Abstract
OBJECTIVES:
To examine the magnitude and sources of inpatient cost variation for kidney transplantation.
METHODS:
We used the 2005-2009 Nationwide Inpatient Sample to identify patients who underwent kidney transplantation. We first calculated the patient level cost of each transplantation admission and then aggregated costs to the hospital level. We fit hierarchical linear regression models to identify sources of cost variation and to estimate how much unexplained variation remained after adjusting for case-mix variables commonly found in administrative datasets.
RESULTS:
We identified 8,866 living donor (LDRT) and 5,589 deceased donor (DDRT) kidney transplantations. We found that higher costs were associated with the presence of complications (LDRT 14%, p<0.001; DDRT 24%, p<0.001), plasmapheresis (LDRT 27%, p<0.001; DDRT 27%, p<0.001), dialysis (LDRT 4%, p<0.001) and prolonged length of stay (LDRT 84%%, p<0.001; DDRT 82%, p<0.001). Even after case-mix adjustment, a considerable amount of unexplained cost variation remained between transplant centers (DDRT 52%, LDRT 66%).
CONCLUSIONS:
While significant inpatient cost variation is present across transplant centers, much of the cost variation for kidney transplantation is not explained by commonly used risk-adjustment variables in administrative datasets. These findings suggest that while there is an opportunity to achieve savings through payment reforms for kidney transplantation, policymakers should seek alternative sources of information (e.g., clinical registry data) to delineate sources of warranted and unwarranted cost variation.
Keywords: kidney transplantation, cost, affordable care act, bundled payments, end stage renal disease
INTRODUCTION
In 2011, ESRD expenditures reached $30 billion and 62% of these costs were paid by the Medicare program.1 In order to curtail the costs of treating ESRD, the Centers for Medicare & Medicaid Services (CMS) implemented a bundled payment for dialysis and recently established the Comprehensive ESRD Care Model. While current ESRD payment reforms are focused on dialysis, they may eventually expand to kidney transplantation as part of rapidly expanding value-based initiatives at CMS. The goal of most payment reforms is to reduce the use of extraneous resources that lead to high costs. In other words, payers are interested in reducing unwarranted cost variation among hospitals.
However, there are two important reasons why designing a fair value-based payment model for kidney transplantation is challenging. First, while the objective of payment reform is to reduce unwarranted cost variation, some patients may have higher costs for reasons that are completely warranted. For example, patients undergoing kidney transplantation often require high rates of specialty consultation, advanced imaging, and laboratory testing during the initial hospitalization.2-6 Moreover, transplant centers vary in their use of expensive (but effective) medical procedures such as desensitization and dual kidney transplantation.5-8 While the aforementioned factors are expected to raise the cost of transplantation at some centers, the resulting cost variation is warranted because it reflects case complexity. Second, it is largely unclear whether standard risk-adjustment methods (i.e., using variables in an administrative database) can adequately capture complex clinical factors that lead to higher costs for kidney transplantation. An understanding of the magnitude of inpatient cost variation and potential sources (warranted vs unwarranted) is crucial to design new and fair payment models for kidney transplantation.
In this context, we used an administrative dataset (and common case-mix variables used by payers) to examine the magnitude and sources of inpatient cost variation across transplant centers. We also assessed the magnitude of cost variation that remains unexplained after accounting for case-mix.
METHODS
Dataset
Our primary dataset was the 2005-2009 Nationwide Inpatient Sample (NIS) linked to the American Hospital Association (AHA) annual survey. The NIS dataset is maintained by the Agency for Healthcare Research and Quality and is part of the Healthcare Cost and Utilization Project (HCUP). For each year of the NIS, data on all discharges is available from a 20% sample of approximately 5,000 hospitals in 44 states.
Cohort identification
Using International Classification of Disease, Clinical Modification (ICD-9-CM) procedure codes, we identified all index hospitalizations for adult (>18 years old) kidney transplant recipients. These hospitalization episodes included the recipient’s kidney transplant procedure (ICD-9-CM 55.6 and 55.69) and post-transplant medical and surgical care within that admission. We classified the transplants as living donor renal transplant (LDRT) (00.91 and 00.92), deceased donor renal transplant (DDRT) (00.93) or missing (5.2%). To ensure that we properly extracted all transplanted patients, we used sampling weights provided by HCUP and found that the national estimates from the NIS were similar to the number of transplants reported by the Scientific Registry of Transplant Recipients and the United States Renal Data System.1 We excluded patients who had concurrent liver (50.5X) or pancreas transplants (52.8X) at time of kidney transplantation (5.6%) and patients who did not have total charge or cost-to-charge ratios available (3.4%). To reduce the chance of using inaccurately recorded charges (i.e., charges that lacked face validity), we excluded the bottom 1% of charges. There was no systematic difference among hospitals where charges were dropped and those that had all charges available for analysis.
Calculation of costs
We calculated total hospitalization cost (THC) by first identifying the total charge reported for each recipient’s hospitalization (e.g, charges for room and board, ICU beds, laboratory services, imaging). Professional charges, deceased donor organ acquisition costs, costs associated with living donor nephrectomy, and charges after the index hospitalization were not included in this analysis.9,10 We multiplied the total charge by the hospital specific all-payer inpatient cost-to-charge (APICC) ratio to arrive at total cost. If the APICC was missing (4.6%), we used the group average all-payer inpatient cost-to-charge (GAPICC) ratio. We performed several adjustments to the calculated costs. First, we adjusted all costs to 2005 dollars, in order to account for the secular growth in the cost of kidney transplant. Second, in order to control for geographic variation in input prices, we adjusted costs by the wage index.9 Finally, we log-transformed the cost variable and achieved a normal distribution.
Identification of patient and hospital characteristics
We identified patient characteristics (i.e., recipient age, recipient race, primary payer, recipient gender, median household income of residents from the patient’s zip code, length of stay) using variables directly available in the NIS. Missing data for these variables ranged from 0 to 2.45%, except for race (20.6% missing). For the race variable, we created a “missing data” category. We used ICD-9-CM diagnosis codes to calculate comorbidity using categories described by Elixhauser et al. and grouped patients into one of 4 categories (0, 1, 2, 3 or more comorbidities).11 We also created dummy variables for patients who had therapeutic plasmapheresis (ICD-9-CM 99.71) and/or inpatient hemodialysis (ICD-9-CM 39.95).
Using ICD-9-CM codes and previously described complication schemes we identified whether a major complication occurred during the hospitalization.12,13 Major complications categories included: surgical site infection, sepsis, wound disruption, pulmonary embolism and/or deep vein thrombosis, stroke, myocardial infarction, cardiac arrest, pneumonia, prolonged ventilator use, other infections and genitourinary complications (Appendix Table 1).
Using variables directly from the NIS/AHA, we identified hospital characteristics (teaching status (teaching/nonteaching), bed size (small is <100 beds, medium is 100-299 beds, and large is >299 beds), location (urban, rural), region (south, northwest, east, midwest). Missing hospital variables ranged from 0% to 0.7%. Average annual hospital volume (low, medium, high) was calculated with the assumption that hospitals that were not included in the NIS sample for a particular year had similar volumes during missing and available years. To account for patient complexity arising from prolonged waiting time for kidney transplant, we included a variable for median time to wait for transplant. This statewide variable was available from the Scientific Registry for Transplant Recipients.14
Assessing total cost variation
Total cost variation was defined as the inter-hospital difference in total hospital costs (THC), which include all inpatient costs attributable to a kidney transplant recipient’s admission to the hospital, transplant operation, and post-operative care. Donor costs were not included. For our multivariable analysis, we fit hierarchical linear regression models (HLMs). We used the HLMs for several reasons. First, HLMs account for the clustering of patients within hospitals. Second, HLMs enable us to perform a reliability adjustment.5,18-20 Reliability adjustment is important because hospitals with a small number of transplants may have misclassified costs (i.e., skewed costs). Finally, HLMs allow us to estimate the magnitude of residual cost variation after risk and reliability-adjustment using interclass correlation coefficient. Separate HLM models were fit for LDRT and DDRT episodes, based on the assumption that these respective groups had inherent differences 3,4,6,15-17. Our primary outcome was adjusted log-transformed THC.
For the HLMs, we selected independent variables based on a combination of the literature, expert opinion, and backwards elimination (significance set at p<0.05). We included both patient-level categorical variables (age, race, gender, comorbidity, median income of ZIP code of residence, primary payer, presence of a complication, length of stay, use of inpatient dialysis and use of plasmapheresis) and hospital characteristics (teaching status, bed size, location, region, annual hospital transplant volume, regional wait time for transplant). We performed a pairwise correlation between the cost of living donor renal transplants and deceased donor renal transplants for each hospital. We then ranked hospitals by cost quartile (very low, low, high, very high) and performed comparisons of patient and hospital characteristics between very low and very high cost hospitals.
Sensitivity and outlier analysis
As a sensitivity analysis, we tested our model with and without the race variable because of the large amount of missing data in that category. Additionally, using univariate statistics we compared patient characteristics of the most expensive five hospitals (i.e., outlier analysis) with patients from all other hospitals to ensure that outliers were not driving our results. Finally, because we expected length of stay and complications to substantially impact cost, we performed a second sensitivity analysis where we only studied cost variation in a cohort of patients who were discharged from the hospital in less than five days and had no complications.
We used STATA version 13 for all statistical analyses and statistical significance was set at p < 0.05. This study was deemed exempt from review by the University of Michigan Institutional Research Board.
RESULTS
Descriptive analysis
From 2005-2009, there were 70,027 kidney transplants were performed in the United States. Of these transplants, 8,866 deceased donor renal transplants (DDRT) and 5,589 living donor renal transplants (LDRT) were included in our sample. At the patient level, the unadjusted cost of kidney transplant varied by donor type (DDRT median $44,893, range $15,674 to $533,097; LDRT median $37,133, range $15,544 to $312,986).
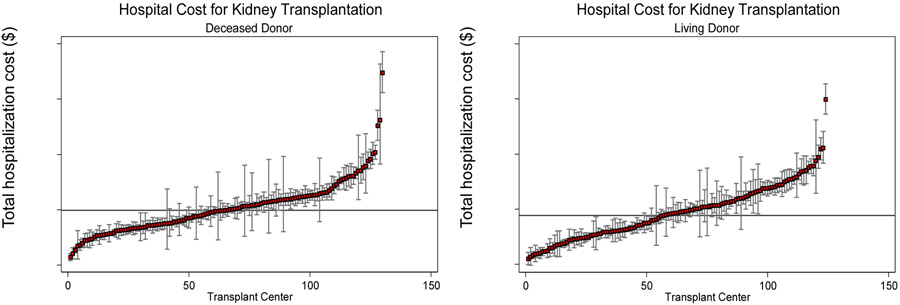
At the hospital level, inpatient costs varied approximately four-fold for both DDRT and LDRT. The adjusted mean cost of DDRT was $39,843 (median $39,740, range $22,782 to $89,442) and LDRT was $38,403 (median $39,029, range $21,907 to $79,772) (Figure 1). We found that hospital cost for living donor renal transplants and deceased donor renal transplants was strongly correlated (0.68, p<0.001) (i.e., high cost hospitals for DDRT were also high cost for DDRT).
Figure 1. Inpatient cost variation for kidney transplantation across transplant centers, deceased and living donor transplants, Nationwide Inpatient Sample, 2005-2009.
Figure illustrates transplant center variation in average hospitalization costs for initial deceased donor and living donor kidney transplant admission with corresponding standard errors. Costs include all hospital expenses from day of hospital admission until discharge. Average cost estimates for each hospital have been risk and reliability-adjusted. Mean cost across all centers is represented by the black line (Deceased donor renal transplant $39,843, Living donor renal transplant $38,403).
Very low and very high cost hospitals were similar with respect to patient and hospital level characteristics (Supplementary Table 2 and 3). Additionally, we found no statistical difference between very low and very high cost hospitals with respect to the individual complications of surgical site infections, wound disruption, sepsis, pulmonary embolism and/or deep vein thrombosis, stroke, acute myocardial infarction, pneumonia, prolonged ventilation, general infections, and genitourinary specific complications (Supplementary Table 4).
Key predictors of high cost for kidney transplant
All predictor variables affected living donor renal transplants and deceased donor renal transplants in a similar fashion (Supplementary Figures 2 and 3). Among other factors, we found that statistically significant higher costs were associated with the presence of complications, inpatient plasmapheresis, inpatient dialysis, prolonged length of stay, and high hospital volume (Table 1). Importantly, after risk-adjustment using the variables available in the NIS, a significant portion of the total cost variation among hospitals remained unexplained (DDRT 52%, LDRT 66%).
Table 1.
Association of total hospitalization cost and selected factors that predict increased cost, Nationwide Inpatient Sample, 2005-2009
| Characteristics | Living donor renal transplant (n=5,568) |
Deceased donor renal transplant (n=8,757) |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | % increase |
p- value* |
Estimate | SE | % increase |
p- value* |
|
| Any complication | 0.131 | 0.012 | 14% | <0.001 | 0.212 | 0.009 | 24% | <0.001 |
| Inpatient dialysis | 0.042 | 0.010 | 4% | <0.001 | 0.001 | 0.005 | 0% | 0.79 |
| Inpatient plasmapheresis | 0.236 | 0.015 | 27% | <0.001 | 0.240 | 0.020 | 27% | <0.001 |
| Length of stay quintiles | <0.001 | |||||||
| 4 or less days (reference) | - | - | - | - | - | - | - | - |
| 5 days | 0.064 | 0.008 | 7% | <0.001 | 0.100 | 0.008 | 10% | <0.001 |
| 6 days | 0.134 | 0.009 | 14% | <0.001 | 0.168 | 0.009 | 18% | <0.001 |
| 7 or 8 days | 0.250 | 0.010 | 28% | <0.001 | 0.275 | 0.009 | 32% | <0.001 |
| More than 8 days | 0.612 | 0.012 | 84% | <0.001 | 0.601 | 0.009 | 82% | <0.001 |
| Hospital volume | ||||||||
| Low (reference) | - | - | - | - | - | - | - | - |
| Medium | 0.172 | 0.064 | 19% | 0.008 | 0.175 | 0.055 | 19% | 0.001 |
| High | 0.182 | 0.067 | 20% | 0.007 | 0.163 | 0.057 | 18% | 0.004 |
Estimates are based on hierarchical linear regression models with primary outcome of log hospitalization cost. Estimates are adjusted for patient level (age, race, gender, comorbidity, median income, primary payer) and hospital level (bed size, location, region, teaching status, median time to transplant) variables. Percent increase was calculated by exponentiating estimates. After removing patients with missing data, 99.6% of living donor renal transplants and 98.7% of deceased donor renal transplants remained in the model.
Sensitivity and outlier analyses
We found no substantial change in the point estimates for key predictor variables with and without the race category included in our model. Additionally, we only found minor differences between outliers and the remaining hospitals in our sample (Supplementary Table 5). When we studied a subgroup of patients who were discharged within five days and had no complications, we found that large portion of variation still remained unexplained (DDRT 75% LDRT 64%).
DISCUSSION
In this study, we found substantial variation in the total hospitalization cost for both living and deceased donor kidney transplants across transplant centers. Complications, the use of inpatient dialysis or plasmapheresis, length of stay, and high hospital volume were associated with higher costs. However, after adjusting for these and other patient and hospital characteristics, we found the majority of variation in total costs of inpatient kidney transplant care among hospitals still remained unexplained.
Unexplained cost variation may be the result of warranted or unwarranted differences in clinical management among transplant centers. For example, transplant centers vary in their patient complexity and their ability to perform advanced therapies within a kidney transplant admission episode. If expensive centers produce high quality care, their higher costs may be justified (i.e., warranted). For example, some aggressive transplant centers may utilize higher risk organs to get their patients transplanted. The utilization of kidneys likely to have delayed graft function, may lead to higher inpatient costs due to longer lengths of stay, but provide patients with excellent long term survival. In contrast to this warranted cost variation, unwarranted variation reflects differences in practice patterns that are not associated with improved outcomes. For example, the use of unnecessary or duplicative imaging and laboratory tests, higher cost immunosuppression for induction, prolonged length of stay for uncomplicated cases21,22 are sources of unwarranted cost variation. Unwarranted variation should be the target of payment reforms such as bundled payments. However, as our study revealed, administrative databases may not sufficiently delineate warranted and unwarranted sources of cost variation (i.e., >50% of the cost variation among centers was unexplained).
While this is the first study to investigate national hospital cost variation for kidney transplant using the NIS, investigators using Medicare claims and have shown that Medicare reimbursement varies considerably among hospitals. 5,24,25 For example, from 2003-2006, 20% of total Medicare reimbursement for kidney transplant was due to outlier and readmission payments. Furthermore, hospitals that have low rates of risk-adjusted mortality tend to have lower Medicare payments and patients who develop complications such as sepsis and/or pneumonia after kidney transplant can increase Medicare payments by up to $48,400. 5,26,27 While from the perspective of Medicare, inpatient hospital cost variation does not directly matter because hospitals are paid by a single diagnosis related group payment, the primary advantage of studying inpatient costs is that it reflects differences in resource utilization of hospitals.
The focus of this analysis was on kidney transplantation, which is a procedure that requires significant resource utilization, is common, and varies in practice across hospitals. Value-based purchasing initiatives are a rapidly gaining traction. All urologists should be mindful of their implications, as these may become payment strategies for even more common urological procedures such nephrectomy, prostatectomy, and cystectomy. With growing support for cost-containment in health care spending and rewarding high quality lean care, it is imperative that surgeons take the lead in informing policymakers of the the benefits and risks of adopting these payment reforms, as well as identifying effective quality improvement strategies that reduce resource utilization without sacrificing quality.
Our study has several limitations. First, while our dataset provided us with general information on the magnitude and sources of cost variation, we cannot sufficiently answer whether the variation is warranted or unwarranted without more clinical data (e.g., from a clinical registry) and without information on processes of care within the transplant center. The latter information that can be elicited from interviews with department administrators and others involved in transplant billing at high and low cost transplant centers. A second limitation is that our dataset does not allow us to calculate costs beyond the initial hospitalization (e.g., costs of readmissions, post-acute care, physician services.) Therefore, a transplant center with low initial hospitalization costs and a high readmission rate would be classified as a low cost center in our study, even though their 30-day costs are high.
The significant cost variation identified in this study has several implications. The finding of cost variation may be the result of inefficiency (unwarranted variation) in the delivery of transplant or the need for better risk adjustment through a clinical registry (warranted variation). CMS is actively testing payment reforms such as bundled payments for many medical diagnoses and surgical procedures. Similar reforms may occur for kidney transplantation. The finding that much of the cost variation among transplant centers is unexplained should encourage CMS policymakers to use transplant-specific risk adjustment with a clinical registry such as the Scientific Registry of Transplant Recipient when designing a new payment model. A better understanding of cost variation is paramount to ensuring optimal patient access/outcomes and the long term viability of transplant programs.
Supplementary Material
Supplementary Figure 1. Number of Kidney Transplants in United States, 2005-2009
Figure illustrates yearly trend in transplants performed from 2005-2009. Yearly numbers are derived from the Nationwide Inpatient Sample (weighted to calculate national estimates), United States Renal Data System (USRDS), and Scientific Registry of Transplant Recipients. Abbreviations: LDRT, Living donor renal transplant, DDRT, deceased donor renal transplant
Supplementary Figure 2. Association between individual patient level factors and cost, Nationwide Inpatient Sample, 2005-2009
Figure illustrates mean predicted cost at each level of the shown categorical variables. OLS regression adjusted for clustering was used to arrive at estimates. Log transformed cost was dependent variable in each model. Exposure of interest and donor type were independent variables. DDRT=deceased donor renal transplant, LDRT=living donor renal transplant. e10.0~$22k, e10.5~$36k, e11.0~$60k, e11.5~$99k
Supplementary Figure 3. Association between individual hospital level factors and cost, Nationwide Inpatient Sample, 2005-2009
Figure illustrates mean predicted cost at each level of the shown categorical variables. OLS regression adjusted for clustering was used to arrive at estimates. Log transformed cost was dependent variable in each model. Exposure of interest and donor type were independent variables. DDRT=deceased donor renal transplant, LDRT=living donor renal transplant. e10.0~$22k, e10.5~$36k, e11.0~$60k, e11.5~$99k
ACKNOWLEDGEMENTS:
Dr. Miller reports serving as a paid consultant for ArborMetrix.
FINANCIAL SUPPORT:
This research was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney (T32 DK 7782-14 to Dr. Ellimoottil)
No funding organization played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST:
All authors: None
This manuscript was presented at the American Society of Transplant Surgeons Winter Meeting, Miami, Florida (Jan 2014) and at the American Urological Association meeting, Naples Florida (May 2014).
REFERENCES
- 1.USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2012. [DOI] [PubMed] [Google Scholar]
- 2.Abecassis M: Organ acquisition cost centers Part I: medicare regulations--truth or consequence. Am. J. Transplant 2006; 6: 2830–5. [DOI] [PubMed] [Google Scholar]
- 3.Axelrod D a, Lentine KL, Salvalaggio PR, et al. : The cost and quality paradox. Am. J. Transplant 2009; 9: 985–6. [DOI] [PubMed] [Google Scholar]
- 4.Beach-Langlois M and Yankasky P: Transplant cost-report tracking at Henry Ford Transplant Institute and other centers nationwide. Prog. Transplant 2011; 21: 169–73. [DOI] [PubMed] [Google Scholar]
- 5.Englesbe MJ, Dimick JB, Fan Z, et al. : Case mix, quality and high-cost kidney transplant patients. Am. J. Transplant 2009; 9: 1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englesbe MJ, Ads Y, Cohn J a, et al. : The effects of donor and recipient practices on transplant center finances. Am. J. Transplant 2008; 8: 586–92. [DOI] [PubMed] [Google Scholar]
- 7.Englesbe MJ, Dubay DA, Gillespie BW, et al. : Risk factors for urinary complications after renal transplantation. Am. J. Transplant 2007; 7: 1536–41. [DOI] [PubMed] [Google Scholar]
- 8.Maggiore U, Oberbauer R, Pascual J, et al. : Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol. Dial. Transplant 2015; 30(2):217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman B, Jiang HJ, Elixhauser A, et al. : Hospital inpatient costs for adults with multiple chronic conditions. Med. Care Res. Rev 2006; 63: 327–46. [DOI] [PubMed] [Google Scholar]
- 10.Makarov DV, Loeb S, Landman AB, et al. : Regional variation in total cost per radical prostatectomy in the healthcare cost and utilization project nationwide inpatient sample database. J. Urol 2010; 183: 1504–9. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med. Care 1998; 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 12.Tan H-J, Norton EC, Ye Z, et al. : Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA 2012; 307: 1629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eappen S, Lane BH, Rosenberg B, et al. : Relationship between occurrence of surgical complications and hospital finances. JAMA 2013; 309: 1599–606. [DOI] [PubMed] [Google Scholar]
- 14.Scientific Registry of Transplant Recipients. http://www.srtr.org. Accesed February 10, 2014.
- 15.Schwartz J, Stegall MD, Kremers WK, et al. : Complications, resource utilization, and cost of ABO-incompatible living donor kidney transplantation. Transplantation 2006; 82: 155–63. [DOI] [PubMed] [Google Scholar]
- 16.McAdams-Demarco M a, Grams ME, Hall EC, et al. : Early hospital readmission after kidney transplantation: patient and center-level associations. Am. J. Transplant 2012; 12: 3283–8. [DOI] [PubMed] [Google Scholar]
- 17.Hurst FP, Abbott KC, Neff RT, et al. : Incidence, predictors and outcomes of transplant renal artery stenosis after kidney transplantation: analysis of USRDS. Am. J. Nephrol 2009; 30: 459–67. [DOI] [PubMed] [Google Scholar]
- 18.Dimick JB, Staiger DO and Birkmeyer JD: Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv. Res 2010; 45: 1614–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimick JB, Ghaferi A a, Osborne NH, et al. : Reliability adjustment for reporting hospital outcomes with surgery. Ann. Surg 2012; 255: 703–7. [DOI] [PubMed] [Google Scholar]
- 20.Adams JL, Mehrotra A, Thomas JW, et al. : Physician cost profiling--reliability and risk of misclassification. N. Engl. J. Med 2010; 362: 1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chughtai B, Abraham C, Finn D, et al. : Fast track open partial nephrectomy: reduced postoperative length of stay with a goal-directed pathway does not compromise outcome. Adv. Urol 2008: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muehling B, Schelzig H, Steffen P, et al. : A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J. Surg 2009; 33: 577–85. [DOI] [PubMed] [Google Scholar]
- 23.Adler JT, Sethi RKV, Yeh H, et al. : Market competition influences renal transplantation risk and outcomes. Ann. Surg 2014; 260: 550–7. [DOI] [PubMed] [Google Scholar]
- 24.Miller DC, Gust C, Dimick JB, et al. : Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health Aff. (Millwood) 2011; 30: 2107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birkmeyer JD, Gust C, Baser O, et al. : Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv. Res 2010; 45: 1783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimick JB, Chen SL, Taheri P a, et al. : Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J. Am. Coll. Surg 2004; 199: 531–7. [DOI] [PubMed] [Google Scholar]
- 27.Kutinova a, Woodward RS, Ricci JF, et al. : The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. Am. J. Transplant 2006; 6: 129–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Number of Kidney Transplants in United States, 2005-2009
Figure illustrates yearly trend in transplants performed from 2005-2009. Yearly numbers are derived from the Nationwide Inpatient Sample (weighted to calculate national estimates), United States Renal Data System (USRDS), and Scientific Registry of Transplant Recipients. Abbreviations: LDRT, Living donor renal transplant, DDRT, deceased donor renal transplant
Supplementary Figure 2. Association between individual patient level factors and cost, Nationwide Inpatient Sample, 2005-2009
Figure illustrates mean predicted cost at each level of the shown categorical variables. OLS regression adjusted for clustering was used to arrive at estimates. Log transformed cost was dependent variable in each model. Exposure of interest and donor type were independent variables. DDRT=deceased donor renal transplant, LDRT=living donor renal transplant. e10.0~$22k, e10.5~$36k, e11.0~$60k, e11.5~$99k
Supplementary Figure 3. Association between individual hospital level factors and cost, Nationwide Inpatient Sample, 2005-2009
Figure illustrates mean predicted cost at each level of the shown categorical variables. OLS regression adjusted for clustering was used to arrive at estimates. Log transformed cost was dependent variable in each model. Exposure of interest and donor type were independent variables. DDRT=deceased donor renal transplant, LDRT=living donor renal transplant. e10.0~$22k, e10.5~$36k, e11.0~$60k, e11.5~$99k