Abstract
Objective
To investigate changes in the median nerve, retinaculum, and carpal tunnel on ultrasound after successful endoscopic carpal tunnel release (ECTR).
Materials and Methods
This prospective study involved 37 wrists in 35 patients (5 male, 30 female; mean age ± standard deviation [SD], 56.9 ± 6.7 years) with primary carpal tunnel syndrome (CTS). An in-house developed scoring system (0–3) was used to gauge the clinical improvement after ECTR. Ultrasound was performed before ECTR, and at 1, 3, and 12 months post-ECTR. Changes in the median nerve, flexor retinaculum, and carpal tunnel morphology on ultrasound after ECTR were analyzed. Ultrasound parameters for different clinical improvement groups were compared.
Results
All patients improved clinically after ECTR. The average clinical improvement score ± SD at 12 months post-ECTR was 2.2 ± 0.7. The median nerve cross-sectional area proximal and distal to the tunnel decreased at all time intervals post-ECTR but remained swollen compared to normal values. Serial changes in the median nerve caliber and retinacular bowing after ECTR were more pronounced at the tunnel outlet than at the tunnel inlet. The flexor retinaculum had reformed in 25 (68%) of 37 wrists after 12 months.
Conclusion
Postoperative changes in median nerve and retinaculum parameters were most pronounced at the tunnel outlet. Even in patients with clinical improvement after ECTR, nearly all ultrasound parameters remain abnormal at one year post-ECTR. These ultrasound parameters should not necessarily be relied upon to diagnose persistent CTS after ECTR.
Keywords: Ultrasonography, Carpal tunnel syndrome, Wrist, Median nerve, Surgery
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most common peripheral nerve entrapment syndrome, which is usually idiopathic rather than secondary in etiology. Typical presenting features include hand numbness, pain, weakness, and thenar muscle atrophy. Conservative treatment is usually undertaken initially followed by surgical carpal tunnel release (CTR), either as an open, endoscopic, or ultrasound-guided procedure, if symptoms persist [1]. Even after CTR, up to one-fifth of patients may have persistent symptoms due to either incomplete release, perineural fibrosis, or flexor tenosynovitis [2,3,4,5,6].
Although nerve conduction testing (NCT) is still considered the diagnostic gold standard for CTS, the use of ultrasound for diagnosis has become increasingly popular over the past two decades due to the clarification of the diagnostic criteria, increased availability, lower cost, and technological advances [7,8,9,10]. NCT is sometimes used to evaluate the changes after CTR, although some abnormal nerve function often persists on NCT despite symptomatic improvement [11,12]. As such, surgical outcomes following CTR are best assessed clinically rather than with NCT [13]. Using ultrasound to evaluate patients with persistent symptoms following CTR is likely to become more popular with the increasing popularity of ultrasound for preoperative evaluation [3]. Before determining the significance of any postoperative ultrasound findings, we first need to have a clear understanding of the expected ultrasound findings following CTR. Several studies have documented changes in median nerve caliber and carpal tunnel size at 1?6 months following CTR [11,14,15,16,17,18], while one study investigated the serial ultrasound changes for up to one year following CTR [18].
The purpose of this study was to document changes in a wide range of ultrasound parameters related to the median nerve, flexor retinaculum, and carpal tunnel before (baseline) and at 1, 3, and 12 months after endoscopic CTR (ECTR) to clarify the expected post-surgical ultrasound findings.
MATERIALS AND METHODS
This prospective study was conducted between April 2013 and December 2017 in primary CTS patients treated with ECTR. Approval was obtained from the local ethics committee with informed consent obtained (CREC Ref. No. 2013.277). Patients at risk of secondary CTS such as those with gout, inflammatory arthropathy, or previous wrist trauma were excluded. Thirty-seven wrists (2 bilateral) in 35 patients (5 men, 30 women; mean age ± standard deviation [SD], 56.9 ± 6.7 years; 25 right and 12 left) were studied.
Clinical Assessment
CTS was diagnosed clinically and confirmed by NCT in all patients. Clinical assessments were performed by one of three orthopedic hand surgeons, each with more than 10 years of clinical experience. ECTR was offered to patients with persistent troublesome symptoms (paraesthesia, numbness, pain, and weakness) after six months of conservative treatment, mainly comprising night splinting and physiotherapy. All ECTR surgeries were performed by one of the same three hand surgeons. A two-portal ECTR technique was used to divide the flexor retinaculum with a retrograde hook knife [19]. Complete retinacular transection was confirmed by tactile and visual endoscopic assessment, as well as by transillumination. An in-house developed scoring system graded the clinical effect of ECTR at 2 weeks, 3 months, and 12 months post-ECTR. This ‘clinician improvement score’ ranged from 0 to 3 with 0 = no improvement; 1 = mild improvement (< 50% improvement in symptoms); 2 = good improvement (> 50% improvement in symptoms); 3 = complete or almost complete (> 90% improvement in symptoms) based on a combined patient-clinician perception of symptom improvement following ECTR. Average time ± SD for the 1st, 2nd, and 3rd follow-up postoperative clinical assessments were 11.9 ± 5.7 days, 140.7 ± 107.6 days, and 361.5 ± 104.3 days, respectively.
Ultrasound Examination
Baseline, 1st, 2nd, and 3rd post-ECTR ultrasound examinations were performed by the same musculoskeletal radiologist (Reader 1), with 20 years of ultrasound experience, on a single ultrasound unit (Affiniti 70 using a high-resolution linear-array 12–17-MHz transducer. The average time (SD) for the baseline ultrasound was 16.7 ± 15.6 days before surgery, while the 1st, 2nd, and 3rd postoperative assessments were performed at 36.2 ± 15.8, 123.6 ± 58.8, and 387.9 ± 59.2 days post-ECTR, respectively.
Examinations were performed with the wrist resting on a table in a supine neutral position, and the fingers were partially flexed. Ultrasound of the volar distal forearm, wrist, and carpal regions was performed mainly in the transverse plane, but also in the longitudinal plane. The circumferential surface area (CSA), major, and minor axes of the median nerve were measured at four different levels, namely immediately proximal to the tunnel inlet (CSAp), at the tunnel inlet (CSAi), at the outlet (CSAo), and immediately distal to the tunnel outlet (CSAd). The flattening ratio (FR) was defined as the major axis divided by the minor axis. The major axis was the maximum width of the median nerve, and the minor axis was the maximum depth of the median nerve perpendicular to the major axis. The CSA was measured using a continuous tracing method along the inner border of the epineurium. Neural epineurium was not included because of the difficulty in defining its outer border [20]. The tunnel inlet and outlet were identified through recognition of the proximal and distal ends of the flexor retinaculum. ‘Proximal to the tunnel’ was immediately proximal to the tunnel just before the nerve dips to pass below the flexor retinaculum. ‘Tunnel inlet’ and ‘outlet’ locations were immediately deep to the proximal and distal margins of the flexor retinaculum respectively. ‘Distal to the tunnel’ was that location immediately distal to the tunnel just after the median nerve emerges from beneath the flexor retinaculum. The median nerve ‘caliber-change ratio’ from proximal to the tunnel inlet was expressed as CSAp/CSAi, while from the outlet to just distal to the tunnel was expressed as CSAd/CSAo.
The flexor retinaculum can be clearly seen in non-operated patients as a thick echogenic band. Following CTR, the retinaculum is less easily seen, although it can still be readily demarcated by slight toggling of the transducer to optimize visualization of the retinacular margins. Palmar retinacular bowing (BR) at the inlet and outlet levels were defined as the height from the deep margin of the retinaculum perpendicular to a tangential line drawn between the most volar aspects of the pisiform and scaphoid bones at the tunnel inlet or the trapezium and hook of the hamate at the tunnel outlet. Positive and negative values indicated a palmar reticulum above and below the tangential line, respectively. The visibility of neural fasciculation and the presence of intraneural vascularity were recorded. The presence of a gap in the flexor retinaculum and the widest distance was measured. Carpal tunnel CSA was measured at the tunnel inlet and outlet by continuous tracing along the bony outline of the carpal tunnel to the undersurface of the retinaculum.
To determine the inter-observer reliability of the measurement, another radiologist (Reader 2, with 2 years of experience in musculoskeletal ultrasound) measured the same parameters on captured images without measurements, and these results were compared to the results of Reader 1. The same parameters were further measured after four weeks on the same captured images by the same radiologist who performed the first ultrasound examination (Reader 1), blinded to the initial findings.
Statistical Analysis
Statistical software was used (SPSS, version 21 for Windows; IBM Corp.) for data analysis. A paired t test was used to compare differences between continuous parameters measured on pre- and post-ECTR ultrasound examinations. ANOVA was used to assess differences in serial ultrasound parameters post-ECTR. Patients were divided into groups according to their clinical scores. ANOVA was used to compare differences in ultrasound parameters among the different clinical improvement score patient groups. Intra- and inter-observer reliability for loss of fasciculation or presence of vascularity were calculated using Cohen's kappa method, while the intra- and inter-observer reliability of continuous data was calculated using the intraclass correlation (ICC) test. The ICC r < 0.20 implied poor agreement; 0.21–0.40 fair agreement; 0.41–0.60 moderate agreement; 0.61–0.80 substantial agreement; and 0.81–1.00 excellent agreement. A probability of p < 0.05 was regarded as a significant difference.
RESULTS
The intra-observer (ICC range 0.83–0.91) agreements were excellent, and the inter-observer (ICC range 0.61–0.97) agreement was substantial to excellent for all continuous parameters. The intra-observer agreement (kappa = 0.85) was excellent, but the inter-observer agreement (kappa = 0.58) was moderate for the grading of neural fasciculation.
Symptoms
No patient had a clinical improvement score of 0 at 12 months post-ECTR. All patients had some symptomatic improvement following ECTR with clinical improvement scores of 1 (n = 6), 2 (n = 17), and 3 (n = 14) at 12 months. Average clinical improvement scores ± SD at 1, 3, and 12 months post-ECTR were 2.0 ± 0.9, 2.1 ± 0.8, and 2.2 ± 0.7, respectively. No significant difference (p = 0.17–0.90) in any of the ultrasound parameters for the three different ‘clinical improvement score’ groups was found.
Median Nerve CSA
Median nerve CSA proximal to the tunnel significantly decreased at 1, 3, and 12 months post-ECTR compared to baseline CSA (Fig. 1, Table 1). Median nerve CSA at the outlet, but not at the inlet, significantly increased at 1, 3, and 12 months post-ECTR when compared to baseline CSA. Distal to the tunnel, median nerve CSA significantly increased at 1 month, but not at 3 and 12 months (Supplementary Fig. 1). At all levels, there was no significant serial change in CSA from 1 to 12 months post-ECTR (Fig. 2).
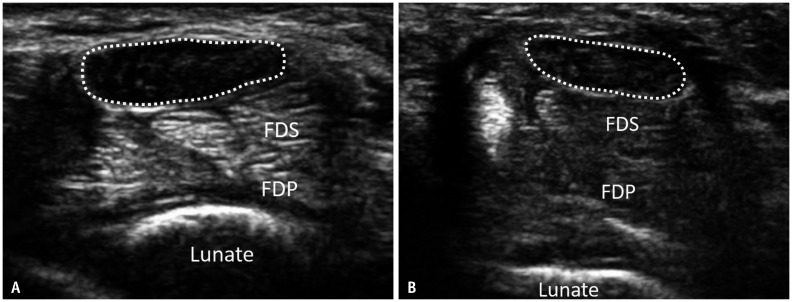
Fig. 1. Transverse ultrasound shows the median nerve proximal to the carpal tunnel in a patient with a clinical improvement score of 2 at baseline (A) and at 3 month (B) post-endoscopic carpal tunnel release.
A. At baseline, the median nerve (dotted lines) is moderately swollen (26.8 mm2) with a loss of normal fasciculation. B. At 3 months, the median nerve remains persistently swollen (25.4 mm2) with loss of normal fasciculation. FDP = flexor digitorum profundus, FDS = flexor digitorum superficialis
Table 1. Values of Each Parameter of the Median Nerve CSA at Different Levels (p, i, o, d) at Baseline, 1, 3, and 12 Months after Endoscopic Carpal Tunnel Release.
| Baseline | 1 Month | 3 Months | 12 Months | ||||
|---|---|---|---|---|---|---|---|
| Measurement | Measurement | P | Measurement | P | Measurement | P | |
| CSAp, mm2 | 19.9 ± 9.6 | 17.9 ± 8.6 (↓ 10.1) | 0.006* | 17.9 ± 7.2 (↓ 10.1) | 0.012* | 17.3 ± 7.8 (↓ 13.1) | 0.001* |
| CSAi, mm2 | 16.1.0 ± 12.9 | 16.3 ± 6.1 (↑ 1.2) | 0.911 | 16.1 ± 5.6 (0) | 0.899 | 15.8 ± 5.0 (↓ 0.2) | 0.915 |
| CSAo, mm2 | 9.5 ± 3.0 | 15.8 ± 6.6 (↑ 66.3) | < 0.001* | 13.0 ± 4.4 (↑ 36.8) | < 0.001* | 13.8 ± 3.8 (↑ 45.3) | < 0.001* |
| CSAd, mm2 | 18.2 ± 8.3 | 20.1 ± 7.9 (↑ 10.4) | 0.047* | 19.1 ± 8.8 (↑ 4.9) | 0.339 | 17.5 ± 5.8 (↓ 3.8) | 0.437 |
| CSAp/CSAi | 1.5 ± 1.0 | 1.1 ± 0.3 (↓ 26.7) | 0.004* | 1.1 ± 0.3 (↓ 26.7) | 0.021* | 1.1 ± 0.6 (↓ 26.7) | 0.003* |
| CSAd/CSAo | 2.0 ± 1.0 | 1.4 ± 0.5 (↓ 63.7) | < 0.001* | 1.5 ± 0.7 (↓ 25.0) | 0.003* | 1.3 ± 0.5 (↓ 35.0) | < 0.001* |
Data are mean ± standard deviation with % change compared to baseline in parentheses. p values are for the comparison with baseline measurements. *Significant (p < 0.05) probabilities. CSA = cross sectional area, CSAd/CSAo = median nerve ‘caliber-change ratio’ at outlet, CSAp/CSAi = median nerve ‘caliber-change ratio’ at inlet, d = distal, i = inlet, o = outlet, p = proximal
Fig. 2. Box-and-whisker plots of serial change in CSA of the median nerve at different levels (p, i, o, d) of carpal tunnel before and after endoscopic carpal tunnel release.
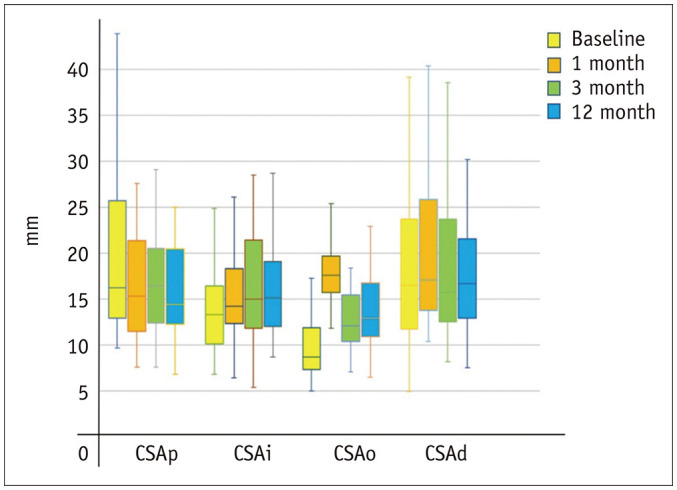
There is a significant decrease in median nerve CSA proximal to the carpal tunnel and a significant increase in CSA of the median nerve at the tunnel outlet one month post-ECTR. CSA = cross-sectional area, d = distal, i = inlet, o = outlet, p = proximal
Median Nerve ‘Caliber-Change Ratio’
Median nerve ‘caliber-change ratio’ significantly decreased at both the inlet and outlet at 1, 3, and 12 months post-ECTR when compared to baseline values (Table 1). This change was most significant at the outlet 12 months post-ECTR with a 35% reduction in the ‘caliber-change ratio’ (outlet), signifying a less abrupt change in nerve caliber. No significant serial changes in the ‘caliber-change ratio’ between 1 to 12 months post-ECTR were apparent (Fig. 3).
Fig. 3. Box-and-whisker plots of serial change of the caliber-change ratio and FR of the median nerve at inlet and outlet levels of the carpal tunnel before and after ECTR.
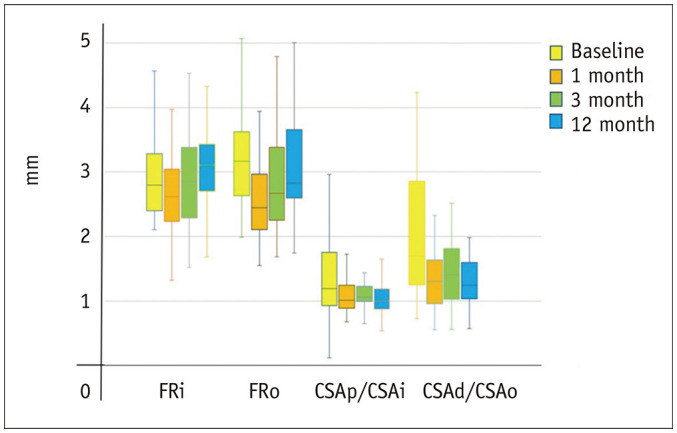
There was a significant decrease in caliber-change ratio and FR one month after ECTR. The changes in caliber-change ratio and FR tended to be more pronounced at the outlet than the inlet. CSA = cross-sectional area, CSAp/CSAi = median nerve ‘caliber-change ratio’ at inlet, CSAd/CSAo = median nerve ‘caliber-change ratio’ at outlet, d = distal, ECTR = endoscopic carpal tunnel release, FR = flattening ratio, i = inlet, o = outlet, p = proximal
Median Nerve Flattening Ratio
The median nerve FR significantly decreased at the inlet and outlet at 1 month post-ECTR compared to baseline values, signifying the expansion of the median nerve (Table 2, Fig. 4). However, the median nerve became increasingly flattened again at the inlet and outlet at 3 and 12 months post-ECTR (Tables 2, 3, Fig. 3).
Table 2. Values of Parameter of Retinaculum and Carpal Tunnel at Different Levels (i, o) at Baseline, 1, 3, and 12 Months after Endoscopic Carpal Tunnel Release.
| Baseline | 1 Month | 3 Months | 12 Months | ||||
|---|---|---|---|---|---|---|---|
| Measurement | Measurement | P | Measurement | P | Measurement | P | |
| FRi | 3.0 ± 0.7 | 2.6 ± 0.7 (↓ 13.7) | 0.029* | 2.9 ± 0.8 (↓ 3.7) | 0.842 | 3.2 ± 0.9 (↑ 6.7) | 0.354 |
| FRo | 3.2 ± 0.7 | 2.6 ± 0.7 (↓ 18.7) | < 0.001* | 2.8 ± 0.8 (↓ 12.5) | 0.021* | 3.1 ± 0.9 (↓ 3.1) | 0.716 |
| RBi, mm | 2.8 ± 1.0 | 4.1 ± 1.1 (↑ 46.4) | < 0.001* | 3.7 ± 1.2 (↑ 32.0) | < 0.001* | 3.5 ± 1.3 (↑ 25.0) | 0.036* |
| RBo, mm | 1.2 ± 0.9 | 2.9 ± 1.2 (↑ 142) | < 0.001* | 2.4 ± 1.2 (↑ 100) | < 0.001* | 1.9 ± 1.2 (↑ 58.3) | 0.050 |
| CTi, cm2 | 1.6 ± 0.35 | 1.8 ± 0.3 (↑ 12.5) | 0.004* | 1.7 ± 0.3 (↑ 6.3) | < 0.001* | 1.7 ± 0.3 (↑ 6.3) | 0.087 |
| CTo, cm2 | 1.3 ± 0.3 | 1.5 ± 0.3 (↑ 15.4) | < 0.001* | 1.4 ± 0.3 (↑ 7.7) | < 0.001* | 1.4 ± 0.2 (↑ 7.7) | < 0.001* |
| Gap, mm | - | 5.4 ± 1.0 | - | 4.7 ± 1.9 | 0.180 | 1.8 ± 2.8 | < 0.001* |
Data are mean ± standard deviation with % change compared to baseline in parentheses. p values are for the comparison with baseline measurements. *Significant (p < 0.05) probabilities. CT = carpal tunnel cross-sectional area, FR = flattening ratio, i = inlet, o = outlet, RB = palmar retinacular bowing
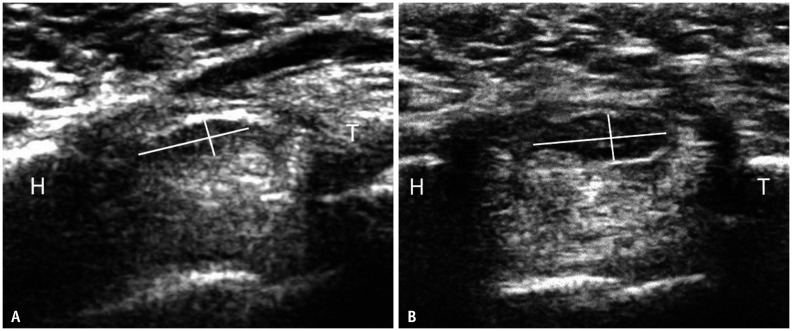
Fig. 4. Transverse ultrasound of a patient with a clinical improvement score of 2 shows the median nerve at the carpal tunnel outlet at baseline (A) and 12 months (B) post-endoscopic carpal tunnel release.
The FR of the median nerve is the ratio of the major and minor axes (white lines). The major axis is the maximum width of the median nerve and the minor axis is the maximum depth of the median nerve perpendicular to the major axis. The median nerve at 12 months appears less flattened (FR = 2.6) when compared to baseline (FR = 3.1). FR = flattening ratio, H = Hook of hamate, T = trapezium
Table 3. Comparison of Different Parameter of the Median Nerve, Retinaculum and Carpal Tunnel at Different Levels (i, o) 1, 3, and 12 Months Post-Endoscopic Carpal Tunnel Release Using ANOVA and Paired t Tests.
| ANOVA between Group | 1 vs. 3 Months | 3 vs. 12 Months | 1 vs. 12 Months | |
|---|---|---|---|---|
| FRi | 0.035* | 0.029* | 0.029* | 0.228 |
| FRo | 0.011* | 0.007* | 0.007* | 0.278 |
| RBo | 0.002* | 0.144 | 0.212 | 0.001* |
| Gap | < 0.001* | 0.182 | < 0.001* | < 0.001* |
*Significant (p < 0.05) probabilities. The parameters with either one of the result has significant difference (p < 0.05) were shown. FR = median nerve flattening ratio, i = inlet, o = outlet, RB = palmar retinacular bowing
Neural Vascularity
Neural vascularity was present in 17 (46%) of 37 wrists at baseline. Vascularity disappeared in 10 (27%) of the 37 patients, while in 7 (19%) of the 37 patients, neural vascularity remained at all time points up to and including 12 months (Fig. 5).
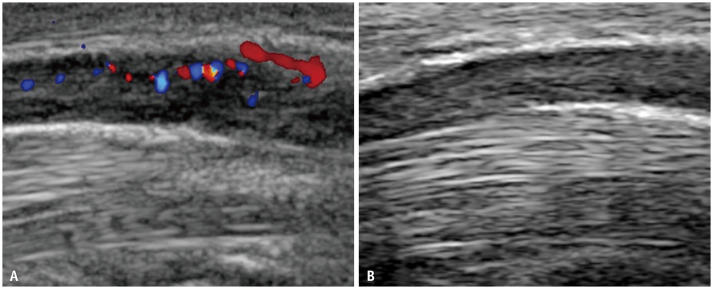
Fig. 5. Longitudinal ultrasound shows the median nerve within the carpal tunnel in a patient with a clinical improvement score of 1 preoperatively (A) and at 12 month (B) post-endoscopic carpal tunnel release.
The hyperemia that was evident at baseline subsided by 12 months.
Neural Fasciculation
Loss of neural fasciculation was evident in 32 (86%) of 37 wrists at baseline. Neural fasciculation returned in two (6%) of these patients, while in 30 (94%) patients, neural fasciculation remained absent at all time points up to and including 12 months (Fig. 1).
Retinacular Bowing
BR increased significantly at the inlet and outlet at all time intervals post-ECTR compared to baseline values, signifying the expansion of the carpal tunnel (Figs. 6, 7, Supplementary Fig. 2, Table 2). This palmar bowing of the retinaculum was maximum at 1 month and progressively decreased thereafter between 1 and 12 months (Tables 2, 3).
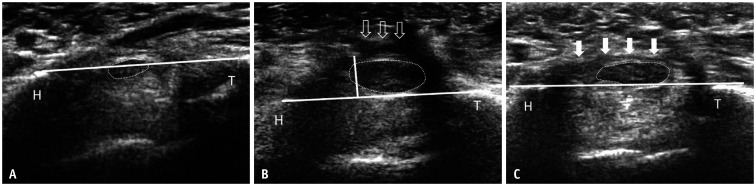
Fig. 6. Transverse ultrasound of the same patient with a clinical improvement score of 2 shows the median nerve at the carpal tunnel outlet at baseline (A), 1 month (B), and 12 months (C) post-ECTR.
A. Tangential line is drawn between the T and H as shown. Palmar bowing of the flexor retinaculum is minimal (0.09 mm). The median nerve is outlined by dots (0.083 cm2). B. At one month post-ECTR, there is a well-defined gap (block arrows) between the retracted ends of the flexor retinaculum. Palmar bowing of the retinaculum is severe as reflected by the vertical line joining the highest point of the undersurface of the flexor retinaculum and the transcarpal line (0.42 mm). Median nerve CSA, as measured by the continuous tracing method, has significantly increased (0.29 cm2) when compared to the preoperative ultrasound (0.083 cm2). C. Ultrasound at 12 months shows less volar bowing (0.27 mm) with a reformed flexor retinaculum (arrows). The CSA of the median nerve has reduced (0.20 cm2) compared to one month post-ECTR. CSA = cross sectional area, ECTR = endoscopic carpal tunnel release, H = Hook of hamate, T = trapezium
Fig. 7. Box-and-whisker plots of serial change of the volar RB at different levels at inlet and outlet levels (i, o) of the carpal tunnel before and after ECTR.
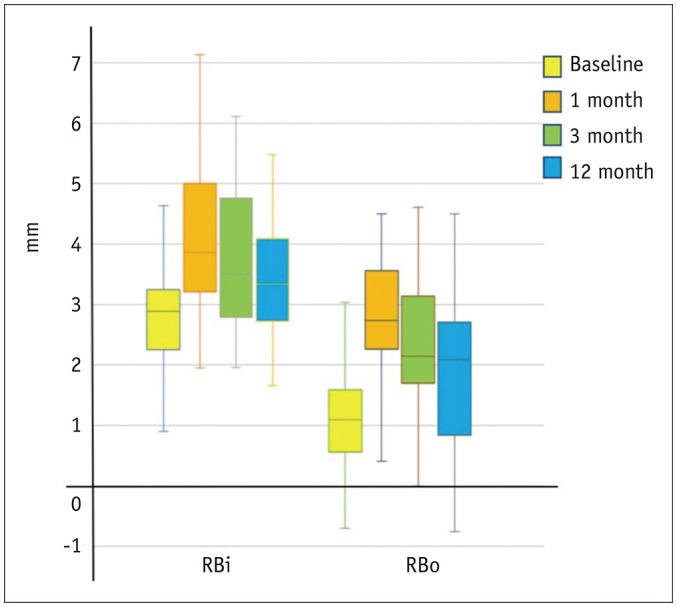
There was a significant increase in retinaculum bowing one month after ECTR, which then gradually flattened in the 3-month and 12-month scans. The changes in RB tended to be more pronounced at the outlet than the inlet. ECTR = endoscopic carpal tunnel release, i = inlet, o = outlet, RB = palmar retinacular bowing
Carpal Tunnel CSA
Carpal tunnel CSA significantly increased at one month post-ECTR at the inlet and outlet, and tended to decrease slightly between one and three months, after which it remained unchanged for 12 months (Table 2).
Retinaculum Gap
A retinaculum gap was seen in all 37 (100%, average gap ± SD, 5.4 ± 1.0 mm) wrists at one month post-ECTR, in 34 (92%, 4.7 ± 1.9 mm) of 37 wrists at 3 months, and in 12 (32%, 1.8 ± 2.8 mm) of 37 wrists at 12 months (Fig. 6, Supplementary Fig. 2, Tables 2, 3). At 12 months, 25 (68%) of the 37 patients had reformed the retinacula, leading to a large SD in gap measurements at 12 months (Table 2).
DISCUSSION
Persistent functional impairment in patients with surgically successful CTR is due to the irreversible or slowly reversible pathological changes of the median nerve as a consequence of a chronic compressive neuropathy [11,12,21,22,23,24]. Surgical outcome is best assessed clinically, though it is limited by pain and numbness at the wound site [13,25]. Ultrasound has been increasingly used to help diagnose CTS, exclude secondary causes, and assess patients with persistent symptoms following CTR [7,8,26,27,28]. However, normal ultrasound findings after CTR have not yet been firmly established. Six previous studies have investigated serial ultrasound changes after CTR; however, these studies focused only on median nerve caliber, but did not, other than the limited mention of the carpal tunnel size by Lee et al. [15], address other parameters used as diagnostic criteria for CTS [11,14,15,16,17,18]. The follow-up period for most studies was 3–6 months, and only one study followed patients for one year [18].
The main finding of the current study was that nearly all the discriminatory criteria for CTS [7,11,29,30], reduced in severity, but remained abnormal for up to 12 months after ECTR, despite good symptomatic improvement in most patients. Based on these findings, care should be taken when using these parameters as an indicator of unsuccessful surgery in patients with continuing symptoms following CTR. The second main finding of this study was that the reduction in median nerve CSA, “caliber-change ratio,” and FR was more pronounced at the tunnel outlet than at the inlet, suggesting that the outlet is more likely to be the main site of neural compression in patients with CTS.
The median nerve CSA proximal to the tunnel significantly decreased by 2–2.6 mm2 (i.e., 10–13%) at 1, 3, and 12 months post-ECTR, which is similar to most previous studies [11,13,16,17,18,31,32,33]. This reduction was evident one month post-ECT [11,30,34], although, similar to previous studies, after one month, no further reduction occurred for up to 12 months post-ECTR [11,18]. This early reduction in median nerve CSA is most likely due to reduced neural venous congestion [12,35], while persistent median nerve swelling thereafter [7,11,29,30], most likely reflects irreversible or slowly reversible endoneural fibrosis due to chronic compressive neuropathy [29,33,36]. Even though most patients had moderate to complete symptom resolution post-ECTR, the median nerve remained swollen from, on average, 13.8 mm2 to 17.3 mm2 at 12 months post-ECTR. This degree of median nerve swelling would be considered indicative of CTS in non-operated wrists, although, as seen in this study, it is an expected feature in patients with successful ECTR [7,8,9].
Compression of the median nerve at the tunnel inlet and outlet leads to a characteristic ‘hour-glass’ configuration, with the nerve being compressed more at the inlet and outlet than just proximal or distal to the tunnel, respectively [37]. A reduction in the median nerve ‘caliber-change ratio’ signifies a reduction of the hour-glass appearance. This ‘caliber-change ratio’ decreased at the inlet and outlet at all time intervals up to 12 months post-ECTR, with the most significant change being at the outlet one month post-ECTR. This parameter may be a useful early and late indicator of successful ECTR, although further study of this parameter over a longer period is needed.
Regarding nerve flattening, the median nerve became less flattened one month post-ECTR, returning thereafter to a level comparable to the preoperative levels, which may be related to the re-constitution of the retinaculum. Following ECTR, approximately 10% of retinacula were seen to be reformed at 3 months and approximately 70% by 12 months.
Intraneural hyperemia persisted in about one-sixth of the patients for at least 12 months post-ECTR. Neural hyperemia is thought to be due to ischemia-induced stimulation of vascular endothelial growth factor and is a diagnostic criterion for CTS [38]. Similarly, loss of neural fasciculation is considered a useful ultrasound sign of CTS [7], although it persists in most patients for at least 12 months post-ECTR.
The normal carpal tunnel is narrowest at the outlet [39]. In CTS, the median nerve is usually compressed at either the inlet or the outlet [40]. As seen previously, following ECTR [11,13,15], both median nerve CSA and BR increased much more at the outlet than at the inlet, indicating how retinacular release had a greater effect at the outlet. This suggests that in CTS, the main site of median nerve compression may be the tunnel outlet rather than the tunnel inlet.
In patients with persistent CTS symptoms, ultrasound has been used to exclude incomplete retinacular resection [41]. As seen in this study, one can expect the retinaculum to be reformed in approximately 10% of symptomatically improved patients by three months and 70% by 12 months post-ECTR.
In addition, it is not clear whether a reduction in median nerve CSA on ultrasound predicts clinical outcome following CTR. Some studies found that patients with reduced median nerve CSA had better clinical outcomes [11,15,31]. Other studies, including the current study, showed no relationship between clinical outcome and a reduction in median nerve CSA [29,32,33,34]. Similarly, we found no relationship between all other parameters assessed and clinical outcome scores. Other methods of assessing the median nerve, such as elastography, may prove more discriminatory in this regard.
This study has some limitations. First, as all ultrasound examinations were performed by one experienced musculoskeletal radiologist with initial measurements made during real-time scanning, inter-observer reliability was only assessed on captured images. While the reliability of CTS measurements for experienced readers has been shown to be high for measurements acquired both in real-time [7,8,9] and on captured images [42], to the best of our knowledge, no study has directly compared the reliability of real-time and captured image measurements. Moreover, one cannot infer that high measurement reliability on captured images translates to high measurement reliability in real-time imaging. Second, the number of patients was small, limiting more in-depth subgroup analysis. Third, clinical improvement scores were used to gauge surgical outcomes rather than NCT, although as mentioned, NCT has known limitations in post-surgical CTS assessment. Fourth, the first postoperative clinical assessment was performed at two weeks, while the first ultrasound assessment was performed at one month as the surgical wound would and, most likely, have limited the complete ultrasound assessment at two weeks post-ECTR.
In conclusion, postoperative changes in the median nerve caliber, BR, and caliber-change ratio are more apparent at the tunnel outlet than at the inlet, suggesting that the tunnel outlet rather than the inlet may be the main site of compression in CTS. Nearly all of the ultrasound features used to diagnose CTS on ultrasound preoperatively remain abnormal one year after ECTR despite good symptomatic improvement. These ultrasound parameters may not necessarily be relied upon to diagnose persistent median nerve compression following ECTR.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Formal analysis: Alex Wing Hung Ng, Raymond Chun Wing Fong.
- Investigation: Alex Wing Hung Ng, Raymond Chun Wing Fong, Carita Tsoi, Michael Chu Kay Mak, Wing Lim Tse.
- Methodology: all authors.
- Project administration: Alex Wing Hung Ng, James Francis Griffith, Pak Cheong Ho.
- Supervision: James Francis Griffith.
- Validation: Alex Wing Hung Ng, Carita Tsoi, James Francis Griffith, Raymond Chun Wing Fong.
- Writing—original draft: Alex Wing Hung Ng.
- Writing—review & editing: all authors.
Supplements
The Supplement is available with this article at https://doi.org/10.3348/kjr.2020.1039.
Transverse ultrasound of the same patient as figure 1 shows the median nerve distal to the carpal tunnel at baseline (A) and 12 months (B) post-endoscopic carpal tunnel release.
Transverse ultrasound shows the median nerve at the carpal tunnel inlet in a patient with a clinical improvement score of 2 at one month (A) and at 12 months (B) post-ECTR.
References
- 1.Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15:1273–1284. doi: 10.1016/S1474-4422(16)30231-9. [DOI] [PubMed] [Google Scholar]
- 2.Mosier BA, Hughes TB. Recurrent carpal tunnel syndrome. Hand Clin. 2013;29:427–434. doi: 10.1016/j.hcl.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Taghizadeh R, Tahir A, Stevenson S, Barnes DE, Spratt JD, Erdmann MW. The role of MRI in the diagnosis of recurrent/persistent carpal tunnel syndrome: a radiological and intra-operative correlation. J Plast Reconstr Aesthet Surg. 2011;64:1250–1252. doi: 10.1016/j.bjps.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Steyers CM. Recurrent carpal tunnel syndrome. Hand Clin. 2002;18:339–345. doi: 10.1016/s0749-0712(01)00005-1. [DOI] [PubMed] [Google Scholar]
- 5.Botte MJ, von Schroeder HP, Abrams RA, Gellman H. Recurrent carpal tunnel syndrome. Hand Clin. 1996;12:731–743. [PubMed] [Google Scholar]
- 6.Hybbinette CH, Mannerfelt L. The carpal tunnel syndrome. A retrospective study of 400 operated patients. Acta Orthop Scand. 1975;46:610–620. doi: 10.3109/17453677508989243. [DOI] [PubMed] [Google Scholar]
- 7.Ng AWH, Griffith JF, Lee RKL, Tse WL, Wong CWY, Ho PC. Ultrasound carpal tunnel syndrome: additional criteria for diagnosis. Clin Radiol. 2018;73:214.e11–214.e18. doi: 10.1016/j.crad.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Wong SM, Griffith JF, Hui AC, Lo SK, Fu M, Wong KS. Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology. 2004;232:93–99. doi: 10.1148/radiol.2321030071. [DOI] [PubMed] [Google Scholar]
- 9.Wong SM, Griffith JF, Hui AC, Tang A, Wong KS. Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum. 2002;46:1914–1921. doi: 10.1002/art.10385. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi A, Ghasemi-Rad M, Mladkova-Suchy N, Ansari S. Correlation between the severity of carpal tunnel syndrome and color Doppler sonography findings. AJR Am J Roentgenol. 2012;198:W181–W184. doi: 10.2214/AJR.11.7012. [DOI] [PubMed] [Google Scholar]
- 11.Mondelli M, Filippou G, Aretini A, Frediani B, Reale F. Ultrasonography before and after surgery in carpal tunnel syndrome and relationship with clinical and electrophysiological findings. A new outcome predictor? Scand J Rheumatol. 2008;37:219–224. doi: 10.1080/03009740801914850. [DOI] [PubMed] [Google Scholar]
- 12.Cudlip SA, Howe FA, Clifton A, Schwartz MS, Bell BA. Magnetic resonance neurography studies of the median nerve before and after carpal tunnel decompression. J Neurosurg. 2002;96:1046–1051. doi: 10.3171/jns.2002.96.6.1046. [DOI] [PubMed] [Google Scholar]
- 13.Tran TA, Williams LM, Bui D, Anthonisen C, Poltavskiy E, Szabo RM. Prospective pilot study comparing pre-and postsurgical CTSAQ and Neuro-QoL questionnaire with median nerve high-resolution ultrasound cross-sectional areas. J Hand Surg Am. 2018;43:184.e1–184.e9. doi: 10.1016/j.jhsa.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Karabaty H, Hetzel A, Galla TJ, Horch RE, Lücking CH, Glocker FX. The effect of carpal tunnel release on median nerve flattening and nerve conduction. Electromyogr Clin Neurophysiol. 2005;45:223–227. [PubMed] [Google Scholar]
- 15.Lee CH, Kim TK, Yoon ES, Dhong ES. Postoperative morphologic analysis of carpal tunnel syndrome using high-resolution ultrasonography. Ann Plast Surg. 2005;54:143–146. doi: 10.1097/01.sap.0000143799.88513.47. [DOI] [PubMed] [Google Scholar]
- 16.Colak A, Kutlay M, Pekkafali Z, Saraçoglu M, Demircan N, Sims¸ek H, et al. Use of sonography in carpal tunnel syndrome surgery A prospective study. Neurol Med Chir (Tokyo) 2007;47:109–115. doi: 10.2176/nmc.47.109. discussion 115. [DOI] [PubMed] [Google Scholar]
- 17.Abicalaf CA, de Barros N, Sernik RA, Pimentel BF, Braga-Baiak A, Braga L, et al. Ultrasound evaluation of patients with carpal tunnel syndrome before and after endoscopic release of the transverse carpal ligament. Clin Radiol. 2007;62:891–894. doi: 10.1016/j.crad.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Inui A, Nishimoto H, Mifune Y, Kokubu T, Sakata R, Kurosaka M. Ultrasound measurement of median nerve cross-sectional area at the inlet and outlet of carpal tunnel after carpal tunnel release compared to electrodiagnostic findings. Arch Orthop Trauma Surg. 2016;136:1325–1330. doi: 10.1007/s00402-016-2514-9. [DOI] [PubMed] [Google Scholar]
- 19.Chow JC, Hantes ME. Endoscopic carpal tunnel release: thirteen years’ experience with the Chow technique. J Hand Surg Am. 2002;27:1011–1018. doi: 10.1053/jhsu.2002.35884. [DOI] [PubMed] [Google Scholar]
- 20.Lee RKL, Griffith JF, Ng AWH, Tipoe GL, Chan AWH, Wong CWY, et al. Cross-sectional area of the median nerve at the wrist: comparison of sonographic, MRI, and cadaveric measurements. J Clin Ultrasound. 2019;47:122–127. doi: 10.1002/jcu.22647. [DOI] [PubMed] [Google Scholar]
- 21.Rivlin M, Kachooei AR, Wang ML, Ilyas AM. Electrodiagnostic grade and carpal tunnel release outcomes: a prospective analysis. J Hand Surg Am. 2018;43:425–431. doi: 10.1016/j.jhsa.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Fowler JR. Nerve conduction studies for carpal tunnel syndrome: gold standard or unnecessary evil? Orthopedics. 2017;40:141–142. doi: 10.3928/01477447-20170419-01. [DOI] [PubMed] [Google Scholar]
- 23.Rempel D, Evanoff B, Amadio PC, de Krom M, Franklin G, Franzblau A, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am J Public Health. 1998;88:1447–1451. doi: 10.2105/ajph.88.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.No authors listed. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome (summary statement). American Academy of Neurology, American Association of Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 1993;43:2404–2405. doi: 10.1212/wnl.43.11.2404. [DOI] [PubMed] [Google Scholar]
- 25.Siegmeth AW, Hopkinson-Woolley JA. Standard open decompression in carpal tunnel syndrome compared with a modified open technique preserving the superficial skin nerves: a prospective randomized study. J Hand Surg Am. 2006;31:1483–1489. doi: 10.1016/j.jhsa.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Yesildag A, Kutluhan S, Sengul N, Koyuncuoglu HR, Oyar O, Guler K, et al. The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol. 2004;59:910–915. doi: 10.1016/j.crad.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Elnady B, Rageh EM, Ekhouly T, Fathy SM, Alshaar M, Fouda ES, et al. Diagnostic potential of ultrasound in carpal tunnel syndrome with different etiologies: correlation of sonographic median nerve measures with electrodiagnostic severity. BMC Musculoskelet Disord. 2019;20:634. doi: 10.1186/s12891-019-3010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klauser AS, Halpern EJ, De Zordo T, Feuchtner GM, Arora R, Gruber J, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250:171–177. doi: 10.1148/radiol.2501080397. [DOI] [PubMed] [Google Scholar]
- 29.Naranjo A, Ojeda S, Rúa-Figueroa I, Garcia-Duque O, Fernández-Palacios J, Carmona L. Limited value of ultrasound assessment in patients with poor outcome after carpal tunnel release surgery. Scand J Rheumatol. 2010;39:409–412. doi: 10.3109/03009741003685632. [DOI] [PubMed] [Google Scholar]
- 30.Chappell CD, Beckman JP, Baird BC, Takke AV. Ultrasound (US) changes in the median nerve cross-sectional area after microinvasive US-guided carpal tunnel release. J Ultrasound Med. 2020;39:693–702. doi: 10.1002/jum.15146. [DOI] [PubMed] [Google Scholar]
- 31.Vögelin E, Nüesch E, Jüni P, Reichenbach S, Eser P, Ziswiler HR. Sonographic follow-up of patients with carpal tunnel syndrome undergoing surgical or nonsurgical treatment: prospective cohort study. J Hand Surg Am. 2010;35:1401–1409. doi: 10.1016/j.jhsa.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Smidt MH, Visser LH. Carpal tunnel syndrome: clinical and sonographic follow-up after surgery. Muscle Nerve. 2008;38:987–991. doi: 10.1002/mus.20982. [DOI] [PubMed] [Google Scholar]
- 33.Tas S, Staub F, Dombert T, Marquardt G, Senft C, Seifert V, et al. Sonographic short-term follow-up after surgical decompression of the median nerve at the carpal tunnel: a single-center prospective observational study. Neurosurg Focus. 2015;39:E6. doi: 10.3171/2015.6.FOCUS15216. [DOI] [PubMed] [Google Scholar]
- 34.Kim JK, Koh YD, Kim JO, Choi SW. Changes in clinical symptoms, functions, and the median nerve cross-sectional area at the carpal tunnel inlet after open carpal tunnel release. Clin Orthop Surg. 2016;8:298–302. doi: 10.4055/cios.2016.8.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleindienst A, Hamm B, Lanksch WR. Carpal tunnel syndrome: staging of median nerve compression by MR imaging. J Magn Reson Imaging. 1998;8:1119–1125. doi: 10.1002/jmri.1880080518. [DOI] [PubMed] [Google Scholar]
- 36.Rempel D, Dahlin L, Lundborg G. Pathophysiology of nerve compression syndromes: response of peripheral nerves to loading. J Bone Joint Surg Am. 1999;81:1600–1610. doi: 10.2106/00004623-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Nakamichi KI, Tachibana S. Enlarged median nerve in idiopathic carpal tunnel syndrome. Muscle Nerve. 2000;23:1713–1718. doi: 10.1002/1097-4598(200011)23:11<1713::aid-mus7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Akcar N, Ozkan S, Mehmetoglu O, Calisir C, Adapinar B. Value of power Doppler and gray-scale US in the diagnosis of carpal tunnel syndrome: contribution of cross-sectional area just before the tunnel inlet as compared with the cross-sectional area at the tunnel. Korean J Radiol. 2010;11:632–639. doi: 10.3348/kjr.2010.11.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aboonq MS. Pathophysiology of carpal tunnel syndrome. Neurosciences (Riyadh) 2015;20:4–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Chammas M, Boretto J, Burmann LM, Ramos RM, Dos Santos Neto FC, Silva JB. Carpal tunnel syndrome - Part I (anatomy, physiology, etiology and diagnosis) Rev Bras Ortop. 2014;49:429–436. doi: 10.1016/j.rboe.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karabay N, Kayalar M, Ada S. Sonographic assessment of transverse carpal ligament after open surgical release of the carpal tunnel. Acta Orthop Traumatol Turc. 2013;47:73–78. doi: 10.3944/aott.2013.2890. [DOI] [PubMed] [Google Scholar]
- 42.Junck AD, Escobedo EM, Lipa BM, Cronan M, Anthonisen C, Poltavskiy E, et al. Reliability assessment of various sonographic techniques for evaluating carpal tunnel syndrome. J Ultrasound Med. 2015;34:2077–2088. doi: 10.7863/ultra.15.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transverse ultrasound of the same patient as figure 1 shows the median nerve distal to the carpal tunnel at baseline (A) and 12 months (B) post-endoscopic carpal tunnel release.
Transverse ultrasound shows the median nerve at the carpal tunnel inlet in a patient with a clinical improvement score of 2 at one month (A) and at 12 months (B) post-ECTR.