Abstract
Choledochal cysts, congenital segmental dilations of the common bile duct, have been reported in few cats, and histologic characterization is lacking. A 20-mo-old spayed female domestic shorthair cat was presented because of vomiting and weight loss. There was progressive elevation of liver enzyme activity (ALT > ALP, GGT) and hyperbilirubinemia. Diagnostic imaging identified focal cystic dilation of the common bile duct, dilation and tortuosity of adjacent hepatic ducts, and a prominent duodenal papilla. A choledochal cyst was suspected, and the animal was euthanized. On postmortem examination, there was a 2-cm, firm, thickened, cystic dilation of the common bile duct, patent with adjacent ducts. Histologically, the cyst wall was expanded by fibroblasts, collagen, and lymphoplasmacytic inflammation. Adjacent bile ducts were markedly dilated and tortuous, with lymphoplasmacytic inflammation and papillary mucosal hyperplasia that extended to the major duodenal papilla. There was chronic neutrophilic cholangitis, suggesting bacterial infection and/or disturbed bile drainage, extrahepatic obstruction, and lymphoplasmacytic pancreatitis with ductular metaplasia. Prominent lymphoid follicles within biliary ducts and duodenum suggested chronic antigenic stimulation. Choledochal cysts can be associated with chronic neutrophilic cholangitis, extrahepatic obstruction, choledochitis, duodenal papillitis, and pancreatitis, and should be a differential for increased hepatic enzymes and hyperbilirubinemia in young cats.
Keywords: bile ducts, cats, cholangitis, choledochal cyst, congenital, liver, pancreatitis
Hepatobiliary anomalies are relatively common in cats, with accessory gallbladder and biliary cysts the most prevalent. 11 There is a reported 12% frequency of accessory gallbladder in cats 11 ; biliary cysts are most common in association with polycystic kidney disease, with affected cats having a 68% rate of concurrent polycystic liver disease. 3 Additionally, solitary biliary cysts, and so-called cystadenomas, are often incidental findings during postmortem examination of older cats.1,11 These lesions, in our opinion, may represent long-standing congenital malformations.
In our experience with feline postmortem examinations at a tertiary care veterinary hospital, hepatobiliary malformations were present in 16 of 124 (12.9%) cases; most of these (10 of 124 cats, 63% of hepatobiliary malformations, or 8% of total cases) were ductal plate malformations (DPMs; unpublished data). DPMs are congenital anomalies of the biliary tree that arise from aberrant development of the embryonic ductal plate that normally develops into tubular biliary ducts.12,20,21 These anomalies can affect different segments of the intrahepatic biliary tree, resulting in a variety of anomalies identified both in humans 20 and animals, including dogs, cats, horses, cattle, rodents, and non-human primates.4,8,10,12,13,21
Congenital choledochal cysts (CC) are dilations predominantly affecting extrahepatic bile ducts, and, in humans, multiple subtypes using different classification schemes have been described.14,16,19 Multiple distinct theories exist regarding the pathomechanisms behind formation of CCs, with different pathogeneses often proposed for different subtypes of choledochal cysts. First, some believe that a subset of CCs represent a purely congenital biliary malformation, with speculated underlying causes including embryologic over-proliferation of extrahepatic biliary epithelium, segmental lack of ganglion cells, interference of biliary mucosa cell-to-cell adhesion, or abnormal rotation and fusion of pancreatic buds during development. 15 Second, given the involvement of the intrahepatic biliary tree in some subtypes of CCs, some believe CCs are related to DPMs; however, the true link between these malformations is unclear given that the extrahepatic biliary tree is not embryologically derived directly from the ductal plate.17,20 Third, others maintain that some subtypes of CCs form as sequela of congenital pancreatobiliary malunion or maljunction, a condition in which the common bile duct and pancreatic duct merge prior to the major duodenal papilla, leading to pancreatic enzyme reflux in the common bile duct and subsequent inflammation and dilation.9,17,19,20 However, the lack of confirmed pancreatobiliary malunion in multiple subtypes of CCs, as well as the presence of pancreatobiliary malunion in patients without CCs, argues against this as an exclusive pathogenesis for all subtypes of CCs.9,16,19 Last, adult-onset CCs have been associated with distal obstruction of extrahepatic ducts, resulting in acquired segmental dilation. Ultimately, given the different subtypes described in humans, it is likely that different pathomechanisms variably contribute to development of CCs in different scenarios.
CCs have been characterized sporadically in cats with variable clinical courses, including association with concurrent cholangitis, pancreatitis, and/or enteritis.2,7,17 Despite these reports, a thorough histologic characterization of feline choledochal cysts as well as affected adjacent organs has not been reported. Here we describe the clinical, gross, and histologic findings in a case of a CC in a young cat.
A 20-mo-old spayed female cat was presented because of vomiting and weight loss. Serial serum biochemistry panels revealed progressive elevation of hepatic enzyme values, with hepatocellular enzymes (alanine aminotransferase, ALT) being more prominently elevated than cholestatic enzymes (alkaline phosphatase, ALP; gamma-glutamyl transferase, GGT; Table 1). Abdominal ultrasound and computed tomography identified focal cystic dilation of the common bile duct that was suspected to represent a CC, with accompanying dilation and tortuosity of adjacent hepatic ducts and a prominent duodenal papilla (Suppl. Figs. 1, 2). The animal was euthanized given the poor prognosis.
Table 1.
Serum biochemistry panel indicates marked hepatocellular injury, mild cholestasis, and moderate hyperbilirubinemia in a young domestic shorthair cat.
| Clinicopathologic parameter | Day | Reference interval | ||
|---|---|---|---|---|
| 1 | 5 | 6 | ||
| ALP | 69 | 56 | NA | 12–59 IU/L |
| ALT | 334 | 502 | 653 | 27–158 IU/L |
| GGT | 8 | 11 | NA | 0–6 IU/L |
| Total bilirubin | 89 | 103 | 118 | 0–5 µmol/L |
ALT was markedly elevated in this patient; only mild cholestasis was present in spite of moderate hyperbilirubinemia. ALP = alkaline phosphatase; ALT = alanine aminotransferase; GGT = gamma-glutamyl transferase; NA = not available.
Figures 1 and 2.
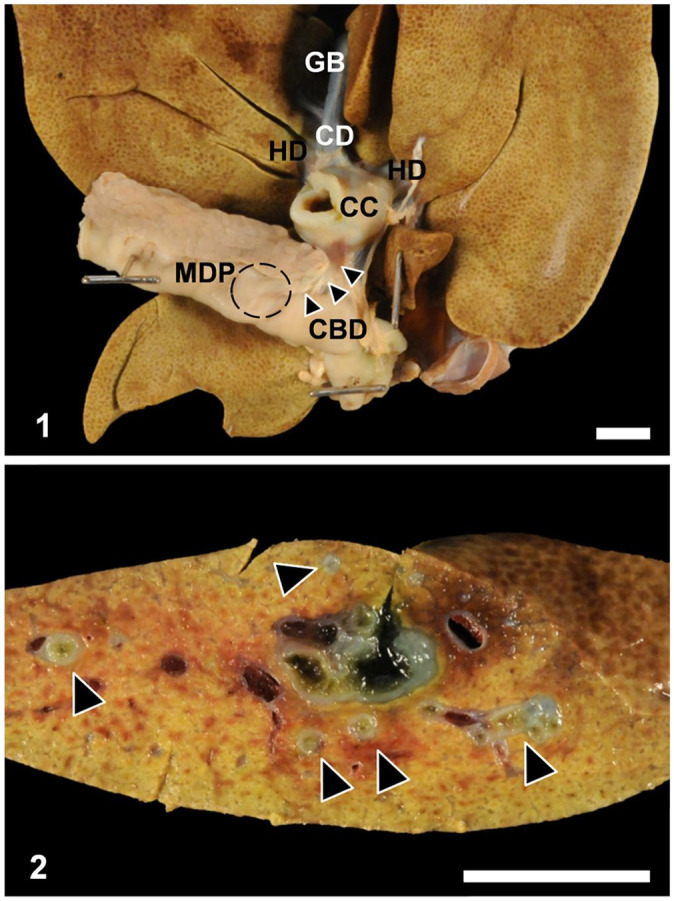
Gross features of choledochal cyst (CC) and associated liver in a young domestic shorthair cat. Figure 1. Midway along the common bile duct there is a thick-walled CC (incised open) patent with hepatic ducts (HD), common bile duct (CBD; arrowheads), and cystic duct (CD) leading to the gallbladder (GB). MDP = major duodenal papilla, indicated with dashed circle. Bar = 1 cm. Figure 2. The CC is associated with intrahepatic and extrahepatic bile duct dilation and inflammation. Fibrosis often accompanied intrahepatic duct dilation (arrowheads). Bar = 1 cm.
On postmortem examination, midway along the common bile duct, distal to the gallbladder, cystic duct, and hepatic ducts, but proximal to the major duodenal papilla, there was a thick-walled, 2-cm diameter cyst that was connected with hepatic ducts, common bile duct, and the cystic duct (Fig. 1). The wall of the cyst was 2–5 mm thick, firm, and pale-tan. The segment of common bile duct distal to the cyst could be traced to the major duodenal papilla and was considered to be of normal diameter, based on our experience; the relationship of this duct with the pancreatic duct was not observed. The liver had a diffuse, mildly enhanced reticular pattern, and, on cut surface, intrahepatic bile ducts, particularly those near the hilus, were multifocally dilated and surrounded by mildly thickened concentric rims of firm tan fibrous tissue (Fig. 2). Given these findings, a diagnosis of choledochal cyst was assigned, and secondary cholangitis and extrahepatic obstruction were suspected. The remainder of the postmortem examination was unremarkable. Samples of the CC, common bile duct, liver, pancreas, duodenum, jejunum, spleen, lymph node, lung, kidney, stomach, and urinary bladder were collected and processed routinely for microscopic evaluation.
Histologic evaluation of the CC revealed a wall comprised of mature spindle cells (presumed fibroblasts and myofibroblasts), collagenous stroma, mild scattered lymphoplasmacytic inflammation, and mild patchy hemorrhage overlain by a simple cuboidal to, less commonly, columnar epithelium (Fig. 3). Submucosal mucous glands were frequently absent. Branches of the cystic duct, hepatic ducts, and common bile duct extending from the cyst were moderately dilated and tortuous. The mucosa of these ducts was convoluted with papillary hyperplasia (Fig. 4), and, within the submucosa, there was mild-to-moderate lymphoplasmacytic inflammation with lymphoid follicle formation. These mucosal and submucosal changes extended to the level of the major duodenal papilla. Within the mucosa and submucosa of the duodenum at the major duodenal papilla, lymphoid follicles were prominent, suggesting chronic antigenic stimulation.
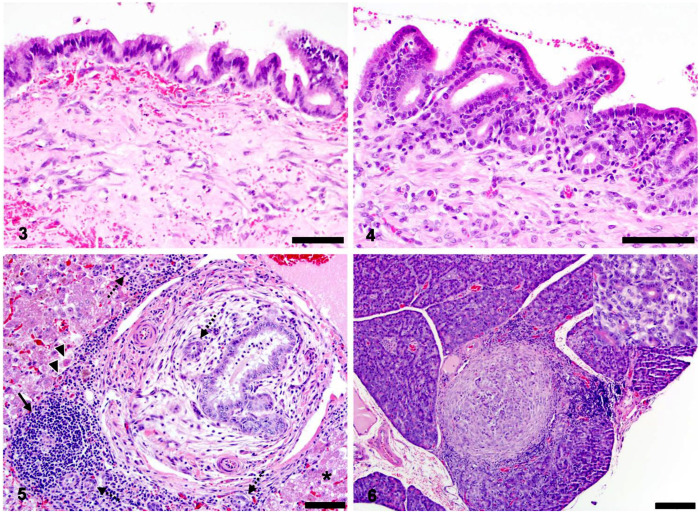
Figures 3–6.
Histologic features of choledochal cyst (CC) and associated liver and pancreas in a young domestic shorthair cat. Figure 3. The CC wall was comprised of mature fibroblasts, scattered mixed inflammatory cells, and patchy hemorrhage below a simple cuboidal-to-columnar mucosa. Bar = 50 µm. Figure 4. The CC was associated with intrahepatic and extrahepatic bile duct mucosal papillary hyperplasia and lymphoplasmacytic-to-neutrophilic inflammation. Bar = 50 µm. Figure 5. The CC was associated with chronic neutrophilic cholangitis, extra-hepatic obstruction, and hepatocellular injury. Intrahepatic bile ducts were tortuous and surrounded by neutrophils, lymphocytes, and fewer plasma cells and macrophages. De novo lymphoid follicles (arrow) and neutrophilic exocytosis were common. Periductular myofibroblasts, edema, and fibrosis indicated extrahepatic obstruction, which was accompanied by proliferation of small caliber bile ducts (ductular reaction; dashed arrows). Hepatocytes often had intracellular lipid (asterisk), and individual necrotic hepatocytes were identified uncommonly at the limiting plate, some of which contained golden brown pigment (lipofuscin; arrowheads). Bar = 50 µm. Figure 6. The CC was associated with lymphoplasmacytic pancreatitis, fibrosis, and ductular metaplasia. Inflammation often cuffed areas of discrete fibrosis, within which there was often loss of zymogen granules and replacement of acini with ductular structures (inset). Bar = 100 µm.
Histologic evaluation of the liver revealed marked dilation, tortuosity, and papillary hyperplasia of hilar intrahepatic bile ducts similar to that seen with extrahepatic bile ducts. Diffusely throughout the parenchyma, biliary ducts were cuffed by variably mild-to-marked numbers of neutrophils admixed with lymphocytes, plasma cells, and macrophages (Fig. 5). There was frequent exocytosis of neutrophils across the biliary epithelium with luminal accumulation of degenerate-to-intact neutrophils and necrotic cellular debris. Surrounding the bile ducts, lymphocytes often formed follicles, suggesting chronic antigenic stimulation. In addition, there was robust periductular proliferation of concentric spindloid cells (myofibroblasts) admixed with collagenous stroma and edema, which, given the antemortem hyperbilirubinemia, was considered most consistent with acute extrahepatic obstruction and cholestasis (Fig. 5).
Obstruction was suspected to be functional in nature and secondary to the extensive fibrosis and inflammation present in the extrahepatic biliary tree and CC. Cholangitis may have contributed directly to periductular edema and myofibroblast proliferation. Mild hemorrhage was also present multifocally in affected portal stroma. Changes in the hepatic parenchyma included mild multifocal random individual hepatocyte necrosis, moderate accumulation of microvesicular lipid within centrilobular hepatocytes (hepatic lipidosis), and moderate multifocal accumulation of golden granular pigment (lipofuscin) within hepatocytes and Kupffer cells. There was also moderate proliferation of small caliber bile ducts that was interpreted as a likely mix of type 1 and type 2A ductular reaction (biliary hyperplasia), secondary to presumed extrahepatic obstruction and chronic cholangitis and cholestasis, respectively. 5
Histologic evaluation of the pancreas revealed moderate lymphoplasmacytic pancreatitis that was accompanied by unusual discrete areas of fibrosis admixed with proliferation of duct structures (Fig. 6), interpreted as reactive ductular metaplasia as a result of chronic inflammation. Additionally, there was occasional pancreatic duct tortuosity similar to that seen in the biliary tree with associated periductular fibrosis. Histologic evaluation of remaining tissues was unremarkable.
In summary, this cat had a thick-walled, fibrotic cyst of the common bile duct consistent with a CC. Further, this was associated with chronic choledochitis, neutrophilic cholangitis, extrahepatic obstruction, pancreatitis, and major duodenal papillitis, as well as hepatocellular injury and necrosis. Collectively, these changes likely contributed to the clinical decline of this animal.
CCs have been characterized sporadically in cats, but specific histologic features are not well described. In contrast, in humans, histologic features of CCs, as well the associated liver, have been characterized more extensively. Common histologic features described in humans include fibrosis of the cyst wall, lymphocytic inflammation, and absence of mucus-producing glands. 16 All of these features were also present in our feline case (Fig. 3). In addition, mucosal hyperplasia has also been described; this feature was generally absent from the CC mucosa but was prominent in the adjacent extrahepatic biliary tree to the level of duodenal papilla as well as some pancreatic ducts (Fig. 4). Lining of the cyst by columnar epithelium is also described 16 and was seen in some areas in our feline choledochal cyst (Fig. 3), although the mucosa more often maintained a typical simple columnar biliary epithelium. Other described features that were not seen in our case include metaplasia of the mucosa to that resembling pyloric and/or duodenal mucosa. 16
In humans, CCs are classified using a variety of schemes, with the Todani classification scheme being the most widely accepted. 19 Using this system, the CC in our case is most consistent with type I, which is characterized by focal-to-segmental, fusiform-to-cystic dilation of the common bile duct. 19 In humans, this subtype has been described as histologically lacking biliary mucosa, 16 but this was not the case in our cat, given that the majority of the examined cyst maintained a simple cuboidal epithelium typical of biliary lining, with occasional transition to a more columnar appearance. However, the significance of this change reported in type I CCs in humans is unclear, given that more severe mucosal changes may simply reflect disease severity (i.e., response to inflammation, cholelithiasis, trauma, etc.). Of the 6 CCs reported previously in cats, 3 were classified as type I, 2 classified as type IV, and 1 was unclassified (suspected to be type I, II, or III).2,7,17 Including our case, type I cysts are therefore the type encountered most commonly in cats (4 of 7, 57%), which is similar to humans, in which type I cysts comprise 60–90% of cases in both adults and children.14,16 Additionally, in type I cysts, mild dilation or enlargement of intrahepatic biliary ducts is also often described. 16 This is consistent with changes seen in our feline case, and may also be considered a direct contributor to concurrent neutrophilic cholangitis and extrahepatic obstruction (i.e., biliary stasis predisposing to bactobilia). In humans with type I cysts, this intrahepatic duct dilation is speculated to occur secondary to biliary stasis. 16 However, as with the uncertainty of the pathogenesis of the CC itself, it is unclear whether these associated bile duct dilations represent a continuation of a congenital dilation or a change secondary to altered bile flow (i.e., as occurs with pancreaticobiliary malunion).
Type I CCs can be further subclassified into multiple subtypes; subtypes IA and IB are both characterized by focal dilations and are distinguished by the presence (IA) or absence (IB) of pancreatobiliary malunion. 19 In our case, definitive gross evaluation for the presence of pancreatobiliary malunion was precluded given the miniscule size of the ducts in cats. Furthermore, histologic identification of these individual ducts is also extremely challenging; in our experience, most cats (104 of 124, 84%) exhibit a complex network of conjoined ductal structures at the major duodenal papilla (unpublished data), and the identification of 2 distinct duct structures is rarely achieved in cats that (presumably) do not have pancreatobiliary malunion. Despite this, the presence of pronounced histologic duct dilation at the major duodenal papilla, which we have not seen previously, and concurrent pancreatic lesions (dilated intrapancreatic ducts, pancreatitis, and atypical fibrosis and ductular metaplasia) support the possibility of pancreatobiliary malunion in our cat.
In human CCs, the presence of pancreatobiliary malunion and the risk of concurrent disease in adjacent organs are not mutually exclusive. In humans, CCs are associated with cholangitis, pancreatitis, and cholelithiasis. 16 In the previous reported CCs in cats, cholangitis was confirmed in 4 of 6 cases, and was most often neutrophilic in nature, with 2 animals having confirmed bactobilia; furthermore, enteritis and pancreatitis were noted in single cats.2,7,17 In our case, cholangitis and pancreatitis were both present, in addition to choledochitis and major duodenal papillitis. Notably, in the general population of cats (i.e., those without CCs), concurrent cholangitis, pancreatitis, and enteritis are thought to be facilitated by feline duodenal papilla anatomy, resulting in a clinical syndrome dubbed “triaditis.” 6 Based on our case and previously published cases of feline CCs and knowledge of feline duodenal papilla anatomy, we postulate that similar to humans, cats with a CC should be considered at elevated risk for not only cholangitis, but also pancreatitis and possibly enteritis. 17 Additionally, CCs predispose human patients to an increased risk of development of biliary and pancreatic neoplasia. 16 Although this progression has not been described in cats with CCs—likely given the low numbers of cases and often young age of patients in which this condition is described—this complication may also be possible in cats.
Interestingly, despite clear histologic evidence of extrahepatic obstruction and cholangitis in our cat, antemortem clinicopathologic evaluation of hepatocellular enzymes was dominated by a pattern of hepatocellular injury rather than cholestasis (Table 1). There was histologic evidence of hepatocellular injury (hepatocyte necrosis, excessive lipofuscin accumulation for the age of the cat, lipidosis) to support ALT elevation, but the lack of concordant ALP and GGT elevation in spite of bilirubin elevation was considered unusual. We hypothesize that this phenomenon may represent spontaneous normalization of cholestatic serum biochemistry values following a period of chronic cholangitis; this has been reported in humans with chronic sclerosing cholangitis, despite evidence of persistent gross lesions. 18 Alternatively, it is possible that the ALT elevation seen in our cat reflected injury of other tissues (e.g., skeletal muscle, thyroid); the cat did have concurrent mild AST elevation on initial presentation (AST 135 IU/L, reference interval: 16–67 IU/L). However, these tissues were not available for histologic evaluation, and this change could not be further evaluated. Ultimately, these theories are purely speculative; the significance of this antemortem finding is ultimately unknown.
Finally, diagnostic imaging (abdominal ultrasound and computed tomography) was helpful in diagnosis of choledochal cyst in our case. However, laparoscopic and/or postmortem examination may be required for definitive diagnosis of CCs in cats and other animals, and in tandem with histopathologic examination, is critical for evaluation of complications in adjacent organs.
Supplemental Material
Supplemental material, sj-pdf-1-jvd-10.1177_10406387211017107 for Choledochal cyst with secondary cholangitis, choledochitis, duodenal papillitis, and pancreatitis in a young domestic shorthair cat by Megan E. Schreeg, Sybille A. Miller and John M. Cullen in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We acknowledge the expertise and contribution of the staff of the NCSU CVM Histology Laboratory.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Megan E. Schreeg  https://orcid.org/0000-0001-5649-8854
https://orcid.org/0000-0001-5649-8854
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Megan E. Schreeg, North Carolina State University College of Veterinary Medicine, Raleigh, NC, USA; The Ohio State University College of Veterinary Medicine, Columbus, OH, USA.
Sybille A. Miller, VCA Leesburg Veterinary Internal Medicine, Leesburg, VA, USA
John M. Cullen, North Carolina State University College of Veterinary Medicine, Raleigh, NC, USA Experimental Pathology Labs, Durham, NC, USA.
References
- 1. Adler R, et al. Biliary cystadenoma of cats. Vet Pathol 1995;32:415–418. [DOI] [PubMed] [Google Scholar]
- 2. Best EJ, et al. Suspected choledochal cyst in a domestic shorthair cat. J Feline Med Surg 2010;12:814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosje JT, et al. Polycystic kidney and liver disease in cats. Vet Q 1998;20:136–139. [DOI] [PubMed] [Google Scholar]
- 4. Brown DL, et al. Congenital hepatic fibrosis in 5 dogs. Vet Pathol 2010;47:102–107. [DOI] [PubMed] [Google Scholar]
- 5. Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011;458:251–259. [DOI] [PubMed] [Google Scholar]
- 6. Fragkou FC, et al. Prevalence and clinicopathological features of triaditis in a prospective case series of symptomatic and asymptomatic cats. J Vet Intern Med 2016;30:1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grand JG, et al. Cyst of the common bile duct in a cat. Aust Vet J 2010;88:268–271. [DOI] [PubMed] [Google Scholar]
- 8. Guerra JM, et al. Congenital hepatic fibrosis and polycystic kidney disease not linked to C > A mutation in exon 29 of PKD1 in a Persian cat. JFMS Open Rep 2015;1:2055116915619191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamisawa T, et al. Pancreaticobiliary maljunction and congenital biliary dilatation. Lancet Gastroenterol Hepatol 2017;2:610–618. [DOI] [PubMed] [Google Scholar]
- 10. Molin J, et al. Congenital hepatic fibrosis in a purebred Spanish horse foal: pathology and genetic studies on PKHD1 gene mutations. Vet Pathol 2018;55:457–461. [DOI] [PubMed] [Google Scholar]
- 11. Otte CM, et al. Feline biliary tree and gallbladder disease: aetiology, diagnosis and treatment. J Feline Med Surg 2017;19:514–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pillai S, et al. Ductal plate malformation in the liver of Boxer dogs: clinical and histological features. Vet Pathol 2016;53:602–613. [DOI] [PubMed] [Google Scholar]
- 13. Roberts ML, et al. Caroli’s-type ductal plate malformation and a portosystemic shunt in a 4-month-old kitten. JFMS Open Rep 2018;4:2055116918812329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ronnekleiv-Kelly SM, et al. Management of choledochal cysts. Curr Opin Gastroenterol 2016;32:225–231. [DOI] [PubMed] [Google Scholar]
- 15. Singham J, et al. Choledochal cysts: part 1 of 3: classification and pathogenesis. Can J Surg 2009;52:434–440. [PMC free article] [PubMed] [Google Scholar]
- 16. Soares KC, et al. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg 2014;219:1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spain HN, et al. Ultrasonographic and clinicopathologic features of segmental dilatations of the common bile duct in four cats. JFMS Open Rep 2017;3:2055116917716881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stanich PP, et al. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis 2011;43:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Todani T. Congenital choledochal dilatation: classification, clinical features, and long-term results. J Hepatobiliary Pancreat Surg 1997;4:276–282. [Google Scholar]
- 20. Venkatanarasimha N, et al. Imaging features of ductal plate malformations in adults. Clin Radiol 2011;66:1086–1093. [DOI] [PubMed] [Google Scholar]
- 21. Wallace S, et al. Ductal plate malformation in a nonhuman primate. Vet Pathol 2009;46:84–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jvd-10.1177_10406387211017107 for Choledochal cyst with secondary cholangitis, choledochitis, duodenal papillitis, and pancreatitis in a young domestic shorthair cat by Megan E. Schreeg, Sybille A. Miller and John M. Cullen in Journal of Veterinary Diagnostic Investigation