Abstract
Introduction
Oral isotretinoin is an effective agent for the treatment of severe cystic acne. Isotretinoin is a teratogen; there is an increased risk of congenital defects in infants exposed to the drug in the uterus. The Saudi Food and Drug Authority (SFDA) has implemented a pregnancy prevention program (PPP) to protect females from those teratogenic effects.
Objectives
To investigate the awareness of women, of reproductive age who were using Isotretinoin or used it previously, about isotretinoin use and the SFDA-approved PPP in Riyadh, Saudi Arabia.
Methods
This cross-sectional study was conducted during the period from June to October 2019. A questionnaire was developed based on the published literature and the PPP recommendations. The study was carried out online among female patients who were on Isotretinoin therapy or have used it previously in Riyadh city. The Statistical Package for Social Sciences (SPSS for Windows, version 24) was used to analyze the study data.
Results
During the study period, 483 patients participated in the study. Among them, 97.3% reported that they used the drug based on a doctor’s prescription, 94.6% were aware of Isotretinoin’s teratogenic effect, and 30.6% confirmed their awareness of the PPP. Amongst the participants, 9.1% (n = 44) used Isotretinoin while being married or planning to get married within a one-month period after using it. Concerning the use of two contraceptive methods according to the PPP guidelines, of the participants, 43.2% reported that they have been informed by their healthcare providers to use two contraceptive methods before starting the medication. Also 43.2% reported that they have been informed to use two contraceptive methods while using the medication, and 50% reported that they have been informed to use two contraceptive methods for one month after stopping the medication. Regardless of the information they had, participants’ actual practice, was as follow: 15.9% used two contraceptive methods before starting the medication, 15.9% used two contraceptive methods during the treatment, and 13.6% used two contraceptive methods for one month after stopping the medication.
Conclusions
Although this study revealed that the vast majority of participants were aware of isotretinoin’s teratogenic effect, still a considerable number of them had no idea about the PPP. This issue needs to greatly be addressed to minimize the risk of teratogenicity.
Keywords: Isotretinoin, Teratogenicity, Pregnancy prevention program, Awareness
1. Introduction
Oral Isotretinoin is a medication that is commonly used to treat severe cystic acne (Layton, 2009). The most common adverse effects are transient worsening of acne, dry and fragile skin, and increased susceptibility to sunburn (Brelsford and Beute, 2008). Isotretinoin is a teratogen; there is about a 20–35% risk for congenital defects in infants exposed to the drug in the uterus, and about 30–60% of children exposed to isotretinoin prenatally have been reported to show neurocognitive impairment (Choi et al., 2013). Studies showed that the teratogenic effect of isotretinoin was associated with a wide spectrum of birth defects including craniofacial, heart, and nervous system malformation as well as psychomotor retardation, mental retardation, learning disabilities, and premature birth (Suuberg, 2019).
In line with US-FDA (Mitchell et al., 1995), the Saudi Food and Drug Authority (SFDA) has implemented, in 2013, a pregnancy prevention program (PPP) to protect females from Isotretinoin’s teratogenic effects (Saudi Food and Drug Authority, 2019a). This program includes signing a medical consent form by the female user, patient guide, and contraceptive guide. The consent form aims to prevent the potential severe birth defects that may be caused by Isotretinoin, and it includes information about Isotretinoin use and warnings (Saudi Food and Drug Authority, 2019b).
The Isotretinoin PPP was initiated in different countries. Published literature showed various levels of the program implementation and different degrees of awareness of the target population about the program. For instance, in Belgium, Biset et al conducted a study aimed at evaluating the concomitant use of contraception during Isotretinoin therapy. The study findings revealed that only a small proportion of studied women adhered to the use of contraception as recommended by the program (Biset et al., 2018). A French study also assessed compliance with the PPP accompanied Isotretinoin use. Among a cohort of 8672 women, the results showed that, overall, adherence to the PPP recommendations was poor (Raguideau et al., 2015). Likewise, a nationwide population-based study carried out in Estonia reported very low effective contraceptive coverage during Isotretinoin treatment among women of reproductive age (Uusküla et al., 2018). In the United Kingdom, a study found that overall; there was good compliance with the standards of the Isotretinoin PPP. Nevertheless, particular aspects of care, such as pregnancy testing post-treatment, were reported as less frequently performed (Exton et al., 2017).
Isotretinoin-related concerns were previously investigated in Saudi Arabia. A study was carried out to explore knowledge of the public regarding the use and safety of Isotretinoin-containing products. The study found that a great percentage of participants knew about the drug’s teratogenicity, yet some women did not know that they must stop the drug before pregnancy (Younis and Al-Harbi, 2019). Similarly, another study targeted patients using Isotretinoin showed that only two-thirds of the patients knew about the drug’s teratogenic effect (Al-Harbi, 2010). On the subject of contraception counseling, the study of Algoblan et al reported that less than half of the studied females received written information about risks, around half signed the Isotretinoin consent form, and a great number did not do pregnancy tests while on Isotretinoin, which highlighted a gap in risk reduction (Algoblan et al., 2019). Furthermore, another study showed that around three-quarters of the study participants were counseled about the side effect by the prescribers, however, aspects of non-adherence to drug usage recommendations were also reported (Albadr et al., 2019).
Previous studies in Saudi Arabia did not investigate female awareness about PPP. Further to the previous studies conducted in Saudi Arabia, investigating issues related to Isotretinoin use, our current study was carried out to portray and update the issue of women’s awareness about isotretinoin use and PPP implemented by the SFDA.
2. Materials and methods
2.1. Study design and setting
This cross-sectional study was conducted during the period from June-October 2019. The questionnaire was distributed online through social media to be filled out by female patients, live in Riyadh city, aged > 18 years old who were on Isotretinoin therapy or have used it within one year before the start of this study. For an acceptable sample size, according to Hair et al, the general rule is to have a minimum of five observations per variable (5:1), and acceptable sample size would have 10 observations per variable (10:1) (Hair et al., 2010). The study questionnaire contains 27 questions, and thus a sample size of 270 was considered acceptable. However, 483 completed surveys were received.
2.2. Recruitment method
The study recruited women who were using Isotretinoin during the time of the study or have used it within one year before the start of this study. The survey was disseminated online and a brief introduction about the topic of the survey and its purpose was provided. The target group was clearly stated to include Saudi nationals or residents of the Riyadh City.
2.3. Questionnaire method
The study was performed using a questionnaire. The questionnaire was developed based on the published literature (Al-Harbi, 2010, Albadr et al., 2019, Younis and Al-Harbi, 2019) and the PPP recommendations (Saudi Food and Drug Authority, 2019b). The questions about the PPP were extracted from the information presented in the consent form. The consent form includes a summary of important information and warnings about the drug. It also emphasizes that there is a high risk that the drug may cause severe birth defects if pregnancy occurred at the start of Isotretinoin treatment, during Isotretinoin treatment, or in a month after stopping Isotretinoin treatment. Questions about pregnancy tests and contraceptives use were targeted participants who were married or will get married during a month of using Isotretinoin.
The final questionnaire involved 27 questions. Two pharmacovigilance experts, who are also co-authors in this manuscript, at SFDA, judged the content validity of the questionnaire. The questionnaire also underwent face validity by administering it to six females, from the College of Pharmacy – Princess Nourah University (PNU), who fulfilled the inclusion criteria of the study participants. Face validity was also enhanced using expert judges; three pharmacovigilance experts at SFDA and two faculty members from the college of Pharmacy – PNU.
2.4. Statistical analysis
Analysis of the data was conducted using the Statistical Package for Social Sciences (SPSS for Windows, version 24). Each question in the questionnaire was coded. Descriptive statistics was carried such as frequencies and percentages.
2.5. Ethical considerations
The IRB at Princess Nourah University, approved the research (IRB Log Number 19–0192). Confidentiality was maintained for all the data collected. Participants were asked about their interest in participating in the study before filling the survey.
3. Results
3.1. Characteristics of patients
During the data collection period, a sample of 483 Isotretinoin female users was studied. Participants’ characteristics and their use of Isotretinoin are displayed in Table 1.
Table 1.
Patients’ characteristics and use of Isotretinoin (n = 483).
| Characteristic | % of participants (n) |
|---|---|
| Age range | |
| 20–30 | 89.8% (n = 433) |
| 31–40 | 7.5% (n = 36) |
| 41–50 | 2% (n = 10) |
| > 50 | 0.7% (n = 4) |
| Use of Isotretinoin | |
| Currently using it | 33.5% (n = 162) |
| have used it within one year before the start of this study | 66.5% (n = 321) |
| Use of Isotretinoin and marital status | |
| Used the medication while married, or planning to get married within a one-month period after using it. | 9.1% (n = 44) |
| Not married | 89.9% (n = 439) |
| Did a doctor prescribe the drug? | |
| Yes | 97.3% (n = 470) |
| No | 2.3% (n = 11) |
| I don't remember | 0.4% (n = 2) |
| Reasons for using isotretinoin | |
| Severe acne affecting the face | 71.0% (n = 343) |
| Acne affecting the torso | 20.1% (n = 102) |
| Other reasons | 7.9% (n = 38) |
3.2. Patients’ information about Isotretinoin
Female patients were asked about some information related to Isotretinoin. These included knowledge of the teratogenic effect of the drug, awareness of the presence of SFDA program, the use of effective contraception method, performing pregnancy tests, and avoiding blood donation. The responses to this inquiry are displayed in the following section.
3.2.1. Patients knowledge of teratogenicity and awareness of SFDA pregnancy prevention program (n = 483)
Concerning the knowledge about the teratogenicity of the drug, the vast majority of the participants (94.6%) reported that they knew about the teratogenic effect of Isotretinoin. The doctor represented a source of information for two-thirds of the participants (60.2%) and the pharmacist reported as a source of information for 7.4%. Moreover, 21.1% reported Social Media as the source of gaining information about teratogenicity. Of the participants, only one-third (30.6%) were aware of the presence of the SFDA program. The rest either had no idea about the program (60%) or did not recall that they heard about this program.
3.2.2. Knowledge of using contraceptive methods and performing pregnancy test (n = 44)
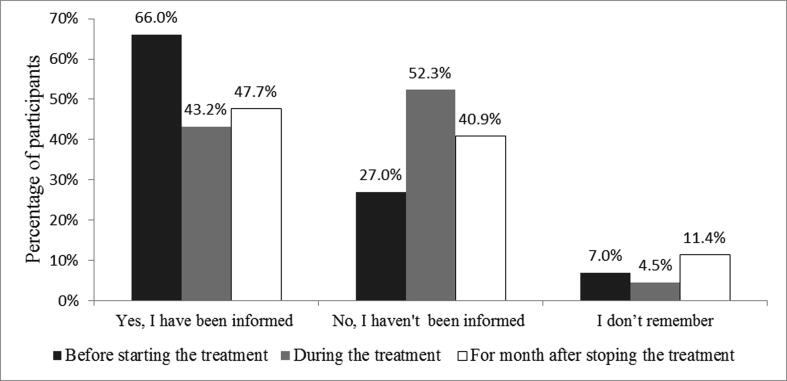
Amongst the participants, 9.1% (n = 44) used Isotretinoin while being married or planning to get married within a one-month period after using it. Within this group, 59.1% (n = 26) stated that they had been provided with materials concerning pregnancy prevention, and 65.9% (n = 29) reported that they had been told to perform a pregnancy test before starting the medication. Also, less than half (43.2%, n = 19) declared that they have been informed to do a monthly pregnancy test while being on Isotretinoin therapy. Likewise, 47.7% (n = 21) stated that the information of performing a pregnancy test, one month after stopping the medication, was communicated to them. The results of this part are displayed in Fig. 1.
Fig. 1.
Information given about performing a pregnancy test (n = 44).
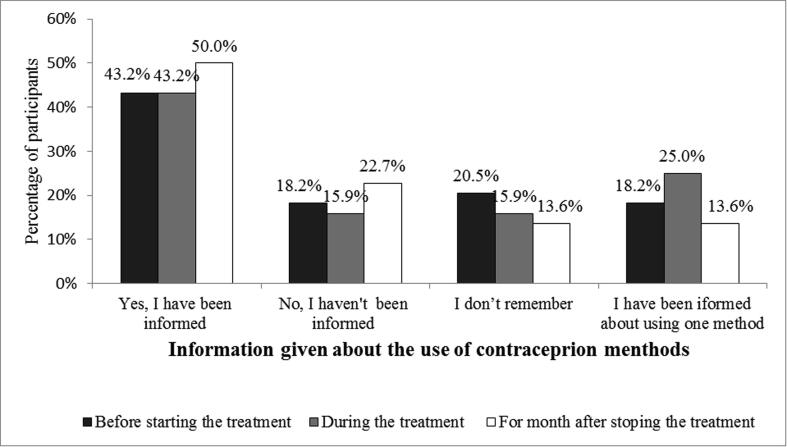
Of the 44 married or planning to get married females, the knowledge of using contraceptives was investigated. Participants were asked if the doctor had told them about the importance of using two methods of contraception before, during, and after stopping the medication. Detailed findings are shown in Fig. 2. Among this group, 43.2% (n = 19) stated that they had been informed of using two contraceptive methods one month before starting the therapy. On the other hand, 18.2% (n = 8) knew about using one method only. Concerning the use of contraception during Isotretinoin treatment, 43.2% (n = 19) declared that they knew, about using two contraceptive methods, from their doctors, and 25% (n = 11) reported their knowledge of one method only. On the subject of using two contraceptive methods for one month after discontinuing the medication, half of the participants (50%, n = 22) stated that they knew about using two methods after stopping the medication course, while 13.6% (n = 6) knew about one method only. The results of this part are displayed in Fig. 2.
Fig. 2.
Response to the question ‘Have you been informed, by the healthcare provider that you have to use two contraceptive methods before using the medication, during the treatment, and one month after stopping the medication? (n = 44).
3.3. The actual use of contraceptive methods (n = 44)
Married or planning to get married participants (n = 44) were asked about the number of contraceptive methods they used (before, during, and after the medication was stopped). Among this group, 15.9% (n = 7) and 56.8% (n = 25) reported using two methods and one method before the course of treatment, respectively. Pertaining to the number of contraceptive methods used during the medication course, 15.9% (n = 7) used two methods, and 70.5% (n = 31) used only one method. With respect to the number of contraceptive methods used after stopping the medication, 13.6% (n = 6) used two methods while 65.9% (n = 29) said that they only used one method.
3.4. Information about blood donation (n = 483)
Of the participants, only 18.4% stated that they had been informed to avoid blood donation while being on Isotretinoin treatment, whereas nearly half of them (44.3%) reported not being informed by their doctors. The rest of the participants declared that they got the information from another source.
3.5. Provision of supporting materials to increase participants’ awareness about Isotretinoin risks (n = 483)
Around half of the participants (47.2%) stated that they had been provided with supporting materials to help them get more information about the medication. Among those participants, two-thirds (62.3%) believed that the supporting materials given, in addition to the consent form, were quite enough for them to have extra information about the medication.
4. Discussion
This study was carried out to investigate the women’s awareness about isotretinoin use and the SFDA program in Riyadh, Saudi Arabia. The current study included female patients aged > 18 years old. The study investigated different issues related to the Isotretinoin treatment including awareness of the teratogenic effect of the medication, prohibiting blood donation while using isotretinoin, signing a medical consent form, and overall awareness of the PPP and its content.
Of great importance to this study was the participants’ awareness of the teratogenic effect of the medication. It seemed that the information is widely disseminated to the target population as almost all participants reported their awareness of this issue. This finding is comparable to what has been found by other studies conducted in different regions of Saudi Arabia (Al-Harbi, 2010, Younis and Al-Harbi, 2019), and it seems that the awareness was enhanced compared to what was reported around a decade ago where only two-thirds of the studied group were aware of the teratogenic effect of the drug (Al-Harbi, 2010). This appeared to be a good point concerning the overall awareness of the target population. However, a great concern remains the source of information, considering that two-thirds of the participants were told by their doctors, few participants were told by a pharmacist, and one-fifth knew from social media. The later source raises concerns about the reliability of such sensitive information.
The current study found that around one-fifth of the participants were informed, by their doctors, to avoid blood donation while using Isotretinoin. Similarly, one-fifth of the participants received this information from other sources. This represents a major health hazard in this area. The information about such an issue should be clearly communicated to the target population from reliable sources.
A cornerstone in this study was the participants’ awareness of the PPP that is approved by the SFDA and disseminated to different beneficiaries. Interestingly, only one-third of the participants reported their awareness of SFDA program that includes signing a medical consent form before starting the medication, patient booklet, and contraception brochure. The participants in this study were, either, using the drug at the time of study or were former users. Additionally, around half of the participants were provided with a patient guide, and only half of them believed that the given material was quite enough for them to have the required information. In fact, the PPP implemented by the SFDA places great emphasis on its contents to be conveyed to target populations by the doctors and other healthcare providers. The program clearly points out the information about carrying out a pregnancy test before treatment and using two contraceptive methods at different time points of the Isotretinoin therapy. This finding highlights that the area of contraception use needs to be addressed. In comparison, a study that was conducted in Riyadh regarding women’s experience with Isotretinoin risk reduction counseling found that only 52% of the patients signed the medical consent form before starting Isotretinoin (Al-Harbi, 2010). Varied awareness level and adherence to PPP were reported among study participants. The varied adherence to the international PPP was also reported in other countries ranging from good adherence (Uusküla et al., 2018) to limited or poor adherence (Biset et al., 2018, Raguideau et al., 2015).
The PPP clearly emphasizes on performing a pregnancy test before, during, and one month after medication discontinuation. Furthermore, using two contraceptive methods in the different time points stated above is necessary for all women with the potential for pregnancy. The current study found that there was a considerable percentage of participants who were unaware of performing pregnancy tests during, and after stopping the treatment. As well, only around half of the participants reported their awareness about using two contraceptive methods. This also highlights a major point in this context. Previous studies performed in Saudi Arabia also identified this gap in risk reduction (Al-Harbi, 2010, Algoblan et al., 2019).
The practice of using contraceptive methods was far from the participants’ awareness as only small percentage used the recommended manner, which is two methods. Noncompliance with contraception was also reported, as common, during Isotretinoin treatment (Collins et al., 2014). This is also a point for improvement when proposing further measures of increasing awareness about the PPP.
5. Limitations of the study:
In this study data were self-reported, thus it is subjected to inaccuracy. The generalizability of the results is limited by the nature of the study design, which introduces different types of biases.. This issue might cause selection or sampling bias. The way participants respond to the questions always remains a concern in any questionnaire. This issue adds to the limitations of the study.
6. Conclusion
In conclusion, the general awareness of medication’ s teratogenicity is noticeable, however; only only one-third of the participants were aware of the PPP, and the measures that should be considered when using Isotretinoin. Further awareness about this program among female patients in Saudi Arabia should be considered.
Authors’ contribution
All authors participated in the development of the research idea, proposal writing and questionnaire development. Alaseeri A, Almonysir A, Alotaibi B, Alrasheed B, and Alfawaz M participated in data collection. Ibrahim A, Almutairi L, and Aljasser N participated in data analysis and interpretation. Ibrahim A, Almotiry Alshatri A, and Mahmoud MA participated in manuscript writing and review. All authors have approved the final m
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Alnada Abdalla Mohamed Ibrahim, Email: alnada44@hotmail.com.
Amal Almotiry Alshatri, Email: asshatri@sfda.gov.sa.
Salem Alsuwaidan, Email: Sa.alsuwaidan@ksmc.med.sa.
Lulu Almutairi, Email: lamutairi@sfda.gov.sa.
Nasser Aljasser, Email: NAJaser@sfda.gov.sa.
References
- Al-Harbi M. Concerns and awareness of acne patients about isotretinoin in qassim region of saudi arabia. Int. J. Health Sci. (Qassim) 2010;4:47–51. [PMC free article] [PubMed] [Google Scholar]
- Albadr T., Alruhaimi D., Cahusac P.B., Rohra D. Knowledge and use of isotretinoin in Saudi female college students: Cross -sectional study. J. Dermatology Dermatologic Surg. 2019;23:76–80. [Google Scholar]
- Algoblan S., Bakhsh S., Alharithy R. Women’s experiences regarding isotretinoin risk reduction counseling in Riyadh. J. Dermatology Dermatologic Surg. 2019;23:13–15. [Google Scholar]
- Biset N., Lelubre M., Senterre C., Amighi K., Bugnon O., Schneider M.P., De Vriese C. Assessment of medication adherence and responsible use of isotretinoin and contraception through belgian community pharmacies by using pharmacy refill data. Patient Prefer. Adherence. 2018;12:153–161. doi: 10.2147/PPA.S149355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsford M., Beute T.C. Preventing and Managing the Side Effects of Isotretinoin. Semin. Cutan. Med. Surg. 2008;27:197–206. doi: 10.1016/j.sder.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Koren G., Nulman I. Pregnancy and isotretinoin therapy. Can. Med. Assoc. J. 2013;185:411–413. doi: 10.1503/cmaj.120729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M.K., Moreau J.F., Opel D., Swan J., Prevost N., Hastings M., Bimla Schwarz E., Korb Ferris L. J. Am. Acad; Dermatol: 2014. Compliance with pregnancy prevention measures during isotretinoin therapy. [DOI] [PubMed] [Google Scholar]
- Exton L.S., Cheung S.T., Brain A.G., Mohd Mustapa M.F., de Berker D.A.R. Compliance with national guidelines on isotretinoin: where are we 2 years since the last audit? Results of the National Isotretinoin Re-Audit 2014. Clin. Exp. Dermatol. 2017;42:381–389. doi: 10.1111/ced.13068. [DOI] [PubMed] [Google Scholar]
- Hair J.F., Black W.C., Babin B.J., Anderson R.E. ed. ed. Vectors; Pearson new international, Harlow: 2010. Multivariate Data Analysis, Seventh. [Google Scholar]
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162–169. doi: 10.4161/derm.1.3.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.A., Van Bennekom C.M., Louik C. A Pregnancy-Prevention Program in Women of Childbearing Age Receiving Isotretinoin. N. Engl. J. Med. 1995;333:101–106. doi: 10.1056/NEJM199507133330206. [DOI] [PubMed] [Google Scholar]
- Raguideau F., Mezzarobba M., Zureik M., Weill A., Ricordeau P., Alla F. Compliance with pregnancy prevention plan recommendations in 8672 French women of childbearing potential exposed to acitretin. Pharmacoepidemiol. Drug Saf. 2015;24:526–533. doi: 10.1002/pds.3763. [DOI] [PubMed] [Google Scholar]
- Saudi Food and Drug Authority, 2020a. https://old.sfda.gov.sa/ar/drug/about/sector_departments/national_pharmacovigilance_center/Documents/curacne%20DHCPL.pdf (accessed 5. 18.2019).
- Saudi Food and Drug Authority, 2020b. Isotretinoin Medical Consent Form. https://www.sfda.gov.sa/en/drug/about/sector_departments/national_pharmacovigilance_center/Documents/isotretinoin-second-version-of-RMM-%283%29.pdf (accessed 5.23.2019).
- Suuberg A. Psychiatric and Developmental Effects of Isotretinoin (Retinoid) Treatment for Acne Vulgaris. Curr. Ther. Res. - Clin. Exp. 2019;90:27–31. doi: 10.1016/j.curtheres.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusküla A., Pisarev H., Kurvits K., Laius O., Laanpere M., Uusküla M. Compliance with Pregnancy Prevention Recommendations for Isotretinoin in Estonia in 2012–2016. Drugs - Real World Outcomes. 2018;5:129–136. doi: 10.1007/s40801-018-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis N.S., Al-Harbi N.Y. Public Understanding and Awareness of Isotretinoin Use and Safety in Al Ahsa, Eastern Saudi Arabia. Ther. Innov. Regul. Sci. 2019;53:618–622. doi: 10.1177/2168479018807677. [DOI] [PubMed] [Google Scholar]