‐continuous feeds by nasogastric feeding tube – ¼ hourly volume fed by gravity every 15 minutes over a 24‐hour period Group B (n = 125): bolus feeds by nasogastric feeding tube – 3 hourly volume by gravity bolus over 15 minutes Both groups: feeding started on day of birth – minimal enteral feeds – 0.5 mL (500 to 750 grams), 1 mL (750 to 1249 grams), 2 mL (1250 to 1749 grams) every 4 hours (own mother's milk or formula) Next day if no PDA or asphyxia – increase feeds to 24 mL/kg/day then equal daily increases to meet 120 mL/kg/day in 6 days Asphyxia – 1 extra day (feeds started 1 day later); SGA/PDA – 2 extra days as slower increases Feeds: EBM or formula. For EBM – fortifier added at 100 mL/kg/day or after 7 days of feeds Note: feedings not truly continuous but acceptable method without using feeding pumps |
|
| Outcomes |
|
|
| Notes |
Funding sources: not reported Declarations of interest: (quote:) “The authors report no conflicts of interest.” Study dates: February 2007 to February 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear: (quote:) “The randomisation (using random numbers) used sealed opaque envelopes that were grouped in an even blocked design” |
| Allocation concealment (selection bias) | Low risk | Quote: “Sealed opaque envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “it was not feasible to blind the caregivers to treatment allocation and outcome” Lack of blinding could have an influence on the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote. "it was not feasible to blind the caregivers to treatment allocation and outcome” Lack of blinding could have an influence on the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Group A ‐ 4/125 not included in analysis (withdrew consent) Group B ‐ all participants included in analysis Low attrition and not differential |
| Selective reporting (reporting bias) | High risk | Some outcomes specified in trial registration are not reported (rates of weight gain, head circumference) |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Schanler 1999.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Texas Children's Hospital Country: USA |
|
| Participants | 171 infants randomised Inclusion: 26 to 30 weeks' gestation, AGA, postnatal age ≤ 96 hours, no congenital anomalies, fraction of inspired oxygen < 0.6 by 72 hours, and written informed consent Removed infants from treatment protocol if unable to adhere to feeding protocol for > 1 week |
|
| Interventions | GI priming vs no enteral intake day 4 to 14 and continuous vs bolus nasogastric tube feedings. 4 Groups: NPO continuous, NPO bolus, GI priming continuous, and GI prime bolus. Bolus feeding given every 3 hours over 20 minutes. Continuous feeding method not described. Feeding protocol for infants. Protocol to manage feeding intolerance based on excess gastric residual volume. Nutrient intakes similar between groups Feeds: undiluted human milk or initially diluted preterm infant formula Timing of feeds
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes | Data comparing continuous vs intermittent groups obtained from investigator
Intention‐to‐treat analysis Sample size calculation based on a 2‐week difference in the time to full oral feeds Infants randomised to early vs late enteral feeds (day 4 vs 14) 11 infants switched protocol (10 continuous and 1 bolus). Observed greater incidence of residuals with continuous feeds, and more infants unable to adhere to feeding protocols. Infants in the intermittent bolus feeding group ‐ tube placement predominantly (> 90%) orogastric (personal communication) Small sample size given the number of effects being examined (NPO, early feeding, and stratification of gestation age and feed type) Have established criteria for transition to oral feeds; however, not well described, some criteria subjective (e.g. favourable oral motor assessment, increased apnoea, or oxygen needs). Methods section states: “The assigned orogastric/nasogastric tube‐feeding method (continuous vs bolus) was maintained throughout the three phases”. There is no further mention of tube placement, and no way to know how the babies in the intermittent group were fed. Awaiting subgroup data Study dates: March 1992 to April 1996 Funding sources: (quote:) “This study was supported by the National Institute of Child Health and Human Development, Grant No. RO‐1‐HD‐28140 and the General Clinical Research Center, Baylor College of Medicine/ Texas Children's Hospital Clinical Research Center, Grant No. MO‐1‐ RR‐00188, National Institutes of Health. Partial funding also has been provided from the USDA/ARS under Cooperative Agreement No. 58‐6250‐1‐003.” Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was performed using sealed opaque envelopes that were grouped, in an uneven blocked design, by stratification variables (gestational age, intent to feed human milk)” No details reported about how random sequence was generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible. Knowledge of treatment allocation may influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | To ensure the objective assessment of the major outcome variable, the time required by the infant to attain full oral feeding (8 breast and/or bottle feedings per day), oral‐motor function was assessed serially, using a method designed specially for this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Infants were removed from the treatment protocol if they were unable to adhere to the feeding protocol for [greater than] 1 week. Infants removed from the study were monitored for as many outcomes as their clinical status permitted and their data were included in the analysis.” Quote: “The primary data analysis was by intent to treat, ie, all subjects were included as randomized” Switched to opposite tube‐feeding method: Continuous group: 10/83 Bolus group: 1/88. These 11 who changed groups seem to have been analysed according to the group they were randomised to. |
| Selective reporting (reporting bias) | Low risk | No published protocol but all outcomes seem to be reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Silvestre 1996.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Children's Hospital of Michigan, Detroit, MI Country: USA |
|
| Participants | 93 infants randomised. 11 post randomisation exclusions. 82 infants analysed (all 93 infants included in analysis of stratified groups) Inclusion: infants AGA with birth weight 750 to 1499 grams, born between 27 to 34 weeks' gestation, had no major congenital malformations and stable to start feeds on day 2 or 3 of life |
|
| Interventions |
Group A (45 infants randomised): continuous feeds administered over 3 hours, every 3 hours by indwelling nasogastric tube Group B (48 infants randomised): intermittent bolus feeds every 3 hours over 15 to 30 minutes by indwelling nasogastric tube Feeding protocol for infants. Criteria to define feeding intolerance predetermined (gastric residual volume ≥ 2 hour feed for continuous or ≥ 2 mL bolus feeds) Feeds: water, initially diluted preterm infant formula Timing of feeds
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes | Clarification of data for head circumference requested (data printed in the article appear to be significant but was reported as insignificant ‐ there may be a typographical error in data). Data on complete study sample not (quote:) “intent‐to‐treat”. Intention‐to‐treat analysis by weight groups Sample size calculation based on ≥ 10% increase rate of weight gain in continuous group Full feeds not defined Criteria for discharge was not provided Initial feed of water given for different durations (2 hours in continuous group vs 6 hours in bolus group) Nipple feeding 34 weeks or 1500 grams Study dates: January 1990 and December 1993 Funding sources: (quote:) “Supported by the Maternal and Child Health Bureau/Crippled Children's Services Research of the Department of Health and Human Services” Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were randomly assigned by means of sealed opaque envelopes; method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible and knowledge of treatment allocation may influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential attrition Excluded from analysis: Continuous group: 3/45 (2 transferred to another hospital for patent ductus arteriosus ligation, 1 protocol violation) Intermittent group: 8/48 (3 feeding tolerance, 1 gastric perforation, 1 suspected galactosaemia, 1 transferred to another hospital for patent ductus arteriosus ligation, 2 protocol violations) |
| Selective reporting (reporting bias) | Low risk | No published protocol, but outcomes seem to have been reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Toce 1987.
| Study characteristics | ||
| Methods |
Study design: quasi‐experimental. Alternate assignment within 16 groups Stratified
Setting: St Mary's Health Center Country: USA |
|
| Participants | 83 infants
(obtained consent). 30 excluded (completed less than 7 days). 53 analysed Inclusion Preterm infants ≤ 1500 grams, no major congenital anomalies, no longer ventilated, and ready for enteral nutrition |
|
| Interventions | Continuous feeds delivered by nasogastric feeding tube by infusion pump. Intermittent feeds every 3 hours by gravity by nasogastric feeding tube which was removed after each feeding. Feeding protocol for infants. Predetermined criteria to manage feeding intolerance (feeds held > 16 hours). Energy intake constant between groups Feeds: sterile water, initially diluted formula Timing of feeds
|
|
| Outcomes |
|
|
| Notes | Subjective eligibility criteria, no sample size calculation, and not intention‐to‐treat Definition of feeding intolerance not described Significant differences in demographic factors between groups: low 1‐minute Apgar scores in the Continuous group, and increase frequency of human milk feeding in the Intermittent bolus gavage feeding method Awaiting subgroup data Study dates: not reported Funding sources: (quote:) “This investigation was supported by a grant from Ross Laboratories, Columbus, Ohio” Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Feeding method was assigned alternately within 16 groups” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Feeding assignment could not be predicted by the caretakers” No details about methods used to achieve allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible. Knowledge of treatment allocation could influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/46 and 14/37 did not complete at least 7 days and were not included in the analysis No differential attrition but almost half of infants randomised were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | No published protocol but most important outcomes are reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
AGA: appropriate for gestational age; BW: birth weight; CI: continuous infusion; CNG: continuous nasogastric gavage; EBM: expressed breast milk;GI: gastrointestinal; IBG: Intermittent bolus by gravity; IBI: Intermittent bolus by infusion; IUGR:intrauterine growth restricted; NEC: necrotising enterocolitis; NICU: neonatal intensive care unit;NPO: nil per os (meaning: nothing by mouth);PDA: patent ductus arteriosus; RCT: randomised controlled trial; SGA: small for gestational age; vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baker 1997 | Not a randomised study |
| Berseth 1992 | Not a randomised study |
| Bozzetti 2012 | Wrong study design |
| Bozzetti 2016 | Wrong study design |
| Cordero Gonzalez 2020 | Wrong intervention |
| IRCT201408205168N7 | Wrong route of administration |
| Jajoo 2013 | Wrong intervention |
| Kempley 2014 | Wrong intervention |
| Nangia 2015 | Wrong intervention |
| NCT01341236 | Wrong study design |
| NCT02915549 | Wrong intervention |
| Ng 2016 | Wrong study design |
| Richmond 2017 | Wrong study design |
| Sokou 2019 | Wrong study design |
| Xu 2014 | Wrong intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Corbin 2011.
| Methods | Single centre, randomised controlled trial Setting: not reported |
| Participants | Quote: “Preterm neonates less than 32 weeks postmenstrual age and birth weight between 500 and 1500 grams” No inclusion or exclusion criteria reported |
| Interventions | Group 1 (“bolus”): infants received intermittent bolus feedings given over no more than 30 minutes. Group 2 (“drip”): infants received intermittent slow infusion feedings over 120 minutes. |
| Outcomes |
Primary outcome
|
| Notes | November 2020: SP contacted author for further information; awaiting reply |
Differences between protocol and review
2020
We made the following changes to the published protocol (Premji 1999).
We updated the methods for the assessment of the certainty of evidence and risk of bias.
-
We included text in these sections:
unit of analysis issues;
assessment of reporting biases;
sensitivity analysis;
summary of main results;
overall completeness and applicability of evidence;
quality of the evidence;
potential biases in the review process; and
agreements and disagreements with other studies or reviews;
We included a PRISMA figure.
We amended the inclusion criteria to clarify that studies with a mix of nasogastric and orogastric feeding were eligible. We deemed it appropriate to include these studies because both methods of feeding are used routinely for feeding preterm infants.
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. RCTs and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see How CENTRAL is created). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL (Ovelman 2020).
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs on the following platforms: ClinicalTrials.gov or from the World Health Organization’s International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see How CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN Registry (at www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
For the 2020 update, we ran searches in the following databases: CENTRAL via CRS Web, MEDLINE via Ovid, and CINAHL via EBSCOhost. The search strategies are available in Appendix 1. The previous search methods are available in Appendix 2. We used Cochrane’s Screen4Me workflow to help assess the search results.
We redefined our key primary outcomes and prioritising, based on their clinical significance and the ability to objectively measure them. We have used GRADE to assess the certainty of evidence for the seven primary outcomes.
Previously our title was 'Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams'. It is now 'Continuous nasogastric milk feeding versus intermittent bolus milk feeding for preterm infants less than 1500 grams'.
Contributions of authors
SS Premji (SSP) and L Chessell (LC): wrote protocol, searched for trials, selected studies, extracted data, assessed risk of bias, wrote review.
SSP: input data into Review Manager 5, performed data analyses, assessed certainty of evidence.
LC: corresponded with authors, double‐checked data entry.
F Stewart (FS): screened search results, extracted data, assessed risk of bias, assessed certainty of evidence, entered data, drafted manuscript.
Sources of support
Internal sources
-
Hamilton Health Sciences Corporation Foundation, Canada
Funding amount: $2000
External sources
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute for Health Research, UK
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
Declarations of interest
SSP declares that Hamilton Health Sciences Foundation funded the systematic review.
LC retired as a Registered Dietitian in the neonatal intensive care unit (NICU) at McMaster Children's Hospital in July 2020.
FS has no interest to declare.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (Sources of support).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akintorin 1997 {published data only}
- Akintorin SM, Kamat M, Pildes RS, Kling P, Andes S, Hill J, et al. A prospective randomized trial of feeding methods in very low birthweight infants. Pediatrics 1997;100(4):E4. [DOI: 10.1542/peds.100.4.e4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dollberg 2000 {published and unpublished data}
- Dollberg S, Kuint J, Mazkereth R, Mimouni FB. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. Journal of the American College of Nutrition 2000;19(6):797-800. [DOI: 10.1080/07315724.2000.10718080] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dsilna 2005 {published and unpublished data}
- Dsilna A, Christensson K, Alfredsson L, Lagercrantz H, Blennow M. Continuous feeding promotes gastrointestinal tolerance and growth in very low birthweight infants. Journal of Pediatrics 2005;147(1):43-9. [DOI: 10.1016/j.jpeds.2005.03.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dsilna A, Christensson K, Gustafsson AS, Lagercrantz H, Alfredsson L. Behavioural stress is affected by the mode of tube feeding in very low birthweight infants. Clinical Journal of Pain 2008;24(5):447-55. [DOI: 10.1097/AJP.0b013e3181633fd6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Macdonald 1992 {published and unpublished data}
- Macdonald PD, Skeoch CH, Carse H, Dryburgh F, Alroomi LG, Galea P, et al. Randomised trial of continuous nasogastric, bolus nasogastric, and transpyloric feeding in infants of birthweight under 1400 g. Archives of Disease in Childhood 1992;67(4 Spec No):429-31. [DOI: 10.1136/adc.67.4_spec_no.429] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neelam 2018 {published data only}
- CTRI/2017/06/008792. Study to compare continuous feeding with bolus feeding in preterm infants less than 32 weeks and less than 1250 g. http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=12803&EncHid=&modid=&compid=%27,%2712803det%27 (first received 8 June 2017).
- Neelam K, Vijay K, Pankaj G, Anup T. Comparison of continuous versus intermittent bolus feeding in preterm infants ≤ 32 weeks and ≤ 1250 g. Journal of Pediatrics and Child Health 2018;54(Supplement 1):37-8. [Google Scholar]
- Thakur A, Kumar V, Kler N, Garg P, Modi M, Soni A, et al. Comparison of continuous versus intermittent bolus feeding in preterm infants ≤ 32 weeks and ≤ 1250 g. Journal of Pediatric Gastroenterology and Nutrition 2018;66(Supplement 2):1103. [Google Scholar]
Rövekamp‐Abels 2015 {published data only}
- NL871 (NTR885). The effect of intermittent bolus nasogastric milk feeding versus semi-continuous milk feeding in preterm infants on TOLerance. trialregister.nl/trial/871 (first received 8 February 2007).
- Rövekamp-Abels LW, Hogewind-Schoonenboom JE, Wijs-Meijler DP, Maduro MD, Jansen-van der Weide MC, Van Goudoever JB, et al. Intermittent bolus or semi-continuous feeding for preterm infants? A randomised controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2015;61(6):659-64. [DOI: 10.1097/MPG.0000000000000888] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schanler 1999 {published and unpublished data}
- Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics 1999;103(2):434-9. [DOI: 10.1542/peds.103.2.434] [DOI: ] [DOI] [PubMed] [Google Scholar]
- Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified milk versus preterm formula. Pediatrics 1999;103(6 Pt 1):1150-7. [DOI: 10.1542/peds.103.6.1150] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatric Research 1998;44(4):519-23. [DOI: 10.1203/00006450-199810000-00009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Silvestre 1996 {published data only}
- Silvestre MA, Morbach CA, Brans YW, Shankaran S. A prospective randomized trial comparing continuous versus intermittent feeding method in very low birthweight neonates. Journal of Pediatrics 1996;128(6):748-52. [DOI: 10.1016/s0022-3476(96)70324-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Toce 1987 {published data only}
- Toce SS, Keenan WJ, Homan SM. Enteral feeding in very-low-birth-weight infants. A comparison of two nasogastric methods. American Journal of Diseases of Children 1987;141(4):439-44. [DOI: 10.1001/archpedi.1987.04460040097025] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baker 1997 {published data only}
- Baker JH, Berseth CL. Duodenal motor responses in preterm infants fed formula with varying concentrations and rates of infusion. Pediatric Research 1997;42(5):618-22. [DOI: 10.1203/00006450-199711000-00012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berseth 1992 {published data only}
- Berseth CL, Baker J, DeVille K, Jadcherla S. Rate of feeding and nutrient concentration affect preterm small intestinal motor responses to orogastric feedings. Gastroenterology 1992;103:1385. [DOI: 10.1016/0016-5085(92)91589-V] [DOI] [Google Scholar]
Bozzetti 2012 {published data only}
- Bozzetti V, Paterlini G, Meroni V, DeLorenzo P, Gazzolo D, Van Bel F, et al. Evaluation of splanchnic oximetry, Doppler flow velocimetry in the superior mesenteric artery and feeding tolerance in very low birthweight IUGR and non-IUGR infants receiving bolus versus continuous enteral nutrition. BMC Pediatrics 2012;12:106. [DOI: 10.1186/1471-2431-12-106] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bozzetti 2016 {published data only}
- Bozzetti V, Paterlini G, De Lorenzo P, Gazzolo D, Valsecchi MG, Tagliabue PE. Impact of continuous vs bolus feeding on splanchnic perfusion in very low birth weight infants: a randomized trial. Journal of Pediatrics 2016;176:86-92.e2. [DOI: 10.1016/j.jpeds.2016.05.031] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cordero Gonzalez 2020 {published data only}
- Cordero González G, Valdés Vázquez NO, Izaguirre Alcántara DD, Michel Macías C, Carrera Muiños S, Morales Barquet DA, et al. Management of abdominal distension in the preterm infant with noninvasive ventilation: comparison of cenit versus 2x1 technique for the utilization of feeding tube. Journal of Neonatal-Perinatal Medicine 2020;13(3):367-72. [DOI: 10.3233/NPM-190301] [PMID: ] [DOI] [PubMed] [Google Scholar]
IRCT201408205168N7 {published data only}
- IRCT201408205168N7. The comparison early outcomes intermittent gavage in preterm infants: randomized clinical trial. en.irct.ir/trial/5520 (first received 15 March 2015).
Jajoo 2013 {published data only}
- Jajoo M, Arora N, Dabas V, Talukdar B, Kumari S. Early total versus gradual advancement of enteral nutrition in healthy preterm very low birth weight neonates in a tertiary care nursery: a randomised controlled trial. In: Pediatric Academic Societies Annual Meeting; 2013 May 4 -7; Washington (DC), USA. 2013.
Kempley 2014 {published data only}
- Kempley S, Gupta N, Linsell L, Dorling J, McCormick K, Mannix P, et al, ADEPT Trial Collaborative Group. Feeding infants below 29 weeks' gestation with abnormal antenatal Doppler: analysis from a randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(1):F6-11. [DOI: 10.1136/archdischild-2013-304393] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nangia 2015 {published data only}
- Nangia S, Vadivel V, Saili A, Thukral A. Comparison of early total enteral feeding (ETEF) vs. conventional enteral feeding (CEF) in stable very low birthweight (VLBW) infants - a randomized control trial. In: Pediatric Academic Societies (PAS) Annual Meeting; 2015 April 25 - 28; San Diego (CA), USA. 2015.
NCT01341236 {published data only}
- NCT01341236. Near infrared spectroscopy (NIRS) and superior mesenteric artery (SMA) Doppler patterns as predictor of feeding tolerance in very low birth weight (VLBW) intra uterine growth restricted (IUGR) and non IUGR Infants. clinicaltrials.gov/ct2/show/NCT01341236 (first received 25 April 2011).
NCT02915549 {published data only}
- NCT02915549. Early progressive feeding in human-milk fed extremely preterm infants: a randomized trial. clinicaltrials.gov/ct2/show/NCT02915549 (first received 27 February 2016).
Ng 2016 {published data only}
- Ng E, Schurr P, Reilly M, Dunn M, Beck J. Impact of feeding method on diaphragm electrical activity and central apnea in preterm infants (FEAdi study). Early Human Development 2016;101:33-7. [DOI: 10.1016/j.earlhumdev.2016.05.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Richmond 2017 {published data only}
- Richmond C, Birch P. Feeding interventions because of respiratory events in preterm infants a randomised triple crossover study. Journal of Paediatrics and Child Health. Special Issue: Abstracts for the RACP Congress 2017. Bringing Specialists Together. Sharing Knowledge. Building Skills. 8–10 May 2017, Melbourne Convention and Exhibition Centre 2017;53(S3):4-5. [DOI: 10.1111/jpc.13572_4] [DOI] [Google Scholar]
Sokou 2019 {published data only}
- Sokou R, Grivea G, Konstantinidi A, Antonogeorgos G, Kokori F, Varhalama E, et al. Feeding mode and gastric emptying time in neonates with gestational age. Journal of Perinatal Medicine 2019;47:eA298. [CENTRAL: CN-02074445] [DOI: 10.1515/jpm-2019-2501] [EMBASE: 630689439] [DOI] [Google Scholar]
Xu 2014 {published data only}
- Xu YM, Zhu XP, Xiao Z, Yu L, Zhao X25551971. Influence of aggressive nutritional support on growth and development of very low birthweight infants. Clinical and Experimental Obstetrics & Gynecology 2014;41(6):717-22. [PMID: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Corbin 2011 {published data only}
- Corbin LL, Smith S. Bolus versus longer infusion of enteral feedings for preterm neonates. Journal of Investigative Medicine 2011;59(1):168-9. [CENTRAL: CN-01738982] [DOI: 10.231/JIM.0b013e31820501bd] [EMBASE: 70524586] [DOI] [Google Scholar]
Additional references
Aynsley‐Green 1982
- Aynsley-Green A, Adrian TE, Bloom SR. Feeding and the development of enteroinsular hormone secretion in the preterm infant: effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatrica Scandinavica 1982;71(3):379-83. [DOI: 10.1111/j.1651-2227.1982.tb09438.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aynsley‐Green 1989
- Aynsley-Green A. New insights into the nutritional management of newborn infants derived from studies of metabolic and endocrine inter-relations during the adaptation of postnatal life. Proceedings of the Nutrition Society 1989;48(2):283-92. [DOI: 10.1079/pns19890040] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aynsley‐Green 1990
- Aynsley-Green A, Lucas A, Lawson GR, Bloom SR. Gut hormones and regulatory peptides in relation to enteral feeding, gastroenteritis, and necrotizing enterocolitis in infancy. Journal of Pediatrics 1990;117(1 Pt 2):S24-32. [DOI: 10.1016/s0022-3476(05)81127-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertoncelli 2012
- Bertoncelli N, Cuomo G, Cattani S, Mazzi C, Pugliese M, Coccolini E, et al. Oral feeding competencies of healthy preterm infants: a review. International Journal of Pediatrics 2012;2012:896257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corvaglia 2014
- Corvaglia L, Martini S, Aceti A, Capretti MG, Galletti S, Faldella G. Cardiorespirtory events with bolus versus continuous enteral feeding in healthy preterm infants. Journal of Pediatrics 2014;165:1255-7. [DOI] [PubMed] [Google Scholar]
Davidson 2020
- Davidson J, Elabiad MT. A simple intervention to decrease nutrient losses in continuous feeds with human milk. Journal of Pediatric Gastroenterology and Nutrition 2020;70(4):e81-3. [DOI: 10.1097/MPG.0000000000002609] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed January 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grant 1991
- Grant J, Denne SC. Effect of intermittent versus continuous enteral feeding on energy expenditure in premature infants. Journal of Pediatrics 1991;118(6):928-32. [DOI: 10.1016/s0022-3476(05)82213-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Krishnan 1981
- Krishnan V, Satish M. Continuous (C) vs. intermittent (I) nasogastric (N/G) feeding in very low birthweight (VLBW) infants. Pediatric Research 1981;15:537. [DOI: 10.1203/00006450-198104001-00596] [DOI] [Google Scholar]
Li 2014
- Li YF, Lin HC, Torrazza RM, Parker L, Talaga E, Neu J. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatrics and Neonatology 2014;55(5):335-40. [DOI: 10.1016/j.pedneo.2014.02.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lucas 1986
- Lucas A, Bloom SR, Aynsley-Green A. Gut hormones and "minimal enteral feeding". Acta Paediatrica Scandinavica 1986;75(5):719-23. [DOI: 10.1111/j.1651-2227.1986.tb10280.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newell 1988
- Newell SJ, Sarkar PK, Durbin GM, Booth IW, McNeish AS. Maturation of the lower esophageal sphincter in the preterm baby. Gut 1988;29(2):167-72. [DOI: 10.1136/gut.29.2.167] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane centralised search service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;S0895-4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: 33476769] [DOI] [PubMed] [Google Scholar]
Ovelman 2020
- Ovelman C, Eckert C, Friesen C. Validating Cochrane Neonatal’s standard search databases: is it okay to stop searching Embase? In: Advances in Evidence Synthesis: special issue. Cochrane Database of Systematic Reviews 2020; (9 Suppl 1): [320]. Available from: cochranelibrary.com/cdsr/doi/10.1002/14651858.CD202001/full.
Raudonis 1995
- Raudonis BM, Talbot LA. Use of critical thinking in research: the research critique. In: Talbot LA, editors(s). Principles and Practice of Nursing Research. St. Louis (MO): Mosby, 1995:513-34. [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rogers 2010
- Rogers SP, Hicks PD, Hamzo M, Veit LE, Abrams SA. Continuous feedings of fortified human milk lead to nutrient losses of fat, calcium and phosphorous. Nutrients 2010;2(3):230-40. [DOI: 10.3390/nu2030240] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rysavy 2020
- Rysavy MA, Watkins PL, Colaizy TT, Das A. Is routine evaluation of gastric residuals for premature infants safe or effective? Journal of Perinatology 2020;40(3):540-3. [DOI: 10.1038/s41372-019-0582-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Thomas 2020
- Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020;S0895-4356(20):31172-0. [DOI: 10.1016/j.jclinepi.2020.11.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Urrutia 1983
- Urrutia J, Poole E. Continuous nasogastric versus intermittent gavage feedings in very low birthweight infants. Pediatric Research 1983;17:203A. [Google Scholar]
Valman 1972
- Valman HB, Heath CD, Brown RJ. Continuous intragastric milk feeds in infants of low birthweight. British Medical Journal 1972;3(5826):547-50. [DOI: 10.1136/bmj.3.5826.547] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI: 10.1186/1471-2288-14-135] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020
- Wang Y, Zhu W, Luo B-R. Continuous feeding versus intermittent bolus feeding for premature infants with low birth weight: a meta-analysis of randomized controlled trials. European Journal of Clinical Nutrition 2020;74(5):775-83. [DOI: 10.1038/s41430-019-0522-x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Premji 1999
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 1999, Issue 4. Art. No: CD001819. [DOI: 10.1002/14651858.CD001819] [DOI] [PubMed] [Google Scholar]
Premji 2001
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No: CD001819. [DOI: 10.1002/14651858.CD001819] [DOI] [PubMed] [Google Scholar]
Premji 2003
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD001819. [DOI: 10.1002/14651858.CD001819] [DOI] [PubMed] [Google Scholar]
Premji 2004
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD001819. [DOI: 10.1002/14651858.CD001819] [DOI] [PubMed] [Google Scholar]
Premji 2011
- Premji SS, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD001819. [DOI: 10.1002/14651858.CD001819.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


