Abstract
The importance and abundance of strict anaerobic bacteria in the respiratory microbiota of people with cystic fibrosis (PWCF) is now established through studies based on high-throughput sequencing or extended-culture methods. In CF respiratory niche, one of the most prevalent anaerobic genera is Prevotella, and particularly the species Prevotella melaninogenica. The objective of this study was to evaluate the antibiotic susceptibility of this anaerobic species. Fifty isolates of P. melaninogenica cultured from sputum of 50 PWCF have been included. Antibiotic susceptibility testing was performed using the agar diffusion method. All isolates were susceptible to the following antibiotics: amoxicillin/clavulanic acid, piperacillin/tazobactam, imipenem and metronidazole. A total of 96% of the isolates (48/50) were resistant to amoxicillin (indicating beta-lactamase production), 34% to clindamycin (17/50) and 24% to moxifloxacin (12/50). Moreover, 10% (5/50) were multidrug-resistant. A significant and positive correlation was found between clindamycin resistance and chronic azithromycin administration. This preliminary study on a predominant species of the lung “anaerobiome” shows high percentages of resistance, potentially exacerbated by the initiation of long-term antibiotic therapy in PWCF. The anaerobic resistome characterization, focusing on species rather than genera, is needed in the future to better prevent the emergence of resistance within lung microbiota.
Keywords: antibiotics susceptibility, Prevotella melaninogenica, cystic fibrosis, resistance
1. Introduction
The importance and abundance of strict anaerobic bacteria in the respiratory microbiota of people with cystic fibrosis (PWCF) is now established. These recent data are based on high-throughput sequencing [1,2] and cultural techniques [3,4,5]. Anaerobes colony count has been evaluated between 1.104 to 9.107 colony forming units per milliliter in sputum culture [3]. Numerous anaerobic genera have been identified as part of the CF core lung microbiota such as Prevotella, Veillonella, Porphyromonas, Fusobacterium, or Peptostreptococcus [2,6,7]. However, these bacteria remain the unknowns of the lung, both in terms of diversity, resistance or impact on the pathophysiology of CF disease [8]. One of the most prevalent and abundant anaerobic genera described in CF lung is Prevotella, and particularly the species Prevotella melaninogenica [4,5,9]. Contradictory hypotheses on the potential role of Prevotella genus have been previously developed [8]. On the one hand, it has been associated with better lung function and less inflammation [2,10,11,12]. On the other hand, Prevotella species are able to enhance other bacteria’s pathogenicity in lung [13], to produce pro-inflammatory short chain fatty acids [14] and are described to be resistant to antibiotics [15]. Focusing on this last point, previous studies have reported resistance acquisition of Prevotella isolates, due to the production of enzyme (mainly beta-lactamase [13,16,17]) or the carrying of the resistance gene [18,19]. Moreover, in CF, an over-expression of antibiotic resistance may be observed due to several reasons: repeated or chronic use of antimicrobial treatment, administration of high doses of antibiotic therapy, or prescription of antibiotic molecules at sub-clinical doses for anti-inflammatory properties. As the issue of antibiotic resistance is crucial, even more in PWCF, antibiotic susceptibility monitoring of this main anaerobic genus is necessary.
The objective of this study was to evaluate the antibiotic resistance rate of Prevotella melaninogenica, the most prevalent anaerobic species of the bronchopulmonary microbiota of PWCF.
2. Materials and Methods
2.1. Patients Characteristics
Fifty PWCF were included in the study. The following sociodemographic and clinical parameters were recorded: age, sex, CFTR (CF transmembrane conductance regulator) gene mutation, lung function, diabetes, chronic antibiotics administration (azithromycin, aztreonam, colistin, and tobramycin), and antibiotic administration one month before the sample. The patients’ lung involvement was assessed by the measurement of the maximum expiratory volume per second (FEV1) by spirometry tests, which was defined by three stages: early (FEV1 > 70%), intermediate (40 < FEV1 < 70%) and advanced (FEV1 < 40%) [20].
2.2. Clinical Isolates and Antibiotic Susceptibility Testing
Fifty isolates of Prevotella melaninogenica cultured from sputum of 50 PWCF collected at the Western Brittany CF center (Roscoff, France) have been included in this study. Cultural methods from sputum (three different media, 21 days of culture at 37 °C in anaerobic atmosphere) have been previously described [5]. These 50 isolates have been identified by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS Biotyper MBT) (Bruker, Billerica, MA, USA); a score of ≥ 2.0 was considered as accurate species-level identification; a score ≥ 1.7 but < 2.0 as a probable genus-level identification; a score < 1.7 as “unidentified”. In order to improve isolates identification, 1 μL of 70% formic acid LC/MS (VWR, Radnor, PA, USA) was added before the addition of 1 μL of portioned IVD-HHCA matrix (Bruker, Billerica, MA, USA) [5]. Antibiotic susceptibility testing was performed using the agar diffusion method according to the recommendations of EUCAST/CASFM 2019 [21]. From a fresh culture, an inoculum of 1 McFarland was prepared in physiological water (ThermoFisher, Waltham, MA, USA). The bacterial suspensions were inoculated onto Brucella agar plates supplemented with vitamin K1, hemin (Sigma-Aldrich, Dorset, England) and sheep blood (ThermoFisher, Waltham, MA, USA). The following antibiotics were tested (ThermoFisher, Waltham, MA, USA; Bio-Rad, Hercules, CA, USA): amoxicillin (20 μg)/clavulanic acid (10 μg), clindamycin (2 μg), imipenem (10 μg), metronidazole (4 μg), moxifloxacin (5 μg), and piperacillin (30 μg)/tazobactam (10 μg). Susceptibility to amoxicillin was assessed by determining the minimum inhibitory concentration (MIC) using an E-test strip (BioMérieux, Marcy-l’Etoile, France). Agars were then incubated under anaerobic conditions at 37 °C for 48 h (Bactron® anaerobic chamber, Sheldon manufacturing, Cornelius, NC, USA). P. melaninogenica isolates with a MIC for amoxicillin greater than or equal to 0.5 mg/L were classified as beta-lactamase producer [21]. Isolates resistant to at least one agent in three or more antimicrobial categories were categorized as multidrug-resistant (MDR) [22].
2.3. Statistical Analysis
Statistical analysis was performed using the Chi-square and Fisher tests. The alpha risk has been reported significant when it was less than 0.05.
3. Results
3.1. Patients Characteristics
The study population was composed of 52.0% of males (26/50) and the median age was 28 years (range: 6–58). Subject characteristics (demographic, clinical and biological) are outlined in Table 1. The stage of lung involvement was early for 22% (11/50), intermediate for 52% (26/50) and advanced for 26% (13/50) of PWCF.
Table 1.
Study cohort characteristics: demographic, clinical and biological data. A total of 50 people with cystic fibrosis (PWCF) were included.
| PWCF Characteristics | Percentage (n) | |
|---|---|---|
| Age group (years) | ≤6 | 4 (2) |
| 7–13 | 8 (4) | |
| 14–18 | 6 (3) | |
| 19–25 | 28 (14) | |
| 26–30 | 22 (11) | |
| ≥30 | 32 (16) | |
| Sex | Female | 48 (24) |
| Male | 52 (26) | |
| p.F508del mutation | Homozygote | 60 (30) |
| Heterozygote | 28 (14) | |
| Other mutations | 12 (6) | |
| Lung function (FEV1 %) | ≤40 | 26 (13) |
| 40–70 | 52 (26) | |
| >70 | 22 (11) | |
| Chronic antibiotic administration |
Azithromycin | 44 (22) |
| Aztreonam | 10 (5) | |
| Colistin | 42 (21) | |
| Tobramycin | 20 (10) | |
| Antibiotics administration one month before the sample | Oral * | 36 (18) |
| Intravenous ** | 16 (8) | |
* amoxicillin/clavulanic acid, cefpodoxime, ciprofloxacin, doxycycline, linezolid, minocycline, pristinamycin, trimetoprim/sulfamethoxazole, ** amikacin, aztreonam, ceftazidime, doxycycline, linezolid, meropenem, piperacillin/tazobactam, tobramycin, trimetoprim/sulfamethoxazole, FEV1: Forced Expiratory Volume in one second.
3.2. Antibiotic Susceptibility Testing
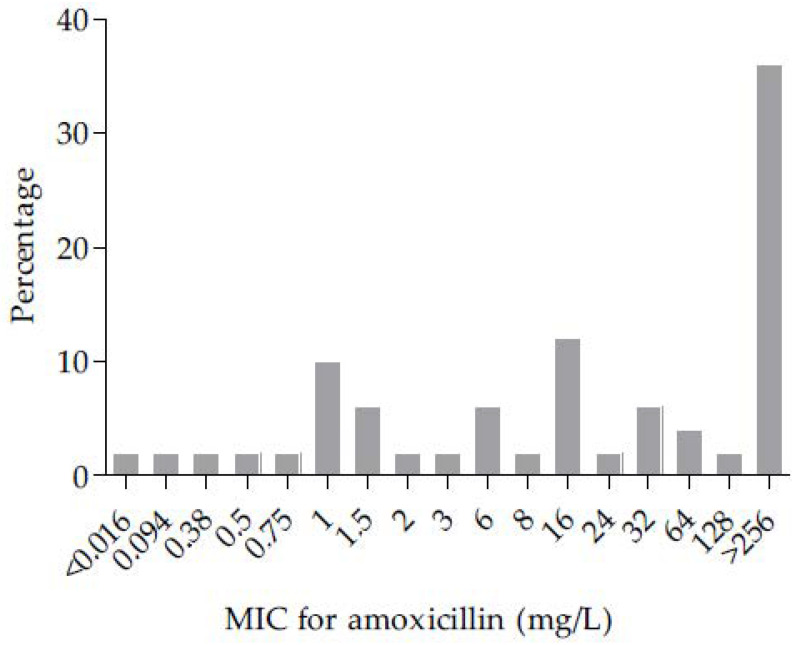
Antibiotic susceptibilities of P. melaninogenica isolates are presented in Table 2. All isolates were susceptible to the following antibiotics: amoxicillin/clavulanic acid, piperacillin/tazobactam, imipenem, and metronidazole. A total of 96% of P. melaninogenica isolates (48/50) were classified as beta-lactamase producers (MIC for amoxicillin > 0.5 mg/L), and 38% of these isolates (18/48) had a MIC for amoxicillin superior to 256 mg/L (Figure 1). The MIC for amoxicillin for the two non-beta-lactamase producers isolates have been evaluated at 0.094 mg/L and inferior to 0.016 mg/L (Figure 1). A total of 34% (17/50) and 24% (12/50) of isolates were resistant to clindamycin and moxifloxacin, respectively. Ten percent of isolates (5/50) were categorized MDR, due to a combined resistance to amoxicillin, clindamycin and moxifloxacin.
Table 2.
Antibiotic susceptibilities of 50 isolates of Prevotella melaninogenica (according to recommendations of EUCAST/CASFM 2019 [21].
| Antibiotic | Interpretative Categories n (%) | |
|---|---|---|
| S | R | |
| Amoxicillin | 2 (4) | 48 (96) |
| Amoxicillin/clavulanic acid | 50 (100) | 0 |
| Piperacillin/tazobactam | 50 (100) | 0 |
| Imipenem | 50 (100) | 0 |
| Clindamycin | 33 (66) | 17 (34) |
| Moxifloxacin | 38 (76) | 12 (24) |
| Metronidazole | 50 (100) | 0 |
S: susceptible, R: resistant.
Figure 1.
Distribution of minimum inhibitory concentration (MIC) for amoxicillin (mg/L) among the 50 isolates of Prevotella melaninogenica.
3.3. Associations between Antibiotic Resistance and Antibiotic Administration
Amoxicillin, clindamycin and moxifloxacin resistance percentages were compared to antibiotic administration one month before the sample (oral or intravenous: yes or no) and chronic antibiotic administration (azithromycin, aztreonam, colistin or tobramycin: yes or no). A significantly positive correlation was found between clindamycin resistance and oral chronic azithromycin administration (p = 0.002, Chi-square test).
4. Discussion
The potential impact of strict anaerobic bacteria in the pathophysiology of CF disease and in the respiratory function of PWCF is still at the hypothesis stage. The important and now undeniable place of these bacteria within the CF respiratory microbiota led to the study of their antibiotic susceptibilities to better understand the issue of resistance. For the genus Prevotella, disparities in resistance rates have been observed among the different studies but all the results agree on an increase in antibiotic resistance from clinical isolates in CF, as well as in other diseases [15,23].
Concerning beta-lactams, resistance is mainly due to the production of beta-lactamases, which induce a loss of susceptibility to penicillin, and that of the first, second, and third (oral) generation cephalosporins [21]. Several beta-lactamase detection methods can be used, combining phenotypic (mostly performed in routine practice) and molecular (cfxA resistance genes detection approaches [18,19]). Indeed, the detection of cfxA genes in Prevotella isolates has been associated with high MICs of amoxicillin [19,24]. However it remains important to determine if the isolates are functionally resistant to amoxicillin [25]. In this study focusing on P. melaninogenica, a high percentage of isolates (96%) were categorized as beta-lactamase producers using phenotypic detection based on the MIC of amoxicillin determination. This result is slightly higher than previous results for this species (Table 3) which can be explained by three main reasons. Firstly, the other studies used the nitrocefin test for phenotypic detection of beta-lactamase. However the recommendation of EUCAST/CASFM 2019 [21] is to avoid nitrocefin, considered as a poor substrate which may underestimate beta-lactamase activity detection. Moreover interference with the isolates pigmentation (as P. melaninogenica) represents a limit to this test based on a colorimetric reaction [26]. As formerly demonstrated, the determination of the MIC of amoxicillin is a more reliable method of detection [27]. Secondly, this study focused only on P. melaninogenica species while others’ studies are based on several Prevotella species, for which resistance rates may differ [26]. Thirdly, in this study, isolates have been obtained from sputum of PWCF, for which antibiotic selective pressure is higher due to chronic antibiotic therapy administration and may lead to diminution of antibiotic susceptibility [15]. Moreover, CF lung has been described as a potential reservoir of resistance genes (as oral sphere) because cfxA genes coding beta-lactamase could be transferred among bacteria by horizontal transfer gene mechanisms [8,28,29]. Fourthly, as demonstrated for other genera known to carry cfxA genes (Capnocytophaga, Bacteroides,…), the expression of beta-lactamase may differ according to major substitutions in the cfxA gene sequence or to the influence of the genetic environment of cfxA [30]. This molecular part has not been evaluated in this study.
Table 3.
Studies reporting the percentage of beta-lactamase activity in Prevotella spp.
| Beta-Lactamase Activity (%) | Number of Isolates | Prevotella Species | Cystic Fibrosis | Method of Detection | |
|---|---|---|---|---|---|
| This study | 96 | 50 | P. melaninogenica | Yes | MIC of amoxicillin |
| Bahar et al., 2005 [32] | 68 | 19 | P. melaninogenica | No | Nitrocefin test |
| Fujita et al., 2019 [33] | 85 | 27 | 5 Prevotella species | No | Nitrocefin test |
| Bancescu et al., 2015 [23] | 33 | 33 | 5 Prevotella species | No | Nitrocefin test |
| Fernandez et al., 2015 [34] | 46 | 41 | 2 Prevotella species | No | Nitrocefin test |
| Montagner et al., 2014 [25] | 20 | 15 | 4 Prevotella species | No | Nitrocefin test |
| Sherrard et al., 2013 [16] | 59 | 157 | Prevotella spp. | Yes and no | Nitrocefin test |
| Tran et al., 2013 [35] | 75 | 16 | Prevotella spp. | No | Nitrocefin test |
| Kuriyama et al., 2007 [36] | 37 | 499 | Prevotella spp. | No | Nitrocefin test |
| Mosca et al., 2007 [37] | 18 | 39 | 3 Prevotella species | No | Nitrocefin test |
| Iwahara et al., 2006 [38] | 31 | 139 | 8 Prevotella species | No | Nitrocefin test |
| Behra-Miellet et al., 2003 [39] | 65 | 40 | Prevotella spp. | No | Nitrocefin test |
| Dubreuil et al., 2003 [40] | 58 | 100 | 12 Prevotella species | No | Nitrocefin test |
| Matto et al., 1999 [41] | 31 | 171 | 3 Prevotella species | No | Nitrocefin test |
For the other beta-lactams, all of the isolates of the present study were susceptible to piperacillin/tazobactam, amoxicillin/clavulanic acid, and imipenem (Table 4). No resistance have been described to date for amoxicillin/clavulanic acid and imipenem, however resistance to piperacillin/tazobactam was previously reported in the literature for Prevotella spp. [3,31].
Table 4.
Studies reporting antibiotic resistance of Prevotella spp.
| Reference | Cystic Fibrosis |
Prevotella Species |
Isolates Identification | Number of Isolates | Location | Percentage of Resistance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amoxicillin or Ampicillin | Amoxicillin/Clavulanic Acid | Piperacillin/Tazobactam | Imipenem | Clindamycine | Moxifloxacine | Metronidazole | ||||||
| This study | Yes | P. melaninogenica | Mass spectrometry | 50 | France | 96.0 | 0 | 0 | 0 | 34.0 | 24.0 | 0 |
| Veloo et al., 2019 [18] | No | P. melaninogenica | Mass spectrometry | 21 | Netherlands | 66.7 | - | - | - | 4.8 | - | 4.8 |
| Ulger Toprak et al., 2018 [26] | No | P. melaninogenica | 16S rRNA gene sequencing | 44 | 11 countries | 67.4 | - | - | - | 16.3 | 20.5 | - |
| Bahar et al., 2005 [32] | No | P. melaninogenica | Biochemical tests | 19 | Turkey | - | - | - | 0 | 10.5 | - | 0 |
| Byun et al., 2019 [45] | No | 8 Prevotella species | Mass spectrometry | 33 | Korea | - | - | 0 | 0 | 45.0 | 9.0 | 3.0 |
| Cobo et al., 2019 [31] | No | Prevotella spp. | Mass spectrometry | 30 | Spain | - | 0 | 3.0 | 0 | 40.0 | 30.0 | 7.0 |
| Bancescu et al., 2015 [23] | No | 5 Prevotella species | Biochemical tests | 33 | Romania | 33.0 | 0 | - | - | 3.0 | - | 0 |
| Shilnikova et al., 2014 [46] | No | 13 Prevotella species | Mass spectrometry | 42 | Russia | - | 0 | - | 0 | 11.9 | - | 4.8 |
| Xie et al., 2014 [47] | No | 10 Prevotella species | 16S rRNA gene sequencing | 42 | China | 30.9 | - | - | 0 | 38.1 | - | 9.5 |
| Tran et al., 2013 [35] | No | Prevotella spp. | 16S rRNA gene sequencing | 16 | Japon | 69.0 | - | 0 | 0 | 19.0 | - | 0 |
| Seifert et al., 2010 [48] | No | 7 Prevotella species | Biochemical tests | 21 | Germany | - | - | - | - | 9.5 | 0 | 0 |
| Papaparaskevas et al., 2008 [42] | No | 10 Prevotella species | Biochemical tests | 141 | Greece | - | - | 0 | 0 | 31.0 | 38.0 | 8.0 |
| Tunney et al., 2008 [3] |
Yes | 4 Prevotella species | Biochemical tests | 14 | Ireland | 36.0 | - | 7.0 | - | 36.0 | - | 46.0 |
| Mosca et al., 2007 [37] | No | 3 Prevotella species | Biochemical tests | 39 | Italy | 18.0 | 0 | - | - | - | 7.7 | 0 |
| Behra-Miellet et al., 2003 [39] | No | Prevotella spp. | Biochemical tests | 40 | France | 65.0 | 0 | - | 0 | 5.0 | - | 0 |
| Mory et al., 1998 [49] | No | 10 Prevotella species | Biochemical tests | 56 | France | 25.0 | 0 | - | 0 | 0 | - | 0 |
As beta-lactams, emerging resistance to macrolides and fluoroquinolone has been described for Prevotella spp. [42,43]. In our study, 24% of P. melaninogenica isolates were resistant to moxifloxacin, which was concordant with the results of Ulger Toprak et al. [26] (Table 4). Thirty-four percent of P. melaninogenica isolates were resistant to clindamycin, which was higher than previously described in non-CF individuals, as no resistance data are available for PWCF [18,26,32]. Chronic azithromycin administration, prescribed at sub-clinical doses for anti-inflammatory properties, was significantly correlated with clindamycin resistance (p < 0.05, Chi-square test), as previously highlighted in PWCF [16]. This result is consistent with the fact that one of the main factors leading to antibiotic resistance identified for anaerobic bacteria is long-term antibiotic use [43,44]. Sherrard et al. [15] showed that the administration of beta-lactam or tetracycline in the year prior to pulmonary sample collection in PWCF was associated with higher MICs of amoxicillin, ceftazidime and tetracycline for Prevotella spp. In the future, the worsening resistance of the Prevotella genus is a threat and should be monitored. Firstly, different studies highlighted the increase of MDR anaerobic isolates. Ulger Toprak et al. [26] have demonstrated that nearly 10% of Prevotella isolates were categorized MDR (antibiotics involved: ampicillin, clindamycin, moxifloxacin and tetracycline). In our study, a similar rate of MDR isolates was determined (five isolates of P. melaninogenica resistant to amoxicillin, moxifloxacin and clindamycin). Secondly, in addition to beta-lactamase activity, Prevotella species are able to produce extended-spectrum beta-lactamase (ESBLs), associated with treatment failure with cephalosporin antibiotics. In a study focused on Prevotella isolates isolated from respiratory samples of PWCF, 76% were found to produce ESBLs [17]. This resistance is rarely investigated in routine practice, as in our study, and may be underestimated. Finally, resistance to metronidazole, an anti-anaerobic molecule, has been reported for P. melaninogenica [18] and Prevotella spp. [31,42,45,46,47] with a resistance rate under 10% (Table 4). Tunney et al. [3] have observed a higher resistance rate evaluated at 46% in Prevotella spp. isolates (including four species: P. corporis, P. disiens, P. melaninogenica and P. salivae). No metronidazole resistant isolates of P. melaninogenica were described in our study.
For all the antimicrobial categories, results obtained in our study compared with previous ones showed the importance of the species level when considering antibiotic resistance and the difficulty to compare antibiotic resistance from isolates of different species belonging to the same genus [26] (Table 4). The targeted study based on species is therefore the most informative. In routine practice, this approach requires equipment (e.g., anaerobic chamber, collecting device for anaerobic atmosphere preservation) for the detection and identification of strict anaerobic bacteria in CF respiratory samples as well as for the realization of antibiogram.
5. Conclusions
This preliminary study of 50 clinical isolates of a predominant species of the lung “anaerobiome” showed a high percentage of resistance and the presence of MDR isolates, potentially exacerbated by the initiation of long-term antibiotic therapy in PWCF. In the future, it will be important to continue to characterize the anaerobic resistome by focusing on species rather than genera in order to better prevent the emergence of resistance within pulmonary microbiota.
Author Contributions
Conceptualization, G.H.-A.; methodology, C.L., C.-A.G., E.C. and S.G.; formal analysis, C.L., C.-A.G., E.C. and S.G.; investigation, C.L., C.-A.G., E.C. and S.G.; resources, C.L., C.-A.G., E.C. and S.G.; data curation, C.L. and G.H.-A.; writing—original draft preparation, C.L.; writing—review and editing, C.L., C.-A.G., C.B. and G.H-A.; visualization, C.L., C.-A.G., C.B. and G.H-A.; supervision, G.H.-A.; project administration, G.H.-A.; funding acquisition, G.H.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the French Cystic Fibrosis Association ‘Vaincre la Mucoviscidose’ (contract n° RC20180502218).
Institutional Review Board Statement
The study was approved by the French Ethical Research Committee in March 2018 (2018-A00624-51). All experiments were performed in accordance with relevant guidelines and regulations.
Informed Consent Statement
Informed consent was obtained from all participants and/or their legal guardians.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brown P.S., Pope C.E., Marsh R.L., Qin X., McNamara S., Gibson R., Burns J.L., Deutsch G., Hoffman L.R. Directly Sampling the Lung of a Young Child with Cystic Fibrosis Reveals Diverse Microbiota. Annals ATS. 2014;11:1049–1055. doi: 10.1513/AnnalsATS.201311-383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernarde C., Keravec M., Mounier J., Gouriou S., Rault G., Férec C., Barbier G., Héry-Arnaud G. Impact of the CFTR-Potentiator Ivacaftor on Airway Microbiota in Cystic Fibrosis Patients Carrying a G551D Mutation. PLoS ONE. 2015;10:e0124124. doi: 10.1371/journal.pone.0124124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunney M.M., Field T.R., Moriarty T.F., Patrick S., Doering G., Muhlebach M.S., Wolfgang M.C., Boucher R., Gilpin D.F., McDowell A., et al. Detection of Anaerobic Bacteria in High Numbers in Sputum from Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 4.Muhlebach M.S., Hatch J.E., Einarsson G.G., McGrath S.J., Gilipin D.F., Lavelle G., Mirkovic B., Murray M.A., McNally P., Gotman N., et al. Anaerobic Bacteria Cultured from Cystic Fibrosis Airways Correlate to Milder Disease: A Multisite Study. Eur. Respir. J. 2018;52:1800242. doi: 10.1183/13993003.00242-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamoureux C., Guilloux C.-A., Beauruelle C., Gouriou S., Ramel S., Dirou A., Le Bihan J., Revert K., Ropars T., Lagrafeuille R., et al. An Observational Study of Anaerobic Bacteria in Cystic Fibrosis Lung Using Culture Dependant and Independent Approaches. Sci. Rep. 2021;11:6845. doi: 10.1038/s41598-021-85592-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einarsson G.G., Zhao J., LiPuma J.J., Downey D.G., Tunney M.M., Elborn J.S. Community Analysis and Co-Occurrence Patterns in Airway Microbial Communities during Health and Disease. ERJ Open Res. 2019;5:00128–02017. doi: 10.1183/23120541.00128-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn B., Wang P.W., Diaz Caballero J., Clark S.T., Brahma V., Donaldson S., Zhang Y., Surendra A., Gong Y., Elizabeth Tullis D., et al. Lung Microbiota across Age and Disease Stage in Cystic Fibrosis. Sci. Rep. 2015;5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamoureux C., Guilloux C.-A., Beauruelle C., Jolivet-Gougeon A., Héry-Arnaud G. Anaerobes in Cystic Fibrosis Patients’ Airways. Crit. Rev. Microbiol. 2019;45:103–117. doi: 10.1080/1040841X.2018.1549019. [DOI] [PubMed] [Google Scholar]
- 9.Bacci G., Taccetti G., Dolce D., Armanini F., Segata N., Di Cesare F., Lucidi V., Fiscarelli E., Morelli P., Casciaro R., et al. Untargeted Metagenomic Investigation of the Airway Microbiome of Cystic Fibrosis Patients with Moderate-Severe Lung Disease. Microorganisms. 2020;8:1003. doi: 10.3390/microorganisms8071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zemanick E.T., Harris J.K., Wagner B.D., Robertson C.E., Sagel S.D., Stevens M.J., Accurso F.J., Laguna T.A. Inflammation and Airway Microbiota during Cystic Fibrosis Pulmonary Exacerbations. PLoS ONE. 2013;8:e62917. doi: 10.1371/journal.pone.0062917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neill K., Bradley J.M., Johnston E., McGrath S., McIlreavey L., Rowan S., Reid A., Bradbury I., Einarsson G., Elborn J.S., et al. Reduced Bacterial Colony Count of Anaerobic Bacteria Is Associated with a Worsening in Lung Clearance Index and Inflammation in Cystic Fibrosis. PLoS ONE. 2015;10:e0126980. doi: 10.1371/journal.pone.0126980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmody L.A., Caverly L.J., Foster B.K., Rogers M.A.M., Kalikin L.M., Simon R.H., VanDevanter D.R., LiPuma J.J. Fluctuations in Airway Bacterial Communities Associated with Clinical States and Disease Stages in Cystic Fibrosis. PLoS ONE. 2018;13:e0194060. doi: 10.1371/journal.pone.0194060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field T.R., Sibley C.D., Parkins M.D., Rabin H.R., Surette M.G. The Genus Prevotella in Cystic Fibrosis Airways. Anaerobe. 2010;16:337–344. doi: 10.1016/j.anaerobe.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Mirković B., Murray M.A., Lavelle G.M., Molloy K., Azim A.A., Gunaratnam C., Healy F., Slattery D., McNally P., Hatch J., et al. The Role of Short-Chain Fatty Acids, Produced by Anaerobic Bacteria, in the Cystic Fibrosis Airway. Am. J. Respir. Crit. Care Med. 2015;192:1314–1324. doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherrard L.J., Tunney M.M., Elborn J.S. Antimicrobial Resistance in the Respiratory Microbiota of People with Cystic Fibrosis. Lancet. 2014;384:703–713. doi: 10.1016/S0140-6736(14)61137-5. [DOI] [PubMed] [Google Scholar]
- 16.Sherrard L.J., Graham K.A., McGrath S.J., McIlreavey L., Hatch J., Muhlebach M.S., Wolfgang M.C., Gilpin D.F., Elborn J.S., Schneiders T., et al. Antibiotic Resistance in Prevotella Species Isolated from Patients with Cystic Fibrosis. J. Antimicrob. Chemother. 2013;68:2369–2374. doi: 10.1093/jac/dkt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherrard L.J., McGrath S.J., McIlreavey L., Hatch J., Wolfgang M.C., Muhlebach M.S., Gilpin D.F., Elborn J.S., Tunney M.M. Production of Extended-Spectrum β -Lactamases and the Potential Indirect Pathogenic Role of Prevotella Isolates from the Cystic Fibrosis Respiratory Microbiota. Int. J. Antimicrob. Agents. 2016;47:140–145. doi: 10.1016/j.ijantimicag.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veloo A.C.M., Baas W.H., Haan F.J., Coco J., Rossen J.W. Prevalence of Antimicrobial Resistance Genes in Bacteroides Spp. and Prevotella Spp. Dutch Clinical Isolates. Clin. Microbiol. Infect. 2019;25:e9–e13. doi: 10.1016/j.cmi.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Sherrard L.J., Schaible B., Graham K.A., McGrath S.J., McIlreavey L., Hatch J., Wolfgang M.C., Muhlebach M.S., Gilpin D.F., Schneiders T., et al. Mechanisms of Reduced Susceptibility and Genotypic Prediction of Antibiotic Resistance in Prevotella Isolated from Cystic Fibrosis (CF) and Non-CF Patients. J. Antimicrob. Chemother. 2014;69:2690–2698. doi: 10.1093/jac/dku192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konstan M.W., Wagener J.S., VanDevanter D.R. Characterizing Aggressiveness and Predicting Future Progression of CF Lung Disease. J. Cyst. Fibros. 2009;8:15–19. doi: 10.1016/S1569-1993(09)60006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Société Française de Microbiologie Comité de L’antibiogramme de la Société Française de Microbiologie/European Committee on Antimicrobial Susceptibility Testing 2019. [(accessed on 8 June 2021)]; Available online: https://www.sfm-microbiologie.org/wp-content/uploads/2019/02/CASFM2019_V1.0.pdf.
- 22.Magiorakos A.-P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 23.Bancescu G., Didilescu A., Bancescu A., Bari M. Antibiotic Susceptibility of 33 Prevotella Strains Isolated from Romanian Patients with Abscesses in Head and Neck Spaces. Anaerobe. 2015;35:41–44. doi: 10.1016/j.anaerobe.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Ulger Toprak N., Akgul O., Sóki J., Soyletir G., Nagy E., Leitner E., Wybo I., Tripkovic V., Justesen U.S., Jean-Pierre H., et al. Detection of Beta-Lactamase Production in Clinical Prevotella Species by MALDI-TOF MS Method. Anaerobe. 2020;65:102240. doi: 10.1016/j.anaerobe.2020.102240. [DOI] [PubMed] [Google Scholar]
- 25.Montagner F., Castilho Jacinto R., Correa Signoretti F.G., Scheffer de Mattos V., Grecca F.S., Figueiredo de Almeida Gomes B.P. Beta-Lactamic Resistance Profiles in Porphyromonas, Prevotella, and Parvimonas Species Isolated from Acute Endodontic Infections. J. Endod. 2014;40:339–344. doi: 10.1016/j.joen.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Ulger Toprak N., Veloo A.C.M., Urban E., Wybo I., Justesen U.S., Jean-Pierre H., Morris T., Akgul O., Kulekci G., Soyletir G., et al. A Multicenter Survey of Antimicrobial Susceptibility of Prevotella Species as Determined by Etest Methodology. Anaerobe. 2018;52:9–15. doi: 10.1016/j.anaerobe.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Gatignol J.P., Poulet P.P., Desse T., Duffaut D. Comparison of Laboratory Methods for Detecting B-Lactamase-Positive Strains in the Species Prevotella Intermedia Sensu Lato Isolated from Periodontal Pockets. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22:389–391. doi: 10.1007/s10096-003-0938-y. [DOI] [PubMed] [Google Scholar]
- 28.Dupin C., Tamanai-Shacoori Z., Ehrmann E., Dupont A., Barloy-Hubler F., Bousarghin L., Bonnaure-Mallet M., Jolivet-Gougeon A. Oral Gram-Negative Anaerobic Bacilli as a Reservoir of β-Lactam Resistance Genes Facilitating Infections with Multiresistant Bacteria. Int. J. Antimicrob. Agents. 2015;45:99–105. doi: 10.1016/j.ijantimicag.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y.J., LiPuma J.J. The Microbiome in Cystic Fibrosis. Clin. Chest Med. 2016;37:59–67. doi: 10.1016/j.ccm.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamanai-Shacoori Z., Monfort C., Oliviero N., Gautier P., Bonnaure-Mallet M., Jolivet-Gougeon A. CfxA Expression in Oral Clinical Capnocytophaga Isolates. Anaerobe. 2015;35:68–71. doi: 10.1016/j.anaerobe.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Cobo F., Rodríguez-Granger J., Pérez-Zapata I., Sampedro A., Aliaga L., Navarro-Marí J.M. Antimicrobial Susceptibility and Clinical Findings of Significant Anaerobic Bacteria in Southern Spain. Anaerobe. 2019;59:49–53. doi: 10.1016/j.anaerobe.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Bahar H., Torun M.M., Demirci M., Kocazeybek B. Antimicrobial Resistance and β-Lactamase Production of Clinical Isolates of Prevotella and Porphyromonas Species. Chemotherapy. 2005;51:9–14. doi: 10.1159/000084017. [DOI] [PubMed] [Google Scholar]
- 33.Fujita K., Takata I., Sugiyama H., Suematsu H., Yamagishi Y., Mikamo H. Antimicrobial Susceptibilities of Clinical Isolates of the Anaerobic Bacteria Which Can Cause Aspiration Pneumonia. Anaerobe. 2019;57:86–89. doi: 10.1016/j.anaerobe.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Canigia L., Cejas D., Gutkind G., Radice M. Detection and Genetic Characterization of β-Lactamases in Prevotella Intermedia and Prevotella Nigrescens Isolated from Oral Cavity Infections and Peritonsillar Abscesses. Anaerobe. 2015;33:8–13. doi: 10.1016/j.anaerobe.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Tran C.M., Tanaka K., Watanabe K., Tanaka K., Watanabe K. PCR-Based Detection of Resistance Genes in Anaerobic Bacteria Isolated from Intra-Abdominal Infections. J. Infect. Chemother. 2013;19:279–290. doi: 10.1007/s10156-012-0532-2. [DOI] [PubMed] [Google Scholar]
- 36.Kuriyama T., Williams D.W., Yanagisawa M., Iwahara K., Shimizu C., Nakagawa K., Yamamoto E., Karasawa T. Antimicrobial Susceptibility of 800 Anaerobic Isolates from Patients with Dentoalveolar Infection to 13 Oral Antibiotics. Oral. Microbiol. Immunol. 2007;22:285–288. doi: 10.1111/j.1399-302X.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 37.Mosca A., Miragliotta L., Iodice M.A., Abbinante A., Miragliotta G. Antimicrobial Profiles of Prevotella Spp. and Fusobacterium Nucleatum Isolated from Periodontal Infections in a Selected Area of Southern Italy. Int. J. Antimicrob. Agents. 2007;30:521–524. doi: 10.1016/j.ijantimicag.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Iwahara K., Kuriyama T., Shimura S., Williams D.W., Yanagisawa M., Nakagawa K., Karasawa T. Detection of CfxA and CfxA2, the -Lactamase Genes of Prevotella Spp., in Clinical Samples from Dentoalveolar Infection by Real-Time PCR. J. Clin. Microbiol. 2006;44:172–176. doi: 10.1128/JCM.44.1.172-176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behra-Miellet J., Calvet L., Mory F., Muller C., Chomarat M., Bézian M.C., Bland S., Juvenin M.E., Fosse T., Goldstein F., et al. Antibiotic Resistance among Anaerobic Gram-Negative Bacilli: Lessons from a French Multicentric Survey. Anaerobe. 2003;9:105–111. doi: 10.1016/S1075-9964(03)00066-0. [DOI] [PubMed] [Google Scholar]
- 40.Dubreuil L., Behra-Miellet J., Vouillot C., Bland S., Sedallian A., Mory F. β-Lactamase Production in Prevotella and in Vitro Susceptibilities to Selected β-Lactam Antibiotics. Int. J. Antimicrob. Agents. 2003;21:267–273. doi: 10.1016/S0924-8579(02)00352-7. [DOI] [PubMed] [Google Scholar]
- 41.Mättö J., Asikainen S., Väisänen M.L., Von Troil-Lindén B., Könönen E., Saarela M., Salminen K., Finegold S.M., Jousimies-Somer H. Beta-Lactamase Production in Prevotella Intermedia, Prevotella Nigrescens, and Prevotella Pallens Genotypes and in Vitro Susceptibilities to Selected Antimicrobial Agents. Antimicrob. Agents Chemother. 1999;43:2383–2388. doi: 10.1128/AAC.43.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papaparaskevas J., Pantazatou A., Katsandri A., Houhoula D.P., Legakis N.J., Tsakris A., Avlamis A. Moxifloxacin Resistance Is Prevalent among Bacteroides and Prevotella Species in Greece. J. Antimicrob. Chemother. 2008;62:137–141. doi: 10.1093/jac/dkn134. [DOI] [PubMed] [Google Scholar]
- 43.Boyanova L., Kolarov R., Mitov I. Recent Evolution of Antibiotic Resistance in the Anaerobes as Compared to Previous Decades. Anaerobe. 2015;31:4–10. doi: 10.1016/j.anaerobe.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Rashid M.-U., Weintraub A., Nord C.E. Development of Antimicrobial Resistance in the Normal Anaerobic Microbiota during One Year after Administration of Clindamycin or Ciprofloxacin. Anaerobe. 2015;31:72–77. doi: 10.1016/j.anaerobe.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Byun J.-H., Kim M., Lee Y., Lee K., Chong Y. Antimicrobial Susceptibility Patterns of Anaerobic Bacterial Clinical Isolates from 2014 to 2016, Including Recently Named or Renamed Species. Ann. Lab. Med. 2019;39:190–199. doi: 10.3343/alm.2019.39.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shilnikova I.I., Dmitrieva N.V. Evaluation of Antibiotic Susceptibility of Bacteroides, Prevotella and Fusobacterium Species Isolated from Patients of the N. N. Blokhin Cancer Research Center, Moscow, Russia. Anaerobe. 2015;31:15–18. doi: 10.1016/j.anaerobe.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Xie Y., Chen J., He J., Miao X., Xu M., Wu X., Xu B., Yu L., Zhang W. Antimicrobial Resistance and Prevalence of Resistance Genes of Obligate Anaerobes Isolated from Periodontal Abscesses. J. Periodontol. 2014;85:327–334. doi: 10.1902/jop.2013.130081. [DOI] [PubMed] [Google Scholar]
- 48.Seifert H., Dalhoff A., PRISMA Study Group German Multicentre Survey of the Antibiotic Susceptibility of Bacteroides Fragilis Group and Prevotella Species Isolated from Intra-Abdominal Infections: Results from the PRISMA Study. J. Antimicrob. Chemother. 2010;65:2405–2410. doi: 10.1093/jac/dkq321. [DOI] [PubMed] [Google Scholar]
- 49.Mory F., Lozniewski A., Bland S., Sedallian A., Grollier G., Girard-Pipau F., Paris M.F., Dubreuil L. Survey of Anaerobic Susceptibility Patterns: A French Multicentre Study. Int. J. Antimicrob. Agents. 1998;10:229–236. doi: 10.1016/S0924-8579(98)00041-7. [DOI] [PubMed] [Google Scholar]