Abstract
Furan-2-carboxylic acid was used as a starting material for the synthesis of dehydro-homopilopic acid. Esterification, hydrogenation and enzymatic hydrolysis followed by the reduction of Weinreb amides and a single-step attachment of a 1-methyl-imidazole residue allowed for the concise synthesis of both enantiomers of pilocarpine.
Keywords: pilocarpine, enzymatic resolution, alkaloids
1. Introduction
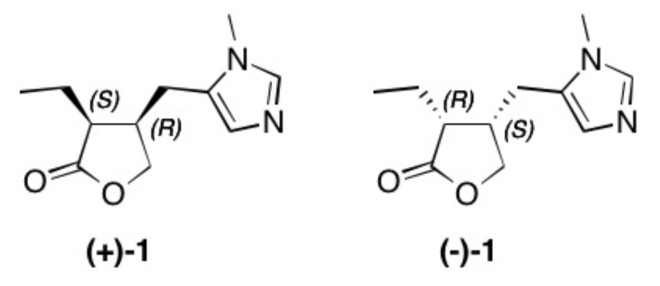
Pilocarpine (+)-1 (Figure 1) is the main alkaloid of the South American tree Pilocarpus jaborandi, and it is usually isolated from its leaves. Pilocarpine is used as a cholinergic [1,2,3] parasympathomimetic [4,5,6,7,8], hidrotic and miotic agent [9,10]; in ophthalmology, it is used against glaucoma [11,12,13] and also acts as an antagonist [14,15,16] to atropine and as an inhibitor of the enzyme carbonic anhydrase II [17,18,19]. Moreover, a deficiency of carbonic anhydrase in humans is linked to osteopetrosis; thereby, osteoclasts are unable to perform bone resorption in a normal way [20]. Recently, some imidazolic alkaloids have been identified as promising targets as possible inhibitors of the main protease of SARS-CoV-2 [21]. The compound was first isolated by Petit and Polonovski [22,23] as well as by Gerrard [24] and Hardy [25], its structure was determined based on the studies of Jowett [26] and Zav’yalov [27], and the absolute configuration of this alkaloid was determined by Hill and Barcza [28]. A biosynthetic pathway has been suggested in 2015 [29].
Figure 1.
Structure of natural occurring pilocarpine (+)-1 and its enantiomer (–)-1.
However, the content of (+)-1 is very low in the leaves of different species of trees of the genus Pilocarpus; it ranges from 0.12–0.6% in P. racemosous [30,31,32] and from 0.45% in P. microphyllus [33,34,35] and P. pennatifolius [36,37] to 0.8% in P. jaborandi [38,39]. Due to the low content of the alkaloid in the plant material as well as a laborious extraction procedure [40], a number of syntheses have been described for this interesting molecule [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Many of them, however, are multi-step and laborious, often of low yield, or they yield a racemic product. In the context of our own studies on inhibitors of carbonic anhydrases, we were interested in an efficient access to (+)-1 but also its enantiomer (–)-1. This approach uses, in part, a chemoenzymatic strategy that we have previously [42] used to synthesize natural occurring (+)-1.
2. Results
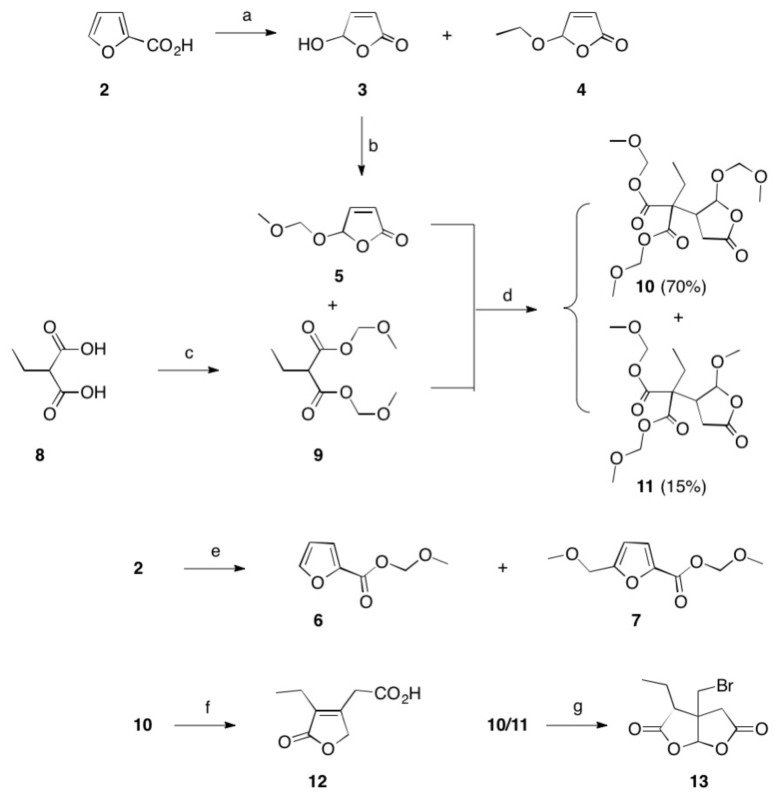
In our own synthetic strategy, the introduction of the 1-methylimidazole residue should be the last step of the synthesis, since this residue—as previously shown [42] —can be introduced in a single step and under very mild conditions using a Leusen imidazole synthesis [58,59,60,61]. However, a prerequisite for carrying out this planned synthetic sequence is that (homo)-pilopaldehyde or (homo)-pilopic acid must be present in pure enantiomeric and diastereomeric forms. Furthermore, these intermediates should be accessible in high yields. Since we were interested in the synthesis of both pilocarpine enantiomers, a synthesis to racemic (homo)-pilopic acid with a subsequent separation of the enantiomers seemed to be a reasonable strategy. A good starting material should be readily accessible furan-2-carboxylic acid (2).
Thus, 2 (Scheme 1) was photo-oxidized [62] in the presence of oxygen and the sensitizer bengal rosa via an intermediately formed hydroperoxide to yield 3. Thereby, 4 was formed as the by-product (by acetalisation). Since acetals are easy to introduce on the one hand, but also easy to cleave off on the other, they should be our preferred protecting groups in the next steps. Hereby, the methoxymethylation of the free hydroxyl groups using formaldehyde dimethyl acetal seems particularly suitable, since these reactions are known to proceed mostly with very high yields. In previous experiments, furfural [63,64,65] had been oxidized as an alternative to 2, but polymerization reactions were observed to a high extent during its photo-oxidation.
Scheme 1.
Reactions and conditions: (a) hν, Bengal rosa, 8 h, 20 °C, 76% (of 3) and 5% (of 4); (b) CH2(OCH3)2, P4O10, DCM, 20 °C, 5 h, 98%; (c) CH2(OCH3)2, P4O10, DCM, 20 °C, 5 h, 99%; (d) THF, Na, 25 °C, 15 h, 72%; (e) CH2(OCH3)2, P4O10, DCM, 20 °C, 5 h, 77% (of 6) and 19% (of 7); (f) HBr, reflux, 2 d, 83%; (g) HBr, reflux, 4 d, 4%.
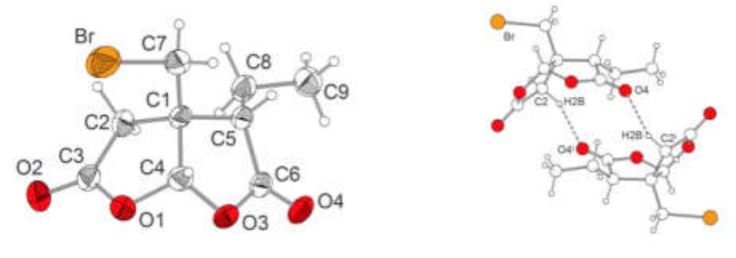
The reaction of 3 with formaldehyde dimethyl acetal in the presence of solid P4O10 [66] produced an almost quantitative yield of methoxymethylated 5. Interestingly enough, applying the same conditions from 2, ester 6 was formed together with 7 as a minor byproduct. These reaction conditions can therefore be used not only to very efficiently introduce a protecting group on a hydroxyl group but also to protect the carboxylic acids. 2-Ethylmalonic acid (8) was also neatly transformed into its bis(methoxymethylester) 9. The Michael addition of the latter with 5 produced 10 in a 70% isolated yield and 15% of 11 as a by-product; the latter product was formed by transacetalisation. A Stobbe condensation [47] of 10 for 2 days produced dehydrohomopilopic acid (12) together with traces of a side product 13. To elucidate the formation and structure of 13, a mixture of 10 and 11 was heated under reflux with aqueous HBr (48%) for 4 days, and 13 was obtained as colorless crystals. The results from the mass spectrometry showed 13 to hold a bromine substituent, and from the interpretation of 1H and the 13C-NMR spectra, 13 was assigned the structure of a 3a-(bromomethyl)-3-ethyl-dihydro-[2,3-b]furan-(3H, 4H)-2,5-dione. For verification of this structure, suitable crystals were grown that were subjected to a single crystal X-ray analysis, whose results are depicted in Table 1 and Figure 2. The formation of 13 remains unclear but should start by a cleavage of the ester moieties of 11 by HBr followed by a decarboxylation of the substituted malonic acid and intramolecular lactonization.
Table 1.
Crystallographic data for compound 13.
| Compound | 13 |
|---|---|
| Molecular formula | C9H11BrO4 |
| Formula weight /g·mol–1 | 263.09 |
| Crystal system | monoclinic |
| Space group | P 21/n |
| a /pm | 633.6(3) |
| b /pm | 1269.4(3) |
| c /pm | 1208.9(3) |
| β/° | 94.30(4)° |
| Cell volume /nm3 | 0.9696(6) |
| Molecules per cell Z | 4 |
| Calc. density ρ /g·cm–3 | 1.802 |
| μ (Mo-Ka) /mm–1 | 4.225 |
| Crystal size/mm | 0.6 × 0.56 × 0.4 |
| Diffractometer | STOE STADI IV |
| T /K | 293(2) |
| θ range /° | 2.33–25.96 |
| Absorption correction | empirical |
| Reflections collected | 5393 |
| Reflections unique | 1887 |
| Reflections with Fo > 4σ(Fo) | 1680 |
| Completeness of dataset /% | 99.9 |
| R int | 0.0435 |
| Parameters | 88 |
| R1 (I > 2σ(I)) | 0.0379 |
| wR2 (all data) | 0.0998 |
| GooF (F2) | 1.080 |
Figure 2.
Molecular structure of compound 13 in the crystal. Thermal ellipsoids with a 50% probability. H atoms with arbitrary radius. C–H…O hydrogen bonds between two symmetrically related enantiomeric molecules. Selected bond lengths (pm) and angles (°): C(1)–C(2) 154.1(4), C(1)–C(4) 154.6(4), C(1)–C(5) 154.6(4), C(1)–C(7) 152.1(4), C(2)–C(3) 149.6(4), C(3)–O(1) 135.9(4), C(3)–O(2) 119.3(4), C(4)–O(1) 141.7(3), C(4)–O(3) 141.7(4), C(5)–C(6) 151.5(4), C(5)–C(8) 153.0(4), C(6)–O(3) 136.4(4), C(6)–O(4) 119.4(4), C(7)–Br 195.4(3), C(8)–C(9) 151.9(4), C(2)–C(1)–C(4) 102.1(2), C(3)–C(2)–C(1) 105.7(3), O(1)–C(3)–C(2) 110.5(2), C(3)–O(1)–C(4) 111.3(2), O(1)–C(4)–C(1) 108.6(2), C(5)–C(1)–C(4) 103.0(2), C(6)–C(5)–C(1) 103.9(2), O(3)–C(6)–C(5) 110.3(2), C(6)–O(3)–C(4) 111.8(2), O(3)–C(4)–C(1) 107.6(2), C(1)–C(7)–Br 112.4(2). Hydrogen bond C(2)–H(2B)–O4i: C(2)…O(4)i 316.7, C(2)–H(2B)–O(4)i 125.98; symmetry operator i: –x, -y+1, -z+1.
Details of the data collection and refinement of the crystal structure of compound 13 are collected in Table 1. Compound 13 (Figure 2) crystallizes in the monoclinic space group P21/n with four formula units per unit cell. Due to its crystallographic symmetry, the crystal structure contains both enantiomers of compound 13 as racemate. Moreover, the enantiomeric pairs are linked by weak C–H…O hydrogen bonds (Figure 2, right). Compound 13 exhibits C–C, C–O and C–Br bond lengths that are in the expected range. Both the lactone rings C1–C2–C3–O1–C4 and C1–C5–C6–O3–C4 exhibit envelope conformation with the central C1 atom at the flap position. However, C1 differs only 8.2 and 11.1 pm, respectively, from the corresponding mean planes. The interplanar angle between both lactone rings is 64.4°.
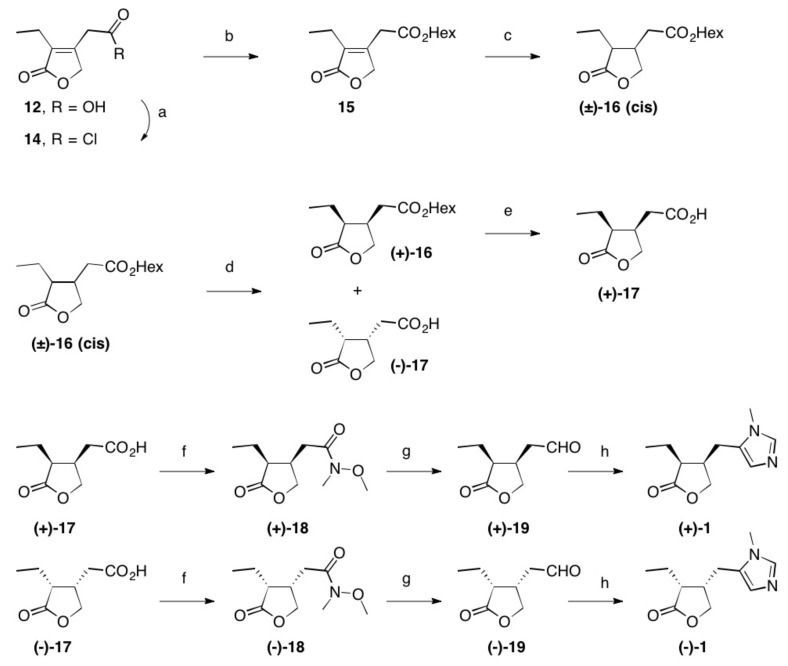
It seems convenient to perform a resolution of the racemate at this stage. An enantioselective hydrolysis of an ester with a suitable enzyme appeared to be particularly attractive. Thus, 12 was activated using thionyl chloride (Scheme 2) to afford in situ the acid chloride 14, the reaction of which with n-hexanol produced ester 15.
Scheme 2.
Reactions and conditions: (a) SOCl2, reflux, 3 h, quant.; (b) Hex-OH, reflux, 16 h, 98%; (c) Rh/Al2O3, H2 (1 at), THF, 5 d, quant.; (d) Lipase PS, pH = 7.0, 2 d, 22 °C, 48% (of (±)-16) and 42% (of (–)-17); (e) PLE, pH = 7.0, 22 °C, 2 d, 96%; (f) N-methylmorpholine, iBu-chloroformate, N,O-dimethylhydroxylamine hydrochloride, 23 °C, 1 d, 84% (of (+)-18) and 85% of (–)-18); (g) LiAlH4, Et2O, 23 °C, 30 min, 95% (of (+)-19) and 95% of (–)-19; (h) CH3NH2, TosMic, DCM, benzene, NEt3, 7 d, 23 °C, 59% (of (+1)-1 and 60% of (–)-1; Hex stands for n-hexyl.
The hydrogenation of 15 using Rh/Al2O3 for 5 days at atmospheric pressure produced cis-configurated (±)-16; its cis-configuration was confirmed by NMR. This ester was reacted under pH-stat conditions at pH=7 with various hydrolytic enzymes (acylase from Aspergillus sp., chymotrypsin A4, Fungamyl 800L®, Lipase from Candida rugosa, Lipase from Candida lipolytica, Lipase from Mucor miehei, Penicillium roqueforti, Rhizopus arrhizus and from Rh. delemar, lipase type II from porcine pancreas, lipase AY (Amano), lipase F AP15 (Amano), lipase M10 (Amano), Lipase OF (Amano), lipase P (Amano), lipase PS (Amano), lipolase 100L®, lipozyme®, novozym 450®, pancreatin, pronase, proteases XXIII, XIV, XXI, pig liver esterase (PLE), subitilisin, Thermo Cat E 003 to 015®). Thereby, hydrolysis by lipase PS of Amano proved suitable, and (+)-16 and (–)-17 were each obtained in excellent yields. Their enantiomeric purity was determined to be > 99% by HPLC using chiral phases (Chiralcel OC and Chiralpak AS).
While hydrolysis of (+)-16 using aq. NaOH resulted in partial isomerization, enzymatic hydrolysis using pig liver esterase (PLE) at pH = 7 readily afforded (+)-17 with an enantiomeric excess of ee > 99.
Thus, both enantiomers of homopilopic acid 17 are readily accessible in pure enantiomeric form. For the synthesis of (–)-1 and (+)-1, 17 was reacted with N-methylmorpholine, isobutyl chloroformate and N,O-dimethylhydroxylamine hydrochloride [67,68,69,70,71,72] to obtain the Weinreb acetamides (–)-18 and (–)-18 in 85% and 84% yields, respectively. Their reduction with LiAlH4 produced aldehydes [44,56] (–)-19 and (+)-19, which, after reaction with methylamine/p-tosylmethylisocyanide (TosMic) [58,59] in the presence of triethylamine, produced (–)-1 and (+)-1. Their enantiomeric purity was determined via HPLC using a chiral phase (Chiralcel OC) with >99% in each case.
3. Conclusions
Furan-2-carboxylic acid served as a valuable starting material for the straightforward synthesis of dehydro-homopilopic acid. Esterification, hydrogenation and enzymatic hydrolysis followed by the reduction of Weinreb amides and the single-step attachment of a 1-methyl-imidazole residue allowed for the convenient synthesis of both enantiomers of pilocarpine in good overall yields.
4. Experimental
NMR spectra were recorded using the Varian spectrometers (Darmstadt, Germany) DD2 and VNMRS (400 and 500 MHz, respectively). MS spectra were taken on a Advion expressionL CMS mass spectrometer (Ithaca, NY, USA); positive ion polarity mode, solvent: methanol, solvent flow: 0.2 mL/min, spray voltage: 5.17 kV, source voltage: 77 V, APCI corona discharge: 4.2 μA, capillary temperature: 250 °C, capillary voltage: 180 V, sheath gas: N2). Thin-layer chromatography was performed on pre-coated silica gel plates supplied by Macherey-Nagel (Düren, Germany). IR spectra were recorded on a Spectrum 1000 FT-IR-spectrometer from Perkin Elmer (Rodgau, Germany). The UV/Vis-spectra were recorded on a Lambda 14 spectrometer from Perkin Elmer (Rodgau, Germany); optical rotations were measured at 20 °C using a JASCO-P2000 instrument (JASCO Germany GmbH, Pfungstadt, Germany) The melting points were determined using the Leica hot stage microscope Galen III (Leica Biosystems, Nussloch, Germany) and are uncorrected. The solvents were dried according to usual procedures. Microanalyses were performed with an Elementar Vario EL (CHNS) instrument (Elementar Analysensysteme GmbH, Elementar-Straße 1, D-63505, Langenselbold, Germany). The crystal structure of compound 13 was solved by direct methods (SHELXS) and refined with the SHELXL (2008) program [73]. OLEX2 (2021) was used as graphical user interface [74]. The hydrogen atoms were positioned geometrically using a riding model. The crystal structure drawings were generated with DIAMOND (4.4.0) [75]. CCDC 2086056 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
4.1. 5-Hydroxyfuran-2(5H)-one (3) and 5-ethoxyfuran-2(5H)-one (4)
Oxygen was bubbled through a solution of 2 (10.0 g, 89.2 mmol) and Bengal rosa (350 mg) in ethanol (600 mL); the reaction mixture was irradiated at 20 °C (Heraeus middle pressure Hg lamp; 450 Watt) for 8 h. The solution was filtered through a pad of activated charcoal, and the solvents were removed under diminished pressure to yield a crude product that was subjected to chromatography (SiO2, n-hexane/ethyl acetate 5:1 → 3:1→ 2:1 → 1:1) to yield 3 (6.8 g, 76%) and 4 (517 mg, 5%); the unreacted starting material 2 (1.32 g, 13%) was recovered.
Data for 3: colorless crystals, m.p. 51–53 °C (lit.: [62] 51–54 °C); Rf = 0.17 (n-hexane/ethyl acetate, 1:1); IR (KBr): ν = 3314brm, 3113m, 2924w, 1794m, 1760s, 1738s, 1733s, 1611w, 1446w, 1329m, 1282m, 1183m, 1131s, 1084m, 996s, 917m, 898m, 828s, 822s, 819m, 791m, 703m, 686m, 668m, 656m, 648m, 639m, 634m and 619m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 7.34–7.24 (m, 1H, 4-H), 6.27–6.13 (m, 2H, 3-H, 5-H), 5.58–5.22 (br s, 1H, OH) ppm; 13C-NMR (100 MHz, CDCl3): δ = 171.7 (C-2), 152.3 (C-4), 124.5 (C-3) and 98.9 (C-5) ppm; MS (ESI, MeOH): m/z = 101.0 (60%, [M+H]+); analysis calcd. for C4H4O3 (100.07): C 48.01, H 4.03; found: C 47.87, H 4.19.
Data for 4: off-white oil; b.p. 84–89 °C/8 mm (lit.: [47] 85–89 °C/mm); Rf = 0.61 (n-hexane/ethyl acetate, 1:1); IR (film): ν = 3105w, 2981m, 2934m, 1793s, 1762s, 1614w, 1445w, 1376m, 1350m, 1137s, 1080m, 1040m, 1010s, 960m, 935m, 894m, 818m, 790m, 700m and 687m, cm−1; 1H-NMR (400 MHz, CDCl3): δ = 7.23 (dd, J = 5.7, 1.1 Hz, 1H, 4-H), 6.23 (dd, J = 5.7, 1.2 Hz, 1H, 3-H), 5.95 (dd, J = 1.2, 1.1 Hz, 1H, 5-H), 4.00–3.70 (m, 2H, 6-H) and 1.29 (t, J = 7.1 Hz, 3H, 7-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 170.6 (C-2), 150.5 (C-4), 125.0 (C-3), 103.3 (C-5), 66.2 (C-6) and 15.0 (C-7) ppm; MS (ESI, MeOH): m/z = 129.1 (25%, [M+H]+); analysis calcd. for C6H8O3 (128.13): C 56.24, H 6.29; found: C 56.01, H 6.44.
4.2. 5-(Methoxymethoxy)-furan-2(5H)-one (5)
P4O10 (10 × 2.0 g, in 30 min intervals) was added to a solution of 3 (4.0 g, 40 mmol) in formaldehyde dimethyl acetal (200 mL, 2.2 mol) and dry DCM (100 mL). The solvents were decanted, and the residue was extracted with diethyl ether (3 × 200 mL). The combined organic phases were washed with a saturated solution of NaHCO3, brine, and dried (MgSO4). The solvents were removed, and pure 5 (4.6 g, 98%) was obtained as a colorless oil pure enough for the next step; an analytical sample showed Rf = 0.28 (n-hexane/ethyl acetate, 3:1); IR (film): ν = 2960m, 2830w, 1794s, 1762s, 1654w, 1612w, 1560w, 1507w, 1370m, 1328m, 1220m, 1155s, 1125s, 1074s, 996s and 697m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 7.28 (dd, J = 5.7, 1.1 Hz, 4-H), 6.24 (dd, J = 5.7, 1.2 Hz, 1H, 3-H), 6.15 (dd, J = 1.2, 1.1 Hz, 1H, 5-H), 4.99 (d, J = 6.6 Hz, 1H, 6-Ha), 4.77 (d, J = 6.6 Hz, 1H, 6-Hb) and 3.47 (s, 3H, 7-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 170.1 (C-2), 150.6 (C-4), 124.5 (C-3), 99.5 (C-5), 95.1 (C-6) and 56.4 (C-7) ppm; MS (ESI, MeOH): m/z = 145.2 (32%, [M+H]+); analysis calcd. for C6H8O4 (144.13): C 50.00, H 5.59; found: C 49.88, H 5.73.
4.3. Methoxymethyl Furan-2-carboxylate (6) and Methoxymethyl 5-(methoxymethyl)furan-2-carboxylate (7)
Following the procedure given for the synthesis of 5, from 2 (3.0 g), formaldehyde dimethylacetal (100 mL, 1132 mmol) and P4O10, 6 (3.2 g, 77%) and 7 (1.0 g, 19%) were obtained each as a colorless oil.
Data for 6: Rf = 0.79 (n-hexane/ethyl acetate, 1:1); IR (film): ν = 3140w, 2960m, 2835w, 1730s, 1642w, 1580m, 1570m, 21475s, 1410m, 1390m, 1298s, 1231m, 1215s, 1162s, 1119s, 1070s, 1015s, 932s, 905s, 885s and 762s cm−1; 1H-NMR (400 MHz, CDCl3): δ = 7.60 (dd, J = 1.7, 0.9 Hz, 1H, 5-H), 7.25 (dd, J = 3.5, 0.9 Hz, 1H, 3-H), 6.55 (dd, J = 3.5, 1.7 Hz, 1H, 4-H), 5.45 (s, 2H, 7-H) and 3.55 (s, 3H, 8-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 158.1 (C-5), 146.8 (C-5), 144.3 (C-2), 118.7 (C-3), 110.0 (C-4), 90.8 (C-7) and 57.8 (C-8) ppm; MS (ESI, MeOH): m/z = 157.2 (82%), [M+H]+); analysis calcd. for C7H8O4 (156.14): C 53.85, H 5.16; found: C 53.70, H 5.33.
Data for 7; Rf = 0.65 (n-hexane/ethyl acetate, 1:1); IR (film): ν = 3127w, 2935m, 2829m, 1795w, 1728s, 1640w, 1595w, 1524m, 1468m, 1452m, 1405m, 1370m, 1300s, 1205s, 1165s, 1130s, 1081s, 1022m, 988m, 968m, 945s and 906s cm−1; 1H-NMR (400 MHz, CDCl3): δ = 7.21 (d, J = 3.4 Hz, 1H, 3-H), 6.46 (d, J = 3.4 Hz, 1H, 4-H), 5.45 (s, 2H, 9-H), 4.67 (s, 2H, 6-H), 3.53 (s, 3H, 7-H) and 3.41 (s, 3H, 10-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 157.8 (C-5), 156.5 (C-8), 143.8 (C-2), 119.3 (d, C-3), 110.6 (C-4), 90.6 (C-9), 66.4 (C-6), 58.8 (C-7) and 57.8 (C-10) ppm; MS (ESI, MeOH): m/z = 201.1 (64%, [M+H]+); analysis calcd. for C9H12O5 (200.19): C 54.00, H 6.04; found: C 53.89, H 6.21.
4.4. Bis(methoxymethyl) Ethylpropanedioate (9)
Following the procedure given for the synthesis of 5, from ethylmalonic acid (8, 3.0 g, 22.7 mmol), formaldehyde dimethyl acetal (60 mL, 680 mmol) and P4O10, 9 (4.95 g, 99%) was obtained as a colorless oil (pure enough for the next step); an analytical sample showed Rf = 0.83 (n-hexane/ethyl acetate, 1:1); IR (film): ν = 3636w, 2970s, 2833m, 2087w, 1736s, 1630w, 1546w, 1463s, 1406m, 21462s, 1071s, 1036s and 958s cm−1; 1H-NMR (400 MHz, CDCl3): δ = 5.30 and 5.29 (each s, 4H, 6-H, 8-H), 3.48 (s, 6H, 7-H and 9-H), 3.37 (virt t, J = 7.5 Hz, 1H, 2-H), 2.00 (virt dq, J = 7.5, 7.5 Hz, 2H, 4-H) and 1.02 (t, J = 7.5, 3H, 5-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 168.6 (C-1 and C-3), 91.0 (C-6 and C-8), 57.7 (C-2), 22.1 (C-4), 14.0 (C-7) and 11.8 (C-5) ppm; MS (ESI, MeOH): m/z = 221.3 (17%, [M+H]+); analysis calcd for C9H16O6 (220.22): C 49.09, H 7.32; found: C 49.00, H 7.49.
4.5. Bis(methoxymethyl) Ethyl-[2-(methoxymethyl)-5-oxooxolan-3-yl] propanedioate (10) and Bis(methoxymethyl) ethyl-(2-methoxy-5-oxooxolan-3-yl) propanedioate (11)
Metallic sodium (2.0 g, 8.7 mmol, 31 mol%) was added to a solution of 5 (4.0 g, 27.8 mmol) and 9 (8.0 g, 36.2 mmol) in dry THF (200 mL) at -45 °C under argon, and the mixture was allowed to warm to -15 °C. Careful control of the temperature is mandatory, and the mixture was stirred between 5–15 °C until all of the sodium was dissolved. Stirring at 25 °C was continued for an additional 15 h. The usual aqueous work-up followed by chromatography (SiO2, n-hexane/ethyl acetate, 10:1 → 7:1 → 5:1) produced 10 (7.25 g, 72%) and 11 (1.4 g, 15%).
Data for 10: colorless oil; Rf = 0.64 (n-hexane/ethyl acetate, 1:1); IR (film): ν = 3555w, 2966m, 2835w, 1790s, 1731s, 1468m, 1455m, 1405m, 1307m, 1213s, 1167s, 1080s, 1008s, 965s and 880s cm−1; 1H-NMR (400 MHz, CDCl3): δ = 5.77 (d, J = 1.6 Hz, 5-H), 5.39 (d, J = 5.9 Hz, 1H, 13-Ha), 5.27 (d, J = 5.9 Hz, 1H, 15-Ha), 5.21 (d, J = 5.9 Hz, 1H, 15-Hb), 5.18 (d, J = 5.9 Hz, 13-Hb), 4.92 (d, J = 6.7 Hz, 1H, 6-Ha), 4.65 (d, J = 6.7 Hz, 1H, 6-Hb), 3.48 (s, 6H, 14-H and 16-H), 3.45 (s, 3H, 7-H), 3.07 (ddd, J = 9.6, 3.0, 1.6 Hz, 1H, 4-H), 2.94 (dd, J = 18.3, 9.6 Hz, 3-Ha), 2.62 (dd, J = 18.3, 3.0 Hz, 1H, 3-Hb), 2.08 (q, J = 7.5 Hz, 1H, 11-H) and 0.96 (t, J = 7.5 Hz, 3H, 12-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 174.9 (C-2), 169.2 and 169.0 (C-7 and C-9), 106.0 (C-5), 91.9 (C-12 and C-14), 59.2 (C-8), 58.1 (C-13 and C-15), 56.9 (C-6), 43.9 (C-4), 30.4 (C-3), 26.4 (C-10), 14.0 (C-6) and 8.7 (C-11) ppm; MS (ESI, MeOH): m/z = 365.4 (49%, [M+H]+); analysis calcd. for C15H24O10 (364.35): C 49.45, H 6.64; found: 49.17, H 6.83.
Data for 11: colorless oil; Rf = 0.65; (n-hexane/ethyl acetate, 1:1); IR (film): ν = 2948s, 2835m, 2088w, 1770s, 1740s, 1730s, 1640w, 1548w, 1465m, 1405m, 1455m, 1415m, 1390m, 1358m, 1310m, 1240s, 1210s, 1168s, 1115s, 1010s, 980s, 941s, 880s, 802m and 702m cm-1; 1H-NMR (400 MHz, CDCl3): δ = 5.40–5.25 (m, 4H, 12-H and 14-H), 5.31 (d, J = 4.7 Hz, 1H, 5-H), 3.50 (s, 3H, 6-H), 3.50 (s, 6H, 13-H, 15-H), 3.02 (ddd, J = 10.1, 4.7, 2.8 Hz, 1H, 4-H), 2.95 (dd, J = 17.3, 10.1 Hz, 1H, 3-Ha), 2.65 (dd, J = 17.3, 2.8 Hz, 1H, 3-Hb), 2.05 (q, J = 7.5 Hz, 2H, 10-H) and 0.95 (t, J = 7.5 Hz, 3H, 11-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 174.9 (C-2), 169.2 and 169.0 (C-7 and C-9), 106.0 (C-5), 91.9 (C-12 and C-14), 59.2 (C-8), 58.1 (C-13 and C-15), 56.9 (C-6), 44.0 (C-4), 30.4 (C-3), 26.4 (C-10) and 8.7 (C-11) ppm; MS (ESI, MeOH): m/z = 335.3 (48%, [M+H]+); analysis calcd. for C14H22O9 (334.32): C 50.30, H 6.63; found: 50.04, H 6.87.
4.6. (4-Ethyl-5-oxo-2,5-dihydrofuran-3-yl) Acetic Acid (12, Dehydro-homopilopic acid)
A solution of 10 (3.0 g, 8.23 mmol) was heated under reflux for 2 days in an aqueous solution of HBr (48%, 20 mL) followed by a continuous extraction with diethyl ether (3 × 50 mL, Kutscher–Steudel apparatus). The organic phase was dried (MgSO4), the solvent removed under diminished pressure, and the residue subjected to chromatography (SiO2, n-hexane/ethyl acetate, 2:1 → 1:1 → 0:1; then methanol/ethyl acetate 1:10 → 1:5) to yield 12 (1.16 g, 83%) as a colorless oil; b.p. 170 °C/3.10−2 mbar (lit.: [76] 172–173.5 °C/0.5 Torr); Rf = 0.42 (methanol/ethyl acetate, 1:1); IR (film): ν = 3507brw, 2976s, 2939s, 2880m, 1732s, 1673m, 1446m, 1380m, 1348m, 1200s, 1177s, 1110m, 1159m, 1035s, 950m and 776m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 8.91 (br s, 1H, CO2H), 4.84 (d, J = 1.0 Hz, 2H, 5-Ha,b), 3.54 (s, 2H, 8-H), 2.33 (q, J = 7.6 Hz, 6-H) and 1.11 (t, J = 7.6 Hz, 3H, 7-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 174.1 (s, C-9), 172.9 (C-2), 150.3 (C-4), 131.8 (C-3), 71.5 (C-5), 32.3 (C-8), 17.1 (C-6) and 12.5 (C-7) ppm; MS (ESI, MS): m/z = 171.3 (44%, [M+H]+); analysis calcd. for C8H10O4 (170.16): C 56.47, H 5.92; found: C 56.18, H 6.13.
4.7. 3a-(Bromomethyl)-3-Ethyl-dihydro-[2,3-b]furan-(3H, 4H)-2,5-dione (13)
A solution of 10 and 11 (ratio 1:5, 15.0 g) in aqu. HBr (48%, 200 mL) was heated under reflux for 4 days followed by an extraction for 1 day (3 × 150 mL, Kutscher–Steudel apparatus) and chromatography (SiO2, n-hexane/ethyl acetate, 1:1) to afford 13 (350 mg, 4%) as colorless crystals; m.p. 132–134 °C; Rf = 0.45 (n-hexane/ethyl acetate 1:1); IR (KBr): ν = 3560m, 2005s, 2975s, 2956m, 2940s, 2881m, 1784s, 1461s, 1442m, 1402s, 1370s, 1354s, 1330s, 1295s, 1274s, 1255m, 1229m, 1188s, 1153s, 1138s, 1095s, 1068s, 1042s, 1011s, 941s, 917s, 883s, 867s, 851s, 780m, 742m, 716m, 707s, 658m, 620s, 572m, 560m, 499m and 488m cm−1; 1H-NMR (400 MHz, CD3OD): δ = 6.10 (s, 1H, 3-Hb), 3.84 (d, J = 1.1 Hz, 2H, 9-H), 2.30 (d, J = 18.7 Hz, 1H, 4-Ha), 2.87 (t, J = 7.2 Hz, 1H, 3-H), 2.68 (d, J = 18.7 Hz, 1H, 4-Hb), 1.94–1.84 (m, 1H, 7-Ha), 1.66–1.57 (m, 1H, 7-Hb) and 1.13 (t = 7.3 Hz, 3H, 8-H) ppm; 13C-NMR (100 MHz, CD3OD): δ = 175.3 (C-2), 174.9 (C-5), 105.7 (C-3b), 52.3 (C-3a), 49.4 (C-3), 37.6 (C-9), 33.6 (C-4), 21.6 (C-7) and 12.7 (C-8) ppm; MS (ESI, MeOH): m/z = 263.0 and 265.1 (47% and 48%, [M+H]+); analysis calcd. for C9H11BrO4 (263.09): C 41.09, H 4.21; found: C 40.79, H 4.32.
4.8. Hexyl (4-Ethyl-5-oxo-2,5-dihydrofuran-3-yl)acetate (15)
The reaction of 12 (1.0 g, 5.88 mmol) with thionyl chloride (2.0 mL, 10 mmol) and hexanol (20 mL, 160 mmol) was performed for 16 h under reflux followed by usual workup and chromatography (SiO2, n-hexane/ethyl acetate, 5:1) and produced 15 (1.45 g, 98%) as a colorless oil (pure enough for the next step); an analytical sample showed Rf = 0.28 (n-hexane/ethyl acetate, 5:1); IR (film): ν = 3630w, 2960s, 2935s, 2861m, 1756s, 1676m, 1654w, 1455m, 1380m, 1341m, 1267m, 1199s, 1178s, 1106m, 1090m, 1060m and 1036s cm−1; 1H-NMR (400 MHz, CDCl3): δ = 4.80 (s, 2H, 5-H), 4.12 (t, J = 6.7 Hz, 2H, 10-H), 3.46 (s, 2H, 8-H), 2.32 (q, J = 7.6 Hz, 6-H), 1.62 (q, 1H, J = 7.0 Hz, 11-H), 1.31 (br s, 6H, 12-H, 13-H, 14-H), 1.11 (t, J = 7.6 Hz, 3H, 7-H) and 0.90 (t, J = 6.7 Hz, 3H, 15-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 174.1 (C-9), 168.4 (C-2), 151.2 (C-4), 131.4 (C-3), 71.6 (C-5), 65.9 (C-10), 32.5 (C-8), 31.4 (C-11), 28.5 (C-12), 25.5 (C-13), 22.5 (C-14), 17.10 (C-6), 13.9 (C-15) and 12.6 (C-7) ppm; MS (ESI, MeOH): m/z = 255.4 (41%, [M+H]+); analysis calcd. for C14H22O4 (254.33): C 66.12, H 8.72; found: C 65.87, H 8.92.
4.9. (±)-Hexyl [(3RS, 4SR)-4-Ethyl-5-oxooxolan-3-yl] acetate [(±)-16]
The hydrogenation (1 atm) of 15 (1.23 g, 4.82 mmol) with Rh/Al2O3 (5%, 500 mg) in dry THF (15 mL) for 5 days followed by usual workup produced (±)-16 (1.23 g, quant.) as a colorless oil; an analytical sample showed Rf = 0.52 (n-hexane/ethyl acetate, 3:1); IR (film): ν = 2960s, 2935s, 2861m, 1775s, 1735s, 1469m, 1375m, 1324m, 1215m, 1175s, 1111m, 1060m, 1030m and 987m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 4.20 (ddd, J = 9.9, 5.9, 0.4 Hz, 1H, 5-Ha), 4.11 (dd, J = 9.6, 3.7 Hz, 1H, 5-Hb), 4.10 (t, J = 6.7 Hz, 2H, 10-H), 3.04–2.98 (m, 1H, 4-H), 2.56 (dd, J = 14.8, 8.0 Hz, 1H, 3-H), 2.47 (dd, J = 16.5, 4.7 Hz, 1H, 8-Ha), 2.30 (dd, J = 16.5, 10.5 Hz, 1H, 8-Hb), 1.85–1.75 (m, 1H, 6-Ha), 1.61 (q, J = 7.0 Hz, 2H, 11-H), 1.53–1.42 (m, 1H, 6-Hb), 1.42–1.30 (m, 6H, 12-H, 13-H, 14-H), 1.05 (t, J = 7.4 Hz, 3H, 7-H) and 0.91 (t, J = 6.7 Hz, 15-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 178.0 (C-9), 171.7 (C-2), 70.9 (C-5), 65.2 (C-10), 44.2 (C-3), 35. 2 (C-4), 32.0 (C-8), 31.4 (C-11), 28.5 (C-12), 25.6 (C-13), 22.5 (C-14), 18.5 (C-6), 14.0 (C-15) and 12.1 (C-7) ppm; MS (ESI, MeOH): m/z = 257.3 (41%, [M+H]+); analysis calcd. for C14H24O4 (256.34): C 65.60, H 9.44; found: C 65.41, H 9.67.
4.10. (+)-Hexyl 2-[(3S, 4R)-4-Ethyl-5-oxooxolan-3-yl]acetate [(+)-16] and (–)-2-[3R, 4S)-4-Ethyl-5-oxooxolan-3-yl] acetic acid [(–)-17]
In a pH-stat (Metrohm), a solution of 16 (1.26 g, 4.92 mmol) in distilled ater (80 mL) was stirred for 2 days at 22 °C with Lipase PS “Amano” (2.4 g), keeping the pH = 7 constant (27.35 mL, 0.1n NaOH). For workup, pH was adjusted to 6.5 with diluted with aqueous HCl (5%), and the reaction mixture was extracted with diethyl ether (3 × 200 mL). The organic phase was dried (MgSO4), the solvent was evaporated under diminished pressure, and the residue was subjected to chromatography (SiO2, n-hexane/ethyl acetate, 7:1 → 5:1 → 3:1 → 0:1) to yield (+)-16 (605 mg, 48%) and (–)-17 (398 mg, 42%).
Data for (+)-16: colorless oil; Rf = 0.52 (n-hexane/ethyl acetate, 3:1), [α]D = +64.6° (c 1.0, CHCl3), [lit.: [42] [α]D = +64.5° (c 1.26, CHCl3)]; ee > 99% by HPLC (Chiralcel OC; n-hexane/ethanol, 95:5, 0.6 mL/min, λ = 225 nm, tR (+) = 22.5 min, tR (–) = 124.7 min; Chiralpak AS (n-hexane/isopropanol, 98:2, 1 mL/min) tR(+) = 17.0 min and tR(–) = 22.3 min; IR (film): ν = 3566br m, 2971m, 1760s, 1700s, 1435m, 1406m, 1375m, 1326m, 1250m, 1177s, 1115s, 1056m, 1031m, 990s, 911m, 731m and 680m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 8.93 (br s, 1H, CO2H), 4.34 (dd, J = 9.5, 5.1 Hz, 5-Ha), 4.12 (dd, J = 9.5, 3.3 Hz, 1H, 5-Hb), 3.06–2.95 (m, 1H, 4-H), 2.60 (dd, J = 14.8, 8.1 Hz, 1H, 3-H), 2.50 (dd, J = 105, 4.6 Hz, 1H, 8-Ha), 2.35 (dd, J = 16.7, 10.5 Hz, 1H, 8-Hb), 1.90–1.75 (m, 1H, 6-Ha), 1.55–1.35 (m, 1H, 6-Hb) and 1.07 (t, J = 7.4 Hz, 3H, 7-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 177.9 (C-9), 171.7 (C-2), 70.8 (C-5), 65.2 (C-10), 44.0 (C-3), 35.0 (C-4), 32.0 (C-8), 31.4 (C-11), 28.5 (C-12), 25.6 (C-13), 22.5 (C-14), 18.5 (C-6), 14.0 (C-15) and 12.1 (C-7) ppm; MS (ESI, MeOH): m/z = 257.3 (56%, [M+H]+); analysis calcd. for C14H24O4 (256.34): C 65.60, H 9.44; found: C 65.44, H 9.61.
Data for (–)-17: colorless oil [53,54]; Rf = 0.90 (methanol/ethyl acetate), 1:4); [α]D = –87.1° (c 1.0, CHCl3); ee > 99% (by HPLC); IR (film): ν = 3566br m, 2970m, 1761s, 1700s, 1435m, 1404m, 1375m, 1250m, 1176s, 1118s, 1056m, 1030m, 990s, 911m, 730m and 680m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 8.91 (br s, 1H, CO2H), 4.35 (dd, J = 9.5, 5.1 Hz, 1H, 5-Ha), 4.12 (dd, J = 9.5, 3.3 Hz, 1H, 5-Hb), 3.03–2.95 (m, 1H, 4-H), 2.58 (dd, J = 14.8, 8.1Hz, 1H, 3-H), 2.50 (dd, J = 10.5, 4.6 Hz, 1H, 8-Ha), 2.35 (dd, J = 16.7, 10.5 Hz, 8-Hb), 1.90–1.75 (m, 1H, 6-Ha), 1.55–1.35 (m, 1H, 6-Hb) and 1.05 (t, J = 7.4 Hz, 1H, 7-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 177.9 (CO2H), 177.0 (CO), 70.8 (C-5), 44.0 (C-3), 35.0 (C-4), 31.7 (C-8), 18.5 (C-6), 12.0 (Me); MS (ESI, MeOH): m/z = 173.2 (27%, [M+H]+); analysis calcd. for C8H12O4 (172.18): C 55.81, H 7.02; found: C 55.68, H 7.17.
4.11. (+)-2-[3S, 4R)-4-Ethyl-5-oxooxolan-3-yl] acetic acid [(+)-17]
In a pH-stat (Metrohm), a solution of (+)-16 (6.7 g, 26.1 mmol) in dist. water (300 mL) was stirred for 8 h at 22 °C with PLE (300 μL, 30 mg/mL, Boehringer), keeping the pH = 7 constant (263.3 mL, 0.1n NaOH). For workup, pH was adjusted to 6.5 with diluted with aq. HCl (5%) and extracted with diethyl ether (3 × 200 mL). The organic phase was dried (MgSO4), the solvent was evaporated under diminished pressure, and the residue was subjected to chromatography (SiO2, n-hexane/ethyl acetate, 7:1 → 5:1 → 3:1 → 0:1) to yield (+)-17 (4.32g, 96%); [α]D = +87.9° (c 1.1, CHCl3) [lit.: [42[α]D = +73.2–81.5° (CHCl3)]; Rf = 0.90 (methanol/ethyl acetate; ee > 99% (by HPLC); IR, 1H-NMR and 13C-NMR as well as MS (ESI, MeOH) identical to its enantiomer (vide supra); analysis calcd. for C8H12O4 (172.18): C 59.81, H 7.02; found: C 59.71, H 7.33.
4.12. (–) 2-[(3R, 4S)-4-Ethyl-5-oxooxolan-3-yl)-N-methoxy-N-methylacetamide [(–)-18]
To a solution of (–)-17 (3.0 g, 17.4 mmol) in ethyl acetate (100 mL) at 0 °C, N-methylmorpholine (NMM, 1.9 mL, 17.45 mmol; in 25 mL dry ethyl acetate) was slowly added followed by the addition of isobutyl chloroformate (2.28 mL, 17.4 mmol, in 3 mL dry ethyl acetate). After stirring for 15 min, N,O-dimethylhydroxylamine hydrochloride (1.9 g, 19.2 mmol) was added followed by the addition of another portion NMM (1.9 mL, 17.45 mmol in 25 mL dry ethyl acetate). After stirring at 0 °C for 30 min and stirring at 23 °C for 1 day, the mixture was washed with water (5 mL), aq. citric acid (10%, 5 mL) and brine (10 mL), the organic layers were dried (MgSO4). The solvents were removed under diminished pressure, and the residue was purified by chromatography (SiO2, n-hexane/ethyl acetate 5:1 → 3:1 → 2:1 → 0:1) to yield (–)-18 (3.18 g, 85%) was a colorless oil; Rf = 0.30 (n-hexane/ethyl acetate, 1:1); [α]D = –113.8° (c 0.9, CHCl3), IR (film): ν = 2970m, 2942m, 2880m, 1770s, 1660s, 1650s, 1435m, 1420m, 1385m, 1260w, 1175s, 1117m, 1055m, 1005m, 985m and 946m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 4.30 (dd, J = 9.3, 5.7 Hz, 1H, 5-Ha), 4.10 (dd, J = 9.3, 2.7 Hz, 1H, 5-Hb), 3.72 (s, 3H, NOMe), 3.21 (s, 3H, NMe), 3.15–3.00 (m, 1H, 4-H), 2.59 (dd, J = 7.3, 7.3 Hz, 1H, 3-H), 2.52 (dd, J = 10.6, 6.2 Hz, 1H, 8-Ha), 2.40 (dd, J = 16.7, 10.6 Hz, 1H, 8-Hb), 1.85–1.74 (m, 21H, 6-Ha), 1.55-1.45 (m, 1H, 6-Hb) and 1.05 (t, J = 7.4 Hz, 3H,7-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 178.2 (CON), 171.8 (CO), 71.3 (C-5), 61.3 (NOMe), 44.2 (C-3), 34.6 (C-4), 34.6 (NMe), 29.3 (C-8), 18.6 (C-6) and 12.1 (C-7) ppm; MS (ESI, MeOH): m/z = 216.4 (63%, [M+H]+); analysis calcd. for C10H17NO4 (215.25): C 55.80, H 7.96, N 6.51; found: C 55.61, H 8.13, N 6.38.
4.13. (+)2-[(3S, 4R)-4-Ethyl-5-oxooxolan-3-yl)-N-methoxy-N-methylacetamide [(+)-18]
Following the procedure given for its enantiomer, from (+)-17 (6.0 g, 34.8 mmol) (+)-18 (6.35 g, 84%) was obtained as a colorless oil; [α]D = +114.1° (c 1.0, CHCl3); Rf = 0.30 (SiO2, n-hexane/ethyl acetate); IR (film), 1H-NMR, 13C-NMR and MS (ESI, MeOH) were identical to the enantiomer (vide supra); analysis calcd. for C10H17NO4 (215.25): C 55.80, H 7.96; found: C 55.63, H 8.17.
4.14. (–)-2-[(3R, 4S)-4-Ethyl-5-oxooxolan-3-yl]-acetaldehyde [(–)-19]
The reduction of (–)-18 (1.96 g, 9.1 mmol) with lithium aluminium hydride (0.42 g, 11.1 mmol) in dry diethyl ether (200 mL) at -45 °C was followed by an additional stirring at 23 °C for 30 min and followed by usual aqueous workup, extraction (4 × 100 mL) and chromatography (SiO2, n-hexane/ethyl acetate, 5:1 → 2:1 → 1:1) to produce (–)-19 (1.35 g, 95%) as a colorless oil; [56] Rf = 0.45 (SiO2, n-hexane/ethyl acetate, 1:1), [α]D = –110.3° (c 0.7, CHCl3); IR (film): ν = 3419s, 2960s, 2879s, 1770m, 1731s, 1463m, 1382m, 1181m, 1155m, 1120s, 1070s, 1036s, 940s, 901m and 861m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 9.82 (s, 1H, 9-H), 4.32 (ddd, J = 9.4, 5.8, 0.9 Hz, 1H, 5-Ha), 4.00 (dd, J = 9.4, 3.2 Hz, 1H, 5-Hb), 3.12–3.05 (m, 1H, 4-H), 2.66 (dd, J = 18.7, 4.5 Hz, 1H, 3-H), 2.56 (dd, J = 10.3, 3.7 Hz, 1H, 8-Ha), 2.51 (dd, J = 10.3, 1.6 Hz, 1H, 8-Hb), 1.85–1.77 (m, 1H, 6-Ha), 1.50–1.34 (m, 1H, 6-Hb) and 1.05 (t, J = 7.4 Hz, 3H, 7-H) ppm; 13C-NMR (100 MHz, CDCl3): δ = 199.2 (CHO), 177.7 (CO), 70.5 (C-5), 44.2 (C-3), 34.6 (C-4), 26.5 (C-8), 18.6 (C-6) and 12.0 (C-7) ppm; MS (ESI, MeOH): m/z = 157.1 (85%, [M+H]+); analysis calcd. for C8H12O3 (156.18): C 61.52, H 7.74; found: 61.39, H 8.04.
4.15. (+)-2-[(3S, 4R)-4-Ethyl-5-oxooxolan-3-yl]-acetaldehyde [(+)-19]
Following the procedure given for the enantiomer, (+)-19 (0.67, 95%) was obtained as a colorless oil; Rf = 0.45 (SiO2, n-hexane/ethyl acetate, 1:1); [α]D = +109.3° (c 0.3, CHCl3); IR (film), 1H-NMR, 13C-NMR and MS (ESI, MeOH) were identical to the enantiomer (vide supra); analysis calcd. for C8H12O3 (156.18): C 61.52, H 7.74; found: 61.35, H 8.11.
4.16. (–)-Pilocarpine [(–)-1]
A solution of methylamine in benzene (3.9 mL, 9.0 mmol) was added to a mixture of (–)-19 (1.2 g, 7.7 mmol) and dry, finely powdered K2CO3 (3.2 g, 38.5 mmol) in dry DCM/benzene (150 mL, 1:1), and the reaction mixture was stirred for 3 h at 23 °C. The solvents were removed under diminished pressure, dry DCM (20 mL) was added, distilled off, and p-tosylmethylisocyanide (3.31 g, 38.5 mmol) and dry triethylamine (5.4 mL, 38.5 mmol) were added. After an additional stirring for 1 week, the solvents were removed and the residue purified by chromatography (SiO2, MeOH/DCM, 1.25% → 5%) to yield (–)-1 (0.96 g, 60%) as a colorless oil; Rf = 0.60 (SiO2, DCM/MeOH/aq NH4OH (25%), 95:4:1); [α]D = –114.7° (c 0.8, CHCl3), ee > 99% (by HPLC, Chiralcel OC, n-hexane/ethanol, 3:7, 0.3 mL/min, UV-detection = 215 nm; tR = (+)-1 47.1 min, tR = (–)-1 = 52.32 min); IR (film): ν = 2965, 2880m, 1770s, 1655w, 1560w, 1505m, 1459m, 1425w, 1375m, 1316w, 1291w, 1270w, 1224m, 1176s, 1109m, 1050m, 1024m and 980m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 7.42 (s, 1H, N=CH-N-CH3), 6.81 (s, 1H, C=CH-N), 4.20 (dd, J = 9.2, 5.6 Hz, 1H, CH2-O), 4.10 (dd, J = 9.2, 2.7 Hz, 1H, -CH2-O), 3.59 (s, 3H, N-CH3), 2.85–2.75 (m, 1H, -CH-), 2.71 (dd, J = 15.3, 3.9 Hz, 1H, -CH2-), 2.64 (dd, J = 8.4, 6.8 Hz, 1H, CO-CH), 2.41 (dd, J = 15.3, 12.0 Hz, 1H, -CH2-), 1.95–1.80 (m, 1H, ethyl), 1.68–1.50 (m, 1H, ethyl) and 1.12 (t, J = 7.5 Hz, 3H, CH3) ppm; 13C-NMR (100 MHz, CDCl3): δ = 177.9 (CO), 138.3 (N=CH-N), 128.6 (=CH), 127.1 (=CH-N), 69.9 (CH2-O), 44.9 (CO-CH), 37.39 (-CH-), 31.2 (N-CH3), 21.4 (-CH2-), 18.3 (CH2) and 12.2 (CH3) ppm; MS (ESI, MeOH): m/z = 209.2 (76%, [M+H]+); analysis calcd. for C11H16N2O2 (208.26): C 63.44, H 7.74, N 13.45; found: C 63.21, H 7.96, N 13.29.
4.17. (+)-Pilocarpine [(+)-1]
Following the procedure given for the synthesis of its enantiomer, (+)-1 (1.92 g, 59%) was obtained as a colorless oil; Rf = 0.60 (SiO2, DCM/MeOH/aq NH4OH (25%), 95:4:1); [α]D = +115.7° (c 0.6, CHCl3), ee > 99% (by HPLC, Chiralcel OC, n-hexane/ethanol, 3:7, 0.3 mL/min, UV-detection λ = 215 nm; tR = (+)-1 47.1 min, tR = (–)-1 = 52.32 min); IR (film), 1H-NMR, 13C-NMR and MS (ESI, MeOH) were identical to the enantiomer (vide supra); analysis calcd. for C11H16N2O2 (208.26): C 63.44, H 7.74, N 13.45; found: C 63.31, H 7.98, N 13.32
Acknowledgments
We like to thank D. Ströhl, Y. Schiller and S. Ludwig for the NMR spectra and the late R. Kluge for recording numerous MS spectra; IR spectra, micro-analyses and optical rotations were measured by M. Schneider. Our thanks are also due to B. Woeste for experimental assistance. We are indebted to H. Wurziger (Merck KGaA, Darmstadt) for the donation of an authentic (+)-pilocarpine reference sample, for helpful discussions and support.
Author Contributions
Conceptualization, R.C., A.A.-H. and H.-P.D.; validation, R.C., A.A.-H. and H.-P.D.; investigation, T.S., N.H. and K.M.; writing—original draft preparation, R.C. writing—review and editing, T.S., N.H., K.M., R.C., A.A.-H. and H.-P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lumley L., Niquet J., Marrero-Rosado B., Schultz M., Rossetti F., de Araujo Furtado M., Wasterlain C. Treatment of acetylcholinesterase inhibitor-induced seizures with polytherapy targeting GABA and glutamate receptors. Neuropharmacology. 2021;185:108444. doi: 10.1016/j.neuropharm.2020.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateos-Hernandez L., Defaye B., Vancova M., Hajdusek O., Sima R., Park Y., Attoui H., Simo L. Cholinergic axons regulate type I acini in salivary glands of Ixodes ricinus and Ixodes scapularis ticks. Sci. Rep. 2020;10:16054. doi: 10.1038/s41598-020-73077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shakeel W., Javaid S., Anjum S.M.M., Rasool M.F., Samad N., Alasmari F. Time course evaluation of lacosamide alone and in polypharmacy on behavioral manifestations and oxidative stress in lithium-pilocarpine-induced model. J. Physiol. Pharmacol. 2020;71:547–564. doi: 10.26402/jpp.2020.4.10. [DOI] [PubMed] [Google Scholar]
- 4.Aboul-Enein H.Y., Ibrahim S.E., Al-Badr A.A., Ismail M., Alsamani F., Alaqil F.A., Alqarni S.A., Alotaib F.M., Alqahtani F., Imran I. Synthesis and pharmacological properties of certain analogs of pilocarpine. Toxicol. Environ. Chem. 1986;11:253–259. doi: 10.1080/02772248609357135. [DOI] [Google Scholar]
- 5.Loprinzi C.L., Balcueva E.P., Liu H., Sloan J.A., Kottschade L.A., Stella P.J., Carlson M.D., Moore D.F., Jr., Zon R.T., Levitt R., et al. A phase III randomized, double-blind, placebo-controlled study of pilocarpine for vaginal dryness: North central cancer treatment group study N04CA. J. Support. Oncol. 2011;9:105–112. doi: 10.1016/j.suponc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Gutierrez R.M., Perez-Gutierrez M.S. Parasympathomimetic activity of salpantiol. A new cyclitol from Salpianthus arenarius. Pharm. Acta Helv. 1992;67:156–158. [PubMed] [Google Scholar]
- 7.Shimura T., Kofunato Y., Yashima R., Hara T., Ito H., Takenoshita S. Pilocarpine Hydrochloride Improves Baseline Image of Magnetic Resonance Cholangiopancreatography. Am. J. Gastroenterol. 2015;110:1735–1736. doi: 10.1038/ajg.2015.330. [DOI] [PubMed] [Google Scholar]
- 8.Singhal S., Powles R., Treleaven J., Rattenbury H., Mehta J. Pilocarpine hydrochloride for symptomatic relief of xerostomia due to chronic graft-versus-host disease or total-body irradiation after bone-marrow transplantation for hematologic malignancies. Leuk. Lymphoma. 1997;24:539–543. doi: 10.3109/10428199709055591. [DOI] [PubMed] [Google Scholar]
- 9.Camp D., Garavelas A., Campitelli M. Analysis of Physicochemical Properties for Drugs of Natural Origin. J. Nat. Prod. 2015;78:1370–1382. doi: 10.1021/acs.jnatprod.5b00255. [DOI] [PubMed] [Google Scholar]
- 10.Wafa H.G., Essa E.A., El-Sisi A.E., El Maghraby G.M. Ocular films versus film-forming liquid systems for enhanced ocular drug delivery. Drug Deliv. Transl. Res. 2021;11:1084–1095. doi: 10.1007/s13346-020-00825-1. [DOI] [PubMed] [Google Scholar]
- 11.Jain N., Verma A. Preformulation studies of pilocarpine hydrochloride as niosomal gels for ocular drug delivery. Asian J. Pharm. Clin. Res. 2020;13:149–155. doi: 10.22159/ajpcr.2020.v13i6.37523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain N., Verma A., Jain N. Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: Intraocular pressure measurement in white albino rabbits. Drug Deliv. 2020;27:888–899. doi: 10.1080/10717544.2020.1775726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umashankar D.D. Plant secondary metabolites as potential usage in regenerative medicine. J. Phytopharm. 2020;9:270–273. doi: 10.31254/phyto.2020.9410. [DOI] [Google Scholar]
- 14.Ancuceanu R., Dinu M., Furtunescu F., Boda D. An inventory of medicinal products causing skin rash: Clinical and regulatory lessons. Exp. Ther. Med. 2019;18:5061–5071. doi: 10.3892/etm.2019.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowanski S., Pacholska-Bogalska J., Rosinski G. Cholinergic agonists and antagonists have an effect on the metabolism of the beetle Tenebrio molitor. Molecules. 2019;24:17. doi: 10.3390/molecules24010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korczynska M., Clark M.J., Valant C., Xu J., Moo E.V., Albold S., Weiss D.R., Torosyan H., Huang W., Kruse A.C., et al. Structure-based discovery of selective positive allosteric modulators of antagonists for the M2 muscarinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 2018;115:E2419–E2428. doi: 10.1073/pnas.1718037115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fajgenbaum M., Ansari E. Prescribing Trends in a Glaucoma Clinic and Adherence to EGS Guidelines: A Retrospective, Non-Interventional, Single-Center UK Study. Adv. Ther. 2017;34:2033–2042. doi: 10.1007/s12325-017-0593-9. [DOI] [PubMed] [Google Scholar]
- 18.Juenemann A., Hohberger B., Rech J., Sheriff A., Fu Q., Schloetzer-Schrehardt U., Voll R.E., Bartel S., Kalbacher H., Hoebeke J., et al. Agonistic autoantibodies to the β 2-adrenergic receptor involved in the pathogenesis of open-angle glaucoma. Front. Immunol. 2018;9:145. doi: 10.3389/fimmu.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thieme H., Renieri G., Schuart C. General substance classification and pharmacology of glaucoma. Ophthalmologe. 2013;110:1149–1154. doi: 10.1007/s00347-012-2676-y. [DOI] [PubMed] [Google Scholar]
- 20.Borthwick K.J., Kandemir N., Topaloglu R., Kornak U., Bakkaloglu A., Yordam N., Ozen S., Mocan H., Shah G.N., Sly W.S., et al. A phenocopy of CAII deficiency: A novel genetic explanation for inherited infantile osteopetrosis with distal renal tubular acidosis. J. Med. Genet. 2003;40:115–121. doi: 10.1136/jmg.40.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Sa E.R.A., Costa A.N., Costa R.K.M., Souza J.L., Ramos R.M., Lima F.d.C.A. In silico study of the interactions of Pilocarpus microphyllus imidazolic alkaloids with the main protease (Mpro) of SARS-CoV-2. Mol. Simul. 2021;47:74–87. doi: 10.1080/08927022.2021.1873321. [DOI] [Google Scholar]
- 22.Petit A., Polonowsky M. Two new alkaloids isolated from a species of Jaborandi. J. Pharm. 1897;5:369–370. [Google Scholar]
- 23.Petit A., Polonowsky M. Isomerism of pilocarpine and pilocarpidine. J. Pharm. 1897;6:8–11. [Google Scholar]
- 24.Gerrard A.W. The alkaloid and active principle of Jaborandi. Pharm. J. 1875;5:865–867. [Google Scholar]
- 25.Hardy E. Sur le jaborandi (Polycarpus pinnatus) Bull. Soc. Chim. Fr. 1875;24:497–501. [Google Scholar]
- 26.Jowett H.A.D. The constitution of pilocarpine. Part 1. J. Chem. Soc. Trans. 1900;77:851–860. doi: 10.1039/CT9007700851. [DOI] [Google Scholar]
- 27.Zav’yalov S.I. Configuration of pilocarpine. Dokl. Akad. Nauk SSSR. 1952;82:257–260. [Google Scholar]
- 28.Hill R.K., Barcza S. Stereochemistry of the jaborandi alkaloids. Tetrahedron. 1966;22:2889–2893. doi: 10.1016/S0040-4020(01)99081-7. [DOI] [Google Scholar]
- 29.Sawaya A.C.H.F., Costa Y.D., Mazzafera P. Unraveling the Biosynthesis of Pilocarpine in Pilocarpus microphyllus. Nat. Prod. Commun. 2015;10:721–724. doi: 10.1177/1934578X1501000506. [DOI] [PubMed] [Google Scholar]
- 30.Hill A.P., Dominicis M.E., Oquendo M., Sarduy R. Isolation of pilocarpine from Pilocarpus racemosus Vahl. Rev. Cubana Farm. 1995;29:123–125. [Google Scholar]
- 31.Jowett H.A.D., Pyman F.L. Note on the Alkaloids of Pilocarpus racemosus. Proc. Chem. Soc. Lond. 1913;28:268. [Google Scholar]
- 32.Sawaya A.C.H.F., Vaz B.G., Eberlin M.N., Mazzafera P. Screening species of Pilocarpus (Rutaceae) as sources of pilocarpine and other imidazole alkaloids. Genet. Resour. Crop. Evol. 2011;58:471–480. doi: 10.1007/s10722-011-9660-2. [DOI] [Google Scholar]
- 33.Caldas Pereira R., Nonato C.d.F.A., Camilo C.J., Melo Coutinho H.D., Rodrigues F.F.G., Xiao J., Martins da Costa J.G. Development and validation of a rapid RP-HPLC-DAD analysis method for the quantification of pilocarpine in Pilocarpus microphyllus (Rutaceae) Food Chem. Toxicol. 2018;119:106–111. doi: 10.1016/j.fct.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Lima D.F., Ianny de Lima L., Rocha J.A., Moreira de Andrade I., Grazina L.G., Villa C., Meira L., Veras L.M.C., Azevedo I.F.S., Biase A.G., et al. Seasonal change in main alkaloids of jaborandi (Pilocarpus microphyllus Stapf ex Wardleworth), an economically important species from the Brazilian flora. PLoS ONE. 2017;12:e0170281/1–e0170281/19. doi: 10.1371/journal.pone.0170281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawaya A.C.F., Abreu I.N., Andreazza N.L., Eberlin M.N., Mazzafera P. HPLC-ESI-MS/MS of imidazole alkaloids in Pilocarpus microphyllus. Molecules. 2008;13:1518–1529. doi: 10.3390/molecules13071518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tunmann O. Microchemical Detection of Alkaloid, Especially in the Leaves of Pilocarpus pennatifolius, Lem. Schweiz. Wochenschr. Chem. Pharm. 1909;47:177–183. [Google Scholar]
- 37.Tunmann O., Jenzer R. Pharmacognostic Investigations of Pilocarpus pennatifolius and Erythroxylon coca with Special Reference to their Alkaloids. Schweiz. Wochenschr. Chem. Pharm. 1910;48:17–24. [Google Scholar]
- 38.Avancini G., Abreu I.N., Saldana M.D.A., Mohamed R.S., Mazzafera P. Induction of pilocarpine formation in jaborandi leaves by salicylic acid and methyljasmonate. Phytochemistry. 2003;63:171–175. doi: 10.1016/S0031-9422(03)00102-X. [DOI] [PubMed] [Google Scholar]
- 39.Holmstedt B., Wassen S.H., Schultes R.E. Jaborandi: An interdisciplinary appraisal. J. Ethnopharmacol. 1979;1:3–21. doi: 10.1016/0378-8741(79)90014-X. [DOI] [PubMed] [Google Scholar]
- 40.Chemnitius F. Zur Darstellung des Pilocarpins. J. Prakt. Chem. 1928;118:20–24. doi: 10.1002/prac.19281180104. [DOI] [Google Scholar]
- 41.Compagnone R.S., Rapoport H. Chirospecific synthesis of (+)-pilocarpine. J. Org. Chem. 1986;51:1713–1719. doi: 10.1021/jo00360a015. [DOI] [Google Scholar]
- 42.Csuk R., Woeste B. A chemoenzymatic approach to (+)-pilocarpine. Tetrahedron. 2008;64:9384–9387. doi: 10.1016/j.tet.2008.07.100. [DOI] [Google Scholar]
- 43.Davies S.G., Roberts P.M., Stephenson P.T., Storr H.R., Thomson J.E. A practical and scaleable total synthesis of the jaborandi alkaloid (+)-pilocarpine. Tetrahedron. 2009;65:8283–8296. doi: 10.1016/j.tet.2009.07.010. [DOI] [Google Scholar]
- 44.Horne D.A., Fugmann B., Yakushijin K., Buchi G. A synthesis of pilocarpine. J. Org. Chem. 1993;58:62–64. doi: 10.1021/jo00053a016. [DOI] [Google Scholar]
- 45.Bermejo Gonzalez F., Perez Baz J., Ruano Espina M.I. Synthesis of (+)-pilocarpine analogs with a 2-oxazolidone structure. Tetrahedron Lett. 1989;30:2145–2148. doi: 10.1016/S0040-4039(01)93734-7. [DOI] [Google Scholar]
- 46.Calmels M., Hardy M. Sur la synthese de la pilocarpine. Compt. Rend. Hepd. Seances Acad. Sci. 1887;105:68–71. [Google Scholar]
- 47.DeGraw J.I. Improved synthesis of pilocarpine. Tetrahedron. 1972;28:967–972. doi: 10.1016/0040-4020(72)80156-X. [DOI] [Google Scholar]
- 48.Dener J.M., Zhang L.H., Rapoport H. An effective chirospecific synthesis of (+)-pilocarpine from L-aspartic acid. J. Org. Chem. 1993;58:1159–1166. doi: 10.1021/jo00057a031. [DOI] [Google Scholar]
- 49.Fairlamb I.J.S. Asymmetric cycloisomerization of 1,6- and 1,7-enynes by transition metal catalysts. Angew. Chem. Int. Ed. 2004;43:1048–1052. doi: 10.1002/anie.200301699. [DOI] [PubMed] [Google Scholar]
- 50.Lei A., He M., Zhang X. Highly Enantioselective Syntheses of Functionalized α-Methylene-γ-butyrolactones via Rh(I)-catalyzed Intramolecular Alder Ene Reaction: Application to Formal Synthesis of (+)-Pilocarpine. J. Am. Chem. Soc. 2002;124:8198–8199. doi: 10.1021/ja020052j. [DOI] [PubMed] [Google Scholar]
- 51.Noordam A., Maat L., Beyerman H.C. Imidazole chemistry. Part VII. Stereoselective synthesis of (+)-pilocarpine, an imidazole alkaloid used in ophthalmology. Recl. Trav. Chim. Pays-Bas. 1979;98:467–468. doi: 10.1002/recl.19790980711. [DOI] [Google Scholar]
- 52.Noordam A., Maat L., Beyerman H.C. Imidazole chemistry. Part IX. Stereoselective synthesis of the imidazole alkaloids (+)-pilocarpine and (+)-isopilocarpine. Recl. J. R. Neth. Chem. Soc. 1981;100:441–446. doi: 10.1002/recl.19811001202. [DOI] [Google Scholar]
- 53.Preobrazhenskii N.A., Polyakova A.M., Preobrazhenskii V.A. Synthesis of pilocarpine alkaloids. Bull. Acad. Sci. URSS Classe Sci. Math. Nat. Ser. Chim. 1936:983–995. (In German) [Google Scholar]
- 54.Preobrazhenskii N.A., Vompe A.F., Preobrazhenskii V.A., Shchukina M.N. Alkaloids of jaborandi leaves. III. Synthesis of pilocarpine and pilocarpidine. Ber. Dtsch. Chem. Ges. B. 1933;66B:1536–1541. doi: 10.1002/cber.19330661017. [DOI] [Google Scholar]
- 55.Shapiro G., Cai C. Asymmetric synthesis of (+)-pilosinine: A formal synthesis of (+)-pilocarpine. Tetrahedron Lett. 1992;33:2447–2450. doi: 10.1016/S0040-4039(00)92211-1. [DOI] [Google Scholar]
- 56.Wang Z., Lu X. Palladium-catalyzed intramolecular alkyne-α,β-unsaturated carbonyl coupling. A formal synthesis of (+)-pilocarpine. Tetrahedron Lett. 1997;38:5213–5216. doi: 10.1016/S0040-4039(97)01114-3. [DOI] [Google Scholar]
- 57.Yoo S.-E., Im M.-N.K., Cho I. Synthesis of pilocarpine analogs via radical cyclization. Bull. Korean Chem. Soc. 1994;15:680–684. [Google Scholar]
- 58.Hoogenboom B.E., Oldenziel O.H., van Leusen A.M. p-Tolylsulfonylmethyl isocyanide. Org. Synth. 1977;57:102–106. [Google Scholar]
- 59.Van Leusen A.M., Wildeman J., Oldenziel O.H. Chemistry of sulfonylmethyl isocyanides. 12. Base-induced cycloaddition of sulfonylmethyl isocyanides to carbon, nitrogen double bonds. Synthesis of 1,5-disubstituted and 1,4,5-trisubstituted imidazoles from aldimines and imidoyl chlorides. J. Org. Chem. 1977;42:1153–1159. doi: 10.1021/jo00427a012. [DOI] [Google Scholar]
- 60.Fodili M., Nedjar-Kolli B., Vedrenne M., Saffon-Merceron N., Lherbet C., Hoffmann P. The first example of an unusual rearrangement in the van Leusen imidazole synthesis. Chem. Heterocycl. Compd. 2015;51:940–943. doi: 10.1007/s10593-015-1801-7. [DOI] [Google Scholar]
- 61.Zheng X., Ma Z., Zhang D. Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals. 2020;13:37. doi: 10.3390/ph13030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White J.D., Carter J.P., Kezar H.S., III Stereoselective synthesis of the macrocycle segment of verrucarin J. J. Org. Chem. 1982;47:929–932. doi: 10.1021/jo00345a005. [DOI] [Google Scholar]
- 63.Schenck G.O. Photochemical reactions. II. The unsensitized and photosensitized autoxidation of furans. Justus Liebigs Ann. Chem. 1953;584:156–176. doi: 10.1002/jlac.19535840111. [DOI] [Google Scholar]
- 64.Schroeter S.H., Appel R., Brammer R., Schenck G.O. Malealdehydic and fumaraldehydic acids. Justus Liebigs Ann. Chem. 1966;697:42–61. doi: 10.1002/jlac.19666970104. [DOI] [Google Scholar]
- 65.Feringa B.L. Photooxidation of furans. Recl. Trav. Chim. Pays-Bas. 1987;106:469–488. doi: 10.1002/recl.19871060902. [DOI] [Google Scholar]
- 66.Fuji K., Nakano S., Fujita E. Improved method for methoxymethylation of alcohols under mild acidic conditions. Synthesis. 1975:276–277. doi: 10.1055/s-1975-23734. [DOI] [Google Scholar]
- 67.Balasubramaniam S., Aidhen I.S. The growing synthetic utility of the Weinreb amide. Synthesis. 2008;2008:3707–3738. [Google Scholar]
- 68.Khlestkin V.K., Mazhukin D.G. Recent advances in the application of N,O-dialkylhydroxylamines in organic chemistry. Curr. Org. Chem. 2003;7:967–993. doi: 10.2174/1385272033486639. [DOI] [Google Scholar]
- 69.Mentzel M., Hoffmann H.M.R. N-Methoxy N-methyl amides (Weinreb amides) in modern organic synthesis. J. Prakt. Chem./Chem. Ztg. 1997;339:517–524. doi: 10.1002/prac.19973390194. [DOI] [Google Scholar]
- 70.Nowak M. Weinreb Amides. Synlett. 2015;26:561–562. doi: 10.1055/s-0034-1380055. [DOI] [Google Scholar]
- 71.Senatore R., Ielo L., Monticelli S., Castoldi L., Pace V. Weinreb Amides as Privileged Acylating Agents for Accessing α-Substituted Ketones. Synthesis. 2019;51:2792–2808. doi: 10.1055/s-0037-1611549. [DOI] [Google Scholar]
- 72.Singh J., Satyamurthi N., Aidhen I.S. The growing synthetic utility of Weinreb’s amide. J. Prakt. Chem. 2000;342:340–347. doi: 10.1002/(SICI)1521-3897(200004)342:4<340::AID-PRAC340>3.0.CO;2-1. [DOI] [Google Scholar]
- 73.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 74.Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009;42:339–341. doi: 10.1107/S0021889808042726. [DOI] [Google Scholar]
- 75.Diamond-Crystal and Molecular Structure Visualization. Crystal Impact—Dr. H. Putz & Dr. K., Brandenburg GbR; Bonn, Germany: 2014. [(accessed on 28 May 2021)]. Available online: https://www.crystalimpact.com/diamond. [Google Scholar]
- 76.Chumachenko A.V., Maurit M.E., Treboganov A.D., Smirnova G.V., Teplinskaya R.B., Volkova L.V., Zvonkova E.N., Preobrazhenskii N.A. Structure of pilocarpine (an alkaloid). A new method of synthesizing cis-α-ethyl-β-carboxymethylbutyrolactone (homopilopic acid) Dokl. Akad. Nauk. SSSR. 1968;178:1352–1355. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.