Abstract
Background
The aim of this systematic review was to summarize the results of trials evaluating interventions for the reduction of sarcopenia in patients undergoing surgery.
Methods
Searches were conducted using the Cochrane Central Register of Controlled Trials, MEDLINE and Embase. RCTs evaluating exercise, dietary or pharmacological interventions to address sarcopenia in the perioperative period were included. Treatment effect estimates were expressed as standardized mean differences (MDs) with confidence intervals, and heterogeneity was expressed as I2 values.
Results
Seventy trials including 3402 participants were selected for the data synthesis. Exercise interventions significantly increased muscle mass (MD 0.62, 95 per cent c.i. 0.34 to 0.90; P < 0.001), muscle strength (MD 0.55, 0.39 to 0.71; P < 0.001), measures of gait speed (MD 0.42, 0.05 to 0.79; P = 0.03), and reduced time for completion of set exercises (MD −0.76, −1.12 to −0.40; P < 0.001) compared with controls. Subgroup analysis showed that interventions in the early postoperative period were more likely to have a positive effect on muscle mass (MD 0.71, 0.35 to 1.07; P < 0.001) and timed tests (MD −0.70, −1.10 to −0.30; P = 0.005) than preoperative interventions. Treatment effects on muscle mass (MD 0.09, −0.31 to 0.49; P = 0.66) and strength (MD 0.46, −0.01 to 0.92; P = 0.05) were attenuated by the presence of cancer. Results of analyses restricted to nine trials at low risk of allocation concealment bias and fourteen trials at low risk of attrition bias were comparable to those of the primary analysis. Risk-of-bias assessment showed that most trials were at high risk of incomplete outcome and attrition bias, thus reducing the estimate of certainty of the evidence according to the GRADE assessment tool.
Conclusion
Exercise interventions appear beneficial in reducing the impact of sarcopenia. Because of the high risk of bias and low certainty of the current evidence, large RCTs using standardized measures of muscle mass should be undertaken.
This review has evaluated the available evidence from trials which have evaluated interventions for the reduction of sarcopenia in patients undergoing surgery. We have found that exercise interventions are likely to improve muscle strength and muscle mass as well as the quality of motor performance especially in the post-operative period. We have also found that the presence of cancer may attenuate the effect of certain interventions.
Resumen
Antecedentes
El objetivo de esta revisión sistemática fue resumir los resultados de los ensayos clínicos que evaluaron las intervenciones para reducir la sarcopenia en pacientes sometidos a cirugía.
Métodos
Las búsquedas se realizaron mediante el Registro Central Cochrane de Ensayos Controlados, MEDLINE y EMBASE. Se incluyeron los ensayos aleatorizados y controlados (randomised controlled trials, RCT) que habían evaluado las intervenciones basadas en el ejercicio físico, dietéticas o farmacológicas para disminuir la sarcopenia en el período perioperatorio. Las estimaciones del efecto del tratamiento se expresaron como diferencias de medias estandarizadas (standardised mean differences, SMD) e intervalos de confianza, y la heterogeneidad se expresó como I2.
Resultados
Se seleccionaron 70 ensayos con 3.402 participantes para la síntesis de los datos. Las intervenciones con ejercicio aumentaron significativamente la masa muscular (SMD 0,62; i.c. del 95%: 0,34-0,90; P < 0,0001), la fuerza muscular (SMD 0,55; i.c. del 95%: 0,39-0,71; P < 0,0001), las medidas de la velocidad de la marcha (SMD 0,42; i.c. del 95% 0,05-0,79; P = 0,02), y disminuyeron el tiempo para completar los ejercicios de la serie (SMD -0,75; i.c. del 95% -1,08 –0,43 P < 0,0001), en comparación con los controles. El análisis de subgrupos mostró que las intervenciones en el período postoperatorio temprano tenían más probabilidades de tener un efecto positivo sobre la masa muscular (SMD 0,71; i.c. del 95%: 0,35-1,07; P < 0,0001) y las pruebas cronometradas (SMD -0,70; i.c. del 95% - 1,10-0,30; P = 0,005 I2 = 59%) en comparación con las intervenciones preoperatorias. Los efectos del tratamiento sobre la masa muscular (SMD 0,09; i.c. del 95%: -0,31-0,49; P = 0,66) y la fuerza (SMD 0,46; -0,01-0,92; P = 0,05), se atenuaron por la presencia de cáncer. Los resultados de los análisis restringidos a cuatro ensayos con bajo riesgo de sesgo de ocultación de la asignación o dos ensayos con bajo riesgo de sesgo de desgaste fueron comparables a los del análisis primario. La evaluación del riesgo de sesgo mostró que la mayoría de los ensayos tenían un alto riesgo de resultado incompleto y sesgo de desgaste, lo que reducía la estimación de la certeza de la evidencia según la herramienta de evaluación GRADE.
Conclusión: Las intervenciones con ejercicios parecen ser beneficiosas para reducir el impacto de la sarcopenia. Debido al alto riesgo de sesgo y la baja certeza de la evidencia actual, se deben realizar grandes ensayos aleatorizados y controlados que utilicen medidas estandarizadas de masa muscular.
Introduction
Sarcopenia, characterized by progressive and generalized muscle loss, is observed in over 20–40 per cent of patients recovering from major surgery, where it has been associated with higher rates of complications in sarcopenic compared with non-sarcopenic patients (45 versus 15 per cent), higher in-hospital mortality rates (23 versus 4 per cent)1, and longer hospital stay2. Sarcopenia is associated with advancing age and frailty, but can occur in younger patients3 who have additional risk factors, including sedentary lifestyle, poor nutrition, chronic disease, and chronic inflammatory states4.
As the population ages, and the numbers of patients with frailty, multiple chronic conditions or cardiometabolic disease referred for surgery increases, sarcopenia will present an increasing challenge to clinicians and health systems. Research points towards a complex and multifactorial pathophysiology characterized by loss of mitochondrial function in skeletal muscle, chronic inflammatory changes, and exposure to oxidative stress5 that may be modified by exercise, diet or pharmacological interventions. The aim of this review was to summarize the results of randomized trials of interventions aiming to attenuate sarcopenia in people undergoing surgery. The secondary aim was to evaluate the effectiveness of these interventions across a range of important subgroups defined by intervention type, age, disease type including cancer, and timing of the intervention. Finally, the strengths and limitations of different definitions of sarcopenia when measuring treatment effects in clinical trials were evaluated.
Methods
A systematic review of RCTs was performed according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions6. A protocol was registered prospectively on PROSPERO (CRD42020165325)7. The study adhered to PRISMA guidelines8.
Study eligibility
Studies were included if they fulfilled the inclusion criteria of RCTs in which an intervention was used to prevent or reverse sarcopenic changes in adult patients (over 18 years old) in a surgical population. Trials were excluded if they reported retrospective or observational studies, or included a significant proportion of people (over 50 per cent) with neuromuscular or neurodegenerative disease, cachexia, or chronic inflammatory conditions. Abstracts were reviewed and were included only if they were of high quality and adhered to CONSORT reporting criteria; recent studies have shown discrepancies between the data presented in conference abstracts and subsequent full-text publications9, or even between the abstract and main article text10. Furthermore, as the adherence of abstracts to the CONSORT reporting criteria has been reported as suboptimal11, these were only included if they adhered to CONSORT reporting guidelines.
Search methods
Electronic searches were conducted in the Cochrane Central Register of Controlled Trials, MEDLINE, and Embase using the following search terms: sarcopenia, muscle mass, dietary proteins, exercise therapy, testosterone or androgen or growth hormone and related terms. A full description of the search terms is available in Appendix S1. The final search was undertaken on 19 December 2019.
Study selection
Title and abstract screening were carried out independently by two authors using the Rayyan QCRI web app (Qatar Computer Research Institute, Hamad Bin Khalifa University, Doha, Qatar). Selected references were managed using Endnote™ X9 (Clarivate Analytics, Philadelphia, Pennsylvania, USA). Full-text screening was carried out and the reference lists of included papers were also screened for suitable articles. Excluded studies and the reason for exclusion were recorded. Disagreements were resolved by discussion or, where this was not possible, by a third author.
Assessment of risk of bias in included studies
Included trials were appraised using the Cochrane risk-of-bias tool version 812. Two authors assessed each outcome of interest as being at either at low, high or unclear risk of bias for each domain. Disagreements were resolved as above.
Data extraction
Data were extracted by two reviewers and managed using Excel™ 2016 (Microsoft, Redmond, Washington, USA). This included year, study type, setting, sample size, participant demographics, baseline characteristics, type of surgery, details of interventions, outcomes, and risk-of-bias assessments. The primary outcome of this review was measures of sarcopenia, evaluated either by functional tests or imaging. A large variety of measures were used in the included trials; direct measures of muscle mass, such as cross-sectional area of lumbar spine or quadriceps muscle assessed by direct imaging (dual-energy x-ray absorptiometry (DEXA), CT, MRI), and muscle strength, such as hand-grip strength or equivalent, were included. Furthermore, in view of the definition of sarcopenia as a decline in muscle quantity and quality, and in keeping with an international consensus guideline3, the measures of muscle function were included. These can be broadly split into two categories: functional assessments which record the time taken to complete a specific task such as the timed-get-up-and go (TUG) test and the sit-to-stand test, and time-based tests in which the numbers of metres walked or repetitions of an exercise were measured, such as the 6-minute-walk test (6MWT).
For the purposes of meta-analyses, these measures were grouped into four categories. Category A comprised measures of muscle mass, including appendicular skeletal muscle mass evaluated by DEXA, CT or MRI; whole-body skeletal muscle mass by DEXA, CT or MRI; mid-thigh cross-sectional area by CT or MRI; lumbar muscle cross-sectional area; bioelectrical impedance analysis; measurement of muscle thickness by ultrasonography; measurements of muscle volume by ultrasound imaging, CT or MRI; or measurement of skinfold thickness. Category B included measures of muscle strength, including hand-grip strength, quadriceps muscle strength, lower limb (any muscle group) resistance test, and upper limb (any muscle group) resistance test not including hand-grip strength. Category C comprised tests for the completion of set exercises including chair stand test (sit to stand), TUG test, and 10-m walk test. As these trials measure the time for completion of the exercises, beneficial treatment effects are negative in these trials. Category D consisted of repetition-based tests in which the number of metres walked or repetitions of an exercise in a set timeframe were assessed, including gait speed, 6MWT, and 30-s chair-rise test.
Secondary outcomes included self-reported quality of life, anaemia, mortality at 30 days, rates of readmission, duration of hospital stay, and rates of admission to a more intensive place of care either upon hospital discharge or from the previous place of residence.
Subgroup analysis was undertaken for type of surgery (orthopaedic, cardiothoracic, general, gynaecological, urological, bariatric, breast, transplant, and trauma), timing of the intervention (preoperative, perioperative, early postoperative, late postoperative), age of the participants (Aged 65 or under or aged over 65), cancer status of the participants.
Statistical analysis
Standardized mean differences (MDs) with 95 per cent confidence intervals and P values were estimated for treatment effect measures as continuous outcomes using an inverse-variance random-effects method. Risk ratios with 95 per cent confidence intervals were estimated for dichotomous outcomes using the Mantel–Haenszel random–effects method. All analyses were carried out using Review Manager (RevMan) version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
The heterogeneity of treatment effects was explored using a prespecified subgroup analysis for the following criteria: type of surgery, cancer status, age, and timing of interventions. The test for subgroup differences in the Cochrane software was used to identify significant treatment–subgroup interactions. Sensitivity analyses excluded studies with high risk of bias in two domains—allocation concealment and incomplete outcome data—as it was predicted that these would be the most likely sources of bias in this review. Heterogeneity within each meta-analysis was explored by using a χ2 test with significance set at a P value of 0.10, and was expressed as percentage heterogeneity due to variation rather than to chance (I2). An I2 value of 0–40 per cent indicated no or mild heterogeneity; 41–80 per cent indicated moderate heterogeneity; and over 80 per cent represented severe heterogeneity.
Publication bias for the primary outcome was assessed using funnel plots, where 10 or more studies contributed to an outcome. The quality of evidence was assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology in GRADEPRO GDT software (https://gdt.gradepro.org)13,14.
Results
Study characteristics
The results of the searches and exclusions are shown in Fig. 1. In total, 70 trials with 3402 participants (mean age 54.6 years), were included in the data synthesis (Table S1)15–73. These included six trials18,38,41 in general surgery, three34,54,59 in bariatric surgery, three15,17 in breast cancer surgery, eleven16,23,31,32,47,48,50,65,70,73 in cardiothoracic surgery, forty-one19–21,24,26–30,33,35–37,39,40,43,45,49,51–53,55,57,58,60–64,66–69,71,72 in orthopaedics, three42,44,46 in transplant surgery, one56 in urology, one25 in gynaecological surgery, and one22 in trauma surgery. Some papers reported results of trial with multiple treatment arms: for the purpose of the meta-analysis, each treatment arm has been considered as a separate trial. The average age of the participants 65 or less in thirty-nine trials and over 65 years in twenty-six trials. Five trials did not report the average age of participants. There were eleven trials involving oncological patients. With regard to timings of intervention, four trials investigated preoperative interventions (interventions introduced at any time before operation), five trials perioperative interventions (interventions introduced before operation and continued in postoperative period), forty-seven trials investigated early postoperative intervention (interventions started within first 6 weeks after surgery) and fourteen trials late postoperative interventions (interventions started 6 weeks after operation). Forty-six trials investigated the effects of exercise, eighteen studied nutritional interventions, and six the effects of medications. A summary of the main findings of interventions are reported in Table 1.
Fig. 1.
PRISMA diagram showing selection of studies for review
Table 1.
Summary of findings with trial interventions for primary and secondary outcomes including intragroup and intergroup heterogeneity
| No. of studies | No. of participants | Treatment effect |
Heterogeneity |
|||
|---|---|---|---|---|---|---|
| Effect size | P | I 2 (%) | P | |||
| Category A: measures of muscle mass | ||||||
| Overall effect | 24 | 852 | MD 0.48 (0.25, 0.70) | < 0.001 | 54 | < 0.001 |
| Exercise | 17 | 606 | MD 0.62 (0.34, 0.90) | < 0.001 | 55 | 0.004 |
| Dietary interventions | 5 | 210 | MD –0.01 (–0.28, 0.27) | 0.96 | 0 | 0.42 |
| Medications | 2 | 36 | MD 0.87 (0.18, 1.57) | 0.01 | 0 | 0.38 |
| Heterogeneity between subgroups | 83.8 | 0.002 | ||||
| Category B: measures of muscle strength | ||||||
| Overall effect | 21 | 793 | MD 0.49 (0.35, 0.63) | < 0.001 | 0 | 0.46 |
| Exercise | 18 | 670 | MD 0.55 (0.39, 0.71) | < 0.001 | 0 | 0.51 |
| Dietary intervention | 3 | 123 | MD 0.19 (–0.17, 0.55) | 0.30 | 0 | 0.75 |
| Medications | 0 | 0 | Not estimable | Not estimable | Not estimable | |
| Heterogeneity between subgroups | 69.5 | 0.07 | ||||
| Category C: timed tests | ||||||
| Overall effect | 9 | 432 | MD –0.75 (–1.08, –0.43) | < 0.001 | 50 | 0.04 |
| Exercise | 8 | 411 | MD –0.76 (–1.12, –0.40) | < 0.001 | 56 | 0.02 |
| Dietary interventions | 1 | 21 | MD –0.76 (–1.66, 0.14) | 0.10 | Not estimable | |
| Medications | 0 | 0 | Not estimable | Not estimable | Not estimable | |
| Heterogeneity between subgroups | 0 | 1.00 | ||||
| Category D: repetition-based tests | ||||||
| Overall effect | 10 | 631 | MD 0.35 (0.06, 0.64) | 0.02 | 51 | 0.03 |
| Exercise | 9 | 490 | MD 0.42 (0.05, 0.79) | 0.03 | 55 | 0.02 |
| Dietary interventions | 1 | 141 | MD 0.13 (–0.20, 0.46) | 0.44 | Not estimable | |
| Medications | 0 | 0 | Not estimable | Not estimable | Not estimable | |
| Heterogeneity between subgroups | 24.3 | 0.25 | ||||
| Self-reported quality of life | ||||||
| Overall effect | 13 | 855 | MD 0.28 (0.10, 0.45) | 0.002 | 30 | 0.15 |
| Exercise | 11 | 719 | MD 0.27 (0.08, 0.47) | 0.006 | 32 | 0.14 |
| Dietary interventions | 1 | 101 | MD 0.49 (0.09, 0.88) | 0.02 | Not estimable | |
| Medications | 1 | 35 | MD –0.10 (–0.80, 0.60) | 0.78 | Not estimable | |
| Heterogeneity between subgroups | 7.5 | 0.34 | ||||
| Discharge to higher level of care | ||||||
| Overall effect | 2 | 139 | OR 0.36 (0.13, 1.04) | 0.06 | 0 | 0.96 |
| Exercise | 2 | 139 | OR 0.36 (0.13, 1.04) | 0.06 | 0 | 0.96 |
| Dietary interventions | 0 | 0 | Not estimable | Not estimable | Not estimable | |
| Medications | 0 | 0 | Not estimable | Not estimable | Not estimable | |
| Heterogeneity between subgroups | Not estimable | |||||
| Mortality at 30 days | ||||||
| Overall effect | 2 | 102 | OR 0.38 (0.07, 2.21) | 0.28 | 0 | 0.41 |
| Exercise | 0 | 0 | Not estimable | Not estimable | Not estimable | |
| Dietary interventions | 1 | 61 | OR 0.97 (0.06, 16.19) | 0.98 | Not estimable | |
| Medications | 1 | 41 | OR 0.21 (0.02, 2.00) | 0.18 | Not estimable | |
| Heterogeneity between subgroups | 0 | 0.41 | ||||
| Readmission rates | ||||||
| Overall effect | 3 | 113 | OR 0.36 (0.10, 1.31) | 0.12 | 0 | 0.95 |
| Exercise | 1 | 32 | OR 0.47 (0.04, 5.73) | 0.55 | Not estimable | |
| Dietary interventions | 1 | 61 | OR 0.34 (0.06, 1.94) | 0.23 | Not estimable | |
| Medications | 1 | 20 | OR 0.25 (0.01, 6.82) | 0.41 | Not estimable | |
| Heterogeneity between subgroups | 0 | 0.95 | ||||
| Duration of hospital stay | ||||||
| Overall effect | 17 | 1154 | MD –0.34 (–0.70, 0.01) | 0.06 | 87 | <0.00001 |
| Exercise | 9 | 632 | MD –0.70 (–1.30, –0.10) | 0.02 | 90 | <0.00001 |
| Dietary interventions | 7 | 481 | MD –0.03 (–0.20, 0.15) | 0.78 | 0 | 0.97 |
| Medications | 1 | 41 | MD 0.06 (–0.56, 0.67) | 0.85 | Not estimable | |
| Heterogeneity between subgroups | 57.2 | 0.10 | ||||
Values in parentheses are 95 per cent confidence intervals. MD, mean difference; OR, odds ratio.
Assessment of bias
The results of the risk-of-bias assessments are shown in Fig. S1. Overall, the methodological quality of the included trials was low. A total of nine trials were at low risk of allocation concealment and fourteen trials were considered to be at low risk of attrition bias.
Data synthesis
For the primary analyses, the studies were grouped by one of the four methods of sarcopenia assessment. In addition, the treatment effect was also determined for each of the three therapeutic approaches to sarcopenia: exercise interventions, dietary interventions, and pharmacological interventions. Twenty-four trials were included in category A (quantitative measures of muscle mass), 21 in category B (measures of muscle strength), nine in category C (timed tests), and 10 in category D (repetition-based tests).
Trial interventions (24 trials, 852 patients) improved quantitative measures of muscle mass (MD 0.48, 95 per cent c.i. 0.25 to 0.70; P < 0.001; I2 = 54 per cent) (Fig. 2 and Table 1). When stratified by type of treatment, exercise (17 trials, 606 patients; MD 0.62, 0.34 to 0.90; P < 0.001; I2 = 55 per cent) and pharmacological strategies (2 trials, 36 patients; MD 0.87, 0.18 to 1.57; P = 0.01; I2 = 0 per cent) increased muscle mass, whereas dietary interventions did not (5 trials, 210 patients; MD −0.01, −0.28 to 0.27; P = 0.96; I2 = 0 per cent).
Fig. 2.
Forest plot of effects of interventions on category A measures (measures of muscle mass) with further subgroup analysis by category of intervention (exercise, dietary intervention or medications)
An inverse-variance random-effects model was used for meta-analysis. Mean differences are shown with 95 per cent confidence intervals. *Values are mean(s.d.). Separate trials within a single publication are referred to as a and b.
Trial interventions (21 trials, 793 patients) improved measures of muscle strength (MD 0.49, 0.35 to 0.63; P < 0.001; I2 = 0 per cent), without heterogeneity (Fig. 3 and Table 1). In analyses stratified by type of treatment, exercise significantly increased muscle strength (18 trials, 670 patients; MD 0.55, 0.39 to 0.71; P < 0.001; I2 = 0 per cent), whereas dietary interventions did not (3 trials, 123 patients; MD 0.19, −0.17 to 0.55; P = 0.30; I2 = 0 per cent). No pharmacological trials reported these outcomes.
Fig. 3.
Forest plot of effects of interventions on category B measures (measures of muscle strength) with further subgroup analysis by category of intervention (exercise, dietary intervention or medications)
An inverse-variance random-effects model was used for meta-analysis. Mean differences are shown with 95 per cent confidence intervals. *Values are mean(s.d.). Separate trials within a single publication are referred to as a and b.
Trial interventions (9 trials, 432 patients) reduced times for completion of sit–stand and other similar tests (MD −0.75, −1.08 to −0.43; P < 0.001; I2 = 50 per cent) with moderate heterogeneity (Fig. 4 and Table 1). In analyses stratified by type of treatment, exercise significantly reduced test times (9 trials, 411 patients; MD −0.76, −1.12 to −0.40; P < 0.001; I2 = 56 per cent), whereas dietary interventions did not (1 trial, 21 patients; MD −0.76, −1.16 to 0.14; P = 0.30). No pharmacological trials reported these outcomes.
Fig. 4.
Forest plot of effects of interventions on category C measures (time needed for completion of a set number of exercises) with further subgroup analysis by category of intervention (exercise, dietary intervention or medications)
An inverse-variance random-effects model was used for meta-analysis. Mean differences are shown with 95 per cent confidence intervals. *Values are mean(s.d.). Separate trials within a single publication are referred to as a and b.
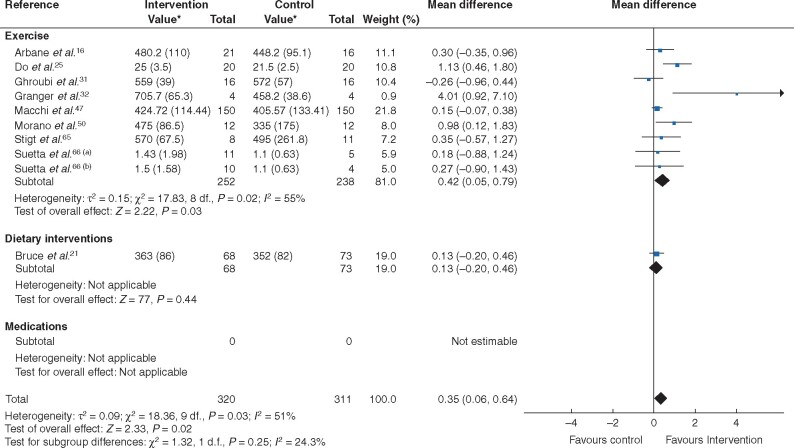
Trial interventions (10 trials, 631 patients) improved measures of walking speed (MD 0.35, 0.06 to 0.64; P = 0.02; I2 = 51 per cent) with moderate heterogeneity (Fig. 5 and Table 1). When stratified by type of treatment, exercise increased walking speed (9 trials, 490 patients; MD 0.42, 0.05 to 0.79; P = 0.03; I2 = 55 per cent), whereas dietary interventions did not (1 trial, 141 patients; MD 0.13, −0.20 to 0.46; P = 0.44). No pharmacological trials reported these outcomes.
Fig. 5.
Forest plot of effects of interventions on category D measures (number of repetitions completed in a set time interval in repetition-based tests) with further subgroup analysis by category of intervention (exercise, dietary intervention or medications)
An inverse-variance random-effects model was used for meta-analysis. Mean differences are shown with 95 per cent confidence intervals. *Values are mean(s.d.). Separate trials within a single publication are referred to as a and b.
Other treatment effects of interventions overall, and stratified by each of three main intervention groups are reported in Table 1. In meta-analyses, trial interventions (13 trials, 855 patients) led to an improvement in self-reported quality of life (MD 0.28, 0.10 to 0.45; P = 0.002; I2 = 30 per cent), with a similar treatment effect estimate for exercise trials (11 trials, 719 patients) and one trial (101 patients) of dietary intervention, but not in one other trial (35 patients) of a pharmacological intervention. Trial interventions (17 trials, 1154 patients) resulted in a non-statistically significant reduction in hospital stay (MD −0.34, −0.70 to 0.01; P = 0.06; I2 = 87 per cent) with severe heterogeneity. For duration of stay there was a significant treatment effect for exercise trials, but not for trials of dietary interventions, or for one trial of a pharmacological intervention (Table 1). Summary treatment estimates for other secondary outcomes were limited by small numbers of trials reporting each outcome (Table 1). No data were reported on levels of anaemia after surgery.
Subgroup analyses
The results of subgroup analyses for the primary outcome are reported in Table S2. The subgroups analysed by type of surgery were limited by small numbers of trials in all specialties except orthopaedic surgery, where treatment effects were similar to those for the primary analysis: category A (16 trials, 453 patients; MD 0.57, 95 per cent c.i. 0.32 to 0.83; P < 0.001; I2 = 38 per cent); category B (12 trials, 472 patients; MD 0.49, 0.27 to 0.72; P < 0.001; I2 =22 per cent); category C (6 trials, 258 patients; MD −0.84, −1.25 to −0.44; P < 0.001; I2 = 49 per cent), and category D (3 trials, n = 171 patients; MD 0.14, −0.16 to 0.45; P = 0.35; I2 = 0 per cent). There was no treatment–subgroup interaction for any of the four measures of the primary outcome for age.
For timing of the intervention (preoperative, perioperative, early postoperative, late postoperative) there was a treatment–subgroup interaction with significant treatment effects for interventions in the early postoperative period aimed at increasing muscle mass (4 trials, 308 patients; MD 0.71, 0.35 to 1.07; P < 0.001; I2 = 49 per cent) and timed tests (7 trials, 367 patients; MD −0.70, −1.10 to −0.30; P = 0.005; I2 = 59 per cent). There was also a treatment–subgroup interaction with significant treatment effects for late postoperative interventions aimed at reducing timed-test scores (2 trials, 65 patients; MD −0.64, −1.17 to −0.11; P = 0.02; I2 = 0 per cent).
There was a significant interaction with cancer status and treatment effects, with no significant treatment effect on muscle mass among patients with cancer (5 trials, 344 patients; MD 0.09, −0.31 to 0.49; P = 0.66; I2 = 62 per cent), whereas there was a significant benefit in patients without cancer (17 trials, 472 patients; MD 0.58, 0.33 to 0.83; P < 0.001; I2 = 36 per cent). Similarly, patients with cancer showed no improvement in muscle strength (3 trials, 91 patients; MD 0.46, −0.01 to 0.92; P = 0.05; I2 = 16 per cent), whereas those without cancer did (18 trials, 702 patients; MD 0.49, 0.34 to 0.65; P < 0.001; I2 = 4 per cent). Such interactions were not seen for timed tests (no significant interaction) or walking speed; for the latter, there was a significant benefit in patients with cancer (5 trials, 128 patients; MD 0.80, 0.22 to 1.39; P = 0.007; I2 = 52 per cent), but not for patients without cancer (5 trials, 503 patients; MD 0.12, −0.05 to 0.30; P = 0.17; I2 = 0 per cent). Subgroup analyses for secondary outcomes are reported in Table S3.
Sensitivity analysis
Sensitivity analyses for the primary outcome (Table S4) restricted to trials with adequate allocation concealment demonstrated a significant treatment effect on muscle mass (4 trials, 322 patients; MD 0.43, 95 per cent c.i. 0.03 to 0.83; P = 0.03; I2 = 91 per cent), muscle strength (2 trials, 209 patients; MD 0.55, 0.05 to 1.55; P = 0.03; I2 = 67 per cent), and for walking speed (1 trial, 24 patients; MD 0.98, 0.12 to 1.83; P = 0.02), but not for timed tests (2 trials, 202 patients; MD −0.85, −1.95 to 0.25; P = 0.13).
Sensitivity analyses restricted to trials at low risk of attrition bias demonstrated beneficial treatment effects for muscle mass (9 trials, 305 patients; MD 0.60, 0.14 to 1.06; P = 0.01; I2 = 71 per cent), and timed tests (4 trials, 228 patients; MD −0.96, −1.48 to −0.45; P = 0.002; I2 = 65 per cent), but not for muscle strength (4 trials, 170 patients; MD 0.40, −0.21 to 1.00; P = 0.20; I2 = 64 per cent), or walking speed (2 trials, 441 patients; MD 0.15, −0.04 to 0.33; P = 0.13; I2 = 0 per cent) (Table S4).
GRADE assessments
GRADE assessment judged the certainty of the effect estimates for exercise interventions on muscle mass or strength as low, and those for timed walking tests, or gait speed or equivalent assessments as moderate (Tables 2–5). The certainty of the effect estimates for pharmacological or dietary interventions were low or very low for all measures of sarcopenia.
Table 2.
Summary of findings for primary and secondary outcomes for interventions aimed at treating or preventing sarcopenia, with summary of GRADE assessment of reliability of available evidence
| Certainty assessment |
No. of patients* |
Effect† |
Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Interventions | Standard management | Relative | Absolute | ||
| Quantitative measures of muscle mass | |||||||||||
| 24 RCTs | Serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | 490 | 362 | MD 0.48 (0.25, 0.70) higher |
⊕⊕◯◯
Low |
Important | |
| Quantitative measures of muscle strength | |||||||||||
| 21 RCTs | Serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | 410 | 383 | MD 0.49 (0.35, 0.63) higher |
⊕⊕◯◯
Low |
Important | |
| Timed tests | |||||||||||
| 9 RCTs | Serious | Not serious | Not serious | Not serious | None | 226 | 206 | MD 0.75 (1.08, 0.43) lower |
⊕⊕⊕◯
Moderate |
Important | |
| Repetition-based tests | |||||||||||
| 10 RCTs | Serious | Not serious | Not serious | Not serious | None | 320 | 311 | MD 0.35 (0.06, 0.64) higher |
⊕⊕⊕◯
Moderate |
Important | |
| Self-reported quality of life | |||||||||||
| 13 RCTs | Very serious | Not serious | Not serious | Not serious | None | 479 | 376 | MD 0.28 (0.10, 0.45) higher |
⊕⊕◯◯
Low |
Important | |
| Mortality at 30 days | |||||||||||
| 2 RCTs | Not serious | Not serious | Not serious | Serious | None | 2 of 49 (4) | 6 of 53 (11) | OR 0.38 (0.07 to 2.21) | 67 fewer (from 104 fewer to 107 more) per 1000 |
⊕⊕⊕◯
Moderate |
Important |
| Duration of hospital stay | |||||||||||
| 17 RCTs | Serious | Not serious | Not serious | Serious | None | 579 | 575 | MD 0.34 lower (from 0.70 lower to 0.01 higher) |
⊕⊕◯◯
Low |
Important | |
Values in parentheses are *percentages and †95 per cent confidence intervals. GRADE, Grading of Recommendations Assessment, Development and Evaluation; MD, mean difference; OR, odds ratio.
Table 5.
Summary of the GRADE assessment of available evidence investigating effects of pharmacological interventions on all measures of sarcopenia and on prespecified secondary outcomes
| Certainty assessment |
No. of patients* |
Effect
†
|
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Medications | Standard management | Relative | Absolute | ||
| Quantitative measures of muscle mass | ||||||||||||
| 2 RCTs | Serious | Not serious | Not serious | Serious | None | 19 | 17 | MD 0.87 (0.18, 1.57) higher |
⊕⊕◯◯
Low |
Important | ||
| Self-reported quality of life: medications | ||||||||||||
| 1 RCT | Serious | Not serious | Not serious | Serious | None | 23 | 12 | MD 0.10 lower (from 0.80 lower to 0.60 higher) |
⊕⊕◯◯
Low |
Important | ||
| Mortality at 30 days: medications | ||||||||||||
| 1 RCT | Not serious | Not serious | Not serious | Very serious | None | 1 of 18 (6) | 5 of 23 (22) | OR 0.21 (0.02, 2.00) | 162 fewer (from 212 fewer to 140 more) per 1000 |
⊕⊕◯◯
Low |
Important | |
| Readmission rates: medications | ||||||||||||
| 1 RCT | Not serious | Not serious | Not serious | Very serious | None | 0 of 11 (0) | 1 of 9 (11) | OR 0.25 (0.01, 6.82) | 81 fewer (from 110 fewer to 349 more) per 1000 |
⊕⊕◯◯
Low |
Important | |
| Duration of hospital stay: medications | ||||||||||||
| 1 RCT | Not serious | Not serious | Not serious | Serious | None | 18 | 23 | MD 0.06 higher (from 0.56 lower to 0.67 higher) |
⊕⊕⊕◯
Moderate |
Important | ||
Values in parentheses are *percentages and †95 per cent confidence intervals. GRADE, Grading of Recommendations Assessment, Development and Evaluation; MD, mean difference; OR, odds ratio.
Table 3.
Summary of GRADE assessment of available evidence investigating effects of exercise interventions on all measures of sarcopenia and on prespecified secondary outcomes
| Certainty assessment |
No. of patients* |
Effect† |
Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Exercise interventions | Standard management | Relative | Absolute | ||
| Quantitative measures of muscle mass | |||||||||||
| 17 RCTs | Serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | 363 | 243 | MD 0.62 (0.34, 0.90) higher |
⊕⊕◯◯
Low |
Important | |
| Quantitative measures of muscle strength | |||||||||||
| 18 RCTs | Serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | 346 | 324 | MD 0.55 (0.39, 0.71) higher |
⊕⊕◯◯
Low |
Important | |
| Timed tests | |||||||||||
| 8 RCTs | Serious | Not serious | Not serious | Not serious | None | 214 | 197 | MD 0.76 (1.12, 0.40) lower |
⊕⊕⊕◯
Moderate |
Important | |
| Repetition-based tests | |||||||||||
| 9 RCTs | Serious | Not serious | Not serious | Not serious | None | 252 | 238 | MD 0.42 (0.05, 0.79) higher |
⊕⊕⊕◯
Moderate |
Important | |
| Self-reported quality of life | |||||||||||
| 11 RCTs | Very serious | Not serious | Not serious | Not serious | None | 404 | 315 | MD 0.27 (0.08, 0.47) higher |
⊕⊕◯◯
Low |
Important | |
| Discharge to higher level of care | |||||||||||
| 2 RCTs | Very serious | Not serious | Not serious | Serious | None | 5 of 69 (7) | 13 of 70 (19) | OR 0.36 (0.13, 1.04) | 110 fewer (from 157 fewer to 6 more) per 1000 |
⊕◯◯◯
Very low |
Critical |
| Duration of hospital stay - exercise | |||||||||||
| 9 RCTs | Serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | 324 | 308 | MD 0.7 (1.30, 0.10) lower |
⊕⊕◯◯
Low |
Critical | |
Values in parentheses are *percentages and †95 per cent confidence intervals. GRADE, Grading of Recommendations Assessment, Development and Evaluation; MD, mean difference; OR, odds ratio.
Table 4.
Summary of GRADE assessment of available evidence investigating effects of dietary interventions on all measures of sarcopenia and on prespecified secondary outcomes
| Certainty assessment |
No. of patients* |
Effect† |
Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Dietary interventions | Standard management | Relative | Absolute | ||
| Quantitative measures of muscle mass | |||||||||||
| 5 RCTs | Serious | Not serious | Not serious | Serious | None | 108 | 102 | MD 0.01 lower (from 0.28 lower to 0.27 higher) |
⊕⊕◯◯
Low |
Important | |
| Quantitative measures of muscle strength | |||||||||||
| 3 RCTs | Serious | Not serious | Not serious | Serious | None | 64 | 59 | MD 0.19 higher (from 0.17 lower to 0.55 higher) |
⊕⊕◯◯
Low |
Important | |
| Timed tests | |||||||||||
| 1 RCT | Very serious | Not serious | Not serious | Very serious | None | 12 | 9 | MD 0.76 lower (from 1.66 lower to 0.14 higher) |
⊕◯◯◯
Very low |
Important | |
| Repetition-based tests | |||||||||||
| 1 RCT | Very serious | Not serious | Not serious | Very serious | None | 68 | 73 | MD 0.13 higher (from 0.20 lower to 0.46 higher) |
⊕◯◯◯
Very low |
Important | |
| Self-reported quality of life: dietary interventions | |||||||||||
| 1 RCT | Very serious | Not serious | Not serious | Serious | None | 52 | 49 | MD 0.49 (0.09, 0.88) higher |
⊕◯◯◯
Very low |
Important | |
| Mortality at 30 day: dietary interventions | |||||||||||
| 1 RCT | Not serious | Not serious | Not serious | Very serious | None | 1 of 31 (3) | 1 of 30 (3) | OR 0.97 (0.06, 16.19) | 1 fewer (from 31 fewer to 325 more) per 1000 |
⊕⊕◯◯
Low |
Critical |
| Readmission rates: dietary interventions | |||||||||||
| 1 RCT | Not serious | Not serious | Not serious | Very serious | None | 2 of 31 (6) | 5 of 30 (17) | OR 0.34 (0.06, 1.94) | 103 fewer (from 155 fewer to 113 more) per 1000 |
⊕⊕◯◯
Low |
Important |
| Duration of hospital stay: dietary interventions | |||||||||||
| 7 RCTs | Serious | Not serious | Not serious | Serious | None | 237 | 244 | MD 0.03 lower (from 0.20 lower to 0.15 higher) |
⊕⊕◯◯
Low |
Critical | |
Values in parentheses are *percentages and †95 per cent confidence intervals. GRADE, Grading of Recommendations Assessment, Development and Evaluation; MD, mean difference; OR, odds ratio.
Discussion
This systematic review of RCTs of interventions that aim to reduce postoperative sarcopenia identified seventy trials, and the vast majority had important methodological limitations; only nine trials were at low risk of allocation concealment bias. Exercise interventions were shown consistently to improve measures of sarcopenia, whether defined by muscle mass, muscle strength, timed tests or gait speed, whereas dietary interventions did not. One analysis of two trials of pharmacological interventions suggested an improvement in muscle mass attributable to the interventions. Further subgroup analyses indicated that interventions in the early and possibly late postoperative periods were most likely to have a positive effect than those undertaken before surgery. Treatment effects were independent of age. Treatment effects on muscle mass and muscle strength, but not timed tests or gait speed, were attenuated by the presence of cancer. The findings of sensitivity analyses restricted to trials at low risk of allocation concealment bias or attrition bias were comparable to the results of the primary analysis.
The review used contemporary, standardized review methods to test a prespecified hypothesis described in a prospectively registered protocol. However, it is limited by the quality and small sample size of the included studies, and the potential heterogeneity of outcome measures included in the analyses. Where possible these outcome measures were grouped according to the type of sarcopenia measure: muscle mass, muscle strength, timed test results, or gait speed (or equivalent). The results provided useful insights into a clinical problem where progress is limited by a lack of standardization and consensus definitions of outcomes.
Exercise interventions, targeted at all patient regardless of age, in the early, or possibly late, postoperative phase could reduce sarcopenia. Postoperative exercise interventions are not part of standard perioperative care, presumably owing to gaps in knowledge. On this basis, a trial of postoperative exercise interventions may be warranted. Preoperative exercise, often as part of a prehabilitation programme, is increasingly being advocated as part of enhanced recovery for people undergoing surgery. However, only two trials with small sample sizes evaluating preoperative interventions were included in the present analysis. The evaluation of treatment effects by surgical specialty was also limited by small numbers of trials in many of the prespecified subgroups. These are further knowledge gaps identified by the present review.
Evaluation of the evidence using the GRADE assessment tool identified the need to downgrade the certainty of evidence to low or very low, except for evidence presented for exercise interventions. Common reasons can be identified across all primary and secondary outcomes. First, there was a serious risk of bias across most trials. This was mainly attributable to the uncertainty around the blinding of participants, personnel and assessors across the included trials; as most of the interventions required that participants completed an exercise or nutritional programme, the lack of blinding of participants could have affected concordance and affected outcomes. Furthermore, as some assessments of muscle mass, such as the 6MWT, require volitional effort, lack of blinding could have affected the results of the assessments of such measures.
Another relevant source of bias was the large proportion of trials reporting incomplete outcomes, with high rates of participants excluded from the final analysis or lost to follow-up. This is a significant issue not only because of the volitional nature of the interventions, but also because exclusion of participants who had developed complications or required readmission to ICU may have led to significant overestimation of the benefits of intervention.
The impact of small numbers of participants on the certainty of the evidence was less severe in trials of exercise interventions, thus increasing confidence in the treatment effects of exercise programmes.
A tertiary aim of the analysis was to review the use of different methods for measurement of sarcopenia in RCTs. Muscle mass and muscle strength offer objective and highly reproducible measures of sarcopenia; however, these may miss more qualitative aspects, including changes in motivation or cognition, that may influence the outcomes of functional and semiquantitative measures such as timed tests or gait speed. Heterogeneity of effect across different measures of sarcopenia was observed in multiple analyses, but this provided limited insights into the best measurement for clinical trials. For example, in the cancer subgroup analysis, there was no treatment effect on muscle mass or strength for people with cancer; however, for measures of gait speed, a benefit was observed for patients with cancer.The reasons for this are unclear. One approach to establishing the value of different measures of sarcopenia is to look for associations with clinically important outcomes. This was not possible in the present analysis owing to the limited number of trials that reported any clinical outcomes. There was general consistency of treatment effects between primary and some secondary outcomes, with agreement between treatment effects on muscle strength and mass in most analyses (primary analysis, type of treatment, timing of intervention, cancer versus no cancer). Discordance in other analyses was generally an issue of precision of the estimate rather than concerning the direction of the treatment effect. These were short-term assessments of well-being, however, and the association between measures of sarcopenia and long-term outcomes and quality of life is a research priority identified by this review.
Although this systematic review of RCTs indicates that exercise interventions are likely to reduce the severity of sarcopenia after surgery, this issue should be evaluated further. Other areas of uncertainty identified by this work include the need for validation of commonly used measures with respect to long-term outcomes, the role of exercise intervention in patients with cancer, and the role of preoperative exercise interventions on sarcopenia and long-term outcomes.
Funding
This study was funded by the Leicester National Institute for Health Research Biomedical Research Centre, and the British Heart Foundation (CH/12/1/29419, AA18/3/34220).
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Supplementary Material
Contributor Information
S Tomassini, Department of Cardiovascular Sciences and National Institute for Health Research Leicester Biomedical Research Unit in Cardiovascular Medicine, University of Leicester, Leicester, UK.
R Abbasciano, Department of Cardiovascular Sciences and National Institute for Health Research Leicester Biomedical Research Unit in Cardiovascular Medicine, University of Leicester, Leicester, UK.
G J Murphy, Department of Cardiovascular Sciences and National Institute for Health Research Leicester Biomedical Research Unit in Cardiovascular Medicine, University of Leicester, Leicester, UK.
References
- 1. Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG; Acute Care and Emergency Surgery (ACES) Group. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery 2014;156:521–527 [DOI] [PubMed] [Google Scholar]
- 2. Wahlen BM, Mekkodathil A, Al-Thani H, El-Menyar A. Impact of sarcopenia in trauma and surgical patient population: a literature review. Asian J Surg 2020;43:647–653 [DOI] [PubMed] [Google Scholar]
- 3. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A et al. Sarcopenia: an overview. Aging Clin Exp Res 2017;29:11–17 [DOI] [PubMed] [Google Scholar]
- 5. Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 2013;45:2288–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins J, SE Green. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.training.cochrane.org/handbook [Google Scholar]
- 7. Tomassini S, Abbasciano R, Murphy G. Interventions to Reduce the Burden of Sarcopenia in a Surgical Adult Population: A Systematic Review. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020165325 [DOI] [PMC free article] [PubMed]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toma M, McAlister FA, Bialy L, Adams D, Vandermeer B, Armstrong PW. Transition from meeting abstract to full-length journal article for randomized controlled trials. JAMA 2006;295:1281–1287 [DOI] [PubMed] [Google Scholar]
- 10. Altwairgi AK, Booth CM, Hopman WM, Baetz TD. Discordance between conclusions stated in the abstract and conclusions in the article: analysis of published randomized controlled trials of systemic therapy in lung cancer. J Clin Oncol 2012;30:3552–3557 [DOI] [PubMed] [Google Scholar]
- 11. Khan MS, Shaikh, Ochani RK, Akhtar T, Fatima K, Khan SU et al. Assessing the quality of abstracts in randomized controlled trials published in high impact cardiovascular journals. Circ Cardiovasc Qual Outcomes 2019;12:e005260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JPT, Savovic J, Chandler J, Cumpston M, Li T, Page MJ et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 [updated July 2019]. The Cochrane Collaboration, 2019 [Google Scholar]
- 13. Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A et al. Going from evidence to recommendations. BMJ 2008;336:1049–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams SC, Segal RJ, McKenzie DC, Vallerand JR, Morielli AR, Mackey JR et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat 2016;158:497–507 [DOI] [PubMed] [Google Scholar]
- 16. Arbane G, Tropman D, Jackson D, Garrod R. Evaluation of an early exercise intervention after thoracotomy for non-small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer Amst Neth 2011;71:229–234 [DOI] [PubMed] [Google Scholar]
- 17. Battaglini C, Bottaro M, Dennehy C, Rae L, Shields E, Kirk D et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J Rev Paul Med 2007;125:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beattie AH, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000;46:813–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benedetti MG, Cavazzuti L, Amabile M, Tassinari E, Valente G, Zanotti G et al. Abductor muscle strengthening in THA patients operated with minimally invasive anterolateral approach for developmental hip dysplasia. Hip Int 2021;31:66–74 [DOI] [PubMed] [Google Scholar]
- 20. Botella-Carretero JI, Iglesias B, Balsa JA, Arrieta F, Zamarrón I, Vázquez C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: a randomized clinical trial. Clin Nutr Edinb Scotl 2010;29:574–579 [DOI] [PubMed] [Google Scholar]
- 21. Bruce D, Laurance I, McGuiness M, Ridley M, Goldswain P. Nutritional supplements after hip fracture: poor compliance limits effectiveness. Clin Nutr Edinb Scotl 2003;22:497–500 [DOI] [PubMed] [Google Scholar]
- 22. Bulger EM, Jurkovich GJ, Farver CL, Klotz P, Maier RV. Oxandrolone does not improve outcome of ventilator dependent surgical patients. Ann Surg 2004;240:472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Busch JC, Lillou D, Wittig G, Bartsch P, Willemsen D, Oldridge N et al. Resistance and balance training improves functional capacity in very old participants attending cardiac rehabilitation after coronary bypass surgery. J Am Geriatr Soc 2012;60:2270–2276 [DOI] [PubMed] [Google Scholar]
- 24. Delitto A, Rose SJ,, McKowen JM, Lehman RC, Thomas JA, Shively RA. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Phys Ther 1988;68:660–663 [DOI] [PubMed] [Google Scholar]
- 25. Do JH, Choi KH, Ahn JS, Jeon JY. Effects of a complex rehabilitation program on edema status, physical function, and quality of life in lower-limb lymphedema after gynecological cancer surgery. Gynecol Oncol 2017;147:450–455 [DOI] [PubMed] [Google Scholar]
- 26. Dreyer HC, Strycker LA, Senesac HA, Hocker AD, Smolkowski K, Shah SN et al. Essential amino acid supplementation in patients following total knee arthroplasty. J Clin Invest 2013;123:4654–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fitzgerald GK, Piva SR, Irrgang JJ. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2003;33:492–501 [DOI] [PubMed] [Google Scholar]
- 28. Flodin L, Cederholm T, Sääf M, Samnegård E, Ekström W, Al-Ani AN et al. Effects of protein-rich nutritional supplementation and bisphosphonates on body composition, handgrip strength and health-related quality of life after hip fracture: a 12-month randomized controlled study. BMC Geriatr 2015;15:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedmann-Bette B, Profit F, Gwechenberger T, Weiberg N, Parstorfer M, Weber MA et al. Strength training effects on muscular regeneration after ACL reconstruction. Med Sci Sports Exerc 2018;50:1152–1161 [DOI] [PubMed] [Google Scholar]
- 30. Gerber JP, Marcus RL, Dibble LE, Greis PE, Burks RT, LaStayo PC. Effects of early progressive eccentric exercise on muscle structure after anterior cruciate ligament reconstruction. J Bone Joint Surg Am 2007;89:559–570 [DOI] [PubMed] [Google Scholar]
- 31. Ghroubi S, Elleuch W, Abid L, Abdenadher M, Kammoun S, Elleuch MH. Effects of a low-intensity dynamic-resistance training protocol using an isokinetic dynamometer on muscular strength and aerobic capacity after coronary artery bypass grafting. Ann Phys Rehabil Med 2013;56:85–101 [DOI] [PubMed] [Google Scholar]
- 32. Granger CL, Chao C, McDonald CF, Berney S, Denehy L. Safety and feasibility of an exercise intervention for patients following lung resection: a pilot randomized controlled trial. Integr Cancer Ther 2013;12:213–224 [DOI] [PubMed] [Google Scholar]
- 33. Grapar Zargi T, Drobnic M, Jkoder J, Strazar K, Kacin A. The effects of preconditioning with ischemic exercise on quadriceps femoris muscle atrophy following anterior cruciate ligament reconstruction: a quasi-randomized controlled trial. Eur J Phys Rehabil Med 2016;52:310–320 [PubMed] [Google Scholar]
- 34. Güneş Y, Karip B, Ergin A, Fersahoğlu MM, Bulut NE, Taşdelen I et al. The impact of protein support on weight loss, sarcopenia and quality of life after sleeve gastrectomy. Clin Nutr 2018;37:S26 [Google Scholar]
- 35. Hasegawa S, Kobayashi M, Arai R, Tamaki A, Nakamura T, Moritani T. Effect of early implementation of electrical muscle stimulation to prevent muscle atrophy and weakness in patients after anterior cruciate ligament reconstruction. J Electromyogr Kinesiol Off J Int Soc Electrophysiol Kinesiol 2011;21:622–630 [DOI] [PubMed] [Google Scholar]
- 36. Hedström M, Sääf M, Brosjö E, Hurtig C, Sjöberg K, Wesslau A et al. Positive effects of short-term growth hormone treatment on lean body mass and BMC after a hip fracture: a double-blind placebo-controlled pilot study in 20 patients. Acta Orthop Scand 2004;75:394–401 [DOI] [PubMed] [Google Scholar]
- 37. Hermann A, Holsgaard-Larsen A, Zerahn B, Mejdahl S, Overgaard S. Preoperative progressive explosive-type resistance training is feasible and effective in patients with hip osteoarthritis scheduled for total hip arthroplasty—a randomized controlled trial. Osteoarthritis Cartilage 2016;24:91–98 [DOI] [PubMed] [Google Scholar]
- 38. Houborg KB, Jensen MB, Rasmussen P, Gandrup P, Schroll M, Laurberg S. Postoperative physical training following colorectal surgery: a randomised, placebo-controlled study. Scand J Surg 2006;95:17–22 [DOI] [PubMed] [Google Scholar]
- 39. Invernizzi M, de Sire A, D’Andrea F, Carrera D, Renò F, Migliaccio S et al. Effects of essential amino acid supplementation and rehabilitation on functioning in hip fracture patients: a pilot randomized controlled trial. Aging Clin Exp Res 2019;31:1517–1524 [DOI] [PubMed] [Google Scholar]
- 40. Iversen E, Røstad V, Larmo A. Intermittent blood flow restriction does not reduce atrophy following anterior cruciate ligament reconstruction. J Sport Health Sci 2016;5:115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jensen MB, Hessov I. Dietary supplementation at home improves the regain of lean body mass after surgery. Nutr Burbank Los Angel Cty Calif 1997;13:422–430 [DOI] [PubMed] [Google Scholar]
- 42. Karelis AD, Hébert MJ, Rabasa-Lhoret R, Räkel A. Impact of resistance training on factors involved in the development of new-onset diabetes after transplantation in renal transplant recipients: an open randomized pilot study. Can J Diabetes 2016;40:382–328 [DOI] [PubMed] [Google Scholar]
- 43. Lambert B, Hedt CA, Jack RA, Moreno M, Delgado D, Harris JD et al. Blood flow restriction therapy preserves whole limb bone and muscle following ACL reconstruction. Orthop J Sports Med 2019;7(Suppl 2):2325967119S00196 [Google Scholar]
- 44. Lattanzi B, Giusto M, Albanese C, Mennini G, D’Ambrosio D, Farcomeni A et al. The effect of 12 weeks of β-hydroxy-β-methyl-butyrate supplementation after liver transplantation: a pilot randomized controlled study. Nutrients 2019;11:2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liao CD, Tsauo JY, Chiu YS, Ku JW, Huang SW, Liou TH. Effects of elastic resistance exercise after total knee replacement on muscle mass and physical function in elderly women with osteoarthritis: a randomized controlled trial. Am J Phys Med Rehabil 2020;99:381–389 [DOI] [PubMed] [Google Scholar]
- 46. Lima PS, de Campos AS, de Faria Neto O, Ferreira TCA, Amorim CEN, Stone WJ et al. Effects of combined resistance plus aerobic training on body composition, muscle strength, aerobic capacity, and renal function in kidney transplantation subjects. J Strength Cond Res 2019 [DOI] [PubMed] [Google Scholar]
- 47. Macchi C, Fattirolli F, Lova RM, Conti AA, Luisi MLE, Intini R et al. Early and late rehabilitation and physical training in elderly patients after cardiac surgery. Am J Phys Med Rehabil 2007;86:826–834 [DOI] [PubMed] [Google Scholar]
- 48. Maiorana AJ, Briffa TG, Goodman C, Hung J. A controlled trial of circuit weight training on aerobic capacity and myocardial oxygen demand in men after coronary artery bypass surgery. J Cardpulm Rehabil 1997;17:239–247 [DOI] [PubMed] [Google Scholar]
- 49. Malafarina V, Uriz-Otano F, Malafarina C, Martinez JA, Zulet MA. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas 2017;101:42–50 [DOI] [PubMed] [Google Scholar]
- 50. Morano MT, Araújo AS, Nascimento FB, da Silva GF, Mesquita R, Pinto JS et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil 2013;94:53–58 [DOI] [PubMed] [Google Scholar]
- 51. Nishizaki K, Ikegami H, Tanaka Y, Imai R, Matsumura H. Effects of supplementation with a combination of β-hydroxy-β-methyl butyrate, l-arginine, and l-glutamine on postoperative recovery of quadriceps muscle strength after total knee arthroplasty. Asia Pac J Clin Nutr 2015;24:412–420 [DOI] [PubMed] [Google Scholar]
- 52. Noyes FR, Mangine RE, Barber S. Early knee motion after open and arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med 1987;15:149–160 [DOI] [PubMed] [Google Scholar]
- 53. Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand 2003;74:62–68 [DOI] [PubMed] [Google Scholar]
- 54. Oppert JM, Bellicha A, Roda C, Bouillot JL, Torcivia A, Clement K et al. Resistance training and protein supplementation increase strength after bariatric surgery: a randomized controlled trial. Obes Silver Spring Md 2018;26:1709–1720 [DOI] [PubMed] [Google Scholar]
- 55. Paternostro-Sluga T, Fialka C, Alacamliogliu Y, Saradeth T, Fialka-Moser V. Neuromuscular electrical stimulation after anterior cruciate ligament surgery. Clin Orthop 1999;368:166–175 [PubMed] [Google Scholar]
- 56. Ritch CR, Cookson MS, Clark PE, Chang SS, Fakhoury K, Ralls V et al. Perioperative oral nutrition supplementation reduces prevalence of sarcopenia following radical cystectomy: results of a prospective randomized controlled trial. J Urol 2019;201:470–477 [DOI] [PubMed] [Google Scholar]
- 57. Ross M. The effect of neuromuscular electrical stimulation during closed kinetic chain exercise on lower extremity performance following anterior cruciate ligament reconstruction. Sports Med Train Rehabil 2000;9:239–251 [Google Scholar]
- 58. Salvarani A, Agosti M, Zanrè A, Ampollini A, Montagna L, Franceschini M. Mechanical vibration in the rehabilitation of patients with reconstructed anterior cruciate ligament. Europa Medicophysica 2003;39:19–25 [Google Scholar]
- 59. Savastano S, Di Somma C, Angrisani L, Orio F, Longobardi S, Lombardi G et al. Growth hormone treatment prevents loss of lean mass after bariatric surgery in morbidly obese patients: results of a pilot, open, prospective, randomized, controlled study. J Clin Endocrinol Metab 2009;94:817–826 [DOI] [PubMed] [Google Scholar]
- 60. Schürch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998;128:801–809 [DOI] [PubMed] [Google Scholar]
- 61. Shaarani SR, O’Hare C, Quinn A, Moyna N, Moran R, O’Byrne JM. Effect of prehabilitation on the outcome of anterior cruciate ligament reconstruction. Am J Sports Med 2013;41:2117–2127 [DOI] [PubMed] [Google Scholar]
- 62. Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc 2012;13:24–30 [DOI] [PubMed] [Google Scholar]
- 63. Sisk TD, Stralka SW, Deering MB, Griffin JW. Effect of electrical stimulation on quadriceps strength after reconstructive surgery of the anterior cruciate ligament. Am J Sports Med 1987;15:215–220 [DOI] [PubMed] [Google Scholar]
- 64. Snyder-Mackler L, Ladin Z, Schepsis AA, Young JC. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament. Effects of electrically elicited contraction of the quadriceps femoris and hamstring muscles on gait and on strength of the thigh muscles. J Bone Joint Surg Am 1991;73:1025–1036 [PubMed] [Google Scholar]
- 65. Stigt JA, Uil SM, van Riesen SJH, Simons FJNA, Denekamp M, Shahin GM et al. A randomized controlled trial of postthoracotomy pulmonary rehabilitation in patients with resectable lung cancer. J Thorac Oncol 2013;8:214–221 [DOI] [PubMed] [Google Scholar]
- 66. Suetta C, Magnusson SP, Rosted A, Aagaard P, Jakobsen AK, Larsen LH et al. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients—a controlled, randomized study. J Am Geriatr Soc 2004;52:2016–2022 [DOI] [PubMed] [Google Scholar]
- 67. Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc 2000;32:2035–2039 [DOI] [PubMed] [Google Scholar]
- 68. Tengstrand B, Cederholm T, Söderqvist A, Tidermark J. Effects of protein-rich supplementation and nandrolone on bone tissue after a hip fracture. Clin Nutr Edinb Scotl 2007;26:460–465 [DOI] [PubMed] [Google Scholar]
- 69. Tsukagoshi R, Tateuchi H, Fukumoto Y, Ibuki S, Akiyama H, So K et al. Functional performance of female patients more than 6 months after total hip arthroplasty shows greater improvement with weight-bearing exercise than with non-weight-bearing exercise. Randomized controlled trial. Eur J Phys Rehabil Med 2014;50:665–675 [PubMed] [Google Scholar]
- 70. Van Meerbeeck J, Salhi B, Haenebalcke C, Perez-Bogerd S, Nguyen MD, Malfait T et al. Resistance training in patients with radically treated respiratory cancer: mature results of a multi-centre randomised phase 3 trial (REINFORCE). J Thorac Oncol 2013;8:S245 [Google Scholar]
- 71. Wigerstad-Lossing I, Grimby G, Jonsson T, Morelli B, Peterson L, Renström P. Effects of electrical muscle stimulation combined with voluntary contractions after knee ligament surgery. Med Sci Sports Exerc 1988;20:93–98 [DOI] [PubMed] [Google Scholar]
- 72. Wu B, Lorezanza D, Badash I, Berger M, Lane C, Sum JC et al. Perioperative testosterone supplementation increases lean mass in healthy men undergoing anterior cruciate ligament reconstruction: a randomized controlled trial. Orthop J Sports Med 2017;5:2325967117722794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ximenes NNPS, Borges DL, Lima RO, Barbosa e Silva MG, da Silva LN, Costa MdAG et al. Effects of resistance exercise applied early after coronary artery bypass grafting: a randomized controlled trial. Braz J Cardiovasc Surg 2015;30:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.