Abstract
AMP-activated protein kinase (AMPK) is a master regulator of energy homeostasis that functions to restore the energy balance by phosphorylating its substrates during altered metabolic conditions. AMPK activity is tightly controlled by diverse regulators including its upstream kinases LKB1 and CaMKK2. Recent studies have also identified the localization of AMPK at different intracellular compartments as another key mechanism for regulating AMPK signaling in response to specific stimuli. This review discusses the AMPK signaling associated with different subcellular compartments, including lysosomes, endoplasmic reticulum, mitochondria, Golgi apparatus, nucleus, and cell junctions. Because altered AMPK signaling is associated with various pathologic conditions including cancer, targeting AMPK signaling in different subcellular compartments may present attractive therapeutic approaches for treatment of disease.
Keywords: AMPK, LKB1, CaMKK2, lysosome, endoplasmic reticulum, mitochondria, nucleus, cell junction
Introduction
Sensing nutrient availability is a key feature of virtually all living organisms (Chantranupong et al., 2015; Efeyan et al., 2015). When nutrients are present in abundance, they are sensed, taken up, and utilized by cells to promote energy-consuming anabolic metabolism. In contrast, under conditions of nutrient scarcity, cells remodel their intracellular metabolic network towards energy-generating catabolic metabolism, with the ultimate goal of surviving under these adverse conditions. Maintaining the balance between anabolic and catabolic metabolism depends mainly on the availability of ATP, the cellular energy currency. In eukaryotes, ATP is generated primarily by oxidative phosphorylation and is utilized not only for building cellular macromolecules (such as fatty acids, nucleic acids, and proteins) but also for supporting many other cellular processes (such as nutrient transport and muscle contraction) (Vander Heiden et al., 2009). Correspondingly, eukaryotes have evolved intricate regulatory mechanisms to sense ATP levels and to modulate cellular metabolism in response to fluctuations in intracellular ATP levels, prominent among which is the AMP-activated protein kinase (AMPK) -mediated energy sensing mechanism (Hardie et al., 2012; Hardie et al., 2016; Herzig and Shaw, 2018).
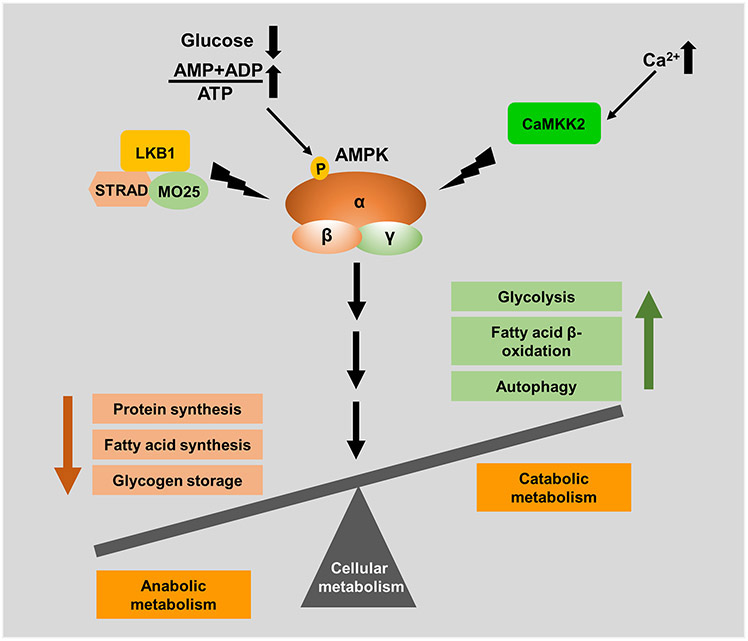
AMPK is well recognized as a critical energy sensor and a master regulator of energy metabolism in eukaryotes. Being at the centre of many metabolic pathways, AMPK is tightly regulated by cellular energy status, which involves both its allosteric activation and its phosphorylation by upstream kinases. AMPK exists as a heterotrimeric complex consisting of AMPK-α, -β and -γ subunits (Figure 1). Mammalian cells possess two isoforms of AMPK-α, two isoforms of AMPK-β, and three isoforms of AMPK-γ (Ross et al., 2016b). The catalytic activity of AMPK resides in its α subunit, whereas both β and γ subunits serve as the regulatory components (Mihaylova and Shaw, 2011). In response to energy stress, an increase in cellular ADP and AMP levels as a result of ATP consumption triggers AMPK activation through direct binding of ADP or AMP to the AMPK-γ subunit (Oakhill et al., 2011). Although both ADP and AMP can bind to AMPK-γ to facilitate AMPK activation, changes in the AMP/ATP ratio are considered a more sensitive indicator of energy stress than the ADP/ATP ratio because newly generated ADP is rapidly converted to AMP by adenylate kinase; therefore, it is proposed that AMP is a true physiological trigger for AMPK activation during energy stress (Gowans et al., 2013). Based on the current model, AMP promotes AMPK activation by at least three mechanisms, which involve (i) promoting allosteric activation of AMPK, (ii) facilitating the phosphorylation of Thr172 located in the activation loop of the AMPK-α subunit by its upstream kinase liver kinase B1 (LKB1) (Gowans et al., 2013; Hawley et al., 2003; Hong et al., 2003; Shaw et al., 2004), and (iii) preventing AMPK Thr172 de-phosphorylation by protein phosphatases (Gowans et al., 2013; Li et al., 2015). Unlike AMP, ADP has only been shown to prevent AMPK dephosphorylation (Xiao et al., 2011). However to mimic the effects of AMP mediated protection against dephosphorylation, ADP has to be present in 10 fold higher concentration, suggesting AMP is a true metabolic regulator of AMPK (Gowans et al., 2013; Ross et al., 2016a).
Figure 1. AMPK activation and function.
Heterotrimeric AMPK complex is activated by its upstream kinases LKB1 and CaMKK2. Following energy stress, LKB1 complex consisting of LKB1, STRAD and MO25 phosphorylates AMPK-α at T172 residue. On the other hand, cytosolic calcium influx activates AMPK through CaMKK2-mediated AMPK phosphorylation at T172. Activated AMPK then acts on its numerous downstream targets and shifts the balance from anabolic processes to catabolic processes.
Once activated by energy stress, AMPK then phosphorylates several downstream targets to restore energy balance by shifting from ATP-consuming anabolic processes (such as fatty acid synthesis and protein synthesis) to ATP-generating catabolic processes (such as glycolysis and autophagy) (Garcia and Shaw, 2017) (Figure 1). For example, in response to energy stress, AMPK activates autophagy, a catabolic process that recycles intracellular nutrients to maintain cell survival under nutrient-deprived conditions, through phosphorylating autophagy regulators such as ULK1 (Egan et al., 2011; Kim et al., 2011), while inhibits mechanistic target of rapamycin complex 1 (mTORC1)-mediated protein synthesis by phosphorylating Raptor (an mTORC1 component) and the TSC1–TSC2 complex (an upstream negative regulator of mTORC1) (Gwinn et al., 2008; Inoki et al., 2003).
In addition to energy-stress–mediated AMPK activation, AMPK can also be activated after a rise in intracellular calcium levels. However, LKB1 does not seem to be required for calcium-induced AMPK activation. Instead, calcium-induced AMPK activation was found to require calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2)-mediated Thr172 phosphorylation in AMPK without affecting cellular ADP or AMP levels (Hawley et al., 2005; Hurley et al., 2005; Woods et al., 2005). Physiologically, increases in cellular calcium levels and activation of CaMKK2-AMPK signaling have been reported during T-cell activation (Tamas et al., 2006), in hypothalamic neurons to regulate appetite (Anderson et al., 2008), and during the mechanosensitive assembly of actin to control cell adhesion and migration (Tojkander et al., 2018). Pathologically, aberrant CaMKK2-AMPK signaling promotes anoikis resistance and cancer metastasis (Jin et al., 2018; Sundararaman et al., 2016).
Apart from the canonical regulation of AMPK through allosteric activation and upstream kinase–mediated phosphorylation, AMPK function and its downstream signaling can be regulated by its protein stability, post-translational modifications (reviewed in (Garcia and Shaw, 2017; Jeon, 2016; Zungu et al., 2011)) and non-coding RNAs (Li et al., 2019b; Liu et al., 2016). Notably, recent studies indicate that AMPK localization at different subcellular compartments also serves to fine-tune its activation and downstream signaling activities in response to energy stress and other upstream stimuli. In this review, we will focus on this emerging theme on AMPK signaling, and discuss in detail the regulation of AMPK signaling at different subcellular compartments, including lysosomes, endoplasmic reticulum (ER), mitochondria, Golgi, nucleus, as well as cellular junctions.
Lysosomes as a hub for glucose-starvation–induced AMPK activation
Lysosomes, once viewed as the “trash bags” of cells, are now recognized as a critical cellular signaling hub (Appelqvist et al., 2013). Lysosomes are membrane-bound organelles with an acidic luminal environment that contain many enzymes including nucleases, hydrolases, and proteases to facilitate the digestion of macromolecules (Lawrence and Zoncu, 2019). Apart from its established role in macromolecule digestion, lysosomes have vital roles in various other cellular processes such as iron homeostasis, cholesterol transport, immune response, plasma membrane repair, and signaling regulation (Kurz et al., 2011; Lim and Zoncu, 2016). Recent studies have established that, similar to AMPK-mediated energy sensing, lysosomes also modulate cellular metabolism by sensing nutrient availability and triggering appropriate signaling responses (Settembre et al., 2013). For example, stimulation by nutrients, particularly amino acids, triggers a Rag GTPase-dependent signaling event that promotes mTORC1 localization on lysosomes, where mTORC1 is further activated by another GTPase, Rheb (Sabatini, 2017; Saxton and Sabatini, 2017). Once activated, in addition to phosphorylating its substrates involved in protein synthesis and autophagy, mTORC1 also phosphorylates the transcription factor EB (TFEB), a master transcriptional regulator of lysosomal biogenesis (Sardiello et al., 2009; Settembre et al., 2011), resulting in TFEB sequestration on lysosomes and inactivation of its transcriptional activity to regulate lysosomal biogenesis; nutrient starvation leads to mTORC1 inactivation, TFEB de-phosphorylation, and translocation to the nucleus to upregulate TFEB-mediated lysosomal biogenesis (Martina et al., 2012; Settembre et al., 2013; Settembre et al., 2012). Alterations in lysosomal function or biogenesis have been linked with various pathological conditions including lysosomal storage diseases, neurodegenerative diseases, and cancer (Fennelly and Amaravadi, 2017; Platt et al., 2018).
Recent studies have also linked lysosomes to energy-stress–induced AMPK activation (Carroll and Dunlop, 2017; Zhang et al., 2014; Zhang et al., 2013). The scaffolding protein axis inhibition protein (AXIN) was originally identified as a negative regulator of the Wnt pathway and is well established as a scaffolding component for Wnt signaling (Kikuchi, 1999; Zeng et al., 1997). Surprisingly, liver-specific knockdown of Axin in mouse led to compromised AMPK activation after fasting (Zhang et al., 2013). Subsequently, AXIN was shown to form a complex with AMPK and LKB1, and AXIN depletion prevents the formation of LKB1-AMPK complexes after glucose starvation. In support of the model that AMP is the main physiological regulator of AMPK activation, only the binding of AMP, but not ADP or ATP, to the AMPK-γ subunit promoted the interaction between AXIN and AMPK (Gowans et al., 2013; Zhang et al., 2013). These findings suggest that AMP has a role in assembling an AXIN-AMPK-LKB1 complex on lysosomes for energy-stress–induced AMPK activation (Lin and Hardie, 2018).
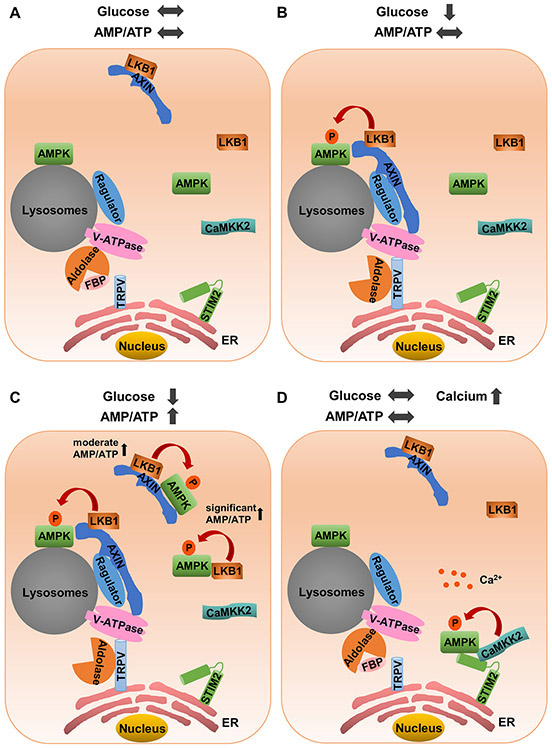
A subsequent study revealed that the v-ATPase-Ragulator complex, which is known to mediate amino-acid–induced mTORC1 activation on lysosomes (Sancak et al., 2010; Zoncu et al., 2011), is also critical for energy-stress–induced AMPK activation. Mechanistically, it was proposed that AMPK is constitutively localized on lysosomes and that, upon glucose starvation, lysosome-localized v-ATPase-Ragulator complex recruits the AXIN-LKB1 complex to the AMPK on lysosomes, resulting in LKB1-mediated phosphorylation and activation of AMPK (Figure 2A-2B) (Zhang et al., 2014). The v-ATPase-Ragulator complex also has critical roles in recruiting mTORC1 to lysosomes via Rag (Sancak et al., 2010; Zoncu et al., 2011). It was further shown that, while promoting AMPK activation in response to energy stress, AXIN binding to the v-ATPase-Ragulator complex releases mTORC1 from lysosomes, resulting in inhibition of mTORC1 activity under energy stress (Zhang et al., 2014). Of note, other studies also support the link between AMPK and lysosomes. For example, studies using a genetically encoded AMPK activity sensor confirmed lysosomal localization of enzymatically active AMPK and further revealed an increase in lysosome-associated AMPK activity after energy stress (Miyamoto et al., 2015b).
Figure 2. Compartmentalized regulation of AMPK on lysosomes and ER.
(A) Under the conditions of glucose sufficiency, FBP (a glycolysis intermediate)-bound aldolase inhibits the interaction of the AXIN-LKB1 complex with the v-ATPase-Ragulator complex on lysosome membrane, thus hampering the activation of lysosomal localized AMPK. (B) In response to glucose starvation (but before obvious AMP/ATP ratio increase), FBP-unoccupied aldolase interacts with and inhibits the TRPV calcium channel present at the local lysosomal-ER contact sites. Aldolase-TRPV interaction promotes the formation of a ternary complex with v-ATPase-Regulator complex that allows the recruitment of the AXIN-LKB1 complex on the lysosomes via v-ATPase-Ragulator to facilitate lysosomal AMPK phosphorylation. (C) Gradient increase in cellular AMP/ATP ratio caused by long-term glucose starvation leads to the activation of both cytosolic and lysosomal pool of AMPK. A moderate increase in AMP/ATP ratio causes cytosolic AMPK activation via AXIN-LKB1, whereas a more significant increase in AMP/ATP ratio causes further AMPK activation via LKB1 independent of AXIN. (D) Under the conditions of calcium influx, ER localized STIM2 recruit AMPK and its upstream kinase CaMKK2 in close proximity to facilitate AMPK phosphorylation. ⬆ ⬇ and ⬌ denote increased, decreased, and unchanged concentrations, respectively.
Metformin is a drug widely used to treat type 2 diabetes, and its anti-diabetic activities have been at least partly linked to its ability to activate AMPK (Foretz et al., 2014). However, exactly how metformin activates AMPK remains incompletely understood. Because metformin inhibits respiratory chain complex I in the mitochondria and suppresses ATP synthesis, it has been suggested that metformin activates AMPK via an increase in the cellular AMP/ATP ratio (Foretz et al., 2014). In contrast, others have reported that clinically recommended concentrations of metformin can activate AMPK without affecting cellular energy status, suggesting that metformin can activate AMPK through AMP-independent mechanisms (He and Wondisford, 2015). In support of this latter point, a recent study proposed that metformin can activate AMPK through the lysosome pathway without affecting cellular energy status (Zhang et al., 2016). It was shown that metformin failed to activate AMPK in AXIN-deficient mouse liver, and metformin was found to act on v-ATPase to facilitate tethering of AXIN-LKB1 to the lysosomes, leading to AMPK activation without compromising cellular energy status (Zhang et al., 2016).
Glucose provides the primary energy source to generate ATP in most cells (Vander Heiden et al., 2009); correspondingly, glucose starvation leads to rapid AMPK activation (Inoki et al., 2003). It is widely accepted that glucose starvation activates AMPK through the increased AMP/ATP ratio. However, short-term glucose deprivation can promote AMPK activation without affecting the cellular AMP/ATP ratio (Zhang et al., 2017). In addition, cells expressing a non-AMP-responsive AMPK-γ subunit (R531G mutant) still exhibited AMPK activation after short-term glucose deprivation, further strengthening an AMP-independent means of activating AMPK in this context (Zhang et al., 2017). As discussed above, AMP promotes the formation of AXIN-AMPK-LKB1 complexes and activates AMPK on lysosome membranes (Zhang et al., 2013). Surprisingly, AMPK activation was found to be compromised in AXIN- or LAMTOR-deficient cells after short-term glucose starvation (despite no obvious change in AMP or ATP levels), suggesting that AXIN-LKB1-mediated AMPK activation can be regulated by sensing glucose availability in addition to AMP (Zhang et al., 2017). This finding led to the hypothesis that either glucose itself or its downstream metabolites might serve as crucial regulators of AMPK activation. Further analyses revealed that fructose-1,6-bisphosphate (FBP), a glycolysis intermediate, has the effect of dissociating AXIN, LKB1, and AMPK from LAMTOR1, leading to compromised AMPK activation. Consistent with the model that FBP is utilized by aldolase during glycolysis, knockdown of aldolase resulted in enhanced AMPK activation even in the presence of glucose. Together, these findings suggest an intriguing model wherein FBP-unoccupied aldolase promotes v-ATPase-Ragulator-AXIN-LKB1 complex formation on lysosomes and AMPK activation in response to acute glucose starvation without obvious changes in the AMP/ATP or ADP/ATP ratio (Figure 2A-2B) (Zhang et al., 2017).
As AMPK can sense both cellular AMP (or ADP) levels and glucose availability and can also be localized at different subcellular compartments (which will be further discussed below), it has remained unclear how different signalling cues regulate differential activation of AMPK at different subcellular compartments. A recent study provides important insights into this question. It was shown that glucose starvation (before obviously decreasing AMP levels) specifically activates lysosomal but not cytosolic or mitochondrial pool of AMPK via aldolase-v-ATPase-Ragulator-AXIN-LKB1 complex (Figure 2B), resulting in phosphorylation of AMPK downstream substrates such as ACC2, Raptor, TSC2, HDAC4, SREBP1, and TBC1D1 to quickly shift the overall metabolic balance from anabolic processes to catabolic processes (Zong et al., 2019). A moderate increase in cellular AMP levels then promotes both lysosomal and cytosolic activation of AMPK, which was found to be independent of v-ATPase-Ragulator-containing complex on lysosomes but dependent on AXIN-mediated tethering of AMPK and LKB1. In contrast, under severe energy stress, high levels of intracellular AMP caused activation of cytosolic, lysosomal, and mitochondrial pools of AMPK, which was found to be independent of AXIN but dependent of LKB1 (Zong et al., 2019) (Figure 2C). Together, this study suggests that differential degrees of energy stress initiates different metabolic responses through regulating different compartmentalized pools of AMPK, therefore further supporting the importance of AMPK subcellular location in mediating AMPK signaling.
Notably, the tethering of AMPK to lysosomes is not only crucial for glucose-starvation–induced AMPK activation but also links AMPK to the regulation of lysosomal biogenesis through TFEB (Young et al., 2016). Functional analyses of AMPK in lineage specification of embryonic stem cells (ESCs) revealed that AMPK-deficient ESCs had impaired differentiation due to dysregulated lysosomal function and biogenesis. Mechanistic studies identified an AMPK-mTORC1-TFEB signaling axis wherein, under AMPK inactivation, mTORC1 promotes nuclear exclusion of TFEB and therefore inhibits lysosomal biogenesis (Young et al., 2016). Recent studies further validated the AMPK-TFEB signaling axis in the context of Kras-mediated lung cancer development. While Lkb1 deletion promotes Kras-driven lung cancer growth (Ji et al., 2007), surprisingly, Ampk deletion markedly inhibited Kras-dependent lung cancer in mouse models (Eichner et al., 2019). Further analyses revealed that AMPK promotes lung cancer development at least partly through regulating lysosomal gene expression mediated by Tfe3, another TFEB transcription factor (Eichner et al., 2019). Reciprocally, overexpression of TFEB in muscle cells has been shown to promote AMPK-mediated glucose uptake (Mansueto et al., 2017). In summary, lysosomes have emerged as a crucial hub to mediate AMPK signaling, not only by serving as a platform for AMPK activation in response to metabolic status alterations but also by providing nutrient-sensing capabilities for AMPK.
Endoplasmic reticulum as a platform for calcium- and glucose-starvation–induced AMPK activation
ER is a dynamic cellular organelle involved in protein folding and secretion (Benham, 2012). Proteins translocated into ER are subjected to correct folding and additional post-translational modifications with the help of ER lumen-localized chaperons (Schwarz and Blower, 2016). Apart from its central role in protein synthesis and trafficking, ER is also involved in numerous other cellular functions including lipid metabolism, calcium signaling, glycosylation, and phagocytosis (Desjardins, 2003; Helenius and Aebi, 2001; Mekahli et al., 2011). Physiological alterations such as imbalances in calcium homeostasis or protein synthesis and folding trigger adaptive responses, collectively called the ER-stress response or unfolded protein response (UPR), which aims to restore cellular homeostasis (Walter and Ron, 2011). Correspondingly, dysregulated UPR is associated with the pathogenesis of various diseases (Ozcan and Tabas, 2012).
Several previous studies have linked AMPK to ER. Analyses using probes for AMPK activity indicated that enzymatically active AMPK is present on ER (Miyamoto et al., 2015b; Qi et al., 2008). Other studies also suggested that AMPK can protect against ER-stress–induced apoptosis through various mechanisms (Amodio et al., 2018; Yang et al., 2013). AICAR-mediated AMPK activation has been shown to prevent ER stress through FOXO1-mediated upregulation of the ER chaperone ORP150 (Jung et al., 2018; Liu et al., 2018). Similarly, AMPK was also found to inhibit low-density lipoprotein (LDL) -induced ER stress (Dong et al., 2010). Activated AMPK has also been demonstrated to phosphorylate transcription factor SREBP-1c (localized on ER) and inhibit its proteolytic processing and nuclear translocation thereby resulting in the decreased fatty acid and cholesterol biosynthesis (Li et al., 2011). In addition to its role in ER stress, AMPK activity has also been linked to the regulation of ER morphology via phosphorylation of GTPase dynamin-related protein 1 (DRP1), a critical regulator of mitochondrial fission (Wikstrom et al., 2013). However, how ER is involved in regulating AMPK activation by upstream stimuli or kinases had remained unclear.
As discussed in a previous section, AMPK is activated by energy stress and increased intracellular calcium levels through different upstream kinases: whereas LKB1 is required for energy-stress–induced AMPK activation, CaMKK2 mainly mediates calcium-induced AMPK activation. Intriguingly, analogous to the role of lysosomes in energy-stress–mediated AMPK activation by LKB1 (Zhang et al., 2017; Zhang et al., 2013), recent studies also revealed that ER has a critical role in calcium-induced AMPK activation by CaMKK2, and this regulation requires stromal interaction molecule 2 (STIM2), an ER-resident transmembrane proteins that can potentially sense calcium levels in the ER lumen through their luminal EF-hand motifs (Chauhan et al., 2018). Based on this model, alterations of calcium levels in the ER lumen lead to conformational changes of STIM2 in its cytoplasmic portion, which promotes STIM2 interaction with both CaMKK2 and AMPK and therefore tethers CaMKK2 to phosphorylate AMPK, resulting in calcium-induced AMPK activation (Figure 2D). Interestingly, a recent study has identified both STIM1 and STIM2 as an AMPK substrate to regulate store operated calcium entry (SOCE), suggesting a reciprocal regulation between AMPK and STIM2 (Stein et al., 2019).
These findings suggest that AMPK uses ER as another docking site to specifically sense cellular calcium fluctuations and regulate its activation and downstream signaling in response to calcium stimulation. Therefore, this model is analogous to the afore-described model wherein energy stress induces AXIN-LKB1-AMPK complex formation on lysosomes, resulting in energy-stress–induced AMPK activation (Figure 2B); in both models, AXIN and STIM2 serve as scaffolding proteins to tether the corresponding kinases (LKB1 or CaMKK2) to phosphorylate AMPK on appropriate intracellular compartments (lysosomes or ER) in response to different stimuli (energy stress or calcium stimulation). The differences between these two models are that, as an ER resident transmembrane protein, STIM2 is sufficient to tether CaMKK2 and AMPK on ER; on the other hand, because AXIN is not a lysosome-resident protein, it requires additional support from lysosome-localized proteins such as v-ATPase and Ragulator to tether LKB1 and AMPK on lysosomes. Considering the versatile role of AMPK in cellular metabolism, it is tempting to speculate that mammalian cells may have evolved these mechanisms to tether AMPK on different intracellular compartments to sense different upstream stimuli and mediate different downstream signaling.
Interestingly, a recent study revealed that ER-lysosomal contact sites also play important roles in glucose-starvation–induced AMPK activation. As discussed in the previous section, acute glucose deprivation can be sensed by AMPK via interaction between FBP-unoccupied aldolase and the v-ATPase-Ragulator-AXIN-LKB1 complex located on lysosomes, although the exact mechanism regulating this interaction had remained elusive (Zhang et al., 2017). A follow-up study from the same group recently showed that under low glucose conditions, FBP-unoccupied aldolase interacts with the ER-localized transient receptor potential channels (TRPV) to inhibit its calcium release activity at ER-lysosome contact sites. It was proposed that the reduced calcium levels at ER-lysosome contact sites somehow promote the interaction between ER-localized TRPV and lysosome-localized v-ATPase, which subsequently recruits the AXIN-LKB1 complex to activate AMPK on lysosomes independently of AMP sensing (Li et al., 2019a) (Figure 2C). In support of this model, TRPV inactivation inhibited glucose-starvation–induced AMPK activation (Li et al., 2019a). Together, these recent studies suggest that ER plays important roles in both calcium- and glucose-starvation–induced AMPK activation: ER directly regulates calcium-induced AMPK activation, while indirectly controls glucose-starvation–induced AMPK activation through communicating with lysosomes.
Regulation of mitochondrial and Golgi dynamics by AMPK
In addition to lysosomes and ER, analyses of AMPK activity probes have revealed the association of enzymatically active AMPK with several other intracellular compartments, including mitochondria and Golgi (Miyamoto et al., 2015a; Miyamoto et al., 2015b). In this section, we discuss recent studies highlighting AMPK signaling at these compartments, with a focus on AMPK function in regulating organelle dynamics at these subcellular compartments.
AMPK has a central role in regulating mitochondrial homeostasis, including mitochondrial biogenesis, mitochondrial fission/fusion, and mitophagy (Herzig and Shaw, 2018). Enhanced mitochondrial biogenesis likely represents another important mechanism to support increased demand for ATP generation during energy stress. AMPK supports mitochondrial biogenesis at least partly by upregulating the expression of mitochondrial proteins via various transcriptional regulators such as peroxisome proliferator-activated receptor-γ (PPARγ) co-activator 1α (PGC-1α) (Jager et al., 2007; Rabinovitch et al., 2017). Mechanistically, AMPK has been shown to both directly phosphorylate PGC-1α, leading to its activation, and upregulate PGC1α expression at least partly by facilitating the nucleosome remodeling via phosphorylating DNA methyltransferase 1 (DNMT1), retinoblastoma binding protein 7 (RBBP7), and histone acetyltransferase 1 (HAT1) (Jager et al., 2007; Marin et al., 2017) (Figure 3).
Figure 3. Mitochondrial regulation by AMPK.
During energy stress conditions, AMPK promotes mitochondrial biogenesis by phosphorylating DNMT1, RBBP7, and HAT1, causing enhanced expression of PGC-1α, as well as directly phosphorylating PGC-1α, a master transcriptional regulator of mitochondrial biogenesis. In addition, AMPK promotes mitochondrial fission to adapt to energy stress by phosphorylating MFF and ARMC10 and facilitating the recruitment of mitochondrial fission-promoting GTPase DRP1. AMPK can also translocate into mitochondria, phosphorylate MCU protein, and regulate energy metabolism in mitochondria.
Recent studies revealed that AMPK also regulates mitochondrial fission/fusion, a dynamic process involved in the maintenance of mitochondrial homeostasis (Herzig and Shaw, 2018; Youle and van der Bliek, 2012). Damaged or defective mitochondria can be toxic to cells because of their ability to generate reactive oxygen species (ROS) and to interfere with cellular metabolic pathways, and therefore need to be eliminated or repaired (Westermann, 2010; Youle and van der Bliek, 2012). Mitochondrial fission and fusion facilitate the clearance of damaged mitochondria by promoting mitophagy or utilization of its unaltered components by mitochondria fusion (Westermann, 2010). Members of the dynamin family of GTPases such as DRP1, mitofusin (Mfn), and optic atrophy 1 (OPA1) are central regulators of mitochondrial fission and fusion (Lee and Yoon, 2016). AMPK deficiency led to impaired mitochondrial fission in response to environmental insults that cause disruption of mitochondrial respiratory chain complexes, whereas pharmacologic activation of AMPK was sufficient to promote mitochondrial fission. Mechanistically, it was further revealed that under mitochondrial stress, AMPK phosphorylates mitochondria-localized mitochondrial fission factor (MFF) (Ducommun et al., 2015; Toyama et al., 2016), which upon phosphorylation promotes mitochondrial fission by recruiting membrane-remodelling GTPase DRP1 to the outer membrane of mitochondria (Kalia et al., 2018). In addition, a recent phosphoproteomic study identified armadillo repeat-containing protein 10 (ARMC10) as another AMPK substrate that localizes on mitochondria and regulates mitochondrial fission (Chen et al., 2019). Proteomic analysis of ARMC10-interacting proteins revealed that ARMC10 may promote mitochondrial fission by interacting with other proteins involved in regulating mitochondrial fission and mitophagy, including MFF (Chen et al., 2019). Both studies showed that depleting MFF (or ARMC10) or mutating MFF (or ARMC10) phosphorylation by AMPK attenuates AMPK-mediated mitochondrial fission, whereas overexpression of their phosphomimetic mutants is sufficient to promote mitochondrial fission even in the absence of AMPK activation, suggesting that AMPK promotes mitochondrial fission at least partly by phosphorylating MFF, ARMC10, or both (Chen et al., 2019; Toyama et al., 2016). Together, these studies identified MFF and ARMC10 as two downstream effectors of AMPK in regulating mitochondrial dynamics (Figure 3).
In the study discussed above, mitochondrial dynamics are controlled by AMPK localized outside of mitochondria (or localized on the mitochondrial surface). A recent study also revealed that AMPK can regulate mitochondrial function through its translocation into mitochondria. It was shown that ATP consumption during mitosis activates AMPK and causes its translocation into mitochondria, where AMPK subsequently phosphorylates mitochondrial calcium uniport (MCU), a calcium channel located in the inner mitochondrial membrane i.e. mitochondrial calcium uniport (MCU) (Zhao et al., 2019). Phosphorylated MCU promotes mitochondrial calcium entry from the cytosol, which in turn activates enzymes involved in cellular respiration (Figure 3). It was proposed that this mechanism serves to boost mitochondrial ATP production and restore energy balance to allow mitotic progression (Zhao et al., 2019).
In addition to mitochondria, AMPK activity has also been reported to regulate the dynamics of the Golgi apparatus. During mitosis, fragmentation of the Golgi apparatus is required for its distribution into daughter cells (Jackson, 2018). Recent studies revealed that AMPK is involved in regulating Golgi fragmentation during mitosis. Both energy stress and mitosis entry promote AMPK-mediated phosphorylation of Golgi-specific brefeldin A resistance factor 1 (GBF1) (Mao et al., 2013; Miyamoto et al., 2008). GBF1 functions as a guanine nucleotide exchange factor (GEF) for Arf GTPases, which regulate protein sorting and maintenance of Golgi apparatus (Bottanelli et al., 2017; Jackson, 2018). AMPK-mediated phosphorylation of GBF1 was shown to result in GBF1 release from Golgi, thereby preventing Arf1 effector recruitment to facilitate Golgi disassembly (Mao et al., 2013). Interestingly, CaMKK2, but not LKB1, has been proposed as the upstream kinase of AMPK to regulate Golgi fragmentation (Jackson, 2018). In summary, increasing evidence indicates that AMPK has an important role in regulating organelle dynamics in response to altered cellular conditions.
AMPK signaling in the nucleus
While AMPK has been shown to be localized on the membrane surface of various intracellular organelles, AMPK can also translocate into the nucleus. For example, both AMPK-α1 and -α2 isoforms were demonstrated to translocate into the nucleus under different contexts (Lamia et al., 2009; Salt et al., 1998; Vara-Ciruelos et al., 2018). Further analyses revealed that AMPK-α contains both nuclear localization signal in its N-terminal catalytic domain and nuclear export signal in its C-terminal regulatory domain to facilitate signal-dependent shuttling between the cytoplasm and the nucleus (Kazgan et al., 2010; Kodiha et al., 2007; Suzuki et al., 2007). Notably, LKB1, the major upstream kinase of AMPK, also exhibits nucleo-cytoplasmic shuttling (Dorfman and Macara, 2008), although it remains unclear whether nuclear-localized LKB1 plays a role in AMPK activation in the nucleus (or AMPK is first activated by LKB1 in the cytoplasm followed by AMPK translocation into the nucleus).
Current studies suggest a model that various signaling cues or pathological conditions modulate AMPK nuclear localization and subsequent transcription alterations. For instance, during myogenic differentiation, AMPK-α2-containing AMPK complex translocates into the nucleus to regulate the expression of PGC-1α, cytochrome C, and muscle creatine kinase (MCK) (Okamoto et al., 2019). Similarly, leptin-induced fatty acid oxidation is shown to be partly mediated by the nuclear translocation of AMPK and subsequent upregulation of PPARα (Suzuki et al., 2007). In patients or mice with Huntington’s disease, AMPK-α1 exhibited enhanced nuclear localization, which correlated with increased huntingtin protein aggregation, neuronal loss, and brain atrophy (Ju et al., 2011). AMPK nuclear localization was also found to be inversely correlated with the nuclear protein levels of circadian component cryptochrome circadian regulator 1 (CRY1). Mechanistically, it was shown that AMPK-mediated phosphorylation of CRY1 causes its destabilization, therefore regulating circadian clock (Lamia et al., 2009).
As mentioned previously, in response to metabolic stress, AMPK activates autophagy by directly phosphorylating ULK1 and other autophagy regulators in the cytoplasm. Recent studies showed that AMPK-mediated autophagy activation in response to metabolic stress also involves transcriptional regulation of autophagy genes in the nucleus (Sakamaki et al., 2017). Bromodomain-containing protein 4 (BRD4), an epigenetic reader for acetylated histones, has been demonstrated to repress the transcription of autophagy genes, therefore maintaining basal autophagy under normal conditions. Following nutrient starvation, AMPK activation triggers histone H4K16 deacetylation by SIRT1. This displaces BRD4 from autophagy gene promoters, leading to their transcriptional activation (Sakamaki et al., 2017). Therefore, in response to nutrient stress, AMPK-mediated autophagy activation involves not only acute phosphorylation and activation of autophagy machinery but also chronic transcriptional regulation of autophagy genes. In addition to the nutrient stress, DNA double-strand breaks also promote the nuclear translocation and activation of AMPK. For example, DNA damage caused by the treatment of etoposide was shown to promote the nuclear translocation of AMPK-α1, which requires CaMKK2 but not LKB1 (Vara-Ciruelos et al., 2018).
Recent studies indicate that AMPK can also rewire the metabolic network by controlling epigenetic regulation of gene transcription in the nucleus. For example, AMPK phosphorylates histone H2B to regulate gene transcription in response to metabolic stress (Bungard et al., 2010). AMPK was also found to phosphorylate EZH2, a histone methyltransferase in the polycomb repressive complex 2 (PRC2), leading to disruption of interactions between EZH2 and other PRC2 components and attenuating PRC2-mediated methylation of histone H3 at Lys27 (Wan et al., 2018). Another recent study revealed that, in response to glucose starvation, AMPK phosphorylates and stabilizes TET2, an epigenetic enzyme that catalyzes the conversion of DNA 5-methylcytosine to 5-hydroxymethylcytosine, and that AMPK-mediated TET2 phosphorylation promotes TET2’s tumour suppressive function (Wu et al., 2018). Collectively, these studies suggest a model that aside from its direct action on metabolic enzymes in the cytoplasm, AMPK can also translocate into the nucleus in response to signaling cues and regulate gene transcription through phosphorylating diverse transcriptional regulators (Figure 4).
Figure 4. Nuclear functions of AMPK.
Cellular insults such as DNA damage or nutrient promote AMPK translocation into the nucleus, where AMPK phosphorylates various downstream transcriptional regulators to control gene transcription as indicated. It remains unclear whether nuclear-localized LKB1 mediates AMPK phosphorylation and activation in the nucleus.
AMPK function at cell junctions
Although cell junctions, such as tight junction or adherens junction, is clearly different from those classic intracellular organelles we have discussed in previous sections, broadly it can be considered a special subcellular location; therefore, in this section, we will briefly discuss AMPK signaling at cell junctions. LKB1 was initially shown to be able to promote polarization of mammalian intestinal epithelial cells (Baas et al., 2004), which is consistent with the notion that mammalian LKB1 is an orthologue of Par-4 polarity gene in C. elegans. This also raised the question of whether AMPK also plays a role in regulating cell polarity and cell junction assembly. Following studies showed that in polarized epithelial cells, E-cadherin promotes LKB1 localization to adherens junction, leading to subsequent AMPK activation (Sebbagh et al., 2009), although the exact role of AMPK in adherens junction remains less clear. Other studies showed that calcium-induced tight junction assembly and cell polarization in epithelial cells promote AMPK phosphorylation in a LKB1-dependent manner; calcium switch-induced tight junction formation is inhibited upon AMPK inactivation whereas AMPK activation by energy stress promotes tight junction assembly in epithelial cells (Zhang et al., 2006; Zheng and Cantley, 2007). Afadin protein, a component of the nectin-afadin complex involved in tight junction regulation, was later identified as an AMPK substrate, indicating that afadin is a potential downstream effector of AMPK in regulating tight junction assembly (Zhang et al., 2011). AMPK can also phosphorylate cingulin, a structural protein localized at the tight junction. Mechanistically, AMPK-mediated cingulin phosphorylation was shown to regulate cingulin binding to microtubules, and thereby facilitates the association of tight junctions with microtubules (Yano et al., 2013; Yano et al., 2018). Together, current studies indicate that cellular junctions are also important subcellular locations for regulating AMPK activity and function.
Conclusion and perspectives
Investigations over the past two decades have put AMPK at the center of metabolic regulatory networks at both cellular and organismal levels. To achieve metabolic homeostasis during altered physiological conditions, AMPK acts not only on its direct metabolic targets but is also involved in cross-talks with other signaling pathways including PI3K-AKT, MAPK/ERK, and mTOR signalling (Hardie, 2014; Mihaylova and Shaw, 2011; Zhao et al., 2017). Owing to the central role of AMPK in cellular physiology, mammalian cells have evolved intricate regulatory mechanisms to govern AMPK activation, thereby enabling cells to appropriately sense and respond to diverse metabolic conditions. In addition to its canonical role in sensing energy status and regulating energy homeostasis, AMPK can also sense other signaling molecules such as calcium and mediate corresponding signaling events. It has long remained elusive how AMPK can sense different upstream stimuli and relay diverse signals to downstream pathways without miscommunications among different upstream stimuli and downstream pathways. Emerging evidence indicates that localization of AMPK to different subcellular compartments provides another dimension to regulate AMPK signaling. For example, calcium stimulation tethers CaMKK2 and AMPK on ER through the formation of the STIM2-CaMKK2-AMPK complex, resulting in AMPK phosphorylation and activation by CaMKK2 but not by LKB1. Considering the central role of ER in mediating calcium signaling, it makes perfect sense that this organelle has been selected to mediate AMPK activation by calcium stimulation. In contrast, current studies indicate that different compartmentalized pools of AMPK are involved in mediating cellular responses to glucose starvation and energy stress, depending on the severity of cellular stress: (i) glucose starvation, before resulting in any obvious increase in AMP levels, promotes v-ATPase-Ragulator-AXIN-LKB1 complex formation on lysosomes and AMPK activation through FBP-unoccupied aldolase, which allows AMPK to initiate downstream signaling to adapt to minor metabolic stress; (ii) further energy stress moderately increases AMP levels, leading to cytosolic AMPK activation in an AXIN-independent manner; (iii) severe energy stress dramatically increases AMP levels, resulting in activation of all compartmentalized pools of AMPK, including mitochondrial localized AMPK; (iv) energy stress also promotes AMPK translocation into the nucleus, although currently, it remains unclear whether nuclear translocation of AMPK is also affected by the severity of energy stress. Presumably, this would help cells adapt to long-term energy stress by regulating the transcription of genes involved in metabolic stress adaptation. In summary, current studies suggest that the spatial control of AMPK signalling allows cells to more precisely coordinate with energy and nutritional status and to achieve better metabolic adaption under different stress conditions.
These intriguing studies also raise several outstanding questions. Because AMPK can exist in possibly 12 complexes with the combinations of different subunit isoforms (including two AMPK-α isoforms, two AMPK-β isoforms, and three AMPK-γ isoforms), whether AMPK functions at different intracellular compartments are mediated by different complexes consisting of combinations of specific subunit isoforms remains an exciting question for future investigation. Addressing this question would require more precise analyses of subcellular localization of all AMPK subunit isoforms. Most current studies have focused on the spatial control of AMPK signaling in response to energy stress (given that AMPK’s major function is to mediate energy stress response). Whether and how AMPK activation by other stress conditions (such as hypoxia (Emerling et al., 2009; Liu et al., 2006) can also be subjected to spatial regulation remains unclear and will be an interesting topic for future studies. In addition, how AMPK dynamically translocate among different subcellular compartments as well as cytoplasm in response to specific stimuli remains to be further studied. Likewise, although multiple AMPK substrates have been identified, not all substrates can be phosphorylated by AMPK under any given condition. The mechanisms underlying AMPK substrate preference still remain poorly understood. It is possible that AMPK phosphorylation of certain substrates requires specific bridging/accessory proteins which only come into play under specific metabolic stress conditions. In addition, it is possible that AMPK-mediated phosphorylation of additional substrates would only occur in specific subcellular compartments (for example, AMPK phosphorylation of H2B or TET2 would only occur under conditions in which AMPK localizes in the nucleus). Further studies are needed to understand this fascinating question. Finally, we envision that additional studies will identify additional AMPK substrates or downstream effectors that mediate AMPK function in regulating organelle dynamics. Altered AMPK signalling has been associated with various diseases including cancer and diabetes, making AMPK an attractive therapeutic target in treating these diseases (Cheng et al., 2016; Coughlan et al., 2014; Goodman et al., 2014; Hardie, 2013; Shackelford and Shaw, 2009; Steinberg and Kemp, 2009). A deeper understanding of the spatial control of AMPK signalling at diverse subcellular compartments may therefore reveal new therapeutic opportunities to target AMPK signaling for the treatment of relevant diseases.
Acknowledgements
We apologize to the many colleagues whose related work cannot be cited due to space limitations. We thank Christine Wogan of the Division of Radiation Oncology at The University of Texas MD Anderson Cancer Center for manuscript editing.
Declaration of interest
Authors have no conflict of interest to disclose. B. Gan is supported by the Andrew Sabin Family Fellow Award and bridge fund from The University of Texas MD Anderson Cancer Center and National Institutes of Health grant R01CA181196.
References
- Amodio G, Moltedo O, Faraonio R, and Remondelli P. 2018. Targeting the Endoplasmic Reticulum Unfolded Protein Response to Counteract the Oxidative Stress-Induced Endothelial Dysfunction. Oxidative medicine and cellular longevity. 2018:4946289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, and Means AR. 2008. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell metabolism. 7:377–388. [DOI] [PubMed] [Google Scholar]
- Appelqvist H, Waster P, Kagedal K, and Ollinger K. 2013. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 5:214–226. [DOI] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, and Clevers HC. 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 116:457–466. [DOI] [PubMed] [Google Scholar]
- Benham AM 2012. Protein secretion and the endoplasmic reticulum. Cold Spring Harbor perspectives in biology. 4:a012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottanelli F, Kilian N, Ernst AM, Rivera-Molina F, Schroeder LK, Kromann EB, Lessard MD, Erdmann RS, Schepartz A, Baddeley D, Bewersdorf J, Toomre D, and Rothman JE. 2017. A novel physiological role for ARF1 in the formation of bidirectional tubules from the Golgi. Molecular biology of the cell. 28:1676–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, Carling D, Thompson CB, Jones RG, and Berger SL. 2010. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science (New York, N.Y.) 329:1201–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B, and Dunlop EA. 2017. The lysosome: a crucial hub for AMPK and mTORC1 signalling. Biochem J. 474:1453–1466. [DOI] [PubMed] [Google Scholar]
- Chantranupong L, Wolfson RL, and Sabatini DM. 2015. Nutrient-sensing mechanisms across evolution. Cell. 161:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan AS, Liu X, Jing J, Lee H, Yadav RK, Liu J, Zhou Y, and Gan B. 2018. STIM2 interacts with AMPK and regulates calcium-induced AMPK activation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology:fj201801225R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lei C, Wang C, Li N, Srivastava M, Tang M, Zhang H, Choi JM, Jung SY, Qin J, and Chen J. 2019. Global phosphoproteomic analysis reveals ARMC10 as an AMPK substrate that regulates mitochondrial dynamics. Nature communications. 10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Zhang T, Ji H, Tao K, Guo J, and Wei W. 2016. Functional characterization of AMP-activated protein kinase signaling in tumorigenesis. Biochim Biophys Acta. 1866:232–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan KA, Valentine RJ, Ruderman NB, and Saha AK. 2014. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes, metabolic syndrome and obesity : targets and therapy. 7:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M 2003. ER-mediated phagocytosis: a new membrane for new functions. Nature reviews. Immunology. 3:280–291. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, and Zou MH. 2010. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 59:1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman J, and Macara IG. 2008. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Molecular biology of the cell. 19:1614–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducommun S, Deak M, Sumpton D, Ford RJ, Nunez Galindo A, Kussmann M, Viollet B, Steinberg GR, Foretz M, Dayon L, Morrice NA, and Sakamoto K. 2015. Motif affinity and mass spectrometry proteomic approach for the discovery of cellular AMPK targets: identification of mitochondrial fission factor as a new AMPK substrate. Cellular signalling. 27:978–988. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Comb WC, and Sabatini DM. 2015. Nutrient-sensing mechanisms and pathways. Nature. 517:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, and Shaw RJ. 2011. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science (New York, N.Y.) 331:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner LJ, Brun SN, Herzig S, Young NP, Curtis SD, Shackelford DB, Shokhirev MN, Leblanc M, Vera LI, Hutchins A, Ross DS, Shaw RJ, and Svensson RU. 2019. Genetic Analysis Reveals AMPK Is Required to Support Tumor Growth in Murine Kras-Dependent Lung Cancer Models. Cell metabolism. 29:285–302 e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, and Chandel NS. 2009. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 46:1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly C, and Amaravadi RK. 2017. Lysosomal Biology in Cancer. Methods Mol Biol. 1594:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Bertrand L, Pollak M, and Viollet B. 2014. Metformin: from mechanisms of action to therapies. Cell metabolism. 20:953–966. [DOI] [PubMed] [Google Scholar]
- Garcia D, and Shaw RJ. 2017. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell. 66:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Liu Z, Zhu P, and Li J. 2014. AMPK Activators as a Drug for Diabetes, Cancer and Cardiovascular Disease. Pharmaceutical regulatory affairs : open access. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans GJ, Hawley SA, Ross FA, and Hardie DG. 2013. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell metabolism. 18:556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, and Shaw RJ. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 30:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG 2013. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 62:2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG 2014. AMPK-Sensing Energy while Talking to Other Signaling Pathways. Cell metabolism. 20:939–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, and Hawley SA. 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews. Molecular cell biology 13:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Schaffer BE, and Brunet A. 2016. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 26:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, and Hardie DG. 2003. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, and Hardie DG. 2005. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell metabolism. 2:9–19. [DOI] [PubMed] [Google Scholar]
- He L, and Wondisford FE. 2015. Metformin action: concentrations matter. Cell metabolism. 21:159–162. [DOI] [PubMed] [Google Scholar]
- Helenius A, and Aebi M. 2001. Intracellular functions of N-linked glycans. Science (New York, N.Y.) 291:2364–2369. [DOI] [PubMed] [Google Scholar]
- Herzig S, and Shaw RJ. 2018. AMPK: guardian of metabolism and mitochondrial homeostasis. Nature reviews. Molecular cell biology 19:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SP, Leiper FC, Woods A, Carling D, and Carlson M. 2003. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proceedings of the National Academy of Sciences of the United States of America. 100:8839–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, and Witters LA. 2005. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. The Journal of biological chemistry. 280:29060–29066. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, and Guan KL. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 115:577–590. [DOI] [PubMed] [Google Scholar]
- Jackson CL 2018. Activators and Effectors of the Small G Protein Arf1 in Regulation of Golgi Dynamics During the Cell Division Cycle. Frontiers in cell and developmental biology. 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, and Spiegelman BM. 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 104:12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM 2016. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 48:e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, and Wong KK. 2007. LKB1 modulates lung cancer differentiation and metastasis. Nature. 448:807–810. [DOI] [PubMed] [Google Scholar]
- Jin L, Chun J, Pan C, Kumar A, Zhang G, Ha Y, Li D, Alesi GN, Kang Y, Zhou L, Yu WM, Magliocca KR, Khuri FR, Qu CK, Metallo C, Owonikoko TK, and Kang S. 2018. The PLAG1-GDH1 Axis Promotes Anoikis Resistance and Tumor Metastasis through CamKK2-AMPK Signaling in LKB1-Deficient Lung Cancer. Mol Cell. 69:87–99 e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju TC, Chen HM, Lin JT, Chang CP, Chang WC, Kang JJ, Sun CP, Tao MH, Tu PH, Chang C, Dickson DW, and Chern Y. 2011. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington's disease. J Cell Biol. 194:209–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TW, Kyung EJ, Kim HC, Shin YK, Lee SH, Park ES, Hacimuftuoglu A, Abd El-Aty AM, and Jeong JH. 2018. Protectin DX Ameliorates Hepatic Steatosis by Suppression of Endoplasmic Reticulum Stress via AMPK-Induced ORP150 Expression. The Journal of pharmacology and experimental therapeutics. 365:485–493. [DOI] [PubMed] [Google Scholar]
- Kalia R, Wang RY, Yusuf A, Thomas PV, Agard DA, Shaw JM, and Frost A. 2018. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature. 558:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazgan N, Williams T, Forsberg LJ, and Brenman JE. 2010. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Molecular biology of the cell. 21:3433–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A 1999. Roles of Axin in the Wnt signalling pathway. Cellular signalling. 11:777–788. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, and Guan KL. 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature cell biology. 13:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiha M, Rassi JG, Brown CM, and Stochaj U. 2007. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK-->ERK1/2 pathway. Am J Physiol Cell Physiol. 293:C1427–1436. [DOI] [PubMed] [Google Scholar]
- Kurz T, Eaton JW, and Brunk UT. 2011. The role of lysosomes in iron metabolism and recycling. Int J Biochem Cell Biol. 43:1686–1697. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, and Evans RM. 2009. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science (New York, N.Y.) 326:437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RE, and Zoncu R. 2019. The lysosome as a cellular centre for signalling, metabolism and quality control. Nature cell biology. 21:133–142. [DOI] [PubMed] [Google Scholar]
- Lee H, and Yoon Y. 2016. Mitochondrial fission and fusion. Biochemical Society transactions. 44:1725–1735. [DOI] [PubMed] [Google Scholar]
- Li M, Zhang CS, Zong Y, Feng JW, Ma T, Hu M, Lin Z, Li X, Xie C, Wu Y, Jiang D, Li Y, Zhang C, Tian X, Wang W, Yang Y, Chen J, Cui J, Wu YQ, Chen X, Liu QF, Wu J, Lin SY, Ye Z, Liu Y, Piao HL, Yu L, Zhou Z, Xie XS, Hardie DG, and Lin SC. 2019a. Transient Receptor Potential V Channels Are Essential for Glucose Sensing by Aldolase and AMPK. Cell metabolism. 30:508–524 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang Y, Wu S, Zhou Z, Ding X, Shi R, Thorne RF, Zhang XD, Hu W, and Wu M. 2019b. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell metabolism. 30:157–173 e157. [DOI] [PubMed] [Google Scholar]
- Li X, Wang L, Zhou XE, Ke J, de Waal PW, Gu X, Tan MH, Wang D, Wu D, Xu HE, and Melcher K. 2015. Structural basis of AMPK regulation by adenine nucleotides and glycogen. Cell Res. 25:50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, and Zang M. 2011. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell metabolism. 13:376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CY, and Zoncu R. 2016. The lysosome as a command-and-control center for cellular metabolism. J Cell Biol. 214:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, and Hardie DG. 2018. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell metabolism. 27:299–313. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Zhang L, Yao J, Yao S, and Yuan T. 2018. AMPK alleviates endoplasmic reticulum stress by inducing the ER-chaperone ORP150 via FOXO1 to protect human bronchial cells from apoptosis. Biochemical and biophysical research communications. 497:564–570. [DOI] [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, and Simon MC. 2006. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 21:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xiao ZD, Han L, Zhang J, Lee SW, Wang W, Lee H, Zhuang L, Chen J, Lin HK, Wang J, Liang H, and Gan B. 2016. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nature cell biology. 18:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansueto G, Armani A, Viscomi C, D'Orsi L, De Cegli R, Polishchuk EV, Lamperti C, Di Meo I, Romanello V, Marchet S, Saha PK, Zong H, Blaauw B, Solagna F, Tezze C, Grumati P, Bonaldo P, Pessin JE, Zeviani M, Sandri M, and Ballabio A. 2017. Transcription Factor EB Controls Metabolic Flexibility during Exercise. Cell metabolism. 25:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Li N, Guo Y, Xu X, Gao L, Xu Y, Zhou L, and Liu W. 2013. AMPK phosphorylates GBF1 for mitotic Golgi disassembly. Journal of cell science. 126:1498–1505. [DOI] [PubMed] [Google Scholar]
- Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S, and Shyy JY. 2017. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Science signaling. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Chen Y, Gucek M, and Puertollano R. 2012. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 8:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De Smedt H, and Missiaen L. 2011. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harbor perspectives in biology. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, and Shaw RJ. 2011. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature cell biology. 13:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Oshiro N, Yoshino K, Nakashima A, Eguchi S, Takahashi M, Ono Y, Kikkawa U, and Yonezawa K. 2008. AMP-activated protein kinase phosphorylates Golgi-specific brefeldin A resistance factor 1 at Thr1337 to induce disassembly of Golgi apparatus. The Journal of biological chemistry. 283:4430–4438. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Rho E, and Inoue T. 2015a. Deconvoluting AMPK dynamics. Oncotarget. 6:30431–30432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Rho E, Sample V, Akano H, Magari M, Ueno T, Gorshkov K, Chen M, Tokumitsu H, Zhang J, and Inoue T. 2015b. Compartmentalized AMPK signaling illuminated by genetically encoded molecular sensors and actuators. Cell reports. 11:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, and Kemp BE. 2011. AMPK is a direct adenylate charge-regulated protein kinase. Science (New York, N.Y.) 332:1433–1435. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Asgar NF, Yokota S, Saito K, and Minokoshi Y. 2019. Role of the alpha2 subunit of AMP-activated protein kinase and its nuclear localization in mitochondria and energy metabolism-related gene expressions in C2C12 cells. Metabolism. 90:52–68. [DOI] [PubMed] [Google Scholar]
- Ozcan L, and Tabas I. 2012. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annual review of medicine. 63:317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM, d'Azzo A, Davidson BL, Neufeld EF, and Tifft CJ. 2018. Lysosomal storage diseases. Nat Rev Dis Primers. 4:27. [DOI] [PubMed] [Google Scholar]
- Qi J, Gong J, Zhao T, Zhao J, Lam P, Ye J, Li JZ, Wu J, Zhou HM, and Li P. 2008. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. The EMBO journal. 27:1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, Andrzejewski S, Raissi TC, Pause A, St-Pierre J, and Jones RG. 2017. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell reports. 21:1–9. [DOI] [PubMed] [Google Scholar]
- Ross FA, Jensen TE, and Hardie DG. 2016a. Differential regulation by AMP and ADP of AMPK complexes containing different gamma subunit isoforms. Biochem J. 473:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross FA, MacKintosh C, and Hardie DG. 2016b. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 283:2987–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM 2017. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proceedings of the National Academy of Sciences of the United States of America. 114:11818–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamaki JI, Wilkinson S, Hahn M, Tasdemir N, O'Prey J, Clark W, Hedley A, Nixon C, Long JS, New M, Van Acker T, Tooze SA, Lowe SW, Dikic I, and Ryan KM. 2017. Bromodomain Protein BRD4 Is a Transcriptional Repressor of Autophagy and Lysosomal Function. Mol Cell. 66:517–532 e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, and Hardie DG. 1998. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 334 ( Pt 1):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, and Sabatini DM. 2010. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 141:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, and Ballabio A. 2009. A gene network regulating lysosomal biogenesis and function. Science (New York, N.Y.) 325:473–477. [DOI] [PubMed] [Google Scholar]
- Saxton RA, and Sabatini DM. 2017. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 169:361–371. [DOI] [PubMed] [Google Scholar]
- Schwarz DS, and Blower MD. 2016. The endoplasmic reticulum: structure, function and response to cellular signaling. Cellular and molecular life sciences : CMLS. 73:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbagh M, Santoni MJ, Hall B, Borg JP, and Schwartz MA. 2009. Regulation of LKB1/STRAD localization and function by E-cadherin. Curr Biol. 19:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, and Ballabio A. 2011. TFEB links autophagy to lysosomal biogenesis. Science (New York, N.Y.) 332:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, and Ballabio A. 2013. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nature reviews. Molecular cell biology 14:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, and Ballabio A. 2012. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. The EMBO journal. 31:1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, and Shaw RJ. 2009. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 9:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, and Cantley LC. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America. 101:3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Calzolari D, Hellberg K, Hu YS, He L, Hung CM, Toyama EQ, Ross DS, Lillemeier BF, Cantley LC, Yates JR 3rd, and Shaw RJ. 2019. Quantitative In Vivo Proteomics of Metformin Response in Liver Reveals AMPK-Dependent and -Independent Signaling Networks. Cell reports. 29:3331–3348 e3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, and Kemp BE. 2009. AMPK in Health and Disease. Physiological reviews. 89:1025–1078. [DOI] [PubMed] [Google Scholar]
- Sundararaman A, Amirtham U, and Rangarajan A. 2016. Calcium-Oxidant Signaling Network Regulates AMP-activated Protein Kinase (AMPK) Activation upon Matrix Deprivation. The Journal of biological chemistry. 291:14410–14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, and Minokoshi Y. 2007. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 27:4317–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, and Cantrell DA. 2006. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 203:1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojkander S, Ciuba K, and Lappalainen P. 2018. CaMKK2 Regulates Mechanosensitive Assembly of Contractile Actin Stress Fibers. Cell reports. 24:11–19. [DOI] [PubMed] [Google Scholar]
- Toyama EQ, Herzig S, Courchet J, Lewis TL Jr., Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, and Shaw RJ. 2016. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science (New York, N.Y.) 351:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, and Thompson CB. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, N.Y.) 324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara-Ciruelos D, Dandapani M, Gray A, Egbani EO, Evans AM, and Hardie DG. 2018. Genotoxic Damage Activates the AMPK-alpha1 Isoform in the Nucleus via Ca(2+)/CaMKK2 Signaling to Enhance Tumor Cell Survival. Mol Cancer Res. 16:345–357. [DOI] [PubMed] [Google Scholar]
- Walter P, and Ron D. 2011. The unfolded protein response: from stress pathway to homeostatic regulation. Science (New York, N.Y.) 334:1081–1086. [DOI] [PubMed] [Google Scholar]
- Wan L, Xu K, Wei Y, Zhang J, Han T, Fry C, Zhang Z, Wang YV, Huang L, Yuan M, Xia W, Chang WC, Huang WC, Liu CL, Chang YC, Liu J, Wu Y, Jin VX, Dai X, Guo J, Liu J, Jiang S, Li J, Asara JM, Brown M, Hung MC, and Wei W. 2018. Phosphorylation of EZH2 by AMPK Suppresses PRC2 Methyltransferase Activity and Oncogenic Function. Mol Cell. 69:279–291 e275. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Westermann B 2010. Mitochondrial fusion and fission in cell life and death. Nature reviews. Molecular cell biology 11:872–884. [DOI] [PubMed] [Google Scholar]
- Wikstrom JD, Israeli T, Bachar-Wikstrom E, Swisa A, Ariav Y, Waiss M, Kaganovich D, Dor Y, Cerasi E, and Leibowitz G. 2013. AMPK regulates ER morphology and function in stressed pancreatic beta-cells via phosphorylation of DRP1. Molecular endocrinology (Baltimore, Md.) 27:1706–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, and Carling D. 2005. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell metabolism. 2:21–33. [DOI] [PubMed] [Google Scholar]
- Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, Rabidou K, Fang R, Tan L, Xu S, Liu H, Argueta C, Zhang L, Mao F, Yan G, Chen J, Dong Z, Lv R, Xu Y, Wang M, Ye Y, Zhang S, Duquette D, Geng S, Yin C, Lian CG, Murphy GF, Adler GK, Garg R, Lynch L, Yang P, Li Y, Lan F, Fan J, Shi Y, and Shi YG. 2018. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 559:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, and Gamblin SJ. 2011. Structure of mammalian AMPK and its regulation by ADP. Nature. 472:230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Sha H, Davisson RL, and Qi L. 2013. Phenformin activates the unfolded protein response in an AMP-activated protein kinase (AMPK)-dependent manner. The Journal of biological chemistry. 288:13631–13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Matsui T, Tamura A, Uji M, and Tsukita S. 2013. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol. 203:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Torisawa T, Oiwa K, and Tsukita S. 2018. AMPK-dependent phosphorylation of cingulin reversibly regulates its binding to actin filaments and microtubules. Sci Rep. 8:15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, and van der Bliek AM. 2012. Mitochondrial fission, fusion, and stress. Science (New York, N.Y.) 337:1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NP, Kamireddy A, Van Nostrand JL, Eichner LJ, Shokhirev MN, Dayn Y, and Shaw RJ. 2016. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes & development. 30:535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, and Costantini F. 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 90:181–192. [DOI] [PubMed] [Google Scholar]
- Zhang CS, Hawley SA, Zong Y, Li M, Wang Z, Gray A, Ma T, Cui J, Feng JW, Zhu M, Wu YQ, Li TY, Ye Z, Lin SY, Yin H, Piao HL, Hardie DG, and Lin SC. 2017. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CS, Jiang B, Li M, Zhu M, Peng Y, Zhang YL, Wu YQ, Li TY, Liang Y, Lu Z, Lian G, Liu Q, Guo H, Yin Z, Ye Z, Han J, Wu JW, Yin H, Lin SY, and Lin SC. 2014. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell metabolism. 20:526–540. [DOI] [PubMed] [Google Scholar]
- Zhang CS, Li M, Ma T, Zong Y, Cui J, Feng JW, Wu YQ, Lin SY, and Lin SC. 2016. Metformin Activates AMPK through the Lysosomal Pathway. Cell metabolism. 24:521–522. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jouret F, Rinehart J, Sfakianos J, Mellman I, Lifton RP, Young LH, and Caplan MJ. 2011. AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3beta (GSK-3beta) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. The Journal of biological chemistry. 286:16879–16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li J, Young LH, and Caplan MJ. 2006. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proceedings of the National Academy of Sciences of the United States of America. 103:17272–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YL, Guo H, Zhang CS, Lin SY, Yin Z, Peng Y, Luo H, Shi Y, Lian G, Zhang C, Li M, Ye Z, Ye J, Han J, Li P, Wu JW, and Lin SC. 2013. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell metabolism. 18:546–555. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li T, Wang K, Zhao F, Chen J, Xu G, Zhao J, Li T, Chen L, Li L, Xia Q, Zhou T, Li HY, Li AL, Finkel T, Zhang XM, and Pan X. 2019. AMPK-mediated activation of MCU stimulates mitochondrial Ca(2+) entry to promote mitotic progression. Nature cell biology. 21:476–486. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, Zhang S, Huang Q, and Shi M. 2017. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Molecular cancer. 16:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, and Cantley LC. 2007. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 104:819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, and Sabatini DM. 2011. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science (New York, N.Y.) 334:678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Zhang CS, Li M, Wang W, Wang Z, Hawley SA, Ma T, Feng JW, Tian X, Qi Q, Wu YQ, Zhang C, Ye Z, Lin SY, Piao HL, Hardie DG, and Lin SC. 2019. Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Res. 29:460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zungu M, Schisler JC, Essop MF, McCudden C, Patterson C, and Willis MS. 2011. Regulation of AMPK by the ubiquitin proteasome system. Am J Pathol. 178:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]