Abstract
Drought and salinity are major constraints to agriculture. In this review, we present an overview of the global situation and the consequences of drought and salt stress connected to climatic changes. We provide a list of possible genetic resources as sources of resistance or tolerant traits, together with the previous studies that focused on transferring genes from the germplasm to cultivated varieties. We explained the morphological and physiological aspects connected to hydric stresses, described the mechanisms that induce tolerance, and discussed the results of the main studies. Finally, we described more than 100 genes associated with tolerance to hydric stresses in the Triticeae. These were divided in agreement with their main function into osmotic adjustment and ionic and redox homeostasis. The understanding of a given gene function and expression pattern according to hydric stress is particularly important for the efficient selection of new tolerant genotypes in classical breeding. For this reason, the current review provides a crucial reference for future studies on the mechanism involved in hydric stress tolerance and the use of these genes in mark assistance selection (MAS) to select the wheat germplasm to face the climatic changes.
Keywords: osmotic adjustment, ionic, redox homeostasis, transcription factors, salt-responsive genes, genetic diversity, germplasm, cross-transferability
1. Global Situation
Water and consequently an adequate hydric balance are essential for life, determining crop production in terms of quantity and quality. In the present century, the main problems are related to hydric stress, including dwindling water resources and excessive salinity of irrigation water and soil. Moreover, it is expected that global warming and climate change would increase the frequency of drought events, leading to a severe threat to food security [1,2,3].
In nature, plants are exposed to various abiotic factors and exhibit complex adaptation responses that depend on their degree of plasticity. In addition, they are facing environmental changes that increase the risk factors, such as rainfall distribution, loss of soil fertility and organic carbon, fluctuation in temperature and light, evaporative intensity, biological stresses, increasing pollution, and declining biodiversity [4]. Water is the most critical factor for the growth and development of living organisms, determining 82% of the variation in grain yield in areas receiving less than 400 mm of annual rainfall [5]. Land plants are anchored in the soil and rely on their root system to ensure water availability and nutrient uptake. About 30% of the Earth’s land is arid or semi-arid, and this percentage is expected to increase due to climate change [6]. The prevision indicates an erratic rainfall distribution, an overall decrease in total annual rainfall, and a more pronounced occurrence of dry periods [7], in addition to an increase in temperatures with a consequent reduction in ecosystem health through species extinction or a reduction in biodiversity, due to migration and changes in behavioral patterns [8].
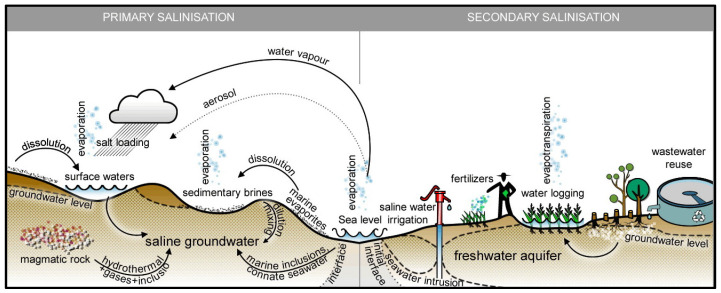
Among all stresses, drought and salinity (soil and water) are the main abiotic factors that reduce a crop’s productivity, causing the most damage for grain production all over the world [9]. Other than grain production, water scarcity also affects the growth rate, leaf size, stem extension, root proliferation, susceptibility to disease, plant color, etc. [10,11]. Particularly in conventional farming, the rainfall water effectively decreases due to increased surface runoff, soil evaporation through unplanted and bare soil surfaces, and evaporation caused by the aeration of soil during intensive tillage [3]. Factors such as rainfall intensity, duration, exact time of rain events, and the soil properties affect the soil moisture status, especially the plant-available water (PAW), which can differ significantly with the same total amount of precipitation under different conditions. In addition, the water availability for plants decreases due to the high soluble salt concentrations in the soil, which causes absorption difficulties; i.e., water deficit or hydric stress. The main causes of soil salinization can be classified as natural or caused by human activity, named primary and secondary salinization, respectively (Figure 1) [12].
Figure 1.
Primary and secondary soil salinity mechanisms (Reprinted with permission from ref. [12]. 2016, Elsevier).
Current estimations indicate that salt issues affect about 20% of global lands and almost 40% of irrigated lands. This phenomenon is increasing and 50% of total cultivated land worldwide will be salinized by 2050 [13,14,15]. Global warming is the increase in temperatures often associated with rainfall decrease, leading to more extreme and unpredictable events. As a conclusion, the need to have a system protecting agriculture from an erratic climate becomes important worldwide.
The global grain production is an important indicator of food security, but unfortunately it grows slower than the human population. This unfavorable situation is exacerbated by abiotic factors such as drought, high salinity, extreme temperatures, flooding, and heavy metals [16,17]. There is an urgent need to increase food, which cannot be done by simply raising the agricultural lands, which causes deforestation and consequently climate change problem. Increasing the production per unit area (i.e., yields) represents a potential solution. Yields can be improved by using genetics methods, appropriate crop rotation, adequate fertilization, and early planting. For example, a six-week delay in planting time resulted in yield reductions of about 42% in barley and 22–32% in wheat [18]. While, early planting, proper fertilization, and appropriate crop rotation were found to increase the water-use efficiency (WUE) [19].
Useful and adequate breeding programs, addressed to reduce crop yield lost under stress conditions, are necessary to face the consequences of climate change. Attention should be focused on the drought-related traits, which include leaf canopy temperature, cell osmotic adjustment, cell membrane stability, leaf water potential, stomatal resistance, leaf rolling index, and leaf waxiness [18]. In wheat, for example, the total aboveground part of the plant (biological yield) in a water-limited production area is directly related to the water supply [20]. Although biological yield offers little hope of modification through breeding, the harvest index, which reflects the ratio of grain to total biological yield, appears to be amenable to genetic improvement for enhanced drought tolerance.
For this reason, hereafter is reported an overview of the sources of genes presents in wheat and in its wild relatives, and the morphological and physiological mechanisms involved in their tolerance. Moreover, this review provides updated information on more than one hundred candidate genes that provide plasticity in wheat tolerance to hydric stresses, providing tools that open possibilities for breeding new tolerant wheat genotypes.
2. Germplasm
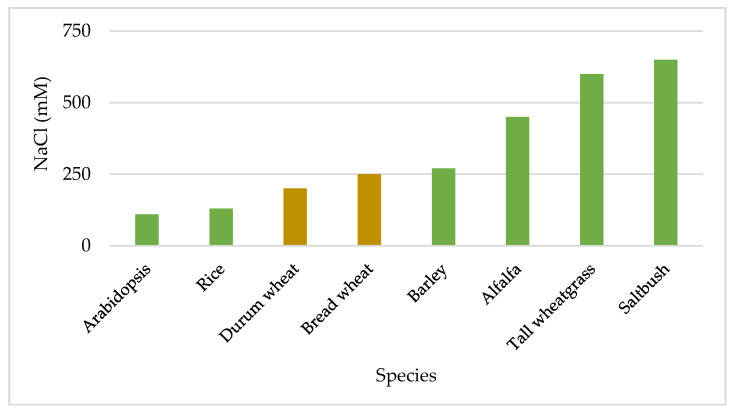
Significant differences in drought and salinity stresses were reported between plant species [21]. Their growth rates were highly different under stress, indicating that germplasm is the main reserve of useful genes that could be used to improve cultivated crops. Figure 2 shows the differences in salt sensitivity among some plant species. Tall wheat grass (Thinopyrum ponticum (Podp.) Z.-W. Liu and R.-C. Wang; (synonymous Agropyron elongatum (Host) P. Beauv.) and saltbush (Atriplex anicola Paul G. Wilson) are extremely tolerant to salinity. Arabidopsis thaliana (L.) is more sensitive to salinity while alfalfa (Medicago sativa L.) is much more tolerant, compared to cereals. Among the cereals, rice (Oryza sativa L.) is the most sensitive while barley (Hordeum vulgare L.) is the most tolerant. Within the intermediate tolerant species, durum wheat (Triticum turgidum subsp. durum (Desf.) Husn) is more sensitive than bread wheat (Triticum aestivum L.) [21].
Figure 2.
Salt tolerance differences among various species, expressed as NaCl concentration to inhibit dry matter production [21].
2.1. Sources of Resistances in Cultivated Species
Genes to improve tolerance to drought and salt stresses could be available in accessions, especially landraces of durum and bread wheat, but also in their wild relatives. Landraces have been successfully used to obtain salt-tolerant varieties by specific breeding programs [22,23]. The tolerance to these stresses could be measured with diverse methodology and observations. Some of these methods focus on the tolerance mechanisms, such as the number and dimension of stomata, or on the evaluation of the Na+ and K+ ion concentrations in leaves, or on looking at the root system and architecture. A more empirical tolerance evaluation looks at the differences in yield under stress levels. This latter approach is more useful for direct utilization and study of the effect on agricultural production. In turn, the former give information about single genes, information useful for gene pyramiding and to understand the physiological mechanism involved in the tolerance.
The homology among genomes is also different: the A, B, and D genomes contain similar loci that in some cases are difficult to be discriminated or to identify the specific locus for a specific function. In addition, the variability in terms of allele number depends of the species population. For example, the Dreb genes, one of the most important gene families conferring drought tolerance, are distributed in the different genomes. However, few alleles have been found related to abiotic stresses, and some allele-specific markers are developed for marker-assisted selection [24,25,26]. Within durum wheat, some alleles due to SNPs and/or INDEL mutations have been identified among varieties for DREB1, HKT1, and WRKY1 genes [27,28]. In spite of the homology among the A, B, and D genome, some higher resistance to salt stress is present in the D genome. Bread wheat, Triticum aestivum L. (genomes AABBDD), accumulates less sodium and more potassium in expanding and young leaves than durum wheat, T. turgidum subsp. durum (Desf.) Husn (genomes AABB) [29].
2.2. Sources of Resistances in Alien Species
Wild relatives are useful sources of genes for stress tolerance. They could be used in breeding programs to transfer a single gene to reduce crop yield loss under stress (see below). The genes conferring resistance were transferred to the amphiploid, which often has very low quality and productivity. This is because the wild relatives, even if having genes able to confer resistance, are lacking in all the other genes selected during 10,000 years of agriculture and breeding, such as to have high yield and high-quality production. Several works [30,31,32,33] focus on the source or resistance available in several genomes of the Triticeae family, from the cultivated species to the donors of the A, B, and D genomes arriving to more distant species having genomes S, C, G, M, N, U, E, and J, up to the Hordeum genera (genomes I, H, and X). Table 1 reports a synthesis of the source of resistance found.
Table 1.
Summary of the studies on a source of genes for tolerance to drought and salt, modified from [30,31].
| Species | Genome | Common Name | Reference |
|---|---|---|---|
| Triticum monococcum L. ssp. aegilopoides (Link) Thell. | AA | Wild einkorn | [22,42] |
| Triticum monococcum L. ssp. monococcum | AA | Einkorn | [43] |
| Triticum urartu Thumanjan ex Gandilyan | AA | [42,44,45] | |
|
Triticum turgidum L. ssp. dicoccoides
(Körn. Ex Asch. and Graebn.) Thell. |
AABB | Wild emmer | [33,40,44,45,46] |
| Triticum turgidum ssp. durum L. (Desf.) Husn | AABB | Durum wheat | [22,47] |
| Triticum aestivum L. ssp. aestivum | AABBDD | Bread wheat | [48,49,50] |
| Aegilops markgrafii (Greuter) K. Hammer | CC | [34] | |
| Aegilops cylindrica Host | CCDD | Jointed goat grass | [34,51,52] |
| Aegilops triuncialis L. | CuCuCC | Barb goat grass | [51] |
| Aegilops tauschii Coss. | DD | Goat grass | [34,36,45,53] |
| Elytrigia elongata Host Nevski | EbEb | Tall wheatgrass | [54,55,56,57] |
| Thinopyrum ponticum (Podp.) Barkworth and DR Dewey | EEEEEEEEEE | [55,58,59] | |
| Thinopyrum bessarabicum (Savul and Rayss) Á. Löve | EjEj | Tall wheatgrass | [60] |
| Triticum timopheevii (Zhuk.) Zhuk. ssp. armeniacum (Jakubz.) Slageren | GGAA | [35] | |
| Triticum timopheevii (Zhuk.) Zhuk. ssp. timopheevii | GGAA | ||
| Aegilops bicornis (Forssk.) Jaub. and Spach | SbSb | [34,51] | |
| Aegilops sharonensis Eig | SjSj | [34,51] | |
| Aegilops longissima Schweinf. and Muschl. | SjSj | [34,51] | |
| Aegilops speltoides Tausch var. speltoides | SS | [45] | |
| Aegilops searsii Feldman and Kislev ex K. Hammer | SSSS | [34] | |
| Aegilops umbellulata Zhuk. | UU | Jointed goat grass | [34,51] |
| Aegilops biuncialis Vis. | UUMM | [34] | |
| Aegilops ovata auct. | UUMM | Ovate goat grass | [34,51] |
| Aegilops variabilis Eig | UUSS | [34,51] | |
| Thinopyrum junceiforme (Á. Löve and D. Löve) Á. Löve | J1J1J2J2 | [61] | |
| Thinopyrum scirpeum (K Presl) DR Dewey | JJJJ | [61] | |
| Thinopyrum junceum L. (Á. Löve) | JJJJEE | Sand couch Sea wheatgrass |
[62] |
| Aegilops comosa Sm. | MM | [34] |
The transferability from the wild relatives to the cultivated ones is certainly different and depends on the genetic distance among species. Therefore, wild emmer, Triticum dicoccoides (Körn. ex Asch. and Graebn.) Schweinf (synonymous Triticum turgidum subsp. dicoccoides), is a useful potential donor for salt-tolerant genes, which can be transferred to cultivated cultivars by classical and/or modern techniques; while, to transfer genes from other more distant species, it would require passing through the amphidiploids. As reported in Table 1, the positive aspect is that there exists variability in salt tolerance amongst members of the Triticeae, with the tribe even containing a number of halophytes. Unfortunately, the amphiploid combines in the same genotypes the donors’ characteristics, which, together with useful tolerance genes, have also several unproductive characteristics. For this reason, few salt-tolerant varieties have been released from previous attempts at employing this approach. Modern technologies for assisted evolution give the possibility to transfer only the useful genes without transferring the whole background of the donor accession.
Gorham and coworkers analyzed several species and, looking at the tolerance of Aegilops tauschii (Coss.) Schmal (genome DD), which store less Na+, postulated the hypothesis that the D genome has the highest involvement in conferring tolerance [34,35]. Nevertheless, Na+ exclusion should not be the only mechanism since some tolerant individuals were found with a high level of sodium stored [36]. The Kna1 locus on chromosome 4D regulated the ratio of accumulated K+/Na+ to leaves under salt stress, for this reason bread wheat is generally a better Na+ ‘excluder’ than durum wheat. To improve durum wheat, the Kna1 locus was transferred from the D genome of hexaploid wheat into tetraploid wheat [29,37]. In the background of the durum cultivar Cappelli, the distal part of the long arm of chromosome 4B was substituted with chromosome 4D, creating some new germplasm with an enhanced K+/Na+ ratio but with not significantly different Na+ concentrations [37,38]; also, the A genome should have greater Na+ exclusion and K+/Na+ discrimination than the B genome, since Triticum urartu Thumanjan ex Gandilyan (AA) is more tolerant than durum wheat (AABB) [30]. This could be because the B genome negatively interact with the A genome, or because the B genome carry genes increasing Na+ entry [30]. Furthermore, within the tetraploid species there are accessions more tolerant to salinity than durum wheat; for example, wild emmer wheat (T. turgidum L. ssp. dicoccoides (Körn. Ex Asch. and Graebn.) Thell.) has lower rates of Na+ [39,40]. In durum wheat, the locus Nax1 mapped on chromosome 2A has considerable variation in capacity to ‘exclude’ Na+ [41].
Farooq and coworkers analyzed tolerance in Triticeae relatives. They specifically assessed the Aegilopes diversity working in Pakistan, which has about 6.8 million hectares of salty land [32,52,63]. Leaf ion concentrations was detected in several Aegilops species exposed to 50 mM of NaCl and the most tolerant and promising sources of tolerance were found in Aegilops cylindrica Host and in Ae. geniculata Roth. [60,61]. Although Cl did not differ significantly in any of the studied Aegilops species, they have differences in the Na+ and K+ concentrations in leaves. Ae. comosa Sm. and Ae. umbellulata Zhuk. (M and U genomes, respectively) had accessions with low Na+ concentrations. Several accessions of Ae. tauschii (DD), Ae. cylindrica (CCDD), and Ae. ovata auct. (UUMM) survived under severe stress [64].
The DRF1 (Dehydration Responsive Factor 1) was isolated and characterized in Ae. speltoides. The DRF1 belongs to the DREB gene family and encodes transcription factors playing a key role in water-stress responses. Studying the variation in DRF1, Thiyagarajan et al. [65] found a high similarity between the B and S genome, but also suggested that the two genomes have evolved independently.
Thinopyrum bessarabicum (Savul and Rayss) Á. Löve (JJ syn, EbEb; 2n = 14) could be much more convenient than polyploids for wheat cytogenetic manipulations. Elytrigia. elongata Host Nevski (EE; syn. Lophopyrum elongatum (Host) D.R. Dewey), the diploid tall wheatgrass, grows in salt marshes around the Mediterranean and survived exposure to 500 mM NaCl [55,66]. The hydric stresses tolerance is often different regarding the type of stress and the mechanisms of resistance. For example, Ae. tauschii is more drought-tolerant, while E. elongata is more salt-tolerant than other species in the Triticeae [54,67]. In addition, Dasypyrum villosum (L.) Borbás could be used as a source of resistance carrying, on chromosomes 5 and 6, loci with significant positive effects on salt tolerance [66]. To transfer tolerance from wild relatives to the cultivated species several interspecific crosses have been performed. T. timopheevii (Zhuk.) Zhuk. ssp. timopheevii (GGAA) has been hybridized with Ae. tauschii (DD) to make the synthetic hexaploid (GGAADD) [68,69]. Synthetic hexaploids have been made crossing durum wheat (AABB) and Triticum monococcum L. ssp. monococcum, T. urartu, and T. monococcum ssp. aegilopoides (Link) Thell. [68,69]. The aim was to have lines able to exclude Na+ and a higher K+/Na+ ratio.
3. Morphological and Physiological Response
When plants suffer from hydric stress, significant changes in their morphology can be observed. Usually, hydric stresses affect plant size, which becomes smaller due to a decreasing number of leaves and decreasing area. However, the roots are lengthened searching for water, which results in an increase in the root-to-shoot ratio. This adverse effect of water scarcity on crop plants causes fresh and dry biomass losses. Early maturity or early flowering is another adaptation strategy where a shorter vegetative phase in wheat can help to avoid stress in further very sensitive flowering and post-anthesis grain filling stages [70].
Drought induces a plethora of negative physiological alterations, such as cell turgor loss, reduction in CO2 assimilation, oxidative stress, and nutritional imbalance [63]. Nutritional imbalances due to drought stress occur by decreasing the water uptake and leaf transpiration, combined with alterations in nutrient uptake. Plants try to counteract these effects by activating drought resistance mechanisms, such as accumulation of salts and water, to improve the cell osmotic adjustment and stomata function. K+ and Cl− ion regulation, as well water transport, have been important mechanism for plants under drought. K+ and Cl− are involved in leaf cells osmotic adjustment, with the consequent water retention in cells; stomatal closure prevents water loss [71]. The presence of high ionic concentrations in soil, especially NaCl, is a great challenge for the plant’s physiology. To balance the high sodium concentrations in the soil, plants cells must maintain high concentration of potassium and low concentration of sodium inside the cells.
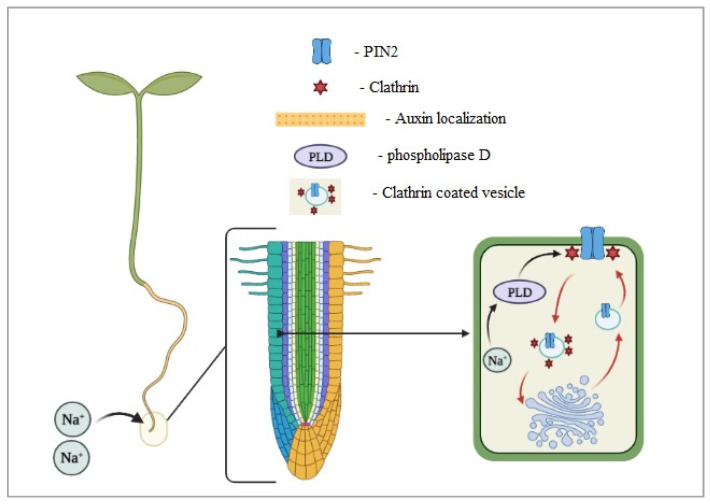
The root system has been studied less than the aboveground morphology. During the juvenile phases, the root architecture is plastic and can adapt to several environmental conditions. It has been detected that roots can escape a high salinity zone, moving to less salty soil. Galvan-Ampudia et al. [72] identified halotropism as the plant’s possibility to reduce their exposure to salinity by moving their root system to an adequate saline environment. The auxin distribution in the root tip affects the root response to salty environments, as summarized in Figure 3 [72]. Furthermore, changes in the PIN subcellular localization affect root auxin transport and can cause various deformities in root architecture, including the size, less lateral roots, root meristem collapse, and others [73].
Figure 3.
Salt-activated phospholipase D (PLD) induces clathrin-mediated endocytosis of PIN2, which allows reorganizing the auxin and roots’ growing direction (modified from Galvan-Ampudia et al. [72]).
Salinity inhibits the root length and reduces the number of roots, but also influences the root growth direction. Sun et al. [74] found that the root direction could change with NaCl increasing; the curved roots could be visible already with a concentration of 100 mM of NaCl in the media. Salt stress reduces gravitropism of root growth, altering the PIN-FORMED2 (PIN2) protein abundance and polar distribution and overly salt-sensitive (sos1 and sos2) genes. Julkowska et al. [75] found that more than 100 genetic loci activated under salt stress are associated with root system architecture changes. Among these, CYP79B2 is correlated with lateral root development under salt stress while HKT1 reduce the lateral root development.
4. Mechanisms of Tolerance
Since plants cannot easily change habitats where conditions are more favorable, to survive and multiply, they evolutionarily adapted, gaining plasticity and specific responses. Generally, plant reactions to abiotic factors include morphological, physiological, biochemical responses, and various adaptation scenarios. In addition, a plant’s response can be either common to many kinds of stresses or specific to each one. Stress-tolerance mechanisms are divided in two macro groups: mechanisms involved in osmotic-stress tolerance and mechanisms involved in ion-stress tolerance [76]. The stomatal adjustment belongs to the first class of mechanisms. Plants under drought and osmotic stresses in non-irrigated systems tend to close their stomata to minimize transpiration, which is reflected in a reduction in growth and production. Exclusion and compartmentalization of toxic ions, avoiding their high concentration in plant cells, are mechanisms of ion-stress tolerance [21]. Each type of physical stress is translated by the plant into a biochemical response, and then into a pathway of interconnected signals with the expression of specific genes that are involved in the stress-tolerance mechanisms. Table 2 lists some of the genes that are candidates for the pathway process and their defense mechanisms for osmotic and ion stresses [21].
Table 2.
Plant salinity-tolerance mechanisms, ordered by processes and their relevance for the three components of salinity tolerance [21].
| Osmotic Stress | Ionic Stress | ||||
|---|---|---|---|---|---|
| Process | Candidate Genes | Osmotic Tolerance | Na+ Excluding | Tissue Tolerance | References |
| Signaling |
SOS3, SnRKs |
Signaling regulation | Activation of ion antiporter | Regulation of vacuolar loading | [77] |
| Photosynthesis |
ERA1, PP2C, AAPK, PKS3 |
Stomatal closure regulation |
Protection of chloroplast from ion toxicity |
Delay Na+ toxicity effect in chloroplast |
[78,79] |
| Accumulation of Na+
in shoots |
HKT, SOS1 | - | Decreasing long distance transport of Na+ |
Decreasing energy used on Na+ exclusion | [80,81,82] |
| Accumulation of Na+in vacuoles | NHX, AVP | - | Increased sequestration of Na+
into root vacuoles |
Increased sequestration of Na+
into leaf vacuoles |
[80,83] |
| Accumulation of organic solutes |
P5CS, OTS, MT1D, M6PR, S6PDH, IMT1 | Increasing osmotic adjustment | Reduction of Na+ accumulation | Accumulation of organic solutes in cytoplasm | [82,84] |
4.1. Osmotic Adjustment
Plants have an initial general response to stress by abscisic acid (ABA) biosynthesis in roots as a signaling molecule due to interactions with different receptors; it may activate the general or very specific response to a particular type of stress [85]. After ABA, the first plant-specific response to hydric stresses is to maintain the cell water content, by increasing osmotic force, named osmotic adjustment (OA), using various mechanisms described by Blum [86]. Molecules involved in osmotic adjustment signaling pathways regulate tolerance against osmotic stress via control of stomatal conduction, with the consequent accumulation of organic solutes in the cytoplasm, in order to decrease the osmotic potential of the cytosol.
The main mechanisms of defense against osmotic stress predict the accumulation of useful solutes and the use of polyamines. Among the first are proline, glycine betaine, sugars, and polyols.
The proline content increases under salt stress at the intracellular level and acts as a reserve of organic nitrogen during the stress period. Deivanai et al. [87] highlighted how rice treated with proline improves its response under salt stress.
Glycine betaine, known also as trimethyl glycine (TMG), is a quaternary ammonium compound with three methyl groups derived from glycine found in many plants and microbes. The TMG is electrically neutral on a wide range of pH and highly water-soluble, but it also contains groups of non-polar methylins. Due to its unique structural characteristics, it interacts with both hydrophobic domains and hydrophilic macromolecules, such as enzymes and protein complexes. Glycine betaine increases the osmolarity of the cell during the period of stress [88], stabilizes the proteins [89], protects the photosynthetic apparatus from stress damage [90], and then plays an important role in stress mitigation [91].
Sugars: Plants under saline stress tend to accumulate carbohydrates that play a role in osmo-protection and energy reserves during the stress phases [92].
Polyols are chemical compounds composed of multiple oxydrilic groups available for organic reactions. They are classified into two types: cyclical (e.g., pinitol) and acyclic (e.g., mannitol). Polyols acts as protectors or enzyme stabilizers when stress related to dehydration occurs [93].
The polyamines have a low molecular weight and are widely spread in the plant kingdom, playing various roles such as the regulation of somatic embryogenesis, cell differentiation, morphogenesis, seed germination, the development of flowers, and fruits and their senescence. Polyamines are associated with gene regulation processes, which are involved in the synthesis of solutes capable to maintain cell membrane integrity and related to the accumulation of Na+ and Cl− ions [94].
4.2. Ionic Homeostasis
The response reaction as ionic homeostasis mainly focuses on the exclusion of the Na+ ion, avoiding toxic concentrations in plant tissue [21]. Plants can avoid the harmful effect of ions by reducing their accumulation in shoots or by transferring them into vacuoles [95]. One of the responses to ion stress was identified in the SOS (Salt Overly Sensitive) stress signaling and tolerance pathway. It has been shown that Arabidopsis thaliana respond to salt mainly through the SOS signal path, which consists of three components involved in ionic homeostasis. SOS1 encodes a plasma membrane Na+/H+ “antiporter” protein (i.e., a protein involved in the secondary active transport of different molecules through the membrane), which plays a critical role in sodium extrusion [96]. SOS2 encodes a kinase protein [97], while SOS3 encodes a Ca2+-binding protein that acts as a calcium sensor for salt tolerance [98].
Salinity produces two independent types of secondary responses in plants. The limited plant growth could depend on the osmotic effect of salt in the soil or to the accumulation of sodium ions within plant cells. Generally, osmotic stress has an immediate and great effect with a decrease in the growth of new shoots. Ionic stress acts secondly with milder effects on plants and only in environments with a high salinity rate, or on species highly sensitive to it, increasing the senescence of older leaves [21]. Munns [99] explained how hydric stress acts over time. As reported in Table 3, after a few minutes and up to a few hours after NaCl was imposed, plants undergo osmotic stress that slows down the growth rate of the leaves and roots, as the salt tends to seize the available Ca2+, seriously compromising root growth.
Table 3.
Timing of the plant’s response to salinity after the stress was imposed. The effects on a salt-tolerant plants are fundamentally identical to those due to soil water deficit (Reprinted with permission from ref. [99]. 2002, John Wiley and Sons).
| Time | Water Stress Effect (Salt-Tolerant Plants) | Salt-Specific Effects Salt-Sensitive Plants |
|---|---|---|
| Minutes | Immediate reduction in leaf and root elongation rate and then rapid partial recovery | |
| Hours | Constant but reduced rate of leaf and root elongation | |
| Days | Leaf growth more affected than root growth; Reduced rate of leaf emergence | Visible injury in the oldest leaf |
| Weeks | Reduced the final size of the leaves and/or the number of side shoots | Death of older leaves |
| Months | Altered flowering time, reduced seed production | Younger leaves dead, plants may die before the seed matures |
Rodriguez et al. [100] revealed that root growth was not compromised if Ca2+ was also administered along with NaCl. After a few days under saline stress conditions, the plants encounter ion stress, due to the accumulation of sodium ions and/or chlorine ions in the leaves’ cells, thus compromising mainly the epigea part of the plants. After weeks or months under saline stress, plants reduce the number and size of the new shoots, and the older ones start to yellow until death. It seems that only plants with an inner capacity to produce more shoots have a better chance to be maintained under adverse conditions, as they still have sufficient photosynthesis tissues. However, after weeks and months under stress, plants may also face death before the maturation of the seed. In cereals, it reduces the number of florets per ear, and alters the time of flowering and hence maturity [99].
4.3. Redox Homeostasis
Water shortages also cause an increase in the reactive oxygen species (ROS) levels and induce cellular redox homeostasis. Therefore, to avoid the harmful effects of these molecules, as a defense mechanism plants increase production of enzymatic and non-enzymatic antioxidants. The genes responsible for antioxidant activity are very important as candidates for breeding programs to increase abiotic stress tolerance. In plants under drought and/or saline stresses, deregulation or even disruption of the electron transport chain in chloroplasts and mitochondria can occur. This involves the formation of oxidizing molecules because oxygen acts as an electron acceptor, creating compounds such as hydrogen peroxide, radical oxydrile, or radical superoxide, which can damage cellular integrity [101]. Plants can defend against the ion stress with antioxidant molecules, including among the most important nitric oxide (NO) [96]. NO not only is involved in the regulation of various processes of plant growth and development, but it also reacts with free lipid radicals, protecting the oxidation of lipids. Furthermore, the NO helps in the activation of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), ascorbate peroxidase (APX), and glutathione reductase (GR) [102].
5. Genes and Transcription Factors Involved
State-of-the-art molecular genetic approaches have provided valuable insights about the plant’s response to environmental change, and signaling pathways to activate mechanisms of adaptation. Moreover, identification of several genes associated with stress adaptation has led to rational breeding programs.
Hereafter, more than 100 genes associated with tolerance to hydric stresses in the Triticeae sp. are described. They are presented in the Supplementary Tables S1–S3, together with characteristics such as Accession Nr., dimension (bp), Annotation, Function, Primers, and reference. These genes belong to several groups of responses with different biological functions, and for easier discussion, we grouped them into osmotic adjustment (Table S1), ionic (Table S2), and redox homeostasis (Table S3).
In plant cells, the adaptation to adverse conditions begins from signal perception to the production of functional proteins through activation of the target genes. Understanding of a given gene function, and expression pattern under hydric stress conditions, is particularly important for efficient selection of new tolerant genotypes in classical breeding.
5.1. Genes Involved in Hydric Stress Tolerance
Sixty-nine genes involved in osmotic adjustment (OA) are reported in Table S1. These genes are responsible for defense mechanisms against osmotic stress via control of stomatal conductance; accumulation of organic solutes in the cytoplasm to decrease the osmotic potential of the cytosol; and ionic balance, i.e., the Na+/K+ ratio (the accumulation of sodium can be used for OA, especially if it is balanced by the accumulation of potassium). The initial general plant response to hydric stress is abscisic acid (ABA) biosynthesis in roots, which, as a signaling molecule due to interactions with different receptors, may activate the different mechanisms of tolerance [85].
The ABA phytohormone accumulation is one of the main and most common ways to inform plant tissues and cells about changes in environmental conditions. Therefore, genes involved in ABA synthesis play an essential role in the perception and transformation of information about adverse conditions. For example, the accumulation of ABA, and therefore increased expression of the TaNCED1 gene isolated from Triticum aestivum, significantly improved drought tolerance in transgenic tobacco plants [103]. In addition, a previous study has shown that oxidative cleavage of cis-epoxycarotenoids catalyzed by NCED (9-cis-epoxycarotenoid dioxygenase) is the critical step in the ABA biosynthesis [104]. Phylogenetic data showed that TaNCED1 has the highest (95%) identity with the barley HvNCED1 gene [103]. The genes involved in the ABA biosynthetic pathway are potential candidates for breeding to improve plants tolerance to various stresses.
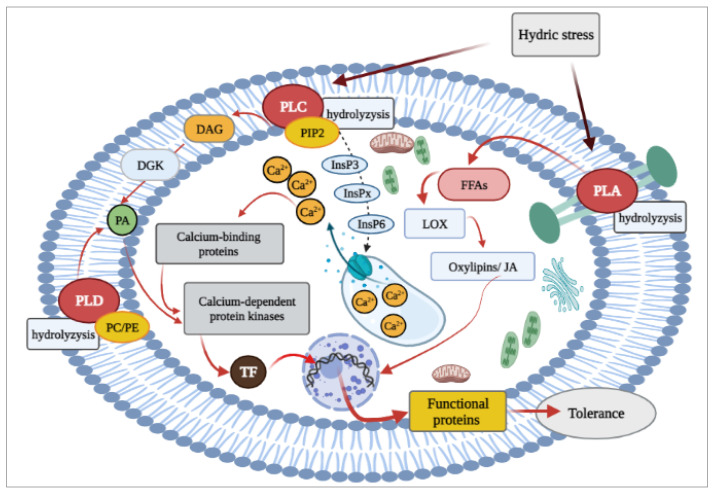
Furthermore, plants can transmit information about stress conditions through the lipid-dependent signaling pathway (Figure 4). Three phospholipases (A, C, and D) involved in this pathway produce secondary signaling messengers, which play important roles in plant response and tolerance under hydric stress [105]. Phospholipases D catalyze the hydrolysis of phospholipids (PC—phosphatidylcholine, and PE—phosphatidylethanolamine) to produce phosphatidic acid (PA), which is a very important second messenger that modulate many cellular processes, including the signaling pathways related to plant defense, and tolerance [106].
Figure 4.
The general scheme of plant cell response to hydric stress in the lipid-dependent pathway: signal perception, transduction, and activation of the target genes. ABA—abscisic acid, PLD—phospholipases D, PC—phosphatidylcholine, PE—phosphatidylethanolamine, PA—phosphatidic acid, PLC—phospholipase C, PIP2—phosphatidylinositol bisphosphate, IP3—inositol triphosphate, DAG—diacylglycerol, DGK—diacylglycerol kinase, PLA—phospholipase A, FFAs—free fatty acids, LOX—lipoxygenase pathway, JA—jasmonic acid, TF—transcription factors.
Another way to produce phosphatidic acid (PA) and transmit information about the environmental changes is through the regulation of the calcium ion concentration in the cytosol, via the phospholipid pathway [107]. The hydrolysis of phosphatidylinositol bisphosphate (PIP2) by phospholipase C produces diacylglycerol (DAG) and inositol triphosphate (InsP3). In animals, IP3 is responsible to release calcium, while in plants this function is related to IP3 and his derivative IP6 (Figure 4) [108,109].
Several studies reported that the TaPLC1 gene is involved in plant response to cold, salt, and drought stresses. It encoded phospholipase C (PLC), a specific enzyme that mediates abscisic acid signal transduction in guard cells via increasing the cytoplasmic Ca2+ [110,111,112]. Moreover, it was found that a reduced level of phosphoinositide-specific phospholipase C in guard cells is associated with the inhibition of stomatal opening by ABA [112]. The importance of this gene for plant tolerance is demonstrated by its prevalence in the plant kingdom from GenBank: Arabidopsis AtPLC1 (AT5G58670) common tobacco NtPLC1 (AF223351.1), rice OsPLC1 (AJ276277.2), cowpea (U85250.1), and Lilium davidii LdPLC1 (AY735314.1).
Another pathway is the lipoxygenase-related (LOX) pathway and its metabolites, oxylipins and jasmonates, produced from free fatty acids (FFAs) that is released free by phospholipase A (PLA) from the membrane phospholipids (Figure 4). The LOX pathway based on polyunsaturated fatty acids oxidation, such as α-linolenic acid, is particularly important since it represents a substrate in the synthesis of the phytohormone jasmonic acid (JA).
Moreover, it has been demonstrated that biotic and abiotic stresses induce the genes involved in α-linolenic acid metabolism [105,113]. The first allene oxide cyclase (AOC) gene was isolated from T. aestivum cultivar SR3 (cross between Cv. JN177 and tall wheatgrass Thinopyrum ponticum). TaAOC1 copy was 720 bp in length and was transcribed in all tissues even with different degrees. Zhao et al. [113] detected the highest levels of this gene at the booting stage, particularly in roots, but also in other aerial organs. Interestingly, during the anthesis, a high level of TaAOC1 transcription was detected in the awn and ears [113]. Moreover, superoxide dismutase (SOD) activity was increased in transgenic plants constitutively expressing TaAOC1, indicating that the regulation of ROS homeostasis is activated by jasmonic acid (JA) to increase salinity tolerance.
As mentioned above, phospholipase C (PLC) activity releases calcium ions into the cytosol (Figure 4) and increases the amount of these ions, raising the interaction with “Ca2+ sensors families” such as calmodulins (CaM), CaM-like proteins, calcineurin B-like proteins (CBLs) and their interacting kinases (CIPKs), and Ca2+-dependent protein kinases (CDPKs) [114]. The Ca2+ ions bind with calcium-binding EF-hand proteins, which consist of a helix–loop–helix structure, and an interhelical loop of 12–14 amino acids that bind calcium ions. One of these calcium-binding EF-hand proteins—TaCab1—was isolated and characterized from wheat leaves, and is upregulated by biotic and abiotic stresses [115]. Moreover, high affinity calcium-binding proteins CRT (calreticulin protein), which was found in several plant species, is responsible for the hydric stress tolerance in crops [116]. In T. aestivum, a full-length cDNA 1446 bp was isolated, encoding calreticulin protein namely TaCRT [117], which has high homology with other plants’ CRT. Jia et al. [117] underline the role of TaCRT in drought tolerance, and found a higher water-use efficiency (WUE), water retention ability (WRA), relative water content (RWC), and lower membrane damaging ratio (MDR) under water-stress conditions. Besides, three TaCRT genes, named TaCRT1, TaCRT2 and TaCRT3-1, have been identified in wheat. The all three genes were strongly induced under salt stress, but exhibited different expression patterns in different tissues. Their localizations were on 2 L, 5 L, and 3 L, respectively, with additional homologous copies in the three genomes A, B, and D [116]. TaCRT1 with an open reading frame of 1287 bp is involved in defense responses and stresses tolerance in wheat. Moreover, Xiang et al. [116] detected an association between TaCRT1 and the superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activities, which suggest that they may be included in redox homeostasis pathways.
Calcium-binding proteins change their conformation after binding calcium ions, and this transformation leads to activation of various calcium-dependent protein kinases [114]. The SNF1-related protein kinase SnRK2 subfamily, belonging to Ser/Thr protein kinase class, has shown to be responsible for signal transductions in various stresses. Overexpression of the SnRK2 subfamily genes, such as TaSnRK2.4, TaSnRK2.8, and W55a, enhanced osmotic potential in transgenic Arabidopsis, and they can be used in breeding to improve osmotic adjustment in wheat species [118,119,120]. Subcellular localization showed that both TaSnRK2.4 and TaSnRK2.8 are presented in the cell membrane, cytoplasm, and nucleus, proving that they are responsible for signal transmission in plant cell. The most highly homologous in the GenBank library for these genes are members of the rice SnRK2 subfamily: W55a-OsSAPK1 (90.38%), TaSnRK2.8-OsSAPK8 (94.8%), and TaSnRK2.4-OsSPAK4 (92.5%).
Other protein kinases, such as TaSK5 and TaGSK1, belong to the glycogen synthase kinase–shaggy kinases family, and they are involved in signal transmission in a stressful condition. The structure of these protein kinases contains both a Ser/Thr protein kinase catalytic domain and phosphorylation site, and they are signal transmitters in plant response to salt stress [121,122]. In common wheat, the Leucine-rich repeat receptor-like serine/threonine-protein kinase TaER-B1 was strongly expressed, and upregulated by numerous environmental stresses, including drought and salinity [123].
Among the protein kinases TaABC1 (Table S1), a member of the ABC1 family was identified in T. aestivum with an activity on the BC1 complex. Members of the kinase family have in common a conserved domain, but they have differences that lead to different localizations of ABC1 proteins and probably cause differences in their functions. Wang et al. [124] studied the effects of TaABC1 protein on transgenic Arabidopsis, underling its role in reducing water loss and increasing osmotic potential, photochemistry efficiency, and chlorophyll content. Moreover, TaABC1 is related to the expression of DREB1A, DREB2A, RD29A, ABF3, KIN1, CBF1, LEA, and P5CS, which are well-known stress-responsive genes.
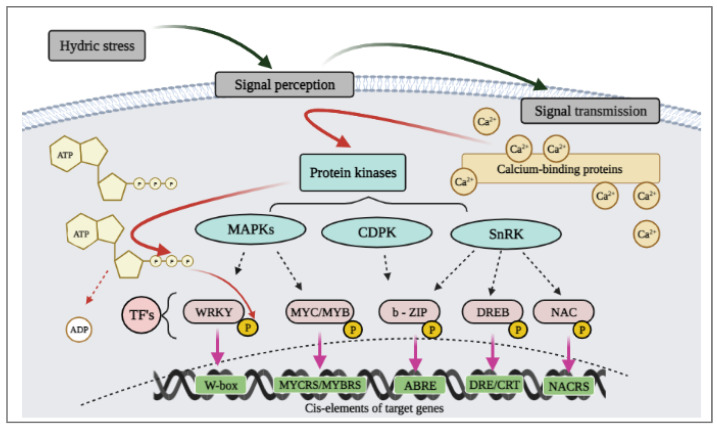
The plants require the expression of a large number of genes and specific transcription factor families (TFs) to activate their tolerance mechanisms. Among these, an important role is played by protein kinases, which use adenosine triphosphate (ATP) as a donor of the phosphate group for transcription factor phosphorylation (Figure 5). This mechanism has been identified as a plant response to abiotic stresses, which allows a fast plant response via fast switching transcription factors from the dephosphorylated state to phosphorylated state and back [125]. Together with protein kinases in TF activation phosphatases, ATP and/or ADP are involved.
Figure 5.
The general scheme for the activation of transcription factors by different protein kinase families in response to hydric stress. TFs—transcription factors, MAPKs—mitogen-activated protein kinases, CDPK—calcium-dependent protein kinases, SnRK2—serine-threonine kinases.
According to the TFs’ DNA-binding domain characteristics, they were divided into multi-gene families (AP2/EREBP, bZIP, MYB/MYC, NAC, and WRKY) [125]. Moreover these families are phosphorylated by various protein kinases families (Figure 5), for example MYB/MYC and WRKY by mitogen-activated protein kinases (MAPKs), while NAC and DREB by serine-threonine kinases (SnRK2), and bZIP also by SnRK2 and some calcium-dependent protein kinases (CDPKs) [125].
The AP2/EREBP family has a domain structure divided into four subfamilies: AP2, RAV, ERF, and DREB. The AP2 transcriptional factor subfamily has two AP2 domains (RAV one AP2) and one B3 domain, only one AP2 domain characterizing DREB and the ethylene-response transcription factor (ERF) subfamilies [126]. The overexpression genes, such as TaDREB1, TaDREB6, and WDREB2, in transgenic plants enhanced the tolerance to abiotic stresses, such as drought, high salinity, and freezing, via the activation of stress-inducible genes (LEA/COR/DHN) with DRE/CRT cis-acting element in their promoter region [126,127,128].
The bZIP proteins belongs to the most large and diverse TF superfamily, classified into 14 subgroups, and carrying a highly conserved bZIP domain, which include a basic region and a leucine zipper. Members of bZIP family that carry ABA response element binding factors (AREBs) belong to subgroup-A, and are involved in the abscisic acid signaling response. These transcription factors regulate the expression of the genes responsible for drought tolerance and have the ABA-responsive cis-element (ABRE), as does numerous of the LEA family genes, such as the cold regulated (COR), responsive to dehydration (RD), early responsive to dehydration (ERD), and responsive to ABA (RAB) genes [129]. For example, in wheat bZIP, the gene WABI5, from group A, regulated the COR/LEA genes in stress responses such as freezing, osmotic, and salt stresses [130]. The other bZIP-type transcription factors from group S, such as Wlip19 and TaOBF1, and their direct protein–protein interaction, also act as a transcriptional regulator of the COR/LEA genes for stress tolerance [131]. However, the bZIP transcription factor TaABL1 regulates the expression of other LEA family genes, such as the osmotic adjustment-related function genes RD29B (responsive to dehydration), RAB18 (responsive to ABA), and other stress-related genes, which control stomatal closure in transgenic Arabidopsis [132]. TFs from the subfamily of basic leucine zipper (bZIP) might be useful genetic resources for breeding tolerant genotypes.
One important and large subfamily of TFs MYB/MYC involved in response and tolerance to various stresses, and characterized by a DNA-binding domain, consist of helix–turn–helix (HTH) or basic helix–loop–helix (bHLH) domains [125]. The overexpression of MYB TFs, such as TaMYB19-B, TaMYB56-B, and TaMYBsdu1, cause the expression of a number of abiotic stress-related genes, and are important regulators involved in wheat adaptation to water scarcity [133,134,135]. A group of MYB genes respond to hydric stress (TaMYB1, TaMYB29, TaMYB34, TaMYB57, and TaMYB72) were isolated and identified in 60 wheat MYB genes during abiotic stress expression analyses [136]. In addition, the overexpression of this family members, such as TaMYB32, TaMYB33, and TaMYB73, which were specific salt-inducible genes, enhanced tolerance to salt stress in transgenic Arabidopsis [136,137,138]. Moreover, TabHLH39, as an MYC transcription factor, consists of the basic helix–loop–helix (bHLH) domain, and overexpression of this TF improved tolerance to drought, salt, and freezing in transgenic Arabidopsis [139]. MYB/MYC family proteins are crucial in plant stress responses and may improve crop tolerance to hydric stress.
A further large superfamily belongs to plant-specific WRKY transcription factors; its specific binding domain consists of a conserved WRKYGQK sequence followed by a zinc-finger motif. The importance of WRKY in abiotic stresses tolerance is shown via the regulation of some stress-responsive genes with specific cis-acting element W-box in their promoters, specific for the WRKY binding domain [140]. For example, TaWRKY2 binds the promoter of gene RD29B (dehydration inducible gene), and TaWRKY19 binds a few more, RD29A and COR6.6, which led to increased tolerance to water deficit in transgenic Arabidopsis [141]. Moreover, the overexpression of TFs TaWRKY10 and TaWRKY44 in transgenic tobacco plants significantly activated the genes involved in osmotic adjustment and ROS scavenging, improved tolerance to hydric stresses via proline and other soluble sugar accumulation, and enhanced the antioxidant system [142,143]. Besides that, WRKY TFs impart tolerance to abiotic stresses, they also control plant development and growth, such as TaWRKY13 and TaWRKY80, which improved the root development characteristics together with an increased proline level in transgenic Arabidopsis [144,145].
Another plant-specific TF superfamily proteins feature is the NAC domain (for NAM, ATAF1/2, and CUC2) in the N-terminal region and various C-terminal sequences [125]. Multiple abiotic stresses in transgenic Arabidopsis cause overexpression of NAC TFs, such as TaNAC2, TaNAC29, TaNAC47, TaNAC67, and TaNAC69, which, in turn, improve dehydration tolerance via controlling expression of some abiotic stress-response genes [146,147,148,149,150]. Moreover, the NAC family members TaNAC4 and TaNAC8 overexpression in wheat were induced not only by abiotic stimuli but also by stripe rust pathogen infection. Both of them contained a plant-specific NAC domain in the N-terminus and transcriptional activity in the C-terminal region [151,152].
5.2. Stress Tolerance-Related Genes and Functional Proteins
The LEA3 proteins belong to the big “late embryogenesis abundant” proteins family, whose function is usually associated with maintaining the water content in plant cells under abiotic stresses [153]. These proteins, according to their structure, are characterized by an 11-mer amino acid motif (TAQAAKEKAGE), which are important in formatting protein α-helical during dehydration [154]. It was shown that transgenic Phellodendron amurense overexpressing wheat TaLEA3 gene were tolerant to water deficit by fast stomatal closure [155]. Moreover, overexpressing LEA3 family genes, WZY3-1 and TaLEA3, effectively enhanced the drought and salinity tolerance in transgenic plants due to protecting the cell membrane from damage, enhancing photosynthetic efficiency, and reducing ROS [156,157,158]. LEA3 family genes Wrab18 and Wrab19 encoded cold-responsive LEA/RAB-related COR proteins, which are induced under salt and drought stresses, too [159]. Both genes contain core ACGT motifs in the promoter regions and are direct target genes for b-ZIP type transcription factor WLIP19 [131].
The late embryogenesis abundant proteins (LEA2) family, also named dehydrins, according to a combination of conserved segments, were subdivided into seven groups: KS, SK3, YSK2, Y2SK2, Kn, Y2SK3, and YSK3 [160]. SK3 dehydrin type genes TaDHN2.1, TaDHN2.2, and TaDHN2.3 encode cold acclimation proteins, which protect the plasma membrane against freezing and dehydration stress via drought or salinity. Genes belonging to the YSK2 group encode dehydrins TaDHN6, TaDHN11, and TaDHN17, which their transcript levels were relatively higher only under dehydration stress. Moreover, dehydrins from the same group, TaDHN9 and TaDHN17, induced expression under salt stress. Dehydration also induced expression of dehydrins from group Kn, such as TaDHN18 and TaDHN23. Furthermore, expression of dehydrin TaDHN7, from the Y2SK2 group, was induced by both dehydration and salt [160]. Dehydrins genes are valuable for breeding programs; for example, overexpression of DHN5 in transgenic plants increases tolerance to drought and salinity stress via osmotic adjustment [161].
Aquaporins belongs to another important group of functional proteins and it is responsible for regulating fast transmembrane water flow in plants. According to the amino acid sequence, homology, and protein subcellular localization, water-selective channel proteins are grouped into subfamilies, such as the nodulin 26-like intrinsic proteins (NIPs), the tonoplast intrinsic proteins (TIPs), the plasma membrane intrinsic proteins (PIPs), and the small basic intrinsic proteins [162]. The overexpression of the gene TaNIP (Triticum aestivum L. nodulin 26-like intrinsic protein) resulted in higher salt tolerance of transgenic Arabidopsis. Gao et al. [163] considered that the TaNIP gene induces plant salt tolerance mostly due to controlling the K+/Na+ ratio and Ca2+ concentrations, but not by proline accumulation. However, the TaTIP2;2 (tonoplast intrinsic protein) overexpression reduced P5CS1 expression and decreased the osmotic tolerance of transgenic Arabidopsis, due to the suppression of proline synthesis [164]. It has also been suggested that this gene may be a negative regulator of stress, due to the downregulation of P5CS1 and other stress-tolerance-related genes [164]. Thus, aquaporin may be involved not only in fast water transport but also as a secondary function–intermediate regulator in processes related to the osmotic adjustment. For example, the PIP2 subgroup genes TaAQP7 and TaAQP8 provide drought and salinity tolerance in transgenic tobacco not only by controlling a better water state but also by inducing expression of antioxidant enzymes, which reduces the ROS levels and membrane damage [165,166]. Such a contrary response of the PIP subfamily genes to stress may be related to their subcellular localization. Osmotic adjustment is positively related to the TaAQP7 and TaAQP8 genes products, which are localized on the cell plasma membrane, while it is negatively related to TaTIP2; two products which are localized on the endomembrane system.
Among the many different mechanisms of defense against osmotic stress, there is the accumulation of useful solutes such as proline, glycine betaine, sugars, and others. The plants can regulate the amount of amino acid L-proline in two ways, either by increasing its synthesis or by inhibiting its degradation. Proline acts not only like an osmoprotectant, but it is also involved in stabilizing membranes, enzymes, cellular structures, and reducing reactive oxygen species (ROS) [167]. Therefore, genes involved in the proline synthesis, such as P5CS (pyrroline-5-carboxylate synthetase), P5CR (pyrroline-5-carboxylate reductase), and degradation PDH (pyrroline-5-carboxylate dehydrogenase), are very useful for drought tolerance and could help to enhance the yield of wheat under salinity stress [84].
5.3. The Specific Genes Involved in Ionic Homeostasis
Twenty-five genes involved in the Salt-Overly-Sensitive (SOS) signal transduction pathway are reported in Table S2. These genes are responsible for the regulation of the ion transport system that facilitates ion homeostasis [168]. Calcineurin B-like (CBL) proteins act as a Ca2+ ion sensor and play an important role in signal transduction in response to various environmental stimuli. Some CBL family proteins (Table S2) are responsible for very specific signaling pathways that ensure ionic homeostasis under salt stress. Moreover, a complex of CBL proteins with their protein-interacting protein kinases, CIPKs, have a crucial regulatory function in ionic homeostasis via the SOS salt stress-signaling pathway. At the beginning of this pathway, calcium-binding protein SOS3, activated by Ca2+ ions, formulate a complex with SOS2 (sucrose non-fermenting-related serine/threonine protein kinase), ready to activate the SOS1 gene. The SOS1 gene encodes a putative Na+/H+ anti-porter [169], which regulate the circulation of the Na+ ion from shoots to roots and accumulate it into the vacuoles [170,171]. Such CBL/CIPK complexes were identified in wheat, and the candidate gene for the calcium-binding protein SOS3 is TaCBL4, the orthologue of AtSOS3, which strongly interacted with six candidates genes for SOS2, TaCIPKs (TaCIPK3, 5, 14, 15, 26, and 31) [77]. It was also found that other calcium-binding proteins, TaCBL1 and TaCBL9, strongly interacted with five (TaCIPK3, 5, 14, 15, and 25) and two (TaCIPK11 and 31) CBL-interacting protein kinases, respectively [77]. In wheat, these complexes can activate H+/Na+ (SOS1) anti-porters, as plasma membrane TaSOS1, or vacuole TNHX1 and TaNHX2 [80,81,83].
In order to keep active the Na+/H+ anti-porters (SOS1), and remove the sodium ions into the vacuole in exchange for H+, the H+ gradient generated by the vacuole H+-ATPase and H+-pyrophosphatase (H+-PPiase) is crucial. In turn, it provides energy for successful exchanges. Among the three classes of H+-ATPase’s in plants, the vacuolar H+-ATPase (V-ATPase) is the most complex one, consisting of two sub-complexes: the peripheral V1, responsible for ATP hydrolysis, and the membrane-integral V0 complex, responsible for proton translocation. In wheat, each sub-complex consists of a number of subunits, whose overexpression evokes tolerance for salinity in transgenic Arabidopsis [172]; for example, subunits A, D, and G from the sub-complex V1, are responsible for ATP hydrolysis and H+ transport, and sub-units C, F, and H are responsible for the stabilization of sub-complex V1 and its connection with V0 [173]. As mentioned earlier, vacuole H+-pyrophosphatase (H+-PPiase) generates a proton gradient and controls Na+/H+ transport, but uses the energy for hydrolysis of pyrophosphate (PPi) molecules [80].
High-affinity K+ transporters (HKT) belong to another gene family that helps regulate potassium transportation, but that are not involved in the SOS pathway. They are common in many plant species, including Arabidopsis [174], rice [175], barley [176], and wheat [28,177]. HKT proteins transport monovalent cations through the electrophysiological gradient, and they have been divided into two subfamilies. The first subfamily in the first pore loop of the protein have a serine (S-G-G-G) and make them more selective for the Na+ ion than subfamily 2 members, which have glycine (G-G-G-G) in the first loop of the protein [178]. Laurie et al. [179] have shown that downregulation of TaHKT2;1 (TaHKT1) in wheat increased the shoot fresh weight from 50% to 100% under 200 mM NaCl treatment and K+ deficiency. TaHKT2;1 expressed in the root, including root hairs, perform as Na+/K+ symporters at low Na+ concentrations, and a Na+ uniporter at high Na+ concentrations [178,179]. One more gene induced by salt stress, and not involved in the SOS pathway, TaSOS4, encodes two pyridoxal kinases (PL), and their function is to convert vitamin B6 to pyridoxal 5′-phosphate (PLP) [180]. PLP in plants is the pivotal cofactor for various enzymes that are involved in biosynthesis of chlorophyll, ethylene, de novo sphingolipid, and metabolism of amino acids and carbohydrates [180]. In wheat, the gene encoding cytoplasmic pyridoxal kinase TaSOS4 was identified with the amino acid sequence that is 78% identical to Arabidopsis AtSOS4 [180].
5.4. The Genes Associated with Reduce Reactive Oxygen Species (ROS)
The plant response to oxidative stress start with producing antioxidant enzymes and a non-enzymatic antioxidant, which reduces the harmful effect of reactive oxygen species (ROS) [181]. Ten genes involved in the ROS are reported in Table S3, with their relative reference, accession number, dimension (bp), annotation, function, and primers usable to amplify. The main enzymatic antioxidants involved in the scavenging of ROS are superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), glutathione peroxidase (GPX), and peroxidase (POX).
Among the enzymes with antioxidant activity, the superoxide dismutase’s (SODs) serve as the first line of defense against abiotic stress and ROS [182]. Moreover, drought and salinity in T. aestivum affect differently mitochondrial TaMn-SOD (manganese superoxide dismutase) and cytosolic TaCu/Zn-SOD (Cu/Zn-superoxide dismutase). After the long-term effects of salt stress on Triticum aestivum, the total SOD activity was the highest in the salt-tolerant line chloroplastic fraction followed by mitochondrial, and the lowest in the cytosolic fraction [183,184].
Ascorbate and glutathione are two essential non-enzymatic compounds, reducing the harmful effect of oxidative stress via detoxification of hydrogen peroxide. Enzymes such as ascorbate peroxidase TaAPX and glutathione peroxidases W69 and W106 play key roles in maintaining the ascorbate and glutathione contents in plants [185]. Sairam and Saxena [186] determined that water-stress-tolerant wheat genotype PBW 175 had the highest ascorbate peroxidase, glutathione reductase, and peroxidase activity, as well had the lowest lipid peroxidation and highest membrane stability under water stress. Glutathione peroxidase (GPX) genes from wheat W69 and W106, localized in chloroplasts, showed their peroxidase activity in vitro, and their overexpression, enhanced tolerance to salt, and H2O2 in transgenic Arabidopsis [187]. To decrease the harmful effects of H2O2, organic hydroperoxide, and lipid hydroperoxide, the GPX uses glutathione (GSH) or thioredoxin (Trx) as the reducing agents. Moreover, the overexpression of Ta-sro1 (poly (ADP ribose) polymerase) in wheat promotes the activity of these ascorbate-GSH cycle enzymes and are involved in maintaining the genomic integrity, which allows plants to regulate redox homeostasis under salinity stress [188].
Peroxidases (PRXs) are also involved in various responses to abiotic stresses. TaPRX-2A gene, which is a member of the wheat class III peroxidase gene family, was recently cloned and characterized by Su et al. [189]. They detected an improved salt tolerance in wheat, with its main expression level located in the root tissues, with 1026 bp an open reading frame (ORF); furthermore, they showed that transgenic wheat plants with TaPRX-2A-overexpressed have also higher activities of other genes involved in the redox mechanisms. Moreover, wheat catalase TaCAT activities in leaves retain their water status, associated with resistance to drought, and preventing the grain yield components from being compromised [190].
Non-enzymatic antioxidant flavonoids belong to a large family of phenolic compounds, protecting plants against various environmental stresses [191]. The flavonoids’ biosynthesis is regulated genetically and controlled by key enzymes, one of them flavanone 3-hydroxylase, which is encoded by gene F3H1. Moreover, the F3H1 belongs to the ‘early’ flavonoid synthesis gene family; its expression patterns under stress conditions can regulate the flavonoid biosynthesis pathway and produce antioxidants with high efficiency [192].
Plant 12-oxo-phytodienoic acid reductases (OPRs) that catalyze the reduction of double bonds in α,β-unsaturated aldehydes, and ketones, do not interact with jasmonate synthesis nor the jasmonate signaling pathway, but are dependent on the abscisic acid (ABA) regulation signaling pathway [193]. In T. aestivum, TaOPR1 (oxophytodienoate reductase), enhanced salinity and drought tolerance, via promoting activity in an ABA-dependent pathway and Reactive Oxygen Species Scavenging [194]. Out of the 1347 bp full-length TaOPR1 includes two untranslated regions at the 5′ and 3′ ends of 60-bp and 69-bp, respectively; while, the central bp open reading frame is of 1110-bp. The analyses with aneuploid stocks revealed its location on chromosome 2BS [193].
6. Conclusions
Wheat, like many other plants, reacts to hydric stress, activating several mechanisms, and these mechanisms involve several genes. The response could vary in accord with (i) the hydric stress typology (drought versus salinity); (ii) the plant stage; and (iii) the stress intensity. Knowledge and the understanding of the mechanisms involved in stresses tolerance, together with the genes activated by the stress, help the selection and use of plants suitable for cultivation in location with water scarcity (quantity and quality). This review aims to be a reference to understand these mechanisms and to have a clear list of the most important genes involved in conferring plant tolerance to hydric stresses. All the information here present are useful to properly run breeding programs to face climatic change either using classical breeding, helped by the specific molecular markers, or by using advanced biotechnological tools, able to transfer specific genes from a species to another.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22126378/s1.
Author Contributions
Drafted different manuscript parts, review and editing of the entire manuscript, I.U., L.B. and M.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mann M.E., Gleick P.H. Climate change and California drought in the 21st century. Proc. Natl. Acad. Sci. USA. 2015;112:3858–3859. doi: 10.1073/pnas.1503667112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam W.H., Hayes M.J., Svoboda M.D., Tadesse T., Wilhite D.A. Drought hazard assessment in the context of climate change for South Korea. Agric. Water Manag. 2015;160:106–117. doi: 10.1016/j.agwat.2015.06.029. [DOI] [Google Scholar]
- 3.Bot A., Benites J. Drought-resistant soils: Optimization of Soil Moisture for Sustainable Plant Production; Proceedings of the Electronic Conference ‘Drought-Resistant Soils. Optimization of Soil Moisture for Sustainable Plant Production.’ FAO Land and Water Development Division; 15 November–18 December 2004; [Google Scholar]
- 4.Cramer W., Guiot J., Fader M., Garrabou J., Gattuso J.P., Iglesias A., Lange M.A., Lionello P., Llasat M.C., Paz S., et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018;8:972–980. doi: 10.1038/s41558-018-0299-2. [DOI] [Google Scholar]
- 5.Lawlor D.W., Mitchell R.A. Crop ecosystem responses to climatic change. In: Nobel P.S., Reddy K.R., Hodges H.F., editors. Climate Change and Global Crop Productivity. CABI Publishing; Wallingford, UK: 2001. pp. 57–80. [Google Scholar]
- 6.Huang J., Zhang W., Zuo J., Bi J., Shi J., Wang X., Chang Z., Huang Z., Yang S., Zhang B., et al. An overview of the semi-arid climate and environment research observatory over the loess plateau. Adv. Atmos. Sci. 2008;25:906–921. doi: 10.1007/s00376-008-0906-7. [DOI] [Google Scholar]
- 7.Houghton J.T., Albritton D.L., Meira Filho L.G., Cubasch U., Dai X., Ding Y., Karl T. Intergovernmental Panel on Climate Change (IPCC), Climate Change 2001: The Scientific Basis. Cambridge University Press; Cambridge, UK: 2001. Technical summary of working group 1; pp. 3–45. [Google Scholar]
- 8.Rani G., Kaur J., Kumar A., Yogalakshmi K.N. Contemporary Environmental Issues and Challenges in Era of Climate Change. Springer; Singapore: 2019. Ecosystem health and dynamics: An indicator of global climate change; pp. 1–32. [Google Scholar]
- 9.Shrivastava P., Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegulo S., Giesler L., Harveson R., Jackson-Ziems T., Liu B., Korus K. 2013 Crop Production Clinic Proceedings, Impacts of Drought on Disease Development and Management. Pap. Plant Pathol. 2013:125–127. [Google Scholar]
- 11.Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009:153–188. [Google Scholar]
- 12.Daliakopoulos I.N., Tsanis I.K., Koutroulis A., Kourgialas N.N., Varouchakis A.E., Karatzas G.P., Ritsema C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016;573:727–739. doi: 10.1016/j.scitotenv.2016.08.177. [DOI] [PubMed] [Google Scholar]
- 13.Allen M.R., Ingram W.J. Constraints on future changes in climate and the hydrologic cycle. Nature. 2002;419:228–232. doi: 10.1038/nature01092. [DOI] [PubMed] [Google Scholar]
- 14.Rozema J., Flowers T.J. Crops for salinized world. Science. 2008;322:1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- 15.Konapala G., Mishra A.K., Wada Y., Mann M.E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-16757-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali S., Rizwan M., Qayyum M.F., Ok Y.S., Ibrahim M., Riaz M., Arif M.S., Hafeez F., Al-Wabel M.I., Shahzad A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017;24:12700–12712. doi: 10.1007/s11356-017-8904-x. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad P., Prasad M.N.V. Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability. Springer Science + Business Media, LLC; New York, NY, USA: 2012. [DOI] [Google Scholar]
- 18.Lawlor D.W. Stress metabolism: Its implication in breeding programmes. In: Srivastava E.J.P., Porceddu E., Acevedo S., editors. Drought Tolerance in Winter Cereals. Varma. Icarda; Aleppo, Syria: 1987. [Google Scholar]
- 19.Hatfield J.L., Dold C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019;10:103. doi: 10.3389/fpls.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards R.A., Passioura J.B. Seminal Root Morphology and Water Use of Wheat I. Environmental Effects 1. Crop Sci. 1981;21:249–252. doi: 10.2135/cropsci1981.0011183X002100020011x. [DOI] [Google Scholar]
- 21.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 22.Munns R., James R.A. Screening methods for salinity tolerance: A case study with tetraploid wheat. Plant Soil. 2003;253:201–218. doi: 10.1023/A:1024553303144. [DOI] [Google Scholar]
- 23.Munns R., James R.A., Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006;57:1025–1043. doi: 10.1093/jxb/erj100. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei B., Jing R., Wang C., Chen J., Mao X., Chang X., Jia J. Dreb1 genes in wheat (Triticum aestivum L.): Development of functional markers and gene mapping based on SNPs. Mol. Breed. 2009;23:13–22. doi: 10.1007/s11032-008-9209-z. [DOI] [Google Scholar]
- 26.Mondini L., Nachit M.M., Pagnotta M.A. Allelic variants in durum wheat (Triticum turgidum L. var. durum) DREB genes conferring tolerance to abiotic stresses. Mol. Genet. Genom. 2015;290:531–544. doi: 10.1007/s00438-014-0933-2. [DOI] [PubMed] [Google Scholar]
- 27.Mondini L., Nachit M.M., Porceddu E., Pagnotta M.A. HRM technology for the identification and characterization of INDEL and SNPs mutations in genes involved in drought and salt tolerance of durum wheat. Plant Genet. Resour. Characterisation Util. 2011;9:166. doi: 10.1017/S1479262111000517. [DOI] [Google Scholar]
- 28.Mondini L., Nachit M., Porceddu E., Pagnotta M.A. Identification of SNP mutations in DREB1, HKT1, and WRKY1 genes involved in drought and salt stress tolerance in durum wheat (Triticum turgidum L. var durum) Omi. A J. Integr. Biol. 2012;16:178–187. doi: 10.1089/omi.2011.0081. [DOI] [PubMed] [Google Scholar]
- 29.Dubcovsky J., Santa María G., Epstein E., Luo M.C., Dvořák J. Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theor. Appl. Genet. 1996;92:448–454. doi: 10.1007/BF00223692. [DOI] [PubMed] [Google Scholar]
- 30.Colmer T.D., Flowers T.J., Munns R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006;57:1059–1078. doi: 10.1093/jxb/erj124. [DOI] [PubMed] [Google Scholar]
- 31.Nevo E., Chen G. Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ. 2010;33:670–685. doi: 10.1111/j.1365-3040.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 32.Farooq S., Farooq E.A. Production of low input and stress tolerant wheat germplasm through the use of biodiversity residing in the wild relatives. Hereditas. 2001;135:211–215. doi: 10.1111/j.1601-5223.2001.t01-1-00211.x. [DOI] [PubMed] [Google Scholar]
- 33.Peleg Z., Fahima T., Abbo S., Krugman T., Nevo E., Yakir D., Saranga Y. Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant Cell Environ. 2005;28:176–191. doi: 10.1111/j.1365-3040.2005.01259.x. [DOI] [Google Scholar]
- 34.Gorham J. Salt tolerance in the triticeae: K/Na discrimination in Aegilops species. J. Exp. Bot. 1990;41:615–621. doi: 10.1093/jxb/41.5.615. [DOI] [Google Scholar]
- 35.Gorham J. Salt tolerance in the triticeae: Ion discrimination in rye and triticale. J. Exp. Bot. 1990;41:609–614. doi: 10.1093/jxb/41.5.609. [DOI] [Google Scholar]
- 36.Schachtman D.P., Munns R., Whitecross M.I. Variation in Sodium Exclusion and Salt Tolerance in Triticum tauschii. Crop Sci. 1991;31:992–997. doi: 10.2135/cropsci1991.0011183X003100040030x. [DOI] [Google Scholar]
- 37.Dvořak J., Noaman M.M., Goyal S., Gorham J. Enhancement of the salt tolerance of Triticum turgidum L. by the Kna1 locus transferred from the Triticum aestivum L. chromosome 4D by homoeologous recombination. Theor. Appl. Genet. 1994;87:872–877. doi: 10.1007/BF00221141. [DOI] [PubMed] [Google Scholar]
- 38.Dvořak J., Gorham J. Methodology of gene transfer by homoeologous recombinatiod into Triticum turgidum: Transfer of K+/Na+ discrimination from Triticum aestivum. Genome. 1992;35:639–646. doi: 10.1139/g92-096. [DOI] [Google Scholar]
- 39.Nevo E., Gorham J., Beiles A. Variation for 11Na uptake in wild emmer wheat, Triticum dicoccoides in israel: Salt tolerance resources for wheat improvement. J. Exp. Bot. 1992;43:511–518. doi: 10.1093/jxb/43.4.511. [DOI] [Google Scholar]
- 40.Nevo E., Krugman T., Beiles A. Genetic Resources for Salt Tolerance in the Wild Progenitors of Wheat (Triticum dicoccoides) and Barley (Hordeum spontaneum) in Israel. Plant Breed. 1993;110:338–341. doi: 10.1111/j.1439-0523.1993.tb00599.x. [DOI] [Google Scholar]
- 41.Lindsay M.P., Lagudah E.S., Hare R.A., Munns R. A locus for sodium exclusion (Nax1), a trait for salt tolerance, mapped in durum wheat. Funct. Plant Biol. 2004;31:1105–1114. doi: 10.1071/FP04111. [DOI] [PubMed] [Google Scholar]
- 42.Gorham J., Bristol A., Young E.M., Wyn Jones R.G. The presence of the enhanced K/Na discrimination trait in diploid Triticum species. Theor. Appl. Genet. 1991;82:236–729. doi: 10.1007/BF00227318. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A., Varma S.K., Angrish R. Differentiation of chloride and sulphate salinity on the basis of ionic distribution in genetically diverse cultivars of wheat. J. Plant Nutr. 1995;18:2199–2212. doi: 10.1080/01904169509365056. [DOI] [Google Scholar]
- 44.Kara Y., Martin A., Souyris I., Rekika D., Monneveux P. Root characteristics in durum wheat (T. turgidum conv. durum) and some wild Triticeae species. Genetic variation and relationship with plant architecture. Cereal Res. Commun. 2000;28:247–254. doi: 10.1007/BF03543601. [DOI] [Google Scholar]
- 45.Valkoun J.J. Wheat pre-breeding using wild progenitors. Euphytica. 2001;119:17–23. doi: 10.1023/A:1017562909881. [DOI] [Google Scholar]
- 46.Peleg Z., Fahima T., Krugman T., Abbo S., Yakir D., Korol A.B., Saranga Y. Genomic dissection of drought resistance in durum wheat × wild emmer wheat recombinant inbreed line population. Plant Cell Environ. 2009;32:758–779. doi: 10.1111/j.1365-3040.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- 47.Forster B.P., Gorham J., Miller T.E. Salt Tolerance of an Amphiploid between Triticum aestivum and Agropyron junceum. Plant Breed. 1987;98:1–8. doi: 10.1111/j.1439-0523.1987.tb01083.x. [DOI] [Google Scholar]
- 48.Islam S., Malik A.I., Islam A.K.M.R., Colmer T.D. Salt tolerance in a Hordeum marinum-Triticum aestivum amphiploid, and its parents. J. Exp. Bot. 2007;58:1219–1229. doi: 10.1093/jxb/erl293. [DOI] [PubMed] [Google Scholar]
- 49.Kingsbury R.W., Epstein E. Selection for Salt-Resistant Spring Wheat1. Crop Sci. 1984;24:310–315. doi: 10.2135/cropsci1984.0011183X002400020024x. [DOI] [Google Scholar]
- 50.Slafer G.A., Araus J.L., Royo C., García Del Moral L.F. Promising eco-physiological traits for genetic improvement of cereal yields in Mediterranean environments. Ann. Appl. Biol. 2005;146:61–70. doi: 10.1111/j.1744-7348.2005.04048.x. [DOI] [Google Scholar]
- 51.Farooq S., Niazi M.L.K., Iqbal N., Shah T.M. Salt tolerance potential of wild resources of the tribe Triticeae-II. Screening of species of the genus Aegilops. Plant Soil. 1989;119:255–260. doi: 10.1007/BF02370417. [DOI] [Google Scholar]
- 52.Farooq S., Iqbal N., Asghar M., Shah T.M. Intergeneric hybridization for wheat improvement-IV: Expression of salt tolerance gene(s) of Aegilops cylindrica in hybrids with hexaploid wheat. Cereal Res. Commun. 1992;20:111–118. [Google Scholar]
- 53.Mujeeb-Kazi A., Rosas V., Roldan S. Conservation of the genetic variation of Triticum tauschii (Coss.) Schmalh. (Aegilops squarrosa auct. non L.) in synthetic hexaploid wheats (T. turgidum L. s.lat. × T. tauschii; 2n = 6x = 42, AABBDD) and its potential utilization for wheat improvement. Genet. Resour. Crop Evol. 1996;43:129–134. doi: 10.1007/BF00126756. [DOI] [Google Scholar]
- 54.Dvořák J., Ross K. Expression of Tolerance of Na+, K+, Mg 2+, Cl− and SO2− 4 Ions and Sea Water in the Amphiploid of Triticum aestivum × Elytrigia elongata 1. Crop Sci. 1986;26:658–660. doi: 10.2135/cropsci1986.0011183X002600040002x. [DOI] [Google Scholar]
- 55.McGuire P.E., Dvôrák J. High Salt-Tolerance Potential in Wheatgrasses1. Crop Sci. 1981;21:702–705. doi: 10.2135/cropsci1981.0011183X002100050018x. [DOI] [Google Scholar]
- 56.Omielan J.A., Epstein E., Dvorak J. Salt tolerance and ionic relations of wheat as affected by individual chromosomes of salt-tolerant Lophopyrum elongatum. Genome. 1991;34:961–974. doi: 10.1139/g91-149. [DOI] [Google Scholar]
- 57.Storey R., Graham R.D., Shepherd K.W. Modification of the salinity response of wheat by the genome of Elytrigia elongatum. Plant Soil. 1985;83:327–330. doi: 10.1007/BF02184304. [DOI] [Google Scholar]
- 58.Suiyun C., Guangmin X., Taiyong Q., Fengnin X., Yan J., Huimin C. Introgression of salt-tolerance from somatic hybrids between common wheat and Thinopyrum ponticum. Plant Sci. 2004;167:773–779. doi: 10.1016/j.plantsci.2004.05.010. [DOI] [Google Scholar]
- 59.Xia G., Xiang F., Zhou A., Wang H., Chen H. Asymmetric somatic hybridization between wheat (Triticum aestivum L.) and Agropyron elongatum (Host) Nevishi. Theor. Appl. Genet. 2003;107:299–305. doi: 10.1007/s00122-003-1247-7. [DOI] [PubMed] [Google Scholar]
- 60.Gorham J., Mcdonnell E., Budrewicz E., Jones R.G.W. Salt tolerance in the Triticeae: Growth and solute accumulation in leaves of Thinopyrum bessarabicum. J. Exp. Bot. 1985;36:1021–1031. doi: 10.1093/jxb/36.7.1021. [DOI] [Google Scholar]
- 61.Gorham J., Budrewicz E., Mcdonnell E., Jones R.G.W. Salt tolerance in the triticeae: Salinity-induced changes in the leaf solute composition of some perennial Triticeae. J. Exp. Bot. 1986;37:1114–1128. doi: 10.1093/jxb/37.8.1114. [DOI] [Google Scholar]
- 62.Wang R.R.C., Li X.M., Hu Z.M., Zhang J.Y., Larson S.R., Zhang X.Y., Grieve C.M., Shannon M.C. Development of salinity-tolerant wheat recombinant lines from a wheat disomic addition line carrying a Thinopyrum junceum chromosome. Int. J. Plant Sci. 2003;164:25–33. doi: 10.1086/344556. [DOI] [Google Scholar]
- 63.Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Sustainable Agriculture. Springer; Cham, Switzerland: 2009. Plant drought stress: Effects, mechanisms and management; pp. 153–188. [Google Scholar]
- 64.Rawson H.M., Richards R.A., Munns R. An examination of selection criteria for salt tolerance in wheat, barley and Triticale genotypes. Aust. J. Agric. Res. 1988;39:759–772. doi: 10.1071/AR9880759. [DOI] [Google Scholar]
- 65.Thiyagarajan K., Latini A., Cantale C., Galeffi P. Structural characterization of the DRF1 gene of Aegilops speltoides and comparison of its sequence with those of B and other Triticeae genomes. Euphytica. 2020;216:1–17. doi: 10.1007/s10681-020-02679-7. [DOI] [Google Scholar]
- 66.Zhong G.-Y., Dvořák J. Evidence for common genetic mechanisms controlling the tolerance of sudden salt stress in the tribe Triticeae. Plant Breed. 1995;114:297–302. doi: 10.1111/j.1439-0523.1995.tb01237.x. [DOI] [Google Scholar]
- 67.Qin P., Lin Y., Hu Y., Liu K., Mao S., Li Z., Wang J., Liu Y., Wei Y., Zheng Y. Genome-wide association study of drought-related resistance traits in Aegilops tauschii. Genet. Mol. Biol. 2016;39:398–407. doi: 10.1590/1678-4685-GMB-2015-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gill R.S., Dhaliwal H.S., Multani D.S. Synthesis and evaluation of Triticum durum-T. monococcum amphiploids. Theor. Appl. Genet. 1988;75:912–916. doi: 10.1007/BF00258053. [DOI] [Google Scholar]
- 69.Limin A.E., Fowler D.B. Cold hardiness expression in interspecific hybrids and amphiploids of the Triticeae. Genome. 1988;30:361–365. doi: 10.1139/g88-063. [DOI] [Google Scholar]
- 70.Shavrukov Y., Kurishbayev A., Jatayev S., Shvidchenko V., Zotova L., Koekemoer F., De Groot S., Soole K., Langridge P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017;8:1950. doi: 10.3389/fpls.2017.01950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nieves-Cordones M., García-Sánchez F., Pérez-Pérez J.G., Colmenero-Flores J.M., Rubio F., Rosales M.A. Coping With Water Shortage: An Update on the Role of K+, Cl-, and Water Membrane Transport Mechanisms on Drought Resistance. Front. Plant Sci. 2019;10:1619. doi: 10.3389/fpls.2019.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galvan-Ampudia C.S., Julkowska M.M., Darwish E., Gandullo J., Korver R.A., Brunoud G., Haring M.A., Munnik T., Vernoux T., Testerink C. Halotropism is a response of plant roots to avoid a saline environment. Curr. Biol. 2013;23:2044–2050. doi: 10.1016/j.cub.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 73.Dai M., Zhang C., Kania U., Chen F., Xue Q., McCray T., Li G., Qin G., Wakeley M., Terzaghi W., et al. A PP6-type phosphatase holoenzyme directly regulates PIN phosphorylation and auxin efflux in Arabidopsis. Plant Cell. 2012;24:2497–2514. doi: 10.1105/tpc.112.098905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun F., Zhang W., Hu H., Li B., Wang Y., Zhao Y., Li K., Liu M., Li X. Salt modulates gravity signaling pathway to regulate growth direction of primary roots in arabidopsis. Plant Physiol. 2008;146:178–188. doi: 10.1104/pp.107.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julkowska M.M., Koevoets I.T., Mol S., Hoefsloot H., Feron R., Tester M.A., Keurentjes J.J.B., Korte A., Haring M.A., De Boer G.J., et al. Genetic components of root architecture remodeling in response to salt stress. Plant Cell. 2017;29:3198–3213. doi: 10.1105/tpc.16.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mondini L., Pagnotta M.A. Drought and Salt Stress in Cereals. Springer; Cham, Switzerland: 2015. pp. 1–31. [Google Scholar]
- 77.Sun T., Wang Y., Wang M., Li T., Zhou Y., Wang X., Wei S., He G., Yang G. Identification and comprehensive analyses of the CBL and CIPK gene families in wheat (Triticum aestivum L.) BMC Plant Biol. 2015;15:1–17. doi: 10.1186/s12870-015-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartels D., Sunkar R. Drought and salt tolerance in plants. CRC. Crit. Rev. Plant Sci. 2005;24:23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- 79.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faïc Xal Brini F., Hanin M., Mezghani I., Berkowitz G.A., Masmoudi K. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt-and drought-stress tolerance in Arabidopsis thaliana plants. J. Exp. Bot. 2007;58:301–308. doi: 10.1093/jxb/erl251. [DOI] [PubMed] [Google Scholar]
- 81.Ramezani A., Niazi A., Abolimoghadam A.A., Zamani Babgohari M., Deihimi T., Ebrahimi M., Akhtardanesh H., Ebrahimie E. Quantitative expression analysis of TaSOS1 and TaSOS4 genes in cultivated and wild wheat plants under salt stress. Mol. Biotechnol. 2013;53:189–197. doi: 10.1007/s12033-012-9513-z. [DOI] [PubMed] [Google Scholar]
- 82.Munns R. Genes and salt tolerance: Bringing them together. New Phytol. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y.M., Zhang H.M., Liu Z.H., Li H.C., Guo X.L., Li G.L. The wheat NHX antiporter gene TaNHX2 confers salt tolerance in transgenic alfalfa by increasing the retention capacity of intracellular potassium. Plant Mol. Biol. 2015;87:317–327. doi: 10.1007/s11103-014-0278-6. [DOI] [PubMed] [Google Scholar]
- 84.Tavakoli M., Poustini K., Alizadeh H. Proline accumulation and related genes in wheat leaves under salinity stress. J. Agric. Sci. Technol. 2016;18:707–716. [Google Scholar]
- 85.Golldack D., Li C., Mohan H., Probst N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014;5:151. doi: 10.3389/fpls.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blum A. Plant Breeding for Water-Limited Environments. Springer; New York, NY, USA: 2011. pp. 27–28. [Google Scholar]
- 87.Deivanai S., Xavier R., Vinod V., Timalata K., Lim O.F. Role of Exogenous Proline in Ameliorating Salt Stress at Early Stage in Two Rice Cultivars. J. Stress Physiol. Biochem. 2011;7:157–174. [Google Scholar]
- 88.Gadallah M.A.A. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999;42:249–257. doi: 10.1023/A:1002164719609. [DOI] [Google Scholar]
- 89.Mäkelä P., Karkkainen J., Somersalo S. Effect of glycinebetaine on chloroplast ultrastructure, chlorophyll and protein content, and RuBPCO activities in tomato grown under drought or salinity. Biol. Plant. 2000;43:471–475. doi: 10.1023/A:1026712426180. [DOI] [Google Scholar]
- 90.Cha-Um S., Kirdmanee C. Effect of glycinebetaine on proline, water use, and photosynthetic efficiencies, and growth of rice seedlings under salt stress. Turkish J. Agric. For. 2010;34:517–527. doi: 10.3906/tar-0906-34. [DOI] [Google Scholar]
- 91.Sakamoto A., Murata N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002;25:163–171. doi: 10.1046/j.0016-8025.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 92.Ahmad R., Lim C.J., Kwon S.Y. Glycine betaine: A versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol. Rep. 2013;7:49–57. doi: 10.1007/s11816-012-0266-8. [DOI] [Google Scholar]
- 93.Bohnert H.J., Nelson D.E., Jensen R.G. Adaptations to environmental stresses. Plant Cell. 1995;7:1099. doi: 10.2307/3870060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tisi A., Angelini R., Cona A. Wound healing in plants: Cooperation of copper amine oxidase and flavin-containing polyamine oxidase. Plant Signal. Behav. 2008;3:204–206. doi: 10.4161/psb.3.3.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roy S.J., Negrão S., Tester M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014;26:115–124. doi: 10.1016/j.copbio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Shi H., Xiong L., Stevenson B., Lu T., Zhu J.K. The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. Plant Cell. 2002;14:575–588. doi: 10.1105/tpc.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu W., Schachtman D.P., Zhang W. Partial deletion of a loop region in the high affinity K+ transporter HKT1 changes ionic permeability leading to increased salt tolerance. J. Biol. Chem. 2000;275:27924–27932. doi: 10.1074/jbc.M002056200. [DOI] [PubMed] [Google Scholar]
- 98.Liu J., Zhu J.K. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- 99.Munns R. Comparative physiology of salt and water stress. Plant. Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 100.Rodríguez H.G., Roberts J.K.M., Jordan W.R., Drew M.C. Growth, water relations, and accumulation of organic and inorganic solutes in roots of maize seedlings during salt stress. Plant Physiol. 1997;113:881–893. doi: 10.1104/pp.113.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Groß F., Durner J., Gaupels F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013;4:419. doi: 10.3389/fpls.2013.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gupta B., Huang B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014;167:645–663. doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang S.J., Song G.Q., Li Y.L., Gao J., Liu J.J., Fan Q.Q., Huang C.Y., Sui X.X., Chu X.S., Guo D., et al. Cloning of 9-cis-epoxycarotenoid dioxygenase gene (TaNCED1) from wheat and its heterologous expression in tobacco. Biol. Plant. 2014;58:89–98. doi: 10.1007/s10535-013-0373-6. [DOI] [Google Scholar]
- 104.Nambara E., Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 105.Laxalt A.M., Munnik T. Phospholipid signalling in plant defence. Curr. Opin. Plant Biol. 2002;5:332–338. doi: 10.1016/S1369-5266(02)00268-6. [DOI] [PubMed] [Google Scholar]
- 106.Canonne J., Froidure-Nicolas S., Rivas S. Phospholipases in action during plant defense signaling. Plant Signal. Behav. 2011;6:13–18. doi: 10.4161/psb.6.1.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pokotylo I., Kolesnikov Y., Kravets V., Zachowski A., Ruelland E. Plant phosphoinositide-dependent phospholipases C: Variations around a canonical theme. Biochimie. 2014;96:144–157. doi: 10.1016/j.biochi.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 108.Gillaspy G.E. The cellular language of myo-inositol signaling. New Phytologist. 2011;192:823–839. doi: 10.1111/j.1469-8137.2011.03939.x. [DOI] [PubMed] [Google Scholar]
- 109.Munnik T., Vermeer J.E.M. Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ. 2010;33:655–669. doi: 10.1111/j.1365-3040.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- 110.Zhang K., Jin C., Wu L., Hou M., Dou S., Pan Y. Expression analysis of a stress-related phosphoinositide-specific phospholipase c gene in wheat (Triticum aestivum L.) PLoS ONE. 2014;9:e105061. doi: 10.1371/journal.pone.0105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aggarwal S., Shukla V., Bhati K.K., Kaur M., Sharma S., Singh A., Mantri S., Pandey A.K. Hormonal regulation and expression profiles of wheat genes involved during phytic acid biosynthesis pathway. Plants. 2015;4:298–319. doi: 10.3390/plants4020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mills L.N., Hunt L., Leckie C.P., Aitken F.L., Wentworth M., McAinsh M.R., Gray J.E., Hetherington A.M. The effects of manipulating phospholipase C on guard cell ABA-signalling. J. Exp. Bot. 2004;55:199–204. doi: 10.1093/jxb/erh027. [DOI] [PubMed] [Google Scholar]
- 113.Zhao Y., Dong W., Zhang N., Ai X., Wang M., Huang Z., Xiao L., Xia G. A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol. 2014;164:1068–1076. doi: 10.1104/pp.113.227595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Batistič O., Kudla J. Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta-Gen. Subj. 2012;1820:1283–1293. doi: 10.1016/j.bbagen.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 115.Feng H., Wang X., Sun Y., Wang X., Chen X., Guo J., Duan Y., Huang L., Kang Z. Cloning and characterization of a calcium binding EF-hand protein gene TaCab1 from wheat and its expression in response to Puccinia striiformis f. sp. tritici and abiotic stresses. Mol. Biol. Rep. 2011;38:3857–3866. doi: 10.1007/s11033-010-0501-8. [DOI] [PubMed] [Google Scholar]
- 116.Xiang Y., Hai Lu Y., Song M., Wang Y., Xu W., Wu L., Wang H., Ma Z. Overexpression of a triticum aestivum calreticulin gene (TaCRT1) improves salinity tolerance in tobacco. PLoS ONE. 2015;10:e0140591. doi: 10.1371/journal.pone.0140591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia X.Y., Xu C.Y., Jing R.L., Li R.Z., Mao X.G., Wang J.P., Chang X.P. Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. J. Exp. Bot. 2008;59:739–751. doi: 10.1093/jxb/erm369. [DOI] [PubMed] [Google Scholar]
- 118.Xu Z.S., Liu L., Ni Z.Y., Liu P., Chen M., Li L.C., Chen Y.F., Ma Y.Z. W55a encodes a novel protein kinase that is involved in multiple stress responses. J. Integr. Plant Biol. 2009;51:58–66. doi: 10.1111/j.1744-7909.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 119.McLoughlin F., Galvan-Ampudia C.S., Julkowska M.M., Caarls L., Van Der Does D., Laurière C., Munnik T., Haring M.A., Testerink C. The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J. 2012;72:436–449. doi: 10.1111/j.1365-313X.2012.05089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang H., Mao X., Wang C., Jing R. Overexpression of a common wheat gene Tasnrk2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS ONE. 2010;5:e16041. doi: 10.1371/journal.pone.0016041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Christov N.K., Christova P.K., Kato H., Liu Y., Sasaki K., Imai R. TaSK5, an abiotic stress-inducible GSK3/shaggy-like kinase from wheat, confers salt and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2014;84:251–260. doi: 10.1016/j.plaphy.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 122.Chen G.P., Ma W.S., Huang Z.J., Xu T., Xue Y.B., Shen Y.Z. Isolation and characterization of TaGSK1 involved in wheat salt tolerance. Plant Sci. 2003;165:1369–1375. doi: 10.1016/S0168-9452(03)00365-0. [DOI] [Google Scholar]
- 123.Huang L., Yasir T.A., Phillips A.L., Hu Y.G. Isolation and characterization of ERECTA genes and their expression patterns in common wheat (Triticum aestivum L.) Aust. J. Crop Sci. 2013;7:381–390. [Google Scholar]
- 124.Wang C., Jing R., Mao X., Chang X., Li A. TaABC1, a member of the activity of bc 1 complex protein kinase family from common wheat, confers enhanced tolerance to abiotic stresses in Arabidopsis. J. Exp. Bot. 2011;62:1299–1311. doi: 10.1093/jxb/erq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gahlaut V., Jaiswal V., Kumar A., Gupta P.K. Transcription factors involved in drought tolerance and their possible role in developing drought tolerant cultivars with emphasis on wheat (Triticum aestivum L.) Theor. Appl. Genet. 2016;129:2019–2042. doi: 10.1007/s00122-016-2794-z. [DOI] [PubMed] [Google Scholar]
- 126.Xu Y., Sun F.Y., Ji C., Hu Q.W., Wang C.Y., Wu D.X., Sun G. Nucleotide diversity patterns at the DREB1 transcriptional factor gene in the genome donor species of wheat (Triticum aestivum L) PLoS ONE. 2019;14:e0217081. doi: 10.1371/journal.pone.0217081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Egawa C., Kobayashi F., Ishibashi M., Nakamura T., Nakamura C., Takumi S. Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes Genet. Syst. 2006;81:77–91. doi: 10.1266/ggs.81.77. [DOI] [PubMed] [Google Scholar]
- 128.Pellegrineschi A., Reynolds M., Pacheco M., Brito R.M., Almeraya R., Yamaguchi-Shinozaki K., Hoisington D. Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome. 2004;47:493–500. doi: 10.1139/g03-140. [DOI] [PubMed] [Google Scholar]
- 129.Wang J., Li Q., Mao X., Li A., Jing R. Wheat transcription factor TaAREB3 participates in drought and freezing tolerances in Arabidopsis. Int. J. Biol. Sci. 2016;12:257. doi: 10.7150/ijbs.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kobayashi F., Maeta E., Terashima A., Takumi S. Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings. Physiol. Plant. 2008;134:74–86. doi: 10.1111/j.1399-3054.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 131.Kobayashi F., Maeta E., Terashima A., Kawaura K., Ogihara Y., Takumi S. Development of abiotic stress tolerance via bZIP-type transcription factor LIP19 in common wheat. J. Exp. Bot. 2008;59:891–905. doi: 10.1093/jxb/ern014. [DOI] [PubMed] [Google Scholar]
- 132.Xu D.B., Gao S.Q., Ma Y.Z., Xu Z.S., Zhao C.P., Tang Y.M., Li X.Y., Li L.C., Chen Y.F., Chen M. ABI-like transcription factor gene TaABL1 from wheat improves multiple abiotic stress tolerances in transgenic plants. Funct. Integr. Genom. 2014;14:717–730. doi: 10.1007/s10142-014-0394-z. [DOI] [PubMed] [Google Scholar]
- 133.Zhang L., Liu G., Zhao G., Xia C., Jia J., Liu X., Kong X. Characterization of a wheat R2R3-MYB transcription factor gene, TaMYB19, involved in enhanced abiotic stresses in Arabidopsis. Plant Cell Physiol. 2014;55:1802–1812. doi: 10.1093/pcp/pcu109. [DOI] [PubMed] [Google Scholar]
- 134.Zhang L., Zhao G., Xia C., Jia J., Liu X., Kong X. Overexpression of a wheat MYB transcription factor gene, TaMYB56-B, enhances tolerances to freezing and salt stresses in transgenic Arabidopsis. Gene. 2012;505:100–107. doi: 10.1016/j.gene.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 135.Rahaie M., Xue G.P., Naghavi M.R., Alizadeh H., Schenk P.M. A MYB gene from wheat (Triticum aestivum L.) is up-regulated during salt and drought stresses and differentially regulated between salt-tolerant and sensitive genotypes. Plant Cell Rep. 2010;29:835–844. doi: 10.1007/s00299-010-0868-y. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L., Zhao G., Jia J., Liu X., Kong X. Molecular characterization of 60 isolated wheat MYB genes and analysis of their expression during abiotic stress. J. Exp. Bot. 2012;63:203–214. doi: 10.1093/jxb/err264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qin Y., Wang M., Tian Y., He W., Han L., Xia G. Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis. Mol. Biol. Rep. 2012;39:7183–7192. doi: 10.1007/s11033-012-1550-y. [DOI] [PubMed] [Google Scholar]
- 138.He Y., Li W., Lv J., Jia Y., Wang M., Xia G. Ectopic expression of a wheat MYB transcription factor gene, TaMYB73, improves salinity stress tolerance in Arabidopsis thaliana. J. Exp. Bot. 2012;63:1511–1522. doi: 10.1093/jxb/err389. [DOI] [PubMed] [Google Scholar]
- 139.Zhai Y., Zhang L., Xia C., Fu S., Zhao G., Jia J., Kong X. The wheat transcription factor, TabHLH39, improves tolerance to multiple abiotic stressors in transgenic plants. Biochem. Biophys. Res. Commun. 2016;473:1321–1327. doi: 10.1016/j.bbrc.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 140.Rushton P.J., Somssich I.E., Ringler P., Shen Q.J. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 141.Niu C.F., Wei W., Zhou Q.Y., Tian A.G., Hao Y.J., Zhang W.K., Ma B., Lin Q., Zhang Z.B., Zhang J.S., et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012;35:1156–1170. doi: 10.1111/j.1365-3040.2012.02480.x. [DOI] [PubMed] [Google Scholar]
- 142.Wang C., Deng P., Chen L., Wang X., Ma H., Hu W., Yao N., Feng Y., Chai R., Yang G., et al. A Wheat WRKY Transcription Factor TaWRKY10 Confers Tolerance to Multiple Abiotic Stresses in Transgenic Tobacco. PLoS ONE. 2013;8:e65120. doi: 10.1371/journal.pone.0065120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X., Zeng J., Li Y., Rong X., Sun J., Sun T., Li M., Wang L., Feng Y., Chai R., et al. Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front. Plant Sci. 2015;6:615. doi: 10.3389/fpls.2015.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou S., Zheng W.J., Liu B.H., Zheng J.C., Dong F.S., Liu Z.F., Wen Z.Y., Yang F., Wang H.B., Xu Z.S., et al. Characterizing the role of TaWRKY13 in salt tolerance. Int. J. Mol. Sci. 2019;20:5712. doi: 10.3390/ijms20225712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qin Y., Tian Y., Liu X. A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015;464:428–433. doi: 10.1016/j.bbrc.2015.06.128. [DOI] [PubMed] [Google Scholar]
- 146.Huang Q., Wang Y., Li B., Chang J., Chen M., Li K., Yang G., He G. TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 2015;15:1–15. doi: 10.1186/s12870-015-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mao X., Zhang H., Qian X., Li A., Zhao G., Jing R. TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J. Exp. Bot. 2012;63:2933–2946. doi: 10.1093/jxb/err462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mao X., Chen S., Li A., Zhai C., Jing R. Novel NAC transcription factor TaNAC67 confers enhanced multi-abiotic stress tolerances in Arabidopsis. PLoS ONE. 2014;9:e84359. doi: 10.1371/journal.pone.0084359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang L., Zhang L., Xia C., Zhao G., Jia J., Kong X. The novel wheat transcription factor TaNAC47 enhances multiple abiotic stress tolerances in transgenic plants. Front. Plant Sci. 2016;6:1174. doi: 10.3389/fpls.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xue G.P., Way H.M., Richardson T., Drenth J., Joyce P.A., McIntyre C.L. Overexpression of TaNAC69 leads to enhanced transcript levels of stress up-regulated genes and dehydration tolerance in bread wheat. Mol. Plant. 2011;4:697–712. doi: 10.1093/mp/ssr013. [DOI] [PubMed] [Google Scholar]
- 151.Xia N., Zhang G., Sun Y.F., Zhu L., Xu L.S., Chen X.M., Liu B., Yu Y.T., Wang X.J., Huang L.L., et al. TaNAC8, a novel NAC transcription factor gene in wheat, responds to stripe rust pathogen infection and abiotic stresses. Physiol. Mol. Plant Pathol. 2010;74:394–402. doi: 10.1016/j.pmpp.2010.06.005. [DOI] [Google Scholar]
- 152.Xia N., Zhang G., Liu X.Y., Deng L., Cai G.L., Zhang Y., Wang X.J., Zhao J., Huang L.L., Kang Z.S. Characterization of a novel wheat NAC transcription factor gene involved in defense response against stripe rust pathogen infection and abiotic stresses. Mol. Biol. Rep. 2010;37:3703–3712. doi: 10.1007/s11033-010-0023-4. [DOI] [PubMed] [Google Scholar]
- 153.Yu Z., Wang X., Tian Y., Zhang D., Zhang L. The functional analysis of a wheat group 3 late embryogenesis abundant protein in Escherichia coli and Arabidopsis under abiotic stresses. Plant Signal. Behav. 2019;14 doi: 10.1080/15592324.2019.1667207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dure L. A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993;3:363–369. doi: 10.1046/j.1365-313X.1993.t01-19-00999.x. [DOI] [PubMed] [Google Scholar]
- 155.Yang J., Zhao S., Zhao B., Li C. Overexpression of TaLEA3 induces rapid stomatal closure under drought stress in Phellodendron amurense Rupr. Plant Sci. 2018;277:100–109. doi: 10.1016/j.plantsci.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 156.Liang Y., Kang K., Gan L., Ning S., Xiong J., Song S., Xi L., Lai S., Yin Y., Gu J., et al. Drought-responsive genes, late embryogenesis abundant group3 (LEA3) and vicinal oxygen chelate, function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnol. J. 2019;17:2123–2142. doi: 10.1111/pbi.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang L., Li X., Chen S., Liu G. Enhanced drought tolerance in transgenic Leymus chinensis plants with constitutively expressed wheat TaLEA 3. Biotechnol. Lett. 2009;31:313–319. doi: 10.1007/s10529-008-9864-5. [DOI] [PubMed] [Google Scholar]
- 158.Koubaa S., Brini F. Functional analysis of a wheat group 3 late embryogenesis abundant protein (TdLEA3) in Arabidopsis thaliana under abiotic and biotic stresses. Plant Physiol. Biochem. 2020;156:396–406. doi: 10.1016/j.plaphy.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 159.Bhagi P., Zhawar V.K., Gupta A.K. Antioxidant response and Lea genes expression under salt stress and combined salt plus water stress in two wheat cultivars contrasting in drought tolerance. Indian J. Exp. Biol. 2013;51:746–757. [PubMed] [Google Scholar]
- 160.Wang Y., Xu H., Zhu H., Tao Y., Zhang G., Zhang L., Zhang C., Zhang Z., Ma Z. Classification and expression diversification of wheat dehydrin genes. Plant Sci. 2014;214:113–120. doi: 10.1016/j.plantsci.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 161.Brini F., Hanin M., Lumbreras V., Amara I., Khoudi H., Hassairi A., Pagès M., Masmoudi K. Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. Plant Cell Rep. 2007;26:2017–2026. doi: 10.1007/s00299-007-0412-x. [DOI] [PubMed] [Google Scholar]
- 162.Maurel C. Plant aquaporins: Novel functions and regulation properties. FEBS Lett. 2007;581:2227–2236. doi: 10.1016/j.febslet.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 163.Gao Z., He X., Zhao B., Zhou C., Liang Y., Ge R., Shen Y., Huang Z. Overexpressing a putative aquaporin gene from wheat, TaNIP, enhances salt tolerance in transgenic Arabidopsis. Plant Cell Physiol. 2010;51:767–775. doi: 10.1093/pcp/pcq036. [DOI] [PubMed] [Google Scholar]
- 164.Xu C., Wang M., Zhou L., Quan T., Xia G. Heterologous expression of the wheat aquaporin gene TaTIP2;2 compromises the abiotic stress tolerance of Arabidopsis thaliana. PLoS ONE. 2013;8:e79618. doi: 10.1371/journal.pone.0079618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou S., Hu W., Deng X., Ma Z., Chen L., Huang C., Wang C., Wang J., He Y., Yang G., et al. Overexpression of the Wheat Aquaporin Gene, TaAQP7, Enhances Drought Tolerance in Transgenic Tobacco. PLoS ONE. 2012;7:e52439. doi: 10.1371/journal.pone.0052439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu W., Yuan Q., Wang Y., Cai R., Deng X., Wang J., Zhou S., Chen M., Chen L., Huang C., et al. Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol. 2012;53:2127–2141. doi: 10.1093/pcp/pcs154. [DOI] [PubMed] [Google Scholar]
- 167.Meena M., Divyanshu K., Kumar S., Swapnil P., Zehra A., Shukla V., Yadav M., Upadhyay R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon. 2019;5:e02952. doi: 10.1016/j.heliyon.2019.e02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yokoi S., Bressan R., Hasegawa P. Salt stress tolerance of plants. JIRCAS Work. Rep. 2002;23:25–33. [Google Scholar]
- 169.Shi H., Ishitani M., Kim C., Zhu J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang S., Spielmeyer W., Lagudah E.S., Munns R. Comparative mapping of HKT genes in wheat, barley, and rice, key determinants of Na+ transport, and salt tolerance. J. Exp. Bot. 2008;59:927–937. doi: 10.1093/jxb/ern033. [DOI] [PubMed] [Google Scholar]
- 171.Horie T., Schroeder J.I. Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol. 2004;136:2457–2462. doi: 10.1104/pp.104.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ma B., Xiang Y., An L. Structural bases of physiological functions and roles of the vacuolar H+-ATPase. Cell. Signal. 2011;23:1244–1256. doi: 10.1016/j.cellsig.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 173.He X., Huang X., Shen Y., Huang Z. Wheat V-H+-ATPase Subunit Genes Significantly Affect Salt Tolerance in Arabidopsis thaliana. PLoS ONE. 2014;9:e86982. doi: 10.1371/journal.pone.0086982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baxter I., Brazelton J.N., Yu D., Huang Y.S., Lahner B., Yakubova E., Li Y., Bergelson J., Borevitz J.O., Nordborg M., et al. A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genet. 2010;6:e1001193. doi: 10.1371/journal.pgen.1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cotsaftis O., Plett D., Shirley N., Tester M., Hrmova M. A two-staged model of Na+ exclusion in rice explained by 3d modeling of HKT transporters and alternative splicing. PLoS ONE. 2012;7:e39865. doi: 10.1371/journal.pone.0039865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ren Z.H., Gao J.P., Li L.G., Cai X.L., Huang W., Chao D.Y., Zhu M.Z., Wang Z.Y., Luan S., Lin H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 177.Munns R., James R.A., Xu B., Athman A., Conn S.J., Jordans C., Byrt C.S., Hare R.A., Tyerman S.D., Tester M., et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012;30:360–364. doi: 10.1038/nbt.2120. [DOI] [PubMed] [Google Scholar]
- 178.Waters S., Gilliham M., Hrmova M. Plant High-Affinity Potassium (HKT) Transporters Involved in Salinity Tolerance: Structural insights to Probe Differences in ion Selectivity. Int. J. Mol. Sci. 2013;14:7660–7680. doi: 10.3390/ijms14047660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Laurie S., Feeney K.A., Maathuis F.J.M., Heard P.J., Brown S.J., Leigh R.A. A role for HKT1 in sodium uptake by wheat roots. Plant J. 2002;32:139–149. doi: 10.1046/j.1365-313X.2002.01410.x. [DOI] [PubMed] [Google Scholar]
- 180.Wang H., Liu D., Liu C., Zhang A. The pyridoxal kinase gene TaPdxK from wheat complements vitamin B 6 synthesis-defective Escherichia coli. J. Plant Physiol. 2004;161:1053–1060. doi: 10.1016/j.jplph.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 181.Trchounian A., Petrosyan M., Sahakyan N. In: Plant Cell Redox Homeostasis and Reactive Oxygen Species in Redox State as a Central Regulator of Plant-Cell Stress Responses. Gupta D., Palma J., Corpas F., editors. Springer; Cham, Switzerland: 2016. pp. 25–50. [Google Scholar]
- 182.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 183.Sairam R.K., Srivastava G.C. Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 2002;162:897–904. doi: 10.1016/S0168-9452(02)00037-7. [DOI] [Google Scholar]
- 184.Sairam R.K., Srivastava G.C., Agarwal S., Meena R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant. 2005;49:85. doi: 10.1007/s10535-005-5091-2. [DOI] [Google Scholar]
- 185.Noctor G., Foyer C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 186.Sairam R.K., Saxena D.C. Oxidative stress and antioxidants in wheat genotypes: Possible mechanism of water stress tolerance. J. Agron. Crop Sci. 2000;184:55–61. doi: 10.1046/j.1439-037x.2000.00358.x. [DOI] [Google Scholar]
- 187.Zhai C.Z., Zhao L., Yin L.J., Chen M., Wang Q.Y., Li L.C., Xu Z.S., Ma Y.Z. Two Wheat Glutathione Peroxidase Genes Whose Products Are Located in Chloroplasts Improve Salt and H2O2 Tolerances in Arabidopsis. PLoS ONE. 2013;8:e73989. doi: 10.1371/journal.pone.0073989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu S., Liu S., Wang M., Wei T., Meng C., Wang M., Xia G. A wheat SIMILAR TO RCD-ONE gene enhances seedling growth and abiotic stress resistance by modulating redox homeostasis and maintaining genomic integrity. Plant Cell. 2014;26:164–180. doi: 10.1105/tpc.113.118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Su P., Yan J., Li W., Wang L., Zhao J., Ma X., Li A., Wang H., Kong L. A member of wheat class III peroxidase gene family, TaPRX-2A, enhanced the tolerance of salt stress. BMC Plant Biol. 2020;20:1–15. doi: 10.1186/s12870-020-02602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Osipova S.V., Permyakov A.V., Permyakova M.D., Pshenichnikova T.A., Börner A. Leaf dehydroascorbate reductase and catalase activity is associated with soil drought tolerance in bread wheat. Acta Physiol. Plant. 2011;33:2169–2177. doi: 10.1007/s11738-011-0756-2. [DOI] [Google Scholar]
- 191.Liu Z., Liu Y., Pu Z., Wang J., Zheng Y., Li Y., Wei Y. Regulation, evolution, and functionality of flavonoids in cereal crops. Biotechnol. Lett. 2013;35:1765–1780. doi: 10.1007/s10529-013-1277-4. [DOI] [PubMed] [Google Scholar]
- 192.Shoeva O.Y., Khlestkina E.K. Differently expressed ‘early’ flavonoid Synthesis genes in wheat seedlings become to be co-regulated under salinity stress. Cereal Res. Commun. 2015;43:537–543. doi: 10.1556/0806.43.2015.025. [DOI] [Google Scholar]
- 193.Wei D., Wang M., Xu F., Quan T., Peng K., Xiao L., Xia G. Wheat oxophytodienoate reductase gene TaOPR1 confers salinity tolerance via enhancement of abscisic acid signaling and reactive oxygen species scavenging. Plant Physiol. 2013;161:1217–1228. doi: 10.1104/pp.112.211854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu C., Li S., Wang M., Xia G. A transcriptomic analysis reveals the nature of salinity tolerance of a wheat introgression line. Plant Mol. Biol. 2012;78:159–169. doi: 10.1007/s11103-011-9854-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.