Abstract
COVID-19 has re-established the significance of analyzing the organism through a metabolic perspective to uncover the dynamic interconnections within the biological systems. The role of micronutrient status and metabolic health emerge as pivotal in COVID-19 pathogenesis and the immune system's response. Metabolic disruption, proceeding from modifiable factors, has been proposed as a significant risk factor accounting for infection susceptibility, disease severity and risk for post-COVID complications. Metabolomics, the comprehensive study and quantification of intermediates and products of metabolism, is a rapidly evolving field and a novel tool in biomarker discovery. In this article, we propose that leveraging insulin resistance biomarkers along with biomarkers of micronutrient deficiencies, will allow for a diagnostic window and provide functional therapeutic targets. Specifically, metabolomics can be applied as: a. At-home test to assess the risk of infection and propose nutritional support, b. A screening tool for high-risk COVID-19 patients to develop serious illness during hospital admission and prioritize medical support, c(i). A tool to match nutritional support with specific nutrient requirements for mildly ill patients to reduce the risk for hospitalization, and c(ii). for critically ill patients to reduce recovery time and risk of post-COVID complications, d. At-home test to monitor metabolic health and reduce post-COVID symptomatology. Metabolic rewiring offers potential virtues towards disease prevention, dissection of high-risk patients, taking actionable therapeutic measures, as well as shielding against post-COVID syndrome.
Keywords: COVID-19, Metabolomics, Metabolic health, Micronutrient deficiencies, Insulin resistance, Infection susceptibility, Post-COVID
Graphical abstract
1. Introduction
In March 2020, the World Health Organization declared the outspread acute respiratory syndrome coronavirus 2 (SARS-COV-2/COVID-19) a global pandemic. At the time of writing, the viral infection accounts for 2.8 million deaths and 132 million infected cases [1]. Although the underlying disease mechanisms have not yet fully unraveled, it seems that common inflammatory cascades, also known as ‘cytokine storm,’ are responsible for the spectrum of clinical manifestations; from asymptomatic cases, loss of smell or/and taste senses, to mild upper respiratory tract infections and acute respiratory distress syndrome (ARDS), characterized by severe organ failure and eventually, death [2,3]. Among the most prominent recruited molecules, IL-1, IL-6, tumor necrosis factor (TNF- α), and interferon (IFN) levels rise abruptly, leading to uncontrolled inflammatory responses and, eventually, tissue damage due to aberrant cellular interactions [4]. Several factors have been acknowledged to affect whether patients will develop severe COVID-19 phenotype, which, as defined by the Centers for Disease Control and Prevention (CDC), includes hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death [5].
Clinical data on COVID-19 have demonstrated that individuals of older age (over 65), immunocompromised, presenting with comorbidities, including cancer, lung and heart disease, as well as patients with metabolic complications, such as type 2 diabetes (T2D), obesity, metabolic syndrome, share major risks in exhibiting a more severe disease course [6,7]. The role of metabolism emerges as pivotal in disease susceptibility and recovery, seeing that, during infections (COVID-19 included), metabolic perturbations influence the effectiveness of immune responses [[8], [9], [10]].
The role of metabolism in COVID-19 can be summarized as:
-
a.
Individuals with poor metabolic health and/or micronutrient deficiencies share a high risk for infections
-
b.
Altered metabolism is a risk factor for the progression to severe disease
-
c.
Metabolic health and/or micronutrient deficiencies defines the time of recovery and post-COVID complications
The aim of the present article is to discuss current advances in the assessment of metabolic health and micronutrient status and their challenges. Here, we present metabolomics as a sensitive, non-invasive and scalable tool to early diagnose unfavorable metabolic conditions that put individuals at risk of infections and severe infection complications, and to monitor the effect and efficacy of interventions.
2. Metabolic health and micronutrient status as a risk factor for COVID-19
Metabolic health has gained increasing attention recently due to its central role in COVID-19 associated mortality. Assessment of the risk factors for hospitalization among 900.000 patients in the U.S. revealed that 63.5% of them were attributed to cardiometabolic factors (diabetes mellitus, obesity, hypertension, and heart failure), thus could have been prevented [11]. Even though metabolic disease, such as T2D, was once thought to be a characteristic of obese people, we now know that it may occur to lean individuals, known as metabolically unhealthy, normal-weight individuals. Metabolic health criteria have not yet been established. A reviewed strict definition includes the absence of a cardiometabolic profile and normal cardiometabolic parameters (levels of triglycerides, High Density Lipoprotein-HDL, fasting glucose, 2 h-oral glucose tolerance test, blood pressure), that can be routinely measured. Additional criteria for an in-depth characterization include insulin sensitivity measured by glucose infusion rate, the Homeostasis Model Assessment (HOMA) obtained from fasting glucose and insulin levels, and intrahepatic lipid content estimated by imaging techniques [12]. A recent sub-cohort study on metabolically unhealthy lean individuals, defined as healthy individuals that developed T2D during follow-up, investigated the T2D risk factors at baseline and their relationship with diabetes onset. Metabolically unhealthy, lean individuals were characterized by a more unfavorable profile of risk factors, but within the normal range compared to healthy, stressing the need to look at the early stage of metabolic disease pathogenesis [13]. Insulin resistance has been proposed to be an early mechanism towards metabolic disruption, consequent low-grade inflammation and health complications including obesity, metabolic syndrome and cardiometabolic disease. However, insulin resistance is asymptomatic until it develops to hyperinsulinemia and subsequent hyperglycemia and increased levels of traditional clinical parameters that reflect diabetes risk. Thus, it is required to establish sensitive markers capable of reflecting the subtle changes that occur on a biochemical level and can predict disease before symptoms occur [[14], [15], [16]]. Research on metabolic biomarker discovery has yielded some promising results regarding insulin resistance, mostly focusing on Branched-Chain Amino Acids (BCAA) and Fatty Acids that will be discussed below.
Poor metabolic health has been associated with a lack of micronutrients, as a result of malnutrition, known as ‘hidden hunger.’ Minerals and vitamins, collectively known as micronutrients, are key regulators of human metabolism and have been implicated in several cardiometabolic and chronic conditions [17,18]. In addition, micronutrient status is emerging as a critical risk factor for COVID-19 infections and progression to severe disease [19].
The adequacy and balance of nutrients are crucial to support the proper function of all the counterparts in the immune system. It is well demonstrated that suboptimal intake of macro- and micronutrients is associated with decreased immune defenses and susceptibility to infections, and also that the severity of disease escalates in the absence of nutritional balance [20]. On the other hand, a debate on the effect of micronutrient deficiencies replenishment on the disease progression remains. Briefly, the development and regulation of physical barrier function, the secretion of anti-microbial proteins and the management of immune cells' growth and response are inclusive responsibilities of certain micronutrients [19].
Thus, maintaining micronutrient adequacies is crucial for reducing the risk of infection and disease progression, either through their roles in the normal function of the immune system or through the promotion of metabolic health. The origin of micronutrient deficiencies may be poor, low-nutrient dietary choices, which in turn alter the microbiota equilibrium, disturbing their production of vitamins and molecules, and promoting gut permeability, further aggravating loss of micronutrients obtained from food [21]. It has been suggested that the immunological responsiveness diminishes from a shift in the food systems, from a nutrient-rich and healthy diet to a high calorie one lacking a range of nutrients, to cover modern lifestyle requirements [22,23]. Collectively, daily exposure to unsustainable diets promotes a metabolic overload and, together with the over activation of nutrient-specific signals, impairs the immune function [23].
The following paragraphs present a summary of specific immune cell activities that are influenced by the presence of nutrients, recent evidence on associations between micronutrient deficiencies and infections, as well as the outcomes from nutrients replenishment and disease course.
3. Micronutrients: the master regulators of the immune system
Among the significant immunity-supporting nutrients are the vitamins A, C, D and E, zinc, magnesium, omega-3 Polyunsaturated Fatty Acids (PUFA's) and probiotics. Importantly, the levels of several of these nutrients are insufficient in cases of respiratory tract infections, including COVID-19 [24]. The exact role of these nutrients has been discussed extensively elsewhere, and here we provide a brief description [20,[25], [26], [27]]. Vitamin A promotes the epithelial barrier function and phagocytic capacity, with lower levels being associated with increased vulnerability to pathogens and reduced responses from dendritic cells (DCs) and macrophages. It also favors adaptive immunity with antibody production and enhances cytotoxic activity [[28], [29], [30]]. Like vitamin A, vitamin C improves cytotoxic T lymphocytes activity and antibody production and is also involved in the epithelial cohesion process. Moreover, it offers antioxidant protection, which has been strongly associated with COVID-19 pathogenesis [[31], [32], [33]]. As demonstrated elsewhere, the immunological memory is hindered in humans deficient in vitamin C, and the incidence of severe respiratory infections increases [34,35]. Due to the therapeutic benefits of vitamin C against respiratory diseases [36,37], it serves as a key interventional mechanism in COVID-19 management [38,39].
Innate immunity is also stimulated by vitamin D levels, which improve resistance to infectious agents by upregulating the production of anti-microbial peptides. The most important immunological benefit of vitamin D, which is generally inhibitory for adaptive immunity, is the monitoring of DCs activity, vital for antigen-presenting signals and acquiring of immunological tolerance. Vitamin D deficiency has been inversely associated with the incidence of respiratory illness in another cohort [41]. In COVID-19 cases, vitamin D administration may alleviate cytokine storm cascades [42] and provide protection from the pro-inflammatory ACE2-mediated pathway as a negative regulator of the angiotensin-II response [43]. An ongoing clinical trial on the use of vitamin D and other nutraceutical compounds at the Queen Mary University of London aspires to elucidate their clinical significance against COVID-19 (NCT04330599). As for vitamin E, it facilitates the communication between DCs and T helper cells [44] and acts as a pro-oxidant scavenger [45]. Vitamin E supplementation has been associated with a reduced risk of infections in the elderly, as well as with lower rates of hospital admissions for tobacco smokers who develop pneumonia [46,47].
Adequate zinc levels help to sustain sufficient populations of B and T precursor cells in the bone marrow and thymus, as well as to preserve the machinery of innate immunity, such as natural killer cells (NKs) and macrophages [48]. Importantly, concerning COVID-19, zinc has been demonstrated to interfere with viral RNAs and to prevent their replication [49]. This implies that zinc levels are associated with enhanced host responses against viral infections. Of note, zinc replenishment reversed NKs activity in patients with sickle cell anemia and improved T cell numbers in the elderly [50,51]. Recent literature on respiratory tract infections shows that zinc supplementation correlates with lower mortality rates and lower incidence in adults and children [52,53].
Magnesium is one of the most significant life-sustaining compounds, mainly required as a cofactor for the optimal function of numerous enzymes, for genomic integrity regulation, as well as for the regulation of secondary messaging and ion channels [54]. Deficiencies have been inherently associated with impaired immune responses, increasing the levels of pro-inflammatory molecules, such as TNF-α and depleting the anti-inflammatory cytokines [55]. Conversely, magnesium replenishment seems to also regulate the hemostasis cascade and tissue factor expression by decreasing NF-κB activation [56]. Recent literature demonstrates that adequacies in intracellular magnesium help to mediate NKs and CD8+ cytotoxic T lymphocytes activities [56]. Together, insufficient levels of magnesium promote cytokine storm mechanisms and increase the risk of intravascular thrombosis. Of note, the enzymes that promote vitamin D catabolism require magnesium as a cofactor [57]. Thus, maintaining optimal levels of magnesium indirectly affects the above-mentioned monitoring of immune responses, coordinated by vitamin D. Data on a COVID-19 observational study have demonstrated the beneficial effect of magnesium, vitamin D and B12 supplementation in lung function deterioration and ICU admission [58].
The immunomodulatory effects of omega-3 PUFAs have been extensively investigated in the area of inflammatory diseases, as they are negative regulators of pro-inflammatory compounds production, namely eicosanoids, cytokines and adhesion molecules [26]. Regarding COVID-19 data, in a recent pilot study of 100 patients, a tendency of reduced mortality risk was correlated with the levels of Eicosapentaenoic (EPA) and Docosahexaenoic (DHA) acids, although more studies are warranted to support the positive effects of omega-3 PUFAs supplementation [59]. Another prophylactic aspect of omega-3 PUFAs concerns the production of their anti-inflammatory metabolites, resolvins and protectins. The therapeutic potential of these compounds has been presented in several cases of infections, and especially in RNA viruses [60,61].
Probiotics are colonies of bacteria and yeasts, localized in various sites across the body (skin, oral mucosa, lung, gastrointestinal tract and other anatomical regions). During infections, the reported fluctuations in their relevant concentrations propose another feature of immune response modulation [[62], [63], [64]]. The gastrointestinal tract supports the largest amount of microbiota, which comes with increased variability among individuals. Nutrition, obesity, as well as disease contribute to the alteration of gut microflora composition. In fact, patients with chronic diseases and metabolic syndrome present with dysbiosis, which means imbalances in the relevant concentrations of the beneficial microflora [65,66]. It has also been postulated that COVID-19 may interfere with the host's microbiome whereas, lung microflora composition regulates immune defenses to viral insults [67,68]. The therapeutic properties of probiotics administration have been demonstrated in the alleviation of ventilator-associated pneumonia, which can be translated in similar cases of pneumonia during COVID-19, adjunctive to the preventive measures [69].
Recently, data evaluating the impact of certain micronutrient replenishment on COVID-19 onset demonstrated that probiotics, omega-3 PUFAs, multivitamins and vitamin D supplementation provided the female population with significantly lower risks of infection [70]. However, despite these promising outcomes, longitudinal interventional clinical trials assessing the effect of multiple micronutrients with an efficient toolset is of the essence, taking into consideration that micronutrients act in synergy to fulfill their biological functions and self-reported data account for subjective contributions in the studies [71]. Overall, even though there is sufficient knowledge on the critical roles of micronutrient status and metabolic health on infectious diseases, targeting the metabolism of the host seems attractive, yet we lack a unified tool for their assessment.
4. Metabolomics: the lens to view metabolic health and disease
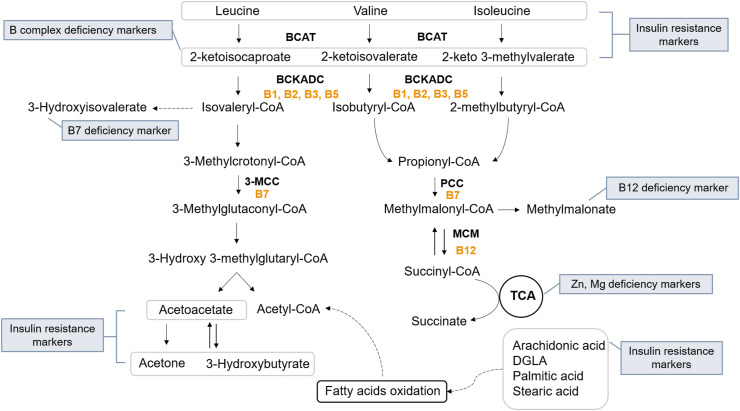
Critical illnesses and infectious disease pandemics, like COVID-19, need to include assessment tools of metabolic health and nutritional status of individuals as a first-line strategy, but also after hospitalization. Metabolomics, an emerging domain in omics, offers the ability to dissect the circulating metabolites of cells, tissues and organs (metabolic profiling) in various biofluids (blood, urine, saliva) [72]. Nutritional deficiencies and metabolism are inherently linked, as metabolic reactions require the presence of cofactors and coenzymes, in order to proceed. Coenzymes are organic molecules that bind to the active site of the enzyme and enhance the binding of the reaction substrate, such as vitamins and amino acids. On the other hand, cofactors may be molecules of organic or inorganic origin, such as metal ions (Mg2+, Zn2+, Fe2+) [36] (Fig. 1 ).
Fig. 1.
The catabolic pathway of BCAAs along with the extracted diagnostic biomarkers for insulin resistance and deficiency of B complex vitamins, zinc (Zn) and magnesium (Mg). BCAT: Branched-chain amino acid aminotransferase, BCKADC: Branched-chain ketoacid dehydrogenase complex, 3-MCC: 3-methylcrotonyl-CoA carboxylase, PCC: Propionyl-CoA carboxylase, MCM: Methylmalonyl-CoA mutase. Vitamins acting as coenzymes in the reactions are marked with yellow.
According to Human Metabolome Database (HMDB), there are already 114.304 metabolite entries, including 8.196 human endogenous metabolites [73]. With the advent of bioinformatics and spectrometric methodologies for metabolomics analysis, it is plausible to extract data on key metabolic processes, such as glycolysis and gluconeogenesis, fatty acid oxidation, oxidative stress, mitochondria function, as well as the metabolism of protein and carbohydrates, neurotransmitters and gut microbiota [72]. The afore-mentioned pathways are affected by nutritional delivery, metabolic perturbations, dysbiosis, disease and environmental pressure. Metabolomics has been applied in several clinical aspects for over three decades exceeding 19.000 published articles in humans. From disease prediction to pharmacometabolomics, metabolites can either predict the onset of a disease years before symptoms appear or reveal the most effective pharmacological drug, coupled with nutritional support against an acute or chronic disease [74]. This technology is not new, considering that biochemical analyses for disease risk assessment in cardiovascular complications and diabetes paradigms utilize the information derived from specific metabolites, such as cholesterol and glucose [75,76]. In addition, inborn errors of metabolism have been diagnosed for decades through the quantification of metabolites [77]. Metabolomics comes to broaden our perspectives on health and disease dynamics by extracting informative biomarkers to illustrate the individual's metabolic health and reveal unnoticed micronutrient deficiencies.
4.1. Metabolomics for micronutrient deficiencies detection
Malnutrition comprises one of the main reasons for ICU admission in cases of acute diseases, serving as a biomarker of disease progress and severity [78]. In addition, respiratory tract infections have been linked to key nutrient deficiencies, as well as an improved disease course upon nutrient replenishment [19,20]. Collectively, the cytokine storm-induced ARDS indicates the similarities in pathogenesis that might be managed through nutritional restoration. For this reason, concerted efforts for the establishment of efficient nutrition support protocols in hospitalized patients have been made, as effective therapeutic ailments targeting disease causalities have not yet been discovered or validated [79,80].
The presence of micronutrient deficiencies comprises a hallmark of metabolic disruption and disease [21]. Patients experience no symptoms until the severe deterioration of tissues or organs. Notably, recent literature describes that more than 20% of global mortality is attributed to poor nutritional habits, with chronic health complications, like cardiovascular disease, cancer and diabetes ranking as the leading causes of death [81]. The diagnosis of micronutrient inadequacies is challenging, regarding the sensitivity of the biomarkers, the optimal levels of nutrient intake and the special populations being at risk. The paradigm of B12 deficiency assessment with the help of metabolomics represents an approach to overcome these barriers.
4.2. The paradigm of B12 deficiency diagnosis
The adequate intake for vitamin B12 (also called cobalamin), relies solely on diet resources, as it cannot be synthesized by the human genome. The main causes of B12 inadequacy are suboptimal dietary intake and malabsorption. In order to be processed, vitamin B12 is transported into the cells via protein transporters, namely haptocorrin (HC), intrinsic factor (IF) and transcobalamin (TC). The functional role of B12 is the promotion of adenosylcobalamin and methylcobalamin-dependent enzymes for the production of succinyl-CoA from methylmalonyl-CoA and of methionine from homocysteine, respectively. The first reaction results in the replenishment of the Tricarboxylic acid cycle (TCA cycle), and as for the second, it yields to the production of tetrahydrofolate, which is a folic acid derivative vital for nucleic acid biosynthesis [82]. Metabolomics might be useful in the diagnosis of B12 deficiency, independently of the origin of the inadequacy, as suboptimal levels of B12 lead to the accumulation of methylmalonic acid and homocysteine in the blood, which can both be captured with sensitivity [83,84], before resulting to methylmalonic acidemia and hyperhomocystinemia [85]. Methylmalonic acidemia has been correlated with cognitive impairments, kidney disease, and pancreatic inflammation, whereas hyperhomocystinemia associates with elevated risk for developing acute coronary syndromes, peripheral vascular disease, myocardial infarction and stroke [86,87]. Although conventional B12 deficiency is assessed via total serum levels, this method evades capturing the bioavailability of B12, as a large amount of B12 (~80%) is bound to HC [88], thus decreasing the diagnostic reliability. The assessment of methylmalonic acid levels in human biofluids with metabolomic-based platforms captures B12 deficiency more efficiently, as the reaction catalyzed by the responsible enzyme (methylmalonyl-CoA synthase) and promoted by B12 does not require the presence of other vitamins [89,90]. Increased methylmalonic acid may also be present in cases of renal dysfunction, requiring careful evaluation and discrimination of distinctive B12 deficiency [90]. The diagnostic value of B12 deficiency increases with the assessment of Holo-TC, which is the bioactive form of B12 that, bound to TC, is furtherly dispensed to systemic circulation for processing. The accuracy increases when employing holo-TC analysis, owing to the ability to capture B12 insufficiencies in special populations [91]. Therefore, combining the conceptions of metabolomics, paired with informative diagnostic algorithms, is of the essence in order to apply accurate, specific and sensitive diagnostics in the field of nutritional insufficiencies.
4.3. Metabolomics to assess metabolic health
A large number of metabolomic studies on T2D and metabolic syndrome patients have identified critical pathways that are implicated in the development of metabolic disease. Specifically, the metabolism of BCAAs, lipids, acylcarnitines and carbohydrates, as well as the metabolites involved in the energy production pathways (TCA cycle) and the microbiome, comprise the most important affected pathways [92]. Of note, some of these pathways have been directly related to insulin resistance and, therefore, may serve as predictive biomarkers of metabolic disease progression. Amino acid metabolism has long been related to insulin function, and several metabolomic studies have concluded that BCAAs including valine, leucine, isoleucine and their downstream metabolites would be useful predictive biomarkers. It has been shown that BCAAs levels are useful to distinguish insulin-resistant from insulin-sensitive individuals, that their levels are related to disease progression from pre-diabetes to diabetes, and they are accurate predictors of therapeutic interventions [15]. The proposed molecular mechanism by which BCAAs levels may reflect insulin resistance involves several aspects (Fig. 1); A. Insulin is a negative regulator of the enzymes that produce a-ketoacids or 2-ketoacids (2-ketoisocaproate, 2-ketoisovalerate, 2-keto-3-methyl valerate), namely BCAA aminotransferase (BCAT) and branched-chain ketoacid dehydrogenase (BCKD) complex. Thus disrupted levels of BCAAs in relation to their downstream metabolites might indicate insulin signaling disruption [93,94]. Of note, these 2-ketoacids are also markers of insufficiency for vitamins B1, B3, B5, which act as coenzymes for BCKD complex, B. BCAAs have been shown to activate the mammalian target of rapamycin 1 (MTORC1), a known mechanism for insulin sensitivity regulation. Thus, increased levels of BCAAs may trigger insulin resistance [95], C. BCAAs levels have been shown to be affected in hyperlipidemic state, playing a key role in the development of insulin resistance. A proposed mechanism includes that, in overnutrition and obese state, glucose and lipids catabolism are promoted, with concurrent suppression of the metabolism of BCAAs in the liver and adipose tissue, but not in the skeletal muscle. BCAAs accumulation in the skeletal muscle leads to increased generation of propionyl-CoA and succinyl-CoA, which supply the TCA cycle continuously, leading to a mitochondrial overload and defective fatty acid oxidation [96]. The accumulation of incomplete oxidized products, mitochondrial overload, cause mitochondrial stress, reduced insulin sensitivity and circulating glucose levels imbalances. D. Besides diet, BCAAs can be endogenously synthesized by gut bacteria, suggesting that gut dysbiosis may contribute to the accumulation of BCAAs and insulin resistance. Taken together, BCAAs have been associated with insulin resistance via multiple pathways, and even though the exact etiological mechanism remains to be elucidated, their predictive value holds promise for clinical application.
Lipidomic patterns have long been associated with metabolic diseases, and the measurement of certain lipids such as cholesterol, triglycerides and total fatty acids are used for the estimation of cardiometabolic risk. Advancements in metabolomics technologies have facilitated the in-depth analysis of fatty acids either free or as part of phospholipids, cholesterol esters and triglycerides, providing a quantitative and qualitative estimation of fatty acids composition. Several fatty acids have been linked to insulin resistance, namely stearic acid, palmitic acid and their monounsaturated derivatives oleic acid and palmitoleic acid and the omega-6 PUFA, linoleic acid, and dihomo-gamma-linoleic acid (DGLA) [[97], [98], [99]]. However, due to the complex mechanism of lipid metabolism, there are several challenges to understand whether free fatty acids changes occur due to specific dietary foods containing these fatty acids or due to increased intake of carbohydrates, that result in de novo lipogenesis. Additional metabolites, such as 3-hydroxybutyric acid and acetoacetate, which are ketone bodies produced through the oxidation of fatty acids in cases of insufficient glucose uptake, might be able to validate insulin resistance. In addition, some reported that the enzymes metabolizing linolenic acid and DGLA, namely delta-6 and delta-5 desaturase, respectively, are regulated by insulin [100,101]. DGLA is the precursor of the pro-inflammatory eicosanoids’ mediator, arachidonic acid. Thus, high enzymatic activity, mediated by insulin signaling dysfunction, might cause excessive production of eicosanoids. Similarly, the enzyme converting myristic, palmitic and stearic to myristoleic, palmitoleic and oleic, respectively, is delta-9 desaturase, and its activity has been shown to be affected upon insulin resistance, a state reflected by the mediated metabolites [102]. Thus, the evaluation of metabolite ratios with a dietary questionnaire might provide valuable insight on the hyperinsulinemia and/or insulin resistance state and as sensitive clinical diagnostic markers of insulin resistance, but also for monitoring the efficacy of applied interventions.
5. Metabolomic-based biomarkers for immune function assessment
The afore-mentioned nutrient deficits, acting on the immune system's performance, require early identification by sensitive and reliable tools. Metabolomics focuses on the accumulation of specific metabolic intermediates, such as methylmalonic acid in the B12 paradigm, to assess the status of vital nutrients.
As described above, methylmalonate is a derivative of the metabolism of the BCAA valine and accumulates in the periphery upon vitamin B12 deficiency. Similarly, 2-ketoisovaleric acid, 2-ketoisocaproic acid and 2-keto 3-methyl valerate are the downstream products of valine, leucine and isoleucine catabolism, respectively, exhibiting elevations in the periphery in response to B complex vitamins inadequacies. The extraction of these metabolomic-based biomarkers provides a functional readout of a host's immunometabolism, as different B vitamins coordinate different immune responses. Specifically, adequate energy production for immune cell activities, gut microbiota composition, fatty acid oxidation, reactive oxygen species (ROS) generation, T cell differentiation and regulation of inflammatory signaling, all comprise key components of B-vitamin mediated monitoring [103]. The sufficiency of beneficial gut microflora can be indirectly evaluated through the levels of vitamin B7 (biotin). This vitamin is endogenously produced by probiotic gastrointestinal bacteria and is required as a coenzyme to promote fatty acid biosynthesis, gluconeogenesis and amino acid metabolism. Biotin-dependent 3-methylcrotonyl-CoA carboxylase promotes leucine catabolism, and decreased enzymatic capacity is associated with the accumulation of 3-methyl crotonic acid and 3-hydroxyisovaleric acid in the biological fluids [104,105].
Apart from the anti-inflammatory properties of vitamin A, it also provides the cells with antioxidant protection. Lipid peroxidation is a sign of cell damage due to oxidative stress and leads to 8-oxo-2′-deoxyguanosine (8-oxo-dG) formation [106]. Vitamin A insufficiencies cannot compensate for the toxic load of ROS, leading to increments in 8-oxo-dG levels. Maintaining redox balance to fight infections is in line with recent data, highlighting that mitochondria dysfunction is correlated with impaired immune responses [107]. Vitamin C acts as a reducing agent, therefore, restoring cellular antioxidant capacity. Together with 8-oxo-dG analysis, vitamin C deficiency is defined by the presence of 4-hydroxyphenylpyruvic and p-hydroxyphenyllactate, which are intermediates of tyrosine metabolism and require ascorbic acid as a cofactor [108]. The measurement of intermediate metabolites in the blood is a more accurate diagnostic approach, as circulating vitamin C is highly degradable [109]. Another biomarker of vitamin C adequacy is adipic acid, a marker of carnitine biosynthesis. Vitamin C is a vital compound of the enzymatic reactions involved in carnitine biosynthesis for successful lipid oxidation in mitochondria [110]. Thus, the presence of adipic acid in the biofluids indicates that fatty acid oxidation is interrupted due to carnitine insufficiency, and omega oxidation occurs in the peroxisomes, leading to elevations of the compound in the periphery [111]. In this context, indirect metabolic biomarkers associated with increased oxidative stress are also employed to assess vitamin E levels, which is a natural antioxidant in the membranes of all cells. The information acquired from the presence of 8-oxo-dG, p-hydroxyphenyllactate and quinolinic acid is translated to increased demands of pro-oxidant scavengers, such as vitamin E [112,113].
Vitamin D is distributed to the tissues and organs after being synthesized in the skin upon UV exposure, in the form of cholecalciferol or in the form of ergocalciferol, of dietary intake. At these stages, vitamin D is biologically inactive and requires two sequential hydroxylations; one in the liver, leading to the formation of 25-hydroxy-vitamin D and one taking place in the kidneys as well as in other cells such as T lymphocytes, to produce 1,25-dihydroxy vitamin D [114]. To exert its immunomodulatory effects, 1,25-dihydroxy vitamin D binds to DNA receptors to initiate protein synthesis [115]. Thus, 25-hydroxy-vitamin D measurement is the most accurate method to test vitamin D supplies in the body [116].
Zinc is involved as a cofactor in several biochemical reactions, enhancing the catalytic activity of enzymes and other proteins [117]. Apart from its role in antioxidant prophylaxis, it restores the metabolic imbalances of the TCA, thus promoting the normal function of mitochondria and oxidative phosphorylation, carbohydrate metabolism as well as fatty acids metabolism [118]. Intermediates of the TCA cycle, such as citrate, pyruvate or succinate, as well as a-linolenic acid of the omega-3 PUFAs catabolism, serve as sensitive indicators of zinc deficiency. Energy production in the form of ATP requires the presence of magnesium in order to be bioavailable, meaning that TCA intermediates including citrate, aconitate and succinate may serve as valuable compounds for the assessment of magnesium deficiency.
Metabolomic-based platforms can directly identify, among others, the omega-3 PUFAs, namely EPA and DHA, the above-mentioned monounsaturated and omega-6 PUFAs, correlated with insulin resistance (stearic acid, palmitic acid, oleic acid, palmitoleic acid, linoleic acid and DGLA), thus providing an overview of an individual's pro-inflammatory and anti-inflammatory eicosanoids. Imbalances in the omega-6 to omega-3 ratios, owing to poor dietary choices, enzymatic dysfunction or malabsorption, result in elevated omega-6 fatty acids that are indicative of a pro-inflammatory state [119].
We conducted a search of the recent PubMed published literature (April 2021) for the topics “metabolomics” and “COVID-19”, which returned 130 results. After narrowing down the search for human studies, the results were 75. The aim was to collect all the research articles on metabolomics-based COVID-19 diagnostic biomarkers, resulting in 26 original article studies summarized in Table 1 . Briefly, the main body of the studied populations consisted of molecularly tested positive COVID-19 patients compared to healthy individuals. The most recurrent candidate biomarkers participated in central metabolic pathways, including the metabolism of TCA, lipids and BCAAs. Interestingly, the presence of the afore-mentioned metabolites both as COVID-19 risk biomarkers and as putative insulin resistance biomarkers indicates the emergence of a pattern between metabolic health regulation and susceptibility to the infection. However, to what extent the impaired metabolic health aggravates the onset of infection or attenuates the immune responses leading to long-term health complications even after recovery, remain to be elucidated.
Table 1.
Summary of the latest clinical studies in metabolomics for the extraction of COVID-19 diagnostic biomarkers.
| Author(s), Year | Population | Group allocation | Specimen | Method | Metabolic profile in CP | Pathways | Conclusions |
|---|---|---|---|---|---|---|---|
| Shen at al. (2020) [120] | N = 102 | 53 H, 49 CP | serum | Targeted, UPLC-MS/MS |
Low sphingolipids, glycerophospholipids, glutamate, arginine, N-(l-arginino)-succinate, citrulline, ornithine, glutamine, 2-oxoglutarate, N-acetyl-L-glutamate, urea, fumarate High 21-hydroxypregnenolone, kynurenine, 8-methoxykynurenate, choline |
Lipid metabolism, arginine metabolism | Severe cases can be classified using a machine learning method based on the proteomic and metabolomic profiling. |
| Thomas et al. (2020) [121] | N = 49 | 16 H, 33 CP | serum | Untargeted, Targeted UHPLC-MS |
Low tryptophan, serotonin, indolepyruvate, gluconeogenic amino acids (alanine, serine, glycine, glutamine, histidine), sulfur amino acids (cysteine, taurine), acylcarnitines High kynurenine, kynurenic acid, picolinic acid, nicotinic acid, creatine, spermidine, fatty acids |
Tryptophan metabolism, glycolysis, pentose phosphate metabolism, fatty acid metabolism | The obtained serum metabolome reflects IL-6 function, providing promising therapeutic targets. |
| Song et al. (2020) [122] | N = 76 | 26 H, 50 CP | plasma | Untargeted, Targeted UPLC-MS/MS |
Low sphingosine-1-phosphate, medium-chain TAG, long-chain TAG, DAG, acylcarnitines, TCA cycle metabolites (itaconic acid), valine, proline, tryptophan, citrulline, isoleucine, glycerophospholipids High sphingolipids, biliverdin, 5-OH tryptophan, lysophosphatidic acid, lyso-phosphatidylcholines, |
Lipid metabolism, β-oxidation, TCA cycle, amino acid metabolism | Exosomes might be associated with COVID-19 pathogenesis. |
| Kimhofer et al. (2020) [123] | N = 42 | 25 H, 19 CP | plasma | Untargeted NMR, LCMS |
Low tryptophan, glutamine, histidine, 3-methylhistidine, apolipoprotein A1 and A2 in HDL, cholesterol in HDL, High kynurenine, a-1-acid glycoprotein, glutamic acid, aspartic acid, glucose, taurine, cystathionine, phenylalanine, triglycerides in HDL, LDL and VLDL, cholesterol in VLDL, phospholipids, apolipoprotein AB in VLDL and IDL, |
Amino acid metabolism, gluconeogenesis, lipid metabolism | The metabolic imbalances indicate multi-organ associations, independently of the respiratory symptoms. |
| Blasco et al. (2020) [124] | N = 100 | 45 H, 55 CP | plasma | Targeted LC-HRMS | Major discriminators: Cytosine, indole-3-acetic acid, 2-aminophenol, isoleucine, asparagine, 1-NH2-cyclopropane-1-carboxylate, leucine, urate, xanthine | BCAAs metabolism, biotin metabolism, purine metabolism, nicotinate nicotinamide metabolism | Involvement of cytosine metabolism and tryptophan-nicotinamide pathway in COVID-19 infection. |
| Zhao et al. (2020) [125] | N = 6 | 2H, 4CP | Colostrum (breastmilk) | Untargeted LC-MS/MS, |
Low indole, indoleacetaldehyde, indole-3-acetic acid, tryptamine, tyrosine, phenylalanine, High Butyryl-carnitine, isobutyryl-carnitine |
aminoacyl-tRNA biosynthesis, aromatic amino acid metabolism, tryptophan metabolism | Breastfeeding with deficiencies in immune-related compounds may not provide adequate defenses to infants. |
| Thomas et al. (2020) [126] | N = 52 | 23 H, 29 CP | serum | Targeted UHPLC-MS |
Low PGM2L1, GAPDH, pyroglutamate, arginine High palmitate, stearate, resolvins, glucose 6-phosphate, fructose bisphosphate, glyceraldehyde 3-phosphate, DPG, phosphoglycerate, phosphoenolpyruvate, pyruvate, lactate, NADH, GSSG, a-ketoglutarate, fumarate, kynurenine |
Glycolysis, Lipid metabolism, protein degradation, ferroptosis, cyclic AMP-AMPK, energy metabolism | Altered RBC's membrane homeostasis suggests that their respond to oxidative stress is compromised. |
| Chen et al. (2020) [127] | N = 83 | 17 H, 66 CP | plasma | Untargeted, NMR |
Low LDL4, LDL5, cholesterol in LDL, Apo-A2 in HDL High LDL1, LDL4, VLDL5, HDL1, HDL4, triglycerides in LDL1, cholesterol in VLDL1, lactate, LDH |
Lipid metabolism, glycolysis, TCA cycle | Identification of biomarkers to assist COVID-19 prognosis and stratification |
| Su et al. (2020) [128] | N = 266 | 133 H, 133 CP | plasma | Untargeted UHPLC-MS/MS | High N1-methyladenosine, mannose, kynurenine, n-palmitoyl-sphingosine | Nucleotide, Amino acid, Carbohydrate, lipid metabolism | Mild to severe: Overactivation of pro-inflammatory mechanisms is accompanied by downregulation of key metabolic pathways and possible hepatic failure. |
| Barberis et al. (2020) [129] | N = 161 | 26 H, 32 CN with symptoms, 103 CP | plasma | Untargeted UPLC-MS/MS, GCxGC-MS |
Low glycerol-phosphocholines, sphingomyelins, glycerol- phosphoethanolamines High lyso-phosphatidylcholine, acylcarnitines, 2-hydroxybutyrate, arachidonic, oleic, palmitic, stearic acids, triglycerides |
Amino acid metabolism, TCA cycle, aminoacyl tRNA degradation, arachidonic acid metabolism | Host's response to the virus involves lipid deregulation and metabolic dysfunction. |
| Smet et al. (2020) [130] | N = 186 | 186 CP | serum | LC-MS | 25-OH-Vitamin D | Vitamin D levels are associated with disease stage. | |
| Grassin Delyle et al. (2020) [131] | N = 40 | 12 ARDS CN, 28CP | EBC | PTR-MS | High Methylpent-2-enal, 2,4-octadiene, 1-chloroheptane, nonanal | COVID-19 entails a metabolic breathprint. | |
| Dogan et al. (2020) [132] | N = 85 | 41 H, 44 CP | serum | Untargeted LC/Q-TOF/MS |
Low R–S lacto-glutathione, glutamine High hypoxanthine, inosine, LTD4 |
purine, glutamine, leukotriene D4, glutathione metabolisms | Antioxidant balance and purine metabolism are targets in COVID-19 therapeutics |
| Lodge et al. (2021) [133] | N = 84 | 34 H, 15 CP, 35 CN with symptoms | plasma | Untargeted NMR |
Low 1-methylhistidine, LDL High glucose, a-1-acid glycoprotein, triglycerides, alanine, lactate, pyruvate, phenylalanine, VLDL |
Lipoprotein metabolism, amino acid metabolism | NMR data indicate systemic disease. |
| Ampudia et al. (2020) [134] | N = 53 | 16 H, 19 recovered, 18 severe CP | serum | Untargeted RP-LC-QTOF-MS | Low sterol lipids, sphingolipids High fatty acyls, fatty acids, glycerophospholipids, glycerolipids | Lipid metabolism | The phenotype of recovered patients was not similar to that of CN patients, with particular deregulation of unsaturated FAs. |
| Wu et al. (2021) [135] | N = 97 | 48 H, 28 CP, 21 CN | plasma | Targeted LC-MS/MS |
High BCAAs and catabolic products, aromatic AAs, acylcarnitines Low methionine |
BCAAs metabolism FAs oxidation, 1-carbon metabolism |
Diabetic complications may accelerate metabolic dysfunction and susceptibility to infection. |
| Lv et al. (2021) [136] | N = 103 | 47 H, 56 CP | serum, stool | Untargeted GC–MS |
Low 2,4-di-tert-butylphenol, deoxyinosine, 7H-purine, behenic acid, orotic acid, arachidic acid, tryptophan, tyrosine High lactic acid, malic acid, oxalic, pantothenic acid, sucrose, ribonic acid, 2-palmitoyl-glycerol, 1,5-anhydroglucitol, D-pinitol |
phenylalanine, tyrosine and tryptophan biosynthesis, aminoacyl-tRNA biosynthesis, glucosinolate biosynthesis, sucrose metabolism |
COVID-19 fecal analysis indicates malnutrition, dysbiosis and inflammation. |
| Bai et al. (2021) [137] | N = 19 | 5 H, 6 Cured severe/elderly 8 Cured mild/young |
plasma | Untargeted UPLC-HRMS |
Low Cholesteryl esters, sphingomyelins, lyso-phosphatidylethanolamines High lyso-phosphatidylcholines, triglycerides, diglycerides |
Lipid metabolism | Hepatic dysfunction is present in recovery, indicating systemic response to infection and the need for assistive treatment. |
| Sindelar et al. (2021) [138] | N = 341 | 67 CN, 274 CP (145 non severe CP, 129 severe CP) | plasma | Untargeted LC-MS/MS | Discriminant metabolites: Lyso-phosphatidylcholines, phosphatidylcholines, triglycerides, gluconate, dimethyl guanosine, bilirubin, kynurenate, nicotinamide, creatinine | Lipid metabolism | A machine learning model can predict disease severity to guide treatment at early stages. |
| Shi et al. (2021) [139] | N = 187 | 78 H, 79 CP, 30 with symptoms | serum | Untargeted GC–MS |
Low citric acid, 2-palmitoyl-glycerol, 1,5-anhydroglucitol High butyric acid, 2-hydroxybutyric acid, succinic acid, L-glutamic, L-phenylalanine, L-serine, L-lactic acid, cholesterol |
TCA cycle, glycolysis, | Distinctive and predictive serum metabolome of COVID-19 reflects systemic implications. |
| Paez-Franco et al. (2021) [140] | N = 92 | 27 H, 65 CP (19 mild CP, 46 severe CP) | serum | Untargeted GC–MS |
Low cysteine, isoleucine, glutamine, and threonine, glyceric and citric acid High α-hydroxyisovaleric acid, α-hydroxybutyric, 2,3-dihydroxybutanoic acid, malic acid, glutamic acid, phenylalanine |
BCAA, glutamate and phenylalanine metabolism, Warburg effect | Lung damage and hypoxia induce deregulated amino acid metabolism, suggesting the potentials of amino acid supplementation. |
| Xu et al. (2021) [141] | N = 130 | 27 H, 103 CP recovered | plasma | Untargeted LC-MS |
Low betain, adenosine High triglycerides, phosphatidylcholines, prostaglandin E2, arginine |
Amino acid metabolism glycerophospholipid metabolism | Metabolomic signature of lung impairments in recovery as a potential therapeutic strategy. |
| Danlos et al. (2021) [142] | N = 99 | 27 CN, 72 CP | Serum/plasma | Untargeted GC–MS, Targeted UHPLC-MS |
Low desaminotyrosine, arginine, tryptophan, indole, indole-acetamide, indole-3-acrylic acid, methyl-3-indole-acetate, arachidonic, carnitine esters, phospholipids, spingosine-1-phosphate, deoxycholic acid High urea, creatine, arabinose, ribose, maltose, arginine, aspartic acid, glutamic acid, phenylalanine, tyrosine, trimethyl-lysine, S -adenosylmethionine, leucylproline, kynurenine |
Glycolysis, sugars metabolism, amino acid metabolism | Metabolic signs of immunosuppression and tryptophan metabolism as therapeutic targets. |
| Schwarz et al. (2021) [143] | N = 57 | 19 H, 18 CP non-ICU, 20 CP ICU | serum | Targeted, LC-MS |
Low PUFA-phosphatidylcholine, phosphatidylserine, plasmalogen High PUFA-phosphatidylethanolamine, lysophospholipids, triacylglycerols Moderate disease: LMs with COX, ALOX12 activity Severe disease: LMs with ALOX5, CYP enzymes |
Lipid metabolism | LMs regulate inflammation that reflects disease onset and progression. |
| Delafiori et al. (2021) [144] | N = 815 | 350 H, 442 CP, 23 CP suspicious | plasma | Untargeted, HESI-Q Orbitrap MS |
Low phosphatidylcholine, cholesterols, unsaturated FAs High triacylglycerols, diacylglycerols, purine |
Lipid metabolism | Mass spectrometry-machine learning provides prognostic markers and treatment targets for COVID-19 with high specificity and sensitivity. |
| Xiao et al. (2021) [145] | N = 84 | 17 H, 14 mild CP, 23 severe CP, 20 CN URTI, 7 mild CP independent cohort | serum | Untargeted, UHPLC quadrupole TOF MS/MS Targeted, UHPLC-MS/MS |
Low citrate, isocitrate, oxalosuccinate, glutamine, citrulline, tryptophan, serotonin, nicotinamide mononucleotide, lyso-phosphatidylcholines High glutamate, succinate, aspartate, kynurenine |
Bile acid biosynthesis, TCA cycle, nicotinate and nicotinamide metabolism, arginine metabolism, nucleic acid metabolism | Cytokine release syndrome is tightly correlated with metabolic regulation. |
H: healthy, CP: COVID-10 positive, CN: COVID-19 negative, MS: Mass Spectrometry, HPLC: High Performance Liquid Chromatography, UPLC: Ultraperformance Liquid Chromatography, DAGs: diglycerides, TAGs: triglycerides, NMR: Nuclear Magnetic Resonance, HDL: high-density lipoprotein, LDL: low-density lipoprotein, VLDL: very low-density lipoprotein, IDL: Intermediate-density lipoprotein, HRMS: High Resolution Mass Spectrometry, PGM2L1: Phosphoglucomutase 2 Like 1, GAPDH: Glyceraldehyde 3-phosphate dehydrogenase, DPG: Dipropylene glycol, NADH: nicotinamide adenine dinucleotide, GSSG: Glutathione disulfide, AMP: Adenosine monophosphate, AMPK: AMP-activated protein kinase, RBCs: red blood cells, EBC: exhaled breath condensate, PTR-MS: Proton-transfer-reaction mass spectrometry, Q-TOF-MS: Quadrupole Time of Flight Mass Spectrometry, LTD4: Leukotriene D4, RP: reverse phase, AAs: amino acids, LMS: Laser Mass Spectrometry, HESI: Heated Electrospray Ionization.
6. Metabolomics in post-COVID syndrome
COVID-19 has been associated with impairments in diverse organ functions, namely lung, cardiovascular, hepatic, neurological and cognitive [146,147]. The major immune responses, inclusive of COVID-19 infection that have been considerably described, are the host's systemic inflammation and the induction of a pro-thrombotic state. Autoimmune complications have also been reported to occur upon the COVID-19 rehabilitation process [148]. The resilience of health issues in the convalescent COVID-19 patients raises the issue of their temporal nature against the induction of chronic health conditions. The example of metabolomics may be useful in the early identification of high-risk populations.
Autoimmunity and viral epidemics interconnection, as well as the causal role of infections in autoimmunity presentation, have been extensively studied in cases of Kawasaki disease [149], Guillain-Barre syndrome [150], type 1 diabetes [151], systemic lupus erythematosus [152], multiple sclerosis [153], autoimmune hemolytic anemia [154] and rheumatoid arthritis development [155]. Nowadays, COVID-19 recuperating patients have already been associated with the aforementioned autoimmune conditions [150,[156], [157], [158], [159]], as well as with the presence of anti-phospholipid antibodies, which increase the risk of thrombosis [160].
The marked depletion in the populations of innate and adaptive immune cells emerges as a key regulator of the immunosuppression and subsequent cell redistribution that promotes loss of cell tolerance and autoimmunity. Specifically, the levels of eosinophils and NKs have been reported to decrease in cases of COVID-19 infection, leading to a compromised IFN-mediated response, and defective defenses against the viral invasion [[161], [162], [163]]. In the same context, CD4+ regulatory and CD8+ effector T and B cells have exhibited reductions in the periphery of acute COVID-19 cases [163]. Additionally, the experimental removal of lymphoid organs in animal models resulted in the infiltration of various tissues with T cells, induction of inflammation and the development of auto-antibodies. It has been postulated that, in order to fill the gap of peripheral immune cell scarcities, the organism seems to be transitioning to an anti-self-renewal state, implicating immune cell lineages that progressively lose their tolerance [164].
The hyperactivation of the cytokine cascade (cytokine storm), the viral breach in the lymphoid organs and the expression of spike protein's ligand, ACE2, by the lymphocytes comprise a suggested biological explanation towards the presence of lymphopenia [165]. Although the role of metabolism has been relatively unvisited in the subject, severe metabolic imbalances, such as metabolic acidosis, have been directly linked to COVID-19 clinical manifestations [166,167]. It has been demonstrated that elevations in serum lactic acid levels compromise T cell metabolism and function, leading to T cell reduction [168]. Taking into consideration that hyperlactatemia is a biomarker of hypoxia [169], monitoring the levels of this particular metabolite might not only serve as a risk assessment strategy but also as a tool to minimize the potentials of autoimmunity appearance.
WHO has reasonably established that individuals with underlying health complications, especially non-communicable diseases (NCDs), share elevated risks of developing a more severe infection and, for that reason, guidelines targeting high-risk populations were published a year ago [170]. Systemic and unresolved inflammation during the onset of the disease has emerged as the central factor, tying NCDs with COVID-19 severity. Interventional strategies targeting modifiable risk factors, that are mainly responsible for the development of NCDs [81], include dietary modifications, micronutrient replenishment, exercise and the adoption of a healthy lifestyle [[171], [172], [173]]. However, consistently to other critically ill recovering patients, convalescent COVID-19 patients may exhibit metabolic abnormalities such as dyslipidemia, cardiovascular complications, lung and kidney injuries, hyperinsulinemia and aberrant glucose uptake [[174], [175], [176]]. These long-term metabolic perturbations drive the development of chronic conditions, such as T2D, even years after recovery [177,178]. The pathophysiology of these issues seems to implicate a combination of psychological, physiological and medical factors, that compose the “metabolic unhealthy” phenotype of post-COVID patients. For instance, impaired metabolic health during the acute ARDS rehabilitation process may result in post-traumatic stress disorders and memory loss, which involve excessive eating habits, reduced motivation for exercise and outdoor activities, thus unfavorable metabolic outcomes. The underlying mechanism suggests that the prevalence of hypoxia and systemic inflammation, treated with ventilatory assistance and brain sedation during the ARDS rehabilitation, leads to post-traumatic stress syndrome and its associated complications [179]. Another potential metabolic burden in the recovery process concerns malnutrition and weight loss in patients exhibiting post-COVID stroke [180]. According to the literature, adipose tissue mass wasting, in an experimentally-induced stroke model, was directly correlated to alterations in lipid metabolism. Specifically, these changes included elevations of free fatty acids and triglycerides, which aggravate cardiovascular manifestations, such as atherosclerosis [181].
Emerging strategies to alleviate ARDS symptomatology have focused on key metabolic pathways. The reported depletions in glucose and folate levels after viral infection have guided the introduction of methotrexate as a recommended rationale for the suppression of the cytokine storm, that mediates systemic inflammation in COVID-19 onset. The drug acts as a folate analog to block the host's folate and one-carbon metabolism used by the virus for viral DNA synthesis and replication [182,183]. Such an approach would be useful both as an anti-viral and as an anti-inflammatory treatment of COVID-19 patients. Merging Metabolomics with Pharmacology, also known as Pharmacometabolomics, is an exciting novel field that offers the ability to address the metabolic impact of a drug, in order to assess its therapeutic efficacy and dosing through pharmacokinetics and pharmacodynamics interactions. The concept of Pharmacometabolomics approaches lies in the extraction of baseline metabolic phenotypes prior to intervention, which will be matched with specific treatment phenotypes. The resulting patterns combined offer information on drug-related individual variations. In this context, patients can be categorized as poor or good responders, according to their metabolic profile before and after drug dosing [184]. Overall, monitoring the levels of the above-mentioned metabolites, as well as integrating the assessment of metabolic health biomarkers in the preventive strategies against post-COVID syndrome, might be of the essence for restoring the metabolic characteristics of the pre-infection state.
7. Expected outcomes
The utmost goal is to employ metabolomics in central healthcare stages to be implemented with current strategies.
-
A.
Metabolomics as a preventive measure for self-protection against infections. In most cases, clinical interventions in critical diseases evade curative effects, as symptoms occur upon advanced stages. In addition, in line with the above, healthcare systems are prompted to manage the symptomatology rather than the origin of disease, which is viruses in this setting. However, the reported inter-individuality in clinical manifestations arises from the diverse deregulations in the immune system function. The combination of metabolomics methodologies with wearable technologies, such as smartphones or smartwatches, could introduce self-testing and obtain real-time health results [185]. Biospecimens of minimal invasiveness, such as blood spots, saliva, urine and exhaled breath condensates, can be obtained with instructive test kits and collected at home or at the workplace to provide mechanistic information on metabolic health, micronutrient status and environmental cues (e.g., viruses). Particularly, breathomics emerges as a convenient and sensitive strategy in the COVID-19-associated diagnostic biomarkers discovery. The method is based on the identification of volatile organic compounds (VOCs) (e.g., acetone, acetic acid, alcohols, methylene chloride, ethylene glycol, carbon disulfides, formaldehyde) or non-volatiles (nucleic acid, DNA, eicosanoids, microbiota metabolites), as well as inorganic compounds (such as nitric oxide) in exhaled breath [186]. Thus, subjects with hidden metabolic disease and at high risk of developing serious illness upon infection will be identified [131]. These people will be instructed to change their lifestyle and diet to reduce the risk, ensure distancing and follow hygiene guidelines.
-
B.
Metabolomics as a tool to identify high-risk groups among infected individuals during hospital admission. In line with the above, metabolic biomarkers extracted from blood, urine or breath, along with predictive tools, such as machine learning technologies, will serve as a stratification method to discriminate high-risk patients to develop serious COVID-19 disease, even in the absence of comorbidities or old age [138]. The metabolic phenotypes will provide the benefit of a diagnostic window before disease progression, will be informative towards patients' allocation, as well as quantitative and representative of each patient's physiological state.
-
C.
Metabolomics in first-line treatment. Early detection of nutritional deficiencies through imbalances in metabolic networks will allow healthcare professionals to provide nutrient-specific guidelines on diet and supplements for low-risk and high-risk individuals. Each category, as well as each patient, will receive a personalized medical nutrition treatment targeting the enforcement of their immune system. Of note, it is essential to establish therapeutic supplement dosages, as special populations, like the elderly, pregnant women, and critically ill patients, usually present with larger-scale deficits, requiring to exceed the recommended dietary allowance in meeting nutritional ends [26,187,188]. In the same context, in the scientific report published by Dietary Guidelines Advisory Committee, it is recommended to increase the intake of certain nutrients whose deficiencies are associated with adverse health conditions [189]. The application of these strategies not only will deal with the acute health consequences from COVID-19 and improve patients’ quality of life, but it will reduce hospital admissions in the overwhelmed healthcare systems.
-
D.
Metabolomics in severe COVID-19 cases. Healthcare professionals will be able to shape their medical nutrition treatment, according to the patient's metabolic health and nutritional deficiencies, in parallel with standard anti-viral treatment. The implementation of a combinatorial approach will expedite patients' recovery time, minimizing the risk of irreversible tissue damage and the length of hospital stay. Another aspect of this approach is the effective prevention of post-COVID complications by maintaining a mild and coordinated immune response level and finetuning the metabolic imbalances.
-
E.
Metabolomics in post-COVID syndrome. Individuals recovered from the infection may take at-home self-tests, which will be remotely assessed by their physicians, in order to take actionable measures. Thus, optimization of the medical nutrition plan during follow-up will prevent the onset or alleviate the severity of COVID-19-related long-term health complications. Overall, metabolic health assessment will serve as a supportive tool for individuals that have recovered from COVID-19 to target post-COVID complications, either metabolic or quality-of-life related.
8. Future steps
Rapid technological advancements have put metabolomics at the center of biomarker discovery of NCDs with proven utility in disorders with known metabolic hallmarks (e.g., cardiovascular disease, cancer, autoimmune diseases). There is strong evidence that COVID-19 should be handled as a disease with metabolic features, and metabolomics is the only tool that can comprehensively study metabolic networks [190]. Now, we are called to combine tools and knowledge from relatively unrelated fields, i.e., virology and biochemistry, to address the COVID-19 pandemic and be prepared for future outspreads. Despite that the assessment of metabolic health and micronutrient status is a widely studied field, COVID-19 is an evolving field that requires additional research. We need to set up a strategy in order to define those biomarkers that will provide the diagnostic advantage over disease progression and serve as therapeutic targets. In this article, we propose a panel of biomarkers (Table 2 ) that are involved in central metabolic pathways, are related to early metabolic derangements, and some of them have been associated with the presence of COVID-19, which could be used as a starting point and be validated in clinical trials (Table 1). However, there needs to be caution as the design of clinical trials is of the highest importance for the generation of conclusive results and the accurate comparability between trials. The ongoing ventures, evaluating nutritional deficits and COVID-19 risk, report considerable discrepancies due to poor study designs, lack of established methodologies and self-reported data [191]. Metabolomic profiling of selected biomarkers needs to be tested in a large number of COVID-19 patients and from different geographical regions, in addition to the medical and nutritional history and clinical laboratory parameters. Special attention should also be given to the methodological details given the sensitivity of metabolite concentrations on handling, storage and method. In addition, we need to establish how this metabolic profile is affected by the metabolic rewiring through medical nutrition treatment and relate it to the clinical phenotype. Thus, metabolite-based, artificial intelligence predictive models will be developed as decision-making tools that will match the metabolic fingerprint to a specific treatment. As described elsewhere, large-scale interventional metabolomic studies harnessing the information on nutritional habits, the optimal dosing of nutritional supplements, as well as the assessment of the synergistic effect of selected nutrients are crucial [71]. Overall, it is becoming apparent that we already have deciphered the scientific and technological background to target the host's metabolism and improve the immune system response; thus, we need to support their implementation in clinical practice.
Table 2.
Potential metabolic biomarkers for insulin resistance and micronutrient deficiency assessment.
| Insulin resistance panel | ||
|---|---|---|
| Metabolite | Metabolic pathway/Enzyme | Correlation with insulin resistance |
| Myristoleic/Myristic | Dehydrogenation of SFA/Δ9-desaturase | – |
| Palmitoleic/Palmitic | Dehydrogenation of SFA/Δ9-desaturase | – |
| Oleic/Stearic | Dehydrogenation of SFA/Δ9-desaturase | – |
| AA/DGLA | Omega 6 metabolism/Δ5-desaturase | – |
| GLA/LA | Omega 6 metabolism/Δ6-desaturase | – |
| AA/EPA | Omega 6 and 3 metabolism/Δ5-desaturase | + |
| Leucine/2-ketoisocaproate | BCAA metabolism/BCAT | + |
| Valine/2-ketoisovalerate | BCAA metabolism/BCAT | + |
| Isoleucine/2-keto-3methylvalerate | BCAA metabolism/BCAT | + |
| Circulating BCAA |
BCAA metabolism |
+ |
| Micronutrient status panel | ||
| Metabolite |
Metabolic pathway |
Micronutrient |
| 8-Oxo-2′-deoxyguanosine | Lipid peroxidation | Vitamin A, Vitamin E, Vitamin C |
| 4-hydroxyphenylpyruvic | Tyrosine metabolism | Vitamin C |
| 4-hydroxyphenyllactate | Tyrosine metabolism | Vitamin C |
| adipic acid | Fatty acids oxidation/carnitine metabolism | Vitamin C |
| Quinolinic acid | Kynurenine pathway | Vitamin E |
| 25-hydroxy-vitamin D | Vitamin D metabolism | Vitamin D |
| Citrate | TCA cycle | Zinc, Magnesium |
| Succinate | TCA cycle | Zinc, Magnesium |
| Pyruvate | TCA cycle | Zinc |
| Methylmalonate | Valine metabolism | Vitamin B12 |
| 2-ketoisocaproic acid | Leucine metabolism | Vitamin B1, B3, B5 |
| 2-ketoisovaleric acid | Valine metabolism | Vitamin B1, B3, B6 |
| 2-keto3-methylvaleric acid | Isoleucine metabolism | Vitamin B1, B3, B7 |
| 3-methylcrotonic acid | Leucine metabolism | Vitamin B7 (dysbiosis) |
| 3-hydroxyisovaleric acid | Leucine metabolism | Vitamin B7 (dysbiosis) |
DGLA: dihomo-gamma linolenic acid, AA: Arachidonic acid, UFA: Unsaturated fatty acids, LA: Linolenic acid, GLA: gamma-linolenic acid, EPA: Eptadecanoic acid, BCAA: Branch chain aminoacids, BCAT: Branched-chain amino acid aminotransferase, TCA: Tricarboxylic acid.
9. Conclusions
At the present time, COVID-19 is in the primal focus, as the need for a successful treatment escalates. The postgenomic era focuses on the emerging field of Metabolomics, the most downstream stage of omics strategies, and the molecular reflection of phenotype. The metabolome is inherently dynamic, as metabolic reactions occur incessantly and engage intracellular, extracellular as well as environmental interactions. COVID-19, although a new infectious disease, shares common pathological mechanisms with other infections, revealing its metabolic perplexity in infection susceptibility, treatment and recovery [190]. Thus, with this article, we propose that metabolic profiling can have multiple applications, including a. At-home risk for infection calculator and nutrition guide, b. A screening tool of high-risk COVID-19 admitted patients, c. First-line treatment guide for mildly ill patients to reduce the risk for hospitalization d. Supportive treatment for critically ill patients to reduce recovery time and risk of post-COVID complications, e. Follow-up metabolic status assessment tool and nutrition guide. As more clinical trials report their findings, the complex interplay between the host's metabolism and COVID-19 progression will be unwound. From a clinical perspective, though, insulin resistance and micronutrient deficiencies are viable targets as they represent early signs of metabolic deregulation and can be easily modulated through medical nutrition treatment. Tuned actions to establish a unified strategy will facilitate the realization of these goals and equip the healthcare system against the current and future pandemics.
Author contributions
All authors contributed to the conception, literature search, figure design and writing of the manuscript.
Sources of funding
No external funding was received.
Declaration of competing interest
The authors have a conflict of interest to declare.
References
- 1.WHO Coronavirus (COVID-19) Dashboard WHO coronavirus (COVID-19) dashboard with vaccination data. https://covid19.who.int/ [Internet]. Available from:
- 2.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020 May;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. https://pubmed.ncbi.nlm.nih.gov/32362390 2020/03/20; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020 Apr;64(5) doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. https://www.frontiersin.org/article/10.3389/fimmu.2020.01446 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira E., Parikh A., Lopez-Ruiz A., Carrilo M., Goldberg J., Cearras M., et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLoS One. 2021 Mar 25;16(3) doi: 10.1371/journal.pone.0249038. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020 Jun;16(6):297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton R. Offline: COVID-19 is not a pandemic. Lancet. 2020 Sep 26;396(10255):874. doi: 10.1016/S0140-6736(20)32000-6. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troha K., Ayres J.S. Metabolic adaptations to infections at the organismal level. Trends Immunol. 2020 Feb;41(2):113–125. doi: 10.1016/j.it.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang A., Huen S.C., Luan H.H., Yu S., Zhang C., Gallezot J.-D., et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016 Sep;166(6):1512–1525. doi: 10.1016/j.cell.2016.07.026. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez K.K., Chen G.Y., Schieber A.M.P., Redford S.E., Shokhirev M.N., Leblanc M., et al. Cooperative metabolic adaptations in the host can favor asymptomatic infection and select for attenuated virulence in an enteric pathogen. Cell. 2018 Sep;175(1):146–158. doi: 10.1016/j.cell.2018.07.016. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meghan O., Junxiu L., Frederick C., Renata M., Dariush M. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021 Mar 2;10(5) doi: 10.1161/JAHA.120.019259. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith G.I., Mittendorfer B., Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019 Oct;129(10):3978–3989. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckel N., Mühlenbruch K., Meidtner K., Boeing H., Stefan N., Schulze M.B. Characterization of metabolically unhealthy normal-weight individuals: risk factors and their associations with type 2 diabetes. Metabolism. 2015 Aug;64(8):862–871. doi: 10.1016/j.metabol.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Roberts L.D., Koulman A., Griffin J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. lancet Diabetes Endocrinol. 2014 Jan;2(1):65–75. doi: 10.1016/S2213-8587(13)70143-8. [DOI] [PubMed] [Google Scholar]
- 15.Newgard C.B. Metabolomics and metabolic diseases: where do we stand? Cell Metabol. 2017 Jan;25(1):43–56. doi: 10.1016/j.cmet.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hameed A., Mojsak P., Buczynska A., Suleria H.A., Kretowski A., Ciborowski M. Altered metabolome of lipids and amino acids species: a source of early signature biomarkers of T2DM. J Clin Med. 2020;9(7):2257. doi: 10.3390/jcm9072257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss J.W.E., Ramji D.P. Nutraceutical therapies for atherosclerosis. Nat Rev Cardiol. 2016 Sep;13(9):513–532. doi: 10.1038/nrcardio.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wessels I., Rink L. Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D. J Nutr Biochem. 2020 Mar;77:108240. doi: 10.1016/j.jnutbio.2019.108240. [DOI] [PubMed] [Google Scholar]
- 19.McAuliffe S., Ray S., Fallon E., Bradfield J., Eden T., Kohlmeier M. Dietary micronutrients in the wake of COVID-19: an appraisal of evidence with a focus on high-risk groups and preventative healthcare. BMJ Nutr Prev Heal. 2020;3(1) doi: 10.1136/bmjnph-2020-000100. bmjnph-2020-000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calder P.C. Nutrition, immunity and COVID-19. BMJ Nutr Prev Heal. 2020;3(1):74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey R.L., West K.P., Jr., Black R.E. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(suppl 2):22–33. doi: 10.1159/000371618. https://www.karger.com/DOI/10.1159/000371618 Available from: [DOI] [PubMed] [Google Scholar]
- 22.Branca F., Lartey A., Oenema S., Aguayo V., Stordalen G.A., Richardson R., et al. Transforming the food system to fight non-communicable diseases. BMJ. 2019 Jan 28;364:l296. doi: 10.1136/bmj.l296. http://www.bmj.com/content/364/bmj.l296.abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Rosa V., La Cava A., Matarese G. Metabolic pressure and the breach of immunological self-tolerance. Nat Immunol. 2017 Oct;18(11):1190–1196. doi: 10.1038/ni.3851. Available from. [DOI] [PubMed] [Google Scholar]
- 24.Chiscano-Camón L., Ruiz-Rodriguez J.C., Ruiz-Sanmartin A., Roca O., Ferrer R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020 Aug;24(1):522. doi: 10.1186/s13054-020-03249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam M., Gómez S., González-Gross M., Marcos A. Possible roles of magnesium on the immune system. Eur J Clin Nutr. 2003;57(10):1193–1197. doi: 10.1038/sj.ejcn.1601689. Available from. [DOI] [PubMed] [Google Scholar]
- 26.Wu D., Lewis E.D., Pae M., Meydani S.N. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2019;10(JAN):1–19. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecora F., Persico F., Argentiero A., Neglia C., Esposito S. The role of micronutrients in support of the immune response against viral infections. Nutrients. 2020;12(10):1–45. doi: 10.3390/nu12103198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villamor E., Fawzi W.W. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005 Jul;18(3):446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown C.C., Noelle R.J. Seeing through the dark: new insights into the immune regulatory functions of vitamin A. Eur J Immunol. 2015 May;45(5):1287–1295. doi: 10.1002/eji.201344398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin A in the immune system. J Clin Med. 2018 Sep;7(9) doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Luo G., Yuan J., Wang Y., Yang X., Wang X., et al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat Inflamm. 2014;2014:426740. doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017 Nov;9(11) doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samir D. Oxidative stress associated with SARS-cov-2 (COVID-19) increases the severity of the lung disease - a systematic review. J Infect Dis Epidemiol. 2020;6(3):1–6. [Google Scholar]
- 34.Jacob R.A., Kelley D.S., Pianalto F.S., Swendseid M.E., Henning S.M., Zhang J.Z., et al. Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr. 1991 Dec;54(6 Suppl):1302S–1309S. doi: 10.1093/ajcn/54.6.1302s. [DOI] [PubMed] [Google Scholar]
- 35.Hemilä H. Vitamin C and infections. Nutrients. 2017 Mar;9(4) doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerullo G., Negro M., Parimbelli M., Pecoraro M., Perna S., Liguori G., et al. The long history of vitamin C: from prevention of the common cold to potential aid in the treatment of COVID-19. Front Immunol. 2020 Oct 28;11:574029. doi: 10.3389/fimmu.2020.574029. https://pubmed.ncbi.nlm.nih.gov/33193359 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr A.C., Rowe S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients. 2020;12 doi: 10.3390/nu12113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiedra R., Lo K.B., Elbashabsheh M., Gul F., Wright R.M., Albano J., et al. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti Infect Ther. 2020 Dec;18(12):1259–1261. doi: 10.1080/14787210.2020.1794819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guglielmetti G., Quaglia M., Sainaghi P.P., Castello L.M., Vaschetto R., Pirisi M., et al. “War to the knife” against thromboinflammation to protect endothelial function of COVID-19 patients. Crit Care. 2020 Jun;24(1):365. doi: 10.1186/s13054-020-03060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berry D.J., Hesketh K., Power C., Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011 Nov;106(9):1433–1440. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 42.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. 2020 Oct;32(10):2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwart S.R., Smith S.M. Vitamin D and COVID-19: lessons from spaceflight analogs. J Nutr. 2020 Oct 12;150(10):2624–2627. doi: 10.1093/jn/nxaa233. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moriguchi S., Muraga M. Vitamin E and immunity. Vitam Horm. 2000;59:305–336. doi: 10.1016/s0083-6729(00)59011-6. [DOI] [PubMed] [Google Scholar]
- 45.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007 Jul;43(1):4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chavance M., Herbeth B., Fournier C., Janot C., Vernhes G. Vitamin status, immunity and infections in an elderly population. Eur J Clin Nutr. 1989 Dec;43(12):827–835. [PubMed] [Google Scholar]
- 47.Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasan R., Rink L., Haase H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013 Jun;19(3):253–264. doi: 10.1177/1753425912458815. [DOI] [PubMed] [Google Scholar]
- 49.Kaushik N., Subramani C., Anang S., Muthumohan R., Shalimar, Nayak B., et al. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J Virol. 2017 Nov;91(21) doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapazoglou E., Prasad A.S., Hill G., Brewer G.J., Kaplan J. Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J Lab Clin Med. 1985 Jan;105(1):19–22. [PubMed] [Google Scholar]
- 51.Barnett J.B., Dao M.C., Hamer D.H., Kandel R., Brandeis G., Wu D., et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016 Mar;103(3):942–951. doi: 10.3945/ajcn.115.115188. [DOI] [PubMed] [Google Scholar]
- 52.Wang L., Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Res J. 2018 Mar;12(3):857–864. doi: 10.1111/crj.12646. [DOI] [PubMed] [Google Scholar]
- 53.Lassi Z.S., Moin A., Bhutta Z.A. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016 Dec;12(12):CD005978. doi: 10.1002/14651858.CD005978.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin H. Magnesium: the missing element in molecular views of cell proliferation control. Bioessays. 2005 Mar;27(3):311–320. doi: 10.1002/bies.20183. [DOI] [PubMed] [Google Scholar]
- 55.Long S., Romani A.M. Role of cellular magnesium in human diseases. Austin J Nutr Food Sci. 2014 Nov 18;2(10):1051. https://pubmed.ncbi.nlm.nih.gov/25839058 [Internet] Available from: [PMC free article] [PubMed] [Google Scholar]
- 56.Diao B., Huang X., Guo S., Yang C., Liu G., Chen Y., et al. MAGT1-mediated disturbance of Mg(2+) homeostasis lead to exhausted of HBV-infected NK and CD8(+) T cells. Sci Rep. 2017 Oct 19;7(1):13594. doi: 10.1038/s41598-017-11522-4. https://pubmed.ncbi.nlm.nih.gov/29051561 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uwitonze A.M., Razzaque M.S. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018 Mar;118(3):181–189. doi: 10.7556/jaoa.2018.037. [DOI] [PubMed] [Google Scholar]
- 58.Tan C.W., Ho L.P., Kalimuddin S., Cherng B.P.Z., Teh Y.E., Thien S.Y., et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B(12) in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asher A., Tintle N.L., Myers M., Lockshon L., Bacareza H., Harris W.S. Blood omega-3 fatty acids and death from COVID-19: a pilot study. Prostaglandins Leukot Essent Fatty Acids. 2021 Mar;166:102250. doi: 10.1016/j.plefa.2021.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandhaus S., Swick A.G. Specialized proresolving mediators in infection and lung injury. Biofactors. 2021 Jan;47(1):6–18. doi: 10.1002/biof.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basil M.C., Levy B.D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016 Jan;16(1):51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazar V., Ditu L.-M., Pircalabioru G.G., Gheorghe I., Curutiu C., Holban A.M., et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. https://www.frontiersin.org/article/10.3389/fimmu.2018.01830 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libertucci J., Young V.B. The role of the microbiota in infectious diseases. Nat Microbiol. 2019;4(1):35–45. doi: 10.1038/s41564-018-0278-4. Available from. [DOI] [PubMed] [Google Scholar]
- 64.Hand T.W. The role of the microbiota in shaping infectious immunity. Trends Immunol. 2016 Oct;37(10):647–658. doi: 10.1016/j.it.2016.08.007. https://pubmed.ncbi.nlm.nih.gov/27616558 2016/09/05; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang R., Ju Z., Zhou P.-K. A gut dysbiotic microbiota-based hypothesis of human-to-human transmission of non-communicable diseases. Sci Total Environ. 2020 Nov;745:141030. doi: 10.1016/j.scitotenv.2020.141030. [DOI] [PubMed] [Google Scholar]
- 66.Tilg H., Zmora N., Adolph T.E., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020 Jan;20(1):40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 67.Khatiwada S., Subedi A. Lung microbiome and coronavirus disease 2019 (COVID-19): possible link and implications. Hum Microbiome J. 2020 Aug;17:100073. doi: 10.1016/j.humic.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bao L., Zhang C., Dong J., Zhao L., Li Y., Sun J. Oral microbiome and SARS-CoV-2: beware of lung Co-infection. Front Microbiol. 2020;11:1840. doi: 10.3389/fmicb.2020.01840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su M., Jia Y., Li Y., Zhou D., Jia J. Probiotics for the prevention of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Respir Care. 2020 May;65(5):673–685. doi: 10.4187/respcare.07097. [DOI] [PubMed] [Google Scholar]
- 70.Louca P., Murray B., Klaser K., Graham M.S., Mazidi M., Leeming E.R., et al. 2021. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445,850 users of the COVID Symptom Study app; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsoukalas D., Sarandi E. Micronutrient deficiencies in patients with COVID-19: how metabolomics can contribute to their prevention and replenishment. BMJ Nutr Prev Heal. 2020;3(2):419–420. doi: 10.1136/bmjnph-2020-000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsoukalas D., Fragoulakis V., Sarandi E., Docea A.O., Papakonstaninou E., Tsilimidos G., et al. Targeted metabolomic analysis of serum fatty acids for the prediction of autoimmune diseases. Front Mol Biosci. 2019;6:120. doi: 10.3389/fmolb.2019.00120. https://www.frontiersin.org/article/10.3389/fmolb.2019.00120 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Human metabolome Database. https://hmdb.ca/ Available from:
- 74.Tsoukalas D., Sarandi E., Thanasoula M., Docea A.O., Tsilimidos G., Calina D., et al. Metabolic fingerprint of chronic obstructive lung diseases: a new diagnostic perspective. Metabolites. 2019;9(12) doi: 10.3390/metabo9120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.VR S. Biomarkers of cardiovascular disease. Circulation. 2006 May 16;113(19):2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. Available from. [DOI] [PubMed] [Google Scholar]
- 76.GS M., CJ I., DS R., DK A., ER H., FB A., et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005 Oct 25;112(17):e285–e290. doi: 10.1161/CIRCULATIONAHA.105.169405. Available from. [DOI] [PubMed] [Google Scholar]
- 77.Guerrero R.B., Salazar D., Tanpaiboon P. Laboratory diagnostic approaches in metabolic disorders. Ann Transl Med. 2018 Dec;6(24):470. doi: 10.21037/atm.2018.11.05. https://pubmed.ncbi.nlm.nih.gov/30740401 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mogensen K.M., Robinson M.K., Casey J.D., Gunasekera N.S., Moromizato T., Rawn J.D., et al. Nutritional status and mortality in the critically ill. Crit Care Med. 2015 Dec;43(12):2605–2615. doi: 10.1097/CCM.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 79.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020 Jun;74:110834. doi: 10.1016/j.nut.2020.110834. https://pubmed.ncbi.nlm.nih.gov/32276799 2020/04/02; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stachowska E., Folwarski M., Jamioł-Milc D., Maciejewska D., Skonieczna-Żydecka K. Nutritional support in coronavirus 2019 disease. Medicina (Kaunas) 2020 Jun 12;56(6):289. doi: 10.3390/medicina56060289. https://pubmed.ncbi.nlm.nih.gov/32545556 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collaborators GBD 2017 D. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2019 May 11;393(10184):1958–1972. doi: 10.1016/S0140-6736(19)30041-8. https://pubmed.ncbi.nlm.nih.gov/30954305 2019/04/04; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hannibal L., Lysne V., Bjørke-Monsen A.L., Behringer S., Grünert S.C., Spiekerkoetter U., et al. Biomarkers and algorithms for the diagnosis of vitamin B 12 deficiency. Front Mol Biosci. 2016;3(JUN) doi: 10.3389/fmolb.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsugawa H., Tsujimoto Y., Sugitate K., Sakui N., Nishiumi S., Bamba T., et al. Highly sensitive and selective analysis of widely targeted metabolomics using gas chromatography/triple-quadrupole mass spectrometry. J Biosci Bioeng. 2014;117(1):122–128. doi: 10.1016/j.jbiosc.2013.06.009. https://www.sciencedirect.com/science/article/pii/S1389172313002284 Available from: [DOI] [PubMed] [Google Scholar]
- 84.Sarvin B., Lagziel S., Sarvin N., Mukha D., Kumar P., Aizenshtein E., et al. Fast and sensitive flow-injection mass spectrometry metabolomics by analyzing sample-specific ion distributions. Nat Commun. 2020;11(1):3186. doi: 10.1038/s41467-020-17026-6. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolffenbuttel B.H.R., Wouters H.J.C.M., Heiner-Fokkema M.R., van der Klauw M.M. The many faces of cobalamin (vitamin B(12)) deficiency. Mayo Clin proceedings Innov Qual outcomes. 2019 May 27;3(2):200–214. doi: 10.1016/j.mayocpiqo.2019.03.002. https://pubmed.ncbi.nlm.nih.gov/31193945 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Management N., Outcomes B., Acidemia M. 2020. Nutritional management and biochemical outcomes methylmalonic acidemia. [Google Scholar]
- 87.BP J., Kerry-Anne R. Homocysteine and cardiovascular disease. Circ Res. 2006 Sep 15;99(6):565–566. doi: 10.1161/01.RES.0000243583.39694.1f. Available from. [DOI] [PubMed] [Google Scholar]
- 88.Guéant J.-L., Namour F. In: Johnson LRBT-E of G, editor. Elsevier; New York: 2004. Vitamin B12: absorption, metabolism, and deficiency; pp. 619–624.https://www.sciencedirect.com/science/article/pii/B0123868602007206 Available from: [Google Scholar]
- 89.Brito A., Grapov D., Fahrmann J., Harvey D., Green R., Miller J.W., et al. The human serum metabolome of vitamin B-12 deficiency and repletion, and associations with neurological function in elderly adults. J Nutr. 2017 Oct 1;147(10):1839–1849. doi: 10.3945/jn.117.248278. https://pubmed.ncbi.nlm.nih.gov/28794205 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clarke R., Refsum H., Birks J., Evans J.G., Johnston C., Sherliker P., et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr. 2003 May;77(5):1241–1247. doi: 10.1093/ajcn/77.5.1241. [DOI] [PubMed] [Google Scholar]
- 91.Valente E., Scott J.M., Ueland P.-M., Cunningham C., Casey M., Molloy A.M. Diagnostic accuracy of holotranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B₁₂ status in the elderly. Clin Chem. 2011 Jun;57(6):856–863. doi: 10.1373/clinchem.2010.158154. [DOI] [PubMed] [Google Scholar]
- 92.Rangel-Huerta O.D., Pastor-Villaescusa B., Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics. 2019 Jun;15(6):93. doi: 10.1007/s11306-019-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hutson S.M., Cree T.C., Harper A.E. Regulation of leucine and alpha-ketoisocaproate metabolism in skeletal muscle. J Biol Chem. 1978 Nov;253(22):8126–8133. [PubMed] [Google Scholar]
- 94.Flakoll P.J., Kulaylat M., Frexes-Steed M., Hourani H., Brown L.L., Hill J.O., et al. Amino acids augment insulin's suppression of whole body proteolysis. Am J Physiol. 1989 Dec;257(6 Pt 1):E839–E847. doi: 10.1152/ajpendo.1989.257.6.E839. [DOI] [PubMed] [Google Scholar]
- 95.Karusheva Y., Koessler T., Strassburger K., Markgraf D., Mastrototaro L., Jelenik T., et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr. 2019 Nov 1;110(5):1098–1107. doi: 10.1093/ajcn/nqz191. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabol. 2009 Apr;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. https://pubmed.ncbi.nlm.nih.gov/19356713 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lovejoy J.C., Champagne C.M., Smith S.R., DeLany J.P., Bray G.A., Lefevre M., et al. Relationship of dietary fat and serum cholesterol ester and phospholipid fatty acids to markers of insulin resistance in men and women with a range of glucose tolerance. Metabolism. 2001 Jan;50(1):86–92. doi: 10.1053/meta.2001.19440. [DOI] [PubMed] [Google Scholar]
- 98.Fernández-Real J.-M., Broch M., Vendrell J., Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003 May 1;26(5):1362. doi: 10.2337/diacare.26.5.1362. http://care.diabetesjournals.org/content/26/5/1362.abstract LP – 1368. Available from: [DOI] [PubMed] [Google Scholar]
- 99.Wanders A.J., Alssema M., de Koning EJP, le Cessie S., de Vries J.H., Zock P.L., et al. Fatty acid intake and its dietary sources in relation with markers of type 2 diabetes risk: the NEO study. Eur J Clin Nutr. 2017;71(2):245–251. doi: 10.1038/ejcn.2016.204. Available from. [DOI] [PubMed] [Google Scholar]
- 100.Warensjö E., Ohrvall M., Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metabol Cardiovasc Dis. 2006 Mar;16(2):128–136. doi: 10.1016/j.numecd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Brenner R.R. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot Essent Fatty Acids. 2003 Feb;68(2):151–162. doi: 10.1016/s0952-3278(02)00265-x. [DOI] [PubMed] [Google Scholar]
- 102.Kurotani K., Sato M., Ejima Y., Nanri A., Yi S., Pham N.M., et al. High levels of stearic acid, palmitoleic acid, and dihomo-γ-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr Res. 2012 Sep;32(9):669–675. doi: 10.1016/j.nutres.2012.07.004. e3. [DOI] [PubMed] [Google Scholar]
- 103.Yoshii K., Hosomi K., Sawane K., Kunisawa J. Metabolism of dietary and microbial vitamin b family in the regulation of host immunity. Front Nutr. 2019;6(April):1–12. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Visconti A., Le Roy C.I., Rosa F., Rossi N., Martin T.C., Mohney R.P., et al. Interplay between the human gut microbiome and host metabolism. Nat Commun. 2019;10(1):4505. doi: 10.1038/s41467-019-12476-z. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cozzolino C., Villani G.R.D., Frisso G., Scolamiero E., Albano L., Gallo G., et al. Vol. 41. Scielo; 2018. Biochemical and molecular characterization of 3-Methylcrotonylglycinuria in an Italian asymptomatic girl; pp. 379–385. (Genetics and Molecular Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanazawa K., Sakamoto M., Kanazawa K., Ishigaki Y., Aihara Y., Hashimoto T., et al. Lipid peroxides as endogenous oxidants forming 8-oxo-guanosine and lipid-soluble antioxidants as suppressing agents. J Clin Biochem Nutr. 2016 Jul;59(1):16–24. doi: 10.3164/jcbn.15-122. https://pubmed.ncbi.nlm.nih.gov/27499574 2016/06/10; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y., Zhou Z., Min W. Mitochondria, oxidative stress and innate immunity. Front Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. https://www.frontiersin.org/article/10.3389/fphys.2018.01487 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.La Du B.N., Zannoni V.G. The role of ascorbic acid in tyrosine metabolism. Ann N Y Acad Sci. 1961 Apr 1;92(1):175–191. doi: 10.1111/j.1749-6632.1961.tb46117.x. Available from. [DOI] [PubMed] [Google Scholar]
- 109.Karlsen A., Blomhoff R., Gundersen T.E. Stability of whole blood and plasma ascorbic acid. Eur J Clin Nutr. 2007;61(10):1233–1236. doi: 10.1038/sj.ejcn.1602655. Available from. [DOI] [PubMed] [Google Scholar]
- 110.Ziegler E.E., Filer L.J. 7th ed. ILSI Press, International Life Sciences Institute; Washington, D.C.: 1996. Present knowledge in nutrition. [Google Scholar]
- 111.Hardwick J.P. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75(12):2263–2275. doi: 10.1016/j.bcp.2008.03.004. https://www.sciencedirect.com/science/article/pii/S0006295208001718 Available from: [DOI] [PubMed] [Google Scholar]
- 112.Coquette A., Vray B., Vanderpas J. Role of vitamin E in the protection of the resident macrophage membrane against oxidative damage. Arch Int Physiol Biochim. 1986 Dec;94(5):S29–S34. [PubMed] [Google Scholar]
- 113.Ribeiro C.A.J., Grando V., Dutra Filho C.S., Wannmacher C.M.D., Wajner M. Evidence that quinolinic acid severely impairs energy metabolism through activation of NMDA receptors in striatum from developing rats. J Neurochem. 2006 Dec;99(6):1531–1542. doi: 10.1111/j.1471-4159.2006.04199.x. [DOI] [PubMed] [Google Scholar]
- 114.Cashman K.D., van den Heuvel E.G., Schoemaker R.J., Prévéraud D.P., Macdonald H.M., Arcot J. 25-Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. 2017 Nov;8(6):947–957. doi: 10.3945/an.117.015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lehmann B., Meurer M. Extrarenal sites of calcitriol synthesis: the particular role of the skin. Recent results cancer Res Fortschritte der Krebsforsch Prog dans les Rech sur le cancer. 2003;164:135–145. doi: 10.1007/978-3-642-55580-0_9. [DOI] [PubMed] [Google Scholar]
- 116.Guo T., Taylor R.L., Singh R.J., Soldin S.J. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006 Oct;372(1–2):76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 117.Jurowski K., Szewczyk B., Nowak G., Piekoszewski W. Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. JBIC J Biol Inorg Chem. 2014;19(7):1069–1079. doi: 10.1007/s00775-014-1139-0. [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang X., Wang H., Huang C., He X., Xu W., Luo Y., et al. Zinc enhances the cellular energy supply to improve cell motility and restore impaired energetic metabolism in a toxic environment induced by OTA. Sci Rep. 2017;7(1):14669. doi: 10.1038/s41598-017-14868-x. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsoukalas D., Alegakis A.K., Fragkiadaki P., Papakonstantinou E., Tsilimidos G., Geraci F., et al. Application of metabolomics part II: focus on fatty acids and their metabolites in healthy adults. Int J Mol Med. 2019 Jan;43(1):233–242. doi: 10.3892/ijmm.2018.3989. https://pubmed.ncbi.nlm.nih.gov/30431095 2018/11/14; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020 Jul 9;182(1):59–72. doi: 10.1016/j.cell.2020.05.032. https://pubmed.ncbi.nlm.nih.gov/32492406 e15. 2020/05/28; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5(14):1–16. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song J.-W., Lam S.M., Fan X., Cao W.-J., Wang S.-Y., Tian H., et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metabol. 2020 Aug;32(2):188–202. doi: 10.1016/j.cmet.2020.06.016. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kimhofer T., Lodge S., Whiley L., Gray N., Loo R.L., Lawler N.G., et al. Integrative modeling of quantitative plasma lipoprotein, metabolic, and amino acid data reveals a multiorgan pathological signature of SARS-CoV-2 infection. J Proteome Res. 2020;19(11):4442–4454. doi: 10.1021/acs.jproteome.0c00519. [DOI] [PubMed] [Google Scholar]
- 124.Blasco H., Bessy C., Plantier L., Lefevre A., Piver E., Bernard L., et al. The specific metabolome profiling of patients infected by SARS-COV-2 supports the key role of tryptophan-nicotinamide pathway and cytosine metabolism. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-73966-5. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao Y., Shang Y., Ren Y., Bie Y., Qiu Y., Yuan Y., et al. Omics study reveals abnormal alterations of breastmilk proteins and metabolites in puerperant women with COVID-19. Signal Transduct Target Ther. 2020;5(1):17–19. doi: 10.1038/s41392-020-00362-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas T., Stefanoni D., Dzieciatkowska M., Issaian A., Nemkov T., Hill R.C., et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J Proteome Res. 2020;19(11):4455–4469. doi: 10.1021/acs.jproteome.0c00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Y., Zheng Y., Yu Y., Wang Y., Huang Q., Qian F., et al. Blood molecular markers associated with COVID-19 immunopathology and multi-organ damage. EMBO J. 2020;39(24):1–23. doi: 10.15252/embj.2020105896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Su Y., Chen D., Yuan D., Lausted C., Choi J., Dai C.L., et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183(6):1479–1495. doi: 10.1016/j.cell.2020.10.037. e20. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barberis E., Timo S., Amede E., Vanella V.V., Puricelli C., Cappellano G., et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to sars-cov-2. Int J Mol Sci. 2020;21(22):1–25. doi: 10.3390/ijms21228623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Smet D., De Smet K., Herroelen P., Gryspeerdt S., Martens G.A. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2021;155(3):381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grassin-Delyle S., Roquencourt C., Moine P., Saffroy G., Carn S., Heming N., et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine. 2021:63. doi: 10.1016/j.ebiom.2020.103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Doğan H.O., Şenol O., Bolat S., Yıldız Ş.N., Büyüktuna S.A., Sarıismailoğlu R., et al. Understanding the pathophysiological changes via untargeted metabolomics in COVID-19 patients. J Med Virol. 2021;93(4):2340–2349. doi: 10.1002/jmv.26716. [DOI] [PubMed] [Google Scholar]
- 133.Lodge S., Nitschke P., Kimhofer T., Coudert J.D., Begum S., Bong S.H., et al. NMR spectroscopic windows on the systemic effects of SARS-CoV-2 infection on plasma lipoproteins and metabolites in relation to circulating cytokines. J Proteome Res. 2021;20(2):1382–1396. doi: 10.1021/acs.jproteome.0c00876. [DOI] [PubMed] [Google Scholar]
- 134.Acosta-ampudia Y., Monsalve D.M., Rojas M., Rodríguez Y., Esteban J., Salazar-uribe J.C., et al. The COVID-19 resource centre is hosted on Elsevier Connect, the Company’ s Public News and Information; 2020. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. (January) [Google Scholar]
- 135.Wu J., Zhao M., Li C., Zhang Y., Wang D.W. The SARS-CoV-2 induced targeted amino acid profiling in patients at hospitalized and convalescent stage. Biosci Rep. 2021;41(3):1–11. doi: 10.1042/BSR20204201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lv L., Jiang H., Chen Y., Gu S., Xia J., Zhang H., et al. The faecal metabolome in COVID-19 patients is altered and associated with clinical features and gut microbes. Anal Chim Acta. 2021;1152:338267. doi: 10.1016/j.aca.2021.338267. https://www.sciencedirect.com/science/article/pii/S0003267021000933 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bai Y., Huang W., Li Y., Lai C., Huang S., Wang G., et al. Lipidomic alteration of plasma in cured COVID-19 patients using ultra high-performance liquid chromatography with high-resolution mass spectrometry. Biosci Rep. 2021;41(3):1–12. doi: 10.1042/BSR20204305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sindelar M., Stancliffe E., Schwaiger-Haber M., Anbukumar D.S., Albrecht R.A., Liu W.-C., et al. Longitudinal metabolomics of human plasma reveals robust prognostic markers of COVID-19 disease severity. medRxiv Prepr Serv Heal Sci. 2021 doi: 10.1016/j.xcrm.2021.100369. http://www.ncbi.nlm.nih.gov/pubmed/33564793%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7872388 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shi D., Yan R., Lv L., Jiang H., Lu Y., Sheng J., et al. The serum metabolome of COVID-19 patients is distinctive and predictive. Metabolism. 2021;118:154739. doi: 10.1016/j.metabol.2021.154739. https://www.sciencedirect.com/science/article/pii/S0026049521000391 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Páez-Franco J.C., Torres-Ruiz J., Sosa-Hernández V.A., Cervantes-Díaz R., Romero-Ramírez S., Pérez-Fragoso A., et al. Metabolomics analysis reveals a modified amino acid metabolism that correlates with altered oxygen homeostasis in COVID-19 patients. Sci Rep. 2021;11(1):6350. doi: 10.1038/s41598-021-85788-0. http://www.ncbi.nlm.nih.gov/pubmed/33737694%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7973513 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu J., Zhou M., Luo P., Yin Z., Wang S., Liao T., et al. Plasma metabolomic profiling of patients recovered from coronavirus disease 2019 (COVID-19) with pulmonary sequelae 3 Months after discharge. Clin Infect Dis. 2021 Feb 17 doi: 10.1093/cid/ciab147. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Danlos F.-X., Grajeda-Iglesias C., Durand S., Sauvat A., Roumier M., Cantin D., et al. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 2021;12(3):258. doi: 10.1038/s41419-021-03540-y. http://www.ncbi.nlm.nih.gov/pubmed/33707411%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7948172 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schwarz B., Sharma L., Roberts L., Peng X., Bermejo S., Leighton I., et al. Cutting edge: severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome, resulting in dysregulation of eicosanoid immune mediators. J Immunol. 2021;206(2):329–334. doi: 10.4049/jimmunol.2001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Delafiori J., Navarro L.C., Siciliano R.F., De Melo G.C., Busanello E.N.B., Nicolau J.C., et al. Covid-19 automated diagnosis and risk assessment through metabolomics and machine learning. Anal Chem. 2021;93(4):2471–2479. doi: 10.1021/acs.analchem.0c04497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao N., Nie M., Pang H., Wang B., Hu J., Meng X., et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat Commun. 2021;12(1):1618. doi: 10.1038/s41467-021-21907-9. http://www.ncbi.nlm.nih.gov/pubmed/33712622 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mokhtari T., Hassani F., Ghaffari N., Ebrahimi B., Yarahmadi A., Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020 Dec;51(6):613–628. doi: 10.1007/s10735-020-09915-3. https://pubmed.ncbi.nlm.nih.gov/33011887 2020/10/04; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.The hidden long-term cognitive effects of COVID-19 - harvard health blog - harvard health publishing. https://www.health.harvard.edu/blog/the-hidden-long-term-cognitive-effects-of-covid-2020100821133 Available from:
- 148.Rodríguez Y., Novelli L., Rojas M., Santis M De, Acosta-ampudia Y., Ansari A.A., et al. the company ’ s public news and information; 2020. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19 . The COVID-19 resource centre is hosted on Elsevier Connect. [January] [Google Scholar]
- 149.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 May 23;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., et al. Guillain-barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Filippi C.M., von Herrath M.G. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008 Nov;57(11):2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen J., Zhang H., Chen P., Lin Q., Zhu X., Zhang L., et al. Correlation between systemic lupus erythematosus and cytomegalovirus infection detected by different methods. Clin Rheumatol. 2015 Apr;34(4):691–698. doi: 10.1007/s10067-015-2868-3. [DOI] [PubMed] [Google Scholar]
- 153.Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020 Oct;45:102377. doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020 Jun 3;369:m2094. doi: 10.1136/bmj.m2094. http://www.bmj.com/content/369/bmj.m2094.abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Costenbader K.H., Karlson E.W. Epstein-Barr virus and rheumatoid arthritis: is there a link? Arthritis Res Ther. 2006;8(1):204. doi: 10.1186/ar1893. https://pubmed.ncbi.nlm.nih.gov/16542469 2006/01/16; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet (London, England) 2020 Jun;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mantovani Cardoso E., Hundal J., Feterman D., Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol. 2020 Sep;39(9):2811–2815. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lazarian G., Quinquenel A., Bellal M., Siavellis J., Jacquy C., Re D., et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020 Jul 1;190(1):29–31. doi: 10.1111/bjh.16794. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020 May;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., et al. Clinical features of 85 fatal cases of COVID-19 from wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020 Jun;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2020 Jul;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Theofilopoulos A.N., Dummer W., Kono D.H. T cell homeostasis and systemic autoimmunity. J Clin Invest. 2001;108(3):335–340. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maeda K., Baba Y., Nagai Y., Miyazaki K., Malykhin A., Nakamura K., et al. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005 Aug 1;106(3):879–885. doi: 10.1182/blood-2005-02-0456. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Papadopoulos V., Koutroulos M.-V., Zikoudi D.-G., Bakola S.-A., Avramidou P., Touzlatzi N., et al. Acute Metabolic Emergencies in Diabetes and COVID-19: a systematic review and meta-analysis of case reports. medRxiv. 2021 Jan 1 doi: 10.1007/s13340-021-00502-9. http://medrxiv.org/content/early/2021/01/14/2021.01.10.21249550.abstract 2021.01.10.21249550. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sarkar S., Rapista N., Jean L.-G. Corona virus disease-19-induced acute liver failure leading to severe metabolic acidosis. Chest. 2020 Oct;158(4):A1002. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7548662/ 2020/10/12; Available from: [Google Scholar]
- 168.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007 Jan 25;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. Available from. [DOI] [PubMed] [Google Scholar]
- 169.Velavan T.P., Kieu Linh L.T., Kreidenweiss A., Gabor J., Krishna S., Kremsner P.G. Longitudinal monitoring of lactate in hospitalized and ambulatory COVID-19 patients. Am J Trop Med Hyg. 2021 Jan 11;104(3):1041–1044. doi: 10.4269/ajtmh.20-1282. https://pubmed.ncbi.nlm.nih.gov/33432902 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Information note on COVID-19 and NCDs. https://www.who.int/publications/m/item/covid-19-and-ncds Available from:
- 171.Yu E., Malik V.S., Hu F.B. Cardiovascular disease prevention by diet modification: JACC health promotion series. J Am Coll Cardiol. 2018;72(8):914–926. doi: 10.1016/j.jacc.2018.02.085. https://www.sciencedirect.com/science/article/pii/S0735109718352926 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsoupras A., Lordan R., Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients. 2018;10 doi: 10.3390/nu10050604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Papamichael M.M., Katsardis C., Lambert K., Tsoukalas D., Koutsilieris M., Erbas B., et al. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: a randomised controlled trial. J Hum Nutr Diet Off J Br Diet Assoc. 2019 Apr;32(2):185–197. doi: 10.1111/jhn.12609. [DOI] [PubMed] [Google Scholar]
- 174.Higgins V., Sohaei D., Diamandis E.P., Prassas I. COVID-19: from3 an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2020:1–23. doi: 10.1080/10408363.2020.1860895. Available from. [DOI] [PubMed] [Google Scholar]
- 175.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2021;2020:5857825. doi: 10.1038/d41586-020-02598-6. https://www.nature.com/articles/d41586-020-02598-6 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 176.Weerahandi H., Hochman K.A., Simon E., Blaum C., Chodosh J., Duan E., et al. medRxiv. medRxiv; 2020. Post-discharge health status and symptoms in patients with severe COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010 Sep;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu Q., Zhou L., Sun X., Yan Z., Hu C., Wu J., et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7(1):9110. doi: 10.1038/s41598-017-09536-z. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang M., Parker A.M., Bienvenu O.J., Dinglas V.D., Colantuoni E., Hopkins R.O., et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016 May;44(5):954–965. doi: 10.1097/CCM.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020 Jul;87:115–119. doi: 10.1016/j.bbi.2020.04.077. https://pubmed.ncbi.nlm.nih.gov/32360439 2020/04/28; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Haley M.J., White C.S., Roberts D., O'Toole K., Cunningham C.J., Rivers-Auty J., et al. Stroke induces prolonged changes in lipid metabolism, the liver and body composition in mice. Transl Stroke Res. 2020;11(4):837–850. doi: 10.1007/s12975-019-00763-2. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rofe A.M., Bourgeois C.S., Washington J.M., Philcox J.C., Coyle P. Metabolic consequences of methotrexate therapy in tumour-bearing rats. Immunol Cell Biol. 1994 Feb;72(1):43–48. doi: 10.1038/icb.1994.7. [DOI] [PubMed] [Google Scholar]
- 183.Zhang Y., Guo R., Kim S.H., Shah H., Zhang S., Liang J.H., et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-21903-z. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Balashova E.E., Maslov D.L., Lokhov P.G. A metabolomics approach to pharmacotherapy personalization. J Personalized Med. 2018;8(3) doi: 10.3390/jpm8030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miller I.J., Peters S.R., Overmyer K.A., Paulson B.R., Westphall M.S., Coon J.J. Real-time health monitoring through urine metabolomics. NPJ Digit Med. 2019;2(1) doi: 10.1038/s41746-019-0185-y. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brusselmans L., Arnouts L., Millevert C., Vandersnickt J., van Meerbeeck J.P., Lamote K. Breath analysis as a diagnostic and screening tool for malignant pleural mesothelioma: a systematic review. Transl Lung Cancer Res. 2018 Oct;7(5):520–536. doi: 10.21037/tlcr.2018.04.09. https://pubmed.ncbi.nlm.nih.gov/30450290 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12 doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med. 2020 Nov 12 doi: 10.1136/postgradmedj-2020-139065. http://pmj.bmj.com/content/early/2020/11/12/postgradmedj-2020-139065.abstract postgradmedj-2020-139065. Available from: [DOI] [PubMed] [Google Scholar]
- 189.Blumberg J.B., Frei B.B., Fulgoni V.L., Weaver C.M., Zeisel S.H. Impact of frequency of multi-vitamin/multi-mineral supplement intake on nutritional adequacy and nutrient deficiencies in U.S. adults. Nutrients. 2017;9(8) doi: 10.3390/nu9080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2(7):572–585. doi: 10.1038/s42255-020-0237-2. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Margină D., Ungurianu A., Purdel C., Tsoukalas D., Sarandi E., Thanasoula M., et al. Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. Int J Environ Res Publ Health. 2020;17(11):1–30. doi: 10.3390/ijerph17114135. [DOI] [PMC free article] [PubMed] [Google Scholar]