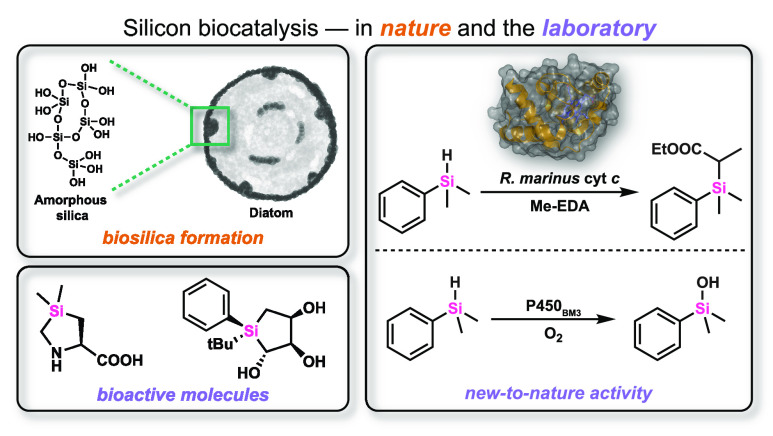
Abstract
Significant inroads have been made using biocatalysts to perform new-to-nature reactions with high selectivity and efficiency. Meanwhile, advances in organosilicon chemistry have led to rich sets of reactions holding great synthetic value. Merging biocatalysis and silicon chemistry could yield new methods for the preparation of valuable organosilicon molecules as well as the degradation and valorization of undesired ones. Despite silicon’s importance in the biosphere for its role in plant and diatom construction, it is not known to be incorporated into any primary or secondary metabolites. Enzymes have been found that act on silicon-containing molecules, but only a few are known to act directly on silicon centers. Protein engineering and evolution has and could continue to enable enzymes to catalyze useful organosilicon transformations, complementing and expanding upon current synthetic methods. The role of silicon in biology and the enzymes that act on silicon-containing molecules are reviewed to set the stage for a discussion of where biocatalysis and organosilicon chemistry may intersect.
Short abstract
This Outlook explores the role of silicon in biology, focusing on natural and engineered enzymes that act on silicon species, and provides a perspective on future directions in silicon biocatalysis.
Introduction
Silicon is found in copious amounts on Earth, where it comprises ∼28% of the lithosphere, is the second most abundant element after oxygen, and is present in teramole quantities in the oceans.1,2 It also holds a privileged position as one of the key elements of human enterprise. Humans have harnessed the unique properties of silicon for centuries, using silica to make structural materials including concrete, brick, and glass. More recent applications have propelled society to capabilities unimaginable a century ago. Leveraging of its electronic properties has laid the foundation for our modern society and innumerable resultant innovations.3 Simultaneously, organosilicon compounds are deployed on the scale of megatons/year for use in sealants and adjuvants for construction, agriculture, cosmetic, automotive, and high-performance aerospace applications.4
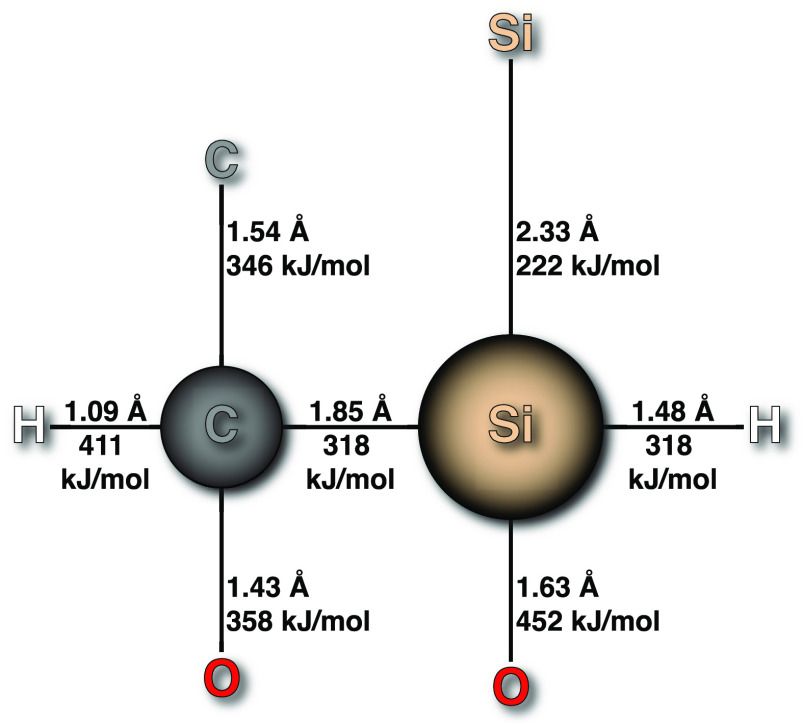
Silicon is also important in the biosphere where its role is dictated by its chemical properties and the scope of reactions it undergoes in the environment. Silicon appears just below carbon in group 14 of the periodic table and shares some similarities with the element that takes center stage throughout the tree of life, but they differ in important ways.5,6 Silicon has a larger covalent radius and a lower Pauling electronegativity than carbon (Figure 1). This leads to Si–E bonds being more polar in general than corresponding C–E bonds.7 Like carbon, silicon generally has a valency of four, but lacks carbon’s ability to form double or triple bonds under mild conditions.8 Silicon can also form hypervalent species, bonding to five—or even six—other atoms. Perhaps the most important difference between carbon and silicon is the ease with which the former forms long chains of the same element, known as catenation. Carbon, as is evident from the most rudimentary inspection of organic molecules, is highly adept at catenation. The predominant forms of silicon in nature contain Si–O bonds, which are substantially more stable than Si–Si bonds and explain the dearth of natural silicon catenation species (Figure 1).9 Polysilanes can be accessed synthetically10 but only under conditions that are wholly inaccessible biologically.
Figure 1.
Diagram of the typical bond lengths and bond energies of carbon and silicon.11 Silicon (covalent radius = 1.17 Å) can coordinate up to six atoms, while carbon (covalent radius = 0.77 Å) is maximally tetravalent.
That is not to say that silicon is absent in biochemistry. Silicon is an important structural building block for the skeletons of marine organisms such as diatoms, sponges, and radiolarians, which take up silicic acid (H4SiO4) and polymerize it to form a variety of intricate cell wall structures.2 In addition to modulating the flux of silicon in marine environments,12 these organisms play a key role in the carbon cycle via the “biological pump” through which photosynthetically fixed carbon from the surface ocean is transferred to the deep ocean, where it either remains sequestered or is eventually remineralized to CO2.13
In addition to its role in the cell walls of marine microbes, silicon plays important structural roles in plants. Silicon is widespread in soil in crystalline forms, and in the form of silicic acid, it is taken up by plants that then precipitate it to silicate species. This pathway has been demonstrated to alleviate biotic and abiotic stresses on plants, partly by structurally stabilizing the plant cell wall.14 Highlighting the importance of silicon for plant growth is the low concentration of silicic acid in soil, which limits the availability of silicon for plant growth and has become an agricultural challenge in certain regions.15 Besides these structural uses of silicon, this element is elusive in the biochemistry of most organisms.11
Myriad organosilicon compounds have been created in laboratory settings, and silicon and silicates are highly abundant and diverse on Earth, yet no organosilicon species have been found in nature. Organosilicon compounds have many uses in pharmaceuticals,9,16 asymmetric synthesis,17 polymers,18,19 and materials science.20 However, production of these compounds is often not easily accomplished and more facile synthetic methods are desirable. For example, most organosilicon compounds are produced from methylchlorosilane monomers, necessitating the use of nucleophilic substitution reactions or energy intensive reductions/direct synthesis reactions for production of useful organosilicon compounds.21 These reactions are stoichiometric in nature, often rely on harsh conditions, have inefficiencies in product yields, and can generate undesired byproducts.22,23 Biocatalysis has many features that can complement more traditional synthetic approaches,24,25 and recent advances in directed evolution have led to enzymes capable of non-natural activities including C–Si and C–B bond formation.26−28 The time is ripe to use enzyme engineering to realize these potential applications and extend the reactivity of enzymes toward organosilicon chemistry.
Biotransformation of Silicon
Biosilicification—Siliceous Marine Microorganisms and Plants
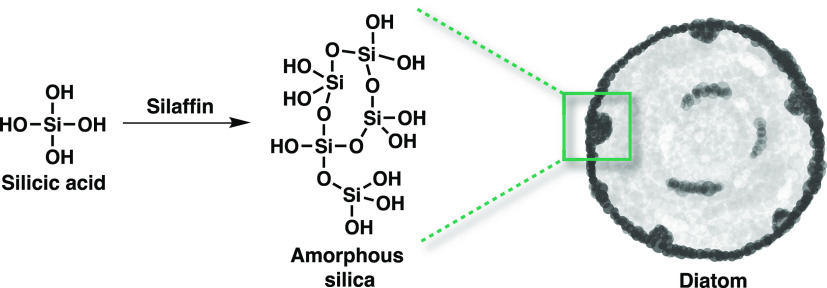
To understand how and why silicon is used by biological systems, consider the global biogeochemical cycle of silicon. Teramoles of silicon per year are processed by organisms as part of the silicon cycle, which mostly occurs in the world’s oceans. Silicon is released from long-term storage in the lithosphere to the oceans by weathering where it dissolves in seawater as silicic acid (H4SiO4).2 This soluble compound is distributed widely throughout the oceans where it is transferred from the hydrosphere to the biosphere. This process is essential for the life cycle of radiolarians, some flagellates, and diatoms, which use dissolved silicic acid to construct their intricate biosilica cell walls (Figure 2).
Figure 2.
Diatoms and other siliceous microorganisms precipitate dissolved silica to generate cell walls composed of biosilica.
The most abundant siliceous marine microorganisms are the photosynthetic diatoms, which synthesize silicified cell walls to provide mechanical protection against grazing. Global biosphere studies have estimated that diatoms conduct as much as 20% of total primary production—the amount of carbon dioxide fixed via photosynthesis—on Earth.13 These quickly growing organisms in turn strongly influence the global silicon and carbon cycles.
Diatoms generate their own inorganic silicified cell walls via a genetically encoded process that results in an array of unique cell wall arrangements. These cell walls are composed of nanopatterned silica with intricately arranged pores. The biosilica mineralization process occurs in silica-deposition vesicles and is directed by polypeptides known as silaffins that are enmeshed in biosilica and precipitate silica. These silaffins generate the regular patterns of silica observed in diatom cell walls.29,30 In order to form patterns that resemble diatom biosilica, extensive post-translational phosphorylation of silaffins is required.31
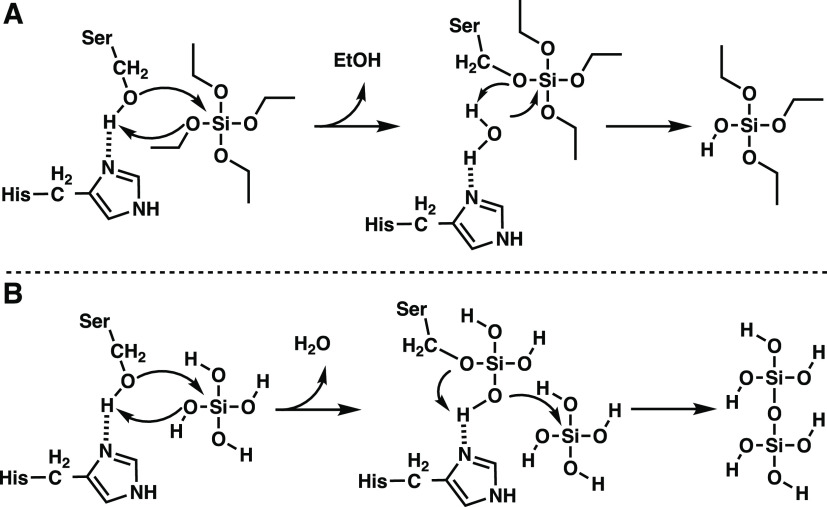
The siliceous marine sponge Tethya aurantia produces siliceous spicules that comprise some 75% of the organism’s dry mass.32 Embedded within the spicules are proteinaceous filaments comprised of three “silica proteins” known as silicateins α, β, and γ. Silicatein α is the most abundant of these proteins, comprising roughly 70% of the total silicatein mass and having approximately 50% sequence similarity to cathepsin L cysteine proteases.32,33 Silicatein α is able to hydrolyze the silicic acid surrogate tetraethyl orthosilicate in vitro (Figure 3A).33,34 The catalytic triad of silicatein α is composed of histidine and asparagine residues, similar to cathepsin L, while the third residue is not the canonical cysteine but rather a serine, which is the motif found in serine proteases.35 To investigate whether silicatein α functions as a catalyst, Morse and colleagues mutated residues Ser26 and His165 to alanine and assayed the variants for hydrolysis of tetraethyl orthosilicate. Mutation of either active site residue nearly abolishes activity, highlighting the importance of both residues and establishing that silicateins act as enzymes and play a catalytic role in templating biosilica formation in T. aurantia spicules.34
Figure 3.
(A) Mechanism of tetraethyl orthosilicate hydrolysis by silicatein.33,34 (B) Proposed mechanism of silicic acid hydrolysis and subsequent polymerization by silicatein.32,36
Recombinantly produced silicateins were demonstrated to act as enzymes and catalyze Si−O bond hydrolysis and condensation.37 The authors demonstrated that these enzymes could catalyze condensation of silanols and alcohols to yield silyl ethers. Silicatein α plays a catalytic role in silica polymerization via a mechanism including nucleophilic attack on the silicon atom, alcohol displacement, and siloxane bond formation (Figure 3B).36
Despite the abundance of silicon on Earth and its critical roles in plants and marine microbes, its known biochemistry does not venture far beyond these roles. Highlighting this is the lack of known natural enzymes that form Si–C bonds or perform biological alkylation of silicon.38
Enzymes That Transform Silicon Species
Biocatalysis has proven to be a useful platform for addressing challenges in synthetic organic chemistry, yet biocatalytic approaches to the formation of organosilicon compounds, including polymers and small molecules, are rare. Reinhold Tacke and colleagues were among the first to use microorganisms and enzymes to mediate such transformations.39,40 Most of these reactions were developed for the enantioselective reduction of silicon compounds and have been summarized in detail in excellent reviews by Frampton and Zelisko.41,42
A variety of enzymes, including lipases and proteases, have been demonstrated to catalyze the formation and hydrolysis of Si–O bonds.43−45 Siloxane bond formation under mild conditions was reported using several lipases and phytases expressed in Escherichia coli.(44) Frampton and Zelisko, along with others, have studied how proteases and lipases hydrolyze alkoxysilanes, form siloxane bonds, and cleave Si–O bonds.46−51 Their 2017 review details these efforts, which have expanded the known scope of siloxane chemistry accessible to enzymes.42
Silicones, which are comprised of siloxane units, are generally engineered to be stable under most environmental conditions. This stability means certain species are subject to long-range transport and persist in aqueous systems for more than a month while experiencing very little degradation.4 Thus, they can serve as a model system with which to study the activities of enzymes toward catalyzing transformations of silicon species. As long as organosilicon compounds have been in production, they have been released into various environmental compartments, and certain organisms may have either evolved or possess latent methods to metabolize some of them.4,52−54 The enzymes that act on these compounds can be used as a case study for what reactivities toward silicon have evolved in nature.
In addition to the enzymes that hydrolyze and condense Si–O bonds in vitro, enzymes also likely cleave Si–O bonds in living organisms.4 Studies claiming cleavage of Si–C bonds by microorganisms exist, but these have largely not been reproducible or have not unequivocally proven Si–C bond cleavage.4,55 In contrast, Si–C cleavage has been demonstrated to occur in higher organisms, including rats52,53,56,57 and humans,58 but the enzymes responsible for these transformations are unknown. Notably, C–H oxidation followed by the Brook rearrangement59 could be responsible for the Si–C cleavage exhibited in these studies.
Engineering Enzymes for Non-Natural Silicon Biocatalysis
The enzymes described above act on Si–O bonds, not Si–C bonds. No enzyme in nature is known that can form Si–C bonds or natively synthesize organosilicon compounds from available precursors. Biocatalysts have been engineered to conduct a myriad of new-to-nature reactions, which inspired Arnold and colleagues to engineer an enzyme that can construct Si–C bonds via carbene insertion into Si–H bonds.26,60
Si–C bond-formation strategies in chemical synthesis can achieve high selectivity but are limited in scope due to lengthy or energy-intensive routes, use of harsh reagents, and poor catalyst total turnover number (TTN).26 Previous demonstrations that enzymes can selectively catalyze non-natural carbene transfer reactions under aqueous conditions led to the hypothesis that heme enzymes might catalyze carbene insertion into Si–H bonds.26,61,62 Since iron was not known to catalyze Si–H carbene insertion, the authors tested whether free heme could catalyze the reaction between phenyldimethylsilane and ethyl 2-diazopropanoate (Me-EDA) and observed the formation of racemic product in aqueous buffer. A panel of cytochromes P450, cytochromes c, and myoglobins catalyzed the reaction with higher turnover number than free heme but generally poor enantioselectivity.
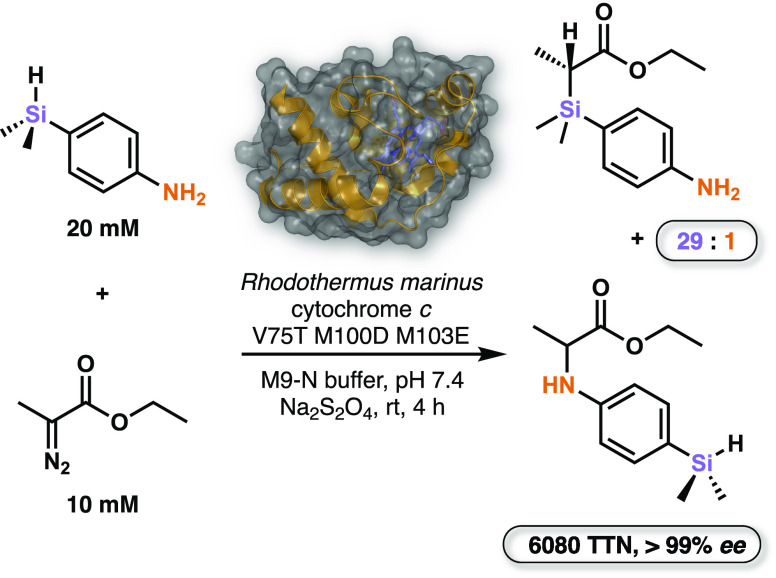
The electron-transfer protein Rhodothermus marinus cytochrome c (Rma cyt c), however, catalyzed the reaction with ∼40 TTN and 97% ee. Three rounds of directed evolution led to variant Rma cyt c V75T M100D M103E that catalyzed the reaction with >1500 TTN and >99% ee. The new enzyme was shown to selectively accept a variety of electronically diverse substituted silanes. It was also highly chemoselective: when challenged with 4-(dimethylsilyl)aniline, a substrate having two possible carbene insertion handles, it catalyzed Si–H insertion preferentially over N–H insertion (Figure 4). Interestingly, over the course of evolution, each variant showed improved chemoselectivity even though the screen only assessed Si–H insertion activity. When tested in a whole-cell reaction, the final variant furnished the Si–H insertion product with 3410 TTN, 70% isolated yield, and 98% ee. These reactions represent the first examples of in vitro and in vivo enzymatic Si–C bond formation and far outperform existing synthetic routes.
Figure 4.
Rma cytochrome c catalyzes Si–H insertion between 4-(dimethylsilyl)aniline and Me-EDA with 29:1 chemoselectivity over N–H insertion, 6080 TTN, and >99% ee.26 The same reaction can also proceed in whole E. coli cells. TTN = total turnover number.
In a further demonstration that enzymes can be engineered to directly functionalize silicon centers, the Arnold group demonstrated that cytochrome P450BM3 variants can hydroxylate silanes to make silanols. Silanols are important compounds for chemical transformations,63,64 polymer synthesis,65 catalysis,66 and synthesis,67 as functional groups in drugs,16 and as antimicrobial agents.68 The primary routes for silanol synthesis involve the oxidation of hydridosilanes, which rely on precious metal catalysts or toxic and commercially unavailable oxidation agents.69−73 Synthetic methods involving the hydrolysis of chlorosilane or alkoxysilane precursors encounter process challenges that include the formation of disiloxane byproducts.74 For the latter approach, extra care must be taken to select suitable reaction conditions (such as pH) and to control reaction byproducts (such as salts) which can affect product stability.75−77
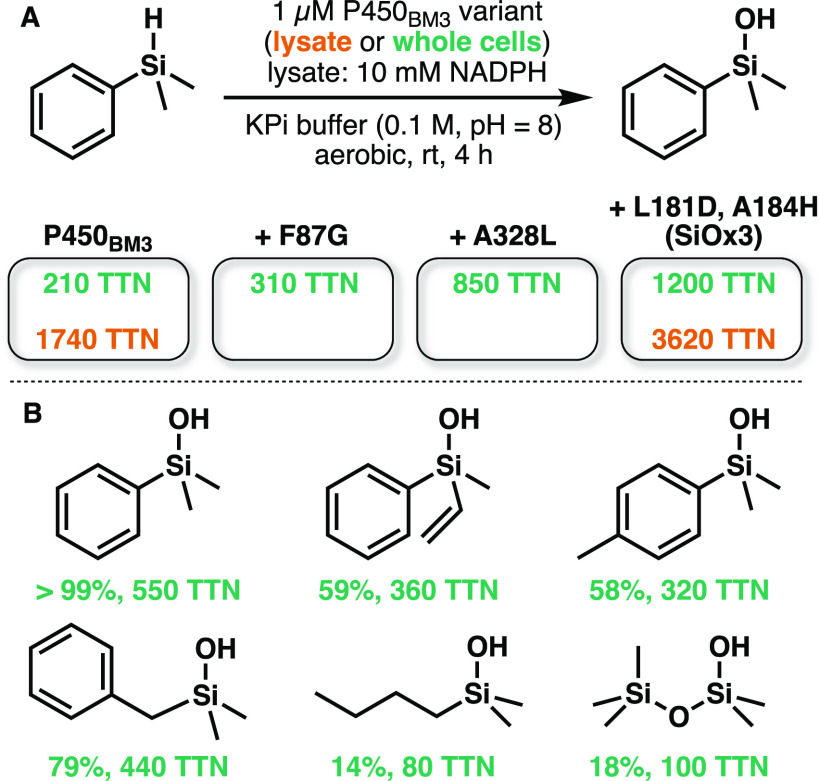
Aiming to develop a biocatalytic route to selectively synthesize silanols, Bähr and colleagues hypothesized that cytochrome P450BM3 could be repurposed to hydroxylate Si–H bonds.74 They found that the wild type enzyme accepted dimethylphenylsilane and formed product with 210 TTN (Figure 5A) in whole-cell reactions. Site-saturation mutagenesis of residue F87—which lies proximal to the heme cofactor and has been demonstrated to control substrate specificity—resulted in the identification of mutation F87G, which improved activity 1.5-fold. Further rounds of mutagenesis and screening identified additional activating mutations A328L, L181D, and A184H.
Figure 5.
(A) Cytochrome P450BM3 variants can hydroxylate the silicon center of dimethylphenylsilane in whole cells and lysate. (B) Variant SiOx3 oxidized dimethylphenylsilane with quantitative yield and can oxidize a variety of substituted, bulky, and aliphatic silanes, as well as one siloxane.74 TTN = total turnover number.
The final variant bearing these mutations catalyzes the formation of the silanol product with 1200 TTN. Using the enzyme in lysate boosted the TTN to 3620, an increase attributed to increased availability of NADPH. The authors then modified the reaction conditions to use higher protein concentration (8.1–9.0 μM) and lower substrate concentration (5 mM), which led to >99% analytical yield and 76% isolated yield of the silanol formed from dimethylphenylsilane (Figure 5B). Under these reaction conditions, the enzyme hydroxylates a variety of silane Si–H bonds (Figure 5B). The P450BM3 variants developed in this study can oxidize silanes in both cellular and in vitro contexts.
These engineering approaches have endowed naturally occurring enzymes with activities that were previously confined to the world of synthetic chemistry; the enzymes provide proof of principle and a platform from which to expand the application of enzymes to organosilicon chemistry. Although the repertoire of enzymes that act on silicon compounds is limited, these few examples suggest the potential for more activities on silicon species to be discovered and optimized by directed evolution.
Opportunities for Biocatalysis to Impact Organosilicon Chemistry
Expanding the ability of enzymes to directly functionalize and transform silicon species may enable more facile syntheses of organosilicon compounds. These molecules are of high value as pharmaceuticals and agrochemicals,9,16 and biocatalytic approaches to their production would complement existing synthetic methods. Traditional methods to form Si–C bonds in silicones rely on energy-intensive processes or require coinage metal catalysts, often making these molecules more expensive to produce than traditional organic polymers. In addition to being potentially more environmentally friendly and less costly than established methods, biocatalysts are exceptional at promoting reactions with high selectivity.28 Engineering proteins to act on organosilicon compounds would lead to new routes to their production and modification and could even expand the realm of accessible organosilicon compounds, resulting in previously unrealized applications.
Using Silicon as a Bioisostere of Carbon in Bioactive Compounds
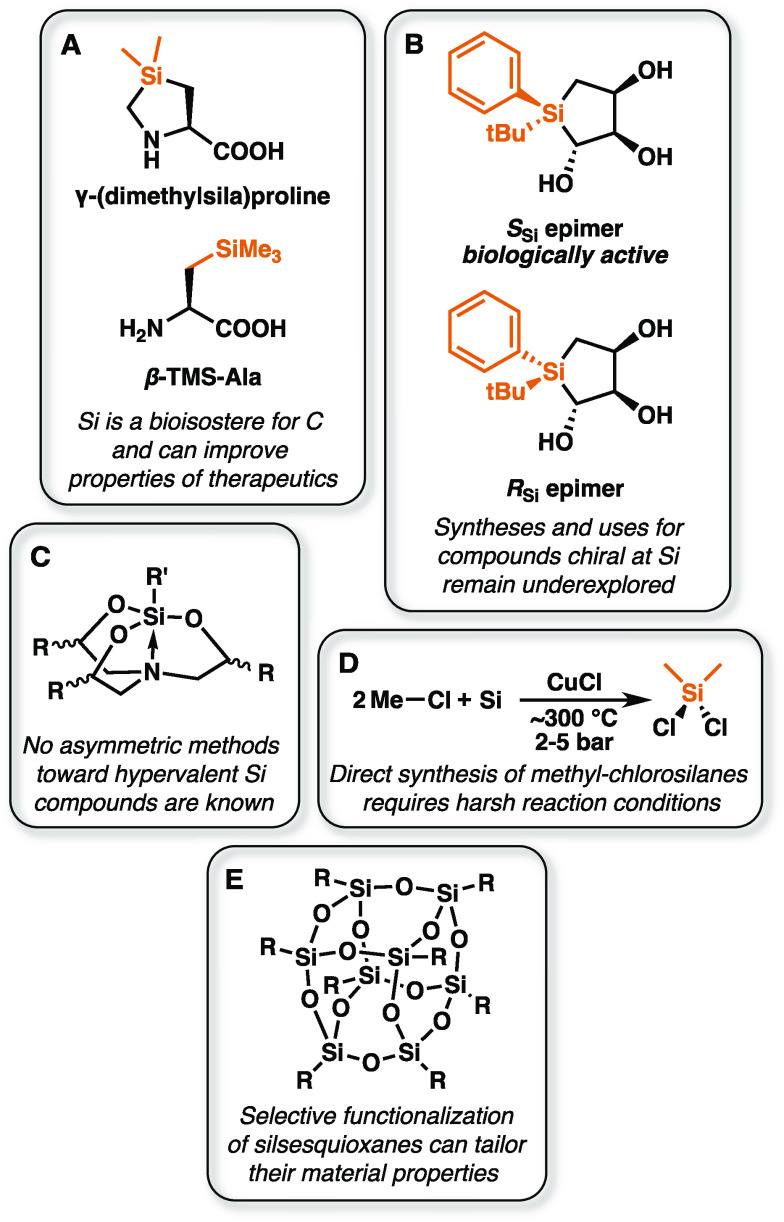
Although both silicon and carbon often display tetrahedral geometries, the longer Si–C bond length and silicon’s lower electronegativity and greater lipophilicity make swapping these elements a powerful way to modulate the properties of molecules.16 Silicon’s use as a bioisostere for carbon can endow molecules with convenient properties, particularly for use in biological systems (Figure 6A). Silicon is not inherently toxic, and its increased lipophilicity can improve its potency in pharmaceuticals.78 Drugs containing silicon are also metabolized differently than their exclusively carbon analogues, making this substitution an attractive tool to influence pharmacokinetics. For example, the antipsychotic drug haloperidol, a treatment for schizophrenia, is known to form a neurotoxic pyridinium metabolite.9,79 On the other hand, the silicon analogue sila-haloperidol, which is formed by sila-substitution of the quaternary carbon atom, is processed via an alternative metabolic pathway that does not include formation of the pyridinium metabolite due to the instability of the Si=C bond.79
Figure 6.
Some opportunities for biocatalysis in organosilicon chemistry. (A) Silicon is a bioisostere for carbon, and substituting Si for C can be a powerful strategy for modulating the properties of pharmaceuticals and other molecules. SiAAs are a key example. (B) Molecules chiral at Si can have important biological properties but can be difficult to synthesize. The SSi epimer of silacyclopentanetriol binds the serotonin receptor 5-HT2B tightly, while the RSi epimer is inactive.85 (C) No methods for the asymmetric synthesis of hypervalent Si complexes, like silatranes, are known despite their unique properties. (D) Dimethyldichlorosilane, the key feedstock for the silicone industry, relies on energy-intensive synthetic routes.21,22 Biomethylation of silicon would present an alternative route to methylated feedstocks. (E) Silsesquioxane materials have numerous emerging applications. Enzymes may complement existing functionalization strategies.
Biocatalysts that can selectively synthesize Si–C or Si–heteroatom bonds could lead to convenient routes to bioactive silicon compounds. As has been reported previously, a key challenge to using silicon in pharmaceuticals is the synthesis of target molecules.9 Entirely different routes are often needed to incorporate Si into druggable small molecules, even in compounds where silicon is used as a simple bioisostere to substitute carbon atoms. The most common route for the formation of Si–C bonds in small molecule synthesis is through the Pt-catalyzed hydrosilylation reaction of hydridosilanes with olefins or through organometallic/Grignard chemistry—two reactions not commonly used in pharmaceutical synthesis and that may present chemoselectivity issues with the polar functionalities commonly found on drugs.80 Additionally, Si is typically limited to being incorporated in place of a quaternary carbon or ketone (as a silane diol). Si–H and other Si–heteroatom bonds are much more reactive than the corresponding C–H or C–heteroatom bonds and thus are not amenable to incorporation into pharmaceuticals.9 The availability of protein biocatalysts capable of synthesizing organosilicon compounds26,74 would open up new areas of medicinal silicon chemistry.
Silicon-Containing Amino Acids (SiAAs)
Noncanonical amino acids have a variety of applications in the life sciences and adjacent industries. In particular, SiAAs are useful for synthesizing bioactive peptides with altered properties (Figure 6A).81 These peptides are, like other peptides containing noncanonical amino acids, more resistant to proteolysis than natural peptides, but SiAAs can endow them with other beneficial effects as well. For instance, replacing a single Pro with γ-(dimethylsila)proline in Pro-rich cell-penetrating peptides was found to dramatically improve cellular uptake.82 β-TMS-Ala has been used as an effective substitute for β-t-butyl-alanine as well as phenylalanine. β-TMS-Ala and β-t-butyl-alanine are identical except for a single C-to-Si substitution, while β-TMS-Ala and Phe have similar lipophilicities.16 Examples of bioactive silicon-containing peptides are described in a recent review.81
Despite the utility of SiAAs, methods for the enantioselective preparation of SiAAs remain difficult.81 Their construction may be facilitated by the exquisite selectivity of enzymes. Indeed, biocatalysts have been used extensively to construct noncanonical amino acids, with tryptophan synthase being a prominent example.83,84 Traditional methods to prepare chiral SiAAs require chiral auxiliaries, which could be avoided with a biocatalytic route.
Chiral Silicon Centers
Silicon often adopts a tetrahedral geometry and thus can be a chiral center. The preparation of molecules chiral at silicon has been reviewed extensively.86 Critically, asymmetric syntheses of these compounds are particularly challenging because sp2 silicon species are exceptionally labile.87,88 Unlike carbon, which readily forms double bonds with many other elements, Si=E bonds (E = Si, C, N, P, and others) are highly reactive; these compounds, when they exist at all, typically require low temperatures, large substituents, and air- and water-free conditions to persist. This is due to the π bonds between Si and other atoms being very weak. As a result, desymmetrization of silicon cannot be achieved by going through an sp2 intermediate, unlike with carbon, where desymmetrization of aldehydes, olefins, and other sp2 carbon atoms is a powerful strategy for creating chiral carbon centers. Biocatalytic methods complementing existing synthetic tools would be invaluable for expanding the chemical space available to researchers.
Examples of bioactive chiral silicon molecules are limited, presumably due to difficulties with their construction. One recent example is a silacyclopentanetriol (Figure 6B). One epimer of this molecule binds tightly to the serotonin receptor 5-HT2B (IC50 = 6.4 μM), while the other epimer does not display significant binding (IC50 > 100 μM).85 This example underscores how effective methods to prepare molecules chiral at silicon could open up an underexplored chemical space for pharmaceuticals. There are no known natural molecules exhibiting chirality at silicon, and no natural enzymes are known to be capable of constructing these compounds.
The “sila-substitution” of drugs is not a new strategy.89 Silicon has long been used as a bioisostere of carbon, and syntheses of sila-substituted compounds go back over 50 years.90 One recent study compared loperamide, an antidiarrheal, with sila-loperamide, a sila-substituted analogue.91 A comparison of these compounds’ pharmacokinetic and pharmacodynamic properties revealed that, despite major differences in their in vitro properties (including clearance and permeability), their in vivo pharmacokinetic profiles are nearly identical. Thorough studies like this example underscore how the ability to access sila-substituted compounds can lead to novel molecules with unique pharmaceutical properties. Critically, the vast majority of sila-substituted drug analogues studied have been achiral at silicon.16 Methods to access molecules chiral at silicon would enable sila-substitutions at other carbons in pharmaceuticals.
Outside of biology, chiral polysilanes are of interest to polymer chemists.92 Polysilanes are polymers containing solely silicon backbones, and this σ-conjugated backbone endows these molecules with unique electronic and optical properties. When their backbone Si atoms are connected to chiral side groups, the resulting chiral polysilanes form helical structures.93 These features make polysilanes of potential use in applications that range from enantioselective separations and molecular recognition to nonlinear optics and chiroptical switches. However, their synthesis from dialkyldichlorosilanes involves a Wurtz reductive coupling and can be challenging to control, limiting the types of chiral polysilanes that can be produced. Methods to construct these molecules more cheaply and with greater selectivity would be invaluable for realizing these applications and more.
Enzymatic Synthesis of Hypervalent Silicon Species
Silicon can form hypervalent species with coordination numbers greater than 4.9,94 Methods to generate penta- and hexavalent silicon species from silica are well established but often require harsh conditions and a stoichiometric strong base. Even more problematic is that no asymmetric syntheses are known: all chiral hypervalent silicon species have been prepared via resolution (Figure 6C). Enzymatic catalysis presents a potential tool to construct chiral species in an asymmetric fashion under milder conditions.
Pentavalent silicon species such as silatranes exhibit valuable biological activities, including antimicrobial, antiviral, and anticancer properties.95 Asymmetric methods to construct these compounds would not only lower production costs but would enable the production of previously inaccessible chiral compounds. Notably, silatranes are reasonably stable to hydrolysis and can be synthesized under mild conditions with (substituted) triethanolamines and alkoxysilanes. The lack of natural enzymes capable of forming these non-natural compounds should not discourage the biocatalysis community from taking on this challenge.
Hydrolytically stable hexacoordinate silicon complexes have been shown to serve as nontoxic DNA intercalators.9,96 Silicon is less toxic and costly than transition metals, and thus, there is value in developing biologically active coordination complexes using Si rather than other metals. However, syntheses of hexavalent Si typically rely on intermediates unstable in water,97 and new chemical strategies would need to be developed to construct these molecules biocatalytically.
Silicon–Carbon Bond Construction
Si–C motifs are of importance to chemical synthesis, pharmaceuticals, and materials. Most methods to prepare these compounds rely on precious metals or start with reduced forms of silicon. Expanding the capability of enzymes to form Si–C motifs may enable more efficient and cost-effective preparations of these compounds.17,98
Biological methylation of many metals and metalloids is performed by organisms using methyltransferases. Of the primordial group 14 elements, Si is the only element that is not known to be methylated (or alkylated) in nature.38,99,100 The lack of enzymatically produced alkylsilanes can probably be attributed to silicon’s form in the biosphere. Silicon is found largely as silica and silicic acid, which are highly stable and of low toxicity. Organisms have little need to evolve enzymes and expend energy to metabolize these compounds, and such pathways have not been found. Alkylsilanes, however, are tremendously valuable industrially. Methyl groups are the most ubiquitous Si–C motifs and are typically installed via the Rochow–Müller “Direct Process” reaction.21,101 In this reaction, elemental Si is reacted with methyl chloride at elevated temperatures (300–320 °C) in the presence of a copper catalyst (Figure 6D). The production of elemental Si itself requires an energy-intensive carbothermic reduction of silica at temperatures above 2000 °C. Biomethylation of Si presents a milder, redox-neutral route to Me–Si bonds, and directed evolution of known methyltransferases may be an approach to access these compounds.
Hydridosilanes have been shown to undergo reactions with engineered enzymes to form Si–C bonds. Hydrosilylation, the reaction of an Si–H bond with an unsaturated C–C bond or carbonyl, is typically catalyzed by Pt or other expensive metals.80 The reaction is also known with more abundant first-row transition metals but is much less robust. It may be possible to generate enzymes that perform this reaction using more earth-abundant metals and access regio- and chemoselectivity for hydrosilylation currently not accessible using traditional homogeneous catalysis. Si–H bonds may also provide access to silylium ions or highly reactive Si–E bonds (E = S, transition metals) under enzymatic catalysis. Such approaches have been demonstrated in homogeneous catalysis using metal complexes approximating [NiFe] hydrogenases. These intermediates could form a number of Si–heteroatom bonds. Interception with arenes in a Friedel–Crafts reaction would produce arylsilanes, which have applications in pharmaceuticals, agrochemicals, and materials. Interception with oxygen nucleophiles would generate silyl ethers or siloxane bonds. Nitrogen nucleophiles would afford silazanes, another common class of silicon compounds. Directed evolution of enzymes may allow access to chemoselectivities not possible under standard reaction conditions.
Biologically Templated Inorganic Materials
Silicatein enzymes are responsible for the formation of vast quantities of inorganic silica structures in nature. Engineering silicateins or enzymes with similar mechanisms may enable the construction of an array of small molecule organosilicon species bearing complex functionalities and may also be employed to synthesize inorganic materials.32,37,102
Recent work has shown that the post-translational modifications that decorate diatom silaffins can be modulated to generate diverse silica morphologies.103 A suite of enzymes was used to decorate the R5 silaffin with modifications including the native phosphorylation as well as methylation, acetylation, and myristoylation. These modifying enzymes and the R5 silaffin, when co-expressed in E. coli, were able to precipitate silica and form nanostructured silica with diverse, controlled physicochemical characteristics and morphologies.
Silsesquioxanes are a class of organosilicon compounds that adopt three-dimensional cage-like conformations via a repeating motif wherein each silicon atom is bonded to three oxygen atoms and a functional group (RSiO3/2).104 Of particular relevance for materials applications are polyoctahedral silsesquioxanes (POSSs) which form cubic cages that can be used as precursors to polymers. The inorganic Si–O–Si backbone of silsesquioxanes is stable, enabling the construction of polymeric compounds, while the functional groups allow installation of potentially diverse reactivities to meet applications in nanocomposites, optoelectronics, catalysis, high-temperature composites, and biomaterials (Figure 6E).104 Controlling the functional substituents on silsesquioxanes remains an outstanding challenge and is an area where the chemo- and regioselectivity of enzymes may aid in precisely functionalizing silicon centers en route to designer silsesquioxanes.
Summary and Outlook
The common refrain has been that organosilicon species are inaccessible to enzymes. However, recent studies have highlighted natural enzymes that act on silicon species and have demonstrated the adaptability of engineered enzymes to perform reactions on silicon species. With the proof-of-principle that enzymes can perform catalytic transformations of silicon species, the doors to merging biocatalysis and organosilicon chemistry have been opened. We encourage researchers in biocatalysis and organosilicon chemistry to use biocatalysis as another tool to access organosilicon transformations with the sustainability and selectivity inherent to biocatalytic platforms.
Acknowledgments
The authors would like to thank Dr. Sabine Brinkmann-Chen (Caltech) for invaluable scientific discussions and feedback. This work was supported by the Dow University Partnership Initiative under grants 227027AO and 227027AU. N.S.S. is also supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1745301. B.J.L. is also supported by a NIH Ruth L. Kirschstein NRSA Postdoctoral Fellowship under Award No. F32GM134566. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
The authors declare no competing financial interest.
References
- Struyf E.; Smis A.; Van Damme S.; Meire P.; Conley D. J. The Global Biogeochemical Silicon Cycle. Silicon 2009, 1 (4), 207–213. 10.1007/s12633-010-9035-x. [DOI] [Google Scholar]
- Tréguer P.; Nelson D. M.; Bennekom A. J. V.; DeMaster D. J.; Leynaert A.; Quéguiner B. The Silica Balance in the World Ocean: A Reestimate. Science 1995, 268 (5209), 375–379. 10.1126/science.268.5209.375. [DOI] [PubMed] [Google Scholar]
- Chelikowsky J.Introduction: Silicon in All Its Forms. In Silicon: Evolution and Future of a Technology; Siffert P., Krimmel E. F., Eds.; Springer: Berlin, Heidelberg, 2004; pp 1–22. 10.1007/978-3-662-09897-4_1. [DOI] [Google Scholar]
- Rücker C.; Kümmerer K. Environmental Chemistry of Organosiloxanes. Chem. Rev. 2015, 115 (1), 466–524. 10.1021/cr500319v. [DOI] [PubMed] [Google Scholar]
- Bertrand G. The Modest Undressing of a Silicon Center. Science 2004, 305 (5685), 783–785. 10.1126/science.1102270. [DOI] [PubMed] [Google Scholar]
- Marschner C.; Tilley T. D. Current Advances in the Chemistry of Silicon: Not Exactly a Carbon Copy. Dalton Trans. 2017, 46 (27), 8699–8700. 10.1039/C7DT90112G. [DOI] [PubMed] [Google Scholar]
- Auner N.; Weis J.. Organosilicon Chemistry III: From Molecules to Materials; John Wiley & Sons, 2008. [Google Scholar]
- Hanusch F.; Groll L.; Inoue S. Recent Advances of Group 14 Dimetallenes and Dimetallynes in Bond Activation and Catalysis. Chem. Sci. 2021, 12 (6), 2001–2015. 10.1039/D0SC03192E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh R.; Reddy D. S. Quest for Novel Chemical Entities through Incorporation of Silicon in Drug Scaffolds. J. Med. Chem. 2018, 61 (9), 3779–3798. 10.1021/acs.jmedchem.7b00718. [DOI] [PubMed] [Google Scholar]
- Miller R. D.; Michl J. Polysilane High Polymers. Chem. Rev. 1989, 89 (6), 1359–1410. 10.1021/cr00096a006. [DOI] [Google Scholar]
- Petkowski J. J.; Bains W.; Seager S. On the Potential of Silicon as a Building Block for Life. Life 2020, 10 (6), 84. 10.3390/life10060084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbrust E. V. The Life of Diatoms in the World’s Oceans. Nature 2009, 459 (7244), 185–192. 10.1038/nature08057. [DOI] [PubMed] [Google Scholar]
- Tréguer P.; Bowler C.; Moriceau B.; Dutkiewicz S.; Gehlen M.; Aumont O.; Bittner L.; Dugdale R.; Finkel Z.; Iudicone D.; et al. Influence of Diatom Diversity on the Ocean Biological Carbon Pump. Nat. Geosci. 2018, 11 (1), 27–37. 10.1038/s41561-017-0028-x. [DOI] [Google Scholar]
- Luyckx M.; Hausman J.-F.; Lutts S.; Guerriero G.. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 10.3389/fpls.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Xiao X.; Xu Y.; Chen B. Environmental Effects of Silicon within Biochar (Sichar) and Carbon–Silicon Coupling Mechanisms: A Critical Review. Environ. Sci. Technol. 2019, 53 (23), 13570–13582. 10.1021/acs.est.9b03607. [DOI] [PubMed] [Google Scholar]
- Franz A. K.; Wilson S. O. Organosilicon Molecules with Medicinal Applications. J. Med. Chem. 2013, 56 (2), 388–405. 10.1021/jm3010114. [DOI] [PubMed] [Google Scholar]
- Chan T. H.; Wang D. Chiral Organosilicon Compounds in Asymmetric Synthesis. Chem. Rev. 1992, 92 (5), 995–1006. 10.1021/cr00013a012. [DOI] [Google Scholar]
- Hurd D. T.; Roedel G. F. Vinyl and Allyl Silicone Polymers and Copolymers. Ind. Eng. Chem. 1948, 40 (11), 2078–2081. 10.1021/ie50467a015. [DOI] [Google Scholar]
- Colas A.Silicones: Preparation, Properties and Performance; Dow Corning, Life Sciences: 2005. [Google Scholar]
- Leitao E. M.; Jurca T.; Manners I. Catalysis in Service of Main Group Chemistry Offers a Versatile Approach to p -Block Molecules and Materials. Nat. Chem. 2013, 5 (10), 817–829. 10.1038/nchem.1749. [DOI] [PubMed] [Google Scholar]
- Seyferth D. Dimethyldichlorosilane and the Direct Synthesis of Methylchlorosilanes. The Key to the Silicones Industry. Organometallics 2001, 20 (24), 4978–4992. 10.1021/om0109051. [DOI] [Google Scholar]
- Roberts J. M.; Pushkarev V. V.; Sturm J. J.; Katsoulis D. E. Toward a New Direct Process: Synthesis of Methylmethoxysilanes from Dimethyl Carbonate and Pentacopper Silicide. Ind. Eng. Chem. Res. 2020, 59 (16), 7457–7465. 10.1021/acs.iecr.0c00505. [DOI] [Google Scholar]
- Rösch L.; John P.; Reitmeier R.. Silicon Compounds, Organic. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: 2000. 10.1002/14356007.a24_021. [DOI] [Google Scholar]
- Sheldon R. A.; Brady D. The Limits to Biocatalysis: Pushing the Envelope. Chem. Commun. 2018, 54 (48), 6088–6104. 10.1039/C8CC02463D. [DOI] [PubMed] [Google Scholar]
- Bornscheuer U. T. The Fourth Wave of Biocatalysis Is Approaching. Philos. Trans. R. Soc., A 2018, 376 (2110), 20170063. 10.1098/rsta.2017.0063. [DOI] [PubMed] [Google Scholar]
- Kan S. B. J.; Lewis R. D.; Chen K.; Arnold F. H. Directed Evolution of Cytochrome c for Carbon–Silicon Bond Formation: Bringing Silicon to Life. Science 2016, 354 (6315), 1048–1051. 10.1126/science.aah6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan S. B. J.; Huang X.; Gumulya Y.; Chen K.; Arnold F. H. Genetically Programmed Chiral Organoborane Synthesis. Nature 2017, 552 (7683), 132–136. 10.1038/nature24996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold F. H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem., Int. Ed. 2018, 57 (16), 4143–4148. 10.1002/anie.201708408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen N.; Sumper M.; Kroger N. Biosilica Formation in Diatoms: Characterization of Native Silaffin-2 and Its Role in Silica Morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (21), 12075–12080. 10.1073/pnas.2035131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H.; Jaeger V.; Schmüser L.; Bonn M.; Pfaendtner J.; Weidner T. The Structure of the Diatom Silaffin Peptide R5 within Freestanding Two-Dimensional Biosilica Sheets. Angew. Chem., Int. Ed. 2017, 56 (28), 8277–8280. 10.1002/anie.201702707. [DOI] [PubMed] [Google Scholar]
- Kröger N.; Poulsen N. Diatoms—From Cell Wall Biogenesis to Nanotechnology. Annu. Rev. Genet. 2008, 42 (1), 83–107. 10.1146/annurev.genet.41.110306.130109. [DOI] [PubMed] [Google Scholar]
- Brutchey R. L.; Morse D. E. Silicatein and the Translation of Its Molecular Mechanism of Biosilicification into Low Temperature Nanomaterial Synthesis. Chem. Rev. 2008, 108 (11), 4915–4934. 10.1021/cr078256b. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Cha J.; Stucky G. D.; Morse D. E. Silicatein α: Cathepsin L-like Protein in Sponge Biosilica. Proc. Natl. Acad. Sci. U. S. A. 1998, 95 (11), 6234–6238. 10.1073/pnas.95.11.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Shimizu K.; Cha J. N.; Stucky G. D.; Morse D. E. Efficient Catalysis of Polysiloxane Synthesis by Silicatein α Requires Specific Hydroxy and Imidazole Functionalities. Angew. Chem., Int. Ed. 1999, 38 (6), 779–782. . [DOI] [PubMed] [Google Scholar]
- Cha J. N.; Shimizu K.; Zhou Y.; Christiansen S. C.; Chmelka B. F.; Stucky G. D.; Morse D. E. Silicatein Filaments and Subunits from a Marine Sponge Direct the Polymerization of Silica and Silicones in Vitro. Proc. Natl. Acad. Sci. U. S. A. 1999, 96 (2), 361–365. 10.1073/pnas.96.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.; Keding R.; Höche T.; Yue Y. Biologically Formed Mesoporous Amorphous Silica. J. Am. Chem. Soc. 2009, 131 (7), 2717–2721. 10.1021/ja808847y. [DOI] [PubMed] [Google Scholar]
- Tabatabaei Dakhili S. Y.; Caslin S. A.; Faponle A. S.; Quayle P.; de Visser S. P.; Wong L. S. Recombinant Silicateins as Model Biocatalysts in Organosiloxane Chemistry. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (27), E5285–E5291. 10.1073/pnas.1613320114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. S. Biological Methylation of Less-Studied Elements. Appl. Organomet. Chem. 2002, 16 (12), 677–691. 10.1002/aoc.375. [DOI] [Google Scholar]
- Ryabov A. D. The Biochemical Reactions of Organometallics with Enzymes and Proteins. Angew. Chem., Int. Ed. Engl. 1991, 30 (8), 931–941. 10.1002/anie.199109311. [DOI] [Google Scholar]
- Tacke R. Milestones in the Biochemistry of Silicon: From Basic Research to Biotechnological Applications. Angew. Chem., Int. Ed. 1999, 38 (20), 3015–3018. . [DOI] [PubMed] [Google Scholar]
- Frampton M. B.; Zelisko P. M. Organosilicon Biotechnology. Silicon 2009, 1 (3), 147–163. 10.1007/s12633-009-9021-3. [DOI] [Google Scholar]
- Frampton M. B.; Zelisko P. M. Biocatalysis in Silicon Chemistry. Chem. - Asian J. 2017, 12 (11), 1153–1167. 10.1002/asia.201700214. [DOI] [PubMed] [Google Scholar]
- Brandstadt K. F. Inspired by Nature: An Exploration of Biocatalyzed Siloxane Bond Formation and Cleavage. Curr. Opin. Biotechnol. 2005, 16 (4), 393–397. 10.1016/j.copbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Abbate V.; Bassindale A. R.; Brandstadt K. F.; Lawson R.; Taylor P. G. Enzyme Mediated Silicon–Oxygen Bond Formation; the Use of Rhizopus Oryzae Lipase, Lysozyme and Phytase under Mild Conditions. Dalton Trans. 2010, 39 (39), 9361. 10.1039/c0dt00151a. [DOI] [PubMed] [Google Scholar]
- Bassindale A. R.; Brandstadt K. F.; Lane T. H.; Taylor P. G. Enzyme-Catalysed Siloxane Bond Formation. J. Inorg. Biochem. 2003, 96 (2–3), 401–406. 10.1016/S0162-0134(03)00177-6. [DOI] [PubMed] [Google Scholar]
- Frampton M. B.; Zelisko P. M. A Comparison of Protease Active Sites and Their Ability to Process Silicon-Based Substrates. Silicon 2012, 4 (1), 51–56. 10.1007/s12633-011-9087-6. [DOI] [Google Scholar]
- Frampton M. B.; Subczynska I.; Zelisko P. M. Biocatalytic Synthesis of Silicone Polyesters. Biomacromolecules 2010, 11 (7), 1818–1825. 10.1021/bm100295z. [DOI] [PubMed] [Google Scholar]
- Frampton M. B.; Simionescu R.; Dudding T.; Zelisko P. M. The Enzymatic Cleavage of Si–O Bonds: A Kinetic Analysis of the Biocatalyzed Hydrolysis of Phenyltrimethoxysilane. J. Mol. Catal. B: Enzym. 2010, 66 (1), 105–112. 10.1016/j.molcatb.2010.04.002. [DOI] [Google Scholar]
- Frampton M. B.; Séguin J. P.; Marquardt D.; Harroun T. A.; Zelisko P. M. Synthesis of Polyesters Containing Disiloxane Subunits: Structural Characterization, Kinetics, and an Examination of the Thermal Tolerance of Novozym-435. J. Mol. Catal. B: Enzym. 2013, 85–86, 149–155. 10.1016/j.molcatb.2012.09.010. [DOI] [Google Scholar]
- Frampton M. B.; Zelisko P. M. Synthesis of Lipase-Catalysed Silicone -Polyesters and Silicone - Polyamides at Elevated Temperatures. Chem. Commun. 2013, 49 (81), 9269–9271. 10.1039/c3cc45380d. [DOI] [PubMed] [Google Scholar]
- Frampton M.; Vawda A.; Fletcher J.; Zelisko P. M. Enzyme-Mediated Sol–Gel Processing of Alkoxysilanes. Chem. Commun. 2008, (43), 5544–5546. 10.1039/b812389f. [DOI] [PubMed] [Google Scholar]
- Varaprath S.; McMahon J. M.; Plotzke K. P. Metabolites of Hexamethyldisiloxane and Decamethylcyclopentasiloxane in Fischer 344 Rat Urine—A Comparison of a Linear and a Cyclic Siloxane. Drug Metab. Dispos. 2003, 31 (2), 206–214. 10.1124/dmd.31.2.206. [DOI] [PubMed] [Google Scholar]
- Varaprath S.; Salyers K. L.; Plotzke K. P.; Nanavati S. Identification of Metabolites of Octamethylcyclotetrasiloxane (D4) in Rat Urine. Drug Metab. Dispos. 1999, 27 (11), 1267–1273. [PubMed] [Google Scholar]
- Boada E.; Santos-Clotas E.; Bertran S.; Cabrera-Codony A.; Martín M. J.; Bañeras L.; Gich F. Potential Use of Methylibium Sp. as a Biodegradation Tool in Organosilicon and Volatile Compounds Removal for Biogas Upgrading. Chemosphere 2020, 240, 124908. 10.1016/j.chemosphere.2019.124908. [DOI] [PubMed] [Google Scholar]
- Sabourin C. L.; Carpenter J. C.; Leib T. K.; Spivack J. L. Biodegradation of Dimethylsilanediol in Soils. Appl. Environ. Microbiol. 1996, 62 (12), 4352–4360. 10.1128/AEM.62.12.4352-4360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaprath S.; Salyers K. L.; Plotzke K. P.; Nanavati S. Extraction of Octamethylcyclotetrasiloxane and Its Metabolites from Biological Matrices. Anal. Biochem. 1998, 256 (1), 14–22. 10.1006/abio.1997.2475. [DOI] [PubMed] [Google Scholar]
- Plotzke K. P.; Crofoot S. D.; Ferdinandi E. S.; Beattie J. G.; Reitz R. H.; McNett D. A.; Meeks R. G. Disposition of Radioactivity in Fischer 344 Rats after Single and Multiple Inhalation Exposure to [14C]Octamethylcyclotetrasiloxane ([14C]D4). Drug Metab. Dispos. 2000, 28 (2), 192–204. [PubMed] [Google Scholar]
- Reddy M. B.; Andersen M. E.; Morrow P. E.; Dobrev I. D.; Varaprath S.; Plotzke K. P.; Utell M. J. Physiological Modeling of Inhalation Kinetics of Octamethylcyclotetrasiloxane in Humans during Rest and Exercise. Toxicol. Sci. 2003, 72 (1), 3–18. 10.1093/toxsci/kfg001. [DOI] [PubMed] [Google Scholar]
- Brook A. G. Molecular Rearrangements of Organosilicon Compounds. Acc. Chem. Res. 1974, 7 (3), 77–84. 10.1021/ar50075a003. [DOI] [Google Scholar]
- Wu Z.; Kan S. B. J.; Lewis R. D.; Wittmann B. J.; Arnold F. H. Machine Learning-Assisted Directed Protein Evolution with Combinatorial Libraries. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (18), 8852–8858. 10.1073/pnas.1901979116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberg O. F.; Fasan R.; Arnold F. H. Exploiting and Engineering Hemoproteins for Abiological Carbene and Nitrene Transfer Reactions. Curr. Opin. Biotechnol. 2017, 47, 102–111. 10.1016/j.copbio.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Arnold F. H. Engineering New Catalytic Activities in Enzymes. Nat. Catal. 2020, 3 (3), 203–213. 10.1038/s41929-019-0385-5. [DOI] [Google Scholar]
- Denmark S. E.; Regens C. S. Palladium-Catalyzed Cross-Coupling Reactions of Organosilanols and Their Salts: Practical Alternatives to Boron- and Tin-Based Methods. Acc. Chem. Res. 2008, 41 (11), 1486–1499. 10.1021/ar800037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A.; Danda Y.; Fujii T.; Hirabayashi K.; Osakada K. Hydroxorhodium Complex-Catalyzed Carbon–Carbon Bond-Forming Reactions of Silanediols with α,β-Unsaturated Carbonyl Compounds. Mizoroki-Heck-Type Reaction vs Conjugate Addition. J. Am. Chem. Soc. 2001, 123 (43), 10774–10775. 10.1021/ja015928l. [DOI] [PubMed] [Google Scholar]
- Ritter U.; Winkhofer N.; Schmidt H.-G.; Roesky H. W. New Cobalt Catalysts for Hydroformylations in Two-Phase Systems. Angew. Chem., Int. Ed. Engl. 1996, 35 (5), 524–526. 10.1002/anie.199605241. [DOI] [Google Scholar]
- Hirabayashi K.; Mori A.; Kawashima J.; Suguro M.; Nishihara Y.; Hiyama T. Palladium-Catalyzed Cross-Coupling of Silanols, Silanediols, and Silanetriols Promoted by Silver(I) Oxide. J. Org. Chem. 2000, 65 (17), 5342–5349. 10.1021/jo000679p. [DOI] [PubMed] [Google Scholar]
- Denmark S. E.; Ober M. H. Cross-Coupling Reactions of Arylsilanols with Substituted Aryl Halides. Org. Lett. 2003, 5 (8), 1357–1360. 10.1021/ol034328j. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Farrah S.; Baney R. H. Structure–Antimicrobial Activity Relationship for Silanols, a New Class of Disinfectants, Compared with Alcohols and Phenols. Int. J. Antimicrob. Agents 2007, 29 (2), 217–222. 10.1016/j.ijantimicag.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Valliant-Saunders K.; Gunn E.; Shelton G. R.; Hrovat D. A.; Borden W. T.; Mayer J. M. Oxidation of Tertiary Silanes by Osmium Tetroxide. Inorg. Chem. 2007, 46 (13), 5212–5219. 10.1021/ic062468u. [DOI] [PubMed] [Google Scholar]
- Limnios D.; Kokotos C. G. Organocatalytic Oxidation of Organosilanes to Silanols. ACS Catal. 2013, 3 (10), 2239–2243. 10.1021/cs400515w. [DOI] [Google Scholar]
- Lee M.; Ko S.; Chang S. Highly Selective and Practical Hydrolytic Oxidation of Organosilanes to Silanols Catalyzed by a Ruthenium Complex. J. Am. Chem. Soc. 2000, 122 (48), 12011–12012. 10.1021/ja003079g. [DOI] [Google Scholar]
- Barnes G. H.; Daughenbaugh N. E. The Preparation of Organosilanols via the Metal-Catalyzed Reaction of Organosilicon Hydrides with Water. J. Org. Chem. 1966, 31 (3), 885–887. 10.1021/jo01341a056. [DOI] [Google Scholar]
- Lee Y.; Seomoon D.; Kim S.; Han H.; Chang S.; Lee P. H. Highly Efficient Iridium-Catalyzed Oxidation of Organosilanes to Silanols. J. Org. Chem. 2004, 69 (5), 1741–1743. 10.1021/jo035647r. [DOI] [PubMed] [Google Scholar]
- Bähr S.; Brinkmann-Chen S.; Garcia-Borràs M.; Roberts J. M.; Katsoulis D. E.; Houk K. N.; Arnold F. H. Selective Enzymatic Oxidation of Silanes to Silanols. Angew. Chem., Int. Ed. 2020, 59 (36), 15507–15511. 10.1002/anie.202002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J. F.; Brown P. L.; Smith A. L. Inductive Effects in the Chlorosilane Hydrolysis Equilibrium. J. Am. Chem. Soc. 1960, 82 (22), 5854–5858. 10.1021/ja01507a022. [DOI] [Google Scholar]
- Lickiss P. D.The Synthesis and Structure of Organosilanols. In Advances in Inorganic Chemistry; Sykes A. G., Ed.; Academic Press: 1995; Vol. 42, pp 147–262. 10.1016/S0898-8838(08)60053-7. [DOI] [Google Scholar]
- Chandrasekhar V.; Boomishankar R.; Nagendran S. Recent Developments in the Synthesis and Structure of Organosilanols. Chem. Rev. 2004, 104 (12), 5847–5910. 10.1021/cr0306135. [DOI] [PubMed] [Google Scholar]
- Bains W.; Tacke R. Silicon Chemistry as a Novel Source of Chemical Diversity in Drug Design. Curr. Opin. Drug Discovery Dev. 2003, 6 (4), 526–543. [PubMed] [Google Scholar]
- Tacke R.; Popp F.; Müller B.; Theis B.; Burschka C.; Hamacher A.; Kassack M. U.; Schepmann D.; Wünsch B.; Jurva U.; et al Sila-Haloperidol, a Silicon Analogue of the Dopamine (D2) Receptor Antagonist Haloperidol: Synthesis, Pharmacological Properties, and Metabolic Fate. ChemMedChem 2008, 3 (1), 152–164. 10.1002/cmdc.200700205. [DOI] [PubMed] [Google Scholar]
- Nakajima Y.; Shimada S. Hydrosilylation Reaction of Olefins: Recent Advances and Perspectives. RSC Adv. 2015, 5 (26), 20603–20616. 10.1039/C4RA17281G. [DOI] [Google Scholar]
- Rémond E.; Martin C.; Martinez J.; Cavelier F. Silicon-Containing Amino Acids: Synthetic Aspects, Conformational Studies, and Applications to Bioactive Peptides. Chem. Rev. 2016, 116 (19), 11654–11684. 10.1021/acs.chemrev.6b00122. [DOI] [PubMed] [Google Scholar]
- Pujals S.; Fernández-Carneado J.; Kogan M. J.; Martinez J.; Cavelier F.; Giralt E. Replacement of a Proline with Silaproline Causes a 20-Fold Increase in the Cellular Uptake of a Pro-Rich Peptide. J. Am. Chem. Soc. 2006, 128 (26), 8479–8483. 10.1021/ja060036c. [DOI] [PubMed] [Google Scholar]
- Watkins-Dulaney E.; Straathof S.; Arnold F. Tryptophan Synthase: Biocatalyst Extraordinaire. ChemBioChem 2021, 22 (1), 5–16. 10.1002/cbic.202000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almhjell P. J.; Boville C. E.; Arnold F. H. Engineering Enzymes for Noncanonical Amino Acid Synthesis. Chem. Soc. Rev. 2018, 47 (24), 8980–8997. 10.1039/C8CS00665B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa K.; Yoshihiro D.; Abe Y.; Tomooka K. Enantioselective Synthesis of Silacyclopentanes. Angew. Chem., Int. Ed. 2016, 55 (19), 5814–5818. 10.1002/anie.201511728. [DOI] [PubMed] [Google Scholar]
- Igawa K.; Tomooka K.Chiral Silicon Molecules. In Organosilicon Chemistry; Hiyama T., Oestreich M., Eds.; Wiley: 2019; pp 495–532. 10.1002/9783527814787.ch14. [DOI]
- Raabe G.; Michl J. Multiple Bonding to Silicon. Chem. Rev. 1985, 85 (5), 419–509. 10.1021/cr00069a005. [DOI] [Google Scholar]
- West R.History of a Paradigm Shift: Multiple Bonds to Silicon. Modern Aspects of Main Group Chemistry; ACS Symposium Series; American Chemical Society: 2005; Vol. 917, pp 166–178. 10.1021/bk-2005-0917.ch012. [DOI] [Google Scholar]
- Tacke R.; Dörrich S.Drug Design Based on the Carbon/Silicon Switch Strategy. In Atypical Elements in Drug Design; Schwarz J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp 29–59. 10.1007/7355_2014_55. [DOI] [Google Scholar]
- Fessenden R. J.; Coon M. D. Silicon-Substituted Medicinal Agents. Silacarbamates Related to Meprobamate. J. Med. Chem. 1965, 8 (5), 604–608. 10.1021/jm00329a012. [DOI] [PubMed] [Google Scholar]
- Geyer M.; Wellner E.; Jurva U.; Saloman S.; Armstrong D.; Tacke R. Can Silicon Make an Excellent Drug Even Better? An in Vitro and in Vivo Head-to-Head Comparison between Loperamide and Its Silicon Analogue Sila-Loperamide. ChemMedChem 2015, 10 (5), 911–924. 10.1002/cmdc.201500040. [DOI] [PubMed] [Google Scholar]
- Kumar V. B.; Leitao E. M. Properties and Applications of Polysilanes. Appl. Organomet. Chem. 2020, 34 (3), e5402 10.1002/aoc.5402. [DOI] [Google Scholar]
- Fujiki M.; Koe J. R.; Terao K.; Sato T.; Teramoto A.; Watanabe J. Optically Active Polysilanes. Ten Years of Progress and New Polymer Twist for Nanoscience and Nanotechnology. Polym. J. 2003, 35 (4), 297–344. 10.1295/polymj.35.297. [DOI] [Google Scholar]
- Vis B. M.; Wen J.; Mellerup S. K.; Merchant R. D.; Mawhinney R. C.; Kinrade S. D. Silicon Forms a Rich Diversity of Aliphatic Polyol Complexes in Aqueous Solution. J. Am. Chem. Soc. 2020, 142 (20), 9188–9202. 10.1021/jacs.9b10701. [DOI] [PubMed] [Google Scholar]
- Puri J. K.; Singh R.; Chahal V. K. Silatranes: A Review on Their Synthesis, Structure, Reactivity and Applications. Chem. Soc. Rev. 2011, 40 (3), 1791–1840. 10.1039/B925899J. [DOI] [PubMed] [Google Scholar]
- Henker J.; Wirmer-Bartoschek J.; Bendel L. E.; Xiang Y.; Fu C.; Harms K.; Schwalbe H.; Meggers E. Progress in the Synthesis and Bioactivity of Hexacoordinate Silicon(IV) Complexes. Eur. J. Inorg. Chem. 2016, 2016 (32), 5161–5170. 10.1002/ejic.201600953. [DOI] [Google Scholar]
- Chuit C.; Corriu R. J. P.; Reye C.; Young J. C. Reactivity of Penta- and Hexacoordinate Silicon Compounds and Their Role as Reaction Intermediates. Chem. Rev. 1993, 93 (4), 1371–1448. 10.1021/cr00020a003. [DOI] [Google Scholar]
- Toutov A. A.; Liu W.-B.; Betz K. N.; Fedorov A.; Stoltz B. M.; Grubbs R. H. Silylation of C–H Bonds in Aromatic Heterocycles by an Earth-Abundant Metal Catalyst. Nature 2015, 518 (7537), 80–84. 10.1038/nature14126. [DOI] [PubMed] [Google Scholar]
- Ridley W.; Dizikes L.; Wood J. Biomethylation of Toxic Elements in the Environment. Science 1977, 197 (4301), 329–332. 10.1126/science.877556. [DOI] [PubMed] [Google Scholar]
- Fatoki O. S. Biomethylation in the Natural Environment: A Review. S. Afr. J. Sci. 1997, 93 (8), 366–370. 10.10520/AJA00382353_73. [DOI] [Google Scholar]
- Rochow E. G. The Direct Synthesis of Organosilicon Compounds. J. Am. Chem. Soc. 1945, 67 (6), 963–965. 10.1021/ja01222a026. [DOI] [Google Scholar]
- Dickerson M. B.; Sandhage K. H.; Naik R. R. Protein- and Peptide-Directed Syntheses of Inorganic Materials. Chem. Rev. 2008, 108 (11), 4935–4978. 10.1021/cr8002328. [DOI] [PubMed] [Google Scholar]
- Wallace A. K.; Chanut N.; Voigt C. A. Silica Nanostructures Produced Using Diatom Peptides with Designed Post-Translational Modifications. Adv. Funct. Mater. 2020, 30, 2000849. 10.1002/adfm.202000849. [DOI] [Google Scholar]
- Cordes D. B.; Lickiss P. D.; Rataboul F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110 (4), 2081–2173. 10.1021/cr900201r. [DOI] [PubMed] [Google Scholar]