INTRODUCTION
Recurrent genetic alterations of fibroblast growth factor receptors (FGFRs) leading to constitutive activation of FGFR signaling have been demonstrated in a variety of pediatric tumors, including gliomas.1-3 Overall, up to 10% of pediatric low-grade gliomas (pLGGs) and 4% of pediatric high-grade gliomas (HGGs) harbor FGFR alterations.4
Debio1347 is an orally available, highly selective, ATP-competitive FGFR1-3 inhibitor with potent antitumor effect in preclinical models.5-7 Debio1347 showed encouraging preliminary clinical activity in tumors harboring FGFR alterations and manageable safety profile in adults in its first in-human phase I study.7 Measurement of Debio1347 unbound drug concentration in the CSF from an adult patient with thalamic glioblastoma receiving Debio1347 suggested significant CNS penetration (43% CSF-plasma ratio, Debiopharm internal data).
METHODS
Five children with progressive or refractory CNS tumors harboring an FGFR gene alteration were treated at Memorial Sloan Kettering Cancer Center with Debio1347 under single patient use protocols approved by the institutional review board. Consents for publication of images were provided by the patients' guardians. The patients were treated using the 20-mg tablet formulation at the approximate body surface area-adjusted adult recommended phase II dose (80 mg/1.73 m2 × body surface area once daily). Tablets were crushed and suspended in water when needed. Toxicities were graded using the National Cancer Institute Common terminology Criteria for Adverse Events version 4.0. Plain radiographs of tibial growth plates, ECG, echocardiogram, and imaging response assessments were performed every 8-12 weeks. Two-dimensional measurements were used to assess response (product of the two largest perpendicular diameters). Partial response (PR) and progressive disease were defined by a > 50% decrease or > 25% increase in tumor measurements, respectively.
RESULTS
Clinical Features
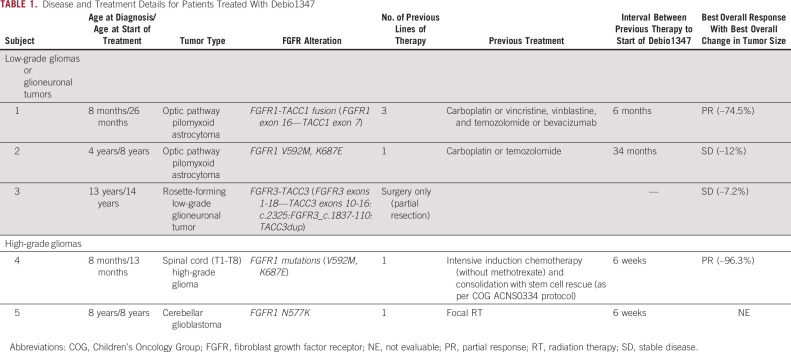
Three patients had low-grade gliomas and two patients had HGGs. Three patients had FGFR1 mutations and two patients had FGFR-TACC fusions. The median age at treatment was 8 years (range, 13 months-14 years). Most participants (4/5) had received ≥ 1 previous chemotherapy or radiotherapy regimen. Clinical characteristics, previous treatments, and tumor FGFR status of enrolled participants are summarized in Table 1.
TABLE 1.
Disease and Treatment Details for Patients Treated With Debio1347
Toxicity
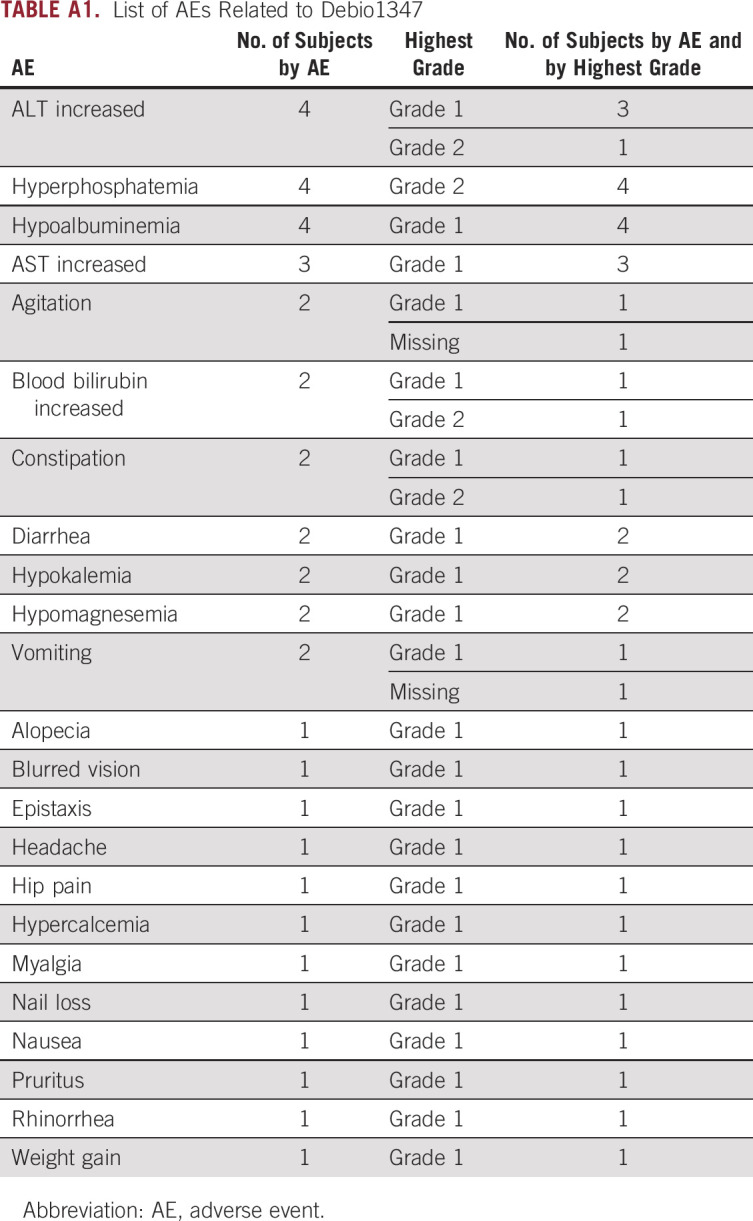
The treatment showed tolerable toxicity and all adverse events (AEs) related to Debio1347 were grade 1-2. No patient required dose modifications. Most common treatment-related AEs were hyperphosphatemia, ALT increase, and hypoalbuminemia (Appendix Table A1).
FGFR signaling plays a role in the regulation of bone development. Toxicities in skeletally immature patients are unknown. Two subjects in our cohort experienced skeletal complications; careful review of growth charts demonstrated increased linear growth velocity as evidenced by an increase in height crossing two percentile lines (from 75th to 97th). Radiographs in both patients demonstrated unfused tibial growth plates. Subject 2 developed bilateral slipped capital femoral epiphysis (SCFE), requiring percutaneous screw stabilization of both hips. The patient had several independent risk factors for SCFE, including a phase of rapid growth, race, and obesity, coupled with endocrine abnormalities.8 Subject 3 developed bilateral knee pain (left > right) following 9 months of drug therapy. Knee magnetic resonance imaging demonstrated osteochondritis dissecans. The event was deemed unrelated, and a decision was made to continue drug therapy.
Response to Therapy
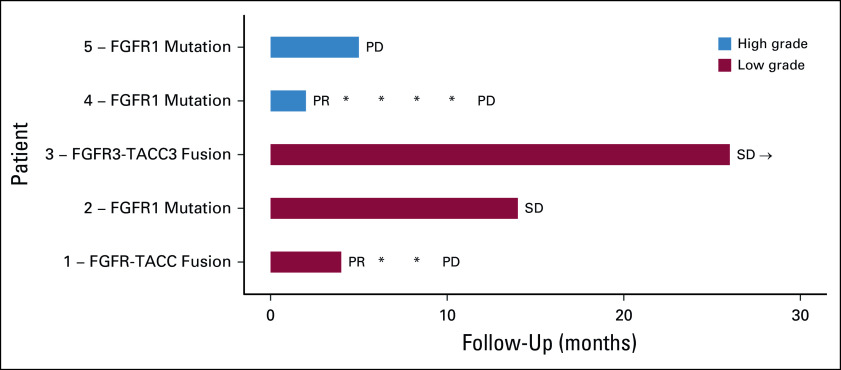
Among the four evaluable patients, two subjects (one pediatric HGG and one pLGG) experienced sustained PRs, whereas two subjects experienced prolonged disease stabilization (stable disease [SD]) (Fig 1). Subject 2 also experienced an objective improvement in visual fields and acuity, along with SD on imaging for 8 months on therapy, which was sustained for an additional 6 months off treatment. Subject 5 had nonmeasurable disease at baseline. Reasons for therapy discontinuation included disease progression (n = 3), family preference (n = 1), and one patient continues on therapy to this date. Individual treatment outcomes are summarized below.
FIG 1.
Swimmers plot demonstrating the duration of responses in the five subjects. *Subjects 1 and 4 had PR, which was maintained at multiple assessments. → Subject 3 continues on therapy with continued SD. PD, progressive disease; PR, partial response; SD, stable disease.
Low-Grade Gliomas
Subject 1 is an 8-month-old infant with a biopsy-confirmed optic pathway pilomyxoid astrocytoma, which was refractory to multiple (4) pLGG chemotherapy regimens. Whole-exome sequencing of the primary tumor showed no significant findings except for a large deletion on chromosome 8p11.2, flanking FGFR1 and TACC, along with an FGFR1 variant of unknown significance (NM_023110.2, c.2294A>G,p.E765G) with a variant allele frequency of 26%. Transcriptome sequencing revealed an FGFR1-TACC1 fusion (FGFR1 exon 16—TACC1 exon 7). Treatment with Debio1347 was initiated, and a PR was achieved following six cycles of therapy (maximal tumor reduction of 74.5%), which was sustained for 9 months (Fig 2). Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) DNA sequencing9 of the recurrent or progressive tumor revealed a newly acquired NF1 deletion in addition to the retained FGFR1-TACC1 fusion detected by MSK-Solid Fusion Assay.10
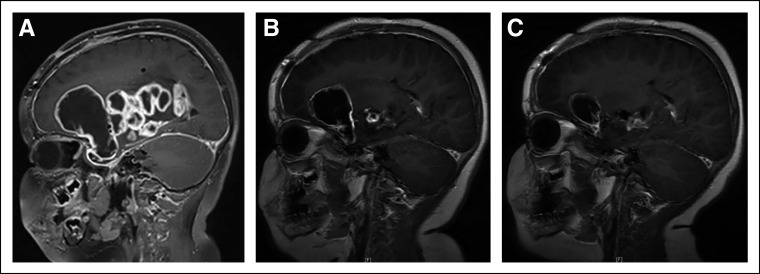
FIG 2.
(A) Sagittal T1 postcontrast MR brain imaging demonstrates a multilobulated cystic and solid, predominantly peripherally enhancing tumor arising in the region of the optic chiasm and hypothalamus and involving the basal ganglia and deep gray matter structures of the right cerebellum in subject 1. (B) Repeat imaging at 20 weeks following initiation of Debio1347 demonstrating decrease in heterogeneously enhancing solid and cystic lesion in the right greater than left optic pathways, decreased heterogeneously enhancing solid components in the inferior medial right basal ganglia and thalamus, decreased large peripherally enhancing cystic component in the right frontal region, and decreased posterior extension into the interpeduncular and prepontine cistern. (C) Repeat imaging at 28 weeks after initiation of Debio1347 demonstrating continued partial response. MR images from subject 2 (consent obtained). MR, magnetic resonance.
Subject 2 is a 4-year-old with a biopsy-confirmed optic pathway pilomyxoid astrocytoma with multiple spinal metastases and chemotherapy refractory residual disease. The patient later developed acute-onset visual decline (20/800 visual acuity bilaterally) prompting emergent ventriculo-peritoneal shunt revision because of concern for overshunting, and a short course of steroids were administered with improvement in vision (visual acuity 20/40 bilaterally). MSK-IMPACT testing performed on CSF demonstrated FGFR1 V592M and K687E mutations. Because of concern for worsening visual decline and CSF seeding, the patient started tumor-directed treatment with Debio1347. Three months after treatment with Debio1347, a clinically significant improvement in visual fields and visual acuity was observed (Fig 3). The patient experienced an serious adverse effect (bilateral SCFE) during course 9, as described above. Although this serious adverse effect was considered unrelated to Debio1347, the family elected to discontinue therapy. Surveillance imaging demonstrated SD (maximal tumor reduction 12.2%). Repeat visual fields and optical coherence tomography 14 months after treatment initiation demonstrated sustained improvement in visual fields and stable structural integrity.
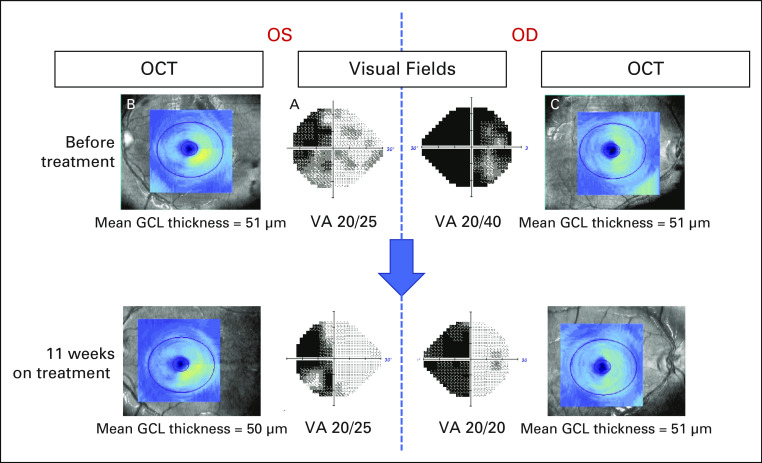
FIG 3.
Improvement in visual fields and acuity, and stable optic nerve structural assessment in subject 2. Ophthalmologic assessment before and 11 weeks after treatment with Debio1347. Humphrey visual fields before therapy (center panels) revealed a combined LHH, from right optic tract involvement, and a bitemporal hemianopia from simultaneous optic chiasmal compression, whereas best-corrected VAs were 20/40 OD and 20/25 OS. Structural assessment of the retinal GCL using OCT demonstrated a thinning of the right retina in both eyes consistent with permanent neuronal loss related to the LHH or optic tract compression (note the lack of normal yellow color on the left side images [B] and [C], reflecting right retinal thinning in the OS and OD, respectively). Mean GCL thicknesses were 51 μm in both eyes. Three months after treatment with Debio1347, repeat visual fields revealed a resolution of the temporal loss OD, reflective of resolved bitemporal hemianopsia, although the LHH persisted, and VAs improved to 20/20 OD and 20/25 OS. OCT demonstrated stable GCL thinning, confirming no further damage to the optic apparatus during that period. Repeat visual fields and OCT 14 months after treatment began (not shown) demonstrated persistent improvement in visual fields and stable structural integrity. GCL, ganglion cell layer; LHH, left homonymous hemianopia; OCT, optical coherence tomography; OD, right eye; OS, left eye; VA, visual acuity.
Subject 3 is a 13-year-old patient with a posterior fossa (right cerebellar hemispheric tumor circumscribing the brainstem and extending into the C2 spine) rosette-forming glioneuronal tumor harboring an FGFR3-TACC3 fusion. Following a partial resection, the patient experienced clinical and radiographic progression. The family declined chemotherapy, and the patient started treatment with Debio1347. Prolonged SD was demonstrated on imaging (26 months), and the patient remains on therapy. Neurologic symptoms (headaches, ataxia, and dysmetria) remained stable to minimally improved.
High-Grade Gliomas
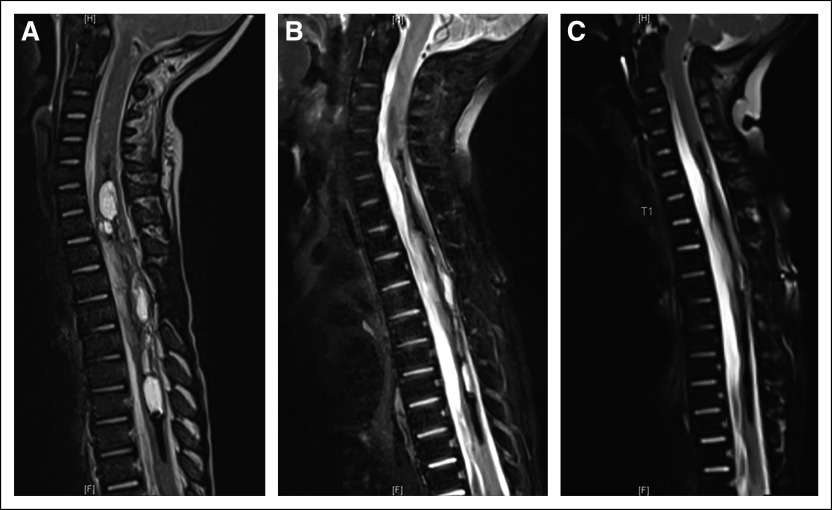
Subject 4 is an 8-month-old infant with a biopsy-confirmed spinal cord glioblastoma extending from T1-T8 and chemotherapy refractory residual disease. MSK-IMPACT DNA sequencing9 demonstrated FGFR1 V592M and K687E mutations. Treatment with Debio1347 was started 6 weeks after completion of intensive chemotherapy with stem-cell rescue and led to a striking rapid imaging response at 2 months and ultimately near complete response (maximal tumor reduction of 96.3%), which was maintained for 11 months (Fig 4).
FIG 4.
Sagittal T2 weight fat-suppressed MR spine imaging demonstrates (A) an expansile cystic and solid tumor in subject 4 centered within the spinal cord spanning from C5-C6 level through T9 level with evidence of hemosiderin deposition and/or mineralization along several of the cystic elements, and (B) marked decrease in the cystic and solid tumor component upon repeat imaging after 8 weeks of therapy. (C) Near-complete response (96.3% reduction in bidimensional measurement) on repeat imaging performed following 18 weeks of therapy. MR images from subject 1 (consent obtained). MR, magnetic resonance.
Subject 5 is an 8-year-old patient with left cerebellar HGG, which belonged to a recently described subclass of histone wild-type glioblastoma with loss of H3K27 trimethylation11 and very poor prognosis. The tumor was found to have an FGFR1 N577K missense mutation, TP53 mutation, and multiple chromosome-level losses and gains. He completed 33 fractions of focal photon radiation therapy to a total dose of 59.4 Gy, followed by adjuvant therapy with Debio1347 after 6 weeks of rest. Disease progression with craniospinal dissemination was noted after 5 months (7.5 months after radiation therapy).
DISCUSSION
FGFR inhibitors have previously been investigated in adult patients with FGFR-altered HGGs. Five patients with primary brain tumors harboring an FGFR3-TACC3 fusion were treated with Debio1347 as part of a phase I study; however, no responses were observed.12 One PR was reported among three patients with recurrent glioblastoma harboring FGFR3-TACC3 fusions treated with erdafitinib.13-15 A phase II trial of infigratinib, an FGFR1-3 inhibitor,16 demonstrated PR or SD in approximately one third of patients with recurrent or refractory FGFR-altered HGGs.16 In our study, subject 4 was an infant with spinal cord glioblastoma who demonstrated a sustained PR on therapy; however, response attribution is confounded by the previous administration of intensive chemotherapy. The FGFR1 K687E mutation in subject 4 is considered a hotspot pathogenic alteration and has been reported in subjects with gliomas,17 including a patient with anaplastic oligodendroglioma who experienced a PR to an FGFR tyrosine kinase inhibitor in a phase I study.18
By contrast, pLGGs predominantly consist of single oncogenic driver entities considered susceptible to molecular targeted therapies. We observed objective imaging responses to Debio1347 in pediatric patients with FGFR-altered low-grade gliomas, as well as functional responses with improved vision in subject 2.
Interestingly, FGFR1 single nucleotide variants frequently co-occur with NF1 alterations or additional mutations in components of RAS/MAPK/PI3K pathway.19,20 Understanding the differential activation of signal transduction pathways using preclinical models will be important in determining whether cancer cells depend primarily on FGFR signaling for growth and survival and lead to the identification of appropriate biomarkers that would be predictive of efficacy.21,22
Our patient series is limited by small numbers, disease heterogeneity, retrospective nature, as well as lack of preclinical data in pediatric glioma models and PK or progressive disease studies. Nonetheless, our results demonstrate several novel findings, which can help guide the development of clinical trials in this population: (1) this is the first report in the literature documenting treatment of pediatric patients with recurrent or refractory brain tumors harboring FGFR alterations with a specific FGFR inhibitor, (2) Debio1347 demonstrated tolerable toxicity and promising antitumor efficacy; however, a phase I dose-escalation study to identify rare AEs and optimal drug dosing is required before a phase II study to evaluate efficacy, (3) we identified candidate biomarkers (FGFR1 K687M and V592M mutations, FGFR-TACC fusions) that may be associated with efficacy of FGFR targeted therapy, and (4) identified NF1 deletion as a novel molecular mechanism of acquired resistance to FGFR inhibition.
ACKNOWLEDGMENT
The authors gratefully acknowledge the members of the Memorial Sloan Kettering Molecular Diagnostics Service in the Department of Pathology. The authors would like to thank Joseph Olechnowicz for editorial assistance and Dr Audrey Mauguen for assistance with biostatistical analysis.
Appendix
TABLE A1.
List of AEs Related to Debio1347
Sameer Farouk Sait
Consulting or Advisory Role: AstraZeneca
Uncompensated Relationships: QED Therapeutics
Sofia Haque
Honoraria: Advance Medical
Consulting or Advisory Role: Advance Medical
Marc J. Dinkin
Employment: CareMount Medical
Research Funding: Serenity Therapeutics
Expert Testimony: Medical Experts Nationwide, Ekblom & Partners LLP
Stephanie Vitolano
Stock and Other Ownership Interests: Pfizer
Daniel E. Prince
Stock and Other Ownership Interests: Modernizing Medicine
Open Payments Link: https://openpaymentsdata.cms.gov/physician/289528https://openpaymentsdata.cms.gov/physician/289528
Ira J. Dunkel
Consulting or Advisory Role: Apexigen, Celgene, Roche/Genentech, AstraZeneca, Fennec Pharma, Bristol Myers Squibb, QED Therapeutics, Day One Therapeutics
Research Funding: Bristol Myers Squibb, Genentech, Novartis
Matthias A. Karajannis
Stock and Other Ownership Interests: Johnson & Johnson (I)
Consulting or Advisory Role: Bayer, Recursion Pharmaceuticals, QED Therapeutics, CereXis, AstraZeneca
Research Funding: Novartis
Travel, Accommodations, Expenses: Bayer, Debiopharm Group
Uncompensated Relationships: Debiopharm Group
Open Payments Link: https://openpaymentsdata.cms.gov/physician/710370/summaryhttps://openpaymentsdata.cms.gov/physician/710370/summary
No other potential conflicts of interest were reported.
SUPPORT
Investigational drug Debio1347 was provided by Debiopharm International SA under Single Patient Use protocols. This work was funded in part by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology and the National Cancer Institute Cancer Center Core Grant No. P30 CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: Sameer Farouk Sait, Krisoula H. Spatz, Ira J. Dunkel, Matthias A. Karajannis
Administrative support: Matthias A. Karajannis
Provision of study materials or patients: Stephen W. Gilheeney, Daniel E. Prince, Ira J. Dunkel, Matthias A. Karajannis
Collection and assembly of data: Sameer Farouk Sait, Stephen W. Gilheeney, Tejus A. Bale, Sofia Haque, Marc J. Dinkin, Stephanie Vitolano, Marc K. Rosenblum, Ira J. Dunkel, Matthias A. Karajannis
Data analysis and interpretation: Sameer Farouk Sait, Tejus A. Bale, Katarzyna Ibanez, Daniel E. Prince, Ira J. Dunkel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sameer Farouk Sait
Consulting or Advisory Role: AstraZeneca
Uncompensated Relationships: QED Therapeutics
Sofia Haque
Honoraria: Advance Medical
Consulting or Advisory Role: Advance Medical
Marc J. Dinkin
Employment: CareMount Medical
Research Funding: Serenity Therapeutics
Expert Testimony: Medical Experts Nationwide, Ekblom & Partners LLP
Stephanie Vitolano
Stock and Other Ownership Interests: Pfizer
Daniel E. Prince
Stock and Other Ownership Interests: Modernizing Medicine
Open Payments Link: https://openpaymentsdata.cms.gov/physician/289528https://openpaymentsdata.cms.gov/physician/289528
Ira J. Dunkel
Consulting or Advisory Role: Apexigen, Celgene, Roche/Genentech, AstraZeneca, Fennec Pharma, Bristol Myers Squibb, QED Therapeutics, Day One Therapeutics
Research Funding: Bristol Myers Squibb, Genentech, Novartis
Matthias A. Karajannis
Stock and Other Ownership Interests: Johnson & Johnson (I)
Consulting or Advisory Role: Bayer, Recursion Pharmaceuticals, QED Therapeutics, CereXis, AstraZeneca
Research Funding: Novartis
Travel, Accommodations, Expenses: Bayer, Debiopharm Group
Uncompensated Relationships: Debiopharm Group
Open Payments Link: https://openpaymentsdata.cms.gov/physician/710370/summaryhttps://openpaymentsdata.cms.gov/physician/710370/summary
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hierro C, Rodon J, Tabernero J: Fibroblast growth factor (FGF) receptor/FGF inhibitors: Novel targets and strategies for optimization of response of solid tumors. Semin Oncol 42:801-819, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Parker BC, Engels M, Annala M, et al. : Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J Pathol 232:4-15, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Dienstmann R, Rodon J, Prat A, et al. : Genomic aberrations in the FGFR pathway: Opportunities for targeted therapies in solid tumors. Ann Oncol 25:552-563, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Wu G, Miller CP, et al. : Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45:602-612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanishi Y, Akiyama N, Tsukaguchi T, et al. : The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol Cancer Ther 13:2547-2558, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Chessex AV, Moulon C, Nicolas-Métral V, et al. : 547 Preclinical activity of Debio 1347, an oral selective FGFR1, 2, 3 inhibitor, in models harboring FGFR alterations. Eur J Cancer 50:177-178, 2014 [Google Scholar]
- 7.Voss MH, Hierro C, Heist RS, et al. : A phase I, open-label, multicenter, dose-escalation study of the oral selective FGFR inhibitor Debio 1347 in patients with advanced solid tumors harboring FGFR gene alterations. Clin Cancer Res 25:2699-2707, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novais EN, Millis MB: Slipped capital femoral epiphysis: Prevalence, pathogenesis, and natural history. Clin Orthop Relat Res 470:3432-3438, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng DT, Mitchell TN, Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251-264, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Z, Liebers M, Zhelyazkova B, et al. : Anchored multiplex PCR for targeted next-generation sequencing. Nat Med 20:1479-1484, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Castel D, Kergrohen T, Tauziède-Espariat A, et al. : Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3-K27M mutation. Acta Neuropathol 139:1109-1113, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Cleary JM, Iyer G, Oh D-Y, et al. : Final results from the phase I study expansion cohort of the selective FGFR inhibitor Debio 1,347 in patients with solid tumors harboring an FGFR gene fusion. J Clin Oncol 38:3603, 2020 [Google Scholar]
- 13.Bahleda R, Italiano A, Hierro C, et al. : Multicenter phase I study of erdafitinib (JNJ-42756493), oral Pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res 25:4888-4897, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Tabernero J, Bahleda R, Dienstmann R, et al. : Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol 33:3401-3408, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Di Stefano AL, Fucci A, Frattini V, et al. : Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma. Clin Cancer Res 21:3307-3317, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassman AB, Sepúlveda-Sánchez JM, Cloughesy T, et al. : OS10.6 infigratinib (BGJ398) in patients with recurrent gliomas with fibroblast growth factor receptor (FGFR) alterations: A multicenter phase II study. Neuro Oncol 21:iii21-iii22, 2019 [Google Scholar]
- 17.Gareton A, Tauziède-Espariat A, Dangouloff-Ros V, et al. : The histomolecular criteria established for adult anaplastic pilocytic astrocytoma are not applicable to the pediatric population. Acta Neuropathol 139:287-303, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahleda R, Meric-Bernstam F, Goyal L, et al. : Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann Oncol 31:1405-1412, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryall S, Zapotocky M, Fukuoka K, et al. : Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37:569-583.e5, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sievers P, Appay R, Schrimpf D, et al. : Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol 138:497-504, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Katoh M: Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol 16:105-122, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Babina IS, Turner NC: Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer 17:318-332, 2017 [DOI] [PubMed] [Google Scholar]