Abstract
Background
Angiotensin receptor blockers (ARBs), such as telmisartan, have been postulated to treat Covid-19-induced lung inflammation.
Methods
This is a parallel-group, randomized, two-arm, open-label, adaptive, multicenter superiority trial with 1:1 allocation ratio. Participants included patients from 18 years of age hospitalized with Covid-19 with 4 or fewer days since symptom onset enrolled at a university and a community hospital in Buenos Aires, Argentina. Exclusion criteria included prior intensive care unit (ICU) admission and use of ARBs/angiotensin converting enzyme inhibitors at randomization. Control arm received standard care alone and treatment arm telmisartan 80 mg twice daily for 14 days. Primary outcomes were C-reactive protein (CRP) plasma levels at day 5 and 8 after randomization. Secondary outcomes included time to discharge within 15 days, admission to ICU and death at 15- and 30-days. NCT04355936 (Completed).
Findings
A pragmatic decision to end the study before the third interim analysis was made on Oct. 30th due to sharp reduction in recruitment. A total of 162 patients were randomized. 158 patients enrolled between May 14 and October 30 2020, were included in the analysis, 80 in the standard care and 78 in the telmisartan added to standard care group. Baseline absolute CRP serum levels were 5.53 ± 6.19 mg/dL (95% CI 6.91 to 4.15, n = 80) and 9.04 ± 7.69 (95% CI 9.04 to 10.82, n = 74) in the standard care and telmisartan added to standard care groups, respectively. Day 5 control-group CRP levels were 6.06 ± 6.95 mg/dL (95% CI 7.79–4.35, n = 66) while telmisartan group were 3.83 ± 5.08 mg/dL (95% CI 5.08–2.59, n = 66, p = 0.038). Day 8 CRP levels were 6.30 ± 8.19 mg/dL (95% CI 8.79–3.81, n = 44) and 2.37 ± 3.47 mg/dL (95% CI 3.44–1.30, n = 43, p = 0.0098) in the control and telmisartan groups, respectively (all values expressed as mean ± SD). Kaplan-Meier analysis showed that telmisartan-treated patients had a lower median time-to-discharge (control=15 days; telmisartan=9 days). Death by day 30 was reduced in the telmisartan-treated group (control 22.54%, 16/71; telmisartan 4.29%, 3/70 participants; p = 0.0023). Composite ICU, mechanical ventilation or death was reduced by telmisartan treatment at days 15 and 30. No adverse events were reported.
Interpretation
Our study suggests that the ARB telmisartan, a widely used antihypertensive drug, is safe and could reduce morbidity and mortality in hospitalized patients infected with SARS -CoV-2 by anti-inflammatory effects. Further studies employing telmisartan are needed for confirmation of our results and to define its true therapeutic value as a tool against Covid-19.
Keywords: Covid-19, ARB, Telmisartan, ACE2
Research in context.
Evidence before this study
SARS-CoV-2 virus enters the airway and binds through protein S (Spike) to the Angiotensin Converting Enzyme 2 (ACE2) of alveolar cells and by endocytosis, internalizes, losing its function transforming angiotensin II into angiotensin 1–7. Consequently, it increases the tissue concentration of angiotensin II (pro-inflammatory stimulating AT1 receptors) and decreases that of angiotensin 1–7 (anti-inflammatory stimulating MAS receptors). Previous experimental studies showed that the classical ACE inhibitor drugs and angiotensin II AT1 receptor blockers increased the expression of ACE2 and it was immediately postulated that they could be harmful, favoring the entry of SARS-CoV-2 and the severity of COVID-19. On the other hand, the opposite was hypothesized considering that these drugs may be beneficial in COVID-19 by antagonizing either the production of angiotensin II or its pro-inflammatory effect via AT1 receptors. We adhere to this last hypothesis and we think that their evaluation in a clinical trial would be possible by choosing a pharmacologically adequate tool (telmisartan), using effective doses (160 mg / day), at an early stage of the disease (≤4 days from the onset of symptoms) in hospitalized patients not admitted to intensive care.
Added value of this study
The marked reduction in serum concentrations of the inflammatory biomarker C-reactive protein (CRP) and the important beneficial clinical effects reducing morbidity and mortality may constitute a significant contribution to the restricted therapeutic arsenal in the face of the COVID-19 pandemic. Furthermore, they represent evidence in favor of the involvement of the Renin Angiotensin System (RAS) in the development of the inflammatory process in COVID-19 patients. However, further studies employing telmisartan are needed for confirmation of our results and to define its true therapeutic value as a tool in Covid-19.
Implications of all the available evidence
Our study suggests that the ARB telmisartan, a widely used antihypertensive drug, is safe and could reduce morbidity and mortality in hospitalized patients infected with SARS -CoV-2 by anti-inflammatory effects. Further studies on telmisartan are needed for confirmation of our results and to define its therapeutic value against COVID-19.
Alt-text: Unlabelled box
1. Introduction
The SARS-CoV-2 virus enters the airway and binds the host cell (alveolar type 2) through the interaction of the structural protein S (Spike) with the protein-membrane ACE2 (angiotensin-converting enzyme 2) [1]. The virus-ACE2 complex is internalized by endocytosis effectively sequestering (apparent down-regulation) ACE2 which in turn loses its function catalyzing the degradation of angiotensin ll to angiotensin 1–7. Angiotensin II acting on AT1 receptors causes vasoconstriction, apoptosis, proinflammatory effects, and fibrosis [2,3]. Angiotensin 1–7 acting on Mas receptors causes opposite effects: it mediates vasodilation and anti-inflammatory actions [4]. Coronavirus disease 2019 (Covid-19), the disease caused by SARS-CoV-2, is associated with respiratory-related morbidity and mortality, as well as elevation of systemic inflammatory biochemical markers [5]. Among them, one of the most relevant is C-reactive protein (CRP) whose serum levels can be used as an independent factor to predict the disease severity and progression [6,7].
Elevation of angiotensin II in other tissues seems to play a role in promoting inflammation and tissue injury (atherosclerosis, myocarditis, renal injury, etc.) [2]. The hypothesis of the involvement of the renin angiotensin system in the inflammatory process triggered by the entry of SARS-CoV-2 into the tissues (lung in first place) considers that the down regulation of ACE2 causes an imbalance which results in an elevation of tissue concentrations of angiotensin II (pro-inflammatory) and a concomitant reduction in angiotensin 1–7 (anti-inflammatory) [8], [9], [10]. This imbalance could induce the development of AT1 receptor-dependent processes, leading to the release of proinflammatory cytokines [6,11], triggering a cascade leading, in severe cases, to Acute Respiratory Distress Syndrome (ARDS) [12].
Among these, IL-6 is particularly relevant since its plasma levels are directly related to the severity of Covid-19 and also induces the CRP gene increasing its production [6,13]. In Covid-19 patients, Liu et al. demonstrated that plasma angiotensin II levels were positively correlated with viral load and lung injury [6]. In line with these results, in Covid-19 patients, Villard et al. showed that plasma levels of aldosterone and CRP at admission were significantly higher in patients with a severe clinical course than those with a mild or moderate clinical course [14]. Considering that aldosterone is synthesized in the adrenal cortex in response to angiotensin II through the stimulation of AT1 receptors, these results strongly suggest that they correspond to an increase in plasma levels of angiotensin II.
Angiotensin receptor blockers (ARBs), a well-known anti-hypertensive drug group that blocks the AT1 receptor, have been postulated as tentative pharmacological agents to treat Covid-19-induced lung inflammation [8]. Data from retrospective studies from Covid-19 patients have provided some evidence to support that hypothesis [15], [16], [17], [18], [19], [20]. However, no conclusive data from a prospective randomized trial on the use of ARBs on Covid-19 patients are available. A pharmacological analysis conducted by Rothlin et al. [21,22] suggested telmisartan as the best candidate to study. Therefore, this study aims to assess whether telmisartan 80 mg twice daily would be effective in reducing lung inflammation and CRP levels at 5 and 8 days of treatment in Covid-19 hospitalized patients.
2. Methods
2.1. Ethics
The study protocol and its modifications were approved by the ethics committee of Hospital de Clínicas “José de San Martín”, Facultad de Medicina, Universidad de Buenos Aires (Argentina) and by the Institutional Review Board at Hospital Español de Buenos Aires (Argentina).
2.2. Study design
We conducted a two-arm, multicenter, randomized, open-label, adaptive, controlled trial at two academic hospitals in Ciudad Autónoma de Buenos Aires, Argentina: Hospital de Clínicas “José de San Martín” or site 1 (HCJSM, University of Buenos Aires main hospital) and Hospital Español de Buenos Aires or site 2 (HEBA, a community hospital). Placebo was not used due to logistical limitations in its provision. The ethics committee approved the protocol at HCJSM and the institutional review board at HEBA. The trial was funded by the participating hospitals. Laboratorio Elea Phoenix S.A. donated and supplied the trial drugs, provided funding for publication and provided administrative support for registration of this trial at www.ClinicalTrials.gov. The authors vouch for the completeness and accuracy of the data and the fidelity of the trial to the protocol. Trial protocol can be found as Supplementary File.
2.3. Participants
All the patients provided written informed consent before randomization. The trial included participants who were 18 years of age or older and who had been hospitalized with PCR-confirmed Covid-19 infection with 4 or fewer days elapsed since symptom onset. Exclusion criteria were: admission to Intensive Care Unit (ICU) prior to randomization, illness symptoms beginning more than 4 days before randomization, pregnancy, breast feeding, major hypersensibility to ARBs (e.g., anaphylaxis or angioedema), systolic blood pressure < 100 mmHg, serum potassium greater than 5.5 mEq/L, AST and/or ALT > 3 times the upper limit of normal, serum creatinine higher than 3 mg/dL, treatment with angiotensin-converting enzyme inhibitor (ACEi) or ARB at admission. Patients already receiving ACEi or ARB were excluded from the study as per protocol. Calcium channel blockers, beta blockers and/or diuretics were continued and no adjustment was made to these drugs.
2.4. Randomization and intervention
Patients were randomly assigned in a 1:1 ratio to receive standard care (control group) or standard care plus telmisartan 80 mg twice daily for 14 days or until discharge. Simple randomization was performed using the GraphPad QuickCalcs Web site by a statistician with no contact with patient care (MVS). LNN and MD accessed the randomization sequence and assigned participants to interventions immediately after independent enrollment by RG, MCR, FW, AA and JC. Patients who received plasma from convalescent patients were censored from the date of plasma administration onwards.
2.5. Outcomes
Reductions of C reactive protein levels at days 5 and 8 were chosen as the primary outcome. Secondary outcomes included admission to ICU within 15 and 30 days from randomization, occurrence of mechanical ventilation (MV) within 15 and 30 days from randomization, death within 15 and 30 days from randomization, composite occurrence of admission to ICU, MV or death within 15 and 30 days from randomization, proportion of patients not requiring supplemental oxygen at day 15, time from randomization to discharge up to day 15 from randomization, and significative differences in serum lactate dehydrogenase levels at day 5 and 8. All the trial outcomes were assessed by the site investigators, who were aware of the trial-group assignments. Database construction was carried out by FP and MVS. Curation was carried out by RR
2.6. Sample size calculation and protocol changes
For sample size calculations, we used our main outcome level as reference (CRP), and a repeated measures model. Calculations were done using the GLIMMPSE (General Linear Mixed Model Power and Sample Size) software [23], freely available at https://glimmpse.samplesizeshop.org/. We determined a 0.80 power and a type I error rate of 0.05, and chose the Hotelling Lawley Trace test. We assumed an initial CRP level of 6 mg/dL in both groups, with an elevation on day 5 in the control group (up to 7.2 mg/dL, 20% more) and a reduction in the telmisartan group to 3.6 mg/dL (40% less). We then assumed that the mean value decreased at day 8 in both groups. Initial standard deviation was set to 3.3 mg/dL. Accounting for variability on these assumptions, we used a scale factor of 0.5 for the mean and 2 for the standard deviation. Although CRP serum levels can be used as an independent factor to predict disease severity and progression [6,7], this level of reduction in CRP was a target difference. We obtained a total population sample of 390 participants (195 in each group), which we roughly approximated to 400 (200 in each group). No allowance was made to adjust sample size based on interim analysis.
Changes in protocol are shown in Table S1. The initial design included CRP level comparison at day 8 and 15. However, given the clinical evolution of the study population (i.e., median time to discharge in the control group) and the dynamics of CRP in Covid-19 patients, measurements were made at day 5 and day 8 to provide a more complete dataset since many patients would be discharged by day 15. Therefore, endpoints were reestablished at day 5 and 8. The composite occurrence of admission to ICU, MV or death between randomization and 15 and 30 days, proportion of patients not requiring supplemental oxygen at day 15 and time to discharge from randomization at 15 days were also added as secondary outcomes at that point.
2.7. Statistical analysis
This is a two-arm, open label, randomized trial testing a superiority hypothesis with a two-sided type I error rate of 0.05. Descriptive analysis was performed using the appropriate summary statistics (e.g., proportions for categorical data, means with 95% confidence intervals for continuous data, median for time-to-event data). Comparison of CRP and lactate dehydrogenase (LDH) levels at day 1, 5 and 8 were analyzed by fitting a mixed model.
Mean changes from baseline were analyzed using a restricted maximum likelihood (REML)‐based repeated measures with an alpha of 0.05. Sphericity was not assumed and the Geisser-Greenhouse correction was used. Analyses included the fixed, categorical effects of treatment, elapsed time of treatment and treatment-by-time interaction [24]. Multiple comparisons were carried out between CRP values at different treatment times and P-values corrected using statistical hypothesis testing using the Holm-Sidak's multiple comparisons test [25]. No allowance for dropout was made. Analysis of time to discharge was done calculating proportions using the Kaplan–Meier method, and the resulting curves were compared by a log-rank test. Differences in proportions (ICU, MV, death, need for oxygen supplementation at day 15) were compared by Fisher's exact test. Using multivariate logistic regression models, we assessed the association between 30-day mortality and age, gender, initial CRP, treatment arm, requirement of O2 at randomization, and presence of comorbidities (defined by the sum of one point per presence of hypertension, coronary heart disease, obesity, diabetes or chronic obstructive pulmonary disease [COPD]). In adjusted models, each independent variable was adjusted for all the others. Analyses were performed using GraphPad Prism version 8.4.3 (686) for Windows.
A first interim analysis was conducted on July 31st 2020 with 82 patients. A second interim analysis was conducted at recruitment of 140 patients on September 12th 2020. A third interim analysis was planned after accrual of 200 patients. Early stopping due to efficacy was defined as achieving significant differences between groups in our main outcome. To control across repeated analyses for Type I error, set at 0.05, critical values for interim testing were defined based on O'Brien-Fleming's boundaries. Interim analysis was carried out by FP and reviewed by RPR and MVS. None of these members took part in on site activities such as data gathering, enrollment and treatment. No data specific to data were discussed on how to manage the trial, manage individual study patients, or make study assessments was shared with onsite investigators. After the second interim analysis, a pragmatic decision was made by RPR, MD, FP and LNN to stop the trial at 162 patients (Oct 30th, 2020) due to a sharp decrease in patient recruitment.
Role of the funding source: The School of Medicine, University of Buenos Aires, provided material support through permission to use Hospital de Clínicas facilities to carry out the trial. Also, all biochemical assays at Hospital de Clínicas were carried out at its Central Laboratory Facility. Hospital Español de Buenos Aires provided material support through permission to use its facilities to carry out the trial. All biochemical assays at this site were carried out at the Central Laboratory Facility at Hospital Español. Laboratorios Elea Phoenix provided the telmisartan tablets used for the study, provided financial support for publishing fees, and assisted in submitting the registration of this trial to www.ClinicalTrials.com.
The sponsors had no role in the design of this study neither had access to the data nor any role during its execution, analyses, interpretation of the data, or decision to submit results. FP, MVS and RPR had access to the dataset. Decision to submit for publication was made by RPR, FP, MD and LNN.
3. Results
3.1. Characteristics of the participants
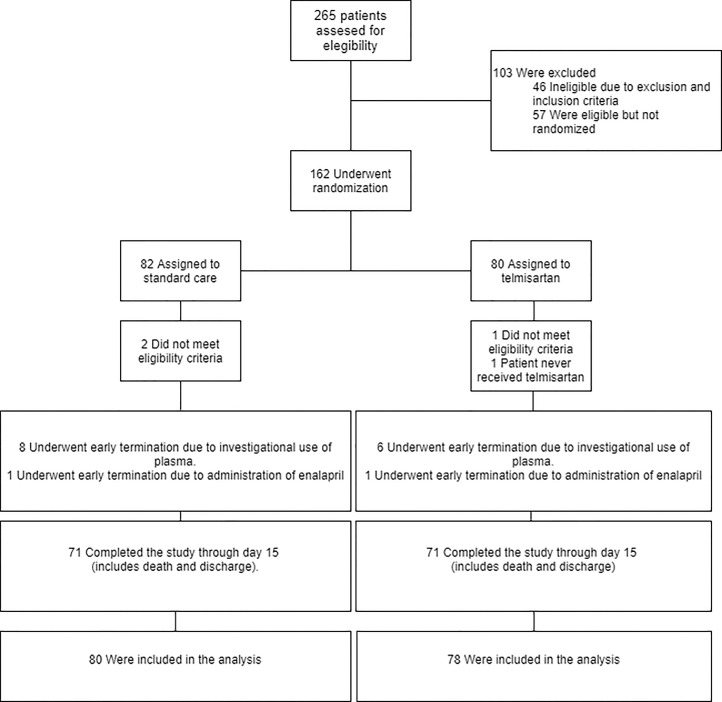
We recruited 162 participants with confirmed Covid-19. The numbers of enrolled patients were 107 and 55 at site 1 and site 2, respectively. A total of 80 patients were randomly assigned to receive telmisartan and 82 patients to receive standard care (control group) (Fig. 1). Four patients were excluded after randomization (3 patients met exclusion criteria and 1 patient did not receive the treatment). The first patient underwent randomization on May 14, 2020. No patients were enrolled after October 30th because of a sharp reduction in cases in Ciudad Autónoma de Buenos Aires, Argentina; Follow up finished on November 30th. At this stage, a pragmatic decision to end the study was made. The demographic and clinical characteristics of participants are depicted in Table 1. Results from first interim analysis were previously presented as a preliminary report [26]. A brief description of preliminary results can be found in the Supplementary Appendix.
Fig. 1.
Trial profile.
Table 1.
Demographic and clinical characteristics at baseline. COPD, Chronic Obstructive Pulmonary Disease; CRP, C-reactive protein; LDH, lactate dehydrogenase. ESR, Erythrocyte Sedimentation Rate; LMWH, low molecular weight heparin.
| Characteristic | Standard care | Telmisartan added to standard care |
|---|---|---|
| Age -yr | 66.9 ± 17.2 [n = 80] | 63.7 ± 17.0 [n = 78] |
| Female – no (%) | 45 (56.3) [n = 80] | 29 (37.2) [n = 78] |
| Coexisting conditions – no (%) | ||
| Hypertension | 35 (43.8) [n = 80] | 35 (44.9) [n = 78] |
| Beta blockers | 19 (23.8) [n = 80] | 16 (20.5) [n = 78] |
| Calcium channel blockers | 12 (15.0) [n = 80] | 12 (15.4) [n = 78] |
| Diuretics | 6 (7.5) [n = 80] | 6 (7.7) [n = 78] |
| COPD | 10 (12.5) [n = 80] | 8 (10.3) [n = 78] |
| Diabetes | 14 (17.5) [n = 80] | 16 (20.5) [n = 78] |
| Oral hypoglycemics | 8 (10.0 [n = 80] | 6 (7.7) [n = 78] |
| Insulin | 12 (15.0) [n = 80] | 13 (16.7) [n = 78] |
| Obesity | 8 (10.0) [n = 80] | 16 (20.5) [n = 78] |
| Dyslipemia | 12 (15.0) [n = 80] | 14 (18.0) [n = 78] |
| Stroke | 4 (5.0) [n = 80] | 7 (9.0) [n = 78] |
| Asthma | 3 (3.8) [n = 80] | 2 (2.6) [n = 78] |
| Chronic kidney disease | 0 (0) [n = 80] | 5 (6.4) [n = 78] |
| Clinical characteristics at admission | ||
| Required supplementary O2 - no (%) | 51 (66.2) [n = 77] | 55 (70.5) [n = 78] |
| Respiratory rate (bpm) mean ± SD |
19.7 ± 3.1[n = 36] | 19.4.5 ± 2.0 [n = 40] |
| CRP (mg/dL) median (Q1 to Q3) |
3.59 (1.27 to 6.23) [n = 80] | 6.53 (3.38 to 12.11 [n = 74] |
| Lymphocyte count (103/µL) median (Q1 to Q3) |
1.09 (0.79 to 1.49) [n = 76] | 1.04 (0.74 to 1.54) [n = 71] |
| Platelet count (103/µL) median (Q1 to Q3) |
214 (177 to 264) [n = 78] | 199 (140 to 297) [n = 71] |
| Neutrophil to lymphocyte ratio median (Q1 to Q3) | 2.91 (1.92 to 7.12) [n = 76] | 3.40 (2.29 to 8.07) [n = 71] |
| LDH (UI/L) median (Q1 to Q3) |
483.5 (375.5 to 565.0) [n = 72] | 513 (420 to 684) [n = 64] |
| ESR (mm/h) median (Q1 to Q3) |
40.0 (27.5–66.0) [n = 76] | 48.0 ± (27.0 to 84.0) [70] |
| D-Dimer (µg/mL) median (Q1 to Q3) |
0.97 (0.55 to 2.19) [n = 42] | 0.79 (0.45 to 1.50) [n = 37] |
| Ferritin (ng/mL) median (Q1 to Q3) |
505.0 (227.0 to 1247.0) [n = 38] | 775 (449.9 to 1479.5) [n = 36] |
| Standard care- no (%) | ||
| Dexamethasone | 41 (51.3) | 39 (50.0) [n = 78] |
| LMWH | 60 (75.0) | 56 (71.8) [n = 78] |
| Antibiotic therapy | 51 (63.8) | 55 (70.5) [n = 78] |
3.2. Primary outcomes
Baseline absolute CRP serum levels were 5.53 ± 6.19 mg/dL (95% CI 6.91 to 4.15, n = 80) and 9.04 ± 7.69 (95% CI 9.04 to 10.82, n = 74) in the standard care and telmisartan added to standard care groups, respectively (all values are expressed as mean ± SD). At day 5, patients in the telmisartan added to standard care group had a lower absolute CRP serum level than patients in the standard care group (standard care 6.06 ± 6.95 mg/dL, 95% CI 7.79 to 4.35, n = 66; telmisartan added to standard care 3.83 ± 5.08 mg/dL, 95% CI 5.08 to 2.59, n = 66, p = 0.038, Table 2 and Fig. S1a). Also, CRP serum levels were lower at day 8 in patients treated with telmisartan than those in the standard care group (control: 6.30 ± 8.19 mg/dL, 95% CI 8.79 to 3.81, n = 44; telmisartan: 2.37 ± 3.47 mg/dL, 95% CI 3.44 to 1.30, n = 43, p = 0.0098, Table 2 and Fig. S1a). Consistently, serum levels of day 5 and 8 expressed as percentage of day 0 are shown in Fig. S1b (day 5: standard care CRP 5.5 ± 122.2%, 95% CI 35.73 to −24.82, n = 66; telmisartan added to standard care −57.6 ± 56.2%, 95% CI −43.83 to −71.46, n = 66; day 8: standard care CRP 13.9 ± 148.2%, 95% CI 58.96 to −31.14, n = 44; telmisartan added to standard care CRP −73.82 ± 38.41%, 95% CI −62.00 to −85.64, n = 43, Fig. S1b). Effects of telmisartan on CRP levels of patients treated with and without dexamethasone are shown in Table S3.
Table 2.
Serum CRP levels in patients treated with telmisartan plus standard care and standard care alone at day 5 and day 8 after randomization. Data expressed as mg/dL.
| Standard care |
Telmisartan added to standard care |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean (mg/dL) | SD | 95% CI | n | mean (mg/dL) | SD | 95% CI | n | means difference (mg/dL) | Standard error of difference | P-value | |
| day 5 | 6.06 | 6.95 | 7.79 to 4.35 | 66 | 3.83 | 5.08 | 5.08 to 2.59 | 66 | 2.23 |
1.06 |
0.038 |
| day 8 | 6.30 | 8.19 | 8.79 to 3.81 | 44 | 2.37 | 3.47 | 3.44 to 1.30 | 43 | 3.93 | 1.34 | 0.0098 |
3.3. Secondary outcomes
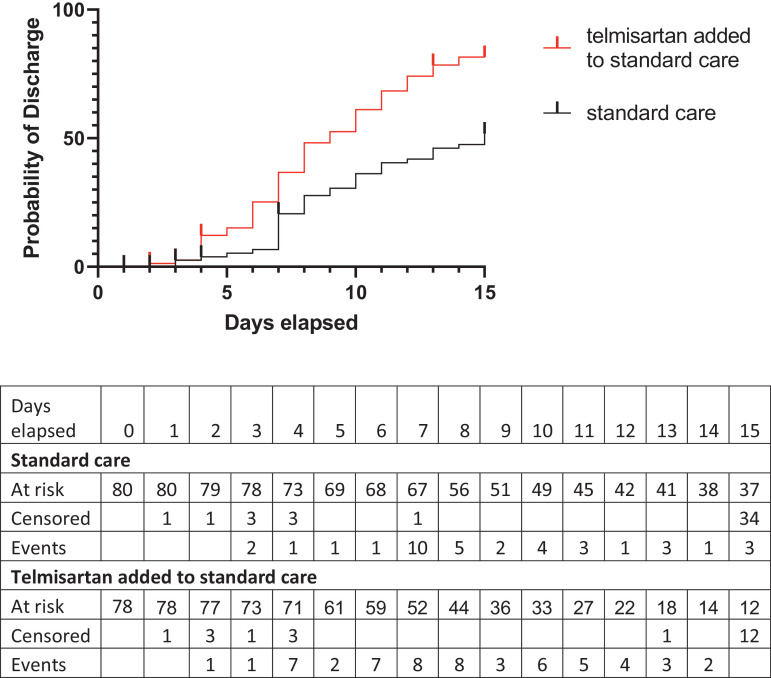
Results from 158 participants analyzed (78 assigned to telmisartan and 80 assigned to standard care) indicated that those who received telmisartan had a median discharge time of 9 days, as compared with 15 days in those who received standard care (log-rank (Mantel-Cox) p < 0.0001), the hazard ratio (log-rank) for discharge telmisartan/control 2.193 (95% CI, 1.46 to 3.31) (Fig. 2, Table 3). Effects of telmisartan on discharge probability of patients treated with and without dexamethasone are shown in Fig. S2. The proportion of inpatients not needing supplementary O2 at day 15 was higher in the telmisartan group (4 out of 6) than in control patients (1 out of 17; relative risk 2.82, 95% CI 1.309 to 9.765; p = 0.0078) (Table 3).
Fig. 2.
Probability of discharge up to day 15 after randomization in standard care (black) and telmisartan added to standard care treated participants (red). Vertical lines indicate censored points. Discharge curve and table were generated by the Kaplan–Meier method (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Table 3.
Clinical evolution at 15 days.
| control | telmisartan | |
|---|---|---|
| No. of hospital discharges by day 15 (%) | 37 (46.3) |
57 (73.1) |
| Median time to discharge (days) | 15 | 9 |
| Hazard ratio - discharge telmisartan/control (95% CI) | 2.193 (1.455 to 3.307) | |
| Proportion of hospitalized patients not needing supplementary O2 at day 15 | 1/17 | 4/6* |
p = 0.0078.
Occurrence of death by day 30 after randomization was reduced in the telmisartan-treated group (control 22.54%, 16 out of 71 participants; telmisartan 4.29%, 3 out of 70 participants; relative risk 5.26, 95% CI 1.741 to 16.42; p = 0.0023). Also, composite occurrence of ICU admission, MV or death was reduced by telmisartan treatment at day 15 (p = 0.025) and day 30 (p = 0.0058) after randomization (Table 4). No differences were observed in absolute and ΔLDH levels at days 5 or 8 between telmisartan and control groups (Fig. S3).
Table 4.
Death, intensive care unit (ICU) admission and mechanical ventilation (MV) in standard care and telmisartan added to standard care at 15 and 30 days after randomization.
| No of events/participants at risk (%) |
|||
|---|---|---|---|
| standard care | telmisartan added to standard care | Relative risk (95% CI) | |
| death by day 15 | 10/71 (14.08) | 3/70 (4.29) | 0.30 (0.09 to 0.97) |
| death by day 30 | 16/71 (22.54) | 3/70 (4.29) | 0.19 (0.06 to 0.57) |
| ICU admission by day 15 | 15/80 (18.75) | 6/78 (7.69) | 0.41 (0.17 to 0.97) |
| ICU admission by day 30 | 15/80 (18.75) | 6/78 (7.69) | 0.41 (0.17 to 0.97) |
| MV by day 15 | 4/80 (5.00) | 4/78 (5.13) | 1.03 (0.29 to 3.63) |
| MV by day 30 | 4/80 (5.00) | 4/78 (5.13) | 1.03 (0.29 to 3.63) |
| death, ICU admission or MV by day 15 | 21/80 (26.25) | 9/78 (11.54) | 0.44 (0.22 to 0.88) |
| death, ICU admission or MV by day 30 | 24/80 (30.00) | 9/78 (11.54) | 0.38 (0.19 to 0.75) |
No differences were observed in blood pressure, serum potassium, serum creatinine or blood urea nitrogen between telmisartan and control groups at day 5 nor day 8 (Tables 5, S4, S5 and S6). Hematological indices and additional biomarkers at day 5 and day 8 are shown in the Supplementary Appendix (Fig. S4 and Table S7).
Table 5.
Blood pressure measured in mmHg of enrolled patients at baseline, day 5 and day 8 after randomization by treatment group.
| Blood pressure | ||||||
|---|---|---|---|---|---|---|
| Baseline | Day 5 | Day 8 | ||||
| Systolic Mean ± SD (n) |
Diastolic Mean ± SD (n) |
Systolic Mean ± SD (n) |
Diastolic Mean ± SD (n) |
Systolic Mean ± SD (n) |
Diastolic Mean ± SD (n) |
|
| Standard care | 120.0 ± 14.5 (73) | 70.5 ± 8.1 (73) | 118.7 ± 10.8 (67) | 70.1 ± 7.2 (67) | 116.8 ± 13.7 (48) | 69.7 ± 8.9 (48) |
| Telmisartan added to standard care | 122.2 ± 11.32 (74) | 72.4 ± 9.1 (74) | 118.7 ± 12.0 (69) | 69.6 ± 7.1 (69) | 115.9 ± 13.4 (49) | 69.4 ± 8.6 (49) |
3.4. Multivariate analysis
An exploratory unadjusted and adjusted logistic regression models assessed the association of age, gender, initial CRP, control arm, number of major comorbidities and requirement of O2 at admission with the 30-days mortality outcome (Table 6). In these models missing data for specific variables reduced the sample size for each model by 7 (final n = 137). Participants were more likely to have died in the 30 days after admission if they were on the control arm (OR= 7.449; 95% CI 2.197 to 34.96).
Table 6.
Unadjusted and adjusted logistic regression model for death at 30 days.
| Death by day 30, OR (95% CI) |
||||
|---|---|---|---|---|
| Variable | Unadjusted | Adjusted | ||
| Age | 1.039 | (1.006 to 1.080) | 1.033 | (0.9969 to 1.077) |
| CPR at randomization | 1.012 | (0.9436 to 1.074) | 1.025 | (0.9459 to 1.104) |
| Standard care | 7.27 | (2.294 to 32.31) | 7.449 | (2.197 to 34.96) |
| Male | 0.7786 | (0.2941 to 2.014) | 1.095 | (0.3748 to 3.169) |
| O2 requirement at randomization | 1.675 | (0.6023 to 5.435) | 1.983 | (0.6380 to 7.093) |
| Sum of comorbidities | 1.184 | (0.7080 to 1.928) | 1.097 | (0.6051 to 1.945) |
4. Discussion
This randomized, two-arm, open, multicenter clinical trial suggested that an ARB therapy might be effective in treating Covid-19. Patients in the telmisartan group (80 mg twice daily) had a lower absolute CRP serum level than patients in the control group at both days 5 and 8 (primary outcome). Considering that baseline CRP was higher in telmisartan arm, treatment with the AT-1 receptor antagonist resulted in an inversion of CRP at days 5 and 8. In the present study, the differences observed in CRP plasma levels between telmisartan and control groups suggest an anti-inflammatory effect of the ARB. This effect may have been clinically relevant considering that patients with high CRP levels are more likely to have severe complications [27]. In line with this hypothesis, patients who received telmisartan added to standard care had a median discharge time of 9 days, compared with 15 days in those who received only standard care. Also, the proportion of inpatients not needing supplementary O2 at day 15 was higher in the telmisartan group than in the control group. More importantly, mortality at day 30 after randomization was reduced in the telmisartan-treated group.
To evaluate the involvement of RAS in systemic inflammation and clinical evolution of hospitalized Covid-19 patients, this protocol was designed using telmisartan, an AT1 receptor blocker [21,22]. The comparative analysis of the pharmacokinetic and pharmacodynamic properties between ARBs clearly distinguishes telmisartan as the best pharmacological tool to obtain a marked and permanent blocking effect of AT1 receptors, depending on the daily dose administered [22]. Telmisartan, which is well absorbed after oral administration, is the ARB with the longest plasma half-life (24 h) among its congeners (losartan 2 h, valsartan 6 h, candesartan 6 h, irbesartan 11–15 h and olmesartan 13 h) [28], promoting a concentration in the effector tissues with less variability between the intervals of each administration; it reaches the highest tissue concentrations due to its high lipid solubility and a high volume of distribution (500 L, markedly higher than those obtained by the remaining ARBs); and dissociates more slowly after binding to the AT1 receptor, causing an apparently irreversible block [28,29]. Furthermore, telmisartan is the only ARB that exhibits a partial agonist activity on Peroxisome Proliferator Activated Receptor gamma (PPAR-gamma) and different researches indicate that their activation has both anti-inflammatory and antifibrotic effects in many organs [30,31].
It has been shown that plasma angiotensin II [5] and aldosterone [14] levels are markedly elevated and are correlated with severity in Covid-19 patients. These findings add support to the rationale of a high dose approach for ARBs in Covid-19 (the higher the biophase concentration of agonist, the higher the antagonist dose). High dose scenario is possible because of the safety profile of this therapeutic class; ARBs are generally well tolerated, with no known class specific adverse events [32]. In this sense, previous studies support the use of "high" doses of telmisartan. Stangier et al. have shown that the incidence of adverse events was low (which were generally non-specific in nature and mild in intensity) in normotensive patients of all ages, even at high single doses of 160 mg i.v. or multiple doses (7 days) of 320 mg/day given orally [33]; Aranda et al. used 80 mg twice daily in non-diabetic hypertensive patients with nephropathies for 2 years, and observed excellent clinical and biochemical tolerability [34], and McGill et al., using 160 mg once daily for 8 weeks as monotherapy or in combination with hydrochlorothiazide, registered that the therapy was safe and well tolerated [35]. In addition to the results presented above, the effect of telmisartan on systolic and diastolic blood pressure on days 5 and 8 (Table 5) observed in our trial are similar to those published by Fogari et al. in hypertensive diabetic patients with microalbuminuria after 48 weeks under treatment with the association of telmisartan 160 mg/day plus amlodipine 2.5 mg [36]. On the other hand, in this study it was observed that the higher urinary albumin excretion rate for higher doses of telmisartan (160 mg/day) was independent of the reduction in systemic blood pressure (the maximum dose for this effect was 80 mg/day). The role of inflammation in chronic kidney disease pathogenesis and progression has been recognized since the late 1990s and microalbuminuria is an indication of an ongoing low-level inflammatory process. Aranda et al. [34] also observed that the decrease in proteinuria was more pronounced with "high" (80 mg twice daily) dose of telmisartan compared with "standard" (80 mg once daily). These results suggest that the maximal anti-inflammatory dose of telmisartan might be superior to its maximal recommended anti-hypertensive dose (80 mg/day), therefore providing additional support for the rationale of high dose telmisartan in our study (160 mg/day).
Main limitations of the study are the lack of blinding and placebo, the exclusion of ICU patients on randomization and the low number of enrolled patients. Another limitation is the restriction to patients with a relatively short time from symptom onset to randomization. It is possible that the clinical efficacy of the application of an ARB may be conditioned by the lapse between the start of the inflammatory process induced by the SARS-CoV-2 and the moment of its administration. Based on our hypothesis, the purpose of the protocol was to treat Covid-19 patients in the early stage of the development of the inflammatory process caused by the increase in tissue concentrations of angiotensin II. Moreover, since symptom reporting is highly subjective, we cannot rule out that some patients might have had a marginally longer disease course at randomization. However, we believe there are no differences between arms in this aspect. Further studies are needed to ascertain whether telmisartan effects are limited to this time window or if its use could be expanded to patients with longer disease course.
Also, patients randomized to telmisartan were more likely to be men and had higher CRP at baseline. These unbalances may have tempered the observed effect of telmisartan, since male sex has been identified as a risk factor for death and ICU admission [37]. Lastly, no prevision was made to account for potential operational bias introduced by a team familiar with the trajectory of the results after the first interim analysis. Therefore, further studies employing telmisartan are needed for confirmation of our results and to define its true therapeutic value as a tool in Covid-19.
In synthesis, the present results support the involvement of the RAS in the inflammatory process observed in hospitalized Covid-19 patients and suggests that the ARB telmisartan, a well-known inexpensive safe antihypertensive drug, administered in high doses, could reduce morbidity and mortality in hospitalized patients infected with SARS -CoV-2.
5. Data sharing
Data of individual participants that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be made available upon publication for 5 years at a third-party website (DOI: 10.5281/zenodo.3970223)
6. Role of contributors
Mariano Duarte Prof MD PhD: Conceptualization, investigation, supervision.
Facundo Pelorosso MD PhD: Data curation, formal analysis, writing - original draft.
Liliana N. Nicolosi Prof MD: Investigation, supervision.
M. Victoria Salgado MD PhD: Formal análisis, writing -review and edit.
Hector Vetulli MD: Conceptualization.
Analia Aquieri MD: Investigation.
Francisco Azzato Prof MD PhD: Resources.
Marcela Castro Bioq: Investigation.
Javier Coyle MD: Investigation.
Ignacio Davolos MD: Investigation.
Ignacio Fernandez Criado MD: Investigation.
Rosana Gregori MD: Investigation.
Pedro Mastrodonato MD: Investigation.
Maria C. Rubio MD: Investigation.
Sergio Sarquis MD: Investigation.
Fernando Wahlmann MD: Investigation.
Rodolfo P. Rothlin MD: Conceptualization, supervision, resources, writing original draft.
The corresponding author and guarantor, Rodolfo P. Rothlin, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Declaration of Competing Interest
Dr. Rothlin reports non-financial support from Laboratorio Elea, during the conduct of the study. All the other authors report no conflicts.
Acknowledgments
Acknowledgments
The authors would like to acknowledge Dr. Carlos R. Rojo, MD. for sharing our hypothesis and promoting institutional support for the conduction of this study. The authors would also like to thank Dr. Raúl Mejía (Centro de Estudios de Estado y Sociedad, Ciudad Autónoma de Buenos Aires, Argentina), Dr. Federico Daray (Institute of Pharmacology, School of Medicine, University of Buenos Aires) and Dr. Carla Rothlin (Dorys McConnell Duberg Professor of Immunobiology and Professor of Pharmacology, School of Medicine, Yale University) for the fruitful discussions and editing of this paper.
Funding
Facultad de Medicina (Universidad de Buenos Aires, Argentina), Hospital Español de Buenos Aires (Argentina) and Laboratorio Elea (Argentina).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100962.
Appendix. Supplementary materials
References
- 1.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):127–147. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dandona P., Dhindsa S., Ghanim H., Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21(1):20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso V.G., Gonçalves G.L., Costa-Pessoa J.M., Thieme K., Lins B.B., Casare F.A.M. Angiotensin II-induced podocyte apoptosis is mediated by endoplasmic reticulum stress/PKC-δ/p38 MAPK pathway activation and trough increased Na + /H + exchanger isoform 1 activity. BMC Nephrol. 2018;19(1) doi: 10.1186/s12882-018-0968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paz Ocaranza M., Riquelme J.A., García L., Jalil J.E., Chiong M., Santos R.A.S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17(2):116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan C., Huang Y., Shi F., Tan K., Ma Q., Chen Y. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;927:856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81(5):537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco R., Rivas-Santisteban R., Serrano-Marín J., Rodríguez-Pérez A.I., Labandeira-García J.L., Navarro G. SARS-CoV-2 as a factor to disbalance the renin–angiotensin system: a suspect in the case of exacerbated IL-6 production. J Immunol. 2020;205(5):1198. doi: 10.4049/jimmunol.2000642. [DOI] [PubMed] [Google Scholar]
- 10.Minato T., Hoshizaki, M., Yamaguchi, T., An, J., Niiyama, M., Nirasawa, S., Asaka, M.N., Fuk-Woo Chan, J., Imai, M., Takahashi, S., Utsumi, D., Kwok-Man Poon, V., Yasuhara, A., Chung-Sing Chan, C., Motoyama, S., Nagata, S., Penninger, J.M., Kamada, H., Yuen, K., Kawaoka, Y., Yasutomi, Y., Imai, Y., Kuba K. ACE2-like carboxypeptidase B38-CAP protects from SARS-CoV-2-induced lung injury. DOI 1021203/rs3rs-124634/v1. 2020.
- 11.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 13.Patra T., Meyer K., Geerling L., Isbell T.S., Hoft D.F., Brien J. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 2020;16(12) doi: 10.1371/journal.ppat.1009128. e1009128-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villard O., Morquin D., Molinari N., Raingeard I., Nagot N., Cristol J.-.P. The plasmatic aldosterone and C-reactive protein levels, and the severity of Covid-19: the dyhor-19 Study. J Clin Med. 2020;9(7) doi: 10.3390/jcm9072315. 2315- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirola C.J., Sookoian S. Estimation of renin-angiotensin-aldosterone-system (RAAS)-inhibitor effect on COVID-19 outcome: a meta-analysis. J Infect. 2020;81(2):276–281. doi: 10.1016/j.jinf.2020.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X., Zhu Y., Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension:a meta-analysis. Hypertension. 2020;76:e13–e14. doi: 10.1161/HYPERTENSIONAHA.120.15572. (Dallas, Tex: 1979) [DOI] [PubMed] [Google Scholar]
- 17.Lam K.W., Chow K.W., Vo J., Hou W., Li H., Richman P.S. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin ii receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222(8):1256–1264. doi: 10.1093/infdis/jiaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan F., Huang F., Xu J., Yang P., Qin Y., Lv J. Antihypertensive drugs are associated with reduced fatal outcomes and improved clinical characteristics in elderly COVID-19 patients. Cell Discov. 2020;6(1):77. doi: 10.1038/s41421-020-00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cippà P.E., Cugnata F., Ferrari P., Brombin C., Ruinelli L., Bianchi G. A data-driven approach to identify risk profiles and protective drugs in COVID-19. Proc Natl Acad Sci. 2021;118(1) doi: 10.1073/pnas.2016877118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baral R., Tsampasian V., Debski M., Moran B., Garg P., Clark A. Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.3594. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothlin R.P., Vetulli H.M., Duarte M., Pelorosso F.G. Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID-19. Drug Dev Res. 2020;81(7):768–770. doi: 10.1002/ddr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothlin R.P., Duarte M., Pelorosso F.G., Nicolosi L., Salgado M.V., Vetulli H.M. Angiotensin receptor blockers for COVID-19: pathophysiological and pharmacological considerations about ongoing and future prospective clinical trials. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.603736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreidler S.M., Muller K.E., Grunwald G.K., Ringham B.M., Coker-Dukowitz Z.T., Sakhadeo U.R. GLIMMPSE: online power computation for linear models with and without a baseline covariate. J Stat Softw. 2013;54(10):i10. doi: 10.18637/jss.v054.i10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Häckl S., Koch A., Lasch F. Empirical evaluation of the implementation of the EMA guideline on missing data in confirmatory clinical trials: specification of mixed models for longitudinal data in study protocols. Pharm Stat. 2019;18(6):636–644. doi: 10.1002/pst.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 26.Duarte M., Pelorosso F.G., Nicolosi L., Salgado M.V., Vetulli H., Aquieri A., et al. Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial. Preliminary report. medRxiv. 2020:2020.08.04.20167205-2020.08.04.
- 27.Chen W., Zheng K.I., Liu S., Yan Z., Xu C., Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19(1):18. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel M.C., Foster C., Brunner H.R., Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. 2013;65:809–848. doi: 10.1124/pr.112.007278. [DOI] [PubMed] [Google Scholar]
- 29.Kakuta H., Sudoh K., Sasamata M., Yamagishi S. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res. 2005;25(1):41–46. [PubMed] [Google Scholar]
- 30.Nobs S.P., Kopf M. PPAR-γ in innate and adaptive lung immunity. J Leukoc Biol. 2018;104(4):737–741. doi: 10.1002/JLB.3MR0118-034R. [DOI] [PubMed] [Google Scholar]
- 31.Villapol S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell Mol Neurobiol. 2018;38(1):121–132. doi: 10.1007/s10571-017-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacher H., Mancia G. The safety profile of telmisartan as monotherapy or combined with hydrochlorothiazide: a retrospective analysis of 50 studies. Blood Press. 2008;17(1):32–40. doi: 10.1080/08038020802144383. SUPPL. [DOI] [PubMed] [Google Scholar]
- 33.Stangier J., Su C.A.P.F., Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res. 2000;28(4):149–167. doi: 10.1177/147323000002800401. [DOI] [PubMed] [Google Scholar]
- 34.Aranda P., Segura J., Ruilope L.M., Aranda F.J., Frutos M.A., López V. Long-term renoprotective effects of standard versus high doses of telmisartan in hypertensive nondiabetic nephropathies. Am J Kidney Dis. 2005;46(6):1074–1079. doi: 10.1053/j.ajkd.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 35.McGill J.B., Reilly P.A. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther. 2001;23(6):833–850. doi: 10.1016/s0149-2918(01)80072-2. [DOI] [PubMed] [Google Scholar]
- 36.Fogari R., Derosa G., Zoppi A., Preti P., Lazzari P., Destro M. Effect of telmisartan–amlodipine combination at different doses on urinary albumin excretion in hypertensive diabetic patients with microalbuminuria. Am J Hypertens. 2007;20(4):417–422. doi: 10.1016/j.amjhyper.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.