Abstract
Background
The SARS-CoV-2 (Severe Acute Respiratory Syndrome coronavirus 2) has led to more than 165 million COVID-19 cases and >3.4 million deaths worldwide. Epidemiological analysis has revealed that the risk of developing severe COVID-19 increases with age. Despite a disproportionate number of older individuals and long-term care facilities being affected by SARS-CoV-2 and COVID-19, very little is understood about the immune responses and development of humoral immunity in the extremely old person after SARS-CoV-2 infection. Here we conducted a serological study to investigate the development of humoral immunity in centenarians following a SARS-CoV-2 outbreak in a long-term care facility.
Methods
Extreme aged individuals and centenarians who were residents in a long-term care facility and infected with or exposed to SARS-CoV-2 were investigated between April and June 2020 for the development of antibodies to SARS-CoV-2. Blood samples were collected from positive and bystander individuals 30 and 60 days after original diagnosis of SARS-CoV-2 infection. Plasma was used to quantify IgG, IgA, and IgM isotypes and subsequent subclasses of antibodies specific for SARS-CoV-2 spike protein. The function of anti-spike was then assessed by virus neutralization assays against the native SARS-CoV-2 virus.
Findings
Fifteen long-term care residents were investigated for SARS-CoV-2 infection. All individuals had a Clinical Frailty scale score ≥5 and were of extreme older age or were centenarians. Six women with a median age of 98.8 years tested positive for SARS-CoV-2. Anti-spike IgG antibody titers were the highest titers observed in our cohort with all IgG positive individuals having virus neutralization ability. Additionally, 5 out of the 6 positive participants had a robust IgA anti-SARS-CoV-2 response. In all 5, antibodies were detected after 60 days from initial diagnosis.
Research in Context.
Evidence before this study
At this time, we are not aware of any reports on the seroconversion ability, durability, antibody function or antibody isotype landscape of centenarians infected with SARS-CoV-2. There are numerous studies investigating the humoral responses of adults and older individuals that are non-centenarians in which neutralizing antibodies have been detected in convalescent participant plasma, with primary focus on IgG and IgM titres post SARS-CoV-2 infection. One study found that the level of neutralizing antibodies correlated with age as well as disease severity, but it is unclear how old the oldest subjects were in the study. Spike-specific memory B cells and antibodies are still present over 6 months after infection in adults. It is well documented that long-term care facilities and older individuals are more susceptible to severe outcomes of SARS-CoV-2 infection and COVID-19, but little is understood regarding the older individual's immune response to the virus during infection.
Added value of this study
To our knowledge, this is the first study investigating seroconversion in SARS-CoV-2 infected centenarians residing in long-term care. Our study demonstrates that an aged immune system is still capable of mounting an antibody response to SARS-CoV-2 infection and that the antibodies elicited have virus neutralizing ability. Our data suggests that older and frail individuals, such as those in our study, have the capacity to elicit antibodies that are capable of neutralizing SARS-CoV-2.
Implications of all the available evidence
The findings demonstrate that serological assays can be used in older and frail individuals to assess exposure to the SARS-CoV-2 virus or after COVID-19 vaccination. Since the older and frail individuals in our study had robust humoral responses to SARS-CoV-2, our data has implications for vaccine responses and possibly effectiveness in older individuals.
Interpretation
Extreme older frail individuals and centenarians were able to elicit robust IgG and IgA antibodies directed toward SARS-CoV-2 spike protein. The antibodies were able to neutralize the virus. Humoral responses were still detectable after 60 days from initial diagnosis. Together, these data suggest that recovered participants who are of extreme old age would be protected if re-exposed to the same SARS-CoV-2 viral variant. Considering the threat of SARS-CoV-2 and COVID-19 to older age groups and long-term care facilities, the humoral responses to SARS-CoV-2 in older age groups is of public health importance and has implications to vaccine responses.
Alt-text: Unlabelled box
1. Introduction
In December 2019, a novel coronavirus was identified in Wuhan, Hubei China, which has led to a devastating pandemic (WHO pandemic) [1,2]. Coronavirus disease 2019 (COVID-19) is used to define the clinical symptoms that are associated with infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. The SARS-CoV-2 is an enveloped positive-sense, single-stranded RNA genome and belongs to the Coronaviridae family in the genus Betacoronavirus, sharing the same lineage (Lineage B) as the 2002 SARS-CoV [4]. The spike protein of SARS-CoV-2 is an external binding protein, which guides the virus to attach to a host cell and bind the angiotensin converting enzyme II receptor (ACE2) [5]. The spike protein is the primary immunogenic target for virus neutralization and vaccine design, due to its critical role in the virus life cycle [5].
As the clinical cases of SARS-CoV-2 infection were analyzed, it was clear that signs and symptoms, clinical manifestations, and disease severity widely varies [6,7]. Certain host factors have been linked to the development of severe COVID-19 resulting in pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ failure [8,9]. In particular, age has been identified as a risk factor for severe illness and death with the elderly population being most susceptible [8]. It is suspected that age-related changes to the immune system, including immunosenescence and ‘inflammaging’ as well as the increased susceptibility to co-morbidities, may contribute to increased risk of severe COVID-19 in older individuals [10], [11], [12]. Historically, the aged immune system was thought to have decreased ability of the immune system to respond in an antigen-specific [13]. We now know constant inflammation or “inflammaging” is a signficant contributor to aberrant immune responses in older people. Additionally, senescent cells can contribute to inflammation and inflammaging, where inflammaging is defined as a constant low level release of inflammatory mediators (C Reactive Protein (CRP) and the inflammatory cytokines IL-6 and IL-8) above baseline contributing to the reduction of antigen-specific immunity [11]. The aberrant levels of inflammatory mediators in older adults is hypothesized to lead to heightened inflammatory responses and immunopathology in this age group during a viral infection such as with SARS-CoV-211. Additionally, those at the highest risk are older adults residing in long-term care homes [14,15] due to the increased number of individuals living in one area [14], asymptomatic transmission [16], [17], [18], atypical symptom presentation in older people [14,16], and the high burden of chronic illnesses [14,19]. Although older individuals are heavily represented in COVID-19 case fatalities, the clinical course and immune responses of older persons to SARS-CoV-2 infection is poorly understood [20].
Here, we conducted a serological study of a cohort of extreme old residents in a long-term care home that experienced a COVID-19 outbreak in Nova Scotia, Canada. We focused on a group of centenarians and nonagenarians who were infected with SARS-CoV-2. The humoral response and durability of antibody production following SARS-CoV-2 infection in these older individuals is currently ill-defined although multiple reports have documented a spectrum of responses in several cohorts. The role of age as a cofactor in establishing protection against future SARS-CoV-2 re-exposure and reinfection is of considerable interest in aged individuals. We investigated the antibody responses to SARS-CoV-2 infection in a small group of extreme aged individuals to better understand how the aged immune system works to protect against SARS-CoV-2 infection.
2. Methods
Our study adhered to STROBE guidelines.
2.1. Study design and participants
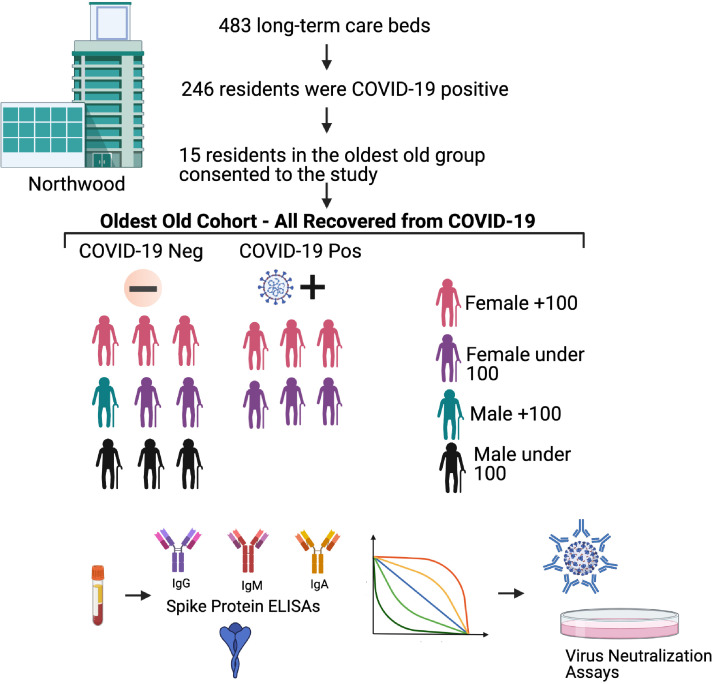
Residents of a non-profit long-term care (LTC) home in Halifax, Nova Scotia, between April and June 2020 where a widespread outbreak of SARS-CoV-2 occurred, were recruited to the study. At the time of the pandemic, Northwood had 483 long-term care beds and over the course of the wave 1 outbreak, 246 residents were found to be COVID-19 positive (Fig. 1). There were 53 deaths attributable to COVID-19. Fewer than 5 residents were transferred to an acute care hospital (none of the participants in this study) and acute care hospital staff were re-deployed to the LTC facility. Low flow nasal oxygen was made available to all residents as indicated. Otherwise, supportive care and medical management for any other decompensating illness (i.e., diuretics for decompensated congestive heart failure) was provided and evaluated daily for all residents. Residents of the facility had single or double rooms. All residents of the LTC facility were considered exposed to the virus due to the widespread nature of the outbreak within the facility. SARS-CoV-2 infection screening was performed regularly throughout the outbreak on all residents regardless of symptom by nasopharyngeal swabbing and subsequent qRT-PCR for the RNA encoding the SARS-CoV-2 nucleoprotein (NP). If a resident was found to be positive on a unit, then all other residents on that unit were screened again. Residents were also swabbed if they developed symptoms potentially attributable to COVID-19 (delirium, fall, ‘taking to bed’, fever, and respiratory and gastroenterologic symptoms). All residents were screened daily for symptoms and all residents who were SARS-CoV-2 positive were assessed by a medical team redeployed from the local tertiary acute care hospital daily. Only participants who gave written consent were enrolled in our study. Testing was performed using routine practices. Briefly, a flocked NP swab was collected in 3 mL universal transport media (Copan Diagnostics Inc., Murrieta, CA), or a combined oropharynx and anterior nares (OP/Na) swab was collected using the Aptima Multitest swab in 2.9 mL of specimen transport medium (Hologic, Inc., San Diego CA). NP or OP/Na swabs were subjected to real-time RT-PCR using the SARS-CoV-2 assay on a Cobas 6800 system (Roche Diagnostics, Mississauga, Ontario, Canada) per the manufacturer's instructions, or using a Total Nucleic Acid (TNA) extraction on a MagNApure LC 2.0 instrument (Roche Diagnostics) followed by real-time RT-PCR using a laboratory-developed test (LDT) designed at the British Columbia centre for Disease Control (BCCDC) (Vancouver, BC) [21]. Twice a year, residents of Northwood each receive an update to their long-term care comprehensive Geriatric Assessment [22]. It includes a Clinical Frailty Scale score [23]. The CFS is a well-validated measure which categorizes frailty from fit to severely frail. Here we extracted information for analysis: age, sex, frailty, comorbidities, BMI (body mass index), and time between identification of the positive SARS-CoV-2 RT-RNA PCR test and blood collection [24]. A clinical description of the cohort is summarized in Table 1.
Fig. 1.
Study Schematic.
Table 1.
Clinical data from SARS-CoV-2 exposed and infected extreme aged residents of a long-term care facility.
| All Participants | SARS-CoV-2 Negative | SARS-CoV-2 Positive | |
|---|---|---|---|
| N | 15 | 9 | 6 |
| Age, mean (range) | 96.9 (84 - 103) | 95.6 (84 - 103) | 98.8 (94 - 102) |
| Women, n (%) | 12 (80.0) | 6 (66.7) | 6 (100.0) |
| Clinical Frailty Scale, mean (SD) | 6.3 (0.6) | 6.3 (0.5) | 6.2 (1.0) |
| Symptoms of Infection, n (%) | 4 (26.7) | 2 (22.2) | 2 (33.3) |
| Died by day 30 (%) | 0 | 0 | 0 |
| Comorbidities: | |||
| Atrial Fibrillation, n (%) | 4 (26.7) | 2 (22.2) | 2 (33.3) |
| Congestive Heart Failure, n (%) | 3 (20.0) | 1 (11.1) | 2 (33.3) |
| Coronary Artery Disease, n (%) | 2 (13.3) | 1 (11.1) | 1 (16.7) |
| Cerebral Vascular Disease, n (%) | 3 (20.0) | 2 (22.2) | 1 (16.7) |
| Chronic Obstructive Pulmonary Disease, n (%) | 5 (33.3) | 3 (33.3) | 2 (33.3) |
| Dementia, n (%) | 13 (86.7) | 8 (88.9) | 5 (83.3) |
| Hypertension, n (%) | 9 (60.0) | 5 (55.6) | 4 (66.7) |
| Diabetes Mellitus, n (%) | 4 (26.7) | 3 (33.3) | 1 (16.7) |
2.2. Blood sample processing
Peripheral blood was collected from study participants in K2EDTA spray coated tubes (Fisher 367,861). Blood samples were immediately transported to the laboratory and centrifuged for 10 m at 200 x g after collection. Plasma was isolated and stored in conical vials (Fisher 1495949B) at −80 °C until assays were performed.
2.3. Enzyme-linked immunosorbent assay
Plasma SARS-CoV-2 antibodies were detected using an indirect enzyme linked immunosorbent assay (ELISA). Microplates (96-well) (Corning® 9018) were coated with 50 μl of 2.5 μg/mL SARS-CoV-2 S protein (Sino Biology 40,591-V08H) diluted in phosphate buffered saline (PBS) overnight at 4 °C. Spike protein concentration was quality control evaluated using The Pierce TM BCA Protein Assay Kit (ThermoFisher 23,225). A positive control polyclonal antibody was used to control the assay (ThermoFisher PA1–41,090). Controls were run on each to plate to ensure variation limits were not reached. Plates were washed three times with 1x PBS (Gibco 70,011,069) supplemented with 0.1% Tween-20 (Sigma P1379) (PBS-T). Plates were blocked with 5% BSA (Bovine Serum Albumin) in PBS-T for one h at room temperature. Blocking solution was removed, and 100 μl of human plasma samples diluted in 5% BSA PBS-T were diluted across plates and incubated for two hours at room temperature. Plates were washed three times with PBS-T. Secondary antibody was diluted in 1% BSA in PBS-T as follows: anti-human IgG-HRP 1:2000 (Invitrogen 31,413), anti-human IgM-HRP 1:2000 (Invitrogen A18835), anti-human IgA-HRP (Invitrogen A18781). Plates were incubated in secondary antibody for one h at room temperature. Plates were then washed five times with PBS-T, and 50 μl TMB substrate (Thermo 34,022) was added to each well. After 15 m, 50 μl of stop solution (Invitrogen SS04) was added to each well and plates were read at 450 nm on a plate reader (Bio Tek Synergy LX Multi-Mode Reader (BTSLXFATS). Samples were considered positive if average optical density (OD) was greater than 0.1 and greater than the mean OD in SARS-CoV-2 unexposed samples plus 3 standard deviations at the same dilution. Negative samples are denoted as “1″ for display on a logarithmic scale.
2.4. SARS-CoV-2 virus and culture
The SARS-CoV-2 strain SARS-CoV-2/Canada/ON/VIDO-01–2020 was used for in vitro neutralization assays. The virus was isolated from the respiratory secretions from an infected 56-year-old man presenting with respiratory symptoms at a hospital in Toronto, Canada, upon returning from Wuhan, China [25]. The viral stock used was from the second passage and the sequence is available at GISAID – EPI_ISL_425,177. The virus was amplified in Vero-76 cells in viral growth media vDMEM (Dulbecco's Modified Eagle Medium (Wisent Bioproducts (Cat # 319–005-CL)), 2% fetal calf serum (Wisent Bioproducts (Cat # 090–150)), 5 mL 100x Penicillin (10,000 U/mL)/Streptomycin (10,000 ug/mL), and 2 mg/mL TPCK-trypsin) at VIDO, Saskatoon, Saskatchewan. All work with SARS-CoV-2 live virus was performed in a CL3 facility at VIDO.
2.5. Neutralization assay
Vero cells (ATCC® CRL-1587) were seeded into 96-well tissue culture plates (Corning) at 20,000 cells per well and incubated overnight at 37 °C in a 5% CO2 chamber. Plasma samples were heat inactivated at 56 °C for 30 m. Heat inactivated plasma was diluted 1:10 in serum-free DMEM and diluted 1:2 across round bottom 96-well plates (Corning). SARS-CoV-2 virus (SARS-CoV-2 strain /Canada/ON/VIDO-01–2020) was diluted to 100 TCID50/17.5μl in serum-free DMEM.
The diluted virus was added to diluted plasma samples and incubated at 37 °C for one hour. A back titer of the virus was also prepared to confirm the titer of the virus dilution used. The plasma-virus mixture was added to plated Vero 76 cells and incubated at 37 °C in a 5% CO2 chamber for 1 h for virus absorption. The mixture was removed and replaced with vDMEM and placed back at 37 °C with 5% CO2 for 3 days. Cells were checked daily for signs of cytopathic effect (CPE), and results were recorded on day 5 post inoculation. Antibody titer was calculated as the inverse of the most diluted sample where no CPE was detected. All work with SARS-CoV-2 live virus was performed in a CL3 facility at VIDO.
2.6. Statistical analysis
Results were analysed using GraphPad Prism8.
2.7. Ethics
Ethics for this study was approved by the IRB at Dalhousie University and is covered under the protocol “Sentinel surveillance for severe outcomes of laboratory-confirmed influenza in adults for the annual influenza season and for confirmed and suspected cases of COVID-19/SARS-CoV-2 acute respiratory disease” REB#1,020,727.
2.8. Role of the funding source
The sponsors had no role in the analysis or interpretation of the data. Alyson Kelvin had full access to all the data analysis and can take responsibility for all aspects of the paper.
3. Results
There were 15 residents of extreme ages that participated in our study. The majority were female (12 women and 3 men) which is consistent with the literature for nonagenarians and centenarians (Table 1) [26]. In total there were 8 centenarian included, representing 9 of the 12 centenarians in the LTC facility at the time of the outbreak and all of the centenarians approached for consent. Additional residents of the LTC facility were not approached for consent due to resource limitations. The additional 6 residents were selected out of the 107 residents enrolled (of 125 approached for consent) in a larger study as they were the next 6 residents enrolled who were, at the time of consent, deemed to be exposed but not yet infected while still in the oldest old of the cohort. Fig. 1 shows the study outline.
The study descriptive characteristics are in found in Table 1. The mean age of the population included in this analysis was 96.9 and the ages of the SARS-CoV-2 negative and positive groups were similar. The SARS-CoV2 positive participants were all women and most participants were asymptomatic. In both the SARS-CoV2 positive and negative groups, two participants had either a fever or cough. None of the centenarians had respiratory symptoms or fever. Three of the SARS-CoV-2 NP PCR positive residents were centenarians, with ages of 100, 102, and 102 years. The ages of the positive non-centenarians were 93, 97, and 94. The average age of the infected centenarians and non-centenarians were 101 (+/−1.15 SD) and 94.7 (+/- 2.08 SD), respectively. The age average for the COVID-19 negative centenarians and non-centenarians were 101 (+/−1.5 SD) and 89 (+/−2.82 SD), respectively. There were no differences in the level of frailty (Clinical Frailty Scale of 6.2 versus 6.3) [23], or comorbidities (dementia, hypertension, chronic obstructive pulmonary disease, etc.) in those who were SARS-CoV-2 positive and SARS-CoV-2 negative, respectively (Table 1). None of the participants enrolled required supplemental oxygen for hypoxia or dyspnea, were transferred to hospital or died in the 30 days following SARS-Cov-2 swab.
For plasma sample collection, the mean time from PCR diagnosis with COVID-19 to sample collection was 30 days. For residents with a second blood sample collected, the mean time between the first and second blood collection was 30 days. Due to the small numbers of nonagenarians and centenarians were we only able to determine trends for the clinical outcome of SARS-CoV-2 infection and were not able to perform multivariable analysis to identify factors that would influence clinical outcome.
For plasma sample collection, the mean time from PCR diagnosis with COVID-19 to sample collection was 30 days. For residents with a second blood sample collected, the mean time between the first and second blood collection was 30 days. Due to the small numbers of nonagenarians and centenarians were we only able to determine trends for the clinical outcome of SARS-CoV-2 infection and were not able to perform multivariable analysis to identify factors that would influence clinical outcome
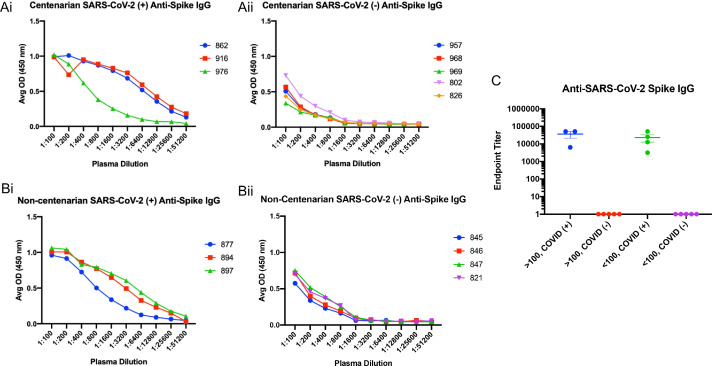
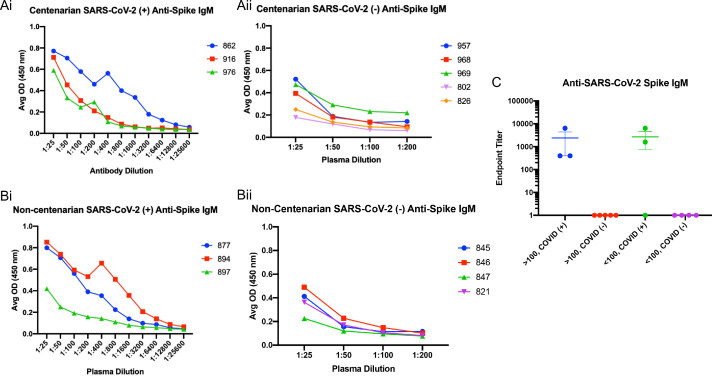
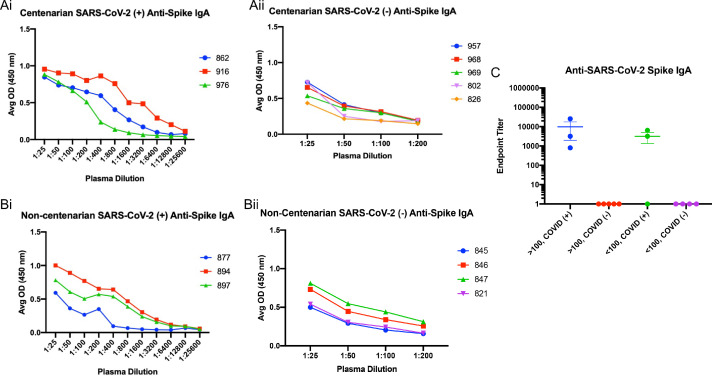
An enzyme-linked immunosorbent assay (ELISA) was used to detect the presence of antibodies directed toward the SARS-CoV-2 S protein present in the first blood collection for each resident enrolled in the study. Subsequently, the isotype and subclasses of S antibodies were identified by ELISA as well. SARS-CoV-2 infected centenarians had high titres of S-directed IgG in their plasma, with endpoint titers ranging from 1:6400–1:52,400 (Fig. 2Ai and C). We were not able to detect S-directed IgG in the plasma of SARS-CoV-2 NP PCR negative participants (Fig. 2Aii and C). Non-centenarians who tested PCR positive also had significant anti-S IgG endpoint titers (1:800) (Fig. 2Bi and C). Antibody IgG subclass analysis indicated that both IgG1 and IgG3 S-directed antibodies were present in centenarian residents 862 and 916 and the non-centenarian residents (Supplemental Figs. 1 and 2). Further isotype characterization revealed that all SARS-CoV-2 PCR positive centenarians had a detectable anti-S IgM response, with titres ranging from 1:400–1:6400 (Fig. 3Ai) similar to positive non-centenarians with the exception of one positive non-centenarian who did not have detectable anti-S IgM (Fig. 3Aii) summarized in Fig. 3C. To complete the isotype analysis, S directed IgA antibodies were also measured in plasma samples where IgA1 anti-S levels were greater than IgA2 (Supplemental Figs. 2 and 3). The SARS-CoV-2 positive centenarians were also positive for S IgA (1:800- 1:25,600) (Fig. 4). To determine if the humoral responses to the SARS-CoV-2 virus were similar in magnitude between infected centenarian and non-centenarian older residents, we directly compared if the titres of S directed IgG, IgM, and IgA of the two groups. The titers of S directed IgG, IgM, and IgA were not statistically different between the groups, demonstrating that advanced age did not alter S-directed antibody elicitation to the SARS-CoV-2 virus.
Fig. 2.
Extreme aged centenarians and non-centenarians infected with SARS-CoV-2 elicit a robust anti-spike IgG response. Peripheral blood samples were collected from 16 residents of a long-term care facility in Halifax, Nova Scotia, who were considered exposed to the SARS-CoV-2 virus. All exposed residents were tested for the presence of virus in the upper respiratory tract by nasopharyngeal swabbing following by PCR to detect the viral N gene. Blood was collected approximately 30 days after a positive PCR test. Serum separated was subjected to a direct ELISA assay to determine the magnitude of SARS-CoV-2 spike protein directed antibodies of the IgG isotype. The ELISA was analyzed by participant group: centenarians PCR positive for SARS-CoV-2 nucleic acid encoding gene N (Ai); centenarians PCR negative for SARS-CoV-2 nucleic acid encoding gene N (Aii); non-centenarians PCR positive for SARS-CoV-2 nucleic acid encoding gene N (Bi); non-centenarians PCR negative for SARS-CoV-2 nucleic acid encoding gene N (Bii). Endpoint titers for each group are summarized (C).
Fig. 3.
Similar profiles of spike IgM antibodies elicited in centenarians and non-centenarians with confirmed SARS-CCoV-2 infection. Plasma samples collected from the long-term care cohort were analyzed by participant group for the presence of anti-S IgM. The endpoint titer results were plotted by group: centenarians PCR positive for SARS-CoV-2 nucleic acid encoding gene N (Ai); centenarians PCR negative for SARS-CoV-2 nucleic acid encoding gene N (Aii); non-centenarians PCR positive for SARS-CoV-2 nucleic acid encoding gene N (Bi); non-centenarians PCR negative for SARS-CoV-2 nucleic acid encoding gene N (Bii). Endpoint titers for each group are summarized and compared (C).
Fig. 4.
Anti-spike IgA is present in the plasma of centenarians and non-centenarians infected with SARS-CoV-2. Anti-S IgA was assessed in plasma samples collected from SARS-CoV-2 positive centenarians (Ai); SARS-CoV-2 negative centenarians (Aii); SARS-CoV-2 positive non-centenarians (Bi); SARS-CoV-2 negative non-centenarians (Bii). Endpoint titers for each group are summarized (C).
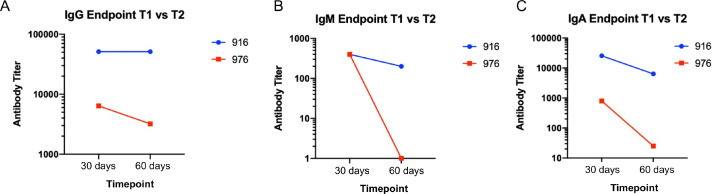
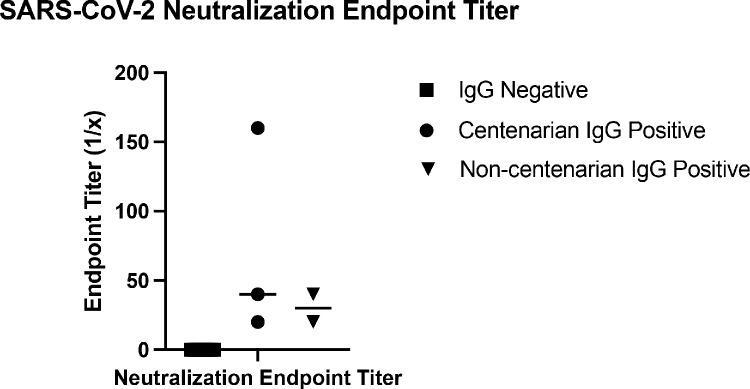
To investigate the durability and function of antibodies elicited toward SARS-CoV-2, we analyzed a second blood sample collected approximately 30 days after the first draw to determine levels of S antibodies and whether antibodies had viral neutralization capacity. For the durability assay, resident 916 had consistently high levels of anti-S IgG titres with levels of 1:52,400 still observed in their second plasma sample (Fig. 5A). Conversely, the titer of resident 976 decreased from 1:6400 to 1:3200 at the second draw. When evaluating anti-spike IgM, both residents had decreased responses (Fig. 5B). Initial titers for both centenarians were 1:400 and decreased in the follow-up sample, with the titer in resident 916 IgM decreasing to 1:200. There was no detectable IgM for resident 976 at either time point. Decreases in antibody titer were also observed at the second timepoint for spike-directed IgA (Fig. 5C). SARS-CoV-2 virus neutralization assays were performed to assess the functional neutralization capabilities of the plasma antibodies elicited by the COVID-19 advanced aged cohort. The plasma samples collected at the first sampling time point were used in standard virus neutralization assays performed in Vero76 cells with SARS-CoV-2 (strain SARS-CoV-2/Canada/ON/VIDO-01–2020) at 10 TCID50. All of the SARS-CoV-2 NP PCR and S IgG positive residents had detectable neutralizing antibody titers, ranging from 1:20 to 1:160 for centenarians and 1:20 to 1:40 for non-centenarians (Fig. 6). Together, our data illustrates that centenarians infected with SARS-CoV-2 were able to elicit SARS-CoV-2 S-directed neutralizing antibodies, demonstrating an intact humoral immune response.
Fig. 5.
Anti-spike immunoglobulins remained robust 60 days after initial SARS-CoV-2 in centenarians. Anti-S IgG (A), IgM (B), and IgA (A) antibodies present in residents 916 and 976 plasma samples collected at 30 days (T1) and 60 days (T2) post a SARS-CoV-2 PCR test were assessed by ELISA.
Fig. 6.
Virus neutralization titers from COVID-19 recovered nonagenarians and centenarians. Plasma samples from COVID-19 positive and negative nonagenarians and centenarians were subjected to a virus neutralization assay using the infectious SARS-CoV-2 virus isolated from a Canadian COVID-19 patient.
4. Discussion
To our knowledge, this is the first study investigating seroconversion in SARS-CoV-2 infected extreme aged adults, some over 100 years, residing in a long-term care facility. Here, a group of highly aged, frail residents (the majority being female), living in a long-term care facility survived infection with SARS-CoV-2, is described. The centenarians were able to elicit a successful S directed antibody response which was able to functionally neutralize the native SARS-CoV-2 virus. This work demonstrates that the extreme aged immune system is capable of responses during SARS-CoV-2 virus infection which are classically associated with convalescence and recovery from acute respiratory virus infection.
The older residents examined in this study represent a particularly vulnerable group in the ongoing global SARS-CoV-2 pandemic. Estimations of infection fatality risk (IFR) of SARS-CoV-2 in Switzerland demonstrated that people older than 65 years had an IFR of 5.6%, compared to 0.0092% in those aged 20–49 y [27]. Moreover, risk of death from COVID-19 has been estimated to be 630 times greater for those over 85 y compared to 19–29 y/o [28]. Older adults, especially those living with frailty, are considered disproportionately susceptible to COVID-19 as they have increased likelihood of chronic health issues and immune dysfunction [15,23]. Historically, aged immune systems were characterized by immunosenescence, where the immune system deteriorates with time [14], and also inflammaging, chronic, low-grade inflammation which can contribute to disease pathogenesis [10]. The aberrant immune function of older adults compared to younger adults led to concern with their ability to mount a successful humoral immune response to SARS-CoV-2 infection considering their increased susceptibility to the virus. With these examples in mind, it is also recognized that people of extreme ages (nonagenarians and centenarians) often display profiles of healthy aging and are equipped to respond in a manner that is beneficial leading to positive outcomes after pathogen infection. As this does not describe all extreme older individuals, some experts in aging hypothesize that nonagenarians and centenarians may be categorized as high-performing or low-performing where those that are high-performing have genetic or immunological signatures that are advantageous for healthy aging [29,30]. These characteristics such as decreased inflammation and inflammatory mediators (such as decreased IL-6), increased telomere length, and deceased redox reactions are associated with the beneficial immune responses and improve the high-performing centenarian outcome to viral infection [30], [31], [32]. Immune responses and immune mediators including NK cell activity, neutrophil microbicidal activity, IL-1bet,a and TNF-alpha, which may be inflammatory as well, have also been associated with positive outcomes for centenarians [31]. High-performing centenarians have been shown to have greater T cell proliferation and telomerase activity when their leukocytes are stimulated in vitro [29]. Although we have found that the older ages in our study all recovered from SARS-CoV-2 infection without complications, all our residents had high frailty scores suggesting they would be low performers. To extend our analysis, future studies should investigate the immune mediator levels in these surviving centenarians to determine if these residents represent high-performing centenarians despite their high frailty score. Evidence from an epidemiological study out of Italy indicated that the oldest old (nonagenarians and centenarians) had similar or greater mortality and morbidity profiles as those within the old age group of 50 to 80 [33]. It would be important to analyze the case fatality rates of high-performing and the low-performing centenarians within this Italian study as well as our own as none of our infected residents succumbed to COVID-19.
An extraordinarily large amount of work has been done over a short amount of time to characterize the evolution of the immune response to SARS-CoV-2 in healthy adults. Antibodies (humoral immunity) and T cells (cell mediated immunity) are essential for eliminating viruses from hosts. Evaluating antibody levels and the antibody isotype are the easiest ways to investigate the involvement of the humoral B cell response and its maturity level, respectively. Antibodies elicited after SARS-CoV-2 infection in healthy adults have been shown to be polyfunctional specifically with virus neutralizing capabilities, ADCC (Antibody-Dependent Cellular Cytotoxicity) activation properties, and complement deposition functions [34]. When investigating the evolution of the antibody response, one study found that RBD (receptor binding domain)-specific IgG and IgA levels correlated more strongly than IgM levels in patients shortly after seroconversion [35]. Furthermore, neutralizing antibody titers were also strongly associated with the IgG and IgA RBD antibody levels [35]. Longitudinal analyses over 6 to 8 months in recovering SARS-CoV-2 individuals found modest decreases in RBD and entire spike antibodies [36,37]. Isotype analysis indicated that RBD IgG and IgM levels had greater declines compared to IgA [36]. Although in our study we only investigated two centenarians over time, we found relative stability in the spike-specific IgG antibodies at 60 days post symptom onset whereas the IgM and IgA levels were more variable. In another study, investigation of the three arms of adaptive immune memory kinetics identified a coordinated response among CD4+ T cells, CD8+ T cells, and antibody longevity over 8 months to be correlated with mild COVID-1935. Interestingly, the authors of this study found that loss of SARS-CoV-2 specificity in any of the immune arms to be more prominent in those over 65 and those experiencing more severe disease [35]. It would be of interest to investigate the three arms of immune memory in the oldest old age group to determine if there is evidence of immunological stability as suggested by Lio and colleagues in their discussion of immunity in nonagenarians and centenarians [30].
Here, we showed that after an average of 30 days after a positive SARS-CoV-2 NP PCR test, some extremely old LTC residents mounted high SARS-CoV-2 S-directed antibody responses across relevant immunoglobulin isotypes. The highest titers were observed with IgG, where centenarian titres ranged from 1:6400 to 1:52,400. All individuals positive for spike-IgG had neutralizing antibody titers ranging from 1:20 to 1:160. Other studies have established ELISA IgG titres of 1:320 as moderate and titres of 1:960 and above as high [38], demonstrating that the centenarians in this study induced a robust anti-spike IgG response after SARS-CoV-2 infection. Interestingly, one study by Klein and colleagues indicated that serum SARS-CoV-2 antibody titers increased with age and male sex in COVID-19 patients [39]. Since most all of our advanced aged participants were female, our data suggests that extreme aged females also elicit high SARS-CoV-2 antibody titers. In younger COVID-19 cohorts, by four weeks post symptom onset, over 90% of participants had neutralizing activity; the older residents in our study appear to have had a similar humoral response [40]. Previous reports also indicate that S-directed IgG antibody titers have the strongest correlation with neutralization antibody titer in younger people infected with SARS-CoV-2 [40], agreeing with our results showing centenarians with anti-S IgG titers were also able to neutralize the virus.
The identified S-directed IgG response was durable and remained high at a second sample collection which was approximately 60 days after an initial diagnostic positive SARS-CoV-2 NP PCR test. Despite media concerns surrounding longevity of the humoral response for SARS-CoV-2 infection, our data, although collected from a small study group, suggest that S IgG antibody levels only slightly decrease 2 months after infection, even in extremely old people. A study investigating the durability of anti-S IgG has shown peak S-directed IgG antibodies 4 weeks after COVID-19 symptom onset which then remained high for 6 months [40]. These results are in agreement with other North American studies that show stable SARS-CoV-2 S antibody levels to 115 days and 90 days post symptom onset (PSO) [38,41].
In contrast to our IgG anti-S findings, we found a robust early increase in S-specific plasma IgA in SARS-CoV-2 infected people studied at 30 days post positive PCR test which declined. Few studies have characterized the onset of IgA after SARS-CoV-2 infection in plasma, most likely due to the mucosal association typically considered for the IgA isotype. One study that examined COVID-19 convalescent plasma found a correlation of S-specific IgG with S-specific IgA in plasma [39], as did we. Also, in agreement with our findings, another study found that plasma anti-S IgA peaked early (16–30 days post symptom onset) and declined to 74.1% of maximum titres by day 115 PSO [41]. It remains unclear at this time how S-directed IgA antibody dynamics may influence COVID-19 disease severity and recovery from SARS-CoV-2 infection among all age groups.
Older people residing in long-term care homes represent an especially vulnerable population. Spread and transmission of the virus within long-term care has been shown to be very rapid [16]. Within long-term care facilities in both Belgium and the USA, high proportions of asymptomatic cases were observed, leading to challenges with isolation of positive individuals within facilities [15,17,18]. Older people, especially those who are frail and have dementia, often develop subtle and atypical illness presentations that present challenges for symptom detection and lead to difficulty in identifying infection [42]. Within the cohort studied here, all but two residents were living with dementia and most residents were at least moderately frail. This may have contributed to the rapid spread of the virus through the long-term care facility. Frequent serological investigation and PCR testing for SARS-CoV-2 antibodies and virus, respectively, provide mechanisms for identifying an outbreak in extreme older individuals who may be unaware of their health status which is confounded by an atypical COVID-19 clinical picture.
Another concern surrounding the immune response to SARS-CoV-2 in adults is the concept of cross-reactivity to the virus due to infection with other coronaviruses previously. Studies using highly-sensitive flow-cytometry based methods have shown that people who were not exposed to SARS-CoV-2 had detectable IgG antibodies against the spike protein of the SARS-CoV-2 virus. These antibodies were especially prevalent in children and adolescents [43]. Other investigations have also found that unexposed people have pre-existing CD4+ memory T cells that are reactive to common cold coronaviruses such as NL63, HKU1, and OC43, as well as the current pandemic coronavirus SARS-CoV-2 [44]. As the participants studied were highly aged and had likely been exposed to many viruses over their lifetime, it was hypothesized that some cross-reactivity to the SARS-CoV-2 spike protein or virus may be observed due to previous infection. In our study, residents who did not test positive for SARS-CoV-2 RNA did not have any detectable plasma IgG against the SARS-CoV-2 spike protein. Our method of detection using indirect ELISA for IgG may suggest that cross-reactive antibodies from previous circulating common cold coronaviruses may not be an issue when screening individuals for serological evidence of a SARS-CoV-2 infection. A previous study investigated the TCR (T cell receptor) repertoire changes across life associated development and aging which included newborns and centenarians [45]. The data indicated that repertoires are more similar among individuals at birth and increase in diversity overtime. It would be of interest to conduct BCR (B cell receptor) repertoire sequencing in our cohort to determine clonal expansion, somatic hypermutation, and isotype switching of the BCR and therefore antibodies. This information may inform if previously established clonotypes from common cold coronavirus exposures were expanded during the infection in SARS-CoV-2. This analysis would also give insight into the specificity of the humoral response in older individuals.
Our study was limited by a small sample size, lack of continued plasma sampling, additional age groups, and uncertain SARS-CoV-2 inoculation/exposure date. The low sample size reflects a low incidence rate in Nova Scotia, even among extremely old people living with frailty (cumulative confirmed cases were 177 per million on April 1 and 1057 by May 15, the period of initial data collection) [46]. Even so, the information gathered here is extremely valuable because of the rare occurrence of centenarians infected with SARS-CoV-2. The second timepoint for blood collection was not available for all residents studied, making it difficult to form more robust and broad conclusions from our data. Although more frequent blood collection would have been beneficial to better model the dynamics of the antibody response following infection in highly aged people and their subsequent outcomes, access to blood samples in this age group will remain a difficult task due to their vulnerability and the pragmatics of venipuncture. Since all of our participants had similar short-term outcomes, follow up analysis may not indicate differences in humoral response correlation to outcome. Long-term follow-up in this cohort may provide a different result for analysis with antibody levels. Additionally, having several age groups for analysis that were infected with SARS-CoV-2 in the long-term care facility as well as in the community setting would have been an important comparator. At the time of the study initiation, we focused on people in their 90 s and 100 s in the long-term care facility as the outcome of infection in these age groups was of particular importance due the epidemiological modeling indicating increased case fatality rates. Since immune mediators are also of interest it would have been important to identify the profiles of specific cytokines such as IL-6 and TNF-alpha in these residents [30,32]. Due to the limited amount of sample we were able to receive from these frail individuals, we were not able to complete additional immune profiling. Finally, as with most viral infections, pinpointing the exact inoculation date or event for an infection is difficult in people. With increased studies following contact tracing and symptom onset as well as studies such as ours describing time from symptom onset and development of SARS-CoV-2 antibodies, a clearer clinical picture of disease progression and immune responses for extreme older adults as well as other age groups will be better defined.
The current COVID-19 pandemic has highlighted the often marked frailty of residents in long-term care which is evident by the high COVID-19 case numbers, COVID-19 fatalities, and SARS-CoV-2 outbreaks in these facilities across several countries [47], [48], [49], [50], [51]. The cause of this may be multifaceted, which may include the inadequacies of infection control measures and gaps in education as well as the difficulty identifying atypical clinical manifestations of COVID-19 in older people. Our study shows that even the oldest people can elicit a strong humoral response to SARS-CoV-2 and recover from infection. These findings are important for developing serological testing protocols as well as investigating the poor COVID-19 outcomes associated with LTC facilities.
Declaration of Competing Interest
Mary K. Foley – Ms. Foley has nothing to disclose.
Samuel D. Searle - Dr. Searle received PhD and fellowship funding from the Canadian Frailty Network, Dalhousie Medical Research Foundation, Dalhousie University Internal Medicine Research Foundation and the QE II Innovation Fund.
Ali Toloue – Toloue has nothing to disclose.
Ryan Booth -Ryan Booth has nothing to disclose.
Alec Falkenham – Dr. Falkenham has nothing to disclose.
Darryl Falzarano – Dr. Falzarano has nothing to disclose.
Salvatore Rubino – Dr. Rubino has nothing to disclose.
Magen E. Francis – Ms. Francis has nothing to disclose.
Mara McNeil – Ms. McNeil has nothing to disclose.
Christopher Richardson – Dr. Richardson has nothing to disclose.
Jason LeBlanc – Dr. LeBlanc has nothing to disclose.
Sharon Oldford – Dr. Oldford has nothing to disclose.
Volker Gerdts – Dr. Gerdts has nothing to disclose.
Melissa K. Andrew – Dr. Andrew reports grants from Sanofi, grants from GSK, grants from Pfizer, grants from Canadian Frailty Network, grants from CIHR, grans from Public Health Agency of Canada, personal fees from Sanofi, from Pfizer, personal fees from Seqirus, outside the submitted work.
Shelly A. McNeil – Dr. McNeil reports grants, personal fees and other from Pfizer, other from Medicago, personal fees and other from Sanofi, grants, personal fees and other from GlaxoSmithKline, grants personal fees and other from Merck, other from CanSino, other from IMV, other from Janssen, outside the submitted work.
Barry Clarke – Dr. Clarke has nothing to disclose.
Kenneth Rockwood – Dr. Rockwood reports personal fees from Clinical Cardio Day – Cape Breton University, personal fees from CRUIGM-Montreal, from Speaker at Jackson Lab, Bar Harbor MA, personal fees from Speaker at MouseAge Rome Italy, personal fees from Frontemporal Dementia Study Group, personal fees from SunLife Insurance Japan, outside the submitted work and Kenneth Rockwood is President and Co-founder of Ardea Outcomes, which in the last three years (as DGI Clinical) has contracts with pharma and device manufacturers (Shire, Hollister, Nutricia, Roche, Otsuka) on individualized outcome measurement. Otherwise any personal fees are for invited guest lectures and academic symposia, received directly from event organizers, chiefly for presentations on frailty. He is Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research, and with additional funding from the Alzheimer Society of Canada and several other charities. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Canadian Institutes of Health Research, The Canadian Frailty Network, the QEII Health Science centre Foundation, the Nova Scotia Health Research Fund and the Fountain Family Innovation Fund of the QEII Health Science centre Foundation.
David J. Kelvin – Dr. Kelvin has nothing to disclose.
Alyson A. Kelvin – Dr. A. Kelvin has nothing to disclose.
Acknowledgments
Acknowledgment
We wish to thank all of the study participants for their involvement. This article is published with the permission of the Director of VIDO-InterVac with article number 932.
Data sharing statement
The data reported in this manuscript are subject to access restriction to protect patient confidentiality. Inquiries can be made with the corresponding authors.
Funding
Canadian Institutes of Health Research (CIHR), NIH/NIAID, Genome Atlantic. VIDO receives operational funding from the Canada Foundation for Innovation through the Major Science Initiatives Fund and by Government of Saskatchewan through Innovation Saskatchewan.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100975.
Contributor Information
David J. Kelvin, Email: dkelvin@dal.ca.
Alyson A. Kelvin, Email: alk308@usask.ca.
Appendix. Supplementary materials
References
- 1.Team TNCPERE Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(WHO) WHO. Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it2020).
- 4.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A., Madhavan M.V., Sehgal K. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domingues R., Lippi A., Setz C., Outeiro T.F., Krisko A. SARS-CoV-2, immunosenescence and inflammaging: partners in the COVID-19 crime. Aging (Albany NY) 2020;12(18):18778–18789. doi: 10.18632/aging.103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbar A.N., Gilroy D.W. Aging immunity may exacerbate COVID-19. Science. 2020;369(6501):256–257. doi: 10.1126/science.abb0762. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Klein S.L., Garibaldi B.T. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65 doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiello A., Farzaneh F., Candore G. Immunosenescence and its hallmarks: how to oppose aging strategically? a review of potential options for therapeutic intervention. Front Immunol. 2019;10:2247. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42(2):505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J Am Geriatr Soc. 2020;68(5):912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 16.Kimball A., Hatfield K.M., Arons M. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feaster M., Goh Y.Y. High proportion of asymptomatic SARS-CoV-2 infections in 9 long-term care facilities, Pasadena, California, USA, April 2020. Emerg Infect Dis. 2020;26(10) doi: 10.3201/eid2610.202694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoxha A., Wyndham-Thomas C., Klamer S. Asymptomatic SARS-CoV-2 infection in Belgian long-term care facilities. Lancet Infect Dis. 2021;21(4):e67. doi: 10.1016/S1473-3099(20)30560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pranata R., Henrina J., Lim M.A. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2020;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koff W.C., Williams M.A. Covid-19 and immunity in aging populations - a new research agenda. N Engl J Med. 2020;383(9):804–805. doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc J.J., Heinstein C., MacDonald J., Pettipas J., Hatchette T.F., Patriquin G. A combined oropharyngeal/nares swab is a suitable alternative to nasopharyngeal swabs for the detection of SARS-CoV-2. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall E.G., Clarke B.S., Varatharasan N., Andrew M.K. A long-term care-comprehensive geriatric assessment (LTC-CGA) tool: improving care for frail older adults? Can Geriatr J. 2015;18(1):2–10. doi: 10.5770/cgj.18.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood K., Song X., MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockwood K., Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23(3):210–215. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchand-Senecal X., Kozak R., Mubareka S. Diagnosis and management of first case of COVID-19 in Canada: lessons applied from SARS-CoV-1. Clin Infect Dis. 2020;71(16):2207–2210. doi: 10.1093/cid/ciaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couderc A.L., Correard F., Nouguerede E. Centenarians in nursing homes during the COVID-19 pandemic. Aging (Albany NY) 2021;13(5):6247–6257. doi: 10.18632/aging.202743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Saez J., Lauer S.A., Kaiser L. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2020;21(4):e69–e70. doi: 10.1016/S1473-3099(20)30584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(CDC) CfDCaP. Coronavirus disease 2019 (COVID-19): older adults. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed 16 September 2020 2020).
- 29.Tedone E., Huang E., O'Hara R. Telomere length and telomerase activity in T cells are biomarkers of high-performing centenarians. Aging Cell. 2019;18(1):e12859. doi: 10.1111/acel.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lio D., Scola L., Giarratana R.M. SARS CoV2 infection _the longevity study perspectives. Ageing Res Rev. 2021;67 doi: 10.1016/j.arr.2021.101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez De Toda I., Vida C., Garcia-Salmones M., Alonso-Fernandez P., De La Fuente M. Immune function, oxidative, and inflammatory markers in centenarians as potential predictors of survival and indicators of recovery after hospital admission. J Gerontol A Biol Sci Med Sci. 2020;75(10):1827–1833. doi: 10.1093/gerona/glz250. [DOI] [PubMed] [Google Scholar]
- 32.Szewieczek J., Francuz T., Dulawa J. Functional measures, inflammatory markers and endothelin-1 as predictors of 360-day survival in centenarians. Age (Dordr) 2015;37(5):85. doi: 10.1007/s11357-015-9822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcon G., Tettamanti M., Capacci G. COVID-19 mortality in Lombardy: the vulnerability of the oldest old and the resilience of male centenarians. Aging (Albany NY) 2020;12(15):15186–15195. doi: 10.18632/aging.103872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dufloo J., Grzelak L., Staropoli I. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. Cell Rep Med. 2021;2(5) doi: 10.1016/j.xcrm.2021.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012. doi: 10.1016/j.cell.2020.09.038. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaebler C., Wang Z., Lorenzi J.C.C. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dan J.M., Mateus J., Kato Y. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wajnberg A., Amanat F., Firpo A. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein S.L., Pekosz A., Park H.S. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J., Liang B., Chen C. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12(1):1813. doi: 10.1038/s41467-021-22034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isho B., Abe K.T., Zuo M. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5(52) doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrew M.K., McElhaney J.E., McGeer A.A. Influenza surveillance case definitions miss a substantial proportion of older adults hospitalized with laboratory-confirmed influenza: a report from the Canadian Immunization Research Network (CIRN) serious outcomes surveillance (SOS) network. Infect Control Hosp Epidemiol. 2020;41(5):499–504. doi: 10.1017/ice.2020.22. [DOI] [PubMed] [Google Scholar]
- 43.Ng K.W., Faulkner N., Cornish G.H. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370(6522):1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mateus J., Grifoni A., Tarke A. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370(6512):89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Britanova O.V., Shugay M., Merzlyak E.M. Dynamics of individual T cell repertoires: from cord blood to centenarians. J Immunol. 2016;196(12):5005–5013. doi: 10.4049/jimmunol.1600005. [DOI] [PubMed] [Google Scholar]
- 46.Authority NSH. Coronavirus (COVID-19): case data. 2020. https://novascotia.ca/coronavirus/data/.
- 47.McMichael T.M., Currie D.W., Clark S. Epidemiology of COVID-19 in a long-term care facility in king county, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z. Use the environment to prevent and control COVID-19 in senior-living facilities: an analysis of the guidelines used in China. HERD. 2020;14(1):130–140. doi: 10.1177/1937586720953519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amagasa S., Kojin H., Inoue S. Long-term care and the coronavirus disease 2019 challenge in Japan. J Gen Fam Med. 2020;21(6):202–203. doi: 10.1002/jgf2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daly M. COVID-19 and care homes in England: what happened and why? Soc Policy Adm. 2020 doi: 10.1111/spol.12645. ;10.1111. doi:10.1111/spol.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vijh R., Ghafari C., Hayden A. Serological survey following SARS-COV-2 outbreaks at long-term care facilities in metro Vancouver, British Columbia: implications for outbreak management and infection control policies. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.10.009. S0196-6553(20): 30927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.