Abstract
Importance:
Osteoarthritis (OA) affects more than 240 million people worldwide and is the most frequent reason for activity limitation in adults. This review focuses on hip and knee OA.
Observations:
OA is the most common type of arthritis. It can involve almost any joint but typically affects the hands, knees, hips and feet. It is characterized by pathologic changes in cartilage, bone, synovium, ligament, muscle, and periarticular fat, leading to joint dysfunction, pain, stiffness, functional limitation, and loss of valued activities. Risk factors include age, female sex, obesity, genetics and major joint injury. Persons with OA have more comorbidities and are more sedentary than those without OA. The reduced physical activity leads to a 20% higher age-adjusted mortality. Several physical examination findings are useful diagnostically, including bony enlargement in knee OA and pain elicited with internal hip rotation in hip OA. Radiographic indicators include marginal osteophytes and joint space narrowing. The cornerstones of OA management are prescribed exercises, weight loss if appropriate, and education—complemented by topical or oral NSAIDs, in those without contraindications. Intraarticular steroid injections provide short-term pain relief and duloxetine has demonstrated efficacy. Opiates should be avoided. Clinical trials have shown promising results for compounds that arrest structural progression (e.g. cathepsin K inhibitors, Wnt inhibitors, anabolic growth factors), or reduce OA pain (e.g. nerve growth factor inhibitors). Persons with advanced symptoms and structural damage are candidates for total joint replacement. Racial and ethnic disparities persist in the utilization and outcomes of joint replacement.
Conclusions and Relevance:
Hip and knee OA are highly prevalent and disabling. Education, exercise and weight loss are cornerstones of management, complemented by NSAIDS (in those who are candidates), corticosteroid injections, and several adjunctive medications. In persons with advanced symptoms and structural damage, total joint replacement effectively relieves pain.
Introduction:
Long characterized as a ‘wear and tear’ disorder, osteoarthritis (OA) is now understood to have a complex pathophysiology affecting multiple joints and joint structures, as captured by the Osteoarthritis Research Society International definition of OA: “The disease manifests first as a molecular derangement (abnormal joint tissue metabolism) followed by anatomic, and/or physiologic derangements (characterized by cartilage degradation, bone remodeling, osteophyte formation, joint inflammation and loss of normal joint function), that can culminate in illness.”1
Worldwide, an estimated 240 million persons have symptomatic, activity-limiting OA.2,3 The knee and hip are two commonly affected joints and are the focus of this review. Nearly 30% of individuals greater than 45 years old have radiographic evidence of knee OA, about half of whom have knee symptoms.4,5 The prevalence of symptomatic, radiographic hip OA is around 10%.6,7
The lifetime risk of symptomatic knee OA is greater in obese persons (BMI ≥ 30 kg/m2) than nonobese persons (19.7% versus 10.9%).8 Prior joint trauma, such as anterior cruciate ligament rupture and ankle fracture, increases risk, accounting for 12% of knee OA cases.9 The prevalence of symptomatic, radiographic knee OA was 11.4% in women and 6.8% in men in one large cohort study4 and 18.7% in women and 13.5% in men in another large cohort study.5 As compared to males with OA, women have more severe radiographic findings and symptoms.10 Older age and female sex are risk factors for hip OA as well as knee OA. In addition, congenital and acquired anatomic abnormalities (e.g. hip dysplasia) are risk factors for hip OA. Regarding race, African Americans and whites have similar prevalence of hip OA (accounting for race, sex and body mass index), while African Americans, especially women, have higher prevalence of knee OA.5,7
OA leads to substantial cost and mortality. Forty-three percent of the 54 million individuals in the US living with arthritis (most of whom have OA) experience arthritis-related limitations in daily activities.11 Wage losses due to OA amount to $65 billion and direct medical costs exceed $100 billion.2,12 Persons with knee OA spend, on average, around $15,000 dollars (discounted) over their lifetimes on direct medical costs of OA.13 OA is commonly associated with comorbidities, which may stem from lack of physical activity, medication toxicity, and the effects of inflammatory cytokines. It has been estimated that 31% of persons with OA have ≥5 comorbid conditions.2 Persons with hip and knee OA have ~20% excess mortality as compared with age-matched controls, due in part to lower levels of physical activity.2
Methods
We searched PubMed for English-language articles on the diagnosis and management of hip and knee OA, using the search terms osteoarthritis and treatment; osteoarthritis and epidemiology; osteoarthritis and diagnosis or imaging; osteoarthritis and disability or comorbidity. We reviewed these publications and relevant references in these papers. We based our conclusions on treatment efficacy primarily using the rigorous systematic literature syntheses and metaanalyses that support the Osteoarthritis Research Society International 2018 OA treatment guidelines.14 The efficacy parameter in these studies is the standardized mean difference (SMD), the mean difference in improvement between active treatment and placebo, divided by the standard deviation of the difference. For questions not addressed by the metaanalyses, we provide results of pivotal trials.
Pathophysiology
OA arises from complex biological processes that include cartilage, bone, synovium, ligaments, periarticular fat, meniscus, and muscle.15 The classic features of OA noted on radiographs include joint space narrowing due to loss of articular cartilage and meniscus, and bony changes including sclerosis of subchondral bone and osteophytes (Figure 1A). The effects of OA on cartilage, meniscus, syovium, subchondral bone and other structures can be appreciated on magnetic resonance imaging (Figure 1B).
Figure 1A:
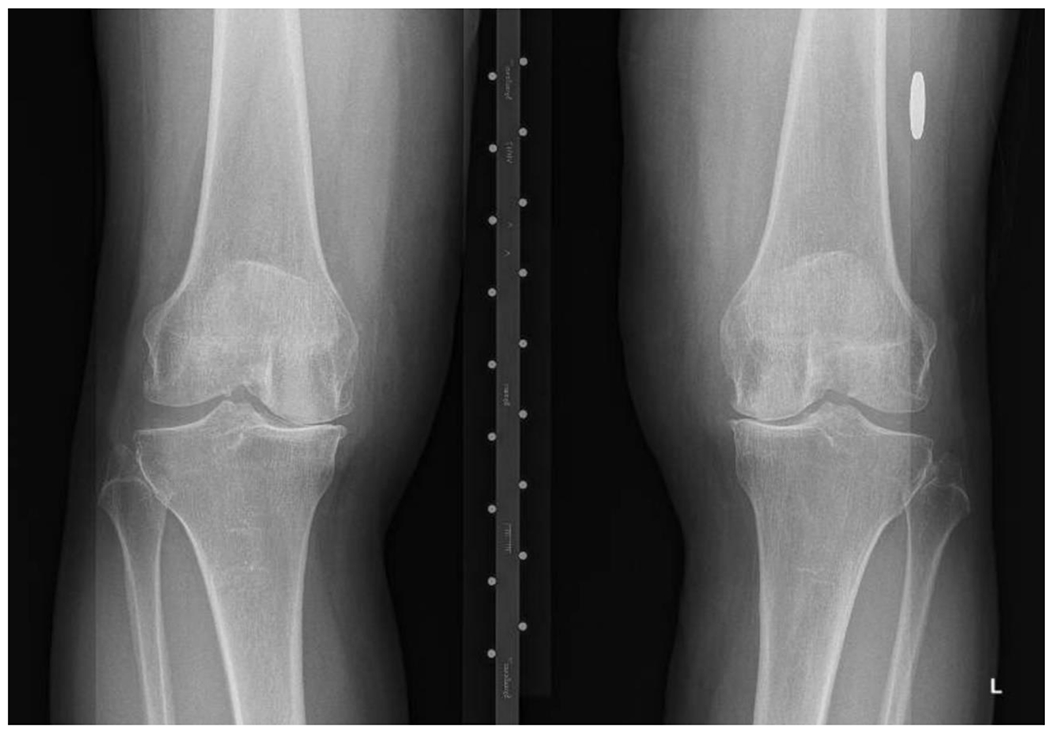
Bilateral varus deformity with medial joint space narrowing (nearly bone on bone) and osteophyte formation. Thin arrows show joint space narrowing and thick arrows medial marginal osteophytes.
Figure 1B:
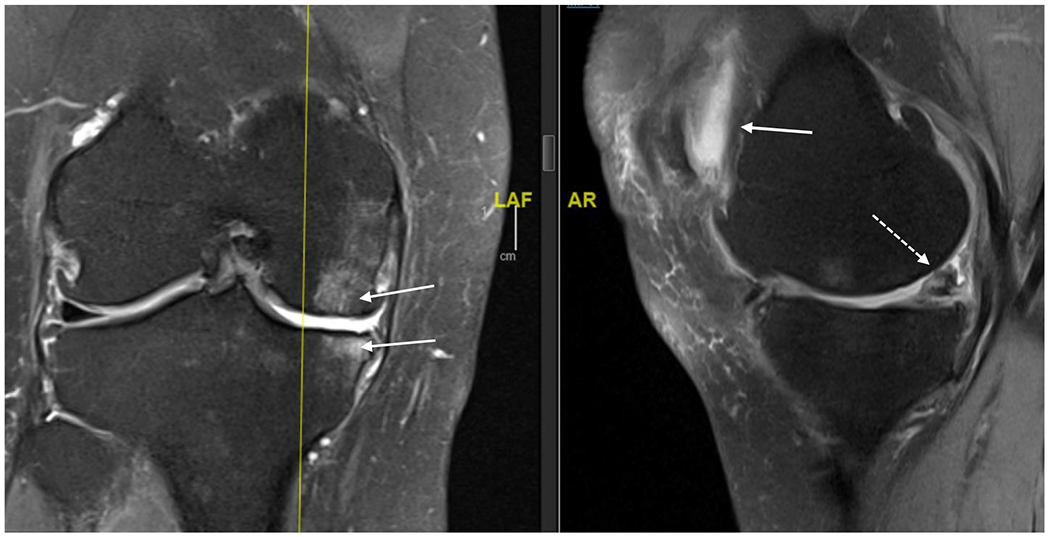
MRI (proton density, fat saturated) of right knee of 63 year old female. Coronal view on left and saggital view on right. Bone marrow lesions are identified with thin, solid white arrows on the coronal view; meniscal damage and cartilage damage are identified with dashed arrow on saggital view and retropatellar effusion as solid arrow on saggital view.
The biomechanical environment influences the disease process. Varus alignment of the lower extremities (“bowlegged”) shifts load medially, increasing risk of medial compartment knee OA, while valgus alignment (“knocked knees”) shifts load laterally leading to lateral compartment OA. These abnormalities in alignment are risk factors for OA incidence and, more importantly, for OA progression.16,17 Excessive loading of bone may result in bone marrow lesions, seen on magnetic resonance imaging (Figure 1B).18 Histologically, bone marrow lesions contain microfractures with bone fragments, necrosis, fibrosis and abnormal adipocytes suggestive of focal areas of damage and remodeling due to abnormal loading.19
Synovitis is commonly noted in OA joints.20 The synovitis seen in OA has a predominance of macrophages while the synovitis of rheumatoid arthritis (RA) has a predominance of T cells.21 This reflects activation of the innate immune response in OA joints, likely due to damage of joint tissues resulting in a chronic wound type of environment.22 OA synovitis is more focal than in RA; in the knee, it is commonly found in the suprapatellar pouch.23 Synovitis plays a prominent role in joint destruction in RA, while its role in the progression of OA may be limited to a subset of individuals.
Many proinflammatory cytokines and growth factors have been identified in the OA joint (Figure 2.) Cytokines present at relatively high levels in OA synovial fluid include IL-6, MCP-1, VEGF, IP-10 and MIG.24 The pro-inflammatory factors are responsible for the progressive destruction and remodeling of the joint through the stimulation of matrix-degrading enzymes, including the matrix metalloproteinases.15,25 The growth factors that normally would stimulate matrix production and repair of joint tissues are overwhelmed by pro-inflammatory mediators. Certain growth factors including TGFβ and BMP-2 promote osteophyte formation and contribute to subchondral sclerosis. The pro-inflammatory mediators and anabolic factors are produced locally by the cells within the affected tissues including the articular chondrocytes, synovial fibroblasts and immune cells in the synovium, inflammatory cells in periarticular fat, as well as cells in bone, including osteoblasts, osteocytes, osteoclasts and bone marrow mesenchymal stem cells (Figure 3).15,26 The cytokines are potential targets for disease modification in OA; however, currently it is not clear which cytokines are primary drivers of joint destruction, and which are involved secondarily.
Figure 2.
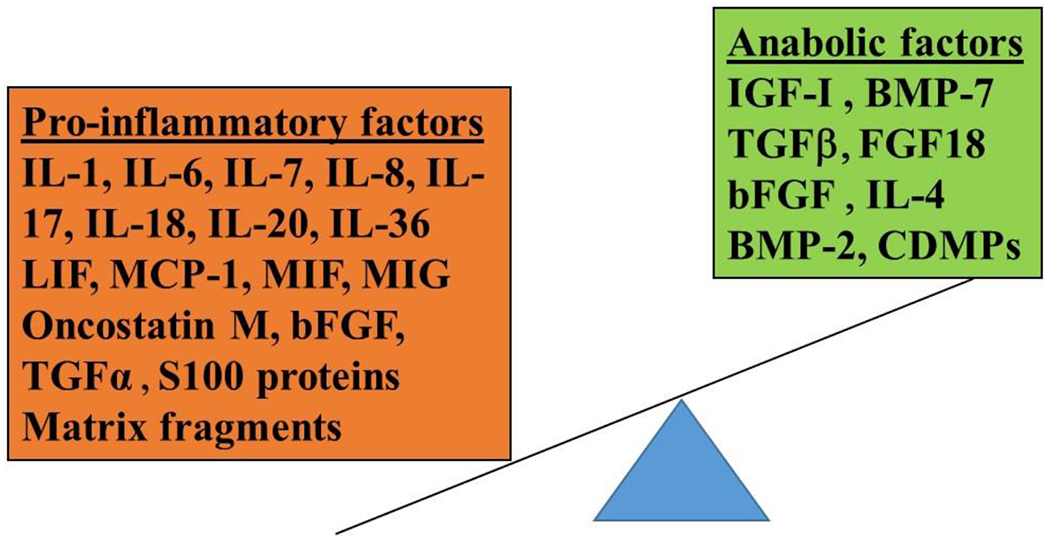
Molecular Mediators of Osteoarthritis. A number of pro-inflammatory factors and anabolic factors are present in joint tissues and in the synovial fluid. Pro-inflammatory mediators contribute to joint tissue destruction in large part by stimulating production of matrix degrading enzymes, including the matrix metalloproteinases, but also through inhibition of matrix synthesis. The anabolic factors stimulate matrix production and, in some cases, also inhibit the catabolic signaling stimulated by pro-inflammatory mediators. Some factors including TGFβ and bFGF are capable of initiating either catabolic or anabolic activity depending on cell type and specific receptors expressed. TGFβ and BMP-2 can also stimulate osteophyte formation. The overall activity in the OA joint is tipped in favor of the pro-inflammatory side. (IL, interleukin; LIF, leukemia inhibitory factor; MCP, monocyte chemoattractant protein, MIF, macrophage migration inhibitory factor; MIG, monokine Induced By Interferon-Gamma; bFGF, basic fibroblast growth factor; TGF, transforming growth factor; IGF, insulin-like growth factor, BMP, bone morphogenetic protein; CDMP; cartilage-derived morphogenetic protein.)
Figure 3.
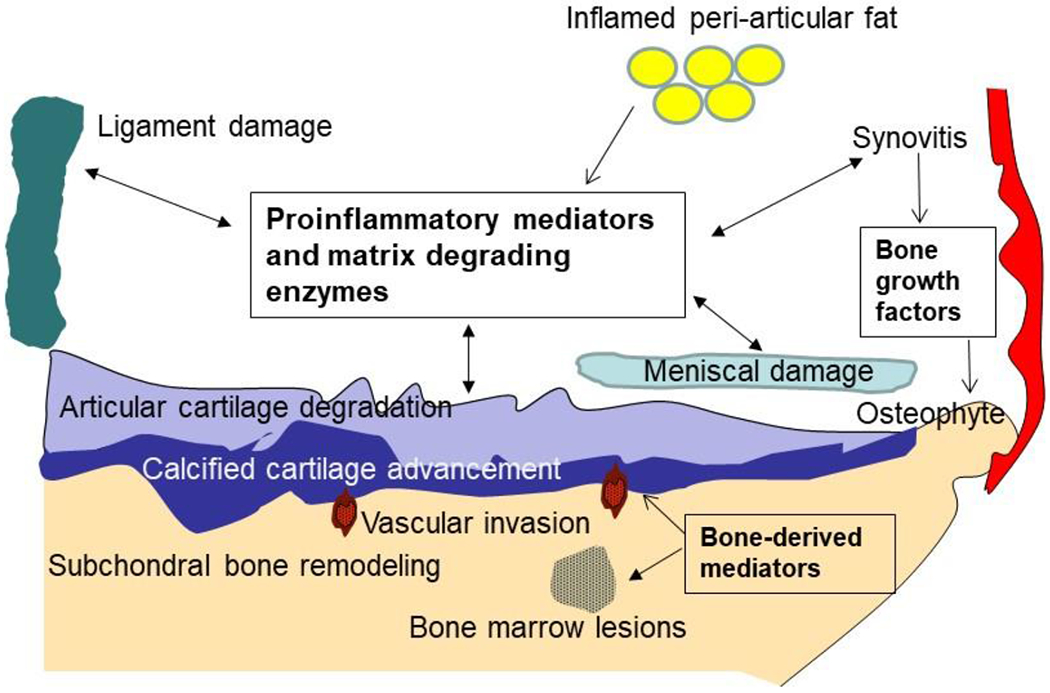
Joint Tissue Involvement in Osteoarthritis. OA can involve all joint structures at some point in the disease process. Although articular cartilage degradation and loss is a central feature, changes in the neighboring bone accompany the cartilage damage. These include subchondral bone remodeling resulting in increased thickness, osteophytes, bone marrow lesions and vascular invasion into the overlying cartilage. Inflammatory cells, primarily macrophages, are present in the synovium and can also be noted in peri-articular fat. Meniscal and ligament damage is often found as well. All of these tissues are capable of producing a host of pro-inflammatory factors and matrix degrading enzymes and thus contribute to the progressive remodeling and destruction of the joint.
Clinical presentation
Patients with OA typically present with pain and stiffness in the affected joint(s). Stiffness is worse in the morning or on arising after prolonged sitting, and improves within 30 minutes. Pain is use-related early in the course, but can become less predictable over time. While sometimes viewed as a disease of inexorable worsening, natural history studies show that most patients report little change in symptoms over six years of observation.27
Assessment and Diagnosis
The clinician must distinguish symptomatic OA from other entities that can cause hip or knee pain, including inflammatory (e.g. rheumatoid and psoriatic) arthritis, infectious and crystalline (e.g. gout, pseudogout) arthritis and soft tissue lesions such as bursitis, tendonitis, and meniscal tear. The stiffness in inflammatory arthritis may last over an hour. The pain of infectious arthritis and crystalline arthritis is typically acute. Individuals with retropatellar pain may have patellofemoral OA, which can exist in isolation or in the presence of tibiofemoral OA. Because the patellofemoral joint is loaded when the knee is bent, patellofemoral OA is especially painful when patients ascend and descend stairs and get in and out of cars or a bath.28 The syndrome of patellofemoral pain is common and often arises from malalignment of the patella in the femoral groove ( due for example to asymmetric tension from the lateral and medial quadriceps) rather than from OA.
On physical exam, knee effusions are generally either absent or small and cool in persons with OA. Those with effusions may have popliteal or “Bakers” cysts, which are extensions of the synovial swelling that can be palpated in the posterior aspect of the knee. In contrast, the knee often has warm, easily palpable effusions in inflammatory, infectious and crystalline arthritis. Soft tissue lesions such as anserine bursitis and trochanteric bursitis are extra-articular and do not cause joint effusions; they are identified by local tenderness. Effusions cannot be detected on physical exam of recessed joints such as the hip. Infectious, crystalline and other inflammatory arthritides can be distinguished incisively from OA because the synovial fluid white blood cells exceed 2000 cells/cc in these disorders.
The sensitivities, specificities and likelihood ratios of various elements of the physical examination and radiographic features for hip and knee OA are shown in Table 1. Bony enlargement on physical examination is specific (95%) for knee OA, though somewhat insensitive (55%), while crepitus is sensitive (89%) though somewhat nonspecific (58%).29 Osteophytes on knee radiographs are both sensitive (91%) and fairly specific (83%). The combination of osteophytes AND knee pain has good sensitivity (83%) and specificity (93%), with likelihood ratio of 11.9.29 (The likelihood ratio = sensitivity / (1 – specificity). If the likelihood ratio is > 1, a positive test indicates that the post-test probability of disease is greater than the pre-test probability.
Table 1:
Performance characteristics* of key physical examination and radiographic features of hip and knee OA
| Feature | Sensitivity | Specificity | Likelihood Ratio |
|---|---|---|---|
| Knee | |||
| Bony enlargement | 55% | 95% | 11.0 |
| Crepitus with passive motion | 89% | 58% | 2.1 |
| Osteophytes | 91% | 83% | 5.4 |
| Knee pain PLUS osteophyte | 83% | 93% | 11.9 |
| Hip | |||
| Internal rotation < 15 deg | 66% | 72% | 2.4 |
| Pain with internal rotation | 82% | 39% | 1.3 |
| Decreased hip adduction | 80% | 81% | 4.2 |
| Femoral or acetabular osteophytes | 89% | 90% | 8.9 |
| Superior joint space narrowing | 85% | 66% | 2.5 |
| Hip pain PLUS osteophyte | 89% | 90% | 8.9 |
A recent review provided detailed data on the utility of physical examination maneuvers in the diagnosis of hip OA, and a video demonstration of the hip examination.30,31 Hip internal rotation <15 degrees is moderately sensitive (66%) and specific (72%), as is limited hip adduction (80% sensitive, 81% specific).30,32 Pain with hip internal rotation is more sensitive (82%) but less specific (39%). Osteophytes on radiographs are both sensitive (89%) and specific (90%). The combination of hip pain PLUS an osteophyte is also quite sensitive (89%) and specific (90%).32
These data suggest a presumptive diagnosis of hip or knee OA can be made on the basis of the history and physical exam. Radiographs portray the severity of structural damage and improve specificity when osteophytes or joint space narrowing are present. Pathologic features and symptoms of OA can occur before osteophytes are present on radiographs. Thus, a normal radiograph does not exclude OA. If the clinical presentation is highly suggestive of OA, clinicians should initiate management (detailed below) despite normal radiographs. Knee radiographs should be performed with the patient standing to reveal the extent of joint space narrowing of the tibiofemoral joint. For research purposes, hip and knee radiographs are typically assessed with the Kellgren-Lawrence grading system, with grade 0 representing no pathologic findings; Grade 1 questionable osteophytes; Grade 2 definite osteophytes; Grade 3, definite joint space narrowing; and Grade 4 advanced joint space narrowing.33,34 The radiograph in Figure 1A is Kellgren-Lawrence Grade 3 and nearly K-L 4 because of the advanced medial joint space narrowing is nearly bone-on-bone.
Hip radiographs typically include an anteroposterior view and a lateral view. Weight-bearing is not necessary. The inter- and intra-rater reliabilities of hip radiographs for detecting joint space narrowing are high.35 Hip radiographs involve greater exposure to ionizing radiation than radiographs of the chest or knee.
MRI is seldom indicated in the assessment or management of knee or hip OA. MRI detects changes in cartilage, meniscus (knee), labrum (hip), bone and synovium, providing a fuller picture of pathological involvement (Figure 1B).36 Because of its high sensitivity36, MRI is useful for research studies to identify early OA and document structural changes over time. In clinical care, MRI can be useful if there is suspicion of conditions such as subchondral insufficiency fracture, tumor or infection that would be treated differently and more urgently than OA.
Ultrasound can visualize joint effusion, osteophytes and other features.37 As compared with MRI, ultrasound has sensitivity and specificity exceeding 85% for detecting osteophytes. Ultrasound is not as accurate as MRI in assessing joint space narrowing.38 Because ultrasound is less expensive and more portable than MRI, it is used frequently in Europe and a growing number of US centers in the diagnosis of OA and assessment of progression.
Treatment
Several professional organizations have developed guidelines for OA management (Table 2). The guidelines suggest that patients with OA should be offered a core set of non-pharmacological interventions including education, weight loss (for those who are overweight), and exercises (strengthening, cardiovascular, and/or mind-body exercises such as Yoga or Tai Chi).14,39–44
Table 2:
Summary of osteoarthritis treatment guidelines from major professional societies
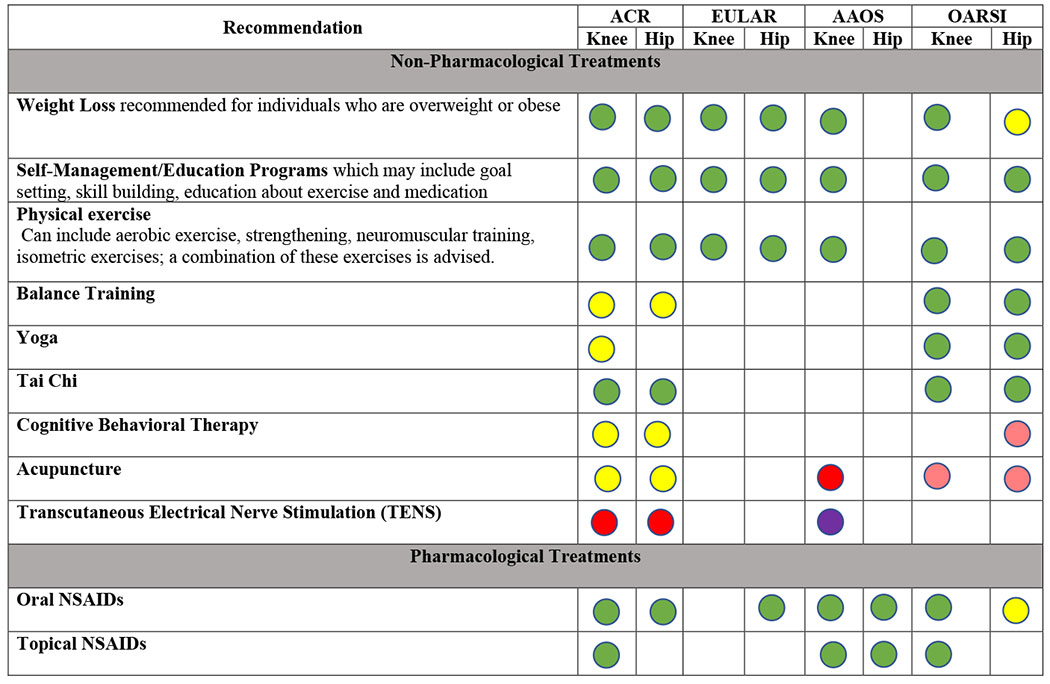 |
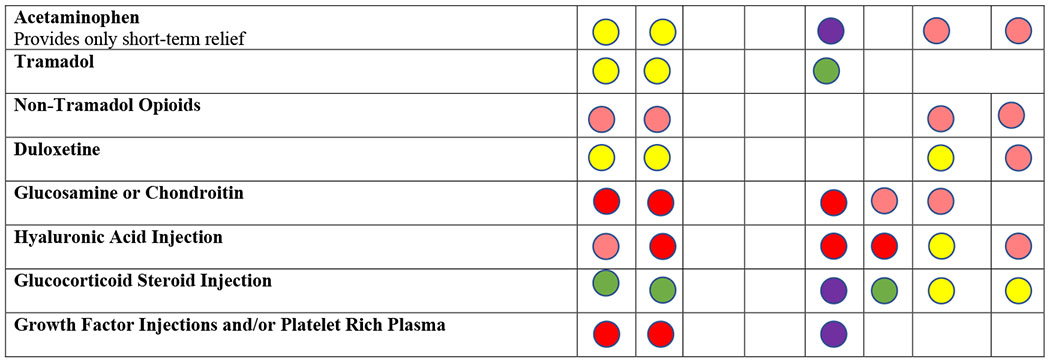 |
Recommendations taken from ACR, EULAR, AAOS, and OARSI Guidelines for the Management of OA.14,43,44,101,102
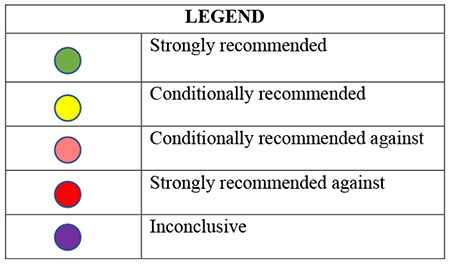
EULAR does not distinguish between strong/conditional recommendations. In this table, any recommendation with a Level of Evidence of 1 (out of 4) and a level of agreement 8.5 (out of 10) or above is considered strongly recommended. AAOS includes 3 levels of evidence: strong, moderate, and limited. In this table, any recommendation that has moderate or limited evidence is considered conditionally recommended.
Structured exercise interventions that typically focus on strengthening of lower extremity muscles offer improvements in pain and functional status (SMD of 0.52 for knee OA and 0.34 for hip OA; Table 3). A randomized controlled trial of a structured walking program showed a reduction in pain scores of 1.38 points (on a 0-10 scale) in the walking group and just 0.1 points in the control group (p=0.003).45 Referral to a physical therapist is appropriate to initiate such a program, or to address lower extremity weakness or limitations in hip or knee range of motion. A combination of diet and exercise can result in substantial weight loss, pain relief, improvement in functional status, and reduction in inflammatory markers, as compared with exercise alone.46
Table 3:
Standardized mean differences in pain score from placebo controlled trials of 4-12 weeks duration
| Knee OA | Hip OA | |||
|---|---|---|---|---|
| SMD | 95% CI | SMD | 95% CI | |
| Structured Exercise Program | 0.52 | 0.37 – 0.68 | 0.34 | 0.19 – 0.49 |
| Mind body programs* | 0.63 | 0.32 – 0.95 | 0.35 | −0.06 – 0.76 |
| Dietary weight management^ | 0.42 | 0.23-0.62 | NT | |
| Acetaminophen | 0.05 | −0.11 – 0.21 | 0.23 | 0.13 – 0.33 |
| Oral NSAID | 0.28 | 0.22 – 0.35 | 0.33 | 0.24 – 0.43 |
| Topical NSAID | 0.20 | 0.11 – 0.29 | NT | |
| Duloxetine | 0.39 | 0.25 – 0.52 | NT | |
| Opioids | 0.20 | 0.05 – 0.35 | 0.21 | 0.10 – 0.32 |
| IA Corticosteroids | 0.41 | 0.21 – 0.61 | 1.65 | 0.16 – 3.47 |
| IA Hyaluronic Acid | 0.34 | 0.26 – 0.42 | 0.18 | −0.13 – 0.50 |
includes Tai-Chi, Yoga
dieteary weght management + exercise vs. exercise alone
From OARSI treatment guidelines Appendix.14
NT= no trials
While trials of lateral wedge shoe inserts have not been efficacious, a recent trial of an individualized external orthotic (attached below the sole) was associated with greater improvement in pain and functional status than a control orthotic.47 This observation should be replicated before being advanced to routine use.
Non-steroidal anti-inflammatory drugs (NSAIDs) are first line pharmacologic treatment for OA. In numerous placebo-controlled trials, NSAIDs have resulted in greater pain relief than placebo, with standardized mean differences in pain and function scores of ~ 0.33 standard deviations, reflecting a moderate effect (Table 3). Many NSAIDs are available over the counter. Topical NSAIDs generally have less gastrointestinal toxicity than oral NSAIDs,14,44 but are less useful in hip OA because the joint is recessed.
NSAIDs have important toxicities, including gastrointestinal irritation and ulceration, bleeding, and decreased renal blood flow with azotemia. Patients on anticoagulants who wish to take an NSAID should use a COX-2 inhibitor (such as celecoxib), which does not increase bleeding. Those with dyspepsia should use proton pump inhibitors and/or a COX-2 inhibitor. Patients with history of bleeding peptic ulcer are typically not prescribed NSAIDs at all. Risk factors for gastrointestinal bleeding from NSAIDs include older age, medical comorbidities, and concomitant use of corticosteroids and anticoagulants.48 Individuals with cardiovascular or renal disease are at risk of renal toxicity; alternatives to NSAIDs should be discussed. Acetaminophen is less efficacious than NSAIDs in management of knee (SMD 0.05) and hip (SMD 0.23) OA.49–53 It is a reasonable, safe alternative for those intolerant to NSAIDs but should not be used in persons with liver disease or risk factors such as heavy alcohol use. The Medical Letter table published in this issue of JAMA provides rich information on formulations, dosages and costs of many of the pharmacologic agents noted in this review.
Patients unable to take NSAIDs, or who do not respond, can try intra-articular corticosteroid injections, which typically relieve pain for a few weeks.54 They are especially helpful in patients with OA of a single joint that can be injected easily, such as the knee. The hip is generally injected under imaging (fluoroscopy or ultrasound) guidance. Corticosteroid injections have no greater effect on pain than placebo after three months,55 and may be inferior to physical therapy at one year.56 A newer formulation of steroid injection (triamcinolone acetonide extended release) appears to have fewer systemic effects than traditional steroid injections.57 Some studies have suggested that intraarticular steroid injections may have deleterious effects on cartilage55,58; the clinical meaning of these findings is not yet known.
Injection of intra-articular hyaluronic acid (HA) products is another option for patients with persistent pain despite NSAIDs. Guidelines differ regarding recommendations of intraarticular HA (Table 2).14,40–44 While efficacy of HA injections is similar to that of NSAIDs (SMD 0.37, Table 3), the highest quality trials showed weaker effects. Injection of growth factors, such as those found in platelet-rich plasma, and injection of stem cell preparations, are increasing in use. However, these products are non-standardized and studies of these agents are weak.
Osteoarthritis pain may be mediated in part by mechanisms in the central nervous system. Several medications have been used to address pain of central origin. Duloxetine, a serotonin-norepinephrine reuptake inhibitor, has been shown in randomized trials to result in greater pain relief than placebo in persons with knee OA (SMD 0.39).59,60 Gabapentin may have efficacy in knee OA, but evidence is limited.61 Opiate analgesics are used by over 20% of patients with OA, but have limited efficacy for hip and knee OA (SMD ~0.20) and considerable toxicity including constipation, falls, somnolence, respiratory depression and potential for addiction. OA treatment guidelines advise against use of stronger opiates, with conditional recommendation of tramadol, a synthetic opioid agonist that also inhibits reuptake of serotonin and norepinephrine.44
To date, trials of biologics to inhibit IL-1 or TNFα in knee OA failed to relieve symptoms or halt structural progression, as compared with placebo.62–64 However, a secondary analysis of the CANTOS trial (canakinumab anti-inflammatory thrombosis outcome study) demonstrated a significant reduction in the incidence of hip and knee replacement in those receiving anti-IL-1β, with a pooled HR of 0.58 (CI 0.42-0.80, p=0.001).65 Some areas of current investigation for disease modification that are being examined in early phase studies include Wnt inhibiton66, intra-articular injection of an anabolic growth factor FGF-1867 and a cathepsin K inhibitor.68
Patients with persistent pain and functional loss and advanced radiographic changes are candidates for total knee or hip replacement (TKR, THR). More than 700,000 primary TKRs and 330,000 primary THRs are done annually in the US, >90% for OA.69 Ninety-day mortality is <1%, and serious complications at 90 days occur in <5%.70–73 About 90% of recipients of THR and 80% of recipients of TKR report little to no residual pain following recovery from these procedures.74 A randomized controlled trial of TKR vs. a rigorous physical therapy program showed that those receiving TKR improved in KOOS Pain score by 35 points (on a 0-100 scale), as compared with 17 points in those receiving PT (difference of 17 points (95% CI 10.4, 23.8).75 Fewer than 10% of TKRs and ~20% of THRs need to be revised over 20 years.76,77 The failure rate is higher in younger and more active recipients, those with comorbidities and those operated upon in low volume centers or by low volume surgeons.78,79 The generally low revision rates mean that persons who receive TKR or THR in their 70’s’s are much more likely to die with their original implants in place than to need revision.80 In the patient with unicompartmental knee OA, surgical options include unicondylar knee replacement and osteotomy as well as TKR. Arthroscopic debridement is not appropriate for treating OA; arthroscopic partical meniscectomy has a limited role in patients with OA and symptomatic meniscal tear, for whom nonoperative therapy was not helpful.81–83
Blacks and Hispanics are ~25% less likely to receive TKR than non-Hispanic whites, even after accounting for age and socioeconomic status.72,84 These patterns are seen for THR as well.85,86 Proposed reasons for these disparities in utilization include less frequent offers of joint replacement to non-Whites,87 less willingness to undergo TJR, implicit bias, and other factors.88,89 Blacks and Hispanics also have higher risk of adverse outcomes including mortality after THR and joint infections following TKR.90
Several innovative interventions for OA have been introduced into clinical use but have not been evaluated with sufficient rigor to be recommended. The include geniculate artery embolization, water-cooled radiofrequency ablation and botox injections.
Evolving concepts in management of OA
OA consists of multiple phenotypes.91 Knee OA developing after anterior cruciate ligament tear might have a mechanism distinct from OA associated with obesity. Individuals may have more than one mechanism at play, requiring multi-modal management. It will be important to determine which individuals with early OA are more likely to progress rapidly and would benefit from an intervention designed to slow disease progression. Machine learning approaches using datasets that include demographic, imaging and biomarker data are being harnessed to identify such subsets.92
Intensive research has identified potential targets for structure-modifying therapies,66–68 including inhibitors of collagenases and aggrecanases that degrade cartilage, and of the cytokines and chemokines that contribute to the pro-inflammatory environment.93 Pre-clinical evidence suggests that senescent cells in the joint contribute to OA by releasing pro-inflammatory mediators and matrix-degrading enzymes. Targeting these cells with senolytics that selectively kill senescent cells could be of value.94 It remains unclear whether arresting progression of structural damage in OA will ultimately result in reduced pain and functional limitation.
In addition to structure modification, research in OA therapeutics has also focused on nerve growth factor (NGF), with several trials showing efficacy in pain relief with injections of anti-NGF antibodies.95–97 However, individuals who received anti-NGF were more likely than those receiving placebo to experience rapid progression of OA requiring joint arthroplasty, especially if they were also taking NSAIDs. 98 If anti-NGF therapy is approved for OA, providers and patients will need to discuss risks and benefits carefully.
Prognosis
While some patients with OA follow a trajectory of steady increase in symptoms, others have waxing and waning pain over many years. There is also variability in the progression of joint damage. Model projections suggest that over 50% of persons in the US with symptomatic knee OA undergo TKR over their lifetimes.13 Several factors influence the rapidity of radiographic and clinical progression including older age, reduced physical activity, the extent of cartilage damage, short term changes of cartilage damage, malalignment and more severe pain.27,99,100
Conclusion
Evolving insights into pathophysiology portend a new age in OA therapeutics, with therapies that can curb structural progression and provide more potent and/or safer pain relief. The efficacy of diet and exercise interventions suggests that breakthroughs in efforts to sustain weight loss could move the field forward. Taken together these advances may change the outlook for patients with this painful, costly, disabling condition.
Table 4:
Approach to the patient with osteoarthritis
| Type of therapy | Specific therapy | Comments |
|---|---|---|
| Non-pharmacologic therapies | Exercise Education Weight loss (if obese) Yoga or Tai Chi |
-Physical therapist can provide structured exercise, especially if patient lacks confidence or knowledge -Weight loss effective but difficult to achieve and sustain -Yoga and Tai Chi beneficial, with few risks |
| Anti-inflammatory agents | Topical NSAIDs PO NSAIDS Cox-2 inhibitors |
-Topical generally less toxic than oral -Cox-2 inhibitors if on anticoagulant or if GI toxicity |
| Intra-articular injections | Corticosteroids Hyaluronic acid compounds |
-Injections most useful in monoarticular presentations -Steroid injections: risk of hyperglycemia, infection; benefits last a few weeks to months -Long-acting steroid compound may offer advantages -HA compounds more costly, conflicting evidence of efficacy -Stem cells, Platelet rich plasma, other growth factors not recommended because of lack of efficacy data |
| Additional medications | Duloxetine Opioids |
-Duloxetine efficacious, though may be difficult to tolerate -Oioid side effects numerous and serious; reserve for short-term use or if no other options; tramadol preferred over stronger opioids |
| Surgery | Arthroscopy Total joint replacement |
-Arthroscopy not indicated for OA per se; reasonable in OA and meniscal tear if no response to PT -Joint replacement effective; cost effective; underutilized in Blacks and Hispanics |
Commonly Asked Questions about Osteoarthritis.
How common is osteoarthritis?
Osteoarthritis (OA) is among the most frequently seen problems in adult office practice. It affects over 240 million persons worldwide and over 32 million in the US..
Who is mostly likely to get osteoarthritis?
The risk of OA rises markedly with age. OA is exceedingly rare in persons less than 30 years old, while one third of individuals over 75 have symptomatic knee OA. OA is more common in women than in men. Other important risk factors of OA include obesity, prior joint injury, genetics and malignment of joints.
How is osteoarthritis diagnosed?
The cardinal symptom of OA is pain, which is typically provoked by load bearing and relieved by rest. Stiffness occurs following inactivity. On physical examination, bony overgrowth can often be appreciated and pain can often be provoked by joint motion. Radiographs typically reveal osteophyte formation and narrowing of the joint space, the latter reflecting loss of cartilage.
Is osteoarthritis a wear and tear disease?
OA was long considered a ‘wear and tear’ disease of articular cartilage caused by prolonged use of joints, but our understanding of the disorder has advanced considerably. Pathologic changes in OA involve cartilage, bone, synovium, ligament, adipose tissue and meniscus, as well as neurologic pathways involving pain processing. These changes can arise from external mechanical loads (including obesity), joint malignment, joint injury and metabolic and genetic factors. Pathologic features include inflammation. These insights have prompted an array of therapies that may soon permit clinicians to arrest the progression of joint damage and attendant symptoms.
What treatments are used for osteoarthritis?
Management of OA begins with educating patients about its natural history, the benefits of excercise and weight loss, and strategies to reduce pain. Weight loss and physical therapy have well documented benefits in persons with knee OA. Nonsteroidal anti-inflammatory drugs, given either topically or orally, are the backbone of pharmacologic treatment. Duloxetine has proven efficacy. Intraarticular injections of corticosteroids provide temporary relief. Injection of hyaluronic acid products is also offered frequently, though evidence of benefit remains disputed. Injections of biologic therapies (such as platelet rich plasma, stem cells) have not been studied rigorously. Joint replacement is highly effective for advanced arthritis of the knee and hip.
How effective is total joint replacement? How dangerous it is? How long does the implant last?
About 90% of recipients of total hip replacement and about 80% of recipients of total knee replacement report substantial improvement in pain following surgery. Mortality following these procedures is less than 1% and serious problems such as pulmonary embolus, myocardial infarction, pneumoinia and infection of the implant occur in less than 5%. The implants are durable with aobut 90% of knee implants and 80% of hip implants lasting 20 years. These procedures appear to be underutilized in African Americans and Hispanics with advanced OA.
Acknowledgement:
Dr. Katz has received research funding for a cohort study of subjects with osteoarthritis from Samumed and for a qualitative study of subjects with osteoarthritis from Flexion Therapeutics. Dr. Loeser has received research funding for a pre-clinical study in osteoarthritis from Bioventus and consulting fees from Unity Biotechnology. Ms. Arant has no disclosures.
Support: NIH/NIAMS P30AR072577, P30 AR072520
References
- 1.Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage. 2015;23(8):1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawker G Osteoarthritis: a serious disease Osteoarthris Research Society International 2016. https://www.oarsi.org/research/oa-serious-disease.
- 3.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30(8):914–918. [DOI] [PubMed] [Google Scholar]
- 5.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of Rheumatology. 2007;34(1):172. [PubMed] [Google Scholar]
- 6.Haugen IK, Englund M, Aliabadi P, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70(9):1581–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 2009;36(4):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken). 2013;65(5):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of orthopaedic trauma. 2006;20(10):739–744. [DOI] [PubMed] [Google Scholar]
- 10.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769–781. [DOI] [PubMed] [Google Scholar]
- 11.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation - United States, 2013-2015. MMWR Morbidity and mortality weekly report. 2017;66(9):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CPI for All Urban Consumers: All items in U.S. city average, all urban consumers, not seasonally adjusted 2019. https://data.bls.gov/pdq/SurveyOutputServlet. Accessed 1 April 2020.
- 13.Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis care & research. 2015;67(2):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. [DOI] [PubMed] [Google Scholar]
- 15.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56(4):1204–1211. [DOI] [PubMed] [Google Scholar]
- 17.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286(2):188–195. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–336. [DOI] [PubMed] [Google Scholar]
- 19.Taljanovic MS, Graham AR, Benjamin JB, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal radiology. 2008;37(5):423–431. [DOI] [PubMed] [Google Scholar]
- 20.Felson DT, Niu J, Neogi T, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood MJ, Leckenby A, Reynolds G, et al. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI insight 2019;4(2):e125325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlowsky EW, Kraus VB. The Role of Innate Immunity in Osteoarthritis: When Our First Line of Defense Goes On the Offensive. The Journal of rheumatology. 2015; 42(3):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohn DH, Sokolove J, Sharpe O, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis research & therapy. 2012;14(1):R7–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Bosch MHJ, van Lent P, van der Kraan PM. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthritis Cartilage. 2020;28(5):532–543. [DOI] [PubMed] [Google Scholar]
- 26.Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22(5):622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. Does isolated patellofemoral osteoarthritis matter? Osteoarthritis Cartilage. 2009;17(9):1151–1155. [DOI] [PubMed] [Google Scholar]
- 29.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. [DOI] [PubMed] [Google Scholar]
- 30.Metcalfe D, Perry DC, Claireaux HA, Simel DL, Zogg CK, Costa ML. Does This Patient Have Hip Osteoarthritis?: The Rational Clinical Examination Systematic Review. JAMA. 2019;322(23):2323–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Does This Patient Have Hip Osteoarthritis: How to Provide a Hip Examination for Hip Osteoarthritis [Video]: JAMA Network 2019. [Google Scholar]
- 32.Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis & Rheumatism. 1991;34(5):505–514. [DOI] [PubMed] [Google Scholar]
- 33.Kohn MD, Sassoon AA, Fernando ND. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gold GE, Cicuttini F, Crema MD, et al. OARSI Clinical Trials Recommendations: Hip imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):716–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter DJ, Zhang W, Conaghan PG, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage. 2011;19(5):557–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet (London, England). 2011;377(9783):2115–2126. [DOI] [PubMed] [Google Scholar]
- 38.Podlipská J, Guermazi A, Lehenkari P, et al. Comparison of Diagnostic Performance of Semi-Quantitative Knee Ultrasound and Knee Radiography with MRI: Oulu Knee Osteoarthritis Study. Scientific Reports. 2016;6:22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–1135. [DOI] [PubMed] [Google Scholar]
- 40.Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2000;59(12):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2005;64(5):669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jevsevar DS. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline, 2nd Edition. JAAOS - Journal of the American Academy of Orthopaedic Surgeons. 2013;21(9):571–576. [DOI] [PubMed] [Google Scholar]
- 44.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis & Rheumatology. 2020;72(2):220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovar PA, Allegrante JP, MacKenzie CR, Peterson MGE, Gutin B, Charlson ME. Supervised Fitness Walking in Patients with Osteoarthritis of the Knee. Annals of internal medicine. 1992;116(7):529–534. [DOI] [PubMed] [Google Scholar]
- 46.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reichenbach S, Felson DT, Hincapié CA, et al. Effect of Biomechanical Footwear on Knee Pain in People With Knee Osteoarthritis: The BIOTOK Randomized Clinical Trial. JAMA. 2020;323(18):1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. The American journal of gastroenterology. 2009;104(3):728–738. [DOI] [PubMed] [Google Scholar]
- 49.Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Annals of internal medicine. 2015;162(1):46–54. [DOI] [PubMed] [Google Scholar]
- 50.Lee C, Straus WL, Balshaw R, Barlas S, Vogel S, Schnitzer TJ. A comparison of the efficacy and safety of nonsteroidal antiinflammatory agents versus acetaminophen in the treatment of osteoarthritis: a meta-analysis. Arthritis Rheum. 2004;51(5):746–754. [DOI] [PubMed] [Google Scholar]
- 51.Leopoldino AO, Machado GC, Ferreira PH, et al. Paracetamol versus placebo for knee and hip osteoarthritis. The Cochrane database of systematic reviews. 2019;2(2):Cd013273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ (Clinical research ed). 2015;350:h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu X, Wu D, Sang L, et al. Comparative effectiveness of glucosamine, chondroitin, acetaminophen or celecoxib for the treatment of knee and/or hip osteoarthritis: a network meta-analysis. Clinical and experimental rheumatology. 2018;36(4):595–602. [PubMed] [Google Scholar]
- 54.Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13(6):740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAlindon TE, LaValley MP, Harvey WF, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA. 2017;317(19):1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deyle GD, Allen CS, Allison SC, et al. Physical Therapy versus Glucocorticoid Injection for Osteoarthritis of the Knee. The New England journal of medicine. 2020;382(15):1420–1429. [DOI] [PubMed] [Google Scholar]
- 57.Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain: A Double-Blinded, Randomized, Placebo-Controlled, Multinational Study. J Bone Joint Surg Am. 2018;100(8):666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng C, Lane NE, Hunter DJ, et al. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27(6):855–862. [DOI] [PubMed] [Google Scholar]
- 59.Hochberg MC, Wohlreich M, Gaynor P, Hanna S, Risser R. Clinically Relevant Outcomes Based on Analysis of Pooled Data from 2 Trials of Duloxetine in Patients with Knee Osteoarthritis. The Journal of Rheumatology. 2012;39(2):352. [DOI] [PubMed] [Google Scholar]
- 60.Osani MC, Bannuru RR. Efficacy and safety of duloxetine in osteoarthritis: a systematic review and meta-analysis. Korean J Intern Med. 2019;34(5):966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enteshari-Moghaddam A, Azami A, Isazadehfar K, Mohebbi H, Habibzadeh A, Jahanpanah P. Efficacy of duloxetine and gabapentin in pain reduction in patients with knee osteoarthritis. Clinical rheumatology. 2019;38(10):2873–2880. [DOI] [PubMed] [Google Scholar]
- 62.Fleischmann RM, Bliddal H, Blanco FJ, et al. A Phase 2 Trial of Lutikizumab, an Anti-Interleukin 1alpha/beta Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis & rheumatology. 2019;71:1056–1069. [DOI] [PubMed] [Google Scholar]
- 63.Kloppenburg M, Peterfy C, Haugen IK, et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1alpha and anti-interleukin-1beta dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann Rheum Dis. 2019;78:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kloppenburg M, Ramonda R, Bobacz K, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77(12):1757–1764. [DOI] [PubMed] [Google Scholar]
- 65.Effects of Interleukin-1β Inhibition on Incident Hip and Knee Replacement. Annals of internal medicine. 0(0):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yazici Y, McAlindon TE, Fleischmann R, et al. A novel Wnt pathway inhibitor, SM04690, for the treatment of moderate to severe osteoarthritis of the knee: results of a 24-week, randomized, controlled, phase 1 study. Osteoarthritis Cartilage. 2017;25(10):1598–1606. [DOI] [PubMed] [Google Scholar]
- 67.Hochberg MC, Guermazi A, Guehring H, et al. Effect of Intra-Articular Sprifermin vs Placebo on Femorotibial Joint Cartilage Thickness in Patients With Osteoarthritis: The FORWARD Randomized Clinical Trial. JAMA. 2019;322(14):1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conaghan PG, Bowes MA, Kingsbury SR, et al. Disease-Modifying Effects of a Novel Cathepsin K Inhibitor in Osteoarthritis: A Randomized, Placebo-Controlled Trial. Annals of internal medicine. 202-;172(2):86–95. [DOI] [PubMed] [Google Scholar]
- 69.Quality AfHRa. Healthcare Cost and Utilization Project 2014. [Google Scholar]
- 70.Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83(11):1622–1629. [DOI] [PubMed] [Google Scholar]
- 71.Memtsoudis SG, Della Valle AG, Besculides MC, Esposito M, Koulouvaris P, Salvati EA. Risk factors for perioperative mortality after lower extremity arthroplasty: a population-based study of 6,901,324 patient discharges. The Journal of arthroplasty. 2010;25(1):19–26. [DOI] [PubMed] [Google Scholar]
- 72.Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87(6):1222–1228. [DOI] [PubMed] [Google Scholar]
- 73.Inacio MCS, Dillon MT, Miric A, Navarro RA, Paxton EW. Mortality After Total Knee and Total Hip Arthroplasty in a Large Integrated Health Care System. Perm J. 2017;21:16–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skou ST, Roos EM, Laursen MB, et al. A Randomized, Controlled Trial of Total Knee Replacement. New England Journal of Medicine. 2015;373(17):1597–1606. [DOI] [PubMed] [Google Scholar]
- 76.Evans JT, Walker RW, Evans JP, Blom AW, Sayers A, Whitehouse MR. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. The Lancet. 2019;393(10172):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Evans JT, Evans JP, Walker RW, Blom AW, Whitehouse MR, Sayers A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet (London, England). 2019;393(10172):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jasper LL, Jones CA, Mollins J, Pohar SL, Beaupre LA. Risk factors for revision of total knee arthroplasty: a scoping review. BMC musculoskeletal disorders. 2016;17:182–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prokopetz JJ, Losina E, Bliss RL, Wright J, Baron JA, Katz JN. Risk factors for revision of primary total hip arthroplasty: a systematic review. BMC musculoskeletal disorders. 2012;13:251–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katz JN, Wright EA, Wright J, et al. Twelve-year risk of revision after primary total hip replacement in the U.S. Medicare population. J Bone Joint Surg Am. 2012;94(20):1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moseley JB, O’Malley K, Petersen NJ, et al. A Controlled Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. New England Journal of Medicine. 2002;347(2):81–88. [DOI] [PubMed] [Google Scholar]
- 82.Katz JN, Wright J, Spindler KP, et al. Predictors and Outcomes of Crossover to Surgery from Physical Therapy for Meniscal Tear and Osteoarthritis: A Randomized Trial Comparing Physical Therapy and Surgery. J Bone Joint Surg Am. 2016;98(22):1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus Physical Therapy for a Meniscal Tear and Osteoarthritis. New England Journal of Medicine. 2013;368(18):1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang W, Lyman S, Boutin-Foster C, et al. Racial and Ethnic Disparities in Utilization Rate, Hospital Volume, and Perioperative Outcomes After Total Knee Arthroplasty. J Bone Joint Surg Am. 2016;98(15):1243–1252. [DOI] [PubMed] [Google Scholar]
- 85.Cavanaugh AM, Rauh MJ, Thompson CA, et al. Racial and ethnic disparities in utilization of total knee arthroplasty among older women. Osteoarthritis Cartilage. 2019;27(12):1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahomed NN, Barrett JA, Katz JN, et al. Rates and Outcomes of Primary and Revision Total Hip Replacement in the United States Medicare Population. JBJS. 2003;85(1). [DOI] [PubMed] [Google Scholar]
- 87.Hausmann LRM, Mor M, Hanusa BH, et al. The effect of patient race on total joint replacement recommendations and utilization in the orthopedic setting. J Gen Intern Med. 2010;25(9):982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Byrne MM, Souchek J, Richardson M, Suarez-Almazor M. Racial/ethnic differences in preferences for total knee replacement surgery. Journal of clinical epidemiology. 2006;59(10):1078–1086. [DOI] [PubMed] [Google Scholar]
- 89.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Understanding ethnic differences in the utilization of joint replacement for osteoarthritis: the role of patient-level factors. Medical care. 2002;40(1 Suppl):I44–51. [DOI] [PubMed] [Google Scholar]
- 90.Nwachukwu BU, Kenny AD, Losina E, Chibnik LB, Katz JN. Complications for Racial and Ethnic Minority Groups After Total Hip and Knee Replacement: A Review of the Literature. JBJS. 2010;92(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deveza LA, Nelson AE, Loeser RF. Phenotypes of osteoarthritis: current state and future implications. Clinical and experimental rheumatology. 2019;37 Suppl 120(5):64–72. [PMC free article] [PubMed] [Google Scholar]
- 92.Nelson AE, Fang F, Arbeeva L, et al. A machine learning approach to knee osteoarthritis phenotyping: data from the FNIH Biomarkers Consortium. Osteoarthritis Cartilage. 2019;27(7):994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Z, Ding C, Li T, Yu SP. Current status and future prospects for disease modification in osteoarthritis. Rheumatology (Oxford). 2017;57(suppl 4):iv108–iv123. [DOI] [PubMed] [Google Scholar]
- 94.Jeon OH, Kim C, Laberge RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. The New England journal of medicine. 2010;363(16):1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanga P, Katz N, Polverejan E, et al. Long-Term Safety and Efficacy of Fulranumab in Patients With Moderate-to-Severe Osteoarthritis Pain: A Phase II Randomized, Double-Blind, Placebo-Controlled Extension Study. Arthritis & rheumatology. 2017;69(4):763–773. [DOI] [PubMed] [Google Scholar]
- 97.Schnitzer TJ, Easton R, Pang S, et al. Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients With Osteoarthritis of the Hip or Knee: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2019;322(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage. 2015;23 Suppl 1:S18–21. [DOI] [PubMed] [Google Scholar]
- 99.Chapple CM, Nicholson H, Baxter GD, Abbott JH. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken). 2011;63(8):1115–1125. [DOI] [PubMed] [Google Scholar]
- 100.Bastick AN, Runhaar J, Belo JN, Bierma-Zeinstra SMA. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Research & Therapy. 2015;17(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. In: Surgeons AAoO, ed. 2nd ed 2013. [DOI] [PubMed] [Google Scholar]
- 102.Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–1135. [DOI] [PubMed] [Google Scholar]


