Abstract
This is Part 1 of an updated follow-up review of a World Allergy Organization (WAO) position paper published in 2012 on the diagnosis and treatment of urticaria and angioedema. Since 2012, there have been advances in the understanding of the pathogenesis of chronic urticaria, and greater experience with the use of biologics, such as omalizumab, in patients with severe refractory disease. For these reasons, the WAO decided to initiate an update targeted to general practitioners around the world, incorporating the most recent information on epidemiology, immunopathogenesis, comorbidities, quality of life, clinical case presentations, and the management of chronic spontaneous and chronic inducible urticaria, including urticaria in special situations such as childhood and pregnancy. A special task force of WAO experts was invited to write the different sections of the manuscript, and the final document was approved by the WAO Board of Directors. This paper is not intended to be a substitute for current national and international guidelines on the management of urticaria and angioedema but to provide an updated, simplified guidance for physicians around the world who manage patients with this common ailment.
Keywords: Angioedema, Chronic inducible urticaria, Chronic spontaneous urticaria, Omalizumab Treatment, Urticaria
Introduction
Chronic spontaneous (idiopathic) urticaria (CSU), defined as the occurrence of wheals, angioedema, or both for more than 6 weeks, affects 1–2% of the population.1 It is more prevalent in women and represents an important burden that compromises patient's quality of life, interferes with routine daily activities,2 and frequently is associated with psychiatric comorbidities (depression and/or anxiety).3 Mean yearly direct and indirect costs of CSU in the United States have been estimated to be $244 million, with medication costs accounting for 62.5% and work absenteeism for 15.7% of the expenses.4 Although the mechanisms leading to CSU are not completely understood at the present time, important pathophysiologic advances have been accomplished in recent years. It is thought that CSU is a chronic inflammatory skin disease in which various inflammatory cells and mediators are involved. This knowledge has permitted the envisioning of precise and personalized approaches for the management of this complex disease. As investigators discern immunologic pathways involved in the pathogenesis of CSU, novel therapeutic agents directed to specific molecular targets are being proposed.
In 2012 WAO published a position paper on the diagnosis and treatment of urticaria with a global vision.5 Since that document was published, there have been advances in the understanding of the pathogenesis of chronic urticaria, and greater experience with the use of biologics, mainly omalizumab, in patients with severe disease. For these reasons, WAO decided to initiate an updated review of that paper targeted to general practitioners around the world, incorporating the most recent information on epidemiology, immunopathogenesis, comorbidities, quality of life, clinical case presentations, and the management of CSU and chronic inducible urticaria (CIndU), including urticaria in special situations such as childhood and pregnancy. This update is a summary of the most current information on urticaria and angioedema and was developed to offer guidance to general practitioners worldwide who must deal with this disease; but is not intended to be a substitute for national and international guidelines that are currently in use.
Epidemiology, classification based in time of evolution and triggering factors. Importance of disease registries
The lifetime prevalence for all types of urticaria is usually described below 10% per different reports, while chronic urticaria (CU) only develops in approximately one-fourth of these individuals.5,6 Point prevalence of CU, based on coding reports in health systems from different countries, ranges from 0.1 to less than 1% globally.7, 8, 9 Currently the point prevalence is the best method to compare the frequency of CU between different populations but the development of a standardized and practical tool for this purpose remains an unmet need.
From the total of CU patients, one-third suffer from both hives and angioedema, 30%–40% present isolated hives, and around 10% show isolated angioedema.10,11
The natural history of the disease has a very wide range. Around half of patients will follow a three-month self-limited evolution and within a year it will resolve in almost 80% of them. However, in more than 10% of patients a duration of 5 years or longer is expected.12, 13, 14 Factors conditioning time to remission will be discussed later.
Females are affected at least twice as often as males, and most patients are over 20 years of age.1,7,14 In children, the prevalence varies from less than 1% to almost 5%, depending largely upon the methodology.15, 16, 17 Ethnic differences, although described as statistically significant in a wide American population sample, do not seem to deserve in depth attention in real world.12 Additionally, a proportion of patients experience exacerbations when taking non-steroidal anti-inflammatory drugs (NSAIDs).
The observation that chronic inducible urticaria (CIU) is much more frequent among first-degree relatives of affected individuals than in the general population suggests the existence of a genetic background for the disease and provides clinical support to the reported association between CIU and human leukocyte antigen DR4.18
The economic burden of the pathology is not negligible. The CU related cost has been reported to be as high as $2050 per year per patient in the United States, having a huge personal and familiar impact, particularly in low to middle income countries.10,19,20 Economic burden analysis using purchasing power parity dollars (PPP$) demonstrated a higher therapy and inpatients costs in France with almost 3000 $, compared to less than 1000 $ in Italy; nonetheless, loss of work productivity was greater in Germany than in France with over PPP$ 1.000 and over PPP$ 500.21
Classification based in time of evolution and triggering factors
CU is generally classified according to the triggers and the reported signs and symptoms.6,22,23 While spontaneous urticaria is more frequent, inducible urticaria accounts for approximately 15% of all chronic urticaria patients; however, up to 75% of patients report having both (Table 1).24, 25, 26, 27, 28, 29, 30, 31, 32, 33
Table 1.
Prevalence of inducible urticarias
| Type of Inducible urticaria | Frequency (Percent of total CIU) | References | |
|---|---|---|---|
| Physical | Dermographism | 2–28.5% | 24, 25, 26 |
| Cold Urticaria | 2–13.4% | 24,26,27 | |
| Heat Urticaria | Unknown | 28, 29, 30 | |
| Solar Urticaria | <0.4% | 31 | |
| Vibratory Urticaria | Unknown | 23,30 | |
| Delayed Pressure Urticaria | 7.3–37% | 24,26 | |
| Non Physical | Cholinergic Urticaria | 5–11% | 26,30 |
| Aquagenic Urticaria | 0,4% | 24 | |
| Contact Urticaria | Unknown | 23,32 |
Importance of disease registries
There has been an evolution in medical care delivery from decision-making based largely upon experience to the use of well documented and validated procedures and interventions which constitutes the backbone of evidence based medicine (EBM).34 Guideline development is also part of this evolution as guideline panels collect and evaluate published scientific evidence and provide recommendations based upon their overall assessment.35
However, registries obtained from real-life patients and databases constitute key instruments for setting health care policies, exploring natural history and endless variables. Additionally registries also provide the background for clinical research. The use of EBM, guideline panels, and patient registries all contribute to providing improved patient care and outcomes.36
Registries in allergology have allowed investigators to obtain data inaccessible with other methodologies. Nevertheless, the use of well-designed and executed registries are essential for producing valid outcomes.36 The use of such registries for CU is invaluable.
An online academia-driven, investigator-initiated registry on chronic urticaria is available from www.urticaria-registry.com. Another registry, the Latin American Chronic Urticaria Registry, has unraveled the understanding and knowledge of CU in that region.37,38 The published data of this last registry confirm the classical pattern of age and gender for this population of 300 patients, besides other peculiar data such as low prevalence of parasite associated infestations, and more than half of these patients having significantly affected their quality of life.
Registries provide useful information not only for having an overview of the situation of those patients, but also for specific variables should they represent true elicitors or just innocent co-factors.
Immunopathogenesis. Endotypes. Biomarkers. Predictive factors
Endotypes
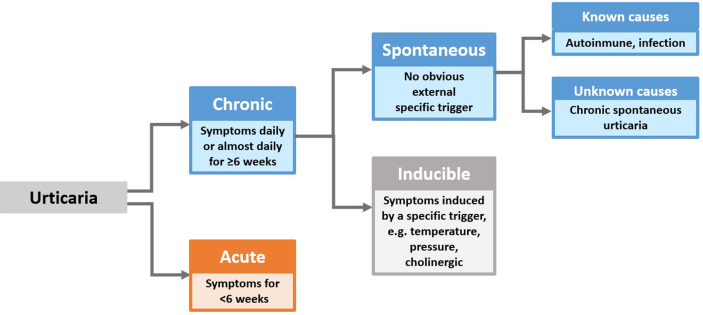
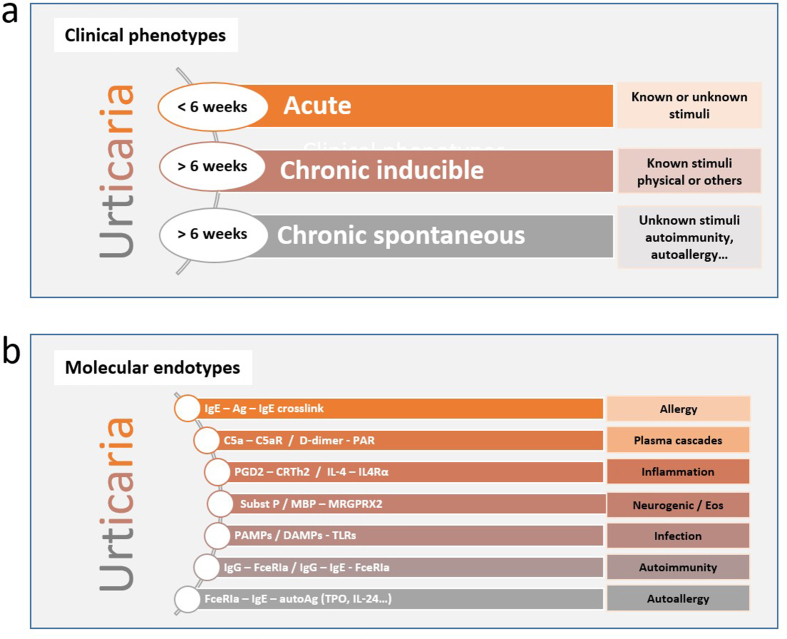
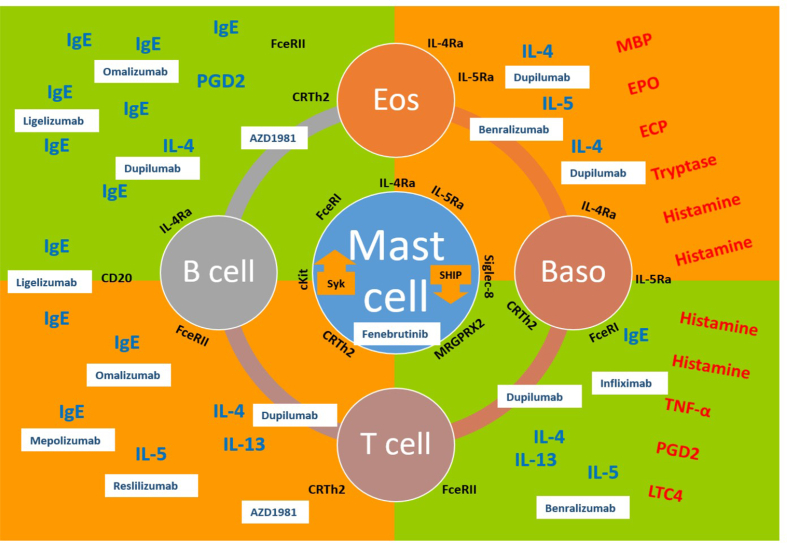
Urticaria can be divided into 3 clinical phenotypes based on its duration (acute vs chronic) and in the presence or absence of inducing factors (inducible vs spontaneous)39,40 (Fig. 1). Formerly referred to as chronic idiopathic urticaria, CSU refers to recurrent urticaria lasting more than 6 weeks that occurs in the absence of an identifiable trigger. Although urticaria has always been considered a mast cell-driven disease, it is now known that it involves dysregulation of both mast cell and basophils with their subsequent activation and degranulation as well as the participation of other cells, eg, eosinophils, T and B lymphocytes, epithelial, and endothelial cells.41 Tissue-resident mast cells can be activated by multiple triggers, immunological or not, highlighting their role in the innate immune response to many skin and mucosal aggressors. Mast cell degranulation can be elicited by the activation of several membrane proteins as a consequence of the crosslinking of allergens recognized by IgE molecules attached to high affinity receptors (FcεRI) on the membrane. There are many other receptors on the mast cell membrane that can also activate it, like C5aR for anaphylotoxins of the complement system (C5a), CRTh2 for PGD2, MRGPRX2 for neuropeptides (like substance P), or proteases and cationic proteins (like MBP and ECP), cKit for stem cell factor (SCF), other important cytokine receptors like IL-4Rα and TSLP-R, different kinds of TLRs to recognize PAMPs and DAMPs, and also some inhibitory receptors like Siglec-8 to downregulate the activation pathways.22 The coagulation pathway has also been implicated with mast cell degranulation through protease-activated receptors (PAR) with thrombin and D-dimer formation. More recently, 2 major mechanisms have been put forward with regards to the pathogenesis of chronic urticaria. The first involves dysregulation of intracellular signaling pathways within mast cells and basophils that lead to defects in trafficking or function of these cells. The second involves the development of IgG autoantibodies to FcεRI or IgE on both mast cells and basophils (autoimmunity type II) or the participation of IgE autoantibodies directed to autoantigens, eg, thyroid peroxidase (TPO), DNA, IL-24 (autoimmunity type I or autoallergy).42 Histamine and other mediators, such as platelet-activating factor (PAF), tryptase, leukotrienes, and cytokines released from activated skin mast cells, result in sensory nerve activation, vasodilatation, and plasma extravasation as well as cell recruitment to the urticarial lesions.22 These multiple ways of mast cell activation and the participation of myriad cells and biomolecules during the process, help us to understand the different endotypes (molecular pathways) that can be seen among the clinical phenotypes discussed above (Fig. 2). A better understanding of these endotypes will help to find relevant molecular targets for biotherapeutic agents (Fig. 3).
Fig. 1.
Urticaria classification
Fig. 2.
Urticaria can be divided into 3 clinical phenotypes on the basis of its duration (less or more than 6 weeks) and in the presence or absence of inducing factors (inducible vs spontaneous). The known stimuli can be a physical agent like temperature, pressure, U/V light, or others as water, chemical irritants or exercise. The unknown stimuli can be related to autoimmunity or autoallergic mechanisms. b. Mast cell activation can be elicited by internal dysregulation and/or external stimuli activation via membrane receptors. Abbreviations. Syk: spleen tyrosine kinase; SHIP = Src homology 2 (SH2)-containing inositol phosphatases; Ag: antigen or allergen (both); C5aR: receptor for C5a; PAR: protease-activated receptor; PGD2: prostaglandin D2; CRTh2: chemoattractant receptor homologous molecule expressed on TH2 cells; IL-4: interleukin 4; IL-4Ra: interleukin 4 receptor alpha chain; Subst P: substance P; MBP: major basic protein; MRGPRX2: mas-related G-protein coupled receptor X2; Eos: eosinophils; PAMPs: pathogen-associated molecular patterns; DAMPs: damage-associated molecular patterns; TLRs: toll-like receptors; FceRIa: type I Fc epsilon receptor alpha chain; TPO: thyroid peroxidase; IL-24: interleukin-24
Fig. 3.
Potential targets for the treatment of chronic urticaria with biotherapeutic agents
Biomarkers
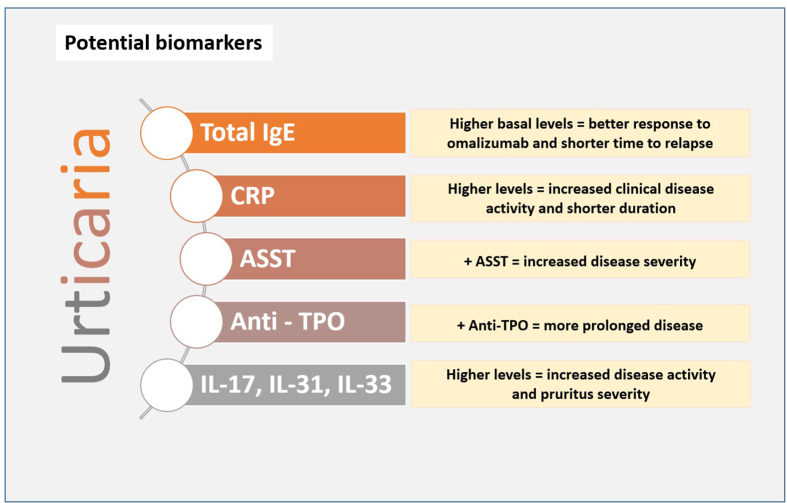
A biomarker is an objectively measured characteristic that can be used as an indicator of a normal or pathogenic biological process, as well as for pharmacologic responses to a therapeutic intervention. In urticaria, and especially in chronic spontaneous urticaria, a reliable biomarker would be very useful not only to evaluate disease activity, severity, and duration but also to predict response to the treatment. Several potential biomarkers for urticaria have been proposed, but only 5 of them have shown good clinical correlation;42,43 whereas for other potential biomarkers current evidence is insufficient (Fig. 4):
-
•
Total serum IgE levels
-
•
C-reactive protein (CRP)
-
•
Autologous serum skin test (ASST)
-
•
Anti-thyroid peroxidase autoantibodies (IgG anti-TPO)
Fig. 4.
Potential biomarkers for chronic spontaneous urticaria
Predictive factors
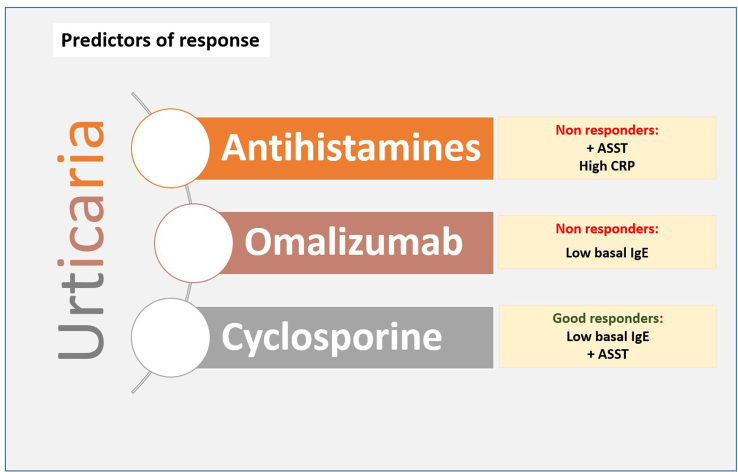
Based on the urticaria activity score in 7 days (UAS7), recent studies have analyzed baseline total IgE levels and the response to omalizumab.44 Unsurprisingly, baseline IgE levels tend to be lower in non-responders, and higher IgE baseline levels predict a better response to omalizumab. Time to relapse was significantly shorter in patients with IgE greater than 100 IU/mL when compared with those who have normal IgE levels.45
CRP levels, a sensitive inflammation marker, have shown a good correlation with disease activity and severity. High CRP levels have been associated with increased clinical disease activity and also with ASST positivity and D-dimer levels, but also with a shorter duration of the disease.46
A positive ASST test correlates also with disease severity (attacks > 4 days/week), but no differences have been demonstrated with time to remission. Patients with positive IgG autoantibodies anti-TPO had a more prolonged disease when compared with negative anti-TPO patients. However, no differences were observed regarding disease severity with the presence of anti-TPO.47
Finally, inflammatory cytokines can be potential biomarkers for disease severity in CSU. Patients with a severe disease (based on UAS7) had higher IL-17 and IL-33 levels when compared with those with mild disease. Pruritus severity has been also associated with higher levels of IL-3148 (Fig. 5).
Fig. 5.
Biomarkers as predictors of response in chronic spontaneous urticaria
A better understanding of the role of these biomarkers in urticaria may provide the rationale for future new treatment strategies and also for guidance with current conventional treatment.
Comorbidities of chronic urticaria.
Chronic urticaria (CU) significantly influences the quality of life of patients, which can be negatively impacted by the association with a wide range of comorbidities. CU has been related to a specific pattern of comorbidities including autoimmune, psychiatric, and atopic diseases, all of which are strongly overrepresented among CU patients. However, the pattern is not so clear when malignancies and cardiovascular and gastrointestinal diseases co-exist with CU.
A systematic review of the literature on autoimmune comorbidities to CU showed that the most common autoimmune comorbidities were autoimmune thyroid diseases and vitiligo, and that the most common circulating auto-antibodies were anti-thyroid and anti-nuclear antibodies (ANA).49,50 In addition, another meta-analysis demonstrated that in systemic lupus erythematosus (SLE) an urticarial rash is common although data for the prevalence of SLE in CU patients does not exist.51
It could be speculated that the increased presence of rheumatoid arthritis in CU patients reflects an increased inflammatory status overall, destabilizing mast cells and thereby causing urticaria, whereas in SLE, vitiligo, and thyroiditis the pathogenesis is mainly caused by specific auto-antibodies.49,50
Thyroid disorders have been the diseases most commonly found among CU patients, with the reported prevalence ranging up to more than 50% depending on the inclusion criteria. Association studies using the presence of anti-thyroid antibodies as the criteria usually obtained higher frequencies.49, 50, 51
In the Scandinavian AWARE-study, a follow up-study of patients with CU refractory to antihistamine treatment, an increased prevalence of atopic diseases including atopic dermatitis, asthma, and rhino-conjunctivitis was demonstrated.52 Mastocytosis and anaphylaxis were also significantly associated with CU.53
Two large registry studies from Korea and Taiwan demonstrated the same pattern of comorbidities but also an increased prevalence of drug allergies, rheumatic and inflammatory diseases, and cancers as well as psychiatric diseases.54,55
In the Taiwan study, the recognition of thyroid disorders was based on the ICD-9-CM classification codes and with the criterion that the study cases must have at least 3 outpatient visit claims with principal/secondary diagnoses of the diseases. The authors found that only 1.78% of the CU patients had thyroid disorders. The lower prevalence found could be due to the strict inclusion criteria or different population/ethnic background.55
Other studies have found that mental disorders and emotional distress including anxiety, depression, and somatoform disorders were the most common comorbidities identified in CU patients.56,57
A few studies have examined the prevalence of cardiovascular diseases but did not find any increased risk of these in CU patients compared to the general population.58 However, hypertension was previously associated with a more persistent duration of CU.59
In a recent nationwide registry study, there was a trend of increasing prevalence of the comorbidities as CU persisted into the second year, but the prevalence of thyroid disorders did not increase steadily as CU persisted longer. In contrast, the prevalence of rheumatic diseases, inflammatory diseases, and psychiatric disorders was higher as CU persisted into the third year. The increased prevalence of these comorbidities in CU patients might reflect a common association of autoimmune-based pathogenesis among CU and rheumatic/thyroid diseases.56,60
Several studies have shown a high prevalence of psychiatric comorbidities in CU patients, with the rates ranging up to 60%. Anxiety, depression, and somatoform disorders have been reported to be the most prevalent mental disorders in CU patients, although depression was most prevalent in some studies.56,60
The prevalence and future risk of depression was indeed increased, which may reflect that many CU patients have had their diagnosis for years before being referred to a hospital setting. Psychological health, itch, and sleep loss are therefore important parameters in the consultation with CU patients, and perhaps the association between CU on the one hand and depression on the other could be prevented, if the guidelines of the EAACI/GA2LEN/EDF/WAO are adhered to when treating CU patients.22
The overall prevalence of any psychiatric comorbidity among CU patients independent of whether studies had a control group was estimated to be 31.6% by Konstantinou et al.60 The most prevalent psychiatric disorders were found to be sleep–wake disorders (36.7%), followed by anxiety disorders (30.6%), mood disorders (29.4%), trauma and stressor-related disorders (17.3%), somatic symptoms and related disorders (17.2%), obsessive–compulsive and related disorders (9.3%) and substance-related and addictive disorders (4%).61
The association of cancer and CU remains controversial. One population-based study reported no association between CU and cancer, and 2 recent studies showed an increased cancer risk in CU patients.5
Ghazanfar et al53 found an increased risk of osteoporosis and diabetes mellitus in CU patients, as both diseases are increased in patients treated with glucocorticoids. Unfortunately, they are still largely used despite the recommendation against their use in current guidelines (EAACI/GA2LEN/EDF/WAO).
The paediatric data on the relation between CU and comorbidities is scarce. In the only available systematic review, Cornillier H et al61 identified 5 comorbidities and laboratory abnormalities: atopy (6 publications, n = 522 patients), positive autologous serum skin test (ASST; 5 publications, n = 304 patients), autoimmunity laboratory abnormalities (6 publications, n = 391 patients), positive seroprevalence for Helicobacter pylori (3 publications, n = 90 patients), 25-OH vitamin D deficiency (2 publications, n = 149 patients), and psychiatric disorders (1 publication, n = 27 patients).
The identified prevalence of atopy was 28.1% in children with CU (15.4% of asthma, 13.8% of allergic rhinitis, and 9.4% of atopic dermatitis).62 Regarding the link between CU and autoimmunity, the ASST was positive in 36.8% of children with CU; antinuclear antibodies were detected in 10.4% of children, and thyroid antibodies (high titers of antithyroperoxidase and/or antithyroglobulin antibody) in 6.4%. The estimated seroprevalence of Helicobacter pylori in children with CSU was 21.1%; 2 patients had suggestive gastrointestinal symptoms associated with chronic urticaria. Vitamin D deficiency, defined by 25-OH vitamin D level <30 ng/mL, was found in 69.1% of children. In only one survey were psychiatric disorders identified in 70.4% (19/27), mainly anxiety disorders (13/27, 48.1%) and disruptive behaviour disorders (7/26, 25.9%).62
The prevalence of different comorbidities observed in patients with chronic urticaria is shown in Table 2.
Table 2.
Prevalence of comorbidities in patients with chronic urticaria
| Most common to least common | Prevalence [references] |
|---|---|
Psychiatric diseases
|
4.4% - depression48 1.0 – psychosis48 8.53%50 35–65%50 31.61%54 |
Atopic diseases
|
2.9% - Rhinoconjunctivitis48 2.5% - Atopic dermatitis48 4.68x higher in patients with CU49 |
Thyroid disease
|
0.3% - Thyroiditis48 1.9x higher in patients with CU - 12.34% in CU patients and 11.34% in CSU patients49 1.78%50 12.1%–57.4%50 |
| Rheumatic diseases | 1.8% - Rheumatoid Arthritis48 0.3% - Lupus Erythematosus48 0.1% - Vitiligo48 2.48% - RA, SLE, AS, PsA/PsO50 |
| Inflammatory diseases | 9.78% - chronic sinusitis, otitis media, periodontitis, diverticulitis, Helicobacter (H.) pylori infection, peptic ulcer, hepatitis B/hepatitis”50 |
| Osteoporosis | 2.9%48 |
| Diabetes mellitus | 2.3%48 |
Cancers
|
1.37 higher in patients with CU49 |
| Hypertension | 48 |
| Obesity | 48 |
Health related quality of life in chronic urticaria
A global assessment of diseases and therapies must include, together with clinical and instrumental parameters, the evaluation of health-related quality of life (HRQoL). Taking into account the patient's perspective allows us to reach a more comprehensive view of the impact of the disease and therapies on daily life.63 This is particularly important in patients with chronic conditions, when the primary objective of treatment is to reduce the impact of the disease on daily life and to improve subjective well-being.
In this scenario, the role of HRQoL assessment in patients with CSU has been well recognized as critical in detecting the disease burden and the effects of treatments:
-
-
The US Food & Drug Administration63 and the European Medicines Agency64 have developed guidances to the healthcare industry on how to include HRQoL in regulatory decision-making.
-
-
The Grading of Recommendations Assessment, Development and Evaluation (GRADE),65, 66 a well defined formal process to rate the quality of clinical evidence and develop evidence-based guidelines, incorporates the patients' perspective as the cornerstone in establishing the strength and direction of recommendations.
-
-
The Global Allergy and Asthma European Network (GA2LEN) consensus report has provided recommendations and suggestions for the use of patient-reported outcomes (PROs) including HRQoL both in clinical trials and routine practice for the evaluation of patients with CSU.67
-
-
The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of chronic urticaria recommends assessing HRQoL at baseline and at each follow-up visit.23 Moreover, it emphasizes the relevance of this outcome in guidance of treatment choice.
While CSU is not a life-threatening condition, it deeply interferes with HRQoL.6 The reason for this impact depends on several aspects that characterize the disease:
-
-
the presence of sudden, recurrent and strongly fluctuating symptoms and the debilitating nature of these symptoms22
-
-
the occurrence of fatigue, pain, and disrupted sleep, and lack of concentration68 as common issues, often related to the constant itching that accompanies urticaria
-
-
the visible lesions can lead to embarrassment, shame and difficulties in social life69,70
-
-
the presence of psychological complaints such as anxiety, depression, irritability,3,71 or emotional distress.22
-
-
the long disease duration22
-
-
the difficulty to identify the causes and/or the triggers22
-
-
the unsatisfactory response to the currently available treatments22
The knowledge about HRQoL in people with CSU has been obtained during the last 20 years. The availability of validated questionnaires, both generic and speciality-specific, and the development of new specific tools for CSU, allowed exploration of how the disease impacts patient's experience72, 73, 74, 75, 76 (Table 3).
Table 3.
Validated questionnaires for the assessment of HRQoL in CSU
| Skin specific | Disease specific | CSU with wheals and angioedema | CSU with angioedema alone | Recommended by EAACI/GA2LEN/EDF/WAO guideline | Minimal Important Difference (MID) | |
|---|---|---|---|---|---|---|
| Dermatology Life Quality Iindex (DLQI) | X | X | X | X | ||
| SKINDEX-29 | X | |||||
| Chronic Urticaria Quality of life Questionnarie (CU-Q2oL) | X | X | X | X | X | |
| Chronic Urticaria Patient Perspective (CUPP) | X | X | X | X | ||
| Angioedema Quality of Life Questionnaire (AE-QoL) | X | X | X | X | X |
The use of generic questionnaires – tools that allow investigators to make comparisons between different health conditions – has shown that, from a subjective viewpoint, CSU represents a major burden. Patients treated for CSU, compared to matched controls, had significantly worse scores in both the Physical and Mental Component of health status.77 The HRQoL impairment does not depend on age, sex, disease duration, or the presence/absence of angioedema.3 The comparison with other chronic diseases further highlights the quality of life impairment with CSU. The effects of CSU on HRQoL were similar to those of coronary artery disease.78 Moreover, CSU patients have significantly worse physical functioning, pain perception, and perceived health than patients with respiratory allergy2 and a more marked impairment in social functioning than those with type 1 diabetes mellitus.79
The use of skin-specific questionnaires (that allow the comparison of dermatological diseases) and, even more, of disease-specific tools has allowed a better determination of the qualitative characteristics of HRQoL in CSU patients. Large or extremely large effect on HRQoL is a common feature80, 81, 82 and the HRQoL impact increases with the severity of the disease.83 Moreover, changes in signs and symptoms are strictly related to changes in HRQoL scores.84 Patients with angioedema reported statistically significantly worse HRQoL than those without angioedema.85
Research activities using specific HRQoL questionnaires, have allowed investigators to capture the subjective experience of CSU patients in all its dimensions: symptoms, impact on life activities, sleep, limits, looks,85 functioning, fatigue/mood, fears/shame, and food.76 The EAACI/GA2LEN/EDF/WAO guidelines recommended the use of chronic urticaria quality of life questionnaire (CU-Q2oL)1 and angioedema quality of life questionnaire (AE-QoL)76 for assessing and monitoring HRQoL in CSU patients. Both tools have been validated and used in clinical research. On the contrary, the chronic urticaria patient perspective (CUPP)75 constitutes a user-friendly tool to assess HRQoL in patients with CU in daily practice. It owns all the characteristics that are required for use with individual patients. The minimal important difference (MID), defined as the smallest difference in a score for an outcome of interest that patients perceive as important, and which would lead to the consideration of a change in the management,86 has been determined for CU-Q2oL, AE-QoL and CUPP.87 MID is crucial to interpreted HRQoL changes of measurement scores and to implement HRQoL assessment in routine practice.
The effect of available treatments on HRQoL of CSU patients has been evaluated in 24 clinical trials.88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100 Although the studies are different with regards to methodology, population, therapy, and questionnaire, the results indicate a HRQoL improvement after treatment.
In view of a patient centered medicine, available knowledge and continuing experience with the assessment of HRQoL allow researchers and clinicians to better understand, monitor, and manage CSU.
Clinical cases: chronic spontaneous urticaria (CSU), with or without angioedema
Chronic spontaneous urticaria (CSU) presents with urticaria only in approximately 40% of patients, with angioedema in 40%, and as isolated angioedema in up to 20% of cases. The latter presentation must differentiate between histaminergic and non-histaminergic angioedema conditions using well established algorithms that are available for physicians to follow (Fig. S1 in supplementary materials).22 When a patient presents with CSU, it is essential to get a thorough history that should query for duration, evanescence, and severity which can be quantified in terms of number of hives over the entire body and severity of itch. Underlying contributing factors such as foods, medications, thyroid disease, autoimmune disorders, chronic infections, and, rarely, malignancies as well as concomitant inducible hives including dermatographism, cold, heat, exercise, delayed pressure, and cholinergic should be determined. In addition, previous response to treatments prescribed is often useful for determining where to begin in the treatment algorithm. Validated instruments are now available that can accurately quantify CSU with or without angioedema severity and assess control and quality of life that are recommended for initial assessment and at each subsequent visit to determine treatment response. Guidelines recommend limiting diagnostic testing unless the history provides clues to an underlying cause.22 Patients must be educated that CSU is due to an internal mechanistic problem resulting in abnormal mast cell and/or basophil activation, rather than an external cause such as allergen or food sensitizations, and that skin or serologic testing to aeroallergens or foods is usually inappropriate unless the history suggests concomitant allergic rhinitis/asthma or food allergy. International and national evidence-based treatment algorithms provide guidance for the clinician for selecting therapies based on the severity of the patient's presentation. There are some distinct differences between international and US guidelines that clinicians should be familiar with, which are summarized in Fig. S2 in supplementary materials. When patients are not responsive to initial therapy with H1-antihistamines, even at doses four times FDA/EMA recommendations, treatment should be advanced to Step 3 therapy with omalizumab, a monoclonal IgG anti-IgE antibody, which has been found to be very effective in up to 45% of H1-antihistamine unresponsive CSU. When patients are unresponsive to omalizumab, options become more limited to Step 4 therapy with immunosuppressants such as cyclosporin, or other anti-inflammatory medications, the latter which are listed in an “Alternative treatment” category in the international guidelines.22 The following two cases illustrate the evaluation and management of CSU with and without angioedema.
Case #1: CSU with angioedema
A 33 year-old female presents with history of CSU since 2013 which resolved in 2017 but recurred in the last 6 weeks. The hives are described as being evanescent and very severe involving over 90% of her body with an itch severity score of 10 on a scale 1–10. An urticaria activity score summed up over 7 days (UAS7) which measures the number of hives over the entire body (0–3 scale) and itch intensity (0–3 scale) with a maximum weekly score of 40 was “40”. The patient has not slept and has experienced absenteeism and presenteeism at work over the past few weeks. She endorses daily lip and tongue swelling associated with urticaria and some throat discomfort with coughing secondary to difficulty in swallowing secretions due to her enlarged tongue. In addition she noted lumps on the bottoms of her feet. The hives were so pruritic that they hurt and were associated with intermittent arthralgias and chills. She has been to the urgent care center on 2 different occasions, once 3 days before her visit, where each time she was treated with a brief course of glucocorticoids and diphenhydramine which helped to reduce the severity of the hives and swelling, but each time she came off the medications they became significantly worse again. She denied any relationship to prescription or over the counter medications or foods. She had no history of thyroid disease, autoimmune disease, or chronic infections. There was no family history of CSU or angioedema. She noted worsening of the hives at pressure points and with cold temperatures. She works as an x-ray tech and is currently nursing an 8-month-old child.
Physical examination revealed a well-developed, well-nourished female in significant distress due to the severity of her hives. Her blood pressure, pulse, and respiratory rate were normal. A UAS score during her visit was “40” and an urticaria control test score (UCT) was 0 (scale 0–16, with 0 being totally uncontrolled). The hives were raised and erythematous with a bluish hue and serpiginous borders that coalesced. Her lower lip was slightly swollen. There was no lymphadenopathy, nor organomegaly, and her respiratory and cardiovascular examination was normal. Her skin did not urticate when stroked with a dermographometer device. A temperature test was negative for cold induced urticaria.
At the time of her visit she had been taking 60 mg of prednisone for 3 days prescribed by the urgent care physician and was still not controlled. Given the chronicity, the visual appearance of the hives and systemic symptoms along with her poor response to oral glucocorticoids, a sedimentation rate, C-reactive protein, complete blood count with differential, thyroid stimulating hormone, autoantibodies to thyroid peroxidase, total IgE, chronic urticaria index, C3, C4, ANA, rheumatoid factor, glucose-6-phosphate dehydrogenase level and a skin biopsy were obtained to identify an underlying vasculitis or autoimmune condition and to obtain biomarkers useful for determining best therapeutic options if conventional therapy with H1-antihistamines was not effective. The patient was instructed to taper the prednisone slowly by 5 mg every 2 days and to start cetirizine 20 mg twice a day, and montelukast 10 mg a day. The latter two treatments which are not primary recommendations in the international guidelines but recommended as Step 2 therapy for the Joint Task Force United States Urticaria guidelines, were started due to their safety, low cost, and the severity of the patients’ hives. She was seen one week later to review the biopsy results and remove the suture. She was still tapering the prednisone, and the hives were now intermittent with a UAS7 of 16 and UCT 10. The biopsy showed perivascular lymphocytes with eosinophils but no evidence of vasculitis. Her laboratory tests revealed a CRP 17.87 (normal ≤ 6), and normal thyroid function, a slight increase in WBC of 11.7 (3.7–10.3 × 103/mol), slight increase in peripheral neutrophils of 8.9 × 103/mol (normal 1.6–6.1103/mol) and a low IgE of 12 kU/L (normal ≤240kU/L) (despite the low IgE levels (12 kU/L) this patient responded brilliantly to omalizumab). She was instructed to continue tapering the prednisone, continue the current Histamine-1/Histamine-2 antagonists and leukotriene modifying agent medication combination, and return in 4 weeks for further assessment. A free T4 and T3 level were ordered to assess for hyperthyroidism and her CRP was repeated. Paperwork for omalizumab insurance authorization was completed in case she required advancement to Step 3 therapy.
Upon return 4 weeks later she was off prednisone; the hives were improved, but she still reported a UCT score of 7 and a UAS7 score of 18. A free T3 and T4 were normal and her CRP was now normal. Omalizumab 300 mg was approved, and the first injection was administered subcutaneously during her visit. She returned 4 weeks later for follow-up and her second injection. She reported now that her UCT score was 16 and her UAS7 score was 4 and was feeling much better overall all without any side effects from the medication. During her third injection one month later she reported a UCT of 16 and a UAS7 = 0.
Discussion: This case represents a severe case of hives with angioedema that was associated with features suggesting an underlying systemic process given the nature of the hive presentation and associated systemic symptoms. The patient was already on prednisone which was continued at a slower taper to provide better control and more comfort while Step 2 therapy was initiated. Testing was performed strictly to investigate clinical suspicions for an underlying vasculitis or systemic condition. In fact, the CRP was increased and the screening TSH was low however further testing failed to reveal evidence of hyperthyroidism. Despite prednisone and Step 2 therapy she was not well controlled, so she was advanced to Step 3 treatment with omalizumab. In preparation for initiating this therapy, the patient was screened with a total IgE as low levels have been associated with slower or poorer response. In addition, the patient was evaluated for the biomarkers TPO and a CU-index, a marker for high affinity IgG anti-FcER1 alpha subunit antibodies that if positive are also predictive of poorer responses to omalizumab. In this patient, these tests were negative and the patient subsequently demonstrated an excellent and rapid response to omalizumab. Of note, she had associated angioedema with the urticaria which was also controlled with omalizumab as has been shown in post-hoc analysis of data from clinical trials. An additional nuance of this case was that the patient was breast feeding while being treated with high dose antihistamines and omalizumab. Although there are no controlled studies demonstrating safety of these agents in pregnant and lactating women, the international guidelines support using these therapies in these special populations based on long-term clinical experience and safety registries.22 In addition, using the validated patient reported outcomes instruments was very helpful for determining control and directing treatment decisions.
Case #2: CSU without angioedema
A 26-year-old female presents with CSU since 2017 when she started to experience spontaneous hives over different parts of her body mostly on her arms, legs and hip. Her UCT score was 8 and UAS7 was 20, but the itch intensity was much lower than the number of hives. There were no inducible triggers for the hives. Her primary care physician had recently prescribed a course of prednisone in conjunction with diphenhydramine, the latter which she was using as needed; however, the hives and swelling recurred when she was off medications. In addition to diphenhydramine which made her tired, she was also prescribed montelukast. Unfortunately she experienced vivid nightmares, and this medication was discontinued. She works as an administrative assistant and noted the medications were interfering with her work performance. She had some previous blood tests including a CBC with differential and TSH which were normal. There was no relationship of the CSU to medications or foods. She had seen another allergist who tested her to aeroallergens and foods which were reportedly negative. During her current visit the physical examination showed scattered erythematous wheals with serpiginous borders over her arms. No angioedema was observed. Otherwise there were no other relevant findings on physical exam.
Based on the chronicity of her CSU and responsiveness to H1-antihistamines, she was started on fexofenadine 180 mg twice a day and scheduled a follow-up visit in 4 weeks. No additional testing was performed. She returned 4 weeks later and had a UCT of 16 and a UAS7 = 0. She was tolerating the medication without side effects, and her quality of life was much improved. She was instructed to continue the medication and return in 3–4 months. At her third visit, her hives were still controlled and the fexofenadine was reduced to once a day.
Discussion: This is a relatively straight forward case of CSU that was not initially evaluated or treated appropriately. She was being treated with a first generation H1-antagonist that was making her tired and affecting her work. Furthermore, she was seen by another allergist who performed skin testing to aeroallergens and foods when there was no history of allergic rhinitis, asthma, or food allergies.22 She was started on fexofenadine at 2 times the recommended FDA dose (Step 2 therapy) which was very effective at preventing recurrent hives. This regimen was continued until it was clear she was not having breakthrough hives (UAS7 = 0) at which point the dose was decreased to once a day. She noted her leisure and work quality of life had significantly improved after starting this medication regimen.
Takeaway Points:
-
1)
Guidelines are useful for guiding clinicians in the appropriate evaluation and management of CSU patients.
-
2)
CSU is rarely caused by external causes.
-
3)
Validated patient reported outcome instruments are valuable for assessing disease severity and monitoring response to treatment.
-
4)
Excessive testing including skin testing to aeroallergens or foods, is not recommended unless the history suggests otherwise.
-
5)
High dose H1-antagonists control over 40% of CSU patients.
-
6)
Patients with and without angioedema and hives respond to omalizumab.
Therapeutics and integral management of CSU and CIndU
General measures
Patient interrogation and physical examination may disclose environmental symptom triggering or aggravating factors of chronic urticaria (CU). When identified, it is mandatory to avoid or reduce the exposure to such factors (physical, drugs: NSAIDs, ACE inhibitors, foods, other allergens, contactants, emotional stress). Treatment of concomitant infections, such as H. pylori, as well as urinary tract, parasites, dental, or gynecological infections is also pertinent.22 Other comorbid conditions such as autoimmune thyroid disease, hypertension, and metabolic syndrome should be treated.11
Up to 40% of patients with chronic spontaneous urticaria (CSU) experience exacerbations when exposed to aspirin and NSAIDs. Ten to fifty percent of patients with CSU also have associated inducible urticaria. In consequence, treatment of comorbid inducible urticaria is necessary.11
Additional measures that contribute to decrease pruritus, the most disturbing symptom of CU, and patient discomfort are the application of physical measures, for example cold compresses (not in patients with cold-induced urticaria).
Concluding remarks
The prevalence of chronic urticaria in the population has been estimated to be between 0.1% and 1.0%. Quality of life of affected patients may be severely compromised, and the costs of the disease for the health system can be substantial. In recent years there have been remarkable advances in the understanding of the pathophysiology of urticaria that have prompted investigators to explore new medications, especially biologics, for patients with severe refractory urticaria. Multiple cell types are involved in the production of symptoms, mainly mast cells, basophils, eosinophils, T and B lymphocytes, and epithelial and endothelial cells. It is proposed that dysregulation of intracellular signaling pathways and autoimmune phenomena play a major role in mast cell/basophil activation leading to inflammatory mediator release in the skin resulting in wheals and angioedema.
Patients may complain of wheals (about 40%), angioedema (40%), or both (20%), whereas no identifiable trigger factors for the symptoms are present in a large proportion of affected subjects (chronic spontaneous urticaria), although in some of them external factors, mainly physical, could be suspected and proven (chronic inducible urticaria). It is also pertinent to mention that some patients may show a combined pattern of spontaneous and inducible urticaria.
Currently biomarkers for the prognosis of CU and therapeutic response to different therapies have been identified which are useful for routine management. Finally, we recommend that clinicians follow the guidelines, utilize validated PRO instruments, and use medications with proven efficacy and safety. In the near future new biologics and small molecules that are currently under investigation will be incorporated into the treatment of severe and refractory CU.
Abbreviations
ACE, angiotensin converting enzyme; AE, angioedema; AE-QoL, angioedema quality of life; ASST, autologous serum skin test; C5a, complement C5a; C5aR, complement C5a receptor; CRP, C reactive protein; CRTh2, chemoattractant receptor-homologous molecule expressed on T helper type 2 cells; CIndU, chronic inducible urticaria; CsA, cyclosporine A; CSU, hronic spontaneous urticaria; CU, chronic urticaria; CUPP, Chronic Urticaria Patient Perspective; CU-Q2oL, chronic urticaria quality of life; DAMPs, damage-associated molecular patterns; DLQI, dermatology quality index; DNA, desoxyribonucleic acid; EBM, evidence-based medicine; ECP, eosinophil cationic protein; FcεRI, high affinity IgE receptor I; GA2 LEN, Global Allergy and Asthma European Network; GRADE, Grading of Recommendations Assessment, Development and Evaluation; EAACI, European Academy of Allergy and Clinical Immunology; EDF, European Dermatology Foundation; HR4, histamine receptor 4; HRQoL, health-related quality of life; IgE, immunoglobulin E; IgG, immunoglobulin G; IgM, immunoglobulin M; IL-1, interleukin-1; IL-2, interleukin-2; IL-2R, interleukin-2 receptor; IL-3, interleukin-3; IL-4, interleukin-4; IL-5, interleukin-5; IL-17, interleukin-17; IL-24, interleukin-24; IL-31, interleukin-31; IL-33, interleukin-33; IL-4Rα, Interleukin-4 receptor alpha chain; IU, inducible urticari; MBP, major basic protein; MRGPRX2, Mas-related G protein-coupled receptor X2; NSAIDs, nonsteroidal anti-inflammatory drugs; NS AHs, nonsedating antihistamines; OMA, omalizumab; PAF, platelet activating factor; AMPs, pathogen-associated molecular patterns; PAR, protease-activated receptors; PGD2, prostaglandin 2; PPP$, power parity dollars; PIDs, Primary immunodeficiency diseases; PROs, patient-reported outcome; SCF, stem cell factor; TNF-α, tumor necrosis factor alpha; TLR, toll-like receptor; TSLP-R, thymic stromal lymphopoietin receptor; UAS-7, urticaria activity score-7; UV-A, ultraviolet light-A; UV-B, ultraviolet light-B; WAO, World Allergy Organization.
Funding
Supported by an unrestricted educational grant from Novartis Pharma AG, Basel, Switzerland.
Author's consent for publication
All authors reviewed and approved the final version of the article.
Author contributions
Mario Sánchez-Borges: Designed the outline of the paper. Wrote the section on Management.
Ignacio J Ansotegui and Maximiliano Gomez: Wrote the section on Epidemiology.
Jose Antonio Ortega Martell: Wrote the section on Immunopathogenesis.
Ilaria Baiardini and Walter Canonica: Wrote the section on Quality of Life.
Mario Morais de Almeida: Wrote the section on Comorbidities.
Jonathan Bernstein: Wote the section on Clinical cases: Chronic spontaneous urticaria (CSU), with or without Angioedema.
Availability of data and materials
Not applicable because this is a review article from the literature including data from previously published articles.
Ethics approval
Not applicable.
Declaration of competing interest
None related to this paper.
Acknowledgements
The authors express their deep appreciation for the late Dr. Mario Sánchez Borges, their highly respected colleague and friend who led the development of this paper and its companion paper.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100533.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zuberbier T., Balke M., Worm M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. 2010;35:869–873. doi: 10.1111/j.1365-2230.2010.03840.x. [DOI] [PubMed] [Google Scholar]
- 2.Baiardini I., Giardini A., Pasquali M. Quality of life and patient's satisfaction in chronic urticaria and respiratory allergy. Allergy. 2003;58:621–623. doi: 10.1034/j.1398-9995.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 3.Staubach P., Eckhardt-Henn A., Dechene M. Quality of life in patients with chronic urticaria is differentially impaired and determined by psychiatric comorbidity. Br J Dermatol. 2006;154:294–298. doi: 10.1111/j.1365-2133.2005.06976.x. [DOI] [PubMed] [Google Scholar]
- 4.Delong L.K., Culler S.D., Saini S.S. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144:35–39. doi: 10.1001/archdermatol.2007.5. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Borges M., Asero R., Ansotegui I.J. WAO Position Paper. Diagnosis and Treatment of urticaria and angioedema: a worldwide perspective. WAO J. 2012;5:125–147. doi: 10.1097/WOX.0b013e3182758d6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell R.J., Leech S.C., Till S. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45:547–565. doi: 10.1111/cea.12494. [DOI] [PubMed] [Google Scholar]
- 7.Moestrup K., Ghazanfar M.N., Thomsen S.F. Patient-reported outcomes (PROs) in chronic urticaria. Int J Dermatol. 2017;56:1342–1348. doi: 10.1111/ijd.13668. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y.S., Park S.H., Han K., Bang C.H., Lee J.H., Park Y.M. Prevalence and incidence of chronic spontaneous urticaria in the entire Korean adult population. Br J Dermatol. 2018;178:976–977. doi: 10.1111/bjd.16105. [DOI] [PubMed] [Google Scholar]
- 9.Maurer M., Weller K., Bindslev-Jensen C. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66:317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 10.Antia C., Baquerizo K., Korman A., Bernstein J.A., Alikhan A. Urticaria: a comprehensive review: epidemiology, diagnosis, and work-up. J Am Acad Dermatol. 2018;79:599–614. doi: 10.1016/j.jaad.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Borges M., Caballero-Fonseca F., Capriles-Hulett A. Factors linked to disease severity and time to remission in patients with chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2017;31:964–971. doi: 10.1111/jdv.14221. [DOI] [PubMed] [Google Scholar]
- 12.Gaig P., Olona M., Muñoz Lejarazu D. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14:214–220. [PubMed] [Google Scholar]
- 13.Stepaniuk P., Kan M., Kanani A. Natural history, prognostic factors and patient perceived response to treatment in chronic spontaneous urticaria. Allergy Asthma Clin Immunol. 2020;16:63. doi: 10.1186/s13223-020-00459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wertenteil S., Strunk A., Garg A. Prevalence estimates for chronic urticaria in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2019;81:152–156. doi: 10.1016/j.jaad.2019.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Pite H., Wedi B., Borrego L.M., Kapp A., Raap U. Management of childhood urticaria: current knowledge and practical recommendations. Acta Derm Venereol. 2013;93:500–508. doi: 10.2340/00015555-1573. [DOI] [PubMed] [Google Scholar]
- 16.Poddighe D. The prevalence of chronic spontaneous urticaria (CSU) in the pediatric population. J Am Acad Dermatol. 2019;81:e149. doi: 10.1016/j.jaad.2019.07.068. [DOI] [PubMed] [Google Scholar]
- 17.Balp M.M., Weller K., Carboni V. Prevalence and clinical characteristics of chronic spontaneous urticaria in pediatric patients. Pediatr Allergy Immunol. 2018;29:630–636. doi: 10.1111/pai.12910. [DOI] [PubMed] [Google Scholar]
- 18.Zazzali J.L., Broder M.S., Chang E. Cost, utilization, and patterns of medication use associated with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012;108:98–102. doi: 10.1016/j.anai.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Arias-Cruz A., González-Díaz S.N., Macías-Weinmann A. Quality of life in chronic urticaria and its relationship with economic impact and disease control in patients attended to at the University Hospital of Monterrey, Mexico. Rev Alerg Mex. 2018;65:250–258. doi: 10.29262/ram.v65i3.398. [DOI] [PubMed] [Google Scholar]
- 20.Maurer M., Abuzakouk M., Bérard F. The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy. 2017;72:2005–2016. doi: 10.1111/all.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuberbier T., Aberer W., Asero R. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 22.Zuberbier T., Aberer W., Asero R. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868–887. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 23.Maurer M., Metz M., Brehler R. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J Allergy Clin Immunol. 2018;141:638–649. doi: 10.1016/j.jaci.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez J., Amaya E., Acevedo A. Prevalence of inducible urticaria in patients with chronic spontaneous urticaria: associated risk factors. J Allergy Clin Immunol Pract. 2017;5:464–470. doi: 10.1016/j.jaip.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Schoepke N., Młynek A., Weller K. Symptomatic dermographism: an inadequately described disease. J Eur Acad Dermatol Venereol. 2015;29:708–712. doi: 10.1111/jdv.12661. [DOI] [PubMed] [Google Scholar]
- 26.Barlow R.J., Warburton F., Watson K. Diagnosis and incidence of delayed pressure urticaria in patients with chronic urticaria. J Am Acad Dermatol. 1993;29:954–958. doi: 10.1016/0190-9622(93)70273-v. [DOI] [PubMed] [Google Scholar]
- 27.Buss Y.L., Sticherling M. Cold urticaria; disease course and outcome--an investigation of 85 patients before and after therapy. Br J Dermatol. 2005;153:440–441. doi: 10.1111/j.1365-2133.2005.06757.x. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Borges M., González-Aveledo L., Caballero-Fonseca F., Capriles-Hulett A. Review of physical urticarias and testing methods. Curr Allergy Asthma Rep. 2017;17:51. doi: 10.1007/s11882-017-0722-1. [DOI] [PubMed] [Google Scholar]
- 29.Pezzolo E., Peroni A., Gisondi P., Girolomoni G. Heat urticaria: a revision of published cases with an update on classification and management. Br J Dermatol. 2016;175:473–478. doi: 10.1111/bjd.14543. [DOI] [PubMed] [Google Scholar]
- 30.Chicharro P., Rodríguez P., de Argila D. Omalizumab in the treatment of chronic inducible urticaria. Actas Dermosifiliogr. 2017;108:423–431. doi: 10.1016/j.ad.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Ferriols A., Barnadas M., Gardeazábal J. Solar urticaria: epidemiology and clinical phenotypes in a Spanish series of 224 patients. Actas Dermosifiliogr. 2017;108:132–139. doi: 10.1016/j.ad.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Wakelin S.H. Contact urticaria. Clin Exp Dermatol. 2001;26:132–136. doi: 10.1046/j.1365-2230.2001.00780.x. [DOI] [PubMed] [Google Scholar]
- 33.Thoma A., Eaves F.F. A brief history of evidence-based medicine (EBM) and the contributions of dr david sackett. Aesthetic Surg J. 2015;35:NP261–NP263. doi: 10.1093/asj/sjv130. [DOI] [PubMed] [Google Scholar]
- 34.Andrews J., Guyatt G., Oxman A.D. GRADE guidelines: going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Gómez R.M., Jares E., Canonica G.W. Why a registry of Chronic Urticaria (CUR) is needed. World Allergy Organ J. 2017;10:16. doi: 10.1186/s40413-017-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jares E.J., Badellino H.A., Ensina L.F. Registries as useful tools in characterization of allergic manifestations. Curr Opin Allergy Clin Immunol. 2016;16:250–256. doi: 10.1097/ACI.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 37.Gómez R.M., Jares E., Sánchez-Borges M. Latin American chronic urticaria registry (CUR) contribution to the understanding and knowledge of the disease in the region. World Allergy Organ J. 2019;12:100042. doi: 10.1016/j.waojou.2019.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan A.P. Treatment of urticaria: a clinical and mechanistic approach. Curr Opin Allergy Clin Immunol. 2019;19:387–392. doi: 10.1097/ACI.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 39.Bracken S.J., Abraham S., MacLeod A.S. Autoimmune theories of chronic spontaneous urticaria. Front Immunol. 2019;10:627. doi: 10.3389/fimmu.2019.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolkhir P., Altrichter S., Munoz M. New treatments for chronic urticaria. Ann Allergy Asthma Immunol. 2020;124:2–12. doi: 10.1016/j.anai.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Kolkhir P., André F., Church M.K. Potential blood biomarkers in chronic spontaneous urticaria. Clin Exp Allergy. 2017;47:19–36. doi: 10.1111/cea.12870. [DOI] [PubMed] [Google Scholar]
- 42.Ensina L.F., Cusato-Ensina A.P., Cardona R. Advances in the pathogenesis representing definite outcomes in chronic urticaria. Curr Opin Allergy Clin Immunol. 2019;19:193–197. doi: 10.1097/ACI.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 43.Ferrer M., Boccon-Gibod I., Gonc¸alo M. Expert opinion: defining response to omalizumab in patients with chronic spontaneous urticaria. Eur J Dermatol. 2017;27:455–463. doi: 10.1684/ejd.2017.3085. [DOI] [PubMed] [Google Scholar]
- 44.Weller K., Ohanyan T., Hawro T. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73:2406–2408. doi: 10.1111/all.13586. [DOI] [PubMed] [Google Scholar]
- 45.Kolkhir P., Altrichter S., Hawro T., Maurer M. C-reactive protein is linked to disease activity, impact, and response to treatment in patients with chronic spontaneous urticaria. Allergy. 2018;73:940–948. doi: 10.1111/all.13352. [DOI] [PubMed] [Google Scholar]
- 46.Chanprapaph K., Iamsumang W., Wattanakrai P., Vachiramon V. Thyroid autoimmunity and autoimmunity in chronic spontaneous urticaria linked to disease severity, therapeutic response, and time to remission in patients with chronic spontaneous urticaria. BioMed Res Int. 2018;2018:1–13. doi: 10.1155/2018/9856843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin W., Zhou Q., Liu C. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci Rep. 2017;7:17797. doi: 10.1038/s41598-017-18187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolkhir P., Borzova E., Grattan C. Autoimmune comorbidity in chronic spontaneous urticaria: a systematic review. Autoimmun Rev. 2017;16:1196–1208. doi: 10.1016/j.autrev.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Kolkhir P., Metz M., Altrichter S., Maurer M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: a systematic review. Allergy. 2017;72:1440–1460. doi: 10.1111/all.13182. [DOI] [PubMed] [Google Scholar]
- 50.Kolkhir P., Pogorelov D., Olisova O., Maurer M. Comorbidity and pathogenic links of chronic spontaneous urticaria and systemic lupus erythematosus – a systematic review. Clin Exp Allergy. 2016;46:275–287. doi: 10.1111/cea.12673. [DOI] [PubMed] [Google Scholar]
- 51.Thomsen S.F., Pritzier E.C., Anderson C.D. Chronic urticaria in the real-life clinical practice setting in Sweden, Norway and Denmark: baseline results from the non-interventional multicentre AWARE study. J Eur Acad Dermatol Venereol. 2017;31:1048–1055. doi: 10.1111/jdv.14210. [DOI] [PubMed] [Google Scholar]
- 52.Ghazanfar M.N., Kibsgaard L., Thomsen S.F., Vestergaard C. Risk of comorbidities in patients diagnosed with chronic urticaria: a nationwide registry study. World Allergy Organization Journal. 2020;13:100097. doi: 10.1016/j.waojou.2019.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim B.R., Yang S., Choi J.W. Epidemiology and comorbidities of patients with chronic urticaria in Korea: a nationwide population-based study. J Dermatol (Tokyo) 2018;45:10–16. doi: 10.1111/1346-8138.14075. [DOI] [PubMed] [Google Scholar]
- 54.Chu C.-Y., Cho Y.T., Jiang J.H. Epidemiology and comorbidities of patients with chronic urticaria in Taiwan: a nationwide population-based study. J Dermatol Sci. 2017;88:192–198. doi: 10.1016/j.jdermsci.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Barbosa F., Freitas J., Barbosa A. Chronic idiopathic urticaria and anxiety symptoms. J Health Psychol. 2011;16:1038–1047. doi: 10.1177/1359105311398682. [DOI] [PubMed] [Google Scholar]
- 56.Staubach P., Dechene M., Metz M. High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol. 2011;91:557–561. doi: 10.2340/00015555-1109. [DOI] [PubMed] [Google Scholar]
- 57.Egeberg A., Kofoed K., Gislason G.H. Cardiovascular risk is not increased in patients with chronic urticaria: a retrospective population-based cohort study. Acta Derm Venereol. 2017;97:261–262. doi: 10.2340/00015555-2516. [DOI] [PubMed] [Google Scholar]
- 58.Nebiolo F., Bergia R., Bommarito L. Effect of arterial hypertension on chronic urticaria duration. Ann Allergy Asthma Immunol. 2009;103:407–410. doi: 10.1016/S1081-1206(10)60360-2. [DOI] [PubMed] [Google Scholar]
- 59.Tzur Bitan D., Berzin D., Cohen A. The association of chronic spontaneous urticaria (CSU) with anxiety and depression: a nationwide cohort study. Arch Dermatol Res. 2021;313:33–39. doi: 10.1007/s00403-020-02064-3. [DOI] [PubMed] [Google Scholar]
- 60.Konstantinou G.N., Konstantinou G.N. Psychiatric comorbidity in chronic urticaria patients: a systematic review and meta-analysis. Clin Transl Allergy. 2019;9:42. doi: 10.1186/s13601-019-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornillier H., Giraudeau B., Munck S. Chronic spontaneous urticaria in children - a systematic review on interventions and comorbidities. Pediatr Allergy Immunol. 2018;29:303–310. doi: 10.1111/pai.12870. [DOI] [PubMed] [Google Scholar]
- 62.Schipper H., Clinch J., Olweny C.L.M. Quality of life studies: definitions and conceptual issues. In: Spilker B., editor. Quality of Life and Pharmacoeconomics in Cinical Trials. Lippincot-Raven Press; Philadelphia: 1990. pp. 11–23. [Google Scholar]
- 63.US Department of Health and Human Services FDA Center for Drug Evaluation and Research US department of health and human services FDA center for biologics evaluation and research and US department of health and human services FDA center for devices and radiological health. Guidance for industry: patient reported outcome measures: use in medical product development to support labelling claims: draft guidance. Health Qual Life Outcome. 2006;4:79. doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.European Medicines Agency Committee for medicinal products for human use (CHMP). Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQoL) measures in the evaluation of medicinal products. European Medicines Agency website. 2005 http://www.emea.europa.eu/pdfs/human/ewp/13939104en.pdf Doc. Ref. EMEA/CHMP/EWP/139391/2004. Available at: [Google Scholar]
- 65.Guyatt G.H., Oxman A.D., Vist G. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baiardini I., Braido F., Bindslev-Jensen C. Recommendations for assessing patient-reported outcomes and health-related quality of life in patients with urticaria: a GA(2) LEN taskforce position paper. Allergy. 2011;66:840–844. doi: 10.1111/j.1398-9995.2011.02580.x. [DOI] [PubMed] [Google Scholar]
- 67.O'Donnell B.F. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin. 2014;34:89–104. doi: 10.1016/j.iac.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Weldon D.R. Quality of life in patients with urticaria. Allergy Asthma Proc. 2006;27:96–99. [PubMed] [Google Scholar]
- 69.Kang M.J., Kim H.S., Kim H.O., Park Y.M. The impact of chronic idiopathic urticaria on quality of life in Korean patients. Ann Dermatol. 2009;21:226–229. doi: 10.5021/ad.2009.21.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niemeier V., Nippesen M., Kupfer J. Psychological factors associated with hand dermatoses: which subgroup needs additional psychological care? Br J Dermatol. 2002;146:1031–1037. doi: 10.1046/j.1365-2133.2002.04716.x. [DOI] [PubMed] [Google Scholar]
- 71.Finlay A., Khan G.K. Dermatology life quality index (DLQI) – a simple practical measure for routine in clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 72.Chren M.M., Lasek R.J., Quinn L.M. Skindex, a quality-of-life measure for patients with skin diseases: reliability, validity and responsiveness. J Invest Dermatol. 1996;107:707–713. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- 73.Baiardini I., Pasquali M., Braido F. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-Q2oL) Allergy. 2005;60:1073–1078. doi: 10.1111/j.1398-9995.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 74.Baiardini I., Braido F., Molinengo G. Chronic urticaria patient perspective (CUPP): the first validated tool for assessing quality of life in clinical practice. J Allergy Clin Immunol Pract. 2018;6:208–218. doi: 10.1016/j.jaip.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 75.Weller K., Groffik A., Magerl M. Development and construct validation of the angioedema quality of life questionnaire. Allergy. 2012;67:1289–1298. doi: 10.1111/all.12007. [DOI] [PubMed] [Google Scholar]
- 76.Balp M.M., Vietri J., Tian H., Isherwood G. The impact of chronic urticaria from the patient's perspective: a survey in five European countries. Patient. 2015;8:551–558. doi: 10.1007/s40271-015-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Donnell B.F., Barr R.M., Kobza Black A., Francis D.M., Kermani F. Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol. 1998;5:101–106. doi: 10.1046/j.1365-2133.1998.02033.x. [DOI] [PubMed] [Google Scholar]
- 78.Brzoza Z., Nabrdalik K., Moos L. Chronic spontaneous urticaria and type 1 diabetes mellitus-does quality of life impairment always reflect health danger? J Clin Med. 2020;9:E2505. doi: 10.3390/jcm9082505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savic S., Leeman L., El-Shanawany T. Chronic urticaria in the real-life clinical practice setting in the UK: results from the noninterventional multicentre AWARE study [published online ahead of print, 2020 Apr 4] Clin Exp Dermatol. 2020 doi: 10.1111/ced.14230. [DOI] [PubMed] [Google Scholar]
- 80.Maurer M., Houghton K., Costa C. Differences in chronic spontaneous urticaria between Europe and Central/South America: results of the multi-center real world AWARE study. World Allergy Organ J. 2018;11:32. doi: 10.1186/s40413-018-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guillet G., Bécherel P.A., Pralong P. The burden of chronic urticaria: French baseline data from the international real-life AWARE study. Eur J Dermatol. 2019;29:49–54. doi: 10.1684/ejd.2018.3495. [DOI] [PubMed] [Google Scholar]
- 82.Lacour J.P., Khemis A., Giordano-Labadie F. The burden of chronic spontaneous urticaria: unsatisfactory treatment and healthcare resource utilization in France (the ASSURE-CSU study) Eur J Dermatol. 2018;28:795–802. doi: 10.1684/ejd.2018.3446. [DOI] [PubMed] [Google Scholar]
- 83.Stull D.E., McBride D., Houghton K. Assessing changes in chronic spontaneous/idiopathic urticaria: comparisons of patient-reported outcomes using latent growth modeling. Adv Ther. 2016;33:214–224. doi: 10.1007/s12325-016-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sussman G., Abuzakouk M., Bérard F. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact: analyses from ASSURE-CSU. Allergy. 2018;73:1724–1734. doi: 10.1111/all.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schunemann H.J., Guyatt G.H. Commentary–goodbye M(C)ID! Hello MID, where do you come from? Health Serv Res. 2005;40:593–597. doi: 10.1111/j.1475-6773.2005.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baiardini I., Canonica G.W., La Grutta S., Braido F. Clinically significant differences in patient-reported outcomes evaluations in chronic spontaneous urticaria. Curr Opin Allergy Clin Immunol. 2020;20:261–267. doi: 10.1097/ACI.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 87.Javaud N., Soria A., Maignan M. Glucocorticoids for acute urticaria: study protocol for a double-blind non-inferiority randomised controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gastaminza G., Azofra J., Nunez-Cordoba J.M. Efficacy and safety of omalizumab (xolair) for cholinergic urticaria in patients unresponsive to a double dose of antihistamines: a randomized mixed double-blind and open-label placebo-controlled clinical trial. J Allergy Clin Immunol Pract. 2019;7:1599–1609.e1. doi: 10.1016/j.jaip.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 89.Staubach P., Metz M., Chapman-Rothe N. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy. 2018;73:576–584. doi: 10.1111/all.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boonpiyathad T., Sangasapaviliya A. Hydroxychloroquine in the treatment of anti-histamine refractory chronic spontaneous urticaria, randomized single-blinded placebo-controlled trial and an open label comparison study. Eur Ann Allergy Clin Immunol. 2017;49:220–224. doi: 10.23822/EurAnnACI.1764-1489.11. [DOI] [PubMed] [Google Scholar]
- 91.Murota H., Azukizawa H., Katayama I. Impact of jumihaidokuto (Shi-Wei-Bai-Du-Tang) on treatment of chronic spontaneous urticaria: a randomized controlled study. Chin J Integr Med. 2019;25:820–824. doi: 10.1007/s11655-017-2950-6. [DOI] [PubMed] [Google Scholar]
- 92.Finlay A.Y., Kaplan A.P., Beck L.A. Omalizumab substantially improves dermatology-related quality of life in patients with chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2017;31:1715–1721. doi: 10.1111/jdv.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maurer M., Sofen H., Ortiz B. Positive impact of omalizumab on angioedema and quality of life in patients with refractory chronic idiopathic/spontaneous urticaria: analyses according to the presence or absence of angioedema. J Eur Acad Dermatol Venereol. 2017;31:1056–1063. doi: 10.1111/jdv.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hide M., Yagami A., Togawa M. Efficacy and safety of bilastine in Japanese patients with chronic spontaneous urticaria: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II/III study. Allergol Int. 2017;66:317–325. doi: 10.1016/j.alit.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Sánchez J., Zakzuk J., Cardona R. Prediction of the efficacy of antihistamines in chronic spontaneous urticaria based on initial suppression of the histamine- induced wheal. J Investig Allergol Clin Immunol. 2016;26:177–184. doi: 10.18176/jiaci.0039. [DOI] [PubMed] [Google Scholar]
- 96.Staubach P., Metz M., Chapman-Rothe N. Effect of omalizumab on angioedema in H1 -antihistamine-resistant chronic spontaneous urticaria patients: results from X-ACT, a randomized controlled trial [published correction appears in Allergy. Allergy. 2017 Sep;72(9):1430. doi: 10.1111/all.12870. 2016; 71: 1135-1144. [DOI] [PubMed] [Google Scholar]
- 97.Saini S.S., Bindslev-Jensen C., Maurer M. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67–75. doi: 10.1038/jid.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maurer M., Rose´n K., Hsieh H.J. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 99.Kaplan A., Ledford D., Ashby M. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 100.Guevara-Gutierrez E., Bonilla-Lopez S., Hernández-Arana S., Tlacuilo-Parra A. Safety and efficacy of cetirizine versus cetirizine plus ranitidine in chronic urticaria: double-blind randomized placebo-controlled study. J Dermatol Treat. 2015;26:548–550. doi: 10.3109/09546634.2015.1025031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable because this is a review article from the literature including data from previously published articles.