Abstract
Cercosporoid fungi (Mycosphaerellaceae, Mycosphaerellales, Ascomycota) are one of the largest and most diverse groups of hyphomycetes causing a wide range of diseases of economically important plants as well as of plants in the wild. Although more than 6000 species are known for this group, the documentation of this fungal group is far from complete. Especially in the tropics, the diversity of cercosporoid fungi is poorly known. The present study aims to identify and characterise cercosporoid fungi collected on host plants belonging to Fabaceae in Benin, West Africa. Information on their morphology, host species and DNA sequence data (18S rDNA, 28S rDNA, ITS and tef1) is provided. DNA sequence data were obtained by a simple and non-culture-based method for DNA isolation which has been applied for cercosporoid fungi for the first time in the context of the present study. Among the loci used for the phylogenetic analysis, tef1 provided the best resolution together with the multigene dataset. Species delimitation in many cases, however, was only possible by combining molecular sequence data with morphological characteristics. Based on forty specimens recently collected in Benin, 18 species are presented with morphological descriptions, illustrations and sequence data. Among these, six species in the genus Cercospora and two species in Pseudocercospora are proposed as species new to science. The newly described species are Cercospora (C.) beninensis on Crotalaria macrocalyx, C. parakouensis on Desmodium tortuosum, C. rhynchophora on Vigna unguiculata, C. vignae-subterraneae on Vigna subterranea, C. tentaculifera on Vigna unguiculata, C. zorniicola on Zornia glochidiata, Pseudocercospora sennicola on Senna occidentalis and Pseudocercospora tabei on Vigna unguiculata. Eight species of cercosporoid fungi are reported for Benin for the first time, three of them, namely C. cf. canscorina, C. cf. fagopyri and C. phaseoli-lunati are new for West Africa. The presence of two species of cercosporoid fungi on Fabaceae previously reported from Benin, namely Nothopassalora personata and Passalora arachidicola, is confirmed.
Keywords: Benin, Cercospora , Fabaceae , Leguminosae , molecular phylogenetic analysis, Nothopassalora , Passalora , Pseudocercospora , West Africa
Introduction
Hyphomycetous anamorphs of Mycosphaerella-like teleomorphs are generally referred to as cercosporoid fungi and are classified in genera with concepts that often changed (Crous and Braun 2003; Braun et al. 2013; Kirschner 2014). Cercosporoid fungi include about 6000 recognized species (Braun et al. 2015), in more than ten genera, with Cercospora Fresen. (C.), Nothopassalora U.Braun, C. Nakash., Videira & Crous (N.), Passalora Fr. (P.) and Pseudocercospora Speg. (Ps.) being the genera relevant for the present publication.
Cercosporoid fungi belonging to Mycosphaerellaceae (Mycosphaerellales, Ascomycota) are one of the largest and most diverse groups of hyphomycetes and cause a wide range of diseases, on numerous economically important plants such as cereals, vegetables and fruits as well as on wild plants. Major diseases include the angular leaf spot of bean caused by Pseudocercospora griseola, black leaf streak of banana caused by Ps. fijiensis (M.Morelet) Deighton, fruit and leaf spot disease of citrus caused by Ps. angolensis (T.Carvalho & O.Mendes) Crous & U.Braun, leaf spot disease of celery (Cercospora apii Fresen.), of sugar beet (C. beticola Sacc.), and foliar diseases of groundnut caused by Nothopassalora personata (Berk. & M.A.Curtis) U.Braun, C. Nakash., Videira & Crous or Passalora arachidicola (Hori) U.Braun (Braun et al. 2013; Videira et al. 2017). Infections by these fungi are mostly evident by leaf spots, but cercosporoid fungi can also cause necrotic lesions on flowers, fruits, seeds and pedicels of numerous hosts in most climatic regions (Agrios 2005). Cercosporoid fungi are known from all parts of the world but they are more abundant and diverse in tropical and subtropical regions (Beilharz et al. 2002; Braun and Freire 2004; Hernández-Gutiérrez and Dianese 2008, 2009).
Cercosporoid fungi are dematiaceous hyphomycetes with conidiophores formed singly or in groups, arranged in sporodochia or in synnemata, with integrated, terminal or intercalary conidiogenous cells (Crous and Braun 2003; Ávila et al. 2005; Braun et al. 2013). Most of the cercosporoid species were previously assigned to a single genus, Cercospora, which was later split into several smaller genera mainly by Deighton (1967, 1973, 1974, 1976, 1979), Braun (1993) and Crous and Braun (2003). Crous and Braun (2003) recognized four genera, namely Cercospora, Passalora, Pseudocercospora and Stenella Syd as important cercosporoid genera. Later, the genus Stenella was assigned to the Teratosphaeriaceae based on the phylogenetic placement of the type species. Stenella-like species remaining in Mycosphaerellaceae were classified in the genus Zasmidium Fr. (Arzanlou et al. 2007; Braun et al. 2013). In the present paper, we follow generic concepts defined by Crous and Braun (2003) and recently updated by Braun et al. (2013), Crous et al. (2013a), Groenewald et al. (2013) and Videira et al. (2017). However, according to recent molecular sequence analyses, most genera of the cercosporoid fungi are not monophyletic (Videira et al. 2017). As many cercosporoid fungi have a strong impact on cultivated plants, a better understanding and stabilisation of the taxonomy of these fungi are urgently needed.
The genus Cercospora was established by Fresenius in 1863 (Fuckel 1863) based on the type species Cercospora apii (Braun and Crous 2016; Videira et al. 2017). It is one of the most species-rich genera of the hyphomycetes and contains numerous important plant pathogenic fungi throughout the world (Crous and Braun 2003). In 1954, the genus was monographed by Chupp (1954), who treated 1419 Cercospora-species using a broad generic concept. Later, several attempts have been made to split Cercospora s. lat. into smaller genera by using characteristics of conidiomatal structure, hyphae, conidiophores, conidiogenous cells, conidiogenous loci and conidia (Ellis 1971, 1976; Deighton 1973, 1979, 1983; Braun 1995a, 1998; Crous and Braun 2003). Currently, Cercospora species are morphologically characterised by pigmented conidiophores, unpigmented conidia, as well as thickened and darkened conidiogenous loci and conidial hila (Crous and Braun 2003; Groenewald et al. 2013). A significant problem in the taxonomy of Cercospora is the host specificity of its species. Most Cercospora species are considered to be distinct based on the host and thus assumed to be specific to a host species or to a host genus (Chupp 1954; Braun 1995a). Some species, such as C. apii and C. beticola, however, were isolated from a high number of host species belonging to several families (Groenewald M et al. 2006). Moreover, phylogenetic approaches based on multi-locus sequences can be problematic for species delimitation in Cercospora due to a high level of conservation in DNA sequences of commonly used loci (i.e., ITS, tef1, actA, cmdA and his3) (Bakhshi et al. 2018).
The genus Pseudocercospora was introduced by Spegazzini (1910) based on the type species Ps. vitis (Lév.) Speg., a foliar pathogen of grapevine. The majority of Pseudocercospora species are known as pathogens occurring on many different plants, mainly in tropical and sub-tropical regions (Chupp 1954; Crous and Braun 2003; Crous et al. 2013). In contrast to Cercospora spp., they are characterised by pigmented conidiophores and conidia, without thickened and darkened conidiogenous loci and conidial hila (Deighton 1976). The monophyly of the genus has not yet been fully resolved (Kirschner 2014). According to molecular sequence data, most species of Pseudocercospora appear to be host specific (Crous et al. 2013).
The genus Passalora Fr. was introduced by Fries (1849) based on the type species Passalora bacilligera (Mont. & Fr.) Mont. & Fr. (≡ Cladosporium bacilligerum Mont. & Fr.) (Videira et al. 2017). Species of Passalora are characterised by pigmented conidiophores and conidia as well as thickened and darkened conidiogenous loci and conidial hila (Crous and Braun 2003).
Several molecular phylogenetic studies are available on species of cercosporoid fungi that are represented by strains in culture collections (Świderska-Burek et al. 2020). These, however, only represent a small fraction of several hundreds of taxa of cercosporoid fungi that are valid species defined by morphological characteristics (Braun et al. 2016; Świderska-Burek et al. 2020). Therefore, the number of cercosporoid species known by detailed morphological characteristics as well as molecular sequence data has to be increased.
Although cercosporoid fungi cause a wide range of diseases on major agricultural crops, the study of cercosporoid fungi in West Africa is still at an early pioneer stage and only very incomplete information is currently available (Piepenbring et al. 2020). To date, approximately 320 species of cercosporoid hyphomycetes are known from 14 West African countries (Piepenbring et al. 2020, Suppl. materials 1, 2). Among these, 12 species of cercosporoid fungi have been reported for Benin (Turner 1971; Marley et al. 2002; Crous and Braun 2003; Houessou et al. 2011; Piątek and Yorou 2018; Soura et al. 2018; Meswaet et al. 2019; Farr and Rossman 2021). Morphological characteristics and molecular sequence data are lacking for most cercosporoid species known for Benin and other West African countries. Although cercosporoid fungi have been investigated for more than 150 years and are important in the agricultural sector, almost no, or only inadequate, studies have been carried out in most West African countries such as Benin. In addition to this lack of species knowledge in tropical regions, many species of cercosporoid fungi are characterised morphologically only. Since many cercosporoid species are known as pathogens on cultivated plants, an accurate diagnosis, identification and documentation of these fungi are a prerequisite and urgent for their control and epidemiological surveys.
As a first step towards a systematic documentation of cercosporoid fungi in tropical Africa, we focus on species infecting hosts belonging to the Fabaceae (Leguminosae) in the present publication. Fabaceae are the third largest family of angiosperms (Gepts et al. 2005). This family includes peas, lentils, beans, peanuts and other plants with pods and/or seeds that are consumed as food (Messina 1999). Several species belonging to Vigna originate from West Africa (Benin, Burkina Faso, Cameroon, Ghana, Niger, Nigeria and Togo) including two important cultivated crops Vigna unguiculata (L.) Walp. and Vigna subterranea (L.) Verdc. (Hepper 1963; Faris 1965; Padulosi and Ng 1990). They provide important nutrients such as proteins, low glycemic index carbohydrates, minerals and vitamins. Legumes are richer in protein than other cultivated plants because of nitrogen-fixing bacteria living in nodules of their roots (Kouris-Blazos and Belski 2016).
We apply an integrative approach that includes sampling in Benin, detailed descriptions and illustrations of collected specimens and herbarium specimens, examination of closely related known species on the same or closely related host species based on herbarium specimens and the isolation, sequencing and analysis of nuclear DNA sequence data. For the isolation of DNA, a new, simple method for DNA isolation has been developed and is presented for the first time for cercosporoid fungi.
Methods
Collections and morphological studies
Samples of leaves infected by cercosporoid fungi were randomly collected in farmlands and fallows in Benin from July–August 2016, July–September 2017 and August–September 2019. Infected leaves were dried in a plant press and deposited in the herbaria Botanische Staatssammlung München (M) and University of Parakou (UNIPAR).
Dried specimens were observed by stereomicroscopy and by light microscopy, using a Zeiss Axioscope 40 microscope. For light microscopy, leaf sections were made with razor blades and mounted in distilled water or 5% KOH without staining. Semi-permanent preparations of sections of the infected leaves were made by a microtome (Leica CM 1510-1) and mounted in lactophenol with cotton blue. For approximately 50 ml lactophenol cotton blue solution we mixed 10 mg phenol, 0.025 mg cotton blue, 10 ml lactic acid, 20 ml glycerin and 10 ml distilled water. Measurements of 30 conidia, conidiophores and other structures have been made for each specimen at a magnification of ×1000. Measurements are presented as mean value ± standard deviation with extreme values in parentheses. Line drawings were made freehand on scaled paper. Images and drawings were edited with Photoshop CS5 (Adobe, San Jose, California). Critical taxa were determined with the help of type specimens and other specimens loaned from the US National Fungus Collections (BPI), the Herbarium of the University of Illinois (ILL) and the New York Botanical Garden (NY).
Host plant identification
Host plants were identified by morphological characteristics and in some cases by molecular methods. Morphological identifications were made by comparison with herbarium specimens, literature (e.g., Akoégninou et al. 2006) and with the help of local botanists. Molecular sequence data for species identifications were obtained by polymerase chain reaction (PCR) for the amplification of the partial region of chloroplast rbcL with the primer pairs rbcLa-F (Levin et al. 2003) and rbcLa-R (Kress et al. 2009). DNA was extracted from approx. 0.05 g of leaf tissue dried with silica gel using the innuPREP Plant DNA Kit (Analytik Jena, Germany) and following the manufacturer’s instructions. Protocols for PCR were carried out as described by Fazekas et al. (2012).
DNA Extraction and PCR amplification of fungal DNA
DNA was isolated from caespituli taken with a needle from dry specimens using the E.Z.N.A Forensic DNA Extraction Kit following the manufacturer’s instructions. Small pieces of leaves containing several clean caespituli, with as little contaminations as possible, were selected under the stereomicroscope. Precautions were taken to avoid picking cells of any other organism (fungi, algae) associated with the leaves. To extract total genomic DNA from caespituli, a small amount of clean hyphae from the leaf surface was transferred into a sterile Eppendorf tube using a sterilized needle or adhesive mini-tapes. The sample was homogenized for 7–10 min. using a Retsch Mixer Mill MM301 with TL buffer and 2.5 mm Zirconia beads. Isolated DNA was re-suspended in elution buffer and stored at -20 °C. DNA concentration was checked by a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, USA).
Four partial nuclear gene regions (three ribosomal loci and one protein-coding gene) were amplified and sequenced: For the large subunit nuclear ribosomal DNA (nrLSU, 28S rDNA) the primers LSU1Fd and LSU3Rd (Crous et al. 2009a), for the small subunit nuclear ribosomal DNA (nrSSU, 18S rDNA) the primers SSU1Fd and SSU1Fd (Crous et al. 2009a), for the internal transcribed spacer region of ribosomal DNA (ITS) the primers V9G (de Hoog and van den Ende 1998) and ITS4 (White et al. 1990) and for the translation elongation factor 1-α (tef1) the primers EF1- 728F and EF1-986R (Carbone and Kohn 1999) were used. PCR amplification and sequencing were conducted following the protocols of Hunter et al. (2006), Crous et al. (2009a, 2012) and Videira et al. (2017). The PCR mixtures consisted of 1 μL genomic DNA, 15× MgCl2 reaction buffer (Bioline, Luckenwalde, Germany), 25 mM MgCl2, 25 μM of each dNTP, 10 μM of each primer and 5 U Taq DNA polymerase (VWR) in a total volume of 25 μL. Cycling parameters of the PCR for LSU, SSU and ITS were as follows: initial denaturation at 94 °C for 3 min, followed by 35 cycles of amplification [denaturation at 94 °C for 30 s, primer annealing at 52 °C for 30 s and primer extension at 72 °C for 45 s] and a final extension at 72 °C for 5 min, followed by storage at 8 °C. The PCR mixture for tef1 contained 2 μL of template DNA and the cycling parameters to obtain the partial tef1 were as follows: an initial denaturation at 96 °C for 2 min; followed by 35 cycles of amplification [denaturation at 94 °C for 30 s, primer annealing at 56 °C for 30 s and primer extension at 72 °C for 30 s] and a final extension at 72 °C for 7 min, followed by storage at 8 °C. PCR-products were checked on 1.5% agarose electrophoresis gels containing HDGreenPlus DNA stain. Amplified PCR products were purified with the Cycle Pure Kit (VWR-Omega, USA). Sequencing was performed at Seqlab GmbH, Germany.
Molecular phylogeny
Amplification of the SSU, LSU, ITS and tef1 gene regions for all isolates used in this study yielded fragments of approximately 1100 bp, 900 bp, 650 bp and 300 bp, respectively. Consensus sequences of trace files were generated with Geneious 10.2.2 (https://www.geneious.com, Kearse et al. 2012) and searched against GenBank (https://www.ncbi.nlm.nih.gov/, Benson et al. 2014) with MegaBLAST. Sequences with a high similarity (65 sequences of LSU, ITS and tef1 regions) were retrieved (Table 1). A total of 148 sequences for 65 specimens were obtained from GenBank (Table 1) and 92 sequences for 28 specimens from Benin were generated in this study (Table 2). They were aligned with MAFFT v. 7 using the L-INS-i algorithm (Nakamura et al. 2018). The alignments were manually checked by using MEGA v. 7 (Kumar et al. 2016). Gblocks v. 0.91b (Talavera and Castresana 2007) was used to remove poorly aligned positions and divergent regions from the DNA alignment using the parameters for a less stringent selection. Subsequently, a four-locus concatenated alignment (SSU, LSU, ITS and tef1) dataset was assembled for phylogenetic analyses using Geneious 10.2.2. Cladosporium sphaerospermum (G402) served as outgroup taxon, because the genus Cladosporium s. str. was shown to be the sister group of Mycosphaerella s. str. (Braun et al. 2003). PartitionFinder2 v.2.1.1 (Lanfear et al. 2014) on XSEDE (Miller et al. 2010) was used to select the best-fit model of evolution for each gene fragment separately. Data were partitioned by gene and by codon position in the case of protein-coding sequences. The TRNEF+G model was applied to 28S rDNA, K80 model to 18S rDNA, K81+G to ITS and TRN+G model to tef1. The alignment and the tree were deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S28032). Phylogenetic analyses of this study were conducted by applying Maximum Likelihood (ML) in RAxML-HPC2 v.8.2.12 (Stamatakis 2014) on XSEDE (Miller et al. 2010) and Bayesian with the program MrBayes 3.2.6 (Ronquist et al. 2012) on XSEDE (Miller et al. 2010) on the CIPRES Science Gateway web portal. (http://www. phylo.org/sub_sections/portal/).
Table 1.
Data of DNA sequences of cercosporoid fungi downloaded from GenBank and used in this study.
| Species | Host | Host family | Country | Source | GenBank Accession Numbers | Reference | ||
|---|---|---|---|---|---|---|---|---|
| nrLSU | ITS | tef1 | ||||||
| Cercospora cf. apii Fresen. | Cajanus cajan (L.) Millsp. | Fabaceae | S. Africa | CBS 115411 | JN941171 | JN942278 | – | Groenewald JZ et al. (2013) |
| Cercospora asparagi Sacc. | Asparagus sp. | Asparagaceae | USA | AS16-02 | KY549100 | KY549098 | KY549102 | Hay et al. (2017) |
| Cercospora canescens Ellis & G.Martin | Vigna radiata (L.) R.Wilczek. | Fabaceae | India | Cer70-18 | – | MN795675 | – | Das et al. 2019 |
| Cercospora capsica Heald & F.A.Wolf | Capsicum annuum L. | Solanaceae | S. Korea | CBS 132622 | – | JX143568 | JX143323 | Groenewald JZ et al. (2013) |
| Cercospora cf. citrulline Cooke | Citrullus lanatus (Thunb.) Matsum. & Nakai | Cucurbitaceae | Japan | MUCC 576 | – | JX143579 | JX143337 | Groenewald JZ et al. (2013) |
| Cercospora dubia Speg. | Chenopodium sp. | Amaranthaceae | Mexico | CPC 15600 | KX286968 | KX287277 | – | Videira et al. (2016) |
| Cercospora kikuchii (Tak. Matsumoto & Tomoy.) M.W.Gardner | Glycine max (L.) Merr. | Fabaceae | USA | DLS5070-3A | AY373573 | AY373582 | Cai and Schneider (2008) | |
| Cercospora lactucae-sativae Sawada | Lactuca sativa L. | Asteraceae | Japan | MUCC 570 | – | JX143623 | JX143382 | Groenewald JZ et al. (2013) |
| Cercospora malayensis F.Stevens & Solheim | Abelmoschus esculentus (L.) Moench | Malvaceae | S. Korea | KACC 47769 | – | MH129519 | MH129517 | Ju et al. (2020) |
| Cercospora cf. maloti Ellis & Everh. | Cucumis melo L. | Cucurbitaceae | Japan | MUCC 575 | – | JX143625 | JX143384 | Groenewald JZ et al. (2013) |
| Cercospora cf. nicotianae Ellis & Everh. | Nicotiana tabacum L. | Solanaceae | – | CBS 570.69 | – | DQ835074 | DQ835100 | Groenewald JZ et al. (2010) |
| Cercospora olivascens Sacc. | Aristolochia clematitis L. | Aristolochiaceae | Romania | CBS 253.67 | – | JX143632 | JX143391 | Groenewald JZ et al. (2013) |
| Cercospora physalidis Ellis | Solanum melongena L. | Solanaceae | India | Cer 69-18 | MK027095 | MK029358 | – | Sinha et al., unpublished |
| Cercospora rodmanii Conway | Eichhornia sp. | Pontederiaceae | Mexico | 15-GTOX | GQ884187 | GQ884185 | – | Montenegro-Calderón et al. (2011) |
| Cercospora sojina Hara | Glycine soja Siebold & Zucc. | Fabaceae | S. Korea | CBS 132615 | – | JX143659 | JX143419 | Groenewald JZ et al. (2013) |
| Cercospora sp. Q JZG-2013 | Acacia mangium Willd. | Fabaceae | Thailand | CPC 10550 | – | AY752139 | AY752172 | Groenewald JZ et al. (2013) |
| Cercospora vignigena C. Nakash., Crous, U.Braun & H.D.Shin | Vigna unguiculata (L.) Walp. | Fabaceae | Japan | MUCC 579 | – | JX143736 | JX143495 | Groenewald JZ et al. (2013) |
| Cercospora zebrina Pass. | Trifolium subterraneum L. | Fabaceae | Australia | CBS 118790 | KF251651 | KF251147 | – | Quaedvlieg et al. (2013) |
| Cladosporium sphaerospermum Penz. | – | – | Russia | G402 | KJ443113 | KJ443245 | KJ443201 | Grum-Grzhimaylo et al. (2016) |
| Mycosphaerella keniensis Crous & T.A.Cout. | Eucalyptus grandis W.Hill | Myrtaceae | Kenya | CMW5147 | DQ246259 | – | DQ235100 | Hunter et al. (2006) |
| Mycosphaerella microsora Syd. | Tilia platyphyllos Scop. | Malvaceae | Romania | CBS 552.71 | MH872022 | MH860260 | Vu et al. (2019) | |
| Mycosphaerella valgourgensis Crous | Yucca sp. | Asparagaceae | France | CPC:18385 | JF951175 | JF951152 | – | Crous et al. (2011) |
| Nothopassalora personata (Berk. & M.A.Curtis) U.Braun, C. Nakash., Videira & Crous | Arachis hypogaea L. | Fabaceae | Australia | CBS 142236 | NG_058496 | NR_156379 | – | Videira et al. (2017) |
| Paracercospora egenula (Syd.) Deighton | Solanum melongena L. | Solanaceae | India | CBS 485.81 | JQ324940 | GU269699 | GU384415 | Crous et al. (2013a) |
| Passalora arctostaphyli Moreno-Rico & Crous | Arctostaphylos pungens Kunth | Ericaceae | Mexico | CPC 22067 | KJ152785 | KJ152782 | – | Moreno-Rico et al. (2014) |
| Neocercosporidium smilacis (Thüm.) U.Braun, C. Nakash., Videira & Crous | Smilax aspera L. | Smilacaceae | Italy | CBS 556.71 | KJ633269 | KJ633265 | – | Collemare et al. (2015) |
| Pseudocercospora abelmoschi (Ellis & Everh.) Deighton | Hibiscus syriacus L. | Malvaceae | S.Korea | CBS 132103 | GU253696 | GU269647 | GU384365 | Crous et al. (2013a) |
| Pseudocercospora atromarginalis (G.F.Atk.) Deighton | Solanum sp. | Solanaceae | New Zealand | CBS 114640 | GU253706 | GU269658 | GU384376 | Crous et al. (2013a) |
| Pseudocercospora cercidicola Crous, U.Braun & C. Nakash. | Cercis chinensis Bunge | Fabaceae | Japan | MUCC 896 | GU253719 | GU269671 | GU384388 | Crous et al. (2013a) |
| Pseudocercospora chengtuensis (F.L.Tai) Deighton | Lycium chinense Mill. | Solanaceae | S. Korea | CBS 131924 | MH877506 | MH866053 | – | Vu et al. (2019) |
| Pseudocercospora chiangmaiensis Cheew., K.D.Hyde & Crous | Eucalyptus camaldulensis Dehnh. | Myrtaceae | Thailand | CBS 123244 | MH874812 | MH863288 | – | Vu et al. (2019) |
| Pseudocercospora cruenta (Sacc.) Deighton | Phaseolus vulgaris L. | Fabaceae | Taiwan | CBS 117232 | GU253730 | GU269689 | GU384405 | Crous et al. (2013a) |
| Pseudocercospora cydoniae (Ellis & Everh.) Y.L.Guo & X.J.Liu | Chaenomeles speciosa (Sweet) Nakai | Rosaceae | S. Korea | CBS 131923 | MH877505 | MH866052 | – | Vu et al. (2019) |
| Pseudocercospora dingleyae U.Braun & C.F.Hill | Haloragis erecta (Murray) Oken | Haloragaceae | New Zealand | CBS 114645 | KX286997 | KX287299 | – | Videira et al. (2016) |
| Pseudocercospora dovyalidis (Chupp & Doidge) Deighton | Dovyalis zeyheri (Sond.) Warb. | Salicaceae | S. Africa | CBS 126002 | MH875338 | MH863877 | – | Vu et al. (2019) |
| Pseudocercospora encephalarti Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. | Encephalartos barteri Carruth. ex Miq. | Zamiaceae | Benin | YMMAS78 | – | MK397016 | – | Meswaet et al. (2019) |
| Pseudocercospora flavomarginata G.C.Hunter, Crous & M.J.Wingf. | Eucalyptus camaldulensis Dehnh. | Myrtaceae | Thailand | CBS 118824 | – | NR_111805 | – | Quaedvlieg et al. (2012) |
| Pseudocercospora fuligena (Roldan) Deighton | Solanum lycopersicum L. | Solanaceae | Japan | MUCC 533 | GU253749 | GU269712 | GU384428 | Crous et al. (2013a) |
| Pseudocercospora griseola f. griseola (Sacc.) Crous & U.Braun | Phaseolus vulgaris L. | Fabaceae | S. Korea | CBS 131929 | MH877495 | MH866046 | – | Vu et al. (2019) |
| Pseudocercospora hakeae (U.Braun & Crous) U.Braun & Crous | Hakea sp. | Proteaceae | Australia | CBS:144520 | MK442553 | MK442617 | MK442708 | Crous et al. (2019) |
| Pseudocercospora humuli (Hori) Y.L.Guo & X.J.Liu | Humulus lupulus L. | Cannabaceae | Japan | MUCC 742 | GU253758 | – | GU384439 | Crous et al. (2013a) |
| Pseudocercospora kaki Goh & W.H.Hsieh | Diospyros kaki L.f. | Ebenaceae | Japan | MUCC 900 | GU253761 | GU269729 | GU384442 | Crous et al. (2013a) |
| Pseudocercospora madagascariensis Crous & M.J.Wingf. | Eucalyptus camaldulensis Dehnh. | Myrtaceae | Madagascar | CBS 124155 | MH874880 | MH863357 | – | Vu et al. (2019) |
| Pseudocercospora metrosideri U.Braun | Metrosideros collina (J.R.Forst. & G.Forst.) A.Gray | Myrtaceae | New Zealand | CBS 118795 | GU253774 | GU269746 | GU384458 | Crous et al. (2013a) |
| Pseudocercospora neriicola Crous, Frisullo & Camele | Nerium oleander L. | Apocynaceae | Italy | CPC 23765 | KJ869222 | KJ869165 | KJ869240 | Crous et al. (2014) |
| Pseudocercospora pallida (Ellis & Everh.) H.D.Shin & U.Braun | Campsis grandiflora (Thunb.) K.Schum. | Bignoniaceae | S. Korea | CBS 131889 | – | – | GU384469 | Crous et al. (2013a) |
| Pseudocercospora paraguayensis (Tak. Kobay.) Crous | Eucalyptus nitens (H.Deane & Maiden) Maiden | Myrtaceae | Brazil | CBS:111286 | KF901945 | KF901619 | KF903205 | Quaedvlieg et al. (2014) |
| Pseudocercospora parapseudarthriae Crous & A.R.Wood | Pseudarthria hookeri Wight & Arn. | Fabaceae | S. Africa | CPC 23449 | KJ869208 | KJ869151 | KJ869238 | Crous et al. (2014) |
| Pseudocercospora pittospori (Plakidas) Y.L.Guo & X.J.Liu | Pittosporum sp. | Pittosporaceae | USA | HI-018 | MK210475 | MK210511 | – | Vaghefi et al. 2021 |
| Pseudocercospora proteae Crous | Protea mundii Klotzsch | Proteaceae | S. Africa | CBS 131587 | – | – | GU384519 | Crous et al. (2013a) |
| Pseudocercospora prunicola (Ellis & Everh.) U.Braun | Prunus sp. | Rosaceae | China | BJFU ZYP141005.9 | KX853057 | KX853048 | KX853066 | Liu et al. (2016) |
| Pseudocercospora ranjita (S.Chowdhury) Deighton | Gmelina sp. | Lamiaceae | Indonesia | CBS 126005 | MH875340 | MH863879 | GU384500 | Crous et al. (2013a) |
| Pseudocercospora ravenalicola G.C.Hunter & Crous | Ravenala madagascariensis Sonn. | Strelitziaceae | India | CBS 122468 | GU253828 | – | GU384521 | Crous et al. (2013a) |
| Pseudocercospora schizolobii (M.J.Wingf. & Crous) M.J.Wingf. & Crous | Eucalyptus camaldulensis Dehnh. | Myrtaceae | Thailand | CBS 124990 | KF251827 | KF251323 | KF253270 | Verkley et al. (2013) |
| Pseudocercospora sennae-multijugae Meir. Silva, R.W.Barreto & Crous | Senna multijuga (Rich.) H.S.Irwin & Barneby | (Fabaceae) | Brazil | CPC 25206 | KT290169 | KT290142 | KT290196 | Silva et al. (2016) |
| Pseudocercospora sp. | Citrus grandis (L.) Osbeck | Rutaceae | China | ZJUM 75 | KP895896 | KP896026 | KP896073 | Huang et al. (2015) |
| Pseudocercospora sp. | Eichhornia azurea (Sw.) Kunth | Pontederiaceae | Brazil | CPC 19537 | KX287003 | KX287304 | – | Videira et al. (2016) |
| Pseudocercospora sp. | Eichhornia azurea (Sw.) Kunth | Pontederiaceae | Brazil | CPC 19535 | KX287001 | KX287303 | – | Videira et al. (2016) |
| Pseudocercospora sp. A MB-2015 | Phaseolus vulgaris L. | Fabaceae | Iran | CCTU 1166 | KP717028 | KM452864 | KM452886 | Bakhshi et al. (2014) |
| Pseudocercospora stizolobii (Syd. & P.Syd.) Deighton | Eucalyptus camaldulensis Dehnh. | Myrtaceae | Thailand | CPC 25217 | KT290170 | KT290143 | KT290197 | Silva et al. (2016) |
| Pseudocercospora tereticornis Crous & Carnegie | Eucalyptus tereticornis Sm. | Myrtaceae | Australia | CBS 125214 | MH874960 | MH863460 | – | Vu et al. (2019) |
| Pseudocercospora vitis (Lév.) Speg. | Vitis vinifera L. | Vitaceae | S. Korea | CPC 11595 | – | – | JX901702 | Quaedvlieg et al. (2012) |
| Pseudocercosporella bakeri (Syd. & P.Syd.) Deighton | Ipomoea indica (Burm.) Merr. | Convolvulaceae | New Zealand | CBS 119488 | KX287005 | KX287306 | KX287862 | Videira et al. (2016) |
| Pseudocercosporella myopori U.Braun & C.F.Hill | Myoporum laetum G.Forst. | Scrophulariaceae | New Zealand | CBS 114644 | KX287000 | KX287302 | JX143491 | Groenewald JZ et al. (2013) |
| Zasmidium daviesiae (Cooke & Massee) U.Braun, C. Nakash., Videira & Crous | Daviesia latifolia R.Br. | Fabaceae | Australia | CBS:116002 | KF901928 | KF901603 | KF903373 | Quaedvlieg et al. (2014) |
Table 2.
Data of sequences of cercosporoid fungi from Benin generated during the present study. Names of species proposed as new in this study are written in bold.
| Species | Voucher | Host | Host family | GenBank Accession Numbers | |||
|---|---|---|---|---|---|---|---|
| nrSSU | nrLSU | ITS | tef1 | ||||
| Cercospora beninensis | YMM11 | Crotalaria macrocalyx Benth. | Fabaceae | MW834445 | MW834433 | MW834437 | MW848615 |
| Cercospora aff. canescens Ellis & G.Martin | YMM07 | Calopogonium sp. | Fabaceae | MW834475 | – | MW834492 | MW848605 |
| YMM01 | Vigna subterranea (L.) Verdc. | Fabaceae | MW834473 | MW834457 | MW834490 | MW848603 | |
| Cercospora cf. canscorina Chidd. | YMM05 | Vigna sp. | Fabaceae | MW834474 | MW834458 | MW834491 | MW848604 |
| Cercospora cf. fagopyri K.Nakata & S.Takim. | YMM23A | Lablab sp. | Fabaceae | – | – | MW861543 | MW848607 |
| Cercospora parakouensis | YMM296A | Desmodium tortuosum (Sw.) DC. | Fabaceae | – | MW834436 | MW834442 | MW848621 |
| Cercospora phaseoli-lunati U.Braun & Crous | YMM289 | Vigna radiata (L.) R.Wilczek | Fabaceae | MW834471 | – | MW834483 | MW848601 |
| Cercospora rhynchophora | YMM03B | Vigna unguiculata (L.) Walp. | Fabaceae | MW834447 | MW834431 | MW834443 | MW848619 |
| Cercospora sp.1 | YMM3S | Sorghum bicolor (L.) Moench | Poaceae | MW834466 | MW834452 | MW834484 | MW848600 |
| Cercospora sp.2 | YMM48S | Sorghum bicolor (L.) Moench | Poaceae | MW834467 | MW834453 | MW834485 | MW848608 |
| Cercospora sp.3 | YMM229 | Spigelia sp. | Loganiaceae | – | MW834462 | MW834500 | MW848599 |
| Cercospora sp.4 | YMM297B | Phaseolus lunatus L. | Fabaceae | MW834481 | MW834464 | MW834501 | MW848612 |
| Cercospora tentaculifera | YMM75 | Vigna unguiculata (L.) Walp. | Fabaceae | MW834448 | – | MW834440 | MW848614 |
| Cercospora vignae-subterraneae | YMM293 | Vigna subterranea (L.) Verdc. | Fabaceae | MW834446 | – | MW834438 | MW848618 |
| Cercospora zorniicola | YMM299 | Zornia glochidiata DC. | Fabaceae | – | – | – | MW848616 |
| Nothopassalora personata (Berk. & M.A.Curtis) U.Braun, C. Nakash., Videira & Crous | YMM49A | Arachis hypogaea L. | Fabaceae | MW834479 | MW844038 | MW834497 | – |
| Passalora arachidicola (Hori) U.Braun | YMM49B | Arachis hypogaea L. | Fabaceae | MW845059 | MW844039 | MW834498 | – |
| Pseudocercospora bradburyae E.Young | YMM275 | Centrosema pubescens Benth. | Fabaceae | MW834465 | – | – | MW848609 |
| Pseudocercospora cruenta (Sacc.) Deighton | YMM288 | Phaseolus sp. | Fabaceae | MW834472 | MW834456 | MW834489 | MW848602 |
| YMM04 | Vigna unguiculata (L.) Walp. | Fabaceae | MW834478 | MW834461 | MW834496 | MW848606 | |
| YMM03A | Vigna unguiculata (L.) Walp. | Fabaceae | MW834482 | MW834460 | MW834495 | MW848613 | |
| YMM294B | Vigna unguiculata (L.) Walp. | Fabaceae | MW834480 | MW834463 | MW834493 | MW848611 | |
| YMM125 | Vigna unguiculata (L.) Walp. | Fabaceae | MW834476 | MW834451 | MW834499 | MW848610 | |
| Pseudocercospora griseola (Sacc.) Crous & U.Braun | YMM297A | Phaseolus lunatus L. | Fabaceae | MW834477 | MW834459 | MW834494 | – |
| Pseudocercospora sennicola | YMM12 | Senna occidentalis (L.) Link | Fabaceae | MW834444 | MW834432 | MW850550 | – |
| Pseudocercospora sp.3 | YMM19 | Abelmoschus sp. | Malvaceae | MW834470 | – | MW834488 | – |
| Pseudocercospora sp.1 | YMM123 | Abelmoschus sp. | Malvaceae | MW834468 | MW834454 | MW834486 | – |
| Pseudocercospora tabei | YMM220 | Vigna unguiculata (L.) Walp. | Fabaceae | MW834450 | MW834434 | MW834439 | MW848617 |
For Maximum Likelihood analyses one thousand nonparametric bootstrap iterations were used with the generalised time-reversible model with a discrete gamma distribution (GTRGAMMA) (Stamatakis et al. 2008). For Bayesian phylogenies, two parallel runs with eight chains of Metropolis-coupled Markov chain Monte Carlo iterations were performed with the heat parameter being set at 0.2. Analyses were run for 100 million generations, with trees sampled every 1000th generation until the average standard deviation of split frequencies reached 0.01 (stop value). The first 25% of saved trees were discarded as the ‘burn-in’ phase. Posterior probabilities (PP) were determined from the remaining trees. Bayesian posterior probabilities (BPP) ≥ 94% and Bootstrap values (BS) ≥ 70% are considered as significant.
Data availability
The specimen data is available through the Dryad Digital Repository https://datadryad.org/ (https://doi.org/10.5061/dryad.73n5tb2x9).
Results
Phylogeny
We isolated DNA from a total of 28 specimens of cercosporoid fungi recently collected in Benin (Table 2). These specimens represent 18 species found on species of Fabaceae for which 76 sequences are provided: 20 sequences of 18S rDNA, 16 of 28S rDNA, 21 of ITS and 19 of tef1. The separately aligned data sets for each marker consisted of 35 sequences/893 base pairs for 18S rDNA, 60/719 for 28S rDNA, 82/437 for ITS and 74/160 for tef1.
For the four-locus data analysis, DNA sequence data from the 18SrDNA, 28SrDNA, ITS and tef1 gene regions were combined and submitted to Bayesian and Maximum Likelihood (ML) analyses. The final concatenated alignment contained a total of 91 specimens including the out-group (65 specimens from NCBI and 26 specimens from this study) and had an aligned length of 2212 characters including alignment gaps. As the ML analyses produced tree topologies mostly identical to results of Bayesian analyses, bootstrap support values of the ML trees were incorporated into the tree that resulted from Bayesian analyses (Fig. 1). In this tree, the cercosporoid fungi are grouped in three major clades: Cercospora (86/76), Pseudocercospora (87/78) and Passalora together with other species of other genera (100/98) (Fig. 1). Phylogenetic analyses of individual loci are deposited in TreeBASE. Details of results concerning the delimitation of species are mentioned and discussed as part of species notes below.
Figure 1.
The Bayesian phylogenetic tree inferred from DNA sequence data from the multigene alignment (SSU rDNA, LSU rDNA, ITS and tef1) of cercosporoid species. Nodes receiving Bayesian PP ≥ 0.94 or MLBS ≥ 70% are considered as strongly supported and are indicated by thickened branches. Names of newly described species are written in bold and red. Species newly reported for Benin are indicated by green letters. Names of host plants are written with blue letters.
Tef1 sequence data showed differences between closely related species in the genera Cercospora and Pseudocercospora and are more informative than ITS and LSU rDNA sequence data. Therefore, we provide molecular phylogenetic analyses based on new tef1 sequences as well as sequences from GenBank for some newly described species, namely Cercospora rhynchophora, C. parakouensis, C. zorniicola and Pseudocercospora tabei. For Ps. sennicola, we provide an analysis based on ITS sequence data, because we were not able to obtain Tef1 sequence data.
Taxonomy
Based on morphological, molecular phylogenetic and host evidence, the cercosporoid fungi recently collected in Benin are assigned to 18 different taxa belonging to four genera. Among these, eight species are proposed as new to science, six in the genus Cercospora and two in Pseudocercospora. Eight species represent new reports for Benin, three of them are new for the whole of West Africa, namely Cercospora cf. canscorina, C. cf. fagopyri and C. phaseoli-lunati. Two species of cercosporoid fungi were previously reported in Benin and are confirmed.
Cercospora beninensis
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
0EC4CB5E-A43D-56A5-B488-FC7786750295
839170
Figure 2.
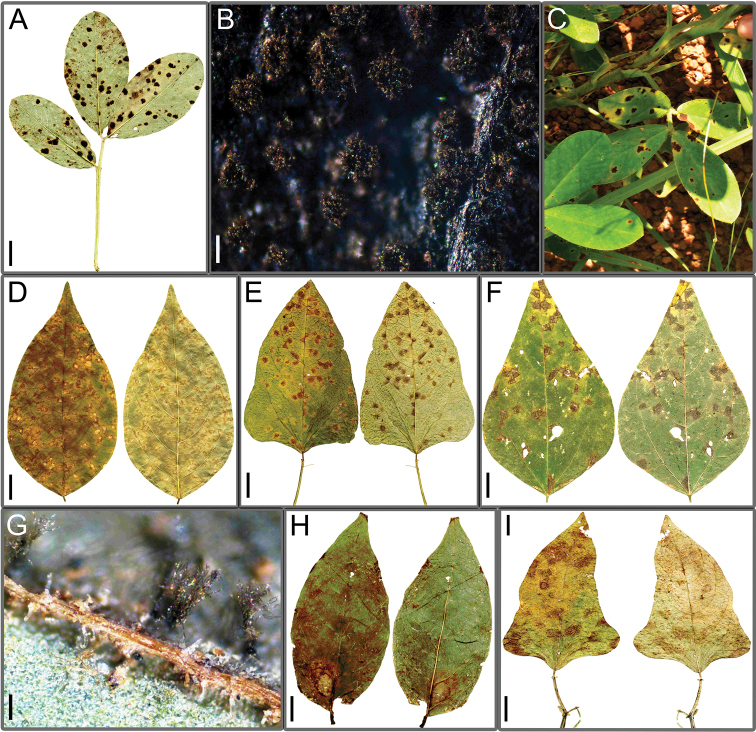
Leaf spot symptoms associated with Cercospora spp. ACercospora beninensis on Crotalaria macrocalyx (YMM11) BCercospora aff. canescens on Calopogonium sp. (YMM07) CCercospora aff. canescens on Vigna subterranea (YMM01) DCercospora fagopyri on Lablab sp. (YMM23A) ECercospora parakouensis on Desmodium tortuosum (YMM296A) FCercospora phaseoli-lunati on Vigna radiata (YMM289) GCercospora rhynchophora on Vigna unguiculata (YMM03B) HCercospora tentaculifera on Vigna unguiculata (YMM75) ICercospora vignae-subterraneae on Vigna subterranea (YMM293) JCercospora zorniicola on Zornia glochidiata (YMM299). Scale bars: 10 mm (A, C, F, G); 12 mm (B, D, E, H, J); 6 mm (I).
Figure 3.
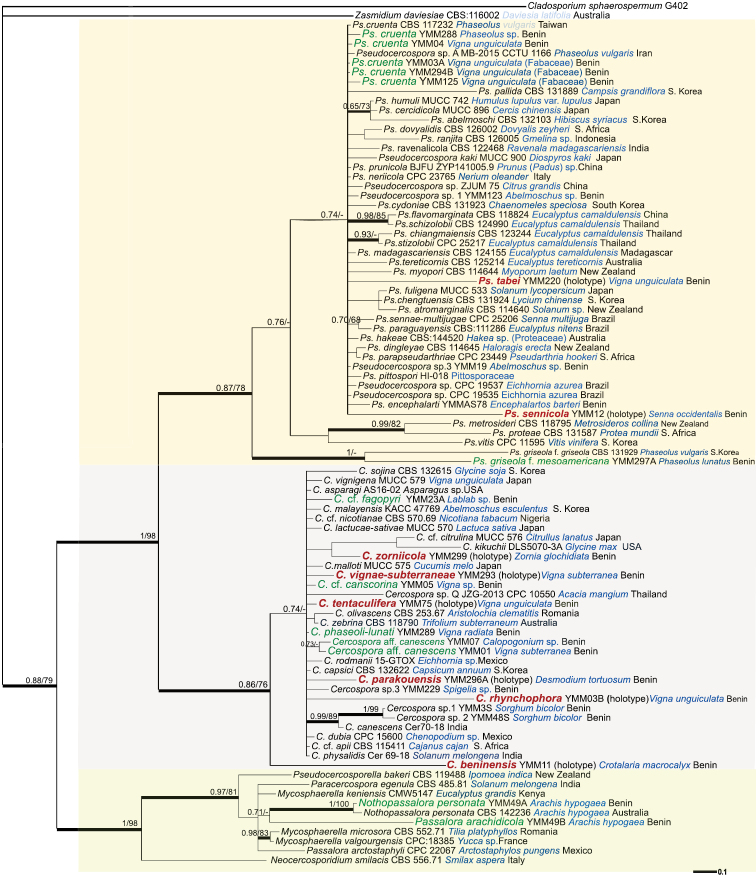
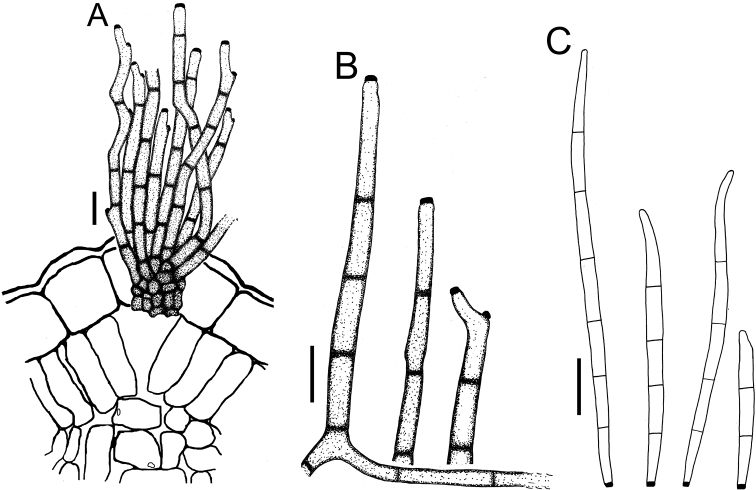
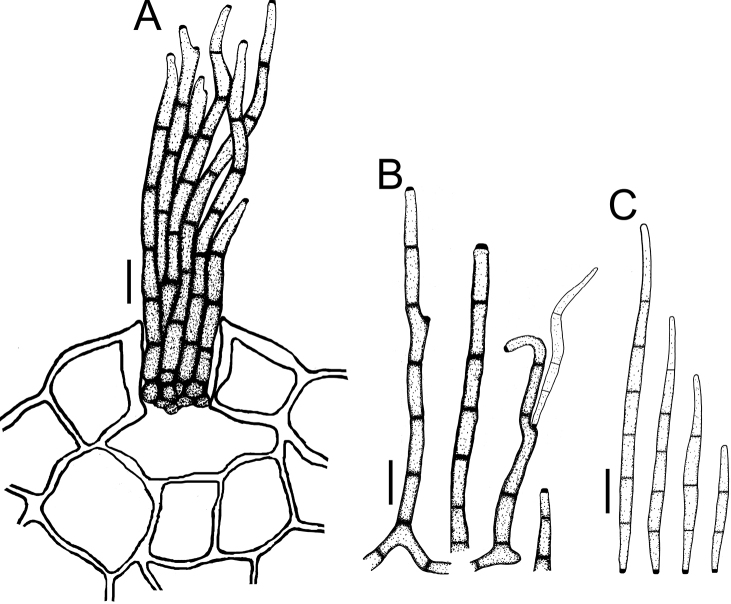
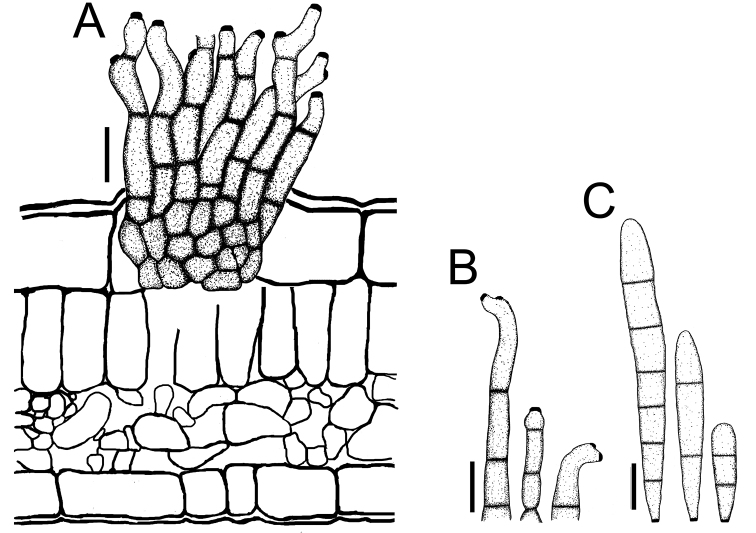
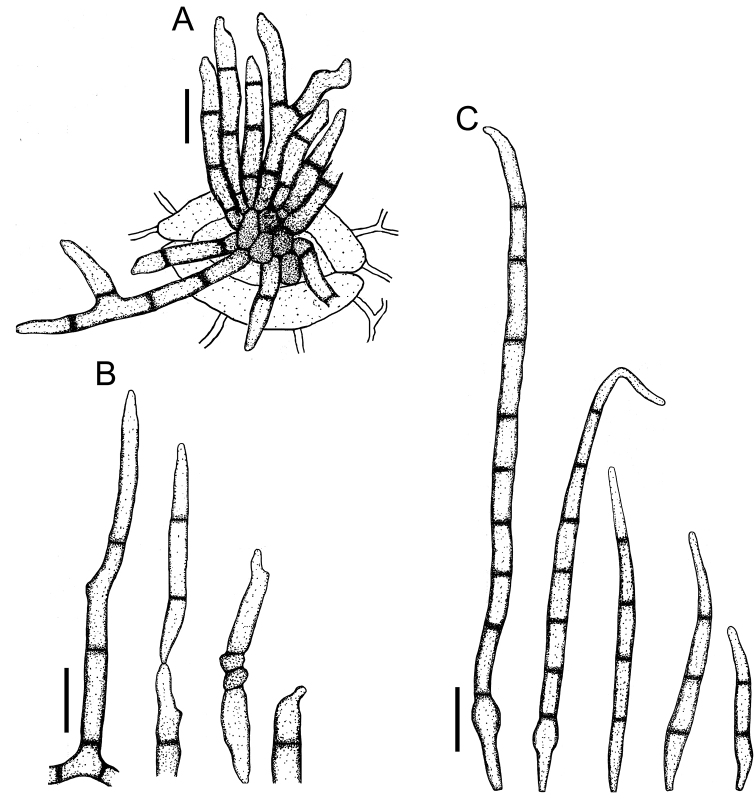
Cercospora beninensis on Crotalaria macrocalyx (YMM11) A fascicle of conidiophores B individual conidiophores C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Etymology.
The epithet beninensis refers to the country of origin of the type specimens, Benin.
Diagnosis.
Cercospora beninensis differs from four Cercospora spp. known on Crotalaria spp. by having only internal hyphae, darker, shorter and narrower conidiophores [(14.5–)28.5–160(–168) × (3–)3.5–4.5(–5) μm] and mostly smaller and narrower conidia [(19–)23.5–122(–150) × (2.5–)3–4(–4.5) μm] (Table 3).
Table 3.
Comparison of Cercospora beninensis (YMM11) on Crotalaria macrocalyx with Cercospora spp. known from Crotalaria spp. based on literature a–f.
| Cercospora species | Leaf spots colour, size | Stromata | Conidiophore size (in μm), branching, septa, colour | Conidium sizes (in μm), septa |
|---|---|---|---|---|
| Cercospora beninensis (YMM11) | Brown to reddish brown, 0.5(1.5–)–5.5 mm diam. | Small or lacking | (14.5–)28.5–160(–168) × (3–)3.5–4.5(–5), branched, 0–6(–8)-septate, dark brown | (19–)23.5–122(–150) × (2.5–)3.5–4(–4.5), 1–7(–9) septa |
| C. apii ab | Present | Often small or lacking, occasionally developed, (up to 50 μm diam.) | 20–300 × 4–6.5, rarely branched, multi-septate, pale brown, uniform in colour and width | 25–315 × 3–6, (0–)3–25(–30) septab |
| C. canescens a | 3–15 mm | Often small | 20–200 × 3–6.5, rarely branched, multi-septate, pale to medium dark brown | 25–300 × 2.5–5.5, indistinctly multi-septate |
| C. demetrioniana cde | Rusty brown to dark brown, 1–1.5 mm. | Present | 40–350 × 4–6 (–7) c or up to 1 mmde, 1–10-septate, unbranched, pale brown | 50–210 × 3.5–5.5 /75–230 × 4–7, 7–16, very closely and indistinctly septate |
| C. demetrioniana f. minor f | No information | No information | 110–130 × 5–6 | 35–70(–170) × 5–5.5 |
Type.
Benin. Borgou: Parakou, c. 363 m a.s.l., 9°20'29"N, 2°37'28"E, on Crotalaria macrocalyx Benth. (Fabaceae), 21 Sep 2019, Y. Meswaet and R. Dramani, YMM11 (Holotype: M-0312640; Isotype: UNIPAR). Ex holotype sequences.MW834445 (SSU), MW834433 (LSU), MW834437 (ITS), MW848615 (tef1).
Description.
Leaf spots amphigenous, subcircular to angular-irregular, (0.5–)1.5–5.5 mm diam., brown to reddish brown, more evident on the adaxial surface of the leaves than on the abaxial side, occasionally with a chlorotic halo, the outermost ring darker than the inner ring, often with indefinite margin. Caespituli amphigenous, mainly epiphyllous, greyish brown to dark brown. Mycelium internal. Internal hyphae conspicuous, branched, 2.5–3.5 μm wide, septate, pale brown. Stromata lacking or formed by few aggregated swollen hyphal cells. Conidiophores in small, loose to moderately dense fascicles of up to approx. 16 conidiophores, occasionally solitary, arising from internal hyphae breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, erect, straight, subcylindrical, 1–2(–3) times geniculate, sometimes attenuated towards the tips, occasionally branched, (14.5–)28.5–160(–168) × (3–)3.5–4.5(–5) μm, 0–6(–8)-septate, brown to dark brown. Conidiogenous cells monoblastic or proliferating sympodially, sometimes distinctly subdenticulate; loci 1.5–2.5(–3.5) μm wide, thickened and darkened. Conidia solitary, acicular to narrowly obclavate, straight to curved, (19–)23.5–122(–150) × (2.5–)3–4(–4.5) μm, 1–7(–9)-septate, hyaline, smooth, tip acute, base truncate to short obconically truncate, 2.5–3(–4) µm wide, hila thickened and darkened.
Additional specimens examined.
Benin. Borgou: Parakou, on the way to Okpara forest, c. 323 m a.s.l., 9°18'11"N, 2°43'50"E, on Crotalaria macrocalyx, 3 Sep 2019, Y. Meswaet and R. Dramani, YMM274 (Paratypes: M-0312641; UNIPAR). Benin. Borgou: N’Dali, c. 380 m a.s.l., 9°52'33"N, 2°41'20"E, same host, 31 Aug 2019, Y. Meswaet and A. Tabé, YMM272 (M-0312642).
Host and distribution.
On Crotalaria macrocalyx (Fabaceae) in Benin.
Notes.
Currently, three species and one form of Cercospora are known on Crotalaria spp., namely C. apii, C. canescens, C. demetrioniana G.Winter and C. demetrioniana f. minor Gonz. Frag. & Cif. (Farr and Rossman 2021). C. beninensis is morphologically distinct from all of them (Table. 3). C. apii differs by conidiophores that are more abundant on the abaxial surface of the leaves, in large and dense fascicles and longer [20–300 µm versus (14.5–)28.5–160(–168) in C. beninensis] as well as by longer and wider conidia [(25–315 × 3–6 µm versus (19–)23.5–122(–150) × (2.5–)3–4(–4.5) in C. beninensis] with more numerous septa (Chupp 1954; Crous and Braun 2003). C. canescens causes larger leaf spots often along the leaf margin, paler conidiophores that are more abundant on the abaxial leaf surface and longer conidia [(30–300) µm versus (19–)23.5–122(–150) μm in C. beninensis] (Chupp 1954). The distinctness is confirmed by molecular data. C. demetrioniana produces unbranched, paler, longer and wider conidiophores [40–350 × 4–6(–7) µm, in the original description a length of up to 1 mm is mentioned, versus (14.5–)28.5–160(–168) × (3–)3.5–4.5(–5) in C. beninensis] and above all, longer and wider conidia (75–230 × 4–7 µm with 7–16 indistinct septa versus (19–)23.5–122(–150) × (2.5–)3–4(–4.5) μm with 1–7(–9) distinct septa in C. beninensis] (Winter 1884; Saccardo 1886; Chupp 1954). C. demetrioniana f. minor differs from the present species by shorter and wider conidiophores (110–130 × 5–6 µm) and wider conidia (5–5.5 µm) (Ciferri and González-Fragoso 1926).
C. beninensis is distinct from all known species for which DNA sequence data are available based on its position in the multi-gene (Fig. 1) and in the tef1 phylogeny (see Suppl. material 4). In the ITS phylogeny, C. beninensis cannot be distinguished from other Cercospora spp. (see Suppl. material 3).
Cercospora aff. canescens
Ellis & G.Martin, Am. Nat. 16(12): 1003 (1882).
C45944C4-8F5B-57C2-A039-D89D88EBFE36
179841
Figure 4.
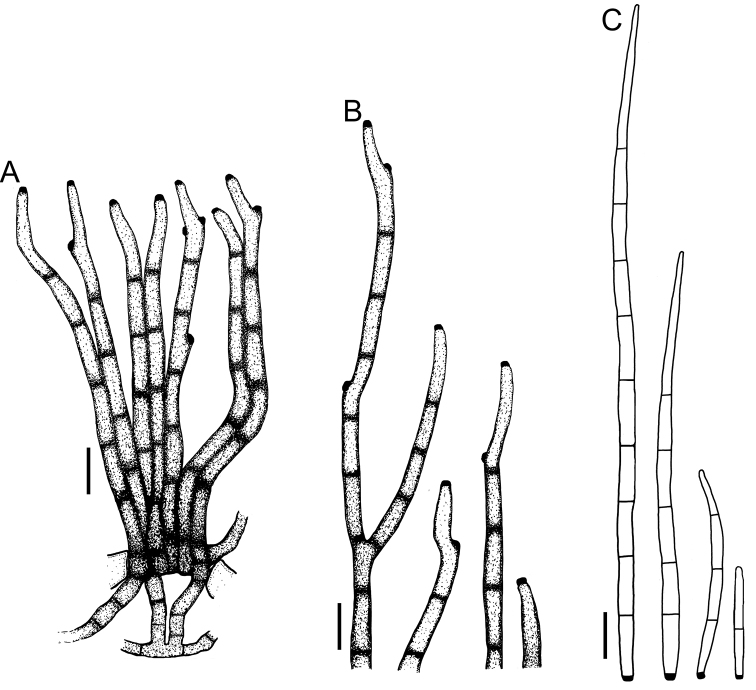
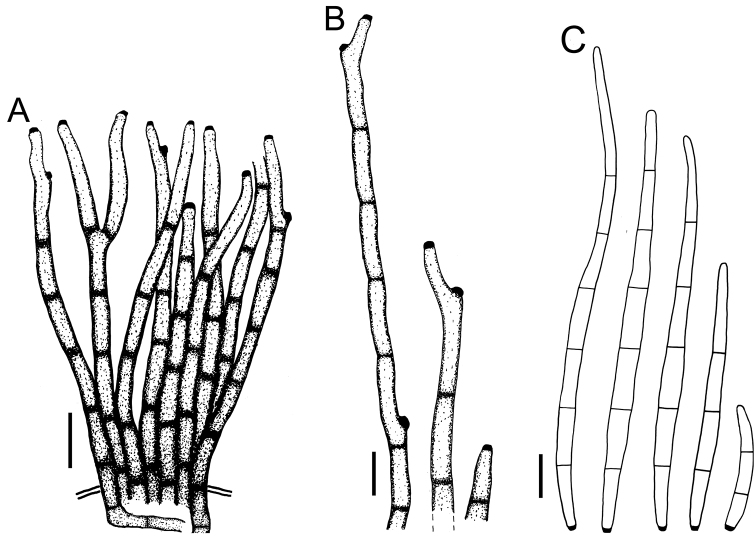
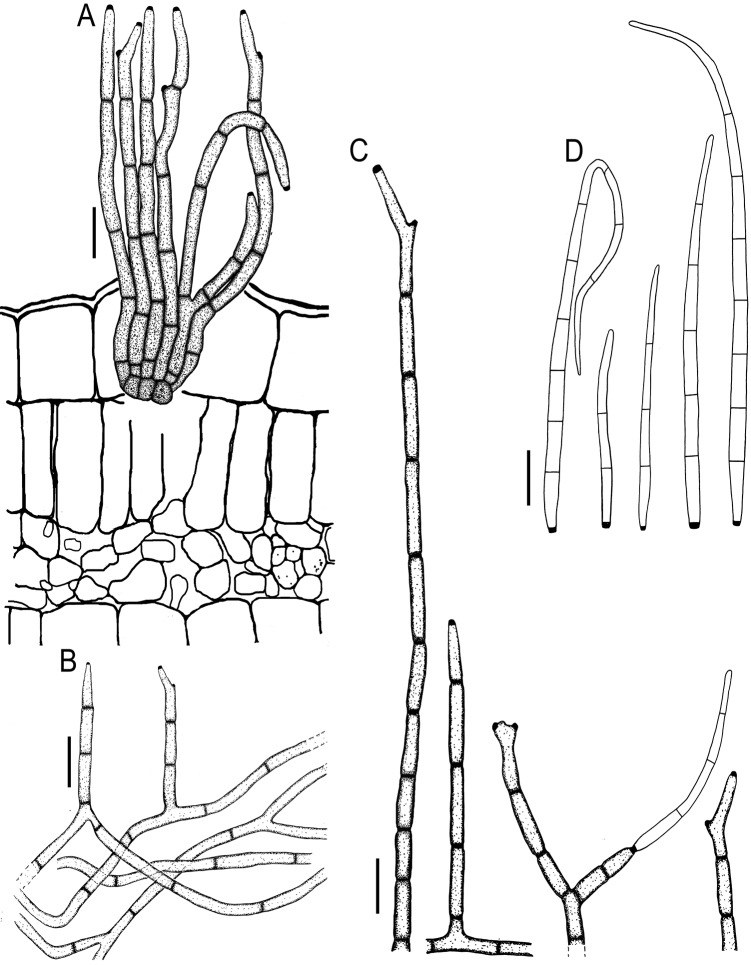
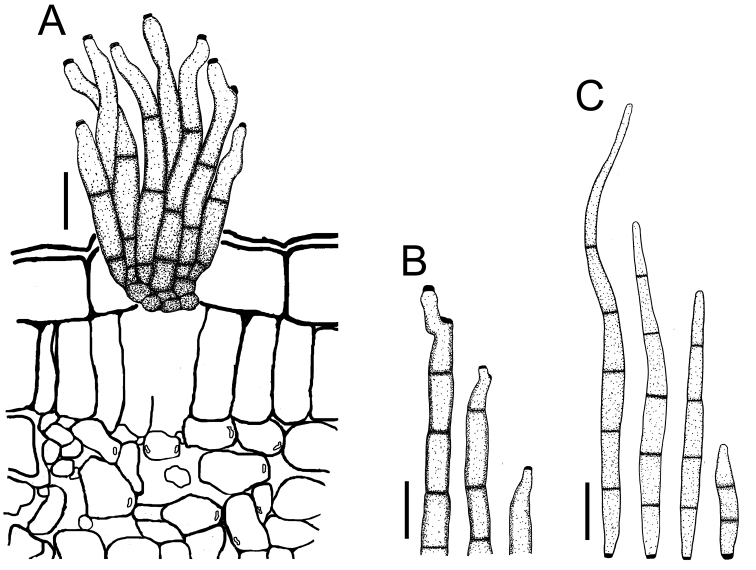
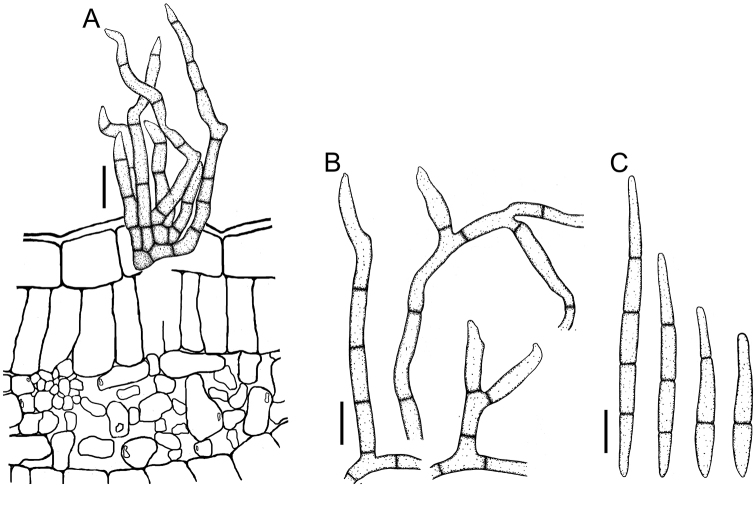
Cercospora aff. canescens on Calopogonium sp. (YMM07) A fascicle of conidiophores protruding from a stomatal opening B solitary conidiophores C conidia. Scale bars: 15 μm (A, C); 10 μm (B).
Type.
USA (no further data available), on Phaseolus sp. (Fabaceae), 1882, s.n. (“Type?” NY, n.v.).
For synonyms see Crous and Braun (2003) or MycoBank.
Description.
Leaf spots amphigenous, subcircular to irregularly angular, 3–11.5(–13) mm diam., occasionally crossing veins, reddish brown to slightly dark brown, with dark margin. Caespituli amphigenous, greyish brown to dark brown. Mycelium internal and external. Internal hyphae often indistinct. External hyphae branched, 2.5–3.5 μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking or formed by few aggregated swollen hyphal cells, immersed in the mesophyll or in substomatal cavities, dark brown. Conidiophores in small, loose fascicles of up to 8, arising from stromata, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, sometimes solitary arising through stomatal openings or erumpent through the cuticle, erect, straight to sinuous or somewhat geniculate, rarely branched, (16.5–)21–152(–165) × (4–)4.5–5.5 μm, 1–6-septate, brown to dark brown. Conidiogenous cells terminal, monoblastic to polyblastic, brown; loci 1.5–2.5 (–3) μm wide, thickened and darkened. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (34–)38–280(–330) × (3–)3.5–4(–4.5) μm, 3–12(–14)-septate, hyaline to subhyaline, smooth, apex subacute or acute, base truncate to short obconically truncate, up to 2.5 μm wide, hila thickened and darkened.
Specimens examined.
Benin. Borgou: Parakou, c. 363 m a.s.l., 9°20'29"N, 2°37'28"E, on Calopogonium sp., 21 Sep 2019, Y. Meswaet and A. Tabé, YMM07 (M-0312643, UNIPAR). Benin. Borgou: Parakou, c. 395 m a.s.l., 9°21'27"N, 2°36'44"E, Calopogonium sp., 17 Sep 2019, Y. Meswaet and A. Tabé, YMM08 (M-0312644). Benin. Borgou: Parakou, c. 395 m a.s.l., 9°21'27"N, 2°36'44"E, on Vigna subterranea, 16 Sep 2019, Y. Meswaet and R. Dramani, YMM01 (M-0312645, UNIPAR).
Herbarium specimens examined for comparison.
C. canescens. On Vigna unguiculata (as V. sinensis L.): El Salvador. Sacocoyo, 3 Jul 1943, Wellman F. L. 140 (BPI 434127B). On V. unguiculata (as V. sinensis): USA. Illinois: Gallatin County, 8 Sep 1932, G.H. Boewe B331 (ILL23703 Holotype of C. vignicaulis Tehon). On V. unguiculata: USA. Illinois: Pulaski, Olmstead, 17 Sep 1933, G.H. Boewe s.n. (ILL24809 Paratype of C. vignicaulis). On V. unguiculata (as V. sinensis): USA. Illinois: White, Carmi., 10 Sep 1934, G.H. Boewe B588 (ILL 25450 Paratype of C. vignicaulis).
Hosts and distribution.
On many species of Fabaceae and of other families (Crous and Braun 2003), known worldwide, from Australia, Bangladesh, Brazil, Bolivia, Brunei, Cambodia, China, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Fiji, Ghana, Guyana, Haiti, Hong Kong, India, Indonesia, Iran, Japan, Kenya, Korea, Malawi, Malaysia, Malawi, Mauritius, Myanmar, Nepal, New Caledonia, New Zealand, Nigeria, Pakistan, Panama, Papua New Guinea, Peru, Philippines, Puerto Rico, Russia, Senegal, Sierra Leone, Solomon Islands, Somalia, South Africa, Saint Vincent and the Grenadines, Sudan, Tadzhikistan, Taiwan, Tanzania, Thailand, Trinidad and Tobago, Togo, Uganda, USA, Uzbekistan, Vanuatu, Venezuela, Zambia, Zimbabwe (Chupp 1954; Ellis 1976; Shin and Kim 2001; Crous and Braun 2003; Farr and Rossman 2021).
Notes.
The present Cercospora sp. on Calopogonium sp. also occurs on Vigna subterranea with different leaf spot appearances and caespituli. The lesions on Calopogonium sp. appear to be associated with a species of Pleosporales, whereas the leaf lesions on V. subterranea apparently are not associated with any other fungus and are dark reddish brown to dark brown with a dark margin, which are typical symptoms caused by Cercospora spp. The lesions on V. subterranea are larger and more abundant than those on Calopogonium sp., with abundant, dense caespituli and with dark greyish brown pigmentation (Fig. 2C).
Cercospora canescens is the only species of Cercospora known for Calopogonium spp. (Farr and Rossman 2021) and has been reported from West Africa (Guinea) on Calopogonium mucunoides (Lenné 1990). Apart from having slightly narrower conidia [(3–)3.5–4(–4.5) μm versus 2.5–5.5(–6) μm in C. canescens] as described by Chupp (1954), Hsieh and Goh (1990) and Mulder and Holliday (1975), the present specimen from Benin is morphologically identical to C. canescens. In the phylogenetic analyses, however, DNA sequences of the two specimens from Benin cluster together but separately from sequences of C. canescens available from India. In the multi-gene tree (Fig. 1), C. canescens is located on a branch in a clade together with sequences of Cercospora spp. YMM3SO and YMM48SO on Sorghum bicolor (Poaceae) from Benin. C. canescens is known to correspond to a species complex that shows diverse morphological characteristics and genetic diversity (Joshi et al. 2006; Groenewald et al. 2013). Although C. canescens is an economically important species, no sequence data from the type or a neotype specimen are available (e.g., Groenewald et al. 2013). These are indispensable to resolve the C. canescens species complex. The specimens collected in Benin are tentatively placed into the species complex of C. canescens until DNA sequence data from the type locality (USA) and from diverse host species are available. C. aff. canescens is cited here for the first time for Benin (Piepenbring et al. 2020).
Cercospora cf. canscorina
Chidd., Sydowia 13 (1–6): 155. 1959.
CB5EDB98-0F7C-5816-9CB8-264ABD41172D
294326
Figure 5.
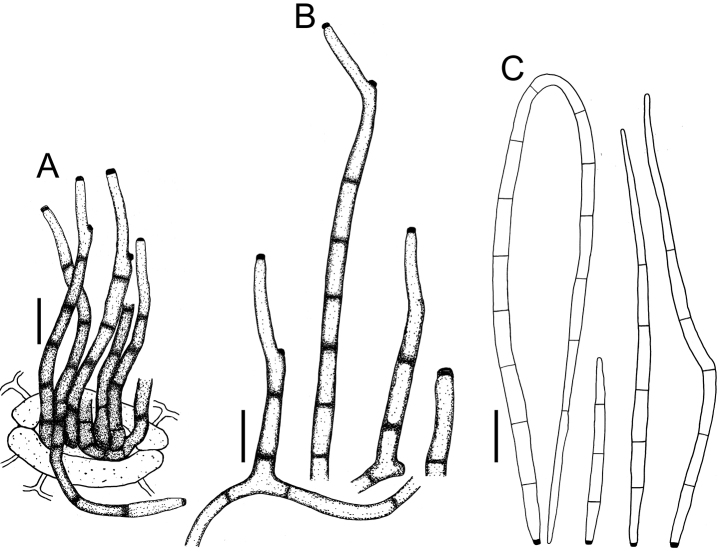
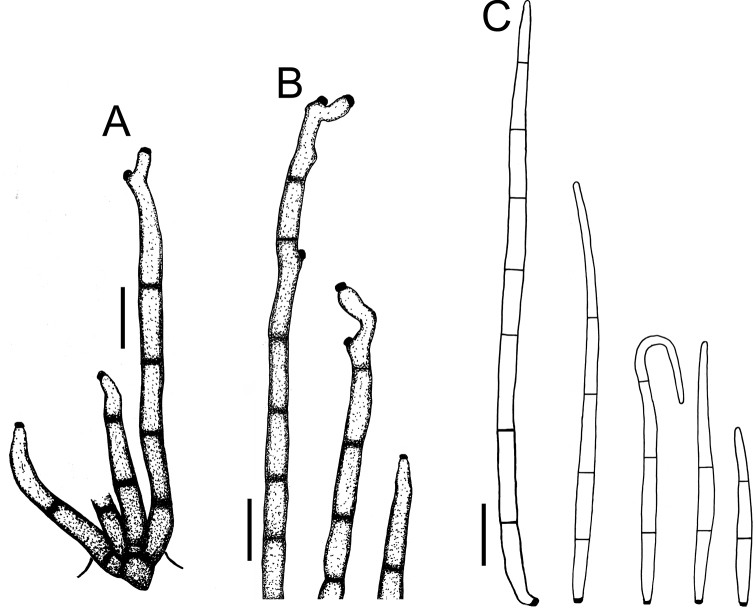
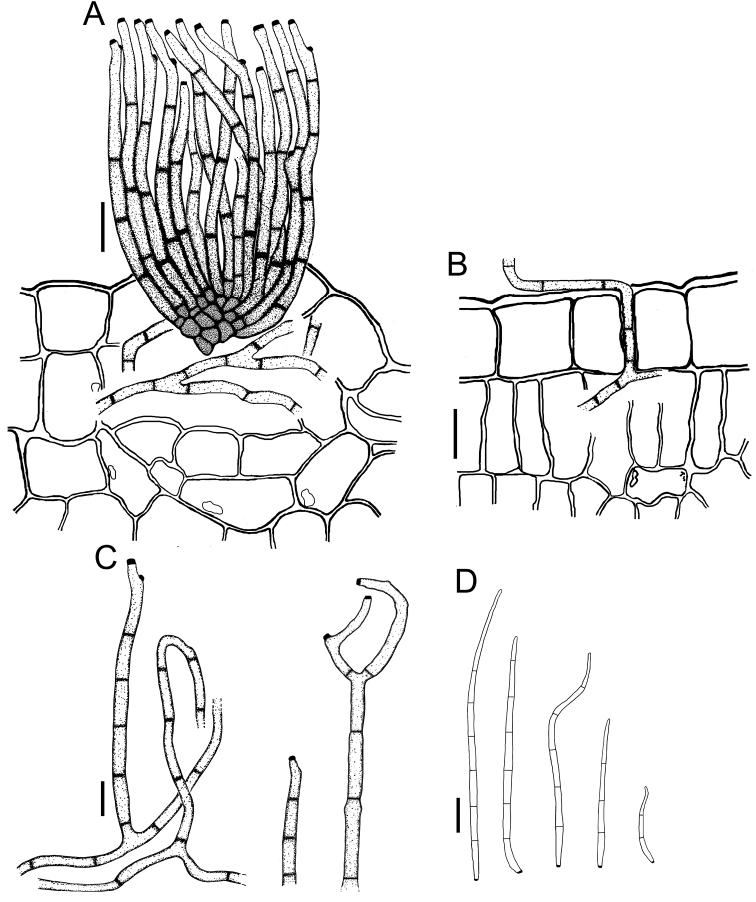
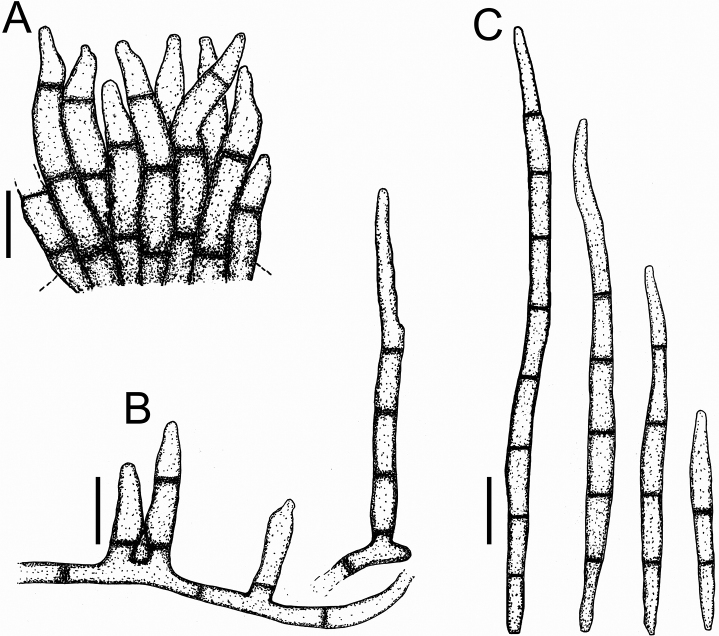
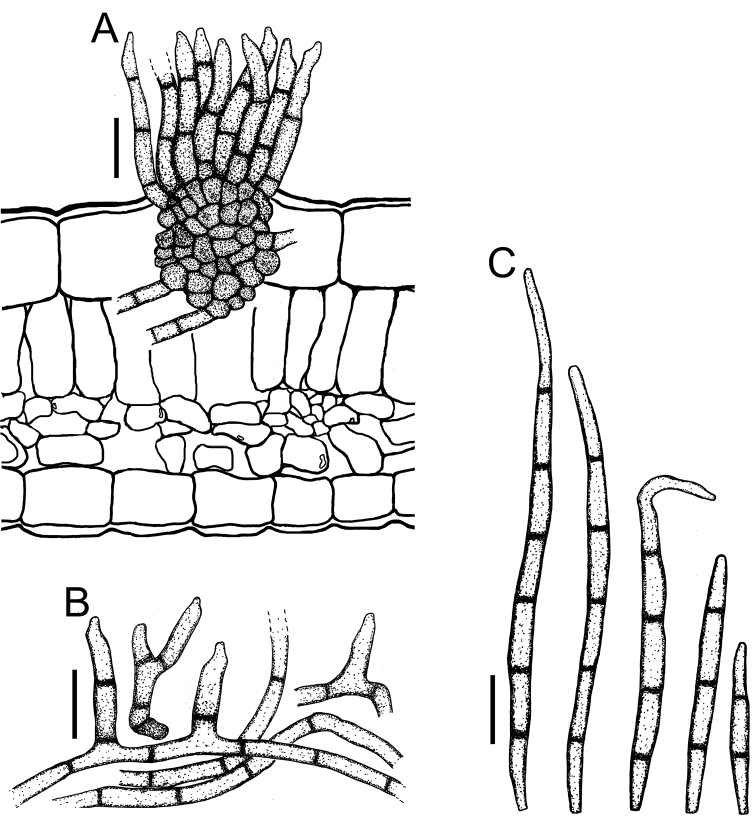
Cercospora cf. canscorina on Vigna sp. (YMM05) A fascicle of conidiophores protruding from a stomatal opening B solitary conidiophores C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Type.
India. R. Br. Khandala (Maharashtra), on Canscora diffusa (Vahl) R.Br. ex Roem. & Schult. (Gentianaceae), 9 Nov 1956, Chiddarwar 4 (Holotype: IMI 83165, n.v.; Isotypes: HCIO, BPI, n.v.).
Description.
Leaf spots amphigenous, subcircular to irregularly angular, 2.5–8 mm diam., brown to reddish brown, with a dark margin. Caespituli amphigenous, greyish brown to brown. Mycelium internal. Stromata lacking or formed by few substomatal aggregated swollen hyphal cells. Conidiophores in small, loose fascicles to moderately large and dense fascicles of up to approx. 22 conidiophores, arising from internal hyphae breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, sometimes solitary, erect, straight, subcylindrical, 1–2 times geniculate, unbranched, (12–)20.5–68(–72) × (3–)3.5–4.5 μm, 0–6-septate, brown to dark brown. Conidiogenous cells terminal, usually monoblastic, sometimes polyblastic; loci apical or sometimes located on the shoulders of geniculations, 1.5–2.5(–3) μm wide, thickened and darkened. Conidia solitary, acicular to narrowly obclavate, straight to curved, 22–76(–80) × 2.5–3.5 μm, 1–7-septate, hyaline, smooth, tip acute, base truncate to short obconically truncate, 2–3 µm wide, hila thickened and darkened.
Specimens examined.
Benin. Borgou: Parakou, c. 386 m a.s.l., 9°20'35"N, 2°36'37"E, on Vigna sp., 14 Sep 2019, Y. Meswaet and R. Dramani, YMM05 (M-0312646; UNIPAR).
Hosts and distribution.
On Canscora diffusa (Gentianaceae) from Khandala, West India (Chiddarwar 1959) and on Vigna unguiculata (as Vigna catjang (Burm.f.) Walp.) from India (Bhat and Pratibha 2010). C. cf. canscorina is reported here for the first time for Benin and for Africa.
Notes.
Seven species of Cercospora have previously been recorded on Vigna spp., namely C. apii, C. canescens, C. canscorina, C. caracallae (Speg.) Vassiljevsky & Karak., C. kikuchii, C. longispora Peck and C. vignigena C. Nakash., Crous, U.Braun & H.D. Shin (Farr and Rossman 2021). The present species from Benin is morphologically identical to C. canscorina (Chiddarwar 1959; Bhat and Pratibha 2010) except for narrower conidiophores with (3–)3.5–4.5 μm versus 3–7 μm in C. canscorina as mentioned by Bhat and Pratibha (2010). The original specimen of C. canscorina was not available for morphological examination and no DNA sequence data are currently published for this species. Therefore, a reliable species identification is not possible. The application of the name for the collections from Benin is tentative and must be verified based on sequences derived from the Indian type specimen or similar samples. C. cf. canscorina differs from all species of Cercospora on other members of Fabaceae from Benin by producing unbranched, relatively pale conidiophores and above all, shorter conidiophores [(12–)20.5–68(–72) μm] and conidia [22–76(–80) μm]. Based on the multi-gene tree (Fig. 1) it is not possible to distinguish Cercospora cf. canscorina from many other Cercospora spp.
Cercospora cf. fagopyri
K.Nakata & S.Takim., J. Agric. Exp. Stat. Gov. Gen. Chosen 15: 29. 1928.
A814C1D6-FEDF-525E-B0AE-A21ACDBB5A21
456931
Figure 6.
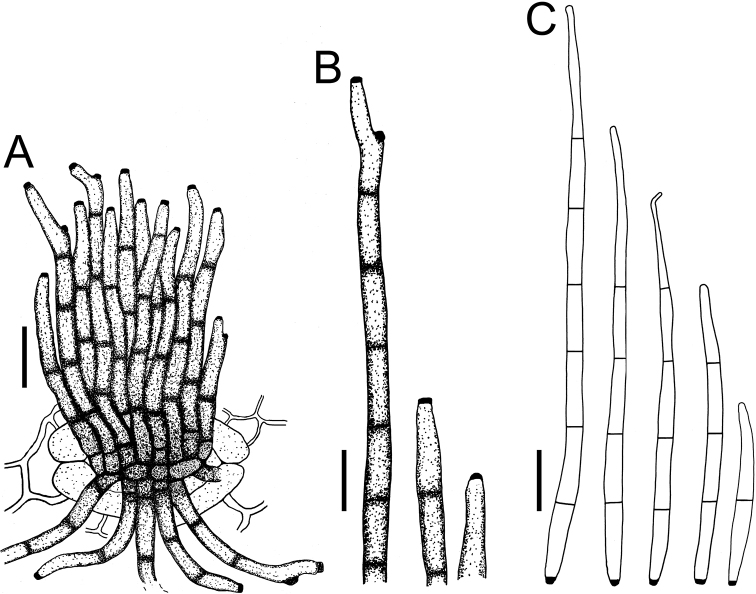
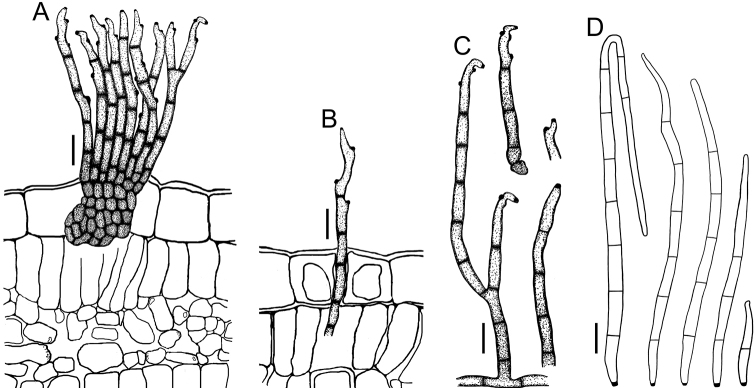
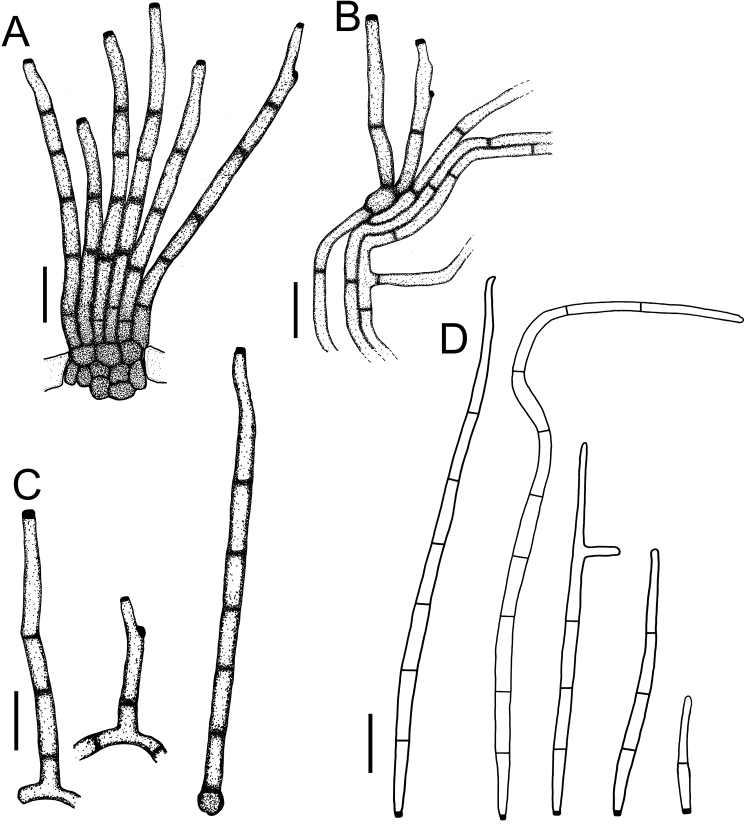
Cercospora cf. fagopyri on Lablab sp. (YMM23A) A fascicle of conidiophores growing out from a slightly developed stroma in the epidermis shown as part of a transverse section of a leaf B solitary conidiophores C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Type.
South Korea. Suwon, on Fagopyrum esculentum Moench (Polygonaceae), Sep 1934, K. Nakata & S. Takimoto (holotype specimen, not located and not preserved according to Groenewald et al. (2013), neotype: CBS H-21008, n.v).
For synonyms see Groenewald et al. (2013) or MycoBank.
Description.
Leaf spots amphigenous, circular to subcircular or rarely irregularly angular, 2–5 mm diam., more or less limited by veins, reddish to pale brown, margin dark brown on the adaxial surface, less conspicuous on the abaxial surface. Caespituli amphigenous, conspicuous, greyish brown to dark brown. Mycelium internal and external. External hyphae branched, often inconspicuous, 1.5–3 μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking to well-developed, 10–45 µm diam., dark brown, substomatal or breaking through the epidermis. Conidiophores in small, loose to moderately dense fascicles of up to approx. 14 conidiophores, arising from stromata breaking through the adaxial epidermis of the leaves or through stomatal openings, sometimes solitary arising from external hyphae, erect, straight, subcylindrical to geniculate, unbranched, (22.5–)36–157(–168) × 3–4(–5) μm, 2–6(–8)-septate, brown to dark brown. Conidiogenous cells terminal, with 1–2 loci; loci mainly apical, sometimes located on the shoulders of geniculations, 1.5–2(–3) μm wide, thickened and darkened. Conidia solitary, acicular to narrowly obclavate, straight to somewhat curved, (24–)27.5–70(–78) × (2–)2.5–3(–4) μm, with 2–5(–6) somewhat indistinct septa, hyaline, smooth, tip acute, base truncate to short obconically truncate, 1.5–2.5 μm wide, hila thickened and darkened.
Specimens examined.
Benin. Donga: Taneka-Koko, c. 441 m a.s.l., 9°51'30"N, 1°29'34"E, on Lablab sp., 29 Jul 2017, Y. Meswaet, M. Piepenbring, N. S. Yorou and participants of the summer school 2017, YMM23A ((M-0312647; UNIPAR). Same locality and host, 03 Aug 2016, Y. Meswaet, M. Piepenbring, N. S. Yorou and participants of the summer school 2016, YMM02 (M-0312648).
Hosts and distribution.
On Cercis chinensis (Fabaceae), Cosmos bipinnata Cav. (Asteraceae), Fallopia dumetorum (L.) Holub and Fagopyrum esculentum (Polygonaceae), Hibiscus syriacus (Malvaceae), Viola mandshurica W. Becker (Violaceae), from China, Japan, South Korea, Taiwan, Uganda and Venezuela (Hsieh and Goh 1990; Groenewald et al. 2013). C. cf. fagopyri is cited here for the first time on Lablab sp. and the first time for Benin and West Africa.
Notes.
Currently there are two species of the genus Cercospora known on hosts belonging to Lablab, namely C. canescens and C. apii. The present Cercospora sp. (YMM23A) differs from C. canescens in leaf spot size, stromata and septation characteristics, as well as unbranched conidiophores. Above all, the sizes of the conidia of the present species are different [(24–)27.5–70(–78) × (2–)2.5–3(–4) μm versus 30–300 × 2.5–5 (–6) µm in C. canescens]. C. apii differs by often small or lacking stromata, dense fascicules of up to 30 conidiophores, branched, longer conidiophores [20–300 μm versus (22.5–)36–157(–168) μm in C. cf. fagopyri] and above all, longer and wider conidia [25–315 × 3–6 μm versus (24–)27.5–70(–78) × (2–)2.5–3(–4) μm in C. cf. fagopyri] (Chupp 1954).
Our sequence of the tef1 region of the specimen YMM23A from Benin is 100% similar to a sequence of Cercospora fagopyri on Fallopia dumetorum (GenBank JX143353) (Identities 233/233, i.e., 100%) and 99% similar to a further sequence of C. fagopyri on Fagopyrum esculentum (GenBank JX143352; Identities; 233/234, i.e., 99%). The identification of the present specimen as C. cf. fagopyri is only based on molecular data. Morphologically, descriptions of specimens of C. fagopyri on diverse host species in the literature differ and are quite confusing (Hsieh and Goh 1990; Groenewald et al. 2013). In order to establish a morphological concept and to know the host range of C. fagopyri, fresh specimens need to be collected once again on Fagopyrum esculentum in Korea, where this species was originally collected and pathogenicity needs to be proven for diverse host species.
Cercospora parakouensis
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
51755D67-071A-52FE-A3BA-6E9489B5660B
839171
Figure 7.
Cercospora parakouensis on Desmodium tortuosum (YMM296A) A fascicle of erumpent conidiophores B solitary conidiophores C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Type.
Benin. Borgou: Parakou, Tankaro, c. 360 m a.s.l., 9°23'01"N, 2°30'36"E, on Desmodium tortuosum (Sw.) DC. (Fabaceae), 20 Sep 2019, Y. Meswaet and R. Dramani, YMM296A (Holotype: M-0312649; Isotype: UNIPAR). Ex holotype sequences.MW834436 (LSU), MW834442 (ITS), MW848621 (tef1).
Etymology.
The epithet parakouensis refers to the city of the type collection, Parakou, Benin.
Diagnosis.
Cercospora parakouensis differs from the two Cercospora species known on Desmodium spp., namely C. canescens and C. kashiensis Bharadwaj by producing almost no stromata, branched, darker and shorter conidiophores [(12.5–)18–178(–190) μm] and non- pigmented and shorter conidia [(14–)19–88(–113.5) × 3.5–4.5(–5) μm].
Description.
Leaf spots almost lacking to well-developed, amphigenous, subcircular to irregularly angular, 1.5–5 mm diam., darkish brown to reddish brown, often with a diffuse whitish centre surrounded by a darker margin. Caespituli amphigenous, greyish brown to dark brown. Mycelium mainly internal. Stromata lacking. Conidiophores in small, loose fascicles, sometimes arising from internal hyphae, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, occasionally solitary, arising through stomatal openings, erect, straight to sinuous or somewhat geniculate, occasionally branched, (12.5–)18–178(–190) × (3.5–)4–5(–5.5) μm, 1–6(–8)-septate, brown to dark brown. Conidiogenous cells terminal or rarely intercalary, usually monoblastic, rarely polyblastic; loci subcircular, 1.5–3 μm wide, thickened and darkened, refractive. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (14–)19–88(–113.5) × 3.5–4.5(–5) μm, 2–6-septate, hyaline, smooth, apex subacute or acute, base truncate to short obconically truncate, 2–3(–3.5) μm wide, hila thickened and darkened.
Additional specimens examined.
Benin. Borgou: Parakou, c. 395 m a.s.l., 9°21'27"N, 2°36'44"E, on Desmodium tortuosum, 17 Sep 2019, Y. Meswaet and A. Tabé, YMM292 (Paratypes: M-0312650; UNIPAR).
Herbarium specimens examined for comparison.
See Cercospora aff. canescens.
Host and distribution.
On Desmodium tortuosum (Fabaceae) from Benin.
Notes.
Currently, two Cercospora species are known from Desmodium spp., namely C. canescens and C. kashiensis (Farr and Rossman 2021). C. canescens differs from the present species by causing large leaf spots often along the margin of the leaf, 3–15 mm in extent, paler conidiophores and above all, longer conidia [30–300 µm versus (14–)19–88(–113.5) µm in C. parakouensis] (Chupp 1954). The distinctness is also confirmed by molecular data (Fig. 1). C. kashiensis described on Desmodium gangeticum (L.) DC. from India causes different leaf spots, has unbranched and longer conidiophores (40–282 µm versus (12.5–)18–178(–190) in C. parakouensis) and above all, pigmented and longer conidia (16–220 µm versus (14–)19–88(–113.5) µm in C. parakouensis) with 2–15 septa (Bharadwaj 1971).
In the multi-gene tree (Fig. 1), the ITS and the tef1 phylogeny (see Suppl. materials 3, 4), C. parakouensis forms part of a polytomy with a relatively large genetic distance (branch length) in relation to other sequences considered in the analysis.
Based on a MegaBLAST search using the tef1 sequence, the closest matches in NCBI’s GenBank nucleotide database were Cercospora nicotianae on Nicotiana tabacum (Solanaceae) from China (GenBank MK881748; Identities 283/291, i.e., 97%), Cercospora cf. sigesbeckiae on Persicaria orientalis L. (Polygonaceae) from South Korea (GenBank JX143412; Identities 283/291, i.e., 97%) and Cercospora aff. canescens on a species of Malvaceae from Mexico (GenBank JX143321; Identities 283/291, i.e., 97%).
Cercospora phaseoli-lunati
U.Braun & Crous, Mycotaxon 92: 396. 2005.
25643304-DB25-5DB4-8E42-0E6E3FDDD0DB
MycoBank No: 500171 Figs 2F , 8
Figure 8.
Cercospora phaseoli-lunati on Vigna radiata (YMM289) A small fascicle of conidiophores B solitary conidiophores C conidia. Scale bars: 12 μm (A); 10 μm (B, C).
Type.
USA. Alabama: Tuskegee, on Phaseolus lunatus (Fabaceae), 5 Jul 1897, G.W. Carver 290 (HolotypeNY, n.v.).
Description.
Leaf spots amphigenous, subcircular to irregularly angular, 2.5–8(–12) mm diam., more or less limited by veins, whitish grey to greyish brown, with a narrow to wide dark brown margin on the adaxial surface, less conspicuous on the abaxial surface. Caespituli amphigenous, mainly epiphyllous, scattered, brown to dark brown. Mycelium internal, indistinct. External hyphae absent. Stromata lacking or formed by few aggregated swollen hyphal cells, immersed in the mesophyll or in substomatal cavities. Conidiophores in small, loose fascicles of up to 6, arising from internal hyphae of small hyphal aggregations, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, or solitarily arising through stomatal openings, erect, rarely branched, straight to geniculate or subcylindrical to mostly attenuated towards the tips, conical or irregularly shaped, (18–)21.5–94(–102) × (3.5–)4–5 μm, 1–6-septate, smooth, brown to dark brown. Conidiogenous cells terminal, monoblastic or polyblastic; loci distinct, up to 2.5 μm wide, thickened and darkened. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (16–)19–94(–105.5) × 2.5–3.5 μm, 2–7-septate, hyaline to subhyaline, smooth, apex subacute or acute, base truncate to short obconically truncate, up 2.5 μm wide, hila thickened and darkened.
Specimen examined.
Benin. Borgou: Parakou, c. 386 m a.s.l., 9°20'35"N, 2°36'37"E, on Vigna radiata, 14 Sep 2019, Y. Meswaet and R. Dramani, YMM289 (M-0312651 UNIPAR).
Hosts and distribution.
On Phaseolus lunatus from USA, Alabama, Tuskegee (type locality) (Braun and Crous 2005). This species is cited here for the first time for Benin. Thereby, it is cited for the first time for West Africa. Vigna radiata is a new host species.
Notes.
Thirteen Cercospora species have previously been recorded on species of Vigna and Phaseolus, namely C. albida Matta & Belliard, C. apii, C. canescens, C. canscorina, C. caracallae, C. kikuchii, C. longispora, C. olivascens, C. phaseoli-lunati, C. phaseolicola U.Braun & Mouch., C. phaseolina Speg., C. vignigena and C. zonata G. Winter (Farr and Rossman 2021). Among these, C. caracallae and C. phaseoli-lunati are morphologically rather similar to the present collection. C. caracallae, however, differs in causing distinct leaf spots and caespituli, dense fascicules composed of unbranched and wider conidiophores [5–6 μm versus (3.5–)4–5 μm in C. phaseoli-lunati] and wider conidia (3–5.5 μm versus 2.5–3.5 μm in C. phaseoli-lunati) with less septa (3–5 versus 2–7 septa) (Chupp 1954; Spegazzini 1910). Except for the presence of distinct leaf spots and sporulation, the morphology of the present collection from Benin fits well to the original description of C. phaseoli-lunati on Phaseolus lunatus from the USA provided by Braun and Crous (2005). Based on the present phylogenies, it is not possible to distinguish C. phaseoli-lunati from numerous other Cercospora spp.
Cercospora rhynchophora
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
B0B89FDF-0245-5ACA-B79E-D26E25E18386
839172
Figure 9.
Cercospora rhynchophora on Vigna unguiculata (YMM03B) A fascicle of conidiophores growing out from a developed stroma embedded in the mesophyll B conidiophore penetrating through a stomatal opening C solitary conidiophores arising from external hyphae D conidia. Scale bars: 20 μm (A, B); 15 μm (C, D).
Type.
Benin. Borgou: Parakou, c. 385 m a.s.l., 9°20'34"N, 2°36'39"E, on Vigna unguiculata (L.) Walp. (Fabaceae), 14 Sep 2019, Y. Meswaet and R. Dramani, YMM03B (Holotype: M-0312652; Isotype: UNIPAR). Ex holotype sequences.MW834447 (SSU), MW834431 (LSU), MW834443 (ITS), MW848619 (tef1).
Etymology.
The epithet rhynchophora refers to the beak- or hook-like tips of the conidiophores, a characteristic of this species.
Diagnosis.
Cercospora rhynchophora differs from other Cercospora spp. known on Vigna spp. by causing distinct leaf spots, often well-developed stromata and up to 4 times geniculate conidiophores with often polyblastic conidiogenous cells with irregular, often beak-shaped tips.
Description.
Leaf spots amphigenous, small to fairly large, subcircular to irregularly angular, (3–)4.5–12.5 mm diam. or confluent and larger, dark brown to reddish brown, mostly with an indefinite margin, or whitish grey to greyish brown, with a narrow to wide dark brown margin on the adaxial surface, occasionally confined by veins. Caespituli amphigenous, scattered to dense, dark brown. Mycelium mainly internal, but some external hyphae also present. External hyphae septate, brown, 2–3.5 μm wide, smooth. Stromata often well-developed, up to 50 μm diam., in substomatal chambers or in the mesophyll, brown to dark brown. Conidiophores in loose to moderately dense fascicles formed by 3–20 conidiophores, arising from internal hyphae or stromata breaking through the adaxial epidermis of the leaves, or penetrating through stomatal openings, or solitary, erect, straight to 1–4 times geniculate or subcylindrical, sometimes branched, mostly attenuated towards the tips that are often irregularly shaped or conical, (12.5–)26–160(–200) × (3.5–)4–5(–5.5) μm, 0–7(–9)-septate, brown to dark brown. Conidiogenous cells terminal or rarely intercalary, proliferating sympodially, mostly polyblastic, frequently distinctly subdenticulate, sometimes with bent tips looking like a beak or a hook; loci (1.5–)2–2.5(–3) μm wide, thickened and darkened. Conidia solitary, acicular to narrowly obclavate, straight to curved, (28–)40–265(–280) × (3–)3.5–4.5(–5) μm, 1–9-septate, hyaline, smooth, tip acute, base truncate to obconically truncate, sometimes long obconically truncate, 2–2.5(–3.5) μm wide, hila thickened and darkened.
Additional specimen examined.
Benin. Borgou: Parakou, c. 395 m a.s.l., 9°21'27"N, 2°36'44"E, on Vigna unguiculata, 17 Sep 2019, Y. Meswaet and R. Dramani, YMM03C (Paratypes: M-0312653; UNIPAR).
Herbarium specimens examined for comparison.
See Cercospora aff. canescens.
Host and distribution.
On Vigna unguiculata (Fabaceae) in Benin.
Notes.
The infection of leaves of Vigna unguiculata by Cercospora rhynchophora was severe and caused dark brown to reddish brown large patches (Fig. 2G). This infection was frequently associated with an infection by Pseudocercospora cruenta (Sacc.) Deighton. Seven species of Cercospora have previously been recorded on Vigna spp. (Table 4). Among these, C. apii, C. canescens, C. kikuchii and C. vignigena have to date been reported as agents of leaf spot diseases on V. unguiculata. Morphologically, C. rhynchophora differs from these species by a specific combination of characteristics (Table 4). C. apii has often small or no stromata, forms non-geniculate, densely fasciculate and longer conidiophores (20–300 μm) that are uniform in colour and width and carry monoblastic conidiogenous cells (Chupp 1954; Hsieh and Goh 1990) versus developed stromata, shorter conidiophores [(12.5–)26–160(–200) μm] that are irregularly shaped with polyblastic conidiogenous cells presenting beak-shaped tips in C. rhynchophora. Additionally, C. apii has pale to olivaceous brown conidiophores (Hsieh and Goh 1990) versus the dark brown ones of Cercospora rhynchophora.
Table 4.
Comparison of Cercospora rhynchophora (YMM03B) on Vigna unguiculata, Cercospora tentaculifera (YMM75) on Vigna unguiculata as well as on Phaseolus vulgaris and C. vignae-subterraneae (YMM293, see below) on Vigna subterranea with Cercospora species known from Vigna spp. based on literature a–f.
| Cercospora species | Leaf spots, colour, size | Stromata | Conidiophore size (in μm), branching, septa, colour | Conidium sizes (in μm), septa |
|---|---|---|---|---|
| Cercospora rhynchophora (YMM03B) | Dark brown to reddish brown, (3–)4.5–12.5 mm diam. | Well-developed | (12.5–)26–160(–200) × (3.5–)4–5(–5.5), branched, 0–7(–9)-septate, dark brown | (28–)40–265(–280) × (3–)3.5–4.5(–5), 1–9 distinct septa |
| C. tentaculifera (YMM75) | Almost absent | Small or lacking | (32.5–)40–400(–435) × (3–)3.5–4.5(–5), rarely branched, (2–)3–8(–10)-septate, brown to dark brown | (29–)38–188(–240) × (2.5–)3–3.5(–4.5), 1–9 septa |
| C. vignae-subterraneae (YMM293) | Brown to reddish brown, 2–6.5 mm diam. | Lacking or small | (28–)35.5–278(–340) × (3.5–)4–5, rarely branched, 2–6-septate, brown to dark brown | (19–)26.5–100(–110.5) × (2.5–)3–4, (2–)3–6 septa |
| C. apii ab | Present | Often small or lacking, occasionally developed, up to 50 μm diam. | 20–300 × 4–6.5, rarely branched, multi-septate, pale brown, uniform in colour and width | 25–315 × 3–6, (0–)3–25(–30) septab |
| C. canescens a | 3–15 mm | Often small | 20–200 × 3–6.5, rarely branched, multi-septate, pale to medium dark brown | 25–300 × 2.5–5.5, indistinctly multi-septate |
| C. canscorina c | Pale brown to brown, 3–6 mm | Developed | 29.8–85.0 × 3.4–4.2, 1–3-septate, or rarely non-septate, pale brown | 31.2–89.9 × 3–3.4, 3–9 septa |
| C. caracallae d | Present | Present | 40–80 × 5–6, unbranched, | 50–75 × 4, 3–5 septa |
| C. kikuchii a | Present | Small | 45–200 × 3–6.5, unbranched, multi-septate | 50–375 × 2.5–5, indistinctly multi-septate |
| C. longispora e | Present | Small | 5–30 × 1.5–3, unbranched, multi-septate, scars indistinct or lacking | 75–170 × 2–3.5, indistinctly multi-septate |
| C. vignigena f | Pale to medium brown, 8–20 mm | Small to well-developed (up to 60 μm diam.) | 40–130 × 5–7(–10), 0–3-septate | (35–)45–70(–150) × (2.5–)4–6(–10), (3–)4–7(–14) septa |
C. canescens causes different leaf spots and caespituli, develops small or no stromata and paler conidiophores that are uniform in colour with often monoblastic, mostly uniform conidiogenous cells (Chupp 1954; Hsieh and Goh 1990) versus irregularly shaped conidiophores with polyblastic, beaked conidiogenous cells in C. rhynchophora. The distinctness is also confirmed by molecular data. C. canscorina forms shorter conidiophores [29.8–85.0 µm versus (12.5–)26–160(–200) μm in C. rhynchophora] and conidia [31.2–89.9 × 3–3.4 μm versus (28–)40–265(–280) μm in C. rhynchophora] with pale brown and 1–3-septate conidiophores (Chiddarwar 1959). C. caracallae has densely fasciculate, unbranched, shorter and wider conidiophores [40–80 × 5–6 µm versus (12.5–)26–160(–200) μm in C. rhynchophora] and shorter conidia [50–75 μm versus (28–)40–265(–280) μm of C. rhynchophora] with 3–5 septa (Spegazzini 1910). C. kikuchii has unbranched conidiophores and longer conidia [50–375 μm versus (28–)40–265(–280) μm in C. rhynchophora] that are 0–22-septate (Hsieh and Goh 1990). C. longispora has shorter and narrower conidiophores [5–30 × 1.5–3 µm versus (12.5–)26–160(–200) × (3.5–)4–5(–5.5) μm in C. rhynchophora] and shorter conidia (75–170 µm versus (28–)40–265(–280) in C. rhynchophora] (Chupp 1954). C. vignigena produces pale brown and wider conidiophores [5–7(–10) µm versus (3.5–)4–5(–5.5) μm in C. rhynchophora] that are 0–3-septate and shorter as well as wider conidia [(35–)45–70(–150) × (2.5–)4–6(–10) μm versus (28–)40–265(–280) × (3–)3.5–4.5(–5) μm of C. rhynchophora] (Groenewald et al. 2013).
In the multi-gene (Fig. 1) and the ITS tree (see Suppl. material 3), C. rhynchophora forms part of a polytomy with a relatively large genetic distance (branch length) in relation to other sequences considered in the analysis. According to a MegaBLAST search using the tef1 sequence, the closest matches in NCBI’s GenBank nucleotide database were Cercospora beticola Sacc. on Tetragonia tetragonoides (Pall.) Kuntze (Aizoaceae) from Brazil (GenBank MN517124; Identities 272 / 279, i.e., 97%), Cercospora kikuchii on Platostoma palustre (Blume) A.J. Paton (Lamiaceae) from Taiwan (GenBank LC488192; Identities 272 / 279, i.e., 97%) and Cercospora sp. RF5 on Brunfelsia hopeana (Hook.) Benth. (Solanaceae) from Thailand (GenBank AB863025; Identities 272 / 279, i.e., 97%).
Cercospora
sp. YMM297B on Phaseolus lunatus L.
76794084-DC54-5B7D-8115-A2890DB9B329
Figure 10.
Cercospora sp. on Phaseolus lunatus (YMM297B) A fascicle of conidiophores emerging through a stomatal opening B solitary conidiophores C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Description.
Leaf spots almost lacking to well-developed, amphigenous, subcircular to irregularly angular, 2.5–8 mm diam., reddish brown, later dark brown by abundant caespituli, finally sometimes greyish brown to dark reddish brown, surrounded by dark margins, often with diffuse whitish centres. Caespituli amphigenous, greyish brown to dark brown. Mycelium mainly internal. External hyphae branched, 2–3(–4) μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking or small, up to 20 μm diam., immersed in the mesophyll or in substomatal cavities, subcircular to irregular, olivaceous brown to darker brown. Conidiophores in small and loose fascicles, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, sometimes solitarily arising through stomatal openings, erect, straight to sinuous, or somewhat geniculate, unbranched, (13–)17.5–195(–220) × (3.5–)4–5 μm, with 2–6(–8) septa each, occasionally slightly constricted and darker at the septa, brown to dark brown. Conidiogenous cells integrated, terminal, mainly monoblastic; loci 2–3.5 μm wide, thickened and darkened. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (27–)36–148(–164) × (2.5–)3–4(–4.5) μm, with 2–7(–9) somewhat indistinct septa each, hyaline to sub-hyaline, smooth, apex subacute or acute, base truncate to short obconically truncate, 2–3(–3.5) μm wide, hila thickened and darkened.
Specimen examined.
Benin. Borgou: Parakou, Tankaro, c. 360 m a.s.l., 9°23'01"N, 2°30'36"E, on Phaseolus lunatus, 20 Sep 2019, Y. Meswaet and R. Dramani, YMM297B (M-0312654; UNIPAR).
Notes.
The infection of leaves of Phaseolus lunatus by Cercospora sp. YMM297B was associated with the infection by Pseudocercospora griseola. Among the Cercospora spp. known on Phaseolus and Vigna, C. olivascens is morphologically close to Cercospora sp. YMM297B. C. olivascens, however, differs from Cercospora sp. YMM297B by hypophyllous caespituli, no external hyphae, conidiophores that are up to five times geniculate and paler (Saccardo 1878; Chupp 1954), as well as hyaline conidia. The present specimen from Benin presents amphigenous caespituli, external hyphae, less geniculate and brown to dark brown conidiophores and often sub-hyaline conidia. C. olivascens also differs from the present species by being originally described from Aristolochia clematitis (Aristolochiaceae). According to Chupp (1954), this species was wrongly reported on Phaseolus vulgaris by Saccardo (1886). This was confirmed by Crous and Braun (2003). In the ITS phylogeny (see Suppl. material 3), Cercospora sp. YMM297B forms part of a polytomy with a relatively large genetic distance (branch length) in relation to other sequences considered in the analysis. In the tef1 phylogeny (see Suppl. material 4), it is not possible to distinguish this collection from several other Cercospora spp. As the description and sequence data are obtained only from a single specimen, the data are not sufficient for a final conclusion and the description as a new species. A reliable species characterisation is not possible until more collections become available.
Cercospora tentaculifera
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
5CFA5669-325A-5B1F-83BE-0E825609FA0C
839173
Figure 11.
Cercospora tentaculifera on Vigna unguiculata (YMM75) A fascicle of conidiophores growing out from a small stroma immersed in the epidermis B external hyphae C solitary conidiophores D conidia. Scale bars: 20 μm (A); 12 μm (B); 15 μm (C, D).
Type.
Benin. Borgou: Parakou, c. 372 m a.s.l., 9°21'43"N, 2°36'04"E, on Vigna unguiculata (L.) Walp. (Fabaceae), 02 August 2017, Y. Meswaet, M. Piepenbring, N. S. Yorou and participants of the summer school 2017, YMM75 (Holotype: M-0312655; Isotype: UNIPAR). Ex holotype sequences.MW834448 (SSU), MW834440 (ITS), MW848614 (tef1).
Etymology.
The epithet tentaculifera refers to the ramified and flexible hyphae.
Diagnosis.
Cercospora tentaculifera differs from other Cercospora spp. on Vigna and Phaseolus in causing inconspicuous or no leaf spots, well-developed external hyphae, mainly adaxial caespituli and up to 435 μm long conidiophores that are constricted at the septa.
Description.
Leaf spots almost lacking or pale brown with reddish brown discolorations. Caespituli amphigenous, mostly epiphyllous, scattered, greyish brown to dark brown. Mycelium internal and external. External hyphae branched, 2–3.5(–4) μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking or formed by few substomatal swollen hyphal cells, immersed in the mesophyll or in substomatal cavities. Conidiophores in small, loose fascicles formed by up to approx. 8 conidiophores, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, solitary when arising from external hyphae, erect, straight, curved or slightly 1–2 times geniculate, often constricted at septa, rarely branched, (32.5–)40–400(–435) × (3–)3.5–4.5(–5) μm, (2–)3–8(–10)-septate, brown to dark brown. Conidiogenous cells terminal, rarely subterminal, mostly monoblastic or with few conidiogenous loci; loci mainly apical, sometimes located on the shoulders of geniculations, 2–2.5(–3.5) μm wide, thickened and darkened, refractive, often subcircular or rarely flattened. Conidia solitary, acicular to narrowly obclavate, straight to curved, (29–)38–188(–240) × (2.5–)3–3.5(–4.5) μm, 1–9-septate, hyaline, smooth, tip acute, base truncate to short obconically truncate, 2.5–3(–3.5) µm wide, hila thickened and darkened.
Additional specimen examined.
Benin. Borgou: Parakou, agricultural research site of the University of Parakou, c. 360 m a.s.l., 9°20'10"N, 2°38'53"E, on Phaseolus vulgaris, 20 Aug 2017, Y. Meswaet and A. Tabé, YMM130 (Paratypes: M-0312656; UNIPAR).
Herbarium specimens examined for comparison.
See Cercospora aff. canescens.
Hosts and distribution.
Known on Phaseolus vulgaris and Vigna unguiculata (Fabaceae) from Benin.
Notes.
Thirteen Cercospora species have previously been recorded on species of Vigna or Phaseolus (Tables 4, 5).
Table 5.
Comparison of Cercospora tentaculifera (YMM75) on Vigna unguiculata and Phaseolus vulgaris with Cercospora species known from Phaseolus spp. based on literature a–g.
| Cercospora species | Leaf spots, colour, size | Stromata | Conidiophore size (in μm), branching, septa, colour | Conidium sizes (in μm), septa |
|---|---|---|---|---|
| C. tentaculifera (YMM75) | Almost absent | Small or lacking | (32.5–)40–400(–435) × (3–)3.5–4.5(–5), rarely branched, (2–)3–8(–10)-septate, brown to dark brown | (29–)38–188(–240) × (2.5–)3–3.5(–4.5), 1–9 septa |
| C. albida a | Almost absent | Small or lacking | 10–60 × 3–6, branched, 1–2-septate | (30–)50–90(–125) × (1.5–)2–3.5(–4), 0–6 septa |
| C. canescens b | 3–15 mm | Often small | 20-200 × 3–6.5, rarely branched, multi-septate, pale to medium dark brown | 25–300 × 2.5–5.5, indistinctly multi-septate |
| C. caracallae c | Present | Present | 40–80 × 5–6, unbranched | 50–75 × 3–4, 3–5 septa |
| C. kikuchii b | Present | Small | 45–200 × 3–6.5, unbranched, multi-septate | 50–375 × 2.5–5, indistinctly multi-septate |
| C. olivascens d | Present | Small | 50–200 × 4–5.5, unbranched, multi-septate | 35–150 × 4–5.5, 3–9 septa |
| C. phaseoli-lunati e | Present | Present | 20–100 × 2.5–5(–6), usually pluri-septate | (20–)30–100 × 1–3, pluri-septate |
| C. phaseolicola f | Present | Absent | 300–600 × 4–7(–10), branched, pluri-septate | 50–200 × 3–5, pluri-septate |
| C. phaseolina g | Present | No information | 50–80 × 4–5, unbranched | 20–45 × 3–3.5, 1–3 septa |
| C. zonata d | Present | Lacking or slightly developed | 10–80 × 3–5, mostly 10–40, 0–2-septate, unbranched | 40–125 × 2.5–4.5, usually 3septa |
Among these, C. apii, C. canescens and C. phaseolicola have a morphology similar to the present collections, particularly by relatively long conidiophores (Tables 4, 5). C. apii, however, differs from the present species in causing distinct leaf spots (brown to fairly dark in colour with darker margin), the place of sporulation (caespituli more abundant on the abaxial surface of leaves versus on the adaxial surface of leaves in the case of C. tentaculifera), paler and shorter conidiophores [20–300 μm versus (32.5–)40–400(–435) μm in C. tentaculifera] that are occasionally arising from developed (up to 50 μm diam.) stromata and somewhat longer and wider conidia [25–315 × 3–6 μm versus (29–)38–188(–240) × (2.5–)3–3.5(–4) μm in C. tentaculifera] (Hsieh and Goh 1990). C. canescens differs from C. tentaculifera in causing different leaf spots and sporulation, producing dense fascicles, paler and shorter conidiophores [20–200 µm versus (32.5–)40–400(–435) µm in C. tentaculifera] and somewhat longer conidia [25–300 μm versus (29–)38–188(–240) μm in C. tentaculifera] (Hsieh and Goh 1990). C. phaseolicola differs from C. tentaculifera in causing zonate leaf spots and producing only internal hyphae, hardly geniculate, much longer and wider conidiophores [300–600 × 4–7 µm, occasionally up to 10 μm wide versus (32.5–)40–400(–435) × (3–)3.5–4.5(–5) µm] (Braun et al. 1999).
Based on the present phylogenies, it is not possible to distinguish this species from many other Cercospora spp. included in this study. Nevertheless, we propose this species as new to science based on a unique combination of morphological characteristics.
Cercospora vignae-subterraneae
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
ADABD0A0-02FA-540B-BBE9-8BD842C15413
839174
Figure 12.
Cercospora vignae-subterraneae on Vigna subterranea (YMM293) A fascicle of conidiophores B external hypha penetrating through a stomatal opening C solitary conidiophores arising from external hyphae D conidia. Scale bars: 20 μm (A, B); 12 μm (C); 10 μm (D).
Type.
Benin. Borgou: Parakou, c. 394 m a.s.l., 9°21'25"N, 2°36'45"E, on Vigna subterranea (L.) Verdc. (Fabaceae), 17 Sep 2019, Y. Meswaet and R. Dramani, YMM293 (Holotype: M-0312657; Isotype: UNIPAR). Ex holotype sequences.MW834446 (SSU), MW834438 (ITS), MW848618 (tef1).
Etymology.
The epithet vignae-subterraneae refers to the host species, Vigna subterranea.
Diagnosis.
Cercospora vignae-subterraneae differs from all other Cercospora spp. known on Vigna spp. in causing often necrotic leaf spots with a pale to white greyish centre, mostly hypophyllous caespituli, external hyphae, flat conidiogenous loci and shorter conidia [(19–)26.5–100(–110.5) µm].
Description.
Leaf spots amphigenous, circular or subcircular to irregularly angular, 2–6.5 mm diam., often limited by veins, brown to greyish brown, later necrotic with a pale to white greyish centre, surrounded by a darker margin, the outermost ring mostly darker than the inner margins. Caespituli amphigenous, but mostly hypophyllous, greyish brown to dark brown. Mycelium internal and external. External hyphae branched, 2–3(–3.5) μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking or small, immersed in the mesophyll or in substomatal cavities. Conidiophores in small to large, loose to dense fascicles or solitary, arising through stomatal openings or breaking through the epidermis, erect, subcylindrical, sinuous or somewhat geniculate, simple or rarely branched, (28–)35.5–278(–340) × (3.5–)4–5 μm, 2–6-septate, smooth, brown to dark brown with slightly paler tips. Conidiogenous cells terminal, usually monoblastic, rarely polyblastic; loci conspicuous, often flat, (1.5–)2–3 μm wide, darkened and thickened. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (19–)26.5–100(–110.5) × (2.5–)3–4 μm, (2–)3–6-septate, hyaline, smooth, apex subacute or acute, base truncate to short obconically truncate, (1.5–)2–2.5(–3) µm wide, hila thickened and darkened.
Additional specimen examined.
Benin. Alibori: Gogounou, c. 333 m a.s.l., 10°50'35"N, 2°49'42"E, on Vigna subterranea Verdc., 2 Sep 2017, Y. Meswaet and A. Tabé, YMM180 (Paratypes: M-0312658; UNIPAR).
Herbarium specimens examined for comparison.
See Cercospora aff. canescens.
Host and distribution.
On Vigna subterranea (Fabaceae) in Benin.
Notes.
Seven species of Cercospora have previously been recorded on Vigna spp. (Table 4) (Farr and Rossman 2021). However, no species of Cercospora is known to occur on Vigna subterranea (Farr and Rossman 2021), a plant species native to West Africa and cultivated mainly in the warm tropics of sub-Saharan Africa (Hepper 1963). Morphologically, C. vignae-subterraneae is distinct from all seven species of Cercospora mentioned above (Table 4). C. apii differs from C. vignae-subterraneae by paler conidiophores occasionally arising from a developed stroma of up to 50 μm diam. and above all, longer and wider conidia [25–300 × 3–6 μm versus (19–)26.5–100(–110.5) × (2.5–)3–4 μm in C. vignae-subterraneae] that are (0–)3–25(–30)-septate (Hsieh and Goh 1990; Crous and Braun 2003). C. canescens causes different leaf spots and caespituli, paler and shorter conidiophores [20–200 µm versus (28–)35.5–278(–340) μm in C. vignae-subterraneae] as well as longer conidia [25–300 μm versus (19–)26.5–100(–110.5) μm of C. vignae-subterraneae] (Hsieh and Goh 1990). C. canscorina forms well-developed stromata as well as paler and shorter conidiophores [29.8–85 µm versus (28–)35.5–278(–340) μm in C. vignae-subterraneae] with 1–3 septa (Chiddarwar 1959).
C. caracallae has densely fasciculate, unbranched and shorter conidiophores [40–80 µm versus (28–)35.5–278(–340) μm in C. vignae-subterraneae] and slightly shorter conidia [50–75 μm versus (19–)26.5–100(–110.5) μm in C. vignae-subterraneae] (Spegazzini 1910). C. kikuchii has unbranched and shorter conidiophores [45–200 × 3–6.5 µm versus (28–)35.5–278(–340) μm in C. vignae-subterraneae] and larger conidia [50–375 μm versus (19–)26.5–100(–110.5) μm in C. vignae-subterraneae] with up to 22 septa (Hsieh and Goh 1990). C. longispora has unbranched, shorter and narrower conidiophores [5–30 × 1.5–3 µm versus (28–)35.5–278(–340) × (3.5–)4–5 μm in C. vignae-subterraneae] with inconspicuous conidiogenous loci and somewhat longer conidia [75–170 × 2–3.5 µm versus (19–)26.5–100(–110.5) μm in C. vignae-subterraneae] (Chupp 1954). C. vignigena has paler, shorter and wider conidiophores [40–130 × 5–7(–10) µm versus (28–)35.5–278(–340) × (3.5–)4–5 μm in C. vignae-subterraneae] that are 0–3-septate, and wider conidia [(2.5–)4–6(–10) μm versus (2.5–)3–4 μm of C. vignae-subterraneae) (Mulder and Holliday 1975).
In the multi-gene (Fig. 1) and in the ITS phylogeny (see Suppl. material 3), C. vignae-subterraneae forms part of a polytomy with a relatively large genetic distance (branch length) in relation to other sequences considered in the analysis. In the tef1 phylogeny (see Suppl. material 4), it is not possible to distinguish C. vignae-subterraneae from other Cercospora spp. Based on the results of our comparative study, we propose C. vignae-subterraneae as a species new to science.
Cercospora zorniicola
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
D4471312-8E68-5DD6-A7D5-4994E8C8395D
839175
Figure 13.
Cercospora zorniicola on Zornia glochidiata (YMM299) A fascicle of conidiophores growing out from a small stroma B external hyphae with two conidiophores C solitary conidiophores arising from external hyphae D conidia. Scale bars: 15 μm (A); 12 μm (B); 10 μm (C, D).
Type.
Benin. Collines: Glazoué, c. 189 m a.s.l., 7°58’ 25"N, 2°14'24"E, on Zornia glochidiata DC. (Fabaceae), 22 Sep 2019, Y. Meswaet, A. Tabé and M. Piepenbring, YMM299 (Holotype: M-0312659; Isotypes: UNIPAR) . Ex holotype sequences.MW848616 (tef1).
Etymology.
The epithet zorniicola refers to the host genus Zornia and “-cola” (lat. colere = to dwell).
Diagnosis.
Cercospora zorniicola is characterised by external hyphae, unbranched conidiophores that are uniform in colour and width, with mostly monoblastic conidiogenous cells (Fig. 13).
Description.
Leaf spots almost lacking or brown to dark brown discolorations, amphigenous, 0.5–2 mm diam., often located along the main veins, surrounded by a yellow discoloration of undefined size and shape. Caespituli amphigenous, greyish brown to dark brown. Mycelium internal and external. External hyphae 2–3 μm wide, septate, branched, subhyaline to pale olivaceous, smooth. Stromata lacking or formed by few substomatal aggregated swollen hyphal cells, up to 22 μm wide, in substomatal chambers or embedded in the mesophyll, dark brown. Conidiophores in small, loose fascicles of up to approx. 14 conidiophores, arising from internal hyphae breaking through the adaxial epidermis of the leaves, or penetrating through stomatal openings, occasionally solitary arising from external hyphae, erect, straight, subcylindrical to geniculate, unbranched, (15–)24.5–134(–158) × 3.5–4.5 μm, 1–5(–6)-septate, brown to dark brown, often uniform in colour and width. Conidiogenous cells usually monoblastic, rarely polyblastic; loci 1.5–3 μm wide, thickened and darkened. Conidia solitary, acicular to narrowly obclavate, straight to curved, (15–)27.5–182.5(–200) × (2–)2.5–3.5(–4) μm, 1–8(–12)-septate, hyaline, tip acute, base truncate to short obconically truncate, 1.5–3 µm wide, hila thickened and darkened.
Additional specimens examined.
Benin. Borgou: Parakou, on the way to N’Dali, c. 367 m a.s.l., 9°27'53"N, 2°37'43"E, on Zornia glochidiata, 17 Sep 2019, Y. Meswaet and R. Dramani, YMM13 (Paratypes: M-0312660; UNIPAR). Benin. Borgou: Parakou, c. 391 m a.s.l., 9°22'56"N, 2°37'33"E, same host, 29 Aug 2019, Y. Meswaet and A. Tabé, YMM233 (M-0312661).
Notes.
The genus Zornia comprises 80 species mainly distributed in tropical regions of the world (Fortuna-Perez et al. 2013). No species of Cercospora are currently known on hosts belonging to Zornia (Farr and Rossman 2021). Pseudocercospora zorniae (J.M. Yen & Gilles) Deighton (≡ Cercospora zorniae J.M. Yen & Gilles) is the only known species of cercosporoid fungi infecting species of Zornia.
In the multi-gene phylogeny (Fig. 1), Cercospora zorniicola grouped closely, but with poor support, with isolates of Cercospora cf. citrullina (MUCC 576) on Citrullus lanatus (Thunb.) Matsum. & Nakai (Cucurbitaceae) and C. kikuchii on Glycine max, Phaseolus spp., Cyamopsis tetragonoloba (L.) Taub., Vigna and other Fabaceae hosts (Mulder and Holliday 1975; Groenewald et al. 2013). However, morphologically, C. zorniicola is clearly distinct from C. cf. citrullina by external hyphae, unbranched, darker and longer conidiophores [(15–)24.5–134(–158) μm] and somewhat longer conidia [(15–)27.5–182.5(–200) μm], while C. cf. citrullina has pale to pale brown and short conidiophores (50–86 μm) and shorter conidia (40–130 μm) (Groenewald et al. 2013). C. zorniicola differs from C. kikuchii in having external hyphae, darker and shorter conidiophores [(15–)24.5–134(–158) μm] and shorter conidia [(15–)27.5–182.5(–200) μm], while C. kikuchii has paler and longer conidiophores (45–200 μm) and above all, much longer conidia (50–375 µm) with numerous indistinct septa (Mulder and Holliday 1975; Hsieh and Goh 1990). In the phylogeny based on tef1 molecular sequence data, it is not possible to distinguish C. zorniicola from other Cercospora spp. (see Suppl. material 4).
Based on a MegaBLAST search in the NCBI GenBank nucleotide database using the tef1 sequence data of C. zorniicola, the closest matches were Cercospora aff. canescens on Dioscorea rotundata Poir. (Dioscoreaceae) from Ghana (GenBank JX143316; Identities 294 / 300, i.e., 98%), Cercospora cf. coreopsidis W.W. Ray on Coreopsis lanceolata L. (Asteraceae) form South Korea (GenBank JX143344; Identities 293 / 300, i.e., 97%) and Cercospora nicotianae on Nicotiana tabacum (Solanaceae) from China (GenBank MK881748; Identities 292 / 300, i.e., 97%). This species is proposed to be new to science based on a distinct combination of morphological characteristics and because no other species of Cercospora is currently known on a species of this host genus.
Nothopassalora personata
(Berk. & M.A. Curtis) U.Braun, C. Nakash., Videira & Crous, Studies in Mycology 87: 333. 2017.
2D60269B-BAD7-5AD9-89B5-6ABE855016BA
822766
Figure 14.
Leaf spot symptoms associated with cercosporoid fungi A, BNothopassalora personata on Arachis hypogaea (YMM49A) B close-up of lesions with caespituli CPassalora arachidicola on Arachis hypogaea (YMM49B) DPseudocercospora bradburyae on Centrosema pubescens (YMM275) EPseudocercospora cruenta on Phaseolus sp. (YMM288) F, GPseudocercospora griseola on Phaseolus lunatus (YMM297A) G close-up of lesions with sporulation HPseudocercospora sennicola on Senna occidentalis (YMM12) IPseudocercospora tabei on Vigna unguiculata (YMM220). Scale bars: 15 mm (A, D, E, F, I); 100 μm (B, G); 12 mm (D, H).
Figure 15.
Nothopassalora personata on Arachis hypogaea (YMM49A) A fascicle of conidiophores growing out from a developed stroma embedded in the epidermis B solitary conidiophores C conidia. Scale bars: 15 μm (A); 12 μm (B, C).
Basionym.
Cladosporium personatum Berk. & M.A. Curtis, Grevillea 3 (27): 106 (1875).
Type.
USA. South Carolina: Santee River, on Arachis hypogaea (Fabaceae), (no date), Ravenel 1612 (Holotype K n.v.; Isotype IMI 104552, n.v.; Epitype CBS H-22946, n.v.).
For more synonyms see Crous and Braun 2003; Videira et al. 2017 or MycoBank.
Description.
Leaf spots amphigenous, subcircular to irregularly angular, 2–8 mm diam., reddish brown, later dark brown by abundant caespituli, finally sometimes greyish brown to blackish brown, margin indefinite. Caespituli amphigenous, greyish brown to dark brown. Mycelium mainly internal. Stromata small to well-developed, up to 48 μm diam., immersed in the mesophyll or in substomatal chambers, subcircular to irregular, brown to dark brown. Conidiophores in moderately dense to dense fascicles, arising from stromata, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, or solitarily arising through stomatal openings, cylindrical, straight to sinuous or geniculate, conically truncate at the apex, unbranched, (12.5–)20–55.5(–58) × 5–7 μm, 1–3(–4)-septate, pale brown to brown, paler towards the apex. Conidiogenous loci 2.5 μm wide, thickened and darkened. Conidia solitary, cylindrical to long-obclavate with round apex, straight to curved, (14–)23–68(–80) × (5–)5.5–8(–9) μm, 2–6-septate, pale brown to olivaceous brown, base obconically truncate, 2–3 μm wide, hila thickened and darkened.
Specimens examined.
Benin. Donga: Taneka-Koko, c. 441 m a.s.l., 9°51'30"N, 1°29'34"E, on Arachis hypogaea, 29 Jul 2017, Y. Meswaet, M. Piepenbring N. S. Yorou and participants of the summer school 2017, YMM49A (M-0312662; UNIPAR). Benin. Borgou: Parakou, c. 354 m a.s.l., 9°20'02"N, 2°38'48"E, same host, 27 Aug 2019, Y. Meswaet and R. Dramani, YMM224A (M-0312663). Benin. Borgou: Parakou, Songhai (farm school), c. 333 m a.s.l., 9°24'42"N, 2°41'24"E, same host, 30 Aug 2019, Y. Meswaet and A. Tabé, YMM247 (M-0312664). Benin. Borgou: Commune of Nikki, Tontarou, c. 452 m a.s.l., 9°50'23"N, 3°14'59"E, same host, 19 Sep 2019, Y. Meswaet, A. Tabé and M. Piepenbring, YMM295 (M-0312665).
Herbarium specimens examined for comparison.
Nothopassalora personata. On Arachis sp.: Democratic Republic of the Congo (Zaire). Kindu, 28 Jan 1920, Shantz H. L., 628 (BPI 439440 as Cercospora personata Berk. & M.A. Curtis). On Arachis sp.: Guinea. 5 Sep 1964, Litzenberger S. C., 91 (BPI 439443 as C. personata). On A. hypogaea: Indonesia, Java, Tegal, 28 Jan 1920, Raciborski, s.n (BPI 407235 “type?” of Septogloeum arachidis Racib.).
Hosts and distribution.
On Arachis glabrata Benth., A. hypogaea (Fabaceae), known in tropical regions where the host is cultivated, including Afghanistan, Angola, Argentina, Australia, Azerbaijan, Bangladesh, Barbados, Benin, Bermuda, Bhutan, Bolivia, Brazil, Brunei, Burkina Faso, Cambodia, Canada, China, Cambodia, Cameroon, Chad, Colombia, Congo, Cuba, Dominican Republic, Egypt, EI Salvador, Ethiopia, Fiji, French Polynesia, Gabon, Gambia, Georgia, Ghana, Greece, Guam, Guatemala, Guinea, Guyana, Haiti, Honduras, Hong Kong, India, Indonesia, Iran, Iraq, Israel, Ivory Coast, Jamaica, Jordan, Kenya, Korea, Laos, Lesser Antilles, Liberia, Libya, Madagascar, Malawi, Malaysia, Mali, Mauritius, Mexico, Morocco, Mozambique, Myanmar, Nepal, New Caledonia, Nicaragua, Niger, Nigeria, Pakistan, Panama, Papua New Guinea, Paraguay, Peru, Philippines, Puerto Rico, Russia, Saint Vincent and the Grenadines, Senegal, Sierra Leone, Singapore, Solomon Islands, Somalia, South Africa, Spain, Sri Lanka, Sudan, Suriname, Taiwan, Tanzania, Thailand, Togo, Tonga, Trinidad and Tobago, Turkmenistan, Turkey, Uganda, Uruguay, USA, Uzbekistan, Venezuela, Vietnam, Zambia, Zimbabwe (Yen and Lim 1980; Hsieh and Goh 1990; Shin and Kim 2001; Crous and Braun 2003; Farr and Rossman 2021).
Notes.
Nothopassalora personata and Passalora arachidicola (Hori) U.Braun are the agents of the two major foliar diseases of Arachis hypogaea worldwide (Jenkins 1938; Kokalis-Burelle et al. 1997; Videira et al. 2017). During the collecting activities in Benin, we observed that both, N. personata and P. arachidicola, are present wherever A. hypogaea is grown and mixed infections are common. In addition, N. personata is occasionally associated with Puccinia sp. N. personata often predominates and is more destructive than P. arachidicola. N. personata differs from P. arachidicola, in forming wider conidiophores (5–7 μm) as well as cylindrical and wider conidia [(5–)5.5–8(–9) µm], while P. arachidicola forms narrower conidiophores [(3.5–)4–5 µm)] and conidia (3.5–4.5 μm). N. personata and P. arachidicola on A. hypogaea were previously reported from Benin (Crous and Braun 2003), but this is the first report of these pathogens in Benin including details of the two species.
Passalora arachidicola
(Hori) U.Braun, New Zealand J. Bot. 37: 303. 1999.
9B59EDBB-2919-5E66-8725-548783FF14B0
459582
Figure 16.
Passalora arachidicola on Arachis hypogaea (YMM49B) A fascicle of conidiophores growing out from a small stroma embedded in the epidermis B solitary conidiophores C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Basionym.
Cercospora arachidicola Hori, Rep. (Annual) Nishigahara Agric. Exp. Sta. Tokyo: 26. 1917.
Type.
Japan. Tokyo, Experiment Station, on Arachis hypogaea (Fabaceae), (no date), S. Hori. s.n. (Holotype HIRO, n.v.).
For more synonyms see Crous and Braun (2003) or MycoBank.
Description.
Leaf spots amphigenous, subcircular to angular-irregular, 2.5–9.5 mm diam., greyish brown to medium dark brown, occasionally limited by veins, margin indefinite. Caespituli epiphyllous, whitish brown to greyish brown. Mycelium mainly internal. Internal hyphae pale brown, smooth, 1.5–3 μm wide. Stromata small, up to approx. 32 μm diam., embedded in the mesophyll or in substomatal chambers, subcircular to irregular, brown to dark brown. Conidiophores in small, loose to moderately dense fascicles, arising from internal hyphae or stromata, or solitary, arising through stomatal openings, erect, straight to sinuous or geniculate, simple, (11.5–)14–42.5(–53) × (3.5–)4–5 μm, 0–5-septate, smooth, olivaceous brown to slightly dark brown, paler towards the tips. Conidiogenous loci 2–2.5(–3) μm wide, thickened and darkened. Conidia solitary, narrowly obclavate to subacicular, straight to slightly curved, (16–)23–76.5(–88) × 3.5–4.5 μm, 2–5-septate, olivaceous brown, apex subacute or acute, base truncate to short obconically truncate, 2–2.5(–3.5) μm wide, hila thickened and darkened.
Specimens examined.
Benin. Donga: Taneka-Koko, c. 441 m a.s.l., 9°51'30"N, 1°29'34"E, on Arachis hypogaea, 29 Jul 2017, Y. Meswaet, M. Piepenbring, N. S. Yorou and participants of the summer school 2017, YMM49B (M-0312666; UNIPAR). Benin. Borgou: Parakou, c. 354 m a.s.l., 9°20'02"N, 2°38'48"E, same host, 27 Aug 2019, Y. Meswaet and R. Dramani, YMM224B (M-0312667).
Herbarium specimens examined for comparison.
Passalora arachidicola. On Arachis sp.: Guinea. Labe, 29 Jul 1964, Litzenberger S. C. 55 (BPI 432987 as Cercospora arachidicola). On Arachis sp.: Guinea. Dubreka, 25 Jul 1964, Litzenberger S. C. 39 (BPI 432989 as C. arachidicola). On Arachis sp.: Guinea. Beyla, 2 Aug 1964, Litzenberger S. C. 47 (BPI 432990A as C. arachidicola). On Arachis sp.: Guinea. Kissidougou, 4 Aug 1964, Litzenberger S. C. 28 (BPI 432991 as C. arachidicola). On Arachis sp.: Guinea. Dabola, 4 Aug 1964, Litzenberger S. C. 26 (BPI 432992 as C. arachidicola).
Host and distribution.
On Arachis hypogaea (Fabaceae) known worldwide where the host is cultivated, including Afghanistan, Angola, Argentina, Australia, Bangladesh, Benin, Bolivia, Brazil, Brunei, Burkina Faso, China, Cuba, Cambodia, Cameroon, Colombia, Comoros, Democratic Republic Congo, Cuba, Dominican Republic, El Salvador, Fiji, Gabon, Gambia, Ghana, Guatemala, Guinea, Guyana, Hong Kong, India, Indonesia, Ivory Coast, Jamaica, Japan, Kenya, Korea, Laos, Lebanon, Libya, Madagascar, Malawi, Malaysia, Mali, Mauritius, Mexico, Mozambique, Myanmar, Nepal, New Caledonia, Nicaragua, Niger, Nigeria, Pakistan, Panama, Papua New Guinea, Philippines, Puerto Rico, Malaysia, Senegal, Sierra Leone, Solomon Islands, Somalia, South Africa, Sudan, Suriname, Taiwan, Tanzania, Thailand, Togo, Uganda, USA, Uruguay, Venezuela, Vietnam, Zambia, Zimbabwe (Chupp 1954; Hsieh and Goh 1990; Shin and Kim 2001; Crous and Braun 2003; Farr and Rossman 2021).
Notes.
Passalora arachidicola was placed into the genus Passalora by Braun (1999) based on morphological characteristics that are confirmed in the context of the present study. Crous et al. (2009b, 2009c, 2013a) showed that the genus Passalora is paraphyletic or polyphyletic. Therefore, the present species most probably does not belong to Passalora. However, we refrain from drawing taxonomic conclusions here because a revision of the genus Passalora is beyond the scope of the present study.
Pseudocercospora bradburyae
(E. Young) Deighton, Mycological Papers 140: 140. 1976
50B23A11-1F0F-5D80-8BF1-D28A2EAEE146
321522
Figure 17.
Pseudocercospora bradburyae on Centrosema pubescens (YMM275) A fascicle of conidiophores B solitary conidiophores arising from external hyphae C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Basionym.
Cercospora bradburyae E. Young, Mycologia 8 (1): 46 (1916).
Type.
Puerto Rico. Rosario, on Centrosema pubescens (as Bradburya pubescens (Benth.) Kuntze (Fabaceae), 15 Feb 1913, F. L. Stevens 446 (Holotype: ILL!).
For more synonyms see Crous and Braun 2003 or MycoBank.
Description.
Leaf spots amphigenous, subcircular to irregularly angular, (2.5–)4–8.5 mm diam., limited by veins, reddish brown to brown, with indefinite margins. Caespituli mainly epiphyllous, olivaceous brown to slightly dark brown. Mycelium internal and external. External hyphae branched, 2.5–3.5 μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking or small, about 10–18 μm diam., immersed in the mesophyll or in substomatal chambers. Conidiophores often in small, loose to slightly dense fascicles of up to approx. 10 conidiophores, arising from stromata or breaking through the adaxial epidermis of the leaves, occasionally solitary arising from external hyphae, straight to sinuous or somewhat geniculate, rarely branched, (11–)13–44(–48.5) × (3.5–)4–5 μm, 0–3(–4)-septate, smooth, olivaceous brown to brown, paler towards the tips. Conidiogenous cells terminal, 10–15 μm long; loci inconspicuous to distinctly denticle-like, not thickened and not darkened, 1.5–3 μm wide. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (30–)38–110(–130) × (2.5–)3–4(–4.5) μm, 3–9-septate, olivaceous brown, smooth, apex subacute to rounded and slightly narrower, base truncate to obconically truncate, 1.5–3 μm wide, hila not thickened and not darkened, occasionally somewhat refractive.
Specimens examined.
Benin. Borgou: N’Dali, c. 380 m a.s.l., 9°52'33"N, 2°41'20"E, on Centrosema pubescens, 31 Aug 2019, Y. Meswaet and A. Tabé, YMM275 (M-0312668; UNIPAR). Same locality and host, 1 Sep 2019, Y. Meswaet and A. Tabé, YMM275B (M-0312669).
Herbarium specimens examined for comparison.
Pseudocercospora bradburyae. On Centrosema pubescens (as Bradburya pubescens): Puerto Rico. Rosario, 15 Feb 1913, Stevens F. L. 446 (ILL14818 Holotype of Cercospora bradburyae). Puerto Rico. Mayagüez, 31 Oct 1913, Stevens F. L. 3930 (ILL10600 Paratype). Puerto Rico. San Germán, 12 Dec 1913, Stevens F. L. 5833 (ILL10606 Paratype). Puerto Rico. Dos Bocas, below Utuado, 30 Dec 1913, Stevens F. L. 6558(ILL10603 Paratype). Puerto Rico. Hormigueros, 14 Jan 1914, Stevens F. L. 225a (ILL10609 Paratype). Guinea. Kindia, May 1963, Kranz J, 2795 (BPI 1112168).
Host and distribution.
On Centrosema acutifolium Benth., C. arenarium Benth., C. brasilianum (L.) Benth., C. macrocarpum Benth., C. plumieri Benth., C. pubescens, C. virginianum (L.) Benth, Centrosema spp. (Fabaceae) from Australia, Barbados, Bolivia, Brazil, Brunei, Cambodia, China, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Fiji, Hong Kong, Ghana, Guinea, Indonesia, Jamaica, Malaysia, Mexico, Micronesia, Mona Island, New Caledonia, Nigeria, Niue, Palau, Papua New Guinea, Peru, Philippines, Puerto Rico, Solomon Islands, St. Thomas, South Africa, Taiwan, Thailand, Togo, Tonga, Trinidad and Tobago, Vanuatu, Venezuela, Virgin Islands (Chupp 1954; Ellis 1976; Hsieh and Goh 1990; Crous and Braun 2003; Farr and Rossman 2021). This species is reported here for the first time for Benin.
Notes.
Three species of Pseudocercospora, namely Ps. bradburyae, Ps. centrosematicola (J.M. Yen & Lim) J.M. Yen and Ps. clitoriae (G.F. Atk.) Deighton are known on Centrosema spp. (Chupp 1954; Farr and Rossman 2021). The present specimens from Benin differ from Ps. clitoriae by having often small fascicles formed by up to approx. 10 conidiophores and longer conidiophores [(11–)13–44(–48) µm] and wider conidia [(2.5–)3–4(–4.5) µm], while Ps. clitoriae has large, dense fascicles formed by 40 or more conidiophores, shorter conidiophores [8–15(–22) µm] and narrower conidia (2.5–3 µm) (Chupp 1954; Deighton 1976). Based on the descriptions made by Chupp (1954), Hsieh and Goh (1990), Young (1916) and the re-examination of the type specimen of Ps. bradburyae, the present specimen from Benin agrees well with Ps. bradburyae. In the tef1 phylogeny (see Suppl. material 4), Ps. bradburyae grouped with low support with isolates of Ps. humuli on Humulus lupulus (Cannabaceae) from Japan, Ps. cercidicola on Cercis chinensis (Fabaceae) from Japan and Ps. abelmoschi on Hibiscus syriacus (Malvaceae) from South Korea.
Pseudocercospora cruenta
(Sacc.) Deighton, Mycol. Pap. 140: 142. 1976
8475F659-D93F-5BF1-98B0-CF500449769D
321556
Figure 18.
Pseudocercospora cruenta on Phaseolus sp. (YMM288) A fascicle of conidiophores protruding from a stomatal opening B solitary conidiophores C conidia. Scale bars: 15 μm (A, C); 10 μm (B).
Basionym.
Cercospora cruenta Sacc., Michelia 2:149 (1880).
Type.
USA. South Carolina: (no further information on the locality), on Phaseolus sp. (Fabaceae), (no date), Ravenel 2156 (Holotype: PAD, n.v.).
For more synonyms see Crous and Braun (2003) or MycoBank.
Description.
Leaf spots amphigenous, subcircular to irregularly angular, (2.5–)4–8.5 mm diam., limited by veins, reddish brown to dark brown, with an indefinite margin. Caespituli amphigenous, denser, darker olivaceous to almost sooty on the abaxial surface of the leaves than on the adaxial side. Mycelium internal and external. External hyphae branched, 2.5–3.5 μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking or small, 8–14 μm diam., immersed in the mesophyll or in substomatal cavities, subcircular to irregular, brown to dark brown. Conidiophores in small, loose, moderately large and dense fascicles formed by up to approx. 10 conidiophores, arising from stromata, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, sometimes solitary, arising from external hyphae, straight to sinuous or somewhat geniculate, rarely branched, (12–)15.5–54(–58.5) × (3.5–)4–5 μm [in YMM125 up to 120 µm long], 1–3-septate, smooth, olivaceous brown to brown, paler towards the tips. Conidiogenous cells terminal or subterminal, a conidiophore can be reduced to a single conidiogenous cell; loci 2–2.5 μm wide, not thickened and not darkened. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (30.5–)42–132(–154) × (3–)3.5–4.5(–5) μm, 2–10-septate, olivaceous brown, smooth, apex subacute to rounded and slightly narrower than the rest of the conidiophore, up to 2.5 μm wide, base truncate to obconically truncate, 2–2.5(–3) μm wide, hila not thickened and not darkened.
Specimens examined.
Benin. Borgou: Parakou, c. 353 m a.s.l., 9°20'02"N, 2°38'48"E, on Phaseolus sp., 12 Sep 2019, Y. Meswaet and A. Tabé, YMM288 (M-0312670, UNIPAR). Benin. Atlantique: Commune of Allada, Sékou, c. 84 m a.s.l., 6°38'18"N, 2°13'09"E, on Vigna unguiculata, 15 August 2017, Y. Meswaet and A. Tabé, YMM125 (M-0312671; UNIPAR). Benin. Borgou: Parakou, c. 385 m a.s.l., 9°20'34"N, 2°36'39"E, same host, 14 Sep 2019, Y. Meswaet and R. Dramani, YMM03A (M-0312672). Borgou: Parakou, c. 394 m a.s.l., 9°21'25"N, 2°36'45"E, same host, 17 Sep 2019, Y. Meswaet and R. Dramani, YMM294B (M-0312673). Benin. Borgou: Parakou, c. 363 m a.s.l., 9°20'29"N, 2°37'28"E, same host, 21 Sep 2019, Y. Meswaet and A. Tabé, YMM04 (M-0312674).
Herbarium specimens examined for comparison.
Pseudocercospora cruenta. On Vigna unguiculata: USA. Mississippi: Starkville, Sep 1888, Tracy S. M. s.n. (BPI 435817 Paratype of Cercospora dolichi Ellis & Everh.); On Phaseolus sp.: USA. South Carolina: Aiken, no date, Ravenel H. W. s.n (BPI 439619 Paratype of C. phaseolorum Cooke). Pseudocercospora stizolobii (Syd. & P. Syd.) Deighton. On Mucuna sp.: Philippines. Los Baños, 6 Apr 1913, Raimundo M. B. 892 (BPI 441666 Holotype of C. stizolobii Syd. & P. Syd.).
Hosts and distribution.
On Calopogonium sp., Canavalia ensiformis (L.) DC., C. gladiata (Jacq.) DC., C. maritima Thouars, Canavalia sp., Cassia lathyroides L., Cicer arietinum L., Clitoria ternatea L., Dolichos biflorus L., D. lablab L., Dolichos sp., Glycine max, Glycine sp., Lablab niger Medik., L. purpureus (L.) Sweet, Mucuna capitata Wight & Arn., M. deeringiana (Bort) Merr., Phaseolus aconitifolius Jacq., P. adenanthus G. Mey., P. aureus Roxb., P. calcaratus Roxb., P. coccineus L., P. lathyroides L., P. lunatus, P. radiatus L., P. sublobatus Roxb., P. vulgaris, Psophocarpus tetragonolobus (L.) DC., Pueraria sp., Strophostyles helvola (L.) Elliott, Vicia faba L., Vigna antillana (Urb.) Fawc. & Rendle, V. catjang (Burm.f.) Walp., V. cylindrica (L.) Skeels, V. luteola (Jacq.) Benth., V. marina (Burm.) Merr., V. mungo (L.) Hepper, V. repens (L.) Kuntze, V. sesquipedalis (L.) Fruwirth, V. sinensis (L.) Savi ex Hausskn., V. unguiculata (L.) Walp., and further species in other genera of Fabaceae. It is widespread in warmer regions, including Afghanistan, Angola, Argentina, Australia, Azerbaijan, Bangladesh, Barbados, Bolivia, Brazil, Brunei, Cambodia, Canada, China, Colombia, Cuba, Dominican Republic, Egypt, EI Salvador, Ethiopia, Fiji, Ghana, Grenada, Guatemala, Guyana, Haiti, Honduras, Hong Kong, India, Indonesia, Iran, Iraq, Italy, Jamaica, Japan, Korea, Liberia, Malawi, Malaysia, Mauritius, Mexico, Mozambique, Myanmar, Nepal, New Caledonia, Niger, Nigeria, Pakistan, Panama, Papua New Guinea, Peru, Philippines, Puerto Rico, Russia, Rwanda, Saint Lucia, Saint Vincent and the Grenadines, Samoa, Saudi Arabia, Senegal, Sierra Leone, Singapore, Solomon Islands, Somalia, South Africa, Sri Lanka, Sudan, Suriname, Taiwan, Tanzania, Thailand, Togo, Tonga, Trinidad and Tobago, Uganda, USA, Venezuela, Virgin Islands, Zambia, Zimbabwe. (Saccardo 1886; Mulder and Holliday 1975; Ellis 1976; Yen and Lim 1980; Shin and Kim 2001; Crous and Braun 2003; Farr and Rossman 2021).
Notes.
Except for the presence of external hyphae and mostly slightly shorter conidiophores, the present specimen from Benin is morphologically identical to Ps. cruenta as known by literature (Chupp 1954; Deighton 1976). This identification is confirmed by results obtained by phylogenetic analyses based on tef1 sequence data (see Suppl. material 4). Ps. cruenta is a well-known pathogen causing leaf spot diseases on species of Vigna and allied genera. It can cause serious yield losses of up to 40% in cowpea (Sivanesan 1990). Ps. cruenta is cited here for the first time for Benin.
Pseudocercospora griseola
(Sacc.) Crous & U.Braun, Studies in Mycology 55: 169. 2006
4371C440-2750-5821-97CB-CCA31604E323
500855
Figure 19.
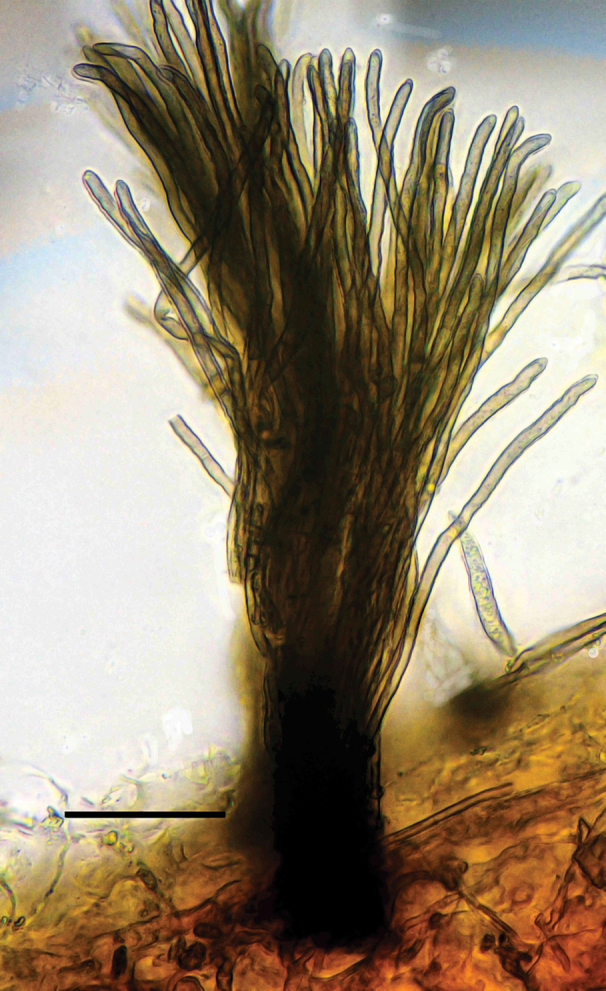
Synnematous fascicle of conidiophores of Pseudocercospora griseola on Phaseolus lunatus (YMM297A). Scale bar: 50 μm.
Basionym.
Isariopsis griseola Sacc., Michelia 1: 273. 1878.
For synonyms see Crous and Braun (2003), Crous et al. (2006) or MycoBank.
Type.
Italy. Selva, on Phaseolus vulgaris L. (Fabaceae), Aug 1877, Saccardo, Mycotheca Veneta 1247 (Lectotype: HAL, designated by Videira et al. 2017: 401, MBT378593, n.v.; Epitype: CBS H-19683, designated by Videira et al. 2017: 401, MBT378594, n.v.).
For illustrations see: Saccardo (1881), Fragoso (1927), Deighton (1990), Shin and Kim (2001) or Crous et al. (2006).
Description.
Leaf spots amphigenous, subcircular to irregularly angular, 2.5–7(–9.5) mm diam., reddish brown to dark brown or sometimes greyish brown to dark reddish brown, surrounded by a narrow darker margin. Caespituli amphigenous, mainly hypophyllous, reddish brown to olivaceous brown. Mycelium internal and external. External hyphae branched, 2.5–3 μm wide, septate, olivaceous brown to brown, smooth. Conidiophores in dense synnematous fascicles, synnemata up to 250 µm high, 20–40(–65) µm wide, emerging through stomatal openings or erumpent, or conidiophores solitary, arising from external hyphae, straight to sinuous or somewhat geniculate, 3–5(–6.5) μm wide, 1–6-septate, smooth, olivaceous brown to brown. Conidiogenous loci not thickened and not darkened, rather inconspicuous. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (22–)30–78(–83) × (4.5–)5–7 μm, 2–6-septate, olivaceous brown, smooth, apex subacute to rounded, base truncate to obconically truncate, (2.5–)3–4(–4.5) µm wide, hila not thickened and not darkened.
Specimen examined.
Benin. Borgou: Parakou, Tankaro, c. 360 m a.s.l., 9°23'01"N, 2°30'36"E, on Phaseolus lunatus, 20 Sep 2019, Y. Meswaet and R. Dramani, YMM297A (M-0312675; UNIPAR).
Herbarium specimens examined for comparison.
Pseudocercospora griseola. On Phaseolus sp.: USA. Pennsylvania: West Chester, Gardens, Sep 1880, W. T. Harris 1363 (NY 00937289 Holotype of Graphium laxum). On Phaseolus sp.: USA. Pennsylvania: West Chester, Gardens, Sep 1880, W. T. Harris s.n (BPI 448758 Paratype of G. laxum). On Phaseolus sp.: USA. New Jersey: Newfield, 27 Sep 1894, Ellis, s.n (BPI 435104 Paratype of Cercospora columnaris Ellis & Everh.). On P. vulgaris: Italy. Venetia, Selva, Aug 1877, Sacc. Mycoth. Ven. s.n (BPI 449390, isolectotype of Isariopsis griseola).
Hosts and distribution.
On Lablab purpureus (L.) Sweet (as Lablab niger Medik.), Lathyrus odoratus L., Macroptilium atropurpureum (DC.) Urb., Phaseolus acutifolius A. Gray, P. coccineus L., P. lunatus, P. vulgaris, Vigna angularis (Willd.) Ohwi & H. Ohashi, V. mungo, V. radiata, V. umbellata (Thunb.) Ohwi & H. Ohashi (as P. pubescens Blume), V. unguiculata (L.) Walp. (Fabaceae) from worldwide, including Angola, Argentina, Armenia, Australia, Austria, Bhutan, Brazil, Bulgaria, Burundi, Cameroon, Canada, China, Colombia, Costa Rica, Croatia, Cuba, Democratic Republic Congo, Dominican Republic, Ecuador, El Salvador, Ethiopia, Fiji, Georgia, Germany, Ghana, Great Britain, Greece, Guatemala, Haiti, Hungary, Jamaica, Japan, India, Indonesia, Iran, Ireland, Israel, Italy, Ivory Coast, Jamaica, Japan, Kenya, Korea, Laos, Latvia, Malawi, Madagascar, Malaysia, Mauritius, Mexico, Mozambique, Nepal, Netherlands, Netherlands Antilles, New Caledonia, New Zealand, Nicaragua, Nigeria, Norfolk Island, Panama, Papua New Guinea, Paraguay, Peru, Philippines, Poland, Portugal, Puerto Rico, Reunion (France), Romania, Russia, Rwanda, Saint Helena (British), Senegal, Sierra Leone, Singapore, Slovenia, Solomon Islands, Somalia, South Africa, Spain, Sudan, Suriname, Swaziland, Switzerland, Taiwan, Tanzania, Thailand, Trinidad and Tobago, Turkey, Uganda, Ukraine, U.S.A., Vanuatu, Venezuela, Virgin Islands, Zambia, Zimbabwe (Crous and Braun 2003; Crous et al. 2006; Farr and Rossman 2021). Ps. griseola is reported here for the first time for Benin.
Notes.
Four species of Pseudocercospora, namely Ps. cruenta, Ps. glycines (Cooke) Deighton, Ps. griseola and Ps. stizolobii are known agents of leaf spot diseases on Phaseolus spp. (Farr and Rossman 2021). The present Pseudocercospora sp. is phylogenetically (Fig. 1) and morphologically well distinguished from Ps. cruenta, Ps. glycines and Ps. stizolobii (Crous et al. 2006) by forming synnematous fascicles, longer and broader conidiophores and broader conidia. The morphology of this collection from Benin on P. lunatus fits well with the description of Ps. griseola.
Angular leaf spot (ALS) caused by Ps. griseola is a serious disease of common bean (P. vulgaris) all around the world (Ddamulira et al. 2014). It is reported for about 80 countries, where it can cause 45% to 80% losses of yield under conditions favourable for the fungus (Guzmán et al. 1999). The disease is also a major problem for bean production (50–60% of yield losses) in Africa, mainly in the Great Lakes Regions (Kenya, Uganda, Tanzania and Rwanda) where bean growing is popular (Golato and Meossi 1972; Wortmann et al. 1998; Aggarwal et al. 2004). According to Guzmán et al. (1995) and Crous et al. (2006), the species includes two major intraspecific groups, Ps. griseola f. griseola (Andean) and Ps. griseola f. mesoamericana (Middle-American) (Crous et al. 2006). Based on ITS sequence data (see Suppl. material 3), the present isolate from Benin clusters with Ps. griseola f. mesoamericana.
Pseudocercospora sennicola
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
1DCC7938-F46A-5AB1-95DF-550CBDB7C9B7
839176
Figure 20.
Pseudocercospora sennicola on Senna occidentalis (YMM12) A fascicle of conidiophores growing out of a small stroma embedded in the epidermis B solitary conidiophores arising from external hyphae C conidia. Scale bars: 15 μm (A); 10 μm (B, C).
Type.
Benin. Atlantique: Cotonou, University of Abomey-Calavi, c. 9 m a.s.l., 6°24'45"N, 2°20'41"E on Senna occidentalis (L.) Link (Fabaceae), 23 Sep 2019, Y. Meswaet and A. Tabé, YMM12 (Holotype: M-0312676; Isotype: UNIPAR). Ex holotype sequences.MW834444 (SSU), MW834432 (LSU), MW850550 (ITS).
Etymology.
The epithet sennicola refers to the host genus Senna and -cola (lat. colere = to dwell).
Diagnosis.
Pseudocercospora sennicola differs from other Pseudocercospora spp. known on Senna spp. by causing often inconspicuous spots and the combination of branched and relatively long conidiophores [16.5–)20.5–92(–98) µm] and relatively short and wide conidia [(16–)22–54.5(–65) × 3–4.5(–5) μm] that are often constricted at the septa (Table 6).
Table 6.
Comparison of Pseudocercospora sennicola on Senna occidentalis (YMM12) with Pseudocercospora species known from Senna spp. based on literature a–g.
| Cercospora species | Leaf spots, colour, size | Stromata | Conidiophore size (in μm), branching, septa | Conidium sizes (in μm), septa |
|---|---|---|---|---|
| Pseudocercospora sennicola (YMM12) | Often lacking or indistinct | Lacking to slightly-developed | (16.5–)20.5–92(–98) × (3–)3.5–4.5, branched, 2–6(–8)- septate | (16–)22–54.5(–65) × 3–4.5(–5) μm, 2–6 septa, slightly constricted at the septa |
| Ps. angustata ab | Brownish to dingy grey, 0.5–3 mm. | Small | 10–50 × 2–3.5, unbranched, rarely septate | 15–75 × 2–4 µm, 3–7 septa |
| Ps. cassiae-alatae c | Present | Small | 3–45 × 2.5–3.5, 0–6-septate | 15–90 × 1.5–2 µm, 1–10 septa |
| Ps. cassiae-fistulae d | Greyish brown to dark, 0.5–2 mm. | Well-developed | 10–30 × 2.5–5, unbranched, 0–2-septate | 25–65 × 3–4 µm, 2–8 distinct septa |
| Ps. cassiae-occidentalis c | Indistinct | Absent | 60–130 × 4–5, unbranched, 2–6-septate | 62–100 × 3.5–4.8, 3–6 septa |
| Ps. cassiae-siameae be | Present | Present | 15.3–27.2 × 3.4–4.2, 0–1-septate | 28.9–93.5 × 3.4–4.2, 2–8 septa |
| Ps. nigricans bdf | Yellowish discoloration to greyish brown, 2–3 mm wide | Small | 15–125 × 3–5, branched, 1–3-septate | 20–80 × 3–5, 1–10 septa |
| Ps. sennae-multijugae g | Grey brown, 2–18 mm in diam. | Well-developed (5–67 µm diam.) | 11–81 × 3–4, unbranched, 0–2-septate | 75–170 × 2–3.5, 2–7 septa |
| Ps. singaporensis c | Yellowish to brownish grey, 0.5–4 mm in diam. | Absent | 31–77 × 4.5–5.5, branched, 0–2(–4)-septate. | 30–67 × 3.5, 3 (rarely 1 or 4) septa |
| Ps. taichungensis d | Greyish brown, 1–5 mm wide | Well-developed | 10–25 × 1–4.3, unbranched, 0–2-septate | 20–55–100 × 1.5–3, 1–6 indistinct septa |
aLenné (1990), bDeighton (1976), cYen and Lim (1980), dHsieh and Goh 1990, eChiddarwar (1959), f Cooke and Massee (1883-1884), gSilva et al. (2016)
Description.
Leaf spots lacking or indistinct to pale brown discolorations, amphigenous, subcircular to irregularly angular, (2–)4.5–10.5 mm diam., occasionally surrounded by a darker margin. Caespituli amphigenous, loose, olivaceous brown. Mycelium internal and external. External hyphae branched, 2.5–3.5 μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking to slightly developed, in substomatal cavities or partly embedded in the mesophyll, 10–20 μm diam., brown to dark brown. Conidiophores in small, loose fascicles of up to approx. 10 conidiophores, arising from internal hyphae or hyphal aggregations, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, or solitary, arising from external hyphae, erect to decumbent, flexuous, simple or occasionally branched, subcylindrical to somewhat clavate, geniculate-sinuous, slightly narrower towards the tips, (16.5–)20.5–92(–98) × (3–)3.5–4.5 μm, 2–6(–8)-septate, smooth, olivaceous brown to slightly dark brown, paler towards the tips. Conidiogenous cells terminal or lateral, medium brown, smooth, proliferating sympodially, with slightly tapering to flat-tipped apical loci; loci 1.5–2.5 μm wide, not thickened and not darkened. Conidia solitary, narrowly obclavate to subacicular, straight to curved, (16–)22–54.5(–65) × 3–4.5(–5) μm, 2–6-septate, often constricted at the septa, olivaceous brown, smooth, apex subacute, base truncate to obconically truncate, 1.5–2.5 μm wide, hila not thickened and not darkened.
Additional specimen examined.
Benin. Atlantique: Cotonou, University of Abomey-Calavi, c. 9 m a.s.l., 6°24'45"N, 2°20'41"E, on Senna occidentalis, 26 Sep 2019, Y. Meswaet and A. Tabé, YMM12B (Paratypes: M-0312677; UNIPAR).
Herbarium specimens examined for comparison.
On Senna occidentalis (as Cassia occidentalis L.): USA. South Carolina: Aiken, 1876, Ravenel H. W. s.n. (BPI 439584, Holotype of Cladosporium personatum var. cassiae Thüm.).
Host and distribution.
On Senna occidentalis (Fabaceae) in Benin.
Notes.
Currently, eleven Pseudocercospora species are known on Senna spp. (Fabaceae), namely Ps. angustata (Chupp & Solheim) Deighton on Senna hirsuta (L.) H.S. Irwin & Barneby, Ps. cassiae-alatae (J.M. Yen & Lim) J.M. Yen on S. alata (L.) Roxb., Ps. cassiae-fistulae Goh & W.H. Hsieh on Cassia fistula L. and S. rizzinii H.S. Irwin & Barneby, Ps. cassiae-occidentalis (J.M. Yen) J.M. Yen on S. occidentalis, Ps. cassiae-siameae (Chidd.) Deighton on S. siamea (Lam.) H.S. Irwin & Barneby, Ps. nigricans (Cooke) Deighton on Senna spp., Ps. sennae-multijugae on S. multijuga (Rich.) H.S. Irwin & Barneby, Ps. sennae-rugosae A. Hern. Gut., Z.M. Chaves & Dianese on S. rugosa (G. Don) H.S. Irwin & Barneby, Ps. singaporensis (J.M. Yen) J.M. Yen on S. occidentalis (L.) Link, Ps. taichungensis Goh & W.H. Hsieh on S. atomaria (L.) H.S. Irwin & Barneby (Hernández-Gutiérrez et al. 2015; Farr and Rossman 2021). Among these eleven species of Pseudocercospora, only Ps. nigricans and Ps. singaporensis have some similarity with the species described here (Table 6). Ps. sennicola, however, differs from Ps. nigricans in causing often indistinct leaf spots, shorter conidiophores [(16.5–)20.5–92(–98) μm versus 15–125 µm in Ps. nigricans] with 6(–8) septa and shorter conidia [(16–)22–54.5(–65) versus 20–80 μm in Ps. nigricans] (Hsieh and Goh 1990). Ps. sennicola differs from Ps. singaporensis by causing often indistinct leaf spots, slightly developed stromata, longer conidiophores [(16.5–)20.5–92(–98) μm versus 31–77 µm in Ps. singaporensis] and shorter conidia [(16–)22–54.5(–65) versus 30–67 μm in Ps. singaporensis] (Yen and Lim 1980). Moreover, the specimen from Benin has conidia with more septa (2–6 versus strictly 3-septate in Ps. singaporensis) and conidial walls constricted at the septa.
In the multi-gene tree (Fig. 1), Ps. sennicola is located in a polytomy at the end of a long branch reflecting a long genetic distance to other species included in the analysis. Morphologically, Ps. sennicola is distinct from all Pseudocercospora species known on species of Fabaceae from Benin by longer conidiophores [(16.5–)20.5–92(–98) μm] and smaller conidia [(16–)22–54.5(–65) μm)].
Based on a MegaBLAST search using the ITS sequence data, the closest matches in NCBI’s GenBank nucleotide database were Pseudocercospora fuligena on Lycopersicon sp. (Solanaceae) from Thailand (GenBank GU214675; Identities 674/687, i.e., 98%), Pseudocercospora chengtuensis on Lycium chinense (Solanaceae) from South Korea (GenBank GU214672; Identities 674/687, i.e., 98%) and Pseudocercospora atromarginalis on Solanum nigrum L. (Solanaceae) from South Korea (GenBank GU214671; Identities 673/687, i.e., 97%). Based on the result of our comparative study, we consider the present Pseudocercospora species on Senna occidentalis from Benin to represent a distinct species, which is described here. However, as sequence data are only available for Ps. sennae-multijugae, more molecular sequence data are needed to clarify the species delimitations among these twelve Ps. species on Senna spp.
Pseudocercospora tabei
Y.Meswaet, Mangelsdorff, Yorou & M.Piepenbr. sp. nov.
C528D5E1-DBFD-5FEA-A628-F245DBA17C68
839177
Figure 21.
Pseudocercospora tabei on Vigna unguiculata (YMM220) A immersed stroma with conidiophores B solitary conidiophores arising from external hyphae C conidia. Scale bars: 15 μm (A); 12 μm (B); 10 μm (C).
Type.
Benin. Borgou: Parakou, c. 360 m a.s.l., 9°20'07"N, 2°38'50"E, on Vigna unguiculata (L.) Walp. (Fabaceae), 2 Sep 2019, Y. Meswaet and A. Tabé, YMM220 (Holotype: M-0312678; Isotype: UNIPAR). Ex holotype sequences.MW834450 (SSU), MW834434 (LSU), MW834439 (ITS), MW848617 (tef1).
Etymology.
The epithet tabei refers to the person who collected the type specimen, Affoussatou Tabé, mycologist at the University of Parakou, Benin.
Diagnosis.
Pseudocercospora tabei differs from other Pseudocercospora spp. known on Vigna spp. by external hyphae, well-developed stromata, as well as the sizes of conidiophores [(20.5–)24–82(–84.5) × 3–4(–4.5) μm] and conidia [(20.5–)24–82(–84.5) × 3–4(–4.5) μm] (Table 7).
Table 7.
Comparison of Pseudocercospora tabei YMM220 on Vigna unguiculata with Pseudocercospora species known from Vigna spp. based on literature a–d.
| Pseudocercospora species | Stromata | Conidiophore size (in μm), branching, septa | Conidia size (in μm), septa |
|---|---|---|---|
| Pseudocercospora tabei (YMM220) | Small or well-developed up to 45 μm diam. | (11.5–)14.5–40(–44.5) × (3–)3.5–4(–4.5), branched, 0–4-septate | (20.5–)24–82(–84.5) × 3–4(4.5), 2–6(–8) septa |
| Ps. cruenta a | Up to 30 μm diam. | 10–75 × 3–5, branched, 0–3-septate | 25–120 × 2–5, 3–14 septa |
| Ps. mungo b | Up to 30 μm diam. | Up to 90(–130) × 4.5–7.5, branched, 1–3-septate | 25–84 × 4.5–7.5, 3–8 septa |
| Ps. phaseolicola a | Absent | 3–25 × 1.5–3 | 20–90 × 1.5–2, indistinctly septate |
| Ps. shihmenensis a | Absent | 35–55 × 4–5, branched, 1–4-septate | 20–52 × 4–5, 3–8 septa |
| Ps. vexillatae ac | Presen t | 10–17 × 4–5, unbranched, continuous or rarely 1-septate | 40–100 × 2.5–4, 3–8 septa |
| Ps. vignae-reticulatae b | Small | 40–250 × 3.5–5.5, branched | 30–95 × 4 –6.5, 1–12 septa |
| Ps. vignicola c | Well-developed | 22–75 × 3–5, branched, 0–1-septate | 30–60 × 2.5–3, 3–6 septa |
| Ps. vignigena d | Small | 22–75 × 3–5, unbranched, 1–3-septate | 33–60 × 4–5.5(–6), 3–6 septa |
Description.
Leaf spots amphigenous, subcircular to irregularly angular, 2.5–7.5 mm diam., occasionally limited by veins, yellowish brown to pale brown, reddish brown to dark brown when old, more evident on the adaxial surface of the leaves, margin indefinite. Caespituli amphigenous, brown. Mycelium internal and external. External hyphae branched, 2–2.5(–3.5) μm wide, septate, olivaceous brown to brown, smooth. Stromata lacking or formed by few aggregated swollen hyphal cells to well-developed, up to approx. 45 μm diam., immersed in the mesophyll or in substomatal chambers, globular to irregular, brown to mostly dark brown. Conidiophores in small, loose to moderately dense fascicles arising from stromata, breaking through the adaxial epidermis of the leaves or penetrating through stomatal openings, or solitary, arising from external hyphae, straight to sinuous or somewhat geniculate, simple or rarely branched, (11.5–)14.5–40(–44.5) × (3–)3.5–4(–4.5) μm, 0–4-septate, smooth, olivaceous brown to brown, paler towards the tips, sometimes a conidiophore is reduced to a single conidiogenous cell. Conidiogenous cells terminal or lateral, rarely up to 20 μm long, pale or olivaceous brown, smooth, proliferating sympodially; loci 2–3.5 μm wide, not thickened and not darkened. Conidia solitary, narrowly cylindrical to obclavate-cylindrical, straight to slightly curved, (20.5–)24–82(–84.5) × 3–4(–4.5) μm, conspicuously 2–6(–8)-septate, olivaceous brown, smooth, apex subacute to rounded and narrower than the rest of the conidium, base truncate, (2–)2.5–3.5 µm wide, hila not thickened and not darkened.
Additional specimens examined.
Benin. Borgou: Parakou, c. 354 m a.s.l., 9°20'02"N, 2°38'48"E, on Vigna unguiculata, 27 Aug 2019, Y. Meswaet and A. Tabé, YMM232A (Paratypes: M-0312679; UNIPAR). Benin. Borgou: Parakou, c. 391 m a.s.l., 9°22'56"N, 2°37'33"E, same host, 29 Aug 2019, Y. Meswaet and A. Tabé, YMM232B (M-0312680).
Herbarium specimens examined for comparison.
Pseudocercospora cruenta. On Vigna unguiculata: USA. Mississippi: Starkville, Sep 1888, Tracy S. M. s.n. (BPI 435817 Paratype of Cercospora dolichi). On Phaseolus sp.: USA. South Carolina: Aiken, Ravenel H. W. s.n (BPI 439619, type of C. phaseolorum). Pseudocercospora stizolobii. On Mucuna sp.: Philippines. Los Baños, 6 Apr 1913, Raimundo M. B. 892 (BPI 441666, Holotype of C. stizolobii).
Host and distribution.
On Vigna unguiculata (Fabaceae) in Benin.
Notes.
On species of Vigna, eight species of Pseudocercospora, namely Ps. cruenta, Ps. mungo Deighton, Ps. phaseolicola Goh & W.H. Hsieh, Ps. shihmenensis (J.M. Yen) J.M. Yen, Ps. vexillatae (J.M. Yen) U.Braun, Ps. vignae-reticulatae Deighton, Ps. vignicola (J.M. Yen, A.K. Kar & B.K. Das) U.Braun and Ps. vignigena J.M. Yen, A.K. Kar & B.K. Das are known (Farr and Rossman 2021). Among these species, Ps. mungo described on Vigna radiata, V. mungo from Tanzania (East Africa) (Deighton 1976) and Ps. phaseolicola on Vigna radiata from China and Taiwan (Hsieh and Goh 1990) are morphologically similar to the present Pseudocercospora specimen from Benin (Table 7). Based on the original description by Deighton (1976), Ps. mungo, however, differs from the present species in causing leaf spots that form only indefinite chlorotic areas on the adaxial surface, hypophyllous caespituli, lack of external hyphae and above all, by longer and wider conidiophores [up to 90(–130) × 4.5–7.5 µm)] and wider conidia (4.5–7.5 µm) (Deighton 1976). Ps. tabei causes yellowish brown to pale brown leaf spots, that are reddish brown to dark brown, when old, forms amphigenous caespituli, often produces well developed stromata, external hyphae and above all, shorter and narrower conidiophores [(11.5–)14.5–40(–44.5) × (3–)3.5–4(–4.5) µm] and narrower conidia (3–4 μm). Ps. phaseolicola differs by producing hypophyllous caespituli, no stromata, non-fasciculate, olivaceous, shorter and narrower conidiophores [3–25 × 1.5–3 µm versus (11.5–)14.5–40(–44.5) × (3–)3.5–4(–4.5) µm in Ps. tabei] and narrower conidia [1.5–2 µm versus 3–4 µm in Ps. tabei] (Hsieh and Goh 1990).
In the multi-gene phylogeny (Fig. 1), Ps. tabei forms part of a polytomy with a large genetic distance (branch length) in relation to other sequences considered in the analysis. In the tef1 phylogeny, Ps. tabei clustered together with the isolates of Ps. cruenta on Vigna and Phaseolus form Benin (see Suppl. material 4). However, morphologically, the present species is clearly distinct from specimens of Ps. cruenta by having darker and shorter conidiophores and above all, shorter conidia [(20.5–)24–82(–84.5) μm] (Table 7). It is not possible to distinguish Ps. tabei from other numerous Pseudocercospora spp. by the phylogenetic analyses based on ITS sequences.
Based on a MegaBLAST search in the NCBI GenBank nucleotide database using the tef1 sequence, the closest matches were Ps. cruenta on Phaseolus vulgaris (Fabaceae) from Taiwan (GenBank GU384405; Identities 283 / 312, i.e., 90%), Pseudocercospora sp. A on P. vulgaris (Fabaceae) from Iran MB-2015(GenBank KM452885; Identities 263 / 292, i.e., 90%) and Ps. madagascariensis on Eucalyptus camaldulensis (Myrtaceae) from Madagascar (GenBank KF253265; Identities 276 / 314, i.e., 88%).
Key 1: Key to species of Cercospora on Fabaceae known for Benin
| 1 | Stromata well-developed, i.e., usually broader than 40 μm diam | 2 |
| – | Stromata lacking or small, i.e., usually less than 20 μm diam | 3 |
| 2 | Conidiophores branched, with polyblastic conidiogenous cells, conidia mostly 26–160 × 4–5 μm. On Vigna | C. rhynchophora |
| – | Conidiophores unbranched, usually with monoblastic conidiogenous cells, conidia mostly 27–70 × 2–3 μm. On Lablab | C. cf. fagopyri |
| 3 | Stromata totally lacking, hyphae mainly internal, conidiophores branched, mostly 18–178 × 4–5 μm, conidia mostly 19–88 × 3.5–4.5. On Desmodium | C. parakouensis |
| – | Stromata often formed by few aggregated swollen hyphal cells with similar morphology | 4 |
| 4 | Conidiophores up to 400 μm long. On Vigna. | 5 |
| – | Conidiophores usually not longer than 150 μm | 6 |
| 5 | Leaf spots inconspicuous or absent, caespituli mostly epiphyllous, conidia mostly 38–188 μm long | C. tentaculifera |
| – | Leaf spots conspicuous, brown to later with necrotic centre, caespituli mostly hypophyllous, conidia mostly 26–100 μm long | C. vignae-subterraneae |
| 6 | Conidia up to 330 μm long. On Calopogonium, Vigna | C. aff. canescens |
| – | Conidia mostly 20–160 μm long | 7 |
| 7 | Only internal hyphae | 8 |
| – | Internal and external hyphae | 9 |
| 8 | Internal hyphae often distinct and developed, conidiophores in loose to moderately large and dense fascicles of up to approx. 16. On Crotalaria | C. beninensis |
| – | Internal hyphae often indistinct, conidiophores in small and loose fascicles of up to approx. 6 conidiophores, conidiophores mostly attenuated towards the tips. On Vigna | C. phaseoli-lunati |
| 9 | Conidiophores unbranched, in small, loose or moderately large and dense fascicles of up to approx. 22. On Vigna | C. cf. canscorina |
| – | Conidiophores branched | 10 |
| 10 | Leaf spots almost lacking or brown discolorations, often uniform in colour and width, conidia hyaline. On Zornia | C. zorniicola |
| – | Leaf spots often developed, reddish brown, later dark brown by abundant caespituli, conidia often sub-hyaline. On Phaseolus | Cercospora sp. YMM297B |
Key 2: Key to the species of Pseudocercospora on Fabaceae known for Benin
| 1 | Conidiophores in synnematous fascicles, synnemata up to 250 µm high, mostly 20–40 µm wide. On Phaseolus | Ps. griseola |
| – | Conidiophores solitary, fasciculate or in sporodochia | 2 |
| 2 | Stromata well-developed | 3 |
| – | Stromata lacking or very small | 4 |
| 3 | Leaf spots often lacking or indistinct, conidiophores often narrower towards the tips, mostly 20–92 μm long, conidia, mostly 22–55 μm long, constricted at the septa. On Senna | Ps. sennicola |
| – | Leaf spots evident, conidiophores, mostly 14–40 μm long, conidia mostly 24–82 μm long. On Vigna | Ps. tabei |
| 4 | Caespituli amphigenous, conidiophores mostly 15–54 μm long, conidia mostly 42–132 μm long. On Phaseolus, Vigna | Ps. cruenta |
| – | Caespituli mainly epiphyllous, conidiophores mostly 13–44 μm long, conidia mostly 38–110 μm long. On Centrosema | Ps. bradburyae |
Discussion
The present study aims to increase the knowledge on the diversity of cercosporoid fungi in tropical Africa. Therefore, cercosporoid fungi collected on fifteen species of plants belonging to ten genera of Fabaceae found in Benin, West Africa, were characterised concerning their morphology, host species and DNA sequence data (18S rDNA, 28S rDNA, ITS and tef1). The specimens of cercosporoid species collected in Benin are attributed to groups corresponding to Cercospora, Pseudocercospora and a heterogeneous group around Passalora. The four-gene phylogenetic tree yielded results consistent with the current knowledge of generic relationships as presented in previous studies (Crous et al. 2013; Groenewald et al. 2013; Nakashima et al. 2016). Species of Cercospora and Pseudocercospora form morphologically distinct groups that receive moderate support in the phylogenetic analysis (Fig. 1). In the Cercospora and Pseudocercospora clades, the lengths of branches of most new species (C. beninensis, C. rhynchophora, C. vignae-subterraneae, C. zorniicola, Ps. sennicola and Ps. tabei) are quite long (Fig. 1). This indicates a relatively large genetic and evolutionary distance from neighbouring species included in the analysis. The partial gene sequences of the protein-coding region tef1 and the combined analysis of four loci provided better results than single gene analyses of ITS and LSU rDNA for the differentiation of species of Cercospora and Pseudocercospora. Consequently, these molecular sequence data only allow to measure phylogenetic distances between the species. A similar situation has been found for Cercospora spp. by Bakhshi et al. (2015, 2018) and for Pseudocercospora spp. by Crous et al. (2013a) and Silva et al. (2016).
Fortunately, most species included in this study differ from each other by their morphology and host range. For example, Cercospora tentaculifera (YMM75) on Vigna unguiculata causes inconspicuous leaf spots and produces adaxial caespituli with large conidiophores (up to 435 μm) that are constricted at the septa (Figs 2H, 11). Thereby, this species is easily distinguishable from other Cercospora spp. known on species of Vigna and Phaseolus. C. zorniicola (YMM299) on Zornia glochidiata produces external hyphae and conidiophores that are unbranched and uniform in colour and width with usually monoblastic conidiogenous cells (Fig. 13). This is the first species of Cercospora known for the host genus Zornia.
For the morphological identification of all species included in this study, we examined about 50 type specimens and other specimens loaned from BPI, ILL and NY. As result of these analyses, dichotomous keys to the species of Cercospora and Pseudocercospora infecting members of Fabaceae known for Benin are presented (see below). The following morphological characteristics are helpful to separate species of Cercospora and Pseudocercospora: characteristics of leaf spots (distinctiveness, colour, size, form) and sporulation (distinctiveness, position on the leaf), the stroma (size, density), the external hyphae (present/absent), conidiophores (form, size, branching, number and position of conidiogenous loci, form of conidiogenous cells), and conidia (form, size range) (comp. Deighton 1976; Crous and Braun 2003; Crous et al. 2013; Groenewald et al. 2013; Videira et al. 2017).
In order to obtain DNA sequence data, up to now, only cercosporoid fungi available as cultures have been used (Groenewald et al. 2013). Due to the fact that most cercosporoid fungi are not available as cultures, molecular sequences are available only for a small fraction of the species diversity of cercosporoid fungi known by morphological characteristics. It is often difficult to cultivate cercosporoid fungi, as this requires living fungal cells and a sterile environment to avoid contamination. As it was not possible to cultivate cercosporoid fungi in Benin, a technique for DNA isolation from dry specimens has been developed and successfully applied in the context of the present study for cercosporoid fungi for the first time. This direct DNA extraction method opens interesting possibilities to obtain DNA data of cercosporoid and other fungal plant pathogens especially in tropical countries.
The present study is the first effort towards generating molecular and morphological data for cercosporoid fungi in Benin, West Africa. We found 18 taxa, representing only a small fraction of the yet unknown species diversity of cercosporoid fungi (Piepenbring et al. 2020; Farr and Rossman 2021). Eight taxa found in this study are proposed as species new to science. Ten known species have been identified, including taxa important for agriculture such as Pseudocercospora cruenta and Ps. griseola on Phaseolus lunatus as well as Nothopassalora personata and Passalora arachidicola on Arachis hypogaea. Eight species are reported for Benin for the first time, with three of them namely, Cercospora cf. canscorina, C. cf. fagopyri and C. phaseoli-lunati, being new for West Africa.
New scientific data, such as species new to science, new records of hosts and for geographic areas, will help plant pathologists to develop efficient and sustainable disease management programs to control these fungal diseases and quarantine officials to take decisions based on scientific evidence. The plethora of novel and newly reported taxa collected on Fabaceae in Benin confirms that mycologists and phytopathologists in Africa have so far not given much attention to the species diversity of fungi occurring on plants, including species of economic relevance, such as those belonging to Fabaceae. Benin and other tropical African countries are likely to harbour highly diverse mycobiomes including cercosporoid fungi that still await discovery (Piepenbring et al. 2020). It is important to investigate them, because these unknown plant pathogens are or may become relevant as agents of emerging diseases that may spread and threaten cultivated plants worldwide. We hope that this study motivates further mycologists to study cercosporoid fungi in Benin, as well as in other countries of tropical Africa, and help to get a better understanding of cercosporoid fungal diversity worldwide.
Conclusions
The present study is a first step for the investigation of the diversity of cercosporoid fungi by an integrative approach including morphological, phylogenetic and ecological information. Taxonomic studies in this work generated eight newly described species, eight new records and the confirmation of two species of cercosporoid fungi that were previously reported from Benin. Previously, 12 cercosporoid fungi were known for Benin. The present work expands this number by adding 16 species of Cercospora and Pseudocercospora to this list, with a total of 28 species. These records together with herbarium specimens and molecular sequence data form a baseline for further studies in the field of systematics, ecology and phytopathology referring to cercosporoid fungi. This information will help plant pathologists to develop effective disease management programs and evidence-based quarantine regulations. The results obtained for a single family (Fabaceae) in easily accessible vegetation close to settlements suggest that many more taxa of cercosporoid fungi remain to be discovered on plants belonging to other family of plants in diverse habitats. In the future, more attention should be directed towards collecting cercosporoid and other pathogenic fungi from Benin as well as other parts of tropical Africa.
Supplementary Material
Acknowledgements
We are grateful to Roland Kirschner, Hermine Lotz-Winter, José Macia-Vicente and Melissa Mardones for fruitful discussions and valuable advice in the context of this study. We thank Carola Glatthorn for her support for the literature search and daily needs. Our special thanks go to Affoussatou Tabé and Ramdan Dramani (Faculty of Agronomy, University of Parakou, Benin) who helped with the sampling of specimens in the field. We acknowledge the support and facilities made available for this study by the Université de Parakou and Université d’Abomey-Calavi, Benin. We are grateful to Adomou Aristide and Paul Yédémohan (National Herbarium of Benin, University of Abomey-Calavi) for their assistance with the identification of host plants. We thank the curators of the US National Fungus Collections (BPI), the Herbarium of the University of Illinois (ILL) and the New York Botanical Garden (NY) for the loans of specimens.
Our participation in summer schools and scientific meetings in tropical mycology 2016, 2017 and 2019 in Benin, along with the field work, has been made possible by financial support from the Volkswagen Foundation (grants Az 90 127 and Az 93 338) and the Freunde und Förderer der Goethe-Universität. A special gratitude goes to the Adolf Messer Foundation for providing a scholarship to the first author. NSY is supported by the German Federal Ministry of Education and Research (BMBF) under the contract number 01DG20015FunTrAf.
Citation
Meswaet Y, Mangelsdorff R, Yorou NS, Piepenbring M (2021) Unravelling unexplored diversity of cercosporoid fungi (Mycosphaerellaceae, Mycosphaerellales, Ascomycota) in tropical Africa. MycoKeys 81: 69–138. https://doi.org/10.3897/mycokeys.81.67850
Supplementary materials
Checklist for cercosporoid fungi in West Africa
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
Checklist
Explanation note
This information is based on the checklist published by Piepenbring et al. (2020) and updated with new results from the present publication and some further publications.
References for the checklist for cercosporoid fungi in West Africa
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
text
Explanation note
References for the checklist for cercosporoid fungi in West Africa (Suppl. material 1).
A Bayesian phylogenetic tree inferred from ITS rDNA sequence data of cercosporoid species
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
phylogenetic
Explanation note
Nodes receiving Bayesian PP ≥ 0.94 are considered as strongly supported and are indicated by thickened branches. Newly described species are denoted in bold and red text, newly reported species are indicated in blue text.
A Bayesian phylogenetic tree inferred from tef1 DNA sequence data of cercosporoid species
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
phylogenetic
Explanation note
Nodes receiving Bayesian PP ≥ 0.94 are considered as strongly supported and are indicated by thickened branches. Newly described species are denoted in bold and red text, newly reported species are indicated in blue text.
References
- Aggarwal VD, Pastor-Corrales MA, Chirwa RM, Buruchara RA. (2004) Andean beans (Phaseolus vulgaris L.) with resistance to the angular leaf spot pathogen (Phaeoisariopsis griseola) in southern and eastern Africa. Euphytica 136: 201–210. 10.1023/B:EUPH.0000030678.12073.a9 [DOI] [Google Scholar]
- Agrios GN. (2005) Plant Pathology (5th ed.). Elsevier Academic Press, Amsterdam, Boston, 922 pp. [Google Scholar]
- Akoégninou A, van der Burg WJ, van der Maesen LJG. (2006) Flore analytique du Bénin. Wageningen University papers, 2. Backhuys Publ, Leiden, 1034 pp. https://edepot.wur.nl/281595 [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57–93. 10.3114/sim.2007.58.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila A, Groenewald JZ, Trapero A, Crous PW. (2005) Characterisation and epitypification of Pseudocercospora cladosporioides, the causal organism of Cercospora leaf spot of olives. Mycological Research 109: 881–888. 10.1017/S0953756205003503 [DOI] [PubMed] [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-Ahari A, Groenewald JZ, Crous PW. (2014) Multi-gene analysis of Pseudocercospora spp. from Iran. Phytotaxa 184: 245–264. 10.11646/phytotaxa.184.5.1 [DOI] [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-Ahari A, Groenewald JZ, Crous PW. (2018) Novel primers improve species delimitation in Cercospora. IMA Fungus 9: 299–332. 10.5598/imafungus.2018.09.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-Ahari A, Groenewald JZ, Braun U, Crous PW. (2015) Application of the consolidated species concept to Cercospora spp. from Iran. Persoonia – Molecular Phylogeny and Evolution of Fungi 34: 65–86. 10.3767/003158515X685698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz V, Pascoe I, Parbery D. (2002) Three new Pseudocercospora species, one with a Mycosphaerella teleomorph, from Kennedia in Australia. Mycotaxon 82: 397–408. [Google Scholar]
- Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. (2014) GenBank. Nucleic Acids Research 42: D32–D37. 10.1093/nar/gkt1030 [DOI] [PMC free article] [PubMed]
- Bharadwaj SD. (1971) Cercospora kashiensis Bharadwaj. Journal of the Indian Botanical Society 49: e119.
- Bhat D, Pratibha J. (2010) Cercospora spp. from Goa and neighbouring areas. Kavaka 37/38: 69–78.
- Braun U. (1993) New genera of phytopathogenic Deuteromycetes. Cryptogamic Botany 4: 1–14. [Google Scholar]
- Braun U. (1995a) A Monograph of Cercosporella, Ramularia, and Allied Genera (Phytopathogenic Hyphomycetes). IHW, Eching bei München, 333 pp. [Google Scholar]
- Braun U. (1995b) Miscellaneous notes on phytopathogenic hyphomycetes (II). Mycotaxon 55: 223–241. [Google Scholar]
- Braun U. (1998) A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes), additions to host range and distribution. Schlechtendalia 1: 41–43. [Google Scholar]
- Braun U, Crous PW. (2005) Additions and corrections to names published in Cercospora and Passalora. Mycotaxon 92: 395–416. [Google Scholar]
- Braun U, Crous PW. (2016) Proposal to conserve the name Cercospora (Ascomycota: Mycosphaerellaceae) with a conserved type. Taxon 65: e185. 10.12705/651.18 [DOI]
- Braun U, Freire F. (2004) Some cercosporoid hyphomycetes from Brazil-III. Cryptogamie Mycologie 25: 221–244. [Google Scholar]
- Braun U, Mouchacca J, McKenzie EHC. (1999) Cercosporoid hyphomycetes from New Caledonia and some other South Pacific islands. New Zealand Journal of Botany 37: 297–327. 10.1080/0028825X.1999.9512636 [DOI] [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JZ, De Hoog GS. (2003) Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3–18. 10.1007/s11557-006-0039-2 [DOI] [Google Scholar]
- Braun U, Nakashima C, Crous PW. (2013) Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. IMA fungus 4: 265–345. 10.5598/imafungus.2013.04.02.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2015) Cercosporoid fungi (Mycosphaerellaceae) 4. Species on dicots (Acanthaceae to Amaranthaceae). IMA Fungus 6: 373–469. 10.5598/imafungus.2015.06.02.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2016) Cercosporoid fungi (Mycosphaerellaceae) 5. Species on dicots (Anacardiaceae to Annonaceae). IMA fungus 7: 161–216. 10.5598/imafungus.2016.07.01.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Schneider RW. (2008) Population structure of Cercospora kikuchii, the causal agent of cercospora leaf blight and purple seed stain in soybean. Phytopathology 98: 823–829. 10.1094/PHYTO-98-7-0823 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. 10.1080/00275514.1999.12061051 [DOI] [Google Scholar]
- Chiddarwar P. (1959) Contributions to our knowledge of the Cercospora of Bombay State I. Sydowia 13: 152–163. [Google Scholar]
- Chupp C. (1954) A Monograph of the Fungus Genus Cercospora. Published by the author, Ithaca.
- Ciferri R, González-Fragoso R. (1926) Hongos parásitos y saprófitos de la República Dominicana (6a serie). Boletín de la Real Sociedad Española de Historia Natural, Biologia 26: 330–341. [Google Scholar]
- Collemare J, Beenen HG, Crous PW, de Wit PJGM, van der Burgt A. (2015) Novel introner-like elements in fungi are involved in parallel gains of spliceosomal introns. PLoS ONE 10: e0129302. 10.1371/journal.pone.0129302 [DOI] [PMC free article] [PubMed]
- Cooke MC, Massee G. (1883–1884) Grevillea. Williams and Norgate, London, 130 pp. https://www.biodiversitylibrary.org/item/188030 [Google Scholar]
- Crous PW, Braun U. (2003) Names Published in Cercospora and Passalora. Centraalbureau voor Schimmelcultures, Utrecht, 571 pp. [Google Scholar]
- Crous PW, Liebenberg MM, Braun U, Groenewald JZ. (2006) Re-evaluating the taxonomic status of Phaeoisariopsis griseola, the causal agent of angular leaf spot of bean. Studies in Mycology 55: 163–173. 10.3114/sim.55.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ. (2009a) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. 10.3114/sim.2009.64.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ. (2009b) Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia – Molecular Phylogeny and Evolution of Fungi 22: 38–48. 10.3767/003158509X424333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Groenewald JZ. (2009c) Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia – Molecular Phylogeny and Evolution of Fungi 23: 119–146. 10.3767/003158509X479531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, Edwards J, Seifert KA, Alfenas AC, Alfenas RF, Burgess TI, Carnegie AJ, Hardy GEStJ, Hiscock N, Hüberli D, Jung T, Louis-Seize G, Okada G, Pereira OL, Stukely MJC, Wang W, White GP, Young AJ, McTaggart AR, Pascoe IG, Porter IJ, Quaedvlieg W. (2011) Fungal planet description sheets: 69–91. Persoonia – Molecular Phylogeny and Evolution of Fungi 26: 108–156. 10.3767/003158511X581723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, Burgess TI, Decock CA, Dreyer LL, Granke LL, Guest DI, Hardy GEStJ, Hausbeck MK, Hüberli D, Jung T, Koukol O, Lennox CL, Liew ECY, Lombard L, McTaggart AR, Pryke JS, Roets F, Saude C, Shuttleworth LA, Stukely MJC, Vánky K, Webster BJ, Windstam ST, Groenewald JZ. (2012) Fungal planet description sheets: 107–127. Persoonia – Molecular Phylogeny and Evolution of Fungi 28: 138–182. 10.3767/003158512X652633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, Shin H-D, Nakashima C, Groenewald JZ. (2013) Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. 10.3114/sim0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, van der Bank M, Zhang Y, Summerell BA, Guarro J, Wingfield MJ, Wood AR, Alfenas AC, Braun U, Cano-Lira JF, García D, Marin-Felix Y, Alvarado P, Andrade JP, Armengol J, Assefa A, den Breeÿen A, Camele I, Cheewangkoon R, De Souza JT, Duong TA, Esteve-Raventós F, Fournier J, Frisullo S, García-Jiménez J, Gardiennet A, Gené J, Hernández-Restrepo M, Hirooka Y, Hospenthal DR, King A, Lechat C, Lombard L, Mang SM, Marbach PAS, Marincowitz S, Marin-Felix Y, Montaño-Mata NJ, Moreno G, Perez CA, Pérez Sierra AM, Robertson JL, Roux J, Rubio E, Schumacher RK, Stchigel AM, Sutton DA, Tan YP, Thompson EH, Vanderlinde E, Walker AK, Walker DM, Wickes BL, Wong PTW, Groenewald JZ. (2014) Fungal planet description sheets: 214–280. Persoonia – Molecular Phylogeny and Evolution of Fungi 32: 184–306. 10.3767/003158514X682395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, Thangavel R, Hernández-Restrepo M, Carnegie AJ, Cheewangkoon R, Wingfield MJ, Summerell BA, Quaedvlieg W, Coutinho TA, Roux J, Wood AR, Giraldo A, Groenewald JZ. (2019) New and interesting fungi. 2. Fungal Systematics and Evolution 3: 57–134. 10.3114/fuse.2019.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Gupta S, Parihar AK, Singh D, Chand R, Pratap A, Singha KD, Kushwaha KPS. (2019) Delineating genotype × environment interactions towards durable resistance in mungbean against Cercospora leaf spot (Cercospora canescens) using GGE biplot. Plant Breeding 139: 639–650. 10.1111/pbr.12789 [DOI] [Google Scholar]
- Ddamulira G, Mukankusi C, Ochwo-Ssemakula M, Edema R, Sseruwagi P, Gepts P. (2014) Distribution and variability of Pseudocercospora griseola in Uganda. Journal of Agricultural Science 6: 1–16. 10.5539/jas.v6n6p16 [DOI] [Google Scholar]
- Deighton FC. (1967) Studies on Cercospora and allied genera. II. Passalora, Cercosporidium, and some species of Fusicladium on Euphorbia. Mycological Papers 112: 1–80. [Google Scholar]
- Deighton FC. (1973) Studies on Cercospora and allied genera. IV. Cercosporella Sacc., Pseudocercosporella gen. nov., and Pseudocercosporidium gen. nov. Mycological Papers 113: 1–62. [Google Scholar]
- Deighton FC. (1974) Studies on Cercospora and allied genera. V. Mycovellosiella Rangel, and a new species of Ramulariopsis. Mycological Papers 137: 1–75. [Google Scholar]
- Deighton FC. (1976) Studies on Cercospora and allied genera. VI. Pseudocercospora Speg., Pantospora Cif., and Cercoseptoria Petr. Mycological Papers 140: 1–168. [Google Scholar]
- Deighton FC. (1979) Studies on Cercospora and allied genera VII. New species and redispositions. Mycological Papers 144: 1–56. [Google Scholar]
- Deighton FC. (1983) Studies on Cercospora and allied genera. VIII. Further notes on Cercoseptoria and some new species and redispositions. Mycological Papers 151: 1–13. [Google Scholar]
- Deighton FC. (1990) Observations on Phaeoisariopsis. Mycological Research 94: 1096–1102. 10.1016/S0953-7562(09)81340-3 [DOI] [Google Scholar]
- Ellis MB. (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, 608 pp. [Google Scholar]
- Ellis MB. (1976) More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, 507 pp. [Google Scholar]
- Faris DG. (1965) The origin and evolution of the cultivated forms of Vigna sinensis. Canadian Journal of Genetics and Cytology 7: 433–452. 10.1139/g65-058 [DOI] [Google Scholar]
- Farr DF, Rossman AY. (2021) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/ [Retrieved January 22, 2021]
- Fazekas AJ, Kuzmina ML, Newmaster SG, Hollingsworth PM. (2012) DNA barcoding methods for land plants. In: Kress WJ, Erickson DL. (Eds) DNA Barcodes.Methods in Molecular Biology. Humana, Totowa, 223–252. 10.1007/978-1-61779-591-6_11 [DOI] [PubMed]
- Fortuna-Perez AP, da Silva MJ, de Queiroz LP, Lewis GP, Simões AO, de Azevedo Tozzi AMG, Sarkinen T, de Souza AP. (2013) Phylogeny and biogeography of the genus Zornia (Leguminosae: Papilionoideae: Dalbergieae). Taxon 62: 723–732. 10.12705/624.35 [DOI] [Google Scholar]
- Fragoso RG. (1927) Estudio Sistemático de los Hifales de la Flora Española Conocidos Hasta Hecha Fecha: Memoria. Real Academia de Ciencias Exactas, Físicas y Naturales, Madrid, 377 pp. [Google Scholar]
- Fries E. (1849) Summa vegetabilium Scandinaviae, Sectio posterior, 259–572.
- Fuckel K. (1863) Fungi Rhenani exsiccati, Fasc. I–IV. Hedwigia 2: 132–136. [Google Scholar]
- Gepts P, Beavis WD, Brummer EC, Shoemaker RC, Stalker HT, Weeden NF, Young ND. (2005) Legumes as a model plant family. Genomics for food and feed report of the cross-legume advances through genomics conference. Plant Physiology 137: e1228. 10.1104/pp.105.060871 [DOI] [PMC free article] [PubMed]
- Golato C, Meossi E. (1972) A serious leaf infection of beans, Phaseolus vulgaris, in Ethiopia. Rivista di Agricoltura Subtropicale e Tropicale 66: 135–138. [Google Scholar]
- Groenewald M, Groenewald JZ, Braun U, Crous PW. (2006) Host range of Cercospora apii and C. beticola and description of C. apiicola , a novel species from celery. Mycologia 98: 275–285. 10.1080/15572536.2006.11832700 [DOI] [PubMed] [Google Scholar]
- Groenewald JZ, Groenewald M, Braun U, Crous PW. (2010) Cercospora speciation and host range. In: Lartey RT, Weiland JJ, Panella L, Crous PW, Windels CE. (Eds) Cercospora leaf spot of sugar beet and related species.American Phytopathological Society (APS Press), St. Paul, Minn, 21–37.
- Groenewald JZ, Nakashima C, Nishikawa J, Shin H-D, Park J-H, Jama AN, Groenewald M, Braun U, Crous PW. (2013) Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75: 115–170. 10.3114/sim0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grum-Grzhimaylo AA, Georgieva ML, Bondarenko SA, Debets AJM, Bilanenko EN. (2016) On the diversity of fungi from soda soils. Fungal Diversity 76: 27–74. 10.1007/s13225-015-0320-2 [DOI] [Google Scholar]
- Guzmán P, Gilbertson R, Nodari R, Johnson WC, Temple SR, Mandala D, Mkandawire AB, Gepts P. (1995) Characterization of variability in the fungus Phaeoisariopsis griseola suggests coevolution with the common bean (Phaseolus vulgaris). Phytopathology 85: 600–607. 10.1094/Phyto-85-600 [DOI] [Google Scholar]
- Guzmán P, Gepts P, Temple S, Mkandawire ABC, Gilbertson RL. (1999) Detection and differentiation of Phaeoisariopsis griseola isolates with the polymerase chain reaction and group-specific primers. Plant Disease 83: 37–42. 10.1094/PDIS.1999.83.1.37 [DOI] [PubMed] [Google Scholar]
- Hay FS, Maloney E, Vaghefi N, Shivas RG, Pethybridge SJ. (2017) First report of Cercospora blight of Asparagus officinalis caused by Cercospora asparagi in New York. Plant Disease 101: e1953. 10.1094/PDIS-05-17-0648-PDN [DOI]
- Hepper FN. (1963) Plants of the 1957–58 West African expedition: II. The Bambara Groundnut (Voandzeia subterranea) and Kersting’s Groundnut (Kerstingiella geocarpa) wild in West Africa. Kew Bulletin 16: 395–407. 10.2307/4114681 [DOI] [Google Scholar]
- Hernández-Gutiérrez A, Dianese JC. (2008) New cercosporoid fungi from the Brazilian Cerrado 1. Species on hosts of the families Anacardiaceae, Araliaceae, Bombacaceae, Burseraceae and Celastraceae. Mycotaxon 106: 41–63. [Google Scholar]
- Hernández-Gutiérrez A, Dianese JC. (2009) New cercosporoid fungi from the Brazilian Cerrado 2. Species on hosts of the subfamilies Caesalpinioideae, Faboideae and Mimosoideae (Leguminosae s. lat.). Mycotaxon 107: 1–24. 10.5248/107.1 [DOI] [Google Scholar]
- Hernández-Gutiérrez A, Chaves ZM, Dornelo-Silva D, Dianese JC. (2015) Additions to the cercosporoid fungi from the Brazilian Cerrado: 1. New species on hosts belonging in family Fabaceae, and reallocations of four Stenella species into Zasmidium. Mycobio 5: 33–64. 10.12664/mycobiota.2015.05.06 [DOI] [Google Scholar]
- de Hoog GS, van den Ende AHGG. (1998) Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189. 10.1111/j.1439-0507.1998.tb00321.x [DOI] [PubMed] [Google Scholar]
- Houessou HJH, Beed F, Sikirou R, Ezin V. (2011) First report of Cercospora beticola on lettuce (Lactuca sativa) in Benin. New Disease Reports 23: 1–16. 10.5197/j.2044-0588.2011.023.016 [DOI] [Google Scholar]
- Hsieh WH, Goh TK. (1990) Cercospora and Similar Fungi from Taiwan. Maw Chang Book Co, Taipei, 376 pp. [Google Scholar]
- Huang F, Groenewald JZ, Zhu L, Crous PW, Li H. (2015) Cercosporoid diseases of citrus. Mycologia 107: 1151–1171. 10.3852/15-059 [DOI] [PubMed] [Google Scholar]
- Hunter GC, Wingfield BD, Crous PW, Wingfield MJ. (2006) A multi-gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Studies in Mycology 55: 147–161. 10.3114/sim.55.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WA. (1938) Two fungi causing leaf spot of peanut. Journal of Agricultural Research 56: 317–332. [Google Scholar]
- Joshi AS, Jegadeesan S, Chand R, Pawar S. (2006) Genetic diversity study of Cercospora canescens (Ellis & Martin) isolates, the pathogen of Cercospora leaf spot in legumes. Current Science 90: 564–568. [Google Scholar]
- Ju HJ, Choi IY, Lee KJ, Shin HD. (2020) First report of Cercospora malayensis causing leaf spot on okra in Korea. Plant Disease 104: 1858–1858. 10.1094/PDIS-11-19-2468-PDN [DOI] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R. (2014) A new species and new records of cercosporoid fungi from ornamental plants in Taiwan. Mycological Progress 13: 483–491. 10.1007/s11557-013-0930-6 [DOI] [Google Scholar]
- Kokalis-Burelle N. (1997) Compendium of peanut diseases. APS Press, American Phytopathological Society, St. Paul, Minn, 94 pp. [Google Scholar]
- Kouris-Blazos A, Belski R. (2016) Health benefits of legumes and pulses with a focus on Australian sweet lupins. Asia Pacific Journal of Clinical Nutrition 25: 1–17. [DOI] [PubMed] [Google Scholar]
- Kress WJ, Erickson DL, Jones FA, Swenson NG, Perez R, Sanjur O, Bermingham E. (2009) Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proceedings of the National Academy of Sciences 106: 18621–18626. 10.1073/pnas.0909820106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A. (2014) Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evolutionary Biology 14: e82. 10.1186/1471-2148-14-82 [DOI] [PMC free article] [PubMed]
- Lenné JM. (1990) A World List of Fungal Diseases of Tropical Pasture Species. Phytopathological Papers (Vol. 31). CAB International, Wallingford.
- Levin RA, Wagner WL, Hoch PC, Nepokroeff M, Pires JC, Zimmer EA, Sytsma KJ. (2003) Family-level relationships of Onagraceae based on chloroplast rbc L and ndh F data. American Journal of Botany 90: 107–115. 10.3732/ajb.90.1.107 [DOI] [PubMed] [Google Scholar]
- Liu Z, Braun U, Crous PW, Si J, Zhang Y. (2016) Taxonomy and phylogeny of cercosporoid fungi (Mycosphaerellaceae) from China 1. Phytotaxa 278: 212–224. 10.11646/phytotaxa.278.3.2 [DOI] [Google Scholar]
- Marley PS, Diourté M, Neya A, Nutsugah SK, Sérémé P, Katilé SO, Hess DE, Mbaye DF, Ngoko Z. (2002) Sorghum and pearl millet diseases in West and Central Africa. In: Leslie JF. (Ed.) Sorghum and millets diseases [based on contributions to the Third Global Conference on Sorghum and Millets Diseases in Guanajuato, Mexico, September 2000] (1st ed.). Iowa State Press, Ames, 419–425. 10.1002/9780470384923.ch70 [DOI]
- Messina MJ. (1999) Legumes and soybeans: overview of their nutritional profiles and health effects. The American Journal of Clinical Nutrition 70: 439s–450s. 10.1093/ajcn/70.3.439s [DOI] [PubMed]
- Meswaet Y, Mangelsdorff R, Yorou NS, Piepenbring M. (2019) A new species of Pseudocercospora on Encephalartos barteri from Benin. Asian Journal of Mycology 2: 101–109. 10.5943/ajom/2/1/4 [DOI] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE). IEEE, New Orleans, 8 pp. 10.1109/GCE.2010.5676129 [DOI] [Google Scholar]
- Montenegro-Calderón J, Martínez Álvarez J, Vieyra-Hernández M, Rangel-Macías L, Razzo-Soria T, Chávez-Herrera R, Ponce-Noyola P, Leal C. (2011) Molecular identification of two strains of Cercospora rodmanii isolated from water hyacinth present in Yuriria lagoon, Guanajuato, Mexico and identification of new hosts for several other strains. Fungal Biology 115: 1151–62. 10.1016/j.funbio.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Moreno-Rico O, Groenewald JZ, Crous PW. (2014) Foliicolous fungi from Arctostaphylos pungens in Mexico. IMA Fungus 5: 7–15. 10.5598/imafungus.2014.05.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder JL, Holliday P. (1975) Cercospora canescens. CMI descriptions of phytopathogenic fungi and bacteria 462.
- Nakamura T, Yamada KD, Tomii K, Katoh K. (2018) Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 34: 2490–2492. 10.1093/bioinformatics/bty121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima C, Motohashi K, Chen C-Y, Groenewald JZ, Crous PW. (2016) Species diversity of Pseudocercospora from Far East Asia. Mycological Progress 15: 1093–1117. 10.1007/s11557-016-1231-7 [DOI] [Google Scholar]
- Padulosi S, Ng NQ. (1990) Wild Vigna species in Africa: their collection and potential utilization. IITA, Ibadan, 58–77.
- Piątek M, Yorou NS. (2018) Pseudocercospora avicenniicola on black mangrove (Avicennia germinans) in Benin: The first report from Africa. Forest Pathology 49: e12478. [1–4.] 10.1111/efp.12478 [DOI]
- Piepenbring M, Maciá-Vicente JG, Codjia JEI, Glatthorn C, Kirk P, Meswaet Y, Minter D, Olou BA, Reschke K, Schmidt M, Yorou NS. (2020) Mapping mycological ignorance – checklists and diversity patterns of fungi known for West Africa. IMA Fungus 11: 1–13. 10.1186/s43008-020-00034-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Groenewald JZ, de Jesús Yáñez-Morales M, Crous PW. (2012) DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia – Molecular Phylogeny and Evolution of Fungi 29: 101–115. 10.3767/003158512X661282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, Barreto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW. (2013) Sizing up Septoria. Studies in Mycology 75: 307–390. 10.3114/sim0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, Summerell BA, Carnegie AJ, Burgess TI, Crous PW. (2014) Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia – Molecular Phylogeny and Evolution of Fungi 33: 1–40. 10.3767/003158514X681981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. (1878) Fungi italici autographice delineati a Prof. PA Saccardo. Patavii 1878. Fascicoli V-VIII sistentes tab. 161–320. Michelia 1: 326–350. [Google Scholar]
- Saccardo PA. (1886) Sylloge fungorum omnium hucusque cognitorum: Additamenta ad volumina I-IV. Sumptibus auctoris Typis seminarii.
- Shin HD, Kim JD. (2001) Cercospora and allied genera from Korea. Plant Pathogens of Korea 7: 1–302. [Google Scholar]
- Silva M, Barreto RW, Pereira OL, Freitas NM, Groenewald JZ, Crous PW. (2016) Exploring fungal mega-diversity: Pseudocercospora from Brazil. Persoonia – Molecular Phylogeny and Evolution of Fungi 37: 142–172. 10.3767/003158516X691078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan A. (1990) Mycosphaerella cruenta. IMI Descriptions of Fungi and Bacteria, 985 pp.
- Soura BH, Gnancadja LSA, Koita K, Gnancadja C. (2018) Distribution of Cercospora oryzae, the fungus causing Cercospora leaf spot or narrow brown leaf spot in southern Benin (advantages and constraints of rice production). Asian Journal of Science and Technology 9(11): 8986–8991. [Google Scholar]
- Spegazzini C. (1881) Fungi argentini additis nonnullis brasiliensibus montevideensibusque. Pugillus quartus (Continuacion). Anales de la Sociedad Científica Argentina 12: 193–227. [Google Scholar]
- Spegazzini C. (1910) Mycetes Argentinenses (Series V) Deuteromycetes. Anales del Museo Nacional de Buenos Aires 3: e364.
- Stamatakis A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. (2008) A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Świderska-Burek U, Daub ME, Thomas E, Jaszek M, Pawlik A, Janusz G. (2020) Phytopathogenic cercosporoid fungi – from taxonomy to modern biochemistry and molecular biology. International Journal of Molecular Sciences 21: e8555. 10.3390/ijms21228555 [DOI] [PMC free article] [PubMed]
- Talavera G, Castresana J. (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56: 564–577. 10.1080/10635150701472164 [DOI] [PubMed] [Google Scholar]
- Turner PD. (1971) Micro-organisms associated with oil palm (Elaeis guineensis Jaco.). Phytopathological Papers 14: 1–58. [Google Scholar]
- Vaghefi N, Shivas RG, Sharma S, Nelson SC, Pethybridge SJ. (2021) Phylogeny of cercosporoid fungi (Mycosphaerellaceae, Mycosphaerellales) from Hawaii and New York reveals novel species within the Cercospora beticola complex. Mycological Progress 20: 261–287. 10.1007/s11557-021-01666-z [DOI] [Google Scholar]
- Verkley GJM, Quaedvlieg W, Shin H-D, Crous PW. (2013) A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305. 10.3114/sim0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, Shin HD, Crous PW. (2016) All that glitters is not Ramularia. Studies in Mycology 83: 49–163. 10.1016/j.simyco.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, Braun U, Barreto RW, de Wit PJGM, Crous PW. (2017) Mycosphaerellaceae – Chaos or clarity? Studies in Mycology 87: 257–421. 10.1016/j.simyco.2017.09.003 [DOI] [PMC free article] [PubMed]
- Vu D, Groenewald M, Vries M de, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM. (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. 10.1016/j.simyco.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR Protocols: A Guide to Methods and Applications.Academic Press, New York, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Winter G. (1884) Repertorium. Rabenhorstii fungi europaei et extraeuraopaei. Cent. XXXI et XXXII. Hedwigia 23: 164–172. [Google Scholar]
- Wortmann CS, Kirkby RA, Eledu CA, Allen DJ. (1998) Atlas of Common Bean (Phaseolus vulgaris L.) Production in Africa. Centro Internacional de Agricultura Tropical, Cali, Colombia, 133 pp. [Google Scholar]
- Yen JM, Lim G. (1980) Cercospora and allied genera of Singapore and the Malay Peninsula. The Gardens’ bulletin, Singapore 33: 151–263. [Google Scholar]
- Yen JM, Kar A, Das B. (1982) Studies on hyphomycetes from West Bengal, India. II. Cercospora and allied genera of West Bengal. 2. Mycotaxon 16: 58–79. [Google Scholar]
- Young E. (1916) Studies in Porto Rican parasitic fungi–II. Mycologia 8: 42–46. 10.1080/00275514.1916.12018861 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Checklist for cercosporoid fungi in West Africa
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
Checklist
Explanation note
This information is based on the checklist published by Piepenbring et al. (2020) and updated with new results from the present publication and some further publications.
References for the checklist for cercosporoid fungi in West Africa
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
text
Explanation note
References for the checklist for cercosporoid fungi in West Africa (Suppl. material 1).
A Bayesian phylogenetic tree inferred from ITS rDNA sequence data of cercosporoid species
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
phylogenetic
Explanation note
Nodes receiving Bayesian PP ≥ 0.94 are considered as strongly supported and are indicated by thickened branches. Newly described species are denoted in bold and red text, newly reported species are indicated in blue text.
A Bayesian phylogenetic tree inferred from tef1 DNA sequence data of cercosporoid species
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Yalemwork Meswaet, Ralph Mangelsdorff, Nourou S. Yorou, Meike Piepenbring
Data type
phylogenetic
Explanation note
Nodes receiving Bayesian PP ≥ 0.94 are considered as strongly supported and are indicated by thickened branches. Newly described species are denoted in bold and red text, newly reported species are indicated in blue text.
Data Availability Statement
The specimen data is available through the Dryad Digital Repository https://datadryad.org/ (https://doi.org/10.5061/dryad.73n5tb2x9).