Abstract
The entorhinal cortex (EC) is especially vulnerable in the early stages of Alzheimer’s disease (AD). In particular, cognitive deficits have been linked to alterations in the upper layers of EC. In the present report, we examined Layers II and III from eight human brain autopsies (four subjects with no recorded neurologic alterations and four AD cases). We used stereological methods to assess cortical atrophy of the EC and possible changes in the volume occupied by different cortical elements (neuronal and glial cell bodies; blood vessels; and neuropil). We performed 3D ultrastructural analyses of synapses using focused ion beam/scanning electron microscopy (FIB/SEM) to examine possible alterations related to AD. At the light microscope level, we found a significantly lower volume fraction occupied by neuronal bodies in Layer III and a higher volume fraction occupied by glial cell bodies in Layer II in AD cases. At the ultrastructural level, we observed that (1) there was a significantly lower synaptic density in both layers in AD cases; (2) synapses were larger and more complex in Layer II in AD cases; and (3) there was a greater proportion of small and simple synapses in Layer III in AD cases than in control individuals. These structural differences may play a role in the anatomic basis for the impairment of cognitive functions in AD.
Keywords: dementia, electron microscopy, FIB/SEM, medial temporal lobe, neuropil, postsynaptic targets
Significance Statement
Analysis of the synaptic characteristics provides critical data on synaptic organization. Using 3D electron microscopy (EM), the present study shows the synaptic organization of the neuropil of the human entorhinal cortex (EC) at the ultrastructural level. The EC is especially vulnerable in the early stages of Alzheimer’s disease (AD). Our present results show structural differences that may contribute as anatomic basis for the impairment of cognitive functions in AD. Thus, these results may help to understand the relationship between alterations of the synaptic circuits and the cognitive deterioration in AD.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease which is considered to be the main cause of dementia. The brain of AD cases shows brain atrophy and, at the neuropathological level, the most characteristic findings include the presence of extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles (NFTs) of filamentous aggregates of hyperphosphorylated tau protein (Alzheimer’s Association, 2020). Aβ plaques and NFTs are mostly found in the cerebral cortex, where both their numbers and the proportion of the cortex affected by them increase progressively as the disease advances (Braak and Braak, 1991; Dickson, 1997; Thal et al., 2002). Studies focusing on AD progression have shown that entorhinal cortex (EC) is one of the first brain regions affected by the presence of altered tau protein (Braak and Braak, 1991).
The EC has been shown to be essential for memory functions and spatial navigation (for review, see Schultz et al., 2015), and alterations in its upper layers have been related to cognitive deficits in AD individuals (Van Hoesen et al., 1991; Gómez-Isla et al., 1996). EC is considered to be an interface between the hippocampal formation and a large variety of association and limbic cortices (Solodkin and Van Hoesen, 1996; Lavenex and Amaral, 2000). In particular, the EC is the origin of the perforant pathway (from Layers II and III), which provides the largest input source to the hippocampal formation, targeting the ammonic fields (CA) CA1, CA2, and CA3, as well as the dentate gyrus (DG) and subiculum. Specifically, Layer II neurons project to CA1 via the trisynaptic circuit, passing through the DG and CA3, while Layer III neurons project directly to CA1 in the monosynaptic pathway (Insausti and Amaral, 2012; Kondo et al., 2009). It has been proposed that the trisynaptic pathway is more susceptible to premature degeneration (for review, see Van Hoesen et al., 2006; Llorens-Martín et al., 2014).
The presence of pathologic forms of Aβ and tau proteins in the cerebral cortex of AD cases has been related to neuronal loss, synapse alterations and dendritic spine degeneration (for review, see Forner et al., 2017; Chen et al., 2019). The loss and dysfunction of synapses have been proposed as the major structural correlates of the cognitive decline associated with AD (Coleman et al., 2004; Dickson et al., 1995; Selkoe, 2002; Sze et al., 1997). It has been proposed that synaptic loss affects the subcortical regions and the EC first and then progresses to other cortical regions (Braak and Braak, 1991; Braak and Del Tredici, 2012, 2020). Therefore, deciphering the changes that affect the normal function of synapses may contribute to better understanding of the pathologic mechanisms of AD.
In the present study, we performed 3D ultrastructural analysis of the EC using focused ion beam/scanning electron microscopy (FIB/SEM) and specific software that allows the segmentation of synapses in a reconstructed 3D volume (Morales et al., 2011). This technology greatly facilitates the analysis of possible alterations at the synaptic level in AD, as previously shown in the human transentorhinal cortex and the CA1 hippocampal region (Domínguez-Álvaro et al., 2019; Montero-Crespo et al., 2021). Our goal was to investigate the possible synaptic changes occurring in Layers II and III of the EC related to AD, not only with regard to numbers, spatial distribution and types of synapses, but also regarding the morphologic characteristics of each synapse (shape and size), as well as possible changes in their postsynaptic targets. For this purpose, we performed an analysis of the neuropil from Layers II and III of the EC, from eight human brain autopsies (four subjects with no recorded neurologic alterations and four AD cases) with short postmortem delays (<3.5 h). Since the presence of Aβ plaques is related to a virtual lack of synapses in their vicinity (Blazquez-Llorca et al., 2013), we focused on the neuropil that was free of plaques. We also used stereological methods to assess cortical atrophy of the EC, and possible changes in the volume occupied by different cortical elements (neuronal and glial cell bodies, blood vessels and neuropil) at the light microscope level.
Materials and Methods
Tissue preparation
Human brain tissue was obtained from eight autopsies (four male and four female subjects) with short postmortem delays (<3.5 h; supplied by Instituto de Neuropatología del IDIBELL-Hospital Universitario de Bellvitge, Barcelona, Spain; Unidad Asociada Neuromax, Laboratorio de Neuroanatomía Humana, Facultad de Medicina, Universidad de Castilla-La Mancha, Albacete and the Laboratorio Cajal de Circuitos Corticales, Universidad Politécnica de Madrid-Consejo Superior de Investigaciones Científicas). The sampling procedure was approved by the Institutional Ethical Committees of each of the institutions involved. Tissue from some of these human brains has been used in previous studies (Domínguez-Álvaro et al., 2018, 2019, 2021; Montero-Crespo et al., 2020, 2021; Cano-Astorga et al., 2021).
Briefly, tissue samples were obtained from four control cases (non-demented subjects with no recorded neurologic or psychiatric alterations) and four AD cases according to the neuropathological criteria provided by the above-mentioned centers (Table 1; Mirra et al., 1991).
Table 1.
Clinical and neuropathological information
|
Case |
Sex | Age (years) |
Cause of death | Postmortem delay (h) |
Braak stage |
CERAD stage |
Neuropsychological diagnosis |
|---|---|---|---|---|---|---|---|
| AB1 | Male | 45 | Lung cancer | <1 | 0 | 0 | No recorded neurologic alterations |
| AB3 | Male | 53 | Bladder carcinoma | 3.5 | 0 | 0 | No recorded neurologic alterations |
| IF10 | Male | 66 | Bronchopneumonia and cardiac failure |
2 | 0 | 0 | No recorded neurologic alterations |
| M16 | Male | 40 | Traffic accident | 3 | 0 | 0 | No recorded neurologic alterations |
| IF1 | Female | 80 | - | 2 | IV | B | No evidence of cognitive impairment and dementia |
| VK11 | Female | 87 | Respiratory inflammation | 1.5 | III−IV | A | Dementia |
| VK16 | Female | 88 | - | 2.5 | VI | C | Dementia |
| VK22 | Female | 86 | - | 2 | V | C | Dementia |
Braak stages (Braak and Braak, 1991): III, NFTs in EC and closely related areas; III−IV, NFTs abundant in amygdala and hippocampus; extending slightly into association cortex; V−VI, NFTs widely distributed throughout the neocortex and ultimately involving primary motor and sensory areas. CERAD stages (Mirra et al., 1991): A, low density of neuritic plaques; B, intermediate density of neuritic plaques; C, high density of neuritic plaques. -, not available.
Data from the ultrastructural analysis of neuropil from Layers II and III of the EC for individual cases. All volume and distance data are corrected for shrinkage. All, includes AS+SS synapses; No., number. Download Table 2-1, DOCX file (48.4KB, docx) .
Upon removal, the brain tissue was fixed in cold 4% paraformaldehyde (Sigma-Aldrich) in 0.1 m sodium phosphate buffer (PB; Panreac, 131965), pH 7.4, for 24–48 h. After fixation, the tissue was washed in PB and coronally sectioned (150 μm thick) in a vibratome (Vibratome Sectioning System, VT1200S Vibratome, Leica Biosystems). Sections containing EC were selected for Nissl staining, immunohistochemistry, and EM processing (Fig. 1).
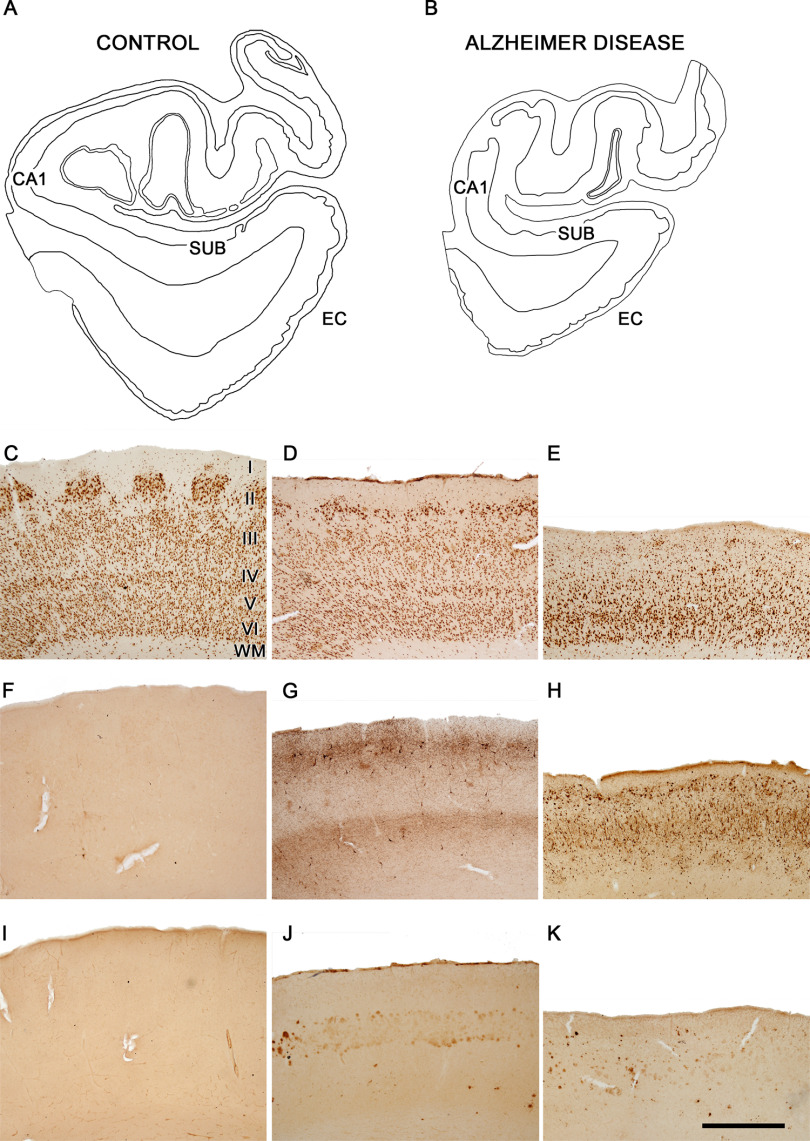
Figure 1.
EC of control and AD cases. Plots of the medial structures including the hippocampal formation and EC from control (A) and AD (B) cases to illustrate the atrophy that occurs in AD. Plots are based on Nissl-stained coronal sections from cases AB3 and IF1. Microphotographs showing the EC in a control case AB3 (C, F, I) and in AD cases IF1 (D, G, J) and VK16 (E, H, K). Layers are indicated to the right of panel C. Sections are immunostained with antibodies anti-NeuN (C–E), anti-PHF-Tau-AT8 (F–H), and anti-Aβ (I–K). Note the intense labeling of PHF-Tau-AT8-positive neurons (G, H) and Aβ-positive plaques (J, K) in AD cases, particularly in case VK16, and the lack of labeling in the control case. CA1, cornu ammonis field 1; SUB, subiculum; WM, white matter. Scale bar in K: 4 mm (A, B) and 1 mm (C−K).
Coronal sections of the human EC at medial level were used for the present study (for review, see Insausti et al., 2017). The delimitation of the EC was established by combining the Nissl and anti-NeuN markers (Fig. 1). The main cytoarchitectural characteristic of the EC that allows it to be recognized is the presence of large islands of modified pyramidal neurons and stellate cells in Layer II (Braak and Braak, 1992; Insausti and Amaral, 2012; Kobro‐Flatmoen and Witter, 2019). Sections containing EC were selected for Nissl staining, immunohistochemistry and EM processing (Fig. 1).
Immunohistochemistry
The selected sections were first rinsed in 0.1 m PB, pretreated in 2% H2O2 for 30 min to remove endogenous peroxidase activity, and then incubated for 1 h at room temperature in a solution of 3% normal horse serum (for monoclonal antibodies; Vector Laboratories Inc.) and 0.25% Triton X-100 (Merck). The sections were then incubated for 48 h at 4°C in the same solution with mouse anti-NeuN (1:2000; Chemicon; MAB377) and anti-human PHF-Tau antibody clone AT8 (1:2000, MN1020, Thermo Scientific); for the sake of clarity, we will refer to this as anti-PHF-Tau-AT8. The sections selected for anti-Aβ were first treated with 88% formic acid (Sigma-Aldrich, #251364) to ensure specific plaque immunostaining, and were then incubated in a solution containing mouse antibody anti-Aβ (clone 6F/3D; 1:50, Dako M0872). The sections were then processed with a secondary biotinylated horse anti-mouse IgG antibody (1:200, Vector Laboratories), and then incubated for 1 h in an avidin-biotin peroxidase complex (Vectastain ABC Elite PK6100, Vector) and, finally, with the chromogen 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich). Finally, the sections were dehydrated, cleared with xylene, and cover-slipped.
Tissue processing for EM
EC sections were postfixed for 24 h in a solution containing 2% paraformaldehyde, 2.5% glutaraldehyde (TAAB, G002) and 0.003% CaCl2 (Sigma, C-2661-500G) in sodium cacodylate (Sigma, C0250-500G) buffer (0.1 m). These sections were washed in sodium cacodylate buffer (0.1 m) and treated with 1% OsO4 (Sigma, O5500), 0.1% potassium ferrocyanide (Probus, 23345) and 0.003% CaCl2 in sodium cacodylate buffer (0.1 m) for 1 h at room temperature. After washing in PB, the sections were stained with 2% uranyl acetate (EMS, 8473), and then dehydrated and flat-embedded in Araldite (TAAB, E021) for 48 h at 60°C (DeFelipe and Fairén, 1993). Embedded sections were glued onto a blank Araldite block and trimmed. Semithin sections (1–2 μm thick) were obtained from the surface of the block and stained with 1% toluidine blue (Merck, 115930) in 1% sodium borate (Panreac, 141644).
The last semithin section (which corresponds to the section immediately adjacent to the block surface) was examined under light microscope and photographed to accurately locate the neuropil regions to be examined (Fig. 2). The blocks containing the embedded tissue were then glued onto a sample stub using conductive adhesive tabs (EMS 77825-09). All the surfaces of the block, except for the one to be studied (the top surface), were covered with silver paint (EMS 12630) to prevent charging artifacts. The stubs with the mounted blocks were then placed into a sputter coater (Emitech K575X, Quorum Emitech) and the top surface was coated with a 10- to 20-nm-thick layer of gold/palladium to facilitate charge dissipation.
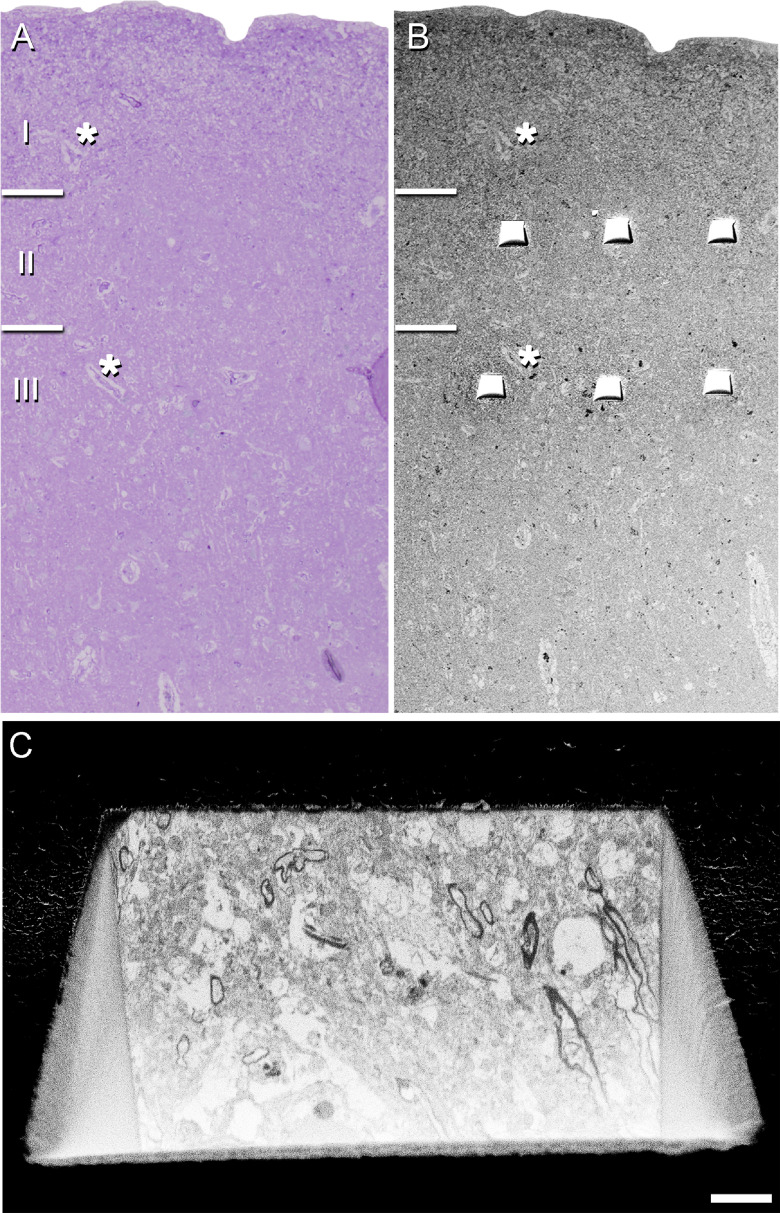
Figure 2.
Correlative light/EM of Layers II and III of the EC. Delimitation of layers is based on the staining pattern of 1-μm-thick semithin section, stained with toluidine blue (A), which is adjacent to the block for FIB/SEM imaging (B). B, SEM image illustrating the block surface with trenches made in the neuropil (three per layer). Asterisks in A, B point to the same blood vessels, showing that the exact location of the region of interest was accurately determined. C, SEM image showing the front of a trench made to expose the tissue and to acquire the FIB/SEM stack of images at the final magnification. Scale bar in C: 70 μm (A), 85 μm (B), and 3 μm (C).
Cortical thickness estimation
In order to estimate the atrophy of the EC, we measured the cortical thickness in three to five toluidine blue-stained semithin sections from each of the cases, obtained in the coronal plane of the cortex and containing the entire cortex, tracing a perpendicular line from the pial surface to the white matter. Measurements of the distance between the pial surface and the boundary with the white matter were performed using Fiji program (ImageJ 1.51; National Institutes of Health; http://imagej.nih.gov/ij/). To average data, three measurements were made per section.
Volume fraction estimation of cortical elements
Three to five semithin sections (1–2 μm thick) stained with 1% toluidine blue were used to estimate the respective volume fractions (Vv) occupied by blood vessels, cell bodies (glia and neurons) and neuropil. This estimation was performed applying the Cavalieri principle (Gundersen et al., 1988) by point counting using the integrated Stereo Investigator stereological package (version 8.0, MicroBrightField Inc.) attached to an Olympus light microscope (Olympus) at 40× magnification. A grid, whose points covered an area of 400 μm2, was overlaid over each semithin section to determine the Vv occupied by the different elements: neurons, glia, blood vessels, and neuropil.
3D EM
The Araldite block containing the tissue was used to obtain images stacks from the EC (Fig. 2) using a dual beam microscope (FIB/SEM; Crossbeam 540 EM, Carl Zeiss NTS GmbH).
The dual beam microscope combines a high-resolution field-emission SEM column with a focused gallium ion beam (FIB), which permits removal of thin layers of material from the sample surface on a nanometer scale. As soon as one layer of material (20 nm thick) is removed by the FIB, the exposed surface of the sample is imaged by the SEM using a backscattered electron detector. The sequential automated use of FIB milling and SEM imaging allowed us to obtain long series of photographs of a 3D sample of selected regions (Merchán-Pérez et al., 2009). FIB/SEM images from the neuropil were obtained avoiding the neuronal and glial somata, blood vessels and also avoiding Aβ plaques to eliminate the effect of alterations of synapses in the vicinity of Aβ-plaques, which has been described previously (Blazquez-Llorca et al., 2013).
In total, 24 stacks of images of the neuropil from Layers II and III of the EC from AD cases were obtained (three stacks for each of the 4 cases, with a total volume studied of 9006 μm3; Table 2). The data obtained from these stacks of images were compared with the data obtained in the same regions of four control cases (Domínguez-Álvaro et al., 2021).
Table 2.
Accumulated data obtained from the ultrastructural analysis of the neuropil from Layers II and III of the EC
| EC layer |
Group | No. AS |
No. SS |
No. all synapses |
% AS (mean ± SD) |
% SS (mean ± SD) |
CF volume (μm3) |
No. AS/μm3 (mean ± SD) |
No. SS/μm3 (mean ± SD) |
No. all synapses/μm3 (mean ± SD) |
Distance to nearest neighbor (nm; mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| II | Control | 1553 | 137 | 1690 | 91.90 ± 2.62 | 8.10 ± 2.62 | 4221 (5445) | 0.37 ± 0.05 (0.38 ± 0.06) | 0.03 ± 0.01 (0.03 ± 0.01) | 0.40 ± 0.03 (0.41 ± 0.06) | 840 ± 35 (811 ± 33) |
| Alzheimer | 954 | 90 | 1044 | 90.53 ± 4.27 | 9.47 ± 4.27 | 4409 (5742) | 0.21 ± 0.09 (0.23 ± 0.09) | 0.02 ± 0.01 (0.02 ± 0.01) | 0.24 ± 0.09 (0.25 ± 0.09) | 938 ± 113 (906 ± 109) | |
| III | Control | 1747 | 130 | 1877 | 92.84 ± 1.97 | 7.16 ± 1.978 | 4371 (5466) | 0.40 ± 0.09 (0.42 ± 0.09) | 0.03 ± 0.01 (0.03 ± 0.01) | 0.43 ± 0.01 (0.45 ± 0.09) | 852 ± 42 (823 ± 41) |
| Alzheimer | 973 | 92 | 1065 | 92.12 ± 5.70 | 7.88 ± 5.70 | 4597 (5653) | 0.22 ± 0.06 (0.23 ± 0.08) | 0.02 ± 0.02 (0.02 ± 0.02) | 0.24 ± 0.08 (0.25 ± 0.09) | 1004 ± 214 (970 ± 206) |
All volume data are corrected for shrinkage factor. Data in parentheses are not corrected with the shrinkage and the fixation artifact factors. The data for individual cases are shown in Extended Data Table 2-1.
Area (nm2) and perimeter (nm) of the SAS in Layers II and III of the EC for individual cases. All data are corrected for shrinkage factor. Download Table 3-1, DOCX file (48.4KB, docx) .
Synaptic 3D analysis
FIB/SEM stacks of images were analyzed using EspINA software (EspINA Interactive Neuron Analyzer, 2.1.9; https://cajalbbp.es/espina/), which allows the segmentation of synapses in the reconstructed 3D volume (Morales et al., 2011; Fig. 3). Since the EspINA software allows navigation through the stack of images (Figs. 3, 4; Movies 1, 2), it was possible to unambiguously identify every synapse as asymmetric synapses (AS) or symmetric synapses (SS), based on the thickness of the PSD (Fig. 4). There is a consensus for classifying cortical synapses into AS (or Type I) and SS (or Type II). The main characteristic distinguishing these synapses is the prominent or thin postsynaptic density, respectively: synapses with prominent PSDs are classified as AS, while thin PSDs are classified as SS (Gray, 1959; Peters and Palay, 1996).
Figure 3.
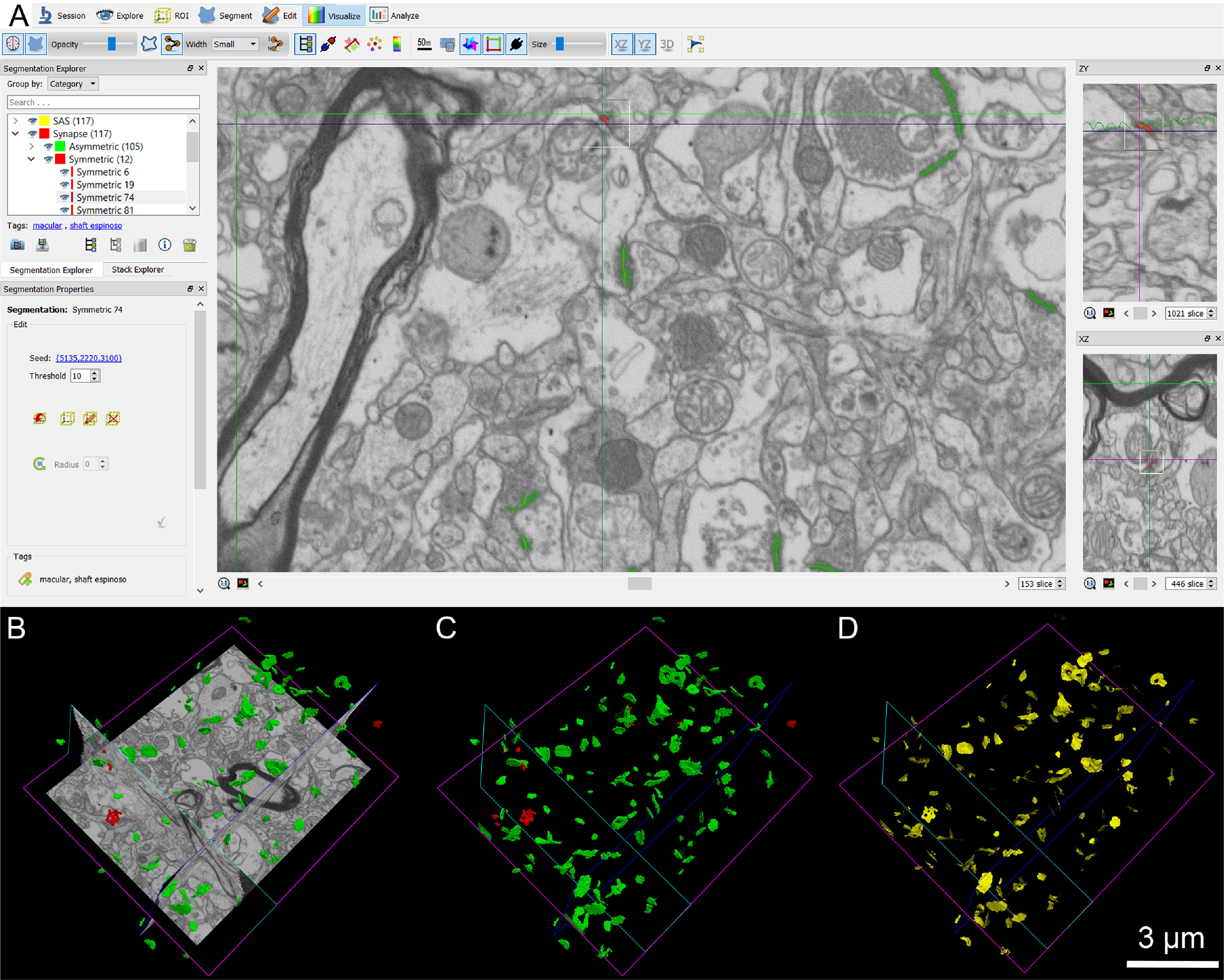
Screenshot of the EspINA software user interface. A, In the main window, the sections are viewed through the xy plane (as obtained by FIB/SEM microscopy). The other two orthogonal planes, yz and xz, are also shown in adjacent windows on the right. B, The 3D windows show the three orthogonal planes and the 3D reconstruction of AS (green) and SS (red) segmented synapses, the reconstructed synapses (C), and the computed SAS for each reconstructed synapse (in yellow; D).
Figure 4.
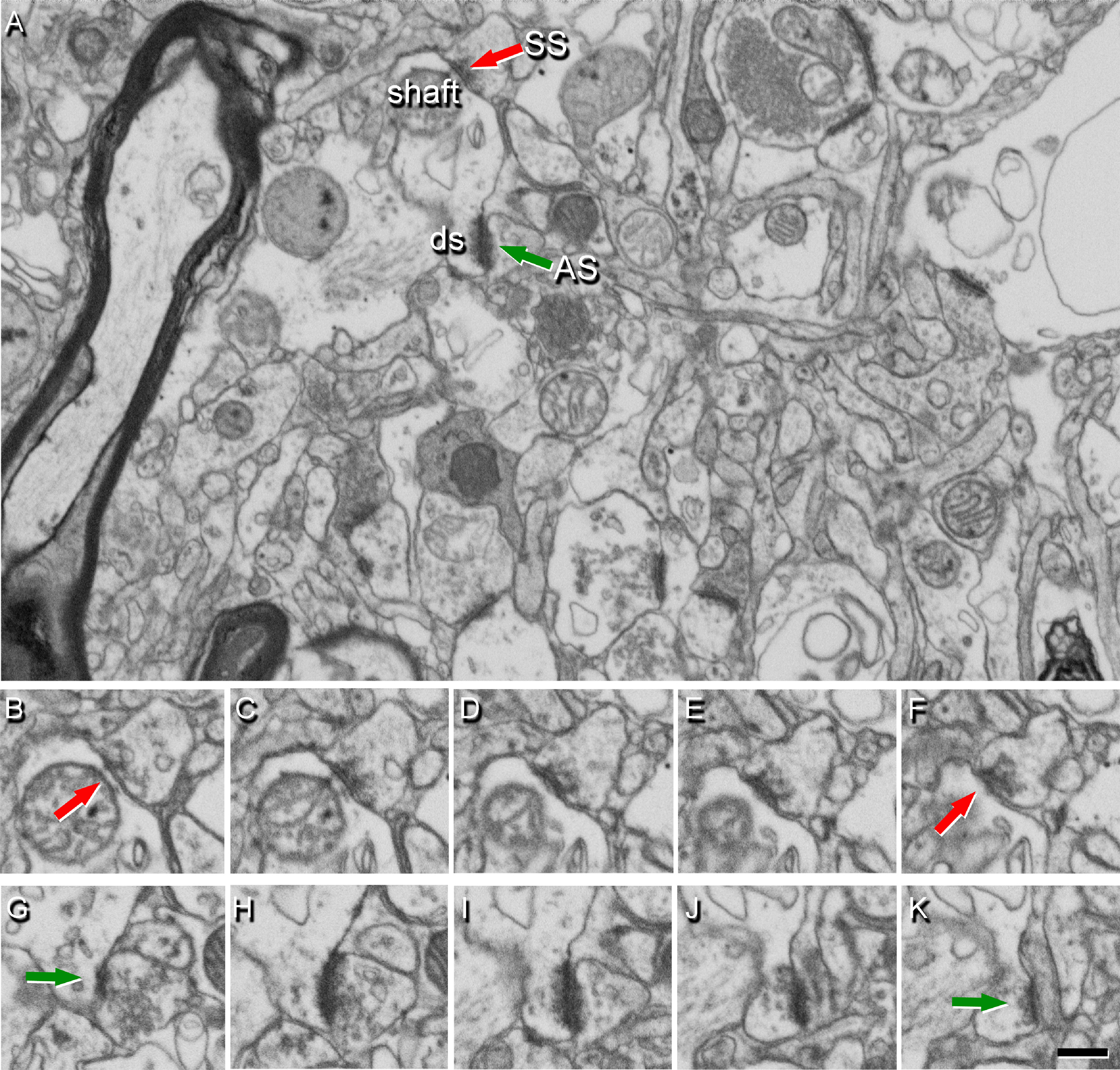
Identification of synapses in serial EM images obtained by FIB/SEM. A, Image from Layer II showing the neuropil from an AD case, with two synapses indicated (arrows) as examples of SS (red) on a dendritic shaft, and AS (green) on a dendritic spine head (ds). Synapse classification was based on examination of the full sequence of serial images; the SS can be visualized in B−F, and the AS in G−K. Scale bar in K: 460 nm (A) and 360 nm (B−K).
EspINA software user interface. FIB/SEM sections are viewed through the xyz planes. AS (green) are shown, established on dendritic spines originating from a dendritic shaft (purple), shown as D529 in Figure 5.
EspINA software 3D viewer. A stack of images is fully represented in the three orthogonal planes (x, y, and z). The video shows the 3D reconstruction of a single dendritic segment (purple; D529 shown in Fig. 5) and the 3D reconstruction of all AS (green) and SS (red) established in this 3D reconstructed fragment.
EspINA also allowed the application of an unbiased 3D counting frame (CF) to obtain the synaptic density per volume (for details, see Merchán-Pérez et al., 2009). The synaptic density values were obtained by dividing the total number of synapses by the total volume of the CF. Geometrical characteristics, such as size, and spatial distribution features (centroids) of each reconstructed synapse were also calculated by EspINA.
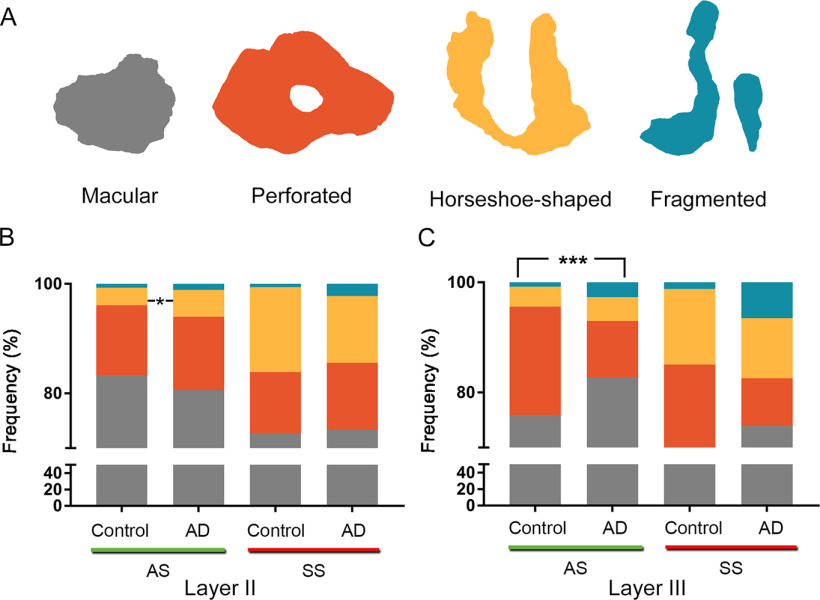
EspINA software extracts the synaptic apposition surface (SAS) and provides its morphologic measurements (Fig. 3). Since the presynaptic and postsynaptic densities are located face to face, their surface areas are comparable (for details, see Morales et al., 2013). Since the SAS comprises both the active zone and the PSD, it is a functionally relevant measure of the size of a synapse (Morales et al., 2013). In addition, the visualization of each 3D reconstructed synapse allowed us to determine the synaptic morphology. Based on the presence of perforations or indentations in their perimeters, the synapses could be classified into four types: macular (with a flat, disk-shaped PSD), perforated (with one or more holes in the PSD), horseshoe (with an indentation in the perimeter of the PSD) or fragmented (with two or more physically discontinuous PSDs; for detailed description, see Domínguez-Álvaro et al., 2019).
To identify the postsynaptic targets of the synapses, we navigated the image stack using EspINA to determine whether the postsynaptic element was a dendritic spine (“spine” or “spines,” for simplicity) or a dendritic shaft (Fig. 5; Movies 1, 2). Unambiguous identification of spines as postsynaptic targets requires the spine to be visually traced to the parent dendrite, yielding what we refer to as fully reconstructed spines. Additionally, when synapses were established on a spine head-shaped postsynaptic element whose neck could not be followed to the parent dendrite, or whose neck was truncated, we identified these elements as non-fully reconstructed spines. These non-fully reconstructed spines were identified on the basis of their size and shape, the lack of mitochondria and the presence of a spine apparatus, or because they were filled with a characteristic fluffy material (used to describe the fine and indistinct filaments present in the spines), a term coined by Peters et al. (1991; see also del Río and DeFelipe, 1995). For simplicity, we will refer to the fully reconstructed and non-fully reconstructed spines as spines, unless otherwise specified. Similarly, for the unambiguous identification of dendritic shafts, it is necessary to be able to visually trace them inside the stack. Accordingly, when the postsynaptic element of a synapse was close to the margins, and it was truncated by the borders of the stack, the identity of the postsynaptic target could not be determined. Therefore, the targets of synapses in each of the stacks were classified into two main categories: spines and dendritic shafts, while truncated elements that could not be safely identified were discarded. When the postsynaptic target was a spine, we further recorded the position of the synapse on the head or neck. Additionally, when the postsynaptic element was identified as a dendritic shaft, it was classified as “with spines” or “without spines.”
Figure 5.
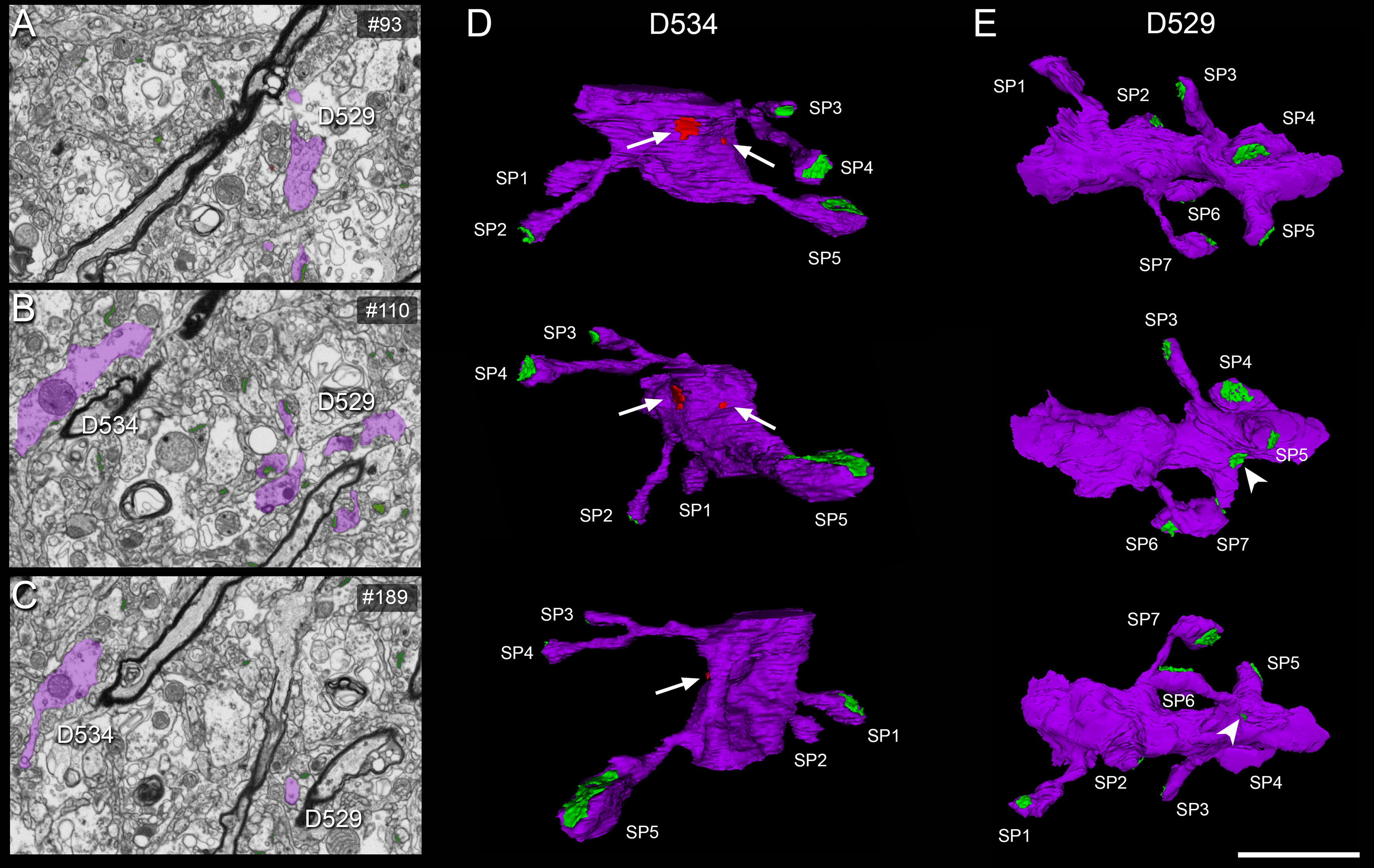
3D reconstruction of dendritic segments from FIB/SEM serial images. A–C, Serial images showing two dendritic segments (D529, D534) partially reconstructed (in purple). 3D reconstructions of these dendritic segments are displayed in panels D, E. D, 3D reconstructed dendritic segment D534 in three views after rotation about the major dendritic axis. Five dendritic spines (SP 1–5) are shown, establishing AS (green), and two SS (arrows) on the shaft (red) are also visible. E, 3D reconstructed dendritic segment D534 in three views after rotation about the major dendritic axis. Seven dendritic spines (SP 1–7) establishing AS (green) are illustrated. Note that the synapse on SP4 can be identified as perforated in the middle panel (E). The arrowhead indicates an AS on a spine neck. Scale bar in E: 3 μm (A–C) and 2 μm (D, E).
Spatial distribution analysis of synapses
To analyze the spatial distribution of synapses, spatial point-pattern analysis was performed as described elsewhere (Merchán-Pérez et al., 2014). For each of the 24 different samples, we calculated three functions commonly used for spatial point pattern analysis: F, G, and K functions (for detailed description, see Blazquez-Llorca et al., 2015). Additionally, we measured the distance of each synapse to its nearest synapse, to compare control and AD samples. This study was conducted using the Spatstat package and R Project program (Baddeley et al., 2015).
Tissue shrinkage
All measurements were corrected for the tissue shrinkage that occurs during osmication and plastic-embedding of the vibratome sections containing the area of interest, as described previously (Merchán-Pérez et al., 2009). We measured the surface area and thickness of the vibratome sections with Stereo Investigator (MBF Bioscience), both before and after they were processed for EM (Oorschot et al., 1991). The surface area after processing was divided by the value before processing to obtain an area shrinkage factor (p2) of 0.933. The linear shrinkage factor for measurements in the plane of the section (p) was therefore 0.966. The shrinkage factor in the z-axis was 0.901. In addition, the total volume was corrected for the presence of fixation artifacts, which did not affect the accurate identification and quantitation of synapses (i.e., swollen neuronal or glial processes). The volume occupied by these artifacts was calculated applying the Cavalieri principle (Gundersen et al., 1988) and was discounted from the volume of the stacks of images to avoid underestimation of the number of synapses per volume. Specifically, a stereological grid with an associated area per point of 400,000 nm2 was superimposed onto each FIB/SEM stack using ImageJ Stereology Toolset (Mironov, 2017). Estimations were made every 20th section in each of the stacks. Every FIB/SEM stack was examined and the volume artifact ranged from 1% to 16% of the volume stacks. Volume fraction estimation was performed by point counting using the Cavalieri principle (Gundersen et al., 1988), in a similar fashion to the volume fraction estimation of cortical elements in semithin sections (see above, Volume fraction estimation of cortical elements).
All parameters measured were corrected to obtain an estimate of the preprocessing values. The shrinkage factor was used to correct the SAS area and perimeter data, while both the shrinkage and the fixation artifact factors were used to correct synaptic density values.
Statistical analysis
To determine possible differences between the control and AD samples, statistical comparisons of the following parameters were conducted using the unpaired Mann–Whitney (MW) nonparametric U test: cortical thickness, synaptic density, type of synapse (AS or SS), SAS size, synaptic shape, postsynaptic targets, and distance to the nearest neighboring synapse. To determine possible differences between control and AD samples regarding volume fractions (neuronal and glial cell bodies, blood vessels and neuropil), parametric t test were conducted. Frequency distribution analyses were performed using Kolmogorov–Smirnov (KS) nonparametric test.
To perform statistical comparisons of AS and SS proportions, χ2 test was used for contingency tables. The same method was used to study whether there were significant differences between groups in relation to the shape of the synaptic junctions and their postsynaptic target. In all the χ2 statistical analyses, we firstly performed an “omnibus test” based on 2 × 4 contingency tables. To further investigate the specific cells driving the significance of the χ2 test, a partitioning procedure was applied to create 2 × 2 contingency tables (Sharpe, 2015).
Statistical analyses were performed using the GraphPad Prism statistical package (Prism 8.4.2 for Windows, GraphPad Software Inc.) and SPSS (IBM SPSS Statistics v24, IBM Corp.).
Availability of data and materials
Most data generated or analyzed during this study are included in the main text and the tables. The control datasets used and analyzed are available on the EBRAINS Knowledge Graph (doi: 10.25493/3GMJ-FEZ). Datasets used during the current study available from the corresponding author on reasonable request.
Results
Data regarding the synaptic organization of the human Layers II and III from the EC in the four control subjects has been previously published and detailed information can be found therein (Domínguez-Álvaro et al., 2021). What follows are the alterations of synapses in AD cases in comparison with control cases.
Cortical thickness
Measurements of the cortical thickness were derived from semi thin toluidine blue (serial sections) using light microscopy. The mean cortical thickness of the EC was 2.0 ± 0.17 mm (mean ± SD) in the control group and 1.6 ± 0.49 mm in the cases with AD. Although ∼20% lower EC cortical thickness in AD cases was observed, we did not find a statistically significant difference between the two groups (MW, p = 0.34; Extended Data Table 1-1).
EC cortical thickness in control and Alzheimer cases. All data are corrected for shrinkage factor. Download Table 1-1, DOCX file (48.4KB, docx) .
Volume fraction of cortical elements
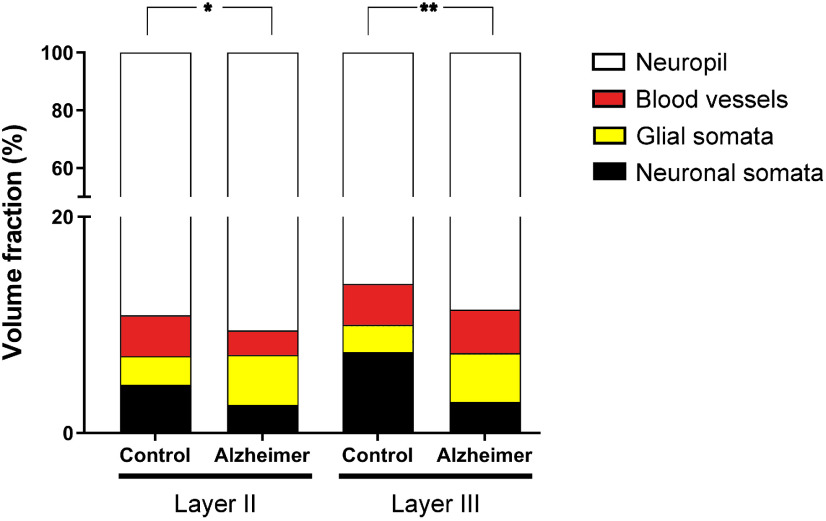
In the control group Layer II, the values of the estimated Vv occupied by neuronal somata, glial somata, blood vessels, and neuropil were 4.5%, 2.6%, 3.8%, and 89.1%, respectively. In the group with AD, these values were 2.6%, 4.6%, 2.3% and 90.5%, respectively. The only statistically significant difference was that of the Vv of glial somata, which was found to be significantly higher in cases with AD (t test, p = 0.03; Fig. 6; Extended Data Table 1-2, Table 1-3).
Figure 6.
Graph showing the Vv occupied by neuronal and glial somata, blood vessels, and neuropil in both Layers II and III of the EC from control subjects and AD cases. Asterisks show the differences between groups. In Layer II, a significantly higher Vv of glial somata was found in AD cases (t test, p = 0.03). In Layer III, a significantly lower Vv of neuronal somata was found in AD cases (t test, p = 0.007). Mean values and case values are detailed in Extended Data Table 1-2, Table 1-3.
Volume fraction occupied by cortical elements in Layers II and III of the EC. Data are given as mean ± SD; Vneu, volume fraction occupied by neurons; Vg, volume fraction occupied by glia; Vbv, volume fraction occupied by blood vessels; Vn, volume fraction occupied by neuropil. Data from individual cases are shown in Extended Data Table 1-3. Download Table 1-2, DOCX file (48.4KB, docx) .
Light microscopy data on volume fraction occupied by cortical elements in Layers II and III of the EC for individual cases. All volume data are corrected for shrinkage. Vneu, volume fraction occupied by neurons; Vg, volume fraction occupied by glia; Vbv, volume fraction occupied by blood vessels; Vn, volume fraction occupied by neuropil. Download Table 1-3, DOCX file (48.4KB, docx) .
In Layer III of the control group, the Vv of neuronal somata, glial somata, blood vessels, and neuropil were 7.5%, 2.5%, 3.8%, and 86.2%. In cases with AD, these values were 2.9%, 4.6%, 4.0%, and 88.6%. Statistical comparisons showed a significantly lower Vv of neuronal somata (t test, p = 0.007) in AD (Fig. 6; Extended Data Table 1-2, Table 1-3).
Synaptic density and AS:SS ratio
A total of 2019 synapses from AD cases (1044 synapses in Layer II and 1065 in Layer III) were identified and reconstructed in 3D, after discarding incomplete synapses or those touching the exclusion edges of the CF, and the total volume analyzed was 9006 μm3 in AD samples (Table 2; Extended Data Table 2-1).
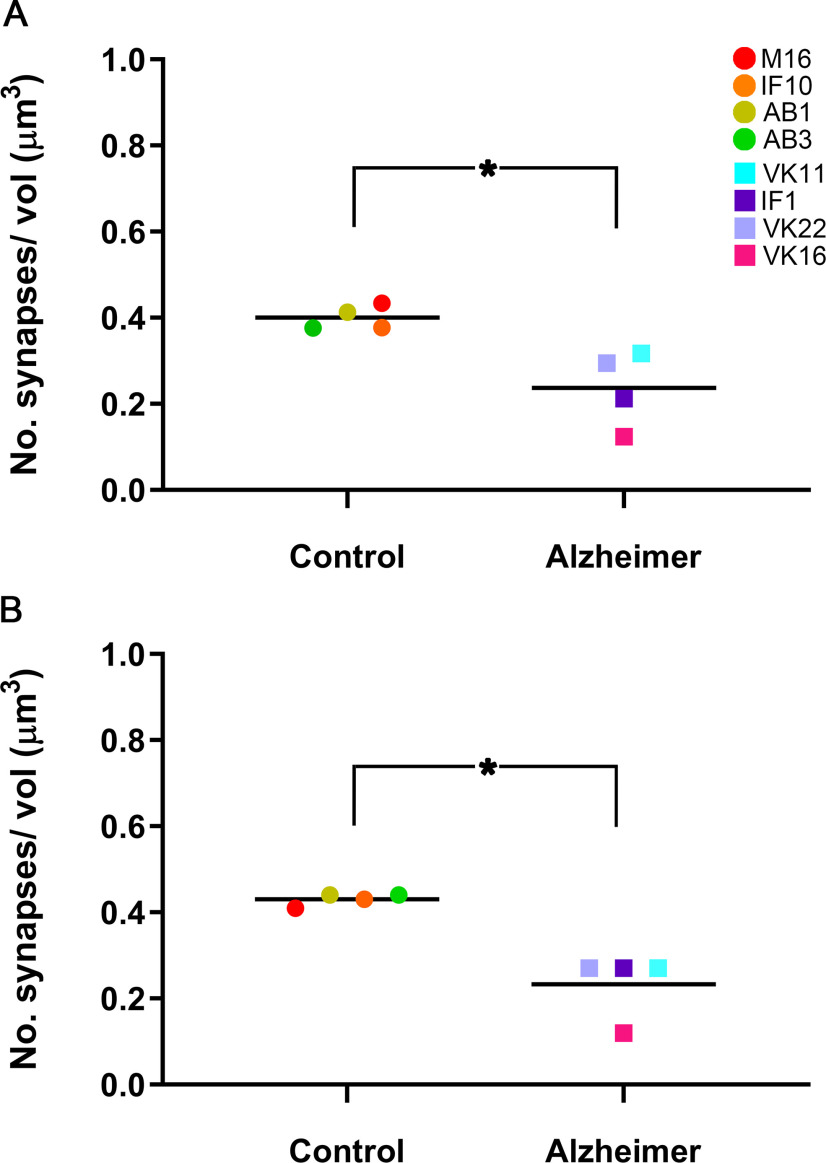
A significantly lower synaptic density (taking both AS and SS together) was observed in AD cases, this value was 40% lower than in control cases in both EC layers. In Layer II, we found 0.24 synapses/μm3 in AD cases and 0.40 synapses/μm3 in control cases (MW, p = 0.03; Fig. 7A). In Layer III, the mean synaptic density in AD was 0.24 synapses/μm3 compared with 0.43 synapses/μm3 found in control cases (MW, p = 0.03; Fig. 7B; Table 2; Extended Data Table 2-1).
Figure 7.
Graphs showing the overall mean synaptic density in Layer II (A) and Layer III (B) of the EC in control and AD cases. Control cases are represented by circles and AD cases are represented by squares. Each color corresponds to each case analyzed, as denoted in the upper right-hand corner. Asterisks show significant differences between groups: in both Layers II and III of the EC, the mean synaptic density was significantly lower in AD cases (MW, p = 0.03).
In Layers II and III, the AS:SS ratio for the AD cases was very similar to that of the control cases (close to 92:8 in both layers and cases; Table 2; Extended Data Table 2-1), and no statistically significant differences were found between the control group and the AD group in any layer (χ2, p > 0.05).
3D spatial synaptic distribution
Analysis indicates a clear fit to a complete spatial randomness (CSR) model, since F, G, and K functions closely resemble the theoretical curve that these functions represent, both in the control and the AD cases. The CSR model defines a situation where a point is equally likely to occur at any location within the study volume, regardless of the locations of other points. Therefore, a CSR model is considered as a reference for a random pattern in spatial point process statistics (for review, see Merchán-Pérez et al., 2014). That is, the spatial distribution of the synapses fitted a random distribution in all subjects and layers. Furthermore, the estimation of the distance from each synapse to its closest synapse showed that although the mean distance was greater in AD cases in both Layer II (840 nm in control; 938 nm in AD; Table 2; Extended Data Table 2-1) and Layer III (852 nm in control, 1004 nm in AD; Table 2; Extended Data Table 2-1), no statistically significant differences were found (MW, p > 0.05).
Study of the synapse characteristics
Synaptic size
The AD samples did not show differences between layers in the SAS area of AS (MW, p = 0.41; Table 3; Extended Data Table 3-1). However, we found significantly larger mean values of SAS area for AS (MW, p = 0.03) from Layer II in AD cases compared with the controls. These differences were not found in SS or in Layer III (MW, p > 0.05; Table 3; Extended Data Table 3-1).
Table 3.
Area (mean ± SEM, in nm2) and perimeter (mean ± SEM, in nm) of the SAS in Layers II and III of the EC
| EC layer | Group | Type of synapse (no. of synapses) | Area of SAS | Perimeter of SAS |
|---|---|---|---|---|
| II | Control | AS (1552) | 110,311 ± 3229 (102,865) | 1631 ± 47 (1575) |
| SS (139) | 65,997 ± 6041 (61,543) | 1404 ± 73 (1356) | ||
| Alzheimer | AS (952) | 136,166 ± 10,773 (126,976) | 1819 ± 52 (1757) | |
| SS (90) | 59,232 ± 6589 (55,234) | 1239 ± 72 (1196) | ||
| III | Control | AS (1746) | 124,183 ± 3265 (115,801) | 1826 ± 67 (1763) |
| SS (129) | 67,445 ± 3243 (62,893) | 1399 ± 75 (1351) | ||
| Alzheimer | AS (973) | 122,727 ± 10,453 (114,444) | 1720 ± 116 (1661) | |
| SS (94) | 57,271 ± 14,132 (56,082) | 1343 ± 272 (1348) |
Data on area and perimeter are corrected for shrinkage factor (data in parentheses are not corrected with the shrinkage and the fixation artifact factors). The data for individual cases are shown in Extended Data Table 3-1.
Proportion of macular, perforated, horseshoe-shaped, and fragmented synapses in Layer II of the EC for individual cases. Data are given as percentages with the absolute number of synapses studied in parentheses. Download Table 4-1, DOCX file (48.4KB, docx) .
Proportion of macular, perforated, horseshoe-shaped, and fragmented synapses in Layer III of the EC for individual cases. Data are given as percentages with the absolute number of synapses studied in parentheses. Download Table 4-2, DOCX file (48.4KB, docx) .
Analysis of the frequency distributions of the area and perimeter of the AS showed significant differences in the frequency distributions of area in Layer II and in the perimeter in Layer III (KS, p < 0.0001), indicating a lower proportion of small SAS area in Layer II in AD cases and higher proportions of small SAS perimeter in Layer III in AD cases (Fig. 8).
Figure 8.
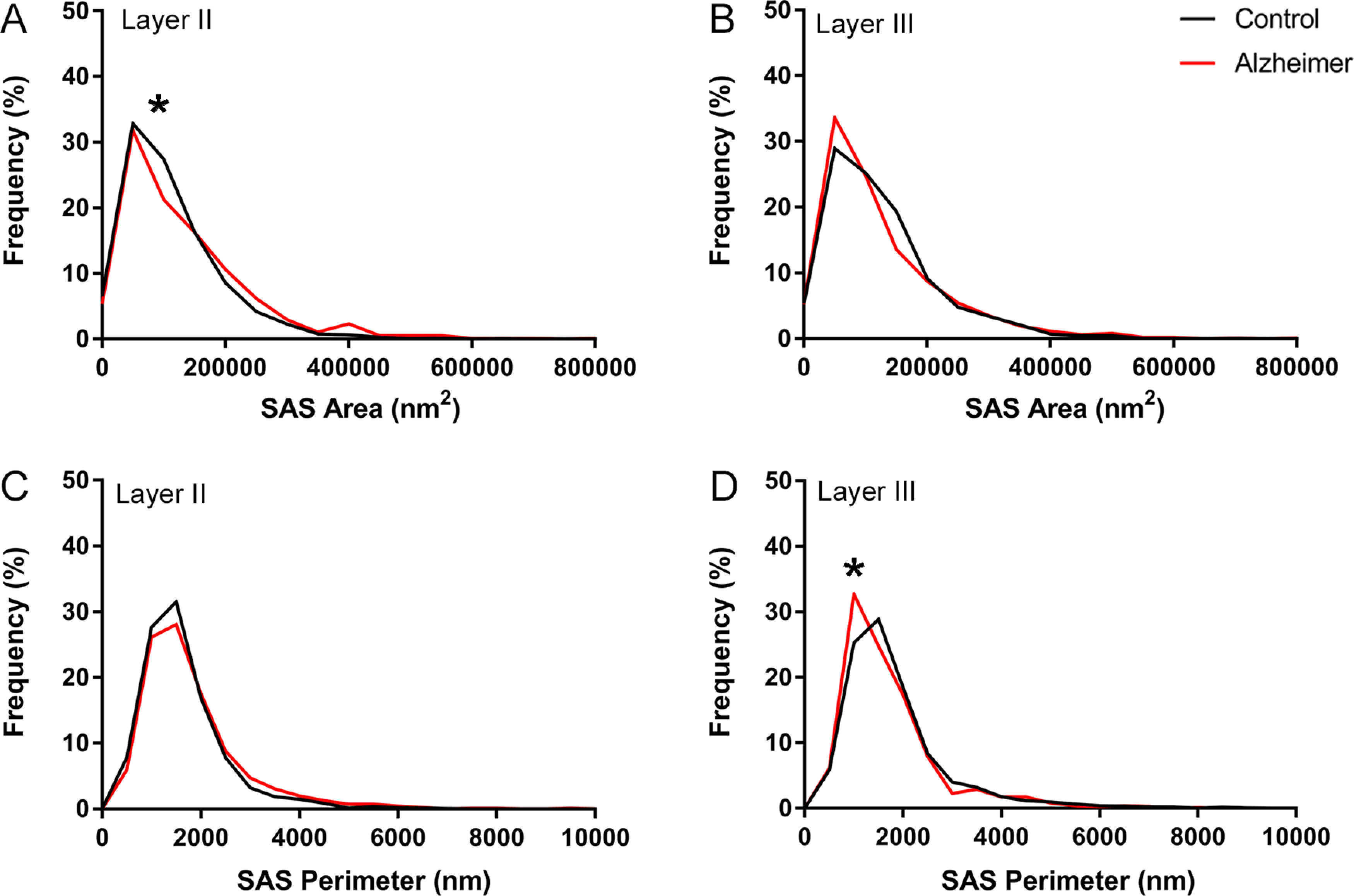
Graph showing the frequency distribution plots of AS SAS area (A, B) and perimeter (C, D), in Layers II and III of the EC in control subjects (black) and AD cases (red). Statistical comparisons between groups showed significant differences (KS, p < 0.0001; indicated with an asterisk) in the frequency distributions of SAS area in Layer II (A), and in the frequency distribution of SAS perimeter in Layer III (D).
Synaptic shape
Most synapses, AS and SS, presented a macular shape in both control and AD cases in Layers II and III (>80%), while synapses with more complex shapes (i.e., horseshoe-shaped, perforated, or fragmented) were less frequent (Fig. 9; Table 4; Extended Data Table 4-1, Table 4-2). Evaluation of the possible differences between the control and the AD group in Layer II showed a slightly higher proportion of horseshoe-shaped AS in AD samples (χ2, p = 0.04; Table 4; Extended Data Table 4-1; Fig. 9B). Analysis of Layer III revealed that fragmented (χ2, p = 0.0004) and macular (χ2, p < 0.0001) AS were more frequent in the AD group, whereas perforated AS were less frequent in AD cases than in the control individuals (χ2, p <0.0001; Table 4; Extended Data Table 4-2; Fig. 9C). Analysis of the SS in Layers II and III did not reveal differences between groups (χ2, p > 0.001).
Figure 9.
Proportions of the different synaptic shapes (A) in Layers II (B) and III (C) of the EC in control subjects and AD cases. A, Schematic representation of the synaptic shapes: macular synapses, with a continuous disk-shaped PSD; perforated synapses, with holes in the PSD; horseshoe-shaped, with a tortuous horseshoe-shaped perimeter with an indentation; and fragmented synapses, with two PSDs with no connections between them. Proportions of macular, perforated, horseshoe-shaped, and fragmented AS and SS are displayed for Layers II (B) and III (C) of the EC in control subjects and AD cases. Statistical differences (indicated with asterisks) showed that in Layer II, horseshoe-shaped AS were more frequent (χ2, p = 0.04) in AD cases; and in Layer III of the AD group, fragmented (χ2, p = 0.0004) and macular (χ2, p < 0.0001) AS were more frequent, whereas perforated AS were less frequent in AD cases than in control individuals (χ2, p <0.0001).
Table 4.
Proportion of the different synaptic shapes in Layers II and III of the EC
| EC layer |
Group | Type of synapse |
Macular synapses |
Perforated synapses |
Horseshoe synapses |
Fragmented synapses |
Total synapses |
|---|---|---|---|---|---|---|---|
| II | Control | AS | 83.0% (1285) | 12.9% (200) | 3.3% (51) | 0.8% (13) | 100% (1549) |
| SS | 73.0% (100) | 11.0% (15) | 15.3% (21) | 0.7% (1) | 100% (137) | ||
| Alzheimer | AS | 80.6% (769) | 13.4% (128) | 4.9% (47) | 1.1% (10) | 100% (954) | |
| SS | 73.4% (66) | 12.2% (11) | 12.2% (11) | 2.2% (2) | 100% (90) | ||
| III | Control | AS | 75.9% (1326) | 19.9% (347) | 3.3% (58) | 0.9% (15) | 100% (1746) |
| SS | 65.9% (85) | 15.5% (20) | 17.0% (22) | 1.6% (2) | 100% (129) | ||
| Alzheimer | AS | 82.8% (806) | 10.2% (99) | 4.3% (42) | 2.7% (26) | 100% (973) | |
| SS | 73.9% (68) | 8.7% (8) | 10.9% (10) | 6.5% (6) | 100% (92) |
Distribution of AS and SS on spines and dendritic shafts in Layer II of the EC for individual cases. Synapses on spines have been subdivided into those that are established on spine heads and those that are established on spine necks. Synapses on spine have been recorded separately. Moreover, we differentiated between aspiny and spiny dendritic shafts. Data are expressed as percentages with the absolute number of synapses studied given in parentheses. Download Table 5-1, DOCX file (48.4KB, docx) .
Distribution of AS and SS on spines and dendritic shafts in Layer III of the EC for individual cases. Synapses on spines have been subdivided into those that are established on spine heads and those that are established on spine necks. Synapses on spines have been recorded separately. Moreover, we differentiated between aspiny and spiny dendritic shafts. Data are expressed as percentages with the absolute number of synapses studied given in parentheses. Download Table 5-2, DOCX file (48.4KB, docx) .
Analysis of synaptic size and shape
Additionally, we determined whether the shape of the synapses was related to their size. For this purpose, the area and perimeter of the SAS of AS were analyzed according to their synaptic shape (SS were not analyzed since the sample size was too small).
When we compared the synaptic size considering the synaptic shape, we found that the mean SAS area from perforated AS in Layer III was larger in AD samples (296,156 ± 24,116 nm2, mean ± SEM) than in the controls (228,057 ± 6993 nm2, mean ± SEM; MW, p = 0.03; Extended Data Tables 4-3, 4-4). No differences were found in the mean area and perimeter of the SAS from macular, horseshoe-shaped, and fragmented synapses (MW, p > 0.05). Analysis of the frequency distribution of the area and perimeter showed no differences between groups (Extended Data Fig. 9-1; KS; p > 0.0001).
Area (nm2) and perimeter (nm) of the macular, perforated, horseshoe-shaped, and fragmented SAS in Layer II of the EC. All data are corrected for shrinkage. Download Table 4-3, DOCX file (48.4KB, docx) .
Area (nm2) and perimeter (nm) of the macular, perforated, horseshoe-shaped, and fragmented SAS in Layer III of the EC. All data are corrected for shrinkage factor. Download Table 4-4, DOCX file (48.4KB, docx) .
Frequency histograms of the SAS area (A, B, E, F) and perimeter (C, D, G, H) of macular, perforated, horseshoe-shaped, and fragmented AS from control cases (A, C, E, G) and from AD cases (B, D, F, H), in Layers II and III of the EC. The SAS area and perimeter of macular synapses were significantly smaller than in perforated, horseshoe-shaped, and fragmented synapses (KW, p < 0.0001). No differences were found between control and AD cases (KS, p > 0.0001). Download Figure 9-1, TIF file (392.7KB, tif) .
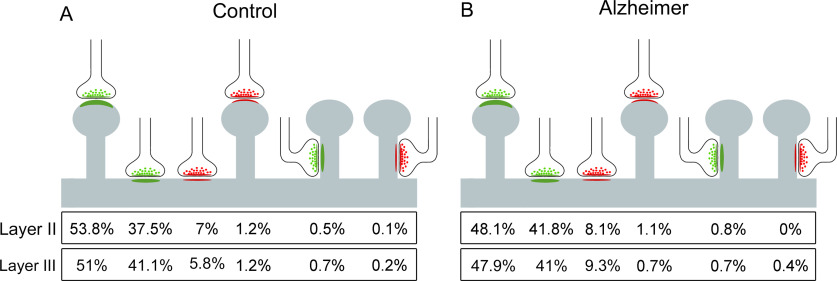
Study of the postsynaptic targets
The postsynaptic targets of 1706 synapses were determined from the AD samples. In Layer II, we identified the postsynaptic targets of a total of 807 AS and 82 SS (Table 5; Extended Data Table 5-1), and in Layer III, the postsynaptic targets for 732 AS and 85 SS were determined (Table 5; Extended Data Table 5-2).
Table 5.
Distribution of AS and SS on spines and dendritic shafts in Layers II and III of the EC
| Layer | Group | Type of synapse |
On spine heads |
On nf. spines |
On spine necks |
On aspiny dendritic shaft |
On spiny dendritic shaft |
Total |
|---|---|---|---|---|---|---|---|---|
| II | Control | AS | 39.68% (567) | 18.89% (270) | 0.56% (8) | 19.87% (284) | 20.99% (300) | 100% (1429) |
| SS | 9.38% (12) | 4.69% (6) | 0.78% (1) | 22.66% (29) | 62.50% (80) | 100% (128) | ||
| Alzheimer | AS | 35.19% (284) | 17.84% (144) | 0.87% (7) | 25.15% (203) | 20.94% (169) | 100% (807) | |
| SS | 10.98% (9) | 1.22% (1) | 0% (0) | 48.78% (40) | 39.02% (32) | 100% (82) | ||
| III | Control | AS | 39.68% (642) | 15.33% (248) | 0.74% (12) | 17.49% (283) | 26.79% (433) | 100% (1618) |
| SS | 11.90% (15) | 4.76% (6) | 2.38% (3) | 22.22% (28) | 58.73% (74) | 100% (126) | ||
| Alzheimer | AS | 41.67% (305) | 11.75% (86) | 0.82% (6) | 28.83% (211) | 16.94% (124) | 100% (732) | |
| SS | 5.88% (5) | 1.18% (1) | 3.53% (3) | 40% (34) | 49.41% (42) | 100% (85) |
Synapses on spines have been subdivided into those that are established on spine heads and those that are established on spine necks. Moreover, we differentiated between aspiny and spiny dendritic shafts. Data are expressed as percentages with the absolute number of synapses studied given in parentheses. Data for each individual case are shown in Extended Data Table 5-1, Table 5-2. nf.: non-fully reconstructed.
The most abundant type of synapse in AD individuals was AS on spine heads (range 47.9–48.1%) closely followed by AS on dendritic shafts (41−41.8%). SS on dendritic shafts were less common (8.1−9.3%), SS on spine heads were very uncommon (0.7–1.1%), and AS or SS on spine necks were very infrequent indeed (<1%; Fig. 10).
Figure 10.
Postsynaptic target distribution in Layers II and III of the EC. Schematic representation of the distribution of AS and SS on different postsynaptic targets from control cases (A) and AD cases (B). In both Layers II and III, the AS preferentially targeted spine heads, while the SS preferentially targeted dendritic shafts. Percentages of postsynaptic targets are indicated, showing, from left to right, the most frequent type (AS on spine heads) to the least frequent type (SS on spine necks). Synapses on spines have been subclassified into those that are established on the spine head and those established on the neck.
When the preference of the synaptic types (AS or SS) for a particular postsynaptic element was analyzed, we found that, in both controls and AD individuals, excitatory contacts (AS) presented a statistically significant preference for spines, whereas inhibitory contacts (SS) preferentially targeted dendritic shafts (χ2, p < 0.001), in both layers, in line with the findings in control EC samples from the same layers (Domínguez-Álvaro et al., 2021). Contingency tables were used to assess possible differences in the distribution of postsynaptic elements of cases with AD. No statistically significant differences were found (χ2, p > 0.001), although a slightly lower proportion of AS established on spine heads (χ2, p = 0.02) and a higher proportion of AS on dendritic shafts were observed in AD samples from Layer II (χ2, p = 0.03).
We also analyzed whether there was a relationship between the synaptic size and the type of postsynaptic element. When we compared the synaptic size of AS associated with the postsynaptic targets between control cases and AD cases, no differences were found in any layer with regard to the area and perimeter of the SAS (MW, p > 0.05) or their frequency distribution (KS, p > 0.001; Extended Data Fig. 10-1).
Frequency histograms of the SAS area (A, B, E, F) and perimeter (C, D, G, H) of AS targeting spine heads, spine necks and dendritic shafts from control cases (A, C, E, G) and from AD cases (B, D, F, H), in both Layers II and III of the EC. No differences were found between groups (KS, p > 0.0001). Download Figure 10-1, TIF file (368.3KB, tif) .
Discussion
In total, there are four main findings in the present study on the human EC: at the light microscope level, (1) a significantly lower volume fraction occupied by neuronal bodies in the Layer III was found in AD cases; at the ultrastructural level, (2) a significantly lower synaptic density was found in both layers in AD cases; (3) synaptic morphology analysis revealed larger and more complex synapses in Layer II in AD cases; and (4) there was a greater proportion of small and simple synapses in Layer III in AD cases than in control individuals.
Cortical thickness and volume fraction analysis
At the light microscopic level, atrophy of the EC and adjacent cortical regions is clearly visible to the naked eye (Fig. 1). However, the difference in the mean cortical thickness was no evident (Extended Data Table 1-1). Macroscopic atrophy of the EC has been described in AD cases (Van Hoesen et al., 1991). This cortical atrophy and, more specifically, the neuronal loss (see below) observed in AD cases have been related to the presence of NFTs and Aβ plaques, with reports indicating an inverse relationship between the number of neurons and the degree of neuropathology (Van Hoesen et al., 1991; Gómez-Isla et al., 1996).
In AD brain samples, we found a significant lower neuronal volume fraction in the Layer III. This is in line with the reported neuronal loss from AD cases in the EC (Van Hoesen and Hyman, 1990; Gómez-Isla et al., 1996; Šimić et al., 2017). Since the neurons of EC Layer III constitute the projection elements of the perforant pathway to the hippocampal CA1 (together with neurons from Layer II), this neuronal loss may contribute to the memory impairment described in AD cases (Hyman et al., 1986; Van Hoesen et al., 1991; von Gunten et al., 2006; Andrade-Moraes et al., 2013). Since our present neuronal volume fraction estimations are based on semithin sections, and different types of neurons have not been identified, we cannot rule out a selective neuronal loss that may affect either to excitatory neurons or to GABAergic interneurons or to both cell types.
The significantly higher volume fraction occupied by glial somata found in Layer II is in line with previous reports showing a larger density of glial cells in human EC from AD cases (Muramori et al., 1998). However, no significant differences were found in Layer III, suggesting a layer-selective change. The effects of gliosis during AD development are still under debate. It has been proposed that gliosis mediates an inflammatory response to prevent the progression of the disease but that this response may in fact contribute to neurodegeneration (for review, see Fakhoury, 2018).
Synaptic changes in AD
Synaptic density, proportions, and spatial distribution
Our main finding was a significantly lower synaptic density (around 40% lower) in the neuropil of both Layers II and III of AD individuals compared with controls.
In brain tissue samples from individuals with AD, a lower density of synapses has been described compared with controls in numerous cortical regions, including the DG and certain frontal, parietal, temporal and cingulate cortical areas (Scheff et al., 1990, 1996; Scheff and Price, 1993, 1998, 2003, 2006). A decrease in the total number of synapses, accompanied by brain atrophy, has been also reported in CA1, the DG and some cingulate and temporal cortices (Scheff et al., 2006, 2007, 2011, 2015; Montero-Crespo et al., 2021). However, other studies have not found differences in synaptic density in layers III and V of EC samples from AD cases (Scheff et al., 1993). These discrepancies may be because of differences in the methodologies used. Furthermore, numerous studies have estimated the synaptic density using indirect methods, either using light microscopy to count immunoreactive puncta for synaptic markers (Masliah et al., 1990; Honer et al., 1992), or employing conventional transmission EM to examine single or few serial ultrathin sections (Scheff et al., 1993). Quantification of synaptic density in single ultrathin sections using transmission EM to infer 3D characteristics of synaptic junctions observed in two dimensions may lead to inaccurate synaptic density estimations (see Merchán-Pérez et al., 2009). Thus, the present results revealing a lower number of synapses per volume of neuropil, along with the atrophy of the EC compared with controls, suggest a dramatic decrease in the absolute number of synapses in AD cases in these EC layers.
Synaptic loss has been widely reported as the characteristic that best correlates with cognitive deficit in AD cases (for review, see Colom-Cadena et al., 2020). Synaptic loss occurs in the early stages of the disease, affecting firstly the subcortical regions and the EC, and progressing to other cortical regions (Braak and Braak, 1991; Braak and Del Tredici, 2012). Tau protein and β-amyloid peptide in pathologic conditions could have toxic effects on synapses leading to synaptic loss and/or dysfunction in AD (Zhou et al., 2017; for review, see Henstridge et al., 2016; Rajmohan and Reddy, 2017). This synaptic loss and impairment may cause dysfunction in the cortical circuits contributing to the decline in cognition (for review, see Colom-Cadena et al., 2020).
Previous studies have shown that the percentage of AS and SS varies, 80–95% and 20–5%, respectively, in all the cortical layers, cortical areas and species examined so far by transmission EM (Beaulieu and Colonnier, 1985; Megías et al., 2001; Bourne and Harris, 2011; DeFelipe, 2011, 2015). Estimations with samples from human brain using the same method have shown that the AS:SS ratio in Layer III of the temporal area 21 is 93:7 (Cano-Astorga et al., 2021), 96:4 in the transentorhinal cortex (Domínguez-Álvaro et al., 2018), and 95:5 in the human CA1 hippocampal field (except in the stratum lacunosum moleculare, in which this ratio was 89:11; Montero-Crespo et al., 2020). Thus, it would appear that the present AS:SS ratio data provide among the highest and lowest proportions previously observed in different brain regions, for AS and SS, respectively. From a functional point of view, the proportion of excitatory and inhibitory synapses is critical, since higher or lower proportions are linked to differences in the excitatory/inhibitory balance of the cortical circuits (for review, see Froemke, 2015; Zhou and Yu, 2018; Sohal and Rubenstein, 2019). Interestingly, no changes in the proportions of AS and SS were observed; therefore, the lower synaptic density in AD cases might affect AS and SS equally, which is in line with our previous results in the transentorhinal cortex (Domínguez-Álvaro et al., 2018). Since most of the synapses are excitatory synapses (∼92% AS), the synaptic decrease may result in a massive loss of AS in particular. As the present results did not reveal differences in the AS:SS ratio between control and AD cases in the neuropil in any layer, it could be interpreted that the proportion of AS and SS on the dendritic arbor of the different types of neurons may be similar. However, it has been shown that there are differences in the number of GABAergic and glutamatergic synaptic inputs in different neuronal types in other cortical regions of a variety of species (DeFelipe and Fariñas, 1992; Freund and Buzsáki, 1996; DeFelipe, 1997; Somogyi et al., 1998; Schubert et al., 2007; Markram et al., 2015; Tremblay et al., 2016; Hsu et al., 2017). Thus, it would be necessary to examine the synaptic inputs on each specific neuronal type to determine actual differences in the AS:SS ratio in particular cell types, although the final general AS:SS ratio does not vary in the neuropil.
Furthermore, it has been reported that GABA inhibitory interneurons in the brains of AD cases are abnormally reduced, along with the inhibitory neurotransmitters (for review, see Xu et al., 2020). Other studies have shown that somatostatin neurons, calretinin neurons, and parvalbumin neurons are decreased in several brain regions including the EC of AD cases (Solodkin and Van Hoesen, 1996; Mikkonen et al., 1999). Therefore, further studies should be performed to examine the GABAergic innervation of neurons in the EC.
Finally, the present results indicate that the distribution of synapses in the neuropil of both control and AD samples is nearly random, only constrained by the fact that synapses cannot physically overlap in space and so their geometric centers or centroids cannot be too close to their neighbors. As we have previously shown in human brain samples from hippocampal CA1, EC, transentorhinal cortex and temporal cortex, there seems to be no limitation to the position of any synapse except the space already occupied by other synapses (Domínguez-Álvaro et al., 2018, 2021; Montero-Crespo et al., 2020; Cano-Astorga et al., 2021). Previous studies in the plaque-free neuropil of AD cases also showed a random distribution pattern of the synapses in other brain regions (Blazquez-Llorca et al., 2013; Domínguez-Álvaro et al., 2018; Montero-Crespo et al., 2021). It has been reported that during cortical development, synapses are randomly added and/or withdrawn from the population, as described by the synaptic turnover (Rakic et al., 1994). It seems that the only constraint for a synapse to form is that this particular spot is not already occupied by a preexisting synapse (for review, see Merchán-Pérez et al., 2014). Interestingly, in the AD cases, the loss of synapses per unit volume does not affect their 3D distribution.
Shape and size of the synapses
There are very few studies on human brain at the ultrastructural level that provide 3D data on the morphologic characteristics of the synaptic junctions to compare with our findings. In the examined EC layers, excitatory AS were larger than inhibitory SS, as previously shown in human transentorhinal and temporal cortex (Domínguez-Álvaro et al., 2018; Cano-Astorga et al., 2021) and in several strata from the hippocampal CA1 (Montero-Crespo et al., 2020). Furthermore, no differences between layers were found regarding the SAS area of AS in AD cases (136,166 nm2 in Layer II and 122,727 nm2 in Layer III), which was in contrast with previous results in control EC showing larger synapses in Layer III (Domínguez-Álvaro et al., 2021). However, the synaptic size in AD samples differs from that of control individuals, with larger and more complex synapses in Layer II and a greater proportion of smaller synapses in Layer III.
Several EM studies conducted with samples of human brain tissue have reported an increase in the size of synaptic apposition, and this has been suggested as a possible compensatory mechanism for the synaptic loss (for review, see Scheff and Price, 2006). Models studying the relationship between the size of synaptic junctions and the probability of neurotransmitter release suggest that larger synapses (with a greater number of postsynaptic receptors, mainly AMPA receptors) may produce more powerful, homogeneous and numerous synaptic responses, while smaller synapses (with fewer receptors, mainly NMDA) may produce weaker and more variable responses (Kharazia and Weinberg, 1999; Montes et al., 2015). Thus, alterations in the normal size of the synaptic junction may change the proportion of postsynaptic receptors, thereby modifying the synaptic response.
Regarding synaptic shape, we found a higher proportion of horseshoe synapses in Layer II from AD cases. In Layer III from these AD individuals, higher proportion of AS with macular and fragmented shape were found along with a lower proportion of perforated AS synapses. Several studies have linked an increase in synaptic complexity to an increase in the efficiency of synaptic transmission, via the insertion of new receptors in the postsynaptic membrane (Lüscher et al., 2000). Perforated synapses have higher immunoreactivity for glutamate receptors than non-perforated synapses (Ganeshina et al., 2004a,b). The larger proportion of macular AS in Layer III (around 83% in AD cases, vs 76% in control cases) and the corresponding lower number of perforated synapses (about half the number found in control cases) supports the evidence for a larger number of simple synapses in this layer in AD cases, which is in turn supported by the finding of a higher proportion of smaller synapses described above. However, the higher proportion of fragmented AS in Layer III from AD cases may indicate the above-mentioned compensatory response to the synaptic loss. Conversely, small macular synapses from Layer III may be more susceptible to damage resulting in a reduction in their numbers. That is, synaptic morphology may be selectively altered by layer, with a non-homogenous pattern of changes, which would differentially affect the circuits in which these layers are involved.
Therefore, synaptic changes in Layers II and III might be part of a remodeling process of the remaining synapses, which overcomes the overall synaptic loss. If the remodeling process involves an increase in the proportion of synapses with more complex morphology, it could be hypothesized that the morphologic changes in AD would trigger compensatory mechanisms during the progression of the disease. However, this type of compensatory mechanism may fail or be deficient, leading to a decline in the synaptic functionality accompanying the progression of the disease.
Postsynaptic targets
Despite the lower synaptic density in AD cases, no changes in the distribution and types of postsynaptic targets were found. In the AD samples, the most frequent combinations were axospinous AS (on dendritic spine heads) and axodendritic AS (established on dendritic shafts). In addition, the majority of the synapses that a pyramidal cell receives are on spines and they represent the vast majority of AS synapses (DeFelipe and Fariñas, 1992). Using the same FIB/SEM technology, in Layer II of the human transentorhinal cortex, it was reported that 60% of all AS were established on spines that were fully reconstructed (Domínguez-Álvaro et al., 2019). However, when we re-analyzed the stacks of images of the TEC to include the non-fully reconstructed spines, the AS percentage established on dendritic spine heads was 75%, which is similar to the case of the AS found in Layer III of the temporal cortex (75%) using the same analysis method (Cano-Astorga et al., 2021). This percentage was much higher in the CA1 hippocampal field, as high as 94% in the superficial part of the CA1 stratum pyramidale (Montero-Crespo et al., 2020). In both Layers II and III of the EC, the percentage of AS on spines was 57%, and 43% in the case of AS on dendritic shafts. This is a higher proportion of AS on shafts than in the other human cortical regions previously analyzed using the same techniques.
In addition, the comparison between control and AD samples did not show a clear reduction in the proportion of AS on spines. Only a slightly lower proportion of AS established on spines was found in Layer II from AD samples. Therefore, the lower synaptic density does not seem to be specifically related to a loss of spines, but rather a general loss of synapses might occur regardless of their postsynaptic targets. It may be that the axospinous synapses in EC from AD cases are functionally altered although no changes were found in their proportions.
Implications of the synaptic changes
EC provides excitatory inputs to the hippocampus from Layer II neurons targeting the DG (trisynaptic pathway) and from Layer III neurons monosynaptically targeting CA1 (for review, see Insausti and Amaral, 2012; Marks et al., 2020). Neuronal projections from both layers give rise to the perforant pathway, which has generally been reported to be weakened in AD (Hyman et al., 1986).
Moreover, in Layer II there are two subpopulations of excitatory neurons: spiny stellate neurons (which project to DG, CA3 and CA2) and modified pyramidal neurons (which project to stratum lacunosum in CA1). Projections from these neurons, constituting the trisynaptic pathway, seem to be related to contextual memories (for review, see Marks et al., 2020). It has been proposed that this pathway is more susceptible to premature degeneration than the monosynaptic pathway (Van Hoesen et al., 2006; Llorens-Martín et al., 2014).
In addition, pyramidal neurons from Layer III directly project to pyramidal neuron dendrites located in the stratum moleculare of CA1, and it has been proposed that these monosynaptic excitatory inputs drive temporal association learning (for review, see Marks et al., 2020).
The present study shows a significant lower number of synapses per volume, as well as morphologic synaptic alterations in the EC from AD cases in Layers II and III. Since the AD cases examined in the present study correspond to advanced stages of the disease, we do not know when the synaptic alterations occurred. Considering that there is neuronal loss, the lower number of synapses may be explained, at least in part, by the loss of local axons of these neurons, at least at the earlier stages of the disease. However, the lower number of synapses may also be explained by a loss of projection neurons that die in distant cortical regions that are also affected by the disease.
Interindividual variability
The present data cannot be generalized to the whole population of patients with AD, which shows clear variability between individuals (Fig. 7; Table 2). Therefore, our study can be considered as a further step to tackle the issue of the synaptic alterations in AD cases, but it would be necessary to validate our results, both in a larger number of individuals and in additional brain regions (which we have already done in the hippocampal CA1 field and in the transentorhinal cortex). Technical effects could be ruled out given that the postmortem delays were all similar and the procedures used were the same. Moreover, all of the AD cases examined were women aged between 80 and 86 years old; therefore, variability because of sex and aging could also be ruled out.
Considering the above-mentioned toxic effect of tau protein and β-amyloid peptide on the synapses, a relationship between the degree of disease progression and the synaptic loss might be expected: the greater the degree of the pathology, the greater the synaptic loss. Subject VK16, who presented a high score for neuropathology and disease progression (Braak/CERAD: VI/C), also had the lowest synaptic density in both layers (0.12 synapse/μm3; Fig. 7; Extended Data Table 2-1). However, subject VK22, who also had a high score for neuropathology and disease progression (V/C), had a synaptic density of more than double (0.29 and 0.27 synapse/μm3, for Layers II and III, respectively) and both cases, VK16 and VK22, suffered dementia. However, subject IF1 did not present evidence of dementia despite the fact that this subject had a high level of pathology (IV/B) and low synaptic density, which was similar to VK22 (0.21 and 0.27 synapse/μm3, for Layers II and III, respectively). However, the cortical thickness of case IF1 was 2.3 mm, whereas in VK16 and VK22, the cortical layers were thinner (1.19 and 1.51 mm, respectively; Extended Data Table 1-1). As pointed out by Ferrer (2012), it should be kept in mind that AD, at least and limited to the entorhinal and transentorhinal cortices (Stages I–II), affects ∼80% of individuals over 65 years, but dementia only occurs in a small percentage of individuals in this age bracket (the prevalence of dementia in AD increases to 25% in 80-year-old individuals). Thus, it is possible that this particular case (IF1) may represent a predementia stage of AD (prodromal AD).
Control cases were on average younger (51 years old) than AD cases (85 years old), and it remains uncertain whether some of the synaptic alterations observed in the present work were because of normal aging or because of the pathology per se. Although no sex-based differences have been reported with aging in the human cerebral cortex (Scheff et al., 2001), previous studies on synaptic density in the human temporal cortex have shown differences depending on the sex (Alonso-Nanclares et al., 2008). Nevertheless, no similar EM studies have been performed in the EC in individuals with different ages and sexes. Thus, the lower synaptic density found in AD cases might also be the results of sex or age effects. In aged rhesus monkey, a lower number of synapses has been reported in prefrontal cortex related to a cognitive decline; however, studies in rats and monkeys have shown no evidence of synaptic loss with age in mesial temporal lobe structures (for review, see Morrison and Baxter, 2012). Further studies using human brain tissue from older control cases would be necessary to determine whether or not these differences in the synaptic density are because of these factors, as opposed to the AD per se. Nevertheless, it is important to point out that only the neuropil without plaques was examined in the present study, whereas it is well established that the neuropil which is adjacent to, and is surrounding, the plaques shows synaptic alterations (Blazquez-Llorca et al., 2013). Thus, it is possible that the neuropil lacking plaques in AD cases displays “normal” characteristics, and differences observed in our study comparing to the control group might be because of factors related to sex and age.
In the present study, we found, in addition to synaptic loss, changes in the morphology of the synapses in AD compared with control cases. These structural changes may contribute to the anatomic basis for the impairment of cognitive functions in AD. Despite the large number of synapses analyzed (5000 synapses), and the proven robustness of the methodology used in this study, it should be kept in mind that (1) the data comes from the analysis of four control cases and four AD cases and (2) we cannot rule out sex-related and age-related factors that may influence the differences observed between the two groups. In order to address these remaining issues, it would be necessary to perform further studies using FIB/SEM or other similar techniques to reconstruct thousands of synapses in aged control cases of both sexes.
Acknowledgments
Acknowledgements: We thank Carmen Álvarez and Lorena Valdés for their technical assistance and Nick Guthrie for his excellent text editing.
Synthesis
Reviewing Editor: Alexey Semyanov, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Maria Medalla.
The authors have addressed the reviewers concerns.
Author Response
Reviewer #1
1) The paper deals with quantitative aspects of synapse parameters using samples of
human entorhinal cortex taken postmortem from normal subjects (published earlier)
and AD patients (present data). The authors chose the FIB/SEM technology to pursue
their goal, which is adequate for revealing statistical aspects of synapse morphology
and numerosity. Clearly, the study is heavily statistically oriented in the sense that
neither the origin of the synapses nor that of the postsynaptic structures were
addressed, so that the emerging picture on the form and distribution of synapses in
the neuropil reflects the sheer frequency occurrence without further knowledge of
their position in the neural circuitry. From a technical point of view the measurements
and statistical evaluation have been done correctly with utmost care. However, the
study seems suffering not only from under-sampling (only 4 cases were considered for
each of the two groups, i.e. control and AD) but heterogeneous since one group
contains only female subjects while the other males. Fixing this problem would be
through to obtain controls of the same sex as that of the AD group (which is more
difficult). For example, authors admit that the lower synaptic density found in AD cases
might also be the attributable to the fact that the control group is restricted to male
subjects vs the AD cases were all females. The discrepancy could have been solved if
the control cases have included females, too.
RESPONSE 1
We would like to point out that when dealing with the study of the human brain,
whether it be in normal conditions or in the altered brain such as in Alzheimer’s
disease, the number of cases examined depends on the type of study. For example,
an MRI study on variations in the size of hippocampus is normally based on hundred
or even thousands of cases, because the level of detail and technical constraints
required for such a study makes it possible to use numerous brain samples.
However, if the question is regarding the synaptic organization of the brain, then the
situation is rather different. First, only relatively small samples of the brain can be
examined because the EM techniques are not only time-consuming and laborious
but also require the processing of the brain for EM to occur with a short postmortem
delay (less than 5 h). Thus, it is difficult to obtain “high-quality human brain tissue",
which is why data on human synaptic organization is so scarce. In addition, it is
important to keep in mind that the structure of the human brain is rather variable
compared with experimental animals, which are reared in laboratories under very
homogeneous conditions (breeding, age, environment, etc.). Thus, when studying
the human brain, “n” is practically always 1 unless thousands of brains in different
conditions are examined. We have used 4 control cases and 4 AD, which in our field
of EM is a reasonable number. Of course, if these 8 cases were more homogeneous
in terms of sex and age the study would be more robust, but we are limited to the 2
tissue that is currently available. We really hope to receive more high-quality brain
tissue in the near future. Furthermore, in the discussion, under the subheading
"Interindividual variability", we have dealt with this issue describing the limitations
of this study. Finally, please note that no detailed EM studies have been performed
previously in this particular cortical region of the human brain. Thus, we think that
our study represents an interesting contribution to the field.
To be clearer, we have included the following paragraphs in the Discussion:
"The present data cannot be generalized to the whole population of patients with
AD, which shows clear variability between individuals. Therefore, our study can be
considered as a further step to tackle the issue of the synaptic alterations in
Alzheimer’s disease cases, but it would be necessary to validate our results, both in a
larger number of individuals and in additional brain regions (which we have already
done in the hippocampal CA1 field and in the trans-entorhinal cortex)”.
"Control cases were on average younger (51 years old) than Alzheimer’s disease
cases (85 years old) and it remains uncertain whether some of the synaptic
alterations observed in the present work were due to normal aging or due to the
pathology per se.”
2) All in all the most surprising and unexpected finding is how little, if any, difference is
observed between the control and AD groups regarding synapse morphologies and
some of the quantitative aspects (e.g. no changes in the distribution and types of
postsynaptic targets were found) apart from synapse density values (see paragraph at
line 448). In relation to the observations the really challenging question is spelled out
at line 482, specifically, to decipher cell type related synaptic changes. Since the study
does not answer such a question neither can tell about the functional meaning of the
morphological observation the implications of the synaptic changes is considerably
reduced.
RESPONSE 2
As we have discussed, one of the limitations of the 3D study of the neuropil is that
we cannot identify to which cell type a synapse belongs. However, our sampling
procedure provides us with data from a large number of synapses of a particular
brain region. These synapses can be characterized as asymmetric or symmetric
synapses, which in general correspond to excitatory and inhibitory synapses,
respectively. Therefore, the present data represent the overall balance of
inhibitory/excitatory synapses. This is rather important since it could be the case
that this balance is impaired, as previously shown in other neurological conditions3
such as epilepsy. Deciphering cell type-related synaptic changes is a different subject
that would need to be examined with other methodologies.
3) A major difficulty of this study is to provide a decent conclusion or, how to interpret
the differences in synapse morphologies between control and AD cases. The inherent
limitation in this regard seems to be overcompensated by the exuberant discussion.
For example, the paragraph on functional considerations of synapse takes up 1.5 pages
alone. Some shortening in the overall 10 pages of the discussion would be beneficial to
the paper.
RESPONSE 3
We have shortened the discussion and removed some references.
4) Minor comments:
at line 294, Regarding a 20% decrease in the thickness of EC in AD samples compared
with control cases how could there be a non-significant difference?
RESPONSE
The statistical analysis applied (non-parametrical Mann Whitney U-test for mean
comparison) found that the data were not significantly different. Nevertheless, the
thickness of the EC in AD cases was 20% less than in control cases. This is suggestive
of a real shrinkage of the EC region, although verifying this would require additional
cases.
at line 323, There is a wealth of studies showing that the distribution of synapses is
non-random when the postsynaptic structure is known - i.e. cell morphology, input
type, neurochemical type. For technical reasons, the present work cannot deal with
the above exemplified attributes and possibly explains why the three-dimensional
spatial synaptic distribution is random.
RESPONSE
Of course, there is specificity and selectivity of synaptic contacts established by
cortical neurons, such as -for example- chandelier cells and double bouquet cells.
What we are analyzing here is the general pattern of the distribution of synapses in
the neuropil. It has been described that some pathological structures, like amyloid
plaques and dystrophic dendrites, modify the available space for the synapses’
location (e.g., Blazquez-Llorca et al., 2013 JAD). Thus, considering the AD pathology,
it was important to verify if the 3D synaptic distribution was affected in the plaque-free regions of the brain.4
Reviewer #2
Review for eNeuro (eN-NWR-0504-20): This study is an ultrastructural 3D EM-analysis
of the synaptic and structural differences in layers II and III of the entorhinal cortex
from the brains of control and Alzheimer disease human subjects. The authors
performed stereological methods to assess both cortical atrophy and volume fraction,
and Focused Ion Beam/Scanning Electron Microscopy (FIB/SEM) to examine synaptic
properties. They found a significantly lower volume of neuronal bodies in layer III and a
higher volume of glial cells in layer II in AD affected individuals. AD cases also had
larger and more complex (including horseshoe shaped, perforated or fragmented)
synapses in layer II and a greater proportion of small and simple (macular) synapses in
layer III. While it is recognized that large-scale 3D EM analyses on human brain tissue is
rare, there are some significant concerns and questions pertinent to their analysis,
results and discussion that need to be addressed.
General/Overall:
1) One rather big concern is the balancing of subjects with regards to age and sex. The
discussion adequately describes these caveats with regards to sex differences in AD
phenotypes. However, the bigger concern is aged. Decades worth of normal aging
studies (from Alan Peters, John Morrison) have shown massive synapse loss in normal
aging in prefrontal cortex. The authors need to discuss this and findings for entorhinal
cortex and hippocampal formation (review Morrison and Baxter, 2012).
RESPONSE 1
As we discussed, we did not rule out the effects of age in our study. Nevertheless, in
the discussion, we have added the following paragraph dealing with the previous
studies on aging (see Discussion, line 644):
"In aged rhesus monkey, a lower number of synapses has been reported in
prefrontal cortex related to a cognitive decline; however, studies in rats and
monkeys have shown no evidence of synaptic loss with age in mesial temporal lobe
structures (reviewed in Morrison and Baxter, 2012).”
Moreover, in our previous studies in human temporal lobe (including the
hippocampal CA1 field and transentorhinal cortex), we did not find differences in the
synaptic density in the AD cases compared to control cases. However, light
microscopy studies showed that the thickness of these regions was severely reduced
in AD samples; thus, the results indicate that there is a dramatic loss in the total
number of synapses (Montero-Crespo e al., 2020b; Domínguez-Álvaro et al., 2020).5
2) While a discussion of the caveats can suffice for most cases, the concern about the
magnitude of synapse loss in normal aging, being close to the effect sizes seen
between AD and control in the current study, is a major concern. The differences can
really just be a result of age. If the tissue is available, the authors should add at one or
two control subject close to the age of the AD patients to support the validity of their
conclusions. It is noted that their data for controls were reanalyzed from a previous
work, and adding control cases would certainly strengthen their current study overall.
RESPONSE 2
We agree with the reviewer; if we had more control cases of the same age as the AD
cases, we would have added them to the present study (please, see response 1 to
reviewer 1 above). Unfortunately, we depend on the donation of high-quality brain
tissue from several hospitals, which has been discontinued due to COVID19.
Methods/Results:
1) Given the excellent quality of this dataset, there is some missed opportunity to
describe qualitatively some ultrastructural neuronal abnormalities in AD cases
compared to controls. One particular important question is whether the authors
observe profiles have some abnormal microtubules (“fibrillar tau”) or
endosomes/multivesicular bodies (increased lysosomal/endosomal activity) in
dendrites in some AD cases.
RESPONSE 1
When analyzing the ultrastructural characteristics of the synapses, Alzheimer’s
disease cases had normal-looking synapses that were similar to those observed in
the control cases (see Domínguez-Álvaro et al., 2020). Further evaluation of the
lysosomal/endosomal activity is being performed, and we expect to gather final data
for publication soon.
2) Statistics: The authors should apply a two-way ANOVA for layer and group to see if
there are independent (group and layer) versus interactive (group*layer) effects. Then
when they find main effect ANOVA is significant (for either group, layer or
group*layer), they can report their MW or paired t-tests as valid post-hocs.
RESPONSE 2
We already performed this test, and no interaction was found; only differences
between groups (AD versus Control) were found. The results presented in the paper
show the statistically significant differences found by the non-parametrical U-Mann
Whitney test.6
3) For the first few Results sections showing Cortical Thickness, and volume fraction
analyses, it is unclear in the Results what tissue/analyses was used. While this is
described in the Methods, it would help if the authors clarify in the Results sections if
analyses were derived from light microscope immune-stained sections or semi-thin
toludine blue (serial sections)
RESPONSE 3
We have modified the Results section as follows:
"Measurements of the cortical thickness were derived from semi-thin toludine blue
(serial sections) using light microscopy.”
4) Cortical thickness: It is unclear how the authors measured this. IT states in the
methods that they measure this on vibratome sections. Did they measure from
multiple points of the pia across the entire EC? Then run a perpendicular line from
each point to the beginning of the white matter?
RESPONSE 4
To address this point, in the Methods section we have changed this section and it
now reads as follows:
"In order to estimate the atrophy of the EC, we measured the cortical thickness in
three to five toluidine blue-stained semithin sections from each of the cases,
obtained in the coronal plane of the cortex and containing the entire cortex, tracing a
perpendicular line from the pial surface to the white matter. Measurements of the
distance between the pial surface and the boundary with the white matter were
performed using Fiji program (ImageJ 1.51; NIH, USA; http://imagej.nih.gov/ij/).To
average data, three measurements were made per section.”
5) Cortical thickness: Results section should site Figure 1
RESPONSE 5
This is now corrected.
6) Lines 297-298: In the control group layer II, the values of the estimated volume
fraction (Vv) occupied by neuronal somata, glial somata
As noted above, it would be helpful if it was clarified that these volume fraction
estimates were from (serial?) semithin sections (1-2μm thick) stained with 1%
toluidine. Further in Figure 5, it would be nice to see an example of the segmentation
on the actual tissue to show how the quantification was done.7
RESPONSE 6
This issue has been dealt with above in responses 3 and 4.
7) Lines 323-321 Three-dimensional spatial synaptic distribution: “Analysis indicates a
clear fit to a CSR model, since F, G and K functions closely resemble the theoretical
curve that these functions represent, both in the control and the AD cases.”
Unclear what this statement is referring too. Please define CSR here, and elaborate on
the rationale of this analyses, include a citation or even a figure to illustrate this
analysis.
RESPONSE 7
We have changed this section and it now reads as follows:
"Analysis indicates a clear fit to a complete spatial randomness (CSR) model, since F,
G and K functions closely resemble the theoretical curve that these functions
represent, both in the control and the AD cases. The CSR model defines a situation
where a point is equally likely to occur at any location within the study volume,
regardless of the locations of other points. Therefore, a CSR model is considered as a
reference for a random pattern in spatial point process statistics (reviewed in
Merchán-Pérez et al., 2014).”
8) The numbers of SS were very low, and perhaps can be due to some of these
methodological constraints of identifying these in FIB/SEM? How do these compare to
previous estimates in EC humans/monkeys?
RESPONSE 8
It is true that the proportion of SS is very low. However, hundreds of synapses were
identified in the images stacks. As described in the Material and Methods section:
"Since the EspINA software allows navigation through the stack of images (Figs. 3, 4), it
was possible to unambiguously identify every synapse as an asymmetric synapse (AS)
or a symmetric synapse (SS), based on the thickness of the PSD (Fig. 4)"
Additionally, we have compared our results with previous estimations, and have
added this paragraph to the discussion:
"Previous studies have shown that the percentage of AS and SS varies -80-95% and
20-5%, respectively- in all the cortical layers, cortical areas and species examined
so far by TEM (Beaulieu and Colonnier, 1985; Megıás et al., 2001; Bourne and Harris,
2011; DeFelipe 2011, 2015). Estimations with samples from human brain using the
same method have shown that the AS:SS ratio in layer III of the temporal area 21 is8
93:7 (Cano-Astorga et al., 2021), 96:4 in the transentorhinal cortex (Dominguez-Alvaro et al., 2018), and 95:5 in the human CA1 hippocampal field (except in the
stratum lacunosum moleculare, in which this ratio was 89:11; Montero-Crespo et al.,
2020). Thus, it would appear that the present AS:SS ratio data provide among the
highest and lowest proportions previously observed in different brain regions, for AS
and SS, respectively.”
Figures and Legends
9) Figure 2-3 should be combined as they represent more methods.
RESPONSE 9
We can see the reviewer's point but think it is preferable to keep them separate so
that they can be printed in larger dimensions.
10) Figure 1 top panel should be labeled as panel A and should have its own scale bar
and axes (M-L, D-V).
RESPONSE 10
Figure 1 plots now have their own scale bar as suggested by the reviewer.
11) Figure 4: Please also label the type of postsynaptic target in EM image (AS on spine,
SS on dendrite). Adding more micrographs to show examples of all synapse
types/targets identified.
RESPONSE 11
Figure 4 has been relabeled as recommended.
We have included a new figure (Fig. 5) of a dendritic shaft segment fully 3D
reconstructed showing diverse postsynaptic targets and their corresponding
synapses. Additionally, two movies of the 3D reconstructed dendrite have been
prepared to illustrate examples of these postsynaptic targets.
12) Figure 8 legend text is truncated.
RESPONSE 12
This problem has been fixed.
13) Minor concern: I am unclear about the organization of the “extended
data/figures”. I am not sure why some “Tables” in the extended data are labeled as
"Fig", specifically:
RESPONSE 139
We are sorry, we probably misunderstood e-Neuro instructions, because the
extended tables/figures must be labeled as related to an original table/figure. We
have re-labeled them.
Table Fig. 5-1. Volume fraction occupied by cortical elements in layers II and III of the
EC
RESPONSE
This has now been corrected.
Table Fig. 5-2. Light microscopy data on volume fraction occupied by cortical elements
in layers II and III of the EC for individual cases.
RESPONSE
This has now been corrected.
Fig. 8-1. Area (nm ) and perimeter (nm) of the macular, perforated, horseshoe shaped
and fragmented SAS in layer II of the EC.
RESPONSE
This has now been corrected.
Fig. 8-2. Area (nm2 ) and perimeter (nm) of the macular, perforated, horseshoe
shaped and fragmented SAS in layer III of the EC.
RESPONSE
This has now been corrected.
Discussion:
14) Lines 392-398: First paragraph of the Discussion should specify/state that the data
is from human Entorhinal Cortex.
RESPONSE
It is now stated that the data are from human Entorhinal Cortex.
15) lines 498-500: “Therefore, the random distribution pattern of synapses in the
neuropil does not appear to be affected by the lower synaptic density in AD cases.”
Please elaborate more in the functional consequence of this finding/statement. It
seems out of place after the previous section describing the highly specific cell-type
and compartmental distribution of excitatory and inhibitory synapses.
RESPONSE 15
We have now included the following paragraphs:10
"The present results indicate that the distribution of synapses in the neuropil of both
control and AD samples is nearly random, only constrained by the fact that synapses
cannot physically overlap in space and so their geometric centers or centroids cannot
be too close to their neighbors. As we have previously shown in human brain
samples from hippocampal CA1, entorhinal cortex, transentorhinal cortex and
temporal cortex, there seems to be no limitation to the position of any synapse
except the space already occupied by other synapses (Montero-Crespo et al., 2020;
Dominguez-Alvaro et al., 2018, 2021; Cano-Astorga et al., 2021).”
"It has been reported that during cortical development, synapses are randomly
added and/or withdrawn from the population, as described by the synaptic turnover
(Rakic et al. 1994). It seems that the only constraint for a synapse to form is that this
particular spot is not already occupied by a preexisting synapse (reviewed in
Merchán-Pérez et al., 2014). Interestingly, in the AD cases, the loss of synapses per
unit volume does not affect their 3D distribution.”
16) Lines 512-522: The following statement has some redundancies: The parenthetical
in Lines 517-520 “..... probability of neurotransmitter release suggest that larger
synapses (with a greater number of postsynaptic receptors, mainly AMPA receptors)
may produce more powerful, homogeneous and numerous synaptic responses, while
smaller synapses (with fewer receptors, mainly NMDA) may produce weaker and more
variable responses.... “ is redundant with the following sentence (lines 521-522). “Also,
larger synapses have a higher number of AMPA receptors, while smaller synapses have
a higher concentration of NMDA receptors (Kharazia and Weinberg, 1999).
RESPONSE 16
The discussion has been reviewed to avoid redundancies.
17) Lines 533-535: “Under normal conditions, macular synapses have the capacity to
progressively increase their perimeter, adopting a more tortuous perimeter with
perforations and indentations (Geinisman et al., 1987; 1991; 1992a, b).” Statement is
unclear, please elaborate and clarify. I presume that the authors are referring to these
studies showing the dynamic nature of synapses transforming from macular to
perforated to eventually splitting into two synapses? Perhaps state how “macular
synapses are thought to have the capacity to change morphologies and develop
perforations and indentations in an activity-dependent manner...”
I am also unclear how this statement fits with the logic of the previous and following
statement/discussions.
RESPONSE 17
This section of the discussion has been edited as suggested by the reviewer.11
18) Lines 561-562: “it could be 561 hypothesized that the morphological changes in AD
would trigger compensatory mechanisms during the development of the disease.”
Perhaps using the word “progression” instead of “development” is more appropriate.
RESPONSE 18
This has now been corrected.
19) In the statement (lines 575-577): “In addition, since the majority of the synapses
that a pyramidal cell receives are on spines and they represent the vast majority of AS
synapses (DeFelipe and Fariñas,1992), the present data do not seem to be related to a
loss of spines.”
The statement “the present data do not seem to be related to a loss of spines.” Is not
consistent with the 40% decrease in density of asymmetric synapses (most of which
are on spines). Their data is indeed consistent with a loss of spines. Their data on
decreased neuronal fraction in layer II of EC also suggest that this spine loss is likely
due to the lower neuron number in layer III of EC, instead of dendritic or spine
regression. To answer this definitively, the authors can do 3D analyses on individual
dendrites and count spines coming off each one of them (a challenging analysis) or
quantify total dendritic length in their volume analyzed and divide this by the total
axospinous asymmetric synapses to estimate spines/dendritic length.
RESPONSE 19
It is true that the majority of the synapses that a pyramidal cell receives are on
spines and, in general, they represent the vast majority of AS synapses. We
completely agree with the reviewer that 3D analyses of individual dendrites would
provide further information and would help to understand the human connectome
and its possible alterations under pathological conditions. However, performing this
study with thousands of synapses, dendrites and axons would be extremely time-consuming and technically challenging. Certainly, we could carry out this study with
a relatively small subset of the reconstructed synapses to accelerate the process, but
the obtained connectivity data would be less robust than if we used all of the
synapses that we were able to reconstruct. For these reasons, our laboratory is
currently developing a software tool that will make it possible to accurately and
reliably trace and reconstruct a large number of axonal and dendritic segments
contained within the FIB/SEM stacks.
In our opinion, this analysis would make it possible to accurately answer important
questions regarding pre- and post-synaptic elements in detail and in relation to their
parental neuronal processes. Thus, we are expecting to answer these questions in
the near future and, respectfully, we would like to leave the answer to this question
for a future study.12
Nevertheless, to improve the accuracy of our postsynaptic target data, all the
FIB/SEM stacks -from both control and Alzheimer’s disease samples- have been
re-examined and classified. The new data have been added to the text and tables.
Accordingly, we have modified the Material & Methods section as follows:
"Unambiguous identification of spines as postsynaptic targets requires the spine to
be visually traced to the parent dendrite, yielding what we refer to as fully
reconstructed spines. Additionally, when synapses were established on a spine head-shaped postsynaptic element whose neck could not be followed to the parent
dendrite, or whose neck was truncated, we identified these elements as non-fully
reconstructed spines. These non-fully reconstructed spines were identified on the
basis of their size and shape, the lack of mitochondria and the presence of a spine
apparatus - or because they were filled with a characteristic fluffy material (used to
describe the fine and indistinct filaments present in the spines) - a term coined by
Peters et al. (1991) (see also del Río and DeFelipe, 1995). For simplicity, we will refer
to the fully reconstructed and non-fully reconstructed spines as spines, unless
otherwise specified.”
The inclusion of these data attempts to provide more accurate results regarding the
proportion of the postsynaptic targets. These new data have been added to tables 5,
5-1 and 5-2, as well as in the results section.
According to the reviewer’s comment, we have modified the discussion, as follows:
"In addition, the majority of the synapses that a pyramidal cell receives are on spines
and they represent the vast majority of AS synapses (DeFelipe and Fariñas, 1992). In
the human transentorhinal cortex, 75% of the synapses were AS on spines, which is
similar to the case of the AS found in the layer III of the temporal cortex (75%) using
the same analysis method (Cano-Astorga et al., 2021). This percentage was much
higher in the CA1 hippocampal field, as high as 94% in the superficial part of the CA1
stratum pyramidale (Montero-Crespo et al., 2020).
In both layers II and III of the entorhinal cortex, the percentage of AS on spines was
57%; 43% in the case of AS on dendritic shafts. This is a higher proportion of AS on
shafts than in the other human cortical regions previously analyzed using the same
techniques.
In addition, the comparison between control and Alzheimer’s disease samples did
not show a clear reduction in the proportion of AS on spines. Only a slightly lower
proportion of AS established on spines was found in layer II from Alzheimer’s disease
samples. Therefore, the lower synaptic density does not seem to be specifically
related to a loss of spines, but rather a general loss of synapses might occur
regardless of their postsynaptic targets.”13
20) Lines 584-586: EC provides excitatory inputs to the hippocampus: the indirect
pathway (trisynaptic), from layer II neurons, and the direct pathway (monosynaptic)
from layer III (reviewed in Insausti and Amaral, 2012; Marks et al., 2020).
This statement is confusing, both the tri-synaptic and monosynaptic perforant
pathways are directly targeting the hippocampus albeit different parts (dentate gyrus
versus CA1 directly). Did the authors mean to say inputs to CA1 instead of
"hippocampus”? Or just remove the words indirect or direct to read something like:
"provides excitatory inputs to the hippocampus from layer II neurons targeting the
dentate gyrus (trisynaptic pathway) and from layer III neurons monosynaptically
targeting CA1 (reviewed in Insausti and Amaral, 2012; Marks et al., 2020).”
RESPONSE 20
Thank you for this comment. We have changed this paragraph as suggested by the
reviewer. It now reads as follows:
"EC provides excitatory inputs to the hippocampus from layer II neurons targeting
the dentate gyrus (trisynaptic pathway) and from layer III neurons monosynaptically
targeting CA1 (reviewed in Insausti and Amaral, 2012; Marks et al., 2020).”
21) Lines 597-598: Layer III receives excitatory inputs from these same regions, but
also receives inputs from the claustrum and the amygdala (reviewed in Insausti and
Amaral, 2012; Schultz et al., 2015). Best to specify in monkeys (including species) when
referring to connectivity. Also, some work from the Barbas lab for the amygdala to
entorhinal connections can be added here. Also, there are inputs from the medial and
orbital prefrontal cortex that maybe worth mentioning (Work from the Barbas
[reviewed in {Barbas 2013}] and Price groups).
RESPONSE 21
To be clearer and shorten the discussion, as suggested by the reviewer, we have
changed this paragraph; it now reads as follows:
"Moreover, in layer II there are two subpopulations of excitatory neurons: spiny
stellate neurons (which project to DG, CA3 and CA2) and modified pyramidal neurons
(which project to stratum lacunosum in CA1). Projections from these neurons,
constituting the trisynaptic pathway, seem to be related to contextual memories
(reviewed in Mark et al., 2020). It has been proposed that this pathway is more
susceptible to premature degeneration than the monosynaptic pathway (Van
Hoesen et al., 2006; Llorens-Martín et al., 2014).
In addition, pyramidal neurons from layer III directly project to pyramidal neuron
dendrites located in the stratum moleculare of CA1, and it has been proposed that 14
these monosynaptic excitatory inputs drive temporal association learning (reviewed
in Mark et al., 2020).”
22) Lines 478-482: However, it has been shown that there are differences in the
number of GABAergic and glutamatergic synaptic inputs in different neuronal types in
other cortical regions of a variety of species (e.g., DeFelipe and Fariñas, 1992; Freund
and Buzsáki, 1996; DeFelipe, 1997; Somogyi et al., 1998; Schubert et al., 2007;
Markram et al., 2015;Tremblay et al., 2016).
Comparative studies from the Medalla/Luebke group have also shown some
differences in 3D ultrastructural features by area and species. Perhaps worth
mentioning here too (Hsu et al. 2017).
RESPONSE 22
This paragraph has been changed to:
"However, it has been shown that there are differences in the number of GABAergic
and glutamatergic synaptic inputs in different neuronal types in other cortical
regions of a variety of species (e.g., DeFelipe and Fariñas, 1992; Freund and Buzsáki,
1996; DeFelipe, 1997; Somogyi et al., 1998; Schubert et al., 2007; Markram et al.,
2015; Tremblay et al., 2016; Hsu et al., 2017).”
Minor points and typos:
23) 376 element was analyzed - space is missing
24) Lines 401-402: “the difference in the mean cortical thickness was no evident (Table
1-1)” - should be “not evident"
25) Lines 415-418:Since our present neuronal volume fraction estimations are based
on semithin sections, and different types of neurons have not been identified, we
cannot rule out a selective neuronal loss that may affect either “to excitatory neurons
or to GABAergic interneurons or to both cell types.” Seems like grammatical use of “to"
is incorrect here, and the sentence is unclear. Are the authors referring to the loss
affecting (selective loss of) either excitatory, inhibitory or both? If so, delete the word
"to"
RESPONSE
The manuscript has been revised and edited according to the reviewer’s comments.
We would like to thank the reviewer for drawing our attention to these points
References
- Alonso-Nanclares L, Gonzalez-Soriano J, Rodriguez JR, DeFelipe J (2008) Gender differences in human cortical synaptic density. Proc Natl Acad Sci USA 105:14615–14619. 10.1073/pnas.0803652105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association (2020) Alzheimer’s disease facts and figures. Alzheimer Dement 16:391. [DOI] [PubMed] [Google Scholar]
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimarães DM, Szczupak D, Parente-Bruno DR, Carvalho LRB, Polichiso L, Gomes BV, Oliveira LM, Rodriguez RD, Leite REP, Ferretti-Rebustini REL, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Lent R (2013) Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain 136:3738–3752. 10.1093/brain/awt273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Rubak E, Turner R (2015) Spatial point patterns: methodology and applications with R. Boca Raton: Chapman and Hall/CRC. [Google Scholar]
- Beaulieu C, Colonnier M (1985) A laminar analysis of the number of round-asymmetrical and flat-symmetrical synapses on spines, dendritic trunks, and cell bodies in area 17 of the cat. J Comp Neurol 231:180–189. 10.1002/cne.902310206 [DOI] [PubMed] [Google Scholar]
- Blazquez-Llorca L, Merchán-Pérez Á, Rodríguez JR, Gascón J, DeFelipe J (2013) FIB/SEM technology and AD: three-dimensional analysis of human cortical synapses. J Alzheimers Dis 34:995–1013. 10.3233/JAD-122038 [DOI] [PubMed] [Google Scholar]
- Blazquez-Llorca L, Woodruff A, Inan M, Anderson SA, Yuste R, DeFelipe J, Merchan-Perez A (2015) Spatial distribution of neurons innervated by chandelier cells. Brain Struct Funct 220:2817–2834. 10.1007/s00429-014-0828-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM (2011) Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus 21:354–373. 10.1002/hipo.20768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1992) The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res 15:6–31. 10.1016/0168-0102(92)90014-4 [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K (2012) Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol 25:708–714. 10.1097/WCO.0b013e32835a3432 [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K (2020) From the entorhinal region via the prosubiculum to the dentate fascia: Alzheimer disease-related neurofibrillary changes in the temporal allocortex. J Neuropathol Exp Neurol 79:163–175. 10.1093/jnen/nlz123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Astorga N, DeFelipe J, Alonso-Nanclares L (2021) Three-dimensional synaptic organization of layer III of the human temporal neocortex. Cereb Cortex. Advance online publication. Retrieved May 17, 2021. doi: 10.1093/cercor/bhab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fu AKY, Ip NY (2019) Synaptic dysfunction in Alzheimer’s disease: mechanisms and therapeutic strategies. Pharmacol Ther 195:186–198. 10.1016/j.pharmthera.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R (2004) A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology 63:1155–1162. 10.1212/01.wnl.0000140626.48118.0a [DOI] [PubMed] [Google Scholar]
- Colom-Cadena M, Spires-Jones T, Zetterberg H, Blennow K, Caggiano A, DeKosky ST, Fillit H, Harrison JE, Schneider LS, Scheltens P, de Haan W, Grundman M, van Dyck CH, Izzo NJ, Catalano SM; Synaptic Health Endpoints Working Group (2020) The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res Ther 12:21. 10.1186/s13195-020-00588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J (1997) Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 14:1–19. 10.1016/s0891-0618(97)10013-8 [DOI] [PubMed] [Google Scholar]
- DeFelipe J (2011) The evolution of the brain, the human nature of cortical circuits and intellectual creativity. Front Neuroanat 5:29. 10.3389/fnana.2011.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J (2015) The anatomical problem posed by brain complexity and size: a potential solution. Front Neuroanat 9:104. 10.3389/fnana.2015.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Fairén A (1993) A simple and reliable method for correlative light and electron microscopic studies. J Histochem Cytochem 41:769–772. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Fariñas I (1992) The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic input. Prog Neurobiol 39:563–607. 10.1016/0301-0082(92)90015-7 [DOI] [PubMed] [Google Scholar]
- del Río MR, DeFelipe J (1995) A light and electron microscopic study of calbindin D-28k immunoreactive double bouquet cells in the human temporal cortex. Brain Res 690:133–140. 10.1016/0006-8993(95)00641-3 [DOI] [PubMed] [Google Scholar]
- Dickson DW (1997) The pathogenesis of senile plaques. J Neuropathol Exp Neurol 56:321–339. 10.1097/00005072-199704000-00001 [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P (1995) Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging 16:285–304. 10.1016/0197-4580(95)00013-5 [DOI] [PubMed] [Google Scholar]
- Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, Insausti R, DeFelipe J, Alonso-Nanclares L (2018) Three-dimensional analysis of synapses in the transentorhinal cortex of Alzheimer’s disease patients. Acta Neuropathol Commun 6:20. 10.1186/s40478-018-0520-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, DeFelipe J, Alonso-Nanclares L (2019) 3D electron microscopy study of synaptic organization of the normal human transentorhinal cortex and its possible alterations in Alzheimer’s disease. eNeuro 6:ENEURO.0140-19.2019–17. 10.1523/ENEURO.0140-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, DeFelipe J, Alonso-Nanclares L (2021) 3D ultrastructural study of synapses in the human entorhinal cortex. Cereb Cortex 31:410–425. 10.1093/cercor/bhaa233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhoury M (2018) Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr Neuropharmacol 16:508–518. 10.2174/1570159X15666170720095240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I (2012) Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog Neurobiol 97:38–51. 10.1016/j.pneurobio.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM (2017) Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci 40:347–357. 10.1016/j.tins.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470. [DOI] [PubMed] [Google Scholar]
- Froemke RC (2015) Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci 38:195–219. 10.1146/annurev-neuro-071714-034002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y (2004a) Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol 468:86–95. 10.1002/cne.10950 [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y (2004b) Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neurosci 125:615–623. 10.1016/j.neuroscience.2004.02.025 [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16:4491–4500. 10.1523/JNEUROSCI.16-14-04491.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG (1959) Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat 93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394. 10.1111/j.1699-0463.1988.tb05320.x [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Pickett E, Spires-Jones TL (2016) Synaptic pathology: a shared mechanism in neurological disease. Ageing Res Rev 28:72–84. 10.1016/j.arr.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Honer WG, Dickson DW, Gleeson J, Davies P (1992) Regional synaptic pathology in AD. Neurobiol Aging 13:375–382. 10.1016/0197-4580(92)90111-a [DOI] [PubMed] [Google Scholar]
- Hsu A, Luebke JI, Medalla M (2017) Comparative ultrastructural features of excitatory synapses in the visual and frontal cortices of the adult mouse and monkey. J Comp Neurol 525:2175–2191. 10.1002/cne.24196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR (1986) Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol 20:472–481. 10.1002/ana.410200406 [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG (2012) Hippocampal formation. In: The human nervous system (Mai JK, Paxinos G, eds), pp 896–942. Cambridge: Academic Press. [Google Scholar]
- Insausti R, Muñoz-López M, Insausti AM, Artacho-Pérula E (2017) The human periallocortex: layer pattern in presubiculum, parasubiculum and entorhinal cortex. A review. Front Neuroanat 11:84. 10.3389/fnana.2017.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ (1999) Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J Comp Neurol 412:292–302. [DOI] [PubMed] [Google Scholar]
- Kobro‐Flatmoen A, Witter MP (2019) Neuronal chemo‐architecture of the entorhinal cortex: a comparative review. Eur J Neurosci 50:3627–3662. 10.1111/ejn.14511 [DOI] [PubMed] [Google Scholar]
- Kondo H, Lavenex P, Amaral DG (2009) Intrinsic connections of the macaque monkey hippocampal formation: II. CA3 connections. J Comp Neurol 515:349–377. 10.1002/cne.22056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG (2000) Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 10:420–430. [DOI] [PubMed] [Google Scholar]
- Llorens-Martín M, Blazquez-Llorca L, Benavides-Piccione R, Rabano A, Hernandez F, Avila J, DeFelipe J (2014) Selective alterations of neurons and circuits related to early memory loss in AD. Front Neuroanat 8:38. 10.3389/fnana.2014.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Nicoll RA, Malenka RC, Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic. Nat Neurosci 3:545–550. 10.1038/75714 [DOI] [PubMed] [Google Scholar]
- Marks WD, Yamamoto N, Kitamura T (2020) Complementary roles of differential medial entorhinal cortex inputs to the hippocampus for the formation and integration of temporal and contextual memory. Eur J Neurosci 00:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso-Nanclares L, Antille N, Arsever S, Kahou GAA, Berger TK, Bilgili A, Buncic N, Chalimourda A, Chindemi G, Courcol JD, Delalondre F, Delattre V, Druckmann S, et al. (2015) Reconstruction and simulation of neocortical microcircuitry. Cell 163:456–492. 10.1016/j.cell.2015.09.029 [DOI] [PubMed] [Google Scholar]
- Masliah E, Terry RD, Alford M, Deteresa R (1990) Quantitative immunohistochemistry of synaptophysin in human neocortex: an alternative method to estimate density of presynaptic terminals in paraffin sections. J Histochem Cytochem 38:837–844. 10.1177/38.6.2110586 [DOI] [PubMed] [Google Scholar]
- Megías M, Emri Z, Freund TF, Gulyás AI (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102:527–540. 10.1016/S0306-4522(00)00496-6 [DOI] [PubMed] [Google Scholar]
- Merchán-Pérez A, Rodríguez JR, Alonso-Nanclares L, Schertel A, DeFelipe J (2009) Counting synapses using FIB/SEM microscopy: a true revolution for ultrastructural volume reconstruction. Front Neuroanat 3:18. 10.3389/neuro.05.018.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchán-Pérez A, Rodríguez JR, González S, Robles V, DeFelipe J, Larrañaga P, Bielza C (2014) Three-dimensional spatial distribution of synapses in the neocortex: a dual-beam electron microscopy study. Cereb Cortex 24:1579–1588. 10.1093/cercor/bht018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen M, Alafuzoff I, Tapiola T, Soininen H, Miettinen R (1999) Subfield- and layer-specific changes in parvalbumin, calretinin and calbindin-D28K immunoreactivity in the entorhinal cortex in Alzheimer’s disease. Neuroscience 92:515–532. 10.1016/S0306-4522(99)00047-0 [DOI] [PubMed] [Google Scholar]
- Mironov A (2017) Stereological morphometric grids for ImageJ. Ultrastruc Pathol 41:126–126. 10.1080/01913123.2016.1272665 [DOI] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The consortium to establish a registry for AD (CERAD). Part II. Standardization of the neuropathologic assessment of AD. Neurology 41:479–486. 10.1212/wnl.41.4.479 [DOI] [PubMed] [Google Scholar]
- Montero-Crespo M, Domínguez-Álvaro M, Rondon-Carrillo P, Alonso-Nanclares L, DeFelipe J, Blazquez-Llorca L (2020) Three-dimensional synaptic organization of the human hippocampal CA1 field. Elife 9:e57013. 10.7554/eLife.57013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Crespo M, Domínguez-Álvaro M, Alonso-Nanclares L, DeFelipe J, Blazquez-Llorca L (2021) Three-dimensional analysis of synaptic organization in the hippocampal CA1 field in Alzheimer’s disease. Brain 144:553–573. 10.1093/brain/awaa406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes J, Peña JM, DeFelipe J, Herreras O, Merchan-Perez A (2015) The influence of synaptic size on AMPA receptor activation: a Monte Carlo model. PLoS One 10:e0130924. 10.1371/journal.pone.0130924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Alonso-Nanclares L, Rodríguez JR, DeFelipe J, Rodríguez Á, Merchán- Pérez Á (2011) Espina: a tool for the automated segmentation and counting of synapses in large stacks of electron microscopy images. Front Neuroanat 18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Rodríguez A, Rodríguez JR, DeFelipe J, Merchán-Pérez A (2013) Characterization and extraction of the synaptic apposition surface for synaptic geometric analysis. Front Neuroanat 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG (2012) The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13:240–250. 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramori F, Kobayashi K, Nakamura I (1998) A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer’s disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatry Clin Neurosci 52:593–599. 10.1111/j.1440-1819.1998.tb02706.x [DOI] [PubMed] [Google Scholar]
- Oorschot D, Peterson D, Jones D (1991) Neurite growth from, and neuronal survival within, cultured explants of the nervous system: a critical review of morphometric and stereological methods, and suggestions for the future. Prog Neurobiol 37:525–546. 10.1016/0301-0082(91)90007-n [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL (1996) The morphology of synapses. J Neurocytol 25:687–700. 10.1007/BF02284835 [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD (1991) The fine structure of the nervous system: neurons and their supporting cells, pp 70–100. New York: Oxford University Press. [Google Scholar]
- Rajmohan R, Reddy PH (2017) Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimers Dis 57:975–999. 10.3233/JAD-160612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS (1994) Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res 102:227–243. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA (1993) Synapse loss in the temporal lobe in Alzheimer’s disease. Ann Neurol 33:190–199. 10.1002/ana.410330209 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA (1998) Synaptic density in the inner molecular layer of the hippocampal dental gyrus in Alzheimer’s disease. J Neuropathol Exp Neurol 57:1146–1153. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA (2003) Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiol Aging 24:1029–1046. 10.1016/j.neurobiolaging.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA (2006) Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis 9:101–115. 10.3233/jad-2006-9s312 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Dekosky ST, Price DA (1990) Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 11:29–37. 10.1016/0197-4580(90)90059-9 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sparks DL, Price DA (1993) Quantitative assessment of synaptic density in the entorhinal cortex in Alzheimer’s disease. Ann Neurol 34:356–361. 10.1002/ana.410340309 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sparks DL, Price DA (1996) Quantitative assessment of synaptic density in the outer molecular layer of the hippocampal dentate gyrus in Alzheimer’s disease. Dementia 7:226–232. 10.1159/000106884 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Sparks LD (2001) Quantitative assessment of possible age related change in synaptic numbers in the human frontal cortex. Neurobiol Aging 22:355–365. 10.1016/S0197-4580(01)00222-6 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384. 10.1016/j.neurobiolaging.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Dekosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68:1501–1508. 10.1212/01.wnl.0000260698.46517.8f [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ (2011) Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer disease. J Alzheimers Dis 24:547–557. 10.3233/JAD-2011-101782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Ansari MA, Roberts KN, Schmitt FA, Ikonomovic MD, Mufson EJ (2015) Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J Alzheimers Dis 43:1073–1090. 10.3233/JAD-141518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kötter R, Staiger JF (2007) Mapping functional connectivity in barrel-related columns reveals layer- and cell type-specific microcircuits. Brain Struct Funct 212:107–119. 10.1007/s00429-007-0147-z [DOI] [PubMed] [Google Scholar]
- Schultz H, Sommer T, Peters J (2015) The role of the human entorhinal cortex in a representational account of memory. Front Hum Neurosci 9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791. 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- Sharpe D (2015) Your chi-square test is statistically significant: now what? Pract Assess Res Evaluation 20:8. [Google Scholar]
- Šimić G, Babić Leko M, Wray S, Harrington CR, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L, de Silva R, Di Giovanni G, Wischik CM, Hof PR (2017) Monoaminergic neuropathology in Alzheimer’s disease. Prog Neurobiol 151:101–138. 10.1016/j.pneurobio.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Rubenstein JLR (2019) Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry 24:1248–1257. 10.1038/s41380-019-0426-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodkin A, Van Hoesen GW (1996) Entorhinal cortex modules of the human brain. J Comp Neurol 365:610–617. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamás G, Lujan R, Buhl EH (1998) Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev 26:113–135. 10.1016/S0165-0173(97)00061-1 [DOI] [PubMed] [Google Scholar]
- Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ (1997) Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. JNeuropathol Exp Neurol 56:933–944. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800. 10.1212/wnl.58.12.1791 [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B (2016) GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91:260–292. 10.1016/j.neuron.2016.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen GW, Hyman BT (1990) Hippocampal formation: anatomy and the patterns of pathology in Alzheimer’s disease. Prog Brain Res 83:445–457. 10.1016/s0079-6123(08)61268-6 [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Hyman BT, Damasio AR (1991) Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1:1–8. 10.1002/hipo.450010102 [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R (2006) The Parahippocampal Gyrus in Alzheimer’s Disease: Clinical and Preclinical Neuroanatomical Correlates. Ann NY Acad Sci 911:254–274. 10.1111/j.1749-6632.2000.tb06731.x [DOI] [PubMed] [Google Scholar]
- von Gunten A, Kövari E, Bussière T, Rivara C-B, Gold G, Bouras C, Hof PR, Giannakopoulos P (2006) Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol Aging 27:270–277. 10.1016/j.neurobiolaging.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhao M, Han Y, Zhang H (2020) GABAergic inhibitory interneuron deficits in Alzheimer’s disease: implications for treatment. Front Neurosci 14:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, McInnes J, Wierda K, Holt M, Herrmann AG, Jackson RJ, Wang YC, Swerts J, Beyens J, Miskiewicz K, Vilain S, Dewachter I, Moechars D, De Strooper B, Spires-Jones TL, De Wit J, Verstreken P (2017) Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun 8:15295. 10.1038/ncomms15295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Yu Y (2018) Synaptic E-I balance underlies efficient neural coding. Front Neurosci 12:46. 10.3389/fnins.2018.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data from the ultrastructural analysis of neuropil from Layers II and III of the EC for individual cases. All volume and distance data are corrected for shrinkage. All, includes AS+SS synapses; No., number. Download Table 2-1, DOCX file (48.4KB, docx) .
Area (nm2) and perimeter (nm) of the SAS in Layers II and III of the EC for individual cases. All data are corrected for shrinkage factor. Download Table 3-1, DOCX file (48.4KB, docx) .
EspINA software user interface. FIB/SEM sections are viewed through the xyz planes. AS (green) are shown, established on dendritic spines originating from a dendritic shaft (purple), shown as D529 in Figure 5.
EspINA software 3D viewer. A stack of images is fully represented in the three orthogonal planes (x, y, and z). The video shows the 3D reconstruction of a single dendritic segment (purple; D529 shown in Fig. 5) and the 3D reconstruction of all AS (green) and SS (red) established in this 3D reconstructed fragment.
EC cortical thickness in control and Alzheimer cases. All data are corrected for shrinkage factor. Download Table 1-1, DOCX file (48.4KB, docx) .
Volume fraction occupied by cortical elements in Layers II and III of the EC. Data are given as mean ± SD; Vneu, volume fraction occupied by neurons; Vg, volume fraction occupied by glia; Vbv, volume fraction occupied by blood vessels; Vn, volume fraction occupied by neuropil. Data from individual cases are shown in Extended Data Table 1-3. Download Table 1-2, DOCX file (48.4KB, docx) .
Light microscopy data on volume fraction occupied by cortical elements in Layers II and III of the EC for individual cases. All volume data are corrected for shrinkage. Vneu, volume fraction occupied by neurons; Vg, volume fraction occupied by glia; Vbv, volume fraction occupied by blood vessels; Vn, volume fraction occupied by neuropil. Download Table 1-3, DOCX file (48.4KB, docx) .
Proportion of macular, perforated, horseshoe-shaped, and fragmented synapses in Layer II of the EC for individual cases. Data are given as percentages with the absolute number of synapses studied in parentheses. Download Table 4-1, DOCX file (48.4KB, docx) .
Proportion of macular, perforated, horseshoe-shaped, and fragmented synapses in Layer III of the EC for individual cases. Data are given as percentages with the absolute number of synapses studied in parentheses. Download Table 4-2, DOCX file (48.4KB, docx) .
Distribution of AS and SS on spines and dendritic shafts in Layer II of the EC for individual cases. Synapses on spines have been subdivided into those that are established on spine heads and those that are established on spine necks. Synapses on spine have been recorded separately. Moreover, we differentiated between aspiny and spiny dendritic shafts. Data are expressed as percentages with the absolute number of synapses studied given in parentheses. Download Table 5-1, DOCX file (48.4KB, docx) .
Distribution of AS and SS on spines and dendritic shafts in Layer III of the EC for individual cases. Synapses on spines have been subdivided into those that are established on spine heads and those that are established on spine necks. Synapses on spines have been recorded separately. Moreover, we differentiated between aspiny and spiny dendritic shafts. Data are expressed as percentages with the absolute number of synapses studied given in parentheses. Download Table 5-2, DOCX file (48.4KB, docx) .
Area (nm2) and perimeter (nm) of the macular, perforated, horseshoe-shaped, and fragmented SAS in Layer II of the EC. All data are corrected for shrinkage. Download Table 4-3, DOCX file (48.4KB, docx) .
Area (nm2) and perimeter (nm) of the macular, perforated, horseshoe-shaped, and fragmented SAS in Layer III of the EC. All data are corrected for shrinkage factor. Download Table 4-4, DOCX file (48.4KB, docx) .
Frequency histograms of the SAS area (A, B, E, F) and perimeter (C, D, G, H) of macular, perforated, horseshoe-shaped, and fragmented AS from control cases (A, C, E, G) and from AD cases (B, D, F, H), in Layers II and III of the EC. The SAS area and perimeter of macular synapses were significantly smaller than in perforated, horseshoe-shaped, and fragmented synapses (KW, p < 0.0001). No differences were found between control and AD cases (KS, p > 0.0001). Download Figure 9-1, TIF file (392.7KB, tif) .
Frequency histograms of the SAS area (A, B, E, F) and perimeter (C, D, G, H) of AS targeting spine heads, spine necks and dendritic shafts from control cases (A, C, E, G) and from AD cases (B, D, F, H), in both Layers II and III of the EC. No differences were found between groups (KS, p > 0.0001). Download Figure 10-1, TIF file (368.3KB, tif) .
Data Availability Statement
Most data generated or analyzed during this study are included in the main text and the tables. The control datasets used and analyzed are available on the EBRAINS Knowledge Graph (doi: 10.25493/3GMJ-FEZ). Datasets used during the current study available from the corresponding author on reasonable request.