Abstract
Background
Chemotherapy efficacy in early-stage hormone receptor-positive (HR+) breast cancer (BC) according to menopausal status needs a biological explanation.
Methods
We compared early-stage HR+ BC biological features before and after (neo)adjuvant chemotherapy or endocrine therapy (ET), and assessed oestrogen receptor (ER) pathway activity in both pre- and post-menopausal patients. The nCounter platform was used to detect gene expression levels.
Findings
In 106 post-menopausal patients with HR+/HER2-negative BC randomized to neoadjuvant chemotherapy or ET (letrozole+ribociclib), a total of 19 oestrogen-regulated genes, including progesterone receptor (PGR), were found downregulated in the ET-based arm-only. We confirmed this finding in an independent dataset of 20 letrozole-treated post-menopausal patients and found, conversely, an up-regulation of the same signature in HR+/HER2-negative MCF7 cell line treated with estradiol. PGR was found down-regulated by 2 weeks of ET+anti-HER2 therapy in pre-/post-menopausal patients with HR+/HER2-positive (HER2+) BC, while anti-HER2 therapy alone increased PGR expression in HR-negative/HER2+ BC. In 88 pre- and post-menopausal patients with newly diagnosed HR+/HER2-negative BC treated with chemotherapy, the 19 oestrogen-regulated genes were found significantly downregulated only in pre-menopausal patients. In progesterone receptor (PR)+/HER2-negative BC treated with neoadjuvant chemotherapy (n=40), tumours became PR-negative in 69.2% of pre-menopausal patients and 14.8% of post-menopausal patients (p=0.001). Finally, a mean decrease in PGR levels was only observed in pre-menopausal patients undergoing anti-HER2-based multi-agent chemotherapy.
Interpretation
Chemotherapy reduces the expression of ER-regulated genes in pre-menopausal women suffering from hormone-dependent BC by supressing ovarian function. Further studies should test the value of chemotherapy in this patient population when ovarian function is suppressed by other methods.
Funding
Instituto de Salud Carlos III, Breast Cancer Now, the Breast Cancer Research Foundation, the American Association for Cancer Research, Fundació La Marató TV3, the European Union's Horizon 2020 Research and Innovation Programme, Pas a Pas, Save the Mama, Fundación Científica Asociación Española Contra el Cáncer, PhD4MDgrant of “Departament de Salut”, exp SLT008/18/00122, Fundación SEOM and ESMO. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the author(s).
Keywords: Pre-menopause, Progesterone receptor, Hormone receptor positive, Breast cancer, Chemotherapy, Oestrogens
1. Introduction
Hormone receptor-positive (HR+) breast cancer is a major cause of cancer death [1]. Early-stage treatment consists of loco-regional therapy and adjuvant endocrine therapy [2]. (Neo)adjuvant chemotherapy is generally indicated for patients with high-risk HR+ tumours that usually have a high-proliferative state, which is a general biomarker of chemotherapy responsiveness [3,4]. In HR+ breast tumours with low-to-intermediate proliferation, however, recent results from three large, randomized adjuvant clinical trials (i.e., TailorX [3], MINDACT [4] and RxPONDER [5]) suggest that chemotherapy improves survival outcomes in pre-menopausal patients yet not in those who are post-menopausal. The biological explanation behind this observation remains unknown. On one hand, intrinsic genomic features of tumour cells in young women might render tumour cells more sensitive to chemotherapy [6]. On the other hand, chemotherapy's ability to induce ovarian failure or premature menopause is well-established [7]; hence, this could lead to a decrease of systemic levels of oestrogen [7], [8], [9], [10]. To optimize the treatment landscape of early-stage HR+ breast cancer, a better understanding of this clinical observation is needed because these alternative hypotheses could mean different treatment options for young women with breast cancer.
2. Methods
To address the potential anti-oestrogen effects of chemotherapy in breast cancer, we analyzed tumour samples from 4 clinical trials, 2 retrospective cohorts from the Hospital Clinic of Barcelona (HCB) and a publicly available cell line-derived dataset, as subsequently described.
Patient cohorts. The SOLTI-1402 CORALLEEN trial is an open-label, phase II parallel, two-arm, neoadjuvant trial, where 106 post-menopausal patients with stage I-IIIA HR+/HER2- negative and luminal B (by the standardized PAM50/Prosigna® assay) breast cancer were randomized 1:1 to receive either six cycles of 28 days of oral ribociclib 600mg once daily on a 3 weeks-on 1week-off schedule plus continuous letrozole 2.5 mg once daily or standard neoadjuvant chemotherapy with four cycles of doxorubicin 60mg/m2 and cyclophosphamide 600mg/m2 intravenous (IV) every 21 days followed by paclitaxel 80mg/m2 during 12 weeks. Tumour samples were collected at baseline and at surgery, and subsequently formalin-fixed paraffin-embedded (FFPE) according to protocol. The main results of the SOLTI-CORALLEEN trial have been previously reported [11]. This study is registered in ClinicalTrials.gov with number NCT03248427 and is completed.
SOLTI-1501 VENTANA trial is a neoadjuvant phase II trial that randomized a total of 61 post-menopausal patients diagnosed with stage I-IIIA HR+/HER2-negative breast to receive either letrozole 2.5 mg daily, oral metronomic vinorelbine 50 mg 3 days a week or letrozole 2.5 mg daily and oral metronomic vinorelbine 50 mg three times a week for 3 weeks. Tumour samples were collected at baseline and after 3 weeks of letrozole treatment and subsequently FFPE according to protocol. The main results of the SOLTI-1501 VENTANA have been previously reported [12]. This study is registered in ClinicalTrials.gov with number NCT02802748 and is completed.
The PAMELA trial is a non-randomized, open label, multicentric phase II study of neoadjuvant dual HER2 blockade therapy without chemotherapy, where 151 patients with early HER2+ breast cancer were treated with neoadjuvant lapatinib (1,000 mg daily) and trastuzumab (8 mg/kg i.v. loading dose followed by 6 mg/kg) for 18 weeks. Patients with HR+ breast cancer received letrozole or tamoxifen according to menopausal status. The main results of the PAMELA trial have been previously reported [13]. Tumour samples were collected at baseline, day 14 and surgery and subsequently formalin-fixed paraffin-embedded (FFPE) according to protocol. This study is registered in ClinicalTrials.gov with number NCT01973660 and is completed.
SOLTI-1007 NEOERIBULIN trial is a neoadjuvant phase II, single-arm trial that included 174 stage II-III HER2-negative breast cancer patients (73 triple-negative and 101 HR+/HER2-negative) to receive 1.4 mg/m2 of eribulin intravenously on days 1 and 8 every 21-day cycle, for 4 cycles. Tumour samples were collected at baseline and at surgery and subsequently FFPE according to protocol. The main results of the SOLTI-1007 NEOERIBULIN study have been previously reported [14]. This study is registered in ClinicalTrials.gov with number NCT01669252 and is completed.
Hospital Clinic HR+/HER2-negative neoadjuvant cohort is a retrospective cohort of 40 patients diagnosed with stage II-III HR+/HER2-negative breast cancer (13 pre-menopausal and 27 post- menopausal), between 2015-2018 at the HCB. All patients received an anthracycline/taxane-based neoadjuvant chemotherapy. Tumour samples were collected at baseline and surgery and subsequently FFPE.
Hospital Clinic HER2+ neoadjuvant cohort is a retrospective cohort of 73 pre-menopausal and post-menopausal patients diagnosed with HER2+ early stage (II/III) breast treated with neoadjuvant anti-HER2-based multi-agent chemotherapy between 2008 and 2017 at the HCB. FFPE tumour samples were collected at baseline and surgery (if residual disease).
2.1. Ethics
Each study was conducted according to the Declaration of Helsinki. For each one of the previously mentioned patient cohorts, an informed consent for study participation had been previously obtained from all patients enrolled in each individual study. HCB cohorts included patients in observational studies for which Ethic approval was granted by the ethics committee of the HCB (IRB: HCB/2021/0007 and HCB/2015/0491). All other cohorts proceeded from clinical trials that were approved by independent ethics committees at each centre, as specified in the respective publications.
RNA extraction. RNA was extracted from formalin-fixed paraffin-embedded tumour samples from the HCB neoadjuvant HER2+ cohort, the SOLTI-CORALLEEN, SOLTI-VENTANA, PAMELA and SOLTI-NEOERIBULIN trials using the High Pure FFPET RNA isolation kit (Roche, Indianapolis, IN, USA). At least 1–5 10 μm FFPE slides were used for each tumour specimen, and macrodissection was performed to avoid contamination with normal breast tissue, if needed. RNA was quantified at the NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Gene expression analysis. A minimum of 100 ng of total RNA was used to measure gene expression across the SOLTI-CORALLEEN, SOLTI-VENTANA, PAMELA and SOLTI- NEOERIBULIN trials and 1 neoadjuvant cohort of the HCB using the nCounter platform (NanoString Technologies, Seattle, Washington, USA). In SOLTI-CORALLEEN and SOLTI-VENTANA, we used the nCounter® Breast Cancer 360™ panel, which includes 771 breast cancer-related genes. In PAMELA, a custom made codeset of 560 breast cancer-related genes was used. In SOLTI-NEOERIBULIN, a custom made codeset of 540 breast cancer-related genes was used. In the neoadjuvant cohort of the HCB, a custom made codeset of 60 genes was used. Across the 5 studies, genomic data were normalized using 5 housekeeping genes (ACTB, MRPL19, PSMC4, RPLP0 and SF3A1) and log2 transformed. Expression counts were then normalized using the nSolver 4.0 software and custom scripts in R 3.6.1.
Immunohistochemistry. Hematoxylin and eosin (H/E) staining was performed to confirm the presence of invasive tumour cells (≥10%) and determine the minimum tumour surface area (4 mm2). Progesterone receptor (PR) expression was identified using the 1E2 rabbit monoclonal primary antibody (Ventana Medical Systems). PR-negative disease was defined as less than 1% of positive tumour cells.
Genomic analysis on MCF7 cell line. Microarray-based gene expression data was obtained from GEO GSE119552 (available at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119552). In the original study, MCF7 breast cancer cell line was cultured for at least 24 h in steroid- and serum-deprived DMEM without phenol red and with 1.5% charcoal/dextran-stripped FBS (Biowest). Cells were treated for 24h with solvent as a control or 10−9 M E2. RNA was extracted and gene expression analysis was performed using Agilent SurePrint G3 Human GE v2 8 × 60 microarrays [15].
Statistical analysis. To identify genes whose expression was significantly different between paired samples (i.e., baseline versus after treatment) or unpaired samples (i.e., different MCF-7 cell lines treated with control or E2) we used a two-class paired or a multiclass Significance Analysis of Microarray (SAM), respectively, with a false discovery rate (FDR)<5% [16]. The SAM provides for each gene analyzed the standardized mean difference between the gene's expression in a class (e.g., a cell line, a patient cohort etc.), versus its overall mean expression in the dataset. The algorithm applies a t-test at the individual gene level to determine whether the expression pattern for that gene is significant and is able to identify up/down-regulated genes by determining the deviations between observed and expected relative differences, considering a prespecified cut-off for the FDR. This is the rate at which a gene will be incorrectly identified as significant [16]. Paired or unpaired Student's t test, Mann-Whitney test and ANOVA were conducted whenever appropriate to compare continuous variables between groups. Univariate logistic regression was performed to evaluate the association between PR expression according to menopausal status in the HER2-negative HCB chemo-treated cohort. All statistical tests were two-sided, and the statistical significance level was set to <0.05. All analyses were performed using R software version 4.0.2.
2.2. Role of funding source
The funders had no role in study design, data collection, data analyses, interpretation, or writing of this report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
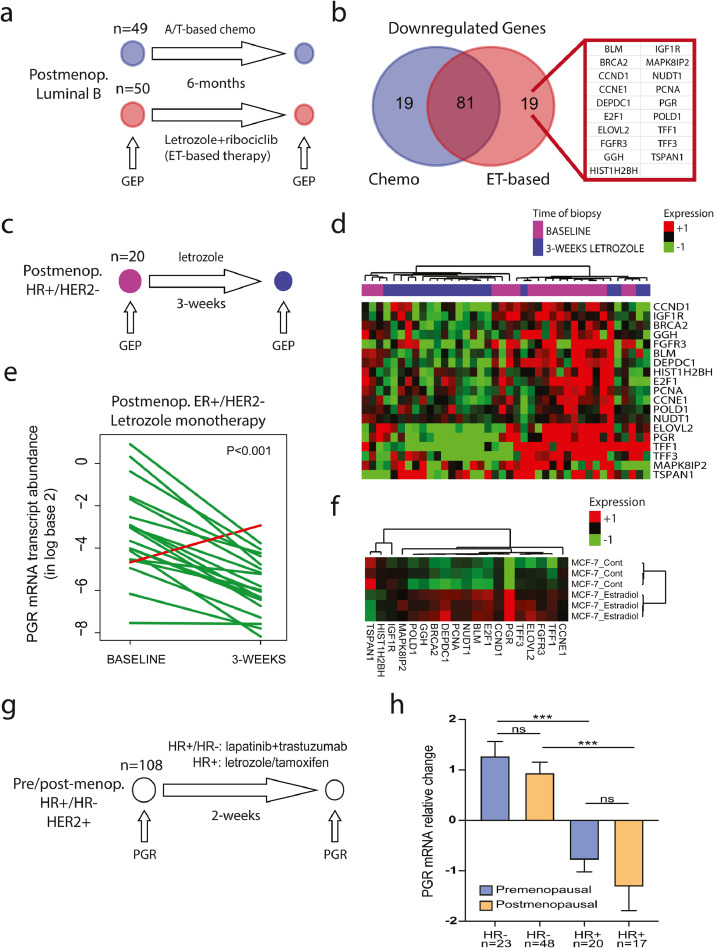
First, we analyzed tumour samples from the SOLTI-CORALLEEN phase II clinical trial [11]. 106 post-menopausal patients with newly diagnosed stage II-III hormone-dependent luminal B breast cancers were randomized to either standard neoadjuvant anthracycline/taxane-based chemotherapy or endocrine therapy (ET) of letrozole in combination with ribociclib, a CDK4/6 inhibitor, for 6 months (Fig 1a). We performed gene expression profiling of 771 breast cancer-related genes in pretreatment tumours, and in residual tumours at surgery, using the nCounter platform (Nanostring Technologies, Seattle, USA). Within each arm, a gene list of the top-100 downregulated genes by each therapy was obtained using a two-class paired SAM. When we compared both gene lists, 81 of 100 genes (81%) were common in both treatment arms including many proliferation-related genes (e.g., MKI67, TOP2A and EXO1). A total of 19 genes were found downregulated in the ET-based arm-only (Fig 1b), including known oestrogen-regulated genes such as progesterone receptor (PGR) [17], cyclin D1 (CCND1) [18], the trefoil factor 1 (TFF1) [19], TFF3 [20], proliferating cell nuclear antigen (PCNA) and E2F transcription factor 1 (E2F1) [21]. Of note, PGR, TFF1 and TFF3 have been previously correlated with plasma estradiol levels in HR+ breast cancer in post-menopausal women [22].
Fig. 1.
Identification of oestrogen-regulated genes in early-stage breast cancer
Legend and captions. (a) Schematic trial design and tumour samples from 99 patients with HR+/HER2-negative disease recruited in the SOLTI-CORALLEEN trial phase II clinical trial(11). (b) Overlap of downregulated genes in SOLTI-CORALLEEN trial between both treatment arms (i.e., anthracycline/taxane [AT]-based chemotherapy and letrozole + ribociclib), and identification of 19 genes found downregulated in the endocrine therapy (ET)-based arm- only. (c) Schematic trial design and tumour samples from the SOLTI-VENTANA phase II clinical trial(12). (d) Unsupervised hierarchical clustering of 19 oestrogen-regulated genes using 20 paired ER+/HER2-negative (i.e., baseline and after 3-weeks of letrozole) from the SOLTI-VENTANA trial(12). Heatmap shows high (red) / low (green) expression of mRNA for each sample and gene. (e) Progesterone receptor (PGR) mRNA expression changes between baseline and after 3-weeks of letrozole monotherapy across 20 patients recruited in the SOLTI-VENTANA trial(12). Each line represents a patient. Increases are represented in red and decreases in green. P-value (P) was determined using a two-tailed paired Student´s t-test. (f) Expression of the 19 oestrogen-regulated genes in the MCF7 HR+/HER2-negative cell line treated with 10−9 M E2 versus control. Gene expression data was obtained from GEO GSE119552 (available at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119552). (f) Schematic trial design and tumour samples from 108 patients with HER2+ early breast cancer, including HER2+/HR- (n=71) and HER2+/HR+/Luminal A or B (n=37) disease recruited in the PAMELA phase II clinical trial(13). (h) Progesterone receptor (PGR) mRNA relative changes after 2 weeks of treatment in pre-menopausal and post-menopausal patients. P-value (P) was determined one-way ANOVA with Tukey's multiple comparisons test. ***: p<0.001; n/s: non-significant.
To confirm these results, we first evaluated the expression of the 19 oestrogen-regulated genes using the nCounter platform in an independent dataset of 20 post-menopausal patients with newly diagnosed HR+/HER2-negative breast cancer (80% Luminal A, 15% Luminal B and 5% normal-like) treated for 3 weeks with letrozole monotherapy in the SOLTI-VENTANA clinical trial (Fig 1c-d) [12]. As expected, all genes, including PGR (Fig 1e), were found significantly downregulated in the post-treatment samples (Fig 1d). Secondly, MCF7 ER+/HER2-negative cell line treated in vitro with 10−9 M estradiol (E2) showed a general up-regulation of the 19-gene signature, including PGR, which showed a 6.1-fold up-regulation (Fig 1f). Finally, PGR was also found down-regulated by 2 weeks of anti-oestrogen therapy (i.e., tamoxifen or letrozole) in combination with anti-HER2-based therapy (i.e. trastuzumab plus lapatinib) in pre-menopausal and post-menopausal patients with HR+/HER2+/Luminal A or B early-stage breast cancer in the PAMELA phase II clinical trial [13]. In contrast, anti-HER2 therapy alone for 2 weeks did not decrease PGR expression but conversely increased its expression in HR-negative/HER2+ early-stage breast cancer, consistent with our previous study showing that anti-HER2 therapy induces a luminal phenotype [23].
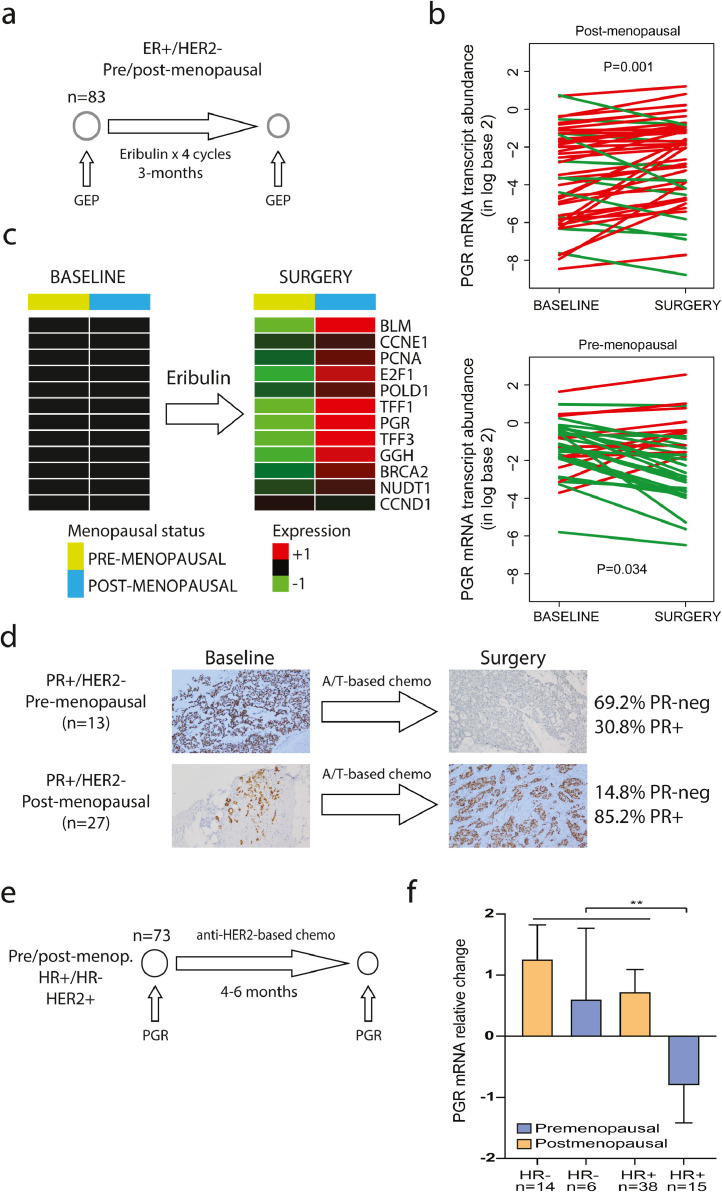
Our previous results identified 19 oestrogen-regulated genes in HR+ breast cancer. To evaluate the oestrogen signaling pathway during chemotherapy, we analyzed tumour samples from the phase II neoadjuvant SOLTI-NEOERIBULIN trial [14], in which 83 patients (35 pre-menopausal and 48 post-menopausal) with newly diagnosed HR+/HER2-negative breast cancer (40% Luminal A, 39% Luminal B, 11% normal-like, 8% Basal-like and 2% HER2-enriched) received 4 cycles (i.e., 12 weeks) of eribulin in monotherapy, an anti-microtubule agent (Fig 2a). We performed gene expression profiling of pretreatment and residual tumours at surgery using the nCounter platform. Strikingly, PGR was found significantly downregulated only in pre-menopausal patients (Fig 2b). We observed similar findings with the other 19 oestrogen-regulated genes (Fig 2c). Conversely, no oestrogen-regulated gene was found differentialy expressed in 55 patients (12 pre-menoapusal and 33 post-menopausal) with triple-negative breast cancer in the SOLTI-NEOERIBULIN according to menopausal status (data not shown). This result provided direct evidence that chemotherapy induces an anti-oestrogen effect in hormone-dependent tumour cells in pre-menopausal patients. Interestingly, 80% (4 of 5) of the pre-menopausal patients with HR+/HER2-negative breast cancer and age <40 had a 3-fold decrease of PGR compared to 23.3% (7 of 30) pre-menopausal patients with age ≥40 (p=0.026).
Fig. 2.
Oestrogen-regulated genes following chemotherapy in early-stage breast cancer per menopausal status
Legend and captions. (a). Schematic trial design and tumour samples from the SOLTI-NEOERIBULIN phase II clinical trial(14). (b) Progesterone receptor (PGR) mRNA expression changes between baseline and after 4 cycles of eribulin monotherapy in 83 patients with residual tumours at surgery (i.e., 35 pre-menopausal and 53 post-menopausal). Each line represents a patient. Increases are represented in red and decreases in green. P- value (P) were determined using a two-tailed paired Student´s t-test. (c). Changes in gene expression after 4 cycles of eribulin in 83 patients included in the SOLTI-NEOERIBULIN trial per menopausal status. Heatmap shows high (red) / low (green) expression of mRNA. (d) Images and summary of progesterone receptor (PR) status by immunohistochemistry in residual tumours of 40 patients (13 pre-menopausal and 27 post- menopausal) with PR+/HER2-negative breast cancer treated with anthracycline/taxane- based neoadjuvant chemotherapy at the Hospital Clinic of Barcelona (Hematoxylin and eosin staining, original magnification x10). (e) Schematic trial design and tumour samples from 73 HER2+ early-stage breast patients treated with neoadjuvant anti-HER2-based multi-agent chemotherapy for 4-6 months, including HER2+/HR- (n=20) and HER2+/HR+/Luminal A or B (n=53) (f) Progesterone receptor (PGR) mRNA relative changes after 4-6 months of treatment in pre-menopausal and post-menopausal patients P-value (P) was determined using a two-tailed unpaired Mann-Whitney test. **: p<0.01.
Progesterone receptor (PR) has been identified as a marker of oestrogenicity in numerous studies spanning 3 decades [22,24]. To further explore the modulation of PR by chemotherapy in residual tumours according to menopausal status, we evaluated the protein levels by immunohistochemistry in an independent dataset of the 40 patients (13 pre-menopausal and 27 post-menopausal) with newly diagnosed ER+/PR+/HER2-negative breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy at the HCB (Fig 2d). The proportion of PR-negative disease in residual tumours at surgery was 9 of 13 (69.2%) in pre-menopausal patients and 4 of 27 (14.8%) in post-menopausal patients (odds ratio=4.70; p-value=0.001). Consistent with this finding, a previous study observed decreased PR expression at residual tumours among pre-menopausal patients treated with neoadjuvant chemotherapy, but not post-menopausal patients [25]. Finally, we explored the ability of chemotherapy to reduce the levels of PGR in 73 pre-menopausal and post-menopausal patients with HER2+ early-stage breast treated with neoadjuvant anti-HER2-based multi-agent chemotherapy at the HCB (Fig 2e). Consistent with previous findings, a mean decrease in PGR levels was only observed in pre-menopausal patients with HR+/HER2+ disease (Fig 2f).
4. Discussion
To our knowledge, this is the first report to provide biological evidence that the benefit of chemotherapy in pre-menopausal patients with a newly diagnosed oestrogen-dependent breast cancer is likely due to the suppression of the ovarian function induced by chemotherapy. This effect is similar to the anti-oestrogen effect induced by an aromatase inhibitor in post-menopausal patients, where oestrogen biosynthesis is suppressed in peripheral tissues through the inhibition of the aromatization of androgens to oestrogens [8]. However, differently from what occurs in menopause, the predominant source of oestrogens in pre-menopausal women is the ovary. Thus, the most likely explanation of our findings is that chemotherapy induces ovarian function suppression in pre-menopausal patients, leading to lower systemic levels of estradiol, which then causes reduced expression of oestrogen/oestrogen-receptor regulated genes in tumour cells. Our findings could explain, in part or in total, the recent clinical findings from TailorX [3], MINDACT [4] and RxPONDER [5] randomized trials in >10,000 patients with HR+/HER2-negative breast cancer with a genomic low-risk by OncotypeDX or MammaPrint, which are biomarkers largely tracking tumour cell proliferation. In these studies, unlike post-menopausal patients, pre-menopausal patients obtained survival benefit from adjuvant chemotherapy. This survival benefit of chemotherapy was also observed in pre-menopausal patients with very low proliferation status (i.e., recurrence score of 0-14 by OncotypeDX in RxPONDER) [5]. Of note, the proportion of patients receiving luteinizing hormone-releasing hormone (LHRH) analogues in these trials was less than 20% [3], [4], [5]. These results reinforce our hypothesis that the benefit induced by chemotherapy in pre-menopausal women is directly related to its ability to induce ovarian function suppression.
Our study present some limitations that need to be acknowledged. Firstly, this is a retrospective and exploratory analyses from available datasets, with all the known limitations and biases concerning sample and dataset availability. Secondly, we had no data regarding patients’ oestradiol (E2), FSH and LH blood levels. Consequently, we could not assess any direct correlation between ovarian function and the molecular findings. Finally, the different patients cohorts proceeded from different prospective interventional and observational multicentre clinical trials, being thus prone to a selection bias. In this regard, it would be useful to expand on the results of the present study by performing confirmatory analyses on tissues from a broader number of patients in wider randomized trials [26], [27], [28], [29], [30], [31]. Nonetheless, results are consistent between similar cohorts and cell lines assays and provide a coherent explanation of the clinical results observed in TailorX [3], MINDACT [4] and RxPONDER [5] trials.
In summary, we have shown that chemotherapy (i.e., eribulin or anthracycline/taxane-based chemotherapy) exerts an anti-oestrogenic biological effect on tumour samples from pre-menopausal patients, which is very similar to that observed with aromatase inhibitors on tumour samples from post-menopausal women. Our chemo-induced endocrine signature, not previously reported, to our knowledge, strongly supports the lengthily accepted hypothesis that at least a substantial proportion of the survival gain obtained from chemotherapy in pre-menopausal women is due to an endocrine effect, most likely via a decrease in global estradiol levels secondary to ovarian function suppression[10, 32]. This is in line with results of several phase 3 trials and one subsequent meta-analysis showing similar outcomes for adjuvant LHRH analogue treatment, alone or added to chemotherapy, versus chemotherapy for all pre-menopausal population with ER+ breast cancer [33]. Interestingly, survival benefits with the addition of LHRH analogues to chemotherapy were only seen in younger women (<40 years), precisely those with lower probability of definitive chemo-induced amenorrhea [33]. Indeed, a non-meaningless proportion of women experience recovery of estradiol and FSH levels to pre-menopausal range in the subsequent months[34, 35]. In this context, pivotal phase 3 trials such as SOFT, TEXT and ASTTRA have established the superiority of adding ovarian function suppression to an aromatase inhibitor/tamoxifen versus tamoxifen alone in pre-menopausal women with HR+ early- stage breast cancer [35, 36]. However, whether LHRH analogues can be used to substitute the endocrine effects of chemotherapy and obtain similar survival outcomes is currently unknown. Nonetheless, our translational study gives consistency to this hypothesis. Finally, a large phase III clinical trial with survival outcome as the primary endpoint is likely needed to demonstrate the endocrine effects of chemotherapy in pre-menopausal patients. This trial could randomize pre-menopausal patients with early-stage HR+/HER2-negative breast cancer to chemotherapy versus no chemotherapy, where all patients are subjected to ovarian function suppression.
To conclude, despite some limitations, our results proffer a strong rationale to develop and/or test effective and more targeted anti-oestrogen treatment strategies in pre-menopausal patients. For example, LHRH modulators could be evaluated to decrease or avoid the use of systemic chemotherapy in hormone-dependent breast cancer. In this context, the capacity of each treatment strategy, including different LHRH modulators and schedules, to afffect ovarian and ER function might be of critical importance [8,37]. For instance, LHRH modulators such as degarelix (i.e., a potent LHRH antagonist) achieves a faster ovarian function suppression, which is more effectively maintained compared to triptorelin (i.e., a LHRH agonist) [38]; thus, the magnitude of ovarian function suppression might be important [8,37]. Another relevant aspect to consider is the existence of a direct cytotoxic effect of chemotherapy on tumour cells, which is likely to be related to their proliferative status (i.e., more tumour proliferation, more benefit from chemotherapy). Future trials in pre-menopausal early-stage hormone-dependent breast cancer to de-escalate and/or escalate systemic therapy should be designed with these results in mind, including hormone-dependent HER2+ breast cancer.
Contributions
AP had the idea for and designed the study. JC and AP were the principal investigator of the SOLTI-NEOERIBULN trial. JG and AP were the principal investigators of the SOLTI-CORALLEEN trial. AP was the principal investigator of the SOLTI-VENTANA trial. NC, FS, ES, FB-M, DM, PG, BG-F, TP contributed to data collection and assembly. NC and FS wrote the manuscript. All authors had full access to all the data in the study, discussed and interpreted the results, revised and approved the final manuscript and accepted responsibility to submit for publication.
Declaration of Competing Interest
Dr. Prat reports grants and personal fees from Pfizer, grants and personal fees from Lilly, personal fees from Nanostring tecnologies, grants and personal fees from Amgen, grants from Roche, personal fees from Oncolytics Biotech, personal fees from Daiichi Sankyo, personal fees from PUMA, personal fees from BMS, from Daiichi Sankyo, outside the submitted work. Dr. Perou reports personal fees from Bioclassifier LLC, outside the submitted work; in addition, Dr. Perou has a U.S. Patent No. 12,995,459 with royalties paid. Dr. Saura reports personal fees from AstraZeneca, Daiichi Sankyo, Eisai, Exact Sciences, Exeter Pharma, F. Hoffmann - La Roche Ltd, MediTech, Merck Sharp & Dohme, Novartis, Pfizer, Philips, Piere Fabre, Puma, Roche Farma, Sanofi-Aventis, SeaGen, and Zymeworks outside the submitted work. Dr. Ciruelos reports personal fees from Roche, Pfizer, Lilly, Novartis, Astra-Zeneca/Daiichy Sankio and from MSD outside the submitted work. Dr. Gavilá reports grants from Novartis, Pfizer, Astra-Zeneca and Lilly, outside the submitted work. Dr. Soberino reports grants and personal fees from MSD, grants from Sanofi and personal fees from Roche outside the submitted work. Dr. Carey reports uncompensed relationship with Sanofi, G1 Therapeutics, Genentech/Roche, AstraZeneca/Daiichi Sankyo, Apitude Health and Exact Sciences; in addition Dr. Carey declared that Companies who have provided research funds to her institution in the past 1–2 years were Syndax, Immunomedics, Novartis, Nanostring technologies, Abbvie, Seattle Genetics and Veracyte, outside the submitted work. Dr Bellett declares advisory board participation for Lilly, Pfizer and Novartis, as well as travel expenses from Pfizer, outside the submitted work. Dr. Muñoz reports expert testimony for Eisai, Novartis and Roche, advisory board for Pierre Fabre and travel grants from Lilly, Pfizer and Roche, outside the submitted work. Dr. Oliveira reports grants from Pfizer, grants, personal fees and non-financial support from Roche, grants and personal fees from Genentech, grants, personal fees and non-financial support from Novartis, grants and personal fees from Seattle Genetics, grants and personal fees from Astra-Zeneca, grants and personal fees from PUMA Biotechnolgy, grants from Immunomedics, grants from Boehringer-Ingelheim, non-financial support from Eisai, Grunenthal, GP Pharma and Pierre Fabre, outside the submitted work. Dr. Pernas reports personal fees and non-financial support from Novartis, personal fees from AstraZeneca, Daiichi-Sankyo, Polyphor, Seattle Genetics, Eisai, Roche and Pierre-Fabre, outside the submitted work. Dr. Cortes reports personal fees and travel expenses from Pfizer, personal fees from Lilly, Servier, Athenex, personal fees and travel expenses and advisory/honoraria from Roche, personal fees from Polyphor, personal fees and travel expenses from Daiichi Sankyo, Novartis and Eisai, personal fees from MSD, GSK, Astra-Zeneca, Celgene, Cellestia, Biothera, Merus, Seattle Genetics, Erytech, Leuko, Bioasis, Clovis Oncology, Boerhinger-Ingelheim, Samsung Bioepis, and stocks from MedSIR, outside the submitted work. The other authors have nothing to declare.
Acknowledgments
Acknowledgments
The authors are grateful to patients who participated in the trials and consented for their tumours to be analyzed for research purposes.
Data sharing statement
All requests for raw and analyzed data and materials will be promptly reviewed by the SOLTI Group to verify whether the request is subject to any intellectual property or confidentiality obligations. Patient-related data not included in the paper were generated as part of the clinical trial and might be subject to patient confidentiality restrictions. Any data and materials that can be shared will be released via a material transfer agreement. Raw counts for gene expression analysis are directly available as Supplementary Material 1.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103451.
[Appendix. Supplementary materials
References
- 1.Carioli G, Malvezzi M, Rodriguez T, Bertuccio P, Negri E, La Vecchia C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89–95. doi: 10.1016/j.breast.2017.06.003. 2017 Dec. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 3.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso F, van ’t Veer L, Poncet C, Lopes Cardozo J, Delaloge S, Pierga J-Y. MINDACT: long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. JCO. 2020;38:506. (15_suppl) [Google Scholar]
- 5.Kalinsky K, Barlow W, Meric-Bernstam F. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy ± chemotherapy in patients with 1-3 positive nodes, hormone receptor-positive and HER2-negative breast cancer with recurrence scores ≤ 25: SWOG S1007 (RxPONDER). 2020 San Antonio Breast Cancer Symposium; San Antonio, TX, USA; 2020 Dec. [Google Scholar]
- 6.Liao S, Hartmaier RJ, McGuire KP, Puhalla SL, Luthra S, Chandran UR. The molecular landscape of premenopausal breast cancer. Breast Cancer Res. 2015 Aug;17:104. doi: 10.1186/s13058-015-0618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid P, Untch M, Kossé V, Bondar G, Vassiljev L, Tarutinov V. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol. 2007 Jun;25(18):2509–2515. doi: 10.1200/JCO.2006.08.8534. [DOI] [PubMed] [Google Scholar]
- 8.Bellet M, Gray KP, Francis PA, Láng I, Ciruelos E, Lluch A. Twelve-Month Estrogen Levels in Premenopausal Women With Hormone Receptor-Positive Breast Cancer Receiving Adjuvant Triptorelin Plus Exemestane or Tamoxifen in the Suppression of Ovarian Function Trial (SOFT): The SOFT-EST Substudy. J Clin Oncol. 2016 May;34(14):1584–1593. doi: 10.1200/JCO.2015.61.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowsett M, Richner J. Effects of cytotoxic chemotherapy on ovarian and adrenal steroidogenesis in pre-menopausal breast cancer patients. Oncology. 1991;48(3):215–220. doi: 10.1159/000226930. [DOI] [PubMed] [Google Scholar]
- 10.Swain SM, Jeong J-H, Geyer CE, Costantino JP, Pajon ER, Fehrenbacher L. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010 Jun;362(22):2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prat A, Saura C, Pascual T, Hernando C, Muñoz M, Paré L. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):33–43. doi: 10.1016/S1470-2045(19)30786-7. 2020 Jan. [DOI] [PubMed] [Google Scholar]
- 12.Adamo B, Bellet M, Paré L, Pascual T, Vidal M, Pérez Fidalgo JA. Oral metronomic vinorelbine combined with endocrine therapy in hormone receptor-positive HER2-negative breast cancer: SOLTI-1501 VENTANA window of opportunity trial. Breast Cancer Res. 2019;18;21(1):108. doi: 10.1186/s13058-019-1195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llombart-Cussac A, Cortés J, Paré L, Galván P, Bermejo B, Martínez N. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017;18(4):545–554. doi: 10.1016/S1470-2045(17)30021-9. [DOI] [PubMed] [Google Scholar]
- 14.Prat A, Ortega V, Villagrasa P, Paré L, Galván P, Oliveira M. Abstract P1-09-09: Efficacy and gene expression results from SOLTI1007 NEOERIBULIN phase II clinical trial in HER2-negative early breast cancer. Cancer Res. 2017 Feb;77(4 Supplement) P1-09–09. [Google Scholar]
- 15.Lecomte S, Demay F, Pham TH, Moulis S, Efstathiou T, Chalmel F. Deciphering the molecular mechanisms sustaining the estrogenic activity of the two major dietary compounds zearalenone and apigenin in ER-positive breast cancer cell lines. Nutrients. 2019 Jan;11(2) doi: 10.3390/nu11020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001 Apr;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay-Schultz J, Gillen AE, Brechbuhl HM, Ivie JJ, Matthews SB, Jacobsen BM. Breast cancer suppression by progesterone receptors is mediated by their modulation of estrogen receptors and RNA polymerase III. Cancer Res. 2017 Sep;77(18):4934–4946. doi: 10.1158/0008-5472.CAN-16-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cicatiello L, Addeo R, Sasso A, Altucci L, Petrizzi VB, Borgo R. Estrogens and progesterone promote persistent CCND1 gene activation during G1 by inducing transcriptional derepression via c-Jun/c-Fos/estrogen receptor (progesterone receptor) complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter. Mol Cell Biol. 2004;24(16):7260–7274. doi: 10.1128/MCB.24.16.7260-7274.2004. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prest SJ, May FEB, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J. 2002;16(6):592–594. doi: 10.1096/fj.01-0498fje. Apr. [DOI] [PubMed] [Google Scholar]
- 20.Miller WR, Larionov AA, Renshaw L, Anderson TJ, White S, Murray J. Changes in breast cancer transcriptional profiles after treatment with the aromatase inhibitor, letrozole. Pharmacogenet Genom. 2007;17(10):813–826. doi: 10.1097/FPC.0b013e32820b853a. Oct. [DOI] [PubMed] [Google Scholar]
- 21.Stender JD, Frasor J, Komm B, Chang KCN, Kraus WL, Katzenellenbogen BS. Estrogen-regulated gene networks in human breast cancer cells: involvement of E2F1 in the regulation of cell proliferation. Mol Endocrinol. 2007 Sep;21(9):2112–2123. doi: 10.1210/me.2006-0474. [DOI] [PubMed] [Google Scholar]
- 22.Dunbier AK, Anderson H, Ghazoui Z, Folkerd EJ, A'hern R, Crowder RJ. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J Clin Oncol. 2010 Mar;28(7):1161–1167. doi: 10.1200/JCO.2009.23.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasó-Maristany F, Griguolo G, Pascual T, Paré L, Nuciforo P, Llombart-Cussac A. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun. 2020 Jan;11(1):385. doi: 10.1038/s41467-019-14111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz KB, Koseki Y, McGuire WL. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology. 1978 Nov;103(5):1742–1751. doi: 10.1210/endo-103-5-1742. [DOI] [PubMed] [Google Scholar]
- 25.Enomoto Y, Morimoto T, Nishimukai A, Higuchi T, Yanai A, Miyagawa Y. Impact of biomarker changes during neoadjuvant chemotherapy for clinical response in patients with residual breast cancers. Int J Clin Oncol. 2016;21(2):254–261. doi: 10.1007/s10147-015-0897-1. Apr. [DOI] [PubMed] [Google Scholar]
- 26.Ishiguro H, Masuda N, Sato N, Higaki K, Morimoto T, Yanagita Y. A randomized study comparing docetaxel/cyclophosphamide (TC), 5-fluorouracil/epirubicin/cyclophosphamide (FEC) followed by TC, and TC followed by FEC for patients with hormone receptor-positive HER2-negative primary breast cancer. Breast Cancer Res Treat. 2020;180(3):715–724. doi: 10.1007/s10549-020-05590-w. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwata H, Sato N, Masuda N, Nakamura S, Yamamoto N, Kuroi K. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer. Jpn J Clin Oncol. 2011 Jul;41(7):867–875. doi: 10.1093/jjco/hyr081. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Noh WC, Lee ES, Jung YS, Kim LS, Han W. Efficacy of neoadjuvant endocrine therapy compared with neoadjuvant chemotherapy in pre-menopausal patients with oestrogen receptor-positive and HER2-negative, lymph node-positive breast cancer. Breast Cancer Res. 2020 May;22(1):54. doi: 10.1186/s13058-020-01288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Minckwitz G, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J Clin Oncol. 2010 Apr;28(12):2015–2023. doi: 10.1200/JCO.2009.23.8303. [DOI] [PubMed] [Google Scholar]
- 30.Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer J-U. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005 Aug;23(22):5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Colleoni M, Sun Z, Martinelli G, Basser RL, Coates AS, Gelber RD. The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up. Ann Oncol. 2009 Aug;20(8):1344–1351. doi: 10.1093/annonc/mdp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain SM, Jeong J-H, Wolmark N. Amenorrhea from breast cancer therapy–not a matter of dose. N Engl J Med. 2010 Dec;363(23):2268–2270. doi: 10.1056/NEJMc1009616. [DOI] [PubMed] [Google Scholar]
- 33.LHRH-agonists in Early Breast Cancer Overview group. Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007 May;369(9574):1711–1723. doi: 10.1016/S0140-6736(07)60778-8. [DOI] [PubMed] [Google Scholar]
- 34.Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006 Mar;24(7):1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 35.Kim H-A, Lee JW, Nam SJ, Park B-W, Im S-A, Lee ES. Adding ovarian suppression to tamoxifen for premenopausal breast cancer: a randomized phase III trial. J Clin Oncol. 2020 Feb;38(5):434–443. doi: 10.1200/JCO.19.00126. [DOI] [PubMed] [Google Scholar]
- 36.Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018 Jul;379(2):122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conforti A, Schettini F, Vallone R, Di Rella F, De Rosa P, De Santo I. Unexpected ovarian activity in premenopausal breast cancer survivors treated with exemestane and GnRH analogues. Breast J. 2019 Nov;25(6):1310–1311. doi: 10.1111/tbj.13474. [DOI] [PubMed] [Google Scholar]
- 38.Dellapasqua S, Gray KP, Munzone E, Rubino D, Gianni L, Johansson H. Neoadjuvant degarelix versus triptorelin in premenopausal patients who receive letrozole for locally advanced endocrine-responsive breast cancer: a randomized phase II trial. J Clin Oncol. 2019 Feb;37(5):386–395. doi: 10.1200/JCO.18.00296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.