Abstract
The human brain is a well-connected, intricate network of neurons and supporting glial cells. Neurodegenerative diseases arise as a consequence of extensive loss of neuronal cells leading to disruption of their natural structure and function. On the contrary, rapid proliferation and growth of glial as well as neuronal cells account for the occurrence of malignancy in brain. In both cases, the molecular microenvironment holds pivotal importance in the progression of the disease. MicroRNAs (miRNA) are one of the major components of the molecular microenvironment. miRNAs are small, noncoding RNAs that control gene expression post-transcriptionally. As compared to other tissues, the brain expresses a substantially high number of miRNAs. In the early stage of neurodegeneration, miRNA expression upregulates, while in oncogenesis, miRNA expression is gradually lost. Neurodegeneration and brain cancer is presumed to be under the influence of identical pathways of cell proliferation, differentiation and cell death which are tightly regulated by miRNAs. It has been confirmed experimentally that miRNA expression can be regulated by nutraceuticals - macronutrients, micronutrients or natural products derived from food; thereby making dietary supplements immensely significant for targeting miRNAs having altered expression patterns during neurodegeneration or oncogenesis. In this review, we will discuss in detail, about the common miRNAs involved in brain cancers and neurodegenerative diseases along with the comprehensive list of miRNAs involved separately in both pathological conditions. We will also discuss the role of nutraceuticals in the regulation of those miRNAs which are involved in both of these pathological conditions.
Keywords: Brain cancer, Neurodegenerative disease, miRNA, Nutraceuticals, Dietary supplements, miR-9, miR-34abc, miR-29, miR-124, Let-7, miR-128, Apigenin, Luteolin, Resveratrol
Brain cancer, Neurodegenerative disease, miRNA, Nutraceuticals, Dietary supplements, ,miR-9, miR-34abc, miR-29, miR-124, Let-7, miR-128, Apigenin, Luteolin, Resveratrol
1. Introduction
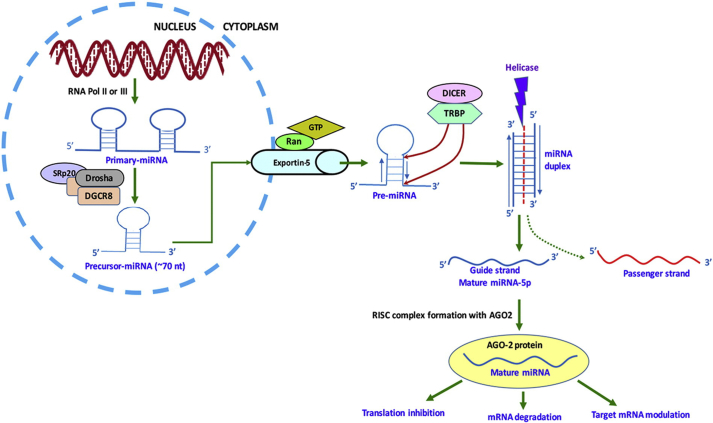
Over a decade ago was discovered a class of small non-coding RNAs of 19–22 nucleotide length, called Micro-RNAs or miRNAs (Lagos-quintana et al., 2001; Lau et al., 2001; Lee and Ambros, 2017) which express endogenously to modulate the expression pattern of several genes concerning various biological functions. Transcription of miRNAs occurs by the formation of a hairpin loop structure from long, single-stranded primary transcripts; with the hairpin being the signal for RNase cleavage. This cleavage results in a short hairpin precursor miRNA which ultimately gives rise to mature miRNA of 17–22 nucleotide length. Figure 1 schematically represents the bio-synthesis and mechanism of action of miRNA. Despite showing distinctive sequence homology and being highly conserved, many of the miRNAs exhibit striking differences in their transcription patterns. They regulate their target messenger RNAs (mRNAs) by rapid destabilization of mRNA and simultaneous repression of translation, thereby being potent regulators of gene expression. miRNAs are important regulators of cellular homeostasis as a single miRNA is capable of modulating hundreds of mRNAs (Lee and Ambros, 2017). These bind partially to the regions with complementarities at the 3′ untranslated region (UTR) of specific mRNAs and thus initiate the formation of inhibitory complexes (Carthew and Sontheimer, 2009). In addition, mechanisms of targeting 5’ UTR or coding sequences (CDS) have been reported as well (Eiring et al., 2010; Fabbri et al., 2012; I. Lee et al., 2009; Miranda et al., 2006; Ørom et al., 2008; Vasudevan et al., 2007).
Figure 1.
Schematic representation of miRNA synthesis and its general mechanism of action. Reprinted with permission from (Kumar and Reddy, 2016). Copyright (2019) Elsevier.
Many diseases target aging populations across the globe, among which the most dreadful are probably cancer and neurodegenerative diseases. Neurodegenerative diseases and cancer may seem very different from each other as in neurodegeneration, post-mitotic neurons die while, in cancer, resistance develops against cell death; but if the molecular genetics and cell biology of these two diseases are closely screened, it would depict a broad overlap between these two. The convergent causal factors of these diseases can be narrowed down to mutations in genes concerning the cell cycle, DNA repair pathways, oxidative stress, protein turnover and autophagy (Morris et al., 2010). Many of the recent discoveries about these diseases have broadened the knowledge about these two age-related conditions; which addresses the dire need for developing alternative therapeutic options for patients with advanced stages of the disease. Studying miRNA dynamics of the diseases has contributed to the advancement in research concerning the underlying molecular mechanisms responsible for their manifestation. miRNA-mediated regulation of gene expression is a potential controlling factor for both cancer and neurodegeneration (Cooper et al., 2009; Saito and Saito, 2012). Specific miRNAs have been found to regulate the initiation and progression of both diseases with the mechanism being the regulation of common pathways or targeting specific genes for each disease. Extensive study of the etiology of the two diseases have been of great significance concerning the development of potential therapies for these two diverse diseases, i.e., neurodegeneration and brain tumor and these include target-based therapies, e.g., neurotransmitter modulators, second messenger modulators, direct receptor agonists/antagonists, stem cell-based therapies, hormone replacement therapy, neurotrophic factors along with regulators of mRNA synthesis and translation into mutant proteins. (Connolly, 2014; Dunkel et al., 2012; Dye et al., 2012; Moraes, 2015; Moreno et al., 2013; Weissmiller and Wu, 2012; Wu et al., 2010; Young, 2009).
While the aforementioned strategies are of immense significance regarding the mitigation of neurodegeneration, they often induce long term adverse consequences (Dye et al., 2012; Morrish, 2012; Young, 2009), leading to the need for a safer alternative to fight the depleting neuronal conditions. A strikingly attractive option can be nutraceuticals/dietary supplements which can act upon multiple targets simultaneously, ensuring wholesome betterment of neuronal health (Essa et al., 2015; Pugh et al., 2015; Sharma et al., 2009; Tripathi and Jena, 2010; Trivedi & Jena, 2013, 2014). Since the last few years, researchers have been thriving over-targeting miRNAs associated with different neurodegenerative diseases and brain tumors by nutraceuticals and this review aims to record the current knowledge of the overlapping miRNAs which have been popularly correlated with the pathophysiology of the two, along with the mechanisms of control of miRNAs by nutraceuticals/dietary supplements.
1.1. Neurodegeneration
The mammalian brain is a complicated construction of billions of neurons and neuroglia working in a co-operative manner with intricate networking among themselves (Rajgor and Hanley, 2016). Disruption of the synaptic network may lead to neurodegeneration through structural and functional disintegration. In neurodegenerative diseases, brain function is progressively lost, and it gives rise to many syndromes with overlapping traits which are greatly influenced by genetic and environmental factors; e.g., deficiency of cognition is observed in a set of diseases, i.e., Alzheimer's disease (AD), Multiple Sclerosis (MS), vascular dementia, frontotemporal dementia (FTD), mixed dementia, and dementia with Lewy bodies (LBD). On a similar note, affected motor system can be found in amyotrophic lateral sclerosis (ALS), Parkinson's disease (PD), spinocerebellar ataxias (SCAs), and Huntington's disease (HD) (Reddy et al., 2010; Reddy and Beal, 2008; Reddy, 2014). These diseases have another factor in common, i.e., aging, which imparts a significant risk. With increasing life expectancy worldwide, the preponderance of these diseases is expected to increase, which may inflict a higher load of socioeconomic inconvenience over the patients, families and communities (Yang et al., 2013). In spite of having a broad range of clinical reflections including perturbation of neural web in certain regions of the brain, these diseases possess overlapping characteristics and mechanisms, among which are disruption of proteasome-ubiquitination mechanism, protofibril accumulation and assembling of misfolded proteins. Clinical manifestations of many slow-progressing neurodegenerative diseases involve excite-toxicity development, nitrosative and oxidative stress, injury of mitochondria, disruption of synaptic transmission and incompetence in axon and dendron transport, as reported by many researchers (Jellinger, 2010).
1.2. Brain cancer
The origin of brain cancer lies in Brain Tumor Initiating Cells (BTICs) which can lead to the development of a complete heterogeneous cancer (Singh et al., 2004). These cells can be differentiated from other cells based on stem-cell-like properties that they possess, as well as specific markers. Several types of brain cancers exist, among which Glioblastoma Multiforme (GBM) is considered as the most destructive one. GBM mean survival rate is only 3.3% and 1.2% at two and three years respectively (Furnari et al., 2007; Ohgaki et al., 2004; Singh et al., 2004). This disease is substantially lethal having an utterly low response to chemotherapy or radiation. The fact that even a single cell level also, GBM shows wide heterogeneity in cellular and genetic composition, makes it challenging to diagnose and treat this dreadful disease (Dirks et al., 2014; Friedmann-Morvinski, 2014; Patel et al., 2014). Despite GBM cells having striking phenotypes, genotypes and epigenetics, molecular analysis mostly converges to the protein-coding transcripts (Patel et al., 2014). Concerning GBM, the effects of non-coding RNAs have not been discovered sufficiently yet (Patel et al., 2014), although it has been reported that non-coding RNA (Du et al., 2013; Mineo et al., 2016) and not miRNA (Godlewski et al., 2017); may be used to distinguish between different subtypes of this disease and therefore may aid in understanding the complexity of the disease. Certain other types of brain tumors include medulloblastoma, atypical teratoid/rhabdoid tumors, pituitary adenoma, pilocytic astrocytoma and ependymoma.
2. miRNAs in brain
Several miRNA regulatory pathways are involved in the development of the human brain and its associated diseases. miRNA expression alters vigorously from cell to cell, which imposes the fact that the expression of miRNA is cell-specific and this specificity essentially confers to the mechanisms of miRNA/mRNA targeting (Godlewski et al., 2017; Li et al., 2018). miRNAome profiling is a significant way to analyze the function or the role of miRNAs in neural pathophysiology which in turn, can bring out remarkable information concerning neurodegeneration and oncogenesis. The brain is rich in miRNAs, with respect to other organs (Patel et al., 2014), which provides significant support to the fact that they regulate many functions of the brain in both pathological and physiological aspects. Extensive modulation of genes can be performed by miRNAs as they have the potential to target multiple mRNAs, i.e., a single miRNA can regulate the expression patterns of many mRNAs. MicroRNAome profiling promotes the cell/tissue-specific functions of them. The altered expression levels of miRNAs have been observed in neurodegenerative disorders as well as brain tumors. Table 1 represents the list of miRNAs that are separately involved in either brain cancers or neurodegenerative diseases.
Table 1.
Differentially regulated miRNAs in brain disorders.
| Disorder | miRNAs |
|---|---|
| Brain cancer | miR-9, miR-21, miR-17–92, Let-7, miR-10b, miR-34a, miR-7, miR-124-3p, miR-124-5p, miR-137, miR-326, miR-99a, miR-524-5p, miR-328, miR-128, miR-101, miR-302–367, miR-143, miR-145, miR-218, miR-93, miR-125b, miR-451, miR-222, miR-339, miR-148a, miR-181d, miR-210, miR-297 |
| Neurodegenerative disorders | miR-7, miR-9, miR-17-p, miR-21, miR-22, miR-26, miR-29, miR-30a-5p, miR-34, miR-101, miR-107, miR-124, miR-128, miR-133, miR-146, miR-153, miR-132, miR-155, miR-196a, miR-197, miR-210, miR-200a, Let-7, miR-221, miR-494, miR-512 |
MiRNAs are currently being extensively studied to figure out the mechanisms of regulation of genes by them. The fact that miRNAs can modulate neuroprotection and neurogenesis, makes them a very significant potential target for therapy (Lewis et al., 2010). However, the information concerning the underlying mechanism of degeneration is still limited as the best possible informers, i.e., the neuronal cells only, degenerate and die leaving no way to scrutinize them for our knowledge. On the contrary, cancer stem cells, being undifferentiated and having exclusive self-renewal capability, provide extensive source of information regarding the etiology of the disease. Hence, finding out the role of miRNAs in brain disorders and the mechanisms involved in it can lead to the identification of specific targets for therapeutic purposes, in both neurodegeneration and cancer (Saugstad, 2010). The acceleration in research regarding the significance of miRNAs on the pathophysiology of neurodegenerative disorders and cancer leading to the discovery of potential therapeutic targets (Kumar and Reddy, 2016; Vijayan et al., 2018, 2019); provides a promising alternative to conventional methods of diagnosis and treatment of the two. Table 2 lists some of the common miRNAs involved in both brain cancers and neurodegenerative diseases.
Table 2.
Common miRNAs involved in brain cancers and Neuro degenerative disorders.
In this review, we will mainly discuss the miRNAs listed in Table 2 as they are directly linked to cancers of the brain and neurogenerative disorders.
2.1. miR-9
DNA methylation at CpG islands holds pivotal importance in Chromatin remodelling and subsequent regulation of gene expression by inhibiting the expression of genes. Many non-coding RNA transcripts tend to get downregulated by this very event that occurs in the CpG island promoters. In case of miR-9 (miR-9 and miR-9∗), downregulation of expression occurs through this mechanism. This accounts for many types of cancers correlating with lowered miR-9 expression, thus conferring miR-9 to be a potential tumor suppressor (Silber et al., 2008). It runs a feedback loop to regulate the expression of genes controlling the proliferation and propagation of GBM cells. In case of glioblastoma, while the cancer stem cells propagate by calmodulin-binding transcription activator 1(CAMTA1), the non-stem cell lines are inhibited by miR-9. It has been reported that miR-9 reduces tumor growth and proliferation by targeting stathmin (STMN1). Cellular targeting of miR-9-STMN1, leads to controlled microtubule formation during cell cycle progression (Song et al., 2013). Since GBM has a complicated and heterogeneous cancer cell population, the primary findings still require validation from tests on a greater number of patient-derived cells having variable phenotypes and transcriptomes. Ectopic expression of miR-9/9∗ (also miR-124) within adult fibroblasts has been established to be responsible for extensive remodelling of chromatin structure. miR-9 mediated repression in EZH2 axis, leads to the opening of chromatin material to provide for RE1 Silencing Transcription Factor (REST) binding sites and thereby directs neuronal conversion (Lee et al., 2018). In accordance with its ability to function as a tumor suppressor, it has been observed that restoration of miRNA expression in glioma cells, driven by mutant epidermal growth factor receptor (EGFR) (Gomez et al., 2014), deregulates target forkhead box protein P1 (FOXP1) and thereby reduces tumorigenicity. It stops proliferation and promotes migration by targeting cyclic AMP response element-binding protein (CREB) and Neurofibrin 1 (NF-1) respectively (Carro et al., 2010; Nowek et al., 2018; Schraivogel et al., 2011; Tan et al., 2012). Expression of miR-9 is observed to be reduced in early HD, by targeting two constituents of the REST system, i.e., miR-9 targets REST while miR-9∗ targets CoREST (Packer et al., 2008). miR-9 controls neurogenesis in adults thereby providing a balance between the proliferation of neural stem cells and their differentiation. miR-9 targeting of SIRT1 and BACE1 has its consequences upon cell survival as well as oxidative response (Rostamian et al., 2018; Schonrock et al., 2012). Upregulation of miR-9 has been associated with PD and AD pathology with its pro-apoptotic functions. It has also been observed that miR-9 plays a pivotal role in the deterministic reprogramming of cells, from their undifferentiated state to functional neurons (Xue et al., 2016). Significantly, conversion of somatic cells towards neurons carries a great deal of potential, from the perspective of regenerative medicine (Xue et al., 2016). The mechanism of miR-9/9∗ directing neurodegeneration and oncogenesis shapes the context of these miRNA operations in the cell (Nowek et al., 2018).
2.2. miR-34abc
miR-34abc is a combination of three miRNAs namely miR-34a, miR-34b, miR-34c. Differential expression of all these miRNAs was reported in different brain pathologies. One of the most widely studied oncogenes is p53 which plays a critical role in neurodegenerative diseases as well, as reported by recent studies (Godar et al., 2008; Zilfou and Lowe, 2009). miR-34a is reported to be the potential target of p53. miR-34a is a tumor suppressor in nature and is involved in cell cycle arrest and apoptosis (He et al., 2004; Tazawa et al., 2007). In GBM, one of the most common types of brain cancers, the promoter region of miR-34a gets inactivated due to the hypermethylation of CpG island (Fabbri et al., 2015; Lodygin et al., 2008; Okada et al., 2014; Pichiorri et al., 2010; Silber et al., 2012). In GBM expressing platelet-derived growth factor receptor α, overexpression of miR-34a results in inhibition of cellular growth, while no such change is seen in case of EGFR.
miR-34a has also been reported to involved in AD. In AD, the neuronal cell cycle is disturbed. miR-34a targets cyclin D1 and prevents cell cycle re-entry to maintain the differentiated state of neurons (Modi et al., 2015). Modi et al. observed that treatment of neurotoxin amyloid β1–42 peptide (Aβ42) in cortical neuron cells diminishes the effect of miR-34a on cyclin D1, resulting in unexpected cell cycle re-entry which in turn results in apoptosis. This inactivation of miR-34a is mediated by MEK extracellular signal-regulated kinase (ERK) pathway (Modi et al., 2015).
Other members, i.e., miR-34bc, were reported to be involved in different pathological stages of PD (Miñones-Moyano et al., 2011; Saito and Saito, 2012). Dj1 and Perkin, two important genes that are involved in PD, were indirectly controlled by miR-34bc (Miñones-Moyano et al., 2011). In general, miR-34bc was downregulated in PD. In dopaminergic neurons downregulation of miR-34bc along with mitochondrial dysfunction caused cellular death (Shanesazzade et al., 2018). Considering the facts, miR-34abc could be a potent therapeutic target in different brain pathologies.
2.3. miR-29 family
miR-29 family of miRNAs (miR-29a, miR-29b, and miR-29c) is another important group taking a principal part in targeting de novo DNA methyltransferases namely DNMT3A and DNMT3B. With the reduction in the expression of miR-29, the methyltransferases get overexpressed. The expression of miR-29 is inhibited in both stem cells as well as differentiated cells. It has been reported that reduction in miR-29 expression may lead to random and aberrant DNA methylation in glioblastoma along with other cancers (Fabbri et al., 2007; Ru et al., 2016; Saito and Saito, 2012; Xu et al., 2015). One of the striking antitumorigenic activities of the cell is the prevention of de novo methylation of target genes. miR-29 targets PDPN gene-encoded membrane sialoglycoprotein podoplanin and thereby inhibits proliferation and propagation of glioblastoma (Cortez et al., 2010). miR-29 having tumor-suppressive function needs to be validated by cell-specific transcriptome profiling to scrutinize the difference between its anti-tumorigenicity and targeting of PTEN, a potent tumor suppressor (Tumaneng et al., 2012). Several researchers have found miR-29 to protect the cellular DNA from those aberrant mutations, i.e., protect the existing pattern of methylation and thereby suppressing tumorigenesis. The correlation between neurodegeneration and cancer is a debatable issue since it has been reported that the patients with neurodegenerative diseases tend to have less chances for cancer, while simultaneously there are reports stating that PD patients have greater risk of brain tumor, and some additional variations could lead to a full-blown malignancy (Godlewski et al., 2019). The mechanism of how PTEN is mutated in cancer and deregulated in neurodegeneration has evoked curiosity about their involvement in the two contrasting cellular fates (Ihle and Abraham, 2017).Both in case of AD and HD, downregulation of miR-29a/b was documented (Roshan et al., 2014). In AD, miR-29 deregulation was found to cause β-secretase 1 (BACE1) enzyme upregulation. This whole event leads to the development of plaques from amyloid precursor protein (APP) (Hebert et al., 2008). miR-29 is a significant factor in brain development as expression of it in the sympathetic nervous system prevents apoptosis and its downregulation leads to death of neuronal cells as well as ataxia (Roshan et al., 2014). Downregulation of miR-29 has also been observed in another disease called Spinocerebral ataxia 17 (SCA17) (Hebert et al., 2008). It is also observed to be extensively expressed in adult mouse brains in primary neurons (Jovic et al., 2013) and regulates cerebral and cortical maturation.
2.4. miR-124
miR-124 is majorly and explicitly expressed in all regions of the brain except the pituitary. According to reports, this miRNA is involved in neurodegeneration, brain cancer, synapse morphology, chronic stress and neurodevelopment (Hou et al., 2015; Soreq and Wolf, 2011). miR-124 helps in the establishment of neuronal identity by repressing hundreds of non-neuronal genes in neuron (Conaco et al., 2006; Lim et al., 2005) while inactivation of this leads to expression of non-neuronal genes (Soreq and Wolf, 2011). Improper balance of miR-124 creates several pathological conditions in the central nervous system. Expression of miR-124 significantly attenuated in brain tumors. Re-introduction of miR-124 in glioblastoma cells induces morphological changes by reducing proliferation, migration and loss of invasion property (Fowler et al., 2011; Silber et al., 2008). This miRNA mediates the differentiation and anti-cancer activity by regulating the expression of SCP1, PTPN12, SNAIL2, and ROCK1 (An et al., 2013; Conti et al., 2012). But the main activity of miRNA depends on tumor type and driver oncogene. In a study, Xie et al. demonstrated that miR-124 suppresses TWIST and SLUG genes to differentiate glioma cell lines, SWO-38 and U251 (Xie et al., 2012). In mice with intracranial xenograft tumors, miR-124 expression was found to be downregulated and upon re-expression of miR-124, tumor cell death and survivability of the mouse were enhanced. miR-124 exerts this activity by regulating TEAD1, MAPK14/p38and SERP1 (Mucaj et al., 2014). Adding to this report, miR-124 plays an important role in medulloblastoma, by acting as a tumor suppressor gene. Over expression of miR-124 inhibits the expression of CDK6 and prevents cellular proliferation in medulloblastoma (Mollashahi et al., 2019).
In case of neurodegenerative diseases, the involvement of miR-124 in AD, PD, HD was also reported. But the function of miR-124 is found to be very contrasting as compared to cancer. In neurodegeneration, its reduced expression induces cellular death. Overexpression of miR-124 induced AD characteristics like memory deficit, synaptic failure (Wang et al., 2018). miR-124 has also been reported to be involved in the processing of amyloid precursor proteins (Smith et al., 2011). In PD models, enhanced brain repair was observed by miR-124 loaded nanoparticles through modulation of neurogenic niche of the subventricular zone. In this case, miR-124 downregulated Sox9 and Jagged1, two stemness-related genes. Significantly, one of the pivotal factors concerned with neuronal death in the striatum of Huntington's patients is REST, a transcription factor that is primarily linked to miR-124 function. This confers to the potential of miRNA in the pathogenesis of HD (Sonntag et al., 2012). The expression of this miRNA was observed to be decreased in the patients diagnosed with epileptic seizures (Wang et al., 2016a, Wang et al., 2016b, Wang et al., 2016c). This miRNA also acts as a biomarker of intracerebral haemorrhage stroke (Wang et al., 2018). Taken together, current information indicates that modulation of miR-124 could be a promising therapeutic target against both brain cancer and neurodegenerative diseases.
2.5. miR-128
miR-128, another brain-dominating miRNA, is involved in neurogenesis and synaptogenesis during normal development of the brain (Franzoni et al., 2015). miR-128 drives the commitment of neural progenitor cells to differentiate into neurons (Bruno et al., 2011; Karam and Wilkinson, 2012). The expression of this miRNA is deregulated in pathologic conditions of the brain (Campbell and Booth, 2015). In case of aggressive brain tumors such as glioblastoma and medulloblastoma, miR-128 is found to be downregulated (Ciafrè et al., 2005; H. Li et al., 2013). In glioblastoma multiforme (GBM) the function of miR128 is completely lost. But according to studies, upon reintroduction of miR-128 in GBM cells, B lymphoma Mo-MLV insertion region 1 homolog (BMI1) gets downregulated and thus tumorigenicity and therapy-resistant ability are ameliorated (Peruzzi et al., 2013). This miRNA correlates to every sub-type of GBM and shows maximum downregulation in mesenchymal tumors (Rooj et al., 2017). Rooj et al. found miR-128 to target GBM sub class-specific mRNA and thus gain/loss of miR-128 contributes to the bidirectional transition between subclasses (Rooj et al., 2017). This property makes miR-128 an excellent therapeutic target for GBM. Several studies correlate the function of miR128 with normal development of the brain and loss of this leads to tumorigenesis (Franzoni et al., 2015; Mondal et al., 2015; Papagiannakopoulos et al., 2012; Wynder et al., 2005).
Deregulation of miR-128 has also been reported in case of neurodegenerative diseases. A comparative study with hippocampus of fetal, adult and AD brains showed differentially expressed miR-128 contributing to the dysfunction of neurons (Lukiw, 2020). In AD patients the expression of miR128 is found to be higher than the normal individuals (Geng et al., 2018; Tiribuzi et al., 2014). In AD patients, upregulation of miR128 in AD mononuclear cells is possibly the cause of lysosomal cathepsin (B, D, S) reduction and upregulated miR128 accounts for reduced Aβ degradation capacity of monocytes (Tiribuzi et al., 2014). This miRNA is also involved in PD since its overexpression ameliorated the progression of PD (Campbell and Booth, 2015). Zhou et al. showed that miR-128 protects dopaminergic neurons from apoptosis and negatively mediates the axis inhibition protein 1 (AXIN1) which is highly expressed in PD patients (Zhou et al., 2018). In HD model, the expression of miR-128 was shown to be downregulated in the frontal cortex of the brain (Campbell and Booth, 2015) while in multiple sclerosis its expression was found to be upregulated (Godlewski et al., 2019). miR-128 overexpressed in immune cells of the brain serves as a proinflammatory miRNA (Guerau-de-Arellano, 2011). The role of miR-128 is also found in neuropsychiatric disorders, such as fear, stress, anxiety, intellectual disability and movement disorder, e.g., epilepsy (Ching and Ahmad-Annuar, 2015; Davis et al., 2012; Lin et al., 2011; Marangi et al., 2013; Tan et al., 2014). Correlating the diseases with miR-128, depicted that a balanced expression of miR-128 should be maintained in physiological system and hence, this miRNA can be an excellent therapeutic target.
2.6. Let-7
Let-7 (lethal-7) is the first known miRNA in animals (Pasquinelli et al., 2000) and its sequence and function are highly conserved among different species (Pasquinelli et al., 2000; Pena et al., 2009). In both embryonic and adult brain, let-7 is extensively expressed and plays a pivotal role in the development and cell maturation (Fairchild et al., 2019; Kapsimali et al., 2007; Miska et al., 2004; Saba et al., 2008; Sempere et al., 2004). In normal condition, Let-7a, a member of let-7 family is reported to be involved in neural cell differentiation (Schwamborn et al., 2009) whereas let-7b has been observed to lower the self-renewal of aging neural stem cells by regulating high mobility group A (HMGA2) expression. Let-7 is also involved in embryonic retinal development through suppressing HMGA2 (Fairchild et al., 2019). The involvement of this miRNA is observed in several pathological conditions of the brain. Buonfiglioli et al. reported the tumor suppressor nature of let-7 miRNA in glioma. They observed that a specific set of let-7 miRNAs regulate the function of microglial cells and forestall glioma progression with the help of toll-like receptor 7 (Buonfiglioli et al., 2019). In another study, let-7 has been shown to prevent the progression of glioblastoma by inhibiting Ras, an oncogene involved in cellular growth, proliferation and invasion (Lee et al., 2011).
Let-7 has been reported to be involved in several neurodegenerative diseases. Spinocerebellar ataxia is a rare genetic neurodegenerative disorder. Wreckage of autophagy contributes to this disease. Let-7 is considered as one of the key regulators of autophagy with special relevance to polyglutamine disorder. Overexpression of let-7 in this disease activates autophagy and prevents the disease (Duarte et al., 2020). In AD, two sets of let-7 miRNAs, i.e., let-7b and let-7e get overexpressed and a high amount of these two miRNAs is found in the cerebrospinal fluid (CSF) of AD patients (Derkow et al., 2018). According to Lehmann et al., let-7 mi-RNA plays an unconventional role in neurodegenerative diseases (Lehmann et al., 2012). It activates TLR-7, a receptor involved in innate immunity and causes neurodegeneration by activating cell death pathways. Let-7 is also involved in cerebral ischemia and reperfusion. A specific set, i.e., let-7a is upregulated in cerebral ischemia and reperfusion (Wang et al., 2016a, Wang et al., 2016b, Wang et al., 2016c). Overall, these studies indicate that let-7 can be a potential therapeutic target and the inverse regulation of let-7 in brain cancer and neurodegenerative diseases suggests the opposite therapeutic approaches in both cases.
3. Nutraceuticals: regulation of miRNAs involved in brain pathology
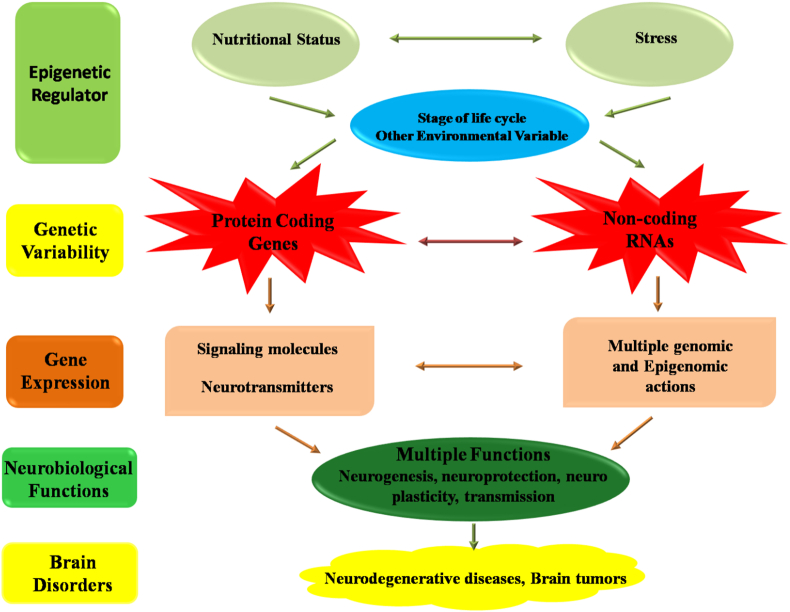
The underlying mechanisms responsible for brain disorders are quite complex. The evolution of genomics and epigenomics has helped researchers to understand these complex mechanisms (Bras et al., 2012; Dunham et al., 2012; Qureshi and Mehler, 2013; Sullivan et al., 2012). Several studies have shown that normal neuronal development and onset of neural diseases are linked via a very critical but intricate interaction between genetic and environmental factors like nutrition (Dauncey, 2012). Figure 2 represents the connection between nutritional status, stress and brain disorders. Throughout life, brain health, to some extent, is maintained by numerous diets, food and nutrition (Christian et al., 2009; Dauncey, 2009; Gomez-Pinilla and Nguyen, 2012; Maher, 2000; Milte et al., 2012; Sinn et al., 2012). Food habits or nutrition influences the development of neurons, their regeneration, functions and maintenance of neural network (Morris, 2012). Food or nutrition is one of the many regulators controlling the expression of genes without any modification in the DNA sequences. Recently, role of miRNAs in the development or disease conditions has gained the attention of researchers. Several studies reported that expressions of miRNAs, involved in different cancers or normal development, could be modulated by nutrition. Even the role of nutrition in regulating the normal nervous system development or associated problems, e.g., aging, neuro degenerative disorders were explored quite extensively. But reports regarding the regulation of miRNAs involved in brain pathology by nutraceuticals are majorly lacking. Very few reports have shown the interaction of miRNAs with nutraceuticals in the context of brain pathology. In this part of the review, we will summarize those nutraceuticals which have been reported to regulate expression of miRNAs involved in several brain pathologies, i.e., brain cancers and neuro degenerative disorders.
Figure 2.
Schematic representation of interconnection between nutritional factors, stress and neurological disorders. Reprinted from (Joy Dauncey, 2013), Copyright Under Creative Commons Attribution v3.0 International License (CC BY 3.0).
3.1. Vitamin A
The main metabolite of vitamin A is retinoic acid (RA). Several studies reported the neural cell differentiation property of RA. Many research groups have established the role of different miRNAs in differentiation of neural cells. Among all the miRNAs involved in neural cell differentiation, several are regulated by RA. One study showed that in RA-induced stem cell differentiation towards neural lineage, let-7 and hsa-miR-10 are overexpressed (Parsons et al., 2012). In RA-induced P19 cells, LIN28A, a highly conserved RNA binding protein, gets phosphorylated through a Let-7 dependent mechanism (Liu et al., 2017). Sirtuin 1 (silent mating type information regulation 2 homolog 1) is a negative regulator of differentiation. During differentiation it is inhibited by miR-34a which is overexpressed upon RA treatment, resulting in continuation of differentiation (Hu et al., 2014). On the contrary, miR-124 has been reported to be involved in inhibiting the differentiation of neurons. miR-124 reduces the RARγ expression within mouse brain and in P19 and N2a cells, resulting in inhibition of neurite extension (Morris, 2012; Wang et al., 2010). Hu et al. observed that treatment of RA in mesenchymal stem cells (adipose-derived) showed altered expression (>2-fold) of 76 miRNAs (Hu et al., 2017). RA treatment in glioblastoma cells enhances the gap junction resulting in overexpression of miR-124-3p, inhibiting its proliferation (Suzhi et al., 2015). MiR-302b and miR-452 are also reported to differentially regulate glioblastoma upon RA treatment (Chen et al., 2014; Liu et al., 2013). In neuroblastoma cells, several miRNAs get affected when treated with RA (Das and Bhattacharyya, 2014). RA treatment on neuroblastoma cells, upregulates miR-9 and miR-103, causing differentiation of the cells by attenuating the inhibitor of DNA-binding 2 (Id2) gene (Annibali et al., 2012). Brain-derived neurotrophic factor (BDNF) and RA treatment to SH-SY-5Y cells caused increased expression of miR-125b and miR-124a, resulting in its differentiation. They are also involved in the neurite extension of ReNcell VM, a human neural progenitor cell type (Le et al., 2009). Luu et al. reported a key role of miR-34a in mutant amyloid precursor protein-containing cells concerning neurite extension (Luu et al., 2019). In NT2 neuroblastoma, RA mediated differentiation causes downregulation of miR-302 whereas let-7, miR-125, -132,-128 are upregulated (Pallocca et al., 2013). miR-128 causes reduction of Reelin and Doublecortin, involved in migration of neural cells (Evangelisti et al., 2009). RA treatment in SK-N-BE neuroblastoma cells induces the expression of miR-34a, which in turn inhibits the E2F3 transcription factor involved in cell cycle progression. miR-34a in neuroblastoma cell lines activates the caspase-dependent apoptotic pathway to prevent cell proliferation (Welch et al., 2007). Apart from these miRNAs, several other miRNAs, like- miR-432, -29b, -664a-5p, -10a/b, -17 and -152 are separately reported by different groups depicting their involvement in differentiation of neuroblastoma cells in presence of RA (Beveridge et al., 2009; Das and Bhattacharyya, 2014; Das et al., 2010; Jauhari et al., 2018; Watanabe et al., 2018).In neurodegenerative disorders, e.g., AD; RA has been reported to tinker miRNA expression. Wang et al. found miR138 to be overexpressed in mutant amyloid precursor protein containing N2a cells. miR-138 directly binds to RARα causing its inactivation and thus promotes subsequent induction of GSK-3β, resulting in phosphorylation of tau (Wang et al., 2016a, Wang et al., 2016b, Wang et al., 2016c).
3.2. Vitamin C
Vitamin C is concerned with multiple biological processes, e.g., synthesis of neurotransmitters, hormones, and collagen. It is widely known that vitamin C deficiency causes scurvy. Its main chemical constituent is ascorbic acid and it is considered as a strong anti-oxidant having multiple favorable effects on the immune system, aging, lipoprotein metabolism and inflammation by regulating the mitochondrial and redox oxidative pathways (Naidu, 2003; Padayatty et al., 2003). It is also used for treating hypertension. Very limited investigations were conducted to check the regulation of brain miRNAs by vitamin C. In a study, ascorbic acid was shown to induce certain sets of miRNAs that negatively regulate post-transcriptional expression of several genes. In the same study it was observed that, after consumption of high dose of ascorbic acid, the expression level of miR-155 decreases, causing a significant anti-inflammatory response (Kim et al., 2015). However, several reports are present regarding miRNA modulation by Vitamin C in cellular differentiation and hormonal regulations. For example, in periodontal ligament cells, ascorbic acid treatment induces the expression of miR-146 which led to their differentiation (Hung et al., 2010). Lack of ascorbic acid, upregulates expression of let7 miRNA family, as seen in murine ovarian follicular cells, where oxidative stress was found to be escalated. These two miRNAs (Let7 and miR-146) play pivotal roles in brain pathology as well. Therefore, their regulation by ascorbic acid in brain pathologic conditions should be investigated thoroughly for therapeutic and diagnostic purposes.
3.3. Vitamin D
Vitamin D is a pro-hormone and steroid that can be taken through diet. It has different subtypes, e.g., vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Vitamin D can be synthesized by skin in presence of sunlight. The active vitamin D metabolite is calcitriol. The deficiency of vitamin D has been linked to several diseases, i.e., cardiovascular diseases, autoimmune diseases, some cancer and neurological disorders like multiple sclerosis. The role of vitamin D in regulation of miRNAs involved in multiple sclerosis has been explored by several groups. In a study by Zeitelhofer et al., 92 miRNAs were identified which were differentially regulated by vitamin D supplementation in CD4+ cells in experimental autoimmune encephalomyelitis (Zeitelhofer et al., 2017). This change in miRNA expression is concerned with T-cell receptor and IL-2 signaling pathways. Among the 92 miRNAs, miR-9-3p, -181b, -483, -134, -30c, -23a, -449c and -377 were observed to be upregulated in CD4+ T cells (Fenoglio et al., 2013; Ghorbani et al., 2017; Jernås et al., 2013; Zeitelhofer et al., 2017). These miRNAs are downregulated in multiple sclerosis patients. Upon vitamin D supplementation, these miRNAs get upregulated and inhibit the progression of multiple sclerosis. Among these miRNAs, miR-9-3p was reported to have a direct link with CNS development. This miRNA is mainly expressed in hippocampus of the brain (Sim et al., 2016). According to Sim et al., this miRNA is essential for the development of hippocampus. The expression of this miRNA regulates the long-term potentiation and memory of the brain. Thus, Vitamin D supplementation could be advantageous for pathological conditions of brain especially in multiple sclerosis as miR-9-3p is upregulated by vitamin D.
3.4. Vitamin B
Vitamin B is extremely important for one-carbon synthesis (methyl groups) which is required for cellular methylation reactions, e.g., DNA repair, methylation of DNA and protein synthesis. Its chemical constituents are folic acid and cobalamin, to name a few. Vitamin B may have an impact on miRNA expression level through methylation reaction. Deprivation of Vitamin B is linked to several diseases- depression, cardiovascular disease, malignancies of breast, colon and pancreas. Several miRNAs, for example- let-7a, miR-34a, -124, -15a/b, -16, -29a/b, -302 has been reported to be differentially regulated in folate deprived mouse embryonic stem cells (Liang et al., 2012). In an earlier study, methyl donor deficient diet was reported to cause hepatocellular carcinoma in rats. The results of this study indicated increased expression of Let-7a, miR-21, -130, -190 and downregulation of miR-34a, -16a, -127, -200b, -181 (Kutay et al., 2006). Among them, Let-7a and miR-34a are differentially regulated in different brain cancers and neurodegenerative diseases. Till date, to the best of our literature search, no reports have been documented regarding the regulation of expression pattern of these two miRNAs in presence or absence of vitamin B. But the expression pattern of three miRNAs, i.e., Let-7a, miR-34a and -23 in normal brain development were observed in absence of methyl donor folic acid by Geoffroy et al. (2019). From their results, no significant change in the expression for let-7a was found. However, the expression levels of miR-34a and -23 were significantly decreased which was completely reversed by folic acid supplementation (Geoffroy et al., 2019). This observation signifies the importance of folic acid in normal development and this demands further detailed analysis on the effect of vitamin B supplementation in various pathological conditions linked to the brain.
3.5. Vitamin E
Vitamin E is a fat-soluble vitamin and its main chemical constituent is tocopherol. It is involved in several biological processes. Vitamin E is reported to be an anti-epileptic agent (Ambrogini et al., 2018). The role of α-tocopherol in regulating miRNAs involved in epileptic seizures is well documented. It impacts epileptic seizures by regulating three miRNAs, namely miR-124, -126 and -146a (Ambrogini et al., 2018). According to the study by Ambrogini et al., in untreated epileptic rats, expression of miR-146a in the hippocampus is upregulated and the situation gets reversed when treated with α-tocopherol. miR-146a has been involved in the astroglia-mediated inflammatory response and it is overexpressed in epileptic patients, causing glioneuronal lesions (Iyer et al., 2012). The expression of miR-124 and 126 were opposite to that of miR-146a in epileptic rats. This situation was induced after the treatment of α-tocopherol. These altered expressions of miRNAs were observed only in presence of tocopherol, without an epileptic insult. This indicates the direct interaction of vitamin E with miRNAs which vouches for further investigation.
3.6. Dietary fat and fatty acid
Fat is an essential component of everyday diet but prolonged intake of fat-containing food without constant energy expenditure may lead to deposition of fat within the body. This affects the body adversely by causing co-morbidities, e.g., fatty liver (without alcohol consumption) and insulin resistance. It was reported previously that a high-fat diet regulates several miRNA expressions in different tissues like skeletal muscle, adipose tissue and brain. Alteration of miRNA expressions in different tissues due to high-fat diet was linked mainly to obesity. In case of the brain, hypothalamus plays a vital role in body weight homeostasis. This homeostasis is critically regulated by several miRNAs expressed in the hypothalamus. Alvarellos et al. identified a set of miRNAs that were differentially regulated in presence of high-fat diet. These miRNAs were miR-9, Let-7a, miR-200a, -132, -218, -30e and -145. These miRNAs have been reported to target several key inflammatory and metabolic pathways, i.e., leptin and insulin pathways (Sangiao-Alvarellos et al., 2014). Interestingly, these miRNAs, especially miR-9 and Let-7a, are also involved in normal brain development as well as pathologic conditions of the brain. Thus, regulation of dietary fat and fatty acid intake in everyday life may prevent or improve the pathologic conditions associated with the brain.
3.7. Olive oil
Olive oil is one of the major food elements in the Mediterranean diet. Consumption of olive oil has several health benefits. A large intake of virgin and total olive oil improves the memory retention capacity and cognition in aged individuals with high risk of cardiovascular diseases (Valls-pedret et al., 2012). Several phenolic components are present in olive oil, e.g., oleocanthal, tyrosol, oleuropein and hydroxytyrosol (Giovannelli, 2012). A study showed that β-amyloid degradation occurs upon treatment with oleocanthal, which makes it a potential treatment strategy against Alzheimer's disease (Pitt et al., 2009). It was also observed that olive oil phenols were found in the brain of experimental animals after consumption of olive oil (D'Angelo et al., 2001; Serra et al., 2012). Olive oil phenolics show improved cognitive and motor function in aged animal models (Farr et al., 2012; Pitozzi et al., 2012). In one study, regulation of brain miRNAs by olive oil was observed in aged mice (Luceri et al., 2017). Luceri et al. used H-EVOO, a commercially available olive oil, to feed experimental mice thereafter examined the miRNA expression pattern in aging conditions (Luceri et al., 2017). From their result, it was observed that H-EVOO fed mice showed downregulation of miR-27 and 137 which controls the NGFR, GLP1R and BMP expression that ultimately leads to improved motor function. Olive oil phenolics also downregulate the expression of miR-124 and -484 resulting in spatial memory enhancement. Contextual memory was also induced upon olive oil consumption which was linked to the downregulation of miR-34a-5p. In several studies, upregulation of the aforementioned miRNAs was found to be involved in aging-associated AD. Thus, the use of olive oil in a regular diet can improve the cognitive behavior of Alzheimer's patients resulting in a better lifestyle.
3.8. Krill oil and buttermilk
ω-3 fatty acid and polar lipids are required for proper functioning, activity and maintenance of the nervous system (Engelborghs et al., 2014). Loss of these from the brain with increasing age along with low intake is concerned with major risk of neurological disorder (Engelborghs et al., 2014). In this regard, krill oil is considered to be a rich source of ω-3 fatty acid which can be incorporated in phosphatidylcholine. Krill oil is extracted from shrimp Euphausia superba found in the Antarctic Ocean (“Krill Oil Monograph,” 2010). In addition, buttermilk, a by-product of butter containing high content of milk fat globule, is enriched with polar lipids especially phosphatidylserine and sphingomyelin. A combination of ω-3 fatty acid and polar lipids regulate the miRNA expression and their target genes in the hippocampus. A study reported that the treatment of krill oil+ Buttermilk modulated the expression of 11 miRNAs in the hippocampus. These major miRNAs included- miR-99a-5p, -148a-3p, -381-3p, 379-5p, -370- 3p, let-7f-5p, -128-3p, -146a-5p, -106b-3p, -30e-3p, and -770-3p. In presence of only buttermilk, miR-29a-3p and -191a-5p showed differential expression in rat model whereas miR-148a-3p, -379-5p, let-7f-5p, -99a-5p and -370-3p showed upregulation in rats consuming only krill oil (Crespo et al., 2018). These miRNAs are involved in synaptic transmission, axon guidance, signalling of neurotrophin, neural differentiation and migration and developmental process of the brain. Considering the effect of krill oil and buttermilk on miRNA expression in the hippocampus, incorporation of these two (in permissible concentrations) in the diet plan of patients with a neurological disorder can disrupt the progression of the disease.
3.9. Green tea
Tea is one of the most consumed beverages worldwide. Except for herbal tea, all types of tea are brewed from Camelia sinensis bush. The oxidation level of leaves determines the type of tea. Green tea is prepared from unoxidized leaves and is most unprocessed. Hence green tea is a rich source of anti-oxidant and polyphenols. In green tea (-) epigallocatechin (EGC) and (-) epigallocatechin-3-gallate (EGCG) polyphenol derivatives are the major constituents. EGCG is the most dominating polyphenol which has great anti-cancer properties and is found to be active against neuroblastoma (Golden et al., 2009). These polyphenolic components are observed to interact with miRNAs to exert their anti-cancer effect. In two consecutive studies by Chakrabarty et al., it was shown that EGC and EGCG differentially regulated six different miRNAs in neuroblastoma cell lines (Chakrabarti et al., 2012, 2013). They reported that upon treatment of these two polyphenols separately, three oncogenic miRNAs namely miR-92, -93 and -106b were downregulated. On the contrary, the three tumor suppressor miRNAs namely miR-7-1, 34a, and -99a were upregulated upon treatment with EGC and EGCG (Chakrabarti et al., 2012, 2013). These miRNAs are affected in several other types of brain tumors and neurodegenerative diseases. Therefore, green tea polyphenols may be helpful in other brain-related pathologies also.
3.10. Panax ginseng
Panax ginseng is perennial plant species whose root is considered as ginseng. It usually grows in the mountains of East Asia. Ginseng is a part of Chinese, Japanese, and Korean traditional medicine. Several secondary metabolites are found in ginseng. Among them, the most important metabolite is ginsenoside Rh2. Ginsenoside Rh2 is a triterpene saponin. It contains a steroid nucleus and sugar moiety. The ginsenoside Rh2 has several biological effects, i.e., prevention of ischemic brain injury, reduction of blood glucose level and anti-proliferative effect on glioma cells (Kim et al., 1999; Zeng and Tu, 2003). It also regulates the expression of miRNAs to exert its effect. Wu et al. showed that ginsenoside Rh2 upregulated the expression of several miRNAs, i.e., Let-7c/d, miR-125, -129, -181a/b/c, and -128. But the most prevalent change was observed in case of miR-128. Treatment of ginsenoside Rh2 in glioma cells resulted in upregulation of miR-128 and inhibited the proliferation of glioma cells. Induction of miR-128 inhibits glioma cell proliferation via activating caspase-3, inhibiting E2F3a activation at the transcriptional level (Wu et al., 2011). As these miRNAs are also functional in other types of brain diseases, the effect of ginsenoside Rh2 can be screened for those diseases as well.
3.11. Apigenin
Apigenin or 4′, 5,7-trihydroxyflavone is a flavonoid and is found in several plants. It is mainly found in tea leaves, vegetables, fruits and beans (Han and Chen, 2015). Several studies explored various biological properties of apigenin including anti-inflammation, anticancer, immunomodulatory, anti-oxidant, and antiviral properties (Palmieri et al., 2012; Zhang et al., 2016). Chen et al. explored the effect of apigenin on glioma cells (Chen et al., 2016). They found that treatment of apigenin inhibited cell proliferation and induced apoptosis in glioma cells and in particular upregulation of miR-16. This upregulation of miR-16 was found to reduce the growth of glioma cells through suppression of BCL2 and NF-κB signalling pathways. Therefore, apigenin could be used as a potential therapeutic choice for the cure and management of various sorts of brain disorders including brain cancer.
3.12. Luteolin
Luteolin or 3′,4’,5,7-tetrahydroxyflavone is a flavonoid and is found in vegetables, fruits and certain medicinal plants. It is reported to have several biological activities, i.e., anti-inflammation, anti-oxidant and anti-cancer activities (Hougee et al., 2005; Romanová et al., 2001; Seelinger et al., 2008). Several studies established luteolin as a neuroprotective agent (Cheng et al., 2008; Dirscherl et al., 2010; Pavlica and Gebhardt, 2010). In a study, luteolin was demonstrated to be a neurotrophic factor as it could promote neurite extension and differentiation in PC12 cells via ERK and PKC pathways (Cheng et al., 2008). Further in their study, they showed the involvement of miRNA (miR-132) in luteolin mediated differentiation of PC12 cells. Treatment of luteolin phosphorylates ERK which in turn phosphorylates CREB. CREB translocate to the nucleus and binds to CRE and mediates the synthesis of pre-miR-132 and upregulates its expression. This helps in neurite extension and differentiation of nerve cells. As miR-132 is upregulated by the treatment of luteolin which in turn results in the differentiation of nerve cells, luteolin mediated differentiation therapy can be explored as a potential therapeutic option against different types of brain cancer.
3.13. Resveratrol
Resveratrol or 3,5,4′-trihydroxytrans-stilbene is an edible natural polyphenolic phytoalexin. It is found in peanuts, red wine, grapes and several other food sources. It has extraordinary medicinal potential. Its role is established in preventing several age-related diseases, i.e., cancers, neurodegenerative diseases and cardiovascular diseases (Albani et al., 2010; Borriello et al., 2010; Harikumar and Aggarwal, 2008). It also has anti-inflammatory and anti-oxidant properties (Baur and Sinclair, 2006; Saiko et al., 2008). Its pathophysiological role in brain cancer, i.e., glioblastoma and neurodegenerative disease like AD is well established (Baur and Sinclair, 2006; Kiskova et al., 2020). Several studies reported that miRNAs are deregulated in glioblastoma or AD and their expressions can be regulated by resveratrol. In glioblastoma, miR-21 gets overexpressed which is known to impart antiapoptotic activity. It inhibits LRRFIP1 and inhibits the synthesis of LRRFIP1 which in turn attenuates the expression of NF-κB (Chan et al., 2005; Li et al., 2009). Downregulation of miR-21 showed reduced cellular proliferation and induced apoptosis in glioblastoma cells (Corsten et al., 2007). Li et al. showed that treatment with resveratrol downregulated the expression of miR-21 resulting in reduced proliferation and escalated apoptosis in glioblastoma cells (Li et al., 2013). Not only in glioblastoma, resveratrol also has its impact on regulating miRNAs involved in AD. In AD, miRNAs, like miR-146a, -155, -21 and -125b have been reported to be upregulated which leads to neuroinflammation. miR-15b is involved in tau-hyperphosphorylation in AD. Furthermore, miR-9, -29c, -186, -107 and -29a get downregulated in AD and affect BASE1 (β-site APP cleaving enzyme 1 or β-secretase) activity. BASE1 is currently the focus of AD because this protease enzyme is involved in abnormal production of Aβ plaques. Expression of all of these miRNAs gets reversed upon treatment of resveratrol, resulting in reduced disease progression. Resveratrol is reported to be effective against both brain cancer and other neurological disorders. Therefore, it can be a good therapeutic option against a variety of brain pathologies.
4. Conclusions
In this review, an attempt was made to decipher the role of various miRNAs in brain pathologies and their regulation by nutraceuticals. Normal brain status can be distinguished from pathologic condition by the differential expression of miRNAs. Few miRNAs are commonly involved in brain tumors and neurological disorders but they act in a contradictory manner in both the pathological conditions. For example, miR-124 prevents cell death in AD whereas induces cellular death in glioma. Likewise, miR-128 gets upregulated in AD and multiple sclerosis whereas the same miRNA is completely lost in glioblastoma. Apart from the differential expression, these miRNAs target several genes and regulate their expression. This unique property makes them potential candidates for therapeutic interventions. In the last few years, research showed substantial pieces of evidence regarding miRNA modulation by diet or nutrition in different tissues either in normal or in diseased conditions. But very few evidences are present regarding the nutrition or diet-mediated regulation of brain-related miRNAs both in vitro and in vivo. In this review, we summarized most of the nutritional components reported so far, which are involved in the regulation of brain pathology-related miRNAs. However, more in vitro and in vivo studies in cellular and animal models of brain cancers and neurological disorders need to be performed to explore the regulatory role of new nutritional components against various miRNA targets. This will provide substantial proof of the dietary regulation of miRNAs in the context of brain carcinomas as well as a plethora of neurological diseases.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the Indian Council of Medical Research (ICMR) (5/3/8/293/2015-ITR).
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would also like to acknowledge the infrastructure provided by Indian Institute of Technology Roorkee for their research activities in this field.
References
- Albani D., Polito L., Forloni G. Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: experimental and genetic evidence. J. Alzheim. Dis. 2010;19(1):11–26. doi: 10.3233/JAD-2010-1215. [DOI] [PubMed] [Google Scholar]
- Ambrogini P., Albertini M.C., Betti M., Galati C., Lattanzi D., Savelli D., Di Palma M., Saccomanno S., Bartolini D., Torquato P., Ruffolo G., Olivieri F., Galli F., Palma E., Minelli A., Cuppini R. Neurobiological correlates of alpha-tocopherol antiepileptogenic effects and MicroRNA expression modulation in a rat model of kainate-induced seizures. Mol. Neurobiol. 2018;55(10):7822–7838. doi: 10.1007/s12035-018-0946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L., Liu Y., Wu A., Guan Y. microRNA-124 inhibits migration and invasion by down- regulating ROCK1 in glioma. PloS One. 2013;8(7):2–11. doi: 10.1371/journal.pone.0069478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annibali D., Gioia U., Savino M., Laneve P., Caffarelli E., Nasi S. A new module in neural differentiation control: two microRNAs upregulated by retinoic acid, miR-9 and -103, target the differentiation inhibitor ID2. PloS One. 2012;7(7):1–12. doi: 10.1371/journal.pone.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Beveridge N.J., Tooney P.A., Carroll A.P., Tran N., Cairns M.J. Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cell. Signal. 2009;21(12):1837–1845. doi: 10.1016/j.cellsig.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Borriello A., Cucciolla V., Della Ragione F., Galletti P. Dietary polyphenols: focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutr. Metabol. Cardiovasc. Dis. 2010;20(8):618–625. doi: 10.1016/j.numecd.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Bras J., Guerreiro R., Hardy J. Use of next-generation sequencing and other whole-genome strategies to dissect neurological disease. Nat. Rev. Neurosci. 2012;13(7):453–464. doi: 10.1038/nrn3271. [DOI] [PubMed] [Google Scholar]
- Bruno I.G., Karam R., Huang L., Bhardwaj A., Lou C.H., Shum E.Y., Song H.W., Corbett M.A., Gifford W.D., Gecz J., Pfaff S.L., Wilkinson M.F. Identification of a MicroRNA that activates gene expression by repressing nonsense-mediated RNA decay. Mol. Cell. 2011;42(4):500–510. doi: 10.1016/j.molcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfiglioli A., Efe I.E., Guneykaya D., Semtner M., Buonfiglioli A., Efe I.E., Guneykaya D., Ivanov A., Huang Y., Orlowski E. let-7 MicroRNAs regulate microglial function and suppress glioma growth through toll-like article let-7 MicroRNAs regulate microglial function and suppress glioma growth through toll-like receptor 7. Cell Rep. 2019;29:3460–3471. doi: 10.1016/j.celrep.2019.11.029. [DOI] [PubMed] [Google Scholar]
- Campbell K., Booth S.A. MicroRNA in neurodegenerative drug discovery: the way forward? Expet Opin. Drug Discov. 2015;10(1):9–16. doi: 10.1517/17460441.2015.981254. [DOI] [PubMed] [Google Scholar]
- Carro M.S., Lim W.K., Alvarez M.J., Bollo R.J., Zhao X., Snyder E.Y., Sulman E.P., Anne S.L., Doetsch F., Colman H., Lasorella A., Aldape K., Califano A., Iavarone A. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R.W., Sontheimer E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti M., Ai W., Banik N.L., Ray S.K. Overexpression of miR-7-1 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem. Res. 2013;38(2):420–432. doi: 10.1007/s11064-012-0936-5. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M., Khandkar M., Banik N.L., Ray S.K. Alterations in expression of specific microRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012;1454:1–13. doi: 10.1016/j.brainres.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.A., Krichevsky A.M., Kosik K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Canc. Res. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- Chen P.H., Shih C.M., Chang W.C., Cheng C.H., Lin C.W., Ho K.H., Su P.C., Chen K.C. MicroRNA-302b-inhibited E2F3 transcription factor is related to all trans retinoic acid-induced glioma cell apoptosis. J. Neurochem. 2014;131(6):731–742. doi: 10.1111/jnc.12820. [DOI] [PubMed] [Google Scholar]
- Chen X.J., Wu M.Y., Li D.H., You J. Apigenin inhibits glioma cell growth through promoting microRNA-16 and suppression of BCL-2 and nuclear factor-κB/MMP-9. Mol. Med. Rep. 2016;14(3):2352–2358. doi: 10.3892/mmr.2016.5460. [DOI] [PubMed] [Google Scholar]
- Cheng H.-Y., Hsieh M.-T., Tsai F.-S., Wu C.-R., Chiu C.-S., Lee M.-M., Xu H.-X., Zhao Z.-Z., Peng W.-H. Neuroprotective effect of luteolin on amyloid b protein (25–35)-induced toxicity in cultured rat cortical neurons. Phytother Res. 2008;22(4):544–549. doi: 10.1002/ptr.2940. [DOI] [PubMed] [Google Scholar]
- Ching A.S., Ahmad-Annuar A. A perspective on the role of microRNA-128 regulation in mental and behavioral disorders. Front. Cell. Neurosci. 2015;9:1–8. doi: 10.3389/fncel.2015.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian A., Grethe S., Harald A. Intake of Flavonoid-Rich Wine , Tea , and Chocolate by Elderly Men and women is associated with better cognitive test performance. J. Nutr. 2009;139(1):120–127. doi: 10.3945/jn.108.095182. [DOI] [PubMed] [Google Scholar]
- Ciafrè S.A., Galardi S., Mangiola A., Ferracin M., Liu C.G., Sabatino G., Negrini M., Maira G., Croce C.M., Farace M.G. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 2005;334(4):1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Conaco C., Otto S., Han J.J., Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc. Natl. Acad. Sci. U. S. A. 2006;103(7):2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B.S. Pharmacological treatment of Parkinson disease. Clin. Rev. Educ. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- Conti L., Crisafulli L., Caldera V., Tortoreto M., Brilli E., Conforti P., Zunino F., Magrassi L., Schiffer D., Cattaneo E. REST controls self-renewal and tumorigenic competence of human glioblastoma cells. PloS One. 2012;7(6):1–13. doi: 10.1371/journal.pone.0038486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T.A., Wan L., Dreyfuss G. RNA and disease. Hhmi. 2009;136(4):777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsten M.F., Miranda R., Kasmieh R., Krichevsky A.M., Weissleder R., Shah K. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell-delivered S-TRAIL in human gliomas. Canc. Res. 2007;67(19):8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- Cortez M.A., Nicoloso M.S., Shimizu M., Rossi S., Gopisetty G., Molina J.R., Carlotti C., Tirapelli D., Neder L., Brassesco M.S., Scrideli C.A., Tone L.G., Georgescu M., Zhang W., Puduvalli V., Calin G.A. miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes, Chrom. Cancer. 2010;49:981–990. doi: 10.1002/gcc.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo M.C., Tomé-Carneiro J., Gómez-Coronado D., Burgos-Ramos E., Garciá-Serrano A., Martín-Hernández R., Baliyan S., Fontecha J., Venero C., Dávalos A., Visioli F. Modulation of miRNA expression in aged rat hippocampus by buttermilk and krill oil. Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-22148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo S., Manna C., Migliardi V., Mazzoni O., Morrica P., Capasso G., Pontoni G., Galletti P., Zappia V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metabol. Dispos. 2001;29(11):1492–1498. [PubMed] [Google Scholar]
- Das E., Bhattacharyya N.P. MicroRNA-432 contributes to dopamine cocktail and retinoic acid induced differentiation of human neuroblastoma cells by targeting NESTIN and RCOR1 genes. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2014;588(9):1706–1714. doi: 10.1016/j.febslet.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Das S., Foley N., Bryan K., Watters K.M., Bray I., Murphy D.M., Buckley P.G., Stallings R.L. MicroRNA mediates DNA demethylation events triggered by retinoic acid during neuroblastoma cell differentiation. Canc. Res. 2010;70(20):7874–7881. doi: 10.1158/0008-5472.CAN-10-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauncey M.J. New insights into nutrition and cognitive neuroscience. Proc. Nutr. Soc. 2009;68(4):408–415. doi: 10.1017/S0029665109990188. [DOI] [PubMed] [Google Scholar]
- Dauncey M.J. Recent advances in nutrition, genes and brain health. Proc. Nutr. Soc. 2012;71(4):581–591. doi: 10.1017/S0029665112000237. [DOI] [PubMed] [Google Scholar]
- Davis M.M., Olausson P., Greengard P., Taylor J.R., Nairn A.C. Regulator of calmodulin signaling knockout mice display anxiety-like behavior and motivational deficits. Eur. J. Neurosci. 2012;35(2):300–308. doi: 10.1111/j.1460-9568.2011.07956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkow K., Rossling R., Schipke C., Kru C., Derkow K., Ro R., Bauer J., Stroux A., Schott E., Ruprecht K., Peters O. Distinct expression of the neurotoxic microRNA family let-7 in the cerebrospinal fluid of patients with Alzheimer’ s disease. PloS One. 2018;13(7):1–18. doi: 10.1371/journal.pone.0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks P.B., Meyer M., Reimand J. ri, Lan X., Head R., Zhu X., Kushida M., Bayani J., Pressey J.C., Lionel A., Clarke I.D., Cusimano M., Squire J., Scherer S., Bernstein M., Woodin M.A., Bader G.D. Single cell derived clonal analysis of human glioblastoma links functional and genomic heterogeneity. Neuro Oncol. 2014;2014 doi: 10.1073/pnas.1320611111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirscherl K., Karlstetter M., Ebert S., Kraus D., Hlawatsch J., Walczak Y., Moehle C., Fuchshofer R., Langmann T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J. Neuroinflammat. 2010;7(3):1–16. doi: 10.1186/1742-2094-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Fei T., Verhaak R.G.W., Su Z., Zhang Y., Brown M., Chen Y., Liu X.S. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat. Struct. Mol. Biol. 2013;20(7):908–913. doi: 10.1038/nsmb.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte S.P., Estremores B., Cunha-santos J., Miranda C.O., Barata J., La A., Almeida L.P. De. Autophagy activation induced by let-7 microRNA overexpression counteracts disease phenotype in Machado-Joseph disease mouse models. Frontiers. 2020:1–2. [Google Scholar]
- Dunham I., Kundaje A., Aldred S.F., Collins P.J., Davis C.A., Doyle F., Epstein C.B., Frietze S., Harrow J., Kaul R., Khatun J., Lajoie B.R., Landt S.G., Lee B.K., Pauli F., Rosenbloom K.R., Sabo P., Safi A., Sanyal A. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel P., Chai C.L.L., Sperla´gh B., Huleatt P.B. Clinical utility of neuroprotective agents in neurodegenerative diseases : current status of drug development for Alzheimer ’ s , Parkinson ’ s and Huntington ’ s diseases , and amyotrophic lateral sclerosis. Expert Opin. Investig. 2012;21(9):1267–1308. doi: 10.1517/13543784.2012.703178. [DOI] [PubMed] [Google Scholar]
- Dye R.V., Miller K.J., Singer E.J., Levine A.J. Hormone replacement therapy and risk for neurodegenerative diseases. Int. J. Alzheim. Dis. 2012;2012:1–18. doi: 10.1155/2012/258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiring A.M., Harb J.G., Neviani P., Garton C., Oaks J.J., Spizzo R., Liu S., Schwind S., Santhanam R., Hickey C.J., Becker H., Chandler J.C., Andino R., Cortes J., Hokland P., Huettner C.S., Bhatia R., Roy D.C., Liebhaber S.A., Perrotti D. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140(5):652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelborghs S., Gilles C., Ivanoiu A., Vandewoude M. Rationale and clinical data supporting nutritional intervention in Alzheimer’s disease. Acta Clin. Belg. 2014;69(1):17–24. doi: 10.1179/0001551213Z.0000000006. [DOI] [PubMed] [Google Scholar]
- Essa M.M., Subash S., Akbar M., Al S. Long-term dietary supplementation of pomegranates , figs and dates alleviate neuroinflammation in a transgenic mouse model of alzheimer ’ s disease. PloS One. 2015;10(3):1–17. doi: 10.1371/journal.pone.0120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelisti C., Florian M.C., Massimi I., Dominici C., Giannini G., Galardi S., Buè M.C., Massalini S., McDowell H.P., Messi E., Gulino A., Giulia Farace M., Ciafre S.A. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. Faseb. J. 2009;23(12):4276–4287. doi: 10.1096/fj.09-134965. [DOI] [PubMed] [Google Scholar]
- Fabbri M., Bottoni A., Shimizu M., Spizzo R., Nicoloso M.S., Rossi S., Barbarotto E., Cimmino A. Association of a MicroRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. J. Am. Med. Assoc. 2015;305(1):59–67. doi: 10.1001/jama.2010.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M., Garzon R., Cimmino A., Liu Z., Zanesi N., Callegari E., Liu S., Alder H., Costinean S., Fernandez-cymering C., Volinia S., Guler G., Morrison C.D., Chan K.K., Marcucci G., Calin A., Huebner K., Croce C.M., Alder H., Guler G. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. PNAS. 2007;104(40):15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., Zanesi N., Crawford M., Ozer G.H., Wernicke D., Alder H., Caligiuri M.A., Nana-Sinkam P., Perrotti D., Croce C.M. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 2012;109(31):12278–12279. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild C.L.A., Cheema S.K., Wong J., Hino K., Simó S., La Torre A. Let-7 regulates cell cycle dynamics in the developing cerebral cortex and retina. Sci. Rep. 2019;9(1):1–21. doi: 10.1038/s41598-019-51703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S.A., Price T.O., Dominguez L.J., Motisi A., Saiano F., Niehoff M.L., Morley J.E., Banks W.A., Ercal N., Barbagallo M. Extra virgin olive oil improves learning and memory in SAMP8 mice. J. Alzheim. Dis. 2012;28(1):81–92. doi: 10.3233/JAD-2011-110662. [DOI] [PubMed] [Google Scholar]
- Fenoglio C., Ridolfi E., Cantoni C., De Riz M., Bonsi R., Serpente M., Villa C., Pietroboni A.M., Naismith R.T., Alvarez E., Parks B.J., Bresolin N., Cross A.H., Piccio L.M., Galimberti D., Scarpini E. Decreased circulating miRNA levels in patients with primary progressive multiple sclerosis. Mul. Scl. J. 2013;19(14):1938–1942. doi: 10.1177/1352458513485654. [DOI] [PubMed] [Google Scholar]
- Fowler A., Thomson D., Giles K., Maleki S., Mreich E., Wheeler H., Leedman P., Biggs M., Cook R., Little N., Robinson B., Mcdonald K. miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion. Eur. J. Cancer. 2011;47(6):953–963. doi: 10.1016/j.ejca.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Franzoni E., Booker S.A., Parthasarathy S., Rehfeld F., Grosser S., Srivatsa S., Fuchs H., Tarabykin V., Vida I., Wulczyn F.G. miR-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene Phf6. ELife. 2015;2015(4):1–23. doi: 10.7554/eLife.04263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann-Morvinski D. Glioblastoma heterogeneity and cancer cell plasticity. Crit. Rev. Oncog. 2014;19(5):327–336. doi: 10.1615/critrevoncog.2014011777. [DOI] [PubMed] [Google Scholar]
- Furnari F.B., Fenton T., Bachoo R.M., Mukasa A., Stommel J.M., Stegh A., Hahn W.C., Ligon K.L., Louis D.N., Brennan C., Chin L., DePinho R.A., Cavenee W.K. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Develop. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Geng L., Zhang T., Liu W., Chen Y. Inhibition of miR-128 abates Aβ-mediated cytotoxicity by targeting PPAR-γ via NF-κb inactivation in primary mouse cortical neurons and neuro2a cells. Yonsei Med. J. 2018;59(9):1096–1106. doi: 10.3349/ymj.2018.59.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy A., Saber-Cherif L., Pourié G., Helle D., Umoret R., Guéant J.L., Bossenmeyer-Pourié C., Daval J.L. Developmental impairments in a rat model of methyl donor deficiency: effects of a late maternal supplementation with folic acid. Int. J. Mol. Sci. 2019;20(4) doi: 10.3390/ijms20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani S., Talebi F., Chan W.F., Masoumi F., Vojgani M., Power C., Noorbakhsh F. MicroRNA-181 variants regulate T cell phenotype in the context of autoimmune neuroinflammation. Front. Immunol. 2017;8(JUL):1–14. doi: 10.3389/fimmu.2017.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli L. Beneficial effects of olive oil phenols on the aging process: experimental evidence and possible mechanisms of action. Nutr. Aging. 2012;1(3–4):207–223. [Google Scholar]
- Godar S., Ince T.A., Bell G.W., Feldser D., Donaher J.L., Bergh J., Liu A., Miu K., Watnick R.S., Reinhardt F., Mcallister S.S., Jacks T. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134(1):62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J., Ferrer-Luna R., Rooj A.K., Mineo M., Ricklefs F., Takeda Y.S., Nowicki M.O., Salińska E., Nakano I., Lee H., Weissleder R., Beroukhim R., Chiocca E.A., Bronisz A. MicroRNA signatures and molecular subtypes of glioblastoma: the role of extracellular transfer. Stem Cell Rep. 2017;8(6):1497–1505. doi: 10.1016/j.stemcr.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J., Lenart J., Salinska E. MicroRNA in brain pathology: neurodegeneration the other side of the brain cancer. Non-Coding RNA. 2019;5(1):1–21. doi: 10.3390/ncrna5010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J., Nowicki M.O., Bronisz A., Otsuki A., Nuovo G., Raychaudhury A., Newton H.B., Chiocca E.A., Lawler S. Targeting of the bmi-1 oncogene/stem cell renewal factor by MicroRNA-128 inhibits glioma proliferation and self-renewal. Canc. Res. 2008;68(22):9125–9131. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- Golden E.B., Lam P.Y., Kardosh A., Gaffney K.J., Cadenas E., Louie S.G., Petasis N.A., Chen T.C., Schönthal A.H. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113(23):5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F., Nguyen T.T.J. Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012;15(3):127–133. doi: 10.1179/1476830511Y.0000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G.G., Volinia S., Croce C.M. Suppression of MicroRNA-9 by mutant EGFR signaling upregulates FOXP1 to enhance glioblastoma tumorigenicity. Canc. Res. 2014;74(5):1429–1439. doi: 10.1158/0008-5472.CAN-13-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerau-de-Arellano M., Smith K.M., Godlewski J., Liu Y., Winger R., Lawler S.E., Whitacre C.C., Racke M.K., Lovett-Racke A.E. Micro-RNA dysregulation in multiple sclerosis favours pro-inflammatory T-cell-mediated autoimmunity. Brain. 2011;134:3575–3586. doi: 10.1093/brain/awr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Chen Q. MiR-16 modulate temozolomide resistance by regulating BCL-2 in human glioma cells. Int. J. Clin. Exp. Pathol. 2015;8(10):12698–12707. [PMC free article] [PubMed] [Google Scholar]
- Harikumar K.B., Aggarwal B.B. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- He F., Song Z., Chen H., Chen Z., Yang P., Li W., Yang Z., Zhang T., Wang F., Wei J., Wei F., Wang Q., Cao J. Long noncoding RNA PVT1-214 promotes proliferation and invasion of colorectal cancer by stabilizing Lin 28 and interacting with miR-128. Oncogene. 2018;38(2):164–179. doi: 10.1038/s41388-018-0432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Hannon G.J., Harbor C.S. MicroRNAs : small RNAs with a big role in gene regulation. Nat. Rev. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hebert S., Horre K., Nicolaï L., Papadopoulou A.S., Mandemakers W., Strooper B. De, Silahtaroglu A.N., Kauppinen S. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer ’ s disease correlates with increased BACE1/β -secretase expression. PNAS. 2008;105(17) doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Ruan H., Gilbert J., Wang G., Ma Q., Yao W.D., Man H.Y. MicroRNA miR 124 is required for the expression of homeostatic synaptic plasticity. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougee S., Sanders A., Faber J., Graus Y.M.F., Van Den Berg W.B., Garssen J., Smit H.F., Hoijer M.A. Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem. Pharmacol. 2005;69(2):241–248. doi: 10.1016/j.bcp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Hu B., Guo Y., Chen C., Li Q., Niu X., Guo S., Zhang A., Wang Y., Deng Z. Repression of SIRT1 promotes the differentiation of mouse induced pluripotent stem cells into neural stem cells. Cell. Mol. Neurobiol. 2014;34(6):905–912. doi: 10.1007/s10571-014-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Xu P., Sun B., Teng G., Xiao Z. Deep sequencing reveals complex mechanisms of microRNA regulation during retinoic acid-induced neuronal differentiation of mesenchymal stem cells. Genomics. 2017;109(3–4):302–311. doi: 10.1016/j.ygeno.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Hung P.S., Chen F.C., Kuang S.H., Kao S.Y., Lin S.C., Chang K.W. MiR-146a induces differentiation of periodontal ligament cells. J. Dent. Res. 2010;89(3):252–257. doi: 10.1177/0022034509357411. [DOI] [PubMed] [Google Scholar]
- Ihle N.T., Abraham R.T. Previews the pten-parkin Axis : at the nexus of cancer and neurodegeneration. Mol. Cell. 2017;65(6):959–960. doi: 10.1016/j.molcel.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Iyer A., Zurolo E., Prabowo A., Fluiter K., Spliet W.G.M., van Rijen P.C., Gorter J.A., Aronica E. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PloS One. 2012;7(9):17–19. doi: 10.1371/journal.pone.0044789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhari A., Singh T., Yadav S. Expression of miR-145 and its target proteins are regulated by miR-29b in differentiated neurons. Molecular Neurobiology. 2018;55(12):8978–8990. doi: 10.1007/s12035-018-1009-9. [DOI] [PubMed] [Google Scholar]
- Jellinger K.A. Basic mechanisms of neurodegeneration: a critical update. J. Cell. Mol. Med. 2010;14(3):457–487. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernås M., Malmeström C., Axelsson M., Nookaew I., Wadenvik H., Lycke J., Olsson B. MicroRNA regulate immune pathways in T-cells in multiple sclerosis (MS) BMC Immunol. 2013;14(1) doi: 10.1186/1471-2172-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Buckley N.J. Gene dysregulation in Huntington ’ s Disease : REST , MicroRNAs and beyond. Neuromol. Med. 2009;11:183–199. doi: 10.1007/s12017-009-8063-4. [DOI] [PubMed] [Google Scholar]
- Jovic A., Roshan R., Moisoi N., Pradervand S., Moser R., Pillai B., Luthi-carter R. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. J. Neurosci. 2013;33(12):5127–5137. doi: 10.1523/JNEUROSCI.0600-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy Dauncey M. Genomic and epigenomic insights into nutrition and brain disorders. Nutrients. 2013;5(3):887–914. doi: 10.3390/nu5030887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimali M., Kloosterman W.P., de Bruijn E., Rosa F., Plasterk R.H.A., Wilson S.W. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8(8) doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam R., Wilkinson M.F. A conserved microRNA/NMD regulatory circuit controls gene expression. RNA Biol. 2012;9(1):22–26. doi: 10.4161/rna.9.1.18010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.E., Oh J.H., Lee S.K., Oh Y.J. Ginsenoside Rh-2 induces apoptotic cell death in rat C6 glioma via a reactive oxygen- and caspase-dependent but Bcl-X(L)-independent pathway. Life Sci. 1999;65(3) doi: 10.1016/s0024-3205(99)00252-0. [DOI] [PubMed] [Google Scholar]
- Kim S.M., Lim S.M., Yoo J.A., Woo M.J., Cho K.H. Consumption of high-dose vitamin C (1250 mg per day) enhances functional and structural properties of serum lipoprotein to improve anti-oxidant, anti-atherosclerotic, and anti-aging effects via regulation of anti-inflammatory microRNA. Food Funt. 2015;6(11):3604–3612. doi: 10.1039/c5fo00738k. [DOI] [PubMed] [Google Scholar]
- Kiskova T., Kubatka P., Büsselberg D., Kassayova M. The plant-derived compound resveratrol in brain cancer: a review. Biomolecules. 2020;10(1):1–19. doi: 10.3390/biom10010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocerha J., Xu Y., Prucha M.S., Zhao D., Chan A.W.S. microRNA-128a dysregulation in transgenic Huntington ’ s disease monkeys. Mol. Brain. 2014;7(46):1–10. doi: 10.1186/1756-6606-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill oil monograph. Alternative Med. Rev. 2010;15(1):84–86. [PubMed] [Google Scholar]
- Kumar S., Reddy P.H. Are circulating microRNAs peripheral biomarkers for Alzheimer ’ s disease ? BBA - Mol. Basis Dis. 2016;1862(9):1617–1627. doi: 10.1016/j.bbadis.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay H., Bai S., Datta J., Motiwala T., Pogribny I., Frankel W., Jacob S.T., Ghoshal K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J. Cell. Biochem. 2006;99(3):671–678. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P., Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294(5543):858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- Le M.T.N., Xie H., Zhou B., Chia P.H., Rizk P., Um M., Udolph G., Yang H., Lim B., Lodish H.F. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol. Cell Biol. 2009;29(19):5290–5305. doi: 10.1128/MCB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., Ajay S.S., Jong I.Y., Hyun S.K., Su H.H., Nam H.K., Dhanasekaran S.M., Chinnaiyan A.M., Athey B.D. New class of microRNA targets containing simultaneous 5′-UTR and 3′-UTR interaction sites. Genome Res. 2009;19(7):1175–1183. doi: 10.1101/gr.089367.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.C., Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2017;294(5543):862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- Lee S.T., Chu K., Oh H.J., Im W.S., Lim J.Y., Kim S.K., Park C.K., Jung K.H., Lee S.K., Kim M., Roh J.K. Let-7 microRNA inhibits the proliferation of human glioblastoma cells. J. Neuro Oncol. 2011;102(1):19–24. doi: 10.1007/s11060-010-0286-6. [DOI] [PubMed] [Google Scholar]
- Lee S.W., Oh Y.M., Lu Y., Kim W.K., Yoo A.S., Lee S.W., Oh Y.M., Lu Y., Kim W.K., Yoo A.S. MicroRNAs overcome cell fate barrier by reducing EZH2-controlled REST stability during neuronal conversion of human adult fibroblasts article MicroRNAs overcome cell fate barrier by reducing EZH2-controlled REST stability during neuronal conversion of hum. Dev. Cell. 2018;46(1):73–84. doi: 10.1016/j.devcel.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S.M., Krüger C., Park B., Derkow K., Rosenberger K., Baumgart J., Trimbuch T., Eom G., Hinz M., Kaul D., Habbel P., Kälin R., Franzoni E., Rybak A., Nguyen D., Veh R., Ninnemann O., Peters O., Nitsch R., Lehnardt S. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012;15(6):827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- Leone M., Bouadma L., Bouhemad B., Brissaud O., Dauger S., Gibot S., Hraiech S., Jung B., Kipnis E., Launey Y., Luyt C.-E., Margetis D., Michel F., Mokart D., Montravers P., Monsel A., Nseir S., Pugin J., Roquilly A., Chanques G. Hospital-acquired pneumonia in icu. Anaesthes. Crit. Care Pain Med. 2017;37(1):83–98. doi: 10.1016/j.accpm.2017.11.006. 1–16. [DOI] [PubMed] [Google Scholar]
- Lewis P.A., Hardy J., Martins L.M., Wood N.W. Cancer and Neurodegeneration : between the devil and the deep blue sea. PLoS Genet. 2010;6(12):1–8. doi: 10.1371/journal.pgen.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Jia Z., Li A., Jenkins G., Yang X., Hu J., Guo W. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Mol. Cell. Biochem. 2013;382(1–2):137–143. doi: 10.1007/s11010-013-1728-1. [DOI] [PubMed] [Google Scholar]
- Li M., Fu W., Wo L., Shu X., Liu F., Li C. miR-128 and its target genes in tumorigenesis and metastasis. Exp. Cell Res. 2013;319(20):3059–3064. doi: 10.1016/j.yexcr.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Li X., Yang L., Chen L.L. The biogenesis, functions, and challenges of circular RNAs. Mol. Cell. 2018;71(3):428–442. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Li Y., Li W., Yang Y., Lu Y., He C., Hu G., Liu H., Chen J., He J., Yu H. MicroRNA-21 targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma multiforme. Brain Res. 2009;1286:13–18. doi: 10.1016/j.brainres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- Liang Y., Li Y., Li Z., Liu Z., Zhang Z., Chang S., Wu J. Mechanism of folate deficiency-induced apoptosis in mouse embryonic stem cells: cell cycle arrest/apoptosis in G1/G0 mediated by microRNA-302a and tumor suppressor gene Lats 2. Int. J. Biochem. Cell Biol. 2012;44(11):1750–1760. doi: 10.1016/j.biocel.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., Johnson J.M. Microarray analysis shows that some microRNAs downregulate large numbers of-target mRNAs. Nature. 2005;433(7027):769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lin Q., Wei W., Coelho C.M., Li X., Baker-Andresen D., Dudley K., Ratnu V.S., Boskovic Z., Kobor M.S., Sun Y.E., Bredy T.W. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat. Neurosci. 2011;14(9):1115–1117. doi: 10.1038/nn.2891. [DOI] [PubMed] [Google Scholar]
- Liu L., Chen K., Wu J., Shi L., Hu B., Cheng S., Li M., Song L. Downregulation of miR-452 promotes stem-like traits and tumorigenicity of gliomas. Clin. Canc. Res. 2013;19(13):3429–3438. doi: 10.1158/1078-0432.CCR-12-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen M., Li L., Gong L., Zhou H., Gao D. Extracellular Signal-regulated Kinases (ERKs) phosphorylate Lin28a protein to modulate P19 cell proliferation and differentiation. J. Biol. Chem. 2017;292(10):3970–3976. doi: 10.1074/jbc.C117.775122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Liu P., Bai H., Li X., Xiao J., Yuan Q., Geng S., Yin H., Zhang H., Wang Z., Li J., Wang S., Wang Y. MicroRNA-128 knockout inhibits the development of Alzheimer’s disease by targeting PPARγ in mouse models. Eur. J. Pharmacol. 2018 doi: 10.1016/j.ejphar.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Lodygin D., Tarasov V., Epanchintsev A., Berking C., Knyazeva T., Körner H., Knyazev P., Diebold J., Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7(16):37–41. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- Luceri C., Bigagli E., Pitozzi V., Giovannelli L. A nutrigenomics approach for the study of anti-aging interventions: olive oil phenols and the modulation of gene and microRNA expression profiles in mouse brain. Eur. J. Nutr. 2017;56(2):865–877. doi: 10.1007/s00394-015-1134-4. [DOI] [PubMed] [Google Scholar]
- Lukiw W.J. Micro-RNA speciation in fetal , adult and Alzheimer ’ s disease hippocampus. Mol. Neurosci. 2020;18(3):297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- Lukiw W.J., Pogue A.I. Induction of specific micro RNA ( miRNA ) species by ROS-generating metal sulfates in primary human brain cells. J. Inorg. Biochem. 2007;101(9):1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu L., Ciccotosto G.D., Vella L.J., Cheng L., Roisman L.C., Multhaup G., Hill A.F., Munter L.M., Cappai R. Amyloid precursor protein dimerisation reduces neurite outgrowth. Mol. Neurobiol. 2019;56(1):13–28. doi: 10.1007/s12035-018-1070-4. [DOI] [PubMed] [Google Scholar]
- Maher T.J. Effects of nutrients on brain function. Prog. Brain Res. 2000;122:187–194. doi: 10.1016/s0079-6123(08)62138-x. [DOI] [PubMed] [Google Scholar]
- Makeyev E.V., Zhang J., Carrasco M.A., Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangi G., Orteschi D., Milano V., Mancano G., Zollino M. Interstitial deletion of 3p22.3p22.2 encompassing ARPP21 and CLASP2 is a potential pathogenic factor for a syndromic form of intellectual disability: a co-morbidity model with additional copy number variations in a large family. Am. J. Med. Genet. Part A. 2013;161(11):2890–2893. doi: 10.1002/ajmg.a.36257. [DOI] [PubMed] [Google Scholar]
- Mi Y., Mahar M., Ewan E.E., Leahy K.M., Zhao G., Cavalli V. Epigenetic regulator UHRF1 inactivates REST and growth suppressor gene expression via DNA methylation to promote axon regeneration. PNAS. 2018;1(24):E12417–E12426. doi: 10.1073/pnas.1812518115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milte C.M., Parletta N., Buckley J.D., Coates A.M., Young R.M., Howe P.R.C. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: a randomized controlled trial. Nutrition. 2012;28(6):670–677. doi: 10.1016/j.nut.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Miñones-Moyano E., Porta S., Escaramı´s G., Rabionet R., Min E., Iraola S., Kagerbauer B., Espinosa-parrilla Y., Ferrer I. MicroRNA profiling of Parkinson ’ s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011;20(15):3067–3078. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- Mineo M., Ricklefs F., Rooj A.K., Lyons S.M., Ivanov P., Ansari K.I., Nakano I., Chiocca E.A., Godlewski J., Bronisz A. The long non-coding RNA HIF1A-AS2 facilitates the maintenance of mesenchymal glioblastoma stem-like cells in hypoxic niches. Cell Rep. 2016;15(11):2500–2509. doi: 10.1016/j.celrep.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K.C., Huynh T., Tay Y., Ang Y.S., Tam W.L., Thomson A.M., Lim B., Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Miska E.A., Alvarez-Saavedra E., Townsend M., Yoshii A., Sestan N., Rakic P., Constantine-Paton M., Horvitz H.R. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5(9):R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi P.K., Jaiswal S., Sharma P. Regulation of neuronal cell cycle and apoptosis by miR-34a. Mol. Cell. Bio. 2015;55(2):936–945. doi: 10.1128/MCB.00589-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollashahi B., Aghamaleki F.S., Movafagh A. The roles of miRNAs in medulloblastoma: a systematic review. J. Cancer Prev. 2019;24(2):79–90. doi: 10.15430/JCP.2019.24.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal T., Subhash S., Vaid R., Enroth S., Uday S., Reinius B., Mitra S., Mohammed A., James A.R., Hoberg E., Moustakas A., Gyllensten U., Jones S.J.M., Gustafsson C.M., Sims A.H., Westerlund F., Gorab E., Kanduri C. MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA-DNA triplex structures. Nat. Commun. 2015;10(1):5290. doi: 10.1038/s41467-019-13200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes W.A.S. Young Perspectives for Old Diseases. 2015. Current pharmacological and non-pharmacological therapies for neurodegenerative diseases; pp. 23–48. [Google Scholar]
- Moreno J.A., Halliday M., Molloy C., Radford H., Verity N., Axten J.M., Ortori C.A., Willis A.E., Fischer P.M., Barrett D.A., Mallucci G.R. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Prion Dis. 2013;5(206):138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- Morita S., Horii T., Kimura M., Ochiya T., Tajima S. miR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int. J. Mol. Sci. 2013;14(7):14647–14658. doi: 10.3390/ijms140714647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L.G.T., Veeriah S., Chan T.A. Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene. 2010;29(24):3453–3464. doi: 10.1038/onc.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.S. The role of B vitamins in preventing and treating. Adv. Nutr. 2012;3(7):801–812. doi: 10.3945/an.112.002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrish P. Prescribing in Parkinson’s disease: a story of hope and adverse events. Practical Neurol. 2012;12(5):335–340. doi: 10.1136/practneurol-2012-000210. [DOI] [PubMed] [Google Scholar]
- Mucaj V., Lee S.S., Skuli N., Giannoukos D.N., Qiu B., Eisinger-mathason T.S.K., Nakazawa M.S., Shay J.E.S., Gopal P.P., Venneti S. MicroRNA- 124 expression counteracts pro-survival stress responses in glioblastoma. Oncogene. 2014;34(17):2204–2214. doi: 10.1038/onc.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu K.A. Vitamin C in human health and disease is still a mystery ? An overview. Nutr. J. 2003;10:1–10. doi: 10.1186/1475-2891-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro F., Lieberman J. miR-34 and p53 : new insights into a complex functional relationship. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowek K., Wiemer E.A.C., Jongen-lavrencic M. The versatile nature of miR-9/9 in human cancer. Oncotarget. 2018;9(29):20838–20854. doi: 10.18632/oncotarget.24889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H., Dessen P., Jourde B., Horstmann S., Nishikawa T., Di Patre P.L., Burkhard C., Schüler D., Probst-Hensch N.M., Maiorka P.C., Baeza N., Pisani P., Yonekawa Y., Yasargil M.G., Lütolf U.M., Kleihues P. Genetic pathways to glioblastoma: a population-based study. Canc. Res. 2004;64(19):6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- Okada N., Lin C., Ribeiro M.C., Okada N., Lin C., Ribeiro M.C., Biton A., Lai G., He X., Bu P., Vogel H., Jablons D.M., Keller A.C., Wilkinson J.E. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes Dev. 2014;28(5):438–450. doi: 10.1101/gad.233585.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørom U.A., Nielsen F.C., Lund A.H. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell. 2008;30(4):460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Packer A.N., Xing Y., Harper S.Q., Jones L., Davidson B.L. The bifunctional microRNA miR-9/miR-9 ∗ regulates REST and CoREST and is downregulated in Huntington ’ s disease. J. Neurosci. 2008;28(53):14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty S.J., Katz A., Wang Y., Eck P., Kwon O., Lee J.H., Chen S., Corpe C., Levine M., Dutta A., Dutta S.K. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- Pallocca G., Fabbri M., Sacco M.G., Gribaldo L., Pamies D., Laurenza I., Bal-Price A. MiRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biol. Toxicol. 2013;29(4):239–257. doi: 10.1007/s10565-013-9250-5. [DOI] [PubMed] [Google Scholar]
- Palmieri D., Perego P., Palombo D. Apigenin inhibits the TNFα-induced expression of eNOS and MMP-9 via modulating Akt signalling through oestrogen receptor engagement. Mol. Cell. Biochem. 2012;371(1–2):129–136. doi: 10.1007/s11010-012-1429-1. [DOI] [PubMed] [Google Scholar]
- Papagiannakopoulos T., Friedmann-Morvinski D., Neveu P., Dugas J.C., Gill R.M., Huillard E., Liu C., Zong H., Rowitch D.H., Barres B.A., Verma I.M., Kosik K.S. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene. 2012;31(15):1884–1895. doi: 10.1038/onc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons X.H., Parsons J.F., Moore D.A. Genome-scale mapping of MicroRNA signatures in human embryonic stem cell neurogenesis. Mol. Med. Ther. 2012;1(2):105–124. doi: 10.4172/2324-8769.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli A.E., Reinhart B.J., Slack F., Martindale M.Q., Kuroda M.I., Maller B., Hayward D.C., Ball E.E., Degnan B., Müller P., Spring J., Srinivasan A., Fishman M., Finnerty J., Corbo J., Levine M., Leahy P., Davidson E., Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., Louis D.N., Rozenblatt-Rosen O., Suvà M.L., Regev A., Bernstein B.E. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1402. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlica S., Gebhardt R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010;86(3–4):79–86. doi: 10.1016/j.lfs.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Pena J.T.G., Sohn-Lee C., Rouhanifard S.H., Ludwig J., Hafner M., Mihailovic A., Lim C., Holoch D., Berninger P., Zavolan M., Tuschl T. miRNA in situ hybridization in formaldehyde and EDC - fixed tissues. Nat. Methods. 2009;6(2):139–141. doi: 10.1038/nmeth.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi P., Bronisz A., Nowicki M.O., Wang Y., Ogawa D., Price R., Nakano I., Kwon C., Hayes J., Lawler S.E., Ostrowski M.C., Chiocca E.A., Godlewski J. MicroRNA-128 coordinately targets Polycomb Repressor Complexes in glioma stem cells. Neuro Oncol. 2013;15(9):1212–1224. doi: 10.1093/neuonc/not055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichiorri F., Suh S., Rocci A., Luca L. De, Taccioli C., Santhanam R., Zhou W., Benson D.M., Hofmainster C., Alder H., Garofalo M., Leva G., Di Volinia S., Lin H., Perrotti D., Kuehl M., Aqeilan R.I., Palumbo A., Croce C.M. Article loop in multiple myeloma development. Cancer Cell. 2010;18(4):367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pitozzi V., Jacomelli M., Catelan D., Servili M., Taticchi A., Biggeri A., Dolara P., Giovannelli L. Long-term dietary extra-virgin olive oil rich in polyphenols reverses age-related dysfunctions in motor coordination and contextual memory in mice: role of oxidative stress. Rejuvenation Res. 2012;15(6):601–612. doi: 10.1089/rej.2012.1346. [DOI] [PubMed] [Google Scholar]
- Pitt J., Roth W., Lacor P., Blankenship M., Velasco P., Felice F. De, Breslin P., Klein W.L. Alzheimer’s-associated Aβ oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol. Appl. Pharmacol. 2009;240(2):1–20. doi: 10.1016/j.taap.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh N.D., Edwall D., Lindmark L., Kousoulas K.G., Iyer A.V., Haron M.H., Pasco D.S. Phytomedicine Oral administration of a Spirulina extract enriched for Braun-type lipoproteins protects mice against influenza A ( H1N1 ) virus infection ✩. Phytomedicine. 2015;22(2):271–276. doi: 10.1016/j.phymed.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Qureshi I.A., Mehler M.F. Epigenetic mechanisms governing the process of neurodegeneration. Mol. Aspect. Med. 2013;34(4):875–882. doi: 10.1016/j.mam.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgor D., Hanley J.G. The ins and outs of miRNA-mediated gene silencing during neuronal synaptic plasticity. Non-Coding RNA. 2016;2(1):1–13. doi: 10.3390/ncrna2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.H. Increased mitochondrial fission and neuronal dysfunction in Huntington ’ s disease : implications for molecular inhibitors of excessive mitochondrial fission. Drug Discov. Today. 2014;19(7):951–955. doi: 10.1016/j.drudis.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.H., Beal M.F. Amyloid beta , mitochondrial dysfunction and synaptic damage : implications for cognitive decline in aging and Alzheimer ’ s disease. Trends Mol. Med. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.H., Reddy T.P., Manczak M., Calkins M.J. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res. Rev. 2010;67(1–2):103–118. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanová D., Vachálková A., Cipák L., Ovesná Z.R.P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48(2):104–107. [PubMed] [Google Scholar]
- Rooj A.K., Ricklefs F., Mineo M., Nakano I., Chiocca E.A., Bronisz A., Cells S., Rooj A.K., Ricklefs F., Mineo M., Nakano I., Chiocca E.A., Bronisz A. MicroRNA-mediated dynamic bidirectional shift between the subclasses of glioblastoma stem-like report MicroRNA-mediated dynamic bidirectional shift between the subclasses of glioblastoma. Cell Rep. 2017;19(10):2026–2032. doi: 10.1016/j.celrep.2017.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan R., Shridhar S., Sarangdhar M.A. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA. 2014;20(8):1–11. doi: 10.1261/rna.044008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostamian M., Baghi M., Safaeinejad Z., Kiani-esfahani A. Di ff erential expression of miR-34a , miR-141 , and miR-9 in MPP + -treated di ff erentiated PC12 cells as a model of Parkinson ’ s disease. Gene. 2018;662:54–65. doi: 10.1016/j.gene.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Ru P., Hu P., Geng F., Mo X., Cheng C., Yoo J.Y., Cheng X., Wu X. Feedback loop regulation of SCAP/SREBP-1 by miR-29 modulates EGFR signaling-driven report feedback loop regulation of SCAP/SREBP-1 by miR-29 modulates EGFR signaling-driven glioblastoma growth. Cell Rep. 2016;16(6):1527–1535. doi: 10.1016/j.celrep.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R., Goodman C.D., Huzarewich R.L.C.H., Robertson C., Booth S.A. A miRNA signature of prion induced neurodegeneration. PloS One. 2008;3(11) doi: 10.1371/journal.pone.0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiko P., Szakmary A., Jaeger W., Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. Rev. Mutat. Res. 2008;658(1–2):68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Saito Y., Saito H. MicroRNAs in cancers and neurodegenerative disorders. Front. Genet. 2012;3:1–5. doi: 10.3389/fgene.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S., Pena-Bello L., Manfredi-Lozano M., Tena-Sempere M., Cordido F. Perturbation of hypothalamic microrna expression patterns in male rats after metabolic distress: impact of obesity and conditions of negative energy balance. Endocrinology. 2014;155(5):1838–1850. doi: 10.1210/en.2013-1770. [DOI] [PubMed] [Google Scholar]
- Saraiva C., Paiva J., Santos T., Ferreira L., Bernardino L. MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson ’ s disease. J. Contr. Release. 2016;235:291–305. doi: 10.1016/j.jconrel.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Saugstad J.A. MicroRNAs as effectors of brain function with roles in ischemia and injury , neuroprotection , and neurodegeneration. J. Cerebr. Blood Flow Metabol. 2010;30(9):1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonrock N., Humphreys D.T., Preiss T., Götz J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid- β. J. Mol. Neurosci. 2012;46:324–335. doi: 10.1007/s12031-011-9587-2. [DOI] [PubMed] [Google Scholar]
- Schraivogel D., Weinmann L., Beier D., Tabatabai G., Eichner A., Zhu J.Y., Anton M., Sixt M., Weller M., Beier C.P., Meister G. miR-9/9 ∗ in glioblastoma stem cells. EMBO J. 2011;30(20):4309–4322. doi: 10.1038/emboj.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamborn J.C., Berezikov E., Knoblich J.A. The TRIM-NHL protein TRIM32 activates MicroRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009;136(5):913–925. doi: 10.1016/j.cell.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelinger G., Merfort I., Wölfle U., Schempp C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13(10):2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere L.F., Freemantle S., Pitha-rowe I., Moss E., Dmitrovsky E., Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5(3):R13.1–R13.11. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A., Rubió L., Borràs X., Macià A., Romero M.P., Motilva M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012;56(3):486–496. doi: 10.1002/mnfr.201100436. [DOI] [PubMed] [Google Scholar]
- Shanesazzade Z., Peymani M., Ghaedi K. miR - 34a/BCL - 2 signaling axis contributes to apoptosis in MPP + - induced SH - SY5Y cells. Mol Genet Genom. Med. 2018;6(6):975–981. doi: 10.1002/mgg3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.S., Kumar A., Arora M., Kaundal R.K. Neuroprotective potential of combination of resveratrol and 4-amino 1 , 8 naphthalimide in experimental diabetic neuropathy : focus on functional , sensorimotor and biochemical changes. Free Radic. Res. 2009;43:400–408. doi: 10.1080/10715760902801509. [DOI] [PubMed] [Google Scholar]
- Silber J., Jacobsen A., Ozawa T., Harinath G., Pedraza A., Sander C., Holland E.C., Huse J.T. miR-34a repression in proneural malignant gliomas upregulates expression of its target PDGFRA and promotes tumorigenesis. PloS One. 2012;7(3) doi: 10.1371/journal.pone.0033844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber J., Lim D.A., Petritsch C., Persson A.I., Maunakea A.K., Yu M., Vandenberg S.R., Ginzinger D.G., James D., Costello J.F., Bergers G., Weiss W.A., Alvarez-buylla A., Hodgson J.G. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;17:1–17. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S.E., Lim C.S., Kim J.I., Seo D., Chun H., Yu N.K., Lee J., Kang S.J., Ko H.G., Choi J.H., Kim T.H., Jang E.H., Han J., Bak M.S., Park J.E., Jang D.J., Baek D., Lee Y.S., Kaang B.K. The brain-enriched microRNA miR-9-3p regulates synaptic plasticity and memory. J. Neurosci. 2016;36(33):8541–8552. doi: 10.1523/JNEUROSCI.0630-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Sinn N., Milte C.M., Street S.J., Buckley J.D., Coates A.M., Petkov J., Howe P.R.C. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br. J. Nutr. 2012;107(11):1682–1693. doi: 10.1017/S0007114511004788. [DOI] [PubMed] [Google Scholar]
- Smith P., Al Hashimi A., Girard J., Delay C., Hébert S.S. In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J. Neurochem. 2011;116(2):240–247. doi: 10.1111/j.1471-4159.2010.07097.x. [DOI] [PubMed] [Google Scholar]
- Song Y., Mu L., Han X. MicroRNA-9 inhibits vasculogenic mimicry of glioma cell lines by suppressing Stathmin expression. J. Neuro Oncol. 2013;115:381–390. doi: 10.1007/s11060-013-1245-9. [DOI] [PubMed] [Google Scholar]
- Sonntag K.C., Woo T.U.W., Krichevsky A.M. Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126. Exp. Neurol. 2012;235(2):427–435. doi: 10.1016/j.expneurol.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Wolf Y. NeurimmiRs: MicroRNAs in the neuroimmune interface. Trends Mol. Med. 2011;17(10):548–555. doi: 10.1016/j.molmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Sullivan P.F., Daly M.J., O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat. Rev. Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzhi Z., Liang T., Yuexia P., Lucy L., Xiaoting H., Yuan Z., Qin W. Gap junctions enhance the antiproliferative effect of MicroRNA-124-3p in glioblastoma cells. J. Cell. Physiol. 2015;230(10):2476–2488. doi: 10.1002/jcp.24982. [DOI] [PubMed] [Google Scholar]
- Tan C.L., Plotkin J.L., Venø M.T., Schimmelmann M., von Feinberg P., Mann S., Handler A., Kjems J., Surmeier D.J., O’Carroll D., Greengard P., Schaefer A. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science. 2014;342(2013):1254–1258. doi: 10.1126/science.1244193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Wang S., Yang B., Zhu L., Yin B., Chao T., Zhao J., Yuan J., Qiang B., Peng X. The CREB-miR-9 negative feedback minicircuitry coordinates the migration and proliferation of glioma cells. PloS One. 2012;7(11):1–11. doi: 10.1371/journal.pone.0049570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa H., Tsuchiya N., Izumiya M., Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. PNAS. 2007;104(39):2–7. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiribuzi R., Crispoltoni L., Porcellati S., Di Lullo M., Florenzano F., Pirro M., Bagaglia F., Kawarai T., Zampolini M., Orlacchio A., Orlacchio A. MiR 128 up-regulation correlates with impaired amyloid β(1-42) degradation in monocytes from patients with sporadic Alzheimer’s disease. Neurobiol. Aging. 2014;35(2):345–356. doi: 10.1016/j.neurobiolaging.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Tripathi D.N., Jena G.B. Mutation Research/Genetic Toxicology and Environmental Mutagenesis Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress , DNA damage and early hepatocarcinogenesis in rat : role of Nrf 2 , p53 , p38 and phase-II enzymes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010;696:69–80. doi: 10.1016/j.mrgentox.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Trivedi P.P., Jena G.B. Role of a -lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice : studies on inflammation , oxidative stress , DNA damage and fibrosis. Food Chem. Toxicol. 2013;(June) doi: 10.1016/j.fct.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Trivedi P.P., Jena G.B. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. Eur. J. Nutr. 2014;54(4):639–652. doi: 10.1007/s00394-014-0745-5. [DOI] [PubMed] [Google Scholar]
- Tumaneng K., Schlegelmilch K., Russell R.C., Yimlamai D., Basnet H., Mahadevan N., Fitamant J., Bardeesy N., Camargo F.D., Guan K. YAP mediates crosstalk between the Hippo and PI ( 3 ) K – TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 2012;14(12):1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-pedret C., Lamuela-ravent R.M., Quintana M., Corella D., Pint X., Angel M. Polyphenol-rich foods in the mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheim. Dis. 2012;29:773–782. doi: 10.3233/JAD-2012-111799. [DOI] [PubMed] [Google Scholar]
- Vasudevan S., Tong Y., Steitz J.A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Verhaak R.G.W., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., Alexe G., Lawrence M., Kelly M.O., Tamayo P., Weir B.A., Gabriel S., Winckler W., Gupta S., Jakkula L. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan M., Alamri F.F., Shoyaib A. Al, Karamyan V.T. Novel miRNA PC-5P-12969 in ischemic stroke. Molec. Neurobiol. 2019;56:6976–6985. doi: 10.1007/s12035-019-1562-x. [DOI] [PubMed] [Google Scholar]
- Vijayan M., Kumar S., Yin X., Zafer D., Chanana V., Cengiz P., Reddy P.H. Identification of novel circulatory microRNA signatures linked to patients with ischemic stroke. Hum. Mol. Genet. 2018;27(13):2318–2329. doi: 10.1093/hmg/ddy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S., Calin G.A., Liu C., Ambs S., Petrocca F., Visone R., Iorio M., Roldo C., Ferracin M., Prueitt R.L., Yanaihara N., Lanza G., Scarpa A., Vecchione A., Negrini M., Harris C.C., Croce C.M., Volinia S., Calin G.A., Yanaiharas N. A microRNA expression signature of human solid tumors defines cancer gene targets. PNAS. 2016;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yao N., Lu C.-L., Li D., Ma X. Mouse microRNA-124 regulates the expression of Hes1 in P19 cells Chong. Front. Biosci. 2010;5:2–4. doi: 10.2741/e74. [DOI] [PubMed] [Google Scholar]
- Wang H., Ye Y., Zhu Z., Mo L., Lin C., Wang Q., Wang H., Gong X., He X., Lu G., Lu F., Zhang S. MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinson ’ s disease by targeting to bim. Int. Soc. Neuropathol. 2016;26:167–176. doi: 10.1111/bpa.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang X., Chen L., Zhang Y., Xu Z., Liu J., Jiang G., Li J., Zhang X., Wang K., Wang J., Chen G., Luo J. The microRNA miR-124 suppresses seizure activity and regulates CREB1 activity. Expet Rev. Mol. Med. 2016;18:1–16. doi: 10.1017/erm.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.K., Liu F.F., Wang Y., Jiang X.M., Yu X.F. Let-7a gene knockdown protects against cerebral ischemia/reperfusion injury. Neural Regen. Res. 2016;11(2):262–269. doi: 10.4103/1673-5374.177734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Lu G., Sze J., Liu Y., Lin S., Yao H., Zhang J., Xie D., Liu Q., Kung H. fu, Lin M. C. mi, Poon W.S. Plasma miR-124 is a promising candidate biomarker for human intracerebral hemorrhage stroke. Mol. Neurobiol. 2018;55(7):5879–5888. doi: 10.1007/s12035-017-0808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Yamaji R., Ohtsuki T. MicroRNA-664a-5p promotes neuronal differentiation of SH-SY5Y cells. Gene Cell. 2018;23(3):225–233. doi: 10.1111/gtc.12559. [DOI] [PubMed] [Google Scholar]
- Weissmiller A.M., Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener. 2012;1:1–9. doi: 10.1186/2047-9158-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch C., Chen Y., Stallings R.L. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26(34):5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- Wu N., Wu G.C., Hu R., Li M., Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol. Sin. 2011;32(3):345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.P., Chen W.S., Teng C., Zhang N. Stem cells for the treatment of neurodegenerative diseases. Molecules. 2010;15(10):6743–6758. [Google Scholar]
- Wynder C., Hakimi M.A., Epstein J.A., Shilatifard A., Shiekhattar R. Recruitment of MLL by HMG-domain protein iBRAF promotes neural differentiation. Nat. Cell Biol. 2005;7(11):1113–1117. doi: 10.1038/ncb1312. [DOI] [PubMed] [Google Scholar]
- Xie Y., Huo S., Zhang G., Zhang F., Lian Z., Tang X., Jin C. CDA-2 induces cell differentiation through suppressing Twist/SLUG signaling via miR-124 in glioma. J. Neuro Oncol. 2012;110(2):179–186. doi: 10.1007/s11060-012-0961-x. [DOI] [PubMed] [Google Scholar]
- Xu H., Sun J., Shi C., Sun C., Yu L., Wen Y. Neuroscience Letters miR-29s inhibit the malignant behavior of U87MG glioblastoma cell line by targeting DNMT3A and 3B. Neurosci. Lett. 2015;590:40–46. doi: 10.1016/j.neulet.2015.01.060. [DOI] [PubMed] [Google Scholar]
- Xue Y., Qian H., Hu J., Zhou B., Zhou Y., Hu X., Karakhanyan A., Pang Z., Fu X. Sequential regulatory loops as key gatekeepers for neuronal reprogramming in human cells. Nat. Neurosci. 2016;19(6):807–815. doi: 10.1038/nn.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Lin P.-J., Levey A. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013;369(5):487–489. doi: 10.1056/NEJMc1305541. [DOI] [PubMed] [Google Scholar]
- Young A.B. Four decades of neurodegenerative disease Research : how far we have Come ! J. Neurosci. 2009;29(41):12722–12728. doi: 10.1523/JNEUROSCI.3767-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitelhofer M., Adzemovic M.Z., Gomez-Cabrero D., Bergman P., Hochmeister S., N’diaye M., Paulson A., Ruhrmann S., Almgren M., Tegnér J.N., Ekström T.J., Guerreiro-Cacais A.O., Jagodic M. Functional genomics analysis of Vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2017;114(9):E1678–E1687. doi: 10.1073/pnas.1615783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.L., Tu Z.G. In vitvo induction of differentiation by ginsenoside Rh2 in SMMC-7721 hepatocarcinoma cell line. Pharmacol. Toxicol. 2003;93(6):275–283. doi: 10.1111/j.1600-0773.2003.pto930605.x. [DOI] [PubMed] [Google Scholar]
- Zhang S., Liu X., Sun C., Yang J., Wang L., Liu J., Gong L., Jing Y. Apigenin attenuates experimental autoimmune myocarditis by modulating Th1/Th2 cytokine balance in mice. Inflammation. 2016;39(2):678–686. doi: 10.1007/s10753-015-0294-y. [DOI] [PubMed] [Google Scholar]
- Zhou L., Yang L., Li Y.J., Mei R., Yu H.L., Gong Y., Du M.Y., Wang F. MicroRNA-128 protects dopamine neurons from apoptosis and upregulates the expression of excitatory amino acid transporter 4 in Parkinson’s disease by binding to AXIN1. Cell. Physiol. Biochem. 2018;51(5):2275–2289. doi: 10.1159/000495872. [DOI] [PubMed] [Google Scholar]
- Zilfou J.T., Lowe S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009;1(5):a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.