Abstract
BACKGROUND & AIMS:
Recent increasing trends in early-onset colorectal cancer (CRC) strongly supports that early-life diet is involved in CRC development. However, data are lacking on the relationship with high sugar intake during early-life.
METHODS:
We prospectively investigated the association of adolescent simple sugar (fructose, glucose, added sugar, total sugar) and sugar-sweetened beverage (SSB) intake with CRC precursor risk in 33,106 participants of the Nurses’ Health Study II who provided adolescent dietary information in 1998 and subsequently underwent lower gastrointestinal endoscopy between 1999 and 2015. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression for clustered data.
RESULTS:
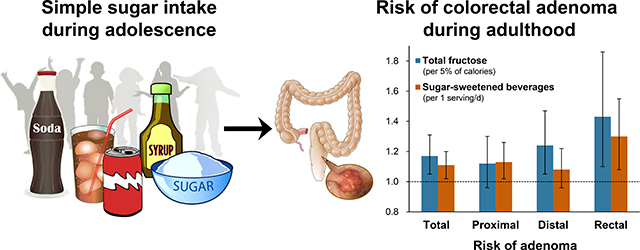
During follow-up, 2,909 conventional adenomas (758 high-risk) and 2,355 serrated lesions were identified (mean age at diagnoses, 52.2±4.3 years). High sugar and SSB intake during adolescence was positively associated with risk of adenoma, but not serrated lesions. Per each increment of 5% of calories from total fructose intake, multivariable ORs were 1.17 (95% CI 1.05–1.31) for total and 1.30 (95% CI 1.06–1.60) for high-risk adenoma. By subsite, ORs were 1.12 (95% CI 0.96–1.30) for proximal, 1.24 (95% CI 1.05–1.47) for distal, and 1.43 (95% CI 1.10–1.86) for rectal adenoma. Per 1 serving/day increment in SSB intake, ORs were 1.11 (95% CI 1.02–1.20) for total and 1.30 (95% CI 1.08–1.55) for rectal adenoma. Contrary to adolescent intake, sugar and SSB intake during adulthood was not associated with adenoma risk.
CONCLUSIONS:
High intake of simple sugars and SSBs during adolescence was associated with increased risk of conventional adenoma, especially rectal adenoma.
Keywords: colorectal adenoma, colorectal polyp, conventional adenoma, cancer epidemiology, fructose
Graphical Abstract
INTRODUCTION
The global burden of colorectal cancer (CRC) is expected to increase to over 2.2 million new cases and 1.1 million cancer deaths per annum by 2030.1 In many high-income countries, the burden of CRC is rapidly shifting to younger individuals.2–4 In the U.S., despite declines in older adults, incidence is increasing in young and middle-aged adults with 22% of CRC cases occurring in those under 55 years in 2013–2017.4,5 CRC incidence has been on the rise among young adults aged 20–39 years since the mid-1980s, among those aged <50 years since the mid-1990s, and among those aged 50–64 years since 2011.4,6 This birth cohort effect (elevated risk in generations born after 1950) strongly indicates that population-level changes in early-life exposures, such as diet and lifestyle factors, may explain the upward trend in early-onset CRC.2,4
Simple sugar, especially added fructose, intake has steeply increased in recent decades largely due to the marked increase in sugar-sweetened beverage (SSB) intake.7,8 SSBs (carbonated and noncarbonated soft drinks, fruit drinks, and sports drinks) are mostly sweetened with high-fructose corn syrup (usually 55% fructose and 45% glucose) or sucrose (half fructose and half glucose).7 In the U.S., SSB availability has risen dramatically since the 1950s.9 Between 1965 and 1996, SSB intake among U.S. adolescents more than doubled (per capita g/day: boys 364 to 1046, girls 303 to 678).10 Compared with other age groups, adolescents had the highest SSB intake with about 10% of daily calories from SSBs in 2011–2014.11 SSB consumption is also rapidly increasing worldwide, particularly in developing countries.12 In 53 low- and middle-income countries, 54% of adolescents consumed carbonated soft drinks at least once per day in 2009–2013.13
High sugar intake can promote colorectal carcinogenesis by causing insulin resistance, obesity, and type 2 diabetes14—established risk factors for CRC.15 Despite the close link between insulin resistance and CRC,16,17 most prospective studies have reported null associations between adult sugar intake and colorectal neoplasia.15,18,19 However, data are lacking on the association of high sugar intake during early-life. Considering the long process of carcinogenesis generally spanning several decades and recent upward trends in early-onset CRC,2,3 early-life diet may be etiologically relevant to CRC development.20 Moreover, adolescence is a unique growth period characterized by physiologically decreased insulin sensitivity and a surge in insulin-like growth factor 1 (IGF1).21 Thus, adolescence may be a critical period of enhanced susceptibility to the adverse effects of high sugar consumption.
Our hypothesis was that high sugar intake during adolescence may play a role in development of CRC precursors, which are the early steps of colorectal carcinogenesis and primary targets of screening colonoscopy for early intervention.3,22 We prospectively investigated the association of adolescent simple sugar and SSB intake with risk of colorectal polyps in a large cohort of young women.
METHODS
Study Population
The Nurses’ Health Study II (NHSII) is an ongoing prospective cohort established in 1989 when 116,430 U.S. female registered nurses aged 25–42 years returned a mailed questionnaire about various lifestyle factors and medical history.23 Follow-up questionnaires were mailed biennially to update the information and newly diagnosed diseases. We included women who had completed a high school Food Frequency Questionnaire (HS-FFQ)24 about adolescent diet in 1998 and subsequently underwent at least one lower gastrointestinal endoscopy between 1999 and 2015. We excluded women who had no lower bowel endoscopy during the follow-up because colorectal polyps are generally asymptomatic and detected during an endoscopy. We also excluded women with a history of any cancer (other than nonmelanoma skin cancer), colorectal polyps, Crohn’s disease, or ulcerative colitis prior to the return of the HS-FFQ, and those reporting implausible adolescent caloric intake (<600 or >5000 kcal/day) or extensive missing responses (>70 for food items or ≥2 sections entirely blank other than dairy and eggs/meat sections), leaving a total of 33,106 women for the current analyses. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Dietary Assessment
Adolescent diet was assessed using a 124-item self-administered HS-FFQ, specifically designed to include food items commonly consumed between 1960 and 1982 when participants were 13–18 years.24 Participants were asked how often, on average, they had consumed a standard portion size of each food or beverage when they were in high school, with 9 possible responses ranging from “never or less than once per month” to “6 or more times per day.” The reproducibility and validity of the HS-FFQ have been previously described in detail.24,25 In brief, reproducibility at a 4-year interval was moderate-to-good (correlation for overall nutrients, r = 0.65; foods, 0.60; total fructose, 0.65; cola, 0.74; orange juice, 0.74).24 In a validation study comparing dietary measures collected in 80 adolescents (aged 13–18 years) with the HS-FFQ completed 10 years later in the same youths, adequate validity was reported (r for overall nutrients, 0.58; total fructose, 0.44).25 Since 1991, adult diet was assessed every 4 years using a validated FFQ with approximately 131-food items.26
Total fructose intake was defined as the sum of free fructose and half of sucrose intake because sucrose consists of half fructose and half glucose.7 Likewise, glucose intake from simple sugars was defined as the sum of free glucose and half of sucrose intake. Added sugar referred to sugar added to foods and beverages during processing or preparation.27 Total sugar represented the sum of free fructose, free glucose, sucrose, and maltose. SSBs were defined as caffeinated and caffeine-free colas (e.g., Coke, Pepsi) and other carbonated (e.g., 7-Up) and non-carbonated sugary beverages (fruit punches, lemonades, or other fruit drinks). Artificially sweetened beverages (ASBs) included carbonated and non-carbonated low-calorie or diet beverages. Standard serving sizes for SSBs and ASBs were 1 glass, a bottle, or a can (12 ounces). Fruit juice included orange, apple, grapefruit, and other fruit juices, with 1 small glass (6 ounces) as a serving size. Dairy products included milk, yogurt, cheese, ice cream, sherbet, milkshake, and frappe.
The nutrient database corresponding to each questionnaire cycle was primarily derived from U.S. Department of Agriculture sources and supplemented with information from manufacturers.24,28 Nutrient intake was adjusted for total energy intake using the residual method.29,30 For sugar intake, we also calculated nutrient density (percentage of daily calories contributed by each sugar) because most current dietary recommendations for added sugar intake are based on percentage of total calorie intake (e.g., the 2015–2020 and 2020–2025 Dietary Guidelines for Americans [DGA] have recommended limiting added sugar intake to <10% of total calories per day).27,31 We considered changes in diet over time during follow-up, including simple sugar and beverage intake. To better represent long-term diet and reduce measurement error due to within-person variability,29 cumulative updated intake was calculated for adult diet by averaging the repeated measures from all available FFQs up to 2 years prior to the most recent endoscopy. As an indicator of overall diet during adolescence, we derived prudent and western dietary patterns using principal component analyses as reported previously (Supplementary Table 1).32 A western dietary pattern was characterized by high intake of desserts, sweets, snacks, red and processed meat, and refined grains; whereas a prudent pattern was characterized by high intake of vegetables, fruits, better-quality grains, fish, and poultry. For analyses of SSBs, ASBs, and fruit juice, dietary patterns were derived after excluding each beverage variable to avoid collinearity with the primary exposure.
Outcome Ascertainment
On each biennial questionnaire, participants were asked whether they underwent a lower bowel endoscopy and the reasons why (screening, family history of CRC, symptoms), and whether CRC or polyps were diagnosed. Self-reported negative colonoscopy was reliable in our cohorts.33,34 In random samples of participants reporting negative colonoscopy (n = 114 in the NHS, 140 in the Health Professionals Follow-Up Study [HPFS]), concordance rate was high between self-reported negative endoscopy and endoscopic record review (97% in the NHS, 100% in the HPFS). Participants who reported a diagnosis of polyps were asked for permission to access medical and pathological records. Physicians blinded to participant exposure information reviewed the records to verify the diagnosis and accrue information on polyp size, number, subtype (adenoma, serrated lesion), subsite (proximal, distal, rectal), and histology (tubular, tubulovillous, villous; with or without high-grade dysplasia). We subdivided adenoma into high risk (≥1 cm, any villous histology, high-grade dysplasia, or ≥3 adenomas) and low risk (1–2 tubular adenomas <1 cm in size).35 Serrated lesions included hyperplastic polyps, sessile serrated adenoma/polyp, and traditional serrated adenoma,36 and were subdivided by size (small, <1 cm; large, ≥1 cm) as a predictor for the malignant potential.33
Assessment of covariates
From the HS-FFQ and biennial questionnaires during follow-up, we collected and updated information on BMI at age 18 years, adult height, current weight, smoking (adolescent, current), alcohol consumption (age 18–22 years, current), family history of CRC in first degree relatives, history of type 2 diabetes, menopausal status and menopausal hormone use, and current aspirin use. Information on physical activity during adolescence was obtained in 1997 as described in detail previously.37,38 In brief, participants reported average time spent per week on walking and a variety of recreational activities during early-life. Each activity was converted to metabolic equivalent of task (MET)-hr/week and then summed up to obtain total physical activity.39 Adolescent physical activity was defined as total physical activity during grades 9–12. Physical activity during adulthood was assessed in 1989, 1991, 1997, 2001, 2005, 2009, and 2013, and cumulative updated averages were calculated.
Statistical Analysis
Sugar intake was categorized into quintiles using either nutrient density or energy-adjusted intake. SSB and other beverage intake was grouped into 4 categories: <1 serving/week, 1–6 servings/week, 1 serving/day, and ≥2 servings/day. Sugar and SSB intake was also treated as continuous variables. Individuals with missing responses for each exposure variable of interest were excluded from analyses (SSBs, n = 666; ASBs, 840; fruit juice, 56). We generated a new dataset for each questionnaire cycle when participants reported an endoscopy. Thus, participants with multiple endoscopies during follow-up could provide multiple records. Once polyp(s) were diagnosed, the participant was censored. Time-varying variables were updated to 2 years prior to most recent endoscopy. To handle individuals with multiple endoscopies and time-varying variables efficiently, the Andersen-Gill data structure was used.40
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression for clustered data (SAS PROC GENMOD) where each participant represented a cluster. We constructed 3 multivariable models with adjustment for various potential confounders during both adolescence and adulthood.15 Model 1 included age, time period of endoscopy, time since most recent endoscopy, number of endoscopies, and reason for endoscopy. Model 2 was additionally adjusted for family history of CRC, menopausal status/menopausal hormone use, current aspirin use ≥2 times/week, history of type 2 diabetes, adult height, body mass index (BMI, at 18 years and current), smoking (adolescent, current), alcohol consumption (18–22 years, current), and physical activity (adolescent, current). In model 3, to assess whether associations were independent of other dietary factors and overall unhealthy dietary pattern, we further adjusted for adolescent and adult intake (total calorie, total calcium, vitamin D, total folate, fiber, fruits, vegetables, and dairy), current total red meat intake, western dietary pattern score during adolescence, and corresponding adult variables to adolescent exposure variables.
Tests for trend were performed by assigning a median value to each category of exposure variables and modeling this value as a continuous variable, using the Wald test to assess statistical significance. Stratified analyses were performed to examine whether associations varied across strata of known CRC risk factors during adolescence (e.g., family history of CRC, BMI, physical activity, fruit and vegetable intake). Tests for interaction were performed by including cross-product terms of exposure and stratification variables in the model and utilizing a Wald test. To examine the effects of dietary changes across different life stages, we examined joint associations of adolescent and adult sugar intake with adenoma risk. According to the 2020–2025 DGA,31 the effects of substituting fruits, fruit juice, or dairy for SSBs were estimated by simultaneously including both SSBs and one of these food items as continuous variables in models; ORs and 95% CIs were calculated from the differences in coefficients and corresponding variances and covariances.41 All tests were two-sided with P < .05 considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of the participants are described in Table 1. During adolescence, 12.6% of women had consumed ≥1 serving/day of SSBs (≥2 servings/day, 4.8%), whereas in adulthood, 11.1% consumed ≥1 serving/day (≥2 servings/day, 3.2%). Adolescent SSB intake contributed to 2.6% of daily calories, on average. When stratified by year of birth, younger birth cohorts tended to have higher fructose and SSB intake during adolescence compared with older birth cohorts, largely consistent with the U.S. national data.10 Participants with higher adolescent fructose intake tended to consume less red meat and more fruits and vegetables, but those with higher SSB intake tended to consume more red meat and less fruits and vegetables. The correlation between total fructose and SSB intake during adolescence was low-to-modest (Spearman correlation, r = 0.38; Supplementary Table 2). Adolescent diet was only weakly correlated with adult diet (total fructose, r = 0.26; SSBs, r = 0.25).
Table 1.
Baseline characteristics of participants by total fructose and sugar-sweetened beverage intake during adolescence in the Nurses’ Health Study IIa
| Intake during adolescence |
||||||
|---|---|---|---|---|---|---|
| Total fructose (% of calorie) |
SSB (serving) |
|||||
| Q1 (<7.9) | Q3 (9.2–10.3) | Q5 (≥11.8) | <1/wk | 1–6/wk | ≥1/d | |
| (n = 6,598) | (n = 6,682) | (n = 6,639) | (n = 13,732) | (n = 14,545) | (n = 4,163) | |
| Age at 1998 questionnaire return (y) | 44.6 (4.4) | 44.2 (4.6) | 43.7 (4.5) | 44.2 (4.5) | 44.3 (4.5) | 43.7 (4.5) |
| Birth year, % | ||||||
| 1946–1949 | 21.8 | 21.1 | 17.3 | 42.0 | 47.0 | 11.0 |
| 1950–1954 | 21.7 | 20.2 | 18.4 | 42.6 | 45.4 | 12.0 |
| 1955–1959 | 18.9 | 19.7 | 21.2 | 42.1 | 44.5 | 13.4 |
| 1960–1965 | 16.9 | 20.4 | 23.2 | 42.5 | 42.5 | 15.0 |
| Number of endoscopies during the study period (n) | 1.2 (0.5) | 1.2 (0.5) | 1.2 (0.5) | 1.2 (0.5) | 1.2 (0.5) | 1.2 (0.5) |
| Time since most recent endoscopy (y) | 1.4 (3.8) | 1.4 (3.8) | 1.4 (3.8) | 1.4 (3.8) | 1.4 (3.8) | 1.5 (4.0) |
| Endoscopy for clinical symptoms or signsb, % | 23.4 | 22.5 | 24.3 | 21.6 | 23.9 | 25.7 |
| Adult height (inches) | 64.8 (2.6) | 64.9 (2.6) | 64.9 (2.6) | 65.0 (2.6) | 64.9 (2.6) | 64.8 (2.6) |
| BMI at age 18 y (kg/m2) | 21.4 (3.3) | 21.1 (3.0) | 21.0 (3.2) | 21.4 (3.2) | 20.9 (3.0) | 21.0 (3.4) |
| Current BMI (kg/m2) | 26.4 (6.0) | 26.0 (5.8) | 26.3 (6.1) | 26.1 (6) | 26.0 (5.8) | 26.9 (6.3) |
| Smoking before 20 y of age, % | 21.9 | 21.9 | 24.0 | 19.4 | 23.2 | 30.3 |
| Current smoker, % | 6.5 | 5.6 | 6.3 | 4.7 | 5.8 | 9.2 |
| Physical activity at grades 9–12 (MET-h/wk) | 49.1 (36.1) | 51.1 (35.1) | 54.5 (37.6) | 50.0 (35.6) | 52.9 (36.5) | 54.7 (37.9) |
| Current physical activity (MET-h/wk) | 17.5 (21.1) | 19.0 (22.6) | 20.5 (26.0) | 20.4 (24.3) | 18.4 (22.0) | 17.4 (24.4) |
| Premenopause, % | 47.0 | 48.7 | 47.5 | 48.6 | 48.5 | 44.4 |
| Family history of CRC, % | 22.4 | 22.8 | 22.2 | 22.6 | 22.0 | 23.9 |
| Diabetes, % | 4.8 | 4.3 | 4.6 | 4.1 | 3.9 | 5.8 |
| Current aspirin use (≥2 d/wk), % | 12.6 | 11.3 | 13.5 | 11.8 | 12.7 | 13.9 |
| Food and nutrient intake during adolescencec | ||||||
| Unprocessed red meat (g/d) | 120 (60) | 109 (48) | 94 (46) | 102 (52) | 110 (50) | 119 (51) |
| Processed red meat (g/d) | 24 (22) | 23 (18) | 20 (17) | 20 (18) | 24 (19) | 26 (21) |
| Fruits (serving/d) | 1.0 (0.6) | 1.6 (0.8) | 2.1 (1.5) | 1.6 (1.1) | 1.5 (1.0) | 1.3 (1.0) |
| Vegetables (serving/d) | 2.7 (1.6) | 3.2 (1.7) | 3.4 (2.1) | 3.3 (1.9) | 3.1 (1.7) | 2.9 (1.7) |
| Total dairy (serving/d) | 3.0 (1.6) | 2.9 (1.4) | 2.3 (1.4) | 2.8 (1.5) | 2.8 (1.5) | 2.7 (1.5) |
| SSBs (serving/wk) | 1.1 (1.5) | 2.4 (2.6) | 7.3 (7.6) | 0.4 (0.4) | 3.2 (1.7) | 12.5 (6.7) |
| ASBs (serving/wk) | 2.8 (5.8) | 2.4 (5.1) | 2.1 (5.1) | 3.4 (6.4) | 1.5 (3.5) | 1.5 (4.5) |
| Fruit juice (serving/wk) | 2.7 (2.8) | 5.1 (3.9) | 6.7 (6.4) | 4.9 (4.7) | 5.1 (4.6) | 4.6 (4.4) |
| Total fructose (% of calorie)d | 6.7 (1.0) | 9.7 (0.3) | 13.6 (1.8) | 9.0 (2.3) | 10.1 (2.1) | 12.6 (2.7) |
| Glucose (from simple sugars, % of calorie)d | 6.7 (1.0) | 9.5 (0.6) | 13.2 (1.9) | 8.7 (2.1) | 9.8 (2.0) | 12.5 (2.7) |
| Added sugar (% of calorie)d | 9.5 (2.3) | 13.6 (2.6) | 19.9 (5.4) | 11.7 (3.6) | 14.6 (3.6) | 20.8 (5.4) |
| Total sugar (% of calorie)d | 18.7 (3.0) | 23.7 (2.0) | 30.3 (3.7) | 22.5 (4.3) | 24.3 (4.0) | 28.7 (5.1) |
| Calcium (mg/d) | 1234 (402) | 1090 (316) | 919 (272) | 1157 (373) | 1065 (320) | 925 (274) |
| Vitamin D (IU/d) | 410 (212) | 347 (178) | 283 (175) | 379 (211) | 341 (174) | 277 (163) |
| Folate (μg/d) | 301 (89) | 328 (96) | 334 (116) | 345 (112) | 316 (90) | 281 (80) |
| Fiber (g/d) | 18.6 (4.6) | 21.1 (4.6) | 22.3 (6.4) | 21.9 (5.9) | 20.4 (4.6) | 18.7 (4.2) |
| Glycemic index | 54.6 (2.9) | 54.7 (2.5) | 55.8 (2.9) | 54.1 (2.7) | 55.1 (2.4) | 57 (2.6) |
| Glycemic load | 148 (22) | 169 (16) | 196 (21) | 165 (25) | 171 (22) | 187 (25) |
| Alcohol (g/d) | 0.3 (2.3) | 0.3 (2.0) | 0.3 (1.8) | 0.2 (1.7) | 0.3 (1.7) | 0.5 (2.5) |
| Current (adult) food and nutrient intakec, e | ||||||
| Unprocessed red meat (g/d) | 58 (35) | 56 (33) | 53 (33) | 51 (33) | 58 (33) | 64 (35) |
| Processed red meat (g/d) | 8 (7) | 8 (7) | 7 (7) | 7 (7) | 8 (7) | 9 (8) |
| Fruits (serving/d) | 1.1 (0.7) | 1.3 (0.8) | 1.4 (1.0) | 1.4 (0.8) | 1.3 (0.8) | 1.1 (0.8) |
| Vegetables (serving/d) | 3.4 (1.8) | 3.7 (1.8) | 3.8 (2.2) | 3.8 (2.0) | 3.6 (1.8) | 3.5 (1.9) |
| Total dairy (serving/d) | 2.1 (1.1) | 2.2 (1.1) | 1.9 (1.1) | 2.1 (1.1) | 2.1 (1.1) | 1.9 (1.1) |
| SSBs (serving/wk) | 2.0 (3.4) | 2.6 (3.9) | 4.1 (6.0) | 1.7 (2.8) | 3.2 (4.2) | 5.4 (7.2) |
| ASBs (serving/wk) | 6.3 (7.9) | 5.9 (7.4) | 6.8 (8.6) | 6.0 (7.7) | 5.6 (7.1) | 8.3 (9.5) |
| Fruit juice (serving/wk) | 3.6 (3.7) | 4.7 (4.2) | 5.3 (5.0) | 4.5 (4.3) | 4.8 (4.3) | 4.3 (4.2) |
| Total fructose (% of calorie)d | 8.4 (2.6) | 9.4 (2.6) | 10.6 (3.2) | 9.1 (2.5) | 9.7 (2.8) | 10.2 (3.7) |
| Glucose (from simple sugars, % of calorie)d | 8.2 (2.5) | 9.2 (2.4) | 10.3 (3.0) | 8.8 (2.4) | 9.4 (2.6) | 10.0 (3.5) |
| Added sugar (% of calorie)d | 9.8 (4.4) | 10.9 (4.5) | 12.6 (6.0) | 9.9 (4.0) | 11.5 (4.9) | 13.3 (6.8) |
| Total sugar (% of calorie)d | 21.4 (5.6) | 23.0 (5.4) | 24.8 (6.4) | 22.6 (5.4) | 23.4 (5.7) | 24.0 (7.2) |
| Calcium (mg/d) | 1239 (421) | 1228 (406) | 1173 (427) | 1274 (418) | 1189 (397) | 1100 (403) |
| Vitamin D (IU/d) | 443 (219) | 432 (212) | 409 (222) | 452 (220) | 418 (206) | 381 (209) |
| Folate (μg/d) | 562 (208) | 573 (204) | 577 (214) | 598 (214) | 562 (200) | 525 (200) |
| Fiber (g/d) | 19.2 (4.7) | 20.0 (4.8) | 20.1 (5.4) | 20.9 (5.2) | 19.4 (4.6) | 18.0 (4.6) |
| Alcohol (g/d) | 4.4 (7.0) | 4.4 (6.5) | 4.0 (6.3) | 4.3 (6.5) | 4.3 (6.4) | 4.5 (7.3) |
CRC, colorectal cancer; SSB, sugar-sweetened beverage; ASB, artificially sweetened beverage; BMI, body mass index; IU, international units; MET, metabolic equivalent of tasks; Q, quintile
Means (SD) are presented for continuous variables; percentages for categorical variables. All variables other than age in 1998 and birth year were standardized to the age distribution of the study population.
Includes bleeding in stool, positive test for occult fecal blood, diarrhea or constipation, and abdominal pain.
Nutrients are energy-adjusted values unless otherwise indicated.
From mono- and disaccharide sugars. Total sugar intake was sum of free fructose, free glucose, sucrose, and maltose intake.
Intake from 1999 food frequency questionnaire.
During follow-up, 4,744 women were diagnosed with at least one colorectal polyp, of whom 2,909 had at least one adenoma (1548 proximal, 1205 distal, 458 rectal, and 758 high-risk adenomas), and 2,355 at least one serrated lesion (196 large serrated lesions). The mean age at diagnoses was 52.2±4.3 years, with the majority of cases diagnosed at relatively young ages (76.5% before 55 years). In adenoma cases, the proportions of rectal adenoma tended to be higher among women born after 1960 (born before 1960, 15.2% vs after 1960, 19.6%).
Sugar and SSB intake and CRC precursor risk
Independent of adult intake, higher intake of total fructose and SSBs during adolescence was significantly associated with increased risk of total adenoma (Tables 2 and 3). For total fructose intake, positive associations were not significant in models 1 and 2. However, additional adjustment for dietary covariables (especially adolescent fruit, fiber, and calcium intake) substantially strengthened the associations in model 3. In fully adjusted models, the ORs of total adenoma were 1.17 (95% CI 1.05–1.31; Ptrend = .006) per each increment of 5% of calories from total fructose intake and 1.11 (95% CI 1.02–1.20; Ptrend = .01) per 1 serving/day of SSB intake. By subsite, higher total fructose intake (per 5% of calories) was associated with increased risk of distal (OR 1.24, 95% CI 1.05–1.47) and rectal (OR 1.43, 95% CI 1.10–1.86) adenoma; higher SSB intake (per 1 serving/day) was associated with increased risk of proximal (OR 1.13, 95% CI 1.02–1.26) and rectal (OR 1.30, 95% CI 1.08–1.55) adenoma. Neither sugar nor SSB intake during adolescence was associated with risk of total and large serrated lesions (all Ptrend ≥ .35).
Table 2.
Odds ratios and 95% confidence intervals of colorectal polyps according to total fructose intake during adolescence in the Nurses’ Health Study II, 1998–2015
| Total fructose intake during adolescence, % of calorie |
Per 5% of calorie increase | ||||||
|---|---|---|---|---|---|---|---|
| Q1 (<7.9) |
Q2 (7.9–<9.2) |
Q3 (9.2–<10.3) |
Q4 (10.3–<11.8) |
Q5 (≥11.8) |
Ptrend | ||
| Total adenoma | |||||||
| Ncases/Ncontrolsa | 573/9976 | 570/9978 | 595/9954 | 591/9958 | 580/9968 | ||
| Model 1b | 1 (ref) | 1.00 (0.89–1.12) | 1.05 (0.93–1.18) | 1.05 (0.93–1.18) | 1.03 (0.92–1.16) | .44 | 1.04 (0.95–1.13) |
| Model 2c | 1 (ref) | 1.00 (0.89–1.13) | 1.06 (0.94–1.20) | 1.07 (0.95–1.20) | 1.06 (0.94–1.20) | .21 | 1.06 (0.97–1.16) |
| Model 3d | 1 (ref) | 1.05 (0.93–1.19) | 1.14 (1.00–1.30) | 1.19 (1.04–1.36) | 1.20 (1.04–1.39) | .006 | 1.17 (1.05–1.31) |
| Proximal adenoma | |||||||
| Ncases/Ncontrols | 316/9795 | 306/9807 | 323/9765 | 310/9776 | 293/9809 | ||
| Model 3 | 1 (ref) | 1.03 (0.87–1.22) | 1.15 (0.96–1.36) | 1.15 (0.96–1.39) | 1.13 (0.92–1.38) | .16 | 1.12 (0.96–1.30) |
| Distal adenoma | |||||||
| Ncases/Ncontrols | 239/9795 | 221/9807 | 241/9765 | 251/9776 | 253/9809 | ||
| Model 3 | 1 (ref) | 0.97 (0.80–1.18) | 1.10 (0.90–1.35) | 1.21 (0.98–1.48) | 1.25 (1.00–1.56) | .014 | 1.24 (1.05–1.47) |
| Rectal adenoma | |||||||
| Ncases/Ncontrols | 77/9795 | 97/9807 | 84/9765 | 101/9776 | 99/9809 | ||
| Model 3 | 1 (ref) | 1.39 (1.01–1.89) | 1.24 (0.90–1.73) | 1.61 (1.15–2.25) | 1.62 (1.14–2.31) | .008 | 1.43 (1.10–1.86) |
| Total serrated lesione | |||||||
| Ncases/Ncontrols | 479/9951 | 442/9993 | 486/9945 | 486/9934 | 462/9983 | ||
| Model 3 | 1 (ref) | 0.93 (0.80–1.07) | 1.03 (0.89–1.20) | 1.06 (0.91–1.23) | 1.01 (0.86–1.19) | .49 | 1.04 (0.92–1.18) |
| Small serrated lesione | |||||||
| Ncases/Ncontrols | 405/9951 | 374/9993 | 420/9945 | 430/9934 | 386/9983 | ||
| Model 3 | 1 (ref) | 0.93 (0.80–1.08) | 1.06 (0.91–1.24) | 1.12 (0.96–1.32) | 1.01 (0.85–1.21) | .41 | 1.06 (0.93–1.21) |
| Large serrated lesione | |||||||
| Ncases/Ncontrols | 46/9951 | 41/9993 | 40/9945 | 29/9934 | 40/9983 | ||
| Model 3 | 1 (ref) | 0.88 (0.57–1.37) | 0.85 (0.54–1.35) | 0.60 (0.36–1.00) | 0.86 (0.54–1.37) | .35 | 0.83 (0.56–1.22) |
| Subtype of polyp | |||||||
| Adenoma only | |||||||
| Ncases/Ncontrols | 472/9598 | 488/9618 | 482/9581 | 469/9594 | 478/9608 | ||
| Model 3 | 1 (ref) | 1.10 (0.96–1.26) | 1.13 (0.98–1.30) | 1.15 (0.99–1.34) | 1.20 (1.03–1.41) | .027 | 1.15 (1.02–1.30) |
| Serrated lesion only | |||||||
| Ncases/Ncontrols | 378/9598 | 360/9618 | 373/9581 | 364/9594 | 360/9608 | ||
| Model 3 | 1 (ref) | 0.96 (0.82–1.12) | 1.00 (0.85–1.17) | 0.99 (0.84–1.17) | 0.99 (0.83–1.18) | .99 | 1.00 (0.87–1.15) |
| Both adenoma and serrated lesion | |||||||
| Ncases/Ncontrols | 101/9598 | 82/9618 | 113/9581 | 122/9594 | 102/9608 | ||
| Model 3 | 1 (ref) | 0.84 (0.62–1.14) | 1.20 (0.89–1.61) | 1.36 (1.01–1.85) | 1.20 (0.85–1.67) | .06 | 1.28 (0.99–1.65) |
N, number of endoscopies; Q, quintile
Due to multiple endoscopies during follow-up per each participant, N is larger than number of participants.
Adjusted for age, time period of endoscopy, number of endoscopies (continuous), time since most recent endoscopy (continuous), and reason for endoscopy (screening/symptoms).
Additionally adjusted for family history of colorectal cancer (yes/no), menopausal status/menopausal hormone use (premenopausal, postmenopausal with never, past, or current hormone therapy), current aspirin use ≥2 times/wk (yes/no), history of type 2 diabetes (yes/no), adult height (continuous), BMI at age 18 y (<18.5, 18.5–<20, 20–<22.5, 22.5–<25, ≥25 kg/m2), current BMI (<22.5, 22.5–<25, 25–<27.5, 27.5–<30, ≥30 kg/m2), smoking status at 19 y (never, 0≤2.5, >2.5 pack-years), current smoking status (never smoker, past smoker <30 pack-years, past smoker ≥30 pack-years, current smoker <30 pack-years, current smoker ≥30 pack-years), alcohol intake at 18–22 y (<0.1, 0.1–4.9, 5–14.9, ≥15 g/d), current alcohol intake (none, 0.1–4.9, 5–9.9, 10–14.9, ≥15 g/d), physical activity during grades 9–12 (quintile), and current physical activity (<21, 21–<30, 30–<39, 39–<54, ≥54 MET hours/wk).
Additionally adjusted for adolescent and current (adult) dietary intake (total calorie, total calcium, vitamin D, total folate, fiber, fruits, vegetables, and dairy; quintile), current total red meat intake (quintile), western dietary pattern score during adolescence (quintile), and current total fructose intake.
Includes hyperplastic polyp, sessile serrated adenoma/polyp, and traditional serrated adenoma; small, <1 cm; large, ≥1 cm.
Table 3.
Odds ratios and 95% confidence intervals of colorectal polyps according to sugar-sweetened beverage intake during adolescence in the Nurses’ Health Study II, 1998–2015
| Sugar-sweetened beverage intake during adolescence, servings |
Per 1 serving/day increase | |||||
|---|---|---|---|---|---|---|
| <1/wk | 1–6/wk | 1/day | ≥2/day | Ptrend | ||
| Total adenoma | ||||||
| Ncases/Ncontrolsa | 1224/20587 | 1201/22103 | 267/3834 | 161/2300 | ||
| Model 1b | 1 (ref) | 0.91 (0.84–0.99) | 1.19 (1.04–1.37) | 1.19 (1.01–1.42) | .007 | 1.10 (1.03–1.17) |
| Model 2c | 1 (ref) | 0.92 (0.84–1.00) | 1.18 (1.03–1.36) | 1.18 (0.99–1.40) | .012 | 1.09 (1.02–1.17) |
| Model 3d | 1 (ref) | 0.92 (0.84–1.00) | 1.20 (1.03–1.39) | 1.21 (1.00–1.48) | .010 | 1.11 (1.02–1.20) |
| Proximal adenoma | ||||||
| Ncases/Ncontrols | 655/20214 | 630/21732 | 147/3757 | 86/2257 | ||
| Model 3 | 1 (ref) | 0.91 (0.81–1.03) | 1.25 (1.03–1.53) | 1.27 (0.96–1.66) | .023 | 1.13 (1.02–1.26) |
| Distal adenoma | ||||||
| Ncases/Ncontrols | 503/20214 | 508/21732 | 110/3757 | 64/2257 | ||
| Model 3 | 1 (ref) | 0.94 (0.82–1.07) | 1.18 (0.94–1.48) | 1.14 (0.84–1.55) | .21 | 1.08 (0.96–1.22) |
| Rectal adenoma | ||||||
| Ncases/Ncontrols | 188/20214 | 174/21732 | 50/3757 | 33/2257 | ||
| Model 3 | 1 (ref) | 0.88 (0.70–1.09) | 1.50 (1.06–2.12) | 1.68 (1.07–2.63) | .005 | 1.30 (1.08–1.55) |
| Total serrated lesione | ||||||
| Ncases/Ncontrols | 917/20667 | 1095/21921 | 179/3881 | 121/2322 | ||
| Model 3 | 1 (ref) | 1.11 (1.01–1.23) | 1.01 (0.84–1.20) | 1.14 (0.91–1.43) | .35 | 1.04 (0.96–1.14) |
| Small serrated lesione | ||||||
| Ncases/Ncontrols | 780/20667 | 940/21921 | 158/3881 | 100/2322 | ||
| Model 3 | 1 (ref) | 1.14 (1.02–1.26) | 1.07 (0.88–1.29) | 1.14 (0.89–1.45) | .32 | 1.05 (0.96–1.15) |
| Large serrated lesione | ||||||
| Ncases/Ncontrols | 84/20667 | 90/21921 | 11/3881 | 9/2322 | ||
| Model 3 | 1 (ref) | 0.96 (0.70–1.32) | 0.63 (0.32–1.22) | 0.88 (0.41–1.87) | .48 | 0.89 (0.64–1.23) |
| Subtype of polyp | ||||||
| Adenoma only | ||||||
| Ncases/Ncontrols | 1010/19884 | 985/21224 | 222/3700 | 126/2214 | ||
| Model 3 | 1 (ref) | 0.94 (0.85–1.03) | 1.24 (1.05–1.46) | 1.19 (0.95–1.48) | .025 | 1.10 (1.01–1.20) |
| Serrated lesion only | ||||||
| Ncases/Ncontrols | 703/19884 | 879/21224 | 134/3700 | 86/2214 | ||
| Model 3 | 1 (ref) | 1.18 (1.05–1.31) | 1.02 (0.84–1.25) | 1.10 (0.85–1.43) | .55 | 1.03 (0.93–1.14) |
| Both adenoma and serrated lesion | ||||||
| Ncases/Ncontrols | 214/19884 | 216/21224 | 45/3700 | 35/2214 | ||
| Model 3 | 1 (ref) | 0.89 (0.73–1.09) | 1.02 (0.73–1.44) | 1.32 (0.87–2.01) | .18 | 1.13 (0.95–1.34) |
N, number of endoscopies
Due to multiple endoscopies during follow-up per each participant, N is larger than number of participants.
Adjusted for age, time period of endoscopy, number of endoscopies, time since most recent endoscopy, reason for endoscopy (screening/symptoms).
Additionally adjusted for family history of colorectal cancer, menopausal status/menopausal hormone use, current aspirin use ≥2 times/wk, history of type 2 diabetes, adult height, BMI (age 18 y, current), smoking status (adolescent, current), alcohol consumption (age 18–22 y, current), physical activity (adolescent, current).
Additionally adjusted for adolescent and current (adult) dietary intake (total calorie, total calcium, vitamin D, total folate, fiber, fruits, vegetables, and dairy), current total red meat intake, western dietary pattern score during adolescence, and current SSB intake.
Includes hyperplastic polyp, sessile serrated adenoma/polyp, and traditional serrated adenoma; small, <1 cm; large, ≥1 cm.
Results for glucose (from simple sugars), added sugar, and total sugar were similar to the results for total fructose, but effect sizes were slightly smaller than total fructose (Supplementary Table 3). Neither ASB nor fruit juice intake was associated with risk of adenoma (Supplementary Table 4). Contrary to adolescent intake, sugar intake during adulthood was not associated with adenoma risk. After adjustment for adolescent intake, the multivariable ORs for total adenoma were 0.96 (95% CI 0.87–1.06) for adult intake of total fructose (per 5% of calories) and 0.94 (95% CI 0.86–1.03) for SSBs (per 1 serving/day).
Sugar and SSB intake and risk of high-risk adenoma
Higher intake of total fructose during adolescence was positively associated with high-risk adenoma (Table 4). The multivariable ORs of high-risk adenoma were 1.30 (95% CI 1.06–1.60; Ptrend = .012) per 5% of calories from total fructose intake. By subsite, higher fructose intake (per 5% of calories) was borderline significantly associated with increased risk of distal (OR 1.33, 95% CI 1.00–1.78; Ptrend = .052) and rectal (OR 1.47, 95% CI 0.99–2.19; Ptrend = .055) high-risk adenoma. Higher adolescent SSB intake (per 1 serving/day) was significantly associated with rectal high-risk adenoma with the OR of 1.34 (95% CI 1.01–1.79; Ptrend = .044).
Table 4.
Multivariable odds ratios and 95% confidence intervals of low- and high-risk colorectal adenoma according to total fructose and sugar-sweetened beverage intake during adolescence in the Nurses’ Health Study II, 1998–2015
|
Total fructose intake during adolescence, % of calorie |
Per 5% of calorie increase | ||||||
|
Q1 (<7.9) |
Q2 (7.9–<9.2) |
Q3 (9.2–<10.3) |
Q4 (10.3–<11.8) |
Q5 (≥11.8) |
Ptrend | ||
| Low-risk adenomaa | |||||||
| Ncases/Ncontrolsb | 346/9795 | 316/9807 | 342/9765 | 307/9776 | 317/9809 | ||
| Multivariablec | 1 (ref) | 0.95 (0.81–1.12) | 1.07 (0.90–1.26) | 1.00 (0.83–1.20) | 1.07 (0.88–1.30) | .41 | 1.07 (0.92–1.24) |
| High-risk adenomaa | |||||||
| Ncases | 131 | 152 | 149 | 168 | 158 | ||
| Multivariable | 1 (ref) | 1.24 (0.97–1.59) | 1.25 (0.97–1.62) | 1.49 (1.15–1.94) | 1.40 (1.06–1.86) | .012 | 1.30 (1.06–1.60) |
| Proximal | |||||||
| Ncases | 82 | 72 | 75 | 78 | 79 | ||
| Multivariable | 1 (ref) | 0.99 (0.71–1.38) | 1.08 (0.77–1.52) | 1.23 (0.85–1.77) | 1.30 (0.89–1.89) | .11 | 1.28 (0.95–1.72) |
| Distal | |||||||
| Ncases | 62 | 71 | 82 | 85 | 89 | ||
| Multivariable | 1 (ref) | 1.17 (0.82–1.68) | 1.37 (0.95–1.98) | 1.46 (1.02–2.10) | 1.44 (0.97–2.14) | .052 | 1.33 (1.00–1.78) |
| Rectal | |||||||
| Ncases | 26 | 39 | 28 | 49 | 35 | ||
| Multivariable | 1 (ref) | 1.65 (0.99–2.77) | 1.23 (0.70–2.15) | 2.35 (1.38–4.02) | 1.65 (0.93–2.92) | .055 | 1.47 (0.99–2.19) |
|
Sugar-sweetened beverage intake during adolescence, servings |
Per 1 serving/day increase |
||||||
| <1/week | 1–6/week | 1/day | ≥2/day | Ptrend | |||
| Low-risk adenoma | |||||||
| Ncases/Ncontrols | 676/20214 | 687/21732 | 151/3757 | 89/2257 | |||
| Multivariable | 1 (ref) | 0.96 (0.86–1.08) | 1.24 (1.02–1.51) | 1.26 (0.98–1.64) | .022 | 1.13 (1.02–1.25) | |
| High-risk adenoma | |||||||
| Ncases | 322 | 305 | 73 | 42 | |||
| Multivariable | 1 (ref) | 0.85 (0.72–1.01) | 1.19 (0.90–1.56) | 1.08 (0.74–1.59) | .41 | 1.07 (0.92–1.24) | |
| Proximal | |||||||
| Ncases | 171 | 149 | 39 | 21 | |||
| Multivariable | 1 (ref) | 0.80 (0.63–1.03) | 1.23 (0.84–1.80) | 1.05 (0.60–1.83) | .58 | 1.06 (0.85–1.33) | |
| Distal | |||||||
| Ncases | 157 | 163 | 39 | 22 | |||
| Multivariable | 1 (ref) | 0.92 (0.72–1.16) | 1.22 (0.84–1.78) | 1.08 (0.64–1.82) | .53 | 1.07 (0.87–1.31) | |
| Rectal | |||||||
| Ncases | 77 | 61 | 21 | 13 | |||
| Multivariable | 1 (ref) | 0.77 (0.54–1.11) | 1.62 (0.96–2.72) | 1.74 (0.85–3.57) | .044 | 1.34 (1.01–1.79) | |
Low-risk: small (<1 cm) 1–2 tubular adenomas; high-risk: large (≥1 cm), any villous histology, high-grade dysplasia, or more than 3 adenomas.
N, number of endoscopies; due to multiple endoscopies during follow-up per each participant, N is larger than number of participants.
Adjusted for age, time period of endoscopy, number of endoscopies, time since most recent endoscopy, reason for endoscopy, family history of CRC, menopausal status/menopausal hormone use, current aspirin use ≥2 times/wk, history of type 2 diabetes, adult height, BMI (age 18 y, current), smoking status (adolescent, current), alcohol consumption (age 18–22 y, current), physical activity (adolescent, current), adolescent and current dietary intake (total calorie, total calcium, vitamin D, total folate, fiber, fruits, vegetables, and dairy), current total red meat intake, western dietary pattern score during adolescence, and current total fructose or SSB intake.
Similar (but somewhat weaker) results were found for glucose (from simple sugars), added sugar, and total sugar (Supplementary Table 3). Sugar and SSB intake during adulthood was not associated with high-risk adenoma with multivariable ORs of 0.97 (95% CI 0.80–1.17) for total fructose (per 5% of calories) and 0.99 (95% CI 0.84–1.16) for SSBs (per 1 serving/day) after adjustment for adolescent intake.
Risk of adenoma by age at diagnosis
We stratified adenoma cases into 3 groups by age at diagnosis: <50, 50–54, and ≥55 years (Supplementary Table 5). Per each increment of 5% of calories, total fructose intake during adolescence was positively associated with risk of total adenoma diagnosed <50 years (Ptrend = .07) and 50–54 years (Ptrend = .02), but no association was found for adenoma diagnosed ≥55 years (Ptrend = .42). In particular, adolescent total fructose intake was significantly associated with increased risk of rectal adenoma diagnosed <50 and 50–54 years (Ptrend ≤ .04) and high-risk adenoma diagnosed <50 years (Ptrend = .004). For adolescent SSB intake, similar, albeit weaker, positive associations were observed with rectal adenoma diagnosed <50 and 50–54 years (per 1 serving/day, Ptrend ≤ .07).
Sensitivity analysis
Overall, sensitivity analysis results were consistent with the principal findings (Supplementary Tables 6–8). In brief, similar results were obtained after further adjustment for ASB and fruit juice intake or prudent dietary pattern, use of energy-adjusted sugar intake (instead of nutrient densities), and restricting analyses to individuals who underwent colonoscopy. When the omitted responses to SSB items (n = 666) on the HS-FFQ were set to zero or estimated intake from regression,42 associations were essentially unchanged (data not presented). We conducted further analyses for serrated lesions in the proximal colon (n = 802) and large (≥1 cm) proximal serrated lesions (n = 145), and found no significant association (Supplementary Table 9).
After additional adjustment for glycemic index and glycemic load as potential mediators, positive associations were substantially attenuated, especially after adjustment for glycemic load: per each increment of 5% of calories from total fructose, ORs were 1.08 (95% CI 0.94–1.25) for total and 1.21 (95% CI 0.94–1.55) for high-risk adenoma (Supplementary Tables 6).
Stratified analysis
Associations of fructose and SSB intake with adenoma risk did not differ appreciably by family history of CRC, birth year, adolescent BMI, physical activity, smoking, or alcohol consumption (all Pinteraction ≥ .15; Figure 1, Supplementary Table 10). Positive associations between sugar intake and adenoma risk were significantly stronger among women with low fruit intake (<1.3 servings/day) during adolescence than women with high intake (≥1.3 servings/day). Among those with low fruit intake, ORs of total adenoma were 1.51 (95% CI 1.26–1.82; Pinteraction < .001) for total fructose (highest vs lowest quintile) and 1.34 (95% CI 1.12–1.60; Pinteraction = .028) for SSBs (≥1 serving/day vs <1 serving/week). Similar differential associations were observed after stratification by vegetable and fiber intake and prudent dietary pattern. In contrast, positive associations with adenoma risk did not differ appreciably by fruit juice intake (Pinteraction ≥ .75). By joint categories of fruit (high/low) and fruit juice (high/low) intake, positive associations were strongest in the ‘low fruit/high fruit juice’ subgroup, with significant differences across subgroups (Pinteraction ≤ .017; Figure 1). Stratified analysis results for high-risk adenoma were similar to those for total adenoma (Supplementary Table 11).
Figure 1. Risk of total adenoma according to (A) total fructose and (B) sugar-sweetened beverage intake during adolescence by lifestyle and dietary factors in the Nurses’ Health Study II, 1998–2015.
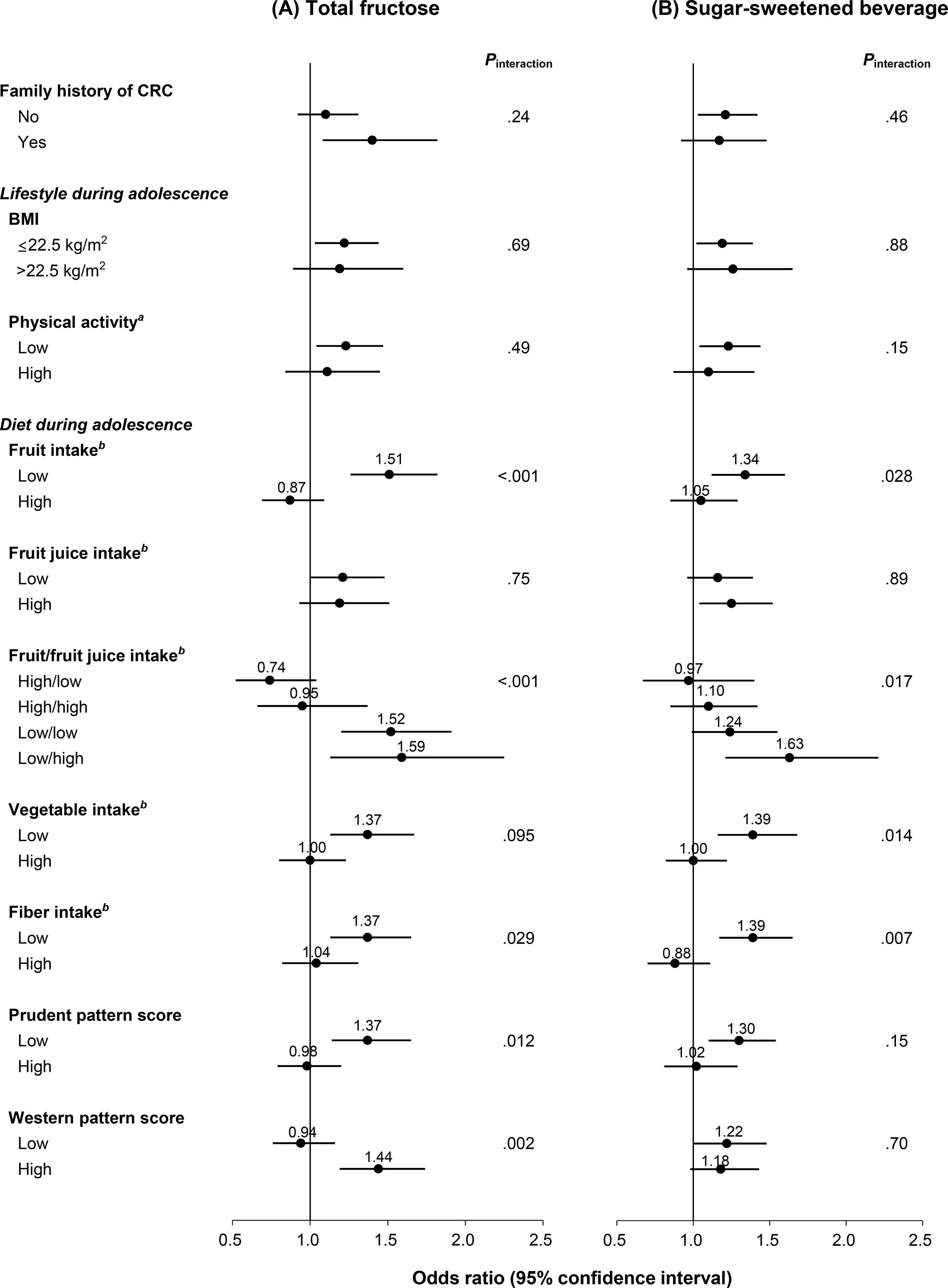
CRC, colorectal cancer; BMI, body mass index
Data were adjusted for age, time period of endoscopy, number of endoscopies, time since most recent endoscopy, reason for endoscopy, family history of CRC, menopausal status/menopausal hormone use, current aspirin use ≥2 times/wk, history of type 2 diabetes, adult height, BMI (age 18 y, current), smoking status (adolescent, current), alcohol consumption (age 18–22 y, current), physical activity (adolescent, current), adolescent and current (adult) dietary intake (total calorie, total calcium, vitamin D, total folate, fiber, fruits, vegetables, and dairy), current total red meat intake, western dietary pattern score during adolescence, and current total fructose or sugar-sweetened beverage intake except for the stratifying variable of each stratum.
(A) highest vs lowest (referent) quintile.
(B) ≥1 serving/d vs <1 serving/wk (referent).
aHigh: highest tertile (≥59 MET-hr/wk); low: two lower tertiles (<59 MET-hr/wk).
bCut-off: median intake (fruits, 1.3 serving/d; fruit juice, 0.4 serving/d; vegetables, 2.8 serving/d; fiber, 20.2 g/d).
Joint analysis of adolescent and adult diet
Compared with women with low fructose or SSB intake during both adolescence and adulthood, women with high intake during adolescence had increased risk of total, rectal, and high-risk adenoma (Supplementary Table 12, Supplementary Figure 1). Associations did not differ significantly between the ‘high adolescent/low adult intake’ and ‘high adolescent/high adult intake’ groups. However, these results should be cautiously interpreted given higher added sugar and calorie intake during adolescence and differences in nutritional/caloric requirements between adolescence and adulthood.
Substitution analysis
The 2020–2025 DGA recommend 2 cup-equivalents of fruits (whole fruits and 100% fruit juice) at the 2000-calorie level and 2–3 cup-equivalents of dairy per day for children and adolescents.31 Substituting 1 serving/day of fruit juice for 1 serving/day of SSBs during adolescence was not associated with lower risk of adenoma (Supplementary Table 13). In contrast, replacement with 2 servings/day of fruits for 2 servings/day of SSBs was marginally associated with reduced risk of proximal (OR 0.75, 95% CI 0.54–1.05) and rectal (OR 0.61, 95% CI 0.35–1.07) adenoma. Substituting 2 servings/day of dairy products for 2 servings/day of SSBs was significantly associated with lower risk of rectal adenoma (OR 0.53, 95% CI 0.30–0.94).
DISCUSSION
In this large cohort of young women, high intake of simple sugars, especially fructose, and SSBs during adolescence was significantly associated with increased risk of colorectal adenoma, particularly rectal adenoma. Results were similar, albeit slightly weaker, for glucose, added sugar, and total sugar. Neither sugar nor SSB intake was associated with risk of serrated lesions. Thus, high sugar intake during adolescence may be etiologically more important for CRC arising from the conventional adenoma-carcinoma sequence, which accounts for approximately 85% of CRC,22 rather than the serrated neoplasia pathway.
To our knowledge, no previous study has investigated the association of adolescent sugar intake with risk of CRC precursors. Previous studies on adult sugar and SSB intake in relation to CRC risk have generally found null associations, including 2 comprehensive pooled analyses of prospective studies as well as a recent large cohort study.18,19,43 In 2018, the World Cancer Research Fund/American Institute for Cancer Research reported that evidence was limited for sugars and foods containing sugars with regard to CRC risk.15,19 However, this conclusion was based on intake during adulthood, mostly capturing mid-to-late adulthood cases. Consistent with previous studies, we observed that adult sugar and SSB intake was not associated with adenoma risk. One possible explanation for the differential associations between adolescent vs adult sugar intake is that adolescence may be a critical developmental period of enhanced susceptibility to the adverse effects of high sugar intake. During adolescence, accompanied by growth and accelerated cell proliferation, distinctive hormonal and metabolic changes occur, including physiological (obesity-unrelated) hyperinsulinemia, decreased insulin sensitivity, and elevated IGF1 levels (up to 4-fold higher than in adulthood).21 Therefore, adolescents may be particularly susceptible to high sugar intake that can further decrease insulin sensitivity.
Several biological mechanisms may explain our findings. First, hyperinsulinemia and insulin resistance may play important roles. The high amount of liquid sugar in SSBs can induce rapid spikes in blood glucose and insulin levels, which over time lead to insulin resistance and elevated free IGF1 levels.12 The insulin/IGF1 system can promote carcinogenesis by activating intracellular signaling pathways related to altered gene expression, stimulating cell proliferation, differentiation, and angiogenesis, and inhibiting apoptosis.3,44 We found that additional adjustment for dietary glycemic load substantially attenuated positive associations of high sugar intake, supporting this hypothesis.
Second, hyperglycemia may exacerbate chronic inflammation that has been implicated in CRC pathogenesis.45 Previous studies have reported that SSB intake was significantly associated with increased circulating inflammatory cytokines and biomarkers (e.g., C-reactive protein, interleukin-6, tumor necrosis factor receptors).14,46
Third, the distinctive metabolism of fructose, a major ingredient of SSBs, can exert additional adverse effects. Unlike glucose, fructose is metabolized predominantly in the liver after absorption in the small intestine.12 When fructose intake chronically exceeds the metabolic capacity of the liver, fructose triggers hepatic de novo lipogenesis, promoting visceral and ectopic fat accumulation, glucose intolerance, and insulin resistance.7 In a recent animal study, fructose was metabolized into glucose in murine small intestine as well, and intestinal fructose-to-glucose conversion was not suppressed by insulin, suggestive of a novel unregulated pathway.47
Furthermore, fructose may affect carcinogenesis by directly acting on colorectal cells or interacting with the gut microbiome. Although fructose is readily absorbed in the small intestine, high doses or rapid flux of fructose could saturate small intestine clearance capacity, with excess fructose reaching the colon.47,48 An 8-week oral administration of high-fructose corn syrup in mice enhanced colorectal tumor cell growth, even at a moderate dose, in the absence of obesity and metabolic syndrome, suggesting direct effects of fructose on tumor cell metabolism.49 Moreover, sugars may change the gut microbiome composition,50,51 which could affect CRC development through modulation of gut immune and metabolic responses and epigenetic alterations.52,53
In stratified analyses, positive associations of high sugar intake were significantly stronger among women with low fruit, vegetable, or fiber intake during adolescence than those with high intake. However, fruit juice intake did not offset the adverse effects of high sugar intake, and substituting fruit juice for SSBs showed no benefits. These results may be explained as follows: although fruits and some vegetables contain naturally occurring sugars,27 many beneficial micronutrients and potential anti-tumorigenic agents (e.g., fiber, folate, vitamins) may offset or dilute the adverse effects of sugars.15 Moreover, whole fruits and fruit juice have different intestinal fructose release rates.47 Fructose in whole fruits is slowly digested due to the fiber content and the need to disrupt cell structure, facilitating gradual and complete intestinal clearance.12 In contrast, rapid flux of liquid fructose from fruit juice may exceed small intestine uptake capacity, resulting in fructose overflow to the liver and colon.12,47,48 We also found stronger associations among women with unhealthy (low prudent and high western) dietary patterns during adolescence than those with healthy patterns. Thus, excessive sugar intake may promote colorectal carcinogenesis particularly when combined with overall unhealthy dietary patterns by further exacerbating underlying chronic insulin resistance.54
If confirmed, our findings may have substantial public health implications for the prevention of CRC. The rising incidence of sporadic CRC among younger adults has been primarily driven by a disproportional increase in distal and rectal cancers.3,4 In our results, positive associations were stronger for distally-located adenoma, especially rectal adenoma. Simple sugar intake during adolescence was more strongly associated with adenoma diagnosed <55 years, further supporting the link between early-life diet and earlier initiation of colorectal carcinogenesis. In recent decades, the global SSB consumption among youths has markedly increased.11–13 In the U.S., 65.4% of adolescents consumed SSBs on a given day in 2013–2014,55 and 72% of male and 76% of female adolescents exceeded the DGA limit of added sugar intake (<10% of total calorie) in 2013–2016.31 Therefore, if applied to the current general population, the impact of high sugar intake may be even larger than observed in our results.
This study has several strengths. To our knowledge, this is the first prospective study investigating the role of high sugar intake during adolescence in risk of colorectal polyps. Dietary data were collected before endoscopic procedures and polyp diagnoses, thus minimizing the potential of recall bias. The large sample size of 33,106 women and 4,744 polyp cases enabled assessment by subtypes and subsites, and stratified analyses with sufficient power. Diet and lifestyle information was validated and obtained throughout different life stages, enabling us to examine both independent and joint associations of adolescent and adult diet. We comprehensively updated information on and adjusted for most of the established CRC risk factors during both adolescence and adulthood. In rigorous sensitivity analyses, the principal results were robust.
Potential limitations of this study need to be considered. First, substantial measurement error is likely in adult recall of adolescent diet. However, the HS-FFQ showed reasonable reproducibility and validity,24,25 supporting the ability to rank individuals adequately. Recalled adolescent diet was only weakly correlated with current diet.24,25 Although recall time period varied between participants (16–35 years later), a previous study showed that adult age was not related to the reproducibility of recalled diet during high school, a distinct time of life.56 In addition, given the prospective design, any measurement error in exposure assessment should be non-differential, which generally attenuates risk estimates towards the null association.24 Second, residual and unmeasured confounding could exist. High sugar intake could be a marker for generally unhealthy diet and lifestyle that might track throughout life. However, we controlled for numerous dietary and lifestyle factors as well as overall dietary patterns during both adolescence and adulthood. Third, we did not have sufficient information to distinguish hyperplastic polyps from sessile serrated adenoma/polyp and traditional serrated adenoma because diagnostic criteria for serrated lesions have changed over time. All endoscopies in this study were performed when standardized diagnostic criteria for serrated lesions were not routinely applied by pathologists.36 Finally, the study population consisted of predominantly white female nurses, and thus results may not be generalizable to other populations. However, secular trends in CRC incidence are similar by sex, and incidence rates under 45 years are comparable between men and women in the U.S.,6 reflecting shared main drivers. In addition, exposure-CRC associations in our cohorts have been highly consistent with findings in diverse populations,15,38,57 suggesting a common underlying biology.
In conclusion, high intake of simple sugars and SSBs during adolescence was significantly associated with increased risk of total and high-risk adenoma, especially rectal adenoma. Given the profound increase in added sugar and SSB intake during the past several decades, our findings may partly explain the current upward trends in early-onset CRC rates. Further prospective studies using valid information on early-life diet in other populations are warranted to confirm our findings.
Supplementary Material
Acknowledgments
The authors would like to thank the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School.
The authors would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA and WY.
The authors assume full responsibility for analyses and interpretation of these data.
Funding
The Nurses’ Health Study II was funded by the National Cancer Institute, National Institutes of Health (U01 CA176726, R01 CA67262, and U01 HL145386) and this project was funded by research grants R03 CA197879 (Wu), R21 CA222940 (Kana Wu and Marios Giannakis), R21 CA230873 (Wu, Ogino), R01 CA151993 (Ogino), R35 CA197735 (Ogino), R01 CA205406 (Ng), K24 DK098311 (Chan), R35 CA253185 (Chan), K99 CA215314 (Song), R00 CA215314 (Song), and K07 CA188126 (Zhang). This work was also in part supported by an Investigator Initiated Grant from the American Institute for Cancer Research (AICR) to Dr. Wu. In addition, this work was supported by American Cancer Society Research Scholar Grant (RSG130476 to Zhang and RSG-20–124-01-CCE to Tabung), the American Cancer Society Research Mentored Research Scholar Grant (MRSG-17–220-01 to Song), and Department of Defense (CA160344 to Ng).
Role of the funding sources
The funders had no role in the study design; in the collection, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. All authors confirm the independence of researchers from funders and have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form and declare support from the National Institutes of Health and American Cancer Society for the submitted work.
Dr. Ng has received institutional research funding from Pharmavite, Revolution Medicines, and Evergrande Group, has served on an advisory board for Seattle Genetics and Array Biopharma, and served as a consultant to X-Biotix Therapeutics.
Dr. Meyerhardt has received institutional research funding from Boston Biomedical, has served as an advisor/consultant to Ignyta and COTA Healthcare, and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical.
Dr. Chan has previously served as a consultant to Bayer Pharma AG, Pfizer Inc., and Boehringer Ingelheim for topics unrelated to this manuscript.
Dr. Fuchs reports consulting role for Agios, Amylin Pharmaceuticals, Bain Capital, CytomX Therapeutics, Daiichi-Sankyo, Eli Lilly, Entrinsic Health, Evolveimmune Therapeutics, Genentech, Merck, Taiho, and Unum Therapeutics. He also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. He is a co-Founder of Evolveimmune Therapeutics and has equity in this private company. He had provided expert testimony for Amylin Pharmaceuticals and Eli Lilly.
The remaining authors report no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Abbreviations
- ASB
Artificially sweetened beverages
- BMI
body mass index
- CI
confidence interval
- CRC
colorectal cancer
- DGA
Dietary Guidelines for Americans
- HPFS
Health Professionals Follow-Up Study
- HS-FFQ
high school food frequency questionnaire
- NHSII
Nurses’ Health Study II
- OR
odds ratio
- SSB
sugar-sweetened beverage
- U.S.
United States
Footnotes
Ethical approval
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Return of the mailed questionnaire was considered to imply informed consent.
References
- 1.Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–691. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Jakubowski CD, Fedewa SA, et al. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book 2020;40:1–14. [DOI] [PubMed] [Google Scholar]
- 3.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16:713–732. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145–164. [DOI] [PubMed] [Google Scholar]
- 5.SEER Cancer Stat Facts: CRC National Cancer Institute. Bethesda, MD. Available at seer.cancer.gov/statfacts/html/colorect.html [Google Scholar]
- 6.American Cancer Society. Colorectal Cancer Facts & Figures 2020–2022. Atlanta: American Cancer Society; 2020. [Google Scholar]
- 7.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 8.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228s–1235s. [DOI] [PubMed] [Google Scholar]
- 9.Healthy Food America. Sugary Drinks in America: Who’s Drinking What and How Much? Available at healthyfoodamerica.org/sugary_drinks_in_america_who_s_drinking_what_and_how_much [Google Scholar]
- 10.Cavadini C, Siega-Riz AM, Popkin BM. US adolescent food intake trends from 1965 to 1996. West J Med 2000;173:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosinger A, Herrick K, Gahche J, et al. Sugar-sweetened Beverage Consumption Among U.S. Youth, 2011–2014. NCHS Data Brief 2017:1–8. [PubMed] [Google Scholar]
- 12.Malik VS, Hu FB. Sugar-Sweetened Beverages and Cardiometabolic Health: An Update of the Evidence. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Bovet P, Liu Y, et al. Consumption of Carbonated Soft Drinks Among Young Adolescents Aged 12 to 15 Years in 53 Low- and Middle-Income Countries. Am J Public Health 2017;107:1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Z, Ley SH, Sun Q, et al. Cross-sectional association between sugar-sweetened beverage intake and cardiometabolic biomarkers in US women. Br J Nutr 2018;119:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018. Available at dietandcancerreport.org, 2018.
- 16.Giovannucci E Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001;131:3109s–20s. [DOI] [PubMed] [Google Scholar]
- 17.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 2000;92:1592–600. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Albanes D, Beeson WL, et al. Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: pooled analysis of prospective cohort studies. J Natl Cancer Inst 2010;102:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imperial College London Continuous Update Project. World Cancer Research Fund International Systematic Literature Review: The Associations between Food, Nutrition and Physical Activity and the Risk of Colorectal Cancer, 2017. Available at wcrf.org/sites/default/files/colorectal-cancer-slr.pdf (accessed July 15, 2020) [Google Scholar]
- 20.Nimptsch K, Wu K. Is Timing Important? The Role of Diet and Lifestyle during Early Life on Colorectal Neoplasia. Curr Colorectal Cancer Rep 2018;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 2006;60:759–63. [DOI] [PubMed] [Google Scholar]
- 22.Strum WB. Colorectal Adenomas. N Engl J Med 2016;374:1065–75. [DOI] [PubMed] [Google Scholar]
- 23.Bao Y, Bertoia ML, Lenart EB, et al. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am J Public Health 2016;106:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruti SS, Feskanich D, Colditz GA, et al. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. American journal of epidemiology 2005;161:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruti SS, Feskanich D, Rockett HR, et al. Validation of adolescent diet recalled by adults. Epidemiology (Cambridge, Mass.) 2006;17:226–229. [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–20. [DOI] [PubMed] [Google Scholar]
- 28.US Department of Agriculture. Composition of foods: raw, processed, and prepared, 1963–1980. (Agricultural handbook no. 8). Washington, DC: US Department of Agriculture, 1980: [Google Scholar]
- 29.Willett W Nutritional Epidemiology. New York: Oxford University Press USA, 2013. [Google Scholar]
- 30.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at DietaryGuidelines.gov. [Google Scholar]
- 32.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 33.He X, Hang D, Wu K, et al. Long-term Risk of Colorectal Cancer After Removal of Conventional Adenomas and Serrated Polyps. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327–34. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158:1131–1153.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill P, Wang LM, Bailey A, et al. Reporting trends of right-sided hyperplastic and sessile serrated polyps in a large teaching hospital over a 4-year period (2009–2012). J Clin Pathol 2013;66:655–8. [DOI] [PubMed] [Google Scholar]
- 37.Maruti SS, Willett WC, Feskanich D, et al. A prospective study of age-specific physical activity and premenopausal breast cancer. J Natl Cancer Inst 2008;100:728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezende LFM, Lee DH, Keum N, et al. Physical activity during adolescence and risk of colorectal adenoma later in life: results from the Nurses’ Health Study II. Br J Cancer 2019;121:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–81. [DOI] [PubMed] [Google Scholar]
- 40.Andersen PK, Gill RD. Cox’s Regression Model for Counting Processes: A Large Sample Study. Ann Statist 1982;10(4):1100–1120. [Google Scholar]
- 41.Bernstein AM, de Koning L, Flint AJ, et al. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr 2012;95:1190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michels KB, Willett WC. Self-administered semiquantitative food frequency questionnaires: patterns, predictors, and interpretation of omitted items. Epidemiology 2009;20:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pacheco LS, Anderson CAM, Lacey JV Jr., et al. Sugar-sweetened beverages and colorectal cancer risk in the California Teachers Study. PLoS One 2019;14:e0223638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vigneri PG, Tirrò E, Pennisi MS, et al. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front Oncol 2015;5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Cai Q, Li H, et al. Circulating C-reactive protein and colorectal cancer risk: a report from the Shanghai Men’s Health Study. Carcinogenesis 2013;34:2799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Koning L, Malik VS, Kellogg MD, et al. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735–41, s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang C, Hui S, Lu W, et al. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab 2018;27:351–361.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao S, Jang C, Liu J, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020;579:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019;363:1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Do MH, Lee E, Oh MJ, et al. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Rienzi SC, Britton RA. Adaptation of the Gut Microbiota to Modern Dietary Sugars and Sweeteners. Adv Nutr 2020;11:616–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 2019;16:690–704. [DOI] [PubMed] [Google Scholar]
- 53.Song M, Chan AT, Sun J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020;158:322–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giovannucci E A framework to understand diet, physical activity, body weight, and cancer risk. Cancer Causes Control 2018;29:1–6. [DOI] [PubMed] [Google Scholar]
- 55.Bleich SN, Vercammen KA, Koma JW, et al. Trends in Beverage Consumption Among Children and Adults, 2003–2014. Obesity (Silver Spring) 2018;26:432–441. [DOI] [PubMed] [Google Scholar]
- 56.Frazier AL, Willett WC, Colditz GA. Reproducibility of recall of adolescent diet: Nurses’ Health Study (United States). Cancer causes & control : CCC 1995;6:499–506. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Keum N, Wu K, et al. Calcium intake and colorectal cancer risk: Results from the nurses’ health study and health professionals follow-up study. Int J Cancer 2016;139:2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


