To the Editor:
We read with interest the systematic review and meta-analysis by Klassen and colleagues,1 recently published in Mayo Clinic Proceedings. The investigators included in their analysis 30 randomized clinical trials (RCTs) and matched control studies, documenting that COVID-19 convalescent plasma (CP) transfusion, especially when it is given within 3 days of hospital admission, is associated with lower mortality of patients with COVID-19 compared with standard treatment. Adverse events analysis, in combination with benefits analysis, is essential to make an informed decision about health intervention. For this reason, we would like to add safety data to the analysis of Klassen and coworkers.1
Through an online systematic search on PubMed and MEDLINE (range, January 1, 2020, to May 15, 2021), we identified 30 studies (14 RCTs and 16 non-RCTs with matched control group) that were downloaded and analyzed for safety data (Supplemental Table, available online at http://www.mayoclinicproceedings.org). Overall, severe (serious and grade 3-4) and thromboembolic adverse reactions were recorded and analyzed. In addition, we collected and evaluated the prevalence of overall and severe adverse reactions to CP transfusion in the selected studies. The study weight was calculated using the Mantel-Haenszel method, and statistical heterogeneity was assessed using the I 2 statistic. Measures of treatment effect were risk difference (RD) together with 95% CI. All calculations were conducted using Review Manager, version 5.4 software (Cochrane Collaboration).
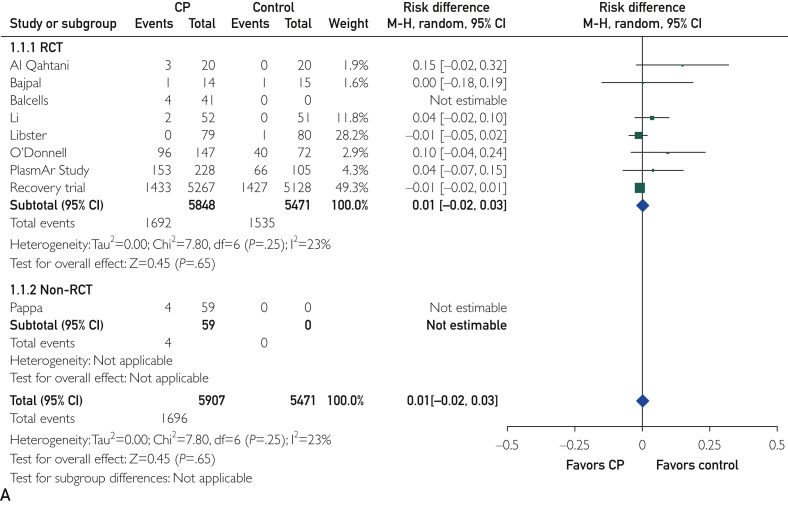
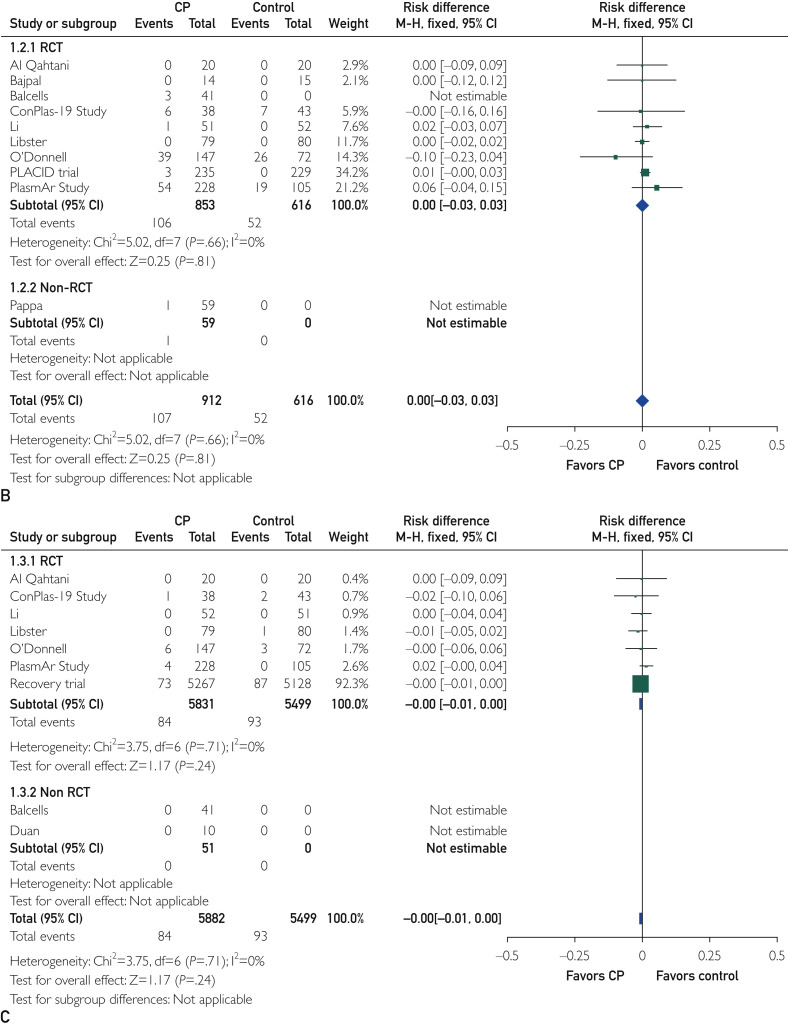
The mean prevalence (standard deviation) of all and severe CP infusion–related adverse events was 2.1% (2.6%) and 0.7% (1.4%), respectively. As reported in Figure A and B, treatment with CP did not increase the risk of overall adverse events (RD, 0.01; 95% CI, −0.02 to 0.03; P=.65) and severe adverse events (RD, 0.00; 95% CI, −0.03 to 0.03; P=.81) compared with standard treatment. Similarly, the rate of thromboembolic events did not differ between the study groups (1.4% in the CP arm vs 1.7% in the control arm; RD, 0.00; 95% CI, −0.01 to 0.00; P=.24; Figure C). In addition, the funnel plot of comparison of all 3 outcomes (all, severe, and thromboembolic adverse reactions; Supplemental Figure, available online at http://www.mayoclinicproceedings.org) appeared to be symmetric, suggesting a substantial homogeneity among the included studies and the lack of publication bias.
Figure.
Forest plots of comparison of convalescent plasma (CP) vs standard treatment. A, Outcome: all adverse reactions. Data are from 8 randomized clinical trials (RCTs) and 1 non-RCT. B, Outcome: severe adverse reactions.
Data are from 9 RCTs and 1 non-RCT. C, Outcome: thromboembolic adverse reactions. Data are from 7 RCTs and 2 non-RCTs. MH, Mantel-Haenszel. Figure continued on next page.
In conclusion, the results of this updated meta-analysis confirm the safety of CP transfusion and, in particular, document the very low rate (0.7%) of CP transfusion–related serious adverse reactions, similar to that reported in the large US Expanded Access Program.2 Differing from the previous systematic reviews, we have focused our analysis on the CP-related thromboembolic risk, considering the particular critical setting of COVID-19, with a hyperinflammatory and hypercoagulative state, and the concerns from some clinicians.3 After a careful analysis of the published literature, we can conclude that the addition of CP to the COVID-19 treatment does not increase the patients’ thromboembolic risk. Finally, we personally think that considering the lack of valid anti–COVID-19 therapies, the relatively low costs, and the high safety profile, CP collection and use should be endorsed and implemented by governments of developing and developed countries, without waiting for conclusive evidence of its efficacy.4
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Klassen S.A., Senefeld J.W., Johnson P.W. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin Proc. 2021;96(5):1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyner M.J., Bruno K.A., Klassen S.A. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marwah V., Choudhary R., Peter D., Bhati G. Pulmonary thromboembolism post-COVID convalescent plasma therapy: adding fuel to a smoldering fire! Adv Resp Med. 2021;89(3):347–349. doi: 10.5603/ARM.a2021.0022. [DOI] [PubMed] [Google Scholar]
- 4.Cruciani M., Bongiovanni G., Franchini M. High-titer convalescent plasma therapy for COVID-19 and mortality. Transfusion. 2021;61(6):1988–1990. doi: 10.1111/trf.16434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.