Summary
Human induced pluripotent stem cells (hiPSCs) can generate a multiplicity of organoids, yet no compelling evidence currently exists as to whether or not these contain tissue-specific, holoclone-forming stem cells. Here, we show that a subpopulation of cells in a hiPSC-derived corneal epithelial cell sheet is positive for ABCB5 (ATP-binding cassette, sub-family B, member 5), a functional marker of adult corneal epithelial stem cells. These cells possess remarkable holoclone-forming capabilities, which can be suppressed by an antibody-mediated ABCB5 blockade. The cell sheets are generated from ABCB5+ hiPSCs that first emerge in 2D eye-like organoids around six weeks of differentiation and display corneal epithelial immunostaining characteristics and gene expression patterns, including sustained expression of ABCB5. The findings highlight the translational potential of ABCB5-enriched, hiPSC-derived corneal epithelial cell sheets to recover vision in stem cell-deficient human eyes and represent the first report of holoclone-forming stem cells being directly identified in an hiPSC-derived organoid.
Subject areas: Bioengineering, Cell biology, Stem cells research, Tissue engineering
Graphical abstract
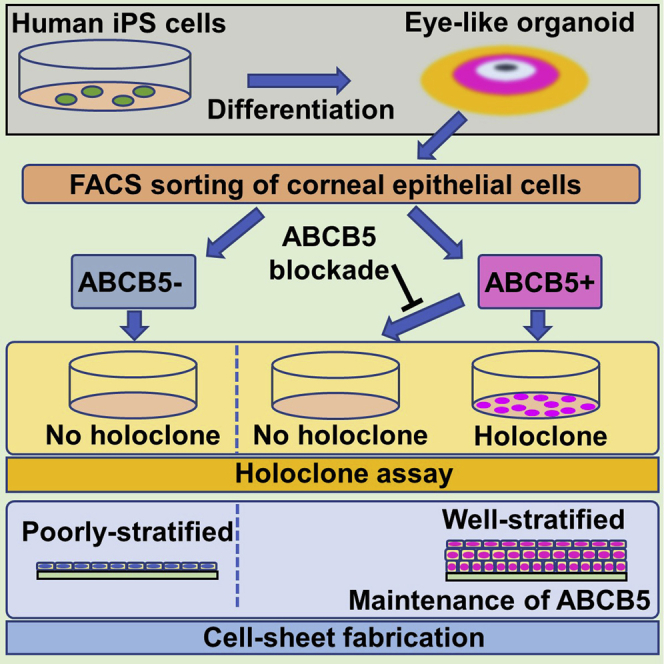
Highlights
-
•
Human iPS cell-derived corneal epithelia contain ABCB5-positive stem cells
-
•
The ABCB5-positive cells possess holoclone-forming capabilities
-
•
An antibody-mediated ABCB5 blockade suppresses holoclone formation
-
•
Holoclone-forming stem cells are present in a human iPS cell-derived tissue construct
Bioengineering; Cell biology; Stem cells research; Tissue engineering;
Introduction
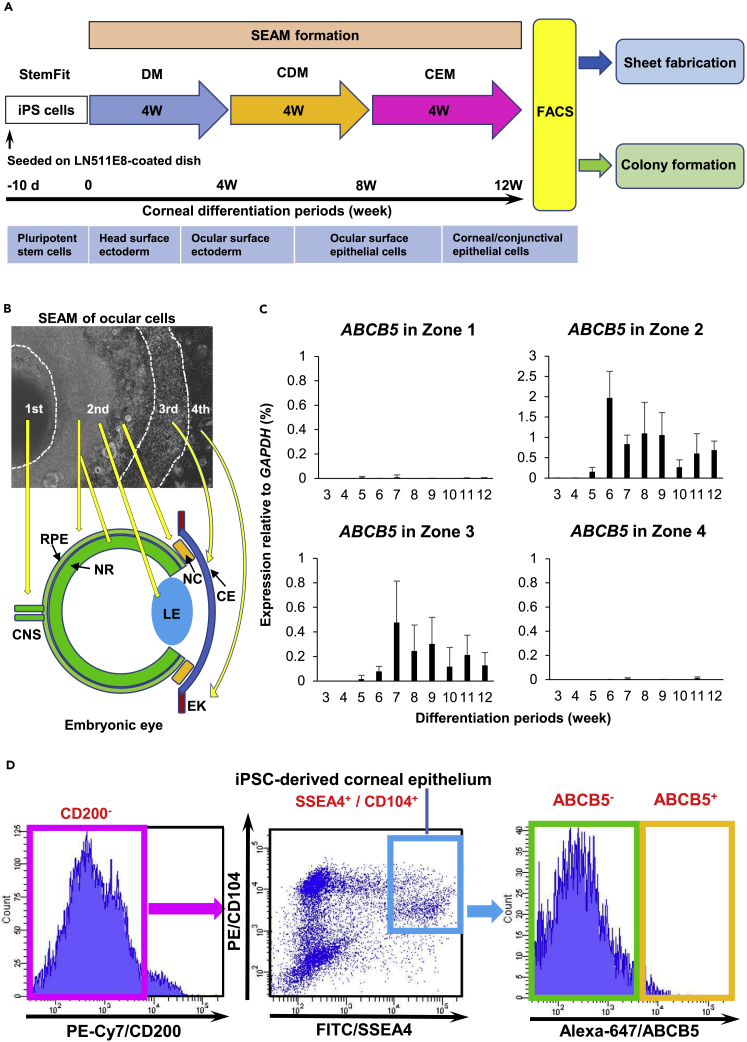
Previously, we reported how human induced pluripotent stem cells (hiPSCs) in defined culture conditions can generate a two-dimensional eye-like organoid, referred to as an SEAM (self-formed, ectodermal, autonomous, multi-zone) (Hayashi et al., 2016, 2017, 2018). In a number of respects, SEAM formation mimics whole-eye development in that the innermost central area (zone 1) forms first, followed by the emergence of three more radially distant, concentric cell populations: zones 2–4 (Figures 1A and 1B). Specifically, cells in zone 1 are akin to presumptive neuroectoderm; zone 2 cells are developmental analogs of neural eye tissues comprising the neuroretina, neural crest, and retinal pigment epithelium (RPE); cells in zone 3 have characteristics of corneal and conjunctival epithelial cells, while zone 4 cells resemble general surface ectodermal cells that will likely differentiate into epidermal keratinocytes. Further investigation of cells in SEAM zone 3 indicated that they could be sorted by flow cytometry and that those that were positive for the cell surface makers, SSEA4 and CD104, and negative for CD200 (i.e. SSEA4+/CD104+/CD200-) possessed corneal epithelial characteristics and could be fashioned into functional corneal epithelial cell sheets (Hayashi et al., 2016, 2017, 2018). Our investigations have also identified ABCB5 as a functional marker of adult corneal epithelial stem cells (Frank et al., 2003; Ksander et al., 2014; Sasamoto et al., 2018, 2020).
Figure 1.
ABCB5+ corneal epithelial cells can be induced and isolated from hiPSCs
(A) Strategy to generate and sort hiPSC-derived cells. CDM, corneal differentiation medium; CEM, corneal epithelium maintenance medium; DM, differentiation medium; d, day(s); w, week(s).
(B) SEAM of hiPSCs mimics ocular development. CNS, central nervous system; NR, neuroretina; RPE, retinal pigment epithelium; NC, neural crest; LE; lens; CE, corneal epithelium; EK, epidermal keratinocyte.
(C) ABCB5 expression in SEAM zones 1–4 during differentiation (each n = 5 independent experiments). Error bars are standard deviation.
(D) Flow cytometric analysis of the differentiated SEAM. 0.71 ± 0.62% of hiPSC-derived corneal epithelial cells (SSEA4+/CD104+/CD200-) were ABCB5+. Data shown are representative of 58 independent cell sorting experiments.
Vision loss arising from a corneal epithelial stem cell deficiency invariably requires surgical intervention if a patient's sight is to be restored. Contemporary treatments often involve the ex vivo expansion of corneal epithelial stem cells obtained from the stem cell niche at the edge of the cornea (a region of the eye known as the limbus) to form a functional corneal epithelial cell sheet that can be grafted onto the eye (Pellegrini et al., 1997; Lindberg et al., 1993; Ang et al., 2007; Schwab et al., 2000). It has been suggested, based on retrospective immunostaining of expanded corneal epithelial cell sheets with the proposed stem cell marker, p63, that a successful surgical outcome correlates with the number of stem cells likely to be present in the expanded sheet (Rama et al., 2010). However, establishing whether or not holoclone-forming stem cells are present in an expanded corneal epithelial cell sheet—or in any other hiPSC-derived organoid for that matter—has not been achieved. Here, we combine the expansion of hiPSCs into SEAMs with cell sorting based on ABCB5 specificity to investigate this issue.
Results
ABCB5+ corneal epithelial cells can be induced and isolated from hiPSCs
ABCB5 mRNA was detected at 6 weeks in SEAM zone 2 and at 7 weeks in zone 3 and was maintained until 12 weeks of differentiation in both zones (Figure 1C). In contrast, no expression was seen in zone 1 or zone 4 throughout the whole cultivation period. Flow cytometry revealed that 0.71 ± 0.62% of SSEA4+/CD104+/CD200- corneal epithelial cells derived from zone 3 were ABCB5+ (Figure 1D). Of note, a similar percentage of ABCB5+ cells were found in corneal limbal epithelial cells isolated from a human cadaveric donor eye (Sasamoto et al., 2020). These findings revealed that ABCB5+ corneal epithelial cells can be induced and isolated from hiPSCs via an SEAM and that the localization of ABCB5 expression in an SEAM mirrors that in human eyes, where it is found in the RPE and limbal epithelium (Ksander et al., 2014; Chen et al., 2005). We also observed that ABCB5 expression first appears midway through SEAM formation, which would be consistent with the notion that ABCB5 is associated with adult stem cell activity during human ocular differentiation.
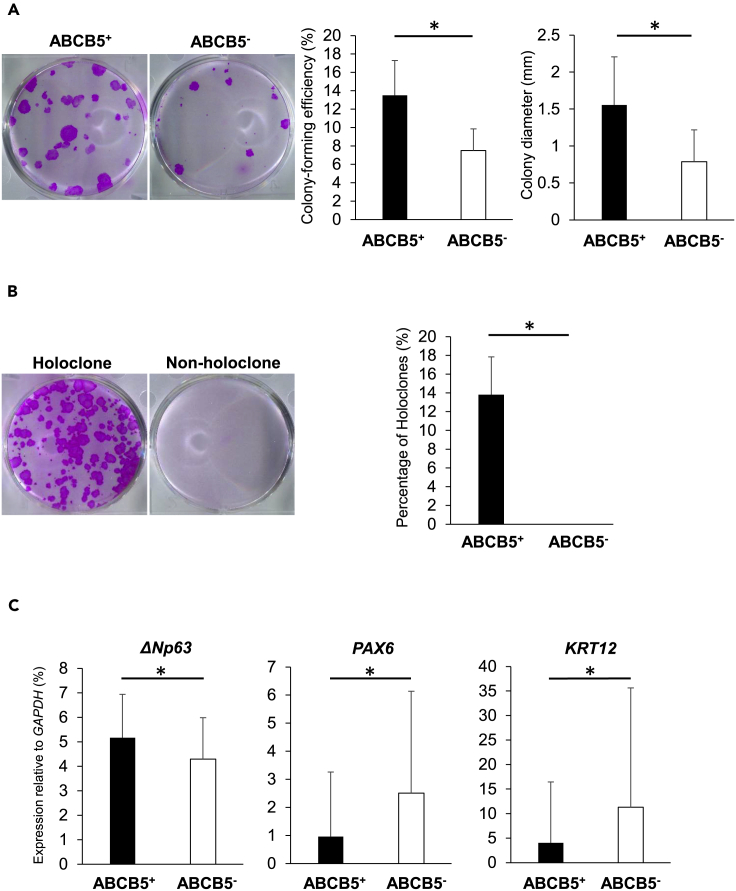
hiPSC-derived ABCB5+ corneal epithelial cells have self-renewal potential
To ascertain the self-renewal potential of ABCB5+ and ABCB5- cells isolated from SEAM-derived corneal epithelial cells, we performed colony-forming assays and holoclone assays (Figures S1A and S1B). These indicated that the colony-forming efficiency was greater and the colony size was larger for ABCB5+ cells when compared to ABCB5- cells (13.5 ± 3.8% vs. 7.5 ± 2.4%, P < 0.05; 1.56 ± 0.65 mm vs. 0.79 ± 0.43 mm, P < 0.05, respectively) (Figure 2A). Moreover, holoclone formation was a characteristic of ABCB5+ cells but not ABCB5- cells (13.8 ± 4.0% vs. 0.0 ± 0.0%, P < 0.05) (Figure 2B), and even though the number of ABCB5+ cells in the SEAM-derived corneal epithelial cells was few, the holoclone-forming capability resided exclusively in this subpopulation. A gene expression analysis by quantitative real-time reverse-transcriptase PCR (qRT-PCR) further showed that ΔNp63, a widely used marker of corneal epithelial stem cells (Rama et al., 2010; Pellegrini et al., 2001), was enriched in ABCB5+ cell-derived colonies compared to ABCB5- cell-derived colonies (5.17 ± 1.77% vs. 4.30 ± 1.69%, P < 0.05) (Figure 2C). The expression of KRT12 and PAX6, known markers of differentiated corneal epithelial cells, was also lower in ABCB5+ cell-derived colonies (4.05 ± 12.41% vs. 11.32 ± 24.31%, P < 0.05; 0.96 ± 2.30% vs. 2.51 ± 3.63%, P < 0.05, for ABCB5+ and ABCB5-, respectively) (Figure 2C). These findings reflect the undifferentiated status of SEAM-derived ABCB5+ corneal epithelial cells and their holoclone-forming capacity.
Figure 2.
hiPSC-derived ABCB5+ corneal epithelial cells have self-renewal potential
(A) Representative colony-forming assays of seven independent experiments for ABCB5+/ABCB5- hiPSC-derived corneal epithelial cells plus colony-forming efficiency and colony diameter (left panel, n = 7 independent experiments; right panel, ABCB5+; n = 172 colonies: ABCB5-; n = 93 colonies from 4 independent experiments).
(B) Representative images of a holoclone and a non-holoclone and percentage of holoclones (n = 4 independent experiments). See also Figure S1.
(C), Gene expression analysis for stem cell marker ΔNp63 and differentiated corneal epithelium-related markers PAX6 and KRT12 in ABCB5+/ABCB5- colonies (ABCB5+; n = 131 colonies: ABCB5-; n = 55 colonies from 6 independent experiments). ∗p < 0.05, Wilcoxon signed-rank test. Error bars are standard deviation.
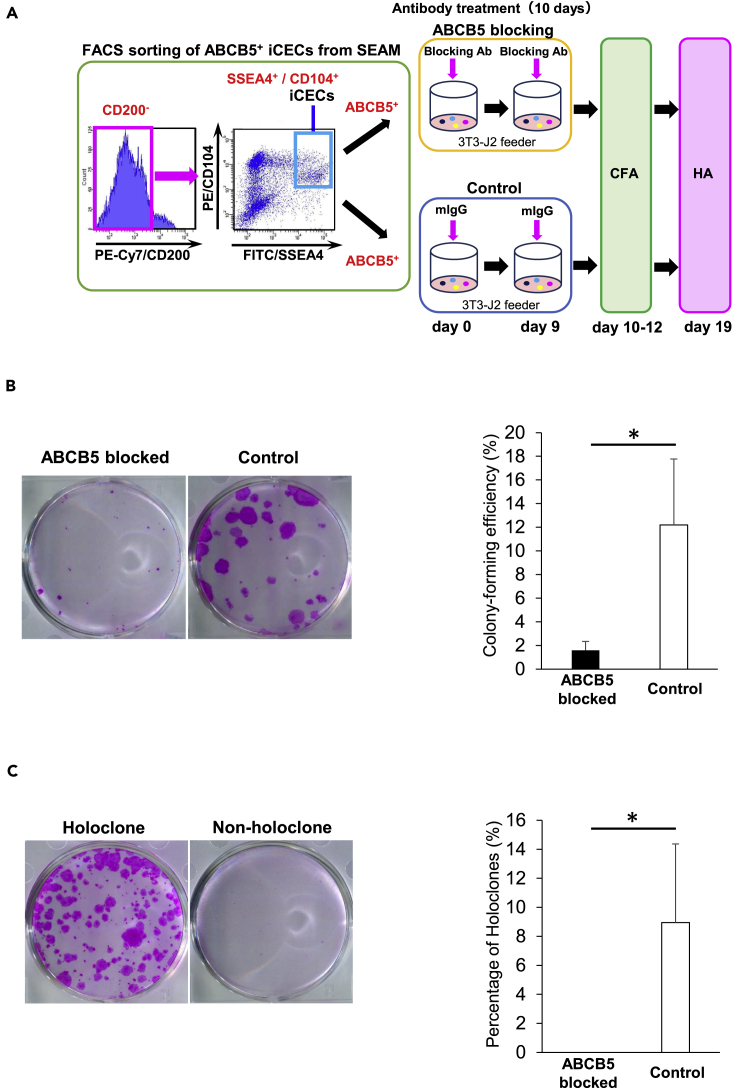
ABCB5 blockade inhibits self-renewal of hiPSC-derived ABCB5+ corneal epithelial cells
To further investigate the connection between ABCB5 and SEAM-derived corneal epithelial cell self-renewal, colony-forming assays and holoclone assays were performed with and without the inclusion of a specific monoclonal antibody (mAb)-mediated ABCB5 blockade (Ksander et al., 2014; Wilson et al., 2014; Lutz et al., 2016; Schatton et al., 2015) (Figure 3A). This revealed that a consecutive 10-day treatment of SEAM-derived ABCB5+ cells with 50 μg/mL of anti-ABCB5 blocking mAb (clone 3C2-1D12) suppressed colony formation to levels that were significantly below those of the control, non-mAb-treated group (1.6 ± 0.7% vs. 12.2 ± 5.6%, P < 0.05) (Figure 3B). Holoclone formation, moreover, was found to be completely inhibited in the anti-ABCB5 mAb-treated group compared with the control group (0.0 ± 0.0% vs. 9.0 ± 5.4%, P < 0.05) (Figure 3C), which is supportive of a defining role for ABCB5 in the self-renewal potential of hiPSC-derived corneal epithelial stem cells.
Figure 3.
ABCB5 blockade inhibits self-renewal in hiPSC-derived ABCB5+ corneal epithelial cells
(A) Schematic representation of mAb-mediated ABCB5 blocking followed by colony-forming assay (CFA) and holoclone assay (HA) for the ABCB5+ hiPSC-derived corneal epithelial cells (iCECs). Sorted ABCB5+ iCECs were treated with anti-ABCB5 blocking mAb or isotype control IgG for 10 consecutive days from the initial day of sorting.
(B) Representative images of CFA after treatment with anti-ABCB5 blocking mAb or isotype control IgG (n = 4 independent experiments) and colony-forming efficiency after the treatments (n = 4 independent experiments).
(C) Representative images of a holoclone and a non-holoclone after antibody treatment and percentage of holoclones after treatments with anti-ABCB5 blocking mAb or isotype control IgG (n = 4 independent experiments). ∗p < 0.05, Wilcoxon signed-rank test. Error bars are standard deviation.
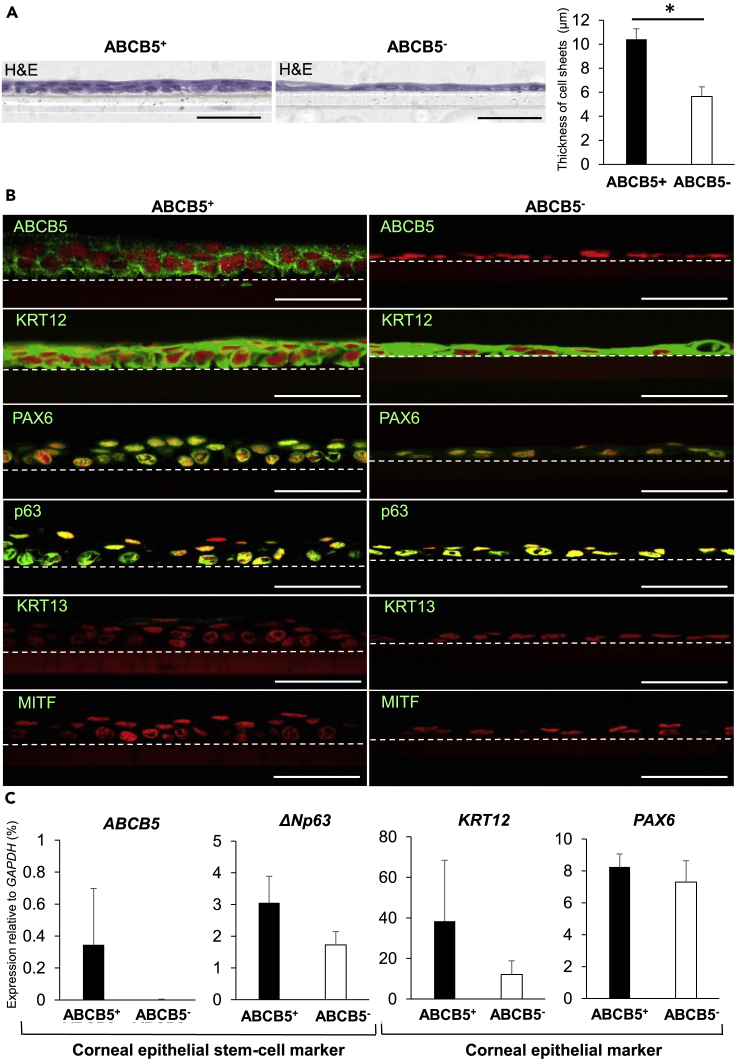
Corneal epithelial cell sheets can be fabricated from hiPSC-derived ABCB5+/ABCB5- cells
It was discovered that ex vivo expanded corneal epithelial cell sheets could be fabricated from both ABCB5+ and ABCB5- cells obtained from SEAM zone 3, although the ABCB5+ cell-derived sheets were more highly stratified and significantly thicker (10.41 ± 0.88 μm vs. 5.65 ± 0.81 μm, P < 0.05) (Figure 4A). The corneal epithelial marker, KRT12, the ocular cell marker, PAX6, and the epithelial/surface ectodermal marker, p63, were all identified in the ABCB5+ and ABCB5- cell-derived sheets (Figure 4B). This represents a typical corneal epithelial immunostaining pattern, the validity of which was reinforced by the results of a gene expression analysis of the cell sheets (Figure 4C). The fact that the pattern of gene expression changes seen in the hiPSC-derived colonies, on the one hand, and in the expanded corneal epithelial cell sheets, on the other hand, differs is perhaps reflective of a process of differentiation/maturation of cells in the cell sheets (21–28 days cultivation) compared to cells in the colonies (10–12 days cultivation). Indeed, the culture medium used for cell sheet fabrication in the current study is the same as that employed previously to generate mature corneal epithelial cell sheets (Miyashita et al., 2013; Hayashi et al., 2016, 2017). Alternative culture conditions more suited to stem cell cultivation would presumably lessen the maturation propensity of cells in the expanded cell sheets. Immunostaining also disclosed that neither the conjunctiva-specific keratin, KRT13, nor the RPE marker, MITF, was detected in the ABCB5+ or ABCB5- cell-derived sheets (Figure 4B), suggesting that no contamination with non-corneal epithelial cells occurred during the fabrication of the hiPSC-derived corneal epithelial cell sheets.
Figure 4.
Corneal epithelial cell sheets can be fabricated from hiPSC-derived ABCB5+/ABCB5- cells
(A) Hematoxylin and eosin (H&E) staining of cell sheets fabricated from ABCB5+/ABCB5- hiPSC-derived corneal epithelial cells (iCECs). Scale bar, 50 μm. Comparative thickness of cell sheets fabricated from hiPSC-derived ABCB5+/ABCB5- cells. (n = 10 measurements from 1 experiment). ∗p < 0.05, Wilcoxon signed-rank test. Error bars are standard deviation.
(B) Immunostaining for ABCB5 and ocular epithelial markers (green) in ABCB5+/ABCB5- iCEC-derived sheets. ABCB5, stem cell marker; KRT12, corneal epithelial marker, PAX6, ocular cell marker; p63, epithelial/surface ectodermal markers; KRT13, conjunctival epithelial marker; MITF, retinal pigment epithelium (RPE) marker. Nuclei, red. Scale bar, 50 μm. Dotted lines indicate border between cell sheets and culture inserts.
(C) Gene expression analysis for corneal epithelial stem cell markers and corneal epithelium-related markers in ABCB5+/ABCB5- iCEC-derived sheets (n = 3 independent experiments). Error bars are standard deviation.
Discussion
Here, we report the successful isolation of ABCB5+ corneal epithelial cells from hiPSCs via an SEAM. Collectively, our data indicate that ABCB5+ corneal epithelial cells possess functional stem cell characteristics, namely a high holoclone-forming propensity accompanied by the expression of a corneal epithelial stem cell marker, ΔNp63, and the capability to differentiate and form the corneal epithelium (Rama et al., 2010; Pellegrini et al., 1999, 2001). The identification of ABCB5+ stem cells in an SEAM-derived corneal epithelium represents the first definitive report of holoclone-forming human stem cells in an organoid originating from hiPSCs. This has theoretical and practical relevance in the fields of stem cell biology and regenerative medicine. In particular, the results suggest that ABCB5+ SEAM-derived corneal epithelial stem cells are similar in nature to the population of adult corneal epithelial stem cells in human eyes, which can differentiate into transient amplifying cells and drive corneal epithelial turnover and repair (Lehrer et al., 1998). Translational relevance lies in the fact that although surgeries to regain vision in eyes blinded by a corneal epithelial stem cell deficiency already exist (Pellegrini et al., 1997; Lindberg et al., 1993; Ang et al., 2007; Schwab et al., 2000), the corneal epithelial cell sheets that are used in contemporary graft surgery are fabricated from corneal epithelial cells obtained from a small biopsy of corneal epithelial cells taken from the limbal stem cell niche of a healthy eye or from the limbus of postmortem donor tissue sourced from an eye bank. The desire to avoid invasive biopsies of healthy eyes, aligned to the worldwide shortage of donated tissue (Sasamoto et al., 2020; Gain et al., 2016), makes the prospect of generating hiPSC-derived corneal epithelial cell sheets for transplant surgery an attractive one. This is especially true when we remember that hiPSC-derived graft tissue containing corneal epithelial stem cells can be applied for patients with bilateral disease whose limbal stem cell niche has been destroyed in both eyes and for whom an autograft using a biopsy of healthy would otherwise not be an option. Previously, we discovered that transplantation of hiPSC/SEAM-derived corneal epithelial cell sheets onto experimental ocular surface wounds in rabbit eyes could recover ocular surface function (Hayashi et al., 2016), and we have recently started to perform a small series of first-in-human corneal epithelial graft surgeries using hiPSC/SEAM-derived corneal epithelial cell sheets as part of a clinical trial (UMIN000036539) that has real potential to revolutionize the treatment of corneal blindness caused by a corneal epithelial stem cell deficiency. The current discovery that the fabricated sheets do, indeed, contain holoclone-forming corneal epithelial stem cells represents a major advance in our knowledge, which provides us with confidence in the concept of the translational approach and demonstrates that functional stem cells can be formed in organoids derived from hiPSCs.
Limitations of the study
We demonstrated that hiPSC-derived ABCB5+ corneal epithelial stem cells possess self-renewal potency within corneal epithelial cell sheets formed in vitro. We reason that the hiPSC-derived ABCB5+ cells will likely contribute to the successful repair of an injured corneal surface after epithelial cell sheet graft surgery, but this has not been proven. In vivo experiments using experimental ocular surface wounds in animal eyes would be required to corroborate this supposition.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-PAX6 | Covance Research Products Inc | Cat#PRB-278P; RRID:AB_291612 |
| Mouse anti-p63 | Santa Cruz Biotechnology | Cat#sc-8431; RRID:AB_628091 |
| Mouse anti-ABCB5 | Ksander et al., 2014 | N/A |
| Goat anti-KRT12 | Santa Cruz Biotechnology | Cat#sc-17098; RRID:AB_639266 |
| Mouse anti-KRT13 | Abcam | Cat#ab16112; RRID:AB_302267 |
| Mouse anti-MITF | Exalpha Biologicals | Cat#X2398M |
| Donkey anti-mouse IgG Alexa Fluor 488 conjugate | Life Technologies | Cat#A-21202; RRID:AB_141607 |
| Donkey anti-rabbit IgG Alexa Fluor 488 conjugate | Life Technologies | Cat#A-21206; RRID:AB_141708 |
| Donkey anti-goat IgG Alexa Fluor 488 conjugate | Life Technologies | Cat#A-11055; RRID:AB_2534102 |
| Mouse anti-SSEA4 FITC conjugate | BioLegend | Cat#330410; RRID:AB_1089204 |
| Mouse anti-CD104 PE conjugate | BioLegend | Cat#327808; RRID:AB_2129146 |
| Mouse anti-CD200 PE-Cy7 conjugate | BD PharMingen | Cat#624052 |
| Chemicals, peptides, and recombinant proteins | ||
| StemFit | Ajinomoto | Cat#AK03N |
| iMatrix-511 (LN511E8) | Nippi | Cat#892012 |
| GMEM | Life Technologies | Cat#11710-035 |
| Cnt-Prime (w/o; EGF and FGF) | CELLnTEC Advanced Cell Systems | Cat#CnT-PR-EF |
| DMEM/F12 (1:1) | Life Technologies | Cat#11320-033 |
| DMEM (high glucose, pyruvate, no glutamine) | Life Technologies | Cat#10313-021 |
| Ham's F-12 Nutrient Mix | Life Technologies | Cat#11765062 |
| Y-27632 | Wako | Cat#034-24024 |
| Recombinant Human KGF | Wako | Cat#112-00813 |
| B27 supplement | Life Technologies | Cat#17504-044 |
| Knockout serum replacement (KSR) | Life Technologies | Cat#10828-028 |
| Sodium pyruvate | Life Technologies | Cat#11360-070 |
| Non-essential amino acids | Life Technologies | Cat#11140-050 |
| Penicillin-streptomycin | Life Technologies | Cat#15140-122 |
| Hydrocortisone sodium succinate | Phizer | Cat#872452 |
| L-Glutamine | Life Technologies | Cat#25030149 |
| 2-Mercaptoethanol | Life Technologies | Cat#21985-023 |
| Monothioglycerol | Wako | Cat# 195-15791 |
| 4% (wt/vol) Paraformaldehyde (PFA) phosphate buffer solution | Wako | Cat# 163-20145 |
| 10% formaldehyde neutral buffer solution | Wako | Cat#062-01661 |
| Methanol | Wako | Cat#131-01826 |
| Hoechst 33342 | Wako | Cat#346-07951 |
| TrypLE Express | Life Technologies | Cat#12605-010 |
| Mitomycin C (Medicine) | Kyowa Hakko Kirin | N/A |
| FBS | Life Technologies | Cat#12483-020 |
| 3,3',5-Triiodo-l-thyronine sodium salt | Sigma-Aldrich | Cat#T5516 |
| Cholera toxin | List Biological Laboratory | Cat#100B |
| Insulin transferrin selenium | Life Technologies | Cat#41400045 |
| Rhodamine B | Wako | Cat#180-00132 |
| Tissue-Tek OCT compound | Sakura finetek Japan | Cat#4583 |
| D-PBS | Wako | Cat#045-29795 |
| TBS(Tris-Buffered Saline)powder | TaKaRa Bio | Cat#T903 |
| Triton X-100 | Sigma-Aldrich | Cat#T8787-100ML |
| Normal donkey serum | Jackson ImmunoResearch | Cat#017-000-121 |
| Experimental models: cell lines | ||
| Human: iPS cell line 201B7 | RIKEN Bio Resource Cente | Cat#HPS0063; RRID:CVCL_A324 |
| Mouse: 3T3-J2 cell line | H. Green, Harvard Medical School, Boston, MA | N/A |
| Oligonucleotides | ||
| Taqman probe ABCB5 (Hs02889060_m1) | Life Technologies | Cat#4331182 |
| Taqman probe KRT12 (Hs00165015_m1) | Life Technologies | Cat#4331182 |
| Taqman probe PAX6 (Hs00240871_m1) | Life Technologies | Cat#4331182 |
| Taqman probe ΔNp63 (Hs00978339_m1) | Life Technologies | Cat#4331182 |
| Taqman probe GAPDH (Hs99999905_m1) | Life Technologies | Cat#4331182 |
| Software and algorithms | ||
| BD FACSDiva Software v8.0.3 | BD Biosciences | RRID:SCR_001456 |
| JMP software program v14.0.0 | SAS institute | RRID:SCR_014242 |
| ImageJ v1.51k | National Institutes of Health | RRID:SCR_003070 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to, and will be fulfilled upon reasonable request, by the lead contact, Ryuhei Hayashi (ryuhei.hayashi@ophthal.med.osaka-u.ac.jp).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The authors declare that all relevant data are available within the article and its Supplemental information files or from the corresponding author upon reasonable request. This study did not generate new code.
Experimental model and subject details
Cell lines
hiPSCs (cell line 201B7) were purchased from the RIKEN Bio Resource Center (Tsukuba, Japan) and cultivated in a feeder-free system in the presence of laminin-511 E8 fragments (LN511E8; Nippi, Tokyo, Japan) and serum-free medium (StemFit; Ajinomoto, Tokyo, Japan) (Hayashi et al., 2016, 2017; Takahashi et al., 2007; Nakagawa et al., 2014; Miyazaki et al., 2012).
Method details
Differentiation of hiPSCs using the SEAM method
The fabrication of SEAMs from hiPSCs was performed according to our previously reported method (Hayashi et al., 2016, 2017). Briefly, hiPSCs were cultivated for 12-14 weeks on LN511E8-coated (0.5 μg/cm2) plates in serum-free differentiation medium (DM), corneal differentiation medium (CDM), and corneal epithelium maintenance medium (CEM) (Hayashi et al., 2016, 2017) (Figure 1A). DM consists of GMEM (Life Technologies, Carlsbad, CA) supplemented with 10% knockout serum replacement (KSR; Life Technologies), 1 mM sodium pyruvate (Life Technologies), 0.1 mM non-essential amino acids (Life Technologies), 2 mM L-glutamine (Life Technologies), 1% penicillin-streptomycin solution (Life Technologies) and 55 μM 2-mercaptoethanol (Life Technologies). CDM is diluted 1:1 in DM and Cnt-PR (without EGF or FGF2) (CELLnTEC Advanced Cell Systems, Bern, Switzerland) supplemented with 10 ng/mL keratinocyte growth factor (KGF) (Wako, Osaka, Japan), 10 μM Y-27632 (Wako) and 1% penicillin-streptomycin (Life Technologies). CEM consists of DMEM:F-12 medium (1:1) (Life Technologies) supplemented with 2% B-27 (Thermo Fisher Scientific, Waltham, MA), 10 ng/mL KGF (Wako), 10 μM Y-27632 (Wako), and 1% penicillin-streptomycin (Life Technologies).
Quantitative real-time reverse-transcriptase PCR
qRT-PCR was performed using the ABI Prism 7500 Fast Sequence Detection System (Life Technologies) in accordance with the manufacturer's instructions. Targets were amplified with TaqMan probe (Life Technologies) assays against ABCB5 (Hs02889060_m1), KRT12 (Hs00165015_m1), PAX6 (Hs00240871_m1), ΔNp63 (Hs00978339_m1), and GAPDH (Hs99999905_m1). The thermocycling program comprised an initial cycle at 95°C for 20 s, followed by 45 cycles at 95°C for 3 s and 60°C for 30 s.
Flow cytometry and cell sorting
Flow cytometry was performed according to a previously published method (Hayashi et al., 2018). Briefly, hiPSC-derived SEAMs were dissociated using TrypLE Express (Thermo Fisher Scientific) and re-suspended in ice-cold keratinocyte culture medium (KCM) (Hayashi et al., 2016, 2017, 2018) supplemented with 5% FBS (Japan Bio Serum, Hiroshima, Japan). Harvested cells were then stained with 5 μg/1×106 cells of mouse anti-ABCB5 mAb (clone 3C2-1D12) (Ksander et al., 2014; Wilson et al., 2014; Lutz et al., 2016; Schatton et al., 2015) for 30 min on ice. Following three washes with PBS they were then stained with 1 μg/1×106 cells of Alexa Fluor 647-conjugated secondary antibody (Thermo Fisher Scientific) for 30 min on ice. Cells were subsequently stained with 0.5 μg/1×106 cells of PE-conjugated anti-CD104 (ITGB4; 58XB4, Biolegend, San Diego, CA), 0.375 μg/1×106 cells of FITC-conjugated anti-SSEA-4 (MC813-70, Biolegend), and 0.25 μg/1×106 cells of PE-Cy7-conjugated anti-CD200 (OX-104, BD Biosciences, San Diego, CA) antibodies for 1 hr on ice, followed by three washes with PBS. Cell sorting was performed with a FACSAria II instrument (BD Biosciences), and the data analyzed using BD FACSDiva Software v8.0.3 (BD Biosciences).
Colony-forming assay (CFA) and holoclone assay (HA)
For the CFA investigation ABCB5+/ABCB5- SEAM-derived corneal epithelial cells that had been isolated by cell sorting were seeded in 6-well plates onto mitomycin C (MMC)-treated 3T3-J2 feeder cells (kindly provided by Prof H. Green, Harvard Medical School, Boston, MA) in corneal epithelium maturation medium (CMM; i.e.10% KCM medium containing 10% FBS, 10 ng/mL KGF and 10 μM Y-27632) at a density of 500 cells/well. Following cultivation for 8-10 days, cells were fixed with 10% formaldehyde neutral buffer solution (Wako) and stained with rhodamine B (Wako). Colony-forming efficiency was ascertained by counting colonies under a dissecting microscope, and colony diameters were measured using ImageJ v1.51k (National Institutes of Health, Bethesda, MD). For the HAs, ABCB5+/ABCB5- SEAM-derived corneal epithelial cells were cultivated in 6-well plates on 3T3-J2 feeder cells in CMM at a density of 500 cells/well. After 10-13 days, single colonies were picked up under a dissecting microscope. After dissociating the colonies using TrypLE Express (Thermo Fisher Scientific), the dissociated cells were again seeded on 3T3-J2 feeder cells and cultivated in CMM for 8-9 days. Colonies were then fixed with 10% formaldehyde neutral buffer solution (Wako) and stained with rhodamine B (Wako), and viewed under a microscope to classified as holoclones or non-holoclones based on a modification of a previously described method (Hayashi et al., 2016; Barrandon and Green, 1987). See also Figure S1.
mAb-mediated ABCB5 blockade
Sorted ABCB5+ hiPSC-derived corneal epithelial cells were treated with 50 μg/mL of anti-ABCB5 blocking mAb (clone 3C2-1D12) (Ksander et al., 2014; Wilson et al., 2014; Lutz et al., 2016; Schatton et al., 2015) for 10 consecutive days from the initial day of sorting. The mAb-mediated ABCB5 blockade was followed by CFAs and HAs for the treated ABCB5+ hiPSC-derived corneal epithelial cells (Figure 3A).
Fabrication of ex vivo expanded cell sheets
Fabrication of corneal epithelial cell sheets was accomplished according to previous reports (Hayashi et al., 2016, 2017). Briefly, ABCB5+/ABCB5- SEAM-derived corneal epithelial cells that had been isolated by cell sorting were seeded on LN511E8-coated dishes at a density of 3,000-9,600 cells/well (96-well plates or cell culture inserts) and cultivated in CEM for 21–28 days.
Hematoxylin and eosin (H&E) staining
Corneal epithelial cell sheets generated from hiPSC-derived ABCB5+/ABCB5- cells were embedded in OCT compound and frozen at −80°C. Thin (10 μm) sections were cut, fixed with 10% formaldehyde neutral buffer solution (Wako), washed with distilled water and stained with hematoxylin and eosin (H&E). Following deparaffinization and hydration, they were imaged on a NanoZoomer-XR C12000 (Hamamatsu Photonics, Hamamatsu, Japan) microscope. The thickness of the H&E stained cell sheets was measured using ImageJ v1.51k (National Institutes of Health) at ten equally spaced positions in individual sheets and the results were averaged (Figure 4A).
Immunofluorescence staining
Immunofluorescence staining of ABCB5 and KRT12 was performed on unfixed material, whereas staining of PAX6 and p63 was performed on tissue that had been fixed in cold methanol (Wako) and washed, three times for 10 min each, with Tris-buffered saline (TBS, TaKaRa Bio, Shiga, Japan). Staining of KRT13 and MITF was conducted on material that had been fixed in 4% paraformaldehyde (PFA, Wako) and washed with TBS three times for 10 min each time. All sections were incubated in TBS containing 5% donkey serum and 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 1 hr to block non-specific reactions. Subsequently they were incubated overnight at 4°C with primary antibodies against ABCB5 (3C2-1D12) (2.5 μg/mL; Ksander et al., 2014; Wilson et al., 2014; Lutz et al., 2016; Schatton et al., 2015), KRT12 (1:200; N-16; Santa Cruz Biotechnology, Santa Cruz, CA), PAX6 (1:1200; PRB-278P; Covance Research Products, Denver, PA), p63 (1:200; 4A4; Santa Cruz Biotechnology), KRT13 (1:200; AE8; Abcam, Cambridge, MA), and MITF (1:1000; C5; Exalpha Biologicals, Watertown, MA). After three more washes with TBS for 10 min each, samples were incubated with AF488-conjugated secondary antibodies (1:200; Life Technologies) for 1 hr at room temperature. Counterstaining was performed with Hoechst 33,342 (1:200; Life Technologies) prior to confocal fluorescence microscopy (FV3000, Olympus Corporation, Tokyo, Japan).
Quantification and statistical analysis
Statistical analysis
The data are expressed as means ± standard deviation (s.d.). The statistical analyses were performed using the Wilcoxon signed-rank test. All of the statistical analyses were performed using the JMP software program v14.0.0 (SAS institute Inc., Cary, NC). No statistical methods were used to predetermine sample size.
Acknowledgments
We thank T Katayama, S Inoue, Y. Yasukawa, M Morita, Y Yamate, S Shibata, Y Kobayashi, and Y Ishikawa of Osaka University for technical assistance. This work was supported, in part, by a project for the realization of regenerative medicine of the Japan Agency for Medical Research and Development (AMED), a Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, an AMED-CREST grant, an Osaka Eye Bank Research Grant 2017, and an International Joint Research Promotion Program of Osaka University.
Author contributions
S.W., R.H., and Y.S. designed the research; S.W. performed the in vitro experiments and acquired the data; M.H.F. and N.Y.F. provided reagents (3C2-1D12); S.W. and R.H. analyzed the data and wrote the respective methods and results; Y.S., M.T., B.R.K, M.H.F, A.J.Q., N.Y.F., and K.N. supervised the project; and S.W., R.H., A.J.Q., and K.N. wrote the paper.
Declaration of interests
M.H.F., B.R.K., and N.Y.F. are inventors or co-inventors of US and international patents assigned to Brigham and Women's Hospital, Boston Children's Hospital, the Massachusetts Eye and Ear Infirmary, and/or the VA Boston Healthcare System, Boston, MA, licensed to TICEBA GmbH (Heidelberg, Germany) and RHEACELL GmbH & Co. KG (Heidelberg, Germany). M.H.F. serves as a scientific advisor for TICEBA GmbH and RHEACELL GmbH & Co. KG.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102688.
Contributor Information
Ryuhei Hayashi, Email: ryuhei.hayashi@ophthal.med.osaka-u.ac.jp.
Kohji Nishida, Email: knishida@ophthal.med.osaka-u.ac.jp.
Supplemental information
References
- Ang L.P., Sotozono C., Koizumi N., Suzuki T., Inatomi T., Kinoshita S. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens-Johnson syndrome. Am. J. Ophthalmol. 2007;143:178–180. doi: 10.1016/j.ajo.2006.07.050. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. U S A. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.G., Szakács G., Annereau J., Rouzaud F., Liang X., Valencia J.C., Nagineni C.N., Hooks J.J., Hearing V.J., Gottesman M.M. Principal expression of two mRNA isoforms (ABCB 5alpha and ABCB 5beta) of the ATP-binding cassette transporter gene ABCB 5 in melanoma cells and melanocytes. Pigment Cell Res. 2005;18:102–112. doi: 10.1111/j.1600-0749.2005.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank N.Y., Pendse S.S., Lapchak P.H., Margaryan A., Shlain D., Doeing C., Sayegh M.H., Frank M.H. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J. Biol. Chem. 2003;278:47156–47165. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- Gain P., Jullienne R., He Z., Aldossary M., Acquart S., Cognasse F., Thuret G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Ishikawa Y., Sasamoto Y., Katori R., Nomura N., Ichikawa T., Araki S., Soma T., Kawasaki S., Sekiguchi K. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531:376–380. doi: 10.1038/nature17000. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Ishikawa Y., Katori R., Sasamoto Y., Taniwaki Y., Takayanagi H., Tsujikawa M., Sekiguchi K., Quantock A.J., Nishida K. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat. Protoc. 2017;12:683–696. doi: 10.1038/nprot.2017.007. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Ishikawa Y., Katayama T., Quantock A.J., Nishida K. CD200 facilitates the isolation of corneal epithelial cells derived from human pluripotent stem cells. Sci. Rep. 2018;8:16550. doi: 10.1038/s41598-018-34845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksander B.R., Kolovou P.E., Wilson B.J., Saab K.R., Guo Q., Ma J., McGuire S.P., Gregory M.S., Vincent W.J.B., Perez V.L. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–357. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer M.S., Sun T.T., Lavker R.M. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 1998;111:2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- Lindberg K., Brown M.E., Chaves H.V., Kenyon K.R., Rheinwald J.G. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest. Ophthalmol. Vis. Sci. 1993;34:2672–2679. [PubMed] [Google Scholar]
- Lutz N.W., Banerjee P., Wilson B.J., Ma J., Cozzone P.J., Frank M.H. Expression of cell-surface marker ABCB5 causes characteristic modifications of glucose, amino acid and phospholipid metabolism in the G3361 melanoma-initiating cell line. PLoS One. 2016;11:e0161803. doi: 10.1371/journal.pone.0161803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita H., Yokoo S., Yoshida S., Kawakita T., Yamagami S., Tsubota K., Shimmura S. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Transl. Med. 2013;2:758–765. doi: 10.5966/sctm.2012-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Futaki S., Suemori H., Taniguchi Y., Yamada M., Kawasaki M., Hayashi M., Kumagai H., Nakatsuji N., Sekiguchi K. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Taniguchi Y., Senda S., Takizawa N., Ichisaka T., Asano K., Morizane A., Doi D., Takahashi J., Nishizawa M. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G., Traverso C.E., Franzi A.T., Zingirian M., Cancedda R., Luca M.D. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- Pellegrini G., Golisano O., Paterna P., Lambiase A., Bonini S., Rama P., Luca M.D. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G., Dellambra E., Golisano O., Martinelli E., Fantozzi I., Bondanza S., Ponzin D., McKeon F., Luca M.D. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P., Matuska S., Paganoni G., Spinelli A., Luca M.D., Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- Sasamoto Y., Ksander B.R., Frank M.H., Frank N.Y. Repairing the corneal epithelium using limbal stem cells or alternative cell-based therapies. Expert Opin. Biol. Ther. 2018;18:505–513. doi: 10.1080/14712598.2018.1443442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamoto Y., Sasamoto N., Tran J., Mishra A., Ksander B.R., Frank M.H., Frank N.Y. Investigation of factors associated with ABCB5-positive limbal stem cell isolation yields from human donors. Ocul. Surf. 2020;18:114–120. doi: 10.1016/j.jtos.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T., Yang J., Kleffel S., Uehara M., Barthel S.R., Schlapbach C., Zhan Q., Dudeney S., Mueller H., Lee N. ABCB5 identifies immunoregulatory dermal cells. Cell Rep. 2015;12:1564–1574. doi: 10.1016/j.celrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab I.R., Reyes M., Isseroff R.R. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Wilson B.J., Saab K.R., Ma J., Schatton T., Putz P., Zhan Q., Murphy G.F., Gasser M., Waaga-Gasser A.M., Frank N.Y. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res. 2014;74:4196–4207. doi: 10.1158/0008-5472.CAN-14-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all relevant data are available within the article and its Supplemental information files or from the corresponding author upon reasonable request. This study did not generate new code.