Abstract
The cosmopolitan subfamily Acidocerinae (Coleoptera: Hydrophilidae) is one of the largest and most taxonomically challenging lineages of water scavenger beetles. Recent phylogenetic studies have substantially advanced our understanding of acidocerine relationships but also illuminated the twin challenges of poorly delineated generic concepts and a classification broadly incompatible with the phylogeny. Here, these two challenges are addressed by providing a comprehensive synthesis and taxonomic tools for the Acidocerinae, including (1) a brief history and the current state of acidocerine classification, (2) a review of acidocerine ecology and collection methods, (3) the current knowledge of larval and fossil acidocerines, (4) a morphological primer on characters of taxonomic and systematic importance within the lineage, (5) a key to the world genera of Acidocerinae, (6) diagnoses, habitus, and aedeagal images, distribution maps, and summary of knowledge for each of the 23 extant genera in the subfamily, and (7) a complete annotated taxonomic catalog including the published distributions, synonyms, and references for all described 541 acidocerine species recognized as of 1 April 2021. The following nomenclatural acts are proposed to bring the phylogeny and classification into alignment: Colossocharesgen. nov. is established to accommodate two African species previously described as Helochares (s. str.); Novocharesgen. nov. is newly established to accommodate 15 Neotropical species previously included in Helochares (s. str.); the remaining Helochares subgenera Helocharimorphus Kuwert syn. nov. and Hydrobaticus MacLeay syn. nov. are synonymized with Helochares Mulsant. Peltochares Régimbart sensu nov. is redefined to include eight Old World species previously included in Helochares (s. str.). A lectotype is designated for Peltochares conspicuus Régimbart, the type species of the genus. The taxonomic and morphological circumscription of Helocharessensu nov. is narrowed and redefined.
Keywords: aquatic beetles, distribution, new taxa, nomenclature, references, water scavenger beetles
Resumen Abstract
La subfamilia Acidocerinae (Coleoptera: Hydrophilidae) es cosmopolita y representa uno de los linajes más diversos y taxonómicamente más desafiantes de escarabajos acuáticos detritívoros. Estudios filogenéticos recientes han incrementado considerablemente nuestro entendimiento sobre las relaciones entre acidocerinos, así como iluminaron los conceptos genéricos pobremente definidos y una clasificación ampliamente incompatible con la filogenia. Aquí se abordan estos dos desafíos proporcionando una síntesis integral y herramientas taxonómicas para Acidocerinae, incluyendo (1) un resumen de la historia y estado actual de la clasificación de Acidocerinae, (2) una revisión de la ecología y los métodos de recolección para acidocerinos, (3) el conocimiento actual de acidocerinos larvales y fósiles, (4) un manual morfológico básico sobre caracteres de importancia taxonómica y sistemática dentro del linaje, (5) una clave para los géneros de Acidocerinae del mundo, (6) diagnosis, imágenes del hábito y del edeago, mapas de distribución y resumen del conocimiento actual para cada uno de los 23 géneros existentes en la subfamilia, y (7) un catálogo taxonómico anotado y completo que incluye las distribuciones publicadas, sinónimos y referencias para todas las 541 especies de Acidocerinae descritas y reconocidas al 1 de abril de 2021. Se proponen los siguientes actos taxonómicos para alinear la clasificación con la filogenia: Colossocharesgen. nov. se establece para incluir dos especies africanas descritas previamente como Helochares (s. str.); Novocharesgen. nov. se establece como nuevo para acomodar 15 especies neotropicales previamente incluidas en Helochares (s. str.); los subgéneros restantes de Helochares Mulsant, Helocharimorphus Kuwert syn. nov. e Hydrobaticus MacLeay syn. nov. se sinonimizan con Helochares. Peltochares Régimbart sensu nov. es redefinido para incluir ocho especies del viejo mundo previamente incluidas en Helochares (s. str.). Se designa un lectotipo para Peltochares conspicuus Régimbart, la especie tipo del género. La circunscripción taxonómica y morfológica de Helocharessensu nov. se reduce y redefine.
Introduction
The water scavenger beetle family Hydrophilidae Latreille, with more than 3,000 described species, is the most diverse family of polyphagan aquatic beetles, and the second largest for all aquatic Coleoptera (Short 2018). This diversity is reflected in their species richness and their ecological habits: members of the family are associated not only with aquatic ecologies, but also various hygropetric and a broad range of terrestrial habitats (Bloom et al. 2014). A comprehensive molecular phylogeny for the family by Short and Fikáček (2013) organized the lineage into six subfamilies: Hydrophilinae Latreille, Chaetarthriinae Bedel, Enochrinae Thomson, Acidocerinae Zaitzev, Cylominae Zaitzev (changed from Rygmodinae d’Orchymont; Seidel et al. 2016), and Sphaeridiinae Latreille. With more than 500 species, the Acidocerinae is the third largest hydrophilid subfamily (after Hydrophilinae and Sphaeridiinae). The Acidocerinae occupies a key position in the evolutionary history and in the broader ecological evolution of water scavenger beetles, as it diverges after the primarily aquatic Hydrophilinae, Chaetarthriinae and Enochrinae, while serving as the sister group to the largely terrestrial Cylominae+Sphaeridiinae (Short and Fikáček 2013).
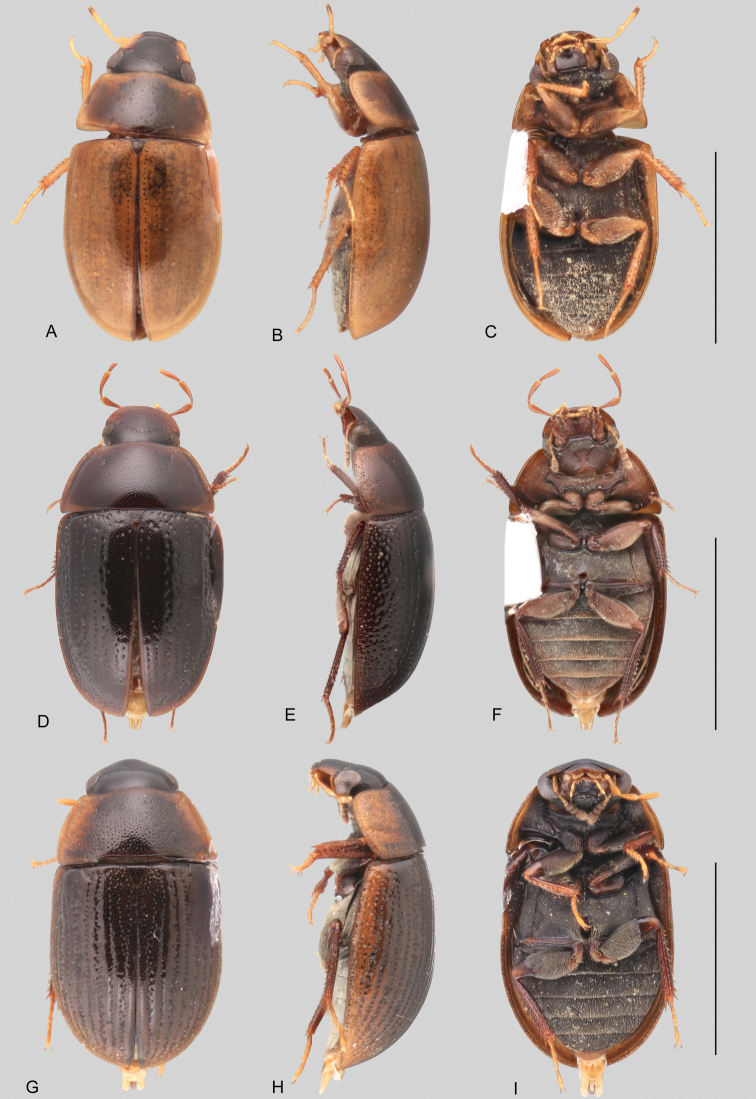
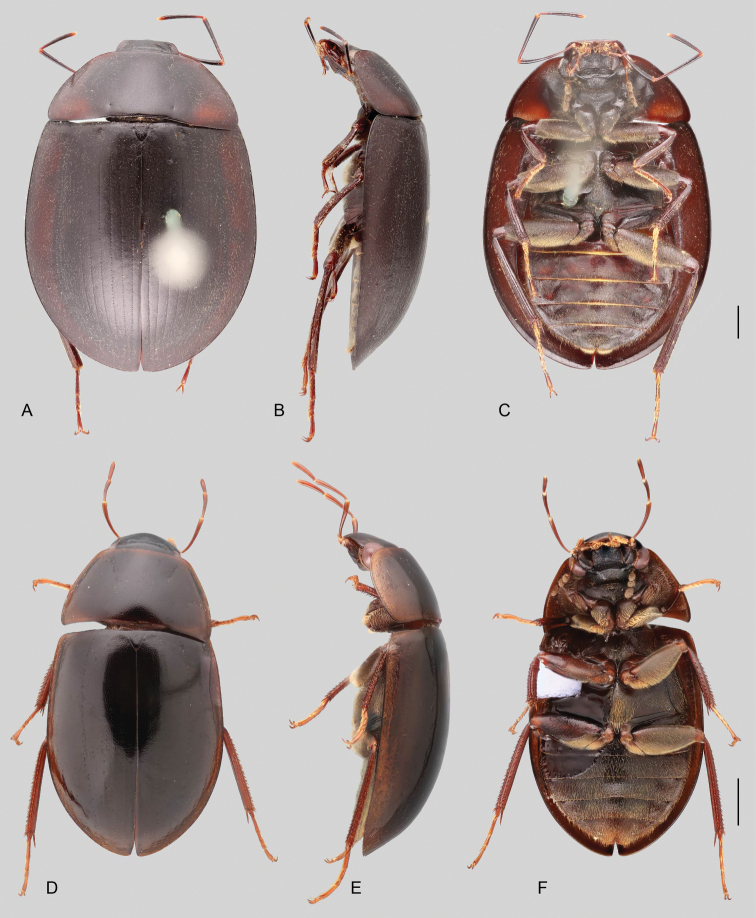
In morphological terms, Acidocerinae is a heterogeneous assemblage of beetles, as a variety of sizes, colorations and body shapes can be found in the group (Fig. 1). Species range in size from 1.1 mm (Nanosaphes Girón & Short; Figs 1L, 41) to 14 mm (Colossochares gen. nov.; Figs 1A, 26) and range in color from pale yellowish and orange brown to nearly black (Fig. 1). Body forms vary from compact and convex (e.g., Globulosis García; Figs 1U, 32) to broadly explanate and dorsoventrally compressed (e.g., Helobata Bergroth, Figs 1J, 33; Helopeltarium d’Orchymont, Figs 1H, 38). Although most genera are relatively easy to tell apart, within a genus, the external morphology ranges from extremely homogeneous (e.g., Aulonochares Girón & Short; Figs 1D, 21) to highly variable (e.g., Primocerus Girón & Short, Figs 1R, 46; Agraphydrus Régimbart, Figs 1S, T, 18, 19). This morphological diversity, which may be a consequence of adapting to the broad range of habitats where acidocerines occur, and compounded by the widespread distribution of some taxa, has resulted in taxonomic confusion. Acidocerine species can be found across a wide variety of environments, spanning almost the full range of habitats that occur in the Hydrophilidae as a whole, including fully aquatic settings like ponds, streams, and river margins, hygropetric habitats like rock seepages, and terrestrial niches such as rotting fruits.
Figure 1.
Variation across Acidocerinae, dorsal and lateral views AColossochares ellipticusBPeltochares sp. CPeltochares conspicuusDAulonochares tubulusEHelochares sp. FHelochares tristisGNovochares sp. HHelopeltarium ferrugineumIBatochares sp. JHelobata larvalisKRadicitus sp. LNanosaphes tricolorMAgraphydrus cf. attenuatusNTobochares luteomargoOTobochares sulcatusPQuadriops similarisQCrucisternum ouboteriRPrimocerus neutrumSAgraphydrus coomaniTAgraphydrus sp. UGlobulosis flavusVCrephelochares nitescens.
Figure 41.
Habitus of Nanosaphes spp. A–CN. tricolor: A dorsal habitus B lateral habitus C ventral habitus D–FN. punctatus: D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 0.5 mm.
Figure 26.
Habitus of Colossochares ellipticusA dorsal habitus B lateral habitus C ventral habitus. Scale bar: 1 mm.
Figure 32.
Habitus of Globulosis flavusA dorsal habitus B lateral habitus C ventral habitus. Scale bar: 1 mm.
FIgure 33.
Habitus of Helobata spp. A–CH. larvalis: A dorsal habitus B lateral habitus C ventral habitus DH. quatipuru (from Clarkson and Almeida 2018) EH. amazonensis (from Clarkson and Almeida 2018) FH. pantaneira (from Clarkson et al. 2016). Scale bars: 1 mm.
Figure 38.
Habitus of Helopeltarium ferrugineumA dorsal habitus B lateral habitus C ventral habitus. Scale bar: 1 mm.
Figure 21.
Habitus of Aulonochares spp. A–CA. tubulus: A dorsal habitus B lateral habitus C ventral habitus DA. lingulatus, dorsal habitus. Scale bar: 5 mm.
Figure 46.
Habitus of Primocerus spp. A–CP. neutrum: A dorsal habitus B lateral habitus C ventral habitus D–FP. maipure: D dorsal habitus E lateral habitus F ventral habitus G–IP. semipubescens: G dorsal habitus H lateral habitus I ventral habitus. Scale bars: 1 mm.
Figure 18.
Habitus of Agraphydrus spp. A–CA. coomani: A dorsal habitus B lateral habitus C ventral habitus D–FA. cf. attenuatus: D dorsal habitus E lateral habitus F ventral habitus G–IA. sp. ex Madagascar: G dorsal habitus H lateral habitus I ventral habitus. Scale bars: 1 mm.
Figure 19.
Habitus of Agraphydrus spp. AA. hanseniBA. jilanzhuiCA. longipalpusDA. contractusEA. anhuianusFA. puzhelongi. Images B–F from Komarek and Hebauer (2018). Scale bars: 1 mm.
Although the circumscription of the subfamily is well supported by several molecular studies (Short and Fikáček 2013; Short et al. 2021) the morphological diversity of acidocerines has befuddled efforts to define the lineage as a whole, as well as many of its historical genera. There is presently no known synapomorphy for the lineage that does not have at least one exception. Additionally, rampant homoplasy in certain characters that have historically been used to circumscribe genera and subgenera (such as the presence of elytral striae and the length of the maxillary palps) have significantly complicated acidocerine taxonomy. A recent comprehensive molecular phylogeny of the subfamily (Short et al. 2021) combined with an explosion of new genera and species from all parts of the world created both the opportunity and the need for a comprehensive taxonomic review of the Acidocerinae. In this work, we provide an integrated synthesis and taxonomic tools for the Acidocerinae, including (1) a brief history and the current state of acidocerine classification, (2) a review of acidocerine ecology and collection methods, (3) the current knowledge of larval and fossil acidocerines, (4) a morphological primer on characters of taxonomic and systematic importance within the lineage, (5) a key to the world genera of Acidocerinae, (6) descriptions, differential diagnoses, habitus and aedeagal images, distribution maps, and summary of knowledge for each of the 23 extant genera in the subfamily, and (7) a complete annotated taxonomic catalog including the published distributions, synonyms, and references for all described acidocerine species.
Taxonomic history and composition of the Acidocerinae
Horn (1873) established the monogeneric tribe Helopeltini for the newly established genus Helopeltis (now Helobata; Figs 1J, 33). Horn (1873) viewed the genus as quite distinct and warranting its own tribe based on the broadly explanate body form, concealed labrum, and long maxillary palps (he retained Helochares, the only other Acidocerinae [in the current sense] in North America at the time, within the Hydrobiini with most other hydrophilids). However, Helopeltini was unavailable due to its type genus Helopeltis being a preoccupied name (Hansen 1999b). Later, Zaitzev (1908) placed the genus Acidocerus Klug (Fig. 17) into its own “subfamily” under the new name Acidocerini without comment. It is unclear why he considered the taxon so unique as to give it such a prominent rank in his classification, which placed it equal to the rank he considered for Epimetopidae, Spercheidae, and other currently recognized hydrophiloid families. A decade later, d’Orchymont (1919c), either unaware or unconcerned with the Acidocerini of Zaitzev, proposed the subtribe Helocharae for Helochares, Enochrus, and their apparent relatives (including Acidocerus). Unlike Helopeltini and Acidocerini, the erection of Helocharae was not done to bestow recognition on a single bizarre taxon, but to unite a morphologically similar collection of genera. The name and concept of the Helocharae (either as a subtribe of Hydrobiini or as the tribe Helocharini (of Hydrobiinae) remained in use for the next 70 years.
Figure 17.
Habitus of Acidocerus aphodioidesA dorsal habitus B lateral habitus C head. Scale bar: 1 mm.
Hansen (1991) was the first to both recognize Zaitzev’s Acidocerini as having priority over Helocharae and to affirm the circumscription of the lineage in a phylogenetic context (as the subtribe Acidocerina of Hydrophilini). Twenty years later, Short and Fikáček (2011), elevated the Acidocerini to tribal level, citing accumulating evidence that the Hydrophilini sensu Hansen was not monophyletic. In a subsequent comprehensive molecular phylogeny and reclassification of the Hydrophilidae, Short and Fikáček (2013) elevated the lineage further to its current subfamily rank, while transferring Enochrus Thomson, Cymbiodyta Bedel, and Helocombus Horn from the Acidocerinae into the newly defined subfamily Enochrinae. This circumscription has remained unchanged to date.
In terms of diversity, Acidocerinae included nearly 300 species grouped in 14 genera when it was first recognized as a subfamily (Acidocerus, Agraphydrus, Chasmogenus Sharp, Dieroxenus Spangler, Globulosis, Helochares, Helobata, Helopeltarium, Horelophopsis Hansen, Megagraphydrus Hansen, Peltochares, Quadriops Hansen, Tobochares Short & García, and Troglochares Spangler; Short and Fikáček 2013). Since then, six genera have been described (Crucisternum Girón & Short, Katasophistes Girón & Short, and Nanosaphes, Girón & Short, 2018; Aulonochares, Primocerus, and Ephydrolithus Girón & Short, 2019), and two genera have been synonymized (Dieroxenus synonym of Chasmogenus; Girón and Short 2018; Horelophopsis synonym of Agraphydrus; Short et al. 2021).
The most comprehensive molecular phylogenetic analysis of the subfamily Acidocerinae was recently conducted by Short et al. (2021). The dataset included DNA sequence data for the mitochondrial gene COI and the nuclear genes 18S, 28S, H3, and CAD, for 206 acidocerine and eleven outgroup terminals (Short et al. 2021). These analyses confirmed the monophyly of the subfamily, as well as of most genera, with the unsurprising exception of a polyphyletic Helochares (Short et al. 2021: figs 1, 2).
The Helochares problem
At the time Acidocerinae was elevated to subfamily, Helochares was its largest and most widespread genus, grouping nearly 2/3 of the species in the lineage. Helochares was traditionally divided into five subgenera: Batochares Hansen (e.g., Figs 1I, 23), Helochares (e.g., Fig. 1B), Helocharimorphus Kuwert (e.g., Fig. 35D–F), Hydrobaticus MacLeay (e.g., Figs 35A–C, 36A–C) and Sindolus Sharp (e.g., Fig. 51), some of which were recognized mostly by the absence [Helochares (s. str.)] or presence [Helochares (Hydrobaticus)] of rows of serial punctures along the elytra.
Figure 23.
Habitus of Batochares sp. A dorsal habitus B lateral habitus C ventral habitus. Scale bar: 1 mm.
Figure 35.
Habitus of Helochares spp. A–CHelochares tristis: A dorsal habitus B lateral habitus C ventral habitus D–FH. sharpi: D dorsal habitus E lateral habitus F ventral habitus. Scale bar: 1 mm.
Figure 36.
Habitus of Helochares spp. A–CH. laevis: A dorsal habitus B lateral habitus C ventral habitus D–FH. sp. (India, Goa): D dorsal habitus E lateral habitus F ventral habitus. Scale bar: 1 mm.
Figure 51.
Habitus of Sindolus optatusA dorsal habitus B lateral habitus C ventral habitus. Scale bar: 1 mm.
The phylogeny presented by Short et al. (2021; figs 1, 2 therein) provided evidence for elevating Batochares and Sindolus to full generic status, as well as for synonymizing Helocharimorphus and Hydrobaticus with Helochares. Nevertheless, there are several taxonomic issues within Helochares left unresolved, which we aim to sort out here. In addition, it is now clear that the presence of rows of serial punctures along the elytra is not necessarily a reliable character to recognize genera (or subgenera) within Acidocerinae, whereas the configuration of the male genitalia, which is much more conserved within clades, is very useful for recognizing allied species.
Updating the classification of the Acidocerinae
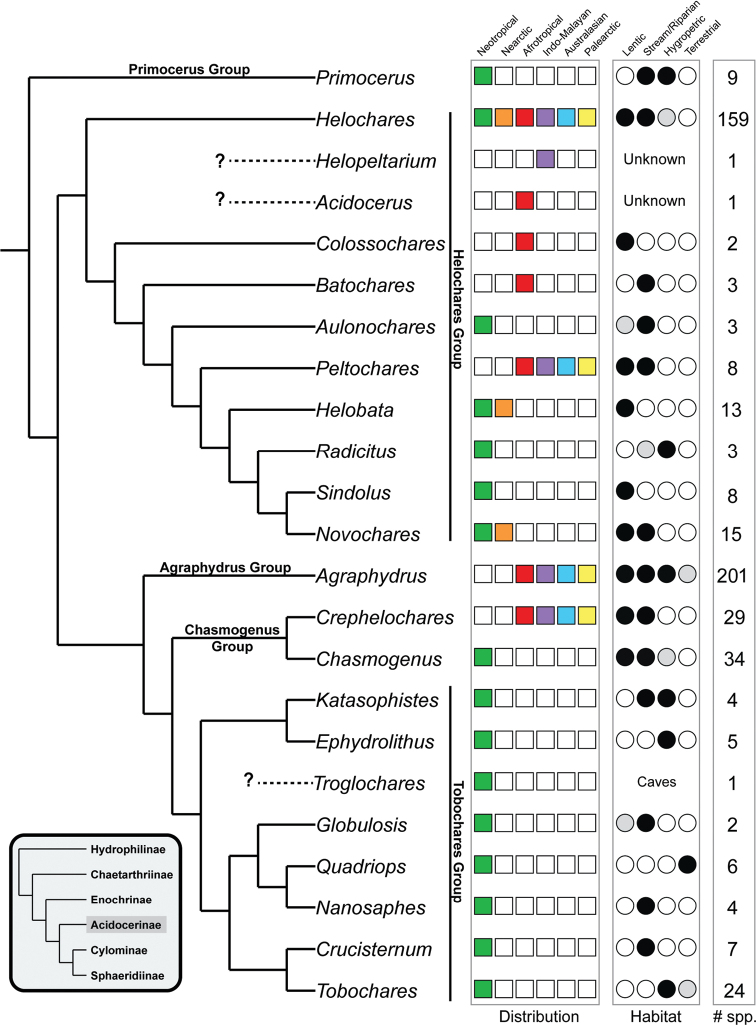
Based on their phylogeny, Short et al. (2021) defined five monophyletic genus groups within the Acidocerinae (Fig. 2): the Primocerus group (including only Primocerus; Helochares group (including Helochares, Colossochares gen. nov., Batochares, Aulonochares, Peltochares, Helobata, Radicitus, Sindolus, and Novochares gen. nov.), Agraphydrus group (including only Agraphydrus), Chasmogenus group (Chasmogenus and Crephelochares), and Tobochares group (Katasophistes, Ephydrolithus, Globulosis, Quadriops, Nanosaphes, Crucisternum, and Tobochares).
Figure 2.
Phylogeny of the Acidocerinae simplified from Short et al. (2021), indicating the distribution, preferred habitat, and currently described number of species for each genus. For habitat, filled black circles indicate that at least some species of the genus are commonly found in this habitat; light grey circles indicate the genus has been found in this habitat, but is rare or not typical for the group; white circles indicate no species have been recorded for the genus in this habitat.
Colossochares gen. nov. is established to accommodate two African species previously described as Helochares (s. str.) (Fig. 2; Helochares Clade B in Short et al. 2021: fig. 2). Peltochares sensu nov. is hereby redefined to include eight Old World species previously described as Helochares (s. str.) (Fig. 2; Helochares Clade C in Short et al. 2021: fig. 2); a lectotype is designated for its type species P. conspicuus Régimbart. Novochares gen. nov. is newly established to accommodate 15 Neotropical species previously described as Helochares (s. str.) (Fig. 2; Helochares Clade D in Short et al. 2021: fig. 2). Helochares sensu nov. is redefined, including 159 species world-wide distributed (Fig. 1; Helochares Clade A in Short et al. 2021: fig. 1). After the publication of a series of revisions of the genus Agraphydrus (Komarek and Hebauer 2018; Komarek 2018, 2019, 2020; Komarek and Freitag 2020), Helochares is now the second largest genus in number of species.
Genus groups within the Acidocerinae
Although the Acidocerinae is the third largest subfamily of Hydrophilidae and is experiencing a rapid growth in diversity, it is not partitioned into tribes as the largest two subfamilies are (Sphaeridiinae and Hydrophilinae). Although there do seem to be reciprocally monophyletic lineages that could serve as tribes, some do not have clear or unambiguous morphological synapomorphies and are therefore very difficult to diagnose. Instead, Short et al. (2021) established five genus groups in place of formal tribes.
Primocerus group
This group contains a single Neotropical genus, Primocerus with nine described species. The group is defined by the lack of a distinct sclerotized gonopore and the presence of a sclerotized projection at the apex of the median lobe. However, it is more readily recognized by the presence of a sharp sutural stria, which is otherwise only found in members of the Chasmogenus group. As such, care must be taken to separate Primocerus and Chasmogenus, as the genera overlap in the Guiana Shield region of South America; the condition of the posterior elevation of the mesoventrite is a useful character to distinguish them.
Helochares group
The Helochares group is the largest lineage of Acidocerinae, which contains 11 genera with a combined 213 species. It is extremely heterogeneous in body form, containing species from very small (e.g., 2 mm in some Helochares) to the largest acidocerine, Colossochares ellipticus (d’Orchymont). The group is distributed worldwide. There is no clear unique morphological synapomorphy for the lineage, but it exhibits a putative behavioral synapomorphy: the females of most (if not all) species in the group carry around their egg case attached to the ventral surface of the abdomen.
Agraphydrus group
The Agraphydrus group contains a single genus (Agraphydrus) that is distributed primarily in the Old World tropics, particularly southeast Asia. The group has exploded in diversity over the last few years, as more than 100 species have been described in a multi-part revision starting in 2018 (Komarek and Hebauer 2018). Potential synapomorphies for the Agraphydrus group include the V-shaped abdominal sternite 9 (Minoshima 2016). Although all placed within a single genus, the morphological variation is rather broad (though perhaps not as broad as Helochares) and includes a variety of forms that have been at times placed in other genera, most notably two species that were not long ago placed in their own subfamily (Horelophopsinae).
Chasmogenus group
The Chasmogenus group contains two genera, the Neotropical-endemic Chasmogenus and the Old-World Crephelochares. The group is most easily distinguished from all others, except the Primocerus group, by the sharply impressed sutural striae. Indeed, in the Old World, it is the only group of Acidocerinae with sutural striae.
Tobochares group
The Tobochares group is comprised of seven Neotropical genera, all of which were described in the last 20 years. Although the group is well-supported as monophyletic by molecular data (Short et al. 2021), there is no clear synapomorphy that identifies membership in the lineage. All species are relatively small (most less than 3 mm), and includes the smallest known acidocerines (e.g., Nanosaphes, at just 1.1 mm in length).
Materials and methods
Morphological methods
Specimen preparation and examination methods are identical to those given in Girón and Short (2017). For each genus, a list of diagnostic character states is provided, followed by notes comparing with similar genera. Morphological terminology largely follows Hansen (1991) except for the use of meso- and metaventrite instead of meso- and metasternum, and the terminology for veins and areas of the hind wings, which follows those of Lawrence and Ślipiński 2013. Diagnoses of genera and species lists are organized in alphabetical order. Figures illustrating each genus are arranged in alphabetical sequence, but within each plate, images are organized to display variation.
Distributional data
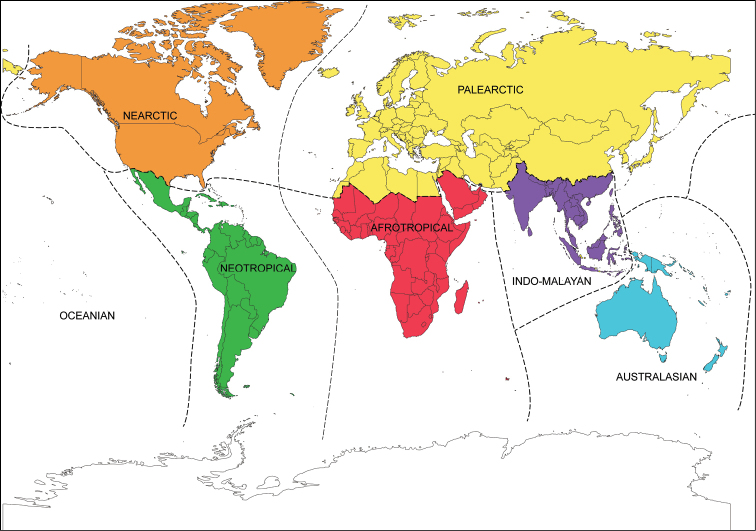
For consistency, we followed the biogeographic regions as delimited by Hansen (1999b) with the following exceptions for convenience: Saudi Arabia is here treated entirely as Afrotropical (rather than split between Afrotropical and Palearctic regions), and India is considered entirely Indo-Malayan (rather than being split between the Indo-Malayan and Palearctic regions) (Fig. 3). To increase precision for several larger countries, records are given for the States/Provinces of Brazil, China, India, and the United States. Specimen data regarding the material examined in this study can be searched by species through the Collection Resources for Aquatic Coleoptera (CReAC) portal at http://creac.kubiodiversityinstitute.org/collections/.
Figure 3.
World map showing the boundaries of the biogeographic regions as used in this work, modified from Hansen (1999b).
Current numbers of species per genus have been consolidated and are presented for each of the regions where acidocerines occur. Known distributional information obtained from the literature has been summarized for each species and included in the catalog.
Catalog
Each current genus or species name is followed by its original name including its full reference. A list of subsequent names and references, in chronological order, is also included where appropriate, indicating in square brackets the kind of reference involved, for example, [checklist], [redescription], [taxonomic treatment], etc. Page numbers where the taxon name appears in the text are given for each reference using colon “:” after the publication year. For the most part, the list of names is based on Hansen’s (1999b) catalog; additional references are also listed. Species described between 15 December 1999 and 1 April 2021 are added to this catalog. The full checklist of valid names is available online via GBIF (https://doi.org/10.15468/ypcrsp; Girón and Short 2021b).
Results
Distribution and regional diversity of Acidocerinae
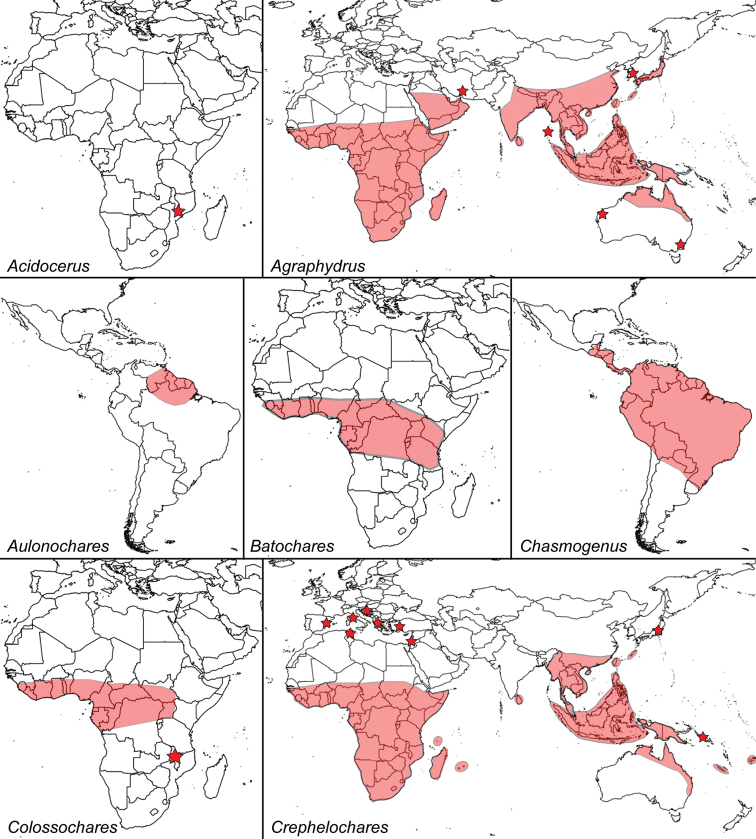
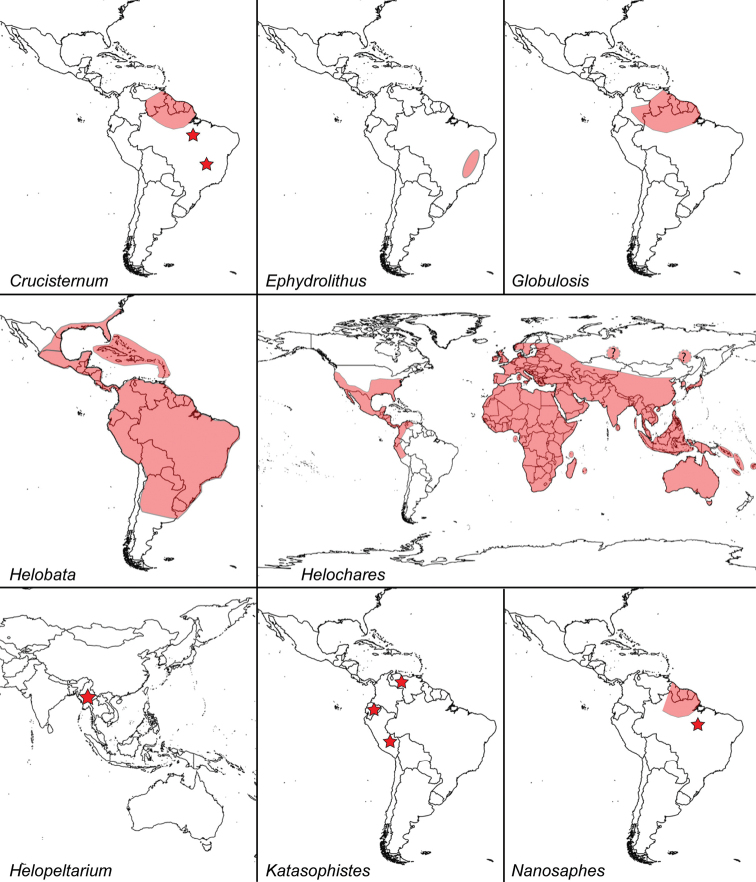
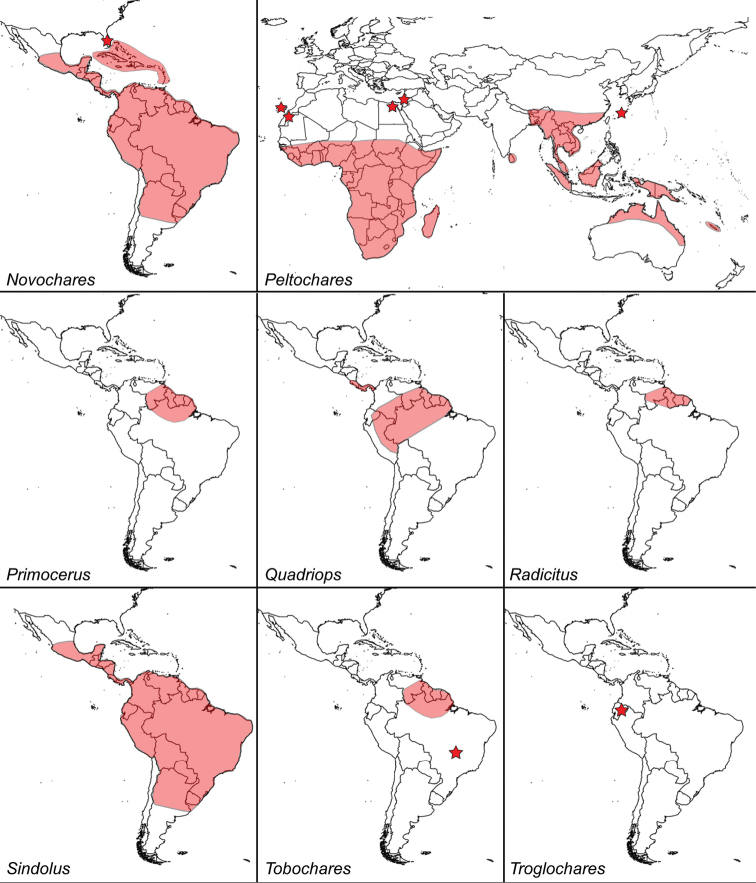
Acidocerines can be found in all biogeographic regions except the Antarctic. A summary of the distributional information of each acidocerine genus is presented in Table 1. Regions correspond to those in Fig. 3. The total number of species are given per genus, per region; in parenthesis the number of species that are shared with other regions. An en-dash is used to indicate that there are no species recorded for a given genus in a given region.
Table 1.
Distributional information for Acidocerinae. Numbers in parentheses correspond to the number of species from the region that are shared with other regions. En-dash (–) indicates that no species of the genus are recorded from that particular region.
| Afrotropical | Australasian | Indo-Malayan | Nearctic | Neotropical | Palearctic | Total | |
|---|---|---|---|---|---|---|---|
| Acidocerus Klug, 1855 | 1 | – | – | – | – | – | 1 |
| Agraphydrus Régimbart, 1903 | 30 (1) | 5 (1) | 162 (13) | – | – | 21 (15) | 201 |
| Aulonochares Girón & Short, 2019 | – | – | – | – | 3 | – | 3 |
| Batochares Hansen, 1991 | 3 | – | – | – | – | – | 3 |
| Chasmogenus Sharp, 1882 | – | – | – | – | 33 | – | 33 |
| Colossochares Girón & Short, gen. nov. | 2 | – | – | – | – | – | 2 |
| Crephelochares Kuwert, 1890 | 18 | 3 | 7 (2) | – | – | 3 (2) | 29 |
| Crucisternum Girón & Short, 2018 | – | – | – | – | 7 | – | 7 |
| Ephydrolithus Girón & Short, 2019 | – | – | – | – | 5 | – | 5 |
| Globulosis García, 2001 | – | – | – | – | 2 | – | 2 |
| Helobata Bergroth, 1888 | – | – | – | 1 (1) | 13 (1) | – | 13 |
| Helochares Mulsant, 1844 | 92 (2) | 16 (3*) | 35 (6) | 2 (2) | 8 (2) | 15 (5) | 159 |
| Helopeltarium d’Orchymont, 1943 | – | – | 1 | – | – | – | 1 |
| Katasophistes Girón & Short, 2018 | – | – | – | – | 4 | – | 4 |
| Nanosaphes Girón & Short, 2018 | – | – | – | – | 4 | – | 4 |
| Novochares Girón & Short, gen. nov. | – | – | – | (1) | 15 | – | 15 |
| Peltochares Régimbart, 1907 | 2 (1) | 3 (1) | 4 (1) | – | – | (1) | 8 |
| Primocerus Girón & Short, 2019 | – | – | – | – | 9 | – | 9 |
| Quadriops Hansen, 1999 | – | – | – | – | 6 | – | 6 |
| Radicitus Short & García, 2014 | – | – | – | – | 3 | – | 3 |
| Sindolus Sharp, 1882 | – | – | – | – | 8 | – | 8 |
| Tobochares Short & García, 2007 | – | – | – | – | 24 | – | 24 |
| Troglochares Spangler, 1981 | – | – | – | – | 1 | – | 1 |
| TOTAL by region | 148 | 27 | 209 | 4 | 146 | 40 | 541 |
* Only one species has been recorded from the Oceanian region (Samoa, Tonga).
Natural history and habitat preferences of Acidocerinae
Acidocerines, as a whole, occupy one of the widest habitat breadths of any aquatic beetle group, although most individual species are fairly narrow and predictable in their ecological preferences. Consequently, collecting in a variety of habitats using multiple methods is often required to adequately survey a locality.
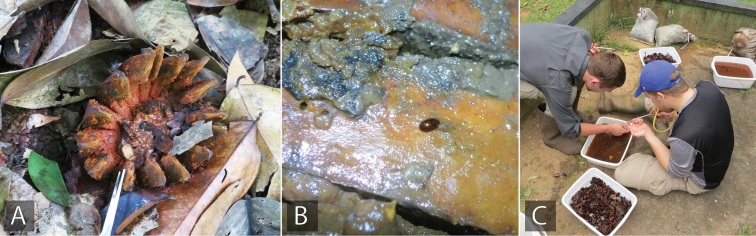
Collecting methods. Members of the subfamily are generally poor swimmers, even those most commonly found in ponds and streams. They primarily move around their habitat by clinging and crawling on substrates of submerged detritus and vegetation. When dislodged, they will float to the surface of the water until they can grab onto something to pull themselves below again. Because of this, the most effective method for collecting acidocerines is typically to agitate the habitat they are living in (e.g., detritus, emergent vegetation, etc.) and collect them either by hand or with a small strainer or sieve when they float to the surface. For example, vigorously treading along the margin of a marsh or pond (Fig. 7C) will cause many non-swimming hydrophilids to rise to the surface for easy collection. In habitats where this is difficult, the vegetation or detritus can be submerged and agitated in a pan or bucket of water to create the same effect (Fig. 8D). Likewise, the pan flotation method is also effective for seepage taxa, where the moss, detritus, or other seepage debris can be put in a pan of water and the specimens floated out of it.
Figure 7.
Examples of open and forested lentic habitat for AcidocerinaeA vegetated ditch B pond margin (Brazil: BR18-0720-04A) C stomping vegetation and substrate in a shallow marsh or ditch (Brazil: BR18-07-01A) D margin of forested swamp (Brazil: BR18-0724-04A) E forested detrital pool (Suriname: SR13-0817-01A) F forested detrital pool (French Guiana: FG20-0307-01D).
Figure 8.
Examples of lotic and riparian habitat for AcidocerinaeA forested stream (Suriname: SR12-0320-02A) B forested stream (Suriname: SR17-0331-01B) C forested stream (Suriname: SR10-0820-01A) D technique of flotation of detritus from stream margin in a white pan, a few small acidocerines can be seen floating on the surface E forested stream (Guyana: GY14-0925-01B) F open gravel stream (USA: California: US16-0908-04A).
Some species readily come to lights, occasionally in large numbers, especially those that live in open marsh and other similar lentic type habitats. Flight intercept traps (FITs) have been effective for collecting select taxa in dense tropical forests. While FITs do not generally produce high volumes of acidocerine specimens, they have been effective at trapping species that are rare or otherwise may miss detection. This is especially true for species that are not found in traditional aquatic habitats. For instance, early collections of the genus Quadriops were almost exclusively known from FIT samples, prior to our knowledge that it was a terrestrial genus. Malaise traps are generally ineffective at surveying acidocerines, and water beetles in general.
Open marsh and pond habitats. Open, exposed lentic habitats such as shallow marshes (Fig. 7C), pond margins (Fig. 7B), and vegetated ditches (Fig. 7A) are perhaps thought of as being the most “classical” habitat for acidocerines. This includes the largely slack-water margins and floating macrophytes of large rivers. Most acidocerines are found in shallow and/or marginal areas, or in areas with abundant emergent vegetation or detritus. Because they are clingers/crawlers, they will not be found in deep water or in areas that are devoid of ample detritus or vegetation in which to hide or cling to. This is a common habitat for many Helochares and Novochares species, and the near-exclusive habitat of Sindolus and Helobata. Other genera such as Chasmogenus, Crephelochares, and Agraphdyrus that are mostly found in other habitats have at least one open lentic species.
Forested lentic habitats. Standing water habitats such as forested pools (Fig. 7E, F) and shallow swamps (Fig. 7D) can be extremely productive for collecting acidocerines, especially when there is abundant detritus. Shallow detrital pools, especially in the early to mid-dry season when they are contracting, can contain abundant acidocerines. In the Neotropics, this is the most common habitat for species of Novochares and Chasmogenus. We presume that similar habitats in Africa and Asia would be productive for Helochares, Crephelochares, and Peltochares.
Stream and riparian habitats. Lotic habitats harbor a broad range of acidocerine taxa, although these can typically be broken into two categories: (1) stream margins that are vegetated or otherwise formed by “banks” with roots (Fig. 8A–C), and (2) stream margins that are composed of sand or gravel, also including sandbars and floodplains (Fig. 8E, F). The vegetated margins of small to medium sized streams, especially those in tropical forests, are the preferred habitat for a number of genera, including Globulosis, Crucisternum, Nanosaphes, and Aulonochares. Other genera such as Helochares, Novochares, Katasophistes, and Agraphydrus have taxa that occur here as well. Sand and gravel margins of streams are also common habitats for certain acidocerinae species, but there is little overlap between the species that prefer gravel margins and those that occur in vegetated/root mat margins. In North and Central America, these sandy margins are frequently home to Helochares normatus (LeConte). In South America, some species of Chasmogenus are common in these habitats, especially in the foothills of the Andes.
Hygropetric and seep habitats. Hygropetric habitats encompass a surprisingly diverse array of microhabitats that are generally characterized by thin water films flowing or seeping over rocky substrate. These habitats most frequently occur in association with (and connected to) rivers and streams, such as in misting or trickle zones adjacent to waterfalls (Fig. 9E, F), or where streams flow over or near expanses of rock (Fig. 9A, B). Others may be isolated or self-contained, such as the seasonal seeps that form on inselbergs and are not necessarily connected to a larger lotic network (Fig. 9C, D). The genera Tobochares, Ephydrolithus, Radicitus, and Primocerus almost exclusively occur in seepage habitats. Many other genera have at least one hygropetric specialist, including Agraphydrus (numerous), Katasophistes (K. merida Girón & Short), and Chasmogenus (C. cremnobates (Spangler)).
Figure 9.
Examples of seepage habitat for AcidocerinaeA, B marginal seepage along river (Guyana: GY14-0312-01B) C, D isolated seep on granite inselberg (Venezuela: VZ10-0710-01A) E, F hygropetric zone next to waterfall (Venezuela: VZ12-0122-03A).
Terrestrial habitats. Although rare within Acidocerinae, several genera contain at least one species that has been collected in terrestrial situations. All species of Quadriops are known or suspected of being entirely terrestrial (Girón and Short 2017). One species, Q. clusia Girón & Short, is reliably found in the rotting fruits of Clusia fruits (Fig. 10), while Q. reticulatus Hansen has been collected from sap flows in freshly cut trees. Other species are known from passive collecting methods such as FITs but were not found in nearby aquatic habitats. Some species of Agraphydrus also appear to be terrestrial, as we have seen series of at least one species from Madagascar from several samples of sifted rainforest litter (Short, pers. obs.). Some other Agraphydrus species have ambiguous or incidental collecting information suggesting they may occur in terrestrial habitats, but more data is needed (e.g., A. vadoni Komarek). Additionally, Tobochares fusus Girón & Short has been collected from both seepage habitats as well as from the rotting fruits of Clusia, suggesting it might have a broad ecological niche (Girón and Short 2021a).
Figure 10.
Examples of terrestrial habitat for AcidocerinaeA, B Rotting Clusia fruit, showing Quadriops clusia crawling on the surface (Suriname: SR17-0322-03A) C collecting specimens by submerging rotting fruits in pans of water and waiting for the beetles to float to the surface.
Other unusual habitats. The blind genus Troglochares is only known from a single cave in Ecuador, where it was found clinging to a stalactite. A few species of Agraphydrus [e.g., A. hanseni (Satô & Yoshitomi)] are associated with the gravel margins of estuarine rivers (Satô and Yoshitomi 2004), however it is not known to what extent they may have any tolerance for salinity.
Karyotypes of Acidocerinae
A paper summarizing the available information on the karyotypes of water scavenger beetles was recently published by Angus et al. (2020). According to Angus et al. (2020), in Acidocerinae “the diploid number of chromosomes is 2n = 18”. Table 2 presents the list of known acidocerine karyotypes.
Table 2.
List of acidocerine species with known karyotypes. Origin refers to the country where the adults were collected according to Angus et al. (2020).
| Species | Origin |
| Agraphydrus decipiens Minoshima, Komarek & Ôhara | Taiwan |
| Agraphydrus variabilis Komarek & Hebauer | Taiwan |
| Helochares lividus (Forster) | United Kingdom |
| Helochares obscurus (Müller) | Sweden |
| Helochares punctatus Sharp | United Kingdom |
| Helochares sauteri d’Orchymont | Taiwan |
Larvae of Acidocerinae
From the 541 acidocerine species, immature stages are only known for 18 species in seven different genera to date. Information is summarized in Table 3.
Table 3.
Summary of information on immature stages of Acidocerinae. Origin refers to the country where the adults, eggs, or larvae were collected according to the provided references.
| Species | Origin | Described stages | References |
| Agraphydrus hanseni (Satô & Yoshitomi) [as Horelophopsis hanseni] | Japan | Third instar larva | Minoshima et al. 2013 |
| Agraphydrus narusei (Satô) | Japan | First and third instar larva | Minoshima and Hayashi 2011 |
| Crephelochares nitescens (Fauvel) [as Helochares nitescens or Chasmogenus nitescens] | Australia | Eggs, egg case, first and third instar larvae, pupa | Anderson 1976; Archangelsky 1997 |
| Helobata larvalis (Horn) | Guatemala | Egg case, first instar larva | Spangler and Cross 1972; Archangelsky 1997 |
| Helochares anchoralis Sharp | Japan | First instar larva | Minoshima and Hayashi 2011 |
| Helochares clypeatus (Blackburn) | Australia | Third instar larva | Watts 2002 |
| Helochares lividus (Forster) [also Helochares griseus (Fabricius)]* | Unknown (Palearctic) – Italy | Unknown stage larva in d’Orchymont 1913b; first, second and third instar larvae in Panzera 1932 | d’Orchymont 1913b; Panzera 1932 |
| Helochares luridus (MacLeay) | Australia | Third instar larva | Watts 2002 |
| Helochares maculicollis Mulsant | USA | Eggs, first and third instar larvae, pupa | Richmond 1920; Archangelsky 1997 |
| Helochares nipponicus Hebauer | Japan | First, second and third instar larvae | Minoshima and Hayashi 2011 |
| Helochares pallens (MacLeay) | Japan | First, second and third instar larvae | Minoshima and Hayashi 2011 |
| Helochares tenuistriatus Régimbart | Australia | Third instar larva | Watts 2002 |
| Helochares tristis (MacLeay) | Australia | Eggs, first, second and third instar larvae, pupa | Anderson 1976; Watts 2002 |
| Novochares pallipes (Brullé) [as Helochares (s. str.) pallipes] | Argentina | Egg sac, first, second and third instar larvae, pupa | Fernández 1983 |
| Peltochares conspicuus Régimbart** | Madagascar | Unknown stage larva | Bertrand 1962 |
| Peltochares foveicollis (Montrouzier) [as Helochares foveicollis] | Australia | Third instar larva | Watts 2002 |
| Sindolus femoratus (Fernández) [as Helochares (Sindolus) femoratus] | Argentina | Egg case, first, second and third instar larvae, pupae | Fernández 2004 |
| Sindolus talarum (Fernández) [as Helochares (Sindolus) talarum] | Argentina | Egg case, first, second and third instar larvae, pupae | Fernández 1983 |
* Panzera (1932) described the larvae of “Helochares griseus” (p. 54) and Helochares lividus (p. 60); “Helochares griseus” is a synonym of Helochares lividus (Forster), with some varieties of “Helochares griseus” synonymized with Helochares obscurus (Müller). This description might correspond to Helochares lividus (Forster) or Helochares obscurus (Müller). **Peltochares conspicuus has never been reported from Madagascar. The species identification is likely incorrect.
Females lay between 18 (Crephelochares nitescens (Fauvel); Anderson 1976) and 103 eggs (Novochares pallipes (Brullé) comb. nov.; Fernández 1983) per egg case or nest. In observations from rearing experiments, it has been described that the larvae emerging from egg sacs carried by the females, the larvae seem to emerge towards the mother’s air bubble to capture their own first air bubble (Anderson 1976). For Crephelochares nitescens, it was described that the females deposit their eggs in cavities built by the adults in damp soil (Anderson 1976). Larvae of Sindolus talarum have been described to perforate and enter the aerenchyma of Spirodella intermedia (Araceae) and staying in the plant tissue for some time, apparently breathing the air stored in the plant tissues (Fernández 1983).
The fossil record of Acidocerinae
Five fossil species have been assigned to Acidocerinae (one of them ambiguously; Table 4). Four of these are compression fossils, one from Australia and three from China. The fifth fossil is a Baltic amber inclusion from Poland, which has been assigned to an extant genus (Helochares fog Arriaga-Varela, Brunke, Girón & Fikáček). Despite the diagnostic features presented by Fikáček et al. (2014) on their subfamily designations, the authors highlight that these compression fossils exhibit a generalized morphology in which only specific combinations of character states (as opposed to the presence of synapomorphic features) support those designations. Unlike compression fossils, where there is no realistic way to recover additional information from what is preserved and visible on the rock, amber inclusions have the possibility of offering more details when studied with techniques such as visualization using X-ray micro-computed tomography (μCT, Arriaga-Varela et al. 2019). Helochares fog has been used as a calibration point to date the phylogeny of Hydrophilidae (Bloom et al. 2014; Toussaint and Short 2018). One additional fossil, Cretocrenis burmanicus Fikáček, Minoshima, Komarek, Short, Huang, & Cai from Burmese amber (ca. 99 ma) has been formally placed in the Anacaenini, although it does have some superficial similarities with Acidocerinae (Fikáček et al. 2017).
Table 4.
Summary of information on fossil species of Acidocerinae.
| Species | Type locality | Geological epoch |
| Alegorius yixianus Fikáček, Prokin, Yan, Yue, Wang, Ren & Beattie, 2014*; Fikáček et al. 2014 | China, Liaoning Province, Shangyuan County, Chaomidian Village, Huangbanjigou. | Yixian Formation: Early Cretaceous, Lower Cretaceous, Aptian, 124.6 Mya; Jurassic–Cretaceous boundary, Late Tithonian–Berriasian, ca. 145–140 Mya |
| Helochares fog Arriaga-Varela, Brunke, Girón & Fikáček, 2019; Arriaga-Varela et al. 2019 | Poland. | Baltic amber: Lower Eocene to Lower Oligocene, ca. 44 Mya |
| Hydroyixia elongata Fikáček, Prokin, Yan, Yue, Wang, Ren & Beattie, 2014; Fikáček et al. 2014 | China, Liaoning Province, Shangyuan County, Chaomidian Village, Huangbanjigou. | Yixian Formation, Early Cretaceous, Lower Cretaceous, Aptian, 124.6 Mya; Jurassic–Cretaceous boundary, Late Tithonian–Berriasian, ca. 145–140 Mya |
| Hydroyixia latissima Fikáček, Prokin, Yan, Yue, Wang, Ren & Beattie, 2014; Fikáček et al. 2014 | China, Liaoning Province, Shangyuan County, Chaomidian Village, Huangbanjigou. | Yixian Formation, Early Cretaceous, Lower Cretaceous, Aptian, 124.6 Mya; Jurassic–Cretaceous boundary, Late Tithonian–Berriasian, ca. 145–140 Mya |
| Protochares brevipalpis Fikáček, Prokin, Yan, Yue, Wang, Ren & Beattie, 2014; Fikáček et al. 2014 | Australia, New South Wales, Talbragar Fossil Fish Bed, ca. 14 km NNW of Ulan, 25 km NE of Gulgong, 32°9.9'S, 149°41.0'E. | Late Jurassic Oxfordian–Tithonian, 161–145 Mya; Kimmeridgian, 155–150 Mya. |
* The genus Alegorius has been assigned in doubt to either Acidocerinae or Enochrinae.
Morphological variation in Acidocerinae and its taxonomic importance
The Acidocerinae have been described as “relatively uniform and difficult to characterize” (Short and Fikáček 2013), mostly because for each proposed synapomorphy, there are taxa that exhibit exceptional character states. The phylogeny presented by Short et al. (2021) revealed a high recurrence of morphological convergence across the phylogeny of the Acidocerinae that seem to track ecologies rather than phylogenetic relationships. Here we present an account of morphological features, how they vary in the subfamily, and their usefulness for recognizing taxonomic units. A summary of the main diagnostic features of each genus is presented in Table 5 at the end of ths section.
Table 5.
Summary of main diagnostic features of acidocerine genera.
| Genus | Size | Antennomeres | Sutural Stria | Serial punctures or striae | 5th Ventrite | Metafemora |
|---|---|---|---|---|---|---|
| Acidocerus | 2.8 mm | 9 | Absent | Present | Emarginated | Mostly pubescent |
| Agraphydrus | 1.4-4.8 mm | 8 or 9 | Absent | Variable | Variable | Variable |
| Aulonochares | 5.8-7.5 mm | 9 | Absent | Absent | Emarginated | Mostly pubescent |
| Batochares | 3-4 mm | 9 | Absent | Present | Truncate | Mostly pubescent |
| Chasmogenus | 2.5-5.0 mm | 8 | Present | Absent | Emarginated (weak) | Mostly Pubescent |
| Colossochares | 8.5-14.0 mm | 9 | Absent | Absent | Emarginated | Mostly pubescent |
| Crephelochares | 2.5-4.8 mm | 9 | Present | Absent | Emarginated (weak) | Mostly pubescent |
| Crucisternum | 2.0-2.5 mm | 9 | Absent | Absent | Rounded | Mostly pubescent |
| Ephydrolithus | 1.8-3.3 mm | 9 | Absent | Variable | Truncate | Mostly glabrous |
| Globulosis | 1.9-2.3 mm | 8 | Absent | Absent | Emarginated | Mostly pubescent |
| Helobata | 4-7 mm | 8 | Absent | Variable | Emarginated | Mostly pubescent |
| Helochares | 2-7 mm | 9 | Absent | Variable | Emarginated | Mostly pubescent |
| Helopeltarium | 3.5 mm | 9 | Absent | Absent | Emarginated | Mostly pubescent |
| Katasophistes | 2.7-4.5 mm | 9 | Absent | Absent | Emarginated (weak) | Mostly pubescent |
| Nanosaphes | 1.1-1.5 mm | 8 | Absent | Absent | Emarginated | Mostly pubescent |
| Novochares | 4.5-9.0 mm | 9 | Absent | Variable | Emarginated | Mostly pubescent |
| Peltochares | 6-14 mm | 9 | Absent | Variable | Emarginated | Mostly pubescent |
| Primocerus | 2.4-4.9 mm | 8 | Present | Variable | Variable | Variable |
| Quadriops | 1.6-2.6 mm | 9 | Absent* | Variable | Rounded | Mostly glabrous |
| Radicitus | 4.5-6.2 mm | 9 | Absent | Variable | Rounded | Pubescent on anterior third |
| Sindolus | 2.5-5.0 mm | 9 | Absent | Absent | Emarginated | Mostly pubescent |
| Tobochares | 1.5-2.6 mm | 8 | Absent* | Variable | Rounded | Mostly glabrous |
| Troglochares | 1.9 mm | 9 | Absent | Absent | Rounded | Pubescent (~half)* |
* When impressed, the stria I on each elytron can be comparatively more strongly impressed, specially along the posterior half of the elytron, which might resemble a well-developed sutural stria.
Size and shape of body. This subfamily includes members among the largest (14.0 mm) and smallest (1.1 mm) hydrophilids (Fig. 1). In general terms, acidocerines can very roughly be grouped by their size: most genera in the Helochares group (sensu Short et al. 2021) are larger than 4 mm (Fig. 1), whereas Agraphydrus, Chasmogenus, Crephelochares, Primocerus, and members of the Tobochares group are smaller than 4.5 mm (Fig. 1). The body is usually oval and parallel-sided, occasionally slightly broader anteriorly or posteriorly; it can also be rather dorsoventrally flattened [e.g., Helobata (Fig. 1J), Peltochares (Fig. 1C), Helopeltarium (Fig. 1H)], or strongly convex [e.g., Globulosis (Fig. 1U), Colossochares (Fig. 1A), Radicitus (Fig. 1K)], but it is generally moderately convex. The outline of the body in dorsal view is continuous (not interrupted between pronotum and elytra) when the specimens are in natural resting position; when a specimen is card-mounted the outline of the body may appear interrupted.
Coloration. Body color ranges from very pale (yellowish) to very dark brown (appearing almost black), and it is usually uniform along the dorsal surfaces of the body, although sometimes the margins of the pronotum and elytra may be slightly paler than the disc (Fig. 1). The ventral surface of the body and the appendages (or parts of appendages) tend to be paler than the dorsum. In Batochares (e.g., Fig. 1I) and Helobata (e.g., Fig. 1J), there are alternating areas of darker/paler colorations along the elytra, giving specimens a flecked or speckled appearance. In some species of Nanosaphes, different regions of the body (head, pronotum, elytra) have different colorations (e.g., Fig. 1L); in some species of Tobochares, the lateral margins of the clypeus are paler (e.g., Fig. 1N); in both cases, coloration can be used for species group recognition. The coloration of the maxillary palps can also be helpful in diagnosing species (e.g., in Tobochares and Helochares), as the apex, or rarely the entire palp can be darkened. In some genera, internal structural reticulations are visible throughout the surface (mostly on the elytra), giving the beetles a “checkered” appearance of darker spots over a paler background, e.g., Aulonochares (Fig. 1D), New World Helochares (Fig. 36A, B; Short and Girón 2018).
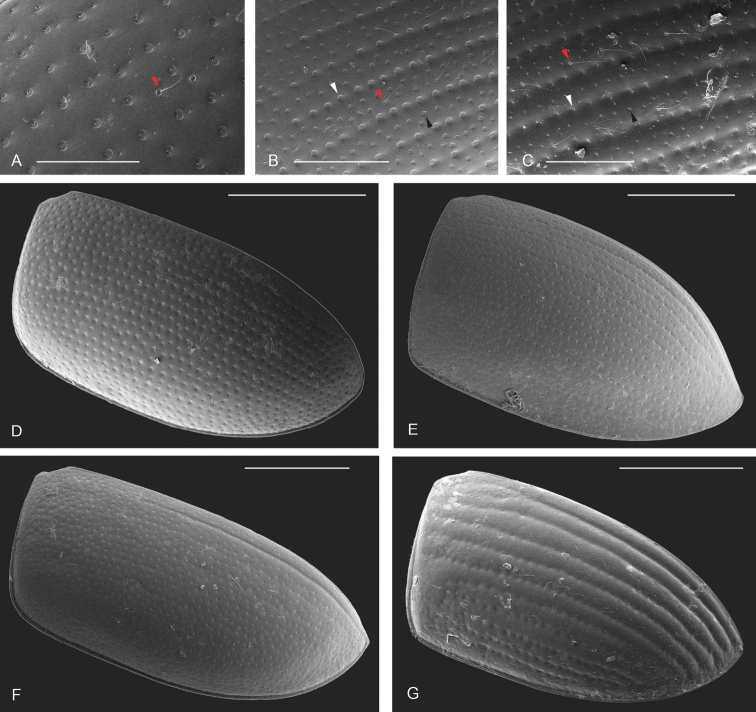
Punctation. Three kinds of punctures can be recognized along the dorsal surface of the body in Acidocerinae that may be shallowly to moderately or sharply (strongly) marked. Ground punctures are usually fine and uniformly distributed along the entire body. Systematic punctures (sensu Hansen 1991), those bearing a seta inserted in a doughnut-shaped socket (thrichobothria sensu Short and Fikáček 2013; Fig. 13A–C, red arrows), are usually well developed and can also be found along the entire body, being more densely distributed in particular areas of the head, pronotum and elytra. The seta on a systematic puncture is usually fine and can be short or long; sometimes these setae may be lost by abrasion but are usually visible along the lateral and posterior areas of the elytra. Systematic punctures usually form well defined rows along the elytra; quite a few species in some genera exhibit four or five rows of systematic punctures clearly enlarged in comparison with the remainder elytral punctation, e.g., Agraphydrus (Fig. 1M, S, T), Ephydrolithus (Fig. 31), Katasophistes (Fig. 39). Serial punctures are only present along the elytra and can only be recognized when well-developed (larger and usually more impressed than ground punctures), as they form usually ten well-defined rows, at least along the posterior third of each elytron (e.g., Radicitus, Fig. 50A, B); some Agraphydrus species have strongly enlarged and irregular elytral series of punctures (e.g., Fig. 18D–F). Serial punctures were traditionally used for the recognition of subgenera within Helochares sensu Hansen (1999b), but it has been shown that the presence or absence of this kind of punctures has taxonomic value only at the species or species group level in certain genera (e.g., Primocerus, Fig. 46; Tobochares, Fig. 52–54). The presence, size, density, degree of impression and development/differentiation of punctures on the dorsal surface of the body are useful for recognition of certain genera and species, but there are no general character states that cover the entire subfamily.
Figure 13.
Elytral punctation ATobochares communis with red arrow pointing to systematic puncture BTobochares sipaliwini with red arrow pointing to systematic puncture, white arrow pointing to serial puncture, and black arrow pointing to ground/interserial puncture CTobochares striatus with red arrow pointing to systematic puncture, white arrow pointing to serial puncture, and black arrow pointing to ground/interserial puncture DTobochares communis elytron with all kinds of punctures similar in size and degree of impression, seemingly evenly distributed (to longitudinally aligned) EQuadriops similaris with serial punctures longitudinally aligned FPrimocerus maipure with sutural stria GTobochares striatus with impressed serial striae. Scale bars: 100 μm (A); 200 μm (B, C); 500 μm (D–G).
Figure 31.
Habitus of Ephydrolithus spp. A–CE. hamadae: A dorsal habitus B lateral habitus C ventral habitus D–FE. ogmos: D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 1 mm.
Figure 39.
Habitus of Katasophistes spp. A–CK. merida: A dorsal habitus B lateral habitus C ventral habitus D–FK. superficialis: D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 1 mm.
Figure 50.
Habitus of Radicitus spp. A–CR. ayacucho: A dorsal habitus B lateral habitus C ventral habitus D–FR. granitum: D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 1 mm.
Figure 52.
Habitus of Tobochares spp. A–CT. sulcatus: A dorsal habitus B lateral habitus C ventral habitus D–FT. luteomargo: D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 0.5 mm.
Figure 54.
Habitus of Tobochares spp. A, BT. kappel: A dorsal habitus B lateral habitus C, DT. akoerio: C dorsal habitus D lateral habitus E, FT. kolokoe: E dorsal habitus F lateral habitus G, HT. goias: G dorsal habitus H lateral habitus. Scale bars: 1 mm.
Eyes. The only known species of hydrophilid lacking eyes (Troglochares ashmolei Spangler, Fig. 56) is a member of the Acidocerinae. Eyes range in shape from subquadrate to oval and are usually of moderate size (Fig. 11E–L), although in some species the eyes are relatively small (e.g., Primocerus ocellatus Girón & Short, Tobochares microps Girón & Short). In some genera, the anterior corners of the frons extend posteriorly forming a canthus that emarginates the anterior margin of the eyes (Fig. 11B), which is more evident in lateral view (e.g., Tobochares, Fig. 11B; Helobata, Fig. 11L). There is only one known acidocerine genus in which the canthus reaches the posterior margin of the eye, thus completely dividing the eye in dorsal and ventral faces (Quadriops; Fig. 11C). In some genera the eyes are protruding, interrupting the outline of the head (e.g., Aulonochares; Fig. 11J). In most cases the proportion between the width of an eye and the distance between eyes remains constant across congeneric species. The shape, size, and degree of protrusion of the eyes are useful for generic recognition.
Figure 56.
Holotype and labels of Troglochares ashmoleiA mount of holotype B head, dorsal view C labels.
Figure 11.
Head of miscellaneous AcidocerinaeA–D anterolateral view: ATobochares luteomargo with white arrow pointing to straight anterior margin of eye BTobochares emarginatus with white arrow pointing to canthus emarginating anterior margin of eye CQuadriops politus with white arrow pointing to canthus fully dividing the eye in dorsal and ventral faces DBatochares sp. black arrow pointing to transverse carina on labrum E–L dorsal view of head: EBatochares sp. FHelochares tristisGCrephelochares nitescens, HChasmogenus australis with black arrow pointing to preclypeal membrane IColossochares ellipticusJAulonochares tubulusKPeltochares conspicuusLHelobata larvalis.
Clypeus. It is usually roughly trapezoid (clearly wider at base; Fig. 11F–I) and relatively flat or antero-medially convex. In some genera, it fully conceals the labrum (e.g., Helobata, Fig. 11L; Helopeltarium, Fig. 38A). The shape of the anterior margin of the clypeus, and the development of a membranous preclypeal area (Fig. 11H) are useful for diagnosing species within some genera (e.g., Chasmogenus). In some Helochares the surfaces along the lateral margins of the clypeus are slightly bent upwards.
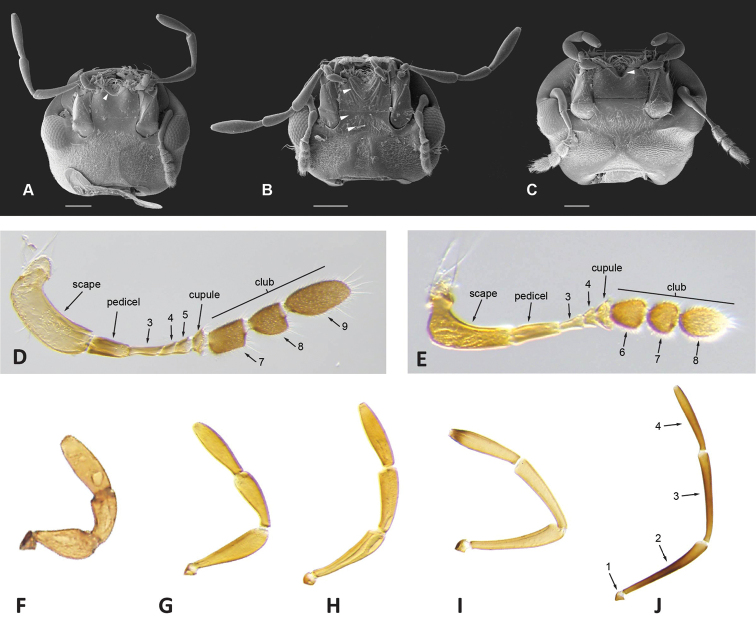
Maxillary palps. In general, the maxillary palps in Acidocerinae have been described as ‘curved inward’ (e.g., Hansen 1991), which means that the outer margin of the maxillary palpomere 2 is apically or medially curved towards the midline of the body, and the apex of palpomere 2 is oblique, so that the palpomere 3 articulates pointing towards the midline of the body. The inner margin of maxillary palpomere 2 ranges from straight (Figs 12F, G) to slightly and uniformly curved (concave; Figs 12H–J). All palpomeres tend to be of somewhat similar proportions among them, and are usually similar in length as well, although it is common that the maxillary palpomere 2 is slightly longer. The comparative length of maxillary palpomeres 3 and 4 may be useful as a supporting diagnostic feature. According to the diagnosis of the Acidocerinae offered by Hansen (1991) and by Short and Fikáček (2013), the maxillary palps are at least as long or usually longer than the width of the head (except for some Agraphydrus and Quadriops). The number of exceptions to this rule keeps growing, the more seepage taxa are found (e.g., Ephydrolithus, Radicitus, some Tobochares). The length of the maxillary palpomeres in Acidocerinae ranges from very short and stout (nearly half width of the head; e.g., Quadriops, Figs 11C, 12G), to very long and slender (nearly 2 × width of the head; e.g., Peltochares conspicuus, Fig. 11K).
Figure 12.
Head structures A–C scanning electron micrographs of ventral view of head: ATobochares pallidus with smooth mentum and white arrow pointing to transverse carina limiting posterior margin of antero-medial depression BNanosaphes tricolor with top white arrow pointing to oblique crenulations of mentum, mid white arrow pointing to flat and smooth anterior surface of submentum, and bottom white arrow pointing to concave posterior surface of submentum CQuadriops reticulatus with white arrow pointing to antero-medial depression of mentum D, E light micrographs of antenna: DAulonochares tubulus (9 antennomeres) EChasmogenus cremnobates (8 antennomeres) F–J light micrographs of maxillary palps: FQuadriops reticulatusGAgraphydrus insidiatorHHelochares sp. IHelochares lividusJAulonochares tubulus. Scale bars: 100 μm (A–C)
Mentum. The anterior margin of the mentum is usually laterally emarginated by the base of the palpigers, mesally emarginated, and deeply depressed in ventral view (projected upwards) (Fig. 12A–C); this antero-medial depression varies in width and depth and may be demarcated by a transverse crest or carina (Fig. 12A). The surface of the mentum may be flat, medially depressed or bear oblique elevations (Fig. 12B); the surface may further range from smooth (Fig. 12A) to punctate, to anteriorly striate, with little or no variation within genera. Characteristics of the mentum and submentum may be useful as supporting diagnostic features.
Antennae. The number of antennomeres is either nine (the ancestral state in Hydrophilidae; Hansen 1991; Fig. 12D) or reduced to eight (Fig. 12E). The cupule (the antennomere right before the club) can be symmetric, or slightly to strongly asymmetric. The three-part pubescent antennal club is always loosely articulated in Acidocerinae; the proportions of the club antennomeres have been used in the past to recognize some groups.
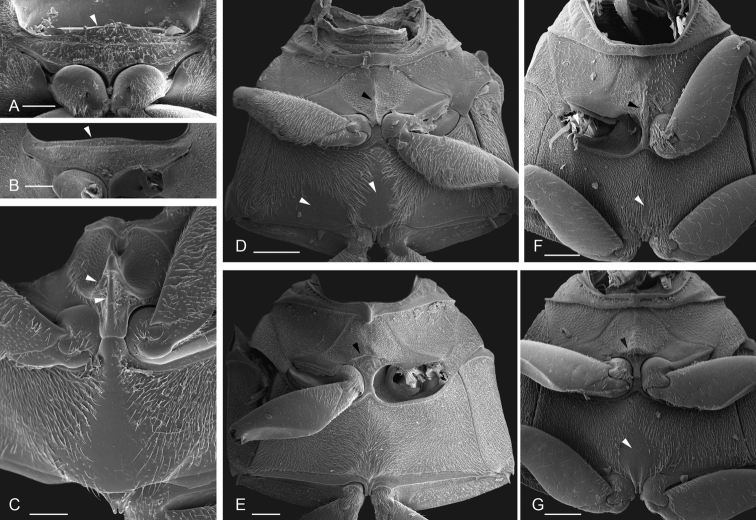
Thoracic venter. The prosternum in Acidocerinae is usually rather flat (Fig. 14A, B), at most medially tectiform or broadly bulging, except in Acidocerus and Crucisternum which bear a medial longitudinal carina. The surface of the posterior elevation of the mesoventrite is taxonomically important; it may be projected in various forms: as a longitudinal carina (Fig. 14D, F), cruciform projection (Fig. 14C), transverse ridge (Fig. 14E, G) or acute spine. The shape of the projection on the posterior elevation of the mesoventrite can sometimes be used for recognition of genera, but it may also vary among congeneric species (e.g., Ephydrolithus, Nanosaphes). The shape of the anapleural sutures ranges from angulate (forming an obtuse angle; e.g., Primocerus, Troglochares (Spangler 1981a: fig. 8) to only slightly curved (e.g., Katasophistes, Nanosaphes (Girón and Short 2018: figs 11A, 17A, respectively); the orientation along their anterior section may be nearly parallel (e.g., Helobata; Clarkson et al. 2016: fig. 8) or anteriorly converging; they may be widely separated anteriorly (anterior margin of mesoventrite nearly as wide as anterior margin of mesepisternum; e.g., Globulosis, Nanosaphes (Girón and Short 2018: fig. 17A), or very closely converging (anterior margin of mesoventrite 0.2 × the width of the anterior margin of mesepisternum; e.g., Ephydrolithus (Girón and Short 2019: fig. 7A), Katasophistes (Girón and Short 2018: fig. 11A). The metaventrite is usually densely and uniformly covered by hydrofuge pubescence; a posteromedian glabrous patch and/or posterolateral glabrous patches may also be present (Fig. 14C–G). The size and shape of the posteromedian glabrous patch is useful for recognition of some genera and subgenera (e.g., Tobochares).
Figure 14.
Scanning electron micrographs of thorax in ventral view A, B prosternum: ATobochares striatus with white arrow pointing to anterior projection BQuadriops reticulatus with white arrow pointing to anterior projection C–G mesoventrite and metaventrite: CCrucisternum ouboteri with white arrows pointing to anteriorly pointed transverse ridge and longitudinal carina of mesoventrite, metaventrite with median glabrous patch DNanosaphes tricolor with black arrow pointing to longitudinal carina along mesoventrite and white arrows pointing to median and postero-lateral glabrous patches of metaventrite EQuadriops reticulatus with black arrow pointing to transverse carina across mesoventrite and metaventrite uniformly pubescent FTobochares communis with black arrow pointing to longitudinal carina along mesoventrite and white arrow pointing to narrow postero-medial glabrous patch on metaventrite GTobochares kasikasima with black arrow pointing to transverse elevation across mesoventrite and white arrow pointing to broad postero-medial glabrous patch on metaventrite. Scale bars: 100 μm.
Elytra. The shape and punctation of the elytra are highly variable in the Acidocerinae. The elytra may be evenly convex (e.g., Radicitus, Fig. 1K) or with nearly flat dorsal outline (e.g., Helopeltarium, Fig. 1H), with outer margins slightly flared or broadly explanate (e.g., Helobata, Fig. 1J); the surface is usually smooth, but can also be granulate (e.g., Acidocerus, Fig. 17; Helobata, Fig. 33). Sutural striae are only present in Chasmogenus (Fig. 24), Crephelochares (Fig. 28), and Primocerus (Figs 13F, 46). The elytral punctation has been traditionally considered as a diagnostic feature at the subgenus level, in Helochares for example, but it is clear now that this character system can be variable among congeneric species (e.g., Ephydrolithus, Fig. 31; Katasophistes, Fig. 39; and Primocerus, Fig. 46). In some cases, all kinds of punctures (ground punctures, systematic punctures, and serial punctures) are well-developed and therefore easily recognized (e.g., Fig. 13B, C), but in other instances they can be virtually indistinguishable from each other (e.g., Fig. 13A, D, F). In some species, or even groups of species within a genus, the serial punctures are impressed forming longitudinal grooves that can extend from the anterior to the posterior margins of the elytra (e.g., Fig. 13G; Tobochares sulcatus Short & García, Fig. 52A, B), or at least along the posterior third of each elytron (e.g., Tobochares akoerio, Fig. 54C, D). When serial punctures are well developed, the ground punctures between series have been called “interserial punctures” (Fig. 13B [black arrows], C [black arrows], G; Girón and Short 2021a), and their distribution may be informative at the species level.
Figure 24.
Habitus of Chasmogenus spp. A–CC. ruidus: A dorsal habitus B lateral habitus C ventral habitus D–FC. cremnobates: D dorsal habitus E lateral habitus F ventral habitus GC. lineatusHC. ampliusIC. itatiaiaJC. fluminensis. G, H from Smith and Short 2020; I, J from Clarkson and Ferreira Jr 2014. Scale bars: 1 mm.
Figure 28.
Habitus Crephelochares spp. A–CCrephelochares nitescens: A dorsal habitus B lateral habitus C ventral habitus DCrephelochares cf. patrizii (image from Bird et al. 2017). Scale bars: 1 mm.
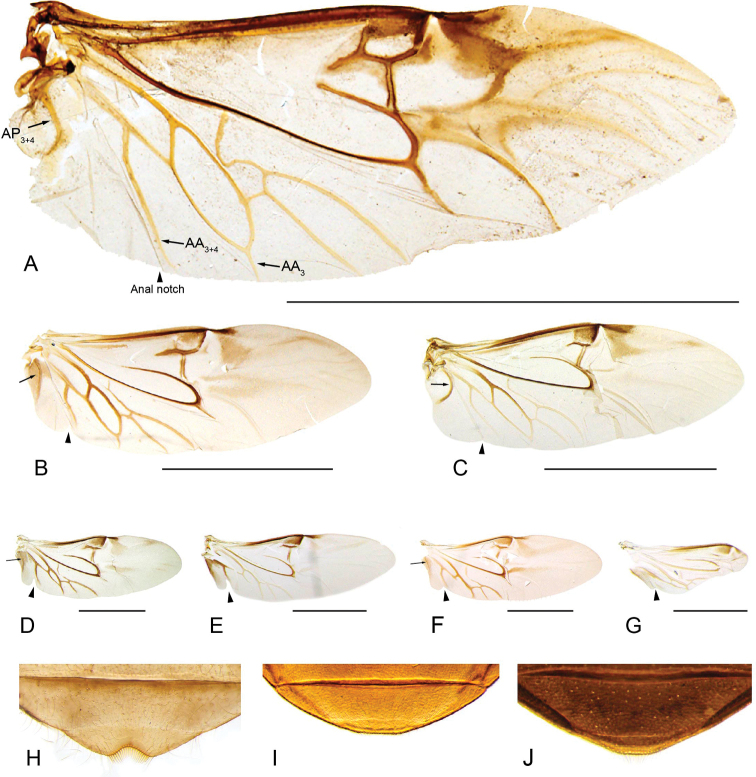
Hind wings. The hind wings of the Acidocerinae are usually well developed, with most of the general venation clearly visible. The posterior margin of the wing usually has a well-defined anal notch, demarcating a noticeable “jugal lobe” (Hansen 1991: fig. 285) that is either broad (Fig. 15B, C) or narrow (Fig. 15D–G). AP3+4 can be either thick and curved (Fig. 15A, C), or evanescent and angulate (Fig. 15B, D–G). Tobochares microps Girón & Short was found to be polymorphic for hind wing development: the reduced hind wing morph (Fig. 15G) has most veins still well developed, but the entire apical region of the wing is reduced (Girón and Short 2021a).
Figure 15.
Hind wing and abdominal ventrite 5 A–G hind wings: AColossochares ellipticusBPrimocerus gigasCHelobata larvalisDCrucisternum ouboteriETobochares sipaliwiniFQuadriops similarisGTobochares micropsH–J abdominal ventrite 5: HAulonochares tubulusIPrimocerus neutrumJEphydrolithus hamadae. Scale bars: 1 cm (A); 3 mm (B, C); 1 mm (D–F); 0.5 mm (G).
Protibiae. Two main features of the protibia are taxonomically relevant: the shape and size of the apical spurs and the characteristics of the spines composing the median longitudinal anterior row. The apical spurs are usually large and slender (longer than protarsomere 1) but can be relatively short and stout (as long as or shorter than protarsomere 1; e.g., Aulonochares). The spines composing the median longitudinal anterior row can be very short, stout, and appressed to the surface of the tibia in most members of the Helochares group (sensu Short et al. 2021), or be long, relatively thick, seta-like, and semi-erect (e.g., Tobochares group).
Metafemora. In Acidocerinae the metafemora are moderate to strongly antero-posteriorly compressed. The anterior surface of the metafemur may be covered to a variable degree with hydrofuge pubescence. Usually, species found in typical fully aquatic habitats (streams, ponds, marshes) have the anterior surface of the metafemora mostly covered by pubescence (e.g., Figs 21C, 26C, 32C, 36C), whereas species found in hygropetric habitats (seepages) exhibit a reduced coverage (about half the surface or less, e.g., Figs 39C, 46I, 50F) and fully terrestrial species (on rotten fruits) lack any pubescence (i.e., Quadriops, Fig. 48C, F). The degree of coverage may be useful for generic identifications in many cases, and it is also known to vary among species of Agraphydrus and Primocerus. The degree of development of the tibial grooves (ventral surface that is either flat or concave) of the metafemora can also be used as a supporting character for identifications; they may be well developed, when at least the posterior edge is sharply marked, or reduced, or absent when the ventral surface of the metafemur is convex or only relatively flattened, without any sharp edges.
Figure 48.
Habitus of Quadriops spp. A–CQ. acroreius: A dorsal habitus B lateral habitus C ventral habitus D–FQ. clusia: D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 1 mm.
Tarsi. The tarsal formula of acidocerine beetles is always 5-5-5, with tarsomeres 1–4 usually similar in shape and length and tarsomere 5 longer and slender; tarsomere 2 is the most variable in length, ranging from similar to tarsomere 1 to as long as tarsomere 5. The coverage of the ventral surface of the tarsomeres is variable. Usually, the protarsomeres will have a dense and uniform coverage of thick setae; the coverage of meso- and metatarsomeres 1 may be asymmetric, with thick setae only along its outer margin. Tarsomeres 2, 3 and 4 may be densely covered ventrally, but more frequently bear a pair of lateral rows of denticles, spines or spiniform setae. Tarsomeres 5 are usually glabrous ventrally, rarely bear a ventral medial row of tiny denticles or fine setae. Very fine and relatively long natatorial setae (swimming hairs sensu Hansen 1991) may be present on the dorsal face of meso- and metatarsomeres but are scarce and do not form a fringe. The length of metatarsomeres 5 relative to the length of all or some of the remaining tarsomeres may be useful as a supporting character to recognize genera.
Apical margin of fifth abdominal ventrite. The apical margin of the fifth abdominal ventrite usually bears a mesal emargination that varies in depth and is usually fringed by flat and stout setae (Fig. 15H). There is a trend for taxa from seepages or terrestrial habitats to have a rounded or truncate posterior margin of the fifth abdominal ventrite (Fig. 15I, J); in these cases, the flat and stout setae are reduced or absent.
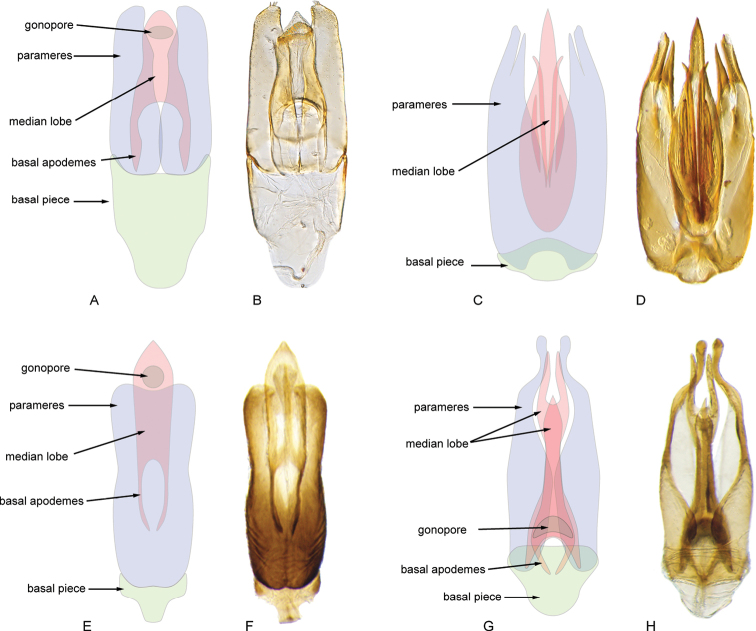
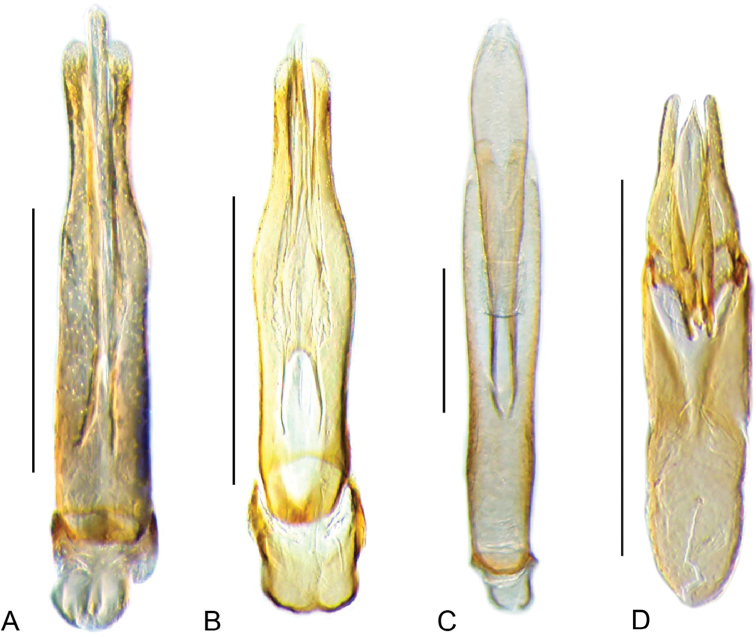
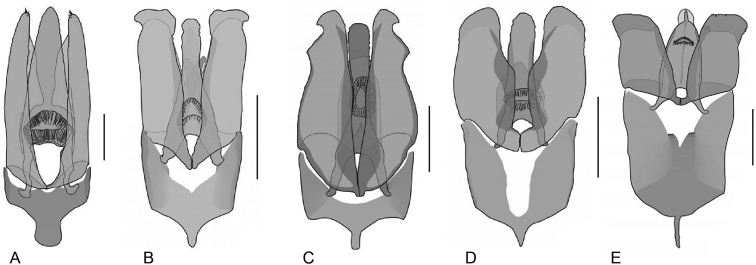
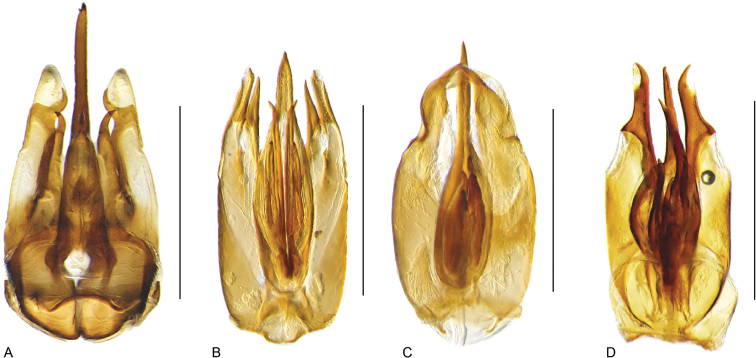
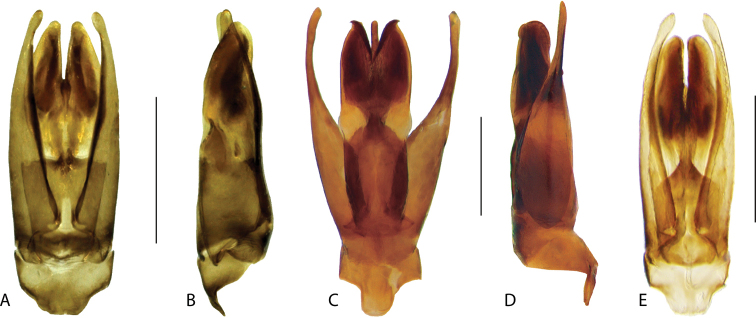
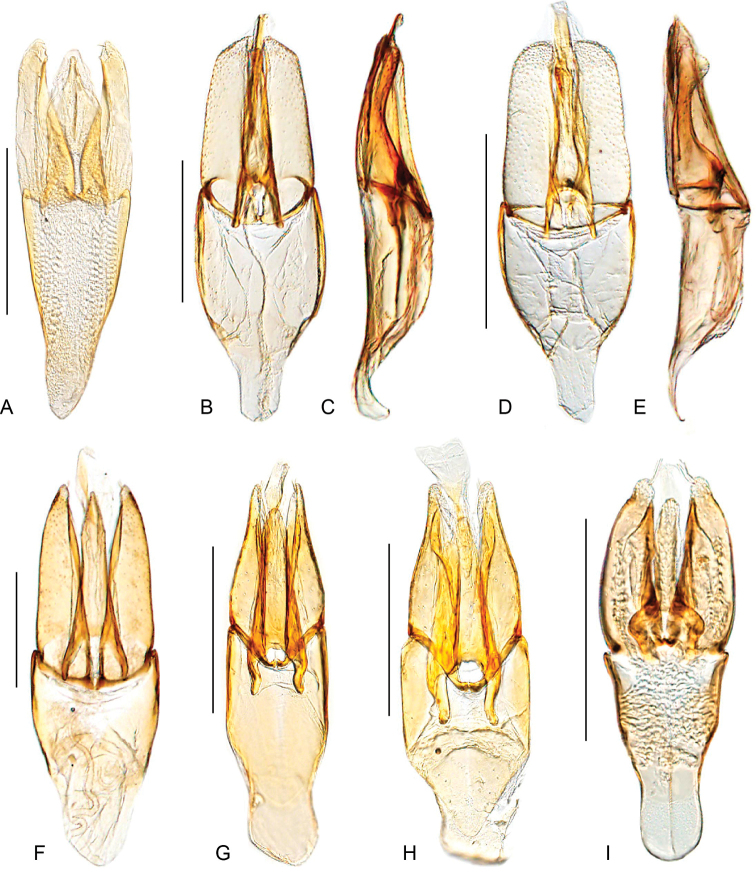
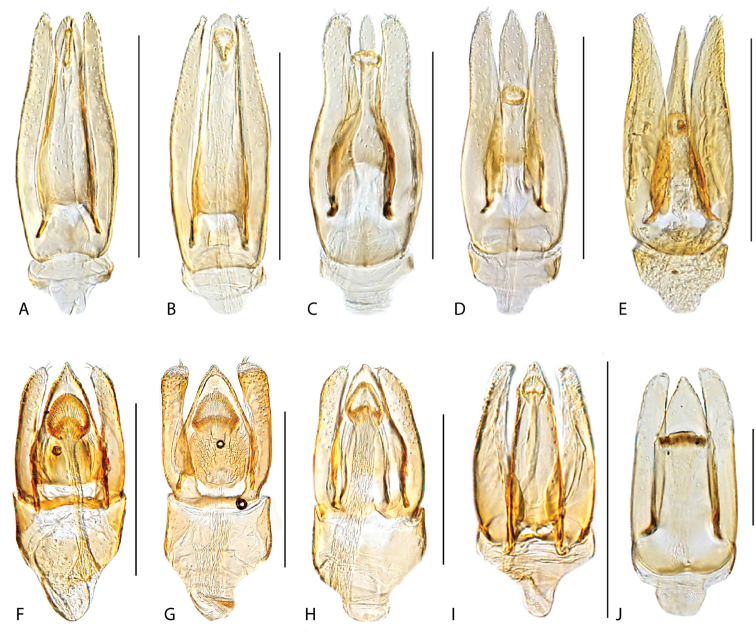
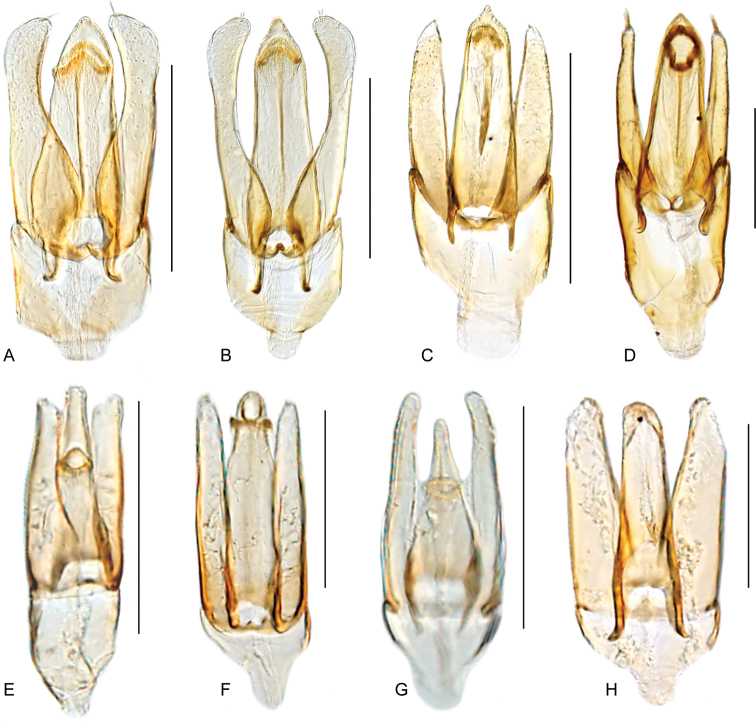
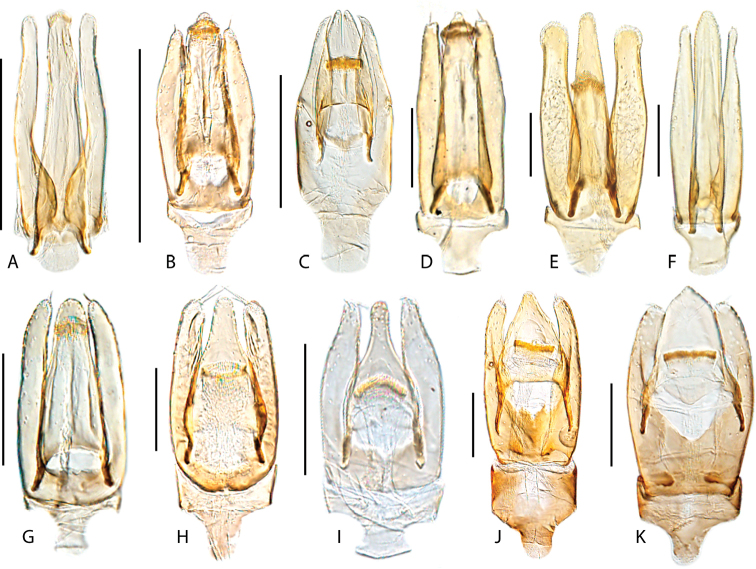
Aedeagus. The general configuration of the aedeagus in acidocerines is highly variable across the subfamily (Fig. 16), yet (usually) strongly conserved within genera and even groups of genera. An attempt to group African species of Helochares (Hydrobaticus) by aedeagal categories was made by Hebauer (1996).
Figure 16.
Aedeagi A–E trilobed: A schematic BChasmogenus schmitsC, D spiked: C schematic DPeltochares foveicollisE, F tubular: E schematic FHelochares politusG, H divided: G schematic HNovochares pallipes.
For merely practical purposes, here we propose four main aedeagal forms in Acidocerinae. These categories are very general and by no means exhaustive or detailed but encompass some of the broad variations we have found. We do not use these categories to convey any phylogenetic meaning, although certainly there is likely very strong phylogenetic signal within the aedeagal morphology of the subfamily.
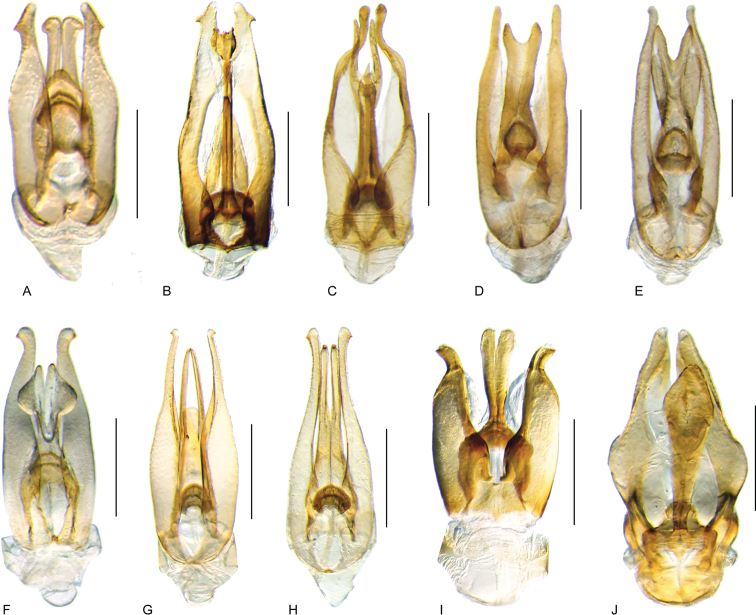
trilobed aedeagus (Fig. 16A, B): parameres separated from each other for most of their lengths; parameres and median lobe simple (without subdivisions); basal piece of variable length; gonopore usually well differentiated, variable in positioning along median lobe. With the exception of the Helochares group, this is the dominant type of aedeagus within the subfamily. All species of the Primocerus (Fig. 47), Tobochares (Figs 30, 40, 49A–D, 55) and Agraphydrus groups (Fig. 20) share this aedeagal form. Batochares (Fig. 22D) and part of Chasmogenus (Fig. 25A–C) do as well.
spiked aedeagus (Fig. 16C, D): main component of median lobe strongly sclerotized, distally elongated and apically acute, usually accompanied by additional shorter slender sclerotizations (these may or may not be symmetrical); apical region of parameres usually partly heavily sclerotized and partly membranous, often bifurcated; basal piece strongly reduced; gonopore usually not clearly visible; e.g., Peltochares (Fig. 45).
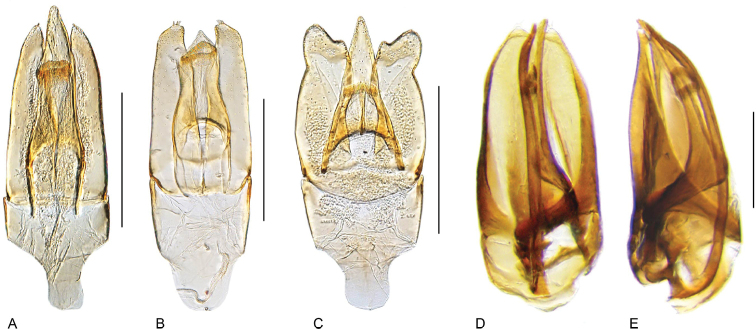
tubular aedeagus (Fig. 16E, F): parameres fused to each other for most of their lengths, forming a tubular structure with apex either simple or bifurcate/bilobate; median lobe with long to very long basal apodemes (as long or longer than main component of median lobe); median lobe either simple (without subdivisions), or with different kinds of sclerotizations of inner membranes; basal piece usually much shorter than parameres; gonopore of variable development; e.g., Aulonochares (Fig. 22A–C), Helochares (Fig. 37).
divided aedeagus (Fig. 16G, H): parameres usually separated from each other for most of their lengths; median lobe divided in dorsal and ventral plates; dorsal and ventral plates may be further bilaterally subdivided, or otherwise shaped; basal piece shorter than parameres, always noticeable; gonopore usually clearly visible, variable in positioning along median lobe. This form is apparent in Helobata (Fig. 34), Novochares (Fig. 43), and Sindolus (Fig. 49E, F).
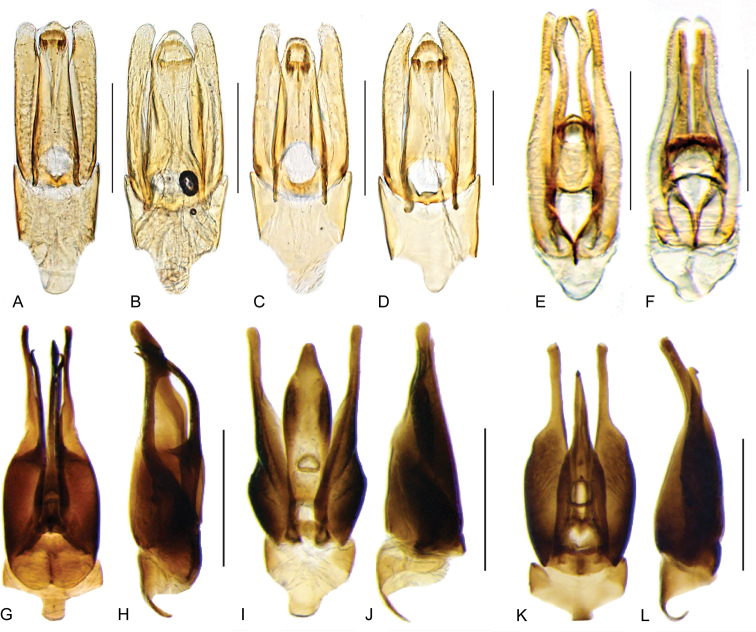
Some of these aedeagal categories are further modified in an incredible array of shapes (e.g., Figs 37, 43), and clearly deserve detailed morphological and functional studies. The particular configuration and relative proportions of parts is, for the most part, genus specific. Even though the median lobe is divided in Crephelochares (Fig. 27B–D), the aedeagal form does not quite conform to any of the described above. Though most genera include species with only one of the forms given above, a few are known to include diverse forms: for example, the genus Radicitus includes forms that are relatively simple and trilobed (Fig. 49I–L) as well as those that are greatly modified, with divided and hooked parameres (Fig. 49G, H). Likewise, the vast majority of Chasmogenus species share a simple trilobed form (e.g., Fig. 25A–C), but a few recently described species exhibit a bizarre and unique aedeagal configuration in which both the parameres and median lobe are enlarged and asymmetrical (Fig. 25D, E).
Figure 37.
Aedeagi A–HHelochares spp.: AH. sp. (Guinea) BH. tristisCH. nr. cresphontesDH. nr. tateiEH. sp. (India, Goa) FH. sp. (Vietnam) GH. politusHH. songi (from Jia and Tang 2018, fig. 48) IHelopeltarium ferrugineum. Scale bars: 0.5 mm.
Figure 43.
Aedeagi of Novochares spp. AN. sp. (Ecuador) BN. abbreviatusCN. pallipesDN. chaquensisEN. atratusFN. pichilingueGN. cf. tectiformisHN. cf. coyaIN. cf. guadelupensisJN. cf. cochlearis. Scale bars: 0.5 mm.
Figure 27.
Aedeagi of Colossochares and Crephelochares spp. AColossochares ellipticusBCrephelochares szeliCCrephelochares sp. (Australia) DCrephelochares abnormalis (Thailand). Scale bars: 0.5 mm.
Figure 49.
Aedeagi of Quadriops, Radicitus and Sindolus spp. AQ. clusiaBQ. depressusCQ. reticulatusDQ. similarisES. sp. (Venezuela) F. S. sp. (Venezuela) G, HR. ayacucho: G dorsal view H lateral view I, JR. cf. granitum (Suriname): I dorsal view J lateral view K, LR. surinamensis: K dorsal view L lateral view. Scale bars: 0.1 mm (A–D); 0.5 mm (E–L).
Figure 25.
Aedeagi of Chasmogenus spp. AC. acuminatusBC. schmitsCC. lineatusD, EC. tafelbergensis: D dorsal view E lateral view. Images from Smith and Short 2020. Scale bars: 0.5 mm.
Key to genera of Acidocerinae of the World
| 1 | Distributed in the Old World | 2 |
| – | Distributed in the New World | 9 |
| 2 | Labrum concealed by clypeus. Only known from the Indo-Malayan region | Helopeltarium (Figs 1H, 37I, 38) |
| – | Labrum not concealed by clypeus | 3 |
| 3 | Elytra with distinctly impressed sutural striae (Fig. 1V) | Crephelochares (Figs 1V, 11G, 27B–D, 28) |
| – | Elytra without sutural striae | 4 |
| 4 | Labrum with apical region anteriorly flattened, thus bearing a fine transverse carina across anterior margin (Fig. 11D, E); pronotum antero-laterally explanate and bent upwards (marginal areas concave; Fig. 23A, B); elytra with margins explanate, especially along anterior third (Fig. 23A); body smaller than 5 mm; basal piece of aedeagus nearly 1.5 × longer than parameres (Fig. 22D). Only known from the Afrotropical region | Batochares (Figs 1I, 22D, 23) |
| – | Labrum with apical region not anteriorly flattened, with even surface (without transverse carina, e.g., Fig. 11H, K); pronotum evenly convex, not laterally explanate (e.g., Fig. 1A, G); elytra with margins not explanate, at most flared (e.g., Fig. 1A, G); if elytra with margins explanate, body approximately 10 mm (e.g., Fig. 1C); basal piece of aedeagus variable in length, usually less than 0.5 × length of parameres (e.g., Fig. 16C–F). Afrotropical or elsewhere in the Old World | 5 |
| 5 | Head and pronotum with granulate surface (Fig. 17); body size small (ca. 3 mm); prosternum with median carina; elytra narrowly explanate laterally, with ten well defined rows of coarse serial punctures impressed into striae (Fig. 17A). Only known from the Afrotropical region | Acidocerus (Fig. 17) |
| – | Head and pronotum shallowly to moderately punctate, without granulations (e.g., Fig. 1A, E, F); body size variable (2–14 mm); prosternum flat to medially broadly bulging, without median carina; elytra at most flared, with or without impressed serial punctures (e.g., Fig. 1A, E, F). Afrotropical or elsewhere in the Old World | 6 |
| 6 | Body length 8.5–14.0 mm; body shape broadly oval in dorsal view, strongly and uniformly convex in lateral view (Fig. 1A); ground punctation extremely fine and shallow; coloration uniformly dark brown (nearly black). Only known from the Afrotropical region | Colossochares gen. nov. (Figs 26, 27A) |
| – | Body length 1.4–14.0 mm; body shape broadly oval in dorsal view, weakly to moderately convex in lateral view (Fig. 1B, C, E, F); ground punctation from fine and shallow to moderately marked; coloration variable, ranging from yellow to dark brown. Widespread in the Old World | 7 |
| 7 | Body length 1.4–4.8 mm; inner margin of maxillary palpomere 2 straight to nearly straight (Fig. 12G); metaventrite with posteromedian glabrous patch (e.g., Figs 18C, F, I); posterolateral glabrous patches absent; antennae with eight or nine antennomeres | Agraphydrus (Figs 1M, S, T, 18–20) |
| – | Body length 2–10 mm; inner margin of maxillary palpomere 2 weakly and evenly curved (e.g., Fig. 12H, I), seldom nearly straight; metaventrite without posteromedian glabrous patch (e.g., Figs 35C, F, 36C, F); posterolateral glabrous patches may be present; antennae with nine antennomeres (Fig. 12D) | 8 |
| 8 | Body length 2–7 mm; dorsal coloration yellow to medium brown (Figs 35, 36); posterior elevation of mesoventrite flat to simply bulging; tibial grooves absent to weakly developed; aedeagus tubular (Figs 16E, F, 37A–H) | Helochares (in part; Figs 1E, F, 35–37) |
| – | Body length 6–14 mm; dorsal coloration dark brown to black (Fig. 44); posterior elevation of mesoventrite longitudinally elevated; tibial grooves sharply marked; aedeagus spiked (Fig. 16C, D) | Peltochares (Figs 1B, C, 44, 45) |
| 9 | Eyes absent. Known only from a cave in Ecuador | Troglochares (Fig. 56) |
| – | Eyes present | 10 |
| 10 | Eyes completely divided into dorsal and ventral sections by a lateral projection of frons (Fig. 11C). Size small (<3 mm). Ranging from Costa Rica to northern South America | Quadriops (Figs 1P, 48, 49A–D) |
| – | Eyes not divided into dorsal and ventral sections by frons (e.g., Fig. 11A, B). Size variable. Anywhere in the New World | 11 |
| 11 | Labrum concealed by clypeus (Fig. 11L), elytral margins broadly explanate (Fig. 33A, D–F). Body extremely dorsoventrally compressed (Fig. 33B) | Helobata (Figs 1J, 11L, 33, 34) |
| – | Labrum not concealed by clypeus (e.g., Fig. 11H, J), elytral margins not or at most weakly explanate (e.g., Fig. 1N–R). Body form variable but rarely dorsoventrally compressed (e.g., Fig. 1N–R) | 12 |
| 12 | Elytra with distinctly impressed sutural striae (e.g., Fig. 1R). Only Neotropical region | 13 |
| – | Elytra without sutural striae (e.g., Figs 1N–Q, U). Both Neotropical and Nearctic | 14 |
| 13 | Posterior elevation of the mesoventrite either flat, broadly elevated or with a longitudinal elevation. Gonopore present and distinct (Fig. 24) | Chasmogenus (Figs 24, 25) |
| – | Posterior elevation of the mesoventrite with a transverse curved ridge, either sharp or reduced, or with a sharp, pyramidal (triangular) spine-like projection. Gonopore absent (Fig. 47) | Primocerus (Figs 1R, 46, 47) |
| 14 | Prosternum with strongly elevated median carina (Fig. 29C) | Crucisternum (Figs 29, 30A–E) |
| – | Prosternum not or only very slightly carinate or at most tectiform medially (e.g., Fig. 14 A, B) | 15 |
| 15 | Posterior elevation of mesoventrite with a large, sharp and strongly elevated laminar longitudinal carina (Fig. 51C); body in lateral view evenly and moderately convex (Fig. 51B) | Sindolus (Figs 49E–F, 51) |
| – | Posterior elevation of mesoventrite variable, but never with a large, sharp and strongly elevated laminar longitudinal carina; body in lateral view variable (Fig. 1L, N, O) | 16 |
| 16 | Elytral systematic punctures very distinct, distinctly larger than surrounding ground punctation, forming five longitudinal rows along each elytron (Figs 31, 39). Antennae with nine antennomeres (Fig. 12D) | 17 |
| – | Elytral systematic punctures indistinct, usually blending with surrounding ground punctation (e.g., Figs 32, 41, 52). Antennae with eight or nine antennomeres (Fig. 12E) | 18 |
| 17 | Metafemora mostly glabrous, with only few scattered setae on anterior surface (Fig. 31C, F). Found in the highlands of eastern Brazil | Ephydrolithus (Figs 30F–I, 31) |
| – | Metafemora at most glabrous along apical third (Fig. 39C, F). Recorded from the Andean region | Katasophistes (Figs 39, 40A–D) |
| 18 | Antennae with eight antennomeres (Fig. 12E). Size small (< 3 mm) | 19 |
| – | Antennae with nine antennomeres (Fig. 12D). Size variable but usually > 4 mm | 21 |
| 19 | Anterior surfaces of metafemora mostly glabrous, with scattered setae (e.g., Fig. 52C, F) | Tobochares (Figs 1N, O, 52–55) |
| – | Anterior surfaces of metafemora densely covered by hydrofuge pubescence along basal 3/4 (e.g., Figs 32C, 41C, F) | 20 |
| 20 | Body form circular, rounded (Fig. 32A). Size very small (1.9–2.3 mm) | Globulosis (Figs 30J, 32) |
| – | Body form ovoid, parallel sided (Fig. 41A, D). Size exceedingly small (1.1–1.5 mm) | Nanosaphes (Figs 1L, 40E–H, 41) |
| 21 | Fifth ventrite entire, without apical emargination or truncation. Maxillary palps shorter than the width of the head | Radicitus (Figs 1K, 49G–L, 50) |
| – | Fifth ventrite with apical emargination. Maxillary palps as long or longer than the width of the head | 22 |
| 22 | Head subquadrate (Fig. 11J); eyes relatively small, separated by a distance nearly 6.5 × the maximum width of an eye; mentum and submentum roughly punctate; pubescence covering abdominal ventrites composed of long golden setae; ventral surface of metatarsomeres 1–4 densely setose. Northern Amazon region | Aulonochares (Figs 1D, 21, 22A–C) |
| – | Head trapezoid; eyes moderate in size, separated by a distance nearly 4 × the maximum width of an eye; mentum obliquely striate, submentum smooth to shallowly punctate; pubescence covering abdominal ventrites composed of short setae; ventral surface of metatarsomeres 1–4 only with paired rows of denticles | 23 |
| 23 | Body size 4.2–7.0 mm; maxillary palps nearly as long as maximum width of the head; internal structural reticulations usually visible along entire dorsal surface of elytra (Fig. 36A, B); metaventrite uniformly covered by hydrofuge pubescence (Fig. 36C); tibial grooves absent to weakly developed; aedeagus tubular (e.g., Fig. 37G). Ranging from USA to Venezuela and Peru (Andean region) | Helochares (in part; Figs 36A–C, 37G) |
| – | Body size 4.5–9.0 mm; maxillary palps 1.1–1.5 × the maximum width of the head; internal structural reticulations of elytra absent (Fig. 42); metaventrite with median glabrous patch, sometimes very narrow and extending along entire length of metaventrite (Fig. 42C, F); tibial grooves well-developed, with sharp margins; aedeagus divided (e.g., Fig. 16G, H) | Novochares gen. nov. (Figs 1G, 42, 43) |
Figure 22.
Aedeagi of Aulonochares and Batochares spp. AA. tubulusBA. novoairensisCA. lingulatusDB. sp. Scale bars: 0.5 mm.
Figure 20.
Aedeagi of Agraphydrus spp. AA. attenuatusBA. gracilipalpisCA. masatakaiDA. chinensisEA. puzhelongi. Scale bars: 0.1 mm. Line drawings taken from Komarek (2018).
Figure 44.
Habitus of Peltochares spp. A–CP. conspicuus: A dorsal habitus B lateral habitus C ventral habitus D–FP. sp. (Tanzania): D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 1 mm.
Figure 45.
Aedeagi of Peltochares spp. AP. conspicuusBP. foveicollisCP. sp. (Australia) DP. sp. (Tanzania;). Scale bars: 1 mm.
Figure 34.
Aedeagi of Helobata spp. A, BH. pantaneira (from Clarkson et al. 2016): A dorsal view B lateral view C, DH. quatipuru (Clarkson and Almeida 2018) A dorsal view D lateral view EH. sp. (Ecuador), dorsal view. Scale bars: 0.5 mm.
Figure 47.
Aedeagi of Primocerus spp. AP. neutrumB, CP. maipure: B dorsal view C lateral view D, EP. pijiguaense: D dorsal view E lateral view FP. gigasGP. petilusHP. striatolatusIP. cuspidis. Scale bars: 0.25 mm.
Figure 29.
Habitus of Crucisternum ouboteriA dorsal habitus B lateral habitus C ventral habitus. Scale bar: 1 mm.
Figure 30.
Aedeagi of Crucisternum, Ephydrolithus, and Globulosis spp. AC. ouboteriBC. toboganensisCC. sinuatusDC. vanessaeEC. queneyiFE. teliGE. spiculatusHE. ogmosIE. minorJG. flavus. Scale bars: 0.25 mm.
Figure 40.
Aedeagi of Katasophistes and Nanosaphes spp. AK. charynaeBK. cuzcoCK. meridaDK. superficialisEN. tricolorFN. hesperusGN. castaneusHN. punctatus. Scale bars: 0.3 mm (A–C); 0.1 mm (E–H).
Figure 55.
Aedeagi of Tobochares spp. AT. benettiiBT. fususCT. luteomargoDT. emarginatusET. kusadFT. kasikasimaGT. anthonyaeHT. auturesIT. communisJT. romanoaeKT. akoreio. Scale bars: 0.5 mm (A–C); 0.1 mm (D–K).
Figure 42.
Habitus of Novochares spp. A–CN. sallaei: A dorsal habitus B lateral habitus C ventral habitus D–FN. sp. (Peru): D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 1 mm.
Taxonomy
Subfamily. Acidocerinae
Zaitzev, 1908
Acidocerini Zaitzev, 1908: 353, as subfamily.
Acidocerina as subtribe Acidocerina [of tribe Hydrophilini, subfamily Hydrophilinae] in Hansen (1991: 282; 1999b: 155).
Acidocerina as tribe [of subfamily Hydrophilinae] in Short and Fikáček (2011: 85).
Acidocerina as subfamily in Short and Fikáček (2013: 741).
Helopeltini Horn, 1873: 118; synonymized by Hansen (1991: 282); unavailable: generic name is preoccupied (ICZN 1999, Code Art. 39).
Type genus: Helopeltis Horn, 1873: 137 [synonym of Helobata Bergroth, 1888: 221].
Helocharae d’Orchymont, 1919c: 147; described as subtribe, synonymized by Hansen (1991: 282).
Type genus: Helochares Mulsant, 1844a: 197.
Horelophopsinae Hansen, 1997: 108.
Type genus: Horelophopsis Hansen, 1997: 109; synonymized by Short and Fikáček (2013: 15, in table, discussed along the text).
Globulina García, 2001: 153; emended to Globulosina by Short and Hebauer (2006: 338); synonymized with tribe Acidocerini by Short and Fikáček 2011: 84.
Type genus: Globulosis García, 2001: 153.
Type genus.
Acidocerus Klug, 1855: 649.
Diagnosis.
Body length 1.2–14.0 mm. Body shape oval in dorsal view, dorsoventrally flattened, or weakly to strongly convex in lateral view (Fig. 1); surface even (without elevations or depressions), granulate (e.g., Figs 17, 33) or smooth on head and pronotum. From yellowish to dark brown in coloration (Fig. 1), usually uniform, sometimes different regions of body colored differently. Shape of head variable (trapezoid, subquadrate, round; Fig. 11E–L). Anterior corners of frons sometimes extended posteriorly forming canthus and emarginating anterior margin of eyes (e.g., Tobochares, Helobata; e.g., Fig. 11B, C). Eyes varying in size, shape, degree of emargination, and degree of projection from outline of head (Fig. 11E–L); absent only in cavernicolous genus Troglochares Spangler, 1981a. Clypeus variable in shape (rectangular to trapezoid; Fig. 11E–L), with anterior margin from straight to mesally emarginate. Labrum usually exposed; concealed by clypeus in Helobata (Fig. 11L) and Helopeltarium (Fig. 1H). Mentum usually wider than long, with strong median anterior depression, may be limited by low transverse carina (Fig. 12A–C); surface of mentum with variable sculpture, ranging from smooth (Fig. 12A) to roughly punctate or obliquely striate (Fig. 12B). Antennae with eight or nine antennomeres (Fig. 12D, E), with cupule varying in symmetry and shape. Maxillary palps curved inward, ranging from very short (nearly half width of the head; e.g., Quadriops reticulatus, Fig. 12C) and stout, to very long and slender (nearly twice the width of the head; e.g., Peltochares, Fig. 11K). Pronotum evenly convex, usually with systematic punctures forming paired anterolateral semicircles and paired short posterolateral transverse bands. Elytra with or without sutural striae, with outer margins simple, slightly flared, or laterally explanate; elytral punctation variable (Fig. 13). Hind wings usually well developed (Fig. 15A–F), seldom reduced along apical region (Fig. 15G). Surface of prosternum flat (e.g., Fig. 14A, B), convex or rarely medially carinate (e.g., Crucisternum; Fig. 29C), with anterior margin straight or anteriorly projected. Posterior elevation of mesoventrite either only weakly bulging or with transverse (e.g., Fig. 14E, G) or longitudinal ridge (e.g., Fig. 14D, F); with strongly produced, anteriorly pointed and longitudinally carinate transverse ridge in Crucisternum (Fig. 14C). Anapleural sutures variable in shape and orientation. Metaventrite rather uniformly covered by hydrofuge pubescence (e.g., Fig. 14E), sometimes with posteromesal glabrous patch (e.g., Fig. 14D, F, G), sometimes also with posterolateral glabrous patches (e.g., Fig. 14D). Protibiae with anterior row of spines varying in shape and development; apical spurs of protibiae varying in development. Metafemora with tibial grooves of varying development; hydrofuge pubescence on anterior surface of metafemora absent, reduced to only basal or dorsal patch, or increasingly covering most of surface. Tarsomeres 5-5-5; tarsomeres variable in size, proportions, and dorsal and ventral coverage. Abdomen with five pubescent ventrites, density of setae ranging from sparse to very dense. Fifth abdominal ventrite with apex either rounded (Fig. 15I), truncate (Fig. 15J), or emarginate (Fig. 15H); apex with or without fringe of flat and stout setae. Aedeagus usually symmetrical (Fig. 16), with basal piece varying in size from longer than parameres (e.g., Primocerus, Fig. 47; Batochares, Fig. 22D), to reduced and virtually absent (e.g., Peltochares, Fig. 45); parameres highly variable in shape, either slender and only connected to each other at base of ventral surface (e.g., Fig. 16A–D, G, H), or fused together forming tube-like structure (e.g., Fig. 16E, F); apex of parameres either simple, or bifurcated and modified with hooks and spines (e.g., Fig. 16C, D); median lobe either simple or with dorsal and ventral lobes, with well-developed lateral basal apodemes; further modifications (longitudinal divisions, presence of internal hooks and spines, development of gonopore) widespread.
Differential diagnosis.
Acidocerines can be generally recognized by their oval and moderately convex body shapes with slender maxillary palps and uniformly slender tibiae (usually strongly convex and sometimes rounded in Cylominae and Sphaeridiinae, with short and stout maxillary palps and stout to apically broadened tibiae). The maxillary palps are always curved inwards in Acidocerinae (maxillary palpomere 2 with inner margin straight to concave; Fig. 12F–J), with palpomeres 2–4 similar in length and proportions (curved outwards, zig-zag oriented, or with shorter palpomere 3 in most Enochrinae and Chaetarthriinae). In addition, Acidocerines always bear five tarsomeres on the meso- and metatarsi (four in some enochrines).
Selected references.
Hansen 1991: diagnosis of the group (at the time as a subtribe, and including some genera now placed in the subfamily Enochrinae), list of genera and subgenera with synonyms, key to genera, and description of each genus (8 out of the 23 recognized in this paper). Hansen 1999b: catalog with full list of species at the time (nearly 300), synonyms and references. Short and Fikáček 2013: Acidocerinae as a subfamily excluding enochrine genera, with Horelophopsinae as synonym, list of genera, general diagnosis. Short et al. (2021): molecular phylogeny and biogeography of the subfamily, groups of genera.
Remarks.
The subfamily Acidocerinae is a group with many contrasts. It includes some of the largest as well as smallest hydrophilids; some genera are either strikingly different from, or extremely similar to others; the external morphology of some genera is extremely uniform and species can only be recognized by characters of the male genitalia, or so variable that is difficult to diagnose the group as a unit; at the species level, the distributions can be very narrow and restricted to one or a few fairly close localities, or very broadly widespread across several continents. There is a trend for species living in the same kind of habitats to have certain shared morphological features. For example, species that live in aquatic habitats tend to have slender and relatively long maxillary palps and metafemora mostly covered by hydrofuge pubescence, whereas species living in hygropetric habitats tend to have shorter and stouter maxillary palps and reduced or absent coverage of hydrofuge pubescence on the metafemora.
Genus. Acidocerus
Klug, 1855
Figure 4.
Known distribution of genera of Acidocerinae: Acidocerus, Agraphydrus, Aulonochares, Batochares, Chasmogenus, Colossochares, and Crephelochares.
Acidocerus Klug, 1855: 649.
Gender.
Masculine.
Type species.
Acidocerus aphodioides Klug, 1855: 649; by monotypy.
Diagnosis.
Small beetles, body length nearly 2.8 mm. Body shape elongate oval in dorsal view, moderately convex in lateral view, with dorsal outline nearly straight along anterior 2/3 of elytra (Fig. 17). Surface of head and pronotum granulate (Fig. 17C). Body pale/yellowish brown, with head slightly darker. Eyes with anterior margin straight in lateral view (not emarginate), in dorsal view slightly projecting from outline of head (Fig. 17C). Labrum not concealed by clypeus (Fig. 17C). Antennae with nine antennomeres, with strongly asymmetric cupule, with longer side acute. Maxillary palps elongate, with palpomere 4 nearly as long as palpomere 3 (d’Orchymont 1943f: 7, in key). Elytra without sutural striae, narrowly explanate laterally, serial punctures strongly marked, arranged in rows (Fig. 17A). Prosternum flat, rather sharply carinate medially, with angulate anteromedian projection. Posterior elevation of mesoventrite only weakly bulging. Metaventrite with hydrofuge pubescence. Metafemora without distinct tibial grooves, mostly pubescent, only glabrous at apex. Metatarsomeres 1–4 similar in length; metatarsomere 5 similar in length to metatarsomeres 1–4 combined. Fifth abdominal ventrite apically emarginate, with stout setae.
Differential diagnosis.
The long fifth metatarsomere (longer than metatarsomeres 1–4 combined) is unusual but not unique in the subfamily (Hansen 1991). The granulate surface of the head and body resembles that of Helobata, but besides their geographic origin, the exposed labrum of Acidocerus (as opposed to concealed in Helobata) allows its recognition. The small size and coarse punctation of the elytra of Acidocerus resemble some of the Old World Helochares (e.g., Fig. 36D–F) and some Agraphydrus (e.g., Agraphydrus hanseni, Fig. 19A), from which it can be differentiated by the medially sharply carinate prosternum (Hansen 1991).
Distribution.
Afrotropical: Mozambique; Fig. 4.
Natural history.
There is no natural history information available for the genus.
Larvae.
Immature stages are not known for the genus.
Taxonomic history.
The taxon was originally described as related to Spercheus Kugelann, with maxillary palps similar to those of Hydraena Kugelann (Klug 1855), and even later afforded its own subfamily (see taxonomic history of the Acidocerinae section, above). d’Orchymont (1943f: 7) provided a list of diagnostic characters in a key, including the relative length of its tarsal segments, specifically that the fifth tarsomere is as long as tarsomeres 1–4 combined. Hansen (1991) redescribed the taxon based on syntypes. Hansen (1991: 149) further commented that he had seen other “typical” species of Helochares that also shared this feature and stated that “although Acidocerus may be somewhat reminiscent of a small Helochares… I prefer to maintain it as a distinct genus at the present stage”. The genus was not included in the molecular phylogeny in Short et al. (2021), and its assignment to the Helochares group is based primarily on its overall dorsal sculpturing, lack of a sutural stria, and Afrotropical distribution.
Remarks.
Only one described species. Hansen (1991) studied Klug’s syntypes housed at the Museum für Naturkunde der Humboldt-Universität in Berlin, Germany (ZMHB), which are the only known specimens for the genus. The diagnostic features listed above include information from d’Orchymont (1943f), Hansen (1991), and our own observations of photographs of the syntypes. Given that the specimens were mounted on cards when photographed, features of the ventral surface were not viewed by us. Characters of the ventral features (as well as the maxillary palps) as described above are based on d’Orchymont (1943f) and Hansen (1991), as the maxillary palps appeared to be missing by the time Hansen examined the syntypes. Until additional specimens are found, it is unlikely there will be a satisfactory resolution on deciding if Acidocerus is in fact a distinct genus or rather another variant of Helochares.
Species examined.
Acidocerus aphodioides (photographs of syntypes).
Selected references.
Klug 1855: 649: original description; d’Orchymont 1943f: 7: offers diagnostic features in a key; Hansen 1991: 149: redescription; Short and Fikáček 2013: 741: Acidocerus listed in subfamily Acidocerinae; Short et al. 2021: phylogenetic position and affinities discussed.
Genus. Agraphydrus
Régimbart, 1903
Figs 1M, S, T , 2 , 4 , 18 , 19 , 20
Agraphydrus Régimbart, 1903a: 33.
Type species: Agraphydrus punctatellus Régimbart, 1903a: 34; by monotypy.
Pseudohelochares Satô, 1960: 77; Satô (1965: 128) [synonymy].
Type species: Pseudohelochares narusei Satô, 1960: 77; by original designation and monotypy.
Pseudopelthydrus Jia, 1998: 225.
Type species: Pseudopelthydrus longipalpus Jia, 1998: 229; by original designation. Komarek (2003: 384) [synonymy].
Megagraphydrus Hansen, 1999a: 137.
Type species: Megagraphydrus siamensis Hansen, 1999a: 140; by original designation. Minoshima et al. (2015: 7) [synonymy].
Gymnhelochares d’Orchymont, 1932: 692; as subgenus of Helochares.
Type species: Helochares (Gymnhelochares) geminus d’Orchymont, 1932: 694; by original designation. Komarek and Hebauer (2018: 17) [synonymy].
Horelophopsis Hansen, 1997: 109.
Type species: Horelophopsis avita Hansen, 1997: 109, by original designation; Short et al. (2021) [synonymy].
Gender.
Masculine.
Type species.
Agraphydrus punctatellus Régimbart, 1903: 34; by monotypy.
Diagnosis.
Small beetles, body length 1.4–4.8 mm. Body shape elongate to broadly oval in dorsal view, weakly to moderately convex in lateral view, rarely strongly convex (Figs 18, 19). Surface of head and pronotum smooth, usually with shallow ground punctation. Body ranging from pale/yellowish to dark brown (Figs 18, 19), either uniform across body regions or with different regions colored differently (e.g., darker head, paler elytra and margins of pronotum; Fig. 18A, B). Eyes with anterior margin straight in lateral view (not emarginate), in dorsal view slightly projecting from outline of head. Clypeus moderately convex, with distinct systematic punctures, with anterior margin slightly to clearly emarginate. Labrum not concealed by clypeus. Mentum nearly 1.5 × wider than long, with variable surface, with wide and moderate median anterior depression limited by low transverse carina. Antennae with eight or nine antennomeres, with slightly asymmetric cupule, round in outline. Maxillary palps elongate, 0.7–1.5 × width of head, with inner margin of palpomere 2 usually straight and palpomere 4 nearly as long to slightly longer than palpomere 3 (Fig. 12G). Pronotum with ground punctation usually moderate. Elytra without sutural striae, not laterally explanate, with serial punctures usually absent; systematic punctures usually rather sparse and aligned in four rows along elytra. Prosternum slightly convex, not carinate medially. Posterior elevation of mesoventrite variable, from simply bulged, to bearing variously shaped elevations; anapleural sutures variable in shape and orientation. Metaventrite with posteromedian glabrous patch. Metafemora without distinct tibial grooves, either mostly pubescent (only glabrous at apex), or with pubescence reduced to small basal area (“Gymnhelochares”). Metatarsomere 1 shorter than 2; metatarsomere 2 slightly shorter than 5; metatarsomere 5 similar in length to metatarsomeres 3 and 4 combined. Fifth abdominal ventrite apically emarginate, sometimes very slightly, or rounded, with or without fringe of stout setae. Aedeagus trilobed in form (Fig. 20); basal piece shorter to longer than parameres; outline of apical region of parameres variable; median lobe triangular, with well-developed lateral basal apodemes, usually rounded at apex; gonopore well developed.
Differential diagnosis.
Agraphydrus can be considered highly variable both morphologically and ecologically. Given their usually small to very small size, in the regions where Agraphydrus is distributed, they may be confused with smaller species of Helochares, from which Agraphydrus can be distinguished by the presence of a posteromesal glabrous patch on the metaventrite (metaventrite uniformly and densely covered by hydrofuge pubescence in Helochares); their size allows to differentiate them from the much larger Colossochares and Peltochares. The lack of sutural stria in Agraphydrus allows to recognize the larger Agraphydrus from similarly sized Crephelochares. The maxillary palps tend to be shorter in Agraphydrus. Most Agraphydrus have moderately punctate head and pronotum and generally lack elytral serial punctures; although they may have very coarse systematic punctures somewhat aligned in rows, these rows are not quite uniform as in many Old World Helochares or Acidocerus. The outer margins of the elytra of Agraphydrus are only slightly flared, as opposed to laterally expanded which differentiates them from Batochares. The most similar genus to Agraphydrus would be the Neotropical genus Tobochares, but they do not co-occur; the body shape in Agraphydrus, in general, tends to be more elongated (1.1–1.4 × longer than wide), whereas in Tobochares it tends to be only slightly longer than wide (1.07–1.15 × longer than wide); in addition, the metafemora in Tobochares are always mostly glabrous, with scattered setae, and their serial punctures are well aligned longitudinally.
Distribution.
Afrotropical: Angola, Botswana, Cameroon, Democratic Republic of the Congo, Djibouti, Eritrea, Eswatini, Ethiopia (in doubt), Gabon, Ghana, Guinea, Ivory Coast, Kenya, Madagascar, Malawi, Mozambique, Namibia, Nigeria, Oman, Republic of South Africa, Saudi Arabia, Sudan, Tanzania, United Arab Emirates, Yemen, Zimbabwe. Australasian: Australia (New South Wales, Northern Territory, Queensland, Western Australia), Indonesia (Java, Papua), Papua New Guinea. Indo-Malayan: Bhutan, Brunei, China (Fujian, Guangdong, Guangxi, Guizhou, Hainan, Himachal, Hong Kong, Hunan, Jiangxi, Taiwan, Yunnan, Zhejiang), India (Arunachal Pradesh, Assam, Goa, Himachal Pradesh, Kerala, Karnataka, Madhya Pradesh, Maharashtra, Meghalaya, North Andaman Island, Sikkim, Tamil Nadu, Uttar Pradesh, Uttarakhand), Laos, Malaysia, Myanmar, Nepal, Philippines, Sri Lanka, Thailand, Vietnam. Palearctic: China (Anhui, Gansu, Hubei, Shaanxi, Shandong, Sichuan, Tibet), Iran, Japan, Korea, Pakistan, South Korea; Fig. 4.
Natural history.
Agraphydrus can be found in an extremely broad range of habitats, from rivers, streams and forest pools, to hygropetric environments around waterfalls or seepages over rocks; a few species have been collected in terrestrial habitats by sifting moss and leaves from near water bodies, or in the gravel along the bank of a river; in many cases specimens have been found associated with floating vegetation, mosses and algae (Komarek 2018, 2019, 2020, Komarek and Freitag 2020, Komarek and Hebauer 2018).
Larvae.
Only the larvae of two species of Agraphydrus are currently known: A. narusei (Satô) (first and third instars; Minoshima and Hayashi 2011), and A. hanseni (Satô and Yoshitomi) (third instar; Minoshima et al. 2013). Minoshima (2016) offers a diagnosis for Agraphydrus larvae.
Taxonomic history.
Originally described as a genus by Régimbart in 1903; downgraded to a subgenus of Enochrus by d’Orchymont (1919c: 155); transferred as a subgenus to Helochares by d’Orchymont (1927a: 250); generic status re-established by Satô (1965: 128). Hansen (1991: 148) placed Gymnhelochares as a subgenus of Agraphydrus; Komarek and Hebauer (2018: 17) placed Gymnhelochares as a synonym of Agraphydrus given that they could not identify any unique morphological traits that allowed the two genera to be differentiated. Minoshima et al. (2015: 7) synonymized Megagraphydrus with Agraphydrus also based on the lack of morphological traits in support of their separation. Short and Fikáček (2013) recovered Horelophopsis and Agraphydrus as sister taxa within the Acidocerinae (Horelophopsis had been described as, and was prior to Short and Fikáček (2013), its own subfamily of Hydrophilidae). These affinities between Agraphydrus and Horelophopsis were also recognized by Minoshima et al. (2013) based on larval characters. Finally, Short et al. (2021), based on their molecular phylogenetic analyses, synonymized Horelophopsis with Agraphydrus, as Horelophopsis was recovered as a lineage nested within Agraphydrus. The genus was redescribed by Komarek (2020). For more details on the taxonomic history of the genus and its synonyms see Minoshima et al. (2015).
Remarks.
With 201 described species, Agraphydrus is currently the largest genus of Acidocerinae, due to a series of recent revisions and monographs (Minoshima et al. 2015; Komarek 2018, 2019, 2020; Komarek and Hebauer 2018; Komarek and Freitag 2020), making it the fifth largest genus of Hydrophilidae (behind Berosus Leach, Laccobius Erichson, Cercyon Leach, and Enochrus Thomson). The condition of the maxillary palpomere 2 being straight (with inner margin straight) is not unique to Agraphydrus but shared with Tobochares and some Helochares. Minoshima et al. (2015) proposed the V-shaped male abdominal sternite 9 as a possible synapomorphy of the genus, but the condition is shared with some members of the Tobochares group.
The genus appears well supported as monophyletic as currently defined, despite its substantial morphological and ecological variation (Short et al. 2021). Although previous decisions to synonymize derived genera (e.g., Megagraphydrus, Pseudopelthydrus, Horelophopsis) were necessary to preserve the monophyly of the broader concept of Agraphydrus, it has rendered the genus unmanageably large and with no internal formal or informal classification system. The lineage would be well-served by further phylogenetic studies to define species groups or to partition into subgenera.
Hebauer (2002a) listed several species of Agraphydrus as “in press”, and some specimens in collections bear associated red and orange holotype or paratype labels bearing these names; however, those were never formally published. Many of these taxa appeared in Komarek and Hebauer (2018) or subsequent revisions by Komarek (2019, 2020), with names different from those proposed by Hebauer (2002a).
Species examined.
Agraphydrus anatinus Komarek, A. attenuatus (Hansen), A. coomani (d’Orchymont), A. decipiens Minoshima, Komarek & Ôhara*, A. hanseni (Satô & Yoshitomi), A. insidiator Minoshima, Komarek & Ôhara*, A. ishiharai (Matsui), A. kempi (d’Orchymont), A. luteilateralis (Minoshima & Fujiwara)*, A. malayanus (Hebauer)*, A. masatakai Minoshima, Komarek & Ôhara*, A. minutissimus (Kuwert), A. narusei (Satô), A. pauculus (Knisch), A. politus (Hansen), A. pygmaeus (Knisch), A. siamensis (Hansen), A. stagnalis (d’Orchymont), A. thaiensis Minoshima, Komarek & Ôhara, and numerous unidentified specimens. For species marked with an asterisk, paratype specimens were studied.
Selected references.
Minoshima et al. 2015: character discussion, taxonomic history, synonymization of Megagraphydrus, description of seven new species; Komarek and Hebauer 2018: 17: synonymized the subgenus Gymnhelochares with Agraphydrus, taxonomic revision for China and Taiwan describing 33 new species; Komarek 2018: taxonomic revision for India describing 36 new species; Komarek 2019: taxonomic revision for South East Asia (except Philippines) and Australasian Region, describing 60 new species; Komarek and Freitag 2020: revision of the species from the Philippines describing nine new species and providing barcodes for the species treated therein; Komarek 2020: revision of the African and Western Asian species, describing 25 new species and redescribing the genus; Short et al. 2021: synonymization of Horelophopsis with Agraphydrus, phylogenetic placement of Agraphydrus.
Genus. Aulonochares
Girón & Short, 2019
Figs 1D , 2 , 4 , 11J , 21 , 22A–C
Aulonochares Girón & Short, 2019: 112.
Gender.
Masculine.
Type species.
Aulonochares tubulus Girón & Short, 2019: 120; by original designation.
Diagnosis.
Medium sized beetles, total body length 5.8–7.5 mm. Body shape elongated oval in dorsal view; weakly convex in lateral view (Fig. 21). Color orange brown to dark brown; ventral surface covered with rather long golden setae, especially on abdominal ventrites, and more densely so (with shorter setae) on surface of femora. Head subquadrate in dorsal view, seemingly constricted at anterior margin of eyes (Fig. 11J). Eyes relatively small, separated by distance nearly 6.5 × the maximum width of an eye (Fig. 11J). Clypeus with lateral margins nearly parallel, slightly convex, with anterior margin only slightly narrower than posterior margin (Fig. 11J). Labrum fully exposed. Mentum and submentum roughly punctate (Fig. 21C). Antennae with nine antennomeres, with cupule slightly asymmetrical and round in outline. Maxillary palps long, nearly 1.5 × longer than maximum width of head, with inner and outer margins of maxillary palpomere 2 evenly curved (Fig. 21A). Pronotum with ground punctation shallow and uniformly sparse. Elytra without sutural striae, with outer margins slightly flared; serial punctures, ground punctures and systematic punctures similar in size, shallowly impressed. Surface of prosternum flat (slightly carinate only along midline of antero-mesal projection of anterior margin). Posterior elevation of mesoventrite simple, without carinae or ridges; anapleural sutures concave, anteriorly converging, anteriorly separated by distance nearly 0.3 × as wide as anterior margin of mesepisternum. Metaventrite densely and uniformly pubescent. Protibiae with spines of anterior row very small and appressed (Fig. 21C); apical spurs of protibiae very short (not exceeding the length of the first tarsomere) and stout. Hydrofuge pubescence covering most surface of metafemora (Fig. 21C). Ventral face of tarsomeres 1–4 densely covered by stiff setae. Apex of fifth abdominal ventrite strongly emarginate; emargination fringed by stout setae. Aedeagus tubular (Fig. 22A–C), somewhat cylindrical, with parameres forming a 5–7 × longer than wide tube; basal piece very short and strongly concave; gonopore reduced, located at apex of median lobe.
Differential diagnosis.
Aulonochares can be easily mistaken with Novochares in the New World, and the two genera can be collected together. The subquadrate shape of the head (Fig. 11J; as opposed to trapezoid as in Fig. 11G), the roughly punctate mentum, the long setae composing the ventral pubescence of the abdominal ventrites, ventrally densely setose tarsomeres, along with the tubular shape of the aedeagus (Fig. 22A–C) are very distinctive and uniquely combined in Aulonochares among Neotropical acidocerines.
Distribution.
Neotropical: Brazil (Amazonas, Roraima), French Guiana, Guyana, Suriname, Venezuela; Fig. 4.
Natural history.
Specimens of Aulonochares have been collected in densely forested sandy streams and detrital pools in forests along creeks. They seem to prefer habitats with abundant detritus or decaying organic matter. Females of A. tubulus and A. ligulatus have been observed carrying their egg cases underneath their abdomen (Girón and Short 2019; pers. obs.).
Larvae.
Immature stages are not known for the genus.
Taxonomic history.
Recently described by Girón and Short (2019).
Remarks.
Only three species are known for the genus (Girón and Short 2019).
Species examined.
Aulonochares lingulatus Girón & Short, A. novoairensis Girón & Short, A. tubulus Girón & Short. Holotypes and paratypes of all three species were available for this study. We have not seen any specimens of the genus from outside the Guiana Shield region of South America.
Selected references.
Girón and Short 2019: original description of the genus and all its currently known species; Short et al. 2021: phylogenetic placement.
Genus. Batochares
Hansen, 1991
Figs 1I , 2 , 4 , 9D, E , 22D , 23
Batochares d’Orchymont, 1939b: 293 [Described as subgenus; unavailable, ICZN (1999) Art. 13.3: no type species designated].
Fixed as subgenus of Helochares by Hansen (1991: 292) [available, granting authorship to Hansen under ICZN (1999) Art. 50.1.].
Elevated to genus by Short et al. (2021).
Gender.
Masculine.
Type species.
Helochares (Batochares) burgeoni d’Orchymont, 1939b: 294; by original designation (Hansen 1991: 292).
Diagnosis.
Body length between 3–4 mm. Body shape oval in dorsal view, moderately convex in lateral view, with dorsal outline nearly straight along basal 2/3 (Fig. 23). Dorsal surfaces smooth, uniformly covered by short setae, brown to pale brown in coloration, either uniform or with yellowish patches along margins of pronotum and elytra, or scattered throughout surface giving spotted appearance (Fig. 23A, B); ground punctation fine and shallow; ventral surfaces rather densely covered by rather long and fine golden setae. Head rather oval in dorsal view, clearly constricted at anterior margin of eyes (Fig. 11E). Eyes not emarginate, moderate in size, separated by nearly 3.8 × width of eye, strongly projected from outline of head (Fig. 11E). Clypeus with anterior margin broadly emarginate, with medial region of emargination nearly straight; anterior corners round. Labrum fully exposed, with apical region anteriorly flattened, thus forming fine transverse carina across anterior region (Fig. 11D). Mentum rather flat, surface laterally punctate, mesally and anteriorly striate, with anteromedial region depressed. Submentum finely and shallowly punctate. Antennae with nine antennomeres, with strongly asymmetric and round cupule. Maxillary palps nearly 1.5 × longer than maximum width of head, with palpomere 4 0.8 × as long as palpomere 3 (Fig. 23C); inner margin of maxillary palpomere 2 nearly straight, outer margin apically slightly curved. Pronotum medially evenly convex, explanate and somewhat bending upwards along antero-lateral areas; posterior margin of pronotum clearly narrower than anterior margin of elytra combined. Elytra without sutural striae, with outer margins explanate, especially along anterior third; serial punctures well developed, forming longitudinal rows, at least well defined along outer areas, or visible along entire length of elytra; seta bearing systematic punctures irregularly distributed. Surface of prosternum slightly elevated along midline, with anterior margin acutely triangular and slightly projected anteriorly. Posterior elevation of mesoventrite rather flat; intercoxal process of mesoventrite broad (nearly as wide as antennal club), apically truncate; anapleural sutures sinuate, separated at anterior margin by distance slightly shorter than anterior margin of mesepisternum. Metaventrite with medial surface elevated as platform, densely covered with hydrofuge pubescence, except for posterolateral patches (Fig. 23C). Protibiae with spines of anterior row very fine and erect; apical spurs of protibiae small (larger spur similar in size and shape to tarsal claws). Metafemora without tibial grooves; metafemora with hydrofuge pubescence covering at least basal 2/3 of anterior surface (Fig. 23C). Metatarsomere 5 1.5 × longer than metatarsomere 2, metatarsomere 2 nearly as long as metatarsomeres 3 and 4 combined; tarsomeres 1 to 4 with sparse long setae on dorsal surface, and spiniform dense setae on ventral surface; tarsomere 5 with few setae along apical margin. Abdomen with five pubescent ventrites. Fifth abdominal ventrite with apex broadly truncate, without stout setae. Aedeagus trilobed, with basal piece nearly as long as parameres (Fig. 22D); parameres somewhat triangular, slender and apically narrowing; median lobe tapering to round apex; gonopore well-developed.
Differential diagnosis.
Batochares differs from all other known acidocerines by its unique labrum (with apical region anteriorly flattened, forming a transverse carina across anterior region; Fig. 11D), combined with oval head which is constricted at the anterior margins of the eyes, anterolaterally explanate pronotum, explanate elytra, rows of serial punctures visible at least along outer margins, broadly truncate posterior margin of fifth abdominal ventrite, and unusually large basal piece of the aedeagus (longer than parameres). These features, especially the configuration of the labrum, pronotum and elytra, along with the yellow spots along the surface of the elytra distinguish Batochares from all other known acidocerines.
Distribution.
Afrotropical: Burundi/Rwanda, Central African Republic, Democratic Republic of the Congo, Gabon, Guinea, Kenya, Republic of the Congo, Uganda; Fig. 4.
Natural history.
Little natural history information is available for the genus. Recent collecting data for a few series suggests it may be associated with the margins of streams and small rivers.
Larvae.
Immature stages for Batochares remain unknown.
Taxonomic history.
Batochares was described as a subgenus of Helochares by d’Orchymont (1939b) who did not explicitly designate a type species; therefore, the subgenus name was unavailable according to article 13.3 of the ICZN (1999). In 1991, Hansen validated Batochares as a subgenus of Helochares by fixing the type species for it; therefore, under article 50.1 of the Code (ICZN 1999), Hansen is granted authorship of the subgenus name. d’Orchymont considered Batochares as a subgenus of Helochares based for the most part in the number of antennomeres, relatively long maxillary palps, characters of the mentum and pubescent femora, even though the author recognized the distinctiveness of the shape of the head and the explanate elytra. Batochares was elevated to full generic status based on the phylogenetic analysis in Short et al. (2021), in which it was resolved as an early-diverging, isolated lineage within the Helochares group.
Remarks.
There are three species of Batochares described to date. In his description of Batochares corrugatusBalfour-Browne (1958a: 183), the author pointed out that his record of B. burgeoni from Mutsora, Parc National Albert (currently Virunga National Park, Democratic Republic of the Congo; Balfour-Browne 1950b) was not actually B. burgeoni, but a larger and likely different species. The author also indicated the existence of a different species from Angola.
Species examined.
Batochares burgeoni (d’Orchymont) and B. byrrhus (d’Orchymont).
Selected references.
d’Orchymont 1939b: 293: original description; Balfour-Browne 1958a: 183: description of one additional species; Hansen 1991: 292: type species designated, subgenus validated; Short et al. 2021: generic status, phylogenetic position and affinities discussed.
Genus. Chasmogenus
Sharp, 1882
Chasmogenus Sharp, 1882: 73; Fernández 1986: 189 [generic status reinstated].
Type species: Chasmogenus fragilis Sharp, 1882: 73; by monotypy.
Helochares (Chasmogenus) Sharp; d’Orchymont 1919c: 149 [as subgenus of Helochares]; Knisch 1924: 195 [catalog].
Dieroxenus Spangler, 1979: 753; Girón and Short 2018: 154 [synonymy].
Type species: Dieroxenus cremnobates Spangler, 1979: 754; by original designation and monotypy.
Gender.
Masculine.
Type species.
Chasmogenus fragilis Sharp, 1882: 73; by monotypy.
Diagnosis.
Body length ranging from 2.5–5.0 mm. Body shape oval in dorsal view, parallel-sided to broader around midlength, dorsoventrally flattened, weakly to moderately convex in lateral view (Fig. 24), either evenly convex or flattened along anterior half. Surface of head, pronotum and elytra smooth, with usually shallow ground punctation. Coloration ranging from yellowish orange to dark brown, usually uniform along body, sometimes darker on head or only frons. Shape of head trapezoid (Fig. 11H). Eyes varying in size, usually subquadrate in dorsal view, only very weakly emarginated anteriorly, and usually projected from outline of head. Clypeus trapezoid, with anterior margin mesally weakly to strongly emarginated; membranous preclypeal area visible when clypeus strongly emarginated (Fig. 11H). Labrum fully exposed, semioval, anteriorly mesally emarginated. Mentum usually rather smooth, with anterior depression often reaching midlength of mentum, sometimes limited by low transverse carina. Antennae with eight antennomeres, with cupule slightly asymmetric and rounded. Maxillary palps usually slender and slightly longer than width of head, with inner margin slightly and evenly curved, and outer margin curved along apical half. Pronotum evenly convex. Elytra with sutural striae, with outer margins slightly flared; ground punctures usually only shallowly marked, serial punctures absent and at least one median row of systematic punctures clearly visible on each elytron (Fig. 24). Surface of prosternum usually flat, only rarely with low medial carina along intercoxal process. Posterior elevation of mesoventrite with an either blunt or sharp longitudinal elevation; anapleural sutures sinuate, separated at anterior margin by distance similar or slightly shorter than anterior margin of mesepisternum. Metaventrite with posteromesal and posterolateral glabrous patches (Fig. 24C). Protibiae with spines of anterior row semi erect, relatively long, thick and sparse; apical spurs of protibiae moderately long and thick, reaching apex of protarsomere 2. Metafemora with tibial grooves moderately developed, with sharp posterior margin; hydrofuge pubescence covering at least basal 3/4 of anterior surface of metafemora (Fig. 24C, F). Metatarsomeres 2–4 with two rows of spiniform setae on ventral surface; metatarsomere 5 nearly as long as 3 and 4 combined; metatarsomere 2 shorter to nearly as long as 5. Apex of fifth abdominal ventrite emarginate, with fringe of flat and stout setae. Aedeagus trilobed (Fig. 25); basal piece shorter to nearly as long as parameres; outline of apical region of parameres variable; sometimes parameres asymmetrical; median lobe triangular, either simple or bearing additional sclerite, with well-developed lateral basal apodemes and gonopore.
Differential diagnosis.
Chasmogenus most closely resembles Crephelochares, although they do not co-occur in the same biogeographic regions (Chasmogenus occurs exclusively in the Neotropical region, whereas Crephelochares occurs throughout the Old World). They can be differentiated by the number of antennomeres (eight in Chasmogenus, nine in Crephelochares) and by the form of the aedeagus (trilobed in most Chasmogenus, Fig. 25), divided and further modified in Crephelochares, Fig. 27B–D). Among New World taxa, Chasmogenus can easily be distinguished by the presence of sutural striae, a character shared only with Primocerus, from which it can be distinguished by the shape of the posterior elevation of the mesoventrite: longitudinally elevated in Chasmogenus, transversally elevated in Primocerus. Although Primocerus is quite rare and has a more restricted range in the Neotropics compared with Chasmogenus, the two genera can co-occur in forested steams in the Guiana Shield region.
Distribution.
Neotropical: Argentina, Brazil (Amapá, Amazonas, Minas Gerais, Pará, Piauí, Rio de Janeiro, Roraima, São Paulo), Costa Rica, Ecuador, French Guiana, Guatemala, Guyana, Panama, Paraguay, Suriname, Venezuela; Fig. 4.
Natural history.
The vast majority of Chasmogenus are known from forested habitats, including the margins of streams and forest pools. A few species are known from open marsh habitats (e.g., Chasmogenus australis García and Chasmogenus sapucay Fernández). They can be found among the vegetation and submerged leaf litter. They are also attracted to lights, though usually not in large numbers. Only one species [Chasmogenus cremobates (Spangler)] has been collected in seepages. See Smith and Short (2020) for more detail on habitat information.
Larvae.
The larvae of Chasmogenus remain unknown. The only descriptions of immature stages were made for Chasmogenus nitescens Fauvel (from Australia), which is now assigned to Crephelochares.
Taxonomic history.
Chasmogenus was originally described by Sharp (1882) as a genus to accommodate one Neotropical species from Guatemala and Panama. d’Orchymont (1919c: 149) synonymized Chasmogenus with Crephelochares (from the Old World) and placed it as a subgenus of Helochares. The generic rank of Chasmogenus was re-established by Fernández (1986: 189), with Crephelochares maintained as a junior synonym. Some authors continued to treat Crephelochares as a valid subgenus (e.g., Hebauer 1992, 1995) while others did not recognize any distinction between the two names (Hansen 1991, 1999). The monotypic genus Dieroxenus was synonymized with Chasmogenus by Girón and Short (2018). The recent phylogeny by Short et al. (2021) offered support considering Chasmogenus and Crephelochares as separate genera and affirmed Dieroxenus as a derived lineage within Chasmogenus.
Remarks.
There are 33 described species of Chasmogenus to date, and we are aware of many yet undescribed species in South America. Chasmogenus is a fairly commonly found group of beetles with very little variation in external morphology. Recent collecting efforts and taxonomic study in the genus have revealed a hidden diversity and interesting biogeographic patterns in South America (Smith and Short 2020).
Species examined.
Chasmogenus australis García*, C. amplius Smith & Short*, C. bariorum García*, C. barrae Short*, C. cremnobates (Spangler), C. lineatus Smith & Short*, C. lorenzo Short*, C. ruidus Short*, C. schmits Smith & Short*. Paratypes of the species marked with an asterisk were available for this study.
Selected references.
Sharp 1882: 73: genus description; Spangler 1979: 753: description of Dieroxenus; Fernández 1986: notes on the genus and one new species; Hebauer 1992: notes, recognition of two subgenera, emphasis on Crephelochares; García 2000: four new species from Venezuela; Short 2005: new species from Costa Rica; Short and Fikáček 2013: inclusion of Chasmogenus species in molecular phylogeny; Clarkson and Ferreira-Jr 2014b: four new species from Brazil; Girón and Short 2018: synonymization of Dieroxenus; Alves et al. 2020: description of a new species from Brazil; Smith and Short 2020: description of 18 new species from northeastern South America; Short et al. 2021: phylogenetic placement.
Genus. Colossochares
Girón & Short gen. nov.
http://zoobank.org/4B774C0E-8A05-4DA7-8392-B809D29DDEE2
Figs 1A , 2 , 4 , 11I , 26 , 27A
Helochares “Clade B”, Short et al. (2021).
Gender.
Masculine.
Type species.
Helochares ellipticus d’Orchymont, 1933: 306; by present designation.
Etymology.
From the Latin word colossus, meaning extremely large, in reference to the comparatively large and robust bodies of the members of the genus, combined with the ending chares, expressing affinity with Helochares. Masculine.
Diagnosis.
Body length 8.5–14.0 mm. Body shape broadly oval in dorsal view, strongly and uniformly convex in lateral view (Fig. 26). Dorsal surfaces even and smooth, uniformly dark brown (nearly black) in coloration with reddish antennae, palps and tarsi; ground punctation extremely fine and shallow (Fig. 26A); ventral surfaces rather densely covered by rather long and fine golden setae (Fig. 26C). Eyes not emarginate, moderate in size, subquadrate in dorsal view, separated by nearly 4 × width of eye, projected from outline of head (Fig. 11I). Frons with large (and somewhat fused together) systematic punctures along inner margin of eye. Clypeus with anterior margin broadly roundly emarginate. Labrum fully exposed, medially convex (Fig. 11I). Antennae with nine antennomeres, with strongly asymmetric and round cupule. Maxillary palps slender, slightly longer than maximum width of head, with palpomere 4 0.7 × as long as palpomere 3 (Fig. 11I). Mentum medially broadly depressed, laterally punctate, mesally and anteriorly striate; sculpture of mentum ranging from shallow to strong. Pronotum evenly convex, and very smooth, with ground punctation very fine and shallow; systematic punctures of pronotum reduced to paired depressions near anterior margin and at midlength of lateral margins. Elytra without sutural striae, with margins slightly flared; serial punctures either absent or only visible along outer lateral area and posterior third of elytra; systematic punctures enlarged, broadly separated longitudinally, forming five rows mostly visible along outer lateral area and posterior third of elytra (Fig. 26A, B). Surface of prosternum flat to broadly convex, with anterior margin slightly projected anteriorly (Fig. 26C). Posterior elevation of mesoventrite with broad longitudinal elevation; anapleural sutures concave, anteriorly converging and separated by distance nearly 1/3 of anterior margin of mesepisternum. Metaventrite uniformly densely covered by with hydrofuge pubescence, medial surface elevated as platform. Protibiae with anterior row of spines extremely reduced to tiny and scanty, appressed denticles; apical spurs of protibiae large, outer nearly as thick and reaching apex of protarsomere 2. Metafemora with tibial grooves well-developed; metafemora with hydrofuge pubescence covering basal 4/5 of anterior surface (Fig. 26C). Metatarsomeres laterally compressed, metatarsomere 2 longer than 5, metatarsomere 5 nearly as long as 3 and 4 combined; all tarsomeres with rows spiniform setae covering ventral surface. Fifth abdominal ventrite with apex emarginate, with fringe of flat and stout setae. Aedeagus symmetrical, either trilobed (C. satoi; Hebauer 2003a: fig. 1) or highly modified (Fig. 27A), with basal piece shorter than parameres; median lobe variable.
Differential diagnosis.
Colossochares groups some of the largest acidocerines. Colossochares species are strongly and uniformly convex and highly polished, with enlarged systematic punctures on the head and elytra; systematic punctures on the pronotum are reduced to a pair of anterior and a pair of lateral depressions, not forming the usual antero-lateral semicircles that are common in acidocerines. Some members of Peltochares may exhibit similar coloration and general highly polished appearance to Colossochares (e.g., compare Fig. 1A vs. 1B); those Peltochares are always dorsoventrally flattened, generally slender, and the pronotum has systematic punctures forming antero-lateral semicircles. Other than general appearance, both genera are very similar to each other in details of the external morphology, except by the sculpture of the submentum, which is smooth in Colossochares and punctate or otherwise sculptured in Peltochares. In addition, the aedeagal form in Peltochares (spiked, Fig. 16C, D) is quite different from the forms present in Colossochares (trilobed or as in Fig. 27A).
Distribution.
Afrotropical: Benin, Burkina Faso, Cameroon, Democratic Republic of the Congo, Ethiopia, Gabon, Ghana, Guinea, Ivory Coast, Liberia, Malawi, Nigeria, Republic of the Congo, Uganda; Fig. 4.
Natural history.
Little is known about the biology of Colossochares, and no museum specimens we examined contained any habitat or collecting information. We have seen some light trap samples from Congo in which C. ellipticus is relatively common.
Larvae.
The larvae of species of Colossochares remain unknown.
Taxonomic history.
Given how large and distinctive Colossochares species are, it is remarkable that it has not been previously recognized as a separate genus, especially given how many other genera and subgenera have been described based on less striking features. The reason may have been due in part to an original identification error: Régimbart (1907: 47) first gave a description for what is now Helochares ellipticus, but mistakenly thought they were conspecific with another already-described central African taxon, Hydrophilus ellipticus Fabricius. Régimbart (1907), based on this incorrect interpretation of his specimens, further recognized that they were not allied with Hydrophilus and instead shared similarities with Helochares, so he transferred Fabricius’ species to Helochares, creating the combination Helochares ellipticus (Fabricius). Later, d’Orchymont (1933) recognized Régimbart’s error and clarified the situation, confirming Helochares ellipticus as a valid species of Helochares, and also different from the original Hydrophilus ellipticus Fabricius.
Hebauer (2003) described Helochares satoi Hebauer and discussed its affinities with Helochares ellipticus. A specimen of Helochares ellipticus was included in the molecular phylogeny by Short et al. (2021), where it was resolved as an early-diverging and isolated member of the Helochares group of genera. Given that it is not nested within Helochares, and it is morphologically distinct, the genus Colossochares is here established to house the two species: Colossochares ellipticus (d’Orchymont) comb. nov. and Colossochares satoi (Hebauer) comb. nov.
Remarks.
Despite the external similarity between the two known species of Colossochares, the male genitalia are quite different from each other. This particularity is quite unusual in the subfamily given that, in general, each genus has a particular aedeagal type shared by all its species (though there are some known exceptions, e.g., Chasmogenus). The genitalia of C. satoi can be categorized as trilobed, whereas that of C. ellipticus is quite uniquely configured (Fig. 27B). More work is needed to confirm the close relationship of these two taxa.
Species examined.
Specimens of Colossochares ellipticus (d’Orchymont) and female paratypes of C. satoi (Hebauer) were available for study.
Selected references.
Régimbart 1907: 47: description of Helochares ellipticus attributed to Fabricius; d’Orchymont 1933: 306: clarification and reaffirmation of species name; Hebauer 2003: new species and discussion of affinities; Short et al. 2021: phylogenetic placement.
Genus. Crephelochares
Kuwert, 1890
Figs 1V , 2 , 4 , 11G , 27B–D , 28
Helochares (Crephelochares) Kuwert, 1890a: 38.
Helochares (Crepidelochares) Ganglbauer, 1904: 248 [unjustified emendation of Crephelochares Kuwert, 1890].
Helochares (Chasmogenus) Kuwert; d’Orchymont 1919c: 148 [taxonomic treatment]; Knisch 1924a: 195 [catalog].
Crephelochares Kuwert; Fernández 1986: 148 [junior synonym of Chasmogenus as genus]; Hansen 1991: 293 [catalog]; Short et al. 2021 [elevated to generic rank].
Chasmogenus (Crephelochares) Kuwert; Hebauer 1992: 62 [as subgenus of Chasmogenus].
Gender.
Masculine.
Type species.
Helochares livornicus Kuwert, 1890: 38; subsequent designation by d’Orchymont (1939a: 154).
Diagnosis.
Body length ranging from 2.5–4.8 mm. Body shape oval in dorsal view, dorsoventrally slightly flattened, moderately convex in lateral view, with dorsal outline nearly evenly convex (Fig. 28); surface even and smooth, with usually shallow ground punctation (Fig. 28). Coloration usually dark brown seldom yellowish, uniform across body regions. Head trapezoid (Fig. 11G). Eyes relatively large, at most only slightly emarginated anteriorly, and not or only slightly projected from outline of head. Clypeus trapezoid, with anterior margin mesally emarginate; membranous preclypeal area visible when clypeus strongly emarginated. Labrum fully exposed. Mentum punctate or punctate laterally and medially obliquely striate; medial surface flat to depressed (Fig. 28C); anteromedial depression sometimes limited by low transverse carina. Antennae with nine antennomeres, with cupule slightly asymmetric and rounded. Maxillary palps slender, 1.2–1.5 × longer than width of head; maxillary palpomere 4 nearly 0.7 × length of maxillary palpomere 3; inner margin of maxillary palpomere 2 nearly straight, and outer margin curved along apical half. Pronotum evenly convex. Elytra with sutural striae, with outer margins slightly flared; ground punctures usually only shallowly marked, serial punctures absent and at least one median row of systematic punctures visible on each elytron (Fig. 28). Surface of prosternum usually flat, sometimes tectiform. Posterior elevation of mesoventrite with longitudinal carina; anapleural sutures sinuate, separated at anterior margin by distance similar to slightly shorter than anterior margin of mesepisternum. Metaventrite with posteromesal and posterolateral glabrous patches (Fig. 28C). Protibiae with spines of anterior row semi erect, relatively long, thick and sparse; apical spurs of protibiae relatively short and stout, not reaching apex of protarsomere 2. Metafemora with tibial grooves moderately developed; hydrofuge pubescence covering basal 4/5 of anterior surface of metafemora (Fig. 28C). Metatarsomeres 2–4 gradually decreasing in size, with two rows of spines on ventral surface; metatarsomere 2 slightly longer than 5, 5 shorter than 3 and 4 combined. Fifth abdominal ventrite emarginate at apex, with fringe of flat and stout setae. Aedeagus (Fig. 27B–D) with parameres at most only fused at base on dorsal surface; median lobe divided in dorsal and ventral plates; dorsal plate sclerotized along margins, medially membranous, membranes with papillae or denticles along apico-medial region; ventral plate as inverted Y, sometimes accompanied by basal median laminar sclerite; basal piece nearly as long as or longer than ventral length of parameres, always noticeable; gonopore not clearly visible.
Differential diagnosis.
Among Old World acidocerines, Crephelochares is unique in the presence of sutural stria. The Neotropical Chasmogenus is the most similar genus, as they both share this character (along with the more distantly related Neotropical genus Primocerus). They can be differentiated by the number of antennomeres (eight in Chasmogenus, nine in Crephelochares) and by the form of the aedeagus (trilobed in Chasmogenus, Fig. 25; divided and further modified in Crephelochares, Fig. 27B–D). The configuration of the aedeagus in Crephelochares is quite unique in Acidocerinae, especially because of the configuration of the median lobe and its inner membranes.
Distribution.
Afrotropical: Angola, Benin, Botswana, Burundi, Cameroon, Democratic Republic of the Congo, Gabon, Gambia, Ghana, Guinea, Kenya, Liberia, Madagascar, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Seychelles (Aldabra), Sierra Leone, Somalia, Republic of South Africa, Sudan, Tanzania, Uganda, Zambia, Zimbabwe. Australasian: Australia (New South Wales, Northern Territory, Queensland), Fiji (Vanua Levu, Viti Levu), New Caledonia, Papua New Guinea. Indo-Malayan: Cambodia, China (Guangdong, Hong Kong, Taiwan, Yunnan), Indonesia (Borneo, Java, Papua, Sulawesi, Sumatra), Laos, Malaysia, Sri Lanka, Thailand, Vietnam. Palearctic: Bosnia, Croatia, Greece, Israel, Italy, Japan, Serbia and Montenegro, Spain, Tunisia, Turkey; Fig. 4.
Natural history.
Archangelsky (1997: 55) reproduced the larval descriptions by Anderson (1976), who reared larvae from adults of Crephelochares nitescens (as Helochares nitescens) in laboratory conditions. According to Anderson (1976: 223), females lay between 18 and 25 eggs, “located below the surface of damp soil, in a mossy hollow constructed by the adult; the hollow was always of the same size and shape and lined inside with loose silk. Eggs were deposited at right angles to base of nest, each covered by strands of fine silk attached to floor, walls and adjacent eggs”. The larvae hatch in 5–7 days and are predaceous (Archangelsky 1997: 55). “The larvae would not pupate in damp tissue paper, but only in moss. […]. The larvae pupated naked in the upper moss or in curled decaying leaves” (Anderson 1976: 223). Complete development lasted 24–33 days. Fikáček (2003) provided a diagnosis, pointed out the incompleteness of the descriptions and drawings offered by Anderson (1976), and commented on the unusualness of the habit of laying eggs on the ground by hydrophilid standards.
As for the adults, ecological information is very scarce. According to Hebauer (1992), C. livornicus (Kuwert) was collected in stagnant water with decaying plants and C. orbus (Watanabe) was collected in a rice field. The recently described C. parorbus (Jia and Tang) was also recorded from stagnant waters (Jia and Tang 2018).
Larvae.
The only species for which immature stages are known is Crephelochares nitescens [from Australia; immature stages were originally described as Helochares nitescens by Anderson (1976)]. Anderson (1976) described the breeding method he used, the eggs and egg case, first and third instar larvae and pupa, as well as the entire life cycle. Archangelsky (1997: 55) reproduced Anderson’s (1976) findings.
Taxonomic history.
Crephelochares was originally described as a subgenus of Helochares by Kuwert (1890: 38). In 1904, Ganglbauer established Crepidelochares without justification or explanation. Later, d’Orchymont (1919c: 148) synonymized Crephelochares with Chasmogenus keeping Chasmogenus as a subgenus of Helochares. In 1986, Fernández reinstated Chasmogenus as a genus, with Crephelochares as a junior synonym. Subsequent authors alternately either treated Crephelochares as a subgenus or junior synonym. Hebauer (1992) removed Crephelochares from synonymy with Chasmogenus, and established it as a subgenus of Chasmogenus, discussing morphological features in support of this view, which he maintained in subsequent works (Hebauer 1995). However, Hansen (1991, 1999b) viewed the differences in antennomeres and the aedeagal complexity as “rather subtle” and maintained the two names as synonymous without subgeneric division. The phylogenetic analysis by Short et al. (2021), together with the morphological evidence offered by Hebauer, resulted in the recognition of the generic status of Crephelochares.
Remarks.
There are 29 species of Chephelochares described to date; some of the older species have long lists of synonyms. The most comprehensive treatment for the genus was by Hebauer (1992); the genus was then considered as a subgenus of Chasmogenus.
Species examined.
Crephelochares abnormalis (Sharp), C. africanus (d’Orchymont), C. balkei (Short)*, C. irianus (Hebauer)*, C. livornicus (Kuwert), C. mauritiensis (Balfour-Browne), C. molinai (Hebauer)*, C. nitescens (Fauvel), C. orbus (Watanabe), C. paramollis (Hebauer)*, C. patrizii (Balfour-Browne), C. punctulatus (Short)*, C. ruandanus (Balfour-Browne), C. rubellus (Hebauer)*, C. rusticus (d’Orchymont), C. rutiloides (d’Orchymont), C. rutilus (d’Orchymont), C. szeli (Hebauer)*. For species marked with an asterisk, paratypes were available.
Selected references.
Hebauer 1992: diagnosis, key to species, diagnoses, descriptions for 22 species, and genitalia drawings for 19 of them; Hebauer 1995: one new species from Namibia; Watts 1995: revision of the Australian species of the genus; Short 2010: revision of the species from the Southwest Pacific islands, describing two new species from Fiji and newly recording C. nitescens (Fauvel) for New Caledonia; Devi et al. 2016: redescription and lectotype designation for C. abnormalis (Sharp) with a discussion on its distribution and morphological variation; Short et al. 2021: generic status and phylogenetic placement.
Genus. Crucisternum
Girón & Short, 2018
Figs 1Q , 2 , 5 , 14C , 29 , 30A–E
Figure 5.
Known distribution of genera of Acidocerinae: Crucisternum, Ephydrolithus, Globulosis, Helobata, Helochares, Helopeltarium, Katasophistes, and Nanosaphes.
Crucisternum Girón & Short, 2018: 116.
Gender.
Masculine.
Type species.
Crucisternum ouboteri Girón & Short, 2018: 121; by original designation.
Diagnosis.
Small beetles, body length 2.0–2.5 mm. Body shape elongated oval in dorsal view; moderately convex in lateral view (Fig. 29). Color orange brown to dark brown. Head trapezoid. Eyes moderate to small, projected from outline of head. Clypeus trapezoid, with anterior margin broadly and roundly emarginate. Labrum fully exposed. Mentum with lateral oblique ridges; anterior median depression marked by transverse carina (Fig. 29C). Antennae with nine antennomeres, with cupule only slightly asymmetrical and rounded. Maxillary palps moderately long, slightly longer than width of head (Fig. 29A). Elytra without sutural striae, with outer margins of elytra slightly flared; serial punctures, ground punctures and systematic punctures similar in size and degree of impression, either shallow or rather sharply marked; all punctures seemingly arranged in rows (Fig. 29A). Prosternum with well-developed median, longitudinal, laminar carina (Fig. 29C). Posterior elevation of mesoventrite with a strongly produced, anteriorly pointed transverse ridge, longitudinally carinate (Fig. 14C); anapleural sutures sinuate, separated by distance nearly 0.6 × width of anterior margin of mesepisternum. Metaventrite densely pubescent, except for median and postero-lateral glabrous patches (Fig. 29C). Protibiae with spines of anterior row long and thick; apical spurs of protibiae short and stout, almost reaching apex of protarsomere 2. Metafemora covered by hydrofuge pubescence along basal 4/5 (Fig. 29C). Metatarsomeres 2–4 gradually slightly decreasing in size; metatarsomere 5 slightly longer than 2; ventral coverage of tarsomeres composed of fine and spiniform setae. Fifth abdominal ventrite apically rounded, truncate, or slightly emarginate, without stout setae. Aedeagus trilobate (Fig. 30A–E); basal piece 0.2–0.25 × the length of parameres; median lobe with well-developed lateral basal apodemes, and acute to narrowly rounded apex; parameres nearly as long as median lobe, with outer margins usually sinuate; gonopore situated distad of midlength of median lobe.
Differential diagnosis.
Although Crucisternum is generally unremarkable dorsally from other small-bodied Neotropical acidocerines, several sternal features are strikingly unique and easily separate the genus from all others. The strongly developed prosternal carina found in the genus, combined with the cruciform shape of the posterior elevation of the mesoventrite (formed by the fusion of both transverse and longitudinal ridges), is unique for this genus in the subfamily. Crucisternum is most likely to be confused in samples as a very small Chasmogenus but can also easily be distinguished from that genus by the lack of sutural striae.
Distribution.
Neotropical: Brazil (Minas Gerais, Pará), French Guiana, Guyana, Suriname, Venezuela; Fig. 5.
Natural history.
All species of the genus are associated with forested streams, usually along margins that contain ample detritus. A single specimen of C. ouboteri was collected at a black light trap.
Larvae.
Immature stages are not known for the genus.
Taxonomic history.
The genus was only recently described.
Remarks.
There are seven species currently known.
Species examined.
Holotypes and paratypes of all the known species were examined for this study.
Selected references.
Girón and Short 2018: original description of the genus and all its known species; Short et al. 2021: phylogenetic placement.
Genus. Ephydrolithus
Girón & Short, 2019
Ephydrolithus Girón & Short, 2019: 122.
Gender.
Masculine.
Type species.
Ephydrolithus hamadae Girón & Short, 2019: 130; by original designation.
Diagnosis.
Small beetles, body length 1.8–3.3 mm. Body shape oval in dorsal view, moderate to strongly convex in lateral view (Fig. 31); with ground punctation usually moderately marked. Color yellowish brown to dark brown, usually uniform across body regions (Fig. 31). Shape of head trapezoid. Eyes relatively small, at most only slightly emarginated anteriorly, usually moderately projected from outline of head. Clypeus trapezoid, with anterior margin from broadly to only slightly emarginate. Labrum fully exposed. Mentum with strong median anterior depression sometimes limited by low transverse carina; surface of mentum mostly smooth and undulated. Antennae with nine antennomeres; cupule slightly asymmetric, with rounded outline. Maxillary palps short, nearly 2/3 width of head, and stout (Fig. 31C); inner margin of maxillary palpomere 2 nearly straight, outer margin strongly curved along apical half. Elytra without sutural striae, and only rarely with impressed striae; ground punctures moderate to sharply marked, uniformly and rather densely distributed; systematic punctures slightly larger and deeper than remainder punctures; serial punctures usually not clearly differentiated; outer margins of elytra only slightly flared (Fig. 31A, D). Prosternum flat, sometimes only slightly elevated along longitudinal midline (Fig. 31C). Posterior elevation of mesoventrite either with transverse ridge, or with well-developed tooth that extends anteriorly as longitudinal carina; anapleural sutures concave, separated at anterior margin by distance nearly 0.3 × anterior margin of mesepisternum. Metaventrite densely pubescent, except for large median teardrop-shaped glabrous patch (Fig. 31C, F); anteromedian area of metaventrite with a deep and narrow transverse depression before anterior intercoxal process. Protibiae with spines of anterior row hair-like, semi erect, relatively long and thick (Fig. 31C). All tarsomeres bearing long apical hair-like setae on dorsal face, and two lateral rows of hair-like spines on ventral face of tarsomeres 2–4. Posterior femora mostly glabrous, with few scattered setae along basal half to basal 2/3, with hydrofuge pubescence along anterodorsal margin (Fig. 31C, F); tibial grooves well-developed, sometimes covered by hydrofuge pubescence. Fifth abdominal ventrite apically truncate, with stout setae. Aedeagus trilobed (Fig. 30F–I), with outer margins convex, straight or sinuate, with basal piece 0.45–0.9 × length of parameres; median lobe somewhat triangular in shape, with well-developed lateral basal apodemes; apex of median lobe widely to narrowly acute, sometimes “pinched”; parameres nearly as long as median lobe; well-developed gonopore, preapically situated.
Differential diagnosis.
Ephydrolithus can be distinguished from most Neotropical acidocerines by their mostly glabrous metafemora. From other genera exhibiting the same condition, such as Quadriops (Girón and Short 2017), Ephydrolithus can be distinguished by the entire (as opposed to divided; Fig. 11C) eyes; from Tobochares (Kohlenberg and Short 2017), Ephydrolithus can be differentiated by the number of antennomeres (nine in Ephydrolithus, eight in Tobochares).
Distribution.
Neotropical: Brazil (Bahía, Minas Gerais); Fig. 5.
Natural history.
All known species are exclusively associated with rock seepages (e.g., Fig. 9; Girón and Short 2019).
Larvae.
Immature stages are not known for the genus.
Taxonomic history.
Ephydrolithus was only recently described.
Remarks.
In the etymology section of the original publication, Girón and Short (2019) indicate that the genus name is neuter, which is erroneous. The name is masculine, which is the gender for the Greek word lithos, the last component of the genus name. Four species of Ephydrolithus have been described until now, all of them from southeastern Brazil.
Species examined.
Holotypes and paratypes of all known species were examined for this study. We have also seen specimens of additional undescribed species.
Selected references.
Girón and Short 2018: original description of the genus and all its known species; Short et al. 2021: phylogenetic placement.
Genus. Globulosis
García, 2001
Globulosis García, 2001: 153.
Gender.
Masculine.
Type species.
Globulosis hemisphericus García, 2001: 153; by original designation.
Diagnosis.
Small beetles, body length 1.9–2.3 mm. Body shape rounded in dorsal view, strongly convex in lateral view (Fig. 32). Surface of head, pronotum and elytra smooth, with moderate to shallow ground punctation. Coloration yellow to dark brown, uniform along body, with paler mouthparts and tarsi (Fig. 32). Shape of head relatively oval. Eyes relatively small, anteriorly emarginated (Fig. 32B), not projected from outline of head. Clypeus trapezoid, with anterior margin mesally broadly emarginate. Labrum fully exposed. Mentum with anterior depression limited by low transverse carina; surface of mentum only slightly striate. Antennae with eight antennomeres, with cupule only slightly asymmetric and rounded in outline. Maxillary palps slender, slightly shorter than width of head (Fig. 32C). Pronotum evenly convex. Elytra without sutural or other distinct striae, with outer margins slightly flared; elytral ground punctation shallow to moderate, uniformly distributed (Fig. 32). Surface of prosternum flat. Mesoventrite with transverse ridge, usually elevated medially into acute tooth (Fig. 32C); anapleural sutures concave, separated at anterior margin by distance nearly as width of anterior margin of mesepisternum. Metaventrite uniformly covered by hydrofuge pubescence, with small, longitudinal posteromesal glabrous patch, and reduced posterolateral glabrous patches (Fig. 32C). Protibiae with spines of anterior row long, thick, semi erect and sparse; apical spurs of protibiae short and of moderate thickness. Metafemora with moderate tibial grooves; hydrofuge pubescence covering basal 4/5 of anterior surface (Fig. 32C). Tarsomeres 1–4 ventrally with rows of long and thick setae. Metatarsomeres 2–4 gradually decreasing in size, 5 nearly as long as 2–4 combined. Fifth abdominal ventrite with small truncation at apex, with fringe of flat and stout setae. Aedeagus trilobed (Fig. 30J); with short basal piece, less than 1/3 length of parameres; median lobe wider than width of parameres; gonopore well differentiated.
Differential diagnosis.
Globulosis is among the smallest acidocerines. Its small size along with very round and convex body shape, sets it apart from all other acidocerines known to date.
Distribution.
Neotropical: Brazil (Amazonas, Pará), Colombia, Guyana, Suriname, Venezuela; Fig. 5.
Natural history.
The genus is most commonly found along the margins of small, sandy forested streams, especially with vegetated margins. However, a few specimens have been taken in shallow swamps.
Larvae.
The immature stages of Globulosis remain unknown.
Taxonomic history.
García (2001) described the genus with one species, and placed it in its own tribe (Globulosina, now synonymized with Acidocerinae). The genus was revised in 2017 by Short et al., who described one new species and examined new material that greatly expanded the range of the previously known species.
Remarks.
There are two described species of Globulosis. One female specimen from Colombia has been left unidentified as it could not be reliably assigned to any species. Because of the extremely uniform external morphology in the genus, the male genitalia is the most reliable feature for species recognition. Based on additional material we have examined the genus appears to be more broadly distributed in the Amazon region than as currently published.
Species examined.
The holotype, along with several additional specimens of Globulosis hemisphericus García, and the holotype and paratypes of G. flavus Short, García & Girón were examined in this study.
Selected references.
García 2001: genus description, monotypic; Short et al. 2017: description of one new species from Venezuela, range expansion for type species; Short et al. 2021: phylogenetic placement.
Genus. Helobata
Bergroth, 1888
Figs 1J , 2 , 5 , 11L , 33 , 34
Helopeltis Horn, 1873: 137.
Type species: Helopeltis larvalis Horn, 1873: 137; by monotypy.
Helobata Bergroth, 1888: 221 – Replacement name for Helopeltis Horn, 1873.
Helopeltina Cockerell, 1906: 240 – Replacement name for Helopeltis Horn, 1873.
Type species: Helopeltis larvalis Horn, 1873: 137.
Gender.
Feminine.
Type species.
Helopeltis larvalis Horn, 1873: 137; by monotypy.
Diagnosis.
Medium sized beetles, body length 4–7 mm. Body shape oval in dorsal view, dorsoventrally flattened, with dorsal outline nearly straight along medial third in lateral view (Fig. 33); surface even and granulate. From yellowish, orange brown to dark brown in coloration, usually with patterns along elytra, with different areas of head and pronotum darkened. Shape of head somewhat trapezoid (Fig. 11L). Anterior corners of frons extended laterally and posteriorly, emarginating anterior margin of eyes. Eyes of moderate size, somewhat oval, anteriorly deeply emarginated, not projected from outline of head. Clypeus somewhat pentagonal, laterally explanate, with anterior margin usually straight (Fig. 11L). Labrum concealed by clypeus (Fig. 11L). Mentum with surface variably sculptured, usually with oblique and transverse striae (Fig. 33C). Antennae with eight antennomeres, with cupule strongly asymmetric and oval in outline. Maxillary palps slender, slightly longer than greatest width of head; inner margin of maxillary palpomere 2 weakly and evenly curved, and outer margin weakly curved along apical third (Fig. 33C). Pronotum with surface of lateral areas flat. Elytra without sutural striae, with outer margins laterally explanate; serial punctures clearly aligned in longitudinal rows (Fig. 33A). Scutellar shield U-shaped. Surface of prosternum flat, to medially bulging, smooth to irregularly sculptured. Posterior elevation of mesoventrite only weakly bulging, with pair of lateral, longitudinal, low ridges; anapleural sutures nearly parallel along anterior section, separated anteriorly by distance slightly shorter than anterior margin of mesepisternum. Metaventrite uniformly covered by hydrofuge pubescence, with medial, narrow, and slightly carinate glabrous patch; posterolateral glabrous patches reduced. Protibiae with spines of anterior row short and semi erect; apical spurs of protibiae reduced, much shorter than protarsomere 1. Metafemora with tibial grooves moderately developed; hydrofuge pubescence covering 5/6 of anterior surface (Fig. 33C). Tarsomeres 1–4 ventrally densely covered by setae; metatarsomere 2 longer than 3 and 4 combined, 1 nearly as long as 3, and 5 nearly as long as 2–4 combined. Fifth abdominal ventrite apically emarginate, with fringe of flat and stout setae. Aedeagus divided (Fig. 34), parameres separated from each other for most of their lengths; median lobe divided in dorsal and ventral plates; dorsal plate usually strongly sclerotized; ventral plate bilaterally bifurcated, forming thick lateral lobes along apical region; basal piece nearly 0.2 × the length of parameres, always noticeable; gonopore not clearly visible.
Differential diagnosis.
Helobata is one of the most conspicuous genera of acidocerines, especially in the New World. The flattened and broadly explanate body shape and concealed labrum, accompanied by granulose surface, long and slender maxillary palps and well-defined elytral serial punctures, are quite unique in the subfamily. The only genus that shares some of these features is Helopeltarium, except that the latter has short maxillary palps, smooth surface and lacks serial punctures along the elytra. The configuration of the aedeagus (Fig. 34), in particular the thickness of the lateral lobes of the ventral plate of the median lobe, is also unique among acidocerines.
Distribution.
Nearctic: United States (California, Florida, Louisiana, Mississippi, North Carolina, South Carolina, Texas, Virginia). Neotropical: Argentina, Bolivia, Brazil (Amazonas, Ceará, Mato Grosso, Mato Grosso do Sul, Pará, Rio de Janeiro, Roraima), Cuba, Guatemala, Mexico, Paraguay, Suriname, Venezuela; Fig. 5.
Natural history.
Species of Helobata occur primarily in open habitats with abundant vegetation. According to Clarkson et al. (2016), specimens of Helobata are uncommonly encountered and occur in marshes, swamps, and ponds, most often in small numbers, although they are rarely found in modest amounts (dozens of individuals; Short, pers. obs.). According to Archangelsky (1997), they can be found in slow moving creeks or rivers, living among the littoral vegetation or on floating plants. They are attracted to lights. Females have been observed carrying their egg cases attached to the ventral side of their abdominal ventrites (Archangelsky 1997).
Larvae.
The larva (first instar) and egg case are only known for Helobata larvalis; these immature stages were described by Spangler and Cross (1972). A differential diagnosis of the first instar larva was provided by Fikáček (2003).
Taxonomic history.
This genus was described by Horn (1873) under the name Helopeltis, which was preoccupied by Helopeltis Signoret, 1858 (Hemiptera). Bergroth (1888) proposed the name Helobata as a replacement name for Helopeltis Horn, whereas Cockerell (1906a) proposed the name Helopeltina. Helobata has priority, so it is the currently valid name for the genus, which was revised by Fernández and Bachmann (1987).
Remarks.
There are 13 species of Helobata described to date. The type species, Helobata larvalis (Horn), has generally been known under the name Helobata striata (originally published as Hydrophilus striatus Brullé, 1841: 58, which is a primary homonym of Hydrophilus striatus Say, 1825 [now Berosus striatus (Say)]; therefore unavailable. The name Helobata larvalis (Horn) was then reinstated by Hansen (1991: 293). Photos of a syntype of Helopeltis larvalis (Horn) are available at https://mczbase.mcz.harvard.edu/guid/MCZ:Ent:101 (accessed 9 January 2021). The external morphology of members of Helobata is very homogeneous. Some variation can be observed in the shape of the clypeus (e.g., Fernández 1987; Clarkson et al. 2016). Helobata is the only Neotropical genus truly widespread in the New World, as it ranges from southeastern North America, all the way to Argentina and Southern Brazil.
Species examined.
Helobata cuivaum García (paratype), H. larvalis (Horn), and H. lilianae García (paratype).
Selected references.
Horn 1873: original description of the genus and the type species; Spangler and Cross 1972: description of egg case and first instar larva; Fernández and Bachmann 1987: review of the genus, description of four new species from Argentina, Brazil and Paraguay; García 2000: three new species from Venezuela; Makhan 2007: two new species from Suriname; Clarkson et al. 2016: two new species from Brazil, review and new country records of Brazilian species; Clarkson and Almeida 2018: new records from Brazil; Short et al. 2021: phylogenetic placement.
Genus. Helochares
Mulsant, 1844
Figs 1E, F , 2 , 5 , 11F , 35 , 36 , 37A–H
Helophilus Mulsant, 1844a: 132 [rejected name no. 1707 (ICZN 1964, Opinion 710)].
Helochares Mulsant, 1844a: 197; replacement name for Helophilus Mulsant, 1844a: 132; official name no. 1601 (ICZN 1964, Opinion 710).
Enhydrus Dahl 1823: 34 [nomen nudum; rejected name no. 1705 (ICZN 1964, Opinion 710)].
Enhydrus MacLeay, 1825: 35 [rejected name no. 1704 (ICZN 1964, Opinion 710)].
Pylophilus Motschulsky, 1845: 32.
Type species: Hydrophilus griseus Fabricius, 1787: 189; fixed by monotypy = Dytiscus lividus Forster, 1771.
Peloxenus Motschulsky, 1845: 549; replacement name for Pylophilus Motschulsky, 1845.
Helophygas Motschulsky, 1853: 11 [rejected name no. 1708 (ICZN 1964, Opinion 710)].
Helocharis Thomson, 1859: 18 [incorrect subsequent spelling].
Hydrobaticus MacLeay, 1871: 131, syn. nov.
Type species: Hydrobaticus tristis MacLeay, 1871: 131; by subsequent designation by d’Orchymont (1943a: 2); originally described as genus; downgraded to subgenus of Helochares by d’Orchymont (1919c: 148).
Helocharimorphus Kuwert, 1890: 306, syn. nov.
Type species: Helocharimorphus sharpi Kuwert, 1890: 307; by monotypy; originally described as genus; downgraded to subgenus of Helochares by d’Orchymont (1919c: 148).
Graphelochares Kuwert, 1890: 38.
Type species: Helophilus melanophthalmus Mulsant, 1844a: 137; by monotypy.
Grapidelochares Ganglbauer, 1904: 248; [unjustified emendation of Graphelochares Kuwert, 1890].
Gender.
Masculine.
Type species.
Dytiscus lividus Forster, 1771: 52; by subsequent designation (Thomson 1859: 18).
Diagnosis.
Small to medium sized beetles, body length 2–7 mm. Body shape oval in dorsal view; slightly to moderately convex in lateral view, with dorsal outline nearly flat along anterior half of elytra, or somewhat evenly curved (Figs 35, 36). Coloration usually yellowish brown, sometimes orange brown, pale brown to medium brown; ground punctation shallow (e.g., Fig. 35D) to strongly marked (e.g., Fig. 36D). Shape of head trapezoid to oval (e.g., Fig. 11F). Eyes medium sized to large, not or moderately emarginated anteriorly, usually projected from outline of head. Clypeus trapezoid, with anterior margin broadly and roundly emarginate; sometimes lateral margins of clypeus slightly bent upwards. Labrum fully exposed. Mentum rather flat, sparsely punctate, coarsely to shallowly, rarely striate (e.g., Figs 35C, 36C); median anterior depression of mentum relatively shallow; submentum shallowly punctate to smooth. Antennae with nine antennomeres; cupule strongly asymmetric, with rounded outline; antennomere 9 slightly, to 3 × longer than antennomere 7. Maxillary palps slender, moderately long, 0.6–1.2 × the width of head (e.g., Figs 35C, 36C); inner margin of maxillary palpomere 2 weakly and evenly curved to nearly straight, outer margin evenly curved to curved along apical 2/3; maxillary palpomere 3 slightly longer than 4. Prosternum flat to medially bulging to tectiform. Elytra without sutural striae, with ground punctures usually moderately marked; often with serial punctures forming ten longitudinal rows along elytra (e.g., Fig. 35A). Posterior elevation of mesoventrite, flat to simply bulging (e.g., Fig. 35C); bulge usually with long fine setae; anapleural sutures strongly concave, nearly parallel along anterior section, separated anteriorly by distance 0.6–1.0 × anterior margin of mesepisternum. Metaventrite densely covered by hydrofuge pubescence, without glabrous patches (e.g., Figs 35C, 36C). Protibiae with spines of anterior row either nearly absent (e.g., Fig. 35C) or as long thick semi-erect setae. Metafemora with tibial grooves weakly developed to absent; hydrofuge pubescence covering basal 6/7 of anterior surface. Tarsomeres 1–4 with pair of lateral rows of long fine spines on ventral face, sometimes ventral face densely covered by hair-like spines; tarsomere 5 with medial row of long fine spines; metatarsomeres variable in proportions (2–4 gradually decreasing in size with 5 nearly as long as 3 and 4 combined; 2 and 5 similar in length, each slightly longer than 3 and 4 combined). Fifth abdominal ventrite apically emarginate, with fringe of stout setae. Aedeagus tubular (Fig. 37A–H); parameres fused to each other for most of their lengths, with apex either simple or bifurcate/bilobate; median lobe with very long basal apodemes (as long or longer than main piece of median lobe), often extending beyond base of parameres in repose; median lobe either simple (without subdivisions), or with multiple and different kinds of sclerotizations of inner membranes; basal piece usually much shorter than parameres; gonopore of variable development, usually visible when median lobe is simple.
Differential diagnosis.
In the present definition, most species of Helochares are yellowish to brown in coloration, ranging in size from 2–7 mm (e.g., Figs 35, 36), usually moderately punctate throughout the dorsal surface, and most diverse in the Old World. Smaller members of the genus may be confused with Agraphydrus, from which Helochares can be distinguished by its uniformly pubescent metaventrite (e.g., 36C, F; Agraphydrus bears a distinct posteromedian glabrous patch on the metaventrite, e.g., Fig. 18F, I). From Peltochares, and Novochares, members of Helochares can be distinguished by their shorter and relatively stout maxillary palps [0.6–1.2 × the width of the head in Helochares (e.g., Fig. 35C), as opposed to slender, 1.3–1.8 × in Peltochares (e.g., Fig. 44C, F), 1.1–1.5 × in Novochares (e.g., Fig. 42C, F)]; and by the development of the tibial grooves (weakly developed to absent in Helochares, well developed in both Novochares and Peltochares). The most problematic species would be those that are dark brown, relatively flattened, highly polished, and 4–5 mm long. In those cases, the most reliable feature for identification would be the male genitalia: Helochares has tubular aedeagi (e.g., Figs 16E, F, 37A–H), Peltochares has spiked aedeagi (e.g., Figs 16C, D, 45), and Novochares has divided aedeagi (e.g., Figs 16G, H, 43); see explanation of aedeagal types under the aedeagus section of Morphological variation in Acidocerinae and its taxonomic importance).
Distribution.
Afrotropical: Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Chad, Democratic Republic of the Congo, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea Bissau, Ivory Coast, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius (incl. Mascarene Is., Rodrigues), Morocco [in doubt], Mozambique, Namibia, Niger, Nigeria, Oman, Republic of the Congo, Réunion, Rwanda, São Tomé and Príncipe, Saudi Arabia, Senegal, Seychelles (incl. Aldabra), Sierra Leone, Republic of South Africa, South Sudan, Sudan, Tanzania, Togo, Uganda, United Arab Emirates, Yemen (incl. Socotra), Zambia, Zimbabwe. Australasian: Australia (Australian Capital Territory, New South Wales, Northern Territory, Queensland, South Australia, Tasmania, Victoria, Western Australia), Fiji, Papua New Guinea (incl. Duke of York), Vanuatu. Indo-Malayan: Bangladesh, Burma, Cambodia, China (Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hong Kong, Hunan, Jiangxi, Macao, Taiwan, Yunnan, Zhejiang), India (Andaman Is., Assam, Bihar, Karnataka, Madhya Pradesh, Nicobar Is., Uttarakhand, Uttar Pradesh, Tamil Nadu, West Bengal), Indonesia (Bali, Borneo, Java, Lombok, Papua, Sumatra), Laos, Malaysia (Peninsula, Sabah), Nepal, Philippines (Manila), Singapore, Sri Lanka, Thailand, Vietnam. Nearctic: U.S.A. (Alabama, Arkansas, Arizona, California, Delaware, District of Columbia, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Mississippi, Missouri, Nevada, North Carolina, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, Tennessee, Texas, Virginia). Neotropical: Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Peru, Venezuela. Oceanian: Samoa, Tonga. Palearctic: Algeria, Austria, Azerbaijan, Belarus, Bosnia Herzegovina, Bulgaria, Canary Islands, China (Chongqing, Jilin, Hubei, Jiangsu, Shanghai, Shaanxi, Sichuan, Xinjiang, Xizang [Tibet]), Croatia, Czech Republic, Denmark, Egypt, Estonia, Finland, France, Germany, Georgia, Great Britain, Greece, Hungary, Iran, Iraq, Ireland, Israel, Italy, Japan, Latvia, Lebanon, Lithuania, Luxembourg, Macedonia, Morocco, Netherlands, Norway, Pakistan, Poland, Portugal, Russia, Serbia and Montenegro, Slovakia, Slovenia, South Korea, Spain, Sweden, Switzerland, Syria, Tunisia, Turkey, Ukraine; Fig. 5.
Natural history.
Most of the older descriptions have no associated ecological information. Species of Helochares are aquatic (Hansen 1991) with a preference for quiet bodies of water (Archangelsky 1997) or slow flowing streams, rivers or pools, with pebbles, and mossy stones (Dong and Bian 2021); some species have been collected in rivers, streams, ponds, stagnant water, along sides of rivers, forest pool margins, usually associated with live or decomposing floating vegetation. They can be occasionally collected at light, sometimes in large numbers (Jia and Tang 2018). Females have been observed carrying their egg cases attached to the ventral side of their abdomen.
Larvae.
Anderson (1976) described the immature stages of Helochares tristis (MacLeay) along with the breeding method he used; the author described the eggs, egg case (25–50 eggs per case), first, second, and third instar larvae and pupa, as well as the entire life cycle. Anderson (1976) recorded observations of the emergence of larvae and adults. As the females carry their eggs attached to the ventral side of their bodies, Anderson (1976: 222) noted: “When hatching from an attached bag, larvae appeared to emerge into the ventral bubble of air. Larvae then rose to the surface of the water and swam away with an alternate head-to-tail movement. They were observed to have bubbles of air in the abdomen. No doubt this was taken from the ventral air bubble and enabled the larvae to become buoyant.” According to Archangelsky (1997) the larvae are predatory and also cannibalistic.
A diagnosis for larvae of Helochares as well as a list of the described immatures are provided in Fikáček (2003), at the time considering Helochares sensu Hansen (1991), including species of Novochares and Peltochares; the known larvae of the redefined Helochares are H. lividus (Forster) (unknown stage larva in d’Orchymont 1913b; first, second and third instar larvae in Panzera 1932), H. maculicollis Mulsant (eggs, first and third instar larvae and pupa in Richmond 1920), H. obscurus (Müller) (first, second and third instar larvae in Panzera 1932, as H. griseus], H. tristis (MacLeay) (eggs, first, second and third instar larvae, and pupa in Anderson 1976), H. clypeatus (Blackburn) (third instar larva in Watts 2002), H. luridus (MacLeay) (third instar larva in Watts 2002), H. tenuistriatus Régimbart (third instar larva in Watts 2002). Minoshima and Hayashi (2011) described H. anchoralis Sharp (first instar larva), H. nipponicus Hebauer (first, second and third instar larvae), and H. pallens (MacLeay) (first, second and third instar larvae); Table 3.
Taxonomic history.
The genus was originally described under the name of Helophilus, which was preoccupied by Helophilus Leach, 1817 (Diptera), therefore Helochares was proposed by Mulsant (1844) as a replacement name. Thomson, in 1859, designated the type species for the genus. Through time Helochares, as well as some of its species, have accumulated multiple synonyms. In 1919, d’Orchymont recognized five subgenera within Helochares: Helochares, Chasmogenus, Helocharimorphus, Hydrobaticus, and Sindolus. Chasmogenus was recognized as a separate genus by Fernández (1986). Hansen (1991) added Batochares as a subgenus of Helochares and commented on the possibility that the recognized subgenera of Helochares at the time, represented actually distinct genera. Short et al. (2021) elevated Batochares and Sindolus to generic status based on their molecular phylogeny, as they were found to indeed represent separate clades. Additionally, Short et al. (2021) found that the type species of Helochares (Helochares lividus (Forster), which is from the Palearctic region) and the type species of Hydrobaticus (Helochares tristis (MacLeay) from Australia) are actually relatively closely related and belong in the same subclade (Clade A3 in Short et al. 2021). Furthermore, both species share morphological details of the male genitalia, therefore, we synonymize Hydrobaticus syn. nov. with Helochares. Conversely, the morphological variation under the new concept of Helochares encompasses the features that were used for recognizing Helocharimorphus: lack of elytral striae, short maxillary palps, mesoventrite only slightly elevated in front of the mesocoxae, and metatibiae slightly curved (d’Orchymont 1919c: 149, in key). In contrast, more distinct and divergent morphotypes [e.g., small size (nearly 3 mm); strongly punctate surface; emarginated eyes; clypeus laterally bent upwards; Fig. 36D–F] are nested within the main Helochares clade. Therefore, despite not knowing the configuration of the aedeagus, we synonymize Helocharimorphus syn. nov. with Helochares.
While the newly defined concept of Helochares is strongly supported as monophyletic (Short et al. 2021), it is a relatively ancient lineage (more than 100 mya) that has accumulated significant morphological variation and deep phylogenetic structure. Short et al. (2021) recovered three strongly supported clades (named A1, A2, and A3), though the relationships among the clades were indecisive among analyses. These clades could potentially serve as a basis for future subgenera. Clade A1 comprises at least two currently described species (H. fuliginosus and H. songi) from southeast Asia that have a tubular form of the aedeagus (Fig. 37H for H. songi), although there appear to be additional undescribed species in the region. Clade A2, which is relatively similar in morphology to Clade A1, comprises all New World species that remain assigned to Helochares, also with a similar tubular aedeagal form (Fig. 37G for H. politus); this lineage was recently revised by Short and Girón (2017). All remaining species fall in Clade A3, which even in this reduced form contains tremendous morphological diversity (Fig. 37A–F). More study is needed for the genus as a whole, and in particular Clade A3, to further refine its classification and reintroduce species groups and subgenera. It is likely that features of the male genitalia will continue to prove useful in any refined classification of the genus.
Remarks.
Helochares has been generally considered the most diverse, most widespread, and most taxonomically challenging genus of acidocerines. Even after the removal of unrelated lineages by Short et al. (2021), there remain 159 described species of Helochares, although Agraphydrus has now eclipsed Helochares as the largest genus, with 201 described species. Efforts have been made to try to make sense of such diversity, by studying local faunas (Hansen 1982; Watts 1995; Hebauer 1996; Short and Girón 2017; Jia and Tang 2018), but traditional character systems used for classification have been inadequate for distinguishing monophyletic groups. Only now, after the phylogenetic study by Short et al. (2021), there is some clarity regarding morphological trends in the genus. Most of the representative specimens available for this study are card-mounted, therefore characters of the ventral surfaces in the diagnosis offered here, are based on observations made on a sample of pin-mounted specimens.
Species examined.
Helochares aethiopicus d’Orchymont,
H. anchoralis Sharp***,
H. alberti d’Orchymont,
H. andreinii d’Orchymont,
H. anthonyae Watts,
H. balfourbrownei Hansen,
H. bohemani d’Orchymont***,
H. camerunensis d’Orchymont,
H. cancellatus Hebauer*,
H. championi Sharp***,
H. clypeatus Blackburn,
H. conformis Hebauer*,
H. congruens d’Orchymont,
H. crenatostriatus Régimbart,
H. crenatuloides d’Orchymont***,
H. crepitus Balfour-Browne,
H. crispus d’Orchymont,
H. densepunctus Régimbart,
H. densus Sharp,
H. depactus d’Orchymont,
H. didymus d’Orchymont,
H. difficilis d’Orchymont,
H. dilutus Erichson***,
H. dimorphus d’Orchymont,
H. dollmani Balfour-Browne,
H. dolus d’Orchymont,
H. egregius Balfour-Browne,
H. endroedyi Hebauer*,
H. fratris Hebauer*,
H. fuliginosus d’Orchymont,
H. insolitus d’Orchymont,
H. itylus Balfour-Browne,
H. ivani Hebauer*,
H. laevis Short & Girón**,
H. lentus Sharp,
H. lepidus d’Orchymont,
H. leptinus d’Orchymont,
H. lividoides Hansen & Hebauer,
H. lividus (Forster),
H. loticus Hebauer*,
H. luridus (MacLeay),
H. maculicollis Mulsant,
H. mecarus d’Orchymont,
H. mediastinus d’Orchymont,
H. melanophthalmus (Mulsant),
H. mentinotus Kuwert,
H. mersus d’Orchymont,
H. minax d’Orchymont,
H. minor d’Orchymont,
H. minusculus d’Orchymont,
H. nebridius d’Orchymont,
H. negatus Hebauer*,
H. neglectus (Hope)***,
H. nexus Short & Girón**,
H. nigrifrons Brancsik,
H. nigripalpis Hebauer & Hendrich*,
H. nigroseriatus Hebauer*,
H. nipponicus Hebauer***,
H. normatus (LeConte),
H. obscurus (Müller)***,
H. pallens (MacLeay)***,
H. percyi Watts,
H. perminutus Hebauer,
H. politus Short & Girón**,
H. punctatus Sharp,
H. salvazai d’Orchymont,
H. schwendingeri Hebauer,
H. scitulus Balfour-Browne,
H. sharpi (Kuwert)***,
H. skalei Hebauer,
H. steffani Hebauer*,
H. stenius d’Orchymont,
H. striatus Boheman,
H. strictus d’Orchymont,
H. strigellus Hebauer*,
H. structus d’Orchymont,
H. subtilis d’Orchymont,
H. tatei (Blackburn)***,
H. tenuistriatus Régimbart,
H. tristis (MacLeay)***,
H. trujillo Short & Girón**,
H. wagneri Hebauer*,
H. wattsi Hebauer & Hendrich*,
H. yangae Hebauer, Hendrich & Balke*,
H. zamora Short & Girón**.
For species marked with one asterisk (*) at least one paratype was available. For species marked with two asterisks (**) the holotype, and in some cases paratypes were examined in this study; all these specimens were card-mounted. For species marked with three asterisks (***) some specimens were pin-mounted, allowing to view ventral structures. For H. championi Sharp one of the available specimens was previously compared with the holotype by A. Short.
Selected references.
d’Orchymont 1939b, 1943a, c, e: miscellaneous taxonomic works focused on Helochares, for the most part describing new species, some of which include aedeagal illustrations; Hansen 1982: notes on European species with morphological clarifications; Hansen 1991: generic diagnosis, synonyms, list of subgenera; Watts 1995: faunistic study for Australia; Hebauer 1996: faunistic study for Africa; Short and Girón 2017: faunistic study for the New World; Jia and Tang 2018: faunistic study for China; Short et al. 2021: phylogenetic placement and main clades within genus.
Genus. Helopeltarium
d’Orchymont, 1943
Helopeltarium d’Orchymont, 1943f: 9.
Gender.
Masculine.
Type species.
Helopeltarium ferrugineum d’Orchymont, 1943f: 10; by original designation and monotypy.
Diagnosis.
Small beetles, body length nearly 3.5 mm. Body broadly oval and explanate in dorsal view, rather flat in lateral view, with dorsal outline nearly straight along median region (Fig. 38). Surface smooth (without granulations or reticulations), with ground punctation strongly marked. Body orange brown, slightly paler along margins (Fig. 38). Shape of head somewhat trapezoid. Anterior corners of frons extended laterally and posteriorly, emarginating anterior margin of eyes. Eyes relatively small, with anterior margin markedly emarginate in lateral view, in dorsal view not projecting from outline of head. Clypeus laterally expanded in front of eyes; anterior margin of clypeus slightly emarginate. Labrum concealed under clypeus. Mentum with surface obliquely striate (Fig. 38C). Antennae with nine antennomeres, cupule strongly asymmetric, with rounded outline. Maxillary palps short and moderately stout, hardly 3/4 as long as width of head; maxillary palpomere 4 nearly as long as palpomere 3; inner margin of maxillary palpomere 2 nearly straight, outer margin curved along apical half (Fig. 38C). Elytra without sutural striae, broadly explanate laterally, serial punctures absent, ground punctures sharply marked, densely and uniformly distributed (Fig. 38A). Prosternum slightly convex, not carinate medially (Fig. 38C). Posterior elevation of mesoventrite only bulging (Fig. 38C); anapleural sutures only slightly concave, separated at anterior margin by distance similar to anterior margin of mesepisternum. Metaventrite uniformly covered by hydrofuge pubescence (Fig. 38C). Protibiae with spines of anterior row long, thick, and semi-erect; apical spurs of protibiae stout, extending to apex of protarsomere 2. Metafemora without distinct tibial grooves; hydrofuge pubescence covering basal 3/4 of anterior surface of metafemora (Fig. 38C). Tarsomeres 2–4 ventrally densely covered by setae; metatarsomere 1 much shorter than 2; metatarsomere 5 nearly as long as metatarsomere 2 or 3 and 4 combined. Fifth abdominal ventrite apically emarginate, with fringe of flat and stout setae. Aedeagus tubular (Fig. 37I); distal region of each paramere diverging; apex of parameres rounded; basal piece nearly half as long as parameres; median lobe broad, apically tapering to rounded tip; gonopore not clearly visible.
Differential diagnosis.
Helopeltarium has a very unique appearance within acidocerines. The flattened and broadly explanate body shape and concealed labrum, accompanied by smooth surface, short and stout maxillary palps, lacking elytral serial punctures is unique in the subfamily. It may appear like a very small Helobata, but besides geographic origin, the lack of serial punctures, smooth surface and short maxillary palps sets Helopeltarium apart very easily. The configuration of the aedeagus in Helopeltarium, is very similar to that of some Helochares, but the external morphology alone allows for its immediate recognition.
Distribution.
Indo-Malayan: Myanmar (formerly Burma); Fig. 5.
Natural history.
There is no natural history information available for the genus.
Larvae.
Immature stages are not known for Helopeltarium.
Taxonomic history.
Originally described by d’Orchymont (1943f: 9). Redescribed by Hansen (1991: 149).
Remarks.
In the original description, d’Orchymont (1943f) compared Helopeltarium with Helobata. As far as we know, the genus is only known from two syntype specimens of the only known species. This genus was not included in the molecular phylogeny by Short et al. (2021). Its assignment to the Helochares group is primarily based on the form of the aedeagus, as well as its distribution in the Old World. Indeed, the genitalia is very similar to those found in some clades of Helochares, and it would not be surprising to us if Helopelatarium is eventually found to be sister to or nested within Helochares.
Species examined.
Syntypes of Helopeltarium ferrugineum d’Orchymont.
Selected references.
d’Orchymont 1943f: 9: original description; Hansen 1991: 149: redescription; Short et al. 2021: phylogenetic position and affinities discussed.
Genus. Katasophistes
Girón & Short, 2018
Katasophistes Girón & Short, 2018: 132.
Gender.
Masculine.
Type species.
Katasophistes merida Girón & Short, 2018: 136; by original designation.
Diagnosis.
Medium to small beetles, body length 2.7–4.5 mm. Body shape oval to elongated in dorsal view; moderately and evenly convex in lateral view (Fig. 39). Color orange brown to dark brown, rather uniform along body regions (Fig. 39). Shape of head trapezoid. Eyes relatively small, subquadrate, at most only slightly emarginated anteriorly, moderately projected from outline of head. Clypeus trapezoid, with anterior margin broadly emarginate. Labrum fully exposed. Mentum with strong median anterior depression sometimes limited by low transverse carina; surface of mentum with lateral oblique ridges (Fig. 39C, F). Antennae with nine antennomeres; cupule slightly asymmetric, with rounded outline. Maxillary palps moderately long, 0.7 × to nearly as long as width of head; inner margin of maxillary palpomere 2 slightly curved near apex, outer margin curved, sometimes strongly, along apical half (Fig. 39C, F). Each elytron with five rows of deep/large systematic punctures; elytra without sutural striae, with outer margins slightly flared; serial punctures absent (Fig. 39A, D). Prosternum slightly convex to tectiform. Posterior elevation of mesoventrite, with a well-defined, curved transverse ridge; anapleural sutures forming an obtuse angle, separated at anterior margin by distance 0.2–0.3 × the width of anterior margin of mesepisternum. Metaventrite densely pubescent, except for large median rhomboid glabrous patch (Fig. 39C, F). Protibiae with spines of anterior row hair-like, semi erect, relatively long and thick. All tarsomeres bearing long apical hair-like setae on dorsal face, and hair-like spines on ventral face of tarsomeres 2–4. Posterior femora glabrous at most along apical third (Fig. 39C, F). Fifth abdominal ventrite apically truncate to slightly emarginate, with fringe of stout setae. Aedeagus trilobed (Fig. 40A–D), nearly parallel sided, with basal piece between 0.5 and 1.1 × length of parameres; median lobe wider than each paramere, gradually narrowing apically, with conspicuous median longitudinal sclerotization, and well-developed lateral basal apodemes; apex of median lobe acute; parameres nearly as long as median lobe, with apical setae; gonopore preapically situated.
Differential diagnosis.
At first glance Katasophistes may appear similar to some species of Chasmogenus, however the lack of sutural striae easily separates the two. The enlargement of the rows of elytral systematic punctures is also rare within the Acidocerinae (found in some Chasmogenus and Agraphydrus) and will separate it from New World Helochares, with which it may also be confused.
Distribution.
Neotropical: Ecuador, Peru, Venezuela; Fig. 5.
Natural history.
One species (K. merida) is known from seepages in the Venezuelan Andes. The other described species are known from forested stream pools with abundant detritus in Ecuador and Peru.
Larvae.
Immature stages are not known for the genus.
Taxonomic history.
Katasophistes was only recently described.
Remarks.
There are four known species of Katasophistes, all of them from Andean or Andean-adjacent localities.
Species examined.
Holotypes and paratypes of all known species were available for this study.
Selected references.
Girón and Short 2018: original description of the genus and all its known species; Short et al. 2021: phylogenetic placement.
Genus. Nanosaphes
Girón & Short, 2018
Nanosaphes Girón & Short, 2018: 143.
Gender.
Masculine.
Type species.
Nanosaphes tricolor Girón & Short, 2018: 151; by original designation.
Diagnosis.
Very small beetles, body length 1.15–1.45 mm. Body shape oval in dorsal view; slightly to moderately, and evenly convex in lateral view (Fig. 41). Coloration uniformly brown, to variable along the body; ground punctation shallow to moderately marked (Fig. 41). Shape of head trapezoid and relatively wide. Eyes moderate in size, slightly emarginated anteriorly, not projected from outline of head. Clypeus trapezoid, with anterior margin broadly emarginate. Labrum fully exposed. Mentum with lateral oblique ridges. Antennae with eight antennomeres; cupule slightly asymmetric, with rounded outline. Maxillary palps slender, moderately long nearly 0.7 × the width of head; inner margin of maxillary palpomere 2 nearly straight, outer margin curved along apical half (e.g., Fig. 41C, F). Each elytron with ground punctures usually only shallowly marked, seemingly forming longitudinal rows, with irregularly distributed systematic punctures bearing rather long setae, denser along lateral and posterior regions; elytra without sutural striae. Prosternum flat, at most only weakly convex. Posterior elevation of mesoventrite, usually projected as low and short longitudinal carina between mesocoxae; anapleural sutures only weakly curved, separated at anterior margin by distance nearly 0.9 × width of anterior margin of mesepisternum. Metaventrite with posterolateral and mesal glabrous patches (e.g., Fig. 41C, F). Protibiae with spines of anterior row hair-like, semi erect, relatively long, thick and sparse. Metafemora mostly densely covered by hydrofuge pubescence (e.g., Fig. 41C, F). All tarsomeres with long and thick spines on ventral faces of tarsomeres 2–4; metatarsomeres 2–4 gradually decreasing in size, metatarsomere 5 as long as 3 and 4 combined, 2 slightly shorter. Fifth abdominal ventrite apically emarginate, with fringe of stout setae. Aedeagus trilobed (Fig. 40E–H), nearly parallel sided, with basal piece 0.3–0.6 × length of parameres; median lobe with well-developed lateral basal apodemes, wider at base than base of each paramere, usually narrower at apex than preapical width of parameres; apex of median lobe rounded; parameres from slightly shorter to longer than median lobe, and only narrowing at apex; gonopore situated beyond midpoint of median lobe.
Differential diagnosis.
The minute size of Nanosaphes make them smaller than any other Acidocerinae in the New World, and about equal in size to the smallest species of Agraphydrus in the Old World. They are among the smallest water scavenger beetles worldwide. The lack of elytral serial or sutural striae and the antennae with eight antennomeres also separate Nanosaphes from all other Neotropical Acidocerinae genera except the co-occurring Globulosis. Nanosaphes can be easily separated from Globulosis by its smaller size and narrower, more parallel sided body form (broader and almost rotund in Globulosis, Fig. 32).
Distribution.
Neotropical: Brazil (Pará), Guyana, Suriname; Fig. 5.
Natural history.
Species are associated with stream margins, particularly where there are marginal banks of sand and roots.
Larvae.
Immature stages are not known for Nanosaphes.
Taxonomic history.
Nanosaphes was only recently described.
Remarks.
There are four known species of Nanosaphes, which can be differentiated from each other by external morphological features (e.g., elytral punctation, coloration, shape of the posterior elevation of the mesoventrite), which is somewhat unusual by acidocerine standards. We have seen additional material of Nanosaphes from other regions within the Guiana Shield.
Species examined.
Holotypes and paratypes of all known species were available for this study.
Selected references.
Girón and Short 2018: original description of the genus and all its known species; Short et al. 2021: phylogenetic placement.
Genus. Novochares
Girón & Short gen. nov.
http://zoobank.org/9E46D713-DA7C-46B6-B407-E99C490CFD32
Figure 6.
Known distribution of genera of Acidocerinae: Novochares, Peltochares, Primocerus, Quadriops, Radicitus, Sindolus, Tobochares, and Troglochares.
Helochares “Clade D”, Short et al. (2021)
Gender.
Masculine.
Type species.
Helochares tectiformis Fernández, 1982b; by present designation.
Etymology.
From the Latin word novus, meaning new, in reference to the genus being restricted to the New World, combined with the ending chares, expressing affinity with Helochares. Masculine.
Diagnosis.
Medium sized beetles, body length 4.5–9.0 mm. Body shape oval in dorsal view; slightly to moderately convex in lateral view, with dorsal outline nearly flat along anterior half of elytra, or somewhat evenly curved (Fig. 42). Coloration usually uniformly dark brown, sometimes orange or pale brown; ground punctation shallow to moderately marked (Fig. 42). Shape of head trapezoid. Eyes relatively large, not emarginated anteriorly, usually projected from outline of head. Clypeus trapezoid, with anterior margin broadly and roundly emarginate. Labrum fully exposed. Mentum with lateral longitudinal crenulations, lateral oblique ridges, and transverse crenulations along antero-medial area (Fig. 42C, F). Antennae with nine antennomeres; cupule strongly asymmetric, with rounded outline; antennomere 9 slightly to 2 × longer than antennomere 7. Maxillary palps slender, moderately long, 1.1–1.5 × the width of head; inner margin of maxillary palpomere 2 weakly and evenly curved to nearly straight, outer margin evenly curved or curved along apical half; maxillary palpomere 3 slightly longer than 4 (Fig. 42C, F). Prosternum flat to weakly convex. Elytra without sutural striae, with ground punctures usually shallowly marked; usually at least one row of systematic punctures visible along midline of each elytron; serial punctures sometimes visible along posterior half of elytra (e.g., Fig. 42D). Posterior elevation of mesoventrite, usually simply bulging, sometimes bulge impressed posteriorly, sometimes bulge extends anteriorly as low, shiny, and glabrous longitudinal ridge; anapleural sutures concave, separated at anterior margin by distance 0.6–0.9 × the width of anterior margin of mesepisternum. Metaventrite with medial glabrous patch, sometimes very narrow and extending along entire length of metaventrite (e.g., Fig. 42C, F). Protibiae with spines of anterior row extremely reduced to tiny appressed denticles. Metafemora with tibial grooves well developed; hydrofuge pubescence covering basal 6/7 of anterior surface. Tarsomeres 1–4 with long, thick, and rather dense setae on ventral face, sometimes with only rows of short spines on metatarsomeres 2–4; metatarsomere 2 as long or slightly longer than 5 and as 3 and 4 combined. Fifth abdominal ventrite apically emarginate, with fringe of stout setae. Aedeagus divided (Fig. 43); parameres separated from each other for most of their lengths; median lobe divided in dorsal and ventral plates; dorsal plate usually strongly sclerotized and elongated, often bifurcated or otherwise shaped along apical region; ventral plate sometimes reduced, usually simple and of variable length; basal piece 0.3 × or less than length of parameres, usually clearly noticeable; gonopore usually clearly visible.
Differential diagnosis.
Novochares includes medium sized, pale to dark brown species that are somewhat dorsoventrally compressed and highly polished (smooth, and often shiny) to the naked eye. In the New World the most similar genus is Aulonochares, from which it can be differentiated by the shape of the head [trapezoid in Novochares, subquadrate in Aulonochares (Fig. 11J)], and the sculpture of the mentum (variously striate in Novochares, punctate in Aulonochares). Some members of the New World Helochares may resemble Novochares in their external features, but the aedeagal form is completely different (tubular in Helochares, Figs 16E, F, 37; divided in Novochares, Figs 16G, H, 43).
From the rest of acidocerines, Novochares externally is strikingly similar to the dark and highly polished members of the Old World genus Peltochares (compare Fig. 1B vs 1G), from which Novochares can be distinguished by the shape of the posterior elevation of the mesoventrite (simply and broadly bulging, often with additional anterior low longitudinal ridge in Novochares, longitudinally elevated in Peltochares), in addition to characteristics of the male genitalia (divided aedeagus in Novochares (Figs 16G, H, 43), spiked aedeagus in Peltochares (Figs 16C, D, 45); see also explanation under the aedeagus section of Morphological variation in Acidocerinae and its taxonomic importance).
To differentiate Novochares from dark brown, relatively flattened, highly polished, and 4–5 mm long species of Helochares, the most reliable feature for identification would be the male genitalia: Novochares always exhibit divided aedeagi (Figs 16G, H, 43; parameres separated from each other for most of their lengths, dorsal plate of the median lobe usually strongly sclerotized, elongated, often bifurcated or otherwise shaped along its apical region), whereas in Helochares the aedeagi are always tubular (Figs 16E, F, 37A–H; parameres fused to each other for most of their lengths, median lobe with very long basal apodemes; see also explanation under the aedeagus section of Morphological variation in Acidocerinae and its taxonomic importance).
Distribution.
Nearctic: U.S.A. (Florida; thought to be introduced). Neotropical: Argentina, Belize, Bolivia, Brazil (Amazonas, Espírito Santo, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Pernambuco, Piauí, Rio de Janeiro, São Paulo), Colombia, Costa Rica, Cuba, Ecuador, French Guiana, Guatemala, Lesser Antilles (Grenada, Guadeloupe, St. Vincent), Mexico, Panama, Paraguay, Suriname, Uruguay, Venezuela; Fig. 6.
Natural history.
Species of Novochares occur in a broad range of both lentic and lotic habitats; we are not aware of any seepage specialists in this lineage. Some species such as the widespread N. abbreviatus (Fabricius) are found in lentic habitats including marshes, swamps, and pond margins (Short 2005). Forest pools with abundant leaf litter detritus are often very productive for a variety of species. Novochares atlanticus (Clarkson and Ferreira-Jr.) was collected at temporary ponds with leaf litter and aquatic vegetation, either covered and shaded in the border of the forest (Clarkson and Ferreira-Jr. 2014), or in open areas. Some species come to lights. Fernández (1983), in describing the immature stages of N. pallipes (Brullé), indicated that the species was found on coastal zones, associated with swamp plants (Spirodela intermedia; Araceae).
Larvae.
The immature stages are only known for Novochares pallipes (Brullé) (described as Helochares (s. str.) pallipes Brullé in Fernández 1983: 444); egg sac, first, second and third instar larvae, and pupa are described and illustrated. From each egg sac, 80–103 larvae emerged (Fernández 1983).
Taxonomic history.
Species of Novochares have been described since as early as 1801, but it was only with the investigations by Fernández in the 1980’s (Fernández 1981, 1982a, 1982b, 1983, 1989) that the group was studied in a comparative taxonomic framework beyond the description of single species.
Remarks.
There are 15 species of Novochares described to date. Species of Novochares tend to have moderate to shallow punctation and serial punctures are usually absent. There is a group of species with serial punctures visible along the posterior half to third of the elytra (Clade D1 in Short et al. 2021).
Species examined.
Novochares abbreviatus (Fabricius), N. carmona (Short), N. chaquensis (Fernández), N. cochlearis (Fernández), N. coya (Fernández), N. guadelupensis (d’Orchymont), N. pallipes (Brullé), N. sallaei (Sharp), N. tectiformis (Fernández). Paratypes of N. carmona were examined for this study.
Selected references.
Fernández 1982a: notes on the taxonomic status of some of the previously described species; Fernández 1982b: description of four new species; Fernández 1983: description of immature stages for Novochares pallipes (Brullé); Fernández 1989: one new species and identification key; Short 2005: one new species with review of Central American species; Clarkson and Ferreira-Jr 2014: one new species and new records from southern Brazil; Short et al. 2021: phylogenetic placement.
Genus. Peltochares
Régimbart, 1907
Figs 1B, C , 4 , 11K , 44 , 45
Peltochares Régimbart, 1907: 49.
Type species. Peltochares conspicuus Régimbart, 1907: 49; by monotypy.
Stagnicola Montrouzier, 1860: 246 [preoccupied name by Stagnicola Gray, 1840 (Mollusca)]
Type species: Stagnicola foveicollis Montrouzier, 1860: 246; by monotypy; Bedel 1880: CXLVIII [synonymy].
Neohydrobius Blackburn, 1898: 221.
Type species: Philhydrus burrundiensis Blackburn, 1890: 447; by monotypy; d’Orchymont 1919b: 228 [synonymy].
Helochares “Clade C” in Short et al. 2021.
Gender.
Masculine.
Type species.
Peltochares conspicuus Régimbart, 1907: 49; by monotypy.
Diagnosis.
Body length 6–14 mm. Body shape oval in dorsal view, weakly to moderately convex in lateral view (Fig. 44). Dorsal surfaces even and smooth, either uniformly covered by short setae (Fig. 44A), or with scarce long setae along particular areas of surface (associated with systematic punctures; Fig. 44D), dark brown in coloration, usually uniform; ground punctation fine and shallow to moderate; ventral surfaces densely covered by fine golden setae (Fig. 44C, F). Head subquadrate (Fig. 11K). Eyes not emarginate, moderate in size, subquadrate, separated by 4.5–5.5 × width of eye, strongly projected from outline of head. Clypeus with anterior margin broadly emarginate, either roundly or acutely, sometimes further medially notched; membranous preclypeal area visible when clypeus strongly emarginated. Labrum fully exposed, often medially convex. Antennae with nine antennomeres, with moderately asymmetric and round cupule; antennomere 9 slightly to 2 × longer than antennomere 7. Maxillary palps slender, 1.3–1.8 × longer than maximum width of head, with palpomere 4 nearly 0.8 × as long as palpomere 3; maxillary palpomere 2 with inner margin slightly and evenly curved, and outer margin curved along apical half (Fig. 44C, F). Mentum slightly depressed mesally, surface laterally punctate, mesally and anteriorly striate, with anteromedial region depressed (Fig. 44C, F). Submentum punctate to crenulate. Pronotum evenly convex, usually with systematic punctures forming distinct anterolateral semicircles. Elytra without sutural striae, with margins usually only slightly flared (explanate in P. conspicuus; Fig. 44A); serial punctures usually absent (visible along entire length of elytra in P. conspicuus; Fig. 44A); ground punctation usually shallow (moderate to strongly marked in P. foveicollis). Surface of prosternum flat to broadly convex, with anterior margin roundly projected anteriorly (Fig. 44C, F). Posterior elevation of mesoventrite usually with longitudinal or somewhat longitudinal elevation, sometimes forming acute posterior point; apical region of elevation usually with long fine setae; anapleural sutures forming obtuse angle, nearly parallel along anterior section, separated anteriorly by distance 0.3–0.7 × anterior margin of mesepisternum. Metaventrite densely covered by hydrofuge pubescence, except for posterolateral patches (Fig. 44C, F). Protibiae with anterior row of spines reduced to extremely reduced (Fig. 44C); apical spurs of protibiae stout, ranging from very large (larger spur considerably larger and thicker than tarsal claws, e.g., P. foveicollis), or very short (barely reaching apex of protarsomere 1, e.g., P. conspicuus); pro- and mesotarsal claws are sexually dimorphic in some species (e.g., P. foveicollis). Metafemora with tibial grooves sharply marked; metafemora with hydrofuge pubescence covering at least basal 3/4 of anterior surface (Fig. 44C, F). Metatarsomeres 5 and 2 similar in length or 2 slightly longer, metatarsomere 2 slightly longer than metatarsomeres 3 and 4 combined; all tarsomeres with ventral surface rather densely covered by long spiniform setae on ventral surface (sparser on tarsomere 5). Abdomen with five pubescent ventrites. Fifth abdominal ventrite with apex emarginate, fringed by stout setae. Aedeagus spiked (Figs 16C, D, 45); main component of median lobe strongly sclerotized, slender, and apically acute, usually accompanied by additional shorter slender sclerotizations; apical region of parameres usually partly heavily sclerotized and partly membranous, often bifurcated; basal piece strongly reduced; gonopore usually not clearly visible.
Differential diagnosis.
The type species of Peltochares is easily recognized by its external morphology alone: laterally explanate pronotum and elytra, well defined serial punctures along elytra (Fig. 44A), which somewhat resembles Helobata (Fig. 33A), from which P. conspicuus can be distinguished by the exposed labrum of Peltochares (Fig. 11K; concealed labrum in Helobata, Fig. 11L). The most common forms of Peltochares more closely resemble Novochares and some Helochares, because of their darkly colored and highly polished dorsal habitus. Besides being distributed (although widespread) in the Old World, Peltochares species can be distinguished from the New World Novochares by the shape of the posterior elevation of the mesoventrite (longitudinally elevated in Peltochares, simply and broadly bulging, often with additional anterior low longitudinal ridge in Novochares), in addition to characteristics of the male genitalia (spiked aedeagus in Peltochares, Figs 16C, D, 45; divided aedeagus in Novochares, Figs 16G, H, 43; see also explanation under the aedeagus section of Morphological variation in Acidocerinae and its taxonomic importance). From dark brown, highly polished, and relatively large species of Helochares, Peltochares can be distinguished by their slender maxillary palps, that are 1.3–1.8 × longer than the width of the head (Fig. 44C, F), as opposed to shorter (0.6–1.2 × the width of the head) and relatively stout maxillary palps in Helochares (Figs 35C, F, 36C, F), in addition to the aedeagal form (spiked in Peltochares, Figs 16C, D, 45; tubular in Helochares, Figs 16E, F, 37A–H; see also explanation under the aedeagus section of Morphological variation in Acidocerinae and its taxonomic importance).
Distribution.
Afrotropical: Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Democratic Republic of the Congo, Ethiopia, Gabon, Gambia, Ghana, Guinea, Ivory Coast, Kenya, Liberia, Madagascar, Malawi, Mozambique, Namibia, Niger, Nigeria, Republic of the Congo, Rwanda, Senegal, Sierra Leone, Somalia, Republic of South Africa, South Sudan, Tanzania, Togo, Uganda, Western Sahara, Zambia, Zimbabwe. Australasian: Australia (Australian Capital Territory, New South Wales, Northern Territory, Queensland, Western Australia), Indonesia (Papua), New Caledonia, Papua New Guinea. Indo-Malayan: Bangladesh, Cambodia, China (Guangdong, Guangxi, Guizhou, Hong Kong, Jiangxi, Macao), Indonesia (Borneo, Sumatra), Laos, Malaysia, Nepal, Sri Lanka, Thailand, Vietnam. Palearctic: Canary Islands, Egypt, Israel, Japan (Nansei Islands); Fig. 6.
Natural history.
Even though species currently placed in Peltochares have been treated in faunistic and taxonomic studies (e.g., Watts 1995, Hebauer 2001b), little is known about their ecology. Jia and Tang (2018) recently reported that P. atropiceus (Régimbart) was living in natural ponds with leaf litter or water grass, sometimes collected on wet ground with plenty of grass; it can be collected at light in May and June in South China and has never been collected from the edges of rivers and streams. The female carries the egg case under the abdominal ventrites (Jia and Tang 2018).
Larvae.
Larval stages of Peltochares conspicuus Régimbart, were described by Bertrand (1962) from larvae collected along with adults on the surface of rocks in Madagascar. Fikáček (2003) provides a diagnosis of the larvae described by Bertrand (1962), but questions their identification, given that P. conspicuus has never been recorded from Madagascar. It seems most probable the description is of another species now placed Peltochares, as P. longipalpis has been recorded from Madagascar, but only future rearing or DNA sequencing of putative larvae will confirm this.
Lectotype designation.
We examined Régimbart’s syntype series for Peltochares conspicuous, consisting of nine specimens, that are deposited in the Muséum national d’Histoire naturelle, Paris, France. We determined all nine to be conspecific. It includes two specimens labeled ‘Cape Lopez’, one of them labeled ‘Peltochares conspicuus Rég.’; five specimens labeled Rembo N’Comi Fernand Vaz, one of them missing prothorax and head, and another one is missing the left elytron; one specimen labeled Rembo N’Comi Fernand Vaz (Gabon), missing prothorax and head; and one specimen labeled ‘Gabon’. All specimens, except the last one, are pinned; the specimen labeled ‘Gabon’ is glued by its abdomen in a small pinned card. To stabilize the identity of the type species of Peltochares, we here designate as the Lectotype the specimen that bears the ‘Peltochares conspicuus Rég.’ label, which even though is not completely clean, has all its appendages complete. The following red label has been attached: “LECTOTYPE/ Peltochares/ conspicuus/ Régimbart/ des. Girón and Short”. The remaining eight specimens are designated as paralectotypes. One of the specimens missing its prothorax and head was dissected to reveal the male genitalia, which is illustrated in Fig. 45A.
Taxonomic history.
The circumscription of Peltochares as used here is changed from its original meaning. Peltochares was originally described as a monotypic genus by Régimbart in 1907, from specimens collected in Gabon of a very unusual species (P. conspicuus) which was a rather large, circular beetle with extremely explanate margins of the pronotum and elytra (Fig. 44A–C). A morphologically similar species was much later described from Indonesia and Malaysia, although that species was placed in the nominal subgenus of Helochares (Helochares (s. str.) discus Hebauer, Hendrich & Balke). In their molecular phylogeny, Short et al. (2021) recovered H. discus in a clade (Helochares Clade C) with some other larger, darkly colored (but not explanate) Old World species that were also placed in Helochares (s. str.), which showed that this clade was not closely related to the “true” Helochares but indeed represented an independent lineage. Examination of the male genitalia of one of the syntypes of P. conspicuus (the type of Pelotochares) and members of “Helochares Clade C” in Short et al. (2021) revealed that they share a quite unique and similar configuration of the male genitalia (spiked genitalia, Figs 16C, D, 45; see also the aedeagus section of Morphological variation in Acidocerinae and its taxonomic importance above), even though they do not share the same extremely explanate body form.
Although the monophyly and morphological circumscription of “Helochares Clade C” is strongly supported, the proper genus name to assign to his lineage is not straightforward, as there are several generic names that had been long synonymized with Helochares that potentially come into play with the new circumscription of the genus. The genus Stagnicola Montrouzier, 1860 was based on what is now Helochares (s. str.) foevicollis, a species which is a definitive member of Helochares Clade C. However, Stagnicola is a preoccupied name and thus unavailable. More complicated is Neohydrobius Blackburn, 1898 and its type species, Philhydrus burrundiensis Blackburn, which is now considered a junior synonym of H. (s. str.) foevicollis. Neohydrobius, although eight years older than Peltochares, had a very short shelf-life, as it was synonymized with Helochares just 21 years after it was proposed by d’Orchymont (1919b) and therefore has not been used in more than a century. Meanwhile, Peltochares has been in continuous usage since 1907 and therefore we believe it is the best and most stable name to apply to this clade.
We had hoped to unilaterally maintain prevailing usage of Peltochares over Neohydrobius by invoking ICZN Article 23.9.1. However, not all the required criteria to apply this article appear to be met in this case. Although Neohydrobius appears to meet the first criterion (the senior synonym not being used as valid since 1899), we were only able to identify 19 works (by more than 10 authors) in the immediately preceding 50 years, but 25 works are required. Therefore, we will formally appeal to the commission for a ruling to maintain Peltochares over Neohydrobius. Accordingly, ICZN Article 82.1 states that prevailing usage is to be maintained until the ruling of the Commission is published and therefore, we use Peltochares in this work.
Remarks.
The group of species previously assigned to Helochares (s. str.), hereby transferred to Peltochares, was first recognized by Hebauer (2001b) as a discrete unit in morphological terms within Helochares. There are currently eight described species of Peltochares, including the following seven species that are transferred from Helochares for the first time: P. atropiceus (Régimbart) comb. nov., P. ciniensis (Hebauer, Hendrich & Balke) comb. nov., P. discus (Hebauer, Hendrich & Balke) comb. nov., P. foveicollis (Montrouzier) comb. nov., P. longipalpis (Murray) comb. nov., P. papuensis (Hebauer) comb. nov., and P. taprobanicus (Sharp) comb. nov.
Species examined.
Peltochares atropiceus, P. ciniensis (including a paratype), P. conspicuus (including syntypes), P. foveicollis, P. longipalpis, and P. taprobanicus.
Selected references.
Régimbart 1907: 49: original description of the genus; Hebauer 2001b: taxonomic treatment of P. taprobanicus (as Helochares taprobanicus) and allied species; Jia and Tang 2018: faunistic review of Chinese species including a redescription and some biological notes on P. atropiceus; Short et al. 2021: phylogenetic placement.
Genus. Primocerus
Girón & Short, 2019
Primocerus Girón & Short, 2019: 133.
Gender.
Masculine.
Type species.
Primocerus neutrum Girón & Short, 2019: 147; by original designation.
Diagnosis.
Small to medium sized beetles, body length 2.4–4.9 mm. Body shape elongated oval in dorsal view; moderate to strongly convex in lateral view; dorsal outline uniformly convex or nearly straight and anteriorly inclined along anterior half (Fig. 46). Color brown, dark brown, reddish brown, or rather orange, usually uniform along body regions, but sometimes with slightly paler margins, pronotum or ventral surfaces and appendages; ground punctation shallow to moderately marked (Fig. 46). Shape of head trapezoid. Eyes small to moderate, seldom very small, not emarginated anteriorly, usually projected from outline of head. Clypeus trapezoid, with anterior margin broadly and roundly emarginate. Labrum fully exposed. Mentum rather flat and smooth, sometimes with lateral oblique ridges, and few crenulations; median anterior depression sometimes marked by a transverse carina (Fig. 46C, F, I). Antennae with eight antennomeres; cupule slightly asymmetric, with rounded outline. Maxillary palps moderately stout, shorter to nearly as long as width of head; inner margin of maxillary palpomere 2 nearly straight, outer margin curved along apical 2/3; maxillary palpomeres 3 and 4 similar in length (Fig. 46C, F, I). Prosternum flat to mesally only slightly produced (Fig. 46C, F, I). Elytra with sutural striae; elytral punctures from shallow to sharply marked; ground punctures rather uniformly distributed; some species with serial punctures; outer margins of elytra slightly flared (Fig. 46A, D, G). Posterior elevation of mesoventrite usually with curved transverse ridge, rather sharp and low, or bearing sharp, pyramidal (triangular) projection; anapleural sutures concave to forming obtuse angle, separated at anterior margin by distance 0.3–0.4 × width of anterior margin of mesepisternum (Fig. 46C, F, I). Metaventrite with posteromesal glabrous patch nearly as wide as long (Fig. 46C, F, I). Protibiae with spines of anterior row as thick, long semi-erect setae; apical spurs of protibiae moderately stout, reaching midlength of protarsomere 3. Metafemora with tibial grooves moderately developed; hydrofuge pubescence coverage ranging from sparse (nearly glabrous metafemora) to dense along basal 3/4 (Fig. 46C, F, I). Tarsomeres 1–4 with long spiniform setae on ventral face; metatarsomere 2 nearly as long as 5 and as 3 and 4 combined. Fifth abdominal ventrite apically rounded, truncate, or slightly emarginate, usually with fringe of stout setae. Aedeagus trilobed (Fig. 47); basal piece as long or longer than parameres; median lobe triangular, nearly as wide at base as basal width of each paramere, with apical projection; gonopore absent.
Differential diagnosis.
At first sight, the smoother members of Primocerus (e.g., Fig. 46A–C) can be mistaken for Chasmogenus (Fig. 24), given that both genera exhibit sutural striae. The presence of a transverse curved ridge (sometimes very low) on the posterior elevation of the mesoventrite distinguishes Primocerus from Chasmogenus, in which the mesoventrite is either flat, broadly elevated or with a longitudinal elevation; maxillary palps of most Chasmogenus species are nearly 1.5 × longer than the maximum width of the head, whereas in Primocerus the maxillary palps are shorter, nearly as long as the width of the head.
Punctate members of Primocerus (e.g., Fig. 46D–F) may resemble some species of Tobochares (Kohlenberg and Short 2017, Girón and Short 2021a); striate Primocerus (e.g., Fig. 46G–I) may resemble Radicitus (Fig. 50; Short and García 2014). In those cases, Primocerus can be easily recognized by the presence of sutural striae. Some species of Primocerus may also superficially resemble certain New World cylomine genera, such as Andotypus Spangler (Fikáček et al. 2014), from which it may be distinguished by the fully exposed labrum of Primocerus.
Distribution.
Neotropical: Brazil (Pará), Guyana, Suriname, and Venezuela; Fig. 6. We have seen additional specimens that slightly expand the range of the genus, but all still fall within the Guiana Shield region of South America.
Natural history.
The habitats occupied by members of Primocerus range from forested pools to seepages. One specimen was collected with a flight intercept trap. Specimens of Primocerus are relatively rare, given that so far have only been found in low numbers of specimens per collecting event (Girón and Short 2019).
Larvae.
Immature stages are not known for Primocerus.
Taxonomic history.
Primocerus was only recently described.
Remarks.
With only nine known species in the genus, Primocerus is one of the most variable genera of New World acidocerines in terms of their external morphology. Additional recent study and collections have revealed that the species described as P. neutrum likely represents a species complex (Short pers. obs.).
Species examined.
Holotypes and paratypes of all known species were examined for this study.
Selected references.
Girón and Short 2019: original description of the genus and all its known species; Short et al. 2021: phylogenetic placement.
Genus. Quadriops
Hansen, 1999
Figs 1P , 2 , 6 , 11C , 48 , 49A–D
Quadriops Hansen, 1999a: 131.
Gender.
Masculine.
Type species.
Quadriops depressus Hansen, 1999a: 136; by original designation.
Diagnosis.
Small to very small beetles, body length 1.6–2.6 mm. Body shape oval in dorsal view; moderate to strongly convex in lateral view, dorsal outline evenly convex or nearly straight along median region (Fig. 48). Color orange brown to dark brown, uniform along body regions; ground punctation shallow to moderately marked (Fig. 48). Shape of head somewhat rectangular. Frons lateral and posteriorly expanded, forming canthus completely dividing eyes in dorsal and ventral portions (Fig. 11C). Eyes very small in dorsal view. Clypeus laterally expanded in front and around outer margin of eyes; anterior margin of clypeus straight (Fig. 11C). Labrum partly exposed. Mentum rather smooth and medially depressed; median anterior depression marked by a transverse carina (Fig. 48C, F). Antennae with nine antennomeres, cupule slightly asymmetric with rounded outline. Maxillary palps rather short and stout, nearly half as long as width of head; maxillary palpomere 4 slightly longer than palpomere 3; inner margin of maxillary palpomere 2 straight to convex, outer margin strongly curved along apical 2/3. Elytra without sutural striae, with punctures either irregularly distributed or forming well defined longitudinal rows; elytra narrowly explanate anteriorly, explanation gradually broader towards apex (Fig. 48). Surface of prosternum flat. Posterior elevation of mesoventrite, usually with well-defined transverse ridge, seldom with acute tooth; anapleural sutures concave, separated at anterior margin by distance nearly 0.7 × width of anterior margin of mesepisternum. Metaventrite usually uniformly densely pubescent, sometimes with reduced posteromedian glabrous patch. Protibiae with spines of anterior row hair-like, semi erect, relatively long, and thick; apical spurs of protibia moderately stout, reaching apex of protarsomere 3. All tarsomeres with thick hair-like spines on ventral face of tarsomeres 2–4; metatarsomeres 1–4 similar in length, 5 nearly as long as 3 and 4 combined. Metafemora with tibial grooves moderately developed; anterior surface of metafemora mostly glabrous, with few very scattered small setae (Fig. 48C, F). Fifth abdominal ventrite apically rounded and without fringe of stout setae. Aedeagus trilobed (Fig. 49A–D), with basal piece about half length of parameres; median lobe wider than base of each paramere, with narrow, triangular, longitudinal sclerite, usually extending along apical third; parameres as long as, to longer than median lobe, and nearly half as wide; gonopore situated preapically; basal piece with lateral margins straight to sinuate, apically slightly diverging.
Differential diagnosis.
Quadriops is the only known acidocerine with fully divided eyes. Species with uniformly distributed punctures along the elytra may resemble Globulosis, but the moderate punctation of Quadriops is very evident (punctation only shallowly marked in Globulosis; Fig. 32). Some species of Tobochares have nearly divided eyes, and lack impressed striae along the elytra (emarginatus species group, Girón and Short 2021a), resembling species of Quadriops with uniformly distributed punctures along the elytra, but they differ in the shape of the posterior elevation of the mesoventrite (sharply elevated as a tooth or a blunt transverse carina in Quadriops, medially bulging in T. canthus Kohlenberg & Short).
Distribution.
Neotropical: Brazil (Amazonas), Costa Rica, Ecuador, French Guiana, Guyana, Panama, Peru, Suriname, Venezuela; Fig. 6.
Natural history.
Specimens have been caught using flight intercept traps, many long series have been collected on decaying Clusia fruits, which can be somewhat used as bait (Fig. 10). Additional specimens have been collected in rotten logs, sap flows on freshly cut trees, and in the refuse piles of leafcutter ants (Girón and Short 2017).
Larvae.
The immature stages of Quadriops remain unknown.
Taxonomic history.
Hansen (1999a) described the genus with five species, differentiated mostly by the presence and degree of impression of reticulation on the head and clypeus. When he originally described it, Hansen (1999a) was unsure of the taxonomic affinity of the genus, as the morphology of the lineage was somewhat unusual. He placed it in the Acidocerina (now Acidocerinae) almost by default as it shared no characters in common with other lineages, but ultimately, he was correct as this placement as verified by Short et al. (2021). García (2000b) described an additional species from Venezuela. The genus was revised by Girón and Short (2017): two species were synonymized with Quadriops depressus Hansen; two new species were described.
Remarks.
Quadriops is the only fully terrestrial genus of Acidocerinae. There are six described species within the genus.
Species examined.
Quadriops acroreius Girón & Short (holotype and paratype), Q. clusia Girón & Short (holotype, paratypes and additional specimens), Q. dentatus Hansen (holotype and additional specimens), Q. depressus Hansen (holotype and additional specimens), Q. reticulatus Hansen (holotype and additional specimens), Q. similaris Hansen (holotype and additional specimens).
Selected references.
Hansen 1999a: original description; García 2000b: description of one additional species from Venezuela; Girón and Short 2017: generic revision including two synonymies and two new species; Short et al. 2021: phylogenetic placement.
Genus. Radicitus
Short & García, 2014
Radicitus Short & García, 2014: 252.
Gender.
Masculine.
Type species.
Radicitus ayacucho Short & García, 2014: 252; by original designation.
Diagnosis.
Medium sized beetles, body length 4.5–6.2 mm. Body shape oval in dorsal view; moderate to strongly convex in lateral view; dorsal outline nearly straight and anteriorly inclined along anterior half (Fig. 50). Color dark brown, usually uniform along body regions, sometimes margins of pronotum and elytra slightly paler; ground punctation fine, moderately marked (Fig. 50A, D). Shape of head trapezoid and rather wide. Eyes moderate in size, not emarginated anteriorly, slightly projected from outline of head. Clypeus trapezoid, with anterior margin broadly, roundly, and weakly emarginate. Labrum fully exposed. Mentum medially rather broadly depressed, laterally longitudinally elevated; median anterior depression marked by transverse nearly straight carina (Fig. 50C, F). Antennae with nine antennomeres; cupule slightly asymmetric, with rounded outline. Maxillary palps short and stout, nearly as long as half width of head (e.g., Fig. 50C); inner margin of maxillary palpomere 2 nearly straight, outer margin strongly curved along apical 2/3; maxillary palpomere 4 slightly shorter than 3. Prosternum flat, only slightly carinate along midline of anterior projection. Elytra without sutural striae; elytral punctures shallow to moderately marked; ground punctures rather uniformly distributed; some species with serial punctures clearly visible along posterior third of elytra; outer margins of elytra slightly flared (Fig. 50A, D). Posterior elevation of mesoventrite with median longitudinal carina elevated and forming posteriorly pointing process; anapleural sutures strongly concave, separated at anterior margin by distance nearly half width of anterior margin of mesepisternum. Metaventrite sometimes with posteromesal glabrous patch. Protibiae with anterior row of spines completely reduced; apical spurs of protibiae stout, reaching apex of protarsomere 3. Metafemora with tibial grooves very sharply marked and covered by hydrofuge pubescence; hydrofuge pubescence restricted to dorsal half on basal three-quarters of anterior surface of metafemora (Fig. 50C, F). Tarsomeres 1–4 with long spiniform setae on ventral face; metatarsomere 2 nearly as long as 5 and as 3 and 4 combined. Fifth abdominal ventrite evenly rounded, without apical emargination or fringe of stout setae. Aedeagus either trilobed (Fig. 49I–L) or divided (Fig. 49G, H), with basal piece short and rather simple parameres separated from each other for most of their lengths; gonopore well developed.
Differential diagnosis.
Radicitus may resemble some punctate Novochares but can be recognized by the short and stout maxillary palps, along with metafemora only partly covered by pubescence (long and slender maxillary palps with metafemora mostly covered by pubescence in Novochares).
Distribution.
Neotropical: Guyana, Suriname, Venezuela; Fig. 6.
Natural history.
Species of Radicitus have been found on a variety of habitats associated with streams and seeps on rock outcrops. Some have been collected by submerging root mats found along streams, and in the roots of vegetation growing on seepage areas on granite outcrops (Short and García 2014).
Larvae.
The immature stages of Radicitus remain unknown.
Taxonomic history.
Radicitus was only recently described.
Remarks.
There are three known species of Radicitus, all currently endemic to the Guiana Shield.
Selected references.
Short and García 2014: original description of the genus and all known species; Short et al. 2021: phylogenetic placement.
Genus. Sindolus
Sharp, 1882
Sindolus Sharp, 1882: 72.
Helochares (Sindolus) Sharp; d’Orchymont 1919c: 148; Knisch 1924: 199; Hansen 1999b: 158.
Gender.
Masculine.
Type species.
Sindolus optatus 1882: 72; by subsequent designation (Hansen 1991: 292).
Diagnosis.
Small to medium sized beetles, body length 2.5–5.0 mm. Body shape oval in dorsal view, moderately to strongly convex in lateral view (Fig. 51); dorsal outline usually evenly curved. Dorsal surfaces even and smooth, yellowish, orange brown to brown and rather uniform in coloration; ground punctation fine and extremely shallow (Fig. 51A). Shape of head trapezoid. Eyes not emarginate, moderate to relatively large in size, subquadrate, separated by nearly 5 × width of eye, only slightly projected from outline of head. Clypeus trapezoid, with anterior margin broadly and slightly emarginate. Labrum fully exposed, convex, and anteriorly emarginate. Mentum rather flat, with few shallow transverse crenulations on anterior region; median anterior depression relatively shallow, sometimes marked by transverse carina (Fig. 51C). Submentum smooth to very shallowly sculptured. Antennae with nine antennomeres, with strongly asymmetric and round cupule; antennomere 9 nearly 3 × longer than antennomere 8. Maxillary palps slender, 1.2–1.5 × longer than maximum width of head; inner margin of maxillary palpomere 2 usually evenly weakly curved, outer margin curved along apical third; palpomere 4 nearly 0.8 × as long as palpomere 3 (Fig. 51C). Pronotum evenly convex, usually with systematic punctures forming distinct anterolateral semicircles. Elytra without sutural striae, with margins only slightly flared; serial punctures absent; scarce systematic punctures, bearing moderately long setae (Fig. 51A). Surface of prosternum somewhat longitudinally elevated, sometimes with low and blunt longitudinal carina; anterior margin acutely to roundly projected anteriorly. Posterior elevation of mesoventrite with sharp and strongly elevated (laminar) longitudinal carina, with the ventral edge of the carina usually straight and parallel to the body (Fig. 51C); anapleural sutures concave, separated at anterior margin by distance nearly half width of anterior margin of mesepisternum. Metaventrite densely and uniformly covered by hydrofuge pubescence (Fig. 51C). Protibiae with anterior row of spines reduced (short appressed spines) to extremely reduced (tiny denticles); apical spurs of protibiae moderate, broad and reaching apex of protarsomere 2. Metafemora with tibial grooves sharply marked, and hydrofuge pubescence covering at least basal four fifths of anterior surface (Fig. 51C). Metatarsomere 2 slightly shorter or similar in length to metatarsomere 5, metatarsomere 2 similar in length to metatarsomeres 3 and 4 combined; ventral surface of all tarsomeres with long setiform setae on ventral surface (tarsomeres 1 and 2 with small stout spines). Abdomen with five pubescent ventrites. Fifth abdominal ventrite emarginate at apex; emargination fringed by stout setae. Aedeagus divided (Fig. 49E, F), somewhat pear-shaped, with basal piece nearly 0.3 × length of parameres; parameres slender, narrowing apically, with outer margins at least slightly sinuated, usually apically rounded; median lobe divided into dorsal and ventral plates; dorsal plate of median lobe medially bifurcate, with narrow, slender and apically rounded lobes; ventral lobe of median lobe varying in width and length, usually very lightly sclerotized; gonopore well-developed, usually positioned at midlength of aedeagus.
Differential diagnosis.
Sindolus is the only known genus of acidocerines that bears a sharp and strongly elevated (laminar) longitudinal carina.
Distribution.
Neotropical: Argentina, Bolivia, Brazil (Amazonas, Mato Grosso do Sul, Rio de Janeiro, Rio Grande do Sul), Colombia [in doubt; d’Orchymont, 1943d: 56], Costa Rica, French Guiana [in doubt; d’Orchymont, 1943d: 56], Guatemala, Lesser Antilles (Antigua), Mexico, Nicaragua, Paraguay, Uruguay; Fig. 6.
Natural history.
Sindolus mundus Sharp and S. optatus Sharp have been collected in stagnant waters at low elevations in dry areas; both species have been collected at mercury vapor lights in a drying lowland marsh where S. optatus Sharp was extremely abundant (Short 2005). Fernández and Kehr studied the annual life cycle (1994) and the spatial and temporal distribution (1995) of a population of S. femoratus in Argentina.
Larvae.
Immature stages are known for Sindolus talarum (Fernández) (as Helochares (Sindolus) talarum); egg case, first, second and third instar larvae and pupae were all described and illustrated by Fernández (1983). From each egg case between 25 and 40 larvae emerged; some larvae perforated and entered the aerenchyma of Spirodella intermedia (Araceae) and spent some time in there, apparently breathing the air stored in the plant tissues (Fernández 1983). In Argentina (Buenos Aires Province) first instar larvae start appearing in September, become abundant in October, and in November and the first two months of the summer all larval stages are abundant; at the end of March third instar larvae are the most common. Fernández (2004) also described the egg case and third instar larva of Sindolus femoratus (Brullé) (as Helochares (Sindolus) femoratus).
Taxonomic history.
Originally described as a genus by Sharp (1882) to accommodate two species from Central America; downgraded to subgenus of Helochares by d’Orchymont (1919c); Hansen (1991): designates type species.
Remarks.
There are eight species of Sindolus described. The genus is among the most easily recognized acidocerines in the New World.
Species examined.
Sindolus femoratus (Brullé), S. mundus Sharp, S. optatus Sharp. One of the available specimens of S. mundus had been previously compared wit the holotype by A. Shohrt.
Selected references.
Sharp 1882: original description of the genus and two species; Fernández 1981: description of two new species; Fernández 1983: description of immature stages for Sindolus talarum (Fernández); Fernández 2004: description of immature stages for Sindolus femoratus (Brullé); Short et al. 2021: phylogenetic placement.
Genus. Tobochares
Short & García, 2007
Figs 1N, O , 2 , 6 , 11A, B , 52 , 53 , 54 , 55
Figure 53.
Habitus of Tobochares spp. A–CT. communis: A dorsal habitus B lateral habitus C ventral habitus D–FT. fusus: D dorsal habitus E lateral habitus F ventral habitus. Scale bars: 1 mm.
Tobochares Short & García, 2007: 2.
Gender.
Masculine.
Type species.
Tobochares sulcatus Short & García, 2007: 4; by original designation.
Diagnosis.
Small beetles, total body length 1.5–2.6 mm. Body shape oval in dorsal view; moderately to strongly convex in lateral view (Fig. 52–54); dorsal outline usually evenly curved. Color yellowish brown, orange brown to dark brown, sometimes with paler spots on head, or paler margins of pronotum and elytra; ground punctation moderate to shallow. Shape of head somewhat oval. Eyes not emarginate (e.g., Fig. 11A) to strongly emarginate (e.g., Fig. 11B), moderate to small in size, somewhat oval, slightly to strongly projected from outline of head. Clypeus trapezoid, with anterior margin broadly emarginate; membranous preclypeal area often visible. Labrum fully exposed, convex, and anteriorly emarginate. Mentum rather smooth, often medially depressed, or anteriorly shallowly crenulated; median anterior depression marked by transverse carina (e.g., Fig. 53C). Submentum anteriorly smooth and shiny. Antennae with eight antennomeres, cupule slightly asymmetric with rounded outline. Maxillary palps from short and slender (slightly shorter than the width of the head; e.g., Fig. 53C) to very short and stout (nearly half the width of the head; Fig. 54E); maxillary palpomere 4 similar in length to slightly longer than palpomere 3; inner margin of maxillary palpomere 2 straight, outer margin strongly curved along apical 2/3. Elytra without sutural striae (in some species, stria 1 more strongly impressed along posterior half of elytra; Fig. 54C); elytral punctures seemingly arranged in rows, in some species more pronounced; interserial punctures occasionally longitudinally aligned; serial punctures sometimes impressed into distinct grooves (e.g., Fig. 52A). Prosternum flat. Posterior elevation of mesoventrite either flat, bulging or with transverse or longitudinal ridge (Fig. 14F, G); anapleural sutures concave, separated at anterior margin by distance nearly 0.3–0.5 × width of anterior margin of mesepisternum. Metaventrite densely pubescent, except for median glabrous patch, either ovoid and broad (Fig. 14G) or longitudinal and narrow (Fig. 14F). Protibiae with spines of anterior row hair-like, semi erect, relatively long and thick; apical spurs of protibia from very short and stout, to enlarged to reach apex of protarsomere 3. Tarsomeres 2–4 densely covered by hair-like spines on ventral face; metatarsomeres 1–4 similar in length, 5 nearly as long as 3 and 4 combined, or metatarsomere 2 similar in length to 5. Metafemora mostly glabrous, with only few scattered setae, sometimes with hydrofuge pubescence along basal half of anterodorsal margin (e.g., Figs 52C, F, 53 C, F). Fifth abdominal ventrite apically evenly rounded, without fringe of stout setae. Aedeagus trilobed (Fig. 55), with basal piece usually very short (nearly 1/3 length of parameres); median lobe usually broader than each paramere; median lobe and parameres apically rounded to truncate; apex of median lobe seldom medially emarginated; gonopore well developed.
Differential diagnosis.
Tobochares are among the smallest acidocerines. Some members of the group are unique in the presence of impressed elytral striae (striatus species group; Girón and Short 2021a). Tobochares without elytral striae may resemble some Agraphydrus (with eight antennomeres and mostly glabrous femora), and other than their distributions (Tobochares in the New World, Agraphydrus in the Old World) and slight differences in overall body shape, they can only be differentiated by the shape of the aedeagus (slender in Tobochares, Fig. 55; overall broader in Agraphydrus, Fig. 20). Within the New World, Tobochares is most likely to be confused with Ephydrolithus, which also contains small, seepage-inhabiting species, although currently the ranges of the two genera do not quite overlap. However, the difference in the number of antennomeres (nine in Ephydrolithus) provides a clear point of separation.
Distribution.
Neotropical: Brazil (Amapá, Amazonas, Goiás, Roraima), French Guiana, Guyana, Suriname, Venezuela; Fig. 6.
Natural history.
Most Tobochares specimens have been collected at hygropetric habitats, including isolated hygropetric seeps as well as wet rock surfaces along rivers and waterfalls. They can sometimes be found in large numbers. One species, T. fusus, has been collected in both seepage habitats as well as terrestrially in the rotten fruits of Clusia (see Kohlenberg and Short 2017 and Girón and Short 2021a for more details).
Larvae.
The immature stages of Tobochares remain unknown.
Taxonomic history.
Short and García (2007) described the genus and one species from Venezuela. Additional species were described from Suriname, one by Short and Kadosoe (2011) and two more by Short (2013). The genus was revised by Kohlenberg and Short (2017), including the description of five new species and the characterization of one specimen from Tobogán de la Selva (Venezuela) left undescribed until additional material can be studied. The genus was reviewed again just a few years later by Girón and Short (2021), in the light of new molecular evidence, describing 15 additional new species and establishing four diagnosable species groups.
Remarks.
There are 24 described species of Tobochares. The genus is rather highly variable in its external morphology: there is variation in coloration, the degree of emargination of the eyes and the degree of development and extension of the elytral striae. The form of the aedeagus is also somewhat variable, although not as extreme as in some genera such as Chasmogenus or Helochares.
The genus is much richer in species and more broadly distributed in the Amazon region than as currently published. We have examined numerous additional specimens from around the Amazonian region, particularly the southern Amazon (e.g., Brazil: Rondonia) from where the genus is currently unknown. We would not be surprised if the genus exceeded 50 species when more attention is paid to seepage habitats in this region.
Species examined.
Holotypes, paratypes, and additional specimens of all described species, as well as several undescribed species were examined for this study.
Selected references.
Short and García 2007: original description of the genus and its type species; Short and Kadosoe 2011: description of one additional species; Short 2013: description of two additional species; Kohlenberg and Short 2017: revision of the genus and description of five new species; Girón and Short 2021a: review of the genus with description of 15 new species and establishment of four species groups; Short et al. 2021: phylogenetic placement.
Genus. Troglochares
Spangler, 1981
Troglochares Spangler, 1981a: 316.
Gender.
Masculine.
Type species.
Troglochares ashmolei Spangler, 1981a: 318; by original designation and monotypy.
Diagnosis.
Small beetles, body length 1.9 mm. Body shape oval in dorsal view; moderately convex in lateral view (Hansen 1991: fig. 39). Color yellowish light brown; ground punctation extremely shallowly marked. Shape of head somewhat oval. Eyes absent (Fig. 56B). Clypeus trapezoidal, with anterior margin broadly emarginate, with medial region of emargination nearly straight (Fig. 56B). Labrum fully exposed, convex. Mentum rather smooth and antero-medially depressed; median anterior depression broad. Antennae with nine antennomeres (Spangler 1981a: fig. 3); cupule slightly asymmetric, with rounded outline. Maxillary palps slender, nearly as long as width of head; inner margin of maxillary palpomere 2 nearly straight, outer margin curved along apical third; maxillary palpomere 3 slightly shorter than 4. Prosternum non carinate, slightly convex. Elytra without sutural striae; ground punctation fine, shallow; outer margins slightly flared (Fig. 56A). Posterior elevation of mesoventrite with curved, transverse ridge (Spangler 1981a: fig. 8); anapleural sutures concave, separated at anterior margin by distance 0.7 × width of anterior margin of mesepisternum. Metaventrite densely pubescent except for median short and narrow posterior glabrous patch; metaventrite short (nearly as long as first abdominal ventrite; Spangler 1981a: fig. 8). Protibiae with spines of anterior row long; apical spurs of protibiae moderately slender, reaching apex of protarsomere 2; metatarsomeres 2–4 slightly decreasing in size; metatarsomere 5 nearly as long as 2–4 combined. Posterior femora densely covered by hydrofuge pubescence along basal 2/3 (Spangler 1981a: fig. 8). Fifth abdominal ventrite apically truncate, without stout setae (Spangler 1981a: fig. 9).
Differential diagnosis.
Troglochares is the only genus of acidocerines (and Hydrophilids) lacking eyes.
Distribution.
Neotropical: Ecuador; Fig. 6.
Natural history.
The only known specimen was collected in a cave on calcite formations and is presumably aquatic (Spangler 1981a).
Larvae.
The immature stages are unknown for Troglochares.
Taxonomic history.
The genus and its only known species were described by Spangler (1981a).
Remarks.
The genus is only known from a single female specimen, which is pin-mounted in pieces (Fig. 56). This species was not included in the molecular phylogeny by Short et al. (2021). Its assignment to the Tobochares group is based primarily on its tiny size (excluding the Helochares group), presence in the Neotropical region (excluding the Agraphydrus group), and lack of a sutural stria (excluding the Primocerus and Chasmogenus groups).
Species examined.
The holotype specimen of Troglochares ashmolei Spangler was examined.
Selected references.
Spangler 1981a: original description; Short et al. 2021: morphological affinities discussed in a phylogenetic context.
Catalog of the subfamily Acidocerinae
The following species list is based for the most part on Hansen (1999b), and therefore follows its format. Species described between 15 December 1999 and 1 April 2021 have been added to the present catalog. Generic synonyms are omitted here as those are listed for each genus above. For each species the currently valid name is provided, followed by the original name with a reference to the original description, including page number and full type locality as provided in the original publication. The full checklist of valid names is available online via GBIF (https://doi.org/10.15468/ypcrsp). For countries which current names are different from those indicated in the original description the name of the country has been updated, leaving in square brackets the country names that have been previously cited (e.g., Sri Lanka [Ceylon]).
For each name that has been used, a list of references including page number and details on the nature/content of the reference in square brackets (e.g., [catalog], [checklist], [new record], etc.) is also provided. ‘Catalog’ refers to publications listing synonyms and references, whereas ‘checklist’ only presents the name of a species for a particular region. ‘Faunistic treatment’ is used for works revising the fauna of a particular country or region, which sometimes include discussions on taxonomic status of certain species, whereas ‘taxonomic treatment’ is used when the reference includes a taxonomic revision for a particular group. ‘New record’ is used for new country records, as opposed to new localities from a previously recorded country. The currently known distribution (extracted from the literature) is summarized for each valid name.
Acidocerus Klug, 1855
Acidocerus aphodioides Klug, 1855
Acidocerus aphodioides Klug, 1855: 649 – Mozambique, Tete [“Mossambique: Tette”]; Knisch 1924: 222 [catalog]; Hansen 1999b: 158 [catalog]; Hebauer 2006a: 25 [checklist].
Distribution: Afrotropical: Mozambique.
Agraphydrus Régimbart, 1903
Agraphydrus abrasus Komarek & Freitag, 2020
Agraphydrus sp. D (in part); Freitag and Zettel 2013: 19, 30.
Agraphydrus abrasus Komarek & Freitag, 2020: 204 – Philippines, Luzon Island, Aurora Province, Maria Aurora Municipality, Barangay Wenceslao, Bingwangan River flowing through extensive coconut plantation, 60 m a.s.l., 15°45'48"N, 121°25'21"E.
Distribution: Indo-Malayan: Philippines (Luzon, Mindoro, Palawan).
Agraphydrus activus Komarek & Hebauer, 2018
Agraphydrus activus Komarek & Hebauer, 2018: 18 – China, Hong Kong Admin. Reg., New Territories, Tai Mo Shan Country Park, SW Tai Po New Town, Lam Tsuen River; Komarek 2019: 157 [new record].
Distribution: Indo-Malayan: China (Fujian, Hong Kong, Guangdong, Jiangxi). Palearctic: China (Anhui), Thailand.
Agraphydrus acutus Komarek, 2020
Agraphydrus acutus Komarek, 2020: 132 – Namibia, Karas Region, Aar Farm Waterhole.
Distribution: Afrotropical: Namibia, Republic of South Africa.
Agraphydrus aethiopicus Komarek, 2020
Agraphydrus aethiopicus Komarek, 2020: 134 – Ethiopia, Amhara Region, Simien Mountains.
Distribution: Afrotropical: Ethiopia.
Agraphydrus agilis Komarek & Hebauer, 2018
Agraphydrus agilis Komarek & Hebauer, 2018: 20 – China, Guangxi Province, Liuzhou Prefecture, 10 km N Liuzhou City, ca. 2 km E Shanmenjiang Forest Station; Komarek 2019: 158 [taxonomic treatment].
Distribution: Indo-Malayan: China (Guangxi, Yunnan), Vietnam.
Agraphydrus albescens (Régimbart, 1903)
Helochares albescens Régimbart, 1903a: 27 – Madagascar, “Centre-Sud”.
Helochares (s. str.) albescens Régimbart, 1903; Knisch 1924a: 196 [catalog].
Helochares (Agraphydrus) albescens Régimbart; d’Orchymont 1939c: 198 [taxonomic discussion; new record].
Agraphydrus (Agraphydrus) albescens (Régimbart, 1903); Hansen 1999b: 156 [new combination; catalog]; Hebauer 2006a: 27 [checklist, new records].
Agraphydrus albescens (Régimbart, 1903); Hebauer 2005: 39 [checklist]; Komarek 2020: 135 [faunistic treatment; lectotype designation; new records].
Distribution: Afrotropical: Botswana, Cameroon, Democratic Republic of the Congo, Kenya, Madagascar, Malawi, Namibia, Republic of South Africa, Tanzania [Zanzibar], Zimbabwe. Sudan is excluded (Komarek 2020).
Agraphydrus ampullatus Komarek & Freitag, 2020
Agraphydrus ampullatus Komarek & Freitag, 2020: 206 – Philippines, Leyte Island and Province, Baybay Municipality, creek 2 km east of Visayas State University, ca. 10°44'46"N, 124°48'50"E, ca. 140 m a.s.l.
Distribution: Indo-Malayan: Philippines (Leyte).
Agraphydrus anacaenoides Komarek, 2019
Agraphydrus anacaenoides Komarek, 2019: 158 – Malaysia, Penang, Southwest Penang Island District, Pantai Aceh Forest Reserve (= Penang N.P.).
Distribution: Indo-Malayan: Malaysia.
Agraphydrus anatinus Komarek, 2018
Agraphydrus anatinus Komarek, 2018: 107 – India, Goa, South Goa District, Salcete (= Salcette or Saxti) Subdivision.
Distribution: Indo-Malayan: India (Goa, Kerala, Maharashtra).
Agraphydrus andamanicus Komarek, 2018
Agraphydrus andamanicus Komarek, 2018: 108 – India, North Andaman Island, Diglipur.
Distribution: Indo-Malayan: India (North Andaman Island).
Agraphydrus andringitra Komarek, 2020
Agraphydrus andringitra Komarek, 2020: 137 – Madagascar, Fianarantsoa Province, Haute Matsiatra Region, Andringitra N.P., Mount Ambatoberger, 22°7'52.0"S, 46°51'51.1"E.
Distribution: Afrotropical: Madagascar.
Agraphydrus angulatus Komarek, 2019
Agraphydrus angulatus Komarek, 2019: 159 – Laos, Khammouan Province, Nakai District, Nakai, 17°43'N, 105°09'E.
Distribution: Indo-Malayan: Laos.
Agraphydrus angustatus Komarek, 2020
Agraphydrus angustatus Komarek, 2020: 138 – Namibia, Kunene Region, Uniab River, Palmwag N.P., near Palmwag Lodge, 19°53'S, 13°50'W.
Distribution: Afrotropical: Angola, Namibia.
Agraphydrus angustipenis Komarek, 2018
Agraphydrus angustipenis Komarek, 2018: 109 – Sri Lanka, “Dambuwa Estate”.
Distribution: Indo-Malayan: Sri Lanka.
Agraphydrus anhuianus (Hebauer, 2000)
Megagraphydrus anhuianus Hebauer, 2000: 15 – China, Anhui, Huang Shan 30 km W Tunxi. Hansen 2004: 52 [catalog]; Short and Hebauer 2006: 337 [catalog]; Fikáček et al. 2015: 62 [catalog].
Agraphydrus (Agraphydrus) anhuianus (Hebauer, 2000); Minoshima et al. 2015: 12 [new combination; redescription; new record].
Agraphydrus anhuianus (Hebauer, 2000); Komarek and Hebauer 2018: 21 [excludes only known specimen from Hong Kong].
Distribution: Indo-Malayan: Thailand. Palearctic: China (Anhui).
Agraphydrus annapurnensis Komarek, 2018
Agraphydrus annapurnensis Komarek, 2018: 110 – Nepal, Western Region, Gandaki Zone, Kaski District, Annapurna Mountains, ca. 10 km ENE Pokhara, tributary of Madi Khola River below Kwinkal (village), ca. 28°13'55"N, 84°5'16"E.
Distribution: Indo-Malayan: Nepal.
Agraphydrus arduus Komarek & Hebauer, 2018
Agraphydrus arduus Komarek & Hebauer, 2018: 22 – China Yünnan Prov., Xishuangbanna Dai Autonomous Prefecture, Mengla County, Wushiwu He River, ca. 10 km NW Menglun Town; Komarek 2019: 160 [new record].
Distribution: Indo-Malayan: China (Guangdong, Yunnan), Laos. Palearctic: China (Hubei).
Agraphydrus ater Komarek, 2018
Agraphydrus ater Komarek, 2018: 111 – Nepal, Western Region, Gandaki Zone, Annapurna, N Pokhara, Kali Khola, below Garlang, ca. 28°17'10"N, 83°59'39"E.
Distribution: Indo-Malayan: Nepal.
Agraphydrus atripalpis Komarek, 2020
Agraphydrus atripalpis Komarek, 2020: 139 – Republic of South Africa: KwaZulu-Natal Province, Port Shepstone, Oribi Gorge.
Distribution: Afrotropical: Republic of South Africa.
Agraphydrus attenuatus (Hansen, 1999)
Megagraphydrus attenuatus Hansen, 1999a: 141 – Vietnam, Vĩnh Phúc Province (N Viertnam), Tam Dao. Hansen 1999b: 157 [catalog]; Hebauer 2000: 15 [taxonomic treatment].
Agraphydrus (Agraphydrus) attenuatus (Hansen, 1999); Minoshima et al. 2015: 16 [new combination; redescription; new records].
Agraphydrus attenuatus (Hansen, 1999); Komarek and Hebauer 2018: 23 [redescription]; Komarek 2019: 161 [taxonomic treatment].
Distribution: Indo-Malayan: China (Yunnan), Laos, Vietnam.
Agraphydrus audax Komarek & Hebauer, 2018
Agraphydrus audax Komarek & Hebauer, 2018: 24 – China Hunan Prov., Xiangxi Prefecture; Dayong County; Zhangjiajie Forest National Park, Suoxiyü Nature Reserve, Wulingyüan section, 30 km N Dayong City.
Distribution: Indo-Malayan: China (Guizhou, Hunan). Palearctic: China (Hubei, Shaanxi, Sichuan).
Agraphydrus avita (Hansen, 1997)
Horelophopsis avita Hansen, 1997: 109 – Indonesia, Papua [New Guinea; Irian Jaya], Japen Island, SSE Sumberbaba, Dawai R. Hansen 1999b: 68 [catalog].
Agraphydrus avita (Hansen); Short et al. 2021: 11 [new combination].
Distribution: Australasian: Indonesia (Papua (Yapen Island)).
Agraphydrus bacchusi Komarek, 2019
Agraphydrus bacchusi Komarek, 2019: 162 – Papua New Guinea, Central Province, road between Port Moresby and Brown River.
Distribution: Australasian: Papua New Guinea (Central Province).
Agraphydrus balkeorum Komarek, 2019
Agraphydrus balkeorum Komarek, 2019: 163 – West Sumatra Province, Solok Regency, Solok – Alahan Panjang road, ca. 0°56'20"S, 100°46'24"E.
Distribution: Indo-Malayan: Indonesia (Sumatra).
Agraphydrus batak Komarek & Freitag, 2020
Agraphydrus sp. I; Freitag and Zettel 2013: 20, 30.
Agraphydrus batak Komarek & Freitag, 2020: 208 – Philippines, Palawan Island and Province, Puerto Princesa City, Barangay Concepcion, Tarabanan River upstream of Batak village, secondary forest, ca. 100 m a.s.l., 10°2'7"N, 119°1'10"E.
Distribution: Indo-Malayan: Philippines (Palawan Island).
Agraphydrus bhutanensis Komarek, 2018
Agraphydrus bhutanensis Komarek, 2018: 113 – Bhutan, Sarpang Province, 11 km NW Sarpang, Bhur Khola, 26°55'23"N, 90°23'51"E.
Distribution: Indo-Malayan: Bhutan.
Agraphydrus bicoloratus Komarek, 2020
Agraphydrus bicoloratus Komarek, 2020: 141 – Gabon, Estuaire Province, near Kinguélé Waterfall.
Distribution: Afrotropical: Gabon.
Agraphydrus bilardoi Komarek, 2020
Agraphydrus bilardoi Komarek, 2020: 142 – Gabon, Ngounié Province, Ndolou Distr., near Mandji, Pény Village, 2°1.804'S, 10°29.372'E.
Distribution: Afrotropical: Gabon.
Agraphydrus biltoni Komarek, 2020
Agraphydrus biltoni Komarek, 2020: 143 – Republic of South Africa: Northern Cape Province, Kamiesberg, 30°23'43.0"S, 18°8'8.4"E.
Distribution: Afrotropical: Republic of South Africa.
Agraphydrus biprojectus Minoshima, Komarek & Ôhara, 2015
Agraphydrus (Agraphydrus) biprojectus Minoshima, Komarek & Ôhara, 2015: 36 – Vietnam, Lào Cai Province, Sa Pa, Ô Quy Hồ; Komarek 2019: 164 [taxonomic treatment].
Distribution: Indo-Malayan: Laos, Vietnam.
Agraphydrus borneensis Komarek, 2019
Agraphydrus borneensis Komarek, 2019: 165 – Malaysia, Sabah, West Coast Division, Kota Kinabalu District, Crocker Range, km 56 of road Kota Kinabalu – Tambunan, near Sunsuron Waterfall.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus boukali Komarek, 2018
Agraphydrus boukali Komarek, 2018: 114 – India, Kerala, Thiruvananthapuram District, Cardamom Hills, 50 km NW Pathanamthitta, near Pambaiyar River, ca. 9°25'N, 77°05'E.
Distribution: Indo-Malayan: India (Kerala, Karnataka, Tamil Nadu).
Agraphydrus brevilobatus Komarek & Freitag, 2020
Agraphydrus brevilobatus Komarek & Freitag, 2020: 209 – Philippines, Negros Occidental, Silay, Patag, small mountain river, downstream Dumalabdab Falls, secondary forest, 800 m a.s.l., 10°41'10"N, 123°10'43"E.
Distribution: Indo-Malayan: Philippines (Negros, Panay).
Agraphydrus brevipenis Komarek, 2019
Agraphydrus brevipenis Komarek, 2019: 167 – Malaysia, Pahang, Cameron Highlands District, Mt. Jasar.
Distribution: Indo-Malayan: Malaysia.
Agraphydrus burmensis Komarek, 2019
Agraphydrus burmensis Komarek, 2019: 168 – Myanmar, Mandalay Region, Pyin Oo Lwin District, Mogok Township, NW Mogok, S Panlin village, west slope of Mt. Taung Mae, 22°57'57"N, 96°27'29"E.
Distribution: Indo-Malayan: Myanmar.
Agraphydrus calvus Komarek & Hebauer, 2018
Agraphydrus calvus Komarek & Hebauer, 2018: 25 – China, Hong Kong Admin. Reg., New Territories, Tai Mo Shan Country Park, SW Tai Po New Town, Lam Tsuen River.
Distribution: Indo-Malayan: China (Guangdong, Guangxi, Hong Kong, Jiangxi).
Agraphydrus camerunensis Komarek, 2020
Agraphydrus camerunensis Komarek, 2020: 144 – Cameroon, Southwest Region, 25 km west of Limbe (City), Bakingili.
Distribution: Afrotropical: Cameroon.
Agraphydrus cantonensis Komarek & Hebauer, 2018
Agraphydrus cantonensis Komarek & Hebauer, 2018: 27 – China, Guangdong Prov., Zhaoqing Pref., Fengkai County, ca. 50 km E of Fengkai, ca. 5 km W of Qixing, Heishiding Nature Reserve, 23°27'04"N, 111°53'53"E.
Distribution: Indo-Malayan: China (Guangdong).
Agraphydrus carinatulus Komarek, 2019
Agraphydrus carinatulus Komarek, 2019: 169 – Indonesia, East Kalimantan Province, Kutai Kartanegara Regency, Tabang District, ca. 200 km NW of Samarinda City near Ritan Baru village.
Distribution: Indo-Malayan: Indonesia.
Agraphydrus cervus Komarek, 2019
Agraphydrus cervus Komarek, 2019: 170 – Malaysia, Sarawak, Kapit Division, Kapit District, ca. 25 km E of Kapit.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus ceylonensis Komarek, 2018
Agraphydrus ceylonensis Komarek, 2018: 115 – Sri Lanka [Ceylon], Sabaragamuwa Province, Kegalle District, a few km E Kitulgala.
Helochares sp.: Jäch 1984: 243.
Distribution: Indo-Malayan: Sri Lanka.
Agraphydrus chinensis Komarek & Hebauer, 2018
Agraphydrus chinensis Komarek & Hebauer, 2018: 27 – China, Fujian Prov., Jianyuan Prefecture, Chong’an City Region, Chong’an Wuyi Shan.
Distribution: Indo-Malayan: China (Fujian, Zhejiang). Palearctic: China (Anhui).
Agraphydrus cinnamum Komarek, 2018
Agraphydrus cinnamum Komarek, 2018: 117 – India, Kerala, Thiruvananthapuram District, Cardamom Hills, 50 km NW Pathanamthitta, near Pambaiyar River, ca. 9°25'N, 77°05'E.
Distribution: Indo-Malayan: India (Kerala).
Agraphydrus clarus Komarek, 2019
Agraphydrus clarus Komarek, 2019: 171 – Malaysia, Sabah, West Coast Division, Kota Kinabalu District, Crocker Range, km 56 of road between Kota Kinabalu and Tambunan, near Sunsuron Waterfall.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus comes Komarek & Hebauer, 2018
Agraphydrus comes Komarek & Hebauer, 2018: 28 – China, Hainan Prov., Ledong County, foot of Jianfeng Mountain, ca. 4 km E Jianfeng Town.
Distribution: Indo-Malayan: China (Hainan).
Agraphydrus communis Komarek, 2018
Agraphydrus communis Komarek, 2018: 118 – Nepal, Central Region, Sindhupalchok District, torrent above Tatobani near Kodari.
Distribution: Indo-Malayan: Bhutan, Nepal, India (Uttarakhand).
Agraphydrus confusus Komarek & Hebauer, 2018
Agraphydrus confusus Komarek & Hebauer, 2018: 29 – China, Hong Kong Admin. Reg., Tai Po Kau Nature Reserve; Komarek 2019: 173 [new record].
Distribution: Indo-Malayan: China (Guizhou, Hong Kong, Yunnan), Laos, Vietnam.
Agraphydrus congolensis Komarek, 2020
Agraphydrus congolensis Komarek, 2020: 145 – Democratic Republic of the Congo, Ituri (former Orientale) Province, Ituri Rainforest, Epulu River.
Distribution: Afrotropical: Democratic Republic of the Congo.
Agraphydrus conicus Komarek & Hebauer, 2018
Agraphydrus conicus Komarek & Hebauer, 2018: 30 – China Jiangxi Prov., Jinggangshan Mountains, Jingzhushan, 26°31.0'N, 114°05.9'E.
Distribution: Indo-Malayan: China (Hunan, Jiangxi). Palearctic: China (Anhui).
Agraphydrus connexus Komarek & Hebauer, 2018
Agraphydrus connexus Komarek & Hebauer, 2018: 31 – Malaysia, Pahang, Kuala Lipis [Town] surround. Komarek 2018: 120 [new records]; Komarek 2019: 173 [taxonomic treatment].
Distribution: Indo-Malayan: Bhutan, China (Hainan), India (Madhya Pradesh), Laos, Malaysia, Myanmar, Nepal, Thailand, Vietnam.
Agraphydrus constrictus Komarek, 2018
Agraphydrus constrictus Komarek, 2018: 121 – India, Uttarakhand, Chamoli District, Nandakini River, below Sedoli, ca. 10 km E Nandaprayag, 30°15'50"N, 79°26'32"E.
Distribution: Indo-Malayan: India (Assam, Uttarakhand), Nepal.
Agraphydrus contractus Komarek & Hebauer, 2018
Agraphydrus contractus Komarek & Hebauer, 2018: 33 – China, Fujian Prov., Jianyuan Prefecture; Yong’an City Region; ca. 20 km SE Yong’an City, 5 km SW Xiyang Village, Ziyungdong Shan.
Distribution: Indo-Malayan: China (Fujian, Guangdong).
Agraphydrus coomani (d’Orchymont, 1927)
Helochares (Agraphydrus) coomani d’Orchymont, 1927a: 248 – Vietnam, [Tonkin], Lac Tho, nr. Hoa Binh Province; d’Orchymont 1928: 108 [faunistic treatment].
Agraphydrus coomani (d’Orchymont, 1927); Watts 1995: 115 [new records]; Komarek and Hebauer 2018: 34 [new records; redescription]; Komarek 2018: 122 [new records]; Komarek 2019: 174 [new records]; Komarek and Freitag 2020: 211 [new records].
Agraphydrus (Agraphydrus) coomani (d’Orchymont, 1927); Hansen 1999b: 156 [catalog].
Enochrus ryukyuensis Matsui, 1994: 217 – Japan, Amami Islands (Kagoshima Pref.), Tokuno-shima Is., Tokunoshima Town, Kamize Dam.
Agraphydrus ryukyuensis (Matsui, 1994); Gentili et al. 1995: 208 [checklist]; Komarek and Hebauer 2018: 34 [synonym of A. coomani (d’Orchymont, 1927)].
Agraphydrus (Agraphydrus) ryukyuensis (Matsui, 1994); Hansen 1999b: 157 [catalog]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 61 [catalog]; Minoshima 2016: 361 [redescription].
Distribution: Indo-Malayan: Brunei, China (Fujian, Guangdong, Hainan, Taiwan), Indonesia, Laos, Malaysia (Peninsula), Myanmar, Philippines (Leyte, Luzon), Sri Lanka, Thailand, Vietnam. Palearctic: Japan. Australasian: Australia (New South Wales, Northern Territory, Queensland, Western Australia), Papua New Guinea.
Agraphydrus coronarius Minoshima, Komarek & Ôhara, 2015
Agraphydrus (Agraphydrus) coronarius Minoshima, Komarek & Ôhara, 2015: 41 – Laos, Bolikhamsai Province, Lak Sao; Komarek 2019: 179 [taxonomic treatment].
Distribution: Indo-Malayan: Laos.
Agraphydrus crassipenis Komarek, 2018
Agraphydrus crassipenis Komarek, 2018: 123 – Nepal, Eastern Region, Kosi (= Koshi) Zone, Sunsari District, Dharan (city) environment.
Distribution: Indo-Malayan: Bhutan, Nepal.
Agraphydrus decipiens Minoshima, Komarek & Ôhara, 2015
Agraphydrus (Agraphydrus) decipiens Minoshima, Komarek & Ôhara, 2015: 44 – Taiwan, Taichung City, Heping District, Basian-shan National Forest Recreation Area, 24°11.55'N, 121°00.83'E.
Agraphydrus decipiens Minoshima, Komarek & Ôhara, 2015; Komarek and Hebauer 2018: 36 [redescription]; Angus et al. 2020: 19 [karyotype].
Distribution: Indo-Malayan: China (Taiwan).
Agraphydrus delineatus Komarek, 2019
Agraphydrus delineatus Komarek, 2019: 180 – Malaysia, Sarawak, Kuching Division, Mt. Serapi, ca. 19 km W Kuching.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus elongatus Ribera, Hernando & Cieslak, 2019
Agraphydrus elongatus Ribera, Hernando & Cieslak, 2019: 264 – Oman, Murri, Wadi Bani Ghafir, N23 29 46.2 E56 53 34.8; Komarek 2020: 146 [faunistic treatment].
Distribution: Afrotropical: Oman, United Arab Emirates.
Agraphydrus engkari Komarek, 2019
Agraphydrus engkari Komarek, 2019: 181 – Malaysia, Sarawak, Sri Aman Division, Lubok Antu District, Batang Ai N.P., E of Bandar Sri Aman, Engkari River.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus excisus Komarek, 2019
Agraphydrus excisus Komarek, 2019: 182 – Malaysia, Sarawak, Kapit Division, Kapit District, ca. 25 km of E Kapit.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus exedis (d’Orchymont, 1937)
Helochares (Agraphydrus) exedis d’Orchymont, 1937a: 29 – India, Maharashtra [Bombay Presidency], Pune distr. [“Poona distr.”], Khandala.
Agraphydrus (Agraphydrus) exedis (d’Orchymont, 1937); Hansen 1999b: 156 [new combination].
Agraphydrus exedis (d’Orchymont, 1937); Komarek 2018: 124 [new records].
Distribution: Indo-Malayan: India (Madhya Pradesh, Maharashtra).
Agraphydrus exiguus Komarek, 2019
Agraphydrus exiguus Komarek, 2019: 183 – Malaysia, Pahang, Cameron Highlands District, Tanah Rata (town), Sungai Ruil near village of Orang Asli.
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus falcatus Komarek, 2018
Agraphydrus falcatus Komarek, 2018: 125 – India, Tamil Nadu, Dindigul District, Palni Hills, Kodaikanal, Pallangi, ca. 10°15'N, 77°30'E.
Distribution: Indo-Malayan: India (Kerala, Tamil Nadu).
Agraphydrus fasciatus Komarek & Hebauer, 2018
Agraphydrus fasciatus Komarek & Hebauer, 2018: 37 – China, Hong Kong Admin. Reg., New Territories, Plover Cove Reservoir.
Distribution: Indo-Malayan: China (Guangdong, Hong Kong, Jiangxi).
Agraphydrus fikaceki Komarek & Hebauer, 2018
Agraphydrus fikaceki Komarek & Hebauer, 2018: 38 – China, Jiangxi Prov., Jinggangshan Mts., Pingshui Shan, 26°30.4'N, 114°06.9'E.
Distribution: Indo-Malayan: China (Hong Kong, Jiangxi).
Agraphydrus flavescens Komarek, 2020
Agraphydrus flavescens Komarek, 2020: 147 – Ghana, Ashanti Region, Bobiri Forest Reserve.
Distribution: Afrotropical: Cameroon, Ghana.
Agraphydrus flavipes Komarek, 2020
Agraphydrus flavipes Komarek, 2020: 148 – Madagascar, Fianarantsoa Province, Vatovavy-Fitovinany Region, Ionilahy (village), Ionilahy River.
Distribution: Afrotropical: Madagascar.
Agraphydrus flavonotus Komarek, 2018
Agraphydrus flavonotus Komarek, 2018: 127 – Bhutan, Sarpang Province, Geylephug – Shemgang road, 26°56'43"N, 90°31'29"E.
Distribution: Indo-Malayan: Bhutan.
Agraphydrus floresinus Komarek, 2019
Agraphydrus floresinus Komarek, 2019: 185 – Indonesia, East Nusa Tenggara Province, East Manggarai Regency, Borong District, Flores Island, Lake Ranamese, between Ruteng and Borong.
Distribution: Indo-Malayan: Indonesia (Flores).
Agraphydrus fontis Komarek, 2020
Agraphydrus fontis Komarek, 2020: 149 – Madagascar, Fianarantsoa Province, Atsimo-Atsinanana Region, Ranomena (town), 21°29'45.9"S, 47°24'7.5"E.
Distribution: Afrotropical: Madagascar.
Agraphydrus forcipatus Komarek & Hebauer, 2018
Agraphydrus forcipatus Komarek & Hebauer, 2018: 39 – China, Anhui Prov., Weizhou Prefecture; Huang Shan NP; 60 km NNW Huang Shan City (= Tunxi), near Tang Kou.
Distribution: Indo-Malayan: (Fujian, Guangdong, Guizhou, Hunan, Jiangxi, Zhejiang). Palearctic: China (Anhui, Hubei).
Agraphydrus fortis Komarek, 2018
Agraphydrus fortis Komarek, 2018: 128 – Sri Lanka [Ceylon], Uva Province, Monaragala District, Gowinda Hela (a giant rock mountain known also as Westminster Abbey).
Distribution: Indo-Malayan: Sri Lanka.
Agraphydrus fujianensis Komarek & Hebauer, 2018
Agraphydrus fujianensis Komarek & Hebauer, 2018: 41 – China, Fujian Prov., Jianyuan Prefecture, Chong’an City Region, Wuyi Shan, 3 km SW Wuyi Gong Village (= Shanqian).
Distribution: Indo-Malayan: China (Fujian).
Agraphydrus geminus (d’Orchymont, 1932)
Helochares (Gymnhelochares) geminus d’Orchymont, 1932: 694 – Indonesia, W. Java, “Tjibodas-Bach”.
Agraphydrus (Gymnhelochares) geminus (d’Orchymont, 1932); Hansen 1991: 292 [subgenus transferred from Helochares to Agraphydrus]; Hansen 1999b: 157 [catalog].
Agraphydrus geminus (d’Orchymont, 1932); Komarek 2019: 186 [taxonomic treatment].
Distribution: Indo-Malayan: Indonesia (Java, Sumatra).
Agraphydrus gereckei Komarek, 2020
Agraphydrus gereckei Komarek, 2020: 150 – Madagascar, Antsiranana Province, Sava Region, Antalaha District, Maromandia (town), above Marofinatra (village), Ankavia River.
Distribution: Afrotropical: Madagascar.
Agraphydrus gilvus Komarek, 2018
Agraphydrus gilvus Komarek, 2018: 129 – India, Kerala, Kallar Valley, 10 km WSW Munnar, 10°3'N, 76°59'E.
Distribution: Indo-Malayan: India (Kerala).
Agraphydrus glaber Komarek, 2018
Agraphydrus glaber Komarek, 2018: 130 – India, Madhya Pradesh, Hoshangabad District, ca. 5 km NE Hoshangabad, ca. 60 km SSE Bhopal, Bandrabhan, Narmada River, 22°48'1"N, 77°46'45"E.
Distribution: Indo-Malayan: India (Madhya Pradesh).
Agraphydrus globipenis Komarek & Hebauer, 2018
Agraphydrus globipenis Komarek & Hebauer, 2018: 41 – China, Hunan Prov., Huaihua Pref., Huitong County, Jinlong Shan, ca. 30 km NE Huitong City.
Distribution: Indo-Malayan: China (Guangxi, Hunan).
Agraphydrus goldschmidti Komarek, 2020
Agraphydrus goldschmidti Komarek, 2020: 151 – Madagascar, Toliara Province, Anosy Region, Tsimelahy, Antarantsa River.
Distribution: Afrotropical: Madagascar.
Agraphydrus gracilipalpis Komarek & Hebauer, 2018
Agraphydrus gracilipalpis Komarek & Hebauer, 2018: 42 – China, Guangdong Prov., Zhaoqing Prefecture, Dinghu Nature Reserve, 23°11'03"N, 112°33'06"E.
Distribution: Indo-Malayan: China (Fujian, Guangdong).
Agraphydrus hamatus Komarek, 2019
Agraphydrus hamatus Komarek, 2019: 187 – Vietnam, Hòa Binh Province, Lac Tho.
Distribution: Indo-Malayan: Vietnam.
Agraphydrus hanseni (Satô & Yoshitomi, 2004)
Horelophopsis hanseni Satô & Yoshitomi, 2004: 42 – Japan, Ôura-gawa Kakou, Okinawa-jima, Ryukyus. Yoshitomi and Nakajima 2005: 376 [new record]; Short and Hebauer 2006: 321 [catalog]; Minoshima et al. 2013: 711 [description of larvae; phylogenetic placement]; Short and Fikáček 2013: 731 [phylogenetic placement]; Fikáček et al. 2015: 62 [catalog].
Agraphydrus hanseni (Satô and Yoshitomi); Short et al. 2021: 11 [new combination].
Distribution: Palearctic: Japan.
Agraphydrus heinrichi Komarek, 2018
Agraphydrus heinrichi Komarek, 2018: 131 – India, Kerala, Thiruvananthapuram District, Cardamom Hills, 50 km NW Pathanamthitta, near Pambaiyar River, ca. 9°25'N, 77°5'E
Distribution: Indo-Malayan: India (Kerala).
Agraphydrus helicopter Komarek, 2019
Agraphydrus helicopter Komarek, 2019: 188 – Malaysia, Johor, Gunung Ledang N.P., Gunung Ledang (= Mt. Ophir), Hutan (= forest) Lipur.
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus hendrichi Komarek, 2019
Agraphydrus hendrichi Komarek, 2019: 189 – Malaysia, Pahang, Taman Negara N.P., surroundings of Nusa Camp.
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus heterochromatus Komarek, 2019
Agraphydrus heterochromatus Komarek, 2019: 190 – Malaysia, Penang, George Town City, Botanic Gardens (= Waterfall Gardens).
Distribution: Indo-Malayan: Malaysia (Peninsula), Thailand.
Agraphydrus hortensis Komarek, 2019
Agraphydrus hortensis Komarek, 2019: 192 – Malaysia, Penang, George Town City, Botanic Garden.
Distribution: Indo-Malayan: Malaysia (Peninsula)
Agraphydrus hygropetricus Komarek, 2018
Agraphydrus hygropetricus Komarek, 2018: 132 – Sri Lanka [Ceylon], Western Province, 24 miles ESE Colombo, Labugama (village).
Distribution: Indo-Malayan: Sri Lanka.
Agraphydrus igneus Komarek & Hebauer, 2018
Agraphydrus igneus Komarek & Hebauer, 2018: 43 – China, Hong Kong, Lantau Island, Ngong Ping village, Po Lin Monastery environment, 22°15.25'N, 113°54.6"E; Komarek 2019: 193 [taxonomic treatment].
Distribution: Indo-Malayan: China (Guangdong, Hong Kong), Laos.
Agraphydrus imitans Komarek, 2019
Agraphydrus imitans Komarek, 2019: 193 – Myanmar, Mandalay Region, ca. 50 km NW Kalaw, Myitsone River, 20°48'27.42"N, 96°21'36.6"E.
Distribution: Indo-Malayan: Laos, Myanmar, Thailand, Vietnam.
Agraphydrus indicus (d’Orchymont, 1932)
Helochares (Gymnhelochares) indicus d’Orchymont, 1932: 694 – India, Uttar Pradesh, Kumaon, Haldwani distr.
Agraphydrus (Gymnhelochares) indicus (d’Orchymont, 1932); Hansen 1999b: 157 [new combination]; Hebauer 2002a: 20 [new records]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 61 [catalog].
Agraphydrus indicus (d’Orchymont, 1932); Komarek 2018: 133 [new records; redescription].
Distribution: Indo-Malayan: Bhutan, India (Arunachal Pradesh, Himachal Pradesh, Meghalaya, Uttarakhand, Uttar Pradesh), Nepal.
Agraphydrus inflatus Komarek, 2018
Agraphydrus inflatus Komarek, 2018: 136 – India, Kerala, Idukki District, Cardamom Hills, Kallar Valley, 15 km SW Munnar, ca. 10°02'N, 76°58'E.
Distribution: Indo-Malayan: India (Kerala, Tamil Nadu).
Agraphydrus infuscatus Komarek, 2019
Agraphydrus infuscatus Komarek, 2019: 195 – Thailand, Phang Nga Province, Khuraburi District, Baan Tumnang, west of Si Phang Nga N.P.
Distribution: Indo-Malayan: Thailand.
Agraphydrus insidiator Minoshima, Komarek & Ôhara, 2015
Agraphydrus (Agraphydrus) insidiator Minoshima, Komarek & Ôhara, 2015: 48 – Taiwan: Taichung City, Heping District, Basian-shan National Forest Recreation Area, 24°11.55'N, 121°00.83'E.
Agraphydrus insidiator Minoshima, Komarek, & Ôhara, 2015; Komarek and Hebauer 2018: 44 [redescription].
Distribution: Indo-Malayan: China (Taiwan).
Agraphydrus ishiharai (Matsui, 1994)
Enochrus ishiharai Matsui, 1994: 215 – Japan, Kyushu, Kumamoto Pref., Ue Village, Menda River.
Agraphydrus (Agraphydrus) ishiharai (Matsui, 1994); Hansen 1999b: 156 [new combination]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 60 [catalog]; Minoshima 2016: 353 [redescription]; Lee and Ahn 2017: 39 [new record].
Distribution: Palearctic: Japan, Korea.
Agraphydrus jaechi (Hansen, 1999)
Megagraphydrus jaechi Hansen, 1999a: 140 – Malaysia, Penang Aceh Forest Reserve 2 km W Telok Bahang; Hansen 1999b: 157 [catalog].
Agraphydrus (Agraphydrus) jaechi (Hansen, 1999); Minoshima et al. 2015: 18 [new combination; redescription].
Agraphydrus jaechi (Hansen, 1999); Komarek 2019: 196 [taxonomic treatment].
Megagraphydrus superans Hebauer, 2000: 16 – Malaysia, Pahang, Taman Negara National Park, Nusa Camp; Short and Hebauer 2006: 337 [catalog]; Komarek 2019 [synonymy].
Agraphydrus (Agraphydrus) superans (Hebauer, 2000); Minoshima et al. 2015: 35 [new combination].
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus jankodadai Komarek, 2019
Agraphydrus jankodadai Komarek, 2019: 197 – Malaysia, Sabah, Interior Division, Nabawan District, near Batu Punggul Resort.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus jilanzhui Komarek & Hebauer, 2018
Agraphydrus jilanzhui Komarek & Hebauer, 2018: 45 – China, Shaanxi Prov., Qin Ling Shan, 33°55'N, 108°49'E.
Distribution: Palearctic: China (Gansu, Hubei, Shaanxi, Sichuan).
Agraphydrus kallar Komarek, 2018
Agraphydrus kallar Komarek, 2018: 137 – India, Kerala, Thiruvananthapuram District, 30 km NNE Thiruvananthapuram, Kallar, ca. 8°45'N, 77°5'E.
Distribution: Indo-Malayan: India (Kerala).
Agraphydrus kathapa Komarek, 2019
Agraphydrus kathapa Komarek, 2019: 198 – Myanmar, Sagaing Region, Alaungdaw Kathapa N.P., 22°19'5.64"N, 94°28'49.38"E.
Distribution: Indo-Malayan: Myanmar.
Agraphydrus kempi (d’Orchymont, 1922)
Helochares (s. str.) kempi d’Orchymont, 1922: 626 – India, Arunachal Pradesh, Abors, “Yembung”.
Helochares (Agraphydrus) kempi (d’Orchymont, 1922); d’Orchymont 1927a: 5 [transferred from subgenus (s. str.) to subgenus (Agraphydrus)]; d’Orchymont 1928: 108 [faunistic treatment].
Agraphydrus (Agraphydrus) kempi (d’Orchymont, 1922); Hansen 1999b: 156 [new combination]; Hebauer 2002a: 21 [new record]; Hansen 2004: 60 [checklist]; Fikáček et al. 2015: 60 [catalog].
Agraphydrus kempi (d’Orchymont, 1922); Komarek 2018: 138 [new records; redescription].
Distribution: Indo-Malayan: Bhutan, India (Arunachal Pradesh, Meghalaya, Uttar Pradesh, Uttarakhand), Nepal.
Agraphydrus khasiensis Komarek, 2018
Agraphydrus khasiensis Komarek, 2018: 141 – India, Meghalaya, Khasi Hills District, Shillong Peak, 25°32.8'N, 91°52.5'E.
Distribution: Indo-Malayan: India (Meghalaya).
Agraphydrus kodaguensis Komarek, 2018
Agraphydrus kodaguensis Komarek, 2018: 142 – India, Karnataka, Kodagu District, Tadiyendamol Mountain, ca. 12°14'N, 75°36'E.
Distribution: Indo-Malayan: India (Karnataka).
Agraphydrus laocaiensis Komarek, 2019
Agraphydrus laocaiensis Komarek, 2019: 200 – Vietnam, Lào Cai Province, Sa Pa District, near Sa Pa (District capital), Cát Cát (village), 22°19'N, 103°50'E.
Distribution: Indo-Malayan: Vietnam.
Agraphydrus latus Komarek, 2019
Agraphydrus latus Komarek, 2019: 201 – Malaysia, Perak, Manjung District, Pangkor Island, Teluk Nipah (village).
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus longipalpus (Jia, 1998)
Pseudopelthydrus longipalpus Jia, 1998: 229 – China, Hainan, Jianfengling, Tianchi; Hansen 1999b: 126 [catalog].
Agraphydrus longipalpis (Jia, 1998) [incorrect subsequent spelling]; Komarek 2003: 384 [new combination]; Short and Hebauer 2006: 330 [catalog].
Agraphydrus (Gymnhelochares) longipalpis (Jia, 1998) [incorrect subsequent spelling]; Hansen 2004: 49 [checklist].
Agraphydrus (Agraphydrus) longipalpus (Jia, 1998); Fikáček et al. 2015: 60 [catalog].
Agraphydrus longipalpus (Jia, 1998); Komarek and Hebauer 2018: 46 [redescription].
Distribution: Indo-Malayan: China (Hainan).
Agraphydrus longipenis Komarek & Hebauer, 2018
Agraphydrus longipenis Komarek & Hebauer, 2018: 47 – Laos, Luang Nam Tha Prov., Luang Nam Tha [City] environment; Komarek 2019: 202 [taxonomic treatment].
Distribution: Indo-Malayan: China (Yunnan), Laos.
Agraphydrus lunaris Komarek, 2019
Agraphydrus lunaris Komarek, 2019: 202 – Laos, Khammouan Province, Khoun Ngeun (village), 18°07'N, 104°29'E.
Distribution: Indo-Malayan: Laos.
Agraphydrus luteilateralis (Minoshima & Fujiwara, 2009)
Megagraphydrus luteilateralis Minoshima & Fujiwara, 2009: 55 – Japan, Okinawa Prefecture, Iriomote-jima Island, Shirahama, 24°21'59"N, 123°45'22"E; Short and Fikáček 2011: 91 [checklist].
Agraphydrus (Agraphydrus) luteilateralis (Minoshima & Fujiwara, 2009); Minoshima et al. 2015: 22 [new combination]; Minoshima 2016: 355 [taxonomic treatment].
Agraphydrus (Agraphydrus) luteimarginalis (Minoshima & Fujiwara, 2009) [incorrect subsequent spelling]; Fikáček et al. 2015: 62 [catalog].
Distribution: Palearctic: Japan.
Agraphydrus madagascarensis Komarek, 2020
Agraphydrus madagascarensis Komarek, 2020: 152 – Madagascar, Toamasina Province, Atsinanana Region, Toamasina (town), Parc Ivoloina.
Distribution: Afrotropical: Madagascar.
Agraphydrus maehongsonensis Komarek, 2019
Agraphydrus maehongsonensis Komarek, 2019: 203 – Thailand, Mae Hong Son Province.
Distribution: Indo-Malayan: Thailand.
Agraphydrus malayanus (Hebauer, 2000)
Megagraphydrus malayanus Hebauer, 2000: 15 – Malaysia, Kedah, SW Langkawi, Telaga Tujuh; Short and Hebauer 2006: 337 [catalog].
Agraphydrus (Agraphydrus) malayanus (Hebauer, 2000); Minoshima et al. 2015: 22 [new combination; record from Thailand in doubt].
Agraphydrus malayanus (Hebauer, 2000); Komarek 2019: 158 [taxonomic treatment; excluded from Thailand].
Distribution: Indo-Malayan: Malaysia.
Agraphydrus malkini Komarek, 2020
Agraphydrus malkini Komarek, 2020: 154 – Cameroon, Southwest Region, Manyu Division, Mamfe.
Distribution: Afrotropical: Cameroon.
Agraphydrus manfredjaechi Komarek, 2019
Agraphydrus manfredjaechi Komarek, 2019: 206 – Indonesia, North Sulawesi Province, Dua Saudara N.P., E of Manado (capital city).
Distribution: Indo-Malayan: Indonesia (Seram, Sulawesi).
Agraphydrus masatakai Minoshima, Komarek & Ôhara, 2015
Agraphydrus (Agraphydrus) masatakai Minoshima, Komarek & Ôhara, 2015: 49 – Houaphanh Province, Xam Neua, Ban Saleui.
Agraphydrus masatakai Minoshima, Komarek & Ôhara, 2015; Komarek and Hebauer 2018: 48 [redescription]; Komarek 2019: 207 [new records].
Distribution: Indo-Malayan: China (Guangdong, Hainan, Hong Kong, Yunnan), Laos, Malaysia, Myanmar, Thailand, Vietnam.
Agraphydrus matoposensis Komarek, 2020
Agraphydrus matoposensis Komarek, 2020: 155 – Zimbabwe, Matabeleland South Province, Matopos N.P., 20°33'S, 28°30'E.
Distribution: Afrotropical: Zimbabwe.
Agraphydrus mazzoldii Komarek, 2019
Agraphydrus mazzoldii Komarek, 2019: 209 – Thailand, Mukdahan Province, Phu Pha Thoep N.P.
Distribution: Indo-Malayan: Thailand.
Agraphydrus meghalayanus Komarek, 2018
Agraphydrus meghalayanus Komarek, 2018: 143 – India, Meghalaya, East Khasi Hills District, 11 km SW Cherrapunjee, Laitkynsew, 25°12'N, 91°40'E.
Distribution: Indo-Malayan: India (Meghalaya).
Agraphydrus microphthalmus Komarek, 2019
Agraphydrus microphthalmus Komarek, 2019: 210 – Malaysia, Sarawak, Kapit Division, Kapit District, ca. 25 km E of Kapit.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus minutissimus (Kuwert, 1890)
Helochares (s. str.) minutissimus Kuwert, 1890b: 304 – Syria; d’Orchymont 1923a: 9 [faunistic treatment]; Hebauer 1994: 112 [faunistic treatment; identification doubtful].
Helochares minutissimus Kuwert, 1890; d’Orchymont 1926a: 379 [as synonym of H. pallens].
Helochares (Agraphydrus) minutissimus Kuwert; d’Orchymont 1939c: 197 [not synonym of Helochares pallens (MacLeay, 1825) as in d’Orchymont 1926a: 379); Balfour-Browne 1951: 213 [new record].
Agraphydrus minutissimus (Kuwert, 1890); Hebauer 1995a: 265 [new combination; new record]; Hebauer 1997: 264 [new record]; Fikáček et al. 2010: 149 [faunistic treatment]; Przewoźny 2019 [checklist]; Komarek 2020: 156 [faunistic treatment; new records].
Agraphydrus (Agraphydrus) minutissimus (Kuwert, 1890); Hansen 1999b: 156 [catalog]; Hansen 2004: 49 [checklist]; Hebauer 2006a: 27 [checklist]; Fikáček et al. 2015: 60 [catalog]; Ribera et al. 2019: 264 [checklist].
Distribution: Palearctic: Syria. Afrotropical: Djibouti, Eritrea, Ethiopia (in doubt), Iran, Oman, Saudi Arabia, Sudan, Yemen. Excluded from Kenya, Madagascar, Namibia, and Republic of South Africa (Komarek 2020).
Agraphydrus mirabilis Komarek, 2019
Agraphydrus mirabilis Komarek, 2019: 212 – Thailand, Chiang Mai Province, Doi (= mountain) Suthep N.P., Huai Sa Lad, 18°48'18.6"N, 98°54'31.2"E.
Distribution: Indo-Malayan: Thailand.
Agraphydrus montanus Minoshima, Komarek & Ôhara, 2015
Agraphydrus (Agraphydrus) montanus Minoshima, Komarek & Ôhara, 2015: 54 – India, West Sikkim, Sikkim State, Yuksom.
Agraphydrus montanus Minoshima, Komarek, & Ôhara, 2015; Komarek 2018: 144 [redescription].
Distribution: Indo-Malayan: India (Sikkim).
Agraphydrus muluensis Komarek, 2019
Agraphydrus muluensis Komarek, 2019: 213 – Malaysia, Sarawak, Miri Division, Gunung Mulu National Park.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus musculus Komarek, 2019
Agraphydrus musculus Komarek, 2019: 214 – Malaysia, Sarawak, Kapit Division, Kapit District, ca. 25 km E of Kapit.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus namthaensis Komarek, 2019
Agraphydrus namthaensis Komarek, 2019: 215 – Laos, Luang Nam Tha Province, Muang Sing District, ca. 20 km SE Muang Sing (town).
Distribution: Indo-Malayan: Laos.
Agraphydrus nanus Komarek, 2019
Agraphydrus nanus Komarek, 2018: 145 – India, Kerala, Thiruvananthapuram District, Cardamom Hills, 50 km NW Pathanamthitta, Pambaiyar River, 9°25'N, 77°05'E.
Distribution: Indo-Malayan: India (Karnataka, Kerala, Madhya Pradesh).
Agraphydrus narusei (Satô, 1960)
Pseudohelochares narusei Satô, 1960: 77 – Japan, Shikoku, Kôchi Pref., Kurosongawa River.
Agraphydrus narusei (Satô, 1960); Satô, 1965: 128 [new combination]; Hansen 1999b: 156 [checklist]; Hansen 2004: 49 [checklist]; Lee and Ahn 2009: 317 [redescription; new record]; Minoshima and Hayashi 2011: 17 [description of larvae]; Fikáček et al. 2015: 60 [catalog]; Minoshima 2016: 356 [redescription].
Distribution: Palearctic: Japan, South Korea.
Agraphydrus nemorosus Komarek, 2019
Agraphydrus nemorosus Komarek, 2019: 216 – Laos, Houaphan Province, 25 km SE (by road) of Vieng Xai City, Kangpabong (village), 20°19'N, 104°25'E.
Distribution: Indo-Malayan: Laos.
Agraphydrus nepalensis Komarek, 2018
Agraphydrus nepalensis Komarek, 2018: 146 – Nepal, Eastern Region, Koshi Zone, 2 km E Mangsingma.
Distribution: Indo-Malayan: Nepal.
Agraphydrus niger Komarek & Hebauer, 2018
Agraphydrus niger Komarek & Hebauer, 2018: 50 – China, Fujian Prov., Jianyuan Prefecture, Chong’an City Region, ca. 1 km W Wuyi Gong Village (= Shanqian, ca. 10 km S Chong’an City).
Distribution: Indo-Malayan: China (Fujian, Zheijang).
Agraphydrus nigroflavus Komarek, 2019
Agraphydrus nigroflavus Komarek, 2019: 217 – Indonesia, North Kalimantan Province [formerly part of East Kalimantan Province], Malinau Regency, Kayan Selatan District, Apokayan Highlands, Sungai Barang (village), Lalut Wai.
Distribution: Indo-Malayan: Indonesia (Borneo).
Agraphydrus obesus Komarek, 2019
Agraphydrus obesus Komarek, 2019: 218 – Vietnam, Central Highlands, Lâm Đồng Province, 12 km N Đà Lạt, Lang Bian.
Distribution: Indo-Malayan: Vietnam.
Agraphydrus obscuratus Komarek, 2018
Agraphydrus obscuratus Komarek, 2018: 148 – India, Kerala, Thiruvananthapuram District, Cardamom Hills, 50 km NW Pathanamthitta, near Pambaiyar River, ca. 9°25'N, 77°5'E.
Distribution: Indo-Malayan: India (Karnataka, Kerala, Maharashtra).
Agraphydrus obsoletus Komarek, 2018
Agraphydrus obsoletus Komarek, 2018: 149 – India, Kerala, Idukki District, 10 km WSW Munnar, Kallar Valley, ca. 10°3'N, 76°58'E.
Distribution: Indo-Malayan: India (Karnataka, Kerala, Tamil Nadu).
Agraphydrus occultus Komarek & Freitag, 2020
Agraphydrus occultus Komarek & Freitag, 2020: 214 – Philippines, Luzon Island, Laguna Province, Majayjay Municipality, Barangay Burgos, Taytay River downstream of Imelda Falls, secondary forest, 510 m a.s.l., 14°6'42"N, 121°30'19"E.
Distribution: Indo-Malayan: Philippines (Luzon, Mindoro, Palawan?, Panay?).
Agraphydrus ogatai Minoshima, 2016
Agraphydrus sp. Inoue et al. 2009: 76 [photo, as an undescribed species similar to A. narusei; in Japanese].
Agraphydrus (Agraphydrus) ogatai Minoshima, 2016: 359 – Japan, Fukuoka Pref., Koga-shi, Taniyama, Taniyamagawa River [about 33°42'N, 130°30'E].
Distribution: Palearctic: Japan.
Agraphydrus orbicularis Komarek, 2019
Agraphydrus orbicularis Komarek, 2019: 219 – Malaysia, Sarawak, Kuching Division, Semengoh, 30 km S Kuching, Semengoh Nature Reserve.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus orientalis (d’Orchymont, 1932)
Helochares (Agraphydrus) orientalis d’Orchymont, 1932: 690 – Indonesia, E. Java, “Ranu Bedali”.
Agraphydrus orientalis (d’Orchymont, 1932); Satô 1965: 128 [Agraphydrus re-established as genus]; Gentili et al. 1995: 208 [checklist]; Komarek and Hebauer 2018: 65 [taxonomic treatment]; Komarek 2019: 220 [taxonomic treatment].
Agraphydrus (Agraphydrus) orientalis (d’Orchymont, 1932); Hansen 1999b: 156 [catalog]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 60 [catalog].
Distribution: Indo-Malayan: China (Yunnan, Taiwan; in doubt, Komarek and Hebauer 2018: 65–66), Indonesia (Bali, Java, Lombok, Siberut, Sumatra).
Agraphydrus palawanensis Komarek & Freitag, 2020
Agraphydrus sp. F; Freitag and Zettel 2013: 19, 30.
Agraphydrus palawanensis Komarek & Freitag, 2020: 216 – Palawan Island and Province, Puerto Princesa City, Barangay Cabayugan, presumably Cabayugan River tributary, primary forest, ca. 100 m a.s.l., ca. 10°9'N, 118°52'E.
Distribution: Indo-Malayan: Philippines (Palawan, Busuanga).
Agraphydrus pallidus Komarek, 2019
Agraphydrus pallidus Komarek, 2019: 222 – Vietnam, Vĩnh Phúc Province, Tam Đảo.
Distribution: Indo-Malayan: Vietnam.
Agraphydrus papuanus Komarek, 2019
Agraphydrus papuanus Komarek, 2019: 223 – Indonesia, West Papua, Pegunungan Bintang Regency, Central Range, Kali Takime, 4°24'S, 140°25'E.
Distribution: Australasian: Indonesia (New Guinea), Papua New Guinea.
Agraphydrus pauculus (Knisch, 1924)
Helochares (Helocharimorphus) pauculus Knisch, 1924b: 36 – India, Uttar Pradesh, Kumaun, W. Almora.
Helochares panculus Knisch, 1924 [incorrect subsequent spelling]; d’Orchymont 1927a: 5 [taxonomic treatment].
Helochares (Agraphydrus) pauculus Knisch, 1924; d’Orchymont 1928: 108 [faunistic treatment].
Agraphydrus pauculus (Knisch, 1924); Hansen 1991: 148 [examined species]; Komarek 2018: 151 [new record; redescription].
Agraphilydrus pauculus Knisch, 1924; Chiesa 1967: 275 [incorrect identification, Komarek 2018: 153]
Agraphydrus (Agraphydrus) pauculus (Knisch, 1924); Hansen 1999b: 156 [catalog]; Hebauer 2002a: 22 [new records]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 60 [checklist].
Distribution: Palearctic: China (Tibet, Komarek 2018: 153). Indo-Malayan: India (Uttarakhand), Nepal.
Agraphydrus pauper Komarek, 2020
Agraphydrus pauper Komarek, 2020: 159 – Madagascar, Antsiranana Province, Sava Region, Andapa District, riparian springs at Masiaposa River, crossing Route National 3b at km 5–6.
Distribution: Afrotropical: Madagascar.
Agraphydrus pelingeni Komarek & Freitag, 2020
Agraphydrus (Agraphydrus) cf. orientalis (d’Orchymont, 1932); Freitag and Zettel 2013: 19, 30.
Agraphydrus pelingeni Komarek & Freitag, 2020: 216 – Philippines, Palawan Island and Province, Puerto Princesa City, Barangay Concepcion, Tarabanan River upstream of Batak village, secondary forest, ca. 30 m a.s.l., ca. 10°1'N, 119°1'E.
Distribution: Indo-Malayan: Philippines (Palawan).
Agraphydrus penangensis Komarek, 2019
Agraphydrus penangensis Komarek, 2019: 225 – Malaysia, Penang, Southwest Penang Island, Pantai Aceh Forest Reserve (= Penang National Park).
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus piceus Komarek, 2019
Agraphydrus piceus Komarek, 2019: 226 – Malaysia, Sabah, West Coast Division, Ranau District, Ranau (town), Liwagu River.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus politus (Hansen, 1999)
Megagraphydrus politus Hansen, 1999a: 138 – Taiwan, Taipei Wulai; Hansen 1999b: 158 [checklist]; Hebauer 2000: 18 [checklist]; Hansen 2004: 52 [checklist]; Fikáček et al. 2015: 62 [catalog].
Agraphydrus (Agraphydrus) politus (Hansen, 1999); Minoshima et al. 2015: 24 [new combination; redescription].
Agraphydrus politus (Hansen, 1999); Komarek and Hebauer 2018: 51 [redescription].
Megagraphydrus wangi Hebauer, 2000: 17 – Taiwan, Taipei Hsien, Sanhsia, 24°51'21"N, 121°24'33"E; Hansen 2004: 52 [checklist]; Short and Hebauer 2006: 337 [catalog]; Fikáček et al. 2015: 63 [catalog]; Minoshima et al. 2015: 25 [synonym with A. politus].
Distribution: Indo-Malayan: China (Taiwan).
Agraphydrus praecipuus (d’Orchymont, 1937)
Helochares (Agraphydrus) praecipuus d’Orchymont, 1937b: 252 – Madagascar, Toliara Province, Androy Region [(Sud), Pays Androy (Nord)].
Agraphydrus (Agraphydrus) praecipuus (d’Orchymont, 1937); Hansen 1999b: 157 [new combination; catalog]; Hebauer 2006a: 27 [checklist].
Agraphydrus praecipuus (d’Orchymont, 1937); Komarek 2020: 160 [faunistic treatment].
Distribution: Afrotropical: Madagascar.
Agraphydrus protentus Komarek, 2018
Agraphydrus protentus Komarek, 2018: 153 – India, Uttarakhand, Nainital.
Distribution: Indo-Malayan: India (Uttarakhand), Nepal.
Agraphydrus pullus Komarek, 2018
Agraphydrus pullus Komarek, 2018: 154 – Nepal, Eastern Region, Koshi Zone, Sunsari District, Dharan (city) environment.
Distribution: Indo-Malayan: Nepal.
Agraphydrus punctatellus Régimbart, 1903
Agraphydrus punctatellus Régimbart, 1903a: 34 – Madagascar [“Diégo-Suarez; forêt de la côte Est de Madagascar”); Komarek 2020: 161 [faunistic treatment; new record].
Enochrus (Agraphydrus) punctatellus Régimbart, 1903; Knisch 1924a: 219 [catalog].
Agraphydrus (Agraphydrus) punctatellus Régimbart, 1903; Satô, 1965: 128 [subgenus transferred from Enochrus to Agraphydrus]; Hansen 1999b: 157 [catalog]; Hebauer 2006a: 27 [checklist; new records].
Distribution: Afrotropical: Eswatini, Madagascar, Mozambique, Republic of South Africa, Tanzania.
Agraphydrus punctulatus Komarek, 2018
Agraphydrus punctulatus Komarek, 2018: 155 – India, Madhya Pradesh, Hoshangabad District, Pachmarhi Wildlife Sanctuary, Satpura Mountain Range, Apsara Vihar (stream), ca. 3 km SSE Pachmarhi, 22°27'7"N, 78°26'39"E.
Distribution: Indo-Malayan: India (Madhya Pradesh).
Agraphydrus puzhelongi (Jia, 2010)
Megagraphydrus puzhelongi Jia, 2010: 65 – China, Jiangxi Province, Shangrao, Sanqingshan mount, Upper Xinjiang river; Short and Fikáček 2011: 91 [catalog]; Fikáček et al. 2015: 63 [catalog].
Agraphydrus (Agraphydrus) puzhelongi (Jia, 2010); Minoshima et al. 2015: 30 [new combination].
Agraphydrus puzhelongi (Jia, 2010); Komarek and Hebauer 2018: 52 [redescription].
Distribution: Indo-Malayan: China (Guizhou, Jiangxi).
Agraphydrus pygmaeus (Knisch, 1924)
Helochares (Helocharimorphus) pygmaeus Knisch, 1924b: 38 – India, Kumaon, W Almora; d’Orchymont 1927a: 5 [taxonomic treatment].
Helochares (Agraphydrus) pygmaeus Knisch, 1924; d’Orchymont 1928: 108 [checklist].
Agraphydrus (Agraphydrus) pygmaeus Knisch, 1924; Hansen 1999b: 157 [new combination]; Hebauer 2002a: 22 [new record]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 60 [catalog].
Agraphydrus pygmaeus (Knisch, 1924); Komarek 2018: 156 [new record].
Distribution: Indo-Malayan: Bhutan, India (Meghalaya, Uttarakhand), Nepal. Palearctic: China (Tibet, Komarek 2018: 158).
Agraphydrus raucus Komarek, 2019
Agraphydrus raucus Komarek, 2019: 227 – Indonesia, West Sumatra Province, Lima Puluh Kota Regency, Lembah Harau Nature Reserve, 15 km NE of Payakumbu City.
Distribution: Indo-Malayan: Indonesia (Sumatra).
Agraphydrus reductus Komarek & Hebauer, 2018
Agraphydrus reductus Komarek & Hebauer, 2018: 53 – China, Yünnan Prov., Xishuangbanna Dai Autonomous Prefecture, Mengla County, Menglun Town, ca. 10 km NW Menglun, Wushiwu He River.
Distribution: Indo-Malayan: China (Yunnan).
Agraphydrus regularis (Hansen, 1999)
Megagraphydrus regularis Hansen, 1999a: 140 – Thailand, Phetchabun, 36 km SE Sila, Ban Pala Yai; Hansen 1999b: 158 [catalog].
Agraphydrus (Agraphydrus) regularis (Hansen, 1999); Minoshima et al. 2015: 30 [new combination; redescription].
Agraphydrus regularis (Hansen, 1999); Komarek 2019: 228 [taxonomic treatment].
Distribution: Indo-Malayan: Thailand.
Agraphydrus reticulatus Komarek, 2019
Agraphydrus reticulatus Komarek, 2019: 230 – Thailand, Surat Thani Province, Khao Sok N.P.
Distribution: Indo-Malayan: Thailand.
Agraphydrus reticuliceps Komarek & Hebauer, 2018
Agraphydrus reticuliceps Komarek & Hebauer, 2018: 53 – China, Hunan Prov., Zhangjiajie Pref., Wulingyuan, N Dayong City, Suoxiyu Nature Reserve.
Distribution: Indo-Malayan: China (Guizhou, Hunan). Palearctic: China (Hubei).
Agraphydrus rhodesiensis Komarek, 2020
Agraphydrus rhodesiensis Komarek, 2020: 163 – Zimbabwe, Mashonaland East Province, Doboshava, 27 km N Harare.
Distribution: Afrotropical: Zimbabwe.
Agraphydrus rhomboideus Komarek, 2019
Agraphydrus rhomboideus Komarek, 2019: 231 – Malaysia, Sarawak, Miri Division, Kelabit Highlands, 5 km E Bario (village community), Pa’Ukat (village).
Distribution: Indo-Malayan: Brunei, Indonesia (Borneo), Malaysia (Borneo).
Agraphydrus rivalis Komarek, 2020
Agraphydrus rivalis Komarek, 2020: 164 – Madagascar, Fianarantsoa Province, Haute Matsiatra Region, Madiorano near Ranomena (villages), stream crossing the railroad at km 51.2.
Distribution: Afrotropical: Madagascar.
Agraphydrus robustus Komarek & Hebauer, 2018
Agraphydrus robustus Komarek & Hebauer, 2018: 55 – China, Yünnan Prov., Simao Pref., 54 km SW Simao, Jian Shan River.
Distribution: Indo-Malayan: China (Guangdong, Yunnan).
Agraphydrus rostratus Komarek, 2018
Agraphydrus rostratus Komarek, 2018: 158 – India, Tamil Nadu, Nilgiris District, Nilgiri Hills, Kotagiri (town) environment, Honnatti, ca. 11°25'N, 76°55'E.
Distribution: Indo-Malayan: India (Kerala, Tamil Nadu).
Agraphydrus rugosus Komarek, 2018
Agraphydrus rugosus Komarek, 2018: 160 – India, Tamil Nadu, Nilgiris District, Nilgiri Hills, 15 km SE Kotagiri (town), Kunjapanai (village), ca. 11°22'N, 76°56'E.
Distribution: Indo-Malayan: India (Kerala, Tamil Nadu).
Agraphydrus sarawakensis Komarek, 2019
Agraphydrus sarawakensis Komarek, 2019: 232 – Malaysia, Sarawak, Kapit Division, Kapit District, 25 km E of Kapit.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus schoedli Komarek, 2019
Agraphydrus schoedli Komarek, 2019: 233 – Indonesia, North Sumatra Province, Toba Samosir Regency, Lumban Julu.
Distribution: Indo-Malayan: Indonesia (Sumatra).
Agraphydrus schoenmanni Komarek & Hebauer, 2018
Agraphydrus schoenmanni Komarek & Hebauer, 2018: 56 – China, Yünnan Prov., Xishuangbanna Dai Autonomous Prefecture, Mengla County, Menglun Town, near Mangmo Village, road Menglun–Ganlanba, ca. 15 km W Menglun.
Distribution: Indo-Malayan: China (Yunnan).
Agraphydrus scintillans Komarek, 2019
Agraphydrus scintillans Komarek, 2019: 235 – Vietnam, Vĩnh Phúc Province, Tam Đảo.
Distribution: Indo-Malayan: Vietnam.
Agraphydrus scutifer Komarek, 2020
Agraphydrus scutifer Komarek, 2020: 165 – Madagascar, Fianarantsoa Province, Haute Matsiatra Region, Andringitra N.P., Amboahisy River, 22°7'54"S, 46°53'30"E.
Distribution: Afrotropical: Madagascar.
Agraphydrus setifer Komarek & Hebauer, 2018
Agraphydrus setifer Komarek & Hebauer, 2018: 57 – Vietnam, Lào Cai Prov., Cat Cat, near Sa Pa, 22°19'43"N, 103°50'E; Komarek 2019: 236 [taxonomic treatment].
Distribution: Indo-Malayan: China (Yunnan), Vietnam.
Agraphydrus shaverdoae Komarek, 2019
Agraphydrus shaverdoae Komarek, 2019: 236 – Myanmar, Shan State, Taunggyi District, NW Kalaw (town), km 23 on road between Kalaw and Thazi, 20°42'22.68"N, 96°30'13.08"E.
Distribution: Indo-Malayan: Myanmar, Thailand.
Agraphydrus siamensis (Hansen, 1999)
Megagraphydrus siamensis Hansen, 1999a: 140 – Thailand, “Prae Siam”; Hansen 1999b: 158 [checklist]; Hebauer 2000: 18 [checklist].
Agraphydrus (Agraphydrus) siamensis (Hansen, 1999); Minoshima et al. 2015: 33 [new combination; redescription].
Agraphydrus siamensis (Hansen, 1999); Komarek 2019: 238 [taxonomic treatment].
Distribution: Indo-Malayan: Thailand.
Agraphydrus sipekorum Komarek, 2018
Agraphydrus sipekorum Komarek, 2018: 161 – India, Meghalaya, East Khasi Hills District, 11 km SW Cherrapunjee, Laitkynsew, 25°12'48"N, 91°39'48"E.
Distribution: Indo-Malayan: India (Meghalaya).
Agraphydrus skalei Komarek, 2019
Agraphydrus skalei Komarek, 2019: 239 – Indonesia, West Papua Province, Raja Ampat Regency, Waigeo Island, Lopintol, Rowery River, ca. 0°7'S, 130°53'E.
Distribution: Australasian: Indonesia (Waigeo Island).
Agraphydrus spadix Komarek, 2019
Agraphydrus spadix Komarek, 2019: 240 – Thailand, Kanchanaburi Province, Sangkhla Buri District, Thung Yai Naresuan Wildlife Sanctuary.
Distribution: Indo-Malayan: Thailand.
Agraphydrus spinosus Komarek, 2019
Agraphydrus spinosus Komarek, 2019: 241 – Malaysia, Selangor, Gombak District, Rawang Subdistrict, Templer Park.
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus splendens Komarek & Hebauer, 2018
Agraphydrus splendens Komarek & Hebauer, 2018: 58 – Laos, Saisombun Special Zone, Mount Phu Bia.
Distribution: Indo-Malayan: China (Yunnan), Laos.
Agraphydrus stagnalis (d’Orchymont, 1937)
Helochares (Agraphydrus) stagnalis d’Orchymont, 1937c: 37 – Pakistan, Punjab, Salt Range, Khewra Gorge.
Agraphydrus (Agraphydrus) stagnalis d’Orchymont, 1937; Hansen 1999b: 157 [new combination]; Hebauer 2002a: 22 [new record]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 60 [catalog].
Agraphydrus stagnalis (d’Orchymont, 1937); Komarek 2018: 162 [new records].
Distribution: Indo-Malayan: Bhutan, India (Himachal, Uttar, Uttarakhand), Nepal. Palearctic: Pakistan.
Agraphydrus stramineus Komarek, 2019
Agraphydrus stramineus Komarek, 2019: 242 – Malaysia, Sarawak, Miri Division, 30 km S Miri, Lambir Hills National Park.
Distribution: Indo-Malayan: Malaysia (Borneo).
Agraphydrus sucineus Komarek, 2019
Agraphydrus sucineus Komarek, 2019: 244 – Malaysia, Pahang, Taman Negara N.P., surroundings of Nusa Camp.
Distribution: Indo-Malayan: Malaysia (Peninsula).
Agraphydrus sundaicus Komarek, 2019
Agraphydrus sundaicus Komarek, 2019: 245 – Indonesia, West Sumatra Province, Padang City, 25 km E Padang, Taman Raya Bung Hatta Nature Reserve.
Distribution: Indo-Malayan: Indonesia (Java, Sumatra).
Agraphydrus tamdao Komarek, 2019
Agraphydrus tamdao Komarek, 2019: 246 – Vietnam, Vĩnh Phúc Province, Tam Đảo.
Distribution: Indo-Malayan: Vietnam.
Agraphydrus taprobanensis Komarek, 2018
Agraphydrus taprobanensis Komarek, 2018: 164 – Sri Lanka, Sabaragamuwa Province, Ratnapura District, Ratnapura (city).
Distribution: Indo-Malayan: Sri Lanka.
Agraphydrus tenuipalpis Komarek & Freitag, 2020
Agraphydrus tenuipalpis Komarek & Freitag, 2020: 216 – Philippines, Leyte Island and Province, Baybay Municipality, secondary forest near Visayas State University, ca. 10°45'N, 124°48'E, ca. 100–200 m a.s.l.
Distribution: Indo-Malayan: Philippines (Leyte, Mindanao).
Agraphydrus thaiensis Minoshima, Komarek & Ôhara, 2015
Agraphydrus (Agraphydrus) thaiensis Minoshima, Komarek & Ôhara, 2015: 56 – Thailand, Songkhla Province, Ton Nga Chang Wildlife Sanctuary.
Agraphydrus thaiensis Minoshima, Komarek, and Ôhara; Komarek 2019: 247 [taxonomic treatment].
Distribution: Indo-Malayan: Thailand.
Agraphydrus tristis Komarek, 2019
Agraphydrus tristis Komarek, 2019: 248 – Myanmar, Mandalay Region, Pyin Oo Lwin District, Mogok Township, S Panlin village, west slope of Mt. Taung Mae, ca. 22°58'9"N, 96°27'11"E.
Distribution: Indo-Malayan: Myanmar.
Agraphydrus tulipa Komarek, 2019
Agraphydrus tulipa Komarek, 2019: 250 – Thailand, Chiang Mai Province, Chiang Dao District, Doi (Luang) Chiang Dao (mountain).
Distribution: Indo-Malayan: Thailand.
Agraphydrus tumidus Komarek, 2020
Agraphydrus tumidus Komarek, 2020: 166 – Madagascar, Toliara Province, Anosy Region, Tsimelahy, Antarantsa River, ca. 1 km upstream from village.
Distribution: Afrotropical: Madagascar.
Agraphydrus tumulosus Komarek, 2018
Agraphydrus tumulosus Komarek, 2018: 165 – India, Kerala, Pathanamthitta District, Cardamom Hills, 50 km NW Pathanamthitta, Pambaiyar River, 9°25'N, 77°5'E.
Distribution: Indo-Malayan: India (Kerala).
Agraphydrus umbrosus Komarek & Hebauer, 2018
Agraphydrus umbrosus Komarek & Hebauer, 2018: 59 – China, Fujian Prov., Jianyuan Prefecture, Yong’an City Region, ca. 20 km SE Yong’an City, 5 km SW Xiyang Village, Ziyungdong Shan.
Distribution: Indo-Malayan: China (Fujian, Guangdong).
Agraphydrus uncinatus Komarek & Hebauer, 2018
Agraphydrus uncinatus Komarek & Hebauer, 2018: 60 – China, Yünnan Prov., Xishuangbanna Dai Autonomous Prefecture, Mengla County, along Mengla–Mengyüan road, ca. 6 km NW Mengla.
Distribution: Indo-Malayan: China (Yunnan).
Agraphydrus usambaraensis Komarek, 2020
Agraphydrus usambaraensis Komarek, 2020: 168 – Tanzania, Tanga Region, East Usambara Mountains, Amani, Sigi River.
Distribution: Afrotropical: Tanzania.
Agraphydrus uvaensis (Hebauer, 2000)
Megagraphydrus uvaensis Hebauer, 2000: 17 – Sri Lanka [Ceylon], Prov. of Uva, Gampaha Estate, 9 miles W Badulla; Short and Hebauer 2006: 337 [catalog].
Agraphydrus (Agraphydrus) uvaensis (Hebauer, 2000); Minoshima et al. 2015: 36 [new combination; redescription].
Agraphydrus uvaensis (Hebauer, 2000); Komarek 2018: 166 [redescription].
Distribution: Indo-Malayan: Sri Lanka.
Agraphydrus vadoni Komarek, 2020
Agraphydrus vadoni Komarek, 2020: 193 – Analanjirofo Region, Toamasina Province, Maroantsetra.
Distribution: Afrotropical: Madagascar.
Agraphydrus variabilis Komarek & Hebauer, 2018
Agraphydrus variabilis Komarek & Hebauer, 2018: 61 – China, Hong Kong, Lantau Island, Pak Kung Au, NW Cheung Sha; Angus et al. 2020: 19 [karyotype].
Distribution: Indo-Malayan: China (Fujian, Guangdong, Guangxi, Guizhou, Hong Kong, Hunan, Jiangxi, Yunnan, Zhejiang). Palearctic: China (Anhui, Gansu, Hubei, Shaanxi, Shandong, Sichuan, Taiwan).
Agraphydrus vietnamensis Komarek, 2019
Agraphydrus vietnamensis Komarek, 2019: 251 – Vietnam, Lâm Đồng Province, 14 km SW Bao Loc.
Distribution: Indo-Malayan: Vietnam.
Agraphydrus villiersi (Balfour-Browne, 1958)
Helochares (Gymnhelochares) villiersi Balfour-Browne, 1958a: 184 – Ivory Coast, Tonkoui.
Agraphydrus (Gymnhelochares) villiersi (Balfour-Browne, 1958); Hansen 1999b: 157 [new combination]; Hebauer 2006a: 27 [checklist; new records].
Agraphydrus villiersi (Balfour-Browne, 1958); Komarek 2020: 194 [redescription, new and corrected records].
Distribution: Afrotropical: Guinea [French Guinea], Ivory Coast, Nigeria (prior records in Cameroon and Gabon are erroneous).
Agraphydrus wangmiaoi Komarek & Hebauer, 2018
Agraphydrus wangmiaoi Komarek & Hebauer, 2018: 63 – China, Hainan Prov., Ledong County, Jianfeng Mountains, ca. 5 km E Tian Chi Village.
Distribution: Indo-Malayan: China (Hainan).
Agraphydrus yunnanensis Komarek & Hebauer, 2018
Agraphydrus yunnanensis Komarek & Hebauer, 2018: 64 – China, Yünnan Prov., Xishuangbanna Dai Autonomous Prefecture, Mengla County, ca. 50 km SSE Menglun, Mengyüan.
Distribution: Indo-Malayan: China (Yunnan).
Agraphydrus zetteli Komarek & Freitag, 2020: 221
Agraphydrus (Agraphydrus) cf. orientalis (d’Orchymont, 1932); Freitag and Zettel 2013: 19, 30.
Agraphydrus zetteli Komarek & Freitag, 2020: 221 – Philippines, Mindoro Island, Province Oriental Mindoro, Victoria Municipality, Barangay Malayas, Malayas Creek (Lake Naujan affluent) flowing through secondary vegetation, ca. 20 m a.s.l., ca. 13°9'26"N, 121°18'29"E.
Distribution: Indo-Malayan: Philippines (Busuanga, Leyte, Luzon, Mindoro, Negros, Panay, Samar, Sibuyan).
Aulonochares Girón & Short, 2019
Aulonochares lingulatus Girón & Short, 2019
Aulonochares lingulatus Girón & Short, 2019: 119 – Suriname, Sipaliwini District; 2.97731N, 55.38500W; Camp 4 (low), Kasikasima; sandy stream on trail to METS camp.
Distribution: Neotropical: French Guiana, Suriname.
Aulonochares novoairensis Girón & Short, 2019
Aulonochares novoairensis Girón & Short, 2019: 119 – Brazil, Amazonas: Novo Airão; 2°41'2.2878"S, 60°56'18.24"W.
Distribution: Neotropical: Brazil (Amazonas).
Aulonochares tubulus Girón & Short, 2019
Aulonochares tubulus Girón & Short, 2019: 120 – Suriname, Sipaliwini District; 2°00.342'N, 55°58.149'W; 337 m; Sipaliwini Savanna nature Res., 4-Brothers Mts.
Distribution: Neotropical: Brazil (Roraima), Guyana, Suriname, Venezuela.
Batochares Hansen, 1991
Batochares burgeoni (d’Orchymont, 1939)
Helochares (Batochares) burgeoni d’Orchymont, 1939b: 293 – Democratic Republic of the Congo [Congo Belge], Haut Uélé, Moto; Balfour-Browne 1950b: 54 [faunistic treatment]; Hebauer 1996: 10 [taxonomic treatment]; Hansen 1999b: 172 [catalog]; Hebauer 2006a: 27 [checklist, new records].
Batochares burgeoni (d’Orchymont, 1939); Short et al. 2021: 11 [new combination].
Distribution: Afrotropical: Burundi/Rwanda, Democratic Republic of the Congo [Congo Belge; Zaire], Guinea, Kenya, Republic of the Congo [Congo/Brazzaville], Uganda.
Batochares byrrhus (d’Orchymont, 1939)
Helochares (Batochares) byrrhus d’Orchymont, 1939b: 294 – Democratic Republic of the Congo [Congo Belge], Mayumbe, Sanzulu; Hebauer 1996: 10 [taxonomic treatment]; Hansen 1999b: 172 [catalog]; Hebauer 2006a: 27 [checklist, new records].
Batochares byrrhus (d’Orchymont, 1939); Short et al. 2021: 11 [new combination].
Distribution: Afrotropical: Central African Republic, Democratic Republic of the Congo [Congo Belge; Zaire], Gabon, Republic of the Congo [Congo/Brazzaville].
Batochares corrugatus (Balfour-Browne, 1958)
Helochares (Batochares) corrugatus Balfour-Browne, 1958a: 183 – Guinea, Mount Nimba, “Camp de Ya”; Hebauer 1996: 10 [taxonomic treatment]; Hansen 1999b: 172 [catalog]; Hebauer 2006a: 27 [checklist].
Batochares corrugatus (Balfour-Browne, 1958); Short et al. 2021: 11 [new combination].
Distribution: Afrotropical: Guinea.
Chasmogenus Sharp, 1882
Chasmogenus acuminatus Smith & Short, 2020
Chasmogenus acuminatus Smith & Short, 2020: 32 – Suriname: Sipaliwini District 2°21.776'N, 56°41.861'W, 237 m, Camp 3 Wehepai.
Chasmogenus sp. X Short 2013: 87 (in part); Short and Kadosoe 2011: 87 (in part); Short et al. 2018: 193 (in part).
Distribution: Neotropical: Brazil (Amapá, Pará), French Guiana, Guyana, Suriname.
Chasmogenus amplius Smith & Short, 2020
Chasmogenus amplius Smith & Short, 2020: 35 – Venezuela, Amazonas State, 4°58.838'N, 67°44.341'W; 95m, Comunidad Caño Gato, on Rio Sipapo.
Distribution: Neotropical: Venezuela.
Chasmogenus australis García, 2000
Chasmogenus australis García, 2000a: 52 – Venezuela, Apure, Samán de Apure, Achaguas, 50 km NW of San Fernando de Apure; Short and Hebauer 2006: 331 [catalog]; Smith and Short 2020: 37 [new records].
Distribution: Neotropical: Brazil (Roraima), French Guiana, Guyana, Venezuela.
Chasmogenus bariorum García, 2000
Chasmogenus bariorum García, 2000a: 49 – Venezuela, Zulia, Machiques de Perijá, Misión Angeles de Tukuko, El Manantial, 36 km SW of Machiques; Short and Hebauer 2006: 331 [catalog]; Smith and Short 2020: 40 [taxonomic treatment].
Chasmogenus occidentalis García, 2000a: 49; Venezuela, Zulia, Machiques de Perijá, Misión Angeles de Tukuko, El Manantial, 35 km SW of Machiques; Short and Hebauer 2006: 331 [catalog]; Smith and Short 2020: 40 [synonym].
Chasmogenus yukparum García, 2000a: 50 – Venezuela, Zulia, Machiques de Perijá, Misión Angeles de Tukuko, El Manantial, 35 km SW of Machiques; Short and Hebauer 2006: 331 [catalog]; Smith and Short 2020: 40 [synonym].
Distribution: Neotropical: Venezuela.
Chasmogenus barrae Short, 2005
Chasmogenus barrae Short, 2005: 194 – Costa Rica, Guanacaste Prov. road to Barra Honda National Park, 6.6 km after junction with route 13; Short and Hebauer 2006: 331 [catalog].
Distribution: Neotropical: Costa Rica.
Chasmogenus berbicensis Smith & Short, 2020
Chasmogenus berbicensis Smith & Short, 2020: 47 – Guyana, Region 6, 4°08.809'N, 58°14.232'W, Upper Berbice, Basecamp 1, margin of Berbice river.
Chasmogenus sp. B Short et al. 2018: 193.
Distribution: Neotropical: Guyana.
Chasmogenus brownsbergensis Smith & Short, 2020
Chasmogenus brownsbergensis Smith & Short, 2020: 48 – Suriname, Brokopondo District, 04°56.871'N, 55°10.911'W, 462 m, Brownsberg Nature Park.
Distribution: Neotropical: Suriname.
Chasmogenus cajuina Alves, Clarkson & Lima, 2020
Chasmogenus cajuina Alves, Clarkson & Lima, 2020: 580 – Brazil, Piauí, Castelo do Piaui, Cachoeira das Arraias, 5°11'28.5"S, 41°42'03.2"W.
Distribution: Neotropical: Brazil (Piauí).
Chasmogenus castaneus Smith & Short, 2020
Chasmogenus castaneus Smith & Short, 2020: 50 – Venezuela, Zulia State, 09°50.490'N, 72°49.310'W, 270m, Perijá National Park, Tukuko, Rio Manantial.
Distribution: Neotropical: Venezuela.
Chasmogenus clavijoi Smith & Short, 2020
Chasmogenus clavijoi Smith & Short, 2020: 53 – Venezuela, Guárico State, 8°8.296'N, 66°24.459'W, San Nicolasito Field Station.
Distribution: Neotropical: Venezuela.
Chasmogenus cremnobates (Spangler, 1979)
Dieroxenus cremnobates Spangler, 1979: 754 – Ecuador, Napo, Baeza, 72 km E; Hansen 1999: 173 [catalog].
Chasmogenus cremnobates (Spangler, 1979); Girón and Short 2018: 155 [new combination].
Distribution: Neotropical: Ecuador.
Chasmogenus cuspifer Smith & Short, 2020
Chasmogenus cuspifer Smith & Short, 2020: 54 – Venezuela, Zulia State, 9°50.490'N, 72°49.310'W, 270 m, Perijá N.P. Tukuko, Río Manantial.
Distribution: Neotropical: Venezuela.
Chasmogenus flavomarginatus Smith & Short, 2020
Chasmogenus flavomarginatus Smith & Short, 2020: 55 – Venezuela, Barinas State, 8°48.424'N, 70°31.139'W, 992m, ca. 13km NW Barinitas.
Distribution: Neotropical: Venezuela.
Chasmogenus fluminensis Clarkson & Ferreira-Jr, 2014
Chasmogenus fluminensis Clarkson & Ferreira-Jr, 2014b: 484 – Brazil Rio de Janeiro, Rio de Janeiro, Parque Nacional da Tijuca, 22°58'13"S, 43°15'25"W.
Distribution: Neotropical: Brazil (Rio de Janeiro).
Chasmogenus fragilis Sharp, 1882
Chasmogenus fragilis Sharp, 1882: 73 – Guatemala, San Gerónimo; Fernández, 1986: 190 [lectotype designation; redescription]; Hansen 1999b: 174 [catalog]; Short 2005: 195 [taxonomic treatment].
Helochares (Chasmogenus) fragilis (Sharp, 1882); Knisch 1924a: 195 [catalog].
Chasmogenus (Chasmogenus) fragilis (Sharp, 1882); Hebauer 1992: 84 [taxonomic treatment].
Distribution: Neotropical: Guatemala, Panama.
Chasmogenus gato Smith & Short, 2020
Chasmogenus gato Smith & Short, 2020: 56 – Venezuela, Amazonas State, 4°58.838'N, 67°44.341'W, 95m, Comunidad Caño Gato on Rio Sipapo.
Distribution: Neotropical: Venezuela.
Chasmogenus guianensis Smith & Short, 2020
Chasmogenus guianensis Smith & Short, 2020: 58 – Suriname, Sipaliwini District, 2.47700°N, 55.62941°W, 275 m, Camp 1, Upper Palumeu.
Chasmogenus sp. X Short 2013: 87 (in part); Short and Kadosoe 2011: 87 (in part).
Distribution: Neotropical: Guyana, Suriname.
Chasmogenus ignotus Smith & Short, 2020
Chasmogenus ignotus Smith & Short, 2020: 60 – Brazil, Amazonas, Manaus, -2.93079, -59.97514, 75 m, Ducke Reserve, near Station.
Distribution: Neotropical: Brazil (Amazonas).
Chasmogenus itatiaia Clarkson & Ferreira-Jr, 2014
Chasmogenus itatiaia Clarkson & Ferreira-Jr, 2014b: 487 – Brazil – Rio de Janeiro, Itatiaia, Parque Nacional de Itatiaia, Poça no caminho das Agulhas Negras, 22°23'05.4"S, 44°40'41.7"W.
Distribution: Neotropical: Brazil (Minas Gerais, Rio de Janeiro).
Chasmogenus ligulatus Smith & Short, 2020
Chasmogenus ligulatus Smith & Short, 2020: 61 – Suriname, Sipaliwini District, 2.97731N, 55.38500W, 200 m, Camp 4 (low), Kasikasima.
Chasmogenus sp. X Short 2013: 87 (in part).
Distribution: Neotropical: Suriname.
Chasmogenus lilianae Clarkson & Ferreira-Jr, 2014
Chasmogenus lilianae Clarkson & Ferreira-Jr, 2014b: 489 – Brazil, Rio de Janeiro, Nova Friburgo, Macaé de Cima, Tributário de 1a Ordem do Rio Macaé, Casa amarela, campo das hortênsias.
Distribution: Neotropical: Brazil (Rio de Janeiro).
Chasmogenus lineatus Smith & Short, 2020
Chasmogenus lineatus Smith & Short, 2020: 64 – Venezuela, Guárico State, 9°46.320'N, 67°21.177'W, 280m, Río San Antonio, N. Dos Caminos.
Distribution: Neotropical: Venezuela.
Chasmogenus lorenzo Short, 2005
Chasmogenus lorenzo Short, 2005: 195; Costa Rica – Alajuela Province, small stream near Rio San Lorenzo, 6km from Los Lagos; Short and Hebauer 2006: 331 [catalog].
Distribution: Neotropical: Costa Rica.
Chasmogenus pandus Smith & Short, 2020
Chasmogenus pandus Smith & Short, 2020: 68 – Suriname, Para District, Zanderij, near Guesthouse, 05°27.5'N, 055°13.0'W.
Distribution: Neotropical: Brazil (Amapá), French Guiana, Suriname.
Chasmogenus rufinasus (Knisch, 1924)
Helochares (Chasmogenus) rufinasus Knisch, 1924c: 124 – Ecuador (Guayaquil).
Chasmogenus rufinasus (Knisch, 1924); Fernández 1986: 193 [new combination; taxonomic treatment]; Hansen 1999b: 175 [catalog].
Distribution: Neotropical: Ecuador.
Chasmogenus ruidus Short, 2005
Chasmogenus ruidus Short, 2005: 196 – Costa Rica, Limón Province, Sector Cerro Cocori, Farm of Elias Rojas, A. C. Tortuguero; Short and Hebauer 2006: 331 [catalog].
Distribution: Neotropical: Costa Rica.
Chasmogenus sapucay Fernández, 1986
Chasmogenus sapucay Fernández, 1986: 192 – Paraguay, Sapucay; Hansen 1999b: 176 [checklist]; Clarkson and Ferreira-Jr 2014b: 492 [new record].
Distribution: Neotropical: Argentina, Brazil (Pará, Rio de Janeiro), Paraguay.
Chasmogenus schmits Smith & Short, 2020
Chasmogenus schmits Smith & Short, 2020: 69 – Suriname, Sipaliwini District, 2°10.521'N, 56°47.244'W, 228 m, on Kutari River.
Chasmogenus sp. X Short and Kadosoe 2011: 87 (in part).
Distribution: Neotropical: Suriname.
Chasmogenus schoedli Short, 2005
Chasmogenus schoedli Short, 2005: 197 – Costa Rica, Guanacaste, 9 km S Santa Cecilia, Pitilla Station; Short and Hebauer 2006: 331 [catalog].
Distribution: Neotropical: Costa Rica.
Chasmogenus sinnamarensis Smith & Short, 2020
Chasmogenus sinnamarensis Smith & Short, 2020: 70 – French Guyana, Road Petit Saut, Crique Eau Claire.
Distribution: Neotropical: French Guyana.
Chasmogenus tafelbergensis Smith & Short, 2020
Chasmogenus tafelbergensis Smith & Short, 2020: 71 – Suriname, Sipaliwini District, 3°55.600'N, 56°11.300'W, 600 m, CSNR: Tafelberg Summit, nr Augustus Creek Camp, pools & creeks on trail into Arrowhead basin.
Distribution: Neotropical: Suriname.
Chasmogenus ubatuba Clarkson & Ferreira-Jr, 2014
Chasmogenus ubatuba Clarkson & Ferreira-Jr, 2014b: 491 – Brasil, São Paulo, Ubatuba, Parque Estadual da Serra do Mar, Núcleo Picinguaba.
Distribution: Neotropical: Brazil (São Paulo).
Chasmogenus undulatus Smith & Short, 2020
Chasmogenus undulatus Smith & Short, 2020: 73 – Guyana, Region VIII, 5°18.261'N, 59°50.257'W, 687 m, Ayanganna Airstrip, trail from airstrip to Ayanganna.
Chasmogenus sp. A Short et al. 2018: 193.
Distribution: Neotropical: Guyana.
Colossochares Girón & Short, gen. nov.
Colossochares ellipticus (d’Orchymont, 1933) comb. nov.
Helochares ellipticus Régimbart, 1907: 47 – Gabon, Lambarené, Cape Lopez, Rembo Nkomi; [misinterpretation of Hydrophilus ellipticus Fabricius, 1801].
Helochares ellipticus Régimbart, 1907; d’Orchymont 1933: 306 [new name]; Hebauer 2003: 129.
Helochares (s. str.) ellipticus d’Orchymont, 1933; Hansen 1999b: 160 [catalog].
Helochares (s. str.) ellipticus Régimbart, 1907; Balfour-Browne 1950b: 59 [faunistic treatment]; Hebauer 1996: 6 [taxonomic treatment]; Hebauer 2006a: 25 [checklist].
Distribution: Afrotropical: Benin, Burkina Faso, Cameroon, Democratic Republic of the Congo, Ethiopia, Gabon, Ghana, Guinea, Ivory Coast, Liberia, Nigeria, Republic of the Congo, Uganda.
Colossochares satoi (Hebauer, 2003) comb. nov.
Helochares (s. str.) satoiHebauer 2003a: 129 – Malawi: “Balaka env.”; Hebauer 2005: 39; Hebauer 2006a: 25 [checklist]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Malawi.
Crephelochares Kuwert, 1890
Crephelochares abnormalis (Sharp, 1890)
Philydrus abnormalis Sharp, 1890: 351 – Sri Lanka, Colombo [“Ceylon: Colombo”]; [specific rank confirmed by d’Orchymont 1937d: 7; not synonym of livornicus Kuwert, as in d’Orchymont 1925: 70].
Helochares (Chasmogenus) abnormalis (Sharp, 1890); Knisch 1921: 68 [catalog].
Helochares (Crephelochares) abnormalis (Sharp, 1890); d’Orchymont 1937d: 7 [checklist]; d’Orchymont 1939a: 159 [taxonomic treatment].
Chasmogenus (Crephelochares) abnormalis (Sharp, 1890); Hebauer 1992: 68 [taxonomic treatment].
Enochrus (Lumetus) abnormicollis (Sharp, 1890); Zaitzev 1908: 385 [catalog – error for abnormalis Sharp, 1890].
Phylhydrus ferrugatus Régimbart, 1903b: 57 – Vietnam [“Cochinchine”] (My Tho); Indonesia (Sumatra); d’Orchymont 1939a: 159 [synonymy; not synonym of livornicus Kuwert, as in d’Orchymont 1925: 70).
Enochrus (Lumetus) ferrugatus Régimbart, 1903; Zaitzev 1908: 386 [catalog].
Helochares (Chasmogenus) ferrugatus Régimbart, 1903; Knisch 1924a: 195 [catalog].
Philhydrus nigritulus Régimbart, 1903b: 57 – Vietnam (Ho Chi Minh [“Saigon”], My Tho); Cambodia (Phnom Penh); Indonesia (Sumatra); Knisch 1924a: 195 [transferred to Helochares, thereby becoming a junior secondary homonym of Helochares nigritulus Kuwert, 1889]. Permanently invalid: replaced before 1961 (ICZN Code Art. 59b); d’Orchymont 1939a: 159 [synonymy].
Enochrus (Lumetus) nigritulus Régimbart, 1903; Zaitzev 1908a: 388 [catalog].
Helochares (Chasmogenus) regimbarti Knisch, 1924a: 195 (replacement name for nigritulus Régimbart); d’Orchymont 1939a: 159 [synonymy].
Chasmogenus abnormalis (Sharp, 1890); Gentili et al. 1995: 210 [checklist]; Hansen 1999b: 173 [catalog]; Hansen 2004: 49 [checklist]; Hebauer and Ryndevich 2005: 46 [new record]; Fikáček et al. 2015: 61 [catalog]; Devi et al. (2016) [redescription; lectotype designation]; Jia and Tang 2018a: 63 [new record].
Crephelochares abnormalis (Sharp, 1890); Short et al. 2021: 12 [new combination].
Distribution: Indo-Malayan: Cambodia, China (Guangdong, Taiwan), Indonesia (Borneo, Java, Sulawesi, Sumatra), Laos, Sri Lanka, Thailand, Vietnam. Palearctic: Japan.
Crephelochares africanus (d’Orchymont, 1937)
Helochares (Crephelochares) africanus d’Orchymont, 1937d: 7 – Mozambique, Nova Chupanga nr Chemba; d’Orchymont 1939a: 163 [taxonomic treatment]; Balfour-Browne 1950b: 58 [faunistic treatment].
Chasmogenus (Crephelochares) africanus (d’Orchymont, 1937); Hebauer 1992: 69 [taxonomic treatment]; Hebauer 1995a: 265 [faunistic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus africanus (d’Orchymont, 1937); Hansen 1999b: 174 [catalog].
Crephelochares africanus (d’Orchymont, 1937); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Botswana, Cameroon, Democratic Republic of the Congo, Gambia, Ghana, Guinea, Mozambique, Namibia, Niger, Nigeria, Senegal, Republic of South Africa, Sudan, Uganda, Zimbabwe.
Crephelochares balkei (Short, 2010)
Chasmogenus balkei Short, 2010: 301 – Fiji (Vanua Levu); Short and Fikáček 2011: 89 [catalog].
Crephelochares balkei (Short); Short et al. 2021: 12 [new combination].
Distribution: Australasian: Fiji (Vanua Levu).
Crephelochares cattienus (Hebauer, 2002)
Chasmogenus cattienus Hebauer, 2002b: 9 – Vietnam, S Cát Tiên, 120 km NNE Ho Chi Minh, Cát Tiên National Park.
Crephelochares cattienus (Hebauer, 2002); Short et al. 2021: 12 [new combination].
Distribution: Indo-Malayan: Vietnam.
Crephelochares irianus (Hebauer, 2001)
Chasmogenus irianus Hebauer, 2001a: 15 – Indonesia, Papua [West New Guinea], Fak-Fak, IR 27, Kali Mati 4 km N of Fak-Fak.
Crephelochares irianus (Hebauer, 2001); Short et al. 2021: 12 [new combination].
Distribution: Indo-Malayan: Indonesia (Papua).
Crephelochares larsi (Hebauer, 1995)
Chasmogenus (Crephelochares) larsi Hebauer, 1995b: 8 – Malaysia, Cameron Highlands, Tanah Rata, G. Jasar track 11.
Chasmogenus larsi Hebauer, 1995; Hansen 1999b: 174 [catalog].
Crephelochares larsi (Hebauer, 1995); Short et al. 2021: 12 [new combination].
Distribution: Indo-Malayan: Malaysia (Peninsula).
Crephelochares livornicus (Kuwert, 1890)
Helochares (Crephelochares) livornicus Kuwert, 1890a: 38 – Italy, Livorno; Heyden 1891: 67 [catalog]; d’Orchymont 1939a: 158 [taxonomic treatment].
Crephelochares livornicus (Kuwert, 1890); Kuwert 1890b: 327 (also as “n. sp.”).
Helochares (Crepidelochares) livornicus Kuwert, 1890; Ganglbauer 1904: 248 [faunistic treatment].
Helochares (Chasmogenus) livornicus Kuwert, 1890; Knisch 1924a: 195 [catalog]; d’Orchymont 1925: 70 [taxonomic treatment]; d’Orchymont 1928: 106 [faunistic treatment].
Chasmogenus (Crephelochares) livornicus (Kuwert, 1890); Hebauer 1992: 70 [taxonomic treatment]
Chasmogenus livornicus (Kuwert, 1890); Hebauer 1994: 111 [faunistic treatment]; Hansen 1999b: 174 [catalog]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 61 [catalog].
Distribution: Palearctic: Bosnia, Croatia, Greece, Israel, Italy, Serbia and Montenegro, Spain, Tunisia, Turkey.
Crephelochares luctuosus (d’Orchymont, 1939)
Helochares (Crephelochares) luctuosus d’Orchymont, 1939a: 164 – Gabon; Hebauer 1988: 157 [faunistic treatment].
Chasmogenus (Crephelochares) luctuosus (d’Orchymont, 1939); Hebauer 1992: 71 [taxonomic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus luctuosus (d’Orchymont, 1939); Hansen 1999: 174 [catalog].
Crephelochares luctuosus (d’Orchymont, 1939); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Cameroon, Democratic Republic of the Congo (in doubt, Hebauer 2006a: 27), Gabon, Ghana (in doubt, Hebauer 2006a: 27), Guinea, Namibia, Senegal.
Crephelochares lycetus (d’Orchymont, 1939)
Helochares (Crephelochares) lycetus d’Orchymont, 1939a: 163; Kenya [“Afrique orientale anglaise”], Taveta.
Chasmogenus (Crephelochares) lycetus (d’Orchymont, 1939); Hebauer 1992: 72 [taxonomic treatment]; Hebauer 1995a: 266 [faunistic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus lycetus (d’Orchymont, 1939); Hansen 1999: 174 [catalog].
Crephelochares lycetus (d’Orchymont, 1939); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Angola, Benin, Botswana, Kenya, Namibia, Republic of South Africa, Tanzania, Zambia, Zimbabwe.
Crephelochares mauritiensis (Balfour-Browne, 1958)
Helochares (Crephelochares) mauritiensis Balfour-Browne, 1958b: 143 – Mauritius, Les Mares.
Chasmogenus (Crephelochares) mauritiensis (Balfour-Browne, 1958); Hebauer 1992: 72 [taxonomic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus mauritiensis (Balfour-Browne, 1958); Hansen 1999: 174 [catalog].
Crephelochares mauritiensis (Balfour-Browne, 1958); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Mauritius.
Crephelochares molinai (Hebauer, 1992)
Chasmogenus (Crephelochares) molinai Hebauer, 1992: 73 – Congo, Loudima; Hebauer 1995a: 266 [faunistic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus molinai Hebauer, 1992; Hansen 1999: 174 [catalog].
Crephelochares molinai (Hebauer, 1992); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Democratic Republic of the Congo, Namibia.
Crephelochares mollis (Régimbart, 1903)
Philhydrus mollis Régimbart, 1903a: 32 – Madagascar, “Baie d’Antongil; pays Androy”; (specific rank confirmed by d’Orchymont, 1937d: 7; not synonym of abnormalis Sharp, as in Scott 1913: 205; not synonym of livornicus Kuwert, as in d’Orchymont 1925: 70).
Enochrus (Lumetus) mollis (Régimbart, 1903); Zaitzev, 1908: 387 [catalog].
Helochares (Crephelochares) mollis (Régimbart, 1903); d’Orchymont, 1937d: 7; d’Orchymont 1939a: 161 [taxonomic treatment]; Hebauer 1988: 157 [faunistic treatment].
Philydrus abnormalis; Scott 1913: 205 [misinterpret. of Philydrus abnormalis Sharp]; d’Orchymont 1939a: 161 [synonymy].
Chasmogenus (Crephelochares) mollis (Régimbart, 1903); Hebauer, 1992: 74 [taxonomic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus mollis (Régimbart, 1903); Hansen 1999: 174 [catalog].
Crephelochares mollis (Régimbart, 1903); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Madagascar, Seychelles (Aldabra).
Crephelochares molluscus (Hebauer, 1992)
Chasmogenus (Crephelochares) molluscus Hebauer, 1992: 75 – Tanzania (Lake Manyara); Hebauer 2006a: 27 [checklist].
Chasmogenus molluscus Hebauer, 1992; Hansen 1999: 175 [catalog].
Crephelochares molluscus (Hebauer), 1992; Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Tanzania.
Crephelochares nitescens (Fauvel, 1883)
Philydrus nitescens Fauvel, 1883: 354 – New Caledonia (Anse Vata).
Enochrus (Lumetus) nitescens Fauvel, 1883; Zaitzev 1908: 388.
Helochares (Crephelochares) nitescens (Fauvel, 1883); d’Orchymont 1939a: 157 [taxonomic treatment].
Helochares (Chasmogenus) nitescens (Fauvel, 1883); Balfour-Browne 1945: 117 [checklist].
Helochares nitescens (Fauvel, 1883); Anderson 1976: 223 [description of immature stages].
Chasmogenus nitescens (Fauvel, 1883); Hansen 1991: 156 [examined species]; Watts 1995: 116 [lectotype designated; redescription]; Archangelsky 1997: 55 [redescription of immature stages]; Hansen 1999: 175 [catalog]; Short (2010) [new record].
Chasmogenus (Crephelochares) nitescens (Fauvel, 1883); Hebauer 1992: 75 [taxonomic treatment].
Crephelochares nitescens (Fauvel, 1883); Short et al. 2021: 12 [new combination].
Distribution: Australasian: Australia (New South Wales, Northern Territory, Queensland), Fiji (Viti Levu), New Caledonia, Papua New Guinea.
Crephelochares omissus (Hebauer, 1995)
Chasmogenus (Crephelochares) omissus Hebauer, 1995a: 266 – Namibia, East Caprivi, Mudumu National Park, Nakatwa, 18°10'S, 23°26'E; Hebauer 1995a: 266 [faunistic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus omissus Hebauer, 1995; Hansen 1999: 175 [catalog].
Crephelochares omissus (Hebauer, 1995); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Namibia.
Crephelochares orbus (Watanabe, 1987)
Helochares (Crephelochares) orbus Watanabe, 1987: 12; Japan, Honshu, Gumma-ken, Tatebayashi-shi, Hanetsuku.
Chasmogenus (Crephelochares) orbus (Watanabe, 1987); Hebauer, 1992: 76 [taxonomic treatment].
Chasmogenus orbus (Watanabe, 1987); Hansen 1999: 175 [catalog]; Hansen 2004: 49 [checklist]; Fikáček et al. 2015: 61 [catalog]; Jia and Tang 2018a: 63 [new record].
Crephelochares orbus (Watanabe, 1987); Short et al. 2021: 12 [new combination].
Distribution: Indo-Malayan: China (Hong Kong). Palearctic: Japan.
Crephelochares paramollis (Hebauer, 1992)
Chasmogenus (Crephelochares) paramollis Hebauer, 1992: 76 – Tanzania, Usa river; Hebauer 1995a: 266 [faunistic treatment; new records]; Hebauer 2006a: 27 [checklist; new records].
Chasmogenus paramollis Hebauer, 1992; Hansen 1999: 175 [catalog].
Crephelochares paramollis (Hebauer, 1992); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Cameroon, Democratic Republic of the Congo, Gabon, Ghana, Guinea, Kenya, Namibia, Republic of South Africa [Transvaal], Zambia, Zimbabwe.
Crephelochares parorbus (Jia & Tang, 2018)
Chasmogenus parorbus Jia & Tang, 2018a: 61 – China, Yünnan Prov., Yingjiang, Tongbiguan, Kaibangyahu, 24.58°N, 97.67°E.
Crephelochares parorbus (Jia & Tang); Short et al. 2021: 12 [new combination].
Distribution: Indo-Malayan: China (Yunnan).
Crephelochares patrizii (Balfour-Browne, 1948)
Helochares (Crephelochares) patrizii Balfour-Browne, 1948: 830 – Somalia [Italian Somaliland], Giuba, Belet Amin.
Chasmogenus (Crephelochares) patrizii (Balfour-Browne, 1948); Hebauer 1992: 77 [taxonomic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus patrizii (Balfour-Browne, 1948); Hansen 1999: 175 [catalog].
Crephelochares patrizii (Balfour-Browne, 1948); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Cameroon, Kenya, Mozambique, Somalia, Republic of South Africa, Sudan, Tanzania, Uganda, Zambia, Zimbabwe.
Crephelochares punctulatus (Short, 2010)
Chasmogenus punctulatus Short, 2010: 303 – Fiji, Viti Levu, Nadarivatu; Short and Fikáček 2011: 89 [checklist].
Crephelochares punctulatus (Short, 2010); Short et al. 2021: 12 [new combination].
Distribution: Australasian: Fiji (Viti Levu).
Crephelochares rhodesiensis (Hebauer, 2006)
Chasmogenus (Crephelochares) rhodesiensis Hebauer, 2006b: 18 – Zambia, Copperbelt, W of Kapiri Mposhi.
Chasmogenus rhodesiensis Hebauer, 2006; Short and Fikáček 2011: 89 [checklist].
Crephelochares rhodesiensis (Hebauer, 2006); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Zambia.
Crephelochares ruandanus (Balfour-Browne, 1957)
Helochares (Crephelochares) ruandanus Balfour-Browne, 1957: 22 – Rwanda, Kibuye.
Chasmogenus (Crephelochares) ruandanus (Balfour-Browne, 1957); Hebauer 1992: 78 [taxonomic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus ruandanus (Balfour-Browne, 1957); Hansen 1999: 175 [catalog].
Crephelochares ruandanus (Balfour-Browne, 1957); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Burundi, Kenya, Rwanda.
Crephelochares rubellus (Hebauer, 1992)
Chasmogenus (Crephelochares) rubellus Hebauer, 1992: 79 – Senegal, village Sare Sara, 21 km ESE Kolda; Hebauer 2006a: 27 [checklist].
Chasmogenus rubellus Hebauer, 1992; Hansen 1999: 175 [catalog].
Crephelochares rubellus (Hebauer, 1992); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Gambia, Senegal.
Crephelochares rubricollis (Régimbart, 1903)
Philhydrus rubricollis Régimbart, 1903b: 58 – Indonesia, Sumatra, Palembang; (specific rank confirmed by d’Orchymont, 1925: 71; not synonym of abnormalis Kuwert, as in Knisch 1921: 68).
Enochrus (Lumetus) rubricollis (Régimbart, 1903); Zaitzev 1908: 389.
Helochares (Chasmogenus) rubricollis (Régimbart, 1903); d’Orchymont 1925: 71 [taxonomic treatment].
Helochares (Crephelochares) rubricollis (Régimbart, 1903); d’Orchymont 1939a: 162 [taxonomic treatment].
Chasmogenus (Crephelochares) rubricollis (Régimbart, 1903); Hebauer 1992: 79 [taxonomic treatment].
Helochares (Chasmogenus) abnormalis Sharp, 1890; Knisch 1921: 68; misinterpret. of Philydrus abnormalis Sharp, 1890; d’Orchymont, 1939a: 162 [synonymy].
Chasmogenus rubricollis (Régimbart, 1903); Hansen 1999: 175 [catalog].
Crephelochares rubricollis (Régimbart, 1903); Short et al. 2021: 12 [new combination].
Distribution: Indo-Malayan: Indonesia (Borneo, Sumatra).
Crephelochares rudis (Hebauer, 1992)
Chasmogenus (Crephelochares) rudis Hebauer, 1992: 80 – Congo, Kindamba, Meya, Bangou forest; Hebauer 2006a: 27 [checklist].
Chasmogenus rudis Hebauer, 1992; Hansen 1999: 175 [catalog].
Crephelochares rudis (Hebauer, 1992); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Congo [Kindamba locality in both Democratic Republic of the Congo and Republic of the Congo].
Crephelochares rusticus (d’Orchymont, 1939)
Helochares (Crephelochares) rusticus d’Orchymont, 1939a: 165 – Gabon.
Chasmogenus (Crephelochares) rusticus (d’Orchymont, 1939); Hebauer 1992: 81 [taxonomic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus rusticus (d’Orchymont, 1939); Hansen 1999: 175 [catalog].
Crephelochares rusticus (d’Orchymont, 1939); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Gabon, Ghana.
Crephelochares rutiloides (d’Orchymont, 1939)
Helochares (Crephelochares) rutiloides d’Orchymont, 1939a: 323 – Gabon.
Chasmogenus (Crephelochares) rutiloides (d’Orchymont, 1939); Hebauer 1992: 82 [taxonomic treatment]; Hebauer 1995a: 266 [faunistic treatment]; Hebauer 2006a: 27 [checklist].
Chasmogenus rutiloides (d’Orchymont, 1939); Hansen 1999: 175 [catalog].
Crephelochares rutiloides (d’Orchymont, 1939); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Botswana, Cameroon, Democratic Republic of the Congo, Gabon, Gambia, Ghana, Namibia, Zambia.
Crephelochares rutilus (d’Orchymont, 1925)
Helochares (Chasmogenus) rutilus d’Orchymont, 1925a: 71. – Gabon; d’Orchymont 1939a: 163 [taxonomic treatment].
Helochares (Crephelochares) rutilus d’Orchymont, 1925; d’Orchymont 1928: 107 [faunistic treatment]; d’Orchymont 1937d: 7 [checklist].
Chasmogenus (Crephelochares) rutilus (d’Orchymont, 1925); Hebauer 1992: 82 [new combination; taxonomic treatment]; Hebauer 2006a: 27 [checklist; new records].
Chasmogenus rutilus (d’Orchymont, 1925); Hansen 1991: 156 [examined species]; Hansen 1999: 176 [catalog].
Helochares (Chasmogenus) abnormalis Sharp, 1890; Knisch 1921a: 68 [misinterpretation of Philydrus abnormalis Sharp, 1890]; d’Orchymont, 1939a: 163 [synonymy].
Crephelochares rutilus (d’Orchymont, 1925); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Cameroon, Democratic Republic of the Congo, Gabon, Ghana, Nigeria, Republic of South Africa.
Crephelochares szeli (Hebauer, 1992)
Chasmogenus (Crephelochares) szeli Hebauer, 1992: 84 – Ghana, Ashanti region, Kumashi, Nhiasu, 6°43'N, 1°36'W; Hebauer 2006a: 27 [checklist; new records].
Chasmogenus szeli Hebauer, 1992; Hansen 1999: 176 [catalog].
Crephelochares szeli (Hebauer, 1992); Short et al. 2021: 12 [new combination].
Distribution: Afrotropical: Democratic Republic of the Congo, Ghana, Liberia, Nigeria, Sierra Leone, Uganda.
Crucisternum Girón & Short, 2018
Crucisternum escalera Girón & Short, 2018
Crucisternum escalera Girón & Short, 2018: 120 – Venezuela, Bolívar State, along La Escalera, 6°2'10.5"N, 61°23'57.8"W.
Distribution: Neotropical: Venezuela.
Crucisternum ouboteri Girón & Short, 2018
Crucisternum ouboteri Girón & Short, 2018: 121 – Suriname, Sipaliwini District, Brownsberg Nature Park, 04°56.871'N, 55°10.911'W.
Distribution: Neotropical: French Guiana, Guyana, Suriname, Venezuela.
Crucisternum queneyi Girón & Short, 2018
Crucisternum queneyi Girón & Short, 2018: 123 – French Guiana, Sinnamary.
Distribution: Neotropical: French Guiana.
Crucisternum sinuatus Girón & Short, 2018
Crucisternum sinuatus Girón & Short, 2018: 124 – Brazil, Minas Gerais, Lassance, Cachoeira da Palmeira, -17.83384, -44.50515.
Distribution: Neotropical: Brazil (Minas Gerais, Pará).
Crucisternum toboganensis Girón & Short, 2018
Crucisternum toboganensis Girón & Short, 2018: 126 – Venezuela, Amazonas, Puerto Ayacucho (40 km S), El Tobogán, Caño Coromoto.
Distribution: Neotropical: Venezuela.
Crucisternum vanessae Girón & Short, 2018
Crucisternum vanessae Girón & Short, 2018: 127 – Suriname, Sipaliwini District, Central Suriname Nature Reserve: Tafelberg Summit, near Caiman Creek Camp, 3°53.942'N, 56°10.849'W.
Distribution: Neotropical: Suriname.
Crucisternum xingu Girón & Short, 2018
Crucisternum xingu Girón & Short, 2018: 131 – Brazil, Pará, Rio Xingu Camp, ca. 60 km S Altamira, 52°22'W, 3°39'S.
Distribution: Neotropical: Brazil (Pará).
Ephydrolithus Girón & Short, 2019
Ephydrolithus hamadae Girón & Short, 2019
Ephydrolithus hamadae Girón & Short, 2019: 130 – Brazil, Minas Gerais, Lassance, Cachoeira da Palmeira; 17.83384S, 44.50515W.
Distribution: Neotropical: Brazil (Minas Gerais).
Ephydrolithus minor Girón & Short, 2019
Ephydrolithus minor Girón & Short, 2019: 130 – Brazil, Bahia, Abaíra, Pico do Barbado W of Catolés, 13.29053S, 41.90489W.
Distribution: Neotropical: Brazil (Bahia).
Ephydrolithus ogmos Girón & Short, 2019
Ephydrolithus ogmos Girón & Short, 2019: 131- Brazil, Brazil, Bahia, Abaíra, Pico do Barbado W of Catolés, 13.29053S, 41.90489W.
Distribution: Neotropical: Brazil (Bahia).
Ephydrolithus spiculatus Girón & Short, 2019
Ephydrolithus spiculatus Girón & Short, 2019: 132 – Brazil, Minas Gerais, Lassance, Cachoeira da Palmeira, 17.83384S, 44.50515W.
Distribution: Neotropical: Brazil (Minas Gerais).
Ephydrolithus teli Girón & Short, 2019
Ephydrolithus teli Girón & Short, 2019: 132 – Brazil, Bahia, Abaíra, Pico do Barbado, W of Catolés; 13.29053S, 41.90489W.
Distribution: Neotropical: Brazil (Bahia, Minas Gerais).
Globulosis García, 2001
Globulosis hemisphericus García, 2001
Globulosis hemisphericus García, 2001: 156 – Venezuela, Bolívar, Municipio Sifontes, Tierra Blanca Pantano; Short et al. 2017: 275 [new records].
Globulosis hemisphaericus García [incorrect subsequent spelling]; Short and Hebauer 2006: 338 [catalog].
Globulosis sp. 1 Short and Kadosoe 2011: 89 [checklist]; Short 2013: 87 [checklist].
Distribution: Neotropical: Venezuela, Guyana, Suriname, Brazil (Amazonas, Pará).
Globulosis flavus Short, García & Girón, 2017
Globulosis flavus Short, García & Girón, 2017: 277 – Venezuela, Amazonas State, nr. Iboruwa: “Tobogancito”, 5 48.141'N, 67 26.313'W.
Distribution: Neotropical: Venezuela.
Helobata Bergroth, 1888
Helobata amazonensis Clarkson, Santos & Ferreira-Jr, 2016
Helobata amazonensis Clarkson, Santos & Ferreira-Jr, 2016: 550 – Brazil, Amazonas, Itacoatiara, Ilha da Trinidade; Clarkson and Almeida 2018 [new records].
Distribution: Neotropical: Brazil (Amazonas, Roraima).
Helobata aschnakiranae Makhan, 2007
Helobata aschnakiranae Makhan, 2007: 1 – Suriname (District Commwijne); Short and Fikáček 2011: 90 [catalog].
Distribution: Neotropical: Suriname.
Helobata bitriangulata García, 2000
Helobata bitriangulata García, 2000c: 244 – Venezuela, Apure State, Achaguas, Samán de Apure; Short and Hebauer 2006: 335 [catalog].
Distribution: Neotropical: Venezuela.
Helobata confusa Fernández & Bachmann, 1987
Helobata confusa Fernández & Bachmann, 1987: 155 – Paraguay (Asunción); Hansen 1999b: 173 [catalog].
Distribution: Neotropical: Argentina, Paraguay.
Helobata corumbaensis Fernández & Bachmann, 1987
Helobata corumbaensis Fernández & Bachmann, 1987: 155 – Brazil (Mato Grosso, Corumbá); Hansen 1999b: 173 [catalog]; Clarkson et al. 2016: 555 [taxonomic treatment].
Distribution: Neotropical: Brazil (Mato Grosso, Mato Grosso do Sul).
Helobata cossyphoides (Bruch, 1915)
Helopeltis cossyphoides Bruch, 1915: 458 – Argentina, Buenos Aires Province, La Plata, “Tiro Federal”; Fernández and Bachmann 1987: 153 [lectotype designation].
Helobata cossyphoides (Bruch, 1915); Fernández and Bachmann 1987: 151 [specific rank confirmed; not synonym of striata Brullé (= larvalis Horn), as in Knisch, 1924a: 223]; Hansen 1999b: 173 [catalog].
Distribution: Neotropical: Argentina.
Helobata cuivaum García, 2000
Helobata cuivaum García, 2000c: 242 – Venezuela (Apure State, Achaguas, Samán de Apure); Short and Hebauer 2006: 335 [catalog].
Distribution: Neotropical: Venezuela.
Helobata larvalis (Horn, 1873)
Helopeltis larvalis Horn, 1873: 137 – U.S.A. (Louisiana, California (Sonora)).
Helopeltina larvalis (Horn, 1873); Cockerell 1906a: 240.
Helobata larvalis (Horn, 1873); Cockerell, 1906b: 349; Hansen 1991: 293 [reinstated as valid name]; Jasper and Vogtsberger 1996: 56 [checklist]; Archangelsky 1997: 50 [description of immature stages]; Clarkson et al. 2016: 557 [taxonomic treatment]; Clarkson and Almeida 2018 [new records].
Hydrophilus (Philydrus) striatus Brullé, 1841: 58 (primary homonym of Hydrophilus striatus Turton, 1802 and Hydrophilus striatus Say, 1825).
Helopeltis striatus (Brullé, 1841); Bedel 1881b: XCIV [new combination].
Enochrus (Lumetus) striatus (Brullé, 1841); Zaitzev 1908: 389 [checklist].
Helobata striata (Brullé, 1841); Knisch, 1924a: 223 [catalog]; Spangler and Cross 1972 [description of eggs, egg case and first instar larva]; Fernández and Bachmann 1987: 53 [taxonomic treatment].
Distribution: Neotropical: Argentina, Bolivia, Brazil (Amazonas, Ceará, Mato Grosso, Mato Grosso do Sul, Minas Gerais), Cuba, Guatemala, Mexico, Paraguay, Venezuela. Nearctic: U.S.A. (California, Florida, Louisiana, Mississippi, North Carolina, South Carolina, Texas, Virginia).
Helobata lilianae García, 2000
Helobata lilianae García, 2000c: 239 – Venezuela, Apure State, Achaguas, Saman de Apure; Short and Hebauer 2006: 335 [catalog].
Distribution: Neotropical: Venezuela.
Helobata pantaneira Clarkson, Santos & Ferreira-Jr, 2016
Helobata pantaneira Clarkson, Santos & Ferreira-Jr, 2016: 553 – Brazil, Mato Grosso, Poconé.
Distribution: Neotropical: Brazil (Mato Grosso).
Helobata perpunctata Fernández & Bachmann, 1987
Helobata perpunctata Fernández & Bachmann, 1987: 156 – Argentina (Chaco Province, San Bernardo); Hansen 1999b: 173.
Distribution: Neotropical: Argentina.
Helobata quatipuru Fernández & Bachmann, 1987
Helobata quatipuru Fernández & Bachmann, 1987: 158 – Brazil, Pará State, Quatipurú; Hansen 1999b: 173 [catalog]; Clarkson et al. 2016: 558 [taxonomic treatment]; Clarkson and Almeida 2018 [new records].
Distribution: Neotropical: Brazil (Minas Gerais, Pará, Rio de Janeiro).
Helobata soesilae Makhan, 2007
Helobata soesilae Makhan, 2007: 3 – Suriname, Nieuw Amsterdam; Short and Fikáček 2011: 90 [catalog].
Distribution: Neotropical: Suriname.
Helochares Mulsant, 1844
Helochares aeacus Balfour-Browne, 1952
Helochares aeacus Balfour-Browne, 1952b: 515 – Mauritania, “Hamdoun”.
Helochares (Hydrobaticus) aeacus Balfour-Browne, 1952; Hebauer 1996: 11 [listed]; Hansen 1999b: 164 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Mauritania.
Helochares aethiopicus d’Orchymont, 1939
Helochares (Hydrobaticus) aethiopicus d’Orchymont, 1939c: 309 – Ethiopia [“Abyssinie”]; Hebauer 1996: 11 [taxonomic treatment]; Hansen 1999b: 164 [catalog]; Hebauer 2006a: 26 [checklist]; Salah and Régil Cueto 2017: 270 [excluded from Egypt checklist].
Distribution: Afrotropical: Ethiopia.
Helochares alberti d’Orchymont, 1943
Helochares (Hydrobaticus) alberti d’Orchymont, 1943a: 10 – Zaire [Congo Belge], Madimba; Hebauer 1996: 11 [taxonomic treatment]; Hansen 1999b: 164 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo [Zaire], Gabon, Republic of the Congo, “West Africa (Uelleburg)”.
Helochares alcimus d’Orchymont, 1943
Helochares (Hydrobaticus) alcimus d’Orchymont, 1943a: 12 – Democratic Republic of the Congo [Zaire; Congo Belge], Haut Uélé, Yebo (Moto); Hebauer 1996: 11 [listed]; Hansen 1999b: 164 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo [Zaire].
Remarks: Based on the general description and the male genitalia drawing presented by d’Orchymont (1943a: 11), this species likely belongs in Agraphydrus.
Helochares alcinous Balfour-Browne, 1948
Helochares (Hydrobaticus) alcinöus Balfour-Browne, 1948: 831 – Kenya, Mombasa; Hebauer 1996: 11 [listed]; Hansen 1999b: 164 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Kenya, Tanzania.
Helochares altus d’Orchymont, 1943
Helochares (Hydrobaticus) altus d’Orchymont, 1943f: 5 – India, Tamil Nadu, Nilgiri, southern border of Lake Oatacamund; Hansen 1999b: 164 [catalog].
Distribution: Indo-Malayan: India (Tamil Nadu).
Helochares anchoralis Sharp, 1890
Helochares anchoralis Sharp, 1890: 352 – Sri Lanka [Ceylon], Colombo; Gentili et al. 1995: 211 [checklist].
Helochares (Grapidelochares) anchoralis Sharp, 1890; Zaitzev 1908: 381 [catalog].
Helochares (Hydrobaticus) anchoralis Sharp, 1890; d’Orchymont 1923a: 9 [faunistic treatment]; d’Orchymont 1928: 105 [faunistic treatment]; d’Orchymont 1943a: 6 [faunistic treatment]; Hebauer 1995b: 4 [faunistic treatment]; Hansen 1999b: 164 [catalog]; Hebauer 2002a: 23 [new record]; Hebauer and Ryndevich 2005: 45 [new record]; Minoshima and Hayashi 2011: 61 [description of larva]; Dong and Bian 2021: 167 [checklist].
Helochares (Hydrovaticus) anchoralis Sharp, 1890; Matsui 1995: 320 [new record; misspelled subgenus name; year in error].
Distribution: Indo-Malayan: Bangladesh, Cambodia, China (Fujian, Guangdong, Hainan, Jiangxi, Taiwan, Yunnan), India, Indonesia (Sumatra), Laos, Philippines, Sri Lanka, Thailand, Vietnam. Palearctic: China (Hubei, Sichuan), Japan.
Helochares anchoralis ssp. expansus Knisch, 1921
Helochares (Hydrobaticus) crenatus ssp. expansus Knisch, 1921: 67 – New Guinea.
Helochares (Hydrobaticus) anchoralis ssp. expansus Knisch, 1921; d’Orchymont 1943a: 6 [taxonomic treatment]; Hansen 1999b: 164 [catalog]; Hansen 2004: 52 [checklist]; Fikáček et al. 2015: 62 [catalog].
Helochares (Hydrobaticus) anchoralis Sharp, 1890; Watts 1995: 119 [faunistic treatment].
Distribution: Australasian: Papua New Guinea.
Helochares ancoroides Hebauer, 2001
Helochares (Hydrobaticus) ancoroidesHebauer 2001a: 13 – Indonesia, Papua, [W. Neuguinea], Paniai Province, Wanggar-Kali Bumi, IR 14; Short and Hebauer 2006: 335 [catalog].
Distribution: Indo-Malayan: Indonesia (Papua).
Helochares andreinii d’Orchymont, 1939
Helochares (Hydrobaticus) andreinii d’Orchymont, 1939f: 320 – Eritrea, Sabarguma; Balfour-Browne 1951: 212 [new records]; Hebauer 1997: 263 [new record]; Hansen 1999b: 165 [catalog]; Hansen 2004: 52 [checklist]; Fikáček et al. 2015: 62 [catalog].
Helochares (Hydrobaticus) andreini d’Orchymont, 1939; Hebauer 1996: 11 [listed; misspelled]; Hebauer 2006a: 26 [checklist; new record; misspelled].
Distribution: Afrotropical: Eritrea, Oman, Saudi Arabia, Yemen, Zimbabwe.
Helochares androgynus Hebauer, 1996
Helochares (Hydrobaticus) androgynus Hebauer, 1996: 11 – Tanzania [“Tanganyika”], 2 mi to Lake Manyara, SE shore; Hansen 1999b: 165 [catalog]; Hebauer 2006a: 26 [new records].
Distribution: Afrotropical: Republic of South Africa, Tanzania, Zambia.
Helochares anthonyae Watts, 1995
Helochares (Hydrobaticus) anthonyae Watts, 1995: 120 – Papua New Guinea, Morobe District, 11 km Lae-Bulolo Rd.; Hansen 1999b: 165 [catalog].
Distribution: Australasian: Australia (Northern Territory), Papua New Guinea.
Helochares balfourbrownei Hansen, 1999
Helochares (Hydrobaticus) balfourbrownei Hansen, 1999b: 165 [nomen novum]; Hebauer 2006a: 26 [checklist].
Helochares (Hydrobaticus) rusticus Balfour-Browne, 1952a: 132 – Ivory Coast, River Lerabara; (primary homonym of Helochares rusticus d’Orchymont, 1939 – currently in Crephelochares); Balfour-Browne 1959: 311 [faunistic treatment]; Hebauer 1996: 21 [new records].
Distribution: Afrotropical: Benin, Burkina Faso, Ghana, Guinea, Ivory Coast, Liberia, Nigeria, Senegal, Sierra Leone.
Helochares basilewskyi Balfour-Browne, 1957
Helochares (Hydrobaticus) basilewskyi Balfour-Browne, 1957: 23 – Rwanda, Rutovu, forêt du Rugege; Hebauer 1996: 12 [faunistic treatment]; Hansen 1999b: 165 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Rwanda.
Helochares bilardoi Hebauer, 2009
Helochares (Hydrobaticus) bilardoi Hebauer, 2009: 4 – Gabon, Monts de Cristal National Park, Andok Village, Foula; Short and Fikáček 2011: 90 [catalog].
Distribution: Afrotropical: Gabon.
Helochares blaesus d’Orchymont, 1936
Helochares (Hydrobaticus) blaesus d’Orchymont, 1936b: 111 (112) – Botswana [Kalahari], Tsotsoroga Pan; Hebauer 1995a: 262 [faunistic treatment]; Hebauer 1996: 12 [faunistic treatment]; Hansen 1999b: 165 [catalog]; Hebauer 2005: 39 [checklist], 2006a: 26 [checklist].
Distribution: Afrotropical: Botswana [Kalahari], Democratic Republic of the Congo, Ethiopia, Kenya, Malawi, Mozambique, Namibia, Republic of South Africa.
Helochares bohemani d’Orchymont, 1936
Helochares (Hydrobaticus) bohemani d’Orchymont, 1936b: 111 – Namibia [“South-West Africa”], Eenfelsbach 25 km SSE Okahandja; Hebauer 1995a: 262 [faunistic treatment]; Hebauer 1996: 12 [faunistic treatment; new records]; Hansen 1999b: 165 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Angola, Botswana, Cameroon, Ethiopia, Kenya, Madagascar, Namibia, Republic of South Africa, Zambia, Zimbabwe.
Helochares camerunensis d’Orchymont, 1939
Helochares (Hydrobaticus) camerunensis d’Orchymont, 1939b: 303 – Cameroon, Douala [Duala]; Balfour-Browne 1952a: 130 [faunistic treatment]; Hebauer 1996: 13 [faunistic treatment]; Hansen 1999b: 165 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Cameroon, Democratic Republic of the Congo, Gabon, Gambia, Ghana, Guinea, Ivory Coast, Nigeria, Republic of the Congo, Senegal.
Helochares cancellatus Hebauer, 1998
Helochares (Hydrobaticus) cancellatus Hebauer, 1998: 42 – Sri Lanka [Ceylon], Labugama, 24 mi ESE of Colombo; Hansen 1999b: 165 [catalog].
Distribution: Indo-Malayan: Sri Lanka.
Helochares championi Sharp, 1882
Helochares (Hydrobaticus) championi Sharp, 1882: 75 – Guatemala (Guatemala City, Dueñas, San Géronimo) and Nicaragua (Chontales); Balfour-Browne, 1939: 293 [faunistic treatment]; Hansen 1999b: 165; Short 2005: 217 [faunistic treatment]; Short and Girón 2018: 34 [new record; faunistic treatment].
Distribution: Neotropical: Costa Rica, Guatemala, Nicaragua.
Helochares chappuisi Balfour-Browne, 1952
Helochares (Hydrobaticus) chappuisi Balfour-Browne, 1952a: 132; Hansen 1999b: 165 [catalog].
Helochares (Hydrobaticus) chappiusi Balfour-Browne, 1952; Hebauer 1996: 13 [listed; misspelled]; Hebauer 2006a: 26 [listed; misspelled].
Distribution: Afrotropical: Benin, Mali, Niger.
Helochares clypeatus (Blackburn, 1891)
Hydrobaticus clypeatus Blackburn, 1891: 305 – Australia, Northern Territory, Burrundie.
Helochares (Hydrobaticus) clypeatus (Blackburn, 1891); Knisch 1924a: 193 [catalog]; d’Orchymont 1943a: 4 [faunistic treatment]; Watts 1995: 120 [redescription]; Hansen 1999b: 165 [catalog]; Watts 2002: 120 [description of larva with Helochares tristis MacLeay].
Distribution: Australasian: Australia (New South Wales, Northern Territory, Queensland, Western Australia).
Helochares collarti d’Orchymont, 1939
Helochares (Hydrobaticus) collarti d’Orchymont, 1939b: 315 – Democratic Republic of the Congo [Congo Belge; Zaire], Blukwa; Balfour-Browne 1950b: 56 [faunistic treatment]; Hebauer 1996: 13 [new record]; Hansen 1999: 165 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Rwanda.
Helochares compactus Hebauer, 2001
Helochares (Hydrobaticus) compactusHebauer 2001a: 13 – Indonesia, Papua [Irian Jaya], Paniai Province, Nabire – Kali Bobo; Short and Hebauer 2006: 336 [catalog].
Distribution: Indo-Malayan: Indonesia (Papua).
Helochares conformis Hebauer, 1995
Helochares (Hydrobaticus) conformis Hebauer, 1995a: 263 – Namibia, East Caprivi, Katima Mulilo, 17°29'S, 24°17'E; Hebauer 1996: 13 [faunistic treatment]; Hansen 1999b: [catalog]; Hebauer 2006a: 26 [new records].
Distribution: Afrotropical: Namibia, Republic of South Africa, Zambia, Zimbabwe.
Helochares congoensis d’Orchymont, 1939
Helochares (Hydrobaticus) congoensis d’Orchymont, 1939b: 304 – Democratic Republic of the Congo [Congo Belge; Zaire], Boma; Hebauer 1996: 13 [faunistic treatment]; Hansen 1999b: 165 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo.
Helochares congruens d’Orchymont, 1939
Helochares (Hydrobaticus) congruens d’Orchymont, 1939b: 304 – Senegal, Thiès; Hebauer 1988: 156 [faunistic treatment]; Hebauer 1996: 13 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hebauer 2005: 39 [checklist]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Ghana, Kenya [in doubt], Madagascar, Malawi, Namibia, Senegal, Republic of South Africa, Tanzania, Uganda, Zambia [in doubt], Zimbabwe.
Helochares conjectus d’Orchymont, 1939
Helochares (Hydrobaticus) conjectus d’Orchymont, 1939b: 305 – Tanzania, Lake Victoria, Ukerewe I.; Balfour-Browne 1950a: 394 [faunistic treatment]; Hebauer 1996: 13 [faunistic treatment]; Hebauer 1996: 14 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Ethiopia, Tanzania, Zambia, Zimbabwe.
Helochares crenatostriatus Régimbart, 1903
Helochares (Graphelochares) melanophthalmus var. crenatostriatus Régimbart, 1903a: 28 – Madagascar; Seychelles (Aldabra).
Helochares (Hydrobaticus) crenatostriatus Régimbart, 1903; d’Orchymont, 1939e: 298; Hebauer 1996: 14 [faunistic treatment]; Hebauer 1996: 14 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Cameroon, Gabon, Ghana, Kenya [in doubt], Madagascar, Republic of the Congo, Seychelles (Aldabra).
Helochares crenatuloides d’Orchymont, 1943
Helochares (Hydrobaticus) crenatuloides d’Orchymont, 1943e: 2 – India, “Bengal, Tetara”; Hebauer 1997: 263; Hansen 1999b: 166 [catalog]; Hansen 2004: 52 [checklist]; Fikáček et al. 2010: 151 [new record]; Fikáček et al. 2015: 62 [catalog]; Ribera et al. 2019: 264 [faunistic treatment].
Distribution: Afrotropical: Oman, United Arab Emirates. Indo-Malayan: India (“Bengal”, Madhya Pradesh, Uttar Pradesh).
Helochares crenatus Régimbart, 1903
Helochares (Graphelochares) crenatus Régimbart, 1903b: 54 – India, Tamil Nadu, Pondicherry; d’Orchymont 1940: 168 [lectotype designation].
Helochares (Hydrobaticus) crenatus Régimbart, 1903; d’Orchymont, 1923a: 9 [faunistic treatment]; d’Orchymont 1928: 105 [faunistic treatment]; Hebauer, 1995b: 4 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hansen 2004: 52 [checklist]; Fikáček et al. 2015: 62 [catalog]; Dong and Bian 2021: 167 [checklist].
Helochares crenatus Régimbart, 1903; Gentili et al. 1995: 211 [checklist].
Distribution: Indo-Malayan: China (Yunnan), India (Tamil Nadu, West Bengal), Thailand.
Helochares crepitus Balfour-Browne, 1950
Helochares (Hydrobaticus) crepitus Balfour-Browne, 1950a: 395 – Zambia [“Northern Rhodesia”], “Mwengwa”; Balfour-Browne 1950a: 395 [faunistic treatment]; Hebauer 1996: 14 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Ghana, Tanzania, Zambia.
Helochares cresphontes d’Orchymont, 1939
Helochares (Hydrobaticus) cresphontes d’Orchymont, 1939b: 313 – Uganda, Kampala; Balfour-Browne 1957: 23 [faunistic treatment]; Hebauer 1996: 14 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Ghana, Rwanda, Tanzania, Uganda.
Helochares crespulus d’Orchymont, 1939
Helochares (Hydrobaticus) crespulus d’Orchymont, 1939b: 313 – Zaire [“Congo Belge”], Haut Uélé, Watsa; Hebauer 1996: 14 [listed]; Hansen 1999b: 166 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Gabon.
Helochares crispus d’Orchymont, 1939
Helochares (Hydrobaticus) crispus d’Orchymont, 1939b: 311 – “Zanguebar”; Hebauer 1996: 14 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hebauer 2005: 39 [new record]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Ethiopia, Kenya, Malawi, Namibia, Republic of South Africa, Rwanda, Tanzania, Zimbabwe.
Helochares dalhuntyi Watts, 1995
Helochares (Hydrobaticus) dalhuntyi Watts, 1995: 121 – Australia, Queensland, Dalhunty River.
Helochares (Hydrobaticus) anthonyae Watts, 1995; Hansen 1999b: 166 [synonym in error].
Distribution: Australasian: Australia (Northern Territory, Queensland).
Helochares densepunctus Régimbart, 1907
Helochares densepunctus Régimbart, 1907: 48 – Guinea Bissau [Guinée Portugaise] (Bolama); Madagascar (Helodrano Antongila [Baie d’Antongil]; “Pays Androy”.
Helochares (Hydrobaticus) densepunctatus Régimbart, 1907; Knisch 1924: 193 [catalog; misspelled]; Hebauer 1996: 14 [faunistic treatment; misspelled].
Helochares (Hydrobaticus) densepunctus Régimbart, 1907; Hansen 1999: 166 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Cameroon, Gabon, Gambia, Guinea, Guinea Bissau, Ivory Coast, Kenya, Liberia, Madagascar, Senegal, Tanzania, Zambia.
Helochares densus Sharp, 1890
Helochares densus Sharp, 1890: 352 – Sri Lanka [Ceylon]: Kandy; Dikoya; Bogawantalawa; d’Orchymont 1943e: 7 [specific rank confirmed: not synonym of lentus Sharp, as in Zaitzev 1908: 381 (as synonym dubious) and d’Orchymont 1913a: 5].
Helochares (Hydrobaticus) densus Sharp, 1890; d’Orchymont 1923a: 9 [faunistic treatment]; d’Orchymont 1943e: 7 [faunistic treatment]; Hebauer 1995b: 4 [faunistic treatment]; Hansen 1999b: 166 [catalog]; Hebauer 2002a: 23 [new record]; Hansen 2004: 52 [checklist]; Fikáček et al. 2015: 62 [catalog]; Dong and Bian 2021: 167 [checklist].
Distribution: Indo-Malayan: China (Fujian, Guangdong, Guangxi, Hainan, Hunan, Jiangxi, Yunnan, Zhejiang), India (Andaman Is., “Bengal”, Madhya Pradesh, Nicobar Is., Tamil Nadu, Uttarakhand, Uttar Pradesh), Nepal, Thailand, Vietnam. Palearctic: China (Sichuan).
Helochares dentalus d’Orchymont, 1943
Helochares (Hydrobaticus) dentalus d’Orchymont, 1943e: 8 – Malaysia, Sabah [“Borneo septentrional”], Bettotan nr Sandakan; Hansen 1999b: 166 [catalog].
Distribution: Indo-Malayan: Malaysia (Sabah).
Helochares denudatus d’Orchymont, 1943
Helochares (Hydrobaticus) denudatus d’Orchymont, 1943e: 9 – Indonesia, Sumatra, Bedagei NE of Tebingtinggi; Hansen 1999b: 166 [catalog].
Distribution: Indo-Malayan: Indonesia (Sumatra), Malaysia (Peninsula).
Helochares depactus d’Orchymont, 1939
Helochares (Hydrobaticus) depactus d’Orchymont, 1939b: 302 – Kenya, Aberdare Ra. (eastside), Kigangop; Hebauer 1996: 15 [faunistic treatment]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Kenya.
Helochares diductus d’Orchymont, 1939
Helochares (Hydrobaticus) diductus d’Orchymont, 1939b: 318 – Gabon, Cape Lopez; Hebauer 1996: 15 [faunistic treatment]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Gabon.
Remarks: Based on original description, probably Agraphydrus: small size, pronotal punctures of two different sizes; aedeagus with median lobe spatulate, arched on the sides and truncated in a straight line at apex.
Helochares didymoides Balfour-Browne, 1947
Helochares (Hydrobaticus) didymoides Balfour-Browne, 1947: 141 – Sudan, Didinga Hills, Nagishot; Hebauer 1996: 15 [faunistic treatment]; Hansen 1999b: 167; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Cameroon, Gabon, Sudan.
Helochares didymus d’Orchymont, 1939
Helochares (Hydrobaticus) didymus d’Orchymont, 1939b: 318 – Uganda, Kampala; Hebauer 1996: 15 [faunistic treatment]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Cameroon, Democratic Republic of the Congo, Gabon, Ghana, Guinea, Kenya, Republic of the Congo, Uganda.
Helochares difficilis d’Orchymont, 1939
Helochares (Hydrobaticus) difficilis d’Orchymont, 1939b: 314 – Uganda (central), “rivière Kizoungou”; Hebauer 1996: 15 [faunistic treatment]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo [Zaire], Kenya, Sudan, Tanzania, Uganda, Zambia.
Helochares dilutus (Erichson, 1843)
Hydrobius dilutus Erichson, 1843: 228 – Angola, Benguela; d’Orchymont, 1943c: 1 [specific rank confirmed: not synonym of Helochares lividus Forster, as in Bedel 1881a: 330).
Philhydrus dilutus (Erichson, 1843); Gemminger and Harold 1868: 481 [catalog].
Helochares dilutus (Erichson), 1843; Reiche and Saulcy 1856: 358 [faunistic treatment]; Heyden 1891: 67 [catalog]; Bird et al. 2017 [faunistic treatment].
Helochares (s. str.) dilutus (Erichson, 1843); d’Orchymont, 1943c: 1 [taxonomic treatment]; Balfour-Browne 1950a: 393 [faunistic treatment]; Balfour-Browne 1950b: 59 [faunistic treatment]; Balfour-Browne 1957: 21 [faunistic treatment]; Hebauer 1988: 156 [faunistic treatment]; Hebauer 1995a: 264 [faunistic treatment]; Hebauer 1996: 5 [faunistic treatment]; Hansen 1999b: 160 [catalog]; Hebauer 2005: 39 [new record]; Hebauer 2006a: 25 [checklist]; Fikáček et al. 2015: 61 [catalog; new record].
Helochares niloticus Sharp, 1903: 7 – Sudan, Jebel Ahmed Agha [Gebel Ahmed Agha]; d’Orchymont, 1943c: 1 [synonymy].
Distribution: Afrotropical: Angola, Botswana, Cameroon, Democratic Republic of the Congo, Ethiopia, Gambia, Ghana, Ivory Coast, Kenya, Liberia, Madagascar, Malawi, Mauritius (incl. Rodrigues), Mozambique, Namibia, Republic of the Congo, Réunion, Rwanda, Senegal, Republic of South Africa, Sudan, Tanzania, Uganda, Yemen (Socotra), Zambia, Zimbabwe.
Helochares dilutus ssp. consputus Boheman, 1851
Hydrobius consputus Boheman, 1851: 598 – Republic of South Africa [Caffraria], Orange river reg. [regione fluvii Gariepis]; Hebauer 1988: 156 [as synonym of dilutus Erichson]; Hebauer 1996: 5 [as synonym of dilutus Erichson].
Helochares consputus (Boheman, 1851); Bedel 1880: CXLVIII [new combination].
Enochrus (Lumetus) consputus (Boheman, 1851); Knisch 1924: 208 [catalog].
Helochares (s. str.) dilutusssp. consputus (Boheman, 1851); d’Orchymont 1943c: 6 [taxonomic treatment]; Hansen 1999b: 160 [catalog]; Salah and Régil Cueto 2017: 269 [excluded from Egypt checklist].
Helochares variabilis Régimbart, 1903a: 25 – Madagascar, pays Androy, Fort-Dauphin, bassin du Mandraré, Centre-Sud, forêts de la côte Est, Tananarive, baie d’Antongil; Mascarene Is., Réunion (Salazie); d’Orchymont, 1926b: 232 [synonymy].
Distribution: Afrotropical: Madagascar, Mauritius (Mascarene Is.), Namibia, Republic of South Africa.
Helochares dimorphus d’Orchymont, 1939
Helochares (Hydrobaticus) dimorphus d’Orchymont, 1939b: 322 – Democratic Republic of the Congo [Congo Belge; Zaire], Lower Uele, Buta; Balfour-Browne 1950b: 57 [faunistic treatment]; Hebauer 1996: 15 [faunistic treatment]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist; new records].
Distribution: Afrotropical: Cameroon [in doubt]; Democratic Republic of the Congo, Ghana, Guinea, Kenya, Liberia, Nigeria, Republic of the Congo, Uganda.
Helochares dollmani Balfour-Browne, 1950
Helochares (s. str.) dollmani Balfour-Browne, 1950a: 393 – Zambia [Northern Rhodesia], Namwala, Kafue River; Hebauer 1995a: 265 [faunistic treatment]; Hebauer 1996: 6 [faunistic treatment]; Hansen 1999b: 160 [catalog]; Hebauer 2005: 39 [checklist; new record]; Hebauer 2006a: 26 [checklist; new record].
Distribution: Afrotropical: Madagascar, Malawi, Namibia, Zambia, Zimbabwe.
Helochares dolus d’Orchymont, 1939
Helochares (Hydrobaticus) dolus d’Orchymont, 1939b: 319 – Mali [Haut Sénégal; Senegal], Khayes; Balfour-Browne 1952a: 130 [faunistic treatment]; Hebauer 1996: 15 [faunistic treatment]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Cameroon, Democratic Republic of the Congo [Zaire], Gambia, Ghana, Ivory Coast, Mali, Nigeria, Republic of the Congo [Congo-Brazzaville], Senegal, Sierra Leone, Sudan, Tanzania.
Helochares egregius Balfour-Browne, 1952
Helochares (Hydrobaticus) egregius Balfour-Browne, 1952a: 131 – Ivory Coast, Toumodi; Hebauer 1995a: 264 [faunistic treatment]; Hebauer 1996: 16 [new records]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist; new record].
Distribution: Afrotropical: Benin, Democratic Republic of the Congo, Ghana, Ivory Coast, Namibia, Nigeria, Republic of the Congo, Senegal.
Helochares endroedyi Hebauer, 1996
Helochares (Hydrobaticus) endroedyi Hebauer, 1996: 16 – Ghana, Ashanti Region, Bobiri forest res., 6°40'N, 1°15'W; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Democratic Republic of the Congo, Ghana, Guinea, Zambia.
Helochares fratris Hebauer, 2003
Helochares (Hydrobaticus) fratris Hebauer, 2003b: 68 – SW Madagascar, Morondave district, Miandrivazo, 246 km W of Antsirabe; Hebauer 2006a: 26 [checklist]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Madagascar.
Helochares fulgurans Hebauer, 1995
Helochares (s. str.) fulgurans Hebauer, 1995b: 7 – Thailand, Chantaburi Khao Sabap NP; Hansen 1999b: 160 [catalog].
Distribution: Indo-Malayan: Thailand.
Remarks: Described from a single female specimen, as similar (related) to Helochares fuliginosus and Agraphydrus.
Helochares fuliginosus d’Orchymont, 1932
Helochares (s. str.) fuliginosus d’Orchymont, 1932: 689 – Indonesia, West Java, Bogor [“Buitenzorg”]; Hebauer 1995b: 7 [faunistic treatment]; Hansen 1999b 160 [catalog]; Jia and Tang 2018: 6 [redescription; new records].
Distribution: Indo-Malayan: China (Fujian, Guangdong, Guangxi, Hong Kong, Macao), Indonesia (Java, Sumatra), Laos, Malaysia (Peninsula).
Helochares goticus Hebauer, 1996
Helochares (Hydrobaticus) goticus Hebauer, 1996: 16 – Democratic Republic of the Congo [Congo-Brazzaville], Kindamba, Meya settlement; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo.
Helochares hainanensis Dong & Bian, 2021
Helochares (Hydrobaticus) hainanensis Dong & Bian, 2021: 168 – China: Hainan Province, Qionghai City, Wanquan Town, 19°11'N, 110°23'E.
Helochares hainanensis Dong & Bian, 2021.
Distribution: Indo-Malayan: China (Hainan).
Helochares hiekei Hebauer, 1995
Helochares (Hydrobaticus) hiekei Hebauer, 1995b: 5 – India, Karnataka, Ablathi; Hansen 1999b: 167 [catalog].
Distribution: Indo-Malayan: India (Karnataka).
Helochares insolitus d’Orchymont, 1925
Helochares (s. str.) pallens-insolitus d’Orchymont, 1925b: 202 (and 1926a: 380) – Philippines, Manila; Short and Hebauer 2006: 336 [catalog].
Helochares (s. str.) insolitus d’Orchymont, 1925; Hebauer 2002b: 15 [elevated to species; not subspecies of Helochares pallens (MacLeay), as in Hansen 1999b: 163]; Hebauer and Ryndevich 2005: 45 [new record].
Distribution: Indo-Malayan: Philippines (Manila), Vietnam.
Helochares interjectus Hebauer, 1998
Helochares (Hydrobaticus) interjectus Hebauer, 1998: 42 – Madagascar, Morarano, “Chrome-Ambakireni”, 10 km W Maheriara; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Madagascar.
Helochares iteratus Hebauer, 1996
Helochares (Hydrobaticus) iteratus Hebauer, 1996: 17 – Republic of the Congo, “Uamgebiet Bosum”; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo [in doubt], Republic of the Congo, Tanzania [in doubt].
Helochares itylus Balfour-Browne, 1952
Helochares (Hydrobaticus) itylus Balfour-Browne, 1952a: 131 – Benin [“Dahomey”], Ketou forest; Hebauer 1996: 17 [faunistic treatment]; Hansen 1999b: 167 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Cameroon, Democratic Republic of the Congo [in doubt], Gambia, Ghana, Ivory Coast, Republic of the Congo [Congo-Brazzaville], Senegal.
Helochares ivani Hebauer, 1996
Helochares (Hydrobaticus) ivani Hebauer, 1996: 18 – Ghana, Kumasi; Hansen 1999b: 167; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Cameroon, Ghana, Ivory Coast, Liberia, Nigeria, Republic of the Congo [Congo-Brazzaville], Zambia [in doubt].
Helochares kerstinneumanni Hebauer, 2009
Helochares (Hydrobaticus) kerstinneumanni Hebauer, 2009: 4 – Gabon, Makokou-Riv. Ivindo Chutes Kongou; Short and Fikáček 2011: 91 [catalog].
Distribution: Afrotropical: Gabon.
Helochares knischi d’Orchymont, 1939
Helochares (Hydrobaticus) knischi d’Orchymont, 1939b: 320 – Democratic Republic of the Congo [Belg. Congo; Zaire]; Hebauer 1996: 18 [faunistic treatment]; Hansen 1999b: 167; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo.
Helochares laevis Short & Girón, 2018
Helochares (Hydrobaticus) laevis Short & Girón, 2018: 36 – Mexico, Chiapas, San Cristobal de las Casas.
Distribution: Neotropical: Mexico.
Helochares lamprus d’Orchymont, 1940
Helochares (Hydrobaticus) lamprus d’Orchymont, 1940: 169 – Indonesia, [Sumatra], Lampong, “Wai Lima”; Hansen 1999b: 167 [catalog].
Distribution: Indo-Malayan: Indonesia (Sumatra).
Remarks: Described as similar to Helochares nebridus and/or H. crenatus; the aedeagal form as illustrated by d’Orchymont (1940: 170, fig. 8) is rather unusual among Helochares.
Helochares lentus Sharp, 1890
Helochares lentus Sharp, 1890: 352 – Sri Lanka [Ceylon], Dikoya; Gentili et al. 1995: 211 [checklist].
Helochares (Grapidelochares) lentus Sharp, 1890; Zaitzev 1908: 381 [catalog].
Helochares (Hydrobaticus) lentus Sharp, 1890; d’Orchymont 1923a: 9 [faunistic treatment]; d’Orchymont 1928: 105 [faunistic treatment]; d’Orchymont, 1943e: 3 [taxonomic treatment]; Hebauer 1995b: 5 [faunistic treatment]; Hansen 1999b: 168 [catalog]; Hebauer 2002a: 23 [faunistic treatment]; Hansen 2004: 52 [checklist]; Hebauer and Ryndevich 2005: 45 [new record]; Fikáček et al. 2015: 62 [catalog]; Dong and Bian 2021: 167 [checklist].
Distribution: Indo-Malayan: Bangladesh, Cambodia, China (Fujian, Guangdong, Guangxi, Guizhou, Hunan, Jiangxi, Taiwan, Yunnan), India, Indonesia (Borneo, Java, Lombok, Sumatra), Laos, Malaysia (Peninsula), Nepal, Sri Lanka, Thailand, Vietnam. Palearctic: China (Sichuan, Tibet).
Helochares lepidus d’Orchymont, 1943
Helochares (Hydrobaticus) lentus lepidus d’Orchymont, 1943e: 5 – Philippines, Luzon, Montalban.
Helochares (Hydrobaticus) lepidus d’Orchymont, 1943; Hebauer 1995b: 4 [elevated to species; not subspecies of lentus as in d’Orchymont, 1943e]; Hansen 1999b: 168 [catalog].
Distribution: Indo-Malayan: Philippines.
Helochares leptinus d’Orchymont, 1943
Helochares (Hydrobaticus) lentus ssp. leptinus d’Orchymont, 1943e: 5 – Philippines, Luzon, Balbalan.
Helochares (Hydrobaticus) leptinus d’Orchymont, 1943; Hebauer 1995b: 5 [specific rank confirmed; not subspecies of lentus as in d’Orchymont, 1943e]; Hansen 1999b: 168 [catalog]; Hebauer 2002a: 23 [new record].
Distribution: Indo-Malayan: Bangladesh, Nepal, Philippines.
Helochares letus d’Orchymont, 1943
Helochares (Hydrobaticus) lentus ssp. letus d’Orchymont, 1943e: 6. – Philippines.
Helochares (Hydrobaticus) letus d’Orchymont, 1943; Hebauer, 1995b: 4 [elevated to species; not subspecies of lentus as in d’Orchymont, 1943e]; Hansen 1999: 168 [catalog].
Distribution: Indo-Malayan: Philippines.
Helochares livianus d’Orchymont, 1939
Helochares (Hydrobaticus) livianus d’Orchymont, 1939b: 317 – Uganda, Kampala, Hoima Rd.; Balfour-Browne, 1950b: [faunistic treatment]; Balfour-Browne 1957: 22 [faunistic treatment]; Hebauer 1996: 18 [faunistic treatment]; Hansen 1999b 168 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Rwanda, Tanzania, Uganda.
Helochares lividoides Hansen & Hebauer, 1988
Helochares (s. str.) lividoides Hansen & Hebauer, 1988: 27 – Israel, Golan, Ein Sha’abanyia; Hebauer 1994: 112 [faunistic treatment]; Hansen 1999b: 160 [catalog]; Hansen 2004: 52 [catalog]; Hebauer and Ryndevich 2005: 45 [new record]; Mart et al. 2010: 298 [faunistic treatment]; Darilmaz and İncekara 2011: 710 [checklist]; Fikáček et al. 2015: 61 [catalog].
Distribution: Palearctic: Israel, Turkey.
Helochares lividus (Forster, 1771)
Dytiscus lividus Forster, 1771: 52 (Official Specific Name No. 1992, cf. ICZN, 1964: 242); England and Germany [Anglia; Gallia].
Hydrophilus lividus (Forster, 1771); Olivier 1792: 127 [faunistic treatment].
Philydrus lividus (Forster, 1771); Solier 1834: 316 [taxonomic treatment].
Helophilus lividus (Forster, 1771); Mulsant 1844b: 134 [faunistic treatment].
Helocharis lividus (Forster, 1771); Thomson 1859: 18 [faunistic treatment; misspelled].
Helophygas lividus (Forster, 1771); Motschulsky 1853: 11 [faunistic treatment].
Philhydrus lividus (Forster, 1771); Fairmaire and Laboulbène 1854: 230 [faunistic treatment].
Hydrophilus fulvus Fourcroy, 1785: 66 – France, Paris [Parisiensis]; Hansen 1982: 203 [synonymy; not synonym of obscurus Müller, as in d’Orchymont 1936a: 10].
Hydrophilus griseus Fabricius, 1787: 188 – Germany, Sachsen [Saxonia]; Illiger 1798: 246 [synonym]; Hansen 1982: 203 [not synonym of obscurus Müller, as in d’Orchymont, 1933: 304).
Dytiscus griseus (Fabricius, 1787); de Villers 1789: 342 [faunistic treatment].
Philydrus griseus (Fabricius, 1787); Solier 1834: 316 [faunistic treatment].
Philhydrus griseus (Fabricius, 1787); Brullé 1835: 278 [faunistic treatment].
Hydrobius griseus (Fabricius, 1787); Erichson 1837: 211 [faunistic treatment].
Phylidrus griseus (Fabricius, 1787); Castelnau 1840: 52 [faunistic treatment].
Pylophilus griseus (Fabricius, 1787); Motschulsky 1845: 32 [faunistic treatment].
Helochares (s. str.) griseus (Fabricius, 1787); Ganglbauer 1904: 249 [faunistic treatment].
Helochares griseus (Fabricius, 1787); Panzera 1932: 54 [description of larvae].
Hydrophilus pallidus Rossi, 1792: 66 – NW Italy [Etruria]; Bedel 1881a: 330 [synonymy]; Hansen 1982: 203 [synonym of griseus Fabricius: Paykull 1798: 183].
Helophilus lividus var. pallidus (Rossi, 1792); Mulsant 1844a: 135 [faunistic treatment].
Philhydrus lividus var. pallidus (Rossi, 1792); Gemminger and Harold 1868a: 481 [catalog].
Helochares dilutus var. pallidus (Rossi, 1792); Rey 1885b: 287 [faunistic treatment].
? Hydrophilus chrysomelinus Herbst, 1797: 313 (primary homonym of Hydrophilus chrysomelinus Müller, 1776); Germany; Schönherr, 1808: 7 [synonymy; sub nom. griseus); Knisch, 1924: 197 [as synonym dubious of Helochares griseus].
Hydrophilus lividus Herbst, 1797: 316 (secondary homonym of Dytiscus lividus Forster, 1771). – Germany; Schönherr, 1808: 7 [synonymy; sub nom. griseus].
Hydrophilus bicolor; Paykull, 1798: 184 [misinterpretation of Hydrophilus bicolor Fabricius, 1792); Bedel 1878a: CLXXVII [synonymy].
Helochares ludovici Schaufuss, 1869: 11 – Spain, Ibiza [Ibiza, Llano de Villa]; Heyden 1891: 67 [catalog]; Ganglbauer 1904: 249 [synonymy]; Hansen 1982: 203 [taxonomic treatment].
Helochares lividus var. pallide-testaceus Stierlin, 1900: 219 [ascribed to Heer, who merely used “pallide” and “testaceus” as the first two adjectives in a description of an unnamed variety [Heer 1841: 485]] – Switzerland [Helvetiae]; Knisch 1924: 198 [synonymy].
Helochares (s. str.) lividus (Forster, 1771); Hansen 1982: 203 [taxonomic treatment]; Hebauer 1994: 111 [faunistic treatment; identification doubtful]; Hebauer 1996: 7 [faunistic treatment]; Ribera et al. 1996: 10 [checklist]; Hansen 1999b: 161 [catalog]; Hansen 2004: 52 [catalog]; Hebauer and Ryndevich 2005: 45 [new record]; Darilmaz and Kiyak 2006: 79 [new record]; Hebauer 2006a: 25 [checklist]; Mart et al. 2010: 298 [faunistic treatment]; Darilmaz and İncekara 2011: 710 [checklist]; Fikáček et al. 2015: 61 [catalog]; Salah and Régil Cueto 2017: 269 [record from Egypt in doubt]; Gentili et al. 2018: 23 [faunistic treatment]; Benamar et al. 2021: 34 [checklist].
Helochares lividus (Forster, 1771); Reiche 1854: 9 [catalog]; Heyden 1891: 67 [catalog]; d’Orchymont 1913b: 200 [description of larva]; Panzera 1932: 60 [description of larvae]; Mabrouki et al. 2018 [faunistic treatment]; Angus et al. 2020: 21 [karyotype].
Distribution: Palearctic: Algeria, Austria, Belarus, Bosnia Herzegovina, Bulgaria, Canary Islands, Croatia, Czech Republic, Egypt [in doubt], France, Germany, Great Britain, Greece, Hungary, Iran, Italy, Luxembourg, Macedonia, Morocco, Netherlands, Poland, Portugal, Serbia and Montenegro, Slovakia, Slovenia, Spain, Switzerland, Syria, Tunisia, Turkey, Ukraine.
Helochares lobatus d’Orchymont, 1948
Helochares (s. str.) lobatus d’Orchymont, 1948: 730 – Ethiopia, Abyssinian Highlands, Muger Wenz, “Mulu”; Hebauer 1996: 7 [faunistic treatment]; Hansen 1999b: 161 [catalog]; Hebauer 2006a: 25 [checklist].
Distribution: Afrotropical: Ethiopia.
Remarks: This species was described as similar to Helochares lividus, but the aedeagus is remarkably different; it needs to be studied in detail, as the drawing provided by d’Orchymont (1948: fig. 5A) is not entirely clear and does not allow to establish affinities with other Helochares groups.
Helochares lollius d’Orchymont, 1939
Helochares (Hydrobaticus) lollius d’Orchymont, 1939b: 321 – Uganda, Kampala; Hebauer 1996: 18 [faunistic treatment]; Hansen 1999b: 168 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Gabon, Uganda.
Helochares loticus Hebauer, 1998
Helochares (Hydrobaticus) loticus Hebauer, 1998: 43 – Thailand (north), Lom Sak, 40 km N Phetchabun; Hansen 1999b: 168 [catalog].
Distribution: Indo-Malayan: Thailand.
Helochares loweryae Watts, 1995
Helochares (Hydrobaticus) loweryae Watts, 1995: 122 – Papua New Guinea, Mt. Lamington; Hansen 1999b: 168 [catalog].
Distribution: Australasian: Australia (Northern Territory), Papua New Guinea.
Helochares luridus (MacLeay, 1871)
Hydrobaticus luridus MacLeay, 1871: 131 – Australia, Queensland, Gayndah.
Hydrobaticus tristis var. luridus MacLeay, 1871; Blackburn, 1893: 99 [faunistic treatment].
Helochares (Hydrobaticus) luridus (MacLeay, 1871); Watts, 1995: 122 [valid species, not synonym of Helochares tristis MacLeay, 1871, as in Zaitzev 1908: 390); Hansen 1999b: 168 [catalog]; Watts 2002: 120 [description of larva with Helochares tristis MacLeay].
Distribution: Australasian: Australia (New South Wales, Northern Territory, Queensland, Western Australia).
Helochares lutulentus Balfour-Browne, 1952
Helochares (Hydrobaticus) lutulentus Balfour-Browne, 1952b: 516 – Mauritania, Kédia d’Idjil; Hebauer 1996: 18 [faunistic treatment]; Hansen 1999b: 168 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Mauritania, Morocco [in doubt].
Helochares maculatus Hebauer, 1988
Helochares (Helocharimorphus) maculatus Hebauer, 1988: 157 – Namibia, Okavango, Nyangana; Hebauer 1995a: 265 [faunistic treatment]; Hebauer 1996: 9 [faunistic treatment]; Hansen 1999b: 164 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Namibia.
Helochares maculicollis Mulsant, 1844
Helochares maculicollis Mulsant, 1844b: 379 – U.S.A., Louisiana [Louisiane]; Richmond 1920: 62 [description of immature stages]; Archangelsky 1997: 49 [redescription of immature stages].
Philhydrus maculicollis (Mulsant, 1844); Lacordaire, 1854: 457 [faunistic treatment].
Philhydrus (s. str.) maculicollis (Mulsant, 1844); LeConte 1855: 370 [faunistic treatment].
Helochares (Grapidelochares) maculicollis Mulsant, 1844; Zaitzev 1908: 381 [catalog].
Helochares (Hydrobaticus) maculicollis Mulsant, 1844; Hansen 1999b: 168 [catalog]; Short 2005: 218 [faunistic treatment]; Short and Girón 2018: 36 [taxonomic review].
? Helochares bipunctatus Sharp, 1882: 76. – Mexico (Cordova) and Guatemala (Torola); d’Orchymont 1943b: 3 [synonymy in doubt].
Helochares (Grapidelochares) bipunctatus Sharp, 1882; Zaitzev, 1908a: 381.
Distribution: Nearctic: U.S.A. (Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Mississippi, Missouri, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia). Neotropical: Guatemala [in doubt], Mexico.
Helochares madli Hebauer, 2002
Helochares (s. str.) madli Hebauer, 2002b: 15 – Madagascar, Mahajanga Katsepi; Hebauer 2006a: 25 [checklist]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Madagascar.
Remarks: This species was described from a single female specimen. According to Hebauer (2002b) it is similar to a small Helochares dilutus, but with shorter maxillary palps and different elytral punctation. Given that the male of this species remains unknown, the placement of this species in Helochares needs to be confirmed.
Helochares marreensis Watts, 1995
Helochares (Hydrobaticus) marreensis Watts, 1995: 123 – Australia, Northern Territory, 7 km NW by N of Cahills Crossing, East Alligator River, 12°23'S, 132°56'E; Hansen 1999b: 168 [catalog].
Distribution: Australasian: Australia (New South Wales, Northern Territory, Queensland, South Australia, Victoria, Western Australia).
Helochares mecarus d’Orchymont, 1939
Helochares (Hydrobaticus) mecarus d’Orchymont, 1939b: 310 – Ethiopia, Arussi Galla, A. Ganale Gudda; Hebauer 1996: 19 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Botswana, Ethiopia, Kenya, Namibia, Zambia.
Helochares mediastinus d’Orchymont, 1939
Helochares (Hydrobaticus) mediastinus d’Orchymont, 1939b: 311 – Ethiopia, Arussi Galla, A. Ganale Gudda; Hebauer 1996: 19 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Angola, Benin, Ethiopia, Kenya, Madagascar, Namibia, Tanzania.
Helochares melanophthalmus (Mulsant, 1844)
Helophilus melanophthalmus Mulsant, 1844a: 137 (ascribed to Dufour) – Sudan [in doubt: type locality probably Sudan (d’Orchymont 1936a), not Spain [Espagne] as stated in the original description].
Hydrobius melanophthalmus (ascribed to Dufour); Dejean 1833: 134 [nomen nudum].
Helochares melanophthalmus (Mulsant, 1844); Rey 1885b: 288 [specific rank confirmed; not synonym of dilutus Erichson, 1843, as in Reiche and Saulcy 1856: 358].
Helochares (Graphelochares) melanophthalmus (Mulsant, 1844); Kuwert 1890: 39 [catalog]; Heyden 1891: 67 [catalog].
Helochares (Hydrobaticus) melanophthalmus (Mulsant, 1844); Hebauer 1996: 19 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hebauer 2006a: 26 [checklist]; Salah and Régil Cueto 2017: 270 [excluded from Egypt].
Distribution: Afrotropical: Cameroon, Ethiopia, Ghana, Ivory Coast, Nigeria, Senegal, Seychelles, Sudan.
Helochares mendosus Hebauer, 1996
Helochares (Hydrobaticus) mendosus Hebauer, 1996: 19 – Ghana, Ashanti region, Bobiri forest reserve 6°40'N, 1°15'W; Hansen 1999b: 19 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Ghana.
Helochares mentinotus Kuwert, 1888
Helochares mentinotus Kuwert, 1888: 292 – Egypt [Aegyptus].
Helochares (Crephelochares) mentinotus Kuwert, 1888; Kuwert 1890a: 38 [faunistic treatment].
Helochares (Chasmogenus) mentinotus Kuwert, 1888; Knisch 1824a: 195 [checklist].
Helochares (Hydrobaticus) mentinotus Kuwert, 1888; d’Orchymont 1936d: 6 [taxonomic treatment]; Balfour-Browne, 1950b: 57 [faunistic treatment]; Hebauer 1994: 112 [faunistic treatment]; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hansen 2004: 52 [catalog]; Hebauer 2006a: 26 [checklist]; Fikáček et al. 2015: 62 [catalog]; Salah and Régil Cueto 2017: [faunistic treatment].
Helochares squalidus Sharp, 1903: 7 – South Sudan (White Nile River; Jebel Ahmed Agha; north of Jebel Ahmed Agha; north of Kaka; d’Orchymont 1936d: 6 [synonymy].
Helochares (Grapidelochares) squalidus Sharp, 1903; Zaitzev 1908: 381 [checklist].
Distribution: Afrotropical: Chad, Democratic Republic of the Congo [Zaire; DR Congo], Ethiopia [Abyssinia], Kenya, South Sudan, Uganda. Palearctic: Egypt, Israel.
Helochares menulus d’Orchymont, 1943
Helochares (Hydrobaticus) menulus d’Orchymont, 1943a: 10 – Democratic Republic of the Congo [Congo Belge; Zaire], Nizi-Blukwa; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hebauer 2006a: 26
Distribution: Afrotropical: Democratic Republic of the Congo [Zaire; DR Congo], Kenya, Nigeria, Tanzania.
Helochares meracus Balfour-Browne, 1950
Helochares (Hydrobaticus) meracus Balfour-Browne, 1950a: 395 – Zambia [Northern Rhodesia], Nama-ula; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hebauer 2005: 39 [checklist]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Ethiopia, Malawi, Republic of South Africa [in doubt], Zambia.
Helochares mersus d’Orchymont, 1939
Helochares (Hydrobaticus) mersus d’Orchymont, 1939b: 307 – Ethiopia [Abyssinie]; Balfour-Browne 1950b: 56 [faunistic treatment]; Hebauer 1988: 156 [faunistic treatment]; Hebauer 1995a: 264 [faunistic treatment]; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hebauer 2005: 39 [checklist]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Botswana [in doubt; “Kalahari”], Democratic Republic of the Congo [Zaire; DR Congo], Ethiopia, Kenya, Malawi, Namibia, Rwanda, Tanzania, Uganda, Zimbabwe.
Helochares minax d’Orchymont, 1939
Helochares (Hydrobaticus) minax d’Orchymont, 1939b: 316 – Uganda, Kampala; Balfour-Browne 1950b: 57 [faunistic treatment]; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Rwanda, Uganda, Gabon [in doubt], Kenya, Tanzania.
Helochares minor d’Orchymont, 1925
Helochares (Hydrobaticus) minor d’Orchymont, 1925c: 293 – Vietnam [Indo-Chine], Cha Pa; d’Orchymont 1928: 106 [faunistic treatment]; d’Orchymont 1943e: 9 [faunistic treatment]; Hansen 1999b: 189 [catalog]; Dong and Bian 2021: 167 [new record].
Distribution: Indo-Malayan: China (Hainan), India (Bihar), Vietnam.
Helochares minusculus d’Orchymont, 1943
Helochares (Hydrobaticus) minusculus d’Orchymont, 1943e: 10 – Indonesia, North Sumatra, Danau Toba region, nr Huta Gindjang; Hansen 1999b: 169 [catalog]; Hebauer and Ryndevich 2005: 46 [new record].
Distribution: Indo-Malayan: Burma, Indonesia (Sumatra), Laos.
Helochares namcatensis Hebauer, 2002
Helochares (Hydrobaticus) namcatensisHebauer 2002b: 12 – Vietnam, Nam Cat Tien National Park; Hebauer 2002b: 12 [faunistic treatment]; Short and Hebauer 2006: 336 [catalog].
Distribution: Indo-Malayan: Vietnam.
Helochares nebridius d’Orchymont, 1940
Helochares (Hydrobaticus) nebridius d’Orchymont, 1940: 169 – Indonesia, Sumatra, Palembang; Hebauer 1995b: 5 [faunistic treatment]; Hansen 1999b: 169 [catalog].
Distribution: Indo-Malayan: Indonesia (Java, Lombok, Sumatra), Singapore.
Helochares negatus Hebauer, 1995
Helochares (Hydrobaticus) negatus Hebauer, 1995b: 5 – Bangladesh, Dinajpur; Hansen 1999b: 169 [catalog]; Hebauer 2002a: 24 [new record]; Hansen 2004: 52 [catalog]; Hebauer and Ryndevich 2005: 46 [new record]; Fikáček et al. 2015: 62 [catalog].
Distribution: Indo-Malayan: Bangladesh, India (Tamil Nadu), Nepal.
Helochares neglectus (Hope, 1845)
Hydrobius neglectus Hope, 1845: 16 – China, Guangdong, Guangzhou, Canton; Gentili et al. 1995: 211 [catalog].
Helochares (Hydrobaticus) neglectus (Hope, 1845); d’Orchymont 1919c: 150 [new combination in doubt]; d’Orchymont 1940b: 166 [new combination confirmed]; Hebauer 1995b: 6 [faunistic treatment]; Hansen 1999b: 169 [catalog]; Hansen 2004: 52 [catalog]; Fikáček et al. 2015: 62 [catalog]; Dong and Bian 2021: 167 [checklist].
Distribution: Indo-Malayan: Cambodia, China (Fujian, Guangdong, Guangxi, Hainan, Jiangxi, Yunnan, Zhejiang), Malaysia (Peninsula), Thailand, Vietnam. Palearctic: China (Hubei, Jiangsu, Shanghai, Sichuan).
Helochares nexus Short & Girón, 2018
Helochares (Hydrobaticus) nexus Short & Girón, 2018: 39 – Panama, Coclé Province, 8°39'05.2"N, 80°35'18.7"W.
Distribution: Neotropical: Ecuador, Panama, Venezuela.
Helochares nigrifrons Brancsik, 1893
Helochares melanophthalmus var. nigrifrons Brancsik, 1893: 219 – Madagascar, Nosy Bé [Nossibé]; Régimbart 1900: 50 [faunistic treatment].
Helochares (Grapidelochares) nigrifrons Brancsik, 1893; Zaitzev 1908: 381 [catalog].
Helochares (Hydrobaticus) melanophthalmus var. nigrifrons Brancsik, 1893; Knisch 1924: 194 [catalog].
Helochares (Hydrocaticus) nigrifrons Brancsik, 1893; d’Orchymont 1939b: 297 [specific rank confirmed; subgeneric name misspelled].
Helochares (Hydrobaticus) nigrifrons Brancsik, 1893; d’Orchymont 1941: 15 [list]; Hebauer 1996: 20 [new records]; Hansen 1999b: 170 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Madagascar, Seychelles (Aldabra), Tanzania.
Helochares nigripalpis Hebauer & Hendrich, 1999
Helochares (Hydrobaticus) nigripalpis Hebauer & Hendrich, 1999: 48 – Australia, Northern Territory, Kakadu National Park, Jim Jim Falls Camp Area, 13°16.218'S, 132°49.276'E; Short and Hebauer 2006: 336 [catalog].
Distribution: Australasian: Australia (Northern Territory).
Helochares nigritulus Kuwert, 1889
Helochares nigritulus Kuwert, 1889: 8 [and 1890a: 34] – Italy, Sicily
Helochares (s. str.) nigritulus Kuwert, 1889; Heyden 1891: 67 [catalog]; Hansen 1999b: 162 [catalog]; Hansen 2004: 52 [catalog]; Fikáček et al. 2015: 61 [catalog].
Distribution: Palearctic: Italy.
Helochares nigroseriatus Hebauer, 1998
Helochares (Hydrobaticus) nigroseriatus Hebauer, 1998c: 43 – Zimbabwe, vicinity of Kotwa, “Broken Causeway”, 17°0'S, 32°45'E; Hansen 1999b: 170 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Zambia, Zimbabwe.
Remarks: Hebauer (2002) indicates that the aedeagus of Helochares nigroseriatus corresponds to fig. 5 in Hebauer 1998.
Helochares niobelus d’Orchymont, 1939
Helochares (Hydrobaticus) niobelus d’Orchymont, 1939b: 308 – Democratic Republic of the Congo [Congo Belge; Zaire], Haut Uélé, Watsa; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 170 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Cameroon [in doubt], Democratic Republic of the Congo, Republic of South Africa, Uganda.
Helochares nipponicus Hebauer, 1995
Helochares striatus Sharp, 1873: 60 [secondary homonym of Hydrobius striatus Boheman, 1851: 599]; Hebauer 1995b: 6 [synonymy; not synonym of Helochares lepidus d’Orchymont, Helochares leptinus d’Orchymont or Helochares lentus Sharp, as in d’Orchymont 1943e: 6].
Helochares (Hydrobaticus) nipponicus Hebauer, 1995b: 6 [replacement name for Helochares striatus Sharp, 1873]; Hansen 1999b: 170 [catalog]; Hansen 2004: 52 [catalog]; Minoshima and Hayashi 2011: 64 [description of immature stages]; Fikáček et al. 2015: 62 [catalog]; Dong and Bian 2021: 167 [new record].
Distribution: Palearctic: China (Jilin), Japan, South Korea.
Helochares normatus (LeConte, 1861)
Philhydrus normatus LeConte, 1861: 341 – U.S.A., California, Bodega.
Helochares normatus (LeConte, 1861); Horn 1890: 252 [faunistic treatment].
Chasmogenus normatus (LeConte, 1861); Zaitzev 1908: 383 [catalog].
Helochares (Hydrobaticus) normatus (LeConte, 1861); Knisch 1924: 194 [catalog]; Hansen 1999b: 170 [catalog]; Short 2005: 218 [new records]; Short and Girón 2018: 42 [taxonomic treatment].
Helochares seriatus Sharp, 1882: 76 – Guatemala (Guatemala City; Pantaleon; Coatepeque; Rio Naranjo; San Gerónimo); d’Orchymont 1943b: 4 [synonymy].
Helochares (Grapidelochares) seriatus Sharp, 1882; Zaitzev 1908: 381 [catalog].
? Helochares regularis Sharp, 1882: 76 – Mexico – d’Orchymont 1943d: 4 [synonymy in doubt].
? Helochares (Grapidelochares) regularis Sharp, 1882; Zaitzev 1908a: 381 [catalog].
Distribution: Nearctic: USA (Arizona, California, Nevada, Oregon, Texas). Neotropical: Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua.
Helochares notaticollis Régimbart, 1906
Helochares melanophthalmus var. notaticollis Régimbart, 1906: 260 – Kenya, Nairobi.
Helochares (Hydrobaticus) notaticollis Régimbart; Balfour-Browne 1950a: 394 [faunistic treatment]; Balfour-Browne 1950b: 54 [faunistic treatment]; d’Orchymont, 1936b: 111 [specific rank confirmed]; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 170 [catalog]; Hebauer 2005: 39 [checklist]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Kenya, Malawi, Rwanda, Tanzania, Uganda.
Helochares notaticollis ssp. curtus Régimbart, 1906
Helochares melanophthalmus var. curtus Régimbart, 1906: 260 – Kenya, Bura.
Helochares (Hydrobaticus) notaticollis var. curtus Régimbart, 1906; d’Orchymont, 1936a: 111.
Helochares (Hydrobaticus) notaticollis ssp. curtus Régimbart, 1906; Hansen 1999b: 170 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Kenya.
Helochares obliquus Mart, İncekara & Karaca, 2010
Helochares obliquus Mart, İncekara & Karaca, 2010: 299 – Turkey, Ordu province, Mesudiye, Lake Ulugöl, 40°24'N, 37°49'E.
Helochares (s. str.) obliquus Mart, İncekara & Karaca, 2010; Darilmaz and İncekara 2011: 711 [checklist]; Short and Fikáček 2011 [catalog]; Fikáček et al. 2015: 61 [catalog].
Distribution: Palearctic: Turkey.
Helochares obscurus (Müller, 1776)
Hydrophilus obscurus Müller, 1776: 69 – Denmark and Norway [Dania et Norvegia].
Helochares (s. str.) obscurus (Müller, 1776); d’Orchymont 1933: 306 [specific rank confirmed; not synonym of Helochares griseus Fabricius, as in Illiger 1798: 246; not synonym of Helochares lividus Forster, as in Mulsant 1844a: 134]; Hebauer 1994: 113 [faunistic treatment]; Hansen 1999b: 162 [catalog]; Hansen 2004: 52 [catalog]; Hebauer and Ryndevich 2005: 45 [new records]; Mart et al. 2010: 299 [faunistic treatment]; Darilmaz and İncekara 2011: 711 [checklist]; Fikáček et al. 2015: 62 [catalog]; Jia and Tang 2018b: 12 [redescription; new record].
Hydrophilus erythrocephalus Fabricius, 1792: 185 – No type locality given; Hansen 1982: 207 [synonymy; not synonym of Helochares griseus Fabricius, as in Erichson 1837: 211].
Helophilus lividus var. erythrocephalus (Fabricius, 1792); Mulsant 1844a: 135 [faunistic treatment].
Philhydrus lividus var. erythrocephalus (Fabricius, 1792); Gemminger and Harold 1868: 481 [catalog].
Helochares (s. str.) erythrocephalus (Fabricius, 1792); Kuwert 1890a: 37 [taxonomic treatment].
Helochares erythrocephalus (Fabricius, 1792); Heyden 1891: 67 [catalog].
Hydrophilus variegatus Herbst, 1797: 304 – Germany [... in hiesigen Gewässern (i.e., German waters)]; Hansen 1982: 207 [synonymy; not synonym of Helochares griseus Fabricius, as in Illiger 1801a: 60].
Hydrophilus griseus var. variegatus Herbst, 1797; Gyllenhal, 1808: 122 [faunistic treatment].
Philhydrus lividus var. variegatus (Herbst, 1797); Gemminger and Harold 1868: 481 [catalog].
Hydrobius lividus; Stephens, 1829: 130 [misinterpretation of Dytiscus lividus Forster].
Philhydrus lividus; Stephens, 1839: 91 [misinterpretation of Dytiscus lividus Forster].
Helochares subcompressus Rey, 1885a: 14 – France, Lille; Hansen 1982: 207 [synonymy; (Fauvel, 1895: 92 [synonym of erythrocephalus Fabricius]); not synonym of Helochares griseus Fabricius, as in Ganglbauer, 1904: 249)]; Heyden 1891: 67 [catalog].
Helochares erythrocephalus var. substriatus Sahlberg, 1903: 20 – Greece, Corfu, Stravopotamos [(Corcyra): prope flumen Stravopotamos]; Hansen 1982: 207 [synonymy].
Helochares (s. str.) griseus (?) var. substriatus Sahlberg, 1903; Zaitzev 1908: 382 [catalog].
Helochares griseus a. Mülleri Reitter, 1909a: 364 [infrasubspecific name; unavailable under ICZN Code Art. 1b (5), 45f)]; Hansen 1982: 207 [synonymy].
? Hydrophilus chrysomelinus; Panzer, 1795: 72 [misinterpretation of Dytiscus chrysomelinus Fabricius]. Hansen, 1982: 202 [synonymy in doubt; not synonym of griseus Fabricius, as in Schönherr 1808: 7 – in doubt; not synonym of Helochares pallidus Rossi, as in Mulsant 1844a: 135].
? Philhydrus lividus var. chrysomelinus (Panzer, 1795); Gemminger and Harold 1868: 481 [catalog].
Helochares obscurus (Müller, 1776); Angus et al. 2020: 21 [karyotype].
Distribution: Palearctic: Austria, Azerbaijan, Belarus, China (Xinjiang), Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Georgia, Great Britain, Greece, Hungary, Iran, Israel, Italy, Latvia, Lithuania, Luxembourg, Montenegro, Netherlands, Norway, Poland, Russia, Slovakia, Sweden, Switzerland, Turkey.
Helochares opacus Hebauer, 2009
Helochares (Hydrobaticus) opacus Hebauer, 2009: 5 – Gabon, Monts de Cristal National Park, Asseng Assala Village; Short and Fikáček 2011: 91 [catalog].
Distribution: Afrotropical: Gabon.
Helochares pallens (MacLeay, 1825)
Enhydrus pallens MacLeay, 1825: 35 – Indonesia, Java.
Philhydrus pallens (MacLeay, 1825); Gemminger and Harold 1868: 482 [catalog].
Enochrus (Lumetus) pallens (MacLeay, 1825); Zaitzev 1908: 388 [catalog].
Helochares pallens (MacLeay, 1825); Gentili et al. 1995: 211 [catalog].
Helochares (s. str.) pallens (MacLeay, 1825); d’Orchymont 1926b: 232 [new combination]; d’Orchymont 1928: 107 [faunistic treatment]; Balfour-Browne 1950b: 59 [faunistic treatment]; Balfour-Browne 1951: 213 [faunistic treatment]; Balfour-Browne 1952a: 129 [faunistic treatment]; Balfour-Browne 1957: 21 [faunistic treatment]; Hebauer 1988: 156 [faunistic treatment]; Hebauer 1994: 113 [faunistic treatment]; Hebauer 1995a: 265 [faunistic treatment]; Hebauer 1995b: 7 [faunistic treatment]; Hebauer 1996: 8 [faunistic treatment]; Hebauer 1997: 263 [faunistic treatment]; Hansen 1999b: 162 [catalog]; Hebauer 2002a: 24 [new record]; Hansen 2004: 52 [catalog]; Hebauer 2005: 39 [checklist]; Hebauer and Ryndevich 2005: 46 [new record]; Hebauer 2006a: 25 [checklist; new records]; Mart et al. 2010: 298 [new record]; Short 2010: 312 [faunistic treatment]; Darilmaz and İncekara 2011: 711 [checklist]; Minoshima and Hayashi 2011: 53 [description of larva]; Fikáček et al. 2015: 62 [catalog]; Jia and Tang 2018: 15 [redescription].
Helochares parvulus Reiche and Saulcy [in Reiche 1854: 9 – nomen nudum].
Helochares parvulus Reiche & Saulcy, 1856: 359 – Lebanon, Beirut [Beyrouth]; d’Orchymont 1927b: 6 [synonymy]; d’Orchymont 1932: 688 [faunistic treatment].
Philhydrus parvulus (Reiche & Saulcy, 1856); Gemminger and Harold 1868: 482 [catalog].
Enochrus (Methydrus) parvulus (Reiche & Saulcy, 1856); Zaitzev 1908: 384 [catalog].
? Helochares simplex Wollaston, 1867: 44 [published in synonymy with dilutus Erichson; unavailable under ICZN Code Art. 11e]; d’Orchymont 1943e: 8 [synonymy in doubt].
Helochares lewisius Sharp, 1873: 60 – Japan (Kyushu (Nagasaki), and Honshu (Hyogo)) [Nagasaki and Hiogo]; Balfour-Browne 1939: 293 [synonymy].
Helochares (s. str.) lewisianus Sharp, 1873; Zaitzev 1908: 382 [catalog; misspelled].
? Philhydrus parvulus Guillebeau, 1896: 226 – “Le Cuire” [secondary homonym of Helochares parvulus Reiche & Saulcy, 1856; possibly synonym of the same, as in Knisch 1924: 219]; Handen 1999b: 162 [synonymy confirmed].
Helochares dispar Sharp, 1903: 7 – Sudan (White Nile River; Jebel Ahmed Agha; north of Jebel Ahmed Agha; north of Kaka); d’Orchymont 1926b: 232 [synonymy].
Helochares laeviusculus Régimbart, 1906: 261 – Kenya, Lake Victoria, Winam Gulf [Baie de Kavirondo]; Hebauer 1996: 8 [synonymy].
Helochares (s. str.) pallensssp. laeviusculus Régimbart, 1906 – Democratic Republic of the Congo, Ishango, Semliki River; Balfour-Browne 1950b: 60 [new combination]; Hebauer 2006a: 25 [checklist].
Distribution: Afrotropical: Benin, Botswana, Cameroon, Chad, Democratic Republic of the Congo, Ethiopia, Ghana, Guinea, Ivory Coast, Kenya, Liberia, Madagascar, Namibia, Rwanda, Republic of South Africa, Sudan, Tanzania, Uganda, Yemen, Zambia, Zimbabwe. Indo-Malayan: Bangladesh, Burma, China (Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hong Kong, Hunan, Jiangxi, Macao, Yunnan), India (Assam, Bihar), Indonesia (Java, Sumatra), Laos, Malaysia (Peninsula), Nepal, Philippines, Sri Lanka, Thailand. Palearctic: China (Chongqing, Hubei, Shaanxi, Sichuan, Xizang [Tibet]), Egypt, Israel, Japan, Lebanon, Pakistan, Syria, Turkey. Australasian: Papua New Guinea (New Guinea), Vanuatu.
Helochares parallelus Hebauer, 1999
Helochares (Hydrobaticus) parallelusHebauer 1999: 11 – Botswana, Kasane Chobe Safari Lodge, Chobe Banks; Hebauer 2006a: 26 [checklist]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Botswana, Republic of South Africa.
Helochares percyi Watts, 1995
Helochares (Hydrobaticus) percyi Watts, 1995: 125 – Australia, Queensland (N.), Boar Pocket Road; Hansen 1999b: 170 [catalog].
Distribution: Australasian: Australia (Australian Capital Territory, New South Wales, Northern Territory, Queensland, Western Australia).
Helochares perminutus Hebauer, 1996
Helochares (Hydrobaticus) perminutus Hebauer, 1996: 20 – Nigeria [Nig.], Pandam W.P. River Li; Hebauer 1996: 20 [faunistic treatment]; Hansen 1999b: 170 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Ghana, Nigeria, Sierra Leone.
Helochares phallicus d’Orchymont, 1936
Helochares (Hydrobaticus) phallicus d’Orchymont, 1936b: 111 – Botswana, Makgadikgadi [Makarikari], Nkate; Hebauer 1995a: 264 [faunistic treatment]; Hebauer 1996: 21 [faunistic treatment]; Hansen 1999b: 170 [checklist]; Hebauer 2005: 39 [checklist]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Angola, Botswana, Malawi, Namibia, Republic of South Africa, Zambia, Zimbabwe.
Helochares politus Short & Girón, 2018
Helochares (Hydrobaticus) politus Short & Girón, 2018: 45 – Guatemala, Departamento de Huehuetenango, 11 km N. Santa Eulalia on road to San Mateo Ixtatán.
Distribution: Neotropical: Guatemala.
Helochares punctatus Sharp, 1869
Helochares punctatus Sharp, 1869: 241 – England (Whittlesea, Mere, Cambridge, London and the New Forest); Hansen 1982: 206 [specific rank confirmed; not synonym of erythrocephalus Fabricius, as in Heyden 1891: 67; not synonym of griseus Fabricius, as in Ganglbauer 1904: 249]; Angus et al. 2020: 21 [karyotype].
Helochares punctulatus Sharp, 1869 [misspelling]; Bedel 1881a: 312 [catalog]; Heyden 1891: 67 [catalog].
Helochares (s. str.) punctatus Sharp, 1869; Hansen 1999b: 163 [catalog]; Hansen 2004: 52 [catalog]; Hebauer and Ryndevich 2005: 45 [new records]; Darilmaz and İncekara 2011: 711 [checklist]; Fikáček et al. 2015: 62 [catalog]; Benamar et al. 2021: 35 [checklist].
Distribution: Palearctic: Belarus, Denmark, France, Germany, Great Britain, Hungary, Ireland, Lithuania, Luxembourg, Netherlands, Portugal, Russia, Spain, Turkey, Ukraine.
Helochares rugipennis Balfour-Browne, 1958
Helochares (Hydrobaticus) rugipennis Balfour-Browne, 1958a: 183 – Mali [“French Sudan”], Source Sanga; Hebauer 1996: 21 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Guinea, Ivory Coast, Mali, Nigeria, Sierra Leone.
Helochares salvazai d’Orchymont, 1919
Helochares (Hydrobaticus) salvazai d’Orchymont, 1919a: 76 (and 1921: 11) – Cambodia; d’Orchymont 1928: 106 [faunistic treatment]; d’Orchymont 1943e: 10 [faunistic treatment]; Hansen 1999b: 171 [catalog].
Distribution: Indo-Malayan: Cambodia.
Helochares sauteri d’Orchymont, 1943
Helochares (Hydrobaticus) sauteri d’Orchymont, 1943e: 6 – Taiwan [Formose], “Kosempo”; Hansen 1999b: 171 [catalog]; Hansen 2004: 52 [catalog]; Fikáček et al. 2015: 62 [catalog]; Dong and Bian 2021: 167 [checklist].
Helochares sauteri d’Orchymont; Gentili et al. 1995 [catalog]; Angus et al. 2020: 21 [karyotype].
Distribution: Indo-Malayan: China (Guangdong, Guizhou, Jiangxi, Taiwan, Zhejiang). Palearctic: China (Hubei, Sichuan).
Helochares schoedli Hebauer, 1996
Helochares (Hydrobaticus) schoedli Hebauer, 1996: 22 – Democratic Republic of the Congo [Zaire; Haut-Zaire], Dungu; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo.
Helochares schwendingeri Hebauer, 1995
Helochares (Hydrobaticus) schwendingeri Hebauer, 1995b: 7 – Thailand, Chiang Mai; Hansen 1999b: 171 [catalog]; Hebauer and Ryndevich 2005: 46 [new record].
Helochares (Hydrobaticus) ubudensis Hebauer, 1998: 44 – Indonesia, Bali, Ubud; Hansen 1999b: 171; Hebauer 2002b: 13 [synonymy]; Short and Hebauer 2006: 337 [catalog].
Distribution: Indo-Malayan: Indonesia (Bali), Laos, Malaysia (Peninsula), Thailand, Vietnam.
Remarks: Hebauer (2002b) indicates that the aedeagus of Helochares schwendingeri (as Helochares ubudensis) corresponds to fig. 4 in Hebauer 1998.
Helochares scitulus Balfour-Browne, 1952
Helochares (Hydrobaticus) scitulus Balfour-Browne, 1952a: 130 – Benin [Dahomey], Bassila; Hebauer 1996: 22 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Benin, Gambia, Ghana, Mali, Senegal, Sudan.
Helochares sechellensis Régimbart, 1903
Helochares (Graphelochares) melanophthalmus var. sechellensis Régimbart, 1903a: 27 – Seychelles [Iles Séchelles].
Helochares (Hydrobaticus) sechellensis Régimbart, 1903; d’Orchymont 1939b: 297 [specific rank confirmed]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Seychelles.
Helochares serpentinus Hebauer, 1998
Helochares (Hydrobaticus) serpentinus Hebauer, 1998: 44 – Republic of South Africa, Wilderness National Park, Lang Wie, 33°59'0"S, 22°40'6"E); Hansen 1999b: 171 [catalog]; Hebauer 2006a: 26 [checklist].
Distribution: Afrotropical: Republic of South Africa.
Helochares sharpi (Kuwert, 1890)
Helocharimorphus sharpi Kuwert, 1890a: 63 (and 1890b: 306) – Egypt [Aegypten]; Syria, Lebanon or Israel [Syria]; Iraq [Mesopotamien].
Helochares (Helocharimorphus) sharpi (Kuwert, 1890); Knisch 1924: 195 [catalog]; Hebauer 1994: 113 [faunistic treatment]; Hansen and Hebauer 1988: 29 [in key]; Hansen 1999b: 164 [catalog]; Hansen 2004: 52 [catalog]; Hebauer 2006a: 27 [checklist]; Fikáček et al. 2015: 62 [catalog]; (İncekara et al. 2016: 22 [new record]; Salah and Régil Cueto 2017: 265 [faunistic treatment].
Distribution: Afrotropical: Ghana, Madagascar, Tanzania, Togo, Uganda, Zambia. Palearctic: Egypt, Iraq, Israel, Turkey.
Helochares silvester Hebauer, 2009
Helochares (Hydrobaticus) silvester Hebauer, 2009: 5 – Republic of the Congo, Brazzaville, d’Odzala Mboko National Park; Short and Fikáček 2011: 91 [catalog].
Distribution: Afrotropical: Republic of the Congo.
Helochares simulator Knisch, 1922
Helochares (Hydrobaticus) simulator Knisch, 1922: 104 – Papua New Guinea, Bismarck Archipelago, Duke of York [not “Duke of York” (= Atafu) in Polynesia]; d’Orchymont 1943a: 7 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Short 2010: 313 [faunistic treatment].
Distribution: Australasian: Fiji, Papua New Guinea (Duke of York). Oceanian: Samoa, Tonga.
Helochares skalei Hebauer, 2002
Helochares (Hydrobaticus) skalei Hebauer, 2002b: 13 – South Africa, Mpumalanga White River, White River behind Staudamm, Quelle; Hebauer 2005: 39 [checklist]; Hebauer 2006a: 27 [checklist]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Malawi, Republic of South Africa, Zimbabwe.
Helochares songi Jia & Tang, 2018
Helochares (s. str.) songi Jia & Tang, 2018b: 3 – China, Guangxi Province, Shiwandashan, Nalin River.
Distribution: Indo-Malayan: China (Guangxi).
Helochares steffani Hebauer, 2002
Helochares (Hydrobaticus) steffani Hebauer, 2002b: 13 – Namibia, Ongongo falls, 19°08'S, 13°49'W, ca. 6 km upp. Warmquelle; Hebauer 2006a: 27 [catalog]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Namibia.
Helochares stenius d’Orchymont, 1943
Helochares (Hydrobaticus) stenius d’Orchymont, 1943a: 8 – Democratic Republic of the Congo [Congo Belge; Zaire], Lubutu nr Kisangani [Stanleyville]; Hebauer 1996: 22 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Gabon, Republic of the Congo.
Helochares striatus (Boheman, 1851)
Hydrobius striatus Boheman, 1851: 599 – Republic of South Africa, Natal [terra Natalensi].
Helochares striatus (Boheman, 1851); Bedel 1880: CXLVIII [new combination].
Helochares (Hydrobaticus) striatus (Boheman, 1851); d’Orchymont 1919c: 150 [faunistic treatment]; d’Orchymont 1943e: 6 [faunistic treatment]; Balfour-Browne 1950a: 394 [faunistic treatment]; Hebauer 1996: 22 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Gambia, Senegal, Sierra Leone, Republic of South Africa, Uganda.
Helochares strictus d’Orchymont, 1939
Helochares (Hydrobaticus) strictus d’Orchymont, 1939b: 306 – Tanzania, Lake Victoria, Ukerewe I; Balfour-Browne 1950b: 55 [faunistic treatment]; Hebauer 1996: 22 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Cameroon, Democratic Republic of the Congo, Ghana, Guinea, Kenya, Rwanda, Senegal, Tanzania, Uganda.
Helochares strigellus Hebauer, 2002
Helochares (Hydrobaticus) strigellus Hebauer, 2002b: 14 – Liberia, Saclepea; Hebauer 2006a: 27 [checklist]; Short and Hebauer 2006: 336 [catalog].
Distribution: Afrotropical: Kenya, Liberia.
Helochares structus d’Orchymont, 1936
Helochares (Hydrobaticus) structus d’Orchymont, 1936b: 112 – Botswana, Kasane; Hebauer 1988: 156 [faunistic treatment]; Hebauer 1995a: 264 [faunistic treatment]; Hebauer 1996: 23 [faunistic treatment]; Hansen 1999b: 171 [catalog].
Distribution: Afrotropical: Benin [in doubt], Botswana, Cameroon [in doubt], Congo, Gambia, Ghana [in doubt], Guinea, Ivory Coast, Liberia, Namibia, Republic of South Africa, Sudan, Tanzania, Zambia.
Helochares sublineatus Hebauer, 2002
Helochares (s. str.) sublineatusHebauer 2002b: 15 – Ghana, Tamale; Hebauer 2006a: 25 [checklist]; Short and Hebauer 2006: 337 [catalog].
Distribution: Afrotropical: Ghana, Nigeria.
Remarks: The aedeagus in this species is quite unusual among Helochares (Hebauer 2002b: fig. 8).
Helochares subseriatus Hebauer, 2009
Helochares (Hydrobaticus) subseriatus Hebauer, 2009: 5 – Gabon, Bateke Plateau National Park, Camp, Mbie; Short and Fikáček 2011: 91 [catalog].
Distribution: Afrotropical: Gabon.
Remarks: The species is described from a single female specimen.
Helochares subtilis d’Orchymont, 1936
Helochares (Hydrobaticus) subtilis d’Orchymont, 1936b: 112 – ? Botswana [“Kalahari”], “Tsotsoroga Pan”; Hebauer 1995a: 264 [faunistic treatment]; Hebauer 1996: 23 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Botswana, Cameroon, Democratic Republic of the Congo, Ethiopia, Namibia, Republic of the Congo, Republic of South Africa, Zimbabwe.
Helochares sufflavus Balfour-Browne, 1952
Helochares (Hydrobaticus) sufflavus Balfour-Browne, 1952a: 131 – Togo, Tohoun; Hebauer 1996: 23 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Togo.
Helochares sylvaticus Balfour-Browne, 1957
Helochares (Hydrobaticus) sylvaticus Balfour-Browne, 1957: 24 – Burundi [“Urundi”], Bururi; Hebauer 1996: 23 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Burundi, Democratic Republic of the Congo, Republic of the Congo.
Helochares tamsi Balfour-Browne, 1947
Helochares (Hydrobaticus) tamsi Balfour-Browne, 1947: 142 – São Tomé and Príncipe [West Africa], São Tomé; Hebauer 1996: 23 [faunistic treatment]; Hansen 1999b: 171 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Gabon, Kenya [in doubt], Republic of the Congo, São Tomé and Príncipe.
Helochares tatei (Blackburn, 1896)
Hydrobaticus tatei Blackburn, 1896: 258 – Australia, Palm Creek; Watts 1995: 126 [Lectotype designated].
Helochares (Hydrobaticus) tatei (Blackburn, 1896); Knisch 1924: 194 [catalog]; d’Orchymont 1943a: 5 [faunistic treatment]; Hansen 1999b: 171 [catalog].
Distribution: Australasian: Australia (New South Wales, Northern Territory, Queensland, South Australia, Western Australia).
Helochares tengchongensis Dong & Bian, 2021
Helochares (Hydrobaticus) tengchongensis Dong & Bian, 2021: 171 – China: Yunnan Province, Tengchong City, Lianghe County, Longhe Village, 1074 m, 24°48'21.158"N, 98°17'51.522"E.
Helochares tengchongensis Dong & Bian, 2021.
Distribution: Indo-Malayan: China (Yunnan).
Helochares tenuistriatus Régimbart, 1908
Helochares (Hydrobaticus) tenuistriatus Régimbart, 1908: 315 – Australia, Western Australia, Perth, Lake Monger [“Mongers Lake, N. de Subiaco”]; Knisch 1924: 194 [catalog]; d’Orchymont 1943a: 5 [faunistic treatment]; Watts 1995: 127 [faunistic treatment]; Hansen 1999b: 172 [catalog]; Watts 2002: 120 [description of larva with Helochares tristis MacLeay].
Distribution: Australasian: Australia (Western Australia).
Helochares tertius Hebauer, 1996
Helochares (Helocharimorphus) tertius Hebauer, 1996: 9 – Republic of the Congo, Mt. Fouari reservation, near Gabon; Hansen 1999b: 172 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Republic of the Congo.
Remarks: The species is described from a unique female.
Helochares thurmerae Watts, 1995
Helochares (Hydrobaticus) thurmerae Watts, 1995: 127 – Papua New Guinea, Morobe District, Gusap Markham Valley ca. 90 ml W of Lae; Hansen 1999b: 172 [catalog].
Distribution: Australasian: Papua New Guinea.
Helochares tristis (MacLeay, 1871)
Hydrobaticus tristis MacLeay, 1871: 131 – Australia, Queensland, Gayndah; Anderson 1976: 220 [description of immature stages]; Watts 2002: 119 [description of larva].
Helochares (Hydrobaticus) tristis (MacLeay, 1871); Knisch 1924: 194 [checklist]; d’Orchymont 1943a: 2 [faunistic treatment]; Hansen 1999b: 172 [catalog].
Hydrobaticus australis Blackburn, 1888: 823 – Australia, South Australia, Port Lincoln; Watts 1995: 128 [lectotype designated; synonymy].
Helochares (Hydrobaticus) australis (Blackburn, 1888); Knisch 1924: 193 [catalog]; d’Orchymont 1943a: 3 [faunistic treatment].
Distribution: Australasian: Australia (Australian Capital Territory, New South Wales, Northern Territory, Queensland, South Australia, Tasmania, Victoria, Western Australia).
Helochares trujillo Short & Girón, 2018
Helochares (Hydrobaticus) trujillo Short & Girón, 2018: 45 – Venezuela, Mérida State, Mérida, Monte Zerpa Area.
Distribution: Neotropical: Venezuela.
Helochares uenoi Matsui, 1995
Helochares (Hydrobaticus) uenoi Matsui, 1995: 317 – Japan, Okinawa Islands, Yonaguni Island, Tindabana; Hansen 1999b: 172 [catalog]; Hansen 2004: 52 [catalog]; Fikáček et al. 2015: 62 [catalog].
Distribution: Palearctic: Japan.
Helochares uhligi Hebauer, 1999
Helochares (s. str.) uhligiHebauer 1999: 11 – Republic of South Africa, Cape Province, Karoo National Park, Mountain View River; Hebauer 2006a: 26 [checklist]; Short and Hebauer 2006: 337 [catalog].
Distribution: Afrotropical: Republic of South Africa.
Helochares vitalisi d’Orchymont, 1919
Helochares (s. str.) vitalisi d’Orchymont, 1919a: 78 (and 1921c: 13) – Cambodia, Phnom Penh; d’Orchymont 1928: 108 [faunistic treatment]; Hansen 1999b: 163 [catalog].
Distribution: Indo-Malayan: Cambodia.
Helochares wagneri Hebauer, 2002
Helochares (Hydrobaticus) wagneri Hebauer, 2002b: 14 – Kenya, Kakamega Forest, 0°22'N, 34°50'E; Hebauer 2006a: 27 [checklist]; Short and Hebauer 2006: 337 [catalog].
Distribution: Afrotropical: Kenya.
Helochares wattsi Hebauer & Hendrich, 1999
Helochares (Hydrobaticus) wattsi Hebauer & Hendrich, 1999: 50 – Australia: Northern Territory, Kakadu National Park, Jim Jim Hwy, Black Jungle Spring; Short and Hebauer 2006: 337 [catalog].
Distribution: Australasian: Australia (Northern Territory).
Remarks: The aedeagus in this species is quite unusual among Helochares (Hebauer and Hendrich 1999: fig. 4).
Helochares wuzhifengensis Dong & Bian, 2021
Helochares (Hydrobaticus) wuzhifengensis Dong & Bian, 2021: 170 – China: Jiangxi Province, Ganzhou City, Shangyou County, Wuzhifeng Town, 25°57'N, 114°05'E.
Helochares wuzhifengensis Dong & Bian, 2021.
Distribution: Indo-Malayan: China (Jiangxi).
Helochares yangae Hebauer, Hendrich & Balke, 1999
Helochares (Hydrobaticus) yangae Hebauer, Hendrich & Balke, 1999: 340 – Malaysia, Pahang, Lake Cini, lakeside near Rimba Resort; Short and Hebauer 2006: 337 [catalog].
Distribution: Indo-Malayan: Malaysia.
Helochares zamora Short & Girón, 2018
Helochares (Hydrobaticus) zamora Short & Girón, 2018: 46 – Ecuador, Zamora-Chinchipe Province, Zamora.
Distribution: Neotropical: Ecuador.
Helopeltarium d’Orchymont, 1943
Helopeltarium ferrugineum d’Orchymont, 1943
Helopeltarium ferrugineum d’Orchymont, 1943f: 10 – Burma, Dawna Range (eastside), “Sukli”.
Distribution: Indo-Malayan: Myanmar [Burma].
Katasophistes Girón & Short, 2018
Katasophistes charynae Girón & Short, 2018
Katasophistes charynae Girón & Short, 2018: 136 – Peru, Madre de Dios, Parque Manu, Pakitza, 12°07'S, 70°58'W.
Distribution: Neotropical: Peru.
Katasophistes cuzco Girón & Short, 2018
Katasophistes cuzco Girón & Short, 2018: 138 – Peru, Cuzco, Quita Calzón, at km 164, 13°09'S, 71°22'W.
Distribution: Neotropical: Peru.
Katasophistes merida Girón & Short, 2018: 138
Katasophistes merida Girón & Short, 2018: 138 – Venezuela, Mérida State, ca. 12 km SE of Santo Domingo, 8°51.933'N, 70°37.131'W.
Distribution: Neotropical: Venezuela.
Katasophistes superficialis Girón & Short, 2018
Katasophistes superficialis Girón & Short, 2018 – Ecuador, Pastaza Province: “AGIP platform Villano B, along transect 1 and 2.
Distribution: Neotropical: Ecuador.
Nanosaphes Girón & Short, 2018
Nanosaphes castaneus Girón & Short, 2018
Nanosaphes castaneus Girón & Short, 2018: 146 – Brazil, Pará, Rio Xingu Camp, Altamira ca. 60 km S, 3°39'S, 52°22'W.
Distribution: Neotropical: Brazil (Pará).
Nanosaphes hesperus Girón & Short, 2018
Nanosaphes hesperus Girón & Short, 2018: 148 – Suriname, Sipaliwini District, Camp 1, on Kutari River, 2°10.521'N, 56°47.244'W.
Distribution: Neotropical: Suriname.
Nanosaphes punctatus Girón & Short, 2018
Nanosaphes punctatus Girón & Short, 2018: 151 – Suriname, Sipaliwini District, Brownsberg Nature Park, 04°56.871'N, 55°10.911'W.
Distribution: Neotropical: Suriname.
Nanosaphes tricolor Girón & Short, 2018
Nanosaphes tricolor Girón & Short, 2018: 151 – Suriname, Sipaliwini District, Camp 4 (low), Kasikasima, trail to Kasikasima, 2.97731°N, 55.38500°W.
Distribution: Neotropical: Suriname.
Novochares Girón & Short gen. nov.
Novochares abbreviatus (Fabricius, 1801) comb. nov.
Hydrophilus abbreviatus Fabricius, 1801: 251 – [America meridionali].
Helochares (s. str.) abbreviatus (Fabricius, 1801); d’Orchymont 1939e: 258 [taxonomic treatment]; d’Orchymont 1943d: 55 [faunistic treatment]; Fernández, 1982a: 34 [taxonomic treatment]; Hansen 1999b: 159 [catalog]; Short 2005: 215 [new record]; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment]; Silva et al. 2018: 9 [faunistic treatment].
Helochares abbreviatus (Fabricius, 1801); Gonzalez-Rodriguez et al. 2017: 606 [checklist].
Philydrus pallidus Castelnau, 1840: 53 – Brazil (secondary homonym of Hydrophilus pallidus Rossi, 1792); d’Orchymont 1936a: 10 [synonymy].
Philhydrus pallidus Castelnau, 1840; Gemminger and Harold 1868: 482 [checklist].
Helochares pallidus (Castelnau, 1840); Fleutiaux and Sallé 1889: 376 [checklist].
Enochrus (Lumetus) pallidus (Castelnau, 1840); Zaitzev 1908: 388 [checklist].
Helochares (Hydrobaticus) rufobrunneus Balfour-Browne, 1939: 293. – Lesser Antilles, Grenada, Balthazar; Spangler 1981b: 158 [synonymy].
Distribution: Neotropical: Argentina, Bolivia, Brazil (Espírito Santo, Pernambuco, Piauí), Colombia, Costa Rica, Cuba, French Guiana, Lesser Antilles, Panama, Paraguay, Suriname, Venezuela.
Novochares atlanticus (Clarkson & Ferreira-Jr., 2014) comb. nov.
Helochares (s. str.) atlanticusClarkson and Ferreira-Jr. 2014a: 401 – Brazil, São Paulo, Ubatuba, Parque Estadual da Serra do Mar, Núcleo Picinguaba.
Distribution: Neotropical: Brazil (Rio de Janeiro, São Paulo).
Novochares atratus (Bruch, 1915) comb. nov.
Helochares atratus Bruch, 1915: 451 – Argentina, Buenos Aires province; Fernández 1982a: 35 [taxonomic treatment]; Hansen 1999b: 159 [catalog]; Gonzalez-Rodriguez et al. 2017: 609 [new record].
Helochares (s. str.) atratus Bruch, 1915; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment].
Helochares (s. str.) parhedrus d’Orchymont, 1939: 259 – Argentina, Chaco de Santiago del Estero; not synonym of Helochares (Sindolus) gibbus Brullé, 1841 (= Helochares ventricosus Bruch), as in d’Orchymont 1926: 236); Fernández 1982a: 35 [synonymy; redescription].
Distribution: Neotropical: Argentina, Brazil (Mato Grosso do Sul, Minas Gerais), Colombia, Ecuador [in doubt]; Paraguay.
Novochares bolivianus (Fernández, 1989) comb. nov.
Helochares (s. str.) bolivianus Fernández, 1989: 146 – Bolivia, Santa Cruz Department, Gutiérrez Province, Nueva Moka; Hansen 1999b: 158 [catalog].
Distribution: Neotropical: Bolivia.
Novochares carmona (Short, 2005) comb. nov.
Helochares (s. str.) carmona Short, 2005: 215 – Costa Rica, Guanacaste Province, Laguna de Cocodrilo, near Carmona, 10°03'31.0"N, 85°14'25.6"W; Short and Hebauer 2006: 335 [catalog].
Distribution: Neotropical: Costa Rica.
Novochares chaquensis (Fernández, 1982) comb. nov.
Helochares (s. str.) chaquensis Fernández, 1982b: 87 – Argentina, Chaco Province, San Bernardo; Hansen 1999b: 159 [catalog]; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment].
Distribution: Neotropical: Argentina, Brazil (Mato Grosso do Sul).
Novochares cochlearis (Fernández, 1982) comb. nov.
Helochares (s. str.) cochlearis Fernández, 1982b: 89 – Argentina, Corrientes, Santo Tomé; Hansen 1999b: 159 [catalog].
Distribution: Neotropical: Argentina, Paraguay.
Novochares coya (Fernández, 1982) comb. nov.
Helochares (s. str.) coya Fernández, 1982b: 87 – Bolivia, Santa Cruz Department, Sara Province, Monteros; Hansen 1999b: 160 [catalog].
Distribution: Neotropical: Bolivia.
Novochares guadelupensis (d’Orchymont, 1926) comb. nov.
Helochares (s. str.) guadelupensis d’Orchymont, 1926b: 233 – Lesser Antilles, Guadeloupe; Hansen 1999b: 160 [catalog].
Distribution: Neotropical: Lesser Antilles (Guadeloupe).
Novochares inornatus (d’Orchymont, 1926) comb. nov.
Helochares (s. str.) inornatus d’Orchymont, 1926b: 235 – French Guiana, “Passoura”; Balfour-Browne 1939: 295 [faunistic treatment]; Hansen 1999: 160 [catalog]; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment].
Distribution: Neotropical: Brazil (Amazonas, São Paulo), French Guiana, Guyana [British Guiana].
Novochares oculatus (Sharp, 1882) comb. nov.
Helochares oculatus Sharp, 1882: 74 – Guatemala, Paso Antonio; Fernández, 1982a: 31 [specific rank confirmed; not synonym of Helochares pallidus Castelnau, as in d’Orchymont 1926b: 232; not a synonym of abbreviatus Fabricius, as in d’Orchymont 1936a: 10; lectotype designated].
Helochares (s. str.) oculatus Sharp, 1882: 74; Hansen 1999b: 162 [catalog]; Short 2005: 216 [new record]; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment].
Distribution: Neotropical: Argentina, Brazil (Mato Grosso do Sul, Pernambuco, Rio de Janeiro), Costa Rica, Guatemala, Panama; according to Hansen (1999b: 162), records from Mexico and the Antilles (Grenada, St. Vincent) need confirmation.
Novochares pallipes (Brullé, 1841) comb. nov.
Hydrophilus (Philydrus) pallipes Brullé, 1841: 58. – Uruguay, Montevideo.
Philhydrus pallipes (Brullé, 1841); Lacordaire 1854: 457.
Helochares pallipes (Brullé, 1841); Bedel, 1881: XCIV.
Helochares (s. str.) pallipes (Brullé, 1841); Fernández 1983: 444 [redescription; description of immature stages]; Hansen 1999b: 163 [catalog]; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment].
Distribution: Neotropical: Argentina, Brazil (Mato Grosso do Sul, Minas Gerais), Paraguay, Uruguay.
Novochares pichilingue (Fernández, 1989) comb. nov.
Helochares (s. str.) pichilingue Fernández, 1989: 147 – Ecuador, Los Ríos, Quevedo, Río Pichilingue; Hansen 1999b: 163 [catalog].
Distribution: Neotropical: Ecuador.
Novochares sallaei (Sharp, 1882) comb. nov.
Helochares sallæi Sharp, 1882: 75 – Mexico, Cordova.
Helochares (s. str.) sellae Sharp, 1882; Knisch, 1924a: 199 [catalog; misspelled].
Helochares (s. str.) sallaei Sharp, 1882; Hansen 1999b: 163 [catalog]; Short 2005: 217 [faunistic treatment].
Philhydrus estriatus Blatchley, 1917: 139. – U.S.A., Florida (west coast); Winters, 1927a: 24 [synonymy].
Enochrus (Lumetus) estriatus (Blatchley, 1917); Knisch 1924a: 208 [catalog].
Distribution: Nearctic: U.S.A. (Florida). Neotropical: Belize, Costa Rica, Mexico.
NOTE: The occurrence of the species in Florida is thought to have been an introduction (Young 1954). If this is the case, the introduction happened more than 100 years ago, as it has been in Florida since at least 1917 when specimens were described as a new species of Enochrus (E. estriatus Blatchley, 1917). We reviewed the holotype of E. estriatus and confirmed this synonymy.
Novochares tectiformis (Fernández, 1982) comb. nov.
Helochares (s. str.) tectiformis Fernández, 1982b: 88. – Argentina, Corrientes, Santo Tomé; Hansen 1999b: 163 [catalog]; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment]; Silva et al. 2018: 9 [faunistic treatment].
Distribution: Neotropical: Argentina, Brazil (Mato Grosso do Sul, Piauí), Paraguay, Venezuela.
Peltochares Régimbart, 1907
Peltochares atropiceus (Régimbart, 1903) comb. nov.
Helochares atropiceus Régimbart, 1903b: 53 – Vietnam [“Cochinchine”] (Ho Chi Minh [“Saigon”]; My Tho); Cambodia (Phnom Penh); Indonesia (Sumatra, Borneo, New Guinea); not synonym of Helochares taprobanicus Sharp, as in d’Orchymont 1923b: 419 and Hansen 1999b: 163.
Helochares (s. str.) atropiceus Régimbart, 1903; Hebauer 2001: 10 [specific rank confirmed; lectotype designated]; Hansen 2004: 52 [checklist]; Hebauer and Ryndevich 2005: 45 [new record]; Fikáček et al. 2015: 61 [checklist]; Jia and Tang 2018b: 9 [redescription; new record].
Helochares (s. str.) atropiceus Sharp; Hebauer 2002a: 24 [author attribution in error; new record].
Helochares (s. str.) ohkurai Satô, 1976: 21 – Japan, Nansei-shoto archipelago [“Ryukyus”], Iriomote-jima Is., Ôhara-Ôtomi; Hansen 1999b: 162 [catalog]; Hebauer 2001: 11 [synonymy].
Distribution: Australasian: Papua New Guinea [“Nouvelle Guinée”]. Indo-Malayan: Bangladesh, Cambodia, China (Guangdong, Guangxi, Guizhou, Hong Kong, Jiangxi, Macao), Indonesia (Borneo, Sumatra), Nepal, Thailand, Vietnam. Palearctic: Japan (Nansei Islands).
Peltochares ciniensis (Hebauer, Hendrich & Balke, 1999) comb. nov.
Helochares (s. str.) ciniensis Hebauer, Hendrich & Balke, 1999: 341 – Malaysia, Pahang, Lake Cini, lakeside nr. Rimba Resort; Short and Hebauer 2006: 335 [catalog].
Distribution: Indo-Malayan: Malaysia.
Peltochares conspicuus Régimbart, 1907
Peltochares conspicuus Régimbart, 1907: 49 – Gabon, Cape Lopez, Rembo N’Comi; Balfour-Browne 1950b: 60 [faunistic treatment]; Bertrand 1962: 1101 [description of larva]; Hansen 1999b: 172 [catalog]; Hebauer 2006a: 27 [checklist].
Distribution: Afrotropical: Democratic Republic of the Congo, Gabon, Ghana, Ivory Coast.
Peltochares discus (Hebauer, Hendrich & Balke, 1999) comb. nov.
Helochares (s. str.) discus Hebauer, Hendrich & Balke, 1999: 342; Hebauer 2001b: 11 [taxonomic treatment]; Short and Hebauer 2006: 336 [catalog].
Distribution: Indo-Malayan: Indonesia (Sumatra), Malaysia.
Peltochares foveicollis (Montrouzier, 1860) comb. nov.
Stagnicola foveicollis Montrouzier, 1860: 247 – New Caledonia, Île Art [“Nouvelle-Calédonie, Art”].
Helochares foveicollis (Montrouzier, 1860); Bedel 1880: CXLVIII [synonymy].
Philhydrus burrundiensis Blackburn, 1890: 447 – Australia, Northern Territory, Burrundie; d’Orchymont 1943b: 6 [synonymy in doubt].
Neohydrobius burrundiensis (Blackburn, 1890); Blackburn 1898: 221 [new genus; new combination].
Helochares (s. str.) burrundiensis (Blackburn, 1890); d’Orchymont 1919b: 228 [synonymy].
Helochares (s. str.) foveicollis (Montrouzier, 1860); d’Orchymont 1937e: 154 [checklist]; Watts, 1995: 118 [taxonomic treatment]; Hansen 1999: 160 [catalog]; Watts 2002: 122 [description of larva]; Short 2010: 312 [catalog].
Distribution: Australasian: Australia (Australian Capital Territory, New South Wales, Northern Territory, Queensland, Western Australia), New Caledonia, Papua New Guinea.
Peltochares longipalpis (Murray, 1859) comb. nov.
Philhydrus (s. str.) longipalpis Murray, 1859: 123 – Nigeria, Calabar [“Old Calabar”].
Helochares longipalpis (Murray, 1859); Régimbart 1903a: 26 [faunistic treatment]; Bird et al. 2017 [faunistic treatment].
Helochares (s. str.) longipalpis (Murray, 1859); Balfour-Browne 1950b: 58 [faunistic treatment]; Balfour-Browne 1952a: 129 [faunistic treatment]; Balfour-Browne 1957: 22 [faunistic treatment]; Hansen and Hebauer 1988: 29 [in key]; Hebauer 1994: 112 [faunistic treatment]; Hebauer 1995a: 265 [faunistic treatment]; Hebauer 1996: 7 [faunistic treatment]; Hansen 1999b: 161 [catalog]; Hebauer 2001: 12 [taxonomic treatment]; Hebauer 2005: 39 [checklist]; Hebauer 2006a: 25 [checklist]; Hansen 2004: 52 [checklist]; Fikáček et al. 2015: 61 [checklist]; Salah and Régil Cueto 2017: 265 [checklist].
Helochares filipalpis Sharp, 1903: 6 – South Sudan [Sudan], Jebel Ahmed Agha [“Gebel Ahmed Agha”]; d’Orchymont 1943c: 7 [synonymy].
Distribution: Afrotropical: Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Democratic Republic of the Congo, Ethiopia, Gabon, Gambia, Ghana, Guinea, Ivory Coast, Kenya, Liberia, Madagascar, Malawi, Mozambique, Namibia, Niger, Nigeria, Republic of the Congo, Rwanda, Senegal, Sierra Leone, Somalia, Republic of South Africa, South Sudan, Tanzania, Togo, Uganda, Western Sahara, Zambia, Zimbabwe. Palearctic: Canary Islands, Egypt, Israel.
NOTE: This species almost certainly represents a species complex. Aside from Peltochares conspicuus, which is much more morphologically divergent, this is the only species of Peltochares currently recorded from sub-Saharan Africa (and Madagascar), although we have seen evidence for multiple species based on aedeagal and molecular data. It is likely that several species exist under this name, and they will need to be teased apart in a future revision of the genus.
Peltochares papuensis (Hebauer, 1995) comb. nov.
Helochares (s. str.) papuensis Hebauer, 1995b: 8 – Indonesia, Papua [W. Neuguinea; Irian Jaya], Paniai province, Wanggar-Kali Bumi; Hansen 1999b: 163 [catalog].
Distribution: Australasian: Indonesia (Papua).
Peltochares taprobanicus (Sharp, 1890) comb. nov.
Helochares (s. str.) taprobanicus Sharp, 1890: 351 – Sri Lanka, Colombo [“(Ceylon): Colombo”]; d’Orchymont 1928: 108 [faunistic treatment]; Hebauer 1995b: 8 [faunistic treatment]; Hansen 1999b: 163 [catalog]; Hebauer 2001: 11 [taxonomic treatment; lectotype designated].
Helochares (s. str.) lacustris Hebauer, Hendrich & Balke, 1999: 342; Hebauer 2001: 11 [synonymy]; Hebauer and Ryndevich 2005: 45 [new record]; Short and Hebauer 2006: 336 [catalog].
Distribution: Indo-Malayan: Indonesia (Sumatra), Laos, Malaysia, Nepal, Sri Lanka, Thailand, Vietnam.
Primocerus Girón & Short, 2019
Primocerus cuspidis Girón & Short, 2019
Primocerus cuspidis Girón & Short, 2019: 144 – Venezuela, Amazonas, Tobogán de la Selva, old “Tobogancito”, 5°23.207'N, 67°36.922'W.
Distribution: Neotropical: Venezuela.
Primocerus gigas Girón & Short, 2019
Primocerus gigas Girón & Short, 2019: 145 – Venezuela, Amazonas, Cerro de la Neblina, camp II, 0°50'N, 65°59'W.
Distribution: Neotropical: Venezuela.
Primocerus maipure Girón & Short, 2019
Primocerus maipure Girón & Short, 2019: 146 – Venezuela, Amazonas, ca. 15 km S of Puerto Ayacucho, 5°30.623'N, 67°36.109'W.
Distribution: Neotropical: Venezuela.
Primocerus neutrum Girón & Short, 2019
Primocerus neutrum Girón & Short, 2019: 147 – Venezuela, Bolívar, along La Escalera, 6°2'10.5"N, 61°23'57.8"W.
Distribution: Neotropical: Guyana, Suriname, Venezuela.
Primocerus ocellatus Girón & Short, 2019
Primocerus ocellatus Girón & Short, 2019: 148 – Venezuela, Amazonas, Cerro de la Neblina, Camp XII, near Pico Phelps.
Distribution: Neotropical: Venezuela.
Primocerus petilus Girón & Short, 2019
Primocerus petilus Girón & Short, 2019: 148 – Brazil, Pará: Alenquer, Vale do Paraíso, ca. 55 km N of Alenquer, 1.49292S, 54.51566W.
Distribution: Neotropical: Brazil (Pará).
Primocerus pijiguaense Girón & Short, 2019
Primocerus pijiguaense Girón & Short, 2019: 149 – Venezuela, Bolívar, Los Pijiguaos, 6°35.617'N, 66°49.238'W
Distribution: Neotropical: Venezuela.
Primocerus semipubescens Girón & Short, 2019
Primocerus semipubescens Girón & Short, 2019: 150 – Guyana, Region VIII, Ayanganna Airstrip, trail from Blackwater Creek Camp to Potaro River, 5°17.823'N, 59°50.000'W.
Distribution: Neotropical: Guyana.
Primocerus striatolatus Girón & Short, 2019
Primocerus striatolatus Girón & Short, 2019: 151 – Suriname, Sipaliwini District, Camp 4 (high) Kasikasima, 2°58'36.7782"N, 55°24'40.986"W.
Distribution: Neotropical: Suriname.
Quadriops Hansen, 1999
Quadriops acroreius Girón & Short, 2017
Quadriops acroreius Girón & Short, 2017: 123 – Suriname, Sipaliwini District, Camp 1: Upper Palemeu, 2°28'37.1994"N, 55°37'45.876"W.
Distribution: Neotropical: Suriname, French Guiana.
Quadriops clusia Girón & Short, 2017
Quadriops clusia Girón & Short, 2017: 125 – Suriname, Brokopondo District, Brownsberg Nature Park, Leo Val trail, nr. pump station, 4.95069'N, -55.18599.
Distribution: Neotropical: Guyana, Suriname, Brazil (Amazonas).
Quadriops dentatus Hansen, 1999
Quadriops dentatus Hansen, 1999a: 134 – Venezuela, Bolivar, 105 km S El Dorado; Hansen 1999b: 155 [catalog]; Girón and Short 2017: 127 [new records].
Distribution: Neotropical: Venezuela, French Guiana, Suriname.
Quadriops depressus Hansen, 1999
Quadriops depressus Hansen, 1999: 136 – Peru, Departamento Loreto, 1.5 km N Teniente Lopez 2°35.66'S, 76°06.92'W; Hansen 1999b: 155 [catalog]; Girón and Short 2017: 128 [new records].
Quadriops amazonensis García, 2000: 59 – Venezuela, Amazonas, Municipio Guinia, Yavita, Caño Chivichi; Girón and Short 2017: 128 [synonymy]; Short and Hebauer 2006: 338 [catalog].
Quadriops politus Hansen, 1999: 135 – Peru, Departamento Loreto, Campamento San Jacinto, 2°18.75'S, 75°51.77'W; Hansen 1999b: 155; Girón and Short 2017: 128 [synonymy]
Distribution: Neotropical: Ecuador, Peru, Venezuela.
Quadriops reticulatus Hansen, 1999
Quadriops reticulatus Hansen, 1999: 135 – Costa Rica, Puntarenas, Las Alturas (Stanford Biological Station), ca. 29 km NE San Vito; Hansen 1999b: 155 [catalog]; Girón and Short 2017: 130 [new records].
Distribution: Neotropical: Costa Rica, Panama.
Quadriops similaris Hansen, 1999
Quadriops similaris Hansen, 1999: 136 – Venezuela, Bolivar, 105 km S El Dorado; Hansen 1999b: 155 [catalog]; Girón and Short 2017: 134 [new records].
Distribution: Neotropical: Venezuela, Guyana, Suriname, French Guiana.
Radicitus Short & García, 2014
Radicitus ayacucho Short & García, 2014
Radicitus ayacucho Short & García, 2014: 252 – Venezuela, Amazonas State, Tobogan de la Selva, 5°23.207'N, 67°36.922'W.
Distribution: Venezuela.
Radicitus granitum Short & García, 2014
Radicitus granitum Short & García, 2014: 254 – Venezuela, Bolívar State, Los Pijiguaos, 6°35.617'N, 66°49.238'W.
Distribution: Venezuela.
Radicitus surinamensis Short & García, 2014
Radicitus surinamensis Short & García, 2014: 257 – Suriname, Sipaliwini Department, Mt. Kasikasima, 2°58.613'N, 55°24.683'W.
Distribution: Suriname.
Sindolus Sharp, 1882
Sindolus femoratus (Brullé, 1841)
Hydrophilus (Philydrus) femoratus Brullé, 1841: 59 – Argentina [“province de Corrientes”].
Hydrobius femoratus (Brullé, 1841); Gemminger and Harold 1868a: 479 [checklist].
Helochares femoratus (Brullé, 1841); Bedel 1881: XCV.
Helochares (Sindolus) femoratus (Brullé, 1841); d’Orchymont 1926b: 236; Fernández and Kehr 1994 [annual life cycle]; Fernández and Kehr 1995 [spatial and temporal distribution]; Hansen 1999: 157 [catalog]; Fernández 2004 [description of immature stages]; Clarkson and Ferreira-Jr 2014a: 403 [faunistic treatment]; Alves et al. 2020: 583 [faunistic treatment].
? Hydrobius spadiceus Dejean, 1833: 134; nom. nud.; Mulsant 1844b: 380 [synonym of Philhydrus spadiceus Mulsant]
? Philhydrus spadiceus Mulsant, 1844b: 380 – French Guiana (Cayenne) and Colombia [“Nouvelle-Grenade”]; d’Orchymont 1929: 95 [synonym doubtful].
? Enochrus (Lumetus) spadiceus (Mulsant, 1844); Zaitzev 1908: 389 [catalog].
Helochares gravidus Bruch, 1915: 452 – Argentina, La Plata (“Tiro Federal”; Formosa (Puerto Bouvier); d’Orchymont 1926b: 236 [synonymy].
Helochares (Sindolus) gravidus Bruch, 1915; Knisch 1924: 199 [catalog].
Sindolus femoratus (Brullé, 1841); Short et al. 2021: 11 [new combination].
Distribution: Neotropical: Argentina, Brazil (Bahía, Pernambuco, Piauí, Rio de Janeiro, Rio Grande do Sul), Colombia [in doubt; d’Orchymont, 1943d: 56], French Guiana [in doubt; d’Orchymont, 1943d: 56], Lesser Antilles (Antigua).
Sindolus mesostitialis (Fernández, 1981)
Helochares (Sindolus) mesostitialis Fernández, 1981: 189 – Argentina, Santa Fe, Dept. Garay, Colonia Mascias; Hansen 1999b: 158 [catalog]; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment].
Sindolus mesostitialis (Fernández, 1981); Short et al. 2021: 11 [new combination].
Distribution: Neotropical: Argentina, Brazil (Mato Grosso do Sul).
Sindolus mini (Fernández, 1982)
Helochares (Sindolus) mini Fernández, 1982b: 89 – Argentina, Santa Fe, Chaco prov., lag. La Cava, Barranqueras; Hansen 1999b: 158 [catalog].
Sindolus mini (Fernández, 1982); Short et al. 2021: 11 [new combination].
Distribution: Neotropical: Argentina, Paraguay.
Sindolus mundus Sharp, 1882
Sindolus mundus Sharp, 1882: 73 – Mexico, Oaxaca.
Helochares (Sindolus) mundus (Sharp, 1882); Knisch 1924: 199 [checklist]; Hansen 1999b: 158 [catalog]; Short 2005: 219 [new records].
Distribution: Neotropical: Costa Rica, Mexico, Nicaragua.
Sindolus optatus Sharp, 1882
Sindolus optatus Sharp, 1882: 72 – Guatemala, Paso Antonio.
Helochares (Sindolus) optatus (Sharp, 1882); Knisch 1924: 199 [checklist]; Hansen 1999b: 158 [catalog]; Short 2005: 220 [new records].
Helochares (s. str.) guatemalensis Knisch, 1921a: 68 – Guatemala; d’Orchymont 1937b: 253 [synonymy].
Helochares (Sindolus) guatemalensis Knisch, 1921; Knisch 1924: 199 [catalog].
Distribution: Neotropical: Costa Rica, Guatemala, Mexico.
Sindolus spatulatus (Fernández, 1981)
Helochares (Sindolus) spatulatus Fernández, 1981: 191 – Argentina, Corrientes.
Sindolus spatulatus (Fernández, 1981); Short et al. 2021: 11 [new combination].
Distribution: Neotropical: Argentina, Paraguay.
Sindolus talarum (Fernández, 1983)
Helochares (Sindolus) talarum Fernández, 1983: 440 – Argentina, Buenos Aires, lag. Los Talas [original description includes description of immature stages].
Sindolus talarum (Fernández, 1983); Short et al. 2021: 11 [new combination].
Distribution: Neotropical: Argentina.
Sindolus ventricosus (Bruch, 1915)
Hydrophilus (Philydrus) gibbus Brullé, 1841: 58 (primary homonym of Hydrophilus gibbus Illiger, 1801 and Hydrophilus gibbus Thunberg, 1820); d’Orchymont, 1926b: 236 (sub nom. gibbus; not synonym of atratus Bruch, as in Balfour-Browne 1939: 293).
Philhydrus gibbus (Brullé, 1841); Lacordaire 1854: 457.
Helochares gibbus (Brullé, 1841); Bedel 1881: XCV.
Helochares (Sindolus) gibbus (Brullé, 1841); d’Orchymont 1926b: 236.
Helochares ventricosus Bruch, 1915: 452; Fernández, 1982a: 36 [specific rank confirmed; lectotype designated; not synonym of atratus Bruch, 1915, as in Balfour-Browne, 1939: 293].
Helochares (Sindolus) ventricosus Bruch, 1915; Clarkson and Ferreira-Jr. 2014: 400 [faunistic treatment].
Sindolus ventricosus (Bruch, 1915); Short et al. 2021: 11 [new combination].
Distribution: Neotropical: Argentina, Bolivia, Brazil (Amazonas, Mato Grosso do Sul, Pernambuco), Paraguay, Uruguay.
Tobochares Short & García, 2007
Tobochares akoerio Girón & Short, 2021
Tobochares akoerio Girón & Short, 2021: 120 – Suriname: Sipaliwini District, 2.46554°N, 55.7700°W, Camp 2, Grensgebergte Rock.
Distribution: Neotropical: Suriname.
Tobochares arawak Girón & Short, 2021
Tobochares arawak Girón & Short, 2021: 122 – Guyana: Region VIII, 5°0.730'N, 59°38.965'W, Upper Potaro Camp I, ca. 7 km NW of Chenapau, top of falls on Potaro River.
Distribution: Neotropical: Guyana.
Tobochares anthonyae Girón & Short, 2021
Tobochares anthonyae Girón & Short, 2021: 125 – Venezuela: Bolívar, 6°13'4.6"N, 67°14'26.4"W; ca. 25 km E of El Burro.
Distribution: Neotropical: Venezuela.
Tobochares atures Girón & Short, 2021
Tobochares atures Girón & Short, 2021: 126 – Venezuela: T.F. Amazonas, Puerto Ayacucho (40 km S), El Tobogán, Caño Coromoto.
Distribution: Neotropical: Venezuela.
Tobochares benettii Girón & Short, 2021
Tobochares benettii Girón & Short, 2021: 106 – Brazil: Amazonas: Rio Preto da Eva, -2.678466, -59.401714, ca. 32 km W of Rio Preto da Eva.
Tobochares sp. B, Short et al. 2021.
Distribution: Neotropical: Brazil (Amazonas).
Tobochares canaima Girón & Short, 2021
Tobochares canaima Girón & Short, 2021: 128 – Venezuela: Bolívar: 5°51'N, 62°33'W, 1700 m, Auyan-tepui.
Distribution: Neotropical: Venezuela.
Tobochares canaliculatus Kohlenberg & Short, 2017
Tobochares canaliculatus Kohlenberg & Short, 2017: 119 – Venezuela, Amazonas State, Tobogan de la Selva, old “tobogancito”, 5°23.207'N, 67°36.922'W.
Distribution: Neotropical: Venezuela.
Tobochares canthus Kohlenberg & Short, 2017
Tobochares canthus Kohlenberg & Short, 2017: 122 – Venezuela, Amazonas State, Tobogan de la Selva, old “tobogancito”, 5°23.207'N, 67°36.922'W.
Distribution: Neotropical: Venezuela.
Tobochares communis Girón & Short, 2021
Tobochares communis Girón & Short, 2021: 129 – Suriname: Sipaliwini District, 4°40.432'N, 56°11.079'W, Raleighvallen Nature Reserve, base of Voltzberg.
Tobochares sp. 1B, Short et al. 2021.
Distribution: Neotropical: Brazil (Amapá, Roraima), Guyana, Suriname, Venezuela.
Tobochares emarginatus Kohlenberg & Short, 2017
Tobochares emarginatus Kohlenberg & Short, 2017: 123 – Suriname: Sipaliwini District, Camp 4 (high) Kasikasima, 2°58.613'N, 55°24.683'W.
Distribution: Neotropical: Suriname.
Tobochares fusus Girón & Short, 2021
Tobochares fusus Girón & Short, 2021: 117 – Brazil: Amapá: Oiapoque, 3.85039, -51.81683, 17 m, Oiapoque (ca. 1 km E).
Distribution: Neotropical: Brazil (Amapá), French Guiana.
Tobochares goias Girón & Short, 2021
Tobochares goias Girón & Short, 2021: 109 – Brazil: Goiás: Cristalina, -16.87004, -47.61716; 947 m; Cristalina Balneario Lajes.
Tobochares sp. C, Short et al. 2021.
Distribution: Neotropical: Brazil (Goiás).
Tobochares kappel Girón & Short, 2021
Tobochares kappel Girón & Short, 2021: 133 – Suriname: Sipaliwini District, 3°47.479'N, 56°8.968'W, CSNR: near Kappel airstrip.
Distribution: Neotropical: Suriname.
Tobochares kasikasima Short, 2013
Tobochares kasikasima Short, 2013: 83 – Suriname, Sipaliwini District, Camp 4 (high) Kasikasima, 2°58.613'N, 55°24.683'W; Kohlenberg and Short 2017: 124 [redescription].
Distribution: Neotropical: Suriname.
Tobochares kolokoe Girón & Short, 2021
Tobochares kolokoe Girón & Short, 2021: 134 – Suriname: Sipaliwini District, CSNR: Tafelberg Summit, Arrowhead Basin.
Distribution: Neotropical: Suriname.
Tobochares kusad Kohlenberg & Short, 2017
Tobochares kusad Kohlenberg & Short, 2017: 126 – Guyana: Region IX, Kusad Mts., Mokoro Creek, 2 48.531'N, 59 51.900'W; Girón and Short 2021a: 114 [new record].
Distribution: Neotropical: Brazil (Roraima), Guyana.
Tobochares luteomargo Girón & Short, 2021
Tobochares luteomargo Girón & Short, 2021: 115 – Venezuela: Bolívar State, 7°41'23.6"N, 64°1'56.0"W, 134 m, ca. 14 km E of Río Aro.
Tobochares sp. 10, Short et al., (2021).
Distribution: Neotropical: Venezuela.
Tobochares microps Girón & Short, 2021
Tobochares microps Girón & Short, 2021: 135 – Suriname: Sipaliwini District, N3 53.359’ W56 10.052’, CSNR: Tafelberg Summit, near South Rim.
Tobochares sp. 2A, Short et al. 2021.
Distribution: Neotropical: Suriname.
Tobochares pallidus Kohlenberg & Short, 2017
Tobochares pallidus Kohlenberg & Short, 2017: 130 – Venezuela: Amazonas State, Tobogan de la Selva, old “tobogancito”, 5°23.207'N, 67°36.922'W.
Distribution: Neotropical: Venezuela.
Tobochares pemon Girón & Short, 2021
Tobochares pemon Girón & Short, 2021: 136 – Venezuela: Bolívar, 5°51'N, 62°33'W, Auyan-tepui.
Distribution: Neotropical: Venezuela.
Tobochares romanoae Girón & Short, 2021
Tobochares romanoae Girón & Short, 2021: 137 – Brazil: Roraima, Amajari, 3°36.381'N, 61°42.878'W, Serra do Tepequém, Igarape Preto Negro, Cachoeira Leje Preta.
Distribution: Neotropical: Brazil (Roraima).
Tobochares sipaliwini Short & Kadosoe, 2011
Tobochares sipaliwini Short & Kadosoe, 2011: 85 – Suriname, Sipaliwini District, Camp 2, on Sipaliwini River, Inselberg, 2 10.973'N, 56 47.235'W; Kohlenberg and Short 2017: 132 [redescription]; Girón and Short 2021a: 115 [new record].
Distribution: Neotropical: Brazil (Roraima), Guyana, Suriname.
Tobochares striatus Short, 2013
Tobochares striatus Short, 2013: 83 – Suriname, Sipaliwini District, 2.24554°N, 55.77000°W, Camp 2 Grensgebergte Rock; Kohlenberg and Short 2017: 136 [redescription]; Girón and Short 2021a: 115 [new record].
Distribution: Neotropical: Suriname.
Tobochares sulcatus Short & García, 2007
Tobochares sulcatus Short & García, 2007: 4 – Venezuela: Amazonas State, Tobogan de la Selva, ca. 40 km S of Puerto Ayacucho, margin of Rio Coromoto; Short and Fikáček 2011: 91 [catalog]; Kohlenberg and Short 2017: 140 [redescription].
Distribution: Neotropical: Venezuela.
Troglochares Spangler, 1981
Troglochares ashmolei Spangler, 1981
Troglochares ashmolei Spangler, 1981a: 318 – Ecuador, Morona-Santiago prov., Los Tayos Cave; Hansen 1999b: 156 [catalog].
Distribution: Neotropical: Ecuador.
Supplementary Material
Acknowledgements
We are grateful for the loan of valuable specimens by curators of collections including Mauricio García (MALUZ), Jesús Camacho (MALUZ), Luis Joly (MIZA), Vanessa Kadosoe and Paul Ouboter (NZCS), Robert Anderson (CMN). We are particularly thankful to the continued assistance of Charyn Micheli (USNM) for access to the Spangler collection and backlog and to Franz Hebauer for exchanging specimens years ago. Martin Fikáček has provided invaluable access to numerous references that would not be accessible otherwise. Rachel Smith dissected and imaged some of the Chasmogenus specimens that are presented here. Alex Kohlenberg produced some of the SEM images used here. Yusuke Minoshima, Albrecht Komarek, Georgina Rodriguez, and Bruno Clarkson provided feedback and caught mistakes and omissions in previous versions of the manuscript, although any remaining errors are our own. This study was supported by US National Science Foundation grant DEB-1453452 to AEZS.
Citation
Girón JC, Short AEZ (2021) The Acidocerinae (Coleoptera, Hydrophilidae): taxonomy, classification, and catalog of species. ZooKeys 1045: 1–236. https://doi.org/10.3897/zookeys.1045.63810
References
- Alves T, Clarkson B, Lima LRC. (2020) A new species of Chasmogenus Sharp, 1882 and new records of Hydrophilidae (Coleoptera) from Northeastern Brazil. Zootaxa 4763: 579–586. 10.11646/zootaxa.4763.4.7 [DOI] [PubMed] [Google Scholar]
- Anderson JME. (1976) Aquatic Hydrophilidae (Coleoptera). The biology of some Australian species with descriptions of immature stages reared in the laboratory. Australian Journal of Entomology 15(2): 219–228. 10.1111/j.1440-6055.1976.tb01696.x [DOI] [Google Scholar]
- Angus RB, Sadílek D, Shaarawi F, Dollimore H, Liu H-C, Seidel M, Sýkora V, Fikáček M. (2020) Karyotypes of water scavenger beetles (Coleoptera: Hydrophilidae): new data and review of published records. Zoological Journal of the Linnean Society zlaa 105: 1–40. 10.1093/zoolinnean/zlaa105 [DOI] [Google Scholar]
- Archangelsky M. (1997) Studies on the biology, ecology, and systematics of the immature stages of New World Hydrophiloidea (Coleoptera: Staphyliniformia). Bulletin of the Ohio Biological Survey 12(1): 1–207. [Google Scholar]
- Arriaga-Varela E, Brunke A, Girón JC, Szawaryn K, Bruthansová J, Fikáček M. (2019) Micro-CT reveals hidden morphology and clarifies the phylogenetic position of Baltic amber water scavenger beetles (Coleoptera: Hydrophilidae). Historical Biology 0: 1–17. 10.1080/08912963.2019.1699921 [DOI] [Google Scholar]
- Balfour-Browne J. (1939) Contribution to the Study of the Palpicornia. Part III. Annals and Magazine of natural History 11(4): 289–310. 10.1080/00222933908526990 [DOI] [Google Scholar]
- Balfour-Browne J. (1945) Aquatic Coleoptera of Oceania (Dytiscidae, Gyrinidae, and Palpicornia). Occasional Papers of the Bernice P. Bishop Museum 18(7): 103–132. [Google Scholar]
- Balfour-Browne J. (1947) New and interesting aquatic Coleoptera from the Sudan. Proceedings of the Royal Entomological Society of London (B) 16: 133–142. 10.1111/j.1365-3113.1947.tb00844.x [DOI] [Google Scholar]
- Balfour-Browne J. (1948) New East African Palpicornia. Annals and Magazine of natural History 11(14): 817–833. 10.1080/00222934708654695 [DOI] [Google Scholar]
- Balfour-Browne J. (1950a) On the aquatic Coleoptera of Northern Rhodesia (Dytiscidae, Gyrinidae and Palpicornia). Occasional Papers of the national Museums of Southern Rhodesia 2(16): 359–399. [Google Scholar]
- Balfour-Browne J. (1950b) Palpicornia. Exploration du Parc National Albert. Mission G. F. de Witte (1933–35) 63: 1–84. http://www.apncb.be/archives/publications/exploration-national-park-albert/exploration-national-park-albert-first-series/mission-g.-f.-de-witte-1933-1935/1950-fascicule-63-palpicornia/balfour-complet.pdf
- Balfour-Browne J. (1951) Coleoptera: Haliplidae, Dytiscidae, Gyrinidae, Hydraenidae, Hydrophilidae. British Museum (Natural History): Expedition to South-west Arabia 1937–1938. The British Museum, London, 504 pp. [pp. 179–220, pl. 10–11.] [Google Scholar]
- Balfour-Browne J. (1952a) Mission A. Villiers au Togo et au Dahomey (1950). VII. Coléoptères Hydrophilides. Bulletin de l’Institut français d’Afrique noire 14: 126–139. [Google Scholar]
- Balfour-Browne J. (1952b) Contribution à l’étude du peuplement de la Mauritanie. Coléoptères Hydrophilides. Bulletin de l’Institut français d’Afrique noire 14(2): 513–517. [Google Scholar]
- Balfour-Browne J. (1957) Contributions à l’étude de la faune entomologique du Ruanda-Urundi (Mission P. Basilewsky 1953). CXVIII. ColeopteraHydrophilidae. Annales du Musée Royal du Congo Belge, Zool. 58: 14–25. [Google Scholar]
- Balfour-Browne J. (1958a) La Reserve naturelle integrale du Mont Nimba, Guinea. VI. Coléoptères Hydrophiloides. Mémoires de l’Institut français d’Afrique noire 53: 169–190. [Google Scholar]
- Balfour-Browne J. (1958b) New species of Malgassic Hydrophilidae (Col.). Mauritius Institute Bulletin 5: 134–147. [Google Scholar]
- Balfour-Browne J. (1959) Dr. Jan Bechyné expedition to French Guinea, 1951. Hydrophilidae. Entomologische Arbeiten aus dem Museum G. Frey 10: 302–320. https://biodiversitylibrary.org/page/45982912 [Google Scholar]
- Bedel L. (1880) (Quelques modifications nouvelles à introduire dans la nomenclature des Hydrophilides). Annales de la Société Entomologique de France 5(10): CXLVII–CXLVIII. https://biodiversitylibrary.org/page/8232411
- Bedel L. (1881a) [(1879–1881)] Faune des Coléoptères du bassin de la Seine (Vol. 1). App. to Annales de la Société entomologique de France 360 pp. https://biodiversitylibrary.org/page/9314722
- Bedel L. (1881b) (Synonymie de quelques Hydrophilidæ et Sphæridiidæ exotiques décrits par Brullé). Annales de la Société Entomologique de France 6(1): XCIV–XCV. https://biodiversitylibrary.org/page/8996339
- Benamar L, Millán A, Sáinz-Cantero CE, Belhaj A, Bennas N. (2021) Annotated checklist of water scavenger beetles (Coleoptera: Polyphaga: Hydrophilidae) of Morocco. Aquatic Insects. 10.1080/01650424.2021.1874422 [DOI]
- Bergroth E. (1888) Fåhraea nov. gen. Hydrophilidarum. Deutsche entomologische Zeitschrift 32: e221. https://biodiversitylibrary.org/page/33177095
- Bertrand H. (1962) Contribution à l’étude des premiers états des Coléoptères aquatiques de la région éthiopienne (4e note). Bulletin de l’Institut Française d’Afrique Noire 24(4): 1065–1114. [Google Scholar]
- Bird MS, Bilton DT, Perissinotto R. (2017) Diversity and distribution of polyphagan water beetles (Coleoptera) in the Lake St Lucia system, South Africa. ZooKeys 656: 51–84. 10.3897/zookeys.656.11622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn T. (1888) Notes on Australian Coleoptera with descriptions of new species. Proceedings of the Linnean Society of New South Wales (2)3 (1889): 805–875. https://biodiversitylibrary.org/page/36009579
- Blackburn T. (1890) Notes on Australian Coleoptera, with descriptions of new species. Part III. Proceedings of the Linnean Society of New South Wales 2(4): 445–482. https://biodiversitylibrary.org/page/6240695 [Google Scholar]
- Blackburn T. (1891) Notes on Australian Coleoptera, with descriptions of new species. Part VII. Proceedings of the Linnean Society of New South Wales (2)5: 303–366. https://www.biodiversitylibrary.org/page/3348578
- Blackburn T. (1893) Notes on Australian Coleoptera, with descriptions of new species. Part XI. Proceedings of the Linnean Society of New South Wales 2(7) (1892): 65–150. https://biodiversitylibrary.org/page/6237149
- Blackburn T. (1896) Coleoptera (exclusive of the Carabidæ) . In: Spencer B (Ed.) Report on the work of the Horn Scientific Expedition to Central Australia. Part II – Zoology. Dulau and Co. , London / Melville, Mullen and Slade, Melbourne, 431 pp. [pp. 254–308, 29 pl.] https://biodiversitylibrary.org/page/57973709 [Google Scholar]
- Blatchley WS. (1917) On some new or noteworthy Coleoptera from the west coast of Florida. Canadian Entomologist 49: 137–143. 10.4039/Ent49137-4 [DOI] [Google Scholar]
- Bloom DD, Fikáček M, Short AEZ. (2014) Clade age and diversification rate variation explain disparity in species richness among water scavenger beetle (Hydrophilidae) lineages. PLoS ONE 9: e98430. 10.1371/journal.pone.0098430 [DOI] [PMC free article] [PubMed]
- Boheman CH. (1851) [(1848–1851)] Insecta Caffrariæ annis 1838–1845 a J. A. Wahlberg collecta (Vol. 1). Coleoptera. Fritze & Norstedt, Holmiæ, 625 pp. [only pp. 299–625 issued in 1851.] https://biodiversitylibrary.org/page/9907494 [Google Scholar]
- Brancsik K. (1893) Beiträge zur Kenntniss Nossibés und dessen Fauna nach Sendungen und Mittheilungen des Herrn P. Frey. Jahresheft des naturwissenschaftlichen Vereins des Trencsiner Komitäts 15–16 (1892–93): 202–258. [pls 6 + 10–12.]
- Bruch C. (1915) Nuevas especies de coleópteros hidrofílidos. Revista del Museo de la Plata 19(2): 447–470. https://publicaciones.fcnym.unlp.edu.ar/rmlp/article/view/1317/1520 [Google Scholar]
- Brullé A. (1835) Histoire naturelle des Insectes (Vol. 5). Coléoptères II. Imprimerie d’Ad. Moëssard, Paris, 436 pp. https://biodiversitylibrary.org/page/24569590 [Google Scholar]
- Brullé A. (1841) Famille des Hydrophiliens. In: d’Orbigny A. Voyage dans l’Amérique méridionale, tome sixiéme, 2e partie, Insectes. P Bertrand, Paris, France, 52–59. https://biodiversitylibrary.org/page/2531016
- Castelnau FL. (1840) Histoire naturelle des Animaux articulés, Histoire naturelle des Insectes Coléoptères (Vol. 2). (Nécrophages-Trimères). P. Duménil, Paris, 565 pp. [38 pls.] https://biodiversitylibrary.org/page/32717751 [Google Scholar]
- Chiesa A. (1967) Compimento di una revisione di Hydrophilidae del Afghanistan (Coleoptera: Hydrophilidae). Annales historico-naturales Musei nationalis hungarici 59: 275–277. [Google Scholar]
- Clarkson B, Ferreira-Jr N. (2014a) A new species and records of Helochares (Insecta: Coleoptera: Hydrophilidae) from Southeastern Brazil. Zoologia 31(4): 400–404. 10.1590/S1984-46702014000400012 [DOI] [Google Scholar]
- Clarkson B, Ferreira-Jr N. (2014b) Four new species and first nominal record of Chasmogenus Sharp, 1882 (Coleoptera: Hydrophilidae) from Brazil. Zootaxa 3765(5): 481–494. 10.11646/zootaxa.3765.5.6 [DOI] [PubMed] [Google Scholar]
- Clarkson B, Santos AD, Ferreira-Jr N. (2016) On Brazilian Helobata Bergroth, 1888 (Coleoptera: Hydrophilidae): description of two new species, new records, and key to species. Zootaxa 4126(4): 548–562. 10.11646/zootaxa.4126.4.6 [DOI] [PubMed] [Google Scholar]
- Clarkson B, Almeida LM. (2018) On Brazilian Helobata Bergroth, 1888 (Coleoptera: Hydrophilidae) II: new distribution data. Papeis Avulsos de Zoologia 58 (e20185835): 1–6. 10.11606/1807-0205/2018.58.35 [DOI]
- Cockerell TDA. (1906a) Preoccupied Generic names of Coleoptera. Entomological News 17: 240–244. https://biodiversitylibrary.org/page/2562995 [Google Scholar]
- Cockerell TDA. (1906b) Notes and News: Names of Coleoptera. Entomological News 17: e349. https://biodiversitylibrary.org/page/2563040
- d’Orchymont A. (1913a) H. Sauter’s Formosa-Ausbeute. Hydrophilidae (Col.). Supplementa entomologica 2: 1–18. [pl. 1.] [Google Scholar]
- d’Orchymont A. (1913b) Contribution à l’etude des larves Hydrophilides. Annales de Biologie Lacustre 6: 173–214. [Google Scholar]
- d’Orchymont A. (1919a) Matériaux pour servir à l’étude de la faune entomologique de l’Indo-Chine. Palpicornia (Col.). Annales de la Société entomologique de Belgique 59: 70–83. [Google Scholar]
- d’Orchymont A. (1919b) Les genres Enochroides Kuw., Neohydrobius Blackb. et Hygrotrophus W. McLeay (Col. Palpicornia). Bulletin de la Société entomologique de France (1919): 226–230.
- d’Orchymont A. (1919c) Contribution à l’étude des sous-familles des Sphaeridiinae et des Hydrophilinae (Col. Hydrophilidae). Annales de la Société entomologique de France 88(1–2): 105–168. https://biodiversitylibrary.org/page/9486175 [Google Scholar]
- d’Orchymont A. (1921) Matériaux pour servir à l’étude de la faune entomologique de l’Indo-Chine. Palpicornia (Col.). Faune entomologique de l’Indo-Chine française 5: 3–22. (= d’Orchymont 1919a, Annales de la Société entomologique de Belgique 59: 70–83).
- d’Orchymont A. (1922) Zoological Results of the Abor Expedition. L. Coleoptera, X: Hydrophilidae. Records of the Indian Museum 8: 623–629. [Google Scholar]
- d’Orchymont A. (1923a) Hydrophilidae of India (Col.). A list of the species in the collection of the Agricultural Research Institute at Pusa (Bihar). Memoirs of the Department of Agriculture in India 7: 1–12. [Google Scholar]
- d’Orchymont A. (1923b) Neue oder interessante Sphaeridiinen und Hydrophilinen der Malayischen Region. Treubia 3: 416–421. [Google Scholar]
- d’Orchymont A. (1925a) Contribution à l’étude des Hydrophilides I. Bulletin et Annales de la Société Entomologique de Belgique 65: 63–77. [Google Scholar]
- d’Orchymont A. (1925b) Hydrophilides des Iles Philippines. Bulletin et Annales de la Société entomologique de Belgique 65: 200–202. [Google Scholar]
- d’Orchymont A. (1925c) Contribution à l’étude des Hydrophilides III. Bulletin et Annales de la Société entomologique de Belgique 65: 261–295. [Google Scholar]
- d’Orchymont A. (1926a) Notes on Philippine Hydrophilidae. Philippine Journal of Science 30(3): 361–385. http://philjournalsci.dost.gov.ph/images/pdf_upload/pjs1926/PJS_Vol_30_No3_Jul_1926.pdf [Google Scholar]
- d’Orchymont A. (1926b) Contribution à l’étude des Hydrophilides VI. Bulletin et Annales de la Société entomologique de Belgique 66: 201–248. [Google Scholar]
- d’Orchymont A. (1927a) Papers on Malayan aquatic biology. V. Notes on the Hydrophilidae in the Federated Malay States Museums. Journal of the Federated Malay States Museums 13(4): 246–252. [Google Scholar]
- d’Orchymont A. (1927b) Coléoptères Hydrophilides recueillis en Egypte. Bulletin de la Société royale entomologique d’Égypte 11: 3–7. [Google Scholar]
- d’Orchymont A. (1928) Catalogue of Indian Insects. Part 14 – Palpicornia. Government of India Central Publication Branch, Calcutta, 146 pp. [Google Scholar]
- d’Orchymont A. (1929) Contribution à l’étude des Palpicornia. VII. Bulletin et Annales de la Société Entomologique de Belgique 69: 79–96. [Google Scholar]
- d’Orchymont A. (1932) Zur Kenntnis der Kolbenwasserkäfer (Palpicornia) von Sumatra, Java und Bali. Archiv für Hydrobiologie, Supplement Band IX: 623–714. [pl. XIV–XVII.]
- d’Orchymont A. (1933) Contribution à l’étude des Palpicornia VIII. Bulletin et Annales de la Société entomologique de Belgique 73: 271–314. [pl. 5.] [Google Scholar]
- d’Orchymont A. (1936a) Quelques synonymies nouvelles d’Hydrophilidae (Col.). Bulletin du Musée royal d’Histoire naturelle de Belgique 12(23): 1–29. http://biblio.naturalsciences.be/rbins-publications/bulletin-of-the-royal-belgian-natural-history-museum/bulletin-of-the-royal-belgian-natural-history-museum-1930-1948/12-36/irscnb_p4087_0126ffp_12_bulletin-23.pdf [Google Scholar]
- d’Orchymont A. (1936b) Scientific results of the Vernay-Lang Kalahari Expedition, March to September, 1930. Hydrophilidae. Annals of the Transvaal Museum 17(2): 109–116. [pl. I.] [Google Scholar]
- d’Orchymont A. (1937a) Descriptions of three new Hydrophilidae from India. Records of the Indian Museum 39: 29–31. [Google Scholar]
- d’Orchymont A. (1937b) Contribution à l’étude des Palpicornia IX. Bulletin et Annales de la Société entomologique de Belgique 77: 213–255. [Google Scholar]
- d’Orchymont A. (1937c) Coleoptera Palpicornia from the Khewra Gorge, Salt Range, Punjab. Records of the Indian Museum 39: 33–41. [Google Scholar]
- d’Orchymont A. (1937d) Contributions à l’étude de la Faune du Mozambique. Voyage de M. P. Lesne (1928–1929). 26. note. Palpicornia. Memorias e Estudos do Museo zoologico da Universidade de Coimbra 96(1): 1–15. [Google Scholar]
- d’Orchymont A. (1937e) Check List of the Palpicornia of Oceania (Coleoptera, Polyphaga). Occasional Papers of the Bernice P. Bishop Museum 13: 147–160. [Google Scholar]
- d’Orchymont A. (1939a) Revision des espèces du sous-genre Crephelochares d’Helochares. Bulletin et Annales de la Société entomologique de Belgique 79: 154–166. [Google Scholar]
- d’Orchymont A. (1939b) Notes sur des Helochares africains. Bulletin et Annales de la Société entomologique de Belgique 79: 293–323. [Google Scholar]
- d’Orchymont A. (1939c) L’Helochares minutissimus Kuwert, vrai. Bulletin et Annales de la Société entomologique de Belgique 79: 196–198. [Google Scholar]
- d’Orchymont A. (1939d) Revision des espèces du sous-genre Crephelochares d’Helochares. Bulletin et Annales de la Société entomologique de Belgique 79: 154–166. [Google Scholar]
- d’Orchymont A. (1939e) Notes sur quelques Palpicornia de la Republique Argentine. Revista de la Sociedad entomológica argentina 10: 253–264. [Google Scholar]
- d’Orchymont A. (1939f) Helochares (Hydrobaticus) Andreinii n. sp. H. melanophthalmus Régimbart, 1905 (nec Mulsant, non Reiche). Redia 25: 319–323. [Google Scholar]
- d’Orchymont A. (1940) Contribution à l’étude des Palpicornia XIV. Bulletin et Annales de la Société entomologique de Belgique 80: 157–197. [Google Scholar]
- d’Orchymont A. (1941) Revision des espèces du genre Régimbartia Zaitzev (ColeopteraHydrophilidae). Bulletin du Musée royal d’Histoire naturelle de Belgique 17(4): 1–15. [Google Scholar]
- d’Orchymont A. (1943a) Nouvelles notes sur les Helochares (Hydrobaticus). (Coleoptera Palpicornia Hydrophilidae). Bulletin du Musée royal d’Histoire naturelle de Belgique 19(20): 1–12. [Google Scholar]
- d’Orchymont A. (1943b) Palpicornia (Coleoptera) V. Bulletin du Musée royal d’Histoire naturelle de Belgique 19(22): 1–8. [Google Scholar]
- d’Orchymont A. (1943c) Notes sur quelques Helochares (s. str.) (Coleoptera Palpicornia Hydrophilidae). Bulletin du Musée royal d’Histoire naturelle de Belgique 19(26): 1–8. [Google Scholar]
- d’Orchymont A. (1943d) Faune du Nord-Est Brésilien (récoltes du Dr. O Schubart). Palpicornia. Mémoires du Musée royal d’Histoire naturelle de Belgique (2)28: 1–85.
- d’Orchymont A. (1943e) Notes complémentaires sur les Helochares (Hydrobaticus) orientaux (Palpicornia Hydrophilidae). Bulletin du Musée royal d’Histoire naturelle de Belgique 19(21): 1–12. [Google Scholar]
- d’Orchymont A. (1943f) Palpicornia (Coleoptera) VI. Bulletin du Musée royal d’histoire naturelle de Belgique 19(60): 1–12. [Google Scholar]
- d’Orchymont A. (1948) Report on the Palpicornia (Coleoptera), Mr. Omer-Cooper’s Investigation of the Abyssinian Fresh Waters (Hugh Scott Expedition). Proceedings of the Zoological Society of London 117(1947–1948): 716–741. 10.1111/j.1096-3642.1948.tb00552.x [DOI] [Google Scholar]
- Darilmaz MC, Kiyak S. (2006) Helochares lividus: new distributional records from Turkey (Coleoptera: Hydrophilidae). Entomological Problems 36(1): 1–79. [Google Scholar]
- Darilmaz MC, İncekara Ü. (2011) Checklist of Hydrophiloidea of Turkey (Coleoptera: Polyphaga). Journal of Natural History 45(11–12): 685–735. 10.1080/00222933.2010.535916 [DOI] [Google Scholar]
- Devi MB, Devi OS, Fikáček M, Minoshima Y, Wanghengbam L. (2016) Redescription and lectotype designation of Chasmogenus abnormalis (Sharp), with notes on its distribution. Zootaxa 4144(2): 296–300. 10.11646/zootaxa.4144.2.12 [DOI] [PubMed] [Google Scholar]
- de Villers CJ. (1789) Caroli Linnaei Entomologia, faunae Suecicae descriptionibus aucta. Vol. 4. Piestre et Delamolliere, Lugduni, 556 pp. [pl. xi.] https://biodiversitylibrary.org/page/12750672 [Google Scholar]
- Dejean PFMA. (1833) (1833–1837) Catalogue des Coléoptères de la collection de M. le Comte Dejean (2. ed.). 443 pp. (only pp. 1–176 issued in 1833; other parts issued in 1834 (pp. 177–256), 1835 (pp. 257–360) and 1837 (pp. 361–443)). Méquignon-Marvis Père et Fils, Paris. https://biodiversitylibrary.org/page/9322478
- Dong X, Bian D. (2021) Three new species and two new records of Helochares (Hydrobaticus) MacLeay, 1871 from China (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 4950: 166–180. 10.11646/zootaxa.4950.1.9 [DOI] [PubMed] [Google Scholar]
- Erichson WF. (1837–1839) Die Käfer der Mark Brandenburg (Vol. 1). F. H. Morin, Berlin, 740 pp. [only pp 1–384 issued in 1837.] https://biodiversitylibrary.org/page/42139591 [Google Scholar]
- Erichson WF. (1843) Beitrag zur Insecten-Fauna von Angola, in besondere Beziehung zur geographischen Verbreitung der Insecten in Afrika. Archiv für Naturgeschichte 9(1): 199–267. https://biodiversitylibrary.org/page/13703230 [Google Scholar]
- Fabricius JC. (1787) Mantissa insectorum sistens eorum species nuper detectas adiectis characteribus genericis, differentiis specificis, emendationibus, observationibus (Vol. 1). C. G. Proft, Hafniae, 348 pp. https://biodiversitylibrary.org/page/12058315 [Google Scholar]
- Fabricius JC. (1792) Entomologia systematica emandata et aucta. Secundum classes, ordines, genera, species adjectis synonimis, locis, observationibus descriptionibus (Vol. 1, pars 1). C. G. Proft, Hafniae, 330 pp. https://biodiversitylibrary.org/page/52755135 [Google Scholar]
- Fabricius JC. (1801) Systema Eleutheratorum: secundum ordines, genera, species, adiectis synonymis, locis, observationibus, descriptionibus. Tomus I. Bibliopolii Academici Novi, Kiliae, 506 pp. [Google Scholar]
- Fairmaire L, Laboulbène A. (1854) [(1854–56)] Faune Entomologique Française ou Description des Insectes qui se trouvent en France (Vol. 1). Coléoptères. Deyrolle, Paris, 665 pp. https://books.google.com/books?id=lBqTM338UHsC&dq=Faune%20Entomologique%20Fran%C3%A7aise%20ou%20Description%20des%20Insectes%20qui%20se%20trouvent%20en%20France&pg=PR3#v=thumbnail& [Google Scholar]
- Fauvel A. (1883) Les Coléoptères de la Nouvelle-Calédonie et dépandances avec descriptions, notes et synonymies nouvelles. Revue d’Entomologie 2: 335–360. https://www.biodiversitylibrary.org/page/25445863 [Google Scholar]
- Fauvel A. (1895) Notes synonymiques. Revue d’Entomologie 14: 92–127. https://biodiversitylibrary.org/page/10977316 [Google Scholar]
- Fernández LA. (1981) Dos especies nuevas del género Helochares (Coleoptera: Hydrophilidae). Limnobios 2(3): 189–192. [Google Scholar]
- Fernández LA. (1982a) Notas sobre el género Helochares (Insecta, Coleoptera: Hydrophilidae). Neotrópica 8(79): 31–40. [Google Scholar]
- Fernández LA. (1982b) Cinco especies nuevas del género Helochares (Coleoptera: Hydrophilidae). Physis (Buenos Aires), secc. b 40(99): 85–90. [Google Scholar]
- Fernández LA. (1983) Helochares (Sindolus) talarum sp. nov., y redescripción de Helochares (Helochares) pallipes (Brullé), con descripción de los estados preimaginales (Coleoptera: Hydrophilidae). Limnobios 2(6): 439–449. [Google Scholar]
- Fernández LA. (1986) Consideraciones sobre el género Chasmogenus Sharp y descripción de Chasmogenus sapucay sp. nov. (Coleoptera: Hydrophilidae). Neotrópica 32: 189–193. [Google Scholar]
- Fernández LA. (1989) Notas sobre el género Helochares. II (Coleoptera: Hydrophilidae). Descripción de dos especies nuevas neotropicales. Clave para determinar las especies argentinas y de áreas vecinas. Revista de la Sociedad Entomológica Argentina 45(1–4): 145–151. [Google Scholar]
- Fernández LA. (2004) Larvae of Neotropical Helochares Mulsant (Coleoptera, Hydrophilidae): Description of H. femoratus (Brullé). Transactions of the American Entomological Society 130(1): 77–83. https://www.jstor.org/stable/25078837 [Google Scholar]
- Fernández LA, Bachmann AO. (1987) Revisión del género Helobata Bergroth (Coleoptera: Hydrophilidae). Revista de la Sociedad Entomológica Argentina 44(2): 149–159. https://www.biotaxa.org/RSEA/article/view/36822 [Google Scholar]
- Fernández LA, Kehr AI. (1994) The annual life cycle of an Argentinean population of Helochares femoratus (Brullé) (Coleoptera: Hydrophilidae). The Coleopterists Bulletin 48(1): 95–98. https://www.jstor.org/stable/4009006 [Google Scholar]
- Fernández LA, Kehr AI. (1995) Disposición espacial y su variabilidad con respecto al tiempo, de una población de Helochares femoratus (Brullé) (Coleoptera: Hydrophilidae). Revista de la Sociedad Entomológica Argentina 54(1–4): 67–73. https://biotaxa.org/RSEA/article/view/33511/29714 [Google Scholar]
- Fikáček M. (2003) Commented review of immature stages of World Hydrophiloidea (Coleoptera: Staphyliniformia). Bachelor Seminar Work, Charles University, Prague.
- Fikáček M, Gentili E, Short AE. (2010) Order Coleoptera, family Hydrophilidae. Arthropod fauna of the UAE 3: 135–165. [Google Scholar]
- Fikáček M, Minoshima YN, Newton AF. (2014) A Review of Andotypus and Austrotypus gen. nov., Rygmodine genera with an Austral disjunction (Hydrophilidae: Rygmodinae). Annales Zoologici 64(4): 557–596. 10.3161/000345414X685893 [DOI] [Google Scholar]
- Fikáček M, Prokin A, Yan E, Yue Y, Wang B, Ren D, Beattie R. (2014) Modern hydrophilid clades present and widespread in the Late Jurassic and Early Cretaceous (Coleoptera: Hydrophiloidea: Hydrophilidae). Zoological Journal of the Linnean Society 170: 710–734. 10.1111/zoj.12114 [DOI] [Google Scholar]
- Fikáček M, Angus RB, Gentili E. (2015) Family Hydrophilidae Latreille, 1802. In: Löbl I, Löbl D. (Eds) Catalogue of Palaearctic Coleoptera (Vol.2). Revised and Updated Edition, Hydrophiloidea – Staphylinoidea. Brill, Leiden, Netherlands, 37–76.
- Fikáček M, Minoshima YN, Komarek A, Short AEZ, Huang D, Cai C. (2017) Cretocrenis brumanicus, the first Mesozoic amber inclusion of a water scavenger beetle (Coleoptera: Hydrophilidae). Cretaceous Research 77: 49–55. 10.1016/j.cretres.2017.04.017 [DOI] [Google Scholar]
- Fleutiaux E, Sallé A. (1889) [(1889–90)] Liste des Coléoptères de la Guadeloupe et descriptions d’espèces nouvelles. Annales de la Société entomologique de France (6)9: 351–484. [only pp. 351–424 issued in 1889.] https://biodiversitylibrary.org/page/32438903
- Forster JR. (1771) Novæ species Insectorum. Centuria I. T. Davies; B. White, London, 100 pp. [Google Scholar]
- Fourcroy AF. (1785) Entomologia Parisiensis; sive, Catalogus insectorum quae in agro Parisiensi reperiuntur. Pars prima. Via et Aedibus Serpentineis, Parisiis, 231 pp. https://biodiversitylibrary.org/page/25563905 [Google Scholar]
- Freitag H, Zettel H. (2013) Aquatic Coleoptera of the Lake Manguao Catchment, Palawan, the Philippines. The Philippine Scientist 50: 1–38. http://journals.usc.edu.ph/index.php/psci/article/view/19 [Google Scholar]
- Ganglbauer L. (1904) Die Käfer von Mitteleuropa (Vol. 4 (part 1)). Karl Gerold’s Sohn, Wien, 286 pp. https://biodiversitylibrary.org/page/9334581 [Google Scholar]
- García M. (2000a) Four new species of Chasmogenus Sharp, 1882 (Coleoptera: Hydrophilidae: Hydrophilinae) from Venezuela. Boletín del Centro de Investigaciones Biológicas Universidad del Zulia 34(1): 45–58. http://www.produccioncientificaluz.org/index.php/boletin/article/view/218 [Google Scholar]
- García M. (2000b) Una nueva especie de Quadriops Hansen, 1999 (Coleoptera: Hydrophilidae: Hydrophilinae) de Venezuela. Boletín del Centro de Investigaciones Biológicas 34(1): 59–65. http://produccioncientificaluz.org/index.php/boletin/article/view/219 [Google Scholar]
- García M. (2000c) Tres nuevas especies de Helobata Bergroth 1888 (Hydrophilidae: Hydrophilinae), de Venezuela. Boletín del Centro de Investigaciones Biológicas 34(2): 237–246. http://www.produccioncientificaluz.org/index.php/boletin/article/view/201 [Google Scholar]
- García M. (2001) Nueva subtribu, género y especie de Hydrophilini (Coleoptera: Hydrophilidae) del extremo suroriental de Venezuela. Boletín del Centro de Investigaciones Biológicas 35(2): 151–160. http://produccioncientificaluz.org/index.php/boletin/article/view/3978 [Google Scholar]
- Gemminger M, Harold B de. (1868) Catalogus Coleopterorum hucusque descriptorum synonymicus et systematicus (Vol. 2). E. H. Gummi, Monachii, 425–752 pp. [+ 6 unn.] https://biodiversitylibrary.org/page/10174177
- Gentili E, Hebauer F, Jäch MA, Ji L, Schödl S. (1995) Hydrophilidae: 1. Checklist of the Hydrophilinae recorded from China (Coleoptera). In: Jäch MA, Ji L. (Eds) Water Beetles of China (Vol.1). Zoologisch-Botanische Gesellschaft in Österreich and Wiener Coleopterologenverein, Vienna, 207–219.
- Gentili E, Ostovan H, Ghahari H, Komarek A. (2018) Annotated checklist of Iranian Hydrophilidae (Coleoptera: Polyphaga: Hydrophiloidea), Aquatic Insects 39(1): 55–88. 10.1080/01650424.2017.1415360 [DOI]
- Girón JC, Short AEZ. (2017) Revision of the Neotropical water scavenger beetle genus Quadriops Hansen, 1999 (Coleoptera: Hydrophilidae: Acidocerinae). ZooKeys 705: 115–141. 10.3897/zookeys.705.19815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón JC, Short AEZ. (2018) Three new genera of acidocerine water scavenger beetles from tropical South America (Coleoptera, Hydrophilidae, Acidocerinae). ZooKeys 768: 113–158. 10.3897/zookeys.768.24423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón JC, Short AEZ. (2019) Three additional new genera of acidocerine water scavenger beetles from the Guiana and Brazilian Shield regions of South America (Coleoptera: Hydrophilidae: Acidocerinae). ZooKeys 855: 109–154. 10.3897/zookeys.855.33013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón JC, Short AEZ. (2021a) New subgenera, new species and new records of the Neotropical water scavenger beetle genus Tobochares Short & García, 2007 (Coleoptera: Hydrophilidae: Acidocerinae). ZooKeys 1019: 93–140. 10.3897/zookeys.1019.59881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón JC, Short AEZ. (2021b) Checklist of accepted names in the water scavenger beetle subfamily Acidocerinae (Coleoptera: Hydrophilidae). ZooKeys. Checklist dataset 10.15468/ypcrsp [accessed via GBIF.org on 2021-06-15.] [DOI]
- Gonzalez-Rodriguez LM, García-Hernández AL, Clarkson B. (2017) First records of water scavenger beetle species (Coleoptera: Hydrophilidae) from Quindío Department, Colombia. Check List 13(5): 605–620. 10.15560/13.5.605 [DOI] [Google Scholar]
- Guillebeau F. (1896) Descriptions de quelques espèces de Coléoptères inédites. Bulletin de la Société entomologique de France (1896): 226–232. [239–245.] https://www.persee.fr/docAsPDF/bsef_0037-928x_1896_num_1_9_21849.pdf
- Gyllenhal L. (1808) Insecta svecica descripta a Leonardo Gyllenhal – Classis I. Coleoptera sive Eleuterata. Tomus 1. F. J. Leverentz, Scaris, 572 pp. https://biodiversitylibrary.org/page/9330983 [Google Scholar]
- Hansen M. (1982) Revisional notes on some European Helochares Muls. (Coleoptera: Hydrophilidae). Entomologica Scandinavica 13: 201–211. 10.1163/187631282X00129 [DOI] [Google Scholar]
- Hansen M. (1991) The hydrophiloid beetles. Phylogeny, classification and a revision of the genera (Coleoptera: Hydrophilidae). Biologiske Skrifter 40: 1–367. [Google Scholar]
- Hansen M. (1997) A new subfamily for a remarkable new genus and species of Hydrophilidae from New Guinea (Coleoptera: Hydrophilidae). Annales Zoologici 47: 107–110. [Google Scholar]
- Hansen M. (1999a) Fifteen new genera of Hydrophilidae (Coleoptera), with remarks on the generic classification of the family. Insect Systematics and Evolution 30(2): 121–172. 10.1163/187631200X00228 [DOI] [Google Scholar]
- Hansen M. (1999b) World catalogue of insects (Volume 2). Hydrophiloidea (s. str.) (Coleoptera). Apollo Books, Stenstrup, 414 pp. [Google Scholar]
- Hansen M. (2004) Family Hydrophilidae. In: Löbl I, Smetana A. (Eds) Catalogue of Palearctic Coleoptera (Vol.2). Hydrophiloidea – Histeroidea – Staphylinoidea. Apollo Books, Stenstrup, 44–68.
- Hansen M, Hebauer F. (1988) A new species of Helochares from Israel, with a key to the European and some Near East species (Coleoptera: Hydrophilidae). Insect Systematics and Evolution 19(1): 27–30. 10.1163/187631289X00023 [DOI] [Google Scholar]
- Hebauer F. (1988) Hydrophiloidea aus Namibia (Coleoptera; Hydrophilidae, Spercheidae). Bonner zoologische Beiträge 39: 153–161. [Google Scholar]
- Hebauer F. (1992) The species of the genus Chasmogenus Sharp, 1882 (Coleoptera, Hydrophilidae). Acta Coleopterologica 8(2): 61–92. [Google Scholar]
- Hebauer F. (1994) The Hydrophiloidea of Israel and the Sinai (Coleoptera, Hydrophiloidea). Zoology in the Middle East 10: 73–138. 10.1080/09397140.1994.10637663 [DOI] [Google Scholar]
- Hebauer F. (1995a) Die Hydrophilidae und Spercheidae Namibias Unter Berücksichtung des Materials der Namibia-Expedition des Museums für Naturkunde Berlin 1992, ergänzt durch Nachweise aus früheren Namibia- und Kalahari-Expeditionen (Coleoptera, Hydrophiloidea: Hydrophilidae et Spercheidae). Mitteilungen aus dem Zoologischen Museum in Berlin 71(2): 251–275. 10.1002/mmnz.19950710206 [DOI] [Google Scholar]
- Hebauer F. (1995b) Neues zu den Acidocerina Hansen (Helocharae d’Orchymont) der indomalaiischen Region (Coleoptera, Hydrophilidae). Acta Coleopterologica 11(3): 3–14. [Google Scholar]
- Hebauer F. (1996) Synopsis der afrikanischen Arten der Gattung Helochares Mulsant (Coleoptera, Hydrophilidae). Acta Coleopterologica 12(2): 3–38. [Google Scholar]
- Hebauer F. (1997) Annotated checklist of the Hydrophilidae and Helophoridae (Insecta: Coleoptera) of the Arabian Peninsula with a description of a new genus and species. Fauna of Saudi Arabia 16: 255–276. [Google Scholar]
- Hebauer F. (1998) Six new species of the genus Helochares Mulsant, 1844, subgenus Hydrobaticus Mac Leay, 1871 from Africa and Asia (Coleoptera: Hydrophilidae). Acta coleopterologica 14(2): 41–46. [Google Scholar]
- Hebauer F. (1999) Neue und wenig bekannte Hydrophiloidea aus dem südlichen Afrika (Coleoptera: Hydrophiloidea). Acta Coleopterologica 15(2): 7–16. [Google Scholar]
- Hebauer F. (2000) The genus Megagraphydrus Hansen, 1999, with description of new species (Coleoptera: Hydrophilidae). Acta Coleopterologica 16(2): 14–22. [Google Scholar]
- Hebauer F. (2001a) Beitrag zur Kenntnis der Hydrophilidae von Neuguinea. Ergebnisse der zoologischen Forschungsreisen von M. Balke und L. Hendrich nach West Neuguinea (Irian Jaya) in den Jahren 1990–1998 (Coleoptera: Hydrophilidae). Acta Coleopterologica 17(1): 3–72. [Google Scholar]
- Hebauer F. (2001b) The real Helochares taprobanicus Sharp 1890 and its allies (Coleoptera: Hydrophilidae). Latissimus 14: 10–16. [Google Scholar]
- Hebauer F. (2002a) Hydrophilidae of Northern India and Southern Himalaya (Coleoptera: Hydrophilidae). Acta Coleopterologica 18(1): 3–72. [Google Scholar]
- Hebauer F. (2002b) New Hydrophilidae of the Old World (Coleoptera, Hydrophilidae). Acta Coleopterologica 18(3): 3–24. [Google Scholar]
- Hebauer F. (2003a) A new species of the genus Helochares (Coleoptera, Hydrophilidae) from Africa. Special Bulletin of the Japanese Society of Coleopterology 6: 129–132. [Google Scholar]
- Hebauer F. (2003b) Checklist of the Malagasy Helochares with description of a new species (Coleoptera: Hydrophilidae). Acta Coleopterologica 19(1): 67–69. [Google Scholar]
- Hebauer F. (2005) Contribution to the knowledge of the Hydrophilidae of Malawi (Coleoptera: Hydrophilidae). Acta Coleopterologica 21(1): 37–40. [Google Scholar]
- Hebauer F. (2006a) Checklist of the Hydrophiloidea of Africa and adjacent archipelagos (Coleoptera: Epimetopidae, Georissidae, Helophoridae, Hydrochidae, Hydrophilidae, Spercheidae). Entomologische Zeitschrift 116(1): 19–36. [Google Scholar]
- Hebauer F. (2006b) Results of the Benin Mission 2001 and the Zambia Mission 2002 of F. and L. Kantner (Coleoptera: Hydrophilidae). Acta Coleopterologica 22(2): 11–24. [Google Scholar]
- Hebauer F. (2009) Five new species of the genus Helochares MacLeay, 1871 from Gabon and the Congo (Coleoptera: Hydrophilidae). Acta Coleopterologica 25(2): 3–8. [Google Scholar]
- Hebauer F, Hendrich L. (1999) Two new species of Helochares from Northern Australia (Coleoptera: Hydrophilidae). Entomological Problems 30(1): 47–51. [Google Scholar]
- Hebauer F, Ryndevich SK. (2005) New data on the distribution of Old World Hydrophilidae (Coleoptera). Acta Coleopterologica 21(1): 43–51. [Google Scholar]
- Hebauer F, Hendrich L, Balke M. (1999) A contribution to the knowledge of the water beetle fauna (Col. Hydradephaga, Hydrophiloidea and Staphylinoidea) of a tropical freshwater lake: Tasek Cini, Pahang, West Malaysia. Raffles Bulletin of Zoology 47(2): 333–348. [Google Scholar]
- Heer O. (1841) [(1838–1841)] Fauna Coleopterorum Helvetica I. Orelii, Fuesslini et Sociorum, Turici, 652 pp. [only pp. 361–652 issued in 1841.] https://books.google.com/books?id=Qow-AAAAcAAJ&pg=PR3#v=onepage&q&f=false [Google Scholar]
- Herbst JFW. (1797) Natursystem aller bekannten in und ausländischen Insekten: als eine Fortsetzung der von Büffonschen Naturgeschichte: nach dem System des Ritters Carl von Linne bearbeitet – Der Käfer, 7. Pauli, Berlin, 346 + xi pp. [26 pl.] 10.3931/e-rara-40522 [DOI]
- Heyden L. (1891) Hydrophilidae. In: Reitter E. (Ed.) Catalogus Coleopterorum Europae, Caucasi et Armeniae rossicae.R. Friesländer & Sohn, Berlin, 66–75. https://archive.org/details/cataloguscoleop00weisgoog
- Hope FW. (1845) On the Entomology of China, with descriptions of the new species sent to England by Dr. Cantor from Chusan and Canton. Transactions of the Entomological Society of London 4(1845–47): 4–17. [pl. 1.] 10.1111/j.1365-2311.1845.tb01326.x [DOI] [Google Scholar]
- Horn GH. (1873) Revision of the genera and species of the tribe Hydrobiini. Proceedings of the American Philosophical Society 13(90): 118–137. https://www.jstor.org/stable/981607 [Google Scholar]
- Horn GH. (1890) Notes on some Hydrobiini of Boreal America. Transactions of the American entomological Society 17: 237–278. [pls 3, 4.] https://www.jstor.org/stable/25076544 [Google Scholar]
- Illiger JCW. (1798) Verzeichniss der Käfer Preussens. J. J. Gebauer, Halle, 510 pp. https://biodiversitylibrary.org/page/52579285 [Google Scholar]
- İncekara Ü, Bektaş M, Taşar GE, Polat A. (2016) A new record for the Turkish fauna (Coleoptera: Hydrophilidae), with further notes on the Laccobius sinuatus Motschulsky, 1849 and Coelostoma transcaspicum Reitter, 1906. Turkish Journal of Science and Technology 11: 21–23. https://dergipark.org.tr/en/pub/tjst/issue/29289/313566. [Google Scholar]
- Inoue D, Nakajima J, Kudo Y, Utsunomiya Y, Kawahara J. (2009) [Illustrated Guide of Aquatic Insects of Fukuoka Prefecture]. Gyobu, a Club of Fukuoka Prefectural Kitakyushu HighSchool, Kitakyushu. [In Japanese]
- International Commission on Zoological Nomenclature [ICZN] (1964) Opinion 710. Enhydrus Laporte, 1834 (Insecta, Coleoptera): validated under the Plenary Powers. Bulletin of Zoological Nomenclature 21: 242–245. https://www.biodiversitylibrary.org/page/12222099 [Google Scholar]
- International Commission on Zoological Nomenclature [ICZN] (1999) International Code of Zoological Nomenclature. International Trust for Zoological Nomenclature, 306 pp. [DOI] [PMC free article] [PubMed]
- Jäch MA. (1984) Die Koleopterenfauna der Bergbäche von Südwest-Ceylon (Col.). Archiv für Hydrobiologie / Supplementum 69(2): 228–332. [Google Scholar]
- Jasper SK, Vogtsberger RC. (1996) First Texas records of five genera of aquatic beetles (Coleoptera: Noteridae, Dytiscidae, Hydrophilidae) with habitat notes. Entomological News 107: 49–60. https://biodiversitylibrary.org/page/2700315 [Google Scholar]
- Jia F. (1998) A new genus Pseudopelthydrus gen. n. from Hainan Island, China (Coleoptera: Hydrophilidae: Hydrophilinae). Chinese Journal of Entomology 18: 225–230. [Google Scholar]
- Jia F. (2010) Megagraphydrus puzhelongi, sp n., a new water scavenger beetle from China (Coleoptera: Hydrophilidae: Hydrophilinae). Zootaxa 2498(1): 65–68. 10.11646/zootaxa.2498.1.6 [DOI] [Google Scholar]
- Jia F, Tang Y. (2018a) A faunistic study of genus Chasmogenus Sharp, 1882 of China (Coleoptera, Hydrophilidae). ZooKeys 738: 59–66. 10.3897/zookeys.738.21711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Tang Y. (2018b) A revision of the Chinese Helochares (s. str.) Mulsant, 1844 (Coleoptera, Hydrophilidae). European Journal of Taxonomy 438: 1–27. 10.5852/ejt.2018.438 [DOI] [Google Scholar]
- Klug JFC. (1855) (Diagnosen neuer Coleoptera aus Mossambique). Bericht über die zur Bekanntmachung geeigneten Verhandlungen der Konigl Preuss Akademie der Wissenschaften zu Berlin 1855: 643–660. https://biodiversitylibrary.org/page/11070837 [Google Scholar]
- Knisch A. (1921) Die exotischen Hydrophiliden des Deutschen Entomologischen Museums (Col.). Archiv für Naturgeschichte. Abteilung A. 85(8): 55–88. https://biodiversitylibrary.org/page/13321515 [Google Scholar]
- Knisch A. (1922) Hydrophiliden-Studien. (Op. 10.). Archiv für Naturgeschichte Abteilung A. 88(5): 87–126. https://biodiversitylibrary.org/page/45506050 [Google Scholar]
- Knisch A. (1924a) Hydrophilidae. In: Junk W, Schenkling S (Eds) Coleopterorum Catalogus (Vol. 14, part 79). W. Junk, Berlin, 306 pp. [Google Scholar]
- Knisch A. (1924b) Neue Palpicornier aus dem südlichen Himalaya. (Col. Hydrophilidae. – Op. 15.). Wiener entomologische Zeitung 41: 29–41. [Google Scholar]
- Knisch A. (1924c) Neue neotropische Palpicornier. (Col. Hydrophilidae. – Op. 16.). Wiener entomologische Zeitung 41: 114–140. [Google Scholar]
- Kohlenberg AT, Short AEZ. (2017) Revision of the Neotropical water scavenger beetle genus Tobochares Short & García, 2007 (Coleoptera, Hydrophilidae, Acidocerinae). ZooKeys 669: 113–146. 10.3897/zookeys.669.11773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarek A. (2003) Hydrophilidae: I. Checklist and key to Palearctic and Oriental genera of aquatic Hydrophilidae (Coleoptera). In: Jäch MA, Ji L. (Eds) Water beetles of China (Vol.3). Zoologisch-Botanische Gesellschaft in Österreich and Wiener Coleopterologenverein, Wien, 383–395.
- Komarek A. (2018) Taxonomic revision of Agraphydrus Régimbart, 1903 II. The Indian Subcontinent. Koleopterologische Rundschau 88: 103–204. http://www.coleoptera.at/uploads/www.coleoptera.at/KOR_88_2018_0103-0204.pdf [Google Scholar]
- Komarek A. (2019) Taxonomic revision of Agraphydrus Régimbart, 1903 III. Southeast Asia (except Philippines) and Australian Region (Coleoptera: Hydrophilidae: Acidocerinae). Koleopterologische Rundschau 89: 151–316. http://www.coleoptera.at/uploads/www.coleoptera.at/KOR_89_2019_0151-0316.pdf [Google Scholar]
- Komarek A. (2020) Taxonomic revision of Agraphydrus Régimbart, IV. Africa, Western Asia, and redescription of the genus (Coleoptera: Hydrophilidae: Acidocerinae). Koleopterologische Rundschau 90: 127–200. http://www.coleoptera.at/uploads/www.coleoptera.at/KOR_90_2020_0127-0200.pdf. [Google Scholar]
- Komarek A, Hebauer F. (2018) Taxonomic revision of Agraphydrus Régimbart, 1903 I. China and Taiwan (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 4452(1): 1–101. 10.11646/zootaxa.4452.1.1 [DOI] [PubMed] [Google Scholar]
- Komarek A, Freitag H. (2020) Taxonomic revision of Agraphydrus Régimbart, 1903 V. Philippine species and their first DNA barcodes. Koleopterologische Rundschau, 90: 201–242. http://www.coleoptera.at/uploads/www.coleoptera.at/KOR_90_2020_0127-0200.pdf [Google Scholar]
- Kuwert A. (1888) Generalübersicht der Philydrus-Arten Europas und der Mittelmeerfauna. Deutsche entomologische Zeitschrift 32: 273–293. https://www.zobodat.at/pdf/Deutsche-Ent-Zeitschrift_32_1888_0273-0293.pdf [Google Scholar]
- Kuwert A. (1890a) Bestimmungs-Tabellen der europäischen Coleopteren. XIX. Heft. Hydrophilidae. I. Abteilung: Hydrophilini. Verhandlungen des naturforschenden Vereins in Brünn 28(1889): 1–121. [Also issued as reprint: 121 pp. – Brünn.] https://biodiversitylibrary.org/page/43763386 [Google Scholar]
- Kuwert A. (1890b) Bestimmungs-Tabellen der europäischen Coleopteren. XX. Heft. Hydrophilidae. II. Abteilung: Sphaeridiini und Helophorini. Verhandlungen des naturforschenden Vereins in Brünn 28(1889): 159–328. [Also issued as reprint: 172 pp. – Brünn.] https://biodiversitylibrary.org/page/43763542 [Google Scholar]
- Lacordaire T. (1854) Histoire naturelle des insectes: Genera des Coléoptères ou exposé méthodique et critique de tous les genres proposés jusqu’ici dans cet ordre d’insectes. Tome Premiere. Contenant les familles des Cicindélètes, Carabiques, Dytiscides, Gyrinides et Palpicornes. Librairie encyclopédique de Roret, Paris, 486 pp. https://biodiversitylibrary.org/page/9623497 [Google Scholar]
- Lawrence J, Ślipiński A. (2013) Australian Beetles: Morphology, Classification and Keys. CSIRO Publishing, Melbourne, Australia, 576 pp. 10.1071/9780643097292 [DOI] [Google Scholar]
- LeConte JL. (1855) Synopsis of the Hydrophilidæ of the United States. Proceedings of the Academy of natural Sciences of Philadelphia 7: 356–375. https://biodiversitylibrary.org/page/1694470 [Google Scholar]
- LeConte JL. (1861) New species of Coleoptera inhabiting the Pacific district of the United States. Proceedings of the Academy of Natural Sciences of Philadelphia 13: 338–359. https://biodiversitylibrary.org/page/1683467 [Google Scholar]
- Lee D-H, Ahn K-J. (2009) Agraphydrus narusei (Satô) and Crenitis apicalis (Reitter) (Coleoptera: Hydrophilidae) new to Korea. Entomological Research 39(5): 317–320. 10.1111/j.1748-5967.2009.00233.x [DOI] [Google Scholar]
- Lee D-H, Ahn K-J. (2017) Three water scavenger beetle species (Coleoptera: Hydrophilidae) new to Korea. Journal of Asia-Pacific Biodiversity 10(1): 39–43. 10.1016/j.japb.2016.12.004 [DOI] [Google Scholar]
- Mabrouki Y, Taybi AF, Chavanon G, Berrahou A, Millán A. (2018) Distribution of aquatic beetles from the east of Morocco (Coleoptera, Polyphaga). Arxius de Miscel·lània Zoològica 16: 185–211. 10.32800/amz.2018.16.0185 [DOI] [Google Scholar]
- MacLeay WS. (1825) Annulosa Javanica or an attempt to illustrate the natural affinities and analogies of the Insects collected in Java by Thomas Horsfield, M.D. F.L. & G.S. and deposited by him in the Museum of the Honourable East-India Company. Kingsbury, Parbury, and Allen, London, 50 pp. [1 pl.] https://biodiversitylibrary.org/page/41989352 [Google Scholar]
- MacLeay WS. (1871) Notes on a collection of insects from Gayndah. Transactions of the Entomological Society of New South Wales 2: 79–205. https://biodiversitylibrary.org/page/16220374 [Google Scholar]
- Makhan D. (2007) Helobata soesilae sp. nov. and Helobata aschnakiranae sp. nov. from Suriname (Coleoptera: Hydrophilidae). Calodema Supplementary Paper 14: 1–3. https://www.researchgate.net/publication/321107032_Helobata_soesilae_sp_nov_and_Helobata_aschnakiranae_sp_nov_from_Suriname_Coleoptera_Hydrophilidae [Google Scholar]
- Mart A, İncekara U, Karaca H. (2010) A new species and new records of Hydrophilidae (Coleoptera) from Turkey. Turkish Journal of Zoology 34: 297–303. https://dergipark.org.tr/tr/download/article-file/134666 [Google Scholar]
- Matsui E. (1994) Three new species of the genus Enochrus from Japan and Taiwan (Coleoptera: Hydrophilidae). Transactions of the Shikoku entomological Society 20: 215–220. [Google Scholar]
- Matsui E. (1995) A new species of the genus Helochares (Coleoptera, Hydrophilidae) from Japan, with a key to the Japanese species of the subgenus Hydrovaticus. Special Bulletin of the Japanese Society of Coleopterology 4: 317–322. [Google Scholar]
- Minoshima YN. (2016) Taxonomic review of Agraphydrus from Japan (Coleoptera: Hydrophilidae: Acidocerinae). Entomological Science 19(4): 351–366. 10.1111/ens.12213 [DOI] [Google Scholar]
- Minoshima Y, Fujiwara J. (2009) Discovery of water scavenger beetle genus Megagraphydrus Hansen (Coleoptera, Hydrophilidae) from Japan, with description of a new species. Elytra 37(1): 53–64. http://coleoptera.sakura.ne.jp/Elytra/37(1)053MinoshimaY_and_FujiwaraJ.pdf [Google Scholar]
- Minoshima Y, Hayashi M. (2011) Larval morphology of the Japanese species of the tribes Acidocerini, Hydrobiusini and Hydrophilini (Coleoptera: Hydrophilidae). Acta Entomologica Musei Nationalis Pragae 51 (Supplementum): 1–118. http://www.aemnp.eu/PDF/51_s/51_s_1.pdf
- Minoshima Y, Hayashi M, Kobayashi N, Yoshitomi H. (2013) Larval morphology and phylogenetic position of Horelophopsis hanseni Satô et Yoshitomi (Coleoptera, Hydrophilidae, Horelophopsinae). Systematic Entomology 38(4): 708–722. 10.1111/syen.12025 [DOI] [Google Scholar]
- Minoshima YN, Komarek A, Ôhara M. (2015) A revision of Megagraphydrus Hansen (Coleoptera, Hydrophilidae): synonymization with Agraphydrus Régimbart and description of seven new species. Zootaxa 3930: 1–63. https://www.biotaxa.org/Zootaxa/article/view/1%E2%80%9363 [DOI] [PubMed] [Google Scholar]
- Montrouzier P. (1860) Essai sur la faune entomologique de la Nouvelle-Calédonie et des îles de Pins, Art, Lifu, etc. Annales de la Société entomologique de France 3(8): 229–308. https://biodiversitylibrary.org/page/33971456 [Google Scholar]
- Motschulsky V. (1845) Remarques sur la collection de Coléoptères Russes. 1. Article. – Bulletin de la Société impériale des Naturalistes de Moscou 18 1(1): 3–127. [pl. I–III (errata: p. 549.] https://biodiversitylibrary.org/page/44164948
- Motschulsky V. (1853) Hydrocanthares de la Russie. Société de Litérature Finnoise, Helsingfors, 15 pp. [Google Scholar]
- Müller OF. (1776) Zoologiae Danicae prodromus, seu Animalium Daniae et Norvegiae indigenarum; characteres, nomina, et synonyma imprimis popularium. Hallager, Havniæ, 274 pp. https://biodiversitylibrary.org/page/13227118 [Google Scholar]
- Mulsant E. (1844a) Histoire Naturelle des Coléoptères de France. Palpicornies. L. Maison, Paris; Ch. Savy Jeune, Lyon, 196 pp. [1 pl (errata et addenda: 197)] https://biodiversitylibrary.org/page/9628710 [Google Scholar]
- Mulsant E. (1844b) Description de quelques Palpicornes inédits. Annales de la Société d’ Agriculture de Lyon 7: 372–382. [Google Scholar]
- Murray A. (1859) List of Coleoptera received from Old Calabar, on the West Coast of Africa. Annals and Magazine of natural History, vol. IV, third series: 116–123. https://www.biodiversitylibrary.org/page/18638031
- Olivier AG. (1792) Encyclopédie méthodique. Dictionnaire des Insectes. Tome septième. Panckoucke, Paris, 827 pp. https://www.biodiversitylibrary.org/page/7609575 [Google Scholar]
- Panzer GWF. (1795) Entomologica Germanica Exhibens Insecta per Germaniam Indigena I. Elevterata. Felsegker, Norimbergae, 32 (unn.) + 368 + 2 (unn.) pp. [12 pl.] https://biodiversitylibrary.org/page/12123973
- Panzera O. (1932) Descrizione delle larve di Helochares griseus Fabr. e H. lividus Forst. (Coleoptera-Hydrophilidae). Memorie della Società entomologica italiana 11(2): 52–63. [Google Scholar]
- Paykull G. (1798) Fauna Svecica, Insecta. Tomus I. Joh. F. Edman, Upsaliæ, 358 pp. https://biodiversitylibrary.org/page/51560628 [Google Scholar]
- Przewoźny M. (2019) Catalogue of Palearctic Hydrophiloidea (Coleoptera). [Internet version 2019–01–01.] http://www.waterbeetles.eu/documents/PAL_CAT_Hydrophiloidea_2019.pdf
- Régimbart M. (1900) Coléoptères aquatiques capturés dans l’île d’Aldabra, près des Comores, par le Dr Voeltzkow, de Strasbourg, et communiqués par le Dr Bergroth. Bulletin de la Société entomologique de France (1900): 49–52. https://books.google.com/books?id=0S9GAQAAMAAJ&pg=PA49#v=onepage&q&f=false
- Régimbart M. (1903a) Coléoptères aquatiques (Haliplidae, Dytiscidae, Gyrinidae et Hydrophilidae) recueillis dans le Sud de Madagascar par M. Ch. Alluaud (Juillet 1900 – Mai 1901). Annales de la Société Entomologique de France 72: 1–51. https://biodiversitylibrary.org/page/8247889 [Google Scholar]
- Régimbart M. (1903b) Contribution a la faune Indo-Chinoise. 19e mémoire. Annales de la Société Entomologique de France 72: 52–64. https://biodiversitylibrary.org/page/8247940 [Google Scholar]
- Régimbart M. (1906) Voyage de M. Ch. Alluaud dans l’Afrique Orientale. Dytiscidae, Gyrinidae, Hydrophilidae. Annales de la Société entomologique de France 75: 235–278. https://biodiversitylibrary.org/page/9207127 [Google Scholar]
- Régimbart M. (1907) Hydrophilides provenant du Voyage de M. L. Fea dans l’Afrique Occidentale. Annali del Museo civico di Storia naturale di Genova 3(3)[43]: 46–62. https://biodiversitylibrary.org/page/7931482
- Régimbart M. (1908) Dytiscidae, Hydrophilidae et Gyrinidae. In: Michaelsen W, Hartmeyer R. (Eds) Die Fauna südwest-Australiens.Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905. Band I, Lieferung 8. Gustav Fischer, Jena, 311–316. https://biodiversitylibrary.org/page/1097855
- Reiche L. (1854) Catalogue des Espèces d’Insectes Coléoptères Recueillies par M. F. de Saulcy Pendant son Voyage en Orient. Gide et J. Baudry, Paris, 19 pp. https://books.google.com/books?id=6fxGAQAAMAAJ&pg=PA1& [Google Scholar]
- Reiche L, Saulcy F de. (1856) Espèces nouvelles ou peu connues de Coléoptères, recueillies par M. F. de Saulcy, membre de l’Institut, dans son voyage en Orient. Annales de la Société entomologique de France 3(4): 353–422. [pl. 12.] https://biodiversitylibrary.org/page/8248901 [Google Scholar]
- Reitter E. (1909) Fauna Germanica. Die Käfer des Deutschen Reiches (Vol. 2). K. G. Lutz, Stuttgart, 392 pp. [pls 41–80.] [Google Scholar]
- Rey C. (1885a) Descriptions de Coléoptères nouveaux ou peu connus de la tribu des Palpicornes. Annales de la Société linnéenne de Lyon 31(1884): 13–32. 10.3406/linly.1886.3987 [DOI] [Google Scholar]
- Rey C. (1885b) Histoire naturelle des Coléoptères de France (suite). Annales de la Société linnéenne de Lyon 31(1884): 213–396. https://books.google.com/books?id=6dNBAQAAMAAJ&pg=RA1-PA213#v=onepage&q&f=false [Google Scholar]
- Ribera I, Hernando C, Cieslak A. (2019) Aquatic Coleoptera of North Oman, with description of new species of Hydraenidae and Hydrophilidae. Acta Entomologica Musei Nationalis Pragae 59(1): 253–272. 10.2478/aemnp-2019-0021 [DOI] [Google Scholar]
- Ribera I, Fresneda J, Aguilera P, Hernando C. (1996) Insecta: Coleoptera 8 (Familias 11–26): Coleópteros acuáticos. HydrophilidaeS: Gyrinidae, Haliplidae, Noteridae, Hygrobiidae, Dytiscidae, Hydraenidae, Helophoridae, Georissidae, Hydrochidae, Hydrophilidae, Elmidae, Dryopidae, Heteroceridae, Psephenidae, Scirtidae, ChrysomelidaeDonaciinae. Catalogus de la Entomofauna Aragonesa 10: 3–22. http://molevol.cmima.csic.es/ribera/pdfs/ribera_etal1996_catalogus.pdf [Google Scholar]
- Richmond EA. (1920) Studies on the biology of the aquatic Hydrophilidae. Bulletin of the American Museum of Natural History 42(1): 1–93. [+ 16 pls.] https://digitallibrary.amnh.org/handle/2246/947 [Google Scholar]
- Rossi P. (1792) Mantissa Insectorum, Exhibens Species Nuper in Etruria collectas. Polloni, Pisis, 148 pp. https://biodiversitylibrary.org/page/33474928 [Google Scholar]
- Sahlberg J. (1903) Messis hiemalis Coleopterorum Corcyraeorum. Enumeratio Coleopterorum mensibus Novembri-Februario 1895–1896 et 1898–1899 nec non primo vere 1896 in insula Corcyra collectorum. Öfversigt av finska Vetenskabssocietetens Förhandlingar 45(11): 1–85. https://biodiversitylibrary.org/page/15315790 [Google Scholar]
- Salah M, Régil Cueto JA. (2017) An Annotated Checklist of the Aquatic Polyphaga (Coleoptera) of Egypt II. Family Hydrophilidae. The Coleopterists Bulletin 71(2): 259–278. 10.1649/0010-065X-71.2.259 [DOI] [Google Scholar]
- Satô M. (1960) One new genus and two new species of the subtribe Helocharae from Japan (Coleoptera: Hydrophilidae). Transactions of the Shikoku Entomological Society 6(5): 76–80. [Google Scholar]
- Satô M. (1965) Some aquatic Coleoptera from Formosa, I. Special Bulletin of the Lepidopterological Society of Japan 1: 126–129. [Google Scholar]
- Satô M. (1976) Two Helochares-species from the Ryukyus (Hydrophilidae). Entomological Review of Japan 29: 21–24. [Google Scholar]
- Satô M, Yoshitomi H. (2004) Discovery of a second representative of the genus Horelophopsis (Coleoptera, Hydrophilidae) from the Ryukyu archipelago, Japan. Elytra 32(1): 41–49. [Google Scholar]
- Schaufuss LW. (1869) Beitrag zur Kenntniss der Coleopteren-Fauna der Balearen. Selbstverlag, Prag, 31 pp. https://books.google.com/books?id=NX-cN95KGtUC&lpg=PA3&ots=4j9hzxNFw5&dq=Beitrag%20zur%20Kenntniss%20der%20Coleopteren-Fauna%20der%20Balearen&pg=PA1#v=onepage&q&f=false [Google Scholar]
- Schönherr CJ. (1808) Synonymia Insectorum, oder: Versuch einer Synonymie Aller bisher bekannten Insecten, nach Fabricii Systema Eleutheratorum &c. geordnet, Erster Band, Eleutherata oder Käfer. Zweiter teil (Spercheus-Cryptocephalus). C. F. Marquard, Stockholm, 424 pp. [1 pl.] https://biodiversitylibrary.org/page/42206652 [Google Scholar]
- Scott H. (1913) The Percy Sladen Trust Expedition to the Indian Ocean in 1905, under the Leadership of Mr. J. Stanley Gardiner, M. A (Vol. 5. No. X). Coleoptera, Hydrophilidae, Histeridae. Transactions of the Linnean Society of London 16(2): 193–235. [pl. 14.] 10.1111/j.1096-3642.1914.tb00121.x [DOI] [Google Scholar]
- Seidel M, Arriaga-Varela E, Fikáček M. (2016) Establishment of Cylominae Zaitzev, 1908 as a valid name for the subfamily Rygmodinae Orchymont, 1916 with an updated list of genera (Coleoptera: Hydrophilidae). Acta Entomologica Musei Nationalis Pragae 56: 159–165. https://www.biotaxa.org/AEMNP/article/view/22807/21741 [Google Scholar]
- Sharp D. (1869) Description of a new species of Philhydrus. Entomologist’s monthly Magazine 5: 240–241. https://www.biodiversitylibrary.org/page/43229950 [Google Scholar]
- Sharp D. (1873) The Water Beetles of Japan. Transactions of the Entomological Society of London (1873): 45–67. 10.1111/j.1365-2311.1873.tb00636.x [DOI]
- Sharp D. (1882) Fam. Hydrophilidae. Biologia Centrali-Americana Insecta. Coleoptera 1(2): 53–80. https://biodiversitylibrary.org/page/577106 [Google Scholar]
- Sharp D. (1890) On some aquatic Coleoptera from Ceylon. Transactions of the Entomological Society of London (1890): 339–359. 10.1111/j.1365-2311.1890.tb03026.x [DOI]
- Sharp D. (1903) Water-beetles (Dytiscidae & Hydrophilidae) of the Swedish Zoological expedition to Egypt and the White Nile (No. 10). In: Jägerskiöld LA (Ed.) Results of the Swedish Zoological expedition to Egypt and the White Nile 1901 under the Direction of L. A. Jägerskiöld. Part I. Library of the Royal University of Uppsala, Uppsala, 10 pp. https://biodiversitylibrary.org/page/18760768 [Google Scholar]
- Short AEZ. (2005) A review of the subtribe Acidocerina of Central America with special reference to Costa Rica (Coleoptera: Hydrophilidae). Koleopterologische Rundschau 75: 191–226. http://www.zobodat.at/pdf/KOR_75_2005_0191-0226.pdf [Google Scholar]
- Short AEZ. (2010) Hydrophilidae: Review of the subtribe Acidocerina of the Southwest Pacific islands (Coleoptera). In: Jäch MA, Balke M (Eds) Water Beetles of New Caledonia, part 1, Wien, 297–318.
- Short AEZ. (2013) Chapter 4. Aquatic Beetles of the Grensgebergte and Kasikasima Regions, Suriname (Insecta: Coleoptera). In: Alonso LE, Larsen TH. (Eds) A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname.RAP Bulletin of Biological Assessment (Vol. 67). Conservation International, Arlington, 79–89. https://bioone.org/ebooks/RAP-Bulletin-of-Biological-Assessment/A-Rapid-Biological-Assessment-of-the-Upper-Palumeu-River-Watershed/Chapter/Aquatic-Beetles-of-the-Grensgebergte-and-Kasikasima-Regions-Suriname-Insecta/10.1896/054.067.0112
- Short AEZ. (2018) Systematics of aquatic beetles (Coleoptera): current state and future directions. Systematic Entomology 43(1): 1–18. 10.1111/syen.12270 [DOI] [Google Scholar]
- Short AEZ, Fikáček M. (2011) World catalogue of the Hydrophiloidea (Coleoptera): additions and corrections II (2006–2010). Acta Entomologica Musei Nationalis Pragae 51(1): 83–122. http://www.aemnp.eu/PDF/51_1/51_1_83.pdf [Google Scholar]
- Short AEZ, Fikáček M. (2013) Molecular phylogeny, evolution and classification of the Hydrophilidae (Coleoptera). Systematic Entomology 38(4): 723–752. 10.1111/syen.12024 [DOI] [Google Scholar]
- Short AEZ, García M. (2007) Tobochares sulcatus, a new genus and species of water scavenger beetle from Amazonas State, Venezuela (Coleoptera: Hydrophilidae). Aquatic Insects 29(1): 1–7. 10.1080/01650420701247869 [DOI] [Google Scholar]
- Short AEZ, García M. (2014) A new genus of egg case-carrying water scavenger beetle from the Guiana Shield (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 3835(2): 251–262. 10.11646/zootaxa.3835.2.5 [DOI] [PubMed] [Google Scholar]
- Short AEZ, Girón JC. (2018) Review of the Helochares (Hydrobaticus) MacLeay of the New World (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 4407(1): 29–50. 10.11646/zootaxa.4407.1.2 [DOI] [PubMed] [Google Scholar]
- Short AEZ, Hebauer F. (2006) World Catalogue of Hydrophiloidea – additions and corrections, 1 (1999–2005) (Coleoptera). Koleopterologische Rundschau 76: 315–359. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.614.3424andrep=rep1andtype=pdf [Google Scholar]
- Short AEZ, Kadosoe V. (2011) Chapter 4. Aquatic Beetles of the Kwamalasamutu Region, Suriname (Insecta: Coleoptera). In: O’Shea BJ, Alonso LE, Larsen TH. (Eds) A Rapid Biological Assessment of the Kwamalasamutu region, Southwestern Suriname.RAP Bulletin of Biological Assessment 63, Conservation International, Arlington, 79–90. http://www.bioone.org/doi/abs/10.1896/054.063.0107
- Short AEZ, García M, Girón JC. (2017) Revision of the Neotropical water scavenger beetle genus Globulosis García, 2001 (Coleoptera: Hydrophilidae: Acidocerinae). Zootaxa 4232(2): 271–281. 10.11646/zootaxa.4232.2.10 [DOI] [PubMed] [Google Scholar]
- Short AEZ, Salisbury S, La Cruz N. (2018) Chapter 7. Aquatic Beetles of the Upper Berbice Region, Guyana. In: Alonso LE, Persaud J, Williams A. (Eds) Biodiversity Assessment Survey of the Upper Berbice Region, Guyana.BAT Survey Report No. 3. WWF-Guianas, Guyana Office, Georgetown, Guyana, 128–135. http://d2ouvy59p0dg6k.cloudfront.net/downloads/biodiversity_assessment_survey_of_the_upper_berbice_region_2018.pdf
- Short AEZ, Girón JC, Toussaint EFA. (2021) Evolution and biogeography of acidocerine water scavenger beetles (Coleoptera: Hydrophilidae) shaped by Gondwanan vicariance and Cenozoic isolation of South America. Systematic Entomology 46(2): 380–395. 10.1111/syen.12467 [DOI] [Google Scholar]
- Silva GR, Clarkson B, Lima LRC. (2018) New distributional records of Hydrophilidae Latreille, 1802 (Coleoptera: Hydrophiloidea) from Brazil. Aquatic Insects 39: 375–388. 10.1080/01650424.2018.1462886 [DOI] [Google Scholar]
- Smith RR, Short AEZ. (2020) Review of the genus Chasmogenus Sharp, 1882 of northeastern South America with an emphasis on Venezuela, Suriname, and Guyana (Coleoptera, Hydrophilidae, Acidocerinae). ZooKeys 934: 25–79. 10.3897/zookeys.934.49359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solier AJJ. (1834) Observations sur la tribu des Hydrophiliens, et principalement sur le genre Hydrophilus de Fabricius. Annales de la Société entomologique de France 3: 299–318. https://biodiversitylibrary.org/page/32515446 [Google Scholar]
- Spangler PJ. (1979) A new genus of madicolous beetles from Ecuador (Coleoptera: Hydrophilidae: Hydrobiinae). Proceedings of the Biological Society of Washington 92(4): 753–761. https://www.biodiversitylibrary.org/page/35514474 [Google Scholar]
- Spangler PJ. (1981a) A new water beetle, Troglochares ashmolei, n. gen., n. sp., from Ecuador; the first known eyeless cavernicolous hydrophilid beetle (Coleoptera: Hydrophilidae). Proceedings of the Entomological Society of Washington 83(2): 316–323. https://biodiversitylibrary.org/page/16364657 [Google Scholar]
- Spangler PJ. (1981b) Supplement to the aquatic and semiaquatic Coleoptera of Cuba collected by the Biospeleological Expeditions to Cuba by the academies of science of Cuba and Romania. In: Orghidan T. (Eds) Résultats des Expéditions Biospéologiques Cubano-Roumaines à Cuba, 3.Academiell Republieli Socialiste România, Bucuresti, 145–171.
- Spangler PJ, Cross JL. (1972) A description of the egg case and larva of the water scavenger beetle Helobata striata (Coleoptera: Hydrophilidae). Proceedings of the Biological Society of Washington 85: 413–418. https://biodiversitylibrary.org/page/34559933 [Google Scholar]
- Stephens JF. (1829) (1828–1829) Illustrations of British entomology; or, A synopsis of indigenous insects: containing their generic and specific distinctions. Mandibulata (Vol. II). Baldwin and Cradock, London, 200 pp. [pl. 10–15. (only pp. 113–200 and pls 13–15 issued in 1829] https://biodiversitylibrary.org/page/35933523 [Google Scholar]
- Stephens JF. (1839) A Manual of British Coleoptera, or Beetles: Containing a Brief Description of all the Species of Beetles Hitherto Ascertained to Inhabit Great Britain and Ireland Together with a Notice of their Chief Localities, Times and Places of Appearances, etc. Longman, Orme, Brown, Green, and Longmans, London, 443 pp. https://biodiversitylibrary.org/page/39160555 [Google Scholar]
- Stierlin G. (1900) Coleoptera Helvetiae. Fauna Coleopterorum helvetica. Die Käfer-Fauna der Schweiz I. Theil. Bolli & Böcherer, Schaffhausen, 667 pp. https://biodiversitylibrary.org/page/58080600 [Google Scholar]
- Thomson CG. (1859) Skandinaviens Coleoptera. I Tom. Berlingska Boktryckeriet, Lund, 290 pp. https://biodiversitylibrary.org/page/54335801 [Google Scholar]
- Toussaint EFA, Seidel M, Arriaga‐Varela E, Hájek J, Král D, Sekerka L, Short AEZ, Fikáček M. (2017) The peril of dating beetles. Systematic Entomology 42: 1–10. 10.1111/syen.12198 [DOI] [Google Scholar]
- Watanabe N. (1987) The Japanese species of Helochares (Crephelochares) (Coleoptera: Hydrophilidae), with description of a new species from Honshu. Aquatic Insects 9: 11–15. 10.1080/01650428709361262 [DOI] [Google Scholar]
- Watts CHS. (1995) Revision of the Australasian genera Agraphydrus Régimbart, Chasmogenus Sharp and Helochares Mulsant (Coleoptera: Hydrophilidae). Records of the South Australian Museum 28: 113–130. https://biodiversitylibrary.org/page/46866161 [Google Scholar]
- Watts CHS. (2002) The larvae of some Australian aquatic Hydrophilidae (Coleoptera: Insects). Records of the South Australian Museum 35(2): 105–138. https://biodiversitylibrary.org/page/40639775 [Google Scholar]
- Winters FC. (1927) Key to the subtribe Helocharæ Orchym. (Coleoptera-Hydrophilidæ) of Boreal America. Pan-Pacific Entomologist 4: 19–29. https://biodiversitylibrary.org/page/53384245 [Google Scholar]
- Wollaston TV. (1867) Coleoptera Hesperidum, being an enumeration of the Coleopterous insects of the Cape Verde Archipelago. J. v. Voorst, London, 285 pp. https://biodiversitylibrary.org/page/33075137 [Google Scholar]
- Yoshitomi H, Nakajima J. (2005) A new record of Horelophopsis hanseni (Coleoptera, Hydrophilidae) from Kyushu. Elytra 33(1): e376.
- Young FN. (1954) The water beetles of Florida. University of Florida Press, Gainesville, 238 pp. [Google Scholar]
- Zaitzev P. (1908) Catalogue des Coléoptères aquatiques des familles des Dryopidae, Georyssidae, Cyathoceridae, Heteroceridae et Hydrophilidae. Horae Societatis Entomologicae Rossicae 38: 283–420. https://biodiversitylibrary.org/page/12380729 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.