Abstract
There are no FDA-approved treatments for cannabis use disorder (CUD). Preclinical research has shown that the 5HT-2c agonist, lorcaserin, attenuates cue-induced reinstatement of THC-seeking and self-administration. The goal of this placebo-controlled, counterbalanced, within-subject human laboratory study was to examine lorcaserin’s effects on cannabis intoxication and self-administration. Lorcaserin (10 mg BID) was administered during one of two 13-day inpatient phases, and placebo during the other; each phase was separated by ≥ 7-days of washout. Inpatient phases comprised: 1) standardized cannabis administration (7.0% THC) at no financial cost (intoxication), counterbalanced with 2) the option to self-administer cannabis following either 0 or 3 days of abstinence. Cognitive task performance, food intake, subjective ratings of drug effects, objective/subjective sleep measures, and tobacco cigarette use were also assessed. Fifteen normal-weight, daily cannabis users (4F, 11M) not seeking treatment for CUD completed the study. Lorcaserin significantly reduced cannabis self-administration following 0 and 3 days of cannabis abstinence, and also reduced craving for cannabis during abstinence. Lorcaserin produced small but significant increases in positive cannabis ratings and body weight relative to placebo. Lorcaserin also reduced tobacco cigarette smoking on days of cannabis administration relative to placebo. During abstinence, subjective but not objective measures of sleep quality worsened during lorcaserin maintenance. Overall, lorcaserin’s ability to decrease drug-taking and cannabis craving in non-treatment seeking cannabis users supports further investigation of 5HT-2C agonists as potential pharmacotherapies for CUD.
Keywords: cannabis use disorder, lorcaserin, marijuana
Introduction
Concurrent to rapidly changing legislation across the United States, cannabis use among adults is increasing. Recent data show that 4.4 million individuals in the U.S. meet criteria for Cannabis Use Disorder (CUD) (1, 2), and over 250,000 individuals sought treatment for their cannabis use in 2017 (2). Yet, outcomes for those who seek treatment for CUD are often poor; the average treatment-seeking cannabis user makes a minimum of six unsuccessful quit attempts (3) and fewer than half are able to maintain long-term abstinence (4). Given that there are no FDA-approved pharmacological treatments for CUD, developing such a medication is imperative.
Human laboratory models offer an efficient way to test the effects of potential therapeutic approaches on discrete behaviors critical to the maintenance of daily cannabis use. Medications that show a positive signal in the laboratory can then be validated in a randomized controlled clinical trial for patients seeking treatment for CUD. In our laboratory model, we have largely focused on testing whether medications decrease symptoms of cannabis withdrawal (e.g., irritability, anxiety, disrupted sleep and food intake) and decrease cannabis self-administration after a period of abstinence, as a laboratory measure of relapse, relative to placebo. Specifically, nontreatment-seeking, daily cannabis smokers undergo abstinence from cannabis for several days in an inpatient setting and withdrawal symptoms are measured. Participants then have the option to ‘relapse,’ defined as purchasing individual puffs of smoked cannabis (4–12). We hypothesize that medications that decrease withdrawal symptoms and the subsequent return to cannabis self-administration would be useful for relapse prevention in clinical settings.
Another strategy for developing a medication to treat CUD is to reduce cannabis’ positive subjective (e.g., ratings of cannabis ‘good drug effect’) and reinforcing effects (13), and thereby help to initiate abstinence in those who are still using cannabis. We hypothesize that medications that decrease ongoing cannabis self-administration could be useful for abstinence initiation in the clinic (13).
The objective of the current study was to assess the selective 5HT-2c agonist, lorcaserin in our laboratory model of CUD. Substance use disorders, similar to obesity, reflect learned behaviors that persist despite negative consequences (e.g., (14–16), and 5HT-2c agonists influence behaviors motivated by a wide range of reinforcers. Preclinical studies show that 5HT-2c agonists reduce the stimulating, discriminative-stimulus, and reinforcing effects of nicotine, ethanol, opioids, and cocaine, while also attenuating reinstatement of nicotine and cocaine seeking behaviors (17–27) and suppressing cocaine self-administration (28). These effects persist with repeated medication administration (28, 29), essential for any medication used to treat a substance use disorder (30).
The mechanism by which 5HT-2c receptor agonists reduce the abuse-related effects of a range of drugs is likely by inhibiting mesocorticolimbic dopamine release (31–33). 5-HT2c receptors overlap with dopaminergic and GABAergic receptors in brain areas relevant to drug reward and seeking, including the prefrontal cortex, the ventral tegmental area, caudate putamen and the nucleus accumbens (32, 33). 5HT-2c agonists appear to improve inhibitory control (30), supporting their utility across a range of drugs of abuse for both reducing the likelihood of relapse or reducing ongoing drug-taking (31).
Lorcaserin has been tested clinically as a potential treatment for smoking cessation (34), and for opioid (35) and cocaine use disorders (36). While lorcaserin did not reduce either opioid or cocaine self-administration in humans (32, 33), the medication (10 mg BID for 3 months) was shown to produce higher rates of tobacco use cessation (15.3%) relative to placebo (5.6%) (34). Little is known about the influence of 5HT-2c agonists on cannabis use. One study in non-human primates showed that lorcaserin dose-dependently reduced the reinforcing effects of Δ9-tetrahydrocannabinol (THC) and reduced cue-induced reinstatement of extinguished THC-seeking behavior (a model of relapse) at doses that did not alter food-maintained behavior (Justinova and colleagues, personal communication). Since these data suggest that a 5HT-2c agonist could both reduce ongoing cannabis self-administration as well as relapse to cannabis use, the objective of this human laboratory study was to assess lorcaserin’s effects under non-abstinent conditions, as a model of relapse (i.e., cannabis self-administration after three days of imposed cannabis abstinence), abstinence initiation (i.e., cannabis self-administration after no days of abstinence) and the abuse-related effects of cannabis (ratings of ‘good drug effect,’ ‘high’). We hypothesized that lorcaserin would attenuate cannabis self-administration under both abstinent and non-abstinent conditions.
Materials and Methods
Participants
Eligible participants were healthy, non-treatment seeking daily cannabis smokers, 21 to 50 years of age. Inclusion criteria required that participants smoke the equivalent of a minimum two cannabis cigarettes/day on at least 6 days a week for the past four weeks, assessed by both self-report and urine toxicology. Exclusion criteria included: (1) repeated use of other illicit substances (verified by urine toxicology); (2) meeting DSM-5 criteria for any current psychiatric disorder requiring medical intervention; (3) daily use of prescription or non-prescription medications; (4) a history of heart disease or cardiac risk factors. Eligibility determination was made by a physical exam and psychiatric evaluation, an electrocardiogram, urinalysis, urine toxicology, and blood chemistry panels. Participants signed a consent form approved by The New York State Psychiatric Institute (NYSPI) Institutional Review Board, which described the study in detail, and outlined potential risks and indicated that one of several FDA-approved medications, or placebo, might be administered. All were compensated for their participation in the study (NCT03253926). Note, lorcaserin had been FDA-approved for the treatment of obesity at the time this study was conducted but has since been removed from the market because of potential cancer risks.
Procedures
Participants, in cohorts of 3–4, were enrolled in a study comprising two 13-day inpatient phases, each of which was preceded by two medication administration outpatient days to achieve steady-state levels prior to key behavioral measures. Each phase tested a different medication condition (lorcaserin 10 mg or placebo BID) in a within-subjects design, with at least one medication-free week between phases to allow for medication clearance, during which participants were free to resume cannabis use. Table 1 illustrates a representative timeline for one phase of the study. On the outpatient medication days, participants came to the laboratory so that we could observe their morning capsule administration, take their vital signs (blood pressure and heart rate), and give them take-home medication for their evening dose. Their recent cannabis use, medication compliance (urinary fluorescence from riboflavin included in capsules) and any related side effects were also assessed. Prior to each inpatient phase, participants were trained on study procedures and also completed a ‘sample’ cannabis administration to familiarize them with (1) the paced-puff procedure of cannabis smoking where inhalation duration, time spent holding smoke in the lungs, and inter-puff interval were standardized (37) for three puffs of the cigarette, and (2) the cannabis strength that would be available for self-administration during the study (7.0% THC, ca. 800 mg; provided by NIDA).
Table 1.
Representative Inpatient Schedule
| Day | 2 Outpatient Days | Move-in | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| Condition | Acute Effects | ‘Abstinence Initiation’* | Acute Effects | ||||
| Cannabis | Exp-Administration | Self-Administration | Exp-Administration | ||||
| Lorcaserin (mg BID) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Day | 6 | 7 | 8 | 9 | 10 | 11 | Move-out |
| Condition | Abstinence | ‘Relapse’ | |||||
| Cannabis | None | Self-Administration | |||||
| Lorcaserin (mg BID) | 10 | 10 | 10 | 10 | 10 | 10 | None |
Cannabis = 7.0% THC. Exp-Administration = Experimenter-administered: Each participant smoked 3 puffs of cannabis at no financial cost. Self-administration: Participants had the option of purchasing individual cannabis puffs. ‘Abstinence Initiation’: Cannabis self-administration following 0 days of cannabis abstinence. ‘Relapse:’ Self-administration following 3 days of cannabis abstinence.
Order of abstinence initiation and withdrawal/relapse conditions was counterbalanced, as were doses of lorcaserin (0, 10 mg BID).
The inpatient phase of the study was conducted in the residential laboratory at NYSPI, equipped with four private bedrooms, a recreational area, two bathrooms, and two vestibules, and continuously monitored by study staff via audio- and video-output. Each morning, vital signs were taken. Throughout the day, participants completed scheduled assessments including a sleep scale, a mood scale, and subjective drug effects and psychomotor task batteries. The recreational area was available during a scheduled lunch (1215–1245), as well as in the evening (1700–2200). At 2330, a final mood scale was completed and ‘lights out’ began at 2400, lasting until 0800 the next morning.
Cannabis Conditions
As shown in Table 1, each inpatient phase began with an experimenter-administered cannabis day (Day 1, “Acute Effects”), which standardized cannabis exposure across participants, while also testing lorcaserin’s influence on the acute subjective effects of cannabis. Days 2–4 assessed self-administration (a model of ‘abstinence initiation’). Day 5 was another day of experimenter-administered cannabis to standardize cannabis exposure. Participants were abstinent from cannabis for the subsequent 3 days (Days 6–8) followed by three days of self-administration (a model of ‘relapse’). Note, the order of the abstinence and relapse phases was counterbalanced.
Each morning, participants were told which of three possible cannabis conditions the day would include: (1) experimenter-administered cannabis at no cost, (2) no cannabis available that day (abstinence); (3) cannabis available for self-administration. On experimenter-administered days, participants were guided through smoking three puffs of cannabis at six timepoints: 1000, 1130, 1300, 1430, 1600, and 2200. On self-administration days modelling abstinence initiation, participants could choose to self-administer up to three puffs of cannabis at these same six timepoints at a cost of $2 per puff, using their study earnings. On self-administration days modelling relapse, participants could self-administer up to three puffs at the same timepoints; on these days, the first self-administered puff of the day cost $7, and each subsequent puff cost $1. This laboratory model of relapse is designed so that the initial return to cannabis use is financially costly, in an effort to model the decision to relapse that occurs clinically in those seeking treatment for CUD (8, 9, 13). All self-administered cannabis was smoked privately so that participants were blind to each other’s choices. Money earned each inpatient day that was not spent on cannabis was given to the participants as part of their study compensation.
Capsule Administration
The NYSPI Research Pharmacy packaged lorcaserin and placebo in size 00 opaque capsules with riboflavin and lactose filler. Medication administration was double-blind, randomized, and counterbalanced across phases.
Mood Scales and Cognitive Tasks
Participants completed a 44-item subjective-effects questionnaire visual analog scale (VAS) eight times per day, comprising a series of 100-mm lines labeled ‘Not at all’ (0 mm) and ‘Extremely’ (100 mm) on either end, which included mood, physical symptom, and drug effect descriptors, meant to probe cannabis’ effects. Participants were told to rate the extent to which each descriptor applied to how they felt at that moment. Based on a cluster analysis, we employed arithmetic means of individual item scores to reduce 34 of the 44 items into eight subscales: miserable (‘miserable,’ ‘depressed’); irritable (‘irritable,’ ‘angry’); anxious (e.g. ‘anxious,’ ‘on edge’); bad effect (e.g. ‘dizzy,’ ‘upset stomach’); tired (e.g. ‘tired,’ ‘sleepy’); social (e.g. ‘friendly,’ ‘talkative’); high (‘high,’ ‘good effect’) and confused (e.g., ‘confused,’ ‘can’t concentrate.’). We also analyzed individual VAS ratings of drug craving: ‘I want…Cannabis,’ ‘Alcohol’ and ‘Cigarettes.’ A Drug-Effect Questionnaire (38) was administered each evening (two hours and 45 minutes after capsule administration) to assess capsule effects, and included “Drug Liking”, “Good Drug Effect”, “Strong Drug Effect”, and “Bad Drug Effects”. Participants also completed a battery of cognitive tasks 6 times throughout each day, which consisted of a 3-minute digit-symbol substitution task, a 3-minute repeated acquisition task, a 10-minute divided attention task, a 10-minute rapid information task and an immediate and delayed digit-recall task prior to first possible cannabis cigarette administration time and 15 to 45 minutes after the later cannabis cigarette administration times.
Food
Each inpatient morning, participants were given a box of food items that included snacks and beverages. Frozen meals and additional units of any item were available by request. To report their food intake throughout the day or to request additional items, participants scanned custom-designed bar codes which would automatically populate the food product and corresponding portion into data files. Food was not available between 2330 and 0815.
Sleep
Objective assessments of sleep latency (the length of time until one falls asleep) and sleep efficiency (the percentage of time spent asleep during the lights-out period [0000–0800]), were obtained using the Actiwatch® Activity Monitoring System (Respironics Company, Bend OR). Subjective ratings of sleep were also collected each morning, including the perceived quality of the previous night’s sleep, using a 7-item VAS sleep questionnaire (10).
Tobacco Cigarette Smoking
Participants who reported smoking cigarettes prior to enrollment were permitted to smoke up to 10 cigarettes ad libitum daily. If participants smoked more than 10 cigarettes in one day, they were charged $0.25/each additional cigarette. This was instituted to discourage participants from increasing their cigarette smoking in the inpatient setting. The number of tobacco cigarettes smoked was recorded by counting the cigarette butts in each participant’s ashtray each evening.
Data Analysis
Repeated measures analyses of variance including two within-subject factors (medication dose, inpatient day) with planned comparisons were used to determine the effects of lorcaserin, relative to placebo, on 1) cannabis self-administration under abstinent and non-abstinent conditions, and 2) subjective effects following controlled cannabis administration. Outcomes included the following: number of cannabis puffs that were self-administered, daily peak subjective-effects ratings, drug craving, objective and subjective sleep measures, food intake [total energy intake, percent macronutrient (fat, protein, carbohydrate)], body weight, blood pressure and heart rate, task performance, and the number of cigarettes smoked per day. Significance was determined at α = 0.02. Huynh-Feldt corrections were used. Note, repeated-measures, within-subjects designs result in substantial correlations between levels, and are a powerful statistical approach. Based on our previous study (7), a sample size of 15 study completers provides > 90 percent power to detect changes in cannabis self-administration and subjective effects as a function of lorcaserin vs. placebo. Effects of lorcaserin on the frequency of side effects were assessed using Sign Tests, a non-parametric test used to identify consistent differences between pairs of observations (lorcaserin vs. placebo). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Participant characteristics
Table 2 presents demographic and substance use data on the 15 daily cannabis smokers (4F, 11M) who completed the study. An additional 5 participants (1F, 4M) started but failed to complete the study (one no-show, one report of migraine after first capsule administration, one death in family, and two had difficulty living inpatient). In addition, to ensure a cohort of four participants for each inpatient study phase, we enrolled six additional volunteers to begin outpatient dosing prior to the first study phase. Those who were reliable for outpatient appointments were selected to continue inpatient.
Table 2.
Demographic characteristics of study completers.
| Number of participants | 15 (11M; 4F) |
|---|---|
| Race (Black/White/Native American/Mixed) | 10/2/2/1 |
| Ethnicity (Hispanic/non-Hispanic) | 6/9 |
| Age (years) | 30.9 ± 7.7 |
| Education (years) | 12.8 ± 1.3 |
| Cannabis frequency (days/week) | 6.9 ± 0.3 |
| Cannabis quantity (grams/day) | 2.9 ± 1.9 |
| Duration regular cannabis use (years) | 13.1 ± 6.7 |
| Tobacco cigarette smokers (#) | 7 |
| Cigarettes/day (#) | 7.6 ± 3.4 |
| Alcohol drinkers (#)a | 8 |
| Alcohol: Drinks/week (#) | 2.5 ± 1.1 |
Note: Data are presented as means (± standard deviation) or as frequency.
Only included those reporting at least 1 drink/week.
Participants used no drugs other than cannabis, alcohol or tobacco, confirmed by urine toxicology.
Cannabis Self-administration
Figure 1 shows the number of puffs of cannabis self-administered as a function of cannabis condition, day of condition and medication. Relative to placebo, lorcaserin significantly decreased the amount of cannabis self-administered on the first [F(1,28) = 22.40, p < 0.0001] and second [F(1,28) = 7.62, p < 0.01] day of cannabis availability following three days of abstinence. By the third day of cannabis availability under these conditions, there was no longer a significant effect of lorcaserin relative to placebo. Under non-abstinent conditions, lorcaserin significantly decreased the amount of cannabis self-administered on the first day it was made available following one day of experimenter-administered cannabis [F(1,28) = 5.98, p < 0.02].
Figure 1.
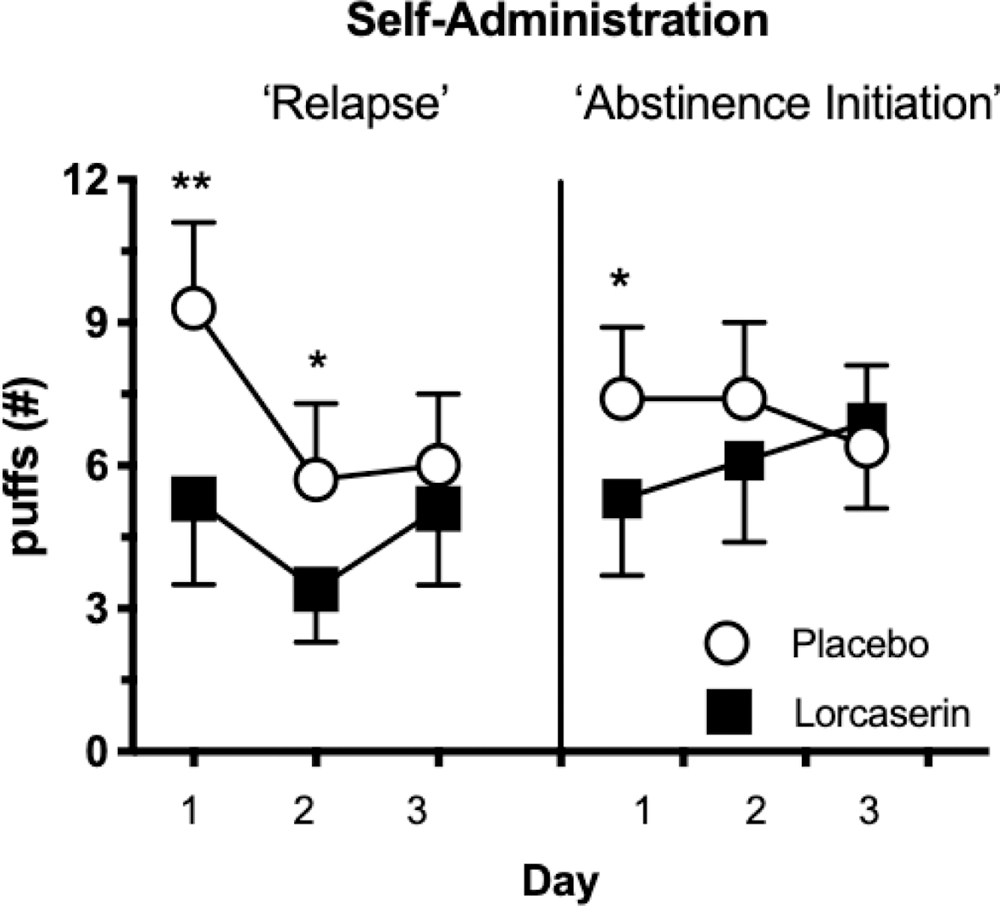
Cannabis self-administration; mean number of cannabis puffs chosen following 3 days of cannabis abstinence (left) and 0 days of cannabis abstinence (right). Error bars represent standard error. Significant differences in puffs chosen are denoted by *(p < 0.01) or ** (p < 0.0001).
Mood Ratings
As shown in Table 3, lorcaserin produced small but significant increases in ratings of sociability on days of cannabis administration [F(1,140) = 6.74, p < 0.02]. During abstinence from cannabis, lorcaserin significantly decreased cannabis craving [F(1,140) = 7.68, p < 0.02]. No other ratings of mood were significantly altered by lorcaserin during abstinence.
Table 3.
Mean peak effects during controlled cannabis administration (2 days of 7.0% THC 6x/day) and during cannabis abstinence (3 days) as a function of lorcaserin dose (0, 10 mg BID).
| Measure | Lorcaserin Dose | Cannabis Administration | Abstinence |
|---|---|---|---|
| Ratings of “Sociable” | 0 mg | 53.6 (2.7) | 47.8 (2.3) |
| 10 mg | ⬆ 57.9 (2.9)* | 46.3 (2.3) | |
| Cannabis Craving | 0 mg | 68.2 (5.6) | 66.2 (5.4) |
| 10 mg | 64.7 (6.3) | ⬇ 57.4 (6.1) | |
| Digit-Recall (#) | 0 mg | 6.9 (0.2) | 7.2 (0.2) |
| 10 mg | ⬇ 6.2 (0.4)* | ⬇ 6.7 (0.2)** | |
| Rapid Information | 0 mg | 81.3 (6.8) | 74.2 (5.0) |
| Task (# misses) | 10 mg | ⬆ 98.8 (8.3)** | ⬆ 86.3 (7.0)* |
| Food intake (kcal) | 0 mg | 4515.4 (446.5) | 2426.0 (255.4) |
| 10 mg | 4237.0 (566.5) | 2331.7 (340.0) | |
| Body weight (kg) | 0 mg | 82.7 (3.5) | 81.7 (2.7) |
| 10 mg | ⬆ 83.2 (3.4)* | ⬆ 82.3 (2.6)* | |
| Fell Asleep Easily | 0 mg | 69.2 (4.9) | 58.6 (5.6) |
| 10 mg | 58.6 (5.6) | ⬇ 35.1 (4.1)** | |
| Slept Well | 0 mg | 63.0 (4.7) | 55.8 (5.3) |
| 10 mg | 59.1 (3.9) | ⬇ 46.2 (3.8)** | |
| “Drug Liking” | 0 mg | 0.37 (0.15) | 0.29 (0.13) |
| 10 mg | ⬆ 0.87 (0.24)* | 0.00 (0.00) | |
| “Strong Drug Effect” | 0 mg | 0.87 (0.22) | 0.58 (0.19) |
| 10 mg | ⬆ 1.3 (2.2)* | 0.44 (016) | |
| “Good Drug Effect” | 0 mg | 0.8 (0.21) | 0.38 (0.19) |
| 10 mg | ⬆ 1.3 (0.27)* | 0.27 (0.16) | |
| Tobacco Cigarettes (# ad lib) | 0 mg | 7.9 (3.7) | 7.8 (1.5) |
| 10 mg | ⬇ 5.9 (2.5)* | 7.0 (1.3) | |
Note: Data in parentheses represent standard error of the mean. Mood/sleep rating range = 0 to100 mm; “Drug Liking” = −4 (dislike very much) to 4 (like very much); “Strong Drug Effect” and “Good Drug Effect” = 0 (none) to 4 (very); subjective drug effects are reflective of capsule effects.
Asterisks represent significant differences between lorcaserin and placebo * p < 0.02, ** p < 0.004, and the arrow signs indicate the direction of the effect.
Task Performance
As shown in Table 3, lorcaserin worsened performance on two of the five tasks relative to placebo regardless of cannabis condition. Specifically, lorcaserin worsened immediate recall scores on the digit-recall task during cannabis administration (F(1,140) = 1 3.08, p < 0.003) and during abstinence (F(1,140) = 11.26, p < 0.005), and increased the number of targets missed on the rapid-information task during cannabis administration (F(1,140) = 10.64, p < 0.006) and during abstinence (F(1,140) = 7.73, p < 0.02).
Food Intake and Body Weight
Lorcaserin did not significantly alter daily caloric intake or the proportion of macronutrients consumed under conditions of cannabis administration or during abstinence relative to placebo. Yet lorcaserin maintenance was associated with small but significant increases in body weight relative to placebo, both during days of cannabis administration [F(1,140) = 8.56, p < 0.02] and during cannabis abstinence [F(1,140) = 15.58, p < 0.005; Table 3].
Sleep
During cannabis administration, lorcaserin had no significant effect on sleep measures relative to placebo. Yet during cannabis abstinence, lorcaserin significantly worsened ratings of ‘Fell Asleep Easily’ [F(1,140) = 10.75, p < 0.005] and ‘Slept Well’ [F(1,140) = 11.23, p < 0.005; Table 3].
Capsule Ratings
Lorcaserin produced small but significant increases in ratings of capsule ‘Liking’ [F(1,140) = 10.24, p < 0.02], ‘Good Drug Effect’ [F(1,140) = 8.11, p < 0.02] and strength of drug effect [F(1,140) = 7.88, p < 0.02] relative to placebo on days of cannabis administration (Table 3). Lorcaserin had no significant effect on capsule ratings during abstinence.
Tobacco Cigarette Smoking
Among tobacco cigarettes smokers (n=7), lorcaserin produced a small but significant decrease in the number of ad libitum tobacco cigarettes smoked as compared to placebo [F(1,60) = 11.33, p < 0.02] on days of active cannabis administration (Table 3). Lorcaserin had no effect on cigarette smoking during cannabis abstinence.
Blood Pressure and Heart Rate
Lorcaserin had no significant effect on baseline cardiovascular measures (taken each morning) relative to placebo, either on days of cannabis administration (128/80 mmHg, HR: 69 bpm [placebo]; 123/77 mmHg, HR: 69 bpm [lorcaserin]) or during abstinence (129/79 mmHg, HR: 70 bpm [placebo]; 127/79 mmHg, HR: 69 bpm [lorcaserin]).
Side Effects
Table 4 shows the frequency and number of individuals reporting side effects as a function of lorcaserin dose. Sign Tests revealed no significant effect of lorcaserin dose on the frequency of side effects reported during either outpatient or inpatient phases.
Table 4.
Frequency of side effects.
| Outpatient | Inpatient | |||
|---|---|---|---|---|
| Lorcaserin dose (mg BID) | 0 | 10 | 0 | 10 |
| Headache | 1 (1) | 3 (3) | 1 (1) | 5 (3) |
| Gastrointestinal upset | 2 (2) | 1 (1) | 4 (4) | 11 (6) |
| Fatigue | 1 (1) | 2 (2) | 0 (0) | 0 (0) |
| Body pain | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
| Jittery/anxiety | 0 (0) | 1 (1) | 1 (1) | 1 (1) |
| Eye-dryness/discomfort | 0 (0) | 0 (0) | 1 (1) | 4 (2) |
| Trouble sleeping | 0 (0) | 0 (0) | 0 (0) | 2 (2) |
Note: Data reflect the number of times each symptom was reported during the outpatient phase (2 days/dose) and inpatient phase (11 days/dose) as a function of lorcaserin dose; the number of participants reporting each symptom is in parentheses (max = 15).
Discussion
This study demonstrates that compared to placebo, the 5HT-2c agonist lorcaserin (10 mg BID) significantly decreased cannabis self-administration both under conditions of ongoing cannabis use and after several days of enforced cannabis abstinence. Lorcaserin also decreased cannabis craving during abstinence conditions. These results are consistent with a study showing that lorcaserin decreased THC self-administration and cue-induced reinstatement in non-human primates (Justinova and colleagues, personal communication). Lorcaserin also decreased ad lib tobacco cigarette smoking on days of cannabis administration, even though participants were not attempting to reduce their tobacco use, consistent with clinical evidence in tobacco smokers (34). Together, these data suggest that 5HT-2c agonists may have potential for both tobacco and cannabis use disorders.
This study utilized a novel design assessing lorcaserin’s effects on self-administration under non-abstinent and abstinent cannabis conditions. As mentioned, the vast majority of efforts in medication development for CUD using human laboratory models have focused on relapse prevention, i.e., assessing whether medications decrease symptoms of cannabis withdrawal and thus reduce the return to cannabis self-administration. This study included a model of abstinence initiation because: (a) preclinical data suggested lorcaserin might decrease the positive and negative reinforcing effects of cannabis, and (b) most patients seeking treatment for their cannabis use initiate treatment while still using cannabis (4). If patients with CUD are still using cannabis while in treatment, medications producing reductions in a laboratory model of relapse may not have wide-ranging applicability. The more urgent clinical need is for medications that can halt or reduce ongoing cannabis use, so the finding that lorcaserin decreased cannabis self-administration in non-abstinent participants is especially relevant.
Note, the effects of lorcaserin on cannabis self-administration in this laboratory model were most marked on the first day of cannabis availability and were no longer significant by the third day of either the ‘relapse’ or ‘abstinence initiation’ condition. Whether this means that lorcaserin’s effects on cannabis reinforcement are short-lived or if it reflects the specific conditions of this inpatient laboratory design remains to be determined. In preclinical studies, lorcaserin’s effects on the abuse-related effects of other drugs of abuse persist with repeated administration (28, 29), suggesting that tolerance to this effect is unlikely to develop within 1–2 days, but confirmation in patients is needed. Based on our experience testing over a dozen medications in non-treatment seeking daily cannabis smokers, with few of those medications able to shift self-administration, our working hypothesis is that any shift in self-administration is a signal worth investigating in a clinical population (4, 39). The fact that lorcaserin reduced cannabis self-administration is noteworthy, even though the effect did not persist in this design.
The 5HT-2c agonist lorcaserin produced few other behavioral effects overall relative to placebo. It did not alter food intake, despite its FDA-approved indication (before being discontinued). It also did not change how participants rated the subjective experience of active cannabis relative to placebo. Lorcaserin slightly worsened performance on certain cognitive tasks and on subjective ratings of sleep during cannabis abstinence. Overall, the medication was well tolerated, with few adverse effects.
Though lorcaserin produced significant increases on measures of abuse liability (e.g., capsule ‘liking’), it is important to note that the effects were small and were present only under conditions of cannabis administration. This finding suggests that lorcaserin may increase the abuse liability of cannabis but given that it had no effects on its own, it is more likely that participants conflated capsule ratings under conditions of cannabis intoxication.
Limitations
There are several limitations to consider in the current study. First, given the study length, only one dose of lorcaserin was tested, though it was the FDA-approved therapeutic dose for obesity and the dose shown to reduce tobacco cigarette use in a clinical study targeting this population (34). Second, the sample comprised mostly men, limiting the generalizability of our conclusions.
Future directions
The present findings would support further investigation of 5HT-2c agonists as medications for treating CUD. Considering the host of preclinical data showing 5HT-2c agonism attenuates a range of behaviors associated with multiple drugs of abuse, along with our current findings that lorcaserin reduces cannabis self-administration and craving, development of alternative 5HT-2c agonists may be a fruitful treatment strategy for a range of substance use disorders including CUD.
Acknowledgements
The US National Institute on Drug Abuse (NIDA) supported this research (5U54DA037842) and supplied the cannabis cigarettes. We would like to acknowledge the support of the Eric D. Hadar Research Fellowship at Columbia University. We are grateful to Philip Kamilar-Britt, Erin Corcoran, Amanda Erakky, Rebecca Denson, Michael Connaughton and Gabriela Fazilov for their expert assistance in data collection and management.
Funding and Disclosures
Dr. Haney’s research was funded by NIDA (5U54DA037842; PI: Levin). She and Dr. Cooper have received partial salary support for an investigator-initiated study from Insys Therapeutics Inc. testing the effects of cannabidiol on laboratory measures of pain. Dr. Cooper has no competing interests in relation to the work described. In the past year, Dr. Cooper has served as a consultant to Beckley Canopy Therapeutics and has served on the scientific advisory board of FSD Pharma. Dr. Levin receives grant support from the NIDA, SAMHSA and US World Meds as well as a consultant for Major League Baseball. She was an unpaid member of a Scientific Advisory Board for Alkermes, Novartis and US WorldMeds but did not personally receive any compensation in the form of cash payments (honoraria/consulting fees) or food/beverage (she declined food/beverages in each circumstance) nor received compensation in the form of travel reimbursement. The other authors (CA, SCR, RF, SC) have no potential conflicts of interest.
References
- 1.SAMHSA. Treatment Episode Data Set (TEDS): 2005–2015. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-91, HHS Publication No. (SMA) 17–5037. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2017. [Google Scholar]
- 2.Administration SAaMHS. Treatment Episode Data Set (TEDS): 2017. Admissions to and Discharges from Publicly-Funded Substance Use Treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2019. [Google Scholar]
- 3.Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addiction science & clinical practice. 2007;4(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brezing CA, Levin FR. The Current State of Pharmacological Treatments for Cannabis Use Disorder and Withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017. [DOI] [PMC free article] [PubMed]
- 5.Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155(2). [DOI] [PubMed] [Google Scholar]
- 6.Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology. 2003;165(2):157–65. [DOI] [PubMed] [Google Scholar]
- 7.Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(8):1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, et al. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2010;211(2):233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197(1):157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(1):158. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology (Berl). 2016;233(13):2469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addiction biology. 2013;18(6):993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haney M, Ramesh D, Glass A, Pavlicova M, Bedi G, Cooper ZD. Naltrexone Maintenance Decreases Cannabis Self-Administration and Subjective Effects in Daily Cannabis Smokers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(11):2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–54. [DOI] [PubMed] [Google Scholar]
- 15.Fields S, Sabet M, Reynolds B. Dimensions of impulsive behavior in obese, overweight, and healthy-weight adolescents. Appetite. 2013;70:60–6. [DOI] [PubMed] [Google Scholar]
- 16.Fields S, Collins C, Leraas K, Reynolds B. Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Experimental and clinical psychopharmacology. 2009;17(5):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins GA, Silenieks LB, Roßmann A, Rizos Z, Noble K, Soko AD, et al. The 5-HT 2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(5):1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins GT, Gerak LR, Javors MA, France CP. Lorcaserin reduces the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2016;356(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohut SJ, Bergman J. Lorcaserin decreases the reinforcing effects of heroin, but not food, in rhesus monkeys. European journal of pharmacology. 2018;840:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins GT, France CP. Effects of lorcaserin and buspirone, administered alone and as a mixture, on cocaine self-administration in male and female rhesus monkeys. Experimental and clinical psychopharmacology. 2018;26(5):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology. 2016;101:237–45. [DOI] [PubMed] [Google Scholar]
- 22.Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT2C receptors on cocaine-and food-maintained behavior. Journal of Pharmacology and Experimental therapeutics. 2000;295(3):1183–91. [PubMed] [Google Scholar]
- 23.Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT 2C receptor agonist Ro60–0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(6):1402. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham KA, Bubar MJ, Anastasio NC. The Serotonin 5-HT 2C Receptor in Medial Prefrontal Cortex Exerts Rheostatic Control over the Motivational Salience of Cocaine-Associated Cues: New Observations from Preclinical Animal Research. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(12):2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munzar P, Justinova Z, Kutkat SW, Goldberg SR. Differential involvement of 5-HT2A receptors in the discriminative-stimulus effects of cocaine and methamphetamine. European journal of pharmacology. 2002;436(1–2):75–82. [DOI] [PubMed] [Google Scholar]
- 26.Gerak LR, Collins GT, Maguire DR, France CP. Effects of lorcaserin on reinstatement of responding previously maintained by cocaine or remifentanil in rhesus monkeys. Experimental and clinical psychopharmacology. 2019;27(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins GA, Fletcher PJ. Therapeutic potential of 5-HT2C receptor agonists for addictive disorders. ACS chemical neuroscience. 2015;6(7):1071–88. [DOI] [PubMed] [Google Scholar]
- 28.Anastasio NC, Sholler DJ, Fox RG, Stutz SJ, Merritt CR, Bjork JM, et al. Suppression of cocaine relapse-like behaviors upon pimavanserin and lorcaserin co-administration. Neuropharmacology. 2020;168:108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamauchi M, Tatebayashi T, Nagase K, Kojima M, Imanishi T. Chronic treatment with fluvoxamine desensitizes 5-HT2C receptor-mediated hypolocomotion in rats. Pharmacology Biochemistry and Behavior. 2004;78(4):683–9. [DOI] [PubMed] [Google Scholar]
- 30.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199(3):403–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76:460–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins GT, Gerak LR, France CP. The behavioral pharmacology and therapeutic potential of lorcaserin for substance use disorders. Neuropharmacology. 2018;142:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacological reviews. 2015;67(1):176–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez-Kam M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine & Tobacco Research. 2016;19(8):944–51. [DOI] [PubMed] [Google Scholar]
- 35.Brandt L, Jones JD, Martinez S, Manubay JM, Mogali S, Ramey T, et al. Effects of lorcaserin on oxycodone self-administration and subjective responses in participants with opioid use disorder. Drug and alcohol dependence. 2020;208:107859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirtle JL, Hickman MD, Boinpelly VC, Surineni K, Thakur HK, Grasing KW. The serotonin-2C agonist Lorcaserin delays intravenous choice and modifies the subjective and cardiovascular effects of cocaine: A randomized, controlled human laboratory study. Pharmacology Biochemistry and Behavior. 2019;180:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug and alcohol dependence. 1987;20(1):87–93. [DOI] [PubMed] [Google Scholar]
- 38.Evans S, Foltin R, Levin F, Fischman M. Behavioral and subjective effects of DN-2327 (pazinaclone) and alprazolam in normal volunteers. Behavioural pharmacology. 1995. [PubMed]
- 39.Arout CA, Herrmann E, Haney M. Human Laboratory Models of Cannabis Use Disorder. Cannabis Use Disorders: Springer; 2019. p. 75–84. [Google Scholar]


