Abstract
Pyropia yezoensis is the most important commercial edible red algae in China, carrying a variety of resident microbes at its surface. To understand microbiome diversity, community structure, interactions and functions with hosts in this regard, thalli and seawater sampleswere collected from Yantai and Rizhao cultivation farms in the Yellow Sea. The thalli and seawater samples (n = 12) were collected and studied using an Illumina NovaSeq 6000 platform and 16S ribosomal RNA (rRNA) gene sequencing, along with the consideration of environmental factors. Bacterial communities in association with P. yezoensis and surrounding seawater were predominated by Cyanobacteria, Proteobacteria, and Bacteroidetes. The variability of bacterial communities related to P. yezoensis and seawater were predominantly shaped by nitrate (NO3), ammonium (NH4), and temperature. Cluster analysis revealed a close relationship between thalli (RTH and YTH) and seawater (RSW and YSW) in terms of the residing bacterial communities, respectively. PICRUSt analysis revealed the presence of genes associated with amino acid transportation and metabolism, which explained the bacterial dependence on algal-provided nutrients. This study reveals that the diversity of microbiota for P. yezoensis is greatly influenced by abiotic factors and algal organic exudates which trigger chemical signaling and transportation responses from the bacterial community, which in turn activates genes to metabolize subsequent substrates.
Keywords: Pyropia yezoensis, microbiome, bacterial communities, Illumina NovaSeq, microbial composition, ribosomal RNA
1. Introduction
P. yezoensis (laver), an economically important red seaweed, is a popular food and condiment that has a long history of cultivation and consumption in Asia [1] and is widely used around the world nowadays [2]. Conventionally, laver seaweeds have been considered as staple foods in limited regions of Asia, but an increased understanding of health benefits and the global availability of processed food products has led to a dramatic increase in consumption worldwide [3]. The growth of global seaweed aquaculture as a source of pharmaceuticals and biomaterials (e.g., by Alga Technologies and Cyanotech, etc.) is expected to contribute to the expansion of the laver industry [2,4]. Worldwide laver production has increased from 517,739 t/USD 945.1 billion in 1987 to 2,563,048 t/USD 2319.7 billion in 2017 [5]. China, South Korea, and Japan were the three largest producers, accounting for almost 99.87% of total world production in 2017 [5]. This represents the typical laver consumption in these countries, as well as the favorable regional aquaculture environmental conditions [6].
Marine microorganisms have been impacted by environmental impacts on several spatial scales, including changes in species distribution, habitat degradation, higher incidences of disease, species extinctions, and additionally alarming effects on the abundance, distribution, and function of marine planktonic microorganisms [7,8,9,10]. Macroalgae normally attract and promote beneficial bacterial species to colonize and multiply on their surface and, in turn, these epiphytic bacteria contribute to the algal life cycle by assisting the algae to counter the intrusion and colonization of harmful microorganisms by secreting secondary metabolites and/or antimicrobials [11]. Since the mid-twentieth century, the strong mutual interactions between bacteria and seaweeds have been studied, and several investigations have shown that green and brown macroalgae axenic cultures grow slowly or display anomalous morphogenesis [12,13,14,15,16,17,18]. Fries firstly documented this dependence for red algae while working with Polysiphonia urceolata [19], furthermore, in recent times, similar findings have been found in red algal species [20,21,22]. Investigations on the microbial makeup and diversity of edible red and brown algae, such as Pyropia haitanensis, Porphyra umbilicalis, and Saccharina japonica, have been undertaken prior to our findings [20,23,24]. Moreover, consumable red alga Kappaphycus alvarezii strains (brown, green) have been examined for the presence or absence of pathogenic bacterial strains [25]; however microbial community investigations of P. yezoensis are limited to a comparison between the healthy and diseased states (red rot), revealing a change in the inhabiting microbial community structure and abundance during infection [21,26]. These studies into the functional and compositional profiles of the microbial communities on and around macroalgae demonstrate the need of developing a comprehensive understanding of the microbiome of the macroalga P. yezoensis, which is both ecologically and economically vital.
The application of high-throughput DNA sequencing techniques to examine a microbial community’s genomic composition in a culture-independent mode has grown tremendously over the last few decades. This technique of high-resolution molecular sequencing, known as metagenomics, can be further sub-divided into two separate methodologies, namely 16S rRNA gene and shotgun metagenomic sequencing. To study bacterial phylogeny and taxonomy, the 16S rRNA gene sequencing method relies on 16S ribosomal RNA (rRNA) gene sequencing as a genetic marker, which consists of conserved hypervariable regions that can be used for bacterial identification [27]. Several research studies have focused on the microbial diversity of Pyropia using marker genes such as 16S rRNA genes, 18S rRNA genes, and internal transcribed spacer (ITS) genes [28,29]. A recent study investigated the change in bacterial population associated with Pyropia using 16S rRNA gene sequencing and found that there were over 300 operating taxonomic units (OTUs) distributed in approximately 15 microbial phyla [21].
Based on the previous studies, the present study aims to explore the microbial communities associated with P. yezoensis and the variations in surrounding seawater in different geographical locations. Moreover, this work aims to explore the contribution of abiotic parameters in driving variation in the structures of microbiota.
2. Materials and Methods
2.1. Study Sites and Sample Collection
The P. yezoensis thalli and seawater samples were collected from two laver farms located in the coastal areas of Yantai (36°38′ N, 121°24′ E) and Rizhao (35°15′ N, 119°26′ E) of Shandong Province, China (Figure 1).
Figure 1.
(A) P. yezoensis thalli. (B) Yantai cultivation farm. (C) Rizhao cultivation farm.
For the analysis of microbial communities, thalli samples (n = 3) and seawater samples (n = 3), were collected in triplicate from each farm, with a total of six thalli (n = 6) samples and six seawater (n = 6) samples from both regions, respectively. To make each of these biological replicates, neighboring triplicate samples (roughly 2 m away) were also collected and pooled (hundreds of pieces in total for thalli and 3 L in total for seawater). All the samples were brought to a laboratory within 4–6 h and were kept at 4 °C by soaking the thalli in collected seawater. Thalli samples were washed three times with sterilized seawater to remove loosely associated microbes and stored at −80 °C until further processing. Moreover, for each seawater replicate, approximately 600 mL was pre-filtered through a nylon mesh (200-μm pore size) to remove debris and subsequently filtered through a 0.22-μm mixed cellulose membrane (MerckMillipore, Darmstadt, Germany) to collect the microbial biomass.
For measuring environmental variables, seawater samples were collected in triplicate from both the Yantai and Rizhao farming sites. These samples were also used to evaluate the environmental stress on bacterial assemblages. The salinity, temperature, dissolved oxygen (DO), and pH values of the seawater were recorded during the sampling process with appropriate sensors at a depth of 50 cm, whereas the concentrations of phosphate, reactive silicate (RS), nitrate, nitrite, and ammonium were calculated using standard methods [30]. Three technical replicates were used for the determination of each variable for each sample.
2.2. DNA Extraction
For the extraction of bacterial community DNA from the thalli and seawater samples, the PrecellysTM-24 (Bertin Corp., Rockville, MD, USA) and DNeasy PowerSoil® kit (Qiagen, Hilden, Germany) were used. Samples were shaken twice at the rate of 6800 shakes min−1 for 30 s with an interval of 5 min prior to DNA extraction [21]. Then, DNA was stored at −20 °C until amplification. DNA concentration and purity were monitored on 1% agarose gel and all DNA samples were diluted with sterile water to adjust the concentration to 1 ng/µL.
2.3. 16.S rRNA Gene Amplification
For the thalli and seawater samples, the V4 hypervariable region of 16S rRNA gene was amplified using the primer pairs of 515F (5′-GTGCCAGCM GCCGCGGTAAT-3′) and 806R (5′-GGACTACHVGGGTWTCTAA-3′) with the barcode. All PCR reactions were performed with 15 µL of the Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 µM of forward and reverse primers, and roughly 10 ng of template DNA. Thermal cycling for initial denaturation was performed at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s, then finally 72 °C for 5 min. Each DNA sample was amplified in triplicate. The same 1× loading buffer (contained SYB green) volume was mixed with PCR products and electrophoresis on 2% agarose gel was used for detection. The PCR products were mixed in equal density ratios, and then the PCR products were purified with a Qiagen gel extraction kit (Qiagen, Hilden, Germany) in correct lengths.
2.4. Library Preparation and Sequencing
Using the TruSeq® DNA PCR-Free sample preparation kit (Illumina, Inc., San Diego, CA, USA), sequencing libraries were generated following the recommendations of the manufacturer and index codes were added. On the Qubit@ 2.0 fluorometer (Thermo Scientific, CA, USA) and Agilent Bioanalyzer 2100 device, the library quality was evaluated. Finally, the library was sequenced on an Illumina NovaSeq 6000 platform.
2.5. Data Processing Based on Bioinformatics and Statistical Analysis
Based on their specific barcode, paired-end reads were assigned to the samples and trimmed by cutting off the barcode and primer sequence. Using the software FLASH v1.2.7 [31], paired end reads were merged and the splicing sequences were called raw tags. In order to obtain clean and high-quality tags, quality filtering of the raw tags was performed under specific filtering conditions [32] according to the QIIME v1.9.1 [33] quality control process. The tags were compared with a reference database (SILVA database) to detect chimera sequences using the UCHIME algorithm [34], and then the chimera sequences were removed [35]. Finally, the effective tags were obtained.
Uparse v7.0.1001 was used to perform the sequence analysis [36]. Sequences with ≥97% similarity were assigned to the same OTUs. For further annotation, representative sequences for each OTU was screened. The SILVA database [37] was used to annotate the taxonomic information for each representative sequence based on Mothur algorithm. In order to study the phylogenetic relationships of different OTUs and differences between the dominant species in different samples (groups), the MUSCLE software v3.8.31 was used to conduct multiple sequence alignment [38]. Finally, the data of each sample were normalized and the smallest amount of data in a sample was used as the standard.
Alpha diversity was applied to analyze the complexity of species diversity through calculating the observed species, Chao1, Shannon, Simpson, ACE, whole-tree PD, and Good’s coverage values with QIIME v1.7.0 and was displayed with R software v2.15.3.
For multi-sample comparative analysis (Beta diversity), QIIME v1.9.1 was used to calculate the UniFrac distance-based species complexity to evaluate differences between samples. Furthermore, R software v2.15.3 was used to draw the PCA and NMDS diagrams. For PCA analysis, the ade4 and ggplot2 packages were used. NMDS analysis was performed using the vegan package. To test the significance of the variations between the compositions of bacterial populations, Bray–Curtis distance-dependent dissimilarity tests (e.g., MRPP, ANOSIM, and Adonis) were used. Clustering with the unweighted pair-group method with arithmetic means (UPGMA) was performed as a type of hierarchical clustering to interpret the distance matrix using average linkage via the QIIME software (Version 1.9.1). For LEfSe analysis, the LEfSe software was used, and the LDA score was set to four as the default.
The PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states) software package was used to understand the potential genetic capabilities of the seaweed bacterial communities [39]. Redundancy analysis (RDA) was applied to reveal the relationships between bacterial communities and environmental variables in R using the vegan package.
Spearman correlation was performed between the abundance of bacterial community composition (top 35 genera) and the environmental factors in R by the correlation test function of the psych package and the pheatmap function in the pheatmap package [40], respectively.
3. Results
3.1. Physico-Chemical Properties of Seawater
The physicochemical properties of seawater samples are illustrated in Table 1. The NO3 and NH4 concentrations were significantly different (p < 0.05) between the regions of Yantai and Rizhao. A significant difference (p = 0.005) in temperature was also recorded, with higher values in Yantai as compared to Rizhao. Most of the other environmental variables were relatively stable and no significant (p < 0.05) differences were recorded (Table 1).
Table 1.
Environmental variables of Yantai and Rizhao seawater samples.
| Environmental Variables | YSW | RSW | p-Value |
|---|---|---|---|
| DO (ppm) | 15.86 ± 0.161 | 16.39 ± 0.44 | 0.123 |
| Temperature (°C) | 6.8 ± 0.205 | 5.9 ± 0.200 | 0.005 * |
| Salinity (%) | 3.28 ± 0.097 | 3.33 ± 0.118 | 0.684 |
| pH | 6.90 ± 0.200 | 7.09 ± 0.262 | 0.639 |
| NH4 (μmol/L) | 2.527 ± 0.260 | 0.85 ± 0.429 | 0.004 * |
| Si (μmol/L) | 5.39 ± 0.263 | 6.30 ± 0.921 | 0.175 |
| NO3 (μmol/L) | 4.70 ± 0.806 | 11.301 ± 0.901 | 0.001 * |
| NO2 (μmol/L) | 0.751 ± 0.032 | 0.836 ± 0.069 | 0.130 |
| Phosphate (μmol/L) | 0.177 ± 0.015 | 0.178 ± 0.009 | 0.911 |
* Data are represented as the mean ± standard deviation. Bold p-values indicate a significant difference between the two regions. Means compared using unpaired t-testing and significance is determined as p < 0.05.
3.2. Bacterial Community Diversities
A total of 792,529 effective sequences of DNA were identified after the quality control process and 62,850–69,872 sequences per sample (mean ± standard deviation = 66,044 ± 1071) were obtained. According to the SILVA database, a total of 1202 OTUs were obtained and 92.43% of reads were classified at the phylum level and could be assigned into 19 and 20 phyla for the P. yezoensis and seawater samples, respectively. A petal diagram revealed that the bacterial OTUs associated with P. yezoensis of the Yantai and Rizhao regions had 55 core OTUs (Figure S1A), while 156 core OTUs were identified in the seawater samples (Figure S1B).
3.2.1. Alpha Diversity
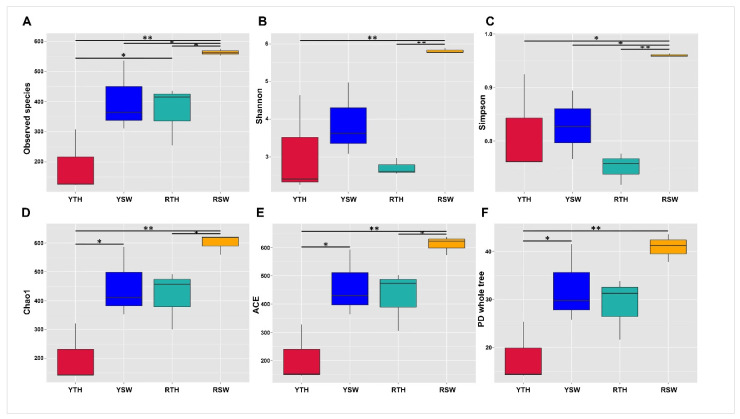
Good’s coverage for all samples was found (greater than 99%), indicating that sequencing captured the majority of bacterial diversity in the samples (Table S1). Meanwhile, 62,452 randomly selected sequences (the lowest number in samples was 62,850) were used to evaluate the alpha diversity (i.e., ACE, Observed species, Shannon, Simpson, Chao1, and PD whole-tree) of each dataset (Figure 2 and Figure S2), whereas the alpha diversity indices showed that seawater features far higher bacterial diversity than P. yezoensis. According to the Wilcoxon rank-sum test community richness (Chao1, ACE), community diversity (Shannon, Simpson), and PD whole-tree results, significant differences (p < 0.05) with stochastic patterns were observed (Table 2); however, in observed species indices, the significant differences (p < 0.05) among all the sample groups were recorded (Table 2).
Figure 2.
Comparisons of alpha diversity indices based on an unpaired Wilcoxon rank-sum test: (A) observed species; (B) Shannon; (C) Simpson; (D) Chao1; (E) ACE; (F) PD whole-tree. YTH, Yantai region thalli of P. yezoensis; YSW, Yantai region seawater sample group; RTH, Rizhao region thalli of P. yezoensis; RSW, Rizhao region seawater sample group. (* p-value < 0.05; ** p-value < 0.01).
Table 2.
Alpha diversity indices differences among the sample groups.
| Sample Groups | Whole-Tree PD | Chao1, ACE | Simpson | Shannon | Observed Species |
|---|---|---|---|---|---|
| RTH-YTH | 0.091 | 0.071 | 0.244 | 0.071 | 0.047 * |
| RSW-YSW | 0.155 | 0.071 | 0.047 * | 0.071 | 0.024 * |
| RTH-RSW | 0.053 | 0.029 * | 0.002 * | 0.004 * | 0.012 * |
| YTH-YSW | 0.031 * | 0.029 * | 0.492 | 0.854 | 0.024 * |
* p-values less than 0.05 are indicated with bold characters.
3.2.2. Beta Diversity
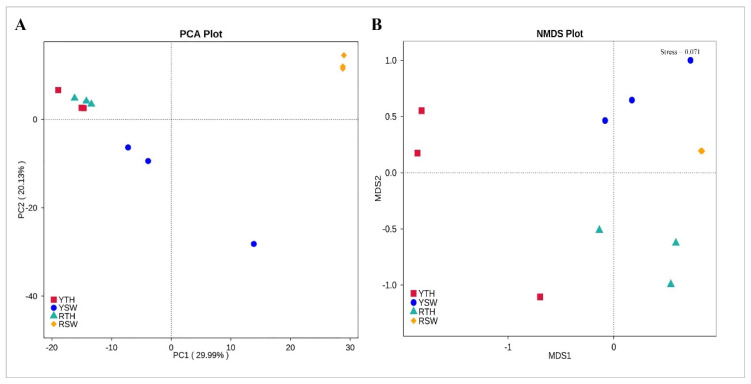
For beta diversity interpretation, PCA-based Euclidean distances were used for the dimensionality reduction of multi-dimensional data, and NMDS ordination based on Bray–Curtis distances was performed to explain the clustering relationships between bacterial community datasets. In the PCA plot, P. yezoensis samples of Rizhao and Yantai regions clustered together, whereas seawater samples were distantly related with each other in both sampling regions (Figure 3A), whereas, in the NMDS plot, it should be noted that P. yezoensis samples of both the regions were clustered distantly from each other while, seawater samples clustered closely to each other (Figure 3B). The results of cluster analysis UPGMA based on UniFrac distance were consistent with the PCA. Thalli samples of both Rizhao and Yantai regions were clustered together, just like the seawater samples of both regions (Figure 4A).
Figure 3.
Relationships between individual datasets illustrated by (A) principal component analysis (PCA) and (B) nonmetric multi-dimensional scaling (NMDS).
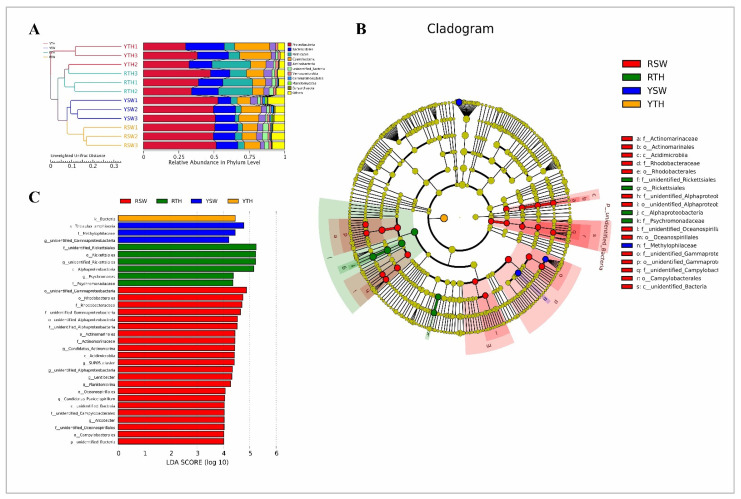
Figure 4.
(A) UPGMA cluster tree analysis based on the unweighted UniFrac distance. The digital number symbolizes three biological replicates for each sample. (B) Evolutionary branch diagram of differential bacterial communities or species. (C) The LDA value (influence value of linear discriminant analysis) distribution histogram shows bacterial communities or species (Biomarker) with a LDA score greater than four.
Bray–Curtis-based Adonis, Anosim, and MRPP analysis values of P. yezoensis thalli and seawater samples were found to be greater than zero, thus indicating non-significant (p > 0.05) differences between groups rather than differences within groups (Table S2).
3.2.3. Linear Discriminant Analysis Effect Size (LEfSe)
LEfSe (LDA effect size) is an analysis tool used to discover and interpret highly dimension biomarkers (genes, pathways, and taxa). Information on the differences in phylum, class, order, family, and genus levels among all bacteria is shown in the pie chart in Figure 4B. The LEfSe analysis used the species abundance data between sample groups to detect the species difference through the method of a rank-sum test and achieved dimensionality reduction through LDA (linear discriminant analysis) to evaluate the impact of the species differences. Overall, 32 biomarkers were identified with a LDA score >4. The maximum effect size in Rizhao P. yezoensis (RTH) was observed for family and order Rickettsiales of the phylum Proteobacteria. In contrast, the microbiota of Rizhao seawater (RSW) was characterized by a preponderance of microorganisms from the phylum Proteobacteria including orders of Gammaproteobacteria and Rhodobacterales, whereas in the Yantai region P. yezoensis (YTH), microbiota were characterized by bacterial biomarkers and the seawater microbiota (YSW) were dominated by the species Teleaulax amphioxia and family Methylophilaceae, belonging to the phyla of Cyanobacteria and Proteobacteria, respectively (Figure 4C).
3.3. Composition of Bacterial Community
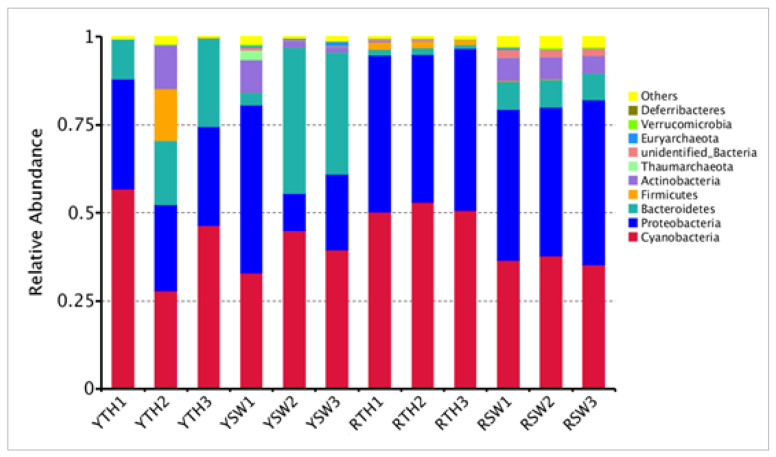
The three taxa of bacterial communities that dominated in P. yezoensis and seawater were Cyanobacteria (42.7%), Proteobacteria (35.7%), and Bacteroidetes (13.6%), respectively (Figure 5). The percentages of dominant phyla associated with P. yezoensis of both regions were the following: Cyanobacteria, 47.5 ± 6.251%; Proteobacteria, 36.05 ± 13.407%; and Bacteroidetes, 9.9 ± 13.818% (Figure 5). The dominant OTUs of bacterial communities from both regions (water and thalli) are summarized in Table S3. For example, the datasets from Yantai P. yezoensis samples were dominated by OTU2 of unidentified Cyanobacteria (22%), followed by OTU1 of unidentified Rickettsiales (21%), and OTU811 of unidentified Cyanobacteria (15%), respectively. In the Rizhao P. yezoensis samples, OTU1 of unidentified Rickettsiales (34%) was the primary contributor, followed by OTU2 of unidentified Cyanobacteria (29%) and OTU811 of family unidentified Cyanobacteria (20%) (Table S3). Furthermore, SIMPER analysis revealed 12.4% dissimilarity index in these OTUs to differentiate the bacterial communities between two sampling regions (Table S4A).
Figure 5.
Bacterial community structures associated with P. yezoensis and seawater at the phylum and class levels. YTH, Yantai region P. yezoensis thalli; YSW, Yantai region seawater; RTH, Rizhao region P. yezoensis thalli; RSW, Rizhao region seawater.
In terms of the relative abundance and taxonomic level, the bacterial communities of seawater showed different profiles as compared to the P. yezoensis related communities of both regions. In seawater population, the dominant taxa were Cyanobacteria (37.8 ± 2.139%), Proteobacteria (35.25 ± 14.229%) and Bacteroidetes (17.25 ± 15.381%) (Figure 5). The prevalent OTUs contributed more than 10% in Yantai region seawater samples were OTU3 (Tenacibaculum, 24%), OTU2 (unidentified Cyanobacteria, 12%), and OTU5 (unidentified Cyanobacteria, 10%). In Rizhao seawater samples the dominated OTUs contributed more than 10% was only OTU4 (Virgulinella fragilis, 13%) while others showed less contribution (Table S3). SIMPER analysis revealed 16.7% dissimilarity index between these OTUs to differentiate each dataset (Table S4B).
The common bacterial taxa from Yantai and Rizhao P. yezoensis were represented by four OTUs with more than 1% contribution in their respective regions, including OTU1 (genus unidentified Rickettsiales), OTU2 (family unidentified Cyanobacteria), OTU4 (genus unidentified Cyanobacteria), and OTU811 (family unidentified Cyanobacteria). Common bacterial taxa from the seawater of more than 1% abundance from both the regions included nine OTUs: OTU1 (genus unidentified Rickettsiales), OTU4 (genus unidentified Cyanobacteria), OTU5 (genus unidentified Cyanobacteria), OUT6 (genus Candidatus Actinobacteria), OTU7 (SUP05 cluster), OTU8 (genus Alphaprotobacteria), OTU11 (order Gammaprotobacteria), OTU15 (family unidentified Gammaprotobacteria), and OTU310 (family Methylophilaceae) (Table S3).
3.4. PICRUSt Analysis on Metagenome Functions Prediction
Metagenome function prediction was carried out based on marker genes (such as 16S rRNA) by PICRUSt. At the primary level, eight categories of biological functional pathways, including metabolism, genetic information processing, environmental processing, organismal system, and human disease were summarized (Figure S3A). The most abundant predicted gene copy numbers were found for metabolism (48–51%), genetic information processing (16–20%), and environmental information processing (11–12%), respectively. It should be noted that genetic information processing and human disease were common in both thalli (RTH > YTH) groups, while cellular process and metabolism were prominent common functions in both seawater (RSW > YSW) groups (Figure S3A).
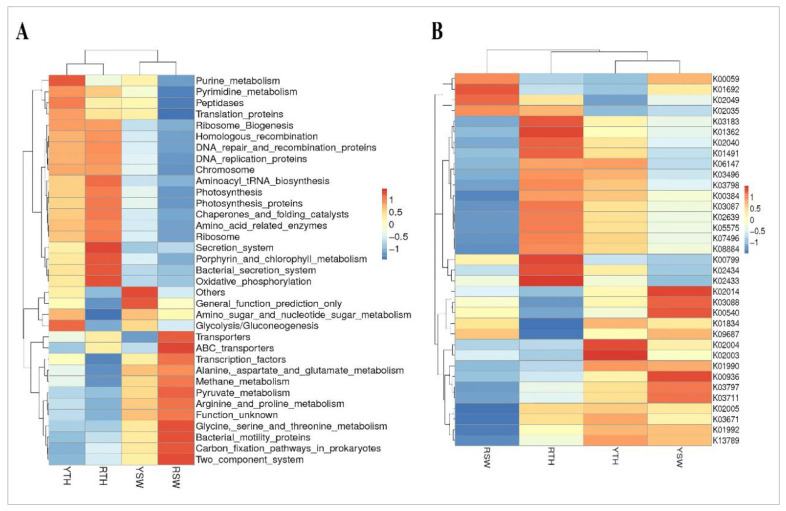
At the secondary level, multiple sub-functions were summarized, including biosynthesis of other secondary metabolites, signaling molecules and interaction, cell motility and amino acid metabolism (Figure S3B). The top 35 subfunctions are under discussion here, and cluster analysis showed that the thalli and seawater samples of both regions were closely related irrespective of the different sampling sites. Overall, 16 out of 35 sub-functions were common in seawater samples with the fluctuations in expression levels such as 5 of 16 sub functions showed decreased gene copy number in RSW as compared to YSW, rest followed revert patterns (Figure S3B). Most functions in thalli samples of both regions (YTH, RTH) were common except, metabolism and environmental adaptations with significant differences (p < 0.05) in the predicted gene copy number (Figure S4A). KO hierarchy level 3 indicated that thalli samples were closely related, and seawater samples were also clustered together. Most of the functions were common between seawater samples with variations in gene copy number following the trend RSW > YSW as shown in Figure 6A. In the thalli samples, there was a significant difference between plant-pathogen interaction (p ≤ 0.001), inositol phosphate metabolism (p = 0.01), and peptidases (p = 0.02). (Figure S4B).
Figure 6.
(A) Heat map display of the hierarchical clustering of the predicted KEGG ortholog (KEGG level 3) gene copy number (log10 transformed) of bacterial microbiota across all samples. (B) Cluster heat map display of KOs based on the predicted gene copy (log10 transformed) of bacterial microbiota across all sample groups. K00059 fabG, K01692 paaF, K02049 ABC.SN.A,K02035 ABC.PE.S, K03183 ubiE, K01362 OVCH, K02040 pstS, K01491 folD, K06147 ABCB-BAC, K03496 parA, K03798 ftsH, K00384 trxB, K03087 rpoS, K02639 petF, K05575 ndhD, K07496 putative transposase, K08884 serine/threonine protein kinase, K00799 GST, K02434 gatB, K02433 gatA, K02014 TC.FEV.OM, K03088 rpoE, K00540 fqr, K01834 PGAM, K09687 Antibiotic transport system, K02004 ABC.CD.P, K02003 ABC.CD.A, K01990 ABC-2.A, K00936 pdtaS, K03797 ctpA, K03711 fur, K02005 ABC.CD.TX, K03671 trxA, K01992 ABC-2.P, K13789 GGPS.
Many studies have shown that the abundance and expression of functional genes in microbial communities can be precisely predicted by PICRUSt [41,42,43,44]. Furthermore, the abundant key genes in Yantai region seawater and thalli samples were K03711 fur (Fur family transcriptional regulator, ferric uptake regulator), K03797 ctpA (carboxyl-terminal processing protease), K00936 pdtaS (two-component system, sensor histidine kinase PdtaS), K02014 FEV (iron complex outer membrane receptor protein), K03088 rpoE (RNA polymerase sigma-70 factor), K00540 fqr (F420H(2)-dependent quinone reductase), K02004 ABC (putative ABC transport system permease protein), K02003 ABC (putative ABC transport system ATP-binding protein),and K01990 ABC-2 (ABC-2 type transport system ATP-binding protein) (Figure 6B); however, it is worth mentioning that a significant difference was observed between YTH and YSW in the pathways of K03497 parB (p = 0.002), K00540 ribBA (p = 0.004), and K11717 sufS (p = 0.006) (Figure S5A).
The most prevalent predicted pathway genes in the Rizhao seawater (RSW) and thalli (RTH) samples were K0059 fabG (3-oxoacyl-[acyl-carrier protein] reductase), K01692 paaF (enoyl-CoA hydratase), K02049 ABC.SN.A (NitT/TauT family transport system ATP-binding protein), K03183 ubiE (demethylmenaquinone methyltransferase/2-methoxy-6-polyprenyl-1,4-benzoquinol methylase), K01362 OVCH (ovochymase), K00799 GST(glutathione S-transferase), K02434 gatB (aspartyl-tRNA(Asn)/glutamyl-tRNA(Gln) amidotransferase subunit B), and K02433 gatA (aspartyl-tRNA(Asn)/glutamyl-tRNA(Gln) amidotransferase subunit A). A significant (p < 0.05) distinction was found in many pathway genes of RTH and RSW (Figure S6). Meanwhile, thalli samples of Rizhao and Yantai (RTH, YTH) were clustered together, and the noticeable predicted common KO were K03183 ubiE (demethylmenaquinone methyltransferase/2-methoxy-6-polyprenyl-1,4-benzoquinol methylase), K01362 OVCH (ovochymase), K00799 GST (glutathione S-transferase), K02040 pstS (phosphate transport system substrate-binding protein), K01491 folD (methylenetetrahydrofolate dehydrogenase (NADP+), and K02005 ABC.CD.TX (HlyD family secretion protein); however, t-testing revealed a significant difference (p < 0.05) between the K00540 fqr (F420H (2)-dependent quinone reductase) and K013789 GGPS (eranylgeranyl diphosphate synthase, type II) pathways of thalli samples (Figure S5B). Overall, there were many common pathways activated in seawater and thalli samples of both regions, but their level of activation or gene copy number were randomly increasing or decreasing, respectively (Figure 6B).
3.5. Linkage between Bacterial Community Structures and Seawater Properties
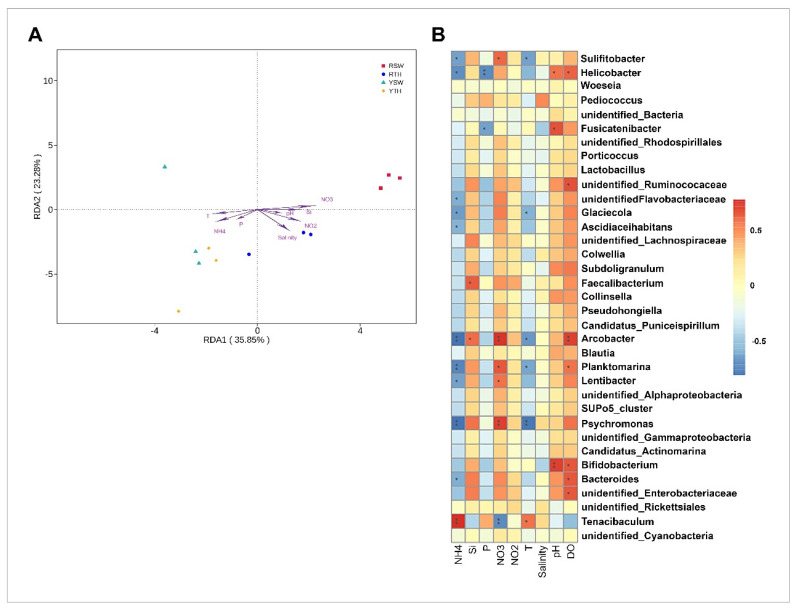
The RDA analysis showed no significant effects (p > 0.05) for the nitrite, phosphate, pH, DO, salinity and reactive silicate concentrations on the bacterial community distributions in the Rizhao and Yantai regions. Ammonium (R2 = 0.646, p = 0.010), nitrate (R2 = 0.642, p = 0.006), and temperature (R2 = 0.411, p = 0.002) were significantly correlated with P. yezoensis and seawater-based bacterial communities (Figure 7A).
Figure 7.
(A) Redundancy analysis (RDA) for bacterial community datasets associated with P. yezoensis and seawater. T, temperature; P, phosphate; RS, reactive silicate. (B) Spearman correlation analysis between seawater environmental factors and bacterial communities. The corresponding intermediate heat map value is the Spearman correlation coefficient r, and r > 0 is the positive correlation, r < 0 is negative correlation. * indicates significance where p < 0.05. ** indicates significance where p < 0.01.
The Spearman correlation analysis showed stronger positive correlation of Tenacibaculum (OTU3) to ammonium concentrations, while Phsychromonas (OTU12), Arcobacter (OTU28), and Planktomarina (OTU13) were negatively correlated with ammonium concentrations, whereas Arcobacter showed a strongly positive correlation with nitrate and dissolved oxygen, which is the same as Psychromonas to nitrate. Other genera which showed strong correlations with seawater properties include Bifidiobacterium within Actinobacteria (OTU27), which was positively correlated with pH. On the other hand, Helicobacter within family Helicobacteraceae (OTU65) exhibited a strong negative correlation with phosphate; however, many other genera were also strongly correlated with seawater properties with positive and negative correlations (Figure 7B).
4. Discussion
Macroalgae perform essential roles in coastal ecosystems [45,46,47] are and known to contain diverse bacterial symbionts on their surfaces [48,49,50]. Microbial biofilm communities have showed effects on the health and normal functions of their hosts in multiple ways [51,52,53,54,55]. Increasing evidence suggests that such microbial community and host interactions and associations are specific [56,57,58]. These specific interactions represent profound implications for the prediction of microbial diversity and functional interactions in marine environments [57,59,60], which highlights the importance of investigating the interactions between hosts and microbial communities [61]. A variety of microbes resides on the surface of P. yezoensis [26] that differ in diversity, composition, and predicted functionality from each other and the surrounding water [62]. Significant scientific research has been aimed at studying the bacterial communities associated with seaweeds in recent decades to understand the interactions between bacteria and seaweed. [63]; however, the understanding of the diversity of microbial biofilm and complex relationships with their hosts is still in its beginnings. In this study, we provide insights into the variations in structure, composition, functional activities and algal-bacterial interactions of P. yezoensis and surrounding seawater associated microbial communities between two different sites, along with the roles of the physicochemical properties in driving the variability in the associated microbiota between the two regions.
4.1. Bacterial Community Structure and Abundance
The profile of the bacterial community on P. yezoensis and the surrounding seawater showed spatial resemblance and variation at the same time. Rizhao seawater showed richer and variable bacterial community structure as compared to Yantai seawater. The microbiota of P. yezoensis thalli showed an almost related bacterial population with slight variations. The alpha diversity indices reveal that seawater held much higher bacterial diversity than P. yezoensis, while the species richness and diversity indices showed significant (p < 0.05) differences among the seawater and thalli microbial communities of the same region (Figure 2). PCA and cluster analysis also confirmed the diversity of microbiota on P. yezoensis surface and seawater, respectively (Figure 3A and Figure 4A). These findings indicate that the bacterial communities may be shaped by the physicochemical properties of seawater. Moreover, the abundant phyla in the Rizhao and Yantai regions in P. yezoensis were Cyanobacteria, Proteobacteria, Bacteriodetes, and Firmicutes (Figure 5). As compared to other microbiota diversity studies, in our study, Cyanobacteria appeared as a dominant phylum, followed by Proteobacteria, which likewise dominated in the red seaweed Laurencia dendroidea [64]. These findings were consistent with Friedrich [65], who observed the bacterial species of the phyla Proteobacteria, Bacteroidetes, and Cyanobacteria in macroalgal species. Furthermore, the bacterial abundance belonging to Firmicutes and Bacteroidetes has been found in different red and green seaweeds, such as Caulerpa racemosa, Bryopsis pennata Caulerpa cylindracea, Bryopsis hypnoides, Monostroma hariotii, and Ulva intestinalis [66,67,68]. Whole-genome sequencing of P. haitanensis microbiota revealed the highest abundance of Proteobacteria (54.64%) and Bacteroidetes (37.92%) [23].
Members of Alphaproteobacteria and Gammaproteobacteria have a worldwide spread in coastal waters [69,70], while other commonly occurring marine taxa include Bacteroidetes, Actinobacteria, Plankomycetes, and Chloroflexi [61,70,71]. In our research, structural alterations were exhibited by bacterial population within seawater, which were particularly revealed by the discrepancies in abundance of prevalent OTUs distributed within the dominant phylum/class. (Table S3). For example, the prevalent OTUs in Yantai and Rizhao seawater samples were OTU3 Tenacibaculum. As the genus Tenacibaculum has a cosmopolitan distribution within saltwater, local distributions of Tenacibaculum spp. are largely unknown [72]. Moreover, non-pathogenic Tenacibaculum spp. has been described for red algae, tunicates, tidal sediments, seawater, mollusks, and crustaceans [72,73,74,75]. While members of the genus Tenacibaculum perform many functions in marine environments, including the decomposition of biopolymers like cellulose derivatives, xylan, agar, and chitin, some have algae-dissolving activities and also play a crucial role in carbon metabolism [22,76,77]. OTU4 (Virgulinella fragilis) and OUT6 (Candidatus Actinomarina), were also dominant in the seawater samples from both regions. Bacterial communities of Candidatus Actinomarina have already been reported in seawater previously [78] and are known to play a key role in organic matter processing (i.e., transport and degradation) in oceans [79]. Rizhao seawater also had a higher abundance for the OTU7 SUP05 cluster. Members of the SUP05 clade reported in marine waters and are supposed to contribute directly by the successive reduction of nitrate (NO3−) to nitrogenous gases (N2O or N2) [80]. Various studies have also identified the occurrence of these genera in algae and surrounding seawater [22,50,81]. Our study results are in agreement with Wei et al., who identified similar profile of bacterial communities in P. yezoensis and surrounding seawater by 16S rRNA gene sequencing approach [82]. Nevertheless, it should be acknowledged that different algal phyla show varying proportions of these bacterial taxa. This may be due to the limitations of the datasets; however, the results pose interesting considerations for the definition of “core” groups of bacterial communities. Additionally, functionally distinct algae (e.g., from different phyla) might not be expected to have a similar core.
4.2. Effects of Abiotic Factors on Community Structure
Apart from biotic variables like holobiont, the presence of grazer species [83,84,85,86] and natural abiotic variables is also responsible for an increase or decrease in the growth and marine diversity of algal species [87]; however, an increasing body of evidence indicates that seaweed interactions with microbiota and epifauna, habitat, growth, development, and abundance depend on the variations of abiotic factors like salinity, pH, temperature, sun radiation, and nutrients [84,85,86,88,89,90,91]. In this study, ammonium (R2 = 0.646, p = 0.010), nitrate (R2 = 0.642, p = 0.006), and temperature (R2 = 0.411, p = 0.002) were strongly associated with P. yezoensis and seawater-borne bacterial assemblages (Figure 7A). Many studies have reported the role of temperature in determining microbial populations and their functional activities by effecting metabolic niches [92,93,94,95]. Similarly, Li et al. stated that regional environmental factors (e.g., pH and temperature) influence the patterns of microbial community in Carassius auratus culture ponds [96]. In this study, ammonium and nitrate showed a significant (p < 0.05) correlation with the identified microbial communities. Shilova et al. reported that the addition of NH4 and NO3 caused a strong shift in the abundance and compositions of microbial communities [97].
4.3. Functional Profiles Of Bacterial Communities
The evolving consensus is that the structures of bacterial communities on macroalgae are primarily determined by functional genes rather than taxonomic or phylogenetic compositions, but also in conjunction with the microenvironment provided by the physiological and biochemical properties of the algal host [61,83,98]; however, the role of functional genes associated with the bacterial community assembly on seaweed surfaces lacks adequate information. In an attempt to gain insight in this regard, we used Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) to analyze and predict the functional capabilities of bacterial communities inhabiting P. yezoensis in thalli and seawater samples. The proteins of seaweed contain huge amounts of essential amino acids such as arginine, alanine, glycine, proline, aspartic, and glutamic acids [99]. This describes the greater abundance of bacterial genes associated with amino acid metabolism in this study (Figure S3B). The presence of genes related with aspartate, alanine, and glutamate metabolism elucidated the bacterial reliance on algae producing amino acids as an adaptive mechanism of bacteria in Pyropia. Furthermore, multiple biosynthetic genes were also identified, including the genes for biosynthesis of amino acids and genes for the production of terpene. Approximately 50,000 terpenoid metabolites have been identified in fungi and terrestrial and marine plants; however, only a few of these broadly occurring metabolites have been isolated from prokaryotes [100]. The synthesis of terpene in Dinoroseobacter and α-Proteobacteria Loktanella has been reported by Schulz and Dickscha [101]. Furthermore, Wei et al. recently stated that the production of terpene in red alga is accomplished through MTPSLs (microbial terpene synthase-like genes), which are found to be phylogenetically distinct from typical plant terpene synthases and their emergence in Rhodophyta can presumably be defined by horizontal gene transfer from bacteria [82].
Quorum sensing (QS) is a common mechanism for communication and works through chemical signaling and has gained and exclusive consideration by marine ecologists [102]. It is understood that quorum sensing induces downstream changes in gene regulation and alters biological functions such as biofilm formation, sporulation, bacterial conjugation, and bioluminescence [103,104,105]. In this study, we have identified genes related to the peptide/nickel transport system (K02035) that are involved in the quorum sensing pathway (K02024). This might be an interesting factor to understand interactions in bacterial communities. Moreover, several studies have reported quorum sensing mechanisms in different marine bacteria [106,107,108]. Meanwhile, side by side to this, we have identified a large number of genes for signaling pathways such as K00936, K02035, and K02040 (Figure 6B). According to previous reports it is thought that component systems are the only recognized channels for environmental sensing in bacteria. Nevertheless, thanks to recent technological advances in genome sequencing, genetic approaches have discovered the presence of eukaryote-like serine/threonine protein kinases (STPKs) and phosphatases in different prokaryotes, which includes many pathogenic bacteria such as Yersinia spp. [109,110], Listeria monocytogenes [111,112], Mycobacterium [113,114], Pseudomonas aeruginosa [115], Enterococcus faecalis [116], and Staphylococcus aureus [117,118,119]. The sensor/signaling proteins of the serine/threonine protein kinase (STPK) family of virulence determining pathogenic bacteria perform the dual role of intuiting the environment and destabilizing the specific host defense system. STPKs have the capability to sense a wide array of signals and coordinate multiple cellular processes in order to generate an appropriate response [120]. A small number of transport genes and vitamin B12 genes were also detected the presence indicate that associated microbiota produces regulatory compounds and transport them to algal host it is stated previously in many studies that algae acquire vitamin B12 through a symbiotic relationship with bacteria [121,122,123].
In our study, the association of complex bacterial communities with algae and seawater is evident. The presence of variable functional profile, community structure and composition can have positive and negative effects on their interaction with macroalgae. Complex interactions have been justified by Goecke et al. [11,124] and Florez et al. [125], whereas the induction of morphological development [126], antimicrobial activity [127,128], and negative effects, for example, of pathogenic bacteria [129], and the presence of bacteria responsible for the degradation of macroalgae, also include in these interactions [130].
5. Conclusions
In summary, our study provides evidence for complex and diverse associations of microbial communities with P. yezoensis and surrounding seawater, displaying an exceptional metabolic, transport, and biosynthetic functions that are essential for favorable interactions and adaptability to the ecological environment. Meanwhile, the microbiota may play a vital role in secondary metabolite production and nutrient cycling, which are substantially influenced by algae metabolites and the physicochemical characteristics of seawater, which triggers the initiation of chemical signaling and transport pathway responses with requisite genes to metabolize subsequent substrates. In return, bacteria produce regulatory compounds and secondary chemical metabolites for algal growth, morphogenesis, and defense. To improve the understanding of bacterial diversity and mutualistic interactions under natural environmental conditions, research using broader sample data based on deep metagenomic sequencing and meta transcriptomes of microbial communities is required. Future research should emphasize the chemical signaling environment, genetic exchange, and metabolic interactions between P. yezoensis and its harboring microbiota to understand the effects of the functional profile of the host and symbiont in shaping the microbial diversity of the niche.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9061291/s1, Figure S1: Petal diagrams showing the total number of OTUs and the number of shared OTUs across all P. yezoensis (A) and seawater (B) datasets. Figure S2: Refraction curves of observed species, Shannon, chao1 and PD whole-tree. Figure S3: KEGG pathway annotation (A) KEGG level 1 functional annotations (B) KEGG level 2 functional annotations. Figure S4: t-test (A) KEGG secondary level YTH-RTH (B) KEGG level 3 YTH-RTH. Figure S5: T-test (A) YTH-YSW KO hierarchy level (B) YTH-RTH KO hierarchy level. Figure S6: t-test of RTH-RSW KO level. Table S1: Average values of alpha diversity indices. Table S2: Significance differences in Pyropia yezoensis and seawater associated bacterial community structures, based on Bray–Curtis dissimilarities. Table S3: Average relative abundance of top 30 OTUs in Pyropia yezoensis and seawater sample groups. Table S4: SIMPER analysis (A) Dissimilarity contribution of bacterial OTUs within Pyropia yezoensis datasets. (B) Dissimilarity contribution of bacterial OTUs within seawater datasets.
Author Contributions
Data curation, A.A.; formal analysis, A.A., J.W., A.K. and T.U.K.; funding acquisition, Y.M.; investigation, A.A., J.W. and X.T.; supervision, Y.M.; writing—original draft, A.A. and A.K.; writing—review and editing, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank for the grants supporting from the Fundamental Research Funds for the Central Universities (202064006), the National Natural Science Foundation of China (Grant No.32060829), the National Key R&D Program of China (2018YFD0900106, 2020YFD0901101), the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (No. 2018SDKJ0302-4), the MOA Modern Agricultural Talents Support Project, and Special Project of Central Government Guiding Local Science and Technology Development (7033204020).
Data Availability Statement
Data will be provided upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tseng C.K. Marine Phycoculture in China. In: Levrig T., editor. International Seaweed Symposium. 10th ed. De Gruyter; Berlin, Germany: Boston, MA, USA: 1981. pp. 123–152. [DOI] [Google Scholar]
- 2.Wells M.L., Potin P., Craigie J.S., Raven J.A., Merchant S.S., Helliwell K.E., Smith A.G., Camire M.E., Brawley S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017;29:949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoyneva-Gärtner M.P., Uzunov B.A. An Ethnobiological Glance on Globalization Impact on the Traditional Use of Algae and Fungi as Food in Bulgaria. J. Nutr. Food Sci. 2015;5:1. doi: 10.4172/2155-9600.1000413. [DOI] [Google Scholar]
- 4.Pereira L. A Review of the Nutrient Composition of Selected Edible Seaweeds. In: Pomin V.H., editor. Ecology, Nutrient Composition and Medicinal Uses. 1st ed. Nova Science Pub.; Coimbra, Portugal: 2011. pp. 15–47. [Google Scholar]
- 5.FAO . FAO Fisheries and Aquaculture Department. FAO; Rome, Italy: 2019. [(accessed on 18 September 2019)]. Fishery and Aquaculture Statistics. Global Aquaculture Production. Available online: http://www.fao.org/fishery/ [Google Scholar]
- 6.Levine I.A., Sahoo D. Porphyra: Harvesting Gold from the Sea. I.K. International Pub. House; New Delhi, India: 2010. [Google Scholar]
- 7.Campbell A.H., Marzinelli E.M., Gelber J., Steinberg P.D. Spatial Variability of Microbial Assemblages Associated with a Dominant Habitat-Forming Seaweed. Front. Microbiol. 2015;6:230. doi: 10.3389/fmicb.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan S., Thomas T. Editorial for: Microbial symbiosis of marine sessile hosts- diversity and function. Front. Microbiol. 2015;6:585. doi: 10.3389/fmicb.2015.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook K., Vanderklift M.A., Poore A.G.B. Strong Effects of Herbivorous Amphipods on Epiphyte Biomass in a Temperate Seagrass Meadow. Mar. Ecol. Prog. Ser. 2011;442:263–269. doi: 10.3354/meps09446. [DOI] [Google Scholar]
- 10.Bulleri F., Chapman M.G. The Introduction of Coastal Infrastructure as a Driver of Change in Marine Environments. J. Appl. Ecol. 2010;47:26–35. doi: 10.1111/j.1365-2664.2009.01751.x. [DOI] [Google Scholar]
- 11.Goecke F., Labes A., Wiese J., Imhoff J.F. Chemical Interactions between Marine Macroalgae and Bacteria. Mar. Ecol. Prog. Ser. 2010;409:267–299. doi: 10.3354/meps08607. [DOI] [Google Scholar]
- 12.Provasoli L., Pintner I.J. Bacteria Induced Polymorphism in an Axenic Laboratory Strain of Ulva Lactuca (Chlorophyceae) 1. J. Phycol. 1980;16:196–201. doi: 10.1111/j.1529-8817.1980.tb03019.x. [DOI] [Google Scholar]
- 13.Pedersen M. The Demand for Iodine and Bromine of Three Marine Brown Algae Grown in Bacteria-Free Cultures. Physiol. Plant. 1969;22:680–685. doi: 10.1111/j.1399-3054.1969.tb07423.x. [DOI] [Google Scholar]
- 14.Kingman A.R., Moore J. Isolation, Purification and Quantitation of Several Growth Regulating Substances in Ascophyllum Nodosum (Phaeophyta) BOT MAR. 1982;25:149–154. doi: 10.1515/botm.1982.25.4.149. [DOI] [Google Scholar]
- 15.Saga N. A New Method for Pure Culture of Macroscopic Algae, the One Step Selection Method. Jpn. J. Phycol. 1982;30:40–45. [Google Scholar]
- 16.Matsuo Y., Imagawa H., Nishizawa M., Shizuri Y. Isolation of an Algal Morphogenesis Inducer from a Marine Bacterium. Science. 2005;307:1598. doi: 10.1126/science.1105486. [DOI] [PubMed] [Google Scholar]
- 17.Ghaderiardakani F., Coates J.C., Wichard T. Bacteria-Induced Morphogenesis of Ulva Intestinalis and Ulva Mutabilis (Chlorophyta): A Contribution to the Lottery Theory. FEMS Microbiol. Ecol. 2017;93:fix094. doi: 10.1093/femsec/fix094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss A., Costa R., Wichard T. Morphogenesis of Ulva Mutabilis (Chlorophyta) Induced by Maribacter Species (Bacteroidetes, Flavobacteriaceae) Bot Mar. 2017;60:197–206. doi: 10.1515/bot-2016-0083. [DOI] [Google Scholar]
- 19.Fries L. Polysiphonia Urceolata in Axenic Culture. Nature. 1964;202:110. doi: 10.1038/202110a0. [DOI] [Google Scholar]
- 20.Aydlett M. Honors Thesis. University of Maine; Orono, Maine: 2019. [(accessed on 4 September 2019)]. Examining the Microbiome of Porphyra Umbilicalis in the North Atlantic. Available online: https://digitalcommons.library.umaine.edu/honors/570. [Google Scholar]
- 21.Yan Y.-W., Yang H.-C., Tang L., Li J., Mao Y.-X., Mo Z.-L. Compositional Shifts of Bacterial Communities Associated with Pyropia Yezoensis and Surrounding Seawater Co-Occurring with Red Rot Disease. Front. Microbiol. 2019;10:1666. doi: 10.3389/fmicb.2019.01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q., Zhi Y., He Y., Ren Z., Chen H., Yang R. Changes in Phycospheric and Environmental Microbes Associated with an Outbreak of Yellow Spot Disease on Pyropia Yezoensis. Aquaculture. 2020;529:735651. doi: 10.1016/j.aquaculture.2020.735651. [DOI] [Google Scholar]
- 23.Wang J., Mao Y., Du G., Li X., Tang X. On Microbial Community of Pyropia Haitanensis by Metagenomic Analysis. J. Oceanol. Limnol. 2020:1–12. doi: 10.1007/s00343-020-0189-0. [DOI] [Google Scholar]
- 24.Zhang R., Chang L., Xiao L., Zhang X., Han Q., Li N., Egan S., Wang G. Diversity of the Epiphytic Bacterial Communities Associated with Commercially Cultivated Healthy and Diseased Saccharina Japonica during the Harvest Season. J. Appl. Phycol. 2020;32:2071–2080. doi: 10.1007/s10811-019-02025-y. [DOI] [Google Scholar]
- 25.Gamolo L., Besagas R.L. Microbiological Profile of Cultured Seaweeds Kappaphycus Alvarezii. Int. J. Biosci. 2018;13:140–145. doi: 10.12692/ijb/13.6.140-145. [DOI] [Google Scholar]
- 26.Khan S., Mao Y., Zou D., Riaz S., Qiu L., Li N. Molecular Analysis of Microbiota Composition and Alterations in Pyropia Yezoensis Infected with Red Rot Disease. Indian J. Geo-Mar. Sci. 2018;47:558–566. [Google Scholar]
- 27.Osman M.-A., Neoh H.-M., Ab Mutalib N.-S., Chin S.-F., Jamal R. 16S RRNA Gene Sequencing for Deciphering the Colorectal Cancer Gut Microbiome: Current Protocols and Workflows. Front. Microbiol. 2018;9:767. doi: 10.3389/fmicb.2018.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang R., Fang W.Y., Shan Y.Y., Chen H.M., Sun X., Ye Y. Genetic Diversity of Epiphytic Bacteria in Porphyra Yezoensis. Acta Oceanol. Sin. 2008;30:161–168. [Google Scholar]
- 29.Shen M., Yang R., Luo Q., Wang S., Ren J. Microbial diversity of Pyropia haitanensis phycosphere during cultivation. Wei Sheng Wu Xue Bao. 2013;53:1087–1102. [PubMed] [Google Scholar]
- 30.Eaton A.D., Clesceri L.S., Rice E.W., Greenberg A.E., Franson M.H.A. STANDARD Methods for the Examination of Water and Wastewater. American Public Health Association; Washington, DC, USA: 2005. [Google Scholar]
- 31.Magoč T., Salzberg S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-Filtering Vastly Improves Diversity Estimates from Illumina Amplicon Sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas B.J., Gevers D., Earl A.M., Feldgarden M., Ward D.V., Giannoukos G., Ciulla D., Tabbaa D., Highlander S.K., Sodergren E., et al. Chimeric 16S RRNA Sequence Formation and Detection in Sanger and 454-Pyrosequenced PCR Amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 37.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langille M.G.I., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Thurber R.L., Knight R., et al. Predictive Functional Profiling of Microbial Communities Using 16S RRNA Marker Gene Sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Algina J., Keselman H.J. Comparing Squared Multiple Correlation Coefficients: Examination of a Confidence Interval and a Test Significance. Psychol. Methods. 1999;4:76–83. doi: 10.1037/1082-989X.4.1.76. [DOI] [Google Scholar]
- 41.Hartman W.H., Ye R., Horwath W.R., Tringe S.G. A Genomic Perspective on Stoichiometric Regulation of Soil Carbon Cycling. ISME J. 2017;11:2652–2665. doi: 10.1038/ismej.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro H., de Sousa T., Santos J.P., Sousa A.G.G., Teixeira C., Monteiro M.R., Salgado P., Mucha A.P., Almeida C.M.R., Torgo L., et al. Potential of Dissimilatory Nitrate Reduction Pathways in Polycyclic Aromatic Hydrocarbon Degradation. Chemosphere. 2018;199:54–67. doi: 10.1016/j.chemosphere.2018.01.171. [DOI] [PubMed] [Google Scholar]
- 43.LeBrun E.S., Kang S. A Comparison of Computationally Predicted Functional Metagenomes and Microarray Analysis for Microbial P Cycle Genes in a Unique Basalt-Soil Forest. F1000Research. 2018;7:179. doi: 10.12688/f1000research.13841.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z.-J., Shao Y., Li Y.-J., Lin L.-A., Chen Y., Tian W., Li B.-L., Li Y.-Y. Rhizosphere Bacterial Community Structure and Predicted Functional Analysis in the Water-Level Fluctuation Zone of the Danjiangkou Reservoir in China during the Dry Period. Int. J. Environ. Res. Public Health. 2020;17:1266. doi: 10.3390/ijerph17041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulleri F., Benedetti-Cecchi L., Acunto S., Cinelli F., Hawkins S.J. The Influence of Canopy Algae on Vertical Patterns of Distribution of Low-Shore Assemblages on Rocky Coasts in the Northwest Mediterranean. J. Exp. Mar. Bio. Ecol. 2002;267:89–106. doi: 10.1016/S0022-0981(01)00361-6. [DOI] [Google Scholar]
- 46.Schiel D.R., Lilley S.A. Gradients of Disturbance to an Algal Canopy and the Modification of an Intertidal Community. Mar. Ecol. Prog. Ser. 2007;339:1–11. doi: 10.3354/meps339001. [DOI] [Google Scholar]
- 47.Alongi D.M. Coastal Ecosystem Processes. 1st ed. CRC Press; Boca Raton, FL, USA: 2019. pp. 1–10. [Google Scholar]
- 48.Lachnit T., Meske D., Wahl M., Harder T., Schmitz R. Epibacterial Community Patterns on Marine Macroalgae Are Host-Specific but Temporally Variable: Epiphytic Communities on Macroalgae. Environ. Microbiol. 2011;13:655–665. doi: 10.1111/j.1462-2920.2010.02371.x. [DOI] [PubMed] [Google Scholar]
- 49.Albakosh M.A., Naidoo R.K., Kirby B., Bauer R. Identification of Epiphytic Bacterial Communities Associated with the Brown Alga Splachnidium Rugosum. J. Appl. Phycol. 2016;28:1891–1901. doi: 10.1007/s10811-015-0725-z. [DOI] [Google Scholar]
- 50.Selvarajan R., Sibanda T., Venkatachalam S., Ogola H.J.O., Christopher Obieze C., Msagati T.A. Distribution, Interaction and Functional Profiles of Epiphytic Bacterial Communities from the Rocky Intertidal Seaweeds, South Africa. Sci. Rep. 2019;9:19835. doi: 10.1038/s41598-019-56269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakanishi K., Nishijima M., Nishimura M., Kuwano K., Saga N. Bacteria that induce morphogenesis in Ulva Pertusa (Chlorophyta) grown under axenic conditions. J. Phycol. 1996;32:479–482. doi: 10.1111/j.0022-3646.1996.00479.x. [DOI] [Google Scholar]
- 52.Lee Y.K., Lee J.H., Lee H.K. Microbial Symbiosis in Marine Sponges. J. Microbiol. 2001;39:254–264. [Google Scholar]
- 53.Lesser M.P., Mazel C.H., Gorbunov M.Y., Falkowski P.G. Discovery of Symbiotic Nitrogen-Fixing Cyanobacteria in Corals. Science. 2004;305:997–1000. doi: 10.1126/science.1099128. [DOI] [PubMed] [Google Scholar]
- 54.Marshall K., Joint I., Callow M.E., Callow J.A. Effect of Marine Bacterial Isolates on the Growth and Morphology of Axenic Plantlets of the Green Alga Ulva Linza. Microb. Ecol. 2006;52:302–310. doi: 10.1007/s00248-006-9060-x. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg E., Koren O., Reshef L., Efrony R., Zilber-Rosenberg I. The Role of Microorganisms in Coral Health, Disease and Evolution. Nat. Rev. Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- 56.Yakimov M.M., Cappello S., Crisafi E., Tursi A., Savini A., Corselli C., Scarfi S., Giuliano L. Phylogenetic Survey of Metabolically Active Microbial Communities Associated with the Deep-Sea Coral Lophelia Pertusa from the Apulian Plateau, Central Mediterranean Sea. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2006;53:62–75. doi: 10.1016/j.dsr.2005.07.005. [DOI] [Google Scholar]
- 57.Longford S.R., Tujula N.A., Crocetti G.R., Holmes A.J., Holmström C., Kjelleberg S., Steinberg P.D., Taylor M.W. Comparisons of Diversity of Bacterial Communities Associated with Three Sessile Marine Eukaryotes. Aquat. Microb. Ecol. 2007;48:217–229. doi: 10.3354/ame048217. [DOI] [Google Scholar]
- 58.Reis A.M.M., Araújo S.D., Jr., Moura R.L., Francini-Filho R.B., Pappas G., Jr., Coelho A.M.A., Krüger R.H., Thompson F.L. Bacterial Diversity Associated with the Brazilian Endemic Reef Coral Mussismilia Braziliensis. J. Appl. Microbiol. 2009;106:1378–1387. doi: 10.1111/j.1365-2672.2008.04106.x. [DOI] [PubMed] [Google Scholar]
- 59.Taylor M.W., Schupp P.J., Dahllöf I., Kjelleberg S., Steinberg P.D. Host Specificity in Marine Sponge-Associated Bacteria, and Potential Implications for Marine Microbial Diversity: Host Specificity and Diversity in Marine Bacteria. Environ. Microbiol. 2004;6:121–130. doi: 10.1046/j.1462-2920.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 60.Egan S., Thomas T., Kjelleberg S. Unlocking the Diversity and Biotechnological Potential of Marine Surface Associated Microbial Communities. Curr. Opin. Microbiol. 2008;11:219–225. doi: 10.1016/j.mib.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Burke C., Thomas T., Lewis M., Steinberg P., Kjelleberg S. Composition, Uniqueness and Variability of the Epiphytic Bacterial Community of the Green Alga Ulva Australis. ISME J. 2011;5:590–600. doi: 10.1038/ismej.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonthond G., Bayer T., Krueger-Hadfield S.A., Barboza F.R., Nakaoka M., Valero M., Wang G., Künzel S., Weinberger F. How Do Microbiota Associated with an Invasive Seaweed Vary across Scales? Mol. Ecol. 2020;29:2094–2108. doi: 10.1111/mec.15470. [DOI] [PubMed] [Google Scholar]
- 63.Singh R.P., Reddy C.R.K. Seaweed-Microbial Interactions: Key Functions of Seaweed-Associated Bacteria. FEMS Microbiol. Ecol. 2014;88:213–230. doi: 10.1111/1574-6941.12297. [DOI] [PubMed] [Google Scholar]
- 64.Oliveira L.S., Gregoracci G.B., Silva G.G.Z., Salgado L.T., Amado Filho G., Alves-Ferreira M., Thompson F.L. Transcriptomic Analysis of the Red Seaweed Laurencia Dendroidea (Florideophyceae, Rhodophyta) and Its Microbiome. BMC Genom. 2012;13:487. doi: 10.1186/1471-2164-13-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedrich M.W. Ecological Studies. Springer; Berlin/Heidelberg, Germany: 2012. Bacterial Communities on Macroalgae; pp. 189–201. [DOI] [Google Scholar]
- 66.Hollants J., Decleyre H., Leliaert F., De Clerck O., Willems A. Life without a Cell Membrane: Challenging the Specificity of Bacterial Endophytes within Bryopsis (Bryopsidales, Chlorophyta) BMC Microbiol. 2011;11:255. doi: 10.1186/1471-2180-11-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizzo L., Pusceddu A., Stabili L., Alifano P., Fraschetti S. Potential Effects of an Invasive Seaweed (Caulerpa Cylindracea, Sonder) on Sedimentary Organic Matter and Microbial Metabolic Activities. Sci. Rep. 2017;7:12113. doi: 10.1038/s41598-017-12556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvarado P., Huang Y., Wang J., Garrido I., Leiva S. Phylogeny and Bioactivity of Epiphytic Gram-Positive Bacteria Isolated from Three Co-Occurring Antarctic Macroalgae. Antonie Van Leeuwenhoek. 2018;111:1543–1555. doi: 10.1007/s10482-018-1044-6. [DOI] [PubMed] [Google Scholar]
- 69.Venter J.C., Remington K., Heidelberg J.F., Halpern A.L., Rusch D., Eisen J.A., Wu D., Paulsen I., Nelson K.E., Nelson W., et al. Environmental Genome Shotgun Sequencing of the Sargasso Sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 70.Rusch D.B., Halpern A.L., Sutton G., Heidelberg K.B., Williamson S., Yooseph S., Wu D., Eisen J.A., Hoffman J.M., Remington K., et al. The Sorcerer II Global Ocean Sampling Expedition: Northwest Atlantic through Eastern Tropical Pacific. PLoS Biol. 2007;5:e77. doi: 10.1371/journal.pbio.0050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giovannoni S.J., Stingl U. Molecular Diversity and Ecology of Microbial Plankton. Nature. 2005;437:343–348. doi: 10.1038/nature04158. [DOI] [PubMed] [Google Scholar]
- 72.Nowlan J.P., Lumsden J.S., Russell S. Advancements in Characterizing Tenacibaculum Infections in Canada. Pathogens. 2020;9:1029. doi: 10.3390/pathogens9121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teramoto M., Zhai Z., Komatsu A., Shibayama K., Suzuki M. Genome Sequence of the Psychrophilic Bacterium Tenacibaculum Ovolyticum Strain Da5A-8 Isolated from Deep Seawater. Genome Announc. 2016;4:e00644-16. doi: 10.1128/genomeA.00644-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Z.-X., Yu P., Mu D.-S., Liu Y., Du Z.-J. Tenacibaculum Agarivorans Sp. Nov., an Agar-Degrading Bacterium Isolated from Marine Alga Porphyra Yezoensis Ueda. Int. J. Syst. Evol. Microbiol. 2017;67:5139–5143. doi: 10.1099/ijsem.0.002432. [DOI] [PubMed] [Google Scholar]
- 75.Petersen J., Colon C., Joyner J. Sediment Microbiomes Associated with Critical Habitat of the Juvenile American Horseshoe Crab; Limulus Polyphemus. Glob. J. Environ. Sci. Manag. 2020;6:309–322. doi: 10.22034/GJESM.2020.03.03. [DOI] [Google Scholar]
- 76.Suzuki M., Nakagawa Y., Harayama S., Yamamoto S. Phylogenetic Analysis and Taxonomic Study of Marine Cytophaga-like Bacteria: Proposal for Tenacibaculum Gen. Nov. with Tenacibaculum Maritimum Comb. Nov. and Tenacibaculum Ovolyticum Comb. Nov., and Description of Tenacibaculum Mesophilum Sp. Nov. and Tenacibaculum Amylolyticum Sp. Nov. Pt 5Int. J. Syst. Evol. Microbiol. 2001;51:1639–1652. doi: 10.1099/00207713-51-5-1639. [DOI] [PubMed] [Google Scholar]
- 77.Habib C., Houel A., Lunazzi A., Bernardet J.-F., Olsen A.B., Nilsen H., Toranzo A.E., Castro N., Nicolas P., Duchaud E. Multilocus Sequence Analysis of the Marine Bacterial Genus Tenacibaculum Suggests Parallel Evolution of Fish Pathogenicity and Endemic Colonization of Aquaculture Systems. Appl. Environ. Microbiol. 2014;80:5503–5514. doi: 10.1128/AEM.01177-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quéméneur M., Bel Hassen M., Armougom F., Khammeri Y., Lajnef R., Bellaaj-Zouari A. Prokaryotic Diversity and Distribution along Physical and Nutrient Gradients in the Tunisian Coastal Waters (South Mediterranean Sea) Front. Microbiol. 2020;11:593540. doi: 10.3389/fmicb.2020.593540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anandan R., Dharumadurai D., Manogaran G.P. Actinobacteria-Basics and Biotechnological Applications. InTech; London, UK: 2018. An Introduction to Actinobacteria; pp. 3–37. [DOI] [Google Scholar]
- 80.Shah V., Chang B.X., Morris R.M. Cultivation of a Chemoautotroph from the SUP05 Clade of Marine Bacteria That Produces Nitrite and Consumes Ammonium. ISME J. 2017;11:263–271. doi: 10.1038/ismej.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nimnoi P., Pongsilp N. Marine Bacterial Communities in the Upper Gulf of Thailand Assessed by Illumina Next-Generation Sequencing Platform. BMC Microbiol. 2020;20:19. doi: 10.1186/s12866-020-1701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei G., Jia Q., Chen X., Köllner T.G., Bhattacharya D., Wong G.K.-S., Gershenzon J., Chen F. Terpene Biosynthesis in Red Algae Is Catalyzed by Microbial Type but Not Typical Plant Terpene Synthases. Plant Physiol. 2019;179:382–390. doi: 10.1104/pp.18.01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Egan S., Harder T., Burke C., Steinberg P., Kjelleberg S., Thomas T. The Seaweed Holobiont: Understanding Seaweed-Bacteria Interactions. FEMS Microbiol. Rev. 2013;37:462–476. doi: 10.1111/1574-6976.12011. [DOI] [PubMed] [Google Scholar]
- 84.Vandendriessche S., Vincx M., Degraer S. Floating Seaweed and the Influences of Temperature, Grazing and Clump Size on Raft Longevity—a Microcosm Study. J. Exp. Mar. Biol. Ecol. 2007;343:64–73. doi: 10.1016/j.jembe.2006.11.010. [DOI] [Google Scholar]
- 85.Macaya E.C., Boltana S., Hinojosa I.A., Macchiavello J.E., Valdivia N.A., Vasquez N.R., Buschmann A.H., Vasquez J.A., Alonso J.M., Thiel M. Presence of Sporophylls in floating kelp rafts of Macrocystis spp. (Phaeophyceae) along the Chillean pacific coast. J. Phycol. 2005;41:913–922. doi: 10.1111/j.1529-8817.2005.00118.x. [DOI] [Google Scholar]
- 86.Vandendriessche S., De Keersmaecker G., Vincx M., Degraer S. Food and Habitat Choice in Floating Seaweed Clumps: The Obligate Opportunistic Nature of the Associated Macrofauna. Mar. Biol. 2006;149:1499–1507. doi: 10.1007/s00227-006-0292-6. [DOI] [Google Scholar]
- 87.Juneja A., Ceballos R., Murthy G. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies. 2013;6:4607–4638. doi: 10.3390/en6094607. [DOI] [Google Scholar]
- 88.Abdel-Gawad K.M., Hifney A.F., Issa A.A., Gomaa M. Spatio-Temporal, Environmental Factors, and Host Identity Shape Culturable-Epibiotic Fungi of Seaweeds in the Red Sea. Egypt. Hydrobiol. 2014;740:37–49. doi: 10.1007/s10750-014-1935-0. [DOI] [Google Scholar]
- 89.Jokiel P.L. Solar Ultraviolet Radiation and Coral Reef Epifauna. Science. 1980;207:1069–1071. doi: 10.1126/science.207.4435.1069. [DOI] [PubMed] [Google Scholar]
- 90.Rybak A.S., Gąbka M. The Influence of Abiotic Factors on the Bloom-Forming Alga Ulva Flexuosa (Ulvaceae, Chlorophyta): Possibilities for the Control of the Green Tides in Freshwater Ecosystems. J. Appl. Phycol. 2018;30:1405–1416. doi: 10.1007/s10811-017-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lemay M.A., Martone P.T., Keeling P.J., Burt J.M., Krumhansl K.A., Sanders R.D., Wegener Parfrey L. Sympatric Kelp Species Share a Large Portion of Their Surface Bacterial Communities. Environ. Microbiol. 2018;20:658–670. doi: 10.1111/1462-2920.13993. [DOI] [PubMed] [Google Scholar]
- 92.Vonshak A., Torzillo G. Environmental Stress Physiology. In: Richmond A., editor. Handbook of Microalgae Culture. Biotechnology and Applied Phycology. Blackwell Science Ltd.; Hoboken, NJ, USA: 2004. pp. 57–82. [DOI] [Google Scholar]
- 93.Joint I., Smale D.A. Marine Heatwaves and Optimal Temperatures for Microbial Assemblage Activity. FEMS Microbiol. Ecol. 2017;93 doi: 10.1093/femsec/fiw243. [DOI] [PubMed] [Google Scholar]
- 94.Dang H., Lovell C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2016;80:91–138. doi: 10.1128/MMBR.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Gestel N.C., Ducklow H.W., Bååth E. Comparing Temperature Sensitivity of Bacterial Growth in Antarctic Marine Water and Soil. Glob. Chang. Biol. 2020;26:2280–2291. doi: 10.1111/gcb.15020. [DOI] [PubMed] [Google Scholar]
- 96.Li T., Li H., Gatesoupe F.-J., She R., Lin Q., Yan X., Li J., Li X. Bacterial Signatures of “Red-Operculum” Disease in the Gut of Crucian Carp (Carassius Auratus) J. Phycol. 2017;74:510–521. doi: 10.1007/s00248-017-0967-1. [DOI] [PubMed] [Google Scholar]
- 97.Shilova I.N., Mills M.M., Robidart J.C., Turk-Kubo K.A., Björkman K.M., Kolber Z., Rapp I., van Dijken G.L., Church M.J., Arrigo K.R., et al. Differential Effects of Nitrate, Ammonium, and Urea as N Sources for Microbial Communities in the North Pacific Ocean: N Effects on Microbial Communities. Limnol. Oceanogr. 2017;62:2550–2574. doi: 10.1002/lno.10590. [DOI] [Google Scholar]
- 98.Cirri E., Pohnert G. Algae− Bacteria Interactions That Balance the Planktonic Microbiome. New Phytol. 2019;223:100–106. doi: 10.1111/nph.15765. [DOI] [PubMed] [Google Scholar]
- 99.Černá M. Seaweed Proteins and Amino Acids as Nutraceuticals. Adv. Food Nutr. Res. 2011;64:297–312. doi: 10.1016/B978-0-12-387669-0.00024-7. [DOI] [PubMed] [Google Scholar]
- 100.Yamada Y., Kuzuyama T., Komatsu M., Shin-Ya K., Omura S., Cane D.E., Ikeda H. Terpene Synthases Are Widely Distributed in Bacteria. Proc. Natl. Acad. Sci. USA. 2015;112:857–862. doi: 10.1073/pnas.1422108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulz S., Dickschat J.S. Bacterial Volatiles: The Smell of Small Organisms. Nat. Prod. Rep. 2007;24:814–842. doi: 10.1039/b507392h. [DOI] [PubMed] [Google Scholar]
- 102.Bassler B.L. Small Talk: Cell-to-Cell Communication in Bacteria. Cell. 2002;109:421–424. doi: 10.1016/S0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 103.Waters C.M., Bassler B.L. Quorum Sensing: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 104.Mangwani N., Dash H.R., Chauhan A., Das S. Bacterial Quorum Sensing: Functional Features and Potential Applications in Biotechnology. J. Mol. Microbiol. Biotechnol. 2012;22:215–227. doi: 10.1159/000341847. [DOI] [PubMed] [Google Scholar]
- 105.Zhou J., Lyu Y., Richlen M., Anderson D.M., Cai Z. Quorum Sensing Is a Language of Chemical Signals and Plays an Ecological Role in Algal-Bacterial Interactions. CRC Crit. Rev. Plant. Sci. 2016;35:81–105. doi: 10.1080/07352689.2016.1172461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Decho A.W., Frey R.L., Ferry J.L. Chemical Challenges to Bacterial AHL Signaling in the Environment. Chem. Rev. 2011;111:86–99. doi: 10.1021/cr100311q. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L.-H., Dong Y.-H. Quorum Sensing and Signal Interference: Diverse Implications: Signal Interference. Mol. Microbiol. 2004;53:1563–1571. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 108.Taylor M.W., Radax R., Steger D., Wagner M. Sponge-Associated Microorganisms: Evolution, Ecology, and Biotechnological Potential. Microbiol. Mol. Biol. Rev. 2007;71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Håkansson S., Galyov E.E., Rosqvist R., Wolf-Watz H. The Yersinia YpkA Ser./Thr Kinase Is Translocated and Subsequently Targeted to the Inner Surface of the HeLa Cell Plasma Membrane. Mol. Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 110.Galyov E.E., Håkansson S., Forsberg A., Wolf-Watz H. A Secreted Protein Kinase of Yersinia Pseudotuberculosis Is an Indispensable Virulence Determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 111.Archambaud C., Gouin E., Pizarro-Cerda J., Cossart P., Dussurget O. Translation Elongation Factor EF-Tu is a Target for Stp, a Serine-Threonine Phosphatase Involved in Virulence of Listeria Monocytogenes: EF-Tu, a Target for the Listeria Phosphatase Stp. Mol. Microbiol. 2005;56:383–396. doi: 10.1111/j.1365-2958.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- 112.Lima A., Durán R., Schujman G.E., Marchissio M.J., Portela M.M., Obal G., Cerveñansky C. Serine/Threonine Protein Kinase PrkA of the Human Pathogen Listeria Monocytogenes: Biochemical Characterization and Identification of Interacting Partners through Proteomic Approaches. J. Proteom. 2011;74:1720–1734. doi: 10.1016/j.jprot.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Alber T. Signaling Mechanisms of the Mycobacterium Tuberculosis Receptor Ser./Thr Protein Kinases. Curr. Opin. Struct. Biol. 2009;19:650–657. doi: 10.1016/j.sbi.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Molle V., Kremer L. Division and Cell Envelope Regulation by Ser/Thr Phosphorylation: Mycobacterium Shows the Way. Mol. Microbiol. 2010;75:1064–1077. doi: 10.1111/j.1365-2958.2009.07041.x. [DOI] [PubMed] [Google Scholar]
- 115.Wang J., Li C., Yang H., Mushegian A., Jin S. A Novel Serine/Threonine Protein Kinase Homologue of Pseudomonas Aeruginosa Is Specifically Inducible within the Host Infection Site and Is Required for Full Virulence in Neutropenic Mice. J. Bacteriol. 1998;180:6764–6768. doi: 10.1128/JB.180.24.6764-6768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kristich C.J., Wells C.L., Dunny G.M. A Eukaryotic-Type Ser/Thr Kinase in Enterococcus Faecalis Mediates Antimicrobial Resistance and Intestinal Persistence. Proc. Natl. Acad. Sci. USA. 2007;104:3508–3513. doi: 10.1073/pnas.0608742104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beltramini A.M., Mukhopadhyay C.D., Pancholi V. Modulation of Cell Wall Structure and Antimicrobial Susceptibility by a Staphylococcus Aureus Eukaryote-like Serine/Threonine Kinase and Phosphatase. Infect. Immun. 2009;77:1406–1416. doi: 10.1128/IAI.01499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Truong-Bolduc Q.C., Ding Y., Hooper D.C. Posttranslational Modification Influences the Effects of MgrA on NorA Expression in Staphylococcus Aureus. J. Bacteriol. 2008;190:7375–7381. doi: 10.1128/JB.01068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Truong-Bolduc Q.C., Hooper D.C. Phosphorylation of MgrA and Its Effect on Expression of the NorA and NorB Efflux Pumps of Staphylococcus Aureus. J. Bacteriol. 2010;192:2525–2534. doi: 10.1128/JB.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Canova M.J., Molle V. Bacterial Serine/Threonine Protein Kinases in Host-Pathogen Interactions. J. Biol. Chem. 2014;289:9473–9479. doi: 10.1074/jbc.R113.529917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Croft M.T., Lawrence A.D., Raux-Deery E., Warren M.J., Smith A.G. Algae Acquire Vitamin B 12 through a Symbiotic Relationship with Bacteria. Nature. 2005;438:90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- 122.Kazamia E., Czesnick H., Nguyen T.T.V., Croft M.T., Sherwood E., Sasso S., Smith A.G. Mutualistic Interactions between Vitamin B12-dependent Algae and Heterotrophic Bacteria Exhibit Regulation. Environ. Microbiol. 2012;14:1466–1476. doi: 10.1111/j.1462-2920.2012.02733.x. [DOI] [PubMed] [Google Scholar]
- 123.Danchin A., Braham S. Coenzyme B12 Synthesis as a Baseline to Study Metabolite Contribution of Animal Microbiota. Microb. Biotechnol. 2017;10:688–701. doi: 10.1111/1751-7915.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goecke F., Thiel V., Wiese J., Labes A., Imhoff J.F. Algae as an Important Environment for Bacteria–Phylogenetic Relationships among New Bacterial Species Isolated from Algae. Phycologia. 2013;52:14–24. doi: 10.2216/12-24.1. [DOI] [Google Scholar]
- 125.Florez J.Z., Camus C., Hengst M.B., Buschmann A.H. A Functional Perspective Analysis of Macroalgae and Epiphytic Bacterial Community Interaction. Front. Microbiol. 2017;8:2561. doi: 10.3389/fmicb.2017.02561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grueneberg J., Engelen A.H., Costa R., Wichard T. Macroalgal Morphogenesis Induced by Waterborne Compounds and Bacteria in Coastal Seawater. PLoS ONE. 2016;11:e0146307. doi: 10.1371/journal.pone.0146307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rao D., Webb J.S., Holmström C., Case R., Low A., Steinberg P., Kjelleberg S. Low Densities of Epiphytic Bacteria from the Marine Alga Ulva Australis Inhibit Settlement of Fouling Organisms. Appl. Environ. Microbiol. 2007;73:7844–7852. doi: 10.1128/AEM.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Z., Xiao T., Pang S., Liu M., Yue H. Isolation and Identification of Bacteria Associated with the Surfaces of Several Algal Species. Chin. J. Oceanol. Limnol. 2009;27:487–492. doi: 10.1007/s00343-009-9165-4. [DOI] [Google Scholar]
- 129.Zozaya-Valdes E., Egan S., Thomas T. A Comprehensive Analysis of the Microbial Communities of Healthy and Diseased Marine Macroalgae and the Detection of Known and Potential Bacterial Pathogens. Front. Microbiol. 2015;6:146. doi: 10.3389/fmicb.2015.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin M., Barbeyron T., Martin R., Portetelle D., Michel G., Vandenbol M. The Cultivable Surface Microbiota of the Brown Alga Ascophyllum Nodosum Is Enriched in Macroalgal-Polysaccharide-Degrading Bacteria. Front. Microbiol. 2015;6:1487. doi: 10.3389/fmicb.2015.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be provided upon request.