Abstract
Abnormalities of neuroinflammation have been implicated in the pathogenesis of depression and suicide. This is primarily based on the observation that cytokines, which are major inflammatory molecules and play an important role in depression and suicide, are increased in both serum and in postmortem brain of depressed and suicidal subjects. Another class of immune mediators are chemokines which are primarily involved in chemotactic properties and trafficking of immune cells in the central nervous system (CNS). Chemokines also play an important role in CNS function. Whereas chemokines have been studied in the serum of depressed and suicidal patients, their role in brain of depressed or suicidal subjects is relatively unexplored. We studied the gene expression of several chemokines in the prefrontal cortex (PFC) obtained from depressed suicidal (DS) and normal control (NC) subjects. We determined the mRNA expression of several chemokines belonging to CXCL and CCL groups of chemokines using qPCR array technique gene expression validation in 24 DS and 24 NC subjects. The postmortem brain samples were obtained from the Maryland Brain Collection. We found that the mRNA expression of chemokines CXCL1, CXCL2, CXCL3 and CCL2 was significantly decreased in the PFC of DS compared with NC subjects. No significant change was observed in CXCL5, CXCL6, CXCL10, CCL8 and CCL19 between DS and NC subjects. Since many of the chemokines are involved in mediating certain important CNS functions, such as neurotrophic effect, neurogenesis, anti-apoptotic growth factor release, modulation of synaptic transmission, brain development and neuronal loss, decreased levels of chemokines can reduce these functions which may be involved in the pathophysiology of depression.
Keywords: suicide, depression, chemokines, postmortem brain, prefrontal cortex (PFC), CCL2, CXCL1
1. Introduction
Abnormalities of the immune function have been implicated in the pathophysiology of depression and suicidal behavior. The major evidence linking the immune function with depression and suicidal behavior is obtained from cytokines studies (Zunszain et al., 2013). There is both direct and indirect evidence linking cytokines with depressive illness (Schiepers et al., 2005). Cytokines, generally known as chemical messengers between immune cells, are a heterogeneous group of messenger molecules produced by immunocompetent cells, such as lymphocytes and macrophages. Cytokines are major mediators of inflammation. The initial evidence suggesting the involvement of cytokines in depressive illness is also based on the observation that administration of cytokines to cancer patients induces depressive symptoms (Capuron et al., 2001). However, the major evidence supporting the involvement of cytokines in depression is obtained from the studies of plasma or serum levels of proinflammatory such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, soluble receptors for TNF-α and IL-1β in depressed patient compared with normal control subjects (Dowlati et al., 2010). The possibility that abnormalities of proinflammatory cytokines may also be present in the central nervous system (CNS) is strengthened by the observation that levels of proinflammatory cytokines IL-1β, IL-6 and TNF-α are increased in the cerebrospinal fluid (CSF) of depressed and suicidal patients (Lindqvist et al., 2009). Recent studies in the postmortem brain (Pandey et al., 2012; Pandey et al., 2018; Tonelli et al., 2008) indicated that levels of IL-1β, IL-6 and TNF-α are increased and the levels of anti-inflammatory cytokine IL-10 are decreased (Pandey et al., 2018) in the prefrontal cortex (PFC) of depressed suicide subjects.
Like cytokines, another group of immune molecules implicated in the pathophysiology of depression are chemokines (also known as chemotactic cytokines), important communication factors that recognize and destroy invading pathogens (Bajetto et al., 2001; Miller et al., 2008; Rollins, 1997). Chemokines are small polypeptides involved in the migration and trafficking of leukocytes. They play an important role in the immune system function under both physiologic and pathologic conditions (Sallusto and Baggiolini, 2008). Chemokines and their receptors are present in and produced by the cells of the CNS, including astrocytes, microglia and neurons (Ambrosini and Aloisi, 2004).
Chemokines family consists of more than 50 chemokines and 20 receptors (Rollins, 1997; Stuart et al., 2015) divided into four main subfamilies: CXC, CC, CX3C and XC. CC and CXC are the two largest groups. Several chemokine receptors have been identified and multiple chemokines may use the same receptors or single receptor can interact with several (Callewaere et al., 2007; Rollins, 1997; Stuart et al., 2015).
Chemokines exert their biological activity by interacting with their receptors and activating several signaling pathways. These include the phospholipase C (PLC) - phosphoinositide (PI) signaling pathway and other signaling pathways including mitogen-activated protein (MAP) kinase pathways (Bajetto et al., 2002). Because of their role in cellular migration and their presence in both the periphery and the CNS, chemokines are an important link between the peripheral and central immune function.
Besides their role in chemotaxis of immune cells, chemokines in the brain also perform many other functions relevant to depression, such as neurogenesis, synaptic plasticity, neuromodulation and proliferation of neural progenitor cells (Stuart and Baune, 2014). Chemokines also regulate neuroendocrine function (Rostene et al., 2011b; Semple et al., 2010). Thus, it appears that chemokines are responsible not only for the regulation of the inflammatory response, but they also control the interaction between the immune system and the CNS and are involved in many brain functions (Quan and Banks, 2007).
Several lines of evidence suggest the involvement of chemokines in depressive disorders. This is primarily based on the observation that several chemokines are altered (generally increased) in the plasma of depressed [see review by Leighton et al. (2018)] and suicidal patients [see meta-analysis by Black and Miller (2015)]. The administration of chemokines can also cause sickness behavior similar to that observed after cytokine administration to cancer patients (Stuart and Baune, 2014). Although there are no human studies of chemokine administration to cancer patients, several animal studies indicate that administration to animals causes sickness behavior. For example, the administration of endotoxin lipopolysaccharide (LPS) to animals causes the release of chemokines and produces symptoms of sickness behavior which may be similar to depression (Corona et al., 2010). When CXCL1 is administered to rats, it reduces sporadic explorative activities on the open field test and learning behavior in a dose-dependent manner (Stuart and Baune, 2014). In a mouse model deficient in fractalkine receptor (CX3CR1), LPS challenge resulted in prolonged duration of depressive-like sickness behavior measured by tail suspension test (Corona et al., 2013). Chemokine receptors have been shown to play a role in cognition, learning behavior, anxiety and other behaviors (Jaehne and Baune, 2014).
Although several studies suggest the presence and important function of chemokines in the CNS (Jaerve and Muller, 2012; Reaux-Le Goazigo et al., 2013), their role in the postmortem brain of depressed patients is not well studied. Several reports using gene array studies suggest abnormalities of some chemokines in the postmortem brain (Pantazatos et al., 2017; Torres-Platas et al., 2014). In general, chemokines have not been systematically studied in the postmortem brain of depressed and/or suicidal subjects. Since we observed abnormalities in the expression of both cytokines and Toll-like receptors (TLRs) in the PFC of depressed and suicidal subjects (Pandey et al., 2019; Pandey et al., 2012; Pandey et al., 2018), it was of interest to examine if other mediators of immunity, such as chemokines, are also dysregulated in the postmortem brain of depressed and suicidal patients. The main aim of this study was to examine the role of chemokines in the postmortem brain [PFC, Brodmann area 9 (BA9)] of depressed and suicide subjects. We therefore determined the mRNA expression of several chemokines in the depressed suicide and normal control subjects. In particular, we determined the expression of chemokines belonging to the CXC and CC families which have been previously studied in the serum of depressed and suicidal patients.
2. Materials and methods
2.1. Subjects and diagnoses
The study was performed on the PFC [Brodmann area 9 (BA9)] of 24 depressed suicide (DS) and 24 non-psychiatric control subjects referred to as normal control (NC) subjects. Brain tissues were obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, Maryland. Tissues were collected only after a family member gave informed consent. All tissue from NC and DS subjects was grossly examined by experienced neuropathologists. Toxicology data were obtained by the analysis of urine and blood samples. All procedures were approved by the University of Maryland Institutional Review Board (IRB) and University of Illinois IRB.
2.2. Criteria for the selection of control subjects
Inclusion criteria:
Male or female accident or murder victims of any ethnic or racial background with no history of psychiatric disorders or substance abuse and/or family history of psychiatric disorders.
Exclusion criteria:
Drivers in car accidents, death due to fire, gunshot wounds (GSW) to the head, major medical or neurological disorders, positive HIV, death after prolonged hospitalization, history of psychiatric disorders.
2.3. Diagnostic method
Subjects diagnoses were based on exhaustive psychological autopsies using the Structured Clinical Interview for DSM-IV (SCID I)(First et al., 1997), after interviews of family members, friends and the review of available medical records. At least one family member and/or a friend, after giving written informed consent, underwent an interview. Diagnoses were made by a consensus of two psychiatrists from the data obtained in the interview, medical records from the case, and records obtained from the Medical Examiner’s office. Normal control subjects were verified to lack mental illnesses using these consensus diagnostic procedures. All the information from psychological autopsies was retained by the Maryland Brain Collection and we do not have access to that information. We obtained only the final diagnoses from the Maryland Brain Collection.
2.4. Dissection of postmortem brain tissue
The brain samples were received from the Maryland Brain Collection already dissected in different areas. All frozen brains were dissected with a Stryker autopsy saw. The brain was hemisected and each side was stored whole in the freezer. To keep the samples frozen, the dissections were performed on a metal plate over a container filled with dry ice, and different brain areas were dissected very quickly with minimum defrosting. The PFC samples were cut out of the coronal sections by a fine microdissecting (Graefe) knife under a stereomicroscope with low magnification. The PFC (BA9) was taken just dorsal to the fronto-polar area, including the most polar portion of the superior and partly the middle frontal gyrus between the superior and intermediate frontal sulci.
2.5. Gene expression using real-time PCR (qPCR) array
The gene expression profiles of 84 genes involved in the Inflammatory Response and Autoimmunity signaling pathway were analyzed using RT2 Profiler PCR Array (PAHS-077A, SABiosciences, Valencia, CA, USA). 500ug of total RNA was reverse transcribed using RT2 First Strand Kit (SABiosciences, Valencia, CA, USA) according to manufactures instructions. Real-Time PCR was performed on the Mx3000P QPCR System (Stratagene, La Jolla CA) and used SYBR green detection with the following thermal profile: segment 1 – 1 cycle: 95°C for 10 minutes, segment 2 – 40 cycles: 95°C for 15 seconds followed by 60°C for 1 minute, segment 3 (dissociation curve) – 95°C for 1 minute, 55°C 30 seconds, and 95°C for 30 seconds. Results of the PCR array experiments were analyzed with SA Bioscience’s PCR Array Data Analysis online software to identify differentially expressed genes. The software calculated fold changes in mRNA using the formula: 2-ΔCt test sample/2-ΔCt control sample. For our analysis, we included GAPDH and ACTB as internal standards for normalization. The gene expression results showed that among the 20 CC chemokines and 8 CXC chemokines, 9 chemokines were down-regulated with >25% fold-change between suicide and control groups. To validate the qPCR array results, we analyzed those nine chemokines (i.e., CCL2, CCL8, CCL19, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL10) by qPCR using TaqMan probes.
2.6. Determination of mRNA levels
Total RNA from 100 mg of frozen tissue was extracted using TRIZOL reagent (Invitrogen, Thermo Fisher Scientific Inc.), followed by DNase treatment according to the manufacturer’s protocol. of the total RNA samples was measured with NanoDrop®ND-1000 (NanoDrop Technologies, LLC) and the purity was determined as 260/280 nm ratio with expected values between 1.8 and 2.0. The RNA quality was assessed using an Agilent Bioanalyzer 2100 (Agilent Technologies, Inc.) and all samples had RNA integrity number (RIN) above 7.0. The RNA samples were stored at −80°C until required for further analysis.
The expression levels of mRNA were determined using a 2-step real-time polymerase chain reaction (qPCR) method as follows: RT in duplicate (25°C, 10 minutes; 37°C, 60 minutes; 70°C, 15 minutes, then hold at 4°C); PCR in duplicate from each RT (95°C, 10 minute; then 95°C, 15 seconds followed by 60°C, 30 seconds and cycled 40 times). For RT, 1 μg of total RNA was reverse transcribed using 50 ng of random hexamers, 2 mM dNTP mix, 10 units of ribonuclease inhibitor and 200 units of MMLV-reverse transcriptase enzyme in a final reaction volume of 20 μl. Real-time PCR was performed using Pre-designed Taqman gene expression assays (Applied Biosystems, Thermo Fisher Scientific Inc.) for all target and housekeeping genes on a MX3005p sequence detection system (Agilent Technologies, Inc.). The TaqMan assay IDs of housekeeping and target genes are listed in Table 1.
Table 1.
TaqMan primers/probes used for real-time polymerase chain reaction (qPCR) analysis
| Primer/probe | TaqMan accession | Probe location (exon boundary) | Assay function |
|---|---|---|---|
| ACTB | Hs99999903_m1 | 1–1 | Housekeeping gene |
| GAPDH | Hs99999905_m1 | 3–3 | Housekeeping gene |
| CCL2 | Hs00234140_m1 | 1–2 | Target gene |
| CCL8 | Hs04187715_m1 | 1–2 | Target gene |
| CCL19 | Hs00171149_m1 | 1–2 | Target gene |
| CXCL1 | Hs00236937_m1 | 3–4 | Target gene |
| CXCL2 | Hs00601975_m1 | 3–4 | Target gene |
| CXCL3 | Hs00171061_m1 | 3–4 | Target gene |
| CXCL5 | Hs00171085_m1 | 3–4 | Target gene |
| CXCL6 | Hs00237017_m1 | 3–4 | Target gene |
| CXCL10 | Hs00171042_m1 | 1–2 | Target gene |
ACTB = β-actin; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; CCL =C-C motif chemokine ligand; CD14 =CD14 molecule; CXCL = C-X-C motif chemokine ligand.
To determine the stability and optimal number of housekeeping genes, we used geNORM version 3.4 (PrimerDesign Ltd.) according to the manufacturer’s instructions. The average gene-stability measure ranked β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the most stable genes in our samples. The PCR efficiency was tested over 5-log dilution series and confirmed that all target genes and housekeeping genes had similar amplification efficiencies. For each primer/probe set, the PCR was performed using μL of cDNA diluted 1:10-fold. Each qPCR plate included “no reverse transcriptase” and “no template” controls to eliminate nonspecific amplification. One sample from each target gene was run on a gel to confirm specificity, and all samples were run in triplicate. Target gene qPCR data is normalized to the geometric mean of β-actin and GAPDH and is expressed relative to the control samples using 2−(ΔΔCt) method.
2.7. Statistical analysis and effects of confounding variables
Statistical analyses were performed using the SAS 9.4 statistical software package. A linear regression model was conducted to determine the differences between two groups – DS and NC subjects. The covariates included in the model were age, gender, postmortem interval (PMI), brain pH and antidepressants treatment. Post-hoc univariate t-tests were also performed to determine the group comparison for all outcome measures separately. False Discovery Rate (FDR) was set at 0.05. FDR-adjusted significant values were reported for t-tests.
3. Results
3.1. Characteristics of study subjects
The demographic and clinical characteristics of DS and NC subjects are summarized in Table 2, and detailed characteristics are provided in Supplementary Table 1. In addition to variables such as age, gender, race, PMI, brain pH, the Supplementary Table 1 also includes the suicide method and the presence of antidepressants or ethanol at the time of death for each DS subject.
Table 2.
Demographic characteristics of subjects
| Parameter | Normal Controls | Depressed Suicide |
|---|---|---|
| Subjects (N) | 24 | 24 |
| Age | 42.08 ± 15.35 | 38.95 ± 15.39 |
| Race | 7 B, 17W | 2 B, 22W |
| Gender | 20 M, 4F | 14 M, 10 F |
| PMI (hours) | 16.54 ± 6.4 | 18.91 ± 6.02 |
| Brain pH | 7.01 ± 0.15 | 6.96 ± 0.25 |
N, number, B, black, W, white; M, male, F, female
Values are presented as means ± SD
3.2. Effect of covariates on chemokines mRNA expression in the PFC of DS and NC subjects
In order to determine the effect of covariates (i.e., confounding variables), we examined the effect on various chemokines mRNA expression using the generalized linear model for each outcome measure. We did not observe any significant effect of confounding variables, such as age, race, gender, PMI, brain pH or presence of antidepressants, on mRNA expression of any chemokines studied between the DS and NC groups.
3.3. Gene expression of chemokines using qPCR array
We first determined the PCR gene array for about 84 genes in 12 DS and 12 NC subjects in order to determine which of the chemokines and other immune genes are differentially regulated in the PFC of DS subjects.
The PCR array results were analyzed with EFFE Bioscience PCR array data analysis online software to identify differentially expressed genes. We found several of the genes differentially regulated in the PFC of DS subjects compared with NC subjects. In addition, we also found several chemokines which were differentially regulated in DS as compared to NC by 25%. Among the differentially regulated genes CCL2, CCL8, CCL19, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6 and CXCL10 were downregulated by 25%, and no chemokine was upregulated. In order to examine which of them are significantly down regulated, we validated the expression of genes by qPCR.
3.4. Determination of mRNA expression of CXCL chemokines in the PFC of DS subjects
In a previous study determined the mRNA expression of IL-8 (also known as CXCL8) in the PFC of DS subjects and found no difference from NC subjects (Pandey et al., 2018). In this study, we determined the mRNA expression of CXCL1, CXCL2, CXCL3, CXCL5, CXCL6 and CXCL10 in the PFC of DS subjects (Fig. 1). When we analyzed the results, we found that the gene expression of CXCL1 (t = − 2.15, p= 0.04), CXCL2 (t = − 2.13, p = 0.04) and CXCL3 (t = − 2.33, p = 0.02) were significantly decreased in the PFC of DS subjects compared with NC subjects. However, there was no change observed in mRNA expression of CXCL5 (t = 0.36, p = 0.72) CXCL6 (t = − 0.24, p = 0.81), and CXCL10 (t = − 0.57, p = 0.57) in the PFC of DS compared to NC subjects.
Figure 1.
Mean mRNA expression levels of CXCL1, CXCL2, CXCL3, CXCL5, CXCL6 and CXCL8 in the prefrontal cortex (PFC) of depressed suicide (DS, n = 24) and normal control (NC, n = 24) subjects. The data are shown as the fold change in mRNA levels ± S.E.M.
*p< 0.05
A post-hoc t-test adjusted with FDR shows that gene expressions of CXCL1 (t = − 2.09, p= 0.04), CXCL2 (t = −2.46, p = 0.02) and CXCL3 (t = −2.36, p = 0.02) were significantly decreased in the DS subjects compared with NC subjects.
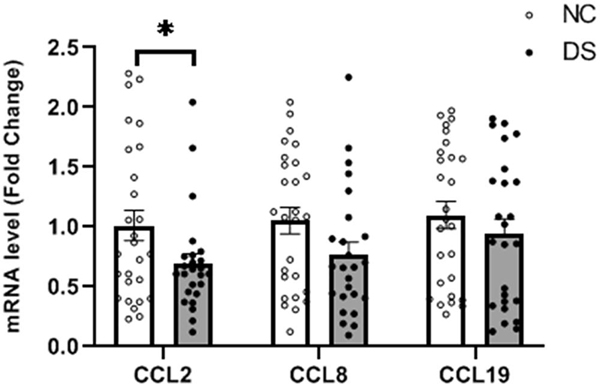
3.5. Determination of mRNA expression of CCL chemokines in the PFC of DS subjects
We then examined if, similar to CXCL chemokines, there was a significant change in CCL chemokines in the PFC of DS group (Fig. 2). Using PCR array, we observed altered gene expression of only three CCL chemokines – CCL2, CCL8 and CCL19. This was further examined using Taqman based qPCR. We found that the mRNA expression of CCL2 (t = −2.1, p = 0.041) was significantly decreased in the PFC of DS group compared with NC group. CCL8 and CCL19 mRNA levels in the PFC of DS subjects were also decreased (t = −1.74, p = 0.088; t = −0.81, p = 0.420, respectively) compared with NC subjects, but this decrease was not statistically significant (Fig. 2).
Figure 2.
Mean mRNA expression levels of CCL2, CCL8 and CCL19 in the PFC of DS (n = 24) and NC (n = 24) subjects. The data are shown as the fold change in mRNA levels ± S.E.M.
*p< 0.05
3.6. Effect of antidepressants on mRNA expression of chemokines in the PFC of DS subjects
Some previous studies showed the effect of antidepressants on the expression of some chemokines. Since we did not observe any significant difference in the gene expression of several chemokines we studied between DS and NC subjects, we examined the possibility that previous exposure to antidepressants may affect mRNA expression of chemokines in DS group. In the DS group, 11 out of 24 subjects were on antidepressant treatment at the time of death, and 13 were free of antidepressant medication. We therefore examined the effect of antidepressant treatment by comparing the mean expression levels of chemokines between antidepressant-free and antidepressant treated DS group, and found no significant difference in the mRNA expression of any of the chemokines.
4. Discussion
We have recently reported that there is no difference in the PFC mRNA expression of CXCL8 (also known as IL-8) between the same DS and NC subjects (Pandey et al., 2018). In this study, we determined the gene expression of several other chemokines in the PFC of DS and NC subjects. We found that the gene expression of CXCL1, CXCL2, CXCL3 and CCL2 was significantly decreased in the PFC of DS subjects compared with NC after FDR correction. We also observed a decrease in gene expression of CCL8, CXCL6 and CXCL10 but the decrease did not reach significance after FDR correction. There were no significant differences in the gene expression of CXCL5 and CCL19 between and NC subjects. These studies thus suggested downregulation of some chemokines we studied in DS subjects.
We studied the CCL and CXCL chemokines in the postmortem brain of DS and NC subjects because these chemokines have been studied earlier in depressed patients and they play an important role in brain function (Eyre et al., 2016; Stuart and Baune, 2014). Although there have not been studies of chemokines in the postmortem brain in depression, some CCL and CXCL chemokines (specifically CCL2 and CXCL8) have been studied in serum of depressed and suicidal subjects (Stuart and Baune, 2014).
There are not many studies of chemokines in the postmortem brain of depressed or suicide subjects, but there are two studies carried out by gene array method. Torres-Platas et al. (2014) determined the gene expression of several genes in order to examine microglial activation and they determined the gene expression of monocyte chemoattractant protein 1 (MCP1/CCL2) in the dorsal anterior cingulate cortex (dACC) obtained from DS and NC subjects. They found significant increase in gene expression of CCL2 in the dACC of DS compared with NC subjects. More recently, Pantazatos et al. (2017) performed brain gene expression profiling using microarrays in dorsal lateral prefrontal cortex (BA9) of DS and NC subjects. They found significant alterations of several cytokines and chemokines in BA9 of DS compared with NC subjects. Among chemokines, they found a decrease in the gene expression of CCL2, CCL4 and CXCL8. However, only the expression of CCL2 was significantly decreased in DS compared with NC subjects, but CCL4 and CXCL8 showed non-significant changes.
Our observation of CCL2 in the PFC of DS subjects is similar to that observed by Pantazatos et al. (2017), both studies using the same brain area (i.e., BA9). However, the inconsistency for CCL2 between our results and those of Pantazatos et al. (2017) with those of Torres-Platas et al. (2014) is not clear but may be due to the use of different brain area (i.e., dACC) by the latter groupRecently, Shinko et al. (2020) determined the protein expression of chemokines in dorsolateral PFC obtained from 16 suicide and 23 control subjects using Bio-Plex Pro™ Human Chemokine Panel 40-Plex. They found that the protein expression of chemokines CCL1, CCL8, CCL13, CCL15, CCL17, CCL19 and CCL20 were significantly decreased in suicide subjects compared with control subjects. Although they found decreases in all these chemokines in suicide subjects, their results appear to be different from ours in terms of decrease in specific chemokines. This discrepancy may be due to two reasons. They determined protein expression and we determined gene expression of chemokines. Also, whereas our subjects included only depressed suicides, their suicide subjects included subjects with mood disorders, psychotic disorders, anxiety disorders and unknown diagnosis. Nonetheless, it was interesting to note that they also found decreased chemokines in their samples.
Although there are only a few studies of chemokines in the postmortem brain of DS subjects there are several studies of chemokines in the serum or plasma obtained from depressed subjects and normal controls. Among the chemokines, the CCL2 and CXCL8 appear to be more widely studied in the plasma/serum of depressed patients. Some of these investigators observed an increase in CXCL8 in serum of depressed patients compared with controls (Simon et al., 2008). On the other hand, some investigators did not find CXCL8 in serum of depressed patients compared with normal controls (Eller et al., 2008, 2009; Hallberg et al., 2010; O’Brien et al., 2007). The increase in CCL2 in depressed patients was observed by Sutcigil et al. (2007) and by Simon et al. (2008). Thus, most but not all studies find increases in serum levels of CCL2 and CXCL8 in MDD subjects. A meta-analysis and the review of these studies has been performed by Leighton et al. (2018), Stuart and Baune (2014), Slusarczyk et al (2016), Eyre et al. (2016), Black and Miller (2015). These meta-analyses generally suggest higher levels of CCL2, CXCL8, CXCL10 and CXCL10 in MDD subjects compared to controls. A brief summary of these studies is provided in Table 3.
Table 3.
Summary of Chemokines Studies in Serum/Plasma of MDD Patients.
| Study / Year | Chemokines | Findings |
|---|---|---|
| Song et al. (1998) | CXCL8 | Increased |
| Simon el al. (2008) | CXCL8, CCL2, CCL3, CCL11 | Increased |
| Sutcigil et al. (2007) | CCL2 | Increased |
| O’Brien et al. (2007) | CXCL8 | No significant difference |
| Eller et al. (2008) | CXCL8 | No significant difference |
| Wang et al. (2008) | CXCL10 | Increased |
| Musselman et al. (2000) | OC3CL1, CLL3 | Increased |
| Lehto et al. (2010) | CCL2, CCL3 | Decreased |
| Oglodek et al. (2014) | CXCL12, CCL5 | Increased |
| Meta-analyses | ||
| Eyre et al. (2016) | CCL | Increased |
| CXCL8 | No significant difference | |
| Leighton et al. (2018) | CCL2, CCL3, CCL11, CXCL7, CXCL8 | Increased |
| CCL4 | Decreased | |
| CCL5 | No significant difference |
There are only a few studies of chemokines in the plasma of suicidal patients. There was one study that found the levels of chemokines CCL2 and CCL5 significantly decreased and CCL11 levels significantly increased in the serum of patients with depression and suicidal ideation compared with those without suicidal ideation (Grassi-Oliveira et al., 2012).
Isung et al. (2012) found a significant decrease in CSF CXCL8 in patients with depression who attempted suicide compared with depressed patients without suicide attempt. Janelidze et al. (2013) also studied several chemokines in the CSF of suicidal patients. They found that patients who had attempted suicide in the previous 2 weeks demonstrated lower levels of chemokines CCL11, CCL2, CCL4, CCL13 and CCL17 in the CSF of suicidal patients compared with healthy control subjects. A meta-analysis of chemokines in suicidal behavior conducted by Black and Miller (2015) found decreased level of CCL2 and CCL5 in suicide patients compared to controls. However, this was based on very few studies.
In summary, the studies of chemokines in the plasma/serum of patients with suicidal behavior generally suggest reduced levels of most of these chemokines in suicidal patients, compared with non-suicidal patients or normal control subjects. This was opposite or in contrast to the generally increased levels of chemokines found in the plasma of depressed subjects. The observation of reduced levels of chemokines in the plasma of suicidal patients appears to be similar to our observation of reduced levels of CCL and CXCL chemokines in the PFC of depressed suicide subjects. This observation raises the possibility that chemokines function or levels in suicidal patients are different or in fact opposite to those observed in depressed patients.
Our observation that there are specific decreases in certain chemokines, such as CCL2, CXCL1, CXCL2 and CXCL3 might suggest that depressed suicide subjects are the immune compromised phenotype. This is also substantiated by our observation and observations of other investigators that the expression of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α is also decreased in the PFC of suicide subjects. It is also reinforced by our observation that innate immunity receptors, for example specific Toll-like receptors like TLR2, TLR3, TLR4 and TLR6 are increased in the PFC of depressed suicide subjects (Pandey et al., 2019; Pandey et al., 2014). Therefore, all these observations do suggest depressed suicide subjects as immune compromised phenotype.
Chemokines play not only an important role in cellular migration and immune coordination but are also important in linking central and peripheral inflammation and orchestrating neuroinflammatory cross-talk (D’Mello et al., 2009; Leighton et al., 2018; Wohleb et al., 2016). and their receptors are present in both the developing and adult CNS (Jaerve and Muller, 2012; Rostene et al., 2011a) under both physiologic and inflammatory conditions and they are important not only in cell trafficking but also in CNS functions such as neurogenesis and neuroinflammation (Banisadr et al., 2002; Rostene et al., 2011a; Rostene et al., 2011b). Some of the chemokines, such as CCL2 and fractalkine have been described as modulators of hippocampal neuronal activity (Marciniak et al., 2015). Some chemokines have been found to affect central neurotransmitter release. For example, CCL3 and CXCL14 may inhibit gamma-aminobutyric acid (GABA) transmission (Heinisch and Kirby, 2009; Reaux-Le Goazigo et al., 2013). The observation that chemokine SDF1/CXCL12 decreases dopamine (DA) content and increases extracellular concentration of DA and its metabolites in ipsilateral striatum supports the idea that chemokines affect neurotransmitter levels and release. Such effect on DA results in CXCR4-mediated contralateral circling behavior (Reaux-Le Goazigo et al., 2013). Besides their effect on neurotransmitter release and function, chemokines can also modulate neuroendocrine functions (Reaux-Le Goazigo et al., 2013; Rostene et al., 2011a).
Since we observed a decrease in some specific chemokines, particularly CCL2, CXCL1, CXCL2 and CXCL3, we examined if a decrease in these chemokines may be related to some of CNS functions in the brain relevant to depression. Although the functions of these chemokines in the CNS are not well understood, the functions of some chemokines, such as CCL2, CXCL1, CXCL8 have been studied in animals. One of the main CNS functions associated with depression and suicide is the disturbance in hypothalamus pituitary adrenal (HPA) axis function (Coryell and Schlesser, 1981). CCL2, CXCL1 and CXCL2 alterations in HPA axis function as it has been shown that the expression of CCL2 and CXCL1 is downregulated by glucocorticoids (Sorrells and Sapolsky, 2007; Zhou et al., 2007). Also, CXCL1 is known to stimulate the release of adrenocorticotropic hormone (ACTH) from cultured pituitary cells and may thus cause an increase in the release of ACTH in suicidal depressed subjects. CXCL1 and CXCL2 deficiency may be involved in the modulation of glucocorticoid receptor (GR)-mediated negative feedback of HPA axis in depressed and suicidal subjects (Licinio et al., 1992; Stuart et al., 2015). Thus, these properties of CCL2 and CXCL1 and CXCL2 strongly suggest that a decrease in the levels of these chemokines in the PFC of DS may alter and cause dysregulation of the HPA axis function in depressed suicidal subjects.
CCL2 and CXCL1–8 chemokines have also been shown to have neurotrophic and neuroprotective effects as they cause the release of growth factors and the inhibition of apoptosis and may thus be involved in regulating neurogenesis. CCL2 promotes glial cell proliferation and growth in vitro (Rezaie et al., 2002) and plays a role in adult neurogenesis, as shown in models of stroke (Liu et al., 2007).
In hippocampal neurons, the ligand CXCL2 was shown to protect against amygdaloid-induced cell death by the activation of mitogen-activated protein kinase (Watson and Fan, 2005). CXCL2 causes dose-dependent neurotoxicity of cultured primary motor neurons (De Paola et al., 2007). One group has shown that CXCL2 treatment of mouse primary cortical neurons stimulated growth factors (Xia and Hyman, 2002). CXCL2 has been shown to be neurotrophic for cerebellar granule neurons (Limatola et al., 2000). We have previously reported a brain-derived neurotrophic factor (BDNF) decrease the PFC of DS subjects (Dwivedi et al., 2003; Pandey et al., 2008), and it may thus appear that decrease may be related to downregulation of CCL2 and CXCL1. The other important function mediated by CCL2 and CXCL1 is in the modulation and release of DA (Guyon et al., 2009). A model for the possible actions of CCL2, CXCL1 and CXCL2 has been proposed by Stuart et al. (2015) and Semple et al. (2010), suggesting a role of these chemokines in depression. The neurotrophic properties of CXCL2 have been shown by its effect on cerebella granule neurons (Limatola et al., 2000) and CXCL2 may also cause survival of neurons and may be anti-apoptotic.
CCL2 and CXCL1 also play important roles in the immune-related function in the brain and are related to neuroinflammation. CCL2 causes activation of microglia and astrocytes, which are the main immune cells in the CNS (Rollins, 1996). Microglia have been reported to be increased in the postmortem brain of suicidal subjects, regardless of the diagnosis (Steiner et al., 2008). Chemokines are involved in chemo-attraction of cells, especially those of the immune system. Chemokines are some of the most important inflammatory factors (Bajetto et al., 2001) and in the CNS they are involved in the recruitment of microglia, astrocytes and also monocytes from the periphery (D’Mello et al., 2009; Rollins, 1996). Therefore, the effect of CCL2 on microglia has been examined. It was shown by Hinojosa et al. (2011) that whereas CCL2 causes migration and proliferation of microglia, CCL2 does not appear to directly activate an inflammatory response in microglial culture. It is therefore possible that the observed increase in microglial activation in suicide PFC is probably related to increases in other factors, such as increased proinflammatory cytokines as we have previously reported (Pandey et al., 2018).
Decreased levels of CXCL1, CXCL2 and CXCL3 we have observed in the brain of DS subjects may lead to increased neurotoxicity, neuronal loss or both and subsequent decreased neurogenesis, altered HPA axis and neurotransmitter function, most of which have been implicated in the pathogenesis of depression. A better understanding of the neurochemokines in depression may aid in the development of novel therapeutic strategies and the knowledge of molecular mechanisms involved in neurotransmission, neuron-glia interaction and neuroimmune regulation.
5. Conclusion
The neuroimmune or neuroinflammatory hypotheses of depression and suicide are primarily based on the observation of abnormal levels of proinflammatory cytokines in the plasma of depressed and suicidal subjects. Besides cytokines, some other members of immune functions such as innate immunity receptors and chemokines, which are significant modulators of immunity in depression, much more so in the brain. Chemokines are present not only in the brain but can also travel from the periphery and are also involved in important functions in the brain relevant to depression and suicide under normal and abnormal physiological and pathological conditions such as psychiatric disorders. Although there are several peripheral chemokines studies there are just a couple of studies in the brain of depressed and suicidal subjects. The present study was undertaken to examine the role of chemokines in the postmortem brain of DS subjects. The current chemokines study suggested decreased levels of some important chemokines, including CCL2 and CXCL1 in the brain. Chemokines are also involved in mediating such important in the brain as neurogenesis, modulation of HPA functions and modulation of neurotransmitters which have all been implicated in the pathophysiology of depression. The dysfunction of chemokines observed in this study may cause changes in many of these functions such as neurogenesis, apoptosis and neuroendocrine functions. These chemokines would be good targets for developing therapeutic agents for treatment for depression or non-responsive depressed patients.
Supplementary Material
Supplementary Table 1. Detailed Demographic Characteristics of Subjects
Highlights.
We found decreased mRNA expression of several chemokines in the postmortem brain of depressed suicide subjects
Decreased mRNA expression of CXCL1, CXCL2, CXCL3, and CCL2 in the prefrontal cortex of depressed suicide subjects suggests an abnormality of chemokines in depressed and suicide brain
These findings suggest that brain chemokines abnormalities may be involved in the pathophysiology of depression and suicide
Acknowledgment
We are thankful to Anuradha Sharma, Ph.D. for her help in the preparation of the manuscript.
Funding
This research was supported by grants RO1MH098554 and RO1MH106565 (Dr. Pandey) from the National Institute of Mental Health, Rockville, MD. The funding source had no role in the study design; collection, analysis and interpretation of data; or writing of the manuscript.
Footnotes
Conflict of interest
All authors declare that they have no financial interests or potential conflicts of interest directly or indirectly related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosini E, Aloisi F, 2004. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res 29, 1017–1038. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G, 2001. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol 22, 147–184. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Schettini G, 2002. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem 82, 1311–1329. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM, 2002. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem 81, 257–269. [DOI] [PubMed] [Google Scholar]
- Black C, Miller BJ, 2015. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol Psychiatry 78, 28–37. [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Rostene W, Parsadaniantz SM, 2007. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J Mol Endocrinol 38, 355–363. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R, 2001. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med 63, 376–386. [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP, 2010. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation 7, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW, Norden DM, Skendelas JP, Huang Y, O’Connor JC, Lawson M, Dantzer R, Kelley KW, Godbout JP, 2013. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun 31, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Schlesser MA, 1981. Suicide and the dexamethasone suppression test in unipolar depression. Am J Psychiatry 138, 1120–1121. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Le T, Swain MG, 2009. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci 29, 2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola M, Buanne P, Biordi L, Bertini R, Ghezzi P, Mennini T, 2007. Chemokine MIP-2/CXCL2, acting on CXCR2, induces motor neuron death in primary cultures. Neuroimmunomodulation 14, 310–316. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN, 2003. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60, 804–815. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E, 2008. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 32, 445–450. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E, 2009. Effects of bupropion augmentation on pro-inflammatory cytokines in escitalopram-resistant patients with major depressive disorder. J Psychopharmacol 23, 854–858. [DOI] [PubMed] [Google Scholar]
- Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ, Baune BT, 2016. A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry 68, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 1997. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). American Psychiatric Publishing, Inc., Arlington, Virginia, USA. [Google Scholar]
- Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, Bauer ME, 2012. chemokine levels in women with recurrent major depression with suicidal ideation. Braz J Psychiatry 34, 71–75. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovere C, Conductier G, Trocello JM, Dauge V, Kitabgi P, Rostene W, Nahon JL, Melik Parsadaniantz S, 2009. Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience 162, 1072–1080. [DOI] [PubMed] [Google Scholar]
- Hallberg L, Janelidze S, Engstrom G, Wisen AG, Westrin A, Brundin L, 2010. Exercise-induced release of cytokines in patients with major depressive disorder. J Affect Disord 126, 262–267. [DOI] [PubMed] [Google Scholar]
- Heinisch S, Kirby LG, 2009. Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuroscience 164, 1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL, 2011. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation 8, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isung J, Aeinehband S, Mobarrez F, Martensson B, Nordstrom P, Asberg M, Piehl F, Jokinen J, 2012. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry 2, e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaehne EJ, Baune BT, 2014. Effects of chemokine receptor signalling on cognition-like, emotion-like and sociability behaviours of CCR6 and CCR7 knockout mice. Behav Brain Res 261, 31–39. [DOI] [PubMed] [Google Scholar]
- Jaerve A, Muller HW, 2012. Chemokines in CNS injury and repair. Cell Tissue Res 349, 229–248. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Ventorp F, Erhardt S, Hansson O, Minthon L, Flax J, Samuelsson M, Traskman-Bendz L, Brundin L, 2013. Altered chemokine levels in the cerebrospinal fluid and plasma of suicide attempters. Psychoneuroendocrinology 38, 853–862. [DOI] [PubMed] [Google Scholar]
- Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J, 2018. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry 23, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Wong ML, Gold PW, 1992. Neutrophil-activating peptide-1/interleukin-8 mRNA is localized in rat hypothalamus and hippocampus. Neuroreport 3, 753–756. [DOI] [PubMed] [Google Scholar]
- Limatola C, Ciotti MT, Mercanti D, Vacca F, Ragozzino D, Giovannelli A, Santoni A, Eusebi F, Miledi R, 2000. The chemokine growth-related gene product beta protects rat cerebellar granule cells from apoptotic cell death through alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors. Proc Natl Acad Sci U S A 97, 6197–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L, 2009. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry 66, 287–292. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Wang L, Yier T, Chopp M, 2007. Chemokine ligand induces migration and differentiation of subventricular zone cells after stroke. J Neurosci Res 85, 2120–2125. [DOI] [PubMed] [Google Scholar]
- Marciniak E, Faivre E, Dutar P, Alves Pires C, Demeyer D, Caillierez R, Laloux C, Buee L, Blum D, Humez S, 2015. The Chemokine MIP-1alpha/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep 5, 15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, White FA, Zhang J, 2008. Chemokine action in the nervous system. J Neurosci 28, 11792–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG, 2007. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res 41, 326–331. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Bhaumik R, Ren X, 2019. Innate immunity in the postmortem brain of depressed and suicide subjects: Role of Toll-like receptors. Brain Behav Immun 75, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Dwivedi Y, Pavuluri MN, 2008. Brain-derived neurotrophic factor gene expression in pediatric bipolar disorder: effect of treatment and clinical response. J Am Acad Adolesc Psychiatry (Under revision). [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y, 2014. Toll-like receptors in the depressed and suicide brain. J Psychiatr Res 53, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y, 2012. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res 46, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X, 2018. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J Psychiatry Neurosci 43, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazatos SP, Huang YY, Rosoklija GB, Dwork AJ, Arango V, Mann JJ, 2017. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry 22, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA, 2007. Brain-immune communication pathways. Brain Behav Immun 21, 727–735. [DOI] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Van Steenwinckel J, Rostene W, Melik Parsadaniantz S, 2013. Current status of chemokines in the adult CNS. Prog Neurobiol 104, 67–92. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Trillo-Pazos G, Everall IP, Male DK, 2002. Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: potential role of chemokines in the developing CNS. Glia 37, 64–75. [DOI] [PubMed] [Google Scholar]
- Rollins BJ, 1996. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today 2, 198–204. [DOI] [PubMed] [Google Scholar]
- Rollins BJ, 1997. Chemokines. Blood 90, 909–928. [PubMed] [Google Scholar]
- Rostene W, Dansereau MA, Godefroy D, Van Steenwinckel J, Reaux-Le Goazigo A, Melik-Parsadaniantz S, Apartis E, Hunot S, Beaudet N, Sarret P, 2011a. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J Neurochem 118, 680–694. [DOI] [PubMed] [Google Scholar]
- Rostene W, Guyon A, Kular L, Godefroy D, Barbieri F, Bajetto A, Banisadr G, Callewaere C, Conductier G, Rovere C, Melik-Parsadaniantz S, Florio T, 2011b. Chemokines and chemokine receptors: new actors in neuroendocrine regulations. Front Neuroendocrinol 32, 10–24. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Baggiolini M, 2008. Chemokines and leukocyte traffic. Nat Immunol 9, 949–952. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M, 2005. Cytokines and major depression. Progress in Neuro-psychopharmacology & Biological Psychiatry 29, 201–217. [DOI] [PubMed] [Google Scholar]
- Semple BD, Kossmann T, Morganti-Kossmann MC, 2010. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab 30, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinko Y, Otsuka I, Okazaki S, Horai T, Boku S, Takahashi M, Ueno Y, Sora I, Hishimoto A, 2020. Chemokine alterations in the postmortem brains of suicide completers. J Psychiatr Res 120, 29–33. [DOI] [PubMed] [Google Scholar]
- Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK, 2008. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol 18, 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarczyk J, Trojan E, Chwastek J, Glombik K, Basta-Kaim A, 2016. A Potential Contribution of Chemokine Network Dysfunction to the Depressive Disorders. Curr Neuropharmacol 14, 705–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM,2007. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun 21, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B, 2008. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of Psychiatric Research 42, 151–157. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Baune BT, 2014. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev 42, 93–115. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Singhal G, Baune BT, 2015. Systematic Review of the Neurobiological Relevance of Chemokines to Psychiatric Disorders. Front Cell Neurosci 9, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A, 2007. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol 2007, 76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Moller HJ, Chen HH, Postolache TT, 2008. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatrica Scandinavica 117, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N, 2014. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42, 50–59. [DOI] [PubMed] [Google Scholar]
- Watson K, Fan GH, 2005. Macrophage inflammatory protein 2 inhibits beta-amyloid peptide (1–42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol 67, 757–765. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, Duman RS, 2016. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 17, 497–511. [DOI] [PubMed] [Google Scholar]
- Xia M, Hyman BT, 2002. GROalpha/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer’s disease? J Neuroimmunol 122, 55–64. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ling EA, Dheen ST, 2007. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. J Neurochem 102, 667–678. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Hepgul N, Pariante CM, 2013. Inflammation and depression. Curr Top Behav Neurosci 14, 135–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Detailed Demographic Characteristics of Subjects