Abstract
Purpose
Two-year follow-up to assess efficacy and safety of high-density autologous chondrocyte implantation (HD-ACI) in patients with cartilage lesions in the ankle.
Design
Twenty-four consecutive patients with International Cartilage repair Society (ICRS) grade 3-4 cartilage lesions of the ankle were included. Five million chondrocytes per cm2 of lesion were implanted using a type I/III collagen membrane as a carrier and treatment effectiveness was assessed by evaluating pain with the visual analogue scale (VAS) and American Orthopaedic Foot & Ankle Society (AOFAS) ankle-hindfoot score at baseline, 12-month, and 24-month follow-up, together with dorsal and plantar flexion. Magnetic resonance observation for cartilage repair tissue (MOCART) score was used to evaluate cartilage healing. Histological study was possible in 5 cases.
Results
Patients’ median age was 31 years (range 18-55 years). Median VAS score was 8 (range 5-10) at baseline, 1.5 (range 0-8) at 12-month follow-up, and 2 (rang e0-5) at 24-month follow-up (P < 0.001). Median AOFAS score was 39.5 (range 29-48) at baseline, 90 (range 38-100) at 12-month follow-up, and 90 (range 40-100) at 24-month follow-up (P < 0.001). Complete dorsal flexion significantly increased at 12 months (16/24, 66.7%) and 24 months (17/24, 70.8%) with regard to baseline (13/24, 54.2%) (P = 0.002). MOCART at 12- and 24-month follow-ups were 73.71 ± 15.99 and 72.33 ± 16.21. Histological study confirmed that neosynthetized tissue was cartilage with hyaline extracellular matrix and numerous viable chondrocytes.
Conclusion
HD-ACI is a safe and effective technique to treat osteochondral lesions in the talus, providing good clinical and histological results at short- and mid-term follow-ups.
Keywords: cell therapy, high-density autologous chondrocyte implantation, osteochondral lesion, talus, hyaline cartilage
Introduction
The ankle joint (talocrural joint) is a synovial joint comprising the tibia, fibula, and talus. Hyaline cartilage covers the joint surface of all bones in the ankle. 1 Prevalence of cartilage damage in the ankle is relatively uncommon, representing less than 2% in comparison with other joints such as the knee in which it could be more than 40%. 2 Indeed, it has been estimated that 50% of ankle sprains will lead to a potential osteochondral injury. 3 Physical or traumatic injuries are the most probable causes leading to lesion development in the ankle joint’s cartilage while in other joints, such as knee or hip, cartilage degeneration is the most common cause for chondral damage.2,4 A limited vascular supply could be the reason for the high risk of posttraumatic osteochondral injuries in the ankle which could be also related to the low healing potential of this tissue. 5 Despite their relatively low prevalence, treatment of chondral and osteochondral talar lesions is mandatory in most cases because they have a huge impact on people’s everyday life, especially in youngsters and those who lead active lives. Nondisplaced, asymptomatic cartilage lesions were conservatively treated whereas displaced, symptomatic injuries were surgically treated. 6
Several surgical options are available when treating ankle cartilage lesions.7-13 There is no consensus about the best option to choose for each particular case. Recent data suggest that lesions less than 10.2 mm in diameter or 107.4 mm2 in area can be treated conservatively (asymptomatic patients) or with bone marrow stimulation techniques such as microfractures9,10 or autologous matrix-induced chondrogenesis (AMIC).7,8 These techniques are based on mesenchymal stem cell migration from bone marrow to the lesion with the aim of repairing it. 11 The difference between both techniques is that in AMIC, a collagen I/III membrane is used to stabilize blood clot. 14 However, in spite of the fact that it might work in some cases, especially when lesions are less than 15 mm, 15 there is significant scientific evidence claiming that bone marrow stimulation techniques are not an effective treatment, leading to cartilage deterioration as observed in mid and long-term follow-up.16,17 The reason is that regenerated tissue is fibrous cartilage instead of hyaline cartilage, which is unable to carry out mechanical and biological articular cartilage functions.18,19 Moreover, magnetic resonance imaging has shown these techniques may well be related to subchondral bone degradation. 20
Cell-based therapies are widely accepted as the best option to treat talar cartilage lesions, especially those larger than 107.4 mm2 in area or 10.2 mm in diameter.13,21 Among them, the different modalities using autologous chondrocytes, with periosteal flap (periosteal autologous chondrocyte implantation: PACI)22,23 and with type I/III collagen membrane (matrix-induced autologous chondrocyte implantation: MACI)24,25 have become the preferential tool for treating these lesions. Although some authors have demonstrated that autologous chondrocyte implantation is superior to other surgical techniques when treating articular cartilage,26,27 various recent publications claim there is no more effective treatment for primary 28 or secondary osteochondral lesions in the talus. 29 PACI and MACI are carried out in 2 steps: first, a biopsy of healthy cartilage from a nonweightbearing area is taken arthroscopically, followed by a second intervention in which previously cultured and expanded chondrocytes are implanted.22-27 There are 2 main differences between PACI and MACI: In the former, the total number of cells obtained after culture (around 20 million cells) are implanted under a periosteal flap while in MACI the number of implanted cells depends on defect size, at a density of 1 million cells per cm2 on the collagen membrane.22-25 Each has its disadvantage: For PACI it is the morbidity due to surgery to harvest periosteum22,23 while for traditional MACI the problem is that not all of the obtained chondrocytes are implanted after culturing.24,25
Recently, a new autologous chondrocyte implantation approach, high-density autologous chondrocyte implantation (HD-ACI), has been described.30,31 In this approach, the same porcine type I/III collagen membrane used in MACI is cut according to defect size and shape after seeding total amount of cultured cells at 5 million cells per cm2 density.30,31 Preliminary results observed in patients with cartilage knee lesions treated with HD-ACI showed that subjective perception of knee functionality, measured by the International Knee Documentation Committee (IKDC) index improved at 1- and 2-year follow-up with regard to baseline, providing to be an effective technique. 31 In this work, we describe the efficacy and safety of HD-ACI in a 2-year follow-up for all patients with cartilage lesions of the ankle, treated in our hospital between 2010 and 2016.
Materials and Methods
Patients
This is a prospective cohort study performed on 24 consecutive patients with cartilage lesions of the ankle treated with HD-ACI between 2010 and 2016. All patients signed an informed consent and the study was approved by the Hospital Education and Research Committee. To be included in this study, following were the patient eligibility criteria: International Cartilage Repair Society (ICRS) grade 3-4 cartilage lesion of the ankle (lateral or medial), diagnosed by imaging test (magnetic resonance or arthro-resonance), 1 to 2 lesions of at least 100 mm2 in size, and an age ranging between 18 and 55 years. The following were the exclusion criteria: arthrosis, misalignment of the limb (more than 10° varus or valgus), allergy to penicillin and/or streptomycin, hypersensitivity to bovine-derived products, active infection, tumoral pathology, and systemic diseases such as rheumatoid arthritis or other autoimmune diseases with articular involvement. We included the first 24 patients fulfilling the inclusion/exclusion criteria who had at least 2 years of follow-up, so a sample of convenience was used to perform this study. Topographic chondral lesion location in ankle was assessed following the Elias et al. 32 classification.
Cell Culture
Included patients underwent a first arthroscopy to harvest a cartilage biopsy from a nonweightbearing area (anterior talar neck), placed in Dulbecco’s modified Eagle medium (DMEM; Lonza Group Ltd., Basel, Switzerland) and immediately processed. Cartilage lesion size is first measured with a caliper during this arthroscopy to estimate the cell number required for high-density implantation. Isolation and culture of cells were carried-out following previously described procedures. 31 Isolated chondrocytes were passaged a maximum of 3 times until 40 to 50 million cells were obtained and time between harvest and implantation was 4 to 6 weeks.
Surgical Procedure
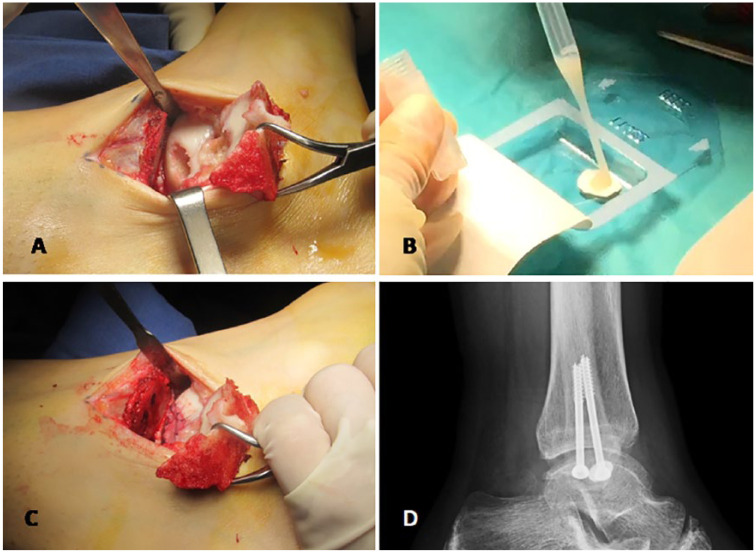
In a second step, we implanted high-density chondrocytes on a resorbable porcine type I/III collagen membrane (Chondro-Gide, Geistlich Biomaterials, Wolhusen, Switzerland). We positioned patients with ankle hanging over a distal silicone support on a radiotransparent surgery table. Additionally, we placed fluoroscopy on the opposite side of operated limb and at right angle to it. When the lesion appeared on zones 3 and 6 of the astragalus, 32 chondrocyte implantation was performed by anterior arthroscopy of the ankle, favored by the posterior position of the fibula with regard to the tibia at the tibiotalar joint ( Fig. 1 ). When the injury appeared on the medial side of the astragalus, 32 zones 1 and 4, a medial malleolus osteotomy was performed ( Fig. 1A ). The osteotomy begins 2 cm away from the proximal tibial malleolus using a 10-mm oscillating saw while locating and protecting posterior tibial tendon. Aided by fluoroscopic imaging, a 1.6-mm Kirschner pin was distally introduced until we reached the center of the osteochondral lesion. To avoid any joint damage, osteotomy was finalized using an osteotome. While carefully handling adjacent soft tissues, tibial malleolus was lifted proximally with a bone clamp and lesion was accessed. The edges and the bed of the osteochondral lesion were debrided, leaving clean healthy perpendicular edges at the perimeter. Once the lesion was cleaned, its definitive size was measured using a rule. In case of lesions deeper than 4 mm, we performed a “sandwich” technique to fill the defect, following an adapted procedure described by Nam et al. 33 to fill osteochondral cysts prior to ACI. Autologous spongious bone taken from the osteotomy itself (this is possible because the amount needed is minimal) was placed onto the defect. To avoid graft going out of the defect, it was compressed and compacted with an “in-house” made compactor. Once the lesion was prepared, the membrane was then cut according to the defect size, chondrocytes were seeded onto it at a density of 5 million cells per cm2 ( Fig. 1B ) and finally fixed to the surrounding cartilage using 5-0 Vicryl resorbable suture or transosseous sutures, followed by fibrin sealing ( Fig. 1C ). Tibial osteotomy was fixed using two 4.5-mm cannulated screws with 16-mm partial threads. X-ray imaging confirmed the correct position of the final osteosynthesis ( Fig. 1D ). Stability of the membrane was checked by flexion-extension movements. See the video of the technique in the supplemental file.
Figure 1.
Surgical procedure of HD-ACI in ankle. Technique starts with a 5-cm incision on medial malleolus (A). Membrane is cut according to lesion size and shape and cells are seeded onto it at a density of 5 million cells per cm2 lesion (B). After waiting 10 minutes, membrane is sutured to adjacent cartilage and sealed with fibrin glue (C). Correct position of the oesteosynthesis is checked by X-ray imaging (D).
Postoperative Treatment
Patients underwent mobilization program with no weightbearing for the first 6 to 8 weeks followed by 6 to 12 weeks of progressive weightbearing with crutches, which also included the start of physical therapy sessions. At 4 months, patients could also be involved in stationary bicycle sessions and swimming. At 9 to 10 months, they were allowed to jog slowly.
Clinical Outcome Assessments
Osteotomy consolidation was evaluated by X-ray at 2 weeks, 2 months, and 3 months after surgery. Treatment effectiveness was assessed by evaluating pain with the visual analogue scale (VAS) and American Orthopaedic Foot & Ankle Society (AOFAS) ankle-hindfoot score 34 at baseline, 12 months, and 24 months follow-up. As considered by other authors, 35 we established the percentage of “good” and “excellent” results according to the following criteria: “good” when AOFAS score was 80 to 90 and “excellent” when it was 91 to 100. Dorsal and plantar flexions were also used to evaluate treatment success. Hence, patients who had 0° to 20° of flexion were considered to have complete dorsal flexion and those who did not reach 20° were considered to have incomplete dorsal flexion. Similarly, patients having 0° to 45° of flexion were considered to have complete plantar flexion, otherwise, plantar flexion was considered incomplete. As per study protocol, magnetic resonance images (MRI) were taken in all patients at 3-, 6-, 12-, and 24-month follow-ups. Images were examined by an independent radiologist, blinded to surgical information and clinical outcomes, who looked for adverse events such as the presence of swelling and bone edema to evaluate treatment safety. Cartilage healing at 12- and 24-month follow-up was assessed by the magnetic resonance observation of cartilage repair tissue (MOCART) score. 36 To avoid bias, patients were examined and followed-up by the same doctor and the same radiologist.
Return to sport and to patient’s normal life (working or studying) was evaluated at 12- and 24-month follow-ups.
Histological Assessment
Osteosynthesis material was removed at least 1 year after the surgery in all patients. At this moment, a second-look arthroscopy was performed. Patients were asked to give their consent for a biopsy to be taken for histological study of neoformed tissue. Five out the 24 included patients accepted. Biopsies from these 5 patients were fixed in 10% buffered neutral formalin and paraffin embedded following standard procedure. Paraffin-embedded samples were serially cut into 4-µm-thick sections, which were subsequently stained with hematoxylin-eosin and Alcian blue.
Statistical Analysis
Statistical analysis was performed using the SPSS 9.0 software for Windows. Continuous variables were expressed as the mean ± standard deviation (SD) or median (minimum and maximum). AOFAS ankle-hindfoot scale differences were expressed as the mean and 95% confidence interval (CI) of the mean. Normality was checked with the Kolmogorov-Smirnov test. Comparison of VAS and AOFAS scores at different moments in time was performed using Friedman’s 2-dimensional analysis of variance (ANOVA) for related samples. Pairwise comparisons were performed using Wilcoxon signed-rank test for related samples. Mean MOCART at 12 and 24 months were compared using Student t test for related samples (normal distribution) or Wilcoxon signed-rank test for related samples (nonnormal distribution). Categorical variables were expressed with the absolute frequency and percentage. Evolution of these variables along the follow-up period was studied using cross-tabs, whose statistical significance was determined using Pearson’s χ2 test. Multivariate linear regression analysis was performed to determine the effects of several epidemiological, lesion- and procedure-related variables on AOFAS improvement at 12-month follow-up and MOCART at 12-month follow-up. Regression coefficient estimations and their 95% CI together with P values were reported. For all comparisons and parameter estimations, a P value <0.05 (2-sided) was considered statistically significant.
Results
Characteristics of the 24 patients included in the present study are depicted in Table 1 . Patients’ median age was 31 years (range 18-55 years). As shown in Table 1 , most patients had at least 1 previous surgery in the same ankle (13 patients, 54.2%). Previous surgeries included microfractures in 7 cases, debridement in 2 patients, debridement plus synovectomy in 1 case, mosaicplasty in 2 patients, and mosaicplasty plus microfractures in the remaining patient. One patient underwent an anterior talofibular ligament (ATFL) surgery, performed arthroscopically during the first step in which the cartilage biopsy was taken.
Table 1.
Included Patients’ Demographics (N = 24).
| Demographic | n (%) |
|---|---|
| Age, a years | 31 (18-55) |
| Gender | |
| Male | 14 (58.3) |
| Female | 10 (41.7) |
| ICRS grade | |
| Grade 3 | 9 (37.5) |
| Grade 4 | 15 (72.5) |
| Laterality | |
| Right | 15 (62.5) |
| Left | 9 (37.5) |
| Number of previous surgeries | |
| 0 | 11 (45.8) |
| 1 | 6 (25.0) |
| 2 | 5 (20.8) |
| 3 | 2 (8.4) |
ICRS = International Cartilage Repair Society.
Age is expressed as median (minimum-maximum).
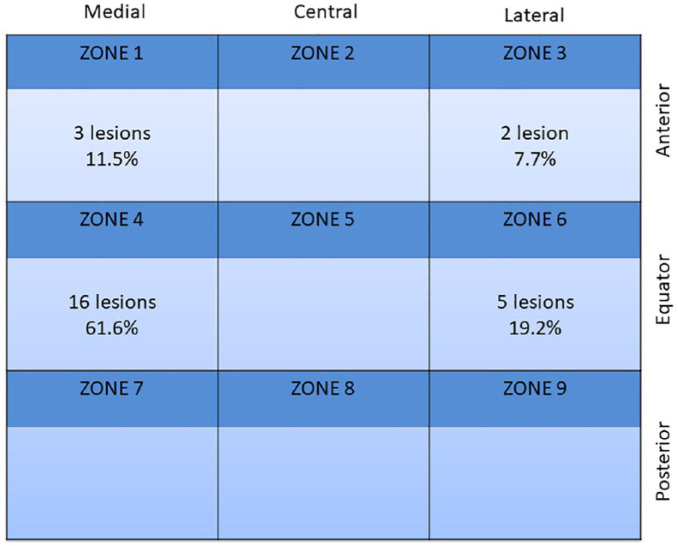
Since 2 patients had 2 lesions, total lesion number was 26. In these patients, both lesions were treated in the same surgical act. Lesion size (mean ± SD), measured during the implantation surgery, was 207.8 ± 59.7 mm2. Nineteen lesions (73.1%) had a medial location while the remaining 7 (22.9%) had a lateral location. Topographical location of lesions is shown in Figure 2 . A large number of lesions were located in the equatorial area of talus, zone 4 being the most frequently located area (16 out of 26 lesions: 61.6%). All lesions were contained. The sandwich technique was carried out in 8 patients (33.3%).
Figure 2.
Topological location of lesions in ankle, according to the Elias et al. 32 classification.
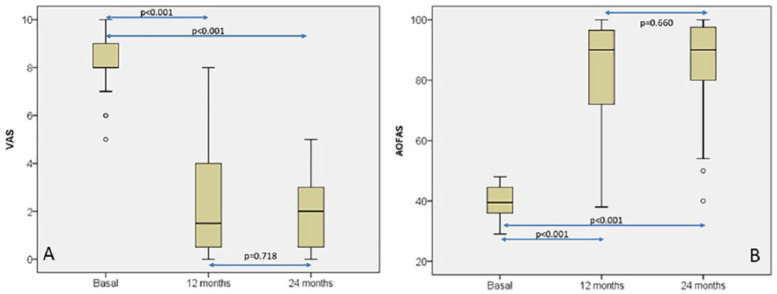
Pain distribution, measured with VAS at baseline, 12-month, and 24-month follow-ups ( Fig. 3A ), was statistically significant [baseline, 8 (5-10); 12-month follow-up, 1.5 (0-8); 24-month follow-up, 2 (0-5); P < 0.001; Friedman’s 2-dimensional ANOVA for related samples]. Pairwise comparisons showed that statistical differences were only found when baseline and 12- or 24-month follow-ups were compared (P < 0.001 in both cases; Wilcoxon signed-rank test) but no differences were found between 12- and 24-month follow-up (P = 0.718; Wilcoxon signed-rank test). Similarly, AOFAS ankle-hindfoot score distribution ( Fig. 3B ) was also statistically significant when the 3 time periods were compared (baseline, 39.5 (29-48); 12-month follow-up, 90 (38-100); 24-month follow-up, 90 (40-100); P < 0.001; Friedman’s 2-dimensional ANOVA for related samples). AOFAS score behavior was similar to that observed in VAS, so when pairwise comparisons were done, statistical differences were found only between baseline and 12 or 24 months (P < 0.001 in both cases; Wilcoxon signed-rank test) whereas no differences were found between 12 and 24 months (P = 0.660; Wilcoxon signed-rank test) ( Fig. 3B ).
Figure 3.
Box plot representations of visual analogue scale (VAS) (A) and American Orthopaedic Foot & Ankle Society (AOFAS) ankle-hindfoot score (B) distributions at baseline, 12-month, and 24-month follow-up. Friedman’s 2-dimensional analysis of variance for related samples was used for statistical comparisons. Pairwise comparisons were carried out with Wilcoxon signed-rank test.
Percentage of patients with “good” or “excellent” results was 75.0% at 12 months (9 out of 24, 37.5% patients with “good” results and 9 out of 24, 37.5% patients with “excellent” results). At 24 months, this percentage increased to 79.2% (8 out of 24, 33.3% patients with “good” results and 11 out of 24, 45.8% patients with “excellent” results).
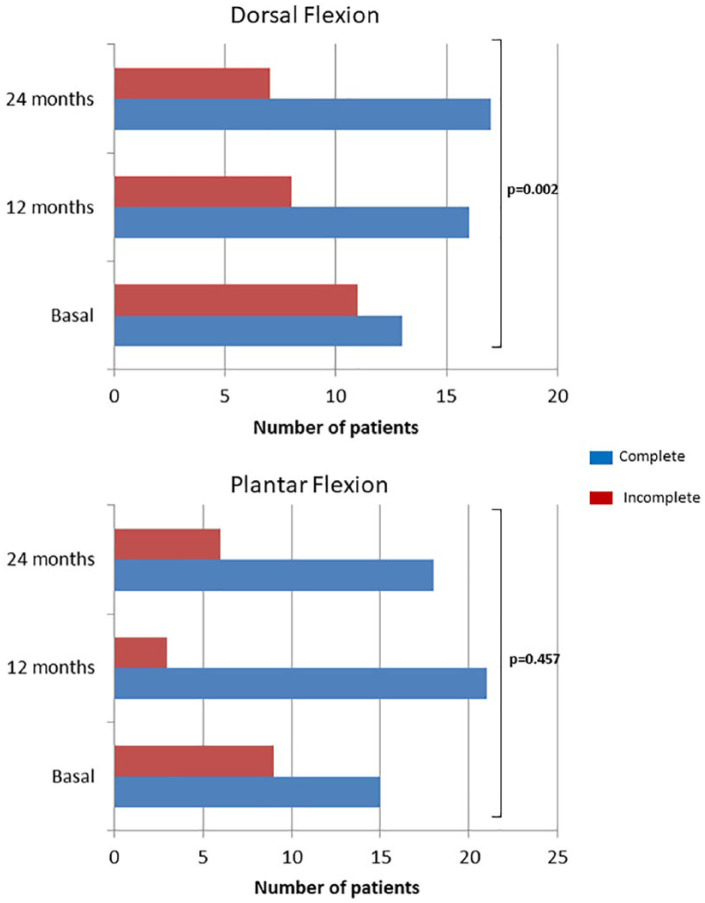
Mean improvement of AOFAS score with regard to basal determination was 42.1 (95% CI of mean: 34.4-49.9) and 44.6 (95% CI of mean: 37.4-51.8) at 12 and 24 months, respectively. No statistical significance was found in AOFAS score improvement at 12 and 24 months when categories of gender, “sandwich” technique, laterality, location, number of lesions, grade, and number of previous surgeries were compared ( Table 2 ). With regard to range of motion, dorsal and plantar flexion distributions are shown in Figure 4 . Percentage of patients with complete dorsal flexion significantly increased at 12 (16/24, 66.7%) and 24 months (17/24, 70.8%) with regard to baseline (13/24, 54.2%) (P = 0.002; Pearson’s χ2 test) ( Fig. 4 ). Otherwise, no differences in plantar flexion were found when baseline (15/24, 62.5%), 12-month follow-up (21/24, 87.5%), and 24-month (18/24, 75.0%) follow-up were compared (P = 0.457; Pearson’s χ2 test) ( Fig. 4 ).
Table 2.
Comparison of American Orthopaedic Foot & Ankle Society (AOFAS) Ankle-Hindfoot Score Improvement at 12- and 24-Month Follow-up among Categories of Epidemiological Factors.
| 12-Month Difference |
24-Month Difference |
|||
|---|---|---|---|---|
| Mean ± SD | P | Mean ± SD | P | |
| Gender | ||||
| Male (n = 10) | 43.7 ± 17.6 | 0.585 a | 44.5 ± 17.9 | 0.625 a |
| Female (n = 14) | 39.9 ± 20.1 | 42.7 ± 16.6 | ||
| Sandwich technique | ||||
| Yes (n = 8) | 48.6 ± 8.7 | 0.452 a | 49.6 ± 7.1 | 0.928 a |
| No (n = 16) | 38.9 ± 21.1 | 42.1 ± 20.0 | ||
| Laterality | ||||
| Right (n = 15) | 44.5 ± 18.2 | 0.238 a | 45.4 ± 15.4 | 0.558 a |
| Left (n = 9) | 38.2 ± 19.1 | 41.0 ± 20.0 | ||
| Location | ||||
| Medial (n = 19) | 45.2 ± 15.5 | 0.199 a | 44.9 ± 16.1 | 0.626 a |
| Lateral (n = 7) | 33.0 ± 24.5 | 40.3 ± 20.7 | ||
| Number of lesions | ||||
| 1 (n = 22) | 43.6 ± 17.6 | 0.181 a | 45.3 ± 16.1 | 0.217 a |
| 2 (n = 2) | 23.6 ± 25.5 | 27.0 ± 24.0 | ||
| ICRS grade | ||||
| Grade 3 (n = 9) | 41.3 ± 21.9 | 1.000 a | 46.4 ± 18.1 | 0.347 a |
| Grade 4 (n = 15) | 42.6 ± 16.7 | 42.1 ± 16.8 | ||
| Number of previous surgeries | ||||
| 0 (n = 11) | 43.5 ± 16.0 | 0.468 b | 46.5 ± 10.2 | 0.737 b |
| 1 (n = 6) | 50.3 ± 12.9 | 48.5 ± 17.1 | ||
| 2 (n = 5) | 34.2 ± 22.8 | 37.4 ± 23.0 | ||
| 3 (n = 2) | 30.0 ± 35.4 | 30.0 ± 35.4 | ||
ICRS = International Cartilage Repair Society.
Mann-Whitney U test.
Kruskal-Wallis test.
Figure 4.
Number of patients with complete or incomplete dorsal (A) and plantar flexion (B) at baseline, at 12- and 24-month follow-up. Statistical comparisons were performed with Pearson’s χ2 test.
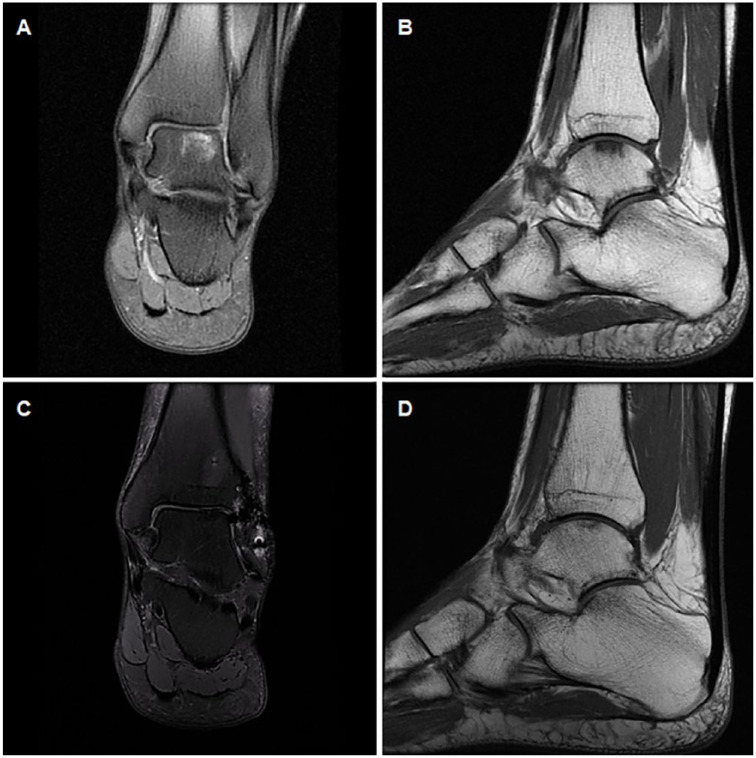
Basline and 24-month follow-up MRIs from a representative case are shown in Figure 5 . Significant differences in the mean MOCART between 12- and 24-month follow-ups were found (73.71 ± 15.99 vs. 72.33 ± 16.21; P = 0.008, Student t test for related samples) ( Fig. 6 ). At 12-month follow-up, 10 patients (41.7%) and 11 (45.8%) had nonintact subchondral lamina and bone, respectively. Twelve (50%) and 11 (45.8%) patients had nonintact subchondral lamina and bone, respectively in 24-month follow-up MRI.
Figure 5.
Coronal and sagittal magnetic resonance imaging (MRI) from a representative case of an International Cartilage Repair Society (ICRS) grade 3 osteochondral lesion located on the talus medial area (144 cm2, American Orthopaedic Foot & Ankle Society [AOFAS] score 48) (A, B). At 24-month follow-up, defect was filled by a smooth tissue very similar to hyaline cartilage (AOFAS score 90; magnetic resonance observation of cartilage repair tissue [MOCART] score 84 (C, D). The persistence of abnormalities in subchondral bone is noteworthy.
Figure 6.
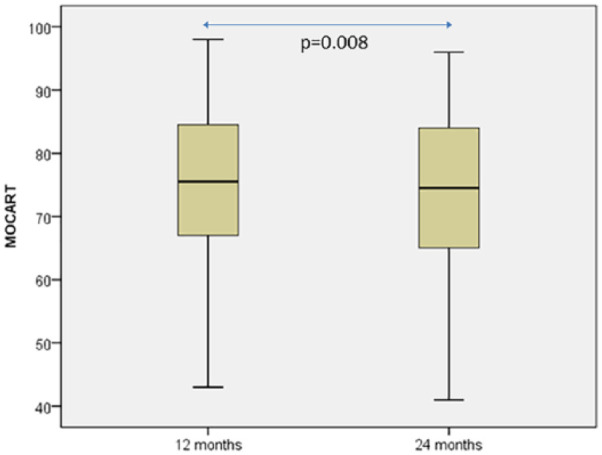
Box plot representation of magnetic resonance observation of cartilage repair tissue (MOCART) distribution at 12- and 24- month follow-up. Statistical comparison was carried out with the Student t test for related samples.
Multivariate linear regression analysis demonstrated that gender, sandwich technique, laterality, location, number of lesions, grade, number of previous surgeries and age have no effects on AOFAS improvement and MOCART at 12-month follow-up ( Table 3 ).
Table 3.
Multivariate Linear Regression Analysis of Different Factor Effects on the AOFAS Difference at 12 Months With Regard to Baseline and on the MOCART at 12-Month Follow-up.
| Variables | 12-Month Improvement AOFAS |
MOCART at 12 Months |
||
|---|---|---|---|---|
| Regression Coefficient (95% CI) | P | Regression Coefficient (95% CI) | P | |
| Gender | ||||
| Female | −1.305 (−22.374, 19.764) | 0.896 | −3.518 (−20.388, 13.352) | 0.660 |
| Laterality | ||||
| Right | 0.142 (−18.802, 19.086) | 0.897 | 1.326 (−13.842, 16.495) | 0.853 |
| Location | ||||
| Medial | 18.719 (−15.017, 52.455) | 0.252 | 21.915 (−5.098, 48.927) | 0.103 |
| Number of lesions | ||||
| 1 | 9.059 (−25.404, 43.522) | 0.580 | −16.636 (−44.231, 10.958) | 0.215 |
| Grade | ||||
| Grade 3 | 22.166 (−11.176, 55.508) | 0.175 | 11.860 (−14.836, 38.557) | 0.355 |
| Sandwich technique | ||||
| Yes | 13.254 (−13.796, 40.304) | 0.309 | −3.291 (−24.950, 18.369) | 0.748 |
| Previous surgeries | ||||
| 0 | 4.968 (−31.305, 41.241) | 0.772 | 16.574 (−12.471, 45.618) | 0.239 |
| 1 | 18.635 (−17.777, 55.047) | 0.289 | 16.353 (−12.793, 45.518) | 0.247 |
| 2 | −7.003 (−47.539, 33.534) | 0.715 | −12.525 (−44.983, 19.934) | 0.420 |
| Age | −0.295 (−1.442, 0.853) | 0.588 | −0.223 (−1.142, 0.695) | 0.608 |
AOFAS = American Orthopaedic Foot & Ankle Society; MOCART = magnetic resonance observation of cartilage repair tissue.
The number of patients with swelling and bone edema are shown in Table 4 . Percentage of patients with swelling significantly decreased along follow-up with regard to baseline (P = 0.003; Pearson’s χ2 test), while no differences in number of patients with bone edema were found along follow-up (P = 0.135; Pearson’s χ2 test) ( Table 4 ). As shown in Table 4 , one patient had swelling 2 years after surgery. This patient had had a previous surgery for a debridement plus synovectomy. No malunions of osteotomy were observed in any patient. No further adverse events were observed during follow-up.
Table 4.
Swelling and Bone Edema Distribution among Patients at Baseline and Follow-up. a
| Swelling |
Bone Edema |
|||||
|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | |
| Baseline | 9/24 (37.5%) | 15/24 (62.5%) | 0.007 | 7/24 (29.2%) | 17/24 (70.8%) | 0.472 |
| 3 months | 3/24 (12.5%) | 21/24 (87.5%) | 2/24 (8.3%) | 22/24 (91.7%) | ||
| 6 months | 2/24 (8.3%) | 22/24 (91.7%) | 4/24 (16.7%) | 20/24 (83.3%) | ||
| 12 months | 2/24 (8.3%) | 22/24 (91.7%) | 3/24 (12.5%) | 21/24 (87.5) | ||
| 24 months | 1/24 (4.2%) | 23/24 (95.8%) | 3/24 (12.5%) | 21/24 (87.5) | ||
Pearson’s χ2 test was used for statistical comparisons.
Patient return to sport was also analyzed. Among the 24 recruited patients, 14 (58.3%) regularly practiced sport before the cartilage lesion. Eleven of these 14 patients (78.6%) returned to sports 12 months after HD-ACI. It is noteworthy that 2 of these 11 could return to professional sports at the same level as before lesion (soccer and judo). Another patient was a professional dancer and although she could not professionally dance anymore, she could continue doing sport. No changes regarding return to sport was observed 24 months after surgery. All patients returned to their normal life (19 patients returned to work and the remaining 5 were students) 12 months after implantation and continued like that at 24-month follow-up.
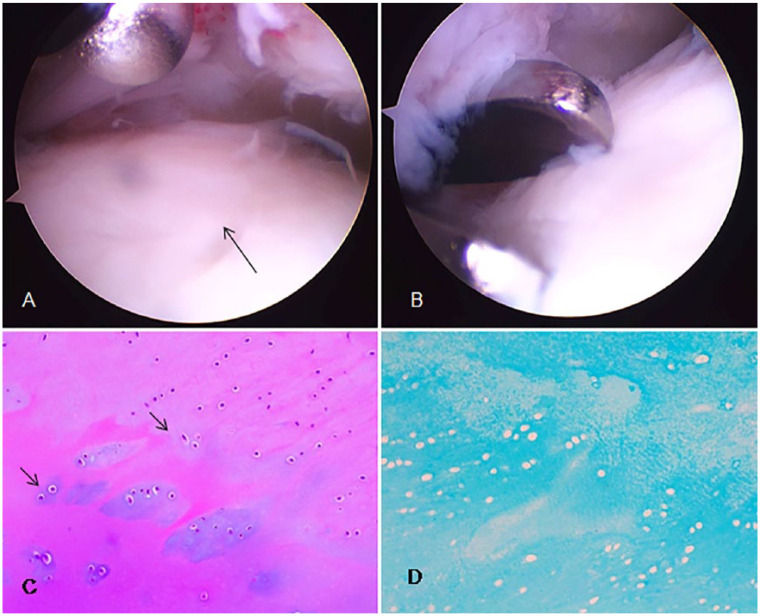
Second-look arthroscopic evaluation showed smooth surface and stable repair tissue. Neoformed tissue appeared to be well-integrated normal or nearly normal cartilage surrounded by normal cartilage ( Fig. 7A and B ). With regard to histologic analysis, in all cases tissue surface was not scuffed, did not have inflammatory cells and was neither calcified nor vascularized ( Fig. 7C and D ). Hematoxylin-eosin staining showed that tissue synthesized was cartilage with hyaline extracellular matrix and numerous viable chondrocytes with rounded nucleus ( Fig. 7C ). Chondrocytes were distributed in lagoons made up of 1 or 2 nuclei. None of the cases showed karyorrhexis, nuclear necrosis, or atypical mitosis. Alcian blue staining was diffusely positive in all cases, showing the presence of acid mucopolysaccharides in extracellular matrix ( Fig. 7D ).
Figure 7.
A representative case of second-look arthroscopy and histological study. Second-look arthroscopic evaluation showed smooth surface and stable repair tissue (indicated by arrows) (A, B). Hematoxylin-eosin (C) and Alcian blue staining (D). Arrows show how chondrocytes are distributed in lagoons, alone or in couples. 200× magnification.
Discussion
In this work, we describe clinical outcome of patients with injured talar cartilage treated with HD-ACI, a new modality of autologous chondrocyte implantation, which consists in increasing the implanted cell amount at least 5-fold and has been successfully used in the knee.30,31 The number of patients with 2-year follow-up included in present study is quite similar to that found in literature. In a systematic review, recently published by Erickson et al. 21 only in 4 of 25 studies considered, the number of enrolled patients was higher than ours.22,24,37,38 Our population had a median age very close to patient’s age in other studies.2,16,39-41 This age-period matches one of the most active periods in life, in which ankle joint cartilage damage due to physical exercise or trauma is highly frequent.2,4
Interestingly, more than a half of included patients (13 out of 24 patients: 54.2%) had at least one surgery with microfractures, combined or not with mosaicplasty, as the most frequent procedure. Long-term studies in ankle and other joints such as knee show that microfractures fail 2 years postoperatively19,20,42 and so ACI is considered a rescue technique for previous procedures. Lesion size of patients included was very close to that reported by Erickson et al. 21 in which the average lesion size was 197 mm2, ranging from 121 to 340 mm2. As with our patients, medial location is the most frequent in all published patient series. 21
Pain, evaluated with VAS, showed a significant improvement at 12 months postoperatively with regard to baseline, which was maintained without statistically significant changes from 12 months to 24 months postoperatively. Pain, as a response variable, is only considered in some studies. Schneider et al. 25 reported an improvement in pain after a 3-year follow-up in patients treated with MACI. AOFAS ankle-hindfoot score was also used to evaluate clinical outcome. We found that AOFAS score significantly improved at 12-month follow-up (mean: 42.1 points) as well as pain. Improvement was maintained 24 months post-op (mean: 44.6 points). Erickson et al. 21 reported an average improvement of 32.9 points (range 27.6-47.0).25,42 It is difficult to compare all these results, including ours, because of the heterogeneity of used procedures for ACI (PACI, open MACI, arthroscopic MACI or PACI and HD-ACI) and different follow-up times (from 36 months to 10 years). Minimum clinically important difference (MCID) is one parameter that could be used to determine whether improvement is perceived by patients as a real clinical improvement. In the case of patients with cartilage damage in talus, treated with ACI, MCID for AOFAS score has not been established yet. Recently, MCID for AOFAS in patients with hallux valgus surgery has been established between 7.9 and 30.2 points. 43 Although, they are different pathologies, it could be suggested that the improvement observed not only in our patients but also in those from other studies,22-35,42,44 could be perceived by patients as clinically important. However, this interpretation should be taken cautiously and deserves further research.
After cartilage repair, MOCART scoring system can be used to evaluate cartilage healing degree through MRI. 36 In our work, we have found similar score values to those reported by other authors after ACI.45,46 Furthermore, MOCART significantly decreased from 12 to 24 months after HD-ACI. We do not have a clear explanation for this fact, but similar results have been published by McCarthy et al. 47 in patients treated with ACI in the knee. In spite of the good AOFAS improvement at 12- and 24-month follow-ups, a relatively high number of patients showed MRI abnormalities, actually related to subchondral lamina and bone. These findings are in accordance with those published by Chan et al. 44 who demonstrated that good clinical outcomes are not always accompanied by normal MRI. They conclude that although MRI is a good tool to evaluate ACI grafts, results should be taken cautiously. 44
Existence of prognostic factors that could predict the response to ACI is controversial21,23,24,41,45,48,49 Several studies have found that age,24,48,50 defect depth, 45 or duration of symptoms 50 could affect response to ACI treatment. In our study, no prognostic factors possibly related to ACI response have been found. Specifically, no statistically significant relations between response at 12 and 24 months with age, lesion size, or symptom duration and lesion depth reflected by the use of “sandwich” technique were found. However, there is not a unanimous opinion about the existence of such factors, which could reflect patient heterogeneity included in published case series.
Range of motion, studied as the dorsal and plantar flexion changes before and after the surgical procedure, was included as 2 secondary response variables. Only significant differences were observed in dorsal flexion at 12 and 24 months with regard to baseline. Return to sports or to patient’s normal life is one way to evaluate efficiency and patient quality of life after implantation. All patients included in this study returned to their normal life after 12 months and a high percentage of patients who used to practice sports returned to sports at the same level as before implantation. Buda et al. 51 have published similar results concerning return to sports in a study comparing ACI with bone marrow–derived mesenchymal cell implantation. These results suggest that HD-ACI and other ACI modalities may be responsible for patient recovery after talus cartilage lesion treatment.
During follow-up, no serious adverse events were observed. Only 1 patient continued having swelling at 24 months postoperatively. Bone edema development has been reported in patients treated with ACI in knee.40,52 In the present study, patients with bone edema at baseline and follow-up were found, but no relationship with improvement was observed. This result is in agreement with that found in knee where no correlation between bone edema and outcome worsening was observed.
Second-look arthroscopy performed 1 year after implantation, at the time of osteosynthesis material removal, revealed a neoformed tissue very close to hyaline cartilage. In 5 patients, it was possible to take a second-look biopsy after the osteosynthesis material was removed, finding hyaline-like cartilage tissue in all cases. Both results suggest that cartilage defect is filled up by hyaline or hyaline-like cartilage 12 months after HD-ACI.
The main weakness of the present study is the small number of included patients (and the few patients with a second-look biopsy). With respect to the small sample size, as discussed above, all publications on ACI have similar case-series due to low prevalence of talar cartilage defects. In relation to the small number of patients with a neoformed tissue biopsy, results found in these 5 patients were consistent to make us think that HD-ACI is able to induce hyaline cartilage synthesis, at least in patients who respond to treatment. This result can be supported by the fact that previous studies performed in sheep animal model demonstrate that increasing chondrocyte dose improves neoformed hyaline cartilage quality. 30 Similar results have been published by other authors 24 who found hyaline cartilage and some components of tissue remodeling in responder patients to ACI but fibrocartilage in patients with treatment failure. The main strength of the present study is that the clinical results obtained were very robust despite heterogeneity of included patients.
Taking all these results together, we can conclude that HD-ACI is a safe and effective technique to treat osteochondral lesions of the talus, providing good clinical and histological results at short- and mid-term follow-ups.
Supplemental Video
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work has been financed by Fundación Pedro Guillén. We would like to thank Mario Wensell for carefully revising the linguistics for this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the Hospital Education and Research Committee.
Informed Consent: All patients signed an informed consent.
Trial Registration: Not applicable.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ray RG. Arthroscopic anatomy of the ankle joint. Clin Podiatr Med Surg. 2016;33:467-80. [DOI] [PubMed] [Google Scholar]
- 2. Kraeutler MJ, Kaenkumchorn T, Pascual-Garrido C, Wimmer MA, Chubinskaya S. Peculiarities in ankle cartilage. Cartilage. 2017;8:12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680-7. [DOI] [PubMed] [Google Scholar]
- 4. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-74. [DOI] [PubMed] [Google Scholar]
- 5. Lomax A, Miller RJ, Fogg QA, Madeley NJ, Kumar CS. Quantitative assessment of the subchondral vascularity of the talar dome: a cadaveric study. Foot Ankle Surg. 2014;20:57-60. [DOI] [PubMed] [Google Scholar]
- 6. Looze CA, Capo J, Ryan MK, Begly JP, Chapman C, Swanson D, et al. Evaluation and management of osteochondral lesions of the talus. Cartilage. 2017;8:19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D’Ambrosi R, Villafañe JH, Indino C, Liuni FM, Berjano P, Usuelli FG. Return to sport after arthroscopic autologous matrix-induced chondrogenesis for patients with osteochondral lesion of the talus. Clin J Sport Med. Epub 2017 Dec 26. [DOI] [PubMed] [Google Scholar]
- 8. Gottschalk O, Altenberger S, Baumbach S, Kriegelstein S, Dreyer F, Mehlhorn A, et al. Functional medium-term results after autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a 5-year prospective cohort study. J Foot Ankle Surg. 2017;56:930-6. [DOI] [PubMed] [Google Scholar]
- 9. Hannon CP, Murawski CD, Fansa AM, Smyth NA, Do H, Kennedy JG. Microfracture for osteochondral lesions of the talus: a systematic review of reporting of outcome data. Am J Sports Med. 2013;41:689-95. [DOI] [PubMed] [Google Scholar]
- 10. Lee KB, Park HW, Cho HJ, Seon JK. Comparison of arthroscopic microfracture for osteochondral lesions of the talus with and without subchondral cyst. Am J Sports Med. 2015;43:1951-6. [DOI] [PubMed] [Google Scholar]
- 11. Yasui Y, Ramponi L, Seow D, Hurley ET, Miyamoto W, Shimozono Y, et al. Systematic review of bone marrow stimulation for osteochondral lesion of talus—evaluation for level and quality of clinical studies. World J Orthop. 2017;8:956-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yasui Y, Wollstein A, Murawski CD, Kennedy JG. Operative treatment for osteochondral lesions of the talus: biologics and scaffold-based therapy. Cartilage. 2017;8:42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramponi L, Yasui Y, Murawski CD, Ferkel RD, DiGiovanni CW, Kerkhoffs GMMJ, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45:1698-705. [DOI] [PubMed] [Google Scholar]
- 14. Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): combining microfracturing and a collagen I/III matrix for articular cartilage resurfacing. Cartilage. 2010;1:65-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polat G, Erşen A, Erdil ME, Kızılkurt T, Kılıçoğlu Ö, Aşık M. Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2016;24:1299-303. [DOI] [PubMed] [Google Scholar]
- 16. Lee KB, Bai LB, Yoon TR, Jung ST, Seon JK. Second-look arthroscopic findings and clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2009;37(Suppl 1):63S-70S. [DOI] [PubMed] [Google Scholar]
- 17. van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GM, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95:519-25. [DOI] [PubMed] [Google Scholar]
- 18. Buckwalter JA, Mow VC, Ratcliffe A. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg. 1994;2:192-201. [DOI] [PubMed] [Google Scholar]
- 19. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;(365):149-62. [DOI] [PubMed] [Google Scholar]
- 20. Shimozono Y, Coale M, Yasui Y, O’Halloran A, Deyer TW, Kennedy JG. Subchondral bone degradation after microfracture for osteochondral lesions of the talus: an MRI analysis. Am J Sports Med. 2018;46:642-8. [DOI] [PubMed] [Google Scholar]
- 21. Erickson B, Fillingham Y, Hellman M, Parekh SG, Gross CE. Surgical management of large talar osteochondral defects using autologous chondrocyte implantation. Foot Ankle Surg. 2018;24:131-6. [DOI] [PubMed] [Google Scholar]
- 22. Kwak SK, Kern BS, Ferkel RD, Chan KW, Kasraeian S, Applegate GR. Autologous chondrocyte implantation of the ankle: 2- to 10-year results. Am J Sports Med. 2014;42:2156-64. [DOI] [PubMed] [Google Scholar]
- 23. Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, et al. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87:179-83. [DOI] [PubMed] [Google Scholar]
- 24. Giannini S, Buda R, Ruffilli A, Cavallo M, Pagliazzi G, Bulzamini MC, et al. Arthroscopic autologous chondrocyte implantation in the ankle joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:1311-9. [DOI] [PubMed] [Google Scholar]
- 25. Schneider TE, Karaikudi S. Matrix-induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 2009;30:810-4. [DOI] [PubMed] [Google Scholar]
- 26. Dekker TJ, Erickson B, Adams SB, Gross CE. Topical review: MACI as an emerging technology for the treatment of talar osteochondral lesions. Foot Ankle Int. 2017;38:1045-8. [DOI] [PubMed] [Google Scholar]
- 27. McGoldrick NP, Murphy EP, Kearns SR. Osteochondral lesions of the ankle: the current evidence supporting scaffold-based techniques and biological adjuncts. Foot Ankle Surg. 2018;24:86-91. [DOI] [PubMed] [Google Scholar]
- 28. Dahmen J, Lambers KTA, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs GMMJ. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26:2142-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambers KTA, Dahmen J, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs GMMJ. No superior surgical treatment for secondary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26:2158-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guillén-García P, Rodríguez-Iñigo E, Guillén-Vicente I, Caballero-Santos R, Guillén-Vicente M, Abelow S, et al. Increasing the dose of autologous chondrocytes improves articular cartilage repair: histological and molecular study in the sheep animal model. Cartilage. 2014;5:114-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopez-Alcorocho JM, Aboli L, Guillen-Vicente I, Guillen-Vicente M, Fernández-Jaén TF, Arauz S, et al. Cartilage defect treatment using high-density autologous chondrocyte implantation: two-year follow-up. Cartilage. 2018;9:363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28:154-61. [DOI] [PubMed] [Google Scholar]
- 33. Nam EK, Ferkel RD, Applegate GR. Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sports Med. 2009;37:274-84. [DOI] [PubMed] [Google Scholar]
- 34. Ceccarelli F, Calderazzi F, Pedrazzi G. Is there a relation between AOFAS ankle-hindfoot score and SF-36 in evaluation of Achilles ruptures treated by percutaneous technique? J Foot Ankle Surg. 2014;53:16-21. [DOI] [PubMed] [Google Scholar]
- 35. Cook JJ, Cook EA, Rosenblum BI, Landsman AS, Roukis TS. Validation of the American College of Foot and Ankle Surgeons Scoring Scales. J Foot Ankle Surg. 2011;50:420-9. [DOI] [PubMed] [Google Scholar]
- 36. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16-23. [DOI] [PubMed] [Google Scholar]
- 37. Dixon S, Harvey L, Baddour E, Janes G, Hardisty G. Functional outcome of matrix-associated autologous chondrocyte implantation in the ankle. Foot Ankle Int. 2011;32:368-74. [DOI] [PubMed] [Google Scholar]
- 38. Magnan B, Samaila E, Bondi M, Vecchini E, Micheloni GM, Bartolozzi P. Three-dimensional matrix-induced autologous chondrocytes implantation for osteochondral lesions of the talus: midterm results. Adv Orthop. 2012;2012:942174. doi: 10.1155/2012/942174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kessler JI, Weiss JM, Nikizad H, Gyurdzhyan S, Jacobs JC, Jr, Bebchuk JD, et al. Osteochondritis dissecans of the ankle in children and adolescents: demographics and epidemiology. Am J Sports Med. 2014;42:2165-71. [DOI] [PubMed] [Google Scholar]
- 40. Niethammer TR, Valentin S, Gülecyüz MF, Roßbach BP, Ficklscherer A, Pietschmann MF, et al. Bone marrow edema in the knee and its influence on clinical outcome after matrix-based autologous chondrocyte implantation: results after 3-year follow-up. Am J Sports Med. 2015;43:1172-9. [DOI] [PubMed] [Google Scholar]
- 41. Pagliazzi G, Vannini F, Battaglia M, Ramponi L, Buda R. Autologous chondrocyte implantation for talar osteochondral lesions: comparison between 5-year follow-up magnetic resonance imaging findings and 7-year follow-up clinical results. J Foot Ankle Surg. 2018;57:221-5. [DOI] [PubMed] [Google Scholar]
- 42. Pestka JM, Bode G, Salzmann G, Südkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40:325-31. [DOI] [PubMed] [Google Scholar]
- 43. Chan HY, Chen JY, Zainul-Abidin S, Ying H, Koo K, Rikhraj IS. Minimal clinically important differences for American Orthopaedic Foot & Ankle Society score in hallux valgus surgery. Foot Ankle Int. 2017;38:551-7. [DOI] [PubMed] [Google Scholar]
- 44. Chan KW, Ferkel RD, Kern B, Chan SS, Applegate GR. Correlation of MRI appearance of autologous chondrocyte implantation in the ankle with clinical outcome. Cartilage. 2018;9:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Battaglia M, Vannini F, Buda R, Cavallo M, Ruffilli A, Monti C, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19:1376-84. [DOI] [PubMed] [Google Scholar]
- 46. Apprich S, Trattnig S, Welsch GH, Noebauer-Huhmann IM, Sokolowski M, Hirschfeld C, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 tesla. Osteoarthritis Cartilage. 2012;20:703-11. [DOI] [PubMed] [Google Scholar]
- 47. McCarthy HS, McCall IW, Williams JM, Mennan C, Dugard MN, Richardson JB, et al. Magnetic resonance imaging parameters at 1 year correlate with clinical outcomes up to 17 years after autologous chondrocyte implantation. Orthop J Sports Med. Epub 2018 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L, et al. Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int. 2010;31:747-53. [DOI] [PubMed] [Google Scholar]
- 49. Quirbach S, Trattnig S, Marlovits S, Zimmermann V, Domayer S, Dorotka R, et al. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009;38:751-60. [DOI] [PubMed] [Google Scholar]
- 50. Aurich M, Bedi HS, Smith PJ, Rolauffs B, Mückley T, Clayton J, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39:311-9. [DOI] [PubMed] [Google Scholar]
- 51. Buda R, Vannini F, Castagnini F, Cavallo M, Ruffilli A, Ramponi L, et al. Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop. 2015;39:893-900. [DOI] [PubMed] [Google Scholar]
- 52. Ebert JR, Smith A, Fallon M, Wood DJ, Ackland TR. Degree of preoperative subchondral bone edema is not associated with pain and graft outcomes after matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2014;42:2689-98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.